Abstract
Adipose Tissue (AT) plays a central role in both metabolic health and pathophysiology. Its expansion in obesity results in increased mortality and morbidity, with contributions to cardiovascular disease, diabetes, fatty-liver disease and cancer. Obesity prevalence is at an all-time high and is projected to be 50% in the US by 2030. AT is home to a large variety of immune cells, which are critical to maintain normal tissue functions. For example, gamma delta T cells are fundamental for adipose tissue innervation and thermogenesis, and macrophages are required for recycling of lipids released by adipocytes. The expansion of visceral white AT (vWAT) promotes dysregulation of its immune cell composition and likely promotes low-grade chronic inflammation, which has been proposed to be the underlying cause for the complications of obesity. Interestingly, weight loss following obesity alters the adipose tissue immune compartment, which may account for the decreased risk of developing these complications. Recent technological advancements that allow molecular investigation on a single-cell level has led to the discovery of previously unappreciated heterogeneity in many organs and tissues. In this review we will explore the heterogeneity of immune cells within the vWAT and their contributions to homeostasis and pathology.
Keywords: inflammation, immune system, obesity
Introduction
Adipose tissue (AT) harbors a plethora of immune cells, including all major leukocyte types of the innate and adaptive immune systems, possibly due to the richness of fatty acids and other energy-related metabolites embedded within AT. Obesity changes the availability and abundance of energy sources in AT, since adipocytes both accumulate and release more lipids. Additionally, obesity alters the production of hormones (adipokines) and mediators of inflammation (cytokines, chemokines and lipid mediators) by adipocytes, which may influence immune responses locally and systemically. Therefore, the composition of AT immune cells, as well as their phenotype and gene expression signatures, are significantly altered in obesity. Low-grade inflammation that is promoted by obesity is thought to be the underlying cause for the associated comorbidities, including cardiovascular disease (reviewed in1). This low-grade inflammation has contributions from both the adipocytes themselves (e.g., by adipokine secretion) as well as from their neighboring immune cells, and includes the cross-talk between the sub-populations of cell types in adipose tissue (see2 for a recent review).
Two major types of AT have been identified and segregated according to their location, morphology and function. The brown AT (BAT) is comprised of multilocular adipocytes that are rich in mitochondria and specialized in energy dissipation through thermogenesis3. White AT (WAT) mainly contains unilocular adipocytes, characterized by single lipid droplets that are rich in triglycerides3 and whose central functions are the storage of energy in the form of fatty acids and regulation of systemic metabolic processes by the production of hormones and adipokines. The WAT is further divided into 2 subtypes: subcutaneous (scWAT), located under the skin, and visceral (vWAT), which is intra-abdominal. vWAT has been intensively studied, due to the association of high vWAT mass with increased risk for development of the metabolic syndrome and cardiovascular risk4–6. One major difference between scWAT and vWAT is that the former demonstrates a greater potential to undergo “beiging”, that is, the process by which white adipocytes become brown-like, with increased mitochondrial function and capacity for energy dissipation7, 8. This may explain the distinct contributions of the two WAT depots to metabolic health, while scWAT is more relevant to temperature control, vWAT has been characterized as playing a more preeminent role in metabolic control9. Of the vWAT sources, epididymal WAT (also known as eWAT or perigonadal) has been most studied in animal models, due to its prominent abundance and clear association with metabolic syndrome development in animal models10. Although other vWAT sources, such as perivascular (surrounding blood vessels), mesenteric (intestine associated) and perirenal (kidney adjacent) may have other distinct features, their immune landscape is less known.
The immune system has emerged as a key regulator of metabolic health. In obesity, several of the leukocyte populations that reside in the vWAT, such as macrophages (Møs)11, 12, T cells13, 14 and Dendritic Cells (DCs)15, 16 have been shown to play a role in the development of insulin resistance in metabolic syndrome models11. Recent technological advances, including single cell RNA-sequencing (scRNA-seq), have enabled the discovery of previously unappreciated cellular heterogeneity. With this in mind, our main focus in this review is to explore the extensive heterogeneity of immune cells within the vWAT, the local contribution of these cells to vWAT physiology and the implications to the development of obesity and metabolic syndrome. Some aspects of the roles of immune cells in AT and obesity are covered in a companion review, and we refer the reader to it for complementary information17. The contributions of other cell types, like adipocytes and stromal cells, and soluble factors such as hormones and adipokines, to the systemic control of body metabolism are extensively reviewed elsewhere18, 19 and will not be further explored in detail here.
I. Innate immune cells-Drivers of the metabolic syndrome or protectors of the visceral white adipose tissue?
WAT is dominated by a rich variety of innate immune cells, which are crucial for tissue maintenance and homeostasis. Obesity changes the immune cell repertoire in the WAT, which is coupled with vast transcriptional alterations. While neutrophils and mast cells are considered harmful in obese conditions, eosinophils are generally regarded as promoting tissue homeostasis, at least in leanness. Møs and DCs have been proposed to have both harmful and protective roles. It is increasingly appreciated that different cell populations are more heterogeneous than previously anticipated, and that each of the major immune cell types (i.e. Møs, DCs, neutrophils, etc.) have multiple subsets. Hence, we postulate that subsets of innate immune cells contribute to the numerous functions attributed to the major cell type. In this section we will describe major innate immune populations and how sub-populations contribute to physiological and pathophysiological processes in the vWAT.
1. Macrophages
Møs are specialized phagocytes found in most, if not all tissues. Møs are the most abundant immune cells in AT and constitute ~5% of total human vWAT and mouse eWAT cells in lean conditions20. In the steady-state, the eWAT releases ~1% of its lipid content daily21. A major role of adipose tissue Møs (ATMs) is the recycling of these fatty acids released from adipocytes21. Møs uptake the lipids as exosomes21 and catabolize them in the lysosome through Lysosomal Acid Lipase (Lipa). Mice deficient for Lipa are born without apparent defects; however, their adipose depots are depleted over time; they develop severe hepatosteatosis; and they die at an age of 7–8 months22. Interestingly, these phenotypes are rescued by expression of Lipa specifically in Møs23. These data suggest that ATMs uptake and recycle lipids secreted from adipocytes, indicating that they actively participate in WAT maintenance and functions. Another function of ATMs is to support adipocyte lipolysis and beiging in response to cold exposure in order to enhance heat production24, 25. Animal models submitted to a cold challenge had their capacity to adjust their body temperature compromised when Møs where not expressing the interleukin (IL)-4 receptor. IL-4 has been described as a fundamental factor for the maintenance of ATM phenotype in steady state25–27. These data indicate that ATMs contributes directly to the physiology of the WAT24.
In a seminal paper published in 2003, Weisberg et al. showed that obesity resulted in a significant accumulation of Møs in the vWAT of humans and eWAT of mice28. ATM accrual is correlated with adiposity28 and can be explained by enhanced monocyte recruitment through C-C Motif Chemokine Ligand 2 (CCL2)- C-C Motif Chemokine Receptor 2 (CCR2) axis29–33, increased Mø retention34, enhanced survival35 and local proliferation36, 37. This may be due, at least in part, to the increased secretion rates of fatty acids by obese adipocytes, which more than double compared to lean conditions21. The distribution of ATMs in the obese WAT is also altered, with ATMs accumulating in crown-like structures (CLSs) around dying adipocytes38.
Several groups have investigated the direct involvement of Møs in obesity and AT inflammation. Deletion of Ccr2, a central chemokine receptor of monocytes and other leukocytes, blocked pro-inflammatory gene expression observed following high fat diet (HFD) feeding in mice31. Inhibition of CCR2 during HFD feeding attenuated weight gain and improved glucose and insulin tolerance in mice39–43. This indicates that the recruitment of monocyte-derived Møs is fundamental to the pathophysiology of the metabolic syndrome. Many of these recruited Møs express high levels of CD11c and this Mø population persists in the eWAT after weight loss44. By fluorescence-activated cell-sorting, it was shown that CD11c+ ATMs in obesity express higher levels of the pro-inflammatory cytokines TNFɑ and IL1ꞵ, relative to the CD11c– population44, indicating a functional heterogeneity of ATMs. Moreover, depletion of CD11c+ after the establishment of obesity caused an improvement of glucose and insulin tolerance45. Taken together, these data suggest that ATMs, especially those expressing CD11c, are drivers of obesity and insulin resistance. However, recent single-cell RNA-seq (scRNA-seq) data from atherosclerotic plaques showed that CD11c is a marker for lipid-laden Møs, and that the non-foamy Mø populations were the ones exhibiting a pro-inflammatory transcriptome46. This has called into question the notion that CD11c expression is a reliable marker of pro-inflammatory Møs, or that the populations marked by it in lipid-rich environments differ in important ways.
Because the approaches mentioned above use tools that are not specific to Møs, a few groups performed studies involving direct ablation of ATMs using chlodronate-containing liposome injections, which promote Mø “suicide”47. These studies show that ATM depletion during HFD feeding does not improve glucose and insulin tolerance48 and may actually increase systemic inflammation and plasma levels of IL-6 and IL-1β49. Short-term (6-day) ATM ablation after obesity was established was shown to be beneficial and improved glucose and insulin tolerance50. With that said, the increased insulin and glucose sensitivity was improved to a greater extent following macrophage ablation in the lean eWAT50. Taken together, these studies suggest an important role of ATMs in obesity, with inconsistencies that may be attributed to the heterogeneous nature of Møs. It is possible that some Mø subpopulations are protective and others pathological in the context of obesity.
There are several hypotheses as to the functional alterations of Møs in obesity. The distribution of ATMs in the vWAT is drastically modified in obesity. While lean WAT is mainly populated by anti-inflammatory/homeostatic (classically termed M2 or alternatively activated Møs (AAMs)), obese/insulin resistant vWAT has a significant increase in the numbers of Møs with cell surface markers classically ascribed to pro-inflammatory Møs (M1 or classically activated). The M2 phenotype in lean eWAT is maintained by the Mø response to type 2 cytokines (IL-4 and IL-13) in a Signal Transducer And Activator Of Transcription (STAT) 6-dependent manner26, 53, 54. In some cases, studies reported that obesity decreased ATM expression of anti-inflammatory genes, such as Il10, Arginase (Arg1) and Cd30155, while increasing the expression of pro-inflammatory factors, such as tumor necrosis factor alpha (TNFɑ, gene name Tnf) and inducible nitric oxide synthase (iNOS, gene name Nos2)28, 31, 55. One explanation for the phenotypic difference between lean and obese ATMs is their divergent origins: In homeostatic conditions, most ATMs are of embryonic origin (also known as tissue-resident Møs), while in obesity there is substantial infiltration of monocytes that differentiate into Møs thereafter51, 52.
Another predominant hypothesis is that ATMs undergo reprogramming under different metabolic perturbations leading to dysfunction. Of note, obesity was shown to activate lysosomal lipid metabolism pathways in ATMs, independent of their immune activation state56. As alluded to above, it has been recently reported that ATMs metabolize lipids secreted by adipocytes in the form of exosomes21. Obesity caused a doubling in the amount of adipocyte-derived exosomes, possibly suggesting that accumulation of Møs in obesity is necessary to handle excess adipocyte-derived lipids. Moreover, lipolysis in adipocytes induced rapid recruitment of monocytes to mouse eWAT57, where ATMs further support differentiation of adipocyte progenitors58, lipid handling21, 59 and clearance of apoptotic adipocytes by ATMs60, 61. Taken together, these data show clearly that ATMs are a fundamental accessory cell that helps the vWAT to perform its primary function62. However, less is known regarding how different ATM subpopulations contribute to vWAT during homeostasis and obesity. Although some progress has been made recently, we believe that many Mø functions are yet unknown.
Next, we will discuss the heterogeneity of Mø subpopulations and how the understanding of the functional role of each subpopulation can be important to uncover mechanism that can modulate the metabolic syndrome. In the past few years, newer technologies, such as High Dimensional flow-cytometry and scRNA-seq, have allowed the identification and characterization of unappreciated subpopulations of many cell types, including Møs. In one paper combining very limited scRNA-seq with more comprehensive bulk RNA-seq of ATMs in obese eWAT, Hill et al.63 reported 3 distinct ATM populations, Ly6C+ and 2 distinct CD11b+Ly6C– (CD11b+CD9+Ly6C–and CD11b+CD9−Ly6C–). One of these Ly6C– subsets had high expression of CD9, as well as genes related to lipid metabolism, and the other, a CD9 low population. The transcriptome of the Ly6C+ population was deep-sequenced with RNA-seq and compared to that of Ly6C– cells. One general observation that the authors made was that “neither obesity-associated ATM subtype shares predominant features of classical M1 or M2 activation by comprehensive transcriptome analysis, consistent with an evolving view that the M1/M2 paradigm is not directly applicable to in vivo ATM populations”. The CD9+Ly6C– ATMs expressed a variety of pro-inflammatory genes, with an enrichment in leukocyte activation and inflammatory response pathways, while the Ly6C+ ATMs were enriched in genes related to angiogenesis and extracellular matrix organization. Both the CD9+Ly6C– and the Ly6C+ ATMs were shown to be monocyte-derived, with the CD9+Ly6C– being the major population to accrue in obesity, reside in CLSs and accumulate lipids. The authors thus concluded that the CD9+Ly6C– ATMs were pro-inflammatory cells that accumulate in the obese AT63. However, a subsequent report (discussed in more detail below) showed that the CD9-expressing ATMs that accrue in obesity also express TREM2 and protect against adipose hypertrophy and exacerbation of the metabolic syndrome52.
In another study, Li et al.64 compared the transcriptome of lean and obese purified eWAT Møs (CD45+CD11b+F4/80+ cells) to bone marrow-derived Møs (BMDMs), stimulated in vitro with either lipopolysaccharide (LPS) and interferon (IFN) γ (M1), IL-4 and IL-13 (M2) or unstimulated (M0). Using controlled in vitro conditions, the authors developed a computation tool, termed MacSpectrum, to stratify Møs according to their “differentiation” and “polarization” states. The authors termed the differentiation index based on the expression of 435 genes previously implicated in terminal maturation, regardless of the polarization cue, and the polarization index as the regression line of transcriptomes to M1 or M2 gene sets (for specific gene sets used see64). Conventional methods of scRNA-seq clustering showed no overlap between BMDMs and ATMs, regardless of the different perturbations, with ATMs from lean and obese mice showing much greater similarities to each other than to any of the BMDM populations. Of note, the analysis found that classical M1/M2 genes were rarely expressed in ATMs, in agreement with the findings noted above. With that said, using MacSpectrum the authors showed higher similarity of lean ATMs to M2 Møs, with high differentiation and low polarization states. On the other hand, obese ATMs were more heterogeneous, and showed similarities to M0, M1 and M2 Møs64.
Overall, we can take from these studies two major points. First, the oversimplified M1/M2 dichotomy established long ago by in vitro experiments does not reliably reflect the in vivo Mø biology complexity. Second, the diversity of Mø subpopulations was largely underestimated and new approaches are needed to evaluate the relevance and contribution of each of these subpopulations to WAT physiology.
Another recent study using RNA-seq analyses of sorted cells examined the heterogeneity of mouse eWAT phagocytes and identified 4 distinct ATM populations, according to the expression of surface markers, such as Tim4, MHCII and CD20651. The authors observed that tissue-resident CD206HIGH ATMs are associated with vessels, which they termed vascular-associated ATMs (VAMs). These specialized Møs are intimately associated with blood vessels of the eWAT and efficiently endocytose macromolecules from the circulation. VAMs are sensitive to perturbations as demonstrated by their rapid decrease in numbers upon fasting and inflammation. However, in obesity, VAM abundance increased by 2–3 fold, while monocyte-derived Møs (defined by the authors as CD11c+CD64+ double positive, DP) increased by approximately 10-fold. Functionally, obesity reduced the endocytic capability of VAMs. Transcriptomic analysis of sorted populations revealed that obesity did not cause a pro-inflammatory program in any of the ATMs and that several anti-inflammatory genes were upregulated compared with lean ATMs. Despite the lack of tools to address the specific function of each population, this report revealed that different subpopulations of Møs are present in the eWAT depending on inflammatory and metabolic conditions and indicated that the subpopulations have distinct functions. Understanding the role of each subpopulation has a great potential for elucidating the roles of Møs in the metabolic syndrome pathophysiology and possibly establish relevant therapies.
Subsequent studies that investigated the heterogeneity of not only myeloid cells or ATMs, but all eWAT leukocytes, revealed that Møs are the most heterogeneous leukocyte type in the eWAT in the lean and obese state52, 65, 66, and following weight loss65. Unbiased clustering of total CD45+ cells sorted from the eWAT resulted in three52 to seven65 distinct Mø populations, with additional monocyte populations. In general, the aforementioned studies make the important point that there are unique functions for distinct ATM populations under different metabolic perturbations. In their study, Jaitin et al.52 performed scRNA-seq on vWAT leukocytes from lean and obese mice and humans and found a monocyte-derived Mø population that is scarce in lean conditions, but that accumulates with obesity, similar to the DP population described by Silva et al51. This ATM population showed gene expression patterns enriched in pathways that are associated with oxidative phosphorylation and lipid metabolism. This provides more evidence that the M1/M2 classification is not appropriate to describe a possible function for Møs in vivo. The highly expressed genes in this population included Cd9, Trem2 and Cd36, which were previously described as pro-inflammatory. Using immunostaining, the authors showed that this ATM population accumulated in CLSs and had elevated lipid content, thus these cells were termed lipid-associated Møs (LAMs).
Monocyte fate-mapping studies showed that LAMs are of monocytic origin52. Deletion of Trem2 in mice resulted in adipocyte hypertrophy and worsening of the metabolic syndrome following high-fat feeding52. Trem2 deficiency dramatically reduced the abundance of LAMs in the obese eWAT and reduced the lipid content of the remaining LAMs. These data suggest that the accumulation of monocyte-derived Møs in the eWAT in obesity is a protective mechanism against worsening of the metabolic syndrome, as opposed to the previous perception that these Møs exacerbate eWAT inflammation and cause insulin resistance. However, further studies using animal models with Trem2 ablation specifically in Møs will be needed to clarify the importance of Trem2 expression in LAMs. The Trem2+CD9+ ATM population was identified in 2 additional scRNA-seq studies as the major ATM population in the lean and obese eWAT65, 66, as well as being the population that robustly accumulated in obesity.
In another study, Weinstock et al.65 examined eWAT leukocyte heterogeneity by scRNA-seq from obese mice before and after weight loss, in which the same diet was continued but food intake was restricted by 30%57. The authors reported 7 distinct ATM populations. In line with other studies described here, the results showed that there was no particular enrichment of pro-inflammatory ATMs in obesity or an anti-inflammatory signature in lean eWAT. Conversely, based on gene expression signatures, the authors postulated that the ATM populations have distinct lipid-handling functions (such as glycerolipid metabolism, arachidonic acid metabolism and oxidative phosphorylation), which resembled Mø populations that were described in other tissues, such as the heart and atherosclerotic lesions. Consistent with this is the aforementioned finding of the TREM2 cluster in vWAT in Jaitin et al.52 in atherosclerotic plaques67. Moreover, the data demonstrated that many of the transcriptional changes seen following different metabolic perturbations (e.g. obesity and weight loss) are shared among the ATM populations. Interestingly, the authors described a unique ATM population following caloric restriction-induced weight loss. This population was the largest among the leukocytes following caloric restriction and was enriched in pathways related to phagocytosis. The authors postulated that these phagocytic ATMs assist in clearing dying adipocytes and leukocytes in the resolution of obesity and its related eWAT inflammation. It is possible that the phagocytic ATMs affect insulin resistance as well, since the calorically restricted mice showed improved glucose tolerance compared to obese mice65. Taken together, these studies suggest the existence of a division of labor between different Mø subpopulations within the vWAT. The development of new tools that allow the modification of specific Mø subpopulations can help in understanding the contribution of these cells to several pathologies, as well as to tailor new therapeutic approaches.
In summary, Møs are the most abundant and diverse of the leukocytes in the adipose tissue. The debate regarding their inflammatory nature in obesity in still ongoing; however, recent investigations of the heterogeneity of ATMs suggest that they undertake metabolic functions (further reviewed in the companion review17), such as lipid handling and recycling and dampen inflammation in obesity and following weight loss (Figure 1). Importantly, the influence of ATMs is systemic, through their secreted factors. For instance, eWAT Møs in obesity were shown to produce IL-1β, which caused proliferation of bone marrow progenitors, resulting in monocytosis and neutrophilia, which, collectively, exacerbated atherosclerosis progression68. Thus, understanding both the local and systemic effects of ATMs will be crucial to fully understand their contributions in obesity and cardiovascular risk.
Figure 1. Adipose tissue macrophage landscape in lean, obese and following caloric restriction-induced weight loss.
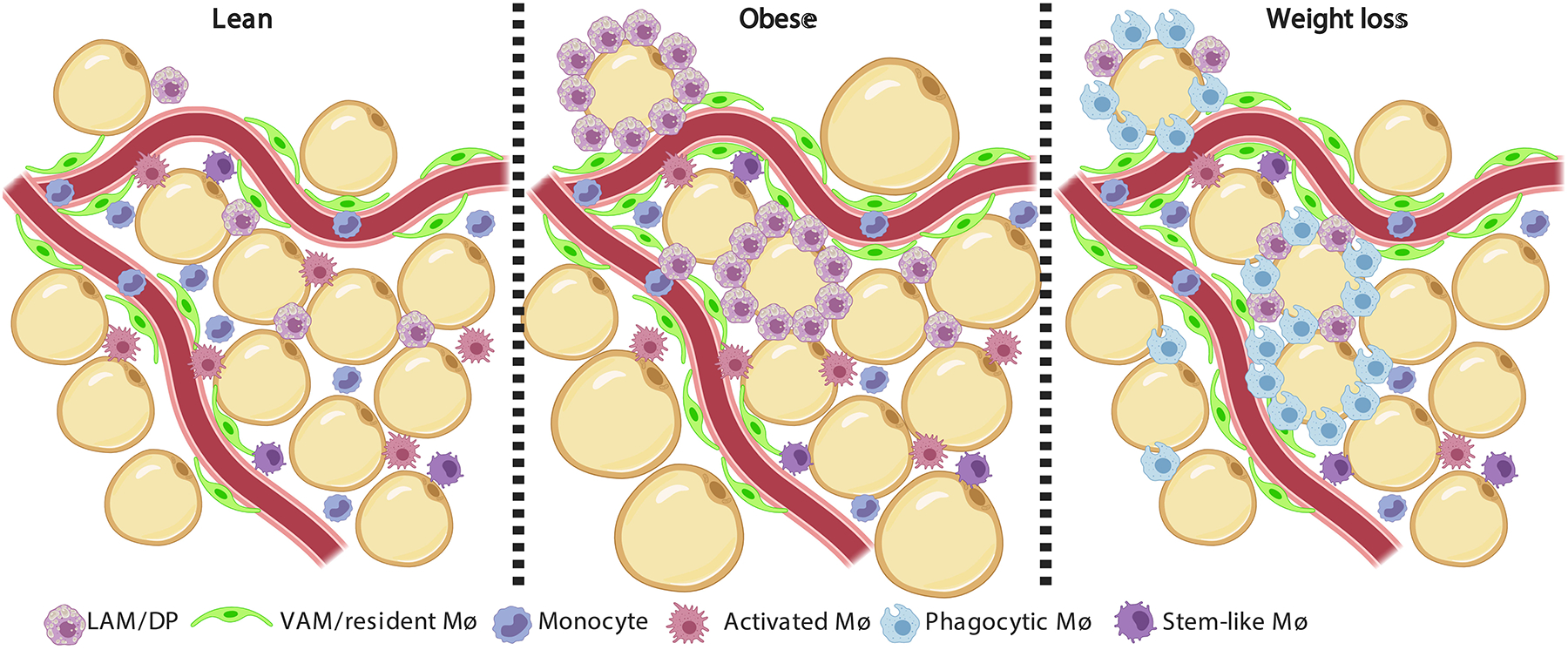
The major leukocyte type in the white adipose tissue (WAT) is the macrophage. Increasing evidence show that adipose tissue macrophages (ATMs) are highly heterogeneous, and their landscape drastically changes in obesity and following weight loss. The lean epididymal WAT (eWAT) is dominated by tissue resident vascular associated macrophages (VAMs)51, which are tightly associated with blood vessels and responsible for the endocytosis of macro-molecules from the circulation. Monocytes and monocyte-derived macrophages also reside in the lean eWAT, namely lipid-associated macrophages (LAMs52, also known as double-positive, DP51). LAMs are thought to assist with lipid handling. Another ATM sub-population was found to express many chemokines, cytokines and their receptors and was named activated macrophage65, 66. Lastly, a macrophage sub-population that expresses many cell cycle genes and resembles a population found in atherosclerotic lesions (termed stem-like)235 was also found in the lean eWAT65. Obesity increases the abundance of macrophages in the eWAT, with specific enrichment of LAMs/DPs and VAMs. While absolute VAM numbers increase by ~2 fold, there is a 10 fold growth in the LAMs/DPs sub-population. LAMs/DPs accumulate especially around adipocytes and form crown-like structures (CLS) and are thought to protect against adipocyte hypertrophy and worsening of the metabolic syndrome52. Weight loss subsequent to obesity causes the accumulation of a novel macrophage sub-population that is rare in lean and obese eWAT57, 65. This ATM sub-population was termed Phagocytic macrophage, due to their expression of genes related with phagocytosis and endocytosis65, and was proposed to participate in clearing dying adipocytes and leukocytes from the shrinking eWAT. Following weight loss, the LAMs/DPs return to their proportions in lean eWAT.
2. Dendritic Cells
Dendritic cells (DCs) are phagocytes, constantly surveying tissues, sampling antigens and instructing adaptive immunity by activating and shaping T cell responses and by releasing chemokines and cytokines. Ralph Steinman and Zanvil Cohn69 discovered these cells in the 1970s and in the past 50 years the ontogeny, function and subsets have been extensively studied and reviewed elsewhere70–72. DCs are conventionally classified as cDC1s (CD11b–CD103+), cDC2s (CD11b+CD103–) and plasmocytoid DCs (pDCs, characterized by their ability to synthesize large amounts of type I interferons, IFN, in response to viral stimulation)71. This section will be focused on cDC1 and cDC2, since pDCs are rare in the vWAT51.
For many years the study of AT DCs was overshadowed by the attention directed at ATMs. Furthermore, the technical difficulties of discriminating DCs from Møs in the eWAT were a major hurdle. DCs and Møs share many surface markers, such as CD11b, F4/80, MHCII, CX3CR1 and CD11c. Some groups relied on the use of CD11c and MHCII as markers of DCs in the eWAT15, 73, however, Møs can express these surface markers as well, which has confounded data interpretation. Another technical hurdle is that cells within the eWAT are intrinsically more autofluorescent, which can complicate the analysis of flow-cytometry data. The recent use of multi parametric flow-cytometry and more advanced genetic tools, such as Zbtb46GFP74–76 (fluorescently marks cDCs specifically) and the CRE-lox77 mice, has enabled direct studies of AT DCs.
The identification of DCs in the mouse eWAT has improved with the use of CD64 as a Mø marker16, 51, since DCs do not express this gene in the eWAT. It was shown that the eWAT is populated by both cDC1s (CD45+CD19–B220–CD11b–CD11c+MHCII+CD64–CD103+) and cDC2s (CD45+CD19–B220–CD11b+CD11c+MHCII+CD64–CD103–)16, 51, 78. At steady-state, the eWAT is enriched in cDC2s, which are the third most abundant immune cell population after Møs and T cells20, while cDC1s represent only a small fraction of immune cells51. These cells express a set of genes related to an immunoregulatory phenotype51. For example, cDC2s express high levels of Aldh1a2, the gene encoding retinol dehydrogenase 2, an enzyme fundamental to the synthesis of retinoic acid from retinaldehyde. Retinoic acid has been shown to be a potent immunoregulatory factor, capable of inducing the differentiation of regulatory T cells79. Single-cell analysis of eWAT leukocytes identified three DC populations, one of which is proposed to be monocyte-derived65. However, the functional differences between the monocyte-derived DCs and the resident populations is unclear. Interestingly, during obesity, the number of DCs per gram of eWAT is not altered while there is a massive expansion in the amount of monocyte-derived Møs51, leading to a proportional decrease in AT DCs. This indicates that DCs contribute to the immunoregulatory environment in steady-state; however, during obesity it is likely that this immunoregulatory function is diluted by the deluge of other leukocyte subpopulations that accumulate in obesity.
Recent studies have begun to shed more light on DC function in the eWAT. Pamir and collaborators have observed that DCs can contribute to adipogenesis and insulin sensitivity80. Using CSF2−/− mice (that presumably has a greater negative impact on DCs than on Møs) they observe that CSF2-deficiency leads to a reduction in the numbers of cDC2s. This reduction led to an increase in total body adiposity and adipocyte size. Furthermore, the researchers observed that metalloproteinase 12 (MMP12) and Fibronectin 1 (FN1), which are highly expressed by cDC2s in the vWAT, impaired adipogenesis in vitro indicating a possible mechanism of regulation of adipogenesis in vivo by DCs. However, CSF2 is also an important cytokine for the development of neutrophils, eosinophils, basophils and monocytes, which can be a confounding factor for the data observed with CSF2−/− mice. However, another report using the FMS like tyrosine kinase 3 ligand (Flt3l)- knockout mice, in which there are low levels of DCs81, 82, showed opposite results, displaying reduced adiposity and increase insulin sensitivity82. Thus, further studies with conditional knockout animals targeting specific subpopulations of DCs are necessary to expand the knowledge about the role of DCs in vWAT function.
It is unclear, however, in these studies if littermate controls and co-housed animals were used to exclude an effect of the microbiome. Furthermore, Macdougall and colleagues have shown that DCs have a tolerogenic phenotype associated with the activation of β–catenin in cDC1s and peroxisome proliferator-activated receptors (PPAR) γ in cDC2s. Deficiency of these transcriptional regulators in DCs rendered mice subjected to HFD more predisposed to the development of insulin resistance. Moreover, it was observed that obesity inhibits the β–catenin and PPARγ pathways in DCs, modulating them towards a more pro-inflammatory phenotype78.
Despite these advances, many outstanding questions remain regarding the role of DCs in the eWAT. Little is known about the trafficking of DCs from the eWAT to the draining lymph nodes and how this influences the differentiation of T cells in homeostasis and obesity. Additionally, how DCs are distributed in eWAT and the cells with which they interact is largely unknown. Do DCs contribute to the maintenance of AT regulatory T cells (adTregs)? If so, which subpopulation of DCs mediates the interaction with adTregs? We expect that in the near future, with the development of better genetic models to specifically manipulate DCs, the contribution of DCs to eWAT homeostasis will be revealed.
3. Monocytes
Monocytes are traditionally stratified into two main populations, based on their expression of Ly6C in mice or CD14 in humans (reviewed in83). Classical monocytes, also termed inflammatory monocytes, highly express Ly6C/CD14 and are the main monocyte type rapidly recruited to sites of inflammation and insult. Non-classical monocytes, characterized by low expression of Ly6C/ CD14 patrol the blood vessels and closely interact with the endothelium65, 83. It has been consistently shown that at least a fraction of the non-classical monocytes arise from classical monocytes84. They exhibit a short lifespan in the circulation (1–7 days)85, 86, but can be recruited to tissues and reside there for long periods of time as tissue monocytes, or more commonly, differentiate into Møs or DCs87. scRNA-seq experiments of mouse peripheral blood monocytes revealed that within each population, Ly6Chi and Ly6Clo monocytes are homogeneous under steady-state conditions. However, a subset of monocytes with intermediate expression of Ly6C was shown to be heterogeneous88. Because circulating monocytes do not show great heterogeneity under physiological conditions, it will be interesting to understand whether their heterogeneity changes with inflammation or in different tissues. Moreover, since tissue microenvironments are critical in shaping the transcriptome of Møs89 and recruited monocytes90, monocyte heterogeneity may be enhanced within eWAT.
To date, scRNA-seq of eWAT leukocytes has not revealed much monocyte heterogeneity (represented by one-two populations), in either lean or obese conditions52, 65, 66. That said, some evidence suggests that the eWAT monocytes give rise to multiple populations of Møs and DCs65, indicating potential functional heterogeneity. Furthermore, pseudotime analysis of scRNA-seq has shown that obesity and subsequent weight loss alters the monocyte’s differentiation trajectories65. While in lean eWAT most monocyte-derived Møs retained transcriptional profiles similar to monocytes, in obesity and caloric restriction the ATMs appeared more differentiated and further away in pseudotime space from monocytes65. Monocytes recruited to the eWAT in obesity primarily differentiate into Møs that co-express CD11c and CD6451. It is likely that these double-positive ATMs consist of a heterogeneous population. Using a fate-mapping mouse model, Jaitin and colleagues showed that LAMs are monocyte-derived52. However, further single cell analysis of fate-mapped monocyte-derived ATMs may shed more light into the function and differentiation capacity of AT monocytes. We postulate that cues in the different microenvironments (e.g. bone marrow, spleen, circulation eWAT) shape the differentiation capacity of monocytes, thus regulating the abundance and function of the monocyte-derived cells- Møs and DCs.
4. Eosinophils
Historically eosinophils were viewed as effector cells mainly involved in allergic inflammation and responses to parasites, but their roles in AT homoeostasis became appreciated in the last decade. The stromal vascular fraction of vWAT contains ~5% eosinophils27. The importance of WAT eosinophils was emphasized in a seminal publication from Locksley and colleagues27, where the authors found that eosinophils are the major source of IL-4 in the WAT, supporting alternative activation of ATMs. As described in the Mø section, IL-4 produced by eosinophils is necessary for heat production upon cold exposure by promoting beiging of white adipocytes25 and increasing the availability of energy from fatty acids by enhancing lipolysis24. Furthermore, IL-4/STAT6 signaling regulates nutrient metabolism and insulin sensitivity. Although STAT6 deficient mice gain less weight on a HFD than their WT counterparts, due to increased energy expenditure, they are more glucose intolerant because of decreased insulin action26.
IL-5 is an important cytokine required for the maintenance of eosinophils and its overproduction resulted in accumulation of eosinophils in the eWAT27, while easonophil recruitment to tissues is mainly governed by eotaxins (reviewed in91). Innate lymphoid cells (ILCs) have been identified as the source of IL-5 in the eWAT that acts to maintain the eosinophil population92, 93 (the contribution of ILCs to AT biology will be further discussed below). eWAT eosinophil counts dropped in a mouse model of diet-induced obesity and eosinophil deficiency enhanced glucose intolerance27 and worsened the metabolic syndrome94. This may be due to impairment of lipid accumulation and adipocyte differentiation in the obese eWAT upon eosinophil deficiency94. Conversely, long term caloric restriction of lean mice resulted in accumulation of eosinophils in the WAT, increased IL-4 production, promoted adipocyte beiging and enhanced glucose tolerance95.
Eosinophil heterogeneity has been recognized for approximately 35 years96, 97, with peripheral blood eosinophils known to have both different appearances microscopically and functionality in patients suffering from allergies98 and parasites99. This altered eosinophil phenotype, termed hypodense, occurred following degranulation of their cytotoxic vesicles. However, it is unclear if hypodense eosinophils represent a distinct population, or whether this is indicative of their activation state. Notably, although eosinophils can be easily detected using flow-cytometry51, all 3 recently published scRNA-seq studies of eWAT leukocytes failed to identify any eosinophil populations52, 65, 66. This may indicate that AT eosinophils do not have a substantially different transcriptome from other leukocytes, thus preventing their identification as a distinct population. Alternatively, there could be a bias introduced by the procedures and instrumentation involved in scRNA-seq100, which precludes the identification of eosinophils in eWAT. It is possible that as more advanced methods are introduced to the field, such as Cellular Indexing of Transcriptomes and Epitopes by Sequencing (CITE)-seq that combines proteomics and transcriptomic data, more light will be shed on the phenotypes and function of eosinophils in eWAT.
5. Neutrophils
Neutrophils are rare in the lean eWAT (<0.01% of the stromal vascular fraction)101, where they have been proposed to be engulfed by Møs and maintain the tissue integrity102. Neutrophils rapidly accumulate in the eWAT upon HFD feeding and their numbers in the stromal vascular fraction increases by ~20 fold within 7 days, after which their proportion is steadily maintained101, 103. The accumulation of neutrophils in the eWAT following HFD is dependent on neutrophil elastase (NE), and its deficiency improves metabolic parameters, such as glucose tolerance and insulin resistance. Mechanistically, it was shown that NE may degrade Insulin receptor substrate 1 (IRS1) in hepatocytes and adipocytes, therefore promoting lipogenesis and insulin resistance101. In the circulation of obese patients, neutrophils produced more pro-inflammatory mediators, such as myeloperoxidase (MPO), IL-8 and CCL2104. MPO deficiency was later shown to enhance insulin sensitivity and induce heat production via upregulation of uncoupling protein 1 (UCP1)105.
It has been proposed that similar to Møs and T cells, neutrophils are heterogeneous in their activation states and responses, with “N1” population referring to a pro-inflammatory neutrophil and “N2” an anti-inflammatory population106–108. Although this stratification of neutrophils is over simplistic, as with M1/M2 Møs, it has remained a convenient “high level” classification.
Similar to eosinophils, neutrophils have been shown to be of variable densities. For instance, three neutrophil populations with varying cellular densities were characterized in the circulation of tumor-bearing mice and humans109. The suggested model for this phenomenon is that immature, low-density neutrophils arise from immature neutrophils in the bone marrow and serve as myeloid-derived suppressor cells (MDSCs), whereas mature low-density neutrophils are derived from conventional circulating mature neutrophils. Both low-density neutrophil populations were found to be immunosuppressive and tumor promoting109.
A recent scRNA-seq analysis of cells positive for Gr1+ cells (also known as Ly-6G/Ly-6C, and expressed on granulocytes and monocytes) in steady-state conditions showed that neutrophils are even more heterogeneous than appreciated previously110. The authors report 3 major neutrophil populations in the bone marrow, and 3 transcriptionally distinct neutrophil populations in peripheral blood and spleen. Interestingly, there was little overlap between neutrophil populations in the bone marrow and peripheral tissues; however blood and spleen neutrophils showed significant overlap110. One caveat of this study is the purification of cells with Gr1 expression, since this is a shared marker for neutrophils, monocytes, natural killer (NK) cells and other cell types. Thus, it is possible that the observed heterogeneity reflects different leukocyte types. It is also possible that the distinct populations reflect varying differentiation states and neutrophil-biased progenitors111. Other support for neutrophil heterogeneity comes from a recent scRNA-seq study of heart neutrophils following myocardial infarction (MI)112. The authors isolated cells from the infarcted heart 0, 1, 3, 5 and 7 days post MI and performed CITE-seq and found 6 distinct neutrophil populations. In the MI model, neutrophil heterogeneity was observed temporally, as different neutrophil clusters populated the heart in different stages post-MI. These data further suggested that neutrophil heterogeneity is, at least in part, due to local phenotypic transition in the ischemic heart112. Since similar numeric changes of neutrophils occur in the eWAT upon high-fat diet feeding, it will be interesting to determine whether populations similar to those observed in the infarcted heart are also present in the eWAT. Neutrophil heterogeneity possibly regulates vWAT inflammatory status via production of Neutrophil Extracellular Traps (further reviewed in17) on one hand, and the suppressive nature of MDSCs on the other.
6. Mast cells
Mast cells home to peripheral tissues as immature cells and undergo their final maturation under the specific control of mucosal and epithelial tissue microenvironments113. The WAT contain mast cell progenitors and can produce mature cells that home to the intestine, skin and WAT upon hematopoietic cell transplantation114. The heterogeneity of mast cells was proposed in the 1980’s. Two major populations of mast cells were described in humans, based on the protease content of their cytoplasmic granules. Connective tissue mast cell granules contain tryptase, chymase and carboxypeptidase, whereas mucosal tissues mast cells produce only tryptase115. A common hypothesis is that mast cell heterogeneity is related to the fact that their final maturation occurs in tissue microenvironments116, and their phenotypes are thus dictated by the specific milieu in which they mature.
Similar to other types of immune cells, mast cells accumulate in the vWAT in obesity117. Mast cell precursors showed enhanced migration following treatment with eWAT conditioned media from pre-obese db/db mice, suggesting that mast cells accumulate in the eWAT before obesity is established118. Genetic and pharmacologic interventions in mice that impair mast cell maturation and function resulted in decreased weight gain on a HFD and subsequently enhanced glucose tolerance117, 119. Strikingly, treatment of obese mice with an inhibitor of mast cell degranulation induced weight loss, even when the high-fat diet feeding continued117. Adoptive transfer of mast cells to mice deficient in mast cells partially restored the obese phenotype, but only if the transferred cells were sufficient for IL-6 and IFNɣ, suggesting a role for these factors in the deleterious effects of mast cells in obesity117. Furthermore, mast cells were shown to produce prostaglandin J2 (PGJ2), an activator of the adipogenic transcription factor PPARɣ, and may promote adipogenic differentiation via this mechanism119. Additionally, obese WAT mast cells seem to be degranulated120 and serum levels of the mast cell protease trypase positively correlates with BMI121. Overall, the preponderance of evidence indicates that vWAT mast cells exacerbate obesity and the metabolic syndrome; however, their heterogeneity in the WAT is yet to be established. It is plausible that, similar to Møs, there are distinct mast cell populations that are responsible for different functions.
II. The adaptive immune system in WAT: lymphoid cells and their tugs of war
Despite the vWAT being a tissue predominantly populated by myeloid cells, it is also home to a diverse population of lymphoid immune cells. At steady state, Tregs, ILC2s, γδ T cells and invariant natural killer T cells (iNKTs) contribute to vWAT homeostasis by inhibiting inflammatory processes and promoting an optimal environment for adipocyte function to control systemic metabolism. On the other side of the spectrum, effector CD4+ T cells (mainly type 1 T helper cells –Th1), CD8+ T cells and natural killer cells (NKs) and/or type 1 innate lymphoid cells (ILC1s) play important roles in inducing an inflammatory environment and consequently, compromising vWAT metabolic function leading to insulin resistance in obesity. This section will explore how these myriad cell populations participate in the activity of vWAT at steady state and in obesity.
7. αβ (conventional) T cells
T cells are the second largest immune population in the WAT20 and can be divided into 2 major subtypes, based on cell surface expression of CD4 or CD8. Upon antigen stimulation in the context of presentation on MHC molecules, T cells are activated and become effector cells, which are crucial for mounting a primary response against pathogens, as well as establishing immunological memory. In obesity, T cells were shown to accumulate in the vWAT in both mice and humans122, 123, possibly through recruitment via CCR5-CCL5 interactions122.
Nagai and colleagues performed a time course examination of the mouse eWAT stromal-vascular fraction124, which revealed that while Mø frequencies begin to increase at 10 weeks post HFD feeding, CD8+ T cells increased proportionally within 6 weeks of HFD124. Other studies showed that T cell accumulation in the eWAT is subsequent to Mø accrual during HFD feeding125, and recent scRNA-seq data showed no marked difference in T cell frequencies in obesity52 or weight loss65. In absolute numbers, CD4+ T cells are much more abundant in the vWAT123 and they increase further in obesity126. However, the distributions of the different CD4+ T cell sub-populations change with obesity, which will be discussed later in this section. In addition, obesity induced the accumulation of memory T cells in the eWAT following viral infection, and mediated acute pancreatitis and eWAT necrosis upon re-challenge14. Weight cycling was also shown to increase T cell abundances in the eWAT, without influencing the number of ATMs127.
Because there is an increase in T cells in the obese eWAT, it is reasonable to speculate that some T cells undergo clonal expansion. Recent T cell Receptor (TCR) sequencing studies found that both CD8+ and CD4+ T cells from obese eWAT are enriched for clones that are shared across many individual mice, indicating a process of selection by similar auto-antigens13, 128, 129. Furthermore, MHCII expression was increased at early times post-HFD feeding (2 weeks) and mice deficient in MHCII showed similar adiposity, with greater insulin sensitivity compared to WT controls130. That said, MHCII inhibition in mice during HFD feeding did not improve glucose tolerance, although it did reduce the eWAT CD4+ T cell abundance131. Importantly, deficiency in conventional T cells (in TCRβ−/− mice) led to improvement of glucose and insulin sensitivity compared to WT mice, albeit lipid accumulation increased in the eWAT and adipocytes hypertrophied after HFD feeding132. Similarly, treatment of obese mice with anti-CD3 antibody (for T cell depletion) led to improvement in glucose and insulin sensitivity129. On balance, these findings suggest that conventional T cell activation is deleterious in obesity. However, other experiments showed that T cell deficiency of the co-stimulatory molecule CD40 exacerbated obesity and insulin resistance133, indicating that some T cell responses may be beneficial.
In general, CD8+ T cells are considered harmful in obesity. Depletion of CD8+ cells throughout HFD feeding or after the establishment of obesity, using a depleting antibody, decreased the expression of IL-6 and TNFα in the eWAT, and modestly improved glucose and insulin tolerance124. Moreover, CD8+ T cell-deficient mice fed HFD were more glucose and insulin tolerant than control mice, a phenotype that was overcome by adoptive transfer of WT CD8+ T cells124. These data suggest that CD8+ T cells are drivers of inflammation in the eWAT. The recruitment of CD8+ T cells to the eWAT was shown to be largely mediated through CD11a, since adoptively transferred CD11a-deficient CD8+ T, but not WT, cells failed to home to the eWAT134. In the obese eWAT, expression of perforin by CD8+ T cells restricted their inflammatory capacity by inhibiting their proliferation and inducing apoptosis135, 136.
CD4+ T cell subtypes, also known as T helpers (Th), have been characterized based on the cytokines they produce and the type of immune response they promote. Of the T helper sub-types, the central population responsible for maintaining WAT integrity is the Tregs, which will be discussed in the following section. The main T helper cell that is thought to have deleterious effects in obesity is the Th1 sub-type, which is regulated by the transcription factor T-bet and produces pro-inflammatory cytokines, including TNFα and IFNγ. Their frequency in the obese human and mouse vWAT is 10–20 fold greater than other T helper populations129, 137, 138, while in lean conditions their proportion is similar to Tregs129. Th1 cells were shown to accumulate in the eWAT and scWAT of mice fed a HFD, as well as in vWAT in humans, which strongly correlated with BMI129.
IFNγ production from eWAT Th1 cells increases in obese compared to lean mice, and IFNγ deficiency moderately improves glucose tolerance following HFD feeding139. TCRβ–/– mice were protected from diet-induced obesity compared to WT mice, and this protection was abolished by adoptive transfer of Th1 cells, providing evidence that Th1 cells are deleterious in obesity. A subset of Th1 cells, which express PD1 and CD44, was shown to produce osteopontin in the obese eWAT that may contribute to worsening of glucose and insulin tolerance. This was confirmed by transferring PD1+CD44+CD4+ T cells from spleens of obese mice into eWAT of lean recipients. This resulted in increased expression of inflammatory markers in the eWAT and in impaired glucose and insulin tolerance, but only when the transferred T cells were sufficient for osteopontin126.
Th17 cells, which are regulated by the transcription factor RAR-related Orphan Receptor (ROR) γt and are the main source of IL-17, are another T helper subtype implicated in pro-inflammatory processes. Their role in obesity has not yet been extensively studied. Current data show that obese, insulin resistant individuals have an accumulation of Th17 in their scWAT, compared with metabolically healthy obese or lean people140. The same study also demonstrated in vitro that IL-17 reduced insulin sensitivity in human liver cells and inhibited glucose uptake by rat skeletal muscle cells140. IL-17 deficient mice had better glucose and insulin tolerance than their WT counterparts, however, this effect was abrogated following HFD feeding and aging141, indicating that IL-17 and Th17 cells may not play a role in obesity. Further investigations using adoptive T cell transfer may shed more light on the role of Th17 cells, since it was also shown that the major producers of IL-17 in eWAT are not Th17, but rather γδ T cells141.
Another T helper subtype is the Th2, which is regulated by the transcription factor GATA Binding Protein (GATA) 3. These cells produce the STAT6 responsive cytokines IL-4 and IL-13, among others. Th2 cells have been shown to be beneficial in mouse models of obesity: Recombination Activating Gene (Rag) deficient mice (lacking T and B lymphocytes) fed HFD were more obese and insulin resistant than their WT counterparts, a phenotype that was reversed via CD4+, but not CD8+, T cell transfer129. Most CD4+ T cells that accumulated in the Rag–/– eWAT post-transfer expressed GATA3, and the improvement in metabolic parameters was impaired when the transferred cells were deficient for STAT6129. With that said, further examination of the contribution of Th2 cells in obesity is needed, since it is currently thought that the main producers of IL-4/13 in the WAT are eosinophils and the target cell population for these cytokines are Møs (see sections above). Moreover, GATA3 can also be expressed by Tregs142, which are critical for maintaining the WAT integrity (see Treg section below).
Despite many studies, the role of T cells in the WAT and their contribution to obesity are still incompletely understood. A plausible unbiased approach to unveiling the different T cell populations in the WAT is scRNA-seq (and preferably CITE-seq) of purified CD3+ cells. Current scRNA-seq datasets of eWAT simply do not have enough T cells to investigate their heterogeneity. Another possibility is to aggregate all of the scRNA-seq data published thus far, in hopes that there is enough information to distinguish between the T cell subtypes.
8. Regulatory T cells
The discovery and characterization of Tregs143, 144, whose differentiation is regulated by the transcription factor Forkhead Box P3 (FOXP3)145–147, have revealed a plethora of functions for these T cells in health and disease. Tregs are essential to maintain homeostasis of most mammalian tissues. Mutations in the Foxp3 gene have been linked to the development of several inflammatory diseases148–151. In addition to their contribution to controlling immune responses, Tregs have been described more recently by several groups as having an active role in the physiology of several organs152, such as muscle153 and eWAT154–157.
Feuere et al. were the first to show that Tregs are highly enriched in eWAT in the steady state155. It was further observed that Tregs accumulate in the WAT of mice with aging, whereas they decline in obesity in both mice and humans, resulting in an exacerbated inflammatory environment that promotes the development of type 2 diabetes155, 158. Recent scRNA-seq data of mouse eWAT confirmed that the relative abundance of Tregs, which clustered together with ILC2 cells, decreased in obesity65. Interestingly, following caloric restriction-induced weight loss, adTreg proportions reverted back to their lean frequencies65.
Following their initial report in WAT, several groups contributed to the characterization and functional analysis of adTregs. It is now well established that adTregs display a unique phenotype. AdTregs express the transcription factors PPARγ154, 159 (the master regulator of adipocyte differentiation), Basic leucine zipper Transcription Factor (BATF), GATA3 and Interferon Regulatory Factor (IRF) 4157. These cells are thymic derived (expressing thymic Treg markers neuropilin-1160 and Helios161), and express high levels of the receptors for the cytokine IL-33 (ST2, IL1RL1 or IL33R) and the Killer cell Lectin like Receptor (KLR) G1154, 156, 157. Elegant work by Vasanthakumar and colleagues demonstrated that adTreg accumulation in the eWAT relies on IL-33 signaling. Tregs deficient for IL-33R, Myeloid differentiation primary response (MyD) 88, BATF and IRF4, all of which are activated by IL-33, displayed impaired development of adTregs and increased susceptibility to the development of insulin resistance when submitted to a hyper-caloric diet157. On the other hand, mice administered IL-33 had their adTreg pool expanded and this correlated with an improvement in insulin sensitivity and glucose tolerance156, 157.
AdTregs are long lived, self-dividing and harbor a restricted TCR repertoire, with the expansion of a few clones, which indicates that these cells are pre-selected to identify limited antigenic repertoire, and do not need constant thymic supply to maintain cell numbers in the eWAT156. More recently Li and colleagues discovered a stepwise acquisition of the adTreg phenotype162. This study clearly showed that FOXP3 and an AT pre-selected TCR are required for adTreg development and accumulation in the eWAT162. They further showed that after homing to the eWAT, adTregs upregulated the expression of the IL33R and that higher abundances of IL-33 in this tissue drove the expansion of adTregs in the eWAT162. Moreover, it was reported that ILC2s (described below) also support the expansion of adTregs in vivo through Inducible T Cell Costimulator (ICOS)- Inducible T Cell Costimulator ligand (ICOSL) interactions163; however, experiments demonstrating this interaction in the eWAT by imaging are still missing.
One of the open questions in the field is how precisely adTregs contribute to the establishment of an immunoregulatory environment in the eWAT that favors insulin sensitivity. Several publications have shown an inverse correlation between adTregs numbers in the eWAT and the appearance of CD11b+CD11c+ Møs in the eWAT154, 156, 157. AdTregs are characterized by the high expression of the immunoregulatory cytokine IL-10157 that has been shown in different settings to contribute to the maintenance of more homeostatic/ tissue-resident Møs164–167. In addition, adTregs express high levels of amphiregulin, a ligand of the epidermal growth factor receptor (EGFR), which promotes tissue repair and resolution of inflammation152, 153. It is also predicted that these cells deploy their regulatory functions to inhibit the activation of CD4+ and CD8+ effector T cells within the eWAT and, therefore, blunting of the initiation of inflammatory responses.
Despite the aforementioned mechanisms, little is known about how this is executed in vivo. A recent study showed that adTregs localize close to mesenchymal stromal cells (MSCs) that are the producers of IL-33 in the eWAT168. However, the interaction of adTregs with other cell types in healthy eWAT is still largely unknown. For example, the fact that adTregs harbor a restricted TCR repertoire suggests an active selection by antigen presenting cells (APCs) within the vWAT, and that these Tregs probably recognize vWAT specific antigens. However, little is known about which APC is responsible for selecting adTregs. As described earlier in this review, the vWAT is populated by diverse and complex populations of antigen presenting cells. The observation that adTregs are located close to MSCs could indicate that these cells could be responsible for the adTreg selection, however, they do not present antigen in a MHCII context. This question goes hand-in-hand with the lack of data regarding antigen recognition by adTregs. What are the antigens recognized by these cells? Are these antigens canonical peptides or could they even be modified peptides harboring lipidated branches due to the high concentrations of triacylglycerol and derivatives present in this tissue? Another open question is how the fat draining lymph node and APCs contribute to the development of adTregs. Li et al. claim that adTregs originate in the thymus, pass through the spleen, and finally home to the vWAT102. However, the vWAT draining lymph node could be a strategic place for drained antigens and/or circulating APCs that had passed through the vWAT and are shaped by the microenvironment to initiate the adTreg program and present vWAT specific antigens.
Finally, one of the most puzzling questions is why the number of adTregs is severely reduced in obesity and whether this is the cause or consequence of obesity/ metabolic syndrome? There are reports in the literature indicating that the fast increase in adipocyte size and numbers cause a hypoxic environment, promoting sterile inflammation169–171, and possibly reducing adTreg cell numbers. However, it is already established in the literature that Tregs are more tolerant of hypoxia and that Hypoxia Inducible Factor (HIF) 1α actually favors Treg development172. Thus, the reason behind adTreg reduction in obesity is still a mystery. If these questions can be addressed, it is possible to foresee the development of new therapeutic approaches to expand adTregs via administration of AT specific antigens and IL-33, to reduce vWAT inflammation and mitigate insulin resistance/ type 2 diabetes.
9. γδ T cells
Although not as prominently studied as αβ T cells, γδ T cells are an important immune cell population involved in response to both microbial and sterile inflammation173. γδ T cells comprise only 5% of the circulating T cells; however they are particularly enriched in barrier surfaces like the skin, intestinal epithelia and reproductive organs174–176. One peculiar characteristic of γδ T cells is that they harbor a restricted TCR repertoire, especially if compared to αβ T cells, with specific clonal expansion in each epithelial barrier that they populate174–176. Interestingly, until today, the antigens recognized by γδ T cells are largely unknown. In the lean eWAT, γδ T cells are as abundant as CD8 T cells, monocytes, mast cells and neutrophils51, although they were not recognized as a separate cell population in recent scRNA-seq experiments65,66. With that said, it was previously shown that scRNA-seq technologies cannot effectively separate T cell subsets, and that integration of proteomic and transcriptomic approaches, such as CITE-seq, are better at distinguishing between different T cell populations177.
Thus far a few groups have investigated the function of γδ T cells in the vWAT. A preliminary study has claimed that these cells promote inflammation and insulin resistance when animals are subjected to HFD. Mice deficient in γδ T cells displayed better insulin sensitivity after 10 weeks of HFD178. However, the fact that TCRδ knockout animals displayed several discrepancies from Vγ4/6 knockout mice (although both mouse models lack γδ T cells), and whether the animals were properly cohoused to normalize the microbiota, precludes a clear conclusion about the role of γδ T cells in the development of metabolic syndrome.
More recently, elegant work by Kohlgruber and collaborators showed that the eWAT is home to two different, self-renewing populations of γδ T cells. One population is characterized as Promyelocytic Leukaemia Zinc Finger protein (PLZF)−, CD3εlow, CD27+, RORγT−, T-bet+ that produces IFNγ, and the second population is PLZF+, CD3εhigh,CD27−, RORγT+, T-bet− and able to produce high levels of IL-17A and TNFα179. Interestingly, the authors observed that animals deficient in γδ T cells, or specifically the PLZF+ γδ population, or IL-17A knockout mice all fail to accumulate adTregs in the eWAT. The authors show that presumably the IL-17A and TNFα produced by PLZF+ γδ T cells positively modulate the number of IL-33 producing stromal cells, which contributes to the accumulation of adTregs. Finally, it was observed that PLZF+ γδ T cells and IL-17A are fundamental for thermogenesis and mice deficient for these cells or cytokine, respectively, fail to control total body temperature179. These phenomena was confirmed in a new report showing that γδ T cells expressing IL-17A are key drivers of organ neuronal innervation that regulates thermogenesis180.
This phenomenon may be linked to the fact that IL-33 is essential for adipocyte beiging and thermogenesis181, 182, hence reduction in IL-17A producing γδ T cells leads to fewer IL-33-producing stromal cells, which in turn reduces the capacity of the AT to control core body temperature. Unfortunately, this study did not explore the impact of PLZF+ γδ T cells in the development of the metabolic syndrome. In addition, studies involving the deletion of IL-17A specifically in γδ T cells can help clarify the direct role of this cytokine produced by γδ T cells. Another question that arises from this study is whether γδ T cells regulate the numbers of ILC2 cells, since it is already established that IL-33 can regulate both adTreg and ILC2 cell numbers157, 181. Overall, it seems that γδ T cells have a clear contribution to vWAT physiology and new studies will clarify their roles.
10. Invariant Natural Killer T cells
Natural Killer T cells (NKTs) are a major evolutionary link between the innate and adaptive immunity. They share several effector innate markers with NK cells but also are able to recognize antigens through an αβ TCR. However, these cells do not recognize peptides. NKTs recognize lipids and glycolipids bound to the MHC-like glycoprotein, CD1d183, 184. Invariant NKT cells (iNKTs) are a subset of NKTs that harbor restricted TCR consisting of one α chain (Vα14Jα18 in mice and Vα24Jα18 in humans) and a limited number of β chains183. Even though the endogenous antigenic ligands are still an enigma, it was discovered that the lipid α-galactosylceramide (α-GC), obtained from marine sponges can strongly stimulate human and mouse iNKT cells when presented in a CD1d-dependent context185. Moreover, a few endogenous ligands have been reported, such as isoglobotrihexosylceramide (iGb-3)186.
iNKT cells have been reported to be important in mediating immunity against tumors187 and bacterial infection, as well as in autoimmunity and allergy183. In addition, due to the nature of the AT, a lipid rich environment harboring a number of cells expressing CD1d (mainly Møs, DCs and adipocytes), several groups have recently evaluated the contribution of iNKTs to vWAT homeostasis. These cells were shown to be enriched in the vWAT, compared to their circulating levels188, 189. Similar to γδ T cells, iNKTs did not form a transcriptionally distinct population in recent scRNA-seq data of mouse eWAT, and were clustered with the conventional T cells65. Interestingly, cell frequency of the T/NKT cluster was the most stable across treatment (i.e. lean, obese and caloric restriction) among all leukocyte clusters in this study65. Because it is difficult to distinguish T cell subsets solely based on transcriptomic data, further investigations are needed to establish transcriptional and functional heterogeneity of vWAT T cells.
In 2011 and 2012 several reports attempted to clarify the contribution of iNKTs in vWAT physiology. The lack of consensus among these studies was obvious: some indicated that iNKTs are protective and help to improve insulin sensitivity190–194, others indicated the opposite- that iNKTs can accelerate the development of insulin resistance195–197, and some claimed that iNKTs do not play a role in systemic metabolic functions198. This variability in the results among different groups was already reviewed elsewhere199–201 and several factors can contribute to it. Overall the studies display several differences in the experimental conditions such as the type of diet used and its duration, mouse age, and the genetic model used to manipulate NKT cells (some used CD1d knockout mice that eliminated all NKTs, others used Jα18 knockout mice that abolished only iNKTs, while others activated NKTs by injecting CD1d-α-GC tetramers). In some studies, it is not clear whether mice were cohoused to normalize their microbiota. In mice, NKT accumulate predominantly in the liver. It is possible that the metabolic phenomena observed upon NKT manipulation are related to their effects on the liver, rather than the vWAT. Although this issue poses a tremendous technical challenge in general.
Overall it was shown that CD1d knockdown (leading to total NKT deficiency) or Jα18 knockdown (iNKTs deficiency) resulted in a more pro-inflammatory environment within the eWAT, leading to glucose and insulin intolerance190, 192, 193. In addition, it was observed that iNKTs in the eWAT express significant levels of IL-4 and IL-10. The activation of these cells by treatment with CD1d-α-GC tetramers augmented the expression of cytokines, leading to an increased Arg1 expression and other factors related to the function of ATMs and ultimately improved insulin and glucose sensitivity190, 192, 193. These data indicate that iNKTs can act in synergy with eosinophils and ILC2s by recognizing the lipid content in eWAT to modulate the activity of resident Møs.
In the aftermath of these studies, Lynch and collaborators unveiled that iNKTs in the vWAT display a unique phenotype. They express low levels of IL7R, downregulate the expression of the transcription factor PLZF (thus far known as a marker of NKTs) after exiting the thymus and homing to the eWAT, and highly express IL-2 and IL-10202. These cytokines produced by iNKT are important for the maintenance of adTregs and to modulate ATMs, respectively202, 203. Deficiency in iNKT cells reduces the numbers of adTregs and the proportion of IL-10+ adTregs in the eWAT202. These data reinforce the idea that iNKTs act together with other regulatory cells in the vWAT to suppress inflammation and maintain its optimal metabolic activity by giving support to ATMs and consequently, to adipocytes. These data were recently reproduced using mice that lacked CD1d expression in Møs, neutrophils, DCs204 and adipocytes205, indicating a cross talk between NKTs, adipocytes and APCs to control the vWAT microenvironment204, 205.
One important observation from these studies is that mice treated with CD1d-α-GC tetramers, which increases the number of iNKTs and improves insulin sensitivity, also induces weight loss. To understand the mechanism underlying this weight loss, a recent publication showed that activation of iNKTs promoted beiging of scWAT by the upregulation of UCP1 and induction of non-shivering thermogenesis. This increase in UCP1 was mediated by increased expression of Fibroblast Growth Factor (FGF) 21 directly induced by iNKT cells206. Interestingly, it was shown that treatment of mice and humans with liraglutide, an agonist of glucagon-like peptide 1 receptor (GLP1R), led to the proliferation of iNKTs, which consequently increased the levels of FGF21, UCP1, thermogenesis and promoted weight loss206. Liraglutide is a commercially available treatment for type 2 diabetes and obesity, which works, at least in part, by modulation of the iNKT-FGF21-UCP1 axis. Understanding how this pathway induces iNKT proliferation may help in the development of new approaches to avoid the side effects caused by GLP1R agonist treatment.
11. Type 2 innate lymphoid cells
The discovery of type 2 innate lymphoid cells (ILC2s) goes back to 2001 when the cytokine IL-25 (also known as IL-17E due to its homology to other members of the IL-17 family) was characterized by two different groups207, 208. Fort and colleagues observed that treating animals with IL-25 induced the expansion and expression of type 2 cytokines by a non-T/non-B cell that is CD11b–NK1.1–Ly6C–Ly6G–207. Later, in 2002 and 2006 these cells were defined as IL-4–, IL-5–, IL-13–producing non–B/non–T (NBNT), c-kit+, FcεR1– cells and shown to be important for the clearance of helminthic infections209, 210. Thereafter several other groups studying these cells coined a diverse nomenclature, such as nuocytes211, natural helper cells (NHCs)212, innate helper type 2 cells (Ih2)213 and multipotent progenitor type 2 (MPP type2)214. These studies found that ILC2s are fundamental at barrier surfaces to promote type 2 responses, important for immunity against helminths, in allergic inflammation and the resolution of pulmonary inflammation. It did not take long to for this population to be renamed type 2 innate lymphoid cells215.
ILC2s are characterized by their lack of expression of CD3, CD4, CD8, CD19, B220, CD11b, FcεRI, TCRβ, TCRδ, CD5, NK1.1, Ter119, Ly6C, Ly6G and CD11c (lineage negative). These cells express CD90, IL-7Rα, c-Kit, Sca-1, IL-2Rβ, IL-2Rγ, CD25, IL-33R, KLRG1, IL-17RA, IL-17RB, CD45, CD38, CD44, GITR, CD69 and several cytokines, especially IL-4, IL-5 and IL-13211–213, 216. In addition, it was shown that ILC2s, similar to other innate lymphoid cells, rely on the transcription factor Inhibitor of DNA Binding (Id) 2 and the common γ chain receptor (IL-2Rγ) for their development, indicating the existence of a shared ILC progenitor211–213. Lastly, it was observed that ILC2 cells depend on the expression of the transcription factors TCF-1, RORα and GATA3 for theirs development and maintenance216–218. Interestingly, the expression of several surface markers and GATA3 is shared by ILC2s and adTregs, indicating that these cells may co-habit the same niche and work together to maintain vWAT homeostasis. Moreover, ILC2s and Tregs clustered together in silico, as demonstrated in scRNA-seq data of mouse eWAT, indicating a high degree of overlap in their transcriptomes65.
Interestingly, one of the first reports to characterize this immune population found their accumulation in the mesenteric adipose tissue (around the intestine)212. This was indicative that ILC2s could contribute to vWAT homeostasis. In 2013, Molofsky et al. revealed that ILC2 cells are resident at the vWAT, are expanded by IL-33, and are a major source of IL-5 and IL-13. This work demonstrated that the IL-5 produced by ILC2s was fundamental for maintenance of eosinophils within the vWAT. Mice deficient for IL-5 or lacking ILC2s have a dramatic reduction in the number of eosinophils93. As mentioned before in this review, eosinophils are an important source of IL-4 in the WAT, which support the maintenance of alternatively activated Møs. Diet-induced obese mice develop faster and more severe insulin resistance in the absence of eosinophils or IL-527, 93. Additionally, the IL-13 produced by ILC2s was shown to contribute to the maintenance of AAMs expressing Arg1 in the vWAT, which is associated with improved insulin sensitivity219, 220.
As was observed for adTregs, IL-33 is fundamental for the maintenance of ILC2s in vWAT. Animals deficient for IL-33 have lower numbers of ILC2s, associated with increased fat content and body weight181. These phenomena were associated with the capacity of ILC2s to promote beiging of white adipocytes by the increase of the opioid peptide methionine-enkephalin, which upregulates UCP1181. Lee and colleagues have shown that the ILC2s promote beiging through the secretion of Type 2 cytokines that activate eosinophils and these 2 cell types can secrete IL-4 and IL-13 that bind to IL4Rα in adipocyte progenitors (expressing PDGFRα), thus inducing their differentiation towards beige adipocytes instead of white adipocytes221.
In addition to their effects on eosinophils and Møs, another study has shown that ILC2s contribute to the maintenance of adTregs in the eWAT through ICOS-ICOSL interactions in steady-state. During inflammation the production of IFNγ inhibits ILC2 cells and consequently the accumulation of adTregs in the eWAT163. This could be a possible mechanism to explain how adTreg numbers are drastically reduced in obesity.
Most recently it was shown that the high levels of TNFα in obese eWAT leads to the upregulation of PD-1 by ILC2. PD-1 can interact with PD-L1 expressed by the infiltrating M1-like Møs, subsequently reducing the expression of IL-5 and IL-13 by the ILC2, and jeopardizing the latter’s homeostatic function222. Of note, this work did not use genetic models to remove the expression of PD-1 specifically in ILC2 to confirm this mechanism. Nonetheless it opens the possibility to utilize PD-1 blockade as a therapy for the metabolic syndrome/insulin resistance. Finally, it was observed also that ILC2s upregulate OX40L upon exposure to IL-33. The authors propose that OX40L can interact with OX40 expressed by adTregs, which expands the adTreg pool, contributing to the control of insulin sensitivity223. Altogether, it seems that the proposed therapeutic approach for adTregs through the administration of IL-33 can have beneficial synergistic effects through the expansion of ILC2 cells. Uncovering the potential side effects of IL-33 administration and if indeed ILC2s interact directly with adTregs can help in the development of approaches to treat type 2 diabetes.
12. B cells
B cells regulate immune responses mainly via the production of antibodies and cytokines. In mice, B cells reside in the lean eWAT and further accumulate in obese conditions224, 225. The absolute number of B cells is similar to that of T cells in both lean and obese eWAT224.
To directly investigate the contribution of B cells to obesity several groups used mouse models lacking B cells (Bnull). On a HFD, Bnull mice had improved glucose and insulin sensitivity compared with WT controls226, albeit similar body weight224. Adoptive transfer of splenic B cells from obese, but not lean, donors to obese Bnull mice worsened glucose tolerance and increased plasma insulin224. Moreover, eWAT CD8+ T cell pro-inflammatory gene expression was attenuated in Bnull mice. Depletion of B cells (using anti-CD20 antibody) in diet-induced obese mice markedly improved metabolic parameters and eWAT inflammation224. It was further shown that B cells from obese mice produce more pro-inflammatory cytokines and less IL-10 in response to toll-like receptor engagement, compared with lean controls227. With that said, B cell deficiency drastically alters the microbiome and impairs intestinal fat absorption, which may contribute to the phenotype in these mice228.
The most abundant antibody isotype in the eWAT is IgM. Compared to lean mice, obesity increases the antibody isotype IgG2c in both the serum and eWAT, without significant changes in other isotypes224, 229. IgG antibodies produced in obesity were shown to be highly pathogenic, since administration of IgGs isolated from obese, but not lean mice, dramatically worsened metabolic parameters of obese Bnull mice. This observation was specific to IgG antibodies, as treatment with IgMs from obese mice did not alter metabolic or inflammatory parameters of Bnull mice. Surprisingly, although IgG antibodies are upregulated in the obese eWAT, deficiency of all IgG receptors worsened insulin and glucose intolerance in obesity230. Taken together these data indicate that B cells in obesity produce diverse IgG antibodies that can be either deleterious or beneficial. It is reasonable that the expression and function of the different IgG receptors on phagocytes will determine the specific response to each antibody.
Similar to other leukocytes, B lymphocytes are stratified into sub-types according to their specific marker expression and function. The most significant difference between B1 and B2 cells is their origin and distribution. B1 cells mostly originate from embryonic tissues and B2 cells arise from adult bone marrow progenitors (reviewed elsewhere231). B1 cells, defined232 as CD19hiB220loIgMhiCD23−, normally reside in the pleural cavity233 and are a major source of IgM antibodies234. In addition to their antimicrobial abilities, IgMs recognize epitopes on dying cells, promoting their uptake by phagocytes, thus helping maintain tissue integrity234. B1 cells are further stratified to 2 distinct sub-types, B1a and B1b, which differ based on their surface expression of CD5 (expressed by B1a, but not B1b)232. Functionally, B1a cells responses are broad, since they are not restricted to B cell receptor engagement, while B1b cells expand and produce IgM in response to specific antigens232 and are thus the major source of memory B1 cells. Most B cells belong to the B2 type, which are the follicular (lymphoid) and marginal-zone (splenic) B cells232. B2 cells are activated following antigen recognition, with the help of T cells in lymphoid tissues. The eWAT was shown to contain predominantly B2 cells, with small amounts of B1a and B1b224. B cells further accumulated in the obese eWAT, with no change to the distribution of the B cell sub-types224. B cells that accumulated in the obese eWAT mainly increased the production of IgM and IgG224. Interestingly, recent scRNA-seq data showed that the frequency of B cells from all eWAT leukocytes did not differ between lean and obese mice, however there were ~2 fold more B cells in the obese eWAT following caloric restriction65. Very little is known about B cells in weight loss and it will be interesting to see whether they accumulate and have a substantial role in this process. Moreover, further investigations are needed to understand the contribution of each B cell sub-population in obesity.
III. Concluding remarks
The WAT microenvironment enables most types of immune cells to reside and cooperate within it, as well as influence systemic processes. Current investigations aim at functionally characterizing the immune populations in the WAT, due to their vital contribution to normal tissue homeostasis (Figure 2), as well as pathophysiology. In this review, we mainly focused on the vWAT, because of the wealth of knowledge in that tissue. However, it is critical to keep in mind that many other tissues (liver, muscle, bone marrow, heart and more) may accumulate excess amounts of lipids, especially in obesity. This fat accrual outside of the AT will, most likely alter the local milieu and possibly change the immune landscape of many tissues.
Figure 2. Major players of immune homeostasis in the lean eWAT.
The eWAT is an organ highly vascularized and replete of leukocytes. Vasculature associated adipose tissue macrophages (VAMs) are normally in intimate contact with the blood vessels. They display a rapid endocytic capacity for blood borne particles and also contain lipid droplets in their cytoplasm51. These cells are regulated by IL-4 and IL-10 produced by iNKT cells that are activated by CD1d expressing cells in the eWAT193. These cytokines contribute to an anti-inflammatory/ homeostatic environment shaping the activity of VAMs. γδ T cells positive for the transcription factor RORγT express high levels of IL-17A. This cytokine regulates the numbers of mesenchymal stromal cells (MSCs or Fibroblastic reticular cells)179. MSCs play an important role by producing IL-33, which is fundamental for the expansion of regulatory T cells (Tregs) within the adipose tissue. eWAT Tregs have a peculiar phenotype, displaying high expression of the receptor for IL-33157 and the transcription factor PPARγ154. Tregs can recognize antigens and be activated by VAMs or Dendritic cells (DCs). Upon activation Tregs release IL-10, which in turn contribute to the physiological eWAT environment. Another important cell population is the innate lymphoid cells type 2 (ILC2s)93. These cells are resident in a healthy eWAT producing high amounts of IL-5, which, in turn attracts and expands eosinophils. Production of IL-4 by eosinophils help to maintain the phenotype and activity of VAMs27. Other leukocytes reside in the eWAT (such as T cells, B cells, monocytes, macrophage and dendritic cells), however, their function in homeostasis remains to be investigated. TCR: T cell receptor; BCR: B cell receptor; MHCII- major histocompatibility complex 2.
Recent technological advances allowing investigations on the single cell level drove the discovery of heterogeneous subpopulations and cell states, which were largely underappreciated. Flow-cytometry based studies were instrumental in finding and isolating cells expressing a distinct set of markers. However, these types of inquiries are biased, since pre-selection of marker genes is necessary. Revolutionizing biological studies was the RNA-seq, and more so scRNA-seq, which allows unbiased identification of sub-populations that may share many marker genes. Using these methods, a large number of immune populations and cell states were found in the vWAT under lean, obese and weight loss conditions. The newly identified sub-populations should be carefully examined and characterized, and to fully understand the processes underlying WAT function, the interactions of the different cell types, immune or others, should be investigated. Additionally, for a more comprehensive picture, such studies should be followed by examination of systemic changes occurring in obesity across multiple tissues. We look forward to such discoveries, which will surely revolutionize our understanding of obesity and its adverse consequences on many tissues and co-morbidities. Such efforts will hopefully enable the development of much needed therapies.
SOURCES OF FUNDING
This work was supported by grants from the National Institutes of Health (P01HL131481 to KJM, AMS and EAF), and the American Heart Association (18POST34080390 to AW). HMS was supported by a fellowship from the National Council for Scientific and Technological Development and a postdoctoral fellowship from Dr. Bernard B. Levine.
Footnotes
Conflict of interest statement: The authors have declared that no conflict of interest exists.
DISCLOSURES
None
References
- 1.Saltiel AR and Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maurizi G, Della Guardia L, Maurizi A and Poloni A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J Cell Physiol. 2018;233:88–97. [DOI] [PubMed] [Google Scholar]
- 3.Schoettl T, Fischer IP and Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol. 2018;221. [DOI] [PubMed] [Google Scholar]
- 4.Foster MT and Pagliassotti MJ. Metabolic alterations following visceral fat removal and expansion: Beyond anatomic location. Adipocyte. 2012;1:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A and Lupien PJ. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–8. [DOI] [PubMed] [Google Scholar]
- 6.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK and Adams PW. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–60. [DOI] [PubMed] [Google Scholar]
- 7.Enerback S. The origins of brown adipose tissue. N Engl J Med. 2009;360:2021–3. [DOI] [PubMed] [Google Scholar]
- 8.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B and Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–8. [DOI] [PubMed] [Google Scholar]
- 10.Chusyd DE, Wang D, Huffman DM and Nagy TR. Relationships between Rodent White Adipose Fat Pads and Human White Adipose Fat Depots. Front Nutr. 2016;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boutens L, Hooiveld GJ, Dhingra S, Cramer RA, Netea MG and Stienstra R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia. 2018;61:942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olefsky JM and Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL and Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misumi I, Starmer J, Uchimura T, Beck MA, Magnuson T and Whitmire JK. Obesity Expands a Distinct Population of T Cells in Adipose Tissue and Increases Vulnerability to Infection. Cell Rep. 2019;27:514–524 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, Blin-Wakkach C, Anty R, Iannelli A, Gugenheim J, Tran A, Bouloumie A, Gual P and Wakkach A. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61:2238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, Geletka L, Meyer KA, O’Rourke RW and Lumeng CN. Adipose Tissue Dendritic Cells Are Independent Contributors to Obesity-Induced Inflammation and Insulin Resistance. J Immunol. 2016;197:3650–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arivazhagan LRH, Wilson R, Manigrasso M, Gugger P, Fisher EA, Moore KJ, Ramasamy R, Schmidt AM. An Eclectic Cast of Cellular Actors Orchestrates Innate Immune Responses in the Mechanisms Driving Obesity and Metabolic Perturbation. Circulation Research. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouchi N, Parker JL, Lugus JJ and Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220:T47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrante AW Jr. The immune cells in adipose tissue. Diabetes Obes Metab. 2013;15 Suppl 3:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flaherty SE 3rd, Grijalva A, Xu X, Ables E, Nomani A and Ferrante AW Jr. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science. 2019;363:989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du H, Heur M, Duanmu M, Grabowski GA, Hui DY, Witte DP and Mishra J. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J Lipid Res. 2001;42:489–500. [PubMed] [Google Scholar]
- 23.Yan C, Lian X, Li Y, Dai Y, White A, Qin Y, Li H, Hume DA and Du H. Macrophage-specific expression of human lysosomal acid lipase corrects inflammation and pathogenic phenotypes in lal−/− mice. Am J Pathol. 2006;169:916–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM and Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD and Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, Mukundan L, Wu D, Locksley RM and Chawla A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107:22617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A and Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL and Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL and Ferrante AW Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh DY, Morinaga H, Talukdar S, Bae EJ and Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61:346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumeng CN, Deyoung SM, Bodzin JL and Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. [DOI] [PubMed] [Google Scholar]
- 32.Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Kloting N, Stumvoll M, Bashan N and Rudich A. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92:2240–7. [DOI] [PubMed] [Google Scholar]
- 33.Coenen KR, Gruen ML, Chait A and Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56:564–73. [DOI] [PubMed] [Google Scholar]
- 34.Ramkhelawon B, Hennessy EJ, Menager M, Ray TD, Sheedy FJ, Hutchison S, Wanschel A, Oldebeken S, Geoffrion M, Spiro W, Miller G, McPherson R, Rayner KJ and Moore KJ. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med. 2014;20:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill AA, Anderson-Baucum EK, Kennedy AJ, Webb CD, Yull FE and Hasty AH. Activation of NF-kappaB drives the enhanced survival of adipose tissue macrophages in an obesogenic environment. Mol Metab. 2015;4:665–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng C, Yang Q, Xu C, Shou P, Cao J, Jiang M, Chen Q, Cao G, Han Y, Li F, Cao W, Zhang L, Zhang L, Shi Y and Wang Y. CD11b regulates obesity-induced insulin resistance via limiting alternative activation and proliferation of adipose tissue macrophages. Proc Natl Acad Sci U S A. 2015;112:E7239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muir LA, Kiridena S, Griffin C, DelProposto JB, Geletka L, Martinez-Santibanez G, Zamarron BF, Lucas H, Singer K, RW OR and Lumeng CN. Frontline Science: Rapid adipose tissue expansion triggers unique proliferation and lipid accumulation profiles in adipose tissue macrophages. J Leukoc Biol. 2018;103:615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS and Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. [DOI] [PubMed] [Google Scholar]
- 39.Tamura Y, Sugimoto M, Murayama T, Ueda Y, Kanamori H, Ono K, Ariyasu H, Akamizu T, Kita T, Yokode M and Arai H. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler Thromb Vasc Biol. 2008;28:2195–201. [DOI] [PubMed] [Google Scholar]
- 40.Ito A, Suganami T, Yamauchi A, Degawa-Yamauchi M, Tanaka M, Kouyama R, Kobayashi Y, Nitta N, Yasuda K, Hirata Y, Kuziel WA, Takeya M, Kanegasaki S, Kamei Y and Ogawa Y. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J Biol Chem. 2008;283:35715–23. [DOI] [PubMed] [Google Scholar]
- 41.Mulder P, van den Hoek AM and Kleemann R. The CCR2 Inhibitor Propagermanium Attenuates Diet-Induced Insulin Resistance, Adipose Tissue Inflammation and Non-Alcoholic Steatohepatitis. PLoS One. 2017;12:e0169740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HM, Lee ES, Lee BR, Yadav D, Kim YM, Ko HJ, Park KS, Lee EY and Chung CH. C-C chemokine receptor 2 inhibitor ameliorates hepatic steatosis by improving ER stress and inflammation in a type 2 diabetic mouse model. PLoS One. 2015;10:e0120711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huh JH, Kim HM, Lee ES, Kwon MH, Lee BR, Ko HJ and Chung CH. Dual CCR2/5 Antagonist Attenuates Obesity-Induced Insulin Resistance by Regulating Macrophage Recruitment and M1/M2 Status. Obesity (Silver Spring). 2018;26:378–386. [DOI] [PubMed] [Google Scholar]
- 44.Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, Amidi A, Watkins SM, Nguyen U and Olefsky JM. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010;285:15333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM and Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim K, Shim D, Lee JS, Zaitsev K, Williams JW, Kim KW, Jang MY, Seok Jang H, Yun TJ, Lee SH, Yoon WK, Prat A, Seidah NG, Choi J, Lee SP, Yoon SH, Nam JW, Seong JK, Oh GT, Randolph GJ, Artyomov MN, Cheong C and Choi JH. Transcriptome Analysis Reveals Nonfoamy Rather Than Foamy Plaque Macrophages Are Proinflammatory in Atherosclerotic Murine Models. Circ Res. 2018;123:1127–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Rooijen N and van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide”. Methods Enzymol. 2003;373:3–16. [DOI] [PubMed] [Google Scholar]
- 48.Bader JE, Enos RT, Velazquez KT, Carson MS, Sougiannis AT, McGuinness OP, Robinson CM and Murphy EA. Repeated clodronate-liposome treatment results in neutrophilia and is not effective in limiting obesity-linked metabolic impairments. Am J Physiol Endocrinol Metab. 2019;316:E358–E372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu CL, McNeill J, Goon K, Little D, Kimmerling K, Huebner J, Kraus V and Guilak F. Conditional Macrophage Depletion Increases Inflammation and Does Not Inhibit the Development of Osteoarthritis in Obese Macrophage Fas-Induced Apoptosis-Transgenic Mice. Arthritis Rheumatol. 2017;69:1772–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng B, Jiao P, Nie Y, Kim T, Jun D, van Rooijen N, Yang Z and Xu H. Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PLoS One. 2011;6:e24358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silva HM, Bafica A, Rodrigues-Luiz GF, Chi J, Santos PDA, Reis BS, Hoytema van Konijnenburg DP, Crane A, Arifa RDN, Martin P, Mendes D, Mansur DS, Torres VJ, Cadwell K, Cohen P, Mucida D and Lafaille JJ. Vasculature-associated fat macrophages readily adapt to inflammatory and metabolic challenges. J Exp Med. 2019;216:786–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, Keren-Shaul H, David E, Zmora N, Eldar SM, Lubezky N, Shibolet O, Hill DA, Lazar MA, Colonna M, Ginhoux F, Shapiro H, Elinav E and Amit I. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell. 2019;178:686–698 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duffen J, Zhang M, Masek-Hammerman K, Nunez A, Brennan A, Jones JEC, Morin J, Nocka K and Kasaian M. Modulation of the IL-33/IL-13 Axis in Obesity by IL-13Ralpha2. J Immunol. 2018;200:1347–1359. [DOI] [PubMed] [Google Scholar]
- 54.Darkhal P, Gao M, Ma Y and Liu D. Blocking high-fat diet-induced obesity, insulin resistance and fatty liver by overexpression of Il-13 gene in mice. Int J Obes (Lond). 2015;39:1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lumeng CN, DelProposto JB, Westcott DJ and Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ and Ferrante AW Jr. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R and Ferrante AW Jr. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takagi M, Uno H, Nishi R, Sugimoto M, Hasegawa S, Piao J, Ihara N, Kanai S, Kakei S, Tamura Y, Suganami T, Kamei Y, Shimizu T, Yasuda A, Ogawa Y and Mizutani S. ATM Regulates Adipocyte Differentiation and Contributes to Glucose Homeostasis. Cell Rep. 2015;10:957–967. [DOI] [PubMed] [Google Scholar]
- 59.Aouadi M, Vangala P, Yawe JC, Tencerova M, Nicoloro SM, Cohen JL, Shen Y and Czech MP. Lipid storage by adipose tissue macrophages regulates systemic glucose tolerance. Am J Physiol Endocrinol Metab. 2014;307:E374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fischer-Posovszky P, Wang QA, Asterholm IW, Rutkowski JM and Scherer PE. Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology. 2011;152:3074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stienstra R, Dijk W, van Beek L, Jansen H, Heemskerk M, Houtkooper RH, Denis S, van Harmelen V, Willems van Dijk K, Tack CJ and Kersten S. Mannose-binding lectin is required for the effective clearance of apoptotic cells by adipose tissue macrophages during obesity. Diabetes. 2014;63:4143–53. [DOI] [PubMed] [Google Scholar]
- 62.Okabe Y and Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2016;17:9–17. [DOI] [PubMed] [Google Scholar]
- 63.Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, Nguyen HCB, Chegireddy K, Kim J, Habertheuer A, Vallabhajosyula P, Kambayashi T, Won KJ and Lazar MA. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A. 2018;115:E5096–E5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li C, Menoret A, Farragher C, Ouyang Z, Bonin C, Holvoet P, Vella AT and Zhou B. Single cell transcriptomics based-MacSpectrum reveals novel macrophage activation signatures in diseases. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinstock A, Brown EJ, Garabedian ML, Pena S, Sharma M, Lafaille J, Moore KJ and Fisher EA. Single-Cell RNA Sequencing of Visceral Adipose Tissue Leukocytes Reveals that Caloric Restriction Following Obesity Promotes the Accumulation of a Distinct Macrophage Population with Features of Phagocytic Cells. Immunometabolism. 2019;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma M, Schlegel M, Brown EJ, Sansbury BE, Weinstock A, Afonso MS, Corr EM, van Solingen C, Shanley LC, Peled D, Ramasamy R, Schmidt AM, Spite M, Fisher EA and Moore KJ. Netrin-1 Alters Adipose Tissue Macrophage Fate and Function in Obesity. Immunometabolism. 2019;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AAJ, Cochain C, Vafadarnejad E, Saliba AE, Zernecke A, Pramod AB, Ghosh AK, Anto Michel N, Hoppe N, Hilgendorf I, Zirlik A, Hedrick CC, Ley K and Wolf D. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ Res. 2018;122:1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ and Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinman RM and Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merad M, Sathe P, Helft J, Miller J and Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schlitzer A, McGovern N and Ginhoux F. Dendritic cells and monocyte-derived cells: Two complementary and integrated functional systems. Semin Cell Dev Biol. 2015;41:9–22. [DOI] [PubMed] [Google Scholar]
- 72.Schlitzer A, Sivakamasundari V, Chen J, Sumatoh HR, Schreuder J, Lum J, Malleret B, Zhang S, Larbi A, Zolezzi F, Renia L, Poidinger M, Naik S, Newell EW, Robson P and Ginhoux F. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat Immunol. 2015;16:718–28. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y, Tian J, Tian X, Tang X, Rui K, Tong J, Lu L, Xu H and Wang S. Adipose tissue dendritic cells enhances inflammation by prompting the generation of Th17 cells. PLoS One. 2014;9:e92450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE and Nussenzweig MC. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med. 2012;209:1153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meredith MM, Liu K, Kamphorst AO, Idoyaga J, Yamane A, Guermonprez P, Rihn S, Yao KH, Silva IT, Oliveira TY, Skokos D, Casellas R and Nussenzweig MC. Zinc finger transcription factor zDC is a negative regulator required to prevent activation of classical dendritic cells in the steady state. J Exp Med. 2012;209:1583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL and Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loschko J, Schreiber HA, Rieke GJ, Esterhazy D, Meredith MM, Pedicord VA, Yao KH, Caballero S, Pamer EG, Mucida D and Nussenzweig MC. Absence of MHC class II on cDCs results in microbial-dependent intestinal inflammation. J Exp Med. 2016;213:517–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Macdougall CE, Wood EG, Loschko J, Scagliotti V, Cassidy FC, Robinson ME, Feldhahn N, Castellano L, Voisin MB, Marelli-Berg F, Gaston-Massuet C, Charalambous M and Longhi MP. Visceral Adipose Tissue Immune Homeostasis Is Regulated by the Crosstalk between Adipocytes and Dendritic Cell Subsets. Cell Metab. 2018;27:588–601 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mucida D, Pino-Lagos K, Kim G, Nowak E, Benson MJ, Kronenberg M, Noelle RJ and Cheroutre H. Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity. 2009;30:471–2; author reply 472–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pamir N, Liu NC, Irwin A, Becker L, Peng Y, Ronsein GE, Bornfeldt KE, Duffield JS and Heinecke JW. Granulocyte/Macrophage Colony-stimulating Factor-dependent Dendritic Cells Restrain Lean Adipose Tissue Expansion. J Biol Chem. 2015;290:14656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD and Peschon JJ. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–97. [PubMed] [Google Scholar]
- 82.Stefanovic-Racic M, Yang X, Turner MS, Mantell BS, Stolz DB, Sumpter TL, Sipula IJ, Dedousis N, Scott DK, Morel PA, Thomson AW and O’Doherty RM. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes. 2012;61:2330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ingersoll MA, Platt AM, Potteaux S and Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32:470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA and Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–7. [DOI] [PubMed] [Google Scholar]
- 85.Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, Macallan D and Yona S. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med. 2017;214:1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E and Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M and Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mildner A, Schonheit J, Giladi A, David E, Lara-Astiaso D, Lorenzo-Vivas E, Paul F, Chappell-Maor L, Priller J, Leutz A, Amit I and Jung S. Genomic Characterization of Murine Monocytes Reveals C/EBPbeta Transcription Factor Dependence of Ly6C(−) Cells. Immunity. 2017;46:849–862 e7. [DOI] [PubMed] [Google Scholar]
- 89.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S and Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sakai M, Troutman TD, Seidman JS, Ouyang Z, Spann NJ, Abe Y, Ego KM, Bruni CM, Deng Z, Schlachetzki JCM, Nott A, Bennett H, Chang J, Vu BT, Pasillas MP, Link VM, Texari L, Heinz S, Thompson BM, McDonald JG, Geissmann F and Glass CK. Liver-Derived Signals Sequentially Reprogram Myeloid Enhancers to Initiate and Maintain Kupffer Cell Identity. Immunity. 2019;51:655–670 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, Doyle AD, Luo H, Zellner KR, Protheroe CA, Willetts L, Lesuer WE, Colbert DC, Helmers RA, Lacy P, Moqbel R and Lee NA. Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol. 2012;130:572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE and Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A and Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee EH, Itan M, Jang J, Gu HJ, Rozenberg P, Mingler MK, Wen T, Yoon J, Park SY, Roh JY, Choi CS, Park WJ, Munitz A and Jung Y. Eosinophils support adipocyte maturation and promote glucose tolerance in obesity. Sci Rep. 2018;8:9894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fabbiano S, Suarez-Zamorano N, Rigo D, Veyrat-Durebex C, Stevanovic Dokic A, Colin DJ and Trajkovski M. Caloric Restriction Leads to Browning of White Adipose Tissue through Type 2 Immune Signaling. Cell Metab. 2016;24:434–446. [DOI] [PubMed] [Google Scholar]
- 96.Prin L, Capron M, Tonnel AB, Bletry O and Capron A. Heterogeneity of human peripheral blood eosinophils: variability in cell density and cytotoxic ability in relation to the level and the origin of hypereosinophilia. Int Arch Allergy Appl Immunol. 1983;72:336–46. [DOI] [PubMed] [Google Scholar]
- 97.Prin L, Charon J, Capron M, Gosset P, Taelman H, Tonnel AB and Capron A. Heterogeneity of human eosinophils. II. Variability of respiratory burst activity related to cell density. Clin Exp Immunol. 1984;57:735–42. [PMC free article] [PubMed] [Google Scholar]
- 98.Fukuda T, Dunnette SL, Reed CE, Ackerman SJ, Peters MS and Gleich GJ. Increased numbers of hypodense eosinophils in the blood of patients with bronchial asthma. Am Rev Respir Dis. 1985;132:981–5. [DOI] [PubMed] [Google Scholar]
- 99.White CJ, Maxwell CJ and Gallin JI. Changes in the structural and functional properties of human eosinophils during experimental hookworm infection. J Infect Dis. 1986;154:778–83. [DOI] [PubMed] [Google Scholar]
- 100.Hicks SC, Townes FW, Teng M and Irizarry RA. Missing data and technical variability in single-cell RNA-sequencing experiments. Biostatistics. 2018;19:562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB and Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Casanova-Acebes M, Nicolas-Avila JA, Li JL, Garcia-Silva S, Balachander A, Rubio-Ponce A, Weiss LA, Adrover JM, Burrows K, N AG, Ballesteros I, Devi S, Quintana JA, Crainiciuc G, Leiva M, Gunzer M, Weber C, Nagasawa T, Soehnlein O, Merad M, Mortha A, Ng LG, Peinado H and Hidalgo A. Neutrophils instruct homeostatic and pathological states in naive tissues. J Exp Med. 2018;215:2778–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elgazar-Carmon V, Rudich A, Hadad N and Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–903. [DOI] [PubMed] [Google Scholar]
- 104.Rouault C, Pellegrinelli V, Schilch R, Cotillard A, Poitou C, Tordjman J, Sell H, Clement K and Lacasa D. Roles of chemokine ligand-2 (CXCL2) and neutrophils in influencing endothelial cell function and inflammation of human adipose tissue. Endocrinology. 2013;154:1069–79. [DOI] [PubMed] [Google Scholar]
- 105.Wang Q, Xie Z, Zhang W, Zhou J, Wu Y, Zhang M, Zhu H and Zou MH. Myeloperoxidase deletion prevents high-fat diet-induced obesity and insulin resistance. Diabetes. 2014;63:4172–85. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS and Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deniset JF and Kubes P. Recent advances in understanding neutrophils. F1000Res. 2016;5:2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, Henry J, Cates CA, Deleon-Pennell KY and Lindsey ML. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res. 2016;110:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, Ariel A, Hovav AH, Henke E, Fridlender ZG and Granot Z. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–73. [DOI] [PubMed] [Google Scholar]
- 110.Xie X, Shi Q, Wu P, Zhang X, Kambara H, Su J, Yu H, Park S-Y, Guo R, Ren Q, Zhang S, Xu Y, Silberstein LE, Cheng T, Ma F, Li C and Luo HR. Single-cell transcriptome profiling reveals neutrophil heterogeneity and orchestrated maturation during homeostasis and bacterial infection. bioRxiv. 2019:792200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhu YP, Padgett L, Dinh HQ, Marcovecchio P, Blatchley A, Wu R, Ehinger E, Kim C, Mikulski Z, Seumois G, Madrigal A, Vijayanand P and Hedrick CC. Identification of an Early Unipotent Neutrophil Progenitor with Pro-tumoral Activity in Mouse and Human Bone Marrow. Cell Rep. 2018;24:2329–2341 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vafadarnejad E, Rizzo G, Krampert L, Arampatzi P, Nugroho VA, Schulz D, Roesch M, Alayrac P, Vilar J, Silvestre J-S, Zernecke A, Saliba A-E and Cochain C. Time-resolved single-cell transcriptomics uncovers dynamics of cardiac neutrophil diversity in murine myocardial infarction. bioRxiv. 2019:738005. [Google Scholar]
- 113.Gurish MF, Pear WS, Stevens RL, Scott ML, Sokol K, Ghildyal N, Webster MJ, Hu X, Austen KF, Baltimore D and et al. Tissue-regulated differentiation and maturation of a v-abl-immortalized mast cell-committed progenitor. Immunity. 1995;3:175–86. [DOI] [PubMed] [Google Scholar]
- 114.Poglio S, De Toni-Costes F, Arnaud E, Laharrague P, Espinosa E, Casteilla L and Cousin B. Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells. 2010;28:2065–72. [DOI] [PubMed] [Google Scholar]
- 115.Irani AA, Schechter NM, Craig SS, DeBlois G and Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986;83:4464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shea-Donohue T, Stiltz J, Zhao A and Notari L. Mast cells. Curr Gastroenterol Rep. 2010;12:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS and Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hirai S, Ohyane C, Kim YI, Lin S, Goto T, Takahashi N, Kim CS, Kang J, Yu R and Kawada T. Involvement of mast cells in adipose tissue fibrosis. Am J Physiol Endocrinol Metab. 2014;306:E247–55. [DOI] [PubMed] [Google Scholar]
- 119.Tanaka A, Nomura Y, Matsuda A, Ohmori K and Matsuda H. Mast cells function as an alternative modulator of adipogenesis through 15-deoxy-delta-12, 14-prostaglandin J2. Am J Physiol Cell Physiol. 2011;301:C1360–7. [DOI] [PubMed] [Google Scholar]
- 120.Divoux A, Moutel S, Poitou C, Lacasa D, Veyrie N, Aissat A, Arock M, Guerre-Millo M and Clement K. Mast cells in human adipose tissue: link with morbid obesity, inflammatory status, and diabetes. J Clin Endocrinol Metab. 2012;97:E1677–85. [DOI] [PubMed] [Google Scholar]
- 121.Fenger RV, Linneberg A, Vidal C, Vizcaino L, Husemoen LL, Aadahl M and Gonzalez-Quintela A. Determinants of serum tryptase in a general population: the relationship of serum tryptase to obesity and asthma. Int Arch Allergy Immunol. 2012;157:151–8. [DOI] [PubMed] [Google Scholar]
- 122.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW and Ballantyne CM. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–38. [DOI] [PubMed] [Google Scholar]
- 123.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Bluher M, Unger T, Wolf AM, Knippschild U, Hombach V and Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–10. [DOI] [PubMed] [Google Scholar]
- 124.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T and Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. [DOI] [PubMed] [Google Scholar]
- 125.Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS and Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring). 2010;18:1918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shirakawa K, Yan X, Shinmura K, Endo J, Kataoka M, Katsumata Y, Yamamoto T, Anzai A, Isobe S, Yoshida N, Itoh H, Manabe I, Sekai M, Hamazaki Y, Fukuda K, Minato N and Sano M. Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest. 2016;126:4626–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Anderson EK, Gutierrez DA, Kennedy A and Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes. 2013;62:3180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McDonnell WJ, Koethe JR, Mallal SA, Pilkinton MA, Kirabo A, Ameka MK, Cottam MA, Hasty AH and Kennedy AJ. High CD8 T-Cell Receptor Clonality and Altered CDR3 Properties Are Associated With Elevated Isolevuglandins in Adipose Tissue During Diet-Induced Obesity. Diabetes. 2018;67:2361–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D and Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, Ren Y, Yin Z, Hamilton DJ, Reardon PR, Sherman V, Wang HY, Phillips KJ, Webb P, Wong ST, Wang RF and Hsueh WA. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, Singer K and Lumeng CN. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khan IM, Dai Perrard XY, Perrard JL, Mansoori A, Smith CW, Wu H and Ballantyne CM. Attenuated adipose tissue and skeletal muscle inflammation in obese mice with combined CD4+ and CD8+ T cell deficiency. Atherosclerosis. 2014;233:419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wolf D, Jehle F, Michel NA, Bukosza EN, Rivera J, Chen YC, Hoppe N, Dufner B, Rodriguez AO, Colberg C, Nieto L, Rupprecht B, Wiedemann A, Schulte L, Peikert A, Bassler N, Lozhkin A, Hergeth SP, Stachon P, Hilgendorf I, Willecke F, von Zur Muhlen C, von Elverfeldt D, Binder CJ, Aichele P, Varo N, Febbraio MA, Libby P, Bode C, Peter K and Zirlik A. Coinhibitory suppression of T cell activation by CD40 protects against obesity and adipose tissue inflammation in mice. Circulation. 2014;129:2414–25. [DOI] [PubMed] [Google Scholar]
- 134.Jiang E, Perrard XD, Yang D, Khan IM, Perrard JL, Smith CW, Ballantyne CM and Wu H. Essential role of CD11a in CD8+ T-cell accumulation and activation in adipose tissue. Arterioscler Thromb Vasc Biol. 2014;34:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Revelo XS, Tsai S, Lei H, Luck H, Ghazarian M, Tsui H, Shi SY, Schroer S, Luk CT, Lin GH, Mak TW, Woo M, Winer S and Winer DA. Perforin is a novel immune regulator of obesity-related insulin resistance. Diabetes. 2015;64:90–103. [DOI] [PubMed] [Google Scholar]
- 136.Wang Q and Wu H. T Cells in Adipose Tissue: Critical Players in Immunometabolism. Front Immunol. 2018;9:2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu R and Nikolajczyk BS. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front Immunol. 2019;10:1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McLaughlin T, Liu LF, Lamendola C, Shen L, Morton J, Rivas H, Winer D, Tolentino L, Choi O, Zhang H, Hui Yen Chng M and Engleman E. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34:2637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH and Libby P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fabbrini E, Cella M, McCartney SA, Fuchs A, Abumrad NA, Pietka TA, Chen Z, Finck BN, Han DH, Magkos F, Conte C, Bradley D, Fraterrigo G, Eagon JC, Patterson BW, Colonna M and Klein S. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145:366–74 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zuniga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB and Butcher EC. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Han JM, Wu D, Denroche HC, Yao Y, Verchere CB and Levings MK. IL-33 Reverses an Obesity-Induced Deficit in Visceral Adipose Tissue ST2+ T Regulatory Cells and Ameliorates Adipose Tissue Inflammation and Insulin Resistance. J Immunol. 2015;194:4777–83. [DOI] [PubMed] [Google Scholar]
- 143.Lafaille JJ, Nagashima K, Katsuki M and Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. [DOI] [PubMed] [Google Scholar]
- 144.Sakaguchi S, Sakaguchi N, Asano M, Itoh M and Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 145.Fontenot JD, Gavin MA and Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. [DOI] [PubMed] [Google Scholar]
- 146.Hori S, Nomura T and Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. [DOI] [PubMed] [Google Scholar]
- 147.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH and Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF and Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. [DOI] [PubMed] [Google Scholar]
- 149.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF and Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. [DOI] [PubMed] [Google Scholar]
- 150.Levy-Lahad E and Wildin RS. Neonatal diabetes mellitus, enteropathy, thrombocytopenia, and endocrinopathy: Further evidence for an X-linked lethal syndrome. J Pediatr. 2001;138:577–80. [DOI] [PubMed] [Google Scholar]
- 151.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M and Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. [DOI] [PubMed] [Google Scholar]
- 152.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM and Rudensky AY. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C and Mathis D. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C and Mathis D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S and Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C and Mathis D. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterle S, Masters SL, Nakae S, Saito H, Wentworth JM, Li P, Liao W, Leonard WJ, Smyth GK, Shi W, Nutt SL, Koyasu S and Kallies A. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16:276–85. [DOI] [PubMed] [Google Scholar]
- 158.Cipolletta D, Cohen P, Spiegelman BM, Benoist C and Mathis D. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARgamma effects. Proc Natl Acad Sci U S A. 2015;112:482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schmidleithner L, Thabet Y, Schonfeld E, Kohne M, Sommer D, Abdullah Z, Sadlon T, Osei-Sarpong C, Subbaramaiah K, Copperi F, Haendler K, Varga T, Schanz O, Bourry S, Bassler K, Krebs W, Peters AE, Baumgart AK, Schneeweiss M, Klee K, Schmidt SV, Nussing S, Sander J, Ohkura N, Waha A, Sparwasser T, Wunderlich FT, Forster I, Ulas T, Weighardt H, Sakaguchi S, Pfeifer A, Bluher M, Dannenberg AJ, Ferreiros N, Muglia LJ, Wickenhauser C, Barry SC, Schultze JL and Beyer M. Enzymatic Activity of HPGD in Treg Cells Suppresses Tconv Cells to Maintain Adipose Tissue Homeostasis and Prevent Metabolic Dysfunction. Immunity. 2019;50:1232–1248 e14. [DOI] [PubMed] [Google Scholar]
- 160.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, Yang Y, Floess S, Huehn J, Oh S, Li MO, Niec RE, Rudensky AY, Dustin ML, Littman DR and Lafaille JJ. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723–42, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y and Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li C, DiSpirito JR, Zemmour D, Spallanzani RG, Kuswanto W, Benoist C and Mathis D. TCR Transgenic Mice Reveal Stepwise, Multi-site Acquisition of the Distinctive Fat-Treg Phenotype. Cell. 2018;174:285–299 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, Bluestone JA and Locksley RM. Interleukin-33 and Interferon-gamma Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity. 2015;43:161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ip WKE, Hoshi N, Shouval DS, Snapper S and Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR and Lindsey ML. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol. 2017;112:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Makita N, Hizukuri Y, Yamashiro K, Murakawa M and Hayashi Y. IL-10 enhances the phenotype of M2 macrophages induced by IL-4 and confers the ability to increase eosinophil migration. Int Immunol. 2015;27:131–41. [DOI] [PubMed] [Google Scholar]
- 167.O’Farrell AM, Liu Y, Moore KW and Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 1998;17:1006–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Spallanzani RG, Zemmour D, Xiao T, Jayewickreme T, Li C, Bryce PJ, Benoist C and Mathis D. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci Immunol. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M and Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–11. [DOI] [PubMed] [Google Scholar]
- 170.Sharma M, Boytard L, Hadi T, Koelwyn G, Simon R, Ouimet M, Seifert L, Spiro W, Yan B, Hutchison S, Fisher EA, Ramasamy R, Ramkhelawon B and Moore KJ. Enhanced glycolysis and HIF-1alpha activation in adipose tissue macrophages sustains local and systemic interleukin-1beta production in obesity. Sci Rep. 2020;10:5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. [DOI] [PubMed] [Google Scholar]
- 172.Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP and Eltzschig HK. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109:E2784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vantourout P and Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Haas W and Tonegawa S. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343:754–7. [DOI] [PubMed] [Google Scholar]
- 175.Itohara S, Nakanishi N, Kanagawa O, Kubo R and Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor gamma delta: analysis of gamma delta T cells during thymic ontogeny and in peripheral lymphoid organs. Proc Natl Acad Sci U S A. 1989;86:5094–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lafaille JJ, DeCloux A, Bonneville M, Takagaki Y and Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989;59:859–70. [DOI] [PubMed] [Google Scholar]
- 177.Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay PK, Swerdlow H, Satija R and Smibert P. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods. 2017;14:865–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mehta P, Nuotio-Antar AM and Smith CW. gammadelta T cells promote inflammation and insulin resistance during high fat diet-induced obesity in mice. J Leukoc Biol. 2015;97:121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kohlgruber AC, Gal-Oz ST, LaMarche NM, Shimazaki M, Duquette D, Koay HF, Nguyen HN, Mina AI, Paras T, Tavakkoli A, von Andrian U, Uldrich AP, Godfrey DI, Banks AS, Shay T, Brenner MB and Lynch L. gammadelta T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat Immunol. 2018;19:464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hu B, Jin C, Zeng X, Resch JM, Jedrychowski MP, Yang Z, Desai BN, Banks AS, Lowell BB, Mathis D and Spiegelman BM. gammadelta T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature. 2020;578:610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P and Artis D. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Odegaard JI, Lee MW, Sogawa Y, Bertholet AM, Locksley RM, Weinberg DE, Kirichok Y, Deo RC and Chawla A. Perinatal Licensing of Thermogenesis by IL-33 and ST2. Cell. 2016;166:841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bendelac A, Savage PB and Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. [DOI] [PubMed] [Google Scholar]
- 184.Brigl M and Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–90. [DOI] [PubMed] [Google Scholar]
- 185.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H and Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. [DOI] [PubMed] [Google Scholar]
- 186.Zhou D, Mattner J, Cantu C 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L and Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–9. [DOI] [PubMed] [Google Scholar]
- 187.Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, Kronenberg M and Seeger RC. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167:3114–22. [DOI] [PubMed] [Google Scholar]
- 188.Caspar-Bauguil S, Cousin B, Galinier A, Segafredo C, Nibbelink M, Andre M, Casteilla L and Penicaud L. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 2005;579:3487–92. [DOI] [PubMed] [Google Scholar]
- 189.Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG and O’Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol. 2009;39:1893–901. [DOI] [PubMed] [Google Scholar]
- 190.Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KS, Gao B, Lee CH, Kersten S and Qi L. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem. 2012;287:13561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kotas ME, Lee HY, Gillum MP, Annicelli C, Guigni BA, Shulman GI and Medzhitov R. Impact of CD1d deficiency on metabolism. PLoS One. 2011;6:e25478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ji Y, Sun S, Xia S, Yang L, Li X and Qi L. Short term high fat diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J Biol Chem. 2012;287:24378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O’Shea D, O’Farrelly C and Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R, Prop S, Meerding J, Hamers N, Besra G, Boon L, Nieuwenhuis EE, Elewaut D, Prakken B, Kersten S, Boes M and Kalkhoven E. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Satoh M, Andoh Y, Clingan CS, Ogura H, Fujii S, Eshima K, Nakayama T, Taniguchi M, Hirata N, Ishimori N, Tsutsui H, Onoe K and Iwabuchi K. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS One. 2012;7:e30568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Strodthoff D, Lundberg AM, Agardh HE, Ketelhuth DF, Paulsson-Berne G, Arner P, Hansson GK and Gerdes N. Lack of invariant natural killer T cells affects lipid metabolism in adipose tissue of diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2013;33:1189–96. [DOI] [PubMed] [Google Scholar]
- 197.Wu L, Parekh VV, Gabriel CL, Bracy DP, Marks-Shulman PA, Tamboli RA, Kim S, Mendez-Fernandez YV, Besra GS, Lomenick JP, Williams B, Wasserman DH and Van Kaer L. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci U S A. 2012;109:E1143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mantell BS, Stefanovic-Racic M, Yang X, Dedousis N, Sipula IJ and O’Doherty RM. Mice lacking NKT cells but with a complete complement of CD8+ T-cells are not protected against the metabolic abnormalities of diet-induced obesity. PLoS One. 2011;6:e19831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Satoh M and Iwabuchi K. Role of Natural Killer T Cells in the Development of Obesity and Insulin Resistance: Insights From Recent Progress. Front Immunol. 2018;9:1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Crosby CM and Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol. 2018;18:559–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, Besra G, Vomhof-Dekrey EE, Tighe M, Koay HF, Godfrey DI, Leadbetter EA, Sant’Angelo DB, von Andrian U and Brenner MB. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat Immunol. 2015;16:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sag D, Krause P, Hedrick CC, Kronenberg M and Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest. 2014;124:3725–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang H, Xue R, Zhu S, Fu S, Chen Z, Zhou R, Tian Z and Bai L. M2-specific reduction of CD1d switches NKT cell-mediated immune responses and triggers metaflammation in adipose tissue. Cell Mol Immunol. 2018;15:506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Huh JY, Park J, Kim JI, Park YJ, Lee YK and Kim JB. Deletion of CD1d in Adipocytes Aggravates Adipose Tissue Inflammation and Insulin Resistance in Obesity. Diabetes. 2017;66:835–847. [DOI] [PubMed] [Google Scholar]
- 206.Lynch L, Hogan AE, Duquette D, Lester C, Banks A, LeClair K, Cohen DE, Ghosh A, Lu B, Corrigan M, Stevanovic D, Maratos-Flier E, Drucker DJ, O’Shea D and Brenner M. iNKT Cells Induce FGF21 for Thermogenesis and Are Required for Maximal Weight Loss in GLP1 Therapy. Cell Metab. 2016;24:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM and Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. [DOI] [PubMed] [Google Scholar]
- 208.Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, Goddard AD, Yansura DG, Vandlen RL, Wood WI and Gurney AL. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem. 2001;276:1660–4. [DOI] [PubMed] [Google Scholar]
- 209.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE and McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G and Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–53. [DOI] [PubMed] [Google Scholar]
- 211.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE and McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H and Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–4. [DOI] [PubMed] [Google Scholar]
- 213.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ and Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF Jr., Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A and Artis D. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Spits H and Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–7. [DOI] [PubMed] [Google Scholar]
- 216.Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M and Diefenbach A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, Radtke F, Hardman CS, Hwang YY, Fallon PG and McKenzie AN. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yang Q, Monticelli LA, Saenz SA, Chi AW, Sonnenberg GF, Tang J, De Obaldia ME, Bailis W, Bryson JL, Toscano K, Huang J, Haczku A, Pear WS, Artis D and Bhandoola A. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hams E, Locksley RM, McKenzie AN and Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013;191:5349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Schipper HS, Prakken B, Kalkhoven E and Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab. 2012;23:407–15. [DOI] [PubMed] [Google Scholar]
- 221.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM and Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Oldenhove G, Boucquey E, Taquin A, Acolty V, Bonetti L, Ryffel B, Le Bert M, Englebert K, Boon L and Moser M. PD-1 Is Involved in the Dysregulation of Type 2 Innate Lymphoid Cells in a Murine Model of Obesity. Cell Rep. 2018;25:2053–2060 e4. [DOI] [PubMed] [Google Scholar]
- 223.Halim TYF, Rana BMJ, Walker JA, Kerscher B, Knolle MD, Jolin HE, Serrao EM, Haim-Vilmovsky L, Teichmann SA, Rodewald HR, Botto M, Vyse TJ, Fallon PG, Li Z, Withers DR and McKenzie ANJ. Tissue-Restricted Adaptive Type 2 Immunity Is Orchestrated by Expression of the Costimulatory Molecule OX40L on Group 2 Innate Lymphoid Cells. Immunity. 2018;48:1195–1207 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM and Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Duffaut C, Galitzky J, Lafontan M and Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun. 2009;384:482–5. [DOI] [PubMed] [Google Scholar]
- 226.Haskell BD, Flurkey K, Duffy TM, Sargent EE and Leiter EH. The diabetes-prone NZO/HlLt strain. I. Immunophenotypic comparison to the related NZB/BlNJ and NZW/LacJ strains. Lab Invest. 2002;82:833–42. [DOI] [PubMed] [Google Scholar]
- 227.DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, Allen J, Bouchard J, Toraldo G, Jasuja R, Obin MS, McDonnell ME, Apovian C, Denis GV and Nikolajczyk BS. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110:5133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shulzhenko N, Morgun A, Hsiao W, Battle M, Yao M, Gavrilova O, Orandle M, Mayer L, Macpherson AJ, McCoy KD, Fraser-Liggett C and Matzinger P. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med. 2011;17:1585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Winer DA, Winer S, Chng MH, Shen L and Engleman EG. B Lymphocytes in obesity-related adipose tissue inflammation and insulin resistance. Cell Mol Life Sci. 2014;71:1033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.van Dam AD, van Beek L, Pronk ACM, van den Berg SM, Van den Bossche J, de Winther MPJ, Koning F, van Kooten C, Rensen PCN, Boon MR, Verbeek JS, van Dijk KW and van Harmelen V. IgG is elevated in obese white adipose tissue but does not induce glucose intolerance via Fcgamma-receptor or complement. Int J Obes (Lond). 2018;42:260–269. [DOI] [PubMed] [Google Scholar]
- 231.Montecino-Rodriguez E and Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. [DOI] [PubMed] [Google Scholar]
- 233.Ansel KM, Harris RB and Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. [DOI] [PubMed] [Google Scholar]
- 234.Chen Y, Park YB, Patel E and Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182:6031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lin JD, Nishi H, Poles J, Niu X, McCauley C, Rahman K, Brown EJ, Yeung ST, Vozhilla N, Weinstock A, Ramsey SA, Fisher EA and Loke P. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]


