Abstract
Cancer growth interferes with local ionic environments, membrane potentials, and transepithelial potentials, resulting in small electrical changes in the tumor microenvironment. Electrical fields (EFs) have significant effects on cancer cell migration (galvanotaxis/electrotaxis), however, their role as a regulator of cancer progression and metastasis is poorly understood. Here, we employed unique probe systems to characterize the electrical properties of cancer cells and their migratory ability under an EF. Subcutaneous tumors were established from a triple-negative murine breast cancer cell line (4T1), electric currents and potentials of tumors were measured using vibrating probe and glass microelectrodes, respectively. Steady outward and inward currents could be detected at different positions on the tumor surface and magnitudes of the electric currents on the tumor surface strongly correlated with tumor weights. Potential measurements also showed the non-homogeneous intratumor electric potentials. Cancer cell migration was then surveyed in the presence of EFs in vitro. Parental 4T1 cells and metastatic sublines in isolation showed random migration in EFs of physiological strength, whereas cells in monolayer migrated collectively to the anode. Our data contribute to an improved understanding of breast cancer metastasis, providing new evidence in support of an electrical mechanism that promotes this phenomenon.
Subject terms: Breast cancer, Cellular motility, Collective cell migration
Introduction
Metastasis accounts for ~90% of mortality in breast cancer patients1,2. The last few decades have seen significant progress in understanding genetic, molecular and signaling mechanisms underpinning cancer cell migration. Despite this knowledge and implementation of advanced detection technologies, the prevalence of metastatic breast cancer at initial diagnosis has remained stagnant since 1975 in the United States3–6. While cancer was long considered a disease defined and driven by genetic evolutions which were mapped to the signaling pathways that regulate cell growth or motility7–9, increasing evidence indicates that the maintenance and expansion of malignant cells also strongly depend on external signals from the tumor microenvironment10–13.
Due to differences in metabolism and segregation of ions, local electrical properties changed and thus induced small direct current electrical fields naturally in live tissues. It has been shown to closely associate with cancer growth and other biological processes, such as wound healing. For instance, electric currents/fields at wounds are readily measurable and can persist from hours to weeks14,15. Similarly, cancer growth interferes with local ionic environments, membrane and transepithelial potentials thus producing local electric fields16,17. Outward current can be detected at the surface of tumor, and this electrical current is significantly greater than the one measured at the surface of intact epithelium18. Besides, it’s been hypothesized that measurement of electrical potential at the skin surface of new growth has potential to provide a reliable index for breast cancer diagnosis and may help to differentiate between malignant and benign growths19,20.
Previous studies by our group and others have demonstrated that galvanotaxis/electrotaxis, directional cell migration in response to extracellular electric gradients, is a powerful mechanism affecting motility and directionality of many cell types14,21. It has been demonstrated that cancer cells change their migratory patterns in electric fields of physiological strength22. Tumor cells from the brain, prostate and lung have all shown galvanotaxis responses23–25, therefore it is likely that most cancer cells exhibit some level of galvanotaxis14,21,26,27. Moreover, the galvanotactic responses of cancer cells may correlate with their metastasis capability. The highly invasive lung cancer subline CL1-5 displayed anodal galvanotaxis with increased cell motility, whereas the less invasive subline, CL1-0, displayed low galvanotaxis28. Highly metastatic breast cancer cells have been shown to respond to EFs with significantly higher speed and migration directionality than less metastatic cells26,27. Electric fields thus may be a fundamental, yet poorly understood, regulator of cancer progression29–31.
Herein, to illustrate the tumor endogenous EFs, we established a cell line derived tumor allograft (CDA) model in NSG mouse with the murine mammary carcinoma cell line (4T1) and systematically measured the electric currents and IntraTumoral potential (ITP) at subcutaneous CDA tumors ex vivo. The galvanotaxis response of 4T1 cells in EFs of physiological strength was also tested in vitro. Lastly, cancer sublines derived from 4T1 metastases to various organs were established and also evaluated for galvanotaxis activity in an EF. Our results demonstrated that electric fields naturally exist at the CDA tumor surface, and 4T1 cells respond to the EFs of physiological strength in monolayer but not in isolation. Metastatic sublines also showed significant galvanotactic movement in EFs with subtle differences.
Results
Electrical current measured at tumor’s surface
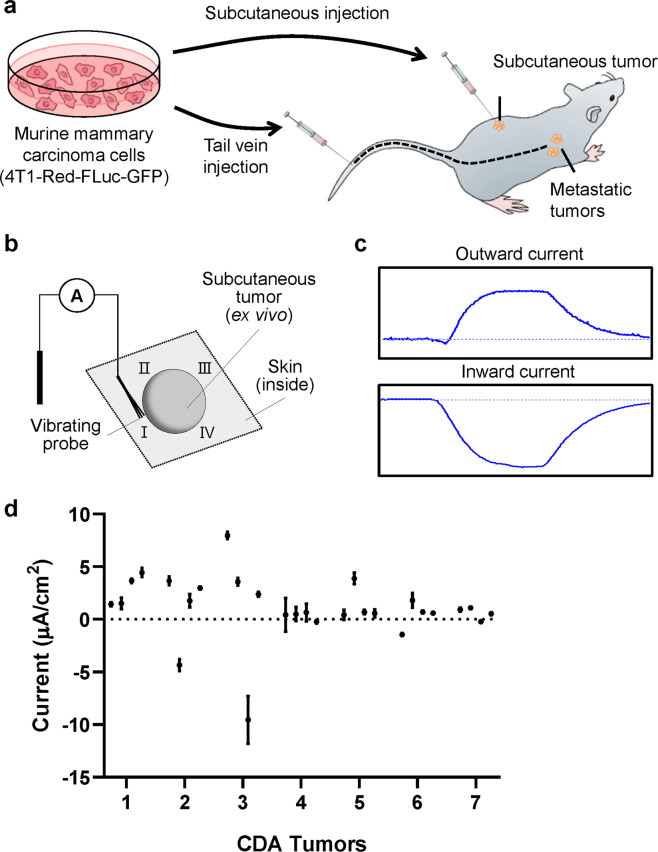
First, the vibrating probe was used to map the electrical currents in transgenic tumors. 1 × 105 4T1-Red-FLuc-GFP cells were delivered subcutaneously in the dorsal flank of NSG mice and tumors were allowed to establish for 3–4 weeks (Fig. 1a). Dissected tumors were immersed in mouse Ringer’s solution and four cardinal points surrounding the tumor were measured to determine the currents (Fig. 1b). Representative measurements of currents measured are shown in Fig. 1c. Signals greater than the background level indicate the outward currents, while signals lower than the background indicate inward currents. The average current measurement from seven subcutaneous tumors was 2.21 µA/cm2 (Fig. 1d). Most tumors had inward and outward currents, suggesting a circuit of current flowing in and out of the tumors and which may be related to tumor growth or polarization. Plotting all tumors together showed a significant linear correlation of current magnitude with tumor weight (r2 = 0.83, P = 0.004; Supplemental Fig. 1). These results revealed that tumor indeed generate an electric field at the tumor surface, and the current intensity appears to increase as the tumors increase in size.
Figure 1.
Non-invasive measurement of electrical currents at tumors ex vivo. (a) Cell Line Derived Tumor Allograft (CDA) Mouse Model (b) Schematic drawing of the electrical current measurement using vibrating probe. (c) Representative measurements of the outward and inward currents. (d) Measurements made at four cardinal points of the tumor surface. Three replicate measurements were made at each point, data are shown as mean ± SEM of each point.
4T1 tumors produce heterogeneous intratumor electric potential
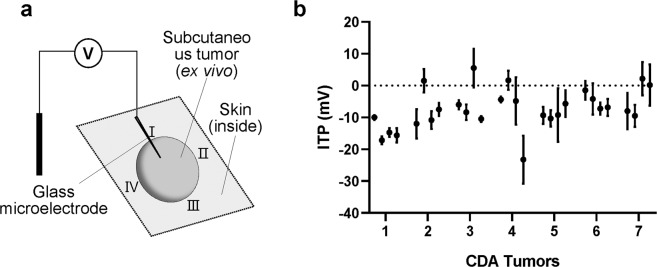
We next used glass microelectrodes to detect the ITP difference at the same positions of the same tumor. For the ITP measurement, the tumor surface needs to be impaled by the glass electrode tip with a diameter of about 1-2 µm to detect the potential difference between the outside surface and inside of the tumor (Fig. 2a). As shown in Fig. 2b, the ITP measurements showed a similar pattern with the current measurements. Majority of the measurements showed ITP varied from 1.5 mV to 23.25 mV across ~50 µm with the negative inside while 5 isolated positions from 4 tumors generated significant ITP with positive inside. This data supports the idea that the electrical property variations at different parts of the tumor may result in the endogenous EFs that flowing inside and outside of the tumors, which may affect cell migration behavior and ultimately contribute to cancer metastasis.
Figure 2.
IntraTumoral potential (ITP) measurements using glass microelectrode. (a) Schematic drawing of the ITP measurement using glass microelectrode. (b) Measurements made at four cardinal points of each tumor. Three replicate measurements were made at each position, data are shown as mean ± SEM of each point.
Cancer cells showed robust and stronger galvanotaxis collectively than cells in isolation
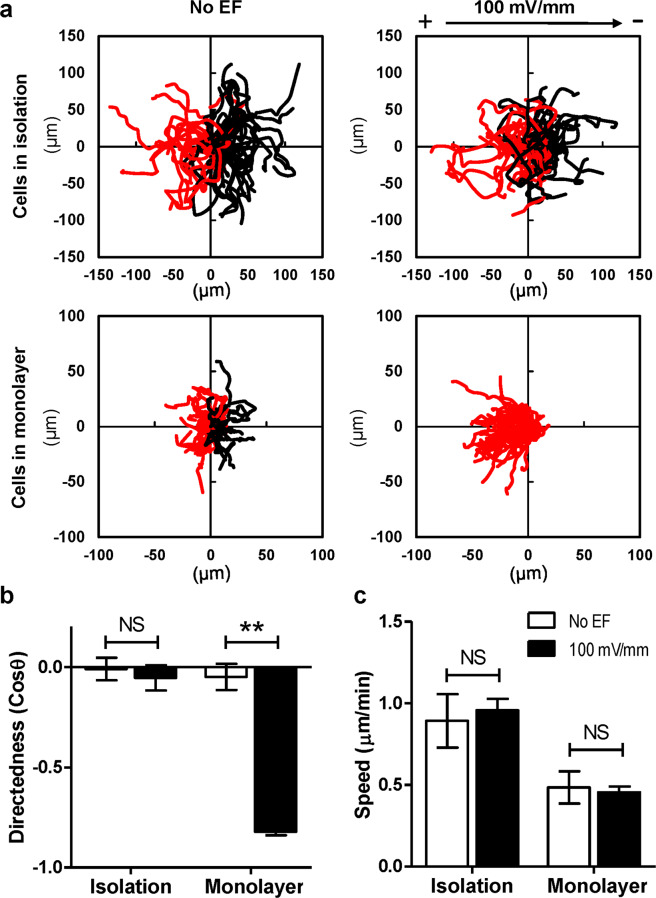
Next, we sought to clarify whether breast cancer cells respond to the electric fields that naturally exist in the tumor microenvironments. Parental 4T1 cells were seeded in galvanotaxis chambers with different density to perform migration assays in vitro. 100 mV/mm EF was applied to the cells and time-lapse images were recorded. As shown in Fig. 3a, parental 4T1 cells in isolation showed random migration in the absence or presence of EF, whereas, cells monolayers in confluent culture responded to the 100 mV/mm EF and migrated to the anode collectively (Supplemental Movie 1). Cells in monolayer showed a significant higher directedness value (−0.86 ± 0.13, P < 0.001) in the EF when compare to the no EF control (0.06 ± 0.79) (Fig. 3b). In addition, cell migration speed was not affected by EF stimulation (Fig. 3c).
Figure 3.
Robust electrotaxis of breast cancer cells in monolayer, not in isolation. (a) Cell migration trajectories of isolated cells and monolayers from one representative experiment were plotted with a common origin. Black and red lines indicate trajectories of cells migrating toward cathode and anode (or left and right in no EF controls), respectively. (b,c) Directedness and migration speed of isolated cells and monolayers in a 100 mV/mm EF. Data are shown as mean ± SEM of three independent experiments. **P < 0.01, student-t test, compared with its no EF control.
Metastatic sublines showed different galvanotaxis threshold
To evaluate the galvanotactic responses of cancer cells metastasized to different organs, we delivered 4T1-Red-FLuc-GFP cells intravenously through the tail vein then isolated 4T1 cells from metastatic tissues 4–6 weeks post-injection and established 4T1 metastatic sublines (m4T1). Cells were then seeded in galvanotaxis chambers and tested in EFs. We established 8 sublines from metastatic sites, including 4 sublines from lung, 2 from heart, 1 from axillary lymph node, and 1 from the spleen. Parental 4T1 cells were used as a control. The cells were exposed to electric fields of 50, 100, and 200 mV/mm in parallel experiments. As shown in Fig. 4a, parental 4T1 cells and metastatic sublines did not respond to electric fields of physiological strength when cultured in a low density with the directedness values close to 0. However, cells in confluent cultures responded to electric fields and migrated to the anode. Parental 4T1 and lung metastatic sublines could respond to EF as low as 50 mV/mm (p < 0.05 compared with its no EF control), while other metastatic sublines from lymph node, spleen, and heart showed weaker responses. The parental cells and all metastatic sublines showed significant anodal migration in a field equal to or greater than 100 mV/mm (Fig. 4b, p < 0.01 compared with its no EF control). When compared to the parental cells, metastatic sublines isolated from lymph node showed significant weaker galvanotaxis in monolayers (p < 0.05 when exposed to 50 mV/mm or 100 mV/mm EF; p < 0.01 in 200 mV/mm EF). In addition, the directedness of the monolayers of spleen-sublines was significantly lower when compare to the parental 4T1 cells in an EF of 100 mV/mm (Fig. 4b, p < 0.01), while that of the lung and heart sublines were significantly lower in an EF of 200 mV/mm (Fig. 4b, p < 0.05).
Figure 4.
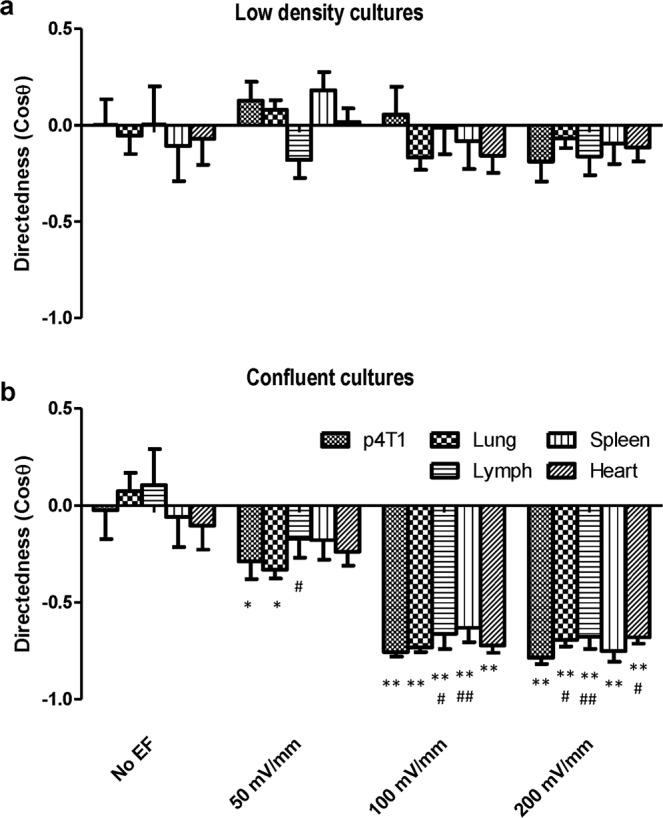
Electrotactic response of metastatic sublines to electric fields of physiological strength. (a) Parental 4T1 cells and cancer cells purified from metastatic sites in different organs, when cultured in very low density, didn’t show significant directional migration in EFs. (b) Cancer cells, in confluent culture, showed significant anodal galvanotaxis in EFs. Data are shown as mean ± SEM and compared using one-way ANOVA followed by Dunnett’s test. *p < 0.05, **p < 0.01 when compared with the no EF controls; #p < 0.05, ##p < 0.01 compared with parental 4T1 cells of the same condition.
In addition, the migration speeds varied among metastatic sublines, but the cells showed a similar pattern in different culture densities. The lung-sublines migrated significantly faster in isolation in the absence or presence of EFs (Fig. 5a), while the heart-sublines migrated significantly faster in monolayer (Fig. 5b). Metastatic cells isolated form spleen showed lower migration speed in most of the conditions (Fig. 5a, p < 0.05 in 200 mV/mm EF; Fig. 5b, p < 0.05 in no EF control and p < 0.01 in 100 mV/mm or 200 mV/mm EF). Migration persistence, namely the ratio of displacement to trajectory length, was also used to evaluate the capacity of cancer cells in maintaining the locomotion direction. As shown in sFig. 2, cancer cell monolayers have higher migration persistence in EFs (100 mV/mm and 200 mV/mm) than that of isolated cells, which suggested that cancer cells migrated more linearly in a certain direction with less turns when responding to EFs in a collective mode.
Figure 5.
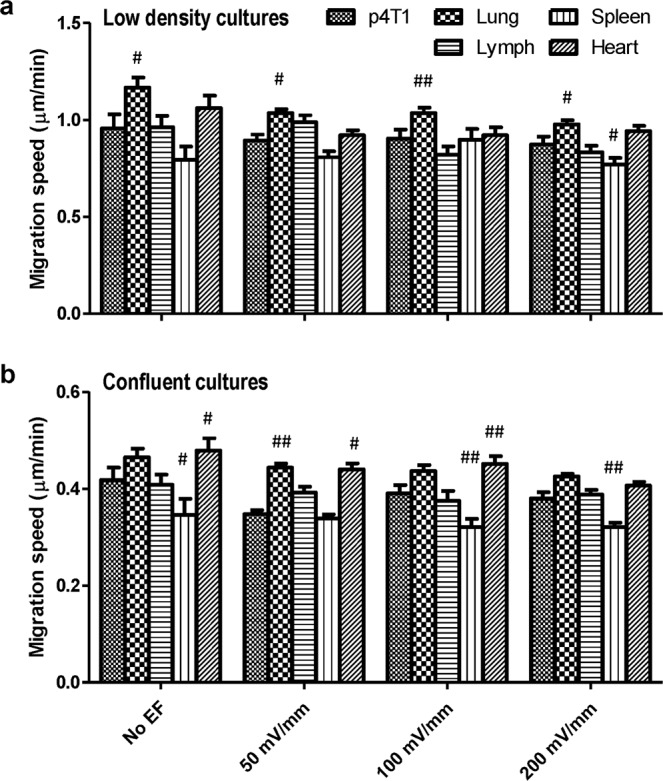
Migration speeds of 4T1 and its metastatic sublines in EFs of physiological strength. (a) Migration speed of parental 4T1 cells and cancer cells purified from metastatic sites in different organs. (b) Migration speed of cancer cells in confluent cultures. Data are shown as mean ± SEM. #p < 0.05, ##p < 0.01 compared with parental 4T1 cells of the same condition.
Discussion
In the present study, we measured the endogenous EFs at breast cancer allografts ex vivo using a non-invasive vibrating probe and glass microelectrode. We demonstrated that the EFs naturally exist at the tumor surface, the direction and magnitude of these currents are inhomogeneous which may be due to the heterogeneity of local tumor tissue. We further tested the galvanotactic responses of the breast cancer cell line and its metastatic sublines in EFs of physiological strength and found that weak applied EFs induced significant collective migration of 4T1 cells, and the metastatic sublines showed different galvanotaxis threshold with some subtle differences.
All cells are able to generate bioelectric signals through their plasma membrane, endogenous EFs thus naturally exist in our body14. Much progress has been made in the EFs enhanced wound healing since it was first reported by Emil Du Bois-Reymond in frog skin wounds about 150 years ago16, however, there is still limited scientific understanding on the tumor EF and its role in cancer progression. Burr32 first reported in 1941 that tumor growth in mice disturbed the voltage gradients across the chest. He found that fast-growing tumor produced a considerable disturbance in the electric fields, whereas slow-growing tumor produced a similar disturbance over a longer period. Later on, several studies demonstrated that the surface electrical potential measurement could be used as a new diagnostic technique for breast cancer19,20,33. These all revealed that the bioelectric characteristics of cancer tissue differs from normal tissue and may change during cancer development. In this study, we detected electric currents of 0–10 μA/cm2 around a tumor, which is comparable to the measurements in corneal or skin wounds of experimental animal models15,34. Both outward and inward currents could be detected. We also used glass microelectrode to detect the ITP difference at tumors, and an EF of 30-465 mV/mm was detected. One could predict that these EFs exist within tumors and between cancerous and normal tissues, thus one or more electrical circuits may exist. Breast cancer is a heterogeneous disease due to the genetic heterogeneity, unstable epigenetic landscape, unstructured and disorganized microenvironment which lead to a highly variable cellular phenotype35,36. In our study, the electric currents and ITP measurements varied or even reversed among different positions suggested that local tumor tissues have diverse electrical activities and the tumor heterogeneity may offer a potential explanation for it. In addition, tumor size is an important factor in breast cancer staging. Studies have reported a correlation between primary tumor size and the likelihood of metastasis37,38. We showed that tumor EFs appear to increase with the tumor growth in size (sFig. 1) which suggested that it may serve as an index for breast cancer diagnosis.
The fact that electrical currents/fields are present at both wounds and cancers tempts us to speculate that electrical abnormalities could be an important factor shared by these two pathologies. In fact, cancers have been described as ‘wounds that never heal’ for decades39,40. Such electrical similarity easily match those well recognized similarities between wounds and tumor growth: the phases of pathology; macrophage polarization and activities; myofibroblasts in tumor stroma; endothelial cell and pericyte reprogramming; epithelial reprogramming; tumor microenvironment; epigenetic reprogramming and cellular plasticity; and gene expression signature41–45. Local electrical currents/fields shared by tumor and wounds warrants further investigation to determine its causal vs. correlative roles in different stage of tumor progression.
Endogenous EFs have emerged as an overriding signal that directs cell migration during wound healing and development14,16,21. These EFs are produced by directional flow of charged ions (Na+, Cl−, K+, Ca2+ and others)46,47 through ion channels and transporters on the cell membrane, which were found to be aberrantly expressed in many types of human cancers. They regulate different aspects of cancer cell behavior and are now considered novel cancer biomarkers48. Ion channels have been implicated in breast cancer proliferation and metastasis, for example, transient receptor potential channels and voltage-gated K+ channels could promote breast cancer cell migration and play a critical role in the development and progression of breast cancer49,50. The ion transport mechanisms thus have been suggested to be novel mechanisms driving the cancer process which could also offer novel clinical possibilities51,52. Moreover, several types of cancer cells have been tested in applied EFs of physiological strength in vitro and showed diverse galvanotaxis responses23,53,54. For instance, highly metastatic lung cancer cells showed significantly higher migration directionality and speed than low metastatic lung cancer cells28. Prostate cancer cell lines with different metastatic potentials, Mat-LyLu cells (strongly metastatic) and AT-2 cells (weakly metastatic), migrated to opposite directions in EFs53. In our study, we used the 4T1 breast cancer cells as the parental cells and injected cells through the tail vein to generate metastatic tumors. All metastatic sublines showed anodal galvanotaxis when grow in monolayer. Metastatic sublines isolated from lymph node showed significant weaker galvanotactic responses in EFs of all voltages, while lung- and heart-sublines in 200 mV/mm EF and spleen-subline in 100 mV/mm EF showed lower directedness respectively when compared with the parental cells of the same condition. Our results support the hypothesis that tumor endogenous EFs could serve as a guidance cue to direct breast cancer cell migration, and the differences of galvanotaxis threshold between metastatic subpopulations imply that abnormal sensing of weak field may have impact on local invasion to help initiate metastatic dissemination. To what extent such a mechanism contributes to metastasis and the underlying molecular pathways will be important future research.
Furthermore, collective cell migration is relevant for many processes in morphogenesis, tissue repair and regeneration, and cancer metastasis. It is prevalent in many cancers in which cells are not completely de-differentiated, including breast cancer55. However, the mechanisms of collective cell movement in cancer are less well studied to date compared with embryogenesis and regeneration, since cancer metastasis is a slow and long-term process. Endogenous EF has various effects including stimulation of the migration of many cell types including fibroblasts, epithelial and endothelial cells, as well as cancer cells56. We here showed the collective galvanotaxis of cancer cells which gives us a new understanding of the role of the tumor EFs. In addition, most epithelial cancers display the hallmarks of collective invasion into surrounding tissues, e.g. E-cadherin55,57. E-cadherin is a well-known tumor suppressor protein. The loss of E-cadherin expression in tumor cells, which frequently occurs during tumor progression, is believed to be one of the important mechanisms that promote cells to dissociate from the primary tumor, invade surrounding tissues, and migrate to distant sites58. However, metastatic cancer tissues often retain E-cadherin expression59, and it greatly contribute to the metastatic spread of breast cancer as E-cadherin is involved in collective cell migration during invasion and metastasis60,61. We previously reported that E-cadherin plays an essential role in collective galvanotaxis of large epithelial sheets62, blocking E-cadherin function abolished the anodal galvanotaxis of cell monolayers. Here, we demonstrated that 4T1 breast cancer cells and metastatic sublines could only respond to the EFs collectively rather that separately. This EF-promoted collective migration may relate to cancer metastasis during tumor development, and E-cadherin may play a vital role in it.
In conclusion, our results revealed that the tumor indeed generate an electric field at the CDA tumor surface, and tumor EFs increase with the size of tumors. The direction and magnitude of the electric currents at the tumor surface are non-homogeneous. Monolayer cancer cells responded to weak applied EFs of physiological strength, while cells in isolation did not. Metastatic sublines of 4T1 cells isolated from different organs also showed significant galvanotactic movement in EFs with subtle differences, which may have impact on local invasion to help initiate metastatic dissemination and colonization.
Materials and Methods
Animals
All the animal procedures were performed in accordance with the National Institutes of Health (NIH) Guide for the Care of Use of Laboratory Animals and approved by Institutional Animal Care and Use Committees (IACUC) of the University of California, Davis and Lawrence Livermore National Laboratory (protocol nos. 20722 and 261). Eight- to ten-week-old immunodeficient mice (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG), Jackson Laboratories, Bar Harbor, ME, USA) were used in this study.
Cell line derived tumor allograft (CDA) mouse model
The transgenically modified murine mammary carcinoma cell line (Bioware Brite Cell Line 4T1-Red-FLuc-GFP) was obtained from Perkin Elmer and used in this study. 4T1 cells were cultured in RPMI 1640 (Gibco, catalog no. 11875093, Thermal Fisher Scientific Inc.) containing 10% fetal bovine serum (FBS) (Gibco, catalog no. 10437028) and antibiotics (Gibco, catalog no. 15140122). Cells then were collected and delivered to the NSG mice subcutaneously and monitored for tumor progression via palpation of the primary tumor for 3–4 weeks. Mice were housed in a temperature-controlled environment (22 ± 0.5 °C) with a 12 h-light-dark cycle and allowed free access to food and water. All efforts were made to minimize animal suffering and reduce the number of animals used. Replicates were generated from two independent mouse cohorts.
Vibrating probe measurement
Tumor current measurement using vibrating probe was performed as previously described with minor changes63. The probes, platinum-electroplated at the tip, vibrated at a frequency between 100–200 Hz. Prior to measurements, probe was calibrated in mouse Ringer under experimental conditions with a current density of 1.5 μA/cm2. When palpable tumors are formed, mice were sacrificed and tumors were dissected and used for examining electrical properties. Under a dissecting microscope, subcutaneous tumors were secured in the non-conductive measuring chamber with the skin-side down. Reference values (baseline) were recorded with probe away from tumor (~1 cm). Plane of probe vibration was perpendicular to the tumor surface at a distance of ~5–10 µm. Current was recorded until plateau peak reached (<1 minute) in the regions of interest – the four cardinal positions of each tumor. Measurements occurred at room temperature. Data were acquired and analyzed using WinWCP V4 (Strathclyde Electrophysiology Software) and analysed using Excel.
Glass microelectrode measurement
ITP was measured invasively by glass microelectrode impalement through the tumor surface as previously described64,65. Briefly, borosilicate glass capillaries without filament were purchased from World Precision Instruments (WPI, Inc., Sarasota, FL, USA; catalog no. TW150-4), and two-step heat-pulled using a Narishige PC-10 electrode puller. Microelectrode (1–2 µm tip diameter; NaCl 3 M electrolyte) that has resistances of ~1–2 MΩ was then placed onto a holder (Warner Instruments, Holliston, MA, USA) containing an Ag/AgCl wire immersed in the NaCl solution. Using the micro-positioners, the tip of the microelectrode mounted on the holder was immersed into the mouse Ringer’s solution. Tumor nodules were secured in the non-conductive measuring chamber and electrode potential offset to 0 mV prior to impalement. The tumor tissue was impaled with microelectrode for 50 µm and ITP was recorded for 1 minute in the same positions as for the current measurement. Since the tumor surface needs to be penetrated by the glass electrode tip which may change the surface potential of the original site, three repetitive measurements were done at nearby locations. Data were acquired (sampling of 100 Hz) and extracted using pClamp 10 (Molecular Devices) and treated using Excel.
Isolation of metastatic sublines
1 × 106 4T1-Red-FLuc-GFP cells were injected into the tail vein. Due to high immunogenicity of GFP protein in immunocompetent BALB/c animals, cancer cells are cleared by the immune system and tumors do not efficiently form in this strain, NSG animals were utilized for this study to permit the use of a system that allows the exclusive isolation of metastatic cancer cells. Cancer cells were allowed to grow in those NSG-mice for 4–6 weeks to allow for metastatic tumors. Upon 6 weeks or moribund behavior of the mice, animals were euthanized via CO2 inhalation then necropsied for observable metastasis. Organs containing metastatic tumors were dissected then homogenized to single cell by first passing the tumor through a syringe without a needle followed by a 1 h digest with shaking at 37 °C in 100 µg/mL DNaseI (Roche, catalog no. 11284932001), 300 U/mL collagenase/ 100 U/mL hyaluronidase (STEMCELL Technologies, catalog no. 07912), 0.6 U/mL Dispase II (Roche, catalog no. 4942078001) in DMEM/F12 (Gibco, catalog no. 11320033) with 10% FBS. Digests were filtered through a 100 µm cell strainer prior to debris removal (Miltenyi Biotec, catalog no. 130-109-398) and resuspended in BD FACS Pre-Sort Buffer (BD, catalog no. 563503) prior to cell sorting on a BD FACS Melody based on GFP gating generated from cell line fluorescence and WT 4T1 cells. Isolated GFP + subpopulations were cultured in complete RPMI media and resorted for GFP expression to ensure a pure population of metastatic cells and assayed for fluorescent intensity via flow cytometry prior to galvanotaxis experiments (sFig. 3).
Galvanotaxis assay
Galvanotaxis experiments were performed as previously described66. Briefly, silicone stencils with 9 wells were placed in the galvanotaxis chambers for multi-spot seeding, which allowed us to simultaneously examine the galvanotactic responses of multiple sublines in the same chamber. Cancer cells isolated from different organs were then seeded singly to the wells with ideal density. 200 µl cell suspension were added in each well at concentrations of 2.2 × 104/mL and 4.5 × 105/mL for low density culture (~150 cells per mm2) and confluent culture (~3 × 103 cells per mm2), respectively. Cells were cultured overnight at 37 °C with 5% CO2 to allow sufficient attachment. Before EF stimulation, the stencils were lifted. Unattached cells were removed and fresh RPMI 1640 medium with 10% FBS and antibiotics were added. Current was applied to the chamber through agar-salt bridges connecting with silver/silver chloride electrodes in Steinberg’s solution as described previously67. Agar gel was pre-prepared in a sterilized condition by dissolving 3% (wt/vol) agar powder (Sigma, catalog no. A1296) into Steinberg’s solution67. 5 mL medium were added into reservoirs to ensure salt bridge contact and support cell viability during EF stimulation. A pair of reference electrodes connecting to a digital multimeter was placed in the two reservoirs to monitor EF strengths at the beginning of the experiment and every 30 minutes afterward to ensure consistent EF application. Cell migration was observed with a Carl Zeiss Observer Z1 inverted microscope with MetaMorph NX program (Molecular Devices, Sunnyvale, CA, USA). The microscope system was able to record serial time-lapse images of multiple locations on the multi-channel galvanotaxis chamber simultaneously. A 10× phase contrast objective lens was used for microscopy. Images were taken at 5-minute intervals for 3 hours.
Quantification of cell migration
Cell migration was analyzed to determine directedness (cos θ) and trajectory speed by using ImageJ software from the National Institutes of Health (http://rsbweb.nih.gov/ij/) with MTrackJ and Chemotaxis tool plugins as previously described14,68. The position of a cell was defined by its centroids. Cells that divided, moved in and out of the field, or merged with other cells during the experiment were excluded from analysis. Directedness was used as an indicator of galvanotaxis which is defined as cosine of the angle between the EF vector and a straight line connecting the start and end positions of a cell. A cell migrating directly toward the cathode would have a directedness of 1 while a cell migrating directly to the anode would have a directedness of −1. Migration speed is the trajectory distance divided by time, and migration persistence is the ratio of displacement distance (the straight-line distance between the start and end positions) to trajectory length traveled by a cell. The persistence would be equal to 1 when cells move persistently along a straight line in a given direction. For each condition, at least 50 cells were analyzed. All experiments were repeated, and data in the bar charts were averaged from three replicates.
Statistical analysis
All data are represented as means ± standard error of the mean (SEM). Statistical analyses were performed using GraphPad Prism 7.0 with one-way ANOVA followed by Dunnett’s test or unpaired two-tailed Student’s t-test. For correlation analysis between tumor weight and electric current density, the Pearson’s correlation coefficient was computed. P value was set at 0.05 for rejecting null hypotheses.
Supplementary information
Acknowledgements
This work is supported by UC Davis Comprehensive Cancer Center pilot grant MZCCC18 (to GGL and MZ). This work is also partially supported by AFOSR-MURI grant FA9550-16-1-0052. Work in Zhao Laboratory is supported by NEI R01EY019101, Core Grant (P-30 EY012576, PI. Jack Werner), the Research to Prevent Blindness, Inc. NRH and GGL performed work under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344 and were supported in part by LDRD-19-SI-003 grant. The authors would like to thank Nan Lin and Kaiwen Liu for assistance in data analysis. The authors are grateful to Drs. Chong-Xian Pan, Fernando Ferreira, and Guillaume Luxardi for some of initial experiments.
Author contributions
K.Z., G.G.L. and M.Z. designed the experiments. N.R.H. generated the tumor mouse model and isolated metastatic sublines. K.Z., B.R. and Q.S. measured the electric features, and K.Z. performed the galvanotaxis experiment and quantified all data. K.Z. drafted the manuscript and all authors contributed to the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gabriela G. Loots, Email: loots1@llnl.gov
Min Zhao, Email: minzhao@ucdavis.edu.
Supplementary information
is available for this paper at 10.1038/s41598-020-65566-0.
References
- 1.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Jin X, Mu P. Targeting Breast Cancer Metastasis. Breast Cancer. 2015;9:23–34. doi: 10.4137/BCBCR.S25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autier P, Boniol M, Koechlin A, Pizot C, Boniol M. Effectiveness of and overdiagnosis from mammography screening in the Netherlands: population based study. BMJ. 2017;359:j5224. doi: 10.1136/bmj.j5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narod S. I. J., AB M. Why have breast cancer mortality rates declined? J. Cancer Policy. 2015;5:10. doi: 10.1016/j.jcpo.2015.03.002. [DOI] [Google Scholar]
- 5.SEER. Stat Database: (1975–2012). Bethesda, MD: National Cancer Institute Surveillance Research Program (2015).
- 6.Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness. N. Engl. J. Med. 2016;375:1438–1447. doi: 10.1056/NEJMoa1600249. [DOI] [PubMed] [Google Scholar]
- 7.Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging Biological Principles of Metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirata Eishu, Sahai Erik. Tumor Microenvironment and Differential Responses to Therapy. Cold Spring Harbor Perspectives in Medicine. 2017;7(7):a026781. doi: 10.1101/cshperspect.a026781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naba A, et al. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell Proteom. 2012;11(M111):014647. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao M, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 15.Reid B, Song B, McCaig CD, Zhao M. Wound healing in rat cornea: the role of electric currents. FASEB journal: Off. Publ. Federation Am. Societies Exp. Biol. 2005;19:379–386. doi: 10.1096/fj.04-2325com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol. Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- 17.McCaig CD, Song B, Rajnicek AM. Electrical dimensions in cell science. J. Cell Sci. 2009;122:4267–4276. doi: 10.1242/jcs.023564. [DOI] [PubMed] [Google Scholar]
- 18.Li L, et al. Caveolin-1-mediated STAT3 activation determines electrotaxis of human lung cancer cells. Oncotarget. 2017;8:95741–95754. doi: 10.18632/oncotarget.21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng EY, Sree SV, Ng KH, Kaw G. The use of tissue electrical characteristics for breast cancer detection: a perspective review. Technol. Cancer Res. Treat. 2008;7:295–308. doi: 10.1177/153303460800700404. [DOI] [PubMed] [Google Scholar]
- 20.Cuzick J, et al. Electropotential measurements as a new diagnostic modality for breast cancer. Lancet. 1998;352:359–363. doi: 10.1016/S0140-6736(97)10002-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhao M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin. Cell developmental Biol. 2009;20:674–682. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Mycielska ME, Djamgoz MB. Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J. Cell Sci. 2004;117:1631–1639. doi: 10.1242/jcs.01125. [DOI] [PubMed] [Google Scholar]
- 23.Huang YJ, et al. Cellular microenvironment modulates the galvanotaxis of brain tumor initiating cells. Sci. Rep. 2016;6:21583. doi: 10.1038/srep21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borys P. On the biophysics of cathodal galvanotaxis in rat prostate cancer cells: Poisson-Nernst-Planck equation approach. Eur. Biophys. J. 2012;41:527–534. doi: 10.1007/s00249-012-0807-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan X, et al. Lung cancer A549 cells migrate directionally in DC electric fields with polarized and activated EGFRs. Bioelectromagnetics. 2009;30:29–35. doi: 10.1002/bem.20436. [DOI] [PubMed] [Google Scholar]
- 26.Pu J, et al. EGF receptor signalling is essential for electric-field-directed migration of breast cancer cells. J. Cell Sci. 2007;120:3395–3403. doi: 10.1242/jcs.002774. [DOI] [PubMed] [Google Scholar]
- 27.Gough N. R. Moving Through an Electrical Field. Science's STKE. 2007;2007(405):tw348–tw348. [Google Scholar]
- 28.Tsai HF, et al. Evaluation of EGFR and RTK signaling in the electrotaxis of lung adenocarcinoma cells under direct-current electric field stimulation. PLoS One. 2013;8:e73418. doi: 10.1371/journal.pone.0073418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morokuma J, et al. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proc. Natl Acad. Sci. U S A. 2008;105:16608–16613. doi: 10.1073/pnas.0808328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levin M. Large-scale biophysics: ion flows and regeneration. Trends Cell Biol. 2007;17:261–270. doi: 10.1016/j.tcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Levin M. The wisdom of the body: future techniques and approaches to morphogenetic fields in regenerative medicine, developmental biology and cancer. Regenerative Med. 2011;6:667–673. doi: 10.2217/rme.11.69. [DOI] [PubMed] [Google Scholar]
- 32.Burr HS. Changes in the Field Properties of Mice with Transplanted Tumors. Yale J. Biol. Med. 1941;13:783–788. [PMC free article] [PubMed] [Google Scholar]
- 33.Faupel M, et al. Electropotential evaluation as a new technique for diagnosing breast lesions. Eur. J. radiology. 1997;24:33–38. doi: 10.1016/s0720-048x(96)01113-8. [DOI] [PubMed] [Google Scholar]
- 34.Reid, B. & Zhao, M. Measurement of bioelectric current with a vibrating probe. Journal of visualized experiments: JoVE, 10.3791/2358 (2011). [DOI] [PMC free article] [PubMed]
- 35.Metzger-Filho O, et al. Dissecting the heterogeneity of triple-negative breast cancer. J. Clin. Oncol. 2012;30:1879–1887. doi: 10.1200/JCO.2011.38.2010. [DOI] [PubMed] [Google Scholar]
- 36.Beca F, Polyak K. Intratumor Heterogeneity in Breast Cancer. Adv. Exp. Med. Biol. 2016;882:169–189. doi: 10.1007/978-3-319-22909-6_7. [DOI] [PubMed] [Google Scholar]
- 37.Sivaramakrishna R, Gordon R. Detection of breast cancer at a smaller size can reduce the likelihood of metastatic spread: a quantitative analysis. Acad. Radiol. 1997;4:8–12. doi: 10.1016/s1076-6332(97)80154-7. [DOI] [PubMed] [Google Scholar]
- 38.Laura S, Coombs NJ, Ung O, Boyages J. Tumour size as a predictor of axillary node metastases in patients with breast cancer. ANZ. J. Surg. 2006;76:1002–1006. doi: 10.1111/j.1445-2197.2006.03918.x. [DOI] [PubMed] [Google Scholar]
- 39.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 40.Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol. Res. 2015;3:1–11. doi: 10.1158/2326-6066.CIR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byun JS, Gardner K. Wounds That Will Not Heal Pervasive Cellular Reprogramming in Cancer. Am. J. Pathol. 2013;182:1055–1064. doi: 10.1016/j.ajpath.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sundaram GM, Quah S, Sampath P. Cancer: the dark side of wound healing. FEBS J. 2018;285:4516–4534. doi: 10.1111/febs.14586. [DOI] [PubMed] [Google Scholar]
- 43.Antsiferova M, Werner S. The bright and the dark sides of activin in wound healing and cancer. J. Cell Sci. 2012;125:3929–3937. doi: 10.1242/jcs.094789. [DOI] [PubMed] [Google Scholar]
- 44.Chang HY, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc. Natl Acad. Sci. U S A. 2005;102:3738–3743. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang HY, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reid B, Zhao M. The Electrical Response to Injury: Molecular Mechanisms and Wound Healing. Adv. Wound Care. 2014;3:184–201. doi: 10.1089/wound.2013.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vieira AC, et al. Ionic components of electric current at rat corneal wounds. PLoS One. 2011;6:e17411. doi: 10.1371/journal.pone.0017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lastraioli E, Iorio J, Arcangeli A. Ion channel expression as promising cancer biomarker. Biochim. Biophys. Acta. 2015;1848:2685–2702. doi: 10.1016/j.bbamem.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 49.Ouadid-Ahidouch H, Dhennin-Duthille I, Gautier M, Sevestre H, Ahidouch A. TRP channels: diagnostic markers and therapeutic targets for breast cancer? Trends Mol. Med. 2013;19:117–124. doi: 10.1016/j.molmed.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Chow LW, Cheng KS, Wong KL, Leung YM, Voltage-gated K. channels promote BT-474 breast cancer cell migration. Chin. J. cancer Res. = Chung-kuo yen cheng yen chiu. 2018;30:613–622. doi: 10.21147/j.issn.1000-9604.2018.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Djamgoz MB, Coombes RC, Schwab A. Ion transport and cancer: from initiation to metastasis. Philos. Trans. R. Soc. London. Ser. B, Biol. Sci. 2014;369:20130092. doi: 10.1098/rstb.2013.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Payne SL, Levin M, Oudin MJ. Bioelectric Control of Metastasis in Solid Tumors. Bioelectricity. 2019;1:114–130. doi: 10.1089/bioe.2019.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Djamgoz MBA, Mycielska M, Madeja Z, Fraser SP, Korohoda W. Directional movement of rat prostate cancer cells in direct-current electric field: involvement of voltagegated Na+ channel activity. J. Cell Sci. 2001;114:2697–2705. doi: 10.1242/jcs.114.14.2697. [DOI] [PubMed] [Google Scholar]
- 54.Levin M. Morphogenetic fields in embryogenesis, regeneration, and cancer: non-local control of complex patterning. Bio Syst. 2012;109:243–261. doi: 10.1016/j.biosystems.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 56.Cortese B, Palama IE, D’Amone S, Gigli G. Influence of electrotaxis on cell behaviour. Integr. biology: Quant. Biosci. nano macro. 2014;6:817–830. doi: 10.1039/c4ib00142g. [DOI] [PubMed] [Google Scholar]
- 57.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. reviews. Mol. Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 58.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Developmental Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Aceto N, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheung KJ, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl Acad. Sci. U S A. 2016;113:E854–863. doi: 10.1073/pnas.1508541113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li L, et al. E-cadherin plays an essential role in collective directional migration of large epithelial sheets. Cell. Mol. life sciences: CMLS. 2012;69:2779–2789. doi: 10.1007/s00018-012-0951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reid B, Nuccitelli R, Zhao M. Non-invasive measurement of bioelectric currents with a vibrating probe. Nat. Protoc. 2007;2:661–669. doi: 10.1038/nprot.2007.91. [DOI] [PubMed] [Google Scholar]
- 64.McCaig CD, Robinson KR. The ontogeny of the transepidermal potential difference in frog embryos. Developmental Biol. 1982;90:335–339. doi: 10.1016/0012-1606(82)90382-7. [DOI] [PubMed] [Google Scholar]
- 65.Luxardi G, Reid B, Maillard P, Zhao M. Single cell wound generates electric current circuit and cell membrane potential variations that requires calcium influx. Integr. biology: Quant. Biosci. nano macro. 2014;6:662–672. doi: 10.1039/c4ib00041b. [DOI] [PubMed] [Google Scholar]
- 66.Nakajima K, et al. KCNJ15/Kir4.2 couples with polyamines to sense weak extracellular electric fields in galvanotaxis. Nat. Commun. 2015;6:8532. doi: 10.1038/ncomms9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song B, et al. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nat. Protoc. 2007;2:1479–1489. doi: 10.1038/nprot.2007.205. [DOI] [PubMed] [Google Scholar]
- 68.Zhao M, Jin T, McCaig CD, Forrester JV, Devreotes PN. Genetic analysis of the role of G protein-coupled receptor signaling in electrotaxis. J. Cell Biol. 2002;157:921–927. doi: 10.1083/jcb.200112070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.