Abstract
BACKGROUND
In the ISCHEMIA-CKD trial, the primary analysis showed no significant difference in the risk of death or myocardial infarction with initial angiography and revascularization plus guideline-based medical therapy (invasive strategy) as compared with guideline-based medical therapy alone (conservative strategy) in participants with stable ischemic heart disease, moderate or severe ischemia, and advanced chronic kidney disease (an estimated glomerular filtration rate of <30 ml per minute per 1.73 m2 or receipt of dialysis). A secondary objective of the trial was to assess angina-related health status.
METHODS
We assessed health status with the Seattle Angina Questionnaire (SAQ) before randomization and at 1.5, 3, and 6 months and every 6 months thereafter. The primary outcome of this analysis was the SAQ Summary score (ranging from 0 to 100, with higher scores indicating less frequent angina and better function and quality of life). Mixed-effects cumulative probability models within a Bayesian framework were used to estimate the treatment effect with the invasive strategy.
RESULTS
Health status was assessed in 705 of 777 participants. Nearly half the participants (49%) had had no angina during the month before randomization. At 3 months, the estimated mean difference between the invasive-strategy group and the conservative-strategy group in the SAQ Summary score was 2.1 points (95% credible interval, −0.4 to 4.6), a result that favored the invasive strategy. The mean difference in score at 3 months was largest among participants with daily or weekly angina at baseline (10.1 points; 95% credible interval, 0.0 to 19.9), smaller among those with monthly angina at baseline (2.2 points; 95% credible interval, −2.0 to 6.2), and nearly absent among those without angina at baseline (0.6 points; 95% credible interval, −1.9 to 3.3). By 6 months, the between-group difference in the overall trial population was attenuated (0.5 points; 95% credible interval, −2.2 to 3.4).
CONCLUSIONS
Participants with stable ischemic heart disease, moderate or severe ischemia, and advanced chronic kidney disease did not have substantial or sustained benefits with regard to angina-related health status with an initially invasive strategy as compared with a conservative strategy. (Funded by the National Heart, Lung, and Blood Institute; ISCHEMIA-CKD ClinicalTrials.gov number, NCT01985360.)
Patients with stable ischemic heart disease and advanced chronic kidney disease represent a high-risk population with unique challenges. Because these patients have been systematically excluded from most clinical trials of invasive treatment strategies,1,2 there is no credible evidence from randomized trials of the benefits of revascularization, when added to medical therapy, on their health status (symptoms, functional status, and health-related quality of life). Several observational studies have suggested that patients who have moderate or advanced chronic kidney disease have worse health status in the context of stable ischemic heart disease3,4 and have less improvement from coronary bypass surgery5 than patients who do not have advanced chronic kidney disease. In contrast, other prospective cohort studies and post hoc analyses from clinical trials have suggested that the health-status benefits of revascularization are similar between patients with moderate chronic kidney disease and those with normal or nearly normal kidney function.6,7 The Society for Cardiovascular Angiography and Interventions has explicitly called for more research into the benefits of revascularization on quality-of-life outcomes in patients with chronic kidney disease.8
To address this gap in knowledge, we assessed participants’ health status at baseline and during follow-up in the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches — Chronic Kidney Disease (ISCHEMIA-CKD) trial. Participants in the trial were randomly assigned to undergo initial coronary angiography and revascularization, if feasible, plus receive guideline-based medical therapy (invasive strategy) or to receive guideline-based medical therapy alone (conservative strategy). The primary analysis of the effects of treatment strategy on the risk of death or myocardial infarction revealed no benefits with an invasive strategy.9 In this article, we report the results from our analysis of health status, a key secondary objective of the ISCHEMIA-CKD trial.
Methods
Trial Population
We randomly assigned participants with stable ischemic heart disease, moderate or severe ischemia, and advanced chronic kidney disease (an estimated glomerular filtration rate of <30 ml per minute per 1.73 m2 of body-surface area or receipt of dialysis) to an invasive or a conservative strategy. The design of the trial has been described previously.10,11
Health-Status Outcomes
To determine the effects of the invasive and conservative strategies on patients’ health status, we administered a survey that was completed by participants before randomization and at 1.5, 3, and 6 months and every 6 months thereafter until termination of the trial. This included the Seattle Angina Questionnaire (SAQ), the Rose Dyspnea Scale,12 and the European Quality of Life–5 Dimensions (EQ-5D) visual analogue scale.13
Linguistically and culturally certified translations of the SAQ (www.cvoutcomes.org) were used in each participating country. The 7-item version of the SAQ was used; this shortened version of the original 19-item SAQ has been shown to be similar to the parent instrument in terms of validity, reliability, and sensitivity to change.14–16 The SAQ captures the frequency of angina (Angina Frequency score) and the disease-specific effect of angina on patients’ physical function (Physical Limitation score) and quality of life (Quality of Life score). All three scores are averaged to calculate the SAQ Summary score, which is used as an overall measure of patients’ stable ischemic heart disease–specific health status; SAQ Summary scores range from 0 to 100, with higher scores indicating less frequent angina, greater physical function, and better quality of life. To convert SAQ results into clinically familiar terms, ranges of scores can be used as benchmarks. For the SAQ Angina Frequency score, ranges of 0 to 30, 31 to 60, 61 to 99, and 100 have been shown to be valid measures of daily, weekly, monthly, and no angina, respectively, as assessed with daily angina diaries.17 These ranges are strongly and independently correlated with risk of subsequent death, risk of myocardial infarction, and health care costs.18,19
The Rose Dyspnea Scale consists of four items that assess whether patients feel breathless with different activities; scores range from 0 to 4, with higher scores indicating shortness of breath with milder activities. On the EQ-5D visual analogue scale, patients rate their current health status along a continuum that ranges from 0 (worst) to 100 (best).
Trial Oversight and Organization
The trial was sponsored by the National Heart, Lung, and Blood Institute. An independent data and safety monitoring board approved the trial protocol (available with the full text of this article at NEJM.org) and monitored participant safety. The protocol was approved by the institutional review boards at New York University Grossman School of Medicine, Duke University, Saint Luke’s Hospital, and all other participating sites. All participants provided written informed consent. Data collection was performed by investigators at 118 sites from 30 countries, with administrative support from the clinical coordinating center at New York University, and data management was performed by the Duke Clinical Research Institute. All health-status analyses were conducted at Saint Luke’s Mid America Heart Institute. The first author vouches for the accuracy and completeness of the data on health status and for the fidelity of this analysis of health-status outcomes to the protocol.
Statistical Analysis
The full statistical analysis plan (available with the protocol) was finalized in September 2019, after the feasibility of the planned analyses had been confirmed in a preliminary pooled data set that did not indicate treatment assignments. All analyses were performed in the intention-to-treat population, which included participants according to the group to which they were randomly assigned, regardless of the treatment received. We prespecified a single primary outcome for these analyses, the SAQ Summary score, but did not specify a time point because we sought to describe the differences in health status throughout follow-up.11,20 No adjustment for multiplicity was planned for these analyses. The prespecified plan was to provide results for both the overall population and the population stratified according to baseline angina frequency, as assessed with the SAQ Angina Frequency score. Although the analyses included all available health-status assessments through 60 months, we present the results through the first 36 months since the median follow-up was 2.2 years. The sample size for this trial was driven by the projected clinical-event rates and not the health-status analyses.
For descriptive purposes, unadjusted mean scores over time are reported. The effect of treatment was evaluated with the use of cumulative probability models (also called “cumulative link models”), which do not impose distributional assumptions on the outcome.21 After exploration of alternative approaches with blinded data, a logit link function was selected that produces odds ratios for having a higher SAQ Summary score.
Blinded review of the trial data revealed nonlinear changes in health-status scores over time, with larger changes early after randomization and with substantial heterogeneity of individual participants’ changes over time. We therefore used mixed models, within the framework of a cumulative probability model, that included fixed effects for baseline health-status score, treatment group, time since randomization, and treatment-by-time interaction, as well as patient-level random intercepts and time effects. Piecewise linear splines were used to model time trends, with knots at 3, 6, 12, 18, and 24 months for the fixed effect of time and with knots at 6 and 12 months for patient-level random effects. Restricted cubic splines were used to allow for nonlinear effects of baseline scores. The mixed models allowed estimation of the effects of treatment assignment on patient-specific health-status outcomes, in addition to marginal or population-averaged outcomes.
All models were fit with the use of Bayesian methods. In addition to facilitating estimation of more complex models than those produced with traditional frequentist methods, Bayesian analysis directly estimates the probability distribution of the treatment effect, which can be interpreted as the probability of different effect sizes given the observed data. Weakly informative prior distributions (e.g., heavy-tailed t distributions around 0 with standard deviations of 10) were used for all fixed and random effects, so that inference was driven predominantly by the observed data. The effect of treatment over time was estimated for a typical patient, with a baseline score equal to the population mean and a random effect of 0. In addition to odds ratios being reported at each time point, effects were transformed back to the scale of the instrument scores by integrating over the predicted probabilities of each possible value for each patient. As prespecified in the protocol, expected time-averaged scores through 48 months were also calculated, with the use of area-under-the-curve analyses. Results are presented as posterior means and 95% highest posterior distributions (credible intervals), which indicate the 95% most plausible values of the parameter being estimated. These distributions are presented for all assessments between 3 months and 36 months and, as prespecified, stratified according to baseline angina frequency.20 Since we used Bayesian methods for analyses of the health-status outcomes, no P values are reported.
Rates of intermittent skipped assessments varied from 8 to 20% over time, with no appreciable differences between treatment groups in rates of missing scores or reasons for missing scores. In the primary analysis of treatment effect, missing scores were assumed to be missing at random, conditional on treatment group and other available scores, because the mixed model implicitly imputes missing data through participants’ estimated health-status trajectories. However, because death may be an informative reason for a missing score, we conducted a prespecified sensitivity analysis of the SAQ Summary score by fitting a joint shared-parameter model of health-status scores and death, in which the patient-level random effects from the health-status model were included as covariates in a Weibull regression model of time to death from any cause.22
Finally, in a post hoc analysis, we assessed differences in the odds ratios for benefit with an invasive strategy between the main ISCHEMIA trial,10 which excluded patients with advanced chronic kidney disease, and the ISCHEMIA-CKD trial. We conducted this analysis by taking 20,000 draws each from the posterior distributions of the ISCHEMIA and ISCHEMIA-CKD trials and calculating the proportion of draws in which the odds ratio from the ISCHEMIA-CKD trial was less than that from the ISCHEMIA trial. This analysis was performed in the overall population and stratified according to baseline angina frequency. All analyses were conducted with the use of SAS software, version 9.4; R software, version 3.5.3; Stan software, version 2.18.1; and R software packages “rstan,” “rstanarm,” “brms,” and “tidyverse.”23–28
Results
Participants
Of the 777 participants included in the trial, 42 were excluded from this analysis because of improper form completion and 30 were excluded for missing either the baseline assessment or all follow-up assessments (Fig. S1 in the Supplementary Appendix, available at NEJM.org). There were no substantial differences in baseline characteristics between participants with survey data and those without survey data (Table S1). Health-status follow-up data were available through 3 years for more than 80% of the participants (Table S2). The baseline characteristics of the 705 participants included in this analysis were well balanced between treatment groups (Table 1). The mean age was 62 years, two thirds of the participants were male, and the majority were white. Hypertension was present in 93% of the participants, and 55% had diabetes. The mean (±SD) baseline SAQ Summary score was 76.0±20 in the invasive-strategy group and 76.7±19 in the conservative-strategy group. A total of 12% of the participants had daily or weekly angina, 39% had angina one to three times per month, and 49% had had no angina during the month before randomization. The median follow-up was 2.2 years.
Table 1.
Characteristics of the Participants at Baseline.*
| Characteristic | Invasive Strategy (N = 347) | Conservative Strategy (N = 358) |
|---|---|---|
| Age — yr | 62.1±10.4 | 63.3±11.2 |
| Male sex — no. (%) | 235 (67.7) | 243 (67.9) |
| White race — no./total no. (%)† | 235/336 (69.9) | 230/343 (67.1) |
| Hypertension — no. (%) | 320 (92.2) | 336 (93.9) |
| Diabetes — no. (%) | 192 (55.3) | 193 (53.9) |
| Previous myocardial infarction — no. (%) | 57 (16.4) | 67 (18.7) |
| New-onset angina during previous 3 mo — no./total no. (%) | 50/335 (14.9) | 61/342 (17.8) |
| History of heart failure — no. (%) | 180 (51.9) | 192 (53.6) |
| Previous cerebrovascular disease — no. (%) | 46 (13.3) | 49 (13.7) |
| History of peripheral artery disease — no. (%) | 25 (7.2) | 23 (6.4) |
| Receipt of dialysis — no. (%) | 180 (51.9) | 206 (57.5) |
| Creatinine level at randomization — mg/dl‡ | 3.3±1.5 | 2.9±1.0 |
| Estimated glomerular filtration rate at randomization — ml/min‡ | 22.5±8.8 | 24.1±8.2 |
| Degree of ischemia on stress test — no./total no. (%) | ||
| Moderate | 218/342 (63.7) | 225/356 (63.2) |
| Severe | 124/342 (36.3) | 131/356 (36.8) |
| SAQ score§ | ||
| Summary score | 76.0±19.9 | 76.7±18.5 |
| Physical Limitation score | 75.0±25.8 | 75.1±24.3 |
| Quality of Life score | 65.8±28.2 | 67.1±26.9 |
| Angina Frequency score | 87.0±17.8 | 87.6±17.0 |
| Angina frequency — % | ||
| Daily or weekly | 11.9 | 11.7 |
| Monthly | 38.2 | 40.2 |
| None | 50.0 | 48.0 |
| Score on Rose Dyspnea Scale¶ | 1.5±1.5 | 1.5±1.4 |
| Score on EQ-5D visual analogue scale‖ | 60.3±18.1 | 61.7±17.4 |
Plus–minus values are means ±SD.
Race was reported by the participant.
These data do not include participants who were receiving dialysis.
On the Seattle Angina Questionnaire (SAQ), the Summary score is an average of the Physical Limitation, Quality of Life, and Angina Frequency scores; SAQ scores range from 0 to 100, with higher scores indicating better health status.
On the Rose Dyspnea Scale, scores range from 0 to 4, with higher scores indicating shortness of breath with milder activities.
On the European Quality of Life–5 Dimensions (EQ-5D) visual analogue scale, scores range from 0 to 100, with higher scores indicating better health status.
Primary Outcome
The unadjusted mean health-status scores in each treatment group are shown in Figure 1; all SAQ scores and the scores on the Rose Dyspnea Scale and the EQ-5D visual analogue scale are similar in the two groups. The odds of a better health-status score at each time point throughout 3 years of follow-up generally favored the invasive strategy over the conservative strategy, as indicated by a mean odds ratio of more than 1, but wide posterior distributions indicated substantial residual uncertainty around those estimates (Table S3). An analysis of treatment effect on the mean SAQ Summary score at 3 months favored the invasive strategy (Table 2, Fig. 2, and Fig. S2), a result that reflects the combination of a large benefit among the 83 participants who had daily or weekly angina at baseline (estimated mean difference between invasive-strategy group and conservative-strategy group in SAQ Summary score, 10.1 points; 95% credible interval, 0.0 to 19.9), a smaller benefit among the 276 participants who had angina one to three times per month at baseline (mean difference in score, 2.2 points; 95% credible interval, −2.0 to 6.2), and no benefit among the 345 participants without angina at baseline (mean difference in score, 0.6 points; 95% credible interval, −1.9 to 3.3). By 12 months, the mean difference in the SAQ Summary score among participants who had daily or weekly angina at baseline was 2.2 points (95% credible interval, −8.0 to 13.1), whereas the mean difference in the overall population was 0.1 points (95% credible interval, −3.0 to 3.1). Posterior distributions of the mean differences in the SAQ Summary score after 12 months were consistent with no appreciable treatment benefit of an initially invasive strategy over a conservative strategy. The absence of a difference between treatment groups was confirmed in an analysis of mean health-status scores over time, with a time-averaged difference in the SAQ Summary score of 0.7 points (95% credible interval, −2.0 to 3.4) (Table S4).
Figure 1. Unadjusted Mean Health-Status Scores Over Time.
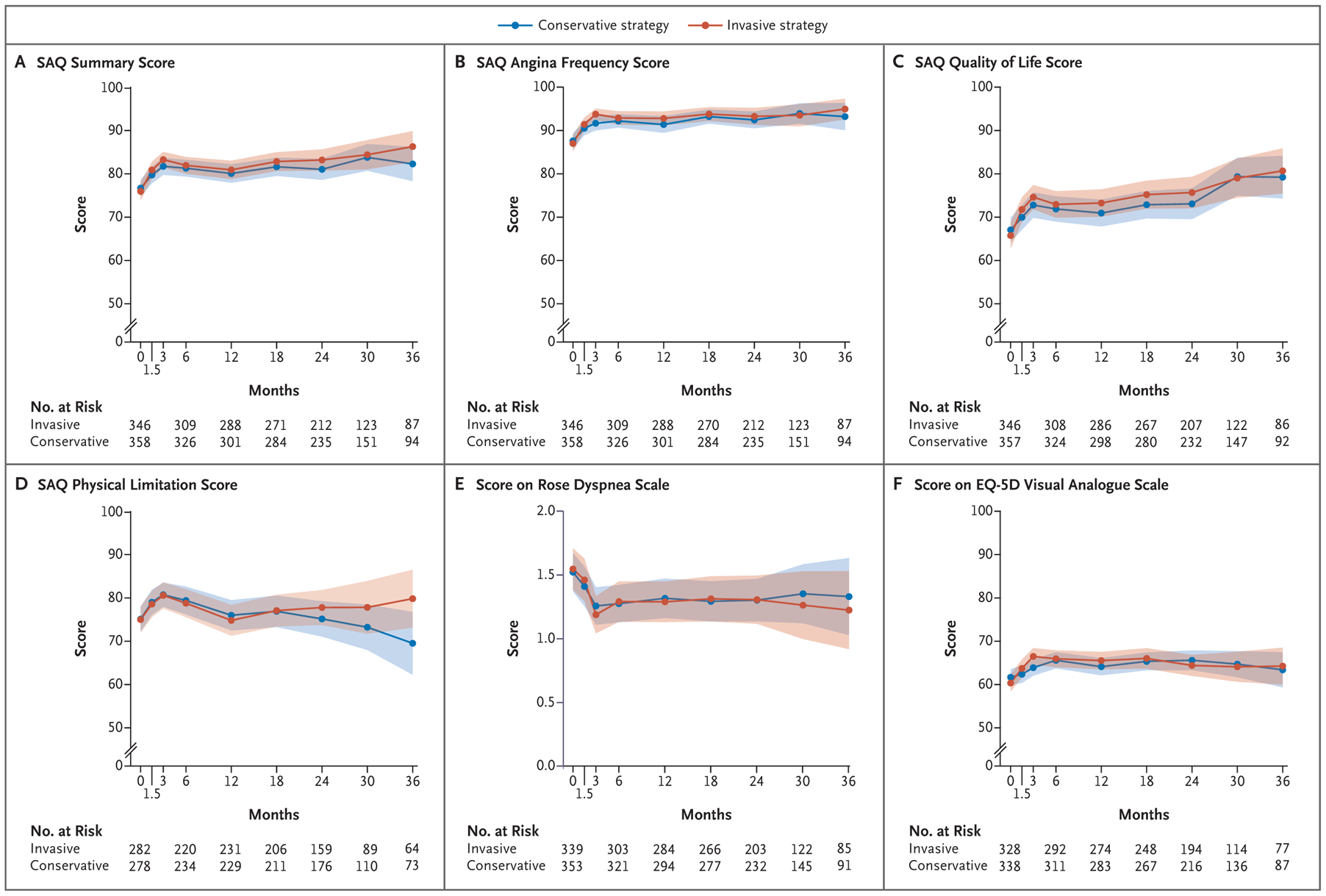
Shown are mean health-status scores among participants who were randomly assigned to undergo initial coronary angiography and revascularization plus receive guideline-based medical therapy (invasive strategy) or to receive guideline-based medical therapy alone (conservative strategy). Shading represents the 95% confidence interval. On the Seattle Angina Questionnaire (SAQ), the Summary score (Panel A) is an average of the Angina Frequency, Quality of Life, and Physical Limitation scores (Panels B, C, and D, respectively); SAQ scores range from 0 to 100, with higher scores indicating better health status. On the Rose Dyspnea Scale (Panel E), scores range from 0 to 4, with higher scores indicating shortness of breath with milder activities. On the European Quality of Life–5 Dimensions (EQ-5D) visual analogue scale (Panel F), scores range from 0 to 100, with higher scores indicating better health status.
Table 2.
Estimated Mean Effect of an Invasive Strategy on SAQ Summary Scores.*
| Month | Overall (N = 705) | Daily or Weekly Angina at Baseline (N = 83) | Monthly Angina at Baseline (N = 276) | No Angina at Baseline (N = 345) |
|---|---|---|---|---|
| points (95% credible interval) | ||||
| 3 | 2.1 (−0.4 to 4.6) | 10.1 (0.0 to 19.9) | 2.2 (−2.0 to 6.2) | 0.6 (−1.9 to 3.3) |
| 6 | 0.5 (−2.2 to 3.4) | 9.2 (−1.6 to 19.7) | 0.3 (−4.3 to 4.8) | −0.8 (−4.0 to 2.3) |
| 12 | 0.1 (−3.0 to 3.1) | 2.2 (−8.0 to 13.1) | 1.0 (−4.0 to 6.2) | −0.8 (−4.2 to 2.8) |
| 18 | 1.3 (−1.8 to 4.2) | 2.0 (−8.7 to 12.9) | 2.4 (−2.7 to 7.5) | 0.2 (−2.9 to 3.4) |
| 24 | 2.4 (−0.7 to 5.7) | −0.5 (−12.0 to 10.2) | 4.3 (−1.0 to 9.6) | 1.4 (−1.9 to 4.7) |
| 30 | 1.4 (−1.7 to 4.6) | 0.1 (−11.3 to 12.0) | 2.9 (−2.2 to 8.2) | 0.7 (−2.5 to 4.0) |
| 36 | 0.5 (−3.6 to 4.5) | 0.7 (−13.8 to 16.2) | 1.5 (−5.1 to 8.2) | 0.0 (−4.2 to 4.3) |
Data are estimated mean differences between the invasive-strategy group and the conservative-strategy group in the SAQ Summary score, according to angina frequency at baseline (as assessed with the SAQ Angina Frequency score) and time point.
Figure 2. Distributions of Differences between Treatment Groups in SAQ Summary Scores.
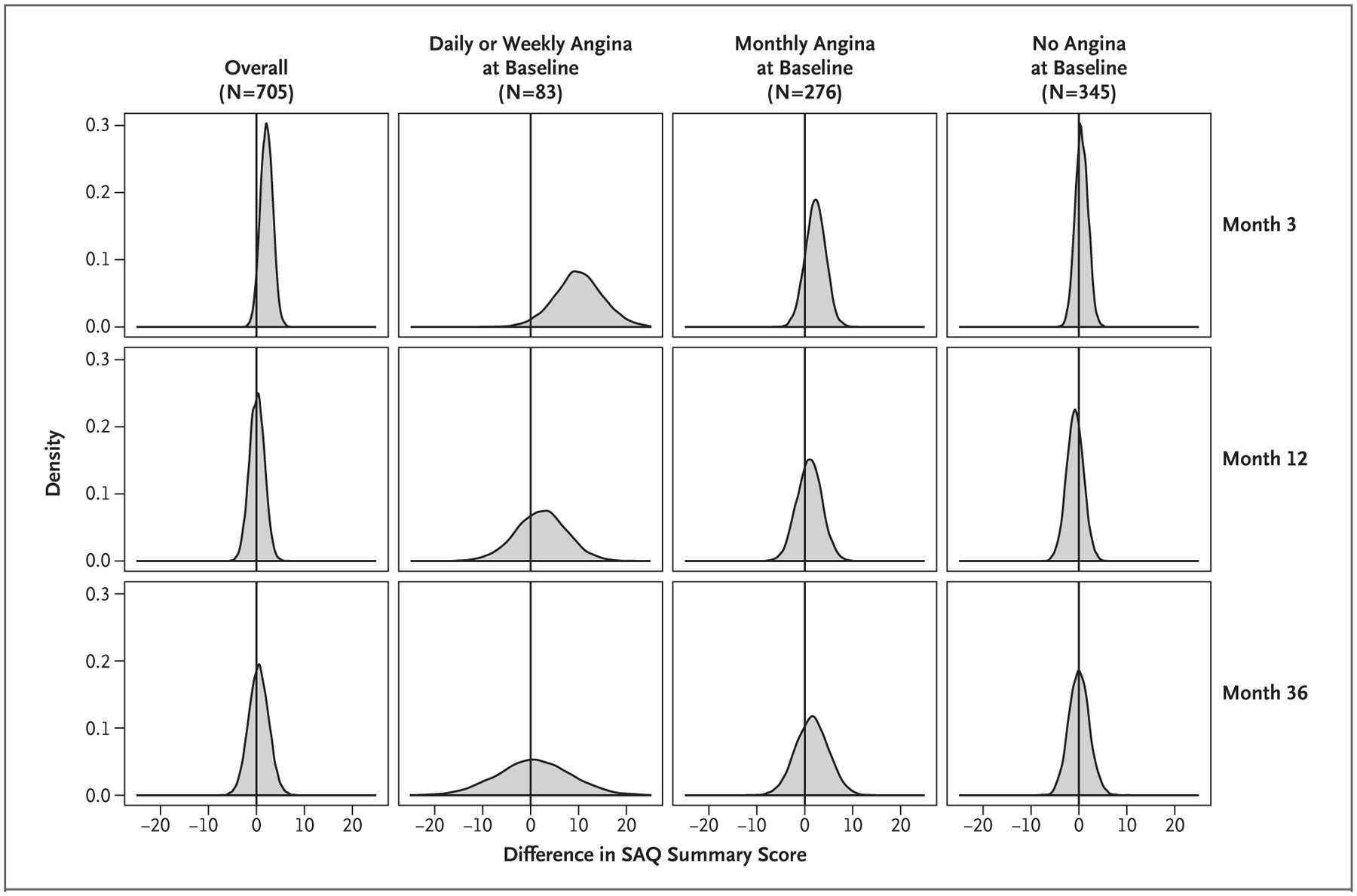
Shown are posterior distributions of estimated mean differences between the invasive-strategy group and the conservative-strategy group in the SAQ Summary score, according to angina frequency at baseline (as assessed with the SAQ Angina Frequency score) and time point. Positive numbers on the x axis show the magnitude of benefits with an invasive strategy, and the y axis shows the probability of those benefits.
Joint Model of Death and Health Status
Because of the high mortality in this population of patients with advanced chronic kidney disease, we performed a sensitivity analysis using joint models of death and health-status scores. Table S5 shows odds ratios for having a higher SAQ Summary score, as well as the probability of any benefit with an invasive strategy, as calculated in models that did not include death and in joint models that included death and health status. Because mortality was similar in the two treatment groups, the findings in the joint models were virtually identical to the findings in the models that did not include death as an outcome.
Benefits of Invasive Strategy According to Kidney Disease Status
To assess the health-status benefits of an invasive strategy in participants with and in those without advanced chronic kidney disease, we obtained samples from the distributions of the odds ratios for having a higher SAQ Summary score in the ISCHEMIA-CKD trial and the ISCHEMIA trial. Our analysis showed an at least 93% probability that the health-status benefits of the invasive strategy would be larger among participants without advanced chronic kidney disease (in ISCHEMIA) than among those with advanced chronic kidney disease (in ISCHEMIA-CKD) (Fig. S3). Table S6 shows data, according to angina frequency at baseline and time point, regarding the probability that the odds ratio for better health status with the invasive strategy in the ISCHEMIA-CKD trial would be less than the odds ratio observed in the ISCHEMIA trial; the results all support a larger benefit in patients without advanced chronic kidney disease.
Discussion
In this large, multicenter trial, which compared an initially invasive strategy with a conservative strategy in participants with stable ischemic heart disease, moderate or severe ischemia, and advanced chronic kidney disease, an analysis of treatment effect on cardiovascular events did not reveal benefits of the invasive strategy. A secondary objective of the trial was to assess health status, since improvement in health status is a major goal of treatment for all patients with stable ischemic heart disease. In the overall trial population, we found little evidence of improvement in angina-related health status with an invasive approach. Nearly half the participants had had no angina during the month before randomization, and this group had no evidence of health-status benefits from an invasive strategy, a finding that heavily weighted the results in the overall intention-to-treat population toward the null. However, even participants with angina at baseline had only transient health-status benefits from an invasive strategy that were less consistent than the benefits seen in the main ISCHEMIA trial,10 which excluded patients with advanced chronic kidney disease. Although participants with advanced chronic kidney disease who had daily or weekly angina during the month before randomization had a substantial estimated mean improvement in their 3-month SAQ Summary scores with the invasive strategy as compared with the conservative strategy, the difference was no longer apparent after 6 months, after which the credible intervals included a substantial possibility of no benefit from an invasive strategy. Combined with the absence of a clinical benefit from the invasive strategy with respect to death or myocardial infarction, the results of this analysis suggest that an initially invasive strategy does not lead to better outcomes than a conservative strategy in patients with stable ischemic heart disease and advanced chronic kidney disease.
Because of the systematic exclusion of patients with advanced chronic kidney disease from most previous trials of management strategies for stable ischemic heart disease, clinicians have had to extrapolate from results of clinical trials involving patients with preserved renal function to treat patients with advanced chronic kidney disease.29–32 In the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial, patients with chronic kidney disease (defined as an estimated glomerular filtration rate of <60 ml per minute per 1.73 m2) had a slight benefit from an invasive approach through 6 months with respect to the SAQ Physical Limitation and Quality of Life scores, and that benefit dissipated by 12 months. No difference in the SAQ Angina Frequency score between the group that received percutaneous coronary intervention plus medical therapy and the group that received medical therapy alone was observed at any time.7 Given the paucity of data from randomized trials, most of the evidence regarding the health-status benefits of invasive treatment for stable ischemic heart disease has come from observational studies, which have had conflicting results.3–7 In the largest of these studies, James et al.6 used an observational registry in Alberta, Canada, to assess the health-status benefits of revascularization in 9394 patients according to their kidney function, although patients receiving dialysis were excluded. In that study, the observed mean improvement in SAQ scores over a 1-year period was greater in patients treated with coronary-artery bypass grafting than in those treated with percutaneous coronary intervention or medical therapy alone. There were no significant interactions of SAQ score with severity of chronic kidney disease, a finding that suggests health-status benefits do not vary according to kidney function. Despite the rigor of these observational analyses, selection bias cannot be ruled out, and the randomized ISCHEMIA-CKD trial provides little confidence in any sustained health-status benefit from an initially invasive treatment strategy.
The results of our analysis should be interpreted in the context of several limitations. First, there were some missing health-status assessments, although less than 20% of assessments were missing at any point in time. Our models included participants with missing follow-up assessments, and only 72 participants were excluded from the entire analysis; 160 patients died before the 36-month assessment, but all available data up to the point of death were included. Second, although participants underwent randomization before angiography, exclusion of participants who had other clinical features that their physicians thought would require an invasive approach may have occurred. Third, a substantial proportion of participants who were randomly assigned to the invasive strategy did not undergo revascularization (a higher proportion than in the main ISCHEMIA trial33), and further analyses are needed to help us understand the effect of this observation on health-status outcomes. It is noteworthy that the participants in the ISCHEMIA-CKD trial had a low angina burden at the time of randomization, which limited the precision of our estimates of a treatment benefit in more symptomatic patients. Finally, the results of this analysis do not apply to patients with advanced chronic kidney disease who meet criteria for exclusion specified in the protocol, such as those with left main coronary artery disease, acute coronary syndromes, or heart failure.
In conclusion, patients with stable ischemic heart disease, moderate or severe ischemia, and advanced chronic kidney disease did not have substantial or sustained benefits with regard to their health status with an initially invasive management strategy as compared with a conservative strategy, regardless of angina frequency at baseline.
Supplementary Material
Acknowledgments
Supported by grants (U01HL117904 and U01HL117905) from the National Institutes of Health. Saint Luke’s Mid America Heart Institute supported these health-status analyses through in-kind support of Dr. Spertus and Mr. Jones.
Footnotes
The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Health and Human Services.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
References
- 1.Brooks MM, Frye RL, Genuth S, et al. Hypotheses, design, and methods for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol 2006; 97: 12A: 9G–19G. [DOI] [PubMed] [Google Scholar]
- 2.Boden WE, O’Rourke RA, Teo KK, et al. Design and rationale of the Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial: Veterans Affairs cooperative studies program no. 424. Am Heart J 2006; 151: 1173–9. [DOI] [PubMed] [Google Scholar]
- 3.Odden MC, Whooley MA, Shlipak MG. Depression, stress, and quality of life in persons with chronic kidney disease: the Heart and Soul Study. Nephron Clin Pract 2006; 103(1): c1–c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odden MC, Whooley MA, Shlipak MG. Association of chronic kidney disease and anemia with physical capacity: the Heart and Soul Study. J Am Soc Nephrol 2004; 15: 2908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh CR, Coca SG, Smith GL, Vaccarino V, Krumholz HM. Impact of chronic kidney disease on health-related quality-of-life improvement after coronary artery bypass surgery. Arch Intern Med 2006; 166: 2014–9. [DOI] [PubMed] [Google Scholar]
- 6.James MT, Wilton SB, Clement FM, et al. Kidney function does not modify the favorable quality of life changes associated with revascularization for coronary artery disease: cohort study. J Am Heart Assoc 2016; 5(7): e003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sedlis SP, Jurkovitz CT, Hartigan PM, et al. Health status and quality of life in patients with stable coronary artery disease and chronic kidney disease treated with optimal medical therapy or percutaneous coronary intervention (post hoc findings from the COURAGE trial). Am J Cardiol 2013; 112: 1703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blankenship JC, Marshall JJ, Pinto DS, et al. Effect of percutaneous coronary intervention on quality of life: a consensus statement from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2013; 81: 243–59. [DOI] [PubMed] [Google Scholar]
- 9.Bangalore S, Maron DJ, O’Brien SM, et al. Management of coronary disease in patients with advanced kidney disease. N Engl J Med 2020; 382:1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spertus JA, Jones PG, Maron DJ, et al. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med 2020; 382:1408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bangalore S, Maron DJ, Fleg JL, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches-Chronic Kidney Disease (ISCHEMIA-CKD): rationale and design. Am Heart J 2018; 205: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ 1968; 56: 1–188. [PubMed] [Google Scholar]
- 13.Group EuroQol. EuroQol — a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 14.Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle Angina Questionnaire. Circ Cardiovasc Qual Outcomes 2014; 7: 640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol 1994; 74: 1240–4. [DOI] [PubMed] [Google Scholar]
- 16.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995; 25: 333–41. [DOI] [PubMed] [Google Scholar]
- 17.Arnold SV, Kosiborod M, Li Y, et al. Comparison of the Seattle Angina Questionnaire with daily angina diary in the TERISA clinical trial. Circ Cardiovasc Qual Outcomes 2014; 7: 844–50. [DOI] [PubMed] [Google Scholar]
- 18.Arnold SV, Morrow DA, Lei Y, et al. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN-TIMI 36 trial. Circ Cardiovasc Qual Outcomes 2009; 2: 344–53. [DOI] [PubMed] [Google Scholar]
- 19.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation 2002; 106: 43–9. [DOI] [PubMed] [Google Scholar]
- 20.Spertus J, Mark D. ISCHEMIA trial update. Am Heart J 2019; 218: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Shepherd BE, Li C, Harrell FE Jr. Modeling continuous response variables using ordinal regression. Stat Med 2017; 36: 4316–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spertus JV, Hatfield LA, Cohen DJ, et al. Integrating quality of life and survival outcomes in cardiovascular clinical trials. Circ Cardiovasc Qual Outcomes 2019; 12(6): e005420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R: a language and environment for statistical computing. Vienna: R Core Team, 2019. [Google Scholar]
- 24.Carpenter B, Gelman A, Hoffman M, et al. Stan: a probabilistic programming language. J Stat Softw 2017; 76(1). abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stan Development Team. RStan: the R interface to Stan. R package version 2182 2018. [Google Scholar]
- 26.Burkner P BRMS: an R package for Bayesian multilevel models using Stan. J Stat Softw 2017; 80: 1–28. [Google Scholar]
- 27.Goodrich B, Gabry J, Ali I, Brilleman S. RStanArm: Bayesian applied regression modeling via Stan. R Package Version 2182 2018. [Google Scholar]
- 28.Wickham H Tidyverse: easily install and lose the ‘Tidyverse.’ R Package Version 121 2017. [Google Scholar]
- 29.Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int 2006; 70: 2021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zannad F, Rossignol P. Cardiovascular outcome trials in patients with advanced kidney disease: time for action. Circulation 2017; 135: 1769–71 [DOI] [PubMed] [Google Scholar]
- 31.The BARI 2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360: 2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Bruyne B, Pijls NHJ, Kalesan B, et al. Fractional flow reserve–guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367: 991–1001. [DOI] [PubMed] [Google Scholar]
- 33.Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020; 382:1395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


