Abstract
Purpose
Depression is the most common psychiatric disorder and the largest contributor to global disability. The Australian Genetics of Depression study was established to recruit a large cohort of individuals who have been diagnosed with depression at some point in their lifetime. The purpose of establishing this cohort is to investigate genetic and environmental risk factors for depression and response to commonly prescribed antidepressants.
Participants
A total of 20 689 participants were recruited through the Australian Department of Human Services and a media campaign, 75% of whom were female. The average age of participants was 43 years±15 years. Participants completed an online questionnaire that consisted of a compulsory module that assessed self-reported psychiatric history, clinical depression using the Composite Interview Diagnostic Interview Short Form and experiences of using commonly prescribed antidepressants. Further voluntary modules assessed a wide range of traits of relevance to psychopathology. Participants who reported they were willing to provide a DNA sample (75%) were sent a saliva kit in the mail.
Findings to date
95% of participants reported being given a diagnosis of depression by a medical practitioner and 88% met the criteria for a lifetime depressive episode. 68% of the sample report having been diagnosed with another psychiatric disorder in addition to depression. In line with findings from clinical trials, only 33% of the sample report responding well to the first antidepressant they were prescribed.
Future plans
A number of analyses to investigate the genetic architecture of depression and common comorbidities will be conducted. The cohort will contribute to the global effort to identify genetic variants that increase risk to depression. Furthermore, a thorough investigation of genetic and psychosocial predictors of antidepressant response and side effects is planned.
Keywords: depression & mood disorders, genetics, anxiety disorders
Strengths and limitations of this study.
This study is one of the largest cohorts in the world for studying genetic and psychosocial risk factors for depression and response to antidepressants.
Wide range of measures collected using the online instrument including diagnostic screening questionnaires for depression and anxiety disorders.
Access to government medical and pharmaceutical records.
Low rates of response to the letters sent through the pharmaceutical benefits scheme and high educational attainment of the cohort may indicate a self-selection bias.
Online assessment allowed for recruitment of a large sample but there may be biases attributable to self-report measures and it was not possible to clarify with participants if there were inconsistencies in their responses.
Introduction
Approximately 20% of Australians will be diagnosed with a depressive disorder in their lifetime. As a consequence of this high prevalence, impact on function and risk to later ill-health and premature death, depressive disorders contribute the largest burden of disease due to common mental disorders1 2 and place a substantial burden on the economy in terms of days lost to disability.
Among psychiatric disorders, depression is moderately heritable, with approximately 40% of the variance in liability to depression attributable to genetic factors.3 Initial efforts to identify depression risk variants using genome-wide association studies (GWASs) did not bear fruit due to insufficient power.4 Common genetic variants for psychiatric disorders have small effect sizes and hence sample sizes in the tens of thousands of individuals are needed in order to robustly detect them.5 Substantial progress has been made in the last few years in identifying genetic variants that increase risk to depressive symptoms and major depression.6–8 These discoveries have been facilitated by the collaboration of researchers worldwide in the Psychiatric Genomics Consortium (PGC). The most recent GWAS for depression which included data from the PGC, the personal genetics company 23andMe, the UK Biobank and DeCODE, identified 102 independent genetic variants that increase risk of depression.9 The identified variants explain only a fraction of the overall liability and larger studies are needed to identify more individual variants and to improve the predictive power of polygenic risk scores, a measure of the genetic vulnerability that an individual possesses. Thus, the psychiatric genomics community aims to collect data on 1 million cases with depression in order to elucidate the genetics of this disorder5
Antidepressants are a frontline treatment for moderate-to-severe depression, but do not provide benefit for all patients and have side effects, leading to poor adherence and reduced quality of life. Variability in response to antidepressants and experiencing side effects have a poorly understood genetic component.10 11 As they are one of the most commonly prescribed medications and many individuals are exposed to several different drugs, or drug classes, before symptoms improve, there is an urgent need to understand the reasons for such wide individual variability in therapeutic response and the experience of side effects. Results from pharmacogenetic studies of response and side effects have been mixed, likely because of insufficient sample sizes.12–16
Large studies of deeply phenotyped patients are needed to reveal the biological underpinnings of this clinically heterogeneous disorder and to better match patients to therapies so as to reduce the time to remission. For these reasons, we established the Australian Genetics of Depression Study (AGDS).
Objectives
This study had three primary objectives. The first was to recruit 10 000 cases with depression in Australia to contribute to the global effort to identify genetic variants conferring risk to depression. The second was to further elucidate genetic and non-genetic risk factors for antidepressant response and side effects. The third was to dissect genetic heterogeneity in depression by leveraging existing GWAS results for depression to investigate whether there are differences among subtypes of depression. Our aim was to contribute to the wider PGC effort by increasing the sample size of cases of depression in order to identify genetic variants that increase risk to the disorder, as well as antidepressant response. Here we describe the aims of the study, the genetic and phenotype data collection procedures and the characteristics of the sample.
Cohort description
Design
The AGDS is an analytical study designed to assess the contribution of genetic variation to risk of depression and therapeutic response to antidepressants. In order to maximise the sample size for genetic analysis, the focus was on recruiting participants who had been diagnosed with depression at some point in their life. An online survey was used to assess history of depression and use and experiences of antidepressants. Controls for genetic analysis come from a separate study conducted in Queensland in which participants were asked if they were ever diagnosed with depression.
Recruitment strategy
Cases
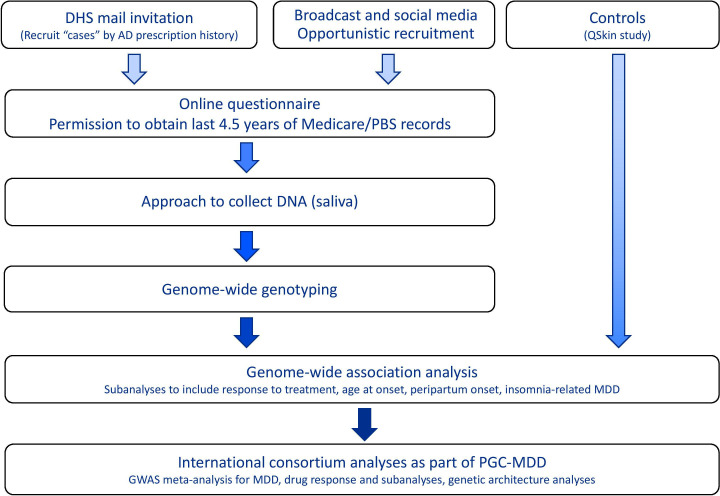
Participants were recruited to the AGDS (www.geneticsofdepression.org.au) using two separate approaches: (1) recruitment based on nationwide, pharmaceutical prescription history in the last 4.5 years and (2) a media publicity campaign throughout Australia. A schematic of the design and aims of the study is shown in figure 1.
Figure 1.
Schematic of the AD, Antidepressant; AGDS. AGDS, Australian Genetics of Depression Study; DHS, Department of Human Services; GWAS, genome-wide association study; MDD, Major Depressive Disorder; PBS, Pharmaceutical Benefits Scheme; PGC, Psychiatric Genomics Consortium.
Recruitment via pharmaceutical prescription history
The Australian Government subsidises certain healthcare services through the Medicare Benefits Scheme (MBS) and prescription medications through the Pharmaceutical Benefits Scheme (PBS). Records for the most recent 4.5 years’ services provided are retained by the Australian Government Department of Human Services (DHS). While these records are not accessible to researchers for the purposes of identifying potential research study participants, DHS is able to send invitations on behalf of researchers to individuals meeting specific selection criteria to invite them to participate in relevant research studies.
After receiving approval from the DHS research ethics committee, two waves of recruitment were undertaken using this method. A pilot study in which DHS sent 10 000 invitation letters to Australian residents aged 18–30 who had received four or more prescriptions in the previous 4.5 years for any of the 10 most commonly prescribed antidepressant medications (single medication or a combination) was initiated in September 2016. Only community patients were selected; individuals with residential locations in the PBS database corresponding to hospitals, aged-care facilities and correctional facilities were excluded as obtaining a saliva sample would not be possible. This group of invitees was 65% female, reflecting the higher prevalence of depression in women. Potential participants were sent a letter by the DHS explaining that they were being contacted on behalf of researchers at QIMR Berghofer to invite them to participate in a study of the genetics of depression. The letter provided details of the study website and also a phone number that they could contact for more information. A total of 294 individuals responded to this invitation over a 6-month period and enrolled in the study.
The second DHS-based recruitment wave started in April 2017 and involved sending 100 000 invitation letters using similar selection criteria to the pilot study, except that the upper age restriction for participants was removed.
Recruitment through media publicity campaign
A Sydney-based public relations company specialising in health sector campaigns (VIVA! Communications) was contracted to manage the media campaign, which was launched on 4 April 2017 and utilised a combination of national broadcast, print and social media to promote knowledge of and interest in the study among the general community. This coincided with the second wave of recruitment through DHS. The campaign encouraged participation among ‘Australian adults who have been, or are continuing to be treated for clinical depression by a doctor, psychologist or psychiatrist’. A second wave of the media campaign was initiated 6 months after the initial one in September 2017 using similar procedures.
Enrolment procedure
In both the DHS recruitment letter and the media public appeal, potential participants were asked to go to the study website which was hosted on the secure QIMR Berghofer server. On going to the website, the information sheet which provided details of the aims of the study as well as a consent form was available for viewing. The information sheet provided telephone and email contact details for the study coordinator and institute ethics committee in case participants had any questions. Those not interested in participating were provided with simple instructions on how to exit the website. The identity of potential participants was not known to the researchers prior to their decision to enrol in the study. The DHS did not provide identifying information to the research team on who was mailed. Before being asked to provide any identifying information, prospective participants were asked to confirm that they had read and understood the information sheet, would be willing to provide a saliva sample for genotyping and sign the study informed consent.
On confirming that they would like to enrol in the study, participants were asked to provide their name, age and contact details which were stored securely on the QIMR server. After providing these details, each participant was assigned a unique link to the questionnaire which was hosted on the Qualtrics website. This transition between websites was seamless to the participant. Participation in this study was not remunerated.
Record linkage—access to Medicare and PBS records
Participants were also asked to consent to provide access to their list of Medicare and PBS records for the previous 4.5 years, and approximately 75% of participants did so. This consent process was separate to the overall consent to participate in the study, and participants could still enrol in the study without allowing access to these records. The consent form had to conform to the requirements of the DHS. Participants were shown an example of what MBS and PBS records look like prior to consenting so they would know what information would be available to researchers. Within the MBS and PBS data, the identifiers for the providing doctor, medical service or pharmacy are randomised so the provider and location are protected. It is possible to identify repeated claims from the same provider but not who the provider is.
Measures
Development and structure of the questionnaire
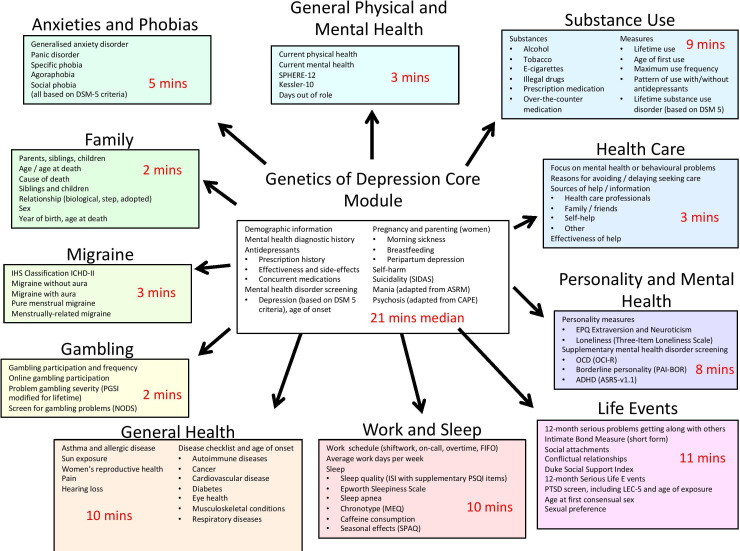
The content of the Australian Genetics of Depression Study online questionnaire was developed over a period of 19 months between January 2015 and September 2016. The object was to maximise the amount of clinically relevant information collected with the shortest time commitment required of participants. To this end, we utilised a modular structure (figure 2), with a core module eliciting essential information on self-report mental health diagnoses, medication response and side effects, depression diagnosis using the relevant section from the Composite International Diagnostic Interview (CIDI), screens for suicidality, mania and psychosis and a question about family history of psychiatric disorders. Several psychiatrists in Australia and internationally with expertise in gene mapping studies and in studies of antidepressant response were consulted about the content of the questionnaire.
Figure 2.
Overview of the structure and content of the AGDS questionnaire with median amount of time taken to complete each module during piloting of the questionnaire. AGDS, Australian Genetics of Depression Study.
Ten additional ‘satellite’ modules assessed a range of complex traits of relevance to mental health using a variety of scales and questionnaires (figure 2). One module screened for clinical anxiety using the CIDI. The questionnaire was administered online using the Qualtrics software. Responses to individual questionnaire items were only required for items critical to phrasing of future questionnaire items and skip functionality (eg, age, sex, number of children). The satellite modules could be completed in any order the participant chose once they had completed the core module. Participants were able to leave the survey and return at their convenience. Rates of completion of the satellite modules are shown in online supplementary table 1. They ranged from 58% for the Games and Gambling module to 76% for the Experiences of Healthcare module.
bmjopen-2019-032580supp002.pdf (2.6MB, pdf)
Extensive beta testing was conducted by research staff at QIMR Berghofer and external consultants to ensure that there were no inconsistencies in the questionnaire and that the appropriate question skips were in place.
Screenshots of the title page, sections of the questionnaire and the module selection page are shown in online supplementary figure 1a-d.
Study measures
As shown in figure 2, a wide range of self-report variables of relevance to mental health were collected. For brevity, we report only on the primary measures of interest. The full questionnaire is available as a online supplementary appendix.
Measures—core module
Mental health history
Participants were asked ‘Have you ever been diagnosed with any of the following’ and were presented with a list of mental health disorders with ‘Depression’ as the first response option. We also evaluated whether participants met the 2013 update to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5, American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (Fifth ed.). Arlington, VA: American Psychiatric Publishing. pp 5–25) criteria for major depressive disorder using the CIDI. The diagnostic questions for depression were focused on the worst period of depression that a participant had experienced. Age at worst episode as well as the age at which the participant had first had a 2-week period of dysphoria or anhedonia as well as age at most recent episode were assessed. Participants were also asked to report the number of periods of at least 2 weeks of dysphoria or anhedonia they had ever had.
Antidepressants
To assess whether participants had taken antidepressants to treat depression, the question ‘Have you ever taken any of the following antidepressants (even if it wasn’t for depression or anxiety)?’ was presented with a list of the 20 most commonly used antidepressants in Australia in addition to their common trade names. If they had taken one or more of the 10 most frequently prescribed antidepressants in Australia according to PBS records (sertraline, escitalopram, venlafaxine, fluoxetine, citalopram, desvenlafaxine, duloxetine, mirtazapine, amitriptyline and paroxetine), they were then asked ‘Why were you prescribed (name of antidepressant)’. The focus on collecting more detailed information on the 10 most common antidepressants was so as to align with the recruitment criteria from the PBS.
Benefits and side effects of the 10 most common antidepressants
Perceived effectiveness of each antidepressant medication was assessed by asking participants ‘How well does/did (name of antidepressant) work for you?’, with response options of ‘very well’, ‘moderately well’, ‘not at all well’ and ‘don’t know’. Participants were also asked to select from a list of all side effects that they experienced from taking each antidepressant. The list of side effects was generated from the ‘very common’ (frequency ≥10%) and ‘common’ (frequency ≥1% and<10%) side effects listed in the Consumer Medication Information for each antidepressant. A total of 24 side effects were included with an ‘other’ option also provided. Participants were also asked if they stopped taking any of the antidepressants because of side effects.
Saliva collection and DNA extraction
Several brands of saliva DNA kits were tested for suitability for use, including cost, ease of handling and yield and quality of extracted DNA. Among those with the best quality reports, the Isohelix GeneFix GFX-02 2 mL saliva collector was selected due to it being the most compact, reliable, easy to use, lightweight, and therefore the least expensive to mail to participants.
After completing the core module of the questionnaire, participants were emailed to confirm their delivery address and their readiness to receive a saliva DNA kit. On confirmation, they were mailed a spit kit, together with a consent form specific to the treatment of genetic information to be signed and returned with the tube. We found that this confirmation step markedly increased compliance. Saliva samples were returned by study participants by prepaid post. If the kit was not returned after 2 months, study personnel followed up by phone or email in order to maximise return rates. On return of the kit, DNA was extracted from the saliva sample and stored in freezers.
Genotyping was conducted using the Illumina Global Screening Array V.2.0 (GSA) and is now ready for analysis. GSA was developed by human genetic disease researchers to maximise utility for gene mapping. It includes a common variant backbone component that maximises information for imputation of common variants in multiple ethnic populations as well as a suite of common and rare variants selected for known or likely association with a range of genetic disorders. Importantly for the purposes of this study, it includes several genetic variants with known pharmacogenetic associations from the Pharmacogenomics Knowledgebase (PharmGKB, https://www.pharmgkb.org/).
Participant and patient involvement
Patients were not consulted directly about the design of the study but a number of psychiatrists were consulted to ensure that the outcome measures reflect the variety of patient experiences seen in clinical practice. Two participants were featured in the promotional material and press conference for the study to encourage others with a history of clinical depression to enrol in the study. All papers that include data from the cohort will be sent to participants via email.
Controls—the QSkin study
The primary aim of the AGDS was to recruit as many individuals with depression as possible. There was no publicity initiated to recruit controls because an appropriate control sample is available at QIMR Berghofer from the QSkin Sun & Health Study. QSkin was established in 2010 to investigate risk factors for melanoma and other skin cancers in a randomly sampled cohort of individuals aged between 40 and 69 years from the state of Queensland.17 To date, more than 40 000 participants have enrolled in QSkin. Recently, a genetics arm of the study was initiated following a similar protocol for collection of DNA using saliva kits returned by mail. At the time of saliva collection, participants were asked about their medical history, including whether they have ever been diagnosed with or treated for depression, bipolar disorder, schizophrenia/psychosis, anxiety, obsessive compulsive disorder, bulimia, anorexia nervosa, autism or attention deficit hyperactivity disorder (ADHD). In addition, women were asked if they experienced either antenatal or postnatal depression. Moreover, participants were consented for access to MBS and PBS records which will permit screening for use of antidepressants in addition to the disease checklist screening items above. QSkin is a separate study to the AGDS and hence the QSkin participants did not complete the detailed questionnaire used in the AGDS.
More than 18 000 participants have been genotyped on the same Single Nucleotide Polymorphism (SNP) microarray chip—the Illumina GSA—and the genotype data will be merged with the AGDS study prior to genome-wide imputation. The QSkin study thus provides a large sample of Australian controls selected at random from the population and genotyped on the same SNP chip.
Results
Sample characteristics
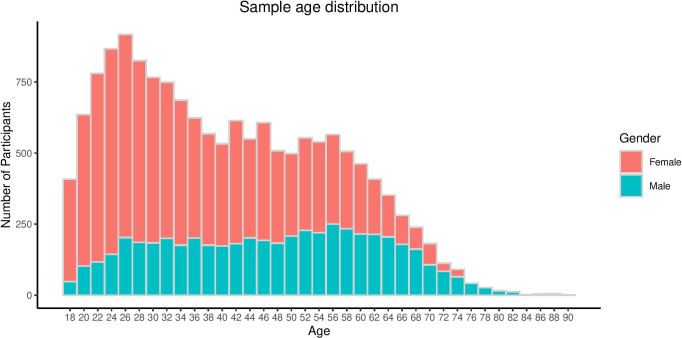
As of 3 September 2018, questionnaire responses had been received from 20 689 participants, 75% of whom were female. The age distribution of participants, by sex, is shown for this recruitment wave in figure 3. By the same date, saliva samples were returned by 15 807 participants (76% of the participant group). The average age of participants was 43 years±15 years (range 18–90 years), with the demographic characteristics of the cohort, as a function of recruitment method, being outlined in table 1.
Figure 3.
Age distribution by sex of participants in AGDS. AGDS, Australian Genetics of Depression Study.
Table 1.
Demographic and study participation characteristics of the study sample
| Prescription history invitation | Public appeal | Total in AGDS | QSkin (genotyped sample) | |
| Number of participants | 2963 | 17 726 | 20 689 | 17 218 |
| Age in years | ||||
| Mean (SD) | 45.5 (16.3) | 42.3 (15.1) | 42.8 (15.3) | 60.8 (8.9) |
| Range | 18 – 89 | 18 – 90 | 18 – 90 | 43 – 87 |
| Sex | ||||
| Female | 2192 (74%) | 13 323 (75%) | 15 515 (75%) | 9469 (55%) |
| Male | 771 (26%) | 4376 (25%) | 5147 (25%) | 7749 (45%) |
| Unspecified | 0 (0%) | 27 (0.2%) | 27 (0.1%) | 0 (0%) |
| Marital status | N/A | |||
| Never married | 788 (27%) | 5604 (32%) | 6392 (31%) | |
| Married/de facto relationship | 1678 (57%) | 9079 (51%) | 10757 (52%) | |
| Separated/divorced | 423 (14%) | 2733 (15%) | 3156 (15%) | |
| Widowed | 64 (2%) | 276 (1.5%) | 340 (1.6%) | |
| Information not provided | 10 (0.3%) | 34 (0.2%) | 44 (0.2%) | |
| Education (completed or partially completed) | ||||
| Junior high school or less | 286 (9%) | 842 (5%) | 1118 (5.4%) | 1003 (6%) |
| Senior high school | 318 (11%) | 1283 (7%) | 1601 (7.7%) | 5568 (31%) |
| Certificate or diploma | 819 (28%) | 3653 (21%) | 4472 (22%) | 5001 (28%) |
| Degree | 772 (26%) | 5837 (33%) | 6609 (32%) | 4960 (28%)* |
| Postgraduate | 556 (19%) | 4448 (25%) | 5004 (24%) | |
| Information not provided | 212 (7%) | 1663 (9%) | 1885 (10%) | 1104 (6%) |
| Provided saliva sample | 2217 (75%) | 13 339 (76%) | 15 616 (76%) | 17 218 (100%) |
| Permitted Medicare and PBS data access | 2637 (89%) | 13 117 (74%) | 15 754 (76%) | 16 482 (95.7%) |
*In the QSkin sample, participants were not asked whether they had a postgraduate degree. Those with postgraduate degrees will be included in the degree category.
PBS, Pharmaceutical Benefits Scheme.
Findings to date
Mental health history
Among respondents, 98.5% reported having discussed mental health problems with a professional and 19 803 (93.4%) respondents reported having received a diagnosis of depression. The next most commonly reported diagnoses were anxiety disorder (55.0%), post-traumatic stress disorder (14.0%) and social anxiety disorder (11.4%). The frequency of all self-reported diagnoses is shown in table 2.
Table 2.
Self-reported mental health diagnostic history of study sample.
| Disorder | Count | Percentage of sample endorsing |
| Depression | 19 603 | 94.7 |
| Anxiety disorder | 11 375 | 55.0 |
| PTSD | 2900 | 14.0 |
| Social anxiety disorder | 2359 | 11.4 |
| Panic disorder | 1960 | 9.5 |
| Bipolar | 1943 | 9.4 |
| Personality disorder | 1200 | 5.9 |
| Obsessive compulsive disorder | 1175 | 5.8 |
| ADD/ADHD | 847 | 4.1 |
| Substance use disorder | 764 | 3.7 |
| Anorexia Nervosa | 731 | 3.6 |
| Specific phobia | 724 | 3.6 |
| Bulimia nervosa | 638 | 3.1 |
| Seasonal affective disorder | 582 | 2.8 |
| Agoraphobia | 448 | 2.2 |
| Autism | 331 | 1.6 |
| Schizophrenia | 184 | 0.9 |
| Hoarding disorder | 100 | 0.5 |
| Tourette's | 27 | 0.1 |
Participants may report more than one diagnosis.
ADD, attention deficit disorder; ADHD, attention-deficit hyperactivity disorder; PTSD, post-traumatic stress disorder.
Depression diagnosed by CIDI
The DSM-5 outlines the following criteria for a depressive episode: dysphoria and/or anhedonia most of the day, nearly every day for at least 2 weeks and experiencing at least five out of nine symptoms (including dysphoria or anhedonia). Consistent with the high rates of self-report diagnosis in the sample, 17 698 out of 20 165 individuals who completed the depression screening section met the criteria for a depressive episode. Additionally, 358 individuals reported not having had a 2-week period of dysphoria or anhedonia; another 1239 reported that their symptoms persisted for less than half the day and 161 did not endorse at least five of the nine symptoms required.
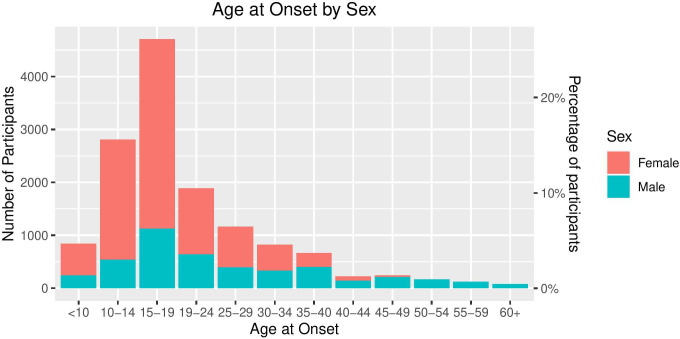
Mean age at onset was 22. The distribution of age at onset by sex is shown in figure 4. Consistent with previous studies, the peaks between ages 10–15 and 16–20 highlight that adolescence is a peak time for developing depression. The proportion of men in each category increases with increasing age, highlighting that men are more at risk to develop depression later in life.
Figure 4.
Age at onset of depression by sex.
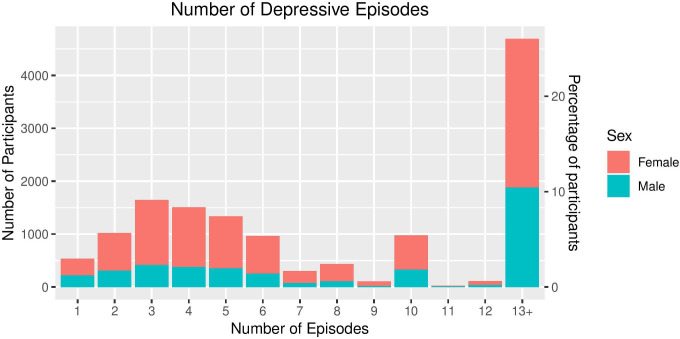
The median number of episodes reported was 6, with the most commonly reported number of periods of at least 2 weeks with depression being 13+. Only 4% of the sample report experiencing only one depressive episode (figure 5), indicating that the sample is enriched for severe, recurrent depression.
Figure 5.
Number of reported depressive episodes among those meeting the criteria for major depressive disorder by sex.
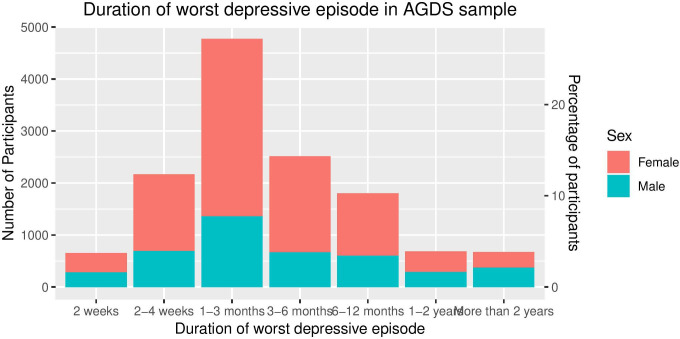
The median duration of the worst episode was 12 weeks. More than 10% of the sample reported that the worst episode that they experienced was longer than a year in duration (figure 6).
Figure 6.
Duration of worst depressive episode by sex. AGDS, Australian Genetics of Depression Study.
Family history
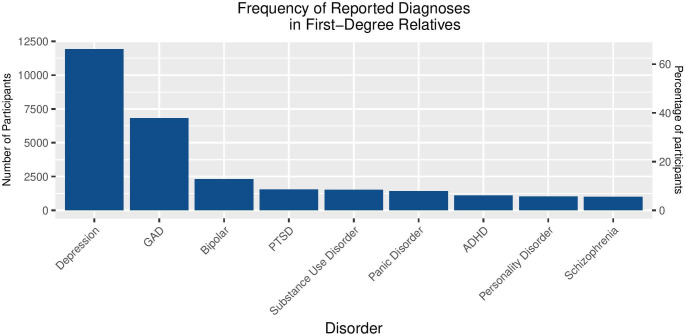
Out of 19 400 individuals who responded to the question about family history, 13 505 (70%) reported that a first-degree relative (parent, sibling or child) had been diagnosed with a mental health disorder. The most commonly reported diagnosis in relatives was depression, (with 11 929 individuals), followed by generalised anxiety disorder and bipolar disorder (figure 7).
Figure 7.
Frequency of reported diagnoses in first-degree relatives of participants. ADHD, attention-deficit/hyperactivity disorder; GAD, generalised anxiety disorder; PTSD, post-traumatic stress disorder.
Antidepressant usage
A total of 95% of the sample (n=19 585) reported taking an antidepressant. Of those reporting antidepressant use, 93% (n=18 174) reported taking the antidepressant for depression and 51% reported taking for anxiety.
Among those taking antidepressants, the mean number of antidepressants taken was 2.75 (SD=2.05, range=1–14). Only 33% of the sample had ever taken only one antidepressant, with 42% reporting having taken three or more different antidepressants (figure 8).
Figure 8.
Distribution of the number of prescribed antidepressants taken by participants. AGDS, Australian Genetics of Depression Study.
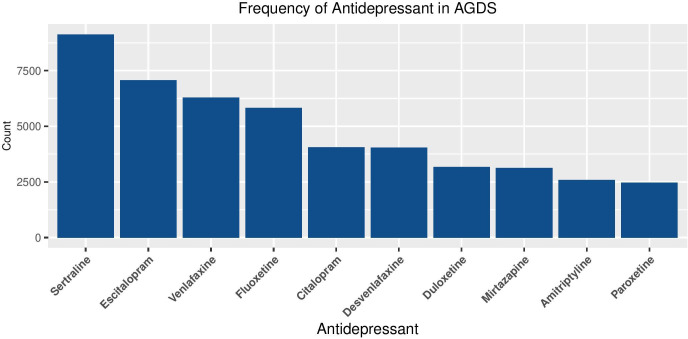
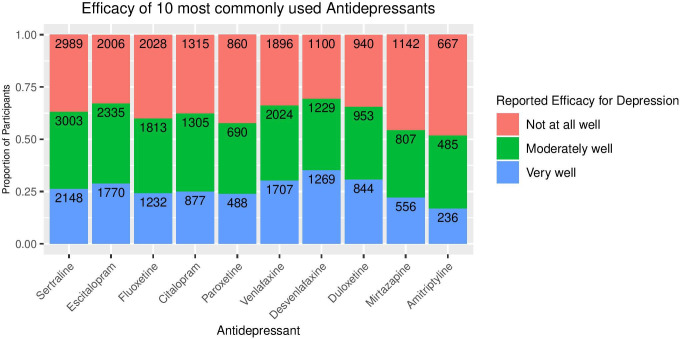
For the 10 most common antidepressants listed, the number and percentage of participants with experiences of each medication are shown in table 3. Reported effectiveness of the 10 most common antidepressants is shown in figure 9. The rates of endorsement of the most common side effects across the 10 most common antidepressants are shown in table 4. More detailed analyses on the therapeutic benefits and side effects of different antidepressants will follow in subsequent papers.
Table 3.
Frequency of antidepressants taken in AGDS.
| Antidepressant | Count | Percentage of sample endorsing |
| Sertraline | 9132 | 44.12 |
| Escitalopram | 7076 | 34.19 |
| Venlafaxine | 6287 | 30.38 |
| Fluoxetine | 5823 | 28.14 |
| Citalopram | 4060 | 19.62 |
| Desvenlafaxine | 4042 | 19.53 |
| Duloxetine | 3168 | 15.31 |
| Mirtazapine | 3134 | 15.14 |
| Amitriptyline | 2593 | 12.53 |
| Paroxetine | 2471 | 11.94 |
| Other | 2212 | 10.69 |
| Fluvoxamine | 793 | 3.83 |
| Moclobemide | 491 | 2.37 |
| Dothiepin | 448 | 2.16 |
| Nortriptyline | 345 | 1.67 |
| Reboxetine | 341 | 1.65 |
| Imipramine | 322 | 1.56 |
| Doxepin | 287 | 1.39 |
| Clomipramine | 228 | 1.1 |
| Tranylcypromine | 212 | 1.02 |
| Phenelzine | 146 | 0.71 |
| Mianserin | 86 | 0.42 |
| Never taken antidepressants | 976 | 4.72 |
Participants may report taking more than one antidepressant.
AGDS, Australian Genetics of Depression Study.
Figure 9.
Reported efficacy of the most commonly prescribed antidepressants (numbers with each response are shown inside the bar).
Table 4.
Proportion of all individuals who have taken one of the top 10 most commonly prescribed antidepressants that endorse each side effect
| Side effect | Percentage of sample endorsing |
| Reduced sex drive | 35.0 |
| Weight gain | 26.3 |
| Dry mouth | 21.6 |
| Nausea | 17.6 |
| Drowsiness | 16.1 |
| Insomnia | 16.0 |
| Dizziness | 15.6 |
| Fatigue | 14.4 |
| Sweating | 14.0 |
| Headache | 14.0 |
| Suicidal thoughts | 12.3 |
| Anxiety | 11.6 |
| Agitation | 11.4 |
| Shaking | 9.3 |
| Constipation | 6.6 |
| Diarrhoea | 4.7 |
| Suicide attempt | 4.3 |
| Blurred vision | 3.9 |
| Muscle pain | 3.4 |
| Vomiting | 2.7 |
| Weight loss | 2.4 |
| Runny nose | 1.3 |
| Rash | 1.0 |
Discussion
The AGDS was established to recruit a large sample of participants in Australia who have experienced depression in order to better understand risk factors for depression, treatment response and side effects. Participants provided extensive information on their experience with depression through a web-based questionnaire and the majority provided a saliva sample for genotyping. Through two modes of recruitment—government medical and pharmaceutical records and a large media campaign—more than 20 000 individuals were recruited to participate over a 2-year period. With extensive follow-up through email and, at the stage of getting saliva samples returned, phone follow-up by experienced interviewers, 76% of those enrolled returned a saliva sample.
The media campaign was the more successful of the two methods as more than 80% of the sample was recruited in this way. Approximately 2.5% of those sent letters by the DHS enrolled in the study. There may be several reasons for the low rate of participation from this method. First, as antidepressants are prescribed for a range of conditions, many of those sent letters may not have had depression and hence decided not to participate. Second, letters may be easily discarded by recipients as unsolicited mail may not be well received. Lastly, the media campaign included interviews with both study investigators and individuals with lived experience of depression who encouraged others to participate. As more information can be conveyed about the importance of the research through a TV or radio interview, it likely had a bigger impact on potential participants.
While the media campaign was more effective for this study, depression is a relatively common disorder and therefore amenable to a media campaign that reaches a substantial proportion of the population. For rarer disorders, recruitment through the PBS could be an efficient method of reaching potential participants, particularly when a drug is used to treat only one disorder and so all those prescribed with it will have a diagnosis.
The primary focus of the study was to recruit cases because of the availability of the QSkin sample for use as controls for genetic analyses. QSkin participants have already been genotyped on the same SNP chip. However, the Qskin participants were not administered the full questionnaire and a single question about a prior diagnosis of psychiatric disorders is used to define controls for inclusion. Some participants may have had depression but did not receive a diagnosis and will be incorrectly included as controls. The Qskin cohort is older than the AGDS cohort (mean age 60.8 years vs 42.8 years). This means that most participants are past the peak age at onset for depression and are unlikely to go on to be diagnosed with depression.
The mean age among those recruited through the media was lower than through the PBS and had higher rates of university completion. This suggests that the former may be closer to a random sample from the population. Likewise, there are differences in the education level (78% with a post high school qualification compared with 56%) between the case sample from AGDS and the controls from QSkin. Some of the differences in education level may be a cohort effect attributable to the age difference between the cohorts, as the proportion of the population with tertiary qualifications is increasing over time. According to Australian census data, the proportion of the population with a post school qualification increased from 46% to 56% between 2006 and 2016.18 However, there may be a response bias whereby participants with higher levels of education are more likely to enrol in a genetic study. Higher levels of education have been found to be associated with participation in the optional components of volunteer studies such as the Avon Longitudinal Study of Parents and Children19 20 and UK Biobank.21 22 These differences could confound genetic association results and therefore we will conduct a number of sensitivity analyses such as comparing only cases and controls with matched education levels to investigate the influence of education differences on the analyses. Likewise, we will compare differences between those who returned a spit kit and those who did not return a kit to assess whether there is response bias that needs to be addressed.
Volunteer participation could also cause bias towards recruiting participants with less severe forms of depression. We will endeavour to investigate this response bias by comparing results from our analyses with those from smaller datasets recruited in clinical settings and to other datasets with a broad spectrum of severity of depression. It has been shown that those with more severe depression have higher mean polygenic risk scores for depression than those with less severe depression. By comparing the distribution of polygenic risk scores to other samples, we can assess the effect of response bias on the severity of depression in AGDS. Our initial analyses suggest that many of the participants have had severe depression as they report large numbers of episodes and nearly 50% report having had symptoms in the past 4 weeks. Likewise, the reported rates of response to the first prescribed antidepressant are nearly identical to those from the STAR*D clinical trial (33%).23Based on the self-report data on number of episodes and other measures of severity, the AGDS sample has high rates of severe depression.
Our results highlight the high rate of comorbidities with depressive disorders in real-world settings.24 Understanding the pattern of comorbidities and how it relates to response to treatment, emergence of side effects (eg, greater anxiety or agitation in those with comorbid anxiety disorders), and underlying genetic variations are aspects of the disorder that this scale of study can address. Specifically it will be of interest to test if there are different genetic or environmental risk factors to onset, course of illness, response to pharmacological treatment or emergence of specific side effects for those with depression and comorbid anxiety compared with depression without anxiety. In addition, we will test specific proposed subtypes of depression (eg, perinatal depression, atypical depression, chronic depression, early-onset vs late-onset depression or depression with hypomanic or brief manic features) that may show evidence of distinct genetic risk factors for onset or treatment response.
Participants reported high rates of mental disorders in their first-degree relatives, highlighting the well-established genetic component of and the covariance between psychiatric disorders.25 High rates of familial disorders may reflect that participants were more likely to participate in a genetic study if they have a family history or that participants shared details of the study with family members. Familial relationships within the participants will be controlled for in future genetic analyses.
Nearly half of participants reported taking three or more antidepressants to treat depression and thus having considerable time to improvement in symptoms. Moreover, side effects are common and in many cases cause individuals to stop taking a drug. These results confirm the urgent need to identify risk factors for non-response to certain drugs and to reduce side effects. Not only will such advances improve the lives of patients but they will also assist to reduce costs attributable to delays in achieving illness remission. When PBS records become available, we will be able to investigate the concordance with self-report information on drug response over the past 4.5 years. In collecting a wide range of environmental, social and genetic data, AGDS will make a significant contribution to our understanding of variability in response and side effects.
Collaboration
We have established a cohort with rich information on history of mental illness and use of antidepressant medication use. A primary motivation for establishing this cohort was to contribute to global efforts to identify genetic risk factors for depression and treatment response through the PGC. Summary genetic association statistics for this cohort will be available through the PGC. We encourage collaborations with researchers from other studies to investigate the aetiology of complex traits ascertained in AGDS to maximise sample sizes for analysis. The full questionnaire is available in the online supplementary material. Researchers are encouraged to contact Nick Martin (nick.martin@qimrberghofer.edu.au) to discuss collaboration. All proposals will be reviewed by the principal investigators of the AGDS.
bmjopen-2019-032580supp001.pdf (797.6KB, pdf)
Supplementary Material
Acknowledgments
We are indebted to all of the participants for giving their time to contribute to this study. We wish to thank all the people who helped in the conception, implementation, beta testing, media campaign and data cleaning. We would specifically like to acknowledge Dale Nyholt for advice on using the PBS for research; Ken Kendler, Patrick Sullivan, Andrew McIntosh and Cathryn Lewis for input on the questionnaire; Lorelle Nunn, Mary Ferguson, Lucy Winkler and Natalie Garden for data and sample collection; Natalia Zmicerevska, Alissa Nichles and Candace Brennan for participant recruitment support. Jonathan Davies, Luke Lowrey and Valeriano Antonini for support with IT aspects; Vera Morgan and Ken Kirkby for help with the media campaign. We would like to thank VIVA! Communications for their effort in promoting the study. We also acknowledge David Whiteman and Catherine Olsen from QSkin.
Footnotes
Twitter: @byrnafyrna, @ColodroConde
Contributors: EB, KK, SEM, JM, RP, NW, IBH and NM designed the AGDS study. DFL, SC, DS, LS and JL revised and tested the online questionnaire and provided intellectual input into the content. EB and KK analysed the data. EB, KK and NM drafted the manuscript. SEM, LCC, JM, NW, IBH, RP, LS and DS revised the article for intellectual content. All authors have read and approved the final version of the manuscript.
Funding: The AGDS was primarily funded by National Health and Medical Research Council (NHMRC) of Australia grant 1086683. This work was further supported by NHMRC grants 1145645, 1078901 and 1087889. LCC is supported by a QIMR Berghofer Institute fellowship.
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: All study protocols were approved by the QIMR Berghofer Medical Research Institute Human Research Ethics Committee. The protocol for approaching participants through the DHS, enrolling them in the study and consenting for all phases of the study (including invitation to future related studies) and accessing MBS and PBS records was approved by the Ethics Department of the Department of Human Services.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Data used in this analysis and described in this article are available to all interested researchers through collaboration. Please contact NGM (Nick.Martin@qimrberghofer.edu.au).
References
- 1.Whiteford HA, Degenhardt L, Rehm J, et al. . Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet 2013;382:1575–86. 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- 2.Ferrari AJ, Charlson FJ, Norman RE, et al. . Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med 2013;10:e1001547. 10.1371/journal.pmed.1001547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000;157:1552–62. 10.1176/appi.ajp.157.10.1552 [DOI] [PubMed] [Google Scholar]
- 4.Wray NR, Pergadia ML, Blackwood DHR, et al. . Genome-Wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol Psychiatry 2012;17:36–48. 10.1038/mp.2010.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan PF, Agrawal A, Bulik CM, et al. . Psychiatric genomics: an update and an agenda. Am J Psychiatry 2018;175:15–27. 10.1176/appi.ajp.2017.17030283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wray NR, Ripke S, Mattheisen M, et al. . Genome-Wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 2018;50:668–81. 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard DM, Adams MJ, Shirali M, et al. . Genome-Wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat Commun 2018;9:1470. 10.1038/s41467-018-03819-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CONVERGE consortium Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature 2015;523:588–91. 10.1038/nature14659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard DM, Adams MJ, Clarke T-K, et al. . Genome-Wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 2019;22:343–52. 10.1038/s41593-018-0326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tansey KE, Guipponi M, Hu X, et al. . Contribution of common genetic variants to antidepressant response. Biol Psychiatry 2013;73:679–82. 10.1016/j.biopsych.2012.10.030 [DOI] [PubMed] [Google Scholar]
- 11.Hodgson K, Uher R, Crawford AA, et al. . Genetic predictors of antidepressant side effects: a grouped candidate gene approach in the genome-based therapeutic drugs for depression (GENDEP) study. J Psychopharmacol 2014;28:142–50. 10.1177/0269881113517957 [DOI] [PubMed] [Google Scholar]
- 12.Biernacka JM, Sangkuhl K, Jenkins G, et al. . The International SSRI pharmacogenomics Consortium (IspC): a genome-wide association study of antidepressant treatment response. Transl Psychiatry 2016;6:e937. 10.1038/tp.2016.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uher R, Perroud N, Ng MYM, et al. . Genome-Wide pharmacogenetics of antidepressant response in the GENDEP project. Am J Psychiatry 2010;167:555–64. 10.1176/appi.ajp.2009.09070932 [DOI] [PubMed] [Google Scholar]
- 14.GENDEP Investigators, MARS Investigators, STAR*D Investigators . Common genetic variation and antidepressant efficacy in major depressive disorder: a meta-analysis of three genome-wide pharmacogenetic studies. Am J Psychiatry 2013;170:207–17. 10.1176/appi.ajp.2012.12020237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tansey KE, Guipponi M, Perroud N, et al. . Genetic predictors of response to serotonergic and noradrenergic antidepressants in major depressive disorder: a genome-wide analysis of individual-level data and a meta-analysis. PLoS Med 2012;9:e1001326. 10.1371/journal.pmed.1001326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li QS, Tian C, Seabrook GR, et al. . Analysis of 23andMe antidepressant efficacy survey data: implication of circadian rhythm and neuroplasticity in bupropion response. Transl Psychiatry 2016;6:e889. 10.1038/tp.2016.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsen CM, Green AC, Neale RE, et al. . Cohort profile: the QSkin sun and health study. Int J Epidemiol 2012;41:929 10.1093/ije/dys107 [DOI] [PubMed] [Google Scholar]
- 18.Australian Bureau of Statistics Australians pursuing higher education in record numbers, 2017. Available: https://www.abs.gov.au/AUSSTATS/abs@.nsf/mediareleasesbyReleaseDate/1533FE5A8541D66CCA2581BF00362D1D
- 19.Taylor AE, Jones HJ, Sallis H, et al. . Exploring the association of genetic factors with participation in the Avon longitudinal study of parents and children. Int J Epidemiol 2018;47:1207–16. 10.1093/ije/dyy060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin J, Tilling K, Hubbard L, et al. . Association of genetic risk for schizophrenia with Nonparticipation over time in a population-based cohort study. Am J Epidemiol 2016;183:1149–58. 10.1093/aje/kww009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams MJ, Hill WD, Howard DM, et al. . Factors associated with sharing e-mail information and mental health survey participation in large population cohorts. Int J Epidemiol 2019;15. 10.1093/ije/dyz134. [Epub ahead of print: 01 Jul 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyrrell J, Zheng J, Beaumont R, et al. . Genetic predictors of participation in optional components of UK Biobank. bioRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howland RH. Sequenced treatment alternatives to relieve depression (STAR*D). Part 2: study outcomes. J Psychosoc Nurs Ment Health Serv 2008;46:21–4. 10.3928/02793695-20081001-05 [DOI] [PubMed] [Google Scholar]
- 24.Plana-Ripoll O, Pedersen CB, Holtz Y, et al. . Exploring comorbidity within mental disorders among a Danish national population. JAMA Psychiatry 2019;76:259. 10.1001/jamapsychiatry.2018.3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cross-Disorder Group of the Psychiatric Genomics Consortium, Lee SH, Ripke S, et al. . Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 2013;45:984–94. 10.1038/ng.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-032580supp002.pdf (2.6MB, pdf)
bmjopen-2019-032580supp001.pdf (797.6KB, pdf)