Summary
Hematogenous metastasis is a multistep, selectin-regulated process whose mechanisms remain poorly understood. To investigate this biological pathway of cancer dissemination and better understand circulating cancer cells, we developed a high-throughput methodology that integrates organ-on-chip-like microfluidic and photoconvertible protein technologies. Our approach can ascribe single cell velocity as a traceable cell property for off-chip analysis of the direct relationships between cell-molecular profiles and adhesive phenotypes in the context of physiologically relevant fluid flow. We interrogated how natively expressed selectin ligands relate to colon cancer cell rolling frequencies and velocities, and provide context for previously reported disparities in in vitro and in vivo models of selectin-mediated adhesion and metastasis. This integrated methodology represents a versatile approach for the development of antimetastatic therapeutics as well as to generate and test mechanistic hypotheses regarding spatiotemporal processes that occur over timescales of seconds to hours with single-cell resolution.
eTOC Blurb
A photoconversion platform, capable of rapidly ascribing the velocity of thousands of single cells as a retainable characteristic for off chip analysis of multidimensional relationships between cell molecular profiles and adhesive phenotypes was developed, characterized, and implemented to study selectin mediated adhesion in the context of cancer metastasis.
Introduction
Metastasis, which is the leading cause of mortality among patients with colon cancer (Massague and Obenauf, 2016; Welch and Donaldson, 1979), is comprised of a highly complex sequence of time- and length-scale regulated steps (Edwards and Thomas, 2017; Lote et al., 2017) that ultimately enable cancer cells to disseminate through the circulation and eventually form secondary tumors (Figure 1A i). Hemodynamic force hence facilitates the transport of blood-borne metastatic cancer cells to distant organs (Figure 1A ii) but, in order to slow cells down for eventual arrest and extravasation, also stipulates that adhesion underpin the process of extravasation (Konstantopoulos and Thomas, 2009). Of the identified extravasation pathways, many are redundant with those of leukocyte homing (Strell and Entschladen, 2008), including interactions between metastatic cell presented selectin ligands (e.g. sialofucosylated CD44 (Hanley et al., 2006; Subramaniam et al., 2007) and carcinembryonic antigen (CEA) (Thomas et al., 2008), amongst others (Thomas et al., 2009)) with corresponding endothelial-, platelet-, or leukocyte- presented E-, P-, or L- selectin (Figure 1A iii, iv). Given the similarities of these mechanisms with physiologically important homeostatic processes, elucidating cancer cell-specific mechanisms of dissemination is thus crucial to the development of effective therapeutic interventions with highly selective anti-metastatic activity (Li and King, 2012; Oh et al., 2015) and also to the to the identification of biomarkers for the differentiation of patients with aggressive disease (Baumann et al., 2005; Lech et al., 2016).
Figure 1). In vitro interrogation of rolling adhesion mechanisms of selectin-mediated metastatic dissemination for the elucidation of cell adhesivity and ligand expression relationships.
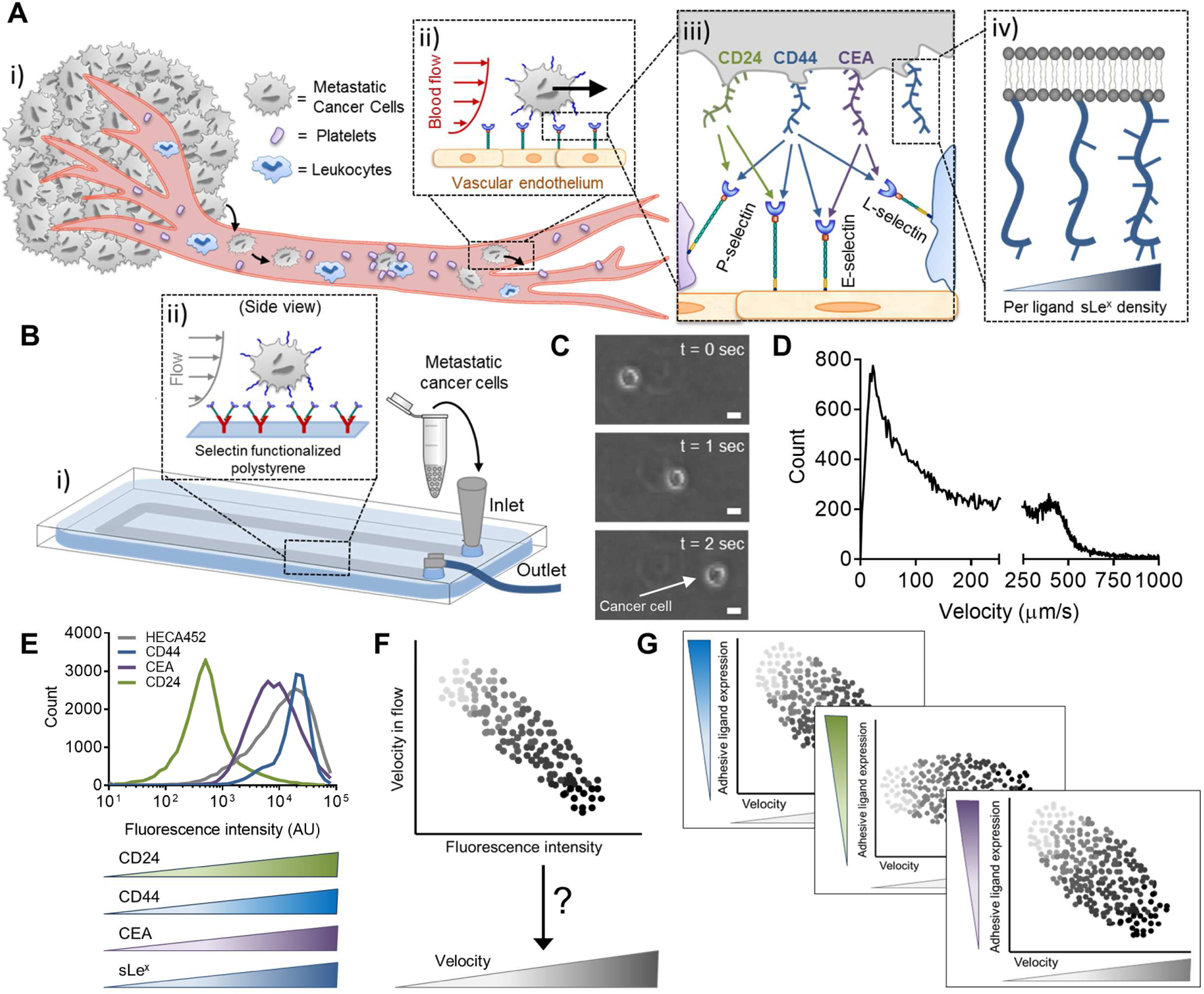
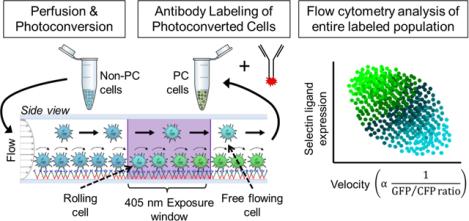
(A) Circulating tumor cells can escape from the vasculature amidst hemodynamic forces to establish secondary tumors through cell-cell interactions mediated by (ii) selectins presented on the vascular endothelium or circulating host cells and (iii) their circulating tumor cell-presented, (iv) sLex-decorated ligands. (B-D) Hemodynamic microenvironment-mimicking, engineered microfluidics (B), (i) wherein metastatic cancer cells are perfused through selectin-functionalized channels (ii), integrated with high speed videomicroscopy (C), enable the in vitro observation of diverse adhesive phenotypes (D). C,D: Representative images (C, scale bars, 10 μm) and manually tracked individual LS174T colon carcinoma cell velocities (D) when perfused over 25 μg/mL P-selectin at a wall shear stress of 0.5 dyn/cm2. (E-G) While adhesive ligands can be labeled in a manner proportional to their expression level and analyzed flow cytometrically (E), no existing techniques facilitate single-cell labeling (F) of the wide distribution of experimentally observed cell velocities (D) that would enable the comparison of selectin ligand expression with frequencies and velocities of rolling cell adhesion (G). F,G: hypothetical data.
Although this shear-flow enforced, selectin-mediated cell adhesion process can be recapitulated using functionalized microfluidic devices (Figure 1B), existing experimental techniques fall short in providing unbiased, high-resolution and high-dimensional insight into the cellular profiles that regulate cancer dissemination. For example, the receptors/ligands implicated in adhesion and consequently hematogenous dissemination of metastatic cells can be isolated, purified, and functionalized on microparticles (Hanley et al., 2006) (Figure 2) or non-endogenously expressed in cell lines, and force probe (Shea et al., 2015) or parallel plate flow chamber assays (Thomas et al., 2008) can be utilized to interrogate their ability to facilitate adhesion in the context of mechanical forces. However, since non-native presentation of receptors/ligands in these approaches may alter their posttranslational modification, other methods have sought to improve upon these techniques by sorting for or perturbing expression levels of endogenously expressed receptors/ligands via fluorescence activated cell sorting (FACS) of cells labeled for a particular receptor/ligand or genetic knockdown of protein ligand backbones (Hanley et al., 2006; Napier et al., 2007; Thomas et al., 2008) (Figure 2). However, these techniques can inadvertently regulate the glycosylation of alternative ligands (Thomas et al., 2008), confounding the interpretation of how adhesion receptors/ligands contribute to adhesive phenotypes in their unperturbed, native context. Moreover, these approaches are limited by technical challenges such as the requirement of non-function blocking antibodies for FACS-based sorting, a lack of persistence of sorted phenotypes in culture, and the infeasibility of generating a myriad of knockdown cell lines (Figure 2). There is therefore a need for methodologies that simultaneously enable the interrogation of cell migration (for example, as mediated by adhesion under the influence of hemodynamic force) in relation to cellular characteristics, including receptor/ligand expression in a native, unperturbed context.
Figure 2).
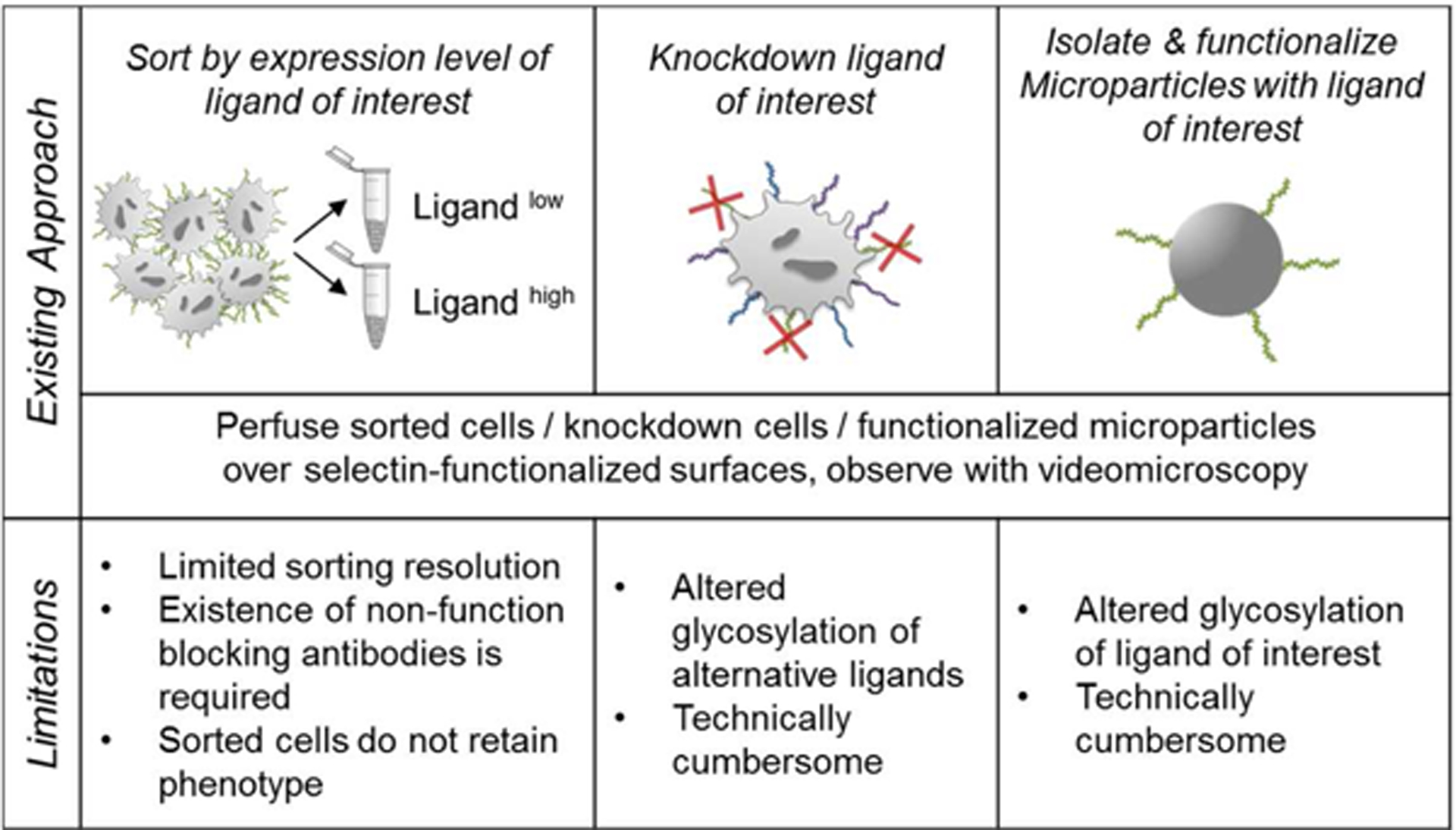
Existing approaches and limitations to assaying selectin ligand function in cancer metastasis under hemodynamic conditions.
Current methods for analyzing single-cell velocities are limited to interrogation via videomicroscopy-based tracking techniques (Figures 1C, 2). These techniques are not only time-consuming but also represent a measured property that cannot be retained by cells for simultaneous off-chip analysis with the molecular mediators that might underlie the heterogeneity of adhesive phenotypes exhibited by metastatic cells that interact with selectins in flow fields (Figure 1D) and are thought to underlie the process of colon cancer hematogenous metastasis (Edwards et al., 2017; Oh et al., 2015; Reyes-Reyes et al., 2006). Fluorescently tagged antibodies offer a mechanism for labeling cells based on expression of any chosen marker, for example various adhesive ligands (Figure 1E), but no such technique exists to “label” cells in proportion to their velocity (Figure 1F). Therefore, there exists a need for a tool to label cells in a manner proportional to their rolling velocity, such that relationships, direct or otherwise, between cellular expression profiles (including but not limited to selectin ligands) and rolling adhesion behavior of metastatic cells can be assayed (Figure 1G) to better understand the molecular mediators that underlie cell adhesion and consequently hematogenous dissemination of metastatic cancer cells.
In order to overcome these numerous challenges, we developed a methodology that integrates microfluidic (Oh et al., 2015) and photoconvertible protein technologies (Matsuda et al., 2008) to fluorescently ascribe single cell velocity as a retainable property of individual cells for off-chip analysis. When used in conjunction with fluorescently tagged antibodies against markers of interest, we demonstrate the ability to simultaneously analyze cell rolling adhesion behavior and molecular characteristics on a single cell level via flow cytometry in an observational rather than interventional approach. This technique leverages the profound transcriptional variability at the single cell level of genetically identical human cancers (Cohen et al., 2008; O’Connell et al., 2013; Shaffer et al., 2017) to reveal multidimensional relationships between expression of adhesive ligands with adhesion phenotype. In particular, since selectins are the molecular brakes that enable slower kinetic mechanisms of cell arrest and chemotaxis along and across the endothelium to occur in the context of hemodynamic flow (Konstantopoulos and Thomas, 2009) and have been implicated in facilitating metastasis in vivo (Borsig et al., 2002; Khatib et al., 2002; Kim et al., 1998), the molecular profiles that underlie rolling adhesion frequencies and velocities on selectins were interrogated herein using this method. The high dimensionality supported by simultaneous fluorescent labeling enabled for the elucidation of relationships between cancer stem cell markers and selectin ligands, providing context for previously reported disparities in in vitro and in vivo models of selectin-mediated adhesion and metastasis, respectively (Dallas et al., 2012). Overall, this high-throughput methodology, which enables the observation of cell properties over continuous rather than discrete scales, facilitates high-resolution analysis of the molecular underpinnings of heterogeneous cell pathophysiological behavior in the context of spatiotemporal processes occurring over timescales of seconds to hours.
Results
Cellular Photoconversion Extent Controlled by 405 nm Light Exposure Time and Power
Activation of the photoconvertible fluorescent protein Phamret protein with a 405 nm light source shifts the excitation maximum of the photoactivatable (PA)-green fluorescent protein (GFP) portion of the fusion protein from 397 nm to 495 nm (Figure 3A) such that when stably transfected in cells, local stimulation with a 405 nm light source can elicit local increases GFP signal (Figure 3B). At the whole cell level, increasing the duration of 405 nm exposure is expected to increase the relative proportion of PA-GFP in its activated versus inactive state (Figure 3A) (Patterson and Lippincott-Schwartz, 2002, 2004). When larger populations of cells are exposed to 405 nm light however, variations in per-cell Phamret expression level necessitate the use of cyan fluorescent protein (CFP) signal from the fusion protein for normalizing measured GFP signal. In this way, the extent of photoconversion can be measured by the ratio of GFP to CFP signal, which increases with increasing duration of cell exposure to 405 nm light (Figure 3C). Moreover, this direct relationship between exposure time and the extent of photoconversion was also observed for cells in suspension and could be measured on a single cell basis using flow cytometry (Figure 3D). Increasing exposure times resulted in a flow cytometry-measured increase in GFP signal, while the mean CFP intensity remained unchanged (Figure 3D), resulting in concomitant increases in mean GFP/CFP ratios as well as fold change in GFP/CFP ratio relative to unphotoconvereted cells (Figure 3E–F). This manner of photoconversion was also tunable by laser power (Figure 3G–I), as evidenced by the effect of laser power setting on the “half-max” (indicated in Figure 3I as EC50), or the exposure time required to achieve half-maximal extents of photoconversion. Phamret expression level, which differs by clone of analyzed LS174T Phamret cells and is determined by CFP expression (indicated in Figure 3J as “CFP Intensity”) altered the rate of photoconversion, as evidenced by the maximum slope of photoconversion (GFP/CFP ratio)-exposure time curves (Figure 3J, Figure S1C), without affecting rolling adhesion behaviors (Figure S1A–B). These adjustable parameters demonstrate the ability to tune the photoconversion response of Phamret transfected cells for a desired kinetic process. Treatment of Phamret-expressing cells with 405 nm light also did not result in any significant change in the extent of total cell death or apoptosis, measured by frequencies of cells positive for SytoxRed or Annexin V, respectively, each of which were induced by treatment with positive controls tert-Butyl hydroperoxide and hydrogen peroxide (Figure 3K). Similarly, photoconversion did not alter reactive oxygen species production (measured by CellRox Orange) relative to untreated cells, both of which were significantly less than those of tert-Butyl hydroperoxide-treated positive controls (Figure 3L). Finally, in characterizing the duration of photoconversion, we found that following 2 min of static exposure to 405 nm light, the GFP/CFP ratio can be sustained at least over several hours and returns to baseline by 48 h after photoconversion (Figure 3M). All together, these experimental data and analyses characterizing this methodology (Table S1, Figure S2 top) suggest that the noninstantaneous photoconversion of Phamret-expressing LS174T cells renders this system an effective means of measuring the extent of cell exposure to a controllable area and intensity of 405 nm light.
Figure 3). Photoconversion of Phamret-expressing LS174T colon carcinoma cells is spatiotemporally controlled.
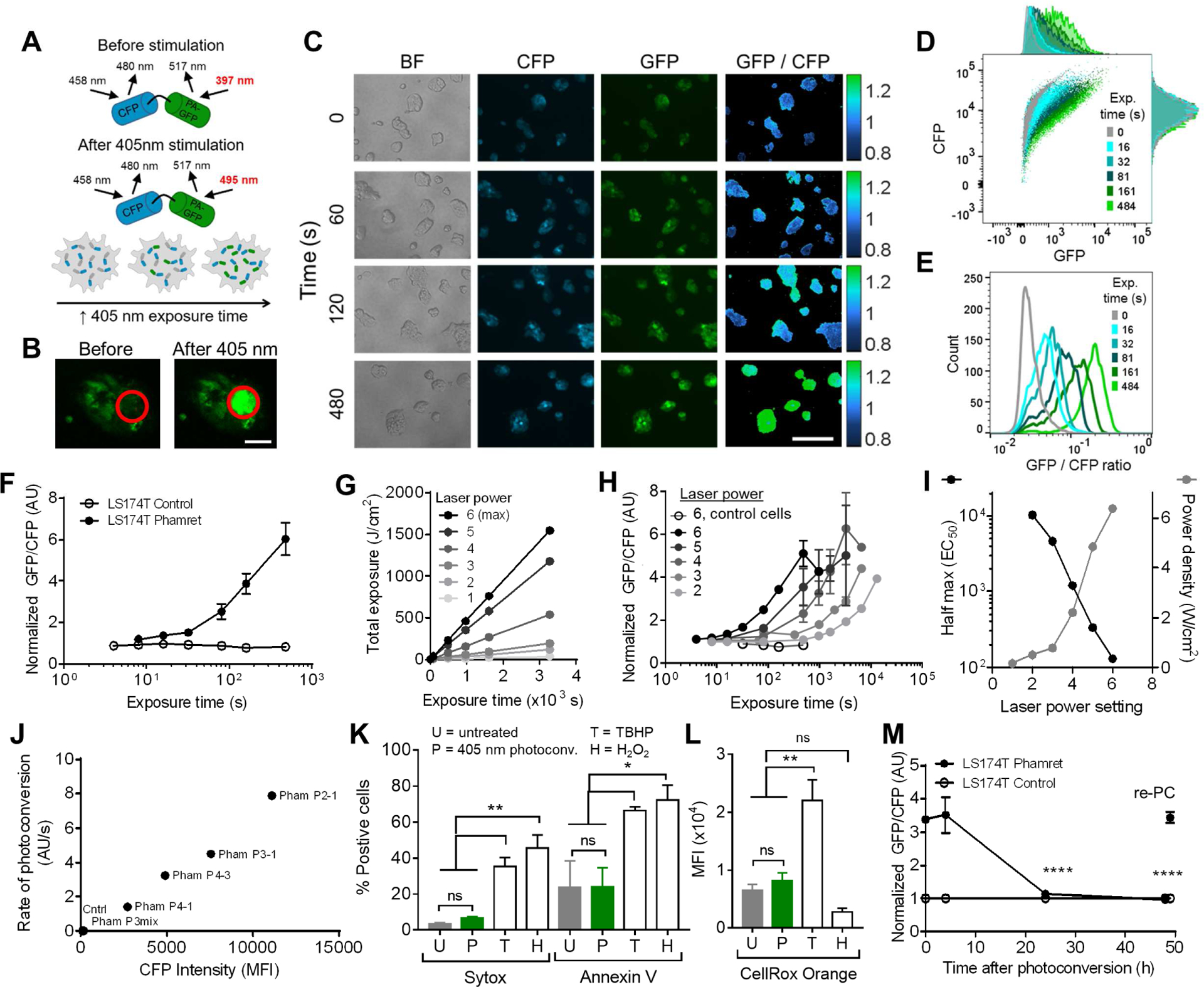
(A) After stimulation with a 405 nm laser, the maximum excitation of photoactivatable (PA)-GFP within Phamret shifts from 397 nm to 495 nm. (b) 405 nm stimulation (red circle) results in locally increased GFP signal by Phamret-expressing LS174T colon carcinoma cells (Phamret LS174T). Scale bar, 50 μm. (C) Fluorescent images of Phamret LS174T cell CFP and GFP levels and GFP/CFP ratios (colorbar) with increasing 405 nm exposure time. Scale bar, 200 μm. (D-E) Flow cytometrically measured Phamret LS174T cell CFP and GFP levels (D) and per cell GFP/CFP ratios (E) after various times of 405 nm exposure. (F) Mean per cell parental (control) and Phamret-expressing (LS174T Phamret) GFP/CFP ratios calculated from flow cytometrically measured per cell CFP and GFP levels with varying with exposure time to a fixed power density (6400 mW/cm2) of 405 nm laser. Data represent mean ± SEM of 3 independent experiments, one-way ANOVA with t-tests with Bonferonni corrections for multiple comparisons, * indicates a multiplicity adjusted p-value <0.05. (G) Laser power and exposure time effects on total 405 nm light exposure. (H) Relationships between 405 nm light exposure time and the GFP/CFP ratios calculated from flow cytometrically measured per cell CFP and GFP levels of Phamret-expressing (closed symbols) or parental control (open symbols) LS174T cells exposed to different powers of 405 nm light. Data represent mean ± SEM of GFP/CFP ratios from n ≥ 3 independently run experiments. (I) Exposure to 405 nm laser at increasing power, which increases the power density of laser exposure (right axis), influences exposure time of half maximum Phamret LS174T cell photoconversion (EC50 obtained from a fit of the data in to a four parameter, variable slope dose-response model, left axis) as determined by the flow cytometrically measured mean per cell GFP/CFP ratio. (J) The rate of Phamret-LS174T photoconversion (calculated from the maximum slope of the mean per cell GFP/CFP ratio versus 405 nm laser exposure time measured in D using a four parameter, variable slope dose-response model) varies with Phamret LS174T cell clone baseline (untreated) Phamret expression level (as determined by CFP expression, “CFP Intensity”). Phamret LS174T clones: Pham P3mix, Pham P4–1, Pham P4–3, Pham P3–1, Pham P2–1. Cntrl, parental LS174T cells. (K-L) 405 nm exposure effects on the frequency of Sytox positive and Annexin V positive cells (K) and the intensity of CellRox Orange staining (L). U, untreated cells; P, 405 nm photoconverted cells; T and H, tert-Butyl hydroperoxide (TBHP)- and hydrogen peroxide (H2O2)-treated cells, respectively. Data represent mean ± SEM of 3 independent experiments, one-way ANOVA with t-tests with Bonferonni corrections for multiple comparisons, * indicates a multiplicity adjusted p-value <0.05. (M) Ratio of flow cytometrically measured GFP and CFP levels is sustained over hours but reversible and repeatable (re-PC). One way ANOVA, * indicates multiplicity-adjusted p<0.05 for post hoc t-tests with Bonferroni correction for 4 comparisons to the 0 h timepoint. See also Figures S1–S2, Table S1.
Photoconversion Residence Time/Velocity Probe Differentiates LS174T Colon Carcinoma Cell Subpopulations with Discrete Selectin Adhesivities and Metastatic Potentials
We sought to exploit the exposure-dependence of photoconversion to measure a rate-based cellular process, specifically applying this method to fluorescently “tag” cells in proportion to the length of time they spent over prescribed lengths interacting with selectin-functionalized surfaces in hemodynamic flow. Human LS174T colon carcinoma cells, which have been used extensively for the interrogation of in vitro selectin binding characteristics of metastatic cells and employed in preclinical tumor models for the investigation of hematogenous metastasis (Hamada et al., 2008; Hanley et al., 2006; Stein and Berger, 1999; Thomas et al., 2009; Thomas et al., 2008), were used. In order to recapitulate salient features of the microvascular microenvironment, we fabricated microfluidic devices (Figure 4A) surface-functionalized with adhesive recombinant selectin protein chimeras over which cell adhesion could be visualized using high speed videomicroscopy. These microfluidic devices also featured a blocked settling region upstream of the selectin-functionalized region (Oh et al., 2015) in which an imaging field of view and 405 nm exposure window are situated (Figure 4B) to allow all cells an equal opportunity to engage in rolling adhesive contact prior to the imaging and photoconversion locations. We hypothesized that this integrative setup would enable LS174T cells to be photoconverted in a manner proportional to the time required to transit the 405 nm exposure window and thus the extent of photoconversion could be used as a proxy for their velocity and to distinguish rolling (i.e. cells mediating selectin adhesion) versus free-flowing cells (Figure 4C). Since wall shear stress is nearly uniform across the bottom width of a parallel plate flow chamber (Yiling et al., 2014), the velocities and frequencies of cells either in free flow or mediating rolling adhesion do not vary across the width of the channel (Figure S1H–I), ensuring equal opportunity for 405 nm exposure regardless of cell position within the channel width. To determine the feasibility of this photoconversion-based approach in estimating single cell velocities, we examined the relationship between manually measured velocities and GFP intensities pre- and post-photoconversion for cells perfused over P-selectin-functionalized surfaces and found an inverse relationship between these measures after photoconversion (Figure 4D, Figure S2 middle, Table S2). Although the variance of photoconversion-based and manual measurements of velocity differed, the mean calculated velocity based on photoconversion was similar to that of manually measured velocities from video image analysis (Figure 4E).
Figure 4). Photoconversion of cells perfused over P-selectin measures rolling velocity and distinguishes slow-rolling, highly metastatic cells.
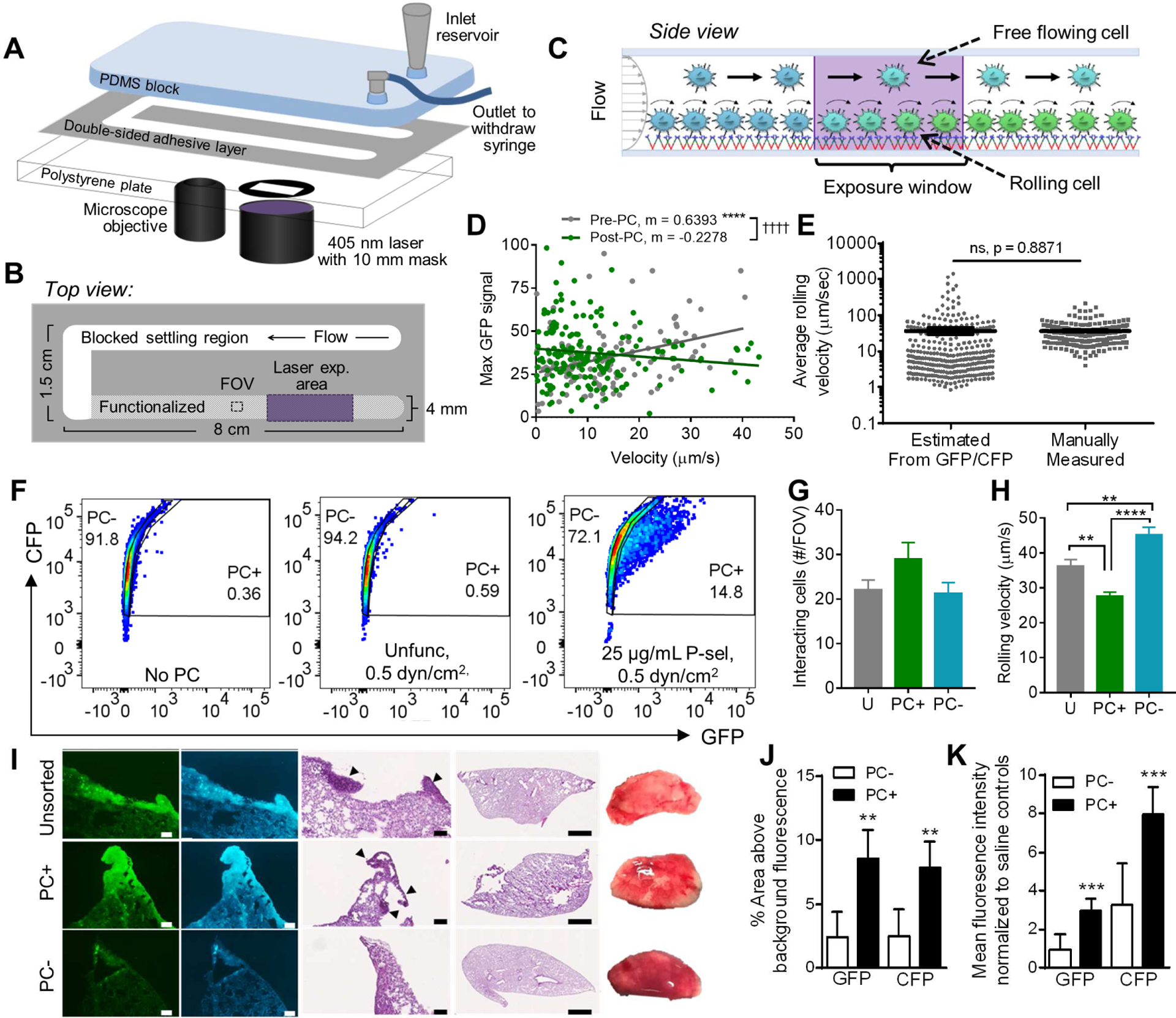
(A-C) Fluidic system schematic (A) and top (B) and side (C) views for perfusion through the 405 nm illumination window to photoconvert cells in proportion to their velocities in flow, either rolling or free flow, on selectin-functionalized substrates. (D) LS174T Phamret cellular GFP levels measured using fluorescence video microscopy versus rolling velocities measured during perfusion prior to photoconversion (Pre-PC) or reperfused (Post-PC) over 25 μg/mL P- selectin at 0.5 dyn/cm2. Points represent individual cells; linear regression, * indicates a significantly non-zero slope, † indicates significant difference between slopes. (E) Individual cellular rolling velocities over 25 μg/mL P- selectin at 0.5 dyn/cm2 estimated from flow cytometrically measured GFP/CFP ratios (using exposure time versus GFP/CFP calibration curve generated under static conditions) or manually measured. Mean ± SEM, p-value from adjusted (Welch’s) t-test for comparison between measurement techniques. (F) Example flow cytometry plots and gating of photoconverted negative (PC−) and positive (PC+) gates established from non-photoconverted Phamret-expressing LS174T cells (No PC) or cells perfused through unfunctionlaized (Unfunc) or P-selectin functionalized channels (25 ug/ml P-sel) at 0.5 dyn/cm2. (G-H) FACS sorted and reperfused PC+, PC−, and unsorted LS174T Phamret cell population rolling adhesion frequencies (G) and velocities (H). Mean ± SEM of 3 FOVs from independently run experiments (G) and mean ± SEM of ≥200 cells from 3 FOVs in independently run experiments (H). (I) Representative fluorescent and chromogenic images of NSG mouse lung sections and whole lobes 28 d post i.v. infusion of 105 unsorted (Unsorted) or sorted PC+ or PC− LS174T Phamret cells. Scale bars, 100 μm or 1 mm in regions of interest and whole lobe images, respectively. (J) Percent area of lung tissue sections at high (above tissue background measured in lung tissues from control mice) CFP and GFP fluorescence, indicating the presence of LS174T Phamret cells. (K) Mean CFP and GFP fluorescence intensities of lung tissue sections. (J-K) T-test, Ho: μ= 0 ; * indicates significantly non-zero mean. All p-values are multiplicity adjusted, α=0.05. See also Figures S1–3, Tables S2–S3.
To determine whether this experimental setup imparted any phenotypic changes to LS174T cells, we performed two control experiments. We compared the adhesive behavior of non-transfected LS174T cells with cells exhibiting a range of Phamret expression that had been perfused over P-selectin functionalized substrates at various physiologically relevant wall shear stresses and found no difference in the frequencies nor velocities of rolling cells (Figure S1A–B). Moreover, perfusion of cells through functionalized microfluidic channels did not alter selectin ligand expression profiles, as evidenced by both the lack of deposition of selectin ligands on the microfluidic plate surface (Figure S1D–E) and the maintenance of flow-cytometrically measured ligand expression on LS174T cells before and after perfusion over functionalized substrates (Figure S1F). Also, the mean cell rolling velocity was unchanged between initially perfused cells and immediately reperfused photoconverted cells (Figure S1G). Taken together, these control experiments demonstrate that our photoconversion platform does not affect adhesive ligand expression profiles or rolling adhesive phenotypes of perfused cells.
To assess the functional significance of LS174T cell photoconversion in vitro in an inflamed microvascular-mimicking microfluidic system, we assayed the rolling adhesion behavior and metastatic potential of cells sorted by their extent of photoconversion on P-selectin in shear flow (Figure S2 middle). Approximately 15% of cells were categorized as photoconverted positive (PC+), with the remaining in the photoconverted negative (PC−) population (gates defined based on the non-photoconverted cell population) when perfused over P-selectin functionalized surfaces at 0.5 dyn/cm2 under exposure to 405 nm light, restricted to a length of 10 mm in the direction of flow (Figure 4F). These frequencies were altered by both adjustments to the laser power, flow rate (e.g. wall shear stress), as well as selectin type (P-, E-, versus L-), the latter mediating rolling adhesion at velocities that vary by orders of magnitude (Edwards et al., 2017; Napier et al., 2007; Oh et al., 2015; Thomas et al., 2008) (Table S3), indicating the sensitivity of this methodology to the kinetic process studied. A PC+ cell population isolated by perfusion through a P-selectin functionalized microchannel at 0.5 dyn/cm2 under exposure to 405 nm light and sorting via FACS exhibited enhanced extents of rolling adhesion upon reperfusion under identical conditions (P-selectin functionalized substrate, 0.5 dyn/cm2 wall shear stress) compared to both PC− and unsorted cell populations (though not to a statistically significant extent as this comparison lacked power at n=6) (Figure 4G). These differences, which reflect an increased propensity of PC+ cells to mediate adhesion to P-selectin in shear flow, were also mirrored by lower velocities of rolling adhesion on P-selectin by PC+ cells compared to PC− and unsorted cell populations (Figure 4H). This highly photoconverted population (PC+) that exhibited an elevated propensity to mediate adhesion to P-selectin in flow in vitro also resulted in greater metastatic tumor burdens in the lung in vivo 28 d post intravenous infusion of 105 LS174T Phamret cells into NSG mice relative to the PC− population. This was quantified within lung sections by assessing both the total fluorescence (Figure 4I,K, Figure S3A–C) and percent area with fluorescence above threshold (Figure 4I,J, Figure S3A) of each GFP and CFP signals (the former of which increases with photoconversion but returns to baseline within 48 h of initial photoconversion (Figure 3M) and the latter of which is unaffected by LS174T Phamret cell photoconversion (Figure 3D)). Moreover, injection of both PC+ and unsorted populations resulted in a higher cell density within lung cryosections (Figure 4I, Figure S3D). In control experiments, no significant difference observed in doubling time of PC+ versus PC− populations was observed (Figure S3E), nor did sorting alter ligand expression (Figure S3F–G)..
Complex Dependencies of Single Cell Velocities of Selectin Adhesion on Ligand Expression Revealed by Photoconversion Residence Time/Velocity Probe
Given that colon carcinoma cells express a variety of selectin ligands with redundant, interdependent, and/or overlapping functions (Konstantopoulos and Thomas, 2009), we implemented the photoconversion platform to explore how these ligands singly or synergistically contribute to cell adhesive interactions with selectins in the context of fluid flow. We perfused LS174T cells over selectin-functionalized surfaces under exposure to 405 nm light and labeled the collected, photoconverted cells with fluorophore-conjugated antibodies specific for different adhesive ligands to quantify the relationships between rolling velocities and the extent of adhesion relative to adhesive ligand expression (Figure 5A, Figure S2 bottom, Table S4). Specifically, relationships between mean cellular GFP/CFP ratio (as a proxy for cell rolling velocity) and cell adhesion molecule expression were assessed within PC+ versus PC− LS174T Phamret cell subpopulations subdivided into bins evenly distributed with respect to the frequency of the parent population (Figure S4A), so as to determine these relationships for cells mediating rolling adhesion on selectin-functionalized surfaces versus those in free flow (Figure S2 bottom). In this analysis, the slope of the relationship between adhesive molecule expression and GFP/CFP ratio quantitatively reflects the relative impact that adhesive molecule expression has on the velocity of cell rolling adhesion. Adhesive molecules whose expression is associated with a significant impact on rolling velocities are revealed by slopes for PC+ subpopulations that are significantly greater than the slopes of the same relationship for PC− subpopulations (p-values reported for this comparison, Table S4).
Figure 5). Photoconversion reveals distinct relationships between single cell velocities and ligand expression levels for rolling adhesion on P-, E-, and L-selectin.
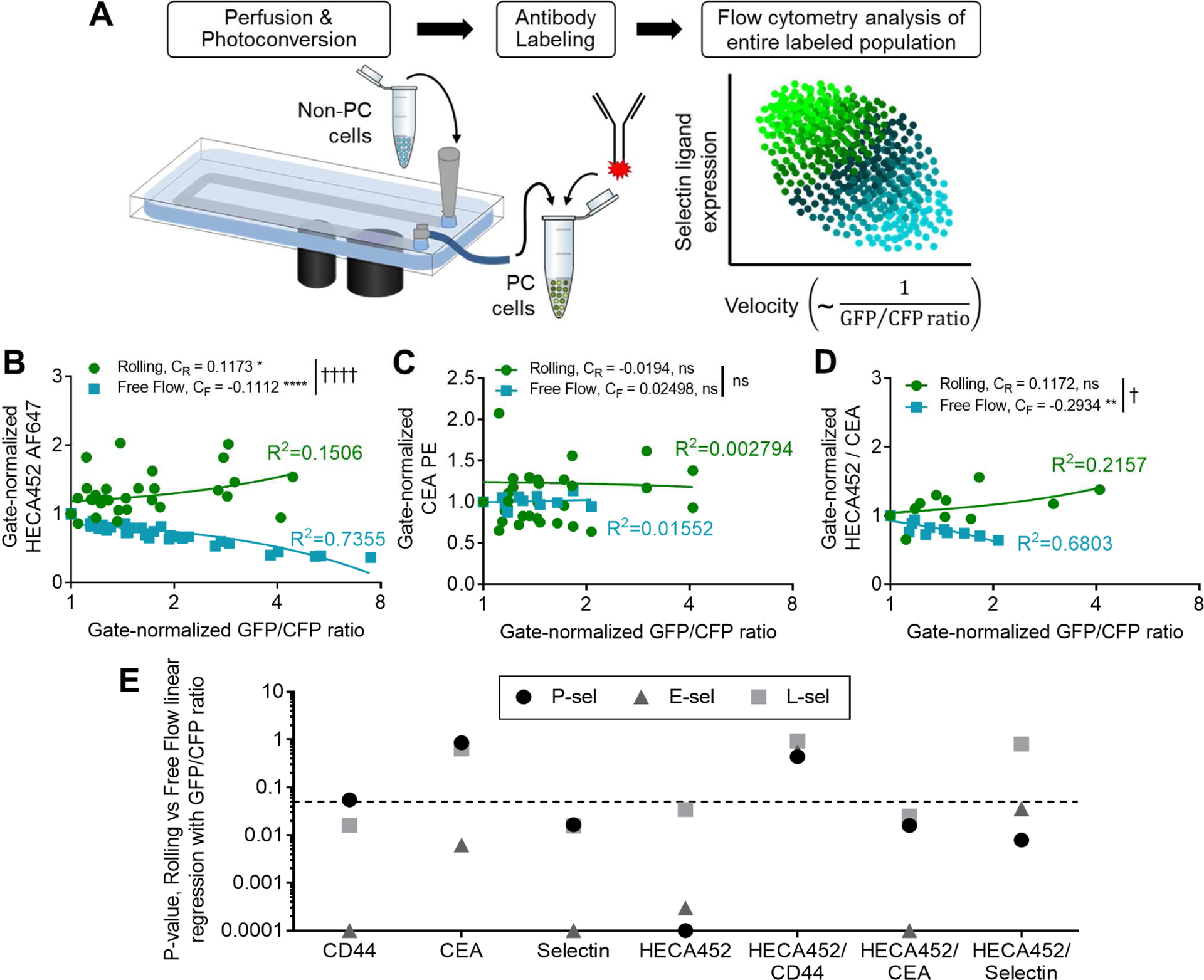
(A) Phamret-expressing LS174T (Phamret LS174T) cells perfused over selectin-functionalized substrates under exposure to 405 nm light can be collected and stained with fluorescently tagged antibodies, allowing within-population trends to be visualized. (B-D) The extent of photoconversion (GFP/CFP ratio) of rolling and free-flowing Phamret LS174T cells perfused over P-selectin at 0.5 dyn/cm2 related to the expression of sLex reactive HECA-452 (B), CEA (C), and the sLex (HECA452)/CEA density (D). Binned, flow cytometry gate-normalized data, pooled from independent experiments and plotted with corresponding linear fits; CR and CF represent the slope of the regression for the rolling and free flowing populations, respectively, * represents non-zero slopes of the linear fit within each population, † represents significance of comparison between CR and CF. (E) P-values for comparisons between the slopes of the linear relationships of interrogated parameters for rolling (CR, PC+) versus free flowing (CF, PC−) populations summarized for all comparisons; dashed line represents p=0.05. See also Figures S4–5, Table S4.
Since the sialofucosylated tetrasaccharides sialyl Lewis x (sLex) and a (sLea) are recognized as the minimal binding structures required to facilitate selectin-mediated adhesion of LS174T cells (St. Hill et al., 2011; Trinchera et al., 2017; Vestweber and Blanks, 1999), we began our interrogation by assessing the relationship between immunoreactivity by HECA452 mAb (which recognizes human sLex and sLea) and the extent of photoconversion (as a proxy for rolling adhesion velocity in flow) of sLex- (but not sLea-) expressing human LS174T cells (Burdick et al., 2003) perfused over P-, E-, and L-selectin. Although control experiments run in the absence of imposed flow revealed no effect of photoconversion on the degree of selectin/ligand staining (Figure S4B–E), sLex expression was proportional to the GFP/CFP ratio of cells perfused and photoconverted on P-, E-, and L-selectin in flow (Figure 5B,E; Figure S5A–C,G). With regard to other known LS174T-expressed selectin ligands and consistent with their previously demonstrated functional roles (Hanley et al., 2006; Napier et al., 2007; Thomas et al., 2008), CD44 expression and the extent of in-solution selectin ligand binding similarly exhibited a direct relationship with the GFP/CFP ratio of cells perfused over P-, E-, and L-selectin (Figure 5E; Figure S5D,G). The relationship between CEA and the extent of photoconversion, on the other hand, only differed between PC+ and PC− populations of cells perfused over E- but not Pselectin (Figure 5C,E; Figure S5E,G), also consistent with LS174T cell expressed CEA’s previously reported E- but not P-selectin ligand activity (Thomas et al., 2008). No relationship between CEA expression and LS174T Phamret cell photoconversion resulting from adhesion on L-selectin in flow was found (Figure 5E), conflicting with the previously demonstrated capacity of LS174T-expressed CEA to mediate L-selectin adhesion in flow (Thomas et al., 2008). Higher expression of sLex relative to CEA, however, did confer slower rolling velocities on all the selectins, including L-selectin, with most pronounced effects on E-selectin (Figure 5D,E; S5H–K). Contrastingly, higher expression of sLex relative to CD44 obfuscated the relationship of CD44 with extent of photoconversion (Figure 5E; S5K). Reflecting these differences in effects of per ligand sLex density, the slope of HECA452 versus CD44 staining was lower in the PC+ relative to PC− population on E-selectin only (Figure S5L–O) whereas a higher slope of HECA452 versus CEA staining in the PC+ relative to PC− populations was observed for P-selectin (Figure S5O).
Photoconversion Residence Time/Velocity Probe-Enabled High Content, Multidimensional Analysis Reveals Dependency of Selectin Adhesion Extent on Ligand Expression
We evaluated the enrichment of cellular features (namely adhesive ligand expression patterns) within rolling (PC+) versus non-rolling (PC−) cell subpopulations (Figure S2 bottom, Figure S4F) in order to reveal the effects of ligand expression on frequencies of rolling adhesion (Table S4). Instead of analyzing the velocity of rolling adhesion, the binned expression levels of selectin ligand within separately gated PC+ or PC− populations were determined via quadrant analysis and compared in order to establish the relative contribution of selectin ligands to photoconversion and thus rolling adhesion frequencies (Figure 6A, Figure S4F). As one example, cells perfused and photoconverted over L-selectin functionalized substrates exhibited PC+ and PC− populations representative of rolling and free-flowing cells, respectively (Figure 6A). Each HECA452hi, CD44hi, and CD44hi/HECA452hi population was enriched within PC+ relative to PC− cells (Figure 6B–D). When comparing across all selectins for different staining combinations, no single selectin ligand glycoprotein was enriched in PC+ populations when perfused over P-selectin (Figure 6D). However, cells expressing both high levels of glycoprotein expression and sLex were enriched in PC+ cells (Figure 6D). We juxtaposed the statistical significance of relationship differences for selectin ligand expression and frequency of rolling adhesion (Figure 6D) to those for rolling velocities (as measured by GFP/CFP ratio) for rolling (PC+) versus free flow (PC−) relationships (Figure 5E). Of selectin ligands assayed here, we found that only HECA452 singly predicted both rolling frequencies and velocities on P-selectin, whereas both CD44 and HECA452 exhibited the ability to singly predict both of these behaviors on E- and L-selectin (Figure 6E). In accordance with this finding, a FACS sorted HECA452hi population (Figure S6A) resulted in more cells interacting with P-selectin at a greatly reduced rolling velocity when compared to HECA452lo or even unsorted populations (Figure 6F, Figure S6B). However, this same HECA452-based sort failed to reveal differences in rolling adhesion on L-selectin (Figure 6G, Figure S6C). Importantly, the staining process used for this sorting procedure itself did not affect the extent or velocity for rolling adhesion (Figure S6D–E).
Figure 6). Selectin ligand glycoprotein expression alone increases Phamret-expressing LS174T cell rolling adhesion on E- and L-selectin, and, in combination with high levels of sLex, increases rolling adhesion on P-selectin.
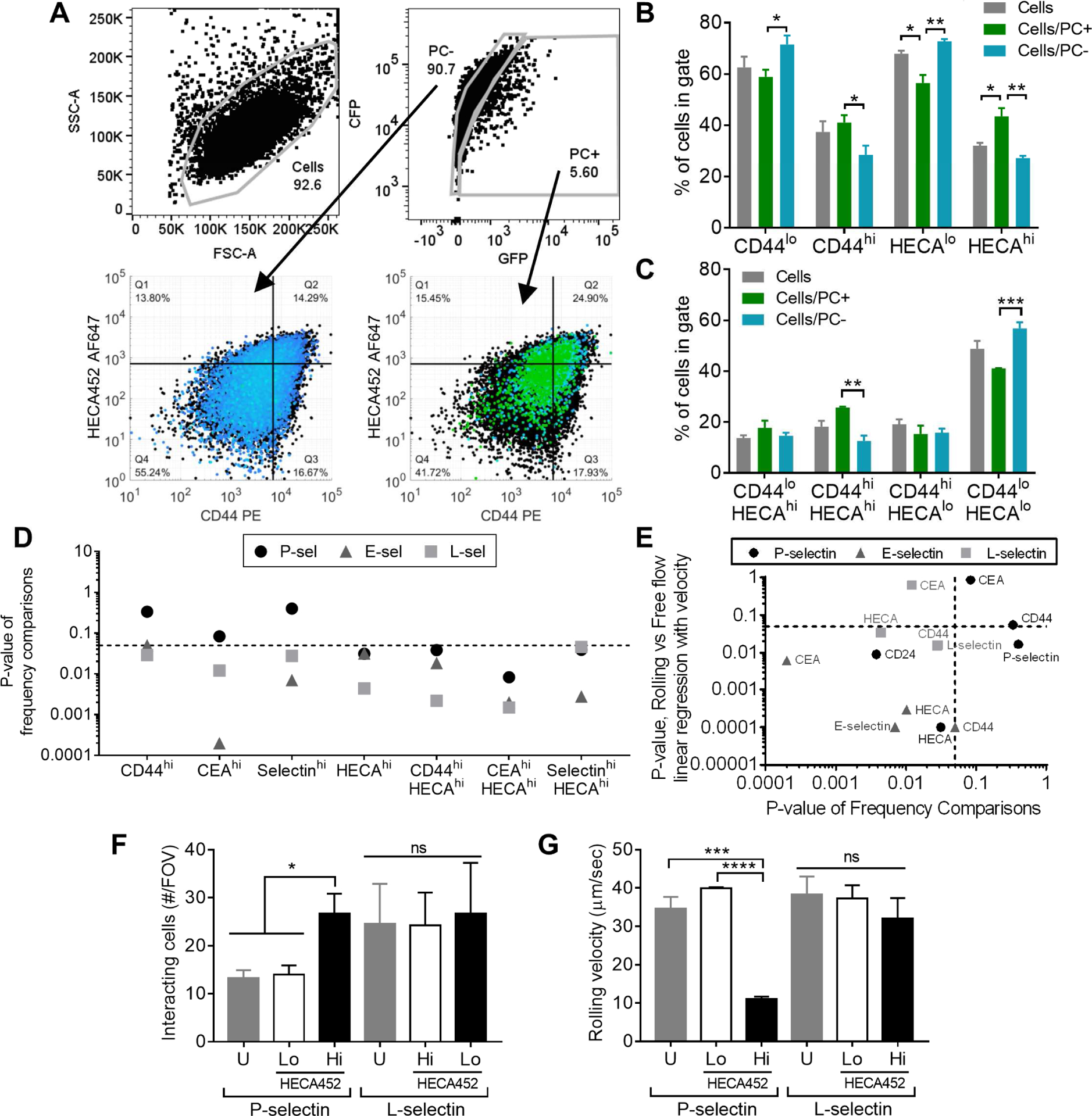
(A-C) Representative flow cytometry gating and analysis strategy. Data from Phamret-expressing LS174T (Phamret LS174T) cells perfused over 25 μg/mL L-selectin at 0.5 dyn/cm2 under exposure to 405 nm light. Total cell population indicated in black. Example comparisons between frequencies of cells in low versus high selectin ligand expression quadrants derived from single stain gating (B) or co-staining (C and depicted in lower panels of A). Frequencies are reported as a percent of parent population (i.e. percent of PC+ cells in the HECA452hi gate is significantly greater than the percent of PC− cells falling within that same gate). Data represent mean ± SEM of independently run experiments. One way ANOVA with post-hoc t-tests and Bonferonni corrections for multiple comparisons between cell populations in each staining group, α=0.05. (D) Compiled p-values from comparison of frequencies of PC+ and PC− Phamret LS174T cells in single and co-staining subgates. (E) Comparison of p-values of linear relationships between the extent of staining and ligand expression to the p-values obtained from comparing frequencies between rolling and free flowing cells in different staining gates. (F-G) Extent (counts from 3 FOVs) of adhesion and rolling velocities (30 cells / FOV in 3 FOVs) on P- versus L-selectin functionalized surfaces of FACS enriched and reperfused HECAhi vs HECAlo LS174T cell subpopulations. U, unsorted stained cells. Data represent mean ± SEM of counts from 3 FOVs from independently run experiments (F) or of pooled rolling velocities of 30 cells / FOV in 3 FOVs from independently run experiments (G), analyzed with a one-way ANOVA with post hoc t-tests with Bonferonni corrections for multiple comparisons between HECA452-sorted groups, α=0.05. See also Figures S4, S6, Table S4.
Photoconversion Residence Time/Velocity Probe-based Analytical Methodology Identifies a Non-Rolling, CD44lo/CD24hi Population of LS174T Cells
The potential of this system to reveal relationships between adhesion/migration phenotypes and other cancer pathways to generate hypotheses regarding the complex molecular underpinnings of cancer metastasis was explored. To this end, relationships between rolling adhesion and expression of LS174T selectin ligands CD44 and CEA as well as with CD24, a cancer stem cell (CSC) marker (Schneider et al., 2012) and a selectin ligand on other cancerous (Aigner et al., 1998; Aigner et al., 1997; Sammar et al., 1994) but not LS174T colon carcinoma cells (McCarty et al., 2000; Napier et al., 2007), were interrogated. Both CD44hi/CD24hi and CEAhi/CD24hi cells were found to be enriched in PC+ cells compared to PC− cells (Figure 7A–F), but PC− cells exhibited higher frequencies of CD44lo/CD24hi cells relative to PC+ rolling cells (Figure 7A–C). Irrespective of photoconversion, the lowest 10% of CD44 expressing cells (Figure S7A–D) demonstrated enriched CD24 positivity with regards to both frequency (Figure 7H) and extent of CD24 expression (Figure 7I), despite exhibiting reduced extents of photoconversion (Figure 7J). In contrast, the lowest 10% of CEA expressing cells (Figure S7E–H) that do not exhibit different extents of photoconversion from the highest 10% (Figure 7J) did enrich for a CD24lo population (Figure 7G–I).
Figure 7). Frequency of CD24hi cells is enriched in CD44low but not CEAhi LS174T subpopulation and is not predicted by rolling adhesion.
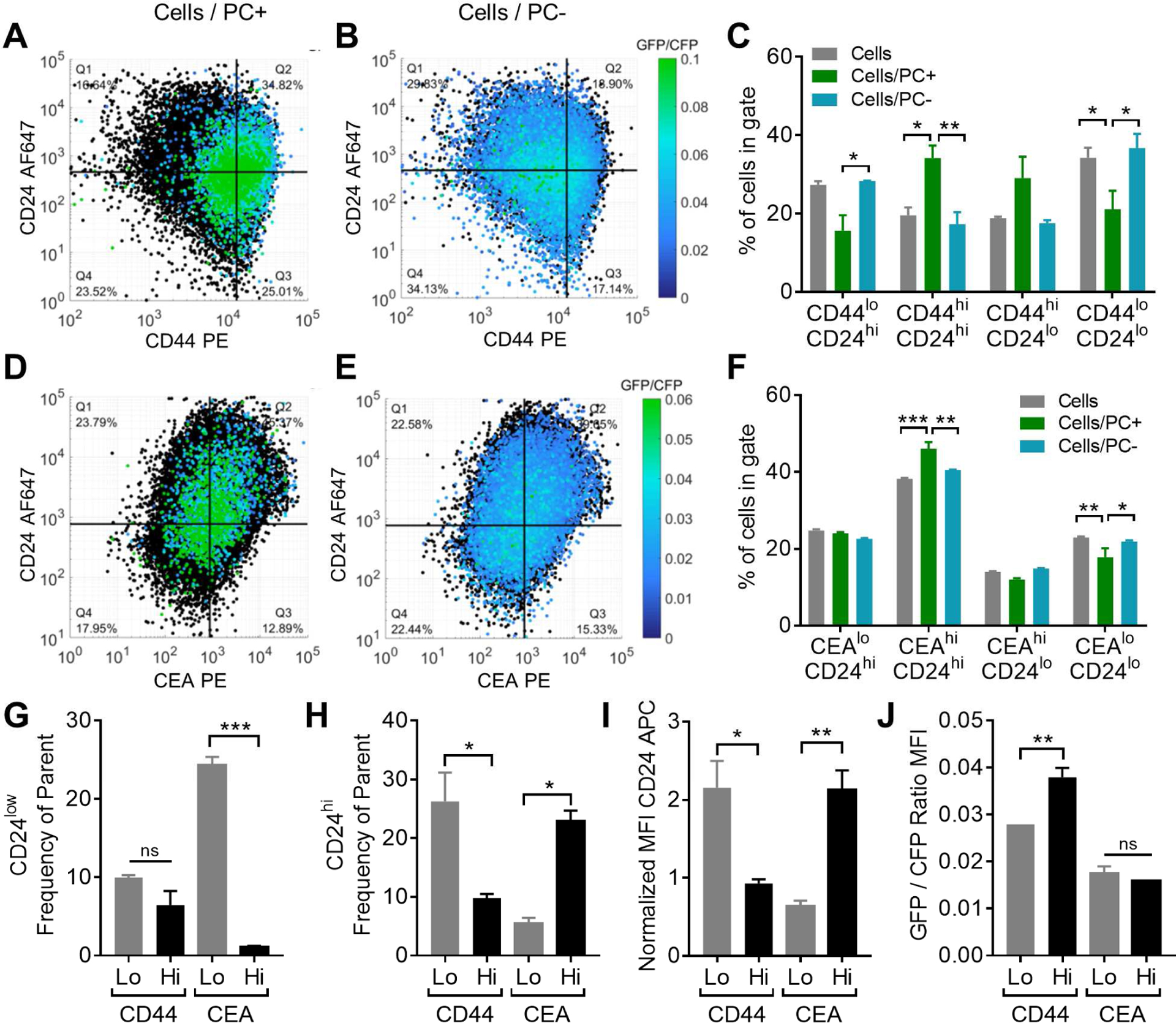
(A-F) Representative flow cytometry gating and analysis from Phamret-expressing LS174T (Phamret LS174T) cells perfused over 25 μg/mL P-selectin at 0.5 dyn/cm2 under exposure to 405 nm light, pre-gated (as shown in Figure 6A) into rolling (PC+) versus non-rolling (PC−) subpopulations and analyzed after antibody staining for co-expression of adhesion and cancer stem cell markers CD44, CEA and CD24. Total cell population indicated in black. (C,F) Frequency of cells in each gated CD44 versus CD24 (C) or CEA versus CD24 (F) quadrant. (G-H) Frequency of low (G) and high (H) CD24 expressing cells in highest versus lowest 10% of CEA and CD44 expressing cells. (I-J) CD24 MFI (I) and GFP/CFP ratio (J) in highest versus lowest 10% of CEA and CD44 expressing cells. Data in all panels represents mean ± SEM of independently run experiments. One-way ANOVA with Bonferonni correction for multiple comparisons between low and high stained populations, α=0.05. See also Figure S7, Table S4.
Discussion
Through the integration of microfluidic approaches to recapitulate salient features of the vascular microenvironment (Oh et al., 2015) with photoconvertible protein technology (Matsuda et al., 2008), we demonstrate the ability to rapidly “label” cells in a manner which directly reflects the extent and velocity with which they mediate physiologically relevant rolling adhesion interactions modeled in vitro that correspond to enhanced metastatic potential in vivo (Figures 1, 3–4). This technique enables the multi-dimensional selectin ligand expression patterns of several thousand single cells to be directly related to their adhesive phenotype via off-chip analysis. The continuous nature by which this platform labels cell velocities facilitates the interrogation of the molecular underpinnings responsible for the diversity of adhesive phenotypes within a single population of cells (Figures 5–6) with a degree of resolution previously inaccessible using existing techniques (Figure 2).
This integrated photoconversion/microfluidic platform enabled analysis of the relationship between endogenous sLex and glycoprotein expression with rolling adhesion behavior of LS174T colon carcinoma cells on selectins in order to expound upon the existing literature regarding the role of these ligands in selectin-mediated adhesion. Our results confirmed, in an endogenously expressed context, a previously revealed direct relationship between sLex expression and rolling adhesion frequencies and velocities on P- (Rodgers et al., 2000), E- (Chang and Hammer, 2000), and L-selectin (Bhatia and Hammer, 2002) (Figure 5B,E; Figure 6D,E). CEA expression was also found to be directly proportional to rolling velocity only on E-selectin and high levels of CEA enriched for a rolling adhesion cell phenotype on E- and L-, but not P-, selectin (Figures 5–6). This contrasts reports of knockdown of this molecule either failing to alter rolling phenotypes (Gebauer et al., 2014) or only increasing velocities on L- selectin (Thomas et al., 2008). We also found CD44 expression to be proportional to rolling velocities on P-, E-, and L-selectin (Figure 5), while previous reports reveal a correlation only between CD44 expression and rolling velocities on L- and P-, but not E-selectin using FACS-sorted cells perfused over selectin-functionalized surfaces (Napier et al., 2007). Importantly, however, we found the level and heterogeneity of sLex expression to be intrinsically tied to selectin ligand expression (Figure S3F–G). Similarly, in knockdown approaches, increased glycan modification and/or alternative ligands may compensate for the absence of a knocked-down ligand being investigated (Thomas et al., 2008). Thus, knockdown or FACS-based methods may convolute the study of concerted versus individual molecular contributions to rolling adhesion behavior. To this end, in addition to relieving the burden imposed by cumbersome generation of knockdown cell lines or continual resorting of cells required by the failure of sorted populations to retain their phenotype in culture (Biddle et al., 2011; Gupta et al., 2011), this photoconversion-based methodology offers the advantage of taking an observational rather than interventional approach to study these complex relationships.
Along these lines, this observational approach enabled the interrogation of more complex, multi-dimensional relationships between sLex and selectin ligand expression levels with the ability of cells to mediate rolling adhesion. For example, while we found that CD44 on its own failed to distinguish a rolling population on P-selectin, despite evidence in the literature for its role in facilitating adhesion on P-selectin (Hanley et al., 2006; Napier et al., 2007; Thomas et al., 2008), co-staining with HECA452 revealed a CD44hi/HECA452hi population enriched for a rolling as opposed to free-flowing population on P-selectin (Figure 6D). The same requirement for high sLex co-expression was noted in CEA and selectin-stained groups (Figure 6D). Consequently, when perfused over P-selectin, cells sorted based on their HECA452 immunoreactivity exhibited distinct rolling phenotypes (Figure 6F–G), supporting this critical role for sLex in the facilitation of rolling adhesion on P-selectin. Alternatively, cells sorted based on HECA452 immunoreactivity and perfused over L-selectin failed to exhibit distinct rolling phenotypes (Figure 6F–G), presumably since, as putative regulators of L-selectin-mediated adhesion irrespective of sLex co-presentation (Figure 6A–E), CD44 or CEA may ameliorate the effects of reduced glycosylation in HECA452lo populations. These findings underscore the utility of this photoconversion residence time/velocity probe-based methodology in interrogating the simultaneous effects of multiple mediators of selectin engagement on rolling adhesion behavior. While multiparameter sorting may be used to achieve a similar ends, such techniques are limited by the number of discrete gates one can sort into (four, given standard FACS equipment), and thus the continuum of expression levels cannot be completely assayed using these methods. In contrast, since this photoconversion platform enables measurement of all parameters (expression levels and velocities) on continuous scales in high replicate numbers, the relationship between selectin ligands and relative to one another (Figures 5D–E, S4, S5H–O) can be interrogated to reveal the more complex regulation of rolling adhesion behavior by both ligand expression and the extent of glycosylation.
By facilitating co-labeling with CSC markers, this in-flow velocity analysis platform also provided additional context for previously reported disparities in in vitro and in vivo models of selectin-mediated adhesion and metastasis. Knockdown of CD44 expression on LS174T cells in previous work (Dallas et al., 2012) resulted in increased lung metastasis, despite selectins’ role in mediating lung metastasis in vivo (Läubli and Borsig, 2010) and the in vitro demonstration of the functional role for CD44 in mediating adhesion with P-, E-, and L-selectin (Hanley et al., 2006). Using the methodology described herein to assay the expression of both CD44 and CEA with the CSC marker CD24, whose expression is also associated with augmented cell proliferation, motility, and invasion (Baumann et al., 2005), we found that selection of CD44lo cells enriches for CD24 positivity with regards to both frequency and intensity of CD24 staining (Figure 7). This suggests that knockdown of CD44 in in vivo experiments (Dallas et al., 2012) may inadvertently enrich a CD24hi CSC subpopulation and CEA knockdown may enrich a non-CSC CD24lo population, potentially leading to the previously reported increase and decrease in lung metastasis, respectively, an effect independent of these molecules’ roles in mediating selectin adhesion.
Overall, we demonstrate a photoconversion platform that can be used to test and generate hypotheses regarding the complex molecular underpinnings of the diversity of cell responses in the context spatiotemporal processes occurring over time scales of seconds to hours. This integrated system could be used to assay the efficacy and cell-subtype selectivity of metabolic inhibitors or other potential drug candidates for their ability to interfere specifically with selectin-mediated adhesion of the most aggressive, chemoresistent, or CSC subpopulations. Separately, while this system is particularly well suited to quantify unidirectional cell movement, the methodology could be adapted to interrogate the mechanisms of other spatiotemporal and rate-based cell-level processes, including, but not limited to wound healing or three dimensional cancer or immune cell migration mechanisms in vitro or in vivo in which residence time during the transit of a particular cell type within a prescribed area of interest has physiological or pathophysiological significance. This photoconversion-based residence time/velocity probe therefore represents a unique and potentially versatile approach for the high-throughput labeling and functional analysis of cells that can be used to generate high content and resolution, single cell-level data for the interrogation of a wide range of spatiotemporal biological processes.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Susan N. Thomas (susan.thomas@gatech.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Generation of a Stable Phamret Expressing LS174T Colon Cancer Cell Lines
The Phamret-containing pcDNA3 expression vector (kindly provided by Dr. Tekaharu Nagai) was transformed into competent DH5α Escherichia coli cells, amplified in the presence of ampicillin, and the plasmid was purified using the EndoFree maxi kit (Qiagen, Valencia, CA). Sequence insertion was verified by restriction digestion. LS174T cells were plated and grown overnight to reach ~50% confluency. Cells were transfected with pcDNA3.neo.Phamret using Lipofectamine 2000 for 24 h after which time the medium was replaced every 24 h by a fresh aliquot (containing 500 ug/ml neomycin starting 48 h post transfection). Upon reaching confluency, transfected cells were passed and subsequently grown continually without passaging for 21 days, replenishing the neomycin-containing medium every 2–3 days. Single cell colonies were isolated and cultured using standard techniques.
Cell Culture
Control and Phamret-expressing LS174T cells (human male) were cultured in Dulbecco’s Modified Eagle’s Medium, supplemented with 10% heat inactivated fetal bovine serum and 1% penicillin-streptomycin. Cells were harvested via mild trypsinization (0.25% trypsin-EDTA at 37°C), centrifuged at 400 g for 5 minutes, re-suspended in complete medium, and either diluted into tissue culture flasks for subculture or maintained in suspension at 37°C for 2h in order to allow surface glycoproteins to regenerate for use in experiments.
In Vivo Metastasis Model
104 LS174T control, LS174T Phamret unsorted, LS174T Phamret PC+, or LS174T Phamret PC− cells were resuspended in 100uL of sterile saline and injected into the tail vein of six week old, female NSG mice (Jackson Labs, Bar Harbor, ME) under isoflurane anesthesia. Saline-only injected animals served as a control. Mice were sacrificed 28 days post injection by CO2 asphyxiation and metastasis quantified in the lungs. Excised lung tissues were flash frozen in optimum cutting temperature compound (Sakura Finetek USA, Inc., Torrance, CA) in a liquid nitrogen-cooled bath of 2-methyl butane (Sigma-Aldrich, St. Louis, MO) and subsequently maintained at −80°C until cryosectioning into 10 μm slices for direct fluorescent imaging or staining with hematoxylin and eosin. All protocols were approved by the Institutional Animal Care and Use Committee.
METHOD DETAILS
Characterization of 405 nm Light Source and Static Photoconversion
The power density of a 405 nm laser (M405L2 - UV Mounted LED, 1000 mA, 410 mW, ThorLabs, Newton, NJ) over a range of power settings was determined by measuring the power output at each setting using a power meter (Model D3MM, ThorLabs, Newton, NJ) and dividing by the total light shedding area, measured at the plane where a plate would rest during an experiment. To measure the effects of power setting on the extent of photoconversion, 5×104 LS174T Phamret cells in suspension in D-PBS or 2.5 ×104 adherent cells were exposed to 405 nm light at a range of power settings and exposure times in a 96 well plate and either imaged using GFP and CFP filters on an EVOS digital microscope (Thermo Fisher Scientific, Rockford, IL, USA) or using a BD LSRII flow cytometer (BD Biosciences, San Jose, CA, USA). The duration of photoconversion sustainment was measured by photoconverting cells in the same manner once for 2 min at maximal power and analysing cellular GFP/CFP ratio via flow cytometry at various time points following photoconversion. The repeatability of photoconversion was confirmed by re-exposure of cells to 405 nm light for 2 min at maximal power 48 h after initial exposure (when previously photoconverted cells had returned to baseline GFP/CFP ratios) and measuring the GFP/CFP level induced by treatment via flow cytometry. The rationale for each quantitative methodology and statistical analysis used is outlined in Table S1.
Cell Viability Assays
Samples of 5 × 105 Phamret LS174T cells in suspension in D-PBS were either left untreated on ice or treated with 100 μM Tert-butyl hydrogen peroxide in complete medium at 37°C for 1 h, 1.5% hydrogen peroxide in D-PBS at room temperature for 10 min, or exposed to 405 nm light at a power density of 6382 mW/cm2 for 5 min in D-PBS. After treatment, cells were centrifuged at 300 × g and resuspended in either 55nM SytoxRed dead cell stain in D-PBS at room temperature for 15 min, 500nM CellRox Orange reactive oxygen species detection reagent in complete medium at 37°C for 1 h, or Annexin V-Alexa Fluor 647 conjugate at a 1:20 dilution in D-PBS at room temperature for 15 min. Cells were analyzed for fluorescent staining immediately using a BD LSRII flow cytometer. A one-way ANOVA with Bonferonni correction for multiple post-hoc t-tests in comparison to untreated control was used to verify that 405 nm light exposure did not induce higher levels of cell death, apoptosis, or reactive oxygen species production in comparison to untreated cells, as outlined in Table S1.
Channel Fabrication and Functionalization
Microfluidic channels were fabricated as previously described (Tran et al., 2017). Briefly, double sided adhesive sheets (2 in wide, 9 cm long, 125 μm thick) were backed with non-silicone release liner into which a U-shaped channel consisting of two parallel 4 mm wide by 8 cm long channels connected by a 4 mm wide by 1.5 cm long perpendicular channel (Figure 4A–B) was cut. Polydimethylsiloxane (PDMS) base and curing agent were mixed at a ratio of 9:1, poured into Pyrex dishes, and cured at 90°C for three hours before being cut to the outer dimensions of the double sided adhesive channel. Adhesive channels were affixed to cured PDMS blocks, a biopsy punch was used to create inlet and outlet ports, and the assembly was affixed to non-tissue culture treated polystyrene plates to complete the chamber fabrication. The 7.6 cm length of channel nearest the outlet was functionalized by incubating with 25 μg/mL anti-IgG (Fc specific) in D-PBS without calcium and magnesium overnight at 4°C, washing with D-PBS, blocking with 1% bovine serum albumin (BSA) in D-PBS for 1 hour at room temperature, washing with D-PBS again, and finally incubating with 2.5 μg/mL of E-selectin or 25 μg/mL of P- or L-selectin in D-PBS with calcium and magnesium for 2 hours at room temperature. After washing again in D-PBS, the entire device was blocked with 1% BSA in D-PBS for 1 hour at room temperature, washed with D-PBS, and stored at room temperature until use in same-day experiments.
Photoconversion Flow Experiment Workflow
An outlet line connected to a withdraw syringe on a PhD Ultra Harvard Apparatus syringe pump (Holliston, MA) was filled with perfusion medium (0.1% BSA in D-PBS) and installed at the channel outlet. An inlet reservoir was installed, filled with perfusion medium and manually withdrawn through the channel until D-PBS in the channel was completely replaced with perfusion medium. The entire apparatus was placed on optical microscope (Eclipse Ti, Nikon, Melville, NY) and a 405 nm light source was placed under the channel, directly downstream of the microscope objective, approximately 1 cm from the channel outlet. To ensure exposure across the width of the channel was not affected by the curvature of the light shedding area, a 10 mm long 8mm wide slit was cut into a mask to occlude 405 nm light exposure outside of this area.
A 500 μL pulse of 5×105 cells/mL cell suspension in perfusion medium (total of 2.5×105 cells) was then added to the inlet reservoir before initiating syringe withdraw at a flow rates calculated to achieve the desired wall shear stress, turning on the 405 nm light source to the appropriate power level, and beginning video acquisition. For all experiments involving P-selectin or E-selectin functionalized substrates, a laser power of 4 was used, and for L-selectin experiments a laser power of 5.5 was used. Laser powers were chosen to ensure the GFP/CFP ratio exhibited greatest sensitivity to exposure time based on the manually measured rolling velocities and used exposure window length (Table S3). Unfunctionalized controls were acquired for each experiment using matched laser powers. Following the cell pulse, the reservoir was continuously refilled with perfusion medium for the remainder of the 1 hr photoconversion experiment. In select experiments, cells were perfused over non-functionalized, blocked channels. NIS-Elements (Nikon, Melville, NY) software was used to acquire videos with a frame rate of 25 frames per second, an exposure time of 0.281 μs, an objective magnification of 10×, and 2×2 binning of a 500 by 376 pixels image. In experiments where the GFP signal was measured during video acquisition, a fluorescein isothiocyanate (FITC) filter (excitation 475–492, emission 505–535, Chroma, Bellows Falls, VT) was used with the similar settings, save for an exposure time of 400 ms.
Cells (photoconverted or otherwise) were collected at the end of experiments and either analyzed or sorted immediately (with and without co-staining) using a BD LSRII or FACS Aria (BD Biosciences, San Jose, CA, USA), respectively. In experiments where selectin ligand and cancer stem cell expression were analysed, cells were centrifuged at 400 g for 5 min and resuspended in antibody solutions in D-PBS for 45 min on ice. Staining dilutions or concentrations were 1:20 for CD44 and CEA, 1:40 for HECA452, 1:10 for CD24, and 20ug/mL premixed 1:1 selectin:anti-IgG for 1 hour at room temperature. Stained cells were washed and resuspended in 0.1% BSA in PBS for analysis via flow cytometry. In select experiments, sorted populations were used in vivo experimentation (described below) or perfused over P- and L-selectin functionalized substrates, observed via videomicroscopy, and quantification of frequencies and velocities of sorted and perfused populations was performed using ImageJ.
QUANTIFICATION AND STATISTICAL ANALYSIS
An outline of quantification methods that were used in this work is provided in Figure S2. The rationale for each quantitative methodology and statistical analysis used in system characterization, validation, and application is described below and outlined in Table S1, S2–2, S4.
Photoconversion quantification
An EVOS digital microscope or BD LSRII flow cytometer was used for all fluorescence quantification. The objective, rationale, and specific methods for each type of analysis are elaborated below and outlined in Table S1.
Image-based GFP/CFP ratio measurements
Fluorescent images were collected as described previously and image math in Matlab was utilized. As a means for normalizing to quantify per-cell Phamret expression since CFP signal does not change with 405 nm exposure time, untransformed GFP channel was divided by untransformed CFP channel and reported as a heat map of the ratios.
Flow cytometry-based GFP/CFP ratio measurements
To quantify per-cell GFP/CFP, a new parameter was derived in FlowJo. Untransformed GFP signal was divided by untransformed CFP signal, calculated on a per-cell basis, and reported in histograms. Frequently, data is reported as normalized to the unphotoconverted control to enable detection of a fold-increase in GFP/CFP ratio for comparisons between cell populations with different baseline GFP/CFP ratios. In select experiments, 405 nm light exposure time vs GFP/CFP curves as presented in Fig. 3F for all laser powers were fit a four parameter, variable slope dose-response model for each power using GraphPad Prism and EC50 values reported. This enabled a quantitative comparison of the exposure time required to achieve half-maximal photoconversion for each laser power and demonstrated the tunability of the system to observe a desired kinetic process (i.e. faster vs slower processes).
Qualification of cell adhesive behavior
Cell adhesive phenotypes were characterized by several approaches that relied on defining subpopulations of “rolling” and “free flowing” cells. For the context of this work, a rolling cell (or a cell “mediating rolling adhesion” or “interacting”) was defined as one that interacted with the selectin-functionalized substrate but continued to translate in the direction of flow at a velocity substantially slower than cells in free flow stream, which were defined as cells not which do not mediate specific, sustained adhesive contact with the substrate throughout the duration of its transit through the channel. Video analysis of cell velocities, both rolling and free flow as well as quantification of GFP signal intensities was performed using ImageJ (National Institutes of Health) with a manual particle tracking plugin (Cordelières, 2005). The objective, rationale, and specific methods for each type of analysis are elaborated below and outlined in Table S2.
Relationship between photoconversion-dependent GFP level and rolling velocity
We sought to demonstrate a relationship between photoconversion-dependent GFP level and rolling velocity. A FITC filter on a Nikon microscope was used to capture videos of Phamret LS174T cells perfused over P selectin functionalized substrates before and after photoconversion. ImageJ was used to manually measure single-cell velocities and GFP intensities from videos. Linear regression with post-hoc test for nonzero slopes and difference between slopes allowed us to estimate the degree of correlation between GFP signal and single cell velocities. Although the CFP signal cannot be simultaneously measured with GFP in the microscopy setup that was used in this study, thereby limiting our ability to normalize the GFP signal to Phamret expression using the CFP signal, this comparison nevertheless substantiated the claim that GFP signal after photoconversion can be used as a proxy for velocity.
Comparison between manual velocity measurement and flow-cytometry-based measurement.
To compare manual velocity measurements to those made using Phamret measured by flow cytometry, Phamret LS174T cells perfused over P-selectin functionalized substrates under exposure to 405 nm light were collected, analyzed via flow cytometry, and their GFP/CFP ratios were converted to velocities using a standard curve of Phamret LS174T cells statically photoconverted for different durations of time. The mean velocity was then compared to the mean manually measured velocity from Fig. 4D. A t-test with Welsh’s correction allowed us to test for unequal variance. The variance presumably differs between these two measurement techniques because the standard curve used to extrapolate velocities from GFP/CFP ratios was generated from statically photoconverted cells, which do not experience the same rotational motion as do cells in free flow. This comparison nevertheless further substantiated the claim, albeit in an indirect fashion, that GFP/CFP ratios can be used to calculate velocities that are in line with those which are manually measured.
Verification that photoconversion distinguishes subpopulations of cells that in vitro behave phenotypically distinct
We sought to verify that photoconversion distinguishes subpopulations of cells that in vitro behave phenotypically distinct. To this end, Phamret LS174T cells were perfused over P-selectin functionalized substrates at 0.5 dyn/cm2 under exposure to 405 nm light, collected, and sorted based on whether or not they exhibited a GFP/CFP ratio indicative of photoconversion and thus a rolling-adhesive state. Sorted cells were then reperfused over P-selectin functionalized substrates at 0.5 dyn/cm2 and the frequency and velocity of rolling adhesion was observed via videomicroscopy (Nikon) and manually quantified using ImageJ. One-way ANOVA with Bonferroni correction for multiple post-hoc t-tests (all means compared to one another) was used to compare groups. With these comparisons between the rolling adhesive behavior of cell populations based on their extents of photoconversion, the ability of this photoconversion-based approach to distinguish phenotypically distinct subpopulations was verified.
Verification that photoconversion distinguishes phenotypically distinct subpopulations of cells that exhibit distinct metastatic potentials in vivo
We sought to demonstrate that photoconversion distinguishes phenotypically distinct subpopulations of cells that exhibit distinct metastatic potentials in vivo. To accomplish this, cells (either from the parental line or after perfusion, photoconversion, and FACS isolation) were injected i.v., allowed to metastasize, and histological sections of flash frozen lungs were acquired post-sacrifice d28 post-injection. An EVOS fluorescent microscope was used to acquire five images from similar locations within each tissue section (four fields of view at distal edges and one at the center of each tissue section) for each of five lung tissue sections per condition using GFP and CFP filters. Alternatively, cryosections were hematoxylin and eosin (H&E) stained and imaged using a Nanozoomer microscope (Hamamatsu Photonics, Hamamatsu, Japan). Images were imported into ImageJ, thresholded using identical parameters across all images, and both the mean fluorescence intensity (in the case of CFP and GFP images) and percent area above the thresholded background was calculated (for CFP, GFP, and H&E images) and normalized to saline controls. Individual t-tests were used to determine if each population mean was greater than 1, Ho: μ= 1. Since differences in metastatic foci counts can be obscured by varying foci size with metastatic stage, this fluorescence-based approach offers a reliable alternative to quantify total metastatic area. A mean of 1 was used for t-test null hypotheses, since all samples were normalized to saline controls.
Determining relationships between selectin ligand expression and frequencies or velocities of rolling adhesion
Gating and analysis of flow cytometry data was performed using a combination of FlowJo (Treestar, Inc., San Carlos, GA) and custom written Matlab scripts (Mathworks, Natick, MA). The objective, rationale, and specific methods for each type of analysis are elaborated below and outlined in Table S4.
Quantitative evaluation of the relationship between rolling velocity and adhesive ligand expression in cells exhibiting adhesion versus those in free flow
To evaluate quantitatively the relationship between rolling velocity and adhesive ligand expression in cells exhibiting adhesion versus those in free flow, Phamret LS174T cells were perfused over P-, E-, or L-selectin functionalized substrates at 0.5 dyn/cm2 under exposure to 405 nm light. Photoconverted cells were collected, labeled with fluorophore-conjugated antibodies specific for different adhesive ligands, and subsequently analyzed on a single cell basis via flow cytometry. All data points for each condition and replicate were pooled and gated into six sub-gates representing increasing extents of photoconversion (gating example Figure S4A). The GFP/CFP ratio as well as the PE or APC signal from the fluorophore-conjugated selectin-ligand antibody were normalized to the least photoconverted subpopulation. Mean GFP/CFP ratios and PE-intensities were computed for each of these gates and linear regression with post-hoc test for non-zero slopes and difference between slopes was performed. The slopes of these linear models were compared between rolling (CR) and free flowing (CF) populations, and regression p-values were reported for interpretation of which selectin-ligands exhibited a relationship with cell rolling velocity. As a note, normalization within each population was conducted for two main reasons: (1) Since experiments of this complexity are often conducted over weeks/months, drift or recalibration of the flow cytometer can make comparisons of raw, non-normalized fluorescence intensities from different experiments impossible. (2) Normalization enables for the relationship between velocity and markers with different baseline expression levels to be directly compared. Furthermore, if comparisons of slopes between markers are desired, normalization is critical. For example, if the PC ratio vs CD44 slope was 1.02 and the PC ratio vs CEA slope was 1.5, CEA expression may be considered more strongly predictive of velocity than CD44. Though not done here, this comparison would be impossible without normalization.
Relationships between marker expression and extent of cell rolling adhesion
To determine relationships between marker expression and extent of cell rolling adhesion, Phamret LS174T cells were perfused over P-, E-, or L-selectin functionalized substrates at 0.5 dyn/cm2 under exposure to 405 nm light. Photoconverted cells were collected, labeled with fluorophore-conjugated antibodies specific for different adhesive ligands, and subsequently analyzed on a single cell basis via flow cytometry. FlowJo was used to gate the total cell population into quadrants such that the center of all quadrants coincided with the mode of the cell population. The cell population was also gated into PC+ and PC− populations and the quadrant divisions from the total cell population were applied to the PC+ and PC− populations to compare frequencies of PC+ versus PC− and unsorted cells within each quadrant gate (Fig. S4F). One way ANOVA with post-hoc t-tests and Bonferonni corrections for multiple comparisons between cell populations in each staining quadrant, α=0.05, was performed. In order to avoid biasing the data based on expression profiles of each of the subpopulations, high and low population gates in Fig. 5–6 were determined based on the distribution of the total cell population density (i.e the cell population mode) rather than on any set fluorescence intensity thresholds.
Graphical display of significant relationships between rolling velocity or frequency with marker expression
In order to graphically display significant relationships between rolling velocity and frequency with marker expression, the slopes of linear regressions displayed in Figs. 5B–D, for example, were compared between PC+ and PC− populations (Fig. 5B–D, S5A–F, S5H–J) and Fig. 6B–C and p-values plotted. Due to the large number of comparisons being made, presenting a summary of the p-values was determined to be a visually compelling way to identify noteworthy relationships between photoconversion (measured GFP/CFP ratio as a proxy for velocity) or rolling frequency with marker expression. The p-values presented in Fig. 5E and 6D were also compiled into one plot, Fig. 6E. Compiling p-values from both Fig. 5E and 6D enabled easy visual identification of markers that correlated with both rolling velocity and frequency or either.
Adhesion and CSC marker relationships with rolling adhesive behavior
To determine relationships between known adhesion markers (CD44, CEA) and the CSC marker CD24 as they relate to rolling adhesive behavior, Phamret LS174T cells perfused and photoconverted as in Fig. 5–6 were co-labeled with antibodies for CD24 and either CEA or CD44. Labeled cells were analyzed via flow cytometry and FlowJo was used to gate the population into the lowest and highest ~10% of CD44, CEA, or CD24 expression. Frequencies of CD24 high cells of parent gate, mean CD24 expression levels within the parent gate, and (d) mean GFP/CFP ratios were calculated using FlowJo. One-way ANOVA with Bonferonni correction for multiple comparisons between low and high stained populations, α=0.05, was performed. By sorting into low- and high-expressing populations, subsets of the total population could be explored for the relationship between selectin ligand expression, cancer stem cell marker expression, and rolling adhesive behavior.
Statistical analyses
For multiple comparisons, one- and two-way ANOVA with post-hoc t-tests with Bonferonni corrections was used. Two-sample t-tests were used when comparing only two experimental groups (PC+ vs PC−, for example). If variances differed between tested groups, an adjusted (Welch’s) test was used. Coefficients of linear models and the significance of differences between linear models were determined using linear regression analysis of data binned into equally sized groups based on the frequency of parent subpopulation. Specifics of statistical tests used, the number of replicates, and the alpha level are reported in figure legend and outlined in Tables S1–2, S4. Throughout all work, one, two, three, and four symbols (* or †) indicates significance at the 0.05, 0.01, 0.001, or 0.0001 level. All statistics, regression analyses, and plots were generated using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA). Power analyses were performed using an alpha of 0.05. Given the high degree of variation (standard deviation) intrinsic to the velocities of LS174T cell adhesive rolling on selectins resulting in low power of comparisons, equivalence between groups in Figures 3E and S4G could not be claimed.
Supplementary Material
Highlights.
Demonstrated implementation of high-throughput, cell-based residence time probe.
Elucidated multidimensional cell molecular and adhesive profile relationships.
Reconciled disparities between in vitro and in vivo adhesion and metastasis models.
Acknowledgments
This work was supported by NSF 1342194, NIH R21 CA202849, and NIH T32 GM-008433, a research partnership between Children’s Healthcare of Atlanta and the Georgia Institute of Technology, and a PHS Grant UL1TR000454 from the NIH National Center for Advancing Translational Sciences CTSA Program. We thank P. Mason McClatchey for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- Aigner S, Ramos CL, Hafezi-Moghadam A, Lawrence MB, Friederichs J, Altevogt P, and Ley K (1998). CD24 mediates rolling of breast carcinoma cells on P-selectin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 12, 1241–1251. [DOI] [PubMed] [Google Scholar]
- Aigner S, Sthoeger ZM, Fogel M, Weber E, Zarn J, Ruppert M, Zeller Y, Vestweber D, Stahel R, and Sammar M (1997). CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood 89, 3385–3395. [PubMed] [Google Scholar]
- Baumann P, Cremers N, Kroese F, Orend G, Chiquet-Ehrismann R, Uede T, Yagita H, and Sleeman JP (2005). CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res 65. [DOI] [PubMed] [Google Scholar]
- Bhatia SK, and Hammer DA (2002). Influence of receptor and ligand density on the shear threshold effect for carbohydrate-coated particles on L-selectin. Langmuir : the ACS journal of surfaces and colloids 18, 5881–5885. [Google Scholar]
- Biddle A, Liang X, Gammon L, Fazil B, Harper LJ, Emich H, Costea DE, and Mackenzie IC (2011). Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer research 71, 5317–5326. [DOI] [PubMed] [Google Scholar]
- Borsig L, Wong R, Hynes RO, Varki NM, and Varki A (2002). Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proceedings of the National Academy of Sciences 99, 2193–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick MM, McCaffery JM, Kim YS, Bochner BS, and Konstantopoulos K (2003). Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. American Journal of Physiology -Cell Physiology 284, C977–C987. [DOI] [PubMed] [Google Scholar]
- Chang K-C, and Hammer DA (2000). Adhesive dynamics simulations of sialyl-Lewis x/E-selectin-mediated rolling in a cell-free system. Biophysical journal 79, 1891–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AA, Geva-Zatorsky N, Eden E, Frenkel-Morgenstern M, Issaeva I, Sigal A, Milo R, Cohen-Saidon C, Liron Y, Kam Z, et al. (2008). Dynamic Proteomics of Individual Cancer Cells in Response to a Drug. Science 322, 1511–1516. [DOI] [PubMed] [Google Scholar]
- Cordelières FP (2005). Manual tracking. Institut Curie, Orsay (France). [Google Scholar]
- Dallas MR, Liu G, Chen W-C, Thomas SN, Wirtz D, Huso DL, and Konstantopoulos K (2012). Divergent roles of CD44 and carcinoembryonic antigen in colon cancer metastasis. The FASEB journal 26, 2648–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EE, Oh J, Anilkumar A, Birmingham KG, and Thomas SN (2017). P-, but not E-or L-, selectin-mediated rolling adhesion persistence in hemodynamic flow diverges between metastatic and leukocytic cells. Oncotarget 8, 83585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EE, and Thomas SN (2017). P-Selectin and ICAM-1 synergy in mediating THP-1 monocyte adhesion in hemodynamic flow is length dependent. Integrative biology : quantitative biosciences from nano to macro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F, Wicklein D, Horst J, Sundermann P, Maar H, Streichert T, Tachezy M, R Izbicki J, Bockhorn M, and Schumacher U (2014). Carcinoembryonic Antigen-Related Cell Adhesion Molecules (CEACAM) 1, 5 and 6 as Biomarkers in Pancreatic Cancer, Vol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta Piyush B., Fillmore Christine M., Jiang G, Shapira Sagi D., Tao K, Kuperwasser C, and Lander Eric S. (2011). Stochastic State Transitions Give Rise to Phenotypic Equilibrium in Populations of Cancer Cells. Cell 146, 633–644. [DOI] [PubMed] [Google Scholar]
- Hamada K, Monnai M, Kawai K, Nishime C, Kito C, Miyazaki N, Ohnishi Y, Nakamura M, and Suemizu H (2008). Liver metastasis models of colon cancer for evaluation of drug efficacy using NOD/Shi-scid IL2Rgammanull (NOG) mice. International journal of oncology 32, 153–159. [PubMed] [Google Scholar]
- Hanley WD, Napier SL, Burdick MM, Schnaar RL, Sackstein R, and Konstantopoulos K (2006). Variant isoforms of CD44 are P- and L-selectin ligands on colon carcinoma cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 20, 337–339. [DOI] [PubMed] [Google Scholar]
- Khatib AM, Fallavollita L, Wancewicz EV, Monia BP, and Brodt P (2002). Inhibition of hepatic endothelial E-selectin expression by C-raf antisense oligonucleotides blocks colorectal carcinoma liver metastasis. Cancer Res 62, 5393–5398. [PubMed] [Google Scholar]
- Kim YJ, Borsig L, Varki NM, and Varki A (1998). P-selectin deficiency attenuates tumor growth and metastasis. Proceedings of the National Academy of Sciences 95, 9325–9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantopoulos K, and Thomas SN (2009). Cancer cells in transit: the vascular interactions of tumor cells. Annual review of biomedical engineering 11, 177–202. [DOI] [PubMed] [Google Scholar]
- Läubli H, and Borsig L (2010). Selectins as Mediators of Lung Metastasis. Cancer Microenvironment 3, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lech G, Słotwiński R, Słodkowski M, and Krasnodębski IW (2016). Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World Journal of Gastroenterology 22, 1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, and King MR (2012). Adhesion receptors as therapeutic targets for circulating tumor cells. Frontiers in Oncology 2, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lote H, Spiteri I, Ermini L, Vatsiou A, Roy A, McDonald A, Maka N, Balsitis M, Bose N, Simbolo M, et al. (2017). Carbon dating cancer: defining the chronology of metastatic progression in colorectal cancer. Annals of Oncology 28, 1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, and Obenauf AC (2016). Metastatic colonization by circulating tumour cells. Nature 529, 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Miyawaki A, and Nagai T (2008). Direct measurement of protein dynamics inside cells using a rationally designed photoconvertible protein. Nature methods 5, 339–345. [DOI] [PubMed] [Google Scholar]
- McCarty OJ, Mousa SA, Bray PF, and Konstantopoulos K (2000). Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood 96, 1789–1797. [PubMed] [Google Scholar]
- Napier SL, Healy ZR, Schnaar RL, and Konstantopoulos K (2007). Selectin ligand expression regulates the initial vascular interactions of colon carcinoma cells: the roles of CD44v and alternative sialofucosylated selectin ligands. The Journal of biological chemistry 282, 3433–3441. [DOI] [PubMed] [Google Scholar]
- O’Connell MP, Marchbank K, Webster MR, Valiga AA, Kaur A, Vultur A, Li L, Herlyn M, Villanueva J, Liu Q, et al. (2013). Hypoxia induces phenotypic plasticity and therapy resistance in melanoma via the tyrosine kinase receptors ROR1 and ROR2. Cancer discovery 3, 1378–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Edwards EE, McClatchey PM, and Thomas SN (2015). Analytical cell adhesion chromatography reveals impaired persistence of metastatic cell rolling adhesion to P-selectin. Journal of cell science 128, 3731–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GH, and Lippincott-Schwartz J (2002). A Photoactivatable GFP for Selective Photolabeling of Proteins and Cells. Science 297, 1873–1877. [DOI] [PubMed] [Google Scholar]
- Patterson GH, and Lippincott-Schwartz J (2004). Selective photolabeling of proteins using photoactivatable GFP. Methods 32, 445–450. [DOI] [PubMed] [Google Scholar]
- Reyes-Reyes EM, George MD, Roberts JD, and Akiyama SK (2006). P-Selectin Activates Integrin-mediated Colon Carcinoma Cell Adhesion to Fibronectin. Experimental cell research 312, 4056–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers SD, Camphausen RT, and Hammer DA (2000). Sialyl Lewis X-Mediated, PSGL-1-Independent RollingAdhesion on P-selectin. Biophysical journal 79, 694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammar M, Aigner S, Hubbe M, Schirrmacher V, Schachner M, Vestweber D, and Altevogt P (1994). Heat-stable antigen (CD24) as ligand for mouse P-selectin. International immunology 6, 1027–1036. [DOI] [PubMed] [Google Scholar]
- Schneider M, Huber J, Hadasc k, B., Siegers GM, Fiebig H-H, and Schüler J (2012). Characterization of colon cancer cells: a functional approach characterizing CD133 as a potential stem cell marker. BMC cancer 12, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer SM, Dunagin MC, Torborg SR, Torre EA, Emert B, Krepler C, Beqiri M, Sproesser K, Brafford PA, Xiao M, et al. (2017). Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature 546, 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea DJ, Wirtz D, Stebe KJ, and Konstantopoulos K (2015). Distinct kinetic and mechanical properties govern mucin 16- and podocalyxin-mediated tumor cell adhesion to E- and L-selectin in shear flow. Oncotarget 6, 24842–24855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Hill CA, Baharo-Hassan D, and Farooqui M (2011). C2-O-sLeX Glycoproteins Are E-Selectin Ligands that Regulate Invasion of Human Colon and Hepatic Carcinoma Cells. PLOS ONE 6, e16281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TN, and Berger MR (1999). Quantification of liver metastases from LS174T human colorectal cancer cells in nude rats by PCR. Anticancer research 19, 3939–3945. [PubMed] [Google Scholar]
- Strell C, and Entschladen F (2008). Extravasation of leukocytes in comparison to tumor cells. Cell Communication and Signaling : CCS 6, 10–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam V, Vincent IR, Gardner H, Chan E, Dhamko H, and Jothy S (2007). CD44 regulates cell migration in human colon cancer cells via Lyn kinase and AKT phosphorylation. Experimental and Molecular Pathology 83, 207–215. [DOI] [PubMed] [Google Scholar]
- Thomas SN, Schnaar RL, and Konstantopoulos K (2009). Podocalyxin-like protein is an E-/L-selectin ligand on colon carcinoma cells: comparative biochemical properties of selectin ligands in host and tumor cells. American journal of physiology Cell physiology 296, C505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SN, Zhu F, Schnaar RL, Alves CS, and Konstantopoulos K (2008). Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin in shear flow. The Journal of biological chemistry 283, 15647–15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran R, Myers DR, Denning G, Shields JE, Lytle AM, Alrowais H, Qiu Y, Sakurai Y, Li WC, Brand O, et al. (2017). Microfluidic Transduction Harnesses Mass Transport Principles to Enhance Gene Transfer Efficiency. Molecular Therapy 25, 2372–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchera M, Aronica A, and Dall’Olio F (2017). Selectin Ligands Sialyl-Lewis a and SialylLewis x in Gastrointestinal Cancers. Biology 6, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D, and Blanks JE (1999). Mechanisms that regulate the function of the selectins and their ligands. Physiological reviews 79, 181–213. [DOI] [PubMed] [Google Scholar]
- Welch J, and Donaldson G (1979). The clinical correlation of an autopsy study of recurrent colorectal cancer. Annals of surgery 189, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiling L, Wei-Q L., Ilias O, and Wen W (2014). Converging Parallel Plate Flow Chambers for Studies on the Effect of the Spatial Gradient of Wall Shear Stress on Endothelial Cells. Journal of Biosciences and Medicines Vol.02 No.02, 7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


