Abstract
Nitric oxide (NO) is a multifunctional signalling molecule and a neurotransmitter that plays an important role in physiological and pathophysiological processes. In physiological conditions, NO regulates cell survival, differentiation and proliferation of neurons. It also regulates synaptic activity, plasticity and vesicle trafficking. NO affects cellular signalling through protein S-nitrosylation, the NO-mediated posttranslational modification of cysteine thiols (SNO). SNO can affect protein activity, protein-protein interaction and protein localization. Numerous studies have shown that excessive NO and SNO can lead to nitrosative stress in the nervous system, contributing to neuropathology. In this review, we summarize the role of NO and SNO in the progression of neurodevelopmental, psychiatric and neurodegenerative disorders, with special attention to autism spectrum disorder (ASD). We provide mechanistic insights into the contribution of NO in diverse brain disorders. Finally, we suggest that pharmacological agents that can inhibit or augment the production of NO as well as new approaches to modulate the formation of SNO-proteins can serve as a promising approach for the treatment of diverse brain disorders.
Keywords: Nitric oxide, S-nitrosylation, Autism spectrum disorder, Alzheimer's disease, Psychiatry, Neurodegeneration, Neurodevelopmental disorders, Brain disorders, SHANK3
1. Introduction
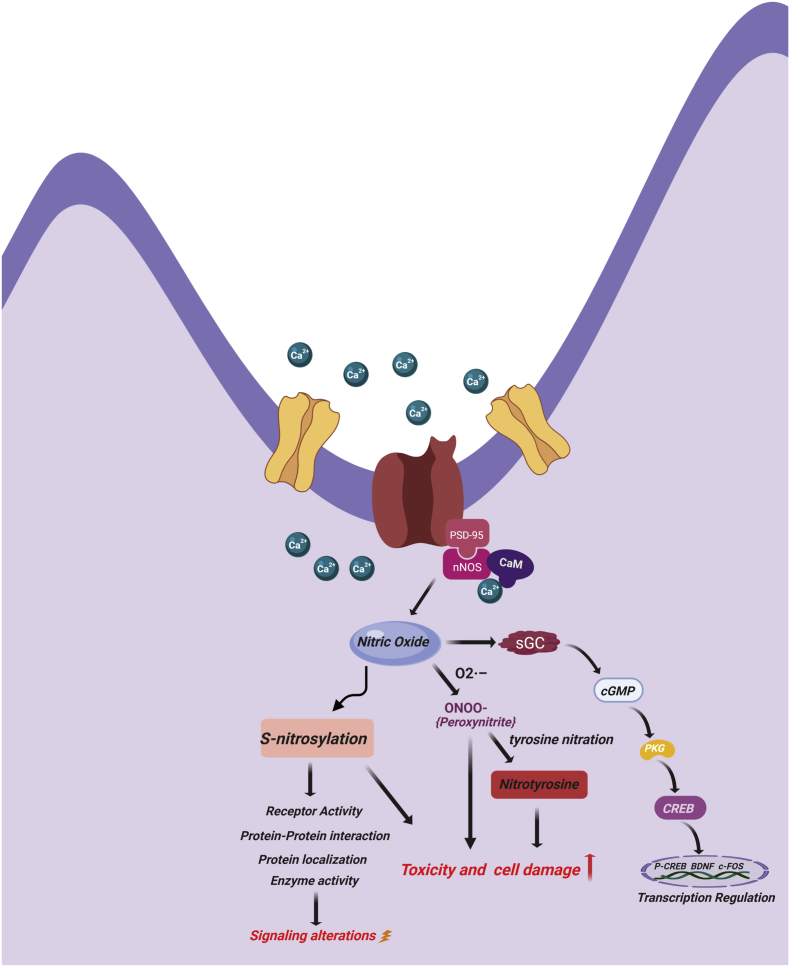
Nitric oxide (NO) is one of the most important signalling molecules of the central nervous system (CNS) and peripheral nervous system (PNS) [[1], [2], [3]]. NO is produced in the brain from l-arginine by three nitric oxide synthase) isoforms (NOS1, NOS2, NOS3) [1]. Neuronal NOS (nNOS or NOS1) is constitutively expressed in the cytosol of neurons and requires Ca2+ for its activity. Inducible NOS (iNOS or NOS2) is found in the cytosol of glial cells and its activity is independent of Ca2+. Endothelial NOS (eNOS or NOS3) is constitutively expressed in endothelial cells in the membrane-bound state and requires Ca2+ for its activity [[4], [5], [6]]. nNOS is attached to N-methyl-d-aspartate receptor (NMDAR), post synaptic density protein-95 (PSD-95) and PSD-93 [7]. When the NMDAR gets activated by extracellular stimuli, it allows entry of Ca2+ inside the cell. Ca2+ can form a complex with calmodulin, and together they initiate the NO formation by activating NOS enzyme [8]. NO is a small gaseous molecule, which diffuses to activate guanosine monophosphate (GMP) cyclase (see Fig. 1). At low concentration, NO acts as a signalling molecule, taking part in the regulation of multiple functions in different organs and systems of the body. In the nervous system, it regulates synaptic activity, plasticity, and vesicle trafficking. However, at higher concentrations, NO may be toxic and could lead to cell death [9]. It reacts with superoxide radical (O2−) and forms peroxynitrite, which ultimately damages DNA, lipid and protein during oxidative stress [10]. NO may affect cellular signalling through proteins S-nitrosylation (SNO), tyrosine nitration, and S-nitrosoglutathione (GSNO) formation [[11], [12], [13], [14], [15]] (see Fig. 1). SNO is the NO-mediated posttranslational modification (PTM) of cysteine thiols, in which a nitrosogroup is incorporated into a reactive cysteine thiol and forms a nitrosothiol group [16,17]. SNO plays a role in protein localization, axonal transport, maintenance of synaptic plasticity and regulation of various neuronal pathways [9,18] (see Fig. 1). However, dysregulation of NO and SNO signalling is involved in progression of many neurodevelopmental, neurobehavioral and neurodegenerative disorders.
Fig. 1.
Schematic representation of NO signalling pathways in physiological conditions. Ca+2 influx activates nNOS by binding with calmodulin, leading to NO production. NO activates soluble guanylate cyclase to produce cGMP which interacts with many intracellular proteins such as PKG. PKG leads to CREB phosphorylation which leads into transcriptional activation of different genes. NO, directly and indirectly, leads to S-nitrosylation (SNO) of many proteins and receptors. SNO modification of proteins can alter the receptor activity, protein-protein interaction and protein localization leading to alteration in signalling. Increased level of NO increases nitrosative stress, proxynitrite formation, tyrosine nitration of proteins, which ultimately may lead into cell death.
In this review, we summarize the role of NO and SNO in the progression of neurodevelopmental disorders, paying special attention to autism spectrum disorder (ASD). We discuss the involvement of SNO in the pathogenesis of ASD (See Fig. 2), which we have recently discovered in our studies [19]. We have summarize the involvement of NO and SNO signalling in Alzheimer's disease (AD) (Fig. 3). We also summarize the involvement of NO and SNO signalling in a number of other brain disorders including psychiatric, neurodevelopment, and neurodegenerative ones. Alterations in NO and other NO-related molecular changes in the different brain disorders are described in detailes (Fig. 4). Further, we list the key SNO-proteins involved in different brain disorders (Table 1). Table 2 summarizes different pharmacological agents used for therapies by manipulating NO. Finally, we provide mechanistic insight into the contribution of NO in diverse brain disorders and suggest potential and promising therapeutic targets for treatment.
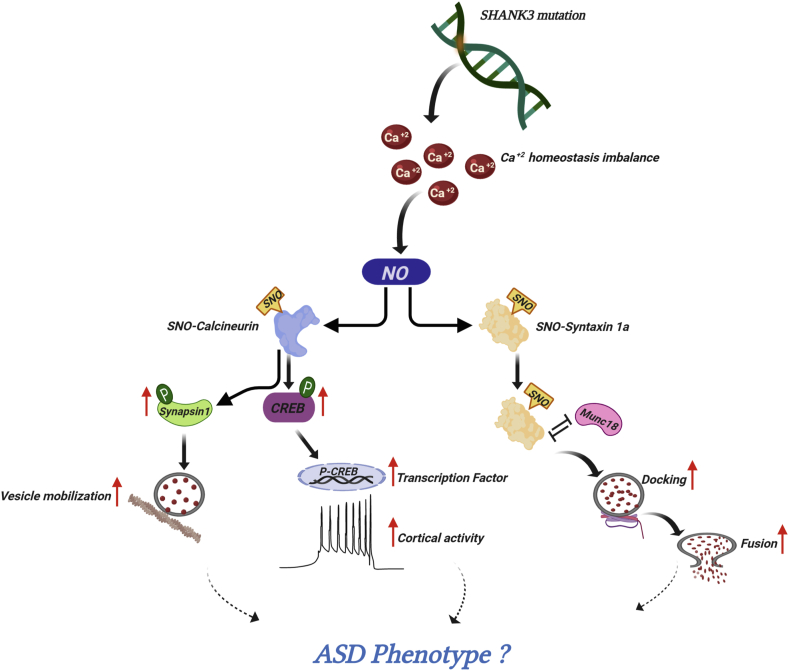
Fig. 2.
NO signalling in autism spectrum disorder (ASD). Schematic representation of NO involvement in ASD. Mutation in SHANK3 gene may cause imbalance in Ca+2 homeostasis. Ca+2 is responsible for intracellular NO production which leads to S-nitrosylation of many proteins. S-nitrosylation of calcineurin inhibited its phosphatase activity which leads to increased levels of phosphorylated (P) synapsin-1 and CREB. P-synapsin-1 increases vesicle mobilization and P-CREB increases the recruitment of transcriptional co-activators and cortical activity. S-nitrosylation of syntaxin1a, inhibited its binding with Munc-18 which ultimately leads to increased vesicle docking and fusion.
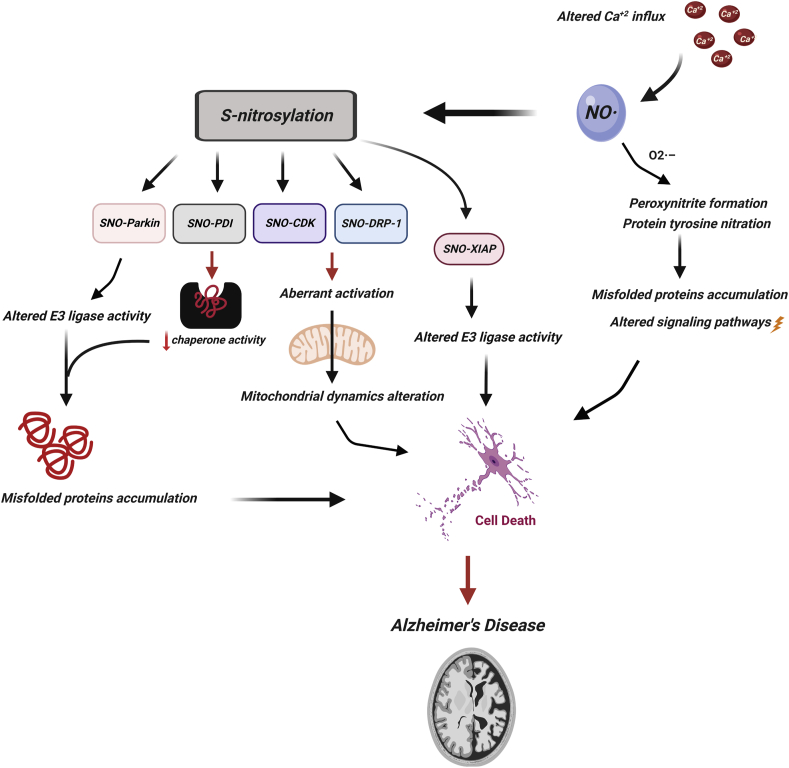
Fig. 3.
NO signalling in Alzheimer's disease (AD). Schematic representation of the involvement of NO in AD progression. Altered Ca+2 influx leads into aberrant NO production in cells, which S-nitrosylates many proteins and increases nitrosative stress, peroxynitrite formation, protein tyrosine nitration, which alters the signalling pathways and lead into cell death in AD. SNO of parkin and XIAP alter their E3 ubiquitin ligase activity. SNO of PDI disrupts its chaperone activity which enhances the accumulation of misfolded proteins in cells. SNO of Cdk and DRP-1 alters the mitochondrial dynamics.
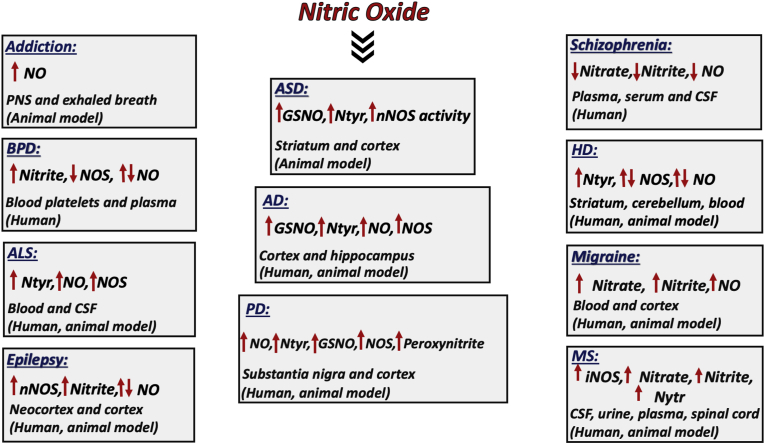
Fig. 4.
The involvement of NO in brain disorders. Alterations in NO and other NO-related molecular changes in the different brain disorders are presented. Abbreviations: NO: nitric oxide; Ntyr: nitrotyrosine; GSNO: S-Nitrosoglutathione; nNOS: neuronal nitric oxide synthase; iNOS: inducible nitric oxide synthase.
Table 1.
List of S-nitrosylated proteins involved in diverse brain disorders.
| Protein | Disease | Sites of SNO modification | Experimental models | Suggested molecular/biological consequences of S-nitrosylation | Reference | ||
|---|---|---|---|---|---|---|---|
| Calcineurin | ASD | – | Mice | Inhibition of the phosphatase activity | [19] | ||
| Syntaxin 1a | ASD | – | Mice | Enhanced binding with SNARE complex | [19] | ||
| mGluR7 | ASD | – | Mice | Increase of Ca2+ influx in presynaptic neurons | [19] | ||
| RNF213 | AD | – | Mice | Inhibition of the ligase activity | [122] | ||
| Drp1 | AD, HD | Cys-644 | Human, mice, cell lines | Increase the rate of mitochondrial fission | [130,178] | ||
| Cdk-5 | AD | Cys-83 and Cys-157 | Human, mice, cell lines | Hyperactivation of the kinase activity | [125,126] | ||
| PSD 95 | AD | Cys-3 and Cys-5 | Animal, cell lines | Blocks translocation of the protein | [205] | ||
| IDE | AD | Cys-110 and Cys 819 | Cell lines | Inhibition of the metalloprotease activity | [9,18,206] | ||
| ApoE | AD | Cys-112 | Human, cell lines | Reduction of affinity to LDL receptors | [136,207] | ||
| MEF2 | AD | Cys-39 | Human, mice, cell lines | Reduction of the binding affinity to DNA | [41] | ||
| Aldolase C, fructose bisphosphate | AD | – | Human | Reduction of the metalloprotease activity | [137] | ||
| MAP1B | AD | Cys-2457 | Mice, cell lines | Enhancement of the binding affinity with microtubules | [36,208] | ||
| Carbonic anhydrase-II (CAH-II) | AD | – | Human | Reduction of the enzymatic activity leading to protein accumulation | [209] | ||
| Caspases | AD | Cys-163 | Human cell lines | Decline in protease activity | [9,210] | ||
| GSK3β | AD | Cys-76, cys-199, cys-317 | Mice, cell lines | Inhibition of the kinase activity and increased translocation into nucleus | [119] | ||
| XIAP | PD, AD, HD | – | Human, mice, cell lines | Inhibition of the anti-apoptotic function | [150] | ||
| GAPDH | AD, ALS, cerebral ischemia, PD | Cys-150, cys-152 | Human, mice, cell line | Enhancement of the binding with Siah complex and activation of p300/CBP resulting in the increased neuronal death | [33,183,184] | ||
| Parkin | PD | – | Human, mice, cell lines | Autoubiquitination and degradation. | [147,152] | ||
| DJ-1 | PD | Cys-46, cys-53, cys106 | Human, cell lines | Reduction of the antioxidant activity | [148,211] | ||
| PTEN | PD, AD | Cys-83 | Human, cell lines | Reduction of the phosphatase activity | [148] | ||
| PDI | PD, ALS | – | Human, mice, cell line | Inhibition of the dithiol isomerase activity | [133,149] | ||
| Prx2 | PD | Cys-51, cys-172 | Human, cell lines | Inhibition of the antioxidant activity | [151] | ||
| Huntingtin | HD | – | Mice, cell lines | Protein aggregate formation leading to cell death | [174] | ||
| NMDAR | AD, Prion disease | Cys −744, cys-798 | Animal | Inhibition of the receptor activity and NO production | [9,18,212] | ||
| MMP9 | Cerebral ischemia | – | Animal, cell lines | Reduction of the metalloprotease activity | [213] | ||
| PLP | MS | – | Animal | Conformational and functional alteration | [195,204] | ||
Table 2.
Summarization of different pharmacological agents used for therapies by manipulating NO.
| Disease name | Pharmacological agents | Protective versus detrimental effects | References |
|---|---|---|---|
| Schizophrenia | Sodium nitroprusside | Improvement in attention, cognitive and working memory | [68,69] |
| Bipolar disorder | Lithium | Increased NO level in plasma and improved symptoms. | [76] |
| Migraine |
|
|
[[87], [88], [89],[94], [95], [96]] |
| Epilepsy |
|
|
[99,100,102,103] |
| Addiction |
|
|
[[108], [109], [110],112] |
| AD | L-NNA | Reduced apoptosis | [126] |
| PD |
|
|
[[164], [165], [166], [167],170] |
| ALS |
|
|
[183,185,191] |
2. Role of nitric oxide in neuronal development
NO plays major roles in neurogenesis and neurodevelopment [20]. Importantly, NO regulates the activity of the brain-derived growth factor (BDNF) [21]. BDNF promotes SNO of many nuclear proteins, including those related to the cAMP response element-binding protein (CREB), a cellular transcription factor that is involved in regulation of neuronal and dendritic development [22]. Sen and Snyder [23] have shown that BDNF, along with other nerve-growth factors, activate nNOS leading to S-nitrosylation of glyceraldehyde 3-phosphate dehydrogenase (GAPDH)/seven in absentia (Siah) homolog complex (SNO-GAPDH-Siah). SNO of GAPDH at Cys-150 promotes its association with Siah, which leads to formation of SNO-GAPDH-Siah complex. Further, SNO-GAPDH-Siah complex translocate into the nucleus [24], initiating the ubiquitination and degradation of the histone-methyltransferase enzyme, suppressor of variegation 3–9 homolog 1 (SUV39H1) protein. SUV39H1 is the principal enzyme responsible for trimethylation of histone H3 at Lys-9, a molecular marker associated with transcriptional silencing. Therefore, inhibition of the trimethylation of histone H3 via SUV39H1 degradation enhances the binding and activation of CREB [23]. In conjunction with BDNF-induced SNO, CREB binds to the cAMP response element of the promoters of its target genes upon phosphorylation at Ser-133 in the KID domain [25] by different receptor-activated protein kinases, such as protein kinase A (PKA), calmodulin-dependent protein kinase (CaMK), mitogen-activated protein kinases (MAPK), and other kinases. These kinases are activated by Ca2+ influx triggered upon depolarization [26,27]. The phosphorylation of Ser-133 leads to a 10- to 20-fold increase in CREB's transcriptional activity [28]. Interestingly that both NO signalling, which produces cGMP, and cAMP signalling could regulate the phosphorylation of CREB (see Fig. 1). cAMP activates CREB through the canonical cAMP/PKA pathway and the exchange protein directly activated by cAMP (Epac) pathway. Epac activates extracellular signal-regulated kinase 1/2 (ERK1/2) signalling, which subsequently leads to Ser-133 phosphorylation of CREB [29]. Meanwhile, cGMP activates the downstream protein cGMP-dependent protein kinase G (PKG), which also phosphorylates the transcription factor CREB at Ser-133 [29]. This dual phosphorylation by cAMP/PKA/Epac and cGMP/PKG pathways may amplify the CREB activity [29]. Once CREB is activated and CREB-binding protein is recruited, transcription is initiated [30].
Further, under normal NO concentration, SNO-GAPDH-Siah translocation is negatively regulated by GAPDH's competitor of Siah protein enhancer life (GOSPEL) protein. SNO of GOSPEL at Cys-47 enhances its ability to bind to GAPDH, which terminates the translocation of SNO-GAPDH-Siah complex. SNO-GAPDH-GOSPEL remains in the cytosol [31]. In contrast, nuclear translocation of SNO-GAPDH promotes transnitrosylation of many proteins, such as histone deacytylase 2 (HDAC2), DNA-activated protein kinase, sirtulin −1, and others [32,33]. BDNF-induced transnitrosylation reaction between SNO-GAPDH and HDAC2 results in SNO of HDAC2 at Cys-262 and Cys-274 [34]. Normally, HDAC2 remains attached to CREB target gene promoters. However, SNO of HDAC2 promotes its dissociation and rapid acetylation of histone H3 and H4, resulting in association of CREB with its target genes. Consequently, BDNF-mediated SNO of HDAC2 plays a role in dendritic development [32,34].
Rearrangement of actin and myosin in cytoskeletons is necessary for axonal growth, axonal guidance, axonal modification and brain development [20] and these processes are also mediated by NO [35]. Furthermore, SNO of microtubule-associated protein B1 (MAPB1) leads to a modification of axon retraction [36]. MAPB1 contains a heavy chain (HC) and a light chain (LC1) domains. LC1 domain can be S-nitrosylated at Cys-2657 of MAPB1, which increases the binding capacity of HC/LC1 MAPB1 complex with microtubules. This complex inhibits the dynein leading to inhibition of axonal extension and increases of axonal retraction [36].
Previous work has shown the involvement of NO in neurogenesis [[37], [38], [39]]. nNOS knockout rats or inhibition of nNOS by pharmacological agents negatively regulate neurogenesis [[37], [38], [39]]. Thus, SNO of myocyte enhancer factor 2 (MEF2), a transcription factor involved in neurogenesis at Cys-39 reduces its binding affinity to DNA and ultimately inhibits its transcriptional activity [40]. SNO-MEF2 also reduces the expression of nuclear receptor tailess (TLX) [41], which is a regulator of adult neurogenesis and is responsible for learning and memory [42]. Lipton and co-workers have found high level of SNO-MEF2 in Alzheimer's disease (AD), leading to neurodegeneration. These changes were present in the post-mortem brains and mutant transgenic mice [41]. Thus, SNO-MEF2 inhibits neurogenesis and neuronal differentiation in brain [41]. Studies on nNOS knockout mice showed abnormal dendritic branching [43] and reduction in neurogenesis [37]. All these reports imply that NO plays an important role in neurodevelopment. Thus, dysregulation of the NO signalling can bring about a variety of neurodevelopmental diseases. Below, we discuss the role of NO in different brain disorders.
3. Autism spectrum disorder (ASD)
ASD is a neurodevelopmental disorder associated with impaired communication, impaired social skills and repetitive behaviour [44]. ASD is caused by genetic mutations, as well as environmental and non-genetic factors [45]. According to the world health organization (WHO), 1–1.5% of children suffer from ASD globally [46,47]. Currently, there is no treatment for ASD and symptomatic features are reduced by different psychiatric medications.
SHANK3 mutation is one of the most promising ASD-associated mutations [48]. Several reports on Shank3 KO mouse models showed defects in biochemical, electrophysiological and cellular pathways [[49], [50], [51]]. As per our knowledge, Amal et al. was the first to report the involvement of NO in the development of ASD [19]. Amal et al. has hypothesized that Shank3 mutation leads to an increase of Ca2+ influx that in turn activates nNOS activity leading to the dramatic NO formation and NO-related molecular changes, including S-nitrosoglutathione (GSNO), 3-nitrotyrosine (Ntyr), and SNO [19]. SNO targets a wide range of prominent intracellular proteins leading to alteration in signalling pathways, which may converge onto synaptic, neuronal and behavioral deficits. The work has reported that in Shank3 mutated mice [InsG3680 (+/+)], the SNO-proteome is reprogrammed and dysregulation of proteins by S-nitrosylation and de-nitrosylation occurs [19]. System biology analysis of both wild type (WT) and Shank3 KO mice revealed 9-fold change in SNO level of proteins involved in the synaptic vesicle cycle (Syntaxin1a (Stx1a), synaptotagmin 1, and N-ethylmaleimide sensitive fusion protein (Nsf)) in cortex of KO mice but not in WT mouse brain. Gene ontology (GO) and KEGG analysis of 6-week-old KO mice showed enrichment of many proteins that involved in neurodevelopment and ASD. Further, systems biology analysis showed the enriched SNO proteins involved in synaptic vesicle cycle and oxidative phosphorylation in Shank3 KO mice. These results convincingly show an association between Shank3 mutation and NO [19]. Further, this work showed that protein-protein interaction analysis in the cortex of KO mice showed a network of S-nitrosylated proteins functionally involved in synaptic vesicle cycle, neurotransmission (protein phosphatase catalystic subunit alpha-Ppp3ca, syntaxin 1a, vesicle associated membrane protein 3 and others) and in glutamatargic pathway (glutamate dehydrogenase 1, mGluR, G protein subunit alpha O1 Gnao-1 and others) [19]. Analyzing the shared SNOed proteins in the cortex of KO mice of both 6-week-old and 4-month-old mice showed an evidence of enriched processes known to be affected in ASD, such as synaptic vesicle cycle. The interactome analysis of the shared proteins in the cortex of KO mice showed protein clusters that function in the synaptic vesicle cycle (syntaxin 1a, Ppp3ca, Nsf and Dnm1) and glutamate regulation (glutamic-oxaloacetic transaminase-Got1, Got2, Gnao-1). This work showed an increase of 3-nitrotyrosine level in different cortical regions. Level of GSNO was found to be increased in the cortex of both KO groups as compared with WT groups. The study also showed that calcineurin was SNOed in the cortex which inhibited its phosphatase activity (see Table 1 and Fig. 2). Inhibition of calcineurin activity increased the levels of p-Synapsin-1 and p-CREB protein [19], (see Fig. 2). Synapsin-1 is involved in regulation of vesicle exocytosis and its phosphorylation increases exocytosis of vesicles [52]. Increase in phosphorylated level of Synapsin-1 in the cortex of the mutant mice may indicate that SNO of calcineurin is responsible for increased vesicle mobilization. The study found a significant increase in p-CREB levels, which is another substrate of calcineurin [19]. Increased level of p-CREB has also been reported in another model of ASD [53]. Syntaxin1a, which enhances the formation of the SNARE complex, was SNOed in Shank3 KO mice [19]. SNO of this protein enhances the formation of the SNARE complex leading to increase of synaptic vesicle docking and fusion [54], (see Fig. 2). SNO of metabotropic glutamate receptor 7 (mGluR7) was found in the cortex of the mutant mice. The study suggested that SNO of mGlur7 may increase the influx of Ca2+ in presynaptic neurons, which in turn increases vesicle fusion [19]. Taken together, the study implies that NO is an important factor in ASD. The insights obtained from the SHANK3 mutation study may likely be applicable to a broader group of patients with genetically diverse but mechanistically related etiology, thus it may imply NO as an important pathological factor in ASD.
4. Schizophrenia
Schizophrenia is a severe and chronic mental disorder, that affects person's thinking, feeling, and behaviour. It accounts for approximately 1% of the total world population [55]. The cause of schizophrenia is unknown and it is considered as a multifactorial disorder [56]. Symptoms of schizophrenia can be divided into three different categories, such as positive symptoms, negative symptoms and cognitive disturbances [56,57]. Hallucinations, catatonic behaviour, delusion and disturbed thought procession are considered as positive symptoms. Avolition, anhedonia and social withdrawal represent negative symptoms [55]. Studies suggested that NO may play a role in the development of schizophrenia [58,59]. Researchers hypothesized that impairments of dopaminergic and cholinergic pathways may be involved in the development of schizophrenia through the involvement of NMDA receptors-dependent NO signalling [[60], [61], [62]]. Polymorphism of nNOS gene is found to be a critical risk factor for the development of schizophrenia [63]. Also, the levels of NO metabolites, such as nitrite and nitrate, were found to be reduced in plasma, serum and cerebrospinal fluid (CSF) of schizophrenic patients (see Fig. 4) [[64], [65], [66]]. Previous work showed that the genetic ablation of nNOS results in cognitive deficit in mice, emphasizing the role of NO in learning and memory [67]. Schizophrenic patients that were treated with NO donor nitroprusside, showed improvement of symptoms (attention, cognitive and working memory) (see Table 2) [68,69]. All these studies suggested that NO deficiency plays a role in the pathology of schizophrenia.
5. Bipolar disorder (BPD)
BPD is a chronic mental illness also referred to as manic depressive illness [70]. Accurate and early diagnosis of BPD is difficult and more than 1% of individuals suffer from this kind of neuropathology globally [71]. On the basis of severity and duration of the manic and depressive episodes, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [72] divide BPD into four categories, bipolar I disorder, bipolar II disorder, cyclothymia and residual category [70]. It has been revealed in some studies that defects in dopaminergic and serotonergic pathways are responsible for the development of BPD [70,73]. The role of NO signalling in BPD has also been reported [74]. NOS activity was reduced in blood platelets of patients suffering from BPD compared to healthy individuals [75]. Another study has shown that lithium treatment increases the level of NO in plasma of patients suffering from this disease, indicating a role of lithium in regulation of NO signalling in subjects with this pathology during depressive episodes (see Table 2) [76]. In this study, plasma NO level in bipolar depression was not different from healthy controls. Other works revealed that NO and nitrite level in plasma of bipolar patients was higher than in healthy controls (see Fig. 4) [77]. A meta-analysis carried out by Andreazza et al. has confirmed an increased activity of the NO signalling in patients with BPD [78]. The controversy of these data can be explained by the differences in the stage of the disease and medications used [76]. Nevertheless, the accumulated data can show the involvement of NO in BPD, although its mechanistic role needs further investigation.
6. Migraine
Migraine is a reversible and chronic neurological disorder with severe or moderate headache. The major symptoms associated with migraine are cutaneous allodynia, phonophobia, photophobia, dizziness, vertigo and different gastrointestinal problems [79]. According to WHO, migraine is the second most common disabling neurological disorder and the third most common medical condition of the world, in which 33% of women and 13% of men are suffering from it [79,80]. The role of NO in migraine was reported many years ago. Blood sample analysis of migraine patients revealed an increased level of cGMP, nitrite, neurokine A and calcitonin gene-related peptide (CGRP), which indicates the association of NO with migraine [81,82]. A variety of factors affect the formation and release of NO, such as bradykinin, NMDA, 5-hydroxytryptamine (5-HT2B/C) receptors, substance P, histamine, acetylcholine, and others [[83], [84], [85]], which indicates its involvement in central pain sensation. Altogether, these data indicate the involvement of NO in the mechanisms of migraine and headache [82,86]. Glyceryl trinitrite (GTN), or sodium nitroprusside, was found to initiate headache and migraine symptoms in workers at explosive industry. This was the first direct evidence of the role of NO in migraine [87]. Further studies of NO donor molecules, such as GTN and isosorbide dinitrate, on migraineurs (people who are suffering from migraine) and non-migraineurs showed that migraineurs are more sensitive to the NO donor compounds than non-migraineurs [88,89]. Nitrate and nitrite concentration in blood was higher in migraineurs compared to control patients (see Fig. 4). These results indicate that NO is involved in initiation and maintenance of pain in migraine [90]. In animal models, headache feature was not investigated, but biochemical analysis has been done. Treatment with GTN in rats resulted in the increase of nNOS in trigeminal nerve ending, NO in cortical regions, and c-fos expression in trigeminal nucleus caudalis [82,91]. Along with this, reduced superoxide dismutase (SOD) expression and increased cortical blood flow were also found in animal models after GTN injections [92,93]. Treatment with cGMP-hydrolyzing phosphodiesterase 5 inhibitor, Sildenafil, led to increased migraine pain in migraineurs compared to controls [94]. Histamine is reported to induce headache in migraineurs and use of a histamine antagonist, Mepyramine, reverted this effect (see Table 2) [95,96]. However, when the NOS-inhibitor L-NG-methylarginine hydrochloride (was administered to histamine-induced migraine patients, no significant changes were found [97]. Nevertheless, in general, the published data indicate that NO signalling may play a role in the development of migraine.
7. Epilepsy
Epilepsy is the most common neurological disorder that affects approximately 50 million people worldwide [98]. It is characterized mainly by recurrent seizures accompanied by loss of consciousness [98]. Here we discuss the involvement of NO in epilepsy. NO regulates excitatory and inhibitory neurotransmission in both physiological and pathological conditions [99]. There is some controversy over the role of NO signalling in the development of epilepsy. Previous reports indicated that NO plays a role of anticonvulsant while other showed that it leads to pro-convulsant effect [99]. Thus, rats with epilepsy induced by NMDA injection were treated with methylene blue, known as a nNOS inhibitor. The methylene blue treatment increased the symptoms of epilepsy [100]. It is worth mentioning that methylene blue appears to be a guanylate cyclase inhibitor that does not interfere with NOS [101]. This casts doubt on the conclusion of the above-mentioned [100] study. Nevertheless, when a precursor of NO l-arginine was given, it reduced the symptoms of epilepsy [99,102]. Also, in DL, homocysteine-thoiolactone (H)-induced seizure model, l-arginine provided protection and the NOS inhibitor N(G)-nitro-l-Arginine methyl ester (l-NAME) treatment potentiated the incidence of seizure (see Table 2) [103]. The researchers concluded that NO is responsible for protection against epilepsy. However, in another study, increased level of NMDA subunit NR2B receptor in epileptic dysplastic human neocortex indicated the involvement of NO [104] and overexpression of nNOS [105] in the pathogenesis of epilepsy was found. In Pentylenetetrazol (PTZ)-induced epilepsy in rats, overexpression of NO and lipid peroxidation was reported in the brain and antioxidant treatment normalised the level of both (see Fig. 4) [106].
8. Addiction
Addiction is a brain disorder distinguished by compulsive engagement in rewarding stimuli. To test the involvement of NO in addiction, morphine addict rats were treated with NOS inhibitors, l-NAME and nitro-l-arginine (L-NNA) and tested the drug withdrawal symptoms. The rats showed reduced morphine withdrawal symptoms and when treated with NO donor compound, isosorbide nitrate, relapse of withdrawal symptoms were detected [108,109]. Immunohistochemical studies in morphine-dependent mice showed increased number of nNOS positive cells in olfactory bulb, cerebellum, medulla oblongata, and locus coeruleus and reduction in the hypothalamus. When treated with an opioid receptor antagonist naloxone, increased nNOS immunoreactivity was found in hypothalamus [110]. These results can explain the role of NO in opioid dependence and withdrawal symptoms [110]. Cocaine is also responsible for induction of nNOS activity in hippocampus of rat brain [111] and tempol treatment, an antioxidant agent, abolished cocaine psychomotor sensitization and condition reward via reduction in oxidative stress [112]. Studies on alcohol addiction showed that ethanol reduces the NO synthesis in peripheral system and also the level of NO in exhaled breath (see Fig. 4) [113]. When l-NAME (an inhibitor of NO) was given to rats, it led to increase in ethanol-induced narcosis; and when isosobarbide dinitrate (an NO donor) was applied, the effect of necrosis was reduced in rats [114]. Further, treatment with 7-nitroindazole(7-NI) (an inhibitor of nNOS) enhances hypnotic effect of ethanol in animals (see Table 2) [115]. Following the mentioned evidence, we suggest that NO signalling plays an important role in addiction.
9. Alzheimer's disease (AD)
Alzheimer's disease (AD) is the most common chronic neurodegenerative disorder [116]. Aging is the main causative factor for the development of AD and memory loss [116]. Cognitive deficits and language impairment are major symptoms of AD [117,118]. Senile plaques and neurofibrillary tangles are the main pathological hallmarks of AD [117]. Tannenbaum and co-workers have tested the involvement of SNO in AD using SNO trapping by TriAryl Phosphine (SNOTRAP) method in conjunction with mass spectrometry [119]. They used the CK-p25-inducible mouse model of AD, and a total of 251 SNO-proteins were detected in the AD model. Among them, 135 SNO proteins were found exclusively for early neurodegeneration in cortex [119]. These proteins found are known to be associated with metabolism, synaptic function and AD progression [119]. According to the GO analysis of CK-p25 mouse model of AD, increased number of SNO proteins were found in cortex and hippocampus compared to control mice. Increased level of amyloid β protein, DNA damage, neuroinflammation and behavioral abnormalities were observed in CK-p25 mice. An increase in GSNO was found in hippocampus and cortex but not in cerebellum of 2-week-old CK-p25 mice. SNO level of PSD95 was also detected in these mice. SNO proteins associated with AD, such as glutamate ionotrophic receptor NMDA 2B (Grin2b), microtubule associated protein (MAPT), glycogen synthase kinase 3β(Gsk3b), lipoprotein receptor-related (LRP), NADH, ubiquinone oxidoreductase core subunit S1 (Ndufs1), cytochrome c oxidase subunit 6B1(Cox6b1) and GAPDH were detected in cortex of Ck-p25 mice brain. Elevated phosphorylation of GSK3β and tau (Mapt) was found in the transgenic mice. It has been shown that these proteins are involved in neuronal cell death in AD [120]. PKCε and PKCγ, isoforms of PKC were found to be SNOed in CK-p25 mice brain. Lipoprotein receptor-related protein (LRP) gene is associated with progression of AD [119,121]. Researchers suggested that SNO-LRP and SNO-PKC in CK-p25 mice disturbed amyloid β processing and clearance, which further lead to amyloid plaque deposition [119]. Amal et al. used P301S mouse model of tauopathy to test the involvement of SNO in the pathology [122]. This work revealed reprogramming of the S-nitroso-proteome in the mutant compared to the control group in both cortex and hippocampus of 2-month-old mice. Increased level of 3-nirotyrosine in the CA1 and entorhinal cortex regions of P301S mice was found. This indicates nitrosative and oxidative stress in the brain of the mutant. This study revealed the role of the noncanonical Wnt/Ca2+ (NC-WCa) signalling in the cortex of P301S mice and found an elevated level of phosphorylated CaMKII. Ring Finger Protein 213 (RNF213), an E3 ubiquitin ligase, was S-nitrosylated, and led to an increase in the level of nuclear factor of activated T-cells 1 (NFAT-1) and FILAMIN-A, which resulted in the potentiation of the NC-WCa signalling [122].
Cyclin dependent kinase (Cdk5) is a serine/threonine kinase enzyme which has diverse functions in the development of brain, synaptic plasticity, regulation of neuronal migration and differentiation [123]. Dysregulation/dysfunction or modification of Cdk5 leads to the development of many neurological disorders [123]. In neuronal cells, Cdk5 scaffolds with the nNOS/PSD95/NMDAR complex which are embedded in the membrane. Cdk5 is S-nitrosylated by interacting with nNOS complex under pathological conditions [124,125]. Post-mortem studies revealed the presence of high level of SNO-Cdk5 protein in the AD brain compared to the normal brain [126]. S-nitrosylation of Cdk5 at Cys-83 and Cys-157 residues activate the function of Cdk5 (see Table 1) [124,125]. Activation of Cdk5 leads to phosphorylation of its substrate ataxia telangiectasia mutated kinase (ATM), which is a proapoptotic protein kinase. In cultured cortical neurons, SNO-Cdk5 is found to enhance the amyloid β (Aβ)-induced synaptic degeneration [126]. Cdk5 mutant protein or treatment with NOS inhibitor, N-nitro-l-arginine (NNA), in the cellular system provided protection against apoptosis (see Table 2) [126]. In summary, aberrant SNO of Cdk5 is responsible for Aβ-induced synaptic degeneration (see Fig. 3) [126]. Importantly, NMDAR plays a role in Aβ-induced synaptic loss [127,128]. Cdk5 activates NMDAR via phosphorylation and p-NMDAR activates nNOS in ischemic insult [129]. The resulting SNO-Cdk5 transnitrosylates Drp-1 protein, a GTPase protein that regulates mitochondrial dynamics such as mitochondrial fission and fusion in cells, with detrimental effects. For example, treatment of the primary cortical neurons with Aβ led to SNO of Drp-1 at Cys-644 residue, which resulted in enhanced mitochondrial fission, impairment of energy homeostasis and dendritic spine loss (see Table 1) [130]. A mutant of Drp-1 (C644A) protected the cells from Aβ-induced synaptic loss [130]. Post mortem studies have confirmed the presence of SNO-Drp-1 in the brain of AD patients and peripheral blood lymphocytes but not in controls [131,132]. The researchers concluded that S-nitrosylation of Drp-1 plays a major role in AD progression (see Fig. 3) [131,132].
Protein disulphide isomerase (PDI) is a chaperon protein, located in endoplasmic reticulum (ER) and plays a major role in protein processing and folding. SNO-PDI was found in post-mortem AD brain [133]. PDI was S-nitrosylated in its active site and inhibited its activity, which led to accumulation of unfolded proteins (see Fig. 3) [133]. Furthermore, a zinc metalloprotease insulin degrading enzyme (IDE) was found to be S-nitrosylated at multiple sites (Cys819, Cys789, Cys966, Cys178) in the presence of GSNO (see Table 1). S-nitrosylation of IDE was found to be involved in the pathogenesis of AD [134]. Mutation in Apo lipoprotein E (ApoE) was reported in late onset of AD [135]. ApoE appears to be S-nitrosylated in patients with AD, and recent studies have reported hippocampal SNOs of ApoE2 and ApoE3. These studies suggest that SNO of ApoE proteins may play a role in AD development by inhibiting lipid homeostasis (see Table 1) [136]. SNO-GAPDH has also found to be S-nitrosylated in various brain regions including cortex, substantia nigra, and hippocampus in postmortem AD brain compared to control brains [137], which suggested the involvement of SNO-GAPDH in AD [137].
Thus, SNO plays a crucial role in the pathogenesis of AD, affecting the activity of numerous proteins. We believe that unravelling the SNO-related signalling pathways will pave the way to new effective therapies against the development of AD.
10. Parkinson's disease (PD)
Parkinson's disease (PD) is the second most common disease after AD in elderly people [138]. It is characterized by four major cardinal features: bradykinesia, rigidity, postural instability and resting tremor [139]. In PD, NO plays both a neuroprotective and neuro-destructive role and was reported in rodent models and human cases of PD [9,140,141]. S-nitrosylation of many proteins was found to be involved in PD progression [9]. Biochemical analysis of postmortem PD brains revealed increase in oxidative/nitrosative stress, which plays a major role in PD pathogenesis [142]. NO forms peroxynitrite in the presence of hydrogen peroxide [143]. Production of peroxynitrite leads to the modification of α-synuclein protein by di-tyrosine synthesis, which further stabilizes the filamentous structure of α-synuclein resulting in aggregate formation [144]. Analysis of postmortem PD brains in substantia nigra and cortical regions showed increased level of nitrated α-synuclein [145]. Nitrated α-synuclein was also found in substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mice model of PD [146]. S-nitrosylation of different proteins, such parkin [147], DJ-1 [148], PDI [133,149], X-linked inhibitor of apoptosis (XIAP) [150] and Prx2 [151] were found to be associated with PD (see Table 1). Previous works have shown that nitrosative stress led to S-nitrosylation of parkin, causing inhibition of its ubiquitination activity, which lead to the formation of Lewy bodies and PD progression. These changes were found both in rodents and in human postmortem PD brain [147,152]. Further, human studies confirmed the SNO modification of parkin in PD pathogenesis [147,152,153] as well as in different mouse models of PD [140,152].
PDI is a chaperone protein residing inside the endoplasmic reticulum and contributing to protein folding and disulfide formation by its isomerase activity [154]. Researchers found that S-nitrosylation of PDI inhibits its activity [133]. They have showed that toxicant rotenone increases the S-nitrosylation of PDI in SH-SY-5Y cell lines [133]. Further, SNO-PDI was also found in the brain of postmortem PD patients, which may emphasize its involvement in the pathology [133,155].
XIAP is an E3 ubiquitin ligase protein which participates in ubiquitination and degradation of caspase 3,7,9 and promotes cell survival [156]. High level of NO leads to SNO modification of XIAP and inhibition of its ubiquitination activity. In rotenone-treated HEK-293 and N2a cell line models of PD, S-nitrosylation of -XIAP is well reported (see Table 1) [150]. Human studies on PD patient's postmortem brain also confirm the SNO modification of XIAP [150,157].
Prx2 has antioxidant properties since it catalyzes the removal of peroxides from cells, and its level is upregulated when oxidative stress is increased in the cells [158]. S-nitrosylation of Prx2 at Cys-51 and Cys-172 inhibited its antioxidant activity [151], which leads to increase of oxidative stress. Importantly, increased level of SNO-Prx2 is reported in PD human postmortem samples and PD animal models (see Table 1) [151].
PD toxicant rodent models, such as MPTP, 6-hydroxydopamine (6-OHDA), lipopolysaccharides (LPS), manganese ethylene bis-dithiocarbamate (maneb) and 1,1′-dimethyl-4,4-bipyridinium dichloride (paraquat), found to have high expression levels of iNOS, nNOS and NO in the brain (see Fig. 4) [[159], [160], [161], [162]]. iNOS-KO mouse model showed resistance to MPTP-induced cell death [163]. Furthermore, NOS inhibitors, 7-nitroindazole and monoamine B oxidase (MAO-B) inhibitor pargyline, protected cell death of dopaminergic neurons in mouse model of PD induced by MPTP(see Table 2) [164]. Together, these studies conclude that NO is involved in MPTP-mediated neurodegeneration. 6-OHDA, is another toxicant used to induce PD in rat, which its intoxication leads to the increased level of iNOS and NO in the rat brain. Pretreatment of animals with NOS inhibitor l-NAME protects from dopaminergic cell death in substantia nigra pars compacta (SNPc) and from dopamine depletion in striatum [165,166]. Intranigral injection of 6-OHDA caused activation of microglial cells, cytokine production, generation of free radicals and increase of NO production, which ultimately led to the death of dopaminergic neurons in PD [160]. GW274150, an iNOS inhibitor, reduced microglial activation, cytokine, ROS and NO production, and provided neuroprotection against the 6-OHDA-induced rodent model of PD (see Table 2) [167]. Rotenone, used as a toxicant to induce PD in rodents, inhibited mitochondrial complex 1 and induced oxidative stress [168]. Furthermore, it reduced the level of glutathione, which further leads to the increase in nitrite levels and apoptosis [169]. Rotenone enhanced NOS enzymatic activity, increased 3-nitrotyrosine (3NT) level, reduced dopamine content and promoted the degeneration of dopaminergic cells [170]. Treatment with the NOS inhibitor 7-NI reduced NOS activity and 3-NT level and provided protection against rotenone-induced toxicity (see Table 2) [170]. Rotenone can induce nitrosative stress by disrupting the redox activity of PDI [171]. In rat striatal synaptosomes treated with methamphetamine, which is also used to produce a toxic model of PD, activated alpha(7) nicotinic receptors and increased the level of intrasynaptosomal calcium, NOS and PKC [172]. All this led to cell death of dopaminergic neurons in the PD rat model [172]. The above data unequivocally point to the involvement of NO and nitrosative stress in the pathogenesis of PD.
11. Huntington disease (HD)
Huntington disease (HD) is a dominantly inherited autosomal neurodegenerative disorder [173]. The main features of HD are motor dysfunction, cognitive impairment and psychiatric disturbances [174]. The main cause of this disease is expansion of CAG repeats in Huntingtin (HTT) protein on chromosome number 4 [173]. The expansion of CAG repeats in Mutant HTT (mHTT) protein results in the formation of long polyglutamine (polyQ) repeats making mHTT a toxic protein. This causes dysfunction of normal homeostasis and cell death of the neurons [175]. The disease starts from the striatum, and when it progresses, it reaches the cortex [173]. Although not enough information is available on the involvement of NO in HD, it is known that the key proteins involved in the progression of HD also become S-nitrosylated. In R6/1 mutant HD mouse, the level of expression and biochemical activity of nNOS was found to be reduced in the striatum and cerebellum (see Fig. 4). Along with this, behavioral abnormalities were also reported in R6/1 HD mice [176]. In another study on SK-N-SH human neuroblastoma cell lines, knockdown of nNOS appeared to be protective for the cells. This can be explained by the fact that inhibition of nNOS may induce autophagy [177]. Mitochondrial dysfunction is the major cause of the development of neurodegenerative disorders. In this context, SNO of dynamin-related protein (Drp-1), the activator of mitochondrial fission, was found to be increased in the human postmortem HD brain and striatum of the transgenic HD mouse model [178]. Further investigation reported that SNO-Drp-1 disrupts the mitochondrial dynamics eventually leading to cell death [179]. Another study has revealed that PTM of HTT protein in N-548 fragment is implicated in the expansion of polyQ repeats. Overexpression of NOS is responsible for SNO of normal HTT protein (see Table 1) [174]. Investigation of the human HD patients indicate that abnormal NO signalling in the peripheral blood tissues is also responsible for the disease progression [178].
12. Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic Lateral Sclerosis (ALS) is an idiopathic neurodegenerative disorder [180]. It is also known as Motor Neuron Disease. This disease is mainly characterized with permanent impairment of lower and upper motor neurons [181]. ALS is sporadic in nature in 85–90% of cases but genetic factors (10–15%) also contribute in the development of this disease. ALS is more common in men than in women [182]. Until now, approximately 30 genes responsible for the development of ALS pathogenesis have been detected [181]. Toxic gain of function due to mutations in SOD1 is one of the major culprits in ALS [180]. Mutant SOD1 G93A is responsible for SNO of GAPDH, which enhances the nuclear translocation of GAPDH-Siah complex in NSC34 motor neurons, which further enhances apoptotic cell death. When deprenyl, a selective inhibitor of Type B monoamine oxidase, was added, it inhibited the SNO of GAPDH and provided protection against apoptosis (see Table 2). This study concludes that mutations of SOD induce SNO of GAPDH, which plays a role in the neuronal apoptosis in ALS model [183]. In contrast, it has been found that mutations of SOD1 increase its denytrosylation activity, and denytrosylation of many mitochondrial proteins can hamper the normal homeostasis in mitochondria, ultimately leading to cell death in ALS models, whilst addition of SNO-donor compounds to the mutant SOD1-containing cells prevents this course of events [184]. This study implies that denytrosylation is important for the cell survival. This conclusion was confirmed by another study, where lymphocyte cells of ALS patients were treated with the NO-releasing agent diethyl NONOate. In this work, the cell death ratio was found to be lower in the diethyl NONOate-treated lymphocytes of ALS patients compared to controls [185].
The role of PDI in the development of ALS is well established. The level of PDI is increased in spinal cord and CSF of ALS patients [186] and spinal cord of SOD1 G93A transgenic rat [187] and mice [188] models of ALS. SNO of PDI in the spinal cord of ALS patients and SOD1 G93A transgenic mice was found to be responsible for the disease progression. Pharmacological agents that mimic the active site of PDI provide protection to SOD1 G93A transgenic mice [155]. Tyrosine nitration of PDI and ERp57 protein has also been reported in SOD1 G93A transgenic mice [189,190]. Treatment with NOS inhibitor, N-nitro-l-Arginine, inhibited both SNO of PDI and the mutant aggregate formation induced by SOD1(see Table 2) [191].
13. Multiple sclerosis (MS)
MS is a chronic neurological disorder in which myelinated exons of CNS started to degenerate by inflammation and autoimmune defects [192]. Epidemiological studies reveal that around 2.3 million people are suffering from this disease worldwide [193]. Environmental and genetic factors are responsible for the development of MS [194]. Role of NO in MS has been established but it is controversial in some cases [195]. Genetic studies have found the association between iNOS gene mutation and MS progression [196]. High level of iNOS RNA is found in the CNS of MS patients and animal models of MS [197]. Further, iNOS immunoreactivity has also been found in active lesions of MS patients compared to the normal human brain [198]. NO metabolites (nitrate and nitrite) level was increased in the CSF, urine and plasma of MS patients (see Fig. 4) [[199], [200], [201]]. These studies indicate that nitrite and nitrate can be used as potent markers for detection of MS. In some studies, nitrotyrosine has also been detected in MS patients [202,203]. Proteolipid protein (PLP) is responsible for maintenance and integrity of myelin sheath. Researchers hypothesized that S-nitrosylation of PLP (SNO-PLP) results in conformational and functional alterations of the protein, which lead to the disease progression (see Table 1) [195,204].
14. Conclusions
NO is one of the most important signalling molecules in the brain. It can play both protective/constitutive and destructive/toxic role, depending upon its regulation/production and interaction with different molecules in the cell. Therefore, NO is also referred to as a “double-edged sword”. A large body of the accumulated evidence suggests that NO is one of the key factors in the genesis of many brain-related disorders. Along with SNO, which targets a wide range of prominent intracellular proteins leading to alteration in signalling pathways, which may converge onto synaptic, neuronal and behavioral deficits. Although NO promotes neurogenesis, aberrant SNO may be responsible for different neurodevelopmental disorders, such as ASD. The SHANK3 study on NO implies it as an important pathological molecule in ASD. As neurons are probably the most vulnerable cell type in the body, oxidative and nitrosative stress and aberrant SNO play a role in the development of a variety of neuroinflammatory and neurodegenerative disorders, including PD, AD, HD, ALS and MS. Further, dysregulation of NO signalling can induce the development of different neuropsychiatric and neurological disorders, such as BPD, migraine, epilepsy, schizophrenia and addiction. Finally, pharmacological agents that can inhibit or augment the production of NO as well as new approaches to pharmacologically modulate formation of SNO-proteins can serve as a potential and promising approach for the treatment of diverse brain disorders.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
We thank Dr. Igor Khaliulin for his contribution in the discussion of this work.
References
- 1.Bredt D.S., Snyder S.H. Nitric oxide: a physiologic messenger molecule. Annu. Rev. Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 2.Bredt D.S., Hwang P.M. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991;351(6329):714. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- 3.Cantu-Medellin N. Effects of T- and R-state stabilization on deoxyhemoglobin-nitrite reactions and stimulation of nitric oxide signaling. Nitric Oxide : Biol. Chem. 2011;25(2):59–69. doi: 10.1016/j.niox.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Förstermann U. Isoforms of nitric oxide synthase characterization and purification from different cell types. Biochem. Pharmacol. 1991;42(10):1849–1857. doi: 10.1016/0006-2952(91)90581-o. [DOI] [PubMed] [Google Scholar]
- 5.Muller U., Bicker G. Calcium-activated release of nitric oxide and cellular distribution of nitric oxide-synthesizing neurons in the nervous system of the locust. J. Neurosci. 1994;14(12):7521–7528. doi: 10.1523/JNEUROSCI.14-12-07521.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Collazo E. Requirement of nitric oxide and calcium mobilization for the induction of apoptosis in adrenal vascular endothelial cells. FEBS Lett. 1997;413(1):124–128. doi: 10.1016/s0014-5793(97)00893-4. [DOI] [PubMed] [Google Scholar]
- 7.Brenman J.E. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84(5):757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 8.Chachlaki K., Garthwaite J., Prevot V. The gentle art of saying NO: how nitric oxide gets things done in the hypothalamus. Nat. Rev. Endocrinol. 2017;13(9):521–535. doi: 10.1038/nrendo.2017.69. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T. Aberrant protein S-nitrosylation contributes to the pathophysiology of neurodegenerative diseases. Neurobiol. Dis. 2015;84:99–108. doi: 10.1016/j.nbd.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deckel A.W. Nitric oxide and nitric oxide synthase in Huntington's disease. J. Neurosci. Res. 2001;64(2):99–107. doi: 10.1002/jnr.1057. [DOI] [PubMed] [Google Scholar]
- 11.Jaffrey S.R. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001;3(2):193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 12.Smith B.C., Marletta M.A. Mechanisms of S-nitrosothiol formation and selectivity in nitric oxide signaling. Curr. Opin. Chem. Biol. 2012;16(5–6):498–506. doi: 10.1016/j.cbpa.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamler J.S., Lamas S., Fang F.C. Nitrosylation: the prototypic redox-based signaling mechanism. Cell. 2001;106(6):675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 14.Stamler J.S. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc. Natl. Acad. Sci. Unit. States Am. 1992;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith B.C., Marletta M.A. Mechanisms of S-nitrosothiol formation and selectivity in nitric oxide signaling. Curr. Opin. Chem. Biol. 2012;16(5):498–506. doi: 10.1016/j.cbpa.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess D.T. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6(2):150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 17.Doulias P.T. Structural profiling of endogenous S-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein S-nitrosylation. Proc. Natl. Acad. Sci. U. S. A. 2010;107(39):16958–16963. doi: 10.1073/pnas.1008036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura T. Aberrant protein s-nitrosylation in neurodegenerative diseases. Neuron. 2013;78(4):596–614. doi: 10.1016/j.neuron.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amal H. Shank3 mutation in a mouse model of autism leads to changes in the S-nitroso-proteome and affects key proteins involved in vesicle release and synaptic function. Mol. Psychiatr. 2018 doi: 10.1038/s41380-018-0113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto S., Lipton S.A. S-Nitrosylation in neurogenesis and neuronal development. Biochim. Biophys. Acta. 2015;1850(8):1588–1593. doi: 10.1016/j.bbagen.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canossa M. Nitric oxide down-regulates brain-derived neurotrophic factor secretion in cultured hippocampal neurons. Proc. Natl. Acad. Sci. U. S. A. 2002;99(5):3282–3287. doi: 10.1073/pnas.042504299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riccio A. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol. Cell. 2006;21(2):283–294. doi: 10.1016/j.molcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Sen N., Snyder S.H. Neurotrophin-mediated degradation of histone methyltransferase by S-nitrosylation cascade regulates neuronal differentiation. Proc. Natl. Acad. Sci. U. S. A. 2011;108(50):20178–20183. doi: 10.1073/pnas.1117820108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hara M.R. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 2005;7(7):665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 25.Guo H. FFPM, a PDE4 inhibitor, reverses learning and memory deficits in APP/PS1 transgenic mice via cAMP/PKA/CREB signaling and anti-inflammatory effects. Neuropharmacology. 2017;116:260–269. doi: 10.1016/j.neuropharm.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 26.West A.E. Calcium regulation of neuronal gene expression. Proc. Natl. Acad. Sci. U. S. A. 2001;98(20):11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng M., McFadden G., Greenberg M.E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990;4(4):571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- 28.Mayr B., Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001;2(8):599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 29.Wang H. cAMP response element-binding protein (CREB): a possible signaling molecule link in the pathophysiology of schizophrenia. Front. Mol. Neurosci. 2018;11(255) doi: 10.3389/fnmol.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyson H.J., Wright P.E. Role of intrinsic protein disorder in the function and interactions of the transcriptional coactivators CREB-binding protein (CBP) and p300. J. Biol. Chem. 2016;291(13):6714–6722. doi: 10.1074/jbc.R115.692020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sen N. GOSPEL: a neuroprotective protein that binds to GAPDH upon S-nitrosylation. Neuron. 2009;63(1):81–91. doi: 10.1016/j.neuron.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornberg M.D. GAPDH mediates nitrosylation of nuclear proteins. Nat. Cell Biol. 2010;12(11):1094–1100. doi: 10.1038/ncb2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S.B. S-nitrosylation of B23/nucleophosmin by GAPDH protects cells from the SIAH1-GAPDH death cascade. J. Cell Biol. 2012;199(1):65–76. doi: 10.1083/jcb.201205015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nott A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455(7211):411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- 35.Wu H.H., Williams C.V., McLoon S.C. Involvement of nitric oxide in the elimination of a transient retinotectal projection in development. Science. 1994;265(5178):1593–1596. doi: 10.1126/science.7521541. [DOI] [PubMed] [Google Scholar]
- 36.Stroissnigg H. S-nitrosylation of microtubule-associated protein 1B mediates nitric-oxide-induced axon retraction. Nat. Cell Biol. 2007;9(9):1035–1045. doi: 10.1038/ncb1625. [DOI] [PubMed] [Google Scholar]
- 37.Packer M.A. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100(16):9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno-Lopez B. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J. Neurosci. 2004;24(1):85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torroglosa A. Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cell. 2007;25(1):88–97. doi: 10.1634/stemcells.2006-0131. [DOI] [PubMed] [Google Scholar]
- 40.Sahay A. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okamoto S. S-nitrosylation-mediated redox transcriptional switch modulates neurogenesis and neuronal cell death. Cell Rep. 2014;8(1):217–228. doi: 10.1016/j.celrep.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murai K. Nuclear receptor TLX stimulates hippocampal neurogenesis and enhances learning and memory in a transgenic mouse model. Proc. Natl. Acad. Sci. U. S. A. 2014;111(25):9115–9120. doi: 10.1073/pnas.1406779111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inglis F.M. The role of nitric oxide and NMDA receptors in the development of motor neuron dendrites. J. Neurosci. 1998;18(24):10493–10501. doi: 10.1523/JNEUROSCI.18-24-10493.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lord C. Autism spectrum disorder. Lancet. 2018;392(10146):508–520. doi: 10.1016/S0140-6736(18)31129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen L. iTRAQ-Based proteomic analysis reveals protein profile in plasma from children with autism. Proteonomics Clin. Appl. 2018;12(3) doi: 10.1002/prca.201700085. [DOI] [PubMed] [Google Scholar]
- 46.Elsabbagh M. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5(3):160–179. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyall K. The changing epidemiology of autism spectrum disorders. Annu. Rev. Publ. Health. 2017;38:81–102. doi: 10.1146/annurev-publhealth-031816-044318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drapeau E. Behavioral phenotyping of an improved mouse model of phelan-McDermid syndrome with a complete deletion of the Shank3 gene. eNeuro. 2018;5(3) doi: 10.1523/ENEURO.0046-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monteiro P., Feng G. SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat. Rev. Neurosci. 2017;18(3):147–157. doi: 10.1038/nrn.2016.183. [DOI] [PubMed] [Google Scholar]
- 50.Peca J. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472(7344):437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y. Mice with Shank3 mutations associated with ASD and schizophrenia display both shared and distinct defects. Neuron. 2016;89(1):147–162. doi: 10.1016/j.neuron.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chi P., Greengard P., Ryan T.A. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron. 2003;38(1):69–78. doi: 10.1016/s0896-6273(03)00151-x. [DOI] [PubMed] [Google Scholar]
- 53.He X. Cytoplasm-predominant Pten associates with increased region-specific brain tyrosine hydroxylase and dopamine D2 receptors in mouse model with autistic traits. Mol. Autism. 2015;6:63. doi: 10.1186/s13229-015-0056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmer Z.J. S-nitrosylation of syntaxin 1 at Cys(145) is a regulatory switch controlling Munc18-1 binding. Biochem. J. 2008;413(3):479–491. doi: 10.1042/BJ20080069. [DOI] [PubMed] [Google Scholar]
- 55.Pitsikas N. The role of nitric oxide donors in schizophrenia: basic studies and clinical applications. Eur. J. Pharmacol. 2015;766:106–113. doi: 10.1016/j.ejphar.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 56.Nasyrova R.F. Role of nitric oxide and related molecules in schizophrenia pathogenesis: biochemical, genetic and clinical aspects. Front. Physiol. 2015;6:139. doi: 10.3389/fphys.2015.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freedman R. Schizophrenia. N Engl J Med. 2003;349(18):1738–1749. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- 58.Yao J.K., Leonard S., Reddy R.D. Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophr. Bull. 2004;30(4):923–934. doi: 10.1093/oxfordjournals.schbul.a007142. [DOI] [PubMed] [Google Scholar]
- 59.Bernstein H.G. Nitric oxide and schizophrenia: present knowledge and emerging concepts of therapy. CNS Neurol. Disord. - Drug Targets. 2011;10(7):792–807. doi: 10.2174/187152711798072392. [DOI] [PubMed] [Google Scholar]
- 60.Lorrain D.S., Hull E.M. Nitric oxide increases dopamine and serotonin release in the medial preoptic area. Neuroreport. 1993;5(1):87–89. doi: 10.1097/00001756-199310000-00024. [DOI] [PubMed] [Google Scholar]
- 61.Brenman J.E., Bredt D.S. Synaptic signaling by nitric oxide. Curr. Opin. Neurobiol. 1997;7(3):374–378. doi: 10.1016/s0959-4388(97)80065-7. [DOI] [PubMed] [Google Scholar]
- 62.Javitt D.C. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int. Rev. Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 63.Reif A. A neuronal nitric oxide synthase (NOS-I) haplotype associated with schizophrenia modifies prefrontal cortex function. Mol. Psychiatr. 2006;11(3):286–300. doi: 10.1038/sj.mp.4001779. [DOI] [PubMed] [Google Scholar]
- 64.Lee B.H., Kim Y.K. Reduced plasma nitric oxide metabolites before and after antipsychotic treatment in patients with schizophrenia compared to controls. Schizophr. Res. 2008;104(1–3):36–43. doi: 10.1016/j.schres.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Ramirez J. Low concentration of nitrite and nitrate in the cerebrospinal fluid from schizophrenic patients: a pilot study. Schizophr. Res. 2004;68(2–3):357–361. doi: 10.1016/S0920-9964(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 66.Nakano Y. Association between plasma nitric oxide metabolites levels and negative symptoms of schizophrenia: a pilot study. Hum. Psychopharmacol. 2010;25(2):139–144. doi: 10.1002/hup.1102. [DOI] [PubMed] [Google Scholar]
- 67.Kirchner L. Impaired cognitive performance in neuronal nitric oxide synthase knockout mice is associated with hippocampal protein derangements. Nitric Oxide. 2004;11(4):316–330. doi: 10.1016/j.niox.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Hallak J.E. Rapid improvement of acute schizophrenia symptoms after intravenous sodium nitroprusside: a randomized, double-blind, placebo-controlled trial. JAMA Psychiatry. 2013;70(7):668–676. doi: 10.1001/jamapsychiatry.2013.1292. [DOI] [PubMed] [Google Scholar]
- 69.Maia-de-Oliveira J.P. The effects of sodium nitroprusside treatment on cognitive deficits in schizophrenia: a pilot study. J. Clin. Psychopharmacol. 2015;35(1):83–85. doi: 10.1097/JCP.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 70.Zhou Q.G. Neuronal nitric oxide synthase and affective disorders. IBRO Rep. 2018;5:116–132. doi: 10.1016/j.ibror.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vieta E. Bipolar disorders. Nat. Rev. Dis.Prim. 2018;4(1):18008. doi: 10.1038/nrdp.2018.8. [DOI] [PubMed] [Google Scholar]
- 72.Force D.-T. Diagnostic and Statistical Manual of Mental Disorders. fifth ed. American Psychiatric Association Publishing; 2013. (DSM-5). Fifith Edition. [Google Scholar]
- 73.Zarate C.A., Singh J., Manji H.K. Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biol. Psychiatr. 2006;59(11):1006–1020. doi: 10.1016/j.biopsych.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 74.Baggott J., Singh A.N. Sildenafil-induced relapse in bipolar disorder: is nitric oxide the mechanism? Int. J. Neuropsychopharmacol. 2004;7(4):525. doi: 10.1017/S146114570400464X. [DOI] [PubMed] [Google Scholar]
- 75.Fontoura P.C. Defective nitric oxide-cyclic guanosine monophosphate signaling in patients with bipolar disorder: a potential role for platelet dysfunction. Psychosom. Med. 2012;74(8):873–877. doi: 10.1097/PSY.0b013e3182689460. [DOI] [PubMed] [Google Scholar]
- 76.de Sousa R.T. Lithium increases nitric oxide levels in subjects with bipolar disorder during depressive episodes. J. Psychiatr. Res. 2014;55:96–100. doi: 10.1016/j.jpsychires.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Savas H.A. Possible role of nitric oxide and adrenomedullin in bipolar affective disorder. Neuropsychobiology. 2002;45(2):57–61. doi: 10.1159/000048677. [DOI] [PubMed] [Google Scholar]
- 78.Andreazza A.C. Oxidative stress markers in bipolar disorder: a meta-analysis. J. Affect. Disord. 2008;111(2):135–144. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 79.Dodick D.W. Migraine. Lancet. 2018;391(10127):1315–1330. doi: 10.1016/S0140-6736(18)30478-1. [DOI] [PubMed] [Google Scholar]
- 80.Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarchielli P. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia. 2000;20(10):907–918. doi: 10.1046/j.1468-2982.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- 82.Neeb L., Reuter U. Nitric oxide in migraine. CNS Neurol. Disord. - Drug Targets. 2007;6(4):258–264. doi: 10.2174/187152707781387233. [DOI] [PubMed] [Google Scholar]
- 83.Fozard J.R. The 5-hydroxytryptamine-nitric oxide connection: the key link in the initiation of migraine? Arch. Int. Pharmacodyn. Ther. 1995;329(1):111–119. [PubMed] [Google Scholar]
- 84.Glusa E., Richter M. Endothelium-dependent relaxation of porcine pulmonary arteries via 5-HT1C-like receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1993;347(5):471–477. doi: 10.1007/BF00166737. [DOI] [PubMed] [Google Scholar]
- 85.Gray D.W., Marshall I. Nitric oxide synthesis inhibitors attenuate calcitonin gene-related peptide endothelium-dependent vasorelaxation in rat aorta. Eur. J. Pharmacol. 1992;212(1):37–42. doi: 10.1016/0014-2999(92)90069-g. [DOI] [PubMed] [Google Scholar]
- 86.Woolf C.J., Thompson S.W. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44(3):293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 87.van der Kuy P.H., Lohman J.J. The role of nitric oxide in vascular headache. Pharm. World Sci. 2003;25(4):146–151. doi: 10.1023/a:1024800512790. [DOI] [PubMed] [Google Scholar]
- 88.Olesen J., Iversen H.K., Thomsen L.L. Nitric oxide supersensitivity: a possible molecular mechanism of migraine pain. Neuroreport. 1993;4(8):1027–1030. doi: 10.1097/00001756-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 89.Bellantonio P. Haemodynamic correlates of early and delayed responses to sublingual administration of isosorbide dinitrate in migraine patients: a transcranial Doppler study. Cephalalgia. 1997;17(3):183–187. doi: 10.1046/j.1468-2982.1997.1703183.x. [DOI] [PubMed] [Google Scholar]
- 90.Shimomura T. Platelet nitric oxide metabolites in migraine. Cephalalgia. 1999;19(4):218–222. doi: 10.1046/j.1468-2982.1999.019004218.x. [DOI] [PubMed] [Google Scholar]
- 91.Knyihar-Csillik E., Vecsei L. Effect of a nitric oxide donor on nitroxergic nerve fibers in the rat dura mater. Neurosci. Lett. 1999;260(2):97–100. doi: 10.1016/s0304-3940(98)00949-5. [DOI] [PubMed] [Google Scholar]
- 92.Read S.J. Enhanced nitric oxide release during cortical spreading depression following infusion of glyceryl trinitrate in the anaesthetized cat. Cephalalgia. 1997;17(3):159–165. doi: 10.1046/j.1468-2982.1997.1703159.x. [DOI] [PubMed] [Google Scholar]
- 93.Read S.J. Effects of sumatriptan on nitric oxide and superoxide balance during glyceryl trinitrate infusion in the rat. Implications for antimigraine mechanisms. Brain Res. 1999;847(1):1–8. doi: 10.1016/s0006-8993(99)01985-x. [DOI] [PubMed] [Google Scholar]
- 94.Kruuse C. The phosphodiesterase 5 inhibitor sildenafil has no effect on cerebral blood flow or blood velocity, but nevertheless induces headache in healthy subjects. J. Cerebr. Blood Flow Metabol. 2002;22(9):1124–1131. doi: 10.1097/00004647-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 95.Lassen L.H., Thomsen L.L., Olesen J. Histamine induces migraine via the H1-receptor. Support for the NO hypothesis of migraine. Neuroreport. 1995;6(11):1475–1479. doi: 10.1097/00001756-199507310-00003. [DOI] [PubMed] [Google Scholar]
- 96.Ayajiki K., Okamura T., Toda N. Involvement of nitric oxide in endothelium-dependent, phasic relaxation caused by histamine in monkey cerebral arteries. Jpn. J. Pharmacol. 1992;60(4):357–362. doi: 10.1254/jjp.60.357. [DOI] [PubMed] [Google Scholar]
- 97.Lassen L.H. Nitric oxide synthase inhibition in migraine. Lancet. 1997;349(9049):401–402. doi: 10.1016/s0140-6736(97)80021-9. [DOI] [PubMed] [Google Scholar]
- 98.Devinsky O. Epilepsy. Nat Rev Dis Primers. 2018;4:18024. doi: 10.1038/nrdp.2018.24. [DOI] [PubMed] [Google Scholar]
- 99.Ferraro G., Sardo P. Nitric oxide and brain hyperexcitability. In Vivo. 2004;18(3):357–366. [PubMed] [Google Scholar]
- 100.Buisson A. Nitric oxide: an endogenous anticonvulsant substance. Neuroreport. 1993;4(4):444–446. [PubMed] [Google Scholar]
- 101.Evora P.R.B. Methylene blue is a guanylate cyclase inhibitor that does not interfere with nitric oxide synthesis. Tex. Heart Inst. J. 2016;43(1) doi: 10.14503/THIJ-15-5629. 103-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Herberg L.J., Grottick A., Rose I.C. Nitric oxide synthesis, epileptic seizures and kindling. Psychopharmacol. (Berl) 1995;119(1):115–123. doi: 10.1007/BF02246062. [DOI] [PubMed] [Google Scholar]
- 103.Hrncic D. The role of nitric oxide in homocysteine thiolactone-induced seizures in adult rats. Cell. Mol. Neurobiol. 2010;30(2):219–231. doi: 10.1007/s10571-009-9444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Najm I.M. Epileptogenicity correlated with increased N-methyl-D-aspartate receptor subunit NR2A/B in human focal cortical dysplasia. Epilepsia. 2000;41(8):971–976. doi: 10.1111/j.1528-1157.2000.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 105.Gonzalez-Martinez J.A. Neuronal nitric oxide synthase expression in resected epileptic dysplastic neocortex. J. Neurosurg. 2009;110(2):343–349. doi: 10.3171/2008.6.17608. [DOI] [PubMed] [Google Scholar]
- 106.Bashkatova V. The influence of anticonvulsant and antioxidant drugs on nitric oxide level and lipid peroxidation in the rat brain during penthylenetetrazole-induced epileptiform model seizures. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2003;27(3):487–492. doi: 10.1016/S0278-5846(03)00037-X. [DOI] [PubMed] [Google Scholar]
- 108.Adams M.L. Inhibition of the morphine withdrawal syndrome by a nitric oxide synthase inhibitor, NG-nitro-L-arginine methyl ester. Life Sci. 1993;52(22):Pl245–P249. doi: 10.1016/0024-3205(93)90472-f. [DOI] [PubMed] [Google Scholar]
- 109.Kimes A.S., Vaupel D.B., London E.D. Attenuation of some signs of opioid withdrawal by inhibitors of nitric oxide synthase. Psychopharmacol. (Berl) 1993;112(4):521–524. doi: 10.1007/BF02244904. [DOI] [PubMed] [Google Scholar]
- 110.Cuellar B. Up-regulation of neuronal NO synthase immunoreactivity in opiate dependence and withdrawal. Psychopharmacol. (Berl) 2000;148(1):66–73. doi: 10.1007/s002130050026. [DOI] [PubMed] [Google Scholar]
- 111.Muriach M. Cocaine causes memory and learning impairments in rats: involvement of nuclear factor kappa B and oxidative stress, and prevention by topiramate. J. Neurochem. 2010;114(3):675–684. doi: 10.1111/j.1471-4159.2010.06794.x. [DOI] [PubMed] [Google Scholar]
- 112.Beiser T. Chronic treatment with Tempol during acquisition or withdrawal from CPP abolishes the expression of cocaine reward and diminishes oxidative damage. Sci. Rep. 2017;7(1):11162. doi: 10.1038/s41598-017-11511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Knych E.T. Ethanol inhibits nonadrenergic, noncholinergic neurotransmission in the anococcygeus muscle of the rat. Alcohol Clin. Exp. Res. 1994;18(3):566–570. doi: 10.1111/j.1530-0277.1994.tb00911.x. [DOI] [PubMed] [Google Scholar]
- 114.Adams M.L. Effects of nitric oxide-related agents on alcohol narcosis. Alcohol Clin. Exp. Res. 1994;18(4):969–975. doi: 10.1111/j.1530-0277.1994.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 115.Vassiljev V. The effects of the nitric oxide synthase inhibitor 7-nitroindazole on ethanol pharmacokinetics in rats after acute and chronic ethanol administration. Alcohol Alcohol. 1998;33(6):609–615. doi: 10.1093/alcalc/33.6.609. [DOI] [PubMed] [Google Scholar]
- 116.Weller J., Budson A. 2018. Current Understanding of Alzheimer's Disease Diagnosis and Treatment; p. 7. F1000Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cruz J.C., Tsai L.H. Cdk5 deregulation in the pathogenesis of Alzheimer's disease. Trends Mol. Med. 2004;10(9):452–458. doi: 10.1016/j.molmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 118.Anggono V., Tsai L.H., Gotz J. Glutamate receptors in alzheimer's disease: mechanisms and therapies. Neural Plast. 2016;2016:8256196. doi: 10.1155/2016/8256196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seneviratne U. S-nitrosation of proteins relevant to Alzheimer's disease during early stages of neurodegeneration. Proc. Natl. Acad. Sci. U. S. A. 2016;113(15):4152–4157. doi: 10.1073/pnas.1521318113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hernandez F., Lucas J.J., Avila J. GSK3 and tau: two convergence points in Alzheimer's disease. J Alzheimers Dis. 2013;33(Suppl 1):S141–S144. doi: 10.3233/JAD-2012-129025. [DOI] [PubMed] [Google Scholar]
- 121.Zerbinatti C.V., Bu G. LRP and Alzheimer's disease. Rev. Neurosci. 2005;16(2):123–135. doi: 10.1515/revneuro.2005.16.2.123. [DOI] [PubMed] [Google Scholar]
- 122.Amal H. S-nitrosylation of E3 ubiquitin-protein ligase RNF213 alters non-canonical Wnt/Ca+2 signaling in the P301S mouse model of tauopathy. Transl. Psychiatry. 2019;9(1):44. doi: 10.1038/s41398-019-0388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Odajima J. Cyclin E constrain Cdk5 activity to regulate synaptic plasticity and memory formation. Dev. Cell. 2011;21(4):655–668. doi: 10.1016/j.devcel.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang P. S-nitrosylation of cyclin-dependent kinase 5 (cdk5) regulates its kinase activity and dendrite growth during neuronal development. J. Neurosci. 2010;30(43):14366–14370. doi: 10.1523/JNEUROSCI.3899-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qu J. S-nitrosylation of Cdk5: potential implications in amyloid-beta-related neurotoxicity in Alzheimer disease. Prion. 2012;6(4):364–370. doi: 10.4161/pri.21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qu J. S-Nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by beta-amyloid peptide. Proc. Natl. Acad. Sci. U. S. A. 2011;108(34):14330–14335. doi: 10.1073/pnas.1105172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shankar G.M. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 2007;27(11):2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Snyder E.M. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 129.Wang J. Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat. Neurosci. 2003;6(10):1039–1047. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]
- 130.Cho D.H. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324(5923):102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J. Neurosci. 2009;29(28):9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang S. Mitochondrial fission proteins in peripheral blood lymphocytes are potential biomarkers for Alzheimer's disease. Eur. J. Neurol. 2012;19(7):1015–1022. doi: 10.1111/j.1468-1331.2012.03670.x. [DOI] [PubMed] [Google Scholar]
- 133.Uehara T. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441(7092):513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 134.Cordes C.M. Nitric oxide inhibits insulin-degrading enzyme activity and function through S-nitrosylation. Biochem. Pharmacol. 2009;77(6):1064–1073. doi: 10.1016/j.bcp.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 135.Lu R.C. TMEM106B and APOE polymorphisms interact to confer risk for late-onset Alzheimer's disease in Han Chinese. J. Neural. Transm. 2014;121(3):283–287. doi: 10.1007/s00702-013-1106-x. [DOI] [PubMed] [Google Scholar]
- 136.Liu C.C. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zahid S. Differential S-nitrosylation of proteins in Alzheimer's disease. Neuroscience. 2014;256:126–136. doi: 10.1016/j.neuroscience.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 138.Mhyre T.R. Parkinson's disease. Subcell. Biochem. 2012;65:389–455. doi: 10.1007/978-94-007-5416-4_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kalia L.V., Lang A.E. Parkinson's disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 140.Gupta S.P. Does restraining nitric oxide biosynthesis rescue from toxins-induced parkinsonism and sporadic Parkinson's disease? Mol. Neurobiol. 2014;49(1):262–275. doi: 10.1007/s12035-013-8517-4. [DOI] [PubMed] [Google Scholar]
- 141.Jimenez-Jimenez F.J. An update on the role of nitric oxide in the neurodegenerative processes of Parkinson's disease. Curr. Med. Chem. 2016;23(24):2666–2679. doi: 10.2174/0929867323666160812151356. [DOI] [PubMed] [Google Scholar]
- 142.Jenner P. Oxidative stress as a cause of nigral cell death in Parkinson's disease and incidental Lewy body disease. The Royal Kings and Queens Parkinson's Disease Research Group. Ann. Neurol. 1992;32(Suppl):S82–S87. doi: 10.1002/ana.410320714. [DOI] [PubMed] [Google Scholar]
- 143.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Souza J.M. Dityrosine cross-linking promotes formation of stable alpha -synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J. Biol. Chem. 2000;275(24):18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- 145.Giasson B.I. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290(5493):985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 146.Przedborski S. Oxidative post-translational modifications of alpha-synuclein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. J. Neurochem. 2001;76(2):637–640. doi: 10.1046/j.1471-4159.2001.00174.x. [DOI] [PubMed] [Google Scholar]
- 147.Chung K.K. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304(5675):1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 148.Choi M.S. Transnitrosylation from DJ-1 to PTEN attenuates neuronal cell death in Parkinson's disease models. J. Neurosci. 2014;34(45):15123–15131. doi: 10.1523/JNEUROSCI.4751-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Conway M.E., Harris M. S-nitrosylation of the thioredoxin-like domains of protein disulfide isomerase and its role in neurodegenerative conditions. Front Chem. 2015;3:27. doi: 10.3389/fchem.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tsang A.H. S-nitrosylation of XIAP compromises neuronal survival in Parkinson's disease. Proc. Natl. Acad. Sci. U. S. A. 2009;106(12):4900–4905. doi: 10.1073/pnas.0810595106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fang J. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson's disease. Proc. Natl. Acad. Sci. U. S. A. 2007;104(47):18742–18747. doi: 10.1073/pnas.0705904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yao D. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc. Natl. Acad. Sci. U. S. A. 2004;101(29):10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lipton S.A. Comment on "S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2005;308(5730):1870. doi: 10.1126/science.1110353. author reply 1870. [DOI] [PubMed] [Google Scholar]
- 154.Andreu C.I. Protein disulfide isomerases in neurodegeneration: from disease mechanisms to biomedical applications. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2012;586(18):2826–2834. doi: 10.1016/j.febslet.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 155.Walker A.K. Protein disulphide isomerase protects against protein aggregation and is S-nitrosylated in amyotrophic lateral sclerosis. Brain. 2010;133(Pt 1):105–116. doi: 10.1093/brain/awp267. [DOI] [PubMed] [Google Scholar]
- 156.Deveraux Q.L. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999;18(19):5242–5251. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nakamura T. Transnitrosylation of XIAP regulates caspase-dependent neuronal cell death. Mol. Cell. 2010;39(2):184–195. doi: 10.1016/j.molcel.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rhee S.G. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 2005;17(2):183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 159.Di Matteo V. Involvement of nitric oxide in nigrostriatal dopaminergic system degeneration: a neurochemical study. Ann. N. Y. Acad. Sci. 2009;1155:309–315. doi: 10.1111/j.1749-6632.2008.03678.x. [DOI] [PubMed] [Google Scholar]
- 160.Yuste J.E. 7-Nitroindazole down-regulates dopamine/DARPP-32 signaling in neostriatal neurons in a rat model of Parkinson's disease. Neuropharmacology. 2012;63(7):1258–1267. doi: 10.1016/j.neuropharm.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 161.Bashkatova V. Chronic administration of rotenone increases levels of nitric oxide and lipid peroxidation products in rat brain. Exp. Neurol. 2004;186(2):235–241. doi: 10.1016/j.expneurol.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 162.Gupta S.P. Involvement of nitric oxide in maneb- and paraquat-induced Parkinson's disease phenotype in mouse: is there any link with lipid peroxidation? Neurochem. Res. 2010;35(8):1206–1213. doi: 10.1007/s11064-010-0176-5. [DOI] [PubMed] [Google Scholar]
- 163.Liberatore G.T. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999;5(12):1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 164.Kurosaki R. Role of nitric oxide synthase against MPTP neurotoxicity in mice. Neurol. Res. 2002;24(7):655–662. doi: 10.1179/016164102101200717. [DOI] [PubMed] [Google Scholar]
- 165.Singh S., Kumar S., Dikshit M. Involvement of the mitochondrial apoptotic pathway and nitric oxide synthase in dopaminergic neuronal death induced by 6-hydroxydopamine and lipopolysaccharide. Redox Rep. 2010;15(3):115–122. doi: 10.1179/174329210X12650506623447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Singh S. Involvement of nitric oxide in neurodegeneration: a study on the experimental models of Parkinson's disease. Redox Rep. 2005;10(2):103–109. doi: 10.1179/135100005X38842. [DOI] [PubMed] [Google Scholar]
- 167.Broom L. Neuroprotection by the selective iNOS inhibitor GW274150 in a model of Parkinson disease. Free Radic. Biol. Med. 2011;50(5):633–640. doi: 10.1016/j.freeradbiomed.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 168.Yadav S. Rodent models and contemporary molecular techniques: notable feats yet incomplete explanations of Parkinson's disease pathogenesis. Mol. Neurobiol. 2012;46(2):495–512. doi: 10.1007/s12035-012-8291-8. [DOI] [PubMed] [Google Scholar]
- 169.Abdin A.A., Sarhan N.I. Intervention of mitochondrial dysfunction-oxidative stress-dependent apoptosis as a possible neuroprotective mechanism of alpha-lipoic acid against rotenone-induced parkinsonism and L-dopa toxicity. Neurosci. Res. 2011;71(4):387–395. doi: 10.1016/j.neures.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 170.He Y. Role of nitric oxide in rotenone-induced nigro-striatal injury. J. Neurochem. 2003;86(6):1338–1345. doi: 10.1046/j.1471-4159.2003.01938.x. [DOI] [PubMed] [Google Scholar]
- 171.Pal R., Miranda M., Narayan M. Nitrosative stress-induced Parkinsonian Lewy-like aggregates prevented through polyphenolic phytochemical analog intervention. Biochem. Biophys. Res. Commun. 2011;404(1):324–329. doi: 10.1016/j.bbrc.2010.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pubill D. Free radical production induced by methamphetamine in rat striatal synaptosomes. Toxicol. Appl. Pharmacol. 2005;204(1):57–68. doi: 10.1016/j.taap.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 173.McColgan P., Tabrizi S.J. Huntington's disease: a clinical review. Eur. J. Neurol. 2018;25(1):24–34. doi: 10.1111/ene.13413. [DOI] [PubMed] [Google Scholar]
- 174.Ni C.L. Polyglutamine tract expansion increases S-nitrosylation of huntingtin and ataxin-1. PloS One. 2016;11(9) doi: 10.1371/journal.pone.0163359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bates G.P. Huntington disease. Nat. Rev. Dis.Prim. 2015;1(1):15005. doi: 10.1038/nrdp.2015.5. [DOI] [PubMed] [Google Scholar]
- 176.Deckel A.W. Altered neuronal nitric oxide synthase expression contributes to disease progression in Huntington's disease transgenic mice. Brain Res. 2002;939(1–2):76–86. doi: 10.1016/s0006-8993(02)02550-7. [DOI] [PubMed] [Google Scholar]
- 177.Sarkar S. Complex inhibitory effects of nitric oxide on autophagy. Mol. Cell. 2011;43(1):19–32. doi: 10.1016/j.molcel.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Haun F. S-nitrosylation of dynamin-related protein 1 mediates mutant huntingtin-induced mitochondrial fragmentation and neuronal injury in Huntington's disease. Antioxidants Redox Signal. 2013;19(11):1173–1184. doi: 10.1089/ars.2012.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nakamura T. S-Nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration. Mitochondrion. 2010;10(5):573–578. doi: 10.1016/j.mito.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kiernan M.C. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 181.Nowicka N. Risk factors and emerging therapies in amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2019;20(11) doi: 10.3390/ijms20112616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Oskarsson B., Gendron T.F., Staff N.P. Amyotrophic lateral sclerosis: an update for 2018. Mayo Clin. Proc. 2018;93(11):1617–1628. doi: 10.1016/j.mayocp.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 183.Lee R. S-nitrosylated GAPDH mediates neuronal apoptosis induced by amyotrophic lateral sclerosis-associated mutant SOD1G93A. Anim. Cell Syst. 2016;20(6):310–316. [Google Scholar]
- 184.Schonhoff C.M. S-nitrosothiol depletion in amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2006;103(7):2404–2409. doi: 10.1073/pnas.0507243103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cereda C. Effect of nitric oxide on lymphocytes from sporadic amyotrophic lateral sclerosis patients: toxic or protective role? Neurol. Sci. 2006;27(5):312–316. doi: 10.1007/s10072-006-0702-z. [DOI] [PubMed] [Google Scholar]
- 186.Atkin J.D. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol. Dis. 2008;30(3):400–407. doi: 10.1016/j.nbd.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 187.Ahtoniemi T. Mutant SOD1 from spinal cord of G93A rats is destabilized and binds to inner mitochondrial membrane. Neurobiol. Dis. 2008;32(3):479–485. doi: 10.1016/j.nbd.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 188.Massignan T. Proteomic analysis of spinal cord of presymptomatic amyotrophic lateral sclerosis G93A SOD1 mouse. Biochem. Biophys. Res. Commun. 2007;353(3):719–725. doi: 10.1016/j.bbrc.2006.12.075. [DOI] [PubMed] [Google Scholar]
- 189.Basso M. Characterization of detergent-insoluble proteins in ALS indicates a causal link between nitrative stress and aggregation in pathogenesis. PloS One. 2009;4(12) doi: 10.1371/journal.pone.0008130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Casoni F. Protein nitration in a mouse model of familial amyotrophic lateral sclerosis: possible multifunctional role in the pathogenesis. J. Biol. Chem. 2005;280(16):16295–16304. doi: 10.1074/jbc.M413111200. [DOI] [PubMed] [Google Scholar]
- 191.Chen X. S-nitrosylated protein disulfide isomerase contributes to mutant SOD1 aggregates in amyotrophic lateral sclerosis. J. Neurochem. 2013;124(1):45–58. doi: 10.1111/jnc.12046. [DOI] [PubMed] [Google Scholar]
- 192.Goldenberg M.M. Multiple sclerosis review. P T. 2012;37(3):175–184. [PMC free article] [PubMed] [Google Scholar]
- 193.Thompson A.J. Multiple sclerosis. Lancet. 2018;391(10130):1622–1636. doi: 10.1016/S0140-6736(18)30481-1. [DOI] [PubMed] [Google Scholar]
- 194.Dobson R., Giovannoni G. Multiple sclerosis - a review. Eur. J. Neurol. 2019;26(1):27–40. doi: 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- 195.Encinas J.M., Manganas L., Enikolopov G. Nitric oxide and multiple sclerosis. Curr. Neurol. Neurosci. Rep. 2005;5(3):232–238. doi: 10.1007/s11910-005-0051-y. [DOI] [PubMed] [Google Scholar]
- 196.Barcellos L.F. Linkage and association with the NOS2A locus on chromosome 17q11 in multiple sclerosis. Ann. Neurol. 2004;55(6):793–800. doi: 10.1002/ana.20092. [DOI] [PubMed] [Google Scholar]
- 197.Kanwar J.R., Kanwar R.K., Krissansen G.W. Simultaneous neuroprotection and blockade of inflammation reverses autoimmune encephalomyelitis. Brain. 2004;127(Pt 6):1313–1331. doi: 10.1093/brain/awh156. [DOI] [PubMed] [Google Scholar]
- 198.Smith K.J., Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002;1(4):232–241. doi: 10.1016/s1474-4422(02)00102-3. [DOI] [PubMed] [Google Scholar]
- 199.Acar G. Nitric oxide as an activity marker in multiple sclerosis. J. Neurol. 2003;250(5):588–592. doi: 10.1007/s00415-003-1041-0. [DOI] [PubMed] [Google Scholar]
- 200.Danilov A.I. Nitric oxide metabolite determinations reveal continuous inflammation in multiple sclerosis. J. Neuroimmunol. 2003;136(1–2):112–118. doi: 10.1016/s0165-5728(02)00464-2. [DOI] [PubMed] [Google Scholar]
- 201.Rejdak K. CSF nitric oxide metabolites are associated with activity and progression of multiple sclerosis. Neurology. 2004;63(8):1439–1445. doi: 10.1212/01.wnl.0000142043.32578.5d. [DOI] [PubMed] [Google Scholar]
- 202.Hooper D.C. Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite: implications for the treatment of multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 1997;94(6):2528–2533. doi: 10.1073/pnas.94.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liu J.S. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am. J. Pathol. 2001;158(6):2057–2066. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bizzozero O.A., DeJesus G., Howard T.A. Exposure of rat optic nerves to nitric oxide causes protein S-nitrosation and myelin decompaction. Neurochem. Res. 2004;29(9):1675–1685. doi: 10.1023/b:nere.0000035802.27087.16. [DOI] [PubMed] [Google Scholar]
- 205.Ho G.P. S-nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of PSD-95. Neuron. 2011;71(1):131–141. doi: 10.1016/j.neuron.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ralat L.A. Protective role of Cys-178 against the inactivation and oligomerization of human insulin-degrading enzyme by oxidation and nitrosylation. J. Biol. Chem. 2009;284(49):34005–34018. doi: 10.1074/jbc.M109.030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Abrams A.J., Farooq A., Wang G. S-nitrosylation of ApoE in Alzheimer's disease. Biochemistry. 2011;50(17):3405–3407. doi: 10.1021/bi200266v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tortosa E. MAP1B regulates microtubule dynamics by sequestering EB1/3 in the cytosol of developing neuronal cells. EMBO J. 2013;32(9):1293–1306. doi: 10.1038/emboj.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sultana R. Identification of nitrated proteins in Alzheimer's disease brain using a redox proteomics approach. Neurobiol. Dis. 2006;22(1):76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 210.Ghavami S. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014;112:24–49. doi: 10.1016/j.pneurobio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 211.Mitsumoto A. Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free Radic. Res. 2001;35(3):301–310. doi: 10.1080/10715760100300831. [DOI] [PubMed] [Google Scholar]
- 212.Meneghetti E. Prions strongly reduce NMDA receptor S-nitrosylation levels at pre-symptomatic and terminal stages of prion diseases. Mol. Neurobiol. 2019;56(9):6035–6045. doi: 10.1007/s12035-019-1505-6. [DOI] [PubMed] [Google Scholar]
- 213.Gu Z. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297(5584):1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]