Abstract
The glucosinolate–myrosinase system is a well-known defense system that has been shown to induce stomatal closure in Brassicales. Isothiocyanates are highly reactive hydrolysates of glucosinolates, and an isothiocyanate, allyl isothiocyanate (AITC), induces stomatal closure accompanied by elevation of free cytosolic Ca2+ concentration ([Ca2+]cyt) in Arabidopsis. It remains unknown whether AITC inhibits light-induced stomatal opening. This study investigated the role of Ca2+ in AITC-induced stomatal closure and inhibition of light-induced stomatal opening. AITC induced stomatal closure and inhibited light-induced stomatal opening in a dose-dependent manner. A Ca2+ channel inhibitor, La3+, a Ca2+chelator, EGTA, and an inhibitor of Ca2+ release from internal stores, nicotinamide, inhibited AITC-induced [Ca2+]cyt elevation and stomatal closure, but did not affect inhibition of light-induced stomatal opening. AITC activated non-selective Ca2+-permeable cation channels and inhibited inward-rectifying K+ (K+in) channels in a Ca2+-independent manner. AITC also inhibited stomatal opening induced by fusicoccin, a plasma membrane H+-ATPase activator, but had no significant effect on fusicoccin-induced phosphorylation of the penultimate threonine of H+-ATPase. Taken together, these results suggest that AITC induces Ca2+ influx and Ca2+ release to elevate [Ca2+]cyt, which is essential for AITC-induced stomatal closure but not for inhibition of K+in channels and light-induced stomatal opening.
Keywords: Allyl isothiocyanate, Arabidopsis, calcium channel, potassium channel, proton pump, stomatal closure, stomatal opening
Allyl isothiocyanate (AITC) induces Ca2+ influx and Ca2+ release to elevate [Ca2+]cyt, which is essential for AITC-induced stomatal closure but not for inhibition of K+in channels or light-induced stomatal opening.
Introduction
Stomata, surrounded by pairs of guard cells, function as the main window for gas exchange and are in the frontline for defense against microbe invasion in the phyllosphere. To deal with the changing growth and environmental cues, guard cells have evolved to be specialist responders to various stimuli, such as light, drought stress, CO2, phytohormones, and microbe-derived signals, resulting in stomatal movement (Murata et al., 2015; Ye & Murata, 2016; Inoue & Kinoshita, 2017; Takahashi et al., 2018; Zhang et al., 2018).
The glucosinolate–myrosinase system is a well-known defense system against herbivores and pathogens in Brassicales (Halkier & Gershenzon, 2006; Bednarek et al., 2009; Clay et al., 2009). Myrosinases are highly abundant proteins in guard cells and required for abscisic acid (ABA)-induced stomatal movement (Zhao et al., 2008; Islam et al., 2009). Recent results suggest that aliphatic glucosinolates are involved in stomatal movement regulated by ABA and auxin (Zhu et al., 2014; Salehin et al., 2019). Isothiocyanates (ITCs) are highly reactive hydrolysates of glucosinolates and an ITC, allyl isothiocyanate (AITC), induces stomatal closure in Arabidopsis (Khokon et al., 2011; Hossain et al., 2014). AITC reversibly induces stomatal closure in Vicia faba, which does not belong to Brassicales (Sobahan et al., 2015). Since many ITCs including AITC are volatile, the results imply that ITCs function as signals for plant–plant interaction (Sobahan et al., 2015). To elucidate the mechanism of AITC-induced stomatal closure, it has been shown that AITC induces production of reactive oxygen species (ROS) and elevation of free cytosolic Ca2+ concentration ([Ca2+]cyt) in guard cells (Khokon et al., 2011). Pharmacological studies indicate that ROS are essential for AITC-induced stomatal closure (Hossain et al., 2014; Sobahan et al., 2015).
ABA-induced stomatal movement has long been studied as a signaling model in guard cells. ABA induces stomatal closure and inhibits light-induced stomatal opening. It was widely thought that the molecular mechanisms underlying these two responses overlap, but recent studies provide evidence that the two signaling pathways can be genetically dissected (Yin et al., 2013). It is important to find out whether other guard cell signaling pathways can be dissected. Though AITC induces stomatal closure, it remains unknown whether it inhibits light-induced stomatal opening.
Cytosolic Ca2+ is a well-known second messenger in both stomatal closure and stomatal opening (Shimazaki et al., 2007; Hubbard et al., 2012; Murata et al., 2015). Exogenous Ca2+ application is known to induce stomatal closure and inhibit light-induced stomatal opening (Peiter et al., 2005; Islam et al., 2010). Elevations of [Ca2+]cyt are triggered by influx of Ca2+ from the apoplast and release of Ca2+ from intracellular stores in guard cell signaling (Leckie et al., 1998; Hamilton et al., 2000; Pei et al., 2000; Lemtiri-Chlieh et al., 2003; Garcia-Mata et al., 2003). The influx of Ca2+ is carried by non-selective Ca2+-permeable cation channels (ICa channels) that are activated by plasma membrane hyperpolarization (Hamilton et al., 2000; Pei et al., 2000). Pharmacological studies using nicotinamide (NA), an inhibitor of cyclic adenosine diphosphate ribose (cADPR) synthesis (Dodd et al., 2007), suggest that the release of Ca2+ is mediated by a cADPR-dependent pathway (Allen et al., 1995; Klüsener et al., 2002; Hossain et al., 2014). It is known that cADPR stimulates ryanodine receptors in the endoplasmic reticulum in animal cells, but homologous genes have not been identified in the Arabidopsis genome (Hetherington and Brownlee, 2004), and therefore, it is likely that cADPR functions via a different mechanism in plant cells. Elevation of [Ca2+]cyt activates S-type anion channels in guard cells and inhibits plasma membrane H+-ATPase and inward-rectifying potassium (K+in) channels (Schroeder & Hagiwara, 1989; Kinoshita et al., 1995; Siegel et al., 2009; Wang et al., 2013a). On the other hand, these transporters are subject to regulation by other components in addition to Ca2+, which probably determine signaling specificity (Allen et al., 2002; Siegel et al., 2009; Xue et al., 2011; Ye et al., 2015). Furthermore, a Ca2+-independent pathway exists to regulate stomatal movement (Roelfsema & Hedrich, 2010; Laanemets et al., 2013). Though AITC induces [Ca2+]cyt elevation, it remains unknown how AITC does this and whether the elevation is essential for AITC-induced stomatal movement. The effects of AITC on ICa channels and K+in channels in guard cells remain to be clarified.
In the present study, we aimed to investigate the role of [Ca2+]cyt elevation in AITC-induced stomatal closure and inhibition of light-induced stomatal opening in Arabidopsis. The presented results reveal that AITC induced influx of Ca2+ through activation of ICa channels and Ca2+ release possibly through a cADPR-dependent pathway, resulting in the elevation of [Ca2+]cyt in guard cells. While [Ca2+]cyt elevation was essential for AITC-induced stomatal closure, it was not essential for inhibition of light-induced stomatal opening. The inhibition was attributed to Ca2+-independent inhibition of K+in channels by AITC in guard cells.
Materials and methods
Plant materials and growth conditions
Arabidopsis wild-type plants (Columbia) were grown in pots containing a mixture of 70% (v/v) vermiculite (Asahi-kogyo, Okayama, Japan) and 30% (v/v) Kureha soil (Kureha Chemical, Tokyo, Japan) in a growth chamber (photon flux density of 80 µmol m−2 s−1 under a 16 h light–8 h dark regime). The temperature and relative humidity in the growth chamber were 22±2 °C and 60±10%, respectively. Twice or three times a week, 0.1% Hyponex solution (Hyponex, Osaka, Japan) as a fertilizer was provided to the plants. All the plants used for experiments were from 4 to 6 weeks old.
Stomatal aperture measurement
Fully expanded young leaves from 4- to 5-week-old plants were excised for stomatal aperture measurements as described previously (Ye et al., 2013). For assays of light-induced stomatal opening, leaves were floated on assay solution containing 5 mM KCl, 50 μM CaCl2, and 10 mM MES–Tris (pH 6.15) with their adaxial surface upward in the dark for 2 h to close the stomata. After adding AITC, the leaves were kept in the light (80 μmol m−2 s−1) for 2 h before measurement. For assays of stomatal closure, leaves were floated on the assay solution in the light for 2 h to open the stomata. Then AITC was added, and the leaves were kept in the light for 2 h before measurement. Inhibitors were added 30 min before AITC treatment. For measurement of stomatal apertures, the leaves were shredded for 30 s, and epidermal tissues were collected using nylon mesh. Thirty stomatal apertures were measured for each sample.
Imaging of [Ca2+]cyt in guard cells
Four- to six-week-old wild-type plants expressing YC3.6 were used for the measurement of [Ca2+]cyt in guard cells as described previously (Ye et al., 2013, 2015). The abaxial side of an excised leaf was gently mounted on a glass slide with a medical adhesive (stock no. 7730; Hollister) followed by removal of the adaxial epidermis and the mesophyll tissue with a razor blade in order to keep the lower epidermis intact on the slide. The remaining abaxial epidermis was incubated in solution containing 5 mM KCl, 50 μM CaCl2, and 10 mM MES–Tris (pH 6.15) in the light for 2 h at 22 °C to promote stomatal opening. Turgid guard cells were used to measure [Ca2+]cyt. The observation chamber was perfused with the solution using a peristaltic pump. Guard cells were incubated in the absence of AITC for 5 min and then in the presence of AITC. Inhibitors were added at the time point indicated. For dual-emission ratio imaging of YC3.6, we used an FF-02-438/24-25 (Semrock) excitation filter, a 445DRLP (Omega) dichroic mirror, an XF3075 480AF30 (Omega) emission filter for cyan fluorescent protein (CFP), and an XF3011 535DF25 (Omega) emission filter for yellow fluorescent protein (YFP). The CFP and YFP fluorescence intensity of guard cells were imaged and analysed using the W-View system and AQUA COSMOS software (Hamamatsu Photonics). CFP and YFP fluorescence were simultaneously monitored.
Patch-clamp measurement
Current measurements of ICa and K+in channels in Arabidopsis guard cells were performed as described previously (Mori et al., 2006; Ye et al., 2013). Arabidopsis guard cell protoplasts were prepared from rosette leaves. For ICa current measurement, the pipette solution contained 10 mM BaCl2, 0.1 mM dithiothreitol, 4 mM EGTA, and 10 mM HEPES–Tris, pH 7.1. The bath solution contained 100 mM BaCl2, 0.1 mM dithiothreitol, and 10 mM MES–Tris, pH 5.6. For K+in channel current measurement, the pipette solution contained 30 mM KCl, 70 mM K-Glu, 2 mM MgCl2, 2.4 mM CaCl2 (free Ca2+ concentration, 150 nM), 6.7 mM EGTA, and 10 mM HEPES–Tris, pH 7.1. The bath solution contained 30 mM KCl, 2 mM MgCl2, 40 mM CaCl2, and 10 mM MES–Tris, pH 5.5. In all cases, osmolality was adjusted to 500 mmol kg−1 (pipette solutions) and 485 mmol kg−1 (bath solutions) with D-sorbitol.
KAT1 current recording in Xenopus laevis oocytes
The expression of KAT1 in Xenopus laevis oocytes and current recording were performed according to our previous method (Islam et al., 2015). Before recording, the microinjected oocytes were incubated in ND96 solution containing 94 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES (pH 7.5) with different concentrations of AITC for 2 h.
Immunohistochemical detection of the plasma membrane H+-ATPase in guard cells using a whole leaf
Immunohistochemistry using a whole leaf was performed according to Sauer et al. (2006) and Hayashi et al. (2011) with modifications. Mature leaves were harvested from dark-adapted plants and floated on the basal buffer (5 mM MES–BTP (pH 6.5), 50 mM KCl, and 0.1 mM CaCl2) containing 50 µM AITC for 20 min in the dark. After AITC treatment, 10 µM fusicoccin was added to the buffer and kept for a further 10 min. For the control, 0.1% (v/v) dimethyl sulfoxide was added to the buffer. After treatment, leaves were put into a syringe with fixative (4% (w/v) formaldehyde freshly prepared from paraformaldehyde and 0.3% (v/v) glutaraldehyde in 50 mM PIPES–NaOH (pH 7.0), 5 mM MgSO4, and 5 mM EGTA), and negative pressure applied several times to infiltrate the fixative, followed by immersion in the solution for 1 h in the dark at room temperature. After washing with phosphate-buffered saline (PBS; 137 mM NaCl, 8.1 mM Na2HPO4, 2.68 mM KCl, and 1.47 mM KH2PO4), chlorophyll was removed by pure methanol (20 min incubation at 37 °C three or four times). Then, central areas of the leaves were cut out, and incubated with xylene at 37 °C for 2 min, pure ethanol at room temperature for 5 min, and 50% (v/v; in PBS) ethanol at room temperature for 5 min, and washed with Milli-Q water twice. The material was transferred to MAS-coated microscope slides (Matsunami) with a droplet of water, where the abaxial side of the leaf was attached to the slide, and freeze–thaw treatment applied followed by complete drying overnight at room temperature. Dried samples were rehydrated by PBS for 5 min at room temperature, and digested with 4% (w/v) Cellulase Onozuka R-10 (Yakult) with 0.5% (w/v) Macerozyme R-10 (Yakult) in PBS for 1 h at 37 °C. After digestion, leaf tissue except for the abaxial epidermis attached on the slide was removed stereomicroscopically in PBS, and the left epidermal tissue was washed four times for 5 min each with PBS, then permeabilized with 3% (v/v) IGEPAL CA-630 (MP Biomedicals) with 10% (v/v) dimethyl sulfoxide in PBS for 1 h at room temperature. Samples were washed five times for 5 min each with PBS and incubated with blocking solution (3% (w/v) bovine serum albumin Fraction V (BSA; Thermo Fisher Scientific) in PBS) for 1 h at room temperature. The primary antibody (anti-pThr; Hayashi et al., 2010) was treated with a dilution of 1:1000 in the blocking solution at 4 °C overnight. Samples were washed five times for 5 min each with PBS; secondary antibody (Alexa Fluor 488-conjugated goat anti-rabbit IgG; Thermo Fisher Scientific) with a dilution of 1:500 in the blocking solution was applied at 37 °C for 3 h, followed by washing five times for 5 min each with PBS. The specimens were covered by a coverglass with 50% (v/v) glycerol. Fluorescence images were obtained (Hayashi et al., 2011) and analysed by ImageJ software (National Institutes of Health).
Statistical analysis
The significance of differences between datasets was assessed by Student’s t-test and analysis of variance (ANOVA) with Tukey’s test. The response of [Ca2+]cyt was assessed by χ 2 test. Differences were considered significant for P<0.05.
Results
Effect of inhibitors of Ca2+ influx and Ca2+ release pathways on allyl isothiocyanate-induced stomatal movement
To investigate AITC-induced stomatal closure, intact leaves were initially incubated in the light to open the stomata followed by AITC treatment in the light. For investigation of AITC inhibition of light-induced stomatal opening, leaves were initially incubated in the dark to close the stomata followed by AITC treatment in the light. AITC induced stomatal closure in a dose-dependent manner (Fig. 1A), which is consistent with previous results (Khokon et al., 2011). In addition, AITC inhibited light-induced stomatal opening in a dose-dependent manner (Fig. 1B).
Fig. 1.
Effect of AITC on stomatal movement. (A) Stomatal closure induced by AITC. (B) Inhibition by AITC of light-induced stomatal opening. Averages from three independent experiments (90 stomata in total per bar) are shown. Error bars represent standard error of the mean (n=3). *Statistical significance compared with 0 μM AITC (P<0.05).
Previous results have shown that AITC induces stomatal closure accompanied by [Ca2+]cyt elevation. However, it remains unknown whether the [Ca2+]cyt elevation is essential for AITC-induced stomatal closure. Application of a Ca2+ channel blocker, La3+, and a Ca2+ chelator, EGTA, inhibited AITC-induced stomatal closure in a dose-dependent manner (Fig. 2A). Nicotinamide, an inhibitor of Ca2+ release, also inhibited AITC-induced stomatal closure in a dose-dependent manner (Fig. 2B). These results suggest that [Ca2+]cyt elevation induced by Ca2+ influx and Ca2+ release is essential for AITC-induced stomatal closure.
Fig. 2.
Effects of La3+, EGTA, and NA on AITC-induced stomatal closure. (A) Effects of La3+ and EGTA on AITC-induced stomatal closure. (B) Effect of NA on AITC-induced stomatal closure. Averages from three independent experiments (90 stomata in total per bar) are shown. Error bars represent standard error of the mean (n=3). Different letters indicate statistical significance between groups (P<0.05).
On the other hand, the three inhibitors even at the highest concentration for stomatal closure assay (Fig. 2) did not significantly affect AITC inhibition of light-induced stomatal opening (Fig. 3). These results suggest that [Ca2+]cyt elevation is not essential for AITC inhibition of light-induced stomatal opening.
Fig. 3.
Effects of La3+, EGTA, and NA on inhibition by AITC of light-induced stomatal opening. Averages from three independent experiments (90 stomata in total per bar) are shown. Error bars represent standard error of the mean (n=3). Different letters indicate statistical significance between groups (P<0.05).
Since intact leaves were used for stomatal movement assay, AITC and inhibitors have to be transported via the petiole and/or penetrate cuticle directly to reach the guard cells. Therefore, it is possible that concentrations of AITC and inhibitors in the apoplast of intact leaves are lower than those in the assay solution.
Effect of inhibitors of Ca2+ influx and Ca2+ release pathways on allyl isothiocyanate-induced [Ca2+]cyt elevation
Previous results have shown that AITC induced [Ca2+]cyt elevations in guard cells (Khokon et al., 2011). Here, we investigated how AITC induces [Ca2+]cyt elevations in guard cells from isolated epidermal tissues. Mock treatment showed [Ca2+]cyt elevations in 16.7% of guard cells (Fig. 4A, F), whereas application of 50 μM AITC induced elevations in 91.4% of wild-type guard cells (Fig. 4B, F), which is consistent with previous results (Khokon et al., 2011). Application of 1 mM La3+, 2 mM EGTA and 50 mM NA abolished the AITC-induced [Ca2+]cyt elevations (Fig. 4C–F). These results indicate that the inhibitors do suppress AITC-induced [Ca2+]cyt elevations and that both Ca2+ influx and Ca2+ release contribute to the [Ca2+]cyt elevations.
Fig. 4.
Effects of La3+, EGTA, and NA on AITC-induced elevation of [Ca2+]cyt in guard cells. (A–E) Representative traces of fluorescence emission ratios (535/480 nm) showing transient [Ca2+]cyt elevations in guard cells; 1 mM La3+, 2 mM EGTA, and 50 mM NA were added 4 min before 50 µM AITC treatment. (F) Percentage of guard cells showing 0, 1 or 2, or ≥3 transient [Ca2+]cyt elevations. [Ca2+]cyt elevations were counted when changes in fluorescence emission ratios were ≥0.1 U from the baseline.
Other Ca2+ channel inhibitors, Gd3+, verapamil (VERA) and ruthenium red (RR) (Allen et al., 1995; McAinsh et al., 1995; Cessna et al., 1998), were used to further investigate AITC-induced [Ca2+]cyt elevations. One millimolar Gd3+ abolished and 1 mM VERA slightly impaired [Ca2+]cyt elevations induced by 50 μM AITC (Fig. S1A, B, D). On the other hand, RR at 100 μM did not affect the [Ca2+]cyt elevations significantly (Fig. S1C, D). These results suggest that Gd3+- and VERA-sensitive Ca2+ channels are involved in AITC-induced [Ca2+]cyt elevations.
Effect of allyl isothiocyanate on ICa currents and K+in currents in guard cell protoplasts
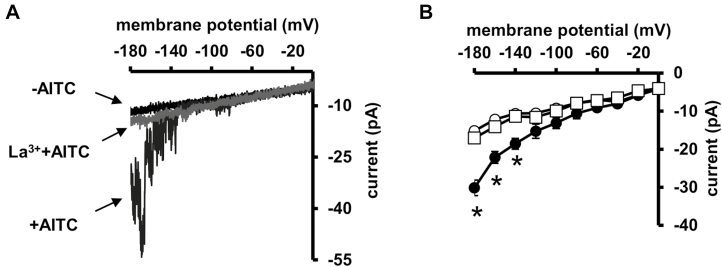
Application of 50 μM AITC significantly activated ICa currents when the membrane was hyperpolarized, and the currents were inhibited by the Ca2+ channel inhibitor La3+ (Fig. 5). These results indicate that AITC induces Ca2+ influx through activation of ICa channels.
Fig. 5.
Activation of ICa channel currents by AITC in guard cell protoplasts (GCPs). (A) Representative ICa current traces in GCPs. (B) Average of current–voltage curves for AITC activation of ICa currents in GCPs (n=5) as recorded in (A) (open circles, control; filled circles, 50 μM AITC; open square, La3++AITC). A ramp voltage protocol from +20 to −180 mV (holding potential, 0 mV; ramp speed, 200 mV s−1) was used. After attaining the whole-cell configuration, GCPs were recorded to obtain control data. To obtain data for ATIC treatment and La3++AITC treatment, recordings were performed sequentially after extracellularly adding AITC and 1 mM La3+. The GCPs were measured 16 times to get averages for each recording. The interpulse period was 1 min. *Statistical significance (P<0.05). Results are from five independent experiments; error bars indicate SE.
Potassium influx through K+in channels is critical for stomatal opening (Kwak et al., 2001; Takahashi et al., 2013). Resting [Ca2+]cyt has been shown to be in the range of 120–150 nM in guard cells (Grabov & Blatt, 1999; Siegel et al., 2009). Since AITC inhibition of light-induced stomatal opening is not dependent on [Ca2+]cyt elevation, the effect of AITC on K+in currents at resting [Ca2+]cyt buffered to 150 nM was investigated. Application of 50 µM AITC significantly suppressed K+in currents in guard cell protoplasts (Fig. 6A, B), indicating that AITC suppresses K+in channels in a Ca2+-independent manner.
Fig. 6.
Effect of AITC on K+in currents in GCPs and KAT1 activity expressed in Xenopus oocytes. (A) K+in currents in GCPs treated without (top trace) or with (bottom trace) 50 μM AITC. (B) Steady-state current–voltage relationship for AITC inhibition of K+in currents in WT GCPs as recorded in (A) (open circles, control; filled circles, AITC). The voltage protocol was stepped up from 0 mV to −180 mV in 20 mV decrements (holding potential, −40 mV). GCPs were treated with AITC for 2 h before recordings. Each data point was obtained from at least seven GCPs in more than five independent experiments. Error bars represent standard errors. *Statistical significance compared with Control (P<0.05). (C) Steady-state current–voltage relationship for KAT1-mediated currents in Xenopus oocytes. Oocytes were treated with AITC for 2 h before recordings. The voltage protocol was stepped up from 0 mV to −180 mV in 20 mV decrements (holding potential, −40 mV) with a pulse duration of 3 s. Each data point was obtained from seven oocytes in more than three independent experiments. Error bars represent standard errors.
The effect of AITC on a major K+in channel in guard cells, KAT1, was investigated using the two-electrode voltage-clamp technique. AITC at 50, 100, and 500 µM had no significant effect on the currents seen in Xenopus oocytes expressing KAT1 (Fig. 6C).
Effect of allyl isothiocyanate on fusicoccin-induced stomatal opening and phosphorylation of penultimate threonine of plasma membrane H+-ATPases
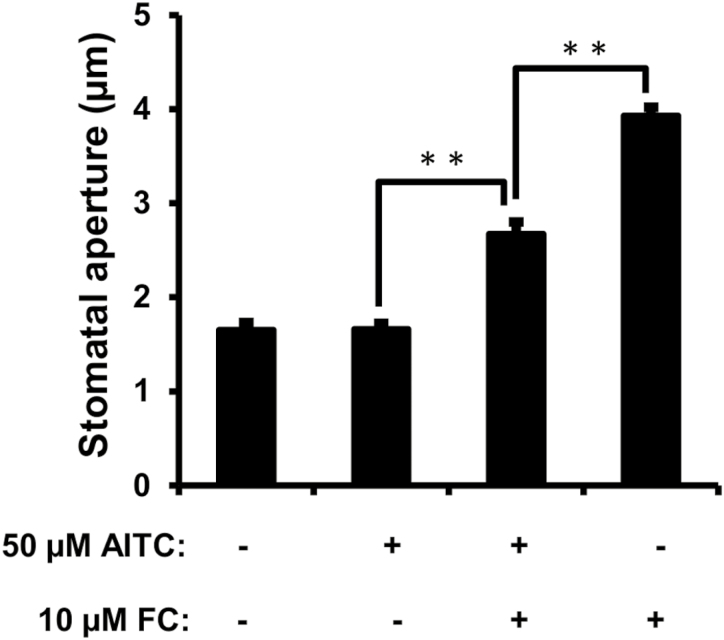
To further investigate how AITC inhibits stomatal opening, the effect of AITC on stomatal opening induced by a plasma membrane H+-ATPase activator, fusicoccin (FC), was investigated. Treatment of 50 μM AITC significantly inhibited FC-induced stomatal opening in the dark (Fig. 7). Since FC induces stomatal opening through activation of H+-ATPases by increasing the phosphorylation level of the penultimate Thr (penThr) of H+-ATPases (Kinoshita & Shimazaki, 2001), the effect of AITC on FC-induced penThr phosphorylation was investigated. Application of 50 μM AITC did not affect FC-induced penThr phosphorylation significantly (Fig. 8). Taken together, these results indicate that AITC inhibits FC-induced stomatal opening without affecting penThr phosphorylation.
Fig. 7.
Effect of AITC on FC-induced stomatal opening in the dark. Averages from three independent experiments (90 stomata in total per bar) are shown. AITC was added 30 min before FC treatment. Error bars represent standard error of the mean (n=3). *Statistical significance (P<0.05).
Fig. 8.
Immunohistochemical detection of FC-induced penultimate Thr phosphorylation of H+-ATPases in guard cell plasma membrane. The vertical scale represents fluorescence levels of guard cells detected using penultimate Thr antiserum as primary antibody and Alexa Fluor 488-conjugated goat anti-rabbit IgG as secondary antibody. The fluorescence level was measured by ImageJ, expressed as relative values normalized to that of mock treatment. Typical fluorescence and the corresponding bright field images are shown at the top. Arrowhead indicates the guard cells. Scale bar: 50 µm. Data represent means with SDs (n=3). NS, no significant difference observed (P>0.1; Student’s t test). Results are from more than three independent experiments.
Discussion
Allyl isothiocyanate induces stomatal movement like many other abiotic and biotic stimuli
The glucosinolate–myrosinase system is widely known to be activated by tissue damage caused by herbivory, and hydrolysis products of glucosinolates have repellent effects on many herbivores (Halkier & Gershenzon, 2006). Studies have also shown that hydrolysis of glucosinolates is induced in live cells by fungal and bacterial infection and plant hormones such as ABA (Zhao et al., 2008; Bednarek et al., 2009; Clay et al., 2009; Fan et al., 2011; Zhu et al., 2014; Andersson et al., 2015). Guard cell responses to ABA were impaired in aliphatic glucosinolate-deficient (Zhu et al., 2014) and myrosinase-deficient (Zhao et al., 2008; Islam et al., 2009) mutants, suggesting that hydrolysis products of glucosinolates function as signaling component in ABA signaling. There is also evidence for involvement of glucosinolate hydrolysis in signaling induced by bacterial flagellin peptide, flg22 (Clay et al., 2009). These results motivate further study of the signaling by the hydrolysis product of glucosinolates. Among the hydrolysis products, isothiocyanates are highly active and their physiological functions in both abiotic and biotic stress are under intensive investigation (Hara et al., 2013; Andersson et al., 2015). Allyl isothiocyanate is biosynthesized in Arabidopsis (Lambrix et al., 2001) and is one of the most studied isothiocyanates so far (Khokon et al., 2011; Hossain et al., 2013; Åsberg et al., 2015; Øverby et al., 2015; Sobahan et al., 2015). While AITC at concentrations above millimolar is detrimental (Hara et al., 2010), AITC at lower concentrations functions as a signal to trigger physiological events (Khokon et al., 2011; Sobahan et al., 2015). It was also suggested that AITC, as a volatile compound, functions as a signal for plant–plant interaction (Sobahan et al., 2015). Previous results have shown that AITC reversibly induces stomatal closure (Khokon et al., 2011; Hossain et al., 2013; Sobahan et al., 2015). In the present study, it was further revealed that AITC also inhibited light-induced stomatal opening (Fig. 1). Many abiotic and biotic stimuli, such as ABA and flg22, also induce both stomatal closure and inhibition of light-induced stomatal opening (Melotto et al., 2006; Zhang et al., 2008; Yin et al., 2013). Therefore, it is likely that there is crosstalk between AITC signaling and signaling induced by other stimuli including ABA and flg22.
Allyl isothiocyanate induces Ca2+ influx and Ca2+ release leading to [Ca2+]cyt elevations
Activation of ICa channels in guard cells is triggered by many stimuli, such as ABA, methyl jasmonate (MeJA), yeast elicitor, and amino acids (Murata et al., 2001; Munemasa et al., 2007; Ye et al., 2013; Yoshida et al., 2016; Kong et al., 2016). In the present study, AITC activated ICa channels in guard cells (Fig. 5). On the other hand, the molecular nature of ICa channels is mostly unknown. It is known that AITC activates transient receptor potential ankyrin 1, a Ca2+ channel, by reacting with several Cys residues in its N-terminus to induce Ca2+ influx in neuron cells (Bautista et al., 2006; Hinman et al., 2006). However, plants do not harbor genes related to transient receptor potential channels in animal cells (Hedrich, 2012). Studies have identified that hyperosmolality-induced [Ca2+]cyt increase 1 (OSCA1) family members, glutamate receptor homologs, and cyclic nucleotide-gated channels are ICa channels activated by different stimuli (Wang et al., 2013b; Yuan et al., 2014; Kong et al., 2016; Tian et al., 2019). It would be interesting to investigate whether these channels are activated by AITC.
Previous studies have shown not only Ca2+ influx but also Ca2+ release from intracellular stores is essential for [Ca2+]cyt elevations induced by ABA, methyl jasmonate and flg22 (Leckie et al., 1998; Hamilton et al., 2000; Pei et al., 2000; Garcia-Mata et al., 2003; Lemtiri-Chlieh et al., 2003; Munemasa et al., 2007; Hossain et al., 2014; Thor and Peiter, 2014). ABA and MeJA recruit a Ca2+ release pathway involving cADPR, which is sensitive to NA (Allen et al., 1995; Klüsener et al., 2002; Hossain et al., 2014). In the present study, NA abolished AITC-induced [Ca2+]cyt elevations, suggesting that the NA-sensitive Ca2+ release mechanism is conserved among different signaling pathways.
Elevation of [Ca2+]cyt is essential for allyl isothiocyanate-induced stomatal closure but not inhibition of light-induced stomatal opening
The driving force for stomatal closure is membrane depolarization induced by activation of S-type anion channels, while the driving force for stomatal opening is membrane hyperpolarization induced by activation of H+-ATPase. Ca2+ positively regulates S-type anion channels but negatively regulates H+-ATPase through Ca2+ sensor-dependent pathways (Schroeder & Hagiwara, 1989; Kinoshita et al., 1995), and thus Ca2+ is essential for stomatal closure induced by many stimuli such as ABA, MeJA, and yeast elicitor (Mori et al., 2006; Munemasa et al., 2011; Ye et al., 2013). Recent studies have shown quadruple loss-of-function mutations of ABA receptors impair ABA-induced stomatal closure and [Ca2+]cyt elevation but not inhibition of light-induced stomatal opening (Wang et al., 2013a; Yin et al., 2013). These results highlight that the signaling pathways underlying stomatal closure and inhibition of stomatal opening can be genetically dissected. In the present study, inhibition of [Ca2+]cyt by three inhibitors impaired AITC-induced stomatal closure but not inhibition of light-induced stomatal opening (Figs 2, 3), indicating [Ca2+]cyt elevation is essential for AITC-induced stomatal closure but not inhibition of light-induced opening. Taken together, these results suggest that signaling leading to stomatal closure does not overlap with signaling leading to inhibition of light-induced stomatal opening in different stress responses.
Inhibition of K+in channels suppresses stomatal opening driven by activation of H+-ATPase (Kwak et al., 2001; Takahashi et al., 2013). In the present study, AITC inhibited K+in channels in the absence of [Ca2+]cyt elevation (Fig. 6). Further results showed that AITC inhibited FC-induced stomatal opening but not phosphorylation of penThr (Figs 7, 8). These results again suggest that inhibition of K+in channels contributes to AITC inhibition of light-induced stomatal opening.
As an electrophile, AITC is prone to forming covalent adducts with amino acid residues, such as Cys, Lys, and His, to modify protein function. It is known that the covalent modification of Cys is reversible under physiological conditions (Nakamura and Miyoshi, 2010; Higdon et al., 2012). It has been shown that two electrophiles, acrolein and methylglyoxal, inhibit K+in channels in guard cells and target KAT1, a main K+in channel in guard cells, expressed in a heterologous system using Xenopus oocytes (Hoque et al., 2012; Islam et al., 2015, 2016). However, our experiments show that AITC at concentration of 50, 100, and 500 µM did not significantly affect KAT1 activity expressed in oocytes (Fig. 6C), suggesting that AITC does not directly modify KAT1. It has been reported that one AITC target is microtubules in Arabidopsis (Øverby et al., 2015) and microtubules are critical for light-induced stomatal opening (Marcus et al., 2001; Eisinger et al., 2012). In the future, it would be interesting to investigate whether AITC modulates K+in channels through a microtubule-dependent pathway.
Conclusion
The presented results suggest that AITC triggers Ca2+ influx and Ca2+ release to induce [Ca2+]cyt elevation, which is essential for AITC-induced stomatal closure but not for inhibition of K+in channels or light-induced stomatal opening.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Effects of Gd3+, VERA, and RR on AITC-induced elevation of [Ca2+]cyt in guard cells.
Acknowledgements
This work was supported by National Natural Science Foundation of China (31901984 to WY), the Start-up Fund from Shanghai Jiao Tong University (WF220515002 to WY), Grants-in-Aid for Japan Society for the Promotion of Science Fellows from the Japan Society for the Promotion of Science (267977 to WY), Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (15H05956 to TK) and the Advanced Low Carbon Technology Research and Development Program from the Japan Science and Technology Agency (to TK).
Author contributions
WY, YN, TK, and YM conceived the research plans; YM and TK supervised the experiments; WY, EA, MSR, MT, and EO performed the experiments; WY wrote the article with the contribution of all the authors.
References
- Allen GJ, Muir SR, Sanders D. 1995. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science 268, 735–737. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Murata Y, Chu SP, Nafisi M, Schroeder JI. 2002. Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. The Plant Cell 14, 1649–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson MX, Nilsson AK, Johansson ON, et al. 2015. Involvement of the electrophilic isothiocyanate sulforaphane in Arabidopsis local defense responses. Plant Physiology 167, 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åsberg SE, Bones AM, Øverby A. 2015. Allyl isothiocyanate affects the cell cycle of Arabidopsis thaliana. Frontiers in Plant Science 6, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. 2006. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282. [DOI] [PubMed] [Google Scholar]
- Bednarek P, Pislewska-Bednarek M, Svatos A, et al. 2009. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323, 101–106. [DOI] [PubMed] [Google Scholar]
- Cessna SG, Chandra S, Low PS. 1998. Hypo-osmotic shock of tobacco cells stimulates Ca2+ fluxes deriving first from external and then internal Ca2+ stores. The Journal of Biological Chemistry 273, 27286–27291. [DOI] [PubMed] [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. 2009. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Gardner MJ, Hotta CT, et al. 2007. The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science 318, 1789–1792. [DOI] [PubMed] [Google Scholar]
- Eisinger W, Ehrhardt D, Briggs W. 2012. Microtubules are essential for guard-cell function in Vicia and Arabidopsis. Molecular Plant 5, 601–610. [DOI] [PubMed] [Google Scholar]
- Fan J, Crooks C, Creissen G, Hill L, Fairhurst S, Doerner P, Lamb C. 2011. Pseudomonas sax genes overcome aliphatic isothiocyanate-mediated non-host resistance in Arabidopsis. Science 331, 1185–1188. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR. 2003. Nitric oxide regulates K+ and Cl −channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proceedings of the National Academy of Sciences, USA 100, 11116–11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR. 1999. A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiology 119, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkier BA, Gershenzon J. 2006. Biology and biochemistry of glucosinolates. Annual Review of Plant Biology 57, 303–333. [DOI] [PubMed] [Google Scholar]
- Hamilton DW, Hills A, Kohler B, Blatt MR. 2000. Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proceedings of the National Academy of Sciences, USA 97, 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Harazaki A, Tabata K. 2013. Administration of isothiocyanates enhances heat tolerance in Arabidopsis thaliana. Plant Growth Regulation 69, 71–77. [Google Scholar]
- Hara M, Yatsuzuka Y, Tabata K, Kuboi T. 2010. Exogenously applied isothiocyanates enhance glutathione S-transferase expression in Arabidopsis but act as herbicides at higher concentrations. Journal of Plant Physiology 167, 643–649. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Inoue S, Takahashi K, Kinoshita T. 2011. Immunohistochemical detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells. Plant & Cell Physiology 52, 1238–1248. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Nakamura S, Takemiya A, Takahashi Y, Shimazaki K, Kinoshita T. 2010. Biochemical characterization of in vitro phosphorylation and dephosphorylation of the plasma membrane H+-ATPase. Plant and Cell Physiology 51, 1186–1196. [DOI] [PubMed] [Google Scholar]
- Hedrich R. 2012. Ion channels in plants. Physiological Reviews 92, 1777–1811. [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Brownlee C. 2004. The generation of Ca2+ signals in plants. Annual Review of Plant Biology 55, 401–427. [DOI] [PubMed] [Google Scholar]
- Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM. 2012. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. The Biochemical Journal 442, 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. 2006. TRP channel activation by reversible covalent modification. Proceedings of the National Academy of Sciences, USA 103, 19564–19568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque TS, Okuma E, Uraji M, Furuichi T, Sasaki T, Hoque MA, Nakamura Y, Murata Y. 2012. Inhibitory effects of methylglyoxal on light-induced stomatal opening and inward K+ channel activity in Arabidopsis. Bioscience, Biotechnology, and Biochemistry 76, 617–619. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Ye W, Munemasa S, Nakamura Y, Mori IC, Murata Y. 2014. Cyclic adenosine 5′ -diphosphoribose (cADPR) cyclic guanosine 3′, 5′ -monophosphate positively function in Ca2+ elevation in methyl jasmonate-induced stomatal closure, cADPR is required for methyl jasmonate-induced ROS accumulation NO production in guard cells. Plant Biology 16, 1140–1144. [Google Scholar]
- Hossain MS, Ye W, Hossain MA, Okuma E, Uraji M, Nakamura Y, Mori IC, Murata Y. 2013. Glucosinolate degradation products, isothiocyanates, nitriles, and thiocyanates, induce stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis thaliana. Bioscience, Biotechnology, and Biochemistry 77, 977–983. [DOI] [PubMed] [Google Scholar]
- Hubbard KE, Siegel RS, Valerio G, Brandt B, Schroeder JI. 2012. Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Annals of Botany 109, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue SI, Kinoshita T. 2017. Blue light regulation of stomatal opening and the plasma membrane H+-ATPase. Plant Physiology 174, 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MM, Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. 2010. Roles of AtTPC1, vacuolar two pore channel 1, in Arabidopsis stomatal closure. Plant & Cell Physiology 51, 302–311. [DOI] [PubMed] [Google Scholar]
- Islam MM, Tani C, Watanabe-Sugimoto M, Uraji M, Jahan MS, Masuda C, Nakamura Y, Mori IC, Murata Y. 2009. Myrosinases, TGG1 and TGG2, redundantly function in ABA and MeJA signaling in Arabidopsis guard cells. Plant & Cell Physiology 50, 1171–1175. [DOI] [PubMed] [Google Scholar]
- Islam MM, Ye W, Matsushima D, Khokon MA, Munemasa S, Nakamura Y, Murata Y. 2015. Inhibition by acrolein of light-induced stomatal opening through inhibition of inward-rectifying potassium channels in Arabidopsis thaliana. Bioscience, Biotechnology, and Biochemistry 79, 59–62. [DOI] [PubMed] [Google Scholar]
- Islam MM, Ye W, Matsushima D, Munemasa S, Okuma E, Nakamura Y, Biswas S, Mano J, Murata Y. 2016. Reactive carbonyl species mediate ABA signaling in guard cells. Plant & Cell Physiology 57, 2552–2563. [DOI] [PubMed] [Google Scholar]
- Khokon MA, Jahan MS, Rahman T, Hossain MA, Muroyama D, Minami I, Munemasa S, Mori IC, Nakamura Y, Murata Y. 2011. Allyl isothiocyanate (AITC) induces stomatal closure in Arabidopsis. Plant, Cell & Environment 34, 1900–1906. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Nishimura M, Shimazaki K. 1995. Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard cells of fava bean. The Plant Cell 7, 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K. 2001. Analysis of the phosphorylation level in guard-cell plasma membrane H+-ATPase in response to fusicoccin. Plant & Cell Physiology 42, 424–432. [DOI] [PubMed] [Google Scholar]
- Klüsener B, Young JJ, Murata Y, Allen GJ, Mori IC, Hugouvieux V, Schroeder JI. 2002. Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiology 130, 2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Hu HC, Okuma E, et al. 2016. L-Met activates Arabidopsis GLR Ca2+ channels upstream of ROS production and regulates stomatal movement. Cell Reports 17, 2553–2561. [DOI] [PubMed] [Google Scholar]
- Kwak JM, Murata Y, Baizabal-Aguirre VM, Merrill J, Wang M, Kemper A, Hawke SD, Tallman G, Schroeder JI. 2001. Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiology 127, 473–485. [PMC free article] [PubMed] [Google Scholar]
- Laanemets K, Brandt B, Li J, Merilo E, Wang YF, Keshwani MM, Taylor SS, Kollist H, Schroeder JI. 2013. Calcium-dependent and -independent stomatal signaling network and compensatory feedback control of stomatal opening via Ca2+ sensitivity priming. Plant Physiology 163, 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrix V, Reichelt M, Mitchell-Olds T, Kliebenstein DJ, Gershenzon J. 2001. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. The Plant Cell 13, 2793–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckie CP, McAinsh MR, Allen GJ, Sanders D, Hetherington AM. 1998. Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proceedings of the National Academy of Sciences, USA 95, 15837–15842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EA, Webb AA, Manison NF, Brownlee C, Skepper JN, Chen J, Prestwich GD, Brearley CA. 2003. Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proceedings of the National Academy of Sciences, USA 100, 10091–10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus AI, Moore RC, Cyr RJ. 2001. The role of microtubules in guard cell function. Plant Physiology 125, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Webb A, Taylor JE, Hetherington AM. 1995. Stimulus-induced oscillations in guard cell cytosolic free calcium. The Plant Cell 7, 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. 2006. Plant stomata function in innate immunity against bacterial invasion. Cell 126, 969–980. [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, et al. 2006. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biology 4, e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y. 2011. The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiology 155, 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Oda K, Watanabe-Sugimoto M, Nakamura Y, Shimoishi Y, Murata Y. 2007. The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiology 143, 1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Mori IC, Munemasa S. 2015. Diverse stomatal signaling and the signal integration mechanism. Annual Review of Plant Biology 66, 369–392. [DOI] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder J. 2001. Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. The Plant Cell 13, 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Miyoshi N. 2010. Electrophiles in foods: the current status of isothiocyanates and their chemical biology. Bioscience, Biotechnology, and Biochemistry 74, 242–255. [DOI] [PubMed] [Google Scholar]
- Øverby A, Silihagen Bævre M, Bones A. 2015. Disintegration of microtubules in Arabidopsis thaliana and bladder cancer cells by isothiocyanates. Frontiers in Plant Science 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. 2000. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. 2005. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434, 404–408. [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R. 2010. Making sense out of Ca2+ signals: their role in regulating stomatal movements. Plant, Cell and Environment 33, 305–321. [DOI] [PubMed] [Google Scholar]
- Salehin M, Li B, Tang M, Katz E, Song L, Ecker JR, Kliebenstein DJ, Estelle M. 2019. Auxin-sensitive Aux/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nature Communications 10, 4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M, Paciorek T, Benková E, Friml J. 2006. Immunocytochemical techniques for whole-mount in situ protein localization in plants. Nature Protocols 1, 98–103. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S. 1989. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338, 427–430. [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T. 2007. Light regulation of stomatal movement. Annual Review of Plant Biology 58, 219–247. [DOI] [PubMed] [Google Scholar]
- Siegel RS, Xue S, Murata Y, Yang Y, Nishimura N, Wang A, Schroeder JI. 2009. Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K channels in Arabidopsis guard cells. The Plant Journal 59, 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobahan MA, Akter N, Okuma E, Uraji M, Ye W, Mori IC, Nakamura Y, Murata Y. 2015. Allyl isothiocyanate induces stomatal closure in Vicia faba. Bioscience, Biotechnology, and Biochemistry 79, 1737–1742. [DOI] [PubMed] [Google Scholar]
- Takahashi F, Suzuki T, Osakabe Y, Betsuyaku S, Kondo Y, Dohmae N, Fukuda H, Yamaguchi-Shinozaki K, Shinozaki K. 2018. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 556, 235–238. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Ebisu Y, Kinoshita T, Doi M, Okuma E, Murata Y, Shimazaki K. 2013. bHLH transcription factors that facilitate K⁺ uptake during stomatal opening are repressed by abscisic acid through phosphorylation. Science Signaling 6, ra48. [DOI] [PubMed] [Google Scholar]
- Thor K, Peiter E. 2014. Cytosolic calcium signals elicited by the pathogen-associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature. New Phytologist 204, 873–881. [DOI] [PubMed] [Google Scholar]
- Tian W, Hou C, Ren Z, et al. 2019. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 572, 131–135. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen ZH, Zhang B, Hills A, Blatt MR. 2013a PYR/PYL/RCAR abscisic acid receptors regulate K+ and Cl −channels through reactive oxygen species-mediated activation of Ca2+ channels at the plasma membrane of intact Arabidopsis guard cells. Plant Physiology 163, 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Munemasa S, Nishimura N, et al. 2013. b Identification of cyclic GMP-activated nonselective Ca2+-permeable cation channels and associated CNGC5 and CNGC6 genes in Arabidopsis guard cells. Plant Physiology 163, 578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Hu H, Ries A, Merilo E, Kollist H, Schroeder JI. 2011. Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. The EMBO Journal 30, 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Adachi Y, Munemasa S, Nakamura Y, Mori IC, Murata Y. 2015. Open stomata 1 kinase is essential for yeast elicitor-induced stomatal closure in Arabidopsis. Plant & Cell Physiology 56, 1239–1248. [DOI] [PubMed] [Google Scholar]
- Ye W, Murata Y. 2016. Microbe associated molecular pattern signaling in guard cells. Frontiers in Plant Science 7, 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Muroyama D, Munemasa S, Nakamura Y, Mori IC, Murata Y. 2013. Calcium-dependent protein kinase CPK6 positively functions in induction by yeast elicitor of stomatal closure and inhibition by yeast elicitor of light-induced stomatal opening in Arabidopsis. Plant Physiology 163, 591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Adachi Y, Ye W, Hayashi M, Nakamura Y, Kinoshita T, Mori IC, Murata Y. 2013. Difference in abscisic acid perception mechanisms between closure induction and opening inhibition of stomata. Plant Physiology 163, 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Mori IC, Kamizono N, Shichiri Y, Shimatani T, Miyata F, Honda K, Iwai S. 2016. Glutamate functions in stomatal closure in Arabidopsis and fava bean. Journal of Plant Research 129, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Yang H, Xue Y, et al. 2014. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514, 367–371. [DOI] [PubMed] [Google Scholar]
- Zhang J, De-Oliveira-Ceciliato P, Takahashi Y, et al. 2018. Insights into the molecular mechanisms of CO2-mediated regulation of stomatal movements. Current Biology 28, R1356–R1363. [DOI] [PubMed] [Google Scholar]
- Zhang W, He SY, Assmann SM. 2008. The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. The Plant Journal 56, 984–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zhang W, Stanley BA, Assmann SM. 2008. Functional proteomics of Arabidopsis thaliana guard cells uncovers new stomatal signaling pathways. The Plant Cell 20, 3210–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Zhu N, Song WY, Harmon AC, Assmann SM, Chen S. 2014. Thiol-based redox proteins in abscisic acid and methyl jasmonate signaling in Brassica napus guard cells. The Plant Journal 78, 491–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.