On Jan 23, 2020 (day 1 of infection), a 38-year-old man developed a cough and dyspnoea, followed by fever and muscle aches. On day 13, he attended the outpatient department of the Union Hospital, Wuhan. A chest CT scan showed multiple ground-glass densities (appendix p 1), a blood test showed normal platelet count (196 × 109 cells per L), and the COVID-19 nucleic acid test was positive. He was diagnosed with moderate COVID-19 and admitted to hospital on day 20. All other laboratory tests were within the normal reference range (except for increased concentration of fibrinogen at admission [5·37 g/L, reference range 2·0–4·0], appendix p 2). After admission, he was given antiviral treatment of interferon-α (5 million units twice daily for 9 days, aerosol inhalation) and umifenovir (0·2 g three times daily for 7 days, orally) according to the COVID-19 diagnosis and treatment plan (trial sixth edition) issued by the National Health Commission of China. The patient's symptoms improved, and the pulmonary lesions appeared to have been absorbed after one week (appendix p 1). When the COVID-19 nucleic acid test showed negative twice (day 27 and day 28), he met the discharge criteria.
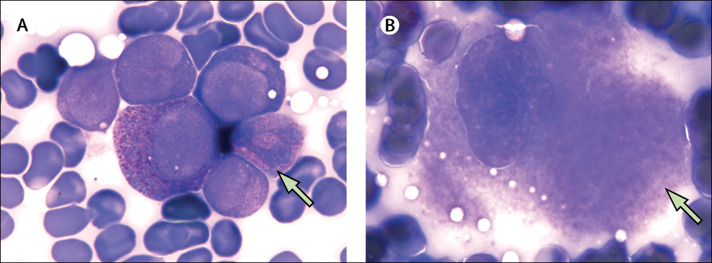
However, on day 29 a blood test showed a decreased platelet count of 2 × 109 cells per L, and fibrinogen concentration remained elevated (4·40 g/L), while other markers were within the normal range (appendix p 2). These results showed a clear, isolated thrombocytopenia. Lymphocyte subset and autoimmune antibody analysis at platelet nadir time showed an increase of the percentage of B cells, from 18·62% on day 21 to 34·80% on day 29 (reference range 4·10–18·31%, appendix p 2) and autoimmune antibodies were negative. Bone marrow aspiration was done (for biosecurity reasons, bone marrow smears were fumigated with formaldehyde for 6 h before Wright's staining); most cellular lineages were normal except for low numbers of platelet-producing megakaryocytes (figure , appendix p 3). A COVID-19 nucleic acid test of the bone marrow aspirate was negative. There were no signs of bleeding or thrombosis during hospital admission or during the time of platelet nadir, and he had never been admitted to the intensive care unit. Our differential diagnosis included complications of acute COVID-19 infection and post-infectious immune thrombocytopenia.
Figure.
Wright's stained bone marrow aspirate smear
(A) Green arrow, a group of immature neutrophils (× 1000). (B) Green arrow, a granular megakaryocyte (× 1000).
We administered intravenous immunoglobulin (400 mg/kg daily) and dexamethasone (10 mg daily). The patient's platelet count increased to 60 × 109 cells per L 3 days later (day 33) and dexamethasone was stopped in case of re-activation of viral replication. The platelet count was normal on day 37, so immunoglobulin was ceased. After the time of platelet nadir (day 29), we monitored the patient for 2 weeks; his platelet count remained in the normal range and B-cell percentage gradually decreased (16·47% on day 45, appendix p 2). The patient recovered and was discharged on day 46, 4 days after the last chest CT scan (day 42, appendix p 1).
Thrombocytopenia has been shown to occur in patients with COVID-19, usually noted on admission to hospital, although here thrombocytopenia occurred later in the disease course. The effectiveness of immunoregulatory treatment, the changing concentrations of B lymphocytes, and the results of the bone marrow aspirate, suggest immune-mediated thrombocytopenia in this patient, and the normal prothombin time and activated partial thromboplastin time suggest that other coagulation abnormalities were not the cause of the severe thrombocytopenia. Post-viral immune thrombocytopenia arising after infection with a range of pathogens, including severe acute respiratory syndrome coronavirus (SARS-CoV), influenza, and Zika virus, has been described in previous case reports (appendix p 4). However, the findings that we present here should be interpreted cautiously. Furthermore, we could not rule out the possibility of drug-induced thrombocytopenia, or increased platelet destruction with or without decreased platelet genesis in the damaged lung tissue, as the lungs also contribute to platelet biogenesis.
Contributors
WC, BY, and ZL drafted and revised the submitted article and contributed equally. BY was the health-care provider of the patient. PW and YC provided constructive criticisms and suggestions. HZ was involved in preparation of the manuscript and revision of the submitted article. This study was approved by the Ethics Committee of the Union hospital, Tongji Medical College, Huazhong University of Science and Technology (No. 2020-0079-1). Written informed consent to publication was obtained.
Declaration of interests
We declare no competing interests.
Supplementary Material
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.