Abstract
Occupational exposure to pesticides leads to the development of cancer. Aberrant DNA methylation plays a crucial role in cancer. The manifestation of the carcinogenic effect of pesticides could be determined by the variation of genes encoding enzyme, including PON1 Q192R and GSTM1. The goal of this study was to find out polymorphism of PON1 Q192R and methylation of p16 gene promoter, and their correlation on Javanese farmers in the agricultural area of Ngablak Subdistrict, Magelang Regency, Central Java. Seventy-eight pesticide-exposed farmers enrolled in the study. Polymorphism of PON Q192R was determined using PCR-RFLP and variation of GSTM1 was examined using conventional PCR. The methylation of the p16 gene promoter was determined using methylation-specific PCR. The result revealed 94.9% polymorphism of PON1 Q192R, which was higher in the R/R (Arg/Arg) genotypes than Q/R (Gln/Arg) and lowest in Q/Q (Gln/Gln) genotypes. We also found 82.1% GSTM1 null genotype among the farmers enrolled in the study. As many as 26.9% methylations of p16 gene promoter were found among farmers. Genetic variation of PON1 Q192R and GSTM1 were not found to be correlated to the methylation status of p16 gene promoter in the Javanese population.
Keywords: Environment, Genetics, Environmental pollution, Environmental toxicology, Public health, Pharmacology, Toxicology, Methylation of p16 gene promoter, Polymorphism of PON1 Q192R, GSTM1 pesticides, Cancer
Environment; Genetics; Environmental Pollution; Environmental Toxicology; Public Health; Pharmacology; Toxicology; Methylation of p16 gene promoter; Polymorphism of PON1 Q192R; GSTM1 Pesticides; cancer
1. Introduction
Cancer is one of the health problems caused by pesticide exposure. Investigators from Agricultural Health Study (AHS) found association between prostate cancer and chlorpyrifos (Lee et al., 2007), lung cancer and diazinon, chlorpyrifos, pendimethalin, metolachlor (Alavanja et al., 2004), dieldrin, parathion, and ethyl chlorimuron (Bonner et al., 2017), along with leukemia and S-ethyl-N,N-dipropylthioarbamate (van Bemmel et al., 2008). Other pesticides were also related to non-Hodgkin lymphoma (NHL), including hexachlorocyclohexane, chlordane, hexachlorobenzene, dichlorodiphenyldichloroethylene (DDE) (Luo et al., 2016), phenoxy acid, and glyphosate (Eriksson et al., 2008).
Genotoxic effects of pesticide exposure were associated with enzymes which metabolize pesticides, i.e. PON1 Q192R (Singh et al., 2011b) and GSTM1 (Singh et al., 2011a). Pesticides are mainly metabolized by various P450 cytochrome enzymes. However, other enzymes also have some roles in their metabolism, including paraoxonase (PON) and glutathione S-transferases (GSTs). PON1 gene in human shows polymorphism in 2 coding regions (Q192R and L55M), promoter region, and 3′-UTR region (Hashemi et al., 2010). Polymorphism also has been found in the GSTM1 gene (Singh et al., 2011a). Genetic variation in GSTs, GSTM1 null genotype, may diminish enzyme activity. Alteration in enzyme activity leads to the risk of cancer development.
Cancer occurs due to uncontrolled cellular proliferation. One of two specific classes of genes that is important as regulatory genes is tumor suppressor genes (TSGs). Tumor suppressor genes protect the integrity of the genome by inhibiting the cell cycle when substantial errors or mutations have occurred and are usually downregulated or inactivated in cancer cells (Vakonaki et al., 2013). In cancer, TSGs are methylated. Gene promoter hypermethylation in CpG islands area suppresses gene expression causes gene silencing and initiates tumor development (Ku et al., 2011). One of the most frequent and earliest TSG which epigenetically loses tumor suppressor function is p16. Silencing of the p16 gene is found in the pre-invasive stadium of breast cancer, colon cancer, lung cancer, and many others (Jones and Baylin, 2007).
DNA methylation is one of the most important epigenetic events in the mammalian genome. DNA methylation is a chemical modification that adds a methyl (CH3) group at the carbon 5 position of the cytosine ring of CpG dinucleotide (Das and Singal, 2004; Ku et al., 2011). This reaction is catalyzed by the DNA methyltransferase enzymes (DNMTs) that transfer the S-adenosyl-L-methionine (AdoMet) as methyl donor forming 5-methylcytosine (5-mC). DNA methylation does not alter the DNA sequence, thus it does not alter information coded by the DNA, but it has a role in modulating gene expression. In the human genome, CpGs dinucleotides are not randomly distributed but are located in CpG islands. CpG islands are generally located in the gene promoter and become the targets of methylation in cancers (Moison et al., 2014).
DNA methylation of specific genes is useful as a cancer biomarker, for example, to identify individuals at increased risk of developing cancer or for the screening of asymptomatic individuals, thus facilitating early diagnosis of cancer. DNA methylation is relatively stable compared to other biomarkers and the materials for examination are easy to derive so that DNA methylation is suggested as cancer biomarker (Martens et al., 2009).
Therefore, we conducted a cross-sectional study in a subdistrict with the vast agricultural area at Magelang Regency, Central Java, to examine the genetic effect of pesticides in farmers and to find out whether polymorphism of PON1 Q192R and variation of GSTM1 correlated with methylation of p16 gene promoter.
2. Materials and methods
2.1. Study population and sampling
This cross-sectional study was done during the year 2018. The study was conducted after it had been approved by the ethical committee of Faculty of Medicine, Public Health, and Nursing Universitas Gadjah Mada Yogyakarta, Indonesia (document number is KE/FK/0424/EC/2018). The DNA samples and interview data were from farmers aged more than 18 years old, both man and woman. Those who used pesticides less than 3 months were excluded.
2.2. Pesticide exposure data
Pesticide exposure data was obtained from an interview based on a questionnaire prepared before the interview. The questionnaire includes details about the subject's demographic factors, pesticide exposure, and medical history. The questionnaire also includes types of pesticides used, use of personal protective equipment, pesticide application methods, pesticide mixing status, equipment repair status, time/period of pesticide application, frequency of pesticide application per week, duration of pesticide application, last pesticide spraying, dosage of pesticide used, pesticide spraying direction, and washing hands and taking bath after spraying pesticide. All participants who completed the interview were physically examined.
2.3. Data processing
Data from the interview was used to estimate the exposure intensity using an algorithm arranged by Dosemeci et al. (2002).
| Exposure intensity score = ([MIX] + [APPLY] + [REPAIR]) × [PPE] |
MIX — mixing status.
APPLY — application method.
REPAIR — repair status.
PPE — personal protective equipment use.
2.4. Sample collection
Blood sample (5 mL) was collected from each subject. The samples were transferred into EDTA vacutainers for DNA extraction. All samples were coded and brought to the laboratory under cold conditions, and stored in -20 °C before use.
2.5. DNA extraction
The DNA was extracted from whole blood using FavorPrep Genomic DNA Mini Kit (Favorgen Biotech Corp., Ping-Tung, Taiwan) according to the manufacturer's protocol. Concentration and purity DNA were measured by NanoDrop spectrophotometer. The extracted DNA was stored at -20 °C until further analysis.
2.6. Polymorphism of PON1 Q192R
Polymorphism of PON1 Q192R was determined using PCR-RFLP. The PCR amplification was carried out with 12.5 μL GoTaq® Green Master Mix (Promega Corp., Madison, U.S.A.), 1 μL 20 mM forward primer, 1 μL 20 mM reverse primer, 10 ng of genomic DNA, and volume adjusted with nuclease-free water, in a total volume of 25 μL. The amplification followed cycling parameters: 95 °C for 6 min; 35 cycles of 95 °C for 1 min, specific annealing temperature (Table 1) for 1 min, 72 °C for 45 s, and a final extension at 72 °C for 4 min. PCR products were digested with aLW1 and incubated at 37 °C for 15 min. The digestion result showed 175 and 63 bp fragments for Arg/Arg mutant allele, in an undigested 238 bp fragment for Gln/Gln allele, and all 238, 175, and 63 fragments for Gln/Arg heterozygous allele.
Table 1.
Specific primer pairs used for PCR analysis and P16 gene promoter methylation detection.
| Primer | Forward | Reverse | Annealing temperature | Amplicon (bp) |
|---|---|---|---|---|
| PON1 Q192R | TATTGTTGCTGTGGGACCTGAG | CCTGAGAATCTGAGTAAATCCACT | 60 °C | 238 |
| GSTM1 | GAACTCCCTGAAAAGCTAAAGC | GTTGGGCTCAAATATACGGTGG | 60 °C | 219 |
| β-globin | CAACTTCATCCACGTTCACC | GAAGAGCCAAGGACAGGTAC | 60 °C | |
| p16-M | TTATTAGAGGGTGGGGCGGATCGC | GACCCCGAACCGCGACCGTAA | 67 °C | 150 |
| p16-U | TTATTAGACGGTGGGGTGGATTGT | CAACCCCAAACCACAACCATAA | 62 °C | 151 |
| p16-W | CAGAGGGTGGGGCGGACCGC | CGGGCCGCGGCCGTGG | 67 °C | 140 |
2.7. Variation of GSTM1
Variation of GSTM1 was examined using Polymerase Chain Reaction (PCR) with the β-globin gene as an internal control. The PCR condition was initial denaturation at of 94 °C for 5 min which was followed by 35 cycles consisting of denaturation at 94 °C for 1 min, annealing for 1 min, and extension at 72 °C for 1 min followed by a final extension at 72 °C for 5 min. Positive genotype resulted in 219 bp fragment.
2.8. Methylation status of p16 gene promoter
2.8.1. Bisulfite modification
Methylation status of the p16 gene promoter was determined by methylation-specific PCR (MSP). 100 nanogram genomic DNA extracted from whole blood was modified using DNA Methylation-GoldTM Kit (Zymo Research Corp., California, U.S.A.) according to the manufacturer's protocol. Final elution was performed with 10 μL M-Elution Buffer.
2.8.2. Polymerase chain reaction (PCR)
Methylation-specific PCR was conducted to detect methylation of P16 gene promoter. Three pairs of primers, described by Herman et al. (1996) were used (Table 1). Primer set used were methylated (M), un-methylated (U) and wild type (W). The W primer is used as control for bisulfite modification. All PCR was performed in a total volume of 25 μL with 2 μL of bisulfite-modified DNA, 1 μL 20 mM forward primer, 1 μL 20 mM reverse primer, 8.5 μL dH2O, and 12.5 μL Go Taq Green master mix, with the following cycling parameters: 95 °C for 5 min; 35 cycles of 94 °C for 45 s, specific annealing temperature (Table 1) for 45 s, 72 °C for 45 s, and a final extension at 72 °C for 7 min. M.SssI modified DNA was used as a positive control and DNA from a healthy donor was used as the negative control. Water (H2O) was used as PCR control. The PCR products were visualized by electrophoresis using an agarose gel.
2.9. Statistical analysis
Results are presented as a percentage with median, maximum, and minimum values for non-normally distributed data. The data of baseline characteristic was classified, then its correlation with methylation of the p16 gene promoter was analyzed using Chi-square. The statistical analysis of differences in pesticide exposures was carried out using Chi-square. However, analysis of differences in exposure intensity score was carried out using Mann-Whitney nonparametric analysis because the data was not normally distributed. Meanwhile, the analysis of differences in personal protective equipment use was carried out using logistic regression. Chi-square was also used to identify the correlation between the variation of GSTM1 and methylation of p16 gene promoter, while the correlation between polymorphism of PON1 Q192R and methylation of p16 gene promoter was performed using logistic regression. The p-value was set as significance at <0.05.
3. Results
As many as 78 pesticide-exposed farmers participated in this study. The demographic data are shown in Table 2. Most of the studied subject was ≤55 years old, male, body mass index <25, and smokers. The frequency of methylation status of the p16 gene promoter does not significantly differ according to age, gender, body mass index, and smoking status (p > 0.05).
Table 2.
Baseline characteristic of the study group.
| Variable | n (%) | P16 gene promoter Methylation specific PCR |
p | |
|---|---|---|---|---|
| (+) | (-) | |||
| Age (years) | 0.278b | |||
| ≤55 | 61 (78.2) | 15 | 46 | |
| >55 | 17 (21.8) | 6 | 11 | |
| Sex | 0.418a | |||
| Male | 58 (74.4) | 17 | 41 | |
| Female | 20 (25.6) | 4 | 16 | |
| Body mass index | 0.208a | |||
| <25 | 59 (79.6) | 18 | 41 | |
| ≥25 | 19 (2.4) | 3 | 6 | |
| Smoking | 0.937a | |||
| Yes | 44 (56.4) | 12 | 32 | |
| No | 34 (43.6) | 9 | 25 | |
Pearson chi square test.
Fisher's exact test.
Pesticide exposure is shown in Table 3. Most of the subjects were exposed for more than 5 years, >1 time per week, and less than 5 h duration of pesticide spraying. Most of the subjects used appropriate dose based on label instruction, observed wind direction while using pesticide, wore personal protective equipment, did hand wash and bath after spraying, and threw pesticide waste at the river, yard, or trash bin. Exposure intensity score on farmers exposed to the pesticide was quite high, with a median score of 9.5 (minimum score was 9.5 and the maximum score was 19.0). All variables on pesticide exposure had p values > 0.05 which meant an insignificant correlation to the methylation status of p16 gene promoter.
Table 3.
Pesticide exposure of the study group.
| Variable | n (%) | P16 gene promoter Methylation specific PCR |
p | |
|---|---|---|---|---|
| (+) | (-) | |||
| Time/period (years) | 0.372b | |||
| <5 | 15 (19.2) | 5 | 10 | |
| >5 | 63 (80.8) | 16 | 47 | |
| Frequency per week (times) | 0.508b | |||
| <1 | 17 (21.8) | 5 | 12 | |
| >1 | 61 (78.2) | 16 | 45 | |
| Duration (hours) | 0.307b | |||
| <5 | 70 (89.7) | 20 | 50 | |
| >5 | 8 (10.3) | 1 | 7 | |
| Last spraying (days ago) | 0.883a | |||
| <14 | 53 (67.9) | 14 | 39 | |
| >14 | 25 (32.1) | 7 | 18 | |
| Dosage | ||||
| Appropriate | 58 (74.4) | 16 | 42 | 0.822c |
| Overdose | 3 (3.8) | 1 | 2 | 0.799c |
| Underdose | 17 (21.8) | 4 | 13 | |
| Observing wind direction | 1.000a | |||
| Yes | 52 (66.7) | 14 | 38 | |
| No | 26 (33.3) | 7 | 19 | |
| Personal protective equipment use | 0.426a | |||
| Always | 42 (53.8) | 11 | 31 | |
| Sometimes | 19 (24.4) | 7 | 12 | |
| Never | 17 (21.8) | 3 | 14 | |
| Washing hands after spraying | 0.469b | |||
| Yes | 76 (97.4) | 20 | 56 | |
| No | 2 (2.6) | 1 | 1 | |
| Bathing after spraying | 0.456b | |||
| Yes | 69 (88.5) | 18 | 51 | |
| No | 9 (11.5) | 3 | 6 | |
| Changing clothes | 0.054b | |||
| Yes | 69 (88.5) | 16 | 53 | |
| No | 9 (11.5) | 5 | 4 | |
| Pesticide dump | 1.000a | |||
| Buried/burned | 26 (33.3) | 7 | 19 | |
| At river/yard/trash bin | 52 (66.7) | 14 | 38 | |
| Exposure intensity score | 9.5 (9.5–19.0)e | 21 | 57 | 0.644d |
Pearson chi square test.
Fisher's exact test.
Logistic regression.
Non-parametric test Mann-Whitney.
Median (maximum-minimum).
The type of pesticides used by the subjects is shown in Table 4. The most common types of pesticide used, that could be defined, were pyrethroids and organophosphates. Unfortunately, most of the subjects were unsure of the type of pesticides they used, they stated unappropriate brand names or even called it as a poison instead. We could not assess the correlation between the type of pesticides and methylation of the p16 gene promoter since the subjects might use more than one type of pesticide.
Table 4.
Type of pesticides used by the subjects.
| Type of pesticides | n (%) |
|---|---|
| Pyrethroids | 38 (48.7) |
| Organophosphates | 16 (20.5) |
| Carbamates | 14 (17.9) |
| Macrocyclic lactones | 7 (9.0) |
| Neonicotinoids | 6 (7.7) |
| Others | 50 (64.1) |
| Not know | 7 (9.0) |
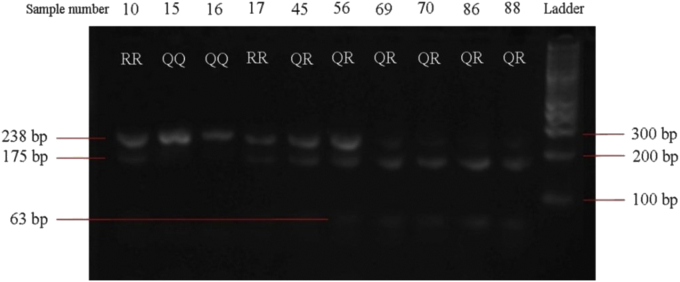
The result of polymorphism of PON1 Q192R examination is shown in Figure 1. Most of the participant had R allele that Q or wild type allele. The distribution of PON1 Q192R was found to agree with Hardy-Weinberg equilibrium.
Figure 1.
The PON1 Q192R. 175 and 63 bp showed mutant allele (RR), undigested 238 bp showed wild type allele (QQ), and all 238, 175, and 63 bp showed heterozygous allele (QR). Please see supplementary file for the corresponding original image.
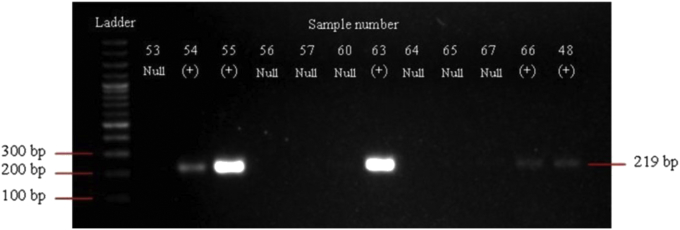
The GSTM1 null genotype examination is shown in Figure 2. As many as 82.1% of the participants had GSTM1 null genotype.
Figure 2.
The GSTM1, 219 bp showed positive genotype. Please see supplementary file for the corresponding original image.
The frequency of p16 gene promoter methylation status based on MSP examination was 21 (26.9%) positive and 57 (73.1%) negative. The representative result is showed in Figure 3.
Figure 3.
Methylation status of p16 gen promoter. U lane shows un-methylation (151 bp). M lane shows methylation (150 bp). W lane is bisulfate modification control (141 bp). Positive control is a M.Sssi treated DNA. Negative control is an unmodified DNA control. Water (H2O) is a control for PCR. Please see supplementary file for the corresponding original image.
The genotype frequencies of PON1 Q192R and GSTM1 and its relation with methylation of the p16 gene promoter are shown in Table 5.
Table 5.
Genetic variation of the study group.
| Variable | n (%) | P16 gene promoter Methylation specific PCR |
p | |
|---|---|---|---|---|
| (+) | (-) | |||
| Polymorphism of PON1 Q192R | ||||
| 4 (5.1) | 2 | 2 | ||
| QR | 34 (43.6) | 10 | 24 | 0.663b |
| RR | 40 (51.3) | 9 | 31 | 0.366b |
| GSTM 1 | 0.557a | |||
| Positive | 14 (17.9) | 4 | 10 | |
| Null | 64 (82.1) | 17 | 47 | |
Fisher's exact test.
Logistic regression.
4. Discussion
Individuals with impaired activity of metabolizing enzymes may have an altered ability to metabolize pesticides. Studies related to genetic polymorphisms of metabolizing enzymes genes and their influence on DNA methylation in workers exposed to pesticides are rare in Indonesia. We described the association of PON1 Q192R and GSTM1 genetic polymorphisms on methylation of p16 gene promoter in farmers occupationally exposed to pesticides in Central Java, Indonesia.
The frequency of methylation of p16 gene promoter in farmers exposed to pesticides was 26.2%. This result is higher compared to the frequency of methylation of p16 gene promoter in polycyclic aromatic hydrocarbons (PAH)-exposed workers (5.71%) (Yang et al., 2012) and in radon-exposed miner (up to 20%) (Su et al., 2006). However, some studies exhibited a higher frequency of p16 gene promoter methylation on arsenic exposure, which reported as high as 32.0% (Banerjee et al., 2013) and 47.5% (Lu et al., 2014). It is alarming since methylation has been proven to be related to various kinds of cancer.
Interestingly, we found that the methylation of the p16 gene promoter did not correlate with age, sex, BMI, and smoking status. However, the functional correlation of epigenetic modification and aging has not been described clearly (Gomes et al., 2012). Studies of the correlation of methylation status and sex also have not been concluded yet (Liu et al., 2006; El-Maarri et al., 2007). Studies on BMI relation with methylation of p16 promoter status showed a positive correlation between them (Ganji et al., 2010; Peters et al., 2007; Wilson et al., 2017). Smoking is also well known to be associated with the increase of DNA methylation. A meta-analysis found that smoking is correlated with methylation of p16 gene promoter (Zhang et al., 2011). Tobacco smoke contains numerous chemicals that may change the DNA methylation machinery. Tobacco smoke is also known as a source of ROS production, and ROS can lead to change the activity of DNA methyltransferase enzyme, hence changes methylation pattern on a certain gene (Deep et al., 2012). Our result is different from the previous study result. We suggest that the type of sample used in the study might cause the difference. Another study was using lung sample meanwhile our study using a blood sample. The different result may also due to the different exposures and methods used. Moreover, we also found that pesticides spraying period, frequency, duration, dosage, last spraying, and spraying direction were not related to methylation of p16 gene promoter. Personal protective equipment use and subjects' hygiene, like washing hands and bathing after spraying and dumping pesticide remnant incorrectly were not related to methylation of p16 gene promoter also. We suggest that it is influenced by the calculation of the exposure intensity score. Exposure intensity score was calculated using simple Dosemeci algorithm. After obtaining the exposure intensity score, cumulative exposure supposed to be calculated. Cumulative exposure was calculated using two kinds of lifetime pesticide exposure (Lee et al., 2007; Alavanja et al., 2004). The first was lifetime exposure days which was calculated based on Dosemeci et al. (2002) algorithm by multiplication between the mean number of days in an average year and years of use. Another is intensity-weighted exposure-days which was calculated by multiplication between lifetime exposure days and exposure intensity score. However, cumulative exposure could not be calculated due to the limitation of the data. Subjects hardly remembered the exact time of pesticide use, and only conveyed the estimated time. Consequently, the cumulative exposure was not calculated and the real exposure was less described. Moreover, the dosage of pesticides was difficult to be determined since subjects measured the dosage of pesticides by inappropriate methods of measurement, such as using a bottle cap or spoon.
Another factor complicates the calculation of exposure was the type of pesticide used by subjects. The most commonly used type of pesticides were pyrethroids, organophosphate, carbamate, macrocyclic lactone, and neonicotinoids. Organophosphate (Bonner et al., 2010; Mahajan et al., 2006; Alavanja et al., 2014; Koutros et al., 2013) and carbamate (Lee et al., 2007; Alexander et al., 2017) were proved to be cancer-related. Meanwhile, pyrethroids were not proved to be cancer-related (Lee et al., 2007; Alavanja et al., 2014; Rusiecki et al., 2009). Macrocyclic lactone (Bansod et al., 2013) and nicotinoids (Cimino et al., 2017) also have not been proved to be cancer-related yet. More than half of the subjects used more than one pesticide. The subjects might use more than two kinds of pesticides, and the type of pesticide used depends on the type of plants they grew and also the climate. The subjects hardly remember the exact duration of the use of specific pesticide, hence the specific cumulative exposure was obscure.
We also aimed to determine the relation of methylation of the p16 gene promoter with the variation of PON1 Q192R and GSTM1. However, we also did not found any correlation between them. Paraoxonase 1 (PON1) enzyme plays an important role in hydrolyzing several organophosphorus (OP) compounds including insecticides and nerve agents (Richter et al., 2009). The name of PON1 is derived from its ability to hydrolyze paraoxon, its first and most studied substrate. PON1 also hydrolyzes the active metabolites of other OP insecticides (e.g. chlorpyrifos oxon, diazoxon). Some other OP and carbamate insecticides are not hydrolyzed by PON1 (Draganov et al., 2005). PON1 genetic variation is one of the factors played a role in xenobiotics metabolizing system in farmer (Kahl et al., 2018a). A glutamine (Q)/arginine (R) substitution at position 192 results in allozymes that affect the hydrolytic efficiency of the enzyme towards different substrates. Certain OPs are metabolized more efficiently by one of the two different PON1 alloforms. For example, PON1 192RR isoform hydrolyzes paraoxon more rapidly than PON1 192QQ isoform and inversely, the PON1 192QQ isoform hydrolyzes diazoxon more rapidly than the PON1 192RR (López-Flores et al., 2009). The presence of the R allele correlates to a gene-dependent increase in PON1 activity, suggesting a biosensor effect during anticholinesterase agent exposure that may upregulate PON1 activity (Sirivarasai et al., 2007). Studies in animal models emerged that PON1 modulates the toxicity of OPs. Rats with increasing plasma PON1 levels would protect against OP toxicity, as indicated by a lower degree of brain and diaphragm acetylcholinesterase (AChE) inhibition, particularly in case of chlorpyrifos oxon (Costa et al., 1990).
Our study found that >90% of the subject had R allele which means that our subjects are more protected towards OPs toxicity. Thus far, the study on the distribution of polymorphism of PON1 Q192R in Indonesia is rare, though there are a great number of studies in other different populations around the world. Zhang et al. (2014) examined polymorphisms of PON1 Q192R in northern Han Chinese workers exposed to OP pesticides and found a similar result which was 15.6%, 40.0%, and 44.4% for QQ, QR, and RR, respectively. While Sunay et al. (2013) found distinct proportion, QQ, QR and RR genotype frequencies were 53.7%, 35.2%, and 11.1% in the OP-exposed Turkish population, respectively.
We have not found other study which assesses if polymorphism of PON1 Q192R associates to methylation of p16 gene promoter. Nonetheless, many reports about polymorphism of PON1 Q192R and several genetic damages were established. A study by da Silva et al. (2008) which evaluated genetic damage in a Brazilian population occupationally exposed to pesticides and its correlation with polymorphisms in metabolizing genes, observed some effects of genetic polymorphisms in PON in the modulation of micronucleus (MN) results. Whereas, Singh et al., 2011a, Singh et al., 2011b asserted that workers with PON1192Q/Q genotype had significantly higher DNA damage as compared with other genotypes (p < 0.001). They observed the inverse correlation between DNA tail moment and PON activity, where the DNA tail moment was lower with increased PON activity and higher with decreased PON activity. In our study, OP was not the most commonly used pesticide. It was the second most commonly used pesticide, with pyrethroids as the most commonly used pesticide among our study subjects. Moreover, PON1 gene variation is not the only factors affecting the DNA methylation in farmer exposed to pesticide. Other study showed that nutritional intake and variation of the gene related to folate metabolism also known to influence methylation in farmer exposed to pesticide (Kahl et al., 2018b). We suggest that those factors might lead to an insignificant correlation between polymorphism of PON1 Q192R and methylation of p16 gene promoter in this study.
Glutathione S-transferases (GSTs) are major phase II detoxification enzymes that act in catalyzing the conjugation of electrophilic substrates to glutathione (GSH) (Sheehan et al., 2001). The GSTs play a central role in the detoxification of pesticides. GST isoenzymes are associated with several types of cancer (Tumer et al., 2016). Polymorphisms in these genes may alter their expression and functional activities and might be involved in the inactivation of procarcinogens that contribute to the progression of cancers (Gong et al., 2012).
In our study, the GSTM1 null genotype was found on 82.1% of the study subjects. Some meta-analyses have shown significant association between the null genotypes of GSTM1 and the increased risk of various types of cancer, including prostate (Gong et al., 2012; Mo et al., 2009; Ntais et al., 2005), bladder (Yu et al., 2016), esophageal (Zheng et al., 2016), and colorectal (Li et al., 2015) cancers. The GSTM1 gene deletions result in complete deletion of the genes and lack of enzyme activity (Singh et al., 2011a, Singh et al., 2011b). The GSTM1 null deletion was found to be significantly associated with DNA damage in individuals exposed to pesticides (Singh et al., 2011a, Singh et al., 2011b, 2012; Tumer et al., 2016). Yet, we found no significance between the GSTM1 null deletion and methylation of the p16 gene promoter. Our result is in contrast with the findings of Sarmishtha et al. (2015) who stated that genetic polymorphism of GSTM1 was significantly associated (p < 0.05) with the degree of methylation in p16 gene promoter region on higher arsenic-exposed people in the southern region of West Bengal, India. The subjects having GSTM1 null genotype have significantly decreased urinary arsenic and increased DNA methylation level relative to those with GSTM1 positive genotype of the same arsenic exposure group.
Individuals with the GSTM1 gene deletion are incapable to detoxify certain carcinogens through the glutathione-conjugation pathway (Rubes et al., 2007). Muniz et al. (2008) asserted that OP pesticides induce oxidative stress and DNA damage in farmworkers by depleting intracellular GSH and increasing ROS production. The GSH has antioxidative properties, which prevents the cell from oxidative injury. The exposure of the mixture of pesticides was found to produce depleted GSH levels which indicate the generation of ROS and to counteract ROS effects, more GSH consumed by related enzymes, like glutathione peroxidase (Shukla et al., 2017).
The limitation of this study is the use of DNA extracted from the blood for DNA methylation examination. The blood cell lifespan in the circulatory system is ranging from hours (granulocytes) to months (lymphocytes) (Hall and Guyton, 2016). Besides, the half-life of pesticide and its metabolites is ranging from hours to days in the plasma. However, they will also be released from tissue deposit when the concentration in the plasma is low that causes their long-lasting presence in the plasma (Chrustek et al., 2018; Hoffmann & Papendorf, 2006). In our study, most of the study subjects had their last pesticide spraying in less than 14 days before the blood sample collection. Therefore, we assumed that some of the blood cells collected in this study were exposed to pesticides within 14 days before blood collection. Moreover, DNA methylation due to pesticide exposure is not only due to direct effect of pesticide exposure but also indirect effect of pesticide exposure. There are several mechanisms of DNA methylation, one of the mechanism is oxidative stress mechanism. Long term pesticide exposure is associated with reduced antioxidant enzymes (Ogut et al., 2011). Moreover, pesticide metabolites also positively correlated with oxidative stress biomarker concentration (Lee et al., 2017). Several studies have shown that pesticide chronic exposure is correlated with oxidative stress and DNA methylation (Benedetti et al., 2018; Kahl et al., 2018c).
5. Conclusions
The frequency of p16 gene promoter methylation in Javanese farmers exposed to pesticides at Magelang Regency, Central Java, Indonesia is more than one fourth. More than three-quarters of them have GSTM1 null genotype and more than 90% of them have PON1 R allele. However, our study showed that PON1 Q192R polymorphism and GSTM1 genotype variation are not associated with methylation of the p16 gene promoter. Further research should be done to reveal the factors affecting the methylation of p16 gene promoter in people exposed with pesticide i.e nutritional intake pattern, the behavior-related pesticide used, types of pesticide used, etc. Revealing the protective factors against the deleterious effect of pesticide to person will be helpful to prevent the effects of pesticides on health.
Declarations
Author contribution statement
Maya G. Ratna: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Dwi A.A. Nugrahaningsih: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Eti N. Sholikhah: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ery K. Dwianingsih: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Rusdy G. Malueka: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Kementrian Riset, Teknologi dan Pendidikan Tinggi Republik Indonesia grant PDUPT 2017 and Dana Masyarakat Faculty of Medicine, Public Health and Nursing Universitas Gadjah Mada 2018.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Dr. Med. dr. Indwiani Astuti for the advice in writing the manuscript.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Koutros S., Freeman L.E.B., Lubin J.H., Heltshe S.L., Andreotti G., Barry K.H., DellaValle C.T., Hoppin J.A., Sandler D.P., Lynch C.F., Blair A., Alavanja M.C.R. Risk of total and aggressive prostate cancer and pesticide use in the Agricultural Health Study. Am. J. Epidemiol. 2013;177(1):59–74. doi: 10.1093/aje/kws225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavanja M.C.R., Dosemeci M., Samanic C., Lubin J., Lynch C.F., Knott C., Barker J. Pesticides and lung cancer risk in the Agricultural Health Study cohort. Am. J. Epidemiol. 2004;160(9):876–885. doi: 10.1093/aje/kwh290. [DOI] [PubMed] [Google Scholar]
- Alavanja M.C.R., Hofmann J.N., Lynch C.F., Hines C.J., Barry K.H., Barker J., Buckman D.W., Thomas K., Sandler D.P., Hoppin J.A., Koutros S., Andreotti G., Lubin J.H., Blair A., Freeman L.E.B. Non-hodgkin lymphoma risk and insecticide, fungicide and fumigant use in the Agricultural Health Study. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M., Koutros S., Bonner M.R., Barry K.H., Alavanja M.C.R., Andreotti G., Byun H.M., Chen L., Freeman L.E.B., Hofmann J.N., Kamel F., Moore L.E., Baccarelli A., Rusiecki J. Pesticide use and LINE-1 methylation among male private pesticide applicators in the Agricultural Health Study. Environ. Epigenet. 2017;3(2):1–9. doi: 10.1093/eep/dvx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee N., Paul S., Sau T.J., Das J.K., Bandyopadhyay A., Banerjee S., Giri A.K. Epigenetic modifications of DAPK and p16 genes contribute to arsenic-induced skin lesions and nondermatological health effects. Toxicol. Sci. 2013;135(2):300–308. doi: 10.1093/toxsci/kft163. [DOI] [PubMed] [Google Scholar]
- Bansod Y.V., Kharkar S.V., Raut A., Choudalwar P. Abamectin: an uncommon but potentially fatal cause of pesticide poisoning. Int. J. Res. Med. Sci. 2013;1(3):285–286. [Google Scholar]
- Benedetti D., Lopes Alderete B., de Souza C.T., Ferraz Dias J., Niekraszewicz L., Cappetta M., Martínez-López W., Da Silva J. DNA damage and epigenetic alteration in soybean farmers exposed to complex mixture of pesticides. Mutagenesis. 2018 Feb 24;33(1):87–95. doi: 10.1093/mutage/gex035. [DOI] [PubMed] [Google Scholar]
- Bonner M.R., Williams B.A., Rusiecki J.A., Blair A., Freeman L.E.B., Hoppin J.A., Dosemeci M., Lubin J., Sandler D.P., Alavanja M.C.R. Occupational exposure to terbufos and the incidence of cancer in the Agricultural Health Study. Cancer Causes Control. 2010;21(6):871–877. doi: 10.1007/s10552-010-9514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner M.R., Freeman L.E.B., Hoppin J.A., Koutros S., Sandler D.P., Lynch C.F., Hines C.J., Thomas K., Blair A., Alavanja M.C.R. Occupational exposure to pesticides and the incidence of lung cancer in the Agricultural Health Study. Environ. Health Perspect. 2017;125(4):544–551. doi: 10.1289/EHP456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrustek A., Hołyńska-Iwan I., Dziembowska I., Bogusiewicz J., Wróblewski M., Cwynar A., Olszewska-Słonina D. Current research on the safety of pyrethroids used as insecticides. Medicina (Kaunas) 2018 Aug 28;(4):54. doi: 10.3390/medicina54040061. pii: E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino A.M., Boyles A.L., Thayer K.A., Perry M.J. Effects of neonicotinoid pesticide exposure on human health: a systematic review. Environ. Health Perspect. 2017;125(2):155–162. doi: 10.1289/EHP515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa L.G., McDonald B.E., Murphy S.D., Omenn G.S., Richter R.J., Motulsky A.G., Furlong C.E. Serum paraoxonase and its influence on paraoxon and chlorpyrifos-oxon toxicity in rats. Toxicol. Appl. Pharmacol. 1990;103:66–76. doi: 10.1016/0041-008x(90)90263-t. [DOI] [PubMed] [Google Scholar]
- da Silva J., Moraes C.R., Heuser V.D., Andrade V.M., Silva F.R., Kvitko K., Emmel V., Rohr P., Bordin D.L., Andreazza A.C., Salvador M., Henriques J.A.P., Erdtmann B. Evaluation of genetic damage in a Brazilian population occupationally exposed to pesticides and its correlation with polymorphisms in metabolizing genes. Mutagenesis. 2008;23(5):415–422. doi: 10.1093/mutage/gen031. [DOI] [PubMed] [Google Scholar]
- Das P.M., Singal R. DNA methylation and cancer. J. Clin. Oncol. 2004;22:4632–4642. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- Deep J.S., Sidhu S., Chandel A., Thapliyal S., Garg C. Aberrant methylation in promoters of GSTP1, p16, p14, and RASSF1A genes in smokers of North India. ISRN Pulmonol. 2012;2012:1–6. [Google Scholar]
- Dosemeci M., Alavanja M.C.R., Rowland A.S., Mage D., Zahm S.H., Rothman N., Lubin J.H., Hoppin J.A., Sandler D.P., Blair A. A quantitative approach for estimating exposure to pesticides in the Agricultural Health Study. Ann. Occup. Hyg. 2002;46(2):245–260. doi: 10.1093/annhyg/mef011. [DOI] [PubMed] [Google Scholar]
- Draganov D.I., Teiber J.F., Speelman A., Osawa Y., Sunahara R., Du B.N.L. Human paraoxonases (PON1, PON2, and PON3) arelactonases with overlapping and distinct substrate specificities. J. Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- El-Maarri O., Becker T., Junen J., Manzoor S.S., Diaz-Lacava A., Schwaab R., Wienker T., Oldenburg J. Gender specific differences in levels of DNA methylation at selected loci from human total blood: a tendency toward higher methylation levels in males. Hum. Genet. 2007;122:505–514. doi: 10.1007/s00439-007-0430-3. [DOI] [PubMed] [Google Scholar]
- Eriksson M., Hardell L., Carlberg M., Akerman M. Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. Int. J. Cancer. 2008;123:1657–1663. doi: 10.1002/ijc.23589. [DOI] [PubMed] [Google Scholar]
- Ganji S.M., Miotto E., Callegari E., Sayehmiri K., Fereidooni F., Yazdanbod M., Rastgar-Jazii F., Negrini M. Associations of risk factors obesity and occupational airborne exposures with CDKN2A/p16 aberrant DNA methylation in esophageal cancer patients. Dis. Esophagus. 2010;23:597–602. doi: 10.1111/j.1442-2050.2010.01059.x. [DOI] [PubMed] [Google Scholar]
- Gomes M.V.M., Toffoli L.V., Arruda D.W., Soldera L.M., Pelosi G.G., Neves-Souza R.D., Freitas E., Castro D.T., Marquez A.S. Age-related changes in the global DNA methylation profile of leukocytes are linked to nutrition but are not associated with the MTHFR C677T genotype or to functional capacities. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M., Dong W., Shi Z., Xu Y., Ni W., An R. Genetic polymorphisms of GSTM1, GSTT1, and GSTP1 with prostate cancer risk: a meta-analysis of 57 studies. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.E., Guyton A.C. Saunders Elsevier; Philadelphia, PA: 2016. Guyton and Hall Textbook of Medical Physiology.https://www.clinicalkey.com/#!/content/book/3-s2.0-B9781455770052000330 [Google Scholar]
- Hashemi M., Moazeni-Roodi A.K., Fazaeli A., Sandoughi M., Bardestani G.R., Kordi-Tamandani D.M., Ghavami S. Lack of association between paraoxonase-1 Q192R polymorphism and rheumatoid arthritis in southeast Iran. Genet. Mol. Res. 2010;9(1):333–339. doi: 10.4238/vol9-1gmr728. [DOI] [PubMed] [Google Scholar]
- Herman J.G., Graff J.R., Myohanen S., Nelkin B.D., Baylin S.B. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc. Natl. Acad. Sc. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann U., Papendorf T. Organophosphate poisonings with parathion and dimethoate. Intensive Care Med. 2006 Mar;32(3):464–468. doi: 10.1007/s00134-005-0051-z. [DOI] [PubMed] [Google Scholar]
- Jones P.A., Baylin S.B. The epigenomics of cancer. Cell. 2007;128(4):683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl V.F.S., da Silva F.R., Alves J.D.S., da Silva G.F., Picinini J., Dhillon V.S., Fenech M., de Souza M.R., Dias J.F., de Souza C.T., Salvador M., Branco C.D.S., Thiesen F.V., Simon D., da Silva J. Role of PON1, SOD2, OGG1, XRCC1, and XRCC4 polymorphisms on modulation of DNA damage in workers occupationally exposed to pesticides. Ecotoxicol. Environ. Saf. 2018;159:164–171. doi: 10.1016/j.ecoenv.2018.04.052. [DOI] [PubMed] [Google Scholar]
- Kahl V.F.S., Dhillon V., Fenech M., de Souza M.R., da Silva F.N., Marroni N.A.P., Nunes E.A., Cerchiaro G., Pedron T., Batista B.L., Cappetta M., Mártinez-López W., Simon D., da Silva J. Occupational exposure to pesticides in tobacco fields: the integrated evaluation of nutritional intake and susceptibility on genomic and epigenetic instability. Oxid. Med. Cell Longev. 2018;2018:7017423. doi: 10.1155/2018/7017423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl V.F.S., Dhillon V.S., Simon D., da Silva F.R., Salvador M., Branco C.D.S., Cappetta M., Martínez-López W.7, Thiesen F.V., Dias J.F., Souza C.T., Fenech M., da Silva J. Chronic occupational exposure endured by tobacco farmers from Brazil and association with DNA damage. Mutagenesis. 2018;33(2):119–128. doi: 10.1093/mutage/gex045. [DOI] [PubMed] [Google Scholar]
- Ku J.L., Jeon Y.K., Park J.G. Methylation-specific PCR. In: Tollefsbol T.O., editor. Epigenetics Protocols: Second Edition, Methods in Molecular Biology. 2011. https://link.springer.com/protocol/10.1007%2F978-1-61779-316-5_3 [serial online] [cited 2017 Jun 19]. Available from: [Google Scholar]
- Lee W.J., Sandler D.P., Blair A., Samanic C., Cross A.J., Alavanja M.C.R. Pesticide use and colorectal cancer risk in the Agricultural Health Study. Int. J. Cancer. 2007;121(2):339–346. doi: 10.1002/ijc.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.M., Park S.Y., Lee K., Oh S.S., Ko S.B. Pesticide metabolite and oxidative stress in male farmers exposed to pesticide. Ann. Occup. Environ. Med. 2017 Feb 28;29:5. doi: 10.1186/s40557-017-0162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Xu W., Liu F., Huang S., He M. GSTM1 polymorphism contribute to colorectal cancer in Asian populations: a prospective meta-analysis. Sci. Rep. 2015;5:1–8. doi: 10.1038/srep12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Lan C., Siegfried J.M., Luketich J.D., Keohavong P. Aberrant promoter methylation of p16 and MGMT genes in lung tumors from smoking and never-smoking lung cancer patients. Neoplasia. 2006;8(1):46–51. doi: 10.1593/neo.05586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Flores I., Lacasana M., Blanco-Munoz J., Aguilar-Garduno C., Sanchez-Villegas P., Pérez-Méndez O.A., Gamboa-Ávila R. Relationship between human paraoxonase-1 activity and PON1 polymorphisms in Mexican workers exposed to organophosphate pesticides. Toxicol. Lett. 2009;188(2009):84–90. doi: 10.1016/j.toxlet.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Lu G., Xu H., Chang D., Wu Z., Yao X., Zhang S., Li Z., Bai J., Cai Q., Zhang W. Arsenic exposure is associated with DNA hypermethylation of the tumor suppressor gene p16. J. Occup. Med. Toxicol. 2014:1–5. doi: 10.1186/s12995-014-0042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Zhou T., Tao Y., Feng Y., Shen X., Mei S. Exposure to organochlorine pesticides and non-Hodgkin lymphoma: a meta-analysis of observational studies. Sci. Rep. 2016:1–11. doi: 10.1038/srep25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan R., Blair A., Lynch C.F., Schroeder P., Hoppin J.A., Sandler D.P., Alavanja M.C.R. Fonofos exposure and cancer incidence in the agricultural health study. Environ. Health Perspect. 2006;114(12):1838–1842. doi: 10.1289/ehp.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens J.W.M., Margossian A.L., Schmitt M., Foekens J., Harbeck N. DNA methylation as a biomarker in breast cancer. Future Oncol. 2009;5(8):1245–1256. doi: 10.2217/fon.09.89. [DOI] [PubMed] [Google Scholar]
- Mo Z., Gao Y., Cao Y., Gao F., Jian L. An updating meta-analysis of the GSTM1,GSTT1, and GSTP1 polymorphisms and prostate cancer: a HuGE review. Prostate. 2009;69:662–688. doi: 10.1002/pros.20907. [DOI] [PubMed] [Google Scholar]
- Moison C., Guieysse-Peugeot A., Arimondo P.B. DNA methylation in cancer. Atlas Genet. Cytogenet. Oncol. Haematol. 2014;18(4):285–292. [Google Scholar]
- Muniz J.F., McCauley L., Scherer J., Lasarev M., Koshy M., Kow Y.W., Nazar-Stewart V., Kisby G.E. Biomarkers of oxidative stress and DNA damage in agricultural workers: a pilot study. Toxicol. Appl. Pharmacol. 2008;227(2008):97–107. doi: 10.1016/j.taap.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Ntais C., Polycarpou A., Ioannidis J.P.A. Association of GSTM1, GSTT1, and GSTP1 gene polymorphisms with the risk of prostate cancer: a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2005;14(1):176–181. [PubMed] [Google Scholar]
- Ogut S., Gultekin F., Kisioglu A.N., Kucukoner E. Oxidative stress in the blood of farm workers following intensive pesticide exposure. Toxicol. Ind. Health. 2011 Oct;27(9):820–825. doi: 10.1177/0748233711399311. [DOI] [PubMed] [Google Scholar]
- Peters I., Vaske B., Albrecht K., Kuczyk M.A., Jonas U., Serth J. Adiposity and age are statistically related to enhanced RASSF1A tumor suppressor gene promoter methylation in normal autopsy kidney tissue. Cancer Epidemiol. Biomarkers Prev. 2007;16(12):2526–2532. doi: 10.1158/1055-9965.EPI-07-0203. [DOI] [PubMed] [Google Scholar]
- Richter R.J., Jarvik G.P., Furlong C.E. Paraoxonase 1 (PON1) Status and substrate hydrolysis. Toxicol. Appl. Pharmacol. 2009;235(1):1–9. doi: 10.1016/j.taap.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubes J., Selevan S.G., Sramc R.J., Evenson D.P., Perreault S.D. GSTM1 genotype influences the susceptibility of men to sperm DNA damage associated with exposure to air pollution. Mutat. Res. 2007;625(2007):20–28. doi: 10.1016/j.mrfmmm.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Rusiecki J.A., Patel R., Koutros S., Beane-Freeman L., Landgren O., Bonner M.R., Coble J., Lubin J., Blair A., Hoppin J.A., Alavanja M.C.R. Cancer incidence among pesticide applicators exposed to permethrin in the Agricultural Health Study. Environ. Health Perspect. 2009;117(4) doi: 10.1289/ehp.11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmishtha C., Uma D., Debendranath G.M., Utpal C., Bhaswati G. Role of glutathione-S-transferase polymorphism on arsenic induced DNA hypermethylation. Expos. Health. 2015;22(3):96–100. [Google Scholar]
- Sheehan D., Meade G., Foley V.M., Dowd C.A. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S., Jhamtani R.C., Dahiya M.S., Agarwal R. Oxidative injury caused by individual and combined exposure of neonicotinoid, organophosphate and herbicide in zebrafish. Toxicol. Rep. 2017;4(2017):240–244. doi: 10.1016/j.toxrep.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Kumar V., Singh P., Thakur S., Banerjee B.D., Rautela R.S., Grovera S.S., Rawata D.S., Pasha S.T., Jain S.K., Rai A. Genetic polymorphisms of GSTM1, GSTT1 and GSTP1 and susceptibility to DNA damage in workers occupationally exposed to organophosphate pesticides. Mutat. Res. 2011;725(2011):36–42. doi: 10.1016/j.mrgentox.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Singh S., Kumar V., Thakur S., Banerjee B.D., Rautela R.S., Grover S.S., Rawat D.S., Pasha S.T., Jain S.K., Ichhpujani R.L., Rai A. Paraoxonase-1 genetic polymorphisms and susceptibility to DNA damage in workers occupationally exposed to organophosphate pesticides. Toxicol. Appl. Pharmacol. 2011;252(2011):130–137. doi: 10.1016/j.taap.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Singh S., Kumar V., Singh P., Banerjee B.D., Rautelaa R.S., Grovera S.S., Rawata D.S., Pasha S.T., Jain S.K., Rai A. Influence of CYP2C9, GSTM1, GSTT1 and NAT2 genetic polymorphisms on DNA damage in workers occupationally exposed to organophosphate pesticides. Mutat. Res. 2012;741(2012):101–108. doi: 10.1016/j.mrgentox.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Sirivarasai J., Kaojarern S., Yoovathaworn K., Sura T. Paraoxonase (PON1) polymorphism and activity as the determinants of sensitivity to organophosphates in human subjects. Chem. Biol. Interact. 2007;168(2007):184–192. doi: 10.1016/j.cbi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Su S., Jin Y.J., Zhang W., Yang L., Shen Y., Cao Y., Tong J. Abberant promoter methylation of p16INK4a and O6-methylguanine-DNA methyltransferase genes in workers at a Chinese uranium mine. J. Occup. Health. 2006;48:261–266. doi: 10.1539/joh.48.261. [DOI] [PubMed] [Google Scholar]
- Sunay S.Z., Kayaalti Z., Bayrak T., Sőylemezoğlu T. Effect of paraoxonase 1 192 Q/R polymorphism on paraoxonase and acetylcholinesterase enzyme activities in Turkish population exposed to organophosphate. Toxicol. Ind. Health. 2013:1–9. doi: 10.1177/0748233713487246. [DOI] [PubMed] [Google Scholar]
- Tumer T.B., Savranoglu S., Atmaca P., Terzioglu G., Sen A., Arslan S. Modulatory role of GSTM1 null genotype on the frequency of micronuclei in pesticide-exposed agricultural workers. Toxicol. Ind. Health. 2016;32(12):1942–1951. doi: 10.1177/0748233715599876. [DOI] [PubMed] [Google Scholar]
- Vakonaki E., Androutsopoulosa V.P., Liesivuori J., Tsatsakisa A.M., Spandidos D.A. Pesticides and oncogenic modulation. Toxicology. 2013;307:42–45. doi: 10.1016/j.tox.2013.01.008. [DOI] [PubMed] [Google Scholar]
- van Bemmel D.M., Visvanathan K., Freeman L.E.B., Coble J., Hoppin J.A., Alavanja M.C.R. S-ethyl-N,N-dipropylthiocarbamate exposure and cancer incidence among male pesticide applicators in the Agricultural Health Study: a prospective cohort. Environ. Health Perspect. 2008;116(11):1541–1546. doi: 10.1289/ehp.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L.E., Harlid S., Xu Z., Sandler D.P., Taylor J.A. An epigenome-wide study of body mass index and DNA methylation in blood using participants from the Sister Study cohort. Int. J. Obes. 2017;41(1):194–199. doi: 10.1038/ijo.2016.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Ma J., Zhang B., Duan H., He Z., Zeng J., Zeng X., Li D., Wang Q., Xiao Y., Liu C., Xiao Q., Chen L., Zhu X., Xing X., Li Z., Zhang S., Zhang Z., Ma L., Wang E., Zhuang Z., Zheng Y., Chenet W. CpG site-specific hypermethylation of p16INK4a in peripheral blood lymphocytes of PAH-exposed workers. Cancer Epidemiol. Biomark. Prev. 2012;21(1):182–190. doi: 10.1158/1055-9965.EPI-11-0784. [DOI] [PubMed] [Google Scholar]
- Yu Y., Li X., Liang C., Tang J., Qin Z., Wang C., Xu W., Hua Y., Shao P., Xu T. The relationship between GSTA1, GSTM1, GSTP1, and GSTT1 genetic polymorphisms and bladder cancer susceptibility: a meta-analysis. Medicine. 2016;95(37):1–12. doi: 10.1097/MD.0000000000004900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zhu W., Yang P., Liu T., Jiang M., He Z., Zhang S., Chen W.Q., Chen W. Cigarret smoking and p16INK4α gene promoter hypermethylation in non-small cell lung carcinoma patients: a meta-analysis. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0028882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Sui H., Li H., Zheng J., Wang F., Li B., Zhang Y. Paraoxonase activity and genetic polymorphisms in northern Han Chinese workers exposed to organophosphate pesticides. Exp. Biol. Med. 2014;239:232–239. doi: 10.1177/1535370213513983. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Ni X., Jiao Y., Shen F., Jiang H., Wang M., Hu G., Tao S. Genetic polymorphisms of glutathione S-transferase (GSTM1, GSTT1 and GSTP1) with esophageal cancer risk: a meta-analysis. Int. J. Clin. Exp. Med. 2016;9(7):13268–13280. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.