Abstract
Whole body exercise tolerance is the consummate example of integrative physiological function among the metabolic, neuromuscular, cardiovascular, and respiratory systems. Depending on the animal selected, the energetic demands and flux through the oxygen transport system can increase two orders of magnitude from rest to maximal exercise. Thus, animal models in health and disease present the scientist with flexible, powerful, and, in some instances, purpose-built tools to explore the mechanistic bases for physiological function and help unveil the causes for pathological or age-related exercise intolerance. Elegant experimental designs and analyses of kinetic parameters and steady-state responses permit acute and chronic exercise paradigms to identify therapeutic targets for drug development in disease and also present the opportunity to test the efficacy of pharmacological and behavioral countermeasures during aging, for example. However, for this promise to be fully realized, the correct or optimal animal model must be selected in conjunction with reproducible tests of physiological function (e.g., exercise capacity and maximal oxygen uptake) that can be compared equitably across laboratories, clinics, and other proving grounds. Rigorously controlled animal exercise and training studies constitute the foundation of translational research. This review presents the most commonly selected animal models with guidelines for their use and obtaining reproducible results and, crucially, translates state-of-the-art techniques and procedures developed on humans to those animal models.
Listen to this article’s corresponding podcast at: https://ajpheart.podbean.com/e/guidelines-for-animal-exercise-and-training-protocols/.
Keywords: critical speed, exercise tolerance, exhaustion, maximal oxygen uptake, oxygen transport system
WHY ARE EXERCISE STUDIES RELEVANT?
Muscular exercise presents the greatest physiological challenge to the organism’s oxygen transport pathway and the coordinated function of the respiratory, cardiovascular, and neuromuscular systems. Just as an automobile needs a test drive, exercise tests represent often the best, or sometimes the only, strategy for determining the presence and severity of organic dysfunction (457). For instance, as determined initially by Karl Weber and colleagues (459, 460), the assessment of heart failure (HF) and recommendations for therapeutic options (287) are based on the maximal oxygen uptake (V̇o2max) as measured most conveniently during a ramp or incremental exercise test. This test is generally performed on a cycle ergometer or treadmill and employs a large muscle mass with the implicit understanding that the patients push themselves to exhaustion and do not quit for some other reason unrelated to the capacity of their O2 transport system and muscle contractile limitation (346).
Not only is exercise invaluable for diagnosis, but also, as recognized as early as 1777 by the English physician William Heberden (471), muscular exertion presents a powerful therapeutic option that reduces the predations of, for example, HF, helping to reverse disease-induced exercise intolerance and improving the patient’s quality of life (173, 364, 365, 371). In addition, scientists and clinicians use exercise tests routinely as a sensitive tool to determine treatment efficacy and increasingly as a mechanism for identifying therapeutic targets to develop novel pharmacological approaches to combat pathological dysfunction. An excellent example of this latter approach is the work of Scott Powers and colleagues, who have identified superoxide dismutase 2 (SOD2) in the myocardium as a key defense mechanism that is upregulated by exercise training and can constrain the myocardial damage consequent to transient ischemia as produced by infarction (381–383).
Although the value of therapeutic approaches often ultimately rests on their ability to reduce the burden of suffering in humans, there are many compelling reasons for the investigator to select an animal over a human model for concept development, unravelling of control mechanisms, and proof of efficacy and also for direct application to the treatment of organic dysfunction in animals.
Paramount among these are the following:
-
1.
Existence of a range of animal models for human diseases in which the disease duration and severity can be controlled.
-
2.
Absence of, or control over, confounding drug treatments.
-
3.
Greater control over subject numbers, diet, and exercise history.
-
4.
Uniformity in genetic background and time course of disease/treatment application.
-
5.
More freedom in invasive procedures such as muscle(s) sampling for structural analyses, oxidative enzymes, respirometry, and in vitro vessel function.
-
6.
Amenability to both acute and chronic exercise interventions.
Kent et al. (223) have presented multiple lines of cardiovascular discovery using exercise models that were initiated in animals as the fundamental step for designing and testing explicit hypotheses in humans. It is no exaggeration to state that the present field of physiology, especially as this relates to exercise, muscle energetics, and metabolism, the O2 transport pathway, and the mechanistic bases for exhaustion, has been founded on animal research. As foreseen by the pioneering comparative physiologists Claude Bernard (33) and, later, August Steenberg Krogh, “for many problems there is an animal model on which it can be most conveniently studied” (245). Well-crafted experimental designs using animals allow an unparalleled vertical integration of methodologies at each level of size and complexity from the subcellular level, to individual muscles/organs, up to the intact functioning organism through a range of oxygen transport, from resting, ~2 mL O2·kg−1·min−1, up to over 200 mL O2·kg−1·min−1 in the exercising horse, for example. Exemplars of key issues addressed in health and disease include defining the following: the upper limits to skeletal muscle blood flow during exercise, the heterogeneity of blood flow among/within muscles at rest and during exercise, and the role of nitric oxide in exercise hyperemia, as well as unravelling the complexities of vascular control and the impact of aging and disease on these processes. Selective gene knockouts and other genetic manipulations, predominantly in mice, have also advanced our understanding of the molecular control of structure and function as it relates to physiological performance.
EXERCISE TESTING AND TRAINING IN HUMANS AND ANIMALS
Exercise testing and training procedures in humans have undergone radical revision in the past three to four decades, in part, due to a greater understanding of physiological function/dysfunction in health and disease, the importance of the kinetics following exercise onset and in recovery, and the availability of more powerful research tools. These tools include rapidly responding gas analyzers, near-infrared spectroscopy, and real-time magnetic resonance spectroscopy (MRS). There has also been a greater appreciation of exercise intensity domains, as defined in the critical power (CP) or critical speed (CS) concept, and the physiological behaviors and fatigue mechanisms operant within each (73, 74, 76, 207, 367). Knowing a subject’s CP or CS [and energy stores component (W′) or the expression of such when running as distance (D′)] allows structuring of exercise tests that are more reproducible and, importantly, can be equated across subjects and studies to better evaluate therapeutic efficacy and outcomes. It has become evident that the percentage V̇o2max reference or peak power achieved on a given test are poor surrogates for the CP or CS-based standard (465). Another crucial issue that has been emphasized by the popularity of the incremental/ramp test is the ability to discriminate that V̇o2max has been measured rather than some motivational or symptom-limited exercise end point that does not reflect the capacity of the O2 transport system (sometimes termed V̇o2peak; 372). Validation of V̇o2max in both human and animal studies is extremely important where this is a criterion outcome.
Given the rediscovery that for many conditions including HF, exercise for patients in recovery produces better outcomes than bed rest, a pertinent question is: What intensity of exercise produces the best outcomes? Despite the longstanding dogma that patients eschew severe or even heavy exercise intensity for more moderate exercise intensities, animal (1, 200, 244) and human (475, 476) exercise investigations have unequivocally demonstrated that high-intensity (i.e., severe) interval exercise training (so-called HIIT) increases V̇o2max and beneficial cardiovascular adaptations far more than moderate-intensity protocols. Moreover, and contrary to enduring medical misconceptions, epidemiological studies to date support that the risk for exercise-related cardiac incidents during rehabilitation is extremely low for both exercise training paradigms (395). This finding has a direct bearing on the selection of animal models based on their ability, willingness, and robustness to undergo HIIT and also facilitate important cardiovascular measurements during exercise.
The subsequent sections will initially review state-of-the-art developments in human exercise testing, present how these may translate to animals, and finally consider a range of animal models by species and protocols broadly suitable for exercise studies. Where possible, common best practices, pros and cons of each species considered, and additional important practical and theoretical considerations will be presented. For further information associated with exercise testing in different species, the reader is referred to the American Physiological Society’s Resource Book for the Design of Animal Exercise Protocols (5).
STATE-OF-THE-ART DEVELOPMENTS IN HUMAN EXERCISE TESTING
In 1986 the accomplished exercise physiologist Charles M. Tipton predicted that within a decade, animal exercise physiologists would have “…progressed to the degree of sophistication exhibited by human exercise physiologists” (442). However, from the perspective of at least two sentinel parameters of O2 transport/utilization and exercise performance, this optimistic aim does not appear to have been unequivocally achieved.
Maximal Oxygen Uptake
The classical measurement of maximal oxygen uptake (V̇o2max) assesses, during large-muscle mass exercises such as running, cycling, cross-country skiing, or swimming, the coordinated capacity of the cardiorespiratory and muscular systems to uptake, transport, and utilize O2. Traditionally measured using a discontinuous series of step increments in work rate or speed, V̇o2max was defined as the highest V̇o2 achieved on a maximal exercise test and was evidenced by the failure of V̇o2 to increase commensurately with the energetic requirements; that is, V̇o2 plateaued when plotted against increasing work rate or speed. However, given the advent of rapidly responding gas analyzers in the 1970s and 1980s and the development of breath-by-breath gas analysis, the rapidly incremented or ramp exercise protocol permitted a compendium of ventilatory, gas exchange, cardiovascular, blood gas, and acid-base and metabolic measurements to be gathered simultaneously across the achievable range of exercise work rates from rest to maximal (exhaustion). Because identification of the gas exchange threshold (GET) is based on the rate of blood [H+] increase (principally from lactic acid), its buffering by bicarbonate, and consequent production of “extra” CO2, a duration of ~10 min or so is optimal, and so the slope of the imposed ramp is set on the basis of the exercise capacity of the subject to achieve the required duration (55). Whereas these protocols yielded a spectrum of submaximal indexes such as the GET and lactate threshold, work efficiency, and, arguably, V̇o2 kinetics, in many instances, subjects stopped exercising before manifesting a V̇o2 plateau, definitive for validation of V̇o2max. Indeed, for most subjects the V̇o2 profile increased linearly as a function of work rate throughout the test or, occasionally, projected upward at a faster rate toward the end of the test. For these subjects, in whom no definitive V̇o2 plateau was evinced, investigators either 1) accepted that it may not be a true V̇o2max and termed it V̇o2peak or 2) developed secondary criteria based on some arbitrary values for respiratory exchange ratio (RER), percentage of age-predicted maximal heart rate, and/or [lactate]. As neither of these procedures was commensurate with rigorous determination of V̇o2max a secondary exhausting validation test performed at a higher work rate or speed than achieved on the preceding ramp has been developed that facilitates a graphical solution to defining an individual’s V̇o2max (Fig. 1; 372).
Fig. 1.
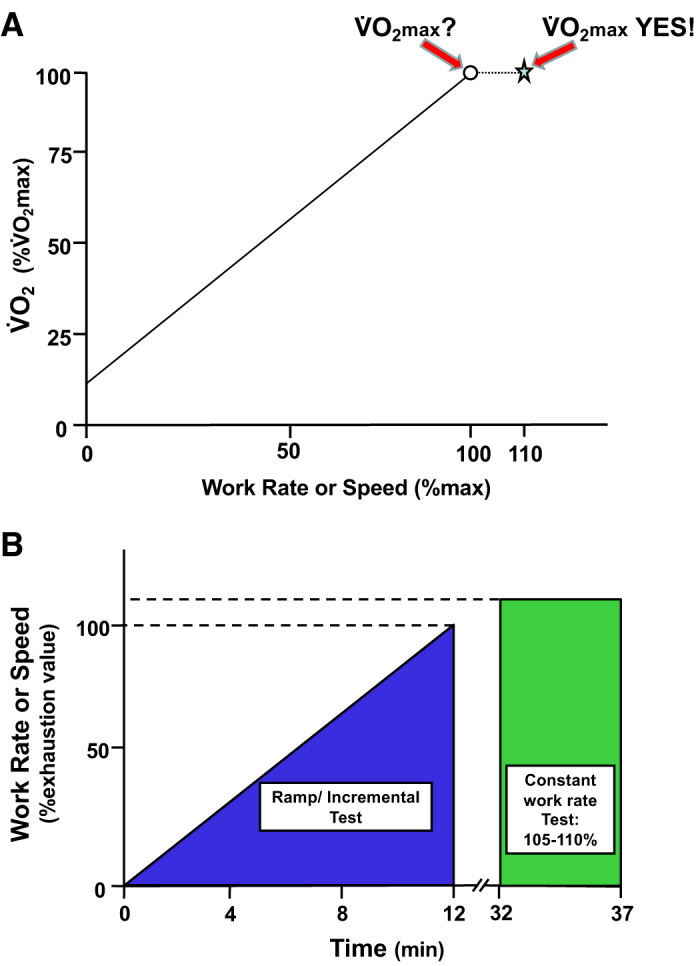
Schematic representation of the combination of the ramp test with the subsequent constant-load or constant-speed test (here at 110% maximum ramp value, star, A) validates that the ramp oxygen uptake (V̇o2; ○, A) was indeed maximal V̇o2 (V̇o2max) as evidenced by the presence of the V̇o2-work rate plateau. Had the V̇o2 increased at 110%, this would mandate that further testing at a still higher work rate or speed would be necessary to validate V̇o2max. Note that the ramp rate and also the constant work rate/speed bout must be scaled to the individual subject/population under investigation. The intervening rest period between tests is shown at 20 min (B), and this may be varied as species/model or protocol demands (372).
Critical Power and Critical Speed
Across populations, V̇o2max does not always correlate well with exercise tolerance, and invariably, exercise training or other perturbations that may increase V̇o2max modestly may translate into substantial increases in exercise performance that are highly variable (197, 377, 465). Thus, notwithstanding the concerns addressed above regarding V̇o2max measurement, a better functional parameter of exercise performance was needed that 1) has a greater translational value to the subject’s exercise tolerance and 2) facilitates setting an exercise intensity domain-specific work rate (i.e., above or below CP) so that changes in performance consequent to therapeutic intervention or the predations of disease are relatable across populations, across individual studies, and within discrete patient cohorts (465). It has been formally recognized, at least since 1925 (170), and probably much earlier [review by Jones et al. (207)], that the maximal duration for which a given power, speed, or, more correctly, metabolic rate (V̇o2; 27) can be sustained decreases hyperbolically as power (P) or speed (S) increases above some critical value (i.e., CP or CS) as described by (Fig. 2).
| (1) |
or, with respect to running speed, for example,
Fig. 2.
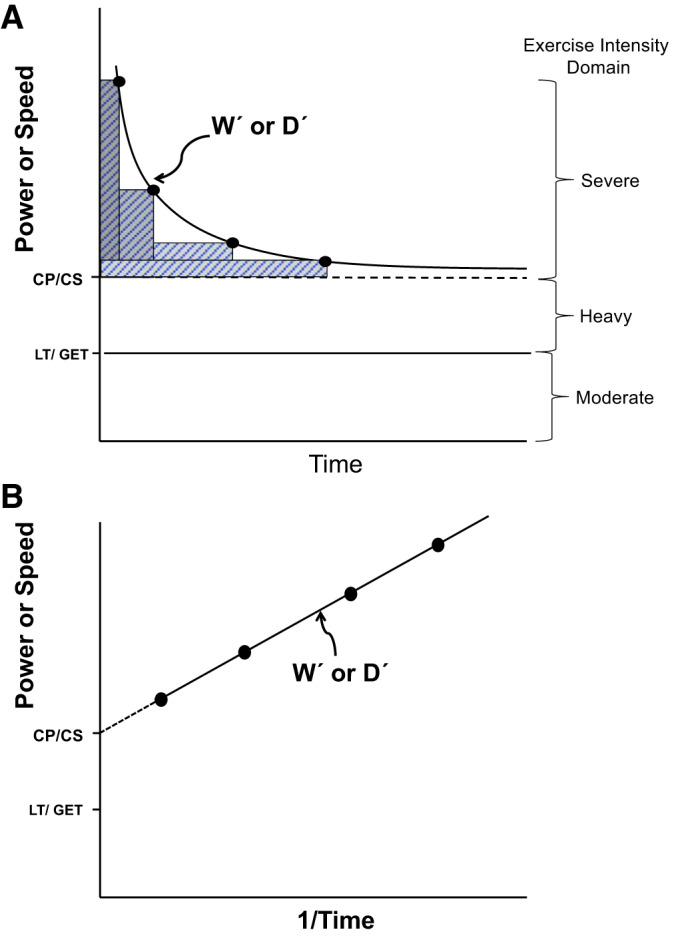
A: the hyperbolic power or speed-time relationship defines the limit of tolerance for whole body exercise such as cycling or running as well as individual muscle, joint, or muscle group exercise (isotonic or isometric). The curve is composed by having the subject (e.g., human, rat, mouse, or horse) exercise at constant power or speed to the point of exhaustion (●; 4 different workloads). Ideally, these bouts are performed on different days and exhaust the subject in 2–15 min. This hyperbolic relationship, which is highly conserved across the realm of human physical activities and exercise modes and also across the animal kingdom, is defined by two parameters: the asymptote for power [critical power (CP), speed (CS), or tension (CT) and their metabolic equivalent, oxygen uptake (V̇o2)] and the curvature constant Wʹ or Dʹ (W′, energy stores component; D′, expression of W′ when running as distance; denoted by the rectangular boxes above CP/CS and expressed in kJ or distance, respectively). Note that CP/CS defines the upper boundary of the heavy-intensity domain and represents the highest power sustainable without drawing continuously upon W′ or Dʹ. Above CP/CS (severe-intensity exercise), exhaustion occurs when Wʹ or Dʹ has been expended. Severe-intensity exercise is characterized by a V̇o2 profile that rises continuously to V̇o2max and blood lactate that increases to exhaustion. GET, gas exchange threshold; LT, lactate threshold. B: the hyperbolic relationship from above is linearized by plotting power or speed against 1/time, where the CP/CS parameter is given by the y-intercept and Wʹ or Dʹ by the slope. Correlative analyses with r > 0.98 provide confidence that maximal performances to exhaustion have been successfully measured.
| (2) |
where P and S are sustained power and speed (above CP or CS), respectively, and W′ (or D′ as distance run) denotes a discrete energy storage component that when depleted, signals exhaustion in time t. CP (or CS) constitutes the highest rate of oxidative energy provision possible without drawing continuously on W′ (or D′). The importance of CP/CS as a physiological parameter is underscored by its high reproducibility (127) and location at the boundary between the heavy- and severe-exercise intensity domains (357, 376) and in close proximity (−4% with respect to the marathon) to the speed (or V̇o2) at or above which most athletic track/running events are contested (206, 372, 418). Physiologically, CP/CS discriminates between exercise intensities that are sustainable for a very long duration versus those that incur exhaustion within a relatively brief duration that is highly predictable from the parameters CP or CS and W′ or D′ (127, 206, 357, 376, 418, 464).
Whereas the precise determinants of muscle exhaustion are likely to be context dependent, it is notable that work rates below versus above CP/CS display profoundly different behavior in that a steady state is attainable below but not above CP/CS [review by Jones et al. (206)]. This is true for pulmonary and leg V̇o2 as well as blood [lactate] [373, 376, 377; review given by Craig et al. (76) and Jones and colleagues (205, 207)], blood flow (73), intramuscular pH, [Pi], and [phosphocreatine] (205), and muscle torque production/neuromuscular fatigue (56). Setting work rate or speed relative to CP/CS helps ensure that changes in exercise capacity consequent to the predations of disease or imposed experimental interventions (e.g., exercise training, hyperthermia, hypoxia, 0-g environments, dietary modifications, etc.) or therapeutic treatments can be compared equitably within and across cohorts and study populations (465).
CONSIDERATIONS FOR ANIMAL MODEL SELECTION
Selection of an appropriate animal model for exercise testing and intervention will often necessitate solving a complex matrix of considerations that includes the following: 1) suitability, availability, and cost for a given experimental paradigm (if a genetic knockout is required, the investigator may be limited to the mouse; although more genetic rat models are becoming available); 2) willingness to exercise (mice, dogs, pigs, horses, and fish, yes; rabbits and cats, not so much) and the availability of required equipment and housing; 3) size of organism for sampling requirements on the continuum from Drosophila to elephant and beyond; 4) response of animal to essential surgical and experimental procedures; 5) presence (or absence) of discrete structures [e.g., contractile spleen in horses (370) and dogs (279, 453) or absence of discrete interpleural space in elephants (194)] or physiology/pathophysiology [e.g., malignant hyperthermia in pigs (78) or exercise-induced pulmonary hemorrhage in horses (108, 370)]; 6) understanding of exercise protocols by local Institutional Animal Care and Use Committee (IACUC) and compliance with reduce, refine, and replace mandates; 7) animal model that is de rigueur in a given field and with which an investigator wishes to compare their results; and 8) cost, availability, and number of animals required, based on appropriate statistical design.
Study Design Considerations: Protocol Development
Testing the impact of exercise on physiology and health outcomes in animals entails addressing multiple complex issues when choosing the best exercise protocol. The Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (342a) covers many issues across different types of research, including exercise research. The team approach to developing performance-based design and implementation standards is key to success for exercise-based studies. The research team, veterinary staff, and IACUC must work in synchrony to develop the animal care and use protocol with effective communication allowing the IACUC to comprehend the scientific issues, key methodologies, and often interpretational limitations that characterize exercise research. For their part, principal investigators (PIs) should recognize the importance of compliance with all policies and regulations governing animal care and use and especially the IACUC’s function in maintaining institutional compliance with regulatory mandates. This is best facilitated by open dialogue and professionalism throughout protocol development and review, which are crucial for establishing that working balance between rigorous science and excellent animal care.
Exercise protocol development faces two paramount challenges. 1) Reliable experimental and performance criteria must be established that match the study’s scientific goals with IACUC guidelines. 2) Humane procedures must be implemented for acute or chronic animal exercise protocols. Protocols and objective criteria must be established for terminating an exercise session and, if necessary, removing or euthanizing an animal. Criteria for such may differ during the training or conditioning period compared with the acute or chronic exercise phases of the study. Within the protocol development phase, it is the responsibility of the PI, IACUC, and laboratory animal veterinarians to develop an intervention plan, with a defined line of authority, that preempts or minimizes animal distress.
GENERAL CONSIDERATIONS FOR EXERCISE STUDY DESIGN
IACUC Review
The National Research Council’s Guide for the Care and Use of Laboratory Animals (the Guide) and other relevant policies and regulations establish the basis for the PI and the IACUC to work together to develop protocols, outcome assessments, and required documentation. Key issues are discussed in the remainder of this section.
Animal Numbers
Good scientific practice and regulatory agencies require that animal numbers be minimized using rigorous statistical design techniques. The importance of rigorous statistical design and power calculations based on accurate effect sizes is essential. Using too few animals and being unable to make any conclusion is also a significant problem. The appendix of the Institute for Laboratory Animal Research (ILAR) document, Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (342a), details procedures for determining animal numbers. Statistical power calculations are de rigueur for determining sample sizes a priori. Effective experimental design, especially for exercise studies where some animals may not perform on a given day, or, indeed, at all, demands inclusion of additional replacement animals.
Animal Use
Specific to exercise studies, the IACUC protocol must detail the need for forced, in contrast to voluntary, exercise: whether that exercise is exhausting and, if so, defined end points/identifiers, workloads imposed including the duration, intensity, and number of individual exercise bouts required; the time interval between repeated bouts; overall study duration; the use/intensity/frequency of aversive stimuli to maintain performance; and the need for any special caging or restraint. Specific justification is necessary for housing or exercising animals under environmental conditions outside the ranges provided in the ILAR, Guide for the Care and Use of Laboratory Animals. Procedures for animal familiarization with the specific environment and exercise equipment must be described along with pertinent animal monitoring procedures during exercise bouts and recovery. Criteria triggering premature termination of an exercise bout must be specified along with assurances that the responsible individual (PI, postdoctoral fellow, student, or technician) has the required training to recognize the relevant criteria. Research staff must be familiar with the normal appearance and behavior of the pertinent animal species to recognize problems promptly. If questions arise regarding animal health, the veterinary staff should be consulted regarding treatment of the animal or removal of the animal from the study. Timely intervention and treatment by the veterinarian may prevent the need to remove the animal from the study permanently.
Whereas exercise protocols have the potential to inadvertently injure experimental animals, implementation of the following will minimize such: For automated exercise equipment, most commonly treadmills, animals should be conditioned initially at low speeds, inclines, and durations with each being increased gradually as animals increase experience and capability. In this regard, selection of species that run willingly such as rats, mice, dogs, horses, pigs, and goats is a significant consideration in subject selection. Safety equipment that may reduce or prevent injury during exercise should be described such as presence of rapid cutoff switches and surcingle for horses that help prevent or minimize injury during a missed step at high speed. Realistic adverse consequences, for instance, drowning, physical injury, or increasing aversive stimuli tolerance, must be identified in the protocol along with applicable mitigation procedures. When historical data are not available, continuous monitoring is always preferable when using automated equipment.
Food and Water
Food and water may be used as exercise motivators. Requiring growing animals to exercise for their food often results in some minimum exercise level that provides low caloric yields and less rapid growth or weight loss compared with their nonexercised peers. Experimentally, these consequences are important when relating experimental data to body mass (e.g., muscle/body mass; 280). For additional information about restriction/reward protocols, refer to Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (342a).
An expanding paradigm in exercise science is strength training with applications to rehabilitation therapy and in particular retention of muscle mass with advanced aging. Muscle hypertrophy is usually achieved through progressive resistance training often using weights (280) and has been induced in multiple animal species by resistance training (weight lifting in conscious animals), muscle(s) electrical stimulation (in anesthetized animals), compensatory overload induced by tenotomy or by surgical ablation of synergistic muscles, imposition of chronic stretch via weight application or casting, and wheel running with high resistance (196, 280, 441).
Surgery Performed Under Anesthesia
As with all protocols, surgery must be justified and performed under anesthesia and needs to include regimens for postsurgical analgesia and monitoring. Omitting analgesia for procedures that would normally be deemed painful for humans requires scientific justification that includes literature citations, if available. Personnel who will provide nursing care should be identified, and a schedule of observation and/or treatment should be explicitly developed.
The standard regulatory benchmark for the use of anesthesia and postsurgical analgesics is the analogous human condition. Thus, if a human patient would request or receive analgesia after a similar procedure, the animal should receive such. For animal survival surgery, the PI must provide appropriate postoperative analgesia as determined by the facility veterinarian unless omission is justified for scientific reasons and approved by the IACUC. Analgesic regimens must be fully detailed in the methods section of resulting publications. Where analgesics cannot be used for scientific reasons, the rationale or justification must be compelling and clearly described with pertinent literature citations. Importantly, the PI must specify the duration between surgery and initiation of the exercise protocol based on demonstrating adequate recovery (see immediately below).
Impact of Surgery/Anesthesia and Euthanasia on Exercise-Related Measurements
Excision of tissue via biopsy or surgical instrumentation is often essential to collect physiological and biochemical data from exercising animals. Such procedures mandate careful selection of anesthesia and instrumentation and especially allowing for adequate recovery before initiating/resuming exercise studies. For instance, liver and muscle glycogen are key metabolic substrates for exercise; yet aortic catheterization under halothane anesthesia reduces liver glycogen by over 50%, and it takes from 2 to 8 days to recover (320). This substantial interanimal variability in recovery times often mandates assessment of recovery in individual animals, which may be predicated on reestablishing a normal food intake relative to body mass (263, 320) and/or the return of the animal’s weight to presurgical levels (57, 123). In addition, it may take as long as 3 days in rats and as long as 7 days in mice to reestablish their normal circadian patterns of activity and/or their diurnal variability in blood pressure (57, 320). It should be noted that Flaim et al. (119) have demonstrated in rats that cardiac or circulatory dynamics, regional blood flow, arterial blood gases, and acid-base status remained unchanged during a 1–6-h recovery period following halothane anesthesia. Furthermore, the values measured at rest over this period of recovery by Flaim et al. (119) were similar to those measured in chronically instrumented counterparts (14, 119, 123, 140, 326, 334, 336, 337). In contrast to that demonstrated by Flaim et al. (119), methoxyflurane anesthesia of mice destabilizes heart rate and other cardiovascular and metabolic variables for between 6 and 12 h and 2,2,2-tribromoethanol for over 24 h (90). Moreover, acute instrumentation can produce a significant reduction in body mass and V̇o2max (123, 140, 141, 171) such that, insofar as possible, the recovery period should not be terminated until an animal has returned to within 10% of its presurgical body mass.
In rats, pentobarbital anesthesia confounds the effects of exercise on acid-base status, whereas euthanasia via decapitation negates exercise-induced changes in muscle metabolites (124). Pentobarbital administered before heart excision, compared with isoflurane and sevoflurane, in an ex vivo working heart model, increases lactate levels and impacts function and stabilization during reperfusion (347). By contrast, enflurane increases the lactate-to-pyruvate ratio in rat hearts and livers during hemorrhage compared with pentobarbital or isoflurane anesthesia (217).
Records
Effective record keeping helps ensure animal care and regulatory compliance and is intrinsic to good science. Whereas some exercise regimens such as high-intensity treadmill training bouts require that an animal’s performance be documented each training session, voluntary wheel running, for example, may not. However, documenting the duration, distance, and intensity on a daily schedule allows the investigator to detect problems with the equipment, the animal, or the environment that can be rapidly addressed. For automated animal exercise equipment that utilizes aversive stimuli, such as mild electric shocks to maintain performance, recording the frequency/number of shocks applied during exercise is an important component of animal monitoring: acute or chronic alterations in administered shock frequency can indicate impending exhaustion, sickness, injury, or equipment problems. The need for aversive stimuli as well as their intensity and frequency will depend on the study design in concert with specific experimental goals. Scientific requirements such as high-intensity exercise, exercise to a predetermined targeted physiological change such as exhaustion, or exercise in obese/sedentary animals may require more frequent stimulation. IACUCs, PIs, and veterinarians must collaboratively review specific protocols to set an acceptable limit for application of aversive stimuli.
Because of the importance of developing effective therapeutic strategies for exercise intolerance in human patient populations, exhaustion is a very valuable end point in animal research. However, end points must be well defined and clearly established. These may entail observation of specific behaviors, circumstances, and/or physiological markers that indicate exercise termination by the investigator. Accurate records of test conditions and of performance are crucial and permit day-to-day adjustment of test parameters dependent on each animal’s performance.
Health Problems
PIs must provide criteria for temporary or permanent removal of animals from a study because of health problems. These include changes in demeanor or willingness to exercise. Common signs of pain, illness, or distress include irritability, decreased appetite, weight loss, reduced spontaneous activity, guarding specific body regions, abnormal posture or gait, porphyrin rings around eyes, and changes in bowel or bladder habits. Decreased body temperature, weak pulse, or reduced or very rapid ventilation are signs of more serious health problems and mandate seeking veterinary evaluation. These criteria can mitigate temporary or permanent removal from the study, which is an essential aspect of quality control and fundamental to scientific rigor and reproducibility. PIs, IACUCs, and veterinarians should be creative, flexible, and compassionate in developing these criteria. Devices that are used for exercising multiple animals, some of which may be immunocompromised by the experimental protocol or disease being investigated, should be appropriately sanitized between animals to negate their providing a locus for infectious disease transmission.
Stopping an Exercise Session
Definition of a humane end point for exhausting exercise is a major responsibility of the PI and IACUC and must be specified and approved in the animal use protocol. Moreover, there are multiple reasons for stopping an exercise session prematurely, which include accidental injury (often damaged toenails in murines; judicious use of an inclined treadmill, which produces higher metabolic rates at slower speeds, is useful for reducing toenail damage), premature exhaustion, innappropriately elevated rectal/core temperature, adverse effects, behavioral issues, and poor performance resulting from the animal’s unwillingness to exercise.
Removal of an Animal from an Exercise Study
Animals that are trained and conditioned for exercise studies are extremely valuable. However, infectious disease and trauma can necessitate temporary or permanent removal from an exercise study. The protocol must specify the circumstances for removal and what criteria must be met for their return to the study. The requirement for excessive motivation to exercise via aversive stimuli mitigates removal from a study.
Animal Reuse
In some circumstances, euthanasia of instrumented and trained animals, especially large animals such as dogs, horses, or cattle, may not be necessary at the end of a study. Depending on the condition and prior use of these animals, use in subsequent studies is permissible and indicated. Reuse that requires additional (i.e., multiple) major survival surgeries requires specific justification and IACUC approval.
SELECTION OF ANIMAL MODEL
Over and above the advantages of animal models presented in the why are exercise studies relevant? vide supra, the investigator must ask themselves a battery of questions when selecting the best animal exercise model for themselves. First and foremost, if their preparation requires conscious “voluntary” running, which species can and will perform the criterion exercise bouts required for testing and/or training? Can the required measurements be made during and following exercise? Are the data transferable to humans? Is the required disease model available in this species? Sometimes, and especially in the case of genetic knockout models, the investigator is limited to a single species (i.e., mouse) and has to adapt or redesign exercise challenges from other species. One example of this would be assessing the performance impact of removing myoglobin from the heart and skeletal muscles. Garry et al. developed an exercise test where the performance criterion was how many times the animals lagged back on the treadmill belt and broke an arbitrary line positioned at the rear of the treadmill in close proximity to the electric grid (134). The strength of their conclusions, that lack of myoglobin did not negatively affect exercise performance, is only as rigorous as the criterion exercise test itself. Notably in this instance, Billat and colleagues (20, 37) have subsequently demonstrated that determination of CS-D′ and also V̇o2max are feasible on the motorized treadmill in multiple strains of mice.
Tipton usefully categorized different species with respect to what aspects of cardiovascular function they may best help investigate, noting that there was a marked paucity of studies, at that time, that had measured V̇o2max, cardiac output (Q̇), and arterial-venous difference (a-vO2diff) simultaneously (442). Specifically, mongrel dogs and rats display proportional changes in Q̇, stroke volume, and a-vO2diff closely akin to those seen in humans despite dog and rat body mass-specific V̇o2max values being far higher than those of their typical human counterparts. Interestingly, the effect of exercise training on the balance between perfusive and diffusive O2 conductances is such that foxhounds increase V̇o2max solely via enhanced Q̇ without elevating a-vO2diff (327). The typical human response is to increase muscle diffusional more than perfusional O2 conductance such that both elevated Q̇ and a-vO2diff contribute to the increased V̇o2max (389). Thus, the foxhound may be valuable for understanding O2 transport limitations in humans who are O2 extraction limited [e.g., healthy aged (303, 304) and patients with mitochondrial myopathy (380)]. The exercising horse and, to a certain extent, the dog (especially the greyhound) have muscular spleens that increase blood O2 carrying capacity during exercise and Q̇ values so high that pulmonary capillary transit times for red blood cells become limiting and exercise-induced arterial hypoxemia and hypercapnia become manifest. Moreover, in both horses and dogs the combination of high intrapulmonary capillary pressure (positive) and very low (negative) alveolar pressures summates across the extremely thin blood-gas barrier causing pulmonary capillary stress failure, leading to exercise-induced pulmonary hemorrhage (EIPH), occasional overt epistaxis, and inevitably long-term lung remodeling (369). The most common species for investigations identified with “physical exercise,” in order of usage (PubMed, June 2019), are as follows: rat (no. 1: 18,875 articles), mouse (no. 2: 10,869 articles), horse (no. 3: 4,096 articles), fish (no. 4: 3,929 articles), dog (no. 5: 3,679 articles), birds (no. 6: 974 articles), pig (no. 7: 867 articles), rabbit (no. 8: 672 articles), cow/cattle (no. 9: 647 articles), cetacean (no. 10: 242 articles), goat (no. 11: 159 articles), camel (no. 12: 37 articles), and pinniped (no. 13: 12 articles).
Exercise Modalities in Rats
Motorized treadmill.
advantages and disadvantages of treadmill exercise.
To investigate physiological, biochemical, behavioral, and, increasingly, molecular responses to acute and chronic (i.e., training) exercise, the treadmill has been the modality of choice. A variety of species have been successfully studied using treadmill running including horses (369, 370, 373, 375, 455), dogs (38, 154, 327, 330, 349), pigs (13, 46, 256, 260, 429, 438), rabbits (92, 274, 275), cattle (218, 410), ducks (226, 227), geese (113), and camels (238). However, the overwhelming choice for such studies has been the rat (Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research; 342a, 187). Notably, selective breeding in the laboratory has been utilized to select spectacularly for high V̇o2max and treadmill running endurance by Britton and colleagues (e.g., 415). In contrast with other exercise modalities, for instance, swimming or spontaneous wheel running, treadmill running in rats facilitates/permits calculation and setting of external work and work rate as well as intensity and duration (51). In addition, although breath-by-breath ventilation and gas exchange measurements are presently intractable in the rat, use of a specially designed metabolic chamber that fits into one lane on the treadmill (51) permits minute-by-minute V̇o2 measurement, and thus work rates can be set according to the rat’s V̇o2max or CS. Using these chambers, V̇o2, V̇co2, and other key exercise variables such as exercise efficiency can be measured (28, 50, 51). Running on the motorized treadmill increases the rat’s metabolic rate in a systematic and quantifiable fashion with respect to V̇o2max or CS and to the external workload (28, 50, 51, 334, 414, 423). Treadmill running imposes potent metabolic stress on the animal, requiring significant increases in O2 delivery to the contracting skeletal muscles in an intensity- and time-dependent manner (10, 12, 71, 211, 252, 253, 375). Akin to that seen in humans, treadmill exercise training elevates the rat’s V̇o2max promoting an array of structural and functional adaptations centrally within the cardiovascular system (increased blood volume, ventricular dimensions, and stroke volume) and peripherally (increased vascular bed, more sensitive vascular control, higher blood flow and microvascular oxygen pressures during exercise, increased oxidative enzyme capacities, faster V̇o2 kinetics, and greater capacity for fat vs. carbohydrate as energetic substrate; 11, 24, 28, 60, 99, 117, 141, 149, 172, 174, 178, 179, 322, 337, 338, 415, 472), all of which are associated with large increases in endurance capacity (80, 247).
Another major advantage of using the rat to study exercise responses and capacity is the degree of instrumentation that is possible. Arterial catheterization studies have permitted determination of cardiovascular function, and using radioactive, colored, or fluorescent microspheres, regional, organ, and muscle(s) blood flow responses to exercise have been resolved to a level not possible in humans (10, 89, 175, 241, 252, 255, 337, 419, 420). This technique has also been used successfully in dogs (150, 327, 330–332), equids (ponies and horses; 15, 288, 358), and pigs (13, 297) running on the motor-driven treadmill. An additional major advantage of the rat is the development of disease models with substantial applicability to humans. These include heart failure (173, 174, 328, 333, 339, 371, 374), diabetes [Type I (29, 232) and Type II (72, 354, 355)], hypertension (48, 294), and pulmonary hypertension (52, 53). In these, and many additional human disease analogs (240), the rat is helping unravel physiological, biochemical, and molecular mechanisms underlying the mechanistic bases for exercise intolerance and increased morbidity/mortality.
Notwithstanding the above, the treadmill exercise mode in the rat is not without its challenges. These include the following: 1) The forced nature of the activity and common requirement for noxious stimuli, most commonly bursts of high-pressure air or electric shock, delivered via prod or grid, for motivation constitute one challenge. The electric shock must be intense enough to provide an incentive to run (10–30 V, 0.5 A) but must not harm the animal. Excessive electric shocks have been defined as >4 per minute and indicate that the exercise intensity should be decreased or that the animal should be removed from the study. As well as high-pressure air, which cannot be used in conjunction with metabolic measurements (V̇o2 and V̇co2), other effective nonnoxious stimuli include the sweeping use of a bottle brush on the tail and also the rattling of keys on the plastic manifold of the treadmill lanes. 2) Running pattern, especially at slow speeds, may be more “stop-and-go” than the continuous movement at close to constant speed desired. This behavior tends to decrease with conditioning. 3) Expense of commercial treadmills and sometimes lack of flexibility for speeds (ideally up to 120 m/min to facilitate high-intensity sprint training, as desired) and a range of inclines/declines are another challenge. Rats can run up inclines of 30° (35% grade; 28, 472, 473) and down declines of 16° (9, 211, 212). 4) Treadmill surface can be problematic. The belt must facilitate good traction (396) while being nonporous (easy to disinfect) and sufficiently soft to minimize toenail and foot problems incurred by daily training. Treadmill lanes typically range from 39 cm (28) up to 70 cm with the longer lanes minimizing contact with the electric grid especially during high-intensity training (472).
conditioning rats to run on the treadmill.
Up to 10% of rats from commercial vendors may refuse to walk or run and must be eliminated from exercise studies (28, 99, 199, 239). To minimize this loss and help rats become proficient runners and reduce foot injuries, they should be familiarized to treadmill exercise gradually. Adequate conditioning, often in bouts as short as 5 min at varying speeds, helps ensure that rats become proficient runners and generally takes between 5 and 14 days (60, 164). The frequency and duration of these sessions must be gauged carefully to avoid training effects such as heat shock protein responses or increased muscle oxidative enzymes or V̇o2max (24, 99, 314). Some investigators have found that positive reinforcement using chocolate (0.5 g) as a reward after the exercise session resulted in rats jumping eagerly out of their cages onto the treadmill, avoiding compliance problems entirely and decreasing the necessity for electric shock encouragement (472). Given institutional restrictions regarding food in laboratories, this important exercise incentive should be mentioned in the application. Foot injuries incurred, usually during running, may require veterinary treatment and temporary removal from training to allow recovery.
Measurements of exercise performance on the treadmill in the rat.
Although tests of “fitness” in rats and other animals have often considered V̇o2max and endurance capacity to be synonymous, as justified under exercise testing and training in humans and animals they clearly are not, with each displaying different weighting of central (cardiovascular O2 transport) and peripheral (i.e., muscle; muscle O2 transport, O2 utilization, mitochondrial oxidative capacity, and substrate selection/availability) capacities.
measurement of V̇o2max in the rat.
For rats running on a motorized treadmill, V̇o2max is normally defined as the point at which V̇o2 does not increase additionally, even though further increases in external workload are imposed on the animal (414). As with humans, a progressive ramp or incremental exercise test can be used to meet this criterion allowing for sufficient exercising time that the expired gas concentrations (O2 and CO2) measured in the metabolic chamber have stabilized at their appropriate levels (51, 140, 334, 472). Again, akin to exercise-conditioned young healthy humans (87, 372), even when a distinct V̇o2 plateau is not evident, the rat’s peak V̇o2 response may not be different from the V̇o2max measured using more strictly defined criteria (28, 117). A validation bout at a faster speed than achieved on the incremental test has been implemented for rats using an abbreviated maximal exercise test 48 h after the initial exercise test (163), which ensures that a true V̇o2max is measured for each animal and serves to minimize the opportunity for exhaustion to prevent V̇o2max being achieved (Fig. 3). The latter test also reduces the possibility for other environmental factors such as high ambient temperatures to negatively impact the rat’s running ability. The importance of a validation bout, in the absence of a defined V̇o2 versus speed/work rate plateau, is likely to be of greater importance in aged and/or disease models where a complex array of factors not related directly to the O2 transport-utilization system may cause the animal to stop running (372).
Fig. 3.
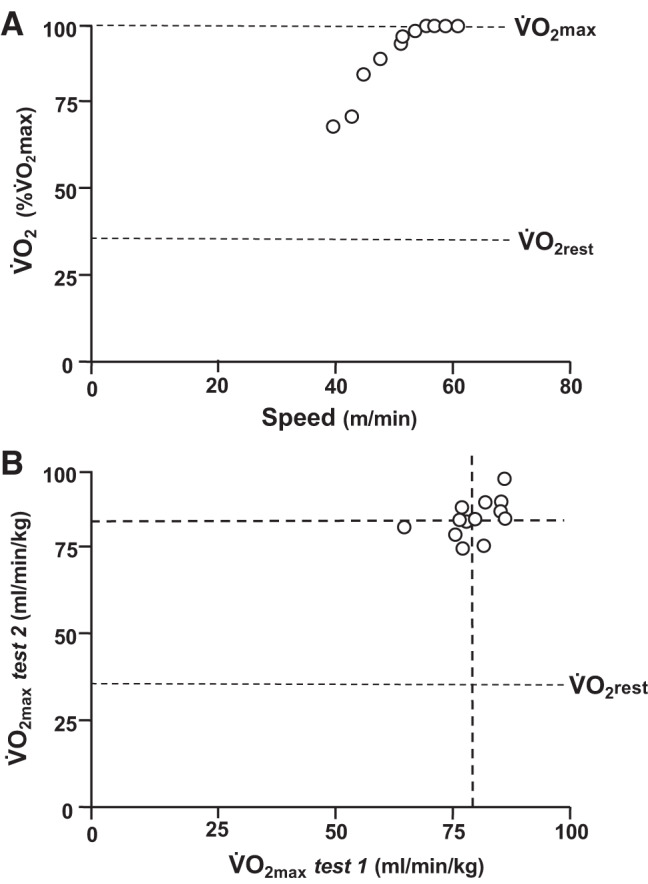
A: maximal exercise test in a male Sprague–Dawley rat demonstrating a distinctive plateau for oxygen uptake (V̇o2) vs. speed definitive for maximal V̇o2 (V̇o2max). V̇o2rest, resting V̇o2. B: individual male Sprague–Dawley rat V̇o2max values from 2 independent tests. Note very similar mean values (dashed lines) from tests 1 and 2, small range of V̇o2max values, and average within-rat coefficient of variation (CV) between tests 1 and 2 of 4 ± 1% (SE) supporting that these two measurements were highly reproducible with this CV falling within the expected measurement error for V̇o2max (71).
Acute instrumentation such as surgical insertion of cannulas into the carotid artery or jugular vein of the animal invariably compromises V̇o2max. Flaim et al. (119) demonstrated that performing the surgery with a short-acting inhalant anesthetic, such as halothane, results in resting cardiac or circulatory dynamics, regional blood flow, arterial blood gases, and acid-base status that remain unchanged during a 1–6-h recovery period with the values measured being similar to those measured in chronically instrumented counterparts (14, 119, 123, 140, 326, 334, 336, 337). Despite this stability in resting cardiac or circulatory dynamics, regional blood flow, arterial blood gases, and acid-base status, acute instrumentation does reduce V̇o2max (123, 140, 141, 171).
A typical progressive exercise test to measure V̇o2max/V̇o2peak utilizes the principles developed in the rat by Brooks and White (51) and Musch et al. (334). Specifically, each rat begins running at 25 m/min up a 10% grade for 2–3 min, which serves as warm-up and familiarization. Subsequently, the treadmill speed is increased to 40 m/min and then by 5–10 m/min each minute until exhaustion. For a cohort of 2–4-mo-old healthy male Sprague–Dawley rats the range of peak treadmill speeds was 46.4–78.9 m/min measured weekly for 5 consecutive weeks (71, 73). Primary criteria defining a successful test were observation of a change in gait immediately preceding termination and/or no further increase of V̇o2. Given the inability of an arbitrary respiratory exchange ratio (RER) value to confirm V̇o2max in humans (378), an RER >1.0 was taken as an ancillary measure of V̇o2max only in the presence of a gait change or V̇o2 plateau (28). These tests typically are of <8-min duration with the majority demonstrating a distinct V̇o2 plateau at 75.7–80.1 mL O2·min−1·kg−1 (71). Using this procedure over 5 consecutive weeks, absolute V̇o2max increased at weeks 2 and 5 in concert with greater body mass, whereas relative V̇o2max was unchanged for weeks 1–3 but decreased slightly thereafter (Fig. 4). Remarkably, there were no differences in the mean within-rat coefficient of variation among consecutive tests (range 3–4%). It is pertinent that Høydal et al. (187) found a more consistent V̇O2 plateau at 25° incline for rats and mice.
Fig. 4.
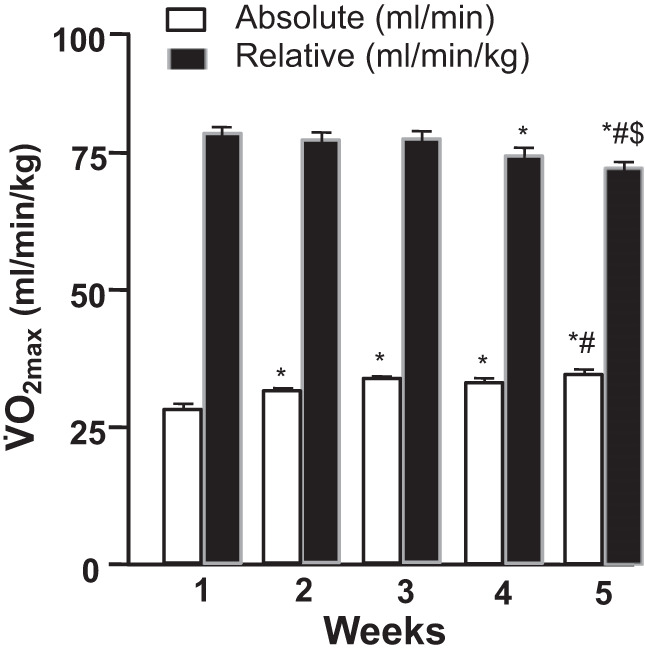
Mean maximal oxygen uptake (V̇o2max; n = 13 male Sprague–Dawley rats) across 5 maximal exercise tests plotted as absolute and relative values (means ± SE; *P < 0.05 vs. test 1, #P < 0.05 vs. test 2, $P < 0.05 vs. test 3; 71).
measurement of endurance capacity in the rat.
The endurance capacity of the rat is measured typically by requiring the animal to run at some submaximal speed/work rate until fatigue or, more correctly, exhaustion ensues. Exhaustion is defined operationally as the inability to keep pace with the treadmill (126, 163, 394, 456, 472). With time at a given speed the animal’s running style changes, and a gradual lowering of the hind haunches becomes apparent as fatigue develops. When the rat is unable to keep pace with the treadmill, even after repeated application of aversive stimuli, the investigator should discontinue the test. Exhaustion is confirmed by placing the rat on its back and observing evidence for a diminished or absent righting reflex (64, 125, 163, 296).
As with humans, rat endurance capacity relates to liver and skeletal muscle glycogen content (64, 70), and because these fluctuate diurnally (64, 70), controlling for the time of day at which endurance exercise tests are conducted is essential. Investigators should be aware that food deprivation significantly compromises liver and skeletal muscle glycogen concentrations with 24 h of fasting decreasing muscle glycogen concentrations by 30–40% and almost completely depleting liver glycogen (70, 461). Therefore, testing in the fed or fasted state will impact the rat’s exercise performance as will chronic instrumentation insofar as they decrease liver and muscle glycogen. Specifically, Moore et al. (320) demonstrated that instrumentation with an aortic cannula decreased both liver and diaphragm glycogen concentrations, which remained depressed until the food intake was reestablished at presurgical levels. Because of the great importance of food intake in glycogen storage the rat’s food intake should be measured before and after surgery with endurance exercise tests delayed pending reattainment of presurgical levels.
Unlike humans and horses, rats do not sweat to regulate body temperature. Exercise performance of the rat is compromised by hot environments, where the total thermal load precludes the rat achieving thermal balance, compared with either thermoneutral (ideally 20–22°C) or cold environments (125, 126, 394, 413). Thus, rats running in hot environments increase both body core and hypothalamic temperatures to the point of exercise intolerance (126, 456). It is therefore incumbent on the investigator to ensure that exercise tests are performed in a thermoneutral environment (379). In this regard, an electric fan placed in front of the rat helps dissipate body heat.
Rigorous adherence to these sources of experimental variability can ensure that tests of exercise capacity and also V̇o2max are highly reproducible across time for up to at least 5 wk despite the increased mass of the rat (71). Thus, Copp et al. (71) utilized a graded exercise tolerance test in male Sprague–Dawley rats (age 2–4 mo) that exhausted the animals in 45.9–52.1 min with consecutive weekly coefficients of variability in endurance capacity in the 6–10% range (Fig. 5). This protocol consists of starting the rat at 25 m/min up a 10% grade for 15 min and subsequently increasing the speed by 5 m/min every 15 min until exhaustion. This protocol has been demonstrated to effectively deplete muscle glycogen stores and elicit an objective index of exhaustion (335, 336).
Fig. 5.
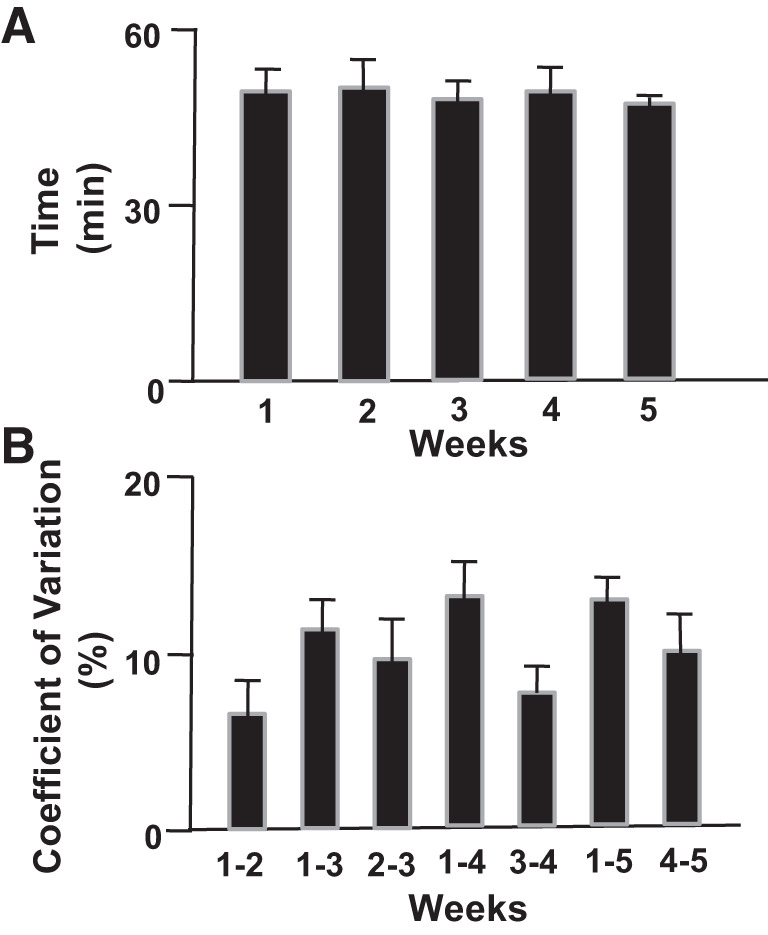
A: average run time to exhaustion (means ± SE) among a cohort of 13 healthy male Sprague–Dawley rats during 5 separate exercise tolerance tests for determination of endurance capacity over successive weeks. B: within-rat coefficient of variation (n = 13 rats) among an increasing number of exercise tolerance (endurance capacity) tests over successive weeks (71).
Copp et al. (73) demonstrated that the CS relationship could be determined in the rat during treadmill exercise. CS was measured via up to five constant-speed runs, performed on separate days, that resulted in exhaustion within 150–500 s (Fig. 6). In rats, as for humans, CS constitutes a crucial performance and metabolic demarcator, below which V̇o2 stabilizes at submaximal values and exercise of a sustained duration is possible. In marked contrast, above CS, V̇o2 rises systematically to V̇o2max, and exhaustion occurs rapidly at a time closely predicted by the CS-D′ parameters of the speed-duration relationship (Fig. 6). These investigators subsequently utilized the CS concept to demonstrate that neuronal nitric oxide synthase plays a major role in regulating muscle(s) blood flow and vascular conductance during severe-intensity (i.e., above CS) but not heavy-intensity (below CS) exercise (74). Moreover, as mentioned previously, a singular advantage of the CS concept is that relating a running bout to CS allows direct comparisons to be made across investigations, which is a huge advantage for judging relative therapeutic efficacy (465). To this end, Craig et al. have demonstrated that CS can be established in rats with HF (76).
Fig. 6.
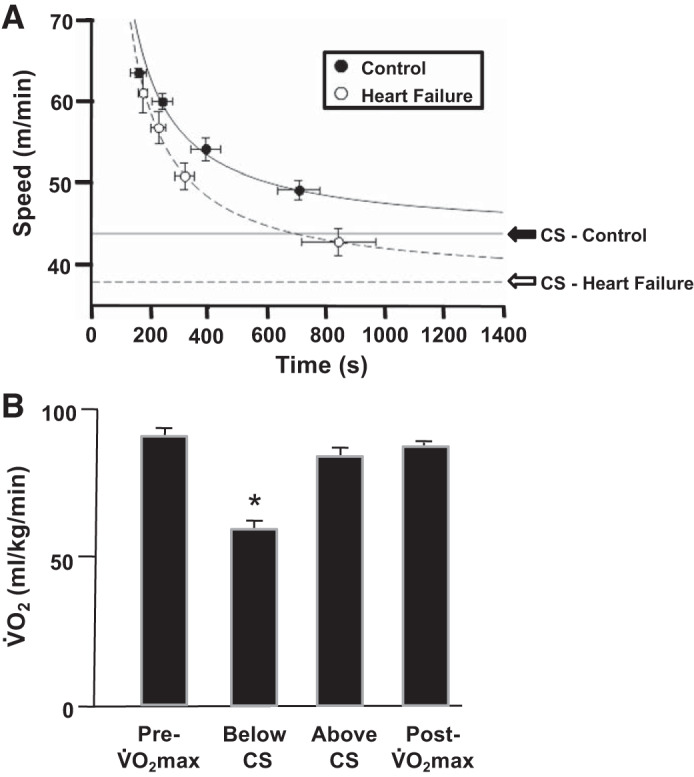
A: hyperbolic models for control (●) and heart failure (HF, ○) female Sprague–Dawley rats (means ± SE). Critical speed (CS) was substantially reduced [indicated by the downward shift of the asymptote, CS, from healthy (solid line) to HF (dashed line)]. Parameter D′ (expression of energy stores component when running as distance, as defined previously in Figure 2) was not impacted by heart failure (76). B: demonstration that CS in the male Sprague–Dawley rat, as for humans (367, 376), does indeed represent the demarcator between the heavy-intensity [oxygen uptake (V̇o2) steady state below maximal V̇o2 (V̇o2max) achieved] and severe-intensity (V̇o2 rises to V̇o2max) domains of exercise (73). Notice that below CS, end-exercise V̇o2 falls significantly below V̇o2max (*P < 0.05), whereas above CS it does not. V̇o2 values are means ± SE.
Wheel running.
advantages and disadvantages of wheel running.
Spontaneous or voluntary running wheels for rats are typically ~1 m in circumference (32–34 cm in diameter) and most commonly made of stainless steel (195). A great advantage of this modality is that the exercise training requires only minimal investigator intervention, does not require aversive stimuli (e.g., electric shocks or air jets) to motivate the animals to run in the wheels, and allows rats to run during the night congruent with their normal active phase. Another compelling reason for selecting this exercise modality is that it offers a low-time investment intervention facilitating long-term investigation of increased physical activity in rodents, which is especially valuable for aging studies (180, 182, 183). Also, in contrast to swimming, in the rat, wheel running does not induce chronic stress as gauged by hypertrophy of the adrenal gland or increased catecholamine content in the left ventricle of the heart (397). However, there is the potential for substantial hypertrophy of hind limb muscles and myocardium, especially in young rats (165–167, 324, 390–392, 397). This is a response not typically observed in most endurance-based treadmill-running investigations and may be either advantageous if the adaptive mechanisms are being investigated or a potential confound contraindicating selection of this training method.
The major disadvantage of wheel running is that regulation of the duration or intensity of the running behavior is challenging although it can be influenced by creative strategies such as dietary interventions (e.g., food restriction; 181, 325) or using variable-resistance wheels. Generally, the animal is in control of the quantity, intensity, and timing of its running behavior, which may be extremely erratic and vary enormously among animals. For instance, spontaneous running varied from 4 to 74 km/wk in a cohort of young healthy male Sprague–Dawley rats (412). Despite these limitations, many physiological and pathophysiological adaptive responses to exercise of cardiovascular, endocrine and metabolic, neuromuscular, neurological, and immunologic variables have been investigated using wheel running. However, this modality is not effective for studies requiring the animals to exercise until fatigue or exhaustion ensues because running rarely lasts longer than 2 min at a time and concludes well before those desired end points. Thus, assessments of “exercise capacity” based on daily wheel-running distances are extremely problematic. With regard to training adaptations, voluntary wheel running does induce elevations in V̇o2max in normal (152, 165, 247, 480) and hypertensive (234, 352) rodent models. Also, within a healthy cohort the most active animals evince the highest locomotory muscle(s) oxidative enzyme activities and vascular transport capacities (412).
Measurements of exercise amount during wheel running in the rat.
Investigators choosing wheel running for an exercise model should measure the total daily distance run by each animal by recording the revolutions completed in each 24-h period, with an attached revolution counter, to calculate the distance run based on the wheel’s circumference. The majority of running generally occurs during the early part of the 12-12-h dark-light cycle in 100–200 bouts of 40–90 s each (319, 390). Wheel odometer records should be documented ideally at the same time each day close to the end of that day’s dark cycle. With implementation of external monitoring devices, the investigator can measure the speed and duration of each individual running bout and thereby estimate exercise intensity (319, 352, 390).
Upon availability of a running wheel an adaptation period of 2–4 wk is evident before the rat’s maximal running activity plateaus (152, 165–167, 177, 318, 390, 393) for several weeks and then steadily declines with advancing age (152, 181, 319, 352). Animals typically stratify themselves into groups attaining high (18–20 km/day), intermediate (6–9 km/day), and low (2–5 km/day) running distances (165, 166, 177, 319, 390). Minimally acceptable running distances have been arbitrarily set with a cutoff value of 2 km/day (165, 318, 390).
If rats are provided adjustable resistance wheels, the amount and rate of work can be measured by calibrating the resistance to rotation of the wheel and measuring revolutions. Running distance decreases above some critical resistance, and as mentioned above, these wheels can induce leg muscle hypertrophy, to a degree that may not occur with freely rotating wheels (195).
As for treadmill exercise, the environmental conditions in which the animals run must be carefully regulated. The distance run is impacted by many variables including 1) the amount of light exposure such that animals housed on the top or bottom shelf of the animal rack are impacted differently, 2) activity of neighboring animals, 3) estrus cycle of female rats, and 4) age. As above, running distances decrease by over 75% through maturation and old age (180, 319).
As with treadmill running, wheel running in rats may break toenails and/or develop hind paw abrasions especially during the first few weeks. Investigators should ensure that toenails are either clipped or protected with a strong adhesive. Paw injuries lasting more than a few days, irrespective of running activity, indicate veterinary evaluation and a brief hiatus (4–7 days) from running for recovery.
Voluntary wheel running may not be appropriate for animals with massive central obesity (e.g., obese Zucker rat) as anecdotal evidence suggests that their spontaneous running activity is very low. However, it is effective for other disease models including hypertension (352, 397) and insulin resistance (155, 342, 366, 482) and induces substantial increases in V̇o2max, as assessed during an incremental treadmill test, in normal (152, 165, 247, 480) and hypertensive (234, 352) rats.
Swimming.
advantages and disadvantages of swimming.
Most terrestrial animals have the innate ability to swim, and thus, although this section focuses primarily on rats, many of the issues presented can be applied to other animal species. Swimming has been the exercise modality of choice across a spectrum of behavioral and exercise studies as reviewed by Dawson and Horvath (84). Rats can swim in nonturbulent isothermal water for as long as 60 h (388). Swimming exercise studies have identified key physiological, biochemical, and molecular responses to acute and chronic exercise (21, 190, 204) and require less expensive and more rudimentary equipment than either treadmill or wheel running. However, the investigator must select carefully the container, water depth, and temperature in which the rats will swim. When applied appropriately, swimming has the potential to provide a more continuous and uniform exercise profile than the stop-and-go pattern that characterizes wheel and, sometimes, treadmill running and avoids foot injuries.
Continuous swimming involves repetitive cycling of the rat’s forelimbs and hind limbs at a rate similar to treadmill running (145, 198, 254), while maintaining its snout above the water. However, ankle and leg extensor muscles are less heavily involved than during running, whereas the ankle and leg flexors are preferentially recruited during swimming (254). Moreover, swimming induces a greater range of motion for both knee and ankle joints compared with running (145). As a result, different muscle recruitment patterns likely affect the effort and intensity of exercise performance. For swimming both the forepaws and hind paws remain submerged, and on occasion, the rat’s head may momentarily submerge. Whereas continuous swimming constitutes only moderate-intensity exercise, adding weight to the rat’s tail increases this to heavy- and even severe-intensity exercise. Rats should not become hypoxemic during continuous swimming (118, 136).
Distinct disadvantages of swimming include the following: 1) Some animals do not swim continuously but resort to diving or bobbing (22, 23, 91) behavior as escape or survival strategies invoked to avoid potential drowning. This behavior introduces intermittent hypoxia as an experimental confound and can be minimized by ensuring adequate water depth. Rats that swim continuously achieve a metabolic rate ~3 times that of resting [i.e., 3 metabolic equivalents (METs)], whereas rats that bob, spending as much as 60% of their time submerged and hypoxic (428), are at ~2 METs (23). It is clear that this type of activity should not be considered exercise. If, in a given rat, diving and bobbing behaviors cannot be eliminated, animals should be removed from the study. 2) Rats will seize any opportunity to avoid swimming such as floating, supporting themselves in corners, climbing out onto the side, or wedging their nails in any available crack. 3) Nonuniform swimming patterns include floating and climbing on one another. 4) Incorrect water temperature will impact swimming ability and metabolic responses.
To minimize these problems, the tank should be round with water deep enough to eliminate bobbing (≥50 cm; 22, 23, 118, 428) and have a vertical distance from waterline to the top of the tank sufficiently great to prevent the rats from extricating themselves from the water. In addition, the tank must provide a sufficient water surface area (~1,000–1,500 cm2 per rat) for the animals to swim (8, 75, 118, 254, 336, 428). Whether rats swim in tanks with clear or opaque sides does not appear to influence performance except that when the rats are swum to exhaustion, opaque sides reduce variability within and between animals (298). Rat swimming movements create water turbulence, which traps air bubbles in their fur increasing buoyancy (283). This process may be so effective that they fall asleep as they float in the water, and rats learn to do this so as to float with very little movement. V̇o2 measured in floating rats is close to that measured during resting conditions on a motorized treadmill and therefore represents a negligible exercise stressor. Consequently, to produce the required exercise pattern and metabolic stress, floating must be prevented. Effective counterstrategies include shaving them, adding a small amount of detergent to the animal’s fur or the water and agitating the water continuously (118, 298), and/or adding weight to the rat’s tail (see selection of animal model, Exercise Modalities in Rats, Measurements of exercise performance during swimming in the rat, below; 298).
Water temperature substantially impacts swimming performance in the rat (22, 23, 26, 54, 85, 86, 450): water warmer than the rat’s core temperature (i.e., 42°C or greater) induces hyperthermia and compromises performance greatly, even causing death (26). On the other hand, water significantly cooler than the rat’s core temperature (i.e., 20°C or lower) promotes hypothermia and also compromises performance (84) and, again, can kill the rat (26). Ideally, setting the water temperature slightly below the animal’s core temperature (specifically, between 33 and 36°C), allows the rat to maintain its core temperature for the duration of exercise (22, 23, 84). Crucially, this thermal range prevents temperature-related decrements in cardiovascular function (i.e., cardiac output, heart rate, and mean arterial pressure) that would influence exercise performance and metabolic regulation (84).
Although more challenging in the aquatic environment, as with treadmill running, and dependent on available technology, the metabolic stress of swimming rats can be defined relative to the maximal heart rate (HRmax) or V̇o2max. V̇o2 must be measured when rats are swimming continuously to provide an accurate measurement of metabolic rate.
A designated observer must be tasked to focus their complete attention on the swimming rats during the exercise testing or training period. Because of their high resting and exercising metabolic rate, rats can drown very quickly. Each observer must define rigorous and humane criteria for exercise session termination such as ~3 s submerged as distinct from bobbing. After swimming, animals must be dried and temporarily placed in a warm environment, such as under a heat lamp.
Measurements of exercise performance during swimming in the rat.
The unweighted continually swimming rat typically achieves a V̇o2 ranging from 46 to 63 mL·min−1·kg body mass−1 (i.e., ~3 METS; 8, 22, 23, 84, 298). For a young healthy rat with V̇o2max of 85–100 mL·min−1·kg−1, nonweighted swimming therefore constitutes a moderate intensity (~45–65% V̇o2; 8, 298, 336). To increase the exercise intensity, weight, relative to body mass, is placed most commonly on the tail (75, 118, 264). Importantly, the positioning of this weight is crucial, and it must not be sufficient to either submerge the animal or prohibit continuous swimming. For instance, a weight equal to 2% of the rat’s body weight attached ~5 cm from the end of its tail increases V̇o2 to ~81 mL·min−1·kg−1 (298) or 80–95% V̇o2max, corresponding to heavy- or severe-intensity exercise. By contrast, 4% of body weight attached at the base of the tail only increases V̇o2 up to 65–70% V̇o2max (336). Thus, placing the weight closer to the end of the tail appears to change swimming biomechanics reducing mechanical efficiency and elevating the V̇o2 cost.
Exercise training in the rat.
The three primary exercise modalities described above, treadmill running, wheel running, and swimming, have each been used for training rats (30, 84, 179, 405). The presence and extent of a training effect have been assessed primarily using one or more of the following criteria: 1) increases in V̇o2max, 2) increases in endurance capacity, 3) training-induced reductions in heart rate measured at rest and during submaximal exercise, 4) reductions in the blood lactate response during submaximal exercise, and 5) increases in skeletal muscle oxidative enzyme activities. Geenen and colleagues compared swim training to treadmill training and found that multiple training-induced cardiovascular and hormonal adaptations produced in the rat differed significantly (136). Notably, ventricular performance increased to a greater degree with swimming even though they attempted to match training workloads as a percentage of V̇o2max (404). Recently, Beleza et al. (30) compared the training-induced adaptations produced by high-intensity aerobic interval treadmill training (HIIT; 4 × 4-min bouts of exercise at 85–90% of V̇o2max interspersed by 2 min of recovery at 60% of V̇o2max) and free-wheel running. Whereas they identified both similarities and differences in the training-induced responses of peripheral skeletal muscles, it was evident that treadmill HIIT increased maximal exercise performance and reduced the blood lactate responses more compared with free-wheel running. These differential cardiovascular, skeletal muscle, and metabolic training responses across the three exercise modalities should be considered carefully as the experimental design is tailored to the scientific question(s) posed.
The impact of exercise training on the motorized treadmill has been assessed most rigorously with respect to relative exercise intensity (relative to V̇o2max) and duration (51, 99, 140, 141, 333). Training regimens have consisted of a variety of intensities and durations varying from moderate running of long duration (62–80% of V̇o2max, 60–120 min/day, 5–6 days/wk) up to high intensity and very short durations (105–116% of V̇o2max or greater, 5–6 repeated 1–2.5-min exercise bouts/day, 5–6 days/wk) termed high-intensity or sprint training (HIST; 80, 81, 99, 147, 148, 171, 257, 261, 411).
Over the last four to five decades these contrasting training paradigms (moderate prolonged continuous or high-intensity sprint training) have unveiled a broad spectrum of training-induced cardiovascular and metabolic adaptations and systems plasticity. One of the earliest studies examining the differences of endurance training versus high-intensity sprint training was performed by Davies et al. (80, 81). Ten weeks of endurance training (27 m/min, 15% grade, 120 min/day, 5 days/wk) increased V̇o2max 14% and endurance capacity 400%, with endurance, but not V̇o2max, being highly correlated with elevated skeletal muscle oxidative capacity. In comparison, a HIST regimen of 4 wk (5 × 1-min bouts of exercise at 97 m/min interspersed with 10 s of rest, 15% grade, 7 days/wk) increased V̇o2max 15% but did not improve either endurance capacity or skeletal muscle oxidative enzyme capacity (81). Using a nearly identical HIST regimen, Hilty et al. demonstrated that the increases in V̇o2max (↑19%) were produced by a higher maximal cardiac output (Q̇max) and maximal stroke volume (SVmax) in the absence of increased maximal arteriovenous O2 difference (i.e., fractional O2 extraction; 171) or muscle (soleus, plantaris, and white gastrocnemius) citrate synthase activities. Collectively, these studies support that high-intensity anaerobic sprint training regimens induce substantial central cardiac adaptations in the absence of the elevated peripheral skeletal muscle oxidative capacity evinced by endurance training regimens at least in the rat (80, 81, 99, 171, 179).
In the late 1980s, Laughlin and colleagues (257, 411) examined the effects of high-intensity interval training (HIIT; 6 × 2.5-min bouts of exercise at 60 m/min interspersed with 4.5 min of rest, 15% grade, 5 days/wk) on the vascular transport capacity of the rat’s hindquarter skeletal muscles along with the blood flow response of these muscles to a given level of treadmill exercise (15% grade, 60 m/min, ~116% of V̇o2max). Unlike the previous HIST, HIIT elevated succinate dehydrogenase activity in the white vastus intermedius and white vastus lateralis muscles and increased total hindquarter skeletal muscle blood flow capacity during supramaximal exercise via fast-twitch glycolytic muscle vascular adaptations of the white gastrocnemius and white vastus lateralis that included a greater capillarity (147). Collectively, the available literature [see review by Laughlin and Roseguini (261)] supports that HIIT increases the blood flow capacity in skeletal muscles with adaptations occurring primarily in fast-twitch glycolytic muscle.
In 2001, Wisløff et al. (472) developed a HIIT regimen where the rats ran 4 × 8-min bouts, instead of the 4 × 4-min bouts used by Beleza et al. (30), at 85–90% of V̇o2max separated by 2 min of recovery at 50–60% of V̇o2max. The unique and important aspect of Wisløff and colleagues’ design was institution of weekly V̇o2max measurement and adjusting running speed accordingly such that all rats trained at 85–90% of V̇o2max throughout the entire training program. This new training regimen has produced the largest increases of V̇o2max (60–70% in both male and female rats) found to date as well as adaptations in the heart and peripheral circulation of both healthy rats and rats suffering from heart failure (HF; 220, 221, 322, 407, 472, 473). In HF rats with reduced ejection fraction (HFrEF) produced by myocardial infarction, Wisløff and colleagues used their new HIIT program to increase V̇o2max ~50% concomitant with multiple cardiac adaptations including increases in contractile function and positive changes in myocyte morphology and excitation-contraction coupling (222, 474, 476). Whereas previous studies demonstrated that HIST produces similar cardiac adaptations in HFrEF rats (340, 472, 473, 484–486), Wisløff et al.’s HIIT regimen also incurs beneficial adaptations in skeletal muscle metabolism and the peripheral vasculature (322, 407), whereas HIST does not (81, 340, 484–486). Accordingly, HIIT has distinct and compelling advantages over previous HIST and also moderate-intensity endurance-type training regimens. Crucially, these HIIT advantages have translated effectively to training humans with HF (6, 475, 476). Because V̇o2max is, perhaps, the best prognostic marker for mortality and morbidity in HF (224, 287, 459, 460), HIIT may be one of the best and most important therapeutic interventions for candidate patients with HF.
Exercise Modalities in Mice
Mice are invaluable for exercise studies from several perspectives including the following: 1) The mouse genome, sequenced in 2002 and published by the Mouse Genome Sequencing Consortium, facilitates identification of the genes controlling/influencing acute exercise responses and training adaptations (158, 159). Complete genetic sequencing data in humans enable homologous human genes to be identified. 2) Their high fertility and short gestation permit rapid and prolific breeding to establish the heritability of a given trait, and decades of inbreeding have produced a plethora of inbred mouse strains with contrasting voluntary and involuntary running exercise performance (269) allowing identification of polymorphisms and genes affecting exercise behavior. Moreover, mice can be selectively bred for high exercise activity on the voluntary running wheel (133, 185, 202, 431, 432). 3) Possibly most importantly, development of transgenic techniques makes it possible to manipulate the mouse genome. The mouse is the dominant choice for creating null transgenic animals with single-gene disruption by means of homologous recombination or overexpression by incorporation of multiple transgene copies into the genome. This proliferation of genetically modified mouse models across multiple disease states has made the mouse second only to the rat in the study of physiology and disease with respect to understanding the acute and chronic exercise responses.
A significant drawback is that traditional techniques for inactivating or overexpressing single genes typically impact all cells over the organism’s life span. Consequently, the presence of secondary consequences to gene inactivation on the primary or some other tissue/organ system or in a preceding developmental phase may compensate for the perturbation and confound interpretation (3). However, innovations such as Cre/Lox recombination, conditional/inducible knockouts, and optigenetics have greatly facilitated the ability of the researcher to control the tissue and temporal specificity of gene inactivation, and these techniques are being increasingly used to more elegantly examine the consequences of inactivation of genes in specific tissues/cells for specific time periods. Moreover, compared with rats, interstrain differences substantially impact exercise performance as detailed below.
Motorized treadmill.
advantages and disadvantages of treadmill exercise.
Use of the CS concept and V̇o2max measurement in mice allows for precise control and, therefore, uniformity of exercise intensity within and across study cohorts (20, 37, 187) with performance decrements used as a sensitive diagnostic indicator of cardiovascular or other defects (116). Major drawbacks are similar to those described above for rats and include necessity for aversive stimuli, foot/toenail injuries, and skilled and labor-intensive use of specialized and expensive equipment. Treadmill exercise performance differs by mouse strain, but no consensus has been reached on which strains perform best. Billat and colleagues measured CS across three different strains and found that with respect to CS, CD1 > FVB/N > C57BL/6J for males and that male and female C57/BL/J6 were not different (20). Other studies have concluded that Swiss Webster mice are excellent treadmill runners, whereas C57BL/6J are some of the poorest (18, 269, 272).
Mice can be run on a rat treadmill. However, so doing is not ideal as mice may run laterally and less efficiently. Also, spaces between lane barriers or between the end of the treadmill and the shock grid may create additional injury risk for mice given their smaller body size. Purpose-built mouse treadmills have steel or Plexiglas lanes ~10–15 cm wide to restrict each animal to its own ~92 cm-long lane. The treadmill ideally is capable of fine control over speeds from <5 up to 40–50 m/min. Adjustable treadmill elevation allows investigators to implement either uphill or downhill (eccentric) running.
conditioning mice to run on the treadmill.
As with rats, mice require familiarization with the treadmill typically over several days before the exercise running protocol begins. This entails placing the mice on the stationary belt to become accustomed to the apparatus sight and smell within the training room. On subsequent days, the treadmill is placed on the lowest speed setting, and the mouse is allowed to walk or run slowly for 5–15 min over several days. Longer durations and/or higher speeds should be avoided so as not to induce training adaptations. These regimens, including training protocols and behavioral definitions of exhaustion, are available in the literature (82, 83), and the investigator should be cognizant that some strains of inbred or genetically manipulated mice may require modified training schedules of shorter duration, lower intensity, or decreased frequency compared with their wild-type counterparts.
Mice can and do run willingly for a few minutes at relatively low speed after familiarization, but some aversive stimulus is often required to sustain this. The three most common stimuli to encourage mice who lag too far back on the treadmill are 1) tapping their tails or hindquarters lightly with a stick, 2) blowing puffs of compressed air on their hindquarters, and 3) placing an electric shock grid at the back of the treadmill where the shock is not sufficient to harm the animal. Here, too, care should be exercised when using a rat treadmill, as the smaller body size of mice may make shocks that are acceptable for rats excessively painful for mice. Mice unwilling to run after several training sessions should be excluded from the study. It is possible that food rewards, as used in rats, may decrease recalcitrance (472).
Running duration may range from a few minutes to a few hours, depending on outcome variables desired, with most training regimens using 30–120 min per bout (217) to achieve typical endurance exercise adaptations. Intensity is usually set via treadmill speed or incline with some maximal treadmill exercise tests increasing both progressively every 5 min until exhaustion ensues. Treadmill training paradigms are often at a constant speed throughout each bout with increasing bout speed as V̇o2max and/or CS improve (219). Training frequency can be as often as once or twice per day or as little as every other day depending on the outcome desired.
Measurements of exercise performance on the treadmill in the mouse.
To evaluate exercise performance, three variables are commonly used: 1) endurance time at a given speed, 2) running speed on a graded exercise test with the speed of the treadmill increased every 2–5 min until the mouse is exhausted, and 3) line or beam breaks per minute during a fixed-speed run. As for rats, measurement of CS may be scientifically indicated (37). Genetic manipulations or natural mutations that impair motor coordination or weight-bearing invariably adversely affect the mouse’s ability to run on the treadmill. Also, as with rats, foot or leg injuries will negatively affect treadmill running performance. Mice should be inspected each training bout for such injuries and treated promptly with palliative care and rest until able to resume the protocol.
Mice display the same stop-and-go running pattern as rats, which increases with exercise time as the mouse fatigues and is unable to maintain a constant speed. This stop-and-go running pattern and “coasting” can artificially inflate actual running time and distance and highlight why investigators need to continuously observe mice during treadmill running. Exhaustion is identified when the mouse requires several aversive stimuli per minute to continue running, and the investigator should discontinue the test.
Aspects of the running environment including ambient temperature (set to minimize heat retention), handling, circadian time, and familiarity with the treadmill conditioning can each impact on mouse cardiovascular control at rest and during exercise (35). As mice are nocturnal, treadmill familiarization and training may be more effective during the dark part of the cycle, and consideration should be given to changing the housing room light-dark cycle for investigator convenience. Exercising mice during their normal sleep period may produce greater stress on animals, interferes with key physiological responses, and may potentially detract from exercise performance. To reduce stress on the animals, further restrict the people in the proximity and also loud or sudden noises and movements. Continuity of handlers and trainers and maintaining a fixed training schedule are also advisable in this regard. Also, toe clipping for any reason including genotyping or identification adversely affects gait and is contraindicated. Finally, mouse instrumentation can impair running performance. For instance, implantation of a heart telemetry device decreased maximal treadmill exercise capacity 33% (35). However, with the continued advancements in telemetry devices (i.e., continued reductions in volume and weight), this effect on exercise capacity may be reduced over time.
As for the rat and other species, during exercise, heart rate and V̇o2 increase close to linearly as a function of either running duration or speed (90). Limb muscle(s) blood flow (295) and glycogen and glucose metabolism (100, 186) are increased by exercise as are multiple blood cytokine concentrations (69). Compared with their wild-type counterparts, exercise performance is changed in some (39, 116, 122, 143, 159, 384), but not all (134, 193), studies of null and transgenic mice. Interestingly, compensatory physiological adaptations to myoglobin knockout such as in vasculogenesis, myocardial hypertrophy, and polycythemia help reduce exercise performance deficits (116, 134, 144).
Wheel running.
advantages and disadvantages of wheel running.
Similar to rat running wheels, running wheels for mice allow them to exercise when and as intensely as they desire and minimize investigator time commitment (250). Running wheels for mice may be upright circular wheels like those used with rats (but smaller) that typically range from around 0.33 to 0.50 m in circumference (11.5–17.5 cm in diameter; 4, 25, 158, 159) or they may be angled, saucer-shaped wheels that may provide a larger footprint for the mouse along with leaving less empty space for the mouse to roam around the cage (142). This modality may also reduce the appearance of depression-like signs in mice (422). Voluntary wheel running exercise in mice provides a behavioral record, over days, weeks, or months, enabling resolution of drug, ergogenic aid, or toxin effects on mammalian physiology and behavior (19, 97, 437). This exercise modality can also measure behavioral/functional defects in both naturally occurring mutant mice, for instance, mdx mice (157), and genetically engineered null mice (158, 159, 444, 463).
With respect to assessing therapeutic efficacy, wheel running can resolve whether exercise reduces disease phenotype and may be valuable for human treatment. It is pertinent that wheel running constrained disease progression across multiple mouse models of human neuromuscular diseases, including amyotrophic lateral sclerosis, Parkinson’s disease, and Duchenne muscular dystrophy (103, 160, 236, 440). A particularly exciting observation is that wheel running enhanced natural cytotoxicity (283) and decreased tumor metastasis/progression across different cancer models (66, 69).
There are multiple disadvantages in using wheel running exercise in mice. One major disadvantage is that to evaluate an individual mouse’s voluntary running, it must be housed singly for an extended period of time. This is contrary to the group housing recommended for mice and may induce stress. In addition, singly housed mice that are not allowed access to wheel running (i.e., a replicate of the physical environment of the exercising mice but with a locked wheel) may demonstrate physical deconditioning due to the large reductions in spontaneous cage activity compared with group-housed mice. This physical deconditioning found in singly housed mice has been associated with vascular endothelial dysfunction, reductions in aortic endothelial nitric oxide synthase (eNOS) protein expression, and reductions in skeletal muscle citrate synthase activity compared with their group-housed counterparts (430).
Additional disadvantages of wheel running include the following: 1) Mice will play with the exterior of the running wheel and may be able to rotate the wheel to introduce false counts of running activity. 2) The amount of bedding and nestlets in the cage may need to be reduced to prevent obstruction of the wheel (i.e., mice may push their bedding and shredded nestlets against the wheel). 3) Specific transgenic mice may refuse to engage in sufficient wheel running to promote training adaptations. 4) Physiological reasons for not running or running less may be inseparable from psychological ones. 5) Mice run intermittently across their active (nocturnal) cycle; hence the precise timing often required to explore acute postexercise adaptations may not be possible. 6) Feet can become injured, and mice should be periodically examined. 7) The wheel can accumulate urine and needs to be cleaned regularly. 8) When animal facility personnel clean the cages, mice may show a small upward spike in running behavior. 9) Accidentally changing the light-dark cycle, especially by leaving the lights on, can reduce running behavior.
Although mice can use larger (i.e., >30 cm diameter) wheels successfully, if the wheels are too big for the mouse, the inertia that must be overcome to turn the wheel will discourage them from running. Mice prefer wheels with a plastic mesh flooring to metal rods (25) but will run effectively in the standard painted-wire mouse wheel (4, 269). Unlike treadmill running, no conditioning process is needed. However, the investigator should allow a day or two for the mouse to access the wheel before officially recording performance metrics.
Measurements of exercise amount during wheel running in the mouse.
Wheel running time, distance, and pattern of running can be recorded by computer (250), or alternatively, a simple bicycle monitor will track distance, time, and maximum speed information (4, 158). Depending on sex, age, and strain, mice run for 1–10 h per night averaging 1–10 km (4, 269). As for rats, mice distribute among high-, intermediate-, and low-running activity levels, with ~10% opting not to run. Over several weeks, mice display a biphasic running pattern: for the first 2–4 wk, average speed and total distance increase with duration unchanged (4) associated with endurance adaptations (159, 269), and from 4 wk onward, average nightly running duration and distance remain fairly constant. Because of the variations in speed and distance run during the first 2–4 wk running, comparisons of short-term wheel running are not likely to be reproducible or informative unless there is a large effect size.
Female mice tend to run longer and farther per night than their male counterparts (243). Older mice are less active than young mice (458).
Swimming.
advantages and disadvantages of swimming.
Swimming recruits a large muscle mass and induces extensive cardiovascular adaptations (214). As was the case for rats, the required apparatus is inexpensive, and the duration and exercise intensity can be controlled better than with wheel running. Disadvantages include the following: 1) There is demand for investigator vigilance. 2) Unless precautions are taken, it may constitute an intermittent hypoxic exercise model. 3) Swimming produces psychological stress (115) that confounds exercise adaptations. 4) There may be significant differences in the ability of mice to adapt to a single acute bout of swimming (null and transgenic mouse lines; 193, 235, 387, 399). 5) Special care must be taken to ensure that null and transgenic mice are capable of swim training without drowning or significant changes in swimming behavior (i.e., increased bobbing, diving, or floating).
Measurements of exercise performance during swimming in the mouse.
Before initiating a study, mice should be placed in the tank and allowed to swim for a few minutes on 3–5 different days to allow them to acclimate. Longer than 5–10 min may produce exercise adaptations.
Water depths ranging from 10 cm (435) to 50 cm (341) have been used, but it is important that the depth of the water is greater than the length of the mouse from nose to tip of tail to prevent diving to the bottom to avoid continuous swimming (160). Having 10–15-cm tank depth above the water surface prevents mice from climbing or jumping out (110). Because mice climb atop one another, increasing the risk of drowning and decreasing continuous swimming, group swimming in a single container is contraindicated. An effective strategy involves fabricating a Plexiglas grid that, when placed on top of a larger tank, defines separate areas for individuals to swim (110). Water temperature influences swimming behavior in mice. Mice are typically swum in water below mouse body temperature [~36°C; i.e., 32–36°C (110, 214)]. However, they can swim in water at room temperature (23°C; 341), whereas below this, core body temperature may decrease and swimming speed in a Morris water maze test is reduced (191).
Swimming behavior found in mice and rats contrasts in two important respects: 1) Mice choose to continuously swim with less time devoted to diving, bobbing, or climbing (5, 214), and as with rats, water bubbling helps prevent these unwanted behaviors (5). 2) Mice minimize use of their forelimbs preferring to swim with their hind limbs (5, 214), which contraindicates use of this species for studies focused on the forelimb musculature.
Swimming time is usually measured as an index of performance and training adaptation. Because mouse swimming really constitutes treading water in a limited area, the distance swum is not measured or useful. After swimming, mice should be dried with a towel. Similar to the rat, mice should be temporarily placed in a warm environment to avoid hypothermia.
Several different mouse strains have been studied with swimming and swimming training, including C57/B6J (192, 214), Swiss Webster (242), C3H (348), NIH-Black Swiss (193), BALB/c (115), and FVBN (307, 308). In addition, swimming studies have used male and female (192, 214, 242) as well as young and old mice (5, 115, 356). Whereas there is limited evidence that old mice swim for shorter times than young mice (356), at present, the effect of differences in sex on mouse swim performance is not well established.
Differences in swimming performance may also occur between several null and transgenic mouse lines as the ability of mice to adapt to a single acute bout of swimming has been documented (193, 235, 387, 399, 445). In addition, Ren et al. (387) have shown that some transgenic mice can sustain up to 3 h of swimming with no differences in swimming performance compared with wild-type mice. However, the transgenic mice evidenced differential postexercise adaptations compared with wild-type controls (387).
Exercise training in the mouse.
Both sex and age impact running performance and adaptation. For intensity-controlled treadmill training, female (vs. male) mice evince a greater adaptive range for V̇o2max, ventricular mass, cardiomyocyte size, and skeletal muscle mass (219). In support of this, for the equivalent exercise intensity, female mice evidence greater adaptational benefit, and as for rats and humans, young mice run longer at a fixed submaximal treadmill speed than old mice (356).
Treadmill training of mice increases V̇o2max (219, 344), maximal running speeds (219), endurance capacity (18, 139, 293), heart and ventricular masses (219), skeletal muscle O2 and substrate utilization including glucose uptake (434), and fatty acid oxidation. Treadmill training will also increase skeletal muscle mitochondrial enzyme content (402), skeletal muscle mass, and capillarization (219, 421). It should be noted that the responses to treadmill training may be highly dependent on the strain of mouse investigated and that appropriate strain-matched controls are used in these types of investigations (18).
A single bout of downhill treadmill running constitutes a model for eccentric contraction-induced muscle damage. In mice, a single bout of downhill running at a 5–20% downward slope in naïve animals elevates serum creatine kinase and decreases specific tetanic force production for up to several days following exercise (61). Training mice with downhill running abolishes this effect (282). However, prolonged running training over the lifetime negatively impacts mouse joints increasing osteoarthritis severity in the knee joints of male C57 mice (249).
Weeks of voluntary wheel running have the following effects: 1) V̇o2max measured during treadmill running is increased (431). 2) Heart mass is elevated via increased ventricular wall thickness (4). 3) Body mass is decreased compared with age-matched sedentary control mice by limiting tissue accumulation. 4) Skeletal muscle mitochondrial enzyme expression is increased (4, 158). 5) Muscle myosin heavy chain (MyHC) expressions shift toward greater MyHC IIa and decreased MyHC IIb isoform expression (4, 158). 6) Like rats, mice evince hind limb skeletal muscle hypertrophy, especially of the soleus muscle (4). 7) Adding resistance to the wheel induces tibialis anterior hypertrophy (196). 8) Neurogenesis and synaptic plasticity are increased. There is increased expression of brain-derived neurotrophic factor (BDNF) in the hippocampus of mice (32, 237) and rats (343) along with increased cell proliferation and neurogenesis in the dentate gyrus (451). In contrast, swimming for 5 min reduces hippocampal BDNF (398) suggesting that increased physical activity alone is not sufficient to increase BDNF expression and supporting the scientific value of voluntary exercise paradigms.
As with any exercise/training paradigm, the length of the training regimen and the frequency, duration, and intensity of each swimming bout can be manipulated as desired. In mice, training regimens can range from days to months depending on the hypotheses tested, with animals swimming 30–180 min at a time (longer as ability increases) once or multiple times per day and every day per week, as indicated. The intensity of swimming increases as the mouse swims continuously in preference to floating or bobbing and, crucially, with the amount of weight (2 or 4% body mass; 5) attached to the animal’s tail and its precise position relative to the base of the tail.
As with other forms of physical exercise, swimming in mice elevates heart rate (235) and V̇o2 (481) and alters insulin and glucagon secretion rates (216). As for most mammals, water immersion in mice instantaneously decreases heart rate, known as diving bradycardia, which lasts for several minutes and often masks early exercise-appropriate elevations of heart rate (65). Conducting chronic swim training sessions (several times per week for several weeks) also produces typical endurance adaptations of the cardiovascular and neuromuscular systems. Specifically, swimming 90 min twice daily, 5 days/wk for a total of 4 wk, increases relative heart size 14–25% (110, 214) and skeletal muscle citrate synthase activity 45–58% (110, 214) and decreases heart rate at submaximal workloads by up to 20% (214) after 1 mo of training. Swim training also results in additional skeletal muscle adaptations that increase exercise tolerance, including increased lipogenic enzyme expression (192), increased muscle capillarity (421), and decreased muscle fiber size.
The recommended swim training protocol for producing maximal cardiac hypertrophy was initially 90 min per bout, twice a day, 5 days/wk for 4 wk, with no weight attached to the mouse (110). However, McMullen et al. demonstrated that extending the training regimen from 5 days/wk to 7 days/wk induced a greater degree of physiological cardiac hypertrophy (increases in heart weight-to-body weight ratios of 35–45%) with the magnitude of the hypertrophy similar to that found in mice with pathological cardiac hypertrophy induced by pressure overload via aortic banding (307). Studies using this training regimen were critical in demonstrating that PI3K, Akt, and IGF1R and other signaling proteins were crucial for exercise-induced cardiac hypertrophy, whereas other signaling proteins were not (i.e., calcineurin and NFAT; 88, 307, 308, 470). In addition, the hypertrophic responses produced with this swimming regimen in FVBN mice are greater than those produced in C57BL/6 mice (FVBN, 307, 308, 470; C57BL/6, 88, 276, 436). See recent review by Bernardo et al. (34). Accordingly, as with treadmill running, investigators need to consider how swimming performance may differ between different strains of mice and how each mouse strain may respond to exercise training using this exercise modality.
LARGE ANIMALS
For larger animals, specifically horses, dogs, pigs, and rabbits, treadmill running is the primary modality chosen by investigators in exercise studies.
Exercise Modalities in Horses
Evaluation of equine cardiopulmonary function and disease detection at rest and during exercise is now possible at a level close to that in humans. This has been driven by improvements in equine treatment and welfare as well as associated industries such as horse racing.
Motorized treadmill.
advantages and disadvantages of treadmill exercise.
The horse is a superlative model of cardiovascular and oxidative function. During peak running speeds, the elite Thoroughbred horse can achieve a V̇o2max well above 70 L/min, which is substantially higher than that of any other animal yet measured. Because blood gases in the galloping horse resemble those in patients with severe lung disease (hypoxemia and hypercapnia; 229, 230, 233, 300, 301, 353), the horse constitutes a model of lung failure and also pulmonary hypertension with mean pulmonary artery pressures during exercise exceeding 90 mmHg (368, 369, 370). With high pulmonary capillary intraluminal and very negative alveolar pressures during exercise the horse experiences exercise-induced pulmonary hemorrhage (EIPH), characterized by failure of the exquisitely thin blood-gas barrier and escape of red blood cells into the alveolar spaces and airways. Although frank epistaxis is rare, all performance horses evaluated by bronchoalveolar lavage after prolonged moderate- (107) or heavy/severe-intensity running experience EIPH (108, 370). The outcome of several millennia of breeding for speed, horses are high-performance runners. Provided there are no negative associations with the treadmill, they quickly become accustomed to running on the level or inclined treadmill at speeds high enough to reproducibly attain their maximal heart rates and V̇o2max (43, 229, 233) and also permit determination of their CS (251). Notably, development of portable equine face masks suitable for respiratory measurements in the field may expand the ecological validity of testing protocols that have, to date, only been possible using the equine treadmill (417).
conditioning horses to run on the treadmill.
Horses can master a standardized treadmill test consisting of walk, trot, canter, and gallop in just one to three treadmill visits. However, they will improve their comfort level and smoothness of locomotory pattern over 10–20 sessions. This is especially true for familiarity with other equipment such as face masks. As with rats, food rewards, in this case, alfalfa pellets, given in a mock-up face mask after treadmill running help build a positive association with equipment. For complex invasive instrumentation such as arterial or pulmonary venous catheters, especially in combination with a face mask, up to 6 wk of familiarization (2–4 times/wk) may be advisable. This is particularly important where true “resting” responses for horses standing on the treadmill (i.e., heart rate ~30 beats/min) are required before running (291).
Measurements of exercise performance on the treadmill in the horse.
Incremental, constant-speed, or intermittent running formats can be selected depending on the research or clinical hypothesis being tested. Most common, perhaps, is the incremental exercise test to measure V̇o2max, which is analogous to that used in humans. When this is performed on the flat treadmill, the horse typically walks or trots at 3 m/s for 800 m (~4–5 min) before moving to the canter at 7 m/s. At this point, the treadmill speed is increased by 1 m·s−1·min−1 until the horse reaches exhaustion. Today, many modern treadmills can achieve 16–18 m/s, which is sufficient to achieve V̇o2max in almost all horses. Because horses evince a slow component of the V̇o2 kinetics (224), even the fastest and fittest horse will eventually reach maximal oxygen uptake before exhaustion at these speeds (373, 376). On an incremental exercise test, most horses peak at 15–17 m/s on the flat, and this peak speed is reduced considerably on the incline (10–12 m/s at 10%).
In constant-speed protocols, analogous to “square-wave” or step work forcing in humans, the horse transitions between rest or a low speed and higher speeds. Similar to horses accelerating rapidly out of the gate at the racetrack, modern treadmills accelerate the horse to the required speed within 5–10 s (137, 248). A typical constant-speed test protocol might incorporate a warm-up comprising walking or trotting for 1–5 min, followed by a slow canter at 7 m/s or a gallop at 13 m/s for 5 or 6 min or until exhaustion, followed by a walk or a trot for a cooldown.
Akin to constant-speed protocols, intermittent running protocols consist of a rest or a low speed to a higher speed transition. Intermittent running protocols are flexible and often involve repeated bouts at a given speed or progressively increasing speeds interspersed with several minutes of walking recovery. Intermittent exercise protocols can induce more extreme physiological and metabolic responses (e.g., blood lactate and body temperature increases) than incremental or constant-speed tests performed to exhaustion.
Other, more customized test protocols may replicate an activity or competitive event such as a 3-day event. Here different environmental conditions may be established on consecutive days, and a constant-speed endurance test is conducted.
After any high-speed or prolonged moderate-speed running, it is vital to cool the horse down. Typically, this is accomplished with several minutes trotting or walking on the treadmill at 3 m/s, followed by 20–30 min of hand walking on grass until resting heart rate approximates baseline conditions. Other options include giving a cool bath, legs first, and allowing moderate water intake.
The treadmill’s rubber belt is harder (disadvantageous with respect to injuries but energetically more efficient; 208) and more even (advantageous) than most other surfaces on which horses normally walk or run. In this regard, the Mustang treadmill is >6-fold stiffer than the Säto treadmill (208). Horses can run on a treadmill inclined up to 12%, and this reduces the concussive impact on their forelimbs. To produce a heart rate response equivalent to carrying a rider on the flat, a treadmill incline of 3.5% is used. It is important to recognize that inclined running produces a higher maximal cardiac output and V̇o2max in the horse (301, 302) as well as exacerbating EIPH (231) and elevating hind limb strain (433). Whereas horses can run up an incline as steep as 12%, prolonged trotting on a 5% incline at 5 m/s can induce lameness (290).
Investigators considering an incremental test using an incline (up to 10 or 12%) should be aware that this may impair the ability to identify a gas exchange or lactate threshold. Specifically, data must be collected at several subthreshold speeds (300, 302), which may be as low as 3–7 m/s on the incline. These slow speeds often produce a choppy, uneven gait, which may compromise the normally smooth cardiorespiratory and metabolic progressions desired for threshold detection.
In horses, most types of exercise risk orthopedic injuries including bowed tendons and lameness; occurrence of either, or any form of ataxia, on the treadmill mandates stopping the test immediately and administering veterinary care. Any exercising horse can suffer from lameness requiring rest, and under veterinary supervision, appropriate diagnostic procedures and therapeutic interventions should be instituted. Additional exercise-related injuries include exertional rhabdomyolysis and are rare. Efficient clinical monitoring ensures early detection of such injuries. Running horses under analgesics is contraindicated as it can incur catastrophic injury. Horses running on a treadmill should be equipped with an overhead safety harness (surcingle) that triggers the emergency treadmill shutoff switch. The surcingle supports the horse’s weight in an emergency such as a misstep or pulling up lame reducing the risk of injury to horse and research staff.
With only ~40% of the body surface area per unit mass of humans, thermoregulation in Thoroughbred horses is more challenging especially in normothermic or hot environments. During exercise at V̇o2max (~180 mL·kg−1·min−1 of oxygen uptake for a fit racehorse) the rate of heat production in the horse is ~3 times that generated by a fit club-level human distance runner and necessitates the horse to lose heat 7.5 times faster per unit surface area than found for humans. Since this rate of heat loss is untenable in running horses, they evince a precipitous rise in body core temperature to ~43°C or higher (228, 233). Well-hydrated horses achieve maximal sustainable sweating rates approaching 15 L/h, or ~3 L·m−1·h−1 (~3 times that of humans). Each minute at V̇o2max, horses store enough heat to raise their body temperatures by 1–1.5°C, and racing Thoroughbred horses may increase their core temperature as much as 3–4°C (290, 291, 312, 313). The likelihood that a horse will experience severe hyperthermia (core body temperature >42–43°C, or 107.6–109.4°F; 290, 312, 313) is heightened at ambient temperatures above 25°C with humidity above 70%. Several additional circumstances exacerbate hyperthermia risk: 1) being overweight, 2) being unconditioned to exercise, 3) having a long/thick coat, and 4) being inadequately hydrated, for example, following a previous run and/or diuretic (Salix/furosemide) treatment for EIPH (229). Salix causes rapid loss of up to 20 kg fluid and lowers pulmonary artery pressures. Whereas 1% loss of body mass as fluids for human athletes reduces performance up to 10%, this relationship in horses is not known. The large hindgut of the horse contains 30–40 L of fluid that can be drawn on during exercise and replaced afterward. Monitoring the return to preexercise body mass can provide a good indication that adequate rehydration has occurred.
Hyperthermia becomes evident within 1–2 min when horses run at high speeds with inadequate airflow (290), which can be exacerbated by considerations 1–4 listed above. In addition, drugs that impair sweating, such as nitro-l-arginine methyl ester (l-NAME), accelerate exercise-induced hyperthermia (228, 233). Horses often simply stop running or refuse to run when they cross some hyperthermic threshold of ~42–43°C. However, maximal running speed tests normally spike body temperature up to ~43°C, which horses can tolerate without injury. IACUCs that do not have a clinician who is familiar with the exercise response in the horse will need to be advised accordingly. Good animal husbandry requires that horses exercised for extended times (i.e., >20 min at >70% V̇o2max) that sustain body temperatures ≥41°C be monitored assiduously for ataxia or sweating cessation. It is recommended that the equine laboratory be equipped with a large fan(s) to blow air over the horse at a speed at least matching that of the treadmill.
For the forelimbs, protective tendon boots that extend past the fetlock and bell boots to cover the hind limb heel bulbs are recommended during treadmill running.
Unless the protocol demands other dietary conditions, food should be withheld 2–3 h before testing and for ~2 h after testing.
The definition of exhaustion in the horse presents as great a challenge as for humans and murines. Working definitions in horses include 1) when the horse drops back >1 m from the treadmill front barrier, 2) when the horse has dropped back but is encouraged humanely to approach the front barrier two or three times, and 3) the time point when the horse cannot be encouraged back to the front barrier. A good indicator that the horse is approaching exhaustion is alternating leads frequently.
Encouragement for the flagging horse may be verbal or with gentle use of a riding crop. In some instances, a fly whisk or the hand is used to pat the gluteal area with two or three light flicks being sufficient to motivate the horse for one final effort. As the horse begins to slow down, the test should be terminated, and cooldown begun at a trot or walk. Ethical treatment demands that the horse should never be struck with spurs, other sharp or damaging objects, or electrical prods. This behavior will prove counterproductive and make the horse skittish and unsuitable for treadmill running.
Exercise training in the horse.
The presence of EIPH and other health-related issues such as soft tissue damage and/or lameness dictate that horses should not be run to exhaustion repeatedly without adequate rest days. As a general rule, maximal exercise tests should not be performed more than once per week or >8 times within a 3-mo time span. Lung lavage red blood cell counts return to control levels within ~1 wk after maximal exercise (311). For laboratory horses, an effective training/conditioning protocol might include short runs to 70–80% of peak treadmill speed at 2- or 3-day intervals each week. In well-controlled laboratory trials, nasal strips and Salix effectively reduce EIPH (229), and unless the treatment interferes directly with the experimental design, one of these treatments, and possibly immunomodulation therapy (107, 368, 369), should be considered for horses suffering from severe EIPH.
Exercise Modalities in Pigs
The selection of the pig as an animal model is dependent on fundamentals in considerations for animal model selection. Consideration of the pig as an exercise model includes the acceptance of its innate behavioral characteristics, goal of the exercise study, and exercise variables of intensity, duration, and frequency, which define the exercise effort or “dose.” The essential objective measures to define the exercise dose are especially important, as the goal is typically to elicit classical training adaptations noted in humans. It has been shown that the association of cardiovascular disease death rates with level of fitness exhibits a strong inverse correlation (40, 41); that is, individuals with higher levels of fitness have lower cardiovascular disease death rates. A key finding is that only moderate fitness is required for a significant association with decreased death and the relationship reaches a plateau between moderate and high fitness levels. The exact amount of exercise required for optimal physical health has evolved somewhat over the years, but the overall view is that maximal exertion may not be required (40, 120, 265, 266, 360).
A special consideration in swine is their innate tendency toward sedentary behavior (98). Given this natural tendency and the need for behavioral modification to elicit running behavior, the pig probably should not be the first animal of choice for studies of extremely high intensity athletic performance involving near-maximal effort. This is in contrast to the horse and dog, which generally appear to thrive on high-intensity exercise. Instead, the pig might be preferred when moderate-intensity exercise (e.g., 60–75% of maximum heart rate) can elicit the acute responses and chronic cardiovascular adaptations of interest. More specifically, the exercise intensity should elicit the health benefits from chronic exercise training. Also, we reiterate that as with other animal models, swine models are essential to study the cellular, molecular, and integrative physiological mechanisms of exercise to provide fundamental knowledge and a basis for therapies, including targets of pharmacotherapy for diseases.
Motorized treadmill.
Exercise studies in the pig have been limited to aerobic and anaerobic running exercise on a motor-driven treadmill. Exercise studies emphasizing resistance and strength training, flexibility, and motor skills in the pig are nonexistent and thus will not be considered. Increases in muscle and skeletal strength are likely, however, to result from exercise of any sort, and it is important to note that future studies may utilize swine to study these adaptations.
advantages and disadvantages of treadmill exercise.
Several important similarities between pigs and humans highlight the advantages in the use of swine for studying responses and adaptations to exercise. 1) Swine have a propensity toward sedentary habits compared with dogs, which are more likely to pace in their cages if not adequately exercised (98). 2) Swine are monogastric and omnivorous; thus they will consume a “human-type” diet (363). 3) Swine lipoprotein metabolism is similar to that of humans (95, 203, 286, 323, 385). The pig has a lipoprotein distribution close to that of humans (67) and on a low-fat/cholesterol diet carries 50–60% of total plasma cholesterol in low-density lipoprotein (LDL) particles (62, 94, 95, 286, 385). This is in contrast to rabbits and mice, which carry almost all of their cholesterol in very low density lipoprotein and high-density lipoprotein (HDL) particles (462), and dogs, which carry fivefold to sevenfold more cholesterol in HDL than in LDL (284, 285, 446). 4) Pigs are sexually mature early in their long life span (447). 5) Most swine are relatively docile and can be handled and restrained in low-stress devices (351, 357, 469), despite the common misconception that the “large” size of even miniature swine (typically ~30–80 kg) is a limitation to their use (215). 6) The humanlike size of organs and transgenic swine technology make xenotransplantation a distinct and practical possibility (246); thus, swine physiology of exercise must be studied to provide a full characterization of organ viability. 7) The “larger” size of pigs is an advantage for adequate blood sampling volumes (typically >20 mL/sample), instrumentation, and longitudinal measures in the same pig (e.g., 242, 429, 447). For example, coronary blood flow can be measured in conscious, exercising swine (44, 309, 310, 429, 448). Sampling of larger blood volumes is essential for studies of coagulation factors, platelets, etc., which is not possible in smaller animals (416). 8) A profound similarity to humans is the cardiovascular system, specifically the heart and coronary circulation and susceptibility to coronary artery disease. Several features are most prominent. 8a) Gross anatomy, including paucity of innate collateral arteries, is similar to that of humans (77, 406, 466, 467). 8b) The pharmacology of coronary artery vasoreactivity is similar to that of humans (114). 8c) Heart rate and, thus, metabolic demand on the heart and cyclic changes in coronary blood flow, are also similar to those of humans (151, 467). For example, resting bradycardia after chronic exercise training can be 50–60 beats/min (316, 317, 477). This is profoundly different from rodents (e.g., mice and rats), which have resting heart rates of 300–600 beats/min (2, 428), and mice have been shown not to increase heart rate further with increasing treadmill workload (2). This indicates that metabolic demands and O2 transport and utilization strategies are dramatically different from those of humans. 8d) Atherosclerotic lesions are morphologically similar to those in humans (31, 138, 184, 203, 299, 362, 386). In contrast, vascular disease occurring in mice results primarily in development of fatty streaks, not full progression to complex lesions, calcification, etc., as in human atherosclerosis (49, 189). 8e) The size of the pig heart is similar to the size of the human heart, thus enabling trials of percutaneous catheter interventions for revascularization with devices identical to those used in humans (104, 105, 128, 201, 277, 281, 409, 443). 9) Finally, and very importantly, the V̇o2max per kilogram of body weight is similar to that of untrained humans and less than that of the rat or dog (13, 63, 112, 403, 469). The cardiovascular adaptations to exercise are also common to those in humans (13, 45, 317, 468, 478). Thus, on the continuum of similarity to humans the pig is an excellent choice of animal model for studying the cardiovascular aspects of physical exercise. On the other hand, the pig would not be the preferred animal model if the purpose of the investigation is to contrast its characteristics with humans and study elite physical exercise performance.
conditioning pigs to run on the treadmill.
The age at which exercise is typically started in miniature swine (Yucatan, Sinclair, or Ossabaw) is 6–8 mo when animals are sexually mature and weigh 30–60 kg or at age ~2–3 mo in juvenile domestic pigs. No studies have been conducted on substantially younger miniature juvenile pigs, but the probability for successful exercise studies at a younger age is very high, based on domestic pigs (58). Similarly, studies with older domestic swine are feasible, but the limitation is the size of the treadmill, since domestic breeds can weigh in excess of 100–150 kg at 6 mo of age. A treadmill with versatility to be used for small horses would clearly be suitable, as shown for a Yucatan miniature pig in Fig. 7. The treadmill should typically be 1.5 times the length of the pig to allow some forward and backward movement on the treadmill. A narrow width of the treadmill is also important to prevent the pig from turning around on the belt, which could lead to injury if this occurs during the exercise bout. To accommodate various sizes of pigs, a simple restraining board can be fashioned out of an inert material (plastic or hardened rubber) for temporary width adjustment. Figure 7 illustrates these features. Treadmills used for humans will suffice if an opaque enclosure is placed on the treadmill to contain the pig. The other main consideration is avoiding slippage on the treadmill due to urination, defecation, and water.
Fig. 7.
Treadmill exercise training of swine. The early phase of the exercise training protocol involves conditioning the pig to the treadmill. A moderate treadmill speed of 4.8 km/h and 3% grade (incline) for this male Yucatan miniature pig with diabetic dyslipidemia is a casual, easily tolerated pace, as shown by the ability to look to the side through the bars. The treadmill is the optimal size to prevent the pig from turning around and has a padded chain at the rear to alert the pig and minimize collision with the rear wall.
Typically, 1–2 wk (5–10 sessions) are used to familiarize the pig to the treadmill and running using standard principles of animal behavior modification (79). It is important that the pigs first be reasonably acclimated to human contact, i.e., socialized, which requires time spent daily in the pig’s cage and physical contact, including scratching and placement of hands at the chest where the heart rate may be monitored. It is helpful to obtain resting heart rates with a stethoscope or by a telemetry device while the pig is in its pen. Simple contact with humans can increase heart rate, thus perhaps making it impossible to obtain a true resting heart rate; however, this is a practical, inexpensive method, and exercise training-induced bradycardia can be observed with these heart rate measures (45). If already appropriately socialized, the pig can be led onto the treadmill by mild application of pressure in the desired direction. Positive reinforcement with a food reward or fruit juice is an effective additional enticement (45).
The first sessions may simply involve placement of the pig on a stationary treadmill and feeding if anxiety toward the treadmill is evident by vocalization, attempts to escape, and excessive heart rate (e.g., >50% of maximum, ~125 beats/min). The percent grade and speed of the treadmill should be at low initial settings before turning on the treadmill to avoid startling the pig. During the familiarization period, exercise time and grade and speed of the treadmill are incrementally increased to reach a workload that elicits a heart rate that is at the intensity desired for optimal exercise training effects (large animals, Exercise Modalities in Pigs, Exercise training in the pig). For example, in week 1 of the familiarization phase the pigs might walk on the treadmill 4 days/wk, only 10 min/day, 0–3% grade, at a slow walking speed of ~2.5 km/h, which elicits a typical heart rate of only 35–40% of maximum heart rate. To put this heart rate in perspective, resting heart rate is 20–30% of maximum; thus, the familiarization heart rate is considered mild-intensity exercise. Week 2 might involve 20 min/day, 5% grade, at a walking speed of ~3.3 km/h, which elicits a heart rate of 45–55% of maximum heart rate. A reward for their compliant behavior given at the end of these early sessions can also aid in successful familiarization.
Although it is to be avoided unless absolutely essential, an aversive stimulus may be used. An electric shock, air spray, or light slap on the hindquarters is virtually 100% effective in eliciting compliance with these low-intensity exercise acclimation regimens. Furthermore, after an initial electric shock the aversive stimulus is immediately associated with noise of the technician behind the pig, such that similar posturing will elicit compliance with the exercise protocol. Electric shock can be administered with a commercially available, handheld device used for livestock. When the session is completed, the pig is rewarded for compliant exercise behavior as soon as possible with a food treat or ~100 mL fruit juice and daily feeding. Delaying the reward long beyond the exercise session will result in minimal association of positive reinforcement with the exercise behavior and thus will not effectively shape the behavior.
Measurements of exercise performance on the treadmill in the pig.
The recommendations in this section are based on general principles in general considerations for exercise study design and ~150 published exercise studies conducted on swine (e.g., 13, 45, 46, 59, 102, 109, 273, 305, 306, 317, 321, 350, 359, 425, 427, 439, 467, 469, 477). It should be noted that the pig is a US Department of Agriculture-regulated species; thus conservative use and more thorough documentation are required and prudent.
measurement of V̇o2max in the pig.
Several excellent studies have documented V̇o2max measures in miniature swine (13, 63, 345, 403, 469). Clearly, this rigorous measure provides evidence of the classic aerobic capacity in pigs, which is correlated with endurance capacity. As will be reinforced in later sections, these measures provide confidence in simpler measures of fitness.
measurement of endurance capacity in the pig.
Figure 8 shows that the submaximal heart rate at an absolute workload was inversely correlated (R = −0.88) with run time to exhaustion, thus confirming that submaximal heart rate is a valid index of exercise performance in pigs. This significant correlation demonstrates the strong predictive value of the submaximal test. This guideline is in compliance with the refinement principle in the “Three Rs” emphasized in the Animal Welfare Act: use of a method that lessens or eliminates pain and/or distress and therefore enhances animal well-being. Basically, if the appropriate physiological hypotheses can be tested, the lowest intensity, duration, frequency, and total length of study should be employed. Other noninvasive measures in conscious pigs, such as decreased resting heart rate (bradycardia) may also be used (e.g., 105, 262). Given the validity of submaximal tests in humans and pigs, if the investigator’s aim is to document efficacy of training that may impact improvement of health, rather than assess elite athletic performance, a submaximal fitness test may be preferred (and more convenient) over a maximal fitness test.
Fig. 8.
Measurement of endurance capacity. Submaximal exercise heart rate predicts run time to exhaustion in Yucatan miniature pigs. Male pigs (n = 15 pigs) were treadmill exercise trained 5 days/wk for 16–20 wk or confined to their pen in a protocol similar to those previously described (46, 371). Data are for the treadmill performance test at the completion of training or sedentary treatments. The correlation (R) of −0.88 was statistically significant (P < 0.05). Here, b, beats.
Exercise training in the pig.
Intensity, duration, and frequency of exercise define the exercise effort or dose. A plethora of literature has established the firm linear relationship between heart rate and V̇o2 in humans (16), and this has been confirmed in pigs (13, 42, 63, 112, 209, 469). Thus, it is recommended that the exercise intensity be determined by a percentage of the maximal heart rate of ~250–275 beats/min (42). Although maximal heart rate has individual variability, in healthy pigs the variability is low (42); thus the direct determination of maximal heart rate in each pig is not imperative. Exercise heart rate for chronic training protocols can be specified as a range, the “target zone,” to ensure appropriate intensity and safety for the pigs. Measurement of heart rate is relatively straightforward in this large animal. Thus, if there is any doubt of the animal’s perceived exertion, heart rate can give an objective measure of intensity. The size and heart rate of the pig enable relatively simple auscultation of the heart rate with a commonly used stethoscope or palpation to provide a noninvasive and simple index of exercise intensity (45). Monitoring heart rate is helpful even though specific grades and speeds of the treadmill have been shown in the literature to elicit specific heart rates (e.g., 42, 44, 45). The heart rate response to a typical exercise training session in Fig. 9 shows resting, gradual increase to the target heart rate zone of 65–75% of maximum and plateau for the 30-min exercise bout in the target zone, followed by a cooldown period in an Ossabaw miniature pig. The treadmill can be stopped to obtain heart rates using auscultation, or a standard human heart rate monitor can be belted on the pig for continuous measures. The individual variability and special considerations (e.g., thermal stress and disease) can have profound effects on the relative intensity, thus sometimes precluding a “one size fits all” approach in these instances.
Fig. 9.
Typical treadmill exercise heart rate responses of an obese Ossabaw miniature pig with metabolic syndrome during phases of a single exercise session. Heart rate [beats (b)/min] is shown during rest on the treadmill and then at 0% grade for 5 min at treadmill speed of 2.2 km/h and 5 min at 6.1 km/h [~40–50% maximum heart rate (Max HR)]. The target exercise heart rate (65–75% of maximum) was elicited by increasing the treadmill speed to 7.7 km/h and grade of 5%. The cooldown period was at 2.2 km/h and 0% grade. Data are from a male pig (see 105).
Because the exercise effort/dose is, in principle, very similar to a drug dose, daily records should be kept and available for IACUC inspection (as noted in general considerations for exercise study design, IACUC Review). For example, an exercise log sheet should include resting, warm-up, training, and warm-down stages. Similar log sheets should be maintained for the treadmill familiarization or conditioning phase. Warm-up stages might be 2.5 and 4 km/h, providing basically an easy walk. Another stage might be a 5 km/h speed at a variable grade to elicit a training (“target”) heart rate of some percentage of maximum, e.g., 65–75%, which is obtained by altering the percent grade of the treadmill. Importantly, after some exercise the treadmill can be stopped and the pig rewarded with fruit juice or water, and the heart rate may be immediately taken and recorded. Again, it is emphasized that records should be maintained for regulatory compliance and scientific rigor. If the pig is achieving the workload (speed and grade) and the heart rate is <65–75% or some other target heart rate, then the percent grade could be increased. Conversely, if heart rate is >230 beats/min, i.e., >85% of maximum, then this objective end point could alert the technician to compromised work capacity and the percent grade could be immediately decreased and the pig could be monitored more closely in case intervention is warranted. Attention to intensity allows for individualization of the exercise regimen to the level of physical fitness of the animal. Finally, note that in this example, a 65–75% maximum heart rate can be readily achieved at a much lower speed (5 km/h) at a variable 5–15% grade (45, 105, 278) compared with the 5–13 km/h variable speeds required at a 0% grade (46). The important point is that both protocols are adequate and proven safe for the animals, thus illustrating that the parameters provided here are guidelines, not absolute mandates for training protocols.
Pigs have been trained in numerous studies at between 65 and 85% of maximum heart rate (180–234 beats/min) for a duration of 30–75 min with almost no major complications (e.g., 45, 259, 469). Pigs can tolerate 5 days of exercise per week very well (262), but for reasons discussed below (large animals, Exercise Modalities in Pigs, Special considerations in the pig), even a lower frequency of 4 days/wk may suffice (45). Note that the exercise effort suggested here is safe and efficacious for eliciting cardiovascular training adaptations. Lower workload is certainly acceptable and may be the most scientifically appropriate in some cases. Low-intensity interval training may be even more effective in eliciting classical training effects (106, 292).
Humane end points that are objectively, quantitatively defined should be used when exercise training pigs, both for the scientific validity of the research and for animal welfare. We define six end points and advise that if any end point is reached, the first treatment is decreasing the exercise intensity (except end point 5) and, if that fails, one may remove the pig from the treadmill and perhaps the entire study. 1) One end point is excessive heart rate: This is largely defined within the goal of conducting submaximal exercise, and this end point is relative to the pig’s previous performance. As indicated above, if the upper limit of the training heart rate is established at 75% of maximum and the pig is tolerating this intensity very well, then deviation 10% in excess of that may be indicative of impairment, e.g., illness or orthopedic problems. See Fig. 10 (429) as an example of satisfactory criteria for training heart rate over a 7-wk chronic study. 2) Another end point involves the number of times electric shock is required: If excessive electric shock must be delivered to the pig to elicit compliance with the exercise intensity, then the pig should be considered noncompliant, and the exercise intensity should be decreased. For example, in Yucatan pigs, use of electric shock >4 times in 1 min has been considered excessive. If the exercise intensity is decreased to a level significantly below the mean intensity tolerated by other pigs in the study and there is no other clinical sign of distress, then the pig may be considered completely noncompliant and removed from the study. 3) A third end point is abnormal gait: Pigs rarely have difficulty ambulating on the treadmill; thus a stagger or limp requires immediate attention. 4) A fourth end point is labored breathing: Pulmonary congestion may be detectable with the stethoscope during heart rate measures before serious impairment. Wheezing and rasping are additional overt signs that indicate veterinary attention. 5) Any fall onto the treadmill requires immediate termination of the treadmill movement. This rarely happens because of poor coordination but may occur if the treadmill has become slippery because of urination and defecation or from water sprayed to ensure cooling of the pig. If the four end points above are monitored, then no pig should be exhausted to the extreme of being unable to right itself if it falls down. 6) Mild skin abrasions on the rump may occur because the pig may try to rest by leaning on the back of the treadmill. Skin abrasions on the hind feet can occur because of rubbing on the rear door/wall of the treadmill. The abrasions are typically treated with topical ointments.
Fig. 10.
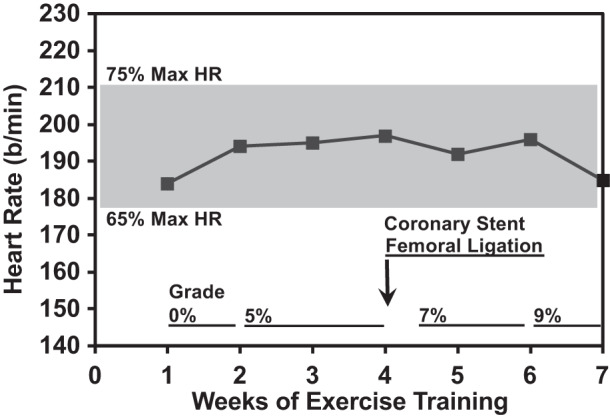
Heart rate responses in exercise training sessions during 7 wk of chronic training of an obese Ossabaw miniature pig with metabolic syndrome. To maintain the exercise heart rate in the target zone of 65–77% of the maximum heart rate (Max HR), the percent grade of the treadmill increases from 0% in week 1 to 5% in weeks 2 and 3. A coronary stent was deployed and femoral artery ligation performed at week 4 followed by several days of recovery before resuming exercise. Midway through week 4 the grade was increased to 7%, and at week 6 it was increased to 9%. Data are from a male pig (see 105). Here, b, beats.
Documentation of the efficacy of training (i.e., increased physical work capacity or underlying biochemical correlate) is important to assess the scientific rigor and validity of the study. Measurements of increased citrate synthase and other skeletal muscle oxidative enzyme activities have been long considered a hallmark as a beneficial exercise training adaptation (179). However, these biochemical measures demonstrating training efficacy require either muscle biopsies before and after training or posttraining and postmortem comparisons of muscle samples between trained and sedentary control animals. Previous studies have demonstrated increases in citrate synthase activity in the skeletal muscle of treadmill-trained pigs compared with their sedentary control counterparts (111, 426); however, there are alternative and less invasive methods of establishing training efficacy in the pig including the use of repeated exercise “stress” tests in conscious pigs (45, 46, 426). A plethora of studies have measured increases in V̇o2max in normal subjects or athletes to demonstrate training efficacy in humans (e.g., 16, 377). In contrast, this is less frequently used in subjects compromised by disease, especially myocardial, coronary, and cerebrovascular diseases that are particularly life-threatening. A large body of evidence also indicates that a decrease in submaximal heart rate at a given absolute workload is sufficient to verify a positive training adaptation (16). Stress tests involving runs to exhaustion have also been used, with pigs showing typically 40–60% increases in run time to exhaustion (e.g., 46). Interestingly, in diabetic and obese pigs there was a clear decrease in submaximal heart rate at a given absolute workload after 16 wk of training at 65–75% of maximum heart rate, 30 min/day, 4 days/wk (45). Efficacy of a moderate 4-wk-long exercise regimen was confirmed in pigs that were fed an atherogenic diet and had bare metal stents deployed in coronary arteries (105, 278, 429) as shown in Fig. 10. The Ossabaw miniature swine were placed on the exercise training regimen after 50 wk of excess calorie atherogenic diet that induced metabolic syndrome. Increased work capacity was clearly shown by the increased grade of the treadmill required to elicit an exercise heart rate in the target heart rate zone of 65–75% of maximum. A brief period of rest after the femoral ligation and coronary stent was included, and then exercise training resumed and produced further increases in functional capacity. These data highlight the potential use of regular short-term aerobic exercise as both a therapeutic modality and a practical answer to exercise training larger animals. This point emphasizes the far-reaching clinically relevant uses of the pig.
The variable effect of exercise on coronary atherosclerosis burden is an example of the need to quantify the efficacy of exercise training. Studies of exercise training of atherosclerotic pigs have shown both an attenuation of plaque burden and peristent stenosis (105, 278) and no effect on plaque burden (438). All studies provided evidence of a training effect: decreased exercise and resting heart rate, increased physical work capacity and exercise tolerance, and increased heart weight-to-body weight ratio (105, 278, 438). These data provide confidence that the exercise stimulus in all studies was sufficient. In other words, the strength of the conclusion regarding an exercise training study is only as valid as the rigor of the criterion exercise test itself. The data pointed to the much greater severity of atherosclerosis in one study (438) versus milder atherosclerosis (105, 278) to explain the differing results.
Special considerations in the pig.
In addition to the abovementioned, special consideration regarding the propensity of swine for sedentary behavior, several other factors may dictate that the investigator adjust the workload and monitor tolerance of the animals. In virtually all special cases the intensity of exercise as measured by heart rate can delineate the severity of the limitation due to the following conditions.
diabetes and obesity.
Autonomic neuropathy may confound the relationship of heart rate to V̇o2; thus other end points must also be monitored closely. Peripheral vascular disease and resulting orthopedic problems have been managed by using lower treadmill speeds and adjusting the grade of the treadmill (45) to provide sufficient workload to elicit exercise training-induced cardiovascular adaptations noted in healthy pigs (46, 59, 258). Resting bradycardia, increased skeletal muscle oxidative enzyme activity, decreased heart rate during submaximal exercise, and increased physical work capacity in diabetic and obese pigs (45, 105, 477) indicate efficacy of a moderate-intensity (dose) exercise training regimen.
coronary ischemia.
Several studies have used reduced workloads to adapt pigs to exercise even with a fully occluded coronary conduit artery (101, 161, 162, 469). Diligent attention to exercise intensity, the gait, and work tolerance of the pig are required continuously during each exercise session. Chronic exercise protocols have elicited classical training effects on cardiac function and skeletal muscle oxidative enzymes.
heart failure and pulmonary hypertension.
Relative studies have shown remarkable exercise effects on pigs having heart failure with preserved ejection fraction (106, 292). These pigs with diastolic dysfunction were tolerant to 15 wk of low-intensity interval training, which elicited increased skeletal muscle oxidative enzyme activity, increasing cardiac extracellular matrix compliance, and improving blood flow/vasoreactivity. These studies are an outstanding example of gradually increasing the workload during only 3 days/wk training. Heart rates during the low-intensity 4.8–6.5 km/h exercise speed at 0% grade treadmill would be very important to report for reference. Mild to moderate exercise challenges are also possible in pigs with robust pulmonary hypertension (58).
heat stress.
As pigs do not sweat, cooling must routinely be achieved by fans directed at the pig’s back and combined with fine water mist applied to the head, ears, and back. Careful attention must be paid to avoid soaking the treadmill belt surface, which can cause slippage and injury to the pig. Body temperature may be monitored with a standard rectal probe if the ambient temperature and humidity are excessive. Routine monitoring of core temperature may be needed if pigs are obese, which further decreases thermal dissipation.
The guidelines provided here for use of the pig combined with the general considerations for selection of an animal model and study design fundamentals will enable broad use of the pig in acute and chronic exercise studies.
Exercise Modalities in Dogs
Dogs have been used for exercise studies for decades using either a treadmill (38, 151, 328) or, less commonly, free running using telemetered instrumentation (452).
Motorized treadmill.
advantages and disadvantages of treadmill exercise.
Similar to the pig, the dog offers a distinct size advantage allowing for chronic instrumentation of a wide variety of transducers, sensors, and catheters for the continuous measurement of cardiac output and regional blood flow, arterial, ventricular, and central venous pressure, cardiac dimensions, devices to manipulate pressures and flows (e.g., vascular occluders), and blood samples. Many dogs are relatively easy to train to run on a treadmill. The dog is a superb athlete; even untrained, the V̇o2max is in excess of 110 mL·min−1·kg−1 (331) and can rise to 150 mL·min−1·kg−1 (331) or higher (~240 mL·min−1·kg−1; 348) with training, over 2 times greater than Olympic caliber cross-country skiers. Dogs have been used to study acute and chronic responses to exercise in the normal state as well as in a number of disease states including heart failure (7, 153, 168, 169, 400), coronary artery disease (47), myocardial infarction (38), hypertension (271, 315, 401, 424), obesity (315), and diabetes (68, 449).
All dogs are now only available from class A vendors. It is important to have effective communication with the vendor to select animals that have had significant human socialization. Some vendors will even do some treadmill pretraining before purchase. Outgoing animals, readily familiar with human handling, responsive to rewards, and unafraid of open spaces and sounds, make the best candidates for training. Aggressive animals should be avoided. Although any animal can become aggressive, there are some tendencies that may aid in predicting a given animal’s behavior (188).
conditioning dogs to run on the treadmill.
A relatively inexpensive treadmill used for humans can be used for dogs with little or no adaptation, although some investigators have enclosed the treadmill in barriers. Most important is that the treadmill should have as low of a starting speed as possible, be as quiet as possible, and be low to the ground. The animal should be very familiar with the laboratory and the sounds of the treadmill. Having a well-trained animal running on the treadmill when a naïve animal enters the laboratory can facilitate adaptation to the treadmill as the new animal will often investigate the behaviors of the trained animal. After several visits to the laboratory, the animal can be placed on the treadmill and given a reward. The treadmill can be moved by hand or at a very low speed (<1 km/h) such that it is barely moving, and the animal can then be led on the treadmill as it slowly moves. The animal should be rewarded often. The speed can then be slowly increased. Negative stimuli such as electric shock, which are often used in rats, are usually not required as well-socialized dogs respond to positive stimuli and rewards. Some animals will splay their legs and slide on the treadmill. Repeated splaying behavior strongly indicates that training will be unsuccessful. The investigator should keep “cleanup” materials readily available as dogs often void urine and feces in the initial periods of treadmill exercise. One member of the laboratory group should always be monitoring the animal during the investigations as the animal may spontaneously try to jump off of the treadmill. A harness attached to a leash is recommended to allow the investigator to rapidly control the animal’s movements. As the animal becomes familiar with walking on the treadmill the speed and grade can be increased progressively. After ~1–2 wk of daily 10–15-min training sessions an animal should be adequately trained. Protocols for exercise training regimes in dogs are available (47, 327, 331) and will provide the normal adaptations to exercise training as observed with humans and other mammals (327, 331).
Measurements of exercise performance on the treadmill in the dog.
Some dogs can be trained to perform very heavy workloads on a treadmill (17, 154, 329–331, 373) and certainly when running outdoors (456). Dogs will trot until speeds of ~8–10 km/h. Above this speed, dogs tend to gallop, which can be more unstable unless a very large treadmill is used or the animal is freely running outdoors. Workloads can be increased by increasing incline. Speeds/incline combinations can be used to obtain at- or near-maximal heart rates (~300 beats/min), which is often achieved at ~8–10 kph/15–20% grade (17, 327, 349).
Exercise Modalities in Rabbits
Rabbits have been used for many years for a variety of purposes in physiological studies. Studies have been performed in both conscious and anesthetized rabbits, and this species has contributed much to our understanding of cardiac and vascular function and cardiac electrophysiology (93, 275). Conscious rabbits are especially well suited for cardiovascular and neural recording studies because of their docile nature, trainability, and relative ease of care. Blood sampling and blood pressure measurements can easily be made using noninvasive or minimally invasive techniques. Of course, this species is not without negative issues such as relatively low arterial pressure and susceptibility to cardiovascular events during stress. Choosing the rabbit for exercise training or acute exercise studies will depend on which variables need to be measured and whether a specific disease model is needed. Users should keep in mind that data acquired from normal laboratory rabbits may result in bigger changes following exercise training than in other species due to their chronic level of inactivity. Mapara et al. (289) have nicely summarized the strengths and weaknesses of using rabbits for research.
Motorized treadmill.
advantages and disadvantages of treadmill exercise.
Unfortunately, the rabbit has not been a favored species for studies involving either acute, short-term exercise or exercise training. This is due, in part, to their sedentary nature (at least in laboratory-reared animals) and the way they locomote, which is not well suited to treadmill exercise studies. It should be mentioned that motivating rabbits to run on a treadmill is challenging. Investigators have used not only an electrical grid situated at the back of the treadmill but also air jet stress, which rabbits are particularly averse to (130, 275). Another technique that was used by Liu et al. (275) was a large motor-driven exercise wheel that forced rabbits to move forward when their position neared the vertical position. In this study, New Zealand White rabbits were trained to run on the wheel at a rate 15–18 m/min for a period of 40 min, 6 days/wk. In this study both normal rabbits and rabbits with heart failure were evaluated. Exercise training reduced both sympathetic outflow and plasma angiotensin II in the heart failure rabbits. Although this technique works well, there are no commercially available running wheels for rabbits.
Measurements of exercise performance on the treadmill in the rabbit.
There are a limited number of studies that have measured the cardiovascular response to exercise in the rabbit. Gaustad et al. (135) measured V̇o2max in female rabbits during maximal treadmill exercise at 0.51 m/s and at inclinations of 0–20°. In this protocol they evaluated V̇o2max at each 5° of incline and exercised to exhaustion. As is standard they used a warm-up period of 10 min at 40–50% of V̇o2max and then ran the rabbits by increasing speed by 0.03 m/s every 2 min until exhaustion. Subsequent trials were performed after 1 day of rest. It should be pointed out that rabbits are reluctant to run, and they excluded one animal due to a failure to run under any conditions. As expected, they found a linear increase in HR and V̇o2 with workload (Fig. 11). They concluded that the rabbit was a good model for studying effects of exercise intensity on cardiovascular function.
Fig. 11.
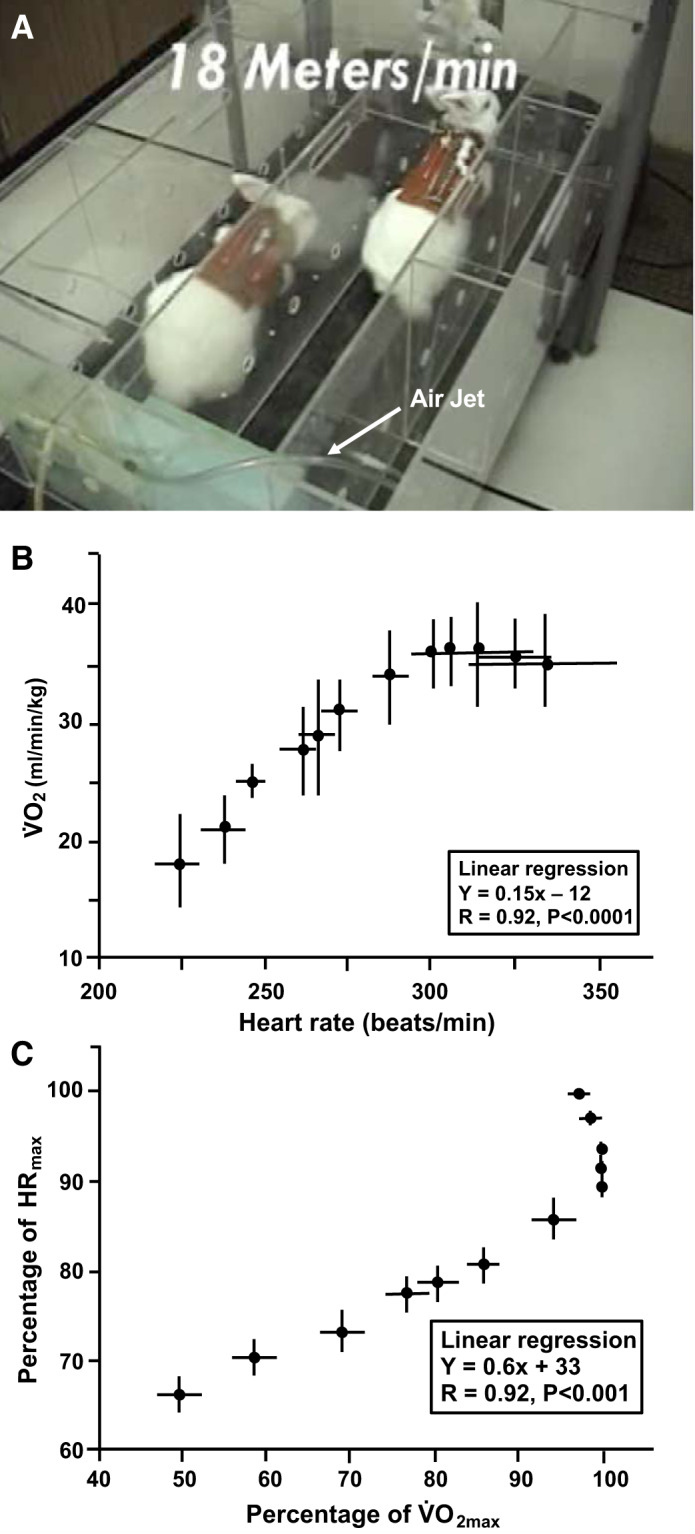
Rabbits exercising on motorized treadmill (A). Relationship between oxygen uptake (V̇o2) and heart rate during treadmill exercise at different inclinations (0–20°) in untrained female rabbits (values expressed as means ± SD, n = 6 rabbits; 135). Note that maximal heart rate (HRmax) was not reached at maximal V̇o2 (V̇o2max; B), but at exercise intensities above those corresponding to V̇o2max (C). [Reprinted from Gaustad SE, Rolim N, Wisløff U. A valid and reproducible protocol for testing maximal oxygen uptake in rabbits. Eur J Cardiovasc Prev Rehabil. 17(1): 83–88, 2010, copyright © 2010 The European Society of Cardiology, with permission of Sage Publications, Ltd.]
Finally, measurements of hemodynamic parameters during exercise in rabbits have not been extensively published. In a study by Noshiro et al. (346), arterial pressure and norepinephrine spillover were measured during treadmill exercise at 6–12 m/min. Although blood pressure can be measured during exercise, it is best to implant arterial catheters as centrally as possible to avoid significant movement artifacts during exercise.
Exercise training in the rabbit.
Studies related to prolonged exercise training in rabbits have been used to assess cardiovascular function and neural control of the circulation. Rabbits are ideal for the recording of renal sympathetic nerve activity in the conscious state because they have a well-definable renal nerve that travels along the length of the renal artery and because they can sit quietly in a restraining box, so that movement artifact is minimized while electrical recording is ongoing (129, 131, 132, 275). For instance, in a study by DiCarlo et al., renal sympathetic nerve activity (RSNA) was evaluated in conscious rabbits subjected to exercise training or a sedentary lifestyle (93). Treadmill exercise training was associated with a lower level of RSNA. Here, daily exercise consisted of running at 7.3 m/min for 15 min for the first week and an additional 5 min of exercise in week 2 at a rate of 12.6 m/min. Thereafter, workload was increased up to 8 wk until the animals could run for 60 min at this level. Importantly, in this study, exercise intensity was documented by recording of rectal temperature. A change of 2.5°C was considered as a measure of adequate intensity.
The rabbit is a good model for cholesterol-induced atherosclerosis (267, 479, 483) and studies on vascular reactivity (225, 270, 479). Yang et al. (479) evaluated the effects of exercise training on aortic vascular reactivity in hypercholesterolemic rabbits. Here they used a commercial Quinton treadmill (Q55), where rabbits hopped at a speed of 0.88 km/h for 5 days/wk, up to 6 wk. Treadmill time was gradually increased to 40 min/day. Exercise training significantly increased citrate synthase activity in the soleus muscle. For this purpose, treadmill exercise of rabbits seems to work particularly well.
CONCLUSIONS
Since the 1901 inception of the Nobel Prize in Physiology or Medicine, animal models have been fundamental to the research success of 180 of the 216 recipients. From Emil Adolf von Behring’s 1901 diphtheria antiserum discoveries using the guinea pig (213) to the present-day (2019) discoveries with the mouse front and center for William G. Kaelin, Gregg L. Semenza, and Peter J. Ratcliffe’s work on hypoxia-inducible factor-1α (HIF-1α; 121), the animal species considered in our review (mouse, rat, horse, pig, dog, and rabbit) have accounted for ~107 of those Nobel awards. With exercise capacity representing, perhaps, the ultimate test of integrative physiological function and certainly for assessing the capacity for systemic oxygen transport, selection of an appropriate animal model amenable to exercise testing is crucial to the progress of physiological and biomedical research (see Table 1). This fact is underscored by the exercise intolerance that arises as a direct consequence of endemic pathologies including heart failure, diabetes, neuromuscular disease, and human immunodeficiency virus (HIV), among many others, and the reduction in morbidity and mortality coupled to improved life quality gained from enhanced exercise tolerance in these patients. Just as important as the selection of the correct animal species for their particular question, the scientist and clinician must embrace the latest developments in human exercise testing such as critical power/speed, V̇o2 kinetics, and correct measurement of V̇o2max to garner the most powerful mechanistic insights possible. We trust that this paper helps provide essential advice and guidelines to investigators across the research spectrum from basic to applied and clinical for whom selection of the most appropriate animal species and testing paradigm will drive the discovery process in biomedical research most effectively.
Table 1.
Selected physiological and anatomical variables for the primary species considered herein
| Human (Athlete) | Horse (Thoroughbred, Quarter Horse, and Standardbred) | Pig (Yucatan or Ossabaw Miniature and Domestic) | Dog (Greyhound) | Rabbit* | Rat | Mouse | |
|---|---|---|---|---|---|---|---|
| Running speed max, km/h | 43 | 72–89 | 20.8 | 72 | 1.8 (inclined treadmill) | 4.3–7.0 | 3.3 |
| V̇o2max | |||||||
| mL·kg−1·min−1 | <95 | >220 | 74 | 240 | 36 | 70–100 | 125–135 |
| L/min | <7.0 | >110 | 1.7 | 7.2 | 0.18 | 0.022–0.031 | 0.0038–0.0041 |
| Critical speed, km/h | 20.7 | NA | NA | NA | NA | 2.4–3.0 | 1.1–1.44 |
| Critical power, W | NA | 2,490 (Standardbred untrained) | NA | NA | NA | NA | NA |
| Body mass, kg | 70–86 | 450–550 | 20–120 | ~30 | ~3.2 | 0.25–0.6 | 0.02–0.05 |
| Heart mass, kg (%body mass) | >0.5 (0.6) | ≤10 (2.0) | 0.145 (0.6) | ≤0.5 (1.7) | 0.0128 (0.4) | 0.0008–0.0012 (0.3–0.4) | 0.00012 (0.6) |
| Heart rate max, beats/min | ~180–200 | 210–250 | 250–275 | >320 | >386 | 530–580 | >600 |
| Hematocrit max exercise, % | ~45 | >65 | 34–42 | >66 | 28–48 (rest) | 35–45 | ~45 |
| EIPH | Rare | Prevalent/severe | NA | Present/mild | NA | NA | NA |
References: human (370), horse (251, 370), pig (36, 105, 176, 345, 429), dog (370), rabbit (135, 156, 210, 268), rat (73, 171, 375, 454), and mouse (20, 37, 96, 344, 361, 408). EIPH, exercise-induced pulmonary hemorrhage; max, maximum; V̇o2max, maximal oxygen uptake.
Caveat: laboratory rabbits, not wild.
GRANTS
T.D.C. was funded by National Heart, Lung, and Blood Institute (NHLBI) Grant F31-HL-145981. J.C.C. was funded by NHLBI Grant 2T32-HL-007576. S.W.C. was funded by NHLBI Grant HL-142877. T.I.M. was funded by NHLBI Grants HL-108328 and HL-142877 and American Heart Association (AHA) Grant-in-Aid Midwest Affiliate 4350011. D.S.O. was funded by NHLBI Grants HL-055473 and HL-126706. D.C.P was funded by NHLBI Grants HL-50306, HL-108328, and HL-137156 and AHA Grant-in-Aid Midwest Affiliate 4350011. M.S. was funded by NIH Grant DK-097512 and the Joshua Diabetes Research Fund. I.H.Z. was funded by NHLBI Grant P01-HL-62222 and the Theodore F. Hubbard Foundation.
DISCLOSURES
M.S. is the Chief Scientific Officer of CorVus Biomedical, LLC, a company that produces Ossabaw miniature swine and does contract research. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
D.C.P., S.W.C., T.D.C., J.C.C., M.S., I.H.Z., and T.I.M. conceived and designed review; D.C.P., S.W.C., T.D.C., J.C.C., M.S., D.S.O., I.H.Z., and T.I.M. prepared figures; D.C.P., S.W.C., T.D.C., J.C.C., D.L.A., M.S., D.S.O., I.H.Z., and T.I.M. drafted manuscript; D.C.P., S.W.C., T.D.C., J.C.C., D.L.A., M.S., D.S.O., I.H.Z., and T.I.M. edited and revised manuscript; D.C.P., S.W.C., T.D.C., J.C.C., D.L.A., M.S., D.S.O., I.H.Z., and T.I.M. approved final version of manuscript.
ACKNOWLEDGMENTS
M.S. thanks Dr. M. Alloosh and J. Byrd for experimental work and reviewing large animals, Exercise Modalities in Pigs. D.C.P. and T.I.M. thank K. S. Hageman for technical assistance during data collection associated with Clarenburg Cardiopulmonary Research Laboratory.
REFERENCES
- 1.Adams V, Alves M, Fischer T, Rolim N, Werner S, Schütt N, Bowen TS, Linke A, Schuler G, Wisløff U. High-intensity interval training attenuates endothelial dysfunction in a Dahl salt-sensitive rat model of heart failure with preserved ejection fraction. J Appl Physiol (1985) 119: 745–752, 2015. doi: 10.1152/japplphysiol.01123.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad F, Arad M, Musi N, He H, Wolf C, Branco D, Perez-Atayde AR, Stapleton D, Bali D, Xing Y, Tian R, Goodyear LJ, Berul CI, Ingwall JS, Seidman CE, Seidman JG. Increased α2 subunit-associated AMPK activity and PRKAG2 cardiomyopathy. Circulation 112: 3140–3148, 2005. doi: 10.1161/CIRCULATIONAHA.105.550806. [DOI] [PubMed] [Google Scholar]
- 3.Allen DL, Harrison BC, Leinwand LA. Molecular and genetic approaches to studying exercise performance and adaptation. Exerc Sport Sci Rev 30: 99–105, 2002. doi: 10.1097/00003677-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse [Erratum in J Appl Physiol 91: 1a, 2001]. J Appl Physiol (1985) 90: 1900–1908, 2001. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- 5.American Physiological Society Resource Book for the Design of Animal Exercise Protocols. https://www.physiology.org/career/policy-advocacy/animal-research/resource-book-for-the-design-of-animal-exercise-protocols. 2006.
- 6.Amundsen BH, Rognmo Ø, Hatlen-Rebhan G, Slørdahl SA. High-intensity aerobic exercise improves diastolic function in coronary artery disease. Scand Cardiovasc J 42: 110–117, 2008. doi: 10.1080/14017430701744477. [DOI] [PubMed] [Google Scholar]
- 7.Ansorge EJ, Augustyniak RA, Perinot ML, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, O’Leary DS. Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. Am J Physiol Heart Circ Physiol 288: H1381–H1388, 2005. doi: 10.1152/ajpheart.00985.2004. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong RB, Saubert CW IV, Sembrowich WL, Shepherd RE, Gollnick PD. Glycogen depletion in rat skeletal muscle fibers at different intensities and durations of exercise. Pflügers Arch 352: 243–256, 1974. doi: 10.1007/BF00590489. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong RB, Laughlin MH, Rome L, Taylor CR. Metabolism of rats running up and down an incline. J Appl Physiol 55: 518–521, 1983. doi: 10.1152/jappl.1983.55.2.518. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong RB, Laughlin MH. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol 344: 189–208, 1983. doi: 10.1113/jphysiol.1983.sp014933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong RB, Laughlin MH. Exercise blood flow patterns within and among rat muscles after training. Am J Physiol Heart Circ Physiol 246: H59–H68, 1984. doi: 10.1152/ajpheart.1984.246.1.H59. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong RB, Laughlin MH. Rat muscle blood flows during high-speed locomotion. J Appl Physiol (1985) 59: 1322–1328, 1985. doi: 10.1152/jappl.1985.59.4.1322. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Distribution of blood flow in muscles of miniature swine during exercise. J Appl Physiol (1985) 62: 1285–1298, 1987. doi: 10.1152/jappl.1987.62.3.1285. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong RB, Hayes DA, Delp MD. Blood flow distribution in rat muscles during preexercise anticipatory response. J Appl Physiol (1985) 67: 1855–1861, 1989. doi: 10.1152/jappl.1989.67.5.1855. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong RB, Essén-Gustavsson B, Hoppeler H, Jones JH, Kayar SR, Laughlin MH, Lindholm A, Longworth KE, Taylor CR, Weibel ER. O2 delivery at Vo2max and oxidative capacity in muscles of standardbred horses. J Appl Physiol (1985) 73: 2274–2282, 1992. doi: 10.1152/jappl.1992.73.6.2274. [DOI] [PubMed] [Google Scholar]
- 16.Astrand PO, Rodahl K. Textbook of Work Physiology. New York: McGraw-Hill, 1986. [Google Scholar]
- 17.Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O’Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001. doi: 10.1152/ajpheart.2001.280.4.H1645. [DOI] [PubMed] [Google Scholar]
- 18.Avila JJ, Kim SK, Massett MP. Differences in exercise capacity and responses to training in 24 inbred mouse strains. Front Physiol 8: 974, 2017. doi: 10.3389/fphys.2017.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avraham Y, Hao S, Mendelson S, Berry EM. Tyrosine improves appetite, cognition, and exercise tolerance in activity anorexia. Med Sci Sports Exerc 33: 2104–2110, 2001. doi: 10.1097/00005768-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Ayachi M, Niel R, Momken I, Billat VL, Mille-Hamard L. Validation of a ramp running protocol for determination of the true Vo2max in mice. Front Physiol 7: 372, 2016. doi: 10.3389/fphys.2016.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16: 1879–1886, 2002. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 22.Baker MA, Horvath SM. Influence of water temperature on heart rate and rectal temperature of swimming rats. Am J Physiol 207: 1073–1076, 1964. doi: 10.1152/ajplegacy.1964.207.5.1073. [DOI] [PubMed] [Google Scholar]
- 23.Baker MA, Horvath SM. Influence of water temperature on oxygen uptake by swimming rats. J Appl Physiol 19: 1215–1218, 1964. doi: 10.1152/jappl.1964.19.6.1215. [DOI] [PubMed] [Google Scholar]
- 24.Baldwin KM, Cooke DA, Cheadle WG. Time course adaptations in cardiac and skeletal muscle to different running programs. J Appl Physiol 42: 267–272, 1977. doi: 10.1152/jappl.1977.42.2.267. [DOI] [PubMed] [Google Scholar]
- 25.Banjanin S, Mrosovsky N. Preferences of mice, Mus musculus, for different types of running wheel. Lab Anim 34: 313–318, 2000. doi: 10.1258/002367700780384681. [DOI] [PubMed] [Google Scholar]
- 26.Bargiel Z, Nowicka H, Wójcikowska J. Swim-stress induced changes of rat adrenal catecholamine level depending on metabolic state and different ambient temperature. Folia Histochem Cytochem (Krakow) 19: 31–37, 1981. [PubMed] [Google Scholar]
- 27.Barker T, Poole DC, Noble ML, Barstow TJ. Human critical power-oxygen uptake relationship at different pedalling frequencies. Exp Physiol 91: 621–632, 2006. doi: 10.1113/expphysiol.2005.032789. [DOI] [PubMed] [Google Scholar]
- 28.Bedford TG, Tipton CM, Wilson NC, Oppliger RA, Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol 47: 1278–1283, 1979. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- 29.Behnke BJ, Kindig CA, McDonough P, Poole DC, Sexton WL. Dynamics of microvascular oxygen pressure during rest-contraction transition in skeletal muscle of diabetic rats. Am J Physiol Heart Circ Physiol 283: H926–H932, 2002. doi: 10.1152/ajpheart.00059.2002. [DOI] [PubMed] [Google Scholar]
- 30.Beleza J, Albuquerque J, Santos-Alves E, Fonseca P, Santocildes G, Stevanovic J, Rocha-Rodrigues S, Rizo-Roca D, Ascensão A, Torrella JR, Magalhães J. Self-paced free-running wheel mimics high-intensity interval training impacts on rat’s functional, physiological, biochemical, and morphological features. Front Physiol 10: 593, 2019. doi: 10.3389/fphys.2019.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell FP, Gerrity RG. Evidence for an altered lipid metabolic state in circulating blood monocytes under conditions of hyperlipemia in swine and its implications in arterial lipid metabolism. Arterioscler Thromb 12: 155–162, 1992. doi: 10.1161/01.ATV.12.2.155. [DOI] [PubMed] [Google Scholar]
- 32.Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci 14: 1992–2002, 2001. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- 33.Bernard C. Introduction à l'étude de la médecine expérimentale. Paris: J. B. Baillière et Fils, Libraires de L’Académie Impériale de Médecine, 1865, 400 p. [Google Scholar]
- 34.Bernardo BC, Ooi JYY, Weeks KL, Patterson NL, McMullen JR. Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: current knowledge and emerging concepts. Physiol Rev 98: 419–475, 2018. doi: 10.1152/physrev.00043.2016. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein D. Exercise assessment of transgenic models of human cardiovascular disease. Physiol Genomics 13: 217–226, 2003. doi: 10.1152/physiolgenomics.00188.2002. [DOI] [PubMed] [Google Scholar]
- 36.Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent K+ channels to metabolic control of coronary blood flow. J Mol Cell Cardiol 52: 912–919, 2012. doi: 10.1016/j.yjmcc.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Billat VL, Mouisel E, Roblot N, Melki J. Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol (1985) 98: 1258–1263, 2005. doi: 10.1152/japplphysiol.00991.2004. [DOI] [PubMed] [Google Scholar]
- 38.Billman GE, Schwartz PJ, Gagnol JP, Stone HL. Cardiac response to submaximal exercise in dogs susceptible to sudden cardiac death. J Appl Physiol (1985) 59: 890–897, 1985. doi: 10.1152/jappl.1985.59.3.890. [DOI] [PubMed] [Google Scholar]
- 39.Binas B, Danneberg H, McWhir J, Mullins L, Clark AJ. Requirement for the heart-type fatty acid binding protein in cardiac fatty acid utilization. FASEB J 13: 805–812, 1999. doi: 10.1096/fasebj.13.8.805. [DOI] [PubMed] [Google Scholar]
- 40.Blair SN, Cooper KH. Dose of exercise and health benefits. Arch Intern Med 157: 153–154, 1997. doi: 10.1001/archinte.1997.00440230019003. [DOI] [PubMed] [Google Scholar]
- 41.Blair SN, Kampert JB, Kohl HW III, Barlow CE, Macera CA, Paffenbarger RS Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 276: 205–210, 1996. doi: 10.1001/jama.1996.03540030039029. [DOI] [PubMed] [Google Scholar]
- 42.Bloor CM, White FC, Sanders TM. Effects of exercise on collateral development in myocardial ischemia in pigs. J Appl Physiol 56: 656–665, 1984. doi: 10.1152/jappl.1984.56.3.656. [DOI] [PubMed] [Google Scholar]
- 43.Bond SL, Greco-Otto P, Sides R, Kwong GPS, Léguillette R, Bayly WM. Assessment of two methods to determine the relative contributions of the aerobic and anaerobic energy systems in racehorses. J Appl Physiol (1985) 126: 1390–1398, 2019. doi: 10.1152/japplphysiol.00983.2018. [DOI] [PubMed] [Google Scholar]
- 44.Borbouse L, Dick GM, Payne GA, Payne BD, Svendsen MC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. Contribution of BKCa channels to local metabolic coronary vasodilation: effects of metabolic syndrome. Am J Physiol Heart Circ Physiol 298: H966–H973, 2010. doi: 10.1152/ajpheart.00876.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boullion RD, Mokelke EA, Wamhoff BR, Otis CR, Wenzel J, Dixon JL, Sturek M. Porcine model of diabetic dyslipidemia: insulin and feed algorithms for mimicking diabetes mellitus in humans. Comp Med 53: 42–52, 2003. [PubMed] [Google Scholar]
- 46.Bowles DK, Hu Q, Laughlin MH, Sturek M. Exercise training increases L-type calcium current density in coronary smooth muscle. Am J Physiol Heart Circ Physiol 275: H2159–H2169, 1998. doi: 10.1152/ajpheart.1998.275.6.H2159. [DOI] [PubMed] [Google Scholar]
- 47.Brandt MA, Gwirtz PA. Exercise training reduces ischemic myocardial dysfunction. Med Sci Sports Exerc 33: 556–563, 2001. doi: 10.1097/00005768-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Braun K, Atmanspacher F, Schreckenberg R, Grgic I, Schlüter KD. Effect of free running wheel exercise on renal expression of parathyroid hormone receptor type 1 in spontaneously hypertensive rats. Physiol Rep 6: e13842, 2018. doi: 10.14814/phy2.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Breslow JL. Mouse models of atherosclerosis. Science 272: 685–688, 1996. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 50.Brooks GA, Donovan CM, White TP. Estimation of anaerobic energy production and efficiency in rats during exercise. J Appl Physiol 56: 520–525, 1984. doi: 10.1152/jappl.1984.56.2.520. [DOI] [PubMed] [Google Scholar]
- 51.Brooks GA, White TP. Determination of metabolic and heart rate responses of rats to treadmill exercise. J Appl Physiol 45: 1009–1015, 1978. doi: 10.1152/jappl.1978.45.6.1009. [DOI] [PubMed] [Google Scholar]
- 52.Brown MB, Chingombe TJ, Zinn AB, Reddy JG, Novack RA, Cooney SA, Fisher AJ, Presson RG, Lahm T, Petrache I. Novel assessment of haemodynamic kinetics with acute exercise in a rat model of pulmonary arterial hypertension. Exp Physiol 100: 742–754, 2015. doi: 10.1113/EP085182. [DOI] [PubMed] [Google Scholar]
- 53.Brown MB, Neves E, Long G, Graber J, Gladish B, Wiseman A, Owens M, Fisher AJ, Presson RG, Petrache I, Kline J, Lahm T. High-intensity interval training, but not continuous training, reverses right ventricular hypertrophy and dysfunction in a rat model of pulmonary hypertension. Am J Physiol Regul Integr Comp Physiol 312: R197–R210, 2017. doi: 10.1152/ajpregu.00358.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruner CA, Vargas I. The activity of rats in a swimming situation as a function of water temperature. Physiol Behav 55: 21–28, 1994. doi: 10.1016/0031-9384(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 55.Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, Whipp BJ. Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol 55: 1558–1564, 1983. doi: 10.1152/jappl.1983.55.5.1558. [DOI] [PubMed] [Google Scholar]
- 56.Burnley M, Vanhatalo A, Jones AM. Distinct profiles of neuromuscular fatigue during muscle contractions below and above the critical torque in humans. J Appl Physiol (1985) 113: 215–223, 2012. doi: 10.1152/japplphysiol.00022.2012. [DOI] [PubMed] [Google Scholar]
- 57.Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics 5: 89–97, 2001. doi: 10.1152/physiolgenomics.2001.5.2.89. [DOI] [PubMed] [Google Scholar]
- 58.Cai Z, van Duin RW, Stam K, Uitterdijk A, van der Velden J, Vonk Noordegraaf A, Duncker DJ, Merkus D. Right ventricular oxygen delivery as a determinant of right ventricular functional reserve during exercise in juvenile swine with chronic pulmonary hypertension. Am J Physiol Heart Circ Physiol 317: H840–H850, 2019. doi: 10.1152/ajpheart.00130.2019. [DOI] [PubMed] [Google Scholar]
- 59.Carey GB. The swine as a model for studying exercise-induced changes in lipid metabolism. Med Sci Sports Exerc 29: 1437–1443, 1997. doi: 10.1097/00005768-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Cartee GD, Farrar RP. Muscle respiratory capacity and VO2 max in identically trained young and old rats. J Appl Physiol (1985) 63: 257–261, 1987. doi: 10.1152/jappl.1987.63.1.257. [DOI] [PubMed] [Google Scholar]
- 61.Carter GT, Kikuchi N, Abresch RT, Walsh SA, Horasek SJ, Fowler WM Jr. Effects of exhaustive concentric and eccentric exercise on murine skeletal muscle. Arch Phys Med Rehabil 75: 555–559, 1994. [PubMed] [Google Scholar]
- 62.Chapman MJ, Goldstein S. Comparison of the serum low density lipoprotein and of its apoprotein in the pig, rhesus monkey and baboon with that in man. Atherosclerosis 25: 267–291, 1976. doi: 10.1016/0021-9150(76)90033-2. [DOI] [PubMed] [Google Scholar]
- 63.Ciccone CD, Lakas CS, Zambraski EJ. Oxygen consumption in treadmill-exercised Yucatan miniature swine. Med Sci Sports Exerc 14: 467–470, 1982. doi: 10.1249/00005768-198206000-00011. [DOI] [PubMed] [Google Scholar]
- 64.Clark JH, Conlee RK. Muscle and liver glycogen content: diurnal variation and endurance. J Appl Physiol 47: 425–428, 1979. doi: 10.1152/jappl.1979.47.2.425. [DOI] [PubMed] [Google Scholar]
- 65.Cogliati T, Good DJ, Haigney M, Delgado-Romero P, Eckhaus MA, Koch WJ, Kirsch IR. Predisposition to arrhythmia and autonomic dysfunction in Nhlh1-deficient mice. Mol Cell Biol 22: 4977–4983, 2002. doi: 10.1128/MCB.22.14.4977-4983.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen LA, Boylan E, Epstein M, Zang E. Voluntary exercise and experimental mammary cancer. Adv Exp Med Biol 322: 41–59, 1992. doi: 10.1007/978-1-4684-7953-9_5. [DOI] [PubMed] [Google Scholar]
- 67.Cohn JS, Rodriguez C, Jacques H, Tremblay M, Davignon J. Storage of human plasma samples leads to alterations in the lipoprotein distribution of apoC-III and apoE. J Lipid Res 45: 1572–1579, 2004. doi: 10.1194/jlr.D300041-JLR200. [DOI] [PubMed] [Google Scholar]
- 68.Coker RH, Lacy DB, Krishna MG, Wasserman DH. Splanchnic glucagon kinetics in exercising alloxan-diabetic dogs. J Appl Physiol (1985) 86: 1626–1631, 1999. doi: 10.1152/jappl.1999.86.5.1626. [DOI] [PubMed] [Google Scholar]
- 69.Colbert LH, Mai V, Perkins SN, Berrigan D, Lavigne JA, Wimbrow HH, Alvord WG, Haines DC, Srinivas P, Hursting SD. Exercise and intestinal polyp development in APCMin mice. Med Sci Sports Exerc 35: 1662–1669, 2003. doi: 10.1249/01.MSS.0000089349.54813.41. [DOI] [PubMed] [Google Scholar]
- 70.Conlee RK. Muscle glycogen and exercise endurance: a twenty-year perspective. Exerc Sport Sci Rev 15: 1–28, 1987. doi: 10.1249/00003677-198700150-00004. [DOI] [PubMed] [Google Scholar]
- 71.Copp SW, Davis RT, Poole DC, Musch TI. Reproducibility of endurance capacity and V̇o2peak in male Sprague-Dawley rats. J Appl Physiol (1985) 106: 1072–1078, 2009. doi: 10.1152/japplphysiol.91566.2008. [DOI] [PubMed] [Google Scholar]
- 72.Copp SW, Hageman KS, Behnke BJ, Poole DC, Musch TI. Effects of type II diabetes on exercising skeletal muscle blood flow in the rat. J Appl Physiol (1985) 109: 1347–1353, 2010. doi: 10.1152/japplphysiol.00668.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Copp SW, Hirai DM, Musch TI, Poole DC. Critical speed in the rat: implications for hindlimb muscle blood flow distribution and fibre recruitment. J Physiol 588: 5077–5087, 2010. doi: 10.1113/jphysiol.2010.198382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Copp SW, Holdsworth CT, Ferguson SK, Hirai DM, Poole DC, Musch TI. Muscle fibre-type dependence of neuronal nitric oxide synthase-mediated vascular control in the rat during high speed treadmill running. J Physiol 591: 2885–2896, 2013. doi: 10.1113/jphysiol.2013.251082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cox RH, Hubbard JW, Lawler JE, Sanders BJ, Mitchell VP. Cardiovascular and sympathoadrenal responses to stress in swim-trained rats. J Appl Physiol (1985) 58: 1207–1214, 1985. doi: 10.1152/jappl.1985.58.4.1207. [DOI] [PubMed] [Google Scholar]
- 76.Craig JC, Colburn TD, Caldwell JT, Hirai DM, Tabuchi A, Baumfalk DR, Behnke BJ, Ade CJ, Musch TI, Poole DC. Central and peripheral factors mechanistically linked to exercise intolerance in heart failure with reduced ejection fraction. Am J Physiol Heart Circ Physiol 317: H434–H444, 2019. doi: 10.1152/ajpheart.00164.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat 193: 105–119, 1998. doi: 10.1046/j.1469-7580.1998.19310105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.D’Allaire S, DeRoth L. Physiological responses to treadmill exercise and ambient temperature in normal and malignant hyperthermia susceptible pigs. Can J Vet Res 50: 78–83, 1986. [PMC free article] [PubMed] [Google Scholar]
- 79.Dantzer R. The pig as a model for behavioral research. Lab Anim Sci 36: 362–365, 1986. [PubMed] [Google Scholar]
- 80.Davies KJ, Packer L, Brooks GA. Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch Biochem Biophys 209: 539–554, 1981. doi: 10.1016/0003-9861(81)90312-X. [DOI] [PubMed] [Google Scholar]
- 81.Davies KJ, Packer L, Brooks GA. Exercise bioenergetics following sprint training. Arch Biochem Biophys 215: 260–265, 1982. doi: 10.1016/0003-9861(82)90303-4. [DOI] [PubMed] [Google Scholar]
- 82.Davis JM, Kohut ML, Colbert LH, Jackson DA, Ghaffar A, Mayer EP. Exercise, alveolar macrophage function, and susceptibility to respiratory infection. J Appl Physiol (1985) 83: 1461–1466, 1997. doi: 10.1152/jappl.1997.83.5.1461. [DOI] [PubMed] [Google Scholar]
- 83.Davis JM, Weaver JA, Kohut ML, Colbert LH, Ghaffar A, Mayer EP. Immune system activation and fatigue during treadmill running: role of interferon. Med Sci Sports Exerc 30: 863–868, 1998. doi: 10.1249/00005768-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 84.Dawson CA, Horvath SM. Swimming in small laboratory animals. Med Sci Sports 2: 51–78, 1970. [PubMed] [Google Scholar]
- 85.Dawson CA, Nadel ER, Horvath SM. Cardiac output in the cold-stressed swimming rat. Am J Physiol 214: 320–325, 1968. doi: 10.1152/ajplegacy.1968.214.2.320. [DOI] [PubMed] [Google Scholar]
- 86.Dawson CA, Roemer RB, Horvath SM. Body temperature and oxygen uptake in warm- and cold-adapted rats during swimming. J Appl Physiol 29: 150–154, 1970. doi: 10.1152/jappl.1970.29.2.150. [DOI] [PubMed] [Google Scholar]
- 87.Day JR, Rossiter HB, Coats EM, Skasick A, Whipp BJ. The maximally attainable V̇o2 during exercise in humans: the peak vs. maximum issue. J Appl Physiol (1985) 95: 1901–1907, 2003. doi: 10.1152/japplphysiol.00024.2003. [DOI] [PubMed] [Google Scholar]
- 88.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation 113: 2097–2104, 2006. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 89.Delp MD, Laughlin MH, Armstrong RB. No relationship between progressive muscle hyperaemia and temperature in exercising rats. J Exp Biol 141: 87–95, 1989. [DOI] [PubMed] [Google Scholar]
- 90.Desai KH, Sato R, Schauble E, Barsh GS, Kobilka BK, Bernstein D. Cardiovascular indexes in the mouse at rest and with exercise: new tools to study models of cardiac disease. Am J Physiol Heart Circ Physiol 272: H1053–H1061, 1997. doi: 10.1152/ajpheart.1997.272.2.H1053. [DOI] [PubMed] [Google Scholar]
- 91.Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res 73: 43–46, 1995. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 92.DiCarlo SE, Bishop VS. Exercise training attenuates baroreflex regulation of nerve activity in rabbits. Am J Physiol Heart Circ Physiol 255: H974–H979, 1988. doi: 10.1152/ajpheart.1988.255.4.H974. [DOI] [PubMed] [Google Scholar]
- 93.DiCarlo SE, Stahl LK, Bishop VS. Daily exercise attenuates the sympathetic nerve response to exercise by enhancing cardiac afferents. Am J Physiol Heart Circ Physiol 273: H1606–H1610, 1997. doi: 10.1152/ajpheart.1997.273.3.H1606. [DOI] [PubMed] [Google Scholar]
- 94.Dixon JL, Shen S, Vuchetich JP, Wysocka E, Sun GY, Sturek M. Increased atherosclerosis in diabetic dyslipidemic swine: protection by atorvastatin involves decreased VLDL triglycerides but minimal effects on the lipoprotein profile. J Lipid Res 43: 1618–1629, 2002. doi: 10.1194/jlr.M200134-JLR200. [DOI] [PubMed] [Google Scholar]
- 95.Dixon JL, Stoops JD, Parker JL, Laughlin MH, Weisman GA, Sturek M. Dyslipidemia and vascular dysfunction in diabetic pigs fed an atherogenic diet. Arterioscler Thromb Vasc Biol 19: 2981–2992, 1999. doi: 10.1161/01.ATV.19.12.2981. [DOI] [PubMed] [Google Scholar]
- 96.Doevendans PA, Daemen MJ, de Muinck ED, Smits JF. Cardiovascular phenotyping in mice. Cardiovasc Res 39: 34–49, 1998. doi: 10.1016/S0008-6363(98)00073-X. [DOI] [PubMed] [Google Scholar]
- 97.Dolinsky ZS, Burright RG, Donovick PJ. Behavioral changes in mice following lead administration during several stages of development. Physiol Behav 30: 583–589, 1983. doi: 10.1016/0031-9384(83)90225-1. [DOI] [PubMed] [Google Scholar]
- 98.Douglas WR. Of pigs and men and research: a review of applications and analogies of the pig, Sus scrofa, in human medical research. Space Life Sci 3: 226–234, 1972. doi: 10.1007/BF00928167. [DOI] [PubMed] [Google Scholar]
- 99.Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol 53: 844–850, 1982. doi: 10.1152/jappl.1982.53.4.844. [DOI] [PubMed] [Google Scholar]
- 100.Dufresne SD, Bjørbaek C, El-Haschimi K, Zhao Y, Aschenbach WG, Moller DE, Goodyear LJ. Altered extracellular signal-regulated kinase signaling and glycogen metabolism in skeletal muscle from p90 ribosomal S6 kinase 2 knockout mice. Mol Cell Biol 21: 81–87, 2001. doi: 10.1128/MCB.21.1.81-87.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duncker DJ, Bache RJ. Inhibition of nitric oxide production aggravates myocardial hypoperfusion during exercise in the presence of a coronary artery stenosis. Circ Res 74: 629–640, 1994. doi: 10.1161/01.RES.74.4.629. [DOI] [PubMed] [Google Scholar]
- 102.Duncker DJ, van Zon NS, Ishibashi Y, Bache RJ. Role of K+ ATP channels and adenosine in the regulation of coronary blood flow during exercise with normal and restricted coronary blood flow. J Clin Invest 97: 996–1009, 1996. doi: 10.1172/JCI118524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dupont-Versteegden EE, McCarter RJ, Katz MS. Voluntary exercise decreases progression of muscular dystrophy in diaphragm of mdx mice. J Appl Physiol (1985) 77: 1736–1741, 1994. doi: 10.1152/jappl.1994.77.4.1736. [DOI] [PubMed] [Google Scholar]
- 104.Edwards JM, Alloosh MA, Long XL, Dick GM, Lloyd PG, Mokelke EA, Sturek M. Adenosine A1 receptors in neointimal hyperplasia and in-stent stenosis in Ossabaw miniature swine. Coron Artery Dis 19: 27–31, 2008. doi: 10.1097/MCA.0b013e3282f262b4. [DOI] [PubMed] [Google Scholar]
- 105.Edwards JM, Neeb ZP, Alloosh MA, Long X, Bratz IN, Peller CR, Byrd JP, Kumar S, Obukhov AG, Sturek M. Exercise training decreases store-operated Ca2+entry associated with metabolic syndrome and coronary atherosclerosis. Cardiovasc Res 85: 631–640, 2010. doi: 10.1093/cvr/cvp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Emter CA, Tharp DL, Ivey JR, Ganjam VK, Bowles DK. Low-intensity interval exercise training attenuates coronary vascular dysfunction and preserves Ca2+-sensitive K+ current in miniature swine with LV hypertrophy. Am J Physiol Heart Circ Physiol 301: H1687–H1694, 2011. doi: 10.1152/ajpheart.00610.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Epp TS, McDonough P, Padilla DJ, Gentile JM, Edwards KL, Erickson HH, Poole DC. Exercise-induced pulmonary haemorrhage during submaximal exercise. Equine Vet J Suppl 38, S36: 502–507, 2006. doi: 10.1111/j.2042-3306.2006.tb05595.x. [DOI] [PubMed] [Google Scholar]
- 108.Erickson HH, Kindig CA, Poole DC. Role of the airways in exercise-induced pulmonary haemorrhage. Equine Vet J 33: 537–539, 2001. doi: 10.2746/042516401776563508. [DOI] [PubMed] [Google Scholar]
- 109.Erickson HH, Faraci FM, Olsen SC. Effect of exercise on cardiopulmonary function in domestic pigs. In: Swine in Biomedical Research, edited by Tumbleson ME. New York: Plenum Press, 1986, p. 709–717. [Google Scholar]
- 110.Evangelista FS, Brum PC, Krieger JE. Duration-controlled swimming exercise training induces cardiac hypertrophy in mice. Braz J Med Biol Res 36: 1751–1759, 2003. doi: 10.1590/S0100-879X2003001200018. [DOI] [PubMed] [Google Scholar]
- 111.Fain JN, Company JM, Booth FW, Laughlin MH, Padilla J, Jenkins NT, Bahouth SW, Sacks HS. Exercise training does not increase muscle FNDC5 protein or mRNA expression in pigs. Metabolism 62: 1503–1511, 2013. doi: 10.1016/j.metabol.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Faraci FM, Olsen SC, Erickson HH. Effect of exercise on oxygen consumption, heart rate, and the electrocardiogram of pigs. Med Sci Sports Exerc 16: 406–410, 1984. doi: 10.1249/00005768-198408000-00014. [DOI] [PubMed] [Google Scholar]
- 113.Fedde MR, Orr JA, Shams H, Scheid P. Cardiopulmonary function in exercising bar-headed geese during normoxia and hypoxia. Respir Physiol 77: 239–252, 1989. doi: 10.1016/0034-5687(89)90010-8. [DOI] [PubMed] [Google Scholar]
- 114.Feletou M, Teisseire B. Vascular pharmacology of the micropig: importance of the endothelium. In: Swine as Models in Biomedical Research, edited by Swindle MM, Moody DC, Phillips LD. Ames, IA: Iowa State University Press, 1992, p. 74–95. [Google Scholar]
- 115.Ferrandez MD, De la Fuente M. Effects of age, sex and physical exercise on the phagocytic process of murine peritoneal macrophages. Acta Physiol Scand 166: 47–53, 1999. doi: 10.1046/j.1365-201x.1999.00535.x. [DOI] [PubMed] [Google Scholar]
- 116.Fewell JG, Osinska H, Klevitsky R, Ng W, Sfyris G, Bahrehmand F, Robbins J. A treadmill exercise regimen for identifying cardiovascular phenotypes in transgenic mice. Am J Physiol Heart Circ Physiol 273: H1595–H1605, 1997. doi: 10.1152/ajpheart.1997.273.3.H1595. [DOI] [PubMed] [Google Scholar]
- 117.Fitzsimons DP, Bodell PW, Herrick RE, Baldwin KM. Left ventricular functional capacity in the endurance-trained rodent. J Appl Physiol (1985) 69: 305–312, 1990. doi: 10.1152/jappl.1990.69.1.305. [DOI] [PubMed] [Google Scholar]
- 118.Flaim SF, Minteer WJ, Clark DP, Zelis R. Cardiovascular response to acute aquatic and treadmill exercise in the untrained rat. J Appl Physiol 46: 302–308, 1979. doi: 10.1152/jappl.1979.46.2.302. [DOI] [PubMed] [Google Scholar]
- 119.Flaim SF, Nellis SH, Toggart EJ, Drexler H, Kanda K, Newman ED. Multiple simultaneous determinations of hemodynamics and flow distribution in conscious rat. J Pharmacol Methods 11: 1–39, 1984. doi: 10.1016/0160-5402(84)90050-0. [DOI] [PubMed] [Google Scholar]
- 120.Fletcher GF, Blair SN, Blumenthal J, Caspersen C, Chaitman B, Epstein S, Falls H, Froelicher ES, Froelicher VF, Pina IL. Statement on exercise. Benefits and recommendations for physical activity programs for all Americans. A statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Association. Circulation 86: 340–344, 1992. doi: 10.1161/01.CIR.86.1.340. [DOI] [PubMed] [Google Scholar]
- 121.Foundation for Biomedical Research Nobel prizes. Lab animals have made important contributions to nearly every Nobel Prize in Medicine (Online). https://fbresearch.org/medical-advances/nobel-prizes/ [30 June 2019].
- 122.Freeman K, Lerman I, Kranias EG, Bohlmeyer T, Bristow MR, Lefkowitz RJ, Iaccarino G, Koch WJ, Leinwand LA. Alterations in cardiac adrenergic signaling and calcium cycling differentially affect the progression of cardiomyopathy. J Clin Invest 107: 967–974, 2001. doi: 10.1172/JCI12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fregosi RF, Dempsey JA. Arterial blood acid-base regulation during exercise in rats. J Appl Physiol 57: 396–402, 1984. doi: 10.1152/jappl.1984.57.2.396. [DOI] [PubMed] [Google Scholar]
- 124.Fregosi RF, Dempsey JA. Effects of exercise in normoxia and acute hypoxia on respiratory muscle metabolites. J Appl Physiol (1985) 60: 1274–1283, 1986. doi: 10.1152/jappl.1986.60.4.1274. [DOI] [PubMed] [Google Scholar]
- 125.Fruth JM, Gisolfi CV. Work-heat tolerance in endurance-trained rats. J Appl Physiol Respir Environ Exerc Physiol 54: 249–253, 1983. doi: 10.1152/jappl.1983.54.1.249. [DOI] [PubMed] [Google Scholar]
- 126.Fuller A, Carter RN, Mitchell D. Brain and abdominal temperatures at fatigue in rats exercising in the heat. J Appl Physiol (1985) 84: 877–883, 1998. doi: 10.1152/jappl.1998.84.3.877. [DOI] [PubMed] [Google Scholar]
- 127.Gaesser GA, Wilson LA. Effects of continuous and interval training on the parameters of the power-endurance time relationship for high-intensity exercise. Int J Sports Med 9: 417–421, 1988. doi: 10.1055/s-2007-1025043. [DOI] [PubMed] [Google Scholar]
- 128.Gal D, Isner JM. Atherosclerotic Yucatan microswine as a model for novel cardiovascular interventions and imaging. In: Swine as Models in Biomedical Research, edited by Swindle MM, Moody DC, Phillips LD. Ames, IA: Iowa State University Press, 1992, p. 118–140. [Google Scholar]
- 129.Gao L, Wang W, Zucker IH. Simvastatin inhibits central sympathetic outflow in heart failure by a nitric-oxide synthase mechanism. J Pharmacol Exp Ther 326: 278–285, 2008. doi: 10.1124/jpet.107.136028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation 115: 3095–3102, 2007. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]
- 131.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95: 937–944, 2004. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- 132.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Simvastatin therapy normalizes sympathetic neural control in experimental heart failure: roles of angiotensin II type 1 receptors and NAD(P)H oxidase. Circulation 112: 1763–1770, 2005. doi: 10.1161/CIRCULATIONAHA.105.552174. [DOI] [PubMed] [Google Scholar]
- 133.Garland T Jr, Morgan MT, Swallow JG, Rhodes JS, Girard I, Belter JG, Carter PA. Evolution of a small-muscle polymorphism in lines of house mice selected for high activity levels. Evolution 56: 1267–1275, 2002. doi: 10.1111/j.0014-3820.2002.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 134.Garry DJ, Ordway GA, Lorenz JN, Radford NB, Chin ER, Grange RW, Bassel-Duby R, Williams RS. Mice without myoglobin. Nature 395: 905–908, 1998. doi: 10.1038/27681. [DOI] [PubMed] [Google Scholar]
- 135.Gaustad SE, Rolim N, Wisløff U. A valid and reproducible protocol for testing maximal oxygen uptake in rabbits. Eur J Cardiovasc Prev Rehabil 17: 83–88, 2010. doi: 10.1097/HJR.0b013e32833090c4. [DOI] [PubMed] [Google Scholar]
- 136.Geenen D, Buttrick P, Scheuer J. Cardiovascular and hormonal responses to swimming and running in the rat. J Appl Physiol (1985) 65: 116–123, 1988. doi: 10.1152/jappl.1988.65.1.116. [DOI] [PubMed] [Google Scholar]
- 137.Geor RJ, Ommundson L, Fenton G, Pagan JD. Effects of an external nasal strip and frusemide on pulmonary haemorrhage in Thoroughbreds following high-intensity exercise. Equine Vet J 33: 577–584, 2001. doi: 10.2746/042516401776563490. [DOI] [PubMed] [Google Scholar]
- 138.Gerrity RG, Natarajan R, Nadler JL, Kimsey T. Diabetes-induced accelerated atherosclerosis in swine. Diabetes 50: 1654–1665, 2001. doi: 10.2337/diabetes.50.7.1654. [DOI] [PubMed] [Google Scholar]
- 139. Gibb AA, McNally LA, Riggs DW, Conklin DJ, Bhatnagar A, Hill BG. FVB/NJ mice are a useful model for examining cardiac adaptations to treadmill exercise. Front Physiol 7: 636, 2016. [Erratum in Front Physiol 8: 800, 2017]. doi: 10.3389/fphys.2016.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gleeson TT, Baldwin KM. Cardiovascular response to treadmill exercise in untrained rats. J Appl Physiol 50: 1206–1211, 1981. doi: 10.1152/jappl.1981.50.6.1206. [DOI] [PubMed] [Google Scholar]
- 141.Gleeson TT, Mullin WJ, Baldwin KM. Cardiovascular responses to treadmill exercise in rats: effects of training. J Appl Physiol 54: 789–793, 1983. doi: 10.1152/jappl.1983.54.3.789. [DOI] [PubMed] [Google Scholar]
- 142.Goh J, Ladiges W. Voluntary wheel running in mice. Curr Protoc Mouse Biol 5: 283–290, 2015. doi: 10.1002/9780470942390.mo140295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Graham BH, Waymire KG, Cottrell B, Trounce IA, MacGregor GR, Wallace DC. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat Genet 16: 226–234, 1997. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- 144.Grange RW, Meeson A, Chin E, Lau KS, Stull JT, Shelton JM, Williams RS, Garry DJ. Functional and molecular adaptations in skeletal muscle of myoglobin-mutant mice. Am J Physiol Cell Physiol 281: C1487–C1494, 2001. doi: 10.1152/ajpcell.2001.281.5.C1487. [DOI] [PubMed] [Google Scholar]
- 145.Gruner JA, Altman J. Swimming in the rat: analysis of locomotor performance in comparison to stepping. Exp Brain Res 40: 374–382, 1980. doi: 10.1007/BF00236146. [DOI] [PubMed] [Google Scholar]
- 147.Gute D, Laughlin MH, Amann JF. Regional changes in capillary supply in skeletal muscle of interval-sprint and low-intensity, endurance-trained rats. Microcirculation 1: 183–193, 1994. doi: 10.3109/10739689409148273. [DOI] [PubMed] [Google Scholar]
- 148.Gute D, Fraga C, Laughlin MH, Amann JF. Regional changes in capillary supply in skeletal muscle of high-intensity endurance-trained rats. J Appl Physiol (1985) 81: 619–626, 1996. doi: 10.1152/jappl.1996.81.2.619. [DOI] [PubMed] [Google Scholar]
- 149.Hahn SA, Ferreira LF, Williams JB, Jansson KP, Behnke BJ, Musch TI, Poole DC. Downhill treadmill running trains the rat spinotrapezius muscle. J Appl Physiol (1985) 102: 412–416, 2007. doi: 10.1152/japplphysiol.00581.2006. [DOI] [PubMed] [Google Scholar]
- 150.Haidet GC, Musch TI, Ordway GA, Mitchell JH. Exercise, dobutamine, and combined atropine, norepinephrine, and epinephrine compared. J Appl Physiol (1985) 58: 2047–2053, 1985. doi: 10.1152/jappl.1985.58.6.2047. [DOI] [PubMed] [Google Scholar]
- 151.Hall JL, Stanley WC, Lopaschuk GD, Wisneski JA, Pizzurro RD, Hamilton CD, McCormack JG. Impaired pyruvate oxidation but normal glucose uptake in diabetic pig heart during dobutamine-induced work. Am J Physiol Heart Circ Physiol 271: H2320–H2329, 1996. doi: 10.1152/ajpheart.1996.271.6.H2320. [DOI] [PubMed] [Google Scholar]
- 152.Halseth AE, Fogt DL, Fregosi RF, Henriksen EJ. Metabolic responses of rat respiratory muscles to voluntary exercise training. J Appl Physiol (1985) 79: 902–907, 1995. doi: 10.1152/jappl.1995.79.3.902. [DOI] [PubMed] [Google Scholar]
- 153.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O’Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000. doi: 10.1152/ajpheart.2000.278.3.H818. [DOI] [PubMed] [Google Scholar]
- 154.Hammond RL, Augustyniak RA, Rossi NF, Lapanowski K, Dunbar JC, O’Leary DS. Alteration of humoral and peripheral vascular responses during graded exercise in heart failure. J Appl Physiol (1985) 90: 55–61, 2001. doi: 10.1152/jappl.2001.90.1.55. [DOI] [PubMed] [Google Scholar]
- 155.Han Y, Oshida Y, Li L, Koshinaka K, Fuku N, Yamanouchi K, Sato Y. Effect of voluntary wheel-running on insulin sensitivity and responsiveness in high-fat-fed rats. Endocr J 48: 551–555, 2001. doi: 10.1507/endocrj.48.551. [DOI] [PubMed] [Google Scholar]
- 156.Hao Q, Zhang F, Wang Y, Li Y, Qi X. Cardiac contractility modulation attenuates chronic heart failure in a rabbit model via the PI3K/AKT pathway. BioMed Res Int 2020: 1625362, 2020. doi: 10.1155/2020/1625362. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 157.Hara H, Nolan PM, Scott MO, Bucan M, Wakayama Y, Fischbeck KH. Running endurance abnormality in mdx mice. Muscle Nerve 25: 207–211, 2002. doi: 10.1002/mus.10023. [DOI] [PubMed] [Google Scholar]
- 158.Harrison BC, Bell ML, Allen DL, Byrnes WC, Leinwand LA. Skeletal muscle adaptations in response to voluntary wheel running in myosin heavy chain null mice. J Appl Physiol (1985) 92: 313–322, 2002. doi: 10.1152/japplphysiol.00832.2001. [DOI] [PubMed] [Google Scholar]
- 159.Haubold KW, Allen DL, Capetanaki Y, Leinwand LA. Loss of desmin leads to impaired voluntary wheel running and treadmill exercise performance. J Appl Physiol (1985) 95: 1617–1622, 2003. doi: 10.1152/japplphysiol.00408.2003. [DOI] [PubMed] [Google Scholar]
- 160.Hayes A, Williams DA. Beneficial effects of voluntary wheel running on the properties of dystrophic mouse muscle. J Appl Physiol (1985) 80: 670–679, 1996. doi: 10.1152/jappl.1996.80.2.670. [DOI] [PubMed] [Google Scholar]
- 161.Heaps CL, Parker JL. Effects of exercise training on coronary collateralization and control of collateral resistance. J Appl Physiol (1985) 111: 587–598, 2011. doi: 10.1152/japplphysiol.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Heaps CL, Sturek M, Price EM, Laughlin MH, Parker JL. Sarcoplasmic reticulum Ca2+ uptake is impaired in coronary smooth muscle distal to coronary occlusion. Am J Physiol Heart Circ Physiol 281: H223–H231, 2001. doi: 10.1152/ajpheart.2001.281.1.H223. [DOI] [PubMed] [Google Scholar]
- 163.Helwig B, Schreurs KM, Hansen J, Hageman KS, Zbreski MG, McAllister RM, Mitchell KE, Musch TI. Training-induced changes in skeletal muscle Na+-K+ pump number and isoform expression in rats with chronic heart failure. J Appl Physiol (1985) 94: 2225–2236, 2003. doi: 10.1152/japplphysiol.00279.2002. [DOI] [PubMed] [Google Scholar]
- 164.Henderson KK, Wagner H, Favret F, Britton SL, Koch LG, Wagner PD, Gonzalez NC. Determinants of maximal O2 uptake in rats selectively bred for endurance running capacity. J Appl Physiol (1985) 93: 1265–1274, 2002. doi: 10.1152/japplphysiol.00809.2001. [DOI] [PubMed] [Google Scholar]
- 165.Henriksen EJ, Halseth AE. Early alterations in soleus GLUT-4, glucose transport, and glycogen in voluntary running rats. J Appl Physiol (1985) 76: 1862–1867, 1994. doi: 10.1152/jappl.1994.76.5.1862. [DOI] [PubMed] [Google Scholar]
- 166.Henriksen EJ, Munoz KA, Aannestad AT, Tischler ME. Cardiac protein content and synthesis in vivo after voluntary running or head-down suspension. J Appl Physiol (1985) 76: 2814–2819, 1994. doi: 10.1152/jappl.1994.76.6.2814. [DOI] [PubMed] [Google Scholar]
- 167.Henriksen EJ, Halseth AE. Adaptive responses of GLUT-4 and citrate synthase in fast-twitch muscle of voluntary running rats. Am J Physiol Regul Integr Comp Physiol 268: R130–R134, 1995. doi: 10.1152/ajpregu.1995.268.1.R130. [DOI] [PubMed] [Google Scholar]
- 168.Higgins CB, Vatner SF, Braunwald E. Postprandial redistribution of regional blood flow at rest and during exercise in conscious dogs with experimental heart failure. Am J Cardiol 34: 318–324, 1974. doi: 10.1016/0002-9149(74)90033-2. [DOI] [PubMed] [Google Scholar]
- 169.Higgins CB, Vatner SF, Franklin D, Braunwald E. Effects of experimentally produced heart failure on the peripheral vascular response to severe exercise in conscious dogs. Circ Res 31: 186–194, 1972. doi: 10.1161/01.RES.31.2.186. [DOI] [PubMed] [Google Scholar]
- 170.Hill AV. The physiological basis of athletic records. Nature 116: 544–548, 1925. doi: 10.1038/116544a0. [DOI] [Google Scholar]
- 171.Hilty MR, Groth H, Moore RL, Musch TI. Determinants of VO2max in rats after high-intensity sprint training. J Appl Physiol (1985) 66: 195–201, 1989. doi: 10.1152/jappl.1989.66.1.195. [DOI] [PubMed] [Google Scholar]
- 172.Hirai DM, Copp SW, Holdsworth CT, Ferguson SK, McCullough DJ, Behnke BJ, Musch TI, Poole DC. Skeletal muscle microvascular oxygenation dynamics in heart failure: exercise training and nitric oxide-mediated function. Am J Physiol Heart Circ Physiol 306: H690–H698, 2014. doi: 10.1152/ajpheart.00901.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hirai DM, Musch TI, Poole DC. Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am J Physiol Heart Circ Physiol 309: H1419–H1439, 2015. doi: 10.1152/ajpheart.00469.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hirai DM, Copp SW, Ferguson SK, Holdsworth CT, Hageman KS, Poole DC, Musch TI. Neuronal nitric oxide synthase regulation of skeletal muscle functional hyperemia: exercise training and moderate compensated heart failure. Nitric Oxide 74: 1–9, 2018. doi: 10.1016/j.niox.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol (1985) 77: 1288–1293, 1994. doi: 10.1152/jappl.1994.77.3.1288. [DOI] [PubMed] [Google Scholar]
- 176.Hodgkin BC, Millard RW, Nelson CV. Effect of hematocrit on electrocardiographic potentials and dipole moment of the pig. Am J Physiol Heart Circ Physiol 232: H406–H410, 1977. doi: 10.1152/ajpheart.1977.232.4.H406. [DOI] [PubMed] [Google Scholar]
- 177.Hokama JY, Streeper RS, Henriksen EJ. Voluntary exercise training enhances glucose transport in muscle stimulated by insulin-like growth factor I. J Appl Physiol (1985) 82: 508–512, 1997. doi: 10.1152/jappl.1997.82.2.508. [DOI] [PubMed] [Google Scholar]
- 178.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242: 2278–2282, 1967. [PubMed] [Google Scholar]
- 179.Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol 38: 273–291, 1976. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- 180.Holloszy JO, Smith EK, Vining M, Adams S. Effect of voluntary exercise on longevity of rats. J Appl Physiol (1985) 59: 826–831, 1985. doi: 10.1152/jappl.1985.59.3.826. [DOI] [PubMed] [Google Scholar]
- 181.Holloszy JO. Exercise and food restriction in rats. J Nutr 122, Suppl 3: 774–777, 1992. doi: 10.1093/jn/122.suppl_3.774. [DOI] [PubMed] [Google Scholar]
- 182.Holloszy JO. Exercise increases average longevity of female rats despite increased food intake and no growth retardation. J Gerontol 48: B97–B100, 1993. doi: 10.1093/geronj/48.3.B97. [DOI] [PubMed] [Google Scholar]
- 183.Holloszy JO. Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol (1985) 82: 399–403, 1997. doi: 10.1152/jappl.1997.82.2.399. [DOI] [PubMed] [Google Scholar]
- 184.Holvoet P, Theilmeier G, Shivalkar B, Flameng W, Collen D. LDL hypercholesterolemia is associated with accumulation of oxidized LDL, atherosclerotic plaque growth, and compensatory vessel enlargement in coronary arteries of miniature pigs. Arterioscler Thromb Vasc Biol 18: 415–422, 1998. doi: 10.1161/01.ATV.18.3.415. [DOI] [PubMed] [Google Scholar]
- 185.Houle-Leroy P, Guderley H, Swallow JG, Garland T Jr. Artificial selection for high activity favors mighty mini-muscles in house mice. Am J Physiol Regul Integr Comp Physiol 284: R433–R443, 2003. doi: 10.1152/ajpregu.00179.2002. [DOI] [PubMed] [Google Scholar]
- 186.Howlett KF, Sakamoto K, Hirshman MF, Aschenbach WG, Dow M, White MF, Goodyear LJ. Insulin signaling after exercise in insulin receptor substrate-2-deficient mice. Diabetes 51: 479–483, 2002. doi: 10.2337/diabetes.51.2.479. [DOI] [PubMed] [Google Scholar]
- 187.Høydal MA, Wisløff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil 14: 753–760, 2007. doi: 10.1097/HJR.0b013e3281eacef1. [DOI] [PubMed] [Google Scholar]
- 188.Hsu Y, Serpell JA. Development and validation of a questionnaire for measuring behavior and temperament traits in pet dogs. J Am Vet Med Assoc 223: 1293–1300, 2003. doi: 10.2460/javma.2003.223.1293. [DOI] [PubMed] [Google Scholar]
- 189.Hsueh W, Abel ED, Breslow JL, Maeda N, Davis RC, Fisher EA, Dansky H, McClain DA, McIndoe R, Wassef MK, Rabadán-Diehl C, Goldberg IJ. Recipes for creating animal models of diabetic cardiovascular disease. Circ Res 100: 1415–1427, 2007. doi: 10.1161/01.RES.0000266449.37396.1f. [DOI] [PubMed] [Google Scholar]
- 190.Iemitsu M, Miyauchi T, Maeda S, Tanabe T, Takanashi M, Irukayama-Tomobe Y, Sakai S, Ohmori H, Matsuda M, Yamaguchi I. Aging-induced decrease in the PPAR-α level in hearts is improved by exercise training. Am J Physiol Heart Circ Physiol 283: H1750–H1760, 2002. doi: 10.1152/ajpheart.01051.2001. [DOI] [PubMed] [Google Scholar]
- 191.Iivonen H, Nurminen L, Harri M, Tanila H, Puoliväli J. Hypothermia in mice tested in Morris water maze. Behav Brain Res 141: 207–213, 2003. doi: 10.1016/S0166-4328(02)00369-8. [DOI] [PubMed] [Google Scholar]
- 192.Ikeda S, Miyazaki H, Nakatani T, Kai Y, Kamei Y, Miura S, Tsuboyama-Kasaoka N, Ezaki O. Up-regulation of SREBP-1c and lipogenic genes in skeletal muscles after exercise training. Biochem Biophys Res Commun 296: 395–400, 2002. doi: 10.1016/S0006-291X(02)00883-5. [DOI] [PubMed] [Google Scholar]
- 193.Ikegami M, Jobe AH, Whitsett J, Korfhagen T. Tolerance of SP-A-deficient mice to hyperoxia or exercise. J Appl Physiol (1985) 89: 644–648, 2000. doi: 10.1152/jappl.2000.89.2.644. [DOI] [PubMed] [Google Scholar]
- 194.Isaza R, Behnke BJ, Bailey JK, McDonough P, Gonzalez NC, Poole DC. Arterial blood gas control in the upright versus recumbent Asian elephant. Respir Physiol Neurobiol 134: 169–176, 2003. doi: 10.1016/S1569-9048(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 195.Ishihara A, Roy RR, Ohira Y, Ibata Y, Edgerton VR. Hypertrophy of rat plantaris muscle fibers after voluntary running with increasing loads. J Appl Physiol (1985) 84: 2183–2189, 1998. doi: 10.1152/jappl.1998.84.6.2183. [DOI] [PubMed] [Google Scholar]
- 196.Ishihara A, Hirofuji C, Nakatani T, Itoh K, Itoh M, Katsuta S. Effects of running exercise with increasing loads on tibialis anterior muscle fibres in mice. Exp Physiol 87: 113–116, 2002. doi: 10.1113/eph8702340. [DOI] [PubMed] [Google Scholar]
- 197.Jacobs RA, Rasmussen P, Siebenmann C, Díaz V, Gassmann M, Pesta D, Gnaiger E, Nordsborg NB, Robach P, Lundby C. Determinants of time trial performance and maximal incremental exercise in highly trained endurance athletes. J Appl Physiol (1985) 111: 1422–1430, 2011. doi: 10.1152/japplphysiol.00625.2011. [DOI] [PubMed] [Google Scholar]
- 198.Jasmin BJ, Gardiner PF. Patterns of EMG activity of rat plantaris muscle during swimming and other locomotor activities. J Appl Physiol (1985) 63: 713–718, 1987. doi: 10.1152/jappl.1987.63.2.713. [DOI] [PubMed] [Google Scholar]
- 199.Jasperse JL, Laughlin MH. Vasomotor responses of soleus feed arteries from sedentary and exercise-trained rats. J Appl Physiol (1985) 86: 441–449, 1999. doi: 10.1152/jappl.1999.86.2.441. [DOI] [PubMed] [Google Scholar]
- 200.Johnsen AB, Høydal M, Røsbjørgen R, Stølen T, Wisløff U. Aerobic interval training partly reverse contractile dysfunction and impaired Ca2+ handling in atrial myocytes from rats with post infarction heart failure. PLoS One 8: e66288, 2013. doi: 10.1371/journal.pone.0066288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Johnson GJ, Griggs TR, Badimon L. The utility of animal models in the preclinical study of interventions to prevent human coronary artery restenosis: analysis and recommendations. Thromb Haemost 81: 835–843, 1999. doi: 10.1055/s-0037-1614578. [DOI] [PubMed] [Google Scholar]
- 202.Johnson RA, Rhodes JS, Jeffrey SL, Garland T Jr, Mitchell GS. Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience 121: 1–7, 2003. doi: 10.1016/S0306-4522(03)00422-6. [DOI] [PubMed] [Google Scholar]
- 203.Jokinen MP, Clarkson TB, Prichard RW. Animal models in atherosclerosis research. Exp Mol Pathol 42: 1–28, 1985. doi: 10.1016/0014-4800(85)90015-2. [DOI] [PubMed] [Google Scholar]
- 204.Jones TE, Baar K, Ojuka E, Chen M, Holloszy JO. Exercise induces an increase in muscle UCP3 as a component of the increase in mitochondrial biogenesis. Am J Physiol Endocrinol Metab 284: E96–E101, 2003. doi: 10.1152/ajpendo.00316.2002. [DOI] [PubMed] [Google Scholar]
- 205.Jones AM, Wilkerson DP, DiMenna F, Fulford J, Poole DC. Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am J Physiol Regul Integr Comp Physiol 294: R585–R593, 2008. doi: 10.1152/ajpregu.00731.2007. [DOI] [PubMed] [Google Scholar]
- 206.Jones AM, Burnley M, Black MI, Poole DC, Vanhatalo A. The maximal metabolic steady state: redefining the ‘gold standard’. Physiol Rep 7: e14098, 2019. doi: 10.14814/phy2.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jones AM, Vanhatalo A, Burnley M, Morton RH, Poole DC. Critical power: implications for determination of V̇o2max and exercise tolerance. Med Sci Sports Exerc 42: 1876–1890, 2010. doi: 10.1249/MSS.0b013e3181d9cf7f. [DOI] [PubMed] [Google Scholar]
- 208.Jones JH, Ohmura H, Stanley SD, Hiraga A. Energetic cost of locomotion on different equine treadmills. Equine Vet J Suppl 38, S36: 365–369, 2006. doi: 10.1111/j.2042-3306.2006.tb05570.x. [DOI] [PubMed] [Google Scholar]
- 209.Jørgensen A, Berge VJ, Brubakk AO, Wisløff U. A reliable and valid protocol for measuring maximal oxygen uptake in pigs. Eur J Cardiovasc Prev Rehabil 16: 628–632, 2009. doi: 10.1097/HJR.0b013e32832e672c. [DOI] [PubMed] [Google Scholar]
- 210.Jover B, McGrath BP, Ludbrook J. Haemodynamic and metabolic responses of laboratory rabbits to near-maximal treadmill exercise. Clin Exp Pharmacol Physiol 14: 811–823, 1987. doi: 10.1111/j.1440-1681.1987.tb02418.x. [DOI] [PubMed] [Google Scholar]
- 211.Kano Y, Padilla DJ, Behnke BJ, Hageman KS, Musch TI, Poole DC. Effects of eccentric exercise on microcirculation and microvascular oxygen pressures in rat spinotrapezius muscle. J Appl Physiol (1985) 99: 1516–1522, 2005. doi: 10.1152/japplphysiol.00069.2005. [DOI] [PubMed] [Google Scholar]
- 212.Kano Y, Padilla D, Hageman KS, Poole DC, Musch TI. Downhill running: a model of exercise hyperemia in the rat spinotrapezius muscle. J Appl Physiol (1985) 97: 1138–1142, 2004. doi: 10.1152/japplphysiol.00334.2004. [DOI] [PubMed] [Google Scholar]
- 213.Kantha SS. A centennial review; the 1890 tetanus antitoxin paper of von Behring and Kitasato and the related developments. Keio J Med 40: 35–39, 1991. doi: 10.2302/kjm.40.35. [DOI] [PubMed] [Google Scholar]
- 214.Kaplan ML, Cheslow Y, Vikstrom K, Malhotra A, Geenen DL, Nakouzi A, Leinwand LA, Buttrick PM. Cardiac adaptations to chronic exercise in mice. Am J Physiol Heart Circ Physiol 267: H1167–H1173, 1994. doi: 10.1152/ajpheart.1994.267.3.H1167. [DOI] [PubMed] [Google Scholar]
- 215.Karasik A, Hattori M. Use of animal models in the study of diabetes. In: Joslin’s Diabetes Mellitus, edited by Kahn CR, Weir GC. Philadelphia, PA: Lea & Febiger, 1994, p. 317–350. [Google Scholar]
- 216.Karlsson S, Ahrén B. Insulin and glucagon secretion in swimming mice: effects of autonomic receptor antagonism. Metabolism 39: 724–732, 1990. doi: 10.1016/0026-0495(90)90108-O. [DOI] [PubMed] [Google Scholar]
- 217.Kashimoto S, Nonaka A, Nakamura T, Kumazawa T. Anesthetic influences on myocardial and hepatic energy metabolism in hemorrhaged spontaneous hypertensive rats. Tohoku J Exp Med 168: 475–481, 1992. doi: 10.1620/tjem.168.475. [DOI] [PubMed] [Google Scholar]
- 218.Kayar SR, Hoppeler H, Jones JH, Longworth K, Armstrong RB, Laughlin MH, Lindstedt SL, Bicudo JE, Groebe K, Taylor CR. Capillary blood transit time in muscles in relation to body size and aerobic capacity. J Exp Biol 194: 69–81, 1994. [DOI] [PubMed] [Google Scholar]
- 219.Kemi OJ, Loennechen JP, Wisløff U, Ellingsen Ø. Intensity-controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J Appl Physiol (1985) 93: 1301–1309, 2002. doi: 10.1152/japplphysiol.00231.2002. [DOI] [PubMed] [Google Scholar]
- 220.Kemi OJ, Haram PM, Wisløff U, Ellingsen Ø. Aerobic fitness is associated with cardiomyocyte contractile capacity and endothelial function in exercise training and detraining. Circulation 109: 2897–2904, 2004. doi: 10.1161/01.CIR.0000129308.04757.72. [DOI] [PubMed] [Google Scholar]
- 221.Kemi OJ, Haram PM, Loennechen JP, Osnes JB, Skomedal T, Wisløff U, Ellingsen Ø. Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res 67: 161–172, 2005. doi: 10.1016/j.cardiores.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 222.Kemi OJ, MacQuaide N, Hoydal MA, Ellingsen O, Smith GL, Wisløff U. Exercise training corrects control of spontaneous calcium waves in hearts from myocardial infarction heart failure rats. J Cell Physiol 227: 20–26, 2012. doi: 10.1002/jcp.22771. [DOI] [PubMed] [Google Scholar]
- 223.Kent JA, Ørtenblad N, Hogan MC, Poole DC, Musch TI. No muscle is an island: Integrative perspectives on muscle fatigue. Med Sci Sports Exerc 48: 2281–2293, 2016. doi: 10.1249/MSS.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 224.Keteyian SJ, Brawner CA, Savage PD, Ehrman JK, Schairer J, Divine G, Aldred H, Ophaug K, Ades PA. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J 156: 292–300, 2008. doi: 10.1016/j.ahj.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 225.Khammy MM, Angus JA, Wright CE. Vascular reactivity of rabbit isolated renal and femoral resistance arteries in renal wrap hypertension. Eur J Pharmacol 773: 32–41, 2016. doi: 10.1016/j.ejphar.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 226.Kiley JP, Fedde MR. Cardiopulmonary control during exercise in the duck. J Appl Physiol 55: 1574–1581, 1983. doi: 10.1152/jappl.1983.55.5.1574. [DOI] [PubMed] [Google Scholar]
- 227.Kiley JP, Fedde MR. Exercise hyperpnea in the duck without intrapulmonary chemoreceptor involvement. Respir Physiol 53: 355–365, 1983. doi: 10.1016/0034-5687(83)90125-1. [DOI] [PubMed] [Google Scholar]
- 228.Kindig CA, McDonough P, Erickson HH, Poole DC. Effect of l-NAME on oxygen uptake kinetics during heavy-intensity exercise in the horse. J Appl Physiol (1985) 91: 891–896, 2001. doi: 10.1152/jappl.2001.91.2.891. [DOI] [PubMed] [Google Scholar]
- 229.Kindig CA, McDonough P, Fenton G, Poole DC, Erickson HH. Efficacy of nasal strip and furosemide in mitigating EIPH in Thoroughbred horses. J Appl Physiol (1985) 91: 1396–1400, 2001. doi: 10.1152/jappl.2001.91.3.1396. [DOI] [PubMed] [Google Scholar]
- 230.Kindig CA, Poole DC, McDonough P, Erickson HH. Nasal strips and EIPH in the exercising Thoroughbred racehorse. J Appl Physiol (1985) 91: 1908–1910, 2001. doi: 10.1152/jappl.2001.91.4.1908. [DOI] [PubMed] [Google Scholar]
- 231.Kindig CA, Ramsel C, McDonough P, Poole DC, Erickson HH. Inclined running increases pulmonary haemorrhage in the Thoroughbred horse. Equine Vet J 35: 581–585, 2003. doi: 10.2746/042516403775467199. [DOI] [PubMed] [Google Scholar]
- 232.Kindig CA, Sexton WL, Fedde MR, Poole DC. Skeletal muscle microcirculatory structure and hemodynamics in diabetes. Respir Physiol 111: 163–175, 1998. doi: 10.1016/S0034-5687(97)00122-9. [DOI] [PubMed] [Google Scholar]
- 233.Kindig CA, Gallatin LL, Erickson HH, Fedde MR, Poole DC. Cardiorespiratory impact of the nitric oxide synthase inhibitor l-NAME in the exercising horse. Respir Physiol 120: 151–166, 2000. doi: 10.1016/S0034-5687(00)00096-7. [DOI] [PubMed] [Google Scholar]
- 234.Kinnick TR, Youngblood EB, O’Keefe MP, Saengsirisuwan V, Teachey MK, Henriksen EJ. Modulation of insulin resistance and hypertension by voluntary exercise training in the TG(mREN2)27 rat. J Appl Physiol (1985) 93: 805–812, 2002. doi: 10.1152/japplphysiol.00236.2002. [DOI] [PubMed] [Google Scholar]
- 235.Kirchhof P, Fabritz L, Fortmuller L, Matherne GP, Lankford A, Baba HA, Schmitz W, Breithardt G, Neumann J, Boknik P. Altered sinus nodal and atrioventricular nodal function in freely moving mice overexpressing the A1 adenosine receptor. Am J Physiol Heart Circ Physiol 285: H145–H153, 2003. doi: 10.1152/ajpheart.01036.2002. [DOI] [PubMed] [Google Scholar]
- 236.Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. Regular exercise is beneficial to a mouse model of amyotrophic lateral sclerosis. Ann Neurol 53: 804–807, 2003. doi: 10.1002/ana.10597. [DOI] [PubMed] [Google Scholar]
- 237.Kitamura T, Mishina M, Sugiyama H. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor ε1 subunit. Neurosci Res 47: 55–63, 2003. doi: 10.1016/S0168-0102(03)00171-8. [DOI] [PubMed] [Google Scholar]
- 238.Knight PK, Rose RJ, Evans DL, Cluer D, Henckel P, Saltin B. Metabolic responses to maximal intensity exercise in the racing camel. Acta Physiol Scand Suppl 617: 61–77, 1994. [PubMed] [Google Scholar]
- 239.Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics 5: 45–52, 2001. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- 240.Koch LG, Britton SL. Rat models of exercise for the study of complex disease. Methods Mol Biol 2018: 309–317, 2019. doi: 10.1007/978-1-4939-9581-3_15. [DOI] [PubMed] [Google Scholar]
- 241.Koga S, Rossiter HB, Heinonen I, Musch TI, Poole DC. Dynamic heterogeneity of exercising muscle blood flow and O2 utilization. Med Sci Sports Exerc 46: 860–876, 2014. doi: 10.1249/MSS.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 242.Konarzewski M, Sadowski B, Jóźwik I. Metabolic correlates of selection for swim stress-induced analgesia in laboratory mice. Am J Physiol Regul Integr Comp Physiol 273: R337–R343, 1997. doi: 10.1152/ajpregu.1997.273.1.R337. [DOI] [PubMed] [Google Scholar]
- 243.Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol 287: H2768–H2776, 2004. doi: 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kraljevic J, Marinovic J, Pravdic D, Zubin P, Dujic Z, Wisløff U, Ljubkovic M. Aerobic interval training attenuates remodelling and mitochondrial dysfunction in the post-infarction failing rat heart. Cardiovasc Res 99: 55–64, 2013. doi: 10.1093/cvr/cvt080. [DOI] [PubMed] [Google Scholar]
- 245.Krebs HA. The August Krogh principle: “For many problems there is an animal on which it can be most conveniently studied”. J Exp Zool 194: 221–226, 1975. doi: 10.1002/jez.1401940115. [DOI] [PubMed] [Google Scholar]
- 246.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295: 1089–1092, 2002. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 247.Lambert MI, Noakes TD. Dissociation of changes in VO2 max, muscle QO2, and performance with training in rats. J Appl Physiol (1985) 66: 1620–1625, 1989. doi: 10.1152/jappl.1989.66.4.1620. [DOI] [PubMed] [Google Scholar]
- 248.Langsetmo I, Weigle GE, Fedde MR, Erickson HH, Barstow TJ, Poole DC. V̇o2 kinetics in the horse during moderate and heavy exercise. J Appl Physiol (1985) 83: 1235–1241, 1997. doi: 10.1152/jappl.1997.83.4.1235. [DOI] [PubMed] [Google Scholar]
- 249.Lapveteläinen T, Nevalainen T, Parkkinen JJ, Arokoski J, Kiraly K, Hyttinen M, Halonen P, Helminen HJ. Lifelong moderate running training increases the incidence and severity of osteoarthritis in the knee joint of C57BL mice. Anat Rec 242: 159–165, 1995. doi: 10.1002/ar.1092420204. [DOI] [PubMed] [Google Scholar]
- 250.Lapveteläinen T, Tiihonen A, Koskela P, Nevalainen T, Lindblom J, Király K, Halonen P, Helminen HJ. Training a large number of laboratory mice using running wheels and analyzing running behavior by use of a computer-assisted system. Lab Anim Sci 47: 172–179, 1997. [PubMed] [Google Scholar]
- 251.Lauderdale MA, Hinchcliff KW. Hyperbolic relationship between time-to-fatigue and workload. Equine Vet J Suppl 30: 586–590, 1999. doi: 10.1111/j.2042-3306.1999.tb05289.x. [DOI] [PubMed] [Google Scholar]
- 252.Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol Heart Circ Physiol 243: H296–H306, 1982. doi: 10.1152/ajpheart.1982.243.2.H296. [DOI] [PubMed] [Google Scholar]
- 253.Laughlin MH, Armstrong RB. Rat muscle blood flows as a function of time during prolonged slow treadmill exercise. Am J Physiol Heart Circ Physiol 244: H814–H824, 1983. doi: 10.1152/ajpheart.1983.244.6.H814. [DOI] [PubMed] [Google Scholar]
- 254.Laughlin MH, Mohrman SJ, Armstrong RB. Muscular blood flow distribution patterns in the hindlimb of swimming rats. Am J Physiol Heart Circ Physiol 246: H398–H403, 1984. doi: 10.1152/ajpheart.1984.246.3.H398. [DOI] [PubMed] [Google Scholar]
- 255.Laughlin MH, Armstrong RB. Muscle blood flow during locomotory exercise. Exerc Sport Sci Rev 13: 95–136, 1985. doi: 10.1249/00003677-198500130-00006. [DOI] [PubMed] [Google Scholar]
- 256.Laughlin MH, Burns JW, Fanton J, Ripperger J, Peterson DF. Coronary blood flow reserve during +Gz stress and treadmill exercise in miniature swine. J Appl Physiol (1985) 64: 2589–2596, 1988. doi: 10.1152/jappl.1988.64.6.2589. [DOI] [PubMed] [Google Scholar]
- 257.Laughlin MH, Korthuis RJ, Sexton WL, Armstrong RB. Regional muscle blood flow capacity and exercise hyperemia in high-intensity trained rats. J Appl Physiol (1985) 64: 2420–2427, 1988. doi: 10.1152/jappl.1988.64.6.2420. [DOI] [PubMed] [Google Scholar]
- 258.Laughlin MH, Overholser KA, Bhatte MJ. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol (1985) 67: 1140–1149, 1989. doi: 10.1152/jappl.1989.67.3.1140. [DOI] [PubMed] [Google Scholar]
- 259.Laughlin MH, McAllister RM. Exercise training-induced coronary vascular adaptation. J Appl Physiol (1985) 73: 2209–2225, 1992. doi: 10.1152/jappl.1992.73.6.2209. [DOI] [PubMed] [Google Scholar]
- 260.Laughlin MH, Schrage WG, McAllister RM, Garverick HA, Jones AW. Interaction of gender and exercise training: vasomotor reactivity of porcine skeletal muscle arteries. J Appl Physiol (1985) 90: 216–227, 2001. doi: 10.1152/jappl.2001.90.1.216. [DOI] [PubMed] [Google Scholar]
- 261.Laughlin MH, Roseguini B. Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: differences with interval sprint training versus aerobic endurance training. J Physiol Pharmacol 59, Suppl 7: 71–88, 2008. [PMC free article] [PubMed] [Google Scholar]
- 262.Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol 302: H10–H23, 2012. doi: 10.1152/ajpheart.00574.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lawson DM, Duke JL, Zammit TG, Collins HL, DiCarlo SE. Recovery from carotid artery catheterization performed under various anesthetics in male, Sprague-Dawley rats. Contemp Top Lab Anim Sci 40: 18–22, 2001. [PubMed] [Google Scholar]
- 264.Leblanc J, Dussault J, Lupien D, Richard D. Effect of diet and exercise on norepinephrine-induced thermogenesis in male and female rats. J Appl Physiol 52: 556–561, 1982. doi: 10.1152/jappl.1982.52.3.556. [DOI] [PubMed] [Google Scholar]
- 265.Lee IM, Paffenbarger RS Jr. Associations of light, moderate, and vigorous intensity physical activity with longevity. The Harvard Alumni Health Study. Am J Epidemiol 151: 293–299, 2000. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- 266.Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc 33, Suppl: S459–S471, 2001. doi: 10.1097/00005768-200106001-00016. [DOI] [PubMed] [Google Scholar]
- 267.Lee SG, Lee SJ, Thuy NVP, Kim JS, Lee JJ, Lee OH, Kim CK, Oh J, Park S, Lee OH, Kim SH, Park S, Lee SH, Hong SJ, Ahn CM, Kim BK, Ko YG, Choi D, Hong MK, Jang Y. Synergistic protective effects of a statin and an angiotensin receptor blocker for initiation and progression of atherosclerosis. PLoS One 14: e0215604, 2019. doi: 10.1371/journal.pone.0215604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Leineweber C, Müller E, Marschang RE. Blood reference intervals for rabbits (Oryctolagus cuniculus) from routine diagnostic samples. Tierarztl Prax Ausg K Klientiere Heimtiere 46: 393–398, 2018. doi: 10.1055/s-0038-1677403. [DOI] [PubMed] [Google Scholar]
- 269.Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol (1985) 92: 2245–2255, 2002. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- 270.Li H, Shin SE, Seo MS, An JR, Choi IW, Jung WK, Firth AL, Lee DS, Yim MJ, Choi G, Lee JM, Na SH, Park WS. The anti-diabetic drug dapagliflozin induces vasodilation via activation of PKG and Kv channels. Life Sci 197: 46–55, 2018. doi: 10.1016/j.lfs.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 271.Liard JF. Regional blood flows in running normotensive and renal hypertensive dogs. J Hypertens 4: 399–406, 1986. doi: 10.1097/00004872-198608000-00002. [DOI] [PubMed] [Google Scholar]
- 272.Lightfoot JT, Turner MJ, Debate KA, Kleeberger SR. Interstrain variation in murine aerobic capacity. Med Sci Sports Exerc 33: 2053–2057, 2001. doi: 10.1097/00005768-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 273.Link RP, Pedersoli WM, Safanie AH. Effect of exercise on development of atherosclerosis in swine. Atherosclerosis 15: 107–122, 1972. doi: 10.1016/0021-9150(72)90044-5. [DOI] [PubMed] [Google Scholar]
- 274.Liu JL, Kulakofsky J, Zucker IH. Exercise training enhances baroreflex control of heart rate by a vagal mechanism in rabbits with heart failure. J Appl Physiol (1985) 92: 2403–2408, 2002. doi: 10.1152/japplphysiol.00039.2002. [DOI] [PubMed] [Google Scholar]
- 275.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: a role for angiotensin II. Circulation 102: 1854–1862, 2000. doi: 10.1161/01.CIR.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 276.Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Boström P, Che L, Zhang C, Spiegelman BM, Rosenzweig A. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab 21: 584–595, 2015. doi: 10.1016/j.cmet.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lloyd PG, Sheehy AJ, Edwards JM, Mokelke EA, Sturek M. Leukemia inhibitory factor is upregulated in coronary arteries of Ossabaw miniature swine after stent placement. Coron Artery Dis 19: 217–226, 2008. doi: 10.1097/MCA.0b013e3282f9d3be. [DOI] [PubMed] [Google Scholar]
- 278.Long X, Bratz IN, Alloosh M, Edwards JM, Sturek M. Short-term exercise training prevents micro- and macrovascular disease following coronary stenting. J Appl Physiol (1985) 108: 1766–1774, 2010. doi: 10.1152/japplphysiol.01014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Longhurst JC, Musch TI, Ordway GA. O2 consumption during exercise in dogs: roles of splenic contraction and α-adrenergic vasoconstriction. Am J Physiol Heart Circ Physiol 251: H502–H509, 1986. doi: 10.1152/ajpheart.1986.251.3.H502. [DOI] [PubMed] [Google Scholar]
- 280.Lowe DA, Alway SE. Animal models for inducing muscle hypertrophy: are they relevant for clinical applications in humans? J Orthop Sports Phys Ther 32: 36–43, 2002. doi: 10.2519/jospt.2002.32.2.36. [DOI] [PubMed] [Google Scholar]
- 281.Lowe HC, Schwartz RS, Mac Neill BD, Jang IK, Hayase M, Rogers C, Oesterle SN. The porcine coronary model of in-stent restenosis: current status in the era of drug-eluting stents. Catheter Cardiovasc Interv 60: 515–523, 2003. doi: 10.1002/ccd.10705. [DOI] [PubMed] [Google Scholar]
- 282.Lynch GS, Fary CJ, Williams DA. Quantitative measurement of resting skeletal muscle [Ca2+]i following acute and long-term downhill running exercise in mice. Cell Calcium 22: 373–383, 1997. doi: 10.1016/S0143-4160(97)90022-1. [DOI] [PubMed] [Google Scholar]
- 283.MacNeil B, Hoffman-Goetz L. Effect of exercise on natural cytotoxicity and pulmonary tumor metastases in mice. Med Sci Sports Exerc 25: 922–928, 1993. doi: 10.1249/00005768-199308000-00007. [DOI] [PubMed] [Google Scholar]
- 284.Mahley RW, Weisgraber KH. Canine lipoproteins and atherosclerosis. I. Isolation and characterization of plasma lipoproteins from control dogs. Circ Res 35: 713–721, 1974. doi: 10.1161/01.RES.35.5.713. [DOI] [PubMed] [Google Scholar]
- 285.Mahley RW, Weisgraber KH, Innerarity T. Canine lipoproteins and atherosclerosis. II. Characterization of the plasma lipoproteins associated with atherogenic and nonatherogenic hyperlipidemia. Circ Res 35: 722–733, 1974. doi: 10.1161/01.RES.35.5.722. [DOI] [PubMed] [Google Scholar]
- 286.Mahley RW, Weisgraber KH, Innerarity T, Brewer HB Jr, Assmann G. Swine lipoproteins and atherosclerosis. Changes in the plasma lipoproteins and apoproteins induced by cholesterol feeding. Biochemistry 14: 2817–2823, 1975. doi: 10.1021/bi00684a005. [DOI] [PubMed] [Google Scholar]
- 287.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 83: 778–786, 1991. doi: 10.1161/01.CIR.83.3.778. [DOI] [PubMed] [Google Scholar]
- 288.Manohar M. Blood flow to the respiratory and limb muscles and to abdominal organs during maximal exertion in ponies. J Physiol 377: 25–35, 1986. doi: 10.1113/jphysiol.1986.sp016174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Mapara M, Thomas BS, Bhat KM. Rabbit as an animal model for experimental research. Dent Res J (Isfahan) 9: 111–118, 2012. doi: 10.4103/1735-3327.92960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Marlin DJ, Nankervis K. Equine Exercise Physiology. Malden, MA: Blackwell Publishing, 2002. [Google Scholar]
- 291.Marlin DJ, Scott CM, Schroter RC, Mills PC, Harris RC, Harris PA, Orme CE, Roberts CA, Marr CM, Dyson SJ, Barrelet F. Physiological responses in nonheat acclimated horses performing treadmill exercise in cool (20°C/40%RH), hot dry (30°C/40%RH) and hot humid (30°C/80%RH). Equine Vet J Suppl 22: 70–84, 1996. doi: 10.1111/j.2042-3306.1996.tb05034.x. [DOI] [PubMed] [Google Scholar]
- 292.Marshall KD, Muller BN, Krenz M, Hanft LM, McDonald KS, Dellsperger KC, Emter CA. Heart failure with preserved ejection fraction: chronic low-intensity interval exercise training preserves myocardial O2 balance and diastolic function. J Appl Physiol (1985) 114: 131–147, 2013. doi: 10.1152/japplphysiol.01059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Massett MP, Berk BC. Strain-dependent differences in responses to exercise training in inbred and hybrid mice. Am J Physiol Regul Integr Comp Physiol 288: R1006–R1013, 2005. doi: 10.1152/ajpregu.00476.2004. [DOI] [PubMed] [Google Scholar]
- 294.Masson GS, Michelini LC. Experimental evidences supporting training-induced benefits in spontaneously hypertensive rats. Adv Exp Med Biol 999: 287–306, 2017. doi: 10.1007/978-981-10-4307-9_16. [DOI] [PubMed] [Google Scholar]
- 295.Maxwell AJ, Schauble E, Bernstein D, Cooke JP. Limb blood flow during exercise is dependent on nitric oxide. Circulation 98: 369–374, 1998. doi: 10.1161/01.CIR.98.4.369. [DOI] [PubMed] [Google Scholar]
- 296.Mazzeo RS, Brooks GA, Horvath SM. Effects of age on metabolic responses to endurance training in rats. J Appl Physiol 57: 1369–1374, 1984. doi: 10.1152/jappl.1984.57.5.1369. [DOI] [PubMed] [Google Scholar]
- 297.McAllister RM, Newcomer SC, Pope ER, Turk JR, Laughlin MH. Effects of chronic nitric oxide synthase inhibition on responses to acute exercise in swine. J Appl Physiol (1985) 104: 186–197, 2008. doi: 10.1152/japplphysiol.00731.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.McArdle WD, Montoye HJ. Reliability of exhaustive swimming in the laboratory rat. J Appl Physiol 21: 1431–1434, 1966. doi: 10.1152/jappl.1966.21.4.1431. [DOI] [PubMed] [Google Scholar]
- 299.McDonald TO, Gerrity RG, Jen C, Chen HJ, Wark K, Wight TN, Chait A, O’Brien KD. Diabetes and arterial extracellular matrix changes in a porcine model of atherosclerosis. J Histochem Cytochem 55: 1149–1157, 2007. doi: 10.1369/jhc.7A7221.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.McDonough P, Kindig CA, Erickson HH, Poole DC. Mechanistic basis for the gas exchange threshold in Thoroughbred horses. J Appl Physiol (1985) 92: 1499–1505, 2002. doi: 10.1152/japplphysiol.00909.2001. [DOI] [PubMed] [Google Scholar]
- 301.McDonough P, Kindig CA, Hildreth TS, Behnke BJ, Erickson HH, Poole DC. Effect of body incline on cardiac performance. Equine Vet J Suppl 34, S34: 506–509, 2002. doi: 10.1111/j.2042-3306.2002.tb05474.x. [DOI] [PubMed] [Google Scholar]
- 302.McDonough P, Kindig CA, Ramsel C, Poole DC, Erickson HH. The effect of treadmill incline on maximal oxygen uptake, gas exchange and the metabolic response to exercise in the horse. Exp Physiol 87: 499–506, 2002. doi: 10.1111/j.1469-445X.2002.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 303.McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH. A 30-year follow-up of the Dallas Bedrest and Training Study: I. Effect of age on the cardiovascular response to exercise. Circulation 104: 1350–1357, 2001. doi: 10.1161/circ.104.12.1350. [DOI] [PubMed] [Google Scholar]
- 304.McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, Mitchell JH. A 30-year follow-up of the Dallas Bedrest and Training Study: II. Effect of age on cardiovascular adaptation to exercise training. Circulation 104: 1358–1366, 2001. doi: 10.1161/hc3701.096099. [DOI] [PubMed] [Google Scholar]
- 305.McKirnan MD, Bloor CM. Clinical significance of coronary vascular adaptations to exercise training. Med Sci Sports Exerc 26: 1262–1268, 1994. doi: 10.1249/00005768-199410000-00013. [DOI] [PubMed] [Google Scholar]
- 306.McKirnan MD, White FC, Guth BD, Bloor CM. Cardiovascular and metabolic responses to acute and chronic exercise in swine. In: Swine in Biomedical Research, edited by Tumbleson ME. New York: Plenum Press, 1986, p. 1379–1394. [Google Scholar]
- 307.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110α) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA 100: 12355–12360, 2003. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, Schinke M, Kong S, Sherwood MC, Brown J, Riggi L, Kang PM, Izumo S. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110α) pathway. J Biol Chem 279: 4782–4793, 2004. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- 309.Melchert PJ, Duncker DJ, Traverse JH, Bache RJ. Role of channels and adenosine in regulation of coronary blood flow in the hypertrophied left ventricle. Am J Physiol Heart Circ Physiol 277: H617–H625, 1999. doi: 10.1152/ajpheart.1999.277.2.H617. [DOI] [PubMed] [Google Scholar]
- 310.Merkus D, Sorop O, Houweling B, Hoogteijling BA, Duncker DJ. KCa+ channels contribute to exercise-induced coronary vasodilation in swine. Am J Physiol Heart Circ Physiol 291: H2090–H2097, 2006. doi: 10.1152/ajpheart.00315.2006. [DOI] [PubMed] [Google Scholar]
- 311.Meyer TS, Fedde MR, Gaughan EM, Langsetmo I, Erickson HH. Quantification of exercise-induced pulmonary haemorrhage with bronchoalveolar lavage. Equine Vet J 30: 284–288, 1998. doi: 10.1111/j.2042-3306.1998.tb04098.x. [DOI] [PubMed] [Google Scholar]
- 312.Mills PC, Marlin DJ, Scott CM, Smith NC. Nitric oxide and thermoregulation during exercise in the horse. J Appl Physiol (1985) 82: 1035–1039, 1997. doi: 10.1152/jappl.1997.82.4.1035. [DOI] [PubMed] [Google Scholar]
- 313.Mills PC, Scott CM, Marlin DJ. Effects of nitric oxide inhibition on thermoregulation during exercise in the horse. Ann N Y Acad Sci 813: 591–599, 1997. doi: 10.1111/j.1749-6632.1997.tb51750.x. [DOI] [PubMed] [Google Scholar]
- 314.Milne KJ, Noble EG. Exercise-induced elevation of HSP70 is intensity dependent. J Appl Physiol (1985) 93: 561–568, 2002. doi: 10.1152/japplphysiol.00528.2001. [DOI] [PubMed] [Google Scholar]
- 315.Mizelle HL, Edwards TC, Montant JP. Abnormal cardiovascular responses to exercise during the development of obesity in dogs. Am J Hypertens 7: 374–378, 1994. doi: 10.1093/ajh/7.4.374. [DOI] [PubMed] [Google Scholar]
- 316.Mokelke EA, Dietz NJ, Eckman DM, Nelson MT, Sturek M. Diabetic dyslipidemia and exercise affect coronary tone and differential regulation of conduit and microvessel K+ current. Am J Physiol Heart Circ Physiol 288: H1233–H1241, 2005. doi: 10.1152/ajpheart.00732.2004. [DOI] [PubMed] [Google Scholar]
- 317.Mokelke EA, Hu Q, Song M, Toro L, Reddy HK, Sturek M. Altered functional coupling of coronary K+ channels in diabetic dyslipidemic pigs is prevented by exercise. J Appl Physiol (1985) 95: 1179–1193, 2003. doi: 10.1152/japplphysiol.00972.2002. [DOI] [PubMed] [Google Scholar]
- 318.Mondon CE, Dolkas CB, Reaven GM. Site of enhanced insulin sensitivity in exercise-trained rats at rest. Am J Physiol Endocrinol Metab 239: E169–E177, 1980. doi: 10.1152/ajpendo.1980.239.3.E169. [DOI] [PubMed] [Google Scholar]
- 319.Mondon CE, Dolkas CB, Sims C, Reaven GM. Spontaneous running activity in male rats: effect of age. J Appl Physiol (1985) 58: 1553–1557, 1985. doi: 10.1152/jappl.1985.58.5.1553. [DOI] [PubMed] [Google Scholar]
- 320.Moore RL, Hilty MR, Musch TI. Effect of aortic arterial catheterization on tissue glycogen content. J Appl Physiol (1985) 65: 2752–2756, 1988. doi: 10.1152/jappl.1988.65.6.2752. [DOI] [PubMed] [Google Scholar]
- 321.Moores WY, White FC, Bloor CM, Willford DC, Guth BD. Hemodynamic measurements in exercising swine. In: Swine in Biomedical Research, edited by Tumbleson ME. New York: Plenum Press, 1986, p. 1371–1377. [Google Scholar]
- 322.Moreira JB, Bechara LR, Bozi LH, Jannig PR, Monteiro AW, Dourado PM, Wisløff U, Brum PC. High- versus moderate-intensity aerobic exercise training effects on skeletal muscle of infarcted rats. J Appl Physiol (1985) 114: 1029–1041, 2013. doi: 10.1152/japplphysiol.00760.2012. [DOI] [PubMed] [Google Scholar]
- 323.Moreland AF, Clarkson TB, Lofland HB. Atherosclerosis in “miniature” swine. Arch Pathol 76: 203–210, 1963. [PubMed] [Google Scholar]
- 324.Munoz KA, Aannestad A, Tischler ME, Henriksen EJ. Skeletal muscle protein content and synthesis after voluntary running and subsequent unweighting. Metabolism 43: 994–999, 1994. doi: 10.1016/0026-0495(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 325.Murphy HM, Wideman CH, Aquila LA, Nadzam GR. Telemetry provides new insights into entrainment of activity wheel circadian rhythms and the role of body temperature in the development of ulcers in the activity-stress paradigm. Integr Physiol Behav Sci 37: 228–241, 2002. doi: 10.1007/BF02734183. [DOI] [PubMed] [Google Scholar]
- 326.Musch TI, Dempsey JA, Smith CA, Mitchell GS, Bateman NT. Metabolic acids and [H+] regulation in brain tissue during acclimatization to chronic hypoxia. J Appl Physiol 55: 1486–1495, 1983. doi: 10.1152/jappl.1983.55.5.1486. [DOI] [PubMed] [Google Scholar]
- 327.Musch TI, Haidet GC, Ordway GA, Longhurst JC, Mitchell JH. Dynamic exercise training in foxhounds. I. Oxygen consumption and hemodynamic responses. J Appl Physiol (1985) 59: 183–189, 1985. doi: 10.1152/jappl.1985.59.1.183. [DOI] [PubMed] [Google Scholar]
- 328.Musch TI, Moore RL, Leathers DJ, Bruno A, Zelis R. Endurance training in rats with chronic heart failure induced by myocardial infarction. Circulation 74: 431–441, 1986. doi: 10.1161/01.CIR.74.2.431. [DOI] [PubMed] [Google Scholar]
- 329.Musch TI, Friedman DB, Haidet GC, Stray-Gundersen J, Waldrop TG, Ordway GA. Arterial blood gases and acid-base status of dogs during graded dynamic exercise. J Appl Physiol (1985) 61: 1914–1919, 1986. doi: 10.1152/jappl.1986.61.5.1914. [DOI] [PubMed] [Google Scholar]
- 330.Musch TI, Friedman DB, Pitetti KH, Haidet GC, Stray-Gundersen J, Mitchell JH, Ordway GA. Regional distribution of blood flow of dogs during graded dynamic exercise. J Appl Physiol (1985) 63: 2269–2277, 1987. doi: 10.1152/jappl.1987.63.6.2269. [DOI] [PubMed] [Google Scholar]
- 331.Musch TI, Haidet GC, Ordway GA, Longhurst JC, Mitchell JH. Training effects on regional blood flow response to maximal exercise in foxhounds. J Appl Physiol (1985) 62: 1724–1732, 1987. doi: 10.1152/jappl.1987.62.4.1724. [DOI] [PubMed] [Google Scholar]
- 332.Musch TI. Skeletal muscle blood flow in exercising dogs. Med Sci Sports Exerc 20, Suppl: S104–S108, 1988. doi: 10.1249/00005768-198810001-00002. [DOI] [PubMed] [Google Scholar]
- 333.Musch TI, Moore RL, Hilty MR. Effects of dynamic exercise training on the metabolic and cardiocirculatory responses to exercise in the rat model of myocardial infarction and heart failure. Am J Cardiol 62: 20E–24E, 1988. doi: 10.1016/S0002-9149(88)80005-5. [DOI] [PubMed] [Google Scholar]
- 334.Musch TI, Bruno A, Bradford GE, Vayonis A, Moore RL. Measurements of metabolic rate in rats: a comparison of techniques. J Appl Physiol (1985) 65: 964–970, 1988. doi: 10.1152/jappl.1988.65.2.964. [DOI] [PubMed] [Google Scholar]
- 335.Musch TI, Moore RL, Riedy M, Burke P, Zelis R, Leo ME, Bruno A, Bradford GE. Glycogen concentrations and endurance capacity of rats with chronic heart failure. J Appl Physiol (1985) 64: 1153–1159, 1988. doi: 10.1152/jappl.1988.64.3.1153. [DOI] [PubMed] [Google Scholar]
- 336.Musch TI, Ghaul MR, Tranchitella V, Zelis R. Skeletal muscle glycogen depletion during submaximal exercise in rats with chronic heart failure. Basic Res Cardiol 85: 606–618, 1990. doi: 10.1007/BF01907895. [DOI] [PubMed] [Google Scholar]
- 337.Musch TI, Terrell JA, Hilty MR. Effects of high-intensity sprint training on skeletal muscle blood flow in rats. J Appl Physiol (1985) 71: 1387–1395, 1991. doi: 10.1152/jappl.1991.71.4.1387. [DOI] [PubMed] [Google Scholar]
- 338.Musch TI, Nguyen CT, Pham HV, Moore RL. Training effects on the regional blood flow response to exercise in myocardial infarcted rats. Am J Physiol Heart Circ Physiol 262: H1846–H1852, 1992. doi: 10.1152/ajpheart.1992.262.6.H1846. [DOI] [PubMed] [Google Scholar]
- 339.Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol 262: H411–H419, 1992. doi: 10.1152/ajpheart.1992.262.2.H411. [DOI] [PubMed] [Google Scholar]
- 340.Musch TI. Effects of sprint training on maximal stroke volume of rats with a chronic myocardial infarction. J Appl Physiol (1985) 72: 1437–1443, 1992. doi: 10.1152/jappl.1992.72.4.1437. [DOI] [PubMed] [Google Scholar]
- 341.Napoli C, Williams-Ignarro S, De Nigris F, Lerman LO, Rossi L, Guarino C, Mansueto G, Di Tuoro F, Pignalosa O, De Rosa G, Sica V, Ignarro LJ. Long-term combined beneficial effects of physical training and metabolic treatment on atherosclerosis in hypercholesterolemic mice. Proc Natl Acad Sci USA 101: 8797–8802, 2004. [Erratum in Proc Natl Acad Sci USA 101: 18262, 2004]. doi: 10.1073/pnas.0402734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Nara M, Kanda T, Tsukui S, Inukai T, Shimomura Y, Inoue S, Kobayashi I. Running exercise increases tumor necrosis factor-α secreting from mesenteric fat in insulin-resistant rats. Life Sci 65: 237–244, 1999. doi: 10.1016/S0024-3205(99)00242-8. [DOI] [PubMed] [Google Scholar]
- 342a.National Research Council (US) Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington, DC: National Academies Press, 2003. doi: 10.17226/10732. [DOI] [PubMed] [Google Scholar]
- 343.Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 726: 49–56, 1996. doi: 10.1016/0006-8993(96)00273-9. [DOI] [PubMed] [Google Scholar]
- 344.Niebauer J, Maxwell AJ, Lin PS, Tsao PS, Kosek J, Bernstein D, Cooke JP. Impaired aerobic capacity in hypercholesterolemic mice: partial reversal by exercise training. Am J Physiol Heart Circ Physiol 276: H1346–H1354, 1999. doi: 10.1152/ajpheart.1999.276.4.H1346. [DOI] [PubMed] [Google Scholar]
- 345.Norton KI, Delp MD, Duan C, Warren JA, Armstrong RB. Hemodynamic responses during exercise at and above VO2max in swine. J Appl Physiol (1985) 69: 1587–1593, 1990. doi: 10.1152/jappl.1990.69.5.1587. [DOI] [PubMed] [Google Scholar]
- 346.Noshiro T, Shimizu K, Way D, Miura Y, McGrath BP. Angiotensin II enhances norepinephrine spillover during sympathetic activation in conscious rabbits. Am J Physiol Physiol Heart Circ 266: H1864–H1871, 1994. doi: 10.1152/ajpheart.1994.266.5.H1864. [DOI] [PubMed] [Google Scholar]
- 347.Oguchi T, Kashimoto S, Yamaguchi T, Nakamura T, Kumazawa T. Is pentobarbital appropriate for basal anesthesia in the working rat heart model? J Pharmacol Toxicol Methods 29: 37–43, 1993. doi: 10.1016/1056-8719(93)90049-K. [DOI] [PubMed] [Google Scholar]
- 348.Oh-ishi S, Kizaki T, Toshinai K, Haga S, Fukuda K, Nagata N, Ohno H. Swimming training improves brown-adipose-tissue activity in young and old mice. Mech Ageing Dev 89: 67–78, 1996. doi: 10.1016/0047-6374(96)01727-7. [DOI] [PubMed] [Google Scholar]
- 349.O’Leary DS, Rossi NF, Churchill PC. Substantial cardiac parasympathetic activity exists during heavy dynamic exercise in dogs. Am J Physiol Heart Circ Physiol 273: H2135–H2140, 1997. doi: 10.1152/ajpheart.1997.273.5.H2135. [DOI] [PubMed] [Google Scholar]
- 350.Oltman CL, Parker JL, Adams HR, Laughlin MH. Effects of exercise training on vasomotor reactivity of porcine coronary arteries. Am J Physiol Heart Circ Physiol 263: H372–H382, 1992. doi: 10.1152/ajpheart.1992.263.2.H372. [DOI] [PubMed] [Google Scholar]
- 351.Otis CR, Wamhoff BR, Sturek M. Hyperglycemia-induced insulin resistance in diabetic dyslipidemic Yucatan swine. Comp Med 53: 53–64, 2003. [PubMed] [Google Scholar]
- 352.Overton JM, Tipton CM, Matthes RD, Leininger JR. Voluntary exercise and its effects on young SHR and stroke-prone hypertensive rats. J Appl Physiol (1985) 61: 318–324, 1986. doi: 10.1152/jappl.1986.61.1.318. [DOI] [PubMed] [Google Scholar]
- 353.Padilla DJ, McDonough P, Kindig CA, Erickson HH, Poole DC. Ventilatory dynamics and control of blood gases after maximal exercise in the Thoroughbred horse. J Appl Physiol (1985) 96: 2187–2193, 2004. doi: 10.1152/japplphysiol.00998.2003. [DOI] [PubMed] [Google Scholar]
- 354.Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hageman KS, Musch TI, Poole DC. Effects of Type II diabetes on capillary hemodynamics in skeletal muscle. Am J Physiol Heart Circ Physiol 291: H2439–H2444, 2006. doi: 10.1152/ajpheart.00290.2006. [DOI] [PubMed] [Google Scholar]
- 355.Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hageman KS, Musch TI, Poole DC. Effects of Type II diabetes on muscle microvascular oxygen pressures. Respir Physiol Neurobiol 156: 187–195, 2007. doi: 10.1016/j.resp.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 356.Pagala MK, Ravindran K, Namba T, Grob D. Skeletal muscle fatigue and physical endurance of young and old mice. Muscle Nerve 21: 1729–1739, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 357.Panepinto LM, Phillips RW, Norden S, Pryor PC, Cox R. A comfortable, minimum stress method of restraint for Yucatan miniature swine. Lab Anim Sci 33: 95–97, 1983. [PubMed] [Google Scholar]
- 358.Parks CM, Manohar M. Distribution of blood flow during moderate and strenuous exercise in ponies (Equus caballus). Am J Vet Res 44: 1861–1866, 1983. [PubMed] [Google Scholar]
- 359.Parsons AH, Smith SC, Kertzer R, Salkovitz IA. Endurance exercising miniature pigs: a review of methodology. In: Swine in Biomedical Research, edited by Tumbleson ME. New York: Plenum Press, 1986, p. 143–151. [Google Scholar]
- 360.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC, , et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 273: 402–407, 1995. doi: 10.1001/jama.1995.03520290054029. [DOI] [PubMed] [Google Scholar]
- 361.Petrosino JM, Heiss VJ, Maurya SK, Kalyanasundaram A, Periasamy M, LaFountain RA, Wilson JM, Simonetti OP, Ziouzenkova O. Graded maximal exercise testing to assess mouse cardio-metabolic phenotypes. PLoS One 11: e0148010, 2016. doi: 10.1371/journal.pone.0148010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Phillips RW, Panepinto LM, Spangler R, Westmoreland N. Yucatan miniature swine as a model for the study of human diabetes mellitus. Diabetes 31, Suppl 1: 30–36, 1982. doi: 10.2337/diab.31.1.S30. [DOI] [PubMed] [Google Scholar]
- 363.Phillips RW, Panepinto LM, Will DH. Genetic selection for diabetogenic traits in Yucatan miniature swine. Diabetes 28: 1102–1107, 1979. doi: 10.2337/diab.28.12.1102. [DOI] [PubMed] [Google Scholar]
- 364.Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corrà U, Dalla Libera L, Emdin M, Mele D, Passino C, Vescovo G, Vigorito C, Villani GQ, Agostoni P; Working Group ‘Exercise Physiology, Sport Cardiology and Cardiac Rehabilitation’, Italian Society of Cardiology . Exercise intolerance in chronic heart failure: mechanisms and therapies. Part I. Eur J Cardiovasc Prev Rehabil 17: 637–642, 2010. doi: 10.1097/HJR.0b013e3283361dc5. [DOI] [PubMed] [Google Scholar]
- 365.Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corrà U, Dalla Libera L, Emdin M, Mele D, Passino C, Vescovo G, Vigorito C, Villani G, Agostoni P; Working Group ‘Exercise Physiology, Sport Cardiology and Cardiac Rehabilitation’, Italian Society of Cardiology . Exercise intolerance in chronic heart failure: mechanisms and therapies. Part II. Eur J Cardiovasc Prev Rehabil 17: 643–648, 2010. doi: 10.1097/HJR.0b013e32833f3aa5. [DOI] [PubMed] [Google Scholar]
- 366.Podolin DA, Wei Y, Pagliassotti MJ. Effects of a high-fat diet and voluntary wheel running on gluconeogenesis and lipolysis in rats. J Appl Physiol (1985) 86: 1374–1380, 1999. doi: 10.1152/jappl.1999.86.4.1374. [DOI] [PubMed] [Google Scholar]
- 367.Poole DC, Burnley M, Vanhatalo A, Rossiter HB, Jones AM. Critical power: an important fatigue threshold in exercise physiology. Med Sci Sports Exerc 48: 2320–2334, 2016. doi: 10.1249/MSS.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Poole DC, Epp TS, Erickson HH. Exercise-induced pulmonary haemorrhage (EIPH): mechanistic bases and therapeutic interventions. Equine Vet J 39: 292–293, 2007. doi: 10.2746/042516407X204078. [DOI] [PubMed] [Google Scholar]
- 369.Poole DC, Erickson HH. Exercise-induced pulmonary hemorrhage: where are we now? Vet Med (Auckl) 7: 133–148, 2016. doi: 10.2147/VMRR.S120421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Poole DC, Erickson HH. Highly athletic terrestrial mammals: horses and dogs. Compr Physiol 1: 1–37, 2011. doi: 10.1002/cphy.c091001. [DOI] [PubMed] [Google Scholar]
- 371.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol 302: H1050–H1063, 2012. doi: 10.1152/ajpheart.00943.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Poole DC, Jones AM. Measurement of the maximum oxygen uptake V̇o2max: V̇o2peak is no longer acceptable. J Appl Physiol (1985) 122: 997–1002, 2017. doi: 10.1152/japplphysiol.01063.2016. [DOI] [PubMed] [Google Scholar]
- 373.Poole DC, Richardson RS. Determinants of oxygen uptake. Implications for exercise testing. Sports Med 24: 308–320, 1997. doi: 10.2165/00007256-199724050-00003. [DOI] [PubMed] [Google Scholar]
- 374.Poole DC, Richardson RS, Haykowsky MJ, Hirai DM, Musch TI. Exercise limitations in heart failure with reduced and preserved ejection fraction. J Appl Physiol (1985) 124: 208–224, 2018. doi: 10.1152/japplphysiol.00747.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Poole DC, Sexton WL, Behnke BJ, Ferguson CS, Hageman KS, Musch TI. Respiratory muscle blood flows during physiological and chemical hyperpnea in the rat. J Appl Physiol (1985) 88: 186–194, 2000. doi: 10.1152/jappl.2000.88.1.186. [DOI] [PubMed] [Google Scholar]
- 376.Poole DC, Ward SA, Gardner GW, Whipp BJ. Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics 31: 1265–1279, 1988. doi: 10.1080/00140138808966766. [DOI] [PubMed] [Google Scholar]
- 377.Poole DC, Ward SA, Whipp BJ. The effects of training on the metabolic and respiratory profile of high-intensity cycle ergometer exercise. Eur J Appl Physiol Occup Physiol 59: 421–429, 1990. doi: 10.1007/BF02388623. [DOI] [PubMed] [Google Scholar]
- 378.Poole DC, Wilkerson DP, Jones AM. Validity of criteria for establishing maximal O2 uptake during ramp exercise tests. Eur J Appl Physiol 102: 403–410, 2008. doi: 10.1007/s00421-007-0596-3. [DOI] [PubMed] [Google Scholar]
- 379.Poole S, Stephenson JD. Body temperature regulation and thermoneutrality in rats. Q J Exp Physiol Cogn Med Sci 62: 143–149, 1977. doi: 10.1113/expphysiol.1977.sp002384. [DOI] [PubMed] [Google Scholar]
- 380.Porcelli S, Grassi B, Poole DC, Marzorati M. Exercise intolerance in patients with mitochondrial myopathies: perfusive and diffusive limitations in the O2 pathway. Curr Opinion Physiol 10: 202–209, 2019. doi: 10.1016/j.cophys.2019.05.011. [DOI] [Google Scholar]
- 381.Powers SK, Smuder AJ, Kavazis AN, Quindry JC. Mechanisms of exercise-induced cardioprotection. Physiology (Bethesda) 29: 27–38, 2014. doi: 10.1152/physiol.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Powers SK, Sollanek KJ, Wiggs MP, Demirel HA, Smuder AJ. Exercise-induced improvements in myocardial antioxidant capacity: the antioxidant players and cardioprotection. Free Radic Res 48: 43–51, 2014. doi: 10.3109/10715762.2013.825371. [DOI] [PubMed] [Google Scholar]
- 383.Powers SK. Exercise: teaching myocytes new tricks. J Appl Physiol (1985) 123: 460–472, 2017. doi: 10.1152/japplphysiol.00418.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H Jr, Christ GJ, Edelmann W, Lisanti MP. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol 22: 2329–2344, 2002. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Reitman JS, Mahley RW. Changes induced in the lipoproteins of Yucatan miniature swine by cholesterol feeding. Biochim Biophys Acta 575: 446–457, 1979. doi: 10.1016/0005-2760(79)90114-0. [DOI] [PubMed] [Google Scholar]
- 386.Reitman JS, Mahley RW, Fry DL. Yucatan miniature swine as a model for diet-induced atherosclerosis. Atherosclerosis 43: 119–132, 1982. doi: 10.1016/0021-9150(82)90104-6. [DOI] [PubMed] [Google Scholar]
- 387.Ren JM, Barucci N, Marshall BA, Hansen P, Mueckler MM, Shulman GI. Transgenic mice overexpressing GLUT-1 protein in muscle exhibit increased muscle glycogenesis after exercise. Am J Physiol Endocrinol Metab 278: E588–E592, 2000. doi: 10.1152/ajpendo.2000.278.4.E588. [DOI] [PubMed] [Google Scholar]
- 388.Richter CP. On the phenomenon of sudden death in animals and man. Psychosom Med 19: 191–198, 1957. doi: 10.1097/00006842-195705000-00004. [DOI] [PubMed] [Google Scholar]
- 389.Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at VO2max. J Appl Physiol (1985) 73: 1067–1076, 1992. doi: 10.1152/jappl.1992.73.3.1067. [DOI] [PubMed] [Google Scholar]
- 390.Rodnick KJ, Reaven GM, Haskell WL, Sims CR, Mondon CE. Variations in running activity and enzymatic adaptations in voluntary running rats. J Appl Physiol (1985) 66: 1250–1257, 1989. doi: 10.1152/jappl.1989.66.3.1250. [DOI] [PubMed] [Google Scholar]
- 391.Rodnick KJ, Holloszy JO, Mondon CE, James DE. Effects of exercise training on insulin-regulatable glucose-transporter protein levels in rat skeletal muscle. Diabetes 39: 1425–1429, 1990. doi: 10.2337/diab.39.11.1425. [DOI] [PubMed] [Google Scholar]
- 392.Rodnick KJ, Reaven GM, Azhar S, Goodman MN, Mondon CE. Effects of insulin on carbohydrate and protein metabolism in voluntary running rats. Am J Physiol Endocrinol Metab 259: E706–E714, 1990. doi: 10.1152/ajpendo.1990.259.5.E706. [DOI] [PubMed] [Google Scholar]
- 393.Rodnick KJ, Henriksen EJ, James DE, Holloszy JO. Exercise training, glucose transporters, and glucose transport in rat skeletal muscles. Am J Physiol Cell Physiol 262: C9–C14, 1992. doi: 10.1152/ajpcell.1992.262.1.C9. [DOI] [PubMed] [Google Scholar]
- 394.Rodrigues LO, Oliveira A, Lima NR, Machado-Moreira CA. Heat storage rate and acute fatigue in rats. Braz J Med Biol Res 36: 131–135, 2003. doi: 10.1590/S0100-879X2003000100018. [DOI] [PubMed] [Google Scholar]
- 395.Rognmo Ø, Moholdt T, Bakken H, Hole T, Mølstad P, Myhr NE, Grimsmo J, Wisløff U. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 126: 1436–1440, 2012. doi: 10.1161/CIRCULATIONAHA.112.123117. [DOI] [PubMed] [Google Scholar]
- 396.Rubin SA, Mickle D. A simply constructed treadmill for rodent exercise studies. J Appl Physiol 52: 505–507, 1982. doi: 10.1152/jappl.1982.52.2.505. [DOI] [PubMed] [Google Scholar]
- 397.Rupp H. Differential effect of physical exercise routines on ventricular myosin and peripheral catecholamine stores in normotensive and spontaneously hypertensive rats. Circ Res 65: 370–377, 1989. doi: 10.1161/01.RES.65.2.370. [DOI] [PubMed] [Google Scholar]
- 398.Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res 120: 87–95, 2001. doi: 10.1016/S0166-4328(00)00364-8. [DOI] [PubMed] [Google Scholar]
- 399.Ryder JW, Kawano Y, Galuska D, Fahlman R, Wallberg-Henriksson H, Charron MJ, Zierath JR. Postexercise glucose uptake and glycogen synthesis in skeletal muscle from GLUT4-deficient mice. FASEB J 13: 2246–2256, 1999. doi: 10.1096/fasebj.13.15.2246. [DOI] [PubMed] [Google Scholar]
- 400.Sala-Mercado JA, Hammond RL, Kim JK, McDonald PJ, Stephenson LW, O’Leary DS. Heart failure attenuates muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 292: H2159–H2166, 2007. doi: 10.1152/ajpheart.01240.2006. [DOI] [PubMed] [Google Scholar]
- 401.Sala-Mercado JA, Spranger MD, Abu-Hamdah R, Kaur J, Coutsos M, Stayer D, Augustyniak RA, O’Leary DS. Attenuated muscle metaboreflex-induced increases in cardiac function in hypertension. Am J Physiol Heart Circ Physiol 305: H1548–H1554, 2013. doi: 10.1152/ajpheart.00478.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Salminen A, Vihko V, Pilström L. Effect of endurance training on the capacity of red and white skeletal muscle of mouse to oxidize carboxyl-14C-labelled palmitate. Acta Physiol Scand 101: 318–328, 1977. doi: 10.1111/j.1748-1716.1977.tb06013.x. [DOI] [PubMed] [Google Scholar]
- 403.Sanders M, White FC, Peterson TM, Bloor CM. Effects of endurance exercise on coronary collateral blood flow in miniature swine. Am J Physiol Heart Circ Physiol 234: H614–H619, 1978. doi: 10.1152/ajpheart.1978.234.5.H614. [DOI] [PubMed] [Google Scholar]
- 404.Schaible TF, Scheuer J. Effects of physical training by running or swimming on ventricular performance of rat hearts. J Appl Physiol 46: 854–860, 1979. doi: 10.1152/jappl.1979.46.4.854. [DOI] [PubMed] [Google Scholar]
- 405.Schaible TF, Scheuer J. Cardiac adaptations to chronic exercise. Prog Cardiovasc Dis 27: 297–324, 1985. doi: 10.1016/S0033-0620(85)80001-3. [DOI] [PubMed] [Google Scholar]
- 406.Schaper W, Schaper J (). Collateral Circulation: Heart, Brain, Kidney, Limbs. Boston, MA: Kluwer, 1993. [Google Scholar]
- 407.Schmederer Z, Rolim N, Bowen TS, Linke A, Wisløff U, Adams V; OptimEx Study Group . Endothelial function is disturbed in a hypertensive diabetic animal model of HFpEF: moderate continuous vs. high intensity interval training. Int J Cardiol 273: 147–154, 2018. doi: 10.1016/j.ijcard.2018.08.087. [DOI] [PubMed] [Google Scholar]
- 408.Schuler B, Arras M, Keller S, Rettich A, Lundby C, Vogel J, Gassmann M. Optimal hematocrit for maximal exercise performance in acute and chronic erythropoietin-treated mice. Proc Natl Acad Sci USA 107: 419–423, 2010. doi: 10.1073/pnas.0912924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Schwartz RS, Edelman ER, Carter A, Chronos N, Rogers C, Robinson KA, Waksman R, Weinberger J, Wilensky RL, Jensen DN, Zuckerman BD, Virmani R; Consensus Committee . Drug-eluting stents in preclinical studies: recommended evaluation from a consensus group. Circulation 106: 1867–1873, 2002. doi: 10.1161/01.CIR.0000033485.20594.6F. [DOI] [PubMed] [Google Scholar]
- 410.Seeherman HJ, Taylor CR, Maloiy GM, Armstrong RB. Design of the mammalian respiratory system. II. Measuring maximum aerobic capacity. Respir Physiol 44: 11–23, 1981. doi: 10.1016/0034-5687(81)90074-8. [DOI] [PubMed] [Google Scholar]
- 411.Sexton WL, Korthuis RJ, Laughlin MH. High-intensity exercise training increases vascular transport capacity of rat hindquarters. Am J Physiol Heart Circ Phyisol 254: H274–H278, 1988. doi: 10.1152/ajpheart.1988.254.2.H274. [DOI] [PubMed] [Google Scholar]
- 412.Sexton WL. Vascular adaptations in rat hindlimb skeletal muscle after voluntary running-wheel exercise. J Appl Physiol (1985) 79: 287–296, 1995. doi: 10.1152/jappl.1995.79.1.287. [DOI] [PubMed] [Google Scholar]
- 413.Shellock FG, Rubin SA. Temperature regulation during treadmill exercise in the rat. J Appl Physiol 57: 1872–1877, 1984. doi: 10.1152/jappl.1984.57.6.1872. [DOI] [PubMed] [Google Scholar]
- 414.Shepherd RE, Gollnick PD. Oxygen uptake of rats at different work intensities. Pflügers Arch 362: 219–222, 1976. doi: 10.1007/BF00581173. [DOI] [PubMed] [Google Scholar]
- 415.Shi M, Ellingsen Ø, Bathen TF, Høydal MA, Koch LG, Britton SL, Wisløff U, Stølen TO, Esmaeili M. Skeletal muscle metabolism in rats with low and high intrinsic aerobic capacity: Effect of aging and exercise training. PLoS One 13: e0208703, 2018. doi: 10.1371/journal.pone.0208703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Shukla SD, Paul A, Klachko DM. Hypersensitivity of diabetic human platelets to platelet activating factor. Thromb Res 66: 239–246, 1992. doi: 10.1016/0049-3848(92)90194-F. [DOI] [PubMed] [Google Scholar]
- 417.Sides RH, Kirkpatrick R, Renner E, Gough K, Katz LM, Evans DL, Bayly WM. Validation of masks for determination of V̇o2max in horses exercising at high intensity. Equine Vet J 50: 91–97, 2018. doi: 10.1111/evj.12711. [DOI] [PubMed] [Google Scholar]
- 418.Smith JC, Dangelmaier BS, Hill DW. Critical power is related to cycling time trial performance. Int J Sports Med 20: 374–378, 1999. doi: 10.1055/s-2007-971147. [DOI] [PubMed] [Google Scholar]
- 419.Smith JR, Ferguson SK, Hageman KS, Harms CA, Poole DC, Musch TI. Dietary nitrate supplementation opposes the elevated diaphragm blood flow in chronic heart failure during submaximal exercise. Respir Physiol Neurobiol 247: 140–145, 2018. doi: 10.1016/j.resp.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Smith JR, Hageman KS, Harms CA, Poole DC, Musch TI. Intercostal muscle blood flow is elevated in old rats during submaximal exercise. Respir Physiol Neurobiol 263: 26–30, 2019. doi: 10.1016/j.resp.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 421.Soares JM, Duarte JA. Effects of training and an anabolic steroid on murine red skeletal muscle. A stereological analysis. Acta Anat (Basel) 142: 183–187, 1991. doi: 10.1159/000147187. [DOI] [PubMed] [Google Scholar]
- 422.Solberg LC, Horton TH, Turek FW. Circadian rhythms and depression: effects of exercise in an animal model. Am J Physiol Regul Integr Comp Physiol 276: R152–R161, 1999. doi: 10.1152/ajpregu.1999.276.1.R152. [DOI] [PubMed] [Google Scholar]
- 423.Sonne B, Galbo H. Simultaneous determinations of metabolic and hormonal responses, heart rate, temperature and oxygen uptake in running rats. Acta Physiol Scand 109: 201–209, 1980. doi: 10.1111/j.1748-1716.1980.tb06587.x. [DOI] [PubMed] [Google Scholar]
- 424.Spranger MD, Kaur J, Sala-Mercado JA, Krishnan AC, Abu-Hamdah R, Alvarez A, Machado TM, Augustyniak RA, O’Leary DS. Exaggerated coronary vasoconstriction limits muscle metaboreflex-induced increases in ventricular performance in hypertension. Am J Physiol Heart Circ Physiol 312: H68–H79, 2017. doi: 10.1152/ajpheart.00417.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Stebbins CL, Symons JD. Role of angiotensin II in hemodynamic responses to dynamic exercise in miniswine. J Appl Physiol (1985) 78: 185–190, 1995. doi: 10.1152/jappl.1995.78.1.185. [DOI] [PubMed] [Google Scholar]
- 426.Stehno-Bittel L, Laughlin MH, Sturek M. Exercise training depletes sarcoplasmic reticulum calcium in coronary smooth muscle. J Appl Physiol (1985) 71: 1764–1773, 1991. doi: 10.1152/jappl.1991.71.5.1764. [DOI] [PubMed] [Google Scholar]
- 427.Stucchi AF, Terpstra AH, Foxall TL, Nicolosi RJ, Smith SC. The effect of exercise on plasma lipids and LDL subclass metabolism in miniature swine. Med Sci Sports Exerc 23: 552–561, 1991. doi: 10.1249/00005768-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 428.Sturek ML, Bedford TG, Tipton CM, Newcomer L. Acute cardiorespiratory responses of hypertensive rats to swimming and treadmill exercise. J Appl Physiol 57: 1328–1332, 1984. doi: 10.1152/jappl.1984.57.5.1328. [DOI] [PubMed] [Google Scholar]
- 429.Sturek M, Tune JD, Alloosh M. Ossabaw Island miniature swine: metabolic syndrome and cardiovascular assessment. In: Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques, edited by Swindle MM. Boca Raton, FL: CRC Press, 2015, p. 451–465. [Google Scholar]
- 430.Suvorava T, Lauer N, Kojda G. Physical inactivity causes endothelial dysfunction in healthy young mice. J Am Coll Cardiol 44: 1320–1327, 2004. doi: 10.1016/j.jacc.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 431.Swallow JG, Carter PA, Garland T Jr. Artificial selection for increased wheel-running behavior in house mice. Behav Genet 28: 227–237, 1998. doi: 10.1023/A:1021479331779. [DOI] [PubMed] [Google Scholar]
- 432.Swallow JG, Garland T Jr, Carter PA, Zhan WZ, Sieck GC. Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus). J Appl Physiol (1985) 84: 69–76, 1998. doi: 10.1152/jappl.1998.84.1.69. [DOI] [PubMed] [Google Scholar]
- 433.Takahashi T, Kai M, Hada T, Eto D, Muka K, Ishida N. Biomechanical implications of uphill training on the aetiology of tendinitis. Equine Vet J Suppl 34, S34: 353–358, 2002. doi: 10.1111/j.2042-3306.2002.tb05447.x. [DOI] [PubMed] [Google Scholar]
- 434.Tan MH, Bonen A. Effect of exercise training on insulin binding and glucose metabolism in mouse soleus muscle. Can J Physiol Pharmacol 65: 2231–2234, 1987. doi: 10.1139/y87-353. [DOI] [PubMed] [Google Scholar]
- 435.Tang T, Reed MJ. Exercise adds to metformin and acarbose efficacy in db/db mice. Metabolism 50: 1049–1053, 2001. doi: 10.1053/meta.2001.25596. [DOI] [PubMed] [Google Scholar]
- 436.Taniike M, Yamaguchi O, Tsujimoto I, Hikoso S, Takeda T, Nakai A, Omiya S, Mizote I, Nakano Y, Higuchi Y, Matsumura Y, Nishida K, Ichijo H, Hori M, Otsu K. Apoptosis signal-regulating kinase 1/p38 signaling pathway negatively regulates physiological hypertrophy. Circulation 117: 545–552, 2008. doi: 10.1161/CIRCULATIONAHA.107.710434. [DOI] [PubMed] [Google Scholar]
- 437.Teramoto K, Horiguchi S, Wakitani F, Tojyo F, Tokimoto T, Kuribara H. Effects of styrene on wheel-running and ambulatory activities in mice. J Toxicol Sci 13: 133–139, 1988. doi: 10.2131/jts.13.133. [DOI] [PubMed] [Google Scholar]
- 438.Tharp DL, Masseau I, Ivey J, Laughlin MH, Bowles DK. Endurance exercise training does not limit coronary atherosclerosis in familial hypercholesterolemic swine. Physiol Rep 7: e14008, 2019. doi: 10.14814/phy2.14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Thomas TR, Pellechia J, Rector RS, Sun GY, Sturek MS, Laughlin MH. Exercise training does not reduce hyperlipidemia in pigs fed a high-fat diet. Metabolism 51: 1587–1595, 2002. doi: 10.1053/meta.2002.36313. [DOI] [PubMed] [Google Scholar]
- 440.Tillerson JL, Caudle WM, Reverón ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience 119: 899–911, 2003. doi: 10.1016/S0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 441.Timson BF. Evaluation of animal models for the study of exercise-induced muscle enlargement. J Appl Physiol (1985) 69: 1935–1945, 1990. doi: 10.1152/jappl.1990.69.6.1935. [DOI] [PubMed] [Google Scholar]
- 442.Tipton CM. Determinants of VO2 max: insights gained from non-human species. Acta Physiol Scand Suppl 556: 33–43, 1986. [PubMed] [Google Scholar]
- 443.Touchard AG, Schwartz RS. Preclinical restenosis models: challenges and successes. Toxicol Pathol 34: 11–18, 2006. doi: 10.1080/01926230500499407. [DOI] [PubMed] [Google Scholar]
- 444.Toyota H, Dugovic C, Koehl M, Laposky AD, Weber C, Ngo K, Wu Y, Lee DH, Yanai K, Sakurai E, Watanabe T, Liu C, Chen J, Barbier AJ, Turek FW, Fung-Leung WP, Lovenberg TW. Behavioral characterization of mice lacking histamine H3 receptors. Mol Pharmacol 62: 389–397, 2002 [Erratum in Mol Pharmacol 62: 763, 2002]. doi: 10.1124/mol.62.2.389. [DOI] [PubMed] [Google Scholar]
- 445.Tsunoda N, Maruyama K, Cooke DW, Lane DM, Ezaki O. Localization of exercise- and denervation-responsive elements in the mouse GLUT4 gene. Biochem Biophys Res Commun 267: 744–751, 2000. doi: 10.1006/bbrc.1999.2031. [DOI] [PubMed] [Google Scholar]
- 446.Tsutsumi K, Hagi A, Inoue Y. The relationship between plasma high density lipoprotein cholesterol levels and cholesteryl ester transfer protein activity in six species of healthy experimental animals. Biol Pharm Bull 24: 579–581, 2001. doi: 10.1248/bpb.24.579. [DOI] [PubMed] [Google Scholar]
- 447.Tumbleson ME. (Editor). Swine in Biomedical Research. New York: Plenum Press, 1986. [Google Scholar]
- 448.Tune JD. Withdrawal of vasoconstrictor influences in local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol 291: H2044–H2046, 2006. doi: 10.1152/ajpheart.00653.2006. [DOI] [PubMed] [Google Scholar]
- 449.Tune JD, Yeh C, Setty S, Zong P, Downey HF. Coronary blood flow control is impaired at rest and during exercise in conscious diabetic dogs. Basic Res Cardiol 97: 248–257, 2002. doi: 10.1007/s003950200018. [DOI] [PubMed] [Google Scholar]
- 450.Vanderwolf CH. Effects of water temperature and core temperature on rat’s performance in a swim-to-platform test. Behav Brain Res 44: 105–106, 1991. doi: 10.1016/S0166-4328(05)80244-X. [DOI] [PubMed] [Google Scholar]
- 451.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266–270, 1999. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 452.Vatner SF, Higgins CB, White S, Patrick T, Franklin D. The peripheral vascular response to severe exercise in untethered dogs before and after complete heart block. J Clin Invest 50: 1950–1960, 1971. doi: 10.1172/JCI106687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Vatner SF, Higgins CB, Millard RW, Franklin D. Role of the spleen in the peripheral vascular response to severe exercise in untethered dogs. Cardiovasc Res 8: 276–282, 1974. doi: 10.1093/cvr/8.2.276. [DOI] [PubMed] [Google Scholar]
- 454.Wadley GD, Laker RC, McConell GK, Wlodek ME. Endurance training in early life results in long-term programming of heart mass in rats. Physiol Rep 4: e12720, 2016. doi: 10.14814/phy2.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Wagner PD, Gillespie JR, Landgren GL, Fedde MR, Jones BW, DeBowes RM, Pieschl RL, Erickson HH. Mechanism of exercise-induced hypoxemia in horses. J Appl Physiol (1985) 66: 1227–1233, 1989. doi: 10.1152/jappl.1989.66.3.1227. [DOI] [PubMed] [Google Scholar]
- 456.Walters TJ, Ryan KL, Tate LM, Mason PA. Exercise in the heat is limited by a critical internal temperature. J Appl Physiol (1985) 89: 799–806, 2000. doi: 10.1152/jappl.2000.89.2.799. [DOI] [PubMed] [Google Scholar]
- 457.Wasserman K, Hansen JE, Sue DY, Whipp BJ, Casaburi R. Principles of Exercise Testing and Interpretation. Philadelphia, PA: Lea & Febiger, 1994. [Google Scholar]
- 458.Wax TM. Runwheel activity patterns of mature-young and senescent mice: the effect of constant lighting conditions. J Gerontol 30: 22–27, 1975. doi: 10.1093/geronj/30.1.22. [DOI] [PubMed] [Google Scholar]
- 459.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation 65: 1213–1223, 1982. doi: 10.1161/01.CIR.65.6.1213. [DOI] [PubMed] [Google Scholar]
- 460.Weber KT, Janicki JS. Cardiopulmonary exercise testing for evaluation of chronic cardiac failure. Am J Cardiol 55: A22–A31, 1985. doi: 10.1016/0002-9149(85)90792-1. [DOI] [PubMed] [Google Scholar]
- 461.Weigand W, Hannappel E, Brand K. Effect of starvation and refeeding a high-protein or high-carbohydrate diet on lipid composition and glycogen content of rat livers in relation to age. J Nutr 110: 669–674, 1980. doi: 10.1093/jn/110.4.669. [DOI] [PubMed] [Google Scholar]
- 462.Weng S, Zemany L, Standley KN, Novack DV, La Regina M, Bernal-Mizrachi C, Coleman T, Semenkovich CF. β3 integrin deficiency promotes atherosclerosis and pulmonary inflammation in high-fat-fed, hyperlipidemic mice. Proc Natl Acad Sci USA 100: 6730–6735, 2003. doi: 10.1073/pnas.1137612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Werme M, Hermanson E, Carmine A, Buervenich S, Zetterström RH, Thorén P, Ogren SO, Olson L, Perlmann T, Brené S. Decreased ethanol preference and wheel running in Nurr1-deficient mice. Eur J Neurosci 17: 2418–2424, 2003. doi: 10.1046/j.1460-9568.2003.02666.x. [DOI] [PubMed] [Google Scholar]
- 464.Whipp BJ, Huntsman DJ, Storer TW. A constant which determines the duration of tolerance to high intensity work. Fed Proc 41: 1591, 1982. [Google Scholar]
- 465.Whipp BJ, Ward SA. Quantifying intervention-related improvements in exercise tolerance. Eur Respir J 33: 1254–1260, 2009. doi: 10.1183/09031936.00110108. [DOI] [PubMed] [Google Scholar]
- 466.White FC, Bloor CM. Coronary collateral circulation in the pig: correlation of collateral flow with coronary bed size. Basic Res Cardiol 76: 189–196, 1981. doi: 10.1007/BF01907957. [DOI] [PubMed] [Google Scholar]
- 467.White FC, Bloor CM. The pig as a model for myocardial ischemia. In: Swine in Biomedical Research, edited by Tumbleson ME. New York: Plenum Press, 1986, p. 481–490. [Google Scholar]
- 468.White FC, Bloor CM, McKirnan MD, Carroll SM. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol (1985) 85: 1160–1168, 1998. doi: 10.1152/jappl.1998.85.3.1160. [DOI] [PubMed] [Google Scholar]
- 469.White FC, Roth DM, Bloor CM. The pig as a model for myocardial ischemia and exercise. Lab Anim Sci 36: 351–356, 1986. [PubMed] [Google Scholar]
- 470.Wilkins BJ, Dai Y-S, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94: 110–118, 2004. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 471.Willius FA, Keys TE. Classics of Cardiology. New York: H. Schuman, 1961. [Google Scholar]
- 472.Wisløff U, Helgerud J, Kemi OJ, Ellingsen O. Intensity-controlled treadmill running in rats: V̇o2max and cardiac hypertrophy. Am J Physiol Heart Circ Physiol 280: H1301–H1310, 2001a. doi: 10.1152/ajpheart.2001.280.3.H1301. [DOI] [PubMed] [Google Scholar]
- 473.Wisløff U, Loennechen JP, Falck G, Beisvag V, Currie S, Smith G, Ellingsen O. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc Res 50: 495–508, 2001. doi: 10.1016/S0008-6363(01)00210-3. [DOI] [PubMed] [Google Scholar]
- 474.Wisløff U, Loennechen JP, Currie S, Smith GL, Ellingsen Ø. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res 54: 162–174, 2002. doi: 10.1016/S0008-6363(01)00565-X. [DOI] [PubMed] [Google Scholar]
- 475.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115: 3086–3094, 2007. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 476.Wisløff U, Ellingsen Ø, Kemi OJ. High-intensity interval training to maximize cardiac benefits of exercise training? Exerc Sport Sci Rev 37: 139–146, 2009. doi: 10.1097/JES.0b013e3181aa65fc. [DOI] [PubMed] [Google Scholar]
- 477.Witczak CA, Sturek M. Exercise prevents diabetes-induced impairment in superficial buffer barrier in porcine coronary smooth muscle. J Appl Physiol (1985) 96: 1069–1079, 2004. doi: 10.1152/japplphysiol.00460.2003. [DOI] [PubMed] [Google Scholar]
- 478.Witczak CA, Sturek M. Training-induced sarcoplasmic reticulum Ca2+ unloading occurs without Ca2+ influx. Med Sci Sports Exerc 37: 1119–1125, 2005. doi: 10.1249/01.mss.0000170125.25749.4d. [DOI] [PubMed] [Google Scholar]
- 479.Yang AL, Jen CJ, Chen HI. Effects of high-cholesterol diet and parallel exercise training on the vascular function of rabbit aortas: a time course study. J Appl Physiol (1985) 95: 1194–1200, 2003. doi: 10.1152/japplphysiol.00282.2003. [DOI] [PubMed] [Google Scholar]
- 480.Yano H, Yano L, Kinoshita S, Tsuji E. Effect of voluntary exercise on maximal oxygen uptake in young female Fischer 344 rats. Jpn J Physiol 47: 139–141, 1997. doi: 10.2170/jjphysiol.47.139. [DOI] [PubMed] [Google Scholar]
- 481.Yoshimura A, Shimomura Y, Murakami T, Ichikawa M, Nakai N, Fujitsuka C, Kanematsu M, Fujitsuka N. Glycogen depletion of the intrafusal fibers in a mouse muscle spindle during prolonged swimming. Am J Physiol Regul Integr Comp Physiol 271: R398–R408, 1996. doi: 10.1152/ajpregu.1996.271.2.R398. [DOI] [PubMed] [Google Scholar]
- 482.Zachwieja JJ, Hendry SL, Smith SR, Harris RB. Voluntary wheel running decreases adipose tissue mass and expression of leptin mRNA in Osborne-Mendel rats. Diabetes 46: 1159–1166, 1997. doi: 10.2337/diab.46.7.1159. [DOI] [PubMed] [Google Scholar]
- 483.Zha S, Wang F, Li Z, Ma Z, Yang L, Liu F. PJ34, a PARP1 inhibitor, promotes endothelial repair in a rabbit model of high fat diet-induced atherosclerosis. Cell Cycle 18: 2099–2109, 2019. doi: 10.1080/15384101.2019.1640008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Zhang XQ, Ng YC, Musch TI, Moore RL, Zelis R, Cheung JY. Sprint training attenuates myocyte hypertrophy and improves Ca2+ homeostasis in postinfarction myocytes. J Appl Physiol (1985) 84: 544–552, 1998. doi: 10.1152/jappl.1998.84.2.544. [DOI] [PubMed] [Google Scholar]
- 485.Zhang LQ, Zhang XQ, Ng YC, Rothblum LI, Musch TI, Moore RL, Cheung JY. Sprint training normalizes Ca2+ transients and SR function in postinfarction rat myocytes. J Appl Physiol (1985) 89: 38–46, 2000. doi: 10.1152/jappl.2000.89.1.38. [DOI] [PubMed] [Google Scholar]
- 486.Zhang LQ, Zhang XQ, Musch TI, Moore RL, Cheung JY. Sprint training restores normal contractility in postinfarction rat myocytes. J Appl Physiol (1985) 89: 1099–1105, 2000. doi: 10.1152/jappl.2000.89.3.1099. [DOI] [PubMed] [Google Scholar]



