Abstract
Background
The prevalence of pre-treatment drug resistance (PDR) to non-nucleoside reverse-transcriptase inhibitor (NNRTI) agents is increasing in sub-Saharan Africa, which may decrease the effectiveness of efavirenz-based antiretroviral therapy (ART) programs. However, due to recent safety concerns, there has been hesitancy to replace efavirenz-based ART with dolutegravir in women of reproductive potential. Our objective was to evaluate whether PDR testing for women not initiating dolutegravir-based ART would be a cost-effective strategy to address the challenges posed by PDR.
Methods
We developed an HIV drug resistance model that simulates the emergence and transmission of resistance mutations, calibrated to the Kenyan epidemic. We modeled three care strategies for PDR testing among women not initiating dolutegravir-based ART: no PDR testing, PDR testing with a low-cost point mutation assay, known as oligonucleotide ligation assay (OLA), and PDR testing with consensus sequencing. Using a health sector perspective, this model was used to evaluate the health outcomes, lifetime costs, and cost-effectiveness under each strategy over a 15-year time horizon starting in 2019.
Findings
OLA and CS PDR testing were projected to have incremental cost-effectiveness ratios (ICER) of $10,741/QALY gained and $134,396/QALY gained, respectively, which are not cost-effective by national income standards. Viral suppression rates among women at 12 months after ART initiation were 87·8%, 89·0%, and 89·3% with no testing, OLA testing, and CS testing, respectively. PDR testing with OLA and CS were associated with a 0.5% and 0.6% reduction in incidence rate compared to no PDR testing. Initial PDR prevalence among women was 13.1% in 2019. By 2034, this prevalence was 17·6%, 17·4%, and 17·3% with no testing, OLA testing, and CS testing, respectively.
Interpretation
PDR testing for women is unlikely to be cost-effective in Kenya whether one uses a low-cost assay, such as OLA, or consensus sequencing.
Funding
National Institutes of Health, Gilead Sciences.
Keywords: HIV, Pretreatment drug resistance, Drug resistance testing, Cost-effectiveness analysis, Dolutegravir-based ART, Efavirenz-based ART, Resource-limited setting, Africa
Research in context.
Evidence before this study
The prevalence of pre-treatment drug resistance (PDR) to non-nucleoside reverse transcriptase inhibitors (NNRTI) is increasing in several countries in sub-Saharan Africa. Individuals with NNRTI-associated PDR have an increased risk of virologic failure on efavirenz-based ART. PDR testing to guide initial choice of ART regimen is a potential strategy to address this risk for women who do not receive dolutegravir-based ART due to concerns about a potential increased risk of neural-tube defects. We searched Web of Knowledge for reports in English and published before Dec 31, 2019, using the following search terms: hiv* AND resistan* AND (efavirenz OR non-nucleoside OR NNRTI) AND cost*. We identified three modeling studies that examined the use of consensus sequencing for PDR testing in low- and middle-income countries (LMICs), but we found no modeling studies that evaluated the potential effectiveness and cost-effectiveness of PDR testing for women who do not receive dolutegravir-based ART in LMICs.
Added value of this study
PDR testing with a low-cost assay, such as an oligonucleotide ligation assay, is more cost-effective than with consensus sequencing. However, consistent with prior modeling studies, PDR testing for women is unlikely to be cost-effective in resource-limited settings with either method.
Implications of all the available evidence
This study provides further evidence in support of WHO's recently updated guidelines strongly recommending dolutegravir-based ART as the preferred empiric first-line ART regimen for people living with HIV, including women. Further research is needed to evaluate the cost-effectiveness of other potential applications for low-cost drug resistance testing methods currently under development.
Alt-text: Unlabelled box
1. Introduction
The expansion of antiretroviral therapy (ART) delivery programs in resource-limited settings has resulted in significant reductions in HIV-related adult mortality [1]. Over time, the prevalence of pre-treatment drug resistance (PDR) to non-nucleoside reverse transcriptase inhibitors (NNRTI)-based ART regimens has increased in sub-Saharan Africa [2]. This may decrease the overall effectiveness of ART programs that use efavirenz-based regimens (most commonly tenofovir/lamivudine/efavirenz), as patients with PDR to their ART regimen have an increased risk of virologic failure and subsequent disease progression and increased transmission risk [3,4]. Recently, WHO recommendations to address high levels of PDR to NNRTI-based regimens have undergone frequent updates in response to rapidly evolving safety evidence regarding use of dolutegravir in women of reproductive potential.
In May 2018, surveillance data from Botswana revealed a potential early signal for increased risk of neural-tube defects in association with use of dolutegravir-based ART from the time of conception [5]. WHO and Kenya Ministry of Health subsequently recommended dolutegravir-based ART as the preferred empiric first-line regimen for women only if they are receiving an effective form of contraception [6,7]. More recent evidence from Botswana suggests that while the potential increased risk of neural-tube defects is still significant it may not be as large as what the 2018 analysis found [8]. In response, WHO has updated its guidelines to provide reassurance and strongly recommend dolutegravir-based ART as the preferred empiric first-line ART regimen for HIV-infected women [9].
Despite this, ongoing uncertainty about the risk of neural tube defects associated with dolutegravir may make some programs and patients hesitant to use this drug in women. PDR testing for women is a potential alternative that could provide protease inhibitor (PI)-based ART to women diagnosed with PDR, thus addressing the increased risk of virologic failure associated with PDR while avoiding the potential risks associated with dolutegravir use. Moreover, it would be consistent with WHO's recommendation to adopt a woman-centered approach that empowers women with information and autonomy in their decision-making [9]. To date, the population-level effectiveness and cost-effectiveness of PDR testing for women has not been evaluated.
The clinical use of drug resistance testing in resource-limited settings has been extremely limited due to the costs of reagents and the infrastructure needed to perform conventional consensus sequencing (CS) [10]. Thus, there has been interest among experts to develop a simple, low-cost drug resistance assay for cost-effective PDR testing to guide selection of initial ART regimens [11]. While prior cost-effectiveness analyses have included PDR testing as a potential strategy to address the high prevalence of PDR [12], [13], [14], none have evaluated simple, low-cost PDR testing assays separately from conventional CS.
The oligonucleotide ligation assay (OLA) is one example of several potential low-cost technologies for drug resistance testing that differ in terms of cost and diagnostic sensitivity [15,16]. OLA is a point-mutation assay that requires relatively simple and inexpensive equipment and is designed to detect “major mutations” that predict virologic failure of NNRTI-based, first-line ART regimens [3,17]. In a recent randomized clinical trial, Kenyan technicians successfully implemented a laboratory-based version of the OLA to guide choice of initial ART regimen [18,19]. Within this trial, OLA was performed at an average cost of $42 per test [19]. A simpler kit is currently being developed, which is likely to have an even lower cost [20]. Evaluating the cost-effectiveness of drug resistance testing with OLA can provide insights about the cost-effectiveness of low-cost technologies more broadly. In this study, we present a cost-effectiveness analysis comparing three testing strategies for women who do not initiate dolutegravir-based ART in Kenya: no PDR testing, PDR testing with OLA, and PDR testing with CS.
2. Methods
2.1. HIV model
We used an individual-based stochastic model that simulates HIV disease, treatment, and transmission [21], as well as the selection and transmission of mutations conferring drug resistance to NNRTI-based first-line ART and their effect on treatment. The model was parameterized and calibrated to simulate an adult population similar to Kenya and tracks individual-level characteristics in monthly intervals. Key model parameter values are shown in Table 1. Each mutation is present in either the majority state (assumed transmissible) or in the minority state (assumed non-transmissible). Mutations can transition from majority to minority state over time in the absence of selective pressure. A full, detailed description of our model is available in the Appendix, and all principle assumptions were tested in sensitivity analyses (see section below).
Table 1.
Model parameters for base-case analysis.
| Parameter | Base-case estimate | Range for sensitivity analyses | Source |
|---|---|---|---|
| Probability of virologic failure | See Appendix Section 2F | ||
| Initial ART (over 12 months) | |||
| Dolutegravir-based ART | 6·2% | ||
| No PDR on efavirenz-based ART | 13·6% | ||
| PDR on PI-based ART | 13·6% | ||
| PDR on efavirenz-based ARTa | 32·0% | 23·9–48·6%a | |
| Second-line, PI-based ART (over 24 months) | 15·2% | 10·1–21·2%a | |
| Drug resistance test performance | |||
| Diagnostic sensitivity of OLA | 80% | 59–100% | Rhee et al. [11], Chung et al. [18] |
| Diagnostic sensitivity of CS | 100% | Assumption | |
| Specificity of OLA and CS | 100% | Beck et al. [17] | |
| ART management decisions | |||
| Probability of HIV-infected woman initiating ART receiving dolutegravir-based ART |
40% | 20–60% | See Appendix Section 1C |
| Probability of switching to second-line ART when virologic failure is diagnosed with viral load testing |
60% | 20–100% | Model calibration, see Appendix Sections 1C and 3C |
| Unit Costs (US$) | |||
| ART annual cost | Global Fund [23] | ||
| Efavirenz-based ART | $70 | ||
| Dolutegravir-based ART | $70 | ||
| PI-based ART | $215 | $70–400 | |
| Inpatient day | $60 | $15–240b | IHME [38]c |
| Outpatient visit | $15 | IHME [38]c | |
| HIV test | $24 | KEMRId | |
| CD4 test | $12 | $6–24 | Primary datae |
| Viral load test | $54 | $10–80 | KEMRId |
| OLA test | $30 | $15–60 | Duarte et al. [19] |
| Consensus sequencing test | $125 | KEMRId | |
| Background health spending (person/year) | $66 | World Bank [31] | |
| Utility weights by health statef | Salomon et al. [39] | ||
| HIV-negative | 1 | ||
| HIV-positive, CD4 > 350 | 0·947 | ||
| HIV-positive, CD4 = 200–350 | 0·779 | ||
| HIV-positive, CD4 < 200 | 0·453 |
In the base-case scenario, the ratio of the odds of virologic failure for those with PDR on efavirenz-based ART compared to those with either no PDR on efavirenz-based ART or those with PDR on PI-based ART is 3·0. The range of 23·9–48·6% probability of virologic failure for those with PDR on efavirenz-based ART corresponds to an odds ratio range of 2·0 to 6·0.
This range is meant to capture uncertainty in both unit cost per inpatient day and the number of inpatient days per opportunistic infection (see Appendix Section 4A for details).
Unit cost estimates for each inpatient day and each outpatient visit in Kenya were originally reported in 2011 US$. These estimates have been adjusted for inflation in Kenya from 2011 to 2019 (see Appendix Section 4B for details).
Unit cost estimates per HIV test, viral load test, and consensus sequencing drug resistance test were obtained through personal communication with Maxwel Majiwa, Ph.D., from the Kenya Medical Research Institute (KEMRI) on 29 March 2019.
Unit cost estimate per CD4 cell count test was obtained based on the price charged at the Coptic Hope Center in Nairobi, Kenya (see Appendix Section 4C for details).
Utility weights are equal to 1 – disability weight from Salomon et al. [39].
2.2. Testing strategies modeled
We modeled comparative clinical and cost-effectiveness outcomes of no PDR testing, PDR testing with OLA, and PDR testing with CS for women who do not initiate dolutegravir-based first-line ART, over a 15-year time horizon starting in 2019 (t0). All individuals diagnosed with HIV, regardless of CD4 cell count, are eligible to initiate ART. In the no PDR testing strategy, efavirenz-based ART is empirically given as the first-line regimen to women who avoid dolutegravir due to safety concerns. In the PDR testing strategies using either OLA or CS, patients initiate ART after test results become available, which we assume occurs within one month [19,22]. PI-based ART is initiated for women in whom PDR is detected, and efavirenz-based ART is used for all other women avoiding dolutegravir. We assume an OLA that tests for mutations K103N, M184V, Y181C, and G190A will detect 80% of PDR cases (diagnostic sensitivity = 80%; see Appendix Section 2E for definition of diagnostic vs. analytical sensitivity). Although a meta-analysis found that only 59% of PDR cases in low- and middle-income countries had at least one mutation detectable by OLA [11], a recent trial found that among subjects with virologic failure at 12 months, 100% of subjects with PDR detected by CS were also detected by OLA [18]. Thus, our assumption that OLA PDR testing has a diagnostic sensitivity of 80% is intended to synthesize data from these two studies. We assume CS PDR testing has diagnostic sensitivity of 100%. We assume OLA and CS testing cost $30 and $125 (Maxwel Majiwa, Ph.D., from Kenya Medical Research Institute, personal communication, 29 March 2019) per test, respectively [19]. For all strategies, initial viral load (VL) testing is performed 6 months after ART initiation and is subsequently performed at 12-month intervals.
2.3. Treatment options
We assume two types of first-line ART are available (efavirenz-based and dolutegravir-based), both at a cost of $70/person/year, and PI-based ART is available as the second-line regimen at a cost of $215/person/year [23]. Our model assumes dolutegravir availability gradually increases to 100% by 2020 (Appendix Section 1C). When available for ART initiation, we assume dolutegravir-based ART is given to all men, as well as to women who are on some form of effective contraception, which we assume is 40% of women (Appendix Section 1C) [24]. In the absence of PDR testing, all other women receive efavirenz-based ART as their empiric first-line ART regimen. We assume individuals with PDR initially treated with efavirenz-based ART have a higher probability of virologic failure compared to those who do not have PDR and are initially treated with efavirenz-based ART and those with PDR given PI-based ART (32·0% vs. 13·6% during first 12 months on ART). These model parameters assume the odds of virologic failure with initial ART are 3·0 times higher (odds ratio = 3·0) for those with PDR receiving an efavirenz-based regimen compared to those without PDR or those with PDR receiving PI-based ART as their initial regimen (Appendix Section 2F). Sixty percent (60%) of patients receiving efavirenz-based ART in whom virologic failure is detected switch to PI-based ART. If a patient experiences virologic failure with a PI-based regimen, we assume no third-line ART is available; however, the patient continues to receive PI-based ART because of the survival advantages associated with a non-suppressive regimen compared with discontinuation of ART [25].
2.4. Health and economic outcomes
For each strategy modeled, we projected several important epidemiologic and health outcomes, including rates of viral suppression, rates of PI use, number of new HIV infections, adult HIV prevalence, and adult PDR prevalence. We projected two primary outcomes for the whole population: total costs incurred, reported in 2019 US$, and total QALYs gained by each strategy, which were then used to calculate incremental cost-effectiveness ratios (ICERs) in terms of US$/QALY gained. We adopted a health sector perspective, discounted future costs and benefits 3% annually, and adhered to the recent recommendations of the Second Panel on Cost-Effectiveness in Health and Medicine, as well as the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) [26,27].
2.5. Sensitivity analyses
To address the uncertainty in the increased risk of virologic failure associated with PDR, we varied the ratio of the odds of virologic failure for those with PDR on efavirenz-based ART compared to those with either no PDR on efavirenz-based ART or those with PDR on PI-based ART from 2·0 to 6·0 [4,18]. One-way sensitivity analyses for the diagnostic sensitivity of OLA and cost per OLA test were conducted to address uncertainty in these parameters, as well as to explore the cost-effectiveness of other potential low-cost technologies that may differ from OLA in terms of diagnostic sensitivity and cost [15,16]. Several additional sensitivity analyses were conducted to further test the robustness of our results (Table 1).
2.6. Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
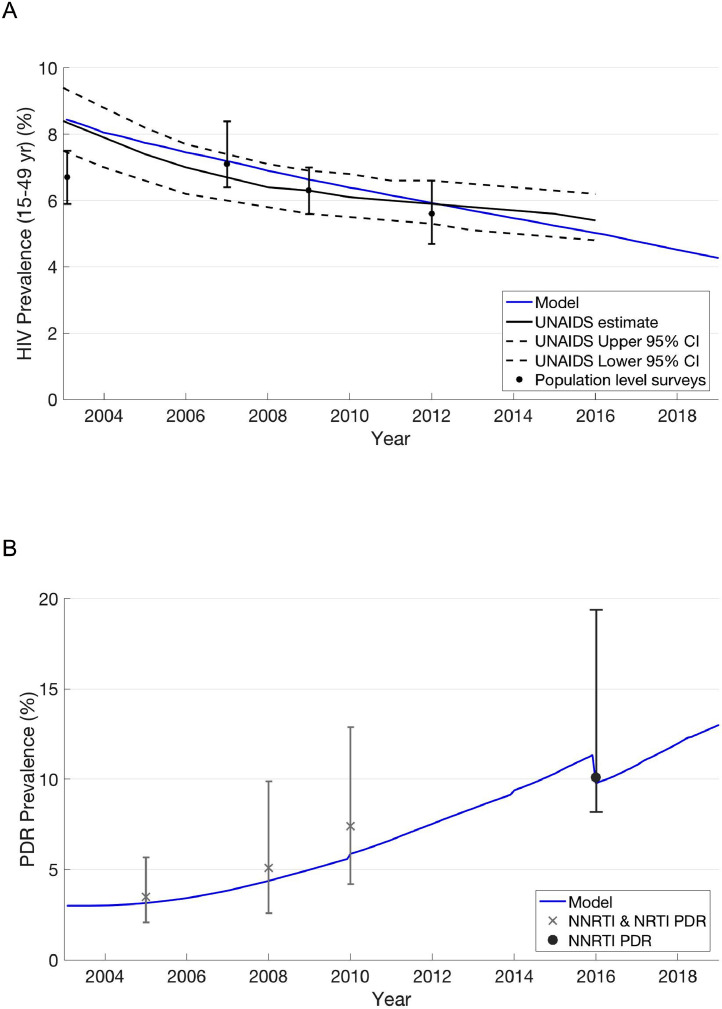
Our model was well-calibrated to multiple epidemiologic and demographic data targets in Kenya. Model outputs matched trends in HIV and PDR prevalence, proportion of HIV-infected individuals on any ART, proportion of HIV-infected individuals on PI-based ART, and population growth (Fig. 1, Table 2, and Appendix Section 3C – Figs. S1 and S2).
Fig. 1.
HIV and PDR prevalence trends and model output.
(A) HIV Prevalence: population-level survey estimates are from the Kenya Demographic and Health Survey (2003 and 2009) and the Kenya AIDS Indicator Survey (2007 and 2012) (see Appendix for references). (B) PDR Prevalence: empirical estimates of PDR prevalence for 2005, 2008, and 2010 include NNRTI and NRTI mutations in ART-naïve individuals in East Africa [2]. 2016 estimate includes only NNRTI mutations in both ART-naïve individuals and those reporting prior exposure to ART who are initiating first-line ART [40]. Model estimate discontinuities in 2010, 2014, and 2016 result from ART coverage expansion and stochastic dynamics, explained in detail in Appendix Section 3C.
Table 2.
Characteristics of simulated population at t0.
| HIV prevalence (15–49 years) | 4·3% |
| PDR prevalence | 13·1% |
| Proportion of HIV-infected people on ART | 82·7% |
| Proportion of all HIV-infected people with viral suppression | 70·6% |
| Of people on ART, proportion now on second-line (PI-based) regimen | 10·9% |
3.1. Health outcomes
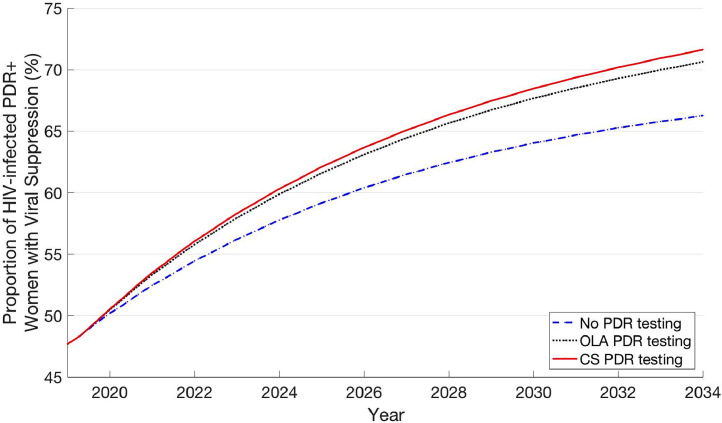
Both PDR testing strategies were associated with higher rates of viral suppression and fewer transmitted infections compared to no PDR testing (Fig. 2; Table 3). Among women on ART, viral suppression rates associated with no PDR testing, PDR testing with OLA, and PDR testing with CS were 87·8%, 89·0%, and 89·3%, respectively (12 months after treatment initiation). Among women with PDR on ART, these strategies were associated with viral suppression rates of 79·8%, 87·1%, and 88·7%, respectively (12 months after treatment initiation). HIV incidence rates for OLA PDR testing and CS PDR testing were 0·5% and 0·6% lower compared to no PDR testing, respectively (12·00 (no testing) vs. 11·94 (OLA) vs. 11·93 (CS) incident infections per 10,000 person-years).
Fig. 2.
Proportion of HIV-infected, PDR+ women with viral suppression over time.
Table 3.
Health and ART outcomes over 15 years from t0.
| No PDR testing | OLA PDR testing | CS PDR testing | |
|---|---|---|---|
| Health outcomes | |||
| Proportion with suppressed VL at month-12 on ARTa | |||
| Among men and women | 88·9% | 89·7% | 89·9% |
| Among women | 87·8% | 89·0% | 89·3% |
| Among women with PDR | 79·8% | 87·1% | 88·7% |
| Proportion with suppressed VL (irrespective of ART status)a | |||
| Among men and women | 76·9% | 77·1% | 77·1% |
| Among women | 76·8% | 77·1% | 77·1% |
| Among women with PDR | 59·7% | 62·3% | 62·8% |
| HIV mortality rateb | 426·8 | 426·5 | 426·5 |
| HIV mortality rate among women with PDRb | 427·2 | 423·1 | 422·4 |
| Incidence rateb | 12·00 | 11·94 | 11·93 |
| ART outcomes | |||
| Proportion on PI-based regimena | 9·3% | 10·2% | 10·4% |
| Person-months of ART use (million) | 212·74 | 212·66 | 212·65 |
| Person-months of PI-based ART use (million) | 21·0 | 23·0 | 23·5 |
Average over 15-year time period.
Per 10,000 person-years over 15-year period.
Improved viral suppression with PDR testing translated to reductions in mortality. Compared to no PDR testing, OLA PDR testing and CS PDR testing were associated with 1·0% and 1·1% reductions in mortality, respectively, among HIV-infected women with PDR (427·2 (no testing) vs. 423·1 (OLA) vs. 422·4 (CS) deaths per 10,000 person-years) and a 0·1% reduction in mortality rate among all HIV-infected patients (426·8 (no testing) vs. 426·5 (OLA) vs. 426·5 (CS) deaths per 100 person-years). Overall, the health benefits associated with PDR testing were marginally greater for CS than for OLA PDR testing. This pattern resulted from our assumption that CS has a higher diagnostic sensitivity than OLA (100% vs. 80%), which leads to more women with PDR receiving PI-based ART as their initial regimen with CS PDR testing than with OLA PDR testing.
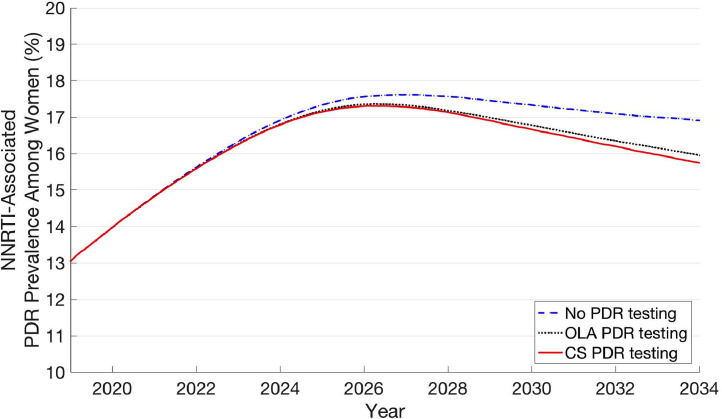
Finally, over time, PDR testing strategies were also associated with a lower PDR prevalence among women (Fig. 3). In 2019, PDR prevalence among women was 13·1%, which increased to a peak of 17·6% in 2026 with no testing and 17·4% and 17·3% in 2026 with OLA and CS PDR testing, respectively. After reaching these peak levels, PDR prevalence among women began to gradually decrease in all three strategies, and by the end of 2034, reached 16·9%, 16·0%, and 15·8% with no testing, OLA PDR testing, and CS PDR testing, respectively.
Fig. 3.
Prevalence of PDR among HIV-infected women over time.
3.2. Costs and cost-effectiveness
Total program costs for the entire Kenyan adult population over a 15-year time horizon were higher for PDR testing strategies compared to no PDR testing (Table 4; Appendix Section 4), with CS having the highest costs. OLA and CS PDR testing required an additional cost of $43 million and $108 million, respectively, compared to no PDR testing over 15 years. The majority of the additional total cost of the OLA PDR testing strategy, relative to no testing, was associated with increased ART costs (56%; $24 million) rather than the cost of drug resistance testing itself (43%; $19 million). The reverse was true for the CS PDR testing strategy as, relative to no testing, 27% ($30 million) of increased costs were due to increased ART costs and 73% ($79 million) were due to cost of drug resistance testing. Averaged over 15 years, the additional annual cost of drug resistance testing alone was $1·3 million for OLA and $5·2 million for CS.
Table 4.
Costs, QALYs, and incremental cost-effectiveness of PDR testing strategies.
| Undiscounted costs (US$) | Undiscounted QALYs | ||
|---|---|---|---|
| No PDR testing | 49,148,153,419 | 569,609,819 | |
| OLA PDR testing | 49,191,581,960 | 569,614,223 | |
| CS PDR testing | 49,256,326,325 | 569,614,723 | |
| Discounted costs (US$) | Discounted QALYs | ICER (US$ per QALY gained) | |
| No PDR testing | 39,129,404,402 | 451,689,577 | N/A |
| OLA PDR testing | 39,163,788,731 | 451,692,778 | 10,741 |
| CS PDR testing | 39,217,003,269 | 451,693,174 | 134,396 |
Costs are reported in 2019 US$. Discounted costs and QALYs were discounted 3% annually. N/A = not applicable. QALYs = quality-adjusted life years. ICER = incremental cost-effectiveness ratio.
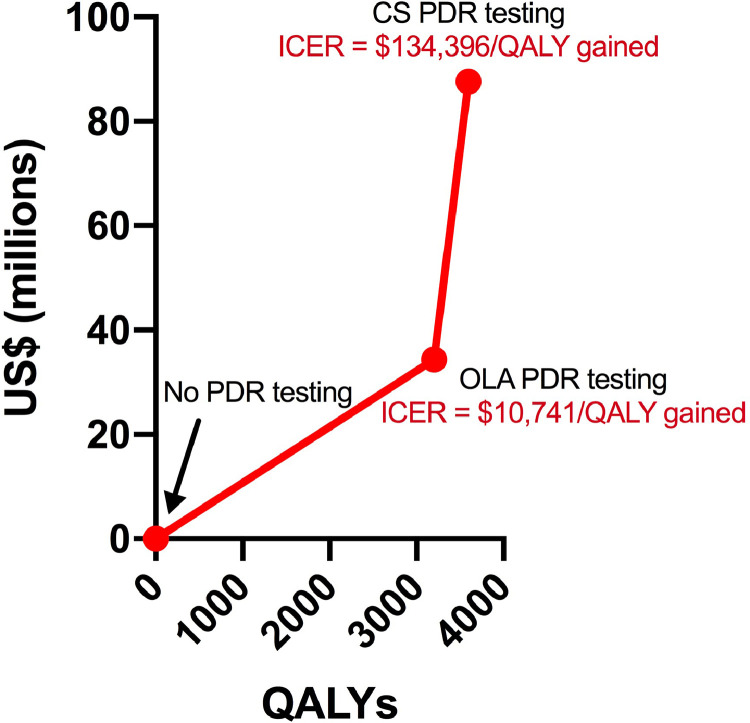
Although both PDR testing strategies were associated with fewer incident HIV infections and therefore incurred fewer total person-months of ART use (81,599 fewer and 96,336 fewer with OLA and CS, respectively) than no PDR testing, the PDR testing strategies also had a higher proportion of patients on ART using a PI-based regimen (10·4% vs. 10·2% vs. 9·3% with CS, OLA, and no PDR testing, respectively), which contributed to higher ART costs (Table 3). Compared to no PDR testing, OLA PDR testing resulted in 3,201 additional discounted QALYs gained over the entire adult population, resulting in an ICER of US$10,741/QALY gained. Compared to OLA PDR testing, CS PDR testing provided 396 additional discounted QALYs over the entire adult population, resulting in an ICER of US$134,396/QALY gained (Fig. 4).
Fig. 4.
Incremental costs and health benefits of PDR testing strategies compared to current policy.
Incremental costs and health benefits are scaled-up to the entire adult Kenyan population over a 15-year time horizon starting in 2019.
3.3. Sensitivity analyses
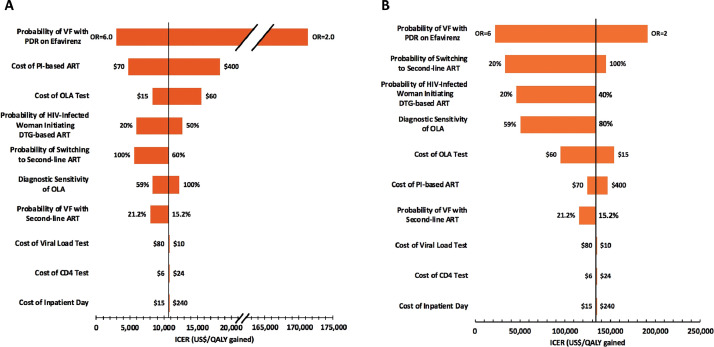
Throughout our sensitivity analyses, OLA PDR testing had lower ICERs compared to CS PDR testing (Fig. 5). The probability of virologic failure for patients on efavirenz-based first-line ART with PDR and the cost of PI-based ART were two key factors in determining the cost-effectiveness of OLA PDR testing. When we assumed the odds of virologic failure on efavirenz-based first-line ART were 6·0 times higher for patients with PDR compared to those without PDR (base-case odds ratio = 3·0), the OLA ICER was $2,968/QALY gained (72% smaller than the base-case). In contrast, when we assumed this odds ratio was 2·0, the OLA ICER was $171,416/QALY gained (1,496% greater than the base-case). When we assumed that PI-based ART cost the same as efavirenz-based ART ($70/person/year), OLA PDR testing had an ICER of $4,775/QALY gained (56% smaller than base-case) and cost only an additional $15 million (compared to an additional $43 million as in the base-case) over 15 years. After these two factors, variability in cost of OLA, probability of an HIV-infected woman initiating dolutegravir-based ART, probability of switching to second-line ART, diagnostic sensitivity of OLA, and probability of virologic failure with second-line ART, had the third through seventh most influential effects on the OLA ICER. Variability in cost of viral load testing, CD4 cell count testing, and inpatient stays were associated with the least variability in the OLA ICER.
Fig. 5.
One-way sensitivity analyses of key model parameters.
(A) OLA PDR testing. ICER values are for OLA PDR testing compared to no PDR testing; the vertical bar represents the OLA PDR testing ICER from the base-case ($10,741/QALY gained). (B) CS PDR Testing. ICER values are for CS PDR testing compared to OLA PDR testing; the vertical bar represents the CS PDR testing ICER from the base-case ($134,396/QALY gained). VF = virologic failure; OR = odds ratio.
Each horizontal bar represents the range of ICER values found for the specified range of values for the corresponding parameter. For each parameter, numbers to the right and left of the horizontal bar indicate the parameter value that corresponds to the high end and low end, respectively, of the ICER range that resulted from the one-way sensitivity analysis (range of values explored for each parameter value defined in Table 1). The ICERs corresponding to each of these parameter values can be found by finding the x-axis value that corresponds to the ends of each bar. In the one-way sensitivity analysis of “probability of VF with PDR on efavirenz”, the odds ratio refers to the ratio of the odds of virologic failure for those with PDR on efavirenz-based ART compared to those with either no PDR on efavirenz-based ART or those with PDR on PI-based ART (“PDR to no PDR odds ratio”). We varied the “probability of VF with PDR on efavirenz-based ART” while holding constant the “probability of VF for patients without PDR on efavirenz-based ART”. The probability of VF with PDR on efavirenz-based ART for cases with an OR equal to 2·0 and 6·0 were 23·9 and 48·6%, respectively.
4. Discussion
Our model predicts that PDR testing improves rates of viral suppression among women with PDR, reduces HIV transmissions, leads to more rapid decrease in PDR prevalence, and leads to increases in quality-adjusted life years in Kenya compared to ART strategies without PDR testing. Relative to the 2016–2017 Kenyan Ministry of Health budget for HIV treatment, care, and support (US$937 million) [28], the average additional annual cost of drug resistance testing by itself would represent 0·1% ($1·3 million) and 0·6% ($5·2 million) increases in annual spending using OLA and CS, respectively. While these spending increases are relatively small and while OLA was consistently more cost-effective than CS, our analysis suggests neither method of conducting PDR testing for women is likely to be cost-effective in Kenya (base-case ICER of $10,741/QALY and $134,396/QALY gained for OLA and CS, respectively).
Although the use of thresholds based on per capita gross domestic product (GDP) (<1× per capita GDP = very cost effective; <3× per capita GDP = cost-effective) has been increasingly criticized for not being based on opportunity costs [29,30], it is worth noting that neither OLA nor CS PDR testing would be considered cost-effective by GDP-based thresholds (Kenyan per capita GDP = US$1,710) [31]. The development of cost-effectiveness thresholds based on opportunity costs is an ongoing area of research [30,32]. Although there is no such established threshold for Kenya, some health economists suggest $500 per disability-adjusted life year averted is likely at the upper end of this threshold for most sub-Saharan African countries [12]. If one uses a threshold of $500/QALY gained, OLA PDR testing would be cost-effective if PI-based ART cost the same as efavirenz-based ART ($70/person/year) and OLA cost $3.65/test (scenario ICER = $494/QALY gained). However, these are highly reduced prices that are unlikely in the foreseeable future, especially for PI-based ART. Furthermore, this scenario assumes the probability of virologic failure with PDR on efavirenz-based ART is 32% (odds ratio = 3·0). Recent studies suggest that the risk of virologic failure associated with NNRTI-associated PDR may be lower with the tenofovir/emtricitabine/efavirenz combination compared to other NNRTI-based regimens used in prior studies [33]. When we assumed an odds ratio of 2·0 (Fig. 5), the OLA ICER was $171,416/QALY gained. Overall, these sensitivity analyses highlight that PDR testing for women not initiating dolutegravir-based ART is unlikely to be cost-effective in Kenya.
Interestingly, the cost of increased use of PI-based ART was a stronger driver of the cost-effectiveness of OLA PDR testing than the cost of OLA testing itself or its diagnostic sensitivity. Thus, while other low-cost drug resistance assays may differ from OLA in terms of their cost and diagnostic sensitivity, their cost-effectiveness, when used for PDR testing for women initiating dolutegravir-based ART, are likely to be similar to our findings in our analysis of OLA.
PDR testing was associated with larger increases in viral suppression rates, compared to no testing, among women with PDR than among all HIV-infected patients as a whole (Table 3), as this intervention had the potential to improve viral suppression only for women not taking dolutegravir. As a result, reductions in incidence (0·5% reduction with OLA and 0·6% reduction with CS) and overall HIV mortality rate (0·1% with OLA and CS) were relatively small with PDR testing. Reductions in mortality rate among HIV-infected women with PDR were also relatively small (1·0% reduction with OLA and 1·1% reduction with CS) for two reasons. First, not everyone with PDR on efavirenz-based ART experiences virologic failure. Second, for some women with PDR who experience virologic failure on efavirenz-based ART, viral load testing provides an opportunity to switch to PI-based ART if they did not undergo PDR testing, thereby reducing their risk of death.
PDR testing strategies resulted in a lower PDR prevalence compared to no testing due to improved viral suppression among HIV-infected women, which made them less likely to transmit drug resistant HIV to others. Even without PDR testing, after an initial increase from 2019 to 2026, there was a gradual decrease in PDR prevalence among women. The primary reason for this is, as use of efavirenz decreases in the population, there is less emergence of acquired drug resistance to efavirenz, and thus, less resistance to transmit to others. However, the absolute decrease in PDR prevalence among women from 2026 to 2034 was only 0·7% (17·6% to 16·9%), reaching higher than current levels 15 years from now. Thus, it remains important to identify effective and affordable strategies to address PDR among women who do not initiate dolutegravir-based ART and to continue PDR prevalence surveillance efforts.
Modeling studies conducted prior to the availability of generic dolutegravir in resource-limited settings [13,14], or prior to concerns about increased risk neural tube birth defects associated with use of dolutegravir [12], concluded that PDR testing was not cost-effective compared to alternative strategies. Since then, two modeling studies have concluded that, for women of reproductive potential in sub-Saharan Africa, the benefits of dolutegravir-based ART outweigh its risks [34,35]. However, to our knowledge, this is the first study to evaluate the potential cost-effectiveness of PDR testing for women who do not initiate empiric dolutegravir-based ART due to safety concerns. Our results are consistent with prior studies and provide further evidence in support of WHO's recent updated guidelines strongly recommending dolutegravir-based ART as the preferred empiric first-line ART regimen for people living with HIV, including women [9].
Our analysis has some limitations. First, waiting for PDR test results could potentially prevent clinicians from offering rapid ART initiation, as recommended by current guidelines [36]. Because our model uses a monthly time cycle and we assume drug resistance test results would be available within one month [19,22], our analysis does not account for potential delays in ART initiation with PDR testing, which could decrease the cost-effectiveness of the intervention through decreased patient retention. However, this would not alter the implications of our analysis, as we found PDR testing for women is already unlikely to be cost-effective without decreased patient retention. Second, we do not account for potential reductions in mother to child transmission that may result from improved rates of viral suppression with PDR testing. Next, we limited our time horizon to 15 years because the landscape of ART programs in resource-limited settings has changed relatively quickly over the last two decades. However, a longer time horizon could potentially alter the cost-effectiveness of PDR testing. Last, we examine the role of PDR testing the first time a patient initiates ART, but we did not simulate PDR testing for patients who had discontinued ART and later re-initiated ART. In practice, it is not always possible to distinguish patients initiating ART for the first time from those who are ART experienced and re-initiating ART. This limitation may underestimate the cost-effectiveness of PDR testing.
In conclusion, we found that while PDR testing for HIV-infected women could improve health outcomes, it is unlikely to be cost-effective in Kenya, even with negotiations to lower the price of ART or drug resistance assays. There may be other roles for low-cost drug resistance assays in resource-limited settings, such as resistance testing in patients with unsuppressed viral load on dolutegravir-based first-line ART to determine whether a regimen switch is needed [37]. Further research is needed to evaluate the cost-effectiveness of this potential application.
5. Contributors
All authors contributed to the conceptualization of the analysis, provided advice on relevant data and assumptions, interpreted results, and commented on manuscript drafts. HAD, DCS, and EB wrote the modeling program. HAD executed the modeling program.
Declaration of Competing Interest
JBB and LGP report personal fees from Roche Molecular Systems outside the submitted work. EE reports personal fees from ViiV Healthcare outside the submitted work. RWS has received grants from Janssen Pharmaceuticals, Vela Diagnostics, and InSilixa, outside the submitted work. All other authors declare no competing interests.
Acknowledgments
We would like to thank the Seattle Children's Research Institute Research Scientific Computing Group, especially Paul Hodor and Bronson Petterson, for their computational support and expertise. This work was supported by the National Institutes of Health through the following grants: R01 AI100037 (LMF, JBB, IAB, MHC), R24AI136618 (RWS), K25AI118476 (EAE), R01 AI127250 (EB), R01 DA015612 (EB), K12 HD000850 (HAD), and T32 AI07140 (HAD). This work was also supported by a Gilead HIV Research Scholars Program Award (HAD).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100355.
Appendix. Supplementary materials
References
- 1.UNAIDS. UNAIDS global AIDS Update. 2016. http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016 (accessed 1 September 2018).
- 2.Gupta R.K., Jordan M.R., Sultan B.J. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet. 2012;380(9849):1250–1258. doi: 10.1016/S0140-6736(12)61038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung M.H., Beck I.A., Dross S. Oligonucleotide ligation assay detects HIV drug resistance associated with virologic failure among antiretroviral-naive adults in Kenya. J Acquir Immune Defic Syndr. 2014;67(3):246–253. doi: 10.1097/QAI.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamers R.L., Schuurman R., Sigaloff K.C. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multicentre cohort study. Lancet Infect Dis. 2012;12(4):307–317. doi: 10.1016/S1473-3099(11)70255-9. [DOI] [PubMed] [Google Scholar]
- 5.Zash R., Makhema J., Shapiro R.L. Neural-tube defects with dolutegravir treatment from the time of conception. N Engl J Med. 2018;379(10):979–981. doi: 10.1056/NEJMc1807653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . World Health Organization; Geneva: 2018. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. 2018. [Google Scholar]
- 7.Kenya Ministry of Health . NASCOP; Nairobi, Kenya: 2018. Guidelines on use of antiretroviral drugs for treating and preventing HIV infection in Kenya. 2018 Edition. [Google Scholar]
- 8.Zash R., Holmes L., Diseko M. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med. 2019;381(9):827–840. doi: 10.1056/NEJMoa1905230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO . World Health Organization; Geneva: 2019. Update of recommendations on first- and second-line antiretroviral regimens. 2019. [Google Scholar]
- 10.Lessells R.J., Avalos A., de Oliveira T. Implementing HIV-1 genotypic resistance testing in antiretroviral therapy programs in Africa: needs, opportunities, and challenges. AIDS Rev. 2013;15(4):221–229. [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee S.Y., Jordan M.R., Raizes E. HIV-1 drug resistance mutations: potential applications for point-of-care genotypic resistance testing. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0145772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips A.N., Cambiano V., Nakagawa F. Cost-effectiveness of public-health policy options in the presence of pretreatment NNRTI drug resistance in sub-Saharan Africa: a modelling study. Lancet HIV. 2018;5(3):e146–ee54. doi: 10.1016/S2352-3018(17)30190-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips A.N., Cambiano V., Miners A. Effectiveness and cost-effectiveness of potential responses to future high levels of transmitted HIV drug resistance in antiretroviral drug-naive populations beginning treatment: modelling study and economic analysis. Lancet HIV. 2014;1(2):e85–e93. doi: 10.1016/S2352-3018(14)70021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols B.E., Sigaloff K.C., Kityo C. Increasing the use of second-line therapy is a cost-effective approach to prevent the spread of drug-resistant HIV: a mathematical modelling study. J Int AIDS Soc. 2014;17:19164. doi: 10.7448/IAS.17.1.19164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inzaule S.C., Ondoa P., Peter T. Affordable HIV drug-resistance testing for monitoring of antiretroviral therapy in sub-Saharan Africa. Lancet Infect Dis. 2016;16(11):e267–ee75. doi: 10.1016/S1473-3099(16)30118-9. [DOI] [PubMed] [Google Scholar]
- 16.Duarte H.A., Panpradist N., Beck I.A. Current status of point-of-care testing for human immunodeficiency virus drug resistance. J Infect Dis. 2017;216(Suppl_9):S824–S8S8. doi: 10.1093/infdis/jix413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck I.A., Crowell C., Kittoe R. Optimization of the oligonucleotide ligation assay, a rapid and inexpensive test for detection of HIV-1 drug resistance mutations, for non-North American variants. J Acquir Immune Defic Syndr. 2008;48(4):418–427. doi: 10.1097/QAI.0b013e31817ed7d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung M.H., McGrath C.J., Beck I.A. Evaluation of the management of pretreatment HIV drug resistance by oligonucleotide ligation assay: a randomised controlled trial. Lancet HIV. 2020;7(2):e104–e112. doi: 10.1016/S2352-3018(19)30337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duarte H.A., Beck I.A., Levine M. Implementation of a point mutation assay for HIV drug resistance testing in Kenya. AIDS. 2018;32(16):2301–2308. doi: 10.1097/QAD.0000000000001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panpradist N., Beck I.A., Chung M.H., Kiarie J.N., Frenkel L.M., Lutz B.R. Simplified paper format for detecting HIV drug resistance in clinical specimens by oligonucleotide ligation. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0145962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bendavid E., Brandeau M.L., Wood R., Owens D.K. Comparative effectiveness of HIV testing and treatment in highly endemic regions. Arch Intern Med. 2010;170(15):1347–1354. doi: 10.1001/archinternmed.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lessells R.J., Stott K.E., Manasa J. Implementing antiretroviral resistance testing in a primary health care HIV treatment programme in rural KwaZulu-Natal, South Africa: early experiences, achievements and challenges. BMC Health Serv Res. 2014;14:116. doi: 10.1186/1472-6963-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Global Fund Pooled Procurement Price List June 2019. https://www.theglobalfund.org/media/5813/ppm_arvreferencepricing_table_en.pdf.
- 24.Kenya Ministry of Health . Kenya National Bureau of Statistics; Nairobi, Kenya: 2014. Kenya demographic and health survey. 2015. [Google Scholar]
- 25.Ledergerber B., Lundgren J.D., Walker A.S. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364(9428):51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 26.Sanders G.D., Neumann P.J., Basu A. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 27.Husereau D., Drummond M., Petrou S. Consolidated health economic evaluation reporting standards (CHEERS) statement. Int J Technol Assess Health Care. 2013;29(2):117–122. doi: 10.1017/S0266462313000160. [DOI] [PubMed] [Google Scholar]
- 28.Kenya Ministry of Health. Kenya AIDS response progress report. 2018 [Google Scholar]
- 29.WHO Commission on Macroeconomics and Health & World Health Organization . World Health Organization; Geneva: 2001. Macroeconomics and health: investing in health for economic development-executive summary. [Google Scholar]
- 30.Woods B., Revill P., Sculpher M., Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–935. doi: 10.1016/j.jval.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Bank. World Bank Open Data. 2019 https://data.worldbank.org/ (accessed 24 September 2019) [Google Scholar]
- 32.Meyer-Rath G., van Rensburg C., Larson B., Jamieson L., Rosen S. Revealed willingness-to-pay versus standard cost-effectiveness thresholds: evidence from the South African HIV investment case. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0186496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shafer R.W., Frenkel L.M. The clinical implications of pretreatment drug resistance—a moving target. Clin Infect Dis. 2018;69(2):215–217. doi: 10.1093/cid/ciy895. [DOI] [PubMed] [Google Scholar]
- 34.Phillips A.N., Venter F., Havlir D. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. Lancet HIV. 2019;6(2):e116–ee27. doi: 10.1016/S2352-3018(18)30317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dugdale C.M., Ciaranello A.L., Bekker L.G. Risks and benefits of dolutegravir- and efavirenz-based strategies for South African women with HIV of child-bearing potential: a modeling study. Ann Intern Med. 2019;170(9):614–625. doi: 10.7326/M18-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO . World Health Organization; Geneva: 2017. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. [PubMed] [Google Scholar]
- 37.Dorward J., Lessells R., Drain P.K. Dolutegravir for first-line antiretroviral therapy in low-income and middle-income countries: uncertainties and opportunities for implementation and research. Lancet HIV. 2018;5(7):e400–e404. doi: 10.1016/S2352-3018(18)30093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Institute for Health Metrics and Evaluation (IHME) IHME; Seattle, WA: 2014. Health service provision in Kenya: assessing facility capacity, costs of care, and patient perspectives. [Google Scholar]
- 39.Salomon J.A., Vos T., Hogan D.R. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta R.K., Gregson J., Parkin N. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis. 2017;18(3):346–355. doi: 10.1016/S1473-3099(17)30702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.