Abstract
Objective
To systematically review and provide information on the incidence of psoriasis and quantify global, regional, and country specific estimates of its prevalence.
Design
Systematic review and meta-analysis.
Data sources
Medline, Embase, Web of Science, SciELO, Korean Journal Databases, Russian Science Citation Index, WPRIM, SaudiMedLit, Informit, IndMed, and HERDIN were searched systematically from their inception dates to October 2019.
Methods
Studies were included if they reported on the incidence or prevalence of psoriasis in the general population. Incidence data were summarised descriptively, whereas bayesian hierarchical models were fitted to estimate the global, regional, and country specific prevalence of psoriasis.
Results
41 164 records were identified and 168 studies met the inclusion criteria. In adults, the incidence of psoriasis varied from 30.3 per 100 000 person years (95% confidence interval 26.6 to 34.1) in Taiwan to 321.0 per 100 000 person years in Italy. The prevalence of psoriasis varied from 0.14% (95% uncertainty interval 0.05% to 0.40%) in east Asia to 1.99% (0.64% to 6.60%) in Australasia. The prevalence of psoriasis was also high in western Europe (1.92%, 1.07% to 3.46%), central Europe (1.83%, 0.62% to 5.32%), North America (1.50%, 0.63% to 3.60%), and high income southern Latin America (1.10%, 0.36% to 2.96%).
Conclusions
Eighty one per cent of the countries of the world lack information on the epidemiology of psoriasis. The disease occurs more frequently in adults than in children. Psoriasis is unequally distributed across geographical regions; it is more frequent in high income countries and in regions with older populations. The estimates provided can help guide countries and the international community when making public health decisions on the appropriate management of psoriasis and assessing its natural history over time.
Systematic review registration
PROSPERO CRD42019160817.
Introduction
Psoriasis is a chronic, immune mediated inflammatory skin disease,1 consisting of red, scaly plaques occurring most commonly on the elbows, knees, scalp, and lower back, but any skin surface can be involved. The condition greatly affects people’s quality of life to the extent that it could be life ruining and stigmatising.2 Psoriasis is now considered a systemic disease; it is associated with psychological, metabolic, arthritic, and cardiovascular comorbidities. Lifespan is reduced as a consequence.3 In addition to the psychological and social burden related to psoriasis, the cost to patients and healthcare systems is high.4 Psoriasis can occur at any age, although most patients present with the condition before 35 years old.5 In 2014 the World Health Organization recognised psoriasis as a serious non-communicable disease6 and the accompanying WHO report (2016)6 emphasised the need to better understand the global burden of the disease. To address this need, the Global Psoriasis Atlas (www.globalpsoriasisatlas.org) was established to conduct further research into the global prevalence and incidence of psoriasis, thereby helping to ensure better access to care for people with the disease.
Studies that report information on the incidence of psoriasis are limited. Current estimates come from Europe and North America, and mainly report on the incidence of psoriasis in adults or in the overall population. Additionally, several studies have reported on the prevalence of psoriasis. Earlier study specific estimates of the prevalence of psoriasis in adults range between 0.27% (95% confidence interval 0.17 to 0.36)7 and 11.4%,8 with age, sex, geography, ethnicity, genetic and environmental factors contributing to the variation in the prevalence of the disease.9 Higher prevalence rates have been reported at higher latitudes and in white people compared with other ethnic groups.10
The Global Burden of Disease group has generated estimates on the prevalence of psoriasis for 21 regions of the world,11 and more recently for the global population.12 These estimates relate to wider regions,11 12 but not to individual countries. Over the past decade there has been an increase in research examining the epidemiology of psoriasis, particularly in countries where estimates were lacking. Recent studies have often been derived from better data quality resources, many of them extracting information from large, routinely collected and electronic health databases, which are more nationally representative than previous studies.
Given the existing gaps in knowledge about the epidemiology of psoriasis, the aim of the study was to perform a systematic review and meta-analysis examining the incidence and prevalence of the disease. Additionally, the study aimed to generate global, regional, and country specific estimates of the prevalence of psoriasis.
Methods
The systematic review and statistical model follow the PRISMA (preferred reporting items for systematic reviews and meta-analyses) and GATHER (guidelines for accurate and transparent health estimates reporting) guidelines (supplementary material 4, eTables 12 and 13, respectively).
Study design
We conducted a systematic review of the incidence and prevalence of psoriasis that involved several steps. These steps included data identification and extraction; a descriptive summary of incidence data; and a statistical analysis to generate estimates of the global, regional, and country specific prevalence of psoriasis by using the information extracted from the included studies examining the prevalence of the disease.
Data identification and extraction
Search strategy
We systematically searched 11 electronic and regional databases (Medline, Embase, Web of Science, SciELO, Korean Journal Databases, Russian Science Citation Index, WPRIM, SaudiMedLit, Informit, IndMed, and HERDIN) from their respective inception dates to October 2019. The main search terms were “psoriasis” (“psoriatic skin,” “pustulosis”), “incidence” (“incident studies” or “cohort studies,” or “longitudinal studies”), and “prevalence” (“prevalent studies” or “cross-sectional studies”). Supplementary material 1 gives full details about the search strategy. We also screened the references of all included studies and published review articles to identify any additional eligible studies.
Inclusion and exclusion criteria
We included studies if they reported on the prevalence or incidence of psoriasis in the general population. No language restrictions were applied. We also included studies that estimated the prevalence or incidence of other skin or autoimmune diseases, but provided data on psoriasis. Studies that used dermatology clinic case series, or specific subgroups of the population, or only focused on psoriatic arthritis were excluded.
Data extraction
In the first stage, two authors (RP and IYKI) independently screened the titles and abstracts identified from searching the databases for eligibility. Eligible papers were critically appraised and those meeting the inclusion criteria were selected for data extraction. We critically appraised all included studies for risk of bias by using the appraisal tool for cross-sectional studies (AXIS).13 This quality assessment tool comprises 20 items that cover several domains: identification of research aim, appropriateness of study design, use of valid measures and statistical analyses, and consideration of bias. Supplementary material 1 (text 2.5) and supplementary material 3 (eTable 14) give full details about the AXIS tool, the items included, and the risk of bias assessment. Studies were classified as having high, medium, or low risk of bias, or were rated as unclear according to the quality of the methods used and results reported in the study. The two authors independently made these judgments, and reached consensus on the final rating if needed.
Analysis of incidence data
Because a limited number of studies reported on the incidence of psoriasis, these were summarised descriptively. We report incidence rates per 100 000 person years (95% confidence intervals). Results were analysed by country and age category (children, adults, or all ages), and if possible, we explored variation in the incidence of psoriasis within each country and age category.
Statistical analysis of prevalence data
After data extraction, we developed a filtering process for inclusion of studies in the statistical model because some studies used the same data resource. To prevent duplication, we included studies with the most recent or complete data on the variable of interest, or the most robust in terms of the methods used (supplementary material 1, eTable 2).
We fitted a bayesian hierarchical linear mixed model to estimate the global, regional, and country specific prevalence of psoriasis. This type of statistical model is considered the gold standard when data are sparse and heterogeneous.14 In the bayesian hierarchical model, estimates of the prevalence of psoriasis were informed by study data from the same country, if available, and by study data from other countries. We briefly describe the model below; supplementary material 2 (text 4) provides a full description.
The outcome was the log transformed prevalence of psoriasis, which allowed us to use a linear model and ensure predictions within the 0-100% range when back transformed. We mapped countries according to the Global Burden of Disease classification: 189 countries were nested in 21 regions, and regions were nested in seven super regions (table 1), the hierarchy of which mainly follows geography and income. High levels of heterogeneity in the global prevalence of psoriasis were expected for several reasons: varying age strata, or whether the prevalence was estimated in children, adults, or the overall population; type of diagnostic method, or whether the diagnosis was made by a physician, a dermatologist, or was self-reported; and type of estimate, or whether the estimate was calculated as point, period, or lifetime prevalence.8 Therefore, the hierarchical model had four levels (global, super regions, regions, and countries), four random intercepts for these, and three fixed covariates: age strata, type of diagnostic method (physician, dermatologist, or self-reported diagnosis), and type of prevalence measure (point, period, or lifetime prevalence). Age strata included children, adults, or the overall population (children and adults combined).
Table 1.
Countries and territories in analysis regions. The table gives the full details of the geographical groups and hierarchy used in the statistical model. The classification is the same as the one used by the Global Burden of Disease and by the United Nations.15 The hierarchy mainly follows geography and income
| Region | Countries |
|---|---|
| Central Europe, eastern Europe, and central Asia | |
| Asia, central | Armenia, Azerbaijan, Georgia, Kazakhstan, Kyrgyzstan, Mongolia, Tajikistan, Turkmenistan, Uzbekistan |
| Europe, central | Albania, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Hungary, Montenegro, Poland, Romania, Serbia, Slovakia, Slovenia, TFYR Macedonia |
| Europe, eastern | Belarus, Estonia, Latvia, Lithuania, Moldova, Russia, Ukraine |
| High income | |
| Asia Pacific, high income | Brunei Darussalam, Japan, Republic of Korea, Singapore |
| Australasia | Australia, New Zealand |
| Europe, western | Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, United Kingdom |
| Latin America, southern | Argentina, Chile, Uruguay |
| North America, high income | Canada, United States of America |
| Latin America and Caribbean | |
| Caribbean | Antigua and Barbuda, Bahamas, Barbados, Belize, Cuba, Dominican Republic, Grenada, Guyana, Haiti, Jamaica, Puerto Rico, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago, Virgin Island (US) |
| Latin America, Andean | Bolivia, Ecuador, Peru |
| Latin America, central | Colombia, Costa Rica, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Venezuela (Bolivarian Republic of) |
| Latin America, tropical | Brazil, Paraguay |
| North Africa and Middle East | |
| North Africa and the Middle East | Afghanistan, Algeria, Bahrain, Egypt, Iran (Islamic Republic of), Iraq, Jordan, Kuwait, Lebanon, Libyan Arab Jamahiriya, Morocco, Occupied Palestinian Territory, Oman, Qatar, Saudi Arabia, Sudan, Syrian Arab Republic, Sudan, Tunisia, Turkey, United Arab Emirates, Yemen |
| South Asia | |
| Asia, south | Bangladesh, Bhutan, India, Nepal, Pakistan |
| South East Asia, east Asia, and Oceania | |
| Asia, east | China, Dem. People’s Republic of Korea, Taiwan |
| Asia, South East | Cambodia, Indonesia, Lao People’s Democratic Republic, Malaysia, Maldives, Mauritius, Myanmar, Philippines, Seychelles, Sri Lanka, Thailand, Timor-Leste, Vietnam |
| Oceania | Fiji, Guam, Kiribati, Marshall Islands, Micronesia (Fed. States of), Papua New Guinea, Samoa, Solomon Islands, Tonga, Vanuatu |
| Sub-Saharan Africa | |
| Sub-Saharan Africa, central | Angola, Central African Republic, Congo, Democratic Republic of the Congo, Equatorial Guinea, Gabon |
| Sub-Saharan Africa, eastern | Burundi, Comoros, Djibouti, Eritrea, Ethiopia, Kenya, Madagascar, Malawi, Mozambique, Rwanda, Somalia, South Sudan, Uganda, United Republic of Tanzania, Zambia |
| Sub-Saharan Africa, southern | Botswana, Lesotho, Namibia, South Africa, Swaziland, Zimbabwe |
| Sub-Saharan Africa, western | Benin, Burkina Faso, Côte d’Ivoire, Cameroon, Cape Verde, Chad, Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Mali, Mauritania, Niger, Nigeria, São Tomé and Príncipe, Senegal, Sierra Leone, Togo |
We used geographical clustering in the model to inform and generate estimates for countries with missing information. In particular, for countries with no data available, the model estimates were based on borrowed evidence from higher levels. For example, the region estimate value was used for the country estimate when no country specific data were available; the super region estimate value was used for countries when no data were available within a region. It is also noteworthy that the region and super region estimates are always obtained from studies identified at the country level; therefore there are no regional or super regional level studies. Supplementary material 2 (eTables 6-11) reports information on countries with observed or missing data.
The statistical model was fitted using bayesian inference, sampling from the posterior distribution over the parameters by using the Hamiltonian Markov chain Monte Carlo method. We used four chains of 2000 iterations each to run the model. After fitting the model, posterior predictions were made for each country and age strata permutation. We provide prevalence estimates in the context of 95% uncertainty intervals. To obtain the number of people affected by psoriasis by country, each country specific prevalence estimate was multiplied by the size of its population; we used the United Nations population structure for the year 2017.15 We assessed the fit of each model by evaluating the measures relative to the effective sample size and autocorrelation, and the trace plots (supplementary material 2, eFig 2). All analyses were performed in R (version 3.6.1) by using the RStanArm package, which relies on Stan.
Patient and public involvement
The International Federation of Psoriasis Associations (IFPA) are a collaborating organisation on the Global Psoriasis Atlas. Members of IFPA include people with psoriasis and our work plans and findings were presented during Global Psoriasis Atlas Steering Group meetings at which IFPA members asked questions about the study design and findings.
Results
We identified 41 164 records by searching the databases. Of the 308 papers that were critically appraised and assessed for eligibility, 168 reported on the incidence or prevalence of psoriasis in the general population (supplementary material 1, eFig 1). Specifically, nine studies focused on the incidence of psoriasis (eTable 1), 145 reported on the prevalence of psoriasis (eTable 2), and 14 studies reported on both incidence and prevalence (eTable 1).
Incidence of psoriasis
The 23 studies that reported on the incidence of psoriasis in the general population were conducted mainly in western and eastern Europe (16 studies), and North America (six studies). Twelve studies reported on the incidence of psoriasis in all ages, with the incidence of the disease varying from 31.4 per 100 000 person years in eastern Europe (Russia)16 to 521.1 per 100 000 person years in western Europe (Germany).17 Springate and colleagues3 reported a slight decline in the incidence of psoriasis in all ages in the United Kingdom (from 159.0 to 129.0 per 100 000 person years between 1999 and 2013). Kubanova and colleagues18 also reported a slight decline in the incidence in Russia (from 69.8 to 65.0 per 100 000 person years between 2010 and 2016). However, the incidence of psoriasis in Denmark has been inconsistent, with a decrease from 140.1 to 104.0 per 100 000 person years between 2003 and 2005, followed by an increase to 181.0 in 2010, which then decreased to 151.2 in 2012 (table 2).19
Table 2.
List of studies providing incidence rates in children, adults, and all ages
| Study, country, study period | Diagnostic method | Age of population | No with psoriasis | Incidence rate per 100 000 person years (95% CI) | ||
|---|---|---|---|---|---|---|
| Entire group | Female population | Male population | ||||
| Children | ||||||
| Cantarutti et al (2015), Italy | ||||||
| 2006-12 | Family paediatrician | ≤14 | — | — | — | — |
| 2006 | 61.0 (50.0 to 80.0)* | — | — | |||
| 2007 | 45.0 (30.0 to 60.0)* | — | — | |||
| 2008 | 54.0 (40.0 to 70.0)* | — | — | |||
| 2009 | 53.0 (40.0 to 70.0)* | — | — | |||
| 2010 | 53.0 (40.0 to 70.0)* | — | — | |||
| 2011 | 40.0 (30.0 to 50.0)* | — | — | |||
| 2012 | 57.0 (40.0 to 80.0)* | — | — | |||
| Tollefson et al (2010), US | ||||||
| 1970-99 | Dermatologist or physician | <18 | 357 | 40.8 (36.6 to 45.1)*† | 43.9 (37.6 to 50.2)*† | 37.9 (32.2 to 43.6)*† |
| 1970-74 | 29.6 (20.9 to 38.3)*† | — | — | |||
| 1975-79 | 35.7 (25.9 to 45.5)*† | — | — | |||
| 1980-84 | 31.4 (22.0 to 40.8)*† | — | — | |||
| 1985-89 | 42.7 (31.8 to 53.7)*† | — | — | |||
| 1990-94 | 40.0 (29.7 to 50.3)*† | — | — | |||
| 1995-99 | 62.7 (50.4 to 65.0)*† | — | — | |||
| Adults | ||||||
| Eder et al (2017), Canada | ||||||
| 2000-15 | Physician | ≥20 | — | — | — | — |
| 2000 | 114.0 (112.0 to 116.0)*† | — | — | |||
| 2001 | 111.0 (109.0 to 113.0)*† | — | — | |||
| 2002 | 103.0 (101.0 to 105.0)*† | — | — | |||
| 2003 | 101.0 (99.0 to 103.0)*† | — | — | |||
| 2004 | 101.0 (99.0 to 103.0)*† | — | — | |||
| 2005 | 97.0 (95.0 to 99.0)*† | — | — | |||
| 2006 | 97.0 (95.0 to 99.0)*† | — | — | |||
| 2007 | 96.0 (94.0 to 98.0)*† | — | — | |||
| 2008 | 96.0 (94.0 to 98.0)*† | — | — | |||
| 2009 | 95.0 (93.0 to 97.0)*† | — | — | |||
| 2010 | 95.0 (93.0 to 97.0)*† | — | — | |||
| 2011 | 98.0 (96.0 to 100.0)*† | — | — | |||
| 2012 | 100.0 (98.0 to 102.0)*† | — | — | |||
| 2013 | 105.0 (103.0 to 107.0)*† | — | — | |||
| 2014 | 102.0 (100.0 to 104.0)*† | — | — | |||
| 2015 | 105.0 (103.0 to 107.0)*† | — | — | |||
| Eder et al (2019), Canada | ||||||
| 2000-15 | Physician | ≥20 | — | — | — | — |
| 2000 | 9873 | 111.1 (108.9 to 113.4)*† | — | — | ||
| 2001 | 9849 | 108.6 (106.5 to 110.8)*† | — | — | ||
| 2002 | 9203 | 99.7 (97.6 to 101.8)*† | — | — | ||
| 2003 | 9111 | 97.3 (95.3 to 99.3)*† | — | — | ||
| 2004 | 9060 | 95.3 (93.3 to 97.3)*† | — | — | ||
| 2005 | 8645 | 89.8 (87.9 to 91.8)*† | — | — | ||
| 2006 | 8979 | 92.7 (90.7 to 94.7)*† | — | — | ||
| 2007 | 8601 | 87.6 (85.7 to 89.6)*† | — | — | ||
| 2008 | 8585 | 85.9 (84.0 to 87.8)*† | — | — | ||
| 2009 | 8610 | 85.2 (83.4 to 87.1)*† | — | — | ||
| 2010 | 8399 | 81.8 (80.0 to 83.7)*† | — | — | ||
| 2011 | 8868 | 85.0 (83.2 to 86.9)*† | — | — | ||
| 2012 | 8883 | 83.9 (82.1 to 85.7)*† | — | — | ||
| 2013 | 8789 | 82.1 (80.3 to 83.9)*† | — | — | ||
| 2014 | 8212 | 76.1 (74.4 to 77.9)*† | — | — | ||
| 2015 | 7541 | 68.7 (67.1 to 70.3)*† | — | — | ||
| Vena et al (2010), Italy | ||||||
| 2001-05 | Physician | ≥18 | 5792 | — | — | — |
| 2001 | 321.0* | 291.0* | 357.0* | |||
| 2005 | 230.0* | 207.0* | 254.0* | |||
| Pezzolo et al (2019), Italy | ||||||
| 2003-04 | Self-reported diagnosis | ≥25 | — | 302 (232 to 392)* | 380 (270 to 535)* | 296 (197 to 443)* |
| Wei et al (2018), Taiwan | ||||||
| 2001 | — | ≥16 | 333 | 42.0 (37.5 to 46.5)*† | — | — |
| 2002 | 320 | 40.1 (35.7 to 44.5)*† | — | — | ||
| 2003 | 329 | 41.7 (37.2 to 46.3)*† | — | — | ||
| 2004 | 370 | 46.9 (42.1 to 51.8)*† | — | — | ||
| 2005 | 272 | 33.8 (29.7 to 37.8)*† | — | — | ||
| 2006 | 325 | 39.7 (35.3 to 44.1)*† | — | — | ||
| 2007 | 295 | 35.7 (31.5 to 39.8)*† | — | — | ||
| 2008 | 291 | 34.1 (30.1 to 38.2)*† | — | — | ||
| 2009 | 322 | 37.8 (33.5 to 42.0)*† | — | — | ||
| 2010 | 316 | 36.7 (32.5 to 40.9)*† | — | — | ||
| 2011 | 338 | 40.3 (35.8 to 44.8)*† | — | — | ||
| 2012 | 327 | 37.2 (33.0 to 41.5)*† | — | — | ||
| 2013 | 273 | 30.3 (26.6 to 34.1)*† | — | — | ||
| Khalid et al (2013), UK | ||||||
| 2007-09 | Physician | ≥18 | 10 832 | 280.0 (280.0 to 290.0)*† | — | — |
| Tillett et al (2017), UK | ||||||
| 1998-2014 | Physician | ≥18 | 88 858 | 183.0 (182.0 to 184.0)* | 186.0 (184.0 to 188.0)* | 179.0 (178.0 to 181.0)* |
| Shbeeb et al (1995), US | ||||||
| 1982-91 | — | — | — | 78.0 (70.0 to 86.0)*† | — | — |
| Icen et al (2009), US | ||||||
| 1970-2000 | Dermatologist or physician | ≥18 | 1633 | 78.9 (75.0 to 82.9)*†‡ | 73.2 (68.0 to 78.4)*†‡ | 85.5 (79.5 to 91.6)*†‡ |
| 1970-74 | 50.8 (41.9 to 59.6)*†‡ | — | — | |||
| 1975-79 | 53.2 (44.8 to 61.6)*†‡ | — | — | |||
| 1980-84 | 80.9 (70.8 to 91.1)*†‡ | — | — | |||
| 1985-89 | 78.9 (69.5 to 88.4)*†‡ | — | — | |||
| 1990-94 | 88.7 (79.1 to 98.3)*†‡ | — | — | |||
| 1995-99 | 100.5 (90.8 to 110.2)*†‡ | — | — | |||
| All ages | ||||||
| Egeberg et al (2017), Denmark | ||||||
| 2003-12 | Physician | 0-70+ | — | — | — | — |
| 2003 | 140.1 (137.1 to 143.2)* | 146.8* | 133.4* | |||
| 2004 | 122.2 (119.4 to 125.1)* | 130.7* | 113.6* | |||
| 2005 | 104.0 (101.4 to 106.7)* | 107.5* | 100.5* | |||
| 2006 | 105.5 (102.9 to 108.2)* | 110.4* | 100.4* | |||
| 2007 | 111.5 (108.7 to 114.2)* | 110.8* | 112.2* | |||
| 2008 | 128.6 (125.7 to 131.6)* | 128.8* | 128.4* | |||
| 2009 | 174.8 (171.4 to 178.3)* | 192.6* | 156.8* | |||
| 2010 | 181.0 (177.5 to 184.5)* | 199.5* | 162.3* | |||
| 2011 | 171.3 (167.9 to 174.7)* | 187.9* | 154.5* | |||
| 2012 | 151.2 (148.0 to 154.5)* | 165.9* | 136.4* | |||
| Jacob et al (2016), Germany | ||||||
| 2007-10 | Physician | — | 14 686 | 521.1* | — | — |
| Sewerin et al (2019), Germany | ||||||
| 2009 | — | — | — | — | 46.3 to 58.2* | 35.4 to 50.3* |
| 2010 | 35.3 to 45.6* | 26.4 to 39.4* | ||||
| 2011 | 21.7 to 30.5* | 17.3 to 29.3* | ||||
| 2012 | 19.1 to 26.4* | 17.1 to 26.3* | ||||
| Shalom et al (2018), Israel | ||||||
| 2016 | — | — | — | 246 (241 to 251)*† | — | — |
| 2017 | 243 (239 to 248)*† | — | — | |||
| Schonmann et al (2019), Israel | ||||||
| 2011 | Dermatologist | 0-85+ | 9770 | 282 (276 to 288)*† | 268 (261 to 276)*† | 296 (287 to 305)*† |
| 2012 | 9796 | 278 (272 to 284)*† | 273 (265 to 281)*† | 283 (275 to 292)*† | ||
| 2013 | 10 430 | 291 (285 to 297)*† | 278 (270 to 286)*† | 304 (295 to 312)*† | ||
| 2014 | 10 072 | 276 (271 to 282)*† | 263 (256 to 271)*† | 290 (281 to 298)*† | ||
| 2015 | 10 033 | 273 (267 to 279)*† | 257 (250 to 265)*† | 289 (280 to 297)*† | ||
| 2016 | 10 505 | 281 (276 to 287)*† | 269 (261 to 276)*† | 294 (286 to 302)*† | ||
| 2017 | 10 489 | 276 (270 to 281)*† | 263 (256 to 271)*† | 288 (280 to 296)*† | ||
| Znamenskaya et al (2012), Russia | ||||||
| 2009 | Physician | 0-18+ | 99 988 | 70.5* | — | — |
| 2010 | 99 348 | 69.8* | — | — | ||
| 2011 | 99 436 | 69.6* | — | — | ||
| Kubanova et al (2017), Russia | ||||||
| 2010 | Physician | 0-18+ | — | 69.8* | — | — |
| 2011 | 69.6* | — | — | |||
| 2012 | 68.4* | — | — | |||
| 2013 | 65.9* | — | — | |||
| 2014 | 64.7* | — | — | |||
| 2015 | 62.8* | — | — | |||
| 2016 | 65.0* | — | — | |||
| Odinets et al (2017), Russia | ||||||
| 2010 | Physician | 0-18+ | 1180 | 42.5* | — | — |
| 2011 | 1136 | 40.8* | — | — | ||
| 2012 | 1257 | 45.1* | — | — | ||
| 2013 | 875 | 31.4* | — | — | ||
| 2014 | 945 | 33.8* | — | — | ||
| 2015 | 941 | 33.6* | — | — | ||
| 2016 | 1094 | 39.0* | — | — | ||
| Donker et al (1998), Netherlands | ||||||
| 1987-88 | Physician | 0-65+ | 106 | 130.0 (120.0 to 140.0)*† | — | — |
| Donker et al (1998), Netherlands | ||||||
| 1995 | Physician | 0-65+ | 24 | 120.0 (70.0 to 190.0)*† | — | — |
| Huerta et al (2007), UK | ||||||
| 1996-97 | Physician | 0-80+ | 3994 | 140.0* | — | — |
| Springate et al (2017), UK | ||||||
| 1999-2013 | Physician | 0-100 | — | — | — | — |
| 1999 | 4279 | 159.0 (155.0 to 164.0)*† | 161.0 (155.0 to 168.0)*† | 158.0 (151.0 to 165.0)*† | ||
| 2000 | 5398 | 163.0 (158.0 to 167.0)*† | 162.0 (156.0 to 169.0)*† | 163.0 (157.0 to 170.0)*† | ||
| 2001 | 6286 | 164.0 (160.0 to 168.0)*† | 163.0 (157.0 to 168.0)*† | 166.0 (160.0 to 172.0)*† | ||
| 2002 | 7259 | 170.0 (166.0 to 174.0)*† | 174.0 (169.0 to 180.0)*† | 166.0 (161.0 to 172.0)*† | ||
| 2003 | 7977 | 172.0 (168.0 to 176.0)*† | 178.0 (173.0 to 183.0)*† | 166.0 (161.0 to 172.0)*† | ||
| 2004 | 8209 | 166.0 (163.0 to 170.0)*† | 170.0 (165.0 to 175.0)*† | 163.0 (158.0 to 168.0)*† | ||
| 2005 | 8522 | 165.0 (162.0 to 169.0)*† | 173.0 (168.0 to 178.0)*† | 158.0 (153.0 to 163.0)*† | ||
| 2006 | 8499 | 161.0 (158.0 to 165.0)*† | 169.0 (164.0 to 174.0)*† | 154.0 (149.0 to 159.0)*† | ||
| 2007 | 8807 | 165.0 (162.0 to 168.0)*† | 170.0 (165.0 to 175.0)*† | 160.0 (155.0 to 165.0)*† | ||
| 2008 | 8964 | 163.0 (160.0 to 167.0)*† | 165.0 (160.0 to 170.0)*† | 162.0 (157.0 to 167.0)*† | ||
| 2009 | 8518 | 155.0 (152.0 to 158.0)*† | 159.0 (154.0 to 163.0)*† | 152.0 (147.0 to 156.0)*† | ||
| 2010 | 7715 | 143.0 (140.0 to 146.0)*† | 145.0 (141.0 to 150.0)*† | 140.0 (136.0 to 145.0)*† | ||
| 2011 | 7499 | 140.0 (137.0 to 143.0)*† | 144.0 (140.0 to 149.0)*† | 135.0 (131.0 to 140.0)*† | ||
| 2012 | 6992 | 131.0 (128.0 to 134.0)*† | 136.0 (132.0 to 141.0)*† | 126.0 (122.0 to 130.0)*† | ||
| 2013 | 6350 | 129.0 (126.0 to 133.0)*† | 131.0 (127.0 to 136.0)*† | 127.0 (123.0 to 132.0)*† | ||
| Bell et al (1991), US | ||||||
| 1980-83 | Dermatologist or physician | 0-70+ | 132 | 59.9 (49.5 to 70.3)*† | 63.6 (48.9 to 78.3)*† | 58.4 (42.8 to 74.1)*† |
Values reported from the study.
Age or sex adjusted.
Rate adjusted with linear interpolation between census years.
Only two studies, from Italy and the United States, explored the incidence of psoriasis in children over a seven year and 30 year period, respectively (table 2).20 21 While Tollefson and colleagues21 reported a steady rise in the incidence of psoriasis in the US between 1970 and 2000, Cantarutti and colleagues20 found that the incidence of psoriasis in Italy was stable between 2006 and 2012 (table 2).
The incidence of psoriasis was higher in adults than in children, varying from 30.3 per 100 000 person years (95% confidence interval 26.6 to 34.1)22 in Taiwan to 321.0 per 100 000 person years in Italy.23 While the data show a steadily increasing trend in incidence of psoriasis in adults in the US between 1970 and 2000 (table 2),24 data from Canada, Italy, and Taiwan show a slightly decreasing trend in incidence over time.22 23 25
Variation in the incidence of psoriasis by age and sex
The incidence of psoriasis in children increased with age from 13.5 per 100 000 person years (0-3 years old) to 53.1 per 100 000 person years (14-18 years old; table 3).21 Despite higher estimates of psoriasis incidence from the UK26 27 than the US,24 28 all of these studies showed a similar trend of increasing psoriasis incidence up to 39 years of age. The incidence then decreased at 40-49 years before increasing again with a second peak at around 50-59 years (UK27) or 60-69 years (UK26 and US24 28). The incidence of psoriasis decreased towards the end of life.
Table 3.
List of studies providing incidence rates -by sex and age groups
| Study, country, and study period | Diagnostic method | Age of population | No with psoriasis | Incidence rate per 100 000 person years (95% CI) | ||
|---|---|---|---|---|---|---|
| Entire group | Female population | Male population | ||||
| Children | ||||||
| Tollefson et al (2010), US | ||||||
| 1970-99 | Dermatologist or physician | <18 | 357 | 40.8 (36.6 to 45.1)*† | 43.9 (37.6 to 50.2)*† | 37.9 (32.2 to 43.6)*† |
| 0-3 | 27 | 13.5* | 13.2* | 13.7* | ||
| 4-7 | 84 | 42.2* | 40.2* | 44.1* | ||
| 8-10 | 69 | 44.0* | 55.7* | 33.2* | ||
| 11-13 | 75 | 52.2* | 49.6* | 54.6* | ||
| 14-17 | 102 | 53.1* | 61.9* | 44.7* | ||
| Adults | ||||||
| Pezzolo et al (2019), Italy | ||||||
| 2003-04 | Self-reported diagnosis | ≥25 | — | 302 (232 to 392)* | 380 (270 to 535)* | 296 (197 to 443)* |
| <35 | — | 186 (95 to 365)* | 236 (101 to 554)* | 137 (45 to 412)* | ||
| 35-44 | — | 342 (193 to 605)* | 251 (98 to 642)* | 433 (211 to 889)* | ||
| 45-54 | — | 219 (110 to 435)* | 339 (156 to 739)* | 96 (22 to 419)* | ||
| 55-64 | — | 385 (223 to 665)* | 492 (252 to 961)* | 269 (105 to 690)* | ||
| 65-74 | — | 420 (253 to 697)* | 395 (199 to 782)* | 456 (214 to 969)* | ||
| ≥74 | — | — | — | — | ||
| Khalid et al (2013), UK | ||||||
| 2007-09 | Physician | ≥18 | 10 832 | 280.0 (280.0 to 290.0)*† | — | — |
| 18-29 | — | 350.0 (320.0 to 380.0)* | 250.0 (230.0 to 280.0)* | |||
| 30-39 | — | 320.0 (280.0 to 350.0)* | 290.0 (260.0 to 320.0)* | |||
| 40-49 | — | 220.0 (200.0 to 240.0)* | 220.0 (200.0 to 250.0)* | |||
| 50-59 | — | 310.0 (280.0 to 340.0)* | 320.0 (300.0 to 360.0)* | |||
| 60-69 | — | 310.0 (280.0 to 350.0)* | 370.0 (330.0 to 400.0)* | |||
| 70-79 | — | 290.0 (250.0 to 320.0)* | 290.0 (250.0 to 330.0)* | |||
| ≥80 | — | 160.0 (130.0 to 190.0)* | 180.0 (140.0 to 230.0)* | |||
| Tillett et al (2017), UK | ||||||
| 1998-2014 | Physician | ≥18 | 88 858 | 183.0 (182.0 to 184.0)* | 186.0 (184.0 to 188.0)* | 179.0 (178.0 to 181.0)* |
| 18-29 | 14 292 | 174.0 (171.0 to 177.0)* | 193.0 (188.0 to 197.0)* | 157.0 (154.0 to 161.0)* | ||
| 30-39 | 15 169 | 176.0 (173.0 to 179.0)* | 182.0 (178.0 to 186.0)* | 170.0 (166.0 to 173.0)* | ||
| 40-49 | 15 423 | 164.0 (162.0 to 167.0)* | 158.0 (155.0 to 162.0)* | 170.0 (166.0 to 174.0)* | ||
| 50-59 | 16 218 | 195.0 (192.0 to 198.0)* | 200.0 (196.0 to 204.0)* | 190.0 (186.0 to 194.0)* | ||
| 60-69 | 14 536 | 216.0 (213.0 to 220.0)* | 217.0 (212.0 to 222.0)* | 216.0 (211.0 to 221.0)* | ||
| 70-79 | 9364 | 196.0 (192.0 to 204.0)* | 196.0 (191.0 to 201.0)* | 197.0 (191.0 to 203.0)* | ||
| 80-89 | 3856 | 149.0 (145.0 to 154.0)* | 145.0 (139.0 to 151.0)* | 157.0 (150.0 to 165.0)* | ||
| Icen et al (2009), US | ||||||
| 1970-2000 | Dermatologist or physician | ≥18 | 1633 | 78.9 (75.0 to 82.9)*†‡ | 73.2 (68.0 to 78.4)*†‡ | 85.5 (79.5 to 91.6)*†‡ |
| 18-29 | 444 | 77.4* | 75.6* | 79.4* | ||
| 30-39 | 391 | 81.1* | 69.2* | 93.3* | ||
| 40-49 | 260 | 71.3* | 69.0* | 73.6* | ||
| 50-59 | 230 | 88.0* | 90.7* | 85.2* | ||
| 60-69 | 174 | 94.2* | 76.2* | 115.3* | ||
| 70-79 | 94 | 73.8* | 71.2* | 77.9* | ||
| ≥80 | 40 | 51.4* | 39.8* | 80.0* | ||
| All ages | ||||||
| Schonmann et al (2019), Israel | ||||||
| 2015 | Dermatologist | <1 | — | 23.0* | — | — |
| 1-4 | — | 58.0* | — | — | ||
| 5-14 | — | 117.0* | — | — | ||
| 15-24 | — | 186.0* | — | — | ||
| 25-34 | — | 315.0* | — | — | ||
| 35-44 | — | 299.0* | — | — | ||
| 45-54 | — | 302.0* | — | — | ||
| 55-64 | — | 347.0* | — | — | ||
| 65-74 | — | 350.0* | — | — | ||
| 75-84 | — | 288.0* | — | — | ||
| ≥85 | — | 173.0* | — | — | ||
| Znamenskaya et al (2012), Russia | ||||||
| 2009 | Physician | 0-18+ | 99 988 | 70.5* | — | — |
| 0-14 | 6069 | 28.8* | — | — | ||
| 15-17 | 5864 | 118.2* | — | — | ||
| ≥18 | 88 055 | 76.0* | — | — | ||
| 2010 | 0-18+ | 99 348 | 69.8* | — | — | |
| 0-14 | 6045 | 28.2* | — | — | ||
| 15-17 | 5873 | 128.2* | — | — | ||
| ≥18 | 87 430 | 75.4* | — | — | ||
| 2011 | 0-18+ | 99 436 | 69.6* | — | — | |
| 0-14 | 6104 | 28.0* | — | — | ||
| 15-17 | 5681 | 126.7* | — | — | ||
| ≥18 | 87 651 | 75.2* | — | — | ||
| Kubanova et al (2017), Russia | ||||||
| 2010 | Physician | 0-18+ | — | 69.8* | — | — |
| 0-14 | — | 27.9* | — | — | ||
| 15-17 | — | 127.2* | — | — | ||
| ≥18 | — | 75.4* | — | — | ||
| 2011 | 0-18+ | — | 69.6* | — | — | |
| 0-14 | — | 28.0* | — | — | ||
| 15-17 | — | 126.7* | — | — | ||
| ≥18 | — | 75.2* | — | — | ||
| 2012 | 0-18+ | — | 68.4* | — | — | |
| 0-14 | — | 27.8* | — | — | ||
| 15-17 | — | 118.8* | — | — | ||
| ≥18 | — | 74.2* | — | — | ||
| 2013 | 0-18+ | — | 65.9* | — | — | |
| 0-14 | — | 27.3* | — | — | ||
| 15-17 | — | 115.6* | — | — | ||
| ≥18 | — | 71.7* | — | — | ||
| 2014 | 0-18+ | — | 64.7* | — | — | |
| 0-14 | — | 25.1* | — | — | ||
| 15-17 | — | 108.4* | — | — | ||
| ≥18 | — | 72.4* | — | — | ||
| 2015 | 0-18+ | — | 62.8* | — | — | |
| 0-14 | — | 24.0* | — | — | ||
| 15-17 | — | 99.3* | — | — | ||
| ≥18 | — | 69.6* | — | — | ||
| 2016 | 0-18+ | — | 65.0* | — | — | |
| 0-14 | — | 24.3* | — | — | ||
| 15-17 | — | 95.6* | — | — | ||
| ≥18 | — | 72.6* | — | — | ||
| Huerta et al (2007), UK | ||||||
| 1996-97 | Physician | 0-80+ | 3994 | 140.0* | — | — |
| 0-19 | 116.0* | 121.0* | 110.0* | |||
| 20-29 | 134.0* | 155.0* | 111.0* | |||
| 30-39 | 155.0* | 131.0* | 174.0* | |||
| 40-49 | 116.0* | 105.0* | 128.0* | |||
| 50-59 | 167.0* | 172.0* | 161.0* | |||
| 60-69 | 164.0* | 144.0* | 186.0* | |||
| 70-79 | 163.0* | 118.0* | 224.0* | |||
| ≥80 | 100.0* | 82.0* | 173.0* | |||
| Bell et al (1991), US | ||||||
| 1980-83 | Dermatologist or physician | 0-70+ | 132 | 59.9 (49.5 to 70.3)*† | 63.6 (48.9 to 78.3)*† | 58.4 (42.8 to 74.1)*† |
| <20 | 21 | 30.9* | 47.1* | 14.8* | ||
| 20-29 | 25 | 49.1* | 41.3* | 59.5* | ||
| 30-39 | 25 | 71.7* | 61.2* | 82.9* | ||
| 40-49 | 12 | 51.4* | 58.6* | 43.8* | ||
| 50-59 | 18 | 94.6* | 109.1* | 78.3* | ||
| 60-69 | 17 | 112.6* | 126.5* | 93.8* | ||
| ≥70 | 14 | 77.4* | 54.9* | 130.6* | ||
Values reported from the study.
Age or sex adjusted.
We found a lack of agreement in the published studies about variations in incidence rates by sex. Although the overall incidence rate was higher in girls than in boys aged less than 18 years (43.9 and 37.9 per 100 000 person years, respectively), this pattern was not consistent across all age bands.21 Some studies reported higher incidence in women than in men,3 19 26 28 whereas other studies presented contrasting results.23 24 When we examined the incidence of psoriasis by sex and age bands, the two peaks for age at onset in women were more frequently around 18-29 and 50-59 years, whereas in men they occurred more frequently around 30-39 and 60-69 or 70-79 years.24 26 27 28
Prevalence of psoriasis
The 159 studies that reported on the prevalence of psoriasis in the general population were identified from 12 regions of the world (table 4). Most studies (107; 67%) were conducted in high income countries; these also contributed the highest number of nationally representative studies. Because of overlapping data sources, we included 129 independent data points in the statistical analysis; these came from 35 out of 189 (19%) countries of the world (fig 1). Most studies included in the statistical analyses were graded as low-medium risk of bias (76%; supplementary material 3, eTable 14). Supplementary material 2 (eTables 6-11) reports estimates of lifetime prevalence of psoriasis for the main analyses for each age stratum according to physician or dermatologist diagnosis, and self-reported lifetime prevalence; however, the number of people affected by psoriasis is reported for the adult population only.
Table 4.
Distribution of studies (n=159) reporting on the prevalence of psoriasis according to regions
| Region | No of studies* |
|---|---|
| Western Europe | 97 |
| High income North America | 20 |
| East Asia | 16 |
| North Africa and the Middle East | 18 |
| Central Europe | 6 |
| Tropical Latin America | 5 |
| High income Asia Pacific | 4 |
| Australasia | 3 |
| Eastern Europe | 4 |
| South Asia | 3 |
| South East Asia | 1 |
| Eastern sub-Saharan Africa | 1 |
The number of studies is higher than 159 because some studies reported data on many countries belonging to different regions.
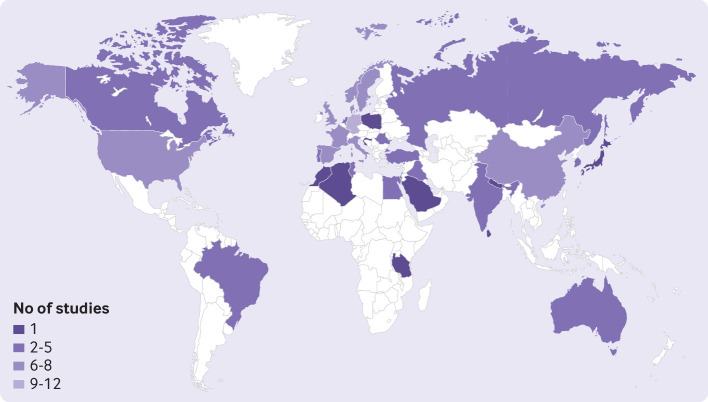
Fig 1.
Distribution of number of studies included in statistical analysis by country. Countries with no observed data are white
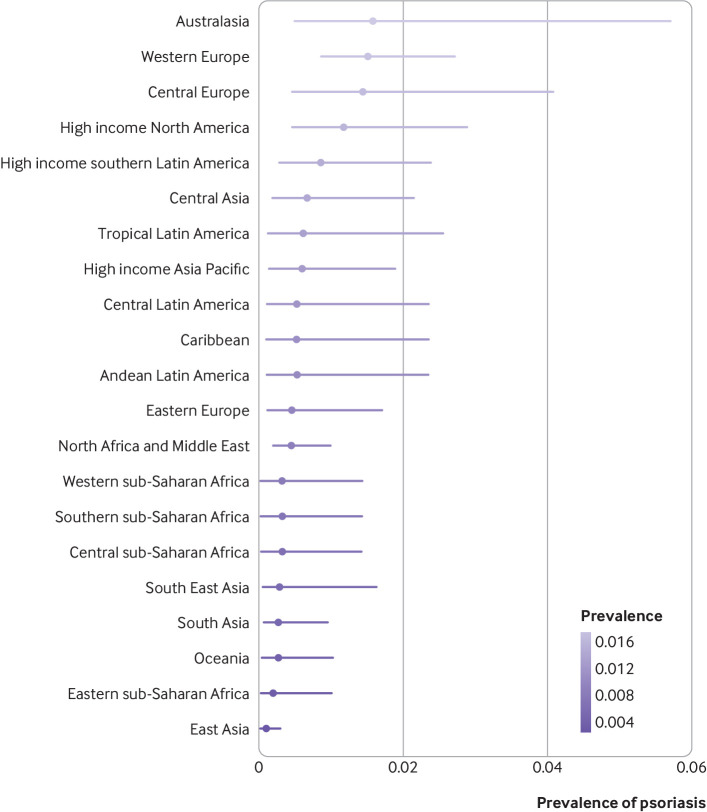
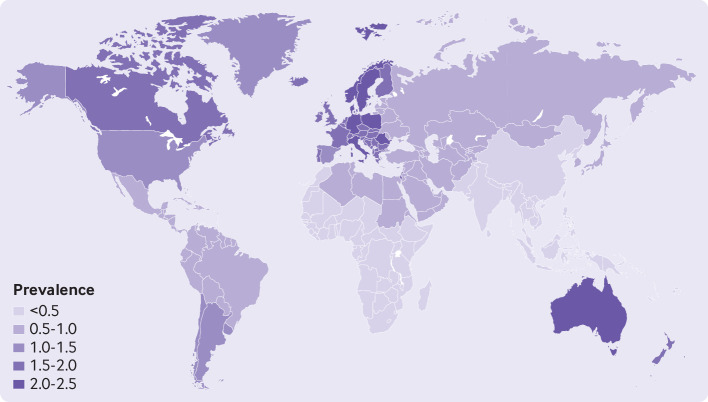
Regionally, the occurrence of the disease for the overall population varied from 0.11% (95% uncertainty interval 0.04% to 0.30%) in east Asia to 1.58% (0.50% to 5.73%) in Australasia, and 1.52% (0.87% to 2.74%) in western Europe (fig 2). Country specific prevalence of psoriasis varied substantially. Considering the estimate for the overall population, Australia (1.88%, 0.59% to 6.10%), Norway (1.86%, 0.94% to 3.97%), Israel (1.81%, 0.83% to 4.44%), and Denmark (1.79%, 0.91% to 3.61%) had the highest estimates of the prevalence of psoriasis (supplementary material 2, eTable 8). The estimated prevalence of psoriasis in countries from east Asia was much lower, Taiwan being the country with the lowest prevalence worldwide (0.05%; 0.02% to 0.16%).
Fig 2.
Crude lifetime (physician or dermatologist diagnosed) prevalence of psoriasis for overall population according to world regions. Regions with observed data: Australasia, central Europe, east Asia, eastern Europe, eastern sub-Saharan Africa, high income Asia Pacific, high income North America, North Africa and the Middle East, south Asia, South East Asia, tropical Latin America, western Europe. Regions with extrapolated data: central Asia, high income southern Latin America, Caribbean, Andean Latin America, central Latin America, Oceania, central sub-Saharan Africa, southern sub-Saharan Africa, western sub-Saharan Africa
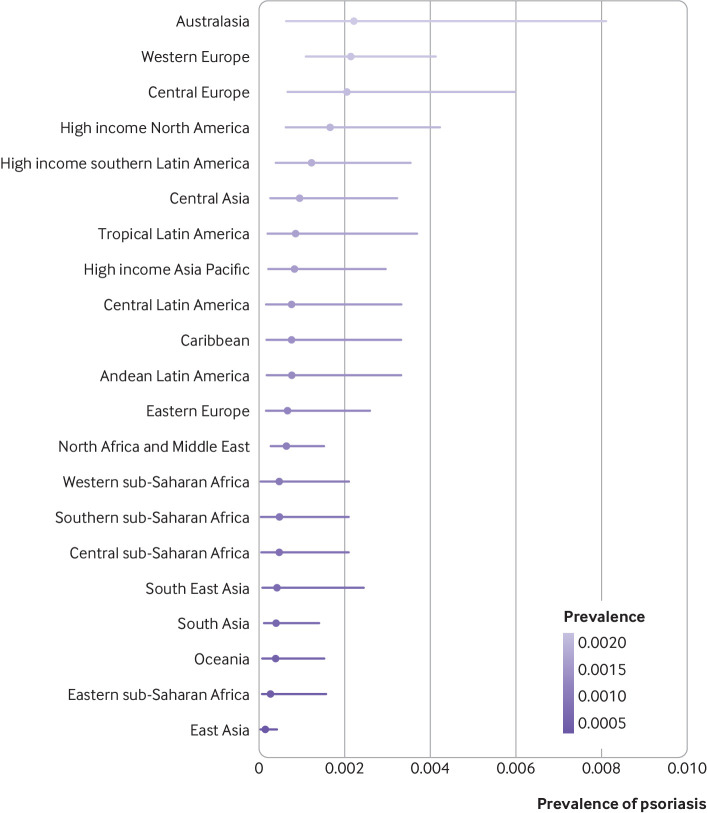
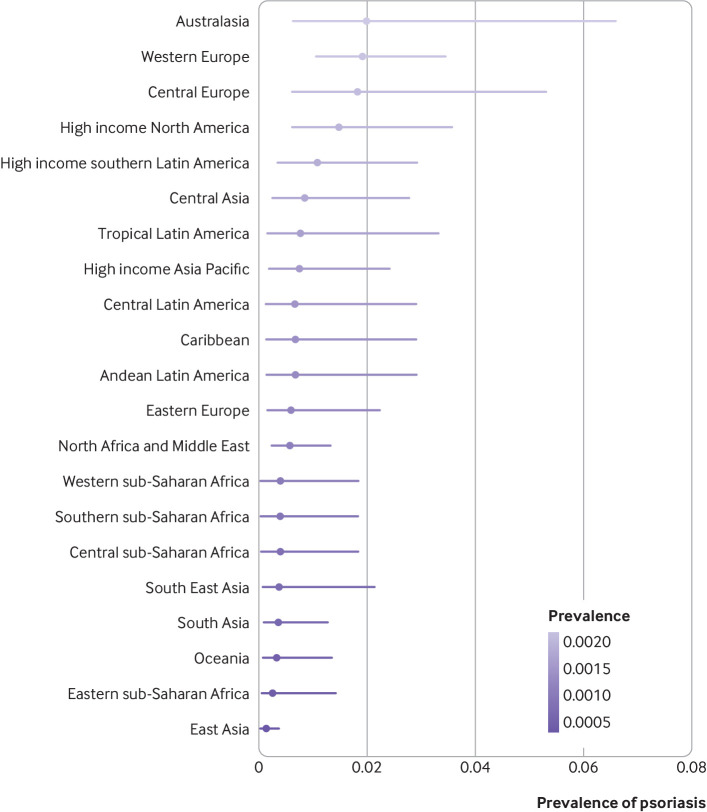
Psoriasis occurred more frequently in adults than in children. The prevalence of psoriasis in children varied from 0.02% (95% uncertainty interval 0.01% to 0.04%) in east Asia to 0.22% (0.06% to 0.81%) in Australasia and 0.21% (0.11% to 0.41%) in western Europe (fig 3). In adults, the disease varied from 0.14% (0.05% to 0.40%) in east Asia to 1.99% (0.64% to 6.60%) in Australasia. Other regions with an occurrence of the disease above 1% were western Europe (1.92%, 1.07% to 3.46%), central Europe (1.83%, 0.62% to 5.32%), high income North America (1.50%, 0.63% to 3.60%), and high income southern Latin America (1.10%, 0.36% to 2.96%; fig 4).
Fig 3.
Crude lifetime (physician or dermatologist diagnosed) prevalence of psoriasis in children according to world regions. Regions with observed data: Australasia, central Europe, east Asia, eastern Europe, eastern sub-Saharan Africa, high income Asia Pacific, high income North America, North Africa and the Middle East, south Asia, South East Asia, tropical Latin America, western Europe. Regions with extrapolated data: central Asia, high income southern Latin America, Caribbean, Andean Latin America, central Latin America, Oceania, central sub-Saharan Africa, southern sub-Saharan Africa, western sub-Saharan Africa
Fig 4.
Crude lifetime (physician or dermatologist diagnosed) prevalence of psoriasis in adults according to world regions. Regions with observed data: Australasia, central Europe, east Asia, eastern Europe, eastern sub-Saharan Africa, high income Asia Pacific, high income North America, North Africa and the Middle East, south Asia, South East Asia, tropical Latin America, western Europe. Regions with extrapolated data: central Asia, high income southern Latin America, Caribbean, Andean Latin America, central Latin America, Oceania, central sub-Saharan Africa, southern sub-Saharan Africa, western sub-Saharan Africa
Given the strong association of psoriasis with age, the prevalence of psoriasis also varied across countries because of differences in regional and country specific age structures. Considering country specific estimates, the physician diagnosed lifetime prevalence of psoriasis in adults was highest in Australia (2.38%, 0.78% to 7.01%), Norway (2.36%, 1.10% to 5.04%), Israel (2.28%, 0.98% to 5.65%), Denmark (2.26%, 1.15% to 4.58%), Romania (2.24%, 0.76% to 6.73%), Germany (2.20%, 1.12% to 4.38%), Sweden (2.10%, 0.94% to 4.74%), Poland (2.06%, 0.61% to 6.58%), and Italy (2.00%, 1.00% to 4.14%; fig 5 and eTable 7, supplementary material 2). However, the countries with the highest number of adults affected were the US (3.4 million, 95% uncertainty interval 1.5 to 7.7 million), India (2.9 million, 0.8 to 10.0 million), China (2.3 million, 0.9 to 6.1 million), Germany (1.5 million, 0.8 to 2.9 million), Brazil (1.2 million, 0.3 to 4.8 million), France (1.0 million, 0.5 to 2.1 million), and the UK (1.0 million, 0.5 to 1.9). Globally, in 2017, an estimated 29.5 million adults had psoriasis, corresponding to a physician diagnosed lifetime prevalence of 0.59% (95% uncertainty interval 0.19% to 1.66%) of the adult population worldwide.
Fig 5.
Lifetime (physician or dermatologist diagnosed) prevalence of psoriasis in adults by country. Details about countries with observed or extrapolated data are given in fig 1 and supplementary material 2 (eTables 6-11)
Additional sources of heterogeneity in the estimates were factors such as type of diagnostic method (self-reported, or diagnosis made by a physician or dermatologist) and type of prevalence estimate (period, point, or lifetime prevalence). Specifically, the self-reported lifetime prevalence of psoriasis for the adult population could be almost twice as high (3.77%, 95% uncertainty interval 1.32% to 12.27% in Australasia, and 3.63%, 2.36% to 5.43% in western Europe) and up to 1.12% (0.38% to 2.84%) globally, affecting approximately 55.8 million adults worldwide. Supplementary material 2 (eTables 6-11) shows the prevalence estimates for the 21 regions and 189 countries, and according to the type of diagnoses (physician, dermatologist, or self-report) and lifetime prevalence.
Discussion
Main findings
This systematic review highlighted that epidemiological data on the incidence of psoriasis are limited, with studies conducted mainly in Europe and North America. Findings revealed consistency across studies about the bimodal age pattern of the onset of the disease. No agreement was found on specific sex differences or trends over time.
The prevalence of the disease was highest in high income countries such as Australasia, western Europe, central Europe, and North America. However, the largest adult populations affected by psoriasis lived in the US, India, and China, followed by Germany, Brazil, France, and the UK. Both the incidence and the prevalence of psoriasis had a strong association with age; the disease was less common in children and occurred more frequently in adults.
Strengths and limitation of the study
The study has several key strengths. Firstly, we undertook an extensive systematic review to search for all available literature since inception by using 11 electronic and regional databases with no restriction on language. Secondly, we used a bayesian framework approach to analyse the data, which is the optimal technique when data are sparse and heterogeneous.14 Thirdly, the statistical model was adjusted for potential sources of heterogeneity, such as age strata, type of diagnostic method, and type of prevalence measure used to calculate the prevalence of the disease. Finally, our analysis generated estimates for 21 regions and 189 countries of the world.
Limitations of the study also need to be acknowledged. Firstly, because of limited information on the incidence of psoriasis, it was not possible to include these data in the statistical model. Secondly, in many low income and middle income countries, only small studies with limited information were available, which resulted in greater uncertainty for some estimates. Thirdly, it was not possible to provide estimates by sex and by five year age bands because of the reporting of the data in the included studies; therefore, no age standardised estimates were calculated. However, by using the information on wide age strata we could estimate the prevalence of psoriasis in children, adults, and the overall population. Fourthly, individual studies provided limited information on their sample sizes; therefore, we could not include this information in the statistical model to give more weight to larger studies. Fifthly, the grouping and hierarchy of the countries within region and super region, which aligns with the Global Burden of Disease methods, are mainly based on geography and income. Therefore, these methods might have influenced the income related patterns highlighted in the findings. Finally, because of the complete lack of information for several countries and regions, in some circumstances we could only use estimates of the wider regions or super regions for countries they were nested in. For this reason, estimates for countries with no data might be helpful in guiding policy makers, healthcare practitioners, and patients, but need to be interpreted with caution. In particular, these estimates might underestimate or overestimate the true values because they have been extrapolated from the regions or super regions the countries are nested in. Nevertheless, given the long term plan of the Global Psoriasis Atlas, future iterations of the model will include new data that will lead to more accurate estimates of the prevalence of psoriasis, particularly in countries with no observed data currently.
Comparison with other studies
Research performing similar analyses is limited.11 12 Hay and colleagues11 provided estimates of the prevalence of psoriasis for 21 regions of the world. Our estimates are lower than their findings, with the main differences in the data sources used. Our systematic review was more extensive and included more recent studies. Furthermore, our statistical model adjusted for important sources of heterogeneity, such as type of diagnostic methods and type of prevalence measure. If we restricted our analysis to the self-reported lifetime prevalence of psoriasis, our estimate of the global prevalence of psoriasis would be similar.12 A major strength of our research compared with Hay and colleagues11 and James and colleagues12 is that we were able to provide a measure of the prevalence of psoriasis for 189 countries. Importantly, data included in our study cover the most comprehensive existing scientific literature identified from 11 electronic and regional databases compared with two electronic databases searched in the studies by Hay and colleagues11 and James and colleagues.12
In contrast to previous suggestions,3 8 we did not find any clear north-south gradient. Conversely, it appeared that the prevalence of psoriasis varied with income, which is similar to the distribution of the disease burden measured as disability adjusted life years (DALYs) by Karimkhani and colleagues.29 Countries located in high income regions had a higher prevalence of psoriasis compared with low income countries and regions. There are several possible explanations for this observed pattern. Firstly, the results might be because high income countries have better healthcare systems, increased awareness of the disease, better data quality, and studies from these countries report data from large population based and nationally representative databases. Secondly, the structure of the hierarchical model used was mainly based on geography and income, which might have exacerbated the income related patterns of the findings. Thirdly, high income countries have a larger proportion of older people in the population, which means life expectancy is higher and so there is an increased prevalence of psoriasis.3 Finally, the lack of access to healthcare for many people with psoriasis will contribute to an underestimate of its prevalence in many least developed countries.
Interpretation of the findings
Our study revealed a high country specific variation in the prevalence of psoriasis. Figures were relatively low in regions with young populations, such as south Asia and sub-Saharan Africa, and figures were relatively high in regions with older populations, such as the high income regions. This finding was because of the strong association between the prevalence of psoriasis and age.
Our main analysis reported estimates of the lifetime prevalence of psoriasis according to physician or dermatologist diagnosis. However, these figures might be underestimates of the true prevalence of the disease because most of the data came from studies using databases that only reflect people with psoriasis who sought healthcare; they might not reflect the underdiagnosed population. Conversely, when the analyses reported lifetime prevalence estimates of self-reported psoriasis, the figures were higher in comparison, but there was a risk of misdiagnosis of the disease. This finding is consistent with a recent study from Denmark, which found that the prevalence of self-reported psoriasis was higher than the physician reported prevalence.30
Conclusions
Data on the incidence and prevalence of psoriasis have increased in recent years. However, considerable gaps exist in the geographical areas that report this information, particularly from low and middle income countries.
Psoriasis is recognised as a chronic and disabling non-communicable disease, and people affected have a highly visible condition that can be stigmatising. Accounting for population growth and ageing, and the fact that psoriasis mainly affects the adult population, the burden associated with psoriasis could continue to rise.
A clear need exists to improve the quality and increase the amount of data on the epidemiology of psoriasis. Methods, diagnostic criteria, and reporting of the incidence and prevalence of the disease should be standardised. An improved understanding of the epidemiology of psoriasis is important so that resources can be allocated to reduce morbidity, disability, and mortality associated with the disease.
What is already known on this topic
Psoriasis is a chronic, disabling skin disease associated with psychological, metabolic, arthritic, and cardiovascular comorbidities
In 2014, the World Health Organization recognised psoriasis as a serious non-communicable disease and its report called for greater understanding of the epidemiology of the disease
The Global Burden of Disease group has produced estimates on the prevalence of psoriasis for 21 regions of the world but not for individual countries
What this study adds
This systematic review and meta-analysis provides global, regional, and country specific estimates of the prevalence of psoriasis
Psoriasis is a common disease that mainly affects the adult population, and is more frequent in high income countries
An improved understanding of the epidemiology of psoriasis is important when allocating resources to reduce morbidity, disability, and mortality associated with the disease
Acknowledgments
The authors are grateful to Seth Flaxman, Imperial College London, for providing helpful advice on the methods. The authors acknowledge the key role played by the Global Psoriasis Atlas (GPA) Collaborating Organisations in the establishment and organisation of the GPA: the International Psoriasis Council; the International Federation of Psoriasis Associations; and the International League of Dermatological Societies. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the GPA Collaborating Organisations. The authors are grateful to Rebekah Swan – GPA Programme Manager. Finally, we acknowledge the enthusiastic collaboration of all of the members of the GPA Board of Governors, Steering Committee, regional coordinators and other dermatologists worldwide who provided the data.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material 1
Web appendix: Supplementary material 2
Web appendix: Supplementary material 3
Web appendix: Supplementary material 4
Contributors: RP and IYKI are joint first authors. IYKI, RP, DMA, and CEMG led on the conception and design of the study. IYKI and RP searched, extracted, and critically appraised the studies included in the systematic review. RP and EK planned and performed the statistical analyses. IYKI and RP wrote the first draft of the manuscript. RP and IYKI contributed equally to the study. All authors contributed to the interpretation of the findings and the final draft of the paper. RP is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The Global Psoriasis Atlas (GPA) project is delivered by the academic project staff based at the University of Manchester and University Medical Center Hamburg-Eppendorf. The GPA is a collaboration between three leading international organisations in the world of dermatology: the International Federation of Psoriasis Associations (IFPA), The International League of Dermatological Societies (ILDS), and the International Psoriasis Council (IPC). The GPA has been supported by grants and sponsorship from the Leo Foundation, Abbvie, Eli Lilly UK, Novartis Pharma AG, UCB, and Almirall. All decisions concerning analysis, interpretation, and publication are made independently of any industrial contribution. DMA and CEMG are funded in part by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Leo Foundation, Abbvie, Eli Lilly UK and Company Ltd, Novartis Pharma AG, UCB, and Almirall for the submitted work; CEMG reports receiving honorariums or research grants from AbbVie, Almirall, Celgene, Eli Lilly, Galderma, Janssen, LEO Pharma, Novartis, Pfizer, Sandoz, Sanofi, and UCB Pharma; DMA reports research grants from AbbVie, Almirall, Celgene, Eli Lilly, Novartis, UCB, and the Leo Foundation; MA reports receiving speakers honorariums or grants from, or participated in clinical trials or health services research projects for Abbott/AbbVie, Almirall, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Forward Pharma, Galderma, GSK, Hexal, Janssen, LEO Pharma, Medac, MSD, Novartis, Pfizer, Sandoz, Teva, TK, Trevi, and Xenoport; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: The raw data are available in the supplementary files.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; and that no important aspects of the study have been omitted; and that any discrepancy from the study as planned have been explained.
Dissemination to participants and related patient and public communities: Findings of the study will be disseminated to patient organisations, specialist and non-specialist audience at international conferences and thorough the Global Psoriasis Atlas website.
Publisher’s note: Published maps are provided without any warranty of any kind, either express or implied. BMJ remains neutral with regard to jurisdictional claims in published maps.
References
- 1. Griffiths CEM, Barker JNWN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370:263-71. 10.1016/S0140-6736(07)61128-3 [DOI] [PubMed] [Google Scholar]
- 2. Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc 2004;9:136-9. 10.1046/j.1087-0024.2003.09102.x [DOI] [PubMed] [Google Scholar]
- 3. Springate DA, Parisi R, Kontopantelis E, Reeves D, Griffiths CEM, Ashcroft DM. Incidence, prevalence and mortality of patients with psoriasis: a U.K. population-based cohort study. Br J Dermatol 2017;176:650-8. 10.1111/bjd.15021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Javitz HS, Ward MM, Farber E, Nail L, Vallow SG. The direct cost of care for psoriasis and psoriatic arthritis in the United States. J Am Acad Dermatol 2002;46:850-60. 10.1067/mjd.2002.119669 [DOI] [PubMed] [Google Scholar]
- 5. Langley RGB, Krueger GG, Griffiths CEM. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis 2005;64(Suppl 2):ii18-23, discussion ii24-5. 10.1136/ard.2004.033217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Global report on psoriasis, 2016. https://apps.who.int/iris/handle/10665/204417
- 7. Li R, Sun J, Ren L-M, et al. Epidemiology of eight common rheumatic diseases in China: a large-scale cross-sectional survey in Beijing. Rheumatology (Oxford) 2012;51:721-9. 10.1093/rheumatology/ker370 [DOI] [PubMed] [Google Scholar]
- 8. Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377-85. 10.1038/jid.2012.339 [DOI] [PubMed] [Google Scholar]
- 9. Enamandram M, Kimball AB. Psoriasis epidemiology: the interplay of genes and the environment. J Invest Dermatol 2013;133:287-9. 10.1038/jid.2012.434 [DOI] [PubMed] [Google Scholar]
- 10. Farber E, Nall M. Epidemiology: natural history and genetics. Dekker, 1998. [Google Scholar]
- 11. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol 2014;134:1527-34. 10.1038/jid.2013.446 [DOI] [PubMed] [Google Scholar]
- 12. James SL, Abate D, Abate KH, et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016;6:e011458. 10.1136/bmjopen-2016-011458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finucane M, Paciorek C, Danaei G, Ezzati M. Bayesian estimation of population-level trends in measures of health status. Stat Sci 2014;29:18-25 10.1214/13-STS427 . [DOI] [Google Scholar]
- 15. United Nations, Department of Economic and Social Affairs, Population Division World population prospects: the 2017 revision. Vol II Demographic Profiles, 2017. [Google Scholar]
- 16. Odinets A. The incidence of skin diseases in Stavropol territory in 2010-2016. Klinicheskaya dermatologiya i venerologiya 2017;16 10.17116/klinderma201716632-37. [DOI] [Google Scholar]
- 17. Jacob C, Meier F, Neidhardt K, et al. Epidemiology and costs of psoriasis in Germany - a retrospective claims data analysis. Value Health 2016;19:A566 10.1016/j.jval.2016.09.1269 . [DOI] [Google Scholar]
- 18. Kubanova A, Kubanov A, Melekhina L, Bogdanova E. The assessment of the incidence of skin disorders in Russian Federation in 2003-2016. Vestn Dermatol Venerol 2017;93:22-33. [Google Scholar]
- 19. Egeberg A, Skov L, Gislason GH, Thyssen JP, Mallbris L. Incidence and prevalence of psoriasis in Denmark. Acta Derm Venereol 2017;97:808-12. 10.2340/00015555-2672 [DOI] [PubMed] [Google Scholar]
- 20. Cantarutti A, Donà D, Visentin F, et al. Pedianet Epidemiology of frequently occurring skin diseases in Italian children from 2006 to 2012: a retrospective, population-based study. Pediatr Dermatol 2015;32:668-78. 10.1111/pde.12568 [DOI] [PubMed] [Google Scholar]
- 21. Tollefson MM, Crowson CS, McEvoy MT, Maradit Kremers H. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol 2010;62:979-87. 10.1016/j.jaad.2009.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wei JC-C, Shi L-H, Huang J-Y, Wu X-F, Wu R, Chiou J-Y. Epidemiology and medication pattern change of psoriatic diseases in Taiwan from 2000 to 2013: a nationwide, population-based cohort study. J Rheumatol 2018;45:385-92. 10.3899/jrheum.170516 [DOI] [PubMed] [Google Scholar]
- 23. Vena GA, Altomare G, Ayala F, et al. Incidence of psoriasis and association with comorbidities in Italy: a 5-year observational study from a national primary care database. Eur J Dermatol 2010;20:593-8. [DOI] [PubMed] [Google Scholar]
- 24. Icen M, Crowson CS, McEvoy MT, Dann FJ, Gabriel SE, Maradit Kremers H. Trends in incidence of adult-onset psoriasis over three decades: a population-based study. J Am Acad Dermatol 2009;60:394-401. 10.1016/j.jaad.2008.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eder L, Widdifield J, Rosen CF, et al. Trends in the prevalence and incidence of psoriasis and psoriatic arthritis in Ontario, Canada: A population-based study. Arthritis Care Res (Hoboken) 2019;71:1084-91. 10.1002/acr.23743 [DOI] [PubMed] [Google Scholar]
- 26. Khalid JM, Globe G, Fox KM, Chau D, Maguire A, Chiou C-F. Treatment and referral patterns for psoriasis in United Kingdom primary care: a retrospective cohort study. BMC Dermatol 2013;13:9. 10.1186/1471-5945-13-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huerta C, Rivero E, Rodríguez LAG. Incidence and risk factors for psoriasis in the general population. Arch Dermatol 2007;143:1559-65. 10.1001/archderm.143.12.1559 [DOI] [PubMed] [Google Scholar]
- 28. Bell LM, Sedlack R, Beard CM, Perry HO, Michet CJ, Kurland LT. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol 1991;127:1184-7. 10.1001/archderm.1991.01680070084010 [DOI] [PubMed] [Google Scholar]
- 29. Karimkhani C, Dellavalle RP, Coffeng LE, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol 2017;153:406-12. 10.1001/jamadermatol.2016.5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Egeberg A, Andersen YMF, Thyssen JP. Prevalence and characteristics of psoriasis in Denmark: findings from the Danish skin cohort. BMJ Open 2019;9:e028116. 10.1136/bmjopen-2018-028116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material 1
Web appendix: Supplementary material 2
Web appendix: Supplementary material 3
Web appendix: Supplementary material 4