Abstract
The current limitations of cancer diagnosis and molecular profiling based on invasive tissue biopsies or clinical imaging have led to the development of the liquid biopsy field. Liquid biopsy includes the isolation of circulating tumor cells (CTCs), circulating free or tumor DNA (cfDNA or ctDNA), extracellular vesicles (EVs), and tumor-educated platelets (TEPs) from body fluid samples and their molecular characterization to identify biomarkers for early cancer diagnosis, prognosis, therapeutic prediction, and follow-up. These innovative biosources show similar features as the primary tumor from where they originated or interacted. This review describes the different technologies and methods used for processing these biosources as well as their main clinical applications with their advantages and limitations.
Cancer diagnosis faces several technological and clinical problems. In most cancer subtypes, tissue biopsy represents the “gold standard” for diagnosis and biomarker profiling. However, this is an invasive procedure. Moreover, the natural cancer course is characterized by the formation of distant metastases and the development of resistance against chemotherapy. These issues challenge the current approach because tissue biopsy only reflects the cancer features at the specific time of the intervention, and obtaining biopsies of metastatic tumors is humanly unviable, although theoretically possible. Medical imaging is a less invasive alternative for real-time follow-up, but it does not allow molecular profiling.
“Liquid biopsy” is becoming a viable alternative for real-time cancer follow-up in patients, and for assessing biomarkers that are usually tested only in tissue biopsies. Liquid biopsy is a minimally invasive procedure based on sampling blood, cerebrospinal fluid, urine, sputum, ascites, and theoretically any other body fluid. The term liquid biopsy was first used for circulating tumor cells (CTCs) in the bloodstream (Pantel and Alix-Panabières 2010), but it has broadened to include circulating free DNA (cfDNA), circulating tumor DNA (ctDNA), extracellular vesicles (EVs), and more recently, tumor-educated platelets (TEPs).
In this review, we describe the different circulating cells that can be analyzed with the liquid biopsy procedure, and their technical, biological, and clinical advantages and limitations.
CIRCULATING TUMOR CELLS
Cancer cell dissemination through the body and the metastatic cascade begin with the active release of the most aggressive tumor cells in the bloodstream and/or lymphatic vessels. These cells are called CTCs (Pantel and Alix-Panabières 2010). CTC enumeration and analysis give important information on the tumor molecular profile, and can lead to the identification of metastasis initiating cells (MICs). There is a direct correlation between CTC number in blood, the expression of specific biomarkers (e.g., cancer stem cell markers), and the formation of distant metastases (Kang and Pantel 2013; Zhang et al. 2013; Alix-Panabières et al. 2017). Therefore, CTC detection/analysis contributes to the early discovery of metastatic lesions and to precision oncology (e.g., prognostic information, patient stratification for targeted therapies, real-time monitoring of treatment efficacy, identification of therapeutic targets, and resistance mechanisms).
CTCs represent the ideal biosource for solid cancer characterization and monitoring because they can be used for genome, proteome, transcriptome, and secretome analyses. Although CTCs are rare in the bloodstream (Pantel and Alix-Panabières 2010), recent high-technology methods allow the detection and characterization of single CTCs (Pantel and Alix-Panabières 2019).
Technological Aspects
As CTC number in blood is limited, CTC detection is always combined with a first enrichment step based on the biological (e.g., expression of surface proteins/receptors) or physical (e.g., size, deformability, density, and electric charge) properties that allow distinguishing CTCs from the other cells in the blood (Pantel and Alix-Panabières 2019). Approaches based on the “biological features” rely on the different expression of membrane proteins between blood cells and CTCs for positive (expression of epithelial markers, such as epithelial cell adhesion molecule—EpCAM—in carcinoma cells) or negative enrichment (Allard et al. 2004; Schulze et al. 2013; Pantel and Alix-Panabières 2019). The CellSearch system (Menarini Silicon Biosystems) is the only method cleared by the United States Food and Drug Administration (FDA) for CTC analysis. In this system, CTCs are enriched based on EpCAM expression and then identified using anti-CK8, 18, and 19, and -CD45 antibodies and DAPI staining. Many other technologies are available (e.g., microfluidic devices [Dong et al. 2013] and intravascular wires [Saucedo-Zeni et al. 2012]) that use antibodies against EpCAM and/or other epithelial surface markers. Very recently, a temporary indwelling intravascular device to capture CTCs from bloodstream and then return erythrocytes and leukocytes to the normal circulation has been developed. This apparatus allows the continuous capture of CTCs in dogs for ∼2 h. It must now be tested in patients with cancer (Kim et al. 2019). Nevertheless, these methods could miss CTCs that undergo epithelial-to-mesenchymal transition (EMT) and that do not express EpCAM. This can be avoided by using negative enrichment methods in which the blood sample is depleted of blood cells, such as leukocytes, by immuno-targeting the CD45 marker that is expressed by all leucocytes but not by solid cancer cells (Iinuma et al. 2000; Bilkenroth et al. 2001).
Numerous marker-independent techniques based on CTC “physical properties” (density, size, deformability, and electric charge) have been developed as well. For example, CTCs, which are epithelial cells, should be bigger than leukocytes and erythrocytes, which have well-defined sizes. Therefore, cell size is used for CTC enrichment by different filtration systems (Hao et al. 2018). Label-free enrichment approaches avoid the biological bias linked to the variability of cell biomarker expression associated with tumor heterogeneity.
After positive/negative enrichment, CTCs need to be clearly differentiated from the remaining leukocytes and endothelial cells. This is usually performed using immunofluorescence methods, similar to the identification step in the CellSearch system. Nucleic acid-based methods can be an alternative to immunological assays. These methods use epithelial gene-specific primers, but their low specificity could lead to false positive results (Pantel et al. 2008; Fehm et al. 2009). Another CTC identification approach focuses on their viability by detecting proteins secreted, released or shed from cancer cells cultured for 24–48 h. This unique functional assay is called Epithelial ImmunoSPOT (EPISPOT) (Soler et al. 2017). A completely new microdroplet technology to detect viable CTCs at the single-cell level is currently under development. This optimized EPISPOT assay is called EPIDROP (Pantel and Alix-Panabières 2019).
After enrichment and identification, CTCs can be characterized at different level: (1) expression of specific surface markers, such as protein death ligand 1 (PD-L1; an immune checkpoint regulator and therapeutic target) (Mazel et al. 2015) and HER2 in breast and gastric carcinomas (Riethdorf et al. 2010; Jaeger et al. 2017); (2) whole-genome or transcriptome analysis in single cells; and (3) identification of metastasis-competent CTCs by in vitro expansion or injection of CTCs in mouse models (Pantel and Alix-Panabières 2019). Single CTCs can be analyzed manually by micromanipulation, or automatically by trapping and moving cells in dielectrophoretic cages (e.g., DEPArray) (Abonnenc et al. 2013).
Biology
Extensive research on the biology of cancer cell dissemination and metastasis formation has brought insights into how CTCs survive in bloodstream. First, EMT is a complex process characterized by down-regulation of epithelial proteins and up-regulation of mesenchymal proteins. This process supports migration of epithelial tumor cells and is thought to play a crucial role in promoting metastasis formation (Nieto et al. 2016). More recent studies have focused on the epithelial-to-mesenchymal plasticity of cells with stem cell characteristics. They showed that cancer epithelial cells may acquire some mesenchymal features and reverse this state in the colonized organs, under the influence of stemness effectors (Alonso-Alconada et al. 2014). Moreover, in normal tissue, loss of adhesion to the extracellular matrix, induces cell death in anchorage-dependent cells, a process called “anoikis” (Kim et al. 2012; Alix-Panabières and Pantel 2014). On the other hand, tumor cells that have acquired anoikis resistance can survive detachment from their primary site and can travel through the circulatory and lymphatic systems and reach ectopic locations (Simpson et al. 2008). Once in the circulation, CTCs face many stresses inherent to this new compartment, particularly the immune system attack. Much research has focused on understanding the immune-suppressive mechanisms that allow CTC escape from the immune system (Mohme et al. 2017). These works led to the discovery of some biomarkers, such as CD47, CTLA4, PD-L1, and PD1 (Steinert et al. 2014), and to the development of immune checkpoint inhibitor-based therapies, with remarkable clinical response in different malignancies (Ohaegbulam et al. 2015). In addition, cancer cells can associate with other cells and form microemboli to survive in the bloodstream environment. Different cell association mechanisms have been described: (1) CTC clusters that show higher metastatic potential through increased cell survival and reduced apoptosis (Aceto et al. 2014; Gkountela et al. 2019; Szczerba et al. 2019); (2) partnership with reactive platelets, used as a shield or camouflage against the immune system assault (Lou et al. 2015); and (3) clustering with white blood cells, mainly neutrophils, that might promote cell cycle progression, leading to more efficient metastasis formation (Szczerba et al. 2019).
Clinical Relevance
The clinical relevance of CTC detection for prognosis and outcome prediction was validated for metastatic breast (Cristofanilli et al. 2019), prostate (Scher et al. 2015), and colorectal cancer (Huang et al. 2015). However, despite the many clinical validation studies, CTCs have not been included in clinical guidelines yet. Although CTC enumeration can improve tumor staging and contribute to the early assessment of therapy effects, their clinical usage needs to be proved (i.e., their usefulness in decision-making concerning the adoption or rejection of a therapeutic action). Currently, two ongoing French interventional studies investigate the clinical usage of CTCs for stratification of patients with metastatic breast and prostate cancer: (1) STIC CTC METABREAST (NCT01710605), and (2) TACTIK (NCT03101046), respectively.
In the clinic, CTCs could be used also for (1) minimal residual disease (MRD) detection and characterization (Pantel and Alix-Panabières 2019), (2) early cancer diagnosis (Ilie et al. 2014; Castro et al. 2018), and (3) prediction of response/resistance to treatment (Mathew et al. 2015).
Moreover, the possibility of real-time monitoring of cancer progression by capturing CTCs in the bloodstream at the exact moment of metastasis initiation gives to this biosource a great potential as liquid biopsy. Finally, CTCs include also MICs that are at the origin of cancer relapse. The main goal is now to identify and specifically eradicate them (Cayrefourcq et al. 2015; Soler et al. 2018).
CIRCULATING TUMOR DNA
CtDNA is DNA that is actively secreted or/and originates from apoptotic or necrotic cancer cells and released directly in the bloodstream (or another biological fluid) (Elazezy and Joosse 2018; Jeppesen et al. 2019; Pantel and Alix-Panabières 2019). It represents only a small fraction of all circulating free DNA (cfDNA) because most cfDNA comes from normal cells in physiological conditions. Mandel and Metais (1948) were the first to describe nucleotide acids in blood in 1948, and Stroun and colleagues identified ctDNA in 1989 (Stroun et al. 1989). Tumor mutations can be detected by sequencing ctDNA, and this allows differentiating ctDNA from cfDNA. Thus, ctDNA is a suitable biosource to analyze the cancer genome (DNA mutations) for diagnosis, prognosis, and prediction of the therapeutic response. Furthermore, as ctDNA can be released from all metastatic sites, it may represent not only the genomic landscape of the primary tumor, but also the intratumor clonal heterogeneity.
Technological Aspects
The preanalytical step (e.g., sample collection) is crucial for ctDNA processing/analysis. Plasma samples should be processed within 6 h after collection to avoid leukocyte degradation that might increase the amount of nontumor cfDNA (Kang et al. 2016; Nikolaev et al. 2018). Leukocyte-stabilizing collection tubes are now commercially available and allow sample storage for up to 48 h (Kang et al. 2016).
There are several cfDNA extraction methods based on centrifugation, immunomagnetic beads, silica column-based enrichment, polymer-mediated enrichment, phenol–chloroform-based extraction, and vacuum generation (Sherwood et al. 2016; Sorber et al. 2017; Lee et al. 2018; Pandoh et al. 2019). However, these methods still require further standardization and harmonization. The choice will depend on the desired DNA purity and the required amount of automatization. As the current DNA extraction methods do not discriminate between ctDNA and cfDNA, DNA extraction must be followed by detection of genomic variations in ctDNA. To this aim, several approaches can be used: next-generation sequencing (NGS), digital polymerase chain reaction (PCR) platforms, real-time PCR, mass-spectrometry, and hypermethylation analysis (Bracht et al. 2018; Elazezy and Joosse 2018; Pantel and Alix-Panabières 2019). The choice is based on the number of genes to be analyzed, the amount of ctDNA and cfDNA in a sample, the nature of the genetic or genomic alteration, and the cost.
Real-time PCR is the most widely used method for liquid biopsy analysis, particularly allele-specific PCR (AS-PCR). These techniques are fast, simple, and cost-effective. However, they display low sensitivity and can detect only few mutations at the same time (Cabel et al. 2018; Pantel and Alix-Panabières 2019). Therefore, there has been a keen interest in improving their sensitivity, and the number of DNA alterations that can be concomitantly tested. NGS allows identifying all point mutations, but is limited by the ctDNA fragment quality, high cost and, and interpretation of complex results. Conversely, digital PCR methods, such as digital-droplet PCR and BEAMing (beads, emulsions, amplification, and magnetics), display high sensitivity and specificity, but detect only a small number of variants and are expensive (Elazezy and Joosse 2018). Recently, mass spectrometry-based methods have been developed: surface-enhanced Raman spectroscopy PCR and UltraSEEK. Although they still require further validation, they show high sensitivity and specificity, requiring low amount of DNA (Mosko et al. 2016; Wee et al. 2016). Finally, the ctDNA methylation status can be assessed by methylation-specific PCR that requires large amounts of ctDNA (Lissa and Robles 2016).
Biology
In normal conditions, cfDNA is mainly released by hematopoietic cells (Sun et al. 2015; Tug et al. 2015), possibly following cell apoptosis and necrosis, but also by active secretion (Diaz and Bardelli 2014; Jeppesen et al. 2019). Most cfDNA fragments are around 143–180 bp in length, which corresponds to the DNA length in a nucleosome (Lo et al. 2010; Thierry et al. 2010), and have a half-life in the bloodstream of 16 min to 2.5 h (Wan et al. 2017). The function of cfDNA (or ctDNA in cancer) is still not clear. It might have a role in the accumulation of macrophages and inflammation after cell necrosis. It might also represent a horizontal gene transfer mechanism between cancer cells (Wan et al. 2017). However, the higher amount of cfDNA found during pregnancy or exercise suggests that its functions might not be only limited to pathological conditions (Lo et al. 2007; Tug et al. 2015).
Clinical Relevance
The only ctDNA-based tests cleared by FDA are the Septin-9 (SEPT9) gene methylation assay for colorectal cancer screening (Song et al. 2017), and the AS-PCR–based assay for the detection of epidermal growth factor receptor (EGFR) mutations in non-small-cell lung cancer (NSCLC) to determine whether a patient is candidate for EGFR tyrosine-kinase inhibitor therapy (Douillard et al. 2014; Weber et al. 2014; Merker et al. 2018). The FDA has also granted the status of “breakthrough device” to CancerSEEK, a method based on the assessment of circulating proteins and ctDNA by mass spectrometry for the detection of early-stage cancer in asymptomatic patients older than 65 years (Cohen et al. 2018). Moreover, ctDNA is used for MRD detection, and a relationship between the tumor stage and amount of ctDNA in blood has been highlighted (Pantel and Alix-Panabières 2019). However, today, ctDNA clinical usage for MRD monitoring has not been established yet, because of the high variability among patients, cancer types, and methods used. Several studies showed the use of ctDNA in different cancer types and at all stages. However, no robust evidence exists on the clinical validity and usage of ctDNA, outside clinical trials (Merker et al. 2018).
Currently, the number of academic and privately funded research projects/studies to find new applications for ctDNA in cancer is increasing worldwide. Nevertheless, cfDNA applications are not limited to cancer, as indicated by its use as a noninvasive prenatal test (Allyse et al. 2015). Therefore, ctDNA might become a common tool in clinical and pathological laboratories worldwide.
EXTRACELLULAR VESICLES
The International Society for Extracellular Vesicles defined extracellular vesicles (EVs) as particles that are naturally released from the cell, delimited by a lipid bilayer and not replicating (Thery et al. 2018). EVs include exosomes, microvesicles, microparticles, oncosomes, apoptotic bodies, lipoprotein particles, and any other nonviral vesicle secreted or shed from cells. They all have specific physiological and pathological functions. Exosomes are the most studied EVs. They are an EV subgroup that has been linked to numerous processes associated with cell-to-cell communications, such as cell proliferation, cell migration, cancer metastasis, and immunomodulatory activity. Exosomes, like other EVs, can be found in blood, urine, saliva, and any other biological fluid. It has been proposed that exosomes can be used as carry-on particles to deliver therapeutic molecules to specific tissues.
In the field of liquid biopsy, the terms EVs and exosomes are often used interchangeably; however, exosomes have an endosomal origin and belong to the small EV (sEV) subgroup, with a size smaller than 100 or 200 nm (Thery et al. 2018). As most EV isolation protocols are based on size and density and not on subcellular origin, it is important to note that exosome is not synonymous of sEV or EV. Nevertheless, it is technically challenging to isolate pure exosomes, and for a practical clinical “liquid biopsy”-based test, many groups call exosomes all particles with a size between 30 and 150 nm (Couto et al. 2018) and with a density between 1.15 and 1.19 g/mL in a gradient (Théry et al. 2006; Chiou and Ansel 2016). In this review, we used the term “exosomes” only for particles of endosomal origin or isolated with high purity methods, whereas we used EVs in all other cases.
Technological Aspects
The gold standard method for EV isolation is ultracentrifugation (Li et al. 2017) that consists in several centrifugation steps (of >100,000 g) of fluids containing EVs. Density gradient ultracentrifugation can be divided in isopycnic and rate-zonal centrifugation. These techniques use a density gradient inside the ultracentrifugation tube to trap specific EVs according to their density (e.g., exosomes) by directly recovering them from the corresponding gradient area (Li et al. 2017). These methods offer the highest EV purity, but a low yield. In addition, they are time-consuming and require the acquisition of an ultracentrifugation equipment (Shao et al. 2016). To improve EV isolation and their clinical applications, other methods have been developed: some are based on size, such as size exclusion chromatography (Böing et al. 2014) and ultrafiltration (Vergauwen et al. 2017), whereas others use immunomagnetic beads against EV surface markers, for instance tetraspanins in exosomes (Oksvold et al. 2015). These methods offer a higher EV yield, can be implemented also in the clinic without the need of special equipment, and are less time-consuming. However, the purity of exosomes and EVs isolated with these methods can be variable (Tauro et al. 2012; Shao et al. 2016; An et al. 2018). Recently, other methods are developed based on microchips or acoustic waves (Wu et al. 2017) to improve the capture of pure EVs. Promising results have been obtained also by combining these different methods (Lobb et al. 2015; Benedikter et al. 2017; Li and Nabet 2019).
Biology
EVs have different functions in cancer and other diseases. Hoshino and colleagues showed that primary tumors secrete exosomes with specific integrin expression profiles at their surface that are associated with formation of metastases in lung or liver. Moreover, their uptake by cells in such organs establishes a premetastatic niche (Hoshino et al. 2015). Exosomes secreted by cultured melanoma or colorectal cancer cells induce higher endothelial permeability (Peinado et al. 2012; Schillaci et al. 2017), and those secreted by fibrosarcoma cells have chemotactic properties (Sung and Weaver 2017). Similarly, exosomes released by the amoeba Dictyostelium discoideum form a kind of pathway that can be directly followed by other cells in vitro (Kriebel et al. 2018).
EV functions are not limited to cancer; indeed, most exosomes in the bloodstream originate from normal physiological events (Johnstone et al. 1987; Italiano et al. 2010; Tao et al. 2017). EVs are also implicated in several inflammatory and chronic diseases (Console et al. 2019) and neurodegenerative diseases (Soria et al. 2017), just to mention a few examples.
EVs and exosomes can contain proteins (Greening et al. 2015), lipids (Skotland et al. 2019), RNA (Janas et al. 2015), and DNA (Thakur et al. 2014). In exosomes, the membrane phospholipid composition/structure reflects that of the membrane of the cell from which they originated (Johnstone et al. 1987; Skotland et al. 2019). Consequently, treatment outcome and prognosis could be predicted by assays based on the detection and analysis of exosomes. Moreover, they may be used to deliver therapeutic agents directly into diseased cells by taking advantage of their specific cell–cell interactions (Luan et al. 2017; Bunggulawa et al. 2018).
Clinical Relevance
The use of EVs in the clinical practice is not established yet; however, EVs and exosomes can bring important information for cancer prognosis and diagnosis and are a candidate predictive biomarker. For instance, EVs in combination with other biomarkers (e.g., ctDNA) have been used to improve the detection of EGFR mutations in lung cancer (Castellanos-Rizaldos et al. 2018; Krug et al. 2018). The National Comprehensive Cancer Network has included EVs (exosomes) in their guidelines (Carroll and Mohler 2018) for early prostate cancer detection (gene expression analysis of EVs from urine) (McKiernan et al. 2016, 2018). The identification of specific biomarkers in exosomes can provide prognostic or diagnostic information. The detection of glypican 1 and macrophage migration inhibitory factor (MIF) in exosomes is associated with colorectal and pancreatic cancer (Costa-Silva et al. 2015; Melo et al. 2015). Also, exosomal miR-19a can predict colorectal cancer recurrence after surgery (Matsumura et al. 2015), whereas the presence of CA125, EpCAM, and CD24 in EVs might be useful for the diagnosis of ovarian cancer (Runz et al. 2007; Zhao et al. 2016). On the other hand, detection of glutathione S-transferase P-1 (GSPT1)-containing exosomes is associated with poor prognosis in breast cancer (Yang et al. 2017). Most of the possible clinical applications of EVs still require validation and standardization, but they reflect the wide range of biomarkers that can be found in such particles.
Besides the identification of biomarkers in exosomes, it has been suggested that other EVs should be also considered. For example, oncosomes and larger EVs contain a higher amount of DNA. As exosome cargos lack double-stranded DNA (dsDNA) and histone proteins (Jeppesen et al. 2019), larger EVs might be a better biosource to identify DNA mutations (Vagner et al. 2018) for clinical use. Recently, exomeres have been described as non-EV nanoparticles. They are smaller than exosomes (diameter of ≤35–50 nm) and are associated with unique cargo profiles (Zhang et al. 2018). Although, more research is necessary to clearly define EV function, in the liquid biopsy field, it would make sense to isolate different EV types in function of the targeted biomarker or disease type, thus using the right EVs for the right application.
TUMOR-EDUCATED PLATELETS
Platelets are anucleated cell fragments that originate from megakaryocytes. They are central players in hemostasis, thrombosis, immunity, inflammation, and metastasis (Rodvien and Mielke 1976; Gros et al. 2014; Ali et al. 2015; Hou et al. 2015; Thomas and Storey 2015; Brass et al. 2016; Leblanc and Peyruchaud 2016; Buettner 2018). In 1865, Trousseau described for the first time, platelet involvement in cancer. He observed that patients with cancer often presented thrombophlebitis and blood clotting far from the tumor site (Trousseau 1865; Menter et al. 2014). Then, Billroth described cancer cell-containing thrombi in the circulation, showing the direct interaction between platelets and tumor cells (Billroth 1877). After a century, Gasic et al. (1973) showed that tumors can induce platelet aggregation, and correlated this with metastases in mice. They suggested that activated/aggregated platelets interact with tumor cells, and increase tumor cell extravasation to the metastatic niche.
High-throughput technology allowed studying the complex interaction between platelets and cancer, leading to the term of TEPs (Nilsson et al. 2011; Sol and Wurdinger 2017; Best et al. 2018). TEPs are a valuable biosource for noninvasive assays. Although platelets are easy to purify, it is important to prevent their activation during handling because it affects their molecular and morphological features. Therefore, strong mechanical or biochemical forces should be avoided (Mustard et al. 1989; Hoffman et al. 1992; Cazenave et al. 2004). Nevertheless, the optimization of a fast and highly efficient method for platelet isolation from a small blood amount is vital. For instance, to minimize platelet activation during blood sampling, blood should be gently collected by using a 1.2 mm intravenous cannula (Amisten 2012). As many drugs interfere with platelet function (Schrör 1997; Scharf 2012), it is important to precisely record all patient's treatments. TEPs can be isolated up to 48 h after blood sampling and allows the obtention of high-quality RNA for molecular tests (Best et al. 2015, 2019).
Platelets contain many growth factors and cytokines that could be involved in the metastatic process through multiple mechanisms (Karpatkin et al. 1988; Trikha and Nakada 2002; Boucharaba et al. 2004). During their lifespan, platelet directly interact with cancer cells via receptors, and indirectly through signaling molecules (Morimoto et al. 2008; Konstantopoulos and Thomas 2009; Labelle et al. 2011; Golebiewska and Poole 2015; Schlesinger 2018; Ward et al. 2018). This interaction leads to platelet activation, resulting in the release different molecules (e.g., PDGF, TGFβ, VEGF-A/C BFGF, EGF, and IGF1) (Erpenbeck and Schön 2010; Radziwon-Balicka et al. 2012; Menter et al. 2014) that can provide a protumor metastatic niche (McAllister and Weinberg 2014) and may induce EMT. Direct platelet–cancer cell interaction and contact-dependent signaling by platelet-derived TGFβ can activate the TGFβ-SMAD and NF-κB pathways, resulting in the acquisition of mesenchymal features by cancer cells and increased metastasis potential (Labelle et al. 2011). TANK-binding kinase 1 (TBK1) is another platelet-induced EMT and metastasis mediator, and a potential therapeutic target to prevent metastasis formation (Zhang et al. 2019). Moreover, platelets can protect tumor cells by forming a protective cloak against natural killer (NK) cell-mediated lysis (Nieswandt et al. 1999) and tumor necrosis factor (TNF)-α (Philippe et al. 1993). Platelets can also disturb the “missing self” recognition by NK cells by conferring a “pseudonormal” phenotype, through transfer of major histocompatibility complex (MHC) class I molecules to the cancer cell surface during aggregation (Placke et al. 2012). Platelet–cancer cell signaling also stimulates matrix metalloproteinases-9 (MMP-9) expression, promoting cancer cell invasion (Alonso-Escolano et al. 2006; Radziwon-Balicka et al. 2014).
Several studies on platelet role in cancer progression consider TEPs as high-potential liquid biopsy biosource. Based on mRNA sequencing of 283 TEP samples, Best and colleagues could differentiate between healthy controls and patients with cancer (96% accuracy). Based on TEP mutational status, they could obtain some information to predict the tumor type. They also developed a highly sensitive algorithm that can classify early-stage (localized) and advanced (metastatic) cancer (Best et al. 2015). TEPs can also be used to predict the treatment response to targeted therapies. The algorithm also predicted the presence of MET amplification, EGFR mutations, and KRAS mutations in TEP samples (Best et al. 2015). The detection of tumor-driving mutations reflects TEP potential use in future clinical trials and to predict the therapeutic response (Joosse and Pantel 2015). In agreement, resistance to chemotherapy was identified by monitoring EM4-ALK rearrangements in TEPs (Nilsson et al. 2016). In addition to transcriptome studies, platelet proteomic analysis allowed differentiating between benign adnexal lesions and late-stage ovarian cancers (Lomnytska et al. 2018). Also, platelets proteome of patients with early stage cancers differs noticeably compared with healthy samples (Sabrkhany et al. 2018).
It has been suggested that CTCs and platelets interact, but the underlying mechanism remains unknown. Jiang et al. (2017) showed the existence of platelet-coated CTCs in patients with metastatic cancer, but they did not molecularly characterize CTCs and platelets to address the interplay between EMT and CTC-TEP interaction. Combinatorial analysis of TEPs with complementary biosources, such as CTCs, EVs, and ctDNA, and protein biomarkers could be the next-generation tools for early cancer diagnosis (In ‘t Veld and Wurdinger 2019).
CONCLUSIONS
In the next decade, cancer diagnosis will focus on early detection and personalized management. Real-time liquid biopsy will play a fundamental role in this progress. CTCs, ctDNA, EVs, and TEPs are complementary and they may become routine clinical laboratory tests in the near future. An index or an algorithm could be developed to combine all these data to obtain a more precise tumor profile. Moreover, more emphasis on technical validation is required, and projects, such as the European CANCER-ID, European Liquid Biopsy Academy (ELBA), European Liquid Biopsy Society (ELBS) networks or the U.S.-based BloodPAC, have been initiated to meet this challenge.
This field is in constant expansion, and it is estimated that the liquid biopsy market will increase from $200 million to $1 billion in the next 5 yr (Budel et al. 2019). The spread of these relatively new biosources will reduce the economic cost of invasive procedures or inefficient therapies, thus improving the cost-efficiency of cancer management with direct implications for the overall survival and quality of life of patients.
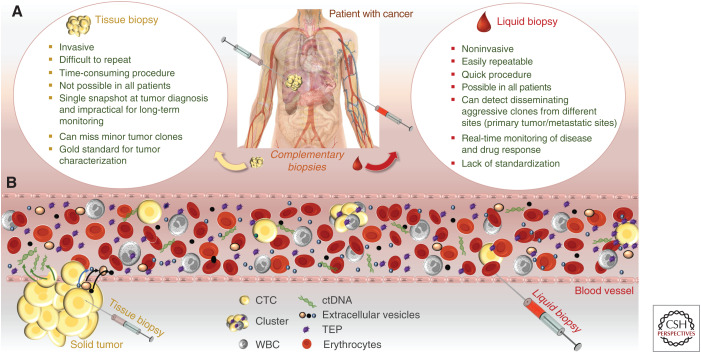
Liquid biopsy should not be seen as a substitute of the histopathological diagnosis in tissue biopsies, but as a complementary tool for diagnosis/characterization, and as part of an innovative tumor management strategy (e.g., CTCs might be used to identify new therapeutic targets to stop metastatic initiator cells; EVs as delivery particles for target therapies in specific tissues or tumors) (Fig. 1). The presence and biological functions of these biosources are not limited to cancer, and future work will open a wide range of applications that might not even be directly related to diseases or human well-being.
Figure 1.
Liquid biopsy potential for cancer monitoring. (A) Complementarity of tissue and liquid biopsies. (B) Overview of liquid biopsy biomarkers that represent the tumor molecular heterogeneity and immunologic phenotype. The primary tumor and metastatic sites release different cells and biomolecules in the bloodstream. Circulating tumor cells (CTCs; as single cells or clusters), circulating tumor DNA (ctDNA), extracellular vesicles (EVs), and tumor-educated platelets (TEPs) can be isolated from whole blood to obtain cancer genome, transcriptome, proteome, and secretome data in real time. Moreover, white blood cells (WBC) can give information on the immunity of the patient with cancer, mainly on their potential to eradicate cancer cells. All these biomarkers are complementary and can be used to build a precise index or an algorithm based on qualitative and quantitative data at different times during the disease course.
“Liquid biopsy” will become a crucial tool for oncologists and physicians in general, and is one example of how cancer management will drastically change in the future years.
COMPETING INTEREST STATEMENT
The authors declare no potential conflicts of interest.
ACKNOWLEDGMENTS
We thank Dr. Elisabetta Andermarcher for assistance with her comments and proofreading that greatly improved the manuscript. This work was supported by (1) the ELBA—Innovative Training Networks (ITN) H2020—European Liquid Biopsies Academy project—Toward widespread clinical application of blood- based diagnostic tools. H2020-MSCA-ITN-2017 (see elba.uni-plovdiv.bg); (2) CANCER-ID, an Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115749, resources of which are from the European Union's Seventh Framework Program (FP7/2007-2013) (see cancer-id.eu) and EFPIA companies’ in-kind contribution; and (3) the National Institute of Cancer (INCa, see e-cancer.fr).
Financial support: The investigators received support from (1) the National Institute of Cancer (INCa, see e-cancer.fr), (2) CANCER-ID, an Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115749, resources of which are composed of financial contribution from the European Union's Seventh Framework Program (FP7/2007-2013) (see cancer-id.eu) and EFPIA companies’ in-kind contribution, and (3) the ELBA—Innovative Training Networks (ITN) H2020—European Liquid Biopsies Academy project—Toward widespread clinical application of blood-based diagnostic tools. H2020-MSCA-ITN-2017 (see elba.uni-plovdiv.bg).
Footnotes
Editors: Jeffrey W. Pollard and Yibin Kang
Additional Perspectives on Metastasis: Mechanism to Therapy available at www.perspectivesinmedicine.org
REFERENCES
- Abonnenc M, Manaresi N, Borgatti M, Medoro G, Fabbri E, Romani A, Altomare L, Tartagni M, Rizzo R, Baricordi O, et al. 2013. Programmable interactions of functionalized single bioparticles in a dielectrophoresis-based microarray Chip. Anal Chem 85: 8219–8224. 10.1021/ac401296m [DOI] [PubMed] [Google Scholar]
- Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al. 2014. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158: 1110–1122. 10.1016/j.cell.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali RA, Wuescher LM, Worth RG. 2015. Platelets: essential components of the immune system. Curr Trends Immunol 16: 65–78. [PMC free article] [PubMed] [Google Scholar]
- Alix-Panabières C, Pantel K. 2014. Challenges in circulating tumour cell research. Nat Rev Cancer 14: 623–631. 10.1038/nrc3820 [DOI] [PubMed] [Google Scholar]
- Alix-Panabières C, Cayrefourcq L, Mazard T, Maudelonde T, Assenat E, Assou S. 2017. Molecular portrait of metastasis-competent circulating tumor cells in colon cancer reveals the crucial role of genes regulating energy metabolism and DNA repair. Clin Chem 63: 700–713. 10.1373/clinchem.2016.263582 [DOI] [PubMed] [Google Scholar]
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AGJ, Uhr JW, Terstappen LWMM. 2004. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10: 6897–6904. 10.1158/1078-0432.CCR-04-0378 [DOI] [PubMed] [Google Scholar]
- Allyse M, Minear MA, Berson E, Sridhar S, Rote M, Hung A, Chandrasekharan S. 2015. Non-invasive prenatal testing: a review of international implementation and challenges. Int J Womens Health 7: 113–126. 10.2147/IJWH.S67124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Alconada L, Muinelo-Romay L, Madissoo K, Diaz-Lopez A, Krakstad C, Trovik J, Wik E, Hapangama D, Coenegrachts L, Cano A, et al. 2014. Molecular profiling of circulating tumor cells links plasticity to the metastatic process in endometrial cancer. Mol Cancer 13: 223 10.1186/1476-4598-13-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Escolano D, Medina C, Cieslik K, Radomski A, Jurasz P, Santos-Martínez MJ, Jiffar T, Ruvolo P, Radomski MW. 2006. Protein kinase Cδ mediates platelet-induced breast cancer cell invasion. J Pharmacol Exp Ther 318: 373–380. 10.1124/jpet.106.103358 [DOI] [PubMed] [Google Scholar]
- Amisten S. 2012. A rapid and efficient platelet purification protocol for platelet gene expression studies. Methods Mol Biol 788: 155–172. 10.1007/978-1-61779-307-3_12 [DOI] [PubMed] [Google Scholar]
- An M, Wu J, Zhu J, Lubman DM. 2018. Comparison of an optimized ultracentrifugation method versus size-exclusion chromatography for isolation of exosomes from human serum. J Proteome Res 17: 3599–3605. 10.1021/acs.jproteome.8b00479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedikter BJ, Bouwman FG, Vajen T, Heinzmann ACA, Grauls G, Mariman EC, Wouters EFM, Savelkoul PH, Lopez-Iglesias C, Koenen RR, et al. 2017. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci Rep 7: 15297 10.1038/s41598-017-15717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best MG, Sol N, Kooi I, Tannous J, Westerman BA, Rustenburg F, Schellen P, Verschueren H, Post E, Koster J, et al. 2015. RNA-seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell 28: 666–676. 10.1016/j.ccell.2015.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best MG, Wesseling P, Wurdinger T. 2018. Tumor-educated platelets as a noninvasive biomarker source for cancer detection and progression monitoring. Cancer Res 78: 3407–3412. [DOI] [PubMed] [Google Scholar]
- Best MG, In ‘t Veld SGJG, Sol N, Wurdinger T. 2019. RNA sequencing and swarm intelligence-enhanced classification algorithm development for blood-based disease diagnostics using spliced blood platelet RNA. Nat Protoc 14: 1206–1234. 10.1038/s41596-019-0139-5 [DOI] [PubMed] [Google Scholar]
- Bilkenroth U, Taubert H, Riemann D, Rebmann U, Heynemann H, Meye A. 2001. Detection and enrichment of disseminated renal carcinoma cells from peripheral blood by immunomagnetic cell separation. Int J Cancer 92: 577–582. 10.1002/ijc.1217 [DOI] [PubMed] [Google Scholar]
- Billroth T. 1877. Lectures on surgical pathology and therapeutics: a handbook for students and practitioners, 8th ed The New Sydenham Society, London. [Google Scholar]
- Böing AN, van der Pol E, Grootemaat AE, Coumans FAW, Sturk A, Nieuwland R. 2014. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles 3: 23430 10.3402/jev.v3.23430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucharaba A, Serre CM, Grès S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clézardin P, Peyruchaud O. 2004. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest 114: 1714–1725. 10.1172/JCI200422123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht JWP, Mayo-de-Las-Casas C, Berenguer J, Karachaliou N, Rosell R. 2018. The present and future of liquid biopsies in non-small cell lung cancer: combining four biosources for diagnosis, prognosis, prediction, and disease monitoring. Curr Oncol Rep 20: 70 10.1007/s11912-018-0720-z [DOI] [PubMed] [Google Scholar]
- Brass LF, Diamond SL, Stalker TJ. 2016. Platelets and hemostasis: a new perspective on an old subject. Blood Adv 1: 5–9. 10.1182/bloodadvances.2016000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budel S, Lee J, Wang M. 2019. Global oncology liquid biopsy manufacturing market (2018-2023) and stakeholder toolkit (founders/entrepreneurs, investors, regulators and payers, biopharma partners). Santa Monica, CA. [Google Scholar]
- Buettner R. 2018. Platelets promoting tumor metastasis: culprits or victims? J Thorac Dis 10: 550–553. 10.21037/jtd.2017.12.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, Wang G. 2018. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol 16: 81 10.1186/s12951-018-0403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabel L, Proudhon C, Romano E, Girard N, Lantz O, Stern MH, Pierga JY, Bidard FC. 2018. Clinical potential of circulating tumour DNA in patients receiving anticancer immunotherapy. Nat Rev Clin Oncol 15: 639–650. 10.1038/s41571-018-0074-3 [DOI] [PubMed] [Google Scholar]
- Carroll PH, Mohler JL. 2018. NCCN Guidelines Updates: Prostate cancer and prostate cancer early detection. J Natl Compr Canc Netw 16: 620–623. 10.6004/jnccn.2018.0036 [DOI] [PubMed] [Google Scholar]
- Castellanos-Rizaldos E, Grimm DG, Tadigotla V, Hurley J, Healy J, Neal PL, Sher M, Venkatesan R, Karlovich C, Raponi M, et al. 2018. Exosome-based detection of EGFR T790M in plasma from non-small cell lung cancer patients. Clin Cancer Res 24: 2944–2950. 10.1158/1078-0432.CCR-17-3369 [DOI] [PubMed] [Google Scholar]
- Castro J, Sanchez L, Nuñez MT, Lu M, Castro T, Sharifi HR, Ericsson C. 2018. Screening circulating tumor cells as a noninvasive cancer test in 3388 individuals from high-risk groups (ICELLATE2). Dis Markers 2018: 4653109 10.1155/2018/4653109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrefourcq L, Mazard T, Joosse S, Solassol J, Ramos J, Assenat E, Schumacher U, Costes V, Maudelonde T, Pantel K, et al. 2015. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res 75: 892–901. 10.1158/0008-5472.CAN-14-2613 [DOI] [PubMed] [Google Scholar]
- Cazenave JP, Ohlmann P, Cassel D, Eckly A, Hechler B, Gachet C. 2004. Preparation of washed platelet suspensions from human and rodent blood. Methods Mol Biol 272: 13–28. [DOI] [PubMed] [Google Scholar]
- Chiou NT, Ansel KM. 2016. Improved exosome isolation by sucrose gradient fractionation of ultracentrifuged crude exosome pellets. Protocol Exchange 10.1038/protex.2016.057 [DOI]
- Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, Douville C, Javed AA, Wong F, Mattox A, et al. 2018. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359: 926–930. 10.1126/science.aar3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Console L, Scalise M, Indiveri C. 2019. Exosomes in inflammation and role as biomarkers. Clin Chim Acta 488: 165–171. 10.1016/j.cca.2018.11.009 [DOI] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et al. 2015. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17: 816–826. 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto N, Caja S, Maia J, Strano Moraes MC, Costa-Silva B. 2018. Exosomes as emerging players in cancer biology. Biochimie 155: 2–10. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Pierga JY, Reuben J, Rademaker A, Davis AA, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, et al. 2019. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): international expert consensus paper. Crit Rev Oncol Hematol 134: 39–45. 10.1016/j.critrevonc.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Diaz LA Jr, Bardelli A. 2014. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 32: 579–586. 10.1200/JCO.2012.45.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Skelley AM, Merdek KD, Sprott KM, Jiang C, Pierceall WE, Lin J, Stocum M, Carney WP, Smirnov DA. 2013. Microfluidics and circulating tumor cells. J Mol Diagn 15: 149–157. 10.1016/j.jmoldx.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Douillard JY, Ostoros G, Cobo M, Ciuleanu T, Cole R, McWalter G, Walker J, Dearden S, Webster A, Milenkova T, et al. 2014. Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status. J Thorac Oncol 9: 1345–1353. 10.1097/JTO.0000000000000263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazezy M, Joosse SA. 2018. Techniques of using circulating tumor DNA as a liquid biopsy component in cancer management. Comput Struct Biotechnol J 16: 370–378. 10.1016/j.csbj.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erpenbeck L, Schön MP. 2010. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood 115: 3427–3436. 10.1182/blood-2009-10-247296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehm T, Hoffmann O, Aktas B, Becker S, Solomayer EF, Wallwiener D, Kimmig R, Kasimir-Bauer S. 2009. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res 11: R59 10.1186/bcr2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic GJ, Gasic TB, Galanti N, Johnson T, Murphy S. 1973. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int J cancer 11: 704–718. 10.1002/ijc.2910110322 [DOI] [PubMed] [Google Scholar]
- Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R, Krol I, Scheidmann MC, Beisel C, Stirnimann CU, et al. 2019. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 176: 98–112.e14. 10.1016/j.cell.2018.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebiewska EM, Poole AW. 2015. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev 29: 153–162. 10.1016/j.blre.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. 2015. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol 1295: 179–209. 10.1007/978-1-4939-2550-6_15 [DOI] [PubMed] [Google Scholar]
- Gros A, Ollivier V, Ho-Tin-Noé B. 2014. Platelets in inflammation: regulation of leukocyte activities and vascular repair. Front Immunol 5: 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao SJ, Wan Y, Xia YQ, Zou X, Zheng SY. 2018. Size-based separation methods of circulating tumor cells. Adv Drug Deliv Rev 125: 3–20. 10.1016/j.addr.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Hoffman M, Monroe DM, Roberts HR. 1992. A rapid method to isolate platelets from human blood by density gradient centrifugation. Am J Clin Pathol 98: 531–533. 10.1093/ajcp/98.5.531 [DOI] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. 2015. Tumour exosome integrins determine organotropic metastasis. Nature 527: 329–335. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Carrim N, Wang Y, Gallant RC, Marshall A, Ni H. 2015. Platelets in hemostasis and thrombosis: novel mechanisms of fibrinogen-independent platelet aggregation and fibronectin-mediated protein wave of hemostasis. J Biomed Res 29: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Gao P, Song Y, Sun J, Chen X, Zhao J, Xu H, Wang Z. 2015. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer 15: 202 10.1186/s12885-015-1218-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iinuma H, Okinaga K, Adachi M, Suda K, Sekine T, Sakagawa K, Baba Y, Tamura J, Kumagai H, Ida A. 2000. Detection of tumor cells in blood using CD45 magnetic cell separation followed by nested mutant allele-specific amplification of p53 and K-ras genes in patients with colorectal cancer. Int J Cancer 89: 337–344. [DOI] [PubMed] [Google Scholar]
- Ilie M, Hofman V, Long-Mira E, Selva E, Vignaud JM, Padovani B, Mouroux J, Marquette CH, Hofman P. 2014. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS ONE 9: e111597 10.1371/journal.pone.0111597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- In ‘t Veld SGJG, Wurdinger T. 2019. Tumor-educated platelets. Blood 133: 2359–2364. 10.1182/blood-2018-12-852830 [DOI] [PubMed] [Google Scholar]
- Italiano JE Jr, Mairuhu ATA, Flaumenhaft R. 2010. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol 17: 578–584. 10.1097/MOH.0b013e32833e77ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger BAS, Neugebauer J, Andergassen U, Melcher C, Schochter F, Mouarrawy D, Ziemendorff G, Clemens M, Abel EV, Heinrich G, et al. 2017. The HER2 phenotype of circulating tumor cells in HER2-positive early breast cancer: a translational research project of a prospective randomized phase III trial. PLoS ONE 12: e0173593 10.1371/journal.pone.0173593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas T, Janas MM, Sapoń K, Janas T. 2015. Mechanisms of RNA loading into exosomes. FEBS Lett 589: 1391–1398. 10.1016/j.febslet.2015.04.036 [DOI] [PubMed] [Google Scholar]
- Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, et al. 2019. Reassessment of exosome composition. Cell 177: 428–445.e18. 10.1016/j.cell.2019.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Wong KHK, Khankhel AH, Zeinali M, Reategui E, Phillips MJ, Luo X, Aceto N, Fachin F, Hoang AN, et al. 2017. Microfluidic isolation of platelet-covered circulating tumor cells. Lab Chip 17: 3498–3503. 10.1039/C7LC00654C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. 1987. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262: 9412–9420. [PubMed] [Google Scholar]
- Joosse SA, Pantel K. 2015. Tumor-educated platelets as liquid biopsy in cancer patients. Cancer Cell 28: 552–554. 10.1016/j.ccell.2015.10.007 [DOI] [PubMed] [Google Scholar]
- Kang Y, Pantel K. 2013. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell 23: 573–581. 10.1016/j.ccr.2013.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Q, Henry NL, Paoletti C, Jiang H, Vats P, Chinnaiyan AM, Hayes DF, Merajver SD, Rae JM, Tewari M. 2016. Comparative analysis of circulating tumor DNA stability in K3EDTA, Streck, and CellSave blood collection tubes. Clin Biochem 49: 1354–1360. 10.1016/j.clinbiochem.2016.03.012 [DOI] [PubMed] [Google Scholar]
- Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. 1988. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest 81: 1012–1019. 10.1172/JCI113411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YN, Koo KH, Sung JY, Yun UJ, Kim H. 2012. Anoikis resistance: an essential prerequisite for tumor metastasis. Int J Cell Biol 2012: 306879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Wang Y, Oliver CR, Thamm DH, Cooling L, Paoletti C, Smith KJ, Nagrath S, Hayes DF. 2019. A temporary indwelling intravascular aphaeretic system for in vivo enrichment of circulating tumor cells. Nat Commun 10: 1478 10.1038/s41467-019-09439-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantopoulos K, Thomas SN. 2009. Cancer cells in transit: the vascular interactions of tumor cells. Annu Rev Biomed Eng 11: 177–202. 10.1146/annurev-bioeng-061008-124949 [DOI] [PubMed] [Google Scholar]
- Kriebel PW, Majumdar R, Jenkins LM, Senoo H, Wang W, Ammu S, Chen S, Narayan K, Iijima M, Parent CA. 2018. Extracellular vesicles direct migration by synthesizing and releasing chemotactic signals. J Cell Biol 217: 2891–2910. 10.1083/jcb.201710170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug AK, Enderle D, Karlovich C, Priewasser T, Bentink S, Spiel A, Brinkmann K, Emenegger J, Grimm DG, Castellanos-Rizaldos E, et al. 2018. Improved EGFR mutation detection using combined exosomal RNA and circulating tumor DNA in NSCLC patient plasma. Ann Oncol 29: 700–706. 10.1093/annonc/mdx765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO. 2011. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20: 576–590. 10.1016/j.ccr.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc R, Peyruchaud O. 2016. Metastasis: new functional implications of platelets and megakaryocytes. Blood 128: 24–31. 10.1182/blood-2016-01-636399 [DOI] [PubMed] [Google Scholar]
- Lee H, Na W, Park C, Park KH, Shin S. 2018. Centrifugation-free extraction of circulating nucleic acids using immiscible liquid under vacuum pressure. Sci Rep 8: 5467 10.1038/s41598-018-23766-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li I, Nabet BY. 2019. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer 18: 32 10.1186/s12943-019-0975-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Kaslan M, Lee SH, Yao J, Gao Z. 2017. Progress in exosome isolation techniques. Theranostics 7: 789–804. 10.7150/thno.18133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissa D, Robles AI. 2016. Methylation analyses in liquid biopsy. Transl Lung Cancer Res 5: 492–504. 10.21037/tlcr.2016.10.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YMD, Lun FMF, Chan KCA, Tsui NBY, Chong KC, Lau TK, Leung TY, Zee BCY, Cantor CR, Chiu RWK. 2007. Digital PCR for the molecular detection of fetal chromosomal aneuploidy. Proc Natl Acad Sci 104: 13116–13121. 10.1073/pnas.0705765104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YMD, Chan KCA, Sun H, Chen EZ, Jiang P, Lun FMF, Zheng YW, Leung TY, Lau TK, Cantor CR, et al. 2010. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci Transl Med 2: 61ra91. [DOI] [PubMed] [Google Scholar]
- Lobb RJ, Becker M, Wen SW, Wong CSF, Wiegmans AP, Leimgruber A, Möller A. 2015. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles 4: 27031 10.3402/jev.v4.27031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomnytska M, Pinto R, Becker S, Engström U, Gustafsson S, Björklund C, Templin M, Bergstrand J, Xu L, Widengren J, et al. 2018. Platelet protein biomarker panel for ovarian cancer diagnosis. Biomark Res 6: 2 10.1186/s40364-018-0118-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou XL, Sun J, Gong SQ, Yu XF, Gong R, Deng H. 2015. Interaction between circulating cancer cells and platelets: clinical implication. Chin J Cancer Res 27: 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. 2017. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin 38: 754–763. 10.1038/aps.2017.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel P, Métais P. 1948. Comptes rendus des seances de la societe de biologie et de ses filiales. C R Seances Soc Biol Fil 142: 241–243. [PubMed] [Google Scholar]
- Mathew A, Brufsky AM, Davidson NE. 2015. Can circulating tumor cells predict resistance in metastatic breast cancer? Clin Cancer Res 21: 2421–2423. 10.1158/1078-0432.CCR-14-2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura T, Sugimachi K, Iinuma H, Takahashi Y, Kurashige J, Sawada G, Ueda M, Uchi R, Ueo H, Takano Y, et al. 2015. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer 113: 275–281. 10.1038/bjc.2015.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel M, Jacot W, Pantel K, Bartkowiak K, Topart D, Cayrefourcq L, Rossille D, Maudelonde T, Fest T, Alix-Panabières C. 2015. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol 9: 1773–1782. 10.1016/j.molonc.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister SS, Weinberg RA. 2014. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 16: 717–727. 10.1038/ncb3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan J, Donovan MJ, O'Neill V, Bentink S, Noerholm M, Belzer S, Skog J, Kattan MW, Partin A, Andriole G, et al. 2016. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol 2: 882–889. 10.1001/jamaoncol.2016.0097 [DOI] [PubMed] [Google Scholar]
- McKiernan J, Donovan MJ, Margolis E, Partin A, Carter B, Brown G, Torkler P, Noerholm M, Skog J, Shore N, et al. 2018. A prospective adaptive utility trial to validate performance of a novel urine exosome gene expression assay to predict high-grade prostate cancer in patients with prostate-specific antigen 2–10 ng/ml at initial biopsy. Eur Urol 74: 731–738. 10.1016/j.eururo.2018.08.019 [DOI] [PubMed] [Google Scholar]
- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, et al. 2015. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523: 177–182. 10.1038/nature14581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. 2014. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev 33: 231–269. 10.1007/s10555-014-9498-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, Lindeman N, Lockwood CM, Rai AJ, Schilsky RL, et al. 2018. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol 36: 1631–1641. 10.1200/JCO.2017.76.8671 [DOI] [PubMed] [Google Scholar]
- Mohme M, Riethdorf S, Pantel K. 2017. Circulating and disseminated tumour cells—mechanisms of immune surveillance and escape. Nat Rev Clin Oncol 14: 155–167. 10.1038/nrclinonc.2016.144 [DOI] [PubMed] [Google Scholar]
- Morimoto K, Satoh-Yamaguchi K, Hamaguchi A, Inoue Y, Takeuchi M, Okada M, Ikeda W, Takai Y, Imai T. 2008. Interaction of cancer cells with platelets mediated by Necl-5/poliovirus receptor enhances cancer cell metastasis to the lungs. Oncogene 27: 264–273. 10.1038/sj.onc.1210645 [DOI] [PubMed] [Google Scholar]
- Mosko MJ, Nakorchevsky AA, Flores E, Metzler H, Ehrich M, van den Boom DJ, Sherwood JL, Nygren AOH. 2016. Ultrasensitive detection of multiplexed somatic mutations using MALDI-TOF mass spectrometry. J Mol Diagn 18: 23–31. 10.1016/j.jmoldx.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Mustard JF, Kinlough-Rathbone RL, Packham MA. 1989. Isolation of human platelets from plasma by centrifugation and washing. Methods Enzymol 169: 3–11. 10.1016/0076-6879(89)69045-3 [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Hafner M, Echtenacher B, Mannel DN. 1999. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59: 1295–1300. [PubMed] [Google Scholar]
- Nieto MA, Huang RYJ, Jackson RA, Thiery JP. 2016. EMT: 2016. Cell 166: 21–45. 10.1016/j.cell.2016.06.028 [DOI] [PubMed] [Google Scholar]
- Nikolaev S, Lemmens L, Koessler T, Blouin JL, Nouspikel T. 2018. Circulating tumoral DNA: preanalytical validation and quality control in a diagnostic laboratory. Anal Biochem 542: 34–39. 10.1016/j.ab.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Nilsson RJA, Balaj L, Hulleman E, van Rijn S, Pegtel DM, Walraven M, Widmark A, Gerritsen WR, Verheul HM, Vandertop WP, et al. 2011. Blood platelets contain tumor-derived RNA biomarkers. Blood 118: 3680–3683. 10.1182/blood-2011-03-344408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson RJA, Karachaliou N, Berenguer J, Gimenez-Capitan A, Schellen P, Teixido C, Tannous J, Kuiper JL, Drees E, Grabowska M, et al. 2016. Rearranged EML4-ALK fusion transcripts sequester in circulating blood platelets and enable blood-based crizotinib response monitoring in non-small-cell lung cancer. Oncotarget 7: 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. 2015. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med 21: 24–33. 10.1016/j.molmed.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksvold MP, Neurauter A, Pedersen KW. 2015. Magnetic bead-based isolation of exosomes BT. In RNA interference: challenges and therapeutic opportunities (ed. Sioud M.), pp. 465–481, Springer, New York. [DOI] [PubMed] [Google Scholar]
- Pandoh PK, Corbett RD, McDonald H, Alcaide M, Kirk H, Trinh E, Haile S, MacLeod T, Smailus D, Bilobram S, et al. 2019. A high-throughput protocol for isolating cell-free circulating tumor DNA from peripheral blood. BioTechniques 66: 85–92. 10.2144/btn-2018-0148 [DOI] [PubMed] [Google Scholar]
- Pantel K, Alix-Panabières C. 2010. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med 16: 398–406. 10.1016/j.molmed.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Pantel K, Alix-Panabières C. 2019. Liquid biopsy and minimal residual disease—latest advances and implications for cure. Nat Rev Clin Oncol 16: 409–424. 10.1038/s41571-019-0187-3 [DOI] [PubMed] [Google Scholar]
- Pantel K, Brakenhoff RH, Brandt B. 2008. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer 8: 329–340. 10.1038/nrc2375 [DOI] [PubMed] [Google Scholar]
- Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar CM, et al. 2012. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18: 883–891. 10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe C, Philippe B, Fouqueray B, Perez J, Lebret M, Baud L. 1993. Protection from tumor necrosis factor-mediated cytolysis by platelets. Am J Pathol 143: 1713–1723. [PMC free article] [PubMed] [Google Scholar]
- Placke T, Orgel M, Schaller M, Jung G, Rammensee HG, Kopp HG, Salih HR. 2012. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res 72: 440–448. 10.1158/0008-5472.CAN-11-1872 [DOI] [PubMed] [Google Scholar]
- Radziwon-Balicka A, Moncada de la Rosa C, Jurasz P. 2012. Platelet-associated angiogenesis regulating factors: a pharmacological perspective. Can J Physiol Pharmacol 90: 679–688. 10.1139/y2012-036 [DOI] [PubMed] [Google Scholar]
- Radziwon-Balicka A, Santos-Martinez MJ, Corbalan JJ, O'Sullivan S, Treumann A, Gilmer JF, Radomski MW, Medina C. 2014. Mechanisms of platelet-stimulated colon cancer invasion: role of clusterin and thrombospondin 1 in regulation of the P38MAPK-MMP-9 pathway. Carcinogenesis 35: 324–332. 10.1093/carcin/bgt332 [DOI] [PubMed] [Google Scholar]
- Riethdorf S, Müller V, Zhang L, Rau T, Loibl S, Komor M, Roller M, Huober J, Fehm T, Schrader I, et al. 2010. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res 16: 2634–2645. 10.1158/1078-0432.CCR-09-2042 [DOI] [PubMed] [Google Scholar]
- Rodvien R, Mielke CHJ. 1976. Role of platelets in hemostasis and thrombosis. West J Med 125: 181–186. [PMC free article] [PubMed] [Google Scholar]
- Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D, Mustea A, Sehouli J, Kristiansen G, Altevogt P. 2007. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol 107: 563–571. 10.1016/j.ygyno.2007.08.064 [DOI] [PubMed] [Google Scholar]
- Sabrkhany S, Kuijpers MJE, Knol JC, Olde Damink SWM, Dingemans A-MC, Verheul HM, Piersma SR, Pham TV, Griffioen AW, Oude Egbrink MGA, et al. 2018. Exploration of the platelet proteome in patients with early-stage cancer. J Proteomics 177: 65–74. 10.1016/j.jprot.2018.02.011 [DOI] [PubMed] [Google Scholar]
- Saucedo-Zeni N, Mewes S, Niestroj R, Gasiorowski L, Murawa D, Nowaczyk P, Tomasi T, Weber E, Dworacki G, Morgenthaler NG, et al. 2012. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int J Oncol 41: 1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf RE. 2012. Drugs that affect platelet function. Semin Thromb Hemost 38: 865–883. 10.1055/s-0032-1328881 [DOI] [PubMed] [Google Scholar]
- Scher HI, Heller G, Molina A, Attard G, Danila DC, Jia X, Peng W, Sandhu SK, Olmos D, Riisnaes R, et al. 2015. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J Clin Oncol 33: 1348–1355. 10.1200/JCO.2014.55.3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillaci O, Fontana S, Monteleone F, Taverna S, Di Bella MA, Di Vizio D, Alessandro R. 2017. Exosomes from metastatic cancer cells transfer amoeboid phenotype to non-metastatic cells and increase endothelial permeability: their emerging role in tumor heterogeneity. Sci Rep 7: 4711 10.1038/s41598-017-05002-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. 2018. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol 11: 125 10.1186/s13045-018-0669-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrör K. 1997. Aspirin and platelets: the antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin Thromb Hemost 23: 349–356. 10.1055/s-2007-996108 [DOI] [PubMed] [Google Scholar]
- Schulze K, Gasch C, Staufer K, Nashan B, Lohse AW, Pantel K, Riethdorf S, Wege H. 2013. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer 133: 2165–2171. 10.1002/ijc.28230 [DOI] [PubMed] [Google Scholar]
- Shao Y, Shen Y, Chen T, Xu F, Chen X, Zheng S. 2016. The functions and clinical applications of tumor-derived exosomes. Oncotarget 7: 60736–60751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood JL, Corcoran C, Brown H, Sharpe AD, Musilova M, Kohlmann A. 2016. Optimised pre-analytical methods improve KRAS mutation detection in circulating tumour DNA (ctDNA) from patients with non-small cell lung cancer (NSCLC). PLoS ONE 11: e0150197 10.1371/journal.pone.0150197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CD, Anyiwe K, Schimmer AD. 2008. Anoikis resistance and tumor metastasis. Cancer Lett 272: 177–185. 10.1016/j.canlet.2008.05.029 [DOI] [PubMed] [Google Scholar]
- Skotland T, Hessvik NP, Sandvig K, Llorente A. 2019. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res 60: 9–18. 10.1194/jlr.R084343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol N, Wurdinger T. 2017. Platelet RNA signatures for the detection of cancer. Cancer Metastasis Rev 36: 263–272. 10.1007/s10555-017-9674-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler A, Cayrefourcq L, Mazel M, Alix-Panabières C. 2017. EpCAM-independent enrichment and detection of viable circulating tumor cells using the EPISPOT assay. Methods Mol Biol 1634: 263–276. 10.1007/978-1-4939-7144-2_22 [DOI] [PubMed] [Google Scholar]
- Soler A, Cayrefourcq L, Mazard T, Babayan A, Lamy PJ, Assou S, Assenat E, Pantel K, Alix-Panabières C. 2018. Autologous cell lines from circulating colon cancer cells captured from sequential liquid biopsies as model to study therapy-driven tumor changes. Sci Rep 8: 15931 10.1038/s41598-018-34365-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Jia J, Peng X, Xiao W, Li Y. 2017. The performance of the SEPT9 gene methylation assay and a comparison with other CRC screening tests: a meta-analysis. Sci Rep 7: 3032 10.1038/s41598-017-03321-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorber L, Zwaenepoel K, Deschoolmeester V, Roeyen G, Lardon F, Rolfo C, Pauwels P. 2017. A comparison of cell-free DNA isolation kits: isolation and quantification of cell-free DNA in plasma. J Mol Diagn 19: 162–168. 10.1016/j.jmoldx.2016.09.009 [DOI] [PubMed] [Google Scholar]
- Soria FN, Pampliega O, Bourdenx M, Meissner WG, Bezard E, Dehay B. 2017. Exosomes, an unmasked culprit in neurodegenerative diseases. Front Neurosci 11: 26 10.3389/fnins.2017.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert G, Schölch S, Niemietz T, Iwata N, García SA, Behrens B, Voigt A, Kloor M, Benner A, Bork U, et al. 2014. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res 74: 1694–1704. 10.1158/0008-5472.CAN-13-1885 [DOI] [PubMed] [Google Scholar]
- Stroun M, Anker P, Maurice P, Lyautey J, Lederrey C, Beljanski M. 1989. Neoplastic characteristics of the DNA found in the plasma of cancer patients. Oncology 46: 318–322. 10.1159/000226740 [DOI] [PubMed] [Google Scholar]
- Sun K, Jiang P, Chan KCA, Wong J, Cheng YKY, Liang RHS, Chan W, Ma ESK, Chan SL, Cheng SH, et al. 2015. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc Natl Acad Sci 112: E5503–E5512. 10.1073/pnas.1508736112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung BH, Weaver AM. 2017. Exosome secretion promotes chemotaxis of cancer cells. Cell Adh Migr 11: 187–195. 10.1080/19336918.2016.1273307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, et al. 2019. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566: 553–557. 10.1038/s41586-019-0915-y. [DOI] [PubMed] [Google Scholar]
- Tao SC, Guo SC, Zhang CQ. 2017. Platelet-derived extracellular vesicles: an emerging therapeutic approach. Int J Biol Sci 13: 828–834. 10.7150/ijbs.19776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ. 2012. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 56: 293–304. 10.1016/j.ymeth.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, Xiang J, et al. 2014. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res 24: 766–769. 10.1038/cr.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Amigorena S, Raposo G, Clayton A. 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 30: 3.22.1–3.22.29. 10.1002/0471143030.cb0322s30 [DOI] [PubMed] [Google Scholar]
- Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. 2018. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7: 1535750 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AR, Mouliere F, Gongora C, Ollier J, Robert B, Ychou M, Del Rio M, Molina F. 2010. Origin and quantification of circulating DNA in mice with human colorectal cancer xenografts. Nucleic Acids Res 38: 6159–6175. 10.1093/nar/gkq421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MR, Storey RF. 2015. The role of platelets in inflammation. Thromb Haemost 114: 449–458. 10.1160/TH14-12-1067 [DOI] [PubMed] [Google Scholar]
- Trikha M, Nakada MT. 2002. Platelets and cancer: implications for antiangiogenic therapy. Semin Thromb Hemost 28: 39–44. 10.1055/s-2002-20563 [DOI] [PubMed] [Google Scholar]
- Trousseau A. 1865. Phlegmasia alba dolens. Clin Medicale L'Hotel dieu Paris 5: 281–332. [Google Scholar]
- Tug S, Helmig S, Deichmann ER, Schmeier-Jurchott A, Wagner E, Zimmermann T, Radsak M, Giacca M, Simon P. 2015. Exercise-induced increases in cell free DNA in human plasma originate predominantly from cells of the haematopoietic lineage. Exerc Immunol Rev 21: 164–173. [PubMed] [Google Scholar]
- Vagner T, Spinelli C, Minciacchi VR, Balaj L, Zandian M, Conley A, Zijlstra A, Freeman MR, Demichelis F, De S, et al. 2018. Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J Extracell Vesicles 7: 1505403 10.1080/20013078.2018.1505403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergauwen G, Dhondt B, Van Deun J, De Smedt E, Berx G, Timmerman E, Gevaert K, Miinalainen I, Cocquyt V, Braems G, et al. 2017. Confounding factors of ultrafiltration and protein analysis in extracellular vesicle research. Sci Rep 7: 2704 10.1038/s41598-017-02599-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R, Rosenfeld N. 2017. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 17: 223–238. 10.1038/nrc.2017.7 [DOI] [PubMed] [Google Scholar]
- Ward Y, Lake R, Faraji F, Sperger J, Martin P, Gilliard C, Ku KP, Rodems T, Niles D, Tillman H, et al. 2018. Platelets promote metastasis via binding tumor CD97 leading to bidirectional signaling that coordinates transendothelial migration. Cell Rep 23: 808–822. 10.1016/j.celrep.2018.03.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B, Meldgaard P, Hager H, Wu L, Wei W, Tsai J, Khalil A, Nexo E, Sorensen BS. 2014. Detection of EGFR mutations in plasma and biopsies from non-small cell lung cancer patients by allele-specific PCR assays. BMC Cancer 14: 294 10.1186/1471-2407-14-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee EJH, Wang Y, Tsao SCH, Trau M. 2016. Simple, sensitive and accurate multiplex detection of clinically important melanoma DNA mutations in circulating tumour DNA with SERS nanotags. Theranostics 6: 1506–1513. 10.7150/thno.15871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Ouyang Y, Wang Z, Zhang R, Huang P-H, Chen C, Li H, Li P, Quinn D, Dao M, et al. 2017. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci 114: 10584–10589. 10.1073/pnas.1709210114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wang D, Li J, Xu H, Shen H, Chen X, Zhou S, Zhong S, Zhao J, Tang J. 2017. Predictive role of GSTP1-containing exosomes in chemotherapy-resistant breast cancer. Gene 623: 5–14. 10.1016/j.gene.2017.04.031 [DOI] [PubMed] [Google Scholar]
- Zhang L, Ridgway LD, Wetzel MD, Ngo J, Yin W, Kumar D, Goodman JC, Groves MD, Marchetti D. 2013. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med 5: 180ra48 10.1126/scitranslmed.3005109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, et al. 2018. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 20: 332–343. 10.1038/s41556-018-0040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Unnithan RVM, Hamidi A, Caja L, Saupe F, Moustakas A, Cedervall J, Olsson AK. 2019. TANK-binding kinase 1 is a mediator of platelet-induced EMT in mammary carcinoma cells. FASEB J 33: 7822–7832. 10.1096/fj.201801936RRR [DOI] [PubMed] [Google Scholar]
- Zhao Z, Yang Y, Zeng Y, He M. 2016. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 16: 489–496. 10.1039/C5LC01117E [DOI] [PMC free article] [PubMed] [Google Scholar]