Abstract
Background:
The role of nutritional supplements and dietary interventions in preventing mortality and cardiovascular disease (CVD) outcomes is unclear.
Purpose:
To examine evidence about the effects of nutritional supplements and dietary interventions on mortality and cardiovascular outcomes in adults.
Data Sources:
PubMed, CINAHL, and the Cochrane Library from inception until March 2019; Clinicaltrials.gov (10 March 2019); journal Web sites; and reference lists.
Study Selection:
English-language, randomized controlled trials (RCTs) and meta-analyses of RCTs that assessed the effects of nutritional supplements or dietary interventions on all-cause mortality or cardiovascular outcomes, such as death, myocardial infarction, stroke, and coronary heart disease.
Data Extraction:
Two independent investigators abstracted data, assessed the quality of evidence, and rated the certainty of evidence.
Data Synthesis:
Nine systematic reviews and 4 new RCTs were selected that encompassed a total of 277 trials, 24 interventions, and 992 129 participants. A total of 105 meta-analyses were generated. Low-certainty evidence showed that omega-3 long-chain polyunsaturated fatty acid (LC-PUFA) was associated with reduced risk for myocardial infarction (RR, 0.92 [CI, 0.85 to 0.99]) and coronary heart disease (RR, 0.93 [CI, 0.89 to 0.98]). Folic acid was associated with lower risk for stroke (RR, 0.80 [CI, 0.67 to 0.96]; low certainty), whereas calcium plus vitamin D increased the risk for stroke (RR, 1.17 [CI, 1.05 to 1.30]; moderate certainty). Other nutritional supplements, such as vitamin B6, vitamin A, multivitamins, antioxidants, and iron and dietary interventions, such as reduced fat intake, had no significant effect on mortality or cardiovascular disease outcomes (very low- to moderate-certainty evidence).
Limitations:
Suboptimal quality and certainty of evidence.
Conclusion:
Use of omega-3 LC-PUFA, and folate supplementation could reduce risk for some cardiovascular outcomes in adults. Combined calcium plus vitamin D might increase risk for stroke.
Primary Funding Source:
None.
Current U.S. dietary guidelines recommend several healthy eating patterns, including U.S., Mediterranean, and vegetarian diets (1). Although the guidelines recognize the occasional need for nutritional supplementation or food fortification for specific nutrients that may be consumed in inadequate amounts, they do not recommend routine use of any dietary supplement to reduce risk for cardiovascular disease (CVD) or other chronic diseases. Despite these recommendations, most U.S. adults use supplements to enhance their diets, with uncertain health benefits (2, 3). From 1999 to 2012, the NHANES (National Health and Nutrition Examination Survey) reported that 52% of participants used at least 1 and 10% used at least 4 dietary supplements (4). From 2011 to 2014, the NHANES reported that among participants aged 60 years or older, 70% used at least 1 and 29% used at least 4 supplements, and 41% of supplement takers reported that they did so to improve their overall health (5).
In 2013, the U.S. Preventive Services Task Force conducted a systematic review of the utility of vitamin and mineral supplements for CVD prevention and found little evidence to support use (6). More recently, Jenkins and colleagues published a meta-analysis of randomized controlled trials (RCTs) of dietary supplements published through October 2017 (7). They found some stroke benefit conferred by folate; no CVD benefit for multivitamins, vitamin C, vitamin D, or calcium; and evidence for mortality harm for niacin and antioxidants. Since then, several landmark RCTs evaluating the efficacy of fish oils (8–10) and vitamin D (11, 12) for CVD prevention have been published, which add to the evidence level. In addition, the quality of the evidence base of these various nutritional supplements and dietary interventions still needs to be evaluated to ascertain the confidence in their efficacy. Thus, we performed a systematic review of existing meta-analyses of RCTs and generated an evidence map for efficacy of nutritional supplements and dietary interventions for CVD prevention.
Methods
Search Strategy
We used PubMed, CINAHL, and the Cochrane Library from inception to March 2019 to find meta-analyses published in the English language about vitamins, minerals, dietary supplements or products, and dietary interventions using the following search terms: (*minerals OR *vitamins OR *diet AND *cardiovascular outcomes) and (meta-analy* OR metaanaly* OR systematic review*). After selecting systematic reviews on the basis of a priori criteria, the search timelines of the systematic reviews were reviewed for recency and an updated search for RCTs published in English was performed starting from the end date of searches from selected systematic reviews until March 2019 (Supplement Table 1, available at Annals.org). Additional sources included Web sites of major cardiovascular and medicine journals (www.onlinejacc.org; https://academic.oup.com/eurheartj; www.ahajournals.org/journal/circ; www.nejm.org; https://jamanetwork.com; and http://annals.org/aim) and bibliographies of relevant studies. We also searched Clinicaltrials.gov (10 March 2019) to check for publication bias and to identify any new or ongoing trials (Supplement Table 2, available at Annals.org).
Study Selection
The prespecified inclusion criteria were meta-analyses of RCTs assessing efficacy of nutritional supplements (vitamins, minerals, dietary supplements) or dietary interventions in adult participants (≥18 years) that report effect estimates for all-cause mortality and cardiovascular outcomes of interest and were written in English. Because the nutritional and dietary recommendations are universal, there were no restrictions on baseline health status, race, or sex of the participants.
Meta-analyses of observational studies or those reporting efficacy of interventions on surrogate or other outcomes, such as blood pressure, lipid values, inflammatory markers, electrolytes, renal values, or quality-of-life indicators, were excluded. Systematic reviews reporting meta-analyses of both clinical trials and observational studies were reviewed for data related to RCTs only. In case of multiple meta-analyses of the same intervention and outcome, we preferred the most recent, largest, and updated meta-analysis. However, the competing meta-analyses were screened for any additional trials not included in the selected meta-analysis.
After removing duplicates and following the selection criteria, we screened the retrieved articles at the title and abstract level and then at the methods level. The search, selection, and abstraction processes were performed independently by 2 authors (M.U.K. and S.V.). Any discrepancies were resolved by discussion and mutual consensus, referring to the original study or third-party review (S.U.K.).
Data Extraction, Outcomes, and Quality Assessment
We first extracted information from eligible meta-analyses on first author, journal, year of publication, interventions, outcomes of interest, number of trials, whether an appropriate study search and selection criteria was reported, method of pooling estimates (fixed or random effects), methods of detecting publication bias, measure of heterogeneity, and risk-of-bias assessment. Second, we generated the pool of clinical trials by identifying trials contained in the selected meta-analyses and screening competing meta-analyses for additional trials and trials published after the selected meta-analyses (Supplement Table 3, available at Annals.org). Among new clinical trials for omega-3 long-chain polyunsaturated fatty acid (LC-PUFA) (8–10), we excluded REDUCE-IT (Reduction of Cardiovascular Events With EPA-Intervention Trial) (9) because icosapent ethyl, a highly purified form of eicosapentaenoic acid (EPA), does not qualify as a dietary supplement according to the Dietary Supplement Health and Education Act of 1994 (13). Third, after removing duplicates, we abstracted data on trial name, first author, year, intervention, outcomes, raw events, and sample sizes for each group.
The main outcome of interest was all-cause mortality. The secondary outcomes were cardiovascular mortality, myocardial infarction (MI), stroke, and coronary heart disease (CHD).
Two independent reviewers (V.O. and M.S.K.) assessed the methodological quality of meta-analyses on specific potential factors that may affect the validity of summary estimates—that is, appropriate search and selection criteria, number of trials and participants included, risk-of-bias assessment of included trials, method of pooling the estimates, assessment of publication bias, and degree of heterogeneity (Supplement Table 4, available at Annals.org).
Data Synthesis and Analysis
We created an evidence map that displays the plausible benefits of each intervention and the certainty of the evidence (14). The certainty of the evidence was evaluated using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach (GRADEpro GDT) (https://gdt.gradepro.org/app/) (14) and was classified as high, moderate, low, or very low (Supplement Table 5, available at Annals.org). Two reviewers (V.O. and M.S.K.) performed these assessments under the supervision of a third reviewer (S.U.K.).
Estimates were pooled according to Mantel-Haenszel random-effects model. The Paule-Mandel method was used for reestimating outcomes. Hartung-Knapp/Sidik-Jonkman (HKSJ) small-sample adjustments were applied when the number of studies was less than 10 (15). We used the modified HKSJ when meta-analyses only included 2 to 4 studies and τ2 = 0 because some of these meta-analyses produced abnormally narrow CIs. Effect sizes were reported as risk ratios (RRs) with 95% CIs. We used I2 statistics to estimate the extent of unexplained heterogeneity; I2 greater than 50% was considered a high degree of between-study heterogeneity. We calculated the Egger regression test as an estimate of publication bias for any reanalysis that included at least 10 studies (16).
Statistical analyses were conducted using “meta,” version 4.9–4 (R Project for Statistical Computing), and “meta” commands from Stata, version 16 (Stata Corp). Statistical significance was set at 0.05 for all analyses except for the Egger regression test, which had a threshold less than 0.10 because of the test’s limited statistical power.
Role of the Funding Source
The study received no funding.
Results
Search Results
Of 942 citations, after removing duplicates and screening at the title and abstract level we reviewed 140 full-text articles for eligibility. We excluded 131 articles because they focused on nonrandomized studies, were not relevant, or were outdated, as well as 5 systematic reviews that assessed intake of nuts (17), fruits and vegetables (18), fiber (19), and green or black tea (20) and those focusing on low-carbohydrate and low-fat diets (21) that did not report cardiovascular outcomes of interest. Ultimately, we included 9 systematic reviews and 4 new RCTs for a total of 105 meta-analyses of 24 interventions (277 RCTs, 992 129 participants) (7, 22–29) (Figure 1). The interventions evaluated in the meta-analyses included 16 types of supplements (antioxidants, β-carotene, vitamin B complex, multivitamins, selenium, vitamin A, vitamin B3 or niacin, vitamin B6, vitamin C, vitamin E, vitamin D, calcium plus vitamin D, calcium, folic acid, iron, and omega-3 LC-PUFA) and 8 types of dietary interventions (Mediterranean diet and intake of reduced saturated fat, modified dietary fat, reduced dietary fat, reduced salt among hypertensive and normotensive participations, increased omega-3 α-linolenic acid [ALA], and increased omega-6 PUFA) (Supplement Table 6, available at Annals.org).
Figure 1.
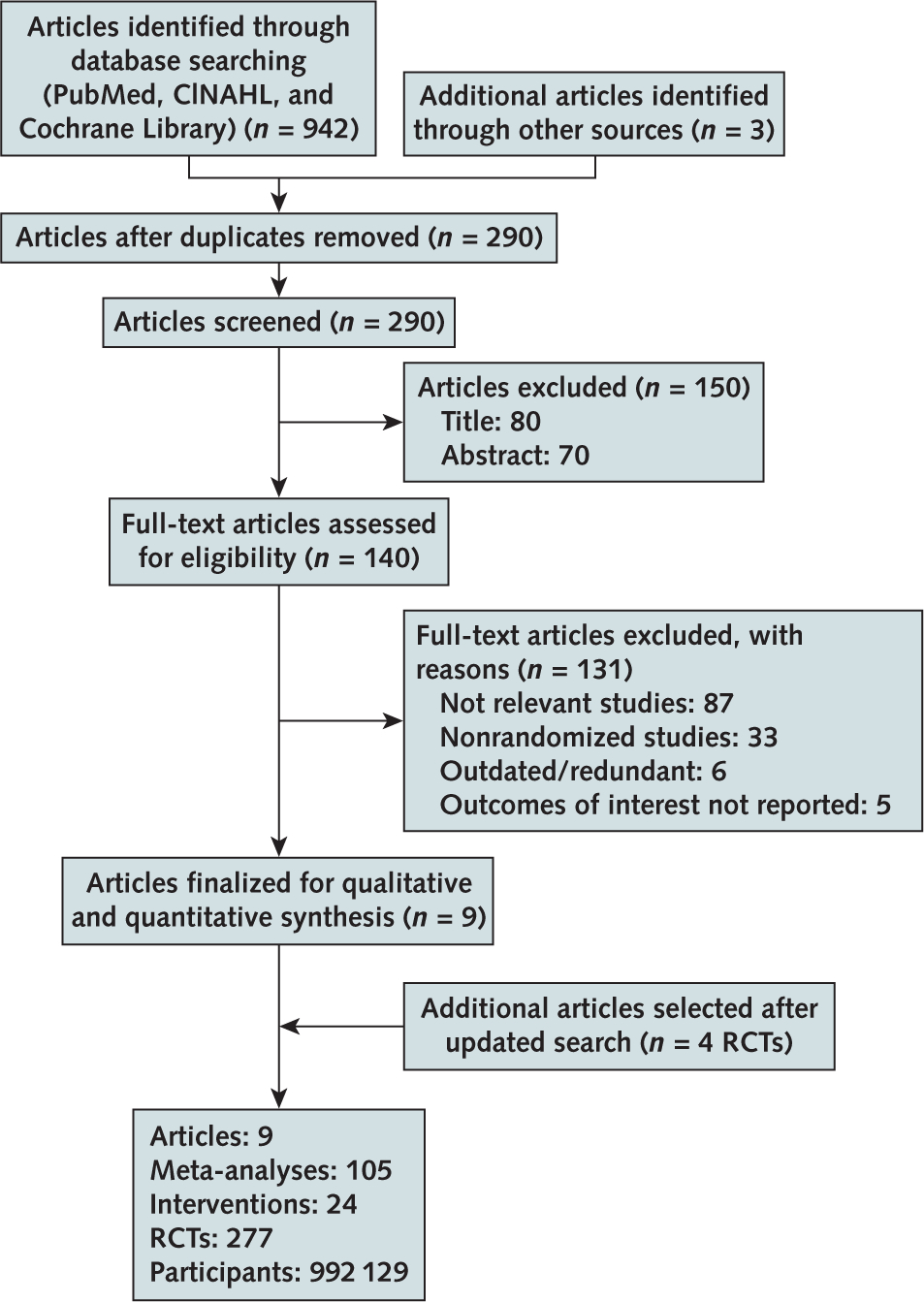
Evidence search and selection.
RCT = randomized controlled trial.
Quality Assessment
All included studies were trial-level meta-analyses (7, 22–28), except the study by Mente and colleagues, which was a patient-level analysis of 4 studies (29) (Supplement Table 4). All trial-level systematic reviews reported comprehensive search and selection criteria as well as quality assessment of studies by using the Cochrane Risk of Bias Tool (30). Six systematic reviews primarily used random-effects models for meta-analyses, of which 4 used fixed-effects models for sensitivity analyses. Two studies primarily used fixed-effects models, of which 1 selected a random-effects model only for estimates with an I2 greater than 50%. Out of all trial-level analyses, only 2 did not assess publication bias, and 1 did not evaluate between-study variance because of the limited number of trials (<10). Eighty-one (77%) meta-analyses included fewer than 10 trials. Thirty-six (34%) meta-analyses included fewer than 84% double-blind RCTs; of these, 3 (2.8%) had a total sample of fewer than 1000 participants, 16 (15%) had I2 greater than 50%, and 4 (3.8%) had significant publication bias.
All-Cause Mortality
All 24 interventions assessed the risk for all-cause mortality. None of the nutritional supplements or dietary interventions had no association with risk for this outcome (Figure 2). The Egger regression test was consistent with publication bias for omega-3 LC-PUFA (P = 0.09) and reduced saturated fat intake for all-cause mortality (P = 0.02) (Supplement Table 7, available at Annals.org).
Figure 2.
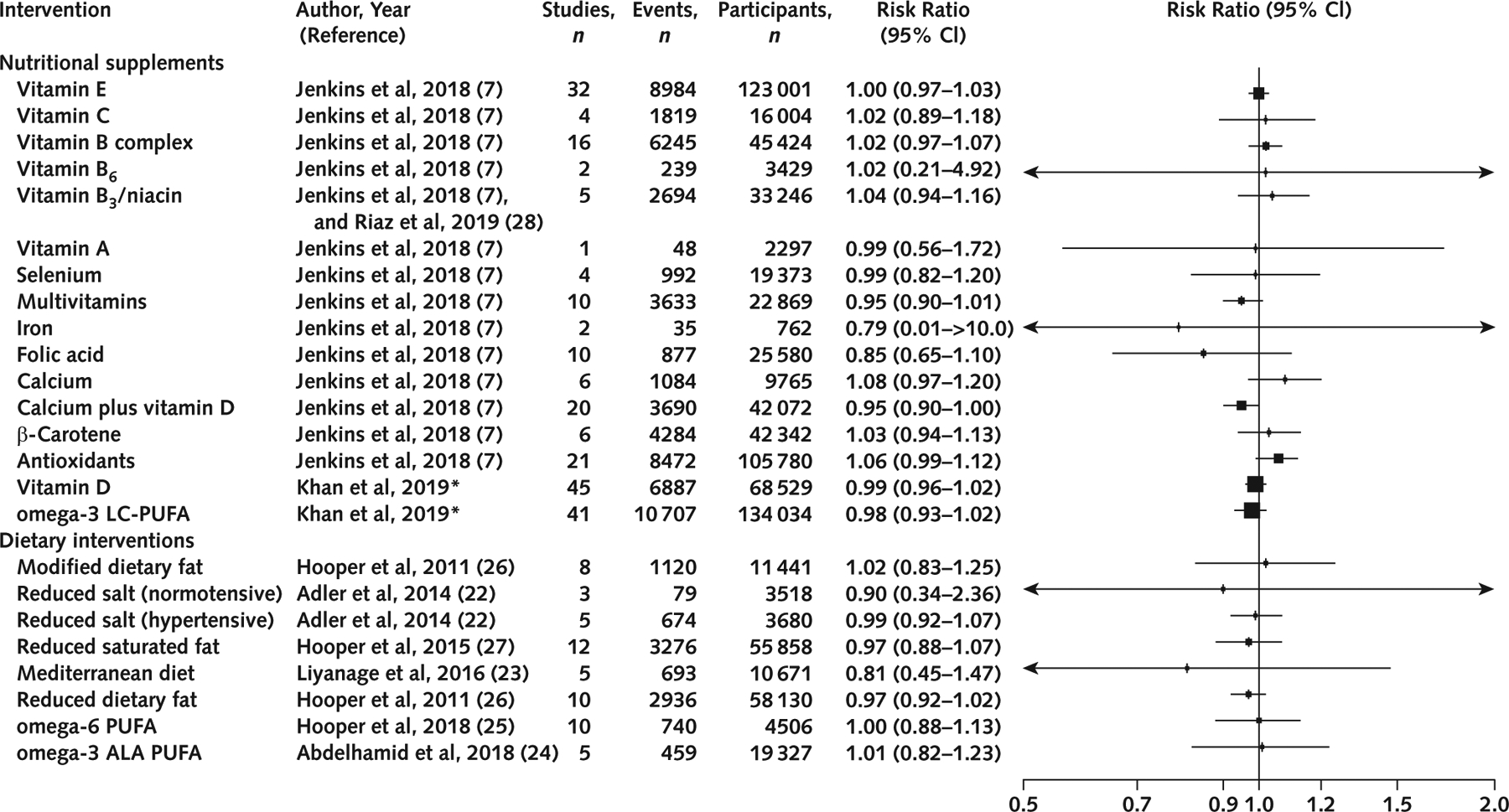
Effects of nutritional supplements and dietary interventions on all-cause mortality.
ALA = α-linolenic acid; LC-PUFA = long-chain polyunsaturated fatty acid. * Updated meta-analysis after inclusion of new clinical trials.
Cardiovascular Mortality
Twenty-one interventions assessed the risk for cardiovascular mortality. None of the nutritional supplements or dietary interventions assessed had any association with risk for this outcome (Figure 3).
Figure 3.

Effects of nutritional supplements and dietary interventions on cardiovascular mortality.
ALA = α-linolenic acid; LC-PUFA = long-chain polyunsaturated fatty acid. * Updated meta-analysis after inclusion of new clinical trials..
MI
Twenty-one interventions assessed risk for MI. Use of omega-3 LC-PUFA was associated with reduced risk (RR, 0.92 [CI, 0.85 to 0.99]; P = 0.03; I2 = 1%; low certainty) (Supplement Figure 1, available at Annals.org). Other nutritional supplements or dietary interventions had no association with risk for this outcome. The Egger regression test was consistent with publication bias for meta-analyses of vitamin E (P = 0.01) (Supplement Table 7).
Stroke
Twenty interventions assessed the risk for stroke. Folic acid was associated with lower risk (RR, 0.80 [CI, 0.67 to 0.96]; P = 0.02; I2 = 0%; low certainty), whereas combined calcium plus vitamin D intake was associated with increased risk (RR, 1.17 [CI, 1.05 to 1.30]; P = 0.01; I2 = 0%; moderate certainty) (Supplement Figure 2, available at Annals.org). Other nutritional supplements or dietary interventions had no association with risk for this outcome. The Egger regression test was consistent with publication bias for meta-analyses of vitamin E (P = 0.08) (Supplement Table 7).
CHD
Nineteen interventions assessed the risk for CHD. Use of omega-3 LC-PUFA was associated with reduced risk (RR, 0.93 [CI, 0.89 to 0.98]; P = 0.01; I2 = 2%; low certainty) (Supplement Figure 3, available at Annals.org). There was no association between other nutritional supplements or dietary interventions with risk for CHD.
Evidence Map
Figure 4 is an evidence map summarizing the findings for included interventions. There is a paucity of data assessing the effects of vitamin B6, vitamin A, multivitamins, iron, antioxidants, and reduced salt intake on certain cardiovascular end points. The map also shows the lack of significant effects on all-cause mortality and cardiovascular outcomes for nutritional supplements, that the certainty of evidence varies from very low to low for most of the interventions, and that none of the interventions have high-quality evidence.
Figure 4.

Evidence map of availability and appraisal of certainty of the evidence.
ALA = α-linolenic acid; LC-PUFA = long-chain polyunsaturated fatty acid.
Discussion
In this overview of 24 nutritional supplements and dietary interventions evaluating data from RCTs and meta-analyses of RCTs, we found some evidence that omega-3 LC-PUFA was protective for MI and CHD, and folic acid was protective for stroke. Conversely, combined calcium plus vitamin D intake increased the risk for stroke. Other supplements, such as multivitamins, selenium, vitamin A, vitamin B6, vitamin C, vitamin E, vitamin D alone, calcium alone, folic acid, and iron, or such dietary interventions as the Mediterranean diet, reduced saturated fat intake, modified fat intake, reduced dietary fat intake, and increased intake of omega-3 ALA or omega-6 PUFA, did not seem to have a significant effect on mortality or CVD outcomes (with very low- to moderate-certainty evidence).
The effects of reduced salt intake on mortality and CVD risk reduction remain a debatable issue. Although some data support lower salt intake to reduce CVD risk (31, 32), other studies have shown a U-shaped relationship between sodium intake and death (33–35). Recently, 2 studies explored the relationship between measures of sodium intake, estimated from urinary sodium excretion and death (29, 32). A patient-level study of 4 prospective studies (133 118 participants) concluded that reduced intake of sodium should be confined to hypertensive patients only who also consume high sodium (29). However, Cook and colleagues reported a higher risk for all-cause mortality with increased sodium intake in participants of the TOHP (Trials of Hypertension Prevention) and showed the benefit of reduced sodium intake on death during a period of 20 years (32).
Clinical trials of omega-3 LC-PUFA have shown conflicting results regarding reduction of cardiovascular outcomes. However, recent randomized data have shown cardiovascular benefits (8–10). Although VITAL (Vitamin D and Omega-3 Trial) (8) and ASCEND (A Study of Cardiovascular Events iN Diabetes) (10) did not find convincing evidence of protective effects of omega-3 LC-PUFA for overall cardiovascular benefits (primary outcomes), VITAL did show a benefit of omega-3 LC-PUFA at 1 g per day for the reduction of MI, a secondary outcome (8). Moreover, VITAL showed a 19% reduction in major CVD outcomes among the subgroup of participants with low dietary fish intake (8).
Even more notable was the recent publication of the landmark REDUCE-IT, that found, compared with placebo, a remarkable 25% reduction in cardiovascular end points with the use of icosapent ethyl, a modified and highly purified form of EPA (9). This trial studied a much higher dose of EPA (4 g/d) than previous studies and included high-risk participants (those with known atherosclerotic CVD or diabetes mellitus and at least 1 additional vascular risk factor) who had controlled low-density lipoprotein cholesterol while receiving statin therapy but had elevated triglyceride levels (135 to 499 mg/dL) (9). As the cardiovascular risk reduction seen with icosapent ethyl exceeded the anticipated benefits from triglyceride reduction alone, other potential beneficial mechanisms, such as anti-inflammatory or anti-thrombotic effects, have been speculated. Icosapent ethyl is proprietary and is available only by prescription. It is unclear whether the effects observed in REDUCE-IT are specific for icosapent ethyl or reflect use of the higher dose of omega-3 LC-PUFA. The results should thus not be generalized to dietary supplement formulations of omega-3 LC-PUFA, which are unregulated and have variable composition (typically EPA plus docosahexaenoic acid).
Folate supplementation was associated with a lower risk for stroke, but this was largely driven by the results of the CSPPT (China Stroke Primary Prevention Trial), which evaluated the efficacy of folic acid therapy for primary prevention of stroke among hypertensive adults in China (36). This benefit might be due to the lack of folate fortification of foods in China (7), and whether these results can be generalized to a population, such as the United States, which has folate fortification, remains unclear.
On the other hand, we found that combined calcium plus vitamin D supplementation resulted in a higher risk for stroke. In a reanalysis of the WHI CaD Study (Women’s Health Initiative Calcium/Vitamin D Supplementation Study), risk for cardiovascular events, including stroke, was higher in women allocated to calcium plus vitamin D administration who were not taking personal calcium supplements (37). Potential biological explanations are hypercalcemia-mediated vascular calcifications, triggering of atherosclerosis, and hypercoagulability (38, 39). Of note, a recent observational analysis from NHANES found that use of calcium supplements was associated with an increased risk for death from cancer (2). Another analysis found an association with increased risk for MI (40). These findings, along with our findings from RCTs regarding stroke risk, raise concerns about harms from calcium supplement use. Regarding vitamin D alone (without calcium), despite new RCT data from the VITAL (11) and ViDA (Vitamin D Assessment Study) (12) trials, there was no evidence found for benefit or harm for vitamin D supplementation and CVD risk reduction.
Regarding multivitamins, our review was consistent with a previous meta-analysis (3) and supports the statements by the U.S. Preventive Services Task Force in 2014 regarding the lack of adequate evidence to support the benefit of multivitamin supplementation for CVD or death (6, 41). The lack of benefit of dietary supplements on death was also seen in a recent observational study from NHANES (2).
Regarding dietary recommendations from food sources, the American Heart Association (42) and the 2015 to 2020 U.S. dietary guidelines suggest limiting saturated fats and trans fats as a “key recommendation” for promoting a healthy lifestyle. The Mediterranean diet has been shown to be effective in reducing cardiovascular risk (23), but concerns have been raised regarding the methodological validity of some of the RCT studies. For instance, the Indo-Mediterranean study generated considerable controversy because of the lack of trained professionals required to run a trial of scientific validity (43). Similarly, the PREDIMED (Prevención con Dieta Mediterránea) (44) study was retracted and republished after errors in random assignment were found, although the conclusions were largely unchanged in the reanalysis. In our analysis, the Mediterranean diet, modified dietary fat, reduced dietary fat, reduced saturated fat intake, omega-6 PUFA, or omega-3 ALA PUFA did not reduce the risk for mortality or cardiovascular outcomes.
We compared our results with previous meta-analyses identified in our searches. Graudal and colleagues (274 683 patients) concluded that both low and high salt intake were associated with higher risk for all-cause mortality (35). However, their results were predominantly based on observational studies (23 cohort studies and 2 follow-up studies of RCTs). Conversely, Adler and colleagues showed little evidence for cardiovascular mortality reduction with lowered salt intake among hypertensive patients (RR, 0.67 [CI, 0.45 to 1.01]), which did not achieve statistical significance (22). We included the same clinical trials, but the discrepancy in results may be due to the different analytic approach used in the meta-analyses. Adler and colleagues used a fixed-effects model to analyze the results, whereas our meta-analysis was conducted using a more robust Paule-Mandel estimator with Hartung-Knapp adjustments (15). The same explanation applies to differences in results related to multivitamins and minerals from a recent meta-analysis by Jenkins and colleagues (7), except for folic acid, where we are in accord with Jenkins and colleagues’ findings. Abdelhamid and colleagues suggested benefit of omega-3 LC-PUFA in reducing CHD risk (RR, 0.93 [CI, 0.88 to 0.97]) but found no statistically significant effect on MI (24). Another meta-analysis by Aung and colleagues (10 RCTs, 77 917 participants) showed that omega-3 LC-PUFA supplementation was not associated with prevention of fatal CHD or CVD events (45). Our analysis is updated with recent data through March 2019, which explains the difference in results for omega-3 LC-PUFA compared with earlier reviews (8, 10, 24, 45). Regarding the higher risk for stroke due to combined calcium plus vitamin D, our results are consistent with a previous meta-analysis (37).
Our study’s strengths included using data only from RCTs and their meta-analyses, considering both dietary interventions and dietary supplements, and incorporating new trial data published in 2018 and 2019 after prior meta-analyses. The U.S. Department of Health and Human Services and the U.S. Department of Agriculture have been criticized for the paucity of sound scientific background behind their dietary recommendations (46). Similarly, the U.S. Preventive Services Task Force report has not been updated since 2014 (41). Our review provides a direct quantitative comparison of various nutritional and dietary interventions for cardiovascular outcomes. Because our generated evidence map is derived from RCTs, this report can assist to cover the “evidence-free zone” in this field (46).
Nevertheless, our findings need to be considered in the context of certain limitations. There are inherent limitations secondary to the shortcomings of included meta-analyses and RCTs (that is, heterogeneity of baseline characteristics of the participants, including age, sex, health and socioeconomic status, and interventions; lack of dose-response analyses; and variable duration of follow-up). Because the focus of our study was to provide broad-based evidence for various nutritional supplements and dietary interventions using existing meta-analyses and trial-level information, we could not analyze interventions according to important subgroups, such as sex, body mass index, lipid values, blood pressure thresholds, diabetes, and history of CVD. Various meta-analyses pooled a smaller number of trials, leading to the risk for small-study effects (47), and were limited by trials that were not double blind, lacked robust methods of pooling estimates, and had publication bias. Using the GRADE system, we found that the certainty of evidence was generally low or very low. Issues related to precision of the estimates, indirectness, quantitative and qualitative heterogeneity, and publication bias resulted in generally low-quality evidence.
In summary, this overview of the efficacy of nutritional supplements and dietary interventions on mortality and cardiovascular outcomes found evidence that supports omega-3 LC-PUFA intake, and folate supplementation for CVD risk reduction. Conversely, combined calcium plus vitamin D showed an increased risk for stroke. Other vitamins, minerals, dietary supplements, and dietary interventions were not associated with survival or cardiovascular benefits. Overall, these findings are limited by suboptimal quality of the evidence. This study can help those who create professional cardiovascular and dietary guidelines modify their recommendations, provide the evidence base for clinicians to discuss dietary supplements with their patients, and guide new studies to fulfill the evidence gap.
From West Virginia University, Morgantown, West Virginia (S.U.K., M.U.K., S.V.); Cleveland Clinic, Cleveland, Ohio (H.R.); Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (D.Z.); East Carolina University, Greenville, North Carolina (L.V., V.O.); Mayo Clinic, Rochester, Minnesota (I.B.R., M.H.M.); John H. Stroger, Jr. Hospital of Cook County, Chicago, Illinois (M.S.K.); Guthrie Robert Packer Hospital, Sayre, Pennsylvania (E.K.); Johns Hopkins School of Medicine, Baltimore, Maryland (M.J.B.); Johns Hopkins Bloomberg School of Public Health and Johns Hopkins School of Medicine, Baltimore, Maryland (E.G.); and Johns Hopkins School of Medicine and Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (E.D.M.).
Supplementary Material
Financial Support:
Drs. Zhao, Guallar, and Michos are funded by the Blumenthal Scholars Fund in Preventive Cardiology at Johns Hopkins University.
Disclosures: Dr. Blaha reports grants from the National Heart, Lung, and Blood Institute, the Food and Drug Administration, the American Heart Association, Amgen, and the Aetna Foundation and personal fees from the Food and Drug Administration, Amgen, Sanofi, Novartis, Novo Nordisk, and Bayer outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M19-0341.
Footnotes
Current author addresses and author contributions are available at Annals.org.
Contributor Information
Safi U. Khan, West Virginia University, One Medical Center Drive,Morgantown, WV 26508..
Muhammad U. Khan, West Virginia University, One Medical Center Drive,Morgantown, WV 26508..
Haris Riaz, Cleveland Clinic, 9500 Euclid Avenue, Cleveland,OH 44195..
Shahul Valavoor, West Virginia University, One Medical Center Drive, Morgantown, WV 26508..
Di Zhao, Johns Hopkins Bloomberg School of Public Health, Welch Center, 2024 East Monument Street,Suite 2600, Baltimore, MD 21205..
Lauren Vaughan, East Carolina University, 2100 Stantonsburg Road, Greenville, NC 27834..
Victor Okunrintemi, East Carolina University, 2100 Stantonsburg Road, Greenville, NC 27834..
Irbaz Bin Riaz, Mayo Clinic, 200 1st Street Southwest,Rochester, MN 55901..
Muhammad Shahzeb Khan, South Ashland Avenue, Apt 1206, Chicago,IL 60607..
Edo Kaluski, Guthrie Robert Packer Hospital, One Guthrie Square, Sayre, PA 18840..
M. Hassan Murad, Mayo Clinic, 200 1st Street Southwest,Rochester, MN 55901..
Michael J. Blaha, Johns Hopkins School of Medicine, 600 North Wolfe Street, Blalock 524-D1, Baltimore, MD 21287..
Eliseo Guallar, Johns Hopkins Bloomberg School of Public Health, Welch Center, 2024 East Monument Street,Suite 2600, Baltimore, MD 21205..
Erin D. Michos, Johns Hopkins School of Medicine, 600 North Wolfe Street, Blalock 524-B, Baltimore, MD 21287..
References
- 1.DeSalvo KB, Olson R, Casavale KO. Dietary guidelines for Americans. JAMA. 2016;315:457–8. doi: 10.1001/jama.2015.18396 [DOI] [PubMed] [Google Scholar]
- 2.Chen F, Du M, Blumberg JB, et al. Association among dietary supplement use, nutrient intake, and mortality among U.S. adults. A cohort study. Ann Intern Med. 2019. doi: 10.7326/M18-2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J, Choi J, Kwon SY, et al. Association of multivitamin and mineral supplementation and risk of cardiovascular disease: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2018;11:e004224. doi: 10.1161/CIRCOUTCOMES.117.004224 [DOI] [PubMed] [Google Scholar]
- 4.Kantor ED, Rehm CD, Du M, et al. Trends in dietary supplement use among US adults from 1999–2012. JAMA. 2016;316:1464–74. doi: 10.1001/jama.2016.14403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gahche JJ, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011–2014. J Nutr. 2017;147:1968–76. doi: 10.3945/jn.117.255984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer. An updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159:824–34. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins DJA, Spence JD, Giovannucci EL, et al. Supplemental vitamins and minerals for CVD prevention and treatment. J Am Coll Cardiol. 2018;71:2570–84. doi: 10.1016/j.jacc.2018.04.020 [DOI] [PubMed] [Google Scholar]
- 8.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 10.Bowman L, Mafham M, Wallendszus K, et al. ; ASCEND Study Collaborative Group. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379:1540–50. doi: 10.1056/NEJMoa1804989 [DOI] [PubMed] [Google Scholar]
- 11.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scragg R, Stewart AW, Waayer D, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the Vitamin D Assessment Study: a randomized clinical trial. JAMA Cardiol. 2017;2:608–16. doi: 10.1001/jamacardio.2017.0175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodge T, Litt D, Kaufman A. Influence of the Dietary Supplement Health and Education Act on consumer beliefs about the safety and effectiveness of dietary supplements. J Health Commun. 2011;16: 230–44. doi: 10.1080/10810730.2010.529493 [DOI] [PubMed] [Google Scholar]
- 14.Farah WH, Alsawas M, Mainou M, et al. Non-pharmacological treatment of depression: a systematic review and evidence map. Evid Based Med. 2016;21:214–21. doi: 10.1136/ebmed-2016-110522 [DOI] [PubMed] [Google Scholar]
- 15.Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7:55–79. doi: 10.1002/jrsm.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin N, Germanò R, Hartley L, et al. Nut consumption for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015:CD011583. doi: 10.1002/14651858.CD011583.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartley L, Igbinedion E, Holmes J, et al. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev. 2013:CD009874. doi: 10.1002/14651858.CD009874.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartley L, May MD, Loveman E, et al. Dietary fibre for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2016:CD011472. doi: 10.1002/14651858.CD011472.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartley L, Flowers N, Holmes J, et al. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD009934. doi: 10.1002/14651858.CD009934.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansoor N, Vinknes KJ, Veierød MB, et al. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr. 2016;115:466–79. doi: 10.1017/S0007114515004699 [DOI] [PubMed] [Google Scholar]
- 22.Adler AJ, Taylor F, Martin N, et al. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014:CD009217. doi: 10.1002/14651858.CD009217.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liyanage T, Ninomiya T, Wang A, et al. Effects of the Mediterranean diet on cardiovascular outcomes: a systematic review and meta-analysis. PLoS One. 2016;11:e0159252. doi: 10.1371/journal.pone.0159252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;11:CD003177. doi: 10.1002/14651858.CD003177.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hooper L, Al-Khudairy L, Abdelhamid AS, et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;7:CD011094. doi: 10.1002/14651858.CD011094.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooper L, Summerbell CD, Thompson R, et al. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev. 2011:CD002137. doi: 10.1002/14651858.CD002137.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hooper L, Martin N, Abdelhamid A, et al. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2015:CD011737. doi: 10.1002/14651858.CD011737 [DOI] [PubMed] [Google Scholar]
- 28.Riaz H, Khan SU, Rahman H, et al. Effects of high-density lipoprotein targeting treatments on cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2019;26:533–43. doi: 10.1177/2047487318816495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mente A, O’Donnell M, Rangarajan S, et al. ; PURE, EPIDREAM and ONTARGET/TRANSCEND Investigators. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388:465–75. doi: 10.1016/S0140-6736(16)30467-6 [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129:981–9. doi: 10.1161/CIRCULATIONAHA.113.006032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook NR, Appel LJ, Whelton PK. Sodium intake and all-cause mortality over 20 years in the Trials of Hypertension Prevention. J Am Coll Cardiol. 2016;68:1609–17. doi: 10.1016/j.jacc.2016.07.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Donnell M, Mente A, Rangarajan S, et al. ; PURE Investigators. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–23. doi: 10.1056/NEJMoa1311889 [DOI] [PubMed] [Google Scholar]
- 34.Kalogeropoulos AP, Georgiopoulou VV, Murphy RA, et al. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: the Health, Aging, and Body Composition (Health ABC) Study. JAMA Intern Med. 2015;175:410–9. doi: 10.1001/jamainternmed.2014.6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graudal N, Jürgens G, Baslund B, et al. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens. 2014;27:1129–37. doi: 10.1093/ajh/hpu028 [DOI] [PubMed] [Google Scholar]
- 36.Huo Y, Li J, Qin X, et al. ; CSPPT Investigators. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313: 1325–35. doi: 10.1001/jama.2015.2274 [DOI] [PubMed] [Google Scholar]
- 37.Bolland MJ, Grey A, Avenell A, et al. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040. doi: 10.1136/bmj.d2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chin K, Appel LJ, Michos ED. Vitamin D, calcium, and cardiovascular disease: A”D”vantageous or “D”etrimental? An era of uncertainty. Curr Atheroscler Rep. 2017;19:5. doi: 10.1007/s11883-017-0637-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA). J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moyer VA; U.S. Preventive Services Task Force. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer. U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:558–64. doi: 10.7326/M14-0198 [DOI] [PubMed] [Google Scholar]
- 42.Lichtenstein AH, Appel LJ, Brands M, et al. ; American Heart Association Nutrition Committee. Diet and Lifestyle Recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. [DOI] [PubMed] [Google Scholar]
- 43.Singh RB, Dubnov G, Niaz MA, et al. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): a randomised single-blind trial. Lancet. 2002;360:1455–61. [DOI] [PubMed] [Google Scholar]
- 44.Estruch R, Ros E, Salas-Salvadó J, et al. ; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389 [DOI] [PubMed] [Google Scholar]
- 45.Aung T, Halsey J, Kromhout D, et al. ; Omega-3 Treatment Trial-ists’ Collaboration. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3:225–34. doi: 10.1001/jamacardio.2017.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nissen SE. U.S. dietary guidelines. An evidence-free zone. Ann Intern Med. 2016;164:558–9. doi: 10.7326/M16-0035 [DOI] [PubMed] [Google Scholar]
- 47.Schwarzer G, Carpenter JR, Rücker G. Small-study effects in meta-analysis In: Schwarzer G, Carpenter JR, Rücker G, eds. Meta-Analysis with R. Cham, Switzerland: Springer Publishing; 2015: 107–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


