Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is characterized by a high mortality of elderly men with age-related comorbidities. In most of these patients, uncontrolled local and systemic hyperinflammation induces severe and often lethal outcomes. The aging process is characterized by the gradual development of a chronic subclinical systemic inflammation (inflamm-aging) and by acquired immune system impairment (immune senescence). Here, we advance the hypothesis that four well-recognized features of aging contribute to the disproportionate SARS-CoV-2 mortality suffered by elderly men: i. the presence of subclinical systemic inflammation without overt disease, ii. a blunted acquired immune system and type I interferon response due to the chronic inflammation; iii. the downregulation of ACE2 (i.e. the SARS-CoV-2 receptor); and iv. accelerated biological aging. The high mortality rate of SARS-CoV-2 infection suggests that clarification of the mechanisms of inflamm-aging and immune senescence can help combat not only age-related disorders but also SARS-CoV-2 infection.
Keywords: SARS-CoV-2, COVID-19, interleukin-6, Cardiovascular diseases, Inflamm-aging, Host-directed therapies
1. Introduction
A novel coronavirus, Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2), has been spreading across the world since December 2019. SARS-CoV-2 causes an atypical pneumonia associated with acute respiratory distress syndrome, acute respiratory failure, and other potentially lethal complications [1] including a deadly "cytokine storm", i.e. a strong increase in the plasma concentration of multiple cytokines [2]. In March 2020 Corona Virus Disease 2019 (COVID-19), the disorder caused by SARS-CoV-2, was declared a pandemic by the World Health Organization [3]. Its high infectivity seems to be mostly due to intrinsic characteristics of the virus [4] and to the lack of previous exposure of the population to the strain. The elderly and patients with pre-existing comorbidities are bearing the brunt of the high case-fatality rate (CFR) of the disease, which is affecting the frailest groups of the population [5]. Data released by the Chinese Center for Disease Control and Prevention [6] suggest that the overall CFR of COVID-19 in China was 2.3%. In particular, whereas there were no fatalities among patients aged up to 9 years, the CFR of those aged 70–79 years and of those aged ≥ 80 years was 8.0% and 14.8%, respectively. Critically, pre-existing comorbidities were associated with a CFR of 10.5% (cardiovascular disease), 7.3% (diabetes), 6.3% (chronic respiratory disease), 6.0% (hypertension), and 5.6% (cancer) [7]. Moreover, men were more likely to die (2.8%) than women (1.7%) [6]. Thus, old age and male gender were among the main risk factors for an adverse outcome [8].
These data are similar to those of Italy, where on March 30th infections were 97,780 [9]. The median age of the deceased was 80 years (interquartile range, 30–103); only 1.1% of those who died were aged less than 50 years. Notably, 70.9% of fatalities were men, whose mean age was 78 years compared to the 82 years of women; men were also 78.5% of deceased patients aged less than 50 years. About 1.4% of the patients who died in Italy suffered from no pre-existing condition, whereas 51.2% had three or more age-related diseases (ARDs), such as cardiac ischemia, hypertension, type II diabetes mellitus, and chronic obstructive pulmonary disease. Similar mortality patterns have been described for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome outbreaks, both of which are due to viral strains of the same family [10]. These data indicate that advanced age and male gender are risk factors for an adverse outcome.
Two Chinese studies comparing the extreme patient phenotypes, i.e. discharged and deceased individuals, found that the most powerful clinical predictors of mortality COVID-19 were the levels of two markers of heart damage, myoglobin and cardiac troponin, and of three major proinflammatory mediators, high-sensitivity C-reactive protein (CRP), interleukin (IL)-6 [11], and d-dimer [8]. In most patients with severe disease the infection was associated with a cytokine storm [[12], [13], [14]]. In particular, higher levels of circulating IL-6 have been reported in patients with more severe disease [8,15].
Aging is characterized by the gradual development of a chronic subclinical systemic inflammation, which has been designated inflamm-aging [16], and by acquired immune system impairment, i.e. immune senescence [17]. The rate of inflamm-aging is higher in men [18]. IL-6 elevation is typical of aging [19]. Persistent IL-6 elevation can promote lung tissue inflammation and injury [20] and foster viral replication [21]. Targeting IL-6, the “cytokine for gerontologists” [22], helps attenuate the cytokine storm [23]. Tocilizumab, a biological drug approved for rheumatoid arthritis, is currently being evaluated for its efficacy against the effects of systemic IL-6 elevation (ClinicalTrial.gov, NCT04317092, NCT04320615, NCT04306705).
The evidence reviewed above suggested to us that the two key features of the aging process – inflamm-aging and immune senescence – and their implications can explain why older men with ARDs are the most prone to the adverse outcomes of SARS−COV2 infection.
2. In older men, accelerated inflamm-aging worsens COVID-19 infection outcomes
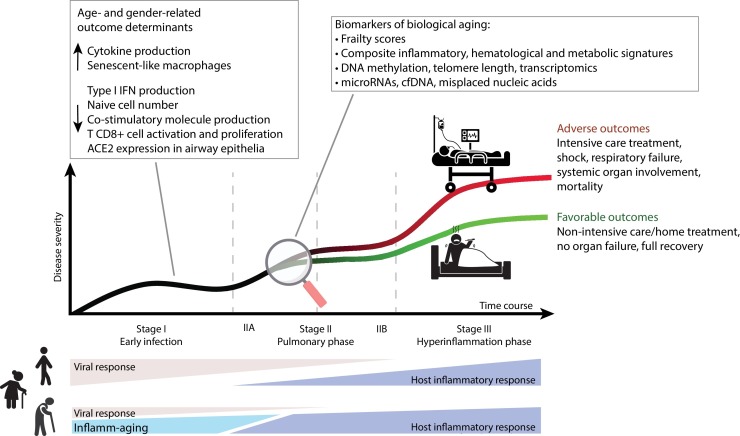
Inflamm-aging affects all individuals irrespective of their health status [16]; inflammation is also a key pathogenic mechanism of COVID-19 disease ( Fig. 1 ). In the elderly, especially men, IL-6 is chronically upregulated [16,18]; its elevation also predicts mortality due to SARS-CoV-2 [8,11]. The gender bias has also been characterized at the molecular level. The stronger age-dependent activation of the innate proinflammatory pathways demonstrated in men compared to women [24] is consistent with men’s higher rate of inflamm-aging [18]. Inflamm-aging is regarded as a major risk factor for the common ARDs, in line with the mounting evidence that an inflammatory pathogenesis is shared by most common ARDs [16]. The situation is different in centenarians, who are characterized by specific prolongevity traits and anti-inflammatory markers that delay ARD onset, which seem to protected them against the adverse outcomes of sustained inflammation [25,26]. In older men with ARDs, who have the highest rate of inflamm-aging, the inflammatory response induced by SARS-CoV2 infection seems to favor a poorer outcome.
Fig. 1.
Overview of the framework where inflamm-aging is the main phenomenon contributing to the high COVID-19 mortality rate seen in male, elderly, and frail patients. In these individuals, acute SARS-CoV-2 infection compounds their chronic, subclinical, aging-related proinflammatory state (inflamm-aging) which, together with immune senescence and the age- and gender-specific distribution of ACE2 in the airway epithelia, could blunt the antiviral response to inflammation. This model could explain the delayed viral clearance and the high rate of adverse outcomes observed in older patients in the late disease stages. The assessment of selected biological and immunological aging markers could be a valuable strategy for COVID-19 patient risk stratification in the earliest disease stage irrespective of their chronological age. Figure based on the classification of COVID-19 disease states proposed by Siddiqi and Mehra and adapted from the accompanying paper [78]. ACE2, angiotensin converting enzyme 2; cfDNA, cell-free DNA; IFN, interferon.
3. In older men, immune senescence worsens COVID-19 infection outcomes
The elderly population is the most vulnerable to infection with influenza virus (IFV) and varicella-zoster virus (VZV), two major pathogens that are responsible for the most common infectious diseases worldwide [27]. The high mortality from seasonal flu affecting the elderly, which most countries address with extensive vaccine campaigns [28], is a consequence of immune senescence, which seems to be the result of lifelong immunogenic stimulation of the immune system by exogenous subclinical infections and endogenous agents [29]. Endogenous factors such as misplaced nucleic acids [30,31] induce a shift of T and B lymphocytes from naïve to memory effector cells and impair the ability of the adaptive immune system to fight pathogens [17]; since the impairment includes the proliferative exhaustion of memory cells, it involves both unencountered and previously encountered antigens [32]. A recent study has found that the depletion of B lymphocyte-driven acquired immunity affects predominantly men [24]. Thus, in older men the chronic inflammatory status is coupled with a dramatic depletion of B lymphocyte-driven acquired immunity. Notably, aging also attenuates the upregulation of co-stimulatory molecules critical for T cell priming and reduces antiviral interferon (IFN) production by alveolar macrophages and dendritic cells (DCs) in response to IFV [33]. Consistent with this finding, the ability of DCs and macrophages to elicit CD8 + T cell response and proliferation and to release antiviral cytokines is impaired in elderly individuals [34]; in parallel, these subjects are characterized by a reduced activity of plasmacytoid DCs, the main sources of type I IFNs, which underpin the antiviral response and provide the first-line sentinels in immune surveillance, also in the lung [35]. An important animal study has found that aged cynomolgus macaques infected with SARS-CoV1 (which was responsible for the SARS-CoV1 epidemic in 2003–2004) developed more severe disease than their young adult counterparts and showed a blunted type I IFN response coupled with an elevated production of proinflammatory factors including IL-6 [36]. Notably, as in other acquired immune system compartments, the impairment of the plasmacytoid DC type I IFN pathway is more pronounced in men compared to women [37]. Both responses help clear the virus: the former is aimed at providing a local/contained response, whereas the latter enhances the local and systemic response also through the recruitment of large numbers of leukocytes. The elevated production of proinflammatory factors may ultimately induce local and systemic damage by unleashing a cytokine storm [38]. In SARS-CoV-1-infected pneumocytes, the antiviral response triggered by plasmacytoid DCs is blunted by proinflammatory cytokines released from pneumocytes [36]. Notably, the gender-biased association of inflamm-aging and immune-senescence is not simply correlative. Indeed, the two phenomena are recognized as being closely coupled in patients suffering from acute critical conditions such as sepsis and cytokine release syndrome (CRS), which is characterized by simultaneous systemic inflammation and immune paralysis [39]. A reciprocal inhibition between antiviral type-I IFN response and inflammation has been demonstrated in viral infections [40]. Therefore, in older men the fine balance between the antiviral response supported by T lymphocytes, B lymphocytes, and plasmacytoid DCs and inflammation is tilted in favor of inflammation.
4. In older men with age-related diseases, the aging-dependent reduction in ACE2 activity worsens SARS-CoV-2 infection outcomes
Angiotensin-converting enzyme (ACE)2, the main SARS-CoV2 host cell receptor, plays a crucial role in virus entry into the cell, as previously demonstrated in SARS and NL63 human coronaviruses [41]. ACE cleaves angiotensin I to generate angiotensin II, whereas ACE2 is a terminal carboxypeptidase that reduces angiotensin II levels, thus playing important functions in the negative regulation of the renin-angiotensin system [42]. ACE2 is expressed in the lung, oral mucosa (epithelial cells of the tongue), intestine (enterocytes), and endothelial cells [43,44]. Mounting evidence indicates that it plays a protective anti-inflammatory role on endothelial and lung function. Notably, ACE2 underexpression in the lung is implicated in respiratory diseases associated to severe infection [45]. In the mouse lung, attenuation of ACE2 activity in the setting of endotoxin inhalation enhanced neutrophil infiltration, exaggerated lung inflammation-induced injury by activating the des-Arg9 bradykinin (DABK)/bradykinin receptor B1 (BKB1R) axis [46], and elicited the release of proinflammatory chemokines (CXCL5, C-X-C motif chemokine 5), macrophage inflammatory protein-2 (MIP2), and TNF-α from airway epithelia [46]. Intriguingly, the spike protein of SARS-CoV induces downregulation of ACE2 expression through an increased release of proinflammatory chemokines and cytokines, thus enhancing inflammation and vascular permeability and promoting neutrophil recruitment to the lung [27]. Accordingly, lung ACE2 underexpression and the consequent impaired inhibition of DABK/BKB1R signaling result in abundant neutrophil infiltration and severe lung inflammation. In mice, in vivo injection of SARS-CoV spike protein worsens acute lung failure, which can be attenuated by blocking the renin-angiotensin pathway [47]. As regards ACE2 expression in endothelial cells, vasculitis has been described during viral infection [48]. Several studies suggest that ACE2 is downregulated in aging [49], explaining, at least in part, the increased susceptibility to vascular injury and cardiovascular disease affecting the elderly [49]. Interestingly, ACE2 overexpression has been reported in Asian females compared to males as well as in other ethnic groups [50]. In contrast, ACE2 is significantly downregulated in patients with type II diabetes as well as in most aged diabetic men with severe COVID-19 outcomes [51].
5. Older men show accelerated biological aging
Several aspects of inflamm-aging are still being investigated [30,52]. The accumulation of senescent cells during aging is one of the most likely contributors to inflamm-aging, due to their acquisition of the senescence-associated secretory phenotype (SASP) [53]. Senescence affects most cell types including macrophages, which serve as sentinels against infection [16]. Inflamm-aging has also been designated as macroph-aging, due to the important role played by this cell type in secreting proinflammatory mediators at the local and systemic level [54]. Notably, studies of the modulation of circulating monocyte/macrophage polarization during aging suggest different age-related trends for the M2 subset [55].
Macrophages are recruited to the lung by SARS-CoV-2-infected cells expressing ACE2 [56]. The stronger inflammatory state described in circulating monocytes from men compared to women [24] suggests that senescent-like macrophages compound the inflammation induced by COVID-19 through the production of proinflammatory cytokines like IL-6 [57,58], in a combination that in older men is especially lethal.
Telomere shortening, i.e. the loss of chromosome ends [59,60], is a hallmark of aging. Older men, particularly those suffering from ARDs, show reduced telomere length (TL) compared to age-matched women [61]. Interestingly, telomeres released outside cells may exert an anti-inflammatory action, while shortened telomeres may favor the production of proinflammatory molecular species [62,63]. Telomere shortening is seen in ARDs and is associated to an impaired response to infective agents, suggesting that leukocyte TL may be a marker of general immune competence [64]. Telomere research supports the notion that TL may play a role in inflamm-aging, which in older men is commonly accelerated. Other measures of biological aging, such as changes in epigenetic patterns, also exhibit a different expression in older men compared to age-matched women [61,65]. The data reviewed above support the notion that the aging process is associated to inflamm-aging and immune senescence and that biological aging is accelerated in men compared to women.
6. Potential implications for SARS-CoV-2 host-directed therapies
The mechanisms underpinning the chronic inflammatory state characterizing older men are the subject of intense research. We speculate that these mechanisms provide crucial insights into COVID-19 pathobiology and natural history and that their further investigation can lead to novel therapeutic interventions.
A variety of repurposed antiviral drugs (e.g. remdesivir, lopinavir/ritonavir) and anti-inflammatory agents, including chloroquine (ClinicalTrial.gov, NCT04323527, NCT04324463, NCT04303507) and tocilizumab (NCT04317092, NCT04320615, NCT04306705) are being tested to treat COVID-19 infection [66]. It is conceivable that drugs that modulate inflamm-aging could help treat elderly and/or frail patients with SARS-CoV-2 infection [67]. In particular, “senolytic” compounds, which selectively promote senescent cell clearance, might help reduce systemic as well as local inflammation in such patients. Pilot trials are already in progress [67,68], but a substantial number of potential senolytics with a low toxicity profile have been identified and can safely be explored [69]. Another approach could rely on harnessing the anti-inflammatory ability of free telomeric end oligonucleotides [70,71], whose airway administration in aerosol form has been studied in a model of mouse lung injury [72]. This approach would not be limited to elderly COVID-19 patients.
The rate of biological aging (including inflamm-aging) varies among subjects as a result of interactions among genetic make-up, epigenetic modulation, environment, and random factors and can be traced and measured by biomarkers such as TL, circulating nucleic acids, epigenetic changes involving extracellular vesicles, and the expression of inflammatory cytokines such as IL-6 [26,59,[73], [74], [75], [76]]. The ability to measure “real” biological age would help determine a person’s aging rate. Attaining this goal clearly requires more accurate and possibly more numerous biomarkers of biological aging capable of predicting functional capacity at a later age than chronological age. Since the variance of aging phenotypes widens over time, owing to different individual trajectories of aging [75,77], immunological/biological age rather than chronological age appears to be a more sensitive approach also to assess individual susceptibility to the adverse outcomes of COVID-19 infection. Consequently, a panel of biomarkers of immunological/biological aging could help identify subjects at risk of adverse COVID-19 outcomes also among the younger population. Thus, young people whose inflamm-aging signature is not consistent with their chronological age – who may therefore be particularly prone to develop not only ARDs, but also a deadly communicable disease such as COVID-19 infection – would also benefit from aging research.
Author contributions
MB, FP, JS, and FO conceived the idea and wrote the manuscript. AG collected the literature and prepared the figure. AG and GS provided critical advice and reviewed the manuscript. All authors approve the final version of the manuscript.
Funding
This work was supported by the Italian Ministry of Health [Ricerca Corrente grants to IRCCS MultiMedica] and by Università Politecnica delle Marche (UNIVPM) [RSA grants to F.O.].
Declaration of Competing Interest
All authors: No conflict.
Acknowledgments
We are grateful to Word Designs for text editing (www.silviamodena.com).
Contributor Information
Francesco Prattichizzo, Email: francesco.prattichizzo@multimedica.it.
Angelica Giuliani, Email: angelica.giuliani@staff.univpm.it.
References
- 1.Wu Y., Ho W., Huang Y., Jin D.Y., Li S., Liu S.L., Liu X., Qiu J., Sang Y., Wang Q., Yuen K.Y., Zheng Z.M. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet. 2020;395(10228):949–950. doi: 10.1016/S0140-6736(20)30557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimabukuro-Vornhagen A., Godel P., Subklewe M., Stemmler H.J., Schlosser H.A., Schlaak M., Kochanek M., Boll B., von Bergwelt-Baildon M.S. Cytokine release syndrome. J. Immunother. Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2020. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19 - 11 March 2020.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Google Scholar]
- 4.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Istituto Superiore di Sanità . 2020. Sorveglianza Integrata COVID-19: I Principali Dati Nazionali.https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati [Google Scholar]
- 6.Vital Surveillances: The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020. 2020. Novel coronavirus pneumonia emergency response epidemiology team.http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51 (Accessed March 16, 2020. [PMC free article] [PubMed] [Google Scholar]
- 7.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., Ng O.T., Marimuthu K., Ang L.W., Mak T.M., Lau S.K., Anderson D.E., Chan K.S., Tan T.Y., Ng T.Y., Cui L., Said Z., Kurupatham L., Chen M.I., Chan M., Vasoo S., Wang L.F., Tan B.H., Lin R.T.P., Lee V.J.M., Leo Y.S., Lye D.C. T. Singapore novel coronavirus outbreak research, epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Istituto Superiore di Sanità . 2020. Characteristics of COVID-19 Patients Dying in Italy Report Based on Available Data on March 24th, 2020.https://www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_24_marzo_eng.pdf [Google Scholar]
- 10.Wu Z., McGoogan J.M. JAMA; 2020. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. [DOI] [PubMed] [Google Scholar]
- 11.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zumla A., Hui D.S., Azhar E.I., Memish Z.A., Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395(10224):e35–e36. doi: 10.1016/S0140-6736(20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L., Liu H.G., Liu W., Liu J., Liu K., Shang J., Deng Y., Wei S. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):203–208. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Franceschi C., Bonafe M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 17.Fulop T., Witkowski J.M., Olivieri F., Larbi A. The integration of inflammaging in age-related diseases. Semin. Immunol. 2018;40:17–35. doi: 10.1016/j.smim.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Bonafè M., Olivieri F., Cavallone L., Giovagnetti S., Marchegiani F., Cardelli M., Pieri C., Marra M., Antonicelli R., Lisa R., Rizzo M.R., Paolisso G., Monti D., Franceschi C. A gender-dependent genetic predisposition to produce high levels of IL-6 is detrimental for longevity. Eur. J. Immunol. 2001;31(8):2357–2361. doi: 10.1002/1521-4141(200108)31:8<2357::aid-immu2357>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Maggio M., Guralnik J.M., Longo D.L., Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61(6):575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu M., Zheng X., Witschi H., Pinkerton K.E. The role of interleukin-6 in pulmonary inflammation and injury induced by exposure to environmental air pollutants. Toxicol. Sci. 2002;68(2):488–497. doi: 10.1093/toxsci/68.2.488. [DOI] [PubMed] [Google Scholar]
- 21.Velazquez-Salinas L., Verdugo-Rodriguez A., Rodriguez L.L., Borca M.V. The role of interleukin 6 during viral infections. Front. Microbiol. 2019;10:1057. doi: 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ershler W.B. Interleukin-6: a cytokine for gerontologists. J. Am. Geriatr. Soc. 1993;41(2):176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M., Grupp S.A., Mackall C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marquez E.J., Chung C.H., Marches R., Rossi R.J., Nehar-Belaid D., Eroglu A., Mellert D.J., Kuchel G.A., Banchereau J., Ucar D. Sexual-dimorphism in human immune system aging. Nat. Commun. 2020;11(1):751. doi: 10.1038/s41467-020-14396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr. Rev. 2007;65(12 Pt 2):S173–6. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 26.Storci G., De Carolis S., Papi A., Bacalini M.G., Gensous N., Marasco E., Tesei A., Fabbri F., Arienti C., Zanoni M., Sarnelli A., Santi S., Olivieri F., Mensa E., Latini S., Ferracin M., Salvioli S., Garagnani P., Franceschi C., Bonafe M. Genomic stability, anti-inflammatory phenotype, and up-regulation of the RNAseH2 in cells from centenarians. Cell Death Differ. 2019;26(9):1845–1858. doi: 10.1038/s41418-018-0255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh S.J., Lee J.K., Shin O.S. Aging and the immune system: the impact of immunosenescence on viral infection, immunity and vaccine immunogenicity. Immune Netw. 2019;19(6):e37. doi: 10.4110/in.2019.19.e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization . 2019. Recommendations on Influenza Vaccination During the 2019–2020 Winter Season.http://www.euro.who.int/en/health-topics/communicable-diseases/influenza/publications/2019/recommendations-on-influenza-vaccination-during-the-20192020-winter-season-2019 [Google Scholar]
- 29.Fulop T., Larbi A., Dupuis G., Le Page A., Frost E.H., Cohen A.A., Witkowski J.M., Franceschi C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018;8 doi: 10.3389/fimmu.2017.01960. 1960-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storci G., Bacalini M.G., Bonifazi F., Garagnani P., De Carolis S., Salvioli S., Olivieri F., Bonafe M. Ribosomal DNA instability: an evolutionary conserved fuel for inflammaging. Ageing Res. Rev. 2020;58 doi: 10.1016/j.arr.2020.101018. [DOI] [PubMed] [Google Scholar]
- 31.Franceschi C., Garagnani P., Vitale G., Capri M., Salvioli S. Inflammaging and’ Garb-aging’. Trends Endocrinol. Metab. 2017;28(3):199–212. doi: 10.1016/j.tem.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Ciabattini A., Nardini C., Santoro F., Garagnani P., Franceschi C., Medaglini D. Vaccination in the elderly: the challenge of immune changes with aging. Semin. Immunol. 2018;40:83–94. doi: 10.1016/j.smim.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs E.J., Boe D.M., Boule L.A., Curtis B.J. Inflammaging and the lung. Clin. Geriatr. Med. 2017;33(4):459–471. doi: 10.1016/j.cger.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montgomery R.R. Age-related alterations in immune responses to West Nile virus infection. Clin. Exp. Immunol. 2017;187(1):26–34. doi: 10.1111/cei.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cervantes-Barragan L., Zust R., Weber F., Spiegel M., Lang K.S., Akira S., Thiel V., Ludewig B. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109(3):1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smits S.L., de Lang A., van den Brand J.M., Leijten L.M., van I.W.F., Eijkemans M.J., van Amerongen G., Kuiken T., Andeweg A.C., Osterhaus A.D., Haagmans B.L. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6(2) doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griesbeck M., Ziegler S., Laffont S., Smith N., Chauveau L., Tomezsko P., Sharei A., Kourjian G., Porichis F., Hart M., Palmer C.D., Sirignano M., Beisel C., Hildebrandt H., Cenac C., Villani A.C., Diefenbach T.J., Le Gall S., Schwartz O., Herbeuval J.P., Autran B., Guery J.C., Chang J.J., Altfeld M. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-alpha production in women. J. Immunol. 2015;195(11):5327–5336. doi: 10.4049/jimmunol.1501684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruse N., Leijte G.P., Pickkers P., Kox M. New frontiers in precision medicine for sepsis-induced immunoparalysis. Expert Rev. Clin. Immunol. 2019;15(3):251–263. doi: 10.1080/1744666X.2019.1562336. [DOI] [PubMed] [Google Scholar]
- 40.Reboldi A., Dang E.V., McDonald J.G., Liang G., Russell D.W., Cyster J.G. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science. 2014;345(6197):679–684. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathewson A.C., Bishop A., Yao Y., Kemp F., Ren J., Chen H., Xu X., Berkhout B., van der Hoek L., Jones I.M. Interaction of severe acute respiratory syndrome-coronavirus and NL63 coronavirus spike proteins with angiotensin converting enzyme-2. J. Gen. Virol. 2008;89(Pt 11):2741–2745. doi: 10.1099/vir.0.2008/003962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87(5):E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 43.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis, J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sodhi C.P., Wohlford-Lenane C., Yamaguchi Y., Prindle T., Fulton W.B., Wang S., McCray P.B., Jr., Chappell M., Hackam D.J., Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg(9) bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;314(1):L17–L31. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imai Y., Kuba K., Penninger J.M. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp. Physiol. 2008;93(5):543–548. doi: 10.1113/expphysiol.2007.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., Cai J., Li X., Kang W., Weng D., Lu Y., Wu D., He L., Yao K. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jia G., Aroor A.R., Jia C., Sowers J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta. Mol. Basis. Dis. 2019;1865(7):1802–1809. doi: 10.1016/j.bbadis.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Chen Q.J.Jiawei, Xia Xian, Liu Kangping, Zhengqing Yu, Tao Wanyu, Gong Wenxuan, Han Jing-Dong J. Individual variation of the SARS-CoV2 receptor ACE2 gene expression and regulation. Preprints. 2020 doi: 10.1111/acel.13168. 2020030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X., Wen F., Huang X., Ning G., Wang W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franceschi C., Zaikin A., Gordleeva S., Ivanchenko M., Bonifazi F., Storci G., Bonafe M. Inflammaging 2018: an update and a model. Semin. Immunol. 2018;40:1–5. doi: 10.1016/j.smim.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Coppe J.P., Patil C.K., Rodier F., Sun Y., Munoz D.P., Goldstein J., Nelson P.S., Desprez P.Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prattichizzo F., Bonafè M., Olivieri F., Franceschi C. Senescence associated macrophages and "macroph-aging": Are they pieces of the same puzzle? Aging. 2016;8(12):3159–3160. doi: 10.18632/aging.101133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costantini A., Viola N., Berretta A., Galeazzi R., Matacchione G., Sabbatinelli J., Storci G., De Matteis S., Butini L., Rippo M.R., Procopio A.D., Caraceni D., Antonicelli R., Olivieri F., Bonafe M. Age-related M1/M2 phenotype changes in circulating monocytes from healthy/unhealthy individuals. Aging (Albany NY) 2018;10(6):1268–1280. doi: 10.18632/aging.101465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qi F., Qian S., Zhang S., Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020 doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J.Y., Souroullas G.P., Diekman B.O., Krishnamurthy J., Hall B.M., Sorrentino J.A., Parker J.S., Sessions G.A., Gudkov A.V., Sharpless N.E. Cells exhibiting strong p16 (INK4a) promoter activation in vivo display features of senescence. Proc Natl Acad Sci U S A. 2019;116(7):2603–2611. doi: 10.1073/pnas.1818313116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rana T., Jiang C., Liu G., Miyata T., Antony V., Thannickal V.J., Liu R.M. PAI-1 regulation of TGF-beta1-induced alveolar type II cell senescence, SASP secretion, and SASP-mediated activation of alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 2020;62(3):319–330. doi: 10.1165/rcmb.2019-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mensà E., Latini S., Ramini D., Storci G., Bonafè M., Olivieri F. The telomere world and aging: analytical challenges and future perspectives. Ageing Res. Rev. 2019;50:27–42. doi: 10.1016/j.arr.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 60.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barrett E.L., Richardson D.S. Sex differences in telomeres and lifespan. Aging Cell. 2011;10(6):913–921. doi: 10.1111/j.1474-9726.2011.00741.x. [DOI] [PubMed] [Google Scholar]
- 62.Storci G., De Carolis S., Olivieri F., Bonafe M. Changes in the biochemical taste of cytoplasmic and cell-free DNA are major fuels for inflamm-aging. Semin. Immunol. 2018;40:6–16. doi: 10.1016/j.smim.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Bonafè M., Sabbatinelli J., Olivieri F. Exploiting the telomere machinery to put the brakes on inflamm-aging. Ageing Res. Rev. 2020;59 doi: 10.1016/j.arr.2020.101027. [DOI] [PubMed] [Google Scholar]
- 64.Helby J., Nordestgaard B.G., Benfield T., Bojesen S.E. Shorter leukocyte telomere length is associated with higher risk of infections: a prospective study of 75,309 individuals from the general population. Haematologica. 2017;102(8):1457–1465. doi: 10.3324/haematol.2016.161943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walker J. Regeneron, Sanofi to Test Arthritis Drug as Coronavirus Treatment. Wall Street. J. 2020 [Google Scholar]
- 67.Justice J.N., Nambiar A.M., Tchkonia T., LeBrasseur N.K., Pascual R., Hashmi S.K., Prata L., Masternak M.M., Kritchevsky S.B., Musi N., Kirkland J.L. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hickson L.J., Langhi Prata L.G.P., Bobart S.A., Evans T.K., Giorgadze N., Hashmi S.K., Herrmann S.M., Jensen M.D., Jia Q., Jordan K.L., Kellogg T.A., Khosla S., Koerber D.M., Lagnado A.B., Lawson D.K., LeBrasseur N.K., Lerman L.O., McDonald K.M., McKenzie T.J., Passos J.F., Pignolo R.J., Pirtskhalava T., Saadiq I.M., Schaefer K.K., Textor S.C., Victorelli S.G., Volkman T.L., Xue A., Wentworth M.A., Wissler Gerdes E.O., Zhu Y., Tchkonia T., Kirkland J.L. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gurau F., Baldoni S., Prattichizzo F., Espinosa E., Amenta F., Procopio A.D., Albertini M.C., Bonafe M., Olivieri F. Anti-senescence compounds: a potential nutraceutical approach to healthy aging. Ageing Res. Rev. 2018;46:14–31. doi: 10.1016/j.arr.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 70.Gursel I., Gursel M., Yamada H., Ishii K.J., Takeshita F., Klinman D.M. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J. Immunol. 2003;171(3):1393–1400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- 71.Yazar V., Kilic G., Bulut O., Canavar Yildirim T., Yagci F.C., Aykut G., Klinman D.M., Gursel M., Gursel I. A suppressive oligodeoxynucleotide expressing TTAGGG motifs modulates cellular energetics through the mTOR signaling pathway. Int. Immunol. 2020;32(1):39–48. doi: 10.1093/intimm/dxz059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheiermann J., Klinman D.M. Suppressive oligonucleotides inhibit inflammation in a murine model of mechanical ventilator induced lung injury. J. Thorac. Dis. 2016;8(9):2434–2443. doi: 10.21037/jtd.2016.08.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jylhava J., Pedersen N.L., Hagg S. Biological age predictors. EBioMedicine. 2017;21:29–36. doi: 10.1016/j.ebiom.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sabbatinelli J., Prattichizzo F., Olivieri F., Procopio A.D., Rippo M.R., Giuliani A. Where metabolism meets senescence: focus on endothelial cells. Front. Physiol. 2019;10:1523. doi: 10.3389/fphys.2019.01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferrucci L., Gonzalez-Freire M., Fabbri E., Simonsick E., Tanaka T., Moore Z., Salimi S., Sierra F., de Cabo R. Measuring biological aging in humans: a quest. Aging Cell. 2020;19(2) doi: 10.1111/acel.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mensà E., Guescini M., Giuliani A., Bacalini M.G., Ramini D., Corleone G., Ferracin M., Fulgenzi G., Graciotti L., Prattichizzo F., Sorci L., Battistelli M., Monsurrò V., Bonfigli A.R., Cardelli M., Recchioni R., Marcheselli F., Latini S., Maggio S., Fanelli M., Amatori S., Storci G., Ceriello A., Stocchi V., De Luca M., Magnani L., Rippo M.R., Procopio A.D., Sala C., Budimir I., Bassi C., Negrini M., Garagnani P., Franceschi C., Sabbatinelli J., Bonafè M., Olivieri F. Small extracellular vesicles deliver miR-21 and miR-217 as pro-senescence effectors to endothelial cells. J. Extracell. Vesicles. 2020;9(1) doi: 10.1080/20013078.2020.1725285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olivieri F., Capri M., Bonafe M., Morsiani C., Jung H.J., Spazzafumo L., Vina J., Suh Y. Circulating miRNAs and miRNA shuttles as biomarkers: perspective trajectories of healthy and unhealthy aging. Mech. Ageing Dev. 2017;165(Pt B):162–170. doi: 10.1016/j.mad.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]