Abstract
Little is known about plastic changes occurring in the brains of patients with severe disorders of consciousness (DOCs) caused by acute brain injuries at rest and during rehabilitative treatment. Brain-derived neurotrophic factor (BDNF) is a neurotrophin involved in neurogenesis and synaptic plasticity whose production is powerfully modulated by physical exercise. In this study, we compared serum BDNF levels in 18 patients with unresponsive wakefulness syndrome (UWS) and in a minimally conscious state (MCS) with those in 16 sex- and age-matched healthy controls. In 12 patients, serum BDNF levels before and after verticalization with ErigoPro robot-assisted lower-limb training were compared. Serum BDNF levels were significantly lower in patients (median, 1141 pg/ml; 25th and 75th percentiles, 1016 and 1704 pg/ml) than in controls (median, 2450 pg/ml; 25th and 75th percentiles, 2100 and 2875 pg/ml; p < 0.001). BDNF levels measured before and after verticalization with robot-assisted lower-limb training did not change (p = 0.5). Moreover, BDNF levels did not differ between patients with UWS and MCS (p = 0.2), or between patients with traumatic and nontraumatic brain injuries (p = 0.6). BDNF level correlated positively with the time since brain injury (p = 0.025). In conclusion, serum BDNF levels are reduced in patients with UWS and MCS and cannot be improved by verticalization associated with passive lower-limb training. Additional studies are needed to better understand the mechanisms underlying BDNF reduction in patients with DOCs and to determine the best rehabilitative strategies to promote restorative plastic changes in these patients.
1. Introduction
To foster the recovery of consciousness in patients in a vegetative state or in a minimally conscious state (MCS) after acute brain injury is among the most challenging tasks in modern rehabilitation. The vegetative state, also referred to as unresponsive wakefulness syndrome (UWS) [1, 2], is a disorder of consciousness (DOC) in which the patient shows no sign of awareness of him- or herself and the external world. The MCS, which may arise directly from acute brain injury or represent the evolution of UWS, is a condition in which the patient shows minimal and fluctuating signs of awareness [3]. UWS and MCS are the results of acute and massive disruption of the brain's ability to subtend normal consciousness; depending on the severity of the brain damage, they may last indefinitely or improve gradually until the patient recovers normal consciousness. Although the mechanisms leading to the recovery of consciousness in patients with DOCs are largely unknown, they necessarily involve plastic changes in the brain, i.e., structural and functional modifications in the circuits subtending the consciousness [4].
Brain-derived neurotrophic factor (BDNF) is a neurotrophin that plays a key role in brain plasticity, promoting structural and functional changes in the brain such as neuro- and synaptogenesis, short- and long-lasting changes in synaptic activity, and the development of neural circuits involved in memory and cognitive functions [5–7]. BDNF expression changes after acute brain injury, suggesting its potential role in neuronal and synaptic reorganization following brain damage. In animal models of traumatic brain injury (TBI), BDNF expression is upregulated in the hippocampus and cerebral cortex [8, 9]; peripheral levels of BDNF after acute brain injury in humans have been scarcely evaluated. Some studies suggest that the secretion of BDNF in the brain is reduced immediately after TBI and that this reduction is more evident in patients with poor outcomes [10, 11], whereas other studies have revealed increased BDNF levels [12]. Few studies have evaluated BDNF levels in patients with brain injuries other than TBI. For example, lower BDNF levels are associated with worse outcomes in patients with neonatal hypoxic-ischemic encephalopathy [13].
A very interesting property of BDNF, especially from a rehabilitative perspective, is that its expression can be upregulated by physical exercise. In healthy humans, short-term exercise increases the BDNF level in the bloodstream, at a magnitude that depends on the exercise intensity [14]. Moreover, the increase in circulating BDNF is more evident after prolonged exercise, possibly as a result of its release from the hippocampus, cortex, and cerebellum [15]. The positive effects of exercise on the BDNF level and cognitive functions have also been demonstrated in elderly patients with cognitive deficits, such as mild cognitive impairment [16]. Thus, the beneficial effects of exercise on cognitive functions may depend, at least in part, on BDNF-mediated mechanisms [17].
Patients with severe DOCs cannot benefit from the BDNF-mediated effects of active exercise because the majority of them cannot cooperate with rehabilitative treatment; only few patients in an MCS have a minimal ability to perform exercises voluntarily. Interestingly, some rehabilitative treatments that do not require active patient participation effectively improve the level of consciousness in patients with DOCs. For example, short periods of standing, achieved using a tilt table, improve arousal and awareness in patients with UWS and those in an MCS [18, 19], although they are associated with a high occurrence of orthostatic hypotension [19]. To reduce orthostatic intolerance, robotic systems that perform patient verticalization in association with passive step training have been developed. These systems, such as the ErigoPro robot (Hocoma, Volketswil, Switzerland), activate the venomuscular pumps of the lower limbs and significantly reduce the occurrence of hypotension in patients with DOCs [20]. However, the neurobiological mechanisms potentially activated by verticalization with robotic lower-limb training have not been investigated in patients with severe DOC.
This study had two main aims: to determine whether the baseline serum BDNF level of patients with DOCs differed from that of sex- and age-matched healthy subjects and to investigate whether the BDNF level can be modulated by verticalization with ErigoPro robot-assisted lower-limb training. The results of this study will aid our understanding of how BDNF expression is modulated after severe brain injury in patients with UWS and in an MCS and whether a rehabilitative treatment based on verticalization associated to passive lower limb robot-assisted training is effective in modifying BDNF levels.
2. Materials and Methods
2.1. Protocol Approval and Informed Consent
The study abided by the Declaration of Helsinki and was approved by the regional ethics review board (Palermo 1 Ethics Committee, Palermo, Italy). Patients' legal guardians and healthy subjects provided written informed consent to all study procedures.
2.2. Participants
Eighteen patients with UWS or in an MCS following acute brain injuries and 16 sex- and age-matched healthy controls participated in this study (see Table 1 for demographic and clinical details). Twelve patients also participated in the evaluation of BDNF levels after treatment with the ErigoPro robot; six patients were excluded from this treatment due to bone fractures (n = 3), the need for mechanical ventilation (n = 1), severe dystonic postures (n = 1), and psychomotor agitation (n = 1). All patients were admitted to our Unit for Severe Acquired Brain Injuries for intensive rehabilitation following acute brain injuries. Inclusion criteria for patients were as follows: (i) diagnosis of UWS or MCS according to the current diagnostic criteria [1, 3] at the time of study inclusion, (ii) study inclusion ≥ 1 month after acute brain injury, and (iii) age 18–65 years (patients aged >65 years were excluded due to the increased risk of neurodegenerative diseases). Exclusion criteria were as follows: (i) previous history of brain injury or psychiatric or neurodegenerative disease and (ii) coexisting neoplasia, severe organ dysfunction, or unstable clinical condition (e.g., hemodynamic instability or severe respiratory failure). Healthy controls were recruited among blood donors and had no previous history of neurological, psychiatric, or neoplastic disease.
Table 1.
Participants' details.
| Variables | Patients (n = 18) | Healthy subjects (n = 16) |
|---|---|---|
| Males | 10 (55.6%) | 9 (56.3%) |
| Females | 8 (44.4%) | 7 (43.7%) |
| Age (years) | 38.9 ± 17 | 38.9 ± 12.6 |
| Time between brain injury and blood sample collection (months) | 3.2 ± 2.2 (range 1–7.3) | |
| Etiology | ||
| TBI | 8 | |
| Cerebral hypoxia | 6 | |
| Subarachnoid hemorrhage | 2 | |
| Brainstem hemorrhage | 1 | |
| Bilateral hemispheric ischemia | 1 | |
| Disorder of consciousness | ||
| UWS (CRS-R score)a | 4.5 ± 1.3 | |
| MCS (CRS-R score)a | 13.8 ± 3.6 |
aCRS-R scores on the day of the blood sample collection. CRS-R: Coma Recovery Scale-Revised; MCS: minimally conscious state; TBI: traumatic brain injury; UWS: unresponsive wakefulness syndrome.
The authors, experts in the clinical evaluation of patients with DOCs (SB, CB, and TVF), diagnosed UWS and MCSs based on assessment using the Coma Recovery Scale-Revised (CRS-R) [21]. The CRS-R, which provides criteria for the diagnosis of UWS, MCS, and emergence from MCS, is the most reliable tool available for the assessment of patients with DOCs following coma [22]. Diagnoses of UWS and MCS were accepted only when confirmed by clinical evaluations performed on 3 consecutive days, including those of blood sample collection.
2.3. Verticalization with Robot-Assisted Stepping
The ErigoPro system (Hocoma) consists of a tilt table with an integrated robotic stepping device that permits early verticalization of uncollaborative patients. The patient's upper body is secured by a harness that fixes the chest and pelvis to the table. The feet are secured to mobile footplates for computer-controlled leg movement. The physiotherapist determines the range of motion at the level of each hip before treatment, and this range is kept stable throughout the exercise period. The inclination of the tilt table can be adjusted gradually from horizontal to vertical. The speed of leg movement can be modified from 0 to 80 steps per minute.
In this study, the number of steps was fixed at 50 per minute and maintained throughout the duration of the experiment. Robot-assisted stepping commenced after the patient had been secured to the table in the horizontal position and lasted for 40 minutes. During the first 10 minutes of stepping, the table was inclined gradually from 0° to 45° at a rate of 15° every 2.5 minutes. Then, the table was inclined to 60° and maintained at this inclination for 30 minutes. These parameters were chosen because they are used most frequently in our center, and most patients tolerate them well. Patients' heart rates, blood pressure, and oxygen saturation were monitored throughout the ErigoPro treatment to prevent complications associated with orthostatism (e.g., hypotension and syncope).
2.4. BDNF Analysis
Blood samples were collected from all patients and healthy controls via venipuncture after ≥30-minute rest. For patients, blood samples were collected immediately before and after verticalization with robot-assisted training. Participants' blood samples were centrifuged at 1500 rpm for 15 minutes, and the serum was aliquoted and frozen at –80°C until analyzed using the Human BDNF PicoKine™ enzyme-linked immunosorbent assay kit (Booster Biological Technology, Pleasanton, CA, USA) according to the manufacturer's instructions. Briefly, this kit is a solid-phase immunoassay designed specifically for the measurement of human BDNF using a 96-well strip plate precoated with BDNF-specific antibody. Biotinylated, polyclonal, BDNF-specific goat antibody was used for detection, and monoclonal mouse antibody was used for capture. Concentrations were calculated to permit evaluation of the mean absorbance of each sample at 450 nm with a microplate reader. Results were determined via an instrument-specific calibration curve and are presented in pg/ml. The test sensitivity and detection range are <15 and 31.2–2000 pg/ml, respectively. Samples with BDNF values > 2000 pg/ml were further diluted and analyzed.
2.5. Statistical Analysis
Demographic and clinical data are expressed as means ± standard deviations; BDNF levels are expressed as medians and 25th and 75th percentiles. We used the Mann–Whitney U test to compare demographic characteristics and BDNF levels between patients with DOCs and controls. The same test was also used to compare patients' BDNF levels before and after verticalization and baseline BDNF levels between patients with different levels of consciousness (UWS and MCS) and DOC etiologies (traumatic and nontraumatic). Finally, we used Spearman's rank-correlation test to examine whether BDNF levels correlated with the time since brain injury. p values < 0.05 were considered to be significant.
3. Results
ErigoPro verticalization did not have adverse effects in any patient. Patients with severe DOCs did not differ from healthy controls in terms of age (U = 134, p = 0.7) or sex (Table 1).
BDNF levels were significantly lower in patients (median, 1141 pg/ml; 25th and 75th percentiles, 1016 and 1704 pg/ml) than in healthy subjects (median, 2450 pg/ml; 25th and 75th percentiles, 2100 and 2875 pg/ml; U = 17, p < 0.001; Figure 1). They did not differ between patients with UWS (n = 10; median, 1480 pg/ml; 25th and 75th percentiles, 120 and 1972 pg/ml) and those in an MCS (n = 8; median, 1179 pg/ml; 25th and 75th percentiles, 833 and 1535 pg/ml; U = 26, p = 0.2) or between patients with TBIs (n = 8; median, 1314 pg/ml; 25th and 75th percentiles, 964 and 2029 pg/ml) and those with non-TBIs (n = 10; median, 1438 pg/ml; 25th and 75th percentiles 1064 and 1598 pg/ml; U = 33, p = 0.6; Figure 2). In the 12 patients who underwent ErigoPro treatment, BDNF levels measured before (median, 1438 pg/ml; 25th and 75th percentiles, 1068 and 1702 pg/ml) and after (median, 1141 pg/ml; 25th and 75th percentiles, 435 and 1680 pg/ml) treatment did not differ (U = 61, p = 0.5; Figure 3). BDNF levels correlated weakly with the time since brain injury (r = 0.53, p = 0.025; Figure 4), as they increased over time.
Figure 1.

Serum BDNF levels in patients with DOCs and healthy controls. BDNF levels were significantly lower in patients with DOCs than in controls. Bars indicate ranges, boxes indicate 25th and 75th percentiles, lines in boxes indicate medians, and +s indicate means. BDNF: brain-derived neurotrophic factor; DOC: disorder of consciousness.
Figure 2.
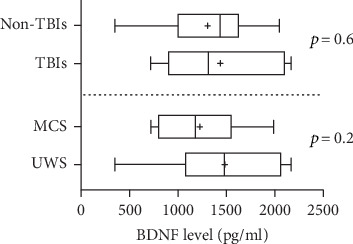
Serum BDNF levels in patients with different DOCs and brain injury etiologies. BDNF levels did not differ between patients with UWS and those in an MCS as well as between patients with TBIs and non-TBIs. Bars indicate ranges, boxes indicate 25th and 75th percentiles, lines in boxes indicate medians, and +s indicate means. BDNF: brain-derived neurotrophic factor; MCS: minimally conscious state; TBI: traumatic brain injury; UWS: unresponsive wakefulness syndrome.
Figure 3.

Serum BDNF levels before and after verticalization with robot-assisted lower-limb training. In patients with DOCs, BDNF levels did not differ before and after ErigoPro training. Bars indicate ranges, boxes indicate 25th and 75th percentiles, lines in boxes indicate medians, and +s indicate means. BDNF: brain-derived neurotrophic factor.
Figure 4.

Correlations between BDNF levels and time since brain injury. Patients' BDNF values (circles) and trend line are showed. BDNF: brain-derived neurotrophic factor.
4. Discussion
In this study, serum BDNF levels were markedly reduced in patients with severe DOCs, to about half the levels found in healthy controls. Moreover, BDNF levels correlated positively with the time since brain injury. Finally, we found that the BDNF level was not modified by verticalization with robot-assisted stepping.
Neurotrophins such as BDNF may promote both neuronal survival and death, depending on different physiological and pathological conditions. Although mature BDNF promotes neurogenesis and activates prosurvival signaling through tropomyosin receptor kinases (Trks) [23–25], its precursor proBDNF binds to the p75 neurotrophin receptor (p75NTR), a tumor necrosis factor receptor-like molecule. Activation of p75NTR by proBDNF can induce neuronal apoptosis in different conditions [26–28]. p75NTR is expressed widely during synaptogenesis and subsequently downregulated in the adult brain. However, it is upregulated after brain injuries, including TBIs and cerebral hypoxia, and the resulting overexpression may cause neuronal death via different intracellular pathways in several conditions [29–32]. The reduction of serum BDNF levels in patients with UWS and those in an MCS may reflect a persistent impaired balance of interactions between BDNF and Trk and between proBDNF and p75NTR, as a consequence of brain injury. After severe brain injury, whether BDNF downregulation reduces neuronal death and apoptosis via less proBDNF–p75NTR binding or mainly impairs neurogenesis and synaptic plasticity via less mature BDNF–Trk binding remains unclear.
Other explanations can be offered for the reduction of serum BDNF levels in patients with DOCs following severe brain injury. In animal models of brain lesions, BDNF levels are increased in the cerebral tissue, in the hippocampus, and in the cerebral cortex [33, 34]. As compelling evidence suggests that BDNF crosses the blood–brain barrier bidirectionally [35–37], the reduction of serum BDNF levels in patients with DOCs may reflect increased use of circulating BDNF by the brain.
Another feasible explanation is that the reduction of the serum BDNF level is caused by the loss of BDNF-secreting neurons as a consequence of acute brain injury and subsequent brain atrophy. Neuroimaging studies have shown that patients with DOCs develop widespread cortical and subcortical atrophy [38–40]. Moreover, the degree to which markers of neuronal injury, such as neuron-specific enolase, are released is below normal in the chronic phases of DOCs [41], suggesting the progressive loss of neurons with brain atrophy [42]. Our results are in line with those of a previous study that documented reduced serum BDNF levels immediately postinjury until 12 months after TBI [43]. However, we also found that the reduction of serum BDNF does not remain constant over time; the BDNF level progressively tends to normalize, not supporting the argument that its reduction is due mainly to the loss of neurons with progressive brain atrophy. The physiological mechanisms that regulate BDNF levels over time in patients with DOCs remain still unknown, as do the clinical implications of changes in these levels.
Serum BDNF levels did not differ in patients with postacute DOCs of different etiologies in this study, suggesting that the reduction of the BDNF level in the postacute phase is not affected significantly by the mechanism of brain injury. Although few studies have evaluated BDNF levels in patients with nontraumatic brain lesions, the lack of difference according to the etiology of brain injury in this study is in agreement with the observation of reduced serum BDNF levels in the acute phase of stroke [44, 45]. In addition, no difference in the serum BDNF level between patients with UWS and those in an MCS was found. Both conditions are severe DOCs, and their clinical differentiation is often very difficult, leading to a high rate of misdiagnosis [46]. In this context, the difference in the BDNF level between patients with UWS and those in an MCS may be subtle, and the sample size in this study may have been inadequate to detect it.
This study failed to find changes in the serum BDNF level after verticalization associated with robot-assisted lower-limb training in patients with DOCs. To induce significant changes in the expression of BDNF, exercise must be sufficiently intense. Aerobic exercise has been associated clearly with increases in serum, plasma, and platelet BDNF levels immediately after exercise in healthy subjects [47, 48]. Under this condition, exercise is associated with changes in brain structure and functions that are believed to be BDNF mediated, such as increased hippocampus size and improved memory [49]. The aerobic threshold likely cannot be reached without voluntary exercise, and our results demonstrate that verticalization with passive stepping is not sufficiently intense to modify BDNF expression.
This study has several limitations. First, we did not determine the distribution of the common BDNF Val66Met polymorphism, which affects neuronal activity-dependent BDNF secretion [50], among patients and controls. Although we showed in a previous study that this polymorphism does not affect the outcomes of patients with posttraumatic UWS [51], we cannot exclude the possibility that its high frequency in patients in this study lowered their serum BDNF levels. Second, although we found that the serum BDNF level increased over time, we did not assess levels in the same patients at different times since brain injury; a more accurate evaluation could be performed in a longitudinal study. Third, we did not correlate BDNF levels with neuroimaging data; it is likely that BDNF levels can be affected by acute and chronic structural changes of the brain, such as loss of tissue, atrophy, or hydrocephalus. Finally, the parameters of the verticalization with robot-assisted stepping (i.e., tilt angle and number of steps per minute) were chosen arbitrarily; thus, we cannot exclude the possibility that different parameters, such as a greater tilt angle and more steps per minute, would induce significant changes in the BDNF level after exercise.
5. Conclusions
Serum BDNF levels are reduced in patients with DOCs, which may reflect decreased BDNF expression, increased cerebral binding, or reduced secretion. This reduction is not affected by DOC etiology or severity, and its magnitude decreases progressively as time since brain injury increases. BDNF plays central roles in synaptic plasticity and neuronal development, and animal models suggest that exogenous BDNF has neuroprotective effects, improving neurological deficits in different brain injuries, including TBI, hypoxic-ischemic brain injury, and subarachnoid hemorrhage [52–54]. Efforts to enhance BDNF expression with rehabilitative strategies are still in the early stage, and this study showed that verticalization with robot-assisted lower-limb training is inadequate for the promotion of BDNF-mediated plastic changes in patients with DOCs. Additional studies are needed to better understand the mechanisms underlying BDNF reduction after severe brain injury, to aid identification of the best rehabilitative strategies to promote restorative plastic changes and recovery in patients with DOCs.
Acknowledgments
The authors would like to thank Teresa Barone from the Immunohematology and Transfusion service of Cefalù, Italy, for the recruitment of healthy controls.
Data Availability
Not available.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Royal College of Physicians. Prolonged Disorders of Consciousness: National Clinical Guidelines. London: R.C.P; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laureys S., Celesia G. G., Cohadon F., et al. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Medicine. 2010;8(1):p. 68. doi: 10.1186/1741-7015-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giacino J. T., Ashwal S., Childs N., et al. The minimally conscious state. Definition and diagnostic criteria. Neurology. 2002;58(3):349–353. doi: 10.1212/WNL.58.3.349. [DOI] [PubMed] [Google Scholar]
- 4.Bagnato S., Boccagni C., Sant’Angelo A., Fingelkurts A. A., Fingelkurts A. A., Galardi G. Emerging from an unresponsive wakefulness syndrome: brain plasticity has to cross a threshold level. Neuroscience and Biobehavioral Reviews. 2013;37(10):2721–2736. doi: 10.1016/j.neubiorev.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez A., Moya-Alvarado G., Gonzalez-Billaut C., Bronfman F. C. Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton. 2016;73(10):612–628. doi: 10.1002/cm.21312. [DOI] [PubMed] [Google Scholar]
- 6.Kowiański P., Lietzau G., Czuba E., Waśkow M., Steliga A., Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cellular and Molecular Neurobiology. 2018;38(3):579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park H., Poo M. M. Neurotrophin regulation of neural circuit development and function. Nature Reviews Neuroscience. 2013;14(1):7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 8.Oyesiku N. M., Evans C. O., Houston S., et al. Regional changes in the expression of neurotrophic factors and their receptors following acute traumatic brain injury in the adult rat brain. Brain Research. 1999;833(2):161–172. doi: 10.1016/S0006-8993(99)01501-2. [DOI] [PubMed] [Google Scholar]
- 9.Kobori N., Clifton G. L., Dash P. K. Altered expression of novel genes in the cerebral cortex following experimental brain injury. Brain Research Molecular Brain Research. 2002;104(2):148–158. doi: 10.1016/S0169-328X(02)00331-5. [DOI] [PubMed] [Google Scholar]
- 10.Korley F. K., Diaz-Arrastia R., Wu A. H. B., et al. Circulating brain-derived neurotrophic factor has diagnostic and prognostic value in traumatic brain injury. Journal of Neurotrauma. 2016;33(2):215–225. doi: 10.1089/neu.2015.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalish H., Phillips T. M. Analysis of neurotrophins in human serum by immunoaffinity capillary electrophoresis (ICE) following traumatic head injury. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2010;878(2):194–200. doi: 10.1016/j.jchromb.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buonora J. E., Yarnell A. M., Lazarus R. C., et al. Multivariate analysis of traumatic brain injury: development of an assessment score. Frontiers in Neurology. 2015;6:p. 68. doi: 10.3389/fneur.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massaro A. N., Wu Y. W., Bammler T. K., et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. The Journal of Pediatrics. 2018;194:67–75.e1. doi: 10.1016/j.jpeds.2017.10.060. [DOI] [PubMed] [Google Scholar]
- 14.Ferris L. T., Williams J. S., Shen C. L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Medicine and Science in Sports and Exercise. 2007;39(4):728–734. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen P., Brassard P., Adser H., et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Experimental Physiology. 2009;94(10):1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 16.Nascimento C. M., Pereira J., Andrade L., et al. Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Current Alzheimer Research. 2014;11(8):799–805. doi: 10.2174/156720501108140910122849. [DOI] [PubMed] [Google Scholar]
- 17.Wang R., Holsinger R. M. D. Exercise-induced brain-derived neurotrophic factor expression: therapeutic implications for Alzheimer’s dementia. Ageing Research Reviews. 2018;48:109–121. doi: 10.1016/j.arr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Elliott L., Coleman M., Shiel A., et al. Effect of posture on levels of arousal and awareness in vegetative and minimally conscious state patients: a preliminary investigation. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76(2):298–299. doi: 10.1136/jnnp.2004.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riberholt C. G., Thorlund J. B., Mehlsen J., Nordenbo A. M. Patients with severe acquired brain injury show increased arousal in tilt-table training. Danish Medical Journal. 2013;60:p. A4739. [PubMed] [Google Scholar]
- 20.Krewer C., Luther M., Koenig E., Müller F. Tilt table therapies for patients with severe disorders of consciousness: a randomized, controlled trial. PLoS One. 2015;10(12, article e0143180) doi: 10.1371/journal.pone.0143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giacino J. T., Kalmar K., Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Archives of Physical Medicine and Rehabilitation. 2004;85(12):2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Seel R. T., Sherer M., Whyte J., et al. Assessment scales for disorders of consciousness: evidence-based recommendations for clinical practice and research. Archives of Physical Medicine and Rehabilitation. 2010;91(12):1795–1813. doi: 10.1016/j.apmr.2010.07.218. [DOI] [PubMed] [Google Scholar]
- 23.Ohira K., Hayashi M. A new aspect of the TrkB signaling pathway in neural plasticity. Current Neuropharmacology. 2009;7(4):276–285. doi: 10.2174/157015909790031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung P. L., Huang C. C., Huang H. M., Tu D. G., Chang Y. C. Thyroxin treatment protects against white matter injury in the immature brain via brain-derived neurotrophic factor. Stroke. 2013;44(8):2275–2283. doi: 10.1161/STROKEAHA.113.001552. [DOI] [PubMed] [Google Scholar]
- 25.Numakawa T., Odaka H., Adachi N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. International Journal of Molecular Sciences. 2018;19(11):p. 3650. doi: 10.3390/ijms19113650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng H. K., Teng K. K., Lee R., et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. The Journal of Neuroscience. 2005;25(22):5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearn M. L., Hu Y., Niesman I. R., et al. Propofol neurotoxicity is mediated by p75 neurotrophin receptor activation. Anesthesiology. 2012;116(2):352–361. doi: 10.1097/ALN.0b013e318242a48c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleitas C., Piñol-Ripoll G., Marfull P., et al. proBDNF is modified by advanced glycation end products in Alzheimer’s disease and causes neuronal apoptosis by inducing p75 neurotrophin receptor processing. Molecular Brain. 2018;11(1):p. 68. doi: 10.1186/s13041-018-0411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shulga A., Rivera C. Interplay between thyroxin, BDNF and GABA in injured neurons. Neuroscience. 2013;239:241–252. doi: 10.1016/j.neuroscience.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Sebastiani A., Granold M., Ditter A., et al. Posttraumatic propofol neurotoxicity is mediated via the pro-brain-derived neurotrophic factor-p75 neurotrophin receptor pathway in adult mice. Critical Care Medicine. 2016;44(2):e70–e82. doi: 10.1097/CCM.0000000000001284. [DOI] [PubMed] [Google Scholar]
- 31.Hota S. K., Barhwal K., Singh S. B., Ilavazhagan G. Chronic hypobaric hypoxia induced apoptosis in CA1 region of hippocampus: a possible role of NMDAR mediated p75NTR upregulation. Experimental Neurology. 2008;212(1):5–13. doi: 10.1016/j.expneurol.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 32.Sharma D., Barhwal K. K., Biswal S. N., et al. Hypoxia-mediated alteration in cholesterol oxidation and raft dynamics regulates BDNF signalling and neurodegeneration in hippocampus. Journal of Neurochemistry. 2019;148(2):238–251. doi: 10.1111/jnc.14609. [DOI] [PubMed] [Google Scholar]
- 33.Hicks R. R., Numan S., Dhillon H. S., Prasad M. R., Seroogy K. B. Alterations in BDNF and NT-3 mRNAs in rat hippocampus after experimental brain trauma. Brain Research Molecular Brain Research. 1997;48(2):401–406. doi: 10.1016/S0169-328X(97)00158-7. [DOI] [PubMed] [Google Scholar]
- 34.Hicks R. R., Li C., Zhang L., Dhillon H. S., Prasad M. R., Seroogy K. B. Alterations in BDNF and trkB mRNA levels in the cerebral cortex following experimental brain trauma in rats. Journal of Neurotrauma. 1999;16(6):501–510. doi: 10.1089/neu.1999.16.501. [DOI] [PubMed] [Google Scholar]
- 35.Poduslo J. F., Curran G. L. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Molecular Brain Research. 1996;36(2):280–286. doi: 10.1016/0169-328X(95)00250-V. [DOI] [PubMed] [Google Scholar]
- 36.Pan W., Banks W. A., Fasold M. B., Bluth J., Kastin A. J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37(12):1553–1561. doi: 10.1016/S0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt H. D., Duman R. S. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35(12):2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guldenmund P., Soddu A., Baquero K., et al. Structural brain injury in patients with disorders of consciousness: a voxel-based morphometry study. Brain Injury. 2016;30(3):343–352. doi: 10.3109/02699052.2015.1118765. [DOI] [PubMed] [Google Scholar]
- 39.Lutkenhoff E. S., Chiang J., Tshibanda L., et al. Thalamic and extrathalamic mechanisms of consciousness after severe brain injury. Annals of Neurology. 2015;78(1):68–76. doi: 10.1002/ana.24423. [DOI] [PubMed] [Google Scholar]
- 40.Morozova S., Kremneva E., Sergeev D., et al. Conventional structural magnetic resonance imaging in differentiating chronic disorders of consciousness. Brain Sciences. 2018;8(8):p. 144. doi: 10.3390/brainsci8080144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagnato S., Andriolo M., Boccagni C., et al. Reduced neuron-specific enolase levels in chronic severe traumatic brain injury. Journal of Neurotrauma. 2020;37(2):423–427. doi: 10.1089/neu.2019.6449. [DOI] [PubMed] [Google Scholar]
- 42.Bagnato S., Boccagni C. Moderate/severe traumatic brain injury as a trigger of chronic neurodegeneration in humans. Neural Regeneration Research. 2020;15(7):1247–1248. doi: 10.4103/1673-5374.272574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Failla M. D., Juengst S. B., Arenth P. M., Wagner A. K. Preliminary associations between brain-derived neurotrophic factor, memory impairment, functional cognition, and depressive symptoms following severe TBI. Neurorehabilitation and Neural Repair. 2015;30(5):419–430. doi: 10.1177/1545968315600525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanne T. M., Åberg N. D., Nilsson S., et al. Low circulating acute brain-derived neurotrophic factor levels are associated with poor long-term functional outcome after ischemic stroke. Stroke. 2016;47(7):1943–1945. doi: 10.1161/STROKEAHA.115.012383. [DOI] [PubMed] [Google Scholar]
- 45.Qiao H. J., Li Z. Z., Wang L. M., Sun W., Yu J. C., Wang B. Association of lower serum brain-derived neurotrophic factor levels with larger infarct volumes in acute ischemic stroke. Journal of Neuroimmunology. 2017;307:69–73. doi: 10.1016/j.jneuroim.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Wade D. T. How often is the diagnosis of the permanent vegetative state incorrect? A review of the evidence. European Journal of Neurology. 2018;25(4):619–625. doi: 10.1111/ene.13572. [DOI] [PubMed] [Google Scholar]
- 47.Cho H. C., Kim J., Kim S., Son Y. H., Lee N., Jung S. H. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO2max performance in healthy college men. Neuroscience Letters. 2012;519(1):78–83. doi: 10.1016/j.neulet.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 48.Nofuji Y., Suwa M., Sasaki H., Ichimiya A., Nishichi R., Kumagai S. Different circulating brain-derived neurotrophic factor responses to acute exercise between physically active and sedentary subjects. Journal of Sports Science and Medicine. 2012;11(1):83–88. [PMC free article] [PubMed] [Google Scholar]
- 49.Erickson K. I., Voss M. W., Prakash R. S., et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egan M. F., Kojima M., Callicott J. H., et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 51.Bagnato S., Minafra L., Bravatà V., et al. Brain-derived neurotrophic factor (Val66Met) polymorphism does not influence recovery from a post-traumatic vegetative state: a blinded retrospective multi-centric study. Journal of Neurotrauma. 2012;29(11):2050–2059. doi: 10.1089/neu.2011.2184. [DOI] [PubMed] [Google Scholar]
- 52.Xu Z., Lv X. A., Dai Q., Lu M., Jin Z. Exogenous BDNF increases mitochondrial pCREB and alleviates neuronal metabolic defects following mechanical injury in a MPTP-dependent way. Molecular Neurobiology. 2018;55(4):3499–3512. doi: 10.1007/s12035-017-0576-5. [DOI] [PubMed] [Google Scholar]
- 53.Huang W., Meng F., Cao J., Liu X., Zhang J., Li M. Neuroprotective role of exogenous brain-derived neurotrophic factor in hypoxia-hypoglycemia-induced hippocampal neuron injury via regulating Trkb/MiR134 signaling. Journal of Molecular Neuroscience. 2017;62(1):35–42. doi: 10.1007/s12031-017-0907-z. [DOI] [PubMed] [Google Scholar]
- 54.Chen H., Dang Y., Liu X., Ren J., Wang H. Exogenous brain-derived neurotrophic factor attenuates neuronal apoptosis and neurological deficits after subarachnoid hemorrhage in rats. Experimental and Therapeutic Medicine. 2019;18(5):3837–3844. doi: 10.3892/etm.2019.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not available.


