Abstract
Human female fetal reproductive tracts 9.5 to 22 weeks of gestation were grown for 1 month in ovariectomized athymic adult female mouse hosts that were either untreated or treated continuously with diethylstilbestrol (DES) via subcutaneous pellet. Normal morphogenesis and normal patterns of differentiation marker expression (KRT6, KRT7, KRT8, KRT10, KRT14, KRT19, ESR1, PGR, TP63, RUNX1, ISL1, HOXA11 and α-ACT2) were observed in xenografts grown in untreated hosts and mimicked observations of previously reported (Cunha et al., 2017) non-grafted specimens of comparable age. DES elicited several notable morphological affects: (a) induction of endometrial/cervical glands, (b) increased plication (folding) of tubal epithelium, (c) stratified squamous maturation of vaginal epithelium and (d) vaginal adenosis. DES also induced ESR1 in epithelia of the uterine corpus, cervix and globally induced PGR in most cells of the developing human female reproductive tract. Keratin expression (KRT6, KRT7, KRT8, KRT14 and KRT19) was minimally affected by DES. Simple columnar adenotic epithelium was devoid of TP63 and RUNX1, while DES-induced mature vaginal epithelium was positive for both transcription factors. Another striking effect of DES was observed in grafts of human uterine tube, in which DES perturbed smooth muscle patterning. These results define for the first time IHC protein markers of DES action on the developing human reproductive tract, which provide bio-endpoints of estrogen-induced teratogenesis in the developing human female reproductive tract for future testing of estrogenic endocrine disruptors.
Keywords: Human female fetal reproductive tract, estrogenic response, diethylstilbestrol, tissue interactions, mesenchyme, epithelium
I. Introduction
The tacit, but unproven assumption inherent in the use of animal models, is that they are representative of human biology, even though substantial differences exist between human versus animal anatomy, development and pathology. One field for which animal/human pathology is particularly congruent concerns the effects of exogenous estrogens on the developing female reproductive tract. Administration of diethylstilbestrol (DES) to pregnant women from the 1940s to the 1970s resulted in a broad spectrum of estrogen-induced malformations of the uterine tubes, uterine corpus, cervix and vagina that include T-shaped uterotubal junctions, malformed incompetent cervix, abnormally shaped endometrial cavity, vaginal adenosis (presence of glandular epithelium in the vagina where normally stratified squamous epithelium should reside) and clear cell vaginal adenocarcinoma (Jefferies et al., 1984; Rennell, 1979; Stillman, 1982; Titus-Ernstoff et al., 2010; Herbst et al., 1971; Herbst et al., 1975b; Robboy et al., 1977; Robboy et al., 1984; Hoover et al., 2011). An immense animal literature preceded/confirmed the effects of exogenous estrogens on female reproductive tract development, and animal studies have provided a molecular underpinning for the teratogenic effects of natural (17β-estradiol [Forsberg, 1972], 17α-estradiol [Forsberg and Kalland, 1981], estradiol benzoate [Plapinger and Bern, 1979] and synthetic estrogens (diethylstilbestrol], dienestrol [Forsberg and Kalland, 1981], clomiphene citrate [Gorwill et al., 1982], tamoxifen [Taguchi and Nishizuka, 1985], nafoxidine [Iguchi et al., 1986], coumestrol [Burroughs et al., 1990] and bisphenol A (BPA) [Newbold et al., 2009) on urogenital development (Herbst and Bern, 1981; Bern and Talamantes, 1981; Bern et al., 1984; McLachlan et al., 1975; McLachlan, 1981; Newbold et al., 1983; Newbold and MaLachlan, 1985; Newbold, 1995; Newbold, 2004; 2008; McLachlan et al., 2001; McLachlan and Newbold, 1996; Kurita et al., 2004; Kurita, 2011; Laronda et al., 2012; Laronda et al., 2013). The animal literature on this topic is replete with observations of vaginal, cervical, uterine and tubal (oviductal) anomalies including cervicovaginal adenosis. While animal models have been useful and relevant to many aspects of human biology/pathology, animal studies frequently are the basis of governmental policy designed to protect human health. Accordingly, whenever possible it is important to establish by experimental means the congruence (or lack thereof) of animal studies to human biology/pathology.
Despite the mouse/human similarities in estrogen-induced anomalies in the female reproductive tract, the molecular mechanisms leading to human malformations are poorly understood even though mouse studies provide some clues (McLachlan and Newbold, 1996; Kurita et al., 2004; Kurita, 2011; Laronda et al., 2012; Laronda et al., 2013). Direct experimentation on human fetal xenografts treated with DES can provide important insights into the genesis of human malformations and can provide essential bio-endpoints of hormonally active endocrine disruptors with direct relevance to human development. In this regard, we pioneered xenograft methods about 35 years ago in which human fetal female reproductive tracts were grown in athymic mouse hosts treated with DES and other hormonally active agents (Cunha et al., 1987b; Cunha et al., 1987a; Robboy et al., 1982; Taguchi et al., 1983). However, the then existing state of technology prevented further exploration of molecular mechanisms. In the current paper we analyze an entirely new set of specimens to (a) revisit our xenograft model of human female fetal reproductive tract development, (b) provide new data to validate normal development in xenografts to untreated hosts and (c) explore the how DES exposure affects the morphogenesis, differentiation and molecular expression within human female fetal reproductive tracts. Our resultant studies provide the essential underpinning for future studies of human female reproductive tract development designed to explore the mechanism of mesenchymal-epithelial interactions in epithelial morphogenesis, differentiation and gene expression, and especially the role of direct versus paracrine signaling in the expression of human uterine epithelial ESR1 and PGR.
II. Materials and Methods
A. General comments.
First and second trimester human fetal specimens devoid of patient identifiers were collected from 2015 to 2017 after elective termination of pregnancy (Committee on Human Research, IRB# 12–08813). Fetal age was estimated using heel-toe length (Drey et al., 2005). Gender was determined by virtue of Wolffian and Müllerian duct morphology. Female internal genitalia were identified and isolated from the abortus specimen using a dissecting microscope. This study is based upon 5 control specimens grafted into untreated hosts and 5 specimens grafted into DES-treated hosts collected over the period of 2015 to 2017 (Table 1). The control group consisted of 4 grafts of intact reproductive tracts (11 to 14 weeks of gestation) as well as 1 additional specimen at 15 weeks of uterine tube only. The DES-treated group consisted of 4 intact specimens at 9.5 and 14 weeks of gestation and one additional specimen of uterine tube only at 22 weeks. The term “intact” means that the specimen contained uterine tubes and the uterovaginal canal. In some cases the lower portion of the uterovaginal canal (the urogenital sinus component) may have been missing, even though the rudiments of the uterine tube, uterine corpus, uterine cervix and vagina were present in all of the “intact” specimens. The uterine tube only specimens (AC297 and AC316) were divided into multiple individual grafts. Since the data from these tube only grafts did not vary, they are registered as a single graft. Fortuitously, the intact 13-week specimens were twins, which provided the most ideal means of comparison between control and DES treatment. Most of the images in this paper were taken from the 13- and 14-week specimens. Immunostaining results within the 5 control specimens were remarkably consistent. DES treatment elicited a distinctly different immunostaining profile that was remarkably similar within this treatment group. The additional specimens of “uterine tube only” were used primarily for examination of α-actin immunostaining.
Table 1.
Grafts of human female fetal reproductive tract specimens
| Specimen # | Treatment | Age | Intact versus tube only |
|---|---|---|---|
| AC115 | Control | 11wks | Intact |
| AC514 | Control | 11wks | Intact |
| AC121* | Control | 13wks | Intact |
| AC159 | Control | 14wks | Intact |
| AC297 | Control | 15wks | Tube only |
| AC509 | DES | 9.5wks | Intact |
| AC513 | DES | 10.5wks | Intact |
| AC122* | DES | 13wks | Intact |
| AC159 | DES | 14wks | Intact |
| AC316 | DES | 22wks | Tube only |
AC121 (Control) and AC122 (DES) are from a twin pregnancy.
Control=untreated ovariectomized host
DES=DES-treated ovariectomized host
B. Grafting of human fetal female reproductive tract organs.
For the younger specimens (9.5 to 15 weeks of gestation) the entire human female reproductive tract with attached uterine tubes was grafted under renal capsules of ovariectomized female athymic mice (Charles River, Holister, CA) as described previously (Cunha and Baskin, 2016). Our xenograft model differs in many respects from human in utero development (absence of a human maternal uterus, liver and absence of the placenta). Therefore, at the onset of this study we considered whether to use ovary-intact or ovariectomized hosts. Both approaches have merit. Use of ovariectomized hosts has the advantage of being able to discern effects of DES independent of other hormonal variables, and thus we chose this condition. DES is known to have far reaching systemic hormonal effects when administered to pregnant or neonatal mice, and presumably also had comparable effects on historic human pregnancies, which cannot be replicated in the xenograft model. Whether the use of ovary-intact hosts may give different results remains to be determined in future studies. Host mice were ovariectomized and either untreated or implanted subcutaneously with a 20mg DES pellet. After 4 weeks of in vivo growth the grafts were harvested for analysis. The effectiveness of the ovariectomy in untreated hosts was determined by examination of hosts’ uteri, which in all cases were atrophic. Likewise, the effectiveness the DES pellet was verified by hypertrophy of the hosts’ uteri (not illustrated).
C. Histologic and immunohistochemical (IHC) analysis.
At harvest, grafts of human fetal female reproductive tracts were dissected from the kidney, fixed for at least 24 hours in 10% buffered formalin, embedded in paraffin and serially sectioned at 7μm. Every 20th section was stained with hematoxylin and eosin (H&E). The remaining paraffin sections were immunostained with the antibodies indicated (Table 2). Immunostaining was detected using horseradish peroxidase based Vectastain kits (Vector Laboratories, Burlingame, CA). Negative controls deleted the primary antibody.
Table 2.
Antibodies used in this study
| Antibody # | Source | Catalogue # | Dilution |
|---|---|---|---|
| Keratin 6 | Acris Antibodies | AM21068PU-S | 1/200 |
| Keratin 7 | EB Lane | LP1K | 1/10 |
| Keratin 8 | EB Lane | LE41 | 1/10 |
| Keratin 10 | Dako | M7002 | 1/50 |
| Keratin 14 | BioGenex | LL002 | 1/100 |
| Keratin 19 | EB Lane | LP2K | 1/100 |
| HOXa11 | Sigma | HPA035623 | 1/400 |
| TP63 | Santa Cruz Biotechnology | Sc-8343 | 1/100 |
| ISL1 | Abcam | Ab20670 | 1/200 |
| ESR1 | Dako | Ab16660 | 1/100 |
| PGR | Abcam | Ab16661 | 1/100 |
| RUNX1 | Abcam | Ab92336 | 1/100 |
| α-ACT2 | Sigma | A-2547 | 1/3000 |
III. Results
A. Epithelial and mesenchymal differentiation in grafts of human fetal female reproductive tracts grown in untreated ovariectomized female hosts.
1. General comments.
Xenografts implanted into untreated ovariectomized hosts were derived from fetuses 9.5 to 22 weeks of gestation. In an earlier report (Cunha et al., 2017) we studied the expression of epithelial and mesenchymal differentiation markers in developing human fetal female reproductive tracts over the time frame of 8 to 21 weeks. Prior to 11 weeks of gestation in non-grafted specimens, many markers are weakly expressed, while at later stages epithelial and mesenchymal differentiation markers are strongly expressed (Cunha et al., 2017). Accordingly, our xenograft experiments using specimens in the age range of (10.5 to 14 weeks) are ideal to assess the effects of DES on expression of protein differentiation markers. Histologic sections of control specimens revealed continued normal development of all organs (uterine tube, uterine corpus, uterine cervix and vaginal plate/vagina) in the untreated xenografts, thus corroborating our earlier xenograft studies (Cunha et al., 1987b; Cunha et al., 1987a; Robboy et al., 1982; Taguchi et al., 1983). Health of the xenografts was verified by prominent MKi67 labeling in both epithelia and mesenchyma in all grafts (Fig. 1). Finally, as indicated in our earlier report (Robboy et al., 2017), boundaries between the uterine corpus, uterine cervix and vagina are indistinct and so stated as “presumed” boundaries. Tables 3 to 5 summarize immunostaining results from non-grafted specimens from Cunha et al., 2017 as well as from untreated and DES-treated xenografts for the uterine tube (Table 3), the uterine corpus (Table 4) and the vagina (Table 5). Results for the cervix are difficult to accurately assess due to the indistinct boundaries between the uterine corpus, cervix and vagina. Qualified cervical results are given in text only.
Figure 1.
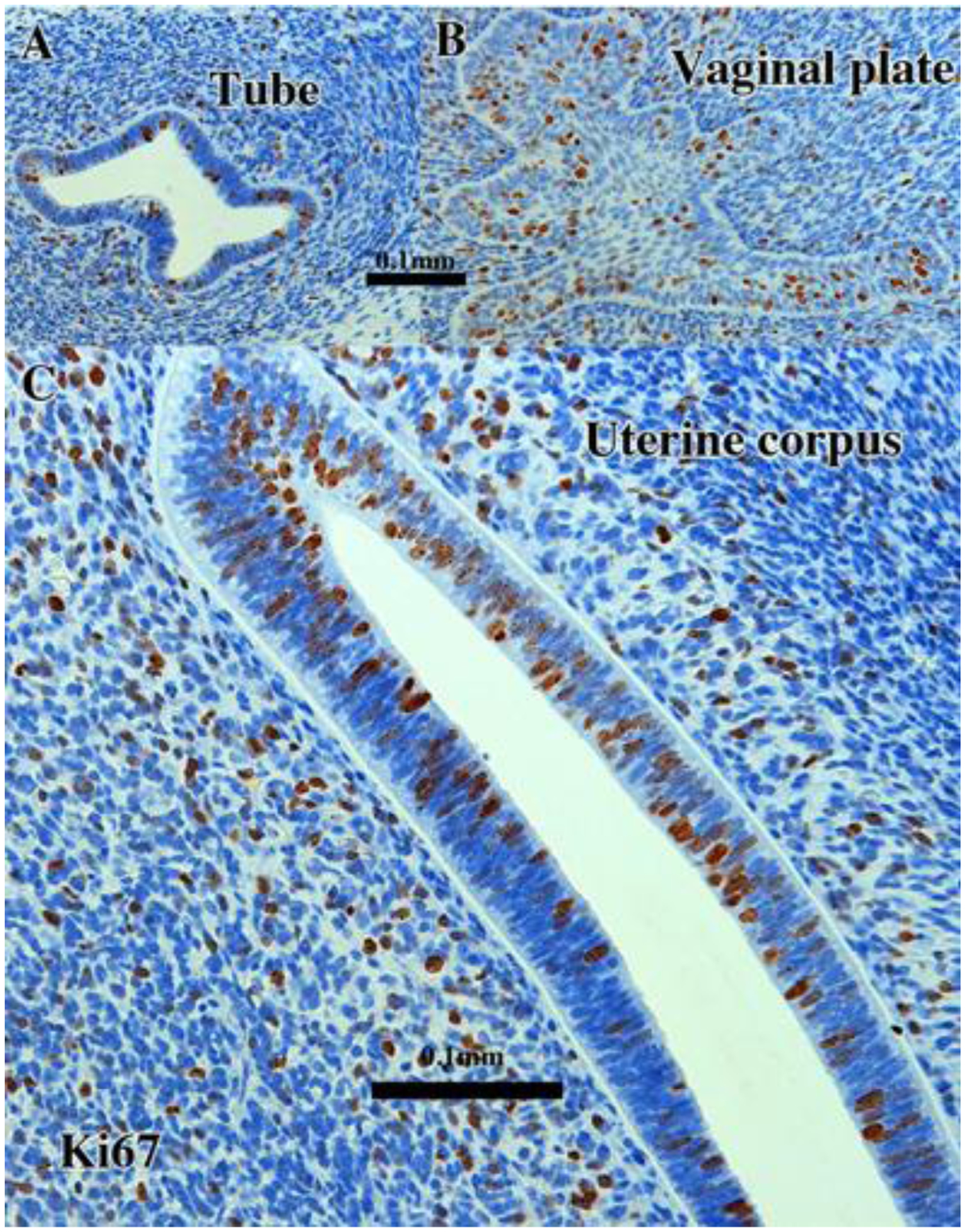
KI67 immunostaining in 13-week intact female fetal reproductive tract (AC121) grown for 4 weeks in an untreated ovariectomized female athymic mouse host. The developing uterine tube (A), vaginal plate (B) and uterus (C) all exhibit considerable numbers of proliferating MKI67-reactive epithelial and mesenchymal cells. Scale bar in (A) also applies to (B).
Table 3.
Differentiation markers in non-grafted, untreated xenografts and diethylstilbestrol-treated human fetal uterine tube
| Non-grafted* | Grafted control-No treatment | Grafted--DES | Control/DES figures | |
|---|---|---|---|---|
| Epithelium | ||||
| KRT7/8 | +++ | + (3/3) | + (4/4) | 4A/13D |
| KRT19 | + | + (2/2) | + (4/4) | NS/14A |
| KRT6 | − | − (2/2) | − (4/4) | NS /15A |
| KRT14 | − | − (2/2) | − (4/4) | 7A/16A |
| KRT10 | − | − (2/2) | − (4/4) | NS /NS |
| RUNX1 | − | − (2/2) | − (4/4) | 9D/18A |
| TP63 | − | − (2/2) | − (4/4) | 8C/17A |
| ESR1 | +++ | + (3/3) | +/− (4/4) | 3B/11A-B |
| PGR | − | − (3/3) | + (4/4) | NS/12A |
| Mesenchyme | ||||
| α-actin | + | + (3/3) | + (2/2) | 20A/20B |
| ISL | − | − (3/3) | − (3/3) | 10A/NS |
| HOXA11 | − | − (3/3) | − (3/3) | 10B/19B |
| ESR1 | + | + (3/3) | + (4/4) | 3A-C/11B |
| PGR | − | − (3/3) | + (4/4) | NS/12A |
Table 5.
Differentiation markers in non-grafted, untreated xenografts and diethylstilbestrol-treated human fetal vagina
| Non-grafted* | Grafted control-No treatment | Grafted-DES Strat. Epi. | Grafted-DES Adenosis | Control/DES figures | |
|---|---|---|---|---|---|
| Epithelium | |||||
| KRT7/8 | + | + (4/4) | + (3/4) | + (2/2) | 4A-C, F/13A-B |
| KRT19 | + | + (4/4) | + (4/4) | + (2/2) | 5A, C/14A-B |
| KRT6 | + | + (4/4) | + (4/4) | − (2/2) | 6D/15A-B |
| KRT14 | − | − (3/4) + (1/1) | + (2/4) | − (2/2) | 7A-B/16A-B |
| KRT10 | − | − (4/4) | − (4/4) | − (2/2) | NS/NS |
| RUNX1 | − | + (4/4) | + (4/4) | − (2/2) | 9B, F/18A-B |
| TP63 | − | + (4/4) | + (4/4) | − (2/2) | 8D-E/17A-C |
| ESR1 | + | − (3/4) MD + (1/1) UGS | + (3/4) | + (2/2) | 3A/11D |
| PGR | − | − (4/4) | + (4/4) | + (2/2) | NS/12B |
| AR | + | − (4/4), | − (4/4) | + (2/2) | |
| Mesenchyme | |||||
| α-actin | + | + (3/3) | + (4/4) | + (2/2) | 20A/20B |
| ISL | − | +(2/3) | + (4/4) | + (2/2) | 10A/19A |
| HOXA11 | − | − (3/3) | − (4/4) | − (2/2) | 10B/NS |
| ESR1 | + | + (4/4) | + (3/4) | + (2/2) | 3A/11D |
| PGR | − | − (4/4) | − (2/3) | − (2/2) | NS/12B |
| AR | + (4/4) | + (4/4) | + (2/2) | NS/NS |
Table 4.
Differentiation markers in non-grafted, untreated xenografts and diethylstilbestrol-treated human fetal uterus
| Non-grafted* | Grafted control-No treatment | Grafted--DES | Control/DES figures | |
|---|---|---|---|---|
| Epithelium | ||||
| KRT7/8 | + | + (3/3) | + (4/4) | 4A, B, D/13A, C |
| KRT19 | + | + (3/3) | + (2/2) | 5A-B/14A |
| KRT6 | − | − (2/2) | − /+ (3/4), − (1/1) | 6A-B/15A |
| KRT14 | − | − (3/3) | − (4/4) | 7A/16A |
| KRT10 | − | − (3/3) | − (3/3) | NS /NS |
| RUNX1 | − | + (3/3) | +/− (4/4) | 9A-B, D/18A, C |
| TP63 | − | +/− (2/3) − (1/1) | − (4/4) | 8C/17A, D |
| ESR1 | + | + (1/1) UGS − (2/2) MD | + (3/3) | 3C/11B-C |
| PGR | − | − (3/3) | + (4/4) | NS/12A |
| AR | + | − (2/2) | + (2/2) | |
| Mesenchyme | ||||
| α-actin | + | + (3/3) | + (2/2) | NS/NS |
| ISL | − | − (2/2) | − (2/2) | 10A/NS |
| HOXA11 | − | + (2/2) | + (2/2) | 10B/19B |
| ESR1 | + | + (3/3) | + (3/3) | 3A-C/11B-C |
| PGR | − | − (3/3) | + (4/4) | NS/12A |
| AR | + (1/2) | + (1/2) | NS /NS |
From Cunha et al., 2017
NS= not shown
ND= Not done
UGS= urogenital sinus-derive vaginal epithelium
MD= Müllerian-derived vaginal epithelium
2. Sex steroid receptors.
a. Estrogen receptor alpha (ESR1).
In grafts of human female fetal reproductive tracts grown in untreated ovariectomized hosts, ESR1 was strongly expressed in the simple columnar epithelium of the uterine tube (Fig. 2A–B) but not in epithelium of the uterine corpus or cervix (Fig. 2B–C and Tables 3–5), thus corroborating the pattern of ESR1 expression observed in non-grafted specimens of comparable age (see the accompanying paper) (Cunha et al., 2017). In the case of the vagina there are two types of stratified vaginal epithelium derived from the urogenital sinus (UGS) and the Müllerian ducts (uterovaginal canal), respectively. The UGS-derived vaginal epithelium is located caudally in the female reproductive tract, whereas the Müllerian-derived vaginal epithelium is located cranially. Each has a distinctive morphology. In grafts grown in untreated ovariectomized hosts, the UGS-derived vaginal epithelium is organized into irregular solid FOXA1-positive cords (Robboy et al., 2017), which are ESR1-positive (Fig. 2A, inset). The Müllerian-derived vaginal epithelium is 3 to 4 cell layers in thickness, lines a lumen, is PAX2-positive (Robboy et al., 2017) and is ESR1-negative (not illustrated). ESR1 was expressed in mesenchymal cells associated with the uterine tube, uterine corpus, and uterine cervix, although in the xenografts grown in untreated ovariectomized hosts, a subset of the mesenchymal cells was ESR1-negative (Fig. 2). ESR1-positive mesenchymal cells were rarely seen in the developing vagina (Fig. 2A).
Figure 2.
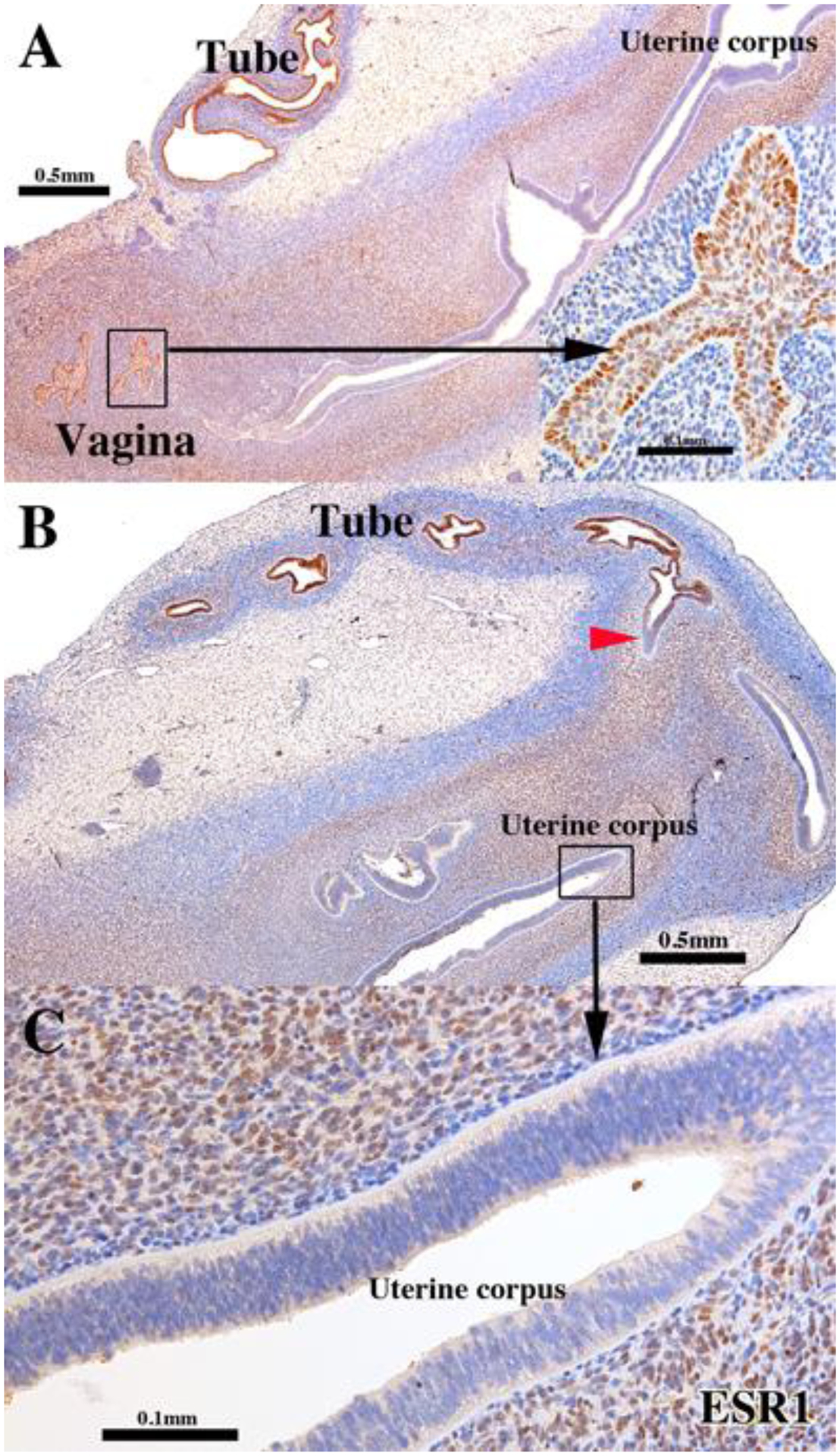
ESR1 immunostaining of a 13-week intact female fetal reproductive tract (AC121) grown for 4 weeks in an untreated ovariectomized female athymic mouse host. The epithelia of the uterine tube and vaginal plate (UGS-derived) are reactive for ESR1, while endometrial/cervical epithelia are not (B). The red arrowhead marks the uterotubal junction remarkable for the sharp transition in ESR1 staining. Detail of ESR1-negative endometrial epithelium (C). Note that the inner layer of mesenchymal cells of the uterine corpus (presumed endometrial stroma) is strongly ESR1-positive in contrast to peripheral mesenchyme (presumptive myometrium), which is weakly ESR1-positive. The stroma of the uterine tube is also ESR1 reactive but to a far lesser degree than in the corresponding area of uterine corpus.
b. Progesterone receptor (PGR).
PGR was not detected in either epithelial or mesenchymal cells of all grafted organs (uterine tube, uterine corpus, uterine cervix and vagina) grown in untreated ovariectomized hosts (not illustrated, Tables 3–5), and thus mimicked observations seen in non-grafted specimens in the accompanying paper epithelium (Cunha et al., 2017).
3. Keratins (KRT)
a. Keratin 7 (KRT7) and Keratin 8 (KRT8).
The staining pattern was similar for KRT7 and KRT8 in human female reproductive tracts that were non-grafted (Cunha et al., 2017) or grown in untreated ovariectomized hosts (Fig. 3, Tables 3–5). Accordingly, KRT7 and KRT8 were strongly expressed in the simple columnar epithelia of the uterine tube, uterine corpus and uterine cervix, and weakly expressed in epithelium of the vagina/vaginal plate (Fig. 3). In areas containing a stratified epithelium interpreted as vagina/cervix, KRT7 and KRT8 were expressed in the apical but not the basal epithelial cells (Fig. 3C, E & F).
Figure 3.
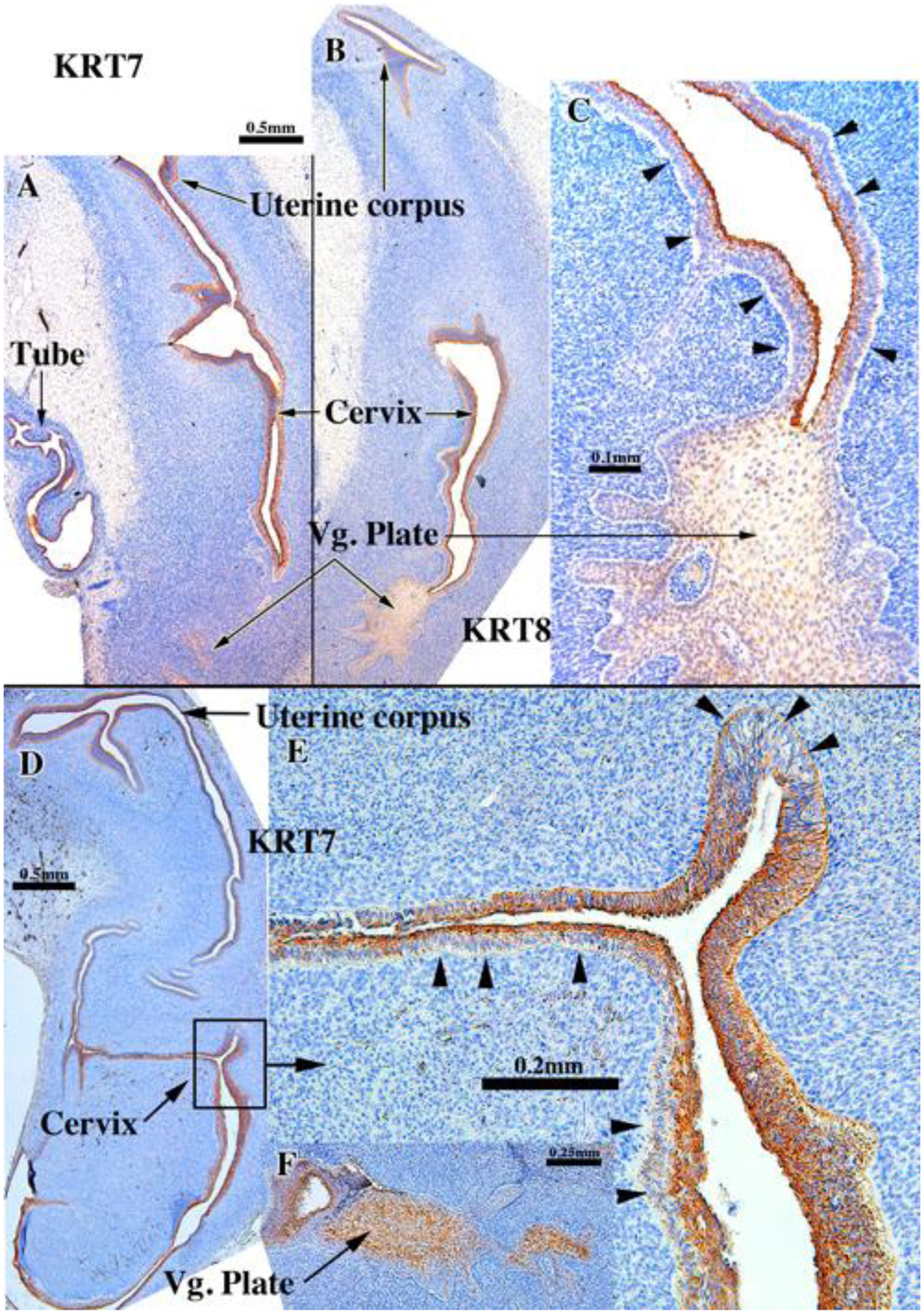
KRT8 (A-C) and KRT7 (D-F) immunostaining in an intact human fetal reproductive tract grown for 4 weeks in an untreated ovariectomized female athymic mouse host. The specimens come from 13-week (A-C & F [AC121]) and 14-week fetuses (D-E [AC159]). In truly stratified epithelium (presumptive exocervix and vagina) the basal epithelial cells (arrowheads) are unstained. KRT7 & KRT8 is expressed in single layered epithelium or apical epithelial cells of multilayered epithelia. Vg. Plate=vaginal plate.
b. Keratin 19 (KRT19).
KRT19 was detected in epithelia of the vaginal plate, vagina, cervix, uterine corpus and uterine tube in human female reproductive tract xenografts grown in untreated ovariectomized hosts (Fig. 4 and Tables 3–5). The pattern of KRT19 expression was virtually identical to that of non-grafted specimens of a similar gestational age (Cunha et al., 2017). The distinguishing feature for KRT19 versus KRT7/KRT8 was that in areas containing a stratified epithelium (vagina/cervix) KRT19 was expressed throughout the full epithelial thickness (basal to apical cells) (Fig. 4C–D).
Figure 4.
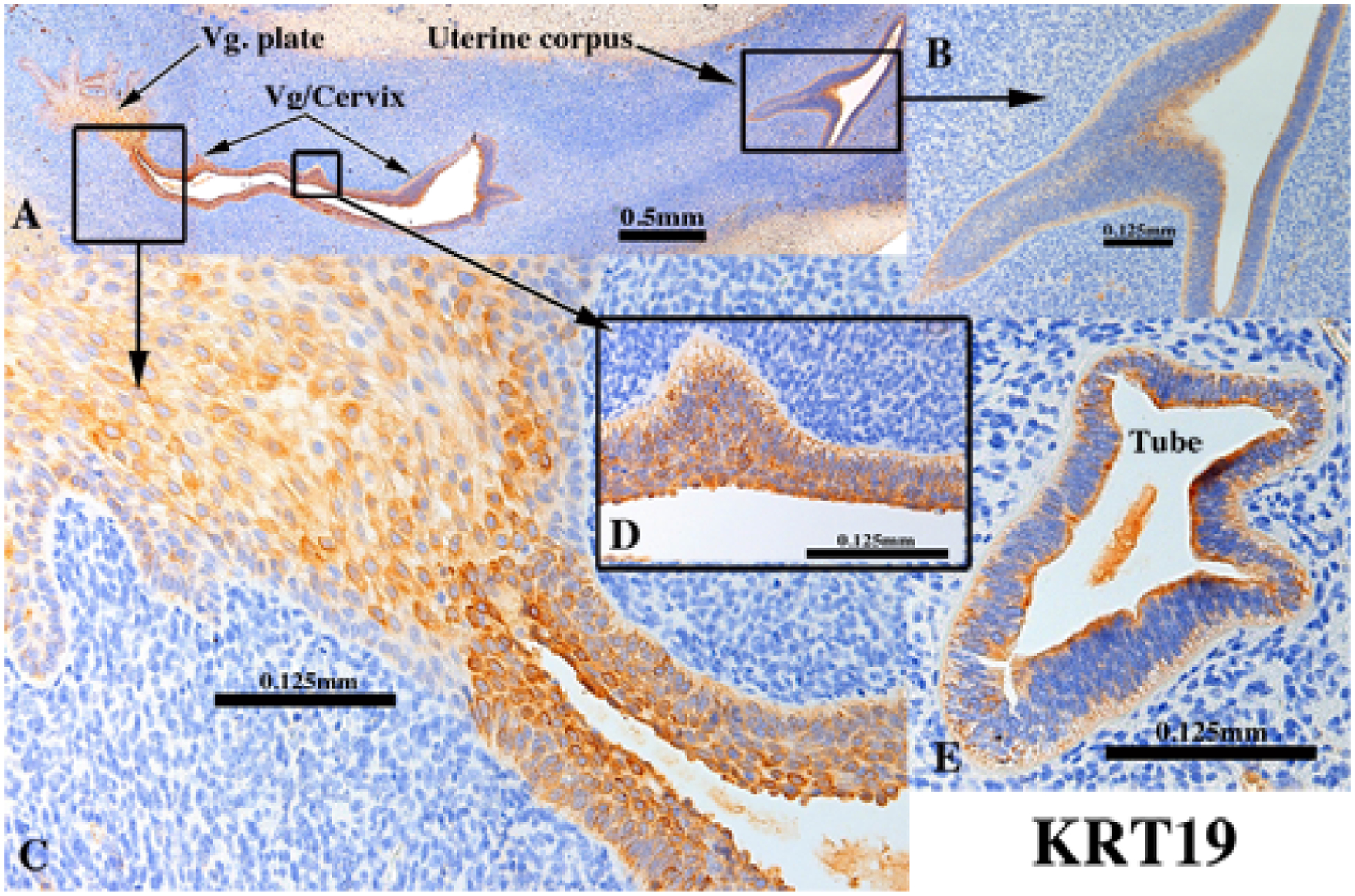
KRT19 immunostaining of an intact 13-week human fetal reproductive tract (AC121) grown for 4 weeks in an untreated ovariectomized female athymic mouse host. KRT19 was detected in simple columnar epithelia of the uterine tube (E), uterine corpus (A-B), endocervix (not illustrated), as well as in stratified epithelia of the presumed exocervix, vagina and vaginal plate (A, C & D) in basal through apical epithelial cells.
c. Keratin 6 (KRT6).
KRT6 is a keratin normally associated with stratified epithelia and accordingly was only detected in the presumed exocervix, vagina (Fig. 5A & C) and vaginal plate (Fig. 5D and Tables 3–5). Simple columnar epithelia of the uterine tube (not illustrated), uterine corpus, and endocervix did not express KRT6 (Fig. 5A & B). The KRT6 staining pattern was similar in xenografts of human female reproductive tracts grown in untreated ovariectomized hosts and in non-grafted specimens of comparable age (Cunha et al., 2017).
Figure 5.
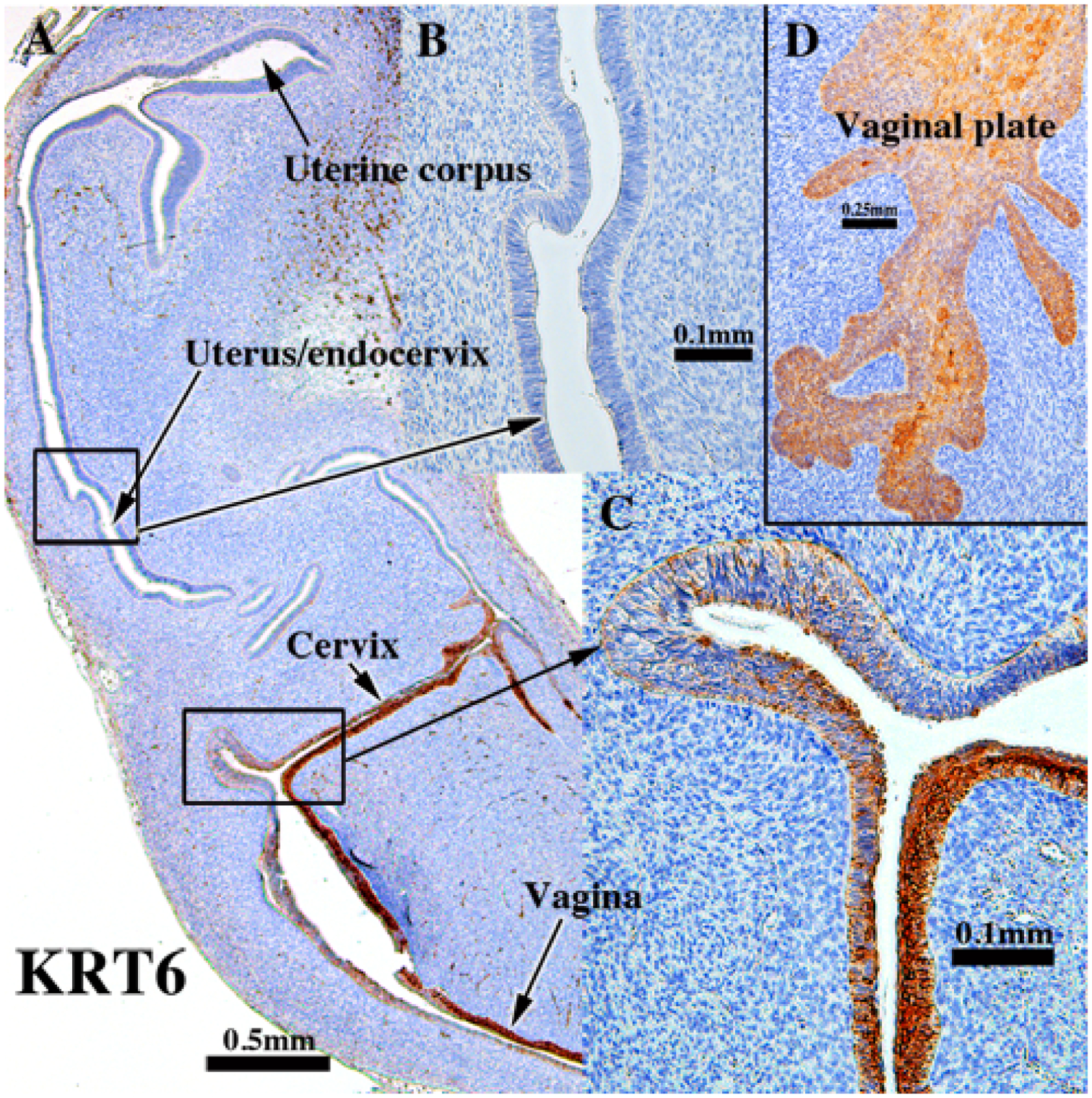
KRT6 immunostaining of an intact 13-week (D) human fetal reproductive tract (AC121) grown for 4 weeks in an untreated ovariectomized female athymic mouse host and immunostained for KRT6. Stratified squamous epithelia of the presumed exocervix, vagina and vaginal plate (A, C & D) express KRT6 in basal through apical epithelial cells. Simple columnar epithelia of the uterine tube (not illustrated), uterine corpus and presumed endocervix do not express KRT6 (A-B).
d. Keratin 14 (KRT14).
KRT14 is normally expressed in basal and supra-basal cells of stratified epithelia (Moll et al., 1982; Moll et al., 2008). In epithelium of the developing mouse Müllerian vagina, KRT14 is a marker of maturing vaginal epithelium whose expression is preceded by and dependent upon prior expression of ΔNp63 (Kurita and Cunha, 2001). In non-grafted human fetal female reproductive tracts, KRT14 was first detected in small patches of basal epithelial cells within the vaginal plate at 14 weeks of gestation (see Fig. 5A–B, in Cunha et al., 2017). In xenografts of 13- and 14-week human fetal female reproductive tracts grown in untreated hosts, KRT14 was likewise detected in FOXA1-positive epithelium of the UGS-derived vaginal plate (Robboy et al., 2017) (Fig. 6A–B and Tables 3–5). For PAX2-positive Müllerian-derived vaginal epithelium (Robboy et al., 2017), KRT14 was rarely detected (Fig. 6A & C). KRT14 was also not detected in epithelia of the uterine tube, uterine corpus and the cervix (Fig. 6A) Thus, the pattern of KRT14 expression in xenografts of human fetal female reproductive tracts was nearly identical to that of non-grafted specimens of a similar gestational age (Cunha et al., 2017).
Figure 6.
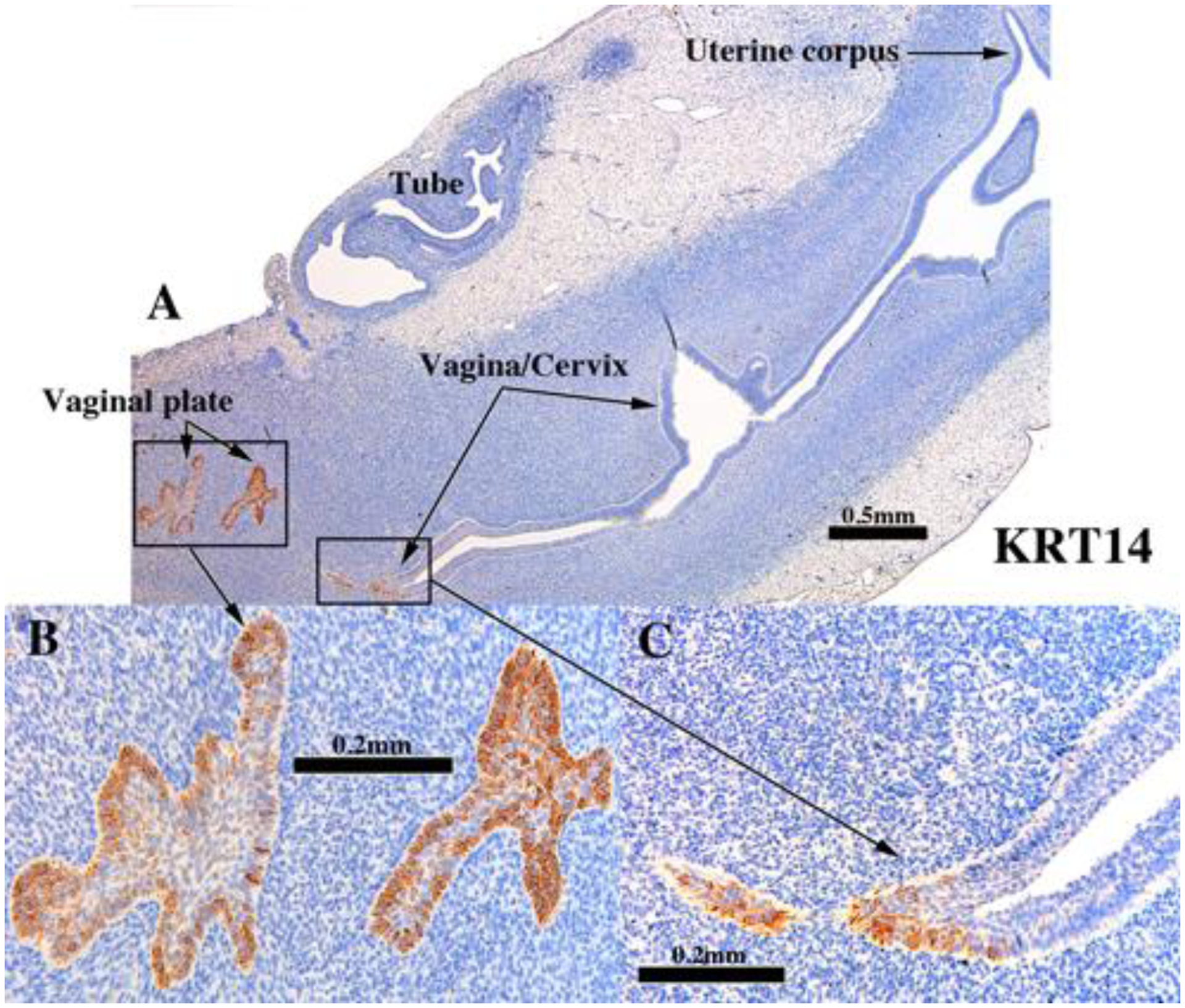
KRT14 immunostaining in an intact 13-week human fetal reproductive tract (AC121) grown for 4 weeks in an untreated ovariectomized female athymic mouse host. KRT14 was detected only in the vagina (A & C)/vaginal plate (A-B). The vaginal plate is FOXA1-positive and derived from UGS epithelium (Robboy et al., 2017), while the stratified PAX2-positive Müllerian-derived vaginal epithelium (Robboy et al., 2017) (A & C) is mostly KRT14-negative. Stratified squamous epithelia of the presumed exocervix (A) as well as the simple columnar epithelia of the endocervix, uterine corpus and uterine tube lack KRT14 reactivity (A).
d. Keratin 10 (KRT10).
As expected, KRT10 was not expressed in epithelia of grafts grown in untreated ovariectomized hosts (not illustrated, Tables 3–5).
4. Epithelial transcription factors.
a. TP63.
The ΔNp63 isoform of TP63 is a vaginal epithelial identity factor that is required for and specifies vaginal differentiation of mouse Müllerian epithelium (Kurita and Cunha, 2001; Kurita et al., 2005a). In non-grafted human fetal reproductive tracts all stratified epithelia (vaginal plate, vagina and cervix) were strongly TP63-positive, while the simple columnar epithelia of the uterine fundus and uterine tube were negative for TP63 (Cunha et al., 2017). The pseudostratified columnar epithelium at the presumed transition between the endocervix and lower uterine corpus exhibited patchy single cell expression of TP63 in non-grafted specimens (Cunha et al., 2017). Similarly, in xenografts of 11- to 14-week human fetal female reproductive tracts grown in untreated hosts, TP63 was strongly expressed in stratified epithelium of the vaginal plate and in epithelia of the presumed exocervix and vagina (Fig. 7B, D–E and Tables 3–5). Conversely, TP63 was not detected in the columnar epithelium of the uterine tube (Fig. 7C), uterotubal junction (Fig. 7C & F) and upper uterine corpus (uterine fundus) (Fig. 7C) in xenografts grown in untreated ovariectomized hosts. A rapid decrease in the percent TP63-reactive epithelial cells was seen at the transition from stratified squamous to simple columnar epithelium at the presumed exocervix/endocervix boundary (Fig. 7D–E). Areas presumed to be the upper endocervix/lower uterine corpus boundary (Fig. 7A) exhibited patchy single cell expression of TP63 in xenografts grown in untreated hosts (Fig. 7A). The pattern of TP63 expression seen in the xenografts was virtually identical to that on non-grafted specimens of comparable gestational age (Cunha et al., 2017).
Figure 7.
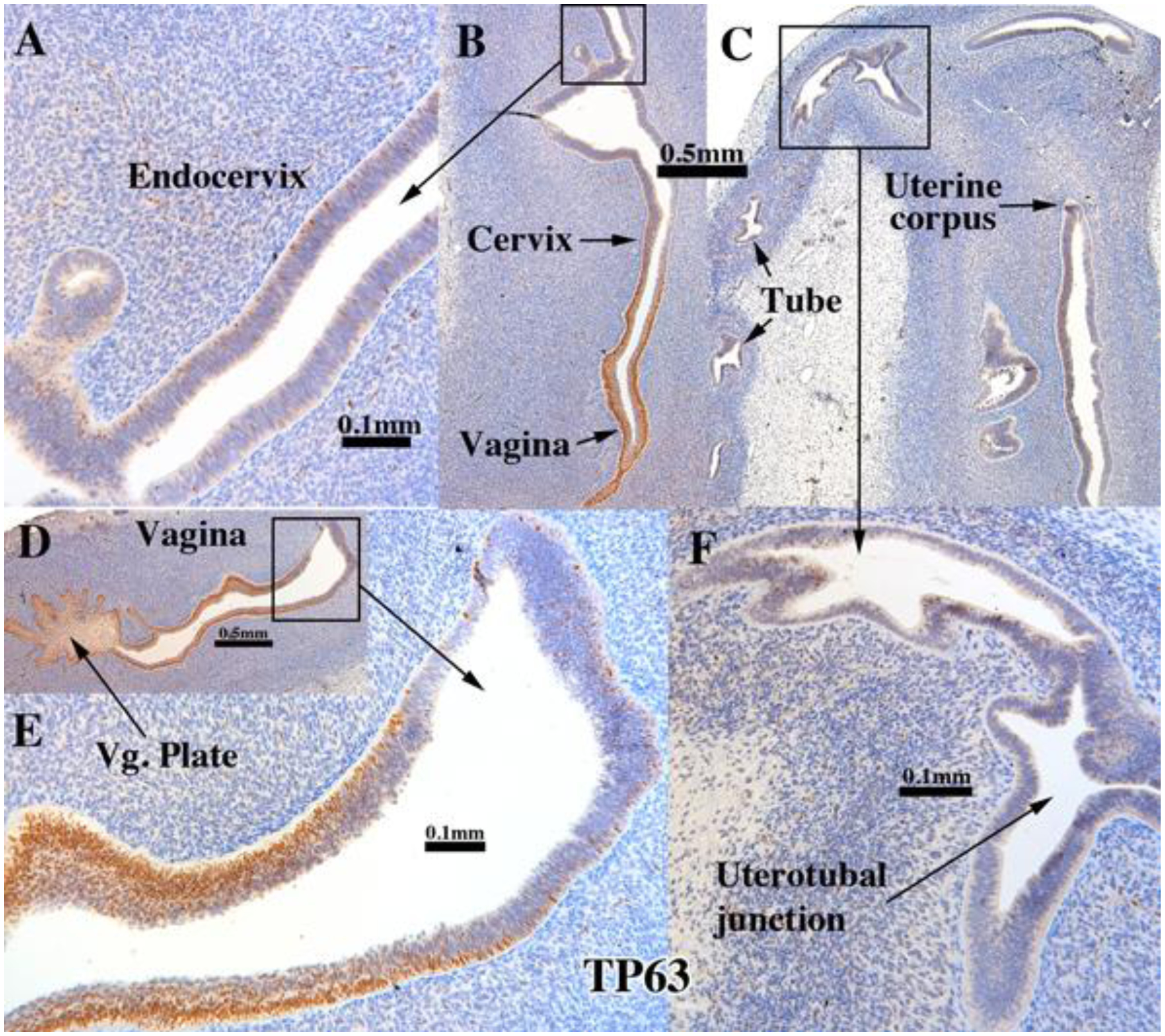
TP63 immunostaining of an intact 13-week human fetal reproductive tract (AC121) grown for 4 weeks in an untreated ovariectomized female athymic mouse host. TP63 was strongly expressed in the stratified epithelium of the vaginal plate (D), vagina (B & D), but not in the simple columnar epithelia of the uterine tube (C), uterotubal junction (F) and upper uterine. Transitions between stratified and simple columnar epithelium (D-E) exhibited a sharp reduction in TP63 to patchy single cell expression, which was also seen in the presumed endocervical region (A).
b. RUNX1.
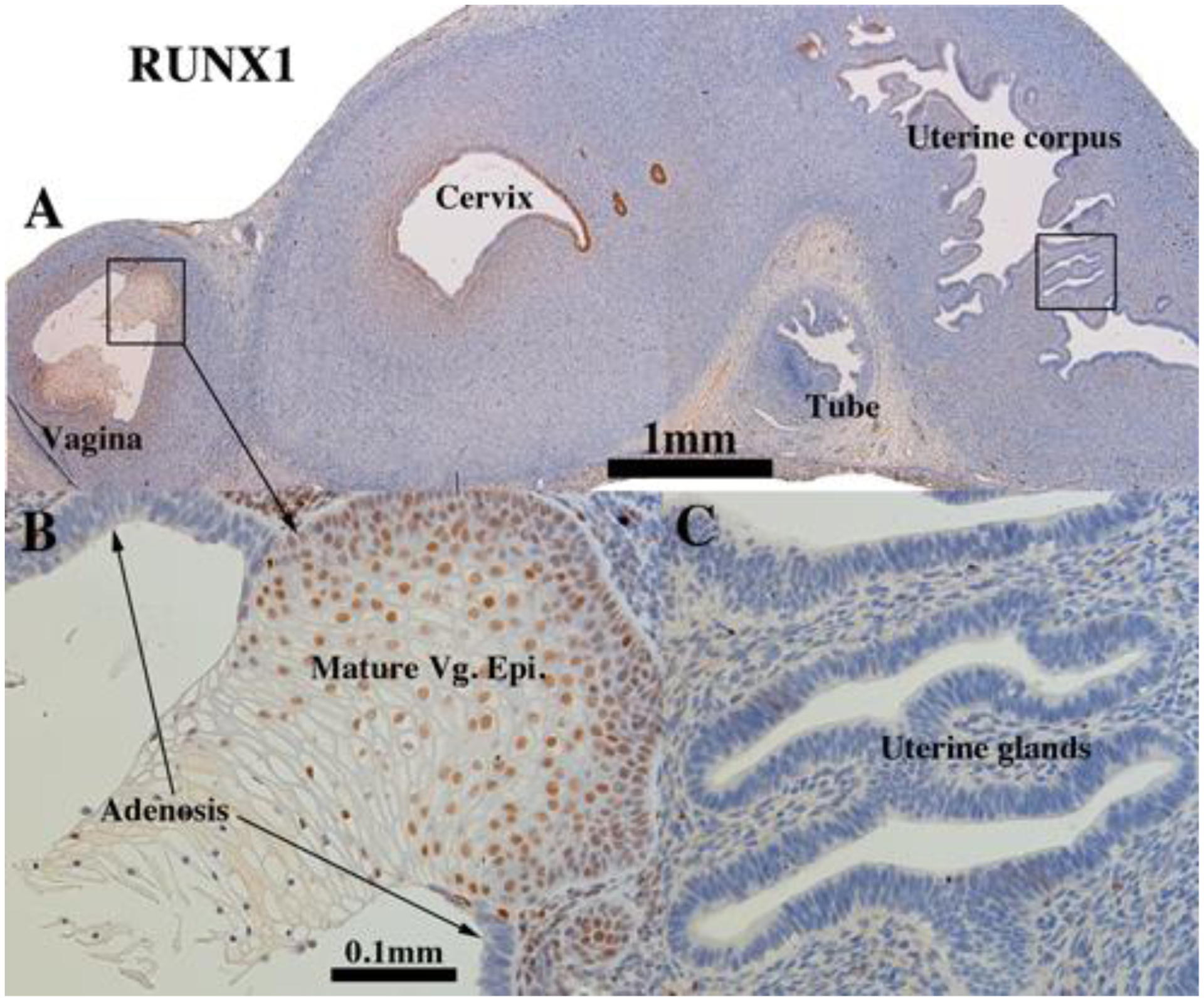
In mice, RUNX1 is essential for expression of ΔNp63 in cervicovaginal epithelial cells. Perinatal exposure to DES represses RUNX1 expression that in turn prevents ΔNp63 expression in Müllerian epithelium, which in turn leads to cervicovaginal adenosis (Kurita et al., 2004; Laronda et al., 2012; Laronda et al., 2013). Given the mechanistic association of RUNX1 and ΔNp63, the pattern of expression of these two transcription factors is fairly congruent in 14 to 16 week human female fetal reproductive tracts in so far as both TP63 and RUNX1 were not detected in the columnar epithelium of the uterine tube and uterotubal junction, but were strongly expressed in the stratified epithelium of the vaginal plate and in epithelium of the presumed vagina and exocervix, with patchy single cell expression in epithelium of the lower uterine corpus/cervix (Cunha et al., 2017). In grafts of 11- to 14-week human female fetal reproductive tracts grown in untreated ovariectomized hosts, RUNX1 was expressed in epithelia of the vaginal plate (Fig. 8B & F and Tables 3–5), vagina, uterine cervix, the uterine corpus, but not in the uterine tube (Fig. 8D). At the uterotubal junction RUNX1 immunostaining abruptly decreased from scattered positive in uterine epithelium to negative in tubal epithelium (Fig. 8E). RUNX1 was also detected in mesenchymal cells, especially in association with stratified vaginal epithelium (Fig. 8B & F) and less so or not at all in mesenchymal cells associated with the uterine corpus and uterine tube (Fig. 8A, C–D). Thus, the pattern of Runx1 expression seen in the xenografts was similar to that in non-grafted specimens of comparable gestational age (Cunha et al., 2017)
Figure 8.
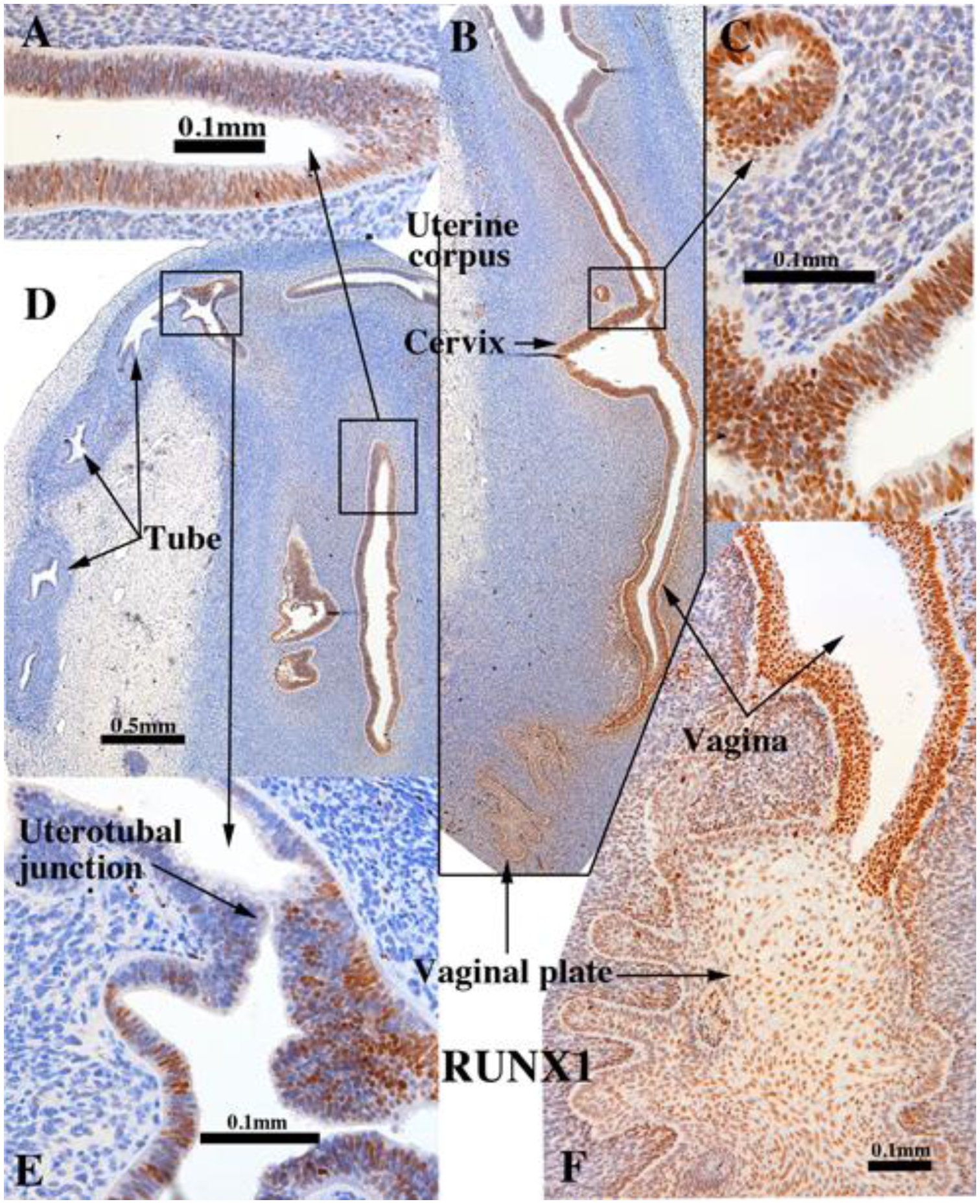
RUNX1 immunostaining in an intact 13-week human fetal reproductive tract (AC121) grown for 4 weeks in an untreated ovariectomized female athymic mouse host and immunostained for RUNX1. RUNX1 was strongly expressed in the stratified epithelium of the vaginal plate (B & F), vagina (B & F), and also in the simple columnar epithelia of the presumed endocervix (B-C) and the uterine corpus (A & D). RUNX1 expression diminished to undetectable at the uterotubal junction (E) and was not expressed in the uterine tube (D).
5. Mesenchymal transcription factors.
a. ISL1 and HOXA11.
ISL1 is expressed in the nuclei of mesenchymal cells in the lower portion of human fetal female reproductive tract, namely in vaginal and cervical mesenchymal cells, but not in mesenchymal cells of the uterus and uterine tube (Cunha et al., 2017). In contrast, HOXA11 is expressed in mesenchymal cells of the developing human uterine corpus, but not in mesenchyme of the uterine tube, vagina and cervix (Cunha et al., 2017). This same pattern of ISL1 and HOXA11 expression was seen in human female fetal reproductive tracts grown for 4 weeks in untreated ovariectomized hosts (Fig. 9 and Tables 3–5).
Figure 9.
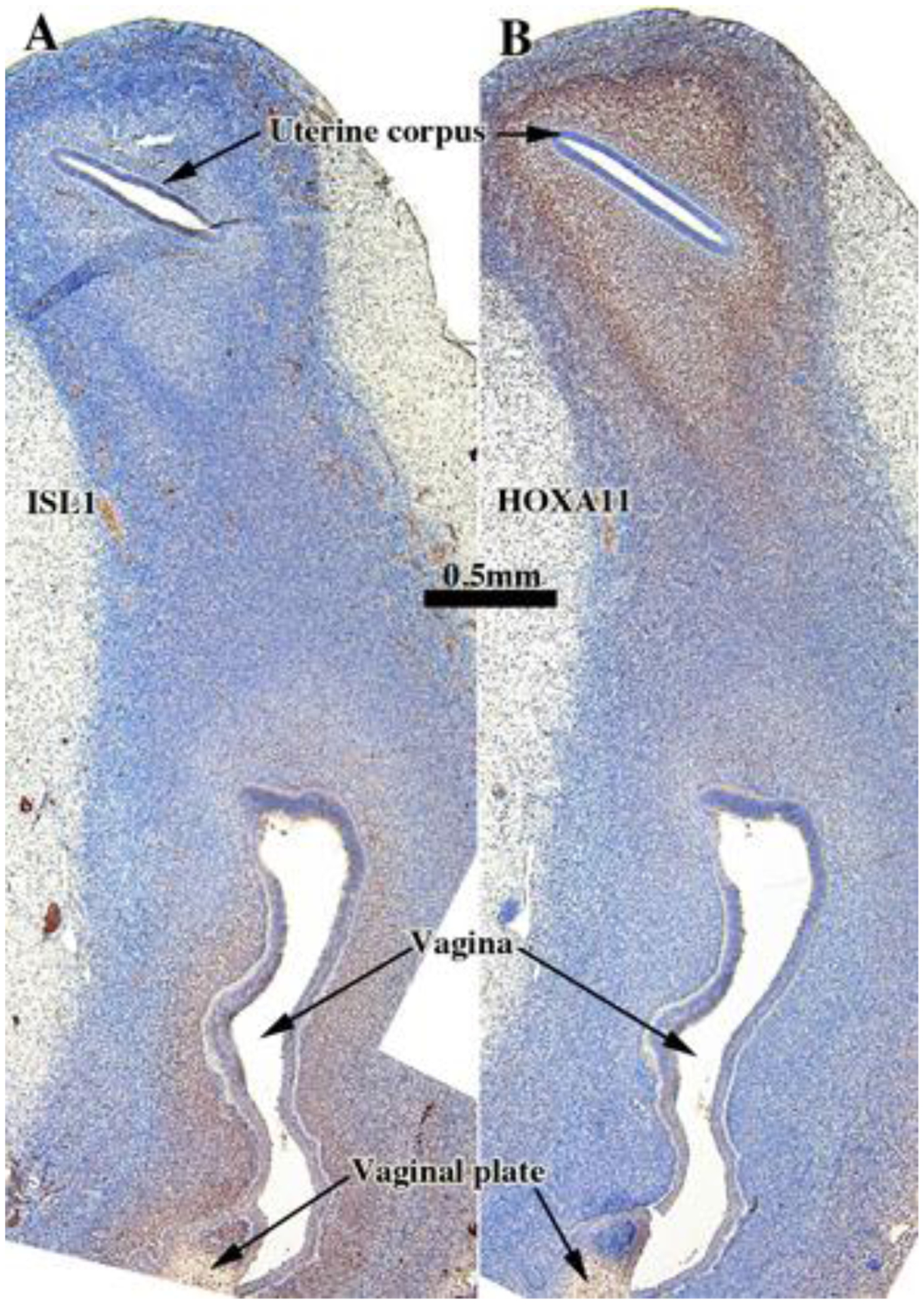
ISL1 (A) and HOXA11 (B) immunostaining in an intact 13-week human fetal reproductive tract (AC121) grown for 4 weeks in an untreated ovariectomized female athymic mouse host. ISL1 is expressed in the mesenchyme associated with the vaginal plate and vagina, whereas HOXA11 is expressed in the mesenchyme associated with the uterine corpus.
6. Summary.
Protein expression was assessed by immunohistochemistry in 5 xenografted human female fetal reproductive tracts of 13 to 17 weeks of gestation grown for an additional 4 weeks in vivo in untreated ovariectomized hosts. The expression of sex steroid receptors, keratins, epithelial and mesenchymal transcription factors was remarkably similar to that of non-grafted specimens of comparable age (Cunha et al., 2017).
B. Epithelial and mesenchymal differentiation in grafts of human fetal female reproductive tracts grown in DES-treated ovariectomized female hosts.
1. General.
Many proteins in developing human fetal reproductive tracts grown in DES-treated ovariectomized hosts exhibit an expression pattern like that of comparable grafts grown in untreated ovariectomized hosts (Tables 3–5). The figures presented below will emphasize patterns of protein expression that differ in DES-treated versus untreated hosts.
2. Morphological effects of DES on human female reproductive tract xenografts.
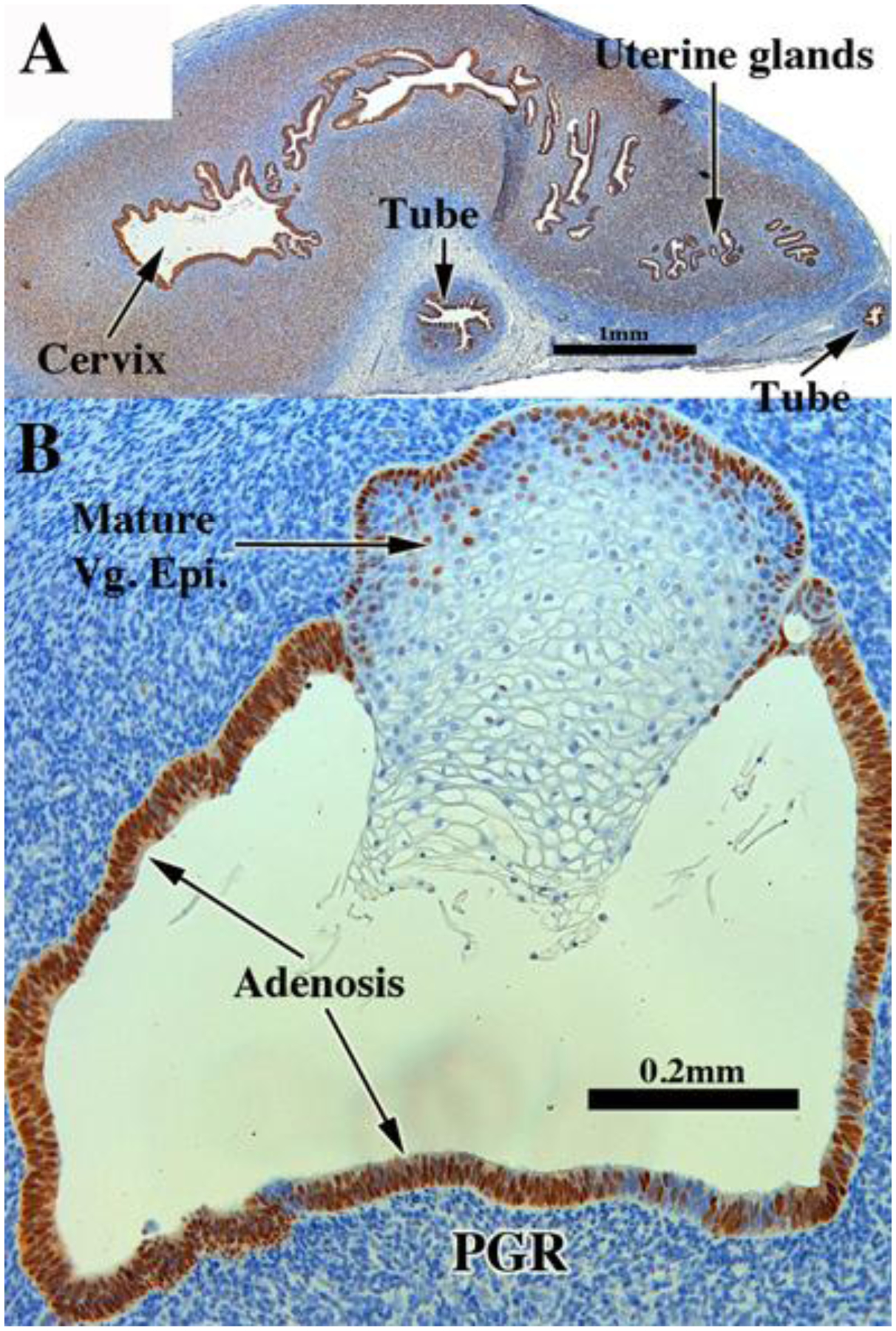
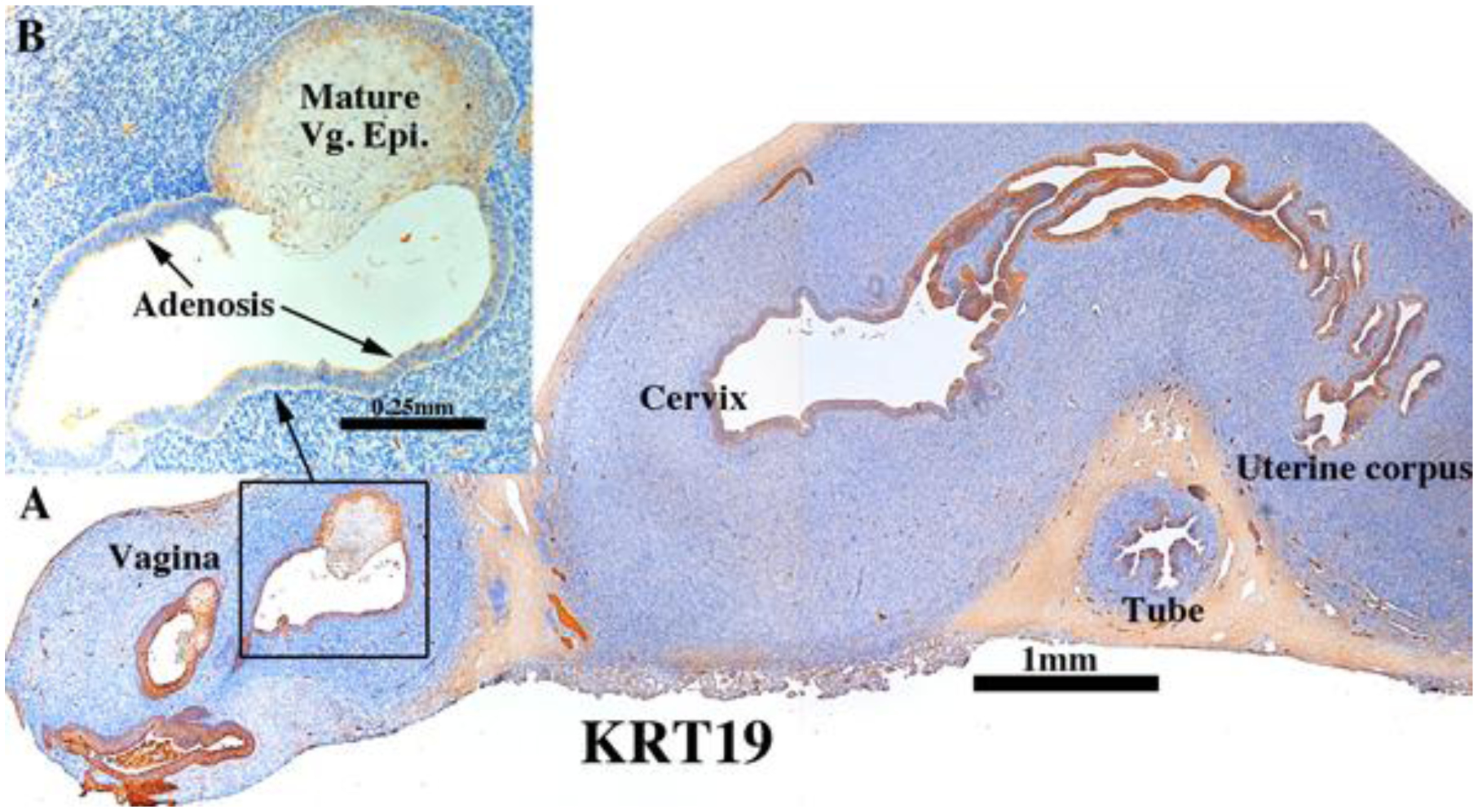
Both non-grafted specimens and specimens grafted into untreated ovariectomized hosts in the age range of 13 to 17 weeks showed few if any endometrial/cervical glands. In contrast, DES stimulated endometrial and cervical gland formation (see Figs. 10, 11, 13, & 14) and increased plication (folding) of tubal epithelium (Figs. 10, 13, 15 & 18). Two DES-treated specimen disclosed the presence of glandular epithelium (adenosis) admixed with mature stratified squamous vaginal epithelium (Figs. 10, 11, 13–15).
Figure 10.
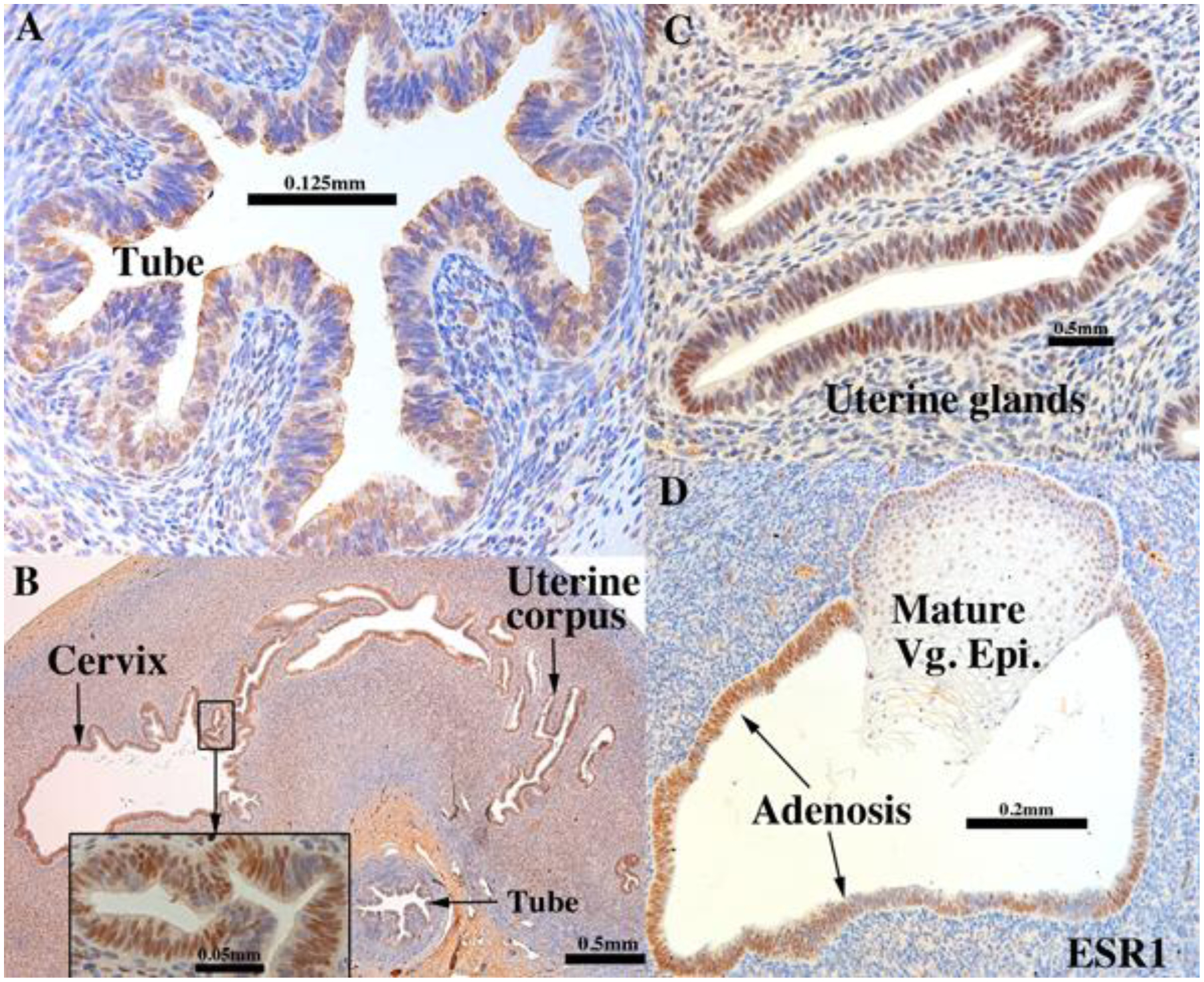
ESR1 immunostaining in an intact 13-week human fetal reproductive tract (AC122) grown for 4 weeks in a DES-treated ovariectomized female athymic mouse host. Tubal epithelium (A) expresses ESR1 but at a level reduced relative to non-grafted and specimens grafted into untreated hosts. ESR1 expression in the uterine tube, glands of the uterine corpus and cervix (B) (low magnification}, (C) (high magnification) of uterine glands. In the vaginal region ESR1 reactivity is seen in both mature squamous epithelium and adenosis, while the mesenchyme is largely unreactive (D).
Figure 11.
PGR immunostaining in an intact 13-week human fetal reproductive tract (AC122) grown for 4 weeks in a DES-treated ovariectomized female athymic mouse host. PGR is expressed strongly in epithelial and inner mesenchymal cells of the female reproductive tract (A). In vagina, PGR is expressed principally in the basal/suprabasal cells and in the adenosis (B).
Figure 13.
KRT19 immunostaining in an intact 13-week human fetal reproductive tract (AC122) grown for 4 weeks in a DES-treated ovariectomized female athymic mouse host. KRT19 is expressed in the adenosis, other glandular epithelia, and in the suprabasal cells in the vaginal squamous epithelium (B).
Figure 14.
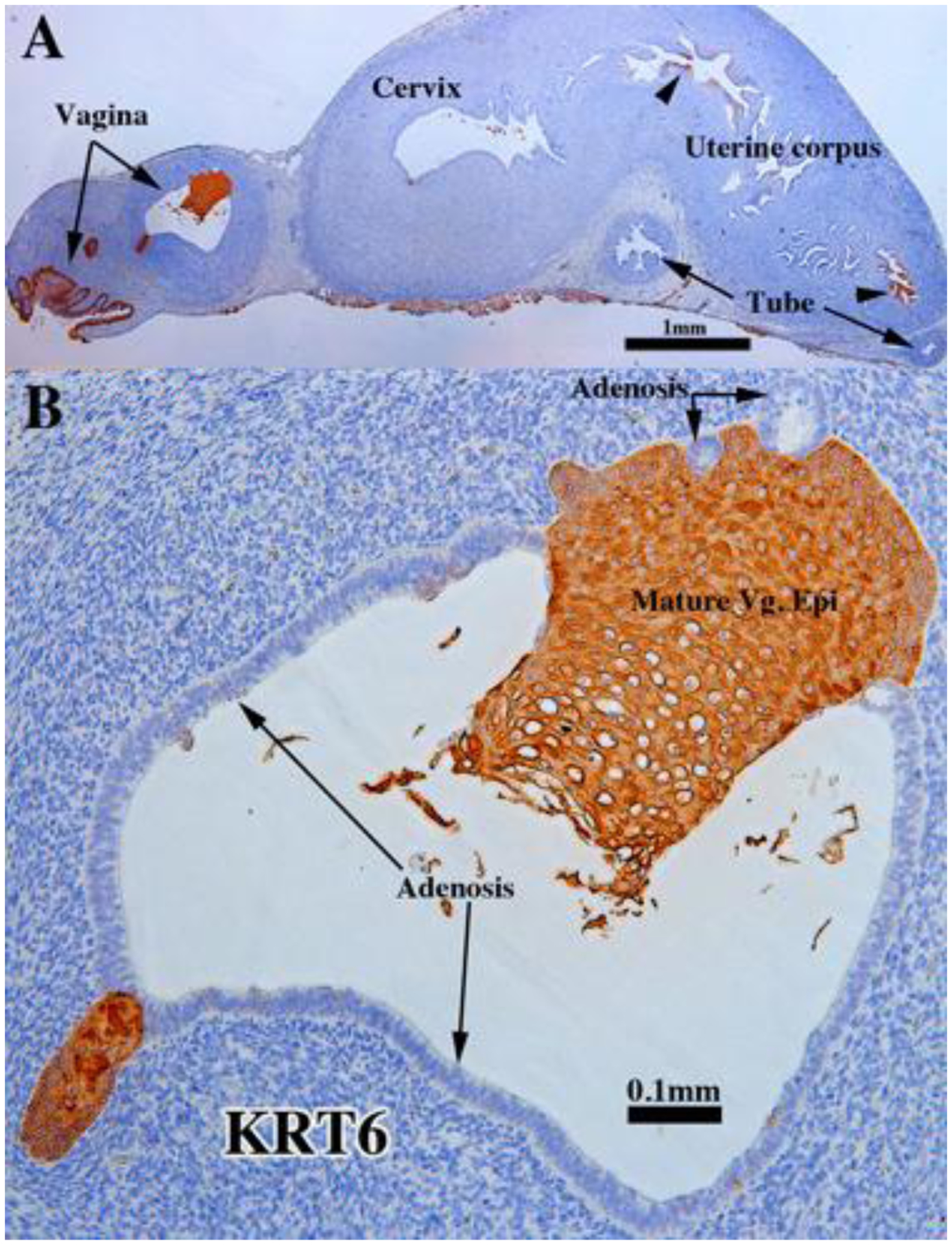
KRT6 immunostaining in an intact 13-week human fetal reproductive tract (AC122) grown for 4 weeks in a DES-treated ovariectomized female athymic mouse host. The vaginal squamous epithelial cells were highly reactive, while adenosis was not (B). The glandular epithelial cells of the cervix, uterine corpus and uterine tube were non-reactive except for small patches (A, arrowheads).
Figure 15.
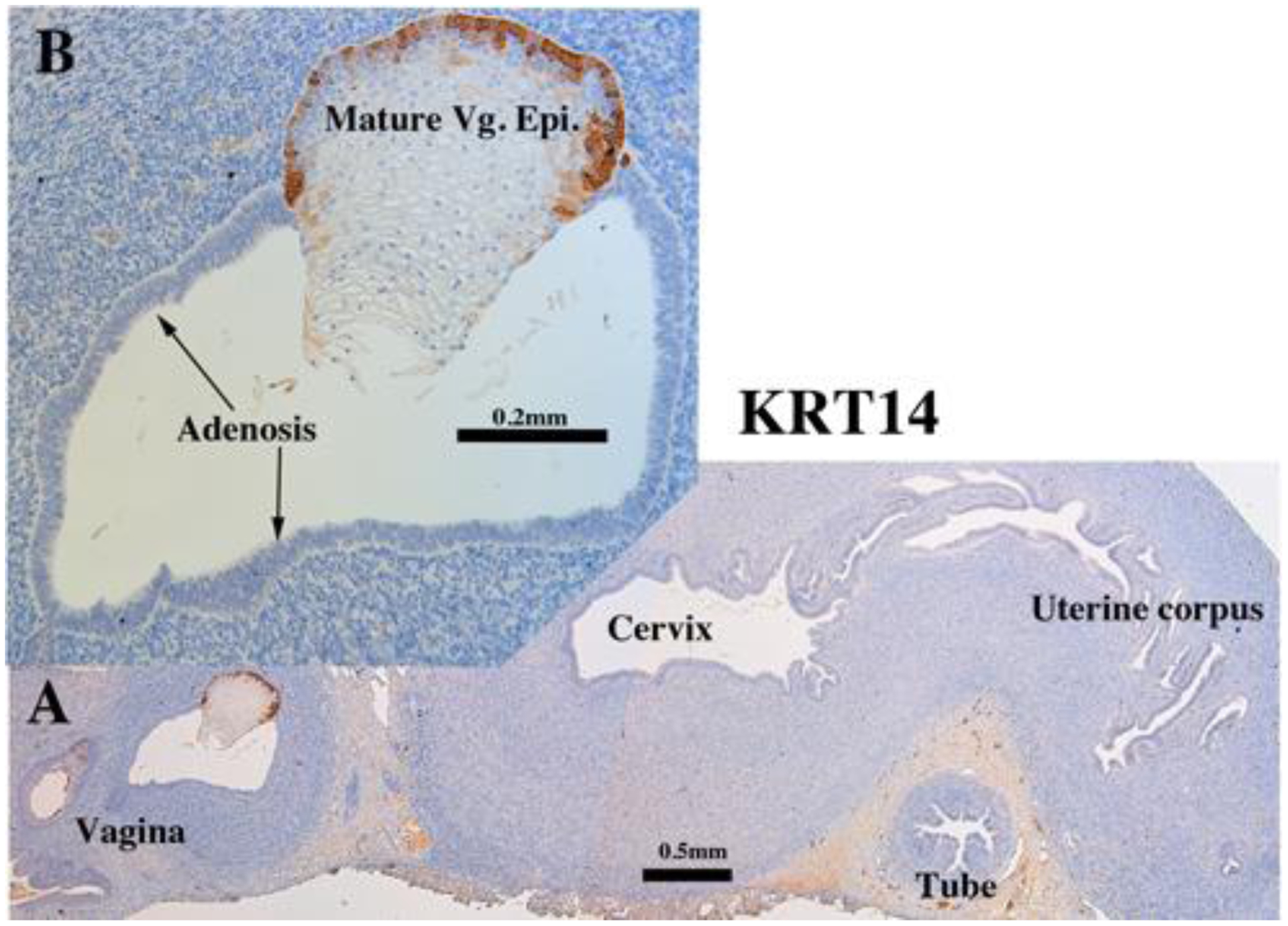
KRT14 immunostaining in an intact 13-week human fetal reproductive tract (AC122) grown for 4 weeks in a DES-treated ovariectomized female athymic mouse host. KRT14 is reactive in the basal epithelial cells of the vagina (A-B), but not in adenosis (B), nor epithelium of the cervix, uterine corpus and uterine tube (A).
Figure 18.
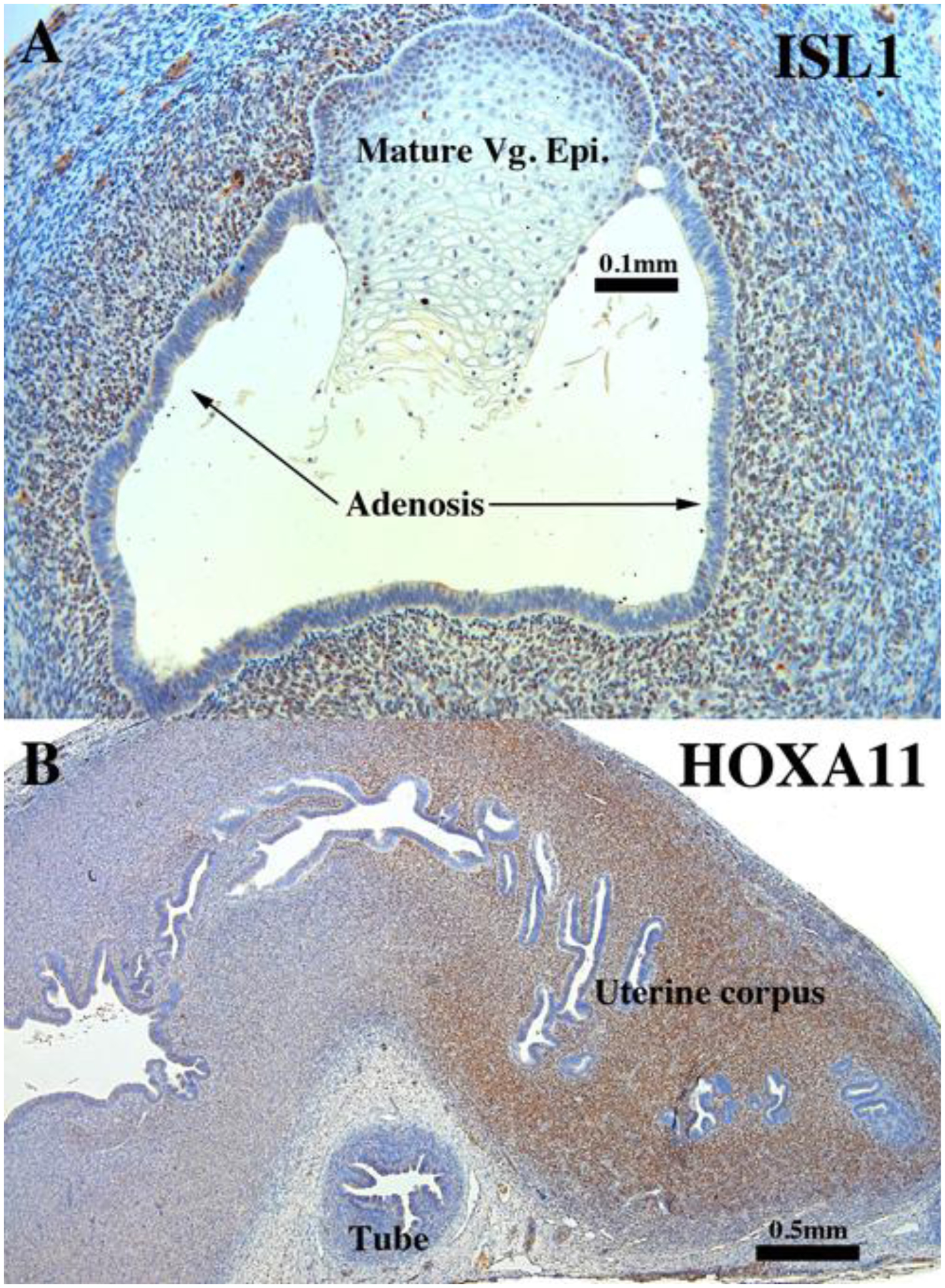
ISL1 (A) and HOXA11 immunostaining (B) in an intact 13-week human fetal reproductive tract (AC122) grown for 4 weeks in a DES-treated ovariectomized female athymic mouse host. ISL1-reactive mesenchyme (A) surrounds vaginal squamous/transitional epithelium. The adenotic epithelium is unreactive. The mesenchyme of the endometrial stroma is highly and diffusely HOXA11 reactive (B).
3. Sex steroid receptors
a. ESR1.
DES had striking effects on ESR1 expression in xenografts of human fetal reproductive tracts (Tables 3–5). DES-treated tubal epithelium consisted of a mixture of ESR1-positive and ESR1-negative cells (Fig. 10A), which contrasted with the homogeneous ESR1 staining in (a) non-grafted specimens (Cunha et al., 2017) and (b) tubal grafts grown in untreated ovariectomized hosts (compare with Fig. 2A–B). Conversely, DES treatment (a) induced prominent ESR1 expression in epithelium of the uterine corpus and endocervix (Fig. 10B and Table 4) and (b) in glands of these areas (Fig. 10C). The strong ESR1 expression in epithelium of the uterine corpus and endocervix of xenografts in DES-treated hosts contrasted with the absence of ESR1 in (a) uterine and cervical epithelium of non-grafted specimens (Cunha et al., 2017) and in (b) specimens grown in untreated ovariectomized hosts (compare with Fig. 2). Two DES-treated vaginal specimens exhibited both mature vaginal epithelium and simple columnar adenotic epithelium, both of which expressed ESR1 (Fig. 10D). ESR1 expression was maintained in a substantial subset of mesenchymal cells in the uterine tube, uterine corpus and uterine cervix at levels/intensities similar to that seen in non-grafted specimens (Cunha et al., 2017) and in specimens grown in untreated ovariectomized hosts (compare with Fig. 2). Vaginal mesenchyme of DES-treated specimens remained devoid of ESR1 or very weakly expressed (Fig. 10D), and thus was similar to that seen in non-grafted specimens (Cunha et al., 2017) and specimens grown in untreated ovariectomized hosts (compare with Fig. 2A, inset).
b. PGR.
PGR was rarely seen in epithelium and mesenchyme of grafts of human fetal reproductive organs grown in untreated ovariectomized hosts (Tables 3–5). In contrast, we found prominent PGR immunostaining in epithelial and mesenchymal cells of the uterine tube, uterine corpus, and cervix grown in DES-treated ovariectomized hosts (Fig. 11A). Two vaginal xenografts grown in DES-treated hosts exhibited mature vaginal epithelium as well as adenosis, and both exhibited intense PGR immunostaining. In DES-treated mature vaginal epithelium, PGR was confined to the basal and the immediate supra-basal cells (Fig. 11 B).
4. Keratins
a. Keratin 7 (KRT7) and Keratin 8 (KRT8).
Expression of KRT7 and KRT8 was detected in epithelia of all organs of the developing human female fetal reproductive tract in xenografts grown in DES-treated ovariectomized hosts even though staining intensity appeared to be generally reduced relative to grafts grown in untreated hosts (Fig. 12 and Tables 3–5). Indeed, in some cases epithelial cells were completely devoid of KRT7 and KRT8 reactivity with patchy expression (Fig. 12 C–D). Mature vaginal epithelium was mostly devoid of KRT8 staining in xenografts grown in DES-treated hosts, while adenotic epithelium was KRT7- and KRT8-reactive (Fig. 12B).
Figure 12.
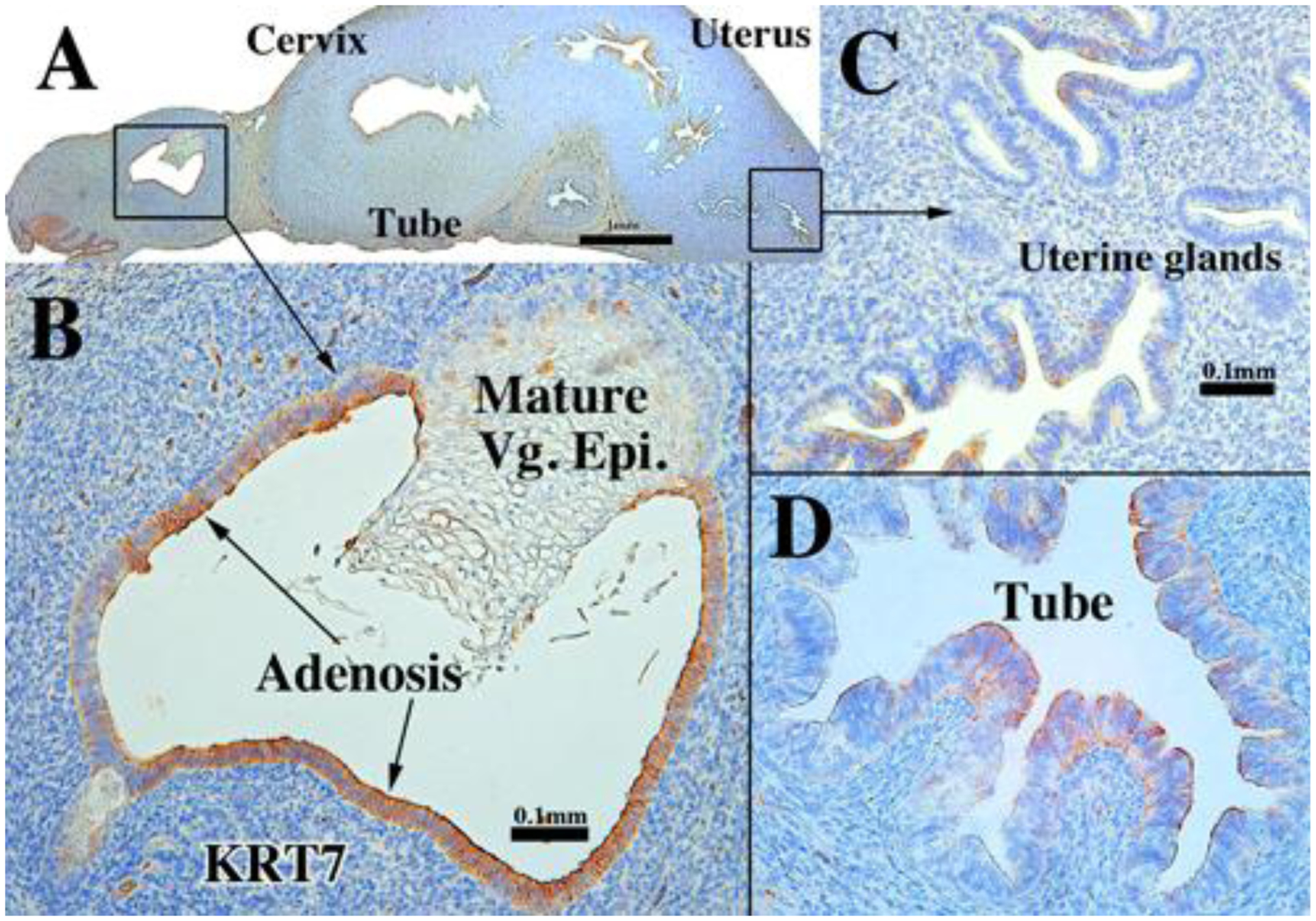
KRT7 immunostaining in a 13-week human fetal reproductive tract (AC122) grown for 4 weeks in a DES-treated ovariectomized female athymic mouse host. KRT7 expression is reduced in the endometrial glands (C) and uterine tube (D), but prominent in the vaginal adenosis (B).
c. Keratin 19 (KRT19).
KRT19 expression in xenografts of DES-treated human female fetal reproductive tracts was virtually identical (Fig. 13, Tables 3–5) to that seen in non-grafted specimens of comparable gestational age (Cunha et al., 2017) and in comparably aged specimens grown in untreated ovariectomized hosts (See figure 4). Areas of mature vaginal epithelial differentiation and adenotic epithelium expressed KRT19 in xenografts grown in DES-treated hosts (Fig. 13).
d. Keratin 6 (KRT6).
KRT6 expression in xenografts grown in DES-treated hosts exhibited a pattern similar to that seen in xenografts grown in untreated ovariectomized hosts and non-grafted specimens of comparable gestational age (Cunha et al., 2017). In this regard, KRT6 was observed throughout the full epithelial thickness in stratified vaginal epithelium (Fig. 14 and Tables 3–5). In addition, patchy expression of KRT6 was seen in epithelial cells of the uterine corpus (arrowheads in Fig. 14A). Adenotic epithelium was devoid of KRT6 staining (Fig. 14B).
d. Keratin 14 (KRT14).
KRT14 expression in grafts of human female fetal reproductive tracts grown in DES-treated ovariectomized hosts exhibited a pattern similar to that seen in xenografts grown in untreated ovariectomized hosts and non-grafted specimens of comparable gestational age (Cunha et al., 2017) in so far as KRT14 was observed in basal cells of mature stratified vagina epithelium (Fig. 15A–B and Tables 3–5). In contrast, simple columnar adenotic epithelium was devoid of KRT14 staining in xenografts grown in DES-treated hosts (Fig. 15B).
e. Keratin 10 (KRT10).
KRT10 was not expressed in any of the organs of the human fetal reproductive tract, including the mature stratified vagina epithelium (Tables 3–5 not illustrated).
5. Epithelial transcription factors.
a. TP63.
TP63 expression was similar in grafts of human female fetal reproductive tracts grown in (a) DES-treated ovariectomized hosts, (b) in untreated ovariectomized hosts and (c) in non-grafted specimens of comparable gestational age (Cunha et al., 2017) in so far as TP63 was observed in basal and suprabasal cells of stratified epithelia in the vagina and cervix (Fig. 16A–C and Tables 3–5). In contrast, adenotic epithelium was devoid of TP63 staining (Fig. 16C). Epithelium of the uterine tube and upper uterine corpus (Fig. 16A) remained non-reactive for TP63, in contrast to patchy TP63 expression in epithelium of the lower uterine corpus/cervix of xenografts grown in DES-treated hosts (Fig. 16D).
Figure 16.
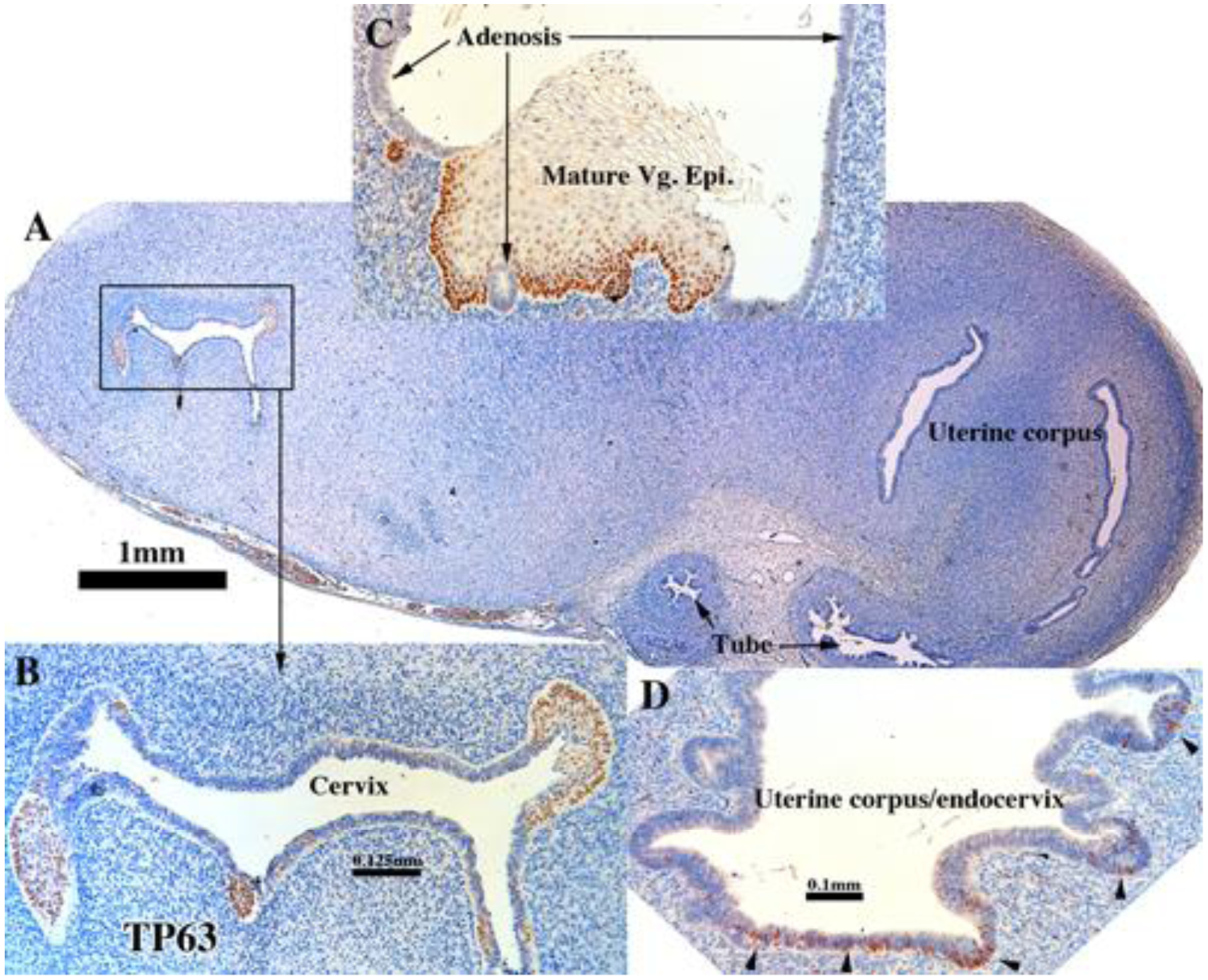
TP63 immunostaining in an intact 13- and 14-week human fetal reproductive tract grown for 4 weeks in a DES-treated ovariectomized female athymic mouse host. TP63 immunostaining (AC159) is absent in the uterine tube and uterine corpus (A,, [AC159]). Early and maturing squamous epithelia in the cervix (B, [AC159]) and mature vaginal epithelium are TP63-positive but not adenotic epithelium in the vagina (C, [AC122]). Foci of epithelium in the region of the endocervix/uterine corpus (D, [AC159]) exhibit patchy TP63 expression (arrowheads).
b. RUNX1.
The RUNX1 staining pattern was similar in (a) xenografts grown in DES-treated ovariectomized hosts, (b) untreated ovariectomized hosts and (c) non-grafted specimens of comparable gestational age (Cunha et al., 2017) with a few exceptions. Uterine epithelium and endometrial glands were devoid of RUNX1 staining in xenografts grown in DES-treated hosts (Fig. 17A & C and Tables 3–5), which contrasted with the presence of RUNX1 in non-grafted specimens of comparable gestational age (Cunha et al., 2017) and in xenografts grown in untreated ovariectomized hosts (compare with figure 8). RUNX1 was expressed in the endocervical region (Fig. 17A) and in mature vaginal epithelium, but not in vaginal adenosis and in uterine tubal epithelium (Fig. 17A–B).
Figure 17.
RUNX1 immunostaining in an intact 13-week human fetal reproductive tract (AC122) grown for 4 weeks in a DES-treated ovariectomized female athymic mouse host. Mature vaginal epithelium (A-B) expresses TP63, but not adenotic epithelium (B). RUNX1 was not detected in the uterine tube, uterine corpus (A) and uterine glands (C).
6. Mesenchymal transcription factors
a. ISL1 and HOXA11.
The expression of ISL1 and HOXA11 did not differ from that seen in non-grafted specimens (Cunha et al., 2017) or in xenografts of untreated or DES-treated ovariectomized hosts. Following DES-treatment HOXA11 was expressed in mesenchymal cells of the uterine corpus and cervix (Fig. 18B and Tables 3–5). ISL1 was expressed in mesenchymal cells associated with vaginal epithelium, whether stratified squamous or glandular (adenosis) (Fig. 18A).
7. α−ACT2.
α-ACT2 is a marker of smooth muscle differentiation. Smooth muscle forms a significant portion of the fibromuscular wall of organs of the female reproductive tract, especially in the uterine corpus/endocervix. For both the uterine corpus and the uterine tube, the epithelium is surrounded by a layer of fibroblastic stromal cells (called endometrial stroma in the uterus). This stromal layer in both the uterine corpus and the uterine tube is in turn surrounded by a smooth muscle layer, which in the uterus is called the myometrium. This pattern (epithelium, stroma and smooth muscle) revealed by αACT2 staining is consistently seen in non-grafted tubal specimens (Cunha et al., 2017) as well as in grafts of human fetal tube grown in untreated ovariectomized hosts (Fig. 19A, Tables 3–5). However, in human fetal uterine tube grown in DES-treated ovariectomized hosts, the αACT2-positive smooth muscle layer was in intimate association with the epithelium and the αACT2-negative mesenchymal layer was absent (Fig. 19B).
Figure 19.
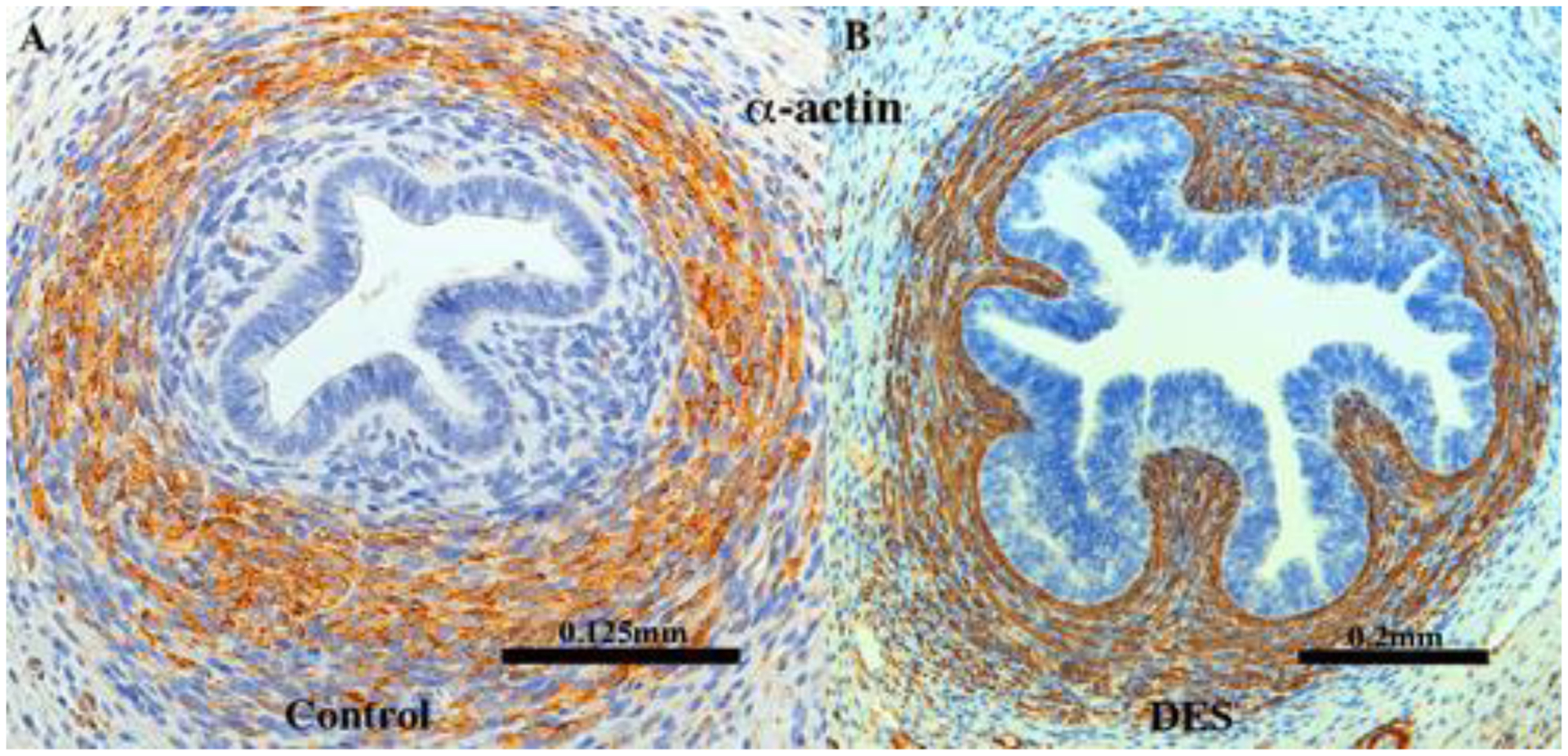
αACT2 immunostaining of 13-week human uterine tubes grown for 4 weeks in (A, [AC121]) an untreated ovariectomized host and (B, [AC122]) in a DES-treated ovariectomized host stained for αACT2. Note that in the control (A), a substantial mesenchymal layer encompasses the tubal epithelium, which DES treatment (B) has obliterated, resulting in a misplaced αACT2 smooth muscle layer, which now comes into a intimate association with the epithelium.
IV. Discussion
The xenograft model we pioneered three decades ago has resulted in a broad array of findings and better understanding of how the human male and female internal and external genitalia develop normally and how drug administration can perturb normal development (Cunha et al., 1987b; Cunha et al., 1987a; Robboy et al., 1982; Sugimura et al., 1988; Taguchi et al., 1983; Taguchi et al., 1984; Yonemura et al., 1995; Cunha et al., 1988; Baskin et al., 1997; Isaacson et al., 2017). This xenograft model is a fully ethical method for in vivo testing of endocrine disruptors on human fetal reproductive tracts. Our earlier xenograft studies were carried out before technical advances in molecular biology and immunohistochemistry were available to explore the molecular mechanisms involved in human female reproductive tract development. With modern methods, we have re-visited this model, have analyzed/confirmed our earlier H&E observations and extended our understanding through analysis of epithelial and mesenchymal protein differentiation markers (Cunha et al., 2017; Robboy et al., 2017). Accordingly, we have demonstrated that epithelial morphology and differentiation markers are remarkably similar in xenografts grown in untreated female hosts to those seen in non-grafted specimens of comparable age (Robboy et al., 2017; Cunha et al., 2017) even though the hormonal conditions provided by the untreated ovariectomized hosts are considerably different from in utero hormonal conditions. In preparation for our studies, we debated whether to use ovary-intact hosts or ovariectomized hosts. We chose the latter so that the effect of DES could be attributed to DES only.
Previously we showed that human female reproductive tract development proceeds normally in grafts grown in untreated female hosts (Cunha et al., 1987a; Robboy et al., 1982; Taguchi et al., 1983). In contrast, human fetal female reproductive tracts grown in DES-treated female hosts exhibited: (a) partial obliteration of the upper genital tract, (b) inhibition of differentiation of utero-tubal mesenchyme into stromal and smooth muscle layers, (c) alteration of plical development in the Fallopian (uterine) tube, (d) inhibition of normal transformation of caudal Müllerian epithelium into a stratified squamous vaginal epithelium (VgE) and (e) induction of vaginal adenosis (Robboy et al., 1982; Taguchi et al., 1983; Robboy, 1983).
Previously we induced adenosis in human female fetal xenografts grown in DES-treated hosts (Robboy et al., 1982; Taguchi et al., 1983). Similarly, adenosis was observed in two of four 14-week xenografts treated with DES. Clinical experience has shown that not all women exposed in utero to DES develop adenosis. In fact, the frequency of vaginal epithelial changes (VEC) and adenosis in DES daughters exposed during the critical window is about 34% (O’Brien et al., 1979) and 15% (Robboy et al., 1979), respectively. VEC changes are a broader definition of vaginal abnormality reflecting cases where the adenosis may have partially healed and only the metaplastic squamous epithelium remains.
In the present study DES elicited multiple effects on epithelial morphology as well as protein differentiation markers in xenografts of human female fetal reproductive tracts. DES induced precocious gland formation in the uterine corpus and cervix. Glands are rudimentary shallow outpouchings at 14 to 16 weeks of gestation in non-grafted specimens (Cunha et al., 2017; Robboy et al., 2017), and gland development is minimal in xenografts grown for 4 weeks in untreated ovariectomized hosts. Human uterine/cervical gland development appears to be stimulated by estrogen. Three observations support this belief: (a) Uterine/cervical glands are absent early in the second trimester before elevation of endogenous estrogens. (b) At 21 weeks uterine/cervical glands are elongated and branched within the endometrial stroma in non-grafted specimens when effects of endogenous estrogens can be readily recognized in vaginal epithelium (Robboy et al., 2017). (c) DES-treated xenografts of comparable age (~14+4 weeks) exhibited considerable gland development. Thus, uterine/cervical gland development correlates with elevated endogenous estrogen or exogenously administered estrogen. These data starkly contrast with the initial appearance of uterine glands in the mice which occurs at ~7 days postnatal when endogenous estrogen levels are exceedingly low (Pang et al., 1979) and serum levels of alpha-fetoprotein are high (Adinolfi et al., 1990). Alpha-fetoprotein binds steroidal estrogens at high affinity (Savu et al., 1981) and thus “neutralizes” serum estrogen. Moreover, analysis of neonatal ESR1 null mice indicates that initial appearance of uterine glands is independent of ESR1 (Nanjappa et al., 2015). Thus, uterine/cervical gland development is stimulated by DES (and perhaps endogenous estrogens) in humans, while uterine gland formation in mice is estrogen independent.
ESR1 is undetectable in epithelium of the human fetal uterine corpus during the second trimester (Cunha et al., 2017), but was induced by DES treatment in xenografts. Estrogen is known to up-regulate ESR1 mRNA and protein (Read et al., 1989; Saceda et al., 1988) presumably via binding of liganded ESR1 to estrogen-response elements associated with the gene encoding ESR1 (Liu et al., 2003). However, human uterine epithelium appears completely ESR1-negative in untreated hosts but is strongly expressed following DES treatment. Hence, it is unclear if DES up-regulates the expression of ESR1 in immature uterine epithelium of human directly via epithelial ESR1 as shown in human breast and endometrial cell lines or via paracrine mechanisms.
The striking DES induction of ESR1 in epithelial cells of the human fetal uterine corpus raises two possibilities: (a) ESR1 may be present in epithelium of the human fetal uterine corpus at levels below the sensitivity of IHC, and thus DES-induction of uterine epithelial ESR1 may be mediated directly via epithelial ESR1. (b) Alternatively ESR1 may be induced in human fetal uterine epithelium via a paracrine mechanism mediated by ESR1 abundantly expressed in adjacent mesenchymal cells (see Fig. 10 in Cunha et al., 2017). Such paracrine regulation of sex steroid receptors in mice (androgen receptor, ESR1 and PGR) is well documented (Cunha et al., 1980; Kurita et al., 1998; Kurita et al., 2000a; Kurita et al., 2000b).
PGR regulation differs vastly in mice and humans. Many studies have shown that human PGR is directly induced by estrogen, a concept verified in both cell lines and in many tissues (Janne et al., 1975; Horwitz and McGuire, 1979). Direct estrogen-induced expression of PGR is furthermore affirmed through analysis of tissue recombinants composed of ESR1-deficient αERKO uterine stroma plus adult human uterine epithelium (Kurita et al., 2005b). In contrast, in adult mice uterine epithelial PGR is strongly expressed in untreated ovariectomized mice and disappears completely following estrogen administration; this regulation of epithelial PGR is mediated via mesenchymal ESR1 (Kurita et al., 2000b). Thus, uterine epithelial PGR regulation differs radically in mice versus humans. The initial expression of uterine epithelial PGR during development also differs in mice versus humans. In mice PGR initially appears at 3 days postnatal in uterine epithelium (Kurita et al., 2001). It is unlikely that this initial PGR expression in mice is estrogen induced since estrogen levels are exceedingly low at this stage and alpha-fetoprotein levels are high as discussed above. In the human fetal uterus PGR is (a) not expressed in the epithelium at 14 to 18 weeks (before evidence of estrogen action), (b) is more broadly expressed at 21 weeks when evidence of estrogen action is apparent (Cunha et al., 2017), (c) is rarely seen in epithelium and mesenchyme of grafts of human fetal reproductive organs grown in untreated ovariectomized hosts, and (d) is globally induced by DES, thus suggesting that during human fetal development the initial appearance of human epithelial and mesenchymal PGR is estrogen induced.
DES induction of proliferation and differentiation of human fetal vaginal epithelium deserves special comment. In humans ESR1 is undetectable in vaginal plate epithelium at 12 weeks, but is expressed in small patches in the vaginal plate/vaginal epithelium at 14 to 18 weeks. The surrounding vaginal mesenchymal cells were devoid of ESR1 at this stage (Cunha et al., 2017). Subsequently at 21 weeks, when the vaginal epithelium had differentiated into a thick mature epithelium, basal and suprabasal vaginal epithelial cells prominently expressed ESR1, and in adulthood estrogen receptors are present in both vaginal epithelial and stromal cells (Gould et al., 1983). In newborn mice ESR1 appears in epithelial cells at about 2 days postnatal (Kurita et al., 2001). In adulthood, estrogen receptors are present in both vaginal epithelium and stroma in mice (Stumpf and Sar, 1976). Thus, the common feature in both mice and humans is the initial absence of ESR1 in vaginal epithelium, followed later by ESR1 expression in vaginal epithelium. The ontogeny of ESR1 expression in vaginal mesenchyme differs in humans and mice. During vaginal development in newborn mice, ESR1 is abundantly expressed throughout vaginal mesenchyme (Kurita et al., 2001). Conversely, during human vaginal development ESR1 is initially expressed at 8 to 14 weeks in a minority of mesenchymal cells associated with the vaginal plate, but only becomes prominent in vaginal mesenchyme at 21 weeks by which time the vaginal epithelium has proliferated into a multi-layer mature epithelium presumably in response to endogenous estrogen (Cunha et al., 2017). The significance of these species differences in the ontogeny of mesenchymal ESR1 remains to be determined.
In response to estrogen, vaginal epithelium of both mice and humans proliferates to form a multi-layered epithelium that undergoes stratified squamous differentiation. In mice the response of vaginal epithelium to estrogenic stimulation involves two separate events: (a) estrogen-induced epithelial proliferation to generate a multi-layered epithelium and (b) stratified squamous differentiation and expression of proteins (KRT14, TP63, KRT10) characteristic of stratified squamous vaginal epithelium. Through analysis of mouse vaginal tissue recombinants composed of epithelium and mesenchyme derived from wild-type mice and ESR1 null mice, we showed that estrogen-induced vaginal epithelial proliferation is mediated indirectly through stromal ESR1 (paracrine mechanism), while epithelial ESR1 was required for estrogen-induced vaginal epithelial cornification and expression of keratin 10 (direct mechanism) (Buchanan et al., 1998). Thus, in mice both mesenchymal and epithelial ESR1 are required to achieve the complete vaginal epithelial response to estrogen. Whether similar mechanisms of vaginal epithelial differentiation occur in humans remain to be determined. Curiously, at 21 weeks ESR1-positive mesenchymal cells were rarely seen near the vaginal introitus, while a high percentage of vaginal mesenchymal cells were ESR1-positive near the vaginal/cervical boundary (Cunha et al., 2017). These two radically different distributions of ESR1-positive vaginal mesenchymal cells in cranial versus caudal zones within the developing human vagina may be indicative of substantially different mechanisms of vaginal epithelial response to estrogen in these two areas. Regardless, substantial mouse/human differences are apparent regarding the ontogeny of vaginal ESR1 and response to estrogen. Additional specimens and further research will be required to understand these differences.
Major DES-induced changes in keratin expression were seen in vaginal epithelium. As a result of DES treatment two vaginal epithelial histo-types formed: (a) mature thick stratified squamous vaginal epithelial differentiation and (b) adenosis (retained simple columnar epithelium associated with the mature vaginal epithelium). These two histo-types presumably emerged from epithelium of the vaginal plate whose Müllerian/urogenital sinus composition changes with gestational age (Robboy et al., 2017). Differentiation of a multi-layered mature glycogenated squamous vaginal epithelium represents the typical response to estrogen. In contrast, adenosis occurs frequently in DES daughters (O’Brien et al., 1979; Robboy et al., 1979) and rarely in the un-exposed human population (Herbst et al., 1975a). From studies in mice, DES-induced adenosis involves the failure of the simple columnar Müllerian epithelium to transform into a stratified squamous epithelium, and in mice the resultant adenotic epithelium expresses a spectrum of differentiation markers indicative of uterine epithelium (Kurita et al., 2004; Kurita, 2011). The adenotic human epithelium induced by DES expresses ESR1, PGR, KRT7, KRT8, KRT19, but not TP63, RUNX1 and KRT14, a pattern of differentiation marker expression consistent with uterine epithelial differentiation (Cunha et al., 2017; Laronda et al., 2013; Terakawa et al., 2016).
DES had a striking effect on differentiation of mesenchyme of the uterine tube, which normally forms an αACT2-negative layer in intimate association with the epithelium, which is in turn surrounded by an αACT2-positive smooth muscle layer. This pattern is seen in non-grafted specimens and in specimens grown in untreated ovariectomized hosts. In DES-treated specimens the αACT2-positive smooth muscle layer is in direct association with the epithelium and the αACT2-negative mesenchymal layer is absent. This effect is presumed to be mediated by ESR1 expressed in the tubal mesenchyme (see figure 12F in Cunha et al., 2017). This observation corresponds to our earlier observation of inhibition of differentiation of utero-tubal mesenchyme into stromal and smooth muscle layers (Robboy et al., 1982) that in part may be the basis of the tubal malformations described in DES daughters as discussed above.
Our observation of abnormal smooth muscle differentiation in the uterine tube, as well as the many other DES-induced alterations in morphogenesis and molecular expression, provide a wealth of bio-end points of estrogen-induced malformation/alteration of the human female reproductive tract. These bio-endpoints can be used in the future for testing of environmental estrogenic endocrine disruptors such as bisphenol-A and its analogues directly on developing human organs, thus addressing concerns of relevance/applicability of animal models to human teratogenesis.
Acknowledgements
This study was supported by NIH grant DK058105 to Dr. Baskin and R01 CA154358 to Dr. Kurita
Abbreviations:
- ESR1
Estrogen receptor alpha
- DES
diethylstilbestrol
- KRT
keratin
- PGR
progesterone receptor
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
References
- Adinolfi M, Beck SE, Seller MJ, Fedor T, and McLaren A (1990) Alpha-fetoprotein levels in different strains of mice during development. Experimental and clinical immunogenetics 7:123–128. [PubMed] [Google Scholar]
- Baskin LS, Sutherland RS, DiSandro MJ, Hayward SW, Lipschutz J, and Cunha GR (1997) The effect of testosterone on androgen receptors and human penile growth. The Journal of urology 158:1113–1118. [DOI] [PubMed] [Google Scholar]
- Bern HA, Mills KT, Ostrander PI, Schoenrock B, Graveline B, and Plapinger L (1984) Cervicovaginal abnormalities in BALB/c mice treated neonatally with sex hormones. Teratology 30:267–274. [DOI] [PubMed] [Google Scholar]
- Bern HA, and Talamantes FJ (1981) Neonatal mouse models and their relation to disease in the humal female In: Herbst A, and Bern HA (eds) Developmental Effects of Diethylstilbestrol (DES) in Pregnancy. Thieme Stratton Inc., New York, pp. 129–147. [Google Scholar]
- Buchanan DL, Kurita T, Taylor JA, Lubahn DL, Cunha GR, and Cooke PS (1998) Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification and cornification. Endocrinology 139:4345–4352. [DOI] [PubMed] [Google Scholar]
- Cunha GR, and Baskin L (2016) Use of sub-renal capsule transplantation in developmental biology. Differentiation; research in biological diversity 91:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Reese BA, and Sekkingstad M (1980) Induction of nuclear androgen-binding sites in epithelium of the embryonic urinary bladder by mesenchyme of the urogenital sinus of embryonic mice. Endocrinology 107:1767–1770. [DOI] [PubMed] [Google Scholar]
- Cunha GR, T., K., Cao M, Shen J, Robboy S, and L., B. (2017) Molecular mechanisms of development of the human fetal female reproductive tract. Differentiaiton (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Taguchi O, Lawrence WD, and Robboy SJ (1988) Absence of teratogenic effects of progesterone on the developing genital tract of the human fetus. Human Path. 19:777–783. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Taguchi O, Namikawa R, Nishizuka Y, and Robboy SJ (1987a) Teratogenic effects of Clomid, tamoxifen, and diethylstilbestrol on the developing human female genital tract. Human Pathol. 18:1132–1143. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Taguchi O, Sugimura Y, Lawrence DW, Mahmood F, and Robboy SJ (1987b) Absence of teratogenic effects of progesterone on the developing gential tract of the human female fetus. Human Pathol 19:777–783. [DOI] [PubMed] [Google Scholar]
- Drey EA, Kang MS, McFarland W, and Darney PD (2005) Improving the accuracy of fetal foot length to confirm gestational duration. Obstetrics and gynecology 105:773–778. [DOI] [PubMed] [Google Scholar]
- Gould SF, Shannon JM, and Cunha GR (1983) The autoradiographic demonstration of estrogen binding in normal human cervix and vagina during the menstrual cycle, pregnancy, and the menopause. Am J Anat 168:229–238. [DOI] [PubMed] [Google Scholar]
- Herbst A, and Bern H (1981) Developmental effects of DES in pregnancy. Thieme Stratton, New York. [Google Scholar]
- Herbst AL, Poskanzer DC, Robboy SJ, Friedlander L, and Scully RE (1975a) Prenatal exposure to stilbestrol. A prospective comparison of exposed female offspring with unexposed controls. The New England journal of medicine 292:334–339. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Scully RE, and Robboy SJ (1975b) Vaginal adenosis and other diethylstilbestrol-related abnormalities. Clin Obstet Gynecol 18:185–194. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Ulfelder H, and Poskanzer DC (1971) Adenocarcinoma of the vagina: Association of maternal stilbestrol therapy with tumor appearance in young women. New Eng. J. Med 284:878–881. [DOI] [PubMed] [Google Scholar]
- Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, Colton T, Hartge P, Hatch EE, Herbst AL, Karlan BY, Kaufman R, Noller KL, Palmer JR, Robboy SJ, Saal RC, Strohsnitter W, Titus-Ernstoff L, and Troisi R (2011) Adverse health outcomes in women exposed in utero to diethylstilbestrol. The New England journal of medicine 365:1304–1314. [DOI] [PubMed] [Google Scholar]
- Horwitz KB, and McGuire WL (1979) Estrogen control of progesterone receptor induction in human breast cancer: role of nuclear estrogen receptor. Adv Exp Med Biol 117:95–110. [DOI] [PubMed] [Google Scholar]
- Isaacson D, Shen J, Cao MS,A, Yue X, Cunha G, and Baskin L (2017) Renal Subcapsular Xenografing of Human Fetal External Genital Tissue – A New Model for Investigating Urethral Development. Differentiation; research in biological diversity (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janne O, Kontula K, Luukkainen T, and Vihko R (1975) Oestrogen-induced progesterone receptor in human uterus. Journal of steroid biochemistry 6:501–509. [DOI] [PubMed] [Google Scholar]
- Jefferies JA, Robboy SJ, O’Brien PC, Bergstralh EJ, Labarthe DR, Barnes AB, Noller KL, Hatab PA, Kaufman RH, and Townsend DE (1984) Structural anomalies of the cervix and vagina in women enrolled in the Diethylstilbestrol Adenosis (DESAD) Project. American journal of obstetrics and gynecology 148:59–66. [DOI] [PubMed] [Google Scholar]
- Kurita T (2011) Normal and abnormal epithelial differentiation in the female reproductive tract. Differentiation; research in biological diversity 82:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Cooke PS, and Cunha GR (2001) Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Developmental biology 240:194–211. [DOI] [PubMed] [Google Scholar]
- Kurita T, and Cunha GR (2001) Roles of p63 in differentiation of Mullerian duct epithelial cells. Ann N Y Acad Sci. 948:9–12. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cunha GR, Robboy SJ, Mills AA, and Medina RT (2005a) Differential expression of p63 isoforms in female reproductive organs. Mechanisms of development 122:1043–1055. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee K-J, Cooke PS, Lydon JP, and Cunha GR (2000a) Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biology of reproduction 62:831–838. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, and Cunha GR (2000b) Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biology of reproduction 62:821–830. [DOI] [PubMed] [Google Scholar]
- Kurita T, Medina R, Schabel AB, Young P, Gama P, Parekh TV, Brody J, Cunha GR, Osteen KG, Bruner-Tran KL, and Gold LI (2005b) The activation function-1 domain of estrogen receptor alpha in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation; research in biological diversity 73:313–322. [DOI] [PubMed] [Google Scholar]
- Kurita T, Mills AA, and Cunha GR (2004) Roles of p63 in the diethylstilbestrol-induced cervicovaginal adenosis. Development 131:1639–1649. [DOI] [PubMed] [Google Scholar]
- Kurita T, Young P, Brody J, Lydon JP, O’Malley BW, and Cunha GR (1998) Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell (UtE) proliferation. Endocrinology 139:4708–4713. [DOI] [PubMed] [Google Scholar]
- Laronda MM, Unno K, Butler LM, and Kurita T (2012) The development of cervical and vaginal adenosis as a result of diethylstilbestrol exposure in utero. Differentiation; research in biological diversity 84:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronda MM, Unno K, Ishi K, Serna VA, Butler LM, Mills AA, Orvis GD, Behringer RR, Deng C, Sinha S, and Kurita T (2013) Diethylstilbestrol induces vaginal adenosis by disrupting SMAD/RUNX1-mediated cell fate decision in the Mullerian duct epithelium. Developmental biology 381:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Zhang Z, Gladwell W, and Teng CT (2003) Estrogen stimulates estrogen-related receptor alpha gene expression through conserved hormone response elements. Endocrinology 144:4894–4904. [DOI] [PubMed] [Google Scholar]
- McLachlan J (1981) Rodent models for perinatal exposure to diethylstilbestrol and their relation to human disease in the male In: Herbst A, and Bern HA (eds) Developmental Effects of Diethylstilbestrol (DES) in Pregnancy. Thieme Stratton Inc., New York, pp. 148–157. [Google Scholar]
- McLachlan JA, and Newbold RR (1996) Cellular and molecular mechanisms of cancers of the uterus in animals. Prog Clin Biol Res 394:175–182. [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, and Bullock BC (1975) Reproductive tract lesions in male mice exposed prenatally to diethylstilbestrol. Science 190:991–992. [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Burow ME, and Li SF (2001) From malformations to molecular mechanisms in the male: three decades of research on endocrine disrupters. APMIS 109:263–272. [DOI] [PubMed] [Google Scholar]
- Moll R, Divo M, and Langbein L (2008) The human keratins: biology and pathology. Histochem Cell Biol 129:705–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, and Krepler R (1982) The catalog of human cytokeratin polypeptides: patterns of expression of specific cytokeratins in normal epithelia, tumors, and cultured cells. Cell 31:11–24. [DOI] [PubMed] [Google Scholar]
- Nanjappa MK, Medrano TI, March AG, and Cooke PS (2015) Neonatal uterine and vaginal cell proliferation and adenogenesis are independent of estrogen receptor 1 (ESR1) in the mouse. Biology of reproduction 92:78. [DOI] [PubMed] [Google Scholar]
- Newbold R (1995) Cellular and molecular effects of developmental exposure to diethylstilbestrol: Implications for other environmental estrogens. Environ. Health Perspect 103:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR (2004) Lessons learned from perinatal exposure to diethylstilbestrol. Toxicology and applied pharmacology 199:142–150. [DOI] [PubMed] [Google Scholar]
- Newbold RR (2008) Prenatal exposure to diethylstilbestrol (DES). Fertility and sterility 89:e55–56. [DOI] [PubMed] [Google Scholar]
- Newbold RR, and MaLachlan JA (1985) Diethylstilbestrol-associated defects in murine genital tract development In: McLachlan JA (ed) Estrogens in the Environment II. Elsevier, New York, pp. 288–318. [Google Scholar]
- Newbold RR, Tyrey S, Haney AF, and McLachlan JA (1983) Developmentally arrested oviduct: a structural and functional defect in mice following prenatal exposure to diethystilbestrol. Teratology 27:417–426. [DOI] [PubMed] [Google Scholar]
- O’Brien PC, Noller KL, Robboy SJ, Barnes AB, Kaufman RH, Tilley BC, and Townsend DE (1979) Vaginal epithelial changes in young women enrolled in the National Cooperative Diethylstilbestrol Adenosis (DESAD) project. Obstetrics and gynecology 53:300–308. [PubMed] [Google Scholar]
- Pang SF, Caggiula AR, Gay VL, Goodman RL, and Pang CS (1979) Serum concentrations of testosterone, oestrogens, luteinizing hormone and follicle-stimulating hormone in male and female rats during the critical period of neural sexual differentiation. J Endocrinol 80:103–110. [DOI] [PubMed] [Google Scholar]
- Read LD, Greene GL, and Katzenellenbogen BS (1989) Regulation of estrogen receptor messenger ribonucleic acid and protein levels in human breast cancer cell lines by sex steroid hormones, their antagonists, and growth factors. Molecular endocrinology 3:295–304. [DOI] [PubMed] [Google Scholar]
- Rennell CL (1979) T-shaped uterus in diethylstilbestrol (DES) exposure. AJR. American journal of roentgenology 132:979–980. [DOI] [PubMed] [Google Scholar]
- Robboy SJ (1983) A hypothetic mechanism of diethylstilbestrol(DES)-induced anomalies in exposed progeny. Hum Pathol 14:831–833. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Kaufman RH, Prat J, Welch WR, Gaffey T, Scully RE, Richart R, Fenoglio CM, Virata R, and Tilley BC (1979) Pathologic findings in young women enrolled in national cooperative diethylstilbestrol adenosis (DESAD) project. Obstet. Gynecol 53:309–317. [PubMed] [Google Scholar]
- Robboy SJ, Kurita T, Baskin L, and Cunha GR (2017) New insights insights into human female reproductive tract development. Differentiation; research in biological diversity (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robboy SJ, Noller KL, R.H., K., Barnes AB, Townsend D, Gunderson JH, Kurland L, and Nash S (1984) An atlas of findings in the human female after intrauterine exposure to diethylstilbestrol. DHEW publication 84–2344. [Google Scholar]
- Robboy SJ, Scully RE, Welch WR, and Herbst AL (1977) Intrauterine diethylstilbestrol exposure and its consequences: pathologic characteristics of vaginal adenosis, clear cell adenocarcinoma, and related lesions. Arch Pathol Lab Med 101:1–5. [PubMed] [Google Scholar]
- Robboy SJ, Taguchi O, and Cunha GR (1982) Normal development of the human female reproductive tract and alterations resulting from experimental exposure to diethylstilbestrol. Human Pathol. 13:190–198. [DOI] [PubMed] [Google Scholar]
- Saceda M, Lippman ME, Chambon P, Lindsey RL, Ponglikitmongkol M, Puente M, and Martin MB (1988) Regulation of the estrogen receptor in MCF-7 cells by estradiol. Molecular endocrinology 2:1157–1162. [DOI] [PubMed] [Google Scholar]
- Savu L, Benassayag C, Vallette G, Christeff N, and Nunez E (1981) Mouse alpha 1-fetoprotein and albumin. A comparison of their binding properties with estrogen and fatty acid ligands. The Journal of biological chemistry 256:9414–9418. [PubMed] [Google Scholar]
- Stillman RJ (1982) In utero exposure to diethylstilbestrol: adverse effects on the reproductive tract and reproductive performance and male and female offspring. American journal of obstetrics and gynecology 142:905–921. [DOI] [PubMed] [Google Scholar]
- Stumpf W, and Sar M (1976) Autoradiographic localization of estrogen, androgen, progestin, and glucocorticosteroid in “target tissues” and “non-target tissues” In: Pasqualini J (ed) Receptors and Mechanism of Action of Steroid Hormones. Marcel Dekker Inc., New York, pp. 41–84. [Google Scholar]
- Sugimura Y, Cunha GR, Yonemura CU, and Kawamura J (1988) Temporal and spatial factors in diethylstilbestrol-induced squamous metaplasia of the developing human prostate. Hum Pathol 19:133–139. [DOI] [PubMed] [Google Scholar]
- Taguchi O, Cunha GR, Lawrence WD, and Robboy SJ (1984) Timing and irreversibility of Mullerian duct inhibition in the embryonic reproductive tract of the human male. Developmental biology 106:394–398. [DOI] [PubMed] [Google Scholar]
- Taguchi O, Cunha GR, and Robboy SJ (1983) Experimental study of the effect of diethylstilbestrol on the development of the human female reproductive tract. International J. Biological Res. in Pregnancy 4:56–70. [PubMed] [Google Scholar]
- Terakawa J, Rocchi A, Serna VA, Bottinger EP, Graff JM, and Kurita T (2016) FGFR2IIIb-MAPK Activity Is Required for Epithelial Cell Fate Decision in the Lower Mullerian Duct. Molecular endocrinology 30:783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus-Ernstoff L, Troisi R, Hatch EE, Palmer JR, Hyer M, Kaufman R, Adam E, Noller K, and Hoover RN (2010) Birth defects in the sons and daughters of women who were exposed in utero to diethylstilbestrol (DES). International journal of andrology 33:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura CY, Cunha GR, Sugimura Y, and Mee SL (1995) Temporal and spatial factors in diethylstilbestrol-induced squamous metaplasia in the developing human prostate. II. Persistent changes after removal of diethylstilbestrol. Acta anatomica 153:1–11. [DOI] [PubMed] [Google Scholar]


