Abstract
Ginkgo biloba extract (GBE) is a popular botanical dietary supplement used worldwide and the safety of use is a public health concern. While GBE is a complex mixture, the terpene trilactones and flavonol glycosides are believed to elicit the pharmacological and/or toxicological effects of GBE. In a National Toxicology Program (NTP) 2-year rodent bioassay with GBE, hepatotoxicity was observed in rodents (≥ 100 mg/kg in rats, ≥ 200 mg/kg in mice). Subsequently, questions arose about whether or not the GBE used in NTP studies was representative of other GBE products and how rodent doses are related to human doses. To address these, we generated systemic exposure data for terpene trilactones in male rats following oral administration of 30, 100, and 300 mg/kg GBE test article from the 2-year bioassay. Dose-normalized Cmax and AUC∞ for terpene trilactones from the current study were within 5-fold of published rodent studies using a standardized GBE preparation. Comparison of our rat systemic exposure data at 100 mg/kg GBE to published human data following ingestion of 240 mg GBE-containing product showed that the rat/human exposure multiple was 3 to 22, for terpene trilactones. These data demonstrate the relevance of NTP rodent toxicity data to humans.
Keywords: Botanical dietary supplements, Ginkgo biloba extract, Terpene trilactones, Flavonolsm, Flavonol glycosides, Systemic exposure
Introduction
The botanical dietary supplement industry is a multibillion-dollar industry worldwide with sales surpassing 8 billion dollars in the U.S. in 2017 (Smith et al., 2018). According to a National Health Information Survey conducted in 2012, approximately 18% of adults in the United States are using these supplements (Clark et al., 2015). Currently, there are over 26,000 supplements in this category in the United States marketplace, according to the Dietary Supplement Label Database (https://dsld.nlm.nih.gov/dsld/). Because of widespread use and limited availability of safety data, these products represent an important public health concern.
Ginkgo biloba leaf extract (GBE) is a popular botanical dietary supplement used worldwide for its purported cognitive and cardiovascular benefits (Brinkley et al., 2010; DeKosky et al., 2008; Snitz et al., 2009). Other reported beneficial activities of GBE include antioxidative (Goh and Barlow, 2002), antitumor (Agha et al, 2001) and anti-inflammatory (Lee et al., 2011) actions. Approximately 3 million people in the U.S. use dietary supplements containing GBE with a recommended daily dose of 120 to 240 mg (DeFeudis, 2003). Some reports indicate that GBE-containing products are generally well-tolerated in humans (Diamond et al., 2000; Liebermann et al., 2017).
GBE is a complex mixture containing different classes of constituents such as flavonol glycosides, terpene trilactones, proanthocyanidines, ginkgolic acids, biflavones, polyflavones and ginkgotoxins (Singh et al., 2008; Leistner and Drewke, 2009). The purported pharmacological properties of GBE are believed to be due to flavonol glycosides and terpene trilactones (Ding et al., 2009), although unlike terpene trilactones which are unique to Ginkgo biloba, flavonol glycosides are ubiquitous in the plant kingdom. It is accepted that GBE should contain 22–27% flavonol glycosides, 5–7% terpene trilactones and ≤ 5 ppm ginkgolic acids (van Beek, 2002). Standardized GBEs in the marketplace should conform to these established levels, including the unofficial industry gold standard, EGb761®, produced by Dr. Wilmar Schwabe Pharmaceuticals (Karlsruhe, Germany). However, a wide range of levels have been reported in GBE products for terpene trilactones (27–358% of standardized) and flavonol glycosides (86–418% of standardized) (Fransen et al., 2010). This variability poses a challenge for selecting a representative GBE product for efficacy and/or toxicity testing.
Absorption, distribution, metabolism and excretion (ADME) studies of GBE have focused mainly on the two purported active classes, terpene trilactones and flavonol glycosides. There is a significant amount of work characterizing the ADME properties of terpene trilactones (e.g., bilobalide, ginkgolides A, B, C, and J). Terpene trilactones are well-distributed in rodents and are highly bioavailable following ingestion in both rodents and humans with short plasma elimination half-lives (rodents, 1–3 hours; humans, 2–11 hours) (Biber and Koach, 1999; Biber 2003; Cheng et al., 2013; Drago et al., 2002; Fourtillian et al., 1995; Kressmann et al., 2002; Mauri et al., 2001, 2006; Rossi et al., 2009; Zheng et al., 2015; Zheng et al., 2016; Woelkart et al., 2010; Ude et al., 2011; summarized in Ude et al., 2013). Data on the metabolism of terpene trilactones are limited. The work by Wang et al. (2008) demonstrated that terpene trilactones are metabolized by cleavage of the lactone ring and/or hydroxylation of the terpene backbone. Flavonol glycosides on the other hand are less studied, likely due to their existence as a variety of glycosides and poor bioavailability (Liu and Hu, 2007; Pietta et al., 1995, 1997). These properties may have led to low systemic concentrations following ingestion, making measurement of compounds in this class analytically challenging. Flavonol glycosides are studied as corresponding aglycone, flavonol, (e.g., quercetin, kaempferol, and isorhamnetin) following hydrolysis of corresponding glycosides. Reported plasma elimination half-lives, depending on the flavonol, were 2–4 hours in humans (Ding et al., 2006; Wójcicki et al., 1995) and 19–24 hours in rodents (Rangel-Ordóñez et al., 2010; Zheng et al., 2015). It has been suggested that neither the flavonol glycosides nor the aglycones, but other downstream metabolites are responsible for pharmacological effects of GBE (summarized in Ude et al., 2013).
There have been a handful of preclinical studies evaluating the toxicity of GBE in rodents. Following oral exposure of rats to 4, 20, and 100 mg/kg EGb761®, no treatment-related effects were reported, although details of the study are not available (Woerdenbag and De Smet, 2000). In another study, following oral exposure of rats and mice to a GBE preparation (details not provided), with doses ranging from 100 to 1600 mg/kg, there was no evidence of treatment-related effects (Salvador, 1995). The lack of toxicity reported in these studies from the literature contrasts with observed toxicity in the 90-day and 2-year GBE studies conducted by the National Toxicology Program (NTP). In the NTP 2-year rodent bioassay, GBE demonstrated clear hepatotoxicity in rats (100, 300, and 1000 mg/kg GBE) and mice (200, 600, and 2000 mg/kg GBE) of both sexes following gavage administration (NTP, 2013; Rider et al., 2014). The GBE preparation used in these studies contained 31.2% flavonol glycosides, 15.4% terpene trilactones, and 10.5 ppm ginkgolic acid (NTP, 2013). During the public reporting of the NTP GBE studies, questions arose regarding the relevance of the findings to human exposure. These questions centered around whether or not: 1) the test article was relevant to other GBE products on the market and 2) the doses used in the rodent bioassays were relevant to human exposure levels.
Data from well-designed ADME studies could help in understanding how different constituent levels among different GBE products might impact observed effects and allow for comparison of animal doses to human exposure levels. Ultimately, these data will aid in understanding the human health risk posed by GBE. As such, a study in rats was designed to generate systemic exposure data to aid in bridging this gap. The rat strain, route of exposure, and the GBE used were the same as those used in the NTP 2-year bioassay (NTP, 2013; Rider et al., 2014). The doses selected were 30, 100, and 300 mg/kg. The two highest doses were the same as those used in the 2-year bioassay; a lower dose was also included to generate data closer to the human recommended daily dose.
Materials and methods
Chemicals and reagents:
GBE (CASRN 90045-36-6; lot # 020703) used in the current study (and NTP 2-year bioassay) was obtained from Shanghai Xing Ling Science and Technology Pharmaceutical Company, Limited (Shanghai, China). The GBE lot was characterized as described elsewhere (Rider et al., 2014; NTP, 2013). The lot contains ~31% flavonol glycosides (16.7% quercetin, 12.2% kaempferol, and 2.4% isorhamnetin), ~15% terpene lactones (6.9% bilobalide, 3.7% ginkgolide A, 1.6% ginkgolide B, 3.1% ginkgolide C), and 10.5 ppm ginkgolic acids. Standards of ginkgolide A, ginkgolide C, isorhamnetin, and quercetin were obtained from Sigma-Aldrich (St. Louis, MO). Standards of bilobalide, ginkgolide B, ginkgolide J, and kaempferol hydrate were obtained from TCI America (Portland, OR). Hesperetin and limonene (used as internal standards) were also obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals and reagents were procured from commercial sources.
Animals:
Male F344/NCrl rats were obtained from Charles River Kingston (Stone Ridge, NY). The animals were quarantined for at least 7 days before they were randomized using Provantis software (Instem, Stone, UK) into dosing groups and used in the study. Animals had ad libitum access to certified, irradiated NTP 2000 wafer feed (Ziegler Bros, Inc., Gardners, PA) and city (West Jefferson, OH) tap water. Feed was used within 120 days of milling. Environmental conditions included: room temperature 69 to 75 °F (22 ± 2 °C), relative humidity 35 to 65% and a 12-h light/dark cycle. Animals were 7–8 weeks old at the time of dosing.
Animal studies were conducted at Battelle (Columbus, OH) and approved by the Institutional Animal Care and Use Committee. Animals were housed in facilities that are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Animal procedures were in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 2011).
Study design and plasma collection:
Oral dose formulations of GBE were prepared in corn oil at 6, 20, and 60 mg/mL and stored at ambient temperature. Formulations were analyzed by liquid chromatography-mass spectrometry (LC-MS) using a Schimadzu LC-20AD LC (Columbia, MD) coupled to an AB Sciex tandem triple quadrupole mass spectrometer (Framingham, MA) and a Phenominex Prodigy ODS 3 column (250 mm × 4.6 mm, 5 um) (Torrance, CA). Mobile phases A (water:methanol, 9:1, with 0.1% formic acid) and B (methanol with 0.1% formic acid) were run at an initial condition of 15% B (5 min) and then changed linearly to 95% B in 10 min. The turbospray ion source was set at 400 °C in negative ion mode. Analytes quantified were bilobalide and quercetin representing terpene trilactone and flavonol classes, respectively. Transitions monitored were m/z 325→163 and m/z 301→151 for bilobalide and quercetin, respectively. All formulations were within 10% of target concentration except for quercetin at the lowest concentration where it was within 20% of target concentration. Prior to study initiation, formulation stability was confirmed for up to 42 days when stored in sealed plastic bottles at approximately 5 °C and at least 3 hours under simulated animal room conditions (within 10% of the day 0 value).
A single dose of GBE was administered by intragastric gavage at 30, 100, and 300 mg/kg (n = 18 animals per dose group) in a volume of 5 mL/kg using a syringe equipped with a ball-tipped 16-gauge gavage needle. Following dose administration, blood was collected using K3EDTA as an anticoagulant from 3 animals per time point at target times of 0 (predose), 5, 10, 15, 30, 60, 90, 120, 240, 480, 720 and 1440 minutes from all dose groups. Two blood samples were obtained from each rat; the first sample (~ 500 μL) was obtained via retro-orbital plexus under CO2/O2 anesthesia after which the animal was returned to its home cage pending a second blood collection. Terminal blood collection was via cardiac puncture following euthanasia with CO2. The blood sampling times were separated by at least 2 hours for each rat. For non-terminal collections the total volume collected did not exceed 2% of body weight. The actual times for blood collection were recorded at each time point. Plasma was isolated from blood within 1 hour of collection. All samples were stored at −70 °C within 2 hours of collection.
Quantitation of terpene trilactones and flavonols:
An LC-MS/MS method based on literature methods was developed to quantify a suite of GBE constituents in plasma (Guan et al., 2014; Zheng et al., 2016). Plasma was hydrolyzed to convert flavonol glycosides to corresponding aglycones. The analytes quantified were bilobalide, ginkgolides A, B, C, and J, quercetin, kaempferol, and isorhamnetin (Figure 1).
Figure 1.
Constituents of Ginkgo biloba extract quantified A) terpene trilactones B) flavonols
Two stock solutions of combined terpene trilactone and flavonol standards were prepared in acetonitrile:[methanol:water (7:3)] (1:1), except isorhamnetin where acetonitrile:water (1:1) was used. The weight of kaempferol hydrate was adjusted to the anhydrous weight prior to preparing solutions. Standards in the working range (20 to 20,000 ng/mL depending on the analyte) were prepared by diluting alternate stock solutions in methanol:water (7:3). Stock solutions of internal standards (hesperetin and limonene) were prepared in methanol:water (7:3) and diluted in water to prepare a combined working internal standard solution at 500 ng/mL with respect to each analyte.
Plasma calibration standards (ginkgolide A, 4.3 to 400 ng/mL; ginkgolides B, C, 1 to 200 ng/mL; ginkgolide J, 10 to 1000 ng/mL; bilobalide, 5 to 1000 ng/mL; quercetin, 3 to 100 ng/mL, kaempferol, 40 to 500 ng/mL; isorhamnetin, 5 to 50 ng/mL) were prepared by adding analytes to plasma using working standard solutions.
For the determination of terpene trilactones and flavonols mentioned above, 100 μL plasma aliquots were transferred to microcentrifuge tubes and 20 μL of internal standard solution were added. To each tube 100 μL 4M hydrochloric acid/20 mM ascorbic acid were added, and the samples were digested at 80 °C for 30 minutes. Following digestion, samples were extracted with 1 mL of ethyl acetate, supernatants were collected and evaporated to dryness under a stream of nitrogen, and residues were reconstituted in 100 μL methanol.
Methanol extracts were analyzed by LC-MS/MS using a Waters Acquity QSM (Milford, MA) LC coupled to a XEVO TQ-S mass spectrometer. Chromatography was performed on a Waters Acquity BEH C18 column (100 × 2.1 mm, 1.7 μm) (Milford, MA). Mobile phases A (0.1% aqueous formic acid) and B (0.1% formic acid in acetonitrile) were run with initial condition of 5% B for 0.5 minutes followed by 5 to 70% B over 4.5 min with a 2 min hold at a flow rate of 0.4 mL/min. The column temperature was maintained at 40 ˚C. The electrospray ion source was operated in negative mode. Due to the large number of analytes to be quantified, samples were injected twice and monitored in 2 groups as given in Table 1; corresponding ion source temperatures were 450 ˚C and 550 ˚C and ion spray voltages were – 3000 or −4500 V. The transitions monitored, corresponding retention times, and the respective internal standard used are given in Table 1.
Table 1.
Transitions monitored for the quantitation of Ginkgo biloba extract constituents and corresponding retention times
| Analyte | Abbreviation | Retention Time (min) | Transition (m/z) | |
|---|---|---|---|---|
| Group 1a | Ginkgolide A | GLA | 4.27 | 407 → 229 |
| Ginkgolide C | GLC | 3.66 | 439 → 383 | |
| Bilobalide | BLL | 3.69 | 325 → 163 | |
| Quercetin | QCT | 4.73 | 301 → 151 | |
| Limonene | LIM | 5.20 | 469 → 229 | |
| Hesperetin | HES | 4.74 | 301 → 164 | |
| Group 2 | Ginkgolide B | GLB | 4.45 | 423 → 367 |
| Ginkgolide J | GLJ | 3.63 | 423 → 349 | |
| Kaempferol | KMF | 4.69 | 285 → 93 | |
| Isorhamnetin | ISR | 4.75 | 315 → 300 | |
| Limonene | LIM | 5.20 | 469 → 229 | |
| Hesperetin | HES | 4.74 | 301 → 164 |
Each sample was injected twice and ions monitored in each injection are given as groups 1 or 2.
A linear regression with 1/X weighing was used to relate LC-MS/MS peak area response ratio of analyte to internal standard and analyte concentration. The concentration of each analyte was calculated using its response ratio, the regression equation, and dilution when applicable. The concentration of analytes was expressed as ng/mL of plasma. The method was linear (≥ 0. 97 for all analytes except kaempferol where it was ≥ 0.87) with limits of quantitation (LOQ) ≤ 10 ng/mL and limits of detection (LOD) ≤ 5 ng/mL for all analytes except kaempferol where LOQ and LOD was 40 ng/mL. Average accuracy determined as relative error (RE) was ± ≤ 16% and precision determined as relative standard deviation (RSD) in general was ≤ 20% for terpene trilactones. Of the flavanols, average RE and RSD for quercetin was ± ≤ 16% and ≤ 23%, respectively. For kaempferol and isorhamnetin RE was ± ≤ 16%, but RSD was higher with values ≤ 33%.
Toxicokinetic analysis:
All concentrations above LODs were used. Individual animal data were evaluated for aberrant concentrations and time points. The actual blood collection times were within 5% from nominal time for timepoints ≤ 4 h and within 15 min for timepoints greater than 4 h and hence, the nominal collection times were used for TK analysis. All concentrations were evaluated to identify outliers by performing Q-tests. Based on these assessments, no values were eliminated from TK analysis.
Group mean plasma concentration versus time curves were analyzed using non-compartmental analysis (NCA) with Phoenix WinNonlin software (Version 7.0, Certara L.P., Princeton, NJ). When using the NCA models, no weighting factors were used to obtain the initial estimates. Also, the software algorithm was allowed to select the points used to calculate slope of the terminal linear phase based on determining an optimal coefficient of determination.
Results
Terpene trilactones:
All terpene trilactones were detected in plasma from rats following oral administration of GBE. Bilobalide, ginkgolide B, and ginkgolide C were quantifiable in all samples except for the 1440 minute time point for the 30 and 100 mg/kg groups. Ginkgolide A was quantifiable in all samples through 720 minutes. For ginkgolide J, all samples from the 30 mg/kg group were below LOD; for the 100 mg/kg, levels were quantifiable from 30 to 240 minutes and for 300 mg/kg from 5 to 480 minutes.
Plasma concentration-versus-time plots are given in Figure 2 for the 300 mg/kg group as an example. Toxicokinetic data for all dose groups are presented in Table 2. For all terpene trilactones, Cmax and AUC∞ increased with the dose but with an apparent less than dose-proportional increase (Table 2). The rank order for Cmax and AUC∞ was bilobalide > ginkgolide A > ginkgolide B > ginkgolide C > ginkgolide J which reflects their abundance in the GBE preparation used in the study (NTP, 2013). Plasma elimination half-lives estimated were similar for terpene trilactones with values ranging from 127 to 230 minutes. The plasma elimination half-life for ginkgolide J could not be estimated since the terminal phase could not be defined due to insufficient timepoints above the LOD for the analyte.
Figure 2.
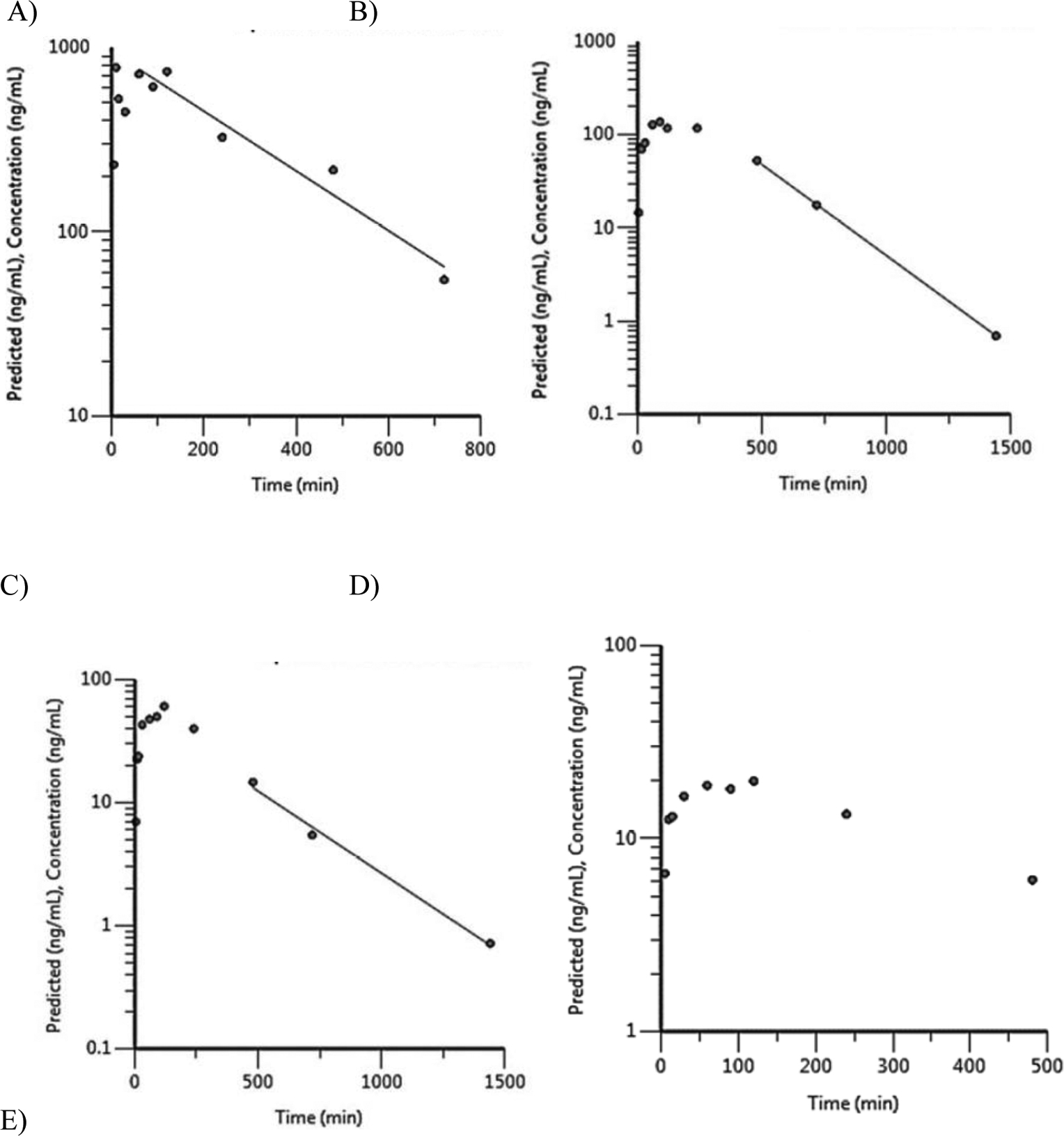
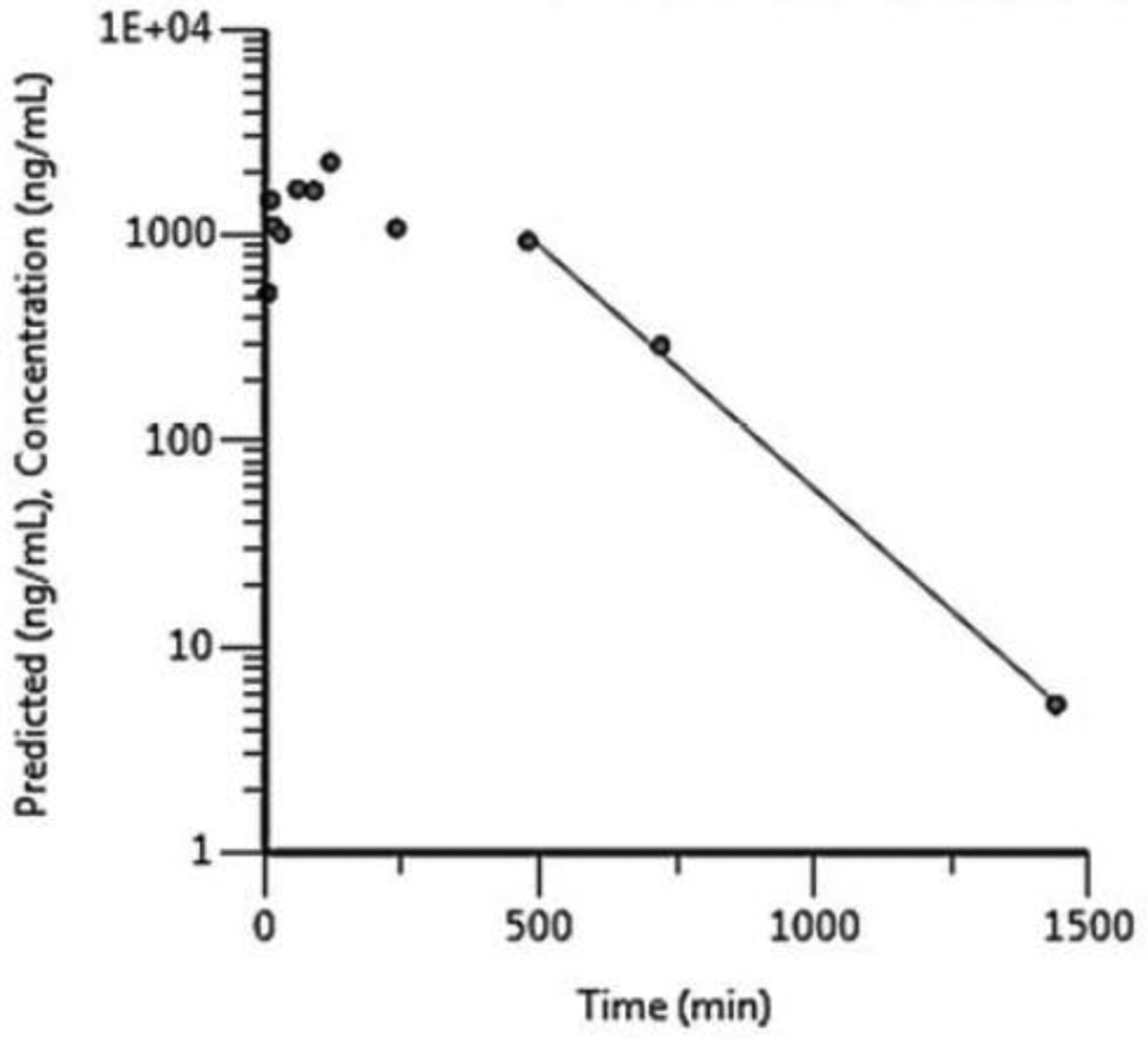
Plasma concentration versus time profiles for terpene trilactones following a single gavage administration of 300 mg/kg Ginkgo biloba extract in male F344/NCrl rats. A) ginkgolide A, B) ginkgolide B, C) ginkgolide C, D) ginkgolide J, and E) bilobalide.
Table 2.
Plasma toxicokinetic parameters for selected constituents following a single gavage administration of Ginkgo biloba extract in male F344/NCrl ratsa
| Analyte | Dose (mg/kg) | Tmax_obsb (min) | Cmax_obs (ng/mL) | Half-life (min) | AUClast (min*ng/mL) | AUC ∞ (min*ng/mL) |
|---|---|---|---|---|---|---|
| BLL | 30 | 15 | 326 | 230 | 132000 | 148000 |
| 100 | 120 | 714 | 162 | 301000 | 319000 | |
| 300 | 120 | 2280 | 127 | 880000 | 881000 | |
| GLA | 30 | 15 | 127 | 230 | 36900 | 41600 |
| 100 | 60 | 277 | 156 | 95100 | 99100 | |
| 300 | 10 | 781 | 186 | 233000 | 251000 | |
| GLB | 30 | 90 | 23.2 | 197 | 9020 | 9820 |
| 100 | 60 | 65.2 | 169 | 24400 | 25800 | |
| 300 | 90 | 138 | 154 | 62600 | 62700 | |
| GLC | 30 | 240 | 9.1 | NDc | 3790 | ND |
| 100 | 60 | 38.3 | 134 | 11500 | 11800 | |
| 300 | 120 | 60.7 | 226 | 22500 | 22700 | |
| GLJd | 100 | 60 | 9.9 | ND | 2000 | ND |
| 300 | 120 | 19.9 | ND | 6350 | ND | |
| ISR | 30 | 15 | 5.92 | 3320 | 6710 | 25700 |
| 100 | 720 | 6.81 | ND | 8430 | ND | |
| 300 | 480 | 24.6 | ND | 18000 | ND | |
| QCT | 30 | 120 | 8.15 | 2120 | 4780 | 12400 |
| 100 | 10 | 13.2 | 570 | 13500 | 17100 | |
| 300 | 480 | 63.4 | ND | 43400 | ND |
Estimated using non-compartmental analysis
Tmax-obs, observed to reach maximum concentration; Cmax-obs, observed maximum concentration; AUClast, area under the concentration versus time curve to last timepoint; AUC∞, area under the concentration versus time curve to infinity; BLL, bilobalide; GLA, ginkgolide A; GLB, ginkgolide B; GLC, ginkgolide C; GLJ, ginkgolide J
ND, not determined. Elimination phase could not be defined and therefore half-life and AUC∞ could not be estimated
Concentrations for 30 mg/kg were below the detection limit of the assay (5 ng/mL)
Flavonols:
All three flavonols were detected in plasma from rats. Quercetin was quantifiable in all samples from the study. Isorhamnetin was also quantifiable in all samples from the study, however, the analytical method was less precise for isorhamnetin than for quercetin (see methods). Kaempferol was not detected in plasma from 30 and 100 mg/kg dose groups; in the 300 mg/kg dose group only 4 time points had quantifiable levels of kaempferol. This was unexpected given that the GBE preparation used had kaempferol levels similar to quercetin and higher than isorhamnetin levels (16.71% quercetin, 12.20% kaempferol, and 2.37% isorhamnetin) and may reflect the lower analytical method sensitivity for kaempferol compared to the other 2 analytes (LOD: quercetin, 1 ng/mL; kaempferol, 40 ng/mL; isorhamnetin, 5 ng/mL). For these reasons, kaempferol data are not reported.
Plasma concentration-versus-time plots for quercetin and isorhamnetin are given in Figure 3 for the 300 mg/kg group as an example. Toxicokinetic data are presented in Table 2. For 300 mg/kg quercetin and 100 and 300 mg/kg isorhamnetin, plasma elimination half-life and AUC∞ could not be estimated because the elimination phase could not be well defined due to insufficient timepoints above the LOD for those analytes. For both analytes, Cmax and AUC∞ increased with dose (AUClast was used in the absence of AUC∞) (Table 2). At 300 mg/kg dose, the rank order for Cmax and AUC∞ was quercetin > isorhamnetin, which is the same as their respective abundance within the GBE preparation used in the study (NTP, 2013).
Figure 3.
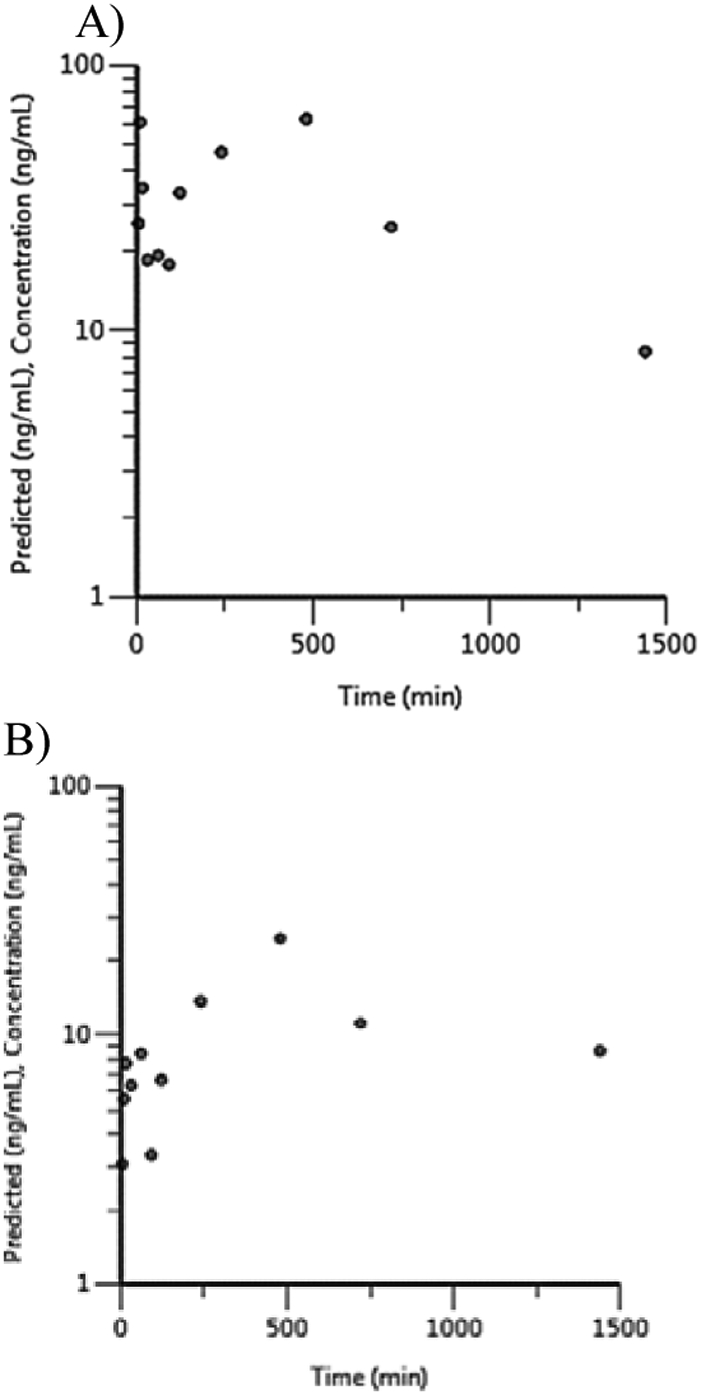
Plasma concentration versus time profiles for flavonols following a single gavage administration of 300 mg/kg Ginkgo biloba extract in male F344/NCrl rats. A) quercetin B) isorhamnetin
Plasma elimination half-lives for quercetin were ~35 and 9.5 h, respectively, following a single administration of 30 and 100 mg/kg, suggesting a decrease in half-life with increasing dose. Plasma elimination half-life for isorhamnetin at 30 mg/kg was ~55 h and was similar to that of quercetin at a similar dose.
Discussion
There are numerous GBE preparations including tinctures, capsules, and tablets, which can contain GBE alone or in combination with other botanical ingredients in the marketplace. Despite the availability of specifications for standardized GBE (22–27% flavonol glycosides, 5–7% terpene trilactones and ≤ 5 ppm ginkgolic acids), the composition varies significantly between different GBE products (Fransen et al., 2010). The variation can arise from numerous sources including differences in raw plant material, storage, extraction procedures, as well as both intentional (e.g., drugs) or economically-motivated (e.g., alternate plant species or less expensive material) adulteration (Shipkowski et al., 2018). The complex chemistry of GBE combined with variability of products in the marketplace have made selecting a representative GBE product for safety testing challenging. In the current investigation we 1) designed a study to evaluate the systemic exposure to GBE constituents following dosing with the GBE preparation used in the NTP 2-year bioassay and compared the data to values from the literature from a study using a standardized GBE preparation, and 2) compared the NTP 2-year bioassay doses to human exposure levels using systemic exposure parameters in our rodent study and human data from the literature to determine the relationship between the NTP 2-year doses and recommended doses in people.
In F344/NCrl rats, terpene trilactones were absorbed rapidly following exposure of rats to a single dose of GBE, with both Cmax and AUC∞ of GBE constituents increasing with dose. Interestingly, systemic exposure parameters for terpene trilactones reflected the abundance of these constituents in the GBE preparation suggesting disposition of these constituents in the rat was similar. Plasma elimination half-lives estimated for terpene trilactones in the current study (~ 2 to 4 hours) were similar to those reported for rats previously (~ 2 to 7 hours) (Zheng et al., 2016; Biber and Koch, 1999; summarized in Ude et al., 2013). The analytical method did not perform as well for the flavonols. The accuracy and precision were lower for kaempferol and hence, data for this analyte were not reported. Based on the data generated for quercetin and isorhamnetin, systemic exposure of flavonols also tracked with the abundance of the flavonols in the GBE preparation fairly well, with quercetin showing higher values than isorhamnetin. The reported plasma elimination half-lives (19–24 h) following a single administration of 600 mg/kg GBE in rats (Rangel-Ordóñez et al., 2010) were in the same range as reported in the current study (9.5 – 55 h).
The systemic exposure data obtained from the current study for terpene trilactones were compared, after normalizing to the dose of GBE administered, with two published studies where male rats were administered a single oral dose of standardized GBE preparations (Biber, 2003; Zheng et al., 2016) (Table 3). In this comparison, the dose-normalized Cmax was ≤ 3-fold for bilobalide, ≤ 2-fold for ginkgolide A and were similar for ginkgolide B between studies. Similarly, dose-normalized AUC∞ was ≤ 5-fold for bilobalide, ≤ 3.5-fold for ginkgoliide A, and ≤ 2-fold for ginkgolide B between studies (Table 3). These findings demonstrate the similarities between the GBE preparations used between studies.
Table 3.
Comparison of dose-normalized systemic exposure parameters for selected terpene trilactones in rats using different Ginkgo biloba extract preparations
| Species | Dose (mg/kg) | Dose-normalized Cmax (ng/mL)/(mg/kg) | Dose-normalized AUC∞ (ng.min/mL)/(mg/kg) | ||||
|---|---|---|---|---|---|---|---|
| BLLa | GLA | GLB | BLL | GLA | GLB | ||
| Ratb | 30 | 10.9 | 4.2 | 0.77 | 4933 | 1387 | 327 |
| 100 | 7.1 | 2.8 | 0.65 | 3190 | 991 | 258 | |
| 300 | 7.6 | 2.6 | 0.46 | 2937 | 837 | 209 | |
| Ratc | 30 | 5.3 | 2.3 | 1.3 | 994 | 441 | 286 |
| 55 | 5.0 | 2.7 | 1.3 | 968 | 425 | 277 | |
| 100 | 4.0 | 3.0 | 1.0 | 1199 | 467 | 221 | |
| Ratd | 25 | 4.1 | 2.1 | 0.8 | 922 | 399 | 179 |
BLL, bilobalide; GLA, ginkgolide A; GLB, ginkgolide B
Data from the current study. GBE used was Shanghai Xing Ling Science and Technology Pharmaceutical Company, Ltd. (lot # GBE-50–001003) (NTP, 2013)
Data from Biber and Koch (1999). GBE used was EGB761®. Dose-normalized values shown are after adjusting for comparable units for Cmax and AUC∞ to the current study
Data from Zheng et al., 2016. GBE used was standardized Ginaton. Dose-normalized values shown are after adjusting for comparable units to the current study
Next, the systemic exposure data obtained from our rat study were compared with data available in the literature following exposure of humans to a clinically-relevant dose of standardized GBE, EGB761®. A single clinical dose of 240 mg in a 70 kg-person translates to 3.4 mg GBE/kg/day. When human and rat doses were compared, based on mg/kg dose, rat doses of 30 and 300 mg/kg/day were 9- and 88-fold higher than the relevant human dose of 3.4 mg/kg/day, respectively (Table 4). To account for different metabolic rates of the two species, the mg/kg/day doses were converted to mg/m2/day, using a Km value of 6 for rats and 37 for humans (Reagan-Shaw et al., 2008). Based on the mg/m2/day dose, a rat dose of 100 mg/kg was only 5 times higher than the clinically relevant daily dose of 240 mg (3.4 mg/kg/day) (Table 4). A similar comparison was made between rats and humans using AUC∞ data available in humans for terpene trilactones (Biber, 2003), which takes into account species differences in ADME. For example, comparison of rat data to human data indicates that a 30 mg/kg dose in rats is equivalent to a typical 240 mg/day dose in humans based on ginkgolide B, but with ginkgolide A and bilobalide, a 30 mg/kg dose in rats is 3- and 10-fold higher, respectively than the 240 mg/day dose in humans. When a similar comparison was made using rat data for 100 mg/kg dose, the rat/human exposure multiple ranged from 3 to 22, depending on the terpene trilactone used for comparison. The estimated rat/human exposure multiple was 3 to 8 when data previously reported by Biber and Koch (1999) in Sprague Dawley rats using EGb761® was used for this comparison, suggesting minimal difference in the 2 types of GBE preparations used (Table 4). Preliminary work that has compared 12 GBE constituents including terpene trilactones, ginkgolic acids, ginkgotoxin and flavonols to hepatotoxicity markers showed that the terpene trilactones correlate with increased liver weight and cholesterol in rats, as well as associated changes in gene expression (unpublished data). Since the observed hepatotoxicity could be due to a combination of the terpene trilactones, it may be more relevant to use total levels of bilobalide, ginkgolide A, ginkgolide B, and ginkgolide C to estimate the totality of exposure because the disposition of individual terpene trilactones in the class may vary between animals and humans. However, it should be noted that other GBE constituents, namely ginkgolic acids have also been shown to elicit cytotoxicity in liver cells in vitro (Qing-Qing, 2018) and hepatotoxicity in rats at doses of 100 or 900 mg/kg ginkgolic acid extract (Qian et al., 2017).
Table 4.
Comparison of exposure multiples between ratsa and humansb for selected terpene trilactones following ingestion of Ginkgo biloba extract
| Rat doses | Exposure multiple (rat/human)c | |||||
|---|---|---|---|---|---|---|
| mg GBE/kg | mg GBE/m2d | mg GBE/kg | mg GBE/m2 | AUC | ||
| BLLe | GLA | GLB | ||||
| 30 | 180 | 9 | 1.4 | 10 | 3 | 1 |
| 100 | 600 | 30 | 5 | 22 | 8 | 3 |
| 300 | 1800 | 88 | 14 | 59 | 20 | 7 |
Data used was from the current study
Data from Biber (2003) following ingestion of 240 mg EGB761® in adults was used. Assuming a 70 kg body weight, this corresponds to 3.4 mg/kg b.wt or 126 mg/m2, using a Km value of 37 for humans (Reagan-Shaw et al., 2008). AUC values reported in this study for BLL, GLA, and GLB, respectively, were: 247.1, 211.1, and 140.69 h*ng/mL (or 14826, 12666 and 8441 min*ng/mL)
Rat/human exposure multiple was estimated 3 ways: mg/kg, mg/m2 and AUC
Dose in mg/m2 was estimated by multiplying mg/kg by a Km value of 6 for rats (Reagan-Shaw et al, 2008)
BLL, bilobalide; GLA, ginkgolide A; GLB, ginkgolide B
Taken collectively, these data demonstrate that systemic exposure to constituents from the GBE preparation used in our studies were similar to those of standardized GBE preparations. In addition, systemic exposure to GBE-specific terpene trilactones, following a 100 mg GBE/kg/day dose in rats, where liver effects were observed, was similar to a single clinically-relevant dose of 240 mg (3.4 mg/kg/day) in humans, demonstrating the relevance of NTP rodent toxicity data to humans. The question of whether the mechanism of toxicity and observed effects in animal studies are relevant to humans requires further consideration by the risk assessment community.
Acknowledgements
The authors are grateful to Dr. Kristen Ryan and Mr. Brad Collins for reviewing the manuscript and Ms. Molly Vallant for managing the Ginkgo biloba extract animal study. Ginkgo biloba extract formulations were prepared by MRIGlobal for the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services, under contract number HHSN273201400022C. Animal study and data analysis was performed by Battelle Memorial Institute (Columbus, OH) for the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services, under contract numbers HHSN273201400027C and HHSN273201400015C.
Footnotes
Declaration of Interest
The authors report no conflicts of interest
References
- Agha AM, El-Fattah AAA, Al-Zuhair HH, Al-Rikabi AC, 2001. Chemopreventive effect of Ginkgo biloba extract against benzo(a)pyrene-induced forestomach carcinogenesis in mice: Amelioration of doxorubicin cardiotoxicity. Journal of Experimental & Clinical Cancer Research 20, 39–50. [PubMed] [Google Scholar]
- Biber A,. 2003. Pharmacokinetics of Ginkgo biloba extracts. Pharmacopsychiatry. 36 Suppl 1:S32–7. [DOI] [PubMed] [Google Scholar]
- Biber A, Koch E, 1999. Bioavailability of ginkgolides and bilobalide from extracts of Ginkgo biloba using GC/MS. Planta Med. 65(2):192–3. [DOI] [PubMed] [Google Scholar]
- Brinkley TE, Lovato JF, Arnold AM, Furberg CD, Kuller LH, Burke GL, Nahin RL, Lopez OL, Yasar S, Williamson JD, 2010. Effect of Ginkgo biloba on Blood Pressure and Incidence of Hypertension in Elderly Men and Women. Am J Hypertens. 23, 528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wan L, Couch L, Lin H, Li Y, Dobrovolsky VN, Mei N, Guo L, 2013. Mechanism study of goldenseal-associated DNA damage. Toxicol Lett. 221(1): 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Li L, Xu F, Sun Y, Du F, Ma X, Zhong C, Li X, Wang F, Zhang N Li C, 2013. Systemic and cerebral exposure to and pharmacokinetics of flavonols and terpene lactones after dosing standardized Ginkgo biloba leaf extracts to rats via different routes of admistration. Britsh J. Pharm 170; 440–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL, 2015. Trends in the use of complementary health approaches among adults: United States, 2002–2012. National Health Statistics Reports. 79:1–16. [PMC free article] [PubMed] [Google Scholar]
- DeFeudis FV, 2003. A brief history of EGb761 and its therapeutic uses. Pharmacopsychiatry 36, S2–S7. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, Lopez OL, Burke G, Carlson MC, Fried LP, Kuller LH, Robbins JA, Tracy RP, Woolard NF, Dunn L, Snitz BE, Nahin RL, Furberg CD, Ginkgo Evaluation of Memory (GEM) Study Investigators, 2008. Ginkgo biloba for Prevention of Dementia: A Randomized Controlled Trial. JAMA. 300, 2730–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond BJ, Shiflett SC, Feiwei N, Matheis RJ, Noskin O, Richards JA, Schoenbergrr NE, 2000. Ginkgo biloba extract: Mechanisms and clinical indications. Arch Phys Med Rehabil. 81, 668–678. [DOI] [PubMed] [Google Scholar]
- Ding S, Dudley E, Chen L, Plummer S, Tang J, Newton RP, Brenton AG, 2006. Determination of active components of Ginkgo biloba in human urine by capillary high-performance liquid chromatography/mass spectrometry with on-line column-switching purification. Rapid Commun Mass Spectr. 20(24):3619–24. [DOI] [PubMed] [Google Scholar]
- Drago F, Floriddia ML, Cro M, Giuffrida S, 2002. Pharmacokinetics and bioavailability of a Ginkgo biloba extract. J Ocul Pharmacol Ther. 18(2):197–202. [DOI] [PubMed] [Google Scholar]
- Fourtillan JB, Brisson AM, Girault J, Ingrand I, Decourt JP, Drieu K, Jouenne P, Biber A, 1995. Pharmacokinetic properties of Bilobalide and Ginkgolides A and B in healthy subjects after intravenous and oral administration of Ginkgo biloba extract (EGb 761). Therapie. 50(2):137–44. [PubMed] [Google Scholar]
- Fransen HP, Pelgrom SM, Stewart-Knox B, de Kaste D, Verhagen H, 2010. Assessment of health claims, content, and safety of herbal supplements containing Ginkgo biloba. Food Nutr Res. 30:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh LM, Barlow PJ, 2002. Antioxidant capacity in Ginkgo biloba. Food Research International 35, 815–820. [Google Scholar]
- Guan H, Qian D, Ren H, Zhang W, Nie H, Shang E, Duan J 2014. Interactions of pharmacokinetic profile of different parts from Ginkgo biloba extracts in rats. Journal of Ethnopharmacology. 155, 758–68. [DOI] [PubMed] [Google Scholar]
- Kressmann S, Biber A, Wonnemann M, Schug B, Blume HH, Müller WE, 2002. Influence of pharmaceutical quality on the bioavailability of active components from Ginkgo biloba preparations. J Pharm Pharmacol. 54(11):1507–14. [DOI] [PubMed] [Google Scholar]
- Lee YW, Lin JA, Chang CC, Chen YH, Liu PL, Lee AW, Tsai JC, Li CY, Tsai CS, Chen TL, Lin FY, 2011. Ginkgo biloba extract suppresses endotoxin-mediated monocyte activation by inhibiting nitric oxide- and tristetraprolin-mediated toll-like receptor 4 expression. J Nutr Biochem 22, 351–359. [DOI] [PubMed] [Google Scholar]
- Leistner E, Drewke C. Ginkgo biloba and ginkgotoxin. 2009. J Nat. Prod, 73(1):86–92. [DOI] [PubMed] [Google Scholar]
- Liebermann HR, Kellogg MD, Fulgoni VL, Agrawak S, 2017. Moderate doses of commercial preparations of Ginkgo biloba do not alter markers of liver function but moderate alcohol intake does: A new approach to identify and quantify biomarkers of ‘adverse effects’ of dietary supplements. Regul Toxicol Pharmacol. 84, 45–53. [DOI] [PubMed] [Google Scholar]
- Liu Z, Hu M, 2007. Natural polyphenol disposition via coupled metabolic pathways. Expert Opin Drug Metab Toxicol. 3:389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri P, De Palma A, Pozzi F, Basilico F, Riva A, Morazzoni P, Bombardelli E, Rossoni G, 2006. LC-MS characterization of terpene lactones in plasma of experimental animals treated with Ginkgo biloba extracts Correlation with pharmacological activity. J Pharm Biomed Anal. 40(3):763–8. [DOI] [PubMed] [Google Scholar]
- Mauri P, Simonetti P, Gardana C, Minoggio M, Morazzoni P, Bombardelli E, Pietta P, 2001. Liquid chromatography/atmospheric pressure chemical ionization mass spectrometry of terpene lactones in plasma of volunteers dosed with Ginkgo biloba L. extracts. Rapid Commun Mass Spectr. 15(12):929–34. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program (NTP), 2013. NTP Technical Report on the Toxicology and Carcinogenesis Studies of Ginkgo biloba Extract (CAS No. 90045-36-6) in F344/N Rats and B6C3F1/N Mice. Natl Toxicol Program Tech Rep Ser. 578. [PubMed] [Google Scholar]
- National Research Council (2011) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press. [Google Scholar]
- Pietta PG, Gardana C, Mauri PL, Maffei-Facino R, Carini M, 1995. Identification of flavonoid metabolites after oral administration to rats of a Ginkgo biloba extract. J Chromatogr B Biomed Appl. 673(1):75–80. [DOI] [PubMed] [Google Scholar]
- Pietta PG, Gardana C, Mauri PL, 1997. Identification of Gingko biloba flavonol metabolites after oral administration to humans. J Chromatogr B Biomed Sci Appl. 693(1):249–55. [DOI] [PubMed] [Google Scholar]
- Qian Y, Peng Y, Shang E, Zhao M, Yan L, Zhu A, Tao J, Su S, Guo S, Duan J (2017). Metabolic profiling of the hepatotoxicity and nephrotoxicity of ginkgolic acids in rats using ultra-performance liquid chromatography-high definitin mass spectrometry. Chemico-Biological Interactions 273: 11–17. [DOI] [PubMed] [Google Scholar]
- Qing-Qing Y, Li L, Ming-Cheng X, Hai-Hong H, Hui Z, Lu-Shan Y and Su Z (2018). The metabolism and hepatotoxicity of ginkgolic acid (17:1) in vitro. Chinese J of Natural Med 16: 829–837. [DOI] [PubMed] [Google Scholar]
- Rangel-Ordóñez L, Nöldner M, Schubert-Zsilavecz M, Wurglics M, 2010. Plasma levels and distribution of flavonoids in rat brain after single and repeated doses of standardized Ginkgo biloba extract EGb 761. Planta Medica. 76(15):1683–90. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N, 2008. Dose translation from animal to human studies revisited. FASEB. 22(3):659–661. [DOI] [PubMed] [Google Scholar]
- Rider CV, Nyska A, Cora MC, Kissling GE, Smith C, Travlos GS, Hejtmancik MR, Fomby LM, Colleton CA, Ryan MJ, Kooistra L, Morrison JP, Chan PC, 2014. Toxicity and carcinogenicity studies of Ginkgo biloba extract in rat and mouse: liver, thyroid, and nose are targets. Toxicol Pathol 42:830–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi R, Basilico F, Rossoni G, Riva A, Morazzoni P, Mauri PL, 2009. Liquid chromatography/atmospheric pressure chemical ionization ion trap mass spectrometry of bilobalide in plasma and brain of rats after oral administration of its phospholipidic complex. J Pharm Biomed Anal. 50(2):224–7. [DOI] [PubMed] [Google Scholar]
- Salvador RL 1995. Herbal medicine - ginkgo. Can. Pharmacists J 52, 39–41. [Google Scholar]
- Shipkowski KA, Betz JM, Birnbaum LS, Bucher JR, Coates PM, Hopp DC, Mackay D, Oketch-Rabah H, Walker NJ, Welch C, Rider CV, 2018. Naturally Complex: Perspectives and Challenges Associated with Botanical Dietary Supplement Safety Assessment. Food Chem Toxicol . ( 10.1016/j.fct.2018.04.007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Kaur P, Gopichand RD, and Ahuja PS. 2008. Biology and chemistry of Ginkgo biloba. Fitoterapia. 79(6):401–18. [DOI] [PubMed] [Google Scholar]
- Smith T, Kawa K, Eckl V, Morton C, and Strednev R, 2018. Herbal supplement sales in US increased 8.5% in 2017 topping $8 billion. HerbalGram. 119:62–71. [Google Scholar]
- Snitz BE, O’merrera ES, Carlson MC, Arnold AM, Ives DG, Rapp SR, Saxton J, Lopez L, Dunn LO, Sink KM, Dekosky ST, Ginkgo Evaluation of Memory (GEM) Study Investigators, 2009. Ginkgo biloba for Preventing Cognitive Decline in Older Adults. A Randomized Trial. JAMA. 302, 2663–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitges M, Aldana BI, Reed RC 2016. Effect of the anti-depressant sertraline, the novel anti-seizure drug vinpocetine and several conventional antiepileptic drugs on the epileptiform EEG activity induced by 4-aminopyridine. Neurochem Res 41: 1365–1374. [DOI] [PubMed] [Google Scholar]
- Ude C, Paulke A, Nöldner M, Schubert-Zsilavecz M, Wurglics M, 2011. Plasma and brain levels of terpene trilactones in rats after an oral single dose of standardized Ginkgo biloba extract EGb 761. Planta Medica. 77(3):259–64 [DOI] [PubMed] [Google Scholar]
- Ude C, Schubert-Zsilavecz M, Wurglics M, 2013. Ginkgo biloba extracts: a review of the pharmacokinetics of the active ingredients. Clin Pharmacokinet. 52: 727–749. [DOI] [PubMed] [Google Scholar]
- van Beek TA, 2002. Chemical analysis of Ginkgo biloba leaves and extracts. Journal of Chromatography A. 967, 21–55. [DOI] [PubMed] [Google Scholar]
- Wang DL, Liang Y, Chen WD, 2008. Identification of ginkgolide B metabolites in urine and liver cytochrome P450 enzymes responsible for their formation in vitro. Acta Paharmacol Sin. 29:376–384. [DOI] [PubMed] [Google Scholar]
- Woelkart K, Feizlmayr E, Dittrich P, Beubler E, Pinl F, Suter A, Bauer R, 2010. Pharmacokinetics of bilobalide, ginkgolide A and B after administration of three different Ginkgo biloba L. preparations in humans. Phytother Res. 24(3):445–50. [DOI] [PubMed] [Google Scholar]
- Woerdenbag HJ, and De Smet PAGM (2000). Adverse effects of and toxicity of Ginkgo extracts In Ginkgo biloba (Beek T.A. van, Ed.), pp. 544–555.Harwood Academic Publishers, Amsterdam. [Google Scholar]
- Wójcicki J, Gawrońska-Szklarz B, Bieganowski W, Patalan M, Smulski HK, Samochowiec L, Zakrzewski J, 1995. Comparative pharmacokinetics and bioavailability of flavonoid glycosides of Ginkgo biloba after a single oral administration of three formulations to healthy volunteers. Mater Med Pol 27(4):141–6. [PubMed] [Google Scholar]
- Zheng B, Teng L, Xing G, Bi Y, Yang S, Hao F, Yan G, Wang X, Lee RJ, Teng L, Xie J, 2015. Proliposomes containing a bile salt for oral delivery of Ginkgo biloba extract: formulation optimization, characterization, oral bioavailability and tissue distribution in rats. Eur J of Pharma Sci. 77:254–264. [DOI] [PubMed] [Google Scholar]
- Zheng B, Xing G, Bi Y, Yan G, Wang J, Cheng Y, Liu Y, Ahraf MA, and Xie J, 2016. Comparative pharmacokinetics of proliposome formulation of Ginkgo biloba extract and Ginton in rats by a sensitive ultra performance liquid chromatography-tandem mass spectrometry method. Saudi J. of Bio Sci 23: 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]


