Abstract
Background
Early and accurate diagnosis of malaria is critical to the success of malaria elimination. However, the current mainstay of malaria diagnosis in the field, such as light microscopy and rapid diagnostic tests (RDTs), have limitations due to low parasite density or mutation in diagnostic markers.
Methods
We evaluated an inexpensive, robust, rapid, malaria diagnostic device, called Gazelle, that employs magneto-optical detection to identify haemozoin crystals (Hz) produced by all species of human malaria parasites in infected individuals. A beam of polarised light is passed through the lysed diluted blood sample under the influence of high (~.55T) and low magnetic fields. The difference in light transmission through the sample between the high and low magnetic fields indicates presence of Hz, suggesting possible malarial infection. A total of 300 febrile patients were screened at the malaria clinic of Indian Council of Medical Research-National Institute of Research in Tribal Health (ICMR-NIRTH), Jabalpur, India, from August 2018 to November 2018. Malaria diagnosis was done using four diagnostic methods: Gazelle, light microscopy, RDT, and malaria specific Polymerase Chain Reaction (PCR). Measures of diagnostic accuracy were compared.
Findings
Out of 300 febrile patients enroled and tested for the presence of malaria parasites, 262 patient samples were included in the final analysis. The sensitivity and specificity of Gazelle was 98% and 97% in comparison to light microscopy, 82% and 99% to PCR and 78% and 99% to RDT, respectively. The results of the four diagnostic methods were comparable and statistically no significant differences in sensitivity or specificity was observed between these methods. Enhanced diagnostic accuracy of Gazelle in malaria patients with no prior history of malaria treatment was observed in this study.
Interpretation
The diagnostic ability of Gazelle was comparable to light microscopy and better than RDTs even in low parasitemia and in presence of pfhrp2/3 deletion mutant parasites. Gazelle may be a novel valuable diagnostic tool in resource poor settings where (i) microscopy is not feasible and (ii) pfhrp2/3gene deleted parasite are present. Its speed, cost-efficiency, and alternative to lack of microscopists makes it an important adjunct in field settings.
Funding
HemexDx, India.
Keywords: P. falciparum, Malaria diagnosis, Rapid diagnostic test, Haemozoin, Gazelle
Research in context.
Evidence before this studyMicroscopy and rapid diagnostic test are widely employed diagnostic tools for malaria in field and resource poor healthcare settings. However, the diagnosis of malaria by microscopy is often not feasible because of lack of infrastructure, unavailability of skilled microscopists and erratic power supply. Further, the performance of RDT is affected by low density parasitemia and Pfhrp-2 gene deletion. We searched PubMed for alternative diagnostic tools which can be used in place or in combination with existing tools for malaria diagnosis in resource poor settings. Even though a number of alternative diagnostic tools are found, yet none were found suitable for field conditions/ CHC/PHC or community surveys.
Added value of this studyIn the present study, we conducted a successful field evaluation of magneto-optical device (Gazelle) for diagnosis of malaria. The performance (sensitivity, specificity and accuracy) of the device Gazelle was similar to microscopy and RDT. Furthermore, it is a quick, battery operated, physically rugged device with digital interface which can be operated with little training in poor resource settings with erratic power supply.
Implications of all the available evidenceThe device Gazelle may be an important alternative diagnostic tool in areas where Pfhrp-2 deletion is prevalent. Further, it may be an important diagnostic tool in mass surveys and poor resource settings in the field. The device may be a useful remedy for lack of skilled microscopists which compromise malaria diagnosis across the globe.
Alt-text: Unlabelled box
1. Introduction
Malaria is a leading cause of death due to parasitic infections worldwide. It is caused by five different species in the Genus Plasmodium (viz. P. falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi). More than 40% of the world's population and nearly 90% of India's population is at risk of malarial infection [1]. About 219 million people were infected globally in 2017 [2]. India contributed to about 85% of all malaria cases in the World Health Organisation's South-East Asia Region in 2017; the majority of these cases were reported from tribal dominated rural areas of the country [2,3]. Even though, Malaria is treatable and curable, 435,000 people died from the disease globally in 2017 [2]. Accurate and timely diagnosis is critically important in the management of malaria.
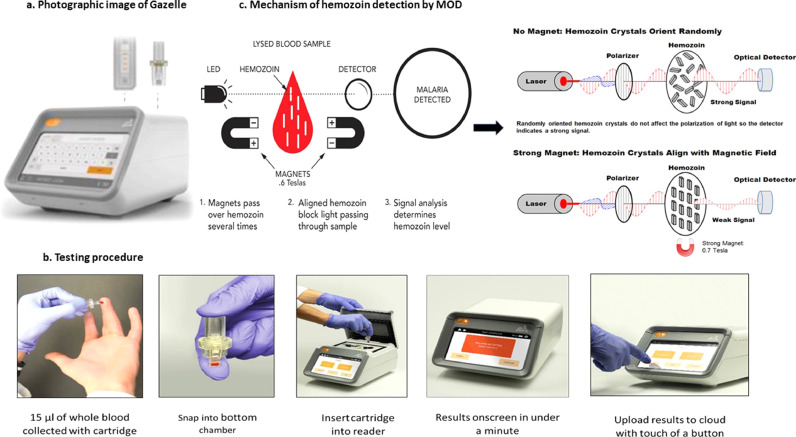
Currently malaria is primarily diagnosed by microscopy and/or rapid diagnostic tests (RDTs) both at primary health centres and community health centres in India. Though microscopy is considered the gold standard for malaria diagnosis, its use depends on the availability of a well-functioning light microscope, clean glass slides, immersion oil with appropriate optical properties, fresh filtered reagents for staining, and most importantly, a skilled microscopist. Further, microscopy is time consuming, labour intensive, and its accuracy is affected by low parasitemia [4]. Due to ease of handling, RDTs are increasingly being used to diagnose malaria in resource-poor settings and field conditions. A total of 276 million RDTs for malaria were sold worldwide in 2017 [2]. RDTs provide qualitative results for malaria and are limited in the number of malaria species that they can diagnose [5]. RDTs also are less effective in cases of low parasitemia and infection with P. falciparum lacking histidine rich protein-2 (Pfhrp2) in malaria endemic regions [6,7]. Loop mediated isothermal amplification (LAMP) is a relatively new technique which employs the rapid amplification and detection of DNA [8]. This method has been successfully demonstrated to detect malaria with promising results in comparison to microscopy, RDT and Polymerase chain reaction (PCR) [9], even though it is not suitable to field conditions. PCR is a highly sensitive and specific technique that can detect low-level parasitemia, but it is relatively expensive and requires advanced laboratory equipment and technologically skilled execution, making it unsuitable for resource-poor settings [10]. Therefore, the currently available repertoire of diagnostic tools are insufficient and inefficient in dealing with diagnostic challenges faced in resource-poor settings and there is an urgent need for alternative malaria diagnostics as India intensifies its efforts toward malaria elimination [7]. In order to address the further demand of point-of-care diagnostic tool for malaria in resource-poor settings, a device, named Gazelle, was designed. Gazelle is a new point-of-care malaria diagnostic device that detects Hz particles in blood and diagnoses malaria in less than a minute (Fig. 1a and b). Previous efforts to use Hz as a biomarker for malaria were largely unsuccessful [11,12] due to lack of adequate sensitivity and specificity, while others involved the use of prohibitively costly machinery [11,13,14]. This study reports the first successful field evaluation of a low-cost, haemozoin-based, battery operated malaria diagnostic device and its comparison with microscopy, RDTs, and PCR.
Fig. 1.
Showing the Gazelle device (1a), testing procedure (1b) and mechanism of haemozoin detection by magneto optical detector (1c).
2. Methods
2.1. Study site
The study was conducted at the malaria clinic of Indian Council of Medical Research-National Institute of Research in Tribal Health (ICMR-NIRTH) located in Late Baliram Kashayap Memorial Medical College, Jagdalpur, Chhattisgarh, India between August 2018 and November 2018. Chhattisgarh has the third highest rate of malaria infection in India; it contributed to about 12% of all malaria cases in India in the year 2017. Four major species (P. vivax, P. falciparum, P. ovale and P. malariae)) of malaria parasites are known to be present here [16,17]. Jagdalpur is a highly malarious, densely forested tribal-dominated region of Bastar district; more than 70% of the inhabitants are ethnic tribes (total population 14,13, 199 as per 2011 Census) [18]. The Late Baliram Kashayap Memorial Medical College in Jagdalpur serves as a referral health facility for six districts adjacent to Jagdalpur: Dantewada, Bijapur, Sukma, Narayanpur, Kondagaon and Kanker. About 50% of Jagdalpur and adjoining areas are made up of forests. Malaria cases are reported throughout the year with predominance of P. falciparum.
2.2. Study population
All febrile patients visiting the malaria clinic at the Medical College were screened for malaria. Written informed consent was obtained from participants before sample collection.
2.3. Sample size
No prior studies are available for reporting sensitivity, specificity of Gazelle for malaria diagnosis. Therefore, the assumed probability of sensitivity was considered 50% with 12% relative precision at 95% confidence limit and 80% power for sample size estimation. This accumulates the minimum required sample as 267. Further 10% samples were added to cover the possible sample losses for any unavoidable reasons. Finally, a total of 300 samples were planned in the study. Further, 5 additional samples were lost from our expected numbers and we were able to analyse the remaining 262 samples in the study.
2.4. Sampling
About one mL of venous blood was collected in sterile vacutainer tube from suspected malaria patients. Thin and thick blood smears were prepared for microscopic examination and the bivalent Malaria RDT Ag Pf/Pv (Catalogue no.- 05FK80-40-0) from SD BIOLINE was used to diagnose malaria infection. This malaria RDT targets the detection of histidine-rich protein II (HRP-II) antigen of P. falciparum and lactate dehydrogenase (pLDH) of P. vivax species in human whole blood. About 15 µL of collected blood sample was used for testing in the Gazelle and rest of the sample was stored for molecular diagnosis. The blood sample was added to 80 µL of 2% Triton buffer, sonicated to lyse all blood cells, and run on the device.
2.5. Magneto-optical detection of malaria parasite
Gazelle device: Gazelle is an in vitro diagnostic device that uses haemozoin as a biomarker for malaria diagnosis. Haemozoin is produced as a byproduct of haemoglobin digestion by all species and strains of malaria parasite circulating in the blood of humans. Gazelle takes advantage of the natural paramagnetic properties of Hz by using magneto-optical technology to detect Hz present in a small blood sample. Gazelle consists of a reader (small, table-top) and single-use disposable cartridges. The reader can be used in challenging tropical environments that may be warm (operating temperature range 5 °C–45 °C) or high in humidity (operating relative humidity range 5%–95%) and operates on either electric or lithium battery power. The reader can be charged with a standard Micro-USB charger, such as those used for Android phones. The reader has internal storage to keep a record of the tests. The Gazelle malaria cartridge has two chambers i.e., upper and lower chambers. A pipette is used to drop 15 µL of whole blood into the lower chamber, along with 80 µL of Gazelle buffer and upper chamber was placed on to it. Now, the sample containing cartridge was sonicated using an external sonicator to lyse the blood and release the haemozoin. Then this cartridge is inserted into the cartridge slot in the reader. When the test is started in the reader, magnets are passed over the sample multiple times. As haemozoin is composed partly of iron, the applied magnetic field aligns the haemozoin; this alignment inhibits the transmission of light through the test solution in the cartridge. An internal LED shines light through the sample and the amount of transmitted light is measured both in presence and absence of the magnetic field. With no magnetic field, the haemozoin crystals assume a random orientation because of the Brownian motion. When the magnetic field is present, the haemozoin crystals are aligned and block the transmission of light and are proportional to the quantity of haemozoin present in the sample. Any haemozoin detected is indicative of malarial infection. The presence or absence of malaria is displayed on the reader within one minute. This test can detect all five species of malaria parasite as they all produce haemozoin. Malaria test results are stored in the reader's memory. From the reader, users can print and save test results to a Wi-Fi or Bluetooth enabled device such as a laptop or printer. The device is calibrated with synthetic Hz (as positive control) and colouring dye (as negative control) once every week or whenever the cartridge of different lot numbers are used to ensure the sensitivity and proper functioning of the device. Current version of Gazelle cannot differentiate between different Plasmodium species.
Mechanism of detection of malaria parasite: When a beam of polarised light is passed through the lysed blood sample in the presence of the high magnetic field (~.55T), the Hz crystals align to the applied field (Fig. 1c) in a manner that increases the opacity of the solution. The opacity is measured with high and with a very low/no magnetic field using a LED detector to determine the Hz concentration. The decrease in light with the high magnetic field is directly proportional to the amount of Hz present. If there is no change with variation in the magnetic field, it would indicate absence of Hz in the sample or the concentration of Hz is below the detection limit of Gazelle. An algorithm uses the optical information and Hz measurement to determine the presence or absence of malaria.
Limit of detection (LoD) of Gazelle: This is defined as the lowest concentration of parasites/µL that is detectable at a 95% or greater accuracy level by Gazelle malaria test. The limit of detection for P. falciparum from cultured parasites was 50 parasites/µL with 95% accuracy (n = 20). The limit of detection for P. vivax from patient samples was 35 parasites/µL with 100% accuracy (n = 20). The established LoD concentration was tested at least 20 times to confirm the accuracy of 95% or greater. The results were confirmed by a WHO certified class-I microscopist.
2.6. Comparative diagnostic methods
The performance of the Gazelle was compared with three current diagnostic methods: microscopy, RDTs and PCR.
Microscopy of blood smear slides was performed as per WHO standards. Thin and thick smears of blood were prepared on pre-cleaned glass slides, dried, fixed, and stained for microscopy. Light microscopy was used to identify and quantify malaria parasites in blood smear slides. The number of malaria parasites per 200 white blood cells (WBCs) were quantified on Jaswant Singh Bhattacharya (JSB) stained thick films [19]. Parasite density, expressed as the number of asexual parasites per μL of blood, was calculated by dividing the number of asexual parasites by the number of WBCs counted, and then multiplying it by an assumed WBC density of 6000 per μL [20]. In cases, where there were fewer than 100 asexual parasites per 200 WBCs in smears, quantification was performed against at least 500 WBCs. Each slide was examined by one microscopist at the study site and one WHO certified level-2 microscopist at ICMR-NIRTH, Jabalpur and their concurrence was crucial for the diagnostic outcome. In case of difference of opinion, the slide was examined by third microscopist (Level-1) and the majoritarian decision prevailed. A blood slide was considered negative when examination of 1000 WBCs or 100 fields containing at least ten WBCs per field showed no asexual parasites [21].
The Plasmodium falciparum histidine-rich protein-2 (PfHRP-2) and common Plasmodium lactate dehydrogenase (PLDH) based bivalent RDTs were used to detect the malaria parasite in whole human blood as per the manufacturer's protocol (Catalogue no- 05FK80-40-0, SD Bio Standard Diagnostics Pvt. Ltd., Gurugram, Haryana). About 5 µL of blood was transferred with sterile capillary pipette to a round specimen well and four drops of diluents was added into the assay diluent well (square). Test results were recorded after 20 min.
The molecular diagnosis of malaria was done by nested PCR following the protocol of Snounou et al. [22]. DNA was isolated from 200 µL of blood sample using QIAamp DNA Blood Mini Kit (Qiagen, Germany) and eluted in 100 µL of elution buffer provided with the kit. The purified DNA (~5 µL) was used as template to detect malaria parasites using genus/species specific primers targeting 18S rRNA gene. The PCR amplified products were resolved in an agarose gel and visualised under UV transilluminator [17]. At the study site, three staff (One technician, one microscopist, one research assistant) were posted to execute the study. Microscopist was responsible for blood smear staining, examination and parasite counting. The technician was responsible for the RDT performance and collection of blood samples for Gazelle as well as PCR analysis. The research assistant mainly handled the device and was also responsible for consent of the patient, filling of case report form and monitoring of all the activities at study site. Microscopist was fully blinded to RDT and Gazelle's outcome. PCR was performed at ICMR-NIRTH laboratory by trained Research assistant.
2.7. Statistical analysis
The results were analysed for sensitivity, specificity, positive and negative likelihood ratios, odds ratio, and positive and negative predictive values (PPV, NPV). The sensitivity, specificity, NPV and PPV were calculated as (a) Sensitivity: True positive / total positive * 100 (b) Specificity: True negative / total negative * 100 (c) Positive Predictive Value: True positive / (true positive + false positive) * 100 (d) Negative Predictive Value: True negative / (true negative + false negative) * 100. The total number of samples are those samples on which all four diagnostic tests were successfully performed. The total number of samples (n) is 262 for all malaria cases. 235 patients had no prior history of malaria treatment. Receiver operating characteristic (ROC) analysis and comparison of area under curve (AUC) was carried out to compare the diagnostic performance of RDT, PCR and microscopy with Gazelle. Measures of diagnostic accuracy were also assessed in a subgroup of patients with no history of malaria treatment.
2.8. Ethics
The study was conducted in accordance with the guiding principles of the Declaration of Helsinki and was approved by the Institutional Ethics Committee of ICMR- National Institute of Research in Tribal Health, Jabalpur, India (IEC ref no. 201,702) and Health Ministry's Screening Committee (HMSC) ICMR, India. Written informed consent was obtained from all patients before sample collection. Data validation and quality control were followed according to the guidelines for good clinical practice. Data was collected at the malaria clinic of ICMR-NIRTH at Jagdalpur and stored in a dedicated computer database of ICMR-NIRTH, Jabalpur.
2.9. Role of the funding source
The funding for the study was provided by HemexDx, India; a subsidiary of Hemex Health, USA. The funding source has no role in design, execution, analyses, interpretation of the data, or decision to submit results as manuscript.
3. Results
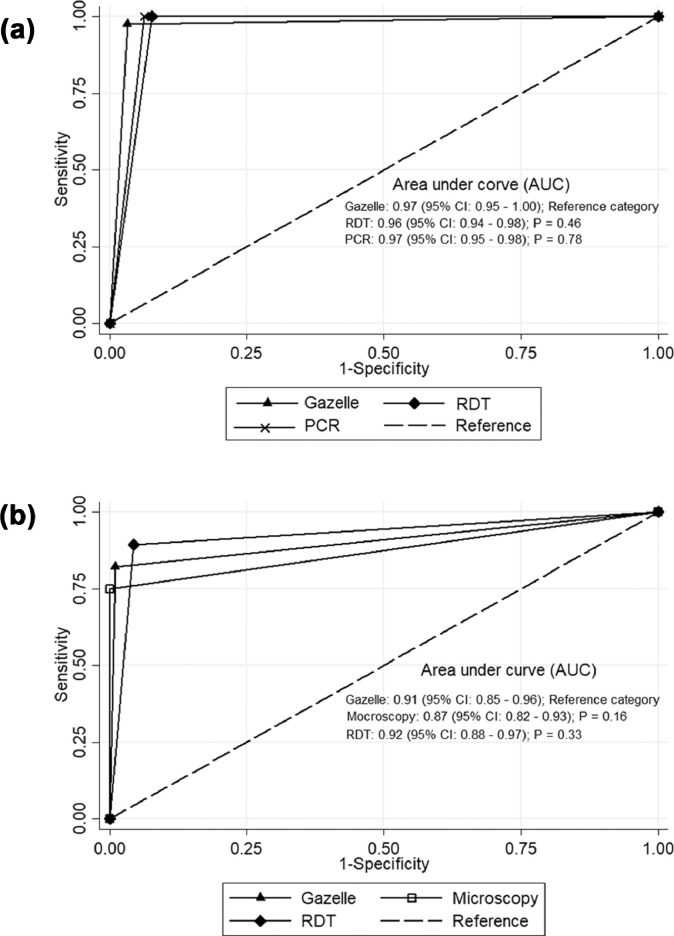
A total of 300 febrile patients were enroled and screened for malaria in the study. Mean patient age was 27.6 ± 14.0 years; 83% were of 14 years or older; 50% were male. Of the 300 samples from enroled patients, the malaria positivity rate by microscopy was 15.3% (46/300), by RDT 22% (66/300) by PCR 21% (63/300) and by Gazelle 18.3% (48/262). A total of 38 samples were excluded from the final analysis because of lysis and inadequate blood sample to perform all the four tests. Of the 262 samples, 42, 59, and 56 samples were identified as malaria positive using microscopy, RDT, and PCR, respectively. The mean parasite density (parasites/µL of blood) of microscopy positive person was 8687 ± 8803 (range 419–33,861). Further, species wise analysis of the data revealed that all microscopically positive P. vivax cases were also detected by Gazzelle. However, a single case of microscopically positive P. falciparum was not detected by the device, despite the parasite density of 500 parasites/µL Measures of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of Gazelle were 97.6, 96.8, 85.4, 99.5 and 96.9 respectively in comparison to microscopy considered as gold standard (Table 1) and 82.1, 99.0, 95.8, 95.3 and 95.4 when compared with PCR as gold standard (Table 1). When RDT was adopted as gold standard, sensitivity, specificity, positive predictive value (PPV), negative predictive values were 77.9, 99.0, 95.8, 93.9 and 94.2 respectively (Table 1). Further, Receiver Operating Characteristics curve analysis revealed that area under curve (AUC) for Gazelle, RDT and PCR against microscopy was 0.97, 0.96 and 0.97, and against PCR as gold standard, it was 0.91, 0.87 and 0.92 for Gazelle, microscopy and RDT, respectively (Fig. 2a and b). The above findings indicate that Gazelle detects positive and negative malaria cases with 97% and 91% accuracy in comparison to microcopy and PCR, respectively. However, the AUC of Gazelle did not differ significantly (p > 0.05) from that of RDT, PCR and microscopy.
Table 1.
Comparison of diagnostic test statistics of Gazelle, RDT and PCR in all patients (n = 262).
| Test diagnostics | Microscopy (Gold standard) |
PCR (Gold standard) |
RDT (Gold standard) |
||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | ||
| A: Gazelle | |||||||
| Positive | 41 | 7 | 46 | 2 | 46 | 2 | |
| Negative | 1 | 213 | 10 | 204 | 13 | 201 | |
| Sensitivity (95% CI) | 97.6 (87.4 - 99.9) | 82.1 (69.6 – 91.1) | 77.9 (65.2–87.7) | ||||
| Specificity (95% CI) | 96.8 (93.6 – 98.7) | 99.0 (96.5 – 99.9) | 99.0 (96.4–99.8) | ||||
| Positive predictive value (95% CI) | 85.4 (72.2 – 93.9) | 95.8 (85.7 – 99.5) | 95.8 (85.1–98.9) | ||||
| Negative predictive value (95% CI) | 99.5 (97.4 – 100) | 95.3 (91.6 – 97.7) | 93.9 (90.5–96.1) | ||||
| Accuracy (95% CI) | 96.9 (94.1 – 98.7) | 95.4 (92.1 – 97.6) | 94.2 (90.7–96.7) | ||||
| B: RDT | |||||||
| Positive | 42 | 17 | 50 | 9 | |||
| Negative | 0 | 203 | 6 | 197 | |||
| Sensitivity (95% CI) | 100 (91.6 – 100) | 89.3 (78.1 – 96.0) | |||||
| Specificity (95% CI) | 92.3 (87.9 – 95.4) | 95.6 (91.9 – 98.0) | |||||
| Positive predictive value (95% CI) | 71.2 (57.9 – 82.2) | 84.7 (73.0 – 92.8) | |||||
| Negative predictive value (95% CI) | 100 (98.2 – 100) | 97.0 (93.7 – 98.9) | |||||
| Accuracy (95% CI) | 93.5 (89.8 – 96.2) | 94.3 (90.7 – 96.8) | |||||
| C: PCR | |||||||
| Positive | 42 | 14 | 50 | 6 | |||
| Negative | 0 | 206 | 9 | 197 | |||
| Sensitivity (95% CI) | 100 (91.6 – 100) | 84.7 (73.0–92.7) | |||||
| Specificity (95% CI) | 93.6 (89.6 – 96.5) | 97.0 (93.6–98.9) | |||||
| Positive predictive value (95% CI) | 75.0 (61.6 – 85.6) | 89.2 (78.9–94.8) | |||||
| Negative predictive value (95% CI) | 100 (98.2 – 100) | 95.6 (92.3–97.5) | |||||
| Accuracy (95% CI) | 94.7 (91.2 – 97.0) | 94.2 (90.7–96.7) | |||||
| D: Microscopy | |||||||
| Positive | 42 | 0 | 42 | 0 | |||
| Negative | 14 | 206 | 17 | 203 | |||
| Sensitivity (95% CI) | 75.0 (61.6 – 85.6) | 71.1 (57.9 –82.2) | |||||
| Specificity (95% CI) | 100 (98.2 – 100) | 100 (98.2–100) | |||||
| Positive predictive value (95% CI) | 100 (91.6 – 100) | 100.0 | |||||
| Negative predictive value (95% CI) | 93.6 (89.6 – 96.5) | 92.2 (88.8–94.6) | |||||
| Accuracy (95% CI) | 94.7 (91.2 – 97.0) | 93.5 (89.8–96.1) | |||||
Fig. 2.
a: Receiver operating characteristic (ROC) curve showing diagnostic performance of Gazelle, RDT and PCR against Microscopy as gold standard. b: Receiver operating characteristic (ROC) curve showing diagnostic performance of Gazelle, RDT and Microscopy against PCR as gold standard.
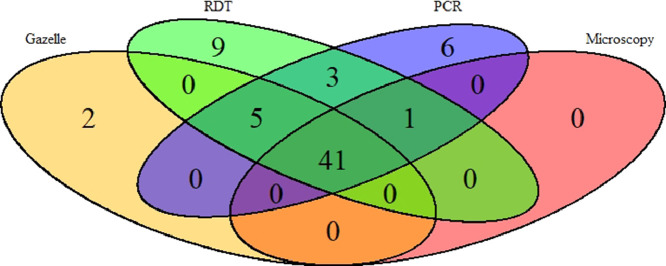
The same validation analyses were also carried out on a subgroup of patients (of the 262) excluding 27 patients who reported a history of malaria treatment within two weeks preceding the study. Sensitivity, specificity, PPV, NPV and accuracy were slightly improved for all the diagnostics methods. However, the trend remained same (Table 2). A total of 41 samples were found to be positive by all four methods (Fig. 3). On the other hand, 2 samples were found positive by Gazelle only and negative by all other methods. This is may be due to electrical fluctuation during processing, defect in cartridge or processing error. Further, 12 samples were RDTs positive but negative by all other methods. This could be due to persistent of PfHRP-2 in their blood as the study was conducted in the high transmission area.
Table 2.
Comparison of diagnostic test statistics of Gazelle, RDT and PCR in patients with no prior history of malaria treatment (n = 235).
| Test diagnostics | Microscopy (Gold standard) |
PCR (Gold standard) |
RDT (Gold standard) |
||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | ||
| A: Gazelle | |||||||
| Positive | 35 | 2 | 35 | 2 | 35 | 2 | |
| Negative | 0 | 198 | 6 | 192 | 0 | 198 | |
| Sensitivity (95% CI) | 100 (90.0 – 100) | 85.4 (70.8 – 94.4) | 100 (90.0–100) | ||||
| Specificity (95% CI) | 99.0 (96.4 – 99.9) | 99.0 (96.3 – 99.9) | 99.0 (96.4–99.8) | ||||
| Positive predictive value (95% CI) | 94.6 (81.8 – 99.3) | 94.6 (81.8 – 99.3) | 94.5 (81.5–98.5) | ||||
| Negative predictive value (95% CI) | 100 (98.2 – 100) | 97.0 (93.5 – 98.9) | 100 | ||||
| Accuracy (95% CI) | 99.1 (97.0 – 99.9) | 96.6 (93.4 – 98.5) | 99.1 (96.9–99.9) | ||||
| B: RDT | |||||||
| Positive | 35 | 0 | 35 | 0 | |||
| Negative | 0 | 200 | 6 | 194 | |||
| Sensitivity (95% CI) | 100 (90.0 – 100) | 85.4 (70.8 – 94.4) | |||||
| Specificity (95% CI) | 100 (98.2 – 100) | 100 (98.1 – 100) | |||||
| Positive predictive value (95% CI) | 100 (90 – 100) | 100 (90.0 – 100) | |||||
| Negative predictive value (95% CI) | 100 (98.2 – 100) | 97.0 (93.6 – 98.9) | |||||
| Accuracy (95% CI) | 100 (98.4 – 100) | 97.4 (94.5 – 99.1) | |||||
| C: PCR | |||||||
| Positive | 35 | 6 | 35 | 6 | |||
| Negative | 0 | 194 | 0 | 194 | |||
| Sensitivity (95% CI) | 100 (90.0 – 100) | 100 (90.0–100) | |||||
| Specificity (95% CI) | 97.0 (93.6 – 98.9) | 97.0 (93.5–98.8) | |||||
| Positive predictive value (95% CI) | 85.4 (70.8 – 94.4) | 85.3 (72.6–92.7) | |||||
| Negative predictive value (95% CI) | 100 (98.1 – 100) | 100 | |||||
| Accuracy (95% CI) | 97.4 (94.5 – 99.1) | 97.4 (94.5–99.0) | |||||
| D: Microscopy | |||||||
| Positive | 35 | 0 | 35 | 0 | |||
| Negative | 6 | 194 | 0 | 200 | |||
| Sensitivity (95% CI) | 85.4 (70.8 – 94.4) | 100 (90.0–100) | |||||
| Specificity (95% CI) | 100 (98.1 – 100) | 100 (98.1–100) | |||||
| Positive predictive value (95% CI) | 100 (90.0 – 100) | 100 | |||||
| Negative predictive value (95% CI) | 97.0 (93.6 – 98.9) | 100 | |||||
| Accuracy (95% CI) | 97.4 (94.5 – 99.1)0.59 | 100 (98.4–100) | |||||
Fig. 3.
Venn diagram depicting the result of four different malaria diagnostic test. The overlap result of diagnostic test showed that 41 samples were found to be positive in all four different tests.
4. Discussion
Majority of malaria cases are reported from rural areas of India. It is important to note that tribal populations constituting 8.6% of total population account for more than 50% of total malaria cases and about 50% death due to malaria in the country [3]. Malaria diagnosis is a challenging task in tribal/rural areas because of poor healthcare infrastructure [7]. The reluctance of these tribal individuals to seek medical care further complicates the issues of malaria diagnosis in tribal areas [23]. We evaluated a new point-of-care, haemozoin-based malaria diagnostic device and compared its performance with conventional microscopy, RDT, and laboratory-based PCR. Hz as a biomarker for malaria has been tried since long, but it remained largely unsuccessful due to a variety of reasons [11,12]. As they were tested in cultured samples as opposed to real-world field conditions, they lacked adequate sensitivity, specificity and minimum detection levels and were prohibitively expensive [11,12,14]. In contrast, the results of current study showed that the performance of Gazelle was equivalent to microscopy or RDTs, the primary diagnostic methods used at the primary/community health centres. Despite being affordable, microscopy is rarely used in remote tribal areas because of lack of infrastructure, skilled personnel, and erratic power supplies [24]. Malaria RDTs are recommended for diagnosis at places where microscopy is not available within 24 h. Most of the RDTs target pfhrp-2 antigen for detection of falciparum malaria. However, the deletion of pfhrp-2/3 genes in P. falciparum in India and other parts of the world [25], [26], [27], [28] underscores the importance and need of non-hrp-2 based diagnostic methods such as magneto-optical detection of malaria parasite employed by Gazelle [29,30]. Moreover, the advantage of Hz as biomarker is that it appears very early during the life cycle of the parasite and gets cleared from the bloodstream within few days. By contrast, PfHRP-2 (histidine rich protein) takes more than a month to clear [15] and results in false positives. Gazelle is a sturdy, battery-operated, magneto-optical point-of-care malaria diagnostic device that is easy to use and quickly provides accurate results in the field. Test result turnaround time is fast (one-minute) compared to microscopy (30 min), RDTs (20 min) and PCR (7–8 h). The diagnostic performance of Gazelle was slightly better in the case of individuals who did not have a history of malaria. The sensitivity and specificity was 100% and 99%, respectively, in patients with no history of malaria as compared to 98% and 97% in those with prior history. It may be noted that many patients reported malaria 2–4 weeks after the initial episode; this may be due to poor compliance of anti-malarial treatment. In such cases, the quantity and quality of Hz may impact the Gazelle performance. Gazelle provides an easily readable result which can be stored and transmitted electronically. The cost of the test on the device is about one dollar per test and it is very much comparable to the cost of a single RDT. This device enables clinicians to perform mass screening affordably and quickly for identification of symptomatic and asymptomatic carriers of malaria. The current Gazelle prototype provides qualitative malaria diagnosis without species differentiation; however the next version of the device, that can also detect the species of malaria parasites, is ready for trial. Several features of Gazelle make it a potential diagnostic tool for field use in control, management and elimination of malaria in malaria endemic areas of the world. Gazelle is a cost-effective point-of-care malaria diagnostic device that may prove very useful for malaria diagnosis in resource-poor settings. It expands and complements the present repertoire of malaria diagnostic tools for use in resource limited settings especially in areas of pfhrp-2 deletion and mixed infection. Overall, this study adds to the increasing evidence that Hz based assay may become a valuable tool for global malaria control and elimination programme. This is the right time to test such devices as 26 countries are planning to eliminate malaria by 2030 including India.
The limitation of current study lies in its ability to detect only malaria positive vs malaria negative cases which create an extra burden where both P. falciparum and P. vivax are present and require different treatment regimens. On the other hand, other human malaria parasites (P. malariae / P. ovale / P. knowlesi) that have low prevalence may be also diagnosed with this device. The next version of the Gazelle is expected to overcome this limitation with efficient diagnosis of Pf, Pv and other malaria parasite species and become one of the best diagnostic tools for malaria elimination globally. Further validation is required for handling of device by unskilled workers.
List of abbreviations
RDT: Rapid diagnostic test
PCR: Polymerase chain reaction
Declaration of Competing Interest
PKB reports grants and non-financial support from HemexDx, India, during the conduct of the study.PT reports personal fees and other from Hemex Health, outside the submitted work; PT is an employee of Hemex Health.RK, AKV,SS, MPS, SR and AD have nothing to disclose.
Acknowledgements
We are thankful to all the study participants, DMO, BMO, CMHO and supporting staff for their support and cooperation during the study. Hemex Health would like to thank Professor Pradip Rathod and John White of University of Washington for their assistance in providing cultured malaria samples for product development testing. The manuscript has been approved by the Publication Screening Committee of ICMR - NIRTH Jabalpur and assigned with the number ICMR-NIRTH/PSC/18/2019.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100347.
Appendix. Supplementary materials
References
- 1.Singh N., Singh P.K. Insecticide treated nets for malaria control: challenges and opportunities. Tribal Health Bull. 2013;19:1–9. [Google Scholar]
- 2.World Health Organisation (WHO), World malaria report 2018. who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf [cited 2019 April 14].
- 3.Sharma R.K., Thakor H.G., Saha K.B., Sonal G.S., Dhariwal A.C., Singh N. Malaria situation in India with special reference to tribal areas. Indian J Med Res. 2015;141:537. doi: 10.4103/0971-5916.159510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukry S.N., Saud M., Sufaida G., Shaikh K., Naz A., Shamsi T.S. Laboratory diagnosis of malaria: comparison of manual and automated diagnostic tests. Can J Infect Dis Med Microbiol. 2017;2017 doi: 10.1155/2017/9286392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell D., Fleurent A.E., Hegg M.C., Boomgard J.D., McConnico C.C. Development of new malaria diagnostics: matching performance and need. Malar J. 2016;15:406. doi: 10.1186/s12936-016-1454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araia Berhane K.A., Mihreteab S., Gresty K. Major threat to malaria control programs by plasmodium falciparum lacking histidine-rich protein 2, eritrea. Emerg Infect Dis. 2018;24:462. doi: 10.3201/eid2403.171723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma A.K., Bharti P.K., Das A. HRP-2 deletion: a hole in the ship of malaria elimination. Lancet Infect Dis. 2018;18:826–827. doi: 10.1016/S1473-3099(18)30420-1. [DOI] [PubMed] [Google Scholar]
- 8.Notomi T., Okayama H., Masubuchi H. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:e63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel J.C., Lucchi N.W., Srivastava P. Field evaluation of a real-time fluorescence loop-mediated isothermal amplification assay, RealAmp, for the diagnosis of malaria in Thailand and India. J Infect Dis. 2014;210:1180–1187. doi: 10.1093/infdis/jiu252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharti A.R., Letendre S.L., Patra K.P., Vinetz J.M., Smith D.M. Malaria diagnosis by a polymerase chain reaction–based assay using a pooling strategy. Am J Trop Med Hyg. 2009;81:754–757. doi: 10.4269/ajtmh.2009.09-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delahunt C., Horning M.P., Wilson B.K., Proctor J.L., Hegg M.C. Limitations of haemozoin-based diagnosis of plasmodium falciparum using dark-field microscopy. Malar J. 2014;13:147. doi: 10.1186/1475-2875-13-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebelo M., Grenho R., Orban A., Hänscheid T. Transdermal diagnosis of malaria using vapor nanobubbles. Emerg Infect Dis. 2016;22:343–344. doi: 10.3201/eid2202.151203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mens P.F., Matelon R.J., Nour B.Y., Newman D.M., Schallig H.D. Laboratory evaluation on the sensitivity and specificity of a novel and rapid detection method for malaria diagnosis based on magneto-optical technology (MOT) Malar J. 2010;9:207. doi: 10.1186/1475-2875-9-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orbán Á., Butykai Á., Molnár A. Evaluation of a novel magneto-optical method for the detection of malaria parasites. PLoS ONE. 2014;9:e96981. doi: 10.1371/journal.pone.0096981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyabayinze D.J., Tibenderana J.K., Odong G.W., Rwakimari J.B., Counihan H. Operational accuracy and comparative persistent antigenicity of HRP2 rapid diagnostic tests for plasmodium falciparum malaria in a hyperendemic region of Uganda. Malar J. 2008;7:221. doi: 10.1186/1475-2875-7-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain V., Basak S., Bhandari S. Burden of complicated malaria in a densely forested Bastar region of Chhattisgarh state (Central India) PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0115266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishna S., Bharti P.K., Chandel H.S. Detection of mixed infections with plasmodium spp. by PCR, India, 2014. Emerg Infect Dis. 2015;21:1853. doi: 10.3201/eid2110.150678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Census 2011, Govt of India. http://www.census2011.co.in/census/state/chhattisgarh.html.
- 19.Singh J., Bhattacharji L.M. Rapid staining of malarial parasites by water soluble stain. Ind Med Gaz. 1944;79:102–104. [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H., Feng G., Zengetal W. A more appropriate white blood cell count for estimating malaria parasite density in plasmodium vivax patients in northeastern Myanmar. Acta Trop. 2016;156:152–156. doi: 10.1016/j.actatropica.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organisation (WHO), Malaria microscopy standard operating procedure. http://www.wpro.who.int/mvp/lab_quality/2096_oms_gmp_sop_09_rev1.pdf. [cited 2019 April 14].
- 22.Snounou G., Viriyakosol S., Zhu X.P. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 23.Saha K.B., Sharma R.K., Mishra R., Verma A., Tiwari B.K., Singh N. Establishing communication mechanism for malaria prevention in Baiga tribal villages in baigachak area of Dindori district, Madhya Pradesh. Indian J Med Res. 2015;141:576. doi: 10.4103/0971-5916.159516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell D., Fleurent A.E., Hegg M.C., Boomgard J.D., McConnico C.C. Development of new malaria diagnostics: matching performance and need. Malar J. 2016;15:406. doi: 10.1186/s12936-016-1454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Q., Gatton M.L., Barnwell J. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar J. 2014;13:283. doi: 10.1186/1475-2875-13-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamboa D., Ho M.F., Bendezu J. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS ONE. 2010;5:e8091. doi: 10.1371/journal.pone.0008091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bharti P.K., Chandel H.S., Ahmad A., Krishna S., Udhayakumar V., Singh N. Prevalence of pfhrp2 and/or pfhrp3 gene deletion in plasmodium falciparum population in eight highly endemic states in India. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0157949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pati P., Dhangadamajhi G., Bal M., Ranjit M. High proportions of pfhrp2 gene deletion and performance of HRP2-based rapid diagnostic test in plasmodium falciparum field isolates of Odisha. Malar J. 2018;17:394. doi: 10.1186/s12936-018-2502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gatton M.L., Dunn J., Chaudhry A., Ciketic S., Cunningham J., Cheng Q. Implications of parasites lacking plasmodium falciparum histidine-rich protein 2 on malaria morbidity and control when rapid diagnostic tests are used for diagnosis. J Infect Dis. 2017;215:1156–1166. doi: 10.1093/infdis/jix094. [DOI] [PubMed] [Google Scholar]
- 30.Rodulfo H., De Donato M., Mora R., Gonzalez L., Contreras C.E. Comparison of the diagnosis of malaria by microscopy, immunochromatography and PCR in endemic areas of Venezuela. Braz J Med Biol Res. 2007;40:535–543. doi: 10.1590/s0100-879x2007000400012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.