Abstract
Improving organ preservation and extending the preservation time would have game-changing effects on the current practice of organ transplantation. Machine perfusion has emerged as an improved preservation technology to expand the donor pool, assess graft viability and ensure adequate graft function. However, its efficacy in extending the preservation time is limited. Subzero organ preservation does hold the promise to significantly extend the preservation time and recent advances in cryobiology bring it closer to clinical translation. In this review, we aim to broaden the perspective in the field from a focus on these individual technologies to that of a systems engineering. This would enable the creation of a preservation process that integrates the benefits of machine perfusion with those of subzero preservation, with the ultimate goal to provide on demand availability of donor organs through organ banking.
Keywords: Organ preservation, Systems Engineering, Process Engineering, Machine perfusion, Supercooling, Cryopreservation, Transplantation
Introduction: The Need for a Better Organ Preservation Process
End stage organ disease contributes to 730,000 deaths per year in the US1. Although transplantation is the only cure of end stage organ failure, this treatment is limited by a severe global donor organ shortage. Surprisingly, the key reason leading to the limited supply of donor organs is not the number of donations; rather, it is time2,3. The current clinical standard for organ preservation is static cold storage (SCS) in an ice box, which limits storage to few hours for vascular and metabolically active tissues such as the liver and the heart2. This very constrained duration of storage is a critical bottleneck for all regenerative medicine therapies employing cells or tissues in any format, ranging from cell transplantation to tissue engineering to organ transplantation2,4. Notably, it also forms a barrier for utilization of human leukocyte antigen (HLA) matching and immune tolerance induction protocols in clinical trials5, and presents a major obstacle toward on-demand tissue availability and global organ sharing.
Static cold storage (SCS) became the gold standard for organ preservation after the first successful organ (kidney) transplantation in 19546. During SCS the organ is preserved in a special preservation solution. Although SCS has truly been an enabling technology for transplantation, metabolism is not entirely halted during SCS. This causes injury due to adenylate triphosphate (ATP) depletion during ongoing vital cell processes, and buildup of ischemia-reperfusion injury (IRI) inducing metabolites7,8. Therefore, SCS only allows transplantation of the highest-quality organs within a matter of hours after procurement.
Improving organ preservation and extending the ex-vivo life of donor organs from hours to days would have game-changing effects on the current practice transplantation. However, the ultimate goal is to stop time and enable organ banking to have on demand replacements for failing organs2,3. Time has come to think outside the (ice-)box9 and reengineer organ preservation.
Herein we review organ preservation and transplantation as a system of medical/biological processes. Therefore, we start with a process flowchart of the organ transplant process as displayed in Figure 1, followed by a review of individual processes and cutting-edge new technologies that can challenge the current dogma in each. Finally, we will discuss the potential systems integration of these individual technologies in the future of organ preservation.
Figure 1. Schematic overview of the changing organ preservation landscape.
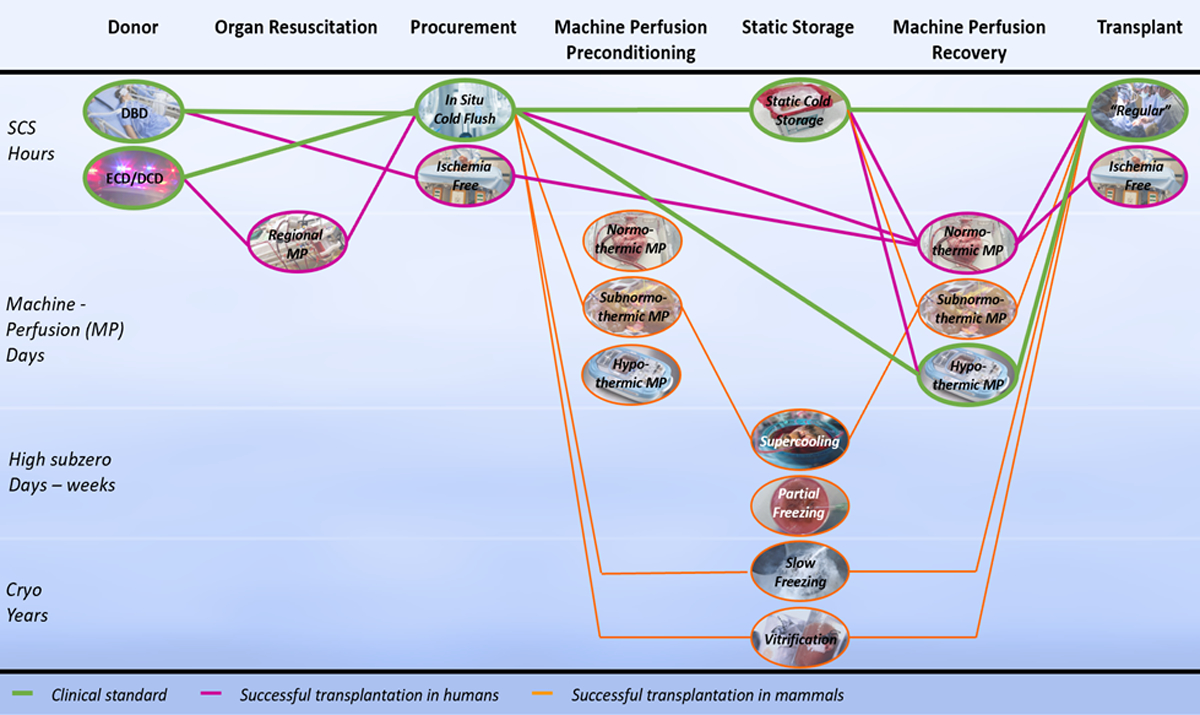
The distinct phases during organ preservation are represented by the columns in chronological order from left to right. The preservation technologies discussed in each of the phases are shown in the ovals of the corresponding column. The diagram should be followed from left to right, using the connecting lines between the preservation technologies of each phase. Different pathways correspond to the combination of technologies as how they are reported in literature. Green paths correspond to the clinical standard, purple paths to strategies that led to successful transplantation in humans, and orange paths to successful transplantation in mammals. Abbreviations: static cold storage (SCS), machine perfusion (MP), cryopreservation (Cryo), donation after brain death (DBD), donation after cardiac death (DCD).
The organ preservation process
Procurement
Organs from brain dead donors (DBD) can be procurement without warm ischemia by initiating in situ cooling of the grafts before cessation of vital functions. Conversely, DCD donation inevitably results in warm ischemia (WI) which significantly injures the grafts and increases IRI during transplantation. In efforts to mitigate the inflicted WI after cardiac death, extracorporeal membrane oxygenation (ECMO) has been used to resuscitate the donor organs in vivo prior to procurement with promising results10–12. Because only the abdominal and thoracic organs are perfused this is called regional perfusion (RP).
Functional Preservation
Contrary to metabolic suppression during SCS, machine perfusion (MP) provides organ support through an extracorporeal artificial circulation. Although first reports of MP date back to 190313, it was outmatched by the simplicity and (cost-)effectiveness of SCS. Over the past decade the donor organ shortage has reinvigorated MP in efforts to render sub optimal donor organs available for transplantation.
Different MP modalities have emerged, mainly differing in perfusion temperature and thus the metabolic rate; normothermic (NMP), sub-normothermic (SNMP) and hypothermic (HMP)14,15.
Although it becomes more complex to meet the organ’s metabolic demands at higher perfusion temperatures, higher metabolic rates enable detailed ex vivo viability assessment and therapeutic intervention. Additional to this tradeoff it is hypothesized that different organs may benefit differently from specific perfusion temperatures. Most often the perfusate is oxygenated, however this can be omitted due to the low metabolic rate during HMP16–20. Perfusate composition is dependent on perfusion temperature and arguably the most complex component of MP. Many different base solutions, oxygen carriers and numerous more additives have been reported, ranging from a close resemblance of blood to acellular serum free solutions21,22.
Another important distinction in machine perfusion modalities can be made with respect to the timing of application during the preservation process. MP can be employed before or after a significant period of SCS (pre-SCS MP and post-SCS MP respectively), but it can also be continuous from procurement until transplantation (preservation MP)15. In a recent report the boundaries were pushed even further: MP was initiated during procurement in DBD donors and continued during transplantation, resulting in complete ischemia free transplantation23.
Trends in application of MP are correlated to the metabolic demands of the organ. Non-oxygenated preservation HMP without oxygenation has become the clinical standard for extended criteria kidneys after randomized controlled trials (RCTs) have shown superior graft survival compared to SCS16–19, but also high-quality kidneys may benefit from HMP24. HMP of livers after SCS has also been proven safe without oxygenation in humans20. However, oxygenation seems to be beneficial for DCD livers25–27. SNMP has been studied on human livers and although promising results were reported it remains in pre-clinical phase28–30. NMP has clinically been applied to harts, lungs and livers. NMP after a period of SCS allows safe transplantation of high risk donor lungs31 and is promising to render high risk livers available for transplantation32. Recent RCTs compared preservation NMP to SCS and showed non-inferior transplant outcome despite significant increase in preservation time for hearts33 and lungs34. In livers, the first RCT demonstrated superiority of preservation NMP over SCS by reduced graft injury and organ discard, even with significantly longer preservation times up to 24 hours14.
Leveraging mathematical modeling and metabolic engineering to improve functional preservation
A very interesting aspect of functional preservation is that it enables the modulation of the organ prior to transplantation in the recipient. A heart that is being machine perfused normothermically is fully functional, beating, pumping perfusate, and performing all the normal tasks it does in the human body. This allows not only the assessment of graft quality (as we’ll review later in the text), but also creates opportunities for viability improvement through organ repair and regeneration. Broadly speaking, such repair can be surgical, genetic35 (such as gene editing), or metabolic.
Of these, metabolic engineering of vascular organs during perfusion is of particular interest to the systems engineering community, in our opinion. For instance, recent literature indicates the short-term viability of clinically transplanted livers is highly correlated to the state of central energy metabolism36 and the same is likely true for other vascular and highly metabolic organs. Optimizing the central energy metabolism fluxes to maximize graft recovery from the ischemic period, replenish ATP stores and therefore allow nominal function of all dependent cellular processes, requires navigating the complex metabolic networks. It is easy to envision that such a process would require carefully tuned perfusates that feed substrates to certain process (such as ketone bodies and fatty acids as a pool for energy substrate). This may also require dynamic protocols where regeneration processes are arrested until the energy stores are optimally recovered for these energy intensive tasks. This is squarely in the interest of systems biology, and new tools such as mapping of metabolic network models in human organs, coupled with models incorporating regulatory networks, can guide such optimization efforts37. Techniques such as Metabolic Control Analysis38, or newer graph-theoretical approaches to perform pathway enrichment analysis37 can be used to identify key points of intervention to achieve the desired effects. This grand challenge also is an ideal experimental model to study each organ in isolation, and elucidate the complex feedback mechanisms which regulate organ metabolism and provide biophysical stimulation at multiple levels.
A related problem of interest for systems biology is creating automated feedback control systems to enable true homeostasis during perfusion and optimize the viability of organs for transplantation. Although clinical studies have shown that NMP can extend the preservation time with valuable hours14,33,34, current perfusion systems lack technology to maintain homeostasis during extended preservation. To date, perfusion devises are limited to simple feedback loops (e.g. for pressure, pH and oxygen levels), often in single input single output configurations, that cannot account for the many interactions between different parameters during perfusion. Use of model predictive control (MPC) algorithms would be crucial for achieving real time optimization of a more advanced multi-input multi-output system39. We previously discussed such automated organ culture systems elsewhere40.
Static Storage
Since sustaining physiological organ function ex-vivo becomes vastly more complex when the preservation duration increases, a more efficient option may be to suppress metabolism and store donor organs in a state of suspended animation at subzero temperatures.
However, ice formation can be severely injurious41–44, in particular below −20°C45,46. Strategies to overcome this issue bifurcate by either controlling, or avoiding ice formation. The field has seen several breakthroughs in recent years in this avenue, which creates new technological possibilities and open this component of preservation to disruptive clinical innovations. Because methods for both strategies are fundamentally depended on the preservation temperature, we present this section divided as high subzero preservation at temperatures above −20°C and cryopreservation at temperatures below −80°C.
High subzero preservation
Since the metabolic rate exponentially decreases with lowering temperature, a reduction of several degrees in storage temperature already can have a significant impact on ATP depletion and buildup of IRI metabolites47. High subzero storage can extend the preservation time to up to weeks and has therefore the potential to be an important enabling technology for transplantation.
High subzero freezing
Probably the most successful reports for high subzero freezing date back to the 1970s: Canine kidneys were frozen to −22°C for 15 minutes and half of them was successfully transplanted48. However, despite several other attempts in kidneys, hearts and livers, freezing injury remains a critical obstacle preventing reproducible long-term transplant survival49. Inspired by the wood frog (Rana sylvatica) that can survive in a frozen state for months by confining ice to the extracellular space, our group has recently introduced a new method for high subzero freezing, coined partial freezing50. We leveraged novel insights in cryoprotective agents (CPAs), ice nucleating strategies and advances in machine perfusion with the goal of confining ice formation to the extracellular space and reduce freezing injury, yielding promising results during ex vivo reperfusion51. (Fig. 3)
Figure 3. Partial freezing.

Frozen Rana sylvatica (Photo by J.M. Storey, Carleton University) and the translation of its ice controlling strategies to rat livers (Photo by C. Pendexter and S.N Tessier, Massachusetts General Hospital and Harvard Medical School).
Supercooling
Ice can be completely avoided at high subzero temperatures by using supercooling to sustain the liquid phase of water below the freezing point. Supercooling resulted in successful preservation of rat livers for up to 4 days, validated by long term survival after orthotopic transplantation52,53. However, the supercooled state becomes more unstable at larger volumes which was thought to limit scalability towards human organs. Nevertheless, we recently overcame these challenges and successfully scaled supercooling preservation to human livers (Fig. 4), and demonstrated no reduction of ex vivo viability after significant durations of supercooling54.
Figure 4. Human liver supercooling.
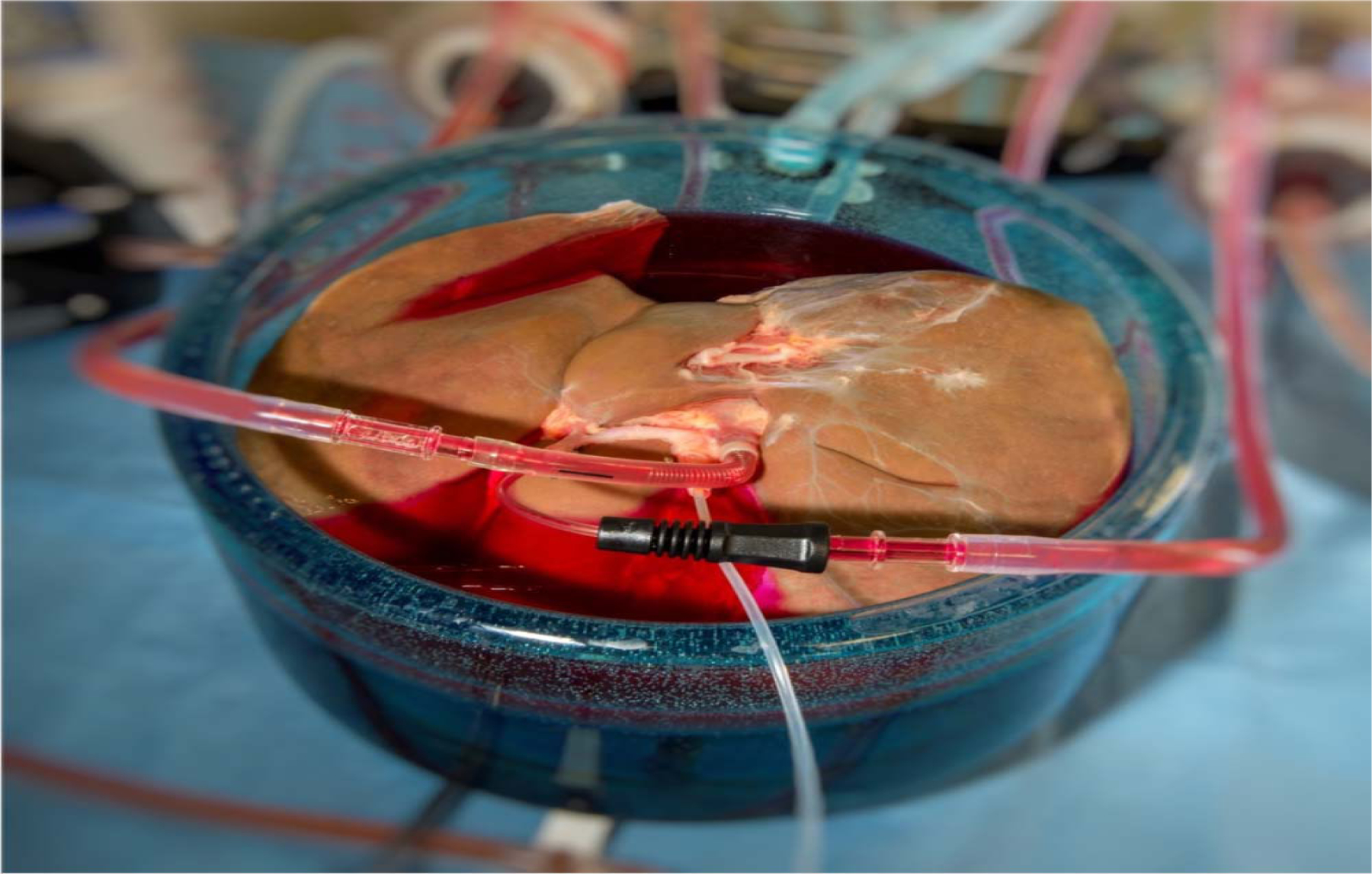
A human liver during machine perfusion to precondition the liver for supercooling.
Cryopreservation
Cryopreservation is considered the holy grail of organ preservation because chemical and biochemical processes practically halt at the temperature of liquid nitrogen (−196°C), allowing (theoretically) indefinite organ banking42. Classic cryopreservation aims to control ice formation whereas ice is completely avoided during vitrification. Both strategies have been highly successful in preservation of single cell suspensions with important clinical applications such as transfusion of blood components, bone marrow transplantation and reproductive technologies55. However, cryopreservation of whole organs is vastly more difficult due to the increased volumes and added complexity of different cell types and tissue architecture42,49,56. Nonetheless, important success in animal studies rise expectations for the coming years.
Classic cryopreservation
Multiple techniques have been tried to control ice formation and reduce freezing injury during cryopreservation. All these methods use CPAs to change the systems freezing properties. Different cooling techniques to control ice formation have also been considered. For instance, ice lattice growth direction can be controlled by changing the direction of the thermal gradient57. Another method to control ice formation is manual ice nucleation which has been used for successful cryogenic freezing of rat ovaries resulting in fertility after transplantation58.
Controlling ice formation is significantly harder for larger volumes, making scale-up of rodent models challenging. Especially the endothelial lining is vulnerable to ice formation and has been noted as the point of failure in many attempts. However, promising techniques have been recently reported to counter this problem. Infusion of ice nucleating agents in the vasculature to control ice nucleation has shown to alleviate endothelial injury50. Also, anticoagulant therapy showed to overcome endothelial injury during transplantation of cryopreserved grafts59. This anticoagulant therapy together with manual ice nucleation and controlled rate cooling enabled successful cryopreservation of whole sheep ovaries; resulting in restored ovarian function and birth of healthy lambs59. Arguably this is the most successful report of solid organ cryopreservation to date.
Vitrification
In contrast to classic cryopreservation, ice formation is completely avoided during vitrification by a direct transition from the liquid to the glass phase. However, extreme fast cooling rates are required to reach the glass transition temperature of water at −137°C while avoiding ice nucleation at higher temperatures56. CPAs can be used to lower the critical cooling rate and increase the glass translon temperature. For whole organs however, very high CPA concentrations are required which can cause significant injury due to toxic and osmotic effects60. Especially rewarming is troublesome as critical warming rates are a magnitude larger and thermomechanical stress causes cracking of the tissue during inhomogeneous outside-in rewarming56,61. To date, the only report of successful organ vitrification is that of a single rabbit kidney62.
Recent progress may overcome the obstacles of vitrification. Isochoric (constant volume) conditions might reduce the required CPA concentrations63 and liquidous tracking could minimize toxicity by incremental addition of CPAs at decreasing organ temperatures64. Even more exciting are magnetic nanoparticles which can be used to very rapidly and homogeneously rewarm vitrified tissues in an alternating magnetic field56,61. These nanoparticles could be perfused into the organ prior to vitrification and prevent ice formation and cracking of the organ during rewarming.
Integration of Preservation - Process Design and Control
Systems engineering in organ preservation
Approaching organ preservation from a systems engineering perspective has the advantage of synergizing the distinct preservation technologies into an enhanced preservation process. These technologies can be divided in sub systems that can form the ‘building blocks’ for the preservation process. In this way, future advances in distinct preservation techniques can be combined and leveraged to improve the overall organ preservation process as seemingly different preservation methods face many of the same difficulties, such as control over ice nucleation, cryoprotectant toxicity, uniform cooling/rewarding and IRI. For example, future freeze avoidance strategies that are developed in supercooling could be leveraged for vitrification, or rewarming strategies to reduce IRI after subzero preservation could be adapted in to improve MP.
MP and subzero preservation can be integrated in many different configurations, as indicated in Figure 1. Given the multiplicity of use cases, MP can be considered a platform technology, being leveraged to precondition the grafts prior to, and recover organs after subzero storage, while simultaneously allowing ex vivo viability assessment. All three steps are likely to be key in extended organ preservation, as they were observed to be in extending liver storage with supercooling52. Using MP to precondition grafts for subzero preservation might be especially beneficial when scaling up to human organs, as we experienced during scale up of supercooling to human livers (Fig. 5). MP can be used to dynamically increase CPA tissue concentrations and because the organ is metabolically active during perfusion, it allows active intracellular uptake of CPAs52. Additionally, ex vivo isolated perfusion of organs allows approaches such as gene-engineering, to pre-condition the grafts. Preclinical studies show promising results for a number of protective molecules reducing IRI, including gene transfer of cryoprotective heat shock proteins65.
A key system component that needs to be integrated in the preservation process will be continuous assessment of organ viability; i.e. integrated quality assurance. Numerous mechanical, physical and biological parameters have been reported to correlate to organ viability; a comprehensive review is provided elsewhere66. Of these parameters, the adenylate energy charge (a relative measure of ATP) seems to be a highly predictive biomarker that can be used without MP36. Clinically however, viability assessment during MP is currently still subjective since definite criteria remain to be established31,33,67. Multiple measurements likely will need to be included to ensure near-perfect accuracy. The sensors necessary could be placed in the in- and outflow, as well as located inside the organ, such as micro- and biological sensors. Our group has demonstrated success in using statistical process control approaches to establish a viability index that is a composite score of different biomarkers as an early proof of concept68. A worthwhile endeavor would be to standardize the different viability metrics to ease such efforts. With more clinical data being generated every day in the ongoing perfusion trials, the prospect is this would allow to develop such viability indices in near future.
It is worth noting that the assessment of viability dovetails strongly with the need for systems biology approaches necessary to understand the preserved organs, as discussed earlier. A key concern here is the need for on-line, if not real-time data acquisition at a cellular level to elucidate the organ function and viability. While sensors can be envisioned at different layers of omics40, many of the omics analysis takes hours or days to produce data, and destructive assays that require biopsies cannot be performed too many times during preservation. Use of mathematical models to interpolate between measurements, i.e. create “soft sensors” that can track organ function real time to allow for organ assessment, and even optimization as aforementioned, would be a key added-value that would be crucial to create vertical advancements in organ preservation. Metabolic flux analysis (MFA) has been created to serve exactly this function; i.e. enable evaluation of fluxes that are difficult to obtain analytically. Dynamic MFA has been used to simulate the evolution of pathology in disease69,70, which would be an ideal starting point to create such “soft sensors”. These could then open the door for multi-omics implementations71, and extend towards quantitative systems pharmacology which would allow modeling the use of pharmacological interventions during preservation72.
Systems integration of organ preservation technologies
Although systems integration has not been a common consideration in the organ transplant chain historically, clearly the process offers opportunities. The current organ preservation process as summarized in Figure 1 is extremely linear. From a systems integration perspective this could be described as “vertical” integration of the different preservation technologies whereby each step in the transplantation chain is consecutively performed. Although this typically is a simple and effective method of integration, this approach might not be suitable for the complexity of organ preservation.
Considering the commonalities in the preservation approaches reviewed above, an alternative and potentially more efficient method to integrate the different preservation technologies is a set of modular devices which specialize in specific “unit operations” that interact with a common device that provides the interface with the organ. This “horizontal” systems integration has the advantage that the preservation process is more flexible and unit processes can be performed simultaneously. For instance, a cassette that is easily exchanged between process steps and equipped with standardized connections may be used to hold the organ. The necessary sensors for viability assessment could be integrated in this cassette while another specialized subsystem interacts with this cassette and continuously computes the organ viability based on the sensors output and dynamic algorithms. Other devices that interact with the cassette could then also be specialized in one task, possibly for multiple types of organs. The cassette storing the organ could be transported over the world while constantly tracking the organs status, whereas the highly specialized equipment and personnel can be concentrated into centralized organ banking facilities for storage and reconditioning units that are adjacent to transplant centers.
As each human donor is unique, each organ may benefit from different preservation methods. For example, it might be favorable to recondition and treat marginal quality grafts during a relatively short preservation period using machine perfusion. Organs with metabolic conditions such as liver steatosis have already been shown to be treatable during perfusion73. On the other hand, high quality organs could be banked to wait for the perfect HLA match and induce immune tolerance, mitigating rejection and the need for immunosuppressants. While such organ-specific perspectives remain in future, these “personalized medicine for the graft” approaches that tailor the preservation protocol for the idiosyncrasies of the donor organ seem a rational convergence point for the future of organ preservation. We believe this dynamic preservation approach would benefit from the flexibility of horizontal integration of the preservation process as opposite to the sequential “vertical” integration of the different preservation technologies. As such, the common device that holds the organ could be easily recombined with multiple specific modular technologies to provide an organ specific preservation method.
Interestingly, this presents an opportunity to go back and recover knowledge from chemical process systems engineering where the design of the system needs to consider the variability in the process; in this case being the specific pathologies present in a donor organ which is rarely perfect. In the ideal case, the organ preservation process would be designed with consideration of complex feedback control systems to accommodate the metabolic needs of the specific organ being preserved based on the sensor readings, and further deliver optimized treatment that is tailored to its specific pathologies. Such an approach would require mathematical models of organ function, prediction of graft viability and transplant outcomes, and sophisticated model-predictive control approaches that can dynamically optimize the organ to maximize predicted clinical outcomes, many of which remain missing. Although the concept of systems engineering in defining health and disease is not new74, its application to organ transplantation certainly is novel. The complexity of the transplant process chain makes it an ideal application and the expected clinical improvements a worthwhile endeavor.
Conclusions
Machine perfusion has advanced to expand the donor pool and ensure adequate graft function. It has been successful for most abdominal and thoracic organs and proved to safely extend the preservation time up to a day in clinical studies. However, progress towards effectively banking organs requires a much more sophisticated approach. Recent advances in cryobiology bring subzero organ preservation closer to clinical application, which perfectly dovetails with machine perfusion in order to develop an integrated solution for long-term organ banking. Still, this is a complex process with issues ranging from the interplay of the biology of mixed-chimerism tolerance protocols to cryobiology and engineering of organ preservation systems. Remarkable progress has been made in terms of individual technologies in each of the processes in organ preservation. Broadening the perspective in the field, from a focus on these individual steps to that of a systems engineering, would enable creating a process that is optimal for clinical outcomes. Moreover, if done correctly, it would lay the foundation for future development of new approaches that could use perfusion and subzero storage as a platform to build on, and enable banking of organs for transplantation.
Figure 2. Commercially available machine perfusion systems that are used in recent clinical trials.
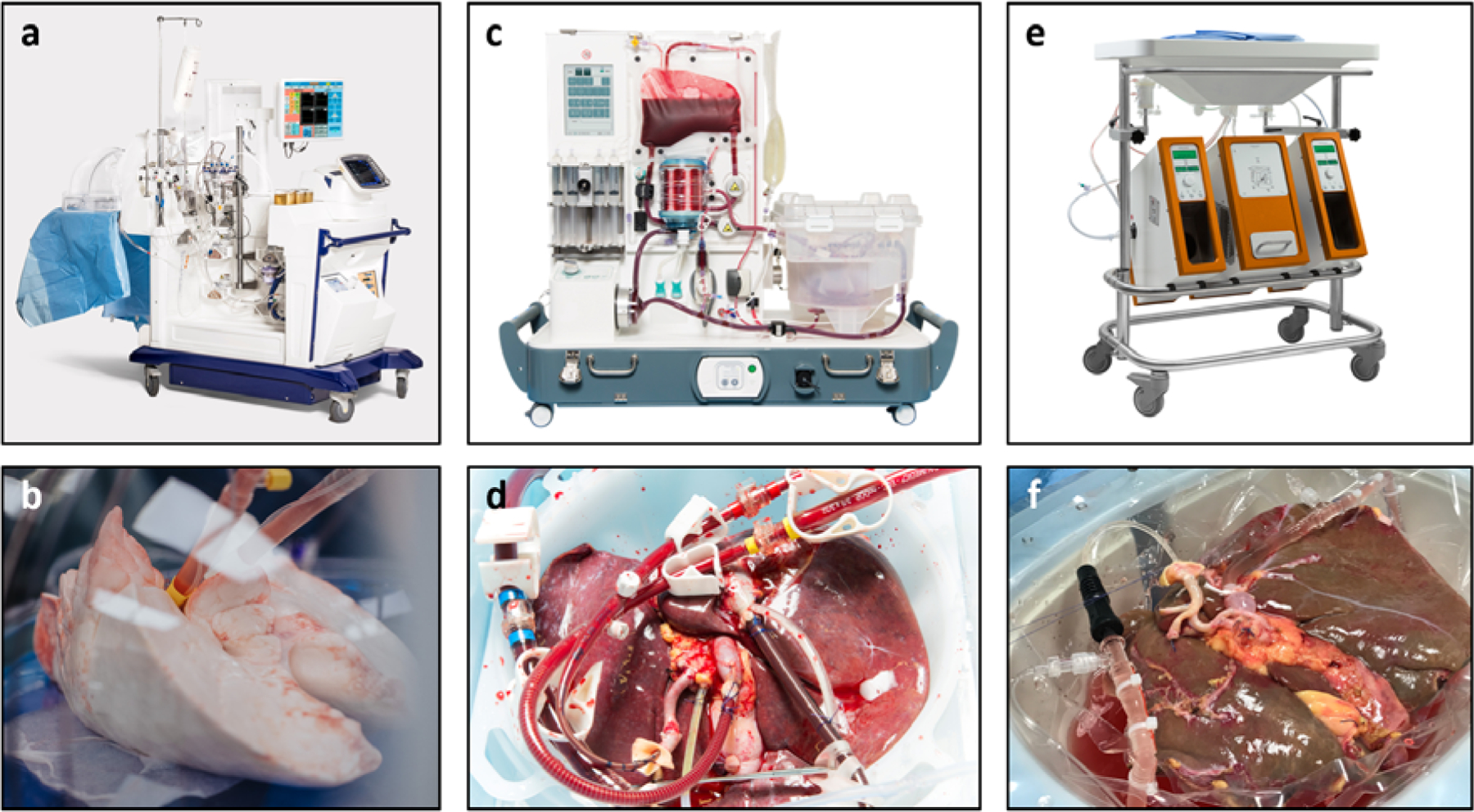
a. The lung perfusion devise of XVIVO Perfusion Systems. b. Lungs during normothermic machine perfusion (NMP) c. The portable perfusion devise of OrganOx for normothermic liver perfusion. d. Liver during NMP. d. The liver perfusion system of Organ Assist c. Liver during oxygenated hypothermic machine perfusion (HMP). Note the clear perfusate color difference as compared to the NMP perfusate that contains red blood cells as oxygen carrier. Photos were provided by the corresponding companies.
HIGHLIGHTS:
Static cold storage is a key bottleneck for all regenerative medicine therapies
Machine perfusion is an important emerging organ preservation technology
Subzero preservation holds the promise to enable organ banking
Machine perfusion and subzero preservation can be used in synergy
Acknowledgements
Funding from the US National Institutes of Health (R01DK096075, R01DK107875, and R01DK114506), the Department of Defense Health Program RTRP W81XWH-17-1-0680, the Shriners Hospitals for Children are gratefully acknowledged. The authors thankfully appreciate the graphical content provided by Janet M. Storey, Casie Pendexter, Shannon. N. Tessier, Organ Assist, OrganOx, and XVIVO.
Footnotes
Conflict of interest statement
The authors declare competing financial interests. Drs. de Vries, Yarmush and Uygun have provisional patent applications relevant to this manuscript. Dr. Uygun has a financial interest in Organ Solutions, a company focused on developing organ preservation technology. The authors interests are managed by the MGH and Partners HealthCare in accordance with their conflict of interest policies.
References
- 1.Hoyert DL & Xu J Deaths: preliminary data for 2011. Natl. Vital Stat. Rep. Cent. Dis. Control Prev. Natl. Cent. Health Stat. Natl. Vital Stat. Syst 61, 1–51 (2012). [PubMed] [Google Scholar]
- **2.Giwa S et al. The promise of organ and tissue preservation to transform medicine. Nat. Biotechnol 35, 530–542 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; A most excellent and comprehensive review about the recent advances in tissue and organ privation.
- 3.Buying time for transplants. Nat. Biotechnol 35, 801 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Fahy GM, Wowk B & Wu J Cryopreservation of complex systems: the missing link in the regenerative medicine supply chain. Rejuvenation Res. 9, 279–291 (2006). [DOI] [PubMed] [Google Scholar]
- *5.Scandling JD, Busque S, Shizuru JA, Engleman EG & Strober S Induced Immune Tolerance for Kidney Transplantation. N. Engl. J. Med 365, 1359–1360 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; A study that demonstrated the feasibility of immune tolerance induction to eliminate the need of antirejection drugs HLA-mismatched kidney transplantation in humans.
- 6.Hatzinger M, Stastny M, Grützmacher P & Sohn M [The history of kidney transplantation]. Urol. Ausg A 55, 1353–1359 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Bruinsma BG, Berendsen TA, Izamis M-L, Yarmush ML & Uygun K Determination and extension of the limits to static cold storage using subnormothermic machine perfusion. Int. J. Artif. Organs 36, 775–780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eltzschig HK & Eckle T Ischemia and reperfusion—from mechanism to translation. Nat. Med 17, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirenne J Time to think out of the (ice) box: Curr. Opin. Organ Transplant 15, 147–149 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Hessheimer AJ, Billault C, Barrou B & Fondevila C Hypothermic or normothermic abdominal regional perfusion in high-risk donors with extended warm ischemia times: impact on outcomes? Transpl. Int. Off. J. Eur. Soc. Organ Transplant 28, 700–707 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Page A, Messer S & Large SR Heart transplantation from donation after circulatory determined death. Ann. Cardiothorac. Surg 7, 75–81 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhital KK, Chew HC & Macdonald PS Donation after circulatory death heart transplantation. Curr. Opin. Organ Transplant 22, 189–197 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Brodie TG The perfusion of surviving organs. J. Physiol 29, 266–275 (1903). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *14.Nasralla D et al. A randomized trial of normothermic preservation in liver transplantation. Nature 557, 50–56 (2018). [DOI] [PubMed] [Google Scholar]; The first randomized controlled trial of normothermic machine perfused human donor livers.
- 15.Karangwa SA et al. Machine Perfusion of Donor Livers for Transplantation: A Proposal for Standardized Nomenclature and Reporting Guidelines. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg 16, 2932–2942 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.Moers C et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med 360, 7–19 (2009). [DOI] [PubMed] [Google Scholar]; The first randomized controlled trial of hypothermic machine perfused human donor kidneys.
- 17.Treckmann J et al. Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transpl. Int. Off. J. Eur. Soc. Organ Transplant 24, 548–554 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Moers C, Pirenne J, Paul A, Ploeg RJ & Machine Preservation Trial Study Group. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med 366, 770–771 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Jochmans I et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann. Surg 252, 756–764 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Guarrera JV et al. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg 10, 372–381 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Matton APM et al. Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc 24, 528–538 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker S et al. Evaluating acellular versus cellular perfusate composition during prolonged ex vivo lung perfusion after initial cold ischaemia for 24 hours. Transpl. Int 29, 88–97 (2016). [DOI] [PubMed] [Google Scholar]
- 23.He X et al. The first case of ischemia-free organ transplantation in humans: A proof of concept. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg 18, 737–744 (2018). [DOI] [PubMed] [Google Scholar]
- 24.De Deken J, Kocabayoglu P & Moers C Hypothermic machine perfusion in kidney transplantation. Curr. Opin. Organ Transplant 21, 294–300 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Dutkowski P et al. HOPE for human liver grafts obtained from donors after cardiac death. J. Hepatol 60, 765–772 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Burlage LC et al. Oxygenated hypothermic machine perfusion after static cold storage improves endothelial function of extended criteria donor livers. HPB 19, 538–546 (2017). [DOI] [PubMed] [Google Scholar]
- 27.van Rijn R et al. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br. J. Surg 104, 907–917 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruinsma BG et al. Subnormothermic Machine Perfusion for Ex Vivo Preservation and Recovery of the Human Liver for Transplantation: Subnormothermic Machine Perfusion of Human Livers. Am. J. Transplant 14, 1400–1409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruinsma BG et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg 14, 1400–1409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruinsma BG et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci. Rep 6, 22415 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Cypel M et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N. Engl. J. Med 364, 1431–1440 (2011). [DOI] [PubMed] [Google Scholar]; The first randomized controlled trial of normothermic machine perfused human donor lungs.
- 32.Mergental H et al. Transplantation of Declined Liver Allografts Following Normothermic Ex-Situ Evaluation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg 16, 3235–3245 (2016). [DOI] [PubMed] [Google Scholar]
- *33.Ardehali A et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. The Lancet 385, 2577–2584 (2015). [DOI] [PubMed] [Google Scholar]; The first randomized controlled trial of normothermic machine perfused human donor hearts.
- *34.Warnecke G et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir. Med 6, 357–367 (2018). [DOI] [PubMed] [Google Scholar]; The first randomized controlled trial of normothermic machine perfused human donor lungs.
- 35.Isolated-organ perfusion for local gene delivery: efficient adenovirus-mediated gene transfer into the liver | Gene Therapy. Available at: https://www.nature.com/articles/3300362#auth-9. (Accessed: 8th May 2019) [DOI] [PubMed]
- 36.Bruinsma BG et al. Peritransplant Energy Changes and Their Correlation to Outcome After Human Liver Transplantation: Transplantation 101, 1637–1644 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sridharan GV et al. Metabolomic Modularity Analysis (MMA) to Quantify Human Liver Perfusion Dynamics. Metabolites 7, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cascante M et al. Metabolic control analysis in drug discovery and disease. Nat. Biotechnol 20, 243 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Capocelli M, Santis LD, Maurizi A, Pozzilli P & Piemonte V Model Predictive Control for the Artificial Pancreas in Biomedical Engineering Challenges 75–95 (John Wiley & Sons, Ltd, 2018). doi: 10.1002/9781119296034.ch5 [DOI] [Google Scholar]
- 40.Bruinsma BG, Yarmush ML & Uygun K Organomatics and organometrics: Novel platforms for long-term whole-organ culture. Technology 2, 13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann NE & Bischof JC The cryobiology of cryosurgical injury. Urology 60, 40–49 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Pegg DE Principles of cryopreservation. Methods Mol. Biol. Clifton NJ 368, 39–57 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Bischof JC Quantitative measurement and prediction of biophysical response during freezing in tissues. Annu. Rev. Biomed. Eng 2, 257–288 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Pegg DE Mechanisms of freezing damage. Symp. Soc. Exp. Biol 41, 363–378 (1987). [PubMed] [Google Scholar]
- 45.Pegg DE, Wang L, Vaughan D & Hunt CJ Cryopreservation of articular cartilage. Part 2: mechanisms of cryoinjury. Cryobiology 52, 347–359 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Taylor MJ & Pegg DE The effect of ice formation on the function of smooth muscle tissue stored at −21 or −60 degrees C. Cryobiology 20, 36–40 (1983). [DOI] [PubMed] [Google Scholar]
- 47.Yoshida K et al. A novel conception for liver preservation at a temperature just above freezing point. J. Surg. Res 81, 216–223 (1999). [DOI] [PubMed] [Google Scholar]
- 48.Dietzman RH, Rebelo AE, Graham EF, Crabo BG & Lillehei RC Long-term functional success following freezing of canine kidneys. Surgery 74, 181–189 (1973). [PubMed] [Google Scholar]
- 49.Bruinsma BG & Uygun K Subzero organ preservation: the dawn of a new ice age? Curr. Opin. Organ Transplant 22, 281–286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tessier SN et al. Effect of Ice Nucleation and Cryoprotectants during High Subzero-Preservation in Endothelialized Microchannels. ACS Biomater. Sci. Eng 4, 3006–3015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tessier SN et al. Partial freezing: A nature-inspired strategy for organ banking. Cryobiology 81, 220 (2018). [Google Scholar]
- *52.Berendsen TA et al. Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nat. Med 20, 790–793 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the first successful results using supercooling preservation to quadruple the preservation time of the rat livers.
- 53.Bruinsma BG et al. Supercooling preservation and transplantation of the rat liver. Nat. Protoc 10, 484–494 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Vries R et al. Extending the Human Liver Preservation Time for Transplantation by Supercooling. Transplantation 102, S396 (2018). [Google Scholar]
- 55.Jang TH et al. Cryopreservation and its clinical applications. Integr. Med. Res 6, 12–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Finger EB & Bischof JC Cryopreservation by vitrification: a promising approach for transplant organ banking. Curr. Opin. Organ Transplant 23, 353–360 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Gavish Z, Ben-Haim M & Arav A Cryopreservation of whole murine and porcine livers. Rejuvenation Res 11, 765–772 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Wang X et al. Fertility after intact ovary transplantation. Nature 415, 385 (2002). [DOI] [PubMed] [Google Scholar]
- **59.Campbell BK et al. Restoration of ovarian function and natural fertility following the cryopreservation and autotransplantation of whole adult sheep ovaries. Hum. Reprod. Oxf. Engl 29, 1749–1763 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; An exciting study showing good and reproducible outcomes after cryopreservation of vascularized solid organs in a large mammal (ovine) model.
- 60.Fahy GM et al. Cryopreservation of organs by vitrification: perspectives and recent advances. Cryobiology 48, 157–178 (2004). [DOI] [PubMed] [Google Scholar]
- *61.Manuchehrabadi N et al. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci. Transl. Med 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that inductive heating of magnetic nanoparticles can be used for extremely rapid and homogeneous rewarming of tissues to overcomes one of the key bottlenecks in vitrification.
- *62.Fahy GM et al. Physical and biological aspects of renal vitrification. Organogenesis 5, 167–175 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; Report of a successful vitrified and transplanted rabbit kidney.
- 63.Zhang Y et al. Isochoric vitrification: An experimental study to establish proof of concept. Cryobiology 83, 48–55 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Puschmann E, Selden C, Butler S & Fuller B Liquidus tracking: controlled rate vitrification for the cryopreservation of larger volumes and tissues. Cryo Letters 35, 345–355 (2014). [PubMed] [Google Scholar]
- 65.Guibert EE et al. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus. Med. Hemotherapy 38, 125–142 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balfoussia D, Yerrakalva D, Hamaoui K & Papalois VV Advances in machine perfusion graft viability assessment in kidney, liver, pancreas, lung, and heart transplant. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant 10, 87–100 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Watson CJE & Jochmans I From ‘Gut Feeling’ to Objectivity: Machine Preservation of the Liver as a Tool to Assess Organ Viability. Curr. Transplant. Rep 5, 72–81 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perk S et al. A metabolic index of ischemic injury for perfusion-recovery of cadaveric rat livers. PloS One 6, e28518 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rozendaal YJW et al. In vivo and in silico dynamics of the development of Metabolic Syndrome. PLoS Comput. Biol 14, e1006145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goffaux G, Hammami I & Jolicoeur M A Dynamic Metabolic Flux Analysis of Myeloid-Derived Suppressor Cells Confirms Immunosuppression-Related Metabolic Plasticity. Sci. Rep 7, 9850 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamisoglu K et al. Understanding Physiology in the Continuum: Integration of Information from Multiple -Omics Levels. Front. Pharmacol 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hartmanshenn C et al. Chapter 1 - Quantitative systems pharmacology: Extending the envelope through systems engineering in Computer Aided Chemical Engineering (ed. Manca D) 42, 3–34 (Elsevier, 2018). [Google Scholar]
- 73.Nagrath D et al. Metabolic preconditioning of donor organs: defatting fatty livers by normothermic perfusion ex vivo. Metab. Eng 11, 274–283 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Androulakis IP A Chemical Engineer’s Perspective on Health and Disease. Comput. Chem. Eng 71, 665–671 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


