Abstract
Aim:
CYP2C19 genotyping is used to guide antiplatelet therapy after percutaneous coronary intervention (PCI). This study evaluated the potential impact of CYP2C19 and multigene pharmacogenomics (PGx) testing on medications beyond antiplatelet therapy in a real-world cohort of PCI patients that underwent CYP2C19 testing.
Methodology & results:
Multiple medications with actionable PGx recommendations, including proton pump inhibitors, antidepressants and opioids, were commonly prescribed. Approximately 50% received a CYP2C19 metabolized medication beyond clopidogrel and 7% met criteria for a CYP2C19 genotype-guided intervention. A simulation analysis projected that 17.5 PGx-guided medication interventions per 100 PCI patients could have been made if multigene PGx results were available.
Conclusion:
This suggests that CYP2C19 and multigene PGx results could be used to optimize medication prescribing beyond antiplatelet therapy in PCI patients.
Keywords: : clopidogrel, CYP2C19, genetic testing, percutaneous coronary intervention, pharmacogenomics, precision medicine
Selecting treatment strategies that account for individual genetic differences offers enormous potential to improve health outcomes and lower healthcare costs. While more than 170 medications have germline genetic information in the drug label approved by the US FDA, pharmacogenomics (PGx) testing is not routinely used to guide prescribing in clinical settings [1,2]. PGx-guided prescribing offers the potential to optimize drug selection and dosing based on patient-specific genetic factors. However, in order for a gene–drug pair to be considered clinically actionable, there must be sufficient supporting evidence, an available genetic test and an alternative dose or drug for individuals with the ‘at-risk’ genotype. Moreover, a structured approach to implement PGx-guided prescribing must be in place [2].
The Clinical Pharmacogenomics Implementation Consortium (CPIC) publishes guidelines that systematically evaluate genotype and drug phenotype association data and translates this information into evidence-based recommendations for clinicians [3]. To date, CPIC has identified over 80 medications impacted by approximately 20 genes with level A or B evidence, whereby a prescribing action is recommended to avoid an adverse medication outcome and alternative therapies or dosing are highly likely to be effective and safe [2–4]. While approximately 7% of US FDA-approved medications have actionable PGx recommendations based on CPIC level A or B evidence, these medications have been estimated to represent 18% of the outpatient medications prescribed in the USA [2]. Furthermore, many of the at-risk genotypes that affect these medications are common, as 99% of individuals carry at least one actionable PGx genotype [5,6].
Currently, when PGx testing is performed, genotyping typically occurs for putatively functional genetic variants in a single gene and in a reactive manner after it has been determined that the patient will be prescribed a drug with actionable PGx recommendations. Notably, implementation of CYP2C19 genotype testing to guide the selection of antiplatelet therapy in patients with coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI) has become increasingly common [7,8]. It is now well established that patients with nonfunctional CYP2C19 variant alleles are at increased risk of adverse cardiovascular events after stent placement when treated with clopidogrel, which remains the most commonly used P2Y12 inhibitor after PCI [9–12]. Consequently, use of alternative therapy (prasugrel or ticagrelor) is recommended in CYP2C19 nonfunctional allele carriers, which comprise approximately 30% of the US population [13].
Multigene preemptive PGx testing, in which a patient is simultaneously tested for multiple PGx actionable genes in advance of medication prescribing, likely offers advantages over single-gene testing due to decreasing genotype costs secondary to technological advancements and the potential benefits associated with PGx-guided prescribing [14]. However, the patient populations in which multigene preemptive PGx testing offers the greatest impact to avoid adverse drug outcomes have not been clearly defined, evaluated and validated. Due to the benefit of CYP2C19 genotype-guided antiplatelet therapy and high prevalence of polypharmacy among CAD patients due to advanced age and common comorbidities such as hyperlipidemia, hypertension, atrial fibrillation and depression, the cardiac catheterization laboratory offers potential to identify a high-risk population in which institutions can implement multigene PGx testing [15–18].
We hypothesize that PCI patients undergoing CYP2C19 genetic testing to guide antiplatelet therapy selection are also prescribed multiple medications, in addition to clopidogrel, that have actionable PGx recommendations for at-risk genotypes in CYP2C19 and other established pharmacogenes. However, it is not known how frequently this patient population is prescribed medications with actionable PGx recommendations and carry an at-risk genotype that increases risk for therapeutic failure or adverse events that could be avoided by preemptive PGx-based prescribing. Therefore, the objective of this study was to: describe the frequency of genetically actionable medication use beyond antiplatelet therapy in a real-world cohort of PCI patients that underwent CYP2C19 genetic testing; determine the proportion of PCI patients at risk for an adverse medication outcome based on their known CYP2C19 metabolizer phenotype; and evaluate the projected impact of multigene PGx testing on medication prescribing in CAD patients undergoing PCI.
Methods
Study design & population
This single-center, retrospective observational cohort study included 646 consecutive adult patients who underwent PCI with coronary artery stent placement at an academic medical center between 1 January 2015 and 31 December 2015. Patients were eligible for clinical CYP2C19 genetic testing at the interventional cardiologist’s discretion, which was clinically implemented in 2012 to guide antiplatelet therapy prescribing in high-risk patients [8,19]. Patients who died before discharge from their PCI hospitalization were excluded from this analysis. The study was approved by the Institutional Review Board. Due to the retrospective nature of the study, informed consent was not required.
Data abstraction
Demographic, clinical, medication and CYP2C19 genotype data were manually abstracted from encounters in the electronic health record (EHR). Physician-documented comorbid conditions were collected from the patient past medical history. CYP2C19 metabolizer phenotypes were assigned based on CPIC guidelines: ultrarapid metabolizer (UM; *17/*17), rapid metabolizer (RM; *1/*17), normal metabolizer (NM; *1/*1), intermediate metabolizer (IM; *1/*2, *1/*3, *2/*17 or *3/*17) or poor metabolizer (PM; *2/*2, *2/*3 or *3/*3) [20]. Medication use was reported for the total study population as well as within the stratum of the cohort that underwent CYP2C19 genotype testing.
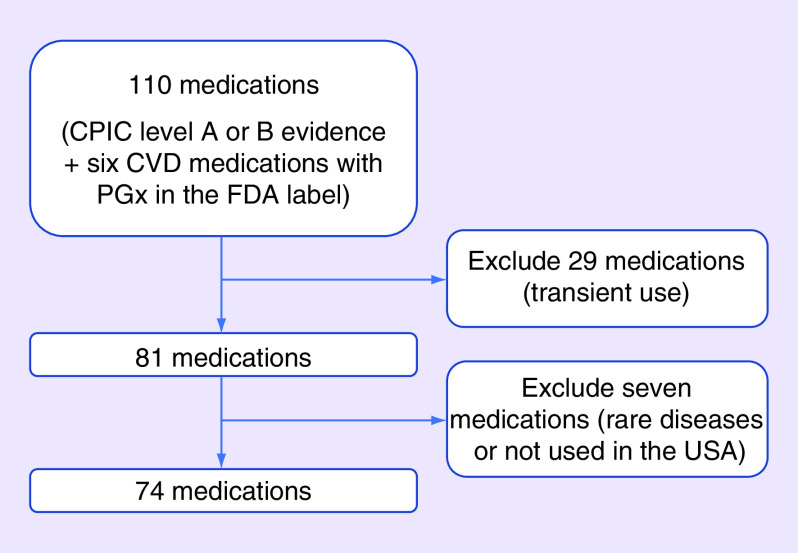
Because antiplatelet therapy with aspirin and a P2Y12 inhibitor (clopidogrel, prasugrel or ticagrelor) is indicated in all PCI patients and CYP2C19 genotype is already used clinically to guide antiplatelet therapy at our institution, the frequency of medication use beyond clopidogrel was the focus of the current analysis. Prescribed medications with CPIC level A or B evidence used to treat chronic medical conditions were collected at discharge from the index PCI hospitalization and at up to two follow-up encounters within 1 year of the index PCI (Supplementary Table 1) [3,4]. Outpatient cardiology and primary care provider (PCP) follow-up visits with a full medication history over 1 year were prioritized for data abstraction. Since PCI patients are treated by cardiologists, six additional medications used to treat cardiovascular diseases (CVD) with genetic information in the FDA label and CPIC level C–D evidence (propafenone, carvedilol, metoprolol, propranolol, rosuvastatin and hydralazine) were collected [1,4]. Medications used for the treatment of rare conditions and medications not commonly used in the USA were excluded due to the low likelihood of use within 1 year after PCI. Medications used transiently, such as antibiotics, antifungals and anesthesiology drugs and drugs with topical formulations, were also excluded because documentation of use in hospital discharge and outpatient encounters would likely underrepresent their actual use. Based on these criteria, five CPIC level A drugs and 31 level B drugs were excluded from data collection and 74 medications in total were included in data collection (Figure 1 & Supplementary Table 1).
Figure 1. . Overview of the number of pharmacogenomics actionable medications included in data collection.
The number of medications included in and excluded from data collection are described. The 74 medications included in data collection and the associated gene and CPIC level of evidence, are listed in Supplementary Table 1.
CPIC: Clinical Pharmacogenomics Implementation Consortium; CVD: Cardiovascular disease; PGx: Pharmacogenomics.
Data analysis
Descriptive statistics were used to assess demographics, clinical characteristics, CYP2C19 metabolizer phenotype and PGx actionable medication use in the full study population as well as the stratum that underwent CYP2C19 genetic testing. Data are reported as counts (%), mean ± standard deviation or median (interquartile range) unless otherwise indicated. Analyses were performed using SAS-JMP version 14.0 (SAS Institute, NC, USA).
The primary end point was the observed number of potential PGx-directed medication interventions that could have been made to optimize prescribing beyond antiplatelet therapy in the stratum of patients with CYP2C19 genotype results available (n = 380). A potential intervention was defined as medication with a clinically actionable recommendation prescribed to an individual that carried an at-risk CYP2C19 metabolizer phenotype (e.g., UM or PM). The total number of potential interventions, as well as the total number of unique patients with a potential intervention, were calculated to account for the possibility of multiple actionable CYP2C19 genotype-guided interventions within the same patient. The proportion of use by drug class and type of adverse outcome avoided (therapeutic failure or adverse event) were also calculated.
A secondary simulation analysis was conducted to evaluate the projected impact of a multigene PGx testing strategy on medication prescribing in the overall PCI patient population (n = 646). The simulation was designed to determine the number of PGx-guided interventions that could have been made based on genotype results in addition to CYP2C19. Actionable gene–drug pairs for which the drug was used in at least 2% of the study population were included. This resulted in inclusion of six genes in addition to CYP2C19 (CYP2C9, CYP2D6, G6PD, HLA-B, SLCO1B1 and VKORC1) and 19 medications in the analysis (Supplementary Table 2). At-risk genotype or phenotype frequencies, stratified by race, were abstracted from CPIC guideline supplements for CYP2C19 (UM, RM or PM), CYP2D6 (UM, IM or PM), SLCO1B1 (intermediate or low function), G6PD (deficient) and HLA-B (*58:01 carrier) [13,21–26]. For VKORC1/CYP2C9 (highly sensitive or sensitive warfarin responders), the ENGAGE AF-TIMI 48 trial was used in addition to the warfarin CPIC guideline to estimate phenotype prevalence [27,28]. For genes without metabolizer phenotypes, in which only allele frequencies were available, the Hardy–Weinberg equation was used to estimate the frequencies of the actionable at-risk genotypes. Because panel-based genotype results were not available among the patients in this cohort, the simulation analysis projected the population genotype/phenotype frequency estimates for each gene onto the PCI study population and assumed a population comprised of 80% Caucasian and 20% African–American patients. A potentially PGx actionable intervention was defined in the same manner as the primary outcome. The projected number of PGx-guided medication interventions that could have been made, if genotype results were available, was calculated by multiplying the observed frequency of medication use in the overall study population by the estimated at-risk genotype/phenotype frequency for each medication. The sum of the total number of potential PGx-directed interventions in the PCI study population (n = 646) and the projected number of interventions per 100 PCI patients were calculated for each gene and overall.
Results
Study population
The mean age of the PCI population was 63 ± 12 years, 70% were male and 20% were African–Americans (Table 1). Approximately half of the patients had an acute coronary syndrome indication for their current PCI, while 53% had a previous revascularization procedure. Common comorbid conditions included hypertension (88%), obesity (46%), diabetes (40%) and psychiatric disorders (18%), while active cancer and end-stage renal disease were prevalent in less than 5% of the cohort.
Table 1. . Study population baseline characteristics.
| Characteristic | N (%)† |
|---|---|
| N | 646 |
| Age, years (mean ± standard deviation) | 63.3 ± 11.9 |
| Male | 453 (70.1%) |
| Race/ethnicity | |
| -Caucasian | 461 (71.4%) |
| -African–American | 128 (19.8%) |
| -Asian | 3 (0.5%) |
| -Hispanic | 22 (3.4%) |
| -Native American | 19 (2.9%) |
| -Other/unknown | 13 (2.0%) |
| Obese (body mass index >30 kg/m2) | 300 (46.4%) |
| Current smoker | 150 (23.2%) |
| Hypertension | 568 (87.9%) |
| Diabetes | 261 (40.4%) |
| Depression or other psychiatric disorder | 117 (18.1%) |
| End-stage renal disease | 20 (3.1%) |
| Active cancer | 31 (4.8%) |
| History of myocardial infarction | 195 (30.2%) |
| History of a prior revascularization procedure | 345 (53.4%) |
| Acute coronary syndrome indication for PCI | 315 (48.8%) |
| CYP2C19 genotype available | 380 (58.8%) |
| -Previously determined genotype‡ | 92 (14.2%) |
| -Genotype during current admission§ | 288 (44.6%) |
Data presented as N (%) unless otherwise stated.
Genotype test performed at a prior PCI admission.
Genotype test performed during the index PCI admission.
PCI: Percutaneous coronary intervention.
Frequency of PGx-actionable medication use in PCI patients
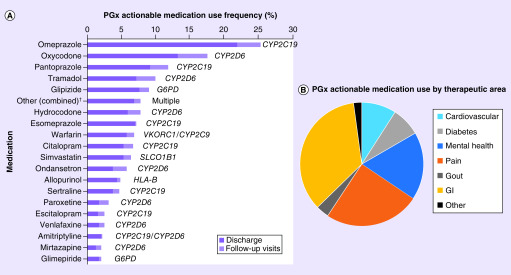
Overall, clopidogrel was prescribed in 68% of the cohort, with an alternative P2Y12 inhibitor (prasugrel or ticagrelor) prescribed in 32% of patients. The most commonly used PGx actionable medications in the study population beyond antiplatelet therapy were omeprazole, oxycodone, pantoprazole, tramadol, glipizide, hydrocodone, esomeprazole, warfarin, citalopram, simvastatin, ondansetron, allopurinol, sertraline, paroxetine, escitalopram, venlafaxine, amitriptyline, mirtazapine and glimepiride (Figure 2A & Supplementary Table 2). In addition, there were 18 PGx actionable medications with observed use in less than 2% of the population that were collectively prescribed to 50 (7.7%) patients. When evaluated by therapeutic area (Figure 2B), the proportion of PGx actionable medications prescribed were most common for gastrointestinal indications (37%, proton pump inhibitors [PPI] and ondansetron), pain (26%, opioids) and mental health disorders (19%, selective serotonin reuptake inhibitors [SSRI] and other antidepressants). Additional disease states with PGx actionable medication use included CVD (9.4%, warfarin and simvastatin), diabetes (7.9%, sulfonylureas) and gout (3.6%, allopurinol). Of the CVD medications with FDA labeling information and CPIC level C–D evidence, metoprolol (59%) and carvedilol (19%) were the most commonly used, followed by rosuvastatin (7.4%), hydralazine (3.7%) and propranolol (1.2%) (Supplementary Figure 1).
Figure 2. . Frequency of multigene pharmacogenomics actionable medication use beyond antiplatelet therapy in percutaneous coronary intervention patients.
(A) Use frequency of PGx actionable medications in the overall study population (n = 646). Medication use reflects the number of patients with a prescription for the given medication at discharge from the index percutaneous coronary intervention hospitalization (dark) or initiation during a follow-up visit up within 1-year post discharge (light). Medications used in ≥2% of patients and the associated gene are presented. (B) The distribution of PGx actionable medication use by medication therapeutic area: GI, pain, mental health, cardiovascular (not including antiplatelet therapy and the CPIC Level C–D drugs described in Supplementary Figure 1), diabetes, gout and other (immunosuppressants, antiviral, cancer, cystic fibrosis and anticonvulsants).
†Other medications is a composite of 18 individual medications with <2% use frequency in the study population (listed in Supplementary Table 2)
CPIC: Clinical Pharmacogenomics Implementation Consortium; GI: Gastrointestinal; PGx: Pharmacogenomics.
Observed number of potential CYP2C19 genotype-guided medication interventions
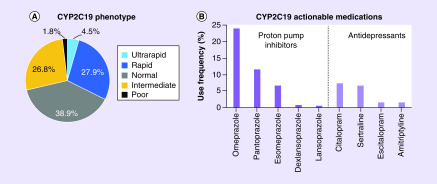
CYP2C19 genotype was available in 380 (59%) patients following the index PCI procedure, with 92 (24%) patients having undergone genotype testing at a prior PCI procedure. Among the 288 patients genotyped at the time of the index PCI, the median (interquartile range) genotype turnaround time was 1 (0–2) day and results were available for 199 (69%) patients by the day after the test was ordered. Overall, the distribution of CYP2C19 metabolizer phenotypes was 5% UM, 28% RM, 39% NM, 27% IM and 2% PM (Figure 3A).
Figure 3. . Observed frequency of CYP2C19 phenotypes and actionable medication use beyond antiplatelet therapy in genotyped percutaneous coronary intervention patients.
(A) CYP2C19 phenotype distribution in 380 genotyped patients: UM (n = 17; 4%), RM (n = 106; 28%), NM (n = 148; 39%), IM (n = 102; 27%) and PM (n = 7; 2%). The IM (*1/*2 = 73 [72%], *1/*3 = 0 [0%], *2/*17 = 29 [28%], *3/*17 = 0 [0%]) and PM (*2/*2 = 7 [100%], *2/*3 = 0 [0%], *3/*3 = 0 [0%]) phenotypes included multiple genotypes. (B) Use frequency of PGx actionable medications affected by CYP2C19 metabolism beyond antiplatelet therapy in CYP2C19 genotyped patients, sorted by medication class. Rabeprazole and clomipramine use was 0% and thus not included in the graph.
IM: Intermediate metabolizer; NM: Normal metabolizer; PGx: Pharmacogenomics; PM: Poor metabolizer; RM: Rapid metabolizer; UM: Ultrarapid metabolizer.
Among the 380 PCI patients with a CYP2C19 genotype result, 194 (51%) were prescribed one or more of nine distinct PGx actionable medications, beyond antiplatelet therapy, affected by CYP2C19 alleles (Figure 3B). As a result of 35 (9.2%) patients taking more than one PGx actionable medication, there were 229 total instances of a CYP2C19 genotype-affected medication being prescribed to a patient with a genotype result available in the EHR. As summarized in Table 2, we observed 30 instances in which a CYP2C19 UM, RM or PM was prescribed CYP2C19-metabolized medication (7.9 per 100 patients). As a result of three patients taking more than one medication, an actionable PGx intervention based on the genotype result could have been made in 27 (7.1%) of the 380 genotyped patients (13.9% of the 194 genotyped patients taking a medication affected by CYP2C19 metabolizer status). The majority of the projected interventions would have served to avoid a potential therapeutic failure (87%) in a CYP2C19 UM or RM patient, with 13% preventing a potential adverse event in a PM (Table 2).
Table 2. . Summary of potential CYP2C19 genotype-guided medication interventions.
| Drug class | Medication | Number of patients prescribed medication | At-risk CYP2C19 phenotype group | At-risk CYP2C19 phenotype frequency | Number of potential actionable PGx interventions† | Avoided clinical outcome |
|---|---|---|---|---|---|---|
| PPI | Esomeprazole, Omeprazole, Lansoprazole, Pantoprazole, Dexlansoprazole and Rabeprazole | 165 | UM PM |
4.5% 1.8% |
7 3 |
Therapeutic failure Adverse event |
| SSRI | Citalopram, Escitalopram and Sertraline | 58 | UM/RM PM |
32.4% 1.8% |
18 1 |
Therapeutic failure Adverse event |
| TCA | Amitriptyline | 6 | UM/RM PM |
32.4% 1.8% |
1 0 |
Therapeutic failure Adverse event |
| Total | 229 | 30 |
The number of potential actionable PGx interventions count the number of observed instances where a CYP2C19 genotyped patient (n = 380) was prescribed a CPIC level A or B evidence medication and carried an actionable CYP2C19 phenotype.
CPIC: Clinical Pharmacogenomics Implementation Consortium; PGx: Pharmacogenomics; PPI: Proton pump inhibitor; PM: Poor metabolizer; RM: Rapid metabolizer; SSRI: Selective serotonin reuptake inhibitor; TCA: Tricyclic antidepressant; UM: Ultrarapid metabolizer.
Projected number of potential PGx-guided interventions following multigene testing
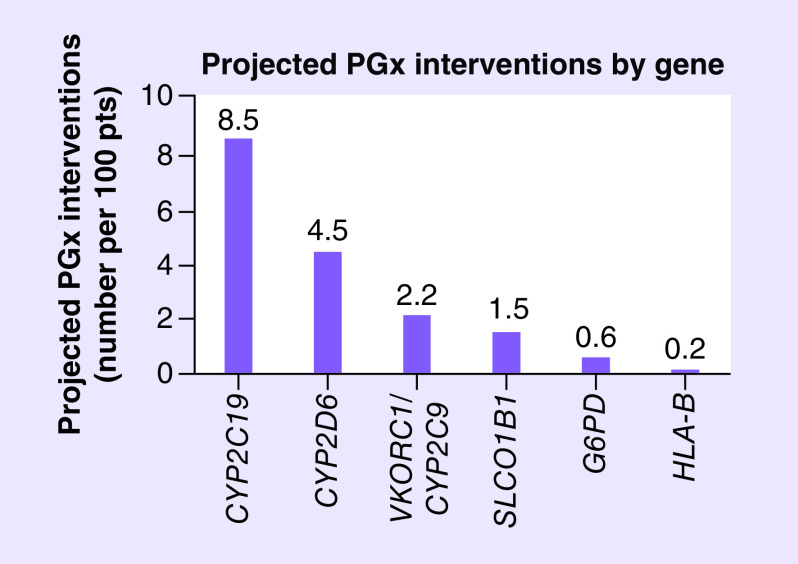
A simulation analysis estimating the potential impact of multigene testing for CYP2C19, CYP2D6, G6PD, VKORC1/CYP2C9, SLCO1B1 and HLA-B in the overall study population (n = 646) projected that 113 total interventions (17.5 per 100 patients) beyond antiplatelet therapy could have been made based on actionable PGx results (Figure 4). A detailed summary, by gene and drug, is provided in Supplementary Tables 3–8.
Figure 4. . Projected number of actionable pharmacogenomics interventions beyond antiplatelet therapy by gene in percutaneous coronary intervention patients.
Based on a simulation analysis including the 19 most frequently prescribed medications and corresponding seven genes with actionable PGx recommendations, the projected number of potential PGx interventions were calculated as summarized in greater detail for each gene in Supplementary Tables 3–8. Data in the figure are presented, by gene, as the projected number of actionable interventions per 100 patients.
PGx: Pharmacogenomics.
Among the 396 instances of CYP2C19 metabolized medication use, we estimated 55 projected PGx interventions beyond antiplatelet therapy (8.5 per 100 patients; Supplementary Table 3). The most common projected interventions involved prevention of a potential SSRI (27 interventions) and PPI (13 interventions) therapeutic failure in UMs or RMs and avoidance of a potential PPI adverse effect in PMs (eight interventions). The number and distribution of projected interventions in the simulation analysis was consistent with our observed data in patients with available CYP2C19 genotype results.
Among the 314 instances of CYP2D6-metabolized medication use, we estimated 29 projected PGx interventions (4.5 per 100 patients; Supplementary Table 4). The projected interventions involved antidepressants (amitriptyline, paroxetine and venlafaxine) in CYP2D6 UMs, IMs and PMs (seven interventions), opioids (tramadol, oxycodone and hydrocodone) in CYP2D6 UMs and PMs (21 interventions) and ondansetron in CYP2D6 UMs (one intervention). Among the 44 and 41 patients taking warfarin and simvastatin, respectively, we projected 14 potential warfarin dose-optimization interventions in VKORC1/CYP2C9 sensitive or highly sensitive responders (2.2 per 100 patients; Supplementary Table 5) and ten potential interventions in patients with a SLCO1B1 intermediate or low function phenotype that could be prescribed a lower dose or alternative statin (1.5 per 100 patients; Supplementary Table 6). We also projected four potential interventions in patients with G6PD deficiency taking glipizide or glimepiride (0.6 per 100 patients; Supplementary Table 7) and one potential intervention for a patient taking allopurinol (0.2 per 100 patients; Supplementary Table 8), which is contraindicated in HLA-B*58:01 allele carriers [29].
Discussion
The projected impact of CYP2C19 and multigene PGx testing on medications beyond clopidogrel was evaluated in a real-world cohort of patients that underwent PCI and CYP2C19 testing. Our results demonstrate that medications with actionable PGx recommendations across multiple drug classes, most notably PPIs, antidepressants and opioids, are commonly prescribed in PCI patients. Among patients with an available CYP2C19 genotype result in the EHR, approximately 50% were prescribed a CYP2C19-metabolized medication beyond clopidogrel and 7% met criteria for a CYP2C19 genotype-guided medication intervention. Further, our simulation analysis projected that approximately 17.5 medication interventions per 100 PCI patients could have been recommended if multigene PGx test results were available. Taken together, these results suggest that multigene PGx testing could be used to optimize prescribing for multiple medications beyond CYP2C19 genotype-guided antiplatelet therapy in PCI patients and warrants further study.
Due to the benefit of CYP2C19 genotype-guided antiplatelet therapy after PCI, use of CYP2C19 testing in clinical practice to optimize antiplatelet therapy after PCI has become increasingly common [7,10,12]. Our results demonstrate that an opportunity exists to optimize the use of medications beyond clopidogrel based on the results of a CYP2C19 genotype test that has already been performed. Approximately 50% of PCI patients in our cohort were prescribed a CYP2C19-metabolized PPI or antidepressant at either discharge or an outpatient encounter within 1 year, with approximately 7% of patients qualifying for an evidence-based CYP2C19 genotype-guided medication intervention. In patients prescribed a PPI, interventions would include increasing the PPI dose in CYP2C19 UM patients to prevent therapeutic failure and selecting an alternative PPI in CYP2C19 PM patients to avoid an adverse event [30]. In patients prescribed a SSRI, interventions would include selecting an alternative agent in CYP2C19 UM or RM patients to avoid therapeutic failure and either a dose reduction or alternative agent in CYP2C19 PM patients to avoid a potential adverse event [23].
Because it has been estimated that approximately 99% of the population carries at least one at-risk PGx genotype, identifying patient populations that are commonly prescribed PGx actionable medications is an important prerequisite for efficient implementation of multigene PGx testing [5,6]. In addition to PPIs and antidepressants, opioids for chronic pain, warfarin for thromboembolic disease and simvastatin for hyperlipidemia were also commonly prescribed in PCI patients. Our simulation analysis projected that multigene testing in our study PCI population could have yielded 17.5 PGx-guided medication interventions per 100 patients, of which approximately half were derived from CYP2C19. The primary genes in addition to CYP2C19 yielding actionable results were CYP2D6 for antidepressants and opioids, VKORC1/CYP2C9 for warfarin and SLCO1B1 for simvastatin. These observations are consistent with two recent analyses that demonstrated multigene testing with CYP2C19, SLCO1B1, VKORC1 and CYP2C9 and multigene testing with CYP2C19 and CYP2D6 was more cost effective than CYP2C19 testing alone in patients undergoing PCI [31,32]. The projected number of PGx interventions in our study population to avoid toxicity in G6PD-deficient patients prescribed a sulfonylurea was low (0.6 interventions per 100 patients); however, most commercial multigene PGx panels do not include testing for G6PD deficiency. Taken together, our results suggest the cardiac catheterization laboratory is a logical ‘foot in the door’ setting for institutions to efficiently implement preemptive multigene PGx testing and can be used to inform the design of prospective studies that evaluate the feasibility and impact of this precision medicine strategy in PCI patients.
The results of our study demonstrates that approximately one of every six PCI patients met qualifications for a PGx-guided medication intervention beyond antiplatelet therapy may underestimate the value of preemptive genotyping since our analysis only considered prescribing during the 12 months after PCI. The number of interventions would be expected to increase over time as patients age and are prescribed additional medications. Furthermore, additional commonly prescribed medications with CPIC level C evidence, such as β-blockers, may be reclassified as clinically actionable as evidence supporting clinical use expands. Considering additional medications with PGx actionable recommendations by the Dutch Pharmacogenetics Working Group would also likely increase the number of potential PGx-guided interventions in PCI patients. Our results also demonstrate that most PGx-guided interventions would serve to prevent a potential therapeutic failure. While the clinical impact of such medication optimization interventions might not be immediate, the long-term effects could be significant to patients, providers and payers.
Notably, SSRIs are the first-line treatment for depression and anxiety and treatment failures can lead to significant health consequences for patients and increased healthcare costs that are especially problematic in the elderly [33]. Moreover, depression is more common in women than men and suboptimal management of depression is associated with adverse cardiovascular outcomes [34]. Given that over a third of the US population carries either a CYP2C19 or CYP2D6 UM or RM phenotype, PGx-guided strategies that enable more precise initial selection and dosing of antidepressants offer enormous potential to prevent or reduce the duration and impact of uncontrolled depressive symptoms [23], particularly in women, the elderly and other subsets of the population with a high frequency of antidepressant prescriptions. Furthermore, CYP2D6 genotype-guided opioid prescribing has been shown to improve pain control, with emerging data also showing benefit in CYP2D6 IMs and PMs [35]. While the effect of CYP2C19 genotype on PPI pharmacokinetics has been well documented, the clinical significance on clinical outcomes remains less clear [36]. It has been suggested that genotype-guided PPI prescribing has clinical benefit in the treatment of gastroesophageal reflux disease and Helicobacter pylori eradication, as well as the prevention of PPI-related adverse events such as infection and acute kidney injury [30]. Given the high prevalence of PPI use in PCI patients receiving antiplatelet therapy, studies evaluating the clinical effectiveness of CYP2C19 genotype-guided PPI prescribing are still needed.
As multigene PGx testing becomes increasingly common in clinical practice, a more comprehensive assessment of multiple medications (rather than a single medication) will be necessary to effectively use each patient’s genetic test results to optimize prescribing decisions and minimize healthcare costs. Notably, our results showed that use of noncardiac medications in PCI patients is common. While this represents an opportunity for medication optimization to occur across a patient’s entire medication list, many PGx actionable medications are used to treat psychiatric and pain conditions that are not typically managed by a cardiologist. Clinical pharmacists, clinical decision support tools and multidisciplinary collaboration are instrumental to improve the adoption and appropriate use of PGx-based medication optimization strategies and facilitate transitions of care in patients with a multitude of comorbidities and medications [37,38]. Innovative precision medicine services, such as a precision medicine pharmacist in cardiology or multiclinic settings, will become especially important as the frequency of PGx testing increases and new evidence linking genetic variation to suboptimal medication outcomes continues to emerge.
There are several limitations to this study that should be acknowledged. The simulation analysis was limited by the use of projected at-risk genotype frequencies and a probability-based estimation of potential PGx interventions in the overall population, rather than patient-level data based on observed genotype results and medication use. However, the simulated CYP2C19 analysis yielded very similar results to the observed analysis in patients with an available CYP2C19 genotype, which increases confidence in our results. While the frequency of patients at risk for a PGx-associated adverse medication outcome were calculated, direct evaluation of therapeutic failures and adverse effects for each medication in individual patients were not completed in the current study, which is a limitation. The impact of PGx-guided prescribing on adverse medication outcomes will require prospective investigation in subsequent studies. Due to the retrospective design, the observed medication use frequency may not reflect more contemporary prescribing patterns. Although medication use was evaluated at multiple encounters over time, misclassification bias cannot be excluded. Further, medications used transiently, such as antibiotics, antifungals and anesthesiology drugs, were not included since our data abstraction strategy would likely underestimate their actual use. Notably, the antifungal voriconazole has actionable prescribing recommendations based on CYP2C19 genotype results [39]. Thus, exclusion of these medications from our analysis likely underestimated the projected impact of PGx testing in this population. Additionally, some patients likely receive some medications for noncardiac disease states at other centers that may not be documented in the EHR, further underestimating the number of actionable PGx recommendations. At last, although the study population was derived from an academic, tertiary referral center with a population that is approximately 20% African–American and representative of surrounding areas, medication use frequency at this single-center may not be generalizable to other PCI populations. Notably, our simulation analysis did not project the impact of multigene PGx testing in Asian populations, which may have further underestimated the projected impact of PGx testing due to the higher prevalence of CYP2C19 IMs and PMs compared with Caucasian and African–American populations. Furthermore, we did not evaluate the impact of gender on the frequency of actionable PGx recommendations, which is an important future direction due to the higher prevalence of antidepressant use in women compared with men. Prospective studies in larger and more diverse multicenter populations and over longer time horizons after PCI are warranted to assess the projected impact of PGx testing on medication prescribing overall and within key demographic subsets such as race/ethnicity, gender and age groups. In addition, future studies evaluating the projected impact of multigene PGx testing on medication optimization beyond PCI patients and across multiple clinical settings are needed.
In summary, the current study demonstrated that medications across multiple drug classes with actionable PGx recommendations are commonly prescribed in PCI patients receiving antiplatelet therapy. The projected impact of PGx testing on optimal medication prescribing beyond clopidogrel in this real-world patient population suggests that multigene PGx testing could be beneficial among PCI patients who are already indicated for CYP2C19 genotyping and the cardiac catheterization laboratory could serve as a logical setting for institutions to efficiently implement multigene PGx testing into routine clinical practice. Future prospective studies evaluating the feasibility and effectiveness of multigene PGx testing in PCI patients are needed.
Summary points.
The patient populations in which pharmacogenomics (PGx) testing offers the greatest potential impact on medication prescribing remain unclear.
We hypothesized that percutaneous coronary intervention (PCI) patients are prescribed multiple medications, in addition to antiplatelet therapy, that have actionable PGx recommendations for at-risk genotypes in CYP2C19 and other pharmacogenes.
This study evaluated the potential impact of CYP2C19 and multigene PGx testing on medications beyond antiplatelet therapy in a real-world cohort of PCI patients that underwent CYP2C19 testing.
Our results demonstrate that medications with actionable PGx recommendations across multiple drug classes, most notably proton pump inhibitors, antidepressants and opioid analgesics, are also commonly prescribed in PCI patients.
Among PCI patients with a CYP2C19 genotype result available, 51% were prescribed one or more CYP2C19-metabolized medications beyond clopidogrel and 7% met criteria for a CYP2C19 genotype-guided medication intervention.
A simulation analysis projected that 113 interventions (17.5 per 100 PCI patients) could have been recommended in the study population if multigene PGx results were available. Approximately half of the interventions would be guided by genes other than CYP2C19.
These results suggest that CYP2C19 and multigene PGx testing could be used to optimize prescribing for multiple medications beyond CYP2C19 genotype-guided antiplatelet therapy in PCI patients and warrants further study.
As new evidence emerges and PGx testing becomes increasingly common in practice, clinical pharmacists, clinical decision support tools and inter-disciplinary collaboration will be instrumental in the adoption and appropriate use of PGx-based prescribing.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://www.futuremedicine.com/doi/suppl/10.2217/pgs-2019-0185
Financial & competing interests disclosure
The project was supported by the National Center for Advancing Translational Sciences (NCATS), the NIH, through grant award number UL1TR002489, and in part by UNC Eshelman Institute for Innovation pilot grant RX03612214 to T Wiltshire. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.US FDA. Table of pharmacogenomic biomarkers in drug labeling | FDA. (2019). https://www.fda.gov/drugs/science-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling
- 2.Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature 526(7573), 343–350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caudle K, Klein T, Hoffman J. et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr. Drug Metab. 15(2), 209–217 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical Pharmacogenetics Implementation Consortium. Genes–drugs – CPIC. (2019). https://cpicpgx.org/genes-drugs/
- 5.Ji Y, Skierka JM, Blommel JH. et al. Preemptive pharmacogenomic testing for precision medicine a comprehensive analysis of five actionable pharmacogenomic genes using next-generation DNA sequencing and a customized CYP2D6 genotyping cascade. J. Mol. Diagn. 18(3), 438–445 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanfreau-Coffinier C, Hull LE, Lynch JA. et al. Projected prevalence of actionable pharmacogenetic variants and level a drugs prescribed among US veterans health administration pharmacy users. JAMA Netw. Open 2(6), e195345 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Empey PE, Stevenson JM, Tuteja S. et al. Multisite investigation of strategies for the implementation of CYP2C19 genotype-guided antiplatelet therapy. Clin. Pharmacol. Ther. 104(4), 664–674 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CR, Sriramoju VB, Cervantes A. et al. Clinical outcomes and sustainability of using CYP2C19 genotype – guided antiplatelet therapy after percutaneous coronary intervention. Circ. Genomic Precis. Med. 11(4), e002069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mega JL, Simon T, Collet JP. et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 304(16), 1821–1830 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavallari LH, Lee CR, Beitelshees AL. et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc. Interv. 11(2), 181–191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dayoub EJ, Seigerman M, Tuteja S. et al. Trends in platelet adenosine diphosphate P2Y12 receptor inhibitor use and adherence among antiplatelet-naive patients after percutaneous coronary intervention, 2008–2016. JAMA Intern. Med. 178(7), 943–950 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claassens DMF, Vos GJA, Bergmeijer TO. et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N. Engl. J. Med. 381(17), 1621–1631 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Scott SA, Sangkuhl K, Stein CM. et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin. Pharmacol. Ther. 94(3), 317–323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunnenberger HM, Crews KR, Hoffman JM. et al. Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu. Rev. Pharmacol. Toxicol. 55(1), 89–106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavallari LH, Beitelshees AL, Blake K V. et al. The IGNITE pharmacogenetics working group: an opportunity for building evidence with pharmacogenetic implementation in a real-world setting. Clin. Transl. Sci. 10(3), 143–146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichtman JH, Bigger JT, Blumenthal JA. et al. Depression and coronary heart disease recommendations for screening, referral and treatment a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation 118(17), 1768–1775 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 4(13), 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong OM, Li A, Suzuki O. et al. Projected impact of a multigene pharmacogenetic test to optimize medication prescribing in cardiovascular patients. Pharmacogenomics 19(9), 771–782 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JA, Lee CR, Reed BN. et al. Implementation and evaluation of a CYP2C19 genotype-guided antiplatelet therapy algorithm in high-risk coronary artery disease patients. Pharmacogenomics 16(4), 303–313 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Caudle KE, Dunnenberger HM, Freimuth RR. et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet. Med. 19(2), 215–223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Relling MV, McDonagh E, Chang T. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype. Clin. Pharmacol. Ther. 96(2), 169–174 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hicks J, Sangkuhl K, Swen J. et al. Clinical Pharmacogenetics Implementation Consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharmacol. Ther. 102(1), 37–44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks JK, Bishop JR, Sangkuhl K. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 98(2), 127–134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell G, Caudle K, Whirl-Carrillo M. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 genotype and use of ondansetron and tropisetron. Clin. Pharmacol. Ther. 102(2), 213–218 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crews KR, Gaedigk A, Dunnenberger HM. et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin. Pharmacol. Ther. 95(4), 376–382 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilke RA, Ramsey LB, Johnson SG. et al. The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin. Pharmacol. Ther. 92(1), 112–117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mega JL, Walker JR, Ruff CT. et al. Genetics and the clinical response to warfarin and edoxaban: findings from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 385(9984), 2280–2287 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Johnson J, Caudle K, Gong L. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin. Pharmacol. Ther. 102(3), 397–404 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito Y, Stamp LK, Caudle KE. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for human leukocyte antigen B (HLA-B) genotype and allopurinol dosing: 2015 update. Clin. Pharmacol. Ther. 99(1), 36–37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Rouby N, Lima JJ, Johnson JA. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin. Drug Metab. Toxicol. 14(4), 447–460 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong OM, Wheeler SB, Cruden G. et al. Cost–effectiveness of multigene pharmacogenetic testing in acute coronary syndrome patients after percutaneous coronary intervention. Value Health 23(1), 61–73 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Hart MR, Garrison LP, Doyle DL, Jarvik GP, Watkins J, Devine B. Projected cost–effectiveness for 2 gene–drug pairs using a multigene panel for patients undergoing percutaneous coronary intervention. Value Health 22(11), 1231–1239 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Aziz R, Steffens DC. What are the causes of late-life depression? Psychiatr. Clin. North Am. 36(4), 497–516 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jha MK, Qamar A, Vaduganathan M, Charney DS, Murrough JW. Screening and management of depression in patients with cardiovascular disease JACC state-of-the-art review. J. Am. Coll. Cardiol. 73(14), 1827–1845 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith DM, Weitzel KW, Elsey AR. et al. CYP2D6-guided opioid therapy improves pain control in CYP2D6 intermediate and poor metabolizers: a pragmatic clinical trial. Genet. Med. 21(8), 1842–1850 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagymási K, Müllner K, Herszényi L, Tulassay Z. Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics 12(6), 873–888 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Owusu-Obeng A, Weitzel KW, Hatton RC. et al. Emerging roles for pharmacists in clinical implementation of pharmacogenomics. Pharmacotherapy 34(10), 1102–1112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hicks JK, Dunnenberger HM, Gumpper KF, Haidar CE, Hoffman JM. Integrating pharmacogenomics into electronic health records with clinical decision support. Am. J. Heal. Syst. Pharm. 73(23), 1967–1976 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moriyama B, Obeng AO, Barbarino J. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP2C19 and voriconazole therapy. Clin. Pharmacol. Ther. 102(1), 45–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.