Abstract
We previously demonstrated that 5’-adenosine monophosphate-activated protein kinase (AMPK) is essential for normal reproductive functions in female mice. Conditional ablation of Prkaa1 and Prkaa2, genes that encode the α1 and α2 catalytic domains of AMPK, resulted in early reproductive senescence, faulty artificial decidualization, uterine inflammation and fibrotic postparturient endometrial regeneration. We also noted a delay in the timing of embryo implantation in Prkaa1/2d/d female mice, suggesting a role for AMPK in establishing uterine receptivity. As outlined in new studies here, conditional uterine ablation of Prkaa1/2 led to an increase in ESR1 in the uteri of Prkaa1/2d/d mice resulting in prolonged epithelial cell proliferation and retention of E2-induced gene expression (e.g., Msx1, Muc1, Ltf) through the implantation window. Within the stromal compartment, stromal cell proliferation was reduced by five-fold in Prkaa1/2d/d mice, and this was accompanied by a significant decrease in cell cycle regulatory genes and aberrant expression of decidualization marker genes such as Hand2, Bmp2, Fst, Inhbb. This phenotype is consistent with our prior study demonstrating a failure of the Prkaa1/2d/d uterus to undergo decidualization. Despite these uterine defects, ovarian function seemed to be normal following ablation of Prkaa1/2 from peri-ovulatory follicles in that ovulation, luteinization and serum progesterone levels were not different on day 5 of pregnancy or pseudopregnancy between Prkaa1/2fl/fl and Prkaa1/2d/d mice. These cumulative findings demonstrate that AMPK activity plays a prominent role in mediating several steroid hormone-dependent events such as epithelial cell proliferation, uterine receptivity and decidualization as pregnancy is established.
Keywords: AMPK, decidualization, endometrium, estrogen, fertility, implantation, pregnancy, progesterone, uterus
INTRODUCTION
Uterine receptivity is established by the sequential actions of estrogen (E2) and progesterone (P4) that act through their nuclear receptors to change the expression of critical genes. Several of these genes have been shown through conditional mutagenesis in mice to be fundamentally required for the establishment of early pregnancy, some of which include leukemia inhibitory factor (Lif), follistatin (Fst), bone morphogenetic protein 2 (Bmp2), heart- and neural crest derivatives-expressed protein 2 (Hand2), Msh homeobox 1 (Msx1), and Indian hedgehog (Ihh) (Stewart et al. 1992, Simon et al. 2009, Daikoku et al. 2011, Li et al. 2011, Li et al. 2013, Bhurke et al. 2016, Cheng et al. 2017, Fullerton et al. 2017). The proliferative and differentiative actions of sex steroid hormones are not direct. Rather, the hormones up-regulate expression of paracrine factors that, in turn, coordinate proliferative events induced by E2 and differentiation events directed by P4. While many of the paracrine ligands and their cognate receptors that mediate female sex steroid hormone actions in the uterus have been characterized, much less is known about the signal transduction pathways linked to these receptors. It is known that the MAPK, AKT and STAT3 signaling pathways play prominent roles in uterine receptivity and decidualization (Cheng et al. 2001, Yoshino et al. 2003, Singh et al. 2011, Yin et al. 2012, Salleh & Giribabu 2014, Makker et al. 2018, Yoo et al. 2018).
Adenosine monophosphate-activated protein kinase (AMPK) is an essential component of the signal transduction pathway that serves as a cellular sensor of energy status (Viollet et al. 2009). AMPK is a highly conserved heterotrimeric protein complex consisting of a catalytic α subunit and regulatory β and γ subunits. The Prkaa1 and Prkaa2 genes encode the α1 and α2 AMPK catalytic domains, respectively. In response to elevated cellular AMP, AMPK initiates catabolic processes while simultaneously inactivating energy depleting anabolic pathways (Hardie et al. 2006, Horman et al. 2012, Hardie & Lin 2017). AMPK plays a vital role in placental development (Kaufman & Brown 2016). Stable shRNA knockdown of Prkaa1 and Prkaa2 (Prkaa1/2) in trophoblast labyrinth cells results in changed morphology, growth rate and nutrient transport (Carey et al. 2014). More specifically, Prkaa1/2 knockdown reduce trophoblast glycolysis, mitochondrial respiration and ATP coupling efficiency (Waker et al. 2017). In contrast, excessive AMPK activity alters fatty acid metabolism and impairs trophoblast invasiveness in preeclampsia (Yang et al. 2018). These collective findings indicate that AMPK activity must be tightly regulated for normal placental development. AMPK may also play an anti-inflammatory role in fetal membranes, as its expression decreases at term. Furthermore, pharmacological activation of AMPK with phenformin or 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) reduced infection-induced expression of pro-inflammatory cytokines in extraembryonic membranes of pregnancies with preterm premature rupture of the membranes (PPROM) (Lim et al. 2015). It is interesting to note that obese women have reduced placental expression and activity of AMPK, which correlates with poor placental and pregnancy outcomes (Martino et al. 2016). Reduced placental expression of the active form of AMPK inversely correlates with birth weight (Jansson et al. 2013). Reduced placental AMPK expression/activity also generates a lipotoxic placental environment and an associated decrease in angiogenesis and increased expression of markers of inflammation and oxidative stress (Saben et al. 2014). The correlation between obesity and reduced placental AMPK expression/activity is also present in other animals. AMPK activity is reduced in cotyledonary tissue in obesogenic ewes (Zhu et al. 2009). Administration of AICAR reduced hypertension and angiogenic imbalance in a rat model of preeclampsia where increased AMPK activity reestablished placental antioxidant activities (Banek et al. 2013).
The known functions of AMPK in the female reproductive system are limited. Based on pharmacological studies, AMPK activation decreases steroidogenesis in rat and bovine granulosa cells (Tosca et al. 2005, Tosca et al. 2006, Tosca et al. 2007a, Tosca et al. 2010). A role for AMPK in oocyte maturation is species-dependent where AMPK catalytic activity results in oocyte activation in mice, but inhibition in pigs and cattle (Mayes et al. 2007, Tosca et al. 2007b, Santiquet et al. 2014, Bertoldo et al. 2015). Within the uterus, P53 was suggested to modulate uterine AMPK activity, which in turn controlled the timing of parturition in a murine model of preterm birth (Deng et al. 2016). Polymorphisms in the PRKAA1 gene are thought to enhance adaptation to high altitudes during pregnancy in Andean women by influencing uterine artery diameter, fetal growth, and birth weight outcomes (Bigham et al. 2014). Steroid hormones were shown to regulate expression of facilitative glucose transporters in murine uterine epithelial tissue in an AMPK-dependent fashion (Kim & Moley 2009). Beyond these limited findings, relatively little is known about the role of AMPK in female reproduction, particularly on the uterine side of the maternal:fetal interface during pregnancy. We recently established that conditional mutagenesis of Prkaa1 and Prkaa2 in the uterus results in subfertility that rapidly progresses to complete infertility and significantly reduced fecundity (McCallum et al. 2018). Additional phenotypes included uterine inflammation and abnormal postparturient deposition of fibrotic tissue in the endometrium, indicative of endometritis. Here, we evaluated AMPK as a mediator of steroid hormone responses and uterine receptivity.
MATERIALS AND METHODS
Animals
All animal experiments were reviewed and approved by the Washington State University Institutional Animal Care and Use Committee. Prkaa1/2d/d mice were developed by crossing Prkaa1/2fl/fl mice with Pgr-cre mice to conditionally ablate genes encoding the α1 and α2 catalytic domains of AMPK within the female reproductive tract (McCallum et al. 2018, Soyal et al. 2005). The exact genetic background of these mice is not known. However, based on the number of crosses we have made in our own lab, the mice are on a greater than 95% C57BL/6 background. The sub/infertility and faulty artificial decidualization observed in Prkaa1/2d/d female mice (McCallum et al. 2018) prompted us to further evaluate the conditional ablation of Prkaa1/2 during the time of uterine receptivity and early decidualization. We were also interested in comparing uterine steroid hormone responses in Prkaa1/2fl/fl and Prkaa1/2d/d female mice. For the early pregnancy studies, female mice were placed with males of proven breeding capacity and were considered day of pregnancy (DOP) 0.5 upon observation of a vaginal plug. Female reproductive tracts were collected for histological analyses on DOP4, 5 and 8. On DOP5, blood was collected from Prkaa1/2fl/fl and Prkaa1/2d/d female mice for serum isolation and the mice were then immediately euthanized. Serum samples were submitted to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for P4 hormone assays.
For evaluating uterine steroid hormone responses around the time of embryo implantation, 6–10 week old Prkaa1/2fl/fl and Prkaa1/2d/d female mice were bred by vasectomized male mice of proven breeding capacity to induce pseudopregnancy. On day of pseudopregnancy (DOPP) 4, a 0.5 inch 30 gauge needle was inserted entirely into the uterine lumen and a scratch was given when the needle was drawn back as 25 µl of sesame oil was injected into the uterine lumen to initiate decidualization. Uteri were collected 24 h later for mRNA isolation and histological evaluation. Ovaries were also collected and processed for paraffin embedding. This model relies on endogenous steroids to prime the uterus for decidualization. In a second pseudopregnancy model, we ovariectomized Prkaa1/2fl/fl and Prkaa1/2d/d female mice and allowed them to recover for two weeks. The mice were then given a steroid hormone regimen consistent with early pregnancy and artificially decidualized on the equivalent of DOPP4 (Zhang et al. 2012, McCallum et al. 2018). Uteri from Prkaa1/2fl/fl and Prkaa1/2d/d female mice were collected 24 h later and processed for mRNA isolation and paraffin embedding.
RNA Isolation and Quantitative Polymerase Chain Reaction (qPCR)
Uterine expression of genes associated with receptivity and decidualization was determined by qPCR using primer sets identified in Table 1. For these studies, RNA was isolated from uteri obtained from Prkaa1/2fl/fl and Prkaa1/2d/d mice using Tri-Reagent (Sigma-Aldrich; St. Louis, MO) following the manufacturer’s instruction. Isolated RNA was DNase treated (Roche Applied Science, Indianapolis, IN) and quantified using a NanoDrop 2000 Spectrophotometer (Thermo Scientific; Waltham, MA) prior to cDNA synthesis. Messenger RNA was then reverse transcribed using BioRad iScript (BioRad; Hercules, CA). Quantitative PCR was completed using BioRad’s C1000 CFX96 Real-Time Thermal Cycler System with an annealing temperature of 56–58°C (1 min) for 40 cycles. Data were analyzed with CFX Manager Software (BioRad; Hercules, CA). Rpl13a was used as an internal control to normalize expression data. A no reverse transcriptase negative control was included to confirm the absence of genomic DNA.
Table 1.
Primers used for genotyping and qPCR
| Gene | Forward Primer | Reverse Primer(s) |
|---|---|---|
| qPCR | ||
| Alox15 | 5’-AGAGTGGCCACACCAAGATG-3’ | 5’-GTAGACCGCTTCAGCACCAT-3’ |
| Bmp2 | 5’-ACGAGAAAAGCGTCAAGCCA-3’ | 5’-CCAGTCATTCCACCCCACAT-3’ |
| Ccna2 | 5’-TAAGCCTTGTCTTGTGGACCT-3’ | 5’-TGTCTCTGGTGGGTTGAGAAG-3’ |
| Ccnb1 | 5’-ACAACGGTGAATGGACACCA-3’ | 5’-TCATGTGCTTTGTGAGGCCA-3’ |
| Ccnb2 | 5’-TGCCTGTCTCAGAAGGTGCT-3’ | 5’-AGCGATGAACTTGGTACGGTT-3’ |
| Ccne1 | 5’-GTTACAGATGGCGCTTGCTC-3’ | 5’-AGCCAGGACACAATGGTCAG-3’ |
| Ccne2 | 5’-CCAGTAACAGTCATCTCCTGGT-3’ | 5’-AGTCGATGGCTAGAATGCACA-3’ |
| Ccnf | 5’-TTCCACGATGATGCACCCAA-3’ | 5’-GTCAGCATAGCTCAGCACCT-3’ |
| Cdk1 | 5’-ACGGCGACTCAGAGATTGAC-3’ | 5’-ACTTCTGGCCACACTTCGTT-3’ |
| E2f8 | 5’-TAACGACATCTGCCTGGACG-3’ | 5’-CCATGTGCAGGCTCTCTAGG-3’ |
| Esr1 | 5’-TGTTTGCTCCTAACTTGCTCCT-3’ | 5’-TCATGCGGAACCGACTTGAC-3’ |
| Follistatin | 5’-GTGACAATGCCACATACGCC-3’ | 5’-ACTTCAAGAAGCACGCCAGA-3’ |
| Gpx3 | 5’-CCGGGGACAAGAGAAGTCTAAG-3’ | 5’-GATGGTGAGGGCTCCATACTC-3’ |
| Hand2 | 5’-TCAAGGCGGAGATCAAGAAGAC-3’ | 5’-TTCTTGTCGTTGCTGCTCACT-3’ |
| Il1b | 5’-TGCCACCTTTTGACAGTGATGA-3’ | 5’-ATCAGGACAGCCCAGGTCAA-3’ |
| Inhbb | 5’-TGTGGCACGAGAGACTCCTA-3’ | 5’-CGCATCCGTTTGCTGGTATC-3’ |
| Ltf | 5’-CCTGCTTGCTAACCAGACCA-3’ | 5’-ACACAGGGCACAGAGATTGG-3’ |
| Msx1 | 5’-CTAGATCGGACCCCGTGGAT-3’ | 5’-TGGTCTTGTGCTTGCGTAGG-3’ |
| Muc1 | 5’-TACCACACTCACGGACGCTA-3’ | 5’-ACTGCCATTACCTGCCGAAA-3’ |
| Pgr | 5’-AGCATGTCGTCTGAGAAAGTGT-3’ | 5’-AACACCGTCAAGGGTTCTCAT-3’ |
| Prss28 | 5’-CAACCTTCTCCAACGTGTGC-3’ | 5’-TTGTGCTCGTCGGATGACTT-3’ |
| Rpl13a | 5’-TTGCTTACCTGGGGCGTCT-3’ | 5’-CCTTTTCCTTCCGTTTCTCCTC-3’ |
| S100a9 | 5’-ACCACCATCATCGACACCTTC-3’ | 5’-AAAGGTTGCCAACTGTGCTTC-3’ |
| Tac2 | 5’-TAGCGTGGGACCAAAGGAGA-3’ | 5’-GGCTGTTCCTCTTGCCCATA-3’ |
| Genotyping | ||
| Pgr-Cre | 5’-ATGTTTAGCTGGCCCAAATG-3’ | 5’-TATACCGATCTCCCTGGACG-3’ |
| 5’-CCCAAAGAGACACCAGGAAG-3’ | ||
| Prkaa1 | 5’-TATTGCTGCCATTAGGCTAC-3’ | 5’-GACCTGACAGAATAGGATATGCCCAACCTC-3’ |
| Prkaa2 | 5’-GCTTAGCACGTTACCCTGGATGG-3’ | 5’-GTTATCAGCCCAACTAATTACAC-3’ |
Immunohistochemistry
All tissues were fixed in 4% paraformaldehyde and stored in 70% ethanol until paraffin embedding. Tissues were processed in an ethanol gradient and xylenes, embedded in paraffin, and sectioned at 5 µm. Tissue sections were heated at 65°C for 60 minutes then deparaffinized in xylenes, rehydrated in a series of decreasing ethanol washes and placed in PBS. Slides were stained with hematoxylin and eosin (Scytek Laboratories Inc., Logan, UT) as per manufacturer’s instructions, or they were used for immunohistochemistry (IHC). For IHC, tissue sections underwent quenching (10 minutes in 3% hydrogen peroxide) and antigen retrieval (brought to a boil in 0.1M sodium citrate followed by incubation in the heated solution for 20 minutes and then cooling to room temperature). Sections were then blocked (0.1% bovine serum albumin, 0.1% normal goat serum or normal donkey serum, and 1% Triton-X100 in phosphate-buffered saline) for 1 h and then incubated overnight at 4°C in blocking solution containing primary antibody shown in Table 2. Slides were washed in phosphate-buffered saline (3×10 minutes) and incubated with secondary antibody diluted at 1:500 in blocking solution for 30 minutes at room temperature. Sections were washed as before and then incubated with horseradish peroxidase-conjugated streptavidin (Vector Laboratories, Burlingame, CA). Washes were again performed and the sections were exposed to 3,3’-diaminobenzidine (BD Biosciences, San Diego, CA) followed by a five-minute inactivation in PBS. Sections were then counterstained with methyl green or hematoxylin, dehydrated and mounted.
Table 2.
Antibodies used for IHC
| Target protein | Manufacturer, Catalog No. | Species, Clonal Status | Dilution |
|---|---|---|---|
| ESR1 | Thermo Fisher Scientific, MA5–13191 | Mouse, Monoclonal | 1:200 |
| HAND2 | Santa Cruz Biotechnology, SC-9409 | Goat, Polyclonal | 1:100 |
| PGR | Thermo Scientific, RM-9102 | Rabbit, Monoclonal | 1:250 |
| PHH3 | Millipore, 06–570 | Rabbit, Polyclonal | 1:500 |
| MUC1 | Abcam, 15481 | Rabbit, Polyclonal | 1:100 |
Data Analysis
Data are presented as a mean ± SEM for n=3–8 independent experiments in which one mouse represented one experimental replicate. The exact number of experimental replicates are indicated in each figure legend. Animals were randomly assigned to each group based on genotype. Differences between treatment groups were analyzed using the Student’s t-test. A p-value ≤0.05 was considered significant. All data were analyzed using GraphPad Prism 5.0 (San Diego, California).
RESULTS
We previously demonstrated that AMPK is required for fertility in the female and that uterine expression of E2 (ESR1) and P4 (PGR) receptors did not differ between Prkaa1/2fl/fl and Prkaa1/2d/d mice following E2 synchronization (McCallum et al. 2018). As E2 and P4 ensure that the uterus is receptive to the implanting embryo as pregnancy is established, we first evaluated the expression of Esr1 and Pgr on DOPP5 by qPCR. A significant increase in Esr1 mRNA levels were observed in the Prkaa1/2d/d uterus compared to the Prkaa1/2fl/fl uterus, whereas Pgr mRNA levels did not differ (Fig. 1A and 1B). This difference in expression at the mRNA level was also evident at the protein level based on IHC analysis, particularly in the epithelial compartment (Fig. 1C).
Figure 1. Disrupted ESR1, but not PGR expression in the DOPP5 Prkaa1/2d/d uterus.
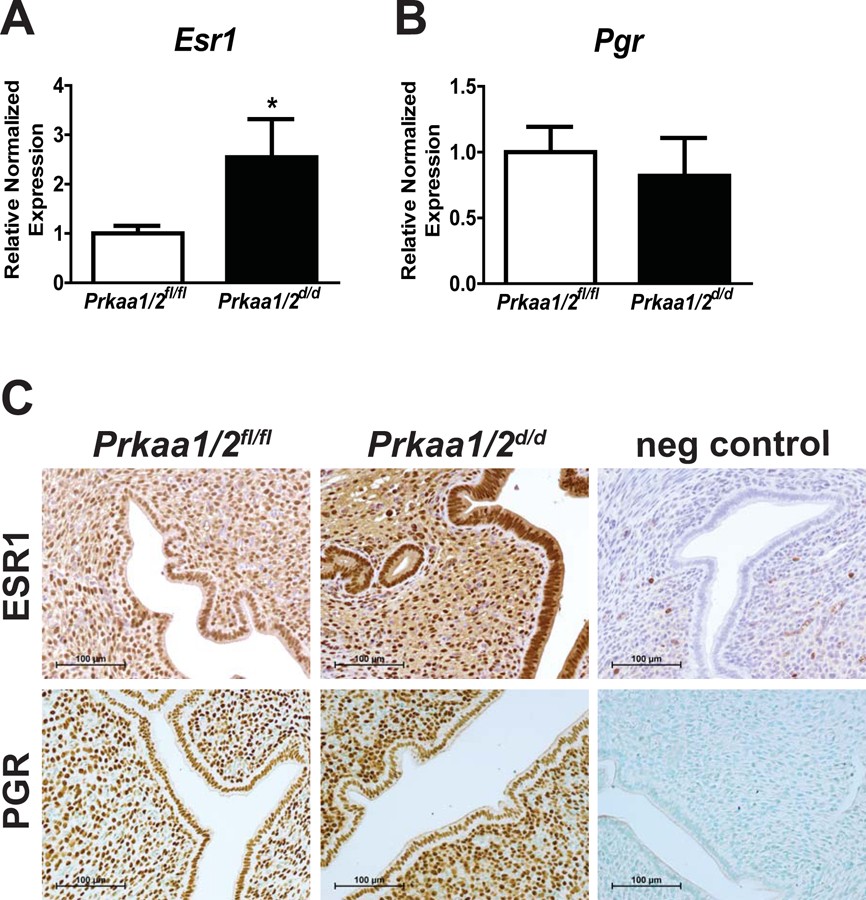
Shown are uterine RT-qPCR (A, B) and immunohistochemical (C) expression data for ESR1 and PGR on day of pseudopregnancy (DOPP) 5 in Prkaa1/2fl/fl and Prkaa1/2d/d mice. Values are expressed as mean ± SEM, *P≤0.05, n=4.
In evaluating our prior breeding trial data (McCallum et al. 2018), we noted that the time to first weaning for nulliparous Prkaa1/2d/d female mice was about twice that of female Prkaa1/2fl/fl mice (Fig. 2A). Many of the pups born to Prkaa1/2d/d female mice appeared smaller at birth than pups from Prkaa1/2fl/fl female mice and this translated to a smaller mean pup mass at the time of weaning (Fig. 2B). Evaluation of ovaries from Prkaa1/2fl/fl and Prkaa1/2d/d mice on DOP5 and DOPP5 revealed no histological differences (Fig. 2C). Furthermore, serum P4 levels were not different on DOP5 (Fig. 2D). Despite no difference in ovarian histology or luteal function, we observed faulty embryo implantation and embryonic growth in Prkaa1/2d/d dams on DOP4, DOP5 and DOP8 (Fig. 3). Embryos within uteri from Prkaa1/2d/d mice were commonly not implanting (DOP4) or were smaller in size or degenerate (DOP4, DOP5 and DOP8) compared with embryos in uteri from Prkaa1/2fl/fl mice. Interestingly, while the initiation of stromal cell decidualization was visible in Prkaa1/2fl/fl mice on DOP4 and DOP5, the decidualization response was commonly blunted or absent during these times in Prkaa1/2d/d mice. Furthermore, the zippering of the luminal epithelium on DOP5 was often incomplete in Prkaa1/2d/d mice.
Figure 2. PRKAA1 and PRKAA2 deficiency disrupts time to first weaning with no impact on ovarian structure and function.
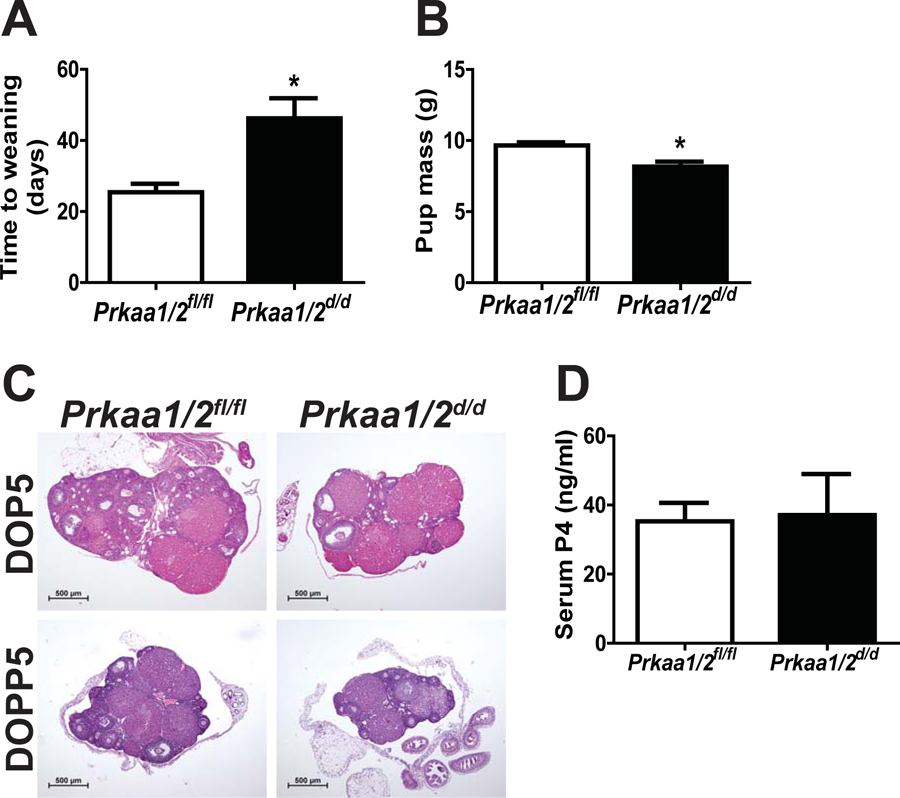
(A) Overall time to weaning of pups is dramatically increased in primiparous Prkaa1/2d/d females (p=0.014). (B) Pups born to Prkaa1/2d/d dams weighed significantly less at the time of weaning than pups born out of Prkaa1/2fl/fl females. (C) A histological analysis demonstrated no difference in ovarian architecture or corpora lutea formation between Prkaa1/2fl/fl and Prkaa1/2d/d mice during early pregnancy (DOP5) or pseudopregnancy (DOPP5) (n=4–6). (D) Serum P4 measured on DOP5 was not different between Prkaa1/2fl/fl (n=6) and Prkaa1/2d/d (n=4) animals demonstrating that post-ovulatory steroidogenesis is not dependent on AMPK activity. Values are expressed as mean ± SEM, *P≤0.05.
Figure 3. Embryo implantation is delayed or disrupted in Prkaa1/2d/d mice.
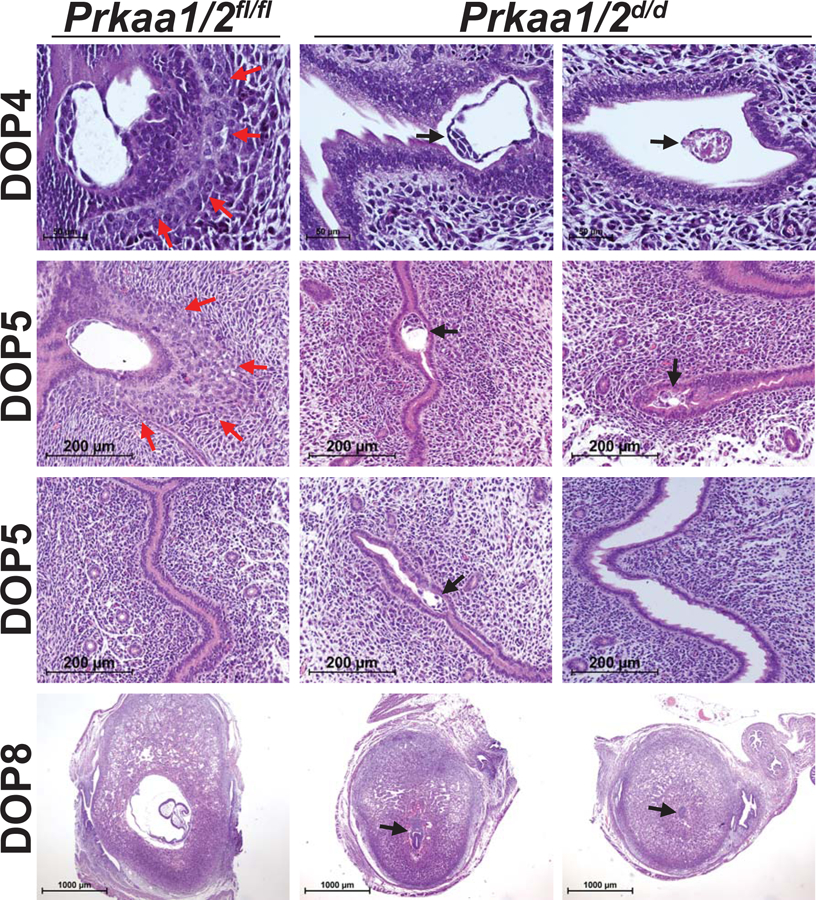
Embryos from Prkaa1/2fl/fl females implanted normally and the stroma decidualized as expected on DOP4, DOP5 and DOP8, (red arrows). Embryos from Prkaa1/2d/d females commonly failed to implant properly, were smaller in size or did not survive the implantation process (black arrows). The luminal epithelium of gravid Prkaa1/2d/d female mice failed to zipper shut on DOP5. While DOP8 embryos had greatly expanded in Prkaa1/2fl/fl mice with a clear presence of an amniotic cavity, DOP8 embryos from Prkaa1/2d/d mice were often small and stunted in development with reduced decidual expansion. n=4–8
At the time of embryo apposition and implantation, endometrial epithelial cells should be non-proliferative and the underlying stromal cells should be proliferating in response to luteal-derived P4. In this next experiment, we generated pseudopregnant Prkaa1/2fl/fl and Prkaa1/2d/d mice by placing intact females with vasectomized male mice to evaluate endometrial cell proliferation. While uteri from Prkaa1/2fl/fl mice displayed normal endometrial cell proliferation, epithelia of Prkaa1/2d/d mice remained proliferative and the stromal cell compartment showed a significant decrease in proliferation on DOPP5 based on expression of the mitosis marker phospho-histone H3 (Fig. 4). This was accompanied by a significant decrease in the expression of the cell cycle regulatory genes Cyclin A2 (Ccna2), Cyclin B1 (Ccnb1), Cyclin B2 (Ccnb2), Cyclin E1 (Ccne1), Cyclin E2 (Ccne2), Cyclin F (Ccnf), and Cyclin Dependent Kinase 1 (Cdk1) (Fig. 5A) and the decidualization marker genes Follistatin (Fst), Inhibin βB (Inhbb), Bone morphogenetic protein 2 (Bmp2), heart-and neural crest derivatives-expressed protein 2 (Hand2), S100 calcium-binding protein A9 (S100a9), and serine protease 28 (Prss28) (Fig. 5B) in Prkaa1/2d/d mice. Interestingly, wingless-type MMTV family member 4 (Wnt4), which is also essential for decidualization, was not differentially regulated. Three common E2-induced genes were up-regulated in uteri of Prkaa1/2d/d mice and these include Msh homeobox 1 (Msx1), Mucin 1 (Muc1), and Lactotransferrin (Ltf, P=0.07) (Fig. 5C). Several other genes such as Tachykinin 2 (Tac2), E2F transcription factor 8 (E2f8), Glutathione peroxidase 3 (Gpx3), Arachidonate 15-lipoxygenase (Alox15) and Interleukin 1 beta (IL1b) were also differentially expressed (Fig. 5D). The Hand2 gene is fundamentally required for uterine receptivity and decidualization in mice (Li et al. 2011). Evaluation of HAND2 protein expression by IHC revealed a significant decrease in stromal cell expression at a time when decidualization should be just underway (Fig. 6). These cumulative findings are consistent with the failure of uteri from Prkaa1/2d/d mice to undergo artificial decidualization (McCallum et al. 2018) and for faulty embryo implantation and luminal closure in these mice, and these defective uterine functions may stem from elevated or unopposed E2-regulated signaling.
Figure 4. Aberrant retention of luminal epithelial proliferation and decreased stromal proliferation in the DOPP5 Prkaa1/2d/d uterus.
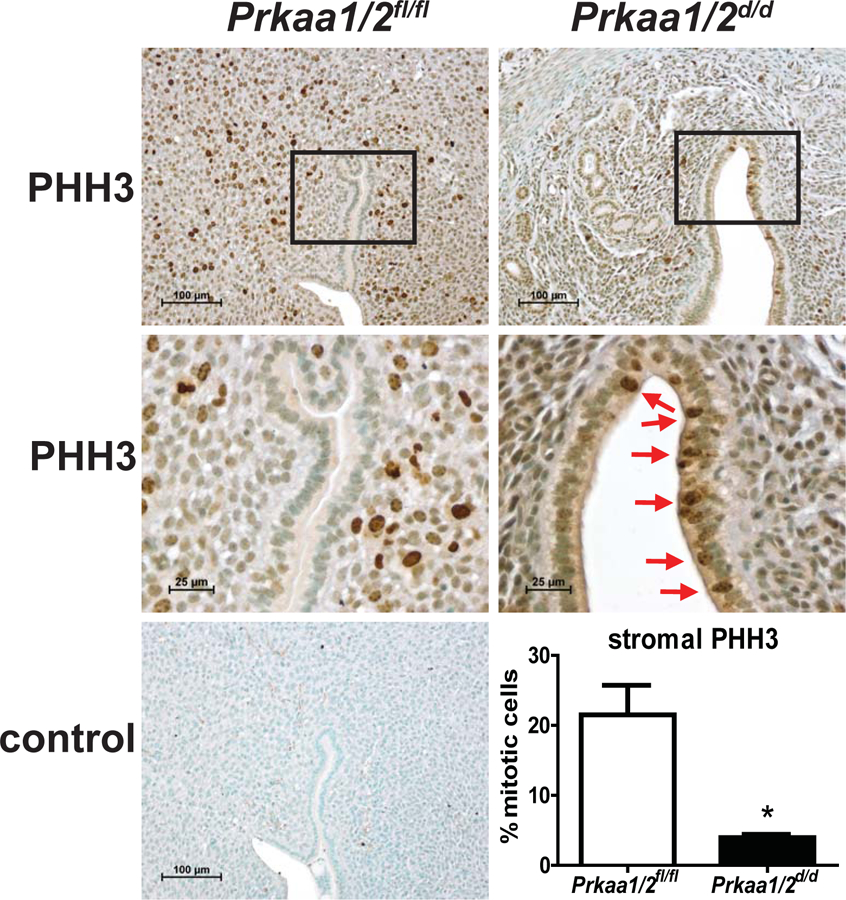
Immunohistochemical staining for the mitosis marker phosphohistone-H3 (PHH3) shows retention of luminal epithelial proliferation in the DOPP5 Prkaa1/2d/d uterus with significantly diminished proliferation in the stromal compartment compared to the Prkaa1/2fl/fl uterus (*P=0.02). Values are expressed as mean ± SEM, n=3–4.
Figure 5. PRKAA1 and PRKAA2 deficiency results in differential expression of genes associated with the cell cycle, decidualization, estrogen-signaling, and inflammation on DOPP5.
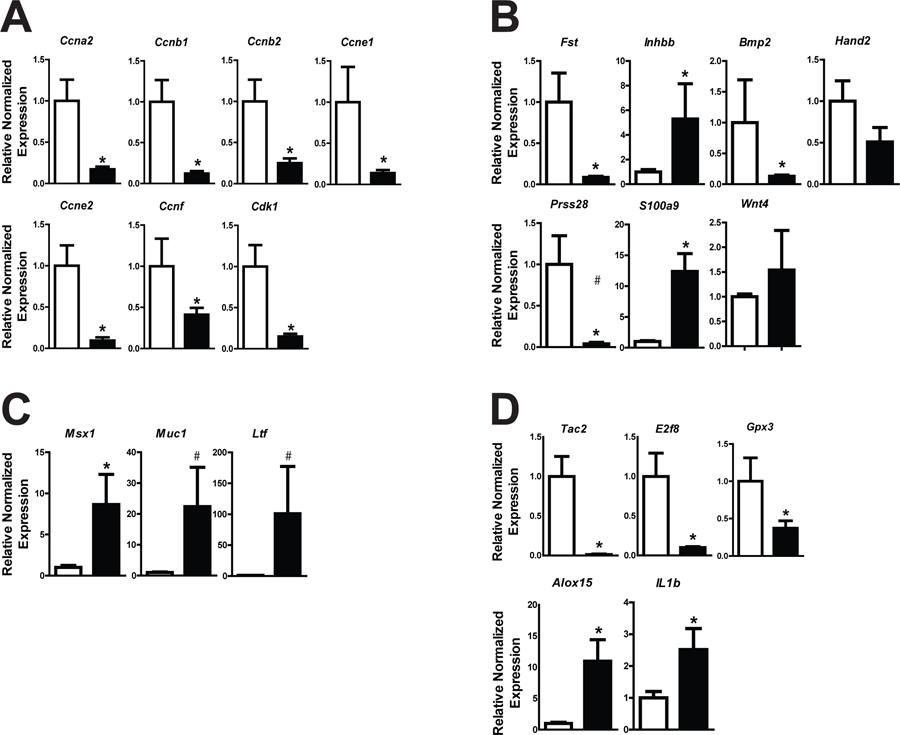
Using an intact pseudopregnancy model, RT-qPCR was used to evaluate expression of cell cycle regulatory genes Ccna2, Ccnb1, Ccnb2, Ccne1, Ccne2, Ccnf, and Cdk1 (A) and genes important for decidualization (B; Fst, Inhbb, Bmp2, Hand2, S100a9, Prss28, Wnt4) in uteri obtained from Prkaa1/2fl/fl (white bars) and Prkaa1/2d/d (black bars) mice. E2 responsive genes (C; Msx1, Muc1 and Ltf), as well as genes commonly associated with inflammation (D; Tac2, E2f8, Gpx3, Alox15 and IL1b) were also differentially regulated between groups. Values are expressed as mean ± SEM, *P≤0.05; #P=0.07, n=3.
Figure 6. HAND2 expression is decreased in the DOPP5 Prkaa1/2d/d uterus.
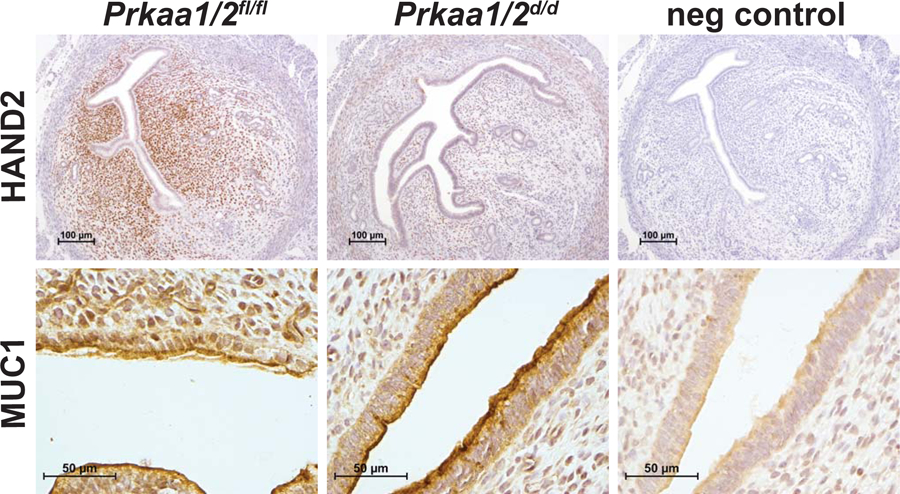
HAND2 and MUC1 were evaluated in intact Prkaa1/2fl/fl and Prkaa1/2d/d mice on DOPP5 using immunohistochemistry. Prkaa1/2fl/fl animals show strong expression of HAND2 in the stroma whereas Prkaa1/2d/d animals show a dramatic decrease in stromal staining. No difference in MUC1 expression was observed between the two groups. n=3
To provide additional evidence that uteri from Prkaa1/2d/d mice fail to properly respond to steroid hormones and establish a receptive uterus, we ovariectomized Prkaa1/2fl/fl and Prkaa1/2d/d mice, provided an exogenous steroid hormone regimen consistent with early pregnancy (McCallum et al. 2018), and then evaluated cell proliferation and gene expression on DOPP5 as before. Unlike uteri from Prkaa1/2fl/fl mice, uteri from Prkaa1/2d/d mice retained mitotic epithelial cells and tended (p=0.07) to weigh more than uteri from Prkaa1/2fl/fl mice (Figure 7). Likewise, we observe the same differential expression for Fst, Inhbb, Muc1, Alox15, and Tac2 as we did in intact mice that were exposed to endogenous steroid hormones.
Figure 7. Aberrant steroid hormone signaling in uteri from ovariectomized Prkaa1/2d/d mice treated with exogenous steroid hormones mimicking early pregnancy.
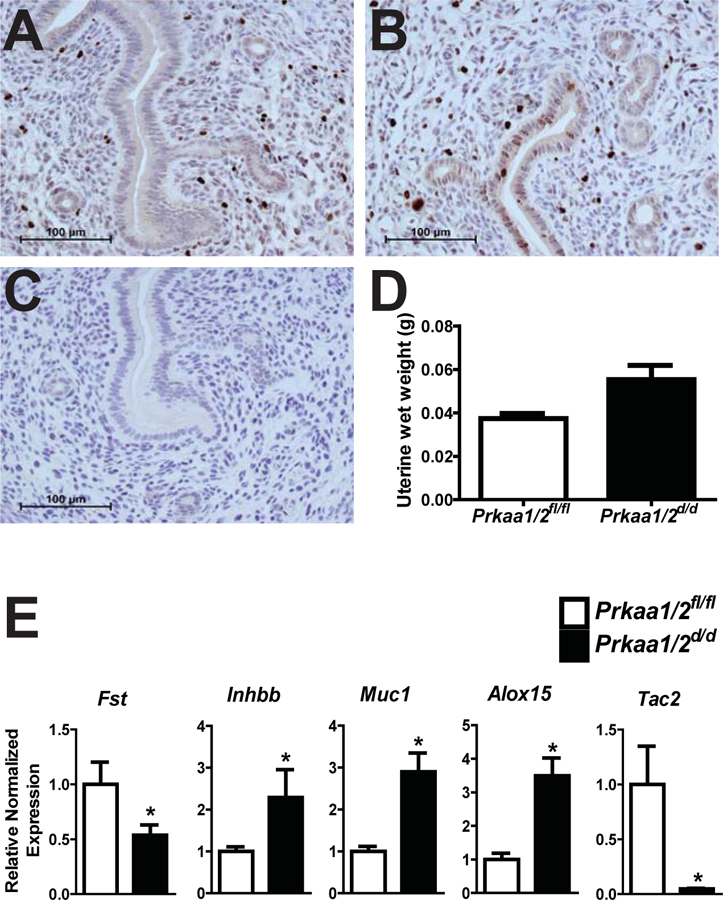
(A) Following ovariectomy and provision of a steroid hormone regimen consistent with early pregnancy (McCallum et al. 2018), immunohistochemical analysis of the mitosis marker phosphohistone-H3 shows some proliferation in the stromal compartment with no proliferation in the luminal epithelium in Prkaa1/2fl/fl uteri. (B) In contrast, Prkaa1/2d/d uteri show retention of proliferation in the luminal epithelium with some proliferation in the stroma. (C) No primary antibody negative control. (D) Prkaa1/2d/d uteri tended (P=0.07) to weight more than Prkaa1/2fl/fl uteri. (E) RT-qPCR analysis of Fst, Inhbb, Muc1, Alox15 and Tac2 in uteri obtained from Prkaa1/2fl/fl and Prkaa1/2d/d mice. Values are expressed as mean ± SEM, *P≤0.05, n=3.
DISCUSSION
We previously established an essential role for the AMPK signal transduction pathway in uterine physiology (McCallum et al. 2018). Ablation of genes encoding the α1 and α2 catalytic subunits of AMPK from the female reproductive tract resulted in subfertility that rapidly progressed to complete infertility. Associated features of this broad phenotype included faulty decidualization, parturition-dependent fibrosis of the stromal compartment (i.e., endometritis), and chronic inflammation. It was noted from breeding trial data that the time to first weaning of pups born to Prkaa1/2d/d dams was twice as long as that observed for Prkaa1/2fl/fl dams despite no difference in estrous cyclicity. Pups born to Prkaa1/2d/d dams were also smaller at the time of weaning, suggesting additional functions for AMPK during pregnancy beyond those previously described (McCallum et al. 2018). As such, the objective of the present study was to evaluate the role of AMPK in establishing the uterine receptive state during early pregnancy. In addition to evaluating early pregnancy, we used two models to appraise AMPK functions around the time of uterine receptivity. The first involved the use of intact Prkaa1/2fl/fl and Prkaa1/2d/d mice that were bred to vasectomized male mice to induce a state of pseudopregnancy. This model relies on endogenous steroid hormones to develop the receptive endometrium. The second model used Prkaa1/2fl/fl and Prkaa1/2d/d mice that were first ovariectomized and then given a series of daily steroid hormone injections that mimicked early pregnancy. Even though estrous cyclicity and steroidogenesis were not different between intact Prkaa1/2fl/fl and Prkaa1/2d/d mice (McCallum et al. 2018, Fig. 2), and a histological evaluation of ovaries on DOP5 and DOPP5 collectively indicated that Prkaa1/2 are dispensable for normal ovarian functions (McCallum et al. 2018), Fig. 2), this second model was implemented to directly evaluate the effects of steroid hormone actions on the uterus under controlled conditions.
Uterine receptivity is established in response to the synchronous secretion of ovarian-derived E2 and P4. These two steroid hormones work in series with E2 first priming the endometrium to undergo epithelial cell proliferation. This is followed by P4, which attenuates epithelial cell proliferation causing them to take on a differentiated secretory function and stimulating stromal cell proliferation (Das & Martin 1973, Wang & Dey 2006). In addition to appropriately timed ligand availability, uterine expression of the classical receptors for E2 and P4 are tightly regulated. Dysregulation of these receptors compromises fertility in the female. Whereas ESR1 expression normally decreases during the window of implantation (Lessey et al. 2006), an increase in ESR1 mRNA and protein was observed in uteri from Prkaa1/2d/d mice during the window of implantation. Elevated ESR1 expression in the endometrium of women during the window of implantation is implicated in some cases of unexplained infertility (Lessey et al. 2006, Dorostghoal et al. 2018).
Because of the increased expression of ESR1 during receptivity, we evaluated implantation and early embryo development on DOP4, 5 and 8. Murine implantation occurs between DOP4 and DOP5. Absorption of uterine fluid and closure, or zippering, of the luminal epithelium promote proper antimesometrial eccentric implantation in mice (Zhang et al. 2013). We previously noted upon gross observation that implantation sites from 4–6 month old nulliparous Prkaa1/2d/d mice appear normal. However, upon histological evaluation of implantation sites at the time of embryo implantation (i.e., DOP 4 and 5; Figure 3) in 6–10 week old Prkaa1/2d/d mice, we did observe clear signs of an unzippered luminal epithelium, lack of formation of an embryonic chamber, and stunted embryonic growth in some sites, all of which are clear signs of dyssynchrony between the mother and implanting embryo. While exhibiting the same gestation length, the delay in implantation likely explains the decrease in pup mass observed in offspring born to Prkaa1/2d/d mice. Use of the Pgr-cre mouse results in peri-ovulatory ablation of floxed genes in ovarian follicles and subsequent corpora lutea given that Pgr is normally expressed for a 12 h window on either side of ovulation in mice. Despite peri-ovulatory follicular ablation of Prkaa1 and Prkaa2, ovulation, luteinization and P4 steroidogenesis were normal in Prkaa1/2d/d mice. In support of our previous findings (McCallum et al. 2018), these data suggest that AMPK is dispensable for late follicular and luteal functions in the ovary and that early pregnancy issues observed in Prkaa1/2d/d mice most likely derive from disrupted uterine functions.
To characterize the faulty uterine receptivity phenotype in more detail, we next evaluated steroid hormone-induced proliferation in the epithelial and stromal compartments, as well as expression of markers of stromal decidualization and inflammation in both models of pseudopregnancy. Coupled with elevated expression of ESR1 in uteri obtained from Prkaa1/2d/d mice on DOPP5 was the prolonged retention of luminal epithelial cell mitosis and elevated expression of the E2 marker genes Msx1, Muc1, and Ltf. While Msx1 expression normally increases in epithelial tissue on DOP4 in the receptive mouse uterus, its expression is almost undetectable following implantation or the equivalent time during pseudopregnancy (Daikoku et al. 2011). Msx1 is fundamentally required for establishing the receptive state in mice through its modulation of epithelial junctional activity in luminal tissue (Sun et al. 2016). Msx1 expression was more recently shown to be widely disrupted in infertile women where reduced Msx1 expression associated with loss of epithelial cell polarity and a receptive uterine state. (Bolnick et al. 2016).
Within the stromal compartment, a 5-fold decrease in stromal cell proliferation was also observed in DOPP5 Prkaa1/2d/d uteri, a phenotype that was accompanied by down-regulation of several cell cycle regulatory genes. This likely contributed to faulty artificial decidualization (McCallum et al. 2018) and the diminished size of implantation sites during early pregnancy (Fig. 3). We also noted decreased expression of decidualization markers, a number of which are discussed below, have been shown through mutagenesis studies in mice and knockdown studies in human endometrial stromal cells to be essential for decidualization. Bone morphogenetic protein 2 (Bmp2), follistatin (Fst), and heart- and neural crest derivatives-expressed protein 2 (Hand2) are among the most well-studied (Lee et al. 2007, Li et al. 2013, Bhurke et al. 2016, Fullerton et al. 2017). The Prkaa1/2d/d uterine phenotype is strikingly similar to the Fst conditional mutant phenotype in which uterine ablation of Fst resulted in subfertility caused by a failure to achieve uterine receptivity, retention of features of E2 signaling such as luminal epithelial cell proliferation at a time when P4 signaling should dominate, impaired embryo implantation, and faulty decidualization (Fullerton et al. 2017). Another interesting feature of the Fstd/d phenotype is the increased expression of inhibin βB. When dimerized, inhibin βB forms activin, which in turn antagonizes BMP signaling. Importantly, BMP signaling, particularly that initiated by BMP2 and BMP7 (Li et al. 2013, Lee et al. 2007, Monsivais et al. 2017), is an essential component of the differentiation program that establishes the receptive state and initiates decidualization in the uterus. The Prkaa1/2d/d uterus similarly shows a pattern of decreased Fst and increased Inhbb during receptivity (Fig. 5b). We suspect that AMPK signaling is essential for reducing Inhbb expression, which then ensures robust BMP signaling that is necessary for initiating the deciduogenic response. The decreased expression of Hand2 mRNA was validated at the protein level by immunohistochemistry. This protein is a mediator of the antiproliferative action of P4 (Li et al. 2011). The significant decrease in HAND2 expression in the Prkaa1/2d/d uterus likely contributes to aberrantly retained epithelial cell expression during receptivity. Of note, the expression of Wnt4, another essential gene required for decidualization, was not different in uterine tissues isolated from Prkaa1/2fl/fl and Prkaa1/2d/d mice. This indicates that AMPK, either directly or indirectly, is necessary for mediating steroid hormone regulated expression of a select cadre of genes essential for implantation and decidualization, but not others.
In summary, this study demonstrates the functional requirement of AMPK signaling in establishing the uterine receptive state for embryo implantation. The actions of AMPK are commonly associated with sensing and regulating available cellular energy. While we have not ruled out the possibility that pregnancy issues observed in Prkaa1/2d/d mice stem specifically from faulty energy sensing, we do show aberrant expression of E2 responsive genes, as well as genes that coordinates stromal decidualization, strongly support the idea that AMPK is functionally linked to normal steroid hormone signaling in the uterus. What now remains to be determined is: 1) identifying the factor(s) that increase AMPK signaling prior to transitioning to the receptive state; and 2) determining the molecular mechanism by which AMPK differentially regulates E2- and P4-dependent genes. With regard to identifying factors that increase AMPK signaling, AMPK activity was recently shown to increase in human stromal cell decidualization assays in an epidermal growth factor receptor-dependent fashion (Large et al. 2014.) As EGFR and several EGF-like ligands (e.g., HB-EGF, amphiregulin) have been shown to be important for implantation and decidualization (Large et al. 2014, Das et al. 1994, Das et al. 1995, Paria et al. 2001, Leach et al. 1999), this would place AMPK near the top of the molecular pyramid that controls establishment of the receptive state should AMPK be confirmed as one of the phosphorylation cascades downstream of the EGFR.
ACKNOWLEGMENTS
We thank The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, supported by the Eunice Kennedy Shriver NICHD/NIH P50 grant (HD28934), for performing the serum P4 hormone assays. We are grateful for funding support from the National Institutes of Health (HD086402 to JKP and HD097087 to WW).
Funding: This study was funded in part by NIH R21HD086402 (JKP) and HD097087 (WW) and USDA National Institute of Food and Agriculture, Functional Genomics in Animal Improvement, Food Safety and Human Health Hatch Project 1014918.
Footnotes
Competing Financial Interests: The authors declare no competing or financial interests.
REFERENCES
- Banek CT, Bauer AJ, Needham KM, Dreyer HC & Gilbert JS 2013. AICAR administration ameliorates hypertension and angiogenic imbalance in a model of preeclampsia in the rat. Am J Physiol Heart Circ Physiol 304 H1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoldo MJ, Faure M, Dupont J & Froment P 2015. AMPK: a master energy regulator for gonadal function. Front Neurosci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhurke AS, Bagchi IC & Bagchi MK 2016. Progesterone-Regulated Endometrial Factors Controlling Implantation. Am J Reprod Immunol 75 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham AW, Julian CG, Wilson MJ, Vargas E, Browne VA, Shriver MD & Moore LG 2014. Maternal PRKAA1 and EDNRA genotypes are associated with birth weight, and PRKAA1 with uterine artery diameter and metabolic homeostasis at high altitude. Physiol Genomics 46 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick AD, Bolnick JM, Kllburn BA, Stewart T, Oakes J, Rodriguez-Kovacs J, Kohan-Ghadr HD, Dai J, Diamond MP, Hirota Y, et al. ; NICHD National Cooperative Reproductive Medicine Network. 2016. Reduced homeobox protein MSX1 in human endometrial tissue is linked to infertility. Hum Reprod 31 2042–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey EA, Albers RE, Doliboa SR, Hughes M, Wyatt CN, Natale DR & Brown TL 2014. AMPK knockdown in placental trophoblast cells results in altered morphology and function. Stem Cells Dev 23 2921–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Rosario G, Cohen TV, Hu J & Stewart CL 2017. Tissue-Specific Ablation of the LIF Receptor in the Murine Uterine Epithelium Results in Implantation Failure. Endocrinology 158 1916–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JG, Chen JR, Hernandez L, Alvord WG & Stewart CL 2001. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc Natl Acad Sci U S A 98 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku T, Cha J, Sun X, Tranguch S, Xie H, Fujita T, Hirota Y, Lydon J, DeMayo F, Maxson R & Dey SK 2011. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev Cell 21 1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK 1995. Amphiregulin is an implanation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol 9 691–705. [DOI] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK 1994. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 120 1071–1083. [DOI] [PubMed] [Google Scholar]
- Das RM, Martin L 1973. Progesterone inhibition of mouse uterine epithelial proliferation. J Endocrinol 59 205–206. [DOI] [PubMed] [Google Scholar]
- Deng W, Cha J, Yuan J, Haraguchi H, Bartos A, Leishman E, Viollet B, Bradshaw HB, Hirota Y & Dey SK 2016. p53 coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. J Clin Invest 126 2941–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorostghoal M, Ghaffari H-a, Moramezi F & Keikhah N 2018. Overexpression of Endometrial Estrogen Receptor-Alpha in The Window of Implantation in Women with Unexplained Infertility. Int J Fertil Steril 12 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton PT, Monsivais D, Kommagani R & Matzuk MM 2017. Follistatin is critical for mouse uterine receptivity and decidualization. Proc Natl Acad Sci U S A 114 E4772–E4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA & Scott JW 2006. AMP-activated protein kinase – development of the energy sensor concept. J Physiol 574 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG & Lin SC 2017. AMP-activated protein kinase - not just an energy sensor. F1000Res 6 1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horman S, Beauloye C, Vanoverschelde JL & Bertrand L 2012. AMP-activated protein kinase in the control of cardiac metabolism and remodeling. Curr Heart Fail Rep 9 164–173. [DOI] [PubMed] [Google Scholar]
- Hu G, Lee H, Price SM, Shen MM, Abate-Shen C 2001. Msx homeobox genes inhibit differentiation through upregulation of cyclin D1. Development 128 2373–2384. [DOI] [PubMed] [Google Scholar]
- Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, Jansson T & Powell TL 2013. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab 98 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MR & Brown TL 2016. AMPK and Placental Progenitor Cells. Exp Suppl 107 73–79. [DOI] [PubMed] [Google Scholar]
- Kim ST & Moley KH 2009. Regulation of facilitative glucose transporters and AKT/MAPK/PRKAA signaling via estradiol and progesterone in the mouse uterine epithelium. Biol Reprod 81 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large MJ, Wetendorf M, Lanz RB, Hartig SM, Creighton CJ, Mancini MA, Kovanci E, Lee KF, Threadquill DW, Lydon JP, et al. 2014. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet 10 e1004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR 1999. Multiple roles for heparin-binding epidermal growth factor-like factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab 84 3355–3363. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong J-W, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP & DeMayo FJ 2007. Bmp2 Is Critical for the Murine Uterine Decidual Response. Mol Cell Biol 15 5468–5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA, Palomino WA, Apparao KB, Young SL, Lininger RA 2006. Estrogen receptor-alpha (ER-alpha) and defects in uterine receptivity in women. Reprod Biol Endocrinol 4 S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, Das A, Demayo FJ, Hornsby PJ, Young SL, Taylor RN, Bagchi MK & Bagchi IC 2013. WNT4 acts downstream of BMP2 and functions via beta-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology 154 446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK & Bagchi IC 2011. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 331 912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Barker G & Lappas M 2015. Activation of AMPK in human fetal membranes alleviates infection-induced expression of pro-inflammatory and pro-labour mediators. Placenta 36 454–462. [DOI] [PubMed] [Google Scholar]
- Makker A, Goel MM, Nigam D, Mahdi AA, Das V, Agarwal A, Pandey A & Gautam A 2018. Aberrant Akt Activation During Implantation Window in Infertile Women With Intramural Uterine Fibroids. Reprod Sci 25 1243–1253. [DOI] [PubMed] [Google Scholar]
- Martino J, Sebert S, Segura MT, Garcia-Valdes L, Florido J, Padilla MC, Marcos A, Rueda R, McArdle HJ, Budge H, Symonds ME & Campoy C 2016. Maternal Body Weight and Gestational Diabetes Differentially Influence Placental and Pregnancy Outcomes. J Clin Endocrinol Metab 101 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes MA, Laforest MF, Guillemette C, Gilchrist RB & Richard FJ 2007. Adenosine 5’-monophosphate kinase-activated protein kinase (PRKA) activators delay meiotic resumption in porcine oocytes. Biol Reprod 76 589–597. [DOI] [PubMed] [Google Scholar]
- McCallum ML, Pru CA, Smith AR, Kelp NC, Foretz M, Viollet B, Du M & Pru JK 2018. A functional role for AMPK in female fertility and endometrial regeneration. Reproduction 156 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsivais D, Clementi C, Peng J, Fullerton PT Jr. Prunskaite-Hyyrylainen R, Vainio SJ, Matzuk MM 2017. BMP7 induces uterine receptivity and blastocyst attachment. Endocrinology 158 979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL 2001. Cellular and molecular responses to the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci 98 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi QR, Zhao XY, Zuo RJ, Wang TS, Gu XW, Liu JL, Yang ZM 2015. Involvement of atypical transcription factor E2F8 in the polyploidization during mouse and human decidualization. Cell Cycle 14 842–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saben J, Lindsey F, Zhong Y, Thakali K, Badger TM, Andres A, Gomez-Acevedo H & Shankar K 2014. Maternal obesity is associated with a lipotoxic placental environment. Placenta 35 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleh N & Giribabu N 2014 Leukemia Inhibitory Factor: Roles in Embryo Implantation and in Nonhormonal Contraception. ScientificWorldJournal 2014. [DOI] [PMC free article] [PubMed]
- Santiquet N, Sasseville M, Laforest M, Guillemette C, Gilchrist RB & Richard FJ 2014. Activation of 5’ adenosine monophosphate-activated protein kinase blocks cumulus cell expansion through inhibition of protein synthesis during in vitro maturation in Swine. Biol Reprod 91 51. [DOI] [PubMed] [Google Scholar]
- Simon L, Spiewak KA, Ekman GC, Kim J, Lydon JP, Bagchi MK, Bagchi IC, DeMayo FJ & Cooke PS 2009. Stromal progesterone receptors mediate induction of Indian Hedgehog (IHH) in uterine epithelium and its downstream targets in uterine stroma. Endocrinology 150 3871–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Chaudhry P & Asselin E 2011. Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. J Endocrinol 210 5–14. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP 2005. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41 58–66. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondazo SJ. 1992. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359 76–79. [DOI] [PubMed] [Google Scholar]
- Sun X, Park CB, Deng W, Potter SS, Dey SK 2016. Uterine inactivation of muscle segment homeobox (Msx) genes alters epithelial cell junction proteins during embryo implantation. FASEB J 30 1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosca L, Chabrolle C, Uzbekova S & Dupont J 2007a. Effects of metformin on bovine granulosa cells steroidogenesis: possible involvement of adenosine 5’ monophosphate-activated protein kinase (AMPK). Biol Reprod 76 368–378. [DOI] [PubMed] [Google Scholar]
- Tosca L, Froment P, Solnais P, Ferre P, Foufelle F & Dupont J 2005. Adenosine 5’-monophosphate-activated protein kinase regulates progesterone secretion in rat granulosa cells. Endocrinology 146 4500–4513. [DOI] [PubMed] [Google Scholar]
- Tosca L, Rame C, Chabrolle C, Tesseraud S & Dupont J 2010. Metformin decreases IGF1-induced cell proliferation and protein synthesis through AMP-activated protein kinase in cultured bovine granulosa cells. Reproduction 139 409–418. [DOI] [PubMed] [Google Scholar]
- Tosca L, Solnais P, Ferre P, Foufelle F & Dupont J 2006. Metformin-induced stimulation of adenosine 5’ monophosphate-activated protein kinase (PRKA) impairs progesterone secretion in rat granulosa cells. Biol Reprod 75 342–351. [DOI] [PubMed] [Google Scholar]
- Tosca L, Uzbekova S, Chabrolle C & Dupont J 2007b. Possible role of 5’AMP-activated protein kinase in the metformin-mediated arrest of bovine oocytes at the germinal vesicle stage during in vitro maturation. Biol Reprod 77 452–465. [DOI] [PubMed] [Google Scholar]
- Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C, et al. 2009. AMPK: Lessons from transgenic and knockout animals. Front Biosci (Landmark Ed) 14 19–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waker CA, Albers RE, Pye RL, Doliboa SR, Wyatt CN, Brown TL & Mayes DA 2017. AMPK Knockdown in Placental Labyrinthine Progenitor Cells Results in Restriction of Critical Energy Resources and Terminal Differentiation Failure. Stem Cells Dev 26 808–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H & Dey SK 2006. Roadmap to embryo implantation: clues from mouse models. Nature Reviews Genetics 7 185. [DOI] [PubMed] [Google Scholar]
- Xu X, Leng JY, Gao F, Zhao ZA, Deng WB, Liang XH, Zhang YJ, Zhang ZR, Li M, Sha AG, Yang ZM 2014. Differential expression and anti-oxidant functio of glutathione peroxidase 3 in mouse uterus during decidualization. FEBS Lett 588 1580–1589. [DOI] [PubMed] [Google Scholar]
- Yang X, Xu P, Zhang F, Zhang L, Zheng Y, Hu M, Wang L, Han TL, Peng C, Wen L, et al. 2018. AMPK Hyper-Activation Alters Fatty Acids Metabolism and Impairs Invasiveness of Trophoblasts in Preeclampsia. Cell Physiol Biochem 49 578–594. [DOI] [PubMed] [Google Scholar]
- Yin X, Pavone ME, Lu Z, Wei J & Kim JJ 2012. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J Clin Endocrinol Metab 97 E35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JY, Yang WS, Lee JH, Kim BG, Broaddus RR, Lim JM, Kim TH & Jeong JW 2018. MIG-6 negatively regulates STAT3 phosphorylation in uterine epithelial cells. Oncogene 37 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Hirota Y, Koga K, Hirata T, Yano T, Ayabe T, Tsutsumi O & Taketani Y 2003. Endometrial stromal cells undergoing decidualization down-regulate their properties to produce proinflammatory cytokines in response to interleukin-1 beta via reduced p38 mitogen-activated protein kinase phosphorylation. J Clin Endocrinol Metab 88 2236–2241. [DOI] [PubMed] [Google Scholar]
- Zhang L, Patterson AL, Teixeira JM & Pru JK 2012. Endometrial stromal beta-catenin is required for steroid-dependent mesenchymal-epithelial cross talk and decidualization. Reprod Biol Endocrinol 10 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Lin H, Kong S, Wang S, Wang H & Armant DR 2013. Physiological and molecular determinants of embryo implantation. Mol Aspects Med 34 939–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MJ, Du M, Nijland MJ, Nathanielsz PW, Hess BW, Moss GE & Ford SP 2009. Down-regulation of growth signaling pathways linked to a reduced cotyledonary vascularity in placentomes of over-nourished, obese pregnant ewes. Placenta 30 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]


