Abstract
How do we decide what we do? This is the essence of action control, the process of selecting the most appropriate response among multiple possible choices. Suboptimal action control can involve a failure to initiate or adapt actions, or conversely it can involve making actions impulsively. There has been an increasing focus on the specific role of the subthalamic nucleus (STN) in action control. This has been fueled by the clinical relevance of this basal ganglia nucleus as a target for deep brain stimulation (DBS), primarily in Parkinson’s disease but also in obsessive-compulsive disorder. The context of DBS has opened windows to study STN function in ways that link neuroscientific and clinical fields closely together, contributing to an exceptionally high level of two-way translation. In this review, we first outline the role of the STN in both motor and nonmotor action control, and then discuss how these functions might be implemented by neuronal activity in the STN. Gaining a better understanding of these topics will not only provide important insights into the neurophysiology of action control but also the pathophysiological mechanisms relevant for several brain disorders and their therapies.
Keywords: subthalamic nucleus, action control, decision making, deep brain stimulation, neuronal oscillations
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is one of only a few clinical treatments that is based on targeted modulation of known pathophysiology in defined circuits, highlighting the translational nature of this field. STN DBS has proven strikingly efficient in addressing motor symptoms (Deuschl and others 2006; Limousin and others 1995), though the effects on nonmotor functions, such as cognition, emotion, and motivation, are less understood (Fasano and others 2012; Kim and others 2015). Driven by the motivation to optimize treatment and minimize side effects, there has been an emerging clinical and neuroscientific interest in understanding the functions and the neural dynamics of the STN. Local recording and stimulation in the context of the operative DBS procedure constitute a unique window to study neural network dynamics of the human brain. In this framework, STN function can be linked to several well-established fields of neuroscience, such as motor control, cognitive control, decision making, and reward. At the same time, STN DBS treatment for different diagnoses is an inherently collaborative technique that spans multiple clinical disciplines across surgical, neurological, and psychiatric fields. This translational relevance and interest largely increases the possibility of understanding the role of STN and developing insights that intersect, connect, and synergize diverse fields of neuroscience and clinical medicine in ways that have been previously difficult.
Functional Anatomy and Dynamics of the STN
The basal ganglia generally exert motor control by keeping the motor cortex under inhibitory control. Processing in the basal ganglia in general, and the STN in particular, leads to adjustments of inhibition from the output nuclei GPI/SNR (internal globus pallidus/substantia nigra pars reticulata), which in turn selectively release the motor cortex from inhibitory control in order to execute actions (Alexander and others 1990).
The Canonical Basal Ganglia Loop and Movement Inhibition
According to the classical view (Alexander and others 1990), the striatum constitutes the main input structure of the basal ganglia, receiving input from the cortex and projecting output either via the direct or indirect (via external segment of the globus pallidus and STN) pathway to the output nuclei GPI/SNR. These nuclei in turn project back to the cortex via the thalamus (Fig. 1A). The direct pathway is thought to facilitate movements by cortical excitation, and the indirect pathway inhibits movements by inhibiting cortical excitation. The balance between the direct and indirect pathways is regulated by dopamine modulation of two striatal cell populations with different dopamine receptor profiles. In this scheme, the STN is a projection nucleus in the indirect pathway, and increased activity of the STN should lead to increased activity of the indirect inhibitory pathway, which would subsequently lead to inhibition of the cortex and therefore inhibition of movement (Alexander and others 1990).
Figure 1.
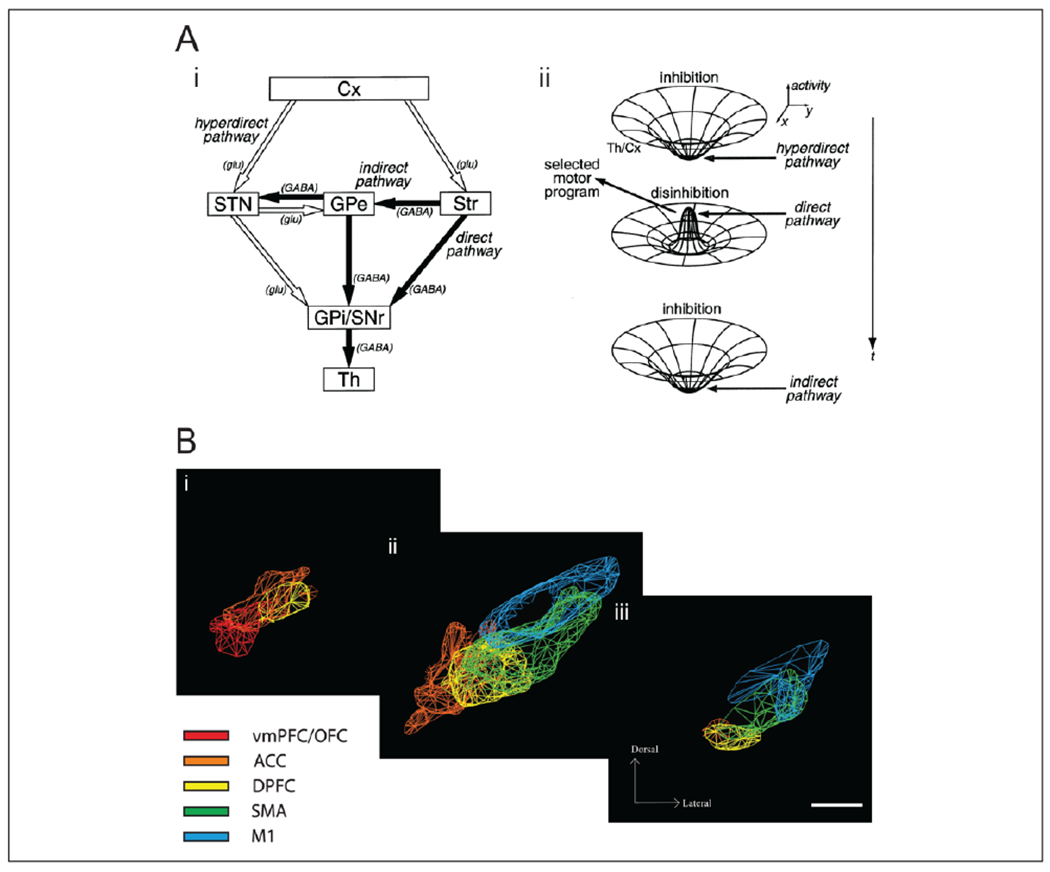
Functional anatomy of the basal ganglia and the STN. (A) (i) The basal ganglia pathways, highlighting both the STN and the striatum as input structures of the basal ganglia: the hyperdirect pathway (Cx-STN-GPI/SNR), the direct pathway (Cx-Str-GPI/SNR), and the indirect pathway (Cx-Str-GPe-GPI/SNR). Filled and empty arrows indicate excitatory glutamatergic (glu) and inhibitory GABAergic (GABA) projections, respectively. Abbreviations: Cerebral cortex (Cx); striatum (Str); subthalamic nucleus (STN); external segment of the globus pallidus (GPe); internal segment of the globus pallidus (GPI); substantia nigra pars reticulata (SNR); thalamus (Th). (ii) Schematic diagram of the “center-surround model” of basal ganglia activity; early activation of the hyperdirect pathway causes a broad inhibition, while subsequent activation of the direct pathway causes a specific activation of motor programs, antagonized by the indirect pathway. Republished with permission from (Nambu and others 2002) and original figure. (B) Topographical organization of the projections from different regions of the frontal cortex to the STN, which partially overlap. Colored meshes represent dense projections from the cortical areas ventromedial prefrontal cortex/orbitofrontal cortex (vmPFC/OFC), anterior cingulate cortex (ACC), dorsal prefrontal cortex (DPFC), supplementary motor areas (SMA), and motor cortex (M1). Coronal view at (i) anterior, (ii) central, and (iii) posterior STN. Republished with permission from Society for Neuroscience, from Haynes and Haber (2013); permission conveyed through Copyright Clearance Center, Inc.
The Hyperdirect Pathway
More recent models of the basal ganglia have highlighted a direct cortical input to the STN, the hyperdirect pathway, which would render the STN an input structure to the BG (Nambu and others 2002) (Fig. 1A). The hyperdirect pathway is faster than the striatal pathways, and the STN projection to the GPI/SNR has been found to be more diffuse than the more specific striatal projection (Hazrati and Parent 1992), but see also Kelly and Strick (2004). This has led to a race model of motor control, involving a center-surround inhibition of the output nuclei (Mink 1996; Nambu and others 2002) (Fig. 1A). In this model, an intended action will cause cortical excitatory input to both the striatum and the STN, but the STN will cause a fast and global activation of the SNR/GPI before the striatal input arrives, causing a broad inhibition of movement. Only after sufficient excitation of one specific action has been built up in the striatum does the direct pathway activate the selected motor program, antagonized by the indirect pathway. A recent experimental study supports central aspects of this model (Schmidt and others 2013).
Parallel Circuits in the Basal Ganglia
The basal ganglia, including the STN, are not only involved in motor control but also in cognitive, motivational, and emotional functions (Haber and Behrens 2014; Weintraub and Zaghloul 2013). Anatomically, this is supported by the existence of parallel circuits throughout the basal ganglia; the canonical circuit between structures is maintained, but the type of information processed in each parallel loop is reflected by the cortical origin of the loop involved in sensorimotor, associative, or limbic processes (Alexander and others 1986). The types of input gradually changes along the dorsolateral-to-ventromedial axis (Haber 2003; Kelly and Strick 2004), matched by a gradient of dopamine input from the more motor-related nigrostriatal system to the more valence-related mesocorticolimbic system (Haber and others 2000).
The same pattern of graded organization has been confirmed in STN afferents, both for the cortical (Haynes and Haber 2013) and pallidal (Karachi and others 2005) inputs (i.e., the hyperdirect and indirect pathway, respectively), in a partially overlapping pattern that suggests both parallel processing and an integration of information (Fig. 1B). Imaging studies have confirmed the existence of different STN subterritories in the human STN, indicating partly overlapping motor, associative, and limbic regions along the longitudinal axis (Lambert and others 2012; Voon and others 2017). These parallel loops raise the possibility that the circuit function proposed for movements is relevant also for nonmotor action control and that cognitive and limbic information may be integrated into action control in the STN.
Anatomically, the STN is thus located at an intersection between the frontal cortex, which is related to cognitive control and decision making (Helfrich and Knight 2016), and the basal ganglia, which is involved in habitual and goal-directed action control (Haber and Behrens 2014). Inhibitory control from the STN could therefore imply a switch between different modes of action control, in particular by inhibiting the default response in favor of a controlled response (Hikosaka and Isoda 2010).
Beyond the cortico-basal ganglia-thalamo-cortical loop outlined above, the basal ganglia and the STN also take part in subcortical circuits, involving areas such as the brainstem (Alexander and others 1990; McHaffie and others 2005). For example, the pedunculopontine nucleus (PPN), which is involved in motor and nonmotor functions and also used as a target for DBS treatment of patients with Parkinson’s disease (Pienaar and others 2016), is connected to the STN (Hammond and others 1983; Lambert and others 2012). The downstream effect of STN processing thus goes beyond feedback to the cortex, and may involve integration of cortical and subcortical processes in action control (McHaffie and others 2005).
Oscillatory Dynamics in the STN
Neural communication in the cortico-basal ganglia loop is however not only characterized by anatomically defined networks but also by dynamic rhythmic activity in the local field potential of specific frequency bands (Fig. 2). The most prominent rhythm of the STN is the beta rhythm (12–30 Hz), which has been particularly linked to sensorimotor functions and movement inhibition (Brittain and Brown 2014). Slower theta and delta rhythms in the STN (1–8 Hz, hereafter collectively described as theta) have been related to cognitive processes and conflict representation (Cavanagh and others 2011; Zavala and others 2014), while some reports link alpha rhythms (8–12 Hz) to emotional processing (Brucke and others 2007; Huebl and others 2011; Kuhn and others 2005). This distinction indicates that motor, cognitive, and emotional functions of the STN may be related to separate spectral bands (Aron and others 2016; Marceglia and others 2011), but this outlined functional separation is far from absolute. Faster oscillations, that is, gamma (60–90 Hz) and high-frequency oscillations (HFO; 200–500 Hz), are thought to be related to active neuronal processing, and are also observed in the STN (Alegre and others 2013; Jenkinson and others 2013; Yang and others 2014). Moreover, oscillations in the STN can be transiently or persistently coupled to cortical oscillations through coherence or phase-amplitude coupling, which may allow for flexible and frequency-specific interactions between different cortical areas and the STN, possibly through the hyper-direct pathway (Aron and others 2016; Yang and others 2014).
Figure 2.
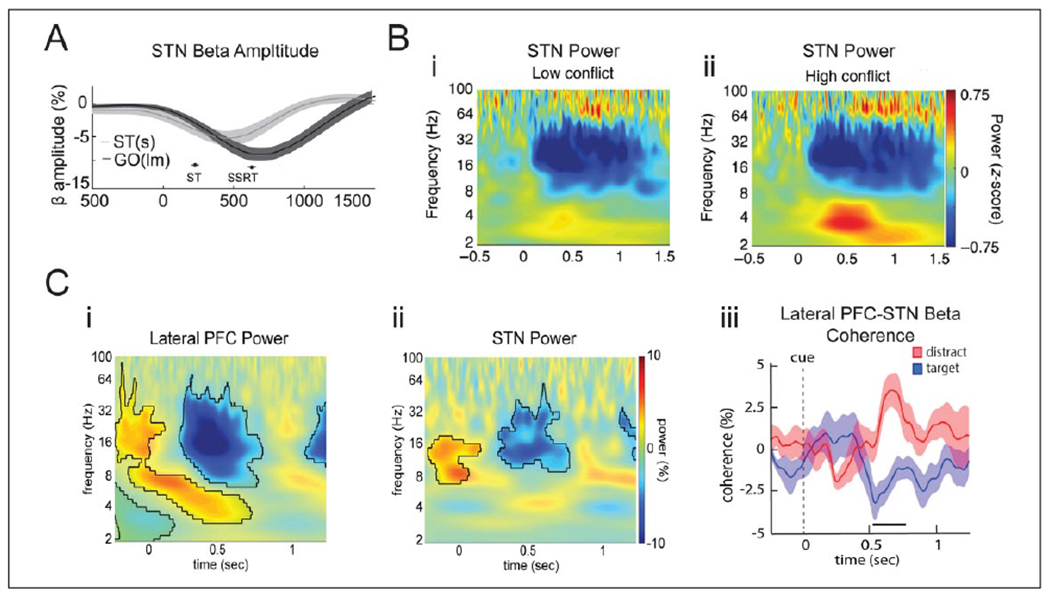
Oscillatory dynamics in the STN during motor and nonmotor inhibition, and conflict. (A) STN beta power decreases from onset of GO cue and during movement. If a STOP signal appears after the GO cue (ST(s) condition), beta power increases relative to the GO trials without STOP signal (GO(lm) condition). (B) STN oscillatory dynamics during sensorimotor decisions with low (i) and high (ii) conflict. Theta power increases only during high conflict trials, while perimotor beta power decreases similarly in both conditions. (C) STN oscillatory dynamics during nonmotor memory decisions. Beta power decreases during memory encoding both in the lateral prefrontal cortex (PFC) (i) and the STN (ii), while theta power increases only in the lateral PFC. PFC-STN beta coherence (iii) decreases during encoding of target trials, which are supposed to be encoded, but increases during distractor trials which are not supposed to be encoded. Reproduced with permission from (A) Benis and others (2014), (B) Zavala and others (2017a), (C) Zavala and others (2017b).
Pathophysiology of the STN and Effects of DBS
Pathological activity in the basal ganglia loop can cause disturbances of action control that are characteristic not only of movement disorders (DeLong 1990) but neuropsychiatric disorders as well (Haber and Behrens 2014). Parkinson’s disease is in particular characterized by hypokinetic motor symptoms but may also involve nonmotor symptoms such as depression, apathy, and cognitive dysfunction (Weintraub and Burn 2011). Obsessive compulsive disorder (OCD) is characterized by intrusive, repetitive thoughts and compulsive actions (Mallet and others 2008). Both diagnoses involve dysfunctions of action control.
In Parkinson’s disease, the loss of dopamine neurons causes an imbalance between activation of the direct and indirect pathways, overexcitation of the STN, and reduced movement (DeLong 1990). The hypokinetic Parkinsonian state is also characterized by an abnormally high synchronization of beta oscillations, which are observed at the neuronal and network levels across the dorsal STN and motor cortex (Brittain and others 2014; de Hemptinne and others 2013; Levy and others 2000; Moran and others 2008). The anti-kinetic effects of beta oscillatory activity are assumed to involve the rhythmic entrainment of neuronal firing and pro-kinetic high-frequency oscillations, which may reduce the information capacity of neuronal processing, and thereby interfere with the process of action selection (Brittain and others 2014; Yang and others 2014). Less is known about the pathophysiology in OCD, though studies indicate hyperconnectivity in associative-limbic cortico-basal ganglia pathways (Figee and others 2013), and increased theta synchronization both in prefrontal areas (Karadag and others 2003) and ventral STN neurons (Welter and others 2011). Thus, the pathophysiology of the two disorders share certain common features of cortico-basal ganglia hypersynchronization, although involve different frequencies and different parallel loops of the basal ganglia circuits.
In Parkinson’s disease, circuit balance can be restored by pharmacological replacement of dopamine, or by DBS of the dorsal STN (Deuschl and others 2006; Limousin and others 1995), putatively by reducing the indirect pathway’s inhibitory control of movements. Similarly, DBS of the ventral STN alleviates symptoms of heightened control in OCD (Mallet and others 2008). Conversely, STN DBS may also induce side effects related to lack of control, such as impulsivity and hypomania (Fasano and others 2012; Jahanshahi and others 2015; Kim and others 2015; Mallet and others 2008). The complex effects of DBS are not well understood and cannot be sufficiently described as a functional inhibition of the STN, as was previously suggested (Limousin and others 1995). Rather, DBS most likely modulates both local and widespread dynamic network activity and plasticity (Hamani and others 2017).
Action Inhibition
While STN firing rate and beta synchrony are related to motor inhibition, action inhibition also involves executive control over prepotent or habitual responses. Such habitual responses play an important role in many cognitive behaviors as well as motivational and emotional regulation. A considerable amount of evidence has shed light on how the STN is involved in various aspects of action inhibition, covering different diagnoses, different species, and different methods (Aron and others 2016; Jahanshahi and others 2015; Zavala and others 2015). In brief, imaging and neurophysiology data suggest that the STN, during proactive and reactive action inhibition, receives controlling signals through dynamic connections with frontal cortical areas. Such communication involves increased local beta power and fronto-subthalamic beta coherence (Alegre and others 2013; Aron and Poldrack 2006; Benis and others 2014) (Fig. 2A and B). Frontal regions particularly involved in action inhibition are the right inferior frontal gyrus (rIFG) and pre-supplementary motor areas (pre-SMA) (Aron and others 2016). Different aspects of inhibition are also represented at the neuronal level (Bastin and others 2014; Benis and others 2016; Isoda and Hikosaka 2008) (Table 1). A causal role of the STN in stopping was recently demonstrated by optogenetic activation and inhibition of the STN in rats (Fife and others 2017).
Table 1.
Neuronal Task Responses in the STN.
| Citation | Task | Feature of Responsive Firing | Type of Change and Localization | |
|---|---|---|---|---|
| Response inhibition tasks | Isoda and Hikosaka (2008) (Monkey) | Response inhibition: Switch/Go-NoGo | Switch cells, Go cells, NoGo cells (eye-movement) | Most ↑, some ↓ rate; Overlapping populations; Ventral STN |
| Schmidt and others (2013) (Rat) | Response inhibition: SSRT | Response to stop-cue, regardless of actual stop | Most ↑ rate; Successful stop depends on relative timing of inputs to SNr | |
| Bastin and others (2014) (Human, OCD) | Response inhibition: SSRT | Go cells, stop cells, and error monitoring cells | Most ↑ rate; Separate populations; Ventral STN | |
| Benis and others (2016) (Human, PD) | Response inhibition: SSRT | Go cells, stop cells | Most ↑ rate; Separate populations; Dorsal STN | |
| Pasquereau and Turner (2017) (Monkey) | Response inhibition: SSRT and Go-NoGo | Switch cells, Go cells, NoGo cells | Most ↑ rate; Distinct and overlapping populations; Switch cells: vm STN, others scattered | |
| Cognitive and conflict tasks | Zaghloul and others (2012) (Human, PD) | Value-based decision task (no feedback), conflict | Response to conflict, only in accurate choices | ↑ rate, relatively consistent across cells; Ventral STN |
| Burbaud and others (2013) (Human, OCD) | Memory decision task, checks allowed | Response to cue, movement, decision, feedback, and doubt/checking | Most ↓, some ↑ rate, ↑ during checking; Multimodal responses, overlapping populations; Ventral STN | |
| Zavala and others (2017a) (Human, PD) | Sensorimotor decision task, conflict | Cue cells: modulated by conflict; Move cells: directionally selective | Most ↑, some ↓ rate; Higher rhythmic entrainment during conflict, across cell types; Cue cells: ventral STN | |
| Zavala and others (2017b) (Human, PD) | Memory decision task (nonmotor) | Response to memory encoding less to decision | ↑ and ↓ rate, overall ↓; Only ↓-cells were beta-locked; No localization | |
| Motivational tasks | Lardeux and others (2009) (Rat) | Instrumental task | Response to reward, dep on reward quality | Specific populations, some overlap; ↑ and ↓ rate |
| Lardeux and others (2013) (Rat) | Instrumental task | Response to sucrose vs. cocaine reward and future error | Distinct populations | |
| Breysse and others (2015) (Rat) | Reward discrimination task | Response to positive/aversive reinforcers, execution and evaluation (missed rewards) | ↑ rate at cue for preferred expected reward; Specialization reset when context changes; Distinct populations | |
| Espinosa-Parrilla and others (2013) (Monkey) | Reward representation, different tasks | Response to task-related reward, reward expectancy, and unexpected reward | Most ↑, some ↓ rate; Overlapping populations respond to movement and reward | |
| Rossi and others (2017) (Human, PD) | Valence-based Go-NoGo task | Response to valence-info, more to reward than loss | ↑ and ↓ rate; Distinct populations, some multimodal; Little localization | |
| Pearson and others (2017) (Human, PD) | Risk decision task, self-paced (BART) | Response to risk, outcome, task context, elapsed time | ↑ and ↓ rate; Sparse response, overlapping population | |
| Emotional tasks | Eitan and others (2013) (Human, PD) | Auditory recognition of emotional stimuli | Response to emotional voices, correlated to recognition and arousal | ↑ RMS density, ↓ alpha band activity of neuronal responses; Right ventral STN |
| Sieger and others (2015) (Human, PD) | Viewing of affective pictures | Response to emotional valence/arousal and eye-movement | ↑ and ↓ alpha band activity; Distinct populations in different locations |
STN = subthalamic nucleus; PD = Parkinson’s disease; OCD = obsessive compulsive disorder.
Neuronal responses to executive, cognitive, motivational, and emotional tasks, recorded in rats, monkeys, or human patients with PD or OCD, from the last decade. Note the range of response types and functional cell types listed in the column “Feature of Responsive Firing.” The column “Type of Change and Localization” illustrates that (1) neuronal responses involve both increases and/or decreases in firing rate, (2) functional cell types can reflect separate and/or overlapping neuronal populations, and (3) these functional cell types can be topographically organized or scattered across the entire STN. Of note, some studies report single unit recording, others multiunit or background neuronal activity.
Many decisions and actions may not involve an explicit motor movement, and one possibility is that the basal ganglia and STN may also play a role in controlling such nonmotor actions. For example, the basal ganglia may participate in deciding which item to store in working memory (O’Reilly and Frank 2006). This possibility was recently confirmed as STN beta power and fronto-subthalamic beta coherence were found to decrease during memory encoding, but less for items that should be ignored, indicating a role of fronto-subthalamic beta oscillations in controlling cognitive actions such as memory encoding (Zavala and others 2017b) (Fig. 2C).
A Switch or a Break?
Inhibition of an action does not necessarily mean stopping all actions, but instead could mean slowing down to select the most appropriate action for the behavioral goals at hand. Consistent with this, the STN can facilitate switching from habitual to controlled behaviors (Hikosaka and Isoda 2010; Isoda and Hikosaka 2008; Jahanshahi and others 2015). Some neurons in the STN respond to behavioral switching rather than inhibition per se (Isoda and Hikosaka 2008; Pasquereau and Turner 2017), and STN DBS generally increases cognitive and behavioral flexibility (Krack and others 2010; Weintraub and Zaghloul 2013), though STN inactivation can also cause perseverative behavior in animal models (Baker and Ragozzino 2014; Baunez and others 2007; Jahanshahi and others 2015). A relevant point in this regard is that beta oscillations may have a broader role than merely inhibiting actions, and may instead promote the maintenance of the ongoing behavior and the status quo (Engel and Fries 2010; Jenkinson and Brown 2011). As such, a decrease in beta power may facilitate behavioral switching. The role of the STN and beta activity in action control may therefore extend beyond action inhibition to also involve control of behavioral switching, though the exact mechanisms are yet to be established.
From Decision to Action
A central purpose of action control is to optimize decisions that we make. This links the field of motor and action control to the field of decision making, where a solid framework of computational modeling based on drift diffusion models has been used to describe discrete choices, both at the behavioral and the neurophysiological levels (Gold and Shadlen 2007; Ratcliff 1978). During a decision between two responses, the perceived evidence for each option is thought to gradually increase, and once the accumulated evidence reaches a certain threshold, that particular response is executed (Fig. 3). A neural signature of this process has been found in cortical areas, where neuronal firing rates gradually increase according to the accumulated evidence for specific responses (Brody and Hanks 2016; Gold and Shadlen 2007). But what decides when the evidence is sufficient and it is time to act?
Figure 3.
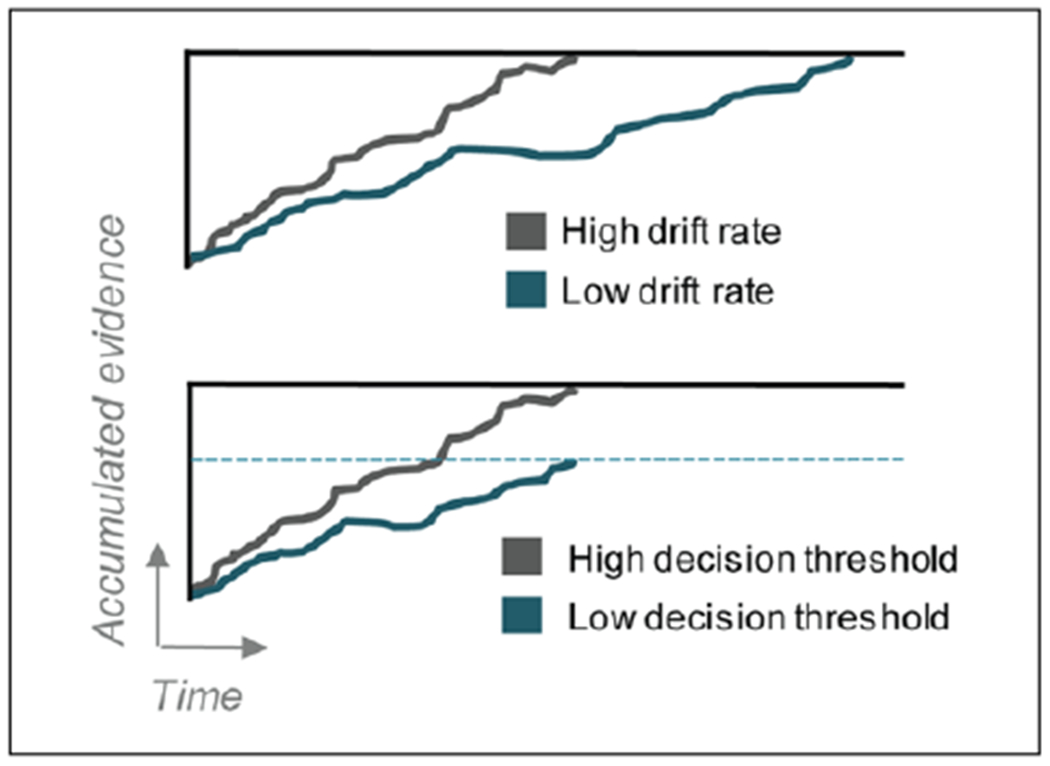
Evidence accumulation and decision thresholds. Schematic illustration of the evidence accumulation process, indicating how accumulated evidence increases over time toward the decision threshold for the correct decision. When the accumulated evidence reaches the decision threshold, a response is made. Upper panel displays high and low drift rates of the accumulated evidence. In this case, both conditions eventually accumulate the same level of evidence and therefore will have similar accuracy, but it takes longer time when the drift rate is low, such as during a difficult task. Lower panel displays high and low decision thresholds. Drift rate is slower in the low-threshold condition, but since the decision threshold is lower, the response is made at the same time as the high-threshold condition, but with less accumulated evidence and therefore lower accuracy level.
Beyond Motor Control: Models of STN as a Decision Threshold
Two influential computational models (Bogacz and Gurney 2007; Frank 2006) point to the basal ganglia as a promising system for adjusting these thresholds. The models agree on their general prediction—evidence is accumulated in cortical areas, which then excite both the striatum and STN through outcome-specific channels. While the striatum conveys the option-specific signals to the GPI/SNR, the STN instead conveys a sum of the options widely to the GPI/SNR, effectively creating a “break” or “decision threshold.” For an action to be executed, this threshold must be exceeded by an option-specific signal from the striatum, initiated by evidence that is sufficiently stronger than the alternative options. Thus, this model follows the same logic as the center-surround-model for movements outlined above.
According to these models, a difficult decision will lead to a slower response than an easy decision, because the alternative responses together will induce a stronger global activation of the STN and hence a higher decision threshold, reflecting the level of conflict (Frank 2006). However, the increased threshold implemented by the STN will ensure that the action is delayed until the best option is selected. These models therefore predict that the role of the STN in action control is to withhold a response during high-conflict decisions in order to improve accuracy (Bogacz and Gurney 2007; Frank 2006).
Evidence for Conflict Detection and Decision Thresholds in the STN
Experimental evidence confirms that the STN is involved in conflict representation during choice between multiple options (Aron and others 2016; Zavala and others 2015) (Figs. 2B and 3B). Behaviorally, STN DBS in patients with Parkinson’s disease has been shown to selectively impair accuracy in high-conflict choices, but not in low-conflict choices (Cavanagh and others 2011; Frank and others 2007). Decisions during high-conflict choices were made faster, indicating problems with slowing down the response in order to select the best option. Consistent with this, DBS of the ventral STN in patients with OCD increase decisional impulsivity by reducing decision thresholds, and thereby shifts decision making toward a less cautious, more healthy style (Voon and others 2017).
Single unit activity in the STN has been found to increase during cognitive (Burbaud and others 2013; Zaghloul and others 2012) and sensorimotor (Zavala and others 2014; Zavala and others 2017a) conflict (Fig. 5B and C), in line with increased action inhibition. While action inhibition involves increased power in STN beta band oscillations, conflict is instead associated with increased oscillatory power in the theta band, which is reported in the context of both sensorimotor(Zavala and others 2014; Zavala and others 2017a), cognitive (Brittain and others 2012; Cavanagh and others 2011), and moral (Fumagalli and others 2011) conflict (Figs. 2B and 3B). Increased STN theta activity co-occurs and is coherent with increased theta power in the prefrontal cortex (Zavala and others 2014; Zavala and others 2016; Zavala and others 2017a). The conflict-induced cortico-subthalamic theta synchrony is not only related to conflicting responses (Zavala and others 2014) but also to difficulty related to sensory uncertainty, and the need to slow down the response until sufficient evidence has been accumulated (Zavala and others 2016).
Figure 5.
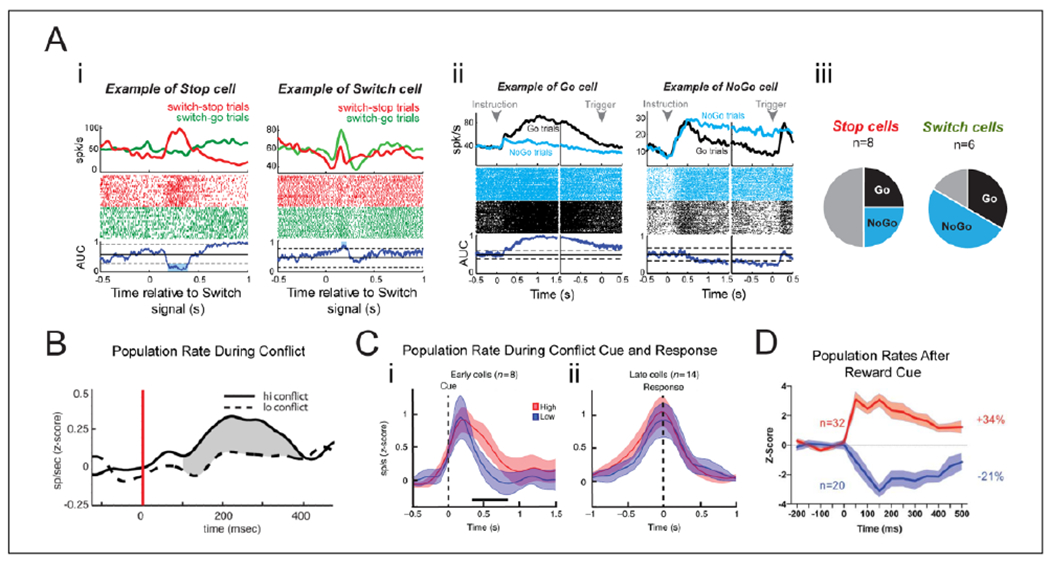
Neuronal activity in the STN during response inhibition, decisional conflict, and reward. (A) Single neuron responses from a monkey performing an inhibitory control task which disentangles proactive Go-NoGo responses and reactive switch-responses: switch-go (stop-to-go) and switch-stop (go-to-stop). (i) Response of two different cells to switch-go trials and switch-stop trials. (ii) Response of two different cells to Go-trials and NoGo-trials in the Go-NoGo task. (iii) Stop and Switch cells were separate populations during reactive trials, but each of these could be both Go and NoGo cells during proactive control trials. (B) STN population rate during associative decisions. Firing rate increases during high conflict trials relative to low conflict trials. (C) During a sensorimotor task, STN cell responses with increased firing rate aligned either to the cue (“Early cells”) or to the response (“Late cells”). The cue-aligned cells increased in firing rate during high conflict trials relative to the low conflict trials. (D) STN neuronal responses following a “Go for Reward” cue for neurons that increased (red) or decreased (blue) in firing rate. (A) Reproduced with permission from Pasquereau and Turner (2017), (B) Republished with permission of Society for Neuroscience, from Zaghloul and others (2012); permission conveyed through Copyright Clearance Center, Inc., (C) Reproduced with permission from Zavala and others (2017a), (D) Reproduced with permission from Rossi and others (2017).
A direct link between conflict-related PFC-STN theta activity and decision thresholds has been formally demonstrated in the context of drift diffusion models (Cavanagh and others 2011; Herz and others 2016; Herz and others 2017) (Fig. 4). Increased medial PFC (mPFC) theta activity indicated increased decision thresholds on a trial-to-trial basis, but STN DBS reversed this relationship while accuracy dropped for high-conflict trials (Cavanagh and others 2011). The level of mPFC-STN theta coupling predicted adjustments of decision thresholds, while threshold-modulation by local theta power in the STN critically depended on level of cautiousness (Herz and others 2016). A recent study demonstrates that STN decision thresholds can be adjusted by instructed tradeoffs between speed and accuracy (Herz and others 2017), and further reveals that cue-induced beta oscillations are also related to the decision threshold. Interestingly, increased STN theta power, coupled to the mPFC, correlated with increased thresholds only after accuracy instructions. Conversely, decreased STN beta power, coupled to the motor cortex, correlated with decreased thresholds when cued by the stimulus itself regardless of instructions (Fig. 4C). Thus, the frontal cortex can implement decision thresholds in the STN through theta or beta synchrony, which can be set both according to internal top-down cognitive control (e.g., according to task instructions) or by external factors related to features of the task stimuli. The relationship between decision thresholds and STN oscillatory power is however more complex than a linear increase in STN theta power and mPFC-STN theta coherence with increased decision thresholds.
Figure 4.
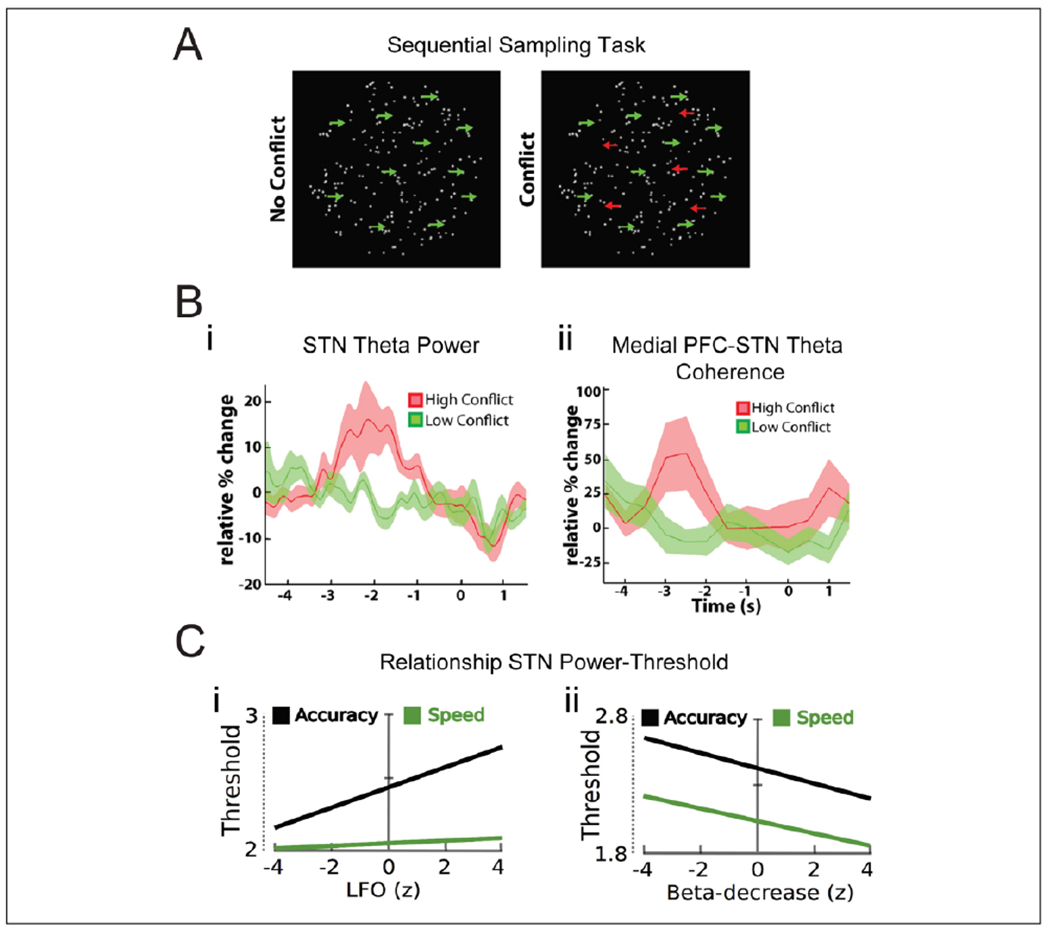
Decision thresholds in the STN. (A, B) Decision making during high and low conflicts. In the moving dot task (A, left), participants are asked to indicate the overall direction of dot movement, which becomes gradually more coherent. Sensory information is sequentially sampled and integrated over time, as evidence for the correct response is accumulated. During high-conflict trials in this study (A, right), some dots moved in the opposite direction of the overall movement direction. STN theta power (B, i) and mPFC-STN theta coherence (B, ii) increased selectively in the high-conflict trials. In this task, increased STN theta power was related to increased decision thresholds in a drift diffusion model, and mPFC-STN theta coupling predicted threshold modulation (Herz and others 2016). (C) Decision making during instructed speed-accuracy tradeoffs. In this task, participants were instructed to be either fast or accurate in their responses, which should indicate the overall direction of moving dots with either high or low movement coherence. Increase in STN theta power predicted decision thresholds only after accuracy instructions (C, left). Decrease in STN beta during high dot moving coherence predicted decreased decision thresholds regardless of instructions (C, right). Reproduced from (A, B) Zavala and others (2014) and (C) Herz and others (2017), licensed under CC BY.
It remains to be determined how conflict-driven cortico-subthalamic theta oscillations interfere with inhibition-related beta oscillations and neuronal spiking in the STN to implement a decision threshold. No study has formally modelled the relation between neuronal spiking in the STN and decision thresholds, though uniform increases in firing rate related to conflict level have been reported (Zaghloul and others 2012). Notably, neuronal activity profiles reflecting evidence accumulation have also been found in the striatum in animal studies (Brody and Hanks 2016; Ding and Gold 2010), which further strengthen the possibility that the basal ganglia serves as a readout structure that transforms cortical evidence into actions. These findings strongly support the above-mentioned models of the STN in action control, which extend the role of STN beyond motor control and into decision making and cognitive control.
Motivational and Emotional Processing in the STN
Beyond its involvement in actions and decisions, the STN is also involved in motivational and emotional functions (Rossi and others 2015; Weintraub and Zaghloul 2013). This is anatomically supported by its connections to ventromedial prefrontal, orbitofrontal, and anterior cingulate cortices and other limbic structures (Alexander and others 1986; Haynes and Haber 2013; Lambert and others 2012; Voon and others 2017), and by the observation of occasional motivational and emotional effects of STN DBS (Fasano and others 2012; Kim and others 2015; Mallet and others 2008). Motivational and emotional processing in the STN has been related to theta (Rosa and others 2013; Zenon and others 2016) and alpha power (Brucke and others 2007; Huebl and others 2011; Kuhn and others 2005), respectively. Neurons in the STN respond to features of reward, risk, outcome, arousal, and emotional valence (Breysse and others 2015; Lardeux and others 2009; Lardeux and others 2013; Pearson and others 2017; Rossi and others 2017) (Table 1).
While drift diffusion models for evidence accumulation are most commonly used in the context of perceptual decisions, a separate line of research has investigated decision making based on value-based choices and reinforcement learning. The former approach focuses on the dynamics of choice processes but assumes static decision values, while the latter focuses on the dynamics of experience but assumes a static choice process (Frank and others 2015). Frank and colleagues demonstrated that the drift diffusion model could describe choices during reinforcement learning with sequential sampling of reward values, involving dynamic adjustments of STN and mPFC activity corresponding to decision thresholds (Frank and others 2015). As the STN also is involved in processing of rewards and risks, it will be an interesting topic for future research to clarify how reward probability, valence, and evidence-value of reinforcers are encoded in the fronto-subthalamic network.
Impulsivity
The role of cortical-subthalamic function in modulating decision thresholds has obvious implications for impulsivity, as lowered decision thresholds may increase impulsive actions, a phenomenon referred to as reflection impulsivity (Box 1). This perspective expands the view of STN in impulsivity beyond “motor impulsivity” (Voon and Dalley 2016) (Box 1) to be relevant also for “decisional impulsivity” (Jahanshahi and others 2015; Voon and others 2017; Zavala and others 2015) (Box 1), though this may be specific for the ventral part of the STN.
Box 1. Definitions of Impulsivity.
“Motor impulsivity includes (i) waiting impulsivity or premature anticipatory responding prior to a cue predicting reward and (ii) response inhibition or stopping inhibition of a prepotent response. Decisional impulsivity includes (iii) delay and probabilistic discounting of reward and (iv) reflection impulsivity—the tendency to make rapid decisions without adequate accumulation and consideration of the available evidence.” Citation from Voon and Dalley (2016)
Dopamine has consistently been found to be important for impulsivity that is related to motivational drive, such as waiting impulsivity (Box 1) and delay discounting, that is, the decline of perceived value for a delayed reward (Voon and Dalley 2016). Since the STN is involved in reward and risk processing (Table 1), it could also potentially be involved in more motivational aspects of impulsivity. Mixed results regarding the effects of STN modulation on delay discounting (Jahanshahi and others 2015; Voon and others 2017; Winstanley and others 2005) call for further investigation of the reward modulating effects of STN DBS, which may also have implications for mood regulation.
Thus, it is well established that the STN generally is involved in inhibiting actions, in particular in high-conflict situations, and that this can be described in the context of “decision thresholds” based on cortical input. Furthermore, rather than solely inhibiting actions, the STN may be involved in the balance between maintenance of the current action plan and behavioral flexibility. Pathologically high decision thresholds can cause problems for initiating or switching movements, behaviors, or thoughts, and may induce hypokinetic symptoms, perseveration, compulsivity, obsessive thinking, and possibly depression. In contrast, inactivation of the STN facilitates movements and flexibility, but may also lead to motor and decisional impulsivity, and potentially hypomania. The behavioral effects of how the STN may modulate decision thresholds seems dependent on dorsoventral topography. Obviously, the context of decision thresholds does not capture all aspects of any of the symptoms discussed here, but this perspective brings a unifying view which can generate hypotheses for future research.
Neuronal Processing in the STN
The effect of STN on action control must ultimately be implemented by neuronal firing in the STN, yet current models often assume a uniform involvement of STN neurons. Here we summarize neuronal activity in the STN during specific tasks, which demonstrate a range of patterned activity (Table 1 and Fig. 5).
Neurons throughout the basal ganglia, including the STN, are active during movement, depending on the body part involved and the direction of movement (Alexander and others 1990; Zavala and others 2017a). STN neurons modulate their firing according to a range of task variables, such as different phases of movement and inhibition, preparation, conflict, switch, risk, elapsed time, reward, and emotions, as summarized in Table 1. General features of STN neuronal activity are comparable across diagnoses and species. A consistent finding is that some neurons respond to cues and premotor decisions, some to the movement itself, while yet others become active after the movement or outcome, and seem involved in postresponse evaluation. Most studies have demonstrated firing modulation that is eventually related to various factors to the motor response, possibly reflecting integration of different types of information toward action. However, firing modulation is also found in tasks where no movement (Zavala and others 2017b), or even no nonmotor action (Eitan and others 2013; Espinosa-Parrilla and others 2013; Sieger and others 2015), is executed. These findings generally confirm that various types of information are integrated in the STN to guide actions, but also that STN neuronal activity reflects cognitive and emotional outcomes in absence of motor actions.
Different task features are in many cases represented by distinct neuronal populations, such as separate populations for go or stop, for cue and movement, for different directions of movement, for different types of reward or valence, or for arousal (Table 1). However, the same neurons can respond to several different features, such as movement and reward (Espinosa-Parrilla and others 2013), or to proactive and reactive stops dependent on task conditions (Isoda and Hikosaka 2008; Pasquereau and Turner 2017). This multimodal response could potentially be confounded by suboptimal spike sorting, in which firing patterns reported as single neuron responses may in fact reflect firing from different neurons. It is likely, however, that the STN indeed does display such multimodal responses, as the prefrontal cortex, upstream to the STN, displays both mixed neuronal selectivity (Rigotti and others 2013) and firing patterns similar to the STN (Hikosaka and Isoda 2010). A confusing but consistent finding is that most transient responses can result in both increases and decreases in firing rates, as is the case for both stop and movement responses (Alexander and others 1990; Isoda and Hikosaka 2008; Pasquereau and Turner 2017).
The complexity of STN activity is well illustrated by a recent report of STN neuronal firing during response inhibition (Pasquereau and Turner 2017) (Fig. 5A), which extends an earlier influential study (Isoda and Hikosaka 2008). This study demonstrated that the neurons within the STN respond to both proactive and reactive inhibition of movement, as well as to switches in the movement plan. The neuronal involvement is not straightforward; one functional cell type, the reactive “switch cells,” had a defined topographical localization and did not overlap with another functional cell type, the “movement cells.” However, the functional classes of proactive “Go” and “NoGo” cells, which one could easily confuse with the reactive “switch-go” and “switch-stop” cells, were scattered across the entire STN. And both these populations overlapped with both the “switch-stop” and “switch-go” cell populations, but not with each other.
Given that different STN neurons behave differently, it is possible that different task-specific functional cell types can be characterized by specific connectivity and molecular properties. Though STN cells often are referred to as a uniform population, differential cell populations in the STN, in terms of transmitter type, connectivity and membrane receptor profile have later been reported (Arcos and others 2003; Kita and others 1983; Levesque and Parent 2005; Sato and others 2000; Takada and others 1988; Xiao and others 2015), suggesting the presence of subcircuits within the STN. However, an in vitro study revealed that single neurons in the STN can function in as many as four stable states (Kass and Mintz 2006), suggesting that different recorded responses do not necessarily correspond to different defined cell types. Future research may clarify how these different properties align or overlap.
Integrative and Parallel Processing in the STN
Based on the above, it seems well established that different territories of the frontal cortex interact with the STN during different motor and nonmotor task demands, that these inputs are anatomically arranged across the longitudinal axis of the STN according to information type, and that the frontal cortex and the STN can communicate via frequency-specific channels. But to what degree are different types of information integrated toward one action versus being processed in parallel toward different outputs?
Though anatomical evidence indicates partially integrative afferents, it remains to be established how afferents from frontal and pallidal subregions monosynaptically connect and integrate on single STN neurons, and whether STN neurons are intrinsically connected, directly or via the GPe. This has been addressed by a recent functional connectivity study in rodents, which confirms that motor and nonmotor inputs partially integrate on to single neurons in the STN (Janssen and others 2017). Moreover, task-related studies (Table 1) demonstrate that different types of information, such as representations of movement and reward outcome (Espinosa-Parrilla and others 2013), converge on to single neurons in the STN. Scattered localization of specific neuronal response profiles further confirms that information to some degree is integrated in the STN.
According to the global inhibition view, the major point of convergence is, however, not the STN, but the output nuclei SNR/GPI, which is thought to increase global inhibition of basal ganglia output in response to STN activation. Supporting evidence for this view is the causal effect of optogenetic activation of the STN on stopping (Fife and others 2017). Furthermore, recent studies have demonstrated an interesting interaction between sensorimotor and cognitive inhibition (Chiu and Egner 2015; Wessel and others 2016). On the other hand, the specific functional connectivity and pathophysiology involved in motor versus cognitive-limbic functions of the STN argues for somewhat distinct downstream routes of different STN sub-regions. It is possible that a “semi-global” signal may be computed by different modules of the STN, such as dorsoventral subregions or specific cell clusters, which would then differentially control specific types of actions.
It seems challenging to reconcile, however, the observed finely patterned but scattered response of different neurons in the STN with the possibility that a (semi)global STN activity exists as an inhibition signal. Various neuronal responses could certainly collectively participate in a (semi)global STN output; examining the average firing rate reported for the studies in Table 1 would be helpful to assess that possibility. Such a global summary of combined features of frontal and striatal processing would then indeed be an informative signal reflecting overall conflict, as suggested by decision models of the STN. However, it appears likely that the information represented by the heterogeneous response is transmitted to downstream networks in a more specific way. Ensembles of specific functional STN cell types could form a uniform downstream signal even if they are not topographically clustered, either by specific anatomical wiring or dynamically by shared state or oscillatory coupling. Different hypothetical schemes for cortico-subthalamic processing is outlined in (Fig. 6).
Figure 6.
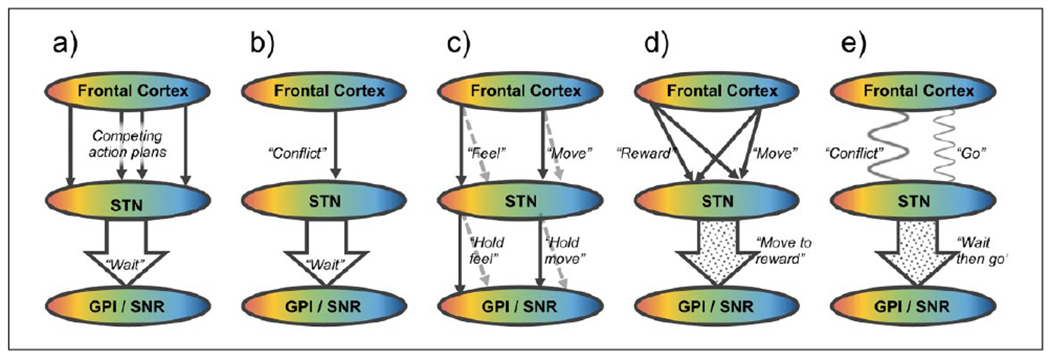
Action control in the STN. Simplified scheme of possible conceptual routes of information flow between the frontal cortex, STN, and GPI/SNR, based on functional anatomical or dynamic connections. Color gradients represent topographical organization of functions from the blue “motor” part in the most posterior dorsolateral STN to the red “limbic” part in the most anterior ventromedial STN, with connected areas in the frontal cortex and GPI/SNR (Alexander and others 1990, Haynes and Haber 2013). Similar principles could be relevant for the striato-pallido-subthalamic (indirect) pathway, but the figure does not address how this input is integrated with cortical input in the STN. All of these schemes may be relevant, in parallel or depending on context. (a) Competing input model: Competing action plans reaching the STN cause a global excitation of GPI/SNR, conveying a “wait” signal to withhold a response until the optimal action is selected by the striatum (Bogacz and Gurney 2007; Frank 2006). (b) Specific input model: A specific signal from the PFC to the STN (conveying for example “Stop” or “Conflict”) causes global excitation of GPI/SNR (Aron and others 2016). (c) Parallel/spiral model: Parallel circuits in the basal ganglia loop process different types of information, though the inhibitory effect of STN on basal ganglia output may be similar regardless of information type (Alexander and others 1986). Open and closed loops across the basal ganglia may allow information to spiral from ventral to dorsal loops (Haber 2003; Haynes and Haber 2013; Kelly and Strick 2004). (d) Integration model: Integration of information modalities to specific neurons across the STN (Espinosa-Parrilla and others 2013; Janssen and others 2017). This could be anatomically supported by nucleus-wide dendrites (Sato and others 2000), intranuclear axon collaterals (Kita and others 1983) or STN-Gpe interactions. STN neurons convey a specific but integrated (modular) signal to the GPI/SNR. (e) Dynamic model: The frontal cortex conveys different signals to the STN through directed oscillatory coupling. For example, a “conflict” signal from the PFC can be transmitted through increased theta coherence, and a “go” signal from sensorimotor cortex can be transmitted through decreased beta coherence. In the STN, parts of the local processing by neurons and high-frequency oscillations can entrain to these rhythms, and the participating ensemble of neurons transmits a collective but patterned signal to the GPI/SNR (Aron and others 2016; Brittain and others 2014; Zavala and others 2015; Zavala and others 2017a).
Interaction between Rhythms and Spikes
Rhythmic activity and coherence are both highly relevant for cortical-subthalamic function in action control. How this framework relates to neuron-specific and global firing rates, however, is an unresolved question in the field (Nambu and others 2015).
One possibility is that, regardless of whether the neuronal firing in the STN transmits a global or patterned signal, STN oscillations may represent global signals distinct from the neuronal firing, which may synchronize with downstream regions and in this way transmit information. Given the importance of synaptic transmembrane currents for shaping the local field potential (Buzsaki and others 2012), oscillatory field activity may reflect global input more directly than global spiking activity necessarily does.
A crucial point for how rhythms and spikes interact is, however, how neuronal firing is modulated by oscillatory activity. As Parkinsonism is characterized by beta band hypersynchrony across neurons and field-potentials in the STN and motor cortex, it has been hypothesized that this hypersynchrony leads to movement inhibition by compromising the processing capacity of STN neurons. This idea is consistent with the finding that oscillatory coupling decreases during a movement (Potter-Nerger and others 2017), potentially reflecting a release from oscillatory inhibition, and that oscillatory entrainment increases during conflict (Zavala and others 2017a), in line with an increased action threshold. Beta entrainment of spiking, however, was not found to change during cognitive inhibition (Zavala and others 2017b), although functional subtypes of the neurons were differently entrained to the beta rhythm, suggesting an alignment between beta locking and task response. Interestingly, only cells with task-specific rate increase were beta locked (Zavala and others 2017a; Zavala and others 2017b), indicating that rhythmic entrainment may selectively control specific task-responsive populations.
Several studies have also reported theta modulation and entrainment of STN neurons at the same time as beta modulation (Moran and others 2008; Zavala and others 2017a). The conflict-related entrainment mentioned above happened both at beta and theta frequency, and both for cue-cells and movement-cells (Zavala and others 2017a). Even if it is unclear how each frequency specifically modulates neuronal firing, the finding that the same or co-localized cells were modulated by two rhythms, indicates a possible convergence point between theta-related conflict, beta-related stop and neuronal firing, although this needs further investigation.
Though broad evidence points to a link between movement inhibition and beta synchrony, and possibly also to conflict-dependent theta synchrony, this does not mean that all rhythmic modulation or entrainment is inhibiting. In the hippocampal system, neuronal phase coding relative to theta oscillations contributes to information coding (O’Keefe and Recce 1993), a principle that also is found in the ventral striatum (van der Meer and Redish 2011). Strong theta power in the local field potential does not necessarily reflect strong theta synchrony between neurons, but rather allows cell assemblies to decorrelate and facilitate information coding (Mizuseki and Buzsaki 2014). This is in line with the idea that Parkinsonian pathophysiology is related to hypersynchronized and maladaptive beta activity rather than beta oscillations per se. To further understand the role of spike-field interactions in the STN, there is a need to clarify how oscillations of different frequencies, levels, and scales interact. Taken together, however, cortico-subthalamic processing by specific rhythms may dynamically entrain or temporally bind specific populations of STN neurons, and by such mechanisms flexibly control how various types of information are linked and transmitted to downstream networks.
Conclusion
In this review, we have outlined the role of STN in motor and nonmotor action control. Behavioral, clinical, and physiological data, combined with computational modelling, converge in many ways toward a unified general understanding of STN function. Still, there are many unresolved questions regarding how different types of information is processed and integrated in the STN, how observed oscillatory and rate-based dynamics interact in this loop, and finally how the STN interacts with other structures in the basal ganglia, frontal cortex, and potentially the brainstem to learn and perform optimal action control.
A focus that deserves attention is the converging evidence that different neurons exhibit differential responses within the STN. This evidence does not strictly align with the view that there is a global and uniform STN output. Given the rich and multidisciplinary reports on human STN function, it is surprising that there are relatively few studies from in vitro physiology work and from rodent models in which high-quality recordings and optogenetic circuit dissections during behavioral tasks could address many of the unresolved issues.
Though it is challenging to disentangle the increasing complexity of the circuits involved in action control, these questions offer a rich opportunity for investigating core features of behavioral control through multidisciplinary approaches and methods across clinical and experimental fields. Ultimately, better insight into these circuits may provide better understanding and treatment of disorders of action control, including but not limited to Parkinson’s disease and OCD.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: TB received a research grant from the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology (NTNU). This work was supported by the Intramural Research Program at the National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Alegre M, Lopez-Azcarate J, Obeso I, Wilkinson L, Rodriguez-Oroz MC, Valencia M, and others 2013. The subthalamic nucleus is involved in successful inhibition in the stop-signal task: a local field potential study in Parkinson’s disease. Exp Neurol 239:1–12. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. 1990. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85:119–46. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–81. [DOI] [PubMed] [Google Scholar]
- Arcos D, Sierra A, Nunez A, Flores G, Aceves J, Arias-Montano JA. 2003. Noradrenaline increases the firing rate of a subpopulation of rat subthalamic neurones through the activation of alpha 1-adrenoceptors. Neuropharmacology 45(8):1070–9. [DOI] [PubMed] [Google Scholar]
- Aron AR, Herz DM, Brown P, Forstmann BU, Zaghloul K. 2016. Frontosubthalamic circuits for control of action and cognition. J Neurosci 36(45):11489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. 2006. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci 26(9):2424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Ragozzino ME. 2014. The prelimbic cortex and subthalamic nucleus contribute to cue-guided behavioral switching. Neurobiol Learn Mem 107:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin J, Polosan M, Benis D, Goetz L, Bhattacharjee M, Piallat B, and others 2014. Inhibitory control and error monitoring by human subthalamic neurons. Transl Psychiatry 4:e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez C, Christakou A, Chudasama Y, Forni C, Robbins TW. 2007. Bilateral high-frequency stimulation of the subthalamic nucleus on attentional performance: transient deleterious effects and enhanced motivation in both intact and parkinsonian rats. Eur J Neurosci 25(4):1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benis D, David O, Lachaux JP, Seigneuret E, Krack P, Fraix V, and others 2014. Subthalamic nucleus activity dissociates proactive and reactive inhibition in patients with Parkinson’s disease. Neuroimage 91:273–81. [DOI] [PubMed] [Google Scholar]
- Benis D, David O, Piallat B, Kibleur A, Goetz L, Bhattacharjee M, and others 2016. Response inhibition rapidly increases single-neuron responses in the subthalamic nucleus of patients with Parkinson’s disease. Cortex 84:111–23. [DOI] [PubMed] [Google Scholar]
- Bogacz R, Gurney K. 2007. The basal ganglia and cortex implement optimal decision making between alternative actions. Neural Comput 19(2):442–77. [DOI] [PubMed] [Google Scholar]
- Breysse E, Pelloux Y, Baunez C. 2015. The good and bad differentially encoded within the subthalamic nucleus in rats(1,2,3). eNeuro 2(5). doi: 10.1523/ENEURO.0014-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JS, Brown P. 2014. Oscillations and the basal ganglia: motor control and beyond. Neuroimage 85(Pt 2):637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JS, Sharott A, Brown P. 2014. The highs and lows of beta activity in cortico-basal ganglia loops. Eur J Neurosci 39(11):1951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JS, Watkins KE, Joundi RA, Ray NJ, Holland P, Green AL, and others 2012. A role for the subthalamic nucleus in response inhibition during conflict. J Neurosci 32(39):13396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody CD, Hanks TD. 2016. Neural underpinnings of the evidence accumulator. Curr Opin Neurobiol 37:149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucke C, Kupsch A, Schneider GH, Hariz MI, Nuttin B, Kopp U, and others 2007. The subthalamic region is activated during valence-related emotional processing in patients with Parkinson’s disease. Eur J Neurosci 26(3):767–74. [DOI] [PubMed] [Google Scholar]
- Burbaud P, Clair AH, Langbour N, Fernandez-Vidal S, Goillandeau M, Michelet T, and others 2013. Neuronal activity correlated with checking behaviour in the subthalamic nucleus of patients with obsessive-compulsive disorder. Brain 136(Pt 1):304–17. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Anastassiou CA, Koch C. 2012. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13(6):407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, and others 2011. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci 14(11):1462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YC, Egner T. 2015. Inhibition-induced forgetting results from resource competition between response inhibition and memory encoding processes. J Neurosci 35(34):11936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, and others 2013. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A 110(12): 4780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. 1990. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13(7):281–5. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, and others 2006. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 355(9):896–908. [DOI] [PubMed] [Google Scholar]
- Ding L, Gold JI. 2010. Caudate encodes multiple computations for perceptual decisions. J Neurosci 30(47):15747–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan R, Shamir RR, Linetsky E, Rosenbluh O, Moshel S, Ben-Hur T, and others 2013. Asymmetric right/left encoding of emotions in the human subthalamic nucleus. Front Syst Neurosci 7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P. 2010. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol 20(2):156–65. [DOI] [PubMed] [Google Scholar]
- Espinosa-Parrilla JF, Baunez C, Apicella P. 2013. Linking reward processing to behavioral output: motor and motivational integration in the primate subthalamic nucleus. Front Comput Neurosci 7:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Daniele A, Albanese A. 2012. Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet Neurol 11(5):429–42. [DOI] [PubMed] [Google Scholar]
- Fife KH, Gutierrez-Reed NA, Zell V, Bailly J, Lewis CM, Aron AR, and others 2017. Causal role for the subthalamic nucleus in interrupting behavior. Elife 6. doi: 10.7554/eLife.27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figee M, Luigjes J, Smolders R, Valencia-Alfonso CE, van Wingen G, de Kwaasteniet B, and others 2013. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci 16(4):386–7. [DOI] [PubMed] [Google Scholar]
- Frank MJ.2006. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw 19(8):1120–36. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Gagne C, Nyhus E, Masters S, Wiecki TV, Cavanagh JF, and others 2015. fMRI and EEG predictors of dynamic decision parameters during human reinforcement learning. J Neurosci 35(2):485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. 2007. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 318(5854):1309–12. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Giannicola G, Rosa M, Marceglia S, Lucchiari C, Mrakic-Sposta S, and others 2011. Conflict-dependent dynamic of subthalamic nucleus oscillations during moral decisions. Soc Neurosci 6(3):243–56. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. 2007. The neural basis of decision making. Annu Rev Neurosci 30:535–74. [DOI] [PubMed] [Google Scholar]
- Haber SN.2003. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 26(4):317–30. [DOI] [PubMed] [Google Scholar]
- Haber SN, Behrens TE. 2014. The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron 83(5):1019–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. 2000. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 20(6): 2369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Florence G, Heinsen H, Plantinga BR, Temel Y, Uludag K, and others 2017. Subthalamic nucleus deep brain stimulation: basic concepts and novel perspectives. eNeuro 4(5). doi: 10.1523/ENEURO.0140-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Rouzaire-Dubois B, Feger J, Jackson A, Crossman AR. 1983. Anatomical and electrophysiological studies on the reciprocal projections between the subthalamic nucleus and nucleus tegmenti pedunculopontinus in the rat. Neuroscience 9(1):41–52. [DOI] [PubMed] [Google Scholar]
- Haynes WI, Haber SN. 2013. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for basal ganglia models and deep brain stimulation. J Neurosci 33(11):4804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazrati LN, Parent A. 1992. Convergence of subthalamic and striatal efferents at pallidal level in primates: an anterograde double-labeling study with biocytin and PHA-L. Brain Res 569(2):336–40. [DOI] [PubMed] [Google Scholar]
- Helfrich RF, Knight RT. 2016. Oscillatory dynamics of prefrontal cognitive control. Trends Cogn Sci 20(12):916–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz DM, Tan H, Brittain JS, Fischer P, Cheeran B, Green AL, and others 2017. Distinct mechanisms mediate speed-accuracy adjustments in cortico-subthalamic networks. Elife 6. doi: 10.7554/eLife.21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz DM, Zavala BA, Bogacz R, Brown P. 2016. Neural correlates of decision thresholds in the human subthalamic nucleus. Curr Biol 26(7):916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Isoda M. 2010. Switching from automatic to controlled behavior: cortico-basal ganglia mechanisms. Trends Cogn Sci 14(4):154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebl J, Schoenecker T, Siegert S, Brucke C, Schneider GH, Kupsch A, and others 2011. Modulation of subthalamic alpha activity to emotional stimuli correlates with depressive symptoms in Parkinson’s disease. Mov Disord 26(3):477–83. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. 2008. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci 28(28):7209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Obeso I, Baunez C, Alegre M, Krack P. 2015. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord 30(2):128–40. [DOI] [PubMed] [Google Scholar]
- Janssen MLF, Temel Y, Delaville C, Zwartjes DGM, Heida T, De Deurwaerdere P, and others 2017. Cortico-subthalamic inputs from the motor, limbic, and associative areas in normal and dopamine-depleted rats are not fully segregated. Brain Struct Funct 222(6):2473–85. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Brown P. 2011. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci 34(12):611–8. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Kuhn AA, Brown P. 2013. Gamma oscillations in the human basal ganglia. Exp Neurol 245:72–6. [DOI] [PubMed] [Google Scholar]
- Karachi C, Yelnik J, Tande D, Tremblay L, Hirsch EC, Francois C. 2005. The pallidosubthalamic projection: an anatomical substrate for nonmotor functions of the subthalamic nucleus in primates. Mov Disord 20(2):172–80. [DOI] [PubMed] [Google Scholar]
- Karadag F, Oguzhanoglu NK, Kurt T, Oguzhanoglu A, Atesci F, Ozdel O. 2003. Quantitative EEG analysis in obsessive compulsive disorder. Int J Neurosci 113(6):833–47. [DOI] [PubMed] [Google Scholar]
- Kass JI, Mintz IM. 2006. Silent plateau potentials, rhythmic bursts, and pacemaker firing: three patterns of activity that coexist in quadristable subthalamic neurons. Proc Natl Acad Sci U S A 103(1):183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. 2004. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res 143:449–59. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Jeon BS, Paek SH. 2015. Nonmotor symptoms and subthalamic deep brain stimulation in Parkinson’s disease. J Mov Disord 8(2):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Chang HT, Kitai ST. 1983. The morphology of intracellularly labeled rat subthalamic neurons: a light microscopic analysis. J Comp Neurol 215(3):245–57. [DOI] [PubMed] [Google Scholar]
- Krack P, Hariz MI, Baunez C, Guridi J, Obeso JA. 2010. Deep brain stimulation: from neurology to psychiatry? Trends Neurosci 33(10):474–84. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Hariz MI, Silberstein P, Tisch S, Kupsch A, Schneider GH, and others 2005. Activation of the subthalamic region during emotional processing in Parkinson disease. Neurology 65(5):707–13. [DOI] [PubMed] [Google Scholar]
- Lambert C, Zrinzo L, Nagy Z, Lutti A, Hariz M, Foltynie T, and others 2012. Confirmation of functional zones within the human subthalamic nucleus: patterns of connectivity and sub-parcellation using diffusion weighted imaging. Neuroimage 60(1):83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardeux S, Paleressompoulle D, Pernaud R, Cador M, Baunez C. 2013. Different populations of subthalamic neurons encode cocaine vs. sucrose reward and predict future error. J Neurophysiol 110(7):1497–510. [DOI] [PubMed] [Google Scholar]
- Lardeux S, Pernaud R, Paleressompoulle D, Baunez C. 2009. Beyond the reward pathway: coding reward magnitude and error in the rat subthalamic nucleus. J Neurophysiol 102(4):2526–37. [DOI] [PubMed] [Google Scholar]
- Levesque JC, Parent A. 2005. GABAergic intemeurons in human subthalamic nucleus. Mov Disord 20(5):574–84. [DOI] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. 2000. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci 20(20):7766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, and others1995. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 345(8942):91–5. [DOI] [PubMed] [Google Scholar]
- Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, and others 2008. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med 359(20):2121–34. [DOI] [PubMed] [Google Scholar]
- Marceglia S, Fumagalli M, Priori A. 2011. What neurophysiological recordings tell us about cognitive and behavioral functions of the human subthalamic nucleus. Expert Rev Neurother 11(1):139–49. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P. 2005. Subcortical loops through the basal ganglia. Trends Neurosci 28(8):401–7. [DOI] [PubMed] [Google Scholar]
- Mink JW.1996. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50(4):381–425. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Buzsaki G. 2014. Theta oscillations decrease spike synchrony in the hippocampus and entorhinal cortex. Philos Trans R Soc Lond B Biol Sci 369(1635): 20120530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Bergman H, Israel Z, Bar-Gad I. 2008. Subthalamic nucleus functional organization revealed by parkinsonian neuronal oscillations and synchrony. Brain 131 (Pt 12):3395–409. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tachibana Y, Chiken S. 2015. Cause of parkinsonian symptoms: firing rate, firing pattern or dynamic activity changes? Basal Ganglia 5(1):1–6. [Google Scholar]
- Nambu A, Tokuno H, Takada M. 2002. Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci Res 43(2):111–7. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Recce ML. 1993. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3(3):317–30. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Frank MJ. 2006. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput 18(2):283–328. [DOI] [PubMed] [Google Scholar]
- Pasquereau B, Turner RS. 2017. A selective role for ventromedial subthalamic nucleus in inhibitory control. Elife 6. doi: 10.7554/eLife.31627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JM, Hickey PT, Lad SP, Platt ML, Turner DA. 2017. Local fields in human subthalamic nucleus track the lead-up to impulsive choices. Front Neurosci 11:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienaar IS, Vernon A, Winn P. 2016. The cellular diversity of the pedunculopontine nucleus: relevance to behavior in health and aspects of Parkinson’s disease. Neuroscientist. Epub 2016. doi: 10.1177/1073858416682471. [DOI] [PubMed] [Google Scholar]
- Potter-Nerger M, Reese R, Steigerwald F, Heiden JA, Herzog J, Moll CKE, and others 2017. Movement-related activity of human subthalamic neurons during a reach-to-grasp task. Front Hum Neurosci 11:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R 1978. Theory of memory retrieval. Psychol Rev 85(2):59–108. [Google Scholar]
- Rigotti M, Barak O, Warden MR, Wang XJ, Daw ND, Miller EK, and others 2013. The importance of mixed selectivity in complex cognitive tasks. Nature 497(7451):585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M, Fumagalli M, Giannicola G, Marceglia S, Lucchiari C, Servello D, and others 2013. Pathological gambling in Parkinson’s disease: subthalamic oscillations during economics decisions. Mov Disord 28(12):1644–52. [DOI] [PubMed] [Google Scholar]
- Rossi PJ, Gunduz A, Okun MS. 2015. The subthalamic nucleus, limbic function, and impulse control. Neuropsychol Rev 25(4):398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi PJ, Peden C, Castellanos O, Foote KD, Gunduz A, Okun MS. 2017. The human subthalamic nucleus and globus pallidus internus differentially encode reward during action control. Hum Brain Mapp 38(4):1952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Parent M, Levesque M, Parent A. 2000. Axonal branching pattern of neurons of the subthalamic nucleus in primates. J Comp Neurol 424(1):142–52. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. 2013. Canceling actions involves a race between basal ganglia pathways. Nat Neurosci 16(8):1118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieger T, Serranova T, Ruzicka F, Vostatek P, Wild J, Stastna D, and others 2015. Distinct populations of neurons respond to emotional valence and arousal in the human subthalamic nucleus. Proc Natl Acad Sci U S A 112(10):3116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Nishihama MS, Nishihama CC, Hattori T. 1988. Two separate neuronal populations of the rat subthalamic nucleus project to the basal ganglia and pedunculopontine tegmental region. Brain Res 442(1):72–80. [DOI] [PubMed] [Google Scholar]
- van der Meer MA, Redish AD. 2011. Theta phase precession in rat ventral striatum links place and reward information. J Neurosci 31(8):2843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Dalley JW. 2016. Translatable and back-translatable measurement of impulsivity and compulsivity: convergent and divergent processes. Curr Top Behav Neurosci 28:5391. [DOI] [PubMed] [Google Scholar]
- Voon V, Droux F, Morris L, Chabardes S, Bougerol T, David O, and others 2017. Decisional impulsivity and the associative-limbic subthalamic nucleus in obsessive-compulsive disorder: stimulation and connectivity. Brain 140(2):442–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Burn DJ. 2011. Parkinson’s disease: the quintessential neuropsychiatric disorder. Mov Disord 26(6):1022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub DB, Zaghloul KA. 2013. The role ofthe subthalamic nucleus in cognition. Rev Neurosci 24(2):125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter ML, Burbaud P, Fernandez-Vidal S, Bardinet E, Coste J, Piallat B, and others 2011. Basal ganglia dysfunction in OCD: subthalamic neuronal activity correlates with symptoms severity and predicts high-frequency stimulation efficacy. Transl Psychiatry 1:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Ghahremani A, Udupa K, Saha U, Kalia SK, Hodaie M, and others 2016. Stop-related subthalamic beta activity indexes global motor suppression in Parkinson’s disease. Mov Disord 31(12):1846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Baunez C, Theobald DE, Robbins TW. 2005. Lesions to the subthalamic nucleus decrease impulsive choice but impair autoshaping in rats: the importance of the basal ganglia in Pavlovian conditioning and impulse control. Eur J Neurosci 21(11):3107–16. [DOI] [PubMed] [Google Scholar]
- Xiao C, Miwa JM, Henderson BJ, Wang Y, Deshpande P, McKinney SL, and others 2015. Nicotinic receptor subtype-selective circuit patterns in the subthalamic nucleus. J Neurosci 35(9):3734–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AI, Vanegas N, Lungu C, Zaghloul KA. 2014. Beta-coupled high-frequency activity and beta-locked neuronal spiking in the subthalamic nucleus of Parkinson’s disease. J Neurosci 34(38):12816–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul KA, Weidemann CT, Lega BC, Jaggi JL, Baltuch GH, Kahana MJ. 2012. Neuronal activity in the human subthalamic nucleus encodes decision conflict during action selection. J Neurosci 32(7):2453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala B, Damera S, Dong JW, Lungu C, Brown P, Zaghloul KA. 2017a. Human subthalamic nucleus theta and beta oscillations entrain neuronal firing during sensorimotor conflict. Cereb Cortex 27(1):496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala B, Tan H, Little S, Ashkan K, Green AL, Aziz T, and others 2016. Decisions made with less evidence involve higher levels of corticosubthalamic nucleus theta band synchrony. J Cogn Neurosci 28(6):811–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala B, Zaghloul K, Brown P. 2015. The subthalamic nucleus, oscillations, and conflict. Mov Disord 30(3):328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala BA, Jang AI, Zaghloul KA. 2017b. Human subthalamic nucleus activity during non-motor decision making. Elife 6. doi: 10.7554/eLife.31007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala BA, Tan H, Little S, Ashkan K, Hariz M, Foltynie T, and others 2014. Midline frontal cortex low-frequency activity drives subthalamic nucleus oscillations during conflict. J Neurosci 34(21):7322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenon A, Duclos Y, Carron R, Witjas T, Baunez C, Regis J, and others 2016. The human subthalamic nucleus encodes the subjective value of reward and the cost of effort during decision-making. Brain 139(Pt 6):1830–43. [DOI] [PMC free article] [PubMed] [Google Scholar]


