Abstract
Rationale: Current practice guidelines recommend pulmonary rehabilitation as an adjunct to standard pharmacologic therapy for individuals with moderate to severe chronic obstructive pulmonary disease (COPD). Whether pulmonary rehabilitation benefits all subjects with COPD independent of baseline disease burden is not known.
Objectives: To test whether pulmonary rehabilitation benefits patients with COPD independent of baseline exercise capacity, dyspnea, and lung function.
Methods: Data from a prospectively maintained database of participants with COPD enrolled in pulmonary rehabilitation at the University of Alabama at Birmingham from 1996 to 2013 were retrospectively analyzed. Subjects were divided into four quartiles based on their baseline level of dyspnea as assessed by the San Diego Shortness of Breath Questionnaire at the initial visit. Similar quartiles were assessed for FEV1 percent predicted as well as the 6-minute-walk distance (6MWD). The primary outcome was the change in quality of life as measured by the 36-item Short Form Health Survey (SF-36). Secondary outcomes were change in dyspnea, 6MWD, and depression scores assessed using the Beck Depression Inventory-II. Differences between baseline and final scores were compared using paired t tests and across quartiles using analysis of variance.
Measurements and Main Results: A total of 229 subjects were included. Their mean age was 66.5 (SD, 9) years. Ninety-one (40%) were female, and 42 (18%) were African American. The mean FEV1 percent predicted was 46.3% (20.0%). On completion of pulmonary rehabilitation, clinically significant improvements were seen in most components of SF-36: physical function, 11.5 (95% confidence interval [CI], 7.4–15.5; P < 0.001); health perception, 2.1 (95% CI, −0.7 to 4.8; P = 0.12); physical role, 16.7 (95% CI, 10.3–23.1; P < 0.001); emotional role, 14.7 (95% CI, 7.1–22.3; P < 0.001); social function, 16.4 (95% CI, 11.3–21.5; P < 0.001); mental health, 5.4 (95% CI, 2.6–8.3; P < 0.001); pain, 5 (95% CI, 1–9.1; P = 0.02); vitality, 12.4 (95% CI, 8.8–16.1; P < 0.001); and depression, 0.01 (95% CI, −0.11 to 0.07; P = 0.54). There was no difference in improvement in SF-36 across quartiles of San Diego Shortness of Breath Questionnaire, 6MWD, and FEV1 percent predicted.
Conclusions: Pulmonary rehabilitation results in significant improvement in quality of life, dyspnea, and functional capacity independent of baseline disease burden.
Keywords: pulmonary rehabilitation, chronic obstructive pulmonary disease, exercise capacity, dyspnea
Chronic obstructive pulmonary disease (COPD) is associated with substantial morbidity, resulting in significant direct and indirect costs (1). Patients with COPD frequently report dyspnea on exertion and fatigue (2), and have high rates of activity limitation (2), disability, and absenteeism from work (2). Recent studies suggest that the proportional indirect costs of COPD are as high as 27–61% of the total costs when working age patients are considered (2). Although pharmacologic therapy with long-acting inhalers is associated with improvement in dyspnea, this is associated with substantial costs (3). Pulmonary rehabilitation has been demonstrated to result in significant improvement in dyspnea, exercise capacity, psychological symptoms, and quality of life (4), and the cost per quality-adjusted life-year is substantially less than for pharmacologic therapy (3). Given the limited resources and infrastructure for pulmonary rehabilitation, predicting which patients will have substantial and meaningful improvement with pulmonary rehabilitation is important. Previous studies have not consistently identified predictors of improvement in clinical outcomes with pulmonary rehabilitation (5–8).
The current guidelines recommend that pulmonary rehabilitation should be considered for patients who have persistent symptoms and activity limitations, and for those who are unable to adjust to their illness despite optimal medical management (9). They also state that it is imperative that patients identified as having the potential to benefit from pulmonary rehabilitation be offered this therapy (10). Recent studies have shown that pulmonary rehabilitation benefits patients with COPD without regard to their level of baseline dyspnea (7, 8). However, dyspnea is strongly influenced by the level of activity, and patient-reported dyspnea scores can be impacted by the degree of exertion asserted by the patient. The influence of the level of baseline exercise capacity on the benefits of pulmonary rehabilitation has not been systematically studied. In addition, it is not known if the same duration of rehabilitation results in equal benefits in patients with relatively preserved exercise capacity compared with those with more severe disease, and a meta-analysis suggested that patients with more severe disease benefit from a longer duration of therapy (11).
The current recommendation is to provide at least 8 weeks of rehabilitation with two or three sessions per week (12). On the basis of data from our well-characterized prospective cohort of patients with COPD participating in pulmonary rehabilitation, we sought to determine whether overall disease burden, based on severity of lung function impairment, dyspnea, baseline quality of life, and exercise capacity, impacted benefits achieved by patients participating in pulmonary rehabilitation. We recently assessed the determinants of completion of pulmonary rehabilitation in this cohort (13), and in the present study, using data for completers only, we hypothesized that a standard duration of pulmonary rehabilitation benefits all subjects with COPD with improvement in quality of life, dyspnea, and exercise capacity, independent of baseline dyspnea, lung function, and exercise capacity.
Methods
Participants
We retrospectively analyzed data from a prospectively maintained database of subjects with COPD who enrolled in pulmonary rehabilitation at the University of Alabama at Birmingham between January 1996 and April 2013. Only those subjects with a primary diagnosis of COPD as identified by International Classification of Diseases codes 491, 492, and 496 at the time of enrollment, and who completed at least 20 sessions, were included in the present analyses. Subjects with other concurrent chronic respiratory diseases, including asthma, bronchiectasis, interstitial lung disease, and post–lung transplant, were excluded.
Information on baseline characteristics, including data on smoking history, oxygen use, and the presence of comorbidities, was obtained prior to beginning the pulmonary rehabilitation program. All participants provided written informed consent to allow inclusion of their demographic, clinical, and outcome data in the pulmonary rehabilitation database for future research, and the study was approved by the University of Alabama at Birmingham Institutional Review Board (assurance number FWA00005960).
Intervention
On the basis of disease severity, usual physical activity as reported by participants, baseline 6-minute-walk distance (6MWD) at enrollment, and comorbidities, each participant was prescribed an exercise plan by clinical exercise physiologists according to standard pulmonary rehabilitation guidelines (9). Participants underwent 2 or 3 sessions per week with a maximum of 36 sessions over 12 weeks. Each exercise session included aerobic exercises, such as treadmill walking and cycle and arm ergometry; resistance training, including machine weights, hand weights, and elastic bands; and breathing techniques, such as pursed-lips breathing and diaphragmatic breathing.
Our protocol was to prescribe cardiovascular exercise in the range of 20–30 minutes of continuous or interval bouts and to gradually increase duration until 30–45 minutes was achieved, usually by 4–8 weeks. The intensity of exercises was based on the estimated metabolic equivalent of task (MET), Rating of Perceived Exertion scale (range, 6–20; desired range, 11–15), Rating of Perceived Dyspnea (range, 0–10; desired range, 0–6), oxygen saturation as measured by pulse oximetry greater than 90%, as well as on the heart rate reserve as calculated by the Karvonen calculation (maximal heart rate in 6-min walk test minus resting heart rate), aiming for 40–80% of this heart rate reserve during exercise (7). Intensity was increased by up to 0.5 METs per week, based on the above-described parameters. Additional stretching and balance exercises were included. Participants also received education sessions lasting 45–60 minutes on understanding their disease, smoking cessation counseling, appropriate use of inhalers, diet and nutrition, as well as on stress management.
Outcomes
To assess changes in outcomes, we administered questionnaires to each participant at enrollment, and these questionnaires were readministered after completion of the program. We used pulmonary function data from tests performed within 2 years of enrollment. The primary outcome was change in quality of life and perception of health status, measured using the 36-item Short Form Health Survey (SF-36). A change of 5 units was considered the minimum clinically important difference for SF-36 (14, 15). Secondary outcomes were changes in dyspnea, functional capacity, and depression. We assessed dyspnea using the San Diego Shortness of Breath Questionnaire (SOBQ) (16). The SOBQ consists of 24 questions and rates dyspnea associated with activities of daily living, with higher scores indicating more severe dyspnea. We considered a change of 5 units in the SOBQ score as the minimum clinically important difference (17). Depression was assessed using the Beck Depression Inventory (BDI)-II, which consists of 21 questions (18). A higher score indicates worse depression, with a minimum clinically important difference of 5 (18). We assessed functional capacity using the 6MWD as per the American Thoracic Society guidelines; a change of 26 meters was considered the minimum clinically important difference (19, 20).
Statistical Analyses
Changes in outcome measures from baseline to completion of pulmonary rehabilitation were compared using paired t tests. We then divided the cohort into quartiles based on baseline SOBQ, 6MWD, and FEV1 percent predicted. For all three variables used to stratify patients, cutoffs were made at the 25th, 50th, and 75th percentiles of decreasing disease burden as measured by 6MWD, SOBQ score, and FEV1 percent predicted. Changes in outcomes were compared across these quartiles using analysis of variance. Between-quartiles differences were assessed using the post hoc Tukey test. All tests of significance were two-tailed, with statistical significance deemed to be at an α-level of less than 0.05. All analyses were performed using IBM SPSS version 22.0 software (IBM, Armonk, NY).
Results
A total of 229 participants who completed at least 20 sessions were included in the analyses. The mean age of the cohort was 66.5 (SD, 9) years. Ninety-one (40%) were female, and 42 (18%) were African American. The mean FEV1 percent predicted was 46.3% (SD 20). On completion of pulmonary rehabilitation, clinically significant improvements were seen in most components of SF-36: physical function, 11.5 (95% confidence interval [CI], 7.4–15.5; P < 0.001); health perception, 2.1 (95% CI, −0.7 to 4.8; P = 0.12); physical role, 16.7 (95% CI, 10.3–23.1; P < 0.001); emotional role, 14.7 (95% CI, 7.1–22.3; P < 0.001); social function, 16.4 (95% CI, 11.3–21.5; P < 0.001); mental health, 5.4 (95% CI, 2.6–8.3; P < 0.001); pain, 5.0 (95% CI, 1–9.1; P = 0.02); vitality, 12.4 (95% CI, 8.8–16.1; P < 0.001); and depression, 0.01 (95% CI, −0.11 to 0.07; P = 0.54). Significant improvements were also seen in the following: 6MWD, 52.4 m (95% CI, 41.0–63.8; P < 0.001); SOBQ, −9.1 (95% CI, −12.0 to −6.1; P < 0.001); and BDI-II, −3.0 (95% CI, −4.2 to −1.8; P < 0.001).
Impact of Baseline Exercise Capacity on Outcomes
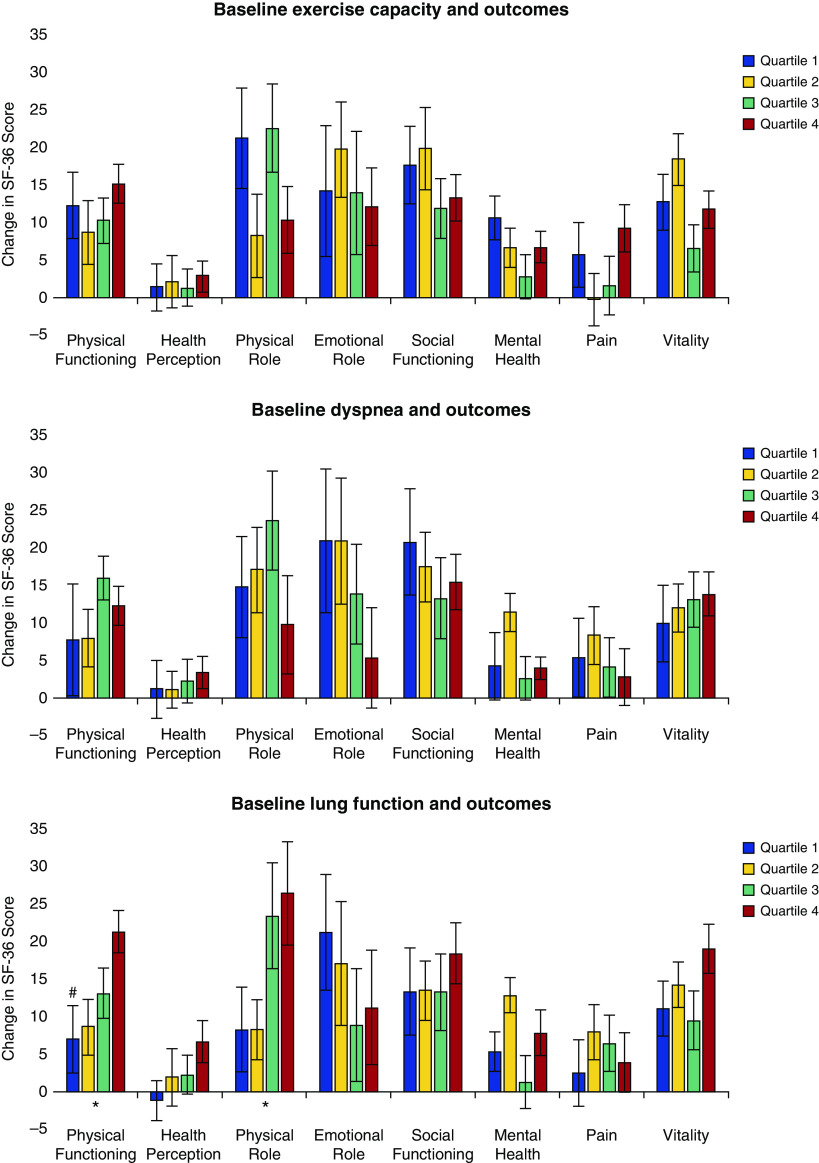
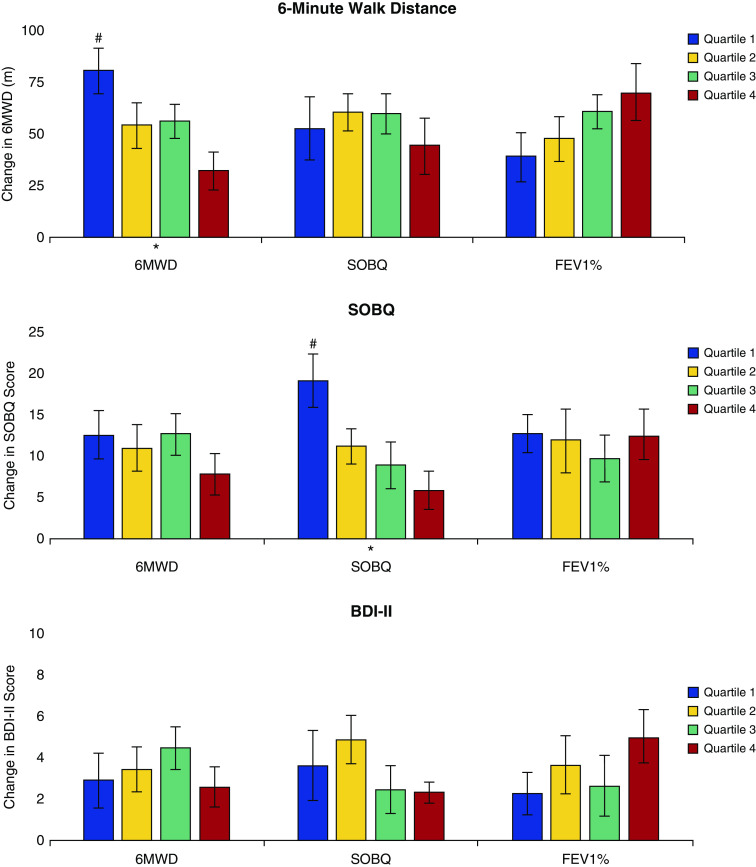
To assess outcomes based on the exercise capacity at enrollment, we divided the cohort into quartiles in increasing order of baseline 6MWD: quartile 1 (≤205 m), quartile 2 (205.1–284 m), quartile 3 (284.1–352 m), and quartile 4 (>352.1 m). Figure 1 and Table E1 in the online supplement show changes in outcomes across the quartiles. There was no difference in the change in any of the domains of the SF-36 (P = NS). There was also no difference in SOBQ (P = 0.298) and BDI-II (P = 0.979) across quartiles. Although the improvement in the 6MWD was greater in those with worse baseline exercise capacity, the changes in each quartile were greater than the minimum clinically important difference: 80.5 (95% CI, 58.5–102.5) m, 54.2 (95% CI, 32.5–76.0) m, 56.2 (95% CI, 39.8–72.6) m, and 32.3 (95% CI, 13.6–50.9) m, respectively (P = 0.001).
Figure 1.
Differences between baseline and final component scores of the 36-item Short Form Health Survey (SF-36) score across the four quartiles of baseline exercise capacity (6-min-walk distance), dyspnea (San Diego Shortness of Breath Questionnaire), and lung function (FEV1 percent predicted). *P < 0.05 for change over quartiles. #P < 0.05 for differences between individual quartiles compared with the reference quartile. The vertical bars represent mean values for each outcome. The error bars represent the SE. To report post hoc comparisons, we used quartile 4 with the best walk distance, dyspnea, and lung function as the reference quartile. SF-36 depression scores are not depicted in the figure, because the changes were minimal compared with other domains of SF-36.
Impact of Baseline Dyspnea on Outcomes
We also assessed outcomes based on baseline dyspnea by dividing the cohort into quartiles in decreasing order of SOBQ: quartile 1 (≥79), quartile 2 (63–78), quartile 3 (48–62), and quartile 4 (≤47). The changes in outcomes with pulmonary rehabilitation across the quartiles are shown in Figure 2 and Table E2. There was no difference between quartiles in the change in SF-36 (P = NS). The changes in 6MWD (P = 0.643) and BDI-II (P = 0.221) were also not different across quartiles. Participants with worse dyspnea at baseline experienced the greatest reduction in dyspnea; however, the mean change in each quartile was greater than the minimum clinically important difference: −5.9 (95% CI, −10.4 to −1.4), −8.9 (95% CI, −14.7 to −3.1), −11.1 (95% CI, −15.4 to −6.9), and −10.6 (95% CI, −13.3 to −8.0), respectively (P = 0.001).
Figure 2.
Differences in secondary outcomes across quartiles of exercise capacity (6-min-walk distance [6MWD]), dyspnea (San Diego Shortness of Breath Questionnaire [SOBQ]), and lung function (FEV1 percent predicted ). BDI-II = Beck Depression Inventory-II. *P < 0.05 for change over quartiles. #P < 0.05 for differences between individual quartiles compared with the reference quartile. The vertical bars represent mean values for each outcome. The error bars represent SE. To report post hoc comparisons, we used quartile 4 with the best walk distance, dyspnea, and lung function as the reference quartile.
Impact of Baseline Lung Function and Outcomes
To assess changes in outcomes based on baseline lung function, we divided the cohort into quartiles in increasing order of FEV1 percent predicted: quartile 1 (≤29), quartile 2 (29.1–40), quartile 3 (40.1–54), and quartile 4 (≥54.1). The changes in outcomes with pulmonary rehabilitation across the quartiles are shown in Figure 2 and Table E3. There was no difference between quartiles in the change in most of the domains of SF-36; there were significant differences only for physical functioning and physical role, as shown in Table E3. The change in SOBQ (P = 0.842) and BDI-II (P = 0.218) were also not different across the quartiles. Participants with worse lung function at baseline experienced the least improvement in 6MWD, although the changes in each quartile were greater than the minimum clinically important difference: 39.0 (95% CI, 15.3–62.6) m, 47.6 (95% CI, 26.1–69.2) m, 61.1 (95% CI, 45.5–76.7) m, and 69.9 (95% CI, 41.3–98.3) m, respectively (P = 0.035). There were also no differences across Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages in change in SOBQ (P = 0.957), 6MWD (P = 0.127), and BDI-II (P = 0.593). There were also no differences across GOLD stages for SF-36 domains, except for physical functioning (P = 0.014).
Discussion
We built on the results of the analyses of our well-characterized cohort of patients participating in pulmonary rehabilitation that aimed to detect predictors of completion of rehabilitation by demonstrating that participating in pulmonary rehabilitation results in significant improvement in metrics of respiratory disease independent of underlying disease severity. Although patients in the worst quartile for dyspnea and exercise capacity experienced the greatest benefit in that specific domain, all quartiles experienced improvements greater than the minimum clinically important difference.
Current guidelines and practice statements from two leading respiratory societies recommend pulmonary rehabilitation for patients with moderate to severe COPD and/or with persistent symptoms (9, 21). Although a combined statement from the leading respiratory societies recently made a strong recommendation for pulmonary rehabilitation for patients with FEV1 less than 50%, the statement made only a weak recommendation for those with better lung function (21). No specific lung function threshold indicates the need for pulmonary rehabilitation, as referral is more often dictated by symptoms and functional limitation.
Although recent insurance reimbursement policies have been changed to reflect these observations, this referral pattern strategy more often results in referral of patients with more long-standing and moderate to severe disease to pulmonary rehabilitation, thus depriving patients with milder disease of substantial preventative strategies, including smoking cessation as well as dietary and nutritional interventions. Current referral practices also potentially limit the range of exercises patients with more severe disease can participate in once exercise limitation has set in. In addition, a substantial number of patients with mild to moderate disease by lung function have unreported symptoms and exercise limitation (22, 23). We showed that baseline lung function did not influence the benefits of pulmonary rehabilitation, and this finding confirms those of previous smaller studies (8, 24–27). The mechanisms underlying this finding are likely multifactorial.
The correlation between lung function and respiratory morbidity in COPD, including dyspnea and exercise capacity, is modest. A number of patients with mild COPD have dynamic hyperinflation, and it is possible that these patients derive significant benefit from the breathing training techniques that are an essential part of pulmonary rehabilitation (28). In addition, mild COPD is also associated with skeletal muscle dysfunction, and this subset of patients might benefit from the cardiovascular training aspect of pulmonary rehabilitation (29).
We also found that baseline dyspnea and exercise capacity did not impact overall benefits of pulmonary rehabilitation. Evans and coworkers found that patients with COPD have substantial improvement in incremental shuttle walk distances following pulmonary rehabilitation, regardless of the baseline level of dyspnea (8). A randomized controlled trial of pulmonary rehabilitation in severe COPD stratified patients by level of dyspnea into moderate and severe groups and found that only those with moderate dyspnea achieved an improvement in exercise capacity (30). Those with severe dyspnea and who were home bound did not show improvements in either exercise capacity or quality of life. This perhaps reflects that these patients had reached a stage where they were significantly debilitated, limiting the range of exercises because the exercise plan was individualized in this study based on baseline functional capacity. In addition, exercise therapy was delivered at home. Indeed, in patients able to attend a hospital-based pulmonary rehabilitation program, we found that those with the lowest 6MWD achieved the greatest benefit in exercise capacity.
Our findings support those of a previous study by ZuWallack and colleagues, which showed that the initial 12-minute-walk distance was inversely related to the magnitude of improvement in walk distance (31). It is well recognized that patients limit their activities with worsening dyspnea, and hence the level of dyspnea reported is strongly linked with the level of activity. Progressive limitation of activity can result in deconditioning, exacerbating disease morbidity, and worsening outcomes (32). We add to the literature by comparing outcomes across quartiles of baseline exercise capacity and showing that patients benefit independent of underlying functional capacity. Perhaps early intervention with pulmonary rehabilitation in milder COPD can interrupt the cycle of symptoms, physical inactivity, deconditioning, and poor exercise capacity.
Strengths and Limitations
Our study has several strengths. Study participants were followed prospectively; a substantial proportion of the cohort was comprised of women and African Americans; and we used numerous validated questionnaires to assess baseline and change in function. Our study has some limitations. We assessed changes in outcomes in only those who participated in at least 20 sessions and completed questionnaires and performed post–pulmonary rehabilitation 6MWT, and hence our findings may not be generalizable to those who dropped out because of limitations caused by disease burden. Although we have previously shown that baseline levels of dyspnea, FEV1, and exercise capacity did not influence dropout rates, this could be a source of bias (13). Generalizability may also be limited by the single center included in the study, but our participants were from both urban and rural settings with relatively equal distributions of sex and race; hence, our findings represent data obtained from a varied patient population that closely approximates real-world settings.
Conclusions
Patients with COPD experience meaningful improvements in quality of life, dyspnea, exercise capacity, and depression, regardless of baseline lung function, dyspnea, and exercise capacity. Current guidelines should be amended to recommend pulmonary rehabilitation to all patients with COPD, regardless of their baseline level of disease burden.
Supplementary Material
Footnotes
Author Contributions: P.S.: study design and manuscript writing; J.H.: collected data and reviewed the manuscript for important intellectual content; C.S.: collected data and reviewed the manuscript for important intellectual content; J.M.W.: reviewed the manuscript for important intellectual content; M.T.D.: manuscript writing and review of the manuscript for important intellectual content; and S.P.B.: study design, data collection, data analysis, and manuscript writing, and also had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Blanchette CM, Dalal AA, Mapel D. Changes in COPD demographics and costs over 20 years. J Med Econ. 2012;15:1176–1182. doi: 10.3111/13696998.2012.713880. [DOI] [PubMed] [Google Scholar]
- 2.Patel JG, Nagar SP, Dalal AA. Indirect costs in chronic obstructive pulmonary disease: a review of the economic burden on employers and individuals in the United States. Int J Chron Obstruct Pulmon Dis. 2014;9:289–300. doi: 10.2147/COPD.S57157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths TL, Phillips CJ, Davies S, Burr ML, Campbell IA. Cost effectiveness of an outpatient multidisciplinary pulmonary rehabilitation programme. Thorax. 2001;56:779–784. doi: 10.1136/thorax.56.10.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793. doi: 10.1002/14651858.CD003793.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott AS, Baltzan MA, Fox J, Wolkove N. Success in pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Can Respir J. 2010;17:219–223. doi: 10.1155/2010/203236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rugbjerg M, Iepsen UW, Jørgensen KJ, Lange P. Effectiveness of pulmonary rehabilitation in COPD with mild symptoms: a systematic review with meta-analyses. Int J Chron Obstruct Pulmon Dis. 2015;10:791–801. doi: 10.2147/COPD.S78607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrod R, Marshall J, Barley E, Jones PW. Predictors of success and failure in pulmonary rehabilitation. Eur Respir J. 2006;27:788–794. doi: 10.1183/09031936.06.00130605. [DOI] [PubMed] [Google Scholar]
- 8.Evans RA, Singh SJ, Collier R, Williams JE, Morgan MD. Pulmonary rehabilitation is successful for COPD irrespective of MRC dyspnoea grade. Respir Med. 2009;103:1070–1075. doi: 10.1016/j.rmed.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, et al. ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 10.Rochester CL, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, Spruit MA, Masefield S, Casaburi R, Clini EM, et al. ATS/ERS Task Force on Policy in Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. 2015;192:1373–1386. doi: 10.1164/rccm.201510-1966ST. [DOI] [PubMed] [Google Scholar]
- 11.Salman GF, Mosier MC, Beasley BW, Calkins DR. Rehabilitation for patients with chronic obstructive pulmonary disease: meta-analysis of randomized controlled trials. J Gen Intern Med. 2003;18:213–221. doi: 10.1046/j.1525-1497.2003.20221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson-Warrington V, Harrison S, Mitchell K, Steiner M, Morgan M, Singh S. Exercise capacity and physical activity in patients with COPD and healthy subjects classified as Medical Research Council dyspnea scale grade 2. J Cardiopulm Rehabil Prev. 2014;34:150–154. doi: 10.1097/HCR.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 13.Brown AT, Hitchcock J, Schumann C, Wells JM, Dransfield MT, Bhatt SP. Determinants of successful completion of pulmonary rehabilitation in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:391–397. doi: 10.2147/COPD.S100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ware JE, Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 15.Mahler DA, Mackowiak JI. Evaluation of the short-form 36-item questionnaire to measure health-related quality of life in patients with COPD. Chest. 1995;107:1585–1589. doi: 10.1378/chest.107.6.1585. [DOI] [PubMed] [Google Scholar]
- 16.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. Chest. 1998;113:619–624. doi: 10.1378/chest.113.3.619. [DOI] [PubMed] [Google Scholar]
- 17.Kupferberg DH, Kaplan RM, Slymen DJ, Ries AL. Minimal clinically important difference for the UCSD Shortness of Breath Questionnaire. J Cardiopulm Rehabil. 2005;25:370–377. doi: 10.1097/00008483-200511000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Hiroe T, Kojima M, Yamamoto I, Nojima S, Kinoshita Y, Hashimoto N, Watanabe N, Maeda T, Furukawa TA. Gradations of clinical severity and sensitivity to change assessed with the Beck Depression Inventory-II in Japanese patients with depression. Psychiatry Res. 2005;135:229–235. doi: 10.1016/j.psychres.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 19.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 20.Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, Hansel NN, Wise RA, Sciurba F National Emphysema Treatment Trial (NETT) Research Group. The minimal important difference of exercise tests in severe COPD. Eur Respir J. 2011;37:784–790. doi: 10.1183/09031936.00063810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schünemann H, Wedzicha W, et al. American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 22.Soumagne T, Laveneziana P, Veil-Picard M, Guillien A, Claudé F, Puyraveau M, Annesi-Maesano I, Roche N, Dalphin JC, Degano B. Asymptomatic subjects with airway obstruction have significant impairment at exercise. Thorax. 2016;71:804–811. doi: 10.1136/thoraxjnl-2015-207953. [DOI] [PubMed] [Google Scholar]
- 23.Chin RC, Guenette JA, Cheng S, Raghavan N, Amornputtisathaporn N, Cortés-Télles A, Webb KA, O’Donnell DE. Does the respiratory system limit exercise in mild chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2013;187:1315–1323. doi: 10.1164/rccm.201211-1970OC. [DOI] [PubMed] [Google Scholar]
- 24.Berry MJ, Rejeski WJ, Adair NE, Zaccaro D. Exercise rehabilitation and chronic obstructive pulmonary disease stage. Am J Respir Crit Care Med. 1999;160:1248–1253. doi: 10.1164/ajrccm.160.4.9901014. [DOI] [PubMed] [Google Scholar]
- 25.Niederman MS, Clemente PH, Fein AM, Feinsilver SH, Robinson DA, Ilowite JS, Bernstein MG. Benefits of a multidisciplinary pulmonary rehabilitation program: improvements are independent of lung function. Chest. 1991;99:798–804. doi: 10.1378/chest.99.4.798. [DOI] [PubMed] [Google Scholar]
- 26.Jácome C, Marques A. Impact of pulmonary rehabilitation in subjects with mild COPD. Respir Care. 2014;59:1577–1582. doi: 10.4187/respcare.03091. [DOI] [PubMed] [Google Scholar]
- 27.Jácome C, Marques A. Pulmonary rehabilitation for mild COPD: a systematic review. Respir Care. 2014;59:588–594. doi: 10.4187/respcare.02742. [DOI] [PubMed] [Google Scholar]
- 28.Casaburi R, Porszasz J. Reduction of hyperinflation by pharmacologic and other interventions. Proc Am Thorac Soc. 2006;3:185–189. doi: 10.1513/pats.200508-095DO. [DOI] [PubMed] [Google Scholar]
- 29.Seymour JM, Spruit MA, Hopkinson NS, Natanek SA, Man WD, Jackson A, Gosker HR, Schols AM, Moxham J, Polkey MI, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36:81–88. doi: 10.1183/09031936.00104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wedzicha JA, Bestall JC, Garrod R, Garnham R, Paul EA, Jones PW. Randomized controlled trial of pulmonary rehabilitation in severe chronic obstructive pulmonary disease patients, stratified with the MRC dyspnoea scale. Eur Respir J. 1998;12:363–369. doi: 10.1183/09031936.98.12020363. [DOI] [PubMed] [Google Scholar]
- 31.ZuWallack RL, Patel K, Reardon JZ, Clark BA, III, Normandin EA. Predictors of improvement in the 12-minute walking distance following a six-week outpatient pulmonary rehabilitation program. Chest. 1991;99:805–808. doi: 10.1378/chest.99.4.805. [DOI] [PubMed] [Google Scholar]
- 32.Waschki B, Kirsten AM, Holz O, Mueller KC, Schaper M, Sack AL, Meyer T, Rabe KF, Magnussen H, Watz H. Disease progression and changes in physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:295–306. doi: 10.1164/rccm.201501-0081OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.