Abstract
PURPOSE:
The role of chemotherapy (CT) and radiotherapy (RT) for management of extraskeletal osteosarcoma (ESOS) remains controversial. We examined disease outcomes for ESOS patients and investigated the association between CT/RT with recurrence and survival.
PATIENTS AND METHODS:
Retrospective review at 25 international sarcoma centers identified patients ≥18 years old treated for ESOS from 1971–2016. Patient/tumor characteristics, treatment, local/systemic recurrence, and survival data were collected. Kaplan-Meier survival and Cox proportional hazards regression and cumulative incidence competing risks analysis were performed.
RESULTS:
370 patients with localized ESOS treated definitively with surgery presented with mainly deep tumors (n=294, 80%). 122 patients underwent surgical resection alone, 96 (26%) also received CT, 70 (19%) RT, and 82 (22%) both adjuvants. Five-year survival for patients with localized ESOS was 56% (95%CI 51% to 62%). Almost half of patients (n=173, 47%) developed recurrence: local 9% (35/370), distant 28% (102/370) or both 10% (36/370). Considering death as a competing event, there was no significant difference in cumulative incidence of local or systemic recurrence between patients who received CT, RT, both, or neither (local p=0.50, systemic p=0.69). Multiple regression Cox analysis showed a significant association between RT and decreased local recurrence (HR 0.46 (95%CI 0.26–0.80), p=0.01).
CONCLUSION:
While the use of RT significantly decreased local recurrences, CT did not decrease the risk of systemic recurrence, and neither CT, RT nor both were associated with improved survival in patients with localized ESOS. Our results do not support the use of CT, however, adjuvant RT demonstrates benefit in patients with locally resectable ESOS.
Keywords: extraskeletal osteosarcoma, soft tissue osteosarcoma, chemotherapy, radiotherapy, radiation therapy
INTRODUCTION
Osteosarcoma (OS) is classically a primary malignant tumor of bone that occurs most commonly in adolescents and young adults. Wilson first described the occurrence of osteosarcoma at an extraskeletal site in 19411. Since then, extraskeletal osteosarcoma (ESOS) has been described in several series2–4; however, a full understanding of optimal treatment remains incomplete5. ESOS represents approximately 4% of osteosarcomas and less than 1% of soft tissue sarcomas (STS)6,7. Unlike the more common form of OS originating in bone, ESOS tends to occur in older patients and is associated with worse outcomes2,7,8.
Chemotherapy (CT) has unequivocally demonstrated decreased risk of metastatic recurrence in patients with localized OS of bone, rendering multi-agent chemotherapy with any combination of doxorubicin, cisplatin +/− methotrexate or ifosfamide as the standard of care in this disease9. In contrast, there is no similar consensus about the role of CT in patients with ESOS, nor guidance for the drugs that should be used3,4,10.
In treating patients with conventional OS of bone, radiation therapy (RT) is rarely utilized. In contrast, both pre-operative and post-operative RT have been used in the management of patients with ESOS, at doses similar to other more conventional types of STS4,10,11.
We therefore sought to review an international experience of treatment for patients with non-metastatic ESOS in order to address the question of whether RT and/or systemic CT offers disease recurrence and/or survival benefit.
METHODS
Patient Information
Twenty-five tertiary-level sarcoma referral centers from Canada, USA, Japan, China, South Korea, France, Taiwan, and Germany participated in this study. Each institution obtained approval for this study by their local research ethics board, and then performed a retrospective review of their prospectively maintained sarcoma database. Inclusion criteria for this study were a diagnosis of high-grade non-metastatic extraskeletal osteosarcoma (ESOS) at an extremity or truncal site and patient age ≥ 18 years, between 1971 and 2016. All patients underwent definitive surgical resection. Retroperitoneal or intra-abdominal tumors were excluded. Patient demographics including age and sex were recorded. Tumor features including size (small = ≤5cm or large >5cm), depth and anatomic location were noted. Treatment details collected included type of surgery and margin status according to the R classification: R0 - negative margin, no tumor at the inked margin; R1 – microscopically positive margin, tumor present at the inked margin; R2 – grossly positive margin12. Patients who underwent only surgical biopsy of their tumor were deemed to have not received definitive surgical treatment. Some of the patients enrolled in this study were previously included in single institution publications assessing the outcomes of patients with ESOS8,13,14.
Information regarding the use of adjuvant therapies including CT and/or RT were also collected. The type of CT regimen was categorized as follows: doxorubicin plus either cisplatin and/or methotrexate (“osteosarcoma-type”) or doxorubicin plus any agent other than cisplatin or methotrexate (“soft-tissue sarcoma type”). If RT was utilized, pre- or post-operative timing was documented. Lastly, local or systemic disease recurrence and survival status were obtained.
Statistical Analysis
An event was defined as first local or distant recurrence from ESOS and was used to calculate the time to disease recurrence from initial surgery date. All-cause deaths were considered events in the analysis of survival. Statistical analysis was performed using R software (version 3.2.2) with the following packages: survival, coxph, coxme, cmprsk, survminer15. Differences in proportions were calculated using the χ2 test, or the Fisher’s exact test in instances of small sample sizes. The cumulative probabilities of local and systemic recurrence were estimated as described by Prentice et al with death as a competing event16,17 The overall survival, from the date of definitive surgery to the date of death (any cause), was estimated with the Kaplan and Meier method18, and differences in survival between subgroups were evaluated using the log-rank test. A Gray’s test was used to compare cumulative incidences between categories19. Univariable and multivariable Cox regression models were computed to look for variables associated with these three outcomes. The following variables of interest were entered in univariable models: age (continuous, in decade), sex, depth of tumor, tumor size (continuous, in centimeter), margin status, surgery type, CT, RT, year of treatment. Variables with p-value <0.10 in the univariable analysis were entered in the multivariable model. Given the objective of the study, RT and CT were forced into the multivariable models regardless their statistical significance in univariable models. A center effect was sought for as a random effect and tested with a permutation test20; when relevant, mixed effects models were performed. Point estimates of hazard ratios with 95% exact confidence intervals are reported for these models.
RESULTS
Patient and Tumor Characteristics
From 1971 to 2016, 451 adults were diagnosed with ESOS. The majority of patients presented with localized disease (379, 84%) (Figure 1). The remainder of this paper focuses on the 370 patients with localized disease who underwent definitive surgery (Table 1). These patients had a median age of 58 years (range 19 to 88 years) with majority being male (n=217, 59%). Primary tumor sites varied with the thigh being the most common location (n=182, 49%). Most tumors were located deep to the fascia (n=294, 80%). Median tumor size in maximal dimension was 8.5 cm (range 1 to 45 cm). The median follow-up time for the 370 patients in this study was 3 years (range 0 to 39.6 years)
Figure 1.
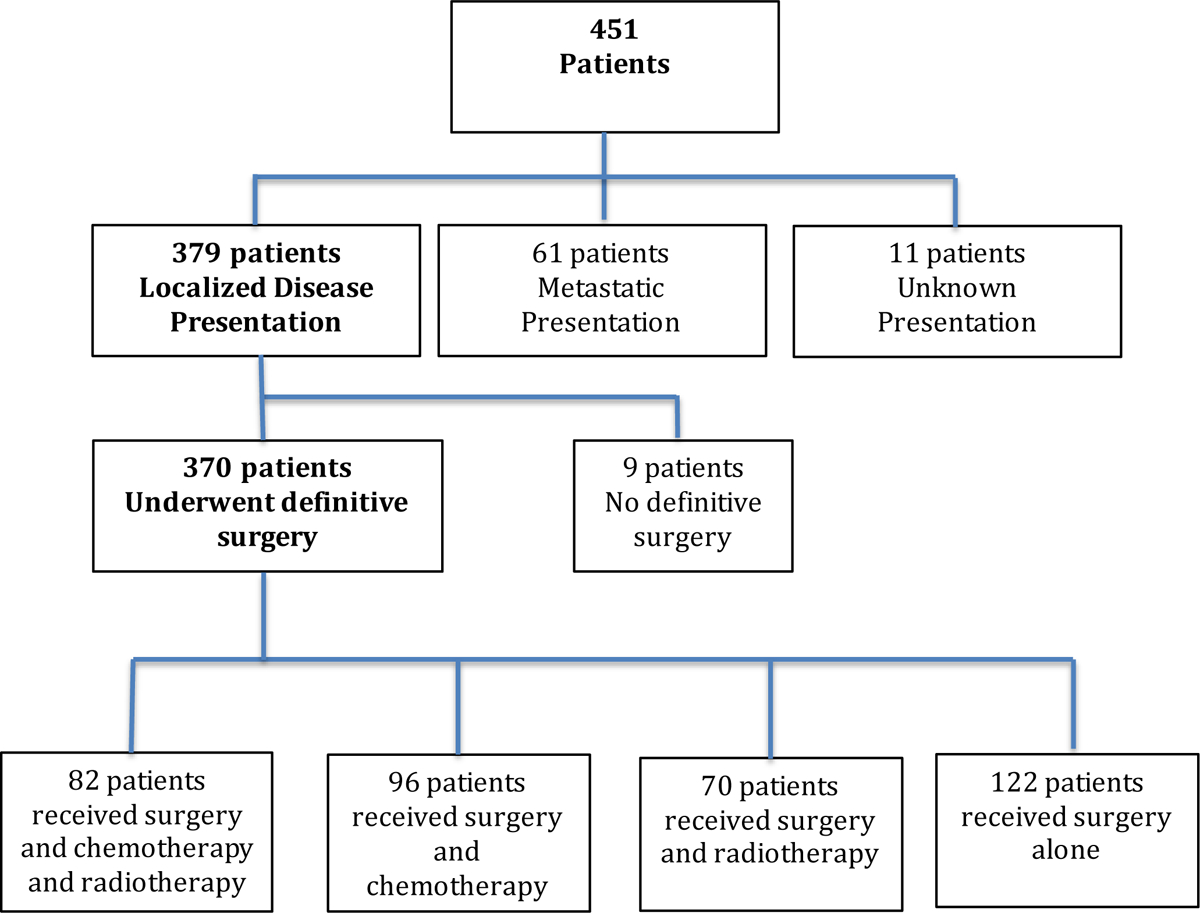
Cohort flow diagram of patients
Table 1.
Demographic, Tumor, and Treatment Characteristics of Patients presenting with localized ESOS who underwent definitive surgery (N=370)
|
Age (yrs) |
Median | 58 |
| Range | 19 – 88 | |
| Age (categorical) | 19 to 40 years | 61 (16.5) |
| 41 to 65 years | 184 (49.7) | |
| 65 years and older | 125 (33.8) | |
| Sex | Male (%) | 217 (58.6) |
| Female (%) | 153 (41.4) | |
| Depth of Tumour | Superficial (%) | 71 (19.2) |
| Deep (%) | 294 (79.5) | |
| Unknown (%) | 5 (1.3) | |
|
Maximal Diameter of Tumour (cm) 19 NAs |
Median | 8.5 |
| Range | 1 – 45 | |
|
Maximal Diameter (categorical) 19 NAs |
<= 5cm | 83 (23.6) |
| 5 to 10cm | 132 (37.6) | |
| > 10cm | 136 (38.7) | |
| Location of Tumour | Thigh | 182 (49.2) |
| Pelvis/Buttocks | 46 (12.4) | |
| Trunk | 42 (11.4) | |
| Shoulder/Arm | 38 (10.3) | |
| Leg | 29 (7.8) | |
| Elbow/Forearm | 14 (3.8) | |
| Knee | 9 (2.4) | |
| Ankle/Foot | 4 (1.1) | |
| Hand | 3 (0.8) | |
| Face | 3 (0.8) | |
| Radiation Therapy | No | 218 (58.9) |
| Yes | 152 (41.1) | |
| Chemotherapy | No | 190 (51.4) |
| Yes | 178 (48.1) | |
| Unknown | 2 (0.5) | |
| Type of Chemotherapy | “osteosarcoma-type”: methotrexate or cisplatin-based | 86 (48.3) |
| “STS-type”: no methotrexate or cisplatin | 58 (32.6) | |
| Other / Unknown | 34 (19.1) | |
| Adjuvant or Neoadjuvant Therapy | Neither Chemo nor Rads | 122 (33.0) |
| Chemo Only | 96 (25.9) | |
| Rads Only | 70 (18.9) | |
| Chemo and Rads | 82 (22.2) | |
| Type of Surgery | Limb Salvage | 345 (93.2) |
| Amputation | 25(6.8) | |
|
Margins 6 NAs |
Negative | 309 (84.9) |
| Micro + | 36 (9.9) | |
| Gross + | 19 (5.2) |
(NA = not available, STS=soft tissue sarcoma)
Treatment
All patients included in the analysis underwent definitive surgical treatment consisting of limb salvage in 345 patients (93%) or amputation in 25 patients (7%). There was a fairly even distribution between patients undergoing surgery alone (122, 33%), or surgery plus CT (96, 26%), surgery plus RT (70, 19%) or surgery plus both (82, 22%) treatments (Table 1).
Chemotherapy
Administration of CT varied according to tumor depth and age at diagnosis (Table 2). A greater proportion of patients treated with chemotherapy had deep tumours (150/178, 87%) compared to those who did not receive chemotherapy (143/190, 75%, p=0.007). 57% of patients aged 19–40 received CT, as did 58% of those 41–65 years, while only 30% of elderly patients greater than 65 years received CT (p<0.001). Different types of CT regimens were administered (Table 1). In addition to doxorubicin, 86/178 (48%) received “osteosarcoma-type” CT that included methotrexate and/or cisplatin, while 58/178 (33%) received CT without methotrexate or cisplatin (“soft tissue sarcoma-type”), and in 34 (19%) patients the CT regimen details were considered as other or unknown. Controlling for age, sex, depth, size, surgery type (limb salvage vs. amputation), margin status, and receipt of RT, there was no significant difference in survival for patients who received either of the CT regimens or no CT (osteosarcoma-type chemo p=0.24, STS-type chemo p=0.34, Other/Unknown chemo p=0.30) (Table 3). Similarly, there was no difference in systemic recurrence in patients who received chemotherapy versus those who did not (p=0.45) (Table 4). Five-year disease-free survival rates were 50.2% (95% confidence interval [CI] 42.9 – 58.8) for patients who received no chemotherapy, 57.4% (95% CI 45.6 – 69.6) for osteosarcoma-type chemotherapy and 43% (95% CI 30.9 – 59.8) for STS-type chemotherapy (p=0.3).
Table 2.
Comparison of demographics for patients with localized ESOS who received CT vs no CT, and patients who received RT vs no RT
| No Chemotherapy N=190 |
Received Chemotherapy N=178 |
p-value |
No Radiotherapy N=218 |
Received Radiotherapy N=152 |
p-value | ||
|---|---|---|---|---|---|---|---|
|
Age (yrs) |
Median | 64 | 55 | <0.001* | 58 | 59 | 0.20 |
| Range | 21 – 88 | 19 – 79 | 19 – 88 | 21 – 88 | |||
| 19–40 years | 26 (42.6) | 35 (57.4) | <0.001* | 46 (75.4) | 15 (24.6) | 0.003* | |
| 41–65 years | 78 (42.4) | 106 (57.6) | 95 (51.6) | 89 (48.4) | |||
| >65 years | 86 (69.9) | 37 (30.1) | 77 (61.6) | 48 (38.4) | |||
| Sex | Male | 108 (56.8) | 108(60.7) | 0.46 | 121 (55.5) | 96 (63.2) | 0.16 |
| Female | 82 (43.2) | 70 (39.3) | 97 (44.5) | 56 (36.8) | |||
| Depth of Tumour | Superficial | 47 (24.7) | 23 (13.3) | 0.007 * | 49 (22.6) | 22 (14.9) | 0.08 |
| Deep | 143 (75.3) | 150 (86.7) | 168 (77.4) | 126 (85.1) | |||
|
Maximal Tumor Diameter (cm) 19 NAs |
Median | 8.1 | 8.8 | 0.32 | 8.4 | 9.1 | 0.40 |
| Range | 1.4 – 45.0 | 1.0 – 42.0 | 1.0 – 45.0 | 1.0 – 42.0 | |||
| Margin Status | Negative (R0) | 156 (83.4) | 151 (86.3) | 0.58 | 187 (87.4) | 122 (81.3) | 0.27 |
| Microscopi c positive (R1) | 19 (10.2) | 17 (9.7) | 17 (7.9) | 19 (12.7) | |||
| Gross positive (R2) | 12 (6.4) | 7 (4.0) | 10 (4.7) | 9 (6.0) |
(NA = not available)
Table 3.
Cox proportional hazards multiple regression analyses for outcomes of cause-specific mortality for patients with localized ESOS at diagnosis based on different chemotherapy regimens
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Specific Chemo Regimen | |||
| No Chemo | Ref | ||
| OSA-type | 0.75 | (0.46 – 1.22) | 0.24 |
| STS-type | 1.28 | (0.78 – 2.10) | 0.34 |
| Other | 0.69 | (0.34 – 1.40) | 0.30 |
OSA=osteosarcoma
STS=soft tissue sarcoma
(†adjusted for age, sex, depth, size, surgery type, margin status, radiation)
Table 4.
Cox proportional hazards multiple regression analyses for outcome of systemic recurrence
| Variable | Univariable regression | Multivariable model | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (per decade increase in age) | 1.13 (1.02 – 1.26) | 0.02 * | 1.09 (0.96 – 1.23) | 0.18 |
| Depth (deep vs superficial) | 3.00 (1.69 – 5.31) | <0.001 * | 2.42 (1.28 – 4.60) | 0.007 * |
| Maximal Diameter (cm) | 1.06 (1.04 – 1.08) | <0.001 * | 1.05 (1.02 – 1.07) | <0.001 * |
| Margin | ||||
| Radiotherapy (yes vs no) | 1.15 (0.82 – 1.61) | 0.41 | 1.05 (0.74 – 1.50) | 0.79 |
| Chemotherapy (yes vs no) | 0.98 (0.70 – 1.37) | 0.90 | 0.86 (0.60 – 1.26) | 0.45 |
| †† Treating Institution | n/a | 0.11 | - | - |
(†Variables in model selected based on a priori determination (CT and RT) and univariable cox analysis with p<0.10)
(†† Controlling for random effects of treating institution)
(n=343, 27 observations deleted due to missingness)
Outcome
Almost half (n=173, 47%) of patients developed recurrent disease, and these were in the form of local recurrence, metastases or both in 9% (35/370), 28% (102/370), and 10% (36/370) of patients, respectively. The 5-year disease-free survival (any recurrence) rate was 50% (95%CI 45% – 56.2%) and overall survival was 56% (95%CI 50.6 – 62.1%). The 2- and 5-year local control rate was 80% (95%CI 75–95%) and 76% (95%CI 71–81%), while the 2- and 5-year distant metastatic-free survival was 64% (95%CI 59–69%) and 58% (95%CI 52–64%). The majority of systemic recurrences were to the lungs. The median time to first recurrence (local or distant) was 7.0 months (range 1 month to 13 years). Median time to systemic recurrence was also 7.0 months (range: less than 1 month to 13 years), while the median time to local recurrence was 9.5 months (range: less than 1 month to 11.6 years). Considering death as a competing event, there was no significant difference in the cumulative incidence of local recurrence or systemic recurrence between patients who received chemotherapy, radiotherapy, both, or neither (local recurrence p=0.50, systemic recurrence p=0.69, Figures 2 and 3). Figure 4 demonstrates that there was not a significant difference in cumulative incidences of local recurrences or systemic recurrences considering competing deaths for RT or CT use, respectively. Administration of RT varied only by age of the patient. A smaller proportion of younger patients aged 19–40 years received RT (25%) compared to patients aged 41–65 years (48%) or older than 65 years (38%, p=0.003; Table 2). In order to account for possible changes in radiotherapy techniques over the study period, year of surgery was tested as a continuous variable and included in the regression model and was not statistically significant, p=0.53 (Table 5).
Figure 2.
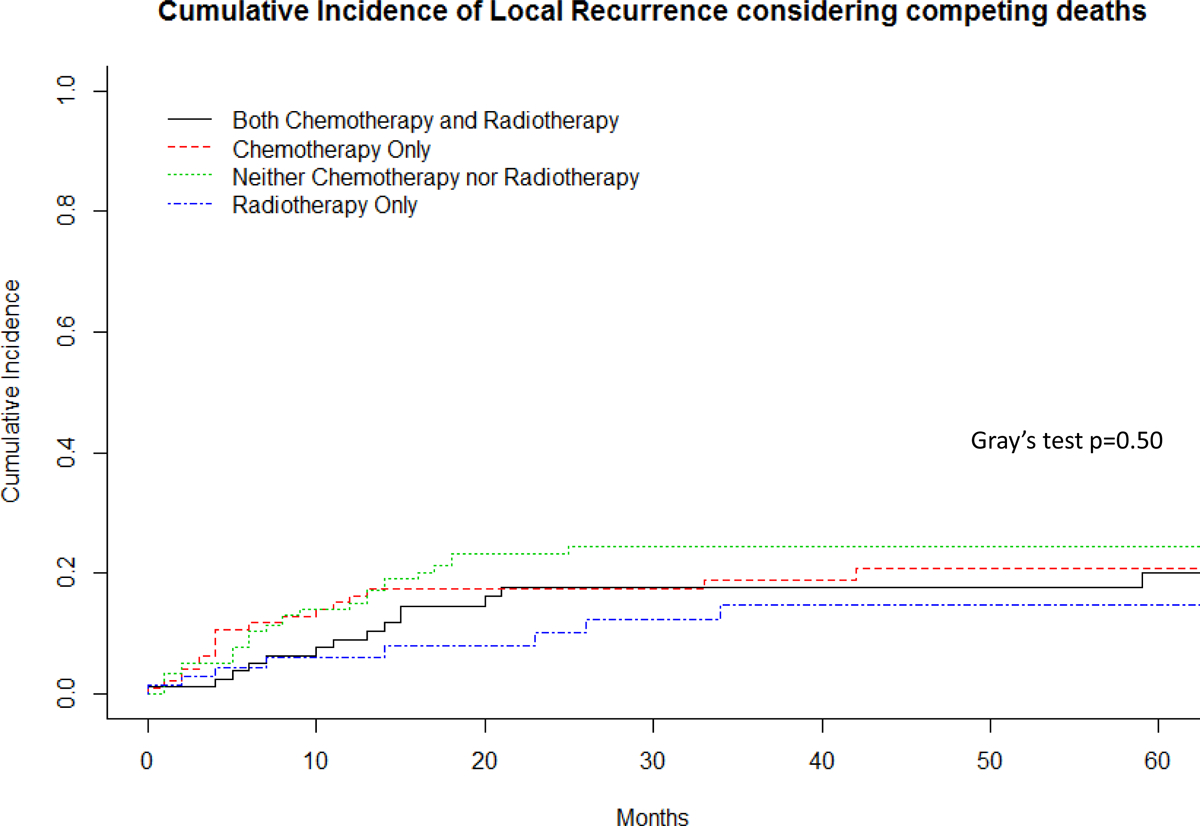
Cumulative incidence of local recurrence considering competing deaths
Figure 3.
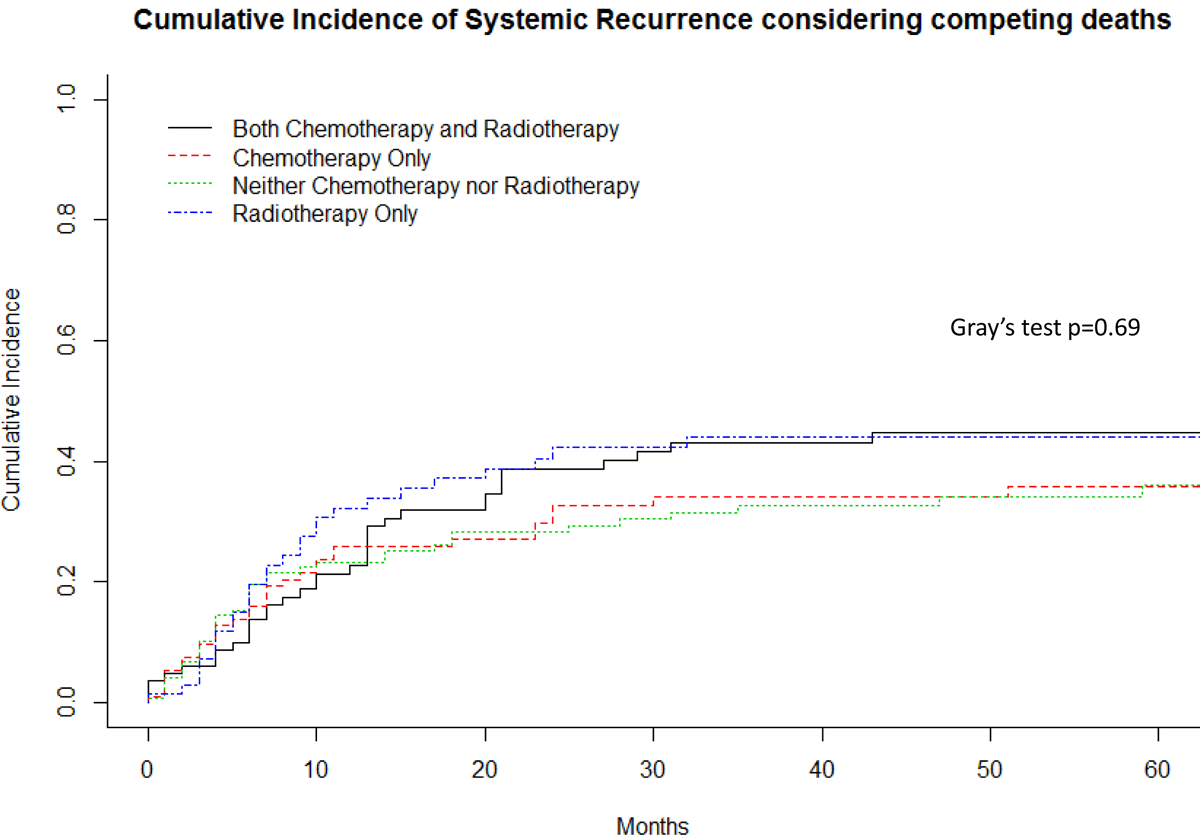
Cumulative incidence systemic recurrence considering competing deaths
Figure 4.
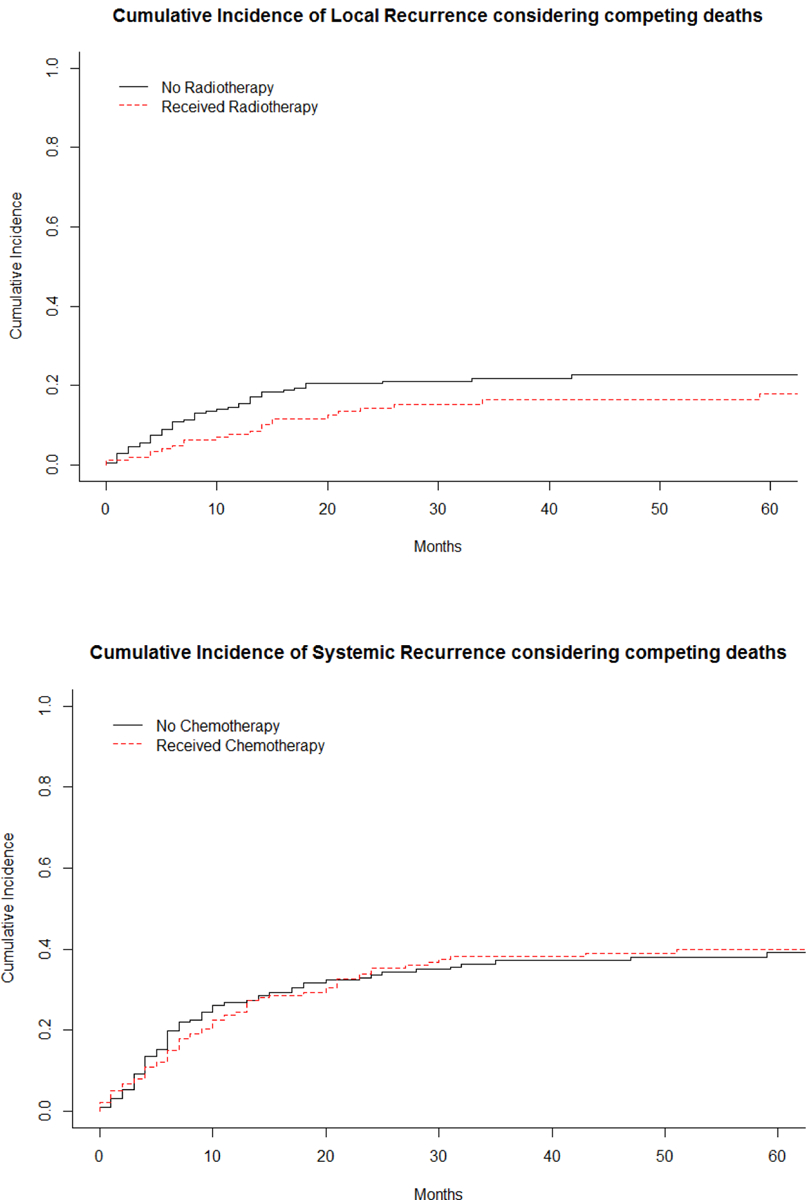
Cumulative incidence curves of local recurrence based on RT and of systemic recurrence based on CT
Table 5.
Cox mixed-effects multiple regression analyses for outcome of local recurrence
| Variable | Univariable regression | Multivariable model | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (per decade increase in age) | 1.05 (0.91 – 1.22) | 0.49 | 1.13 (0.95 – 1.34) | 0.18 |
| Depth (deep vs superficial) | 3.37 (1.46 – 7.80) | 0.004 * | 3.27 (1.20 – 8.21) | 0.02 * |
| Maximal Diameter (cm) | 1.05 (1.02 – 1.08) | 0.002 * | 1.03 (0.99 – 1.07) | 0.15 |
| Margin | ||||
| Radiotherapy (yes vs no) | 0.64 (0.39 – 1.05) | 0.07 | 0.46 (0.26 – 0.80) | 0.01 * |
| Chemotherapy (yes vs no) | 1.00 (0.63 – 1.59) | 0.99 | 1.14 (0.69 – 2.01) | 0.64 |
| Year of Treatment | 1.03 (0.99 – 1.06) | 0.12 | 1.01 | 0.53 |
| ††Treating Institution | n/a | 0.004 | n/a | 1 |
(†Variables in model selected based on a priori determination (CT and RT) and univariable cox analysis with p<0.10), ††Controlling for random effects of treating institution)
(n=343, 27 observations deleted due to missingness)
Two-year overall survival by Kaplan-Meier was 76% (95%CI 72% to 81%). Five-year survival was 56% (95%CI 51% to 62%) (Figure 5). The median time to death for the 159 patients who died throughout the entire study period was 25 months (2.1 years, range 1 month to 27.7 years). Patients who were not known to have died during the study period (n=211) had a median follow-up of 54 months (4.5 years, range 0 to 39.6 years).
Figure 5.
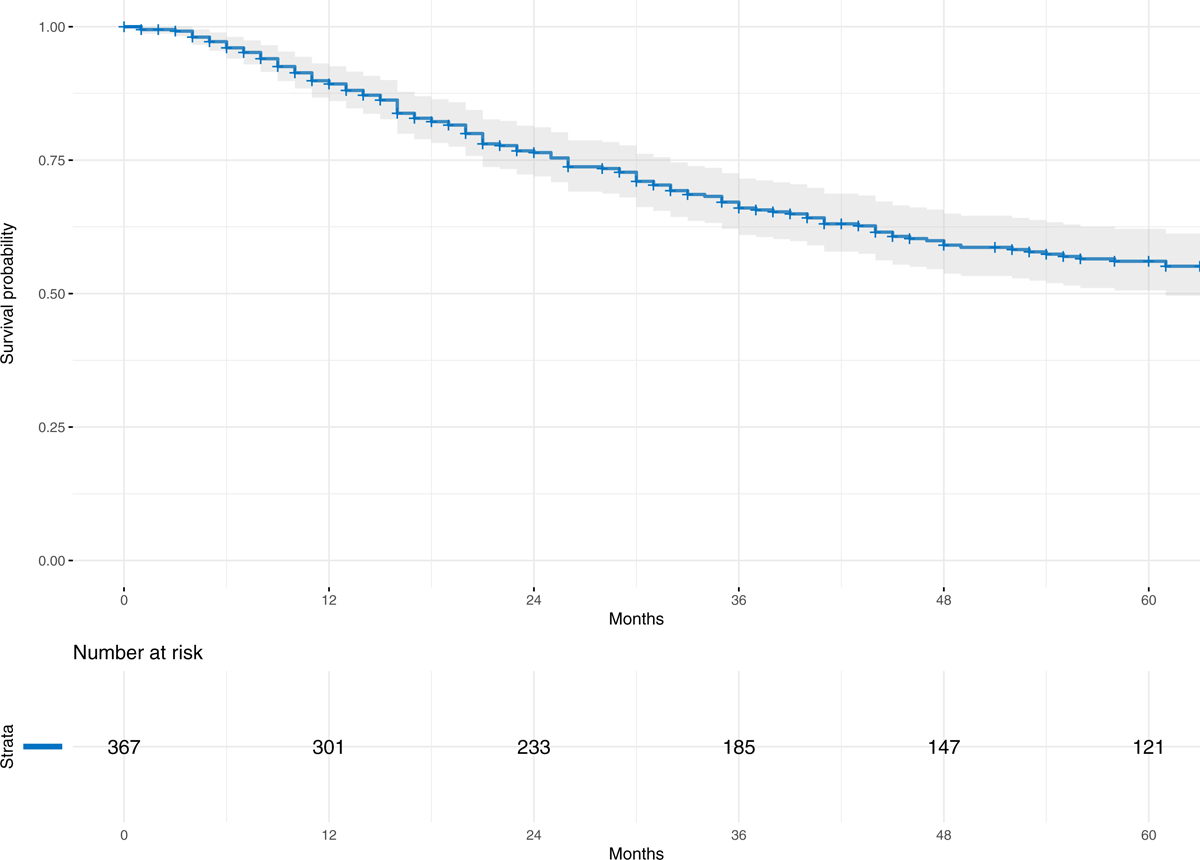
Kaplan-Meier survival curve for the entire cohort of 370 patients with localized ESOS who underwent definitive surgical resection
Factors Predicting Outcome
For the 370 patients who underwent definitive surgical resection, margin status was as follows: negative (R0), 309 (85%); microscopically positive (R1), 36 (10%); and grossly positive (R2), 19 (5%) (Table 1). Six patients had unknown margin status. Local recurrence occurred in 68/370 (18%) patients, which was correlated with margin status (R0: 45/309 (15%) vs R1: 13/36 (36%) vs.R2: 10/19 (53%) , p<0.001).
An effect of the treating institution was found in univariate analysis for local recurrence (p=0.004) and overall survival (p=0.015), but not for systemic recurrence (p=0.11) or disease-free survival (p=0.26), and not by multivariate analysis for any of these outcomes (Tables 4–7). Controlling for age, depth of tumor, size of tumor, margin status, chemotherapy, year of treatment, and treating institution, there was a significant association between RT and local recurrence (HR 0.46 (95%CI 0.26–0.80), p=0.01) with an unadjusted 5-year local control rate of 82% in patients receiving RT compared to 77%). In this model, depth of tumor and margin status were also significant factors associated with risk of local recurrence (Table 5). Use of adjuvant CT was not associated with decreased local recurrence (HR 1.14 (95%CI 0.69 – 2.01 p=0.64).
Table 7.
Kaplan Meier and Cox Proportional Hazards Analysis for Survival
| % Survival (95%CI) | ||
|---|---|---|
|
Overall (3 NAs) |
1-year | 89.3 (86.1 – 92.6) |
| 3-year | 66.0 (61.0 – 71.5) | |
| 5-year | 56.1 (50.6 – 62.1) |
| Variable | Univariable regression | Multivariable model | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (per decade increase in age) | 1.27 (1.14 – 1.41) | <0.001 * | 1.27 (1.13 – 1.44) | <0.001* |
| Depth (deep vs superficial) | 2.79 (1.66 – 4.68) | <0.001 * | 2.58 (1.43 – 4.68) | 0.002 * |
| Maximal Diameter (cm) | 1.05 (1.03 – 1.07) | <0.001 * | 1.04 (1.02 – 1.06) | <0.001 * |
| Margin | ||||
| Micro + vs Negative | 1.81 (1.14 – 2.89) | 0.01 * | 1.95 (1.20 – 3.17) | 0.007 * |
| Gross + vs Negative | 1.97 (1.06 – 3.67) | 0.03 * | 2.01 (1.03 – 3.89) | 0.04 * |
| Radiotherapy (yes vs no) | 1.18 (0.86 – 1.61) | 0.30 | 0.99 (0.71 – 1.39) | 0.96 |
| Chemotherapy (yes vs no) | 0.90 (0.66 – 1.23) | 0.51 | 0.85 (0.60 – 1.21) | 0.38 |
| Treating institution | n/a | 0.015 | n/a | 1 |
(†Variables in model selected based on a priori determination (CT and RT) and univariable cox analysis with p<0.10), ††Controlling for random effects of treating institution)
(n=343, 27 observations deleted due to missingness)
Conversely, after controlling for age, depth of tumor, size of tumor, margin status, and RT, the use of CT did not show a significant association with the risk of systemic recurrence, p=0.45. Only depth of tumor and size of tumor was associated with systemic recurrence (Table 4).
Neither RT nor CT demonstrated a statistically significant association with disease-free or overall survival (Tables 6–7). Tumor size, depth and margin status were associated with both disease-free and overall survival, whereas increasing patient age was only associated with worse survival (Table 7).
Table 6.
Kaplan Meier and Cox Proportional Hazards Analysis for Disease Free Survival (DFS)
| % DFS (95%CI) | ||
|---|---|---|
|
Disease Free Survival (3 NAs) |
1-year | 68.8 (64.1 – 73.9) |
| 3-year | 53.0 (47.8 – 58.7) | |
| 5-year | 50.3 (45.0 – 56.2) |
| Variable | Univariable regression | Multivariable model | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (per decade increase in age) | 1.08 (0.98 – 1.19) | 0.09 | 1.06 (0.95 – 1.18) | 0.29 |
| Depth (deep vs superficial) | 2.55 (1.58 – 4.11) | <0.001 * | 2.15 (1.25 – 3.68) | 0.006 * |
| Maximal Diameter (cm) | 1.06 (1.04 – 1.08) | <0.001 * | 1.04 (1.02 – 1.07) | <0.001 * |
| Margin | ||||
| Micro + vs Negative | 1.68 (1.06 – 2.66) | 0.03 * | 1.63 (1.01 – 2.64) | 0.04 * |
| Gross + vs Negative | 1.81 (0.98 – 3.36) | 0.06 | 1.67 (0.88 – 3.16) | 0.11 |
| Radiotherapy (yes vs no) | 0.99 (0.73 – 1.34) | 0.96 | 0.92 (0.66 – 1.27) | 0.61 |
| Chemotherapy (yes vs no) | 0.96 (0.72 – 1.30) | 0.81 | 0.87 (0.62 – 1.22) | 0.42 |
| Treating institution | n/a | n/a | 0.26 | |
(†Variables in model selected based on a priori determination (CT and RT) and univariable cox analysis with p<0.10), ††Controlling for random effects of treating institution)
(n=343, 27 observations deleted due to missingness)
DISCUSSION
In this largest series to date of patients with localized ESOS, we were unable to demonstrate any positive effect of systemic CT on local or systemic recurrence, or survival. However, the use of RT decreased local recurrences. Considering the well known resistance of conventional osteosarcoma of bone to radiotherapy, our observations lend credence to the ethos that ESOS behaves more similar to STS, rather than OS of bone. ESOS is known to be somewhat different from classical bone OS, and our study suggests that the benefits of CT which are well documented for conventional bone osteosarcoma do not translate to ESOS9. Interestingly, patients with non-osteogenic spindle cell sarcomas of bone (e.g. undifferentiated pleomorphic sarcoma, leiomyosarcoma) have improved survival following treatment with chemotherapy compared to their histologically similar STS counterparts which do not21–23. These examples support the idea that tumor site is a more important determinant of biological behavior than histology alone, and that the same histological type of sarcoma can demonstrate a different biological behavior depending on its site of origin.
In our series of 370 patients with localized ESOS the 5-year survival was 56%, comparable to 51% in a recent study of 211 patients by the European Musculoskeletal Oncology Society (EMSOS)24, and similar to prior smaller reports on the outcome of patients with ESOS3,4,11.
Since there is disparity between the roles of CT and RT in ESOS and OS of bone, understanding the role for CT and/or RT in the treatment of patients with ESOS was central to our study. Our most important result was that treatment with RT did decrease local recurrence of tumor. In the univariate Cox analysis, the hazard ratio for RT showed a large effect size at 0.64; this effect size became greater with multivariable analysis (HR = 0.46) and was statistically significant. This would seem to indicate that RT is given to patients more at risk of having a local recurrence, so when the effect of other covariates is removed, the true effect is the result of our multivariable analysis. Similarly, the likelihood that patients treated with RT had more aggressive tumors explains the finding that the cumulative incidences did not show a significant difference between RT/No RT groups as likely the competition from death is more pronounced for the RT group.
It is important to compare the results of our study with 370 ESOS patients with localized disease to the previously published EMSOS study of 266 patients of which only 211 presented without metastases. Although RT did confer a significant reduction in the risk of local recurrence in our study, we were unable to demonstrate a benefit of CT for either systemic recurrence or survival. While the EMSOS study did not find a reduction in overall local relapse with radiation treatment, there was close statistical significance for RT decreasing local relapse in patients with tumours greater than 5cm +/− R0 margins. RT was used in approximately 40% of patients in both studies. In addition, the EMSOS investigation reported a significant benefit of CT in disease-free and overall survival24. In contrast, the EMSOS investigation included a proportion of pediatric patients younger than in our study - 7.5% of patients in the EMSOS group were under the age of 18 years whereas our cohort included only adults starting at age 19 years. A greater proportion of younger patients in the EMSOS study received CT compared to older patients (87% for those <=18 years and 78 % for ages 19–40 years compared with 69% for those 41–65 years and 20.6% in those >65 years). In our study, the age-related differences in chemotherapy administration were not as striking (19–40 years 57%, 41–65 years 57%, >65 years 29%). Consequently, the EMSOS analysis suggested a survival benefit with CT in contrast to the negative result found in our study.
Three recent single institution studies evaluating the outcomes of patients with ESOS each contributed individual patient-level data to this investigation. Comparing their results demonstrates the limitations of studies with small sample sizes and limited power. Paludo et al., assessed 43 patients with ESOS including 37 with localized disease and found 5-year overall survival of the entire cohort to be 45%14. Chemotherapy was found to significantly improve survival only if it included cisplatin. Although RT did not significantly improve local control, local recurrences occurred less commonly in patients treated with RT (2/14, 14%) compared to those who did not receive RT (3/8, 37%). Choi et al. examined 53 patients with ESOS including 42 with localized disease who had a 3-year disease-specific survival of 62%8. Neither CT nor RT reduced metastases or local recurrences. In 36 patients with localized ESOS, Fan et al. found 5-year disease-specific survival of 53%13. Radiotherapy significantly improved local control. Interestingly, although CT treatment with doxorubicin and ifosfamide also significantly decreased local recurrences for patients with AJCC stage III disease, it did not improve disease-specific survival. The reported survival in these three studies is similar to our results showing 56% at 5 years.
Our series includes patients treated at high volume sarcoma specialty centers which all maintain prospective databases. With an international representation, this study demonstrates a collaboration to determine a collective result that would not have been possible with a single or even a few institutions. Nonetheless, treatment decisions at each center on whether or not to offer CT or RT to individual patients were made based on clinical judgements and/or institutional treatment policies which were not captured in this retrospective review, thereby limiting our interpretations. Our study is limited by its retrospective nature including biases of selection, recall, and outcomes that are inherent to these types of investigations. Diagnosis of ESOS was at the judgment of each individual institution based on histopathological observation of an osteosarcoma located in the soft tissues and not in continuity with any bone. Central pathology review was not performed for this study, and thus is a limitation. However, all institutions in this study are tertiary sarcoma centers where multidisciplinary review of diagnoses is routine. Similarly, evaluating the intensity of treatment for patients who received CT and the response to CT was beyond the scope of this study. However, we did attempt to control for the different CT regimens used in our study by classifying each as either osteosarcoma-type or soft tissue sarcoma-type, in line with the definitions used in the EMSOS study. Given the rarity of ESOS, our study encompasses a time frame over 45 years that may have included treatment variations with the passage of time, especially in relation to the quality of pre-operative imaging, pathology and radiotherapy techniques. A limitation of our study is that we did not have complete information on radiation therapy doses. We examined for the possibility of temporal influences such as the development of newer RT techniques such as intensity-modulated radiation therapy (IMRT); however, analysis including year of treatment revealed that it did not have a significant effect.
Between this study and the recent EMSOS investigation, we have likely gathered the majority of ESOS patients treated in the last 40 years. Encouragingly, this study has demonstrated the feasibility of a multi-institutional collaborative with a commitment for investigating a rare entity in the sarcoma community.
CONCLUSION
This series of 370 patients with localized ESOS who underwent definitive surgical treatment is the largest to date in the literature. Combined modality therapy with surgery and RT resulted in a significantly decreased risk of local recurrence. Furthermore, CT did not decrease the risk of systemic recurrence, and neither chemotherapy, radiation therapy nor both were associated with improved survival in patients with localized ESOS. Thus our results do not support the routine use of CT for patients with ESOS, but rather combined modality local therapy with surgery and RT should be considered for patients with locally resectable disease.
ACKNOWLEDGEMENTS:
No research support applicable.
FUNDING STATEMENT: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Appendix 1. Corporate Authors and Affiliations:
Japanese Musculoskeletal Oncology Group (JMOG):
Osaka National Hospital: Takafumi Ueda MD, Shigeki Kakunaga MD; National Cancer Center Hospital: Akira Kawai MD PhD; Aichi Cancer Center Hospital: Hideshi Sugiura MD; Ehime University: Teruki Kidani MD; Okayama University: Toshiyuki Kunisasa MD, Toshifumi Ozaki MD; Cancer Institute Hospital: Keisuke Ae MD; Gifu University: Akihito Nagano MD, Takatoshi Ohno MD; Kurume University: Koji Hiraoka MD; Kanazawa University Hospital: Norio Yamamoto MD, Hiroyuki Tsuchiya MD; Kyushu University: Yoshihiro Matsumoto MD; Gunma University Hospital: Takashi Yanagawa MD; Keio University: Robart Nakayama MD, Hideo Morioka MD; Hiroshima University: Tadahiko Kubo MD, Shoji Simose MD; Kagawa University: Yoshiki Yamagami MD, Tetsuji Yamamoto MD; Kochi Medical School: Motohiro Kawasaki MD; Saitama International Medical Center: Tomoaki Torigoe MD, Yasuo Yazawa MD; Saitama Medical Center, Jichi Medical University: Toru Akiyama MD; Saitama Cancer Center: Tabu Gokita MD, Jun Manabe MD; Sapporo Medical University: Mitsunori Kaya MD, Makoto Emori MD; Mie University: Tomoki Nakamura MD, Akihiko Matsumine MD; Shikoku Cancer Center: Shinsuke Sugihara MD; Kagoshima University: Masahiro Yokouchi MD, Setsuro Komiya MD; Juntendo University: Yoshiyuki Suehara MD, Tatsuya Takagi MD; Kobe University: Teruya Kawamoto MD; Shizuoka Cancer Center: Junji Wasa MD; Chiba Cancer Center: Tsukasa Yonemoto MD, Takeshi Ishii MD; Osaka Medical College: Ichiro Baba MD; Osaka City University: Manabu Hoshi MD; Osaka University: Kenichiro Hamada MD, Norifumi Naka MD; Osaka Medical Center for Cancer: Tsukasa Sotobori MD, Nobuhito Araki MD; Komagome: Tomotake Okuma MD, Takahiro Goto MD; Tokyo University Hospital: Hiroshi Kobayashi MD, Hirotaka Kawano MD; Tohoku University Hospital: Masami Hosaka MD; Hyogo Medical College: Hiroyuki Futani MD; Hokkaido Cancer Center: Hiroaski Hiraga MD; Nagoya University: Yoshihiro Nishida MD
Soft Tissue Osteosarcoma International Collaborative (STOIC):
Mount Sinai Hospital/University of Toronto: Anthony Griffin MSc; Princess Margaret Cancer Centre/University of Toronto: Albiruni R Abdul Razak MD, David Benjamin Shultz MD PhD, Charles Catton MD FRCPC; Mayo Clinic: Steven Robinson MBBS; MD Anderson Cancer Center: Shreyaskumar R. Patel MD, Valerae O. Lewis MD, B. Ashleigh Guadagnolo MD, MPH; Massachusetts General Hospital/ Harvard Medical School: Thomas DeLaney MD, Haotong Wang MD, Kevin Raskin MD; University of Texas Southwestern Medical Center: Alexandra K. Callan, MD; Medstar Georgetown Orthopaedic Institute: Robert Henshaw MD; Universite de Montreal: Marc Isler MD FRCSC, Sophie Mottard, MD; Taipei Veterans General Hospital: Wei-Ming Chen, MD; University of Tuebingen: Frank Traub MD PhD; National Taiwan University Hospital: Tom Wei-Wu Chen MD; McGill University: Robert E. Turcotte, MD FRCSC; University of Washington: Darin Davidson MD MHSc; Helios-Klinikum Berlin-Buch: Per-Ulf Tunn MD PhD; The Chinese University of Hong Kong: Herbert Loong, MBBS FRCP; McMaster University: Michelle Ghert MD FRCSC; The Ottawa Hospital: Joel Werier MD FRCSC; BC Cancer Agency: Paul Clarkson, MD; Rothman Institute at Jefferson University: John A. Abraham, MD
Footnotes
PRESENTATIONS: This study has been presented as a podium presentation at the Musculoskeletal Tumor Society Annual Meeting in Detroit, Michigan, USA on October 6, 2016 and as a panel discussion at the 21st Annual Meeting of the Connective Tissue Oncology Society (CTOS) in Lisbon, Portugal on November 12, 2016.
CONFLICT OF INTEREST: none declared
REFERENCES
- 1.Wilson H Extraskeletal ossifying tumours. Annals of Surgery. 1941;113:95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76(11):2253–2259. [DOI] [PubMed] [Google Scholar]
- 3.Torigoe T, Yazawa Y, Takagi T, Terakado A, Kurosawa H. Extraskeletal osteosarcoma in Japan: multiinstitutional study of 20 patients from the Japanese Musculoskeletal Oncology Group. J Orthop Sci. 2007;12(5):424–429. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol. 2002;20(2):521–527. [DOI] [PubMed] [Google Scholar]
- 5.Miller BJ. CORR Insights(®): Should High-grade Extraosseous Osteosarcoma Be Treated With Multimodality Therapy Like Other Soft Tissue Sarcomas? Clin Orthop Relat Res. 2015;473(11):3612–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allan C, Soule E. Osteogenic sarcoma of the somatic soft tissue: clinicopathologic study of 26 cases and review of literature. Cancer. 1971;27:1121–1133. [DOI] [PubMed] [Google Scholar]
- 7.Sordillo PP, Hajdu SI, Magill GB, Golbey RB. Extraosseous osteogenic sarcoma. A review of 48 patients. Cancer. 1983;51(4):727–734. [DOI] [PubMed] [Google Scholar]
- 8.Choi LE, Healey JH, Kuk D, Brennan MF. Analysis of outcomes in extraskeletal osteosarcoma: a review of fifty-three cases. J Bone Joint Surg Am. 2014;96(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari S, Bielack SS, Smeland S, et al. EURO-B.O.S.S.: A European study on chemotherapy in bone-sarcoma patients aged over 40: Outcome in primary high-grade osteosarcoma. Tumori. 2017:0. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Lee MR, Lee SJ, et al. Extraosseous osteosarcoma: single institutional experience in Korea. Asia Pac J Clin Oncol. 2010;6(2):126–129. [DOI] [PubMed] [Google Scholar]
- 11.Bishop AJ, Livingston JA, Araujo DM, et al. Extraskeletal Osteosarcomas: A Case Made for Combined Modality Local Therapy With Radiation and Surgery. Am J Clin Oncol. 2019;42(3):238–242. [DOI] [PubMed] [Google Scholar]
- 12.Gundle KR, Kafchinski L, Gupta S, et al. Analysis of Margin Classification Systems for Assessing the Risk of Local Recurrence After Soft Tissue Sarcoma Resection. J Clin Oncol. 2018;36(7):704–709. [DOI] [PubMed] [Google Scholar]
- 13.Fan Z, Patel S, Lewis VO, Guadagnolo BA, Lin PP. Should High-grade Extraosseous Osteosarcoma Be Treated With Multimodality Therapy Like Other Soft Tissue Sarcomas? Clin Orthop Relat Res. 2015;473(11):3604–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paludo J, Fritchie K, Haddox CL, et al. Extraskeletal Osteosarcoma: Outcomes and the Role of Chemotherapy. Am J Clin Oncol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Team RC. R: A language and environment for statistical computing. In. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 16.Prentice RL, Kalbfleisch JD, Peterson AV, Flournoy N, Farewell VT, Breslow NE. The Analysis of Failure Times in the Presence of Competing Risks. Biometrics. 1978;34(4):541–554. [PubMed] [Google Scholar]
- 17.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40(4):381–387. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 19.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16(3):1141–1154. [Google Scholar]
- 20.Biard L, Porcher R, Resche-Rigon M. Permutation tests for centre effect on survival endpoints with application in an acute myeloid leukaemia multicentre study. Statistics in Medicine. 2014;33(17):3047–3057. [DOI] [PubMed] [Google Scholar]
- 21.Waddell AE, Davis AM, Ahn H, Wunder JS, Blackstein ME, Bell RS. Doxorubicin-cisplatin chemotherapy for high-grade nonosteogenic sarcoma of bone. Comparison of treatment and control groups. Can J Surg. 1999;42(3):190–199. [PMC free article] [PubMed] [Google Scholar]
- 22.Bielack SS, Schroeders A, Fuchs N, et al. Malignant fibrous histiocytoma of bone: a retrospective EMSOS study of 125 cases. European Musculo-Skeletal Oncology Society. Acta Orthop Scand. 1999;70(4):353–360. [DOI] [PubMed] [Google Scholar]
- 23.Callegaro D, Miceli R, Bonvalot S, et al. Impact of perioperative chemotherapy and radiotherapy in patients with primary extremity soft tissue sarcoma: retrospective analysis across major histological subtypes and major reference centres. Eur J Cancer. 2018;105:19–27. [DOI] [PubMed] [Google Scholar]
- 24.Longhi A, Bielack SS, Grimer R, et al. Extraskeletal osteosarcoma: A European Musculoskeletal Oncology Society study on 266 patients. Eur J Cancer. 2017;74:9–16. [DOI] [PubMed] [Google Scholar]


