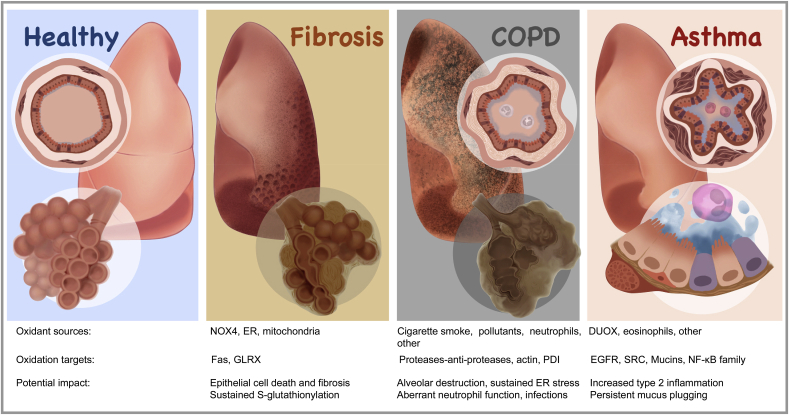
1. Introduction
The lung is a unique organ, as it is constantly exposed to air and airborne pollutants including oxidant gases such as highly reactive nitrogen dioxide and ozone, particulates, viruses, other microbes, immune activators and irritants. Defense from these pollutants is provided by a number of cell types that include epithelial cells and macrophages. Differentiated ciliated airway epithelial cells contain cilia, specialized organelles that beat in waves to propel pathogens and inhaled particles trapped in the mucous layer out of the airways [1]. Secretory epithelial cells produce mucins, and an array of anti-microbial products [2]. Airway epithelial cells also express hydrogen peroxide (H2O2)-producing dual oxidases (DUOX) providing defense from microbes and other irritants [3]. Located in the distal alveolar lung regions, type II epithelial cells produce surfactant proteins with anti-microbial or surfactant lowering activities [4]. Type II epithelial cells have been estimated to constitute 60% of alveolar epithelial cells and 10–15% of all lung cells. These cells are highly bioenergetically active due to their role in surfactant production, barrier protection, metabolism of xenobiotics, and as progenitor cells to replace damaged alveolar epithelial cells. The functions of distal epithelial cells and other cell types allow the lung to function to exchange oxygen and carbon dioxide to allow for normal aerobic function [5].
The high metabolic demand on airway and alveolar type II epithelial cells which secrete mucins, surfactant proteins, cytokines, etc., can create stress in the endoplasmic reticulum (ER). A tightly regulated, evolutionary conserved stress response termed the unfolded protein response (UPR), is triggered to rectify the stress, thereby allowing adaptation. However, when the stress is excessive, apoptosis occurs [6]. ER stress has become a well-recognized feature of chronic lung diseases. Less well recognized is the fact that protein folding within the ER creates oxidants, and that the ER environment is oxidizing to allow disulfide bond formation in proteins to occur. Therefore, altered redox-based processes that accompany ER stress have the potential to contribute to chronic lung diseases.
Changes in the redox environment have long been implicated in the pathophysiology of a myriad of lung diseases that include cancer, asthma, acute respiratory distress syndrome, chronic obstructive pulmonary disease and pulmonary fibrosis. The low MW thiol molecule, glutathione (GSH) has been at the forefront of investigation in these chronic diseases, and oxidation of glutathione to its disulfide, (glutathione disulfide, GSSG) is a frequently used oxidative stress marker. While an increased oxidative burden originally was believed to contribute to lung injury, for instance by damaging epithelial cells, changes in the redox environment are now believed to promote lung disease via the oxidation of proteins, notably via the oxidation of reactive cysteines that typically occur in highly conserved regions. S-glutathionylation, the covalent attachment of glutathione to protein cysteines, is one of the numerous protein cysteine oxidations that has received considerable attention recently, given that S-glutathionylation affects numerous regulatory proteins and other redox systems. Dysregulation of S-glutathionylation is speculated to contribute to the pathogenesis of chronic lung diseases by affecting cellular pathways that govern death, proliferation, migration, plasticity, inflammation and production and profibrogenic factors [[7], [8], [9]]. Herein we will describe glutathione biochemistry, processes that regulate S-glutathionylation, and provide evidence that dysregulation of S-glutathionylation is associated with ER stress in settings of chronic lung diseases. Lastly, we provide a rationale for developing small molecules or biologics that harness S-glutathionylation chemistry for the treatment of chronic lung disease. Parts of this review were recently published as a book chapter entitled: “Redox Mechanisms in pulmonary disease: Emphasis on pulmonary fibrosis”, in “Oxidative eustress”, edited by Helmut Sies (Elsevier), and is expanded here with additional focus on ER stress, chronic obstructive pulmonary disease and asthma.
2. Chronic lung diseases: Pulmonary fibrosis, asthma and chronic obstructive pulmonary disease
2.1. Pulmonary fibrosis
Pulmonary fibrosis is an unrelenting progressive disease, characterized by a loss of normal alveolar architecture, repopulation of alveolar spaces with extracellular matrix, an overpopulation of activated myofibroblasts, and loss of alveolar epithelia [10]. While the causes of pulmonary fibrosis might be suspected in some cases, for instance as a result of exposure to asbestos or silica, in other cases the causes remain unknown. The diagnosis idiopathic pulmonary fibrosis (IPF) is made when other potential etiological factors have been excluded, and is based upon strict radiological criteria, sometimes also requiring histo-pathological confirmation of a pattern of usual interstitial pneumonia. Pulmonary fibrosis presents in patients in their 50–70's, and affects more men than women [10]. Each year, pulmonary fibrosis kills 40,000 people in the USA alone, and it affects over 5 million people worldwide [11]. The age-associated enhanced susceptibility to fibrosis is believed to reflect the lack of proper repair in an aging individual following repeated cumulative stresses [10]. Defective autophagy, telomere attrition, altered proteostasis, and cell senescence have been observed in epithelial cells and fibroblasts from IPF lungs, as compared to age-matched controls [12]. IPF is a dismal diagnosis, and current FDA-approved therapies, pirfenidone and nintedanib (marketed as Esbriet and OFEV, respectively), have limited effectiveness to halt progression of idiopathic pulmonary fibrosis (IPF), which typically leads to death of patients with IPF within 3–5 years from the time of diagnosis [10]. A number of environmental insults have been shown or are speculated to cause pulmonary fibrosis, that include inhalation of the aforementioned particulates, asbestos or silica, smoking, viral infections, radiation, etc. The chemotherapeutic drug, bleomycin, causes pulmonary fibrosis as a side effect, which limits its use as a chemotherapeutic modality. Radiotherapy for the treatment of lung cancer can also induce pulmonary fibrosis, one of its most common side effects [13]. Pulmonary fibrosis also can have a genetic component, and in cases of familial IPF, patients are known to have germline mutations in certain genes, including surfactant protein C (SFTPC) [14,15]. These genes encode proteins that are highly expressed in epithelia, and mutations in these genes result in defects in protein folding, leading to ER stress and mitochondrial dysfunction as will be further discussed below [16]. Besides IPF, a number of other disorders can result in lethal fibrosis, including systemic sclerosis (SS) and Hermansky-Pudlak syndrome (HPS).
3. Asthma
Asthma is a complex spectrum of diseases that affects approximately 7% of the US population [17], and is characterized by symptoms such as wheezing, frequent cough, or shortness of breath, and is typically diagnosed by spirometry and methacholine challenge tests as well as allergen skin tests. Asthma is a chronic inflammatory disease characterized by increased numbers of eosinophils, T-Helper 2 (TH2) lymphocytes and activated mast cells and by structural changes in the airways, including epithelial cell mucus metaplasia, smooth muscle hypertrophy and hyperplasia, subepithelial fibrosis (with increased deposition of collagens, fibronectin, and tenascin), changes in submucosal gland cells, and increased blood vessel formation [18,19]. The subepithelial fibrosis in the airways of asthmatics differs anatomically from the parenchymal fibrosis seen in IPF, but the molecular pathways that underlie fibrotic remodeling may be quite similar [20]. The majority of asthmatics are hyperresponsive to common allergens such as house dust mite, cat dander, pollen, fungal allergens, etc. but a large subgroup of asthmatic patients displays a different phenotype not characterized by allergy. Asthma severity varies greatly and can be grouped into 4–5 phenotype clusters based on systematic analysis of age, gender, atopy, lung function, or obesity. These include large subgroups of early-onset asthma typically characterized by allergic sensitization and exacerbations (T2 High Asthma), and distinct groups of adult-onset asthma which are often independent of allergy and atopy (T2 Low Asthma) and are associated with obesity, smoking, and other factors [18,19] in addition to frequently being characterized by severe airway obstruction and exacerbations. Efforts to define asthma based on specific functional or pathobiological mechanisms also revealed different asthma endotypes, which may benefit from treatments specifically targeted at these causative molecular mechanisms. Treatment and management of asthma typically involves bronchodilators (e.g. long-acting beta agonists) and/or anti-inflammatory treatments that target eosinophilic inflammation (corticosteroids or biologics that directly target eosinophils or TH2 lymphocytes). However, certain subgroups of asthma do not benefit from such treatments, probably due to alternative underlying mechanisms such as neutrophilic inflammation and exacerbations due environmental pollutants or infections [21,22].
4. Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is one of the most common lung diseases in the world, currently affecting over 251 million people. In 2015, it caused 5% of all deaths and the World Health Organization estimates that COPD will become the third leading cause of death by 2030. Symptoms include breathlessness, chronic cough and sputum production, which hinder patients in their daily activities as the disease worsens and greatly impact their quality of life. COPD is defined as a common, preventable and treatable disease characterized by persistent airflow limitation, due to airway and/or alveolar abnormalities caused by significant exposure to noxious particles or gasses [23]. The lack of reversal of airflow limitation after the administration of a short-acting bronchodilator distinguishes COPD from asthma. Current treatments are aimed at relieving symptoms and can slow down the progressive loss of lung function, but they fail to halt disease progression, and do not recover lung capacity that is lost. The loss of lung function in COPD is attributed to airflow obstruction, which is caused by chronic bronchitis, small airway remodeling and emphysema in varying combinations and severities. It is therefore not a single disease, but rather a set of complex conditions, each consisting of various components and clinical presentations [24]. Chronic bronchitis is an inflammatory state of the airways associated with bronchial wall thickening and excess mucus production arising from mucus hyperplasia. The small airways (<2 mm) are affected by mucus plugging and inflammation, and the mucosal thickening and increased deposition of extracellular matrix (ECM), a hallmark of fibrosis [25]. This sub-epithelial fibrosis is again different from IPF, but also in COPD molecular pathways that underlie fibrotic remodeling might be very similar [20]. Emphysema on the other hand, is defined by a loss of alveolar septae, which reduces the surface area available for gas exchange and can lead to hypoxia. In addition, it makes small airways more likely to collapse upon exhalation, resulting in further air trapping. Cellular damage to pneumocytes and degradation of alveolo-capillary membranes by an excess of proteases, are thought to underlie emphysema development. Importantly, the remaining alveolar walls in emphysema have been shown to display matrix alterations that are similar to the ones described in small airways. Increases in collagens, glycoproteins and glycosaminoglycans, and loss of elastin have been shown in both compartments [[25], [26], [27], [28]]. The presence of chronic pulmonary inflammation, which includes neutrophils, macrophages, B-cells, lymphoid aggregates and CD8+ T-cells, and which persists after smoking cessation is the driving force for the pathological alterations in the airways and alveoli [24]. Although they represent very different pathological entities, chronic bronchitis, small airways remodeling and lung emphysema can each be considered the result of defective tissue repair ensued by the inhalation of noxious particles or gasses, in which chronic inflammation plays a major role. Unfortunately, the inflammatory reaction in COPD is unresponsive to corticosteroid treatment, in which mechanisms similar as in the unresponsive subgroups of asthmatics mentioned earlier likely play a role [29]. The disease is considered preventable, because the main risk factor for COPD is (second-hand) exposure to tobacco smoke. Additional risk factors which are not all avoidable, include exposures to other environmental pollutants, and dusts and chemicals in an occupational setting [24]. Many genetic factors have been shown to contribute to the risk of a smoker to develop COPD. Only a genetic α1-anti-trypsin deficiency has been causally linked to early onset emphysema. Because the normal decline in lung function with advancing age is accelerated in COPD patients, and because of the similarities between normal structural alterations occurring in lung tissue upon aging which lead to the development of senile emphysema and smoke-induced emphysema, COPD has been considered a disease of accelerated lung aging. At a molecular level many hallmarks of aging have been shown to be involved in the pathogenesis of COPD [30,31]. These include, among others, telomere attrition, a loss of proteostasis and cellular senescence. These aging hallmarks represent a marked overlap between COPD and IPF [31].
5. Glutathione and glutathione-mediated protein oxidation
Glutathione is considered a major redox buffer that exists in the reduced (GSH) and oxidized (GSSG, glutathione disulfide) forms [32], in addition to the chemical forms described below. Glutathione consists of the three amino acids, glutamate, cysteine and glycine, with a unique gamma glutamyl linkage of glutamate and cysteine. Cysteine, glycine and glutamate are derived from dietary sources or can be synthesized via the conversion of methionine, through the transsulfuration pathway [33]. Intriguingly, cysteine can have additional sulfur atoms, with cysteine persulfide (CysSSH) representing a cysteine derivative having one additional sulfur atom bound to a cysteinyl thiol group. Consequently, GSH can also exist as glutathione persulfide (GSSH), which has been reported to exist at concentrations as high as 100 μM in vivo [34]. GSSH has higher nucleophilicity than parental glutathione, and therefore GSSH exhibits strong scavenging activities against oxidants [34,35]. GSH can also exist in conjunction with various lipid-derived mediators, such as leukotriene C4 (LTC4), a leukotriene isoform that is generated by enzymatic coupling of GSH to LTA4 [36], and has important biological implications as a vaso- and bronchoconstrictor [37]. Another modified version of glutathione, S-geranylgeranyl-l-glutathione (GGG) was recently identified and shown to be a ligand for the P2RY8 receptor critical to the biology of germinal centers and B-cells, and GGG was shown to be metabolized by gamma-glutamyltransferase-5 [38]. Lastly, methylglyoxal is reactive metabolite formed as a byproduct of glycolysis. Conjugation of glutathione with methylglyoxal results in the formation of lactoyl-glutathione (LGSH) in a reaction catalyzed by glyoxalase, important in the detoxification of methylglyoxal. This metabolic pathway was recently shown to be important in the survival of non small cell lung cancer cells and tumor growth in mice [39]. Interestingly, glyoxyase II also has been implicated in promoting S-glutathionylation reactions [40]. The extent to which some of these modified versions of glutathione contribute to lung physiology and disease represents an unknown domain that warrants further exploration. The enzymes in the γ-glutamyl cycle that regulate GSH synthesis and degradation include glutamate cysteine ligase (GCL, also known as γ-glutamylcysteine synthetase); glutathione synthase (GS); γ-glutamyltranspeptidase (GGT); γ-glutamylcyclotransferase (GGCT); 5-oxoprolinase (OPLAH); and dipeptidase (DP). The recently identified cytosolic glutathione-specific gamma-glutamylcyclotransferase (ChaC)1 and ChaC 2, can metabolize GSH directly into 5-oxoproline and Cys-Gly [41]. Thus, the existence of multiple and redundant enzyme systems that precisely maintain GSH and its precursor amino acids point to exquisite regulation of GSH.
The oxidized disulfide form of glutathione, GSSG can be reduced to 2 molecules of GSH by glutathione reductase (GR) oxidizing NADPH to NADP+. The pentose phosphate pathway is a major pathway that reduces NADP+ to NADPH, thereby maintaining the glutathione redox buffer [32,42] (Fig. 1). GSH concentrations in cells range from ~ 1 mM–10 mM [42] with cytosolic GSH:GSSG ratios ranging from 100:1 to >500:1 [42]. GSH levels in extracellular fluids such as blood plasma are much lower (only several μM) but extracellular levels ranging from 100 μM to 400 μM have been measured in the lung lining fluid [43,44], demonstrating the unique regulation of GSH in lung tissue. In contrast to the cytosol, the endoplasmic reticulum is far more oxidizing (see below) and the reported GSH:GSSG ratio is notably lower and ranges from 1:1 to 7:1. Reported variations in GSH:GSSG ratios likely stem from variations in sample preparation, and methods used to block the thiol in reduced GSH. Lack of adequate blocking, in addition to auto-oxidation, may result in an overestimation of GSSG values, and these concerns continue to plague the field [45]. GSH is utilized in multiple processes. It is consumed by glutathione peroxidases to reduce oxidized lipids [46]. GSH contributes to the excretion of xenobiotics wherein glutathione S-transferases (GSTs) conjugate GSH to electrophiles or other toxic compounds leading to their excretion via mercapturic acids [47]. Lastly, and the focus of this review, GSH can be conjugated to proteins in a process called protein S-glutathionylation (PSSG). PSSG protects protein cysteines from being overoxidized to sulfinic and sulfonic species that are not readily regenerated. S-glutathionylation also changes the protein structure and function as will be further described next.
Fig. 1.
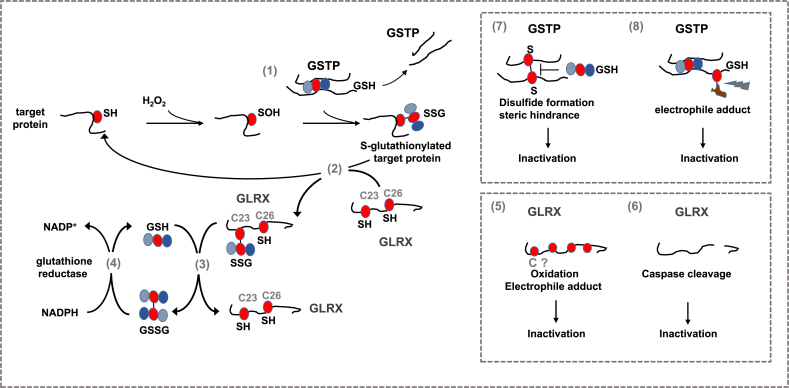
Steps in the catalytic cycle of protein S-glutathionylation (PSSG) and deglutathionylation believed to be relevant in the pathogenesis of chronic lung diseases. A number of biochemical events can induce PSSG. The mode of PSSG is likely to be target and context specific, dependent upon the proximity of oxidant producing enzymes, redox relays, the oxidation state of the GSH/GSSG redox couple etc. In epithelial cells in settings of a pro-fibrotic environment, oxidants originating from multiple sources lead to a sulfenic acid intermediate (SOH) which can be a platform for subsequent PSSG, which can happen spontaneously or catalyzed by glutathione S- transferases (GST), notably GSTP [1]. Conversely GLRX acts to deglutathionylate proteins [2], restoring the original sulfhydryl group. GLRX induces deglutathionylation via the monothiol mechanism requiring only the N-terminal cysteine in the thioredoxin (TXN) domain [2], which can thereafter be reduced by reduced glutathione (GSH) [3] re-establishing the reduced thiol group of the N-terminal cysteine in GLRX. Glutathione disulfide (GSSG), formed in this process can be reduced back to GSH through the action of glutathione reductase (GR) consuming reducing equivalents form NADPH [4]. GLRX can be inactivated via oxidations of one or more cysteines outside of the TXN domain [5] including electrophiles from cigarette smoke. GLRX can also directly cleaved by caspases 8 and 3 [6]. Similarly, GSTP also is subject to oxidant-mediated inactivation, potentially involving a disulfide which creates steric hindrance that interferes with GSH binding [7], or electrophile-induced modification [8]. We refer the reader to the body of text for detailed information. Red circle: protein cysteine or cysteine in glutathione. SH: reduced cysteine, SOH: Sulfenic acid, SSG: S-glutathionylated protein, GSH: Reduced glutathione, GSSG: glutathione disulfide, comprised of two glutathione molecules with a disulfide bond between the cysteines of each glutathione molecule, S–S: disulfide. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Cysteines in proteins have emerged as important biological sensors for redox perturbations that in turn regulate biological responses. Reactive, low pKa cysteines can be oxidized in specific, reversible, and regulated manners, in order to permit cells to sense and respond to redox changes. The specificity of redox modifications of target proteins is controlled via compartmentalization of oxidant formation, and the presence of enzymes that regulate protein cysteine redox status in subcellular domains [8,48,49]. As stated earlier, protein S-glutathionylation (PSSG) represents a cysteine oxidation wherein GSH is conjugated covalently to the protein cysteine. PSSG has emerged as a critical regulatory mechanism that regulates protein structure and function. The conjugation of glutathione to protein cysteines changes protein conformation (addition of three amino acids) as well as charge (glutamic acid), thereby affecting function [8,[50], [51], [52]]. The precise pathways that lead to PSSG in biological settings are not completely known, but it is thought that an oxidant, such as H2O2, can first lead to a sulfenic acid intermediate (-SOH) (or sulfenylamide (-SN)), a gateway oxidation, that can subsequently be S-glutathionylated. While such GSH conjugation can occur spontaneously, it can also be catalyzed by glutathione-S-transferases (GSTs) (Fig. 1). The majority of studies conducted thus far on GST-catalyzed PSSG have centered on glutathione S-transferase π (GSTP) [53,54], and therefore we will focus on GSTP herein. GSTP is a dual function enzyme that has both chaperone activity and can catalyze GSH conjugation to biological and xenobiotic electrophiles as well as S-glutathionylation reactions. Separate domains within GSTP regulate these functions. The N-terminal thioredoxin-like G-site binds GSH and activates the cysteinyl moiety of GSH via tyrosine 7 whereas the C-terminal alpha-helical domain shapes the co-substrate binding site, termed the H-site, based upon the hydrophobic (H) character of co-substrates [55,56]. Although the role of GSTP in protein S-glutathionylation is well recognized, the exact mechanisms whereby GSTP induces PSSG still remain unclear. Studies of the 1 cysteine-containing PRDX6 demonstrated that oxidation of PRDX6 to a sulfenic acid intermediate is associated with formation of a heterodimer with GSH-containing GSTP, which in turn leads to S-glutathionylation of PRDX6, followed by GSH-mediated reduction of the disulfide to regenerate the active form of PRDX6 [57,58]. An array of proteins are now known to be S-glutathionylated by GSTP. However, S-glutathionylation of these proteins in some cases has been demonstrated in cell lines under conditions of overt stress. The repertoire of proteins S-glutathionylated by GSTP and other GSTs in settings of lung (patho)physiology remains poorly understood. Interestingly, GSTP also can be oxidatively inactivated, and it has been suggested that cysteines-47 and -101 regulate the binding of GSH, and that disulfide bond formation between these cysteines results in steric hindrance and resultant inactivation of GSTP [59] (Fig. 1).
The family of glutaredoxins (GLRX) under physiological conditions acts to specifically deglutathionylate proteins restoring the reduced sulfhydryl group [60] (Fig. 1). It is worthy of mention that under conditions where GSSG levels are high, GLRX can catalyze S-glutathionylation. Four mammalian GLRX have been described, the dithiol GLRXs, GLRX (active site; CPYC) and GLRX2 (active site; CSYC) and monothiol GLRX3 (also known as PICOT, active site; CGFS) and GLRX5 (active site; CGFS). GLRX exert specificity toward the γ-linkage-glutamyl moiety of glutathionylated proteins (or free GSH) [61,62]. Mammalian GLRX catalyzes deglutathionylation in a two-step mechanism in which the first step involves the nucleophilic displacement of the GSH moiety in the S-glutathionylated protein target by the N-terminal active site cysteine, resulting in S-glutathionylation of GLRX itself. In the second step, the thiolate ion of the N-terminal active site cysteine is regenerated by consuming one molecule of GSH, forming GSSG [63] (Fig. 1). Mammalian GLRX has 5 reactive cysteines. Only the active site N-terminal cysteine is required for the deglutathionylation reaction, as the mutation of individual or all four cysteines other than active site cysteine 23 does not affect its activity [[64], [65], [66]]. However, oxidation, including S-glutathionylation, of the cysteines outside of the catalytic site results in inactivation of GLRX [67,68] (Fig. 1), and suggests that GLRX is subject to negative feed-back regulation by its substrate, GSH. In summary, regulatory pathways comprised of GSTs and GLRX, among others, that collectively control protein S-glutathionylation and deglutathionylation operate to precisely control the oxidation state of target cysteines through utilization of glutathione. These observations point to the importance of GSH as a critical regulator of biological pathways, beyond the traditional view that GSH is a mere quencher of oxidants.
6. ER stress
The endoplasmic reticulum (ER) is an organelle essential to normal cellular function that plays key roles in the regulation of proteostasis, calcium storage, lipid synthesis, mitochondrial function, among others. Protein misfolding and ensuing ER stress triggers the activation of a signaling network known as the unfolded protein response (UPR), mentioned above, through distinct effector pathways that involve activation of PKR-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1α (IRE1α). These pathways are controlled by a chaperone protein, immunoglobulin heavy-chain-binding protein (Bip, also known as glucose regulated protein 78 (GRP78) or heat shock 70 kDa protein 5, (HSPA5)), and their activation is restricted by binding of Bip to these ER sensors (PERK, ATF6, and IRE1α). Bip also binds to misfolded proteins in the ER. As misfolded proteins accumulate, Bip binding to the three UPR sensors is reduced and resultant UPR signaling in turn modulates new protein synthesis, increases production of ER chaperones, and induces components of the ER-associated degradation (ERAD) system [14,69]. The UPR response is triggered to rectify the stress, but severe stress can result in cell death. In epithelial cells, ER stress can lead to apoptosis, pro-inflammatory signaling, and epithelial-mesenchymal transition (EMT), features that have all been linked to chronic lung diseases, reviewed in Refs. [14,70,71]. During the UPR, various chaperones including oxidoreductases such as protein disulfide isomerases (PDIs) are upregulated in order to restore ER homeostasis [72,73]. Twenty members exist in the PDI family of proteins. PDIs exhibit varying numbers of thioredoxin-like domains (CXXC) which catalyze oxidative folding and disulfide bond isomerization of proteins in the ER lumen [74]. It has been speculated that a large amount of redundancy in client proteins of the various PDIs exists. However, recent studies using mutants to trap PDI-associated proteins indicate more nuanced roles for the individual PDIs toward regulating specific client proteins [75]. The environment of the endoplasmic reticulum (ER) is more oxidizing than the cytosol, allowing disulfide bridge formation [76]. As mentioned earlier, the GSH/GSSG ratio is substantially lower in the ER than the cytosol, and a majority of glutathione in the ER is present as mixed disulfides with proteins (S-glutathionylated proteins) [77]. Not surprisingly, it has therefore been suggested that GSH regulates oxidative folding processes [78].
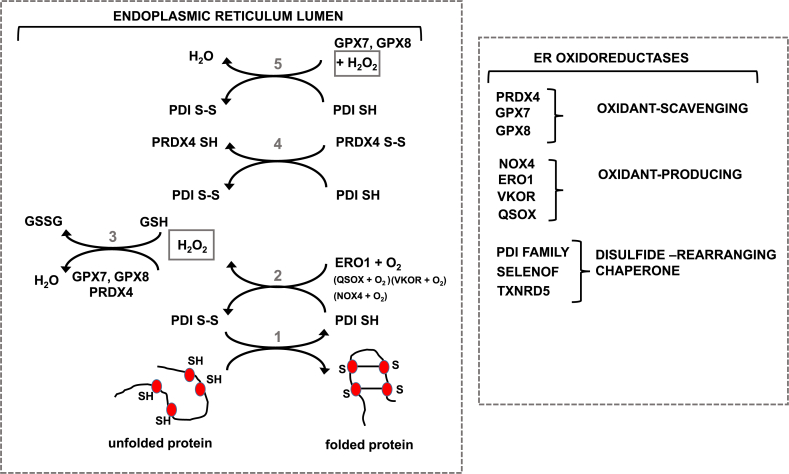
Many proteins that enter the secretory pathway, including the aforementioned surfactant proteins, mucins (79) etc. contain cysteines that are oxidatively processed into disulfide bonds in the ER in order to stabilize the protein. Disulfide bridge formation in proteins is regulated by the PDI family. Oxidized PDI (S–S) will covalently bind its reduced client protein (SH) to catalyze disulfide bridge formation, via a sulfhydryl-disulfide exchange reaction in which the client protein will be oxidized (S–S), while PDI will be reduced (SH). In order to regenerate catalytically active PDI, it has to be re-oxidized by ER oxidoreductin-1 (ERO1). During this regeneration cycle of PDI by ERO1, H2O2 is formed [80] (Fig. 2). Thus, oxidative processing of proteins in the ER leads to production of H2O2. Steady state levels of H2O2 are precisely controlled in part via oxidation of GSH to GSSG, and H2O2 metabolism by ER-resident peroxiredoxin-4 (PRDX4). Besides ERO1, other mechanisms have emerged to regenerate oxidized PDI, which are speculated to involve H2O2 produced by the NADPH oxidase, NOX4, acceptance of electrons by oxidized PRDX4, reduction of vitamin K with concomitant oxidation of PDI by Vitamin K epoxide hydrolase (VKOR), quiescin-sulfhydryl oxidase (QSOX), and use of H2O2 generated by ERO1 by glutathione peroxidases (GPX) 7 and 8 [[80], [81], [82], [83]] (Fig. 2). Similarly, additional quality control systems exist the ER. For example, Selenof is an ER-resident thioredoxin-like oxidoreductase that acts as a gatekeeper for immunoglobins and likely other disulfide-rich glycoproteins, thereby providing redox quality control of secreted glycoproteins [84]. The import of cystine via system Xc has emerged as a pathway that controls ER homeostasis. System Xc imports cystine, while exporting glutamate, increasing intracellular levels of cystine (the oxidized version of cysteine), which is rapidly reduced to cysteine, the rate limiting amino acid required for GSH biosynthesis. Inhibition of system Xc results in decreases in GSH, and induces ER stress as manifested by activation of the eIF2α-ATF4 branch of the ER stress response pathway, and increased expression of notably of ATF4, ATF3 and CHOP [85]. These observations collectively suggest important links between glutathione homeostasis and ER stress.
Fig. 2.
ER oxidoreductases and their roles in protein folding and maintenance of ER redox homeostasis. Left: Role of protein disulfide isomerase (PDI) in oxidative disulfide bond (S–S) formation in proteins [1]. Oxidized PDI (containing S–S disulfide) oxidizes its client protein by introducing disulfide bonds, while PDI itself as a result becomes reduced (SH) [2]. ER oxidoreductin (ERO1) plays a major role in re-oxidizing PDI, a reaction that generates hydrogen peroxide (H2O2) [3]. H2O2 can be detoxified by GSH, or the actions of peroxiredoxin 4 (PRDX4), or glutathione peroxidases (GPX) 7 or 8. Alternative pathways for the regeneration of oxidized PDI are the abstraction of electrons by oxidized PRDX4 [4], or a H2O2-dependent GPX7/8 catalyzed reaction. Right: Overview of the most studied ER-oxidoreductases and their potential roles in oxidant-production/scavenging or other functions. We refer the reader to the body of text for detailed descriptions.
Relevant to this review are findings that, among other cellular compartments, GSTP is found in the ER [86,87], where it promotes S-glutathionylation of a number of ER resident proteins, including calnexin, calreticulin, endoplasmin, sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), PDI and Bip in settings of overt oxidative stress [86] (Fig. 3). Genetic ablation of Gstp1/2 increased sensitivity of mouse embryonic fibroblasts to death induced by the UPR inducers tunicamycin or thapsigargin, in association with increased expression of UPR proteins [86], suggesting that GSTP is critical in the regulation of ER stress responses. It remains unclear to date whether the chaperone and/or S-glutathionylation functions of GSTP regulate ER stress responses and whether GSTP contributes to enhanced ER stress in patients with chronic lung diseases.
Fig. 3.
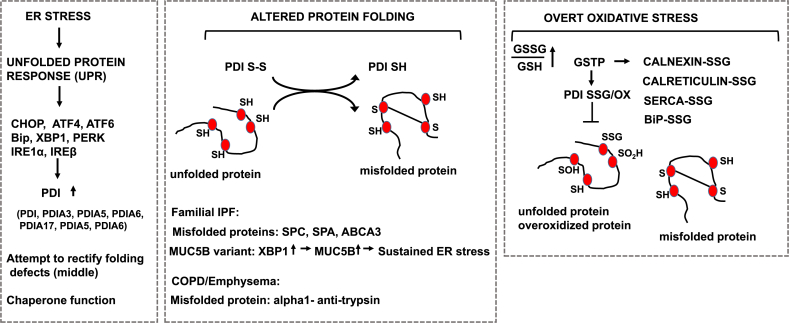
Disregulation of the Unfolded Protein Response (UPR) and protein disulfide isomerase in settings of chronic lung diseases. Left: Schematic overview of the link between ER stress, the resultant unfolded proteins response, and the effector pathways that are increased in chronic lung diseases. Middle: Illustrations of misfolded proteins relevant to IPF (SPC, SPA, ABCA3) or COPD (α1 anti-trypsin). Also illustrated is MUC5B which has been linked to ER stress in settings of familial IPF. Observed increases in PDI in chronic lung diseases may be to rectify the burden of ER stress and/or misfolded proteins. Right: In settings of overt oxidative stress, the function of PDI, and other ER proteins shown, may be compromised through oxidations and/or other modifications, allowing misfolded and/or overoxidized proteins to accumulate. Note that thus far, data to support this scenario was obtained in cell lines and/or settings of overt oxidative stress. Relevance of these putative events to chronic lung disease will require detailed analyses of human tissue specimens. We refer the reader to the body of text for detailed descriptions.
7. ER stress and lung fibrosis
Chronic ER stress is associated with the development of fibrotic disorders in the lung, liver and kidney. ER stress within epithelial cells has been strongly implicated in the pathogenesis of lung fibrosis, based upon discoveries of germline mutations in genes expressed exclusively in epithelial cells that result in defects in folding and/or processing of a nascent peptide, causing prolonged ER stress and subsequent fibrosis in patients with familial IPF [14,88]. Evidence that ER stress occurs in patients with IPF was first reported by the laboratory of Dr. Timothy Blackwell in 2008, documenting that expression of various markers of ER stress was increased in airway epithelial cells of patients with IPF, in association with the presence of herpes virus [15]. These markers include ATF4, ATF6, and CCAAT-enhancer-binding protein homologous protein (CHOP), Bip, and X-box binding protein 1 (XBP-1) [14]. Some patients with familial IPF have germline mutations in surfactant protein C (SFTPC), surfactant protein A2 (SFTPA2), or the lipid transporter, ATP binding cassette class A3 (ABCA3) genes. These genes encode proteins highly expressed in epithelia, and mutations in these genes result in defects in protein folding, leading to ER stress [14] (Fig. 3). Some affected individuals with familial IPF shared a rare missense variant (L188Q) containing a mutation adjacent to a cysteine residue in pro–SP-C that is required for proper folding [4]. Similarly, a mutation of cysteine 121 within the so-called BRICHOS domain of pro-SPC has been reported in a child with interstitial lung disease [89]. Misfolded pro–SP-C arising from these mutations aggregates in the ER and activates the UPR. The relevance of these mutations for the pathogenesis of lung fibrosis has been corroborated in mouse models where it was demonstrated that transgenic overexpression the L188Q mutant variant of SFTPC in type II alveolar lung epithelial cells increased the sensitivity to the development of fibrosis [90]. Knock-in mice expressing of an isoleucine-to-threonine substitution at codon 73 (I73T) of SFTPC, or a cysteine-to-glycine substitution at codon 121 (C121G), developed spontaneous fibrosis, [89,91]. The mucin, MUC5B, has been shown to play a key role on the obstruction of peripheral airspaces and honeycomb cysts in patients with IPF, and a MUC5B promoter polymorphism (rs35705950) has been identified as a strong risk factor for development of IPF. A recent study demonstrated that the UPR is critical in inducing expression of the MUC5B gene. Notably, the endoplasmic reticulum to nucleus signaling 2 protein (ERN2, also known as IRE1β) and its downstream target, spliced XBP1, in cooperation with the mucus cell transcription factor, SAM pointed domain containing ETS transcription factor (SPDEF), bind the MUC5B promoter to induce its expression in a rs35705950-specific manner [92] (Fig. 3). PDIs also are emerging as potential contributors to pulmonary fibrosis. As will be described below, PDIA3 promotes disulfide formation of FAS, leading to epithelial cell death [87]. PDIA3 also has been implicated in the trans-differentiation of murine type II alveolar epithelial cells into type I cells, in association with increased Wnt/β-catenin signaling [93]. Furthermore, recent studies have highlighted that PDIs interact with and potentially regulate disulfide bonds in numerous pro-apoptotic and extracellular matrix regulating proteins including BAK [94], FAS [87], collagen 1a1 (95), transglutaminase 2 [96] matrix metalloproteinase 9 [97], and the collagen crosslinking enzyme lysyl oxidase like 2 (LOXL2) [75]. PDI also plays a role in integrin-mediated cell adhesion [98]. Intriguingly, fibronectin itself contains protein-disulfide isomerase activity thought to be relevant to the disulfide-mediated cross linking of fibronectin in the extracellular matrix [99]. Relevant to fibrosis, it is worthy to mention that PDI also acts as a non-catalytic component of the enzyme prolyl 4-hydroxylase, important in the hydroxylation of collagen [100,101].
Links between ER stress, loss of proteostasis and mitochondrial dysfunction in settings of IPF also have become apparent [12]. The ER tightly controls the calcium pool available for mitochondrial uptake through a number of proteins that include the mitochondrial calcium uniporter via sarcoendoplasmic reticulum Ca2+ ATPase and the inositol triphosphate receptor, providing a mechanism whereby the ER regulates cellular bioenergetics (reviewed in Refs. [14]) and cell death [102]. Specialized regions of interaction and communication between ER and mitochondria have been demonstrated and represent an area of active investigation [103]. Notably, aging and enhanced ER stress during aging have been shown to lead to mitochondrial dysfunction in type II alveolar epithelial cells [12]. A higher frequency of enlarged mitochondria with a bias toward mitochondrial fusion and an increased mitochondrial area occurred in aging type II alveolar epithelial cells [104]. Additional findings of mitochondrial abnormalities in the aging lung include increases in mitochondrial reactive oxygen species, decreases in mitophagy, impaired respiration, mitochondrial DNA deletions, and decreased expression of sirtuin 3 [12,105]. ER stress in epithelial cells has been linked to mitochondrial perturbations via decreases in levels of PTEN-induced putative kinase 1 (PINK1), a regulator of mitochondrial homeostasis. A role for PINK1 in lung fibrosis was corroborated based upon findings demonstrating that PINK1-deficient mice exhibited increased susceptibility to apoptosis, and spontaneous transforming growth factor beta (TGFB)-dependent fibrosis, in association with abnormal mitochondria in type II alveolar epithelial cells [104]. Epithelial cells expressing the I73T-mutant variant of human SPC showed defects in autophagy in association with increases in mitochondria biomass and decreases in mitochondrial membrane potential [11]. Mitochondrial dysfunction and ER stress also have been linked to the senescent fibroblast phenotype present in the IPF lung [106]. Fibroblasts from IPF patients have shorter telomers, express a number of senescence-associated proteins and produce cytokines related to the senescence-associated secretory phenotype. Upon TGFB stimulation, markers of ER stress increased to greater extents in IPF fibroblasts compared to age-matched cells from control lungs [107]. TGFB was furthermore shown to stimulate increases in mitochondrial mass in human lung fibroblasts via the SMAD2/3 (SMAD represents an acronym from the fusion of Caenorhabditis elegans Sma genes and the Drosophila Mad, Mothers against decapentaplegic) and CCAAT/enhancer binding protein β (CEBPB) pathways [108]. Interestingly a role of oxidants has been suggested in promoting the senescence phenotype in fibroblasts and this had been linked to the activation of signal transducer and activator of transcription (STAT)-3, a transcription factor implicated in premature senescence [109,110]. Human lung fibroblasts exposed to H2O2 displayed increases in expression of senescence markers and expression of interleukin-6 (IL6), in association with translocation of STAT3 to the nucleus and mitochondria. These observations were accompanied by increased basal respiration, proton leak and an associated increase in oxidant production in senescent fibroblasts. Using a small molecule inhibitor of STAT3, the authors demonstrated attenuated IL6 production, senescence markers and restoration of normal mitochondrial function [109]. Collectively, these findings strongly support the concept that ER dysfunction/loss of proteostasis in alveolar type II cells play key roles in driving the pathogenesis of IPF. ER stress is not limited to those individuals with familial IPF, and ER stress is now recognized as a common feature of sporadic IPF. Disruptions in proteostasis are also linked to mitochondrial dysfunction and the senescence phenotype of fibroblasts, relevant to pulmonary fibrosis.
A few studies have been conducted to suggest a link between dysregulated oxidative processing in the ER and altered extracellular matrix biology. Mice with combined loss-of-function mutations in genes encoding ERO1α, ERO1β, and PRDX4 display a compromised extracellular matrix due to defects in the intracellular maturation of procollagen. Combined absence of these enzymes resulted in increased cysteinyl sulfenic acid modification of ER proteins. Intriguingly, the increased cysteinyl sulfenic acids led to oxidation of ascorbate to dihydroascorbate. Consistent with the requirement of ascorbate in maintaining the function of ER-localized prolyl-4 hydroxylases, the decreases in ascorbate in the ER of mouse embryonic fibroblasts lacking ERO1α, ERO1β, and PRDX4 resulted in a strong decrease in procollagen 4-hydroxyproline content [111]. Thioredoxin domain containing 5 (TXNDC5), an ER-localized protein disulfide isomerase, was shown to facilitate ECM protein folding, and to contribute to the stability of ECM proteins. Enhanced expression of TXNDC5 promoted redox-sensitive activation of cardiac fibroblasts and augmentation of cardiac fibrosis [112]. Quiescin sulfhydryl oxidase 1 (QSOX1) is an atypical disulfide catalyst, as it is localized to the Golgi and can be secreted from cells. QSOX1 activity was shown to be essential for the incorporation of laminin into the extracellular matrix synthesized by fibroblasts [113]. A role of VKOR in organ fibrosis has is emerging based upon a study showing that inhibition of VKOR with 3-acetyl-5-methyltetronic acid (AMT) attenuated the progression of cisplatin-induced renal fibrosis in rats [114]. Although GPX7 has not been linked to fibrosis, GPX7 expression was decreased in senescent cells, and knock down of GPX7 promoted premature senescence. Moreover, the ability of metformin to extend life span in worms has been linked to the upregulation of GPX7 [115]. GPX7 was also shown to be essential to promote Bip's chaperone function [116]. As fibrosis is associated with aging, it will be interesting to explore the function of GPX7 in settings of age-associated fibrosis. Lastly, overexpression of PRDX4 elicited protective effects against nonalcoholic steatohepatitis and type 2 diabetes in a mouse model [117]. Based upon these observations, it is highly plausible that dysregulation of proteostasis in the lung, linked to altered functions in ER-redox processes, contribute to the pathogenesis of lung fibrosis (Table 1).
Table 1.
Implications of ER oxidoreductases and/or ER stress in lung fibrosis or extracellular matrix biology.
| ER oxidoreductase | Putative function | Model | References |
|---|---|---|---|
| PDIA3 | Fas-SS formation, Epithelial cell apoptosis AEC2- AEC1 differentiation, regulation of Wnt/β-catenin signaling |
Epithelial cells, Mouse models, IPF Primary lung epithelial cells |
[87] [93] |
| PDI | Disulfide bond formation in LOXL2, collagen 1a1, transglutaminase 2, matrix metalloproteinase 9, Non-catalytic component of prolyl-4 hydroxylase Integrin mediated adhesion |
Cell lines, mouse models Human renal fibrosis Rat liver, chick embryos, C. Elegans Platelets |
[75,[95], [96], [97]] [100,101] [98] |
| ERO1α, ERO1β PRDX4 | Prolyl-4 hydroxylation of collagen | Triple knock out mice Mouse embryonic fibroblasts |
[111] |
| PRDX4 | Protection of NASH and diabetes | Mouse model | [117] |
| TXNDC5 | Stabilization of ECM proteins, Augmentation of cardiac fibrosis |
Cardiac fibroblasts, mice | [112] |
| QSOX1 | Laminin incorporation in the ECM | mice, fibroblasts | [113] |
| GPX7 | Regulation for longevity, protection from senescence | Mouse models Fibroblasts, mesenchymal stem cells C.Elegans |
[116] |
| Fibronectin | Intrinsic PDI activity | In vitro | [99] |
| VKOR | Promotes cisplatin-induced hepatic fibrosis | rats | [114] |
8. Glutathione biochemistry, S-glutathionylation and lung fibrosis
Numerous studies in patients with IPF and rodent models have demonstrated that perturbations of the redox status occur and are linked to disease pathogenesis [[118], [119], [120]]. Disruptions in mitochondria and, as mentioned earlier, the ER have been implicated in the pathogenesis of IPF [12,14,121], although the extent to which redox perturbations originating from dysfunctional mitochondria or ER, respectively, or other sources contribute to IPF remain unclear. In rodent models of fibrosis, genetic ablation of the antioxidant enzyme extracellular superoxide dismutase (SOD3) increased the susceptibility to fibrogenesis [122], and similar findings were observed in mice lacking the transcription factor, nuclear factor erythroid 2–related factor (NRF2) [123], which regulates numerous antioxidant and cytoprotective responses including expression of enzymes important in GSH synthesis. Conversely, administration of various antioxidants has been suggested to be protective against development of fibrosis (for review [124,125]). NOX4, which produces H2O2, has been shown to play a causal role in myofibroblast activation and resultant fibrosis in the bleomycin and fluorescein isothiocyanate models [126]. NOX4 dampens mitochondrial bioenergetics and biogenesis via an NRF2-dependent pathway. Interestingly, NOX4 silencing increased the levels of NRF2 mRNA, while NRF2 silencing led to a decrease in NOX4 mRNA expression, showing counter-regulation between NOX4 and NRF4. A NOX4/NRF2 redox imbalance also was demonstrated to contribute pulmonary fibrosis in aged mice where persistent fibrosis was linked to upregulation of NOX4 and failure to induce expression of NRF2 [127]. Levels of GSH were reported to be markedly decreased in lung lining fluids, induced sputum and plasma of patients with IPF, in association with increases in GSSG in plasma [118,128,129]. Increased levels of H2O2 occur in exhaled breath condensates from patients with IPF, compared to control groups [130]. To address the potential impact of GSH on the biology of lung fibroblasts, studies as early as 1990 found that extracellular GSH in the 500 μM range, similar to the concentration of GSH in the lung lining fluid, decreased lung fibroblast proliferation, suggesting that lowered levels of reduced GSH might promote fibroblast activation. The effect of GSH was mimicked by other sulfhydryl-containing molecules including cysteine and N-acetylcysteine (NAC), precursors of GSH, 2-mercaptoethanol, and low concentrations of dithiothreitol, but not by GSSG [131]. Transforming growth factor beta-1 (TGFB1) is growth factor critical to organ fibrogenesis, and overexpression of active TGFB1 is sufficient to induce lung fibrosis in rodent models [132]. TGFB1 alters the GSH status in the lung. Intranasal instillation of AdTGFB1(223/225), an adenovirus expressing constitutively active transforming growth factor beta 1 (hereafter referred to as AdTGFB1) suppressed the expression of catalytic and modifier subunits of glutamate-cysteine ligase (GCL), the rate-limiting enzyme in GSH synthesis, resulting in decreases of GSH. This resulted in increases in epithelial apoptosis, which occurred prior to the development of lung fibrosis [133]. The microRNA, miR-433, has been shown to directly target GCL. In vivo models of renal and hepatic fibrosis were associated with a TGFB1-mediated reduction of GCL levels that were miR-433 dependent, resulting in decreases in GSH levels, increases in S-glutathionylation and enhanced fibrogenesis [134]. It remains unclear whether miR-433 has a similar impact on lung fibrosis. TGFB1 is one of the major repressors of GCL expression leading to GSH depletion [133,135], suggesting that in a pro-fibrogenic environment, depletion and/or oxidation of GSH occurs through multiple mechanisms, which in turn promote fibrosis. Using the bleomycin model of fibrosis, the redox potential (E(h)) of plasma GSH and Cys redox systems was shown to change uniquely as a function of progression of bleomycin-induced lung injury. Plasma E(h) GSH/GSSG was shown to be selectively oxidized during the proinflammatory phase, whereas oxidation of E(h) Cys/CySS occurred at the later fibrotic phase. Increased oxidation of E(h) Cys/CySS in the lung lining fluid was also detected and was linked due to decreased food intake in mice exposed to bleomycin [136].
To address the role of glutathione disruption in pulmonary fibrosis, a number of studies involving the GSH precursor, N-acetyl-l-cysteine (NAC), were conducted with the goal to augment pulmonary GSH levels and to alleviate oxidative stress. NAC was shown to dampen bleomycin-induced inflammation and subsequent fibrosis in mice in association with decreases in lysyl oxidase [137,138]. A number of clinical trials have been also been conducted to determine whether increases in GSH in the lung would attenuate the progression of disease in patients with IPF and maintain lung function. One clinical trial that included the GSH precursor, NAC, along with another drug, initially showed therapeutic effects associated with NAC [139,140]. However, re-evaluation of NAC in a clinical trial with over 130 IPF patients in the NAC or placebo groups failed to show a clinical benefit of NAC on disease progression or lung function decline [141]. However, a pharmacogenomic interaction involving toll interacting protein (TOLLIP) and MUC5B single nucleotide polymorphisms (SNPs) and a response of IPF patients to NAC has become apparent [142].
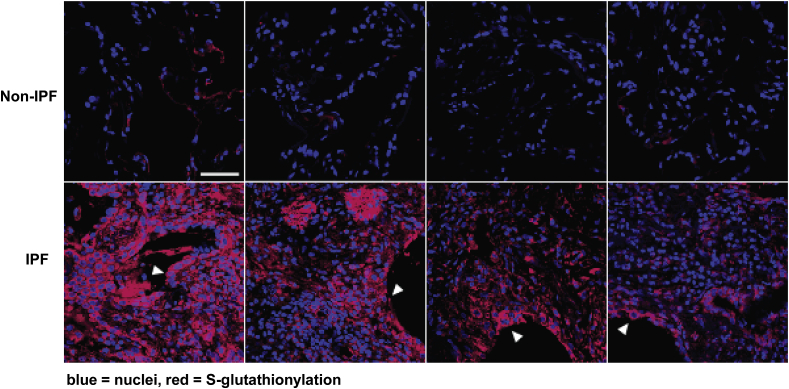
Although changes in glutathione homeostasis were first demonstrated in lungs from IPF patients over 30 years ago, the extent to which changes in S-glutathionylation occurred in fibrotic tissues had remained unclear. In order to assess the potential implications of PSSG for the pathophysiology of lung fibrosis, the highly specific catalytic activity of mammalian GLRX toward reduction of PSSG was used to visualize PSSG in formalin-fixed, paraffin-embedded (FFPE) tissues in situ using microscopy approaches [143]. Notable PSSG immunoreactivity was apparent in bronchiolar epithelial cells and alveolar macrophages under physiological conditions. In mice with bleomycin-induced fibrosis increases in PSSG occurred in bronchiolar epithelial cells, as well as parenchymal regions [143]. Increases in PSSG also occurred in lungs from patients with IPF (Fig. 4), and increases in PSSG inversely correlated with lung function in these patients [68]. Airway epithelial cells showed noticeable PSSG. Interestingly, GSTP was shown to be highly expressed in bronchiolar epithelial cells and type II alveolar epithelial cells [144], consistent with a role of these cells in protection against environmental insults and metabolism of xenobiotics. In lungs from patients with IPF, GSTP was prominently increased in bronchiolar epithelia as was as in the distal lung epithelial cells, in areas of re-bronchiolarization, and in reactive type II epithelial cells, including those type II pneumocytes at the leading edge of disease progression [144]. In contrast to increases in GSTP immunoreactivity in lungs from patients with IPF, the enzymatic activity of GLRX was decreased in IPF lungs, in a partial dithiothreitol-specific manner, indicative of oxidative inactivation of GLRX. As mentioned above, increases in GLRX S-glutathionylation were observed in lungs from patients with IPF, and in mice with bleomycin-induced fibrosis [68], suggestive of negative feedback inhibition of GLRX via S-glutathionylation. Earlier studies reported decreases in GLRX content in lungs from patients with IPF and COPD [145,146] and downregulation of GLRX by TGFB1 [147], consistent with a putative role of enhanced PSSG in promoting lung fibrosis.
Fig. 4.
Increases in S-glutathionylation in lung tissues from patients with IPF. Lung sections were deparrafinized, rehydrated, permeabilized and reduced protein thiols blocked with N-ethyl maleamide (NEM). Sections were then subjected to GLRX-catalyzed protein cysteine labeling in order to detect regions of PSSG, as described in the text. Red = PSSG, Blue = DAPI counterstain Note the increases in PSSG in lungs from IPF patients (n = 4), compared to non-IPF controls (n = 4). This image was first published in Nature Medicine. 2018 Aug; 24 (8):1128–1135. https://doi.org/10.1038/s41591-018-0090-y. Epub 2018 Jul 9. by Anathy V et al. and was reproduced with permission from Nature Medicine [68]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
9. S-glutathionylation, the death receptor FAS, and lung fibrosis
A critical role of epithelial cell death in the pathogenesis of pulmonary fibrosis has clearly emerged. In fibrotic lung disease, including idiopathic pulmonary fibrosis (IPF), loss of or damage to distal conducting airway or alveolar epithelial cells represent common histopathological features that are believed to contribute to diminished lung function. Chronic injury leads to death of epithelial cells, and lack of normal epithelial restitution is a cardinal driving process for fibrosis, with concomitant activation and proliferation of myofibroblasts [148,149]. Numerous preclinical studies strongly support the importance of epithelial cell death per se in subsequent fibrogenesis [150]. For example, upon administration of diphtheria toxin to transgenic mice expressing the diphtheria toxin receptor in type II epithelial cells or Club cells (epithelial cells with secretory functions that exist in the proximal airways of the lung were gas exchange does not occur), marked death of these cells occurred with resultant prolonged fibrosis [151,152]. The functional role of the death receptor, FAS (also known as CD95) in the development of pulmonary fibrosis is evident from studies showing that agonistic FAS antibody (which mimics the crosslinking between FAS ligand (FASL) and FAS) induces apoptosis of bronchial and alveolar epithelial cells leading to fibrosis. Conversely, bleomycin-induced fibrosis could be prevented using soluble anti-FAS, or anti-FASL antibodies, and did not occur in mice that lack functional FAS or FASL [148,153,154]. Apoptosis of epithelial cells has been shown following co-culture with myofibroblasts isolated from patients with IPF which express FASL, while these myofibroblasts themselves are resistant to FASL-induced apoptosis [154,155]. Epithelial cell death by IPF-derived fibroblasts has been linked to H2O2 produced by myofibroblasts [156]. Low immunoreactivity of FAS was found in fibroblasts within fibroblastic foci, and upregulation of surface FAS in fibroblasts sensitized them to FASL-induced apoptosis [157]. A recent study showed that expression of protein tyrosine phosphatase-N13 mediated the resistance of human lung (myo)fibroblasts to FAS-induced apoptosis and promoted pulmonary fibrosis in mice [158]. Collectively, these findings demonstrate the importance of FAS and epithelial cell apoptosis in the pathogenesis of lung fibrosis, and suggest that avenues that dampen the extent of epithelial cell death and target the biology of the FAS receptor may attenuate fibrotic remodeling.
Work from our laboratories has demonstrated that FAS is subject to S-glutathionylation which amplifies its apoptosis-inducing function. Specifically, S-glutathionylation of FAS at cysteine 294 (murine FAS) augments the ability of FASL to induce epithelial cell death. S-glutathionylation of FAS coincided with a loss of GLRX enzymatic activity. Inhibition of caspases 8 and 3, or knock-down of caspase 8 prevented the loss of GLRX activity, FAS-SSG, and abolished cell death. Consistent with a role of S-glutathionylation of FAS in promoting apoptosis, absence of GLRX enhanced FASL-induced apoptosis, in association with more FAS accumulating in lipid rafts and FASL binding to FAS, while overexpression of GLRX protected against epithelial cell apoptosis, and diminished accumulation of FAS onto lipid rafts [159]. The demonstration that caspases 8 or 3 can directly cleave GLRX [159] suggests a protease-dependent mechanism towards inactivation of GLRX, in addition to aforementioned mechanism of oxidative inactivation of GLRX (Fig. 1).
A functional link between FAS, ER redox stress, epithelial cell death and lung fibrosis is emerging. In this regard, we have demonstrated that the molecular events that culminate in FAS-SSG originate within the ER [87]. FAS contains 24 cysteines, 20 of which occur as disulfide bridges, formed in the ER, that stabilize the receptor and enable binding of FASL. Epithelial cells were shown to contain a pool of latent FAS, not in the disulfide-bonded state. Administration of FASL triggers a calcium-dependent signal that promotes disulfide bridge formation of FAS within the ER. The protein disulfide isomerase, PDIA3 was shown to be responsible for disulfide bridge formation in FAS (FAS S–S). Absence of PDIA3, or its pharmacological inhibition, maintained FAS cysteines in a sulfhydryl state, and resulted in attenuated FAS-SSG and attenuated epithelial cell death. Incubation of cells with the sulfenic acid trapping agent, dimedone, also prevented FAS-SSG, suggesting that formation of a sulfenic acid intermediate preceded the formation of FAS-SSG. In epithelial cells stimulated with FASL, GSTP was found to bind to FAS, and interaction between FAS and GSTP was first observed in the ER-enriched fraction [87].
A role of FAS-SSG in lung fibrosis also has emerged based upon the use of lung tissues from patients with IPF. In these tissues, an interaction between FAS and GSTP was also observed [144], along with increases in FAS-SSG [68]. SiRNA-mediated ablation of GSTP or its pharmacological inhibition attenuated FAS-SSG and decreased cell death. The active GSTP inhibitor, TLK117, is an analog of GSH and the active metabolite of TLK199 has been used clinically [160,161]. TLK117 is a highly specific inhibitor of GSTP, having binding affinity greater than GSH itself and a selectivity for GSTP over 50-fold greater than other GSTs (inhibition constant [Ki] = 0.4 μM) [162,163]. TLK117 was shown to attenuate both Fas-SSG as well as cell death [87,144].
To address the role of PSSG chemistry in lung fibrosis, our laboratories conducted a number of studies to modulate GSTP and GLRX. Mice that lacked functional Gstp1 and Gstp2 were subjected to bleomycin- or AdTGFB1-induced fibrosis, and were shown to have attenuations in FAS-SSG, decreased caspases 8 and 3 activity and diminished fibrosis, compared to WT controls. Direct administration of TLK117 into airways of mice with existing bleomycin- or AdTGFB1-induced fibrosis blocked the progression of fibrosis, in association with decreases in overall PSSG, decreases in S-glutathionylation of FAS, and decreased activities of caspases 3 and 8, compared to mice receiving vehicle control [144]. The importance of PSSG in lung fibrosis was further corroborated by studies that addressed the role of GLRX, using transgenic mice overexpressing Glrx in lung epithelia, as well as mice that globally lack Glrx. Mice that lack Glrx were more sensitive to AdTGFB1- or bleomycin-induced fibrosis, whereas mice that overexpress Glrx in lung epithelial cells showed increased resistance to fibrosis. Absence of Glrx augmented FAS-SSG in mice with bleomycin- or AdTGFB1-induced fibrosis, compared to WT mice, in association with enhanced caspase 3 activities, consistent with increases in cell death. Overexpression of Glrx in epithelial cells dampened FAS-SSG and caspase 3 activation. Furthermore, ablation of caspase-8 which is activated following activation of the FAS pathway, also attenuated FAS-SSG, caspase-3 activation and fibrosis [68]. Collectively, these data demonstrate that attenuation of epithelial cell death confers protection from bleomycin- or AdTGFB1-induced fibrosis, and that S-glutathionylation of FAS in epithelial cells is an important death-inducing signal that promotes fibrogenesis. In addition to the aforementioned findings, direct administration of recombinant GLRX into airways of mice augmented GLRX activity in the lung tissue, dampened PSSG, and reversed increases in collagen content, while inducing collagenolytic activity within the lung. Similar protective effects of GLRX were observed in lungs from aging mice, which were more prone to bleomycin-induced fibrosis. A mutant of GLRX lacking cysteine 23, which is critical in the deglutathionylation reaction (Fig. 1), failed to elicit protective responses, demonstrating that the catalytic activity of GLRX is important in the anti-fibrotic responses [68]. These collective observations point to the importance not of GSH per se, but a unique facet of GSH chemistry, which involves its covalent incorporation into proteins (i.e. PSSG), catalyzed by GSTP, and reversed by GLRX, a biochemical pathway regulated by enzymes that had not previously been recognized in settings of pulmonary fibrosis (Fig. 1). Thus, the consideration of clinical studies utilizing the clinically relevant GSTP inhibitor, TLK199, or a variant thereof, in conjunction with active GLRX appears well warranted and may yield new insights into the functional importance of S-glutathionylation chemistry in the pathogenesis and progression of this deadly disease.
10. ER stress in COPD
Like IPF, activation of the UPR also has been detected in lung tissue of patients with COPD and has predominantly been associated with the emphysema phenotype [[164], [165], [166]]. Chronic ER stress was shown to promote alveolar epithelial cell death, although ER stress has also been demonstrated in airway epithelial cells, lung endothelial cells [167] and fibroblasts [168] as well. Moreover, ER stress in COPD is not limited to lung tissue, but was also shown to be involved in COPD-related comorbidities. In skeletal and respiratory muscles for instance, increased ER stress was associated with muscle dysfunction [169]. In this review only the evidence for ER stress in the lungs will be further elaborated on. The induction of the UPR has been linked with the exposure to cigarette smoke per se, as many harmful components in cigarette smoke lead to oxidative damage of proteins, which tend to misfold, aggregate and accumulate. In fact, the evidence for the presence of ER stress in COPD itself is rather limited, as more studies have examined ER stress in smokers compared to non-smokers, or in in vitro or in vivo models of smoke-exposure. Since the animal models of smoke exposure use emphysema development as their main endpoint, ER stress has been solely linked to this disease phenotype. The few studies that used patient-derived material have confirmed the link between ER stress and emphysema.
In vitro exposure of lung epithelial cells to smoke rapidly and dose-dependently activated the PERK and ATF6 arms of the UPR, whereas inconsistent induction of the IRE1α arm occurred [[170], [171], [172]]. The effects of smoke on the UPR could be largely inhibited by compounds with antioxidant-like activities suggesting the involvement of smoke-induced oxidation of target proteins [172,173]. Importantly, the vapor phase appeared to be a more potent activator of the UPR as compared to the particulate phase of cigarette smoke [171]. Acrolein, a particular component of the vapor phase, was shown to cause UPR activation and ER stress in vitro, as well as in acute and chronic exposure models in rats [174,175]. Mechanistically, other thiol-reactive compounds present in smoke, such as acrolein and hydroxyquinones were shown to oxidize and inhibit PDI located in the ER, thereby limiting protein folding [176]. When comparing the induction of the UPR by smoke in air liquid interface cultures of primary bronchial epithelial cells isolated from COPD patients compared to cells isolated from controls, it appeared that an induction of all three branches of the UPR could only be observed in cells isolated from COPD patients. Glutathione peroxidase 1 (GPX1), which is known to be deficient in COPD patients, was downregulated in the COPD-derived cultures in response to smoke [166], and a role for the tyrosine kinase SRC in promoting the accelerated decay of GPX1 mRNA in airway epithelial cells from COPD patients was shown. Chemical inhibitors of SRC prevented the loss of lung GPX-1 expression in COPD-derived epithelial cells and in response to chronic smoke exposure in vivo [177], although in this study, the impact of SRC inhibition on the UPR was not evaluated. However, the reintroduction of GPX1 in the COPD-derived cultures was sufficient to reduce the UPR. Furthermore, ER stress markers were more prominently induced by chronic smoke exposure in GPX1 deficient mice, in association with enhanced cell death, inflammation and emphysema development. These findings further confirm that an altered redox environment, possibly linked to SRC activation and loss of GPX1 is involved in the induction of the UPR in response to smoke [166]. In a variety of murine smoke-exposure models of COPD, ER stress has been documented. In mice, exposure to a single cigarette was sufficient to induce changes to the organization of the ER network, to induce phosphorylation of eIF2α, and to increase nuclear ATF6 levels [178]. ER stress was still evident when extending the exposure to 3 months or even a year [166,179]. No time-course studies have been performed to evaluate whether ER stress progressively increases with the duration of the exposure, when a maximal level is reached, or when the adaptive UPR fails and/or becomes pathological. As in in vitro experiments, curcumin, a compound with an electrophilic character present in the diet which has been attributed antioxidant-like properties in part through activation of NRF2 [180], reversed the increased expression of ER stress markers, as well as apoptosis in smoke models [181].
In addition to cigarette smoke itself, other smoke- or disease-related factors are implicated in the activation of ER stress in COPD. The depletion of calcium from the ER triggered by smoke exposure for instance possibly also contributes to UPR activation [182]. In air liquid interface cultures of epithelial cells from COPD patients, calcium release from the ER and calcium signaling were found to be disrupted, but this has not yet been linked to ER stress [183]. Factors related to mitochondrial dysfunction, of which the contribution to COPD pathogenesis and the dysregulation of epithelial cell function was recently reviewed [184], include low energy levels and mitochondrial-derived ROS. Hypoxia is another important factor in COPD that could trigger the UPR [185], but for which there is no direct evidence yet. Plasma heme, which is released from damaged red blood cells and which is a potent trigger of oxidative stress, was found to be increased in patients with very severe COPD. In a ferret model of fibrosis and emphysema induced by the inhalation of Br2, heme scavenging inhibited ER stress and the development of both pathological features of COPD, fibrosis and emphysema. Inhibition of ER stress-mediated apoptosis using salubrinal or genetic deficiency of ATF4, similarly prevented the development of fibrosis and emphysema in this injury model. Reduced numbers of macrophages and neutrophils and elastase activity were implicated in these protective effects. Collectively findings from this study shows that ER stress induced by free heme could play a role in the development of airway fibrosis and pulmonary emphysema as observed in COPD [186].
The only monogenetic cause underlying COPD is α1-antitrypsin deficiency, which leads to early onset emphysema. The liver is the main source of circulating α1-antitrypsin, a secretory glycoprotein that counterbalances trypsin activity. Interestingly, the deficiency which is caused by a lysine to glutamate 342 substitution (E342K), leads to the misfolding of α-antitrypsin and subsequently to its polymerization and hyper-aggregation. The protein can no longer be secreted and accumulates in all components of the secretory pathway, but mostly in the early part. In the liver, the proteotoxic effects of misfolded protein accumulation causes an ER storage disease. Accumulation of mutated α1-antitrypsin activates NF-κB and autophagy, but modest UPR activation [187]. In the lungs on the other hand, emphysema results mainly from the lack of trypsin inhibition and consequent excessive breakdown of connective tissue. However, small quantities of α1-antitrypsin are also produced by epithelial cells. In a mouse model of the α1-antitrypsin E342K mutation, accumulation of the α1-antitrypsin Z variant occurred also in pneumocytes, through a process similar as in the liver. In these mice the UPR was induced, and promoted inflammation [188]. Transgenic mice expressing the α1-antitrypsin Z variant also spontaneously developed pulmonary fibrosis as a consequence of the ineffectiveness of proteostasis mechanisms, which include autophagy, to counteract the proteinopathy [189]. It was furthermore shown that α1-antitrypsin mutation carriers displayed a dysregulated expression of various miRNAs that target ER protein folding responses, which was associated with enhanced expression of UPR activation markers. miR-199a-5p in particular was upregulated in asymptomatic mutation carriers, likely in response to ER stress. A downregulation was on the other hand found in symptomatic mutation carriers, which appeared to be driven by hypermethylation of the miR-199a-5p promotor. Importantly, overexpression of pre-miRNA-199a-5p significantly reduced Bip, ATF6, CHOP, GADD34 and XBP1 levels in monocytes. RELA expression was also attenuated, in concert with lower LPS-induced production of various inflammatory cytokines [190]. In COPD, as in IPF however, miR-199a-5p was upregulated [191], but to date its association with ER stress has not been examined.
Triggers of COPD exacerbations, including viral and bacterial infections, are also known inducers of ER stress and might further amplify existing stress [166,192,193]. A normal ER stress response is required for an effective innate immune response to infection, but in COPD the increased susceptibility to infection and defective clearance mechanisms might be related to the pre-existing aberrant stress responses [194,195]. As was described above, dissociation of the chaperone Bip from the ER luminal surface triggers the dimerization and autophosphorylation of the triad of UPR sensors PERK, ATF6 and IRE1α. In small airway epithelial and type II alveolar epithelial cells of lung tissue of smokers, in rats, as well as in epithelial cells exposed to smoke in vitro, Bip expression was found to be increased in association with ER stress [179,196,197]. In lung tissue, Bip was present in higher levels in alveolar epithelial cells and macrophages from emphysema patients compared to controls [164]. Bip could furthermore be detected in BALF, and levels were shown to be elevated in smokers. The secretion of Bip occurs through a non-classical mechanism, and could be induced from human airway epithelial cells by smoke exposure, by pharmacologically-induced ER stress, as well as inhibition of histone deacetylases (HDAC) [198]. Increased levels of Bip have also been reported in plasma and serum of subjects with COPD, and are considered a potential biomarker for the disease, correlating with disease severity and the degree of emphysema [186,199]. Whereas Bip located in the ER acts as the main trigger that activates the three UPR sensors, extracellular Bip exerts anti-inflammatory effects by augmenting the release of anti-inflammatory molecules, such as IL1RA and soluble TNFα receptor II [200]. In addition to increased levels of Bip, plasma of COPD patients was found to contain anti-Bip IgG autoantibodies. Bip is an antigen that has been associated with autoimmune diseases [201]. A specific association with the presence and severity of emphysema was noted for these auto-antibodies in COPD, as for the auto-antigen itself. The auto-Bip antibody increased baseline production of various inflammatory mediators by macrophages, and treatment of PBMCs from patients in whom auto-antibodies were measured, with recombinant Bip induced CD4+ T-cell proliferation [164]. This finding potentially connects ER stress to the paradigm of an autoimmune component of COPD development. It is furthermore of interest that the HLA-DRB1*15 haplotype appears to protect smokers from auto-Bip antibody production, whereas the haplotype is overrepresented in IPF patients with anti-HSP70 autoantibodies [202]. Disease-specific reactions seem thus to underlie these auto-immune responses.
In lung tissue of smokers, increased protein expression of PDI was observed compared to non-smokers, likely as an adaptive response to smoke exposure. It is not known if this adaptive response fails in smokers who develop COPD. In mice, exposure to a single cigarette did not affect PDI expression, whereas protein expression was increased in airway lining cells when exposure was extended to 6 weeks. This was not found to be related to enhanced PDI mRNA levels. Moreover, a reduced turnover rate of PDI protein is unlikely as PDI is a long-lived protein. It was speculated to occur rather through preferential loading of PDI mRNA into ribosomes, as was reported for other effectors of the ER stress response [203]. On the other hand, the increased protein level of PDI in a smoke-exposure model of COPD was associated with high levels of oxidized, including sulfenic acid-containing forms of the protein. These observations are in line with the reported alkylation of PDI by acrolein and hydroxyquinones described above. PDI oxidation was furthermore found to progressively increase with age. The consequent inhibition of its reductase and isomerase activity would limit its capacity to restore ER homeostasis, and potentially contribute to fueling chronic ER stress responses [176,178] (Table 2).
Table 2.
Implications of ER stress in COPD.
| ER stress features | Putative function | Model | References |
|---|---|---|---|
| PDI | Inhibition and oxidation of PDI by cigarette smoke, diminished protein folding Increased expression reflecting potential adaptive response |
Mouse models In vitro Humans |
[176,178] |
| Bip | Increased in smokers and COPD; Extracellular BiP: Anti-inflammatory effect Anti-BiP antibodies: Linked with enhanced inflammation, emphysema and auto-immunity |
Humans Human airway epithelial cells, lung lavages Humans Mouse model |
[164,186,[197], [198], [199],201] |
| PERK/ATF6 | Induced by cigarette smoke vapor phase, acrolein, hydroxyquinones Impact: adaptive response or cell death |
Cell lines Human airway epithelial cells |
[[170], [171], [172],175] |
| UPR | Induced by cigarette smoke in epithelial cells from COPD patients Impact: cell death, regulated by GPX1 |
Patient-derived cells | [166] |
| ATF4/ER stress | Induced by free heme following Br2 inhalation Impact: Emphysema and fibrosis |
Mouse and ferret models | [186] |
| ATF4/PERK | mutant alpha 1 anti-trypsin > Inflammation | Mouse model Human lung explants from COPD patients |
[188] |
| Bip/ATF6 | Controlled by miRNA-199a-5p protease activation > inflammation, fibrosis and emphysema | Blood monocytes from patients with αanti-trypsin mutation | [190] |
Because of the chronic nature of the insult, and the self-perpetuating mechanisms of injury that occur in COPD, the UPR is unable to repair or dispose of damaged proteins through the ERAD. Likewise, other proteostasis processes such as autophagy fail. The processes of ER stress and autophagy have both been found to contribute to COPD and are interrelated. For instance, inhibiting autophagy in a murine smoke model was shown to limit apoptotic cell death and attenuated the expression of ER stress proteins PERK and CHOP. In concert, this led to limited emphysema development as well as attenuated bronchial wall thickening [181,204]. Failure of these adaptive mechanisms results in aberrant cell functioning or excessive cell death. ER stress in response to smoke indeed contributes to epithelial cell death and this has been put forward as a mechanism underlying emphysema development. In primary alveolar epithelial cells smoke-induced apoptotic cell death was associated with caspase 12 activity, CHOP expression, and phosphorylation of PERK, eIF2α and IRE1. These effects could be attenuated by overexpression of HERD1, a specific ubiquitin E3 ligase located in the ER and part of ERAD [205]. Other reports also demonstrated that a dysfunction of the proteasomal system in smoke-exposed conditions contributed to ER stress and excessive epithelial cell death [206]. Inhibition of ER stress in smoke-treated epithelial cells and a mouse model using the chemical chaperone, 4-phenylbutyric acid (4-PBA), reduced the amount of epithelial cell death and inflammation, and could partially suppress emphysema development [179]. Protection against ER stress and consequent cell death therefore provides a plausible strategy to prevent the development of emphysema (Table 2).
In addition to inducing cell death, ER stress results in pro-inflammatory signaling which is demonstrated in many of the COPD-related studies described above. Activation of antioxidant defense via concerted actions of NRF2 and ATF4, fails in COPD because of defects in NRF2 signaling, further discussed below. A role for ER stress in the induction of airway fibrosis in COPD is inferred from only a limited number of studies conducted so far. Likewise, it has not been examined whether ER stress in airway epithelial cells could contribute to airway features of COPD, such as mucus metaplasia and ciliary dysfunction. In this regard, a recent study demonstrated that cigarette smoke exposure caused internalization of cystic fibrosis transmembrane conductance regulator (CFTR) and promoted its retrograde trafficking to the ER. Inhibition of CFTR has been linked to decreases in airway hydration and mucus stasis in COPD patients [207].
11. COPD and S-glutathionylation
Oxidative stress is considered a major driver of the development of COPD [208]. Cigarette smoke itself of course contains a myriad of oxidants and the state of chronic inflammation further challenges the pulmonary anti-oxidant defenses. In recent years, the contribution of NADPH oxidases in epithelial cells to the local redox imbalances have furthermore become known. The downregulation of DUOX1 in patients with COPD [209], for instance could contribute to diminished epithelial responses to injury [210]. Furthermore, increased NOX4 levels co-localized with αSMA and TGFβ in airway smooth muscle cells, and correlated with small airway remodeling and COPD disease severity [211,212]. As part of the adaptive response to these oxidant exposures, GSH levels increase in smokers and in early stages of COPD. Increased GSH levels were shown in epithelial lining fluid, sputum, as well as plasma of COPD patients compared to controls [129]. GSSG was elevated as well in sputum, indicating a more oxidizing environment of the lungs [213]. The excess oxidant levels are indeed insufficiently counteracted by the pulmonary defense systems. The antioxidant defenses naturally decline with aging [214], and GSH is not an exception to this phenomenon [215,216]. In COPD, NRF2 constitutes an important mechanism underlying the failing antioxidant defense. Indeed, lower levels of NRF2 were present in lung tissue of emphysema patients, in association with a disequilibrium between NRF2 and its inhibitors KEAP1 and BACH [217]. Moreover, genetic ablation of NRF2 enhanced the susceptibility to smoke-induced emphysema in mice [218]. Pharmacological activation of NRF2 on the other hand attenuated emphysema development in mice [219]. Short duration clinical trials have however not been successful at even restoring NRF2-target gene expression or inflammation [220]. Prior to targeting NRF2, clinical trials have examined the therapeutic potential of increasing GSH levels in COPD. The GSH precursor, NAC, the main compound investigated in most studies failed to demonstrate clinical improvement [[221], [222], [223], [224], [225]]. However, a meta-analysis showed that NAC, and related compounds erdosteine and carbocysteine, lowered the number of disease exacerbations, their duration and severity [226]. These effects are however likely to be effectuated though NACs mucolytic activity, which involves reducing cysteine oxidations in mucin polymers increasing their solubility, rather than its GSH precursor function per se [227].
In contrast to the large number of studies that have examined oxidative stress and GSH responses in general in COPD, the potential importance of PSSG and its regulatory enzymes is only recently emerging. GLRX protein levels were decreased in lung tissue of patients with COPD, paralleling decreases in the number of GLRX-positive macrophages. Macrophages were shown to be the main cell type expressing GLRX and the number of GLRX-positive macrophages positively correlated with lung function. Furthermore, during disease exacerbations, significantly increased GLRX protein could be detected in sputum [146]. Our unpublished observations confirm decreases in protein level of GLRX in lung tissue of COPD patients, which was also found to correlate with lung function (N.L. Reynaert). Moreover, the lower protein level of GLRX was associated with attenuated GLRX activity and overall increased PSSG in lung tissue of COPD patients compared to smoking controls. Other unpublished observations confirm the increased level of GLRX in sputum during a disease exacerbation in COPD patients, which was also associated with a higher GLRX activity level and an attenuated level of sputum PSSG. Although we were unable to determine the cellular source of GLRX in sputum supernatant, there was a positive correlation with the total number of viable cells present in the sputum (N.L Reynaert, unpublished). A non-classical export mechanism was proposed for GLRX [228], which remains to be further elucidated, as is the cellular source, as well as its particular functions.
Contrasting aforementioned findings with GLRX, GSTP1 levels were found to be increased in lung tissue lysates of patients with mild COPD. However, using immunohistochemistry methods, no differences were observed when examining GSTP1 positive cell populations between controls and COPD patients. Like GLRX, GSTP1 could be detected in sputum, but here the levels were not different between patients and controls [229]. The causal role of GSTP in COPD pathogenesis has been investigated. Deletion of GSTP1 in fibroblasts induced apoptosis [230], whereas overexpression protected against smoke-induced cell death [230], findings that contrast the aforementioned death promoting effects of GSTP in FASL-exposed lung epithelial cells [87]. At a genetic level, GSTP1 has been studied in COPD more frequently, because it is thought that variations in the gene could contribute to COPD susceptibility by attenuated detoxification of, and antioxidant protection against cigarette smoke and associated products. The GSTP1 I105V polymorphism in particular has been studied frequently in COPD, but a recent meta-analysis concluded that that there is no significant association between this polymorphism and disease risk [231]. No studies to date have examined the putative protective role of GSTP1 to smoke-induced emphysema, nor has GSTP1 been related to PSSG in this context.
Exposure of lung epithelial cells to cigarette smoke extract reduced GLRX protein levels and activity, and increased PSSG [232]. In mice, exposure to cigarette smoke also reduced GLRX expression and activity, but here PSSG was decreased, as were free protein thiol levels, with a concomitant increase in protein carbonylation [233]. In bronchoalveolar lavage fluid, as well as in macrophages isolated from smoke-exposed mice, an increase in PSSG was however observed [232]. PSSG seems thus to be distinctly regulated in different regions and cellular compartments of the lungs in response to cigarette smoke. Importantly, recombinant GLRX was found to be irreversibly oxidized and inhibited by the electrophilic compound acrolein present in smoke [232]. Extracellular GLRX could be more prone to such modification by smoke. Overall, the attenuated activity of GLRX in lung tissue of COPD patients, and in lungs and cells treated with cigarette smoke is likely the result of decreased mRNA expression in combination with smoke-induced post-translational modifications of the protein.
GLRX and PSSG play a role in regulating epithelial cell death in response to cigarette smoke. Overexpression of GLRX protected epithelial cells from smoke-induced cell death, which was associated with attenuated PSSG, while the converse was observed in the absence of GLRX [232]. The mode of cell death triggered by smoke was not specifically examined and could involve ER stress. In mice, the absence of Glrx decreased neutrophil influx into lung tissue in response to 4 weeks of cigarette smoke exposure, whereas the number of macrophages was increased. Bronchoalveolar lavage fluid of these mice contained lower KC levels and epithelial cells from Glrx −/− mice produced less KC in response to smoke as well [232]. Macrophages from GLRX −/− mice were however smaller, expressed lower levels of PU.1 and showed a diminished phagocytic capacity, and lower inflammatory responses to LPS [234]. Another study on the other hand found that 3 days after smoke exposure the neutrophil influx was increased in Glrx−/− mice, with a concordant increase in pro-inflammatory cytokines [235]. These contrasting results are likely related to the different durations of the exposure to cigarette smoke. Interestingly, the protective effects of the absence of Glrx on smoke-induced inflammation we showed using a 4 week exposure regimen is in line with our previous study in which the absence of Glrx in epithelial cells, through enhanced protein S-glutathionylation of IKKβ was shown to limit the activation of NF-κB in response to both TNFα or LPS [236]. Conversely, in the 3 day exposure regimen NF-κB activity was enhanced in Glrx-deficient mice, in association with S-glutathionylation of IKKα as well as IKKβ [235]. The duration of the exposure to smoke thus also seems to divergently affect the regulation of NF-κB by the GLRX/PSSG axis, which should be examined in more detail. It has not been examined whether Glrx −/− mice are be more or less susceptible to smoke-induced emphysema. The positive correlation between GLRX and lung function reported in the clinical studies points to a putative protective role of GLRX towards COPD, a scenario supported by the demonstrated protection that GLRX confers against smoke-induced cell death, which is an important driver of emphysema development.
When considering additional pathways that could be involved in COPD pathogenesis through differential S-glutathionylation, neutrophil migration and phagocytic capacity are prominent candidates. Negative influences of PSSG were shown on actin polymerization in neutrophils, which limited migration and phagocytic capacity [237,238], effects which were exacerbated in Glrx-deficient neutrophils [238]. Conversely, S-glutathionylation of α4 integrin positively affected the migration of neutrophils by increasing the binding to VCAM1 on endothelial cells, thereby increasing the egression of neutrophils from the bone-marrow [239]. Glrx-deficient neutrophils in addition showed defects in formation of neutrophil extracellular traps (NET). In COPD, NET formation was increased and implicated in the enhanced susceptibility of patients to develop disease exacerbations [240]. Pathogens that colonize the airways of COPD patients on the other hand can evade trapping and killing in NETs. A bacterial peroxiredoxin-glutaredoxin bifunctional enzyme in nontypeable Haemophilus influenzae has been shown to promote such resistance and to be involved in the colonization and recurrence of infections by this pathogen in COPD [241]. It remains to be determined to what extent PSSG and the regulation by GLRX is involved in the dysregulation of neutrophil trafficking and functioning in COPD. Therefore, the GLRX/PSSG axis in pathogens should not be overlooked as a potential contributor to COPD pathogenesis and progression.
Lastly, S-glutathionylation of α1-antitrypsin was shown to attenuate its anti-protease function, whereas S-glutathionylation of MMP-1,-8 and -9 was associated with enhanced activity [242]. If α1-antitrypsin and MMPs were found to be more S-glutathionylated in lung tissue of COPD patients, this scenario would have the potential to contribute to enhanced tissue destruction by disrupting both sides of the protease-antiprotease imbalance.
12. The unfolded protein response and protein disulfide isomerases in allergic asthma
Like IPF and COPD, activation of the UPR also has been observed the airway epithelium of patients with asthma and indicates that ER stress also accompanies asthma. Activation of the UPR transducer, IRE1β, subsequent XBP-1 splicing and transcriptional increases of chaperone anterior gradient homolog 2 (AGR2, also known as PDIA17) were shown to be important for mucin production in human primary bronchial epithelial cells [79,243] in addition to the aforementioned role of XBP-1 in the transcriptional upregulation of MUC5B relevant to IPF [92]. The increases in expression of IRE1β in human asthmatic epithelium as compared to the non-asthmatic epithelium were also correlated with increased mucus production and goblet cell metaplasia [79]. Consequently, depletion in IRE1β or deletion of AGR2 significantly attenuated mucus metaplasia in a mouse model [79,243] and suggests that the IRE1β axis is important in allergen-induced mucus metaplasia in the lung epithelial cells.
Genetic association studies have linked polymorphisms of Orosomucoid-like (ORMDL)3 with asthma [[244], [245], [246]]. ORMDL3 is an ER–localized protein with homologs in yeast that decreases the activity of serine palmitoyltransferase thereby inhibiting de novo sphingolipid biosynthesis [246]. Expression of ORMDL3 was increased in mouse models of allergic asthma [247] and ORMDL3 over-expressing mice showed selective activation of ATF6 in association with increases in airway remodeling, including increased airway smooth muscle, subepithelial fibrosis, and mucus metaplasia [247]. Furthermore, ATF6 was found to regulate airway hyperresponsiveness, smooth muscle proliferation and contractility in a mouse model of asthma [248]. In addition to ATF6, allergic asthmatics as well as mouse models of asthma exhibited increases in expression of CHOP [249]. CHOP in association with ATF6 exacerbated allergic airway inflammation by enhancing M2 programming in macrophages in a mouse model of asthma [249]. These reports collectively highlight that the canonical UPR transducer pathways impact various facets of allergic asthma [250] and have led to the hypothesis heterogeneous severe asthma could be potentally be endotyped based upon the UPR, moving beyond type 2 high/low paradigm [[251], [252], [253]] (Table 3).
Table 3.
Implications of ER oxidoreductases and/or ER stress in asthma.
| ER oxidoreductase | Putative function | Model | References |
|---|---|---|---|
| PDIA3 | Apoptosis, chemokine/growth factor folding | Cell culture, mouse models, human epithelial cells | [254,256] |
| AGR2 (PDIA17) | Mucus metaplasia | Cell culture, mouse models, human epithelial cells | [243] |
| IREβ | Mucus metaplasia linked to upregulation of XBP1 and AGR2 | Cell culture, mouse models human samples | [79] |
| XBP1 | Mucus, Eosinophil differentiation, type II inflammation and IFN-related gene expression | Cell culture, mouse models, human samples | [243,250,304] |
| ATF6α | Linked to OMRDL3 and SERCA2b, macrophage activation, epithelial cell death airway hyperresponsiveness, smooth muscle hypertrophy | Cell culture, mouse, human samples | [247,248,256,305] |
| CHOP | IL-4/macrophage signaling, ATF6 expression | Cell culture, mouse, human samples | [249] |
PDIs are upregulated in asthma, however their function in disease pathophysiology is not well understood [254,255]. We have identified that increases in expression of various PDIs correlated with a higher bronchodilator response or blood eosinophilic counts in allergic asthmatics, indicative of worse disease [254,255]. The protein disulfide isomerase A3 (PDIA3) can associate with Bak and promote its oligomerization thereby triggering intrinsic apoptosis under conditions of ER stress. In a house dust mite model of allergic inflammation, genetic ablation of PDIA3 specifically in lung epithelial cells decreased proapoptotic Bak oligomerization, and caspase-3 activity in mice, in association with decreases in airway fibrosis [254], and similar results were obtained following siRNA mediated ablation of ATF6-α [256]. Ablation of PDIA3 resulted in more exposed sulfhydryls (-SH) in the glycoproteins periostin and epidermal growth factor (EGF), both of which are involved in airway remodeling, suggesting a potential mechanism whereby PDIA3 promotes airways remodeling. PDIA1, PDIA5, PDIA6, and ERp18 are known to modulate the activity of PERK, ATF6 and IRE1 through oxidoreductase activities in various biological settings [[257], [258], [259], [260]]. In addition to PDIA3, we found that both PDIA5 and PDIA6 were also upregulated in epithelial cells from allergic asthmatics and airway epithelial cells using mouse models of asthma [255]. Furthermore, in mouse models of asthma, therapeutic administration of the UPR modulators, tauroursodeoxycholic acid (TUDCA) or Alanine β-Muricholic acid (AβM), decreased the UPR and subsequently decreased PDIA5 and PDIA6 expression [255]. Furthermore, these decreases were associated with an attenuation of various parameters of allergic airway disease in mice. Retrospective analysis of patient data revealed that increases in PDIA5 significantly correlated with increased eosinophilic and diminished bronchodilator response in allergic asthmatics, again being indicative of worse disease [255].
To date, no FDA-approved therapeutics are available to inhibit PDIs; however, decades of research from various laboratories have identified numerous inhibitors that have shown in vivo and in vitro efficacy in inhibiting PDIs. Interestingly, rutinosides (plant flavonoids) that are known to inhibit PDIs are now being used in different clinical studies [261]. It also is interesting to note that Kaplan et al. have identified LOC14 as a specific inhibitor of PDIA1 and PDIA3 [262]. These observations suggest that the UPR and subsequent induction of PDIs regulate the pathophysiology of asthma, among other diseases, and that inhibition of an oxidoreductases such as PDIs may be a potential therapeutic approach that would benefit patients with chronic lung diseases (Table 3). One of the modulators of UPR, TUDCA is being increasingly used in clinical studies (Clinicaltrials.gov) for various indications. A few studies show that TUDCA administration is safe in humans and potentially effective in alleviating symptoms in patients with amyotrophic lateral sclerosis [263](NCT00877604), and in increasing insulin sensitivity in obese men and women [264](NCT00771901). These results suggest that modulating the UPR may have potential beneficial effects in neuro-degenerative and metabolic diseases. Therefore, future studies using TUDCA in patients with chronic lung diseases appear well warranted.
13. Glutathione biochemistry in asthma
Many studies have addressed potential alterations in GSH redox homeostasis in patients with asthma, and these have been summarized in several recent reviews [33,265,266]. Not surprisingly, given the high diversity in asthma subtypes and severity, different methodologies to measure GSH and/or GSSG, and variable impact of corticosteroids or other medications, reported values vary dramatically [265]. Some reports indicate that total levels of GSH are increased in asthmatics, which has been attributed to increased oxidative stress and resultant adaptive responses in asthmatic patients [266], for example by activation of NRF2 and induction of genes involved in GSH biosynthesis [219,267]. However, increased activation of NRF2 pathways in severe asthmatics was not associated with increased GSH or cysteine levels in either BAL or plasma, and GSH redox status was in fact more oxidizing, suggesting dysregulation of NRF2 signaling [268]. The importance of cellular GSH status in asthma pathology has been supported by animal studies showing that disruption of cellular cysteine uptake by deletion or inhibition of GGT [269,270], or inhibition of GSH synthesis using buthionine sulfoximine [271], worsened allergen-induced airway reactivity and/or inflammation, and have promoted studies with GSH administration or precursors such as NAC to alleviate disease phenotypes, with variable success [271]. GSH may also be protective in asthma based on its involvement in formation of nitrosoglutathione (GSNO), which acts as endogenous bronchodilator [272]. In fact, GSNO levels were found to be decreased asthmatic airways compared to airways of healthy subjects, which was related to increased expression of GSNO reductase (GSNOR) [273]. Mice lacking the Gsnor gene showed increases in S-nitrosothiols and were protected from airways hyperresponsiveness in models of allergic asthma [274], prompting development of GSNOR inhibitors as novel therapeutic agents in therapeutic management of asthma [275]. Other biochemical forms of GSH, such as LTC4 and related cysteinyl leukotrienes have been found to be elevated in asthma, and are thought to contribute to asthma pathology due to their broncho-constrictive properties [276]. Redox alterations in asthma are also manifested in the form of enhanced disulfide cross-linking in mucus proteins, by oxidative pathways likely involving eosinophil activation, thereby contributing to formation of mucus plugs and airflow obstruction [277]. The beneficial effects of thiol-based antioxidants such as N-acetylcysteine, often thought to act by fueling GSH synthesis, likely involve its ability to break such disulfide bonds and act as a mucolytic, and novel strategies are being developed to promote such a mucolytic action [227].
Alterations in some GSH-dependent enzymes have also be documented in asthma. For example, various isoforms of glutathione peroxidase, critical in GSH-mediated detoxification of H2O2 or related peroxides, are upregulated rodent models of allergic airway disease or in patients with asthma [278,279]. Glutathione S-transferases have also been implicated in the pathogenesis of asthma, and common genetic variants of a number of GSTs have been linked asthma, with variable correlations to disease severity [280,281]. This variability likely relates to the diverse functions of GSTP1, as well as the varying asthma endotypes. GSTP1 may be protective against environmentally-induced or occupational asthma by contributing to metabolism of environmental toxicants, but may also enhance features of asthma through its role in protein S-glutathionylation (see below). Interestingly, GSTP1 has also been reported to enhance the proteolytic activity of the HDM allergen Der p1, and may thereby enhance allergen-induced immune responses [282]. Gstp1 was found to be downregulated in mouse models of allergic inflammation and in children with asthma [283], and studies in mice showed that Gstp1 deletion enhanced OVA-induced allergic airways inflammation and hyperresponsiveness in mice, and also enhanced indices of collagen deposition and smooth muscle proliferation [284]. However, it is unclear to what extent these latter findings are relevant for alternative models of asthma and exacerbations, or whether these effects of GSTP1 are related to S-glutathionylation.
Relevant to the main topic of this review, emerging evidence also suggests changes in protein S-glutathionylation in asthma. Analysis of sputum samples from asthmatics revealed a decrease in PSSG compared to controls, which was attributed to increased levels of extracellular GLRX and increased GLRX mRNA in bronchial epithelial cells [285]. However, studies of nasal brushings from asthmatic patients indicated a decrease in GLRX mRNA expression [286], possibly reflecting the different sites of sampling. Studies in mouse models of allergic airway disease indicate increased Glrx mRNA during allergic inflammation induced by ovalbumin (OVA) or house dust mite (HDM), whereas Glrx2 mRNA was unchanged. In spite of these increases in GLRX, overall lung tissue levels of PSSG were also increased in models of allergic asthma [147,287,288]. Evaluation of temporal changes of GLRX, GSH and PSSG in airways and lung tissues from mice subjected to the OVA model showed transient increases in BAL GSH and GLRX protein after OVA challenge, and more persistent increases in lung tissue PSSG and GLRX. Observed increases in GLRX levels in the BAL were found to positively correlate with increases in cytokines [288], suggesting a link between GLRX and airway inflammation [288,289]. Studies with Glrx deletion showed somewhat disparate findings, depending on the mouse strain and allergen model used. OVA-induced allergic airway inflammation and remodeling in BalB/C mice developed similarly in Glrx−/− mice but appeared to resolve faster [288], suggesting that PSSG may actually contribute to resolution of allergic airways disease. In a HDM-induced model of allergic airways disease, different patterns of inflammatory profiles and cytokine secretion were observed in BalB/C Glrx−/− mice, with a decrease in Th2 cytokines and airway eosinophils, but an increase in airway macrophages and neutrophils, which was associated with increased production of the Th17 cytokine, IL17. Glrx−/− mice displayed altered profiles of airway hyperresponsiveness in this model, with increases in central airway resistance but with dampened peripheral elastance in response to HDM, indicating variable impact of GLRX and PSSG on airway and parenchymal lung function [287,288].
While the critical protein targets for S-glutathionylation in the context of allergic airway inflammation have not been identified, several proteins relevant for asthma pathobiology are known to be regulated by S-glutathionylation. Among these are proteins involved in the NF-κB signaling pathway, which plays a central role in immune responses and inflammation [290] and in asthma pathophysiology [291,292]. As was described in the previous section, S-glutathionylation can regulate the NF-κB pathway at multiple levels, and S-glutathionylation of IKKβ, IKKα and RelA have been reported, in association with decreases in or alterations of expression various epithelial cell-derived pro-inflammatory mediators in epithelial cells exposed to LPS, TNFα or IL17A [236,293,294]. The adaptor protein, MyD88 adaptor-like (MAL or TIRAP), critical in signaling induced by various Toll-like receptors, can also be targeted by S-glutathionylation of Cys91, which appears to enhance pro-inflammatory signaling [295]. A recently identified redox-based mechanism relevant to asthma pathology involves epithelial secretion of the alarmin IL33, as an innate response mechanism to allergens in activation of type 2 cytokines such as IL-13, and subsequent mucus metaplasia and fibrotic remodeling. Allergen-induced secretion of epithelial IL33 was found to require activation of the H2O2 producing NADPH oxidase homolog dual oxidase 1 (DUOX) [296]. Epithelial DUOX1 expression and activation is enhanced in the airways of asthmatic subjects, in associated with increased cysteine oxidation in tyrosine kinases such as SRC and EGFR [296,297], which have been previously found to be involved in asthma pathology. Cell-based studies indicate that DUOX1 activation can promote S-glutathionylation of several target proteins [298], including SRC and EGFR [299], but their activation is enhanced by oxidation of conserved cysteines within their kinase domains to a sulfenic acid, whereas the importance of S-glutathionylation for activation of these kinases is less clear [300,301]. Induction of IL33 in macrophages is also subject to regulation by S-glutathionylation, based on observations that LPS-induced increases in IL33 mRNA is diminished in macrophages lacking Glrx, in association with enhanced S-glutathionylation of TRAF6 resulting in attenuated NF-κB activation. Conversely, IL33 was found to enhance Glrx induction in response to cockroach, based upon observations demonstrating that increases in Glrx were attenuated in lungs from mice lacking IL33 [302]. Collectively, these findings indicate reciprocal actions of GLRX and IL33 in settings of allergen exposure and suggest a role of GLRX and PSSG in the regulation of type 2 responses in asthma in part via the regulation of IL33. Lastly, in addition to regulating inflammatory signaling pathways in diverse ways, S-glutathionylation can also directly regulate the biological activity of several asthma-relevant cytokines, such as IL1β, IL6, or IL17 [303]. For example, the activity of IL1β can be inhibited by overoxidation of its Cys188, and S-glutathionylation of Cys188 protects against such inhibition and helps sustain IL1β activity [303].
Collectively, these various findings illustrate diverse mechanisms by which S-glutathionylation can impact inflammatory signaling and other signaling pathways that contribute to asthma pathobiology, which likely depends on cell-specific actions of different oxidant sources (e.g. NOX enzymes, mitochondria, ER stress).
14. Concluding comments and challenges for future research and development
Although the etiology of IPF, COPD and asthma highly complex, in the present review we highlighted the importance of altered GSH redox homeostasis and ER stress, particularly in the development of fibrotic remodeling that is common to all three diseases, despite differences in the anatomical location of fibrosis in asthma/COPD (airways) and IPF (parenchyma). We presented arguments that avenues to normalize redox homeostasis warrant targeted approaches to alleviate ER stress and/or activation of PDIs, dampen oxidant production from mitochondria, or NOX enzymes such as NOX4 or DUOX1, as these diverse processes provide important sources of oxidants in settings of chronic lung diseases (Table 4). The demonstration of increased protein S-glutathionylation, related to altered expression and/or enzymatic function of GSTP or GLRX, points to the importance not of GSH per se, but a unique facet of GSH chemistry, protein S-glutathionylation, which involves its covalent incorporation into proteins, catalyzed by GSTP, and reversed by GLRX. The present review highlights the importance of S-glutathionylation as a critical enzymatically-controlled biochemical pathway that had not previously been recognized in settings of chronic lung diseases, and that is amenable to unique therapeutic intervention, although alternative redox-based thiol modifications may also be important in these pathologies.
Table 4.
Proposed development of GSH-based/redox therapeutics for chronic lung diseases.
|
|
|
|
|
Note: TLK199 and TUCDA have already been used clinically, but the undesirable intellectual property domain along with concerns for future profit margins makes it unattractive to invest in costly clinical trials and re-development of these compounds.
A number of signaling molecules, structural proteins, ER resident proteins, mitochondrial proteins, the death receptor Fas, proteases, the protease inhibitor A1AT, and cytokines are targets for S-glutathionylation, and therefore have the potential to affect cell death, migration, mitochondrial function, release of alarmins, innate and adaptive immune responses, epithelial and immune cell activation, and protease activities. Many of these aforementioned processes are involved in or altered in IPF, severe asthma and COPD (Fig. 5). Therefore, additional translational studies to elucidate the exact PSSG targets in these diseases will be critical. Notably redox proteomics studies to unravel alterations in the oxidized cysteine proteome including the S-glutathionylated proteome remain to be performed in lung tissue specimens or epithelial and other cells directly isolated from patients. Our hope is that the recent discoveries pertaining to glutathion(e)ylation chemistry provide a rapid path to drug development. The consideration of clinical studies utilizing the clinically relevant GSTP inhibitor, TLK199, or a variant thereof, potentially in conjunction with local administration of recombinant active GLRX, appears well warranted and may yield new insights into the functional importance of S-glutathionylation chemistry in the pathogenesis and progression of chronic lung diseases. Similarly, approaches to inhibit PDI, NOX4 and DUOX1 also come to the forefront as new avenues to treat chronic lung diseases, based upon the compelling observations made in pre-clinical models (Table 4). We are hopeful that such strategies, perhaps in conjunction with standard of care treatment, have the potential to increase the survival and quality of life of the millions of patients living with chronic lung diseases.
Fig. 5.
Illustration highlighting the defining histopathological features of fibrosis, COPD and asthma, and redox-controlled events that may control these disease manifestations. Healthy: Normal lung tissue depicted with healthy bronchiole, alveolar ducts and alveoli. Fibrosis: Shown is the honey-comb appearance visualized by CT cans. Also shown are the disruption of alveolar structures. The death receptor Fas, a critical player in epithelial cell death as well as lung fibrosis is S-glutathionylated in the ER. NOX4 derived from fibroblasts is one of the oxidant sources important in fibrosis. COPD: COPD classically has been linked to inhalation of cigarette smoke or other environmental pollutants, although it also is linked to α1 antitrypsin deficiency. The bronchioles of COPD patients are inflamed and neutrophils are depicted. Mucus plugging and subepithelial collagen deposition also are features of COPD. Narrowing of small airways and loss of alveoli lead to gas trapping and poor oxygen exchange. An imbalance in protease-antiprotease activity is believed to contribute in part to alveolar destruction. Pollutants in cigarette smoke induce ER stress, linked to alkylation of PDI. The UPR reflects an adaptive response that in some cases contributes to epithelial cell death. Mutant α1 antitrypsin also induces the UPR. Oxidation of the proteases, antiproteinases contribute to their imbalance and resultant tissue destruction. Asthma: Depicted is an inflamed airway, characterized by increases in eosinophils, and mucus plugging in type-2 high asthmatics. An expansion of smooth muscle cell mass contributes to constriction of the airways. Severe asthma also is characterized by sub-epithelial fibrosis. ER-redox processes are implicated in mucus metaplasia, cytokine disulfide content, and type-2 responses. Oxidation of mucins contributes to persistent mucus plugging. Although numerous oxidation targets have been identified in cell cultures and mouse models, for the most part these remain to be verified in human lung tissues. We refer the reader to the text for more detailed information.
Declaration of competing interest
Yvonne Janssen-Heininger, Niki L. Reynaert, and Vikas Anathy hold patents: United States Patent No. 8,679,811, “Treatments Involving Glutaredoxins and Similar Agents” (YJ-H, VA), United States Patent No. 8,877,447, “Detection of Glutathionylated Proteins” (YJ-H, NLR), United States Patent, 9,907,828, “Treatments of oxidative stress conditions” (YJ-H, VA) Yvonne Janssen-Heininger and Vikas Anathy have received consulting fees from Celdara Medical LLC for their contributions with the commercialization of glutaredoxin for the treatment of pulmonary fibrosis.
Acknowledgements
This work was supported by grants NIH R35HL135828 (Y-JH), NIH R01HL137268, R01HL085646, R01HL138708, R21AG055325 (AvdV) and NIH R01HL122383, R01HL141364 and RO1HL136917 (VA). The authors thank Dr. Isabelle Tian for creating Fig. 5.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101516.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bustamante-Marin X.M., Ostrowski L.E. Cilia and mucociliary clearance. Cold Spring Harbor Perspect. Biol. 2017;(4):9. doi: 10.1101/cshperspect.a028241. Epub 2016/11/20, PubMed PMID: 27864314; PMCID: PMC5378048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiemstra P.S. Parallel activities and interactions between antimicrobial peptides and complement in host defense at the airway epithelial surface. Mol. Immunol. 2015;68(1):28–30. doi: 10.1016/j.molimm.2015.07.030. Epub 2015/11/26, PubMed PMID: 26597202. [DOI] [PubMed] [Google Scholar]
- 3.van der Vliet A., Danyal K., Heppner D.E. Dual oxidase: a novel therapeutic target in allergic disease. Br. J. Pharmacol. 2018;175(9):1401–1418. doi: 10.1111/bph.14158. Epub 2018/02/07, PubMed PMID: 29405261; PMCID: PMC5900994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulugeta S., Nureki S., Beers M.F. Lost after translation: insights from pulmonary surfactant for understanding the role of alveolar epithelial dysfunction and cellular quality control in fibrotic lung disease. Am. J. Physiol. 2015;309(6):L507–L525. doi: 10.1152/ajplung.00139.2015. Epub 2015/07/19, PubMed PMID: 26186947; PMCID: PMC4572416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersson J., Glenny R.W. Gas exchange and ventilation-perfusion relationships in the lung. Eur. Respir. J. 2014;44(4):1023–1041. doi: 10.1183/09031936.00037014. Epub 2014/07/27, PubMed PMID: 25063240. [DOI] [PubMed] [Google Scholar]
- 6.Hetz C., Papa F.R. The unfolded protein response and cell fate control. Mol. Cell. 2018;69(2):169–181. doi: 10.1016/j.molcel.2017.06.017. Epub 2017/11/07, PubMed PMID: 29107536. [DOI] [PubMed] [Google Scholar]
- 7.Janssen-Heininger Y.M., Aesif S.W., van der Velden J., Guala A.S., Reiss J.N., Roberson E.C., Budd R.C., Reynaert N.L., Anathy V. Regulation of apoptosis through cysteine oxidation: implications for fibrotic lung disease. Ann. N. Y. Acad. Sci. 2010;1203:23–28. doi: 10.1111/j.1749-6632.2010.05553.x. Epub 2010/08/19, PubMed PMID: 20716279; PMCID: 2943339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen-Heininger Y.M., Mossman B.T., Heintz N.H., Forman H.J., Kalyanaraman B., Finkel T., Stamler J.S., Rhee S.G., van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic. Biol. Med. 2008;45(1):1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. Epub 2008/04/22, PubMed PMID: 18423411; PMCID: 2453533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen-Heininger Y.M., Nolin J.D., Hoffman S.M., van der Velden J.L., Tully J.E., Lahue K.G., Abdalla S.T., Chapman D.G., Reynaert N.L., van der Vliet A., Anathy V. Emerging mechanisms of glutathione-dependent chemistry in biology and disease. J. Cell. Biochem. 2013 doi: 10.1002/jcb.24551. Epub 2013/04/05, PubMed PMID: 23554102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez F.J., Collard H.R., Pardo A., Raghu G., Richeldi L., Selman M., Swigris J.J., Taniguchi H., Wells A.U. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. doi: 10.1038/nrdp.2017.74. Epub 2017/10/21, PubMed PMID: 29052582. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins A., Guttentag S.H., Deterding R., Funkhouser W.K., Goralski J.L., Chatterjee S., Mulugeta S., Beers M.F. A non-BRICHOS SFTPC mutant (SP-CI73T) linked to interstitial lung disease promotes a late block in macroautophagy disrupting cellular proteostasis and mitophagy. Am. J. Physiol. 2015;308(1):L33–L47. doi: 10.1152/ajplung.00217.2014. Epub 2014/10/26, PubMed PMID: 25344067; PMCID: PMC4281696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mora A.L., Bueno M., Rojas M. Mitochondria in the spotlight of aging and idiopathic pulmonary fibrosis. J. Clin. Invest. 2017;127(2):405–414. doi: 10.1172/JCI87440. Epub 2017/02/02, PubMed PMID: 28145905; PMCID: PMC5272191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Son B., Kwon T., Lee S., Han I., Kim W., Youn H., Youn B. CYP2E1 regulates the development of radiation-induced pulmonary fibrosis via ER stress- and ROS-dependent mechanisms. Am. J. Physiol. 2017;313(5) doi: 10.1152/ajplung.00144.2017. L916–l29. Epub 2017/08/12, PubMed PMID: 28798253. [DOI] [PubMed] [Google Scholar]
- 14.Kropski J.A., Blackwell T.S. Endoplasmic reticulum stress in the pathogenesis of fibrotic disease. J. Clin. Invest. 2018;128(1):64–73. doi: 10.1172/JCI93560. Epub 2018/01/03, PubMed PMID: 29293089; PMCID: PMC5749533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawson W.E., Crossno P.F., Polosukhin V.V., Roldan J., Cheng D.S., Lane K.B., Blackwell T.R., Xu C., Markin C., Ware L.B., Miller G.G., Loyd J.E., Blackwell T.S. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am. J. Physiol. 2008;294(6):L1119–L1126. doi: 10.1152/ajplung.00382.2007. Epub 2008/04/09, PubMed PMID: 18390830. [DOI] [PubMed] [Google Scholar]
- 16.Korfei M., Ruppert C., Mahavadi P., Henneke I., Markart P., Koch M., Lang G., Fink L., Bohle R.M., Seeger W., Weaver T.E., Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008;178(8):838–846. doi: 10.1164/rccm.200802-313OC. PubMed PMID: 18635891; PMCID: PMC2566794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eder W., Ege M.J., von Mutius E. The asthma epidemic. N. Engl. J. Med. 2006;355(21):2226–2235. doi: 10.1056/NEJMra054308. Epub 2006/11/25, PubMed PMID: 17124020. [DOI] [PubMed] [Google Scholar]
- 18.Holgate S.T., Wenzel S., Postma D.S., Weiss S.T., Renz H., Sly P.D. Asthma. Nat Rev Dis Primers. 2015;1:15025. doi: 10.1038/nrdp.2015.25. Epub 2015/01/01, PubMed PMID: 27189668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavord I.D., Beasley R., Agusti A., Anderson G.P., Bel E., Brusselle G., Cullinan P., Custovic A., Ducharme F.M., Fahy J.V., Frey U., Gibson P., Heaney L.G., Holt P.G., Humbert M., Lloyd C.M., Marks G., Martinez F.D., Sly P.D., von Mutius E., Wenzel S., Zar H.J., Bush A. After asthma: redefining airways diseases. Lancet (London, England) 2018;391(10118):350–400. doi: 10.1016/s0140-6736(17)30879-6. Epub 2017/09/16, PubMed PMID: 28911920. [DOI] [PubMed] [Google Scholar]
- 20.Boorsma C.E., Dekkers B.G., van Dijk E.M., Kumawat K., Richardson J., Burgess J.K., John A.E. Beyond TGFbeta–novel ways to target airway and parenchymal fibrosis. Pulm. Pharmacol. Therapeut. 2014;29(2):166–180. doi: 10.1016/j.pupt.2014.08.009. Epub 2014/09/10, PubMed PMID: 25197006. [DOI] [PubMed] [Google Scholar]
- 21.Fahy J.V. Type 2 inflammation in asthma–present in most, absent in many. Nat. Rev. Immunol. 2015;15(1):57–65. doi: 10.1038/nri3786. Epub 2014/12/24, PubMed PMID: 25534623; PMCID: PMC4390063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esteban-Gorgojo I., Antolin-Amerigo D., Dominguez-Ortega J., Quirce S. Non-eosinophilic asthma: current perspectives. J. Asthma Allergy. 2018;11:267–281. doi: 10.2147/jaa.S153097. Epub 2018/11/23, PubMed PMID: 30464537; PMCID: PMC6211579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh D., Agusti A., Anzueto A., Barnes P.J., Bourbeau J., Celli B.R., Criner G.J., Frith P., Halpin D.M.G., Han M., Lopez Varela M.V., Martinez F., Montes de Oca M., Papi A., Pavord I.D., Roche N., Sin D.D., Stockley R., Vestbo J., Wedzicha J.A., Vogelmeier C. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur. Respir. J. 2019;53(5) doi: 10.1183/13993003.00164-2019. Epub 2019/03/09, PubMed PMID: 30846476. [DOI] [PubMed] [Google Scholar]
- 24.Agusti A., Hogg J.C. Update on the pathogenesis of chronic obstructive pulmonary disease. N. Engl. J. Med. 2019;381(13):1248–1256. doi: 10.1056/NEJMra1900475. Epub 2019/09/26, PubMed PMID: 31553836. [DOI] [PubMed] [Google Scholar]
- 25.Higham A., Quinn A.M., Cancado J.E.D., Singh D. The pathology of small airways disease in COPD: historical aspects and future directions. Respir. Res. 2019;20(1):49. doi: 10.1186/s12931-019-1017-y. Epub 2019/03/06, PubMed PMID: 30832670; PMCID: PMC6399904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eurlings I.M., Dentener M.A., Cleutjens J.P., Peutz C.J., Rohde G.G., Wouters E.F., Reynaert N.L. Similar matrix alterations in alveolar and small airway walls of COPD patients. BMC Pulm. Med. 2014;14:90. doi: 10.1186/1471-2466-14-90. Epub 2014/06/03, PubMed PMID: 24886452; PMCID: PMC4055380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kranenburg A.R., Willems-Widyastuti A., Moori W.J., Sterk P.J., Alagappan V.K., de Boer W.I., Sharma H.S. Enhanced bronchial expression of extracellular matrix proteins in chronic obstructive pulmonary disease. Am. J. Clin. Pathol. 2006;126(5):725–735. doi: 10.1309/jc477fael1ykv54w. Epub 2006/11/23, PubMed PMID: 17111536. [DOI] [PubMed] [Google Scholar]
- 28.Annoni R., Lancas T. Yukimatsu Tanigawa R, de Medeiros Matsushita M, de Morais Fernezlian S, Bruno A, Fernando Ferraz da Silva L, Roughley PJ, Battaglia S, Dolhnikoff M, Hiemstra PS, Sterk PJ, Rabe KF, Mauad T. Extracellular matrix composition in COPD. Eur. Respir. J. 2012;40(6):1362–1373. doi: 10.1183/09031936.00192611. Epub 2012/04/13, PubMed PMID: 22496324. [DOI] [PubMed] [Google Scholar]
- 29.Barnes P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013;131(3):636–645. doi: 10.1016/j.jaci.2012.12.1564. Epub 2013/01/31, PubMed PMID: 23360759. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. Epub 2013/06/12, PubMed PMID: 23746838; PMCID: PMC3836174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meiners S., Eickelberg O., Konigshoff M. Hallmarks of the ageing lung. Eur. Respir. J. 2015;45(3):807–827. doi: 10.1183/09031936.00186914. Epub 2015/02/07, PubMed PMID: 25657021. [DOI] [PubMed] [Google Scholar]
- 32.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30(11):1191–1212. doi: 10.1016/s0891-5849(01)00480-4. Epub 2001/05/23, PubMed PMID: 11368918. [DOI] [PubMed] [Google Scholar]
- 33.Chia S.B., Elko E.A., Aboushousha R., Manuel A.M., van de Wetering C., Druso J.E., van der Velden J., Seward D.J., Anathy V., Irvin C.G., Lam Y.W., van der Vliet A., Janssen-Heininger Y. Dysregulation of the glutaredoxin/S-glutathionylation redox axis in lung diseases. Am. J. Physiol. Cell Physiol. 2019 doi: 10.1152/ajpcell.00410.2019. Epub 2019/11/07, PubMed PMID: 31693398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasamatsu S., Nishimura A., Morita M., Matsunaga T. Abdul hamid H, akaike T. Redox signaling regulated by cysteine persulfide and protein polysulfidation. Molecules. 2016;21(12) doi: 10.3390/molecules21121721. Epub 2016/12/17, PubMed PMID: 27983699; PMCID: PMC6273478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawa T., Ono K., Tsutsuki H., Zhang T., Ida T., Nishida M., Akaike T. Reactive cysteine persulphides: occurrence, biosynthesis, antioxidant activity, methodologies, and bacterial persulphide signalling. Adv. Microb. Physiol. 2018;72:1–28. doi: 10.1016/bs.ampbs.2018.01.002. Epub 2018/05/21, PubMed PMID: 29778212. [DOI] [PubMed] [Google Scholar]
- 36.Lam B.K., Austen K.F. Leukotriene C4 synthase: a pivotal enzyme in cellular biosynthesis of the cysteinyl leukotrienes. Prostag. Other Lipid Mediat. 2002;68–69:511–520. doi: 10.1016/s0090-6980(02)00052-7. Epub 2002/11/16, PubMed PMID: 12432940. [DOI] [PubMed] [Google Scholar]
- 37.Kim D.C., Hsu F.I., Barrett N.A., Friend D.S., Grenningloh R., Ho I.C., Al-Garawi A., Lora J.M., Lam B.K., Austen K.F., Kanaoka Y. Cysteinyl leukotrienes regulate Th2 cell-dependent pulmonary inflammation. J. Immunol. 2006;176(7):4440–4448. doi: 10.4049/jimmunol.176.7.4440. Epub 2006/03/21, PubMed PMID: 16547282. [DOI] [PubMed] [Google Scholar]
- 38.Lu E., Wolfreys F.D., Muppidi J.R., Xu Y., Cyster J.G. S-Geranylgeranyl-L-glutathione is a ligand for human B cell-confinement receptor P2RY8. Nature. 2019;567(7747):244–248. doi: 10.1038/s41586-019-1003-z. Epub 2019/03/08, PubMed PMID: 30842656; PMCID: PMC6640153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luengo A., Abbott K.L., Davidson S.M., Hosios A.M., Faubert B., Chan S.H., Freinkman E., Zacharias L.G., Mathews T.P., Clish C.B., DeBerardinis R.J., Lewis C.A., Vander Heiden M.G. Reactive metabolite production is a targetable liability of glycolytic metabolism in lung cancer. Nat. Commun. 2019;10(1):5604. doi: 10.1038/s41467-019-13419-4. Epub 2019/12/08, PubMed PMID: 31811141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ercolani L., Scire A., Galeazzi R., Massaccesi L., Cianfruglia L., Amici A., Piva F., Urbanelli L., Emiliani C., Principato G., Armeni T. A possible S-glutathionylation of specific proteins by glyoxalase II: an in vitro and in silico study. Cell Biochem. Funct. 2016;34(8):620–627. doi: 10.1002/cbf. Epub 2016/12/10, 3236. PubMed PMID: 27935136. [DOI] [PubMed] [Google Scholar]
- 41.Bachhawat A.K., Yadav S. The glutathione cycle: glutathione metabolism beyond the gamma-glutamyl cycle. IUBMB Life. 2018;70(7):585–592. doi: 10.1002/iub. Epub 2018/04/19, 1756. PubMed PMID: 29667297. [DOI] [PubMed] [Google Scholar]
- 42.Giustarini D., Galvagni F., Tesei A., Farolfi A., Zanoni M., Pignatta S., Milzani A., Marone I.M., Dalle-Donne I., Nassini R., Rossi R. Glutathione, glutathione disulfide, and S-glutathionylated proteins in cell cultures. Free Radic. Biol. Med. 2015;89:972–981. doi: 10.1016/j.freeradbiomed.2015.10.410. Epub 2015/10/18, PubMed PMID: 26476010. [DOI] [PubMed] [Google Scholar]
- 43.Cantin A.M., North S.L., Hubbard R.C., Crystal R.G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 1987;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. Epub 1987/07/01, PubMed PMID: 3040659. [DOI] [PubMed] [Google Scholar]
- 44.van der Vliet A., O'Neill C.A., Cross C.E., Koostra J.M., Volz W.G., Halliwell B., Louie S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am. J. Physiol. 1999;276(2):L289–L296. doi: 10.1152/ajplung.1999.276.2.L289. Epub 1999/02/10, PubMed PMID: 9950891. [DOI] [PubMed] [Google Scholar]
- 45.Giustarini D., Tsikas D., Colombo G., Milzani A., Dalle-Donne I., Fanti P., Rossi R. Pitfalls in the analysis of the physiological antioxidant glutathione (GSH) and its disulfide (GSSG) in biological samples: an elephant in the room. Journal of chromatography B. Analytical technologies in the biomedical and life sciences. 2016;1019:21–28. doi: 10.1016/j.jchromb.2016.02.015. Epub 2016/02/26, PubMed PMID: 26905452; PMCID: PMC4829456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brigelius-Flohe R., Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta. 2013;1830(5):3289–3303. doi: 10.1016/j.bbagen.2012.11.020. Epub 2012/12/04, PubMed PMID: 23201771. [DOI] [PubMed] [Google Scholar]
- 47.Hinchman C.A., Ballatori N. Glutathione conjugation and conversion to mercapturic acids can occur as an intrahepatic process. J. Toxicol. Environ. Health. 1994;41(4):387–409. doi: 10.1080/15287399409531852. Epub 1994/04/01, PubMed PMID: 8145281. [DOI] [PubMed] [Google Scholar]
- 48.Chen K., Kirber M.T., Xiao H., Yang Y., Keaney J.F., Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 2008;181(7):1129–1139. doi: 10.1083/jcb.200709049. Epub 2008/06/25, PubMed PMID: 18573911; PMCID: 2442210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winterbourn C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4(5):278–286. doi: 10.1038/nchembio.85. Epub 2008/04/19, PubMed PMID: 18421291. [DOI] [PubMed] [Google Scholar]
- 50.Anathy V., Roberson E.C., Guala A.S., Godburn K.E., Budd R.C., Janssen-Heininger Y.M. Redox-based regulation of apoptosis: S-glutathionylation as a regulatory mechanism to control cell death. Antioxidants Redox Signal. 2012;16(6):496–505. doi: 10.1089/ars.2011.4281. Epub 2011/09/21, PubMed PMID: 21929356; PMCID: 3304251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Georgiou G. How to flip the (redox) switch. Cell. 2002;111(5):607–610. doi: 10.1016/s0092-8674(02)01165-0. PubMed PMID: 12464172. [DOI] [PubMed] [Google Scholar]
- 52.Gallogly M.M., Mieyal J.J. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr. Opin. Pharmacol. 2007;7(4):381–391. doi: 10.1016/j.coph.2007.06.003. Epub 2007/07/31, PubMed PMID: 17662654. [DOI] [PubMed] [Google Scholar]
- 53.Tew K.D., Manevich Y., Grek C., Xiong Y., Uys J., Townsend D.M. The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free Radic. Biol. Med. 2011;51(2):299–313. doi: 10.1016/j.freeradbiomed.2011.04.013. Epub 2011/05/12, PubMed PMID: 21558000; PMCID: 3125017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Townsend D.M., Manevich Y., He L., Hutchens S., Pazoles C.J., Tew K.D. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J. Biol. Chem. 2009;284(1):436–445. doi: 10.1074/jbc.M805586200. PubMed PMID: 18990698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kong K.H., Nishida M., Inoue H., Takahashi K. Tyrosine-7 is an essential residue for the catalytic activity of human class PI glutathione S-transferase: chemical modification and site-directed mutagenesis studies. Biochem. Biophys. Res. Commun. 1992;182(3):1122–1129. doi: 10.1016/0006-291x(92)91848-k. Epub 1992/02/14, PubMed PMID: 1540159. [DOI] [PubMed] [Google Scholar]
- 56.Allocati N., Masulli M., Di Ilio C., Federici L. Glutathione transferases: substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis. 2018;7(1):8. doi: 10.1038/s41389-017-0025-3. Epub 2018/01/25, PubMed PMID: 29362397; PMCID: PMC5833873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ralat L.A., Manevich Y., Fisher A.B., Colman R.F. Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase pi with activity changes in both enzymes. Biochemistry. 2006;45(2):360–372. doi: 10.1021/bi0520737. Epub 2006/01/13, PubMed PMID: 16401067. [DOI] [PubMed] [Google Scholar]
- 58.Manevich Y., Feinstein S.I., Fisher A.B. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc. Natl. Acad. Sci. U. S. A. 2004;101(11):3780–3785. doi: 10.1073/pnas.0400181101. PubMed PMID: 15004285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen H., Tsuchida S., Tamai K., Sato K. Identification of cysteine residues involved in disulfide formation in the inactivation of glutathione transferase P-form by hydrogen peroxide. Arch. Biochem. Biophys. 1993;300(1):137–141. doi: 10.1006/abbi.1993.1019. Epub 1993/01/01, PubMed PMID: 8424645. [DOI] [PubMed] [Google Scholar]
- 60.Lillig C.H., Berndt C., Holmgren A. Glutaredoxin systems. Biochim. Biophys. Acta. 2008;1780(11):1304–1317. doi: 10.1016/j.bbagen.2008.06.003. Epub 2008/07/16, PubMed PMID: 18621099. [DOI] [PubMed] [Google Scholar]
- 61.Yu J., Zhang N.N., Yin P.D., Cui P.X., Zhou C.Z. Glutathionylation-triggered conformational changes of glutaredoxin Grx1 from the yeast Saccharomyces cerevisiae. Proteins. 2008;72(3):1077–1083. doi: 10.1002/prot.22096. Epub 2008/05/14, PubMed PMID: 18473363. [DOI] [PubMed] [Google Scholar]
- 62.Sun C., Berardi M.J., Bushweller J.H. The NMR solution structure of human glutaredoxin in the fully reduced form. J. Mol. Biol. 1998;280(4):687–701. doi: 10.1006/jmbi.1998.1913. Epub 1998/07/25, PubMed PMID: 9677297. [DOI] [PubMed] [Google Scholar]
- 63.Srinivasan U., Mieyal P.A., Mieyal J.J. pH profiles indicative of rate-limiting nucleophilic displacement in thioltransferase catalysis. Biochemistry. 1997;36(11):3199–3206. doi: 10.1021/bi962017t. Epub 1997/03/18, PubMed PMID: 9115997. [DOI] [PubMed] [Google Scholar]
- 64.Gan Z.R., Wells W.W. Identification and reactivity of the catalytic site of pig liver thioltransferase. J. Biol. Chem. 1987;262(14):6704–6707. Epub 1987/05/15. PubMed PMID: 3571279. [PubMed] [Google Scholar]
- 65.Yang Y.F., Wells W.W. Identification and characterization of the functional amino acids at the active center of pig liver thioltransferase by site-directed mutagenesis. J. Biol. Chem. 1991;266(19):12759–12765. Epub 1991/07/05. PubMed PMID: 2061338. [PubMed] [Google Scholar]
- 66.Yang Y.F., Wells W.W. Catalytic mechanism of thioltransferase. J. Biol. Chem. 1991;266(19):12766–12771. Epub 1991/07/05. PubMed PMID: 2061339. [PubMed] [Google Scholar]
- 67.Hashemy S.I., Johansson C., Berndt C., Lillig C.H., Holmgren A. Oxidation and S-nitrosylation of cysteines in human cytosolic and mitochondrial glutaredoxins: effects on structure and activity. J. Biol. Chem. 2007;282(19):14428–14436. doi: 10.1074/jbc.M700927200. Epub 2007/03/16, PubMed PMID: 17355958. [DOI] [PubMed] [Google Scholar]
- 68.Anathy V., Lahue K.G., Chapman D.G., Chia S.B., Casey D.T., Aboushousha R., van der Velden J.L.J., Elko E., Hoffman S.M., McMillan D.H., Jones J.T., Nolin J.D., Abdalla S., Schneider R., Seward D.J., Roberson E.C., Liptak M.D., Cousins M.E., Butnor K.J., Taatjes D.J., Budd R.C., Irvin C.G., Ho Y.S., Hakem R., Brown K.K., Matsui R., Bachschmid M.M., Gomez J.L., Kaminski N., van der Vliet A., Janssen-Heininger Y.M.W. Reducing protein oxidation reverses lung fibrosis. Nat. Med. 2018;24(8):1128–1135. doi: 10.1038/s41591-018-0090-y. Epub 2018/07/11, PubMed PMID: 29988126; PMCID: PMC6204256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Senft D., Ronai Z.A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015;40(3):141–148. doi: 10.1016/j.tibs.2015.01.002. Epub 2015/02/07, PubMed PMID: 25656104; PMCID: PMC4340752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blumental-Perry A. Unfolded protein response in chronic obstructive pulmonary disease: smoking, aging and disease: a SAD trifecta. Curr. Mol. Med. 2012;12(7):883–898. doi: 10.2174/156652412801318764. Epub 2012/06/16, PubMed PMID: 22697343. [DOI] [PubMed] [Google Scholar]
- 71.Chen A.C., Burr L., McGuckin M.A. Oxidative and endoplasmic reticulum stress in respiratory disease. Clinical & translational immunology. 2018;7(6):e1019. doi: 10.1002/cti2.1019. Epub 2018/06/22, PubMed PMID: 29928501; PMCID: PMC5999202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Z., Zhang L., Zhou L., Lei Y., Zhang Y., Huang C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox biology. 2019;25:101047. doi: 10.1016/j.redox.2018.11.005. Epub 2018/11/25, PubMed PMID: 30470534; PMCID: PMC6859529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niforou K., Cheimonidou C., Trougakos I.P. Molecular chaperones and proteostasis regulation during redox imbalance. Redox biology. 2014;2:323–332. doi: 10.1016/j.redox.2014.01.017. Epub 2014/02/25, PubMed PMID: 24563850; PMCID: PMC3926111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soares Moretti A.I., Martins Laurindo F.R. Protein disulfide isomerases: redox connections in and out of the endoplasmic reticulum. Arch. Biochem. Biophys. 2017;617:106–119. doi: 10.1016/j.abb.2016.11.007. Epub 2016/11/28, PubMed PMID: 27889386. [DOI] [PubMed] [Google Scholar]
- 75.Jessop C.E., Watkins R.H., Simmons J.J., Tasab M., Bulleid N.J. Protein disulphide isomerase family members show distinct substrate specificity: P5 is targeted to BiP client proteins. J. Cell Sci. 2009;122(Pt 23):4287–4295. doi: 10.1242/jcs.059154. Epub 2009/11/06, PubMed PMID: 19887585; PMCID: PMC2779130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hwang C., Sinskey A.J., Lodish H.F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science (New York, NY) 1992;257(5076):1496–1502. doi: 10.1126/science.1523409. Epub 1992/09/11. PubMed PMID: 1523409. [DOI] [PubMed] [Google Scholar]
- 77.Bass R., Ruddock L.W., Klappa P., Freedman R.B. A major fraction of endoplasmic reticulum-located glutathione is present as mixed disulfides with protein. J. Biol. Chem. 2004;279(7):5257–5262. doi: 10.1074/jbc.M304951200. Epub 2003/11/25, PubMed PMID: 14630926. [DOI] [PubMed] [Google Scholar]
- 78.Appenzeller-Herzog C. Glutathione- and non-glutathione-based oxidant control in the endoplasmic reticulum. J. Cell Sci. 2011;124(Pt 6):847–855. doi: 10.1242/jcs.080895. Epub 2011/03/08, PubMed PMID: 21378306. [DOI] [PubMed] [Google Scholar]
- 79.Martino M.B., Jones L., Brighton B., Ehre C., Abdulah L., Davis C.W., Ron D., O'Neal W.K., Ribeiro C.M. The ER stress transducer IRE1beta is required for airway epithelial mucin production. Mucosal Immunol. 2013;6(3):639–654. doi: 10.1038/mi.2012.105. Epub 2012/11/22, PubMed PMID: 23168839; PMCID: PMC4031691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kakihana T., Nagata K., Sitia R. Peroxides and peroxidases in the endoplasmic reticulum: integrating redox homeostasis and oxidative folding. Antioxidants Redox Signal. 2012;16(8):763–771. doi: 10.1089/ars.2011.4238. Epub 2011/12/08, PubMed PMID: 22146055. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen V.D., Saaranen M.J., Karala A.R., Lappi A.K., Wang L., Raykhel I.B., Alanen H.I., Salo K.E., Wang C.C., Ruddock L.W. Two endoplasmic reticulum PDI peroxidases increase the efficiency of the use of peroxide during disulfide bond formation. J. Mol. Biol. 2011;406(3):503–515. doi: 10.1016/j.jmb.2010.12.039. Epub 2011/01/11, PubMed PMID: 21215271. [DOI] [PubMed] [Google Scholar]
- 82.Tavender T.J., Springate J.J., Bulleid N.J. Recycling of peroxiredoxin IV provides a novel pathway for disulphide formation in the endoplasmic reticulum. EMBO J. 2010;29(24):4185–4197. doi: 10.1038/emboj.2010.273. Epub 2010/11/09, PubMed PMID: 21057456; PMCID: 3018787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fass D. Hunting for alternative disulfide bond formation pathways: endoplasmic reticulum janitor turns professor and teaches a lesson. Mol. Cell. 2010;40(5):685–686. doi: 10.1016/j.molcel.2010.11.034. Epub 2010/12/15, PubMed PMID: 21145477. [DOI] [PubMed] [Google Scholar]
- 84.Yim S.H., Everley R.A., Schildberg F.A., Lee S.G., Orsi A., Barbati Z.R., Karatepe K., Fomenko D.E., Tsuji P.A., Luo H.R., Gygi S.P., Sitia R., Sharpe A.H., Hatfield D.L., Gladyshev V.N. Role of Selenof as a gatekeeper of secreted disulfide-rich glycoproteins. Cell Rep. 2018;23(5):1387–1398. doi: 10.1016/j.celrep.2018.04.009. Epub 2018/05/03, PubMed PMID: 29719252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dixon S.J., Patel D.N., Welsch M., Skouta R., Lee E.D., Hayano M., Thomas A.G., Gleason C.E., Tatonetti N.P., Slusher B.S., Stockwell B.R. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 2014;3 doi: 10.7554/eLife.02523. e02523. Epub 2014/05/23, PubMed PMID: 24844246; PMCID: PMC4054777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ye Z.W., Zhang J., Ancrum T., Manevich Y., Townsend D.M., Tew K.D. Glutathione S-transferase P-mediated protein S-glutathionylation of resident endoplasmic reticulum proteins influences sensitivity to drug-induced unfolded protein response. Antioxidants Redox Signal. 2017;26(6):247–261. doi: 10.1089/ars.2015.6486. Epub 2016/02/04, PubMed PMID: 26838680; PMCID: PMC5312626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anathy V., Roberson E., Cunniff B., Nolin J.D., Hoffman S., Spiess P., Guala A.S., Lahue K.G., Goldman D., Flemer S., van der Vliet A., Heintz N.H., Budd R.C., Tew K.D., Janssen-Heininger Y.M. Oxidative processing of latent Fas in the endoplasmic reticulum controls the strength of apoptosis. Mol. Cell Biol. 2012;32(17):3464–3478. doi: 10.1128/mcb.00125-12. Epub 2012/07/04, PubMed PMID: 22751926; PMCID: PMC3422013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Romero F., Summer R. Protein folding and the challenges of maintaining endoplasmic reticulum proteostasis in idiopathic pulmonary fibrosis. Ann. Am. Thoracic Soc. 2017;14(Supplement_5) doi: 10.1513/AnnalsATS. S410–s3. Epub 2017/11/22, 201703-207AW. PubMed PMID: 29161089; PMCID: PMC5711273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katzen J., Wagner B.D., Venosa A., Kopp M., Tomer Y., Russo S.J., Headen A.C., Basil M.C., Stark J.M., Mulugeta S., Deterding R.R., Beers M.F. A SFTPC BRICHOS mutant links epithelial ER stress and spontaneous lung fibrosis. JCI insight. 2019 doi: 10.1172/jci.insight.126125. Epub 2019/02/06, PubMed PMID: 30721158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lawson W.E., Cheng D.S., Degryse A.L., Tanjore H., Polosukhin V.V., Xu X.C., Newcomb D.C., Jones B.R., Roldan J., Lane K.B., Morrisey E.E., Beers M.F., Yull F.E., Blackwell T.S. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc. Natl. Acad. Sci. U. S. A. 2011;108(26):10562–10567. doi: 10.1073/pnas.1107559108. Epub 2011/06/15, PubMed PMID: 21670280; PMCID: 3127925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nureki S.I., Tomer Y., Venosa A., Katzen J., Russo S.J., Jamil S., Barrett M., Nguyen V., Kopp M., Mulugeta S., Beers M.F. Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. J. Clin. Invest. 2018;128(9):4008–4024. doi: 10.1172/jci99287. Epub 2018/06/20, PubMed PMID: 29920187; PMCID: PMC6118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen G., Ribeiro C.M.P., Sun L., Okuda K., Kato T., Gilmore R.C., Martino M.B., Dang H., Abzhanova A., Lin J.M., Hull-Ryde E.A., Volmer A.S., Randell S.H., Livraghi-Butrico A., Deng Y., Scherer P.E., Stripp B.R., O'Neal W.K., Boucher R.C. XBP1S regulates MUC5B in a promoter variant-dependent pathway in idiopathic pulmonary fibrosis airway epithelia. Am. J. Respir. Crit. Care Med. 2019;200(2):220–234. doi: 10.1164/rccm.201810-1972OC. Epub 2019/04/12, PubMed PMID: 30973754; PMCID: PMC6635783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mutze K., Vierkotten S., Milosevic J., Eickelberg O., Konigshoff M. Enolase 1 (ENO1) and protein disulfide-isomerase associated 3 (PDIA3) regulate Wnt/beta-catenin-driven trans-differentiation of murine alveolar epithelial cells. Disease Models Mech. 2015;8(8):877–890. doi: 10.1242/dmm.019117. Epub 2015/06/03, PubMed PMID: 26035385; PMCID: PMC4527283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao G., Lu H., Li C. Proapoptotic activities of protein disulfide isomerase (PDI) and PDIA3 protein, a role of the Bcl-2 protein Bak. J. Biol. Chem. 2015;290(14):8949–8963. doi: 10.1074/jbc.M114.619353. Epub 2015/02/24, PubMed PMID: 25697356; PMCID: PMC4423685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dihazi H., Dihazi G.H., Bibi A., Eltoweissy M., Mueller C.A., Asif A.R., Rubel D., Vasko R., Mueller G.A. Secretion of ERP57 is important for extracellular matrix accumulation and progression of renal fibrosis, and is an early sign of disease onset. J. Cell Sci. 2013;126(Pt 16):3649–3663. doi: 10.1242/jcs.125088. Epub 2013/06/20, PubMed PMID: 23781031. [DOI] [PubMed] [Google Scholar]
- 96.Yi M.C., Melkonian A.V., Ousey J.A., Khosla C. Endoplasmic reticulum-resident protein 57 (ERp57) oxidatively inactivates human transglutaminase 2. J. Biol. Chem. 2018;293(8):2640–2649. doi: 10.1074/jbc.RA117.001382. Epub 2018/01/07, PubMed PMID: 29305423; PMCID: PMC5827427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khan M.M., Simizu S., Suzuki T., Masuda A., Kawatani M., Muroi M., Dohmae N., Osada H. Protein disulfide isomerase-mediated disulfide bonds regulate the gelatinolytic activity and secretion of matrix metalloproteinase-9. Exp. Cell Res. 2012;318(8):904–914. doi: 10.1016/j.yexcr.2012.02.021. Epub 2012/03/13, PubMed PMID: 22406264. [DOI] [PubMed] [Google Scholar]
- 98.Rosenberg N., Mor-Cohen R., Sheptovitsky V.H., Romanenco O., Hess O., Lahav J. Integrin-mediated cell adhesion requires extracellular disulfide exchange regulated by protein disulfide isomerase. Exp. Cell Res. 2019;381(1):77–85. doi: 10.1016/j.yexcr.2019.04.017. Epub 2019/05/03, PubMed PMID: 31042499. [DOI] [PubMed] [Google Scholar]
- 99.Langenbach K.J., Sottile J. Identification of protein-disulfide isomerase activity in fibronectin. J. Biol. Chem. 1999;274(11):7032–7038. doi: 10.1074/jbc.274.11.7032. Epub 1999/03/06, PubMed PMID: 10066758. [DOI] [PubMed] [Google Scholar]
- 100.Koivu J., Myllyla R., Helaakoski T., Pihlajaniemi T., Tasanen K., Kivirikko K.I. A single polypeptide acts both as the beta subunit of prolyl 4-hydroxylase and as a protein disulfide-isomerase. J. Biol. Chem. 1987;262(14):6447–6449. Epub 1987/05/15. PubMed PMID: 3032969. [PubMed] [Google Scholar]
- 101.Winter A.D., McCormack G., Page A.P. Protein disulfide isomerase activity is essential for viability and extracellular matrix formation in the nematode Caenorhabditis elegans. Dev. Biol. 2007;308(2):449–461. doi: 10.1016/j.ydbio.2007.05.041. Epub 2007/06/26, PubMed PMID: 17586485. [DOI] [PubMed] [Google Scholar]
- 102.Marchi S., Patergnani S., Missiroli S., Morciano G., Rimessi A., Wieckowski M.R., Giorgi C., Pinton P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018;69:62–72. doi: 10.1016/j.ceca.2017.05.003. Epub 2017/05/19, PubMed PMID: 28515000. [DOI] [PubMed] [Google Scholar]
- 103.Csordas G., Weaver D., Hajnoczky G. Endoplasmic reticulum-mitochondrial contactology: structure and signaling functions. Trends Cell Biol. 2018;28(7):523–540. doi: 10.1016/j.tcb.2018.02.009. Epub 2018/03/29, PubMed PMID: 29588129; PMCID: PMC6005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bueno M., Lai Y.C., Romero Y., Brands J. St croix CM, kamga C, corey C, herazo-maya JD, sembrat J, lee JS, duncan SR, rojas M, shiva S, chu CT, Mora AL. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J. Clin. Invest. 2015;125(2):521–538. doi: 10.1172/JCI74942. Epub 2015/01/07, PubMed PMID: 25562319; PMCID: PMC4319413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bratic A., Larsson N.G. The role of mitochondria in aging. J. Clin. Invest. 2013;123(3):951–957. doi: 10.1172/jci64125. Epub 2013/03/05, PubMed PMID: 23454757; PMCID: PMC3582127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schuliga M., Pechkovsky D.V., Read J., Waters D.W., Blokland K.E.C., Reid A.T., Hogaboam C.M., Khalil N., Burgess J.K., Prele C.M., Mutsaers S.E., Jaffar J., Westall G., Grainge C., Knight D.A. Mitochondrial dysfunction contributes to the senescent phenotype of IPF lung fibroblasts. J. Cell Mol. Med. 2018;22(12):5847–5861. doi: 10.1111/jcmm.13855. Epub 2018/09/27, PubMed PMID: 30255990; PMCID: PMC6237609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alvarez D., Cardenes N., Sellares J., Bueno M., Corey C., Hanumanthu V.S., Peng Y., D'Cunha H., Sembrat J., Nouraie M., Shanker S., Caufield C., Shiva S., Armanios M., Mora A.L., Rojas M. IPF lung fibroblasts have a senescent phenotype. Am. J. Physiol. 2017;313(6) doi: 10.1152/ajplung.00220.2017. L1164–l73. Epub 2017/09/02, PubMed PMID: 28860144; PMCID: PMC6148001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun Q., Fang L., Tang X., Lu S., Tamm M., Stolz D., Roth M. TGF-beta upregulated mitochondria mass through the SMAD2/3-->C/EBPbeta-->PRMT1 signal pathway in primary human lung fibroblasts. J. Immunol. 2019;202(1):37–47. doi: 10.4049/jimmunol.1800782. Epub 2018/12/12, PubMed PMID: 30530593; PMCID: PMC6305796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Waters D.W., Blokland K.E.C., Pathinayake P.S., Wei L., Schuliga M., Jaffar J., Westall G.P., Hansbro P.M., Prele C.M., Mutsaers S.E., Bartlett N.W., Burgess J.K., Grainge C.L., Knight D.A. STAT3 regulates the onset of oxidant-induced senescence in lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2019 doi: 10.1165/rcmb.2018-0328OC. Epub 2019/01/05, PubMed PMID: 30608861. [DOI] [PubMed] [Google Scholar]
- 110.Kojima H., Inoue T., Kunimoto H., Nakajima K. IL-6-STAT3 signaling and premature senescence. JAK-STAT. 2013;2(4) doi: 10.4161/jkst.25763. Epub 2014/01/15, PubMed PMID: 24416650; PMCID: PMC3876432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zito E., Hansen H.G., Yeo G.S., Fujii J., Ron D. Endoplasmic reticulum thiol oxidase deficiency leads to ascorbic acid depletion and noncanonical scurvy in mice. Mol. Cell. 2012;48(1):39–51. doi: 10.1016/j.molcel.2012.08.010. Epub 2012/09/18, PubMed PMID: 22981861; PMCID: PMC3473360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shih Y.C., Chen C.L., Zhang Y., Mellor R.L., Kanter E.M., Fang Y., Wang H.C., Hung C.T., Nong J.Y., Chen H.J., Lee T.H., Tseng Y.S., Chen C.N., Wu C.C., Lin S.L., Yamada K.A., Nerbonne J.M., Yang K.C. Endoplasmic reticulum protein TXNDC5 augments myocardial fibrosis by facilitating extracellular matrix protein folding and redox-sensitive cardiac fibroblast activation. Circ. Res. 2018;122(8):1052–1068. doi: 10.1161/circresaha.117.312130. Epub 2018/03/15, PubMed PMID: 29535165; PMCID: PMC5899016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ilani T., Alon A., Grossman I., Horowitz B., Kartvelishvily E., Cohen S.R., Fass D. A secreted disulfide catalyst controls extracellular matrix composition and function. Science (New York, NY) 2013;341(6141):74–76. doi: 10.1126/science.1238279. Epub 2013/05/25, PubMed PMID: 23704371. [DOI] [PubMed] [Google Scholar]
- 114.Uchida M., Miyoshi T., Miyamoto Y. Pharmacological effects of a vitamin K1 2,3-epoxide reductase (VKOR) inhibitor, 3-acetyl-5-methyltetronic acid, on cisplatin-induced fibrosis in rats. J. Vet. Med. Sci. 2017;79(9):1507–1515. doi: 10.1292/jvms.17-0216. Epub 2017/07/19, PubMed PMID: 28717059; PMCID: PMC5627320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fang J., Yang J., Wu X., Zhang G., Li T., Wang X., Zhang H., Wang C.C., Liu G.H., Wang L. Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell. 2018;17(4):e12765. doi: 10.1111/acel.12765. Epub 2018/04/17, PubMed PMID: 29659168; PMCID: PMC6052468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wei P.C., Hsieh Y.H., Su M.I., Jiang X., Hsu P.H., Lo W.T., Weng J.Y., Jeng Y.M., Wang J.M., Chen P.L., Chang Y.C., Lee K.F., Tsai M.D., Shew J.Y., Lee W.H. Loss of the oxidative stress sensor NPGPx compromises GRP78 chaperone activity and induces systemic disease. Mol. Cell. 2012;48(5):747–759. doi: 10.1016/j.molcel.2012.10.007. Epub 2012/11/06, PubMed PMID: 23123197; PMCID: PMC3582359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nabeshima A., Yamada S., Guo X., Tanimoto A., Wang K.Y., Shimajiri S., Kimura S., Tasaki T., Noguchi H., Kitada S., Watanabe T., Fujii J., Kohno K., Sasaguri Y. Peroxiredoxin 4 protects against nonalcoholic steatohepatitis and type 2 diabetes in a nongenetic mouse model. Antioxidants Redox Signal. 2013;19(17):1983–1998. doi: 10.1089/ars.2012.4946. Epub 2013/03/13, PubMed PMID: 23477499; PMCID: PMC3869472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cantin A.M., Hubbard R.C., Crystal R.G. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1989;139(2):370–372. doi: 10.1164/ajrccm/139.2.370. Epub 1989/02/01. PubMed PMID: 2913886. [DOI] [PubMed] [Google Scholar]
- 119.Cantin A.M., North S.L., Fells G.A., Hubbard R.C., Crystal R.G. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J. Clin. Invest. 1987;79(6):1665–1673. doi: 10.1172/JCI113005. PubMed PMID: 3034979; PMCID: PMC424497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kinnula V.L., Fattman C.L., Tan R.J., Oury T.D. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am. J. Respir. Crit. Care Med. 2005;172(4):417–422. doi: 10.1164/rccm.200501-017PP. Epub 2005/05/17, PubMed PMID: 15894605; PMCID: PMC2718525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lederer D.J., Martinez F.J. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 2018;378(19):1811–1823. doi: 10.1056/NEJMra1705751. Epub 2018/05/10, PubMed PMID: 29742380. [DOI] [PubMed] [Google Scholar]
- 122.Fattman C.L., Tan R.J., Tobolewski J.M., Oury T.D. Increased sensitivity to asbestos-induced lung injury in mice lacking extracellular superoxide dismutase. Free Radic. Biol. Med. 2006;40(4):601–607. doi: 10.1016/j.freeradbiomed.2005.09.030. PubMed PMID: 16458190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cho H.Y., Reddy S.P., Yamamoto M., Kleeberger S.R. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18(11):1258–1260. doi: 10.1096/fj.03-1127fje. PubMed PMID: 15208274, Epub 2004/06/23. [DOI] [PubMed] [Google Scholar]
- 124.Day B.J. Antioxidants as potential therapeutics for lung fibrosis. Antioxidants Redox Signal. 2008;10(2):355–370. doi: 10.1089/ars.2007.1916. PubMed PMID: 17999627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu R.M. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxidants Redox Signal. 2008;10(2):303–320. doi: 10.1089/ars.2007.1903. PubMed PMID: 17979497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hecker L., Vittal R., Jones T., Jagirdar R., Luckhardt T.R., Horowitz J.C., Pennathur S., Martinez F.J., Thannickal V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15(9):1077–1081. doi: 10.1038/nm. Epub 2009/08/25, 2005. PubMed PMID: 19701206; PMCID: PMC2743335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hecker L., Logsdon N.J., Kurundkar D., Kurundkar A., Bernard K., Hock T., Meldrum E., Sanders Y.Y., Thannickal V.J. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci. Transl. Med. 2014;6(231) doi: 10.1126/scitranslmed.3008182. 231ra47. Epub 2014/04/11, PubMed PMID: 24718857; PMCID: PMC4545252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Beeh K.M., Beier J., Haas I.C., Kornmann O., Micke P., Buhl R. Glutathione deficiency of the lower respiratory tract in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2002;19(6):1119–1123. doi: 10.1183/09031936.02.00262402. Epub 2002/07/11. PubMed PMID: 12108866. [DOI] [PubMed] [Google Scholar]
- 129.Rahman I., MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am. J. Physiol. 1999;277(6):L1067–L1088. doi: 10.1152/ajplung.1999.277.6.L1067. Epub 1999/12/22, PubMed PMID: 10600876. [DOI] [PubMed] [Google Scholar]
- 130.Psathakis K., Mermigkis D., Papatheodorou G., Loukides S., Panagou P., Polychronopoulos V., Siafakas N.M., Bouros D. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur. J. Clin. Invest. 2006;36(5):362–367. doi: 10.1111/j.1365-2362.2006.01636.x. Epub 2006/04/26, PubMed PMID: 16634841. [DOI] [PubMed] [Google Scholar]
- 131.Cantin A.M., Larivee P., Begin R.O. Extracellular glutathione suppresses human lung fibroblast proliferation. Am. J. Respir. Cell Mol. Biol. 1990;3(1):79–85. doi: 10.1165/ajrcmb/3.1.79. Epub 1990/07/01, PubMed PMID: 2363938. [DOI] [PubMed] [Google Scholar]
- 132.Sime P.J., Xing Z., Graham F.L., Csaky K.G., Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta 1 induces prolonged severe fibrosis in rat lung. J. Clin. Invest. 1997;100(4):768–776. doi: 10.1172/jci119590. Epub 1997/08/15, PubMed PMID: 9259574; PMCID: PMC508247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu R.M., Vayalil P.K., Ballinger C., Dickinson D.A., Huang W.T., Wang S., Kavanagh T.J., Matthews Q.L., Postlethwait E.M. Transforming growth factor beta suppresses glutamate-cysteine ligase gene expression and induces oxidative stress in a lung fibrosis model. Free Radic. Biol. Med. 2012;53(3):554–563. doi: 10.1016/j.freeradbiomed.2012.05.016. Epub 2012/05/29, PubMed PMID: 22634145; PMCID: PMC3432394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Espinosa-Diez C., Fierro-Fernandez M., Sanchez-Gomez F., Rodriguez-Pascual F., Alique M., Ruiz-Ortega M., Beraza N., Martinez-Chantar M.L., Fernandez-Hernando C., Lamas S. Targeting of gamma-glutamyl-cysteine ligase by miR-433 reduces glutathione biosynthesis and promotes TGF-beta-dependent fibrogenesis. Antioxidants Redox Signal. 2015;23(14):1092–1105. doi: 10.1089/ars.2014.6025. Epub 2014/10/30, PubMed PMID: 25353619; PMCID: PMC4657521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ryoo I.G., Shin D.H., Kang K.S., Kwak M.K. Involvement of Nrf2-GSH signaling in TGFbeta1-stimulated epithelial-to-mesenchymal transition changes in rat renal tubular cells. Arch Pharm. Res. 2015;38(2):272–281. doi: 10.1007/s12272-014-0380-y. Epub 2014/05/23, PubMed PMID: 24849033. [DOI] [PubMed] [Google Scholar]
- 136.Iyer S.S., Ramirez A.M., Ritzenthaler J.D., Torres-Gonzalez E., Roser-Page S., Mora A.L., Brigham K.L., Jones D.P., Roman J., Rojas M. Oxidation of extracellular cysteine/cystine redox state in bleomycin-induced lung fibrosis. Am. J. Physiol. 2009;296(1):L37–L45. doi: 10.1152/ajplung.90401.2008. Epub 2008/10/22, PubMed PMID: 18931052; PMCID: PMC2636957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hagiwara S.I., Ishii Y., Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am. J. Respir. Crit. Care Med. 2000;162(1):225–231. doi: 10.1164/ajrccm.162.1.9903129. Epub 2000/07/21, PubMed PMID: 10903246. [DOI] [PubMed] [Google Scholar]
- 138.Li S., Yang X., Li W., Li J., Su X., Chen L., Yan G. N-acetylcysteine downregulation of lysyl oxidase activity alleviating bleomycin-induced pulmonary fibrosis in rats. Respiration. international review of thoracic diseases. 2012;84(6):509–517. doi: 10.1159/000340041. Epub 2012/09/26, PubMed PMID: 23006535. [DOI] [PubMed] [Google Scholar]
- 139.Demedts M., Behr J., Buhl R., Costabel U., Dekhuijzen R., Jansen H.M., MacNee W., Thomeer M., Wallaert B., Laurent F., Nicholson A.G., Verbeken E.K., Verschakelen J., Flower C.D., Capron F., Petruzzelli S., De Vuyst P., van den Bosch J.M., Rodriguez-Becerra E., Corvasce G., Lankhorst I., Sardina M., Montanari M. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2005;353(21):2229–2242. doi: 10.1056/NEJMoa042976. PubMed PMID: 16306520. [DOI] [PubMed] [Google Scholar]
- 140.Behr J., Demedts M., Buhl R., Costabel U., Dekhuijzen R.P., Jansen H.M., MacNee W., Thomeer M., Wallaert B., Laurent F., Nicholson A.G., Verbeken E.K., Verschakelen J., Flower C.D., Petruzzelli S., De Vuyst P., van den Bosch J.M., Rodriguez-Becerra E., Lankhorst I., Sardina M., Boissard G. Lung function in idiopathic pulmonary fibrosis–extended analyses of the IFIGENIA trial. Respir. Res. 2009;10:101. doi: 10.1186/1465-9921-10-101. Epub 2009/10/29, PubMed PMID: 19860915; PMCID: 2774307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martinez F.J., de Andrade J.A., Anstrom K.J., King T.E., Jr., Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370(22):2093–2101. doi: 10.1056/NEJMoa1401739. Epub 2014/05/20, PubMed PMID: 24836309; PMCID: PMC4116664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oldham J.M., Ma S.F., Martinez F.J., Anstrom K.J., Raghu G., Schwartz D.A., Valenzi E., Witt L., Lee C., Vij R., Huang Y., Strek M.E., Noth I. TOLLIP, MUC5B, and the response to N-acetylcysteine among individuals with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2015;192(12):1475–1482. doi: 10.1164/rccm.201505-1010OC. Epub 2015/09/04, PubMed PMID: 26331942; PMCID: PMC4731723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aesif S.W., Anathy V., Havermans M., Guala A.S., Ckless K., Taatjes D.J., Janssen-Heininger Y.M. In situ analysis of protein S-glutathionylation in lung tissue using glutaredoxin-1-catalyzed cysteine derivatization. Am. J. Pathol. 2009;175(1):36–45. doi: 10.2353/ajpath.2009.080736. Epub 2009/06/27, PubMed PMID: 19556513; PMCID: PMC2708792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McMillan D.H., van der Velden J.L., Lahue K.G., Qian X., Schneider R.W., Iberg M.S., Nolin J.D., Abdalla S., Casey D.T., Tew K.D., Townsend D.M., Henderson C.J., Wolf C.R., Butnor K.J., Taatjes D.J., Budd R.C., Irvin C.G., van der Vliet A., Flemer S., Anathy V., Janssen-Heininger Y.M. Attenuation of lung fibrosis in mice with a clinically relevant inhibitor of glutathione-S-transferase pi. JCI insight. 2016;1(8) doi: 10.1172/jci.insight.85717. Epub 2016/07/01, PubMed PMID: 27358914; PMCID: PMC4922427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peltoniemi M., Kaarteenaho-Wiik R., Saily M., Sormunen R., Paakko P., Holmgren A., Soini Y., Kinnula V.L. Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor-beta in vitro and in interstitial lung diseases in vivo. Hum. Pathol. 2004;35(8):1000–1007. doi: 10.1016/j.humpath.2004.04.009. PubMed PMID: 15297967. [DOI] [PubMed] [Google Scholar]
- 146.Peltoniemi M.J., Rytila P.H., Harju T.H., Soini Y.M., Salmenkivi K.M., Ruddock L.W., Kinnula V.L. Modulation of glutaredoxin in the lung and sputum of cigarette smokers and chronic obstructive pulmonary disease. Respir. Res. 2006;7:133. doi: 10.1186/1465-9921-7-133. PubMed PMID: 17064412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reynaert N.L., Wouters E.F., Janssen-Heininger Y.M. Modulation of glutaredoxin-1 expression in a mouse model of allergic airway disease. Am. J. Respir. Cell Mol. Biol. 2007;36(2):147–151. doi: 10.1165/rcmb.2006-0259RC. PubMed PMID: 16980552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Matute-Bello G., Wurfel M.M., Lee J.S., Park D.R., Frevert C.W., Madtes D.K., Shapiro S.D., Martin T.R. Essential role of MMP-12 in Fas-induced lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2007;37(2):210–221. doi: 10.1165/rcmb.2006-0471OC. PubMed PMID: 17446527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kuwano K., Kunitake R., Maeyama T., Hagimoto N., Kawasaki M., Matsuba T., Yoshimi M., Inoshima I., Yoshida K., Hara N. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am. J. Physiol. 2001;280(2):L316–L325. doi: 10.1152/ajplung.2001.280.2.L316. PubMed PMID: 11159011. [DOI] [PubMed] [Google Scholar]
- 150.Selman M., Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc. Am. Thorac. Soc. 2006;3(4):364–372. doi: 10.1513/pats.200601-003TK. Epub 2006/06/02, PubMed PMID: 16738202. [DOI] [PubMed] [Google Scholar]
- 151.Perl A.K., Riethmacher D., Whitsett J.A. Conditional depletion of airway progenitor cells induces peribronchiolar fibrosis. Am. J. Respir. Crit. Care Med. 2011;183(4):511–521. doi: 10.1164/rccm.201005-0744OC. Epub 2010/09/28, PubMed PMID: 20870756; PMCID: 3056226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sisson T.H., Mendez M., Choi K., Subbotina N., Courey A., Cunningham A., Dave A., Engelhardt J.F., Liu X., White E.S., Thannickal V.J., Moore B.B., Christensen P.J., Simon R.H. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010;181(3):254–263. doi: 10.1164/rccm.200810-1615OC. Epub 2009/10/24, PubMed PMID: 19850947; PMCID: 2817814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kuwano K., Hagimoto N., Kawasaki M., Yatomi T., Nakamura N., Nagata S., Suda T., Kunitake R., Maeyama T., Miyazaki H., Hara N. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J. Clin. Invest. 1999;104(1):13–19. doi: 10.1172/JCI5628. PubMed PMID: 10393694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wallach-Dayan S.B., Golan-Gerstl R., Breuer R. Evasion of myofibroblasts from immune surveillance: a mechanism for tissue fibrosis. Proc. Natl. Acad. Sci. U. S. A. 2007;104(51):20460–20465. doi: 10.1073/pnas.0705582104. PubMed PMID: 18077384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Golan-Gerstl R., Wallach-Dayan S.B., Zisman P., Cardoso W.V., Goldstein R.H., Breuer R. Cellular FLICE-like inhibitory protein deviates myofibroblast fas-induced apoptosis toward proliferation during lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2012;47(3):271–279. doi: 10.1165/rcmb.2010-0284RC. Epub 2012/05/15, PubMed PMID: 22582174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Waghray M., Cui Z., Horowitz J.C., Subramanian I.M., Martinez F.J., Toews G.B., Thannickal V.J. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. Off. Pub. Feder. Am. Soc. Exp. Biol. 2005;19(7):854–856. doi: 10.1096/fj.04-2882fje. Epub 2005/04/29, PubMed PMID: 15857893. [DOI] [PubMed] [Google Scholar]
- 157.Wynes M.W., Edelman B.L., Kostyk A.G., Edwards M.G., Coldren C., Groshong S.D., Cosgrove G.P., Redente E.F., Bamberg A., Brown K.K., Reisdorph N., Keith R.C., Frankel S.K., Riches D.W. Increased cell surface Fas expression is necessary and sufficient to sensitize lung fibroblasts to Fas ligation-induced apoptosis: implications for fibroblast accumulation in idiopathic pulmonary fibrosis. J. Immunol. 2011;187(1):527–537. doi: 10.4049/jimmunol.1100447. Epub 2011/06/03, PubMed PMID: 21632719. [DOI] [PubMed] [Google Scholar]
- 158.Bamberg A., Redente E.F., Groshong S.D., Tuder R.M., Cool C.D., Keith R.C., Edelman B.L., Black B.P., Cosgrove G.P., Wynes M.W., Curran-Everett D., De Langhe S., Ortiz L.A., Thorburn A., Riches D.W.H. Protein tyrosine phosphatase-N13 promotes myofibroblast resistance to apoptosis in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2018;198(7):914–927. doi: 10.1164/rccm.201707-1497OC. Epub 2018/05/05, PubMed PMID: 29727583; PMCID: PMC6173065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Anathy V., Aesif S.W., Guala A.S., Havermans M., Reynaert N.L., Ho Y.S., Budd R.C., Janssen-Heininger Y.M. Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas. J. Cell Biol. 2009;184(2):241–252. doi: 10.1083/jcb.200807019. Epub 2009/01/28, PubMed PMID: 19171757; PMCID: PMC2654302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Raza A., Galili N., Callander N., Ochoa L., Piro L., Emanuel P., Williams S., Burris H., 3rd, Faderl S., Estrov Z., Curtin P., Larson R.A., Keck J.G., Jones M., Meng L., Brown G.L. Phase 1-2a multicenter dose-escalation study of ezatiostat hydrochloride liposomes for injection (Telintra, TLK199), a novel glutathione analog prodrug in patients with myelodysplastic syndrome. J. Hematol. Oncol. 2009;2:20. doi: 10.1186/1756-8722-2-20. Epub 2009/05/15, PubMed PMID: 19439093; PMCID: PMC2694211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Raza A., Galili N., Smith S.E., Godwin J., Boccia R.V., Myint H., Mahadevan D., Mulford D., Rarick M., Brown G.L., Schaar D., Faderl S., Komrokji R.S., List A.F., Sekeres M. A phase 2 randomized multicenter study of 2 extended dosing schedules of oral ezatiostat in low to intermediate-1 risk myelodysplastic syndrome. Cancer. 2012;118(8):2138–2147. doi: 10.1002/cncr.26469. Epub 2011/09/03, PubMed PMID: 21887679. [DOI] [PubMed] [Google Scholar]
- 162.Laborde E. Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ. 2010;17(9):1373–1380. doi: 10.1038/cdd.2010.80. Epub 2010/07/03, PubMed PMID: 20596078. [DOI] [PubMed] [Google Scholar]
- 163.Morgan A.S., Ciaccio P.J., Tew K.D., Kauvar L.M. Isozyme-specific glutathione S-transferase inhibitors potentiate drug sensitivity in cultured human tumor cell lines. Canc. Chemother. Pharmacol. 1996;37(4):363–370. doi: 10.1007/s002800050398. Epub 1996/01/01. PubMed PMID: 8548883. [DOI] [PubMed] [Google Scholar]
- 164.Bon J., Kahloon R., Zhang Y., Xue J., Fuhrman C.R., Tan J., Burger M., Kass D.J., Csizmadia E., Otterbein L., Chandra D., Bhargava A., Pilewski J.M., Roodman G.D., Sciurba F.C., Duncan S.R. Autoreactivity to glucose regulated protein 78 links emphysema and osteoporosis in smokers. PloS One. 2014;9(9) doi: 10.1371/journal.pone.0105066. Epub 2014/09/13, PubMed PMID: 25216103; PMCID: PMC4162538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Min T., Bodas M., Mazur S., Vij N. Critical role of proteostasis-imbalance in pathogenesis of COPD and severe emphysema. J. Mol. Med. 2011;89(6):577–593. doi: 10.1007/s00109-011-0732-8. Epub 2011/02/15, PubMed PMID: 21318260; PMCID: PMC3128462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Geraghty P., Baumlin N., Salathe M.A., Foronjy R.F., D'Armiento J.M. Glutathione peroxidase-1 suppresses the unfolded protein response upon cigarette smoke exposure. Mediat. Inflamm. 2016 doi: 10.1155/2016/9461289. 2016:9461289. Epub 2017/01/11, PubMed PMID: 28070146; PMCID: PMC5187475 has an interest in the subject of this manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sakhatskyy P., Gabino Miranda G.A., Newton J., Lee C.G., Choudhary G., Vang A., Rounds S., Lu Q. Cigarette smoke-induced lung endothelial apoptosis and emphysema are associated with impairment of FAK and eIF2alpha. Microvasc. Res. 2014;94:80–89. doi: 10.1016/j.mvr.2014.05.003. Epub 2014/05/24, PubMed PMID: 24853558; PMCID: PMC4185927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Weidner J., Jarenback L., Aberg I., Westergren-Thorsson G., Ankerst J., Bjermer L., Tufvesson E. Endoplasmic reticulum, Golgi, and lysosomes are disorganized in lung fibroblasts from chronic obstructive pulmonary disease patients. Physiological reports. 2018;(5):6. doi: 10.14814/phy2.13584. Epub 2018/02/28, PubMed PMID: 29484832; PMCID: PMC5827558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Barreiro E., Salazar-Degracia A., Sancho-Munoz A., Aguilo R., Rodriguez-Fuster A., Gea J. Endoplasmic reticulum stress and unfolded protein response in diaphragm muscle dysfunction of patients with stable chronic obstructive pulmonary disease. J. Appl. Physiol. 2019;126(6):1572–1586. doi: 10.1152/japplphysiol.00670.2018. Epub 2019/04/19, PubMed PMID: 30998124. [DOI] [PubMed] [Google Scholar]
- 170.Kelsen S.G., Duan X., Ji R., Perez O., Liu C., Merali S. Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am. J. Respir. Cell Mol. Biol. 2008;38(5):541–550. doi: 10.1165/rcmb.2007-0221OC. Epub 2007/12/15, PubMed PMID: 18079489. [DOI] [PubMed] [Google Scholar]
- 171.Jorgensen E., Stinson A., Shan L., Yang J., Gietl D., Albino A.P. Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Canc. 2008;8:229. doi: 10.1186/1471-2407-8-229. Epub 2008/08/13, PubMed PMID: 18694499; PMCID: PMC2527015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tagawa Y., Hiramatsu N., Kasai A., Hayakawa K., Okamura M., Yao J., Kitamura M. Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP) Free Radic. Biol. Med. 2008;45(1):50–59. doi: 10.1016/j.freeradbiomed.2008.03.003. Epub 2008/04/09, PubMed PMID: 18394432. [DOI] [PubMed] [Google Scholar]
- 173.Tagawa Y., Hiramatsu N., Kato H., Sakoh T., Nakajima S., Hayakawa K., Saito Y., Johno H., Takahashi S., Gu L., Yao J., Kitamura M. Induction of CCAAT/enhancer-binding protein-homologous protein by cigarette smoke through the superoxide anion-triggered PERK-eIF2alpha pathway. Toxicology. 2011;287(1–3):105–112. doi: 10.1016/j.tox.2011.06.005. Epub 2011/06/28, PubMed PMID: 21703327. [DOI] [PubMed] [Google Scholar]
- 174.Kitaguchi Y., Taraseviciene-Stewart L., Hanaoka M., Natarajan R., Kraskauskas D., Voelkel N.F. Acrolein induces endoplasmic reticulum stress and causes airspace enlargement. PloS One. 2012;7(5):e38038. doi: 10.1371/journal.pone.0038038. Epub 2012/06/08, PubMed PMID: 22675432; PMCID: PMC3364999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hengstermann A., Muller T. Endoplasmic reticulum stress induced by aqueous extracts of cigarette smoke in 3T3 cells activates the unfolded-protein-response-dependent PERK pathway of cell survival. Free Radic. Biol. Med. 2008;44(6):1097–1107. doi: 10.1016/j.freeradbiomed.2007.12.009. Epub 2008/01/22, PubMed PMID: 18206657. [DOI] [PubMed] [Google Scholar]
- 176.Kenche H., Ye Z.W., Vedagiri K., Richards D.M., Gao X.H., Tew K.D., Townsend D.M., Blumental-Perry A. Adverse outcomes associated with cigarette smoke radicals related to damage to protein-disulfide isomerase. J. Biol. Chem. 2016;291(9):4763–4778. doi: 10.1074/jbc.M115.712331. Epub 2016/01/06, PubMed PMID: 26728460; PMCID: PMC4813498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dabo A.J., Ezegbunam W., Wyman A.E., Moon J., Railwah C., Lora A., Majka S.M., Geraghty P., Foronjy R.F. Targeting c-src reverses accelerated GPX-1 mRNA decay in COPD airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2019 doi: 10.1165/rcmb.2019-0177OC. Epub 2019/12/05, PubMed PMID: 31801023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kenche H., Baty C.J., Vedagiri K., Shapiro S.D., Blumental-Perry A. Cigarette smoking affects oxidative protein folding in endoplasmic reticulum by modifying protein disulfide isomerase. FASEB J. Off. Pub. Feder. Am. Soc. Exp. Biol. 2013;27(3):965–977. doi: 10.1096/fj.12-216234. Epub 2012/11/22, PubMed PMID: 23169770. [DOI] [PubMed] [Google Scholar]
- 179.Wang Y., Wu Z.Z., Wang W. Inhibition of endoplasmic reticulum stress alleviates cigarette smoke-induced airway inflammation and emphysema. Oncotarget. 2017;8(44):77685–77695. doi: 10.18632/oncotarget.20768. Epub 2017/11/05, PubMed PMID: 29100417; PMCID: PMC5652808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Aggarwal B.B., Deb L., Prasad S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules. 2014;20(1):185–205. doi: 10.3390/molecules20010185. Epub 2014/12/31, PubMed PMID: 25547723; PMCID: PMC6272158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tang F., Ling C. Curcumin ameliorates chronic obstructive pulmonary disease by modulating autophagy and endoplasmic reticulum stress through regulation of SIRT1 in a rat model. J. Int. Med. Res. 2019;47(10):4764–4774. doi: 10.1177/0300060519869459. Epub 2019/09/13, PubMed PMID: 31510839; PMCID: PMC6833429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang Y.M., Liu G.T. Damaging effect of cigarette smoke extract on primary cultured human umbilical vein endothelial cells and its mechanism. Biomedical and environmental sciences. BES. 2004;17(2):121–134. Epub 2004/09/25. PubMed PMID: 15386938. [PubMed] [Google Scholar]
- 183.Petit A., Knabe L., Khelloufi K., Jory M., Gras D., Cabon Y., Begg M., Richard S., Massiera G., Chanez P., Vachier I., Bourdin A. Bronchial epithelial calcium metabolism impairment in smokers and chronic obstructive pulmonary disease. Decreased ORAI3 signaling. Am. J. Respir. Cell Mol. Biol. 2019;61(4):501–511. doi: 10.1165/rcmb.2018-0228OC. Epub 2019/04/04, PubMed PMID: 30943377. [DOI] [PubMed] [Google Scholar]
- 184.Aghapour M., Remels A.H.V., Pouwels S.D., Bruder D., Hiemstra P.S., Cloonan S.M., Heijink I.H. Mitochondria: at the crossroads of regulating lung epithelial cell function in chronic obstructive pulmonary disease. Am. J. Physiol. 2019 doi: 10.1152/ajplung.00329.2019. Epub 2019/11/07, PubMed PMID: 31693390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Delbrel E., Uzunhan Y., Soumare A., Gille T., Marchant D., Planes C., Boncoeur E. ER stress is involved in epithelial-to-mesenchymal transition of alveolar epithelial cells exposed to a hypoxic microenvironment. Int. J. Mol. Sci. 2019;20(6) doi: 10.3390/ijms20061299. Epub 2019/03/17, PubMed PMID: 30875855; PMCID: PMC6470993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Aggarwal S., Ahmad I., Lam A., Carlisle M.A., Li C., Wells J.M., Raju S.V., Athar M., Rowe S.M., Dransfield M.T., Matalon S. Heme scavenging reduces pulmonary endoplasmic reticulum stress, fibrosis, and emphysema. JCI Insight. 2018;3(21) doi: 10.1172/jci.insight.120694. Epub 2018/11/06, PubMed PMID: 30385726; PMCID: PMC6238745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Perlmutter D.H. alpha 1-antitrypsin deficiency: a misfolded secretory protein variant with unique effects on the endoplasmic reticulum. Endoplasmic Reticulum Stress Diseases. 2016;3(1):63–72. doi: 10.1515/ersc-2016-0004. Epub 2017/02/22, PubMed PMID: 28217691; PMCID: PMC5310618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Alam S., Li Z., Atkinson C., Jonigk D., Janciauskiene S., Mahadeva R. Z alpha 1-antitrypsin confers a proinflammatory phenotype that contributes to chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014;189(8):909–931. doi: 10.1164/rccm.201308-1458OC. Epub 2014/03/07, PubMed PMID: 24592811; PMCID: PMC4098095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hidvegi T., Stolz D.B., Alcorn J.F., Yousem S.A., Wang J., Leme A.S., Houghton A.M., Hale P., Ewing M., Cai H., Garchar E.A., Pastore N., Annunziata P., Kaminski N., Pilewski J., Shapiro S.D., Pak S.C., Silverman G.A., Brunetti-Pierri N., Perlmutter D.H. Enhancing autophagy with drugs or lung-directed gene therapy reverses the pathological effects of respiratory epithelial cell proteinopathy. J. Biol. Chem. 2015;290(50):29742–29757. doi: 10.1074/jbc.M115.691253. Epub 2015/10/24, PubMed PMID: 26494620; PMCID: PMC4705969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hassan T., Carroll T.P., Buckley P.G., Cummins R., O'Neill S.J., McElvaney N.G., Greene C.M. miR-199a-5p silencing regulates the unfolded protein response in chronic obstructive pulmonary disease and alpha 1-antitrypsin deficiency. Am. J. Respir. Crit. Care Med. 2014;189(3):263–273. doi: 10.1164/rccm.201306-1151OC. Epub 2013/12/05, PubMed PMID: 24299514. [DOI] [PubMed] [Google Scholar]
- 191.Mizuno S., Bogaard H.J., Gomez-Arroyo J., Alhussaini A., Kraskauskas D., Cool C.D., Voelkel N.F. MicroRNA-199a-5p is associated with hypoxia-inducible factor-1 alpha expression in lungs from patients with COPD. Chest. 2012;142(3):663–672. doi: 10.1378/chest.11-2746. Epub 2012/03/03, PubMed PMID: 22383663; PMCID: PMC3435138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.van 't Wout Ef van Schadewijk A, van Boxtel R, dalton LE, clarke HJ, tommassen J, marciniak SJ, hiemstra PS. Virulence factors of Pseudomonas aeruginosa induce both the unfolded protein and integrated stress responses in airway epithelial cells. PLoS Pathog. 2015;11(6) doi: 10.1371/journal.ppat.1004946. e1004946. Epub 2015/06/18, PubMed PMID: 26083346; PMCID: PMC4471080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zarcone M.C., van Schadewijk A., Duistermaat E., Hiemstra P.S., Kooter I.M. Diesel exhaust alters the response of cultured primary bronchial epithelial cells from patients with chronic obstructive pulmonary disease (COPD) to non-typeable Haemophilus influenzae. Respir. Res. 2017;18(1):27. doi: 10.1186/s12931-017-0510-4. Epub 2017/01/29, PubMed PMID: 28129777; PMCID: PMC5273858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Richardson C.E., Kooistra T., Kim D.H. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature. 2010;463(7284):1092–1095. doi: 10.1038/nature08762. Epub 2010/02/26, PubMed PMID: 20182512; PMCID: PMC2834299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Clavarino G., Claudio N., Couderc T., Dalet A., Judith D., Camosseto V., Schmidt E.K., Wenger T., Lecuit M., Gatti E., Pierre P. Induction of GADD34 is necessary for dsRNA-dependent interferon-beta production and participates in the control of Chikungunya virus infection. PLoS Pathog. 2012;8(5):e1002708. doi: 10.1371/journal.ppat.1002708. Epub 2012/05/23, PubMed PMID: 22615568; PMCID: PMC3355096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.He B., Luo B., Chen Q., Zhang L. Cigarette smoke extract induces the expression of GRP78 in A549 cells via the p38/MAPK pathway. Mol. Med. Rep. 2013;8(6):1683–1688. doi: 10.3892/mmr.2013.1724. Epub 2013/10/16, PubMed PMID: 24126384. [DOI] [PubMed] [Google Scholar]
- 197.Kelsen S.G. Respiratory epithelial cell responses to cigarette smoke: the unfolded protein response. Pulm. Pharmacol. Therapeut. 2012;25(6):447–452. doi: 10.1016/j.pupt.2012.07.005. Epub 2012/08/01, PubMed PMID: 22846757. [DOI] [PubMed] [Google Scholar]
- 198.Aksoy M.O., Kim V., Cornwell W.D., Rogers T.J., Kosmider B., Bahmed K., Barrero C., Merali S., Shetty N., Kelsen S.G. Secretion of the endoplasmic reticulum stress protein, GRP78, into the BALF is increased in cigarette smokers. Respir. Res. 2017;18(1):78. doi: 10.1186/s12931-017-0561-6. Epub 2017/05/04, PubMed PMID: 28464871; PMCID: PMC5414124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Merali S., Barrero C.A., Bowler R.P., Chen D.E., Criner G., Braverman A., Litwin S., Yeung A., Kelsen S.G. Analysis of the plasma proteome in COPD: novel low abundance proteins reflect the severity of lung remodeling. COPD. 2014;11(2):177–189. doi: 10.3109/15412555.2013.831063. Epub 2013/10/12, PubMed PMID: 24111704; PMCID: PMC4497366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Corrigall V.M., Bodman-Smith M.D., Brunst M., Cornell H., Panayi G.S. Inhibition of antigen-presenting cell function and stimulation of human peripheral blood mononuclear cells to express an antiinflammatory cytokine profile by the stress protein BiP: relevance to the treatment of inflammatory arthritis. Arthritis Rheum. 2004;50(4):1164–1171. doi: 10.1002/art.20134. Epub 2004/04/13, PubMed PMID: 15077298. [DOI] [PubMed] [Google Scholar]
- 201.Purcell A.W., Todd A., Kinoshita G., Lynch T.A., Keech C.L., Gething M.J., Gordon T.P. Association of stress proteins with autoantigens: a possible mechanism for triggering autoimmunity? Clin. Exp. Immunol. 2003;132(2):193–200. doi: 10.1046/j.1365-2249.2003.02153.x. Epub 2003/04/18, PubMed PMID: 12699405; PMCID: PMC1808692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kahloon R.A., Xue J., Bhargava A., Csizmadia E., Otterbein L., Kass D.J., Bon J., Soejima M., Levesque M.C., Lindell K.O., Gibson K.F., Kaminski N., Banga G., Oddis C.V., Pilewski J.M., Sciurba F.C., Donahoe M., Zhang Y., Duncan S.R. Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses. Am. J. Respir. Crit. Care Med. 2013;187(7):768–775. doi: 10.1164/rccm.201203-0506OC. Epub 2012/12/25, PubMed PMID: 23262513; PMCID: PMC3678112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Labunskyy V.M., Gerashchenko M.V., Delaney J.R., Kaya A., Kennedy B.K., Kaeberlein M., Gladyshev V.N. Lifespan extension conferred by endoplasmic reticulum secretory pathway deficiency requires induction of the unfolded protein response. PLoS Genet. 2014;10(1):e1004019. doi: 10.1371/journal.pgen.1004019. Epub 2014/01/07, PubMed PMID: 24391512; PMCID: PMC3879150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tang Y., Cai Q.H., Wang Y.J., Fan S.H., Zhang Z.F., Xiao M.Q., Zhu J.Y., Wu D.M., Lu J., Zheng Y.L. Protective effect of autophagy on endoplasmic reticulum stress induced apoptosis of alveolar epithelial cells in rat models of COPD. Biosci. Rep. 2017;37(6) doi: 10.1042/bsr20170803. Epub 2017/10/01, PubMed PMID: 28963374; PMCID: PMC5686393. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 205.Tan S.X., Jiang D.X., Hu R.C., Dai A.G., Gan G.X., Fu D.Y., Kong C.C., Chen Y.R., Wang L.L., Li J. Endoplasmic reticulum stress induces HRD1 to protect alveolar type II epithelial cells from apoptosis induced by cigarette smoke extract. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry. pharmacology. 2017;43(4):1337–1345. doi: 10.1159/000481845. Epub 2017/10/11, PubMed PMID: 28992619. [DOI] [PubMed] [Google Scholar]
- 206.Somborac-Bacura A., van der Toorn M., Franciosi L., Slebos D.J., Zanic-Grubisic T., Bischoff R., van Oosterhout A.J. Cigarette smoke induces endoplasmic reticulum stress response and proteasomal dysfunction in human alveolar epithelial cells. Exp. Physiol. 2013;98(1):316–325. doi: 10.1113/expphysiol.2012.067249. Epub 2012/08/01, PubMed PMID: 22848082. [DOI] [PubMed] [Google Scholar]
- 207.Marklew A.J., Patel W., Moore P.J., Tan C.D., Smith A.J., Sassano M.F., Gray M.A., Tarran R. Cigarette smoke exposure induces retrograde trafficking of CFTR to the endoplasmic reticulum. Sci. Rep. 2019;9(1):13655. doi: 10.1038/s41598-019-49544-9. Epub 2019/09/22, PubMed PMID: 31541117; PMCID: PMC6754399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kirkham P.A., Barnes P.J. Oxidative stress in COPD. Chest. 2013;144(1):266–273. doi: 10.1378/chest.12-2664. Epub 2013/07/25, PubMed PMID: 23880677. [DOI] [PubMed] [Google Scholar]
- 209.Nagai K., Betsuyaku T., Suzuki M., Nasuhara Y., Kaga K., Kondo S., Nishimura M. Dual oxidase 1 and 2 expression in airway epithelium of smokers and patients with mild/moderate chronic obstructive pulmonary disease. Antioxidants Redox Signal. 2008;10(4):705–714. doi: 10.1089/ars.2007.1941. Epub 2008/01/08, PubMed PMID: 18177232. [DOI] [PubMed] [Google Scholar]
- 210.van der Vliet A. NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic. Biol. Med. 2008;44(6):938–955. doi: 10.1016/j.freeradbiomed.2007.11.016. Epub 2008/01/01, PubMed PMID: 18164271; PMCID: PMC2323509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu X., Hao B., Ma A., He J., Liu X., Chen J. The expression of NOX4 in smooth muscles of small airway correlates with the disease severity of COPD. BioMed Res. Int. 2016 doi: 10.1155/2016/2891810. 2016:2891810. Epub 2016/09/23, PubMed PMID: 27656649; PMCID: PMC5021463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hollins F., Sutcliffe A., Gomez E., Berair R., Russell R., Szyndralewiez C., Saunders R., Brightling C. Airway smooth muscle NOX4 is upregulated and modulates ROS generation in COPD. Respir. Res. 2016;17(1):84. doi: 10.1186/s12931-016-0403-y. Epub 2016/07/21, PubMed PMID: 27435477; PMCID: PMC4950777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Beeh K.M., Beier J., Koppenhoefer N., Buhl R. Increased glutathione disulfide and nitrosothiols in sputum supernatant of patients with stable COPD. Chest. 2004;126(4):1116–1122. doi: 10.1378/chest.126.4.1116. Epub 2004/10/16, PubMed PMID: 15486372. [DOI] [PubMed] [Google Scholar]
- 214.Elko E.A., Mahoney J.M., Vacek P., van der Vliet A., Anathy V., van der Velden J., Janssen-Heininger Y.M.W., Seward D.J. Age-dependent dysregulation of redox genes may contribute to fibrotic pulmonary disease susceptibility. Free Radic. Biol. Med. 2019;141:438–446. doi: 10.1016/j.freeradbiomed.2019.07.011. Epub 2019/07/18, PubMed PMID: 31315063; PMCID: PMC6820706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhou L., Zhang H., Davies K.J.A., Forman H.J. Aging-related decline in the induction of Nrf2-regulated antioxidant genes in human bronchial epithelial cells. Redox biology. 2018;14:35–40. doi: 10.1016/j.redox.2017.08.014. Epub 2017/09/02, PubMed PMID: 28863281; PMCID: PMC5576992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gould N.S., Min E., Huang J., Chu H.W., Good J., Martin R.J., Day B.J. Glutathione depletion accelerates cigarette smoke-induced inflammation and airspace enlargement. Toxicol. Sci. Off. J. Soc. Toxicol. 2015;147(2):466–474. doi: 10.1093/toxsci/kfv143. Epub 2015/07/08, PubMed PMID: 26149495; PMCID: PMC4707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Goven D., Boutten A., Lecon-Malas V., Marchal-Somme J., Amara N., Crestani B., Fournier M., Leseche G., Soler P., Boczkowski J., Bonay M. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax. 2008;63(10):916–924. doi: 10.1136/thx.2007.091181. Epub 2008/06/19, PubMed PMID: 18559366. [DOI] [PubMed] [Google Scholar]
- 218.Rangasamy T., Cho C.Y., Thimmulappa R.K., Zhen L., Srisuma S.S., Kensler T.W., Yamamoto M., Petrache I., Tuder R.M., Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Invest. 2004;114(9):1248–1259. doi: 10.1172/jci21146. Epub 2004/11/03, PubMed PMID: 15520857; PMCID: PMC524225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sussan T.E., Rangasamy T., Blake D.J., Malhotra D., El-Haddad H., Bedja D., Yates M.S., Kombairaju P., Yamamoto M., Liby K.T., Sporn M.B., Gabrielson K.L., Champion H.C., Tuder R.M., Kensler T.W., Biswal S. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc. Natl. Acad. Sci. U. S. A. 2009;106(1):250–255. doi: 10.1073/pnas.0804333106. Epub 2008/12/24, PubMed PMID: 19104057; PMCID: PMC2629210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wise R.A., Holbrook J.T., Criner G., Sethi S., Rayapudi S., Sudini K.R., Sugar E.A., Burke A., Thimmulappa R., Singh A., Talalay P., Fahey J.W., Berenson C.S., Jacobs M.R., Biswal S. Lack of effect of oral sulforaphane administration on Nrf2 expression in COPD: a randomized, double-blind, placebo controlled trial. PloS One. 2016;11(11) doi: 10.1371/journal.pone.0163716. e0163716. Epub 2016/11/11, PubMed PMID: 27832073; PMCID: PMC5104323 and AstraZeneca/Medimmune, and personal fees from Bristol-Myers-Squibb, GlaxoSmithKline, Mylan, Novartis, Pfizer, Pulmonx, Spiration, Roche/Genentech, Janssen, Merck, Teva, Forest Labs, and Sanofi/Mannkind, outside of submitted work. Drs. Fahey and Talalay report multiple US and foreign issued patents related to broccoli sprouts and sulforaphane. Dr. Biswal reports a pending patent on methods for targeting Nrf2 pathway for oxidative stress related disease. The relevant patents held by Drs. Fahey, Talalay and Biswal are listed below. Drs. Fahey and Talalay have relinquished all ownership and any fiduciary or management involvement in Brassica Protection Products (BPP). They are no longer either Board Members or equity holders. In addition, they have instructed JHU NOT to collect the 'inventor's share' of any royalties that may accrue to JHU from the patents originating with their work on broccoli sprouts, licensed to BPP or anyone else. This does not alter our adherence to PLOS ONE policies on sharing data and materials. 1998 Fahey JW, P Talalay."Method of Preparing a Food Product from Cruciferous Seeds". U.S. Patent 5,725,895. 1999 Fahey JW, P Talalay. "Cancer Chemoprotective Food Products." U.S. Patent 5,968,505. 1999 Fahey JW, P Talalay. "Method of Preparing a Food Product From Cruciferous Sprouts." U.S. Patent 5,968,567. 2001 Fahey JW, P Talalay. "Method of Preparing a Food Product From Cruciferous Sprouts." U.S. Patent 6,177,122. 2001 Fahey JW, P Talalay. "Cancer Chemoprotective Food Products." U.S. Patent 6,242,018. 2001 Fahey JW, P Talalay, J Troyer. Airlift Bioreactor for Harvesting Sprouts. U.S. Patent Application 09/450,753 and PCT WO 01/39591 A2. 2003 Fahey JW. Development of Novel, Highly Chemoprotectant Crucifer Germplasm. U.S. Patent 6,521,818. 2004 Fahey JW. Treatment of Helicobacter with Isothiocyanates. U.S. Patent 6,737,441. 2004 Fahey JW. Method for the direct extraction of isothiocyanates into the seed oil from glucosinolate-containing plant seeds. U.S. Provisional Patent Application # 60/635,998 and U.S. Patent Application (2005). 2007 Fahey JW and P Talalay. "Cancer Chemoprotective Food Products." U.S. Patent 7,303,770 B2. 2008 Fahey JW. Treatment of Helicobacter with Isothiocyanates. U.S. Patent 7,402,569. 2010 Talalay P, JW Fahey, A Talalay. Formulations for Treatment with Glucosinolates. U.S. Provisional Patent Application #61/383,853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Grassi C., Morandini G.C. A controlled trial of intermittent oral acetylcysteine in the long-term treatment of chronic bronchitis. Eur. J. Clin. Pharmacol. 1976;9(5–6):393–396. doi: 10.1007/bf00606554. Epub 1976/03/22, PubMed PMID: 786665. [DOI] [PubMed] [Google Scholar]
- 222.Black P.N., Morgan-Day A., McMillan T.E., Poole P.J., Young R.P. Randomised, controlled trial of N-acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344] BMC Pulm. Med. 2004;4:13. doi: 10.1186/1471-2466-4-13. Epub 2004/12/08, PubMed PMID: 15581425; PMCID: PMC539269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tse H.N., Raiteri L., Wong K.Y., Yee K.S., Ng L.Y., Wai K.Y., Loo C.K., Chan M.H. High-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest. 2013;144(1):106–118. doi: 10.1378/chest.12-2357. Epub 2013/01/26, PubMed PMID: 23348146. [DOI] [PubMed] [Google Scholar]
- 224.Tse H.N., Raiteri L., Wong K.Y., Ng L.Y., Yee K.S., Tseng C.Z.S. Benefits of high-dose N-acetylcysteine to exacerbation-prone patients with COPD. Chest. 2014;146(3):611–623. doi: 10.1378/chest.13-2784. Epub 2014/05/17, PubMed PMID: 24833327. [DOI] [PubMed] [Google Scholar]
- 225.Papi A., Zheng J., Criner G.J., Fabbri L.M., Calverley P.M.A. Impact of smoking status and concomitant medications on the effect of high-dose N-acetylcysteine on chronic obstructive pulmonary disease exacerbations: a post-hoc analysis of the PANTHEON study. Respir. Med. 2019;147:37–43. doi: 10.1016/j.rmed.2018.12.014. Epub 2019/02/02, PubMed PMID: 30704697. [DOI] [PubMed] [Google Scholar]
- 226.Rogliani P., Matera M.G., Page C., Puxeddu E., Cazzola M., Calzetta L. Efficacy and safety profile of mucolytic/antioxidant agents in chronic obstructive pulmonary disease: a comparative analysis across erdosteine, carbocysteine, and N-acetylcysteine. Respir. Res. 2019;20(1):104. doi: 10.1186/s12931-019-1078-y. Epub 2019/05/28, PubMed PMID: 31133026; PMCID: PMC6537173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yuan S., Hollinger M., Lachowicz-Scroggins M.E., Kerr S.C., Dunican E.M., Daniel B.M., Ghosh S., Erzurum S.C., Willard B., Hazen S.L., Huang X., Carrington S.D., Oscarson S., Fahy J.V. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci. Transl. Med. 2015;7(276) doi: 10.1126/scitranslmed.3010525. 276ra27. Epub 2015/02/27, PubMed PMID: 25717100; PMCID: PMC4403633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lundberg M., Fernandes A.P., Kumar S., Holmgren A. Cellular and plasma levels of human glutaredoxin 1 and 2 detected by sensitive ELISA systems. Biochem. Biophys. Res. Commun. 2004;319(3):801–809. doi: 10.1016/j.bbrc.2004.04.199. Epub 2004/06/09, PubMed PMID: 15184054. [DOI] [PubMed] [Google Scholar]
- 229.Harju T., Mazur W., Merikallio H., Soini Y., Kinnula V.L. Glutathione-S-transferases in lung and sputum specimens, effects of smoking and COPD severity. Respir. Res. 2008;9:80. doi: 10.1186/1465-9921-9-80. Epub 2008/12/17, PubMed PMID: 19077292; PMCID: PMC2654438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ishii T., Fujishiro M., Masuda M., Nakajima J., Teramoto S., Ouchi Y., Matsuse T. Depletion of glutathione S-transferase P1 induces apoptosis in human lung fibroblasts. Exp. Lung Res. 2003;29(7):523–536. doi: 10.1080/01902140303777. Epub 2004/01/09, PubMed PMID: 14710442. [DOI] [PubMed] [Google Scholar]
- 231.Yang L., Li X., Tong X., Fan H. Association between glutathione S-transferase P1 Ile (105) Val gene polymorphism and chronic obstructive pulmonary disease: a meta-analysis based on seventeen case-control studies. Meta gene. 2015;6:59–64. doi: 10.1016/j.mgene.2015.08.007. Epub 2015/10/28, PubMed PMID: 26504746; PMCID: PMC4576405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kuipers I., Bracke K.R., Brusselle G.G., Aesif S.W., Krijgsman R., Arts I.C., Wouters E.F., Reynaert N.L. Altered cigarette smoke-induced lung inflammation due to ablation of Grx1. PloS One. 2012;7(6):e38984. doi: 10.1371/journal.pone.0038984. Epub 2012/06/23, PubMed PMID: 22723915; PMCID: PMC3377591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kuipers I., Bracke K.R., Brusselle G.G., Wouters E.F., Reynaert N.L. Smoke decreases reversible oxidations S-glutathionylation and S-nitrosylation in mice. Free Radic. Res. 2012;46(2):164–173. doi: 10.3109/10715762.2011.647011. Epub 2011/12/08, PubMed PMID: 22145974. [DOI] [PubMed] [Google Scholar]
- 234.Aesif S.W., Anathy V., Kuipers I., Guala A.S., Reiss J.N., Ho Y.S., Janssen-Heininger Y.M. Ablation of glutaredoxin-1 attenuates lipopolysaccharide-induced lung inflammation and alveolar macrophage activation. Am. J. Respir. Cell Mol. Biol. 2011;44(4):491–499. doi: 10.1165/rcmb.2009-0136OC. Epub 2010/06/12, PubMed PMID: 20539014; PMCID: PMC3095922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chung S., Sundar I.K., Yao H., Ho Y.S., Rahman I. Glutaredoxin 1 regulates cigarette smoke-mediated lung inflammation through differential modulation of I{kappa}B kinases in mice: impact on histone acetylation. Am. J. Physiol. 2010;299(2):L192–L203. doi: 10.1152/ajplung.00426.2009. Epub 2010/05/18, PubMed PMID: 20472709; PMCID: PMC2928604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Reynaert N.L., van der Vliet A., Guala A.S., McGovern T., Hristova M., Pantano C., Heintz N.H., Heim J., Ho Y.S., Matthews D.E., Wouters E.F., Janssen-Heininger Y.M. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc. Natl. Acad. Sci. U. S. A. 2006;103(35):13086–13091. doi: 10.1073/pnas.0603290103. Epub 2006/08/19, PubMed PMID: 16916935; PMCID: PMC1559757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dubey M., Singh A.K., Awasthi D., Nagarkoti S., Kumar S., Ali W., Chandra T., Kumar V., Barthwal M.K., Jagavelu K., Sanchez-Gomez F.J., Lamas S., Dikshit M. L-Plastin S-glutathionylation promotes reduced binding to beta-actin and affects neutrophil functions. Free Radic. Biol. Med. 2015;86:1–15. doi: 10.1016/j.freeradbiomed.2015.04.008. Epub 2015/04/18, PubMed PMID: 25881549. [DOI] [PubMed] [Google Scholar]
- 238.Sakai J., Li J., Subramanian K.K., Mondal S., Bajrami B., Hattori H., Jia Y., Dickinson B.C., Zhong J., Ye K., Chang C.J., Ho Y.S., Zhou J., Luo H.R. Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity. 2012;37(6):1037–1049. doi: 10.1016/j.immuni.2012.08.017. Epub 2012/11/20, PubMed PMID: 23159440; PMCID: PMC3525814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.You Y., Chen J., Zhu F., Xu Q., Han L., Gao X., Zhang X., Luo H.R., Miao J., Sun X., Ren H., Du Y., Guo L., Wang X., Wang Y., Chen S., Huang N., Li J. Glutaredoxin 1 up-regulates deglutathionylation of alpha 4 integrin and thereby restricts neutrophil mobilization from bone marrow. J. Biol. Chem. 2019;294(8):2616–2627. doi: 10.1074/jbc.RA118.006096. Epub 2019/01/02, PubMed PMID: 30598505; PMCID: PMC6393595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dicker A.J., Crichton M.L., Pumphrey E.G., Cassidy A.J., Suarez-Cuartin G., Sibila O., Furrie E., Fong C.J., Ibrahim W., Brady G., Einarsson G.G., Elborn J.S., Schembri S., Marshall S.E., Palmer C.N.A., Chalmers J.D. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018;141(1):117–127. doi: 10.1016/j.jaci.2017.04.022. Epub 2017/05/17, PubMed PMID: 28506850; PMCID: PMC5751731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Juneau R.A., Pang B., Armbruster C.E., Murrah K.A., Perez A.C., Swords W.E. Peroxiredoxin-glutaredoxin and catalase promote resistance of nontypeable Haemophilus influenzae 86-028NP to oxidants and survival within neutrophil extracellular traps. Infect. Immun. 2015;83(1):239–246. doi: 10.1128/iai.02390-14. Epub 2014/10/29, PubMed PMID: 25348637; PMCID: PMC4288874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Okamoto T., Akaike T., Sawa T., Miyamoto Y., van der Vliet A., Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J. Biol. Chem. 2001;276(31):29596–29602. doi: 10.1074/jbc.M102417200. Epub 2001/06/08, PubMed PMID: 11395496. [DOI] [PubMed] [Google Scholar]
- 243.Schroeder B.W., Verhaeghe C., Park S.W., Nguyenvu L.T., Huang X., Zhen G., Erle D.J. AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am. J. Respir. Cell Mol. Biol. 2012;47(2):178–185. doi: 10.1165/rcmb.2011-0421OC. Epub 2012/03/10, PubMed PMID: 22403803; PMCID: PMC3423459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Das S., Miller M., Broide D.H. Chromosome 17q21 genes ORMDL3 and GSDMB in asthma and immune diseases. Adv. Immunol. 2017;135:1–52. doi: 10.1016/bs.ai.2017.06.001. Epub 2017/08/23, PubMed PMID: 28826527. [DOI] [PubMed] [Google Scholar]
- 245.Verlaan D.J., Berlivet S., Hunninghake G.M., Madore A.M., Lariviere M., Moussette S., Grundberg E., Kwan T., Ouimet M., Ge B., Hoberman R., Swiatek M., Dias J., Lam K.C., Koka V., Harmsen E., Soto-Quiros M., Avila L., Celedon J.C., Weiss S.T., Dewar K., Sinnett D., Laprise C., Raby B.A., Pastinen T., Naumova A.K. Allele-specific chromatin remodeling in the ZPBP2/GSDMB/ORMDL3 locus associated with the risk of asthma and autoimmune disease. Am. J. Hum. Genet. 2009;85(3):377–393. doi: 10.1016/j.ajhg.2009.08.007. Epub 2009/09/08, PubMed PMID: 19732864; PMCID: PMC2771592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.James B., Milstien S., Spiegel S. ORMDL3 and allergic asthma: from physiology to pathology. J. Allergy Clin. Immunol. 2019;144(3):634–640. doi: 10.1016/j.jaci.2019.07.023. Epub 2019/08/04, PubMed PMID: 31376405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Miller M., Rosenthal P., Beppu A., Mueller J.L., Hoffman H.M., Tam A.B., Doherty T.A., McGeough M.D., Pena C.A., Suzukawa M., Niwa M., Broide D.H. ORMDL3 transgenic mice have increased airway remodeling and airway responsiveness characteristic of asthma. Journal of immunology (Baltimore, Md. 1950) 2014;192(8):3475–3487. doi: 10.4049/jimmunol.1303047. Epub 2014/03/14, PubMed PMID: 24623133; PMCID: PMC3981544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Unno H., Miller M., Rosenthal P., Beppu A., Das S., Broide D.H. Activating transcription factor 6 alpha (ATF6alpha) regulates airway hyperreactivity, smooth muscle proliferation, and contractility. J. Allergy Clin. Immunol. 2018;141(1):439–442. doi: 10.1016/j.jaci.2017.07.053. e4. Epub 2017/09/30, PubMed PMID: 28958904; PMCID: PMC5758405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wang Y., Zhu J., Zhang L., Zhang Z., He L., Mou Y., Deng Y., Cao Y., Yang P., Su Y., Zhao J., Zhang S., Yu Q., Hu J., Chen Z., Ning Q., Xiang X., Xu Y., Wang C.Y., Xiong W. Role of C/EBP homologous protein and endoplasmic reticulum stress in asthma exacerbation by regulating the IL-4/signal transducer and activator of transcription 6/transcription factor EC/IL-4 receptor alpha positive feedback loop in M2 macrophages. J. Allergy Clin. Immunol. 2017;140(6):1550–1561. doi: 10.1016/j.jaci.2017.01.024. e8. Epub 2017/02/28, PubMed PMID: 28238747. [DOI] [PubMed] [Google Scholar]
- 250.Bhakta N.R., Christenson S.A., Nerella S., Solberg O.D., Nguyen C.P., Choy D.F., Jung K.L., Garudadri S., Bonser L.R., Pollack J.L., Zlock L.T., Erle D.J., Langelier C., Derisi J.L., Arron J.R., Fahy J.V., Woodruff P.G. IFN-stimulated gene expression, type 2 inflammation, and endoplasmic reticulum stress in asthma. Am. J. Respir. Crit. Care Med. 2018;197(3):313–324. doi: 10.1164/rccm.201706-1070OC. Epub 2017/10/25, PubMed PMID: 29064281; PMCID: PMC5811952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kim S.R., Lee Y.C. Endoplasmic reticulum stress and the related signaling networks in severe asthma. Allergy, asthma & immunology research. 2015;7(2):106–117. doi: 10.4168/aair.2015.7.2.106. Epub 2015/03/03, PubMed PMID: 25729617; PMCID: PMC4341331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Jeong J.S., Kim S.R., Cho S.H., Lee Y.C. A novel insight on endotyping heterogeneous severe asthma based on endoplasmic reticulum stress: beyond the "type 2/non-type 2 dichotomy". Int. J. Mol. Sci. 2019;20(3) doi: 10.3390/ijms20030713. Epub 2019/02/10, PubMed PMID: 30736433; PMCID: PMC6386842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bonser L.R., Erle D.J. The airway epithelium in asthma. Adv. Immunol. 2019;142:1–34. doi: 10.1016/bs.ai.2019.05.001. Epub 2019/07/13, PubMed PMID: 31296301. [DOI] [PubMed] [Google Scholar]
- 254.Hoffman S.M., Chapman D.G., Lahue K.G., Cahoon J.M., Rattu G.K., Daphtary N., Aliyeva M., Fortner K.A., Erzurum S.C., Comhair S.A., Woodruff P.G., Bhakta N., Dixon A.E., Irvin C.G., Janssen-Heininger Y.M., Poynter M.E., Anathy V. Protein disulfide isomerase-endoplasmic reticulum resident protein 57 regulates allergen-induced airways inflammation, fibrosis, and hyperresponsiveness. J. Allergy Clin. Immunol. 2016;137(3):822–832. doi: 10.1016/j.jaci.2015.08.018. e7. Epub 2015/10/06, PubMed PMID: 26435004; PMCID: PMC4597791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nakada E.M., Bhakta N.R., Korwin-Mihavics B.R., Kumar A., Chamberlain N., Bruno S.R., Chapman D.G., Hoffman S.M., Daphtary N., Aliyeva M., Irvin C.G., Dixon A.E., Woodruff P.G., Amin S., Poynter M.E., Desai D.H., Anathy V. Conjugated bile acids attenuate allergen-induced airway inflammation and hyperresponsiveness by inhibiting UPR transducers. JCI insight. 2019;4(9) doi: 10.1172/jci.insight.98101. Epub 2019/05/03, PubMed PMID: 31045581; PMCID: PMC6538331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hoffman S.M., Tully J.E., Nolin J.D., Lahue K.G., Goldman D.H., Daphtary N., Aliyeva M., Irvin C.G., Dixon A.E., Poynter M.E., Anathy V. Endoplasmic reticulum stress mediates house dust mite-induced airway epithelial apoptosis and fibrosis. Respir. Res. 2013;14:141. doi: 10.1186/1465-9921-14-141. Epub 2013/12/25, PubMed PMID: 24364984; PMCID: PMC3877992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kranz P., Neumann F., Wolf A., Classen F., Pompsch M., Ocklenburg T., Baumann J., Janke K., Baumann M., Goepelt K., Riffkin H., Metzen E., Brockmeier U. PDI is an essential redox-sensitive activator of PERK during the unfolded protein response (UPR) Cell Death Dis. 2017;8(8):e2986. doi: 10.1038/cddis.2017.369. Epub 2017/08/11, PubMed PMID: 28796255; PMCID: PMC5596557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Eletto D., Eletto D., Dersh D., Gidalevitz T., Argon Y. Protein disulfide isomerase A6 controls the decay of IRE1alpha signaling via disulfide-dependent association. Mol. Cell. 2014;53(4):562–576. doi: 10.1016/j.molcel.2014.01.004. Epub 2014/02/11, PubMed PMID: 24508390; PMCID: PMC3977204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Higa A., Taouji S., Lhomond S., Jensen D., Fernandez-Zapico M.E., Simpson J.C., Pasquet J.M., Schekman R., Chevet E. Endoplasmic reticulum stress-activated transcription factor ATF6alpha requires the disulfide isomerase PDIA5 to modulate chemoresistance. Mol. Cell Biol. 2014;34(10):1839–1849. doi: 10.1128/mcb.01484-13. Epub 2014/03/19, PubMed PMID: 24636989; PMCID: PMC4019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Oka O.B., van Lith M., Rudolf J., Tungkum W., Pringle M.A., Bulleid N.J. ERp18 regulates activation of ATF6alpha during unfolded protein response. EMBO J. 2019;38(15) doi: 10.15252/embj.2018100990. Epub 2019/08/02, PubMed PMID: 31368601; PMCID: PMC6670016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Stopa J.D., Neuberg D., Puligandla M., Furie B., Flaumenhaft R., Zwicker J.I. Protein disulfide isomerase inhibition blocks thrombin generation in humans by interfering with platelet factor V activation. JCI insight. 2017;2(1):e89373. doi: 10.1172/jci.insight.89373. Epub 2017/01/18, PubMed PMID: 28097231; PMCID: PMC5214567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kaplan A., Gaschler M.M., Dunn D.E., Colligan R., Brown L.M., Palmer A.G., 3rd, Lo D.C., Stockwell B.R. Small molecule-induced oxidation of protein disulfide isomerase is neuroprotective. Proc. Natl. Acad. Sci. U. S. A. 2015;112(17):E2245–E2252. doi: 10.1073/pnas.1500439112. Epub 2015/04/08, PubMed PMID: 25848045; PMCID: PMC4418888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Elia A.E., Lalli S., Monsurro M.R., Sagnelli A., Taiello A.C., Reggiori B., La Bella V., Tedeschi G., Albanese A. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 2016;23(1):45–52. doi: 10.1111/ene.12664. Epub 2015/02/11, PubMed PMID: 25664595; PMCID: PMC5024041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kars M., Yang L., Gregor M.F., Mohammed B.S., Pietka T.A., Finck B.N., Patterson B.W., Horton J.D., Mittendorfer B., Hotamisligil G.S., Klein S. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59(8):1899–1905. doi: 10.2337/db10-0308. Epub 2010/06/05, PubMed PMID: 20522594; PMCID: PMC2911053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Fitzpatrick A.M., Jones D.P., Brown L.A. Glutathione redox control of asthma: from molecular mechanisms to therapeutic opportunities. Antioxidants Redox Signal. 2012;17(2):375–408. doi: 10.1089/ars.2011.4198. Epub 2012/02/07, PubMed PMID: 22304503; PMCID: PMC3353819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Reynaert N.L. Glutathione biochemistry in asthma. Biochim. Biophys. Acta. 2011;1810(11):1045–1051. doi: 10.1016/j.bbagen.2011.01.010. Epub 2011/02/02, PubMed PMID: 21281701. [DOI] [PubMed] [Google Scholar]
- 267.Rangasamy T., Guo J., Mitzner W.A., Roman J., Singh A., Fryer A.D., Yamamoto M., Kensler T.W., Tuder R.M., Georas S.N., Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 2005;202(1):47–59. doi: 10.1084/jem.20050538. Epub 2005/07/07, PubMed PMID: 15998787; PMCID: PMC2212893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Fitzpatrick A.M., Stephenson S.T., Hadley G.R., Burwell L., Penugonda M., Simon D.M., Hansen J., Jones D.P., Brown L.A. Thiol redox disturbances in children with severe asthma are associated with posttranslational modification of the transcription factor nuclear factor (erythroid-derived 2)-like 2. J. Allergy Clin. Immunol. 2011;127(6):1604–1611. doi: 10.1016/j.jaci.2011.03.031. Epub 2011/04/26, PubMed PMID: 21514635; PMCID: PMC3105207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lowry M.H., McAllister B.P., Jean J.C., Brown L.A., Hughey R.P., Cruikshank W.W., Amar S., Lucey E.C., Braun K., Johnson P., Wight T.N., Joyce-Brady M. Lung lining fluid glutathione attenuates IL-13-induced asthma. Am. J. Respir. Cell Mol. Biol. 2008;38(5):509–516. doi: 10.1165/rcmb.2007-0128OC. Epub 2007/12/08, PubMed PMID: 18063838; PMCID: PMC2335334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tuzova M., Jean J.C., Hughey R.P., Brown L.A., Cruikshank W.W., Hiratake J., Joyce-Brady M. Inhibiting lung lining fluid glutathione metabolism with GGsTop as a novel treatment for asthma. Front. Pharmacol. 2014;5:179. doi: 10.3389/fphar.2014.00179. Epub 2014/08/19, PubMed PMID: 25132819; PMCID: PMC4116799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nadeem A., Siddiqui N., Alharbi N.O., Alharbi M.M., Imam F., Sayed-Ahmed M.M. Glutathione modulation during sensitization as well as challenge phase regulates airway reactivity and inflammation in mouse model of allergic asthma. Biochimie. 2014;103:61–70. doi: 10.1016/j.biochi.2014.04.001. Epub 2014/04/20, PubMed PMID: 24742380. [DOI] [PubMed] [Google Scholar]
- 272.Barnett S.D., Buxton I.L.O. The role of S-nitrosoglutathione reductase (GSNOR) in human disease and therapy. Crit. Rev. Biochem. Mol. Biol. 2017;52(3):340–354. doi: 10.1080/10409238.2017.1304353. Epub 2017/04/11, PubMed PMID: 28393572; PMCID: PMC5597050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Que L.G., Yang Z., Stamler J.S., Lugogo N.L., Kraft M. S-nitrosoglutathione reductase: an important regulator in human asthma. Am. J. Respir. Crit. Care Med. 2009;180(3):226–231. doi: 10.1164/rccm.200901-0158OC. Epub 2009/04/28, PubMed PMID: 19395503; PMCID: PMC2724715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Que L.G., Liu L., Yan Y., Whitehead G.S., Gavett S.H., Schwartz D.A., Stamler J.S. Protection from experimental asthma by an endogenous bronchodilator. Science (New York, NY) 2005;308(5728):1618–1621. doi: 10.1126/science.1108228. Epub 2005/05/28, PubMed PMID: 15919956; PMCID: PMC2128762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Que L.G., Yang Z., Lugogo N.L., Katial R.K., Shoemaker S.A., Troha J.M., Rodman D.M., Tighe R.M., Kraft M. Effect of the S-nitrosoglutathione reductase inhibitor N6022 on bronchial hyperreactivity in asthma. Immunity, inflammation and disease. 2018;6(2):322–331. doi: 10.1002/iid3.220. Epub 2018/04/12, PubMed PMID: 29642282; PMCID: PMC5946144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Yamamoto T., Miyata J., Arita M., Fukunaga K., Kawana A. Current state and future prospect of the therapeutic strategy targeting cysteinyl leukotriene metabolism in asthma. Respiratory investigation. 2019;57(6):534–543. doi: 10.1016/j.resinv.2019.08.003. Epub 2019/10/09, PubMed PMID: 31591069. [DOI] [PubMed] [Google Scholar]
- 277.Dunican E.M., Elicker B.M., Gierada D.S., Nagle S.K., Schiebler M.L., Newell J.D., Raymond W.W., Lachowicz-Scroggins M.E., Di Maio S., Hoffman E.A., Castro M., Fain S.B., Jarjour N.N., Israel E., Levy B.D., Erzurum S.C., Wenzel S.E., Meyers D.A., Bleecker E.R., Phillips B.R., Mauger D.T., Gordon E.D., Woodruff P.G., Peters M.C., Fahy J.V. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J. Clin. Invest. 2018;128(3):997–1009. doi: 10.1172/jci95693. Epub 2018/02/06, PubMed PMID: 29400693; PMCID: PMC5824874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Comhair S.A., Bhathena P.R., Farver C., Thunnissen F.B., Erzurum S.C. Extracellular glutathione peroxidase induction in asthmatic lungs: evidence for redox regulation of expression in human airway epithelial cells. FASEB journal. official publication of the Federation of American Societies for Experimental Biology. 2001;15(1):70–78. doi: 10.1096/fj.00-0085com. Epub 2001/01/10, PubMed PMID: 11149894. [DOI] [PubMed] [Google Scholar]
- 279.Dittrich A.M., Meyer H.A., Krokowski M., Quarcoo D., Ahrens B., Kube S.M., Witzenrath M., Esworthy R.S., Chu F.F., Hamelmann E. Glutathione peroxidase-2 protects from allergen-induced airway inflammation in mice. Eur. Respir. J. 2010;35(5):1148–1154. doi: 10.1183/09031936.00026108. Epub 2009/11/10, PubMed PMID: 19897562; PMCID: PMC2911780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Piacentini S., Polimanti R., Simonelli I., Donno S., Pasqualetti P., Manfellotto D., Fuciarelli M. Glutathione S-transferase polymorphisms, asthma susceptibility and confounding variables: a meta-analysis. Mol. Biol. Rep. 2013;40(4):3299–3313. doi: 10.1007/s11033-012-2405-2. Epub 2013/01/12, PubMed PMID: 23307299. [DOI] [PubMed] [Google Scholar]
- 281.Dai X., Bowatte G., Lowe A.J., Matheson M.C., Gurrin L.C., Burgess J.A., Dharmage S.C., Lodge C.J. Do glutathione S-transferase genes modify the link between indoor air pollution and asthma, allergies, and lung function? A systematic review. Curr. Allergy Asthma Rep. 2018;18(3):20. doi: 10.1007/s11882-018-0771-0. Epub 2018/03/21, PubMed PMID: 29557517. [DOI] [PubMed] [Google Scholar]
- 282.Lopez-Rodriguez J.C., Manosalva J., Cabrera-Garcia J.D., Escribese M.M., Villalba M., Barber D., Martinez-Ruiz A., Batanero E. Human glutathione-S-transferase pi potentiates the cysteine-protease activity of the Der p 1 allergen from house dust mite through a cysteine redox mechanism. Redox biology. 2019;26:101256. doi: 10.1016/j.redox.2019.101256. Epub 2019/06/24, PubMed PMID: 31229842; PMCID: PMC6597738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Schroer K.T., Gibson A.M., Sivaprasad U., Bass S.A., Ericksen M.B., Wills-Karp M., Lecras T., Fitzpatrick A.M., Brown L.A., Stringer K.F., Hershey G.K. Downregulation of glutathione S-transferase pi in asthma contributes to enhanced oxidative stress. J. Allergy Clin. Immunol. 2011;128(3):539–548. doi: 10.1016/j.jaci.2011.04.018. Epub 2011/05/17, PubMed PMID: 21570714; PMCID: PMC3164907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zhou J., Wolf C.R., Henderson C.J., Cai Y., Board P.G., Foster P.S., Webb D.C. Glutathione transferase P1: an endogenous inhibitor of allergic responses in a mouse model of asthma. Am. J. Respir. Crit. Care Med. 2008;178(12):1202–1210. doi: 10.1164/rccm.200801-178OC. Epub 2008/09/13, PubMed PMID: 18787219. [DOI] [PubMed] [Google Scholar]
- 285.Kuipers I., Louis R., Manise M., Dentener M.A., Irvin C.G., Janssen-Heininger Y.M., Brightling C.E., Wouters E.F., Reynaert N.L. Increased glutaredoxin-1 and decreased protein S-glutathionylation in sputum of asthmatics. Eur. Respir. J. 2013;41(2):469–472. doi: 10.1183/09031936.00115212. Epub 2013/02/02, PubMed PMID: 23370801; PMCID: PMC3855363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Pandey G., Pandey O.P., Rogers A.J., Ahsen M.E., Hoffman G.E., Raby B.A., Weiss S.T., Schadt E.E., Bunyavanich S. A nasal brush-based classifier of asthma identified by machine learning analysis of nasal RNA sequence data. Sci. Rep. 2018;8(1):8826. doi: 10.1038/s41598-018-27189-4. Epub 2018/06/13, PubMed PMID: 29891868; PMCID: PMC5995932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Hoffman S.M., Qian X., Nolin J.D., Chapman D.G., Chia S.B., Lahue K.G., Schneider R., Ather J.L., Randall M.J., McMillan D.H., Jones J.T., Taatjes D.J., Aliyeva M., Daphtary N., Abdalla S., Lundblad L.K., Ho Y.S., Anathy V., Irvin C.G., Wouters E.F., Reynaert N.L., Dixon A.E., van der Vliet A., Poynter M.E., Janssen-Heininger Y.M. Ablation of glutaredoxin-1 modulates house dust mite-induced allergic airways disease in mice. Am. J. Respir. Cell Mol. Biol. 2016;55(3):377–386. doi: 10.1165/rcmb.2015-0401OC. Epub 2016/04/02, PubMed PMID: 27035878; PMCID: PMC5023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Hoffman S.M., Tully J.E., Lahue K.G., Anathy V., Nolin J.D., Guala A.S., van der Velden J.L., Ho Y.S., Aliyeva M., Daphtary N., Lundblad L.K., Irvin C.G., Janssen-Heininger Y.M. Genetic ablation of glutaredoxin-1 causes enhanced resolution of airways hyperresponsiveness and mucus metaplasia in mice with allergic airways disease. Am. J. Physiol. 2012;303(6):L528–L538. doi: 10.1152/ajplung.00167.2012. Epub 2012/07/04, PubMed PMID: 22752969; PMCID: PMC3468477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Maki K., Nagai K., Suzuki M., Inomata T., Yoshida T., Nishimura M. Temporal changes in glutaredoxin 1 and protein s-glutathionylation in allergic airway inflammation. PloS One. 2015;10(4) doi: 10.1371/journal.pone.0122986. e0122986. Epub 2015/04/16, PubMed PMID: 25874776; PMCID: PMC4395207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhang Q., Lenardo M.J., Baltimore D. 30 Years of NF-kappaB: A Blossoming of Relevance to Human Pathobiology. Cell. 2017;168(1–2):37–57. doi: 10.1016/j.cell.2016.12.012. Epub 2017/01/14, PubMed PMID: 28086098; PMCID: PMC5268070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Tully J.E., Hoffman S.M., Lahue K.G., Nolin J.D., Anathy V., Lundblad L.K., Daphtary N., Aliyeva M., Black K.E., Dixon A.E., Poynter M.E., Irvin C.G., Janssen-Heininger Y.M. Epithelial NF-kappaB orchestrates house dust mite-induced airway inflammation, hyperresponsiveness, and fibrotic remodeling. Journal of immunology (Baltimore, Md. 1950) 2013;191(12):5811–5821. doi: 10.4049/jimmunol.1301329. Epub 2013/11/15, PubMed PMID: 24227776; PMCID: PMC3858534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Schuliga M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules. 2015;5(3):1266–1283. doi: 10.3390/biom5031266. Epub 2015/07/02, PubMed PMID: 26131974; PMCID: PMC4598751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Aesif S.W., Kuipers I., van der Velden J., Tully J.E., Guala A.S., Anathy V., Sheely J.I., Reynaert N.L., Wouters E.F., van der Vliet A., Janssen-Heininger Y.M. Activation of the glutaredoxin-1 gene by nuclear factor kappaB enhances signaling. Free Radic. Biol. Med. 2011;51(6):1249–1257. doi: 10.1016/j.freeradbiomed.2011.06.025. Epub 2011/07/19, PubMed PMID: 21762778; PMCID: PMC3181077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Nolin J.D., Tully J.E., Hoffman S.M., Guala A.S., van der Velden J.L., Poynter M.E., van der Vliet A., Anathy V., Janssen-Heininger Y.M. The glutaredoxin/S-glutathionylation axis regulates interleukin-17A-induced proinflammatory responses in lung epithelial cells in association with S-glutathionylation of nuclear factor kappaB family proteins. Free Radic. Biol. Med. 2014;73:143–153. doi: 10.1016/j.freeradbiomed.2014.04.028. Epub 2014/05/13, PubMed PMID: 24816292; PMCID: PMC4111997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Hughes M.M., Lavrencic P., Coll R.C., Ve T., Ryan D.G., Williams N.C., Menon D., Mansell A., Board P.G., Mobli M., Kobe B., O'Neill L.A.J. Solution structure of the TLR adaptor MAL/TIRAP reveals an intact BB loop and supports MAL Cys91 glutathionylation for signaling. Proc. Natl. Acad. Sci. U. S. A. 2017;114(32) doi: 10.1073/pnas.1701868114. E6480–e9. Epub 2017/07/26, PubMed PMID: 28739909; PMCID: PMC5559006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hristova M., Habibovic A., Veith C., Janssen-Heininger Y.M., Dixon A.E., Geiszt M., van der Vliet A. Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses. J. Allergy Clin. Immunol. 2016;137(5):1545–1556. doi: 10.1016/j.jaci.2015.10.003. e11. Epub 2015/11/26, PubMed PMID: 26597162; PMCID: PMC4860024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Habibovic A., Hristova M., Heppner D.E., Danyal K., Ather J.L., Janssen-Heininger Y.M., Irvin C.G., Poynter M.E., Lundblad L.K., Dixon A.E., Geiszt M., van der Vliet A. DUOX1 mediates persistent epithelial EGFR activation, mucous cell metaplasia, and airway remodeling during allergic asthma. JCI insight. 2016;1(18):e88811. doi: 10.1172/jci.insight.88811. Epub 2016/11/05, PubMed PMID: 27812543; PMCID: PMC5085603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hristova M., Veith C., Habibovic A., Lam Y.W., Deng B., Geiszt M., Janssen-Heininger Y.M., van der Vliet A. Identification of DUOX1-dependent redox signaling through protein S-glutathionylation in airway epithelial cells. Redox biology. 2014;2:436–446. doi: 10.1016/j.redox.2013.12.030. Epub 2014/03/14, PubMed PMID: 24624333; PMCID: PMC3949091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Heppner D.E., Hristova M., Dustin C.M., Danyal K., Habibovic A., van der Vliet A. The NADPH oxidases DUOX1 and NOX2 play distinct roles in redox regulation of epidermal growth factor receptor signaling. J. Biol. Chem. 2016;291(44):23282–23293. doi: 10.1074/jbc.M116.749028. Epub 2016/10/30, PubMed PMID: 27650496; PMCID: PMC5087744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Heppner D.E., Dustin C.M., Liao C., Hristova M., Veith C., Little A.C., Ahlers B.A., White S.L., Deng B., Lam Y.W., Li J., van der Vliet A. Direct cysteine sulfenylation drives activation of the Src kinase. Nat. Commun. 2018;9(1):4522. doi: 10.1038/s41467-018-06790-1. Epub 2018/10/31, PubMed PMID: 30375386; PMCID: PMC6207713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Paulsen C.E., Truong T.H., Garcia F.J., Homann A., Gupta V., Leonard S.E., Carroll K.S. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 2011;8(1):57–64. doi: 10.1038/nchembio.736. Epub 2011/12/14, PubMed PMID: 22158416; PMCID: PMC3528018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Weinberg E.O., Ferran B., Tsukahara Y., Hatch M.M.S., Han J., Murdoch C.E., Matsui R. IL-33 induction and signaling are controlled by glutaredoxin-1 in mouse macrophages. PloS One. 2019;14(1) doi: 10.1371/journal.pone.0210827. e0210827. Epub 2019/01/27, PubMed PMID: 30682073; PMCID: PMC6347181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Zhang X., Liu P., Zhang C., Chiewchengchol D., Zhao F., Yu H., Li J., Kambara H., Luo K.Y., Venkataraman A., Zhou Z., Zhou W., Zhu H., Zhao L., Sakai J., Chen Y., Ho Y.S., Bajrami B., Xu B., Silberstein L.E., Cheng T., Xu Y., Ke Y., Luo H.R. Positive regulation of interleukin-1 beta bioactivity by physiological ROS-mediated cysteine S-glutathionylation. Cell Rep. 2017;20(1):224–235. doi: 10.1016/j.celrep.2017.05.070. Epub 2017/07/07, PubMed PMID: 28683316; PMCID: PMC5758045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Bettigole S.E., Lis R., Adoro S., Lee A.H., Spencer L.A., Weller P.F., Glimcher L.H. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat. Immunol. 2015;16(8):829–837. doi: 10.1038/ni. Epub 2015/07/07, 3225. PubMed PMID: 26147683; PMCID: PMC4577297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Miller M., Tam A.B., Cho J.Y., Doherty T.A., Pham A., Khorram N., Rosenthal P., Mueller J.L., Hoffman H.M., Suzukawa M., Niwa M., Broide D.H. ORMDL3 is an inducible lung epithelial gene regulating metalloproteases, chemokines, OAS, and ATF6. Proc. Natl. Acad. Sci. U. S. A. 2012;109(41):16648–16653. doi: 10.1073/pnas.1204151109. Epub 2012/09/27, PubMed PMID: 23011799; PMCID: PMC3478632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.