Abstract
Stinging nettles provide low-cost quality nutrition for alleviating malnutrition. Previous research on stinging nettles focused mainly on the nutritional quality of fresh leaves. In this study, the effect of drying method on macronutrients, mineral content, ascorbic acid, β-carotene content and total phenols content and antioxidant activity were investigated. The contribution of fresh, oven dried or freeze dried stinging nettle leaves to the required daily value for the nutrients were also determined. Oven drying of nettle leaves resulted in a higher loss of β-carotene and ascorbic acid content compared to freeze drying. In contrast, the total phenols content and total antioxidant activity were higher in oven dried stinging nettle leaves compared to freeze dried leaves. Overall, freeze dried and oven dried nettle leaves can be considered as a rich source of Ca, Mg and vitamin A; a good source of vitamin C, Fe, and Mn; and a source for Mg and K. Stinging nettle leaves could potentially be used as a cheap natural source of antioxidants and for addressing micronutrient malnutrition.
Keywords: Food science, Agriculture, Stinging nettle, Ascorbic acid, β-carotene, Antioxidant activity, Total phenols content, Dietary value, Oven drying, Freeze drying, Minerals
Food Science; Agriculture; Stinging nettle; Ascorbic acid; β-carotene; Antioxidant activity; Total phenols content; Dietary value; Oven drying; Freeze drying; Minerals
1. Introduction
Stinging nettle, Urtica dioica L., provides vitamins and minerals needed to maintain health in humans (Kavalali, 2003). The plant may help to combat malnutrition or nutrition-related health problems due to its bioactive compounds (Adhikari et al., 2016). Fresh nettle leaves contain phenolic compounds (Orčić et al., 2014; Farag et al., 2013; Otles and Yalcin, 2012; Pinelli et al., 2008) and polyunsaturated fatty acids (Rutto et al., 2013; Guil-Guerrero et al., 2003), essential amino acids (Hughes et al., 1980; Rutto et al., 2013) and ascorbic acid (Ioana et al., 2013). Nettle leaves contain nine carotenoids of which lutein and lutein isomers, and β-carotene are the basic carotenoids (Guil-Guerrero et al., 2003). Nettle leaves are good sources of minerals such as calcium, iron, magnesium, manganese, zinc, phosphorus, potassium, copper and selenium (Pytlakowska et al., 2012; Kara, 2009; Özcan et al., 2008). Popov et al. (2020) investigated the application of contemporary extraction techniques for elements and minerals from stinging nettle leaves. The authors found that microwave and ultrasonically-assisted extraction techniques could possibly be used for obtaining extracts as a secondary source of minerals. Nettle leaves are good sources of protein and dietary fiber (Hughes et al., 1980).
Fresh stinging nettle leaves are reported to contain phenolic acid (e.g. hydroxycinnamic acid, hydroxybenzoic acid), tannins and flavonoids (e.g. flavones, flavonols, iso-flavonols, anthocyanins, catechins, lignin) (Pinelli et al., 2008; Farag et al., 2013; Orčić et al., 2014). Gião et al. (2007) evaluated the antioxidant and phenolic content of 48 Portuguese herbal plants for their dependence on extraction features [e.g. powder infusion (dried leaves), fresh leaf infusion and fresh leaf boiling (decoction)]. The authors recommended infusion as the most effective mode of extraction of antioxidants from medicinal plants.
Stinging nettle leaves add variety to the menu, thus could be used as a supplementary, spinach-like vegetable in human diet (Hughes et al., 1980). Naude, 2013a, Naude, 2013b also emphasized that the need to include dark green vegetables at least one serving per day to reduce the burden of nutrition-related disease. Nettle plant is widely used as food in early spring when young leaves are added to soups, salads, herbal tea or decocted tea as well as in dried form for winter use (Guil-Guerrero et al., 2003).
Drying of stinging nettle leaves not only grants their use when the plants are not physiologically active but also extends their consumption period. Additionally, the irritating contents of the stinging hairs are dissipated upon drying (Upton, 2013).
The drying method chosen can have a major impact on nutrient degradation and retention (Shilton, 2003). Ambient air-drying (such as well-ventilated air drying and sun drying) was mentioned as a common method of drying stinging nettle leaves (Maanda and Bhat, 2010; Upton, 2013). However, the slow drying process involved in ambient air-drying methods may lead to a loss of quality of the leaves (e.g. colour changes, losses of ascorbic acid and carotenoids) (Harbourne et al., 2009). Freeze-drying is the most common method of drying nettle for medicinal purposes such as for the production of encapsulated nettle products (Dey, 2013; Ait Haj Said et al., 2015). During freeze-drying, very few chemical changes occur whereas oven drying at 45 °C–140 °C can cause faster degradation of colour and loss of primary metabolites (Shilton, 2003).
Drying processes involving high temperature such as oven drying result in protein denaturation, ascorbic acid and β-carotene degradation (Shilton, 2003) and might affect antioxidants in food products (Chang et al. 2006; Rodrigues et al. 2009). When drying herbs the amount of phenolic compounds and antioxidant activity of herb extracts increased (Suhaj, 2006; Hossain et al., 2010). Differences in aroma, flavour and colour of leaf infusions and cooked leaves were noted when oven-dried leaves used compared to fresh leaves (Shonte and de Kock, 2017). Colour changes during oven drying of stinging nettle leaves were also reported (Alibas, 2006; Shonte and de Kock, 2017). Ascorbic acid and β-carotene are better retained in freeze-dried food products compared to oven-dried (Gupta et al., 2013; Abascal et al., 2005).
In general, the low temperature of the freeze drying process more likely slow down degradation reactions and preserves the nutrient content of food more efficiently than oven or solar drying (Ratti, 2001; Shilton, 2003). However, the cost of freeze drying equipment limits its application to pharmaceutical products and production of highly valued healthy products such as nettle leaves, nettle leaf powder; nettle leaf tea bags, etc. These products are expensive and therefore only affordable to high economic end consumers (developed market, rich) where such consumers demand a higher value and natural products.
Oven drying is used more often in food processing industries due to its lower production costs leading to products that are more affordable to consumers at the lower end of the market (developing market, poor).
However, information on the effect of oven drying and freeze drying on vitamins, total phenolics content and antioxidant activity of nettle leaves are lacking. This study was carried out to determine the effect of oven drying and freeze drying of nettle leaves on macronutrient, minerals, β-carotene, ascorbic acid and total phenolics content, as well as antioxidant activity. The findings of this study could enable the consumers or food industry to choose either an oven drying or freeze drying method for maximum retention of nutritional properties of stinging nettle leaves.
2. Materials and methods
2.1. Drying processes
Stinging nettle young and tender shoots were produced and harvested in October (spring season) 2014 at the University of Pretoria Experimental Farm Station, South Africa. Spinach leaves (as a control) were purchased from a supermarket. Twenty units (500 g each) of young nettle and spinach leaves were sorted and washed. Treatments, each replicated three times, were prepared as follows. Oven dried leaves (70 °C for 15 h) and freeze dried leaves (-40 °C for 5 days) were prepared. Dried leaves were ground to a powder using a coffee grinder and sealed in polyethylene bags. All samples were kept frozen (- 4 °C) until analysis.
2.2. Nutritional properties
2.2.1. Proximate composition
AACC International (2000) methods were used to determine moisture (method 44-15A), fats (method 30-25), proteins content (N x 6.25) by Dumas combustion (method 46-30), ash (method 08-01) and crude fibre (method 32-10.01) while available carbohydrate content was calculated by difference.
2.2.2. Mineral analyses
AACC International (2000)method No.40–70.01 was used for mineral analyses (Ca, Fe, Mg, Mn, Zn, P, K, and Na) using Ion Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) (SpectroAcros, SPECTRO Analytical Instruments GmbH, Kleve, Germany).
2.2.3. β-Carotene content
The quantitative analysis of β-carotene content of fresh, freeze dried, and oven dried stinging nettle leaves was done using an auto-sampler Shimadzu Ultra-Fast Liquid Chromatography.. Sample extraction and mobile phase preparation were done following the method of Rodriguez-Amaya and Kimura (2004).
The detection of β-carotene was performed at 450 nm and UV/Vis spectra of between 200 to 600 nm, 25 °C on a YMC carotenoid-C30 column (250 × 4.6 mm i.d., 5 μm pore size) through isocratic elution with a methanol:methyl-tert-butyl ether (80:20, v/v) mobile phase at a flow rate of 0.8 ml/min (Kimura et al., 2007). Identification of the all-trans-β-carotene was carried out through the combined use of the retention time, visible absorption spectrum obtained with a photodiode array detector and co-injection with an all-trans-β-carotene standard at four different concentration levels (calibration curve of the all-trans-β-carotene standard, Figure 1)). The chromatogram is presented in Figure 2, and all-trans-β-carotene (peak 1) was identified by comparing the spectrum (λ max) (Figure 3) with those given in literature (Mercadante et al., 1997; Rodriguez-Amaya and Kimura, 2004) and retention time of the all-trans-β-carotene standard. Vitamin A content of fresh and dried stinging nettle leaves was calculated using retinol activity equivalents (RAE) conversion factor of 12 μg β-carotene to 1 μg retinol (Joint FAO/WHO, 2001).
Figure 1.
Standard curve of the all-trans-β-carotene standard.
Figure 2.
HPLC chromatograms of the all-trans-β-carotene standard
Figure 3.
HPLC UV/Vis spectra of the all-trans-β-carotene standard.
2.2.4. Ascorbic acid content
The quantitative analysis of ascorbic acid content of fresh, freeze-dried, and oven-dried leaves was carried out using high-performance liquid chromatography-HPLC (Waters Alliance, Milford, Massachusetts, USA) equipped with 1525 Binary pump system, 2487 Dual λ Absorbance detector (operated at 254 nm) and manual injection valve with 20-μL sample loop.
Sample extraction and mobile phase preparation was done following Maia et al. (2007).The components were filtered through 0.45μm Nylon filters (Millipore) before analysis by the HPLC.
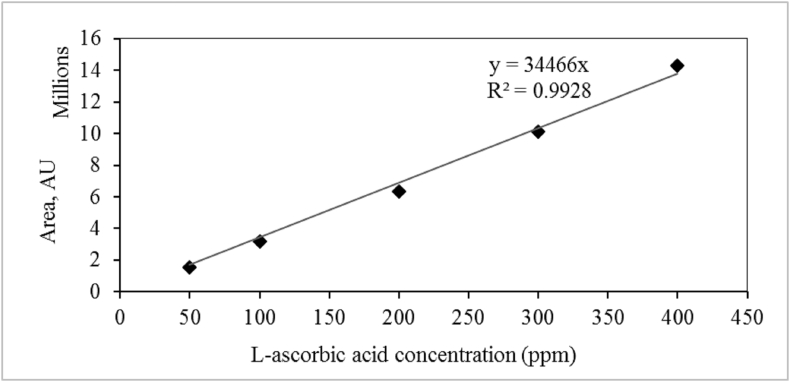
The separation of ascorbic acid was performed on a 4.6 mm × 250 mm i.d., 5μm pore size Phenomenex-C18 column by isocratic elution with a mobile phase consisting of 0.2 % metaphosphoric acid/methanol/acetonitrile (90:8:2, v/v/v) at a flow rate of 0.9 ml/min. The quantification of ascorbic acid was done using a calibration curve of L-ascorbic acid standard (Figure 4). The determination of ascorbic acid content was carried out by HPLC through the combined use of the retention time and co-injection with L-ascorbic acid standard (Figure 5).
Figure 4.
Standard curve of L-ascorbic acid standard.
Figure 5.
L-ascorbic acid HPLC chromatograms
2.2.5. Total antioxidant activity and phenols content
Sample extraction was performed as described by Otles and Yalcin (2012). Fresh, freeze-dried, and oven-dried leaves (1 g each) were extracted in covered test tubes in a drying oven (PROLAB, Model: IDS 160, Switzerland) for 1 h at 50 °C using 10 mL of 80 % methanol-water mixture. The extracts were centrifuged at 3000 rpm for 10 min and the supernatants were recovered for analysis. Total phenolics content was determined using the Folin-Ciocalteau (FC) method (Singleton et al., 1999). Total antioxidant activity of fresh, freeze-dried, and oven-dried leaves were determined using the DPPH Radical Scavenging Activity Assay according to Brand-Williams et al. (1995).
2.2.6. The contribution of fresh or dried stinging nettle leaves to dietary intakes of protein, minerals and vitamins content
The % contribution of a serving of fresh (100 g), freeze dried (15 g) or oven dried (15 g) leaves (see Table 1) to the daily value (DV) (i.e. Daily values of nutrients provided in Table 2) [based on a caloric intake of 2, 000 Cal for adults and children four or more years of age as described by US FDA (2013) of a specific nutrient including minerals (Ca, Fe, Mg, Mn, Zn, P, K, and Na), protein, vitamin A and vitamin C was determined as follows (1):
| (1) |
Table 1.
Recommended serving sizes for green leafy vegetables and stinging nettle leaf food products.
| Food products | Serving sizes (g) | References |
|---|---|---|
| Green leafy vegetables | 80–100 | (Joint WHO/FAO, 2003; Naude, 2013b, 2013a) |
| Fresh nettle leaves | 100 | (Danesi et al., 2013; Rutto et al., 2013) |
| Dried nettle leaves | 15–18 | (Ait Haj Said et al., 2015) |
| Infusion or decoction | 250 | (Gallaher et al., 2006) |
Table 2.
Daily values (DVs∗) and conditions for nutrient content claims in food products as described by US FDA (2013).
| Nutrients | DVs | Unit | Conditions for nutrient contents |
|
|---|---|---|---|---|
| Conditions (% DV per serving) | Claim | |||
| Vitamin A | 870 | μg | ||
| Vitamin C | 60 | mg | ||
| Calcium | 1000 | mg | ≤5% | Low |
| Iron | 18 | mg | 6–9 % | Source |
| Magnesium | 400 | mg | 10–19 % | Good source |
| Manganese | 2 | mg | ≥20 % | High source or ‘rich in’ |
| Phosphorus | 1000 | mg | ||
| Zinc | 15 | mg | ||
| Potassium | 3.5 | g | ||
| Protein | 50 | g | ||
| Sodium | 2.4 | g | 0.12 g per 100 g or per serving | Low |
| 0.04 g per 100 g or per serving | Very Low | |||
| 0.005 g per 100 g or per serving | Free | |||
DVs based on a caloric intake of 2,000 cal, for adults and children four or more years of age.
2.3. Data analyses
Analysis of variance (ANOVA) in XLSTAT 2015 (AddinSoft™ SARL, Paris, France) was applied to assess the effects of species (viz. stinging nettle, spinach (Spinacia oleracea) included as a control) and drying method (viz. freeze drying and oven drying) on macronutrients, minerals content, ascorbic acid, β-carotene content, total phenols content and antioxidant activity. Fisher's Least Significant Difference (LSD) test was applied to separate statistically significant means (at the 5 % level).
3. Results
3.1. Proximate composition
Results for the proximate composition of the fresh, freeze-dried and oven-dried leaves are presented in Table 3. The moisture, fat, crude fibre, ash, crude protein and available carbohydrate contents were significantly affected by species and drying method, whereas only moisture and available carbohydrates were significantly affected by the species and drying method interaction effect. For fresh leaves, crude fibre and crude protein content were higher (p < 0.01) in nettle leaves compared to spinach. For freeze-dried and oven-dried samples; fat, crude fibre, ash, and crude protein were higher (p < 0.01) in nettle leaves compared to spinach, whereas available carbohydrates and moisture content were higher (p < 0.01) in spinach.
Table 3.
Effect of drying method on the mean proximate composition (g/100 g ± SD) and percent daily value (% DV∗) of stinging nettle and spinach∗∗leaves.
| Drying method (DM) |
Fresh |
Freeze dried |
Oven dried |
p-values |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Species (SP) | Nettle | Spinach | Nettle | Spinach | Nettle | Spinach | SP | DM | SP x DM |
| Moisture | 85.0b (1.5) | 89.6a (0.6) | 6.4c (0.2) | 6.6c (0.0) | 3.4d (0.1) | 4.5d (0.0) | 0.01 | 0.01 | 0.01 |
| Ash | 3.1c (0.3) | 1.8c (0.2) | 19.5a (0.2) | 16.7b (0.5) | 19.2a (1.0) | 17.0b (0.7) | 0.01 | 0.01 | 0.23 |
| Macronutrients | |||||||||
| Fats | 0.6d (0.1) | 0.4d (0.0) | 3.5a (0.1) | 3.2b (0.1) | 3.4ab (0.1) | 2.8c (0.1) | 0.01 | 0.01 | 0.15 |
| Available carbohydrates | 2.2e (0.2) | 2.4e (0.1) | 14.7d (0.7) | 22.1b (0.6) | 18.9c (2.2) | 26.1a (0.4) | 0.01 | 0.01 | 0.01 |
| Fibres | 4.6d (0.5) | 2.9e (0.2) | 27.5a (0.5) | 25.4b (0.3) | 26.3ab (1.0) | 23.8c (0.1) | 0.01 | 0.01 | 0.50 |
| Proteins | 4.6c (0.4) | 2.9d (0.1) | 28.3a (0.4) | 26.0b (0.4) | 28.8a (0.4) | 25.8b (0.1) | 0.01 | 0.01 | 0.08 |
| Proteins, g/serving | 4.6 | 2.9 | 4.2 | 3.9 | 4.3 | 3.9 | |||
| % DV | 9.2 | 5.8 | 8.5 | 7.8 | 8.6 | 7.7 | |||
a-d Means within same rowwith different superscripts are different (p < 0.05) when analysed using analysis of variance.
Spinach leaves were used as anexternal reference sample.
3.2. Minerals content
The concentrations of eight minerals (Ca, Fe, Mg, Mn, P, Zn, K, and Na) determined in fresh, freeze dried and oven dried stinging nettle and spinach leaves are shown in Table 4. Nettle leaves were found to contain significantly higher (p < 0.01) Fe, K, Mg, Mn, Ca and Zn compared to spinach leaves. The drying processes did not affect the mineral content of the leaves.
Table 4.
Effect of drying method on the mean minerals content (mg/100 g, db ± SD) and percent daily value (% DV∗, as is basis) of stinging nettle and spinach∗∗leaves.
| Drying method (DM) |
Fresh |
Freeze dried |
Oven dried |
p-values |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Species (SP) | Nettle | Spinach | Nettle | Spinach | Nettle | Spinach | SP | DM | SP X DM |
| Ca | 2136a (182) | 500b (4) | 2283a (198) | 536b (4) | 2065a (133) | 543b (26) | 0.01 | 0.31 | 0.32 |
| Ca, mg/serving | 323 | 52 | 320 | 75 | 299 | 78 | |||
| % DV | 32.3 | 5.2 | 32 | 7.5 | 29.9 | 7.8 | |||
| Fe | 16.7a (0.7) | 7.5b (1.8) | 17.9a (0.7) | 8.0b (1.9) | 17.6a (1.8) | 8.3b (1.6) | 0.01 | 0.57 | 0.93 |
| Fe, mg/serving | 2.5 | 0.8 | 2.5 | 1.1 | 2.6 | 1.2 | |||
| % DV | 14 | 4.3 | 14 | 6.3 | 14.2 | 6.6 | |||
| Mg | 692a (13) | 430b (95) | 740a (14) | 460b (102) | 726a (32) | 411b (93) | 0.01 | 0.60 | 0.80 |
| Mg, mg/serving | 104 | 45 | 104 | 64 | 105 | 59 | |||
| % DV | 26 | 11.2 | 26 | 16.1 | 26.3 | 14.7 | |||
| Mn | 2.5ab (0.1) | 2.3c (0.1) | 2.7a (0.1) | 2.5bc (0.1) | 2.6ab (0.0) | 2.3bc (0.2) | 0.01 | 0.09 | 0.97 |
| Mn, mg/serving | 0.4 | 0.2 | 0.4 | 0.3 | 0.4 | 0.3 | |||
| % DV | 19.0 | 11.9 | 18.9 | 17.2 | 18.5 | 16.8 | |||
| Zn | 3.5a (0.1) | 2.4b (0.6) | 3.8a (0.1) | 2.6b (0.7) | 3.8a (0.2) | 2.5b (0.7) | 0.01 | 0.71 | 0.94 |
| Zn, mg/serving | 0.5 | 0.2 | 0.5 | 0.4 | 0.6 | 0.4 | |||
| % DV | 3.5 | 1.7 | 3.5 | 2.4 | 3.7 | 2.4 | |||
| P | 550a (31) | 543a (62) | 588a (33) | 582a (66) | 584a (47) | 558a (53) | 0.60 | 0.44 | 0.93 |
| P, mg/serving | 82.6 | 56.7 | 82.5 | 81.5 | 84.6 | 80 | |||
| % DV | 8.3 | 5.7 | 8.2 | 8.2 | 8.5 | 8.0 | |||
| K | 1266a (60) | 610b (73) | 1354a (62) | 653b (78) | 1278a (43) | 663b (91) | 0.01 | 0.30 | 0.58 |
| K, g/serving | 0.2 | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | |||
| % DV | 5.4 | 1.8 | 5.4 | 2.6 | 5.3 | 2.7 | |||
| Na | 3.1b (0.8) | 110.9a (25) | 3.3b (0.9) | 118.7a (26) | 3.4b (0.9) | 107.9a (26) | 0.01 | 0.87 | 0.87 |
| Na, g/serving | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| % DV | 0.0 | 0.5 | 0.0 | 0.7 | 0.0 | 0.6 | |||
ab Means within same rowwith different superscripts are different (p < 0.05) when analysed using analysis of variance.
Spinach leaves were used as anexternal reference sample.
3.3. The effects of drying methods on β-carotene, ascorbic acid, total phenols content and antioxidant activities
The result for the effects of drying on β-carotene, ascorbic acid, total phenols content and total antioxidant activities of nettle and spinach leaves can be found in Table 5. Ascorbic acid, β-carotene, total phenols content and total antioxidant activities were significantly (p < 0.01) affected by species and drying methods.
Table 5.
Effect of drying method on the mean values of β-carotene (μg/100 g), ascorbic acid (mg/100 g), total phenols content (TPC, mg GAE/g), total antioxidant activity (TAA, % DPPH inhibition) (±SD) and percent daily value (%DV∗, as is basis) of stinging nettle and spinach∗∗∗ leaves.
| Drying method (DM) |
Fresh |
Freeze dried |
Oven dried |
p-values |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Species (SP) | Nettle | Spinach | Nettle | Spinach | Nettle | Spinach | SP | DM | SP x DM |
| β-carotene | 58059a (243) | 44160d (256) | 56341b (453) | 43537d (62) | 52504c (447) | 40847e (479) | 0.01 | 0.01 | 0.01 |
| Vitamin A, μg RAE∗∗/serving | 728 | 383 | 659 | 508 | 634 | 488 | |||
| % DV | 83.7 | 44.0 | 75.7 | 58.4 | 72.9 | 56.0 | |||
| Ascorbic acid | 94.7a (3.3) | 77.0c (0.5) | 83.8b (2.3) | 75.7c (0.6) | 68.5d (0.6) | 65.3d (2.1) | 0.01 | 0.01 | 0.01 |
| Vitamin C, mg/serving | 14.2 | 8.0 | 11.8 | 10.6 | 9.9 | 9.4 | |||
| % DV | 23.7 | 13.3 | 19.6 | 17.7 | 16.5 | 15.6 | |||
| TPC | 118.4b (0.8) | 87.3d (3.9) | 121.5b (3.8) | 111.0c (1.5) | 128.7a (1.3) | 117.8b (0.2) | 0.01 | 0.01 | 0.01 |
| TAA | 65.1b (1.6) | 52.9c (1.6) | 66.6b (1.7) | 55.1c (0.5) | 70.6a (0.9) | 65.1b (0.6) | 0.01 | 0.01 | 0.01 |
a-d Means within same row with different superscripts are different (p < 0.05) when analysed using analysis of variance.
Without ‘db’ represent dry basis.
Spinach leaves were used as anexternal reference sample.
represent vitamin A content of fresh and dried leaves was calculated as retinol activity equivalents (RAE) using an RAE conversion factor of 12 μg β-carotene to 1 μg retinol (Joint FAO/WHO, 2001).
Stinging nettle leaves contained significantly (p < 0.01) more ascorbic acid, β-carotene, total phenols content and total antioxidant activities compared to spinach. Oven drying of nettle leaves resulted in a higher loss of β-carotene and ascorbic acid content compared to freeze drying.
3.4. The contribution of fresh or dried stinging nettle leaves to dietary intakes of proteins, minerals and vitamins
The percent daily value of proteins from a serving of fresh (100 g) or dried (15 g) nettle leaves was found to be 9 % (Table 3). Similarly, a typical serving of fresh or dried nettle leaves provided approximately 30–32 % Ca, 14 % Fe, 26 % Mg, 19 % Mn, 4 % Zn, 9 % P, and 5 % K to the daily values of the respective mineral elements (e.g. 1000 mg Ca, 18 mg Fe, 400 mg Mg, 2 mg Mn, 15 mg Zn, 1000 mg P and 3.5 g K per day) (Table 4). The percent daily value of β-carotene (calculated as RAE, vitamin A) and vitamin C to the daily requirement of these nutrients (870 μg/day for vitamin A and 60 mg/day for vitamin C) were found to be higher in fresh (83.7 %, 23.7 %), followed by freeze-dried (75.7 %, 19.6 %) leaves compared to oven-dried (72.9 %, 16.5 %) leaves, respectively (Table 5).
4. Discussion of results
The fat, fibre, ash and protein content of freeze dried and oven dried nettle leaves were found to be significantly higher than spinach leaves. The variability in preharvest conditions (e.g. growth conditions, type of fertilizers, climatic conditions, and genotypic difference) could have an immense role leading to variability in nutrients accumulated by nettle leaves and spinach. For example, the same species of stinging nettle produced at different agro-ecological conditions were reported to contain different concentrations of nutrients (Otles and Yalcin, 2012; Farag et al., 2013). According to Allen et al. (2006) and Nishida et al. (2004), a food may be described as a part of a healthy diet if the food carries a statement describing the conditions (e.g. rich source and good source) of the nutrient content claims per 100 g or per serving as provided in the dietary guidelines. For vegetables, the recommendation for school children and adults is at least 400 g/day of vegetables (80–100 g per serving or 4 to 5 servings) (World Health Organization, 2003). Recommended serving sizes was reported to be 80–100 g for green leafy vegetables (Danesi et al., 2013; Naude, 2013a; Joint WHO/FAO, 2003), 100 g for fresh nettle leaves (Danesi et al., 2013; Rutto et al., 2013) and 15–18 g for dried nettle leaves (Ait Haj Said et al., 2015).
Accordingly, a serving size of 100 g for fresh leaves and 15 g dried nettle leaves were used to determine the potential contribution of fresh or dried nettle leaves to the dietary intakes of the specific nutrient. As described by US FDA (2013), a food product with 5 % DV or less is considered low for that specific nutrient, 6–9 % DV a source, 11–19 % DV a good source and 20 % DV or more indicates a rich source (Table 2). Fresh or dried stinging nettle leaves were found to be a source of proteins; one will need to consume at least two servings of fresh or dried nettle leaves to reach 10 % of the DV (300 g/day for protein).
The high ash content of nettle leaves explains their higher concentration of Fe, K, Mg, Mn, Ca and Zn compared to spinach leaves. This could be attributed to differences in cultural practices such as frequency of irrigation, fertilizer type, rate and time of application as well as fertility status of the soil during production (Lee and Kader, 2000; Walker et al., 2010). Pytlakowska et al. (2012) reported that medicinal plants (such as nettle, senna leaves) strongly vary in mineral elements concentration (e.g. Fe, Zn, Mn, Mg, K, Na, P, and Ca) because of differing absorption of mineral elements from the soil. Other authors reported variation in macro and micro mineral contents of moringa leaves sampled from different areas (Gyamfi et al., 2011).
Fresh and dried nettle leaves can be considered as a “good source” of Fe and Mn because the contribution from a serving of the leaves was more than 10 % of the DV for these nutrients and rich source for Ca and Mg (US FDA, 2013; Joint FAO/WHO, 2007). In developing countries, anaemia was reported to be a serious problem in pregnant woman and preschool children (World Health Organization, 2003). Anaemia also contributes to 20 % of all maternal deaths. A nettle leaf in both fresh and dried forms was found to be a good source of iron. Therefore, integrating stinging nettle leaves in the diet could help to combat anaemia which was reported to affect more than 60 % of children in Africa (Standing Committee on Nutrition, 2010).
A person will need to consume at least two to three servings of fresh or dried nettle to reach the 10 % DV for Zn, P and K. Fresh and dried stinging nettle leaves could be categorized as ‘free’ for sodium content of the leaves, as the contribution of the sodium per serving of the leaves to the daily value of sodium (e.g. 2.4 g per day) is not more than 0.005 g (US FDA, 2013; Joint FAO/WHO, 2007). The low level of sodium in stinging nettle leaves could be beneficial for persons on a restricted sodium diet. According to the World Health Organization (2014) an estimated 2.5 million deaths could be prevented each year if global salt consumption was reduced to the recommended level (2.4 g per day).
The high potassium/sodium ratio (K/Na ratio ranging from 381 to 494) of stinging nettle leaves could be also another potential indicator of the protective powers of the stinging nettle leaves foliage against cardiovascular and neoplastic diseases (Kavalali, 2003; World Health Organization, 2014).
However, Jimoh et al. (2010) reported that stinging nettle leaves contained antinutrients such as alkaloids (0.6 mg/100 g), phytates (4.39 mg/100 g) and saponins (3.25 mg/100 g). The phytates in the green leafy vegetables can be reduced by soaking, boiling or frying (Akubugwo and Obasi, 2007). Kruger et al. (2015) reported that cooking maize meal fortified with green leafy vegetable (porridge) resulted in a decrease in phytates content and concomitant increase in bio-accessibility of Fe and Zn in the porridge. Bravo (2009) pointed out that tannins have the ability to chelate Fe and Zn by binding with their hydroxyl and carbonyl groups, and thereby reduces the bioavailability of these minerals. Therefore, cooking of stinging nettle leaves could potentially decrease the phytates content and increase the bio-accessibility of Fe and Zn in the cooked leaves.
The β-carotene concentrations determined in fresh stinging nettle leaves samples were higher than those reported by Guil-Guerrero et al. (2003) and Rutto et al. (2013) (for fresh nettle leaves) and Adhikari et al. (2016) (for dried nettle leaves). Similarly, the concentration of ascorbic acid found in fresh stinging nettle leaves samples was higher than those reported by Ioana et al. (2013) and Rutto et al. (2013) (in fresh nettle leaves). This is because the accumulation of vitamins and minerals by the plant tissues is a function of its genotype, pruning and thining, frequency of irrigation, temperature, temperature, sunlight, fertilizers used and fertility status of the soil (Lee and Kader, 2000a). For example, the vitamin C content in plant tissues is highly correlated with the intensity of sunlight and whereas it is negatively correlated with the frequency of irrigation and the rates of nitrogen fertilizer during the growing season of vegetables (Lee and Kader, 2000a; Walker et al., 2010). Furthermore, the β-carotene and ascorbic acid content of stinging nettle leaves could be influenced by postharvest practices (e.g. drying and cooking). Drying conditions could have a great effect on heat and light labile β-carotene and ascorbic acid.
The ascorbic acid and β-carotene content of freeze-dried nettle leaves were higher compared to oven dried leaves. This can be attributed to the higher temperature of the oven drier (>50 °C). During the freeze-drying process the temperature of the product is low (-45 °C), which limits degradation reactions (Ratti, 2001).
The higher loss of β-carotene during oven drying could be because of the highly unsaturated β-carotene structure which can lead to photooxidation and autooxidative reactions (Bernhardt and Schlich, 2006, Di Cesare et al., 2004, Kidmose et al., 2000, Owuor, 2003 ). Chang et al. (2006) reported heating causes increase in conversion of trans-isomers to cis-isomers of β-carotene by 50%. Previous research also confirmed that ascorbic acid and β-carotene are better retained in freeze dried food products compared to oven dried (Abascal et al., 2005; Gupta et al., 2013; Shilton, 2003).
The high loss of ascorbic acid during oven drying could be due to the two hydroxyl groups in its structure which could be oxidized to dehydro-ascorbic acid at high temperature (Ajayi et al., 1980, Sanmartin et al., 2000, Waheed Uz et al., 2013).
The loss of vitamin C in food products can range from 10 % to 50 % depending on temperature of drying processes (Shilton, 2003). This is in agreement with the findings of the present study where the loss of ascorbic acid was found to be 12 % in freeze dried and 22 % loss in oven dried nettle leaves.
Even though oven drying of stinging nettle leaves resulted in a higher loss of β-carotene and ascorbic acid content compared to freeze drying, a serving of either fresh, freeze dried or oven dried nettle leaves provided more than 20 % of the DV of vitamin A (870 μg per day). Therefore, nettle leaves in all forms can be considered as a “rich sources” of vitamin A. In contrast, oven dried nettle leaves found to be a ‘good source for vitamin C while fresh and freeze-dried nettle leaves can be considered as “rich sources” of vitamin C. This could imply that consumption of fresh or dried nettle leaves might help to reduce vitamin A deficiency, which has been estimated to affect about 2.5 million preschool children in Africa (World Health Organization, 2009).
Furthermore, inclusion of β-carotene-rich food in the daily diets, instead of costly synthetic vitamin A supplementation, may be a more successful strategy for improving vitamin A status of at risk or malnourished populations (Gopalan, 1992). Hence, integrating either fresh or dried stinging nettle leaves in the diet or utilization of dried nettle leaves for fortifying cereal-based foods would help address vitamin A and C deficiencies. The high β-carotene content of fresh and dried stinging nettle leaves could also help to address health related problems due to their antioxidant activity (Guo et al., 2008).
Interestingly, the consumption of stinging nettle leaves could provide a double impact as a provitamin A, protein and vitamin C dietary source, and also as an enhancer of Fe absorption. Amagloh et al. (2017) emphasized that Fe bio-availability in dark green leafy vegetables is influenced by protein, ascorbic acid, β-carotene and total polyphenols content in the plant tissues. The authors reported that the high content of ascorbic acid and β-carotene in moringa leaves increased its Fe bio-availability.
The higher antioxidant activity of nettle leaves compared to spinach may be attributed to its β-carotene, phenolic compounds, ascorbic acid, Mn and Zn. Phenolic compounds, carotenoids and ascorbic acid (Rincσn-Leσn et al., 2003, Velioglu et al., 1998), Mn and zinc (Caballero et al., 2015) are nutrient antioxidants.
Additionally, the higher total antioxidant activity and phenols content of nettle leaves compared to spinach leaves found in this study could also be due to variability in genotype and environmental conditions (Vagiri, 2014). Although genetic factors are the main determinants for the content of phenolics and antioxidants, these contents can also be affected by light and temperature conditions of the environment (Tiwari and Cummins, 2013).
Genetic diversity in content of phenolic compounds and antioxidant activity was also reported in nettle leaves. For example, Otles and Yalcin (2012) reported a wide variability in total phenols content (151–1941mg GAE/g DM) and antioxidant activity (ranging from 60.62 to 320.38 mg GAE/g DM) of fresh stinging nettle leaves collected from the Mediterranean, Aegean, Black Sea and Marmara coastal parts in Turkey.
The higher total phenols content of oven-dried compared to freeze-dried leaves could be linked to more efficient extraction of the insoluble phenolic compounds such as condensed tannins, and phenolic acids (Farag et al., 2013; Komes et al., 2011; Pinelli et al., 2008) bound to cell wall polysaccharides or proteins (Giada, 2013; Singleton et al., 1999). Because during oven drying processes phenolic-sugar glycosidic bonds may be cleaved with heat treatment leading to the formation of phenolic aglycons, which react better with the Folin-Ciocalteu reagent leading to higher values of total phenolics (Singleton et al., 1999).
Similarly, the higher total antioxidant activity in oven-dried nettle leaves compared to fresh and freeze dried leaves could probably be due to: 1) release of antioxidant nutrients by thermal (heating effects of the oven dryer) destruction of cell walls and subcellular compartments; 2) formation of antioxidants by thermal chemical reaction; 3) suppression of the oxidation of antioxidants by thermal inactivation of oxidative enzymes. As an example, the increase in total antioxidant activity after heat treatment could be due to the increased release of phytochemicals, such as lycopene, from the matrix (Gahler et al., 2003). Various studies reported to that fresh stinging nettle leaves contain phenolic acid (e.g. hydroxycinnamic acid, hydroxybenzoic acid), tannins and flavonoids (e.g. flavones, flavonols, iso-flavonols, anthocyanins, catechins, lignin) using HPLC method (Farag et al., 2013; Orčić et al., 2014; Pinelli et al., 2008). To this end, HPLC method could enable to accurately track the phenolic compounds as well as the changes in total phenol content and antioxidant activity of dried stinging nettle leaves using different parameters of the selected drying techniques.
The high total phenols content and antioxidant activity in both freeze-dried and oven-dried leaves could present dried nettle leaf powders as good natural antioxidants for application in health-promoting foods and as a food preservative. For example, natural sources of antioxidants are replacing synthetic antioxidants (such as butylated hydroxy anisole, butylated hydroxy toluene, teriary butyl hydroquinone, and propyl gallate) to reduce toxicological and carcinogenic effects (Kumar et al., 2015). Lindsey, Motsei and Jäger (2002) suggested that nettle leaves may not only be a good dietary source but could also be used as a natural antioxidant in the food industry. Applications of the use of extracts from herbs like rosemary and oregano (Rojas and Brewer, 2008) and sage (Mariutti et al., 2011) in meat and poultry products have been well demonstrated.
A voucher specimen of the plant materials used was not included in this research. Given that the focus was on investigating the effect of the drying methods on response variables and not on botanical aspects (such as identification of the taxon and location of the stinging nettle), priority was given to including an external reference sample (spinach). Nonetheless, in future research of this nature, it would be useful to include a stinging nettle voucher specimen if available.
5. Conclusions
Even though, oven drying of nettle leaves results in a higher loss of β-carotene and ascorbic acid content compared to freeze drying, approximately 90 % and 72 %, respectively, of the nutrients are retained in the oven-dried leaves. In contrast, oven drying increases the total antioxidant activity and phenol content of nettle leaves more than freeze drying does. Overall, fresh stinging nettle leaves can be considered as a rich source of antioxidants, Ca, Mg, vitamins A and C, a good source of Fe and Mn and a source of P and K. Whereas, freeze-dried and oven-dried stinging nettle leaves can be considered as a rich source of antioxidants, Ca, Mg, and vitamin A, while it is a good source of vitamin C, Fe and Mn. These benefits present possible avenues for utilization of dried nettle leaves or leaf powder by the food industry and consumers for addressing micronutrient deficiencies and for providing a healthy diet. Further research should consider more drying methods and/or different parameters of the selected drying techniques and determine/track the change in TPC and antioxidant activity using the HPLC method.
Declarations
Author contribution statement
Tigist T. Shonte: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Geybi K. Duodu, Henriëtte L. de Kock: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Tigist T. Shonte was supported by the Organization for Women in Sciences for Developing Countries (OWSD), Trieste, Italy, which contributed towards a PhD Scholarship.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The intellectual and material contributions of OWSD and Sida (Swedish International Development Cooperation Agency) are acknowledged. We appreciate and thank the personnel of the Crop Production and Soil Science Field Trial Section and the Experimental Farm Station of University of Pretoria that facilitated the field work.
References
- AACC International . AACC International; St. Paul, MN, U.S.A.: 2000. AACC International Approved Methods of Analysis. [Google Scholar]
- Abascal Kathy, Ganora Lisa, Yarnell Eric. The effect of freeze-drying and its implications for botanical medicine: a review. Phytother Res. 2005;19(8):655–660. doi: 10.1002/ptr.1651. [DOI] [PubMed] [Google Scholar]
- Adhikari Bhaskar Mani, Bajracharya Alina, Ashok K., Shrestha Comparison of nutritional properties of stinging nettle (Urtica dioica) flour with wheat and barley flours. Food Sci. Nutr. 2016;4(1):119–124. doi: 10.1002/fsn3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait Haj Said, Amal, El Otmani Ibrahim Sbai, Derfoufi Sanae, Benmoussa Adnane. Highlights on nutritional and therapeutic value of stinging nettle (Urtica dioica) Int. J. Pharm. Pharmaceut. Sci. 2015;7(10):8–14. [Google Scholar]
- Ajayi S.O. Vitamin C Losses in Cooked Fresh Leafy Vegetables. Food Chem. 1980;5(3):243–247. [Google Scholar]
- Akubugwo I.E., Obasi N.A. Nutritional and Chemical Value of Amaranthus Hybridus L. Leaves from Afikpo, Nigeria. Afr. J. Biotechnol. 2007;6(24):2833–2839. [Google Scholar]
- Alibas Ilknur. Characteristics of chard leaves during microwave, convective, and combined microwave-convective drying. Dry. Technol. 2006;24(11):1425–1435. [Google Scholar]
- Allen Lindsay, de Benoist Bruno, Dary Omar, Hurrell Richard. Lindsay Allen, Bruno de Benoist, Omar Dary, and Richard Hurrell. France: WHO/FAO of United Nations. 2006. Guidelines on food fortification with micronutrients. [Google Scholar]
- Amagloh Francis Kweku. Nutrient and Total Polyphenol Contents of Dark Green Leafy Vegetables, and Estimation of Their Iron Bioaccessibility Using the In Vitro Digestion/Caco-2 Cell Model. Foods. 2017;6(7) doi: 10.3390/foods6070054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt Simone, Schlich Elmar. Impact of Different Cooking Methods on Food Quality: Retention of Lipophilic Vitamins in Fresh and Frozen Vegetables. J. Food Eng. 2006;77(2):327–333. [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 1995;28(1):25–30. [Google Scholar]
- Bravo Laura. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 2009;56(11):317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Caballero Benjamin. Encyclopedia of Food and Health. Elsevier Science Ltd; Amsterdam, Netherlands: 2015. Antioxidant activity of foods. [Google Scholar]
- Chang Ching Hui, Lin Hsing Yu, Chang Chi Yue, Liu Yung Chuan. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 2006;77(3):478–485. [Google Scholar]
- Danesi Francesca, Pasini Federica, Caboni Maria Fiorenza, D’Antuono Luigi Filippo, Bordoni Alessandra. Traditional foods for health: screening of the antioxidant capacity and phenolic content of selected Black Sea area local foods. J. Sci. Food Agric. 2013;93(14):3595–3603. doi: 10.1002/jsfa.6339. [DOI] [PubMed] [Google Scholar]
- Dey Tilottama. Department of Chemical Engineering Jadavpur University; 2013. Freeze Drying of Novel Materials. [Google Scholar]
- Di Cesare L.F. Influence of Drying Techniques on the Volatile Phenolic Compounds, Chlorophyll and Colour of Oregano (Origanum Vulgare L. Ssp. Prismaticum Gaudin) Ital. J. Food Sci. 2004;16(2):165–175. [Google Scholar]
- Farag Mohamed A., Weigend Maximilian, Luebert Federico, Brokamp Grischa, Wessjohann Ludger A. Phytochemical, phylogenetic, and anti-inflammatory evaluation of 43 Urtica accessions (stinging nettle) based on UPLC-Q-TOF-MS metabolomic profiles. Phytochemistry. 2013;96:170–183. doi: 10.1016/j.phytochem.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Gahler Susan. Alterations of Vitamin C, Total Phenolics, and Antioxidant Capacity as Affected by Processing Tomatoes to Different Products. J. Agric. Food Chem. 2003;51(27):7962–7968. doi: 10.1021/jf034743q. [DOI] [PubMed] [Google Scholar]
- Gallaher R.N., Gallaher K., Marshall A.C.J., Marshall A.C.J. Mineral analysis of ten types of commercially available tea. J. Food Compos. Anal. 2006;19(SUPPL):53–57. [Google Scholar]
- Giada Maria de Lourdes Reis. Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power. In: José A. Morales-González., editor. Oxidative Stress and Chronic Degenerative Diseases – A Role for Antioxidants. InTech; Croatia - European Union: 2013. pp. 87–112. [Google Scholar]
- Gião Maria S., González-Sanjosé Maria L., Rivero-Pérez Maria D., Pereira Cláudia I., Pintado Manuela E., Xavier Malcata F. Infusions of Portuguese medicinal plants: dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007;87(14):2638–2647. doi: 10.1002/jsfa.3023. [DOI] [PubMed] [Google Scholar]
- Gopalan C. Kwashiorkor and Marasmus: Evolution and Distinguishing Features. Natl. Med. J. India. 1992;5(3):145–151. [PubMed] [Google Scholar]
- Guil-Guerrero J.L., Rebolloso-Fuentes M.M., Torija Isasa M.E. Fatty acids and carotenoids from stinging nettle (Urtica dioicaL.) J. Food Compos. Anal. 2003;16(2):111–119. [Google Scholar]
- Guo Wen Hsin. Cis-Trans Isomerizations of β -Carotene and Lycopene: A Theoretical Study. J. Phys. Chem. 2008;112(38):12158–12167. doi: 10.1021/jp8019705. [DOI] [PubMed] [Google Scholar]
- Gupta Sheetal, Gowri B.S., Lakshmi A. Jyothi, Prakash Jamuna. Retention of nutrients in green leafy vegetables on dehydration. J. Food Sci. Technol. 2013;50(5):918–925. doi: 10.1007/s13197-011-0407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamfi E.T., Kwarteng I.K., Ansah M.O., Anim A.K., Ackah M., Kpattah Lebene, Bentil Nash O. Effects of processing on Moringa oleifera. Proc. Int. Acad. Ecol. Environ. Sci. 2011;1:179–185. [Google Scholar]
- Harbourne Niamh, Marete Eunice, Jacquier Jean Christophe, O’Riordan Dolores. Effect of drying methods on the phenolic constituents of meadowsweet (Filipendula ulmaria) and willow (Salix alba) LWT - Food Sci. Technol. 2009;42(9):1468–1473. [Google Scholar]
- Hossain M.B.B., Barry-Ryan C., Martin-Diana A.B.B., Brunton N.P.P. Effect of drying method on the antioxidant capacity of six lamiaceae herbs. Food Chem. 2010;123(1):85–91. [Google Scholar]
- Hughes R. Elwyn, Ellery Peter, Harry Tim, Jenkins Vivian, Jones Eleri. The dietary potential of the common nettle. J. Sci. Food Agric. 1980;31(12):1279–1286. doi: 10.1002/jsfa.2740311210. [DOI] [PubMed] [Google Scholar]
- Ioana Nencu. Preliminary Research Regarding the Therapeutic Uses of Urtica dioica l Note Ii. The Dynamics of Accumulation of Total Phenolic Compounds and Ascorbic Acid. Farmacia. 2013;61(2):276–283. [Google Scholar]
- Jimoh Florence, Adedapo Adeolu, Aliero Adamu, Anthony Afolayan. Polyphenolic and biological activities of leaves extracts of Argemone subfusiformis(papaveraceae) and Urtica urens(urticaceae) Rev. Biol. Trop. 2010;58(4):1517–1531. doi: 10.15517/rbt.v58i4.5428. [DOI] [PubMed] [Google Scholar]
- Joint FAO/WHO . 2001. Human Vitamin and Mineral Requirements. Geneva, Switzerland. [Google Scholar]
- Joint FAO/WHO . World Health Organization; Geneva, Switzerland: 2007. Food Labelling. [Google Scholar]
- Joint WHO/FAO . 2003. Diet, Nutrition and the Prevention of Chronic Diseases. Geneva, Switzerland. [PubMed] [Google Scholar]
- Kara Derya. Evaluation of trace metal concentrations in some herbs and herbal teas by principal component analysis. Food Chem. 2009;114(1):347–354. [Google Scholar]
- Kavalali Gulsel M. Taylor & Francis Ltd; New York, USA: 2003. Urtica: Therapeutic and Nutritional Aspects of Stinging Nettles. [Google Scholar]
- Kidmose U. Colour Stability in Vegetables. In: Douglas B Kidmose., editor. Colour in Food: Improving Quality. oodhead Publishing Limited and CRC Press LLC; Sawston, Cambridge: 2000. pp. 179–218. [Google Scholar]
- Kimura Mieko, Kobori Cintia N., Rodriguez-Amaya Delia B., Nestel Penelope. Screening and HPLC methods for carotenoids in sweetpotato, cassava and maize for plant breeding trials. Food Chem. 2007;100(4):1734–1746. [Google Scholar]
- Komes Draženka. Phenolic Composition and Antioxidant Properties of Some Traditionally Used Medicinal Plants Affected by the Extraction Time and Hydrolysis. Phytochem. Anal. 2011;22(2):172–180. doi: 10.1002/pca.1264. [DOI] [PubMed] [Google Scholar]
- Kruger Johanita, Mongwaketse Tiyapo, Faber Mieke, van der Hoeven Marinka, Cornelius M., Smuts Potential contribution of african green leafy vegetables and maize porridge composite meals to iron and zinc nutrition. Nutrition. 2015;31(9):1117–1123. doi: 10.1016/j.nut.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Kumar Yogesh. Recent Trends in the Use of Natural Antioxidants for Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2015;14(6):796–812. [Google Scholar]
- Lee Seung K., Kader Adel A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000;20(20):207–220. [Google Scholar]
- Lindsey K.L., Motsei M.L., Jäger A.K. Screening of South African Food Plants for Antioxidant Activity. J. Food Sci. 2002;67(6):2129–2131. [Google Scholar]
- Maanda M.Q., Bhat R.B. Wild vegetable use by vhavenda in the Venda region of limpopo province, South Africa. In. J. Exp. Bot. 2010;79:189–194. [Google Scholar]
- Maia Adriana M., Baby André Rolim, Yasaka Wilson J., Suenaga Eunice, Kaneko Telma M., Maria Valéria R. Velasco. Validation of HPLC stability-indicating method for vitamin C in semisolid pharmaceutical/cosmetic preparations with glutathione and sodium metabisulfite, as antioxidants. Talanta. 2007;71(2):639–643. doi: 10.1016/j.talanta.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Mariutti Lilian Regina Barros. Lipid and Cholesterol Oxidation in Chicken Meat Are Inhibited by Sage but Not by Garlic. J. Food Sci. 2011;76(6):c909–c915. doi: 10.1111/j.1750-3841.2011.02274.x. [DOI] [PubMed] [Google Scholar]
- Mercadante Adriana Z., Rodriguez-Amaya Délia B., George Britton. HPLC and mass spectrometric analysis of carotenoids from mango. J. Agric. Food Chem. 1997;45:120–123. [Google Scholar]
- Naude Celeste E. “Food-Based dietary guidelines for South Africa: the ‘eat plenty of vegetables and fruit every day. S. Afr. J. Clin. Nutr. 2013;26(3):46–56. [Google Scholar]
- Naude Celeste E. Would an increase in vegetable and fruit intake help to reduce the burden of nutrition-related disease in South Africa? An umbrella review of the evidence. S. Afr. J. Clin. Nutr. 2013;26(3):104–114. [Google Scholar]
- Nishida Chizuru, Uauy Ricardo, Kumanyika Shiriki, Shetty Prakash. The Joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Publ. Health Nutr. 2004;7(1A):245–250. doi: 10.1079/phn2003592. [DOI] [PubMed] [Google Scholar]
- Orčić Dejan, Francišković Marina, Bekvalac Kristina, Svirčev Emilija, Beara Ivana, Lesjak Marija, Mimica-Dukić Neda. Quantitative determination of plant phenolics in Urtica dioicaextracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem. 2014;143(January):48–53. doi: 10.1016/j.foodchem.2013.07.097. [DOI] [PubMed] [Google Scholar]
- Otles Semih, Yalcin Buket. Phenolic compounds analysis of root, stalk, and leaves of nettle. Sci. World J. 2012;2012:1–12. doi: 10.1100/2012/564367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owuor P.O. Tea: Analysis and Tasting. In: Caballero Benjamin, Trugo Luiz, Finglas Paul M., editors. Encyclopedia of Food Sciences and Nutrition. Elsevier Science Ltd; Amsterdam, Netherlands: 2003. pp. 5757–5762. [Google Scholar]
- Özcan Musa, Mehmet, Ünver Ahmet, Uçar Tolga, Arslan Derya. Mineral content of some herbs and herbal teas by infusion and decoction. Food Chem. 2008;106(3):1120–1127. [Google Scholar]
- Pinelli Patrizia, Ieri Francesca, Vignolini Pamela, Bacci Laura, Baronti Silvia, Romani Annalisa. Extraction and HPLC analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica dioicaL. J. Agric. Food Chem. 2008;56(19):9127–9132. doi: 10.1021/jf801552d. [DOI] [PubMed] [Google Scholar]
- Popov Saša, Skeledžija Suzana, Šorgić Saša, Zeković Zoran, Micić Darko, Radulović Aleksandra, Đurović Saša. Application of contemporary extraction techniques for elements and minerals recovery from stinging nettle leaves. Appl. Sci. 2020;10(3):793. [Google Scholar]
- Pytlakowska K., Kita A., Janoska P., Połowniak M., Kozik V. Multi-element analysis of mineral and trace elements in medicinal herbs and their infusions. Food Chem. 2012;135(2):494–501. doi: 10.1016/j.foodchem.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Ratti Cristina. Hot air and freeze-drying of high value foods: a review. J. Food Eng. 2001;49(4):311–319. [Google Scholar]
- Rincón-León F. Encyclopedia of Food Sciences and Nutrition. Elsevier Science Ltd; 2003. pp. 5737–5743. [Google Scholar]
- Rodrigues A.S., Pérez-Gregorio M.R., García-Falcón M.S., Simal-Gándara J. Effect of curing and cooking on flavonols and anthocyanins in traditional varieties of onion bulbs. Food Res. Int. 2009;42(9):1331–1336. [Google Scholar]
- Rodriguez-Amaya D.B., Kimura M. International Food Policy Research Institute (IFPRI) and International Center for Tropical Agriculture (CIAT); Washington, DC and Cali: 2004. HarvestPlus Handbook for Carotenoid Analysis. HarvestPlus Technical Monographs. [Google Scholar]
- Rojas Martha C, Brewer Susan M. Effect of Natural Antioxidants on Oxidative Stability of Frozen, Vacuum-Packaged Beef and Pork. J. Food Qual. 2008;31(2):173–188. [Google Scholar]
- Rutto Laban K., Xu Yixiang, Ramirez Elizabeth, Brandt Michael. Mineral properties and dietary value of raw and processed stinging nettle (Urtica dioicaL.) Int. J. Food Sci. 2013;2013:1–9. doi: 10.1155/2013/857120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartin Maite. Plant L-Ascorbic Acid: Chemistry, Function, Metabolism, Bioavailability and Effects of Processing: Review. J. Sci. Food Agric. 2000;80:825–860. [Google Scholar]
- Shilton N. Drying:Chemical changes. In: Caballero B., Trugo L., Finglas P., editors. In Encyclopaedia of Food and Health. Second. Elsevier Science Ltd; Amsterdam, Netherlands: 2003. pp. 1947–1950. [Google Scholar]
- Shonte T.T., de Kock H.L. Descriptive sensory evaluation of cooked stinging nettle (Urtica dioicaL.) leaves and leaf infusions: effect of using fresh or oven-dried leaves. South Afr. J. Bot. 2017;110:167–176. [Google Scholar]
- Singleton Vernon L., Orthofer Rudolf, Lamuela-Raventós Rosa M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In: Kumar Challa Vijaya., editor. In Methods in Enzymology. Elsevier Inc; Amsterdam, Netherlands: 1999. pp. 152–178. [Google Scholar]
- Standing Committee on Nutrition . 2010. 6th Report on the World Nutrition Situation. Switzerland. [Google Scholar]
- Suhaj Milan. Spice antioxidants isolation and their antiradical activity: a review. J. Food Compos. Anal. 2006;19(6–7):531–537. [Google Scholar]
- Tiwari U., Cummins E. Factors Influencing Levels of Phytochemicals in Selected Fruit and Vegetables during Pre- and Post-Harvest Food Processing Operations. Food Res. Int. 2013;50(2):497–506. [Google Scholar]
- Upton Roy. Stinging nettles leaf (Urtica dioicaL.): extraordinary vegetable medicine. J. Herb. Med. 2013;3(1):9–38. [Google Scholar]
- US FDA . Center for Food Safety and Applied Nutrition: Office of Nutrition, Labeling, and Dietary Supplements; Maryland, United States: 2013. A Food Labeling Guide: Guidance for Industry. Silver Spring. [Google Scholar]
- Vagiri Michael Rajeev. Phenolic Compounds and Ascorbic Acid in Black Currant (Ribes Nigrum L.) Variation Due to Genotype, Ontogenetic Stage, Harvest Date and Location, Thesis. Swedish University of Agricultural Sciences; 2014. [Google Scholar]
- Velioglu Y.S. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables and Grain Products. J. Agric. Food Chem. 1998;46(10):4113–4117. [Google Scholar]
- Waheed Uz. Effect of Temperature Variations during Cooking and Storage on Ascorbic Acid Contents of Vegetables: A Comparative Study. J. Chem. Soc. Pakistan. 2013;35(1):1–4. [Google Scholar]
- Walker Paul G., Viola Roberto, Woodhead Mary, Jorgensen Linzi, Gordon Sandra L., Brennan Rex M., Hancock Robert D. Ascorbic acid content of blackcurrant fruit is influenced by both genetic and environmental factors. Funct. Plant Sci. Biotechnol. 2010;4(1):40–52. [Google Scholar]
- World Health Organization . WHO Fruit and Vegetable Promotion Initiative – Report of the Meeting,. Geneva. 2003. Fruit and vegetable promotion initiative/a meeting report. [Google Scholar]
- World Health Organization . Global Prevalence of Vitamin A Deficiency in Populations at Risk WHO Global Database on Vitamin A Deficiency WHO Global Database on Vitamin A Deficiency. World Health Organization Report; Geneva: 2009. [Google Scholar]
- World Health Organization . Vol. 1. France; 2014. (Salt Reduction and Iodine Fortification Strategies in Public Health). [Google Scholar]