Abstract
Background:
Lyme disease (LD) is a tick-borne infection caused by Borrelia burgdorferi sensu lato. The current therapeutic approach to this disease is limited to antibiotics. However, after their administration, about 20% of patients experience delayed onset of this illness manifesting as lingering persistent symptoms.
Methods:
To determine a suitable approach that would help reduce this number, we examined the efficacy of a composition of polyphenolic compounds (baicalein, luteolin, and rosmarinic acid) with fatty acids (monolaurin and cis-2-decenoic acid), and iodine/kelp in a Lyme disease animal model and volunteers.
Results:
The results showed that 4 weeks of dietary intake of this composition reduced the spirochete burden in animal tissues by about 75%. Basic and differential blood parameters did not show significant differences between control animals and the animals fed with this composition. Also, hepatic and renal toxicity markers were not changed and apoptosis was not observed. Relevant inflammatory cytokines such as IL-6, IL-17, TNF-α, and INF-γ, were elevated in infected animals but normalized in infected and treated animals. A small observational study revealed that after administration of this composition to 17 volunteers three times per day for 6 months, 67.4% of the volunteers with late or persistent LD, and not receptive to previous antibiotic application, responded positively, in terms of energy status as well as physical and psychological wellbeing to supplementation with this composition, while 17.7% had slight improvement, and 17.7% were none responsive.
Conclusion:
We concluded that this specific composition revealed feasible benefits in late or persistent LD management, although double-blind controlled clinical trials are warranted.
Keywords: fatty acids, inflammation, Lyme disease, polyphenols, spirochetes
Introduction
Lyme disease (LD) is a systemic zoonosis prevalent worldwide.1,2 It is spread by ticks which transmit bacteria of the genus Borrelia while feeding on animals and humans.3,4 The number of reported LD cases has systematically grown over the past 20 years with the latest estimates reaching 300,000 cases annually in the USA alone.5 Its causative pathogen, Borrelia burgdorferi sensu lato, is prevalent on the east and west coasts of the USA as well as in the central and eastern parts of Europe. LD affects people of all ages and both genders, although the highest rates have been documented in children aged 10–14 years and in adults over 45 years old.5–7 The clinical manifestations of LD vary, however common symptoms have been identified. The early signs of LD account for a skin lesion called erythema migrans (EM) and/or flu-like symptoms, whereas the systemic symptoms include arthritis, neurologic problems, and cardiac abnormalities which can appear approximately 4–6 weeks after a tick’s bite. Persistent fatigue and aches/pain may develop in about 20% of those individuals who followed the recommended antibiotic treatment and can last beyond 6 months. This phenomenon has been described as PTLDS (post-treatment Lyme disease syndrome).5,8–10
Several US Food and Drug Administration (FDA)-approved antibiotics are used as primary therapeutics in patients with LD. The first choice for early stages of LD is usually a 2–4-week administration of doxycycline for adults and amoxicillin for children. For late-stage LD, ceftriaxone or cefotaxime are recommended for about the same treatment period. Although some clinical trials have brought contradictory results, it is generally agreed that prolonged antibiotic treatment is not recommended for patients with PTLDS.5,11,12
The efficacy of naturally occurring and biologically active substances as anti-borreliae agents is still not well explored, although the number of research investigations with such agents has been growing.13–16 Our previous in vitro study showed that a specific combination of polyphenols with fatty acids and iodine has significant bactericidal effect against two species of Borrelia that have been recognized as a pathogenic factor of LD in the USA and Europe. Moreover, this defined composition of phytochemicals worked synergistically and was shown to affect the membrane but not the DNA of the bacteria, demonstrating significant anti-oxidative and anti-inflammatory properties at the same time.17
In this study, we report the efficacy of this specific composition of plant-derived compounds against Borrelia burgdorferi in an animal model of LD and volunteer patients with a late or persistent form of LD. We attempt here to provide a more comprehensive evaluation of this composition as a potential alternative or perhaps adjunct approach to LD, which needs to be further validated by large double-blind controlled clinical trials.
Materials and methods
Compounds such as baicalein, luteolin, rosmarinic acid, and cis-2-decenoic acid (10-HAD), with a purity between 90% and 95% according to the manufacturer, were obtained from Baoji GuoKang Bio-Technology Co. Ltd (Baoji City, China). Organic kelp with standardized iodine content (i.e. 150 µg/ml as 100% of recommended daily allowance, and 60 minerals, vitamins, protein, fats, carbohydras, and dietary fibers as approximately 25% of daily values) was purchased from Thorvin Inc. (New Castle, VA, USA), and monolaurin as a pure sn-1 monolaurin (glycerol monolaurate) was from Purem Biological (Xi’an, China). Additional purity analysis of compounds to potentially exclude the presence of antibiotics all of which were purchased from Cayman Chemical (Ann Arbor, MI, USA), such as doxycycline and amoxicillin and the anti-inflammatory drug methotrexate used in LD treatment1,9 was performed at the mass spectroscopy laboratory of Oregon State University and analyzed at the mass spectroscopy laboratory of Stanford University (Supplementary Figures 1Sa–c and Figures 2Sa–d).
Test microorganisms
A clonal derivative of Borrelia burgdorferi B31 strain, MSK5, containing all plasmids, was used in this study.18 It was cultured in commonly used conditions, that is, Barbour-Stoenner-Kelly H (BSK-H) medium supplemented with 6% rabbit serum (Sigma, St. Louis, MO, USA) without antibiotics at 33°C with 5% CO2, in sterile screw-cap 15 ml polystyrene test tubes.
Test diet
Standard rodent diet was purchased from LabDiet Inc. (St. Louis, MO, USA). The test diet was prepared by LabDiet Inc. and was composed of standard diet enriched with baicalein (650 mg/kg diet), luteolin (300 mg/kg diet), rosmarinic acid (500 mg/kg diet), monolaurin (500 mg/kg diet), 10-HAD (500 mg/kg diet), and iodine 1 mg/kg diet.
Animal study
A total of 32 C3H/HeN inbred female mice weighing approximately 20 g were obtained from Charles River Laboratories (Wilmington, MA, USA). The 4-week-old mice were housed at the animal facility located at the Dr. Rath Research Institute at an ambient temperature of 21°C. Food and water were provided ad libitum during a light and dark cycle of 12 h. Experimental animal protocol No. 01/B052014 was reviewed by and approved in 2014 by the Animal Care and Use Committee at the Dr. Rath Research Institute. Mice were randomly divided into four experimental groups (eight animals per group): WT (not infected animals fed with the standard diet), LD (infected animals fed with the standard diet), WT + T (not infected animals fed with the test diet), and LD + T (infected animals fed with the test diet). Low passage of virulent Borrelia burgdorferi, grown at 33°C in a BSK-H medium until reaching a concentration of approximately 1 × 107 spirochetes/ml, were inoculated intradermally (104 spirochetes/mouse) into animals from two experimental groups, LD and LD + T, as previously reported.19 Mice from groups WT and WT + T received mock injections of 1 × phosphate buffered saline (PBS). After 3 weeks, mice from the WT + T and LD + T groups were transferred from the standard diet to the test diet and fed for a subsequent 4 weeks. The rest of the animals were kept on the standard diet. At the end of the study all animals were sacrificed and the tissue samples, including blood, were collected for further testing. Control animals were mice fed with the standard diet and not infected (WT) as well as mice fed with the standard diet and infected with Borrelia burgdorferi (LD). Blood was aseptically obtained from all experimental mice by an intracardiac puncture using a 1-ml tuberculin syringe with a 27-G needle and separated into two samples. One sample of the collected blood was used for basic and differential hematology, and the second sample was used to prepare serum for biochemical analysis. Tissue samples from the ears, heart base, tibiotarsal joints, liver, kidneys, and spleen were collected, immediately snap-frozen in liquid nitrogen, and subjected to quantitative polymerase chain reaction (qPCR Bio-Rad, Hercules, CA, USA) analysis and/or immunohistochemistry. In addition, blood and tissue cultures (from the ear, heart base, spleen, and joints) were processed and scored for spirochetal growth in BSK-H medium after 3–5 weeks as previously described.19
Quantitative analysis of Borrelia burgdorferi DNA
To confirm the presence of infection and quantify spirochete tissue burdens, all samples were analyzed by qPCR as previously reported.18,19 Briefly, whole genomic DNA was extracted from tissue samples (ears, heart base, spleen, and tibiotarsal joints) using DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Before DNA extraction, all tissue samples were snap-frozen in liquid nitrogen, weighed, and homogenized using a QIA shredder kit (Qiagen). Optimized primer sets of Borrelia burgdorferi flagellin (flaB, bb0147) gene (F-5' TCTTTTCTCTGGTGAGGGAGCT; R-5' TCCTTCCTGTTGAACACCCTCT) and murine β-actin (ACTB) (F-5' CAAGTCATCACTATTGGCAACGA; R-5' CCAAGAAGGAAGGCTGGAAAA) gene from published sequences were used.19 All qPCR reagents were purchased from Qiagen. DNA templates [i.e. 1 × 105/µl B. burgdorferi equivalents from American Type Culture Collection (ATCC) (Manassas, VA, USA) and mouse from Promega (Madison, WI, USA) served as known amounts of spirochetal and mouse genomic DNA], were included in each qPCR experiment, and used to compile the standard curves, and to determine the total number of spirochetes (flaB copy number) in collected mouse tissue samples. Also, a no-template reaction control was included. QuantiTect SYBR Green PCR Kit (Qiagen) was utilized to determine the borrelial flaB target gene, expressed as per 1 mg of tissue weight. It contained QuantiTect SYBR Green PCR Buffer, pH = 8.7, as the source of 2.5 mM Mg2+, deoxynucleotides and HotStarTaq DNA polymerase, in addition to each primer used at a concentration of 0.3 μM in a 50 μl qPCR mixture. The qPCR cycler conditions (BioRad CFX96) were programmed according to manufacturer’s recommendations and were as follows: an activation step at 95°C for 15 min, 40 cycles of: denaturation at 94°C for 15 s, annealing at 59°C for 30 s, and extension at 72°C for 30 s. To verify the specificity and identity of the qPCR product, melting curve analyses were performed. Additional validation was conducted by subjecting the tissue samples to an independent qPCR analysis performed by a certified laboratory (IDEXX BioResearch, Sacramento, CA, USA). Each sample was analyzed in triplicate.
Blood hematology
Blood samples obtained from all experimental mice were submitted to a certified analytical laboratory (IDEXX BioResearch) where the basic (RBC, Red Blood Cells; WBC, White Blood Cells; HGB, Hemoglobin; HCT, Hematocrit; MCV, Mean Corpuscular Volume; MCH, Mean Corpuscular Hemoglobin; and MCHC, Mean Corpuscular Hemoglobin Concentration) and differential (neutrophils, monocytes, lymphocytes, and platelets counts/levels) hematologic analysis was performed and supported by pathological evaluation. Each sample was analyzed in triplicate.
Biochemical analysis
Sera from all experimental animals were analyzed by a certified analytical laboratory (IDEXX BioResearch) for alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), creatinine, cholesterol, glucose, blood urea nitrogen (BUN), bilirubin, haptoglobin, and kidney injury molecule-1 (KIM-1) levels. KIM-1, a 30 KDa, type 1 membrane protein, has been considered as a potential biomarker for renal injury since it is highly upregulated in the proximal tubule of the injured kidney, but exists in very low levels in normal uninjured kidneys.20 In addition, 50 µl serum from each mouse was subjected to pro-inflammatory cytokines and chemokines analysis using the Analyte Profiler ELISArray assay kit (SA Biosciences, Germantown, MD, USA) according to the manufacturer’s protocol. Each sample was analyzed in triplicate.
Immunohistochemistry
Samples from kidney, liver, and joints, collected at the end of the experiment, were fixed in 10% neutral-buffered formalin for 24–48 h and subjected to immunohistochemistry evaluation performed by an independent certified laboratory (HistoTox Labs, Boulder, CO, USA). The 5 µm sections, placed on glass slides, were stained with hematoxylin and eosin, anti-F4/80 (macrophage marker), and anti-ACS3 (activated caspase-3, apoptotic marker) antibodies. Caspase-3 is activated by the upstream caspase-8 and caspase-9, serving as a convergence point for intrinsic and extrinsic apoptotic signaling pathways. Thus activation of the caspase-3 pathway is a hallmark of apoptosis and can be used in cellular assays to quantify activators and inhibitors of the ‘death cascade’.21
F4/80, also known as EGF-like module-containing mucin-like hormone receptor-like 1 (Epidermal Growth Factor-like module-containing mucin-like hormone receptor-like 1) is a member of the adhesion G-protein coupled receptor (GPCR) family. F4/80 antigen is a mature mouse cell surface glycoprotein expressed at high levels on various macrophages including: Kupffer cells, splenic red pulp macrophages, microglia, gut lamina propria, and Langerhans cells in the skin. F4/80 antigen is also expressed on the macrophages of the connective tissue, heart, kidney, and reproductive and neuroendocrine systems, thus it is a well-known and widely used marker of murine macrophage populations.22 Sections were cryptically coded and an additional unbiased histopathological examination was performed by an onsite board-certified pathologist.
Human observational study
Between 2014 and 2015, eligible adult patients (n = 17) were recruited through referrals from physicians in Germany where the study was conducted at the Private Praxisklinik H. Baltin, Bavaria, Germany. The study followed the protocol adhering to the regulations of the 1975 Declaration of Helsinki and was approved by the institutional Ethics Committee (No. DRRI/2014) at the participating center (Private Praxisklinik H. Baltin, Bavaria, Germany), and supported by written informed consent from each patient. Patients were classified as eligible for the study if they were at least 18 years old, with a history of acute LD acquired in Germany, and with at least one of the following: a history of single or multiple EM, neurologic symptoms attributed to LD, or Lyme arthritis. Additional medical documentation proving that the patients currently suffered from late or chronic LD (manifesting in the form of fatigue, musculoskeletal pain, arthritis, cardiac arrhythmia, neuropathies, cognitive dysfunction/paralysis) and that they previously had undergone treatments with a recommended antibiotic regimen was mandatory.5,23 At the time of enrollment, all patients had the following symptoms that interfered with their regular functioning and ability to work: widespread musculoskeletal pain and/or cognitive impairment with accompanying profound fatigue. The chronic symptoms had to have begun within 6 months after the initial application of the recommended antibiotic regimen and had to have persisted beyond 6 months.23 Patients received the test composition in the form of capsules containing baicalein 250 mg/day, luteolin 75 mg/day, rosmarinic acid 100 mg/day, monolaurin 250 mg/day, 10-HAD 100 mg/day, and iodine 0.15 mg/day (in the form of kelp), three times per day for 6 months. During the oral treatment phase, compliance and safety were monitored through monthly patient visits at the doctor’s clinic. Clinical and laboratory evaluations were performed on day 90 and day 180 and included a complete medical history, a detailed physical examination, neuropsychological testing, and sampling of peripheral blood, if deemed necessary.
Statistical analysis
All the data are presented as means ± standard deviation (SD) (n = 8 for the animal study, and n = 17 for the human study). Student’s two-tailed t test was used to determine statistically significant differences set at 0.05 levels. Statistical analysis was performed using GraphPad software.
Results
Effect of the test composition in vivo
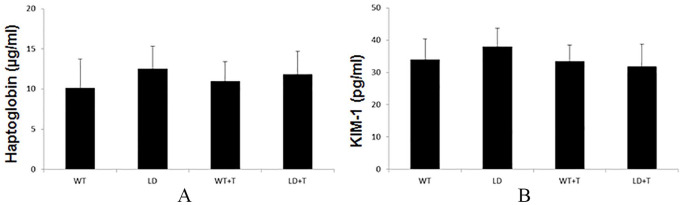
After completion of 4 weeks of treatment with the test diet, there were no significant variations in the weight as well as food and water consumption between all experimental groups (Table 1). Evaluation of blood revealed no alterations in hematology parameters between experimental groups, except that there was a significantly increased level of monocytes in the animals infected with Borrelia burgdorferi (LD) (Table 2). Also in the same animal group an increased level of neutrophils was noticed, but it did not reach statistical significance (p = 0.053). Pathologist analysis further revealed no signs of hemolysis and lipemia, nor any anisocytosis or poikilocytosis. Also, the level of (meta/pro)-myelocytes was classified as ‘undetectable’. Furthermore, biochemical analysis of sera from all experimental animals showed no changes in the levels of liver enzymes and basic clinical metabolic markers such as cholesterol, glucose, bilirubin, BUN, and creatinine. The LD group displayed an increased level of ALP, however, it was not statistically significant (p = 0.055) (Table 3). In addition, haptoglobin and KIM-1 levels showed no changes, and pathologist evaluation reported no toxicity in the tissues (Figure 1).
Table 1.
Summary of mice physiological parameters after 4 weeks of treatment.
| Parameters | WT (n = 8) |
LD (n = 8) |
WT + T (n = 8) |
LD + T (n = 8) |
|---|---|---|---|---|
| RBC (× 106/mm3 ± SD) | 8.11 ± 2.2 | 7.48 ± 2.6 | 8.56 ± 2.8 | 8.78 ± 2.5 |
| WBC (× 103/mm3 ± SD) | 6.3 ± 1.9 | 6.8 ± 2.3 | 6.1 ± 2.4 | 6.4 ± 2.2 |
| HGB (g/L ± SD) | 142 ± 23 | 146 ± 27 | 147 ± 35 | 134 ± 39 |
| HTC (% ± SD) | 41.4 ± 6.5 | 43.2 ± 5.6 | 41.8 ± 5.8 | 41.9 ± 7.6 |
| MCV (fL ± SD) | 49.3 ± 7.5 | 49.3 ± 5.9 | 46.0 ± 6.7 | 49.4 ± 6.9 |
| MCH (pg ± SD) | 14.8 ± 5.9 | 13.4 ± 7.1 | 14.9 ± 5.8 | 14.6 ± 5.6 |
| MCHC (mmol/L ± SD) | 18.9 ± 4.2 | 18.9 ± 4.2 | 20.6 ± 4.3 | 20.0 ± 4.7 |
| Neutrophils (% ± SD) | 25.8 ± 6.8 | 29.6 ± 7.5 | 24.4 ± 6.7 | 24.5 ± 7.9 |
| Monocytes (% ± SD) | 1.9 ± 0.6 | 4.1 ± 0.5* | 1.6 ± 0.2 | 2.7 ± 0.3 |
| Lymphocytes ( % ±SD) | 66.3 ± 8.6 | 84.9 ± 7.5 | 74.7 ± 9.1 | 75.8 ± 8.2 |
| Platelets | Adequate | Adequate | Adequate | Adequate |
p < 0.01; HGB, hemoglobin; HTC, hematocrit; LD, mice infected with Borrelia burgdorferi (intradermal injection with 104 spirochetes/mouse); LD + T, mice infected with Borrelia burgdorferi (intradermal injection with 104 spirochetes/mouse) and fed with plant-based diet for 4 weeks; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume, RBC, red blood cells; SD, standard deviation; WBC, white blood cells; WT; control mice; WT + T, control mice fed with plant-based diet for 4 weeks.
Table 2.
Basic and differential blood parameters of mice after 4 weeks of treatment.
| Parameters | WT (n = 8) |
LD (n = 8) |
WT + T (n = 8) |
LD + T (n = 8) |
|---|---|---|---|---|
| Weight (g/mouse ± SD) | 25.3 ± 3.1 | 24.3 ± 2.9 | 25.2 ± 3.1 | 25.1 ± 3.1 |
| Food (g/mouse/day ± SD) | 3.3 ± 0.6 | 3.2 ± 1.1 | 3.1 ± 1.0 | 3.4 ± 0.8 |
| Water (ml/mouse/day ± SD) | 2.4 ± 0.3 | 2.6 ± 0.7 | 2.8 ± 0.8 | 2.5 ± 0.4 |
LD, mice infected with Borrelia burgdorferi (intradermal injection with 104 spirochetes/mouse); LD + T, mice infected with Borrelia burgdorferi (intradermal injection with 104 spirochetes/mouse) and fed with plant-based diet for 4 weeks; SD, standard deviation; WT, control mice; WT + T, control mice fed with plant-based diet for 4 weeks.
Table 3.
Biochemical parameters of mice sera after 4 weeks of treatment.
| Parameters | WT (n = 8) |
LD (n = 8) |
WT + T (n = 8) |
LD + T (n = 8) |
|---|---|---|---|---|
| ALP (U/L ± SD) | 83.1 ± 8.7 | 100.9 ± 7.6 | 89.1 ± 7.3 | 70.9 ± 7.9 |
| ALT (U/L ± SD) | 40.3 ± 5.5 | 35.7 ± 4.9 | 31.3 ± 5.9 | 36.9 ± 7.6 |
| AST (U/L ± SD) | 182.5 ± 10.2 | 257.8 ± 14.3 | 162.7 ± 11.5 | 141.9 ± 9.9 |
| GGT (U/L) | <3 | <3 | <3 | <3 |
| Total bilirubin (µmol/L) | <3.4 | <3.4 | <3.4 | <3.4 |
| Cholesterol (mmol/L ± SD) | 3.8 ± 0.3 | 3.8 ± 0.3 | 3.9 ± 0.3 | 3.3 ± 0.2 |
| Glucose (mmol/L ± SD) | 6.3 ± 0.6 | 6.5 ± 0.8 | 5.9 ± 0.7 | 5.8 ± 0.8 |
| BUN (mmol/L ± SD) | 8.6 ± 1.1 | 8.2 ± 0.8 | 7.9 ± 0.9 | 9.5 ± 1.1 |
| Creatinine (µmol/L) | <26.5 | <26.5 | <26.5 | <26.5 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; GGT, gamma-glutamyl transferase; LD, mice infected with Borrelia burgdorferi (intradermal injection with 104 spirochetes/mouse); LD + T, mice infected with Borrelia burgdorferi (intradermal injection with 104 spirochetes/mouse) and fed with plant-based diet for 4 weeks; SD, standard deviation; WT, control mice; WT + T, control mice fed with plant-based diet for 4 weeks.
Figure 1.
Levels of haptoglobin (a) and KIM-1 (b) in mice sera after completion of 4 weeks of treatment. n = 8.
KIM-1, kidney injury molecule-1; LD, mice infected with Borrelia burgdorferi (intradermal injection with 104 spirochetes/mouse); LD + T, mice infected with Borrelia burgdorferi (intradermal injection with 104 spirochetes/mouse) and fed with plant-based diet for 4 weeks; WT, control mice; WT + T, control mice fed with plant-based diet for 4 weeks.
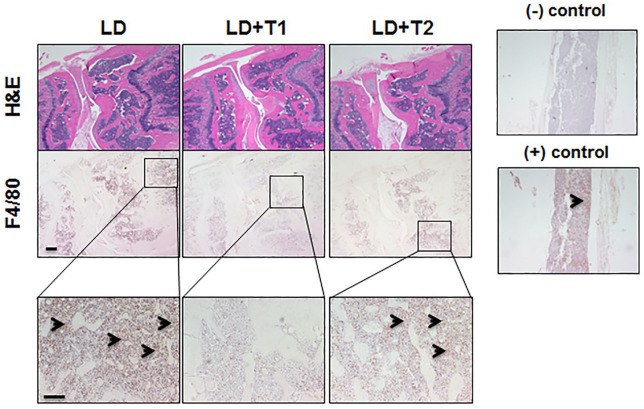
Pro-inflammatory cytokines such as IL-6, IL-17, INT-γ, and TNF-α, were elevated in the LD group, however their levels were normalized in the LD + T group (Figure 2). None of the control groups (WT and WT + T) tested for Borrelia burgdorferi DNA showed detectable levels. In the LD group of mice, the borrelial DNA was detected in the ears, heart base, spleen, and joints of all mice. In the LD + T group borrelial DNA was found only in the joints of 2/8 animals (25%) and at a significantly decreased level compared with the LD group (Table 4 and Figure 3). Results were confirmed by an independent qPCR examination performed by a certified outside laboratory. Immunohistochemistry analysis supported by the pathological evaluation revealed no signs of apoptosis in the liver and kidneys of animals in any of the experimental groups (Figure 4). However, a noticeable increase in staining with anti-F4/80 antibodies was detected in the joints of mice in the LD group, as well as in two animals from the LD + T group in which borrelial DNA was detected, although at markedly reduced intensity (Figure 5).
Figure 2.
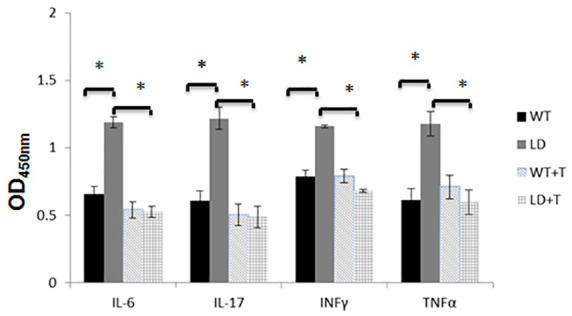
OD readings of mice serum cytokines after completion of the 4-week treatment. n = 8, *p < 0.001.
IL, interleukin; INT-γ, interferon gamma; LD, mice intradermally infected with 104 spirochetes/mouse and not fed with plant-based diet; LD + T, mice infected intradermally with 104 spirochetes/mouse and fed for 4 weeks with plant-based diet; OD, optical density; TNF-α, tumor necrosis factor alpha; WT, mice not infected and not fed with plant-based diet; WT + T, mice not infected and fed for 4 weeks with plant-based diet.
Table 4.
Comparison of culture and flaB DNA qPCR for individual infected mice tissue samples collected after 4 weeks of treatment.
| LD culture* |
Ear | Heart base | Spleen | Joints |
|---|---|---|---|---|
| LD qPCR | ||||
| + | + | + | + | + |
| + | + | + | + | + |
| + | + | + | + | + |
| + | + | + | + | + |
| + | + | + | + | + |
| + | + | + | + | + |
| + | + | + | + | + |
| + | + | + | + | + |
| LD + T culture* |
LD + T qPCR |
|||
| – | – | – | – | – |
| – | – | – | – | + |
| – | – | – | – | – |
| – | – | – | – | – |
| – | – | – | – | – |
| – | – | – | – | + |
| – | – | – | – | – |
| – | – | – | – | – |
qPCR, quantitative polymerase chain reaction; LD, mice infected with Borrelia burgdorferi (intradermal injection with 104 spirochetes/mouse); LD + T, mice infected with Borrelia burgdorferi (intradermal injection with 104 spirochetes/mouse) and fed with plant-based diet for 4 weeks, n = 8. * Culture of ear, heart base, spleen, and joints tissue. (–), flaB DNA-negative result in all subjected tissues; (+), flaB DNA-positive result in all subjected tissues.
Figure 3.
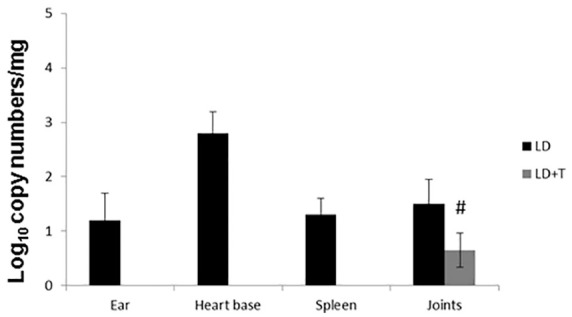
Estimation of spirochete burden by quantitative polymerase chain reaction in mice per mg of tissue after completion of 4 weeks of treatment. n = 8, #p < 0.05 compared with LD group. CT values <40 were considered positive.
LD, mice infected intradermally with 104 spirochetes/mouse and not fed with plant-based diet; LD + T, mice infected intradermally with 104 spirochetes/mouse and fed for 4 weeks with plant-based diet.
Figure 4.
Representative images of AC3 detection in mouse kidney and liver tissues by immunohistochemistry using rabbit polyclonal antibody. Magnifications 4×, 10× for inserts; (–) negative and (+) positive control of mouse tumor.
AC3, active caspase-3; H&E, hematoxylin and eosin staining; LD, mice infected intradermally with 104 spirochetes/mouse and not fed with plant-based diet; LD + T, mice infected intradermally with 104 spirochetes/mouse and fed for 4 weeks with plant-based diet; WT, mice not infected and not fed with plant-based diet; WT + T, mice not infected and fed for 4 weeks with plant-based diet.
Figure 5.
Representative images of anti-F4/80 antibody detection in mice joints tissues by immunohistochemistry using rabbit polyclonal antibody. Magnifications 4×, 10× for inserts; (–) negative and (+) positive control of mouse bone.
H&E, hematoxylin and eosin staining; LD, mice intradermally with 104 spirochetes/mouse and not fed with plant-based diet; LD + T1, mouse no. 2 infected intradermally with 104 spirochetes/mouse and fed for 4 weeks with plant-based diet with increased staining with anti-F4/80; LD + T2, mouse no. 1 infected intradermally with 104 spirochetes/mouse and fed for 4 weeks with plant-based diet with no increased staining with anti-F4/80.
Effect of the test composition in human subject
Evaluation of the dietary supplementation with the six plant-based agents in 17 patients affected by LD revealed that three (3/17) patients became completely symptom-free after 6 months. The patients reported physical and psychological improvements of their symptoms and were able to resume working. Eight patients (8/17) also experienced significant physical and psychological improvement and were able to work again; however, further evaluation was scheduled to follow up on whether the improvements would change after discontinuation of the treatment. Two patients (2/17) experienced only slight improvements, while four patients (4/17) showed no improvement and reported abnormal tiredness, pain in the limbs, headache, and visual disturbances. After discontinuing the supplementation for 2 weeks, these symptoms subsided. One of these four patients (1/4) made a new attempt and resumed the supplementation starting with a lower dose of one capsule per day gradually increasing to three capsules per day, which resulted in no negative effects observed with a slight improvement in physical condition. Further diagnostics for co-infections often associated with LD as well as neurological issues with a change in therapy approach were necessary for patients with no improvement, and that took place outside of this observation study (Table 5).
Table 5.
Summary of the observational study of 17 patients with late or chronic LD after 6 months of supplementation with the test composition.
| No. patient | MS | SS | PSY/US | No improvement | Slight improvement | Marked improvement | Complete improvement |
|---|---|---|---|---|---|---|---|
| 1 | +++ | +++ | + | – | x | – | – |
| 2 | +++ | +++ | – | x | – | – | – |
| 3 | – | + | +++ | – | – | – | x |
| 4 | +++ | + | +++ | – | x | – | – |
| 5 | ++ | ++ | ++ | – | – | x | – |
| 6 | +++ | +++ | ++ | x | – | – | – |
| 7 | +++ | +++ | ++ | x | – | – | – |
| 8 | + | + | +++ | – | – | x | – |
| 9 | +++ | ++ | + | x | – | – | – |
| 10 | +++ | +++ | ++ | – | – | – | x |
| 11 | +++ | ++ | – | – | – | – | x |
| 12 | +++ | + | +++ | – | – | x | – |
| 13 | +++ | ++ | + | – | – | x | – |
| 14 | +++ | +++ | ++ | – | – | x | – |
| 15 | +++ | + | + | – | – | x | – |
| 16 | +++ | – | + | – | – | x | – |
| 17 | +++ | ++ | +++ | – | – | x | – |
Score: +++, severe symptoms, unable to work; ++, less severe symptoms, ability to work not permanently restricted; +, intermittent slight symptoms.
LD, Lyme disease; MS, main symptoms: lack of energy/tiredness/low stamina/fatigue/ musculoskeletal pain/forgetfulness/concentration disorder; PSY/US, psychological symptoms/unusual symptoms: depression/irritability/panic and anxiety attacks/aggression/neuropathies/paralysis; SS, subsidiary symptoms: vertigo/visual disorders/cardiac arrhythmia.
Discussion
Here we demonstrated the results from animal and human observational studies after the application of the test composition consisting of six compounds such as baicalein, luteolin, rosmarinic acid, monolaurin, 10-HAD, and kelp/iodine. The present research extends the findings from our previous study demonstrating significant in vitro efficacy of this composition against pleomorphic forms of Borrelia burgdorferi s.s. and B. garinii.17 We found there that the effect of this combination was comparable to in vitro effectiveness of the triple combination of antibiotics (i.e. doxycycline with daptomycin and cefoperazone), and it demonstrated anti-oxidative and anti-inflammatory effects at the same time.17,24 Results from the animal study concurred with observations on human subjects demonstrating the general safety of this composition when applied in a diet. They revealed no significant changes in basic and differential hematology. Only the infected, but not treated mice (LD group), had an increased level of monocytes and borderline elevated neutrophils, however, these parameters were normalized in animals infected and fed with the test composition (LD + T). Also clinical physiological and biochemical parameters in sera of the experimental groups of animals showed no significant variability. There was an observed increase in inflammatory cytokine levels such as IL-6, IL-17, INT-γ, and TNF-α, in the LD group which corroborates other reports and points to ongoing chronic inflammation and might signal ongoing pathophysiological processes.25 However, the cytokine levels were normalized in the LD + T group of mice which consumed the test diet. Consumption of the diet enriched with the test composition also did not cause toxic effects in mice tissues since the hepatic and renal injury markers were not elevated in the control (WT + T) and the infected (LD + T) animals. This was confirmed by activated caspase-3 and pathologist evaluation of the results.
The infected animals (LD group) showed the presence of detectable borrelial DNA in different tissues, which is consistent with previously published data. However, it is worth mentioning that an in vivo study published by Pahl et al. and others showed that dissemination of Borrelia burgdorferi does not proceed evenly and its distribution can vary among tissues of infected animals.26–29 Our qPCR results indicated that very low copy numbers of spirochetal DNA were present in the joint tissues from two infected mice fed with the test diet; however, they could not be cultured and were significantly decreased compared with the LD group. The issue of detection of noncultivable Borrelia burgdorferi after antibiotic treatment in vitro and in vivo is not new and was reported previously.30,31 Interestingly, an increased level of F4/80 marker in the joint tissue was found in the same two mice, although, likewise, at a visibly reduced level compared with the LD group. These results cannot exclude the possibility that spirochetes could retain a low level of infectivity and be a cause of inflammation, but their ability to replicate was altered. Potentially, the retained ability to express borrelial lipoproteins could contribute, since it has been shown that they can potentiate a plethora of pro-inflammatory responses.32–36 Therefore, the fate of Borrelia burgdorferi and its infectivity aspects in the joints needs to be established. However, the presence of enhanced inflammatory areas in the analyzed tissue sections could correspond to the presence of spirochetes in the persistent stage, which should be confirmed by more detailed study. Joint tissue is extracellular matrix (ECM)-rich and, similar to bone, it is difficult to penetrate by antibacterial agents. The reports about the existence of live spirochetes after antibiotic treatment implicating collagen as a key factor of persistence contributing to antibiotic treatment failure have been reported earlier.37–40
Treatment with different FDA-approved antibiotics on small and large mammals also seems to reduce the Borrelia burgdorferi burden but fails to clear the infection as also documented by many other research groups.41–50 These in vivo reports were further substantiated by clinical trials findings.5,11,12,46,49 It is also worth noting that a declining antibody titer has been observed after antibiotic treatment, despite the presence of low levels of persisting spirochetes.41,50 However, others reported that after antibiotic therapy in dogs, the antibody titers in some animals remained at constant levels, which would argue for the persistence of the antigenic stimulus rather than complete elimination of pathogens.51
It is being increasingly accepted that conventional therapy with antibiotics for late and persistent LD brings limited results. Monotherapy especially has minimal long-term success and, although it is still used there has been no solid evidence documenting its efficacy in late and persistent stages of LD to date.5,11,12 Our previously published in vitro results showed that a composition of baicalein, luteolin, rosmarinic acid, monolaurin, 10-HAD, and iodine at their 1/8 minimum inhibitory concentration (MIC) values has significant synergistic effect against Borrelia spp. This composition revealed anti-oxidative properties affecting the membrane of Borrelia but not its DNA. We also noticed its inhibitory effect on the release of IL-1α, IL-1β, and IL-6 by human CD14+ monocytes stimulated with live Borrelia sp.17 To validate these results we performed a small observational study limited, however, to number of patients and clinical and laboratory evaluations. In our pilot study of 17 patients with LD who had previously undergone several rounds of antibiotic treatment but whose health had not improved after the finished therapy, a 6-month intake of a specific combination of phenolic compounds with fatty acids and kelp/iodine improved the health conditions of 64.7% of the patients allowing them to conduct their normal daily activities and resume working. Nonetheless, observed results, although encouraging, need to be further confirmed in a larger double-blind controlled clinical study to ensure that patients improved health conditions are also significant and consistent in a larger population of patients with late or chronic LD, and substantiated by more in-depth clinical evaluation. Only then, may a more confident conclusion about the efficacy of this composition in humans be drawn. Perhaps further health benefits may be observed with an increased dosage and longer time of intake of the composition. However, as the study indicated the dose adjustment should be made gradually. This study also revealed that those patients with little or no improvement should be subjected to further diagnosis for accompanying infections and neurologic dysfunction to exclude the lack of response as the reason.
In summary, this study documented the efficacy of the oral intake of a composition composed of six biologically active plant-derived agents in an animal model of LD and volunteer patients with late or chronic LD. Further studies investigating their efficacy as an adjunct to antibiotic treatment, and as well as in a larger controlled clinical setting, seems to be reasonable and merits further study. Also, it should be mentioned that the natural compounds used in our in vivo study reached 90–95% purity, although additional testing performed by us showed similar or even greater purity and lack of antibiotics and anti-inflammatory agents relevant in LD treatment. However, the presence of small amounts of residues could be influential and in a limited way affect experimental outcomes.
Supplemental Material
Supplemental material, TAJ922005_Supplemental_Material_CLN for Specific composition of polyphenolic compounds with fatty acids as an approach in helping to reduce spirochete burden in Lyme disease: in vivo and human observational study by Anna Goc, Gebhard Gehring, Hartmut Baltin, Aleksandra Niedzwiecki and Matthias Rath in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors thank Cathy Flowers and Waldemar Sumera for valuable input during preparation of the manuscript.
Footnotes
Author contributions: AG conceived, designed, and performed the in vivo experiments, analyzed data, wrote the paper and had primary responsibility for final content; GG and HB designed and performed the human study, validated and had primary responsibility for final content; AN and MR validated, wrote the paper, and had primary responsibility for final content. All authors read and approved the final manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funds were provided by the nonprofit Dr. Rath Health Foundation, a separate entity from the Dr. Rath Research Institute BV.
Conflict of interest statement: AN is a member of the Dr. Rath Health Foundation and receives no revenues from it. The founding sponsors had no role in the study design, performance, data collection and analysis, decision to publish, or preparation/writing of the manuscript. No conflict of interest is declared.
ORCID iD: Anna Goc  https://orcid.org/0000-0001-8736-2941
https://orcid.org/0000-0001-8736-2941
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Anna Goc, Department of Infectious Diseases, Dr. Rath Research Institute BV, 5941 Optical Ct, San Jose, CA 95138, USA.
Gebhard Gehring, Private Praxisklinik H. Baltin, Aschau/Chiemsee, Bavaria, Germany.
Hartmut Baltin, Private Praxisklinik H. Baltin, Aschau/Chiemsee, Bavaria, Germany.
Aleksandra Niedzwiecki, Department of Infectious Diseases, Dr. Rath Research Institute BV, 5941 Optical Ct, San Jose, CA, USA.
Matthias Rath, Department of Infectious Diseases, Dr. Rath Research Institute BV, San Jose, CA, USA.
References
- 1. Shapiro ED. Lyme disease. N Engl J Med 2014; 370: 1724–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stricker RB, Johnson L. Lyme disease: the next decade. Infect Drug Resist 2011; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson JF, Magnarelli LA, Burgdorfer W, et al. Spirochetes in Ixodes dammini and mammals from Connecticut. Am J Trop Med Hyg 1983; 32: 818–824. [DOI] [PubMed] [Google Scholar]
- 4. Dryden MW, Hodgkins E. Vector-borne diseases in pets: the stealth health threat. Compend Contin Educ Vet 2010; 32: E1–E4. [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Lyme disease website, http://www.cdc.gov/lyme/. (2014, accessed 13 September 2014)
- 6. Robinson S. Lyme disease in Maine: a comparison of NEDSS surveillance data and Maine health data organization hospital discharge data. Online J Public Health Inform 2014; 5: e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strle F, Wormser GP, Mead P, et al. Gender disparity between cutaneous and non-cutaneous manifestations of Lyme borreliosis. PLoS One 2013; 8: e64110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murray TS, Shapiro ED. Lyme disease. Clin Lab Med 2010; 30: 311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arvikar SA, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29: 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steere AC, Sikand VK, Schoen RT, et al. Asymptomatic infection with Borrelia burgdorferi. Clin Infec Dis 2003; 37: 528–532. [DOI] [PubMed] [Google Scholar]
- 11. Klempner MS, Baker PJ, Shapiro ED, et al. Treatment trials for post-Lyme disease symptoms revisited. Am J Med 2013; 126: 665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fallon BA, Keilp JG, Corbera KM, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2008; 70: 992–1003. [DOI] [PubMed] [Google Scholar]
- 13. Brorson O, Brorson SH. Grapefruit seed extract is a powerful in vitro agent against motile and cystic forms of Borrelia burgdorferi sensu lato. Infection 2007; 35: 206–208. [DOI] [PubMed] [Google Scholar]
- 14. Goc A, Niedzwiecki A, Rath M. In vitro evaluation of antibacterial activity of phytochemicals and micronutrients against Borrelia burgdorferi and Borrelia garinii. J Appl Microbiol 2015; 119: 1561–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goc A, Rath M. The anti-borreliae efficacy of phytochemicals and micronutrients: an update. Ther Adv Infec Dis 2016; 3: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Theophilus PA, Victoria MJ, Socarras KM, et al. Effectiveness of Stevia Rebaudiana whole leaf extract against the various morphological forms of Borrelia burgdorferi in vitro. Eur J Microbiol Immunol 2015; 5: 268–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goc A, Niedzwiecki A, Rath M. Synergistic anti-borreliae efficacy of a composition of naturally-occurring compounds: an in vitro study. J Nutr Biol 2019; 5: 350–363. [Google Scholar]
- 18. Labandeira-Rey M, Skare JT. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect Immun 2001; 69: 446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Laar TA, Hole C, Rajasekhar Karna SL, et al. Statins reduce spirochetal burden and modulate immune responses in the C3H/HeN mouse model of Lyme disease. Microbes Infect 2016; 18: 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 2002; 62: 237–244. [DOI] [PubMed] [Google Scholar]
- 21. Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest 2005; 115: 2665–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gordon S, Hamann J, Lin HH, et al. F4/80 and the related adhesion-GPCRs. Eur J Immunol 2011; 41: 2472–2476. [DOI] [PubMed] [Google Scholar]
- 23. Stricker RB, Fesler MC. Chronic Lyme disease: a working case definition. Am J Infect Dis 2018; 14: 1–44. [Google Scholar]
- 24. Feng J, Auwaerter PG, Zhang Y. Drug combinations against Borrelia burgdorferi persisters in vitro: eradication achieved by using daptomycin, cefoperazone and doxycycline. PLoS One 2015; 10: e0117207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Forsberg P, Ernerudh J, Ekerfelt C, et al. The outer surface proteins of Lyme disease borrelia spirochetes stimulate T cells to secrete interferon-gamma (IFN-gamma): diagnostic and pathogenic implications. Clin Exp Immunol 1995; 101: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pahl A, Kühlbrandt U, Brune K, et al. Quantitative detection of Borrelia burgdorferi by real-time PCR. J Clin Microbiol 1999; 37: 1958–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang L, Weis JH, Eichwald E, et al. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun 1994; 62: 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu Q, Liu Z, Wang J, et al. Pathogenic analysis of Borrelia garinii strain SZ isolated from northeastern China. Parasit Vectors 2013; 6: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strother KO, Hodzic E, Barthold SW, et al. Infection of mice with Lyme disease spirochetes constitutively producing outer surface proteins A and B. Infec Immun 2007; 75: 2786–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iyer R, Mukherjee P, Wang K, et al. Detection of Borrelia burgdorferi nucleic acids after antibiotic treatment does not confirm viability. J Clin Microbiol 2013; 51: 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hodzic E, Feng S, Holden K, et al. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob Agents Chemother 2008; 52: 1728–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bradley JF, Johnson RC, Goodman JL. The persistence of spirochetal nucleic acids in active Lyme arthritis. Ann Int Med 1994; 120: 487–489. [DOI] [PubMed] [Google Scholar]
- 33. Giambartolomei GH, Dennis VA, Lasater BL, et al. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun 1999; 67: 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang LM, Ma Y, Schoenfeld R, et al. Evidence for lymphocyte-B mitogen activity in Borrelia burgdorferi-infected mice. Infect Immun 1992; 60: 3033–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tai KF, Ma Y, Weis JJ. Normal human B lymphocytes and mononuclear cells respond to the mitogenic and cytokine-stimulatory activities of Borrelia burgdorferi and its lipoprotein OspA. Infect Immun 1994; 62: 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berende A, Oosting M, Kullberg BJ, et al. Activation of innate host defense mechanisms by Borrelia. Eur Cytokine Netw 2010; 21: 7–18. [DOI] [PubMed] [Google Scholar]
- 37. Liang FT, Brown EL, Wang T, et al. Protective niche for Borrelia burgdorferi to evade humoral immunity. Am J Pathol 2004; 165: 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Priem S, Burmester GR, Kamradt T, et al. Detection of Borrelia burgdorferi by polymerase chain reaction in synovial membrane, but not in synovial fluid from patients with persisting Lyme arthritis after antibiotic therapy. Ann Rheum Dis 1998; 57: 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kvasnicka HM, Thiele J, Ahmadi T. Bone marrow manifestation of Lyme disease (Lyme Borreliosis). Br J Haematol 2003; 120: e723. [DOI] [PubMed] [Google Scholar]
- 40. Berndtson K. Review of evidence for immune evasion and persistent infection in Lyme disease. Int J Gen Med 2013; 6: 291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bockenstedt LK, Mao J, Hodzi E, et al. Detection of attenuated, non-infectious spirochetes after antibiotic treatment of Borrelia burgdorferi-infected mice. J Infect Dis 2002; 186: 1430–1437. [DOI] [PubMed] [Google Scholar]
- 42. Preac-Mursic V, Pfister HW, Wilske B, et al. Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection 1989; 17: 355–359. [DOI] [PubMed] [Google Scholar]
- 43. Schmidli J, Hunziker T, Moesli P, et al. Cultivation of Borrelia burgdorferi from joint fluid three months after treatment of facial palsy due to Lyme borreliosis. J Infect Dis 1988; 158: 905–906. [DOI] [PubMed] [Google Scholar]
- 44. Straubinger RK, Summers BA, Chang YF, et al. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J Clin Microbiol 1997; 35: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Embers ME, Barthold SW, Borda JT, et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PloS One 2012; 7: e29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kadam P, Gregory NA, Zelger B, et al. Delayed onset of the Jarisch-Herxheimer reaction in doxycycline-treated disease: a case report and review of its histopathology and implications for pathogenesis. Am J Dermatopathol 2015; 37: e68–e74. [DOI] [PubMed] [Google Scholar]
- 47. Marques A, Telford SR, Turk SP, et al. Xenodiagnosis to detect Borrelia burgdorferi infection: a first-in-human study. Clin Infec Dis 2014; 58: 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bockenstedt LK, Gonzalez DG, Haberman AM, et al. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Invest 2012; 122: 2652–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krupp LB, Hyman LG, Grimson R, et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology 2003; 60: 1923–1930. [DOI] [PubMed] [Google Scholar]
- 50. Straubinger RK, Straubinger AF, Summers BA, et al. Status of Borrelia burgdorferi infection after antibiotic treatment and the effects of corticosteroids: an experimental study. J Infect Dis 2002; 181: 1069–1081. [DOI] [PubMed] [Google Scholar]
- 51. Straubinger RK. PCR-Based quantification of Borrelia burgdorferi organisms in canine tissues over a 500-day post-infection period. J Clin Microbiol 2000; 38: 2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, TAJ922005_Supplemental_Material_CLN for Specific composition of polyphenolic compounds with fatty acids as an approach in helping to reduce spirochete burden in Lyme disease: in vivo and human observational study by Anna Goc, Gebhard Gehring, Hartmut Baltin, Aleksandra Niedzwiecki and Matthias Rath in Therapeutic Advances in Chronic Disease