Abstract
Following injury to the adult mammalian central nervous system, regenerative growth of severed axons is very limited. The lack of neuronal repair is often associated with significant functional deficits, and depending on the severity of injury, may result in permanent paralysis distal to the site of injury. A detailed understanding of the molecular mechanisms that limit neuronal growth in the injured spinal cord is an important step toward the development of specific strategies aimed at restoring functional connectivity lost as a consequence of injury. While rapid progress is being made in defining the molecular identity of CNS growth inhibitory constituents, comparatively little is known about their receptors and downstream signaling mechanisms. Emerging new evidence suggests that the mechanisms for myelin inhibition are likely to be complex, involving multiple and distinct receptor systems that may operate in a redundant manner. Furthermore, the relative contribution of a specific ligand-receptor system to bring about growth inhibition may greatly vary among different neuronal cell types. Myelin-associated glycoprotein (MAG), for example, employs different mechanisms to inhibit neurite outgrowth of cerebellar, sensory, and retinal ganglion neurons in vitro. Nogo-A harbors distinct growth inhibitory regions, which employ different signaling mechanisms. The Nogo-66 receptor 1 (NgR1), a shared ligand binding component in a receptor complex for Nogo-66, MAG, and OMgp, participates in neuronal growth cone collapse to acutely presented myelin inhibitors, but is dispensable for longitudinal neurite outgrowth inhibition on substrate-bound Nogo-66, MAG, OMgp, or crude CNS myelin in vitro. Consistent with the idea of cell-type specific mechanisms for myelin inhibition, different types of CNS neurons possess very different regenerative capacities and respond differently to experimental treatment strategies in vivo. We speculate that differences in regenerative axonal growth among different fiber systems are a reflection of their intrinsic ability to elongate axons and their distinct cell surface receptor profiles to respond to the growth inhibitory extracellular milieu. The existence of cell type specific mechanisms to impair regenerative axonal growth in the CNS may have important implications for the development of treatment strategies. Depending on the fiber tract injured, different ligand-receptor systems may need to be targeted in order to elicit robust and long-distance regenerative axonal growth.
Keywords: Spinal cord injury, myelin associated glycoprotein, Nogo-A, receptor, NgR1, NgR2, integrin, ganglioside
1. Overview
In higher vertebrates, including humans, the regenerative capacity of severed axons in the adult central nervous system (CNS) is extremely limited. Over the past several years a growing number of CNS inhibitory cues has been identified. These include the prototypic myelin inhibitors Nogo-A, myelin-associated glycoprotein (MAG), oligodendrocyte myelin glycoprotein (OMgp) and several types of chondroitin sulfate proteoglycans (CSPGs). In addition, there is now good evidence that canonical axon guidance molecules belonging to the semaphorin, ephrin and netrin families contribute to the growth-hostile environment of injured CNS tissue (Yiu and He, 2006; Xie and Zheng, 2007). When combined with the observation that mature CNS neurons show an intrinsic loss of their ability to rapidly elongate axons (Goldberg et al., 2002), the expanding list of CNS inhibitors imposes a major hurdle for regenerative axonal growth.
In the brain and spinal cord, myelin is produced by oligodendrocytes, the myelinating glia of the CNS. Myelin sheaths are wrapped around axons to ensure rapid propagation of action potentials. Mature oligodendrocytes adopt complex morphologies with extended membrane structures that typically ensheath multiple axons. Traumatic injuries to brain or spinal cord often result in substantial axonal damage. While the neuronal cell body and axon proximal segment of an injured neuron survive, the axon distal segment undergoes progressive degeneration, a process known as Wallerian degeneration. Axonal loss disrupts a delicate biochemical balance between axons and oligodendrocytes and indirectly leads to death of oligodendrocytes and the accumulation of cellular debris rich in degenerating myelin. In the CNS, Wallerian degeneration is very slow compared to the peripheral nervous system (PNS) and degenerating myelin in the CNS persist for months to years (Miklossy and Van der Loos, 1991; George and Griffin, 1994). Because CNS myelin contains multiple inhibitors of growth, the protracted clearance of degenerating CNS myelin is likely to be a major contributor to the regenerative failure of severed axons (Vargas and Barres, 2007).
While a growing number of myelin-associated inhibitors has been identified and significant inroads are beginning to be made in defining the molecular basis of CNS myelin inhibition, the exact composition of inhibitors and their relative contribution to the regenerative failure of injured CNS axons remains largely unknown. With an increasing number of CNS inhibitors identified, an important next question is to define their mechanisms of action. The search for the neuronal surface receptors of myelin inhibitors and their down-stream signaling intermediates is of great interest both biologically and clinically. Cell surface binding partners for MAG, Nogo, and OMgp implicated in neuronal inhibition include the Nogo receptor family members NgR1 and NgR2, as well as complex brain gangliosides (Vyas and Schnaar, 2001; Liu et al., 2006). Membrane proteins that participate directly or indirectly in CNS myelin inhibition include the leucine-rich repeat protein Lingo-1, select members of the TNF receptor superfamily, integrins, and the receptor tyrosine kinase EGFR (Yiu and He, 2006; Hu and Strittmatter, 2008). Many of these ligand-receptor systems share common down-stream signaling components, including RhoA, PKC, and appear to be antagonized by cAMP/PKA signaling. A detailed understanding of the molecular and cellular mechanisms that limit axonal growth and regeneration following CNS injury or disease is of fundamental importance for the rational design of new interventions aimed at promoting CNS repair. Our current knowledge of the molecular mechanisms of MAG, Nogo-A and OMgp mediated neuronal inhibition is the subject of this review, with a primary focus on ligand-receptor interactions and their importance for neuronal growth inhibition in vitro and axonal regeneration in vivo. For a detailed discussion on intracellular signaling mechanisms of neuronal growth inhibition we refer to several excellent reviews that recently have been published (Huber et al., 2003; Yiu and He, 2006; Schmandke and Strittmatter, 2007).
2. CNS inhibitors
Over the past several years numerous CNS inhibitory cues have been identified. These include the prototypic myelin inhibitors MAG, Nogo-A, and OMgp (Filbin, 2003; Schwab, 2004; Liu et al., 2006; Yiu and He, 2006). Furthermore, chondroitin sulfate proteoglycans (CSPGs) which are expressed throughout the brain and spinal cord, inhibit neuronal growth and are upregulated following traumatic CNS injury, particularly near the site of astroglial scar formation (McKeon et al., 1995; Davies et al., 1999; Bradbury et al., 2002). In addition, there is now good evidence that canonical axon guidance molecules belonging to the semaphorin (Pasterkamp et al., 1999; Moreau-Fauvarque et al., 2003; Kaneko et al., 2006), ephrin (Benson et al., 2005; Du et al., 2007), and netrin (Low et al., 2008) families of guidance cues also contribute to the growth hostile environment of injured CNS tissue. Expression levels of these different inhibitors often change following injury and there is currently no clear information available about their relative contribution to the growth inhibitory milieu of intact or injured adult mammalian CNS tissue. It appears likely, however, that robust regenerative axonal growth will depend on strategies directed at neutralizing multiple inhibitors of growth simultaneously. When combined with approaches to boost cell intrinsic growth mechanisms (Song et al., 1997; Cai et al., 2001), this may result in significant axonal regeneration, an important first step toward reestablishing meaningful neuronal connectivity lost as a consequence of CNS injury.
3. Myelin-associated glycoprotein
One of the best-characterized inhibitors of axonal growth is myelin-associated glycoprotein (MAG). A biochemical approach aimed at the purification and molecular characterization of myelin-associated growth inhibitory activity led to the identification of MAG, a previously known myelin constituent (McKerracher et al., 1994; Mukhopadhyay et al., 1994).
MAG (Siglec-4) is a sialic acid-recognizing Igsuperfamily lectin comprised of 5 immunoglobulin (Ig)-like domains, a single transmembrane domain, and a cytoplasmic domain that is variable in length. Alternative splicing in rodents generates two MAG splice forms with identical ectodomains and either a short (S-MAG) or large (L-MAG) cytoplasmic portion (Quarles et al., 1992; Kelm et al., 1994; Crocker and Varki, 2001). A soluble fragment of MAG (dMAG), the product of matrix metalloproteinase activity, retains neurite outgrowth inhibitory activity in vitro (Tang et al., 1997b; Milward et al., 2008). The MAG lectin activity is located within its N-terminal V-set Ig domain and binds with high specificity to certain types of terminal sialic acids. Soluble MAG ectodomain binds to the neuronal cell surface and inhibits growth in a sialic acid-dependent, Vibrio cholerae neuraminidase (VCN) sensitive manner (Kelm et al., 1994; DeBellard et al., 1996). Characterization of the MAG lectin activity revealed strong binding to α2,3-linked but not to α2,6- or α2,8-linked terminal sialic acids. Moreover, α2,3-linked sialic acids on a core structure of Galβ1–3GalNAc, found on O-glycosylated proteins and some gangliosides, bind to MAG very strongly (Vyas and Schnaar, 2001). In addition, MAG also binds to α2,3-linked sialic acids on glycoconjugates that are N-linked to the protein core (Strenge et al., 1998; Strenge et al., 1999). Together this suggests the existence of numerous ligands recognized by the MAG lectin activity, and similar to other siglecs, MAG function may be regulated by overall levels of both cis- and trans-sialic acid associations (Varki and Angata, 2006).
MAG is a bi-functional molecule that regulates neurite outgrowth in an age-dependent manner; it promotes growth of many types of young neurons and at more mature stages, strongly inhibits neurite outgrowth (Johnson et al., 1989; McKerracher et al., 1994; Mukhopadhyay et al., 1994; Hasegawa et al., 2004). The molecular basis of the switch from growth promotion to growth inhibition is not yet fully understood (Hasegawa et al., 2004) and depending on the neuronal cell type examined, occurs at different developmental stages. Late embryonic rat hippocampal and cortical neurons already show a growth inhibitory response toward MAG (Vinson et al., 1996; Chivatakarn et al., 2007). Postnatal day (P) 1 DRG neurons on the other hand, grow longer neurites when cultured on MAG. After P6 all neuronal cell types examined thus far are strongly inhibited by MAG (Mukhopadhyay et al., 1994; Cai et al., 2001).
MAG is expressed by myelinating glia, Schwann cells in the PNS and oligodendrocytes in the CNS, and is strongly enriched in myelin membranes juxtaposed to the axon and on apposing myelin membranes in the non-compact myelin compartments such as the Schmidt–Lanterman incisures and the paranodal loops (Trapp et al., 1989; Quarles, 2002; Erb et al., 2006). S-MAG is the predominant isoform in the PNS, and both isoforms, S-MAG and L-MAG, are abundantly found in the mature CNS (Erb et al., 2006). Expression levels of MAG in CNS myelin are much greater than in PNS myelin (Quarles, 1989).
MAG is a strong inhibitor of neurite outgrowth in vitro, however, loss of MAG is not sufficient to improve regenerative axonal growth in spinal cord injured mice (Bartsch et al., 1995). Nevertheless, there is good evidence that MAG has growth inhibitory activity towards regenerating neurons in vivo (Schafer et al., 1996; Sicotte et al., 2003). When crossed into Wlds mice, a strain in which Wallerian degeneration is greatly delayed (Lunn et al., 1989), MAG null mice show faster axonal regrowth in crushed femoral nerves compared to MAG wild-type controls, indicating that MAG is an inhibitor of axonal regeneration in vivo (Schafer et al., 1996). Also, acute inactivation of MAG in chicken retina-optic nerve cultures leads to enhanced regeneration of injured retinal ganglion cell axons (Wong et al., 2003). Because MAG is much more abundant in CNS myelin compared to the PNS myelin (Quarles, 1989), it is conceivable that MAG significantly contributes to the growth inhibitory nature of adult mammalian CNS white matter.
While MAG’s role as an inhibitor of axonal growth and regeneration has received most attention, analysis of the MAG null mouse revealed defects in myelin stability (Li et al., 1994; Montag et al., 1994). In addition to mild deficits in myelination, MAG null mice exhibit late-onset progressive PNS axonal atrophy and increased Wallerian degeneration. More recent studies revealed clear evidence for CNS axon degeneration in MAG null mice (Loers et al., 2004; Pan et al., 2005). For a more detailed discussion of the MAG mutant phenotype see (Quarles, 2007).
4. Nogo
Since the identification of MAG as a myelin inhibitor in 1994, several additional myelin-associated growth inhibitors have been cloned and characterized at the molecular level. Pioneering work by Schwab and Caroni demonstrated the existence of specific neurite growth inhibitory factors in CNS myelin (Caroni and Schwab, 1988). A monoclonal antibody (IN-1) raised against a 35/250-kDa inhibitory activity was found to promote neurite outgrowth in the presence of CNS myelin in vitro, and more importantly, to also promote regenerative axonal growth following spinal cord injury in vivo (Schnell and Schwab, 1990; Bregman et al., 1995; Brosamle et al., 2000; Liebscher et al., 2005). Based on subsequently deduced peptide sequences of the 250-kDa IN-1 antigen (Spillmann et al., 1998), three groups independently cloned Nogo-A and demonstrated its growth inhibitory activity (Chen et al., 2000; GrandPre et al., 2000; Prinjha et al., 2000). Nogo-A/RTN4 is a member of the reticulon (RTN) family of membrane-associated proteins. All RTN family members contain a carboxy-terminal RTN homology domain that consists of two hydrophobic regions flanking a hydrophilic loop of 60–70 amino acids. The RTN amino-terminal domains are more divergent and display little or no similarity to each other (Yang and Strittmatter, 2007). The Nogo gene gives rise to three different splice forms, Nogo-A, Nogo-B, and Nogo-C. The largest of which, Nogo-A, is comprised of at least two distinct and dissociable neurite outgrowth inhibitory domains (Fournier et al., 2001; Oertle et al., 2003). The Nogo-A specific inhibitory activity located toward the N-terminal domain of Nogo-A (Amino-Nogo), inhibits neurite outgrowth and fibroblast spreading in vitro (Caroni and Schwab, 1988; Oertle et al., 2003). A neuron-specific inhibitory activity, shared by all three Nogo isoforms, is a 66 amino acid hydrophilic loop (Nogo-66) flanked by two transmembrane domains and part of the carboxy-terminal RTN homology domain.
Nogo-A is strongly expressed by developing and mature oligodendrocytes but is not found in PNS myelin. In addition, Nogo-A is also expressed by many neuronal cell types in the developing and mature nervous system (Huber et al., 2002; Wang et al., 2002c). Inhibitory regions of Nogo-A are present on the cell surface; however, the membrane topology of Nogo-A is variable and may be regulated in a developmental manner (Oertle et al., 2003). A large part of Nogo-A is located intracellularly and may only be exposed following injury induced death of oligodendrocytes, further contributing to the growth inhibitory environment of injured CNS tissue. When coupled with the protracted clearance of Nogo-A from degenerating tracts of spinal cord injured rats (Buss et al., 2005), this suggests that Nogo-A inhibition persists for a long time following injury.
5. Oligodendrocyte myelin glycoprotein
Oligodendrocyte myelin glycoprotein (OMgp) is a 110-kDa leucine rich repeat (LRR) protein linked to the cell surface via a glycosylphosphatidylinositol (GPI) anchor (Vourc’h and Andres, 2004). OMgp is a well-known CNS myelin constituent (Mikol and Stefansson, 1988). Its neurite outgrowth inhibitory activity, however, was only described more recently (Wang et al., 2002b). OMgp appears to be largely identical with arretin, a previously reported growth inhibitory activity found in mouse and human CNS myelin (Kottis et al., 2002). Similar to Nogo-A, OMgp is expressed by oligodendorcytes and neurons in the developing and adult CNS (Habib et al., 1998). OMgp has been reported to be present at Nodes of Ranvier where it is thought to function as a growth inhibitor that limits aberrant axonal sprouting at nodes (Huang et al., 2005). Whether functional ablation of OMgp leads to enhanced regenerative axonal growth following SCI in vivo has not yet been examined.
6. Mechanisms of myelin inhibition
6.1. MAG inhibition – Importance of terminal sialic acids
MAG is a siglec family member that binds to the neuronal cell surface in a sialic acid-dependent manner (Kelm et al., 1994; DeBellard et al., 1996). To what extent the presence of neuronal sialoglycans is required for MAG inhibition, however, is still an ongoing debate. Knowledge on the role of MAG’s lectin activity is of considerable interest because it offers a potential target to antagonize MAG inhibition. Initially, the importance of MAG’s lectin activity for growth inhibition was probed by neuraminidase shedding of terminal sialic acids or the use of a MAG mutant (MAGR118A) with greatly decreased lectin activity (Tang et al., 1997a). If cerebellar granule neurons (CGNs) are desialylated before the neurite outgrowth assay, inhibition by soluble MAG is completely lost, while inhibition by membrane bound MAG, expressed on the surface of CHO-MAG feeder cells, is only partially reversed. Moreover, soluble MAG-Fc but not MAGR118A-Fc inhibits neurite outgrowth of CGNs (Tang et al., 1997a). At high doses, however, MAGR118A-Fc still inhibits neurite outgrowth of embryonic hippocampal neurons (Vinson et al., 2001). Interestingly, when stably expressed on the surface of CHO cells, MAGR118A inhibits neurite outgrowth similarly to wild-type MAG (DeBellard et al., 1996; Tang et al., 1997a). The differences observed in outgrowth inhibition between neurons grown on CHO-MAGR118A cells and desialylatated neurons grown on CHO-MAG cells may be the result of residual lectin activity that remains present in MAGR118A. Alternatively, MAG’s lectin activity may primarily function in the binding and proper presentation of MAG to a signal-transducing component in the MAG holoreceptor complex – and when presented at high concentration on the cell surface of CHO-MAG feeder cells, MAG’s lectin activity may be dispensable for growth inhibition. At lower concentration or in soluble form, however, the MAG lectin activity is necessary for neurite outgrowth inhibition (Vinson et al., 2001). Consistent with the idea that the MAG lectin activity does not directly participate in growth inhibition, MAG(1–3)-Fc binds to the neuronal surface in a sialic-acid dependent manner but fails to inhibit neurite outgrowth (Tang et al., 1997a). Based on these observations Filbin and colleagues proposed a model in which there are two binding sites on MAG, a sialic acid dependent site and a growth inhibitory site (Tang et al., 1997a). In support of their model, a growth inhibitory site on MAG was recently found to be located in the fifth Ig domain (Cao et al., 2007). The binding partner(s) of the MAG growth inhibitory site in the fifth Ig domain, however, remains elusive. As discussed below, the importance of MAG’s lectin activity for neuronal growth inhibition does not only depend on the form in which MAG is presented (soluble or membrane bound), but also varies greatly among different neuronal cell types.
6.2. Gangliosides
If sialoglycans are important for aspects of MAG inhibition, what is their molecular identity? Complex gangliosides, including GD1a and GT1b, have been identified as sialic acid-dependent MAG ligands that function in neurite outgrowth inhibition of soluble MAG-Fc and substrate adsorbed MAG (Yang et al., 1996; Collins et al., 1997; Vinson et al., 2001; Vyas et al., 2002). The most compelling evidence for the importance of complex gangliosides in MAG inhibition stems from the greatly reduced inhibition of CGNs isolated from GalNacT mutant mice (Vyas et al., 2002; Fujitani et al., 2005). GalNacT mice lack all major brain gangliosides, including the MAG binding gangliosides GD1a and GT1b, however, other MAG binding gangliosides such as GM3 are still present. To what extent gangliosides contribute to MAG inhibition in neuronal cell types other than CGNs was only examined very recently. Consistent with mouse genetic studies, pharmacological inhibition of ganglioside biosynthesis largely abrogates inhibition of CGNs cultured on substrate adsorbed dMAG (Mehta et al., 2007). In marked contrast to CGNs, gangliosides are largely dispensable for MAG inhibition of DRG neurons and also play a less prominent role in MAG inhibition of hippocampal neurons compared to CGNs. This suggests that the importance of gangliosides for MAG inhibition greatly depends on the neuronal cell type examined (Mehta et al., 2007). Consistent with this conclusion, we found that MAG inhibition of CGNs, but not retinal ganglion cells (RGCs) or DRG neurons is sensitive to neuraminidase treatment (Venkatesh et al., 2007), further arguing for the existence of cell type specific mechanisms for MAG inhibition.
Because MAG-Fc binding to primary neurons and neuroblastoma cells is both neuraminidase and trypsin sensitive (DeBellard et al., 1996; DeBellard and Filbin, 1999; Strenge et al., 1999), it has been argued that in addition to gangliosides, cell surface protein(s) contribute to high affinity MAG binding. Consistent with this idea, affinity precipitation with immobilized MAG-Fc identified specific protein interactions, some of which are sialic acid-dependent (DeBellard and Filbin, 1999; Strenge et al., 1999). Whether the neuraminidase and trypsin sensitivity of MAG binding is a reflection of MAG binding to neuronal sialoglyco-protein(s) or a protein-ganglioside complex, however, remains unknown.
7. Nogo receptors
7.1. NgR1
In addition to gangliosides, several other MAG binding partners have been described. These include extracellular matrix components such as heparin (Fahrig et al., 1987), collagen type 1 (Probstmeier et al., 1992), fibronectin (Strenge et al., 2001), and tenascin-R (Yang et al., 1999). The functional significance of MAG binding to different extracellular matrix components is not clear, however, it was recently found that extracellular matrix components greatly influence MAG’s ability to inhibit neurite outgrowth. In the presence of laminin, MAG inhibition of DRGs and CGNs is reduced in a dose dependent manner (Laforest et al., 2005).
Neuronal binding partners of MAG include a glycosylated form of MAP1B (Franzen et al., 2001), the Nogo receptor family members NgR1 and NgR2 (Domeniconi et al., 2002; Liu et al., 2002; Venkatesh et al., 2005) and OMgp (Lauren et al., 2007). In Xenopus spinal neurons, a homologue of mammalian TRPC1 is required for proper growth cone turning in response to microscopic gradients of MAG. However, MAG is not thought to directly bind to TRPC1, and it is not known how TRPC1 is activated in the presence of MAG (Shim et al., 2005).
The first MAG binding protein directly implicated in neuronal growth inhibition was the Nogo-66 receptor NgR1. Initially identified as a high affinity receptor for Nogo-66 (Fournier et al., 2001), NgR1 was subsequently found to also directly bind MAG (Domeniconi et al., 2002; Liu et al., 2002), and OMgp (Wang et al., 2002b). NgR1 is the founding member of the Nogo receptor gene family comprised of three members NgR1, NgR2, and NgR3 (Fig. 1). Nogo receptors are leucine rich repeat (LRR) proteins linked to the surface by a glycosylphosphatidyl inositol (GPI) anchor. The LRR cluster of Nogo receptor family members is composed of 8.5 canonical LRRs flanked N-terminally and C-terminally by cysteine-rich LRRNT and LRRCT capping domains. The LRR cluster of NgR1 adopts a helical quaternary structure and is connected to the cell membrane via a ∼ 100 amino acid stalk region and a GPI anchor (Barton et al., 2003; He et al., 2003). The NgR1 LRR cluster is necessary and sufficient to support binding of Nogo-66, MAG, and OMgp. The stalk region of NgR1 is required for growth cone collapse in retinal ganglion cells but does not participate in ligand binding (Fournier et al., 2002).
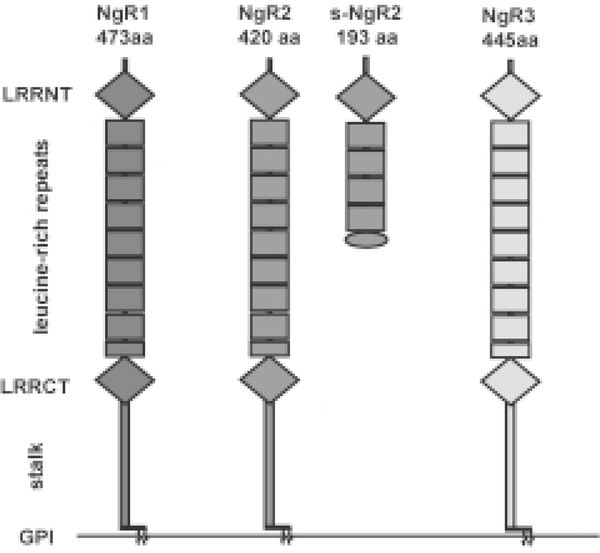
Fig. 1.
The Nogo receptor family. The Nogo receptors NgR1, NgR2, and NgR3 comprise a small subfamily of leucine-rich repeat (LRR) proteins. The three receptors share an identical overall domain organization composed of a cluster of 8.5 canonical LRRs flanked N-terminally by a LRRNT capping domain and C-terminally by a LRRCT capping domain. The LRRNT-LRR-LRRCT domains are connected via a stalk region to a glycosylphosphatidyl inositol (GPI) anchor. We identified s-NgR2, a soluble form of NgR2 that is the product of alternative splicing of exon III of the NgR2 gene. The first 171 amino acid residues of s-NgR2 are identical with full-length NgR2 and are followed by 22 amino acids unique to s-NgR2. NgR1 is a 65-kDa glycoprotein that supports binding of Nogo, MAG, and OMgp. NgR2 is a 65-kDa glycoprotein and supports binding of MAG but not Nogo or OMgp. Thus far no binding partners for NgR3 have been identified.
Two independent approaches were pursued to assess the functional significance of the NgR1-MAG association for neuronal growth inhibition. One study showed that neurite outgrowth inhibition of CGNs plated on CHO-MAG feeder layers is blocked in the presence of anti-NgR1 antibody (Domeniconi et al., 2002), and a second study found that ectopic expression of NgR1 in embryonic chick DRG neurons is sufficient to confer MAG responsiveness (Liu et al., 2002). These findings would argue that NgR1 is both necessary and sufficient for MAG inhibition. However, when later challenged with primary neurons isolated from NgR1 null mice, it was found that in CGNs and other primary neuronal cell types, NgR1 is not necessary for MAG elicited neurite outgrowth inhibition (Chivatakarn et al., 2007; Venkatesh et al., 2007).
7.2. Impact of inhibitor presentation – Chronic versus acute exposure
One of the most stringent and direct tests to examine to what extent any binding partner or receptor candidate contributes to neuronal growth inhibition is the use of primary neurons isolated from mice null for the binding partner(s) under investigation. Two independent NgR1 null mouse lines have been generated (Kim et al., 2004; Zheng et al., 2005) and primary neurons from these mice were used to examine the importance of neuronal NgR1 for growth inhibitory responses toward crude CNS myelin or individual inhibitors in vitro. In one set of experiments DRG neurons isolated from two-week old NgR1 wild-type pups were shown to exhibit a mild but significant (∼20%) increase in growth cone collapse following acute application of soluble Nogo-66, OMgp, and MAG to the culture medium. A similar increase in growth cone collapse was not observed with DRG neurons from age matched NgR1 mutant pups treated under the same conditions (Kim et al., 2004). It thus came as a surprise when subsequent studies reported that CNS myelin, Nogo-66, and MAG inhibition of CGNs, RGCs, or DRG neurons is NgR1-independent (Zheng et al., 2005; Venkatesh et al., 2007). At face value, these studies seemed to come to opposite conclusions, but upon closer examination it became clear that there were important differences in the way the studies were performed. While Kim et al. (2004) studied the modest growth cone collapsing activity of NgR1 ligands when applied acutely to DRG neurons, Zheng et al. (2005) and Venkatesh et al. (2007) examined the neurite growth-inhibitory activity of Nogo-66, MAG, and crude CNS myelin when presented as substrates or in membrane bound form to postnatal CGNs, RGCs, and DRG neurons in chronic culture experiments. These experiments suggest that NgR1 is necessary for growth cone collapse if inhibitors are presented acutely (Kim et al., 2004) but not for neurite outgrowth inhibition on substrate bound inhibitors (Zheng et al., 2005; Venkatesh et al., 2007).
To independently show that NgR1 is important for DRG neuron growth cone collapse in response to acutely presented inhibitor, the experiments by Kim et al. (2004) were repeated with an independently generated NgR1 null mouse (Zheng et al., 2005; Chivatakarn et al., 2007). Furthermore, because NgR1 has been proposed to represent a convergence point for multiple inhibitors, NgR1 null DRG neurons were also examined for OMgp responsiveness. Similar to substrate bound Nogo-66 (Zheng et al., 2005) and membrane bound MAG (Venkatesh et al., 2007), neurite outgrowth inhibition on substrate bound OMgp is NgR1 independent (Chivatakarn et al., 2007). Consistent with Kim and colleagues it was found that growth cone collapse of postnatal-day 21 DRG neurons in response to acutely applied OMgp or MAG is attenuated in NgR1 mutants compared to age-matched wild-type controls (Chivatakarn et al., 2007). There was a ∼20% decrease in collapsed growth cones in NgR1 mutants compared to NgR1 wild-type DRG cultures treated with ligand. Consistent with this observation, only a subpopulation of adult DRG neurons is expressing NgR1 (Hunt et al., 2002). Because germline ablation of NgR1 may lead to compensatory changes in gene expression, an RNAi-based approach was used to independently confirm the results with NgR1 null neurons. Similar to experiments with NgR1 null neurons, RNAi knock-down of NgR1 does not result in enhanced neurite outgrowth on membrane bound MAG (Chivatakarn et al., 2007). Collectively, these results provide unexpected evidence that the growth cone collapsing activities and substrate growth-inhibitory activities of inhibitory ligands can be dissociated. These results also demonstrate that chronic axon growth inhibition by myelin is mediated by NgR1-independent mechanisms.
Our results demonstrate that NgR1 is not necessary for neurite outgrowth inhibition of substrate bound inhibitor and imply that some other receptor(s) must exist that can bind Nogo-66, MAG and OMgp to mediate the actions of these factors. If so, how does NgR1 fit in with this alternate receptor(s)? A first possibility is that NgR1 pairs with this receptor(s) to increase its affinity for the inhibitory ligands. In this model, loss of NgR1 would reduce the sensitivity of the receptor, which could lead to loss of collapse but not substrate inhibition if the concentration of each ligand was limiting in the collapse assay but saturating in the substrate assay. Arguing strongly against this possibility, however, is the finding from a substrate dilution series that the degree of inhibition by different concentrations of MAG is the same for wild-type and NgR1 knockout neurons (Chivatakarn et al., 2007). Instead, these results appear to imply that different mechanisms are required for collapse and for growth inhibition.
Based on these recent findings, the originally proposed model in which Nogo, MAG, and OMgp all converge on neuronal NgR1 to exert their axon growth inhibitory action is most likely an oversimplification of the mechanisms employed by these inhibitors and implies the existence of additional receptor(s).
7.3. NgR2
In addition to NgR1, a close homologue, NgR2, associates with MAG directly (Venkatesh et al., 2005; Lauren et al., 2007). Ectopic expression of NgR2 in neonatal DRG neurons is sufficient to confer MAG inhibition and in P7 CGNs ectopic NgR2 augments MAG inhibition of neurite outgrowth (Venkatesh et al., 2005). NgR2 binds MAG-Fc with an approximately five-fold greater affinity than NgR1, however, NgR2 does not support binding of Nogo-66 or OMgp (Venkatesh et al., 2005). Furthermore, the previously reported interactions of NgR1 with Lingo-1, p75 and TROY are not shared with NgR2. We found no association of NgR2 with p75, TROY, or Lingo-1 (K. Venkatesh and R. Giger, unpublished observations). With respect to MAG inhibition, an obvious question is to what extent neuronal NgR2 functions as a MAG receptor that contributes to MAG inhibition, either following acute or chronic presentation of MAG. Because NgR1 and NgR2 show similar and largely overlapping expression patterns in the adult CNS, this question has not yet been addressed and awaits the availability of NgR1 and NgR2 double mutant mice.
We identified a truncated soluble form of human NgR2 (called s-NgR2) that is generated by alternative splicing and comprised of the first 171 amino acids of full-length NgR2 followed by 22 amino acids unique to s-NgR2 (Fig. 1). S-NgR2 is expressed in the adult human brain, however, its functional significance is unknown. One possibility is that s-NgR2 competes with full-length NgR2 for specific binding sites, and thus, may have NgR2 antagonistic function. While s-NgR2 is the only splice variant we identified in the NgR family (our unpublished observation), soluble forms of NgR1 and NgR2, generated by metalloproteinase-dependent ectodomain shedding, have been identified in vitro (Walmsley et al., 2005).
NgR1 and NgR2 are both expressed by many projection neurons in the adult CNS, including pyramidal neurons of the neocortex, principal neurons in the hippocampus, retinal ganglion cells, and subsets of DRG neurons (Hunt et al., 2002; Wang et al., 2002c; Barrette et al., 2007; Funahashi et al., 2008). Postnatal CGNs appear to form an exception, as they express NgR1 but not NgR2 (Venkatesh et al., 2005). When coupled with the observation that CGNs null for NgR1 are strongly inhibited by MAG, this shows that MAG signals growth inhibition in CGNs in an NgR1 and NgR2 independent manner. NgR3 is expressed by CGNs but does not support MAG binding, and thus, is likely not a functional substitute for NgR1. Thus in CGNs, complex brain gangliosides, including GD1a and GT1b appear to be the main MAG binding partners and an important component of the MAG receptor complex (Vyas et al., 2002). The mechanisms of how MAG binding to gangliosides triggers intracellular signaling events, such as activation of RhoA, however, are not well understood. Cell types other than CGNs, including DRG neurons, RGCs, and hippocampal neurons appear to depend much less or not at all on the presence of gangliosides or terminal sialic acids to show a growth inhibitory response to MAG (Mehta et al., 2007; Venkatesh et al., 2007). Therefore, it will be interesting to examine whether and to what extent RGCs and neocortical or hippocampal pyramidal neurons – cells that express very high levels of NgR1 and NgR2 – depend on the presence of NgR1 and NgR2 for MAG inhibition.
7.4. Redundant mechanisms for MAG inhibition
Clues about the existence of redundant mechanisms for MAG inhibition stem from recent experiments in which the association of MAG with multiple neuronal cell surface binding partners was impaired simultaneously (Fig. 2). For example, neither neuraminidase treatment nor genetic ablation of NgR1 is sufficient to attenuate MAG inhibition of RGCs cultured on CHO-MAG feeder layers, however, the combined loss of terminal sialic acids and NgR1 leads to a significant decrease in MAG inhibition (Venkatesh et al., 2007). In a similar vein, combined pharmacological blockade of ganglioside biosynthesis and NgR1 antagonism (with the Nogo peptide NEP1–40), leads to significantly more neuronal growth of hippocampal and DRG neurons cultured on substrate adsorbed MAG than either treatment alone (Mehta et al., 2007). Taken together, these studies strongly argue for the existence of cell type specific mechanisms for MAG inhibition. Furthermore, there appears to be a significant degree of redundancy among different MAG receptor systems, suggesting that they function largely independently (Mehta et al., 2007; Venkatesh et al., 2007), an observation that may have important implications for the development of treatment strategies aimed at overcoming MAG inhibition in vivo.
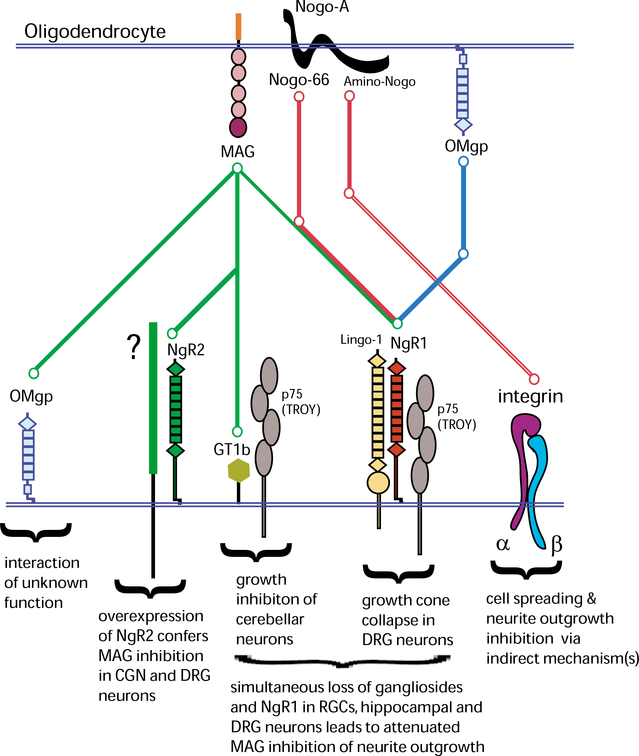
Fig. 2.
Molecular players of neurite outgrowth inhibition in the CNS. “Metro map” of ligand-receptor interactions of the prototypic myelin inhibitors Nogo-A, MAG, and OMgp. The “Green line” depicts the associations of MAG with its receptors, the “Red line” depicts the associations of Nogo-A (Amino-Nogo and Nogo-66) with its receptors, and the “Blue line” depicts the association of OMgp with its receptor. Nogo-66, MAG, and OMgp associate with the NgR1/p75/Lingo-1 complex. MAG also binds to gangliosides (including GT1b), NgR2, and axonal OMgp. NgR2 does not associate with p75, TROY, or Lingo-1 and the signal-transducing component (green box) in the NgR2 complex is not known. Amino-Nogo interacts with neuronal integrins, however, this interaction is thought to be indirect. See main text for more details on the functional significance of these interactions.
7.5. MAG binding sialoglycans: Gangliosides, NgR1 and NgR2
Because MAG binding to NgR1 expressed on the surface of CHO or COS-7 cells is not sensitive to neuraminidase treatment, it was proposed that NgR1 is a sialic acid independent MAG receptor (Domeniconi et al., 2002; Liu et al., 2002). Subsequent studies showed that NgR1 and NgR2 are sialoglycoproteins (Venkatesh et al., 2005; Wen et al., 2005) and when ectopically expressed in neonatal DRG neurons, NgR2 and to a lesser extent NgR1, associates with MAG-Fc in a neuraminidase sensitive manner (Venkatesh et al., 2005). Differences observed in MAG binding to NgR1 and NgR2 expressed in primary neurons (neuraminidase sensitive) and cell lines (neuraminidase insensitive) may be a reflection of different glycosylation patterns of Nogo receptors in different cell types. When compared to mock transfected DRG neurons, desialylated neurons transfected with NgR1 and NgR2 still show greater binding of MAG-Fc. This suggests that in primary neurons NgR1 and NgR2 harbor sialic acid dependent, as well as sialic acid independent MAG binding sites (Venkatesh et al., 2005). Because NgR1 and NgR2 are primarily expressed by neurons, it has been proposed that MAG engages in a multivalent recognition complex with axonal NgR1 or NgR2. For high affinity MAG binding to occur, the presence of terminal sialic acids associated with NgR1 or NgR2 are necessary. Consistent with this model, in the absence of sialic acids MAG still binds to neuronal NgR1 and NgR2, though with greatly reduced affinity (Venkatesh et al., 2005). NgR1 and NgR2 endogenously expressed in postnatal rat brain undergo a neuraminidase dependent molecular weight shift, indicating that NgR1 and NgR2 are sialoglycopoteins (Venkatesh et al., 2005). Direct binding of MAG to sialoglycans covalently linked to NgR1 and NgR2 appears likely, however, more indirect mechanisms such as binding to a NgR1- or NgR2- ganglioside complex can formally not be ruled out.
7.6. Lingo-1, p75 and TROY
Because the neuronal cell surface binding partners for MAG – gangliosides, NgR1 and NgR2 – are lipid linked to the plasma membrane, an important question concerns the signal transduction mechanism of MAG upon binding to the neuronal cell surface. Yamashita and colleagues reported that DRG neurons and CGNs deficient for the low affinity neurotrophin receptor p75 are no longer inhibited by MAG (Yamashita et al., 2002). p75 does not directly associate with MAG and a complex between p75 and GT1b has been proposed to signal MAG inhibition (Yamashita et al., 2002). Subsequent studies found an interaction between p75 and NgR1 that signals growth inhibition in the presence of different myelin inhibitors (Wang et al., 2002a; Wong et al., 2002). Upon MAG binding to the neuronal cell surface, p75 undergoes α- and γ-secretase-dependent proteolytic cleavage, and processing of p75 is important for RhoA activation and subsequent inhibition of neurite outgrowth (Domeniconi et al., 2005). In addition to NgR1 and p75, Lingo-1/Lern-1, a nervous system specific type-I membrane protein (Carim-Todd et al., 2003), is an essential component of the NgR1 receptor complex in vitro (Mi et al., 2004). Similar to MAG, neurite outgrowth inhibition by Nogo-66 and OMgp was proposed to be mediated by a tripartite NgR1/Lingo-1/p75 receptor complex (He and Koprivica, 2004). Analysis of Lingo-1 and NgR1 expression revealed overlapping, yet distinct neural distribution patterns (Barrette et al., 2007; Llorens et al., 2008). For example, Lingo-1, but not NgR1 is robustly expressed in immature oligodendrocytes. In the mature CNS, Lingo-1 and NgR1 show largely overlapping neuronal expression patterns in the neocortex, hippocampus, and several thalamic structures. Consistent with the idea that Lingo-1 and NgR1 are part of the same receptor complex, Lingo-1 and NgR1 were found to interact in P7 mouse brain extracts (Llorens et al., 2008). Lingo-1 has a short cytoplasmic domain that was found to be necessary for neuronal growth inhibition in the presence of CNS myelin (Mi et al., 2004). The cytoplasmic portion of Lingo-1 associates with the zinc finger protein Myt1l, the functional significance of the interaction, however, has not yet been addressed (Llorens et al., 2008).
In vitro studies revealed that NgR1 and p75 function can be dissociated with respect to neurite outgrowth inhibition. In DRG neurons, p75 is important for neurite outgrowth inhibition on substrate bound CNS myelin, Nogo-66, and membrane bound MAG, while NgR1 function is not necessary (Zheng et al., 2005; Venkatesh et al., 2007). In marked contrast to DRG neurons, neurite outgrowth inhibition of CGNs on substrate bound CNS myelin or membrane bound MAG neither depend on p75 nor NgR1 (Zheng et al., 2005; Venkatesh et al., 2007). Thus, similar to experiments with neuraminidase or ganglioside synthesis inhibitors (Mehta et al., 2007; Venkatesh et al., 2007), studies using p75 mutant neurons argue for the existence of neuronal cell type specific mechanisms for growth inhibition.
Many CNS neuron types that are inhibited by myelin, such as retinal ganglion cells, do not express p75 in the adult CNS (Roux and Barker, 2002). Furthermore, although neurons isolated from p75 mutant mice are less inhibited by myelin inhibitors in vitro, a significant inhibitory activity remains (Wang et al., 2002a), arguing for the existence of p75-independent mechanisms for the myelin inhibitors MAG, Nogo-66, and OMgp. Because p75 is a member of the tumor necrosis factor (TNF) receptor superfamily (TNFRSF), a class of type I transmembrane proteins which contain one or more cysteine-rich domains (CRDs), other members of this family may serve as functional substitutes for p75. In a screen for neural TNFRSF members that bind to NgR1, TROY was identified. NgR1 interacts with TROY eight-fold stronger than with p75, and functional studies showed that ectopic expression of dominant negative TROY (lacking the cytoplasmic portion) in DRG neurons attenuates Nogo66 inhibition (Park et al., 2005). Moreover, DRG neurons and CGNs isolated from mice null for TROY are significantly more resistant to Nogo-66, OMgp and CNS myelin inhibition of neurite outgrowth in vitro (Shao et al., 2005).
Because p75 is absent from most RGCs (Park et al., 2005), postnatal RGCs from TROY mutant mice were assayed for MAG inhibition to ask whether TROY is a functional substitute in neurons that normally do not express p75. We found that similar to TROY wild-type RGCs, TROY null RGCs are strongly inhibited by MAG. Likewise, CGNs from TROY mutant and wild-type mice are strongly inhibited by membrane bound MAG in vitro (Venkatesh et al., 2007). These experiments indicate that TROY is not necessary for MAG inhibition of RGCs and CGNs. While it has been reported that TROY mRNA is expressed in DRG neurons and Purkinje neurons of the cerebellum (Park et al., 2005; Shao et al., 2005), and in pyramidal neurons of the cerebral cortex (Shao et al., 2005), these findings were not confirmed by two subsequent studies. In situ hybridization for TROY mRNA and immunolabeling with two different sera specific for TROY protein failed to detect TROY expression in neurons (Hisaoka et al., 2006). The lack of neuronal TROY expression in the mature CNS was recently confirmed by an independent study (Barrette et al., 2007). While additional studies are needed to define more conclusively the contribution of TROY in CNS myelin elicited growth inhibition, the studies thus far argue for the existence of p75 and TROY independent mechanisms for Nogo-66, MAG, and OMgp to bring about neurite outgrowth inhibition.
7.7. Integrins
Nogo-A is comprised of multiple inhibitory domains. The Nogo-66 loop together with adjacent sequences (Nogo-A-24) forms a high affinity complex with NgR1 (Hu et al., 2005) and more N-terminal sequences (residues 181–864, the X-fragment of Amino-Nogo) inhibit neurite outgrowth and fibroblast spreading in an NgR1 independent manner (Fournier et al., 2001; Oertle et al., 2003). A first mechanism for the X-fragment of Nogo-A has recently been identified – cell adhesion and axonal outgrowth by the X-fragment is inhibited in an integrin specific manner (Hu and Strittmatter, 2008). Integrins are a large family of heterodimeric cell-surface receptors for extracellular matrix (ECM) proteins including laminin, collagen, and fibronectin. Integrin receptors are expressed in all cell types, including neurons, functioning as both cell adhesion and signaling molecules. Integrins mediate a vast array of biological effects, ranging from proliferation and survival to cell migration, angiogenesis, axon guidance, and synaptic function (Pasterkamp et al., 2003; Nikolopoulos and Giancotti, 2005; Huang et al., 2006). Integrin function is regulated both by extracellular (outside-in) and intracellular (inside-out) signaling. Canonical axon guidance molecules regulate integrin function (Condic and Letourneau, 1997) and integrin signaling has also been implicated in nervous system regeneration (Lemons and Condic, 2008). Indirect evidence for a role of integrins in axonal regeneration stems from the observation that following conditioning lesions (axotomy of DRG peripheral axons), expression of the integrin subunits α5 and α7 is upregulated (Gardiner et al., 2005; Gardiner et al., 2007). Conversely, sensory and motor neurons lacking functional α7 show impaired peripheral regeneration (Ekstrom et al., 2003; Gardiner et al., 2005). Also, more recent work shows that fibrinogen, a novel inhibitor of regeneration, inhibits neurite outgrowth via β-integrin-mediated phosphorylation of the EGF receptor (Schachtrup et al., 2007).
Based on the observation that the X-fragment of Amino-Nogo attenuates spreading of non-neuronal cells on different ECM components in a cell type specific manner, Hu and Strittmatter examined whether the X-fragment of Amino-Nogo blocks specific integrin signaling pathways (Hu and Strittmatter, 2008). Interestingly, activation of integrins partially overcomes Amino-Nogo inhibition in non-neuronal cells and primary neurons. Furthermore, biochemical studies revealed that in brain Nogo-A indirectly interacts with α5 or αv integrin subunits. Taken together, integrins have been implicated in promotion and inhibition of neurite outgrowth in vitro, as well as the regenerative failure of axon growth in vivo (Lemons and Condic, 2008). Mounting evidence suggests an important role for integrins in CNS myelin inhibition (Laforest et al., 2005; Hu and Strittmatter, 2008) as well as CSPG inhibition of neuronal growth (Zhou and Snider, 2006). Additional work is needed to define more precisely the role and contribution of specific integrin receptors in the regenerative failure of damaged CNS axons. For a schematic summary of the known myelin inhibitor-receptor interactions, see Fig. 2.
7.8. In vivo regeneration studies
In vitro neurite outgrowth assays are a powerful approach to study growth inhibitory activities and elucidate their mechanisms of inhibition. A long-term goal is to evaluate the significance and relative contribution of different growth inhibitory mechanisms to the regenerative failure in vivo. Antibody-based strategies directed against myelin inhibitory constituents have been successful in achieving anatomical and functional regeneration following CNS injury (Schnell and Schwab, 1990; Bregman et al., 1995; Huang et al., 1999; Ellezam et al., 2003; Sicotte et al., 2003). In addition, positive behavioral outcomes have been reported by antagonism of NgR1 with peptide inhibitors of Nogo (NEP1–40) (GrandPre et al., 2002; Li and Strittmatter, 2003) or following application of soluble NgR1 ligand binding domain [NgR(310)-Fc] (Li et al., 2004; Wang et al., 2006). As discussed below, some of these pharmacological studies lead to robust regenerative phenotypes following SCI and stand in contrast to the more modest phenotypes reported for spinal cord injured mice following germline ablation of individual inhibitors or receptor components.
7.9. Antibody and peptide-based strategies
Following SCI, treatment with anti-Nogo-A antibody leads to long-distance regenerative growth of severed CST axons into the distal spinal cord (Schwab et al., 1993). In addition to longitudinal growth, enhanced collateral axonal sprouting of injured and spared axons is observed, both above and below the injury site (Thallmair et al., 1998). Over the past years, several different fiber systems have been analyzed and reported to show enhanced neuronal growth and sprouting following injury if treated with anti-Nogo-A. Studies focusing on CST and serotonergic fiber regeneration were most successful (Gonzenbach and Schwab, 2008). Other fiber systems, such as ascending sensory fibers in rat spinal cord do not exhibit enhanced regenerative growth in the presence of anti-Nogo-A (Oudega et al., 2000). This suggests that Nogo-A is an important inhibitor of growth in vivo, however, the contribution of Nogo-A to the regenerative failure of different CNS fiber systems may be variable.
Soluble NgR(310)-Fc binds and neutralizes Nogo-66, MAG, and OMgp in vitro and has been used successfully to promote anatomical fiber regeneration and behavioural improvement following spinal cord injury in vivo. In one study, purified NgR(310)-Fc protein was delivered intrathecally after midthoracic dorsal over-hemisection (Li et al., 2004). Axonal sprouting of CST and raphespinal fibers was observed in NgR(310)-Fc-treated animals and found to correlate with improved spinal cord electrical conduction and improved locomotion (Li et al., 2004). MacDermid and colleagues reported that intrathecal application of NgR(310)-Fc results in differential growth effects on distinct populations of spinally projecting axons (MacDermid et al., 2004). Specifically it was found that myelin antagonism with NgR1(310)-Fc differentially affects plasticity of uninjured primary afferent and descending monoaminergic projections in the rat spinal cord following dorsal rhizotomy (MacDermid et al., 2004). Furthermore, it was found that delayed therapy with NgR(310)-Fc following SCI is as efficacious as acute therapy in a contusion injury model (Wang et al., 2006).
Some of the most robust regenerative axonal growth was reported for spinal cord injured animals treated with the NgR1 antagonistic peptide NEP1–40 (GrandPre et al., 2002). Following dorsal over-hemisection at T8 in mice, NEP1–40 was administered immediately after injury (early treatment) or after 7 days (delayed treatment). It was found that NEP1–40 results in enhanced growth of CST and serotonergic axons and enhanced hindlimb locomotor recovery, both when delivered early or delayed (GrandPre et al., 2002). However, caution needs to be exercised with the interpretation of the NEP1–40 efficacy, as a more recent study by Steward and colleagues failed to independently replicate the robustness of the NEP1–40 effects (Steward et al., 2008). Using identical procedures as GrandPre and colleagues, and focusing strictly on the delayed subcutaneous treatment with NEP1–40, experiments by Steward did not support the interpretation that treatment with NEP1–40 robustly enhances regenerative growth of CST or serotonergic axons, however fiber growth is slightly enhanced in mice treated with NEP1–40. Moreover, assessment of hindlimb motor function did not support the interpretation that treatment with NEP1–40 consistently enhanced recovery of motor function (Steward et al., 2008).
In sum, these in vivo studies show that antibody-based neutralization of Amino-Nogo or antagonism of Nogo-66, MAG and OMgp with NgR(310)-Fc each leads to substantial functional repair in spinal cord injured animals. Based on the observation that Amino-Nogo functions independently of NgR1 it would be interesting to examine whether the positive effects of anti-Nogo-A and NgR(310)-Fc on SCI repair are additive when applied simultaneously.
7.10. Mouse genetic approaches
In contrast to CNS injured animals treated with anti-Nogo-A, germline ablation of Nogo-A (Simonen et al., 2003), Nogo-A and Nogo-B (Kim et al., 2003), or all three Nogo isoforms, Nogo-A, Nogo-B, and Nogo-C (Zheng et al., 2003), revealed either modest or no enhanced regenerative axonal growth of injured CST fibers. Differences in Nogo gene targeting strategies (resulting in the ablation of different isoforms), compensatory upregulation of other Nogo proteins, differences in the genetic background (Dimou et al., 2006), age of injured animals (Kim et al., 2003) and differences in the lesion paradigms used (Cafferty and Strittmatter, 2006) do not allow for a direct comparison of the different Nogo regeneration phenotypes reported by different groups. The consensus from these studies is that Nogo mutant mice show a less impressive CST regenerative phenotype than animals treated with anti-Nogo-A antibody. While the differences in Nogo gene targeting strategies, genetic background, age, and surgical procedures may account for variable regeneration phenotypes in Nogo mutant mice, the authors also note that much greater variability will likely be encountered in any clinical trial assessing SCI repair in humans – making it more challenging to clearly identify treatment strategies that are beneficial.
Similar to studies with Nogo knockout mice, germline ablation of p75 does not lead to improved anatomical or functional outcomes following SCI (Song et al., 2004). In addition, two independent studies showed that germline ablation of NgR1 does not result in a significant axonal regeneration of CST fibers following dorsal hemisection at T6 or complete transection at T8 (Kim et al., 2004) or dorsal hemisection at T8 (Zheng et al., 2005). Kim et al. reported regeneration of some raphespinal and rubrospinal fibers in NgR1 mutants following dorsal hemisection, suggesting that loss of NgR1 differentially affects regenerative growth of different spinal cord fiber populations (Kim et al., 2004). β-galactosidase reporter gene expression analysis confirmed NgR1 promoter activity in neurons that give rise to the CST, however, much less NgR1 promoter activity was detected in raphespinal and rubrospinal tract (RST) fibers compared to CST fibers in spinal cord sections of one month old mice (Fig. 3). In a more recent study, it was reported that pyramidotomy in NgR1 mutants does not lead to long-distance axonal regeneration but induced growth of CST axons into denervated gray matter proximal and distal to the injury site – and a very similar regenerative phenotype was found in Nogo-A/B null mice following pyramidotomy (Cafferty and Strittmatter, 2006). This suggests that loss of NgR1-NogoA/B function in upper motoneurons does not lead to long-distance regenerative axonal growth but increases collateral sprouting of injured and uninjured fibers.
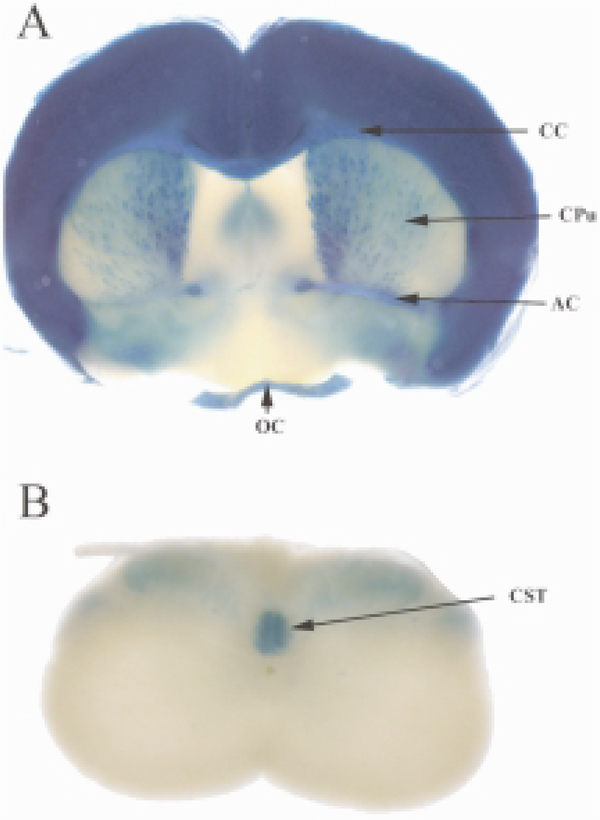
Fig. 3.
NgR1 expression in brain and spinal cord. β-galactosidase reporter gene expression analysis in NgR1tauLacZ mice (Zheng et al., 2005), as assessed by X-gal histochemistry. A. In brain tissue sections of one-month-old mice, strongest β-galactosidase activity is observed in the cerebral cortex, the corpus callosum (CC), anterior commissure (AC), and optic chiasm (OC). Weaker β-galactosidase activity is found in the medial septum and the striatum/caudate putamen (CPu). B. In the spinal cord of one-month-old mice, β-galactosidase activity is most robust in the corticospinal tract (CST). Weaker labeling is detected in the dorso-lateral spinal while matter including presumptive fiber tracts such as the rubrospinal or raphespinal tract. Also weak labeling is detected in the dorsal gray matter of the spinal cord, and may arise from DRG neuron afferent projections. No reporter gene expression was detected in motoneuron pools or the ventral gray and white matter of the spinal cord. This suggests that robust NgR1 expression in the spinal cord is restricted to a small number of fiber tracts.
8. Conclusions and future directions
A long standing goal of spinal cord injury research is to develop tools and strategies that lead to robust, directed, and long-distance regenerative growth of injured CNS axons in order to restore neuronal connectivity lost as a consequence of injury. A prerequisite for the design of specific intervention strategies is a solid knowledge basis of the molecular players of axonal growth inhibition and a detailed understanding of their mechanisms of action, including neuronal cell surface receptors and down-stream signaling cascades. In addition, the basic biology and physiological function of ligand-receptor systems that limit growth and regeneration needs to be understood in some detail. This will indicate what type of side effects may potentially occur following their perturbation in an attempt to promote regenerative axonal growth and may have important implications for how a potential drug(s) will be applied.
Over the past years enormous progress has been made in identifying growth inhibitors present in the mature CNS and their mechanisms of action are now rapidly beginning to be defined. Emerging evidence points to multiple and cell type specific mechanisms for growth inhibition. Furthermore, individual inhibitors may employ distinct and at least partially redundant mechanisms. Nogo receptors bind myelin inhibitors and have been implicated in aspects of neuronal growth inhibition, however, the concept of a tripartite NgR1/p75/Lingo-1 receptor system that serves as a convergence point for multiple CNS myelin inhibitors is an oversimplification of the complex mechanisms of CNS myelin elicited growth inhibition.
Mechanistic findings based on studies carried out in vitro need to be confirmed in vivo and also scrutinized for their importance in regenerative failure of axonal growth in vivo. Regeneration studies in animal models will benefit from genetic tools enabling the visualization of specific fiber tracts. “Genetic tracing” of fiber tracts instead of tracer injections will reduce the risk of potential artifacts associated with this technique (Cafferty et al., 2007; Steward et al., 2007). Specifically, it would be advantageous to have mice in which specific fiber tracts such as the CST or RST are genetically labeled with distinct reporters. Also, the technology for the generation of transgenic animals other than mice is available. The rat, a commonly used model for SCI research, is more accessible surgically than the mouse and genetic tracing of the CST and other fiber tracts would help to standardize how fiber regeneration is assessed. If combined with the identification of recent biomarkers specific for regenerating fibers (Bonilla et al., 2002), this would allow for an unambiguous identification of “truly” regenerating axons. Fluorescent tagging of specific fibers tracts can be combined with in vivo imaging of regenerating axons to observe regenerative axonal growth over time in live animals (Kerschensteiner et al., 2005). This technique would be particularly helpful to test different treatment strategies as well as combination therapies in animal models of SCI over a prolonged time range. When combined with recent advances at the molecular level of axon growth inhibition, new imaging tools to observe regenerating axons in vivo will greatly accelerate progress in this rapidly evolving and fascinating field of research.
Acknowledgments
We thank Marc Tessier-Lavigne for the NgR1taulacZ mice. Current support, the New York State Spinal Cord Injury Research Program (R.J.G. and C.R.), National Research Service Award Ruth Kirschstein Fellowship F31NS056558 (O.C.) and F31NS07489 (K.V.), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation’s Adelson Program in Neural Repair and Rehabilitation (APNRR), National Institute of Neurological Disorders and Stroke NS047333 (R.J.G.).
References
- Barrette B, Vallieres N, Dube M, & Lacroix S (2007). Expression profile of receptors for myelin-associated inhibitors of axonal regeneration in the intact and injured mouse central nervous system. Mol Cell Neurosci, 34, 519–538. [DOI] [PubMed] [Google Scholar]
- Barton WA, Liu BP, Tzvetkova D, Jeffrey PD, Fournier AE, Sah D, et al. (2003). Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J, 22, 3291–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, et al. (1995). Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron, 15, 1375–1381. [DOI] [PubMed] [Google Scholar]
- Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, & Parada LF (2005). Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci USA, 102, 10694–10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla IE, Tanabe K,& Strittmatter SM(2002). Small prolinerich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci, 22, 1303–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LDF, Popat RJ, King VR, Bennett GS, Patel PN, et al. (2002). Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature, 416, 636–640. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, & Schwab ME (1995). Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature, 378, 498–501. [DOI] [PubMed] [Google Scholar]
- Brosamle C, Huber AB, Fiedler M, Skerra A, & Schwab ME (2000). Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 anatibody fragment. J Neurosci, 20, 8061–8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A, Pech K, Merkler D, Kakulas BA, Martin D, Schoenen J, et al. (2005). Sequential loss of myelin proteins during Wallerian degeneration in the human spinal cord. Brain, 128, 356–364. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, & Strittmatter SM (2006). The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci, 26, 12242–12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Kim JE, Lee JK, & Strittmatter SM (2007). Response to correspondence: Kim et al., “axon regeneration in young adult mice lacking Nogo-A/B.” Neuron, 38, 187–199. Neuron, 54, 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, & Filbin MT (2001). Neuronal Cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci, 21, 4731–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Qiu J, Domeniconi M, Hou J, Bryson JB, Mellado W, et al. (2007). The inhibition site on myelin-associated glycoprotein is within Ig-domain 5 and is distinct from the sialic acid binding site. J Neurosci, 27, 9146–9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carim-Todd L, Escarceller M, Estivill X, & Sumoy L (2003). LRRN6A/LERN1 (leucine-rich repeat neuronal protein 1), a novel gene with enriched expression in limbic system and neocortex. Eur J Neurosci, 18, 3167–3182. [DOI] [PubMed] [Google Scholar]
- Caroni P,& Schwab ME(1988). Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol, 106, 1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, et al. (2000). Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature, 403, 434–439. [DOI] [PubMed] [Google Scholar]
- Chivatakarn O, Kaneko S, He Z, Tessier-Lavigne M, & Giger RJ (2007). The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J Neurosci, 27, 7117–7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BE, Yang LJ, Mukhopadhyay G, Filbin MT, Kiso M, Hasegawa A, et al. (1997). Sialic acid specificity of myelin-associated glycoprotein binding. J Biol Chem, 272, 1248–1255. [DOI] [PubMed] [Google Scholar]
- Condic ML, & Letourneau PC (1997). Ligand-induced changes in integrin expression regulate neuronal adhesion and neurite outgrowth. Nature, 389, 852–856. [DOI] [PubMed] [Google Scholar]
- Crocker PR, & Varki A (2001). Siglecs, sialic acids and innate immunity. Trends Immunol, 22, 337–342. [DOI] [PubMed] [Google Scholar]
- Davies SJA, Goucher DR, Doller C, & Silver J (1999). Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci, 19, 5810–5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBellard ME, & Filbin MT (1999). Myelin-associated glycoprotein MAG, selectively binds several neuronal proteins. J Neurosci Res, 56, 213–218. [DOI] [PubMed] [Google Scholar]
- DeBellard ME, Tang S, Mukhopadhyay G, Shen YJ, & Filbin MT (1996). Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol Cell Neurosci, 7, 89–101. [DOI] [PubMed] [Google Scholar]
- Dimou L, Schnell L, Montani L, Duncan C, Simonen M, Schneider R, et al. (2006). Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J Neurosci, 26, 5591–5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Zampieri N, Spencer T, Hilaire M, Mellado W, Chao MV, et al. (2005). MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron, 46, 849–855. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, et al. (2002). Myelin-associated glycoprotein interacts with the Nogo 66 receptor to inhibit neurite outgrowth. Neuron, 35, 283–290. [DOI] [PubMed] [Google Scholar]
- Du J, Tran T, Fu C, & Sretavan DW (2007). Upregulation of EphB2 and ephrin-B2 at the optic nerve head of DBA/2J glaucomatous mice coincides with axon loss. Invest Ophthalmol Vis Sci, 48, 5567–5581. [DOI] [PubMed] [Google Scholar]
- Ekstrom PA, Mayer U, Panjwani A, Pountney D, Pizzey J, & Tonge DA (2003). Involvement of alpha7beta1 integrin in the conditioning-lesion effect on sensory axon regeneration. Mol Cell Neurosci, 22, 383–395. [DOI] [PubMed] [Google Scholar]
- Ellezam B, Bertrand J, Dergham P, & McKerracher L (2003). Vaccination stimulates retinal ganglion cell regeneration in the adult optic nerve. Neurobiol Dis, 12, 1–10. [DOI] [PubMed] [Google Scholar]
- Erb M, Flueck B, Kern F, Erne B, Steck AJ, & Schaeren Wiemers N (2006). Unraveling the differential expression of the two isoforms of myelin-associated glycoprotein in a mouse expressing GFP-tagged S-MAG specifically regulated and targeted into the different myelin compartments. Mol Cell Neurosci, 31, 613–627. [DOI] [PubMed] [Google Scholar]
- Fahrig T, Landa C, Pesheva P, Kuhn K, & Schachner M (1987). Characterization of binding properties of the myelin-associated glycoprotein to extracellular matrix constituents. EMBO J, 6, 2875–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT (2003). Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci, 4, 703–713. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, & Strittmatter SM (2001). Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature, 409, 341–346. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Gould GC, Liu BP, & Strittmatter SM (2002). Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci, 22, 8876–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen R, Tanner SL, Dashiell SM, Rottkamp CA, Hammer JA, & Quarles RH (2001). Microtubule-associated protein 1B: a neuronal binding partner for myelin-associated glycoprotein. J Cell Biol, 155, 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani M, Kawai H, Proia RL, Kashiwagi A, Yasuda H, & Yamashita T (2005). Binding of soluble myelin-associated glycoprotein to specific gangliosides induces the association of p75NTR to lipid rafts and signal transduction. J Neurochem, 94, 15–21. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Hasegawa T, Nagano A, & Sato K (2008). Differential expression patterns of messenger RNAs encoding nogo receptors and their ligands in the rat central nervous system. J Comp Neurol, 506, 141–160. [DOI] [PubMed] [Google Scholar]
- Gardiner NJ, Fernyhough P, Tomlinson DR, Mayer U, von der Mark H, & Streuli CH (2005). Alpha7 integrin mediates neurite outgrowth of distinct populations of adult sensory neurons. Mol Cell Neurosci, 28, 229–240. [DOI] [PubMed] [Google Scholar]
- Gardiner NJ, Moffatt S, Fernyhough P, Humphries MJ, Streuli CH, & Tomlinson DR (2007). Preconditioning injury-induced neurite outgrowth of adult rat sensory neurons on fibronectin is mediated by mobilisation of axonal alpha5 integrin. Mol Cell Neurosci, 35, 249–260. [DOI] [PubMed] [Google Scholar]
- George R, & Griffin JW (1994). Delayed macrophage responses and myelin clearance during Wallerian degeneration in the central nervous system: the dorsal radiculotomy model. Exp Neurol, 129, 225–236. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Klassen MP, Hua Y, & Barres BA (2002). Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science, 296, 1860–1864. [DOI] [PubMed] [Google Scholar]
- Gonzenbach RR,&Schwab ME(2008). Disinhibition of neurite growth to repair the injured adult CNS: Focusing on Nogo. Cell Mol Life Sci, 65, 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Li S, & Strittmatter SM (2002). Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature, 417, 547–551. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, & Strittmatter SM (2000). Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature, 403, 439–444. [DOI] [PubMed] [Google Scholar]
- Habib AA, Marton LS, Allwardt B, Gulcher JR, Mikol DD, Hognason T, et al. (1998). Expression of the oligodendrocyte-myelin glycoprotein by neurons in the mouse central nervous system. J Neurochem, 70, 1704–1711. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Fujitani M, Hata K, Tohyama M, Yamagishi S, & Yamashita T (2004). Promotion of axon regeneration by myelin-associated glycoprotein and Nogo through divergent signals downstream of Gi/G. J Neurosci, 24, 6826–6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XL, Bazan JF, McDermott G, Park JB, Wang K, Tessier-Lavigne M, et al. (2003). Structure of the Nogo receptor ectodomain: a recognition module implicated in myelin inhibition. Neuron, 38, 177–185. [DOI] [PubMed] [Google Scholar]
- He Z, & Koprivica V (2004). The nogo signaling pathway for regeneration block. Annu Rev Neurosci, 27, 341–368. [DOI] [PubMed] [Google Scholar]
- Hisaoka T, Morikawa Y, & Senba E (2006). Characterization of TROY/TNFRSF19/TAJ-expressing cells in the adult mouse forebrain. Brain Res, 1110, 81–94. [DOI] [PubMed] [Google Scholar]
- Hu F,, & Strittmatter SM. (2008). The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J Neurosci, 28, 1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Liu BP, Budel S, Liao J, Chin J, Fournier A, & Strittmatter SM (2005). Nogo-A interacts with the Nogo-66 receptor through multiple sites to create an isoform-selective subnanomolar agonist. J Neurosci, 25, 5298–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, McKerracher L, Braun PE, & David S (1999). A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron, 24, 639–647. [DOI] [PubMed] [Google Scholar]
- Huang JK, Phillips GR, Roth AD, Pedraza L, Shan W, Belkaid W, et al. (2005). Glial membranes at the node of Ranvier prevent neurite outgrowth. Science, 310, 1813–1817. [DOI] [PubMed] [Google Scholar]
- Huang Z, Shimazu K, Woo NH, Zang K, Muller U, Lu B, et al. (2006). Distinct roles of the beta 1-class integrins at the developing and the mature hippocampal excitatory synapse. J Neurosci, 26, 11208–11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD,, & Cloutier JF (2003). Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci, 26, 509–563. [DOI] [PubMed] [Google Scholar]
- Huber AB, Weinmann O, Brosamle C, Oertle T, & Schwab ME (2002). Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci, 22, 3553–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D, Mason MR, Campbell G, Coffin R, & Anderson PN (2002). Nogo receptor mRNA expression in intact and regenerating CNS neurons. Mol Cell Neurosci, 20, 537–552. [DOI] [PubMed] [Google Scholar]
- Johnson PW, Abramow-Newerly W, Seilheimer B, Sadoul R, Tropak MB, Arquint M, et al. (1989). Recombinant myelin-associated glycoprotein confers neural adhesion and neurite outgrowth function. Neuron, 3, 377–385. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, et al. (2006). A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med, 12, 1380–1389. [DOI] [PubMed] [Google Scholar]
- Kelm S, Pelz A, Schauer R, Filbin MT, Tang S, de Bellard ME, et al. (1994). Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr Biol, 4, 965–972. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, & Misgeld T (2005). In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med, 11, 572–577. [DOI] [PubMed] [Google Scholar]
- Kim JE, Liu BP, Park JH, & Strittmatter SM (2004). Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron, 44, 439–451. [DOI] [PubMed] [Google Scholar]
- Kim JE, Li S, GrandPre T, Qiu D, & Strittmatter SM (2003). Axon regeneration in young adult mice lacking Nogo-A/B. Neuron, 38, 187–199. [DOI] [PubMed] [Google Scholar]
- Kottis V, Thibault P, Mikol D, Xiao Z-C, Zhang R, Dergham P, et al. (2002). Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J Neurochem, 82, 1566. [DOI] [PubMed] [Google Scholar]
- Laforest S, Milanini J, Parat F, Thimonier J, & Lehmann M (2005). Evidences that beta1 integrin and Rac1 are involved in the overriding effect of laminin on myelin-associated glycoprotein inhibitory activity on neuronal cells. Mol Cell Neurosci, 30, 418–428. [DOI] [PubMed] [Google Scholar]
- Lauren J, Hu F, Chin J, Liao J, Airaksinen MS, & Strittmatter SM (2007). Characterization of myelin ligand complexes with neuronal Nogo-66 receptor family members. J Biol Chem, 282, 5715–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemons ML, & Condic ML (2008). Integrin signaling is integral to regeneration. Exp Neurol, 209, 343–352. [DOI] [PubMed] [Google Scholar]
- Li C, Tropak MB, Gerlai R, Clapoff S, Abramow-Newerly W, Trapp B, et al. (1994). Myelination in the absence of myelin-associated glycoprotein. Nature, 369, 747–750. [DOI] [PubMed] [Google Scholar]
- Li S, & Strittmatter SM (2003). Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci, 23, 4219–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu BP, Budel S, Li M, Ji B, Walus L, et al. (2004). Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci, 24, 10511–10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, et al. (2005). Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol, 58, 706–719. [DOI] [PubMed] [Google Scholar]
- Liu BP, Fournier AE, GrandPre T, & Strittmatter SM (2002). Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science, 297, 1190–1193. [DOI] [PubMed] [Google Scholar]
- Liu BP, Cafferty WB, Budel SO, & Strittmatter SM (2006). Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci, 361, 1593–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens F, Gil V, Iraola S, Carim-Todd L, Marti E, Estivill X, et al. (2008). Developmental analysis of Lingo-1/Lern1 protein expression in the mouse brain: Interaction of its intracellular domain with Myt1l. Dev Neurobiol, 68, 521–541 [DOI] [PubMed] [Google Scholar]
- Loers G, Aboul-Enein F, Bartsch U, Lassmann H, & Schachner M (2004). Comparison of myelin, axon, lipid, and immunopathology in the central nervous system of differentially myelin-compromised mutant mice: a morphological and biochemical study. Mol Cell Neurosci, 27, 175–189. [DOI] [PubMed] [Google Scholar]
- Low K, Culbertson M, Bradke F, Tessier-Lavigne M, & Tuszynski MH (2008). Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci, 28, 1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn ER, Perry VH, Brown MC, Rosen H, & Gordon S (1989). Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur J Neurosci, 1, 27–33. [DOI] [PubMed] [Google Scholar]
- MacDermid VE, McPhail LT, Tsang B, Rosenthal A, Davies A, & Ramer MS (2004). A soluble Nogo receptor differentially affects plasticity of spinally projecting axons. Eur J Neurosci, 20, 2567–2579. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Hoke A, & Silver J (1995). Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol, 136, 32–43. [DOI] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, & Braun PE (1994). Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron, 13, 805–811. [DOI] [PubMed] [Google Scholar]
- Mehta NR, Lopez PH, Vyas AA, & Schnaar RL (2007). Gangliosides and Nogo receptors independently mediate myelin-associated glycoprotein inhibition of neurite outgrowth in different nerve cells. J Biol Chem, 282, 27875–27886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, et al. (2004). LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci, 7, 221–228. [DOI] [PubMed] [Google Scholar]
- Miklossy J, & Van der Loos H (1991). The long-distance effects of brain lesions: visualization of myelinated pathways in the human brain using polarizing and fluorescence microscopy. J Neuropathol Exp Neurol, 50, 1–15. [DOI] [PubMed] [Google Scholar]
- Mikol DD, & Stefansson K (1988). A phosphatidylinositollinked peanut agglutinin-binding glycoprotein in central nervous system myelin and on oligodendrocytes.J Cell Biol, 106, 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milward E, Kim KJ, Szklarczyk A, Nguyen T, Melli G, Nayak M, et al. (2008). Cleavage of myelin associated glycoprotein by matrix metalloproteinases. J Neuroimmunol, 193, 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag D, Giese KP, Bartsch U, Martini R, Lang Y, Bluthmann H, et al. (1994). Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron, 13, 229–246. [DOI] [PubMed] [Google Scholar]
- Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, et al. (2003). The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci, 23, 9229–9239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, & Filbin MT (1994). A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron, 13, 757–767. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos SN, & Giancotti FG (2005). Netrin-integrin signaling in epithelial morphogenesis, axon guidance and vascular patterning. Cell Cycle, 4, e131–e135. [PubMed] [Google Scholar]
- Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, et al. (2003). Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci, 23, 5393–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega M, Rosano C., Sadi D, Wood PM, Schwab ME, & Hagg T (2000). Neutralizing antibodies against neurite growth inhibitor NI-35/250 do not promote regeneration of sensory axons in the adult rat spinal cord. Neuroscience, 100, 873–883. [DOI] [PubMed] [Google Scholar]
- Pan B, Fromholt SE, Hess EJ, Crawford TO, Griffin JW, Sheikh KA, et al. (2005). Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: neuropathology and behavioral deficits in single- and double-null mice. Exp Neurol, 195, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Yiu G, Kaneko S, Wang J, Chang J, He XL, et al. (2005). A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron, 45, 345–351. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Peschon JJ, Spriggs MK, & Kolodkin AL (2003). Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature, 424, 398–405. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ, Ruitenberg MJ, Holtmaat AJ, De Wit J, De Winter F, et al. (1999). Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci, 13, 143–166. [DOI] [PubMed] [Google Scholar]
- Prinjha R, Moore SE,Vinson M, Blake S, Morrow R, Christie G, et al. (2000). Inhibitor of neurite outgrowth in humans. Nature, 403, 383–384. [DOI] [PubMed] [Google Scholar]
- Probstmeier R, Fahrig T, Spiess E, & Schachner M (1992). Interactions of the neural cell adhesion molecule and the myelin-associated glycoprotein with collagen type I: involvement in fibrillogenesis. J Cell Biol, 116, 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles RH (1989). Glycoproteins of myelin and myelin-forming cells Neurobiology of glycoconjugates (Margolis RU and Margolis RK, eds). Plenium Publishing Corporation, New York:243–275. [Google Scholar]
- Quarles RH (2002). Myelin sheaths: glycoproteins involved in their formation, maintenance and degeneration. Cell Mol Life Sci, 59, 1851–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles RH (2007). Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem, 100, 1431–1448. [DOI] [PubMed] [Google Scholar]
- Quarles RH, Coleman D, Salzer JL, & Trapp BD (1992). Myelin-associated glycoprotein: Structure-function relationships and involvement in neurological diseases Myelin: Biology and Chemistry Martenson R, ed. CRC Press, Boca Raton:4413–4448. [Google Scholar]
- Roux PP, & Barker PA (2002). Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol, 67, 203–233. [DOI] [PubMed] [Google Scholar]
- Schachtrup C, Lu P, Jones LL, Lee JK, Lu J, Sachs BD, et al. (2007). Fibrinogen inhibits neurite outgrowth via beta 3 integrin-mediated phosphorylation of the EGF receptor. Proc Natl Acad Sci USA, 104, 11814–11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Fruttiger M, Montag D, Schachner M, & Martini R (1996). Disruption of the gene for the myelin-associated glycoprotein improves axonal regrowth along myelin in C57BL/Wlds mice. Neuron, 16, 1107–1113. [DOI] [PubMed] [Google Scholar]
- Schmandke A, & Strittmatter SM (2007). ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist, 13, 454–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, & Schwab ME (1990). Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature, 343, 269–272. [DOI] [PubMed] [Google Scholar]
- Schwab ME (2004). Nogo and axon regeneration. Curr Opin Neurobiol, 14, 118–124. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Kapfhammer JP, & Bandtlow CE (1993). Inhibitors of neurite growth. Annu Rev Neurosci, 16, 565–595. [DOI] [PubMed] [Google Scholar]
- Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, et al. (2005). TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron, 45, 353–359. [DOI] [PubMed] [Google Scholar]
- Shim S, Goh EL, Ge S, Sailor K, Yuan JP, Roderick HL, et al. (2005). XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat Neurosci, 8, 730–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicotte M, Tsatas O, Jeong SY, Cai CQ, He Z, & David S (2003). Immunization with myelin or recombinant Nogo-66/MAG in alum promotes axon regeneration and sprouting after corticospinal tract lesions in the spinal cord. Mol Cell Neurosci, 23, 251–263. [DOI] [PubMed] [Google Scholar]
- Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, et al. (2003). Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron, 38, 201–211. [DOI] [PubMed] [Google Scholar]
- Song HJ, Ming GL, & Poo MM (1997). cAMP-induced switching in turning direction of nerve growth cones. Nature, 388, 275–279. [DOI] [PubMed] [Google Scholar]
- Song XY, Zhong JH, Wang X, & Zhou XF (2004). Suppression of p75NTR does not promote regeneration of injured spinal cord in mice. J Neurosci, 24, 542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillmann AA, Bandtlow CE, Lottspeich F, Keller F, & Schwab ME (1998). Identification and characterization of a bovine neurite growth inhibitor (bNI-220). J Biol Chem, 273, 19283–19293. [DOI] [PubMed] [Google Scholar]
- Steward O, Zheng B, Banos K, & Yee KM (2007). Response to: Kim et al., “axon regeneration in young adult mice lacking Nogo-A/B.” Neuron, 38, 187–199. Neuron, 54, 191–195. [DOI] [PubMed] [Google Scholar]
- Steward O, Sharp K, Yee KM, & Hofstadter M (2008). A re-assessment of the effects of a Nogo-66 receptor antagonist on regenerative growth of axons and locomotor recovery after spinal cord injury in mice. Exp Neurol, 209, 446–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenge K, Schauer R, & Kelm S (1999). Binding partners for the myelin-associated glycoprotein of N2A neuroblastoma cells. FEBS Lett, 444, 59–64. [DOI] [PubMed] [Google Scholar]
- Strenge K, Brossmer R, Ihrig P, Schauer R, & Kelm S (2001). Fibronectin is a binding partner for the myelin-associated glycoprotein (siglec-4a). FEBS Lett, 499, 262–267. [DOI] [PubMed] [Google Scholar]
- Strenge K, Schauer R, Bovin N, Hasegawa A, Ishida H, Kiso M, et al. (1998). Glycan specificity of myelin-associated glycoprotein and sialoadhesin deduced from interactions with synthetic oligosaccharides. Eur J Biochem, 258, 677–685. [DOI] [PubMed] [Google Scholar]
- Tang S, Shen YJ, DeBellard ME, Mukhopadhyay G, Salzer JL, Crocker PR, et al. (1997a). Myelin-associated glycoprotein interacts with neurons via a sialic acid binding site at ARG118 and a distinct neurite inhibition site. J Cell Biol, 138, 1355–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Woodhall RW, Shen YJ, deBellard ME, Saffell JL, Doherty P, et al. (1997b). Soluble myelin-associated glycoprotein (MAG). found in vivo inhibits axonal regeneration. Mol Cell Neurosci, 9, 333–346. [DOI] [PubMed] [Google Scholar]
- Thallmair M, Metz GA, Z’Graggen WJ, Raineteau O, Kartje GL, & Schwab ME (1998). Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat Neurosci, 1, 124–131. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Andrews SB, Cootauco C, & Quarles R (1989). The myelin-associated glycoprotein is enriched in multivesicular bodies and periaxonal membranes of actively myelinating oligodendrocytes. J Cell Biol, 109, 2417–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas ME, & Barres BA (2007). Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci, 30, 153–179. [DOI] [PubMed] [Google Scholar]
- Varki A, & Angata T (2006). Siglecs–the major subfamily of I-type lectins. Glycobiology, 16, 1R–27R. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Sheu SS, & Giger RJ (2007). Molecular dissection of the myelin-associated glycoprotein receptor complex reveals cell type-specific mechanisms for neurite outgrowth inhibition. J Cell Biol, 177, 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, et al. (2005). The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci, 25, 808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson M, van der Merwe AP, Kelm S, May A, Jones EY, & Crocker PR (1996). Characterization of the Sialic Acidbinding Site in Sialoadhesin by Site-directed Mutagenesis. J Biol Chem, 271, 9267–9272. [DOI] [PubMed] [Google Scholar]
- Vinson M, Strijbos PJ, Rowles A, Facci L, Moore SE, Simmons DL, et al. (2001). Myelin-associated glycoprotein interacts with ganglioside GT1b. A mechanism for neurite outgrowth inhibition.J Biol Chem, 276, 20280–20285. [DOI] [PubMed] [Google Scholar]
- Vourc’h P,, & Andres C (2004). Oligodendrocyte myelin glycoprotein (OMgp): evolution, structure and function. Brain Res Brain Res Rev, 45, 115–124. [DOI] [PubMed] [Google Scholar]
- Vyas AA, & Schnaar RL (2001). Brain gangliosides: Functional ligands for myelin stability and the control of nerve regeneration. Biochem J, 83, 677–682. [DOI] [PubMed] [Google Scholar]
- Vyas AA, Patel HV, Fromholt SE, Heffer-Lauc M, Vyas KA, Dang J, et al. (2002). Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proc Natl Acad Sci USA, 99, 8412–8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley AR, Mir AK, & Frentzel S (2005). Ectodomain shedding of human Nogo-66 receptor homologue-1 by zinc metalloproteinases. Biochem Biophys Res Commun, 327, 112–116. [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, & He Z (2002a). p75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature, 420, 74–78. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, et al. (2002b). Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature, 417, 941–944. [DOI] [PubMed] [Google Scholar]
- Wang X, Baughman KW, Basso DM, & Strittmatter SM (2006). Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol, 60, 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chun S, Treloar H, Vartanian T, Greer CA, & Strittmatter SM (2002c). Localization of Nogo-A and Nogo-66 Receptor Proteins at Sites of Axon-Myelin and Synaptic Contact. J Neurosci, 22, 5505–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D, Wildes CP, Silvian L, Walus L, Mi S, Lee DH, et al. (2005). Disulfide structure of the leucine-rich repeat C-terminal cap and C-terminal stalk region of Nogo-66 receptor. Biochemistry, 44, 16491–16501. [DOI] [PubMed] [Google Scholar]
- Wong EV, David S, Jacob MH, & Jay DG (2003). Inactivation of myelin-associated glycoprotein enhances optic nerve regeneration. J Neurosci, 23, 3112–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, & Poo MM (2002). A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci, 5, 1302–1308. [DOI] [PubMed] [Google Scholar]
- Xie F, & Zheng B, (2007). White matter inhibitors in CNS axon regeneration failure. Exp Neurol, 209, 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T,Higuchi H, &Tohyama M(2002). The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol, 157, 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xiao ZC, Becker B, Hillenbrand R, Rougon G, & Schachner M (1999). Role for myelin-associated glycoprotein as a functional tenascin-R receptor. J Neurosci Res, 55, 687–701. [DOI] [PubMed] [Google Scholar]
- Yang LJ, Zeller CB, Shaper NL, Kiso M, Hasegawa A, Shapiro RE, et al. (1996). Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc Natl Acad Sci USA, 9(3), 814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YS, & Strittmatter SM (2007). The reticulons: a family of proteins with diverse functions. Genome Biol, 8, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G, & He Z (2006). Glial inhibition of CNS axon regeneration. Nat Rev Neurosci, 7, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Ho C, Li S, Keirstead H, Steward O, & Tessier-Lavigne M (2003). Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron, 38, 213–224. [DOI] [PubMed] [Google Scholar]
- Zheng B, Atwal J, Ho C, Case L, He XL, Garcia KC, et al. (2005). Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci USA, 102, 1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FQ, & Snider WD (2006). Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci, 361, 1575–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]