Abstract
Progesterone’s ability to maintain pregnancy in eutherian mammals highlighted this steroid as the “hormone of pregnancy”. It was the unique “pro-gestational” bioactivity of progesterone that enabled eventual purification of this ovarian steroid to crystalline form by Willard Myron Allen in the early 1930s. While a functional connection between normal progesterone responses (“progestational proliferation”) of the uterus with the maintenance of pregnancy was quickly appreciated, an understanding of progesterone’s involvement in the early stages of pregnancy establishment was comparatively less well understood. With the aforementioned as historical backdrop, this review focusses on a selection of key advances in our understanding of the molecular mechanisms by which progesterone, through its nuclear receptor (the progesterone receptor), drives the development of endometrial receptivity, a transient uterine state that allows for embryo implantation and the establishment of pregnancy. Highlighted in this review are the significant contributions of advanced mouse engineering and genome-wide transcriptomic and cistromic analytics to revealing the pivotal molecular mediators and modifiers that are essential to progesterone-dependent endometrial receptivity and decidualization. With a clearer understanding of the molecular landscape that underpins uterine responsiveness to progesterone during the periimplantation period, we predict that common gynecologic morbidities due to abnormal progesterone responsiveness will be more effectively diagnosed and/or treated in the future.
Keywords: Progesterone, nuclear receptor, isoforms, endometrium, receptivity, decidualization, mediators, modifiers
Introduction
Sitting on top of the world…
May 1933 was a “glorious month” for a young Willard Myron Allen (1904–1993) (Allen 2005). For the first time, the newly minted physician not only became a father but successfully crystallized the corpus luteum hormone, progesterone—in his words “My friends gave me double congratulations and I was sitting on top of the world” (Allen 2005). Only a few years previously, during his medical training (and as part of his two-year research fellowship for a Master’s degree (1927–1929)), Allen along with George Washington Corner (1889–1981), his anatomy professor and mentor at the University of Rochester, demonstrated through a series of landmark experiments that alcohol extracts of the ovarian corpus luteum were sufficient to maintain pregnancy in rabbits that were ovariectomized eighteen hours following copulation (Allen and Corner 1929; Corner and Allen 1929). With the isolation, bioassay and official naming of progesterone, the first chapter of the progesterone story was concluded. The next chapter would focus on identifying and characterizing the “receptor” for this hormone and elucidating its mechanism of action, this chapter of study remains a relentless preoccupation for many molecular endocrinologists to this day. Inspired by the pioneering research of Jacob and Monod in the early 1960s, which established the basic regulatory principles of gene expression (Jacob and Monod 1961), investigations on the rat uterus by Jensen and Gorski (Jensen 1962; Jensen, et al. 1968; Toft and Gorski 1966) and on the chick oviduct model by O’Malley (O’Malley, et al. 1969; O’Malley and Schrader 1972; O’Malley, et al. 1970) would provide the necessary evidential support for the existence of a specific intracellular binding protein (“the receptor”) for estrogen and progesterone respectively.
In honor of the 90th anniversary commemorating the discovery of progesterone, this review focusses on recent advances in our understanding of progesterone action through its receptor in the uterus that enables embryo implantation and the establishment of pregnancy. As a prequel, however, a review of the fundamental structural/functional properties of the intracellular receptor for progesterone is instructive.
The Progesterone Receptor: An Isoform Duo
Whether during the luteal (or secretory) phase of the menstrual cycle or with pregnancy onset, most of the physiological responses to ovarian-derived progesterone are mediated by its specific intracellular receptor, the progesterone receptor (PGR) (Graham and Clarke 1997). Early structural characterization of the PGR demonstrated that the receptor belongs to the nuclear receptor superfamily of transcription factors (Conneely, et al. 1986; Jeltsch, et al. 1986), which includes cognate receptors for other steroid hormones (including estrogen) as well as for retinoids, prostanoids, thyroid hormone, and vitamin D3 (Mangelsdorf, et al. 1995; Tsai and O’Malley 1994). As a nuclear receptor superfamily member, the PGR is modular in structure with clearly demarcated functional domains (Fig. 1). The PGR is composed of an N-terminal domain (NTD), a central DNA binding domain (DBD) with two highly conserved zinc fingers, a hinge (H) region, followed by a ligand binding domain (LBD) near the C-terminus; reviewed in (Diep, et al. 2015; Grimm, et al. 2016) (Fig. 1). Within this functional domain organization, the PGR also contains three activational domains or functions (AFs) that interface with coregulator proteins within the transcriptional complex; AF1 and AF3 are located within the NTD whereas the AF2 resides in the LBD (Fig. 1). The PGR comprises two primary receptor isoforms: the full-length PGR-B (~116 kDa) and the truncated PGR-A (~94 kDa); the PGR-A lacks the first 164 amino acid residues of the NTD (termed the B upstream segment (BUS) (Bain, et al. 2000)) (Fig. 1). Absence of the AF3 in the PGR-A isoform is thought to explain in part the different transactivational properties reported for these two receptor isoforms in vitro. The PGR isoforms are frequently co-expressed in the majority of target tissues and are encoded by a single gene that uses a PGR-B distal promoter and a PGR-A proximal promoter in tandem to regulate expression of the respective receptor isoforms (Kastner, et al. 1990). Given the cellular coexistence of both isoforms, the physiological response of a target tissue to progesterone exposure is considered to be dependent on the net stoichiometry of the PGR-B and PGR-A isoforms within a cell population, in which the formation of homodimers and/or heterodimers project distinct transactivational controls on the expression of select gene-sets.
Figure 1.
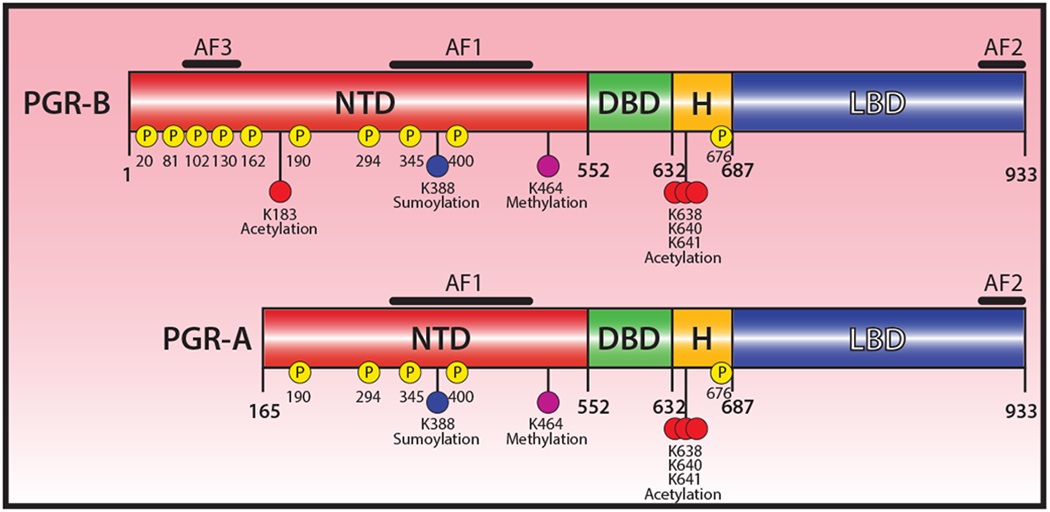
Functional domain organization of the human progesterone receptor. The progesterone receptor (PGR), a member of the NR3C3 subfamily of the nuclear receptor superfamily (Mangelsdorf et al. 1995; Tsai and O’Malley 1994), is comprised of two isoforms: the full-length PGR-B and the truncated PGR-A; the same gene—located on human chromosome 11 (11q22-q23)—encodes both isoforms. The N-terminal domain (NTD), DNA binding domain (DBD), hinge (H) region, and ligand binding domain (LBD) are indicated. The positions of activation functional domains (AF1, AF2, and AF3) are shown; note: the PGR-A isoform lacks AF-3 and multiple phosphorylation events. Phosphorylation sites are denoted by (P); locations of sumoylation, acetylation and methylation sites are also highlighted. Detailed information on these PTMs is comprehensively described in previous excellent reviews, such as (Diep et al. 2015; Grimm et al. 2016; Hagan and Lange 2014). The schematic was reproduced and modified from (Grimm et al. 2016) with permission from the Journal of Molecular Biology (license number: 4591510498146).
The classical mechanism of PGR action entails progesterone traversing the lipid bilayer of the target cell plasma membrane, interacting with the LBD of the PGR to trigger a conformational change (“the activation step” (O’Malley et al. 1969; O’Malley and Schrader 1972; O’Malley et al. 1970)), which results in ligand-bound receptor translocating to the nucleus and directly binding as a dimer to a canonical progesterone response element (PRE) within the promoter or distant enhancer of a target gene (Diep et al. 2015; Grimm et al. 2016). As part of this process, coregulators (i.e. coactivators or corepressors) and associated cofactors are recruited to the nucleating transcriptional complex to activate or silence gene expression, the net result of which manifests the progesterone physiological response at the molecular level. In many physiological contexts, PGR expression is induced by estrogen through its nuclear receptor, estrogen receptor-α (ESR1) (Graham and Clarke 1997).
Studies on human breast cancer cell lines in particular revealed that PGR’s expression levels, transcriptional activity, trafficking and target gene selection are modulated by post-translational modifications (PTMs) (Hagan and Lange 2014), which include phosphorylation, ubiquitination, sumoylation, acetylation, and methylation (Fig. 1).
Related studies have shown that PGR can also indirectly modulate gene expression through a non-classical mode of action which dispenses with the need for direct DNA binding. Instead, PGR tethers to other transcription factors bound to DNA, such as activator protein 1 (AP1), specificity protein 1 (SP1), NFkB and signal transducer activator of transcription 3 (Stat3) (Grimm et al. 2016). In addition to genomic actions, PGR has been implicated in rapid non-genomic effects that operate beyond the strictures of the nucleus. In this non-classical or extranuclear signaling context, PGR has been shown to bind kinases sequestered with growth factor receptors at the plasma membrane, which trigger rapid activation of kinase signaling cascades that frequently phosphorylate a myriad of transcription factors (including PGR) to influence downstream gene expression programs (Grimm et al. 2016; Hagan and Lange 2014). Intriguingly, recent genome topology studies support the involvement of PGR (along with ESR1) in local and global three-dimensional organization of the chromatin, which can control transcriptional output both in ligand dependent and independent context (Le Dily, et al. 2019).
Collectively, these mechanisms of action studies have furnished critical conceptual advances—within an in vitro context—on the diverse mechanisms by which PGR markedly expands its signaling bandwidth beyond the classical mode of action. However, findings from these studies raise important questions to be addressed in the future: (1) How are extranuclear and nuclear actions of PGR integrated and coordinated? (2) Is this regulatory complexity observed in non-cancerous cells, such as uterine cells? And (3) Can these various PGR signaling events be functionally linked to in vivo physiological and/or pathophysiological endpoints?
Having provided an abbreviated account of the structural and functional properties of the PGR (much of the information gleaned from in vitro studies), the ensuing sections will focus on the significant advances in our understanding of uterine PGR action in an in vivo context, thanks in large part to studies on the mouse and on the genetically engineered mouse in particular.
Progesterone and Its Uterine Receptor are required for Pregnancy Establishment
With a perhydrocyclopentanophenanthrene ring structure, progesterone received its apt appellation by Allen and colleagues (Allen, et al. 1935) primarily because the steroid hormone maintained gestation (hence “pro-gestation”) in pregnant rabbits previously ovariectomized (Allen and Corner 1929; Corner and Allen 1929). In the decades since, however, rodent studies in particular demonstrated that progesterone via PGR in the uterus is essential not only for the maintenance but also the establishment of pregnancy. In viviparous placental mammals (eutherians, including human), pregnancy establishment is achieved by successful implantation of the activated blastocyst into the receptive endometrium of the uterus (Enders 1976; Psychoyos 1973; Schlafke and Enders 1975). Held within the pelvis by the endopelvic fascia, the uterus is a complex composite organ, which is composed of the inner endometrium and outer myometrium. The endometrium, which comprises the luminal and glandular epithelium as well as the stromal compartment, supports embryo implantation and pregnancy progression to term. At parturition, the contractile force of the surrounding myometrial circular and longitudinal smooth muscle layers is required for live birth delivery.
Advancing our understanding of progesterone’s involvement in early pregnancy establishment is of utmost clinical importance as embryo implantation is an inefficient reproductive process, with implantation failure and early embryo miscarriage still representing significant etiologic factors for preclinical pregnancy loss even for healthy women (Macklon, et al. 2002). Previous studies estimate that for each menstrual cycle in a healthy woman, the chance for natural conception is only 30% (Wilcox, et al. 1999; Zinaman, et al. 1996), the low conception rate due in large part to implantation failure. While advancements in our understanding of oocyte and embryo development have significantly improved pregnancy success rates using assisted reproductive technologies (ARTs), periimplantation failure due to a nonreceptive endometrium continues to limit the full potential of these technologies (Blesa, et al. 2014). Moreover, implantation failure is frequently implicated in recurrent pregnancy loss after parental chromosomal abnormalities, maternal thrombophilic disorders, and anatomical uterine defects are first eliminated as causal factors (Rai and Regan 2006). In addition to the clear clinical challenges, such early pregnancy failures contribute to serious emotional distress and economic hardship for women and their families worldwide.
Given progesterone is the quintessential “hormone of pregnancy”, a comprehensive understanding of the pivotal signaling mechanisms by which progesterone through its receptor governs endometrial periimplantation biology is essential if future goals to develop more effective diagnostics, prognostics and/or therapeutics to manage early pregnancy loss are to be realized. However, due to obvious ethical restrictions on investigative studies on the human endometrium, most of our mechanistic understanding of endometrial periimplantation responses to progesterone at the cellular and molecular level has primarily been obtained from investigations on the murine uterus.
The mouse: A surrogate for the human
In the human and mouse, embryo implantation is dependent on the endometrium transitioning from a “pre-receptive” to a “receptive” state within a short time-frame (the window of receptivity) during which the luminal epithelium of the endometrium is transiently permissive to blastocyst attachment, adhesion and subsequent invasion (Cha, et al. 2012b; Pawar, et al. 2014). In both species, synchronized development of the endometrial receptive state with the on-time arrival of the activated blastocyst is controlled by ovarian-derived progesterone in tight coordination with estrogen. Although the endometrial cellular changes that enable embryo implantation in the human are interstitial as opposed to eccentric in the murine endometrium, the execution of the basic developmental steps that lead to uterine receptivity are shared by both species, suggesting that many of the key molecular signaling mechanisms, which mediate progesterone (and estrogen) control of these developmental steps are also conserved (Lim and Wang 2010).
Endometrial Receptivity and Decidualization Require Progesterone
Prior to embryo implantation in the mouse, proliferation of the endometrial luminal epithelium occurs in response to elevating serum levels of preovulatory estrogen (Cha et al. 2012b; Namiki, et al. 2018). Following ovulation, fertilization and progressive development of the resultant zygote to the activated blastocyst-stage with its two distinct cell-lineages (the inner cell mass and outer trophectoderm), rising levels of systemic progesterone synthesized and secreted from newly formed ovarian corpora lutea result in endometrial stromal cell proliferation, which is further augmented by a small nidatory spike in estrogen levels. In parallel, progesterone suppresses estrogen-induced proliferation of the endometrial epithelia, which results in the transition of the epithelium from a proliferative to a differentiative state to become temporarily permissive to embryo attachment and invasion. In response to embryo attachment (“the attachment reaction”), underlying fibroblastic stromal cells undergo extensive localized progesterone-dependent proliferation and differentiation to become polygonal epithelioid decidual cells, a cellular transformation process termed decidualization. As endometrial decidualization manifests, luminal epithelial cells undergo entosis at the implantation site thereby allowing the blastocyst’s trophoblastic cells to breach the underlying basal lamina and invade the decidualizing stromal compartment of the endometrium. Early studies by Lydon and colleagues using the Pgr knockout (PRKO) mouse model, in which both Pgr isoforms were simultaneously ablated (Lydon, et al. 1995), underscored the indispensability of nuclear receptor-mediated progesterone signaling in the development of the receptive and decidualized uterus. Subsequent studies using Pgr isoform specific knockout mice demonstrated that PGR-A alone—and not PGR-B or PGR isoform heterodimerization—is required for murine pregnancy establishment and maintenance (Mulac-Jericevic, et al. 2000). In humans, however, both PGR isoforms are important for pregnancy success, with the PGR-B isoform playing a predominant role in decidualization (Kaya, et al. 2015).
Surrounding the invading conceptus, decidual cells furnish histotrophic nutrition, an immunotolerant microenvironment, a negative selection process for non-viable embryos, and protection against physiological stressors as well as excessive invasion by the embryo into the uterine compartment (Gellersen and Brosens 2014). Progesterone-driven decidualization is accompanied by angiogenesis and the influx of leukocytes, such as uterine natural killer (uNK) cells and monocytes. Furthermore, molecular studies provide strong support for progesterone induction of interleukin 15 (and its receptor) in endometrial stromal cells as an essential trans-presentation signal in the differentiation of immature uNK cells to mature cells (Okada, et al. 2000; Wilkens, et al. 2013). Concentrated around maternal spiral arterioles, uNK cells are important innate immune cells that promote an immunosuppressive milieu for the invading hemiallogeneic conceptus and promote remodeling of spiral arterioles to facilitate endovascular trophoblast invasion, which ultimately establishes the hemochorial and endotheliochorial placenta in the mouse and human respectively. Importantly, uNK cells curtail excessive trophectodermal invasion into the uterine compartment by triggering apoptosis of the advancing trophoblastic cellular front of the invading conceptus. While the mouse requires the conceptus or an artificial stimulus to trigger endometrial stromal decidualization, the human endometrial stromal compartment can decidualize during the progesterone-dominant early secretory phase of a non-conception cycle (Gellersen and Brosens 2014). Also, cultured human endometrial stromal cells require only estrogen, progesterone, and cAMP to decidualize in vitro (Brosens, et al. 1999).
Progesterone-dependent uterine receptivity and decidualization represent critical reproductive events along a tightly choreographed procession of uterine cellular changes in which the successful execution of one event is predicated on the successful completion of events that came before. Because the establishment of the fetoplacental interface is inextricably linked to successful decidualization, defective decidualization has been causally connected to a number of obstetric disorders, such as early fetal miscarriage due to placental insufficiency, placenta accreta, preeclampsia, fetal growth restriction and preterm birth (Cha et al. 2012b).
Although progesterone’s pivotal role as an apex hormone regulator of uterine peri-implantation biology is well recognized, our fundamental understanding of the nuclear receptor-mediated signaling mechanisms by which progesterone exerts its action in the endometrium that lead to pregnancy establishment is only now emerging. Without question, the ongoing efforts to identify the key molecular mediators and modifiers of uterine progesterone action will provide deeper mechanistic insight into early pregnancy establishment and uncover new molecular signals that may be used in novel and more efficient clinical solutions to address periimplantation failure, gestational complications that occur in later trimesters, and common reproductive clinicopathologies arising due to defective responsiveness to progesterone.
Molecular Mediators and Modifiers of Uterine Progesterone Receptor Action
Just as signaling crosstalk between the blastocyst and uterine compartment is essential for embryo-implantation, so too is the reciprocal communication between the epithelial and stromal cellular compartments of the endometrium critical for the development of uterine receptivity and subsequent decidualization (Cha et al. 2012b; Hantak, et al. 2014). Using genome-wide expression analysis in conjunction with advanced engineered mouse models, molecular mediators and modifiers of endometrial PGR signaling, which are indispensable during the periimplantation period, are now being revealed.
The Indian hedgehog-signaling axis is a pivotal PGR molecular mediator in the endometrium
Separate investigations disclosed that Indian hedgehog (Ihh) is transcriptionally induced by progesterone in the luminal epithelium of the murine endometrium, just prior to embryo implantation (Matsumoto, et al. 2002; Takamoto, et al. 2002). Later cistromic studies would demonstrate that Ihh is a direct molecular target of PGR (Wang, et al. 2018). A secreted morphogen, IHH is a member of the highly conserved hedgehog family (Ng and Curran 2011), which includes sonic and desert hedgehog. The hedgehog triad of diffusible morphogens regulates cell proliferation, differentiation and short-range cell-cell communication, cellular processes that are essential for organogenesis, tissue homeostasis, and oncogenesis. Investigations on the PRKO and a conditional Ihh knockout mouse respectively demonstrated that progesterone induction of Ihh was via the PGR (Takamoto et al. 2002), and that IHH was essential for uterine receptivity and decidualization (Lee, et al. 2006). Absence of Ihh in the murine endometrium resulted in derailed cell-cycle progression, significantly decreased epidermal growth factor (EGF) signaling, and the display of overt histological hallmarks of a persistent estrogenized uterus (i.e. the presence of numerous cystic glandular ducts and a hylalinized stroma) (Franco, et al. 2010; Lee et al. 2006).
Crossing the epithelial-stromal divide as a paracrine secreted factor, epithelial-derived IHH activates the canonical hedgehog effector pathway in the underlying stroma (Fig. 2A); the hedgehog pathway includes: the cognate IHH receptor patched-1 (PTCH1), the activated intracellular transducer: Smoothened (SMO) and the glioma-associated oncogene homolog (GLI) transcription factors (Fig. 2A). Activation of the hedgehog pathway promotes the expression of the orphan nuclear receptor: chicken ovalbumin upstream promoter transcription factor II (COUP-TFII; NR2F2) in the stroma ((Takamoto et al. 2002) (Fig. 2A)). A key mesenchymal differentiation factor, COUP-TFII modulates a myriad of cellular processes from angiogenesis, organogenesis, inflammation and metabolism to cell adhesion and cell-fate specification (Wu, et al. 2016). Conditional ablation of Coup-tfII results in a block in embryo-implantation and stromal cell decidualization (Kurihara, et al. 2007; Lee, et al. 2010), which is caused by a persistent estrogenized uterus (Kurihara et al. 2007; Lee et al. 2010). Of clinical significance, aberrant downregulation of both IHH and COUP-TFII in the human endometrium is linked to endometriosis (Lin, et al. 2014), a uterine pathology that is dependent in part on a heightened estrogenic response. Recent clinical data also reveal that selective progesterone receptor modulators can significantly perturb the normal expression levels of these progesterone-responsive factors in the human endometrium (Whitaker, et al. 2017).
Figure 2.
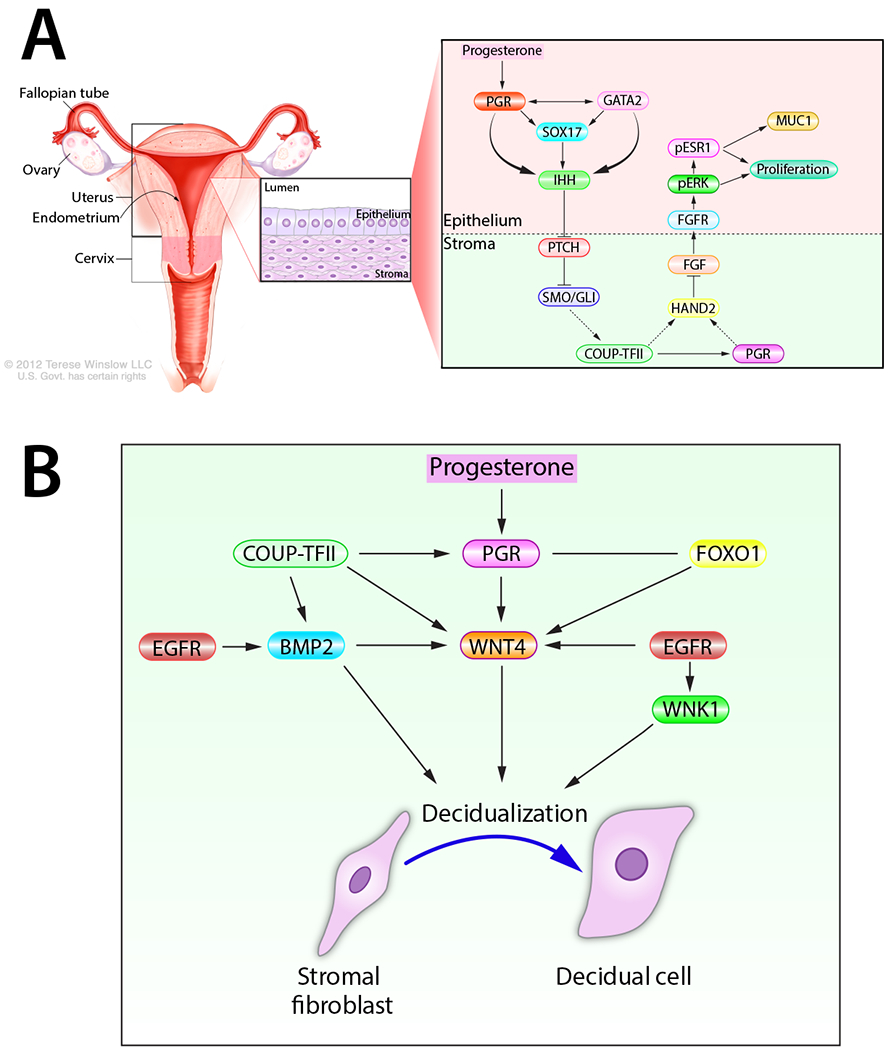
A propensity for complexity: molecular signaling required for progesterone-dependent uterine receptivity and decidualization. (A) The progesterone-PGR-IHH-COUP-TFII signaling pathway, which spans the epithelial and stromal cellular compartments of the endometrium, controls ESR1 activity in the endometrial epithelium. Such ESR1 control is required for epithelial differentiation and development of the receptive endometrium for embryo implantation. For clarity, other signals (i.e. Msx 1 and 2), which are important for uterine receptivity, are not be included in this schematic. (B) A selection of progesterone-induced signaling pathways required for endometrial stromal cell decidualization during the periimplantation period. Adapted with permission in modified form from (Wu, et al. 2018).
Enhanced COUP-TFII expression by the PGR-IHH axis is associated with increased stromal PGR expression as well as the induction of stromal heart and neural crest derivatives expressed transcript 2 (Hand2) (Li, et al. 2011), a basic-helix-loop-helix transcription factor. While it’s not clear whether COUP-TFII directly or indirectly (i.e. through stromal PGR) increases stromal Hand2 levels, HAND2 normally inhibits the expression of several stromal fibroblast growth factor (fgf) family members (fgf1, −2, −9, and −18) in the endometrium (Li et al. 2011). Accordingly, molecular phenotyping of the conditional Hand2 knockout mouse demonstrated that absence of Hand2 results in constitutive induction of stromal fgf expression in the endometrium (Li et al. 2011). As paracrine signals within the normal endometrium, stromal FGFs engage their transmembrane tyrosine kinase receptors (FGFRs with associated docking factors) located in epithelial cells to trigger phosphorylation (and activation) of extracellular signal regulated kinases 1 and 2 (ERK1/2), which in turn phosphorylate, stabilize and activate the ESR1 (Li et al. 2011). Apart from epithelial proliferation, activated ESR1 maintains expression of Muc1 (MUC1), a glycoprotein expressed on the apical surface of luminal epithelial cells that prevents embryo attachment (Surveyor, et al. 1995). Moreover, persistent proliferation of the glandular epithelium results in a block in the expression of the leukemia inhibitory factor ((LIF) an interleukin 6 family cytokine member) and the Forkhead box A1 (Fox A1) transcription factor, both pivotal signaling cues for embryo implantation (Kelleher, et al. 2017; Stewart, et al. 1992). Therefore, uncoupling of the progesterone-PGR-IHH-COUP-TFII-HAND2 regulatory axis can cause persistent activation of epithelial ESR1 (Fig. 2A), which results in failure of the luminal epithelial cell to exit the cell cycle and undergo differentiation, the net result of which is to prevent the development of the receptive state.
Uncovering the complex molecular circuitries that mediate progesterone-driven endometrial receptivity also revealed how uterine receptivity is tightly coordinated with progesterone-dependent endometrial decidualization at the molecular level. For example, induction of stromal COUP-TFII by the PGR-IHH axis results in the induction of bone morphogenetic protein 2 (Bmp2) (Kurihara et al. 2007), a member of the transforming growth factor beta (TGFβ) superfamily of cytokines. As a consequence of elevated Bmp2 levels, wingless-type MMTV integration site (WNT) family member 4 (Wnt 4) is induced in the stroma. Both BMP2 and WNT 4—along with COUP-TFII and IHH—are essential for PGR-dependent endometrial stromal cell decidualization in both human and mouse (Franco, et al. 2011; Lee, et al. 2007; Li, et al. 2013; Li, et al. 2007) (Fig. 2B). This regulatory complexity is further increased by the finding that BMP2 upregulates members of the muscle segment homeobox (Msx) family of transcription factors (Msx1 and 2) (Nallasamy, et al. 2019), which have previously been shown to be important for epithelial-stromal cross-talk required for the elaboration of the receptive uterus (Daikoku, et al. 2011; Nallasamy, et al. 2012). In parallel, enhanced stromal EGFR-mediated signaling phosphorylates and activates the serine/threonine protein kinase: With-No-Lysine (K) 1 (WNK1) (Large, et al. 2014), a kinase which is essential for decidualization of human endometrial stromal cells in culture (Adams, et al. 2017).
In a remarkably short period of time, the confluence of independent molecular investigations has furnished invaluable insights into the tremendous molecular complexity that underlies progesterone regulation of endometrial receptivity and decidualization. At a minimum, the innumerable genes, pathways and networks identified to date are merely a prologue for the immense molecular complexity that is to come. Given the predicted scale and complexity of the multicomponent molecular networks that mediate progesterone control of uterine receptivity and decidualization, an immediate challenge will be to delineate the molecular mechanisms by which these various signal transduction pathways are spatiotemporally integrated and coordinated in a hierarchical manner. As detailed above, many of these progesterone mediators are critical for endometrial stromal cell decidualization, a type of mesenchymal-epithelial transformation (MET) cellular process. However, which factors are also important for other cellular processes that are required for successful stromal decidualization—i.e. angiogenesis, local immunosuppression and immune cell influx—remain an open question. Because premature senescence of decidual cells is an established cause of preterm birth (Cha, et al. 2012a), another open question is whether all or a subset of these factors are continually required to maintain healthy decidual tissue throughout gestation. While much remains unknown, expanding our knowledge in this area will undoubtedly provide a broader conceptual foundation for understanding progesterone action not only in normal endometrial function but also in common gynecologic morbidities in which normal progesterone responsiveness is markedly attenuated or abrogated (Al-Sabbagh, et al. 2012).
Modifiers of Endometrial PGR Action
Loosely defined, transcriptional modifiers of PGR are factors that regulate the transcriptional output mediated by ligand-activated PGR through control of PGR levels and/or by participating directly or indirectly as a coregulator in PGR-containing transcriptional complexes that govern target gene expression. Modifier control of PGR functionality operates in tandem with PGR PTMs, another regulatory process that modifies PGR transcriptional activity and turnover (Fig. 1). Recent transcriptomic and cistromic analyses together with follow-up in vivo validation in engineered mouse models have identified crucial modifiers of uterine PGR activity that modulate the levels and/or the transcriptional activity of PGR in the endometrium.
The GATA2 transcription factor is a master modifier of PGR expression and transcriptional activity in the uterine epithelium
Through their highly conserved dual zinc finger domains, the six member GATA-binding transcription factor family bind the evolutionary conserved consensus sequence 5’-(A/T)GATA(A/G)-3’ to control diverse physiological processes from hematopoiesis, adipocyte differentiation to pituitary function (Tremblay, et al. 2018). To execute these processes at the molecular level, GATA transcription factors can act as “pioneer factors” endowed with special properties that allow for their binding to heterochromatin to promote—through recruitment of epigenetic modifiers—chromatin opening. In turn, open chromatin facilitates local combinatorial assembly at a promoter or an enhancer of other transcriptional regulators (i.e. coregulators and tissue-specific transcription factors) that ultimately modulate target gene expression programs.
Expression studies in the murine uterus revealed that the temporal expression profile of the Gata2 transcription factor and Pgr is similar in the epithelium, in which expression levels for both transcription factors peak just before and precipitously decline immediately after entry into the window of receptivity (Rubel, et al. 2012a). That GATA2 and PGR might be mechanistically linked is also supported by similar infertility phenotypes—a block in uterine receptivity and decidualization—exhibited by female mice in which the uterine epithelium is selectively devoid of Gata2 or Pgr (Franco, et al. 2012; Rubel, et al. 2016). Abrogation of Gata2 also results in uterine epithelial signatures of unopposed estrogen signaling (Rubel et al. 2016), such as aberrant epithelial integrity and stratification. Further mouse phenotypic investigations, in conjunction with data sourced from genome-wide chromatin occupancy and transcriptomic analytics of murine and human uterine tissue, revealed that GATA2 is responsible for the expression of epithelial PGR as well as the ability of PGR to activate its downstream transcriptional program that drives uterine receptivity (Rubel et al. 2016). In particular, gene signature and pathway analysis of both human and mouse uterine datasets highlighted the existence of a conserved GATA2-PGR regulatory network that relies on a cooperative relationship between GATA2 and PGR (as well as with other transcription factors important for uterine receptivity (i.e. SOX17 (see below)) in controlling normal endometrial epithelial transcriptional responsiveness to progesterone during the periimplantation period. As a prototypic example, chromatin co-occupancy of GATA2 and PGR has been mapped to a putative enhancer located 19kb upstream of the promoter controlling Ihh transcription (Rubel et al. 2016), which is required for uterine receptivity. Note: Ihh is not induced by progesterone in the murine uterine epithelium in the absence of GATA2 (Rubel et al. 2016).
Similar to findings from studies on the androgen receptor ((AR); a close relative of PGR) in the prostate (Wu, et al. 2014), data support epithelial GATA2 serving as a pioneer factor as well as a coregulator of PGR target gene expression in the uterine epithelium (Rubel et al. 2016) (Fig. 2A). Indeed, extensive studies on the prostate demonstrate that GATA2 can: (a) bind upstream regulatory elements to increase AR expression; (b) establish chromatin accessibility at AR enhancer regions through recruitment of p300 histone acetyltransferase that creates active chromatin by acetylating lysine 27 in histone 3 (H3K27); and (c) generate and maintain regulatory chromatin loops between AR-bound distal enhancers and AR target gene promoters through enlistment of the mediator coregulator complex (MED1) (Wu et al. 2014). Whether GATA2 modifies Pgr expression and its signaling program through similar mechanisms in the uterine epithelium awaits further investigation. What governs GATA2 expression in the uterine epithelium and whether there are fundamental commonalities between the GATA2-PGR network in the uterus and GATA regulatory networks operative in other physiological systems also remain open questions.
A potent coregulator of uterine PGR action: SOX17
Mining the PGR cistrome dataset from the mouse uterus (Rubel, et al. 2012b), the SOX17 transcription factor was quickly identified as a putative direct target of PGR and GATA2 and possibly important for mediating progesterone-dependent uterine receptivity. Initial in vivo support for this proposal came from the observation that female mice with whole-body Sox17 haploinsufficiency display a severe subfertility defect due to impaired implantation (Hirate, et al. 2016). Subsequent investigations on mice with Sox17 selectively ablated in PGR positive cells of the uterus or in the uterine epithelium not only confirmed the essential role of epithelial SOX17 in the development of the receptive endometrium but also the requirement for this transcription factor in postnatal endometrial gland development (adenogenesis) (Guimaraes-Young, et al. 2016; Wang et al. 2018).
At least twenty members comprise the SRY-determining region Y-related high-mobility group (HMG) box (SOX) family of transcription factors, which are critical determinants of cell fate reprogramming by pioneering epigenetic remodeling of the genome (Grimm, et al. 2019). Mechanistically, SOX transcription factors can bind DNA through the HMG domain to cause DNA to bend over long distances in the genome that enables the juxtaposition of enhancer bound factors to interact with members of the transcriptional holocomplex at target gene promoters. Accordingly, the fundamental regulatory functions of SOX family members (including SOX17) encompass the specification and morphogenesis of the primitive and definitive endoderm, the ontogenesis of germ cells, maintenance of fetal and neonatal hematopoietic stem cells, and the development and function of the cardiovascular system.
Comparative analyses of transcription factor cistrome datasets from the mouse uterus disclosed a discrete regulatory element containing a cluster of binding sites for PGR, GATA2, FOXA2, and SOX17 (Wang et al. 2018). Importantly, this cis regulatory element is associated with over 700 uterine genes, many of which are important for uterine epithelial function (Wang et al. 2018). As described above, this cis-regulatory element was identified 19kb from the promoter of the Ihh gene, a gene that is critical for progesterone-dependent uterine receptivity (Fig. 2A). As a testament to the singular importance of SOX17 as a coregulator of PGR driven Ihh gene transcription, Crispr/Cas9 mediated in vivo deletion of the SOX17 binding site within this element renders the murine endometrial epithelium incapable of exiting the cell cycle to enter the differentiation program that is necessary for uterine receptivity (Wang et al. 2018). Collectively, these studies underscore a pivotal role for SOX17 in uterine epithelial transcriptional responses required for progesterone controlled uterine receptivity. To be addressed by future studies is the cellular and molecular mechanisms by which SOX17 regulates postnatal adenogenesis, the role of SOX17 in the uterine vasculature during the embryo implantation process, and the involvement SOX17 as a mutated cancer driver gene in endometrial carcinomas (Senna Tan, et al. 2019).
The PGR and FOXO1 relationship: a “Yin-Yang” dynamic
A member of the O-class of Forkhead box (FOX) proteins, FOXO1 is critical for a broad spectrum of fundamental cellular processes, including metabolism, oxidative stress avoidance, cell proliferation and apoptosis (Link 2019). Conditional Foxo1 knockout (Foxo1d/d) mouse studies demonstrated that FOXO1 is essential for embryo implantation (Vasquez, et al. 2018). Absence of FOXO1 in the uterine epithelium resulted in an aberrant alteration in epithelial cell polarity and an impaired apoptotic program that blocked embryo invasion through the luminal epithelial compartment (Vasquez et al. 2018). Transcriptomic analysis revealed that epithelial FOXO1 regulates expression programs associated with cell invasion, molecular transport, apoptosis, β−catenin signaling, and an increase in PGR signaling (Vasquez et al. 2018). Intriguingly, the enhanced PGR signaling persisting in the endometrial epithelium of the Foxo1d/d mouse is reflected by the constitutive expression of PGR during the window of receptivity; note: previous studies showed that epithelial PGR levels must markedly decrease to enable normal uterine receptivity to occur (Wetendorf, et al. 2017) (Fig. 3). That there may be a mutually exclusive or “Yin-Yang” reciprocal relationship between FOXO1 and PGR expression in the murine uterine epithelium is further supported by the absence of FOXO1 expression in the uterine epithelium of mice engineered to continually express epithelial PGR (Wetendorf et al. 2017). Importantly, this Yin-Yang relationship between FOXO1 and PGR was observed in the glandular epithelium of human endometrial tissue biopsied during the proliferative and secretory phase of the cycle (Vasquez et al. 2018). These studies showed that while FOXO1 is not expressed in the glandular epithelium during the proliferative and late-secretory phase of the cycle, FOXO1 is strongly expressed in the glandular epithelial compartment during the mid-secretory phase of the cycle (considered the window of receptivity). In contrast, glandular epithelial PGR expression is strongest during proliferative phase of the cycle and undetectable during the cycle’s mid-secretory phase. Interestingly, this reciprocal expression relationship between PGR and FOXO1 in the glandular epithelium of the human endometrium was also reported by the Critchley group (Whitaker et al. 2017). Together, these findings support the proposal that the gain and loss of FOXO1 and PGR expression respectively are interrelated and that the expression switch of these two transcription factors represents an evolutionary conserved and pivotal determinant of endometrial receptivity.
Figure 3.
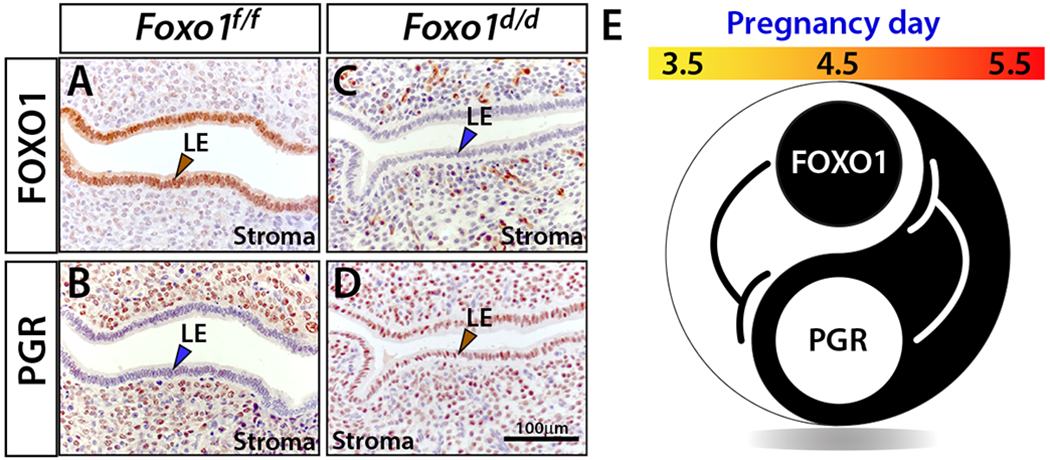
Progesterone receptor and FOXO1 display a “Yin Yang” expression relationship in the epithelium of the murine endometrium during the window of implantation. (A) Robust luminal epithelial (LE) expression of FOXO 1 (brown arrowhead) in the uterus of a control mouse (Foxo1f/f) at day 4.5 of pregnancy (the morning of vaginal plug detection is designated pregnancy day 0.5). (B) Serial section of uterine tissue shown in (A) stained for PGR. As expected (Wetendorf et al. 2017), note the absence of PGR expression in the luminal epithelium (blue arrowhead) with marked expression in the stroma. (C) Staining for FOXO1 expression in the uterus of a Foxo1d/d mouse at 4.5 days following the detection of the vaginal plug. Unlike control uterus (A), note the clear absence of FOXO1 expression in the luminal epithelium (blue arrowhead) in the Foxo1d/d uterus. (D) Staining for PGR expression in a serial section of uterine tissue shown in (C) reveals abnormal constitutive PGR expression in the luminal epithelium (brown arrowhead) in the Foxo1d/d uterus. (E) The immunohistochemical findings shown in (A-D) are schematically summarized as a proposed model for the suppression of PGR expression in the endometrial luminal epithelium by FOXO1 within the window of receptivity. The immunohistochemical data shown in panels (A-D) and the modified schematic model shown in (E) are presented in adapted form from (Vasquez et al. 2018).
A critical question to be addressed in the future is: what is the regulatory mechanism(s) that orchestrate the expression switch of FOXO1 and PGR in the endometrial epithelium just prior to the window of receptivity? Also, previous in vitro studies using cultured human endometrial stromal cells revealed an important coregulator role for FOXO1 in progesterone-dependent transcriptional programs necessary for human endometrial stromal cell decidualization (Vasquez, et al. 2015). Although the mouse studies did not provide support for FOXO1 in the initiation of progesterone-dependent endometrial stromal cell decidualization in vivo (Vasquez et al. 2018), these studies did not rule out a role for FOXO1 in maintaining a normal decidual response over time. In the future, it will be interesting to address whether continued FOXO1 expression is necessary to prevent premature senescence of the decidua as decidual premature senescence causes adverse gestational outcomes in later trimesters (Cha et al. 2012a; Cha et al. 2012b). Related to the above, recent studies revealed a key role for FOXO1 in maintaining a healthy human decidual cell population by removing a subset of endometrial stromal cells exhibiting replication stress through programming these cells for senescence and ultimately for uNK mediated clearance (Brighton, et al. 2017).
It should be noted that there are numerous important PGR modifiers—i.e. the FKBP52 immunophilin (Tranguch, et al. 2005) and the steroid receptor coactivator (SRC) 2 (Kommagani, et al. 2013) just to name two—of endometrial PGR action that could not be described here due to space limitations.
Perspectives
Engineering the murine Pgr gene as a null allele (Lydon et al. 1995), a floxed allele (Fernandez-Valdivia, et al. 2010) or a cre driver (Soyal, et al. 2005) has significantly expanded our understanding of uterine PGR action in vivo. With the advent of precise Crispr/Cas9 gene editing (Doudna and Charpentier 2014), high-resolution structure functional analysis is now possible in vivo not only for the PGR but also for its mediators and modulators. Application of this fast and effective mouse engineering methodology is predicted to allow expeditious functional validation of PTM events on the PGR in an in vivo context. Also, this technological advance will provide opportunities to elucidate the functional importance in vivo of defined protein-protein interaction sites on PGR and its modifiers within a transcriptional complex. In addition to protein structure-function analysis, this mouse technology will be invaluable in validating the in vivo functional relevance of cis-regulatory elements within enhancers or promoters of predicted target genes of PGR or of its mediators and modifiers; the first example of applying this approach in uterine studies was recently published (Wang et al. 2018). In addition to the mouse, this powerful and versatile gene-editing tool will enable broad application of precise genome manipulation in other experimental models of uterine biology and pathobiology, such as the simple monolayer cell culture model, the more recent three dimensional organoid cell culture system and animal models that are closer to human on the evolutionary scale. Especially noteworthy are the technological advances in three-dimensional organoid cultures of human endometrium that can recapitulate—in response to hormone—the dynamic endometrial cellular and molecular changes that occur throughout the menstrual cycle and early pregnancy (Turco, et al. 2017). From a clinical perspective, this powerful cell-culture approach in combination with Crispr/Cas9 gene-editing is predicted to accelerate and expand our understanding of endometrial PGR signaling in human uterine receptivity and decidualization that is free from ethical constraints.
To date, the use of engineered mouse models, human biopsy tissue and primary cells in conjunction with the analysis and integration of genome-wide transcriptome and cistrome datasets has now exposed to molecular dissection the complex signaling cascades—comprising transcription factors, growth factors, morphogens, and cytokines—that underpin progesterone-driven uterine receptivity and decidualization. Due to space limitations, this review showcased only a select number of molecular mediators and modifiers along the chain of signal transmission that starts with initial progesterone exposure and ends with the development of the uterine receptive state. A formidable challenge going forward will be to understand how these numerous progesterone-responsive genes, pathways, and networks operate dynamically and spatially in concert with signaling networks governed by other extrinsic cues such as systemic estrogen. Meeting this challenge will be a clinical imperative as derailment of the majority of mediators and modifiers of progesterone signaling lead to a persistent estrogenized uterus, an abnormal uterine state that (if persistent) can cause not only infertility but also the etiopathogenesis of common uterine morbidities, such as endometriosis, endometrial hyperplasia and cancer (Al-Sabbagh et al. 2012).
In conclusion, our review ends as it began, with Willard Myron Allen. Toward the end of his scientific career, Willard Myron Allen authored a brief career retrospective, entitled “Recollections of My Life with Progesterone” (Allen 1974). Within the concluding paragraph, Allen wrote: “These were golden years for both of us [referring to Allen and Corner]. For a few fleeting years progesterone was ours. My story ends on a happy note; there are still unsolved problems relating to progesterone”. Written many decades ago, the concluding line is prescient as it is understated…
Acknowledgements
The authors apologize in advance to investigators whose exceptional research did not get cited due to space limitations set by the journal.
Funding
Supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences: Project Z1AES103311-01 (FJD) and by National Institutes of Health/NICHD: R01HD042311 (JPL).
Footnotes
This review forms part of a special section on 90 years of progesterone. The guest editors for this section are Dr. Simak Ali, Imperial College London, UK and Dr. Bert W. O’Malley, Baylor College of Medicine, USA
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
REFERENCES
- Adams NR, Vasquez YM, Mo Q, Gibbons W, Kovanci E & DeMayo FJ 2017. WNK lysine deficient protein kinase 1 regulates human endometrial stromal cell decidualization, proliferation, and migration in part through mitogen-activated protein kinase 7. Biol Reprod 97 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sabbagh M, Lam EW & Brosens JJ 2012. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol 358 208–215. [DOI] [PubMed] [Google Scholar]
- Allen WM 1974. Recollections of my life with progesterone. Gynecol Invest 5 142–182. [DOI] [PubMed] [Google Scholar]
- Allen WM 2005. My life with progesterone. 1970 Am J Obstet Gynecol 193 1575–1577. [DOI] [PubMed] [Google Scholar]
- Allen WM, Butenandt A, Corner GW & Slotta KH 1935. Nomenclature of Corpus Luteum Hormone. Science 82 153. [DOI] [PubMed] [Google Scholar]
- Allen WM & Corner GW 1929. Physiology of the corpus luteum, III: normal growth and implantation of embryos after early ablation of the ovaries, and under the influence of extracts of the corpus luteum. . Am J Physiol 88 340–346. [Google Scholar]
- Bain DL, Franden MA, McManaman JL, Takimoto GS & Horwitz KB 2000. The N-terminal region of the human progesterone A-receptor. Structural analysis and the influence of the DNA binding domain. J Biol Chem 275 7313–7320. [DOI] [PubMed] [Google Scholar]
- Blesa D, Ruiz-Alonso M & Simon C 2014. Clinical management of endometrial receptivity. Semin Reprod Med 32 410–413. [DOI] [PubMed] [Google Scholar]
- Brighton PJ, Maruyama Y, Fishwick K, Vrljicak P, Tewary S, Fujihara R, Muter J, Lucas ES, Yamada T, Woods L, et al. 2017. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosens JJ, Hayashi N & White JO 1999. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 140 4809–4820. [DOI] [PubMed] [Google Scholar]
- Cha J, Hirota Y & Dey SK 2012a. Sensing senescence in preterm birth. Cell Cycle 11 205–206. [DOI] [PubMed] [Google Scholar]
- Cha J, Sun X & Dey SK 2012b. Mechanisms of implantation: strategies for successful pregnancy. Nat Med 18 1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely OM, Sullivan WP, Toft DO, Birnbaumer M, Cook RG, Maxwell BL, Zarucki-Schulz T, Greene GL, Schrader WT & O’Malley BW 1986. Molecular cloning of the chicken progesterone receptor. Science 233 767–770. [DOI] [PubMed] [Google Scholar]
- Corner GW & Allen WM 1929. Physiology of the corpus luteum, II: production of a special uterine reaction (progestational proliferation) by extracts of the corpus luteum. Am J Physiol 88 326–399. [Google Scholar]
- Daikoku T, Cha J, Sun X, Tranguch S, Xie H, Fujita T, Hirota Y, Lydon J, DeMayo F, Maxson R, et al. 2011. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev Cell 21 1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep CH, Daniel AR, Mauro LJ, Knutson TP & Lange CA 2015. Progesterone action in breast, uterine, and ovarian cancers. J Mol Endocrinol 54 R31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna JA & Charpentier E 2014. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346 1258096. [DOI] [PubMed] [Google Scholar]
- Enders AC 1976. Anatomical aspects of implantation. J Reprod Fertil Suppl 1–15. [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Jeong J, Mukherjee A, Soyal SM, Li J, Ying Y, Demayo FJ & Lydon JP 2010. A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland. Genesis 48 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, et al. 2011. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J 25 1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Lee KY, Broaddus RR, White LD, Lanske B, Lydon JP, Jeong JW & DeMayo FJ 2010. Ablation of Indian hedgehog in the murine uterus results in decreased cell cycle progression, aberrant epidermal growth factor signaling, and increased estrogen signaling. Biol Reprod 82 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, Jeong JW, Spencer TE, Behringer RR, Lydon JP & Demayo FJ 2012. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J 26 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B & Brosens JJ 2014. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev 35 851–905. [DOI] [PubMed] [Google Scholar]
- Graham JD & Clarke CL 1997. Physiological action of progesterone in target tissues. Endocr Rev 18 502–519. [DOI] [PubMed] [Google Scholar]
- Grimm D, Bauer J, Wise P, Kruger M, Simonsen U, Wehland M, Infanger M & Corydon TJ 2019. The role of SOX family members in solid tumours and metastasis. Semin Cancer Biol. [DOI] [PubMed] [Google Scholar]
- Grimm SL, Hartig SM & Edwards DP 2016. Progesterone Receptor Signaling Mechanisms. J Mol Biol 428 3831–3849. [DOI] [PubMed] [Google Scholar]
- Guimaraes-Young A, Neff T, Dupuy AJ & Goodheart MJ 2016. Conditional deletion of Sox17 reveals complex effects on uterine adenogenesis and function. Dev Biol 414 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan CR & Lange CA 2014. Molecular determinants of context-dependent progesterone receptor action in breast cancer. BMC Med 12 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantak AM, Bagchi IC & Bagchi MK 2014. Role of uterine stromal-epithelial crosstalk in embryo implantation. Int J Dev Biol 58 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirate Y, Suzuki H, Kawasumi M, Takase HM, Igarashi H, Naquet P, Kanai Y & Kanai-Azuma M 2016. Mouse Sox17 haploinsufficiency leads to female subfertility due to impaired implantation. Sci Rep 6 24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F & Monod J 1961. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol 3 318–356. [DOI] [PubMed] [Google Scholar]
- Jeltsch JM, Krozowski Z, Quirin-Stricker C, Gronemeyer H, Simpson RJ, Garnier JM, Krust A, Jacob F & Chambon P 1986. Cloning of the chicken progesterone receptor. Proc Natl Acad Sci U S A 83 5424–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EV 1962. On the mechanism of estrogen action. Perspect Biol Med 6 47–59. [DOI] [PubMed] [Google Scholar]
- Jensen EV, Suzuki T, Kawashima T, Stumpf WE, Jungblut PW & DeSombre ER 1968. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A 59 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H & Chambon P 1990. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9 1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya HS, Hantak AM, Stubbs LJ, Taylor RN, Bagchi IC & Bagchi MK 2015. Roles of progesterone receptor A and B isoforms during human endometrial decidualization. Mol Endocrinol 29 882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher AM, Peng W, Pru JK, Pru CA, DeMayo FJ & Spencer TE 2017. Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc Natl Acad Sci U S A 114 E1018–E1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommagani R, Szwarc MM, Kovanci E, Gibbons WE, Putluri N, Maity S, Creighton CJ, Sreekumar A, DeMayo FJ, Lydon JP, et al. 2013. Acceleration of the glycolytic flux by steroid receptor coactivator-2 is essential for endometrial decidualization. PLoS Genet 9 e1003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ & Tsai SY 2007. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet 3 e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large MJ, Wetendorf M, Lanz RB, Hartig SM, Creighton CJ, Mancini MA, Kovanci E, Lee KF, Threadgill DW, Lydon JP, et al. 2014. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet 10 e1004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Dily F, Vidal E, Cuartero Y, Quilez J, Nacht AS, Vicent GP, Carbonell-Caballero J, Sharma P, Villanueva-Canas JL, Ferrari R, et al. 2019. Hormone-control regions mediate steroid receptor-dependent genome organization. Genome Res 29 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Kurihara I, Jeong JW, Lydon JP, DeMayo FJ, Tsai MJ & Tsai SY 2010. Suppression of ERalpha activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol 24 930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, et al. 2006. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet 38 1204–1209. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP & DeMayo FJ 2007. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol 27 5468–5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, Das A, Demayo FJ, Hornsby PJ, Young SL, Taylor RN, Bagchi MK & Bagchi IC 2013. WNT4 acts downstream of BMP2 and functions via beta-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology 154 446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK & Bagchi IC 2011. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 331 912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK & Bagchi IC 2007. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem 282 31725–31732. [DOI] [PubMed] [Google Scholar]
- Lim HJ & Wang H 2010. Uterine disorders and pregnancy complications: insights from mouse models. J Clin Invest 120 1004–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Li YH, Wu MH, Chang YF, Lee DK, Tsai SY, Tsai MJ & Tsai SJ 2014. Suppression of COUP-TFII by proinflammatory cytokines contributes to the pathogenesis of endometriosis. J Clin Endocrinol Metab 99 E427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link W 2019. Introduction to FOXO Biology. Methods Mol Biol 1890 1–9. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr., Shyamala G, Conneely OM & O’Malley BW 1995. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9 2266–2278. [DOI] [PubMed] [Google Scholar]
- Macklon NS, Geraedts JP & Fauser BC 2002. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update 8 333–343. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. 1995. The nuclear receptor superfamily: the second decade. Cell 83 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Zhao X, Das SK, Hogan BL & Dey SK 2002. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol 245 280–290. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP & Conneely OM 2000. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289 1751–1754. [DOI] [PubMed] [Google Scholar]
- Nallasamy S, Kaya Okur HS, Bhurke A, Davila J, Li Q, Young SL, Taylor RN, Bagchi MK & Bagchi IC 2019. Msx Homeobox Genes Act Downstream of BMP2 to Regulate Endometrial Decidualization in Mice and in Humans. Endocrinology 160 1631–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallasamy S, Li Q, Bagchi MK & Bagchi IC 2012. Msx homeobox genes critically regulate embryo implantation by controlling paracrine signaling between uterine stroma and epithelium. PLoS Genet 8 e1002500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki T, Ito J & Kashiwazaki N 2018. Molecular mechanisms of embryonic implantation in mammals: Lessons from the gene manipulation of mice. Reprod Med Biol 17 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JM & Curran T 2011. The Hedgehog’s tale: developing strategies for targeting cancer. Nat Rev Cancer 11 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley BW, McGuire WL, Kohler PO & Korenman SG 1969. Studies on the mechanism of steroid hormone regulation of synthesis of specific proteins. Recent Prog Horm Res 25 105–160. [DOI] [PubMed] [Google Scholar]
- O’Malley BW & Schrader WT 1972. Progesterone receptor components: identification of subunits binding to the target-cell genome. J Steroid Biochem 3 617–629. [DOI] [PubMed] [Google Scholar]
- O’Malley BW, Sherman MR & Toft DO 1970. Progesterone “receptors” in the cytoplasm and nucleus of chick oviduct target tissue. Proc Natl Acad Sci U S A 67 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Nakajima T, Sanezumi M, Ikuta A, Yasuda K & Kanzaki H 2000. Progesterone enhances interleukin-15 production in human endometrial stromal cells in vitro. J Clin Endocrinol Metab 85 4765–4770. [DOI] [PubMed] [Google Scholar]
- Pawar S, Hantak AM, Bagchi IC & Bagchi MK 2014. Minireview: Steroid-regulated paracrine mechanisms controlling implantation. Mol Endocrinol 28 1408–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychoyos A 1973. Hormonal control of ovoimplantation. Vitam Horm 31 201–256. [DOI] [PubMed] [Google Scholar]
- Rai R & Regan L 2006. Recurrent miscarriage. Lancet 368 601–611. [DOI] [PubMed] [Google Scholar]
- Rubel CA, Franco HL, Jeong JW, Lydon JP & DeMayo FJ 2012a. GATA2 is expressed at critical times in the mouse uterus during pregnancy. Gene Expr Patterns 12 196–203. [DOI] [PubMed] [Google Scholar]
- Rubel CA, Lanz RB, Kommagani R, Franco HL, Lydon JP & DeMayo FJ 2012b. Research resource: Genome-wide profiling of progesterone receptor binding in the mouse uterus. Mol Endocrinol 26 1428–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel CA, Wu SP, Lin L, Wang T, Lanz RB, Li X, Kommagani R, Franco HL, Camper SA, Tong Q, et al. 2016. A Gata2-Dependent Transcription Network Regulates Uterine Progesterone Responsiveness and Endometrial Function. Cell Rep 17 1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlafke S & Enders AC 1975. Cellular basis of interaction between trophoblast and uterus at implantation. Biol Reprod 12 41–65. [DOI] [PubMed] [Google Scholar]
- Senna Tan D, Holzner M, Weng M, Srivastava Y & Jauch R 2019. SOX17 in cellular reprogramming and cancer. Semin Cancer Biol. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ & Lydon JP 2005. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41 58–66. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F & Abbondanzo SJ 1992. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359 76–79. [DOI] [PubMed] [Google Scholar]
- Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK & Carson DD 1995. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology 136 3639–3647. [DOI] [PubMed] [Google Scholar]
- Takamoto N, Zhao B, Tsai SY & DeMayo FJ 2002. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol 16 2338–2348. [DOI] [PubMed] [Google Scholar]
- Toft D & Gorski J 1966. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci U S A 55 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF & Dey SK 2005. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A 102 14326–14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M, Sanchez-Ferras O & Bouchard M 2018. GATA transcription factors in development and disease. Development 145. [DOI] [PubMed] [Google Scholar]
- Tsai MJ & O’Malley BW 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63 451–486. [DOI] [PubMed] [Google Scholar]
- Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, Hollinshead M, Marsh SGE, Brosens JJ, Critchley HO, et al. 2017. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol 19 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez YM, Mazur EC, Li X, Kommagani R, Jiang L, Chen R, Lanz RB, Kovanci E, Gibbons WE & DeMayo FJ 2015. FOXO1 is required for binding of PR on IRF4, novel transcriptional regulator of endometrial stromal decidualization. Mol Endocrinol 29 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez YM, Wang X, Wetendorf M, Franco HL, Mo Q, Wang T, Lanz RB, Young SL, Lessey BA, Spencer TE, et al. 2018. FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. PLoS Genet 14 e1007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li X, Wang T, Wu SP, Jeong JW, Kim TH, Young SL, Lessey BA, Lanz RB, Lydon JP, et al. 2018. SOX17 regulates uterine epithelial-stromal cross-talk acting via a distal enhancer upstream of Ihh. Nat Commun 9 4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetendorf M, Wu SP, Wang X, Creighton CJ, Wang T, Lanz RB, Blok L, Tsai SY, Tsai MJ, Lydon JP, et al. 2017. Decreased epithelial progesterone receptor A at the window of receptivity is required for preparation of the endometrium for embryo attachment. Biol Reprod 96 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker LH, Murray AA, Matthews R, Shaw G, Williams AR, Saunders PT & Critchley HO 2017. Selective progesterone receptor modulator (SPRM) ulipristal acetate (UPA) and its effects on the human endometrium. Hum Reprod 32 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD & Weinberg CR 1999. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 340 1796–1799. [DOI] [PubMed] [Google Scholar]
- Wilkens J, Male V, Ghazal P, Forster T, Gibson DA, Williams AR, Brito-Mutunayagam SL, Craigon M, Lourenco P, Cameron IT, et al. 2013. Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J Immunol 191 2226–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Sunkel B, Chen Z, Liu X, Ye Z, Li Q, Grenade C, Ke J, Zhang C, Chen H, et al. 2014. Three-tiered role of the pioneer factor GATA2 in promoting androgen-dependent gene expression in prostate cancer. Nucleic Acids Res 42 3607–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SP, Li R & DeMayo FJ 2018. Progesterone Receptor Regulation of Uterine Adaptation for Pregnancy. Trends Endocrinol Metab 29 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SP, Yu CT, Tsai SY & Tsai MJ 2016. Choose your destiny: Make a cell fate decision with COUP-TFII. J Steroid Biochem Mol Biol 157 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinaman MJ, Clegg ED, Brown CC, O’Connor J & Selevan SG 1996. Estimates of human fertility and pregnancy loss. Fertil Steril 65 503–509. [PubMed] [Google Scholar]


