Abstract
Here we begin by briefly reviewing landmark structural studies on the nicotinic acetylcholine receptor. We highlight challenges that had to be overcome to push through resolution barriers, then focus on what has been gleaned in the past few years from crystallographic and single particle cryo-EM studies of different nicotinic receptor subunit assemblies and ligand complexes. We discuss insights into ligand recognition, ion permeation, and allosteric gating. We then highlight some foundational aspects of nicotinic receptor structural biology that remain unresolved and are areas ripe for future exploration.
Introduction
The nicotinic acetylcholine receptor has played a prominent role in numerous landmark studies since the turn of the 20th century. As the subject of John Newport Langley’s revolutionary theory on “receptive substances” (Langley, 1905), the nicotinic receptor provided a foundation for modern pharmacological research. Electrophysiological studies on nicotinic receptors at the neuromuscular junction contributed to the initial discovery of postsynaptic potentials (Eccles et al., 1941; Eccles and O’Connor, 1939; Fatt and Katz, 1950; Göpfert and Schaefer, 1938). Furthermore, these receptors were the first ion channels to be purified (Changeux et al., 1970; Karlsson et al., 1972; Klett et al., 1973; Meunier et al., 1971; Miledi et al., 1971; Olsen et al., 1972), cloned (Claudio et al., 1983; Devillers-Thiery et al., 1983; Noda et al., 1983), and studied on a single-channel level (Neher and Sakmann, 1976). It is thus difficult to overstate the impact that the nicotinic receptor has had throughout the history of neuroscience. One discipline in which research on these proteins has lagged is structural biology. Until recently, high-resolution information on intact nicotinic receptors was absent, forcing the field to rely on structures of homologous proteins and lower-resolution electron microscopy (EM) reconstructions for insights. Over the past few years, several structures of neuronal nicotinic receptors have emerged that provide a blueprint for a more comprehensive understanding of the molecular mechanisms underlying their function. Recent advances in structure determination with single particle cryo-EM, as well as tomography, will undoubtedly assist in resolving further outstanding questions. In this review, we discuss subunit and subtype diversity in the nicotinic receptor family, historical advances in the pursuit of structural information, and conclusions drawn from available data with an emphasis on recently published structures.
Cys-loop receptors
Nicotinic acetylcholine receptors belong to a superfamily of pentameric ligand-gated ion channels, commonly referred to as Cys-loop receptors. In humans, this superfamily also includes the γ-aminobutyric acid type A (GABAA) receptor, glycine receptor, serotonin type 3 (5-HT3) receptor and the zinc-activated ion channel (ZAC) (Nemecz et al., 2016). Cys-loop receptor homologs are also prevalent in prokaryotes and invertebrates (Tasneem et al., 2005). Notable examples include ion channels from Erwinia chrysanthemi (ELIC), Gloebacter violaceus (GLIC), and a glutamate-activated chloride channel from Caenorhabditis elegans (GluCl). These members have been studied extensively through X-ray crystallography and provided initial high-resolution insights into the overall topology, pore profiles, and allosteric gating mechanisms within the family (Althoff et al., 2014; Bocquet et al., 2009; Hibbs and Gouaux, 2011; Hilf and Dutzler, 2008, 2009; Nemecz et al., 2016; Sauguet et al., 2013; Sauguet et al., 2014).
Cys-loop receptors form homo- or heteropentameric assemblies with five subunits arranged in an approximately symmetric manner about the central channel axis. Individual subunits share a modular design with a large N-terminal extracellular domain (ECD) where orthosteric ligands bind, a transmembrane domain (TMD) that surrounds the ion-conducting pore, and an intracellular domain (ICD) of variable length and secondary structure (Thompson et al., 2010). Remarkably, within the constraints of this generally conserved architecture, members of this family have evolved differential selectivities for cations and anions (Cymes and Grosman, 2016). Accordingly, Cys-loop receptors represent the only family of ion channels that facilitate both excitatory and inhibitory signaling, allowing them to perform a wide range of important physiological functions. In vertebrates, anionic members such as GABAA and glycine receptors mediate the majority of fast inhibitory neurotransmission in the central nervous system (Bowery and Smart, 2006). In contrast, cationic members of the family, including 5-HT3 and nicotinic receptors, largely play a modulatory role in the brain (Miquel et al., 2002; Picciotto et al., 2012; Wonnacott, 1997) and are directly involved in fast excitatory synaptic transmission in the periphery (Browning, 2015; Lummis, 2012; Skok, 2002).
Subtype diversity of nicotinic receptors
The nicotinic receptor family consists of 17 subunits (α1-α10, β1-β4, γ, δ, and ε) that can assemble in limited combinations to generate a large but restricted number of distinct pentameric subtypes (Albuquerque et al., 2009) (Fig. 1). Broadly, nicotinic receptors can be divided into two main classes, muscle-type and neuronal-type, based on subunit composition and physiological function. Muscle-type receptors are found on the motor endplate at the neuromuscular junction, and activation of these receptors produces depolarizing end plate potentials that lead to muscle contraction. Neuronal nicotinic receptors are expressed throughout the central and peripheral nervous systems and play important roles in cognition (Ji et al., 2001; Levin, 2002; Maskos et al., 2005; Ohno et al., 1993; Picciotto et al., 1995), addiction (Azam et al., 2002; Berrettini et al., 2008; Leslie et al., 2013; Picciotto et al., 1998), and homeostatic function of the autonomic nervous system (Skok, 2002; Zoli et al., 2015).
Figure 1:

Nicotinic acetylcholine receptor subunit assemblies and sequence diversity. (A) Subunit arrangement of the muscle-type nicotinic receptor. Orthosteric binding sites are denoted by arrows at α1-γ/ε and α1-δ interfaces. (B) Subunit arrangement of a homomeric neuronal nicotinic receptor, α7. α9 can also form homomers. (C) Subunit arrangement of heteromeric neuronal nicotinic receptors. αX positions can be occupied by α2, α3, α4, or α6 subunits, βY positions can be occupied by β2 or β4 subunits, and the last position (αZ/βZ) can be occupied by any of the above as well as α5 and β3. (D) Sequence identity matrix of all human nicotinic receptor subunits. α8 is not found in mammals and is not included. Principal α subunits are indicated in green, complementary β subunits in blue, complementary muscle-type subunits in yellow and orange, and auxiliary subunits in teal. Highlighted boxes show sequence identities between interchangeable subunits.
Muscle-type nicotinic receptors form heteromers with a stoichiometry of α12β1γδ (Karlin et al., 1983) (Fig. 1A). In adult human muscle, the γ subunit is replaced by an ε subunit to form α12β1εδ assemblies (Mishina et al., 1986). While γ and ε protomers share high sequence identity (Fig. 1D), receptor subtypes containing either subunit display varying functional properties (Mishina et al., 1986; Sakmann and Brenner, 1978) and contribute differently to neuromuscular development and maintenance (Koenen et al., 2005; Liu et al., 2008; Takahashi et al., 2002; Witzemann et al., 1996). Muscle-type receptors have classically served as the prototype not just for nicotinic receptors, but for all ligand-gated ion channels, due to their abundance at muscle fiber endplates and in other natural sources such as the electrocytes of electric fish (Nachmansohn et al., 1941). Historical perspectives on this receptor subtype and its involvement in significant breakthroughs in neuroscience have been discussed in great detail in (Changeux, 2012; Karlin, 2002; Unwin, 2013).
Neuronal-type receptors are composed of various combinations of α2-α10 and β2-β4 subunits (Dani and Bertrand, 2007; Zoli et al., 2015). The large diversity in subunits in this class produces an apparently overwhelming number of possible permutations of neuronal-type assemblies. However, these subunits can be further grouped into clades that dictate the permitted subtypes they can form, greatly limiting the number of potential subtypes. α7 and α9 subunits typically assemble as homopentamers (Elgoyhen et al., 1994; Seguela et al., 1993) (Fig. 1B), although α9-containing receptors can also incorporate α10 subunits (Elgoyhen et al., 2001; Sgard et al., 2002), and there is growing evidence of an α7β2 subtype expressed in restricted tissues (Liu et al., 2009; Moretti et al., 2014). In contrast, α2-α6 and β2-β4 subunits form obligate heteromers. Ligand binding sites in these subtypes require contributions from a principal α (α2, α3, α4, or α6) subunit and a complementary β (β2 or β4) subunit (Le Novere et al., 2002). Thus, neuronal assemblies consist of at least two α subunits and two β subunits. The fifth subunit in the pentamer does not directly contribute to the classical agonist binding site and thus its identity is not as restricted (Fig. 1C). Due to tissue-dependent expression differences, certain assemblies are more prevalent than others. Receptors containing α4 and β2 subunits constitute the most abundant subtype in the central nervous system and serve as the major high-affinity binding site for nicotine (Flores et al., 1992; Wada et al., 1989; Whiting and Lindstrom, 1988). α3β4-containing receptors are the predominant nicotinic receptors in the autonomic ganglia (Conroy and Berg, 1995; Vernallis et al., 1993) and adrenal medulla (Campos-Caro et al., 1997; Free et al., 2002) and are also expressed in abundance in certain brain areas that modulate reward, such as the medial habenula and the interpeduncular nucleus (Grady et al., 2009; Mulle et al., 1991; Quick et al., 1999). Both α4β2 and α3β4 are expected to co-assemble with accessory subunits such as α5 (Boulter et al., 1990; Conroy et al., 1992; Ramirez-Latorre et al., 1996) and β3 (Broadbent et al., 2006; Grady et al., 2009; Jain et al., 2016), although the extent and functional consequences of accessory subunit incorporation remain unclear. Differential incorporation of accessory subunits may allow for further diversity and fine-tuning of functional responses in different brain regions.
A brief history of structural studies
Structural studies on the nicotinic receptor date back to the 1970’s. Negatively-stained electron micrographs of purified receptors and subsynaptic membranes from Torpedo marmorata and Torpedo californica electrocytes revealed distinct rosettes 80–90 Å in diameter (Cartaud et al., 1973; Cartaud et al., 1980; Klymkowsky and Stroud, 1979). Later, Unwin and colleagues were able to coax membrane fragments containing a high density of receptors into forming well-ordered tubular crystals that were then suitable for examination through electron crystallography (Brisson and Unwin, 1984). Early attempts at structure determination through this method yielded relatively low-resolution reconstructions that provided an initial glimpse at the overall architecture of the receptor (Brisson and Unwin, 1985; Toyoshima and Unwin, 1988, 1990; Unwin et al., 1988). Systematic improvements in data quality through averaging (Unwin, 1993), correction of image distortions by computational segmentation of tubular crystals (Beroukhim and Unwin, 1997), and advances in electron microscope hardware led to substantial enhancement of map quality (Miyazawa et al., 2003; Unwin, 2005). However, the overall resolutions of reconstructions attained from this work were still insufficient for accurate atomic modeling of the entire receptor, limiting the detailed molecular insights that could be gleaned. This review will mainly focus on recently determined higher-resolution structures; however, a thorough examination of this transformative series of Torpedo receptor studies can be found in (Unwin, 2013).
The dawn of the new millennium provided the opportunity for higher-resolution information on the ligand-binding site of the nicotinic receptor with the identification of a synaptic protein released by glial cells in mollusks (Smit et al., 2001). This molecule, appropriately dubbed the acetylcholine-binding protein (AChBP), is structurally homologous to the extracellular domain of nicotinic receptors, sharing 24% sequence identity with the N-terminal region of the α7 nicotinic receptor subunit. The relatively small size (~120 kDa pentamer) and soluble nature of this protein make it particularly amenable to crystallization (Brejc et al., 2001). Consequently, numerous X-ray structures of AChBPs, mostly from Lymnaea stagnalis and Aplysia californica, have been determined, ranging in resolutions from 4.2 Å – 1.75 Å (Brejc et al., 2001; Rucktooa et al., 2009; Shahsavar et al., 2016). Further progress was made with the design of chimeras between AChBP and the human α7 subunit (Delbart et al., 2018; Li et al., 2011; Nemecz and Taylor, 2011). A couple of these humanized receptors share >60% sequence identity with the α7 ECD, with the ligand-binding site and surrounding regions entirely consisting of α7 residues. Structures of AChBP and the related chimeras as well as emerging crystal structures of isolated extracellular domains of human α1 (Dellisanti et al., 2007; Noridomi et al., 2017), α9 (Zouridakis et al., 2014; Zouridakis et al., 2019), and α2 (Kouvatsos et al., 2016) provided unprecedented structural insights into the fold of the extracellular domain and local conformational changes in the ligand-binding site upon binding of agonists and antagonists that rationalized prior biochemical studies (Bourne et al., 2005; Celie et al., 2004; Damle and Karlin, 1980; Hansen et al., 2005; Karlin, 1969). However, these structures came with several limitations. AChBP is not functionally coupled to a pore domain (Bouzat et al., 2004); therefore, these structures could not elucidate how the conformational changes seen in the binding pocket are propagated to the transmembrane domain. The isolated nicotinic receptor extracellular domains, on the other hand, did not form pentamers, except in the case of α2, which is not thought to assemble physiologically as a homopentamer. Perhaps the most glaring issue was the lack of transmembrane and intracellular domains, thus precluding any conclusions that could be drawn about these regions.
The need for high-resolution structural information on intact nicotinic receptors was thus apparent. A recent series of structures of α4β2 (Morales-Perez et al., 2016b; Walsh et al., 2018) and α3β4 (Gharpure et al., 2019) subtypes have begun to address outstanding questions about these proteins, including detailed examinations into structural bases for ion permeation, lipidic interactions, and differences in ligand affinity, as well as queries into the nature of heteromeric assemblies. The remainder of this review will describe the technical developments required to achieve these structures, and then highlight key findings from them in the context of earlier biochemical and functional studies.
Technical hurdles
Several technical difficulties thwarted attempts to obtain high-resolution structures of intact nicotinic receptors for many years. These receptors, like many other membrane proteins, suffer from low expression in heterologous systems and poor biochemical stability when extracted from the plasma membrane. Nicotinic receptors are also heavily glycosylated, which can impede traditional structural approaches such as X-ray crystallography, as the flexible and chemically heterogeneous sugar moieties can hinder growth of well-ordered crystals. Furthermore, the heteromeric nature of the vast majority of physiologically relevant subtypes presents important problems for structure determination. Heteropentamers can often assemble in multiple stoichiometries (usually as 2α:3β or 3α:2β) (Nelson et al., 2003), complicating the isolation of a homogeneous sample required for crystallography. Heteromeric assemblies are also inherently pseudo-symmetric, with nearly identical tertiary structure between different subunits in a pentamer. This pseudosymmetry can pose a significant challenge in structural biology by making difficult the correct assignment of subunits.
Advances in membrane protein biochemistry and cell biology contributed to overcoming these obstacles associated with expression, purification, and structure determination of nicotinic receptors. Concurrent developments of an HEK 293-derived cell line deficient in N-acetylglucosaminyltransferase I (GnTI−) (Reeves et al., 2002), and technology utilizing baculovirus mediated gene transfer in mammalian cells (BacMam expression system) (Dukkipati et al., 2008; Goehring et al., 2014), allowed for overexpression of human membrane proteins containing homogeneous N-linked glycans that could be more efficiently cleaved by endoglycosidases. Rapid and cost-efficient biochemical screening in order to identify tractable constructs and optimal conditions for receptor stability was enabled by the popularization of fluorescence-detection size exclusion chromatography (FSEC) (Kawate and Gouaux, 2006). By attaching a fluorophore such as GFP to the protein of interest, hundreds of constructs and solubilization conditions could be screened in a matter of days, requiring only minute quantities of protein, circumventing the need for purification in the initial optimization of sample biochemistry. A related approach was used to address the mixed stoichiometry problem and streamline titration of the viruses used to express the heteromer (Morales-Perez et al., 2016a). Fusion of different fluorescent proteins to α4 and β2 genes allowed for optimization of expression conditions to bias production of one heteromeric assembly of α4β2, which was a critical step in determining the initial crystal structure of this subtype (Morales-Perez et al., 2016b).
Perhaps the most powerful and far-reaching developments in high-resolution structure determination were associated with the “resolution revolution” in cryo-EM (Cao, 2020; Cheng, 2015; Cheng et al., 2015). Improvements in direct electron detectors and related data-processing software that could correct beam-induced motions of individual particles resulted in a substantial increase in achievable resolutions through single-particle analysis. This new technology has been a boon for structural biology by removing the requirement of crystallization, which is often the rate-limiting step in structural studies of membrane proteins. Single-particle cryo-EM has now become the preferred method for studies on integral membrane proteins and large complexes and structural information for such proteins is being uncovered at an unprecedented rate. However, as mentioned above, pseudo-symmetric molecules such as nicotinic receptors are not ideal specimens for this technique. 3D reconstructions of cryo-EM maps rely on low-resolution information for initial particle alignment (Henderson et al., 2011; Scheres, 2016), and in heteromeric nicotinic receptors, subunit identities may not be obvious at low resolutions. To resolve this problem, subtype-specific Fab fragments were used as fiducial markers to distinguish subunits in the initial stages of particle alignment. This method enabled the determination of both stoichiometries of α4β2 from a single heterogeneous sample (Walsh et al., 2018), as well as the 2α:3β stoichiometry of α3β4 (Gharpure et al., 2019).
Global architecture of heteromeric assemblies
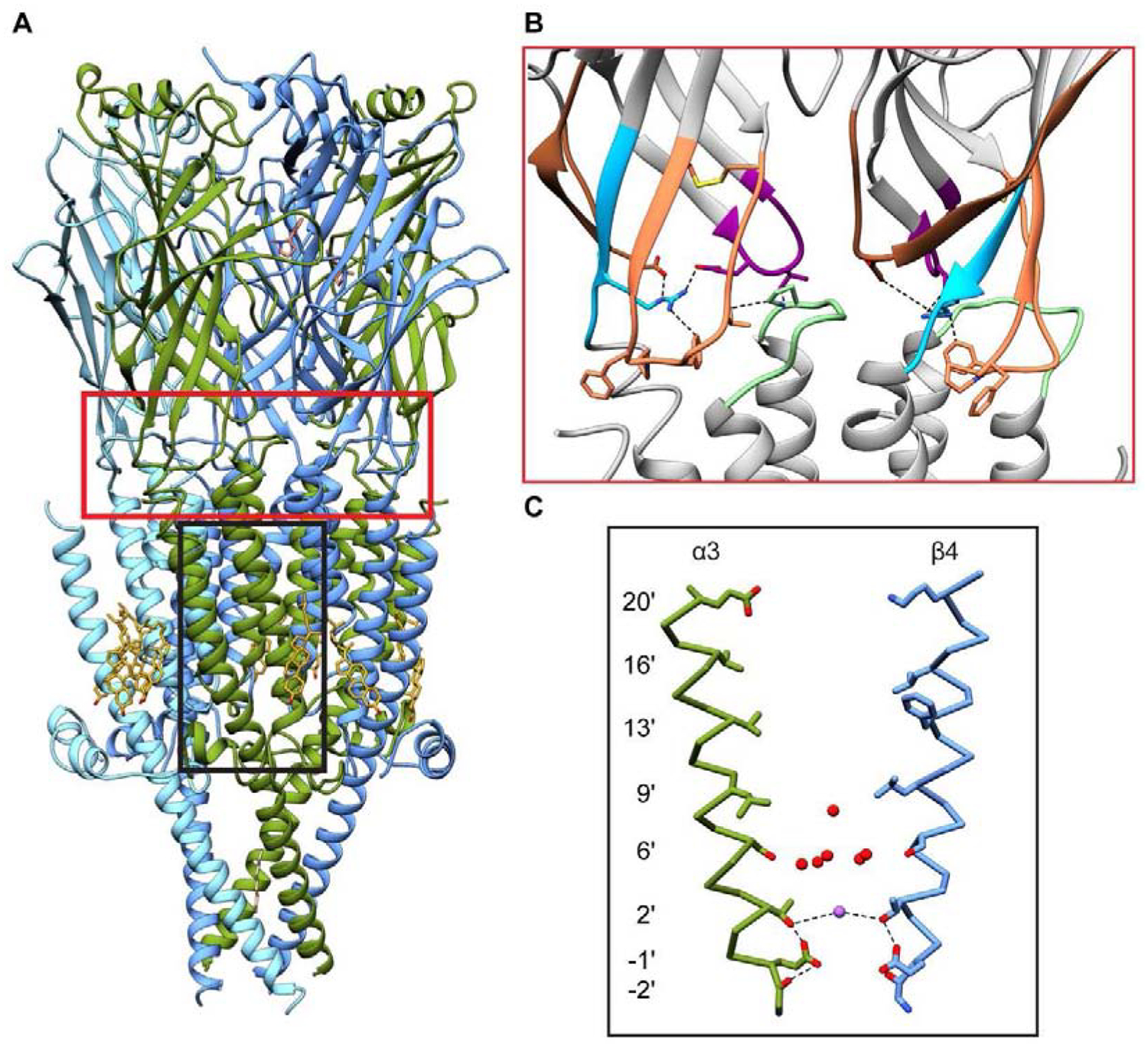
The overall topology of nicotinic receptor subunits is consistent with that of other Cys-loop receptors (Fig. 2). Following an N-terminal α-helix, the extracellular domain folds as a β-sandwich comprising ten β-strands (β1-β10). Between strands β6 and β7 lies the Cys-loop, from which the superfamily derives its name. This loop, which has been implicated in transducing the signal from agonist binding to channel opening, comprises 13 amino acids flanked by a pair of disulfide-bonded cysteine residues. These cysteines are absent in the prokaryotic orthologs, but the architecture of the loop is the same. The transmembrane domain consists of four α-helices (M1–M4) arranged in a pseudo-rhombic bundle. The M2 helices are situated proximally to the central axis and line the channel pore, while the M4 helices are most distal and lipid-exposed, leading to a proposed role as a lipid sensor (Henault et al., 2015). The M3 and M4 helices are connected by a cytoplasmic substructure that constitutes the intracellular domain. This domain contains a stretch of ~10 residues known as the post-M3 loop, an amphipathic helix, MX, that rests at the interface between the plasma membrane and cytosol, and an intracellular helix, MA, that feeds directly into M4. The MX and MA helices are connected by a poorly conserved and disordered loop, ranging in length from 78–259 amino acids. Receptor constructs used for structure determination of both α4β2 and α3β4 subtypes utilized deletions in this region to promote biochemical stability. However, these construct modifications did not significantly alter protein function and structures of related Cys-loop receptors (Basak et al., 2018a; Basak et al., 2018b; Polovinkin et al., 2018) show no clear density corresponding to this region, so these structures are not expected to deviate significantly from full-length proteins. Crystallization of the α4β2 receptor required a more aggressive deletion that removed most of the MA helix. Subsequent EM studies of this subtype used the same constructs; thus, high-resolution structural information on this portion of the intracellular domain is only available from the α3β4 receptor.
Figure 2:

Nicotinic receptor architecture. (A) Cryo-EM structure of the α4β2 subtype (PDB: 6CNJ). α4 subunits are in green, β2 subunits in blue, nicotine in salmon, and CHS in gold. (B) Cryo-EM structure of the α3β4 subtype (PDB:6PV7). α3 subunits are in green, β4 subunits in blue, nicotine in salmon, and CHS in gold. (C) Architecture of an α subunit, with key regions labeled.
Despite these differences in the intracellular loop, nicotine-bound structures of α4β2 (Walsh et al., 2018) and α3β4 (Gharpure et al., 2019) are remarkably similar. Superposition of the two models reveals that the root mean square deviation (rmsd) for the Cα atoms in the full pentamers is only 1.1 Å. Individual α subunits have rmsd values of about 0.9 Å, whereas values for β subunits range from 0.9 to 1.4 Å. This high conservation of backbone positioning is perhaps unsurprising as the two subtypes share high sequence identity (67% between α3 and α4, 65% between β2 and β4) (Fig. 1B). The remaining two principal subunits in neuronal nicotinic receptors for which there is currently no structural information also share high sequence identity with α3 and α4 (α2:α3, 57%; α2:α4, 70%; α6:α3, 65%; α6:α4, 54%). It is therefore likely that they will also adopt a tertiary structure similar to the observed α subunits, suggesting that differences in functional properties between various subtypes may be governed by local differences in key regions of the protein, as will be discussed later, rather than by large-scale rearrangements.
The α4β2 and α3β4 subtype structures further helped probe the nature of heteromeric assemblies. The elucidation of both expected stoichiometries of the α4β2 receptor was particularly enlightening, as it provided direct structural information on all possible interface classes. Using this information, Walsh and Roh et al. sought to understand why formation of 2α:3β and 3α:2β receptors were more favorable than other possible stoichiometries. Analysis of these structures revealed that α-α interfaces buried the most protein surface area and β-β buried the least, with α-β and β-α intermediate. These differences resulted in varying interfacial angles between subunits and suggested that optimal packing in a pentamer only permitted the presence of one α-α or β-β interface. More specifically, hypothetical pentamers containing four or five α4 subunits would incur significant clashes, while pentamers containing four or five β2 subunits would result in an incomplete pentamer with a substantial gap. In reality, formation of these aberrant stoichiometric assemblies would likely involve some compensatory rearrangement in order to avoid such clashes or gaps. However, these structural changes may prove to be too energetically costly to allow for efficient production of these stoichiometries. Preferential formation of 2α:3β and 3α:2β assemblies is probably important physiologically as well, as it only allows for a receptor population with two high-affinity neurotransmitter binding sites per pentamer at either α-β interface.
Neurotransmitter-binding site
High-resolution structural information for the orthosteric binding site has been of interest from a basic-science perspective and as a rational framework for developing improved therapeutics for drug addiction, schizophrenia, and cognitive and neurodegenerative disorders (Hogg and Bertrand, 2004; Lloyd and Williams, 2000). The considerable diversity of nicotinic receptors poses a conceptual challenge for drugability of this site, as different subtypes play diverse physiological roles throughout the nervous system, increasing the risk of potential side effects. Although residues lining the ligand-binding pocket are well conserved, differences in the pharmacological properties between subtypes suggest subtle structural changes, thus emphasizing the need for structures from numerous members of the family. Studies on agonist-bound complexes of α4β2 and α3β4 have begun to reveal the extensive similarities in different classes of ligand-binding sites, as well as the minor rearrangements that give rise to varying functional properties.
The neurotransmitter-binding site of nicotinic receptors is located in the extracellular domain at the interface between a principal (+) and a complementary (−) subunit and is encompassed by six canonical loops designated A-F (Fig. 3A). The principal face of the pocket, which must be contributed by an α subunit, contains loops A, B, and C, while the complementary face provides loops D, E, and F. In the available agonist-bound structures, residues on loops A-E directly contact the ligand whereas loop F potentially plays a scaffolding role in shaping the pocket. The hallmark feature of the binding site is loop C with its characteristic vicinal disulfide at the tip. This loop undergoes a large conformational change upon agonist binding, wherein it tightly caps the neurotransmitter site and makes direct interactions with agonists (Hansen et al., 2005). The core of the pocket is defined by a cage of five highly conserved aromatic residues on loops A-D, which stabilize agonists through hydrophobic and cation-π interactions. Notable among these is the tryptophan on loop B. This residue lines the back wall of the binding pocket and makes essential cation-π and hydrogen bonding interactions with a basic nitrogen on the ligand (Xiu et al., 2009), a nearly strictly-conserved pharmacophore of nicotinic receptor agonists (Beers and Reich, 1970; Camacho-Hernandez et al., 2019; Glennon and Dukat, 2000). Another common, although less essential pharmacophore, is a hydrogen bond acceptor (Blum et al., 2010) that interacts with mainchain atoms on loop E at the top of the binding site. In nicotine complexes, this interaction is indirect and mediated by a water molecule that serves as a hydrogen bonding bridge. Density for this water is likely resolution-limited and thus only seen in the highest-resolution α3β4 structure, but related structures of AChBP (Celie et al., 2004) and mutagenesis studies in α4β2 (Blum et al., 2010) suggest its presence in this subtype as well. Residues on loop E whose sidechains are in close proximity to the ligand are not well conserved, and this variability may contribute to affinity differences at various interface classes (Fig. 3E).
Figure 3:
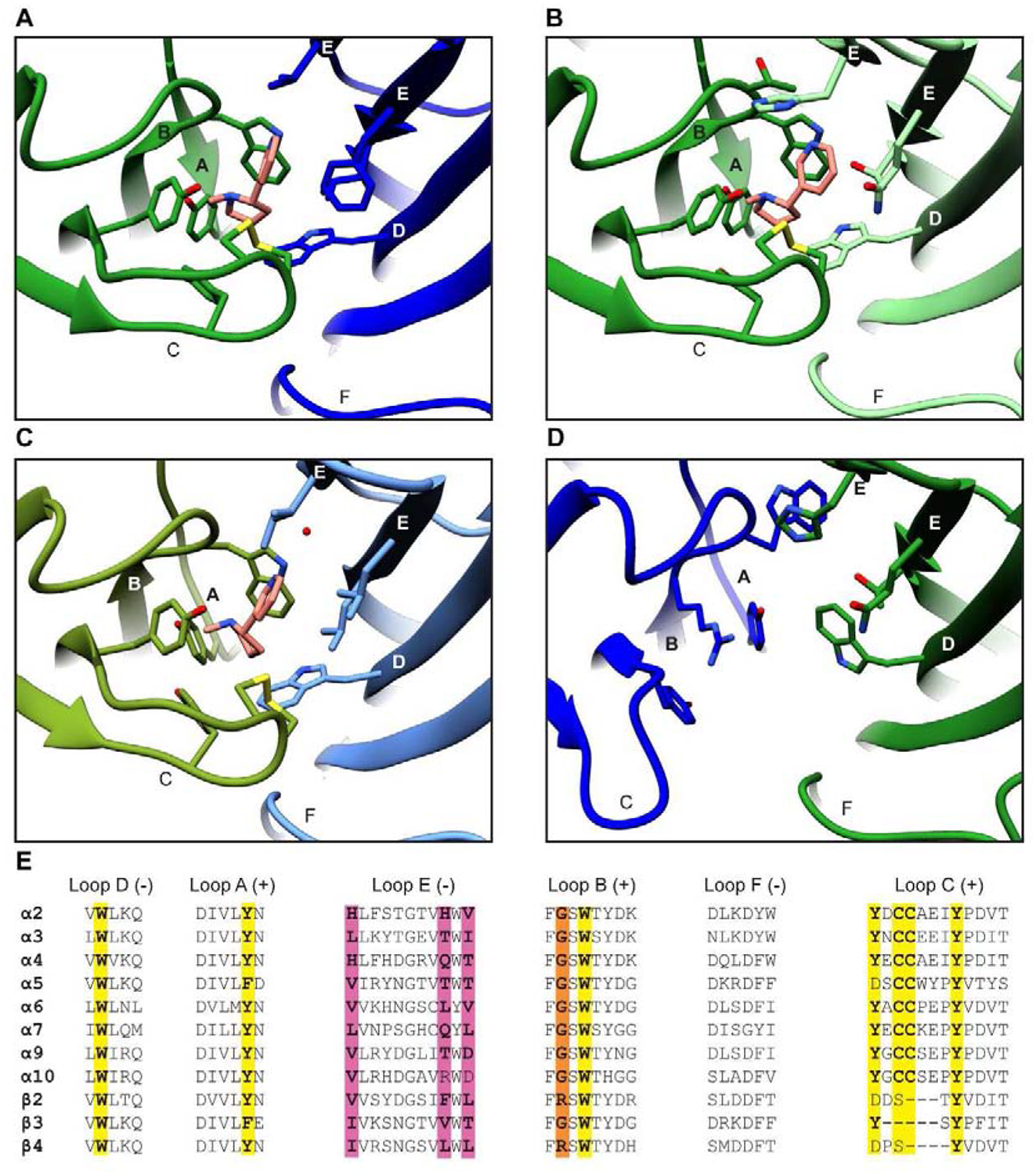
Neurotransmitter-binding site of neuronal nicotinic receptors. (A) Binding of nicotine at the α4-β2 interface (PDB: 6CNJ). Loops A-F are labeled and residues contacting the ligand are shown as sticks. (B) Binding of nicotine at the α4-α4 interface (PDB: 6CNK). (C) Binding of nicotine at the α3-β4 interface (PDB: 6PV7). (D) Pseudo-agonist binding site at the β2-α4 interface (PDB: 6CNJ). (E) Sequence alignments of loops A-F in all human neuronal nicotinic receptor subunits. Bolded residues are shown as sticks in panels A-D. Residues comprising the aromatic cage and vicinal disulfide are highlighted in yellow. Variable residues in close proximity to the ligand on loop E are highlighted in magenta. Residues in β subunits that may preclude agonist binding on loop B are highlighted in orange.
Structures of both stoichiometries of α4β2 and the 2α:3β stoichiometry of α3β4 in complex with the same agonist (nicotine), allow for direct comparison of three distinct ligand-binding sites: α4-β2, α4-α4, and α3-β4. Among these, the α4-β2 site has the highest affinity for nicotine (Eaton et al., 2003; Harpsoe et al., 2011; Mazzaferro et al., 2011; Xiao et al., 1998). Structural deviations in the other two sites may provide insights into their lowered sensitivity to this and other classical nicotinic receptor agonists such as acetylcholine and epibatidine. The α4-α4 site shares its principal face with α4-β2 so substitutions on the complementary face are likely responsible for its weaker affinity (Harpsoe et al., 2011). Three hydrophobic residues on loop E of β2-V111, F119, and L121, are replaced with a histidine, glutamine, and threonine respectively in α4 (Ahring et al., 2015; Shahsavar et al., 2015). The longer phenylalanine and leucine residues in β2 may optimally orient nicotine in the aromatic cage by providing a hydrophobic scaffold of van der Waals contacts. In contrast, the shorter and polar residues in these positions of α4 allow the pyridine ring of nicotine to tilt away from the core of the pocket toward the complementary face, in what may be a less favorable pose (Fig. 3B). Analysis of sequences of loop E in all subunits suggest that this difference in polarity may be a conserved distinction between α-β and α-α interfaces, as the hydrophobic nature of these residues in all β subunits is conserved, while most α subunits, including homomer-forming α7 and α9, contain at least one polar residue at these positions.
Consistent with this observation, the nicotine-binding site of α3-β4 looks similar to that of α4-β2, with nicotine assuming a similar orientation. The only two differences directly in the agonist-binding pocket are V111I and F119L substitutions. While hydrophobicity at these positions is maintained, the loss of the aromatic group of F119 may contribute to the lower affinity in the α3-β4 pocket by removing a potential π-stacking interaction with the pyridine ring of nicotine (Fig. 3C). Another intriguing hypothesis for the weaker sensitivity at this site relates to restructuring of loop C. In α3β4, this loop is located about 2.1 Å farther away from nicotine, potentially weakening van der Waals contacts with the ligand. This rearrangement may be explained by differences in loops C (E198N and A201E), D (T59K), and E (S113R and D115N) between the two subtypes. Together, these substitutions create a network of hydrogen bonds in α3β4 that cannot form in α4β2, potentially supporting loop C in the observed retracted position. The more spacious binding pocket of α3β4 may also explain the higher affinity of this subtype to larger agonists such as AT-1001 (Tuan et al., 2015). Importantly, these observations suggest that residues not in direct proximity to the ligand may influence binding by structurally rearranging the pocket. This discovery further highlights the need for structures of more subtypes, as changes like these distinct conformations of loop C would not be easily predicted from sequence alignments.
These heteromeric structures also clarified why orthosteric ligands do not bind at β-α and β-β interfaces (Fig. 3D). Unique to canonical β subunits (β2 and β4) is an arginine in loop B, which is a glycine in all other neuronal nicotinic subunits. The bulkier sidechain of arginine settles at the bottom of the pocket, forcing a rearrangement of the surrounding aromatic cage. Two tyrosine residues on loops A and C rotate downward and upward respectively to accommodate and form cation-π interactions with the guanidinium group of arginine. The movement of the tyrosine on loop A forces the aforementioned tryptophan on loop B back and out of the binding pocket. This disruption of the aromatic cage, along with the shorter loop C of β subunits that would be unable to fully cap a putative ligand, likely explain why interfaces with β2 or β4 as the principal face are not conducive to neurotransmitter binding. Interfaces where β2 or β4 are the principal subunits remain of interest in development of allosteric modulators, with the strikingly different chemistry at these interfaces explaining why efforts building off the classical basic nitrogen pharmacophore have failed to hit these potential sites.
Channel gating and pore conformation
The binding of agonists to orthosteric sites is coupled to a conformational change in the transmembrane domain ~50 Å away, resulting in the opening of an ion permeable pore and allowing for flux of cations across the membrane. A functional chimera of AChBP and the 5-HT3 TMD (Bouzat et al., 2004), as well as structures of other Cys-loop receptors in multiple conformations (Althoff et al., 2014; Basak et al., 2018a; Du et al., 2015; Polovinkin et al., 2018; Sauguet et al., 2014) have implicated several key loops at the ECD-TMD interface to be important in this signal transduction. These include the β1-β2 loop, the β8-β9 loop, and the Cys-loop on the extracellular side and the pre-M1 linker and the M2–M3 loop in the transmembrane domain. The existing nicotinic receptor structures are all bound to agonists and adopt similar conformational states, thus limiting insights into the specific molecular details of this process. However, they do show that key residues identified by functional studies are poised to communicate signals from the ECD to pore domain. These include an arginine on the pre-M1 loop (Lee and Sine, 2005; Purohit and Auerbach, 2007) and a proline on the M2–M3 loop (Lee et al., 2008) which interact with residues on the β1-β2 and Cys-loops, and a conserved “FPF” motif on the Cys-loop (Alcaino et al., 2017; Lee et al., 2009) that forms substantial contacts with the pre-M1 loop and the extracellular end of the M1 helix (Fig. 4B).
Figure 4:
Gating regions of the desensitized nicotinic receptor. (A) Overview of the α3β4 receptor in a putatively desensitized state. The red box highlights the hinge region, and the black box highlights the channel pore. (B) Closeup of the hinge region. The β1-β2 loop is shown in magenta, β8-β9 loop in brown, Cys-loop in orange, pre-M1 linker in blue, and M2–M3 linker in green. Key residues are shown as sticks. (C) Pore architecture. Representative M2 helices from an α3 and a β4 subunit are shown. Waters and sodium ions are shown as red and purple spheres respectively. Dashed lines indicate putative electrostatic interactions.
Upon prolonged exposure to agonists, nicotinic receptors will desensitize and enter one or more classes of non-conducting states (Feltz and Trautmann, 1982; Karlin, 2002; Katz and Thesleff, 1957). Decades of functional, biochemical, and structural data support the notion that the slow desensitized state is structurally distinct from the resting, or antagonist-bound state (Auerbach and Akk, 1998; Gielen and Corringer, 2018; Keramidas and Lynch, 2013; Purohit and Grosman, 2006; Unwin et al., 1988; Wilson and Karlin, 2001). In the resting state, the primary gate is formed by a hydrophobic constriction at the 9ʹ position on the pore-forming M2 helix near the midpoint of the membrane. Although at the time of acceptance of this review, there are no high-resolution structures of nicotinic receptors in this conformation, mutation of the conserved leucine at this position to a polar residue alters fundamental channel properties of nicotinic receptors (Filatov and White, 1995; Labarca et al., 1995; Revah et al., 1991). Furthermore, structures of other Cys-loop receptors in conditions that would presumably favor the resting state show an impermeable constriction at 9ʹ (Basak et al., 2018b; Du et al., 2015; Huang et al., 2015; Masiulis et al., 2019; Pan et al., 2012; Polovinkin et al., 2018). In contrast, the consensus location for the desensitization gate is at the intracellular end of the pore (Gielen and Corringer, 2018). The available nicotinic receptor structures are consistent with this idea, with a minimum pore radius of ~1.7 Å formed by the sidechains of glutamates at the −1ʹ position (Fig. 4C).
It may be initially surprising to think of a gate for a cation-selective channel to be defined by acidic sidechains, as is suggested by the presumed desensitized-state nicotinic receptor structures. The negatively-charged carboxyl groups of the sidechains and their ability to adopt a variety of rotameric conformations (Cymes and Grosman, 2012; Harpole and Grosman, 2014) would appear to be conducive to ion flux, rather than forming a robust obstruction. A gate formed by charged residues also contrasts with familiar hydrophobic gating mechanisms in voltage-gated and glutamate-gated channels, and with the hydrophobic resting-state gate likely conserved across the Cys-loop receptor superfamily (Aryal et al., 2015; Twomey et al., 2017). The highest-resolution nicotinic receptor structure of the α3β4 subtype provides some clues into this hypothetical ionic desensitization gate. In the α3β4 nicotinic receptor structure bound to nicotine, clear density was observed for all five −1ʹ glutamates, confirming that they position their sidechains, on average, toward the central pore axis. At first glance, this orientation appears energetically unfavorable, however nearby structural features allow for dispersal of the electrostatic potential. Density for a putative cation was observed near the 2ʹ position. This ion, modeled as Na+, in conjunction with the hydroxyl groups on Thr2ʹ sidechains and backbone carbonyls of Gly-2ʹ, may form a network of electrostatic interactions that stabilize the constriction-forming orientation of the −1ʹ sidechains (Fig. 4C). The positive charge of the sodium ion is likely important to counterbalance the concentrated negative charges from the glutamates and thus a divalent cation, such as calcium, may be even more effective in this role
We would be remiss to discuss the pore conformation of an ion channel without mentioning the membrane mimetic used for structural studies. Historically, purification and crystallization of membrane proteins typically required the use of detergents. Detergent micelles are capable of stabilizing transmembrane regions while maintaining solubility but are far from perfect substitutes for the natural lipid bilayer that encompasses membrane proteins. The advent of cryo-EM has allowed for structural studies in more native-like lipidic environments through the use of nanodisc technology. Nanodiscs are ~100–200 Å diameter lipid bilayers that are rendered soluble through the use of amphipathic protein or polymer belts, (Denisov and Sligar, 2016; Frauenfeld et al., 2016; Knowles et al., 2009). Reconstitution into such lipidic environments is particularly important for nicotinic receptors, which have been shown to have a strong functional dependence on the surrounding lipid composition (Epstein and Racker, 1978; Fong and McNamee, 1986; Lindstrom et al., 1980; McNamee et al., 1975; Nelson et al., 1980). The absence of essential components, such as anionic lipids (daCosta et al., 2002) and cholesterol (Criado et al., 1982), can force nicotinic receptors into an uncoupled state where agonist binding is no longer coupled to conformational changes in the pore (daCosta and Baenziger, 2009).
To address the lipid issue, the study on the α3β4 subtype utilized a functional reconstitution approach where a lipid mixture that was shown to support channel activity was used for structure determination. Importantly, the selected lipids included phosphatidic acid, a common anionic lipid, and the sample was supplemented with cholesteryl hemisuccinate (CHS), a soluble cholesterol analog. In this study, another agonist-bound complex was determined in detergent (dodecylmaltoside) micelles, also supplemented with CHS, allowing for direct comparison of detergent- and nanodisc- bound structures. The pore architectures of the two complexes are remarkably similar, resembling the observed conformations of the α4β2 structures, also in detergent. Furthermore, there are not substantial structural differences in M4, which has been proposed to play a role as a lipid sensor through putative interactions with surrounding lipids and the Cys-loop (Henault et al., 2015). Densities corresponding to CHS are seen along the periphery of the TMD in both detergent and nanodisc structures, supporting and more clearly defining the presence of cholesterol binding sites (Corbin et al., 1998; Hamouda et al., 2006), but do not clearly explain the lipid dependence of nicotinic receptors. It is possible that lipids may be more influential in stabilizing the open state, and structural information on an open channel may reveal other important lipid-binding sites. Obtaining nicotinic receptor structures in additional conformational states is essential in understanding the mechanism of allosteric gating and should shed light on how differences in lipid composition affect the gating process.
Intracellular domain
The cytoplasmic portion of nicotinic receptors provides an interface for communication with the cellular milieu. As such, this domain is the site of post-translational modifications and protein-protein interactions, which can modulate cellular trafficking and channel properties (Paulo et al., 2009; Stokes et al., 2015; Swope et al., 1999; Talwar and Lynch, 2014; Williams et al., 1998; Williams et al., 2005). Besides the amphipathic MX and intracellular MA helices, the M3–M4 substructure or “loop” is largely disordered, frustrating efforts to understand the structural bases underlying these processes. The intracellular domain also contributes to ion permeation by providing an exit pathway for cations in the cytosol. This function appears to be primarily facilitated by the MA helices, and structures of the α3β4 receptor have shed light on how the chemical environment of this region may affect channel conductance.
The five MA helices converge to form a narrow constriction at the bottom (cytosolic extremity) of the receptor (Fig. 5A). This constriction has a diameter of ~5.4 Å, which would be large enough to permit the passage of a hydrated sodium ion. However, this axial pathway is highly hydrophobic in nature, with several conserved rings of aliphatic residues lining the central axis of the ICD. Interestingly, a strong density, probably corresponding to the hydrocarbon tail of a lipid or detergent molecule, was observed in this hydrophobic patch, indicating that a physical obstruction may also prevent ion flux through the bottom tip of the receptor. Instead, early structural studies from Unwin (Miyazawa et al., 1999), and work on the 5-HT3 receptor (Hales et al., 2006; Kelley et al., 2003), suggested that cations are likely to diffuse into the cytosol through the five lateral portals located at the interface of MA helices. These portals contain fenestrations in the α3β4 receptor of about ~6 Å diameter and are primarily surrounded by acidic and other polar sidechains, making them ideal cation exit pathways (Fig. 5). The high concentration of negatively charged residues in this region has been shown to influence channel conductance. In the α3β4 study, acidic residues whose sidechains lined the portals were mutated to lysines, thereby decreasing the electronegativity of the fenestrations. The mutant receptor yielded significantly reduced single-channel currents than WT receptors, confirming that the electronegative nature of the portals plays a key role in ion permeation.
Figure 5:

Intracellular domain. (A) Side view of the α3β4 ICD. Acidic residues lining the portals are shown as sticks. The hydrophobic plug is shown in tan. (B) Side view shown as a surface colored by electrostatic potential.
Structural studies on the 5-HT3 receptor have suggested that the ICD may also contribute to pentameric assembly (Pandhare et al., 2019) as well as channel gating. Structures of this receptor in a putative open conformation suggest that the MA helices may develop a kink or even become disordered in the open state (Basak et al., 2018a; Polovinkin et al., 2018). It is not immediately clear why such drastic conformational changes would be necessary in a conductive state, as the fenestrations seen in the putative desensitized conformations are large enough to accommodate hydrated ions. Nevertheless, structures of nicotinic receptors in alternative conformations will be helpful in visualizing potential roles of the ICD in channel gating.
Looking forward
Despite serving as the paradigm for ligand-gated ion channels for well over a century, the nicotinic acetylcholine receptor was one of the last families to be characterized structurally at high resolution. The recent series of structures of two neuronal nicotinic receptor subtypes have helped contextualize decades of functional and biochemical data and have laid groundwork for new insights into quaternary assembly, subtype-specific ligand selectivity, gating, and ion permeation. However, this work is merely the beginning of a new age of studies on nicotinic receptors- many unanswered questions remain that can be addressed structurally.
Structural information on other subtypes will be imperative in understanding the mechanisms governing their idiosyncratic properties. The muscle-type receptor and the homomeric α7 receptor are of utmost importance, considering their crucial roles in human physiology and disease (Kalamida et al., 2007; Lindstrom, 2000; Pohanka, 2012). The muscle-type receptor is perhaps the best characterized subtype and high-resolution structures of this receptor would allow for a direct interpretation of many historical studies. α7 displays several unique properties, including a high calcium permeability (Castro and Albuquerque, 1995; Seguela et al., 1993) and rapid desensitization (Peng et al., 1994). Other notable targets are subtypes containing accessory subunits, such as α5 and β3, as it is unclear whether or not these subunits contribute to neurotransmitter binding sites (Groot-Kormelink et al., 2001; Jain et al., 2016; Kuryatov et al., 2008). Structures of α5- and/or β3- containing pentamers would definitively clarify this issue and may also reveal a novel class of binding sites with potential for allosteric ligand binding akin to the benzodiazepine sites of GABAA receptors (Kuryatov et al., 2008; Sigel and Buhr, 1997). Single-particle cryo-EM can also provide insights into the relative proportions of nicotinic receptor subtypes found in native tissues. Up until this point, the identification of receptor populations has relied on bulk measurement techniques such as in situ hybridization (Boulter et al., 1990; Wada et al., 1989), immunoprecipitation (Conroy and Berg, 1995; Vernallis et al., 1993), and immunohistochemistry (Graham et al., 2002). Isolation and cryo-EM analysis of receptors from native sources, as was done in a recent study on native AMPA receptors (Zhao et al., 2019), could help define the exact subunit composition and stoichiometries of native receptors. Finally, the pursuit of structures in other conformational states will be essential to fully understand the gating cycle of nicotinic receptors. While it is probable that the overall features of gating are likely broadly conserved throughout the Cys-loop receptor superfamily (Nemecz et al., 2016), many specific details are likely receptor-family and even subtype specific. Therefore, structures of nicotinic receptors in resting-like and open conformations will further help identify important regions involved in channel function.
Acknowledgements
We thank Leah Baxter for assistance with illustrations and are grateful for support from the National Institutes of Health (DA037492, DA042072, and NS095899 to REH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
Authors declare no competing interests associated with the manuscript. All authors read and approved the final manuscript.
References
- Ahring PK, Olsen JA, Nielsen EO, Peters D, Pedersen MH, Rohde LA, Kastrup JS, Shahsavar A, Indurthi DC, Chebib M, et al. (2015). Engineered alpha4beta2 nicotinic acetylcholine receptors as models for measuring agonist binding and effect at the orthosteric low-affinity alpha4-alpha4 interface. Neuropharmacology 92, 135–145. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, and Rogers SW (2009). Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89, 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaino C, Musgaard M, Minguez T, Mazzaferro S, Faundez M, Iturriaga-Vasquez P, Biggin PC, and Bermudez I (2017). Role of the Cys Loop and Transmembrane Domain in the Allosteric Modulation of alpha4beta2 Nicotinic Acetylcholine Receptors. J Biol Chem 292, 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althoff T, Hibbs RE, Banerjee S, and Gouaux E (2014). X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors. Nature 512, 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal P, Sansom MS, and Tucker SJ (2015). Hydrophobic gating in ion channels. J Mol Biol 427, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A, and Akk G (1998). Desensitization of mouse nicotinic acetylcholine receptor channels. A two-gate mechanism. J Gen Physiol 112, 181–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, and Leslie FM (2002). Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol 444, 260–274. [DOI] [PubMed] [Google Scholar]
- Basak S, Gicheru Y, Rao S, Sansom MSP, and Chakrapani S (2018a). Cryo-EM reveals two distinct serotonin-bound conformations of full-length 5-HT3A receptor. Nature 563, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S, Gicheru Y, Samanta A, Molugu SK, Huang W, Fuente M, Hughes T, Taylor DJ, Nieman MT, Moiseenkova-Bell V, et al. (2018b). Cryo-EM structure of 5-HT3A receptor in its resting conformation. Nat Commun 9, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers WH, and Reich E (1970). Structure and activity of acetylcholine. Nature 228, 917–922. [DOI] [PubMed] [Google Scholar]
- Beroukhim R, and Unwin N (1997). Distortion correction of tubular crystals: improvements in the acetylcholine receptor structure. Ultramicroscopy 70, 57–81. [DOI] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, and Mooser V (2008). Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry 13, 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum AP, Lester HA, and Dougherty DA (2010). Nicotinic pharmacophore: the pyridine N of nicotine and carbonyl of acetylcholine hydrogen bond across a subunit interface to a backbone NH. Proc Natl Acad Sci U S A 107, 13206–13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, and Corringer PJ (2009). X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457, 111–114. [DOI] [PubMed] [Google Scholar]
- Boulter J, O’Shea-Greenfield A, Duvoisin RM, Connolly JG, Wada E, Jensen A, Gardner PD, Ballivet M, Deneris ES, McKinnon D, et al. (1990). Alpha 3, alpha 5, and beta 4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J Biol Chem 265, 4472–4482. [PubMed] [Google Scholar]
- Bourne Y, Talley TT, Hansen SB, Taylor P, and Marchot P (2005). Crystal structure of a Cbtx-AChBP complex reveals essential interactions between snake alpha-neurotoxins and nicotinic receptors. EMBO J 24, 1512–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzat C, Gumilar F, Spitzmaul G, Wang HL, Rayes D, Hansen SB, Taylor P, and Sine SM (2004). Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature 430, 896–900. [DOI] [PubMed] [Google Scholar]
- Bowery NG, and Smart TG (2006). GABA and glycine as neurotransmitters: a brief history. Br J Pharmacol 147 Suppl 1, S109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, and Sixma TK (2001). Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411, 269–276. [DOI] [PubMed] [Google Scholar]
- Brisson A, and Unwin PN (1984). Tubular crystals of acetylcholine receptor. J Cell Biol 99, 1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson A, and Unwin PN (1985). Quaternary structure of the acetylcholine receptor. Nature 315, 474–477. [DOI] [PubMed] [Google Scholar]
- Broadbent S, Groot-Kormelink PJ, Krashia PA, Harkness PC, Millar NS, Beato M, and Sivilotti LG (2006). Incorporation of the beta3 subunit has a dominant-negative effect on the function of recombinant central-type neuronal nicotinic receptors. Mol Pharmacol 70, 1350–1357. [DOI] [PubMed] [Google Scholar]
- Browning KN (2015). Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front Neurosci 9, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Hernandez GA, Stokes C, Duggan BM, Kaczanowska K, Brandao-Araiza S, Doan L, Papke RL, and Taylor P (2019). Synthesis, Pharmacological Characterization, and Structure-Activity Relationships of Noncanonical Selective Agonists for alpha7 nAChRs. J Med Chem 62, 10376–10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Caro A, Smillie FI, Dominguez del Toro E, Rovira JC, Vicente-Agullo F, Chapuli J, Juiz JM, Sala S, Sala F, Ballesta JJ, et al. (1997). Neuronal nicotinic acetylcholine receptors on bovine chromaffin cells: cloning, expression, and genomic organization of receptor subunits. J Neurochem 68, 488–497. [DOI] [PubMed] [Google Scholar]
- Cao E (2020). Structural mechanisms of transient receptor potential ion channels. J Gen Physiol 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartaud J, Benedetti EL, Cohen JB, Meunier JC, and Changeux JP (1973). Presence of a lattice structure in membrane fragments rich in nicotinic receptor protein from the electric organ of Torpedo marmorata. FEBS Lett 33, 109–113. [DOI] [PubMed] [Google Scholar]
- Cartaud J, Popot JL, and Changeux JP (1980). Light and heavy forms of the acetylcholine receptor from Torpedo marmorata electric organ: morphological identification using reconstituted vesicles. FEBS Lett 121, 327–332. [DOI] [PubMed] [Google Scholar]
- Castro NG, and Albuquerque EX (1995). alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys J 68, 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celie PH, van Rossum-Fikkert SE, van Dijk WJ, Brejc K, Smit AB, and Sixma TK (2004). Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41, 907–914. [DOI] [PubMed] [Google Scholar]
- Changeux JP (2012). The nicotinic acetylcholine receptor: the founding father of the pentameric ligand-gated ion channel superfamily. J Biol Chem 287, 40207–40215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP, Kasai M, Huchet M, and Meunier JC (1970). [Extraction from electric tissue of gymnotus of a protein presenting several typical properties characteristic of the physiological receptor of acetylcholine]. C R Acad Hebd Seances Acad Sci D 270, 2864–2867. [PubMed] [Google Scholar]
- Cheng Y (2015). Single-Particle Cryo-EM at Crystallographic Resolution. Cell 161, 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Grigorieff N, Penczek PA, and Walz T (2015). A primer to single-particle cryoelectron microscopy. Cell 161, 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio T, Ballivet M, Patrick J, and Heinemann S (1983). Nucleotide and deduced amino acid sequences of Torpedo californica acetylcholine receptor gamma subunit. Proc Natl Acad Sci U S A 80, 1111–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy WG, and Berg DK (1995). Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J Biol Chem 270, 4424–4431. [DOI] [PubMed] [Google Scholar]
- Conroy WG, Vernallis AB, and Berg DK (1992). The alpha 5 gene product assembles with multiple acetylcholine receptor subunits to form distinctive receptor subtypes in brain. Neuron 9, 679–691. [DOI] [PubMed] [Google Scholar]
- Corbin J, Wang HH, and Blanton MP (1998). Identifying the cholesterol binding domain in the nicotinic acetylcholine receptor with [125I]azido-cholesterol. Biochim Biophys Acta 1414, 65–74. [DOI] [PubMed] [Google Scholar]
- Criado M, Eibl H, and Barrantes FJ (1982). Effects of lipids on acetylcholine receptor. Essential need of cholesterol for maintenance of agonist-induced state transitions in lipid vesicles. Biochemistry 21, 3622–3629. [DOI] [PubMed] [Google Scholar]
- Cymes GD, and Grosman C (2012). The unanticipated complexity of the selectivity-filter glutamates of nicotinic receptors. Nat Chem Biol 8, 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cymes GD, and Grosman C (2016). Identifying the elusive link between amino acid sequence and charge selectivity in pentameric ligand-gated ion channels. Proc Natl Acad Sci U S A 113, E7106–E7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- daCosta CJ, and Baenziger JE (2009). A lipid-dependent uncoupled conformation of the acetylcholine receptor. J Biol Chem 284, 17819–17825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- daCosta CJ, Ogrel AA, McCardy EA, Blanton MP, and Baenziger JE (2002). Lipid-protein interactions at the nicotinic acetylcholine receptor. A functional coupling between nicotinic receptors and phosphatidic acid-containing lipid bilayers. J Biol Chem 277, 201–208. [DOI] [PubMed] [Google Scholar]
- Damle VN, and Karlin A (1980). Effects of agonists and antagonists on the reactivity of the binding site disulfide in acetylcholine receptor from Torpedo californica. Biochemistry 19, 3924–3932. [DOI] [PubMed] [Google Scholar]
- Dani JA, and Bertrand D (2007). Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47, 699–729. [DOI] [PubMed] [Google Scholar]
- Delbart F, Brams M, Gruss F, Noppen S, Peigneur S, Boland S, Chaltin P, Brandao-Neto J, von Delft F, Touw WG, et al. (2018). An allosteric binding site of the alpha7 nicotinic acetylcholine receptor revealed in a humanized acetylcholine-binding protein. J Biol Chem 293, 2534–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellisanti CD, Yao Y, Stroud JC, Wang ZZ, and Chen L (2007). Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 A resolution. Nat Neurosci 10, 953–962. [DOI] [PubMed] [Google Scholar]
- Denisov IG, and Sligar SG (2016). Nanodiscs for structural and functional studies of membrane proteins. Nat Struct Mol Biol 23, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A, Giraudat J, Bentaboulet M, and Changeux JP (1983). Complete mRNA coding sequence of the acetylcholine binding alpha-subunit of Torpedo marmorata acetylcholine receptor: a model for the transmembrane organization of the polypeptide chain. Proc Natl Acad Sci U S A 80, 2067–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Lu W, Wu S, Cheng Y, and Gouaux E (2015). Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature 526, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukkipati A, Park HH, Waghray D, Fischer S, and Garcia KC (2008). BacMam system for high-level expression of recombinant soluble and membrane glycoproteins for structural studies. Protein Expr Purif 62, 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JB, Peng JH, Schroeder KM, George AA, Fryer JD, Krishnan C, Buhlman L, Kuo YP, Steinlein O, and Lukas RJ (2003). Characterization of human alpha 4 beta 2-nicotinic acetylcholine receptors stably and heterologously expressed in native nicotinic receptor-null SH-EP1 human epithelial cells. Mol Pharmacol 64, 1283–1294. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Katz B, and Kuffler SW (1941). NATURE OF THE “ENDPLATE POTENTIAL” IN CURARIZED MUSCLE. Journal of Neurophysiology 4, 362–387. [Google Scholar]
- Eccles JC, and O’Connor WJ (1939). Responses which nerve impulses evoke in mammalian striated muscles. The Journal of Physiology 97, 44–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, and Heinemann S (1994). Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79, 705–715. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, and Boulter J (2001). alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A 98, 3501–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M, and Racker E (1978). Reconstitution of carbamylcholine-dependent sodium ion flux and desensitization of the acetylcholine receptor from Torpedo californica. J Biol Chem 253, 6660–6662. [PubMed] [Google Scholar]
- Fatt P, and Katz B (1950). Membrane potentials at the motor end-plate. J Physiol 111, 46p–47p. [PubMed] [Google Scholar]
- Feltz A, and Trautmann A (1982). Desensitization at the frog neuromuscular junction: a biphasic process. The Journal of Physiology 322, 257–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov GN, and White MM (1995). The role of conserved leucines in the M2 domain of the acetylcholine receptor in channel gating. Mol Pharmacol 48, 379–384. [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, and Kellar KJ (1992). A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol 41, 31–37. [PubMed] [Google Scholar]
- Fong TM, and McNamee MG (1986). Correlation between acetylcholine receptor function and structural properties of membranes. Biochemistry 25, 830–840. [DOI] [PubMed] [Google Scholar]
- Frauenfeld J, Loving R, Armache JP, Sonnen AF, Guettou F, Moberg P, Zhu L, Jegerschold C, Flayhan A, Briggs JA, et al. (2016). A saposin-lipoprotein nanoparticle system for membrane proteins. Nat Methods 13, 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free RB, Bryant DL, McKay SB, Kaser DJ, and McKay DB (2002). [3H]Epibatidine binding to bovine adrenal medulla: evidence for alpha3beta4* nicotinic receptors. Neurosci Lett 318, 98–102. [DOI] [PubMed] [Google Scholar]
- Gharpure A, Teng J, Zhuang Y, Noviello CM, Walsh RM Jr., Cabuco R, Howard RJ, Zaveri NT, Lindahl E, and Hibbs RE (2019). Agonist Selectivity and Ion Permeation in the alpha3beta4 Ganglionic Nicotinic Receptor. Neuron 104, 501–511 e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen M, and Corringer PJ (2018). The dual-gate model for pentameric ligand-gated ion channels activation and desensitization. J Physiol 596, 1873–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, and Dukat M (2000). Central nicotinic receptor ligands and pharmacophores. Pharm Acta Helv 74, 103–114. [DOI] [PubMed] [Google Scholar]
- Goehring A, Lee CH, Wang KH, Michel JC, Claxton DP, Baconguis I, Althoff T, Fischer S, Garcia KC, and Gouaux E (2014). Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat Protoc 9, 2574–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert H, and Schaefer H (1938). Über den direkt und indirekt erregten Aktionsstrom und die Funktion der motorischen Endplatte. Pflüger’s Archiv für die gesamte Physiologie des Menschen und der Tiere 239, 597–619. [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, and Gotti C (2009). Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci 29, 2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Court JA, Martin-Ruiz CM, Jaros E, Perry R, Volsen SG, Bose S, Evans N, Ince P, Kuryatov A, et al. (2002). Immunohistochemical localisation of nicotinic acetylcholine receptor subunits in human cerebellum. Neuroscience 113, 493–507. [DOI] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Boorman JP, and Sivilotti LG (2001). Formation of functional α3β4α5 human neuronal nicotinic receptors in Xenopus oocytes: a reporter mutation approach. British Journal of Pharmacology 134, 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales TG, Dunlop JI, Deeb TZ, Carland JE, Kelley SP, Lambert JJ, and Peters JA (2006). Common determinants of single channel conductance within the large cytoplasmic loop of 5-hydroxytryptamine type 3 and alpha4beta2 nicotinic acetylcholine receptors. J Biol Chem 281, 8062–8071. [DOI] [PubMed] [Google Scholar]
- Hamouda AK, Chiara DC, Sauls D, Cohen JB, and Blanton MP (2006). Cholesterol interacts with transmembrane alpha-helices M1, M3, and M4 of the Torpedo nicotinic acetylcholine receptor: photolabeling studies using [3H]Azicholesterol. Biochemistry 45, 976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, and Bourne Y (2005). Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J 24, 3635–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpole TJ, and Grosman C (2014). Side-chain conformation at the selectivity filter shapes the permeation free-energy landscape of an ion channel. Proc Natl Acad Sci U S A 111, E3196–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpsoe K, Ahring PK, Christensen JK, Jensen ML, Peters D, and Balle T (2011). Unraveling the high- and low-sensitivity agonist responses of nicotinic acetylcholine receptors. J Neurosci 31, 10759–10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henault CM, Sun J, Therien JP, daCosta CJ, Carswell CL, Labriola JM, Juranka PF, and Baenziger JE (2015). The role of the M4 lipid-sensor in the folding, trafficking, and allosteric modulation of nicotinic acetylcholine receptors. Neuropharmacology 96, 157–168. [DOI] [PubMed] [Google Scholar]
- Henderson R, Chen S, Chen JZ, Grigorieff N, Passmore LA, Ciccarelli L, Rubinstein JL, Crowther RA, Stewart PL, and Rosenthal PB (2011). Tilt-pair analysis of images from a range of different specimens in single-particle electron cryomicroscopy. J Mol Biol 413, 1028–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs RE, and Gouaux E (2011). Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf RJ, and Dutzler R (2008). X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452, 375–379. [DOI] [PubMed] [Google Scholar]
- Hilf RJ, and Dutzler R (2009). Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 457, 115–118. [DOI] [PubMed] [Google Scholar]
- Hogg RC, and Bertrand D (2004). Nicotinic acetylcholine receptors as drug targets. Curr Drug Targets CNS Neurol Disord 3, 123–130. [DOI] [PubMed] [Google Scholar]
- Huang X, Chen H, Michelsen K, Schneider S, and Shaffer PL (2015). Crystal structure of human glycine receptor-alpha3 bound to antagonist strychnine. Nature 526, 277–280. [DOI] [PubMed] [Google Scholar]
- Jain A, Kuryatov A, Wang J, Kamenecka TM, and Lindstrom J (2016). Unorthodox Acetylcholine Binding Sites Formed by alpha5 and beta3 Accessory Subunits in alpha4beta2* Nicotinic Acetylcholine Receptors. J Biol Chem 291, 23452–23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Lape R, and Dani JA (2001). Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron 31, 131–141. [DOI] [PubMed] [Google Scholar]
- Kalamida D, Poulas K, Avramopoulou V, Fostieri E, Lagoumintzis G, Lazaridis K, Sideri A, Zouridakis M, and Tzartos SJ (2007). Muscle and neuronal nicotinic acetylcholine receptors. Structure, function and pathogenicity. FEBS J 274, 3799–3845. [DOI] [PubMed] [Google Scholar]
- Karlin A (1969). Chemical modification of the active site of the acetylcholine receptor. J Gen Physiol 54, 245–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A (2002). Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci 3, 102–114. [DOI] [PubMed] [Google Scholar]
- Karlin A, Holtzman E, Yodh N, Lobel P, Wall J, and Hainfeld J (1983). The arrangement of the subunits of the acetylcholine receptor of Torpedo californica. J Biol Chem 258, 6678–6681. [PubMed] [Google Scholar]
- Karlsson E, Heilbronn E, and Widlund L (1972). Isolation of the nicotinic acetylcholine receptor by biospecific chromatography on insolubilized Naja naja neurotoxin. FEBS Lett 28, 107–111. [DOI] [PubMed] [Google Scholar]
- Katz B, and Thesleff S (1957). A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol 138, 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate T, and Gouaux E (2006). Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14, 673–681. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Dunlop JI, Kirkness EF, Lambert JJ, and Peters JA (2003). A cytoplasmic region determines single-channel conductance in 5-HT3 receptors. Nature 424, 321–324. [DOI] [PubMed] [Google Scholar]
- Keramidas A, and Lynch JW (2013). An outline of desensitization in pentameric ligand-gated ion channel receptors. Cell Mol Life Sci 70, 1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klett RP, Fulpius BW, Cooper D, Smith M, Reich E, and Possani LD (1973). The acetylcholine receptor. I. Purification and characterization of a macromolecule isolated from Electrophorus electricus. J Biol Chem 248, 6841–6853. [PubMed] [Google Scholar]
- Klymkowsky MW, and Stroud RM (1979). Immunospecific identification and three-dimensional structure of a membrane-bound acetylcholine receptor from Torpedo californica. J Mol Biol 128, 319–334. [DOI] [PubMed] [Google Scholar]
- Knowles TJ, Finka R, Smith C, Lin YP, Dafforn T, and Overduin M (2009). Membrane proteins solubilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer. J Am Chem Soc 131, 7484–7485. [DOI] [PubMed] [Google Scholar]
- Koenen M, Peter C, Villarroel A, Witzemann V, and Sakmann B (2005). Acetylcholine receptor channel subtype directs the innervation pattern of skeletal muscle. EMBO Rep 6, 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouvatsos N, Giastas P, Chroni-Tzartou D, Poulopoulou C, and Tzartos SJ (2016). Crystal structure of a human neuronal nAChR extracellular domain in pentameric assembly: Ligand-bound alpha2 homopentamer. Proc Natl Acad Sci U S A 113, 9635–9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, and Lindstrom J (2008). Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol 74, 132–143. [DOI] [PubMed] [Google Scholar]
- Labarca C, Nowak MW, Zhang H, Tang L, Deshpande P, and Lester HA (1995). Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature 376, 514–516. [DOI] [PubMed] [Google Scholar]
- Langley JN (1905). On the reaction of cells and of nerve-endings to certain poisons, chiefly as regards the reaction of striated muscle to nicotine and to curari. J Physiol 33, 374–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novere N, Corringer PJ, and Changeux JP (2002). The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol 53, 447–456. [DOI] [PubMed] [Google Scholar]
- Lee WY, Free CR, and Sine SM (2008). Nicotinic receptor interloop proline anchors beta1-beta2 and Cys loops in coupling agonist binding to channel gating. J Gen Physiol 132, 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Free CR, and Sine SM (2009). Binding to gating transduction in nicotinic receptors: Cys-loop energetically couples to pre-M1 and M2–M3 regions. J Neurosci 29, 3189–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, and Sine SM (2005). Principal pathway coupling agonist binding to channel gating in nicotinic receptors. Nature 438, 243–247. [DOI] [PubMed] [Google Scholar]
- Leslie FM, Mojica CY, and Reynaga DD (2013). Nicotinic receptors in addiction pathways. Mol Pharmacol 83, 753–758. [DOI] [PubMed] [Google Scholar]
- Levin ED (2002). Nicotinic receptor subtypes and cognitive function. J Neurobiol 53, 633–640. [DOI] [PubMed] [Google Scholar]
- Li SX, Huang S, Bren N, Noridomi K, Dellisanti CD, Sine SM, and Chen L (2011). Ligand-binding domain of an alpha7-nicotinic receptor chimera and its complex with agonist. Nat Neurosci 14, 1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J, Anholt R, Einarson B, Engel A, Osame M, and Montal M (1980). Purification of acetylcholine receptors, reconstitution into lipid vesicles, and study of agonist-induced cation channel regulation. J Biol Chem 255, 8340–8350. [PubMed] [Google Scholar]
- Lindstrom JM (2000). Acetylcholine receptors and myasthenia. Muscle Nerve 23, 453–477. [DOI] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G, Zhang J, Lucero L, Wang M, Sierks M, et al. (2009). A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J Neurosci 29, 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Padgett D, Takahashi M, Li H, Sayeed A, Teichert RW, Olivera BM, McArdle JJ, Green WN, and Lin W (2008). Essential roles of the acetylcholine receptor gamma-subunit in neuromuscular synaptic patterning. Development 135, 1957–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd GK, and Williams M (2000). Neuronal nicotinic acetylcholine receptors as novel drug targets. J Pharmacol Exp Ther 292, 461–467. [PubMed] [Google Scholar]
- Lummis SC (2012). 5-HT(3) receptors. J Biol Chem 287, 40239–40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiulis S, Desai R, Uchanski T, Serna Martin I, Laverty D, Karia D, Malinauskas T, Zivanov J, Pardon E, Kotecha A, et al. (2019). GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature 565, 454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, et al. (2005). Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436, 103–107. [DOI] [PubMed] [Google Scholar]
- Mazzaferro S, Benallegue N, Carbone A, Gasparri F, Vijayan R, Biggin PC, Moroni M, and Bermudez I (2011). Additional acetylcholine (ACh) binding site at alpha4/alpha4 interface of (alpha4beta2)2alpha4 nicotinic receptor influences agonist sensitivity. J Biol Chem 286, 31043–31054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee MG, Weill CL, and Karlin A (1975). Purification of acetylcholine receptor from Torpedo californica and its incorporation into phospholipid vesicles. Ann N Y Acad Sci 264, 175–182. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Huchet M, Boquet P, and Changeux JP (1971). [Separation of the receptor protein of acetylcholine and acetylcholinesterase]. C R Acad Hebd Seances Acad Sci D 272, 117–120. [PubMed] [Google Scholar]
- Miledi R, Molinoff P, and Potter LT (1971). Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature 229, 554–557. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Emerit MB, Nosjean A, Simon A, Rumajogee P, Brisorgueil MJ, Doucet E, Hamon M, and Verge D (2002). Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. Eur J Neurosci 15, 449–457. [DOI] [PubMed] [Google Scholar]
- Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, and Sakmann B (1986). Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature 321, 406–411. [DOI] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Stowell M, and Unwin N (1999). Nicotinic acetylcholine receptor at 4.6 A resolution: transverse tunnels in the channel wall. J Mol Biol 288, 765–786. [DOI] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, and Unwin N (2003). Structure and gating mechanism of the acetylcholine receptor pore. Nature 423, 949–955. [DOI] [PubMed] [Google Scholar]
- Morales-Perez CL, Noviello CM, and Hibbs RE (2016a). Manipulation of Subunit Stoichiometry in Heteromeric Membrane Proteins. Structure 24, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Perez CL, Noviello CM, and Hibbs RE (2016b). X-ray structure of the human alpha4beta2 nicotinic receptor. Nature 538, 411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti M, Zoli M, George AA, Lukas RJ, Pistillo F, Maskos U, Whiteaker P, and Gotti C (2014). The novel alpha7beta2-nicotinic acetylcholine receptor subtype is expressed in mouse and human basal forebrain: biochemical and pharmacological characterization. Mol Pharmacol 86, 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Vidal C, Benoit P, and Changeux JP (1991). Existence of different subtypes of nicotinic acetylcholine receptors in the rat habenulo-interpeduncular system. J Neurosci 11, 2588–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmansohn D, Coates CW, and Cox RT (1941). Electric Potential and Activity of Choline Esterase in the Electric Organ of Electrophorus Electricus (Linnaeus). J Gen Physiol 25, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, and Sakmann B (1976). Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 260, 799–802. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, and Lindstrom J (2003). Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol 63, 332–341. [DOI] [PubMed] [Google Scholar]
- Nelson N, Anholt R, Lindstrom J, and Montal M (1980). Reconstitution of purified acetylcholine receptors with functional ion channels in planar lipid bilayers. Proc Natl Acad Sci U S A 77, 3057–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemecz A, Prevost MS, Menny A, and Corringer PJ (2016). Emerging Molecular Mechanisms of Signal Transduction in Pentameric Ligand-Gated Ion Channels. Neuron 90, 452–470. [DOI] [PubMed] [Google Scholar]
- Nemecz A, and Taylor P (2011). Creating an alpha7 nicotinic acetylcholine recognition domain from the acetylcholine-binding protein: crystallographic and ligand selectivity analyses. J Biol Chem 286, 42555–42565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Takahashi H, Tanabe T, Toyosato M, Kikyotani S, Furutani Y, Hirose T, Takashima H, Inayama S, Miyata T, et al. (1983). Structural homology of Torpedo californica acetylcholine receptor subunits. Nature 302, 528–532. [DOI] [PubMed] [Google Scholar]
- Noridomi K, Watanabe G, Hansen MN, Han GW, and Chen L (2017). Structural insights into the molecular mechanisms of myasthenia gravis and their therapeutic implications. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Yamamoto T, and Watanabe S (1993). Blockade of hippocampal nicotinic receptors impairs working memory but not reference memory in rats. Pharmacol Biochem Behav 45, 89–93. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Meunier JC, and Changeux JP (1972). Progress in the purification of the cholinergic receptor protein from Electrophorus electricus by affinity chromatography. FEBS Lett 28, 96–100. [DOI] [PubMed] [Google Scholar]
- Pan J, Chen Q, Willenbring D, Yoshida K, Tillman T, Kashlan OB, Cohen A, Kong XP, Xu Y, and Tang P (2012). Structure of the pentameric ligand-gated ion channel ELIC cocrystallized with its competitive antagonist acetylcholine. Nat Commun 3, 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandhare A, Pirayesh E, Stuebler AG, and Jansen M (2019). Triple arginines as molecular determinants for pentameric assembly of the intracellular domain of 5-HT3A receptors. J Gen Physiol 151, 1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo JA, Brucker WJ, and Hawrot E (2009). Proteomic analysis of an alpha7 nicotinic acetylcholine receptor interactome. J Proteome Res 8, 1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Katz M, Gerzanich V, Anand R, and Lindstrom J (1994). Human alpha 7 acetylcholine receptor: cloning of the alpha 7 subunit from the SH-SY5Y cell line and determination of pharmacological properties of native receptors and functional alpha 7 homomers expressed in Xenopus oocytes. Mol Pharmacol 45, 546–554. [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, and Mineur YS (2012). Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76, 116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, Vincent P, Pich EM, Brulet P, and Changeux JP (1995). Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature 374, 65–67. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, and Changeux JP (1998). Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391, 173–177. [DOI] [PubMed] [Google Scholar]
- Pohanka M (2012). Alpha7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology. Int J Mol Sci 13, 2219–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polovinkin L, Hassaine G, Perot J, Neumann E, Jensen AA, Lefebvre SN, Corringer PJ, Neyton J, Chipot C, Dehez F, et al. (2018). Conformational transitions of the serotonin 5-HT3 receptor. Nature 563, 275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit P, and Auerbach A (2007). Acetylcholine receptor gating at extracellular transmembrane domain interface: the “pre-M1” linker. J Gen Physiol 130, 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit Y, and Grosman C (2006). Block of muscle nicotinic receptors by choline suggests that the activation and desensitization gates act as distinct molecular entities. J Gen Physiol 127, 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, and Lester RA (1999). Alpha3beta4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology 38, 769–783. [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, and Role L (1996). Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature 380, 347–351. [DOI] [PubMed] [Google Scholar]
- Reeves PJ, Callewaert N, Contreras R, and Khorana HG (2002). Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc Natl Acad Sci U S A 99, 13419–13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revah F, Bertrand D, Galzi JL, Devillers-Thiery A, Mulle C, Hussy N, Bertrand S, Ballivet M, and Changeux JP (1991). Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature 353, 846–849. [DOI] [PubMed] [Google Scholar]
- Rucktooa P, Smit AB, and Sixma TK (2009). Insight in nAChR subtype selectivity from AChBP crystal structures. Biochem Pharmacol 78, 777–787. [DOI] [PubMed] [Google Scholar]
- Sakmann B, and Brenner HR (1978). Change in synaptic channel gating during neuromuscular development. Nature 276, 401–402. [DOI] [PubMed] [Google Scholar]
- Sauguet L, Poitevin F, Murail S, Van Renterghem C, Moraga-Cid G, Malherbe L, Thompson AW, Koehl P, Corringer PJ, Baaden M, et al. (2013). Structural basis for ion permeation mechanism in pentameric ligand-gated ion channels. EMBO J 32, 728–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauguet L, Shahsavar A, Poitevin F, Huon C, Menny A, Nemecz A, Haouz A, Changeux JP, Corringer PJ, and Delarue M (2014). Crystal structures of a pentameric ligand-gated ion channel provide a mechanism for activation. Proc Natl Acad Sci U S A 111, 966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres SH (2016). Processing of Structurally Heterogeneous Cryo-EM Data in RELION. Methods Enzymol 579, 125–157. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, and Patrick JW (1993). Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci 13, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgard F, Charpantier E, Bertrand S, Walker N, Caput D, Graham D, Bertrand D, and Besnard F (2002). A novel human nicotinic receptor subunit, alpha10, that confers functionality to the alpha9-subunit. Mol Pharmacol 61, 150–159. [DOI] [PubMed] [Google Scholar]
- Shahsavar A, Ahring PK, Olsen JA, Krintel C, Kastrup JS, Balle T, and Gajhede M (2015). Acetylcholine-Binding Protein Engineered to Mimic the alpha4-alpha4 Binding Pocket in alpha4beta2 Nicotinic Acetylcholine Receptors Reveals Interface Specific Interactions Important for Binding and Activity. Mol Pharmacol 88, 697–707. [DOI] [PubMed] [Google Scholar]
- Shahsavar A, Gajhede M, Kastrup JS, and Balle T (2016). Structural Studies of Nicotinic Acetylcholine Receptors: Using Acetylcholine-Binding Protein as a Structural Surrogate. Basic Clin Pharmacol Toxicol 118, 399–407. [DOI] [PubMed] [Google Scholar]
- Sigel E, and Buhr A (1997). The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci 18, 425–429. [DOI] [PubMed] [Google Scholar]
- Skok VI (2002). Nicotinic acetylcholine receptors in autonomic ganglia. Auton Neurosci 97, 1–11. [DOI] [PubMed] [Google Scholar]
- Smit AB, Syed NI, Schaap D, van Minnen J, Klumperman J, Kits KS, Lodder H, van der Schors RC, van Elk R, Sorgedrager B, et al. (2001). A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature 411, 261–268. [DOI] [PubMed] [Google Scholar]
- Stokes C, Treinin M, and Papke RL (2015). Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol Sci 36, 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swope SL, Moss SJ, Raymond LA, and Huganir RL (1999). Regulation of ligand-gated ion channels by protein phosphorylation. Adv Second Messenger Phosphoprotein Res 33, 49–78. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kubo T, Mizoguchi A, Carlson CG, Endo K, and Ohnishi K (2002). Spontaneous muscle action potentials fail to develop without fetal-type acetylcholine receptors. EMBO Rep 3, 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar S, and Lynch JW (2014). Phosphorylation mediated structural and functional changes in pentameric ligand-gated ion channels: implications for drug discovery. Int J Biochem Cell Biol 53, 218–223. [DOI] [PubMed] [Google Scholar]
- Tasneem A, Iyer LM, Jakobsson E, and Aravind L (2005). Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys-loop ion channels. Genome Biol 6, R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Lester HA, and Lummis SC (2010). The structural basis of function in Cys-loop receptors. Q Rev Biophys 43, 449–499. [DOI] [PubMed] [Google Scholar]
- Toyoshima C, and Unwin N (1988). Ion channel of acetylcholine receptor reconstructed from images of postsynaptic membranes. Nature 336, 247–250. [DOI] [PubMed] [Google Scholar]
- Toyoshima C, and Unwin N (1990). Three-dimensional structure of the acetylcholine receptor by cryoelectron microscopy and helical image reconstruction. J Cell Biol 111, 2623–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan EW, Horti AG, Olson TT, Gao Y, Stockmeier CA, Al-Muhtasib N, Bowman Dalley C, Lewin AE, Wolfe BB, Sahibzada N, et al. (2015). AT-1001 Is a Partial Agonist with High Affinity and Selectivity at Human and Rat alpha3beta4 Nicotinic Cholinergic Receptors. Mol Pharmacol 88, 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey EC, Yelshanskaya MV, Grassucci RA, Frank J, and Sobolevsky AI (2017). Channel opening and gating mechanism in AMPA-subtype glutamate receptors. Nature 549, 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N (1993). Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol 229, 1101–1124. [DOI] [PubMed] [Google Scholar]
- Unwin N (2005). Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol 346, 967–989. [DOI] [PubMed] [Google Scholar]
- Unwin N (2013). Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: insights from Torpedo postsynaptic membranes. Q Rev Biophys 46, 283–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N, Toyoshima C, and Kubalek E (1988). Arrangement of the acetylcholine receptor subunits in the resting and desensitized states, determined by cryoelectron microscopy of crystallized Torpedo postsynaptic membranes. J Cell Biol 107, 1123–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernallis AB, Conroy WG, and Berg DK (1993). Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron 10, 451–464. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, and Swanson LW (1989). Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol 284, 314–335. [DOI] [PubMed] [Google Scholar]
- Walsh RM Jr., Roh SH, Gharpure A, Morales-Perez CL, Teng J, and Hibbs RE (2018). Structural principles of distinct assemblies of the human alpha4beta2 nicotinic receptor. Nature 557, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, and Lindstrom JM (1988). Characterization of bovine and human neuronal nicotinic acetylcholine receptors using monoclonal antibodies. J Neurosci 8, 3395–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BM, Temburni MK, Levey MS, Bertrand S, Bertrand D, and Jacob MH (1998). The long internal loop of the alpha 3 subunit targets nAChRs to subdomains within individual synapses on neurons in vivo. Nat Neurosci 1, 557–562. [DOI] [PubMed] [Google Scholar]
- Williams ME, Burton B, Urrutia A, Shcherbatko A, Chavez-Noriega LE, Cohen CJ, and Aiyar J (2005). Ric-3 promotes functional expression of the nicotinic acetylcholine receptor alpha7 subunit in mammalian cells. J Biol Chem 280, 1257–1263. [DOI] [PubMed] [Google Scholar]
- Wilson G, and Karlin A (2001). Acetylcholine receptor channel structure in the resting, open, and desensitized states probed with the substituted-cysteine-accessibility method. Proc Natl Acad Sci U S A 98, 1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzemann V, Schwarz H, Koenen M, Berberich C, Villarroel A, Wernig A, Brenner HR, and Sakmann B (1996). Acetylcholine receptor epsilon-subunit deletion causes muscle weakness and atrophy in juvenile and adult mice. Proc Natl Acad Sci U S A 93, 13286–13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S (1997). Presynaptic nicotinic ACh receptors. Trends Neurosci 20, 92–98. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, and Kellar KJ (1998). Rat alpha3/beta4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Mol Pharmacol 54, 322–333. [DOI] [PubMed] [Google Scholar]
- Xiu X, Puskar NL, Shanata JA, Lester HA, and Dougherty DA (2009). Nicotine binding to brain receptors requires a strong cation-pi interaction. Nature 458, 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Chen S, Swensen AC, Qian WJ, and Gouaux E (2019). Architecture and subunit arrangement of native AMPA receptors elucidated by cryo-EM. Science 364, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Pistillo F, and Gotti C (2015). Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 96, 302–311. [DOI] [PubMed] [Google Scholar]
- Zouridakis M, Giastas P, Zarkadas E, Chroni-Tzartou D, Bregestovski P, and Tzartos SJ (2014). Crystal structures of free and antagonist-bound states of human alpha9 nicotinic receptor extracellular domain. Nat Struct Mol Biol 21, 976–980. [DOI] [PubMed] [Google Scholar]
- Zouridakis M, Papakyriakou A, Ivanov IA, Kasheverov IE, Tsetlin V, Tzartos S, and Giastas P (2019). Crystal Structure of the Monomeric Extracellular Domain of alpha9 Nicotinic Receptor Subunit in Complex With alpha-Conotoxin RgIA: Molecular Dynamics Insights Into RgIA Binding to alpha9alpha10 Nicotinic Receptors. Front Pharmacol 10, 474. [DOI] [PMC free article] [PubMed] [Google Scholar]


