Abstract
In this position statement, developed by The International College of Obsessive-Compulsive Spectrum Disorders, a group of international experts responds to recent developments in the evidence-based management of obsessive-compulsive disorder (OCD). The article presents those selected therapeutic advances judged to be of utmost relevance to the treatment of OCD, based on new and emerging evidence from clinical and translational science. Areas covered include refinement in the methods of clinical assessment, the importance of early intervention based on new staging models and the need to provide sustained well-being involving effective relapse prevention. The relative benefits of psychological, pharmacological and somatic treatments are reviewed and novel treatment strategies for difficult to treat OCD, including neurostimulation, as well as new areas for research such as problematic internet use, novel digital interventions, immunological therapies, pharmacogenetics and novel forms of psychotherapy are discussed.
Keywords: evidence based, obsessive-compulsive disorder, position statement, treatments
Introduction
Once a neglected illness, obsessive-compulsive disorder (OCD) is now recognized as a common, highly disabling and potentially treatable early-onset brain disorder. Clinical and translational research in OCD grows apace, and over the past 10 years has contributed to substantial advances in understanding of the phenomenology, brain-based biology and treatment response, leading to innovations in nosological conceptualizations, therapeutic interventions and services. Recent changes in the DSM-5 (American Psychiatric Association, 2013) and ICD-11 (WHO, 2018) diagnostic classification systems have set OCD at the head of a new family of obsessive-compulsive spectrum disorders [otherwise known as Obsessive-Compulsive or Related Disorders, or, Obsessive-Compulsive and Related Disorders (OCRDs)], including body dysmorphic, hoarding, hair-pulling, skin picking and olfactory reference disorders and hypochondriasis, all sharing compulsive behaviour as a cardinal characteristic. Serotonin reuptake inhibitors [selective serotonin reuptake inhibitors (SSRIs), clomipramine] or cognitive behavioural therapy (CBT) involving exposure and response prevention (ERP), represent the mainstay of contemporary treatment for OCD, with emerging evidence suggesting that early intervention produces better outcomes (Fineberg et al., 2019). However, a substantial minority of patients still fail to respond either in any meaningful way, or in terms of residual symptoms. Treatment-resistant OCD has become a fruitful research focus for clinical treatment and specialist services development, worldwide.
A number of evidence-based clinical guidelines for managing OCD have been published (Bandelow et al., 2012; Baldwin et al., 2014; Sookman et al., 2015). However, recent feedback from topic experts and stakeholders (National Institute for Health and Care Excellence, 2019) has identified the need for an update, highlighting that clinical practice has progressed in many areas. This includes evidence of efficacy for new pharmacological interventions and augmentation therapies among treatment-resistant groups, advances in invasive and noninvasive neurostimulation technology as well as rapid advances in information technology and telecommunications and the introduction of technology-enhanced interventions. Yet, in many parts of the world, access to recommended treatments and specialist care services, in particular for children, remains limited.
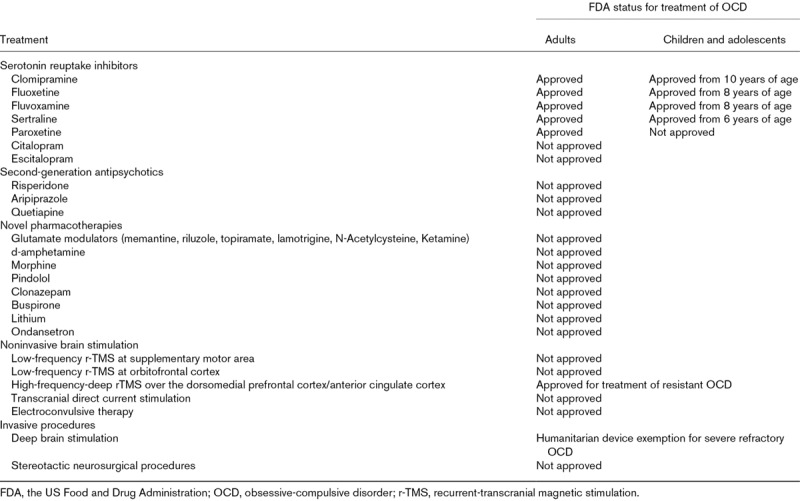
The International College of Obsessive-Compulsive Spectrum Disorders (ICOCS; www.ICOCS.org) is a global network of expert clinicians, researchers and ‘experts by experience of OCD’, whose principal objective is to support and stimulate the study and treatment of obsessive-compulsive spectrum disorders. In recognition of the need for updated clinical guidance on the treatment of OCD, the ICOCS has developed this position statement, based on expert consensus and including a balanced representation of genders, child versus adult psychiatrists and early career scientists, with global and ethnic diversity. Agreement was reached on the key issues to be covered at a series of meetings, and the authors of each section were chosen based on their expertise in that area. An initial draft was prepared, based on a literature review, and circulated first among the authors and then to all ICOCS members and iterative edits were incorporated. In sum, we have selected those recent therapeutic advances judged by a range of experts to be of most relevance to the treatment of OCD, including products that are not licensed or labelled for treatment of OCD by the US Food and Drug Administration (FDA) (Table 1), which are marked with an asterisk (*) throughout the article, based on new and emerging evidence from clinical and translational science
Table 1.
The US Food and Drug Administration status of treatments covered in this article
Global assessment of obsessive-compulsive disorder
A comprehensive assessment of OCD requires trained clinicians who perform direct interviews with the patient and, whenever possible, with family members, so that an accurate diagnosis can be determined and individualized treatment can be tailored. The hallmarks of OCD are obsessions (recurrent, intrusive, unwanted thoughts, images or impulses and compulsions (repititive behaviours or mental acts that the individual feels compelled to perform), these can present together or separately.The most common symptom dimensions of OCD are contamination/washing, aggression/checking, symmetry/ordering/arranging, sexual/religious (also known as ‘taboo thoughts’) and hoarding (Rosario-Campos et al., 2006). Importantly, according to DSM-5, a diagnosis of Hoarding Disorder should be assigned when symptoms pertain to this single dimension (American Psychiatric Association, 2013). The presence and severity of symptoms can be measured by validated instruments (Goodman et al., 1989; Rosario-Campos et al., 2006; Storch et al., 2010), which is relevant to tailoring the behavioural treatment and monitoring treatment response objectively. For the OCD diagnosis, while free-form interviews by clinicians are commonly used, structured interviews offer advantages in terms of objectivity and psychometric properties (Rapp et al., 2016). Suitable interviews for the diagnosis of OCD in adulthood include the Structured Clinical Interview for DSM-5 Disorders (First et al., 2016), or the Mini International Neuropsychiatric Interview (Sheehan et al., 1998). The Yale–Brown Obsessive-Compulsive Scale (Y-BOCS) is the gold-standard for assessing symptom severity in diagnosed adult patients, and incorporates a detailed checklist for individual symptoms (Goodman et al., 1989). For initial screening, six brief questions can be used. These include as follows: (1) Do you wash or clean a lot? (2) Do you check things a lot? (3) Is there any thought that keeps bothering you that you would like to get rid of but can’t? (4) Do your daily activities take a long time to finish? (5) Are you concerned about orderliness or symmetry? (6) Do these problems trouble you? Positive response to one or more statements would indicate a need for more detailed assessment (Fineberg et al., 2008).
Obsessions and compulsions tend to occur concomitantly in the vast majority of people with OCD (Shavitt et al., 2014). In addition, compulsions are commonly preceded not only by obsessions but also by subjective experiences of incompleteness, or ‘not feeling just-right’, or so-called sensory phenomena (perceptual experiences that precede or accompany compulsions) (Shavitt et al., 2014). We could expect these phenomena to be targeted by cognitive-behavioural techniques in a way similar to the premonitory urges in the behavioural treatment of tic disorders (McGuire et al., 2015).
Another relevant clinical feature that merits attention when assessing subjects with OCD is the degree of insight, meaning the extent to which the person recognizes that his/her beliefs are not true (Eisen et al., 1998). Insight (good or fair insight, poor insight, absent insight/delusional beliefs) is a diagnostic specifier for OCD, body dysmorphic disorder (BDD) and hoarding disorder in the DSM-5 (American Psychiatric Association, 2013). In general, subjects with OCD have at least good insight, with only a minority presenting poor insight or delusional OCD (Shavitt et al., 2014). The presence of tic symptomatology represents another clinically relevant diagnostic specifier in the DSM-5, as tic may predict a more favourable response to dopamine antagonist agents (Bloch et al., 2006). Finally, the clinician must obtain information regarding avoidance behaviours, which commonly occurs as a means to handle the distress evoked by the obsessions and constitutes one of the main targets of the cognitive-behavioural treatment for this disorder (Drummond, 2014). Functional impairment varies in OCD. It is an important domain that reflects clinical severity and constitutes an indirect measure of improvement during treatment. Impairment can be measured indirectly with OCD severity scales or with specific measures [e.g., the WHO Disability Assessment Schedule 2.0 (Üstün et al., 2010) or the Cognitive Assessment Instrument of Obsessions and Compulsions (Dittrich et al., 2011)].
Comorbidity is almost always present with OCD and is often ‘phase-specific’ (Pallanti and Grassi, 2014). Assessment of specific comorbidities, like tic disorders, anxiety and depressive disorders, disruptive disorders, eating disorders, autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD) and schizophrenia (Zohar, 1997), is essential in guiding the formulation of an effective treatment strategy. Comorbidity has also been a focus of emerging genetic studies of OCD. For example, a recent study in 4645 OCD patients found different genotypes to be associated with different OCD comorbidities. Thus, OCD comorbid with bipolar disorders was associated with COMT, OPRM1 and GRIK1 genotypes; OCD and depressive disorders were associated with OPRM1 and CYP3A4/5 genotypes; OCD comorbid with ADD/ADHD was associated with 5HT2C genotypes; and OCD comorbid with anxiety was associated with CYP3A4/5 genotypes (Nezgovorova et al., 2018). However, these findings should be viewed with caution, as the ‘candidate gene’ approach, in which specific genes are tested for association with specific disorders, chosen for the biological plausibility of their relationship, using relatively small samples of affected subjects and healthy controls, has been criticized for overestimating statistical associations. Attempts to replicate the findings have tended to produce disappointing results. Therefore, more unbiased forms of the association study, such as genome-wide association studies (GWAS), which test the association between a disease and multiple genetic variants across the whole genome, are to be preferred (Gordon, 2018; National Advisory Mental Health Council Workgroup on Genomics, 2019). A recent meta-analysis of GWAS of eight psychiatric disorders identified a common genetic factor linking OCD, anorexia nervosa and Tourette’s syndrome (Lee et al., 2019b).
Interestingly, comorbid disorders that start before the onset of OCD symptoms seem to influence the occurrence of additional comorbidities over time. In a cohort of 1001 patients with OCD, separation anxiety disorder preceded OCD in 17.5% of individuals and was associated with a higher lifetime frequency of posttraumatic stress disorder; ADHD preceded OCD in 5.0% of subjects, and was associated with higher lifetime frequencies of substance abuse and dependence; tic disorders preceded OCD in 4.4% of subjects and were associated with higher lifetime frequencies of OCD spectrum disorders (de Mathis et al., 2013). In children and adolescents, in addition to the considerations for the adult subjects, a history of paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) should be taken, as this could also have treatment implications (Wilbur et al., 2019). Taken together, these findings emphasize the importance of identifying comorbid disorders, as they may serve as markers of different biological or clinical substrates of potential relevance for treatment planning (see section Future directions for research).
OCD needs to be differentiated from: anxiety disorders presenting with recurrent fears (as in the phobias) and excessive worry (as in generalized anxiety disorder); ruminations accompanying depressive mood in depressive disorders; OCD-related disorders like BDD (where there are specific concerns with one’s appearance), hair-pulling disorder (the only compulsion); tic disorders; eating disorders (concerns focussed on weight and shape and food); psychotic disorders (especially in poor-insight OCD and so-called schizo-obsessive disorder) and obsessive-compulsive personality disorder (with the hallmarks of enduring rigidity and perfectionism over the lifetime) (American Psychiatric Association, 2013).
Along with the identification of the most bothersome symptoms, the clinician should investigate the age of onset of symptoms and the age when a diagnosis of OCD has been determined, because these data can help to predict the prognosis (Fineberg et al., 2019). OCD frequently emerges in childhood, in which group accurate diagnosis is essential for care planning. Paediatric clinicians can ask simple screening questions such as ‘do you ever have unwanted thoughts or worries that won’t go away? Are there things you have to do over and over again, even though you don’t want to or that don’t make sense?’ The formal diagnosis should be made with a structured interview and the nationwide translated versions of the standardized Children’s Y-BOCS (CY-BOCS), which has good reliability (López-Pina et al., 2015a,b).
Awareness of other conditions associated with the onset and course of OCD symptoms can also be of help in treatment planning, because OCD frequently follows a chronic course, with most patients reporting residual symptoms, or present an episodic course with long symptom-free periods (Skoog and Skoog, 1999). For example, a cross-cultural study has shown an association between reproductive cycle events and the onset (mostly menarche) or exacerbation of OCD during the premenstruum, pregnancy, postpartum and menopause (Guglielmi et al., 2014). Relevant to prevention strategies, exacerbation during or after first pregnancy posed a significant risk to exacerbation in or after a subsequent pregnancy. The underlying factors responsible for triggering exacerbation remain to be understood, especially the role of oestrogen and oxytocin (Guglielmi et al., 2014).
Information on the family history of OCD, tics and other psychiatric disorders and the understanding of OCD among family members and family accommodation are also relevant to treatment-planning and adherence. Evidence shows that successful treatment depends on the reduction of the participation of the family members in the patient’s compulsive behaviours (i.e., reduction of accommodation) (Gomes et al., 2017). Moreover, a recent analysis suggested that children with a family history of OCD have a six times lower response to CBT (Garcia et al., 2010).
Suicidality should be included when assessing people with OCD (Dell’Osso et al., 2018). A recent meta-analysis (Angelakis et al., 2015) found that OCD patients showed relatively increased risk of ‘suicidality’, when compared with healthy controls. In terms of absolute risk, estimates vary. Among 582 patients with OCD, 36% reported lifetime suicidal thoughts, 20% had made suicidal plans, 11% had already attempted suicide and 10% presented with current suicidal thoughts (Torres et al., 2011). In another study of 425 outpatients, recruited by the ICOCS network, 14.6% of the sample reported at least one suicide attempt during their lifetime (Dell’Osso et al., 2018). In the study by Torres et al. (2011), comorbid depressive disorder and posttraumatic stress disorder were associated with a range of suicidal behaviours. Sexual/religious symptoms and comorbid substance use disorders were associated with suicidal thoughts and plans, while impulse control disorders were associated with current suicidal thoughts, suicide plans and attempts. In the study of Dell’Osso et al. (2018), comorbid tic disorders as well as medical disorders and a previous history of hospitalization were also associated with increased suicidality.
Neuropsychological assessment of patients with OCD suggests that there are deficits across a broad range of domains (Fineberg et al., 2018a). For example, a recent meta-analysis found that patients with symptoms related to symmetry and orderliness were more likely to have poor performance on memory, visuospatial ability, verbal working memory and cognitive flexibility tests, whereas patients with doubting and checking were more likely to perform poorly on memory and verbal memory tasks (Bragdon et al., 2018). Other meta-analyses have found cognitive flexibility and response inhibition to be impaired in OCD in general (all literature pooled), with medium–large effect sizes (Lipszyc and Schachar, 2010; Chamberlain et al., 2019). It must be considered that comorbid neurodevelopmental disorders, such as ASD (Postorino et al., 2017), or ADHD are expected to influence performance on distinct tests, especially but not exclusively in youth.
Behavioural analysis of OCD involves obtaining a history to ascertain the specific situations that provoke obsessions, anxious thoughts or uncomfortable feelings and then separating out the compulsions or anxiolytic behaviours. This is important, as during therapy the patient needs to face up to the anxiety-provoking thoughts or uncomfortable feelings while resisting the urge to ‘put this right’ using compulsive thoughts, behaviours or avoidance. Full descriptions of behavioural analysis are given elsewhere (Drummond, 2014). From a cognitive perspective, there have been several theories about the underlying beliefs that may trigger OCD, such as the failure to challenge underlying beliefs sufficiently (Emmelkamp et al., 1988), inflated responsibility and guilt if compulsions were not acted upon and negative consequences occurred (Salkovskis, 1985, 1999), or an overinflated idea of danger (Jones and Menzies, 1998) (see section Novel forms of psychotherapy below).
Early intervention in obsessive-compulsive disorder
OCD frequently has an onset early in life (Fineberg et al., 2019). Childhood or adolescent onset accounted for more than 50% of the sample in a recent international multisite report (Dell’Osso et al., 2016). Unfortunately, early onset is all too often not associated with early help-seeking and recognition of the illness. OCD has been consistently associated with a long duration of untreated illness (DUI) – around 7 years on average (Dell’Osso et al., 2019) – with this period accounting, in many cases, for more than half of the overall duration of illness (Albert et al., 2019; Dell’Osso et al., 2019). Longer DUI implies late interventions and poor therapy response, particularly in relation to pharmacological treatment (Dell’Osso et al., 2010; Albert et al., 2019). The need for service investment in early intervention for OCD is further highlighted by studies indicating that OCD is among the top 10 most disabling of all disorders, accounting for 2.2% of all years lost to disability (Ayuso-Mateos, 2006), with economic costs to society including those associated with lost productivity, which are long-lasting and profound. It has been estimated that in the USA, over $10 billion dollars per year are spent on treatments for OCD alone (Hollander et al., 2016).
OCD has been traditionally viewed as a secretive illness with some phenotypes (e.g., with sexual, religious or aggressive content) being particularly associated with reluctance to seek help (Dell’Osso et al., 2015). There may also be difficulty detecting the disorder in childhood (Storch et al., 2014). Nonetheless, a greater effort needs to be made at multiple levels (e.g., education, service development and screening of ‘at risk’ individuals) to implement effective strategies for prevention, early diagnosis and intervention. For instance, there have been reports indicating that the earliest symptoms shown by OCD patients belong to the symmetry and ordering dimension (Kichuk et al., 2013) and these may represent a red flag for early detection of subthreshold/early symptoms.
Children of individuals with OCD represent another high-risk group deserving attention and potentially needing preventive interventions. The presence of tic, paediatric acute-onset neuropsychiatric syndrome, obsessive-compulsive personality disorder and impulse control disorders may be indicators of comorbid OCD or herald the subsequent development of OCD (Fineberg et al., 2019). Staging models may also be useful (Fineberg et al., 2019; Fontenelle and Yücel, 2019), with four major stages proposed (from stage 0 ‘increased risk, asymptomatic’ to stage 4 ‘severe illness’). However, their clinical utility and applicability remain to be investigated. Interventions such as psychoeducation and reduction of family accommodation represent promising areas for prevention and early intervention when OCD is at its early stages in high-risk groups (Brakoulias et al., 2018). One Australian health service (Brakoulias, 2018) has recently begun using existing early intervention services for psychosis to provide early intervention to patients with OCD (Brakoulias, 2018) (Western Sydney Obsessive-Compulsive and Related Disorders Service).
Cognitive behavioural therapy, selective serotonin reuptake inhibitor or their combination as a first-line treatment for adults with obsessive-compulsive disorder
Pharmacological therapies (SSRIs and the tricyclic clomipramine) (Zohar et al., 1996; Fineberg et al., 2012) and psychological therapies (ERP, CBT) (Abramowitz, 2006) are often efficacious in treating OCD in adults. As SSRIs and CBT have been thought to have broadly similar efficacy in acute treatment, current guidelines recommend taking account of patients’ clinical features, needs and preference as well as service availability when choosing a first-line treatment (Baldwin et al., 2014). Monotherapy with CBT involving ERP is particularly recommended as an initial treatment in those with mild–to-moderate OCD, in the absence of severe depression, in those who do not prefer medications and where this form of treatment is accessible, available and preferred by patients (National Institute for Health and Clinical Excellence, 2005a; Koran et al., 2007; Katzman et al., 2014; Janardhan Reddy et al., 2017). In contrast, SSRIs are particularly recommended as a first-line treatment option in more severe OCD, in those who have comorbid depression, in those with previous history of good response to SSRIs, in those who are uncooperative with CBT or in situations where ERP/CBT is not available, accessible or preferred by patients. A combination of CBT involving ERP and SSRIs is often recommended in severe OCD, in the presence comorbid depression and in poor responders to CBT or SSRIs alone (National Institute for Health and Clinical Excellence, 2005b; Skapinakis et al., 2016b; Hirschtritt et al., 2017; Janardhan Reddy et al., 2017). In essence, most guidelines recognize SSRIs and CBT involving ERP as first-line monotherapies, but prefer CBT involving ERP over SSRIs.
Several meta-analyses and systematic reviews have demonstrated SSRIs and clomipramine (Ackerman and Greenland, 2002; Soomro et al., 2008; Skapinakis et al., 2016b) and CBT involving ERP to be more effective than placebo (frequently waiting list in CBT trials) in the treatment of OCD (Gava et al., 2007; Rosa-Alcázar et al., 2008). Although an earlier meta-analysis suggested superiority of clomipramine over SSRIs (Ackerman and Greenland, 2002), a recent network meta-analysis failed to demonstrate the superiority of clomipramine over SSRIs (Skapinakis et al., 2016b). Direct head-to-head comparisons of various medications are few and there seems to be no individual differences in efficacy among SSRIs (Skapinakis et al., 2016b), although, of course, they may differ in side effect profiles.
Most studies of CBT involving ERP included symptomatic patients stabilized on antidepressants (Skapinakis et al., 2016b). Although the observed effect size of CBT was larger than the SSRIs and clomipramine, this superiority could well be attributed to the additive or synergistic effects of two effective treatment modalities. Therefore, it is not clear whether the efficacy data attributed to CBT with ERP can be generalized to patients who are not taking medication for OCD. The efficacy of CBT as monotherapy still needs to be established clearly in drug-naïve or drug-free patient population for it to be recommended as initial monotherapy in this population.
Some studies suggest that a combination of CBT and an SSRI may be superior to SSRI monotherapy (Foa et al., 2005; Liu et al., 2005; Franklin et al., 2011; Romanelli et al., 2014; Meng et al., 2019), exposure monotherapy (Cottraux et al., 1990, Fineberg et al., 2018a) or multimodal CBT (Hohagen et al., 1998). However, it is uncertain whether combining ab-initio CBT and an SSRI is advantageous compared to either treatment used alone (Albert et al., 2012). Confidence in the superiority of the combination of medications and psychotherapy partly stems from the fact that, as described above, most psychotherapy trials are considered variants of combination trials because most patients in these studies were stabilized on SSRI or clomipramine (Skapinakis et al., 2016b). Most guidelines and literature recommend a combination of SSRIs and CBT involving ERP in severe OCD, but the recommendation is based on evidence of its efficacy as an augmenting strategy in patients who have clinically significant symptoms despite treatment with medications and not necessarily in severe OCD (Simpson et al., 2008, 2013). A recent randomized feasibility study that included patients treated in primary care found that although combined treatment with SSRI and ERP was associated with the largest improvement after 16 weeks, SSRI monotherapy was the most efficacious and cost effective treatment after 52 weeks (Fineberg et al., 2018b). If replicated, this finding would carry major implications for health services planning, especially where resources are limited, such as lower and middle income countries.
The critical importance of adequate treatment of obsessive-compulsive disorder in children and young people
For children and young people, CBT should always be the first-line approach (Sánchez-Meca et al., 2014; Skapinakis et al., 2016a), with ERP as core elements (Lewin et al., 2014). ERP is both highly effective and also an acceptable intervention for youth ages 3–8 years with OCD (Lewin et al., 2014). Children with a strong family history of OCD are reported to respond less well to conventional CBT (Garcia et al., 2010), possibly owing to family accommodation of their symptoms. Key adaptations for younger children include extensive parental involvement targeting family accommodation and frequent family meetings while delivering a full course of ERP. According to the study of Sánchez-Meca et al. (2014), effect sizes were large for CBT (d+ = 1.742) and combined (medication plus CBT) interventions (d+ = 1.710) and moderate for pharmacological only treatments (d+ = 0.746). Family-based CBT (Piacentini et al., 2011; Freeman et al., 2014) is also effective for children and adolescents with OCD, especially when there is a high degree of accommodation. The extant literature also supports CBT when delivered in group settings. More recently, the use of technical devices (smart phones and tablets) using App-delivered CBT seems promising.
Medication is, however, indicated for children and young people when symptoms are more severe, CBT has failed, skilled CBT is unavailable, and there is a comorbid disorder (e.g., depression) that may respond to medication, or when, in the judgement of the parent or clinician, earlier introduction of medicines is clinically indicated. SSRIs have been shown in randomized controlled trials to be well tolerated and effective in youth (Geller et al., 2004; Skarphedinsson et al., 2015). Sertraline and fluvoxamine have been approved for children from 6 to 8 years of age. Dosing schedules should include low starting doses, slow titration schedules and maximum recommended doses. Following adequate response and stabilization, treatment should be reviewed after 6–12 months.
In the case of nonresponse or inadequate response, another SSRI should be tried (Geller et al., 2004, 2012; Locher et al., 2017). Treatment with SSRIS in CBT-resistant patients may improve OCD symptoms. Although clomipramine may be effective, it is not recommended as a first-line treatment because of its potential side effects. However, if there are no cardiac contraindications, clomipramine* is also an option in youth but requires electrocardiogram monitoring. In the case of insufficient efficacy of drug treatment with several SSRIs and clomipramine, or in the presence of tic disorder, augmentation with antipsychotics, for example, aripiprazole* or risperidone* in low dosage may be used. Minimal duration on antipsychotics (these medications are not approved or indicated for paediatric use) is encouraged and close monitoring is required.
Relapse prevention
Relapse prevention strategies play an essential role for the optimal clinical management of OCD, considering its frequently chronic course and relapsing nature. Recovery occurs in only about one-fifth of adult cases, while for children, the mean persistence rates for full or subthreshold OCD have been estimated at around 60% (Maina et al., 2001; Stewart et al., 2004). Earlier age of OCD onset, increased illness duration, inpatient status, the presence of comorbidities and a positive family history seem to predict greater rates of persistence (Geller et al., 2003; Stewart et al., 2004). Furthermore, relapses in OCD are associated not only with considerable distress, significant functional impairment and impairment of quality of life (Hollander et al., 2010) but also with a decreased response to a previous efficacious treatment (Maina et al., 2001).
To date, relapse prevention studies in OCD have mainly investigated SSRIs and clomipramine as the maintenance treatment, with the duration of treatment under placebo-controlled conditions extending up to 12 months. Studies with a longer follow-up period or investigating relapse following CBT are relatively scarce. In the case of adults, the majority of relapse prevention studies have shown an overall superiority of SSRI compared with placebo in preventing relapse (Fineberg et al., 2007) and that discontinuation of maintenance treatment, even after a period of prolonged well-being under SSRI, is associated with a heightened relapse risk. Relapse was particularly prominent in patients with comorbidities, which is the rule rather than the exception in children with OCD. As childhood and adolescence are critical periods for achievement of social, educational and occupational milestones, relapse prevention is particularly relevant for the younger patient population (Fineberg et al., 2019). There has been one randomized controlled relapse prevention study in paediatric OCD, which showed an advantage for paroxetine* over placebo (Geller et al., 2004). As there is no available evidence suggesting a duration of treatment beyond which treatment can be discontinued safely, more recent guidelines emphasized the importance of maintaining medication for at least 12 months to reduce relapse risk (Baldwin et al., 2014).
The clinician’s role in enabling an informed choice about whether or not to discontinue medication at any particular time is challenging, considering the limitations of the available relapse prevention studies. Strategies for safely managing emerging relapse, such as reinstating either ‘booster’ CBT or medication at the first sign of symptoms, do not have established evidence of efficacy. Nevertheless, it is advisable to establish a relapse-management plan, in cooperation with patients and their families based on vigilance for emergent symptoms and rapid access to treatment previously known to be effective. If medication is to be discontinued, this should be done gradually, after a careful explanation of the potential consequences, such as withdrawal symptoms and relapse risk. SSRI tapering over a period of months, rather than weeks, may reduce the risk of withdrawal symptoms (Horowitz and Taylor, 2019).
Treatment-resistant obsessive-compulsive disorder – novel pharmacotherapies tested in adults
After well supported first- and second-line treatments and strategies have been exhausted, some patients will continue to experience impairing OCD symptoms. Next-step treatment strategies may include continuing with the chosen SRI for an extended period of time, switching to another SRI, augmenting the SRI with a second-generation antipsychotic agent* or raising the dose of SRI to the highest tolerated level* (Fineberg and Craig, 2007; Bandelow et al., 2008; Fineberg et al., 2012; Stein et al., 2012).
Although switching to another SRI often is recommended, there is little evidence to support this approach in OCD. When a partial or moderate response has been achieved following the adequate first-line treatment, there is randomized controlled trial (RCT) and meta-analytic evidence to support augmentation with an second-generation antipsychotic (Brakoulias and Stockings, 2019; Dold et al., 2015; Stein et al., 2012; Zhou et al., 2019); however, the use of these agents would be considered off-label. Of these agents, risperidone* is supported by the greatest number of studies, which have generally been positive (Brakoulias and Stockings, 2019). Two RCTs (Muscatello et al., 2011; Sayyah et al., 2012), several open-label studies (Connor et al., 2005; Pessina et al., 2009; Ak et al., 2011), and multiple case reports have demonstrated the efficacy in OCD of aripiprazole* as an augmentation agent (Matsunaga et al., 2011; Higuma et al., 2012; Hou and Lai, 2014; Ercan et al., 2015; Akça and Yilmaz, 2016; Patra, 2016; Brakoulias and Stockings, 2019). One meta-analysis also found a larger effect size for aripiprazole than for risperidone: Cohen’s d = 1.11 (aripiprazole) versus d = 0.53 (risperidone) (Veale et al., 2014). Quetiapine* has also been examined as an augmentation agent in OCD, but the evidence is conflicting. Despite several positive studies (Atmaca et al., 2002; Denys et al., 2004; Vulink et al., 2010; Diniz et al., 2011), negative results have been found in many placebo-controlled trials (Carey et al., 2005; Kordon et al., 2008; Fineberg et al., 2013).
Contrary to the depression literature, a meta-analysis of SSRIs in OCD found that high doses (high end of recommended dosage, not higher than recommended doses) were more effective than medium or low doses in the first-line treatment of OCD (Bloch et al., 2010). Response was more robust for patients with comorbid tics and in individuals who had received more than 12 weeks of maximal SSRI monotherapy (Bloch et al., 2008). However, tolerability is a significant issue as compared with lower doses so that this strategy requires caution in primary care settings (Stein et al., 2012). The Food and Drug Administration in the USA raised a safety warning in 2011 against citalopram doses higher than 40 mg/day due to a modest but probable risk of arrhythmias (US Food and Drug Administration, 2012). However, a more recent meta-analysis identified only 18 cases where electrocardiogram QTc prolongation or torsades de pointes was associated with citalopram at doses between 20 and 60 mg/day. The authors concluded that these cardiac adverse events were infrequent (Tampi et al., 2015).
When an inadequate treatment response persists, less well supported treatment strategies (lacking multiple randomized, controlled trials or meta-analyses) may be considered (Koran et al., 2007; Koran and Simpson, 2013), including use of glutamate modulators*, d-amphetamine* or oral morphine sulfate*.
Glutamate modulators such as memantine*, riluzole*, topiramate*, lamotrigine*, N-acetylcysteine* and ketamine* have varying levels of support (Koran et al., 2007; Pittenger et al., 2011; Koran and Simpson, 2013; Pittenger, 2015). Memantine augmentation showed benefit in case studies and open-label trials (Poyurovsky et al., 2005; Pasquini and Biondi, 2006; Aboujaoude et al., 2009; Feusner et al., 2009; Stewart et al., 2010; Bakhla et al., 2013). In addition, two RCTs of memantine showed exceptionally high response rates (100% in one study), inconsistent with the literature (Ghaleiha et al., 2013; Haghighi et al., 2013). Riluzole augmentation showed promise in a case series and open-label trial (Coric et al., 2003, 2005). Subsequent small controlled studies have been mixed (Pittenger et al., 2008; Emamzadehfard et al., 2016). While topiramate augmentation showed promise in case studies and open-label trials (Rubio et al., 2006; Van Ameringen et al., 2006; Van Ameringen and Patterson, 2015), small RCTs have also produced mixed results (Mowla et al., 2010; Berlin et al., 2011; Afshar et al., 2014). Lamotrigine augmentation showed mixed results in case reports (Kumar and Khanna, 2000; Uzun, 2010; Arrojo-Romero et al., 2013; Hussain et al., 2015) and benefits in two small RCTs (Bruno et al., 2012; Khalkhali et al., 2016). Limited data suggest that N-acetylcysteine is of benefit in some cases of refractory OCD (Lafleur et al., 2006), with mixed data in four RCTs (Afshar et al., 2012; Sarris et al., 2015; Paydary et al., 2016; Costa et al., 2017). N-acetylcysteine is generally well tolerated. A single intravenous dose of ketamine has been reported to be of rapid (in hours) and robust benefit in unmedicated adults with OCD in case report and open-label studies (Rodriguez et al., 2011, 2016) and a randomized controlled cross-over study (Rodriguez et al., 2013). In an open-label trial of medicated OCD adults with multiple comorbidities, depression improved on ketamine but improvement in OCD symptoms was minimal, and two patients developed new-onset irritability and suicidal ideation (Bloch et al., 2012; Niciu et al., 2013). Experience with intranasal ketamine in OCD is very limited (Adams et al., 2017; Rodriguez et al., 2017). Ketamine should only be administered at sites with expertise in this approach, with appropriate precautions including monitoring for side effects and screening individuals who have a current or past substance abuse problem (Sanacora et al., 2017).
In two double-blind, placebo-controlled studies, d-amphetamine was superior to placebo in unmedicated OCD adults (Insel et al., 1983; Joffe et al., 1991). A subsequent double-blind comparison of SSRI augmentation with d-amphetamine versus high-dose caffeine showed benefit of both drugs (Koran et al., 2009). Oral morphine showed benefit in a case series (Warneke, 1997) and in a double-blind crossover study (Koran et al., 2005) in adults with OCD. Precautions should be taken in the case of both d-amphetamine and morphine to screen out individuals who have current or past substance abuse (Koran et al., 2007).
Other drugs, such as pindolol*, clonazepam*, buspirone*, or lithium*, have been tested, but the results have been mixed and some of the placebo-controlled trials have not found positive results. Some promising results have been found with the 5HT3 antagonist ondansetron* (Serata et al., 2015) and a clinical trial is currently underway (ClinicalTrials.gov, 2017) though a double-blind placebo-controlled trial of low daily dosages of odansetron* (0.5 and 0.75 mg) in a relatively large sample was negative (ClinicalTrials.gov, 2015).
Treatment-resistant obsessive-compulsive disorder – noninvasive neurostimulation
Noninvasive neuromodulatory interventions targeting the corticostriatothalamocortical (CSTC) circuits hold promise as augmenting intervention for treatment-resistant OCD (Lusicic et al., 2018). Repetitive transcranial magnetic stimulation (rTMS)* is the best studied noninvasive modulatory intervention in OCD. rTMS delivered at low-frequency rTMS (≤1 Hz) (LF-rTMS) is thought to inhibit the activity of underlying cortical regions, while high-frequency rTMS, provided at ≥5 Hz, is thought to enhance cortical activity (Lefaucheur et al., 2014). Conventional rTMS, provided through the figure-8 coil, is relatively focal, modulating superficial cortical regions over a depth of around 2 cm (Lefaucheur et al., 2014). LF-rTMS* protocols targeting the supplementary motor area (SMA) have been found to be helpful for OCD in multiple RCTs and meta-analyses (Mantovani et al., 2010; Gomes et al., 2012; Hawken et al., 2016; Zhou et al., 2017; Rehn et al., 2018). This effect has been found to last up to 3 months (Gomes et al., 2012). A recent trial demonstrated superior efficacy of this protocol over antipsychotic augmentation in treatment-resistant OCD subjects (Pallanti et al., 2016). However, given recent inconsistent reports on inhibitory rTMS protocols targeting the SMA (Arumugham et al., 2018; Harika-Germaneau et al., 2019; Pelissolo et al., 2016), there is a need for large multicentre trials to confirm its efficacy at this location.
LF-rTMS targeting the orbitofrontal cortex (OFC)* has also shown promise in small RCTs (Ruffini et al., 2009; Nauczyciel et al., 2014). There is a need for larger trials targeting the OFC to confirm its efficacy and tolerability. RCTs targeting the dorsolateral prefrontal cortex have, in contrast – and unlike in major depressive disorder – shown highly inconsistent findings in OCD (Lusicic et al., 2018). A multisite randomized sham-controlled trial found high-frequency deep rTMS, using an H7 coil, over the dorsomedial prefrontal cortex/anterior cingulate cortex to be efficacious and well tolerated in a treatment resistant OCD population (Carmi et al., 2019). This FDA approval and CE (Conformité Européene) certification device for the treatment of resistant OCD. However, considering the cost of this device, there is a need for replication studies confirming the efficacy of the above protocol, which included personalized symptom provocation as an interventional component. Less-expensive deep coils, which have shown promise in targeting the dorsomedial prefrontal cortex in open-label trials on OCD (Modirrousta et al., 2015; Dunlop et al., 2016), are yet to be evaluated under controlled conditions.
Transcranial direct current stimulation (tDCS)* involves administration of low-amplitude (1–2 mA) electric current to the brain between a cathode and anode. Anodal tDCS is thought to enhance cortical excitability and cathodal tDCS to have an inhibitory effect (Rachid, 2019). The SMA and OFC are key targets. A randomized sham-controlled trial (n = 24 treatment-resistant OCD subjects) demonstrated efficacy for anodal tDCS administered over bilateral pre-SMA and cathodal tDCS over right supraorbital regions (Gowda et al., 2019). However, another randomized crossover trial (n = 12) found clinical improvement with cathodal tDCS over pre-SMA, while anodal tDCS was ineffective (D’urso et al., 2016). Thus, replication studies are needed to determine the optimal stimulation protocol for tDCS over SMA in OCD*. Another randomized sham-controlled trial (n = 21 treatment-resistant OCD patients) showed efficacy for cathodal tDCS delivered over the OFC and the anode over the right cerebellum, but the effect was not sustained at follow-up (Bation et al., 2019). Other promising results in treatment-resistant OCD for protocols targeting OFC and other cortical regions, such as dorsolateral prefrontal cortex and dorsomedial prefrontal cortex, are found in case reports and small uncontrolled studies and have to be confirmed in well designed trials (Brunelin et al., 2018; Rachid, 2019). Furthermore, studies present significant heterogeneity and methodological differences in sample selection criteria, concomitant treatment and tDCS stimulation protocols (da Silva et al., 2019; Rachid, 2019). Some authors suggest that overall cathodal tDCS may be better than anodal in treating OCD (Rapinesi et al., 2019).
Currently, there are no RCTs to support the efficacy of electroconvulsive therapy* (ECT) in OCD (Fontenelle et al., 2015). Hence, ECT may be recommended only for acute treatment of comorbid conditions such as depression or psychosis*.
To summarize, LF-rTMS delivered over the SMA (with figure-8 coil) and HF-deep-rTMS over the dorsomedial prefrontal cortex/anterior cingulate cortex (with H7 coil) appear promising interventions in treatment-resistant OCD. There is a pressing need for large replication studies and evaluation of long-term effects/maintenance protocols. The evidence for tDCS is highly preliminary and further exploratory studies are encouraged.
Treatment-resistant obsessive-compulsive disorder – deep brain stimulation and ablative neurosurgery
A significant number (10–40%) of patients do not respond to any available therapy and suffer from severe, enduring symptoms and dysfunction (Fineberg and Gale, 2005; Denys, 2006; Gupta et al., 2019). For this highly refractory patient group, ablative neurosurgery* and deep brain stimulation* (DBS) remain modalities to be considered. These procedures are usually delivered as an adjunct to existing pharmacological treatments, and CBT is frequently also administered, either during the acute treatment phase or follow-up. DBS is considered an experimental treatment, but has an FDA ‘humanitarian device exemption’ for severe refractory OCD (US Food and Drug Administration, 2009).
Stereotactic neurosurgical procedures for intractable OCD have been available for >50 years (Miguel et al., 2019). The procedures include dorsal anterior cingulotomy and anterior capsulotomy and are reserved for the most severe, treatment nonresponsive patients. A systematic review involving 10 studies and 193 participants suggested both procedures were efficacious (Brown et al., 2016). The authors reported a mean Y-BOCS reduction of 37% for cingulotomy and 57% for capsulotomy. The rates of serious or permanent adverse events were 5.2% in the cingulotomy studies and 21.4% in the capsulotomy studies. Another recent review of publications on anterior capsulotomy spanning over five decades (Pepper et al., 2019) reported ‘significant clinical response’ in 73–90% of patients and ‘remission’ in 24–39% of patients with treatment-resistant OCD.
DBS was investigated as a partially reversible alternative to surgical ablation (Nuttin et al., 1999). The original stimulation target was similar to the site of anterior capsulotomy, that is, ventral capsule/ventral striatum (VC/VS). Three reasonably sized studies have provided evidence in favour of the acute efficacy of DBS in the VC/VS. The first involved 24 patients who were followed up to four years and reported a 37% median improvement in baseline Y-BOCS scores (Luyten et al., 2016). ‘ON’ phases of stimulation were compared with ‘OFF’ phases (no stimulation), demonstrating that improvements were unlikely to represent ‘placebo’ effects. The second study investigated 16 patients, initially as open label, reporting a 46% reduction in baseline Y-BOCS at 8 months as well as a significant difference (25%) in Y-BOCS scores when compared with sham stimulation in a subsequent month-long double-blind phase (Denys et al., 2010). A recent 12-month multicentre study of 30 patients given VC/VS DBS (Menchón et al., 2019) reported a mean reduction of baseline Y-BOCS of 42%. Sixty percent of patients were responded (reduction in baseline Y-BOCS > 40%).
The long-term benefits of VC/VS DBS are less certain. An open-label follow up study of 10 patients (Greenberg et al., 2006) reported a reduction in mean Y-BOCS from 34.67 at baseline (severe) to 22.37 (moderate) at 36 months. In addition, significant improvements in global functioning, depression and anxiety persisted.
The anteromedial subthalamic nucleus (amSTN) has been identified as another promising target for DBS in OCD. Sixteen patients were randomized according to a crossover design to either 3 months active or sham treatment, resulting in a significantly greater reduction in mean Y-BOCS in the stimulation versus sham group (endpoint 19 ± 8 versus 28 ± 7) (Mallet et al., 2008). It remains unclear whether VC/VS holds any advantage over amSTN DBS. A recent ‘mechanism of effect’ study of six OCD patients, in which electrodes were implanted in both these sites, found differential improvements in mood (VC/VS) and cognitive flexibility (amSTN), suggesting that DBS exerts therapeutic effects at these targets via different brain networks (Tyagi et al., 2019).
There have been no head-to-head trials comparing ablative neurosurgery with DBS. A recent review (Pepper et al., 2015) retrospectively evaluated 20 studies of varying methodological quality involving 62 patients who underwent DBS of the VC/VS or the nucleus accumbens and 108 patients who underwent anterior capsulotomy. The capsulotomy group showed a significantly higher (51%) mean reduction in Y-BOCS than the DBS group (40%). No difference in surgical complication rates was observed. Adverse events across both modalities included intracranial haemorrhage (2–5%), persisting postoperative side effects (5–7%), cognitive and personality changes (7–13%) and suicide (1–2%). Weight gain (defined by an increase >10%) was significantly higher in the capsulotomy group (29 versus 3%). In other studies (Mallet et al., 2008; Menchón et al., 2019), hypomania after electrode implantation is commonly (6%) reported.
In summary, studies of both DBS and ablative neurosurgery have shown these techniques are clinically effective for this highly refractory and extremely chronically disabled patient group. However, there is as yet insufficient evidence to determine which technique to choose at an individual patient level. Further clarification of the differential effects of ablation and stimulation across the different candidate neural targets, as well as better understanding of the interaction between somatic, pharmacological and psychological interventions, have the potential to advance the field towards a personalized approach. Agreement over standardized patient selection and treatment protocols that would allow clinical outcomes data to be collected and compared across treatment centres, represents an achievable milestone towards this goal (Menchón et al., 2019). Meanwhile, technological innovations, for example, MRI-guided focussed ultrasound, laser interstitial thermal therapy (Miguel et al., 2019), offer potential for safer and more cost-effective surgical approaches.
Future directions for research
Problematic usage of the internet
Problematic use of the internet (PUI) is an umbrella term for a range of repetitive functionally impairing compulsive behaviours including gambling, gaming, sexual behaviour, shopping, video-streaming or social media use. While advances have been made in defining diagnostic criteria and developing rating scales for some forms of PUI (e.g., Gaming Disorder) (Király et al., 2015), a considerable amount of research is needed to understand better the broad range of PUI phenomena and translate the known behavioural phenotypes into valid and reliable diagnostic criteria and assessment tools, to facilitate the systematic investigation of aetiological factors and brain-based mechanisms, as a platform for the development of preventive and therapeutic interventions (Fineberg et al., 2018c).
Significant cross-sectional associations between PUI and OCD symptoms have been found (Carli et al., 2013). For example, in a two-site international online survey, ADHD and social anxiety disorder were associated with high PUI scores in young participants, whereas OCD and generalized anxiety disorder were associated with high PUI scores in older participants (Ioannidis et al., 2018).
Novel digital interventions in obsessive-compulsive disorder
Digital technology offers new opportunities for monitoring and interventions. The extensive use of smartphones and the vast amounts of information they contain has positioned them as a proxy for behavioural and social interactions (WHO, 2016). Harnessing smartphone technology along with smart wearables (e.g., smart watches) is expected to be a valuable source of continuous, objective and reliable data for clinical characterization, behavioural monitoring and treatment support (Marzano et al., 2015). This is true for several disorders, but especially true for obsessive-compulsive problems such as PUI, as the digital media that is directly linked to the disorder is the same one that can accurately monitor the behaviour (Ferreri et al., 2019).
Accordingly, using digital technology along with big data analyses may enable the potential to characterize the ‘digital phenotype’ of the disorder (Ferreri et al., 2019) and to identify those individuals most at risk (e.g., by monitoring online internet usage in comparison with changes in diurnal variation, lack of human contact, lack of geographical movement, restricted circles of friends, etc.). A research avenue in this direction is to use (real time) big data analysis, alongside machine learning algorithms, to establish identifiable OCRD-specific illness patterns and use those real-time results to create an immediate feedback loop with the patient, which could then be used therapeutically by providing direct feedback on their behaviour and progress.
Other forms of active online intervention have become increasingly available for OCRD (Whiteside et al., 2013) and may potentially enhance and facilitate treatment adherence (Andersson et al., 2014; Marzano et al., 2015). For example, WhatsApp group interventions, in which the patient reports to their clinician, in real time, their difficulties, daily achievement and progress, enable continual communication, real-time reporting, prompt responses and rapid intervention when needed. In addition, the digital intervention may serve as a platform for continuous monitoring of tasks delivered in face-to-face meetings. Another example of existing digital interventions is the proactive use of webcams and smartphone cameras. Using this domain and with patient’s consent, the clinician has the opportunity to monitor patients in their natural environment. As the digital platform bridges the elapsed time between therapeutic sessions, it overcomes geographical distances and enables therapeutic practice in the patient’s natural environment (WHO, 2016), where symptoms are manifested daily. In addition to enriching the clinical picture by direct observation of symptoms, it confers the general assertive outreach benefits of telemedicine, which can be critical for otherwise difficult to treat socially isolated patients who cannot access help otherwise.
In practice, this approach breaks down the traditional terminology of ‘outpatient’, ‘in-patient’ and ‘day hospitalization’, by allowing real time, objective and continuous monitoring (WHO, 2016). The combination of digital monitoring and online communication produces a form of ‘virtual hospitalization’, enabling comprehensive and intensive treatment by offering continued monitoring and delivery of therapy in the patient’s natural environment, where the OCD usually occurs, and not within the artificial setting of the clinic. While such approaches are still under development, digital tools seem to bear great potential and may change the landscape of treatment in OCRDs, providing potentially cost-effective alternatives to hospitalization or outpatient clinics.
Immunological therapies
Inflammation and release of inflammatory cytokines affect brain circuitry involving both reward and threat-sensitivity, producing potentially adaptive and beneficial behavioural responses (Raison and Miller, 2013). There is growing evidence of dysfunctional immunological function in the pathogenesis of a significant subset of OCD patients. Elevated levels of basal ganglia antibodies have been detected in adult OCD patients’ plasma compared with psychiatric control groups (Nicholson et al., 2012). In addition, significantly increased levels of CSF autoantibodies directed against basal ganglia and thalamus were found among drug-naive OCD patients, and were associated with increased levels of CSF glutamate and glycine, indicating underpinning abnormalities in excitatory neurotransmission and correlating with hyperactivity in the ventral cognitive circuit (Bhattacharyya et al., 2009). Translocator protein distribution volume, a marker of the microglial component of neuroinflammation, was found to be significantly elevated in the CSTC circuit of OCD subjects compared with healthy controls, demonstrating inflammation within the neurocircuitry extending beyond the basal ganglia, and affecting the adult population rather than solely childhood OCD (Attwells et al., 2017).
A common genetic link may explain an excess of some autoimmune comorbidities. For example, in the acute paediatric onset subset of children (PANDAS), there is immunological cross-reactivity with epitopes associated with streptococcal infection expressed on the surface of basal ganglia neurons. About 20% of the mothers of children fulfilling criteria for PANDAS (Chang et al., 2015) had at least one autoimmune disease. Multigenerational studies also show that OCD patients’ relatives are more likely to have an autoimmune disease such as Sjögren’s syndrome, coeliac disease, Guillian Barrè, Crohn’s disease, Hashimotos Thyroiditis, type I diabetes mellitus, ulcerative colitis, multiple sclerosis and psoriasis vulgaris (Mataix-Cols et al., 2018). A subset of patients with PANDAS with motor symptoms demonstrated antineural antibodies against dopamine (D1) receptors as well as elevated antibodies against tubulin, lysoganglioside and higher activation of calmodulin-dependent protein kinase II (Cox et al., 2015).
Immunomodulatory therapy represents a new field of investigation in OCD. While treatment with antimicrobials has delivered inconsistent results (Burchi and Pallanti, 2018), other immunological modulators, such as celecoxib* (Shalbafan et al., 2015) and nonspecific nonsteroidal anti-inflammatory drugs (Spartz et al., 2017), have produced some positive findings, the latter only in a subset of young people. Thus, evidence of the usefulness of this approach in OCD remains insufficient. Nevertheless, researchers and clinicians should consider genetic and immunological profile differences in the search for precise individualized therapy for OCD.
Novel forms of psychotherapy
Although it may seem logical to try to tackle OCD using cognitive therapy, little evidence suggests that it offers any advantage to graded exposure and self-imposed response prevention (Tyagi et al., 2010; Ougrin, 2011). Poorly applied cognitive therapy, such as that expecting patients to re-evaluate actual dangers, may make some patients with OCD worse. This is because the process of looking for evidence to confirm or refute the obsessions can become incorporated into rituals. Cognitive therapy may also turn out to be less cost-efficient, as it requires more training and supervision for the therapist and usually takes more time in therapy. It is therefore probably best used in situations where there is OCD refractory to ERP therapy (Drummond and Edwards, 2018).
Rational emotive therapy, on the other hand, has been shown to have some possible beneficial effects in OCD (Emmelkamp et al., 1988). Australian researchers have developed danger ideation reduction therapy (DIRT), using rational emotive therapy but with instructions not to undergo exposure for patients with contamination fears; good outcomes in case reports and some small controlled trials have been found (Jones and Menzies, 1998; Krochmalik et al., 2001; Maqbool et al., 2017). The techniques used in DIRT include cognitive restructuring using rational emotive therapy (Ellis, 1962); filmed interviews with people who work in feared situations; corrective information about the real risks of ‘contamination’ as opposed to the deleterious effects of overzealous hand-washing and attentional focussing whereby patients are taught to focus the mind away from the danger-related intrusive thoughts.
In recent years, the so-called Third Wave Therapies have been used in a number of psychiatric conditions (Pérez Álvarez, 2012). The therapy of this type most commonly used in OCD is mindfulness, which teaches an individual to focus on the world around them rather than their internal dialogue. A recent study demonstrated that both cognitive restructuring and also mindfulness led to a small improvement in Y-BOCS score when compared with waiting list controls. However, the strength of efficacy for both treatments appeared to be less than that generally found with ERP (Rupp et al., 2019). Despite promising results for metacognitive therapy in patients with OCD in case series, a full controlled trial has yet to be performed (Melchior et al., 2019).
Many OCD patients describe their compulsions as habitual, that is, fixed ‘stimulus-response ’acts that, through habit learning, occur automatically in response to a specific environmental trigger. Habit reversal therapy (HRT) (Azrin and Nunn, 1973) is a long-established form of therapy that helps patients alter habitual performance through a variety of behavioural methods. HRT is reported to be efficacious for the treatment of Tourette syndrome and Tic Disorders and has more recently been applied with success in OCRDs such as trichotillomania and skin picking behaviours. However, there remains a scarcity of evidence from controlled trials to support the efficacy of HRT in OCRDs in general and OCD in particular (Lee et al., 2019a). Emerging neurosciences evidence identifying faulty habit learning in OCD (Fineberg et al., 2018a) suggests further study of HRT in OCD would be worthwhile.
Pharmacogenetics
Pharmacogenetics or pharmacogenomics define genetic variants that influence either drug metabolism, delivery, affinity to receptors or transporters may contribute to the prediction of drug efficacy or toxicity, promoting precision medicine (Hess et al., 2015). Because approximately one-quarter of OCD patients do not respond to treatment with either SSRIs or CBT alone, or their combination (Hirschtritt et al., 2017), it has been suggested that pharmacogenetics may contribute to better drug-response prediction and side effect tolerance (Zai et al., 2014).
Currently, several pharmacogenetic approaches using hypothesis-free GWAS have been conducted into the association between candidate genes and drug response in OCD patients (Di Bella et al., 2002; Denys et al., 2007; Van Nieuwerburgh et al., 2009; Miguita et al., 2011; Grünblatt et al., 2014; Zai et al., 2014; Umehara et al., 2015; Mas et al., 2016; Qin et al., 2016; Umehara et al., 2016; Taj et al., 2018; Lisoway et al., 2018; Sina et al., 2018; Abdolhosseinzadeh et al., 2019a,b; Alizadeh et al., 2019). The candidate genes investigated belong to: (1) pharmacokinetic regulating genes, such as the CYP450 liver enzymes such as CYP2D6 and CYP2C19; (2) serotonergic systems, such as SLC6A4 and its promoter, HTTLPR, HTR2A, HTR2C, HTR1B and TPH2; (3) glutamatergic systems, such as SLC1A1, DLGAP2, DLGAP2, GRIN2B, GRIK2, SLIT, SLITRK5; (4) dopaminergic systems, such as COMT, MAOA, DRD2 and DRD4 and (5) other systems, such as BDNF, NTRK3, MOG, OLIG2 and DISP1.
Currently, no consensus with sufficiently robust results exists in the pharmacogenetics of OCD, due to the fact that many studies used naturalistic approaches, did not employ double blinded designs or crossed over with the tested drug, used a variety of drugs and doses, as well as used various cutoffs and measures determining response. Although there is still a need systematically to assess the pharmacogenetic link between treatment response (to SSRIs, tricyclics, antipsychotics, clomipramine, etc.) and certain genes, some data are already available, though very limited, on the Internet (e.g., https://www.pharmgkb.org; Whirl-Carrillo et al., 2012) summarizing some findings on pharmacogenetics of some drugs and giving some recommendations aligning with those of the FDA, European Medicine Agency, Pharmaceutical and Medical Devices Agency, Japan and Health Canada (Sante Canada).
Conclusion
Until just 40 years ago, OCD was considered rare, of psychological origin and without effective treatment. Now, all have changed; the finding in the 1970s and 1980s that serotonergic medication (clomipramine, followed by SSRIs) was effective (Montgomery, 1980; Zohar et al., 1987; Zohar and Insel, 1987) opened the door to great interest in OCD (Zohar, 2012). This led to the development of specific forms of psychological intervention (ERP) which replaced the dynamic approach and to a focus on the serotonergic system in the treatment and pathophysiology of OCD. As a result of neuroscience insights including endophenotype-based approaches (reviewed in Fineberg et al., 2018a), OCD has been removed from the anxiety disorder grouping in the DSM-5 (American Psychiatric Association, 2013) and ICD-11 (WHO, 2018) and now stands at the head of a new family of OCRDs.
The realization that OCRDs as a group are different from other anxiety disorders has led to significant changes in understanding their impact (the prevalence of OCRD in the population is more than 9%) (Carmi et al., 2019, in submission) and to refinement of the treatment approach (e.g., focussing on the urge to perform compulsions and the need for higher doses of serotonergic medication).
This position statement highlights the major changes that have been taking place in the last few years in the field of OCD, in terms of conceptualization, diagnosis, assessment, intervention (with focus on early intervention), strategies for optimizing the efficacy of specific pharmacological intervention (SRI) with specific psychological intervention (ERP), the critical role of treatment of children and young adults and the importance of maintenance of well-being.
As new neuroscience insights are revealed, new therapeutic interventions are being explored (e.g., glutamatergic agents, dopaminergic modulators, etc.). This position statement also covers invasive and noninvasive neuromodulation as experimental interventions, including deep TMS (achieving FDA indication for OCD in 2018) (US Food and Drug Administration, 2018).
Looking to the future, other exciting avenues for investigation include the use of digital tools to monitor (and eventually to diagnose OCRDs), better understanding of links between excessive Internet use and OCRDs, advanced genetic methods and new pharmacological domains (e.g., immunological systems). Indeed, it seems that the future was never so bright for OCRD patients. We trust that this position statement has managed to capture, describe, explain and shed light on many of these developments, including those in the front line of understanding and treatment of OCRD in the future.
Acknowledgements
The authors wish to acknowledge the members of the International College of Obsessive-Compulsive Disorders (www.ICOCS.org), who have contributed to the development of this article. With particular thanks for critically reviewing the statement and editing the manuscript, to Rajshekhar Bipeta, Julius Burkauskas, Artemisa Dores, Giacomo Grassi, Donatella Marazziti, Pedro Morgado and Humberto Nicolini. We grateful to the European College of Neuropsychopharmacology (ECNP) Obsessive-Compulsive and Related Disorders Research Network (OCRN) for providing monetary support for the open access article processing charges for this article. We are also grateful to the ECNP OCRN, American College of Neuropsychopharmacology and the World Psychiatric Association Scientific Section for Anxiety and Obsessive-Compulsive and Related Disorders, for providing networking support.
This article refers to studies funded by the National Institute for Health Research (NIHR) RFPB (Grant Reference Number PB-PG-0712-28044, NIHR RfPB PB-PG-1216-20005). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
N.A.F. was supported by a COST Action Grant (CA16207; European Network for Problematic Usage of the Internet; European Cooperation in Science and Technology; www.cost.eu.) and a NIHR grant [NIHR RfPB PB-PG-1216-20005; FEasibility and Acceptability of Transcranial Stimulation in Obsessive-Compulsive Symptoms (FEATSOCS)]. S.R.C. was supported by a Wellcome Trust Clinical Fellowship. E.H. was funded by DOD, and OPD-FDA. E.G. was funded by the University of Zurich.
All authors were involved in drafting the manuscript and agreed to its publication. All authors read and approved their sections of the final version of the manuscript.
Conflicts of interest
N.A.F. declares that in the past 3 years, she had held research or networking grants from the ECNP, UK NIHR, EU H2020, MRC, University of Hertfordshire. In the past 3 years, she had either accepted travel or hospitality expenses or both from the BAP, ECNP, RCPsych, CINP, International Forum of Mood and Anxiety Disorders, World Psychiatric Association and Indian Association for Biological Psychiatry. In the past 3 years, she had received payment from Taylor and Francis and Elsevier for editorial duties. In the past 3 years, she had accepted a paid speaking engagement in a webinar sponsored by Abbott. Previously, she had accepted paid speaking engagements in various industry supported symposia and have recruited patients for various industry-sponsored studies in the field of OCD treatment. She leads an NHS treatment service for OCD. She holds Board membership for various registered charities linked to OCD. She gives expert advice on psychopharmacology to the UK MHRA and NICE. S.W. has received royalties from Thieme, Hogrefe, Kohlhammer, Springer, Beltz in the last 5 years. Her work was supported by the Swiss National Science Foundation (SNF), diff. EU FP7s, HSM Hochspezialisierte Medizin of the Kanton Zurich, Switzerland, Bfarm Germany, ZInEP, Hartmann Müller Stiftung, Olga Mayenfisch, Gertrud Thalmann and Vontobel Fonds in the last 5 years. She received no honoraria from pharmaceutical or other industrial companies in the last 36 months. Outside professional activities and interests are declared under the link of the University of Zurich, https://www.uzh.ch/prof/ssl-dir/interessenbindungen/client/web. V.B. has received lecture honoraria from Lundbeck and Otsuka. V.B. is a clinical investigator in a clinical trial funded by Boeringher Ingelheim and has obtained competitive grant funding from a Pfizer Neuroscience Grant, the Nepean Medical Research Foundation, the University of Sydney, Western Sydney University, Western Sydney Local Health District and the Better Foundation. C.I.R. has served as a consultant for Allergan, BlackThorn Therapeutics, Rugen Therapeutics and Epiodyne, receives research grant support from Biohaven Inc. and a stipend from APA Publishing for her role as Deputy Editor at The American Journal of Psychiatry. J.M.M. has received honoraria and travel grants from Exeltis, Janssen, Servier, AbBiotics and Medtronic in the last 36 months. B.M.D. has received lecture honoraria from Lundbeck, Angelini, Janssen, Neuraxpharma, Arcapharma and Livanova. Y.C.J.R. has received grants from the Department of Biotechnology (DBT) and the Indian Council of Medical Research (ICMR) of the Government of India and is currently involved in a study funded by the National Institute of Mental Health (NIMH), USA. Y.C.J.R. is the lead author of the Indian Psychiatric Society (IPS) Clinical Practice Guidelines for Obsessive-Compulsive Disorder and is also the lead author of the Clinical Practice Guidelines for Cognitive Behaviour Therapies in Anxiety Disorders and Obsessive-Compulsive Related Disorders (in press). D.J.S. has received either research grants or consultancy honoraria from Lundbeck and Sun or both. S.P. declares funding from the National Institute of Mental Health, USA; R21 NCTID NCT03669315. J.Z. received grants and research support from Lundbeck, Servier, Brainsway, Pfizer and the DOD, honoraria and consultation fees from Lundbeck, Roche, Lilly, Servier, Pfizer. S.R.C. consults for Promentis and Ieso Digital Health. S.S.A. has received research funding grant from the Wellcome Trust-DBT India alliance and Indian Council of Medical Research. M.V.A. reports being on the Advisory Boards of Allergan, Almatica, Brainsway, Janssen, Lundbeck, Myriad Neuroscience, Otsuka and Purdue Pharma (Canada); M.V.A. is on the Speaker’s Bureau for Allergan, Lundbeck, Otsuka, Pfizer, Purdue Pharma (Canada) and Takeda; and has received research support from Janssen, Purdue Pharma (Canada), the Canadian Foundation for Innovation and Hamilton Academic Health Sciences Organization (HAHSO). D.A.M.D.G. has received grant or research support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development subcontract with Duke Clinical Research Center Pediatric Trials Network, Biohaven Pharmaceuticals, Neurocrine Biosciences, Nuvelution Pharma, Peace of Mind Foundation, Syneos Health and Teva Pharmaceutical Industries. He has served as a consultant to the Arlington Youth Counseling Center. He has served on the editorial board of Annals of Clinical Psychiatry. He has received honoraria from the Massachusetts Psychiatry Academy and the American Academy of Child and Adolescent Psychiatry. He has held stock options/ownership in Assurex Health and Revolutionary Road. R.G.G.S. receives a productivity grant from the National Council for Scientific and Technological Development, Brazilian Federal Government (CNPq). L.D. declares that she holds small investments in pharmaceutical and biotechnology firms, including AstraZeneca, Bioventic, Hikma Pharmaceutical, International Biotech Trust, Reneuron, Syncona and Yourgene. U.A. has received lecture honoraria from Lundbeck, Angelini, Janssen, Neuraxpharma and Innova Pharma. There are no conflicts of interest for the remaining authors.
References
- Abdolhosseinzadeh S, Alizadeh N, Shams J, Asadi S, Ahmadiani A. BDNF association study with obsessive-compulsive disorder, its clinical characteristics, and response to fluvoxamine-treatment in Iranian patients. 2019a; 28:216–224 [DOI] [PubMed] [Google Scholar]
- Abdolhosseinzadeh S, Sina M, Ahmadiani A, Asadi S, Shams J. Genetic and pharmacogenetic study of glutamate transporter (SLC1A1) in Iranian patients with obsessive-compulsive disorder. J Clin Pharm Ther. 2019b; 44:39–48 [DOI] [PubMed] [Google Scholar]
- Aboujaoude E, Barry JJ, Gamel N. Memantine augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. J Clin Psychopharmacol. 2009; 29:51–55 [DOI] [PubMed] [Google Scholar]
- Abramowitz JS. The psychological treatment of obsessive – compulsive disorder. can. J Psychiatry. 2006; 51:407–416 [DOI] [PubMed] [Google Scholar]
- Ackerman DL, Greenland S. Multivariate meta-analysis of controlled drug studies for obsessive-compulsive disorder. J Clin Psychopharmacol. 2002; 22:309–317 [DOI] [PubMed] [Google Scholar]
- Adams T, Bloch M, Pittenger C. Intranasal ketamine and cognitive-behavioral therapy for treatment refractory obsessive-compulsive disorder. J Clin Psychopharmacol. 2017; 37:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar H, Akuchekian S, Mahaky B, Zarean E. Topiramate augmentation in refractory obsessive-compulsive disorder: a randomized, double-blind, placebo-controlled trial J Res Med Sci. 2014; 19:976–981 [PMC free article] [PubMed] [Google Scholar]
- Afshar H, Roohafza H, Mohammad-Beigi H, Haghighi M, Jahangard L, Shokouh P, et al. N-acetylcysteine add-on treatment in refractory obsessive-compulsive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2012; 32:797–803 [DOI] [PubMed] [Google Scholar]
- Ak M, Bulut SD, Bozkurt A, Ozsahin A. Aripiprazole augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a 10-week open-label study Adv Ther. 2011; 28:341–348 [DOI] [PubMed] [Google Scholar]
- Akça ÖF, Yilmaz S. Aripiprazole in the treatment of obsessive compulsive disorder and aggressive behaviors in a child with Prader Willi syndrome: a case report J Clin Psychopharmacol. 2016; 36:526–528 [DOI] [PubMed] [Google Scholar]
- Albert U, Barbaro F, Aguglia A, Maina G, Bogetto F. Combined treatments in obsessive-compulsive disorder: current knowledge and future prospects. Riv Psichiatr. 2012; 47:255–268 [DOI] [PubMed] [Google Scholar]
- Albert U, Barbaro F, Bramante S, Rosso G, De Ronchi D, Maina G. Duration of untreated illness and response to SRI treatment in obsessive-compulsive disorder. Eur Psychiatry. 2019; 58:19–26 [DOI] [PubMed] [Google Scholar]
- Alizadeh N, Nosrat N, Jahani Z, Ahmadiani A, Asadi S, Shams J. Association of HTR1A gene polymorphisms with obsessive-compulsive disorder and its treatment response: the influence of sex and clinical characteristics. Int J Neurosci. 2019; 129:264–272 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association ; Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). 2013, Arlington, VA: American Psychiatric Pub [Google Scholar]
- Andersson G, Cuijpers P, Carlbring P, Riper H, Hedman E. Guided internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: a systematic review and meta-analysis. World Psychiatry. 2014; 13:288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakis I, Gooding P, Tarrier N, Panagioti M. Suicidality in obsessive compulsive disorder (OCD): a systematic review and meta-analysis. Clin Psychol Rev. 2015; 39:1–15 [DOI] [PubMed] [Google Scholar]
- Arrojo-Romero M, Tajes Alonso M, de Leon J. Lamotrigine augmentation of serotonin reuptake inhibitors in severe and long-term treatment-resistant obsessive-compulsive disorder. Case Rep Psychiatry. 2013; 2013:612459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugham SS, Vs S, Hn M, B V, Ravi M, Sharma E, et al. Augmentation effect of low-frequency repetitive transcranial magnetic stimulation over presupplementary motor area in obsessive-compulsive disorder: arandomized controlled trial. J ECT. 2018; 34:253–257 [DOI] [PubMed] [Google Scholar]
- Atmaca M, Kuloglu M, Tezcan E, Gecici O. Quetiapine augmentation in patients with treatment resistant obsessive–compulsive disorder: a single-blind, placebo-controlled study Int Clin Psychopharmacol. 2002; 17:115–119 [DOI] [PubMed] [Google Scholar]
- Attwells S, Setiawan E, Wilson AA, Rusjan PM, Mizrahi R, Miler L, et al. Inflammation in the neurocircuitry of obsessive-compulsive disorder. JAMA Psychiatry. 2017; 74:833–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso-Mateos JL. Global Burden of Obsessive-Compulsive Disorder in the Year 2000. 2006World Health Organization [Google Scholar]
- Azrin NH, Nunn RG. Habit-reversal: a method of eliminating nervous habits and tics. Behav Res Ther. 1973; 11:619–628 [DOI] [PubMed] [Google Scholar]
- Bakhla AK, Verma V, Soren S, Sarkhel S, Chaudhury S. An open-label trial of memantine in treatment-resistant obsessive-compulsive disorder. Ind Psychiatry J. 2013; 22:149–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DS, Anderson IM, Nutt DJ, Allgulander C, Bandelow B, den Boer JA, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014; 28:403–439 [DOI] [PubMed] [Google Scholar]
- Bandelow B, Sher L, Bunevicius R, Hollander E, Kasper S, Zohar J, Möller H J; WFSBP Task Force on Mental Disorders in Primary Care; WFSBP Task Force on Anxiety Disorders, OCD and PTSD. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012; 16:77–84 [DOI] [PubMed] [Google Scholar]
- Bandelow B, Zohar J, Hollander E, Kasper S, Möller HJ, Zohar J, et al. ; WFSBP Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disoders. World federation of societies of biological psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders – first revision. World J Biol Psychiatry. 2008; 9:248–312 [DOI] [PubMed] [Google Scholar]
- Bation R, Mondino M, Le Camus F, Saoud M, Brunelin J. Transcranial direct current stimulation in patients with obsessive compulsive disorder: arandomized controlled trial. Eur Psychiatry. 2019; 62:38–44 [DOI] [PubMed] [Google Scholar]
- Berlin HA, Koran LM, Jenike MA, Shapira NA, Chaplin W, Pallanti S, Hollander E. Double-blind, placebo-controlled trial of topiramate augmentation in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2011; 72:716–721 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Khanna S, Chakrabarty K, Mahadevan A, Christopher R, Shankar SK. Anti-brain autoantibodies and altered excitatory neurotransmitters in obsessive-compulsive disorder. Neuropsychopharmacology. 2009; 34:2489–2496 [DOI] [PubMed] [Google Scholar]
- Bloch MH, Landeros-Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry. 2006; 11:622–632 [DOI] [PubMed] [Google Scholar]
- Bloch MH, Landeros-Weisenberger A, Rosario MC, Pittenger C, Leckman JF. Meta-analysis of the symptom structure of obsessive-compulsive disorder. Am J Psychiatry. 2008; 165:1532–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, Pittenger C. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010; 15:850–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Wasylink S, Landeros-Weisenberger A, Panza KE, Billingslea E, Leckman JF, et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2012; 72:964–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragdon LB, Gibb BE, Coles ME. Does neuropsychological performance in OCD relate to different symptoms? A meta-analysis comparing the symmetry and obsessing dimensions. Depress Anxiety. 2018; 35:761–774 [DOI] [PubMed] [Google Scholar]
- Brakoulias V. NSW Health [WWW Document]; 2018. Western Sydney Obsessive-Compulsive and Related Disorders Service. http://wslhdintranet.wsahs.nsw.gov.au/Obsessive-Compulsive-and-Related-Disorders-Service/wslhd-obsessive-compulsive-and-related-disorders-service. [Accessed 16 Augest 2019] [Google Scholar]
- Brakoulias V, Perkes IE, Tsalamanios E. A call for prevention and early intervention in obsessive-compulsive disorder. Early Interv Psychiatry. 2018; 12:572–577 [DOI] [PubMed] [Google Scholar]
- Brakoulias V, Stockings E. A systematic review of the use of risperidone, paliperidone and aripiprazole as augmenting agents for obsessive-compulsive disorder. Expert Opin Pharmacother. 2019; 20:47–53 [DOI] [PubMed] [Google Scholar]
- Brown LT, Mikell CB, Youngerman BE, Zhang Y, McKhann GM, II, Sheth SA. Dorsal anterior cingulotomy and anterior capsulotomy for severe, refractory obsessive-compulsive disorder: a systematic review of observational studies. J Neurosurg. 2016; 124:77–89 [DOI] [PubMed] [Google Scholar]
- Brunelin J, Mondino M, Bation R, Palm U, Saoud M, Poulet E. Transcranial direct current stimulation for obsessive-compulsive disorder: a systematic review. Brain Sci. 2018; 8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno A, Micò U, Pandolfo G, Mallamace D, Abenavoli E, Di Nardo Lamotrigine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive–compulsive disorder: a double-blind, placebo-controlled study. J Psychopharmacol. 2012; 26:1456–1462 [DOI] [PubMed] [Google Scholar]
- Burchi E, Pallanti S. Antibiotics for PANDAS? Limited evidence: review and putative mechanisms of action Prim Care Companion CNS Disord. 2018; 20(3) 10.4088/pcc.17r02232 [DOI] [PubMed] [Google Scholar]
- Carey PD, Vythilingum B, Seedat S, Muller JE, Van Ameringen M, Stein DJ. Quetiapine augmentation of SRIs in treatment refractory obsessive-compulsive disorder: a double-blind, randomised, placebo-controlled study [ISRCTN83050762] BMC Psychiatry. 2005; 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli V, Durkee T, Wasserman D, Hadlaczky G, Despalins R, Kramarz E, et al. The association between pathological internet use and comorbid psychopathology: a systematic review. Psychopathology. 2013; 46:1–13 [DOI] [PubMed] [Google Scholar]
- Carmi L, Fineberg N, Ben Arush O, Zohar J. Geddes J, Andreasen N, Goodwin G. Obsessive compulsive disorder New Oxford Textbook of Psychiatry. Oxford: Oxford University Press [Google Scholar]
- Carmi L, Tendler A, Bystritsky A, Hollander E, Blumberger DM, Daskalakis J, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019; 176:931–938 [DOI] [PubMed] [Google Scholar]
- Chamberlain S, Solly J, Hook R, Vaghi M, Robbins T. Fineberg NA, Robin T. Cognitive inflexibility in OCD and related disorders Future Trends In Obsessive-Compulsive And Related Disorders Research. 2019, Berlin: Springer Publishing [Google Scholar]
- Chang K, Frankovich J, Cooperstock M, Cunningham MW, Latimer ME, Murphy TK, et al. ; PANS Collaborative Consortium. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS consensus conference. J Child Adolesc Psychopharmacol. 2015; 25:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov ; Efficacy and safety study of low-dose ondansetron for adjunctive therapy in adult patients with obsessive-compulsive disorder [WWW document] 2015. https://clinicaltrials.gov/ct2/show/NCT01275248. [Accessed 12 January 2020]
- ClinicalTrials.gov ; Effects of ondansetron in obsessive-compulsive and tic disorders [WWW document] 2017. https://clinicaltrials.gov/ct2/show/record/NCT03239210 [Accessed 12 January 2020]
- Connor KM, Payne VM, Gadde KM, Zhang W, Davidson JRT. The use of aripiprazole in obsessive-compulsive disorder: preliminary observations in 8 patients J Clin Psychiatry. 2005; 66:49–51 [DOI] [PubMed] [Google Scholar]
- Coric V, Milanovic S, Wasylink S, Patel P, Malison R, Krystal JH. Beneficial effects of the antiglutamatergic agent riluzole in a patient diagnosed with obsessive-compulsive disorder and major depressive disorder. Psychopharmacology (Berl). 2003; 167:219–220 [DOI] [PubMed] [Google Scholar]
- Coric V, Taskiran S, Pittenger C, Wasylink S, Mathalon DH, Valentine G, et al. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biol Psychiatry. 2005; 58:424–428 [DOI] [PubMed] [Google Scholar]
- Costa DLC, Diniz JB, Requena G, Joaquim MA, Pittenger C, Bloch MH, et al. Randomized, double-blind, placebo-controlled trial of N-acetylcysteine augmentation for treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2017; 78:e766–e773 [DOI] [PubMed] [Google Scholar]
- Cottraux J, Mollard E, Bouvard M, Marks I, Sluys M, Nury AM, et al. A controlled study of fluvoxamine and exposure in obsessive-compulsive disorder. Int Clin Psychopharmacol. 1990; 5:17–30 [DOI] [PubMed] [Google Scholar]
- Cox CJ, Zuccolo AJ, Edwards EV, Mascaro-Blanco A, Alvarez K, Stoner J. Antineuronal antibodies in a heterogeneous group of youth and young adults with tics and obsessive-compulsive disorder et al. J Child Adolesc Psychopharmacol. 2015; 25:76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys D, de Geus F, van Megen HJGM, Westenberg HGM. A double-blind, randomized, placebo-controlled trial of quetiapine addition in patients with obsessive-compulsive disorder refractory to serotonin reuptake inhibitors J Clin Psychiatry. 2004 [DOI] [PubMed] [Google Scholar]
- Diniz JB, Shavitt RG, Fossaluza V, Koran L, de Bragança Pereira CA, Miguel EC. A double-blind, randomized, controlled trial of fluoxetine plus quetiapine or clomipramine versus fluoxetine plus placebo for obsessive-compulsive disorder J Clin Psychopharmacol. 2011; 31:763–768 [DOI] [PubMed] [Google Scholar]
- D’urso G, Brunoni AR, Mazzaferro MP, Anastasia A, de Bartolomeis A, Mantovani A. Transcranial direct current stimulation for obsessive–compulsive disorder: a randomized, controlled, partial crossover trial. Depress Anxiety. 2016; 33:1132–1140 [DOI] [PubMed] [Google Scholar]
- da Silva RMF, Brunoni AR, Miguel EC, Shavitt RG. Transcranial direct current stimulation for obsessive-compulsive disorder: patient selection and perspectives. Neuropsychiatr Dis Treat. 2019; 15:2663–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mathis MA, Diniz JB, Hounie AG, Shavitt RG, Fossaluza V, Ferrão Y, et al. Trajectory in obsessive-compulsive disorder comorbidities. Eur Neuropsychopharmacol. 2013; 23:594–601 [DOI] [PubMed] [Google Scholar]
- Dell’Osso B, Benatti B, Arici C, Palazzo C, Altamura AC, Hollander E, et al. Prevalence of suicide attempt and clinical characteristics of suicide attempters with obsessive-compulsive disorder: a report from the International College of Obsessive-Compulsive Spectrum Disorders (ICOCS). CNS Spectr. 2018; 23:59–66 [DOI] [PubMed] [Google Scholar]
- Dell’Osso B, Benatti B, Grancini B, Vismara M, De Carlo V, Cirnigliaro G, et al. Investigating duration of illness and duration of untreated illness in obsessive compulsive disorder reveals patients remain at length pharmacologically untreated. Int J Psychiatry Clin Pract. 2019; 23:311–313 [DOI] [PubMed] [Google Scholar]
- Dell’Osso B, Benatti B, Hollander E, Fineberg N, Stein DJ, Lochner C, et al. Childhood, adolescent and adult age at onset and related clinical correlates in obsessive–compulsive disorder: a report from the International College of Obsessive–Compulsive Spectrum Disorders (ICOCS). Int J Psychiatry Clin Pract. 2016; 20:210–217 [DOI] [PubMed] [Google Scholar]
- Dell’Osso B, Benatti B, Oldani L, Spagnolin G, Altamura AC. Differences in duration of untreated illness, duration, and severity of illness among clinical phenotypes of obsessive-compulsive disorder. CNS Spectr. 2015; 20:474–478 [DOI] [PubMed] [Google Scholar]
- Dell’Osso B, Buoli M, Hollander E, Altamura AC. Duration of untreated illness as a predictor of treatment response and remission in obsessive-compulsive disorder. World J Biol Psychiatry. 2010; 11:59–65 [DOI] [PubMed] [Google Scholar]
- Denys D. Pharmacotherapy of obsessive-compulsive disorder and obsessive-compulsive spectrum disorders. Psychiatr Clin North Am. 2006; 29:553–84, xi [DOI] [PubMed] [Google Scholar]
- Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010; 67:1061–1068 [DOI] [PubMed] [Google Scholar]
- Denys D, Van Nieuwerburgh F, Deforce D, Westenberg HG. Prediction of response to paroxetine and venlafaxine by serotonin-related genes in obsessive-compulsive disorder in a randomized, double-blind trial. J Clin Psychiatry. 2007; 68:747–753 [DOI] [PubMed] [Google Scholar]
- Di Bella D, Erzegovesi S, Cavallini MC, Bellodi L. Obsessive-compulsive disorder, 5-HTTLPR polymorphism and treatment response. Pharmacogenomics J. 2002; 2:176–181 [DOI] [PubMed] [Google Scholar]
- Dittrich WH, Johansen T, Fineberg NA. Cognitive assessment instrument of obsessions and compulsions (CAIOC-13) – a new 13-item scale for evaluating functional impairment associated with OCD. Psychiatry Res. 2011; 187:283–290 [DOI] [PubMed] [Google Scholar]
- Dold M, Aigner M, Lanzenberger R, Kasper S. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: an update meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2015; 18:pyv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond LM. CBT for Adults: A Practical Guide for Clinicians. 2014, RCPsych Publications London: Royal College of Psychiatrists; 93–111 [Google Scholar]
- Drummond LM, Edwards LJ. Obsessive Compulsive Disorder: All you Want to Know About OCD for People Living With OCD, Carers, and Clinicians. 2018, Cambridge, UK: Cambridge University Press [Google Scholar]
- Dunlop K, Woodside B, Olmsted M, Colton P, Giacobbe P, Downar J. Reductions in cortico-striatal hyperconnectivity accompany successful treatment of obsessive-compulsive disorder with dorsomedial prefrontal rtms. Neuropsychopharmacology. 2016; 41:1395–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The Brown assessment of beliefs scale: reliability and validity. Am J Psychiatry. 1998; 155:102–108 [DOI] [PubMed] [Google Scholar]
- Ellis A. Reason and Emotion in Psychotherapy. 1962, USA: Stuart Lyle (Inc.) [Google Scholar]
- Emamzadehfard S, Kamaloo A, Paydary K, Ahmadipour A, Zeinoddini A, Ghaleiha A, et al. Riluzole in augmentation of fluvoxamine for moderate to severe obsessive-compulsive disorder: randomized, double-blind, placebo-controlled study. Psychiatry Clin Neurosci. 2016; 70:332–341 [DOI] [PubMed] [Google Scholar]
- Emmelkamp PMG, Visser S, Hoekstra RJ. Cognitive therapy vs exposure in vivo in the treatment of obsessive-compulsives. Cognit Ther Res. 1988; 12:103–114 [Google Scholar]
- Ercan ES, Ardic UA, Ercan E, Yuce D, Durak S. A promising preliminary study of aripiprazole for treatment-resistant childhood obsessive-compulsive disorder J Child Adolesc Psychopharmacol. 2015; 25:580–584 [DOI] [PubMed] [Google Scholar]
- Ferreri F, Bourla A, Peretti CS, Segawa T, Jaafari N, Mouchabac S. How new technologies can improve prediction, assessment, and intervention in obsessive-compulsive disorder (e-OCD). JMIR Ment Heal. 2019; 6:e11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Kerwin L, Saxena S, Bystritsky A. Differential efficacy of memantine for obsessive-compulsive disorder vs. generalized anxiety disorder: an open-label trial. Psychopharmacol Bull. 2009; 42:81–93 [PubMed] [Google Scholar]
- Fineberg NA, Apergis-Schoute AM, Vaghi MM, Banca P, Gillan CM, Voon V, et al. Mapping compulsivity in the DSM-5 obsessive compulsive and related disorders: cognitive domains, neural circuitry, and treatment. Int J Neuropsychopharmacol. 2018a; 21:42–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Baldwin DS, Drummond LM, Wyatt S, Hanson J, Gopi S, et al. Optimal treatment for obsessive compulsive disorder: a randomized controlled feasibility study of the clinical-effectiveness and cost-effectiveness of cognitive-behavioural therapy, selective serotonin reuptake inhibitors and their combination in the mana. Int Clin Psychopharmacol. 2018b; 33:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Brown A, Reghunandanan S, Pampaloni I. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2012; 15:1173–1191 [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Craig KJ. Pharmacological treatment for obsessive–compulsive disorder. Psychiatry. 2007; 6:234–239 [Google Scholar]
- Fineberg NA, Dell’Osso B, Albert U, Maina G, Geller D, Carmi L, et al. Early intervention for obsessive compulsive disorder: an expert consensus statement. Eur Neuropsychopharmacol. 2019; 29:549–565 [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Demetrovics Z, Stein DJ, Ioannidis K, Potenza MN, Grünblatt E, et al. ; COST Action Network. Manifesto for a European research network into problematic usage of the internet. Eur Neuropsychopharmacol. 2018c; 28:1232–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Gale TM. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2005; 8:107–129 [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Krishnaiah RB, Moberg J, O’Doherty C. Clinical screening for obsessive-compulsive and related disorders. Isr J Psychiatry Relat Sci. 2008; 45:151–163 [PubMed] [Google Scholar]
- Fineberg NA, Pampaloni I, Pallanti S, Ipser J, Stein DJ. Sustained response versus relapse: the pharmacotherapeutic goal for obsessive–compulsive disorder. Int Clin Psychopharmacol. 2007; 22:313–322 [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Reghunandanan S, Brown A, Pampaloni I. Pharmacotherapy of obsessive-compulsive disorder: evidence-based treatment and beyond Aust N Z J Psychiatry. 2013; 47:121–141 [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5 Disorders: SCID-5-CV Clinician Version. 2016, Arlington, VA: American Psychiatric Association Publishing [Google Scholar]
- Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005; 162:151–161 [DOI] [PubMed] [Google Scholar]
- Fontenelle LF, Coutinho ES, Lins-Martins NM, Fitzgerald PB, Fujiwara H, Yücel M. Electroconvulsive therapy for obsessive-compulsive disorder: a systematic review. J Clin Psychiatry. 2015; 76:949–957 [DOI] [PubMed] [Google Scholar]
- Fontenelle LF, Yücel M. A clinical staging model for obsessive-compulsive disorder: is it ready for prime time? Eclinicalmedicine. 2019; 7:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin ME, Sapyta J, Freeman JB, Khanna M, Compton S, Almirall D, et al. Cognitive behavior therapy augmentation of pharmacotherapy in pediatric obsessive-compulsive disorder: the pediatric OCD treatment study II (POTS II) randomized controlled trial. JAMA. 2011; 306:1224–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J, Sapyta J, Garcia A, Compton S, Khanna M, Flessner C, et al. Family-based treatment of early childhood obsessive-compulsive disorder: the pediatric obsessive-compulsive disorder treatment study for young children (POTS jr)–a randomized clinical trial. JAMA Psychiatry. 2014; 71:689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AM, Sapyta JJ, Moore PS, Freeman JB, Franklin ME, March JS, Foa EB. Predictors and moderators of treatment outcome in the pediatric obsessive compulsive treatment study (POTS I). J Am Acad Child Adolesc Psychiatry. 2010; 49:1024–1033; quiz 1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gava I, Barbui C, Aguglia E, Carlino D, Churchill R, De Vanna M, McGuire H. Psychological treatments versus treatment as usual for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev. 2007. (2): CD005333. [DOI] [PubMed] [Google Scholar]
- Geller DA, Biederman J, Stewart SE, Mullin B, Farrell C, Wagner KD, et al. Impact of comorbidity on treatment response to paroxetine in pediatric obsessive-compulsive disorder: is the use of exclusion criteria empirically supported in randomized clinical trials? J Child Adolesc Psychopharmacol. 2003; 13Suppl 1S19–S29 [DOI] [PubMed] [Google Scholar]
- Geller DA, March J. Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. Focus (Madison). 2012; 10:360–373 [Google Scholar]
- Geller DA, Wagner KD, Emslie G, Murphy T, Carpenter DJ, Wetherhold E, et al. Paroxetine treatment in children and adolescents with obsessive-compulsive disorder: a randomized, multicenter, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2004; 43:1387–1396 [DOI] [PubMed] [Google Scholar]
- Ghaleiha A, Entezari N, Modabbernia A, Najand B, Askari N, Tabrizi M, et al. Memantine add-on in moderate to severe obsessive-compulsive disorder: randomized double-blind placebo-controlled study. J Psychiatr Res. 2013; 47:175–180 [DOI] [PubMed] [Google Scholar]
- Gomes JB, Cordioli AV, Heldt E. Obsessive-compulsive disorder and family accommodation: a 3-year follow-up. Psychiatry Res. 2017; 253:107–109 [DOI] [PubMed] [Google Scholar]
- Gomes PV, Brasil-Neto JP, Allam N, Rodrigues de Souza E. A randomized, double-blind trial of repetitive transcranial magnetic stimulation in obsessive-compulsive disorder with three-month follow-up. J Neuropsychiatry Clin Neurosci. 2012; 24:437–443 [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown obsessive compulsive scale. I. development, use, and reliability. Arch Gen Psychiatry. 1989; 46:1006–1011 [DOI] [PubMed] [Google Scholar]
- Gordon J.Towards a genomic psychiatry: recommendations of the genomics workgroup of the NAMHC [WWW Document] 2018. https://www.nimh.nih.gov/about/director/messages/2018/towards-a-genomic-psychiatry-recommendations-of-the-genomics-workgroup-of-the-namhc.shtml. [Accessed 24 December 2019]
- Gowda SM, Narayanaswamy JC, Hazari N, Bose A, Chhabra H, Balachander S, et al. Efficacy of pre-supplementary motor area transcranial direct current stimulation for treatment resistant obsessive compulsive disorder: a randomized, double blinded, sham controlled trial. Brain Stimul. 2019; 12:922–929 [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy P F, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006; 31:2384–2393 [DOI] [PubMed] [Google Scholar]
- Grünblatt E, Tschakarjan S, Brezinka V, Walitza S. Extraordinarily fast response to low-dose sertraline in a child with severe obsessive-compulsive disorder and high functioning serotonin transporter genotype. J Child Adolesc Psychopharmacol. 2014; 24:102–104 [DOI] [PubMed] [Google Scholar]
- Guglielmi V, Vulink NC, Denys D, Wang Y, Samuels JF, Nestadt G. Obsessive-compulsive disorder and female reproductive cycle events: results from the OCD and reproduction collaborative study. Depress Anxiety. 2014; 31:979–987 [DOI] [PubMed] [Google Scholar]
- Gupta A, Shepard MJ, Xu Z, Maiti T, Martinez-Moreno N, Silverman J, et al. An international radiosurgery research foundation multicenter retrospective study of gamma ventral capsulotomy for obsessive compulsive disorder. Neurosurgery. 2019; 85:808–816 [DOI] [PubMed] [Google Scholar]
- Haghighi M, Jahangard L, Mohammad-Beigi H, Bajoghli H, Hafezian H, Rahimi A, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant memantine improved symptoms in inpatients suffering from refractory obsessive-compulsive disorders (OCD). Psychopharmacology (Berl). 2013; 228:633–640 [DOI] [PubMed] [Google Scholar]
- Harika-Germaneau G, Rachid F, Chatard A, Lafay-Chebassier C, Solinas M, Thirioux B, et al. Continuous theta burst stimulation over the supplementary motor area in refractory obsessive-compulsive disorder treatment:a randomized sham-controlled trial. Brain Stimul. 2019; 12:1565–1571 [DOI] [PubMed] [Google Scholar]
- Hawken ER, Dilkov D, Kaludiev E, Simek S, Zhang F, Milev R. Transcranial magnetic stimulation of the supplementary motor area in the treatment of obsessive-compulsive disorder: a multi-site study Int J Mol Sci. 2016; 17:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess GP, Fonseca E, Scott R, Fagerness J. Pharmacogenomic and pharmacogenetic-guided therapy as a tool in precision medicine: current state and factors impacting acceptance by stakeholders Genet Res. 2015; 97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuma H, Kanehisa M, Maruyama Y, Ishitobi Y, Tanaka Y, Tsuru J. Aripiprazole augmentation in 13 patients with refractory obsessive-compulsive disorder: a case series et al. World J Biol Psychiatry. 2012; 13:14–21 [DOI] [PubMed] [Google Scholar]
- Hirschtritt ME, Bloch MH, Mathews CA. Obsessive-compulsive disorder: advances in diagnosis and treatment. JAMA. 2017; 317:1358–1367 [DOI] [PubMed] [Google Scholar]
- Hohagen F, Winkelmann G, Rasche-Räuchle H, Hand I, König A, Münchau N, et al. Combination of behaviour therapy with fluvoxamine in comparison with behaviour therapy and placebo: results of a multicentre study. Br J Psychiatry. 1998; 173:71–78 [PubMed] [Google Scholar]
- Hollander E, Doernberg E, Shavitt R, Waterman RJ, Soreni N, Veltman DJ, et al. The cost and impact of compulsivity: a research perspective. Eur Neuropsychopharmacol. 2016; 26:800–809 [DOI] [PubMed] [Google Scholar]
- Hollander E, Stein DJ, Fineberg NA, Marteau F, Legault M. Quality of life outcomes in patients with obsessive-compulsive disorder: relationship to treatment response and symptom relapse. J Clin Psychiatry. 2010; 71:784–792 [DOI] [PubMed] [Google Scholar]
- Horowitz MA, Taylor D. Tapering of SSRI treatment to mitigate withdrawal symptoms - authors’ reply. Lancet Psychiatry. 2019; 6:562–563 [DOI] [PubMed] [Google Scholar]
- Hou Y-C, Lai C-H. Rapid responses of high-dose combined therapy of escitalopram and aripiprazole in a case of severe obsessive compulsive disorder with delusion J Neuropsychiatry Clin Neurosci. 2014; 26:E44–E45 [DOI] [PubMed] [Google Scholar]
- Hussain A, Dar MA, Wani RA, Shah MS, Jan MM, Malik YA, et al. Role of lamotrigine augmentation in treatment-resistant obsessive compulsive disorder: a retrospective case review from South Asia. Indian J Psychol Med. 2015; 37:154–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Hamilton JA, Guttmacher LB, Murphy DL. D-amphetamine in obsessive-compulsive disorder. Psychopharmacology (Berl). 1983; 80:231–235 [DOI] [PubMed] [Google Scholar]
- Ioannidis K, Treder MS, Chamberlain SR, Kiraly F, Redden SA, Stein DJ, et al. Problematic internet use as an age-related multifaceted problem: evidence from a two-site survey. Addict Behav. 2018; 81:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janardhan Reddy YC, Sundar AS, Narayanaswamy JC, Math SB. Clinical practice guidelines for obsessive-compulsive disorder. Indian J Psychiatry. 2017; 59:S74–S90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe RT, Swinson RP, Levitt AJ. Acute psychostimulant challenge in primary obsessive-compulsive disorder. J Clin Psychopharmacol. 1991; 11:237–241 [PubMed] [Google Scholar]
- Jones MK, Menzies RG. Danger ideation reduction therapy (DIRT) for obsessive-compulsive washers. A controlled trial. Behav Res Ther. 1998; 36:959–970 [DOI] [PubMed] [Google Scholar]
- Katzman MA, Bleau P, Blier P, Chokka P, Kjernisted K, Van Ameringen M, et al. ; Canadian Anxiety Guidelines Initiative Group on behalf of the Anxiety Disorders Association of Canada/Association Canadienne des troubles anxieux and McGill University. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014; 14Suppl 1S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalkhali M, Aram S, Zarrabi H, Kafie M, Heidarzadeh A. Lamotrigine augmentation versus placebo in serotonin reuptake inhibitors-resistant obsessive-compulsive disorder: a randomized controlled trial. Iran J Psychiatry. 2016; 11:104–114 [PMC free article] [PubMed] [Google Scholar]
- Kichuk SA, Torres AR, Fontenelle LF, Rosário MC, Shavitt RG, Miguel EC, et al. Symptom dimensions are associated with age of onset and clinical course of obsessive–compulsive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013; 44:233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Király O, Griffiths MD, Demetrovics Z. Internet gaming disorder and the DSM-5: conceptualization, debates, and controversies. Curr Addict Reports. 2015; 2:254–262 [Google Scholar]
- Koran LM, Aboujaoude E, Bullock KD, Franz B, Gamel N, Elliott M. Double-blind treatment with oral morphine in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2005; 66:353–359 [DOI] [PubMed] [Google Scholar]
- Koran LM, Aboujaoude E, Gamel NN. Double-blind study of dextroamphetamine versus caffeine augmentation for treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2009; 70:1530–1535 [DOI] [PubMed] [Google Scholar]
- Koran LM, Hanna GL, Hollander E, Nestadt G, Simpson HB; American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007; 164:5–53 [PubMed] [Google Scholar]
- Koran LM, Simpson HB. Guideline watch (March 2013): practice guideline for the treatment of patients with obsessive-compulsive disorder. Arlington VA Am Psychiatr Assoc. 2013 [PubMed] [Google Scholar]
- Kordon A, Wahl K, Koch N, Zurowski B, Anlauf M, Vielhaber K. Quetiapine addition to serotonin reuptake inhibitors in patients with severe obsessive-compulsive disorder: a double-blind, randomized, placebo-controlled study et al. J Clin Psychopharmacol. 2008; 28:550–554 [DOI] [PubMed] [Google Scholar]
- Krochmalik A, Jones MK, Menzies RG. Danger ideation reduction therapy (DIRT) for treatment-resistant compulsive washing. Behav Res Ther. 2001; 39:897–912 [DOI] [PubMed] [Google Scholar]
- Kumar TC, Khanna S. Lamotrigine augmentation of serotonin re-uptake inhibitors in obsessive-compulsive disorder. Aust N Z J Psychiatry. 2000; 34:527–528 [DOI] [PubMed] [Google Scholar]
- Lafleur DL, Pittenger C, Kelmendi B, Gardner T, Wasylink S, Malison RT, et al. N-acetylcysteine augmentation in serotonin reuptake inhibitor refractory obsessive-compulsive disorder. Psychopharmacology (Berl). 2006; 184:254–256 [DOI] [PubMed] [Google Scholar]
- Lee MT, Mpavaenda DN, Fineberg NA. Habit reversal therapy in obsessive compulsive related disorders: a systematic review of the evidence and CONSORT evaluation of randomized controlled trials. Front Behav Neurosci. 2019a; 13:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Anttila V, Won H, Feng YCA, Rosenthal J, Zhu Z, et al. Genomic Relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 2019b; 179:1469–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rtms). Clin Neurophysiol. 2014; 125:2150–2206 [DOI] [PubMed] [Google Scholar]
- Lewin AB, Park JM, Jones AM, Crawford EA, De Nadai AS, Menzel J, et al. Family-based exposure and response prevention therapy for preschool-aged children with obsessive-compulsive disorder: a pilot randomized controlled trial. Behav Res Ther. 2014; 56:30–38 [DOI] [PubMed] [Google Scholar]
- Lipszyc J, Schachar R. Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. J Int Neuropsychol Soc. 2010; 16:1064–1076 [DOI] [PubMed] [Google Scholar]
- Lisoway AJ, Zai G, Tiwari AK, Zai CC, Wigg K, Goncalves V, et al. Pharmacogenetic evaluation of a DISP1 gene variant in antidepressant treatment of obsessive–compulsive disorder Hum Psychopharmacol Clin Exp. 2018; 33:e2659. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu J, Long J. Paroxetine combined with cognitive behavior therapy in treatment of obsessive-compulsive disorder. Chinese J Heal Psychol. 2005; 2:86–87 [Google Scholar]
- Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, et al. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta-analysis. JAMA Psychiatry. 2017; 74:1011–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Pina JA, Sanchez-Meca J, López-López JA, Marin-Martinez F, Núñez-Núñez RM, Rosa-Alcazar AI, et al. Reliability generalization study of the Yale–Brown obsessive–compulsive scale for children and adolescents. J Pers Assess. 2015a; 97:42–54 [DOI] [PubMed] [Google Scholar]
- López-Pina JA, Sánchez-Meca J, López-López JA, Marín-Martínez F, Núñez-Núñez RM, Rosa-Alcázar AI, et al. The Yale–Brown obsessive compulsive scale: a reliability generalization meta-analysis. Assessment. 2015b; 22:619–628 [DOI] [PubMed] [Google Scholar]
- Lusicic A, Schruers KRJ, Pallanti S, Castle DJ. Transcranial magnetic stimulation in the treatment of obsessive–compulsive disorder: current perspectives. Neuropsychiatr Dis Treat. 2018; 14:1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, Hendrickx S, Raymaekers S, Gabriëls L, Nuttin B. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry. 2016; 21:1272–1280 [DOI] [PubMed] [Google Scholar]
- Maina G, Albert U, Bogetto F. Relapses after discontinuation of drug associated with increased resistance to treatment in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2001; 16:33–38 [DOI] [PubMed] [Google Scholar]
- Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, et al. ; STOC Study Group. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008; 359:2121–2134 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Simpson HB, Fallon BA, Rossi S, Lisanby SH. Randomized sham-controlled trial of repetitive transcranial magnetic stimulation in treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2010; 13:217–227 [DOI] [PubMed] [Google Scholar]
- Maqbool M, Sengar KS, Vikas Kumar M, Uparikar PD. Efficacy of danger ideation reduction therapy in obsessive-compulsive disorder washer with poor insight: a case study and literature review. Indian J Psychol Med. 2017; 39:523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzano L, Bardill A, Fields B, Herd K, Veale D, Grey N, Moran P. The application of mhealth to mental health: opportunities and challenges. Lancet Psychiatry. 2015; 2:942–948 [DOI] [PubMed] [Google Scholar]
- Mas S, Blázquez A, Rodríguez N, Boloc D, Lafuente A, Arnaiz JA, et al. Pharmacogenetic study focused on fluoxetine pharmacodynamics in children and adolescent patients: impact of the serotonin pathway Pharmacogenet Genomics. 2016; 26:487–496 [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Frans E, Pérez-Vigil A, Kuja-Halkola R, Gromark C, Isomura K, et al. A total-population multigenerational family clustering study of autoimmune diseases in obsessive-compulsive disorder and Tourette’s/chronic tic disorders. Mol Psychiatry. 2018; 23:1652–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga H, Hayashida K, Maebayashi K, Mito H, Kiriike N. A case series of aripiprazole augmentation of selective serotonin reuptake inhibitors in treatment-refractory obsessive compulsive disorder Int J Psychiatry Clin Pract. 2011; 15:263–269 [DOI] [PubMed] [Google Scholar]
- McGuire JF, Piacentini J, Scahill L, Woods DW, Villarreal R, Wilhelm S, et al. Bothersome tics in patients with chronic tic disorders: characteristics and individualized treatment response to behavior therapy. Behav Res Ther. 2015; 70:56–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior K, Franken I, Deen M, van der Heiden C. Metacognitive therapy versus exposure and response prevention for obsessive-compulsive disorder: study protocol for a randomized controlled trial. Trials. 2019; 20:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menchón JM, Real E, Alonso P, Aparicio MA, Segalas C, Plans G, et al. A prospective international multi-center study on safety and efficacy of deep brain stimulation for resistant obsessive-compulsive disorder. Mol Psychiatry. 2019; 24:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng FQ, Han HY, Luo J, Liu J, Liu ZR, Tang Y, et al. Efficacy of cognitive behavioural therapy with medication for patients with obsessive-compulsive disorder: a multicentre randomised controlled trial in china. J Affect Disord. 2019; 253:184–192 [DOI] [PubMed] [Google Scholar]
- Miguel EC, Lopes AC, McLaughlin NCR, Norén G, Gentil AF, Hamani C, et al. Evolution of gamma knife capsulotomy for intractable obsessive-compulsive disorder. Mol Psychiatry. 2019; 24:218–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguita K, Cordeiro Q, Shavitt RG, Miguel EC, Vallada H. Association study between genetic monoaminergic polymorphisms and OCD response to clomipramine treatment. Arq Neuropsiquiatr. 2011; 69:283–287 [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Shams E, Katz C, Mansouri B, Moussavi Z, Sareen J, Enns M. The efficacy of deep repetitive transcranial magnetic stimulation over the medial prefrontal cortex in obsessive compulsive disorder: results from an open-label study. Depress Anxiety. 2015; 32:445–450 [DOI] [PubMed] [Google Scholar]
- Montgomery SA. Clomipramine in obsessional neurosis: a placebo-controlled trial. Pharm Med. 1980; 1:189–192 [Google Scholar]
- Mowla A, Khajeian AM, Sahraian A, Chohedri AH, Kashkoli F. Topiramate augmentation in resistant OCD: a double-blind placebo-controlled clinical trial. CNS Spectr. 2010; 15:613–617 [DOI] [PubMed] [Google Scholar]
- Muscatello MR, Bruno A, Pandolfo G, Micò U, Scimeca G, Romeo VM, et al. Effect of aripiprazole augmentation of serotonin reuptake inhibitors or clomipramine in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. J Clin Psychopharmacol. 2011; 31:174–179 [DOI] [PubMed] [Google Scholar]
- National Advisory Mental Health Council Workgroup on Genomics ; Report of the National Advisory Mental Health Council Workgroup on Genomics [WWW Document] 2019. https://www.nimh.nih.gov/about/advisory-boards-and-groups/namhc/reports/report-of-the-national-advisory-mental-health-council-workgroup-on-genomics.shtml#acknowledgement. [Accessed 24 December 2001]
- National Institute for Health and Clinical Excellence ; Overview | Obsessive-Compulsive Disorder and Body Dysmorphic Disorder: Treatment. 2005a, London: Guidance | NICE [Google Scholar]
- National Institute for Health and Clinical Excellence ; Obsessive-Compulsive Disorder: Core Interventions in the Treatment of Obsessive-Compulsive Disorder and Body Dysmorphic Disorder. Quick Reference Guide. 2005b, London: National Institute for Clinical Excellence; [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (2019). ; CG31), 2019 surveillance of obsessive-compulsive disorder and body dysmorphic disorder: treatment (NICE guideline [WWW Document] https://www.nice.org.uk/guidance/cg31/resources/2019-surveillance-of-obsessivecompulsive-disorder-and-body-dysmorphic-disorder-treatment-nice-guideline-cg31-6713804845/chapter/Surveillance-decision?tab=evidence. [Accessed 12 December 2019] [PubMed]
- Nauczyciel C, Le Jeune F, Naudet F, Douabin S, Esquevin A, Vérin M, et al. Repetitive transcranial magnetic stimulation over the orbitofrontal cortex for obsessive-compulsive disorder: a double-blind, crossover study. Transl Psychiatry. 2014; 4:e436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezgovorova V, Rigby N, Battles J, Krause D, Fineberg N, Ameringen M, et al. Genetic Variants of OCD Phenotypes and Comorbid Conditions, ACNP Poster, 2018. 2018, Hollywood, Florida: The American College of Neuropsychopharmacology [Google Scholar]
- Niciu MJ, Grunschel BDG, Corlett PR, Pittenger C, Bloch MH. Two cases of delayed-onset suicidal ideation, dysphoria and anxiety after ketamine infusion in patients with obsessive–compulsive disorder and a history of major depressive disorder. J Psychopharmacol. 2013; 27:651–654 [DOI] [PubMed] [Google Scholar]
- Nicholson TR, Ferdinando S, Krishnaiah RB, Anhoury S, Lennox BR, Mataix-Cols D, et al. Prevalence of anti-basal ganglia antibodies in adult obsessive–compulsive disorder: cross-sectional study. BrJ Psychiatry. 2012; 200:381–386 [DOI] [PubMed] [Google Scholar]
- Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999; 354:1526. [DOI] [PubMed] [Google Scholar]
- Ougrin D. Efficacy of exposure versus cognitive therapy in anxiety disorders: systematic review and meta-analysis BMC Psychiatry. 2011; 11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanti S, Grassi G. Pharmacologic treatment of obsessive-compulsive disorder comorbidity. Expert Opin Pharmacother. 2014; 15:2543–2552 [DOI] [PubMed] [Google Scholar]
- Pallanti S, Marras A, Salerno L, Makris N, Hollander E. Better than treated as usual: transcranial magnetic stimulation augmentation in selective serotonin reuptake inhibitor-refractory obsessive–compulsive disorder, mini-review and pilot open-label trial. J Psychopharmacol. 2016; 30:568–578 [DOI] [PubMed] [Google Scholar]
- Pasquini M, Biondi M. Memantine augmentation for refractory obsessive–compulsive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2006; 30:1173–1175 [DOI] [PubMed] [Google Scholar]
- Patra S. Treat the disease not the symptoms: successful management of obsessive compulsive disorder in bipolar disorder with aripiprazole augmentation Aust N Z J Psychiatry. 2016; 50:809–810 [DOI] [PubMed] [Google Scholar]
- Paydary K, Akamaloo A, Ahmadipour A, Pishgar F, Emamzadehfard S, Akhondzadeh S. N-acetylcysteine augmentation therapy for moderate-to-severe obsessive–compulsive disorder: randomized, double-blind, placebo-controlled trial. J Clin Pharm Ther. 2016; 41:214–219 [DOI] [PubMed] [Google Scholar]
- Pelissolo A, Harika-Germaneau G, Rachid F, Gaudeau-Bosma C, Tanguy ML, BenAdhira R, et al. Repetitive transcranial magnetic stimulation to supplementary motor area in refractory obsessive-compulsive disorder treatment: a sham-controlled trial. Int J Neuropsychopharmacol. 2016; 19:pyw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper J, Hariz M, Zrinzo L. Deep brain stimulation versus anterior capsulotomy for obsessive-compulsive disorder: a review of the literature. J Neurosurg. 2015; 122:1028–1037 [DOI] [PubMed] [Google Scholar]
- Pepper J, Zrinzo L, Hariz M. Anterior capsulotomy for obsessive-compulsive disorder: a review of old and new literature. J Neurosurg. 2019; 122:1–10 [DOI] [PubMed] [Google Scholar]
- Pérez Álvarez M. Third-generation therapies: achievements and challenges. Int J Clin Heal Psychol. 2012; 12:291–310 [Google Scholar]
- Pessina E, Albert U, Bogetto F, Maina G. Aripiprazole augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive–compulsive disorder: a 12-week open-label preliminary study Int Clin Psychopharmacol. 2009; 24:265–269 [DOI] [PubMed] [Google Scholar]
- Piacentini J, Bergman RL, Chang S, Langley A, Peris T, Wood JJ, McCracken J. Controlled comparison of family cognitive behavioral therapy and psychoeducation/relaxation training for child obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2011; 50:1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C. Glutamatergic agents for OCD and related disorders. Curr Treat Options Psychiatry. 2015; 2:271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacol Ther. 2011; 132:314–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Kelmendi B, Wasylink S, Bloch MH, Coric V. Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: a series of 13 cases, with long-term follow-up. J Clin Psychopharmacol. 2008; 28:363–367 [DOI] [PubMed] [Google Scholar]
- Postorino V, Kerns CM, Vivanti G, Bradshaw J, Siracusano M, Mazzone L. Anxiety disorders and obsessive-compulsive disorder in individuals with autism spectrum disorder. Curr Psychiatry Rep. 2017; 19:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyurovsky M, Weizman R, Weizman A, Koran L. Memantine for treatment-resistant OCD. Am J Psychiatry. 2005; 162:2191–2192 [DOI] [PubMed] [Google Scholar]
- Qin H, Samuels JF, Wang Y, Zhu Y, Grados MA, Riddle MA, et al. Whole-genome association analysis of treatment response in obsessive-compulsive disorder. Mol Psychiatry. 2016; 21:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachid F. Transcranial direct current stimulation for the treatment of obsessive-compulsive disorder? A qualitative review of safety and efficacy. Psychiatry Res. 2019; 271:259–264 [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Malaise, melancholia and madness: the evolutionary legacy of an inflammatory bias. Brain Behav Immun. 2013; 31:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapinesi C, Kotzalidis GD, Ferracuti S, Sani G, Girardi P, Del Casale A. Brain stimulation in obsessive-compulsive disorder (OCD): a systematic review. Curr Neuropharmacol. 2019; 17:787–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp AM, Bergman RL, Piacentini J, McGuire JF. Evidence-based assessment of obsessive–compulsive disorder. J Cent Nerv Syst Dis. 2016; 8:JCNSD–S38359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehn S, Eslick GD, Brakoulias V. A meta-analysis of the effectiveness of different cortical targets used in repetitive transcranial magnetic stimulation (rtms) for the treatment of obsessive-compulsive disorder (OCD). Psychiatr Q. 2018; 89:645–665 [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Lapidus K, Zwerling J, Levinson A, Mahnke A, Steinman S, et al. Challenges testing intranasal ketamine in obsessive-compulsive disorder (OCD). Neuropsychopharmacology. 2017; 43:S128–S129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Flood P, Simpson HB. Rapid resolution of obsessions after an infusion of intravenous ketamine in a patient with treatment-resistant obsessive-compulsive disorder: a case report. J Clin Psychiatry. 2011; 72:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013; 38:2475–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Levinson A, Zwerling J, Vermes D, Simpson HB. Open-label trial on the effects of memantine in adults with obsessive-compulsive disorder after a single ketamine infusion. J Clin Psychiatry. 2016; 77:688–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli RJ, Wu FM, Gamba R, Mojtabai R, Segal JB. Behavioral therapy and serotonin reuptake inhibitor pharmacotherapy in the treatment of obsessive–compulsive disorder: a systematic review and meta-analysis of head-to-head randomized controlled trials. Depress Anxiety. 2014; 31:641–652 [DOI] [PubMed] [Google Scholar]
- Rosa-Alcázar AI, Sánchez-Meca J, Gómez-Conesa A, Marín-Martínez F. Psychological treatment of obsessive–compulsive disorder: a meta-analysis. Clin Psychol Rev. 2008; 28:1310–1325 [DOI] [PubMed] [Google Scholar]
- Rosario-Campos MC, Miguel EC, Quatrano S, Chacon P, Ferrao Y, Findley D, et al. The dimensional Yale-Brown obsessive-compulsive scale (DY-BOCS): an instrument for assessing obsessive-compulsive symptom dimensions. Mol Psychiatry. 2006; 11:495–504 [DOI] [PubMed] [Google Scholar]
- Rubio G, Jiménez-Arriero MA, Martínez-Gras I, Manzanares J, Palomo T. The effects of topiramate adjunctive treatment added to antidepressants in patients with resistant obsessive-compulsive disorder. J Clin Psychopharmacol. 2006; 26:341–344 [DOI] [PubMed] [Google Scholar]
- Ruffini C, Locatelli M, Lucca A, Benedetti F, Insacco C, Smeraldi E. Augmentation effect of repetitive transcranial magnetic stimulation over the orbitofrontal cortex in drug-resistant obsessive-compulsive disorder patients: a controlled investigation. Prim Care Companion J Clin Psychiatry. 2009; 11:226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp C, Jürgens C, Doebler P, Andor F, Buhlmann U. A randomized waitlist-controlled trial comparing detached mindfulness and cognitive restructuring in obsessive-compulsive disorder. PLoS One. 2019; 14:e0213895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkovskis PM. Obsessional-compulsive problems: a cognitive-behavioural analysis. Behav Res Ther. 1985; 23:571–583 [DOI] [PubMed] [Google Scholar]
- Salkovskis PM. Understanding and treating obsessive-compulsive disorder. Behav Res Ther. 1999; 37Suppl 1S29–S52 [PubMed] [Google Scholar]
- Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, et al. ; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017; 74:399–405 [DOI] [PubMed] [Google Scholar]
- Sánchez-Meca J, Rosa-Alcázar AI, Iniesta-Sepúlveda M, Rosa-Alcázar Á. Differential efficacy of cognitive-behavioral therapy and pharmacological treatments for pediatric obsessive–compulsive disorder: a meta-analysis. J Anxiety Disord. 2014; 28:31–44 [DOI] [PubMed] [Google Scholar]
- Sarris J, Oliver G, Camfield DA, Dean OM, Dowling N, Smith DJ, et al. N-acetyl cysteine (NAC) in the treatment of obsessive-compulsive disorder: a 16-week, double-blind, randomised, placebo-controlled study. CNS Drugs. 2015; 29:801–809 [DOI] [PubMed] [Google Scholar]
- Sayyah M, Sayyah M, Boostani H, Ghaffari SM, Hoseini A. Effects of aripiprazole augmentation in treatment-resistant obsessive-compulsive disorder (a double blind clinical trial). Depress Anxiety. 2012; 29:850–854 [DOI] [PubMed] [Google Scholar]
- Serata D, Kotzalidis GD, Rapinesi C, Janiri D, Di Pietro S, Callovini G, et al. Are 5-HT3 antagonists effective in obsessive–compulsive disorder? A systematic review of literature. Hum Psychopharmacol Clin Exp. 2015; 30:70–84 [DOI] [PubMed] [Google Scholar]
- Shalbafan M, Mohammadinejad P, Shariat SV, Alavi K, Zeinoddini A, Salehi M, et al. Celecoxib as an adjuvant to fluvoxamine in moderate to severe obsessive-compulsive disorder: a double-blind, placebo-controlled, randomized trial. Pharmacopsychiatry. 2015; 48:136–140 [DOI] [PubMed] [Google Scholar]
- Shavitt RG, de Mathis MA, Oki F, Ferrao YA, Fontenelle LF, Torres A R, et al. Phenomenology of OCD: lessons from a large multicenter study and implications for ICD-11. J Psychiatr Res. 2014; 57:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998; 59Suppl 2022–33;quiz 34–57 [PubMed] [Google Scholar]
- Simpson HB, Foa EB, Liebowitz MR, Huppert JD, Cahill S, Maher M J, et al. Cognitive-behavioral therapy vs risperidone for augmenting serotonin reuptake inhibitors in obsessive-compulsive disorder: a randomized clinical trial. JAMA Psychiatry. 2013; 70:1190–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HB, Foa EB, Liebowitz MR, Ledley DR, Huppert JD, Cahill S, et al. A randomized, controlled trial of cognitive-behavioral therapy for augmenting pharmacotherapy in obsessive-compulsive disorder. Am J Psychiatry. 2008; 165:621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sina M, Ahmadiani A, Asadi S, Shams J. Association of serotonin receptor 2a haplotypes with obsessive–compulsive disorder and its treatment response in iranian patients: a genetic and pharmacogenetic study. Neuropsychiatr Dis Treat. 2018; 14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapinakis P, Caldwell D, Hollingworth W, Bryden P, Fineberg N, Salkovskis P, et al. A systematic review of the clinical effectiveness and cost-effectiveness of pharmacological and psychological interventions for the management of obsessive-compulsive disorder in children/adolescents and adults. Health Technol Assess. 2016a; 20:1–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapinakis P, Caldwell DM, Hollingworth W, Bryden P, Fineberg NA, Salkovskis P, et al. Pharmacological and psychotherapeutic interventions for management of obsessive-compulsive disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2016b; 3:730–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarphedinsson G, Weidle B, Ivarsson T. Sertraline treatment of nonresponders to extended cognitive-behavior therapy in pediatric obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2015; 25:574–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog G, Skoog I. A 40-year follow-up of patients with obsessive-compulsive disorder [see comments]. Arch Gen Psychiatry. 1999; 56:121–127 [DOI] [PubMed] [Google Scholar]
- Sookman D, Fineberg NA; Accreditation Task Force of the Canadian Institute for Obsessive Compulsive Disorders. Specialized psychological and pharmacological treatments for obsessive-compulsive disorder throughout the lifespan: a special series by the accreditation task force (ATF) of the Canadian Institute for Obsessive Compulsive Disorders (CIOCD, www.ciocd.ca). Psychiatry Res. 2015; 227:74–77 [DOI] [PubMed] [Google Scholar]
- Soomro GM, Altman DG, Rajagopal S, Browne MO. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane database Syst Rev. 2008; 1:CD001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz EJ, Freeman GM, Jr, Brown K, Farhadian B, Thienemann M, Frankovich J. Course of neuropsychiatric symptoms after introduction and removal of nonsteroidal anti-inflammatory drugs: a pediatric observational study. J Child Adolesc Psychopharmacol. 2017; 27:652–659 [DOI] [PubMed] [Google Scholar]
- Stein DJ, Koen N, Fineberg N, Fontenelle LF, Matsunaga H, Osser D, Simpson HB. A 2012 evidence-based algorithm for the pharmacotherapy for obsessive-compulsive disorder. Curr Psychiatry Rep. 2012; 14:211–219 [DOI] [PubMed] [Google Scholar]
- Stewart SE, Geller DA, Jenike M, Pauls D, Shaw D, Mullin B, Faraone SV. Long-term outcome of pediatric obsessive–compulsive disorder: a meta-analysis and qualitative review of the literature. Acta Psychiatr Scand. 2004; 110:4–13 [DOI] [PubMed] [Google Scholar]
- Stewart SE, Jenike EA, Hezel DM, Stack DE, Dodman NH, Shuster L, Jenike MA. A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. J Clin Psychopharmacol. 2010; 30:34–39 [DOI] [PubMed] [Google Scholar]
- Storch EA, De Nadai AS, Jacob ML, Lewin AB, Muroff J, Eisen J, et al. Phenomenology and correlates of insight in pediatric obsessive–compulsive disorder. Compr Psychiatry. 2014; 55:613–620 [DOI] [PubMed] [Google Scholar]
- Storch EA, Rasmussen SA, Price LH, Larson MJ, Murphy TK, Goodman WK. Development and psychometric evaluation of the Yale–Brown obsessive-compulsive scale—second edition. Psychol Assess. 2010; 22:223. [DOI] [PubMed] [Google Scholar]
- Taj MJRJ, Ganesh S, Shukla T, Deolankar S, Nadella RK, Sen S, et al. BDNF gene and obsessive compulsive disorder risk, symptom dimensions and treatment response. Asian J Psychiatr. 2018; 38:65–69 [DOI] [PubMed] [Google Scholar]
- Tampi RR, Balderas M, Carter KV, Tampi DJ, Moca M, Knudsen A, May J. Citalopram, qtc prolongation, and torsades de pointes. Psychosomatics. 2015; 56:36–43 [DOI] [PubMed] [Google Scholar]
- Torres AR, Ramos-Cerqueira AT, Ferrão YA, Fontenelle LF, do Rosário MC, Miguel EC. Suicidality in obsessive-compulsive disorder: prevalence and relation to symptom dimensions and comorbid conditions. J Clin Psychiatry. 2011; 72:17–26; quiz 119 [DOI] [PubMed] [Google Scholar]
- Tyagi H, Apergis-Schoute AM, Akram H, Foltynie T, Limousin P, Drummond LM, et al. A randomized trial directly comparing ventral capsule and anteromedial subthalamic nucleus stimulation in obsessive-compulsive disorder: clinical and imaging evidence for dissociable effects. Biol Psychiatry. 2019; 85:726–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi H, Drummond LM, Fineberg NA. Treatment for obsessive compulsive disorder. Curr Psychiatry Rev. 2010; 6:46–55 [Google Scholar]
- Umehara H, Numata S, Kinoshita M, Watanabe S, Nakaaki S, Sumitani S, Ohmori T. No association between BDNF val66met polymorphism and treatment response in obsessive-compulsive disorder in the Japanese population. Neuropsychiatr Dis Treat. 2016; 12:611–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara H, Numata S, Tajima A, Kinoshita M, Nakaaki S, Imoto I, et al. No association between the COMT Val158Met polymorphism and the long-term clinical response in obsessive–compulsive disorder in the Japanese population Hum Psychopharmacol Clin Exp. 2015; 30:372–376 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration ; Humanitarian Device Exemption (HDE) [WWW Document] 2009. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfhde/hde.cfm?id=H050003. [Accessed 27 January 2020]
- US Food and Drug Administration ; FDA Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses | FDA [WWW Document] 2012. http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. [Accessed 19 December 2019]
- US Food and Drug Administration ; FDA permits marketing of transcranial magnetic stimulation for treatment of obsessive compulsive disorder [WWW Document] 2018. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-transcranial-magnetic-stimulation-treatment-obsessive-compulsive-disorder. [Accessed 27 January 2019]
- Üstün TB, Kostanjsek N, Chatterji S, Rehm J. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule WHODAS 2.0. 2010, Geneva: World Health Organization [Google Scholar]
- Uzun Ö. Lamotrigine as an augmentation agent in treatment-resistant obsessive-compulsive disorder: a case report. J Psychopharmacol. 2010; 24:425–427 [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Mancini C, Patterson B, Bennett M. Topiramate augmentation in treatment-resistant obsessive–compulsive disorder: a retrospective, open-label case series. Depress Anxiety. 2006; 23:1–5 [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Patterson B. Topiramate augmentation in a patient with obsessive–compulsive disorder. J psychiatry Neurosci JPN. 2015; 40:E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nieuwerburgh FC, Denys DA, Westenberg HG, Deforce DL. Response to serotonin reuptake inhibitors in OCD is not influenced by common CYP2D6 polymorphisms. Int J Psychiatry Clin Pract. 2009; 13:345–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veale D, Miles S, Smallcombe N, Ghezai H, Goldacre B, Hodsoll J. Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and meta-analysis BMC Psychiatry. 2014; 14:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulink NCC, Denys D, Fluitman SB, Meinardi JCM, Westenberg HGM. Quetiapine augments the effect of citalopram in non-refractory obsessive-compulsive disorder: a randomized, double-blind, placebo-controlled study of 76 patients J Clin Psychiatry. 2009; 70:1001. [DOI] [PubMed] [Google Scholar]
- Warneke L. A possible new treatment approach to obsessive–compulsive disorder. Can J Psychiatry. 1997; 42:667–668 [DOI] [PubMed] [Google Scholar]
- Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012; 92:414–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside S, Ale C, Vickers K, Tiede M, Dammann J. Case examples of enhancing pediatric OCD treatment with a smartphone application. Clin Case Stud. 2013; 13:80–94 [Google Scholar]
- Wilbur C, Bitnun A, Kronenberg S, Laxer RM, Levy DM, Logan WJ, et al. PANDAS/PANS in childhood: controversies and evidence Paediatr Child Health. 2019; 24:85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization ; International Classification of Diseases for Mortality and Morbidity Statistics (11th Revision). 2018, Geneva: World Health Organization. [Google Scholar]
- World Health Organization ; Monitoring and Evaluating Digital Health Interventions: A Practical Guide to Conducting Research and Assessment, Who. 2016, Geneva: World Health Organization. [Google Scholar]
- Zai G, Brandl EJ, Müller DJ, Richter MA, Kennedy JL. Pharmacogenetics of antidepressant treatment in obsessive-compulsive disorder: an update and implications for clinicians. Pharmacogenomics. 2014; 15:1147–1157 [DOI] [PubMed] [Google Scholar]
- Zhou DD, Wang W, Wang GM, Li DQ, Kuang L. An updated meta-analysis: short-term therapeutic effects of repeated transcranial magnetic stimulation in treating obsessive-compulsive disorder. J Affect Disord. 2017; 215:187–196 [DOI] [PubMed] [Google Scholar]
- Zhou DD, Zhou XX, Lv Z, Chen XR, Wang W, Wang GM, et al. Comparative efficacy and tolerability of antipsychotics as augmentations in adults with treatment-resistant obsessive-compulsive disorder: a network meta-analysis. J Psychiatr Res. 2019; 111:51–58 [DOI] [PubMed] [Google Scholar]
- Zohar J. Is there room for a new diagnostic subtype – the schizo-obsessive subtype? CNS Spectr. 1997; 2:49–50 [Google Scholar]
- Zohar J. Obsessive Compulsive Disorder: Current Science and Clinical Practice. 2012, Cambridge: John Wiley & Sons [Google Scholar]
- Zohar J, Insel TR. Obsessive-compulsive disorder: psychobiological approaches to diagnosis, treatment, and pathophysiology. Biol Psychiatry. 1987; 22:667–687 [DOI] [PubMed] [Google Scholar]
- Zohar J, Judge R; Investigators, O.C.D.P.S. Paroxetine versus clomipramine in the treatment of obsessive–compulsive disorder. Br J Psychiatry. 1996; 169:468–474 [DOI] [PubMed] [Google Scholar]
- Zohar J, Mueller EA, Insel TR, Zohar-Kadouch RC, Murphy DL. Serotonergic responsivity in obsessive-compulsive disorder. Comparison of patients and healthy controls. Arch Gen Psychiatry. 1987; 44:946–951 [DOI] [PubMed] [Google Scholar]


