Abstract
A new coronavirus emerged in December 2019 in Wuhan city of China, named as the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), and the disease was called coronavirus disease‐2019 (COVID‐19). The infection due to this virus spread exponentially throughout China and then spread across >205 nations, including the United States (US). Gastrointestinal (GI) endoscopies are routinely performed in the US and globally. Previous reports of isolated infection outbreaks were reported with endoscopes acting as potential vectors. While multidrug‐resistant organisms have been reported to be spread by endoscopes, few cases of viruses such as hepatitis B and C are noted in the literature. COVID‐19 is predominately spread by droplet transmission, although recent evidence has showed that shedding in feces and feco‐oral transmission could also be possible. It is unclear if COVID‐19 could be transmitted by endoscopes, but it could theoretically happen due to contact with mucous membranes and body fluids. GI endoscopies involve close contact with oral and colonic contents exposing endoscopy staff to respiratory and oropharyngeal secretions. This can increase the risk of contamination and contribute to virus transmission. Given these risks, all major GI societies have called for rescheduling elective non‐urgent procedures and perform only emergent or urgent procedures based on the clinical need. Furthermore, pre‐screening of all individuals prior to endoscopy is recommended. This article focuses on the risk of COVID‐19 transmission by GI shedding, the potential role of endoscopes as a vector of this novel virus, including transmission during endoscopies, and prevention strategies including deferral of elective non‐urgent endoscopy procedures.
Keywords: coronavirus, COVID‐19, endoscopy, pandemic, SARS‐CoV‐2
INTRODUCTION
Coronaviruses are a group of medium‐sized positive‐sense single‐stranded RNA viruses with crown‐like structure due to projections (S protein projections) noted over the surface of the virus. 1 There are four genera (α, β, γ, δ) of coronaviruses. Of these, α genera (229 E, NL63) and β genera (HKU1, OC43, MERS, severe acute respiratory syndrome coronavirus‐2 [SARS‐CoV‐2]) affect humans. 2 Some of the coronaviruses (229E, HKU1, NL63, OC43) are known to cause mild disease in humans. The novel 2019 coronavirus identified in China was called SARS‐CoV‐2 and infection caused by it was declared as a pandemic by the World Health Organization (WHO) in March 2020. There have been more than 1,340,500 reported cases of coronavirus disease 2019 (COVID‐19), with 74,442 deaths as of April 06, 2020, and counting. 3 Of note, two previous outbreaks have been reported with coronaviruses. One in 2002–2003, a β genera of coronavirus was identified in Guangdong province of China. 4 This origin of this virus was from bats with civet cats acting as an intermediate host. The SARS infection caused by this genus (SARS‐CoV‐1) affected 8422 individuals with 916 deaths. 4 , 5 Similarly, in 2012, a different coronavirus called Middle East respiratory syndrome coronavirus (MERS‐CoV) emerged in Saudi Arabia. 6 This virus originated from bats with camels as an intermediate host. MERS‐CoV affected 2494 individuals with 858 deaths (Fig. 1). 7 However, the origin of the novel SARS‐CoV‐2 is unclear so far. The structure of this virus is similar to one reported in bats and SARS1CoV‐1. Reports indicate that this virus first affected in the Hubei province of China in individuals exposed to a seafood market. This market traded live animals as well. While the intermediary hosts for SARS‐CoV‐2 are unclear, pangolins and snakes are suspected. 8
Figure 1.
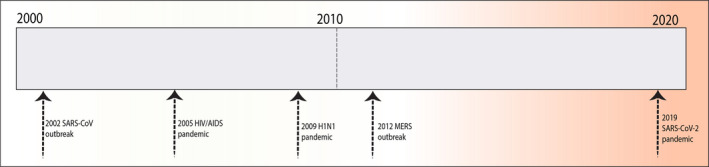
Major global viral outbreaks in the last 20 years. [Information sources: Center for Disease Control and Prevention (CDC) and the World Health Organization (WHO)].
Though the epicenter of this pandemic was in the city of Wuhan, in the Hubei province of China, it soon spread to various other countries quickly (Fig. 2). 9 Now, the primary source of the spread of the virus is in Europe, possibly because of tourism. Initially, the virus was thought to spread from bats via an unknown intermediary to humans, but a human to human spread has become rampant, leading to an exponential increase in the number of cases. 10 Cases continue to rise with a significant number in Japan, South Korea, the United Kingdom, and throughout the rest of the world. COVID‐19 has affected more than 135 countries, and this number is only expected to rise. 10 The exact number of cases is unknown but is likely underrepresented due to a delay in the diagnosis, lack of availability of testing kits, and minor symptoms in 80% of cases.
Figure 2.
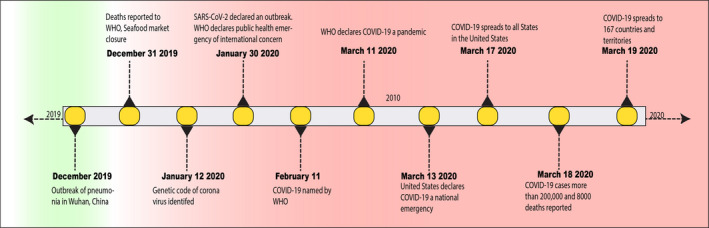
Timeline of the global spread of COVID‐19. [Information sources: Center for Disease Control and Prevention (CDC) and the World Health Organization (WHO)].
Furthermore, in China, the definition of a positive case has been defined as any patient with clinical, radiological, and epidemiological features of COVID‐ 19 rather than based on positive nucleic acid testing. 11 This makes it a formidable task to assess the exact number of cases in China. The doubling time of SARS‐CoV‐2 has been reported as 1.8 days, which is likely to change as human to human transmission rises. 12 The incubation period of the virus is 2–14 days, with a median period of 5 days. The infection transmission occurs from virus droplets by either symptomatic or asymptomatic individuals, and it can remain viable for 2–3 days on plastic and metallic surfaces. The transmission occurs with the contact of these droplets with mucous membranes (nose, mouth, and eyes). However, reports of infection transmission with contaminated water, stools, with subsequent feco‐oral route have also been reported.
COVID‐19 IN GASTROINTESTINAL SECRETIONS
Data on the presence of SARS‐CoV‐2 in stool specimens are sparse. As it is predominately a respiratory pathogen, most of the data is restricted to nasopharyngeal testing. However, few reports of stool testing are noted. 13 Viral shedding in stools is reported both in symptomatic and asymptomatic patients. For example, the virus was present in stool samples at day 17 in the asymptomatic individuals with prior exposure. This positivity was again noted for an additional nine days. 14 Stool specimen positive rate was identified as high as 29%. 15 This concept is critical in understanding the transmission of COVID‐19 as human to human transmission, which could occur between asymptomatic individuals not only from respiratory droplets but potentially from stool specimens too. 16 This indirect transmission could pose a significant challenge to infection control and spread to non‐infected individuals. Reports of positive stool PCR were observed in the 2002–2003 SARS‐CoV‐1 pandemic, making it a potential route of transmission. 17 SARS‐CoV‐1 has been transmitted indirectly through contaminated surfaces and fomites with the survival of the virus noted on these surfaces for hours to days. 5 Intestinal tropism is noted with SARS‐CoV‐1 and could be one of the mechanisms of the gastrointestinal (GI) manifestations of diarrhea. Active viral replication was noted in both small and large intestine biopsies obtained via colonoscopy, which could last for more than 10 weeks after the onset of symptoms. 18 Multiple outbreaks were reported of this virus among healthcare workers, including physicians, nurses, and healthcare assistants. 19 These findings of stool or sewage as a potential source of viral shedding and transmission among previous corona viral pandemics and the current finding of positive stools specimens among asymptomatic COVID‐19 patients raises concern. Further studies are needed to know if stool samples could be utilized as a screening tool for identification of asymptomatic carriers.
Fomites (inanimate objects) are important and have the potential for carrying coronavirus and could play a role in the transmission. MERS‐CoV demonstrated high affinity and survival ex‐vivo and retained its infectivity for up to 60 min after aerosolization. 7 Similarly, SARS‐CoV‐2 survival has been reported on confined public spaces such as restrooms, elevators, and doorknobs, etc. 20 In general, human coronaviruses can remain infectious on fomites for up to 9 days at room temperature (based on the data from 229 E coronavirus). 21 Unfortunately, this duration increases with a fall in temperature. Veterinary coronaviruses (such as mouse hepatitis virus [MHV], canine coronavirus [CCV]) can persist up to 28 days. 21 This duration also increases with a higher inoculum of the virus and higher humidity (50% compared to 30%). 22 During active shedding of the virus, any non‐living object could be contaminated by infected respiratory droplets, which could be transmitted to other individuals. Endoscopes come into direct contact with body fluids and oral‐pharyngeal mucosa, which can contaminate them, especially during active viral shedding. Though the transmissibility of coronaviruses from fomites to hands is unknown, data has been extrapolated from experience with other viruses such as influenza A and parainfluenza. Fomite transmission with influenza A is 31.6% to the hands compared to about 1.5% with parainfluenza 3. 23 , 24 While no reports of viral culture of endoscopic surface or tip have been reported, the possibility of transmission is yet to be researched.
ENDOSCOPY STAFF AND COVID‐19
Gastrointestinal endoscopy suites are a conglomeration of endoscopists, nursing staff, technicians, anesthesia providers, and multiple types of equipment. Due to multiple procedures performed every day involving getting the luminal access at a close distance such as the oral cavity, esophagogastroduodenoscopy, biliary, and colorectum, endoscopy staff get exposed to plentiful respiratory and oropharyngeal microbial flora. In the 2002–2003 SARS‐CoV‐1 pandemic, 21% of individuals affected were healthcare workers. 25 Furthermore, aggressive suctioning, multiple exchanges of catheters via endoscope working channels during these procedure increases the splash rates, which puts the endoscopy personnel at risk. 26 Studies have shown an increased exposure to microorganisms of endoscopists’ face, eyes, and skin. 27 Besides, the contamination has also been observed on endoscopy suite walls and post‐op areas. 28 Some of the procedures can induce coughing (such as esophagogastroduodenoscopy [EGD]) and increase the spread of aerosolized respiratory droplets. In most cases, these splashes are not recognized by the endoscopists. This can put the entire endoscopy suite and staff at risk of transmission since SARS‐CoV‐1, and potentially SARS‐CoV‐2 spread has been estimated to up to 6 feet from the infected individuals via droplets. 29 , 30 Furthermore, the presence of multiple patients for procedures can put them at risk of human to human transmission. Biopsy samples obtained from the infected person can also be a source of infection as active viral replication has been identified during the SARS‐CoV‐1 pandemic in 2002–2003. 18
GASTROINTESTINAL ENDOSCOPY AND VIRAL TRANSMISSION
Contaminated flexible endoscopes have been reported as the vector for transmission of infections for many years. 31 The exact number of infections though unclear, are likely underreported, possibly due to asymptomatic infections, incomplete surveillance, and prolonged latency. Healthcare‐associated infection outbreaks have been reported in the past, forcing the FDA and Centers for Disease Control (CDC) to recommend comprehensive endoscope cleaning strategies with high‐level disinfection and reprocessing. 32 Infections related to the duodenoscopes and echoendoscopes are more frequent than colonoscopes and EGD scopes. While most of these infections occur due to multidrug resistant organisms, the transmission of other microbial organisms has also been reported. 33 The sophisticated design of the elevator channel of duodenoscopes can harbor microorganisms and might be challenging to disinfect. 34 Microbials form a biofilm with a matrix around these endoscopes, which might protect them from drying and penetration of disinfectants. 35 Historically, salmonellae were the most commonly reported organisms from 1974 to 1988. Gradually this trend was replaced by other virulent organisms such as Pseudomonas aeruginosa and carbapenem‐resistant organisms. 36
Infrequently, viral transmission through endoscopes has been reported, especially with hepatitis B and hepatitis C viruses. 37 Hepatitis B transmission occurred after endoscopy with an instrument used in an HBV‐positive patient and immediately after gastroscopy. 38 , 39 Hepatitis C has been shown to be transmitted by infected scopes. Enteroviruses and HIV can also be theoretically transmitted via endoscopes. 40 Artificially contaminated endoscopes with enteroviruses were disinfectants such as glutaraldehyde. 41 Similarly, infectious agents such as prions (proteins without nucleic acid) can be transmitted by the GI tract (given accumulation in the lymphoid tissue), although rare. 42
Though COVID‐19 is predominately spread by respiratory droplets, the virus is also present in infected stools and contaminated water supply. This can result in viral transmission via aerosolization and fecal‐oral route of contamination. 21 SARS‐CoV‐2 virus is detected in the stool samples of infected patients in up to 50% of cases from day 1 to day 7 13 , 14 with or without diarrhea. This is similar to other coronaviruses such as SARS‐CoV‐1 and MERS‐CoV. In SARS‐CoV‐1 infection, persistent stool viral shedding could occur beyond the second week. 17 Viral shedding in stool can also be a potential route of transmission among COVID‐19 patients. As endoscopes are frequently affected by gut flora, this could pose a risk not only to the endoscopists, nurses, and other endoscopy staff but also could be a vector for potential transmission to other patients. 31 While no cases of endoscope‐related COVID‐19 transmission have been reported so far, this risk exists, especially with previous experiences with hepatitis B and C viruses. It is reassuring that post‐cleaning samples of SARS‐CoV‐2 became negative, indicating that current endoscope disinfection techniques are sufficient. 21
NON‐URGENT ELECTIVE ENDOSCOPIES AND COVID‐19
Screening colonoscopy is one of the most common procedures performed in the endoscopy suite on a daily basis. 43 Most of these patients are 50 years or older. This unique subset of patients (often with comorbidities) is at the highest risk due to their increased susceptibility to SARS‐CoV‐2. 10 Elderly individuals with pre‐existing conditions are at the highest risk of developing severe disease and mortality. The fatality rate of 4–11% was observed in adults, but it can be as high as 50–75% in the elderly population with the overall fatality of 2–3%. 44 As stated earlier, the disease symptoms can vary from asymptomatic or minimal symptoms to severe disease with multiorgan dysfunction and death. About 80% of the individuals have a milder form of sickness, and 20% develop severe disease. This can make these asymptomatic patients go unnoticed during the pre‐operative, endoscopy suites, and post‐operative area with increased risk of transmission of disease. 45 Because of these risks, there is a need to call for increased vigilance and careful screening of individuals who are at risk and deferring their procedures until this pandemic is over, especially if they are elective non‐urgent in nature.
In the light of an exponential rise in the COVID‐19 cases in Italy, Spain and other parts of Europe and anticipation of a further rise in the number of cases in the US, the American College of Surgeons have called for minimizing, postponing or canceling elective surgeries, endoscopies and other invasive procedures until the peak of the COVID‐19 transmission has passed. 46 In addition, they recommended minimal use of ICU beds with maximum personal protective equipment. The Surgeon General in the US advised hospitals to postpone elective surgeries. 46 On 15th March 2020, the American Association of Study of Liver Diseases, American College of Gastroenterology, American Gastroenterological Association and the American Society of Gastrointestinal Endoscopy (ASGE) announced a joint message about the evolving COVID‐19 situation. In their message, societies urged GI physicians to reschedule the elective non‐urgent endoscopic procedures. They also advised to further classify the procedures into non‐urgent/postpone and non‐urgent/perform depending on the need of the endoscopy. 45 European Society of Gastrointestinal Endoscopy (ESGE) also issued a position statement about risk of transmission among health care professionals in endoscopy units. 47 , 48 According to this statement, patients should be risk stratified based on their symptoms, travel history, and contact with other COVID‐19 patients. Similarly, use of personal protective equipement (PPE) should be based on the risk status of the patient (high‐risk individuals require two pairs of gloves, respiratory mask and related equipment). A strong consideration should be given to contact patient at least 24 h prior to procedure. In addition, post‐procedure risk management by contacting patients 1–2 weeks after procedure to assess for development of any symptoms. A similar recommendation were made by the Indian Society of Gastroenterology and some of the states in the United States such as New York Society of Gastrointestinal Endoscopy (Table 1).
Table 1.
Recommendation from various gastrointestinal societies around the world
| Recommendation | Joint GI American society † | ESGE position statement | Indian society of gastroenterology |
|---|---|---|---|
| Prescreening | All patients with high‐risk symptoms should be screening and procedures should be deferred if possible | All patients should be screened per local human resources and policies and temporarily postpone elective procedures | All patients should be screened prior to endoscopy |
| Classification and scheduling of procedures |
Reschedule elective non‐urgent procedures Some non‐urgent procedures which of high priority may need to be performed Classification of procedures into non‐urgent postpone and non‐urgent perform is useful |
Reschedule elective non‐urgent procedures. A triage should be applied to health professionals Each GI unit should have a detailed plan for cleaning and disinfecting endoscopy procedure rooms For patients in ICU, GI endoscopy should be performed at bedside |
Procedures should be divided into emergent (life‐saving such as acute upper or lower GI bleeding, impacted foreign body, cholangitis), urgent (conditions that a significant impact may be achieved on outcome in 1‐month time by an endoscopic procedure such as GI cancers, nutritional support for the enteral route, draining malignant biliary obstruction, stenting of malignant luminal obstruction), routine (procedures other than urgent or emergent) |
| Screening on arrival to endoscopy suit | Prescreen all patients for high‐risk exposure or symptoms. History about contact or travel to high‐risk areas or symptoms should be asked |
Triage the patients and staff based on the symptoms. Low‐risk patient (No symptoms, no travel history and no contact with COVID‐individual), high‐risk (positive symptoms with no travel or contact history, positive symptoms with contact/ travel, no symptoms but contact/recent travel to the high‐risk area) Encourage telemedicine care for prescreening |
All patients should be considered low‐risk (no symptoms/ travel/contact with COVID‐19 individuals), intermediate‐risk (symptoms but no contact or stay during last 14 days, absent symptoms but contact or travel present), high‐risk (symptoms with contact/ travel/ stay in high‐risk areas) |
| Personal protective equipment (PPE) | All endoscopy team members should wear PPE | Infection prevention and control measures should be considered. If possible negative‐pressure room should be used. For high‐risk patients recommend respiratory PPE and two pairs of gloves | Recommend minimal stay in the endoscopy rooms, use of PPE throughout the procedure, use of chemical disinfectants. Use of disposable endoscopic accessories as much as possible. Use of standard endoscopy room disinfection policy |
| Follow‐up | Phone follow‐up 7–14 days after the procedure | Phone follow‐up 7–14 days after the procedure for any new diagnosis | Non‐urgent procedures should be reevaluated as outpatient. Use of digital media |
| Special groups | Patients on immunosuppressive drugs should continue medications and assess risk‐benefit ratio after discussing with their health care provider | N/A | Patients with immunosuppression (chemotherapy/ steroids) should contact health care provider. Postponement of primary prophylaxis for variceal ligation for 4–6 weeks |
ESGE, European society of gastrointestinal endoscopy; GI, Gastrointestinal.
Joint GI American societies‐ American Society of Gastrointestinal Endoscopy (ASGE), American Association for the Study of Liver Diseases (AASLD), American College of Gastroenterology (ACG) and American Gastroenterology Association (AGA).
The American Society of Gastrointestinal Endoscopy also released recommendations for endoscopy units in the era of COVID‐19 patients recently. 49 The CDC and WHO provided information on stratifying patients into the highest and high risk based on their recent travel. 50 Endoscopy centers have started to stratify the patients based on the symptoms and risk with nurse‐directed triage protocol. One of the most important aspects is the proper use of PPE with the use of gloves, face shield, gowns, and respiratory protective equipment. 51 The standard face shield might be ineffective in blocking small viral particles and hence a respirator (N95) is recommended to filter airborne particles. Safe distancing of accompanying personnel including caregivers and relatives, is recommended as well. 49 Disinfection of the endoscopy equipment should be performed as they can potentially become vectors. Strict implementation of guidelines on the infection control in the endoscopy unit is strongly recommended. 41 , 52 If possible, procedures should be performed in negative‐pressure rooms to minimize transmission. 52
PRE‐SCREENING OF THE INDIVIDUALS BEFORE ENDOSCOPY‐ IS IT ENOUGH?
Pre‐screening of patients undergoing elective endoscopy has been suggested by ASGE. Patients are classified into low, intermediate, or high risk based on their history (contact with an individual with positive SARS‐COV‐2, visitation to highest or high‐risk country), symptoms (fever, shortness of breath, cough, diarrhea). 49 While these actions minimize the risk of individuals undergoing endoscopy, the success rate in preventing transmission is unknown. Until more data is available, these recommendations should be used at the full extent to minimize the transmission risk. Besides, evaluation of endoscopy staff for clinical symptoms and exposure history is paramount to decrease the spread from staff to patients and among staff. Some of the characteristics of COVID‐19, which make the transmission prevention challenging include: asymptomatic individuals could shed the virus, onset of transmission before the start of symptoms, relatively non‐specific symptoms, and ongoing shedding after the resolution of symptoms. 53 If a member of staff develops symptoms of fever and upper respiratory infection, or exposure to a positive case, the local healthcare safety protocols should be followed while keeping them off work. Currently, all contacts are being closely monitored for the development of symptoms of COVID‐19. 50
HAND HYGIENE AND COVID‐19
Coronaviruses can persist on different types of fomites, including steel, aluminum, wood, paper, glass, plastic, silicon rubber, disposable gown, and ceramic. Viral presence and duration are dependent on the size of inoculum, temperature, and type of strain. 21 Multiple biocidal agents such as ethanol, 2‐propanol, sodium hypochlorite, glutaraldehyde, povidone‐iodine, and hydrogen peroxide have been used for disinfection. 54 Ethanol (62–71% concentration), 2% glutaraldehyde, and 0.1–0.5% sodium hypochlorite are commonly used and can reduce the concentrations of coronavirus within 1 min exposure time. Universal hand washing and the use of alcohol‐based hand rubs while entering and exiting patient rooms are highly recommended. Effective hand hygiene decreases the transmission of infection and has proven benefit in SARS‐CoV‐1. 55 , 56
CONCLUSION
COVID‐19 emerged as a small outbreak in China in December 2019 and soon spread to more than 205 countries worldwide. It has become a pandemic, and the community spread from human to human continues to rise. GI endoscopes could pose a potential risk for transmission of viruses, and they could also theoretically transmit COVID‐19, especially in patients with fecal shedding. Even individuals with minimal or no symptoms have shown to shed virus in feces, which can last many days after resolution of symptoms. As the COVID‐19 pandemic continued to rise and spread across nations, risk stratification of individuals undergoing endoscopy is paramount. The use of objective data to stratify patients needing endoscopy and deferral of elective procedures to decrease the spread of this virus could assist in decreasing the peak of this virus spread. Finally, adherence to high‐level disinfection, safe distancing, and strict hand hygiene are warranted.
Conflicts of Interest
Authors declare no conflicts of interest for this article.
Funding Information
None.
Acknowledgment
None.
References
- 1. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet 2020; 395: 470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East respiratory syndrome coronavirus: Another zoonotic betacoronavirus causing SARS‐like disease. Clin Microbiol Rev 2015; 28: 465–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Coronavirus Death .Coronavirus Death Toll and Trends ‐ Worldometer 2020. [Cited 6 Apr 2020.] Available from URL: https://www.worldometers.info/coronavirus/coronavirus‐death‐toll/.
- 4. Chan‐Yeung M, Xu RH. SARS: Epidemiology. Respirology 2003; 8(Suppl): S9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen YC, Huang LM, Chan CC et al. SARS in hospital emergency room. Emerg Infect Dis 2004; 10(5): 782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MERS .WHO | Middle East respiratory syndrome coronavirus (MERS‐CoV). World Health Organization, 2020. [Cited 10 Mar 2020 01:10:51]. Available from URL: https://www.who.int/emergencies/mers‐cov/en/. [Google Scholar]
- 7. Fehr AR, Channappanavar R, Perlman S. Middle east respiratory syndrome: Emergence of a pathogenic human coronavirus. Annu Rev Med 2017; 68: 387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu P, Chen W, Chen JP. Viral metagenomics revealed sendai virus and coronavirus infection of Malayan Pangolins (Manis javanica). Viruses 2019; 11: 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. COVID ‐19 S . Situation Summary | CDC, 2020. [Cited 1 Apr 2020.] Available from URL: https://www.cdc.gov/coronavirus/2019‐ncov/cases‐updates/summary.html.
- 11. Reports WS . Novel Coronavirus (2019‐nCoV) situation reports, 2020. [Cited 1 Apr 2020.] Available from URL: https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports/.
- 12. Li Q, Guan X, Wu P et al. Early transmission dynamics in wuhan, china, of novel coronavirus‐infected pneumonia. N Engl J Med 2020; 382: 1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young BE, Ong SWX, Kalimuddin S et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. JAMA 2020; 323(15): 1488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang A, Tong ZD, Wang HL et al. Detection of novel coronavirus by RT‐PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis 2020; 26(6): 1337–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang W, Xu Y, Gao R et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA 2020;e203786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gu J, Han B, Wang J. COVID‐19: Gastrointestinal manifestations and potential fecal‐oral transmission. Gastroenterology 2020; 158(6): 1518–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang XW, Li JS, Guo TK et al. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital. J Virol Methods 2005; 128: 156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leung WK, To KF, Chan PK et al. Enteric involvement of severe acute respiratory syndrome‐associated coronavirus infection. Gastroenterology 2003; 125: 1011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ho AS, Sung JJ, Chan‐Yeung M. An outbreak of severe acute respiratory syndrome among hospital workers in a community hospital in Hong Kong. Ann Intern Med 2003; 139: 564–7. [DOI] [PubMed] [Google Scholar]
- 20. Cai J, Sun W, Huang J, Gamber M, Wu J, He G. Indirect virus transmission in cluster of COVID‐19 cases, Wenzhou, China, 2020. Emerg Infect Dis 2020; 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 2020; 104: 246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ijaz MK, Brunner AH, Sattar SA, Nair RC, Johnson‐Lussenburg CM. Survival characteristics of airborne human coronavirus 229E. J Gen Virol 1985; 66(Pt 12): 2743–8. [DOI] [PubMed] [Google Scholar]
- 23. Bean B, Moore BM, Sterner B, Peterson LR, Gerding DN, Balfour HH Jr. Survival of influenza viruses on environmental surfaces. J Infect Dis 1982; 146: 47–51. [DOI] [PubMed] [Google Scholar]
- 24. Ansari SA, Springthorpe VS, Sattar SA, Rivard S, Rahman M. Potential role of hands in the spread of respiratory viral infections: Studies with human parainfluenza virus 3 and rhinovirus 14. J Clin Microbiol 1991; 29: 2115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang XuH, Rebaza A, Sharma L, Dela Cruz CS. Protecting health‐care workers from subclinical coronavirus infection. Lancet Respir Med. 2020; 8: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perisetti A, Garg S, Inamdar S, Tharian B. Role of face mask in preventing bacterial exposure to the endoscopist's face. Gastrointest Endosc 2019; 90: 859. [DOI] [PubMed] [Google Scholar]
- 27. Mohandas KM, Gopalakrishnan G. Mucocutaneous exposure to body fluids during digestive endoscopy: The need for universal precautions. Indian J Gastroenterol 1999; 18: 109–11. [PubMed] [Google Scholar]
- 28. Johnston ER, Habib‐Bein N, Dueker JM et al. Risk of bacterial exposure to the endoscopist's face during endoscopy. Gastrointest Endosc 2019; 89: 818–24. [DOI] [PubMed] [Google Scholar]
- 29. Wong TW, Lee CK, Tam W et al. Cluster of SARS among medical students exposed to single patient, Hong Kong . Emerg Infect Dis 2004; 10: 269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am J Infect Control 2007; 35(10 Suppl 2): S65–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rahman MR, Perisetti A, Coman R, Bansal P, Chhabra R, Goyal H. Duodenoscope‐associated infections: Update on an emerging problem. Dig Dis Sci 2019; 64: 1409–18. [DOI] [PubMed] [Google Scholar]
- 32. US_FDA . Infections associated with reprocessed duodenoscopes | FDA: @US_FDA, 2020. [Cited 1 Apr 2020.] Available from URL: https://www.fda.gov/medical‐devices/reprocessing‐reusable‐medical‐devices/infections‐associated‐reprocessed‐duodenoscope.
- 33. Rutala WA, Weber DJ. Outbreaks of carbapenem‐resistant enterobacteriaceae infections associated with duodenoscopes: What can we do to prevent infections? Am J Infect Control 2016; 44(5 Suppl): e47–51. [DOI] [PubMed] [Google Scholar]
- 34. Muscarella LF. Automatic flexible endoscope reprocessors. Gastrointest Endosc Clin N Am 2000; 10: 245–57. [PubMed] [Google Scholar]
- 35. Pajkos A, Vickery K, Cossart Y. Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? J Hosp Infect 2004; 58: 224–9. [DOI] [PubMed] [Google Scholar]
- 36. Nelson DB, Muscarella LF. Current issues in endoscope reprocessing and infection control during gastrointestinal endoscopy. World J Gastroenterol 2006; 12: 3953–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kovaleva J, Peters FT, van der Mei HC, Degener JE. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clin Microbiol Rev 2013; 26: 231–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Birnie GG, Quigley EM, Clements GB, Follet EA, Watkinson G. Endoscopic transmission of hepatitis B virus. Gut 1983; 24: 171–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seefeld U, Bansky G, Jaeger M, Schmid M. Prevention of hepatitis B virus transmission by the gastrointestinal fibrescope. Successful disinfection with an aldehyde liquid. Endoscopy 1981; 13: 238–9. [DOI] [PubMed] [Google Scholar]
- 40. Mele A, Spada E, Sagliocca L et al. Risk of parenterally transmitted hepatitis following exposure to surgery or other invasive procedures: Results from the hepatitis surveillance system in Italy. J Hepatol 2001; 35: 284–9. [DOI] [PubMed] [Google Scholar]
- 41. Beilenhoff U, Biering H, Blum R et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) ‐ Update 2018. Endoscopy 2018; 50: 1205–34. [DOI] [PubMed] [Google Scholar]
- 42. Bramble MG, Ironside JW. Creutzfeldt‐Jakob disease: Implications for gastroenterology. Gut 2002; 50: 888–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Joseph DA, Meester RG, Zauber AG et al. Colorectal cancer screening: Estimated future colonoscopy need and current volume and capacity. Cancer 2016; 122: 2479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Worldometer . Coronavirus Update (Live): 162,685 Cases and 6,069 Deaths from COVID‐19 Virus Outbreak – Worldometer, 2020. [Cited 6 Apr 2020.] Available from URL: https://www.worldometers.info/coronavirus/.
- 45. Bezzara J, Pochapin M, El‐Serag H, Vargo J. Joint GI society message on COVID‐19 ‐ American College of Gastroenterology, 2020. [Cited 15 Mar 2020]. Available from URL: https://gi.org/2020/03/15/joint‐gi‐society‐message‐on‐covid‐19/.
- 46. Hoyt DB. Information for surgeons, 2020. [Cited 1 Apr 2020.] Available from URL; https://www.facs.org/about‐acs/covid‐19/information‐for‐surgeons.
- 47. ESGE . ESGE and ESGENA position statement on gastrointestinal endoscopy and the COVID‐19 pandemic. European Society of Gastrointestinal Endoscopy (ESGE), 2020. [Cited 5 Apr 2020.] Available from URL: https://www.esge.com/esge‐and‐esgena‐position‐statement‐on‐gastrointestinal‐endoscopy‐and‐the‐covid‐19‐pandemic/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. SGE . Society of Gastrointestinal Endoscopy of India, 2020. [Cited 5 Apr 2020.] Available from URL: https://www.sgei.co.in/#.
- 49. Repici A, Maselli R, Colombo M et al. Coronavirus (COVID‐19) outbreak: What the department of endoscopy should know. Gastrointest Endosc 2020. 10.1016/j.gie.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. COVID ‐19 C . Travel | CDC: @CDCgov, 2020. [Cited 14 Mar 2020 T12:58:15Z]. Available from URL: https://www.cdc.gov/coronavirus/2019‐ncov/travelers/index.html.
- 51. @CDCgov PPE . Healthcare supply of personal protective equipment | CDC: @CDCgov, 2020. [Cited 12 Mar 2020 T08:26: 28Z]. Available from URL: https://www.cdc.gov/coronavirus/2019‐ncov/hcp/healthcare‐supply‐ppe‐index.html.
- 52. Calderwood AH, Day LW, Muthusamy VR et al. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc 2018; 87: 1167–79. [DOI] [PubMed] [Google Scholar]
- 53. Jin YH, Cai L, Cheng ZS et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019‐nCoV) infected pneumonia (standard version). Mil Med Res 2020; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Siddharta A, Pfaender S, Vielle NJ et al. Virucidal activity of World Health Organization‐recommended formulations against enveloped viruses, including Zika, Ebola, and Emerging Coronaviruses. J Infect Dis 2017; 215: 902–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Al Awaidy ST, Khamis F. Middle east respiratory syndrome coronavirus (MERS‐CoV) in Oman: Current situation and going forward. Oman Med J 2019; 34: 181–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wong TW, Tam WW. Handwashing practice and the use of personal protective equipment among medical students after the SARS epidemic in Hong Kong. Am J Infect Control 2005; 33: 580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


