Abstract
Concurrent advances in information technology infrastructure and mobile computing power in many low and middle-income countries (LMICs) have raised hopes that artificial intelligence (AI) might help to address challenges unique to the field of global health and accelerate achievement of the health-related sustainable development goals. A series of fundamental questions have been raised about AI-driven health interventions, and whether the tools, methods, and protections traditionally used to make ethical and evidence-based decisions about new technologies can be applied to AI. Deployment of AI has already begun for a broad range of health issues common to LMICs, with interventions focused primarily on communicable diseases, including tuberculosis and malaria. Types of AI vary, but most use some form of machine learning or signal processing. Several types of machine learning methods are frequently used together, as is machine learning with other approaches, most often signal processing. AI-driven health interventions fit into four categories relevant to global health researchers: (1) diagnosis, (2) patient morbidity or mortality risk assessment, (3) disease outbreak prediction and surveillance, and (4) health policy and planning. However, much of the AI-driven intervention research in global health does not describe ethical, regulatory, or practical considerations required for widespread use or deployment at scale. Despite the field remaining nascent, AI-driven health interventions could lead to improved health outcomes in LMICs. Although some challenges of developing and deploying these interventions might not be unique to these settings, the global health community will need to work quickly to establish guidelines for development, testing, and use, and develop a user-driven research agenda to facilitate equitable and ethical use.
Introduction
AI is changing how health services are delivered in many high-income settings, particularly in specialty care (eg, radiology and pathology).1, 2, 3 This development has been facilitated by the growing availability of large datasets and novel analytical methods that rely on such datasets. Concurrent advances in information technology (IT) infrastructure and mobile computing power have raised hopes that AI might also provide opportunities to address health challenges in LMICs.4 These challenges, including acute health workforce shortages and weak public health surveillance systems, undermine global progress towards achieving the health-related sustainable development goals (SDGs).5, 6 Although not unique to such countries, these challenges are particularly relevant given their contribution to morbidity and mortality.7, 8
AI-driven health technologies could be used to address many of these and other system-related challenges.4 For example, in some settings, AI-driven interventions have supplemented clinical decision making towards reducing the workload of health workers.9 New developments in AI have also helped to identify disease outbreaks earlier than traditional approaches, thereby supporting more timely programme planning and policy making.10 Although these interventions provide promise, there remain several ethical, regulatory, and practical issues that require guidance before scale-up or widespread deployment in low and middle-income settings.4
The global health community, including several large donor agencies, has increasingly recognised the urgency of addressing these issues towards ensuring that populations in low and middle-income settings benefit from developments in digital health and AI.11 Several global meetings have taken place since 2015.12, 13, 14 For example, in May, 2018, the World Health Assembly adopted a resolution on digital technologies for universal health coverage.15 In 2019, the United Nations Secretary General's High-Level Panel on Digital Cooperation recommended that “by 2030, every adult should have affordable access to digital networks, as well as digitally-enabled financial and health services, as a means to make a substantial contribution to achieving the SDGs”.16 In October, 2019, The Lancet and Financial Times inaugurated a joint Commission focused on the convergence of digital health, AI, and universal health coverage.17 A report from this Commission is expected in 2021.
In the context of these efforts to achieve the health-related SDGs and ensure universal health coverage, we aim to assess current AI research related to health in LMICs. We identified the types of health issues being addressed by AI, types of AI used in these interventions (eg, machine learning, natural language processing, signal processing), and whether there is sufficient evidence that such interventions could improve health outcomes in LMICs. In this Review we aim to highlight additional research requirements, inform national and global policy discussions, and support efforts to develop a research and implementation agenda for AI in global low-income and middle-income countries.
Current research on AI in LMICs
A full list of studies included in this narrative Review is provided in the appendix (pp 8–11). AI interventions focus on a broad range of health issues common to LMICs. Most AI studies focused on communicable diseases, including tuberculosis, malaria, dengue, and other infectious diseases. Other AI studies focused on non-infectious diseases in children and infants, preterm birth complications, and malnutrition. Some interventions aimed to address non-communicable diseases, including cervical cancer. AI studies in LMICs addressed public health from a broader perspective, particularly, health policy and management. These studies include AI research aimed at improving the performance of health facilities, improving resource allocation from a systems perspective, reducing traffic-related injuries, and other health system issues.
The types of AI deployed in health research in LMICs are described in the table . Most AI-driven health interventions used some form of machine leaning or signal processing, or both. Studies often evaluated the use of machine learning together with other AI approaches, most often with signal processing. In addition, several types of machine learning methods were frequently used together. For example, a common approach used in machine learning and signal processing was the use of convolutional neural networks for feature extraction, and support-vector machines for classification. A few research studies assessed interventions based on natural language processing, data mining, expert systems, or advanced planning.
Table.
Public health functions and associated types of AI
| Types of AI* | Example | |
|---|---|---|
| Diagnosis | Expert system; machine learning; natural language processing; signal processing | Researchers applied machine learning and signal processing methods to digital chest radiographs to identify tuberculosis cases18 and drug-resistant tuberculosis cases19 |
| Mortality and morbidity risk assessment | Data mining; machine learning; signal processing | To quantify the risk of dengue fever severity, researchers applied machine learning algorithms to administrative datasets from a large tertiary care hospital in Thailand20 |
| Disease outbreak prediction and surveillance | Data mining; machine learning; natural language processing; signal processing | Remote sensing data and machine learning algorithms were used to characterise and predict the transmission patterns of Zika virus globally21 |
| Health policy and planning | Expert planning; machine learning | Machine learning models were applied to administrative data from South Africa to predict length of stay among health-care workers in underserved communities22 |
AI=artificial intelligence.
Many types AI were implemented together.
AI-driven interventions for health
AI-driven health interventions broadly fit into four categories described in the table. The automation or support of diagnosis for communicable and non-communicable diseases emerged from studies as one of the main uses of AI. Signal processing methods are often used together with machine learning to automate the diagnosis of communicable diseases. Signal processing interventions focused specifically on the use of radiological data for tuberculosis18, 23 and drug-resistant tuberculosis,19 ultrasound data for pneumonia,24 microscopy data for malaria,25, 26, 27 and other biological sources of data for tuberculosis.28, 29, 30 Most diagnostic interventions using AI in LMICs reported either high sensitivity, specificity, or high accuracy (>85% for all), or non-inferiority to comparator diagnostic tools. Machine learning aids clinicians in diagnosing tuberculosis,31 and expert systems are used for diagnosing tuberculosis32 and malaria.27 Studies mostly reported high diagnostic sensitivity, specificity, and accuracy; however, at least one study reported low accuracy when attempting to identify asymptomatic cases of malaria.27
AI-driven interventions also focused on the diagnosis of non-communicable diseases in LMICs, primarily using signal processing methods for disease detection, including cervical cancer and pre-cervical cancer using microscopy,33, 34, 35, 36 or data from photos of the cervix called cervigrams.37 The accuracy has been reported to be greater than 90%. One study aimed to evaluate a low-cost, point-of-care oral cancer screening tool using cloud-based signal processing and reported high sensitivity and specificity relative to that of an onsite specialist.38
Morbidity and mortality risk assessment is another area for which AI driven interventions have been assessed in the global health context. These interventions are based largely on machine learning classification tools and typically compare multiple machine learning approaches with the aim of identifying the optimal approach to characterise risk. This approach has also been used at health facilities to predict disease severity in patients with dengue fever20 and malaria,39 and children with acute infections.40 Researchers have used this approach to quantify the risk of tuberculosis treatment failure41 and assess the risk of cognitive sequelae after malaria infection in children.42
Machine learning classification tools were also used to estimate the risk of non-infectious disease health outcomes. For example, studies have focused on estimating anaemia risk in children using standardised household survey data,43 identifying children with the greatest risk of missing immunisation sessions,44 and detecting high-risk births using cardiotocography data.45 A study from Brazil aimed to assess the behavioural risk classification of sexually active teenagers.46 The reported accuracy of these tools ranged from moderate (approximately 65%) to high (almost 99%).
Signal processing and machine learning have also been used to estimate perinatal risk factors—eg, to automatically estimate gestational age using data from ultrasound images and other patient variables.47, 48, 49 Studies reported high accuracy (>85%) relative to trained experts and other standard gestational age estimation techniques.
Researchers are using AI for public health surveillance to predict disease outbreak and evaluate disease surveillance tools. Researchers have evaluated prediction models using machine learning algorithms and remote (ie, data collected by satellite or aircraft sensors) or local (ie, data measured on site such as rainfall) sensing data to estimate outbreaks of dengue virus. Although one study reported high sensitivity and specificity for identifying dengue outbreaks using a data-driven epidemiological prediction method,50 other researchers51 found that machine learning approaches for predicting dengue outbreaks outperformed approaches based on linear regression. Researchers have also used remote sensing data and machine learning methods to predict malaria52, 53 and Zika virus21 outbreaks with accuracy greater than 85%.
Another common approach to disease prediction and surveillance is the use of machine learning and data mining, together with data from online social media networks and search engines. One study used this approach to predict dengue outbreaks54 and other studies to track and predict influenza outbreaks.55, 56 All studies reported high accuracy compared with observed data. Social media data and machine learning using artificial neural networks were also used to improve surveillance of HIV in China.57
AI-driven health interventions can also be used to support programme policy and planning. One such study used data from a health facility in Brazil and an agent-based simulation model to compare programme options aimed at increasing the overall efficiency of the health workforce.58 In another study, researchers used several government datasets—including health system, environmental, and financial data—together with machine learning (ie, artificial neural networks) to optimise the allocation of health system resources by geography based on an array of prevalent health challenges.59 Expert planning methods and household survey data to optimise community health-worker visit schedules were reported in the literature; however, no results have yet been published.60
Additionally, AI methods aimed at informing programme planning efforts within facilities have been evaluated in low and middle-income settings. Some examples include forecasting the number of outpatient visits at an urban hospital61 and the length of health -worker retention,22 using machine learning methods and large administrative datasets from health facilities. In another example, researchers used expert systems and administrative data to design a system for measuring the performance of hospital managers.62
Researchers are also using machine learning and data mining methods to improve road safety in LMICs. In one study, researchers used street imagery available online and machine learning to estimate helmet use prevalence.63 In another study, a large government dataset of road injuries and data mining techniques were used to predict road injury severity.64
Accelerating access to AI
Numerous data are available to show how AI is being tested to address health challenges relevant to the achievement of SDGs. Such interventions include disease-specific applications and those aimed at strengthening health systems. Many AI health interventions have shown promising preliminary results, and could soon be used to augment existing strategies for delivering health services in LMICs. Especially in disease diagnosis, where AI-powered interventions could be used in countries with insufficient numbers of health providers, and in risk assessment, where tools based largely on machine learning could help to supplement clinical knowledge.9
Although the research identified in this Review indicates that AI-driven health interventions can help to address several existing and emerging health challenges, many issues are not sufficiently described in these studies and warrant further exploration. These issues relate to the development of AI-driven health interventions; how efficacy and effectiveness are assessed and reported; planning for deployment at scale; and the ethical, regulatory, and economic standards and guidelines that will help to protect the interests of communities in LMICs. Although these issues have been described elsewhere,4, 11, 65, 66, 67 they have not been systematically or explicitly addressed in research published to date. We highlight these areas and suggest a framework for consideration in future development, testing, and deployment.
From development to deployment
One of the most important challenges facing AI in LMICs relates to appropriate development and design. Although none of the articles we reviewed here have explained the impetus for project development, there are most likely multiple reasons that explain why particular health challenges in LMICs have been targeted by AI developers. Communicable diseases—including malaria and tuberculosis—continue to account for a pronounced burden of disease in LMICs5 and attract substantial donor funding.68 In addition, the characteristics of some common health challenges in LMICs are able to be addressed by AI—eg, the use of ultrasound data to diagnose respiratory diseases and identify preterm birth risk factors. The availability and portability of digital ultrasound units and large datasets that can be used to train AI algorithms (including in high-income settings), have contributed to the development and testing of such interventions in LMICs.
Although interventions such as those identified in this Review might be beneficial, it is important that the research agenda and development of interventions is driven by local needs, health system constraints, and disease burden rather than availability of data and funding. A global research agenda for AI interventions relevant to LMICs would help to ensure that new tools are developed to respond to population needs. Step should also be taken during the development of AI applications to avoid ethnic, socioeconomic, and gender biases found in some AI applications.
Another major challenge relates to comparative performance of algorithms—including benchmarking against any current standard care—and for continuously assessing performance after deployment. Although processes to enable benchmarking and assessment have begun, including a collaboration between WHO and the UN International Telecommunications Union (ITU),12, 69 this type of testing will require adequate and representative datasets from observational and surveillance studies, electronic medical records, and social media platforms. Open access to diverse datasets representing different populations is particularly important, considering that most AI-driven health interventions from the research literature we identified are based on machine learning. Enabling access across borders will require new types of data sharing protocols and standards on inter-operability and data labelling. This global movement could be facilitated by an international collaboration so that data are rapidly and equitably available for the development and testing of AI-driven health interventions. Such collaborations are already being developed in the UK by initiatives such as the Health Data Research Alliance70 and the Confederation of Laboratories for Artificial Intelligence Research in Europe.71
Reporting and methodological standards are also required for AI health interventions in LMICs, particularly those used for diagnostic tools. Although the epidemiological and statistical methods used in studies that we identified seem largely appropriate for the research questions addressed, results were not reported consistently. For example, some studies assessing diagnostic tools provide estimates of sensitivity, specificity, and overall accuracy—ie, the probability of an individual being correctly identified by a diagnostic test, which is mathematically equivalent to a weighted average of the sensitivity and specificity of the test. However, other studies provided only a subset of these measurements. The use of comparators was also inconsistently reported. The Standards for Reporting of Diagnostic Accuracy Studies72 provide guidelines for diagnostic assessments and could be a starting place for standardising of research in AI diagnostics.
None of the reviewed studies described whether health technology assessments for an AI-driven health intervention had been done. Standardised methods for these assessments, including the extent to which these interventions add value over current standards of care, are urgently needed. Such methods should show how well AI tools work outside study settings and highlight related health system costs, including unintended clinical, psychological, and social consequences. The costs associated with false positive and false negative results are also important to assess.
Although many studies reviewed here used statistical methods that follow classic epidemiology methods, basing their hypotheses on plausible models of causality, some new AI-driven health interventions—particularly those applying machine learning algorithms—identify disease patterns and associations without a priori hypotheses. Such approaches hold promise because they are not necessarily affected by developer-introduced bias. However, there remains a threat that false associations could be identified and integrated into new AI-driven health interventions.
The successful deployment of many AI-driven health interventions will require investment to strengthen the underlying health system. In addition to ethical concerns related to diagnosing disease when treatment is not available, the effectiveness of new diagnostic tools will be limited if access to treatment is not expanded for all patients. Similarly, tools that aim to predict outbreaks and supplement surveillance would need to be supported and complemented by robust surveillance systems to guide an adequate public health emergency response if an outbreak is accurately predicted.
Recommendations
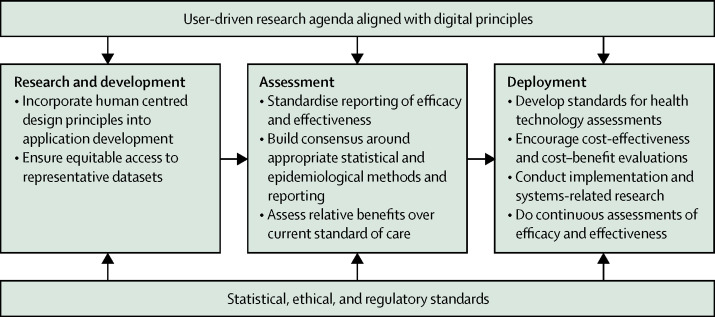
Given the nascent stage of research on AI health interventions in LMICs, global standards and guidelines are needed to inform the development and evaluate performance of tools in these settings. To support such efforts, we provide several recommendations for research and development of AI-driven health interventions in low and middle-income settings using the AI application value chain (figure ).
Figure.
Recommendations for development of artificial intelligence driven health applications in low and middle-income countries
Throughout the development and deployment phases, we propose that researchers consider the principles for digital development (panel ).13 These principles provide guidance on the best practice for development of digital health technologies. Although none of the studies reviewed here explicitly acknowledge digital principles, we believe that they are helpful for development of AI-driven health technologies. However, the digital principles alone are insufficient. Institutional structures also have an important role to play in the development and deployment of new health technologies. Such structures include appropriate regulatory and ethical frameworks, benchmarking standards, pre-qualification mechanisms, guidance on clinical and cost-effective approaches, and frameworks for issues related to data protection, in particular for children and youth, many of whom now have a digital presence from birth. The impact of AI tools on gender issues is another important consideration and an area in which global guidance is currently lacking.
Panel. Digital principles for artificial intelligence driven interventions in global health.
-
•
User-centred design starts with getting to know the people you are designing for by conversation, observation, and co-creation
-
•
Well designed initiatives and digital tools consider the particular structures and needs that exist in each country, region, and community
-
•
Achieving a larger scale requires adoption beyond a pilot population and often necessitates securing funding or partners that take the initiative to new communities and regions
-
•
Building sustainable programmes, platforms, and digital tools is essential to maintain user and stakeholder support, and to maximise long-term effect
-
•
When an initiative is data driven, quality information is available to the right people when they need it, and those people will use data to act
-
•
An open approach to digital development can help to increase collaboration in the digital development community and avoid duplicating work that has already been done
-
•
Reusing and improving is about taking the work of the global development community further than any organisation or programme can do alone
-
•
Addressing privacy and security in digital development involves careful consideration of which data are collected and how data are acquired, used, stored, and shared
-
•
Being collaborative means sharing information, insights, strategies, and resources across projects, organisations, and sectors, leading to increased efficiency and effect
AI does not need to be held to a higher standard of research; however, its unique complexities, including the requisite use of large datasets and the opaque nature of some AI algorithms, will require approaches specifically tailored to interventions and consideration of how efficacy and effectiveness are assessed. Guidelines, such as those from the EQUATOR network including the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis—statement specific to Machine Learning (TRIPOD-ML), Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT)-AI, and Consolidated Standards of Reporting Trials (CONSORT)-AI, that aim to harmonise terminologies and reporting standards in prediction research,66 might help to guide researchers as they design and assess AI interventions. Agencies in high-income countries, including the US Food and Drug Administration, have begun to develop separate regulatory pathways for AI-driven health intereventions.67 In addition to the UN ITU benchmarking initiative, WHO has recently created a new digital health department and released new guidelines on digital health.73 These efforts can help to provide valuable insight for LMICs.
Current AI research highlights additional areas for strengthening standards and guidelines for AI research in LMICs. Although most AI investigators report necessary approvals by institutional review boards, indicating that the studies were all done ethically, only a few described how the research teams addressed issues of informed consent or ethical research design in tools that used large datasets and electronic health records. Reporting on ethical considerations would help future researchers to address these complex yet essential issues.
Similarly, only a few studies reported on the usability or acceptability of AI tools from the provider or patients' perspective, despite acknowledging that usability is an important factor for AI interventions, particularly in LMICs. Human-centred design, an approach to programme and product development frequently cited in technology literature, considers human factors to ensure that interactive systems are more usable. Human-centered design is acknowledged as an important factor for the development of new technologies in LMICs.65
There was also an absence of randomised clinical trials (RCTs) identified in the literature. Clinical trials help to establish clinical efficacy in LMICs. Given the challenges associated with conducting RCTs for new health technologies,74 new approaches such as the Idea, Development, Exploration, Assessment, and Long Term (IDEAL) follow-up framework75 recommended for the evaluation of novel surgical practices, could serve to provide relevant learning. This framework provides guidance on clinical assessment for surgical interventions, in the context of challenges that make clinical trials difficult, including variation in setting, disparities in quality, and subjective interpretation.
There were only a few references to any type of implementation research to assess questions related to adoption or deployment at scale. Assessing implementation-related factors could help to identify potential unintended consequences at an individual and system level of AI interventions. Further, there was no description of the costs related to patients, providers, or systems. A thorough assessment of these costs is crucial to inform cost-effectiveness analyses and the potential for scalability.
Limitations and conclusions
First, relevant articles might have been published before 2010. However, The field of AI, particularly in global health, is rapidly evolving and any articles that were not included as a result of being published before 2010 are unlikely to be representative of this field as it is today. In addition, our Review included only English-language articles. Given the prominence of AI research around the world, excluding articles published in languages other than English could be a limitation.
As with all reviews, publication bias is another potential limitation. There are two probable sources of this bias in AI research. First, studies with null results are less likely to be published.76 For that reason, AI-driven health interventions that have not shown statistically significant results might be under-represented in our literature Review. Furthermore, investments in AI and health were forecasted to have reached US$1·7 billion in 2018,77 and are increasingly dominated by private equity firms78 and driven by so-called big tech companies such as Google and Baidu ventures.79 Given that many interventions are developed in the private sector for commercial use, some AI developers might not place a high priority on publishing the results in academic literature.80
AI is already being developed to address health issues in LMICs. Current research is addressing a range of health issues and using various AI-driven health interventions. The breadth and promising results of these interventions emphasise the urgency for the global community to act and create guidance to facilitate deployment of effective interventions. This point is particularly crucial given the rapid deployment of AI-driven health interventions which are being rolled out at scale as part of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic response. In many cases this roll-out is being carried out without adequate evidence or appropriate safeguards.
In accordance with our recommendations, the global health community will need to work quickly to: incorporate aspects of human-centred design into the development process, including starting from a needs-based rather than a tool-based approach; ensure rapid and equitable access to representative datasets; establish global systems for assessing and reporting efficacy and effectiveness of AI-driven interventions in global health; develop a research agenda that includes implementation and system related questions on the deployment of new AI-driven interventions; and develop and implement global regulatory, economic, and ethical standards and guidelines that safeguard the interests of LMICs. These recommendations will ensure that AI helps to improve health in low and middle-income settings and contributes to the achievement of the SDGs, universal health coverage, and to the coronavirus disease 2019 (COVID-19) response.
Search strategy and selection criteria
We reviewed PubMed, MEDLINE, and Google Scholar. This Review included peer-reviewed research articles published in English between Jan 1, 2010, and Dec 31, 2019. Relevant articles were identified using search terms that included low and middle-income country names (appendix pp 2–7) and “artificial intelligence”, “augmented intelligence”, “computational intelligence”, and “machine learning”. The titles and abstracts of identified articles were initially reviewed by a study reviewer to assess whether the study was done in a low-income or middle-income country, according to the World Bank Atlas country classification method, and focused on health or health system challenges that could be addressed with artificial intelligence (AI) interventions. We synthesised key themes and trends, using a previously described classification for AI-driven health interventions (ie, expert systems, machine learning, natural language processing, automated planning and scheduling, and image and signal processing) and broad categories of health interventions (ie, diagnosis, risk assessment, disease outbreak prediction and surveillance, and health policy and planning). We excluded studies done in LMICs where AI might have been used to develop a drug or diagnostic, but was not a central component of the final health tool being studied.
Acknowledgments
Acknowledgments
Fondation Botnar funded the data collection and supported an initial synthesis of the literature which provided the basis for this Review. The funder had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication. All authors had full access to all the data used in the study and the corresponding author had final responsibility for the decision to submit for publication.
Contributors
NS and BW are joint first authors. NS and BW reviewed the literature and wrote the manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts HJWL. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500–510. doi: 10.1038/s41568-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang HY, Jung CK, Woo JI. Artificial intelligence in pathology. J Pathol Transl Med. 2019;53:1–12. doi: 10.4132/jptm.2018.12.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jha S, Topol EJ. Adapting to artificial intelligence: radiologists and pathologists as information specialists. JAMA. 2016;316:2353–2354. doi: 10.1001/jama.2016.17438. [DOI] [PubMed] [Google Scholar]
- 4.Wahl B, Cossy-Gantner A, Germann S, Schwalbe NR. Artificial intelligence (AI) and global health: how can AI contribute to health in resource-poor settings? BMJ Glob Health. 2018;3 doi: 10.1136/bmjgh-2018-000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lozano R, Fullman N, Abate D. Measuring progress from 1990 to 2017 and projecting attainment to 2030 of the health-related Sustainable Development Goals for 195 countries and territories: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:2091–2138. doi: 10.1016/S0140-6736(18)32281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartolomeos KK. The case for investing in public health surveillance in low- and middle-income countries. Afr J Emerg Med. 2018;8:127–128. doi: 10.1016/j.afjem.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alkire BC, Peters AW, Shrime MG, Meara JG. The economic consequences of mortality amenable to high-quality health care in low- and middle-income countries. Health Aff (Millwood) 2018;37:988–996. doi: 10.1377/hlthaff.2017.1233. [DOI] [PubMed] [Google Scholar]
- 8.Kruk ME, Gage AD, Joseph NT, Danaei G, García-Saisó S, Salomon JA. Mortality due to low-quality health systems in the universal health coverage era: a systematic analysis of amenable deaths in 137 countries. Lancet. 2018;392:2203–2212. doi: 10.1016/S0140-6736(18)31668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo J, Li B. The application of medical artificial intelligence technology in rural areas of developing countries. Health Equity. 2018;2:174–181. doi: 10.1089/heq.2018.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lake IR, Colón-González FJ, Barker GC, Morbey RA, Smith GE, Elliot AJ. Machine learning to refine decision making within a syndromic surveillance service. BMC Public Health. 2019;19:559. doi: 10.1186/s12889-019-6916-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.USAID Center for Innovation and Impact Artificial intelligence in global health: defining a collective path forward. 2019. https://www.usaid.gov/sites/default/files/documents/1864/AI-in-Global-Health_webFinal_508.pdf
- 12.International Telecommunication Union AI for good global summit. 2017. https://www.itu.int/en/ITU-T/AI/Pages/201706-default.aspx
- 13.Digital Principles Principles for digital development. 2017. https://digitalprinciples.org/
- 14.Digital Investment Principles The principles of donor alignment for digital health. 2018. https://digitalinvestmentprinciples.org/
- 15.WHO Seventy-first world health assembly. Digital health. 2018. https://apps.who.int/gb/ebwha/pdf_files/WHA71/A71_R7-en.pdf
- 16.UN Secretary-General's High-level Panel on Digital Cooperation The age of digital interdependence. 2018. https://www.un.org/en/pdfs/DigitalCooperation-report-for%20web.pdf
- 17.Kickbusch I, Agrawal A, Jack A, Lee N, Horton R. Governing health futures 2030: growing up in a digital world-a joint The Lancet and Financial Times Commission. Lancet. 2019;394 doi: 10.1016/S0140-6736(19)32181-6. [DOI] [PubMed] [Google Scholar]
- 18.Lopes UK, Valiati JF. Pre-trained convolutional neural networks as feature extractors for tuberculosis detection. Comput Biol Med. 2017;89:135–143. doi: 10.1016/j.compbiomed.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Jaeger S, Juarez-Espinosa OH, Candemir S. Detecting drug-resistant tuberculosis in chest radiographs. Int J CARS. 2018;13:1915–1925. doi: 10.1007/s11548-018-1857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phakhounthong K, Chaovalit P, Jittamala P. Predicting the severity of dengue fever in children on admission based on clinical features and laboratory indicators: application of classification tree analysis. BMC Pediatr. 2018;18:109. doi: 10.1186/s12887-018-1078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang D, Hao M, Ding F, Fu J, Li M. Mapping the transmission risk of Zika virus using machine learning models. Acta Trop. 2018;185:391–399. doi: 10.1016/j.actatropica.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Moyo S, Doan TN, Yun JA, Tshuma N. Application of machine learning models in predicting length of stay among healthcare workers in underserved communities in South Africa. Hum Resour Health. 2018;16:68. doi: 10.1186/s12960-018-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aguiar FS, Torres RC, Pinto JV, Kritski AL, Seixas JM, Mello FC. Development of two artificial neural network models to support the diagnosis of pulmonary tuberculosis in hospitalized patients in Rio de Janeiro, Brazil. Med Biol Eng Comput. 2016;54:1751–1759. doi: 10.1007/s11517-016-1465-1. [DOI] [PubMed] [Google Scholar]
- 24.Correa M, Zimic M, Barrientos F. Automatic classification of pediatric pneumonia based on lung ultrasound pattern recognition. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Go T, Kim JH, Byeon H, Lee SJ. Machine learning-based in-line holographic sensing of unstained malaria-infected red blood cells. J Biophotonics. 2018;11 doi: 10.1002/jbio.201800101. [DOI] [PubMed] [Google Scholar]
- 26.Torres K, Bachman CM, Delahunt CB. Automated microscopy for routine malaria diagnosis: a field comparison on Giemsa-stained blood films in Peru. Malar J. 2018;17:339. doi: 10.1186/s12936-018-2493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrade BB, Reis-Filho A, Barros AM. Towards a precise test for malaria diagnosis in the Brazilian Amazon: comparison among field microscopy, a rapid diagnostic test, nested PCR, and a computational expert system based on artificial neural networks. Malar J. 2010;9:117. doi: 10.1186/1475-2875-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan S, Ullah R, Shahzad S, Anbreen N, Bilal M, Khan A. Analysis of tuberculosis disease through Raman spectroscopy and machine learning. Photodiagn Photodyn Ther. 2018;24:286–291. doi: 10.1016/j.pdpdt.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Mohamed EI, Mohamed MA, Moustafa MH. Qualitative analysis of biological tuberculosis samples by an electronic nose-based artificial neural network. Int J Tuberc Lung Dis. 2017;21:810–817. doi: 10.5588/ijtld.16.0677. [DOI] [PubMed] [Google Scholar]
- 30.Kuok CP, Horng MH, Liao YM, Chow NH, Sun YN. An effective and accurate identification system of Mycobacterium tuberculosis using convolution neural networks. Microsc Res Tech. 2019;82:709–719. doi: 10.1002/jemt.23217. [DOI] [PubMed] [Google Scholar]
- 31.Elveren E, Yumuşak N. Tuberculosis disease diagnosis using artificial neural network trained with genetic algorithm. J Med Syst. 2011;35:329–332. doi: 10.1007/s10916-009-9369-3. [DOI] [PubMed] [Google Scholar]
- 32.Osamor VC, Azeta AA, Ajulo OO. Tuberculosis-Diagnostic Expert System: an architecture for translating patients information from the web for use in tuberculosis diagnosis. Health Informatics J. 2014;20:275–287. doi: 10.1177/1460458213493197. [DOI] [PubMed] [Google Scholar]
- 33.Zhao M, Wu A, Song J, Sun X, Dong N. Automatic screening of cervical cells using block image processing. Biomed Eng Online. 2016;15:14. doi: 10.1186/s12938-016-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chankong T, Theera-Umpon N, Auephanwiriyakul S. Automatic cervical cell segmentation and classification in pap smears. Comput Methods Programs Biomed. 2014;113:539–556. doi: 10.1016/j.cmpb.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Kumar R, Srivastava R, Srivastava S. Detection and classification of cancer from microscopic biopsy images using clinically significant and biologically interpretable features. J Med Eng. 2015;2015 doi: 10.1155/2015/457906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su J, Xu X, He Y, Song J. Automatic detection of cervical cancer cells by a two-level cascade classification system. Anal Cell Pathol (Amst) 2016;2016 doi: 10.1155/2016/9535027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu L, Bell D, Antani S. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;111:923–932. doi: 10.1093/jnci/djy225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uthoff RD, Song B, Sunny S. Point-of-care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston IG, Hoffmann T, Greenbury SF. Precision identification of high-risk phenotypes and progression pathways in severe malaria without requiring longitudinal data. NPJ Digit Med. 2019;2:63. doi: 10.1038/s41746-019-0140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwizera A, Kissoon N, Musa N. A machine learning-based triage tool for children with acute infection in a low resource setting. Pediatr Crit Care Med. 2019;20:e524–e530. doi: 10.1097/PCC.0000000000002121. [DOI] [PubMed] [Google Scholar]
- 41.Hussain OA, Junejo KN. Predicting treatment outcome of drug-susceptible tuberculosis patients using machine-learning models. Inform Health Soc Care. 2019;44:135–151. doi: 10.1080/17538157.2018.1433676. [DOI] [PubMed] [Google Scholar]
- 42.Veretennikova MA, Sikorskii A, Boivin MJ. Parameters of stochastic models for electroencephalogram data as biomarkers for child's neurodevelopment after cerebral malaria. J Stat Distrib Appl. 2018;5:8. doi: 10.1186/s40488-018-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meena K, Tayal DK, Gupta V, Fatima A. Using classification techniques for statistical analysis of Anemia. Artif Intell Med. 2019;94:138–152. doi: 10.1016/j.artmed.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Chandir S, Siddiqi DA, Hussain OA. Using predictive analytics to identify children at high risk of defaulting from a routine immunization program: feasibility study. JMIR Public Health Surveill. 2018;4:e63. doi: 10.2196/publichealth.9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoodbhoy Z, Noman M, Shafique A, Nasim A, Chowdhury D, Hasan B. Use of machine learning algorithms for prediction of fetal risk using cardiotocographic data. Int J Appl Basic Med Res. 2019;9:226–230. doi: 10.4103/ijabmr.IJABMR_370_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waleska Simões P, Cesconetto S, Toniazzo de Abreu LL. A data mining approach to identify sexuality patterns in a Brazilian university population. Stud Health Technol Inform. 2015;216 [PubMed] [Google Scholar]
- 47.van den Heuvel TLA, Petros H, Santini S, de Korte CL, van Ginneken B. Automated fetal head detection and circumference estimation from free-hand ultrasound sweeps using deep learning in resource-limited countries. Ultrasound Med Biol. 2019;45:773–785. doi: 10.1016/j.ultrasmedbio.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Rittenhouse KJ, Vwalika B, Keil A. Improving preterm newborn identification in low-resource settings with machine learning. PLoS One. 2019;14 doi: 10.1371/journal.pone.0198919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papageorghiou AT, Kemp B, Stones W. Ultrasound-based gestational-age estimation in late pregnancy. Ultrasound Obstet Gynecol. 2016;48:719–726. doi: 10.1002/uog.15894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buczak AL, Koshute PT, Babin SM, Feighner BH, Lewis SH. A data-driven epidemiological prediction method for dengue outbreaks using local and remote sensing data. BMC Med Inform Decis Mak. 2012;12:124. doi: 10.1186/1472-6947-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scavuzzo JM, Trucco F, Espinosa M. Modeling Dengue vector population using remotely sensed data and machine learning. Acta Trop. 2018;185:167–175. doi: 10.1016/j.actatropica.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Haddawy P, Hasan AHMI, Kasantikul R. Spatiotemporal Bayesian networks for malaria prediction. Artif Intell Med. 2018;84:127–138. doi: 10.1016/j.artmed.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Kabaria CW, Molteni F, Mandike R. Mapping intra-urban malaria risk using high resolution satellite imagery: a case study of Dar es Salaam. Int J Health Geogr. 2016;15:26. doi: 10.1186/s12942-016-0051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Althouse BM, Ng YY, Cummings DA. Prediction of dengue incidence using search query surveillance. PLoS Negl Trop Dis. 2011;5 doi: 10.1371/journal.pntd.0001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Q, Gel YR, Ramirez Ramirez LL, Nezafati K, Zhang Q, Tsui KL. Forecasting influenza in Hong Kong with Google search queries and statistical model fusion. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clemente L, Lu F, Santillana M. Improved real-time influenza surveillance: using internet search data in eight Latin American countries. JMIR Public Health Surveill. 2019;5 doi: 10.2196/12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nan Y, Gao Y. A machine learning method to monitor China's AIDS epidemics with data from Baidu trends. PLoS One. 2018;13 doi: 10.1371/journal.pone.0199697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yousefi M, Yousefi M, Ferreira RPM, Kim JH, Fogliatto FS. Chaotic genetic algorithm and Adaboost ensemble metamodeling approach for optimum resource planning in emergency departments. Artif Intell Med. 2018;84:23–33. doi: 10.1016/j.artmed.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Rosas MA, Bezerra AF, Duarte-Neto PJ. Use of artificial neural networks in applying methodology for allocating health resources. Rev Saude Publica. 2013;47:128–136. doi: 10.1590/s0034-89102013000100017. [DOI] [PubMed] [Google Scholar]
- 60.Brunskill E, Lesh N. Routing for rural health: optimizing community health worker visit schedules. Association for the Advancement of Artificial Intelligence. 2010. https://www.cs.cmu.edu/~ebrun/brunskilllesh.pdf
- 61.Huang D, Wu Z. Forecasting outpatient visits using empirical mode decomposition coupled with back-propagation artificial neural networks optimized by particle swarm optimization. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shafii M, Hosseini SM, Arab M, Asgharizadeh E, Farzianpour F. Performance analysis of hospital managers using fuzzy AHP and fuzzy TOPSIS: Iranian experience. Glob J Health Sci. 2015;8:137–155. doi: 10.5539/gjhs.v8n2p137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merali HS, Lin LY, Li Q, Bhalla K. Using street imagery and crowdsourcing internet marketplaces to measure motorcycle helmet use in Bangkok, Thailand. Inj Prev. 2019 doi: 10.1136/injuryprev-2018-043061. published online March 4. [DOI] [PubMed] [Google Scholar]
- 64.Beshah T, Hill S. Mining road traffic accident data to improve safety: role of road-related factors on accident severity in Ethiopia. 2010. AAAI Spring Symposium Series.
- 65.The Lancet Artificial intelligence in global health: a brave new world. Lancet. 2019;393 doi: 10.1016/S0140-6736(19)30814-1. [DOI] [PubMed] [Google Scholar]
- 66.Collins GS, Moons KGM. Reporting of artificial intelligence prediction models. Lancet. 2019;393:1577–1579. doi: 10.1016/S0140-6736(19)30037-6. [DOI] [PubMed] [Google Scholar]
- 67.He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25:30–36. doi: 10.1038/s41591-018-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chapman N, Doubell A, Oversteegen L. Neglected disease research and development: reaching new heights. G-FINDER Report. 2018. https://www.vaccineresources.org/details.php?i=2597
- 69.Wiegand T, Krishnamurthy R, Kuglitsch M. WHO and ITU establish benchmarking process for artificial intelligence in health. Lancet. 2019;394:9–11. doi: 10.1016/S0140-6736(19)30762-7. [DOI] [PubMed] [Google Scholar]
- 70.Health Data Research UK UK health data research alliance. 2019. https://www.hdruk.ac.uk/infrastructure/uk-health-data-research-alliance/
- 71.Confederation of Laboratories for Artificial Intelligence Research in Europe A European vision for AI. https://claire-ai.org/?lang=de
- 72.Cohen JF, Korevaar DA, Altman DG. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.WHO WHO guideline recommendations on digital interventions for health system strengthening. 2019. https://apps.who.int/iris/bitstream/handle/10665/311941/9789241550505-eng.pdf?ua=1 [PubMed]
- 74.Lorenzo C, Garrafa V, Solbakk JH, Vidal S. Hidden risks associated with clinical trials in developing countries. J Med Ethics. 2010;36:111–115. doi: 10.1136/jme.2009.031708. [DOI] [PubMed] [Google Scholar]
- 75.McCulloch P, Altman DG, Campbell WB. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105–1112. doi: 10.1016/S0140-6736(09)61116-8. [DOI] [PubMed] [Google Scholar]
- 76.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 77.Franklin Templeton Artificial intelligence: real opportunity. 2019. https://www.franklintempleton.com/investor/article?contentPath=html/ftthinks/en-us-retail/featured/artificial-intelligence-real-opportunity.html
- 78.OECD Private Equity Investment in Artificial Intelligence. 2018. https://www.oecd.org/going-digital/ai/private-equity-investment-in-artificial-intelligence.pdf
- 79.Hui M, Lahiri T. This Chinese tech giant was 2018's most active corporate investor in AI startups. Quartz. 2019. https://qz.com/1555973/baidu-ventures-was-2018s-most-active-investor-in-ai/
- 80.Breschi S, Lassébie J, Menon C. OECD; 2018. A portrait of innovative start-ups across countries.https://www.oecd-ilibrary.org/docserver/f9ff02f4-en.f?expires=1580483659&id=id&accname=guest&checksum=3BD05CBBFA0446D064FFFA10A059C23D [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.