Abstract
Cardiovascular disease (CVD) is the most common disease to increase as life expectancy increases. Most high-profile pharmacological treatments for age-related CVD have led to inefficacious results, implying that novel approaches to treating these pathologies are needed. Emerging data have demonstrated that senescent cardiovascular cells, which are characterized by irreversible cell cycle arrest and a distinct senescence-associated secretory phenotype, accumulate in aged or diseased cardiovascular systems, suggesting that they may impair cardiovascular function. This review discusses the evidence implicating senescent cells in cardiovascular ageing, the onset and progression of CVD, and the molecular mechanisms underlying cardiovascular cell senescence. We also review eradication of senescent cardiovascular cells by small-molecule-drug–mediated apoptosis and immune cell-mediated efferocytosis and toxicity as promising and precisely targeted therapeutics for CVD prevention and treatment.
Keywords: Cellular senescence, Quiescence, Vascular ageing, Cardiovascular disease, Senotherapy, Immune surveillance
1. Introduction
Human life expectancy is significantly increasing due to the better quality of water, food, hygiene, housing, and lifestyle, as well as vaccine usage and improved medical care (Foreman et al., 2018). As projected, the percentage of the global population of age ≥ 65 years will increase from 13% in 2010 to 19% in 2030, whereas those age ≥ 85 years will increase from approximately 0.03% in 2010 to approximately 1.4% in 2030 (Kontis et al., 2017). Advanced age has been well recognized as the leading unmodifiable risk factor for chronic fatal diseases (Niccoli and Partridge, 2012), including cardiovascular disease (CVD) (Shakeri et al., 2018), cancer, and neurodegenerative diseases (Baker and Petersen, 2018). Among these, CVD is the most common disease to increase globally as populations continue to age (Partridge et al., 2018). CVD is the leading cause of death in the elderly (Roth et al., 2017). However, the mechanisms underlying development of age-related CVD are largely unknown. Cellular senescence, a state of permanent cell-cycle arrest despite continued viability and metabolic activity, presents in diseased cardiovascular tissues and is strongly associated with cardiovascular ageing (Shakeri et al., 2018). Senescence is different from ageing, which is characterized by progressive functional decline. Senescence generally happens at the cellular level, whereas ageing occurs on the tissue or organ level. Cell senescence drives tissue ageing (McHugh and Gil, 2018) and is also different from cell quiescence characterized by reversible cell cycle arrest. Cell senescence and quiescence have distinct features and roles in the pathophysiology of CVD. Growing evidence indicates that senescent cardiovascular cells tightly trigger or exacerbate the onset and progression of numerous CVDs, including atherosclerosis (Childs et al., 2016), arterial stiffening (Schellinger et al., 2019), aortic aneurysms (Chen et al., 2016), (re) stenosis, myocardial fibrosis (Sawaki et al., 2018), and heart failure. Here, we discuss the unique features of senescent cardiovascular cells, molecular mechanisms underlying cardiovascular cell senescence, and emerging roles of senescent vascular cells in CVD initiation and progression. We also summarize whether and how senotherapy targeting elimination of senescent cardiovascular cells by senolytics or the immune system could be used to improve cardiovascular function with normal ageing-, disease-, or cancer therapy-induced damage, ideally resulting in healthy longevity (Campisi et al., 2019; Ovadya and Krizhanovsky, 2018; van Deursen, 2019).
2. Cellular senescence or quiescence and development of CVD
Senescent cardiovascular cells are especially abundant at sites of diseased or impaired cardiovascular systems, and accumulating evidence from human samples and mouse models demonstrates a causal role for senescent cells in the pathogenesis of age-related CVD, including atherosclerosis (Matthews et al., 2006), abdominal aortic aneurysm (AAA) (Chen et al., 2016), arterial stiffness (Roos et al., 2016), hypertension (Boe et al., 2013), and heart failure (Gude et al., 2018). We will review a body of work that, taken together, strongly suggests that cardiovascular cell senescence may have a significant role in the pathogenesis of CVD.
2.1. Cardiovascular cell senescence and quiescence
Cardiovascular cell senescence is defined as irreversible and permanent cell cycle arrest while cells remain metabolically active. Vascular cell senescence can be triggered by various detrimental stimuli, including but not limited to, radiation, oxidative stress, shortened telomeres (Matthews et al., 2006; Minamino et al., 2002), DNA damage, mitochondrial dysfunction, abnormal metabolism, and gene mutation. There are two kinds of vascular cell senescence (Bennett et al., 2016; Chi et al., 2019). The first is replicative senescence, irrevocable cell proliferation arrest after multiple cell divisions, which is generally mediated by telomere shortening (Kuilman et al., 2010). The second is stress-induced premature senescence (SIPS), a stable cell cycle arrest in the absence of any detectable telomere loss or dysfunction, which is usually induced by distinct endogenous or exogenous stresses (Kuilman et al., 2010). Cell senescence is a strategy used generally by mitotic cells to prevent dysregulated cell division. Emerging evidence demonstrates that cell senescence also occurs in post-mitotic cells, including cardiomyocytes and mature adipocytes (Sapieha and Mallette, 2018). In general, DNA damage in telomere regions drives post-mitotic cardiomyocyte senescence (Anderson et al., 2019). p53 induction mediates the senescence of post-mitotic adipocytes (Minamino et al., 2009). Upregulation of pro-senescence factor p21 triggers cell senescence in post-mitotic dopaminergic neurons (Riessland et al., 2019). Cardiovascular cell senescence is vital for the maintenance of cardiovascular tissue homeostasis during embryonic development, tissue regeneration, and wound healing (Demaria et al., 2014). However, persistent accumulation of senescent cells in cardiovascular tissues will impair cardiovascular function and has been implicated in the pathogenesis of age-related CVD. In contrast, cardiovascular cell quiescence with reversible cell cycle arrest usually occurs due to a lack of nutrition or growth factors (Blagosklonny, 2011).
2.1.1. Hallmarks of cardiovascular cell senescence
Senescent cardiovascular cells usually differ greatly from non-senescent cardiovascular cells, including proliferating cells and quiescent cells (Table 1). Senescent cardiovascular cells present several morphological and molecular features (Table 2) that may serve as suitable markers and therapeutic targets for these cells. Senescent cardiovascular cells generally display a characteristic flattened and enlarged morphology (Coleman et al., 2010; Meijles et al., 2017), increased senescence-associated beta-galactosidase (SA β-gal) activity (Matthews et al., 2006), telomere attrition, and accumulation of cyclin-dependent kinase inhibitor p16ink4a or p21 (Morgan et al., 2013). The prominent feature of senescent cardiovascular cells is the senescence-associated secretory phenotype (SASP). Senescent vascular cells secrete a variety of pro-inflammatory cytokines (e.g. IL-6, IL-8), growth factors (e.g. vascular endothelial growth factor [VEGF], platelet-derived growth factor AA [PDGF-AA]) (Demaria et al., 2014), chemokines, and matrix metalloproteinases (MMPs). Senescent vascular cells exhibit a SASP that enables them to communicate with other cells, as well as the microenvironment, and to promote the senescence of neighboring cells, tissue regeneration, and embryonic development (Munoz-Espin et al., 2013). A critical feature of senescent cells is that they are more resistant than non-senescent cells to both extrinsic and intrinsic pro-apoptotic stimuli, which may be due to the transcriptional and cap-independent translational upregulation of pro-survival BH2 family proteins (BCL-W, BCL-XL, and BCL-2) (Yosef et al., 2016). Another surrogate marker of vascular cell senescence is the induction of telomere-associated foci (TAF) of DNA damage (Roos et al., 2016). DNA methylation may function as a biomarker for vascular cell senescence and biological ageing (Field et al., 2018).
Table 1.
Cardiovascular cell senescence versus quiescence
| Characteristics | Cardiovascular cell senescence | Cardiovascular cell quiescence |
|---|---|---|
| Cell-cycle arrest | Permanent cell-cycle arrests at G1, early S, G2, or M phase (Komaravolu et al., 2019; Mao et al., 2012; Soriani et al., 2009) | Reversible cell cycle arrest at G0 phase (Kalucka et al., 2018) |
| DNA content | 2N, 4 N (Yang et al., 2007), or 8 N (Komaravolu et al., 2019) | 2N |
| Morphology | Enlarged nucleoli (Buchwalter and Hetzer, 2017; Tiku et al., 2017) and nucleus (Mammoto et al., 2019), flattened and enlarged cell morphology (Kim et al., 2017; Mammoto et al., 2019) | N/A |
| Hallmarks | p16↑ (Baker et al., 2011), p21↑ (Chen et al., 2016), SA β-gal↑, prelamin A↑ (Ragnauth et al., 2010), LamA/C↓, LaminB1↓ (Freund et al., 2012; Han et al., 2018), SASP↑, SAHF (H3K9me3, HP1γ)↑ (Boumendil et al., 2019; Ding et al., 2016; Zhang et al., 2007), ROS↑, apoptosis↓ (Baar et al., 2017; Childs et al., 2014), lysosomal content↑/aggregate (lipofuscins), NAD+↓ (Fang et al., 2017), Ki67↓, pRB↓, caveolin-1↑ (Farhat et al., 2008; Voghel et al., 2007; Zou et al., 2011), autophagy↓, mitochondria↑. | p27↑, Oct4↑, LamB1↑ (Han et al., 2018), repressive E2Fs↑, autophagy↑ (Cho and Hwang, 2012), eNOS↑ (Kalucka et al., 2018), PTGS1↑ (Kalucka et al., 2018), FoxO1↑ (Savai et al., 2014; Wilhelm et al., 2016), Dll4↑ (Zhang et al., 2011), Notch signaling activation, glycolysis↓ (Kalucka et al., 2018), BMP9↑ (David et al., 2008). |
BMP9, bone morphogenetic protein-9; Dll4, delta-like 4; FoxO1, forkhead box O1; PTGS1, prostaglandin G/H synthase 1; SA β-gal, senescence-associated β-galactosidase; SAHF, senescence-associated heterochromatin foci; SASP, senescence-associated secretory phenotype; ↑, increase; ↓, decrease; N/A, not available. For definitions of other abbreviations, please see the main text.
Table 2.
Senescent cardiovascular system contributes to cardiovascular disease
| Senescent cell types | Features of cellular senescence | Cardiovascular disease or dysfunction |
|---|---|---|
| Endothelial cells | ICAM-1↑, DPP4↑ (Kim et al., 2017), eNOS↓ (Minamino et al., 2002), TAF↑ (Roos et al., 2016), PAI-1↑ (Comi et al., 1995; Xu et al., 2000), TSP1↑ (Meijles et al., 2017), telomere attrition (Cafueri et al., 2012) | Atherosclerosis (Minamino et al., 2002), HfpEF (Gevaert et al., 2017), hematopoietic ageing (Poulos et al., 2017), AAA, vascular stiffness (Durik et al., 2012) |
| Vascular smooth muscle cells | prelamin A↑ (Ragnauth et al., 2010), SA β-gal↑ (Matthews et al., 2006), p16↑ (Matthews et al., 2006), p21↑ (Chen et al., 2016), Cyclin D1↑ (Burton et al., 2007), Sirt1↓ (Thompson et al., 2014), PDGFRα↑ (Vazquez-Padron et al., 2004), TRF2↓ (Wang et al., 2015), telomere attrition (Cafueri et al., 2012), glycolysis↑ (Docherty et al., 2018) | Atherosclerosis and plaque vulnerability (Kunieda et al., 2006; Matthews et al., 2006; Wang et al., 2015), neointima formation (Vazquez-Padron et al., 2004), AAA (Chen et al., 2016; Liao et al., 2000), TAA (Watson et al., 2017), vascular stiffness (Durik et al., 2012), artery calcification (Nakano-Kurimoto et al., 2009) |
| Cardiomyocytes | p16↑ (Chimenti et al., 2003), MMP 9↑, TAF↑ (Anderson et al., 2019) | Cardiac ageing (myocardial hypertrophy and fibrosis) (Anderson et al., 2019; Walaszczyk et al., 2019) and heart failure (Chimenti et al., 2003) |
| Myofibroblasts | SA β-gal↑, p21↑, p16↑ (Meyer et al., 2016) | Anti-myocardial fibrosis (Meyer et al., 2016) |
| Fibroblasts | FoxO4↑ (Baar et al., 2017), DPP4↑ (Kim et al., 2017), PAI-1↑ (Goldstein et al., 1994; Murano et al., 1991), Sirt1↓, DNase2↓, TREX1↓ (Takahashi et al., 2018) | HPGS/Atherosclerosis |
| Adipocytes | Osteopontin↑ (Sawaki et al., 2018), TAF↑ (Xu et al., 2018), γ-H2AX↑, p21↑, Sirt1↓, Sirt3↓ (Lefranc et al., 2019) | Myocardial fibrosis/dysfunction↑ (Sawaki et al., 2018), anticontractile capacity↓ (Lefranc et al., 2019) |
| Macrophages | SA β-gal↑ | Atherosclerosis (Childs et al., 2016) |
| T cell | Telomere shortening | Atherosclerosis (Samani et al., 2001) and myocardial infarction (Brouilette et al., 2003) |
| Endothelial progenitor cells | SA β-gal↑ (Hill et al., 2003) | Endothelial dysfunction (Hill et al., 2003) |
| Cardiac progenitor cells | p16↑, SA β-gal↑, γH2AX↑ (Lewis-McDougall et al., 2019) | Impaired regeneration and cardiac function in infarcted or aged heart (Lewis-McDougall et al., 2019) |
AAA, abdominal aortic aneurysm; DNase2, deoxyribonuclease 2; DPP4, dipeptidyl peptidase 4; HFpEF, heart failure with a preserved ejection fraction; PAI-1, plasminogen activator inhibitor-1; TAF, telomere-associated foci; TREX1, DNA 3’ repair exonuclease 1; TRF2, telomeric repeat-binding factor-2; TSP1, thrombospondin 1. Refer to the text for the expanded form of abbreviations.
Notably, one type of cardiovascular cell may have its unique senescent hallmarks with different kinds of senescence. For example, passaged vascular smooth muscle cells (VSMCs) exhibit p16, but not p21, elevation in replicative senescence, whereas p21, but not p16, is expressed in oxidative SIPS (Matthews et al., 2006). Endothelial cell (EC) SENEX is upregulated in SIPS, but not in replicative senescence (Coleman et al., 2010). Upregulation of fibroblast senescence marker dipeptidyl peptidase 4 (DPP4, also known as CD26) is much stronger in replicative senescence than in ionizing radiation (IR)-induced premature senescence (Kim et al., 2017). Middle-aged wild-type lung ECs show elevation of p53 and p21, but not p16, compared with younger counterparts (Meijles et al., 2017). Cyclin D1 reactivity (upregulation) is a more accurate marker than SA β-gal activity for replicative senescence in human VSMCs (Burton et al., 2007). Thrombospondin 1 (TSP1) protein levels are increased in senescent ECs, but not in VSMCs (Meijles et al., 2017). Thus, different cardiovascular cells have distinct molecular signatures of senescence, which may serve as potential therapeutic targets for selective elimination of different senescent cells.
2.1.2. Features of cardiovascular cell quiescence
Most cardiovascular cells in a healthy adult are quiescent (Eelen et al., 2018). Quiescent cardiovascular cells are characterized by reversible cell cycle arrest at G0 (Kalucka et al., 2018) and responsiveness to external stimuli, including both growth factors and apoptotic agents, which is distinct from senescent cells (Table 1). Different cardiovascular cells may have unique features of quiescence. EC quiescence has been well studied. Generally, Notch signaling induces endothelium quiescence (Harrington et al., 2008), which increases fatty acid β-oxidation (FAO) via elevation of Notch1-mediated carnitine palmitoyltransferase 1A (CPT1A) up to levels 3- to 4-fold greater than in proliferating ECs to sustain the tricarboxylic acid cycle for redox homeostasis through regeneration of the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH). Quiescent ECs also have upregulated endothelial nitric oxide synthase (eNOS) and prostaglandin G/H synthase 1 (PTGS1), as well as downregulated glycolysis (Kalucka et al., 2018). Also, forkhead box O1 (FoxO1) activation enhances EC quiescence by downregulating Myc protein levels and triggering consequent glycolysis inhibition, whereas FoxO1 activation does not induce EC senescence and apoptosis (Wilhelm et al., 2016). FoxO1 activation also mediates quiescence of pulmonary artery smooth muscle cells (Savai et al., 2014). Supplementation with acetate (metabolized to acetyl-coenzyme A) restores endothelial quiescence and counters oxidative stress-mediated EC dysfunction in EC-specific CPT1A-deleted mice (Kalucka et al., 2018), offering therapeutic opportunities. Quiescent ECs stimulated by β-hydroxybutyrate (β-HB) present upregulated Oct4 and Lamin B1 (Han et al., 2018). Bone morphogenetic protein-9 (BMP9) can function as a vascular (endothelial) quiescence factor (David et al., 2008).
2.2. Cellular senescence contributes to CVD
Dysregulation of cardiovascular cell senescence is tightly linked to many human CVDs, such as heart failure, coronary artery disease, atherosclerosis, aortic aneurysm, and vessel (re)stenosis. The role of senescent cardiovascular cells in the etiology of these pathologies was recently established. It was reported that p16-positive cells are major drivers of the age-related cardiac phenotype that results in decreased lifespan in mice (Baker et al., 2016). Removal of senescent cells with p16 promoter activity inhibits both atherosclerotic plaque onset and progression and enhances plaque stability (Childs et al., 2016).
2.2.1. Endothelium senescence and CVD
ECs line the inner vascular wall, and their phenotype, fate, and function alternately depend on the organs and tissues in which they reside and the niches. Not only do ECs form the barrier of vessel walls, they also communicate via signals with neighboring cells to promote tissue regeneration and growth, as well as to control low-density lipoprotein (LDL) transcytosis and consequent atherogenesis (Huang et al., 2019). EC senescence is tightly linked to EC dysfunction (Kim et al., 2018b) and subsequent CVD development and progression (Table 2) (Bochenek et al., 2016; Pantsulaia et al., 2016; Regina et al., 2016). Minamino et al. first demonstrated that senescent ECs with strong SA β-gal activity are present in atherosclerotic lesions of human coronary arteries (Minamino et al., 2002). Atherosclerotic ECs have shortened telomeres compared with the ECs in the normal vessel wall (Ogami et al., 2004). ECs from the aneurysmal region also present a senescent phenotype with shorter telomeres and more severe oxidative DNA damage (Cafueri et al., 2012). Importantly, in a mouse ageing model, EC senescence contributes to heart failure without systolic dysfunction, specific heart failure with preserved ejection fraction (HFpEF), which occurs in approximately 50% of all patients with heart failure (Gevaert et al., 2017). Also, EC senescence mediates thrombosis (complete vena cava occlusion) via elevation of plasminogen activator inhibitor-1 (PAI-1), an established marker and key mediator of cellular senescence (McDonald et al., 2010). EC premature senescence due to sirtuin deacetylase 1 (Sirt1) inhibition (Ota et al., 2007; Zu et al., 2010) may reversibly lead to vascular ageing and age-related decrease in exercise endurance (Das et al., 2018). Senescence of bone ECs (type H ECs with high expression of CD31 and endomucin) may trigger dysfunctional vascular niches for hematopoietic stem cells (Kusumbe et al., 2016), which may accelerate atherosclerosis development in mice (Fuster et al., 2017).
2.2.2. Senescence of vascular smooth muscle cells and CVD
VSMC senescence is profoundly associated with and contributes to numerous CVDs, including atherosclerosis (Bennett et al., 2016; Gardner et al., 2015; Grootaert et al., 2018), aortic aneurysm (Cafueri et al., 2012), and fibrotic neointima formation (Komaravolu et al., 2019). VSMCs from aged thoracic aortas express higher levels of platelet-derived growth factor receptor-alpha (PDGFR-α) and are resistant to apoptosis induced by serum starvation or nitric oxide (Vazquez-Padron et al., 2004). VSMCs derived from human atherosclerotic plaques have a lower level of proliferation compared with cells from the regular arterial media, suggesting that plaque VSMCs are prematurely senescent (Bennett et al., 1998). Human plaque VSMCs are characterized by higher p16 and p21 expression, hypophosphorylation of retinoblastoma (RB), stronger SA β–gal activity, and sizeable flattened cell morphology, when compared with normal VSMCs (Gorenne et al., 2006). Matthews et al. reported that senescent VSMCs are present in the fibrous cap of human advanced carotid atherectomies (Matthews et al., 2006), and VSMCs within the fibrous cap demonstrate remarkable telomere loss compared with medial VSMCs of the same lesion. Furthermore, telomere shortening of intimal VSMCs is tightly linked to increasing severity of atherosclerosis (Matthews et al., 2006). Angiotensin II (Ang II) has been reported to accelerate the development of atherosclerosis via induction of premature senescence by the p53/p21-dependent pathway in VSMCs, but not bone marrow cells (Kunieda et al., 2006). VSMC senescence due to Sirt1 inactivation increases atherosclerosis (Gorenne et al., 2013). Also, VSMC senescence contributes to plaque vulnerability, leading to myocardial infarction and stroke (Wang et al., 2015). VSMC-specific TRF2 overexpression in apolipoprotein E knockout (ApoE−/−) mice prevents senescence and consequently improves several features of plaque vulnerability (Wang et al., 2015).
Medial VSMCs derived from patient AAAs demonstrate accelerated replicative senescence compared to VSMCs from the corresponding adjacent (non-aneurysmal) inferior mesenteric artery of the same patient (Liao et al., 2000). Ang II induces VSMC senescence and resultant AAA formation via Sirt1 reduction (Chen et al., 2016). Medial VSMC senescence due to NAD+ reduction by inhibition of the rate-limiting enzyme nicotinamide phosphoribosyltransferase (NAMPT) leads to human thoracic aorta (ascending aorta) aneurysm (Watson et al., 2017). VSMC senescence in the aorta also increases vascular stiffness (Durik et al., 2012). VSMC senescence induced by nicotine (Suner et al., 2004) may drive nicotine-mediated aortic and arterial stiffness (Ding et al., 2019). Replicative senescence of VSMCs instigates age-related medial artery calcification that is not concomitant with lipid or cholesterol deposit via runt-related transcription factor-2 (RUNX-2)-mediated osteoblastic transdifferentiation (Nakano-Kurimoto et al., 2009). Ageing exacerbates neointimal formation by wire injury in carotid arteries in mice (Vazquez-Padron et al., 2004). However, it is unknown if age-enhanced neointimal formation is due to VSMC senescence.
2.2.3. Immune cell senescence in CVD
Immune cell senescence (immunosenescence) plays a pivotal role in CVD initiation and progression (Alpert et al., 2019; Yu et al., 2016). Macrophages are the primary type of immune cells that play critical roles in CVD development. Employing CD11b-driving diphtheria toxin (DT) receptor (DTR) transgenic mice, Stoneman et al. showed that monocyte/macrophage content positively contributes to atherosclerotic plaque development, collagen content, and necrotic core formation. However, monocyte reduction has minor effects on the established plaques (Stoneman et al., 2007). Mouse ageing is associated with the accumulation of senescent macrophages that can be induced in young mice by senescent fibroblasts (Hall et al., 2016). Senescent macrophages accumulate in the sub-endothelial space during early atherogenesis (Childs et al., 2016). In advanced atherosclerotic plaques, senescent macrophages promote features of plaque instability, including diminished collagen content, elastic fiber fragmentation, and fibrous cap thinning, in descending aorta and brachiocephalic artery, by elevating MMP3 and MMP13 formation. Interestingly, selective removal of these p16-positive senescent cells without interfering with the senescence program by genetic or pharmacological strategies reverses atherosclerosis in mice (Childs et al., 2016). It was reported that older persons (over the age of 60 years) with the senescent marker of shorter telomeres in leukocyte DNA have a 3.18-fold higher mortality rate from heart disease (Cawthon et al., 2003), implying that senescent immune cells may lead to heart disease. Accelerated telomere shortening also presents in leukocytes of patients with severe coronary artery disease (Samani et al., 2001) and myocardial infarction (Brouilette et al., 2003). Plasmacytoid dendritic cells (pDCs, uniquely produce type I interferon) and regulatory T cells (Tregs) are concomitantly induced and co-localized in mouse atherosclerotic intima (Yun et al., 2016). Although the accumulation of intimal DCs increases in aged mice with accelerated atherogenesis (Liu et al., 2008), the causal function of senescent DCs and T cells in CVD development remains an unmet challenge. Recently, it was reported that human carotid artery plaques contain immune cells, including CD4+ or CD8+ T cells, natural killer (NK) cells, and macrophages (Fernandez et al., 2019). However, it is totally unknown whether the patient’s plaque immune components are senescent and the role of senescent immune components in human atherogenesis.
2.2.4. Senescent myofibroblasts and fibroblasts in CVD
Senescence of cardiac myofibroblasts is increased in perivascular fibrotic areas after transverse aortic constriction (TAC) compared with the sham-treated heart. Inhibition of premature senescence by genetic deletion of both p53 and p16 leads to enhanced fibrosis and cardiac dysfunction after TAC compared with the wild-type control heart. In contrast, induction of premature senescence by cardiac-specific adeno-associated virus serotype 9 (AAV9) (Suckau et al., 2009) gene transfer-mediated expression of cysteine-rich angiogenic inducer 61 (CYR61) (Jun and Lau, 2010) results in an approximately 50% reduction of perivascular fibrosis and improved cardiac function after TAC (Meyer et al., 2016). These data imply that premature senescence of myofibroblasts functions as an essential anti-fibrotic mechanism and is a promising therapeutic target for myocardial fibrosis (Condorelli et al., 2016). The role and regulation of senescent fibroblasts and myofibroblasts in the development of CVD, including AAA, cardiac fibrosis, and arterial stiffness, warrant further investigation.
2.2.5. Senescence of vascular stem/progenitor cells and CVD
Ageing is frequently associated with dysfunction of stem or progenitor cells. Although cellular senescence of progenitor cells (PCs) contributes to multiple diseases (Nicaise et al., 2019), senescence of cardiovascular PCs in CVD progression has been less investigated. Circulating endothelial progenitor cells (EPCs) from human subjects at high risk for cardiovascular events or older subjects have higher percentages of in vitro senescence (Hill et al., 2003) or functional impairment (e.g. decreased migration and proliferation) (Heiss et al., 2005), which is correlated with vascular or EC dysfunction, a key trigger of atherogenesis. Depletion of growth differentiation factor 11 (GDF11) or telomerase reverse transcriptase (TERT) causes senescence of young VEGFR2+/CD133+ EPCs, leading to impaired vascular function and angiogenesis in vitro and in vivo (Zhao et al., 2019). However, it is unknown whether EPC senescence contributes to the onset and progression of CVD.
Although the endogenous cardiomyocyte renewal capacity of adult cardiac stem/progenitor cells (CSCs/CPCs) is still a matter of debate (van Berlo et al., 2014; Vicinanza et al., 2018), they exert a beneficial effect on cardiac function in animal models of cardiac ischemic injury (Vagnozzi et al., 2020). Age affects the senescence of human CSCs from older patients (Lewis-McDougall et al., 2019; Nakamura et al., 2016), and it also enhances mouse CSC senescence (Torella et al., 2004). Indeed, c-kit+ cardiac CPCs from aged (24 months) C57BL/6 mice have increased senescent phenotype, decreased stemness, and impaired ability to upregulate paracrine factors for angiogenesis (Castaldi et al., 2017). Overall, CSC senescence mediates cardiac ageing and heart failure (Cianflone et al., 2019; Torella et al., 2004). Interestingly, elimination of senescent CPCs using dasatinib + quercetin (D + Q) senolytics attenuates the SASP and its effect on promoting senescence of healthy non-senescent CPCs in vitro. Moreover, systemic ablation of senescent cells in aged mice in vivo using senolytics (D + Q) leads to resident CPC activation and enhanced heart regenerative capacity (Lewis-McDougall et al., 2019). Ageing induces senescence of cardiac mesenchymal stem cells (MSCs) associated with decreased CD90 expression, resulting in impaired EC differentiation potentials and enhanced SASP (Martini et al., 2019), which may contribute to cardiac disease. Additionally, CVD risk factors, such as type 2 diabetes, depletes circulating pro-vascular PCs characterized by high aldehyde dehydrogenase activity and CD34+ (Terenzi et al., 2019). Importantly, in patients with their first acute myocardial infarction, tight glycemic control reduces senescent myocyte precursor cells, thus increasing the regenerative potential of the ischemic myocardium (Marfella et al., 2012).
3. Molecular mechanisms of cardiovascular cell senescence
There are multiple mechanisms involved in cardiovascular cell senescence. Here, the review summarizes several key underlying molecular mechanisms.
3.1. Progeria and vascular cellular senescence in cardiovascular ageing and diseases
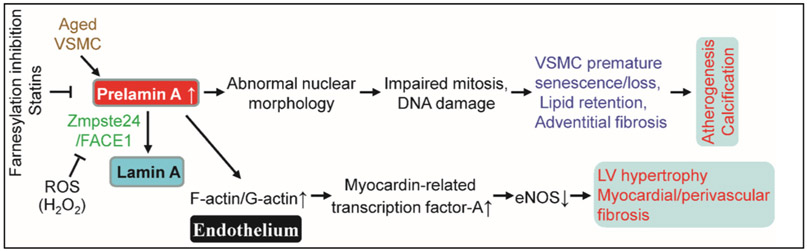
The homeostasis of the cell nucleus is profoundly modified during cellular senescence. Defects of the nuclear lamina have been associated with several different diseases of accelerated ageing, including Hutchinson-Gilford progeria syndrome (HGPS) (Gonzalo et al., 2017; Gordon et al., 2014), mandibuloacral dysplasia (Novelli et al., 2002), and atypical Werner syndrome (Bonne and Levy, 2003). HGPS is an ultra-rare, early-onset, and severe genetic disease of premature ageing caused by a point mutation (C1824 T) in Lmna (G608 G) or Zmpste24 that disrupts nuclear lamin A processing, leading to the formation of mutated (truncated and farnesylated) prelamin A, generally referred to as progerin (50 amino acids deleted from the tail of prelamin A) (Kim et al., 2018a; Lee et al., 2016). Prelamin A elevation is linked to oxidative stress-mediated reduction of the lamin A-processing enzyme Zmpste24/FACE1 (Fig. 1) (Ragnauth et al., 2010). HGPS patients exhibit severe premature arteriosclerosis characterized by VSMC calcification and attrition, as well as prominent adventitial fibrosis, and die in their early teens (younger than 15 years), mainly due to myocardial infarction or stroke (Olive et al., 2010).
Fig. 1.
Prelamin A accumulation leads to vascular cell senescence and multiple cardiovascular diseases. ⊥, inhibits. Refer to the text for the expanded form of abbreviations.
Prelamin A accumulation in multiple cardiovascular cells contributes to their senescence. For example, senescent VSMCs rapidly accumulate prelamin A and present defective nuclear morphology in vitro, both of which are reversible by treatment with farnesylation inhibitors and statins (Fig. 1) (Ragnauth et al., 2010). In human arteries, prelamin A does not accumulate in young and healthy vessels but is prevalent in medial VSMCs from aged individuals or in atherosclerotic lesions, where it often colocalizes with senescent and degenerative VSMCs. Knockdown of FACE1 recapitulates the prelamin A-induced defects of nuclear morphology in aged VSMCs, whereas prelamin A overexpression promotes VSMC senescence through disrupting mitosis and inducing DNA damage in VSMCs, leading to premature senescence (Ragnauth et al., 2010). Selective overexpression of progerin in VSMCs, but not macrophages, leads to VSMC loss and promotes LDL retention in the aorta and the resultant atherogenesis and death in a mouse model of HGPS (Hamczyk et al., 2018). Disruption of the linker of the nucleoskeleton and cytoskeleton (LINC) complex in VSMCs ameliorates progerin-induced VSMC apoptosis and limits the accompanying adventitial fibrosis (Kim et al., 2018a). Furthermore, VSMC-derived progerin accelerates atherogenesis via inducing endoplasmic reticulum (ER) stress in the aorta (Hamczyk et al., 2019). Mice with progerin overexpression in ECs (progerinecTg) develop perivascular and cardiac fibrosis, cardiac hypertrophy (Fig. 1), and premature death without VSMC depletion (Osmanagic-Myers et al., 2019). Also, progerin expression is increased in human hearts with dilated cardiomyopathy and is strongly associated with left ventricular remodeling and myocardial ageing (Messner et al., 2018). Left ventricular diastolic dysfunction is the most prevalent echocardiographic abnormality in HGPS patients, and its prevalence increases with age (Prakash et al., 2018). Recently, Beyret and colleagues employed a single-dose systemic administration of AAV9-delivered CRISPR-Cas9 components with lamin A/progerin reduction via facial vein injection to repress HGPS in a mouse model (Beyret et al., 2019). At the same time, another group using intraperitoneal injection of AAV9-mediated CRISPR-Cas9 to ameliorate HGPS in LmnaG609G/G609G mice (Santiago-Fernandez et al., 2019). All the results indicate that prelamin A accumulation in different cardiovascular cells due to impaired lamin A processing is a novel biomarker of cardiovascular ageing and contributes to CVD development (Fig. 1) and therefore represents a novel therapeutic target to ameliorate the effects of age-induced cardiovascular dysfunction.
3.2. Impaired autophagy leads to cardiovascular cell senescence
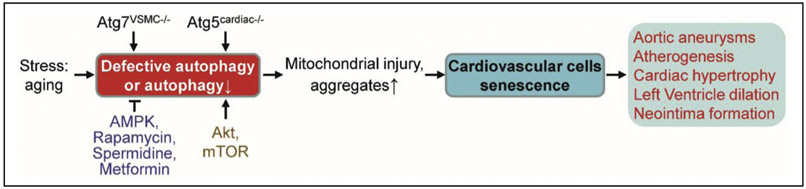
Autophagy is a “housekeeping” cellular process recognized as a mechanism for cell survival when cells encounter stress, including nutrient deprivation or hypoxia, in which cells degrade their dysfunctional proteins, macromolecules, or sub-organelles in lysosomes and recycle them to produce the required raw materials for biosynthesis or energy generation (Anding and Baehrecke, 2017; Grootaert et al., 2018). In general, autophagy appears to be constitutively active in the cardiovascular system, but its activity decreases with age (Kroemer, 2015; Shirakabe et al., 2016). Importantly, inhibited general autophagy or special autophagy of mitochondria (mitophagy) leads to or accelerates cardiovascular ageing (Abdellatif et al., 2018). Dysfunctional autophagy in ECs, VSMCs, and macrophages, plays a detrimental role in atherogenesis (Fig. 2). Growing evidence implies that decreased autophagy results in cardiovascular cell senescence (Sasaki et al., 2017). For instance, VSMC-specific deficiency of the essential autophagy factor autophagy-related 7 (ATG7) causes accumulation of SQSTM1/p62 and accelerates SIPS. ATG7 deletion in VSMCs of ApoE−/− mice promotes ligation-induced neointima formation and Western diet-induced atherogenesis in mice (Grootaert et al., 2015). Interestingly, moderate activation of autophagy by rapamycin has been shown to repress VSMC replicative senescence (Tan et al., 2016) and stabilize progressed atherosclerotic plaques (Luo et al., 2017). Inhibition of autophagic adaptor p62-mediated selective autophagy stabilizes and increases GATA4 protein, which initiates and maintains the SASP, thus triggering senescence of fibroblasts (Kang et al., 2015).
Fig. 2.
Defective autophagy and cardiovascular cell senescence. ⊥, inhibits. Refer to the text for the expanded form of abbreviations.
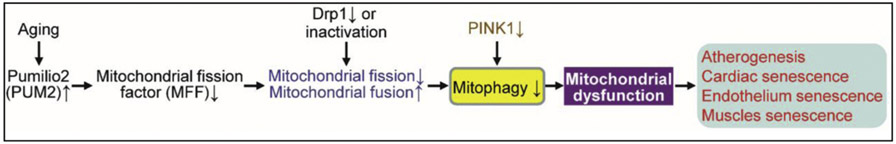
3.3. Mitochondrial dysfunction causes cardiovascular cell senescence
Mitochondrial dysfunction usually drives cellular senescence (Chapman et al., 2019; Wiley et al., 2016), which is characterized by lower NAD+/NADH ratios (Mouchiroud et al., 2013; Watson et al., 2017; Wiley et al., 2016), excluding RAS oncogene-induced fibroblast senescence (Nacarelli et al., 2019). In general, mitochondrial fission reduction-caused inhibition of mitophagy contributes to senescence in multiple cell types by mitochondrial dysfunction (Fig. 3). For example, mouse heart with mitochondrial imbalance between fission (fragmentation) and fusion develops mitochondrial senescence and heart failure due to the impaired mitophagy (Song et al., 2017). Furthermore, increased mitochondrial fission associated with elevation of mitochondrial reactive oxygen species (ROS), but not ER stress, triggers EC senescence and dysfunction, including impaired EC-dependent vasorelaxation and angiogenesis (Kim et al., 2018b). Kim and colleagues recently identified protein disulfide isomerase A1 (PDIA1) as a thiol reductase for the mitochondrial fission protein dynamin-related GTPase1 (Drp1) at Cys644. Diabetic reduction of PDIA1 induces Drp1 sulfenylation (oxidation) at Cys644, promoting Drp1 GTPase activity, which leads to mitochondrial fission contributing to EC senescence (Kim et al., 2018b). On the other hand, ageing also leads to mitochondrial dysfunction. For example, ageing elevates RNA-binding protein Pumilio2 (PUM2) in mouse muscle, which translationally downregulates mitochondrial fission factor (MFF, an outer mitochondrial membrane protein) and thereby inhibits mitochondrial fission and mitophagy, resulting in mitochondrial dysfunction (D’Amico et al., 2019). Interestingly, NAD+ replenishment restores defective mitophagy and mitochondrial function in fibroblasts and consequently restrains the accelerated ageing in Caenorhabditis elegans and Drosophila melanogaster models of Werner syndrome (Fang et al., 2019), a human premature ageing disease. It is unknown whether clearance of dysfunctional fragmented mitochondria by guanine derivative-targeted cargo-mediated mitophagy (Takahashi et al., 2019) attenuates cardiovascular cell senescence.
Fig. 3.
Possible mechanisms for mitochondrial dysfunction leading to cardiovascular cell senescence. For definitions of other abbreviations, please see the main text.
Mitochondrial dysfunction may induce cell senescence through the following mechanisms: 1) instigation of oxidative stress, triggering activation of DNA damage response or telomere damage in cardiomyocytes (Anderson et al., 2019; Chapman et al., 2019); 2) leakage of mitochondrial DNA into the cytoplasm of tubular cells (Chung et al., 2019; Maekawa et al., 2019) or triggering of cytoplasmic chromatin fragmentation in fibroblasts (Vizioli et al., 2020) and consequently driving activation of the cGAS-STING (stimulator of interferon genes) pathway to mediate SASP and senescence; and 3) AMPK-p53 activation-mediated mitochondrial dysfunction-associated senescence with distinct SASP profiles in fibroblasts (Wiley et al., 2016). Mitochondrial DNA polymerase (PolG)-mutated (POLGD257A) mice showing mitochondrial dysfunction with lower NAD+/NADH ratios in inguinal adipose tissue demonstrate more senescent cells in adipose tissue and skin compared to that of age-matched wild-type mice (Wiley et al., 2016). Moreover, overexpression of mitochondria-targeted catalase partially reverses cell senescence in heart and age-related cardiomyopathy in POLGD257A mice in vivo (Dai et al., 2010).
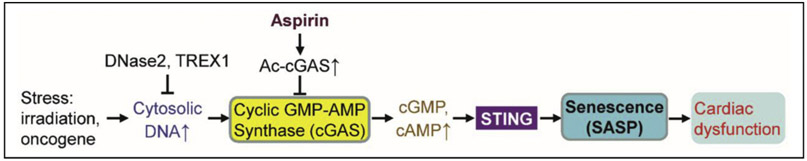
3.4. cGAS-STING signaling in cardiovascular cell senescence and disease
Although DNA damage responses have been tightly linked to cardiovascular cell senescence (Gray et al., 2015; Matthews et al., 2006), the underlying mechanism remains incompletely understood. Damaged or stressed cells usually have increased chromatin fragmentation and cytosolic DNA, which binds and activates cyclic guanosine monophosphate-adenosine monophosphate (GMP-AMP) synthase (cGAS) (Ablasser and Chen, 2019). The activation of cGAS, in turn, increases the second messenger molecule 2′3′ cyclic GMP-AMP (cGAMP), which binds and activates the ER protein STING (Motwani et al., 2019), which triggers the production of SASP factors (including IL-6 and TNF-α) and paracrine senescence (Gluck et al., 2017). Numerous stimuli (including oxidative stress) of cellular senescence engage the cGAS-STING pathway in fibroblasts in vitro (Gluck et al., 2017). In pre-senescent hepatic stellate cells and human diploid fibroblasts, transcriptional downregulation of E2F-mediated cytoplasmic DNases (DNase2 and DNA 3’ repair exonuclease 1 [TREX1]) results in cytoplasmic accumulation of nuclear DNA, which provokes aberrant activation of cGAS-STING signaling and resultant SASP and cellular senescence (Takahashi et al., 2018). The cGAS-STING pathway mediates irradiation- and NRasV12 oncogene-induced senescence and SASP in mice in vivo (Gluck et al., 2017). Interestingly, cGAS activity can be post-translationally regulated. Dai et al. reported that aspirin-induced cGAS acetylation at one of three lysine residues (K384, K394, or K414) robustly suppresses cGAS activity and self DNA-induced autoimmunity in a mouse model of Aicardi-Goutières syndrome (AGS) (Dai et al., 2019). Whether senescence stimuli lead to deacetylation of cGAS in the cardiovascular system remains undetermined. It has been reported that cGAS-STING signaling from ischemic cell death results in a fatal response to myocardial infarction (MI). Inhibition of the cGAS-STING-IRF3-type I interferon axis blocks pathological myocardial remodeling, maintains cardiac function, and improves post-MI cardiac repair and survival in mice (Fig. 4) (Cao et al., 2018; King et al., 2017). These studies suggest a novel molecular mechanism for cellular senescence and suggest that modulation of cGAS activity may be a new strategy to treat senescence-associated cardiovascular disease. Cytosolic DNA from dysfunctional mitochondria and nuclei of senescent cardiovascular cells would activate cGAS-STING signaling. Whether and how cGAS-STING signaling plays causative roles in cardiovascular cell senescence warrants further exploration. It remains to be determined whether the regulation of cGAS or STING is beneficial in CVD prevention and therapy.
Fig. 4.
Possible roles of cGAS-STING pathway in cardiovascular cell senescence. ⊥, inhibits. For definitions of other abbreviations, please see the main text.
3.5. Other mechanisms
There are other mechanisms underlying cardiovascular cell senescence. Epigenetic events, including DNA methylation, regulate cell senescence (known as an epigenetic clock) (Cheng et al., 2017; Ermolaeva et al., 2018). For example, hypermethylation of DNA cytosine-preceding-guanosine (CpG) islands in the NAMPT promoter is present within both dilated thoracic aortas and VSMCs, is inversely associated with NAMPT mRNA level, leading to NAD+ reduction and consequent VSMC premature senescence (Watson et al., 2017). Recently, a high-throughput screen of a library of short hairpin RNAs for targeted silencing of all known epigenetic proteins showed that histone acetyltransferase p300 positively controls replicative senescence of IMR-90 lung fibroblasts via inducing a dynamic hyper-acetylated chromatin state (Sen et al., 2019).
Noncoding RNAs (ncRNAs) also play crucial roles in cell senescence. Notably, long ncRNAs (lncRNAs; > 200 nt in length) have recently been demonstrated to play critical roles in ageing and age-related diseases (Kour and Rath, 2016; Zhang et al., 2018). Abdelmohsen et al. used RNA sequencing and reported that lncRNA MALAT1 (metastasis-associated lung adenocarcinoma transcript 1) is decreased in senescent fibroblasts (Abdelmohsen et al., 2013). lncRNA MALAT1 may be reduced in senescent ECs as proliferating human ECs have higher levels of lncRNA MALAT1 (Michalik et al., 2014). lncRNA Meg3 (maternally expressed gene 3) is upregulated in senescent human umbilical vein endothelial cells (HUVECs). Meg3 reduction in HUVECs blocks age-induced inhibition of sprouting angiogenesis in vitro. Meg3 silencing restores blood flow impaired in an aged mouse ischemic hind limb in vivo (Boon et al., 2016). Recently, it was reported that oncogene HRas-induced senescent fibroblasts had increased lncRNA-OIS1, which transcriptionally upregulates DPP4 protein (Li et al., 2018). lncRNA-OIS1 may also be elevated in senescent ECs because senescent ECs have higher DPP4 levels (Kim et al., 2017). However, the functions and regulation of lncRNAs implicated in cardiovascular senescence are largely unknown.
4. Clearance of senescent cardiovascular cells alleviates CVD
Compelling data indicate that senescent cardiovascular cells lead to and accelerate CVD onset and development; thus, senescent cells are an emerging target for age-related disease, including CVD (Childs et al., 2017). Targeting senescent cardiovascular cells is a potential strategy to prevent or cure CVDs. For example, inhibiting vascular cell senescence by β-hydroxybutyrate (Han et al., 2018), which is elevated by fasting and calorie restriction, may be beneficial for prevention of CVD having diverse risk factors (Chakraborty et al., 2018). Rapamycin (Flynn et al., 2013; Singh et al., 2016) or metformin (Barzilai et al., 2016; Yin et al., 2011), acting on the senescent cell property of SASP, also attenuates or reverses CVD development. Interestingly, therapeutic removal of senescent cells is emerging as a promising and innovative strategy to delay cardiovascular ageing or disease progression. Currently, several approaches are being used for the elimination of senescent cardiovascular cells in in vitro and in vivo models.
4.1. Induction of apoptosis in senescent cardiovascular cells by small-molecule drugs
Because senescent cells have a pivotal feature, resistance to apoptosis due to elevation of pro-survival molecules, the B cell lymphoma 2 (BCL-2) family proteins (BCL-2, BCL-W, and BCL-XL) (Singh et al., 2019), the development of novel small-molecule inhibitors of these proteins, known as BH3 mimetics, has been used to selectively induce apoptosis of senescent cells (Yosef et al., 2016), preparing for elimination of apoptotic cells by phagocytosis. Senotherapeutic agents are used to target features of cellular senescence (Table 3). For example, senolytics are used to target anti-apoptotic signaling molecules and induce cell death of senescent vascular cells (Chang et al., 2016; Zhu et al., 2016). Elegant experiments by Childs and colleagues demonstrated that clearance of senescent cells by ABT-263 (navitoclax) dramatically inhibits atherogenesis onset in the aortic arch of high-fat diet (HFD)-fed Ldlr−/− mice (Childs et al., 2016). Treatment of aged (2-year-old) mice with the senolytic drug ABT-263 eliminates senescent cardiomyocytes and consequently reduces fibrosis and cardiomyocyte hypertrophy (Anderson et al., 2019). Importantly, clearance of senescent cells by ABT-263 attenuates myocardial remodeling and improves diastolic function, as well as overall survival in aged mice following myocardial infarction mimicked by ligation of the left anterior descending coronary artery (Walaszczyk et al., 2019). BH3 mimetics ABT-737 and ABT-199 targeting BCL-2 specifically eliminate senescent pancreatic beta cells without effect on the abundance of the immune cell (lymphoid or myeloid) types in a non-obese diabetic mouse model and prevent type 1 diabetes (Thompson et al., 2019).
Table 3.
Major compounds for eliminating senescent cells
| Compounds | Molecular targets | Animal models | Treatments | Outcomes | Clinical trials |
|---|---|---|---|---|---|
| ABT263 (Navitoclax) | BCL-2, BCL-XL (Chang et al., 2016; Zhu et al., 2016) | HFD-fed Ldlr−/− mice (Childs et al., 2016); 24-month old male C57BL/6 mice (Anderson et al., 2019). | 100 mg/kg ABT263 in PBS with 15% DMSO/7% Tween-20 (Childs et al., 2016); Oral gavage (50 mg/kg BW/day) for 7 days per cycle for 2 cycles with a 1-week interval between cycles (Anderson et al., 2019) | Inhibits atherogenesis onset and stabilizes atherosclerotic plaques (Childs et al., 2016); rejuvenates aged hematopoietic stem cells in mice (Chang et al., 2016); promotes cardiomyocyte regeneration (Anderson et al., 2019) | NCT00406809 (for relapsed or refractory lymphoid malignancies); NCT00445198 (for SCLC or other non-hematological malignancies |
| ABT737 | BCL-W, BCL-XL (Yosef et al., 2016) | 8 Gy-irradiated male mice; p14ARF-expressing mice | i.p. injection with 75 mg/kg for 2–4 days | Elimination of senescent cells in lungs and from the epidermis (Yosef et al., 2016) | N/A |
| Dasatinib (D) + Quercetin (Q) | Tyrosine protein kinases (Guo et al., 2018) | Aged and ApoE−/− mice (Roos et al., 2016); senescent cell-transplanted mice or aged (20–27-month old, 27.7 ± 2.7 months) mice. | Oral gavage (D, 5 mg/kg + Q, 10 or 50 mg/kg) single dose (Zhu et al., 2015), once monthly for 3 months or once weekly for 2 months) (Roos et al., 2016); D (5 mg/kg) + Q (50 mg/kg) (Xu et al., 2018); D (5 mg/kg) + Q (50 mg/kg) 3 consecutive days every 2 weeks for 2 months (Lewis-McDougall et al., 2019) | Improves systolic cardiac function and vascular relaxation (Zhu et al., 2015); decreases aortic calcification (Roos et al., 2016); alleviates physical dysfunction (Xu et al., 2018); activates resident CPCs (Lewis-McDougall et al., 2019) | NCT02874989 (for idiopathic pulmonary fibrosis) (Justice et al., 2019); NCT02848131, phase 2 (for chronic kidney disease; improve function of ADMSC) (Hickson et al., 2019) |
| 17-DMAG | HSP90 | Ercc1−/Δ mice (Fuhrmann-Stroissnigg et al., 2017); STZ-treated ApoE−/− mice (Lazaro et al., 2015) | 3x weekly with 1 week on followed by 2 weeks off, at 10 mg/kg by oral gavage beginning at 6 weeks of age (Fuhrmann-Stroissnigg et al., 2017); 2–4 mg/kg i.p., every other day for 10 weeks (Lazaro et al., 2015) | Extends health span (Fuhrmann-Stroissnigg et al., 2017), decrease atherosclerotic lesions and induces a more stable plaque phenotype (Lazaro et al., 2015) | NCT00088868 (for solid tumor or lymphoma) |
| Cardiac glycosides | ATP1A1 of Na+/K+ ATPase | Aged (24-month-old) or ApoE−/− mice (Shi et al., 2016) | Digoxin (2 mg/kg, i.p., twice weekly) (Triana-Martinez et al., 2019); Ouabain (1 mg/kg, i.p.) (Guerrero et al., 2019) | Reduce lung fibrosis, inhibits atherogenesis (Shi et al., 2016); tumor suppression (Guerrero et al., 2019) | Cardiac disease treatment, cancer therapy |
| FoxO4-DRI peptide | FoxO4-p53 interaction (Baar et al., 2017) | Doxorubicin-treated, fast- ageing XpdTTD/TTD, and naturally aged mice. | Injection at 5 mg/kg every other day for 2 weeks. | Induces the apoptosis of senescent cells, neutralizes doxorubicin-induced chemotoxicity, improves fitness, fur growth, and renal function in both fast ageing XpdTTD/TTD and naturally aged mice. | N/A (Cleara Biotech in UMC Utrecht is optimizing the drugs). |
17-DMAG, 17-dimethylaminoethylamino-17-demethoxygeldanamycin; ADMSC, adipose-derived mesenchymal stem cells; BCL-W, B cell lymphoma W; BCL-XL, B cell lymphoma extra large; DRI, D-retro inverso; i.p., intraperitoneal; N/A, not available; STZ, streptozotocin. For definitions of other abbreviations, please see the main text.
As senescent cells share common SASP and apoptosis-resistance features with cancer cells, dasatinib (D), which is used in the cancer treatment, may have a role in clearing senescent cells. Zhu et al. demonstrated that oral gavage administration of single-dose dasatinib + quercetin (D + Q) dramatically decreases senescent cell number and improves cardiac function of 24-month-old mice as shown by improved left ventricular ejection fraction and fractional shortening (Zhu et al., 2015). A single D + Q treatment significantly improves vascular endothelial function and vascular smooth muscle sensitivity to nitroprusside. However, senescent cell elimination does not change smooth muscle contractile function (Zhu et al., 2015). Intermittent treatment with D + Q by oral gavage reduces the number of TAF-positive senescent VSMCs in the aorta media of aged (24-month old) and atherosclerotic ApoE−/− mice (fed a western diet for two months), but not in established intimal atherosclerotic plaques. Treatment with D + Q also improves vasomotor function in aged mice, as well as reduced aortic calcification in ApoE−/− mice. However, D + Q treatment does not affect intimal plaque size (Roos et al., 2016). Additionally, clearance of senescent glial cells from HFD-fed or leptin receptor-deficient obese mice by D + Q restores neurogenesis and alleviates neuropsychiatric disorders, including anxiety and depression (Ogrodnik et al., 2019). D + Q senolytic treatment selectively clears amyloid beta (Aβ)-triggered senescent oligodendrocyte progenitor cells (OPCs) characterized by upregulation of p21, p16, and SA β-gal activity, and decreases Aβ plaque load and subsequent cognitive improvement in Alzheimer's disease mice (Zhang et al., 2019). In clinical trial, D + Q treatment (D, 100 mg/day plus Q, 1250 mg/day, 3 times per week for three weeks) improves physical function of patients with idiopathic pulmonary fibrosis (Justice et al., 2019). Another D + Q phase 2 pilot study (oral D 100 mg and Q 1000 mg for three days) on subjects with diabetic kidney disease decreases adipose tissue senescence and circulating key SASP factors (Hickson et al., 2019). It is noteworthy that dasatinib treatment increases susceptibility to experimental pulmonary hypertension development in rats (Guignabert et al., 2016).
More approaches have been used to induce apoptosis of senescent cells. Compared with healthy cells, senescent cells upregulate transcription factor forkhead box protein O4 (FoxO4), which interacts with p53. FoxO4-DRI peptide, designed to interfere with the interaction of FoxO4 and p53, thus directs p53 from the nucleus to mitochondria for apoptosis induction. Selective downregulation of FoxO4 by inhibitory RNA triggers apoptosis in senescent, but not healthy, cells via release and activation of p53 (Baar et al., 2017).
Intriguingly, senolytic drugs seem to exert their effects in a cell type-specific manner. For example, dasatinib is more effective in selectively killing senescent human pre-adipocytes than HUVECs, whereas quercetin (polyphenol, PI3K inhibitor) is more effective in killing senescent HUVECs and mouse bone marrow-derived mesenchymal stem cells (BM-MSCs) than senescent adipocytes (Zhu et al., 2015). ABT-263, targeting the anti-apoptotic BCL-2 family, selectively increases apoptosis and decreases cell viability of senescent but not proliferating HUVECs, while does not affectprimary human preadipocytes (Zhu et al., 2016). D + Q does not affect the viability of proliferating or quiescent cells. The HSP90 inhibitor Ganetespib exhibits senolytic activity in IR-induced senescent HUVECs, but not in pre-adipocytes (Fuhrmann-Stroissnigg et al., 2017).
4.2. Immune clearance of senescent or apoptotic cells
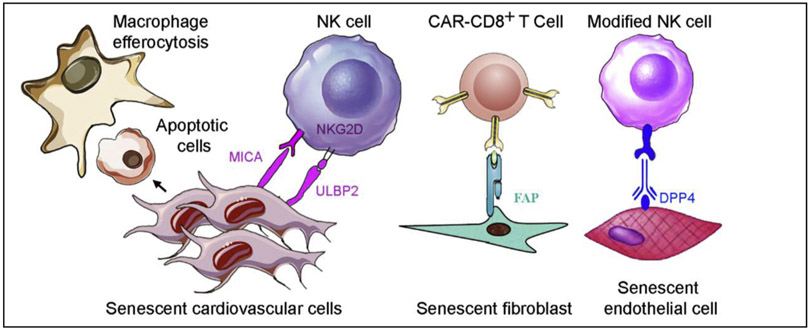
Accumulating data indicate that immune surveillance of senescent cells is mediated by immune cells, such as macrophages, natural killer (NK) cells, neutrophils, and cytotoxic T cells in tumors (Burton and Krizhanovsky, 2014; Kang et al., 2011; Xue et al., 2007) and liver cirrhosis (Krizhanovsky et al., 2008). Different senescent cells generate unique ligands that attract different immune cells. For example, senescence-related hepatic stellate cells elevate cell surface MICA and ULBP2, ligands of activating receptor NKG2D, on NK cells (Krizhanovsky et al., 2008). Senescent cells may express specific surface antigens, such as major histocompatibility complex class II (MHCII) molecules that will be recognized by distinct cells (such as CD4 + T) of the immune system and subsequently killed (Kang et al., 2011). At present, senescence immunotherapy is an emerging research field (Burton and Stolzing, 2018; Hoenicke and Zender, 2012; Krizhanovsky et al., 2008; Sagiv et al., 2013). Senescence immunotherapy strategies are also a promising alternative to senolytics for removing senescent cardiovascular cells in CVD prevention and therapy (Fig. 5).
Fig. 5.
Proposed immunotherapies targeting senescent cardiovascular cells. Refer to the text for the expanded form of abbreviations.
4.2.1. Macrophages engulf apoptotic or senescent cells
It was reported that macrophages engulf senescent cells in cancer. Kang and colleagues presented that CD4+ T cells need monocytes or- macrophages, but not NK cells, to clear pre-malignant senescent hepatocytes and subsequently restrain liver cancer development (Kang et al., 2011). Interestingly, p53 restoration induces liver tumor cell senescence with upregulated p16 and SA β-gal activity, but not apoptosis, in mice in vivo. The senescent tumor cells attract innate immune cells, including macrophages, neutrophils, and NK cells, resulting in clearance of senescent tumor cells and resultant tumor regression (Xue et al., 2007). Whether macrophages remove senescent cardiovascular cells in aged or diseased cardiovascular systems remains to be elucidated.
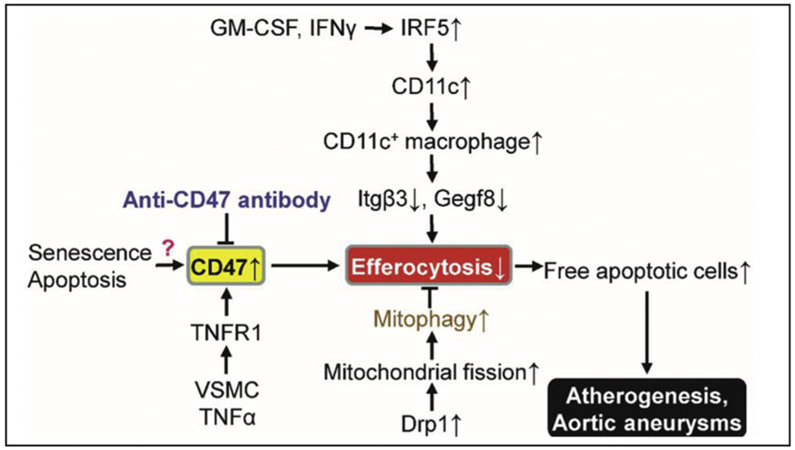
It is well known that macrophages can clear apoptotic cells in a process known as efferocytosis, which prevents apoptotic cells from becoming necrotic or acquiring pro-inflammatory activity (Henson, 2017; Roberts et al., 2017). Impaired macrophage efferocytosis would enhance atherosclerotic lesion development (Kojima et al., 2017; Proto et al., 2018; Schrijvers et al., 2005) and vulnerable plaque formation (Seneviratne et al., 2017; Thorp et al., 2008; Yurdagul et al., 2017). For example, transcription factor interferon regulatory factor (IRF)-5 enhances fragile plaque formation through maintenance of pro-inflammatory CD11c+ macrophages within atherosclerotic lesions and by stimulating the expansion of the necrotic core by impairing macrophage efferocytosis mediated by downregulated integrin-β3 and its ligand, milk fat globule-epidermal growth factor 8 (Fig. 6) (Seneviratne et al., 2017)
Fig. 6.
Efferocytosis regulation and cardiovascular disease. For definitions of other abbreviations, please see the main text.
Both the macrophage itself and the features of apoptotic or senescent cells regulate macrophage efferocytosis capability. Tissue-resident macrophages silently eradicating apoptotic cells with limited recognition of nucleic acids within the apoptotic cells are characterized by a lack of Toll-like receptor 9 (TLR9) expression (Roberts et al., 2017). Recently, Yang et al. reported that C-type lectin receptor LSECtin (Clec4g) in colon macrophages is needed for macrophage engulfment and elimination of apoptotic cells (Yang et al., 2018). It is noteworthy that Treg cells secrete interleukin-13 (IL-13), thus stimulating IL-10 production in macrophages. The upregulated IL-10 signaling elevates macrophage Vav1 (a guanine nucleotide exchange factor), which activates GTPase Rac1 to promote apoptotic cell engulfment by macrophages (Proto et al., 2018). Continued clearance of multiple apoptotic cells by macrophages requires Drp1-mediated macrophage mitochondrial fission, which is initiated by the first uptake of apoptotic cells (Wang et al., 2017). Drp1-deficient macrophages show defective efferocytosis and subsequently increased plaque necrosis in western diet-fed Ldlr1−/− mice (Wang et al., 2017). On the other hand, apoptotic cell fate also affects macrophage efferocytosis. For example, apoptotic cells expressing cell-surface protein CD47, a “don’t eat me” signal, impair macrophage efferocytosis. Antibodies against CD47 markedly recover efferocytosis without cellular apoptosis alternation, as well as reduce atherosclerosis in both aortic sinus and en face aorta (Kojima et al., 2016). Moreover, the anti-CD47 antibody ameliorates AAA formation in an ApoE−/−/AngII model and a porcine pancreatic elastase model (Kojima et al., 2018). Cyclin-dependent kinase inhibitor 2B (CDKN2B)-deficient apoptotic cells are resistant to efferocytosis leading to accelerated atherogenesis due to the reduction of calreticulin, a principal phagocyte receptor ligand (Gardai et al., 2005). Supplementation with exogeneous calreticulin normalizes the engulfment of CDKN2B-deficient apoptotic cells (Kojima et al., 2014). Thus, it is critical for us to know the molecular mechanisms regulating the phagocytic ability and senescent cell clearance by macrophages in CVD progression and therapy.
4.2.2. NK cells eradicate senescent cells
The human NK cell line YT selectively targets etoposide-induced senescent and activated hepatic stellate cells, but not proliferating cells, in vitro. Also, YT cells preferentially attack senescent IMR-90 cells, which then undergo apoptosis and detach from the surface of the culture dish (Krizhanovsky et al., 2008). This selectivity is because expression of NKG2D ligands MICA and ULBP2 is selectively upregulated in senescent IMR-90 fibroblasts, but not in growing or quiescent cells (Sagiv et al., 2016). Furthermore, NK cell activation with polyinosinic-polycytidylic acid (Radaeva et al., 2006) decreases senescent cell number in the liver in vivo resulting in the resolution of liver fibrosis (Krizhanovsky et al., 2008). NKG2D receptor deletion enhances the accumulation of senescent stellate cells leading to increased liver fibrosis in mice (Sagiv et al., 2016). Chemotherapeutic agents, including doxorubicin, melphalan, and bortezomib, increase both DNAM-1 (DNAX accessory molecule-1; CD 226) ligand PVR (poliovirus receptor; CD155) and NKG2D ligands (MICA and MICB) on multiple myeloma cells exhibiting a senescent phenotype. These ligands promote NK cell susceptibility (Soriani et al., 2009). Interestingly, PVR and Nectin-2 are expressed at cell junctions on primary vascular ECs. Moreover, the specific binding of DNAM-1-Fc molecule was detected at endothelial junctions. This binding is almost completely abrogated by anti-PVR monoclonal antibodies (mAbs), but is not modified by - mAbs anting Nectin-2, which demonstrates that PVR is the major DNAM-1 ligand on ECs. Both anti-DNAM-1 and anti-PVR mAbs strongly block the transmigration of monocytes through the endothelium (Reymond et al., 2004). Moreover, granule exocytosis, but not death-receptor-mediated apoptosis, is required for NK cell-mediated killing of senescent cells. Accordingly, mice with defects in granule exocytosis accumulate senescent stellate cells and display more liver fibrosis in response to a fibrogenic agent (Sagiv et al., 2013). Unfortunately, the roles of NK cell-mediated depletion of senescent cardiovascular cells in CVD progression remain unknown.
Senescent human diploid fibroblasts selectively and robustly elevate expression of DPP4 on the cell surface, but not in the cytosol, compared with proliferating fibroblasts (Kim et al., 2017). Anti-DPP4 antibodies have been used to recognize the specific antigen DPP4 on the cell surface of senescent cells and guide NK cells to selectively destroy the antibody-labeled senescent cells in vitro (Fig. 5). Because senescent HUVECs and HAECs also express higher levels of DPP4 mRNA (Kim et al., 2017), whether we can use a DPP4-based mechanism to eradicate senescent cardiovascular cells needs further exploration. Whether senescent cardiovascular cells generate specific surface ligands recognized by NK cell receptors, such as NKG2D and DNAM-1, is another exciting research arena.
4.2.3. Dendritic cells and senescent or apoptotic vascular cells
Dendritic cells (DCs), one kind of professional phagocytic cells, can also recognize and remove apoptotic cells (Albert et al., 1998). For example, DCs exclusively traffic mouse apoptotic intestinal epithelial cells (IECs) to mesenteric lymph nodes, which serve as crucial determinants for the induction of tolerogenic regulatory CD4+ T-cell differentiation and activation (Cummings et al., 2016). DC accumulation in aorta intima of aged wild-type mice, but not of young mice, is associated with increased atherosclerosis (Liu et al., 2008). CD11b+ DCs with impaired autophagy as a result of ATG16l1 deficiency expand aortic CD4+ Treg cells and inhibit atherosclerosis in Ldlr−/− mice (Clement et al., 2019). Chemokine (C-C motif) receptor 9 (CCR9)+ pDCs expressing indoleamine 2,3-dioxygenase 1 (IDO1) in aorta locally induce aortic Treg cells, which produce IL-10 and subsequently prevent atherogenesis (Yun et al., 2016). However, it is largely unknown whether and how DCs eliminate apoptotic or senescent cells in cardiovascular systems.
4.2.4. Chimeric antigen receptor T cells eliminate senescent cardiovascular cells
Redirecting cytotoxic T cells to recognize the particular antigens on cancer cells using either a modified T-cell receptor or a chimeric antigen receptor (CAR) has been successfully used for certain cancer therapies (June et al., 2018). Fibroblast activation protein (FAP), a cell-surface glycoprotein (Scanlan et al., 1994), is selectively and highly expressed in activated cardiac fibroblasts, but not cardiomyocytes (Aghajanian et al., 2019). High FAP expression contributes to cardiac fibrosis and resultant myocardial disease. Recently, adoptive transfer of engineered antigen-specific CD8+ T cells specifically targeting FAP dramatically ablated cardiac fibrosis and restored both systolic and diastolic cardiac function in Ang II- and phenylephrine-exposed mice (Aghajanian et al., 2019). Because senescent cells produce specific cell-surface antigens, such as band 3 (Kay, 1993) and an oxidized form of membrane-bound vimentin (Frescas et al., 2017), developing particular CAR T cells to selectively deplete senescent cardiovascular cells is a promising strategy.
5. Conclusions and perspectives
Homeostasis of senescent cardiovascular cells is required for a healthy cardiovascular system. Multiple complex molecular pathways regulate cardiovascular cell senescence in vitro and in vivo. Emerging evidence suggests that permanent accumulation of senescent cardiovascular cells is responsible for the initiation and development of various CVDs and cardiovascular ageing. Senolytics and senescence immunotherapy are developing strategies for CVD prevention and therapy. However, there is insufficient understanding of the molecular mechanisms that precisely drive the deregulation of cardiovascular cell senescence during CVD onset. Currently, there are no highly selective markers for senescent cardiovascular cells in vivo (Gorgoulis et al., 2019). It is still challenging to spatiotemporally identify and quantify individual senescent cardiovascular cells in vivo in a noninvasive manner (Biran et al., 2017). All of these circumstances have prevented the development of effective treatments for CVD. Development of novel therapeutic approaches to target senescent cardiovascular cells and reduce significant clinical consequences such as MI or stroke, will depend on a rigorous understanding of the senescence biology of each of the major cell types that contribute to the pathogenesis of CVD. So far, only D + Q has been assessed in the clinical setting, and none of the current clinical trials is testing whether senolytic agents can prevent cardiovascular disorders. A more in-depth understanding of molecular mechanisms underlying activation of the immune response, as well as special recognition and targeting of a senescent cardiovascular cell, is warranted. Taken together, to target the senescent cardiovascular cells accurately, effectively, and safely, it is essential to do the following research: 1) identify the unique spatiotemporal biomarkers (particularly the cell surface markers) and targets for senescence of different cardiovascular cells in vivo; 2) investigate the mechanism underlying cardiovascular cell senescence and its function in CVD onset and progression; 3) validate the efficiency and potential side effects of known senolytics in animal models and the cardiovascular clinic; 4) explore novel senolytic agents or local delivery methods that can act on specific senescent cardiovascular cells or tissues and optimize the dosage, mode of administration, and combinations for the treatment of various CVDs; and 5) develop a novel strategy for clearance of senescent cardiovascular cells by immunosurveillance.
Acknowledgments
This study was supported in part by the following agencies: National Institute on Ageing (AG047776) and National Heart, Lung, and Blood Institute (HL140954). M.-H. Zou is an eminent scholar of the Georgia Research Alliance.
Footnotes
Disclosures
The authors declare that there are no conflicts of interest.
References
- Abdellatif M, Sedej S, Carmona-Gutierrez D, Madeo F, Kroemer G, 2018. Autophagy in Cardiovascular Aging. Circ Res 123, 803–824. [DOI] [PubMed] [Google Scholar]
- Abdelmohsen K, Panda A, Kang MJ, Xu J, Selimyan R, Yoon JH, Martindale JL, De S, Wood WH 3rd, Becker KG, Gorospe M, 2013. Senescence-associated lncRNAs: senescence-associated long noncoding RNAs. Aging Cell 12, 890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablasser A, Chen ZJ, 2019. cGAS in action: Expanding roles in immunity and inflammation. Science 363, eaat8657. [DOI] [PubMed] [Google Scholar]
- Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, Wang T, Bedi K, Morley MP, Linares Saldana RA, Bolar NA, McDaid K, Assenmacher CA, Smith CL, Wirth D, June CH, Margulies KB, Jain R, Pure E, Albelda SM, Epstein JA, 2019. Targeting cardiac fibrosis with engineered T cells. Nature 573, 430–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N, 1998. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med 188, 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert A, Pickman Y, Leipold M, Rosenberg-Hasson Y, Ji X, Gaujoux R, Rabani H, Starosvetsky E, Kveler K, Schaffert S, Furman D, Caspi O, Rosenschein U, Khatri P, Dekker CL, Maecker HT, Davis MM, Shen-Orr SS, 2019. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med 25, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, Birch J, Salmonowicz H, Ogrodnik M, Jurk D, Proctor C, Correia-Melo C, Victorelli S, Fielder E, Berlinguer-Palmini R, Owens A, Greaves LC, Kolsky KL, Parini A, Douin-Echinard V, LeBrasseur NK, Arthur HM, Tual-Chalot S, Schafer MJ, Roos CM, Miller JD, Robertson N, Mann J, Adams PD, Tchkonia T, Kirkland JL, Mialet-Perez J, Richardson GD, Passos JF, 2019. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J 38, e100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anding AL, Baehrecke EH, 2017. Cleaning House: Selective Autophagy of Organelles. Dev Cell 41, 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IWF, Houtsmuller AB, Pothof J, deBruin RWF, Madl T, Hoeijmakers JHJ, Campisi J, deKeizer PLJ, 2017. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 169 (132-147), e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM, 2016. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Petersen RC, 2018. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest 128, 1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, vande Sluis B, Kirkland JL, van Deursen JM, 2011. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA, 2016. Metformin as a Tool to Target Aging. Cell Metab 23, 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Macdonald K, Chan SW, Boyle JJ, Weissberg PL, 1998. Cooperative interactions between RB and p53 regulate cell proliferation, cell senescence, and apoptosis in human vascular smooth muscle cells from atherosclerotic plaques. Circ Res 82, 704–712. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Sinha S, Owens GK, 2016. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res 118, 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyret E, Liao HK, Yamamoto M, Hernandez-Benitez R, Fu Y, Erikson G, Reddy P, Izpisua Belmonte JC, 2019. Single-dose CRISPR-Cas9 therapy extends lifespan of mice with Hutchinson-Gilford progeria syndrome. Nat Med 25, 419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biran A, Zada L, Abou Karam P, Vadai E, Roitman L, Ovadya Y, Porat Z, Krizhanovsky V, 2017. Quantitative identification of senescent cells in aging and disease. Aging Cell 16, 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV, 2011. Cell cycle arrest is not senescence. Aging (Albany NY) 3, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochenek ML, Schutz E, Schafer K, 2016. Endothelial cell senescence and thrombosis: Ageing clots. Thromb Res 147, 36–45. [DOI] [PubMed] [Google Scholar]
- Boe AE, Eren M, Murphy SB, Kamide CE, Ichimura A, Terry D, McAnally D, Smith LH, Miyata T, Vaughan DE, 2013. Plasminogen activator inhibitor-1 antagonist TM5441 attenuates Nomega-nitro-L-arginine methyl ester-induced hypertension and vascular senescence. Circulation 128, 2318–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne G, Levy N, 2003. LMNA mutations in atypical Werner’s syndrome. Lancet 362, 1585–1586 author reply 1586. [DOI] [PubMed] [Google Scholar]
- Boon RA, Hofmann P, Michalik KM, Lozano-Vidal N, Berghauser D, Fischer A, Knau A, Jae N, Schurmann C, Dimmeler S, 2016. Long Noncoding RNA Meg3 Controls Endothelial Cell Aging and Function: Implications for Regenerative Angiogenesis. J Am Coll Cardiol 68, 2589–2591. [DOI] [PubMed] [Google Scholar]
- Boumendil C, Hari P, Olsen KCF, Acosta JC, Bickmore WA, 2019. Nuclear pore density controls heterochromatin reorganization during senescence. Genes Dev 33, 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ, 2003. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol 23, 842–846. [DOI] [PubMed] [Google Scholar]
- Buchwalter A, Hetzer MW, 2017. Nucleolar expansion and elevated protein translation in premature aging. Nature Communications 8, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DG, Krizhanovsky V, 2014. Physiological and pathological consequences of cellular senescence. Cell Mol Life Sci 71, 4373–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DG, Sheerin AN, Ostler EL, Smith K, Giles PJ, Lowe J, Rhys-Williams W, Kipling DG, Faragher RG, 2007. Cyclin D1 overexpression permits the reproducible detection of senescent human vascular smooth muscle cells. Ann N Y Acad Sci 1119, 20–31. [DOI] [PubMed] [Google Scholar]
- Burton DGA, Stolzing A, 2018. Cellular senescence: Immunosurveillance and future immunotherapy. Ageing Res Rev 43, 17–25. [DOI] [PubMed] [Google Scholar]
- Cafueri G, Parodi F, Pistorio A, Bertolotto M, Ventura F, Gambini C, Bianco P, Dallegri F, Pistoia V, Pezzolo A, Palombo D, 2012. Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition. PLoS One 7, e35312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E, 2019. From discoveries in ageing research to therapeutics for healthy ageing. Nature 571, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao DJ, Schiattarella GG, Villalobos E, Jiang N, May HI, Li T, Chen ZJ, Gillette TG, Hill JA, 2018. Cytosolic DNA Sensing Promotes Macrophage Transformation and Governs Myocardial Ischemic Injury. Circulation 137, 2613–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaldi A, Dodia RM, Orogo AM, Zambrano CM, Najor RH, Gustafsson AB, Heller Brown J, Purcell NH, 2017. Decline in cellular function of aged mouse c-kit (+) cardiac progenitor cells. J Physiol 595, 6249–6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA, 2003. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361, 393–395. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Galla S, Cheng X, Yeo JY, Mell B, Singh V, Yeoh B, Saha P, Mathew AV, Vijay-Kumar M, Joe B, 2018. Salt-Responsive Metabolite, beta-Hydroxybutyrate, Attenuates Hypertension. Cell Rep 25 (677-689), e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D, 2016. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J, Fielder E, Passos JF, 2019. Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett 593, 1566–1579. [DOI] [PubMed] [Google Scholar]
- Chen HZ, Wang F, Gao P, Pei JF, Liu Y, Xu TT, Tang X, Fu WY, Lu J, Yan YF, Wang XM, Han L, Zhang ZQ, Zhang R, Zou MH, Liu DP, 2016. Age-Associated Sirtuin 1 Reduction in Vascular Smooth Muscle Links Vascular Senescence and Inflammation to Abdominal Aortic Aneurysm. Circ Res 119, 1076–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LQ, Zhang ZQ, Chen HZ, Liu DP, 2017. Epigenetic regulation in cell senescence. J Mol Med (Berl) 95, 1257–1268. [DOI] [PubMed] [Google Scholar]
- Chi C, Li DJ, Jiang YJ, Tong J, Fu H, Wu YH, Shen FM, 2019. Vascular smooth muscle cell senescence and age-related diseases: State of the art. Biochim Biophys Acta Mol Basis Dis 1865, 1810–1821. [DOI] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Kirkland JL, Campisi J, vanDeursen JM, 2014. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep 15, 1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, vanDeursen JM,2016. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, vanDeursen JM, 2017. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov 16, 718–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P, 2003. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res 93, 604–613. [DOI] [PubMed] [Google Scholar]
- Cho S, Hwang ES, 2012. Status of mTOR activity may phenotypically differentiate senescence and quiescence. Mol Cells 33, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KW, Dhillon P, Huang S, Sheng X, Shrestha R, Qiu C, Kaufman BA, Park J, Pei L, Baur J, Palmer M, Susztak K, 2019. Mitochondrial Damage and Activation of the STING Pathway Lead to Renal Inflammation and Fibrosis. Cell Metab 30 (784-799), e785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianflone E, Torella M, Chimenti C, De Angelis A, Beltrami AP, Urbanek K, Rota M, Torella D, 2019. Adult Cardiac Stem Cell Aging: A Reversible Stochastic Phenomenon? Oxid Med Cell Longev 2019, 5813147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement M, Raffort J, Lareyre F, Tsiantoulas D, Newland S, Lu Y, Masters L, Harrison J, Saveljeva S, Ma MKL, Ozsvar-Kozma M, Lam BYH, Yeo GSH, Binder CJ, Kaser A, Mallat Z, 2019. Impaired Autophagy in CD11b(+) Dendritic Cells Expands CD4(+) Regulatory T Cells and Limits Atherosclerosis in Mice. Circ Res 125, 1019–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PR, Hahn CN, Grimshaw M, Lu Y, Li X, Brautigan PJ, Beck K, Stocker R, Vadas MA, Gamble JR, 2010. Stress-induced premature senescence mediated by a novel gene, SENEX, results in an anti-inflammatory phenotype in endothelial cells. Blood 116, 4016–4024. [DOI] [PubMed] [Google Scholar]
- Comi P, Chiaramonte R, Maier JA, 1995. Senescence-dependent regulation of type 1 plasminogen activator inhibitor in human vascular endothelial cells. Exp Cell Res 219, 304–308. [DOI] [PubMed] [Google Scholar]
- Condorelli G, Jotti GS, Pagiatakis C, 2016. Fibroblast Senescence as a Therapeutic Target of Myocardial Fibrosis: Beyond Spironolactone? J Am Coll Cardiol 67, 2029–2031. [DOI] [PubMed] [Google Scholar]
- Cummings RJ, Barbet G, Bongers G, Hartmann BM, Gettler K, Muniz L, Furtado GC, Cho J, Lira SA, Blander JM, 2016. Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature 539, 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico D, Mottis A, Potenza F, Sorrentino V, Li H, Romani M, Lemos V, Schoonjans K, Zamboni N, Knott G, Schneider BL, Auwerx J, 2019. The RNA-Binding Protein PUM2 Impairs Mitochondrial Dynamics and Mitophagy During Aging. Mol Cell 73 775–787 e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, Ngo CP, Prolla TA, Rabinovitch PS, 2010. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 9, 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Huang YJ, He X, Zhao M, Wang X, Liu ZS, Xue W, Cai H, Zhan XY, Huang SY, He K, Wang H, Wang N, Sang Z, Li T, Han QY, Mao J, Diao X, Song N, Chen Y, Li WH, Man JH, Li AL, Zhou T, Liu ZG, Zhang XM, Li T, 2019. Acetylation Blocks cGAS Activity and Inhibits Self-DNA-Induced Autoimmunity. Cell 176 1447–1460 e1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Trevino-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA, 2018. Impairment of an Endothelial NAD(+)-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell 173 (74-89), e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S, 2008. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res 102, 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J, 2014. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31, 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Han Y, Lu Q, An J, Zhu H, Xie Z, Song P, Zou MH, 2019. Peroxynitrite-Mediated SIRT (Sirtuin)-1 Inactivation Contributes to Nicotine-Induced Arterial Stiffness in Mice. Arterioscler Thromb Vasc Biol 39, 1419–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Zhang M, Zhang W, Lu Q, Cai Z, Song P, Okon IS, Xiao L, Zou MH, 2016. AMP-Activated Protein Kinase Alpha 2 Deletion Induces VSMC Phenotypic Switching and Reduces Features of Atherosclerotic Plaque Stability. Circ Res 119, 718–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty CK, Carswell A, Friel E, Mercer JR, 2018. Impaired mitochondrial respiration in human carotid plaque atherosclerosis: A potential role for Pink1 in vascular smooth muscle cell energetics. Atherosclerosis 268, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durik M, Kavousi M, van der Pluijm I, Isaacs A, Cheng C, Verdonk K, Loot AE, Oeseburg H, Bhaggoe UM, Leijten F, van Veghel R, deVries R, Rudez G, Brandt R, Ridwan YR, van Deel ED, deBoer M, Tempel D, Fleming I, Mitchell GF, Verwoert GC, Tarasov KV, Uitterlinden AG, Hofman A, Duckers HJ, van Duijn CM, Oostra BA, Witteman JC, Duncker DJ, Danser AH, Hoeijmakers JH, Roks AJ, 2012. Nucleotide excision DNA repair is associated with age-related vascular dysfunction. Circulation 126, 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eelen G, de Zeeuw P, Treps L, Harjes U, Wong BW, Carmeliet P, 2018. Endothelial Cell Metabolism. Physiol Rev 98, 3–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolaeva M, Neri F, Ori A, Rudolph KL, 2018. Cellular and epigenetic drivers of stem cell ageing. Nat Rev Mol Cell Biol 19, 594–610. [DOI] [PubMed] [Google Scholar]
- Fang EF, Hou Y, Lautrup S, Jensen MB, Yang B, SenGupta T, Caponio D, Khezri R, Demarest TG, Aman Y, Figueroa D, Morevati M, Lee HJ, Kato H, Kassahun H, Lee JH, Filippelli D, Okur MN, Mangerich A, Croteau DL, Maezawa Y, Lyssiotis CA, Tao J, Yokote K, Rusten TE, Mattson MP, Jasper H, Nilsen H, Bohr VA, 2019. NAD(+) augmentation restores mitophagy and limits accelerated aging in Werner syndrome. Nat Commun 10, 5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Lautrup S, Hou Y, Demarest TG, Croteau DL, Mattson MP, Bohr VA, 2017. NAD(+) in Aging: Molecular Mechanisms and Translational Implications. Trends Mol Med 23, 899–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat N, Thorin-Trescases N, Voghel G, Villeneuve L, Mamarbachi M, Perrault LP, Carrier M, Thorin E, 2008. Stress-induced senescence predominates in endothelial cells isolated from atherosclerotic chronic smokers. Can J Physiol Pharmacol 86, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir ED, Amadori L, Khan NS, Wong CK, Shamailova R, Hill CA, Wang Z, Remark R, Li JR, Pina C, Faries C, Awad AJ, Moss N, Bjorkegren JLM, Kim-Schulze S, Gnjatic S, Ma’ayan A, Mocco J, Faries P, Merad M, Giannarelli C, 2019. Single-cell immune landscape of human atherosclerotic plaques. Nat Med 25, 1576–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AE, Robertson NA, Wang T, Havas A, Ideker T, Adams PD, 2018. DNA Methylation Clocks in Aging: Categories, Causes, and Consequences. Mol Cell 71, 882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, O’Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, Kapahi P, Nelson MD, Kennedy BK, Melov S, 2013. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 12, 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, Pletcher MA, Smith AE, Tang K, Yuan CW, Brown JC, Friedman J, He J, Heuton KR, Holmberg M, Patel DJ, Reidy P, Carter A, Cercy K, Chapin A, Douwes-Schultz D, Frank T, Goettsch F, Liu PY, Nandakumar V, Reitsma MB, Reuter V, Sadat N, Sorensen RJD, Srinivasan V, Updike RL, York H, Lopez AD, Lozano R, Lim SS, Mokdad AH, Vollset SE, Murray CJL, 2018. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 392, 2052–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frescas D, Roux CM, Aygun-Sunar S, Gleiberman AS, Krasnov P, Kurnasov OV, Strom E, Virtuoso LP, Wrobel M, Osterman AL, Antoch MP, Mett V, Chernova OB, Gudkov AV, 2017. Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc Natl Acad Sci U S A 114, E1668–E1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Laberge RM, Demaria M, Campisi J, 2012. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 23, 2066–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann-Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW, Grassi D, Gregg SQ, Stripay JL, Dorronsoro A, Corbo L, Tang P, Bukata C, Ring N, Giacca M, Li X, Tchkonia T, Kirkland JL, Niedernhofer LJ, Robbins PD, 2017. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun 8, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA, Walsh K, 2017. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM, 2005. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123, 321–334. [DOI] [PubMed] [Google Scholar]
- Gardner SE, Humphry M, Bennett MR, Clarke MC, 2015. Senescent Vascular Smooth Muscle Cells Drive Inflammation Through an Interleukin-1 alpha-Dependent Senescence-Associated Secretory Phenotype. Arterioscler Thromb Vasc Biol 35, 1963–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevaert AB, Shakeri H, Leloup AJ, Van Hove CE, De Meyer GRY, Vrints CJ, Lemmens K, Van Craenenbroeck EM, 2017. Endothelial Senescence Contributes to Heart Failure With Preserved Ejection Fraction in an Aging Mouse Model. Circ Heart Fail 10, e003806. [DOI] [PubMed] [Google Scholar]
- Gluck S, Guey B, Gulen MF, Wolter K, Kang TW, Schmacke NA, Bridgeman A, Rehwinkel J, Zender L, Ablasser A, 2017. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol 19, 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Moerman EJ, Fujii S, Sobel BE, 1994. Overexpression of plasminogen activator inhibitor type-1 in senescent fibroblasts from normal subjects and those with Werner syndrome. J Cell Physiol 161, 571–579. [DOI] [PubMed] [Google Scholar]
- Gonzalo S, Kreienkamp R, Askjaer P, 2017. Hutchinson-Gilford Progeria Syndrome: A premature aging disease caused by LMNA gene mutations. Ageing Res Rev 33, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon LB, Rothman FG, Lopez-Otin C, Misteli T, 2014. Progeria: a paradigm for translational medicine. Cell 156, 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenne I, Kavurma M, Scott S, Bennett M, 2006. Vascular smooth muscle cell senescence in atherosclerosis. Cardiovasc Res 72, 9–17. [DOI] [PubMed] [Google Scholar]
- Gorenne I, Kumar S, Gray K, Figg N, Yu H, Mercer J, Bennett M, 2013. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation 127, 386–396. [DOI] [PubMed] [Google Scholar]
- Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M, 2019. Cellular Senescence: Defining a Path Forward. Cell 179, 813–827. [DOI] [PubMed] [Google Scholar]
- Gray K, Kumar S, Figg N, Harrison J, Baker L, Mercer J, Littlewood T, Bennett M, 2015. Effects of DNA damage in smooth muscle cells in atherosclerosis. Circ Res 116, 816–826. [DOI] [PubMed] [Google Scholar]
- Grootaert MO, daCosta Martins PA, Bitsch N, Pintelon I, De Meyer GR, Martinet W, Schrijvers DM, 2015. Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis. Autophagy 11, 2014–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer GRY, 2018. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res 114, 622–634. [DOI] [PubMed] [Google Scholar]
- Gude NA, Broughton KM, Firouzi F, Sussman MA, 2018. Cardiac ageing: extrinsic and intrinsic factors in cellular renewal and senescence. Nat Rev Cardiol 15, 523–542. [DOI] [PubMed] [Google Scholar]
- Guerrero A, Herranz N, Sun B, Wagner V, Gallage S, Guiho R, Wolter K, Pombo J, Irvine EE, Innes AJ, Birch J, Glegola J, Manshaei S, Heide D, Dharmalingam G, Harbig J, Olona A, Behmoaras J, Dauch D, Uren AG, Zender L, Vernia S, Martínez-Barbera JP, Heikenwalder M, Withers DJ, Gil J, 2019. Cardiac glycosides are broad-spectrum senolytics. Nature Metabolism 1, 1074–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignabert C, Phan C, Seferian A, Huertas A, Tu L, Thuillet R, Sattler C, Le Hiress M, Tamura Y, Jutant EM, Chaumais MC, Bouchet S, Maneglier B, Molimard M, Rousselot P, Sitbon O, Simonneau G, Montani D, Humbert M, 2016. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest 126, 3207–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K, Bu X, Yang C, Cao X, Bian H, Zhu Q, Zhu J, Zhang D, 2018. Treatment Effects of the Second-Generation Tyrosine Kinase Inhibitor Dasatinib on Autoimmune Arthritis. Front Immunol 9, 3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, Rydkina E, Vujcic S, Balan K, Gitlin I, Leonova K, Polinsky A, Chernova OB, Gudkov AV, 2016. Aging of mice is associated with p16(Ink4a)- and beta-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging (Albany NY) 8, 1294–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamczyk MR, Villa-Bellosta R, Gonzalo P, Andres-Manzano MJ, Nogales P, Bentzon JF, Lopez-Otin C, Andres V, 2018. Vascular Smooth Muscle-Specific Progerin Expression Accelerates Atherosclerosis and Death in a Mouse Model of Hutchinson-Gilford Progeria Syndrome. Circulation 138, 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamczyk MR, Villa-Bellosta R, Quesada V, Gonzalo P, Vidak S, Nevado RM, Andres-Manzano MJ, Misteli T, Lopez-Otin C, Andres V, 2019. Progerin accelerates atherosclerosis by inducing endoplasmic reticulum stress in vascular smooth muscle cells. EMBO Mol Med 11, e9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YM, Bedarida T, Ding Y, Somba BK, Lu Q, Wang Q, Song P, Zou MH, 2016. beta-Hydroxybutyrate Prevents Vascular Senescence through hnRNP A1-Mediated Upregulation of Oct4. Mol Cell 71 (1064-1078), e1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LS, Sainson RC, Williams CK, Taylor JM, Shi W, Li JL, Harris AL, 2008. Regulation of multiple angiogenic pathways by Dll4 and Notch in human umbilical vein endothelial cells. Microvasc Res 75, 144–154. [DOI] [PubMed] [Google Scholar]
- Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C, 2005. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol 45, 1441–1448. [DOI] [PubMed] [Google Scholar]
- Henson PM, 2017. Cell Removal: Efferocytosis. Annu Rev Cell Dev Biol 33, 127–144. [DOI] [PubMed] [Google Scholar]
- Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL, Kellogg TA, Khosla S, Koerber DM, Lagnado AB, Lawson DK, LeBrasseur NK, Lerman LO, McDonald KM, McKenzie TJ, Passos JF, Pignolo RJ, Pirtskhalava T, Saadiq IM, Schaefer KK, Textor SC, Victorelli SG, Volkman TL, Xue A, Wentworth MA, Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL, 2019. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T, 2003. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348, 593–600. [DOI] [PubMed] [Google Scholar]
- Hoenicke L, Zender L, 2012. Immune surveillance of senescent cells-biological significance in cancer- and non-cancer pathologies. Carcinogenesis 33, 1123–1126. [DOI] [PubMed] [Google Scholar]
- Huang L, Chambliss KL, Gao X, Yuhanna IS, Behling-Kelly E, Bergaya S, Ahmed M, Michaely P, Luby-Phelps K, Darehshouri A, Xu L, Fisher EA, Ge WP, Mineo C, Shaul PW, 2019. SR-B1 drives endothelial cell LDL transcytosis via DOCK4 to promote atherosclerosis. Nature 569, 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF, 2010. Cellular senescence controls fibrosis in wound healing. Aging (Albany NY) 2, 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC, 2018. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365. [DOI] [PubMed] [Google Scholar]
- Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, Prata L, Masternak MM, Kritchevsky SB, Musi N, Kirkland JL, 2019. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalucka J, Bierhansl L, Conchinha NV, Missiaen R, Elia I, Bruning U, Scheinok S, Treps L, Cantelmo AR, Dubois C, de Zeeuw P, Goveia J, Zecchin A, Taverna F, Morales-Rodriguez F, Brajic A, Conradi LC, Schoors S, Harjes U, Vriens K, Pilz GA, Chen R, Cubbon R, Thienpont B, Cruys B, Wong BW, Ghesquiere B, Dewerchin M, De Bock K, Sagaert X, Jessberger S, Jones EAV, Gallez B, Lambrechts D, Mazzone M, Eelen G, Li X, Fendt SM, Carmeliet P, 2018. Quiescent Endothelial Cells Upregulate Fatty Acid beta-Oxidation for Vasculoprotection via Redox Homeostasis. Cell Metab 28 (881-894), e813. [DOI] [PubMed] [Google Scholar]
- Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L, Lu T, Yankner BA, Campisi J, Elledge SJ, 2015. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 349 aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, Iken M, Vucur M, Weiss S, Heikenwalder M, Khan S, Gil J, Bruder D, Manns M, Schirmacher P, Tacke F, Ott M, Luedde T, Longerich T, Kubicka S, Zender L, 2011. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551. [DOI] [PubMed] [Google Scholar]
- Kay MM, 1993. Generation of senescent cell antigen on old cells initiates IgG binding to a neoantigen. Cell Mol Biol (Noisy-le-grand) 39, 131–153. [PubMed] [Google Scholar]
- Kim PH, Luu J, Heizer P, Tu Y, Weston TA, Chen N, Lim C, Li RL, Lin PY, Dunn JCY, Hodzic D, Young SG, Fong LG, 2018a. Disrupting the LINC complex in smooth muscle cells reduces aortic disease in a mouse model of Hutchinson-Gilford progeria syndrome. Sci Transl Med 10, eaat7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Noh JH, Bodogai M, Martindale JL, Yang X, Indig FE, Basu SK, Ohnuma K, Morimoto C, Johnson PF, Biragyn A, Abdelmohsen K, Gorospe M, 2017. Identification of senescent cell surface targetable protein DPP4. Genes Dev 31, 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Youn SW, Sudhahar V, Das A, Chandhri R, Cuervo Grajal H, Kweon J, Leanhart S, He L, Toth PT, Kitajewski J, Rehman J, Yoon Y, Cho J, Fukai T, Ushio-Fukai M, 2018b. Redox Regulation of Mitochondrial Fission Protein Drp1 by Protein Disulfide Isomerase Limits Endothelial Senescence. Cell Rep 23, 3565–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KR, Aguirre AD, Ye YX, Sun Y, Roh JD, Ng RP Jr, Kohler RH, Arlauckas SP, Iwamoto Y, Savol A, Sadreyev RI, Kelly M, Fitzgibbons TP, Fitzgerald KA, Mitchison T, Libby P, Nahrendorf M, Weissleder R, 2017. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat Med 23, 1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Downing K, Kundu R, Miller C, Dewey F, Lancero H, Raaz U, Perisic L, Hedin U, Schadt E, Maegdefessel L, Quertermous T, Leeper NJ, 2014. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J Clin Invest 124, 1083–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt EE, Quertermous T, Betancur P, Maegdefessel L, Matic LP, Hedin U, Weissman IL, Leeper NJ, 2016. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536, 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Weissman IL, Leeper NJ, 2017. The Role of Efferocytosis in Atherosclerosis. Circulation 135, 476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Werner N, Ye J, Nanda V, Tsao N, Wang Y, Flores AM, Miller CL, Weissman I, Deng H, Xu B, Dalman RL, Eken SM, Pelisek J, Li Y, Maegdefessel L, Leeper NJ, 2018. Proefferocytic Therapy Promotes Transforming Growth Factor-beta Signaling and Prevents Aneurysm Formation. Circulation 137, 750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaravolu RK, Waltmann MD, Konaniah E, Jaeschke A, Hui DY, 2019. ApoER2 (Apolipoprotein E Receptor-2) Deficiency Accelerates Smooth Muscle Cell Senescence via Cytokinesis Impairment and Promotes Fibrotic Neointima After Vascular Injury. Arterioscler Thromb Vasc Biol 39, 2132–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M, 2017. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet 389, 1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kour S, Rath PC, 2016. Long noncoding RNAs in aging and age-related diseases. Ageing Res Rev 26, 1–21. [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW, 2008. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, 2015. Autophagy: a druggable process that is deregulated in aging and human disease. J Clin Invest 125, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS, 2010. The essence of senescence. Genes Dev 24, 2463–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, Katsuno T, Miyauchi H, Orimo M, Okada S, Takamura M, Nagai T, Kaneko S, Komuro I, 2006. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation 114, 953–960. [DOI] [PubMed] [Google Scholar]
- Kusumbe AP, Ramasamy SK, Itkin T, Mae MA, Langen UH, Betsholtz C, Lapidot T, Adams RH, 2016. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532, 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro I, Oguiza A, Recio C, Mallavia B, Madrigal-Matute J, Blanco J, Egido J, Martin-Ventura JL, Gomez-Guerrero C, 2015. Targeting HSP90 Ameliorates Nephropathy and Atherosclerosis Through Suppression of NF-kappaB and STAT Signaling Pathways in Diabetic Mice. Diabetes 64, 3600–3613. [DOI] [PubMed] [Google Scholar]
- Lee JM, Nobumori C, Tu Y, Choi C, Yang SH, Jung HJ, Vickers TA, Rigo F, Bennett CF, Young SG, Fong LG, 2016. Modulation of LMNA splicing as a strategy to treat prelamin A diseases. J Clin Invest 126, 1592–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc C, Friederich-Persson M, Braud L, Palacios-Ramirez R, Karlsson S, Boujardine N, Motterlini R, Jaisser F, Nguyen Dinh Cat A, 2019. MR (Mineralocorticoid Receptor) Induces Adipose Tissue Senescence and Mitochondrial Dysfunction Leading to Vascular Dysfunction in Obesity. Hypertension 73, 458–468. [DOI] [PubMed] [Google Scholar]
- Lewis-McDougall FC, Ruchaya PJ, Domenjo-Vila E, Shin Teoh T, Prata L, Cottle BJ, Clark JE, Punjabi PP, Awad W, Torella D, Tchkonia T, Kirkland JL, Ellison-Hughes GM, 2019. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 18, e12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, van Breugel PC, Loayza-Puch F, Ugalde AP, Korkmaz G, Messika-Gold N, Han R, Lopes R, Barbera EP, Teunissen H, deWit E, Soares RJ, Nielsen BS, Holmstrom K, Martinez-Herrera DJ, Huarte M, Louloupi A, Drost J, Elkon R, Agami R, 2018. LncRNA-OIS1 regulates DPP4 activation to modulate senescence induced by RAS. Nucleic Acids Res 46, 4213–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Curci JA, Kelley BJ, Sicard GA, Thompson RW, 2000. Accelerated replicative senescence of medial smooth muscle cells derived from abdominal aortic aneurysms compared to the adjacent inferior mesenteric artery. J Surg Res 92, 85–95. [DOI] [PubMed] [Google Scholar]
- Liu P, Yu YR, Spencer JA, Johnson AE, Vallanat CT, Fong AM, Patterson C, Patel DD, 2008. CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler Thromb Vasc Biol 28, 243–250. [DOI] [PubMed] [Google Scholar]
- Luo Z, Xu W, Ma S, Qiao H, Gao L, Zhang R, Yang B, Qiu Y, Chen J, Zhang M, Tao B, Cao F, Wang Y, 2017. Moderate Autophagy Inhibits Vascular Smooth Muscle Cell Senescence to Stabilize Progressed Atherosclerotic Plaque via the mTORC1/ULK1/ATG13 Signal Pathway. Oxid Med Cell Longev 2017, 3018190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa H, Inoue T, Ouchi H, Jao TM, Inoue R, Nishi H, Fujii R, Ishidate F, Tanaka T, Tanaka Y, Hirokawa N, Nangaku M, Inagi R, 2019. Mitochondrial Damage Causes Inflammation via cGAS-STING Signaling in Acute Kidney Injury. Cell Rep 29 (1261-1273), e1266. [DOI] [PubMed] [Google Scholar]
- Mammoto T, Torisawa YS, Muyleart M, Hendee K, Anugwom C, Gutterman D, Mammoto A, 2019. Effects of age-dependent changes in cell size on endothelial cell proliferation and senescence through YAP1. Aging (Albany NY) 11, 7051–7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Ke Z, Gorbunova V, Seluanov A, 2012. Replicatively senescent cells are arrested in G1 and G2 phases. Aging (Albany NY) 4, 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfella R, Sasso FC, Cacciapuoti F, Portoghese M, Rizzo MR, Siniscalchi M, Carbonara O, Ferraraccio F, Torella M, Petrella A, Balestrieri ML, Stiuso P, Nappi G, Paolisso G, 2012. Tight glycemic control may increase regenerative potential of myocardium during acute infarction. J Clin Endocrinol Metab 97, 933–942. [DOI] [PubMed] [Google Scholar]
- Martini H, Iacovoni JS, Maggiorani D, Dutaur M, Marsal DJ, Roncalli J, Itier R, Dambrin C, Pizzinat N, Mialet-Perez J, Cussac D, Parini A, Lefevre L, Douin-Echinard V, 2019. Aging induces cardiac mesenchymal stromal cell senescence and promotes endothelial cell fate of the CD90 + subset. Aging Cell 18, e13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M, 2006. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res 99, 156–164. [DOI] [PubMed] [Google Scholar]
- McDonald AP, Meier TR, Hawley AE, Thibert JN, Farris DM, Wrobleski SK, Henke PK, Wakefield TW, Myers DD Jr, 2010. Aging is associated with impaired thrombus resolution in a mouse model of stasis induced thrombosis. Thromb Res 125, 72–78. [DOI] [PubMed] [Google Scholar]
- McHugh D, Gil J, 2018. Senescence and aging: Causes, consequences, and therapeutic avenues. J Cell Biol 217, 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijles DN, Sahoo S, Al Ghouleh I, Amaral JH, Bienes-Martinez R, Knupp HE, Attaran S, Sembrat JC, Nouraie SM, Rojas MM, Novelli EM, Gladwin MT, Isenberg JS, Cifuentes-Pagano E, Pagano PJ, 2017. The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1. Sci Signal 10, eaaj1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner M, Ghadge SK, Goetsch V, Wimmer A, Dorler J, Polzl G, Zaruba MM, 2018. Upregulation of the aging related LMNA splice variant progerin in dilated cardiomyopathy. PLoS One 13, e0196739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Hodwin B, Ramanujam D, Engelhardt S, Sarikas A, 2016. Essential Role for Premature Senescence of Myofibroblasts in Myocardial Fibrosis. J Am Coll Cardiol 67, 2018–2028. [DOI] [PubMed] [Google Scholar]
- Michalik KM, You X, Manavski Y, Doddaballapur A, Zornig M, Braun T, John D, Ponomareva Y, Chen W, Uchida S, Boon RA, Dimmeler S, 2014. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res 114, 1389–1397. [DOI] [PubMed] [Google Scholar]
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I, 2002. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105, 1541–1544. [DOI] [PubMed] [Google Scholar]
- Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I, 2009. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15, 1082–1087. [DOI] [PubMed] [Google Scholar]
- Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, Richardson RS, Donato AJ, 2013. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am J Physiol Heart Circ Physiol 305, H251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motwani M, Pesiridis S, Fitzgerald KA, 2019. DNA sensing by the cGAS-STING pathway in health and disease. Nat Rev Genet 20, 657–674. [DOI] [PubMed] [Google Scholar]
- Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J, 2013. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 154, 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M, 2013. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118. [DOI] [PubMed] [Google Scholar]
- Murano S, Thweatt R, Shmookler Reis RJ, Jones RA, Moerman EJ, Goldstein S, 1991. Diverse gene sequences are overexpressed in werner syndrome fibroblasts undergoing premature replicative senescence. Mol Cell Biol 11, 3905–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarelli T, Lau L, Fukumoto T, Zundell J, Fatkhutdinov N, Wu S, Aird KM, Iwasaki O, Kossenkov AV, Schultz D, Noma KI, Baur JA, Schug Z, Tang HY, Speicher DW, David G, Zhang R, 2019. NAD(+) metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol 21, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Hosoyama T, Kawamura D, Takeuchi Y, Tanaka Y, Samura M, Ueno K, Nishimoto A, Kurazumi H, Suzuki R, Ito H, Sakata K, Mikamo A, Li TS, Hamano K, 2016. Influence of aging on the quantity and quality of human cardiac stem cells. Sci Rep 6, 22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano-Kurimoto R, Ikeda K, Uraoka M, Nakagawa Y, Yutaka K, Koide M, Takahashi T, Matoba S, Yamada H, Okigaki M, Matsubara H, 2009. Replicative senescence of vascular smooth muscle cells enhances the calcification through initiating the osteoblastic transition. Am J Physiol Heart Circ Physiol 297, HI673–1684. [DOI] [PubMed] [Google Scholar]
- Nicaise AM, Wagstaff LJ, Willis CM, Paisie C, Chandok H, Robson P, Fossati V, Williams A, Crocker SJ, 2019. Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc Natl Acad Sci U S A 116, 9030–9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli T, Partridge L, 2012. Ageing as a risk factor for disease. Curr Biol 22, R741–752. [DOI] [PubMed] [Google Scholar]
- Novelli G, Muchir A, Sangiuolo F, Helbling-Leclerc A, D’Apice MR, Massart C, Capon F, Sbraccia P, Federici M, Lauro R, Tudisco C, Pallotta R, Scarano G, Dallapiccola B, Merlini L, Bonne G, 2002. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am J Hum Genet 71, 426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, Yoshimi N, Hai E, Shirai N, Ehara S, Komatsu R, Naruko T, Ueda M, 2004. Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol 24, 546–550. [DOI] [PubMed] [Google Scholar]
- Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Kruger P, Fielder E, Victorelli S, Ruswhandi RA, Giorgadze N, Pirtskhalava T, Podgorni O, Enikolopov G, Johnson KO, Xu M, Inman C, Palmer AK, Schafer M, Weigl M, Ikeno Y, Burns TC, Passos JF, von Zglinicki T, Kirkland JL, Jurk D, 2019. Obesity-Induced Cellular Senescence Drives Anxiety and Impairs Neurogenesis. Cell Metab 29 (1061-1077), e1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive M, Harten I, Mitchell R, Beers JK, Djabali K, Cao K, Erdos MR, Blair C, Funke B, Smoot L, Gerhard-Herman M, Machan JT, Kutys R, Virmani R, Collins FS, Wight TN, Nabel EG, Gordon LB, 2010. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol 30, 2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmanagic-Myers S, Kiss A, Manakanatas C, Hamza O, Sedlmayer F, Szabo PL, Fischer I, Fichtinger P, Podesser BK, Eriksson M, Foisner R, 2019. Endothelial progerin expression causes cardiovascular pathology through an impaired mechan-oresponse. J Clin Invest 129, 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y, 2007. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol 43, 571–579. [DOI] [PubMed] [Google Scholar]
- Ovadya Y, Krizhanovsky V, 2018. Strategies targeting cellular senescence. J Clin Invest 128, 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantsulaia I, Ciszewski WM, Niewiarowska J, 2016. Senescent endothelial cells: Potential modulators of immunosenescence and ageing. Ageing Res Rev 29, 13–25. [DOI] [PubMed] [Google Scholar]
- Partridge L, Deelen J, Slagboom PE, 2018. Facing up to the global challenges of ageing. Nature 561, 45–56. [DOI] [PubMed] [Google Scholar]
- Poulos MG, Ramalingam P, Gutkin MC, Llanos P, Gilleran K, Rabbany SY, Butler JM, 2017. Endothelial transplantation rejuvenates aged hematopoietic stem cell function. J Clin Invest 127, 4163–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A, Gordon LB, Kleinman ME, Gurary EB, Massaro J, D’Agostino R Sr, Kieran MW, Gerhard-Herman M, Smoot L, 2018. Cardiac Abnormalities in Patients With Hutchinson-Gilford Progeria Syndrome. JAMA Cardiol 3, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proto JD, Doran AC, Gusarova G, Yurdagul A Jr, Sozen E, Subramanian M, Islam MN, Rymond CC, Du J, Hook J, Kuriakose G, Bhattacharya J, Tabas I, 2018. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity 49 (666-677), e666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B, 2006. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 130, 435–452. [DOI] [PubMed] [Google Scholar]
- Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, Shroff R, Skepper G, Shanahan CM, 2010. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation 121, 2200–2210. [DOI] [PubMed] [Google Scholar]
- Regina C, Panatta E, Candi E, Melino G, Amelio I, Balistreri CR, Annicchiarico-Petruzzelli M, Di Daniele N, Ruvolo G, 2016. Vascular ageing and endothelial cell senescence: Molecular mechanisms of physiology and diseases. Mech Ageing Dev 159, 14–21. [DOI] [PubMed] [Google Scholar]
- Reymond N, Imbert AM, Devilard E, Fabre S, Chabannon C, Xerri L, Farnarier C, Cantoni C, Bottino C, Moretta A, Dubreuil P, Lopez M, 2004. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J Exp Med 199, 1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riessland M, Kolisnyk B, Kim TW, Cheng J, Ni J, Pearson JA, Park EJ, Dam K, Acehan D, Ramos-Espiritu LS, Wang W, Zhang J, Shim JW, Ciceri G, Brichta L, Studer L, Greengard P, 2019. Loss of SATB1 Induces p21-Dependent Cellular Senescence in Post-mitotic Dopaminergic Neurons. Cell Stem Cell 25 (514-530), e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, Barton GM, 2017. Tissue-Resident Macrophages Are Locally Programmed for Silent Clearance of Apoptotic Cells. Immunity 47 (913-927), e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD, 2016. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Arnlov J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Barnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castaneda-Orjuela CA, Castillo-Rivas J, Catala-Lopez F, Choi JY, Christensen H, Cirillo M, Cooper L Jr., Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang YH, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin MJ, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C, 2017. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol 70, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv A, Biran A, Yon M, Simon J, Lowe SW, Krizhanovsky V, 2013. Granule exocytosis mediates immune surveillance of senescent cells. Oncogene 32, 1971–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv A, Burton DG, Moshayev Z, Vadai E, Wensveen F, Ben-Dor S, Golani O, Polic B, Krizhanovsky V, 2016. NKG2D ligands mediate immunosurveillance of senescent cells. Aging (Albany NY) 8, 328–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH, 2001. Telomere shortening in atherosclerosis. Lancet 358, 472–473. [DOI] [PubMed] [Google Scholar]
- Santiago-Fernandez O, Osorio FG, Quesada V, Rodriguez F, Basso S, Maeso D, Rolas L, Barkaway A, Nourshargh S, Folgueras AR, Freije JMP, Lopez-Otin C, 2019. Development of a CRISPR/Cas9-based therapy for Hutchinson-Gilford progeria syndrome. Nat Med 25, 423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapieha P, Mallette FA, 2018. Cellular Senescence in Postmitotic Cells: Beyond Growth Arrest. Trends Cell Biol 28, 595–607. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Ikeda Y, Iwabayashi M, Akasaki Y, Ohishi M, 2017. The Impact of Autophagy on Cardiovascular Senescence and Diseases. Int Heart J 58, 666–673. [DOI] [PubMed] [Google Scholar]
- Savai R, Al-Tamari HM, Sedding D, Kojonazarov B, Muecke C, Teske R, Capecchi MR, Weissmann N, Grimminger F, Seeger W, Schermuly RT, Pullamsetti SS, 2014. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat Med 20, 1289–1300. [DOI] [PubMed] [Google Scholar]
- Sawaki D, Czibik G, Pini M, Ternacle J, Suffee N, Mercedes R, Marcelin G, Surenaud M, Marcos E, Gual P, Clement K, Hue S, Adnot S, Hatem SN, Tsuchimochi I, Yoshimitsu T, Henegar C, Derumeaux G, 2018. Visceral Adipose Tissue Drives Cardiac Aging Through Modulation of Fibroblast Senescence by Osteopontin Production. Circulation 138, 809–822. [DOI] [PubMed] [Google Scholar]
- Scanlan MJ, Raj BK, Calvo B, Garin-Chesa P, Sanz-Moncasi MP, Healey JH, Old LJ, Rettig WJ, 1994. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci U S A 91, 5657–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellinger IN, Mattern K, Raaz U, 2019. The Hardest Part. Arterioscler Thromb Vasc Biol 39, 1301–1306. [DOI] [PubMed] [Google Scholar]
- Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W, 2005. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol 25, 1256–1261. [DOI] [PubMed] [Google Scholar]
- Sen P, Lan Y, Li CY, Sidoli S, Donahue G, Dou Z, Frederick B, Chen Q, Luense LJ, Garcia BA, Dang W, Johnson FB, Adams PD, Schultz DC, Berger SL, 2019. Histone Acetyltransferase p300 Induces De Novo Super-Enhancers to Drive Cellular Senescence. Mol Cell 73 (684-698), e688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne AN, Edsfeldt A, Cole JE, Kassiteridi C, Swart M, Park I, Green P, Khoyratty T, Saliba D, Goddard ME, Sansom SN, Goncalves I, Krams R, Udalova IA, Monaco C, 2017. Interferon Regulatory Factor 5 Controls Necrotic Core Formation in Atherosclerotic Lesions by Impairing Efferocytosis. Circulation 136, 1140–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeri H, Lemmens K, Gevaert AB, De Meyer GRY, Segers VFM, 2018. Cellular senescence links aging and diabetes in cardiovascular disease. Am J Physiol Heart Circ Physiol 315, H448–H462. [DOI] [PubMed] [Google Scholar]
- Shi H, Mao X, Zhong Y, Liu Y, Zhao X, Yu K, Zhu R, Wei Y, Zhu J, Sun H, Mao Y, Zeng Q, 2016. Digoxin reduces atherosclerosis in apolipoprotein E-deficient mice. Br J Pharmacol 173, 1517–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakabe A, Ikeda Y, Sciarretta S, Zablocki DK, Sadoshima J, 2016. Aging and Autophagy in the Heart. Circ Res 118, 1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Jensen MD, Lerman A, Kushwaha S, Rihal CS, Gersh BJ, Behfar A, Tchkonia T, Thomas RJ, Lennon RJ, Keenan LR, Moore AG, Kirkland JL, 2016. Effect of Low-Dose Rapamycin on Senescence Markers and Physical Functioning in Older Adults with Coronary Artery Disease: Results of a Pilot Study. J Frailty Aging 5, 204–207. [DOI] [PubMed] [Google Scholar]
- Singh R, Letai A, Sarosiek K, 2019. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol 20, 175–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Franco A, Fleischer JA, Zhang L, Dorn GW 2nd, 2017. Abrogating Mitochondrial Dynamics in Mouse Hearts Accelerates Mitochondrial Senescence. Cell Metab 26 (872-883), e875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, Foa R, Santoni A, 2009. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood 113, 3503–3511. [DOI] [PubMed] [Google Scholar]
- Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M, 2007. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res 100, 884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskamper J, Westermann D, Bisping E, Ly H, Wang X, Kawase Y, Chen J, Liang L, Sipo I, Vetter R, Weger S, Kurreck J, Erdmann V, Tschope C, Pieske B, Lebeche D, Schultheiss HP, Hajjar RJ, Poller WC, 2009. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation 119, 1241–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suner IJ, Espinosa-Heidmann DG, Marin-Castano ME, Hernandez EP, Pereira-Simon S, Cousins SW, 2004. Nicotine increases size and severity of experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 45, 311–317. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Loo TM, Okada R, Kamachi F, Watanabe Y, Wakita M, Watanabe S, Kawamoto S, Miyata K, Barber GN, Ohtani N, Hara E, 2018. Downregulation of cytoplasmic DNases is implicated in cytoplasmic DNA accumulation and SASP in senescent cells. Nat Commun 9, 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D, Moriyama J, Nakamura T, Miki E, Takahashi E, Sato A, Akaike T, Itto-Nakama K, Arimoto H, 2019. AUTACs: Cargo-Specific Degraders Using Selective Autophagy. Mol Cell 76, 797–810. [DOI] [PubMed] [Google Scholar]
- Tan P, Wang YJ, Li S, Wang Y,He JY, Chen YY, Deng HQ, Huang W, Zhan JK, Liu YS, 2016. The PI3K/Akt/mTOR pathway regulates the replicative senescence of human VSMCs. Mol Cell Biochem 422, 1–10. [DOI] [PubMed] [Google Scholar]
- Terenzi DC, Al-Omran M, Quan A, Teoh H, Verma S, Hess DA, 2019. Circulating Pro-Vascular Progenitor Cell Depletion During Type 2 Diabetes: Translational Insights Into the Prevention of Ischemic Complications in Diabetes. JACC Basic Transl Sci 4, 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AM, Wagner R, Rzucidlo EM, 2014. Age-related loss of Sirt1 expression results in dysregulated human vascular smooth muscle cell function. Am J Physiol Heart Circ Physiol 307, H533–541. [DOI] [PubMed] [Google Scholar]
- Thompson PJ, Shah A, Ntranos V, Van Gool F, Atkinson M, Bhushan A, 2019. Targeted Elimination of Senescent Beta Cells Prevents Type 1 Diabetes. Cell Metab 29 (1045-1060), e1010. [DOI] [PubMed] [Google Scholar]
- Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I, 2008. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol 28, 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiku V, Jain C, Raz Y, Nakamura S, Heestand B, Liu W, Späth M, Suchiman HED, Müller R-U, Slagboom PE, Partridge L, Antebi A, 2017. Small nucleoli are a cellular hallmark of longevity. Nature Communications 8, 16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A, 2004. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res 94, 514–524. [DOI] [PubMed] [Google Scholar]
- Triana-Martinez F, Picallos-Rabina P, Da Silva-Alvarez S, Pietrocola F, Llanos S, Rodilla V, Soprano E, Pedrosa P, Ferreiros A, Barradas M, Hernandez-Gonzalez F, Lalinde M, Prats N, Bernado C, Gonzalez P, Gomez M, Ikonomopoulou MP, Fernandez-Marcos PJ, Garcia-Caballero T, Del Pino P, Arribas J, Vidal A, Gonzalez-Barcia M, Serrano M, Loza MI, Dominguez E, Collado M, 2019. Identification and characterization of Cardiac Glycosides as senolytic compounds. Nat Commun 10, 4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, Sadayappan S, Molkentin JD, 2020. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 577, 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD, 2014. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen JM, 2019. Senolytic therapies for healthy longevity. Science 364, 636–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Padron RI, Lasko D, Li S, Louis L, Pestana IA, Pang M, Liotta C, Fornoni A, Aitouche A, Pham SM, 2004. Aging exacerbates neointimal formation, and increases proliferation and reduces susceptibility to apoptosis of vascular smooth muscle cells in mice. J Vasc Surg 40, 1199–1207. [DOI] [PubMed] [Google Scholar]
- Vicinanza C, Aquila I, Cianflone E, Scalise M, Marino F, Mancuso T, Fumagalli F, Giovannone ED, Cristiano F, Iaccino E, Marotta P, Torella A, Latini R, Agosti V, Veltri P, Urbanek K, Isidori AM, Saur D, Indolfi C, Nadal-Ginard B, Torella D, 2018. Kitcre knock-in mice fail to fate-map cardiac stem cells. Nature 555, E1–E5. [DOI] [PubMed] [Google Scholar]
- Vizioli MG, Liu T, Miller KN, Robertson NA, Gilroy K, Lagnado AB, Perez-Garcia A, Kiourtis C, Dasgupta N, Lei X, Kruger PJ, Nixon C, Clark W, Jurk D, Bird TG, Passos JF, Berger SL, Dou Z, Adams PD, 2020. Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence. Genes Dev 34, 428–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voghel G, Thorin-Trescases N, Farhat N, Nguyen A, Villeneuve L, Mamarbachi AM, Fortier A, Perrault LP, Carrier M, Thorin E, 2007. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech Ageing Dev 128, 662–671. [DOI] [PubMed] [Google Scholar]
- Walaszczyk A, Dookun E, Redgrave R, Tual-Chalot S, Victorelli S, Spyridopoulos I, Owens A, Arthur HM, Passos JF, Richardson GD, 2019. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell 18, e12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Uryga AK, Reinhold J, Figg N, Baker L, Finigan A, Gray K, Kumar S, Clarke M, Bennett M, 2015. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation 132, 1909–1919. [DOI] [PubMed] [Google Scholar]
- Wang Y, Subramanian M, Yurdagul A Jr, Barbosa-Lorenzi VC, Cai B, de Juan-Sanz J, Ryan TA, Nomura M, Maxfield FR, Tabas I, 2017. Mitochondrial Fission Promotes the Continued Clearance of Apoptotic Cells by Macrophages. Cell 171 (331-345), e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A, Nong Z, Yin H, O’Neil C, Fox S, Balint B, Guo L, Leo O, Chu MWA, Gros R, Pickering JG, 2017. Nicotinamide Phosphoribosyltransferase in Smooth Muscle Cells Maintains Genome Integrity, Resists Aortic Medial Degeneration, and Is Suppressed in Human Thoracic Aortic Aneurysm Disease. Circ Res 120, 1889–1902. [DOI] [PubMed] [Google Scholar]
- Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, Campisi J, 2016. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab 23, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm K, Happel K, Eelen G, Schoors S, Oellerich MF, Lim R, Zimmermann B, Aspalter IM, Franco CA, Boettger T, Braun T, Fruttiger M, Rajewsky K, Keller C, Bruning JC, Gerhardt H, Carmeliet P, Potente M, 2016. FoxO1 couples metabolic activity and growth state in the vascular endothelium. Nature 529, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Neville R, Finkel T, 2000. Homocysteine accelerates endothelial cell senescence. FEBS Lett 470, 20–24. [DOI] [PubMed] [Google Scholar]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL, 2018. Senolytics improve physical function and increase lifespan in old age. Nat Med 24, 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW, 2007. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, McCrann DJ, Nguyen H, St Hilaire C, DePinho RA, Jones MR, Ravid K, 2007. Increased polyploidy in aortic vascular smooth muscle cells during aging is marked by cellular senescence. Aging Cell 6, 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Li Q, Wang X, Jiang X, Zhao D, Lin X, He F, Tang L, 2018. C-type lectin receptor LSECtin-mediated apoptotic cell clearance by macrophages directs intestinal repair in experimental colitis. Proc Natl Acad Sci U S A 115, 11054–11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M, van der Horst IC, van Melle JP, Qian C, van Gilst WH, Sillje HH, deBoer RA, 2011. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am J Physiol Heart Circ Physiol 301, H459–468. [DOI] [PubMed] [Google Scholar]
- Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, Ben-Porath I, Krizhanovsky V, 2016. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun 7, 11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HT, Park S, Shin EC, Lee WW, 2016. T cell senescence and cardiovascular diseases. Clin Exp Med 16, 257–263. [DOI] [PubMed] [Google Scholar]
- Yun TJ, Lee JS, Machmach K, Shim D, Choi J, Wi YJ, Jang HS, Jung IH, Kim K, Yoon WK, Miah MA, Li B, Chang J, Bego MG, Pham TN, Loschko J, Fritz JH, Krug AB, Lee SP, Keler T, Guimond JV, Haddad E, Cohen EA, Sirois MG, El-Hamamsy I, Colonna M, Oh GT, Choi JH, Cheong C, 2016. Indoleamine 2,3-Dioxygenase-Expressing Aortic Plasmacytoid Dendritic Cells Protect against Atherosclerosis by Induction of Regulatory T Cells. Cell Metab 23, 852–866. [DOI] [PubMed] [Google Scholar]
- Yurdagul A Jr, Doran AC, Cai B, Fredman G, Tabas IA, 2017. Mechanisms and Consequences of Defective Efferocytosis in Atherosclerosis. Front Cardiovasc Med 4, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fukuhara S, Sako K, Takenouchi T, Kitani H, Kume T, Koh GY, Mochizuki N, 2011. Angiopoietin-1/Tie2 signal augments basal Notch signal controlling vascular quiescence by inducing delta-like 4 expression through AKT-mediated activation of beta-catenin. J Biol Chem 286, 8055–8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, Misra Sen J, Gorospe M, Mattson MP, 2019. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nature Neuroscience 22, 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Chen W, Adams PD, 2007. Molecular dissection of formation of senescence-associated heterochromatin foci. Mol Cell Biol 27, 2343–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Salisbury D, Sallam T, 2018. Long Noncoding RNAs in Atherosclerosis: JACC Review Topic of the Week. J Am Coll Cardiol 72, 2380–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zhang S, Cui J, Huang W, Wang J, Su F, Chen N, Gong Q, 2019. TERT assists GDF11 to rejuvenate senescent VEGFR2(+)/CD133(+) cells in elderly patients with myocardial infarction. Lab Invest 99, 1661–1688. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL, 2016. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL, 2015. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Stoppani E, Volonte D, Galbiati F, 2011. Caveolin-1, cellular senescence and age-related diseases. Mech Ageing Dev 132, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Y, Liu L, Lee MY, Xu C, Liang Y, Man RY, Vanhoutte PM, Wang Y, 2010. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ Res 106, 1384–1393. [DOI] [PubMed] [Google Scholar]