Abstract
Background
Repurposing hydroxychloroquine (HCQ) and chloroquine (CQ) as antiviral agents is a re-emerging topic with the advent of new viral epidemics.
Aims
To summarize evidence from human clinical studies for using HCQ or CQ as antiviral agents for any viral infection.
Sources
PubMed, EMBASE, Scopus, Web of Science for published studies without time or language restrictions; Cochrane Clinical Trial Registry and Chinese Clinical Trials Registry for trials registered after 2015; MedRxiv for preprints within the last 12 months.
Content
Study eligibility criteria were interventional and prospective observational studies (with or without a control group). Participants were adults and children with a confirmed viral infection. Interventions included the use of CQ or HCQ as antiviral agent in one or more groups of the study. Two authors independently screened abstracts, and all authors agreed on eligible studies. A meta-analysis was planned if studies were available which were similar in terms of participants, intervention, comparator and outcomes. Nineteen studies (including two preprints) were eligible (HIV 8, HCV 2, dengue 2, chikungunya 1, COVID-19 6). Nine and ten studies assessed CQ and HCQ respectively. Benefits of either drug for viral load suppression in HIV are inconsistent. CQ is ineffective in curing dengue (high-certainty evidence) and may have little or no benefit in curing chikungunya (low-certainty evidence). The evidence for COVID-19 infection is rapidly evolving but at this stage we are unsure whether either CQ or HCQ has any benefit in clearing viraemia (very-low-certainty evidence).
Implications
Using HCQ or CQ for HIV/HCV infections is now clinically irrelevant as other effective antivirals are available for viral load suppression (HIV) and cure (HCV). There is no benefit of CQ in dengue, and the same conclusion is likely for chikungunya. More evidence is needed to confirm whether either HCQ or CQ is beneficial in COVID-19 infection.
Keywords: Antivirals, Chikungunya, Chloroquine, COVID-19, Dengue, Hydroxychloroquine, Pneumonia
Introduction
With the advent of the pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease (COVID-19), repurposing cheap and accessible drugs for off-label use as antivirals if they have demonstrated antiviral properties in vitro or in animal studies is gaining popularity. Chloroquine (CQ), a well-established antimalarial agent, and hydroxychloroquine (HCQ), a similarly established disease-modifying anti-rheumatic drug (DMARD), have both received increased attention in recent days for their purported efficacy as antiviral agents in the context of COVID-19. Both drugs are out-of-patent, cheap, and widely available in high-, middle- and low-income countries.
The antiviral properties of CQ were first explored against viral hepatitis as far back as 1963 [1]. Since then many observations from in vitro and animal experiments have suggested a beneficial role of HCQ and CQ in viral infections [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. Yet the ultimate test for their benefit as antivirals comes from human clinical studies, and to our knowledge neither of these drugs is used as a mainstream antiviral agent for any viral infection. This review focuses on the clinical evidence for using CQ and HCQ as antiviral agents against any viral infection. This reflection and summary of evidence is needed in the current context for judicious, evidence-based recommendations for off-label use of these drugs. Even when such evidence may be incomplete or unavailable for emerging infections such as COVID-19, historical attempts for repurposing these agents for other emerging viral infections from time to time may draw parallels with the current scenario to inform a rational approach to clinical trials and guideline recommendations.
Methods
Study selection criteria
Types of studies
Interventional and observational studies (controlled and non-controlled), including case series, were considered, but case reports limited to single patients were excluded. Retrospective studies and animal or in vitro experiments were excluded.
Participants
Adults or children with a confirmed viral infection were included.
Intervention and comparator
All participants (non-controlled studies) or one study arm (controlled studies) must have received either CQ or HCQ as an antiviral agent (as stand-alone therapy or in combination with other treatments). The comparators for controlled studies were standard treatment, no treatment, or placebo.
Outcomes
Primary outcome was viral load suppression for chronic infections and clearance of viraemia for acute infections. Any other significant outcomes (depending on the type of infection) are discussed narratively as reported by authors given the broad scope of this review.
Data sources and search strategy
We searched PubMed, EMBASE, Scopus, Web of Science, Cochrane Clinical Trials Registry (CENTRAL), Chinese Clinical Trials Registry and MedRxiv (for preprints) according to the search strategy detailed in Table 1 . Bibliographies of eligible articles were also searched. The date of the last search was 30th April 2020. Two authors searched and selected abstracts independently, and all authors identified studies for full-text review by consensus. The following data items were extracted from each included study: study design and location, viral infection and diagnostic criteria, participant demographics, intervention and control groups (if any), drug doses, primary and secondary outcomes, and adverse events attributable to therapy. A meta-analysis was planned if any clinical trials were comparable in terms of participants, interventions, comparators and outcomes. Risk of bias for all randomized controlled studies, regardless of whether published or deposited as preprints, were assessed according to Cochrane guidelines [14]. Risk of bias in non-randomized trials with more than one intervention was assessed with the ROBINS-I tool [15]. All other study designs were considered to have an inherent high risk of bias. Certainty of evidence was assessed for each infection (only from randomized controlled trials, RCTs) according to the GRADE recommendations [16]. Uncontrolled studies and RCTs available only as preprints were not considered for assessing certainty of evidence.
Table 1.
Search strategy (last date of search 30th April 2020)
| Database | Search terms | Field limits | Language limits | Time limits | Comments |
|---|---|---|---|---|---|
| PUBMED | ‘hydroxychloroquine AND antivir∗’, ‘chloroquine AND antivir∗’, ‘Hydroxychloroquine AND virus’ and ‘chloroquine AND virus’ | None | None | None | |
| Scopus | As above | Title, Keywords or Abstract | None | None | |
| Web of Science | As above | None | None | None | |
| EMBASE | As above | None | None | None | |
| CENTRAL | “hydroxychloroquine” or “chloroquine” | None | None | 2015–2020 | Any unpublished/incomplete trial registered prior to 6 years was considered as unlikely to be completed |
| Chinese Clinical Trials Registry | “hydroxychloroquine” or “chloroquine” | None | None | 2015–2020 | As above |
| MedRxiv | “hydroxychloroquine” or “chloroquine” | Title or Abstract | None | Within 12 months | Once a preprint is deposited, if the study met peer-review standards for publication, it is likely to have been published within this time frame |
Results
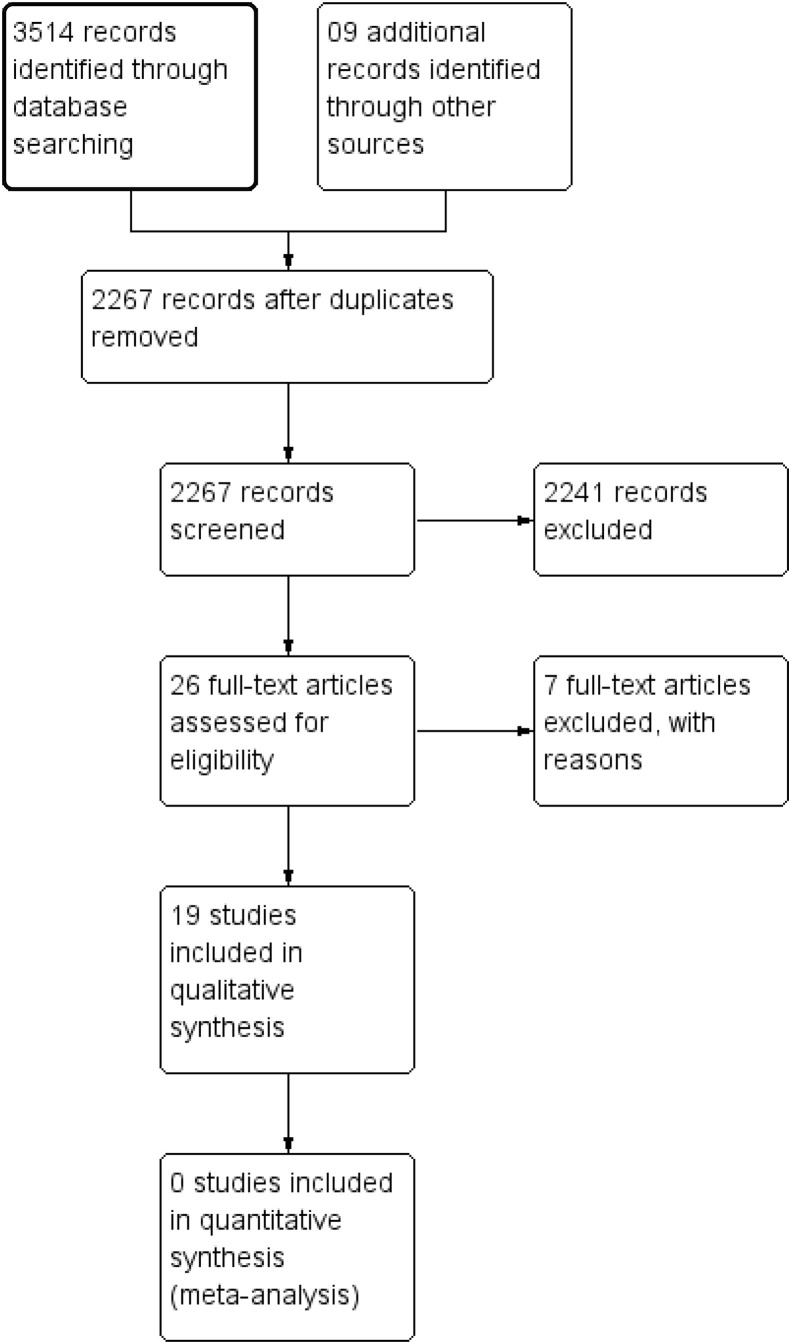
A total of 2267 abstract results were found from all databases after removing duplicates (Fig. 1 ). The majority of these (n = 2026) were published after 1990. After removing retrospective studies, in vitro and animal studies, reviews, letters, opinion papers and editorials, only 209 abstracts remained. Further examination identified 26 articles for full-text review, revealing a significant disparity with the large number of articles demonstrating in vitro evidence for antiviral effects of HCQ and CQ. Only 19 publications (including two preprints) from this subset met the inclusion criteria. One full-text paper was in Mandarin but had an English abstract. The studies included randomized and non-randomized controlled trials and uncontrolled prospective studies equivalent to case series. These had tested the efficacy of CQ and HCQ in human immunodeficiency virus (HIV), dengue, chikungunya, hepatitis C and COVID-19 infections. A meta-analysis was not performed due to unavailability of similar studies. A summary of risk of bias for all controlled clinical trials are given in Fig. 2 . The results are discussed separately for each infection and under each section; randomized clinical trials are discussed first, followed by other study designs. Chronic infections (HIV/HCV) are discussed first as the aim of treatment is viral load suppression rather than cure. Details of study design and setting, participants, interventions, comparators, outcomes and risk of bias for each included study are given in Table 2 . There are 23 trials still in progression, all examining the role of HCQ and CQ in COVID-19 infection (Table 3 ).
Fig. 1.
PRISMA flow diagram of study selection process.
Fig. 2.
Risk of bias summary for randomized clinical trials.
Table 2.
Summary of included studies
| Study and location | Design | Inclusion criteria | Treatment regimens | Results—highlights | Risk of biasa and other comments |
|---|---|---|---|---|---|
| HIV | |||||
| Sperber et al., 1995, USA [17] | Randomized, double-blind, placebo-controlled trial | HIV-infected patients with CD4+ cell count 200–500/μL and not taking antiretroviral (ART) therapy | 1) HCQ 800 mg/d for 8 weeks (n = 20) or 2) Placebo for same duration (n = 20) | Significant decrease in viral load in HCQ group (p 0.022) No change in absolute CD4+ cell count |
Method of sample size calculation or randomization is not given HCQ was well tolerated |
| Sperber et al., 1997, USA [18] | Randomized, double-blind, masked trial | HIV-infected patients with CD4+ cell count 200–500/μL | 1) HCQ 800 mg/d for 16 weeks (n = 35) or 2) Zidovudine 500 mg/d for same duration (n = 37) | Significant decrease in viral load in both HCQ (p 0.02) and zidovudine groups (p 0.001) No change in absolute CD4+ cell count |
Method of sample size calculation or randomization not given |
| Paton et al., 2012, United Kingdom [20] | Randomized, double-blind, placebo-controlled trial | HIV-infected asymptomatic patients, not on ART, CD4+ count> 400 cells/μL | HCQ 400 mg/d (n = 42) or placebo (n = 41) for 48 weeks | No significant difference in CD8+ cell activation Greater decline of CD4+ cell count in HCQ group (p 0.03) Greater increase in HIV viral load in HCQ group (p < 0.003) |
High risk of attrition bias – 31% attrition rate HCQ was well tolerated despite more flu-like episodes |
| Paton et al., 2002, Singapore [24] | Non-controlled prospective study | HIV-infected patients with a viral load <105 copies/μL and CD4+ cell count > 150/μL | HCQ 200 mg/bid, hydroxyurea 500 mg/bid and didanoside 125–200 mg/bid according to bodyweight for 48 weeks (n = 22) | Decline in viral load by an average of 1.3 logs with sustained CD4+ cell level | Neutropenia and elevated amylase levels noted in a majority of patients with two severe adverse events |
| Piconi et al., 2011, Italy [23] | Non-controlled prospective study | HIV-infected, adult, immunological non-responders (CD4+ cell count <200/μL or <5% increase in preceding 12 months) | HCQ 400 mg/d for 6 months (n = 20) | No significant change in absolute CD4+ cell count Significant reduction in Ki67-expressing (activated) CD4+ cells Significant reduction in TLR-2-, TLR-4- and TLR-5-expressing CD14+ cells Significant increase in TLR-2- and TLR-4-expressing regulatory T cells Plasma IL-6 was significantly decreased Significant reduction in plasma lipopolysaccharide (LPS) levels |
Observations supportive of an immunomodulatory effect of HCQ to reduce immune activation in HIV |
| Engchanil et al., 2006, Thailand [19] | Prospective, randomized open-label clinical trial | HIV-infected children (<14 years of age); CDC clinical category A,B or C; CDC immunological category 2 or 3 | 1) Zidovudine (ZDV)+ didanosine (ddI) (n = 25) 2) ZDV + ddI + CQ (n = 30, includes nine children where CQ was added to an existing ZDV + ddI therapy) ZDV 150 mg/m2 body surface area/dose every 8 h; ddI 90–100 mg/m2 body surface area/dose every 12 h; and CQ 4 mg/kg/dose daily Treatment continued for 24 weeks |
No significant difference in the drop of viral load or increase in CD4+ cell count between the groups without CQ and with CQ | High risk of performance and detection bias due to open-label design High risk of attrition bias – 22% attrition rate More gastrointestinal adverse events noted in the group with CQ |
| Jacobson et al., 2016, USA [21] | Randomized, double-blind, placebo-controlled cross-over trial | Two cohorts: a) off ART for at least 6 months, HIV RNA >1000 copies/mL, CD4+ count >400/μL b) on ART for at least 24 months, HIV RNA level undetectable, CD4+ count >350/μL |
Each cohort had two arms that received either CQ 250 mg (150 mg base) per day for 12 weeks followed by placebo for 12 weeks or vice versa 37 patients were on ART, 33 patients were off ART |
No significant immune activation of CD4+ and CD8+ cells in off-ART cohort Significant reduction in activated CD8+ cells but not in CD4+ cells with CQ in the on-ART cohort. CQ also led to an increase in viral RNA load |
High risk of attrition bias – 13% attrition rate No significant increase in adverse events reported for CQ |
| Murray et al., 2010, USA [22] | Randomized, double-blind, placebo-controlled trial | HIV-infected adults with CD4+ counts >250/μL and either ART naïve or off ART for 16 months | 250 mg (150 mg base) CQ daily (n = 6, CQ 500 mg daily (n = 3), or placebo (n = 4) for 2 months | Percentage of CD38+ and HLA DR + CD8 cells were significantly reduced with CQ treatment (p 0.016) | Small clinical trial with 3–6 patients per trial arm. Method for sample size calculation is not mentioned CQ may reduce immune activation in HIV |
| HCV | |||||
| Helal et al., 2016, Egypt [27] | Randomized, prospective, single-blind, controlled study | Patients with chronic active HCV (genotype 4) infection with chronic hepatitis on liver biopsy but without decompensated liver disease | Group 1: pegylated interferon (IFN) 160 μg subcutaneously weekly + oral ribavirin 1000–1200 md/d for 12 weeks (n = 60) Group 2: as above + 400 mg/d HCQ (n = 60) |
Significantly higher rate of early virological response in group 2 (p 0.011) | High risk of selection bias—method of allocation concealment not clear High risk of performance or detection bias—single-blind study design No additional adverse events due to HCQ |
| Peymani et al., 2016, Iran [26] | Randomized, triple-blind, placebo-controlled pilot trial | Patients with HCV (genotype 1) not responding to standard IFN and ribavirin therapy | CQ 150 mg (base)/d for 8 weeks or placebo (n = 10) | Significant decrease in viral RNA (p 0.04) after 8 weeks | This is a small clinical trial and method of sample size calculation is unclear Rebound of viral RNA noted after stopping CQ |
| Dengue | |||||
| Tricou et al., 2010, Vietnam [28] | Randomized, double-blind, placebo-controlled trial | Patients with clinically suspected dengue, enrolled within first 3 days of fever and later confirmed with diagnostic testing (NS1 antigen test and RT-PCR) | Group 1: CQ 600 mg/d (base) for 2 days and 300 mg for 1 day (n = 153) Group 2: placebo (n = 153) |
No statistically significant difference in clearance of antigenaemia (NS1), clearance of viraemia or incidence of dengue haemorrhagic fever | Low risk of bias CQ group had significantly more adverse events |
| Borges et al., 2013, Brazil [29] | Randomized double-blind study | Patients with clinically suspected dengue, later confirmed with diagnostic testing | Group 1: CQ 600 mg (base) for 3 days (n = 19) Group 2: placebo (n = 18) |
No statistically significant difference in the duration of fever | High risk of bias as most recruited patients were not confirmed to have dengue. Sample size calculation is unclear. Subjective improvement of symptoms was noted with CQ which reversed upon ceasing the regimen |
| Chikungunya | |||||
| De Lamballerie et al. French Reunion Islands 2008 [30] | Randomized, double-blind, placebo-controlled trial | Patients with confirmed acute chikungunya infection by RT-PCR and seroconversion between day 1 and 16 of illness | Group 1: CQ 600 mg/d (base) for 3 days, 300 mg/d for 2 days (n = 27) Group 2: placebo (n = 27) |
No statistically significant difference in fever clearance time or viraemia clearance time | Patients in CQ group were more likely to complain of persistent symptoms at day 200. However, they might have had more severe disease at enrolment as revealed later by an analysis of inflammatory markers of stored blood samples—high risk of selection bias |
| COVID-19 | |||||
| Chen et al. China, 2020 [36] | Randomized controlled clinical trial | Adult patients with virologically confirmed (RT-PCR) mild COVID-19 infection | Group 1: HCQ 400 mg/d for 5 days plus ‘standard treatment’ (n = 15) Group 2: ‘standard treatment’ (n = 15) |
No statistically significant difference in clearance of viraemia by day 7, fever clearance time or total duration of hospitalization | Article is published in Mandarin Only the English abstract was reviewed Unable to assess risk of bias |
| Gautret et al. France, 2020 [35] | Open-label, non-randomized, controlled trial | Adult patients with virologically confirmed (RT-PCR) COVID-19 infection as test group Similar patients treated at a different institution or those refusing HCQ treatment at the same institute |
Group 1: HCQ 600 mg/d for 10 days (n = 26) Group 2: no HCQ treatment (n = 16) |
Statistically significant rate of clearance of viraemia by day 6 of illness (70% in HCQ group versus 12.5% in placebo group, p 0.001) | Serious risks of bias due to open-label design, small sample size, non-random allocation and confounding effects due to recruiting control patients from a different institution, azithromycin administration in some patients only, and baseline age difference in test and control groups |
| Chen et al. China, 2020 [38] | Randomized double-blind controlled clinical trial | Adult patients with virologically confirmed (RT-PCR) mild COVID-19 infection | Group 1: HCQ 400 mg/d for 5 days plus ‘standard treatment’ (n = 31) Group 2: ‘standard treatment’ (n = 31) |
Statistically significant faster fever recovery and cough relief in HCQ group | This was a non-peer reviewed preprint High risk of reporting bias—the details of statistical interpretation and effect size are not explained The ‘standard treatment’ according to authors included antibiotics, antivirals, steroids and immunoglobulins Until more details are provided, the results cannot be interpreted |
| Tang et al. China, 2020 [37] | Open-label randomized trial | Adult patients with virologically confirmed (RT-PCR) mostly mild COVID-19 infection | Group 1: HCQ 200 mg/d for 3 days followed by 800 mg/d for 14–21 days + supportive care Group 2: supportive care only |
No statistically significant difference in the proportion of aviraemic patients by day 28, time to aviraemia, or symptom resolution by day 28 | This was a non-peer reviewed preprint High risk of performance and detection bias |
| Huang et al. China, 2020 [40] | Randomized controlled clinical trial (open label?) | Adult patients with virologically confirmed (RT-PCR) COVID-19 infection | Group 1: CQ 600 mg base/d for 10 days Group 2: lopinavir/ritonavir 400/100 mg orally twice daily for 10 days |
No statistically significant difference in viraemia clearance by day 14 Faster radiological resolution and reduced hospital stay in CQ group |
It is not clear whether this was a double-blind study and, if not, there is a high risk of detection and performance bias Method of sample size calculation is unclear |
| Borba et al. Brazil, 2020 [41] | Randomized controlled double-blind study | Adult patients with clinically suspected COVID-19 infection | Group 1: CQ 1200 mg base/d for 10 days Group 2: CQ 900 mg base on day 1 and 45 0mg base/d for 4 days |
Significantly higher mortality in high-dose group by day 13 (15% versus 39%) | Study terminated due to safety concerns—risk of bias not evaluated |
ART, antiretroviral therapy; CQ, chloroquine; HCQ, hydroxychloroquine.
Risk of bias not assessed for uncontrolled studies as results from these studies were not used to grade evidence. For non-randomized trials with more than one intervention, risk of bias assessed with ROBINS-I tool.
Table 3.
Clinical trials/studies in progression on using chloroquine (CQ) or hydroxychloroquine (HCQ) as antiviral agents
| Namea | Reference ID | Source |
|---|---|---|
| Post-exposure prophylaxis for SARS-Coronavirus-2 | NCT04308668 | ClinicalTrials.gov |
| Comparison of lopinavir/ritonavir or hydroxychloroquine in patients with mild coronavirus disease (COVID-19) | NCT04307693 | ClinicalTrials.gov |
| Chloroquine prevention of coronavirus disease (COVID-19) in the healthcare setting | NCT04303507 | ClinicalTrials.gov |
| Treatment of mild cases and chemoprophylaxis of contacts as prevention of the COVID-19 epidemic | NCT04304053 | ClinicalTrials.gov |
| Various combination of protease inhibitors, oseltamivir, favipiravir, and chloroquine for treatment of COVID19: a randomized control trial | NCT04303299 | ClinicalTrials.gov |
| New treatment for radical cure of dengue fever with antiviral and anti-cytokine | CTRI/2017/12/010834 | WHO ICTRP |
| A prospective, open label, randomized, control trial for chloroquine or hydroxychloroquine in patients with mild and common novel coronavirus pulmonary (COVID-19) | ChiCTR2000030054 | Chinese Clinical Trials Registry |
| A prospective, randomized, open-label, controlled trial for chloroquine and hydroxychloroquine in patients with severe novel coronavirus pneumonia (COVID-19) | ChiCTR2000029992 | Chinese Clinical Trials Registry |
| Evaluation the efficacy and safety of hydroxychloroquine sulfate in comparison with phosphate chloroquine in mild and common patients with novel coronavirus pneumonia (COVID-19): a randomized, open-label, parallel, controlled trial | ChiCTR2000029899 | Chinese Clinical Trials Registry |
| Evaluation the efficacy and safety of hydroxychloroquine sulfate in comparison with phosphate chloroquine in severe patients with novel coronavirus pneumonia (COVID-19): a randomized, open-label, parallel, controlled trial | ChiCTR2000029898 | Chinese Clinical Trials Registry |
| A prospective, randomized, open-label, controlled clinical study to evaluate the preventive effect of hydroxychloroquine on close contacts after exposure to the novel coronavirus pneumonia (COVID-19) | ChiCTR2000029803 | Chinese Clinical Trials Registry |
| A multicenter, single-blind, randomized controlled clinical trial for chloroquine phosphate in the treatment of novel coronavirus pneumonia (COVID-19) | ChiCTR2000031204 | Chinese Clinical Trials Registry |
| A randomized controlled trial for favipiravir tablets combine with chloroquine phosphate in the treatment of novel coronavirus pneumonia (COVID-19) | ChiCTR2000030987 | Chinese Clinical Trials Registry |
| Randomized controlled trial for chloroquine phosphate in the treatment of novel coronavirus pneumonia (COVID-19) | ChiCTR2000030718 | Chinese Clinical Trials Registry |
| Clinical study of chloroquine phosphate in the treatment of severe novel coronavirus pneumonia (COVID-19) | ChiCTR2000029988 | Chinese Clinical Trials Registry |
| Single arm study for exploration of chloroquine phosphate aerosol inhalation in the treatment of novel coronavirus pneumonia (COVID-19) | ChiCTR2000029975 | Chinese Clinical Trials Registry |
| A single-blind, randomized, controlled clinical trial for chloroquine phosphate in the treatment of novel coronavirus pneumonia 2019 (COVID-19) | ChiCTR2000029939 | Chinese Clinical Trials Registry |
| A single-arm clinical trial for chloroquine phosphate in the treatment of novel coronavirus pneumonia 2019 (COVID-19) | ChiCTR2000029935 | Chinese Clinical Trials Registry |
| Efficacy of chloroquine and lopinavir/ritonavir in mild/general novel coronavirus (CoVID-19) infections: a prospective, open-label, multicenter randomized controlled clinical study | ChiCTR2000029741 | Chinese Clinical Trials Registry |
| A prospective, open-label, multiple-center study for the efficacy of chloroquine phosphate in patients with novel coronavirus pneumonia (COVID-19) | ChiCTR2000029609 | Chinese Clinical Trials Registry |
| Study for the efficacy of chloroquine in patients with novel coronavirus pneumonia (COVID-19) | ChiCTR2000029542 | Chinese Clinical Trials Registry |
The names were extracted as they appeared on relevant databases without any language corrections.
HIV
There were six RCTs (five double-blind studies and one open-label study) [[17], [18], [19], [20], [21], [22]] and two prospective non-controlled studies [23,24] on HIV that met the eligibility criteria; five studies used HCQ (including both non-controlled studies) [17,18,20,23,24] and three used CQ [19,21,22].
HCQ for HIV
Enrolments in these RCTs varied from 40 patients [17] to 83 patients [20]. Two RCTs enrolled adults with a CD4 cell count >200/μL [17,18] and the other enrolled adults with a CD4 cell count >400/μL [20]. HCQ doses used ranged from 400 mg/d to 800 mg/d and comparators included placebo [17,20] or zidovudine [18]. Two RCTs reported a significant decrease in viral load with no change in absolute CD4 cell count with HCQ [17,18], while the third RCT [20] recorded a significant increase in viral load and a decrease in the CD4 cell count with HCQ. The two uncontrolled studies were small (n = 20–22) and used HCQ 400 mg/d alone [23] or in combination with hydroxyurea plus didanosine [24]. There was a modest reduction in viral load after 48 weeks with combination therapy [24], while markers of HIV immune activation were reduced with HCQ in the other study after 6 months of treatment [23], suggesting an immunomodulatory role of HCQ. HCQ was well tolerated in all studies.
CQ for HIV
Two double-blind RCTs enrolled adult patients with HIV (CQ 150–300 mg base/d, 8–12 weeks) [21,22] and one open-label trial enrolled paediatric patients (CQ 4 mg/kg/d for 24 weeks) [19]. The smallest trial had 13 patients [22] while the largest had 70 patients [21]. The comparators were either placebo [21,22] or zidovudine plus didanosine (paediatric trial) [19]. Both adult trials enrolled patients with a CD4 cell count of at least 250/μL and demonstrated a modestly beneficial immunomodulatory effect of CQ (a significant decline in activated CD8 cells) [21,22]. However, in one trial which had a complex cross-over design, the HIV viral load increased when CQ was administered without any other antiretroviral therapy [21]. There was no significant virological or immunological improvement with CQ in the third (paediatric) trial [19] but significantly more gastrointestinal adverse events were observed with CQ.
Hepatitis C
Use of CQ or HCQ for HCV infection has been evaluated in two RCTs, one a single-blinded study (n = 120, HCV genotype 4) and another a double-blinded study (n = 10, HCV genotype 1) [[25], [26], [27]]. The first trial used HCQ 400 mg/d for 12 weeks added to interferon (IFN) and ribavirin therapy, while the control group received IFN and ribavirin only. HCQ was well tolerated and a significantly higher rate of early virological response was noted in the HCQ group (p 0.011). The other trial used CQ 150 mg base/d versus placebo in patients who had already failed IFN and ribavirin therapy, and noted a significant drop in viral RNA levels following 8 weeks of therapy (p 0.04), but this effect was transient.
IFN and ribavirin therapy for HCV is largely outdated nowadays as highly effective direct-acting antiviral agents are now available. Neither HCQ nor CQ has been evaluated in combination with these agents.
Dengue
Two studies had assessed CQ for dengue infection (none assessed HCQ). A double-blind RCT (n = 307) in Vietnam administered CQ 1500 mg over 3 days (dosage regimen the same as that used for malaria: Table 2) or placebo to clinically suspected dengue patients within the first 3 days of fever. A large proportion of the enrolments (84%) were later confirmed to have dengue. CQ failed to demonstrate a clinical benefit in any of the following—clearance of viraemia, clearance of NS1 antigen positivity, fever clearance time, prevention of dengue haemorrhagic fever, prevention of a decrease in platelet count or increase in haematocrit (surrogate markers of dengue-associated plasma leakage)—but significantly increased gastrointestinal adverse events (p < 0.05) [28]. The second study enrolled 129 patients with clinically suspected dengue to a two-arm randomized controlled trial to receive either 600 mg CQ base/d for 3 days or placebo. Only 37 participants were later confirmed to have dengue (19 in the CQ group, 18 in the placebo group) [29]. There was no statistically significant difference in the total duration of illness or adverse events.
Chikungunya
Chikungunya is a viral illness endemic in the tropics. Acute infection mimics a viral flu and sometimes leads to a debilitating, persistent inflammatory arthritis. There was only one RCT that had evaluated CQ as an antiviral agent (none evaluated HCQ) in acute chikungunya infection. This double-blind RCT, conducted in French Reunion Islands in 2006, enrolled 54 adult patients with virologically confirmed acute chikungunya infection to receive either CQ (600 mg of base/day for 3 days followed by 300 mg/d for 2 days) or placebo [30,31]. There was no statistically significant difference in time for viraemia clearance or fever clearance in the CQ-treated group. The adverse effects were minor but all (n = 7) were reported in the CQ group. When followed up at day 200 (since onset of symptoms) by a telephone interview, the CQ-treated group was significantly more likely to have persistent arthralgia. However, a later analysis of cytokines and chemokines from plasma samples revealed higher levels of IFNα, IL-6, IL-8 and MCP-1 in the group that received CQ (more severe disease at baseline and hence increased likelihood of persistent arthralgia) [32].
Given its established role as a DMARD, HCQ is potentially useful to treat chikungunya-induced chronic arthritis. A randomized controlled trial evaluated HCQ for this purpose after acute chikungunya infection [33]. However, as HCQ was not ‘repurposed’ as an antiviral agent, this trial was outside the scope of this review.
SARS-CoV-2
The ongoing epidemic of SARS-CoV-2 disease (referred to as COVID -19) is rapidly evolving. Potential use of CQ for COVID-19 associated pneumonia first emerged in February 2020 based on several Chinese studies, and an expert consensus from Guangdong Province of China (abstract in English) recommends using CQ 500 mg (presumably 300 mg of CQ base) two times daily for 10 days to treat patients with COVID-19-associated pneumonia [34]. We could not verify the evidence for this recommendation independently as the original full-text articles were not available. We have summarized the evidence for using HCQ or CQ for COVID-19 from full-text articles that were available within our search strategy by 30th April 2020 below. Preprints from MedRxiv are also discussed, but these are not peer-reviewed publications and hence not considered in certainty-of-evidence assessment. Ongoing trials are listed in Table 3.
HCQ for COVID-19
There are two peer-reviewed publications (one RCT and a non-randomized controlled study) [35,36] and two non-peer-reviewed preprints (two RCTs) on this topic [37,38]. A third study (from the USA) available on MedRxiv as a preprint was excluded as it was a retrospective analysis [39]. The only peer-reviewed publication of an RCT (in Mandarin, abstract in English) was not indexed in any of the databases searched and was identified from a secondary bibliography search. It describes a randomized clinical trial (NCT04261517) that recruited 15 non-severe, confirmed COVID-19 patients to an HCQ arm (400 mg/d for 5 days) and a no-HCQ arm [36]. Clearance of viraemia (RT-PCR of a throat swab) by day 7, fever clearance time, and total duration of hospital stay were similar between the two groups. A non-randomized controlled trial from Marseille, France (full-paper reviewed) enrolled 26 patients with virologically confirmed COVID-19 to receive HCQ 600 mg/d [35]. Twenty patients completed HCQ treatment (six patients also received azithromycin) and all had radiological evidence of pneumonia (CT scan). The control group (n = 16) was younger (mean age; 51.2 versus 37.3 years) and had patients treated at another institution or those treated at the same institution but refusing HCQ. More patients in the HCQ group were virologically cured by day 6 (70% versus 12.5%, p 0.001), including all patients who received azithromycin.
Results of two more RCTs from China on using HCQ in COVID-19 are available as preprints [37,38]. The largest was a multicentre open-label clinical trial (ChiCTR2000029868) that enrolled 150 virologically confirmed COVID-19 patients (75 per group) to receive HCQ plus standard care (as defined by Chinese guidelines for COVID-19 management) or standard care only [37]. The HCQ group received 200 mg/d for 3 days followed by 800 mg/d for 14–21 days. Only two patients (one in each group) had severe illness. There was no statistically significant difference in the proportion of aviraemic patients by day 28 (85.4% in the HCQ group versus 81.3% in the control group, p > 0.05). There was also no difference in time to aviraemia or symptom resolution by day 28. Many secondary outcomes proposed in methods—including all-cause mortality—are not reported in results, presumably due to lack of events. The second study (ChiCTR2000029559) enrolled and randomized 62 virologically confirmed COVID-19 patients with evidence of pneumonia on CT scan to receive HCQ (400 mg/d for 5 days) or no HCQ treatment (31 in each group). The respiratory distress was not severe (PaO2/FIO2 >300 or SaO2/SPO2 >93%) at enrolment in all recruits. The authors report faster time to clinical recovery (defined as normalization of body temperature and cough relief maintained for 72 h) and earlier radiological improvement in the HCQ group, but the calculations and radiological reporting standards for these conclusions are unclear. Four patients in the control group versus none in the HCQ group progressed to severe illness.
CQ for COVID-19
There are two published RCTs on using CQ for COVID-19. The first study from China randomized 22 virologically confirmed COVID-19 patients to receive either a lopinavir/ritonavir combination or CQ 600 mg (base)/day for 10 days [40]. There was no statistically significant difference for having a negative RT-PCR by day 14 of illness (10/10 in the CQ group versus 11/12). However, on average, the lopinavir/ritonavir group had started treatment later than those in the CQ group (2.5 versus 6.5 days from the onset of illness, p < 0.001). The second RCT was a dose-ranging study for CQ which did not have a no-CQ comparator group [41]. It randomized 81 patients clinically suspected of having COVID-19 to receive either CQ 12 g over 10 days or CQ 2.7 g over 5 days. Diagnosis was later confirmed (virologically) in 31 patients in each group. The recruits had severe infection at the time of enrolment as defined by tachycardia, tachypnoea, oxygen saturation <90%, and hypotension. Recruitment to the high-dose arm was terminated early (by day 13) as mortality was significantly higher in this group (15% versus 39%, p 0.03).
Discussion
Despite being researched for several decades and being supported by a large volume of in vitro and animal study data for plausible mechanisms for antiviral effects, neither HCQ nor CQ is currently recommended as an antiviral agent for any of the infections for which they were tested in clinical trials (with the exception of COVID-19 which is an evolving situation at the time of writing). The most researched infection in this regard is HIV. However, mainstream antiretroviral treatment in HIV is highly successful in viral load suppression, allowing reasonable control of the disease though it is not a cure. These newer combinations have not been tested against CQ/HCQ, and given the inconsistent evidence from existing trials in this regard there is no need to do such tests. The same can be said of HCV infection where pan-genotypic direct-acting antiviral agents can now achieve >90% cure rates with minimum adverse events [42]. Repurposing HCQ/CQ for these infections is largely redundant and of historical interest only.
In contrast, for acute viral infections such as dengue, chikungunya or COVID-19, effective antivirals are not available. These infections cause epidemics in vulnerable populations, exerting an enormous financial burden on resource-limited healthcare systems. For dengue fever, given the evidence presented here we conclude that CQ is of no benefit (high-certainty evidence). This conclusion is supported by a single well-designed RCT with an adequate sample size and a low risk of bias. CQ may have little or no effect in curing acute chikungunya infection (low-certainty evidence) and this conclusion is supported by a small clinical trial which may have inadvertently recruited people with more severe disease to the CQ arm despite randomization.
For COVID-19 we are unsure whether either CQ or HCQ is of any benefit as per currently available evidence (very-low-certainty evidence). The only peer-reviewed publication describing an RCT on using HCQ for COVID-19 was a small trial that did not demonstrate any benefit of HCQ. The other peer-reviewed publication which did show a benefit was an open-label, non-randomized trial with a serious risk of bias in sample selection, confounders and assessing outcomes. The sample size was small, and there was a marked age discrepancy (of approximately 14 years) between test and control groups. Administration of azithromycin to six subjects who also received HCQ does not seem to be based on an a priori hypothesis or a study protocol. Some control-group patients were from a different centre, and it is unclear whether management protocols in the two institutions were the same. More importantly, it is now established that most patients with COVID-19 (>95%) will recover after a mild infection regardless of antiviral treatment. Therefore, the primary outcome assessed in this study (clearance of viraemia) is less useful for patient management compared to outcomes that demonstrate a benefit in the more severe end of the disease spectrum, such as mortality benefit, reduction in intensive care unit admissions, or faster discharge from intensive care (or high dependency units), faster recovery from assisted ventilation or prevention of the need for assisted ventilation. Examining such outcomes requires a well-coordinated multicentre study (preferably with a randomized, double-blind study design) with similar management protocols across all centres. The other two studies from China (available as preprints) mentioned in the results section also does not help to resolve this issue because of methodological issues and conflicting conclusions. For example, in one of these studies [38] the control group is said to have received ‘standard’ treatment which includes ‘antivirals, antibiotics and steroids or immunoglobulins’. These may have a serious confounding effect on the interpretation of results.
The role of CQ in COVID-19 is also unclear and not supported by evidence at the moment. One small RCT reported no benefit of CQ in terms of achieving negative viraemia by day 14 compared to treatment with lopinavir and ritonavir [40]. The authors report faster radiological improvement and reduced hospital stay with CQ, but on average the CQ group received treatment at an earlier stage of the illness than the comparator group. This plus the small sample size make interpretation of the results difficult. The other RCT did not have a no-CQ control group, which is rather surprising. Instead of proving the efficacy of CQ, the authors investigated whether a higher dose of CQ was safe in patients with severe infection. Notably this CQ dose (1200 mg/d for 10 days) was much higher than that used for cure of malaria which is the standard indication for CQ. Mortality was significantly higher in the high-dose CQ group, and patient recruitment was halted prematurely. Thus, this trial also doesn't help to confirm whether CQ is beneficial for COVID-19.
Conclusion
CQ and HCQ have been examined for their antiviral properties in many in vitro and animal studies for more than five decades and in a limited number of human clinical studies spanning over 25 years. For HIV and HCV infections, the benefit of either drug is doubtful and perhaps no longer relevant as other effective treatments are now available for viral load suppression (HIV) or cure (HCV). There is good evidence that CQ is ineffective in curing dengue infection or preventing dengue haemorrhagic fever. CQ also may not have any benefit in curing acute chikungunya infection. A role for HCQ or CQ in COVID-19 is as yet unclear and needs to be assessed by well-designed randomized double-blind clinical trials.
Author contributions
SR conceived the study. CR, SR and SDF independently performed the literature review. CR did the preliminary analysis and wrote the first draft which was double checked by other authors. All authors approved the final version.
Transparency declaration
None of the authors have any conflicts of interest regarding the content of this manuscript. No grants or funding were received for this study.
Editor: M. Paul
References
- 1.Pareja-Coronel A. Treatment of viral hepatitis with chloroquine. Am J Gastroenterol. 1963;39:288–298. [PubMed] [Google Scholar]
- 2.Keyaerts E., Vijgen L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2 doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Clercq E. Potential antivirals and antiviral strategies against SARS coronavirus infections. Expert Rev Anti-Infect Ther. 2006;4:291–302. doi: 10.1586/14787210.4.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolain J.M., Colson P., Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Ag. 2007;30:297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han Y., Pham H.T., Xu H., Quan Y., Mesplède T. Antimalarial drugs and their metabolites are potent Zika virus inhibitors. J Med Virol. 2019;91:1182–1190. doi: 10.1002/jmv.25440. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A., Liang B., Aarthy M., Singh S.K., Garg N., Mysorekar I.U. Hydroxychloroquine inhibits Zika virus NS2B-NS3 protease. ACS Omega. 2018;3:18132–18141. doi: 10.1021/acsomega.8b01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiryaev S.A., Mesci P., Pinto A., Fernandes I., Sheets N., Shresta S. Repurposing of the anti-malaria drug chloroquine for Zika virus treatment and prophylaxis. Sci Rep. 2017;7 doi: 10.1038/s41598-017-15467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akpovwa H. Chloroquine could be used for the treatment of filoviral infections and other viral infections that emerge or emerged from viruses requiring an acidic pH for infectivity. Cell Biochem Funct. 2016;34:191–196. doi: 10.1002/cbf.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barclay W., Long J., Wright E., Molesti E., Temperton N. Antiviral therapies against Ebola and other emerging viral diseases using existing medicines that block virus entry. F1000 Res. 2015;4 doi: 10.12688/f1000research.6085.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broder C.C. Henipavirus outbreaks to antivirals: the current status of potential therapeutics. Curr Opin Virol. 2012;2:176–187. doi: 10.1016/j.coviro.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne J.A.C., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperber K., Louie M., Kraus T., Proner J., Sapira E., Lin S. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin Ther. 1995;17:622–636. doi: 10.1016/0149-2918(95)80039-5. [DOI] [PubMed] [Google Scholar]
- 18.Sperber K., Chiang G., Chen H., Ross W., Chusid E., Gonchar M. Comparison of hydroxychloroquine with zidovudine in asymptomatic patients infected with human immunodeficiency virus type 1. Clin Ther. 1997;19:913–923. doi: 10.1016/s0149-2918(97)80045-8. [DOI] [PubMed] [Google Scholar]
- 19.Engchanil C., Kosalaraksa P., Lumbiganon P., Lulitanond V., Pongjunyakul P., Thuennadee R. Therapeutic potential of chloroquine added to zidovudine plus didanosine for HIV-1 infected children. J Med Assoc Thai. 2006;89:1229–1236. [PubMed] [Google Scholar]
- 20.Paton N.I., Goodall R.L., Dunn D.T., Franzen S., Collaco-Moraes Y., Gazzard B.G. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA. 2012;308:353–361. doi: 10.1001/jama.2012.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson J.M., Bosinger S.E., Kang M., Belaunzaran-Zamudio P., Matining R.M., Wilson C.C. The effect of chloroquine on immune activation and interferon signatures associated with HIV-1. AIDS Res Hum Retroviruses. 2016;32:636–647. doi: 10.1089/aid.2015.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray S.M., Down C.M., Boulware D.R., Stauffer W.M., Cavert W.P., Schacker T.W. Reduction of immune activation with chloroquine therapy during chronic HIV infection. J Virol. 2010;84:12082–12086. doi: 10.1128/JVI.01466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piconi S., Parisotto S., Rizzardini G., Passerini S., Terzi R., Argenteri B. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood. 2011;118:3263–3272. doi: 10.1182/blood-2011-01-329060. [DOI] [PubMed] [Google Scholar]
- 24.Paton N.I., Aboulhab J., Karim F. Hydroxychloroquine, hydroxycarbamide, and didanosine as economic treatment for HIV-1. Lancet. 2002;359:1667–1668. doi: 10.1016/S0140-6736(02)08557-4. [DOI] [PubMed] [Google Scholar]
- 25.Peymani P., Ghavami S., Yeganeh B., Tabrizi R., Sabour S., Geramizadeh B. Effect of chloroquine on some clinical and biochemical parameters in non-response chronic hepatitis C virus infection patients: pilot clinical trial. Acta Biomed. 2016;87:46–53. [PubMed] [Google Scholar]
- 26.Peymani P., Yeganeh B., Sabour S., Geramizadeh B., Fattahi M.R., Keyvani H. New use of an old drug: chloroquine reduces viral and ALT levels in HCV non-responders (a randomized, triple-blind, placebo-controlled pilot trial) Can J Physiol Pharmacol. 2016;94:613–619. doi: 10.1139/cjpp-2015-0507. [DOI] [PubMed] [Google Scholar]
- 27.Helal G.K., Gad M.A., Abd-Ellah M.F., Eid M.S. Hydroxychloroquine augments early virological response to pegylated interferon plus ribavirin in genotype-4 chronic hepatitis C patients. J Med Virol. 2016;88:2170–2178. doi: 10.1002/jmv.24575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tricou V., Minh N.N., Van T.P., Lee S.J., Farrar J., Wills B. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis. 2010;4 doi: 10.1371/journal.pntd.0000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borges M.C., Castro L.A., Fonseca B.A. Chloroquine use improves dengue-related symptoms. Mem Inst Oswaldo Cruz. 2013;108:596–599. doi: 10.1590/0074-0276108052013010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Lamballerie X., Boisson V., Reynier J.C., Enault S., Charrel R.N., Flahault A. On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis. 2008;8:837–839. doi: 10.1089/vbz.2008.0049. [DOI] [PubMed] [Google Scholar]
- 31.de Lamballerie X., Ninove L., Charrel R.N. Antiviral treatment of chikungunya virus infection. Infect Disord Drug Targets. 2009;9:101–104. doi: 10.2174/187152609787847712. [DOI] [PubMed] [Google Scholar]
- 32.Roques P., Thiberville S.D., Dupuis-Maguiraga L., Lum F.M., Labadie K., Martinon F. Paradoxical effect of chloroquine treatment in enhancing chikungunya virus infection. Viruses. 2018;10 doi: 10.3390/v10050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravindran V., Alias G. Efficacy of combination DMARD therapy vs. hydroxychloroquine monotherapy in chronic persistent chikungunya arthritis: a 24-week randomized controlled open label study. Clin Rheumatol. 2017;36:1335–1340. doi: 10.1007/s10067-016-3429-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhonghua Jie, He He, Hu Xi, Za Zhi. Multicenter collaboration group of department of science technology of Guangdong Province health commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia. Expert Consensus Chloroquine Phosphate Treat Novel Coronavirus Pneumonia. 2020;43:185–188. [Google Scholar]
- 35.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Jun L.D., Li L., Ping L., Qingnian X., Lu X., Yun L. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejian Univ. 2020;49 doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial. medRxiv. 2020;2020 04.10.20060558. [Google Scholar]
- 38.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020;2020 03.22.20040758. [Google Scholar]
- 39.Magagnoli J., Narendran S., Pereira F., Cummings T., Hardin J.W., Sutton S.S. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. 2020;2020 doi: 10.1016/j.medj.2020.06.001. 04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang M., Tang T., Pang P., Li M., Ma R., Lu J. Treating COVID-19 with chloroquine. J Mol Cell Biol. 2020 doi: 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 42.Spengler U. Direct antiviral agents (DAAs)—a new age in the treatment of hepatitis C virus infection. Pharmacol Ther. 2018;183:118–126. doi: 10.1016/j.pharmthera.2017.10.009. [DOI] [PubMed] [Google Scholar]