Abstract
Over‐the‐counter supplements, such as Alipotec which often purported to amplify weight loss and readily available in naturopathic shops, can have clinically significant patient outcomes including severe cytopenia, and even inducing immune thrombocytopenic purpura.
Keywords: Alipotec, immune thrombocytopenic purpura, root of tejocote, thrombocytopenia
Over‐the‐counter supplements, such as Alipotec which often purported to amplify weight loss and readily available in naturopathic shops, can have clinically significant patient outcomes including severe cytopenia, and even inducing immune thrombocytopenic purpura.

1. INTRODUCTION
Root of Tejocote is often purported to amplify weight loss and marketed under the brand name Alipotec®. This agent is readily available in naturopathic shops and online for purchase without any US Food and Drug Administration (FDA) indication for medicinal merit. We present the first known case in the literature of a patient who consumed Alipotec® and developed immune thrombocytopenia purpura (ITP).
2. CASE PRESENTATION
A 51‐year‐old female with the history of hypothyroidism presented to the primary care office with complaint of generalized malaise for roughly 1 month duration. Hemogram revealed new thrombocytopenia with a platelet count of 62 × 103/μL. The remainder of her complete blood count (CBC) showed a normal hemoglobin of 12.7 g/dL and white blood cell count (WBC) of 4.7/μL. Her most recent CBC from 6 months prior showed a hemoglobin of 12.8 g/dL, WBC of 6.3/μL, and platelet count of 406 × 103/μL. Repeat CBC, ordered by the primary care physician 72 hours later, showed worsening thrombocytopenia with platelet count of 43 × 103/μL, and the patient was instructed to seek care in the local emergency department (ED). She presented to the ED the following day, and her platelet count had decreased further to 25 × 103/μL. After discussion with the on‐call hematologist, the patient was administered 1 mg/kg of prednisone (60 mg) and discharged on this dose with an outpatient hematology appointment.
The patient was seen in the hematology clinic 5 days after discharge. During her initial visit, she had no active bruising or bleeding and denied any prior bleeding tendency. She denied any antecedent coryza, fever, or pharyngitis. Her medications included levothyroxine, an over‐the‐counter multi‐vitamin, and a weight loss supplement, Alipotec® (Tejocote). She drank alcohol socially. She denied any nicotine use or illicit drug use. She did not have a family history of blood diathesis. She works as a professional horse jockey. On physical examination, she had a small ecchymosis on her left elbow. No hepatosplenomegaly or lymphadenopathy was identified. The patient did not exhibit any overt gingival bleeding or petechial rash. Testing for Hepatitis B and C, HIV, Helicobacter Pylori serology, and heterophile antibodies was negative. TSH and T4 were checked and were 0.2 and 7.6, respectively. ESR, CRP, and ANA were negative.
A blood smear from her initial CBC in the ED was analyzed in the office and did not reveal pseudothrombocytopenia; there was no evidence of platelet clumping or platelet satellitism. Rare large platelets were appreciated on the index smear. There was no evidence of dysplasia in the red blood cell (RBC) or WBC cell lineages. Her platelet count was rechecked in the office and improved to 365 × 103/μL on the 60 mg of prednisone. The patient was presumed to have ITP. Upon further questioning in the office, the patient admitted to starting a new weight loss supplement, Alipotec 6 weeks prior to ED evaluation in an attempt to lose weight for her aforementioned employment as a professional horse jockey.
The patient was started on a prednisone taper which was gradually reduced by 10 mg weekly. Four weeks later, at a dose of 20 mg of prednisone, her platelet count dropped from 266‐14 × 103/μL. Prednisone was increased back to 60 mg. Despite this increase in steroids, her platelet count increased to only 62 × 103/μL after 1 week.
Discussion was had about pursuing a bone marrow biopsy to exclude less common entities such amegakaryocytic thrombocytopenia, aplastic anemia, myelodysplastic syndrome, and overt leukemia, but the patient declined and continued on prednisone. Unfortunately, the patient had poor tolerance to steroids with frequent headaches, labile mood, and insomnia. Her platelet count continued to fluctuate between 50 and 87 × 103/μL on glucocorticoids. Given the intolerance to prednisone and the relatively modest increase in her platelet count, a bone marrow biopsy was again suggested but patient declined.
Instead, the patient was next started on the Thrombopoietin Receptor Agonists (TPO‐RAs), Eltrombopag, at 50 mg daily. Unfortunately, after a singular dose, she developed nausea and vomiting and refused further treatment with the agent. At this time, both the patient and the treating hematologist began suspecting that her Alipotec® may be the cause for her thrombocytopenia. She was advised to discontinue it, but she was reluctant to do so given the weight loss she was experiencing on the supplement.
At her next hematology appointment, which was 9 weeks from her index hematology visit, the patient was given the option to proceed with curative intent splenectomy or Rituxan treatment. The patient was informed that these remedies would only be attempted if she would agree to a diagnostic bone marrow biopsy. The patient again refused invasive testing.
A hemogram the following week, surprisingly, revealed a platelet count of 342x103/μL, despite the patient admitting to self‐reducing her prednisone from 40 to 10 mg. Upon further inquiry, she also admitted to stopping her Alipotec in hopes that the over‐the‐counter supplement was the cause of the ITP. Her platelet count the following 3 weeks continued to remain over 200 × 103/μL, despite the patient self‐discontinuing all prednisone products.
Four weeks after stopping the Alipotec® and prednisone, her platelet count decreased to 46 × 103/μL. She confided that she resumed the Alipotec in preparation to a critical horse race, 4 days prior to the blood draw. She refused resuming any steroid products, and she again discontinued the offending agent once she was informed of her recurrent thrombocytopenia. She had a repeat hemogram the following week after discontinuing the Alipotec®, which again showed correction of her thrombocytopenia with platelet count of 336 × 103/μL. Repeated CBC 1 month later off any treatment continued to show normal platelet counts, and the patient stated she had completely stopped the Alipotec.
3. DISCUSSION
Thrombocytopenia is defined as a platelet count below the lower limit of normal (<150 × 103/μL). While there are many etiologies of thrombocytopenia, including peripheral destruction, decreased bone marrow production, and increased splenic sequestration, a frequent entity for severe isolated thrombocytopenia is ITP. ITP, formerly known as idiopathic thrombocytopenia purpura, is an acquired autoimmune condition where IgG type antibodies are directed against platelet membrane glycoproteins IIb‐IIIa leading to peripheral destruction.1 While antibody and complement mediated platelet destruction by macrophage clearance in the reticuloendothelial system is felt to best explain the disease process, the pathophysiology remains incompletely understood.2 In fact, up to 50% of patients with ITP will not have detectable autoantibodies and such antibody testing is not felt to be part of standard diagnostic workup.3 The lack of identifiable antibody formation might be due to a second mechanism of platelet clearance, namely, that platelet destruction is also mediated by cytotoxic T cells and cellular immunity rather than humoral immunity.2 Thrombocytopenia from ITP often manifests as mucocutaneous bleeding, petechial rash, spontaneous bruising, and rarely, intracranial hemorrhage.4
ITP in adults and children manifests differently, most notably for the high rates of spontaneous resolution in the pediatric population compared to the adult population.5 Adults with ITP have spontaneous resolution rates of only 9%, and therefore, often require prolonged immunosuppression.6 The cornerstone of ITP treatment remains glucocorticoids; administered either with daily prednisone at 1‐2 mg/kg for 1‐2 weeks followed by gradual taper or as high pulse dose of dexamethasone at 40 mg for 4‐5 days.7 Compared to intravenous immunoglobulin (IVIG), prednisone has demonstrated similar efficacy and is associated with lower cost and feasibility of administration.8 Despite the efficacy of glucocorticoids, adult patients uncommonly can be weaned off completely with high rates of disease relapse.9 As such, further management with TPO‐RAs, monoclonal antibody immune suppression treatment with rituximab, or splenectomy is often employed for long‐term control.10, 11
Many causes for ITP have been established, including non‐Hodgkin's lymphoma such as chronic lymphocytic leukemia (CLL), viral infections such as HIV, Hepatitis C, or infectious mononucleosis, medications such as Quinine, Trimethoprim‐Sulfamethoxazole, and Vancomycin, and autoimmune conditions including hypothyroidism, rheumatoid arthritis, and systemic lupus erythematosus.3, 12, 13, 14, 15, 16, 17 While the data on prescription drugs are robust with respect to thrombocytopenia, over‐the‐counter supplements, which are often not scrutinized by the FDA, may have unintended consequences that are not readily reported by the manufacturers.
Tejocote (Crataegus Species) is a genus of fruit‐bearing Hawthorn trees (Figure 1) distributed mostly in Mexico and in Latin America.18 Thirteen species are identified and classified according to different shoot types, fruits, and flowers.19 Tejocote fruit (Figure 2) which are rich in Vitamin C, antioxidants, and pectin fibers are often collected for direct consumption and processed into jams, jelly, or sirup.18 Medicinally, Crataegus ingredients were historically reported to be used in treating diarrhea, cough suppression, and even managing kidney pain and cardiac ailments.19, 20
Figure 1.

Tejocote Tree. Source Ref. 26
Figure 2.

Tejocote fruit. Source Ref. 26
Alipotec (Figure 3) is a brand name product derived from Root of Tejocote. It is a non‐FDA approved over‐the‐counter supplement that is promoted as a weight loss remedy.One presumed mechanism for the weight loss is the high pectin content of the Tejocote root, which has been associated with early satiety.21 Side‐effects reported by the supplier include GI upset and myalgias, the former presumably from the high pectin content.22 Other side‐effects noted in the literature include possible cardiotoxicity and drug‐drug interactions, including false negative elevation in digoxin assay.23
Figure 3.

Alipotec supplement. Source Ref. 26
ITP has established criteria to gauge treatment response.24 Following holding of the Alipotec, our patient had a complete response of her ITP, as defined by a platelet count of greater than 100 × 103/μL measured on two occasions 7 days apart. At the time of writing this case, the patient has remained with a platelet count of greater than 300 000 over the past 12 months with her last platelet count of 415 × 103/μL while remaining off the Alipotec (Graph 1).
Graph 1.
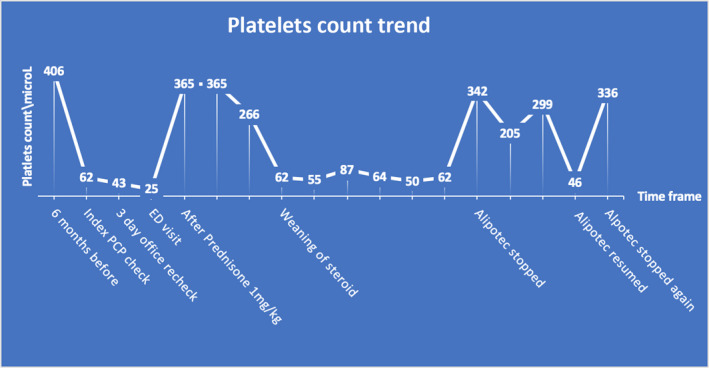
Platelet count trends during the course of the Immune thrombocytopenia (ITP) from diagnosis to resolution
In our patient, drugs‐dependent antibodies were not checked as negative does not rule out the diagnosis with low sensitivity.25 Also, our patient tested negative to HIV, HCV and had negative heterophile antibodies. Also, she denied temporal exposure to medications known to cause ITP including Quinine. She is a known hypothyroidism on Levothyroxine and had mildly low T4 and, however, her platelets count remained to fluctuate rapidly. Thyroid function remained mildly low upon recheck after her platelets consistently normalized. Inadvertently due to repeated use of Alipotec by patient, she demonstrated positive drug rechallenge which exhibit high quality evidence that her Alipotec use was responsible for her thrombocytopenia.
Thrombocytopenia is an unknown, but a possible serious, adverse effect of Alipotec that has never been reported in the literature. Our patient showed immediate, dramatic recovery of her platelet count following discontinuation of Alipotec®. The mechanism of how Alipotec causes presumed immune‐mediated thrombocytopenia is currently unknown.
4. CONCLUSION
Detailed history taking including medication and over‐the‐counter supplement use in patients with newly unexplained thrombocytopenia is essential. When there is high clinical suspicion that a nonprescription agent might be responsible for adverse events, such as ITP, these agents should be empirically held, and the patient's clinical status and laboratory evaluation should be reassessed.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
HA drafted and took the lead in writing the manuscript as well as created the graph and figures. EM revising manuscript critically for important intellectual content. HA and EM both contributed to and approved the final version of the manuscript.
Alhasson H, Muchnik E. Immune thrombocytopenic purpura caused by the over‐the‐counter weight supplement Root of Tejocote (Crataegus species). Clin Case Rep. 2020;8:872–876. 10.1002/ccr3.2804
REFERENCES
- 1. Godeau B. Immune thrombocytopenic purpura: major progress in knowledge of the pathophysiology and the therapeutic strategy, but still a lot of issues. Presse Med. 2014;43(4 Pt 2):e47‐e48. [DOI] [PubMed] [Google Scholar]
- 2. Cooper N, Ghanima W. Immune thrombocytopenia. N Engl J Med. 2019;381(10):945‐955. [DOI] [PubMed] [Google Scholar]
- 3. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190‐4192. [DOI] [PubMed] [Google Scholar]
- 4. Lee MS, Kim WC. Intracranial hemorrhage associated with idiopathic thrombocytopenic purpura: report of seven patients and a meta‐analysis. Neurology. 1998;50(4):1160‐1163. [DOI] [PubMed] [Google Scholar]
- 5. Despotovic JM, Grimes AB. Pediatric ITP: is it different from adult ITP?. Hematology Am Soc Hematol Educ Program. 2018;2018(1):405‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stasi R, Stipa E, Masi M, et al. Long‐term observation of 208 adults with chronic idiopathic thrombocytopenic purpura. Am J Med. 1995;98:436‐442. [DOI] [PubMed] [Google Scholar]
- 7. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168‐186. [DOI] [PubMed] [Google Scholar]
- 8. Ou CY, Hsieh KS, Chiou YH, Chang YH, Ger LP. A comparative study of initial use of intravenous immunoglobulin and prednisolone treatments in childhood idiopathic thrombocytopenic purpur. Acta Paediatr Taiwan. 2006;47(5):226‐231. [PubMed] [Google Scholar]
- 9. George JN, Kojouri K, Perdue JJ, Vesely SK. Management of patients with chronic, refractory idiopathic thrombocytopenic purpura. Semin Hematol. 2000;37:290‐298. [DOI] [PubMed] [Google Scholar]
- 10. Giagounidis AA, Anhuf J, Schneider P, et al. Treatment of relapsed idiopathic thrombocytopenic purpura with the anti‐CD20 monoclonal antibody rituximab: a pilot study. Eur J Haematol. 2002;69(2):95‐100. [DOI] [PubMed] [Google Scholar]
- 11. Siegal D, Crowther M, Cuker A. Thrombopoietin receptor agonists in primary ITP. Semin Hematol. 2013;50(01):S18‐S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hauswirth AW, Skrabs C, Schützinger C, et al. Autoimmune thrombocytopenia in non‐Hodgkin's lymphomas. Haematologica. 2008;93(3):447‐450. [DOI] [PubMed] [Google Scholar]
- 13. Fallah M, Liu X, Ji J, Försti A, Sundquist K, Hemminki K. Autoimmune diseases associated with non‐Hodgkin lymphoma: a nationwide cohort study. Ann Oncol. 2014;25(10):2025‐2030. [DOI] [PubMed] [Google Scholar]
- 14. Sloand EM, Klein HG, Banks SM, Vareldzis B, Merritt S, Pierce P. Epidemiology of thrombocytopenia in HIV infection. Eur J Haematol. 1992;48:168‐172. [DOI] [PubMed] [Google Scholar]
- 15. Chiao EY, Engels EA, Kramer JR, et al. Risk of immune thrombocytopenic purpura and autoimmune hemolytic anemia among 120 908 US veterans with hepatitis C virus infection. Arch Intern Med. 2009;169(4):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bougie DW, Wilker PR, Aster RH. Patients with quinine‐induced immune thrombocytopenia have both "drug‐dependent" and "drug‐specific" antibodies. Blood. 2006;108(3):922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnold DM, Kukaswadia S, Nazi I, et al. A systematic evaluation of laboratory testing for drug‐induced immune thrombocytopenia. J Thromb Haemost. 2013;11(1):169‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karpp D. The favored ingredient of a seasonal Latino punch cannot be imported, so San Diego County farmers came up with a solution. LA Times 12/9/2009.
- 19. Núñez‐Colín CA, Sanchez D. Ethnobotanical, cultural, and agricultural uses of tejocote (Crataegus species) in Mexico. Acta Horticulturae. 2011;918:901‐910. [Google Scholar]
- 20. Crataeguspubescens (Kunth) Steud. (1841). NomenclatorBotanicus. 2: 433. 1841.
- 21. Bray JK, Chiu GS, McNeil LK, et al. Switching from a high‐fat cellulose diet to a high‐fat pectin diet reverses certain obesity‐related morbidities. Nutr Metab (Lond). 2018;15:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alipotec Tejocote Root Review ‐ 9 Things You Need to KnowBy Summer Banks FNS, SPT, Natalie Rizzo, MS, RD (expert review). https://www.dietspotlight.com/alipotec-tejocote-root-review/
- 23. Palmer K, Lebin J, Burns R, Mazor S. Tejocote Raíz: a root for weight‐loss or digoxin doppelgänger? ACMT 2019 annual scientific meeting abstracts—San Francisco, CA. J Med Toxicol. 2019;15(2):53‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386‐2393. [DOI] [PubMed] [Google Scholar]
- 25. Aster RH, Curtis BR, McFarland JG, Bougie DW. Drug‐induced immune thrombocytopenia: pathogenesis, diagnosis, and management. J Thromb Haemost. 2009;7(6):911‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Núñez‐Colín CA. The tejocote (Crataegus Species): a Mexican plant genetic resource that is wasted. Acta Horticulturae. 2009/01/01. 10.17660/ActaHortic.2009.806.42.


