Abstract
Background
Amnioinfusion is thought to dilute meconium present in the amniotic fluid and so reduce the risk of meconium aspiration.
Objectives
To assess the effects of amnioinfusion for meconium‐stained liquor on perinatal outcome.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (1 December 2013).
Selection criteria
Randomised trials comparing amnioinfusion with no amnioinfusion for women in labour with moderate or thick meconium staining of the amniotic fluid.
Data collection and analysis
Three review authors independently assessed eligibility and trial quality, and extracted data.
Main results
Fourteen studies of variable quality (4435 women) are included.
Subgroup analysis was performed for studies from settings with limited facilities to monitor the baby's condition during labour and intervene effectively, and settings with standard peripartum surveillance.
Settings with standard peripartum surveillance: there was considerable heterogeneity for several outcomes. There was no significant reduction in the primary outcomes meconium aspiration syndrome, perinatal death or severe morbidity, and maternal death or severe morbidity. There was a reduction in caesarean sections (CSs) for fetal distress but not overall. Meconium below the vocal cords diagnosed by laryngoscopy was reduced, as was neonatal ventilation or neonatal intensive care unit admission, but there was no significant reduction in perinatal deaths or other morbidity. Planned sensitivity analysis excluding trials with greater risk of bias resulted in an absence of benefits for any of the outcomes studied.
Settings with limited peripartum surveillance: three studies were included. In the amnioinfusion group there was a reduction in CS for fetal distress and overall; meconium aspiration syndrome (three studies, 1144 women; risk ratio (RR) 0.17, 95% confidence interval (CI) 0.05 to 0.52); perinatal mortality (three studies, 1151 women; RR 0.24, 95% CI 0.11 to 0.53) and neonatal ventilation or neonatal intensive care unit admission. In one of the studies, meconium below the vocal cords was reduced and, in the other, neonatal encephalopathy was reduced.
Authors' conclusions
Amnioinfusion is associated with substantive improvements in perinatal outcome only in settings where facilities for perinatal surveillance are limited. It is not clear whether the benefits are due to dilution of meconium or relief of oligohydramnios.
In settings with standard peripartum surveillance, some non‐substantive outcomes were improved in the initial analysis, but sensitivity analysis excluding trials with greater risk of bias eliminated these differences. Amnioinfusion is either ineffective in this setting, or its effects are masked by other strategies to optimise neonatal outcome.
The trials reviewed are too small to address the possibility of rare but serious maternal adverse effects of amnioinfusion.
Plain language summary
Amnioinfusion for meconium‐stained liquor in labour
Amnioinfusion is not beneficial for babies releasing medium to heavy meconium during labour, except in settings with limited facilities to monitor the baby's condition during labour.
A bowel movement (meconium) from the unborn baby during labour can enter the baby's lungs, causing breathing difficulties after birth. Extra liquid can be injected through the woman's vagina or abdomen into the womb (amnioinfusion) to provide more liquid to dilute the meconium and surround the baby. The review of 14 trials (4435 women) found that amnioinfusion with a salt (saline) solution is beneficial for babies only in settings in which babies are at high risk due to limited monitoring facilities. Further research into the effects on women is needed.
Background
Amnioinfusion has been used to improve neonatal outcomes in cases complicated by meconium aspiration (Sadovsky 1989; Wenstrom 1989). Meconium aspiration may occur before birth, or during the birth process, and is associated with significant mortality. Airways suctioning of the neonate has been suggested to reduce, but does not eliminate, the occurrence of meconium aspiration (Davis 1985). Strategies have therefore, been sought to reduce fetal meconium aspiration before birth.
The presence of thick meconium staining of the amniotic fluid is an indication of oligohydramnios, as meconium passed into a normal volume of amniotic fluid will usually appear thin. Amnioinfusion may thus at the same time dilute the meconium and correct oligohydramnios, relieving umbilical cord compression. It is difficult to distinguish effects of amnioinfusion due to these two mechanisms.
Readers are referred to other reviews of the subject (Gelfand 2004; Gramellini 2003; Hofmeyr 1996; Hofmeyr 2000; Lameier 1993; Pierce 2000; Velaphi 2006; Xu 2007), and to related Cochrane reviews (Hofmeyr 2012; Novikova 2012).
Description of the condition
Meconium aspiration refers to fetal aspiration of meconium‐stained amniotic fluid (MSAF) during the antepartum or intrapartum period. Meconium aspiration syndrome (MAS) refers to newborn respiratory distress secondary to the presence of meconium in the tracheobronchial airways. Passage of fetal meconium before birth occurs in 8% to 20% of pregnancies (Nathan 1994; Woods 1994). It occurs mainly in term and post‐term pregnancies. It may be associated with fetal compromise, but is also common in uncompromised labours. Possible causes that have been proposed include fetal hypoxia, a normal physiological function of the mature gastro‐intestinal tract, vagally‐mediated peristalsis in response to cord compression, and a direct effect on the fetal gastro‐intestinal tract of maternal medication such as misoprostol (Hofmeyr 2003), castor oil and certain herbal remedies (Mitri 1987). Thick, but not thin meconium staining of the amniotic fluid is associated with poor perinatal outcome (Mahomed 1994; Ziadeh 2000). The association between severe meconium aspiration syndrome and aspiration of meconium has been challenged (Ghidini 2001).
Amnioinfusion has been described as a method of preventing or relieving umbilical cord compression during labour (Miyazaki 1983), eliciting fetal heart rate accelerations during labour (Wax 2004), or of diluting meconium in the amniotic fluid to try to reduce the risk of meconium aspiration. Saline or Ringer's lactate is infused transcervically through a catheter into the uterine cavity, or transabdominally through a 'spinal' needle when membranes are intact. Amnioinfusion has also been used transabdominally for antepartum indications (Gramellini 2003b) and to facilitate external cephalic version at term (Benifla 1995).
Description of the intervention
The technique for amnioinfusion has been clearly described (Weismiller 1998). Saline or Ringer's lactate is usually infused through a purpose‐designed intrauterine pressure catheter using an infusion pump. However, studies from low‐income countries where such catheters are unaffordable have demonstrated that amnioinfusion can be successfully achieved using inexpensive infant feeding tubes or suction catheters, and gravity infusion (Abdel‐Aleem 2005; Ashfaq 2004; Mahomed 1998; Moodley 1998). A study in which women with meconium‐stained amniotic fluid were randomly allocated to receive amnioinfusion with saline or lactated Ringer's solution found no effect on neonatal plasma concentrations or pH, compared with a non‐randomised normal control group (Gonzalez 2002). While randomised trials have not found increased risks associated with amnioinfusion, isolated case reports have documented complications following amnioinfusion (Dorairajan 2005). It is difficult to know whether there was a causal relationship in such reports. Amnioinfusion in women with previous caesarean section has not been found to carry risks of uterine rupture, though larger studies are needed (Hicks 2005).
How the intervention might work
Amnioinfusion has been used to improve neonatal outcomes in cases complicated by meconium aspiration (Sadovsky 1989; Wenstrom 1989). Amnioinfusion has also proven effective for reducing overall complication rates in meconium aspiration syndrome. The success of amnioinfusion for prevention of meconium aspiration syndrome may be secondary to a dilutional effect. The amnioinfusion dilutes the meconium thereby reducing complications following meconium aspiration. Other investigators have shown a significant reduction in meconium concentration when amnioinfusion is used (Ashfaq 2004; Weismiller 1998).
Why it is important to do this review
Amnioinfusion has been shown to be beneficial in reducing the complications following meconium‐stained amniotic fluid in several randomised clinical trials. However, there has been no systematic review to address this topic, hence this review.
In several perinatal interventions, differences in effectiveness have been reported in studies conducted in low‐income compared with high‐income settings. Possible explanations include less stringent methodology in under‐resourced settings; or the fact that in well‐resourced settings outcomes are close to optimal, leaving little scope for further improvement with additional interventions. The latter concept has been discussed recently with respect to the potential benefits of early pregnancy ultrasound (Hofmeyr 2009). For this reason we have undertaken subgroup analyses for settings with limited and with standard peripartum surveillance.
Objectives
To assess the effects of amnioinfusion for meconium‐stained liquor in labour on maternal and perinatal morbidity and mortality.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing the effect of amnioinfusion for meconium‐stained liquor on clinically meaningful outcomes, with a control group (no or sham amnioinfusion). Quasi‐randomised (alternate allocation) were considered eligible, but cross‐over and cluster‐randomised trials were not considered.
Types of participants
Women in labour with moderate or thick meconium staining of the amniotic fluid.
Types of interventions
Amnioinfusion (the infusion of physiological saline or lactated Ringer's solution into the amniotic cavity) compared with no or sham amnioinfusion.
Types of outcome measures
Method of delivery, neonatal outcome and maternal complications. Two additional composite outcomes were added post hoc to accommodate data from Fraser 2005: perinatal death or severe morbidity; and maternal death or severe morbidity.
Primary outcomes
1. Meconium aspiration syndrome 2. Perinatal death or serious morbidity (post hoc) 3. Maternal death or serious morbidity (post hoc)
Secondary outcomes
4. Heavy meconium staining of amniotic fluid 5. Variable fetal heart rate decelerations 6. Caesarean section for fetal distress 7. Caesarean section overall 8. One‐minute Apgar score less than four 9. One‐minute Apgar score less than seven 10. Five‐minute Apgar score less than seven 11. Umbilical arterial blood pH less than 7.20 12. Meconium below the vocal cords 13. Neonatal ventilation/neonatal intensive care unit admission 14. Neonatal encephalopathy 15. Perinatal death 16. Puerperal pyrexia (as defined by trial authors) 17. Puerperal endometritis 18. Oxytocin augmentation of labour 19. Narcotic analgesia 20. Epidural analgesia 21. First stage of labour (minutes) 22. Instrumental vaginal delivery for fetal distress 23. Instrumental vaginal delivery overall 24. Second stage of labour (minutes) 25. Respiratory distress syndrome (post hoc) 26. Mean maternal hospital stay (post hoc)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (1 December 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, see Hofmeyr 2010.
For this update we used the following methods when assessing the report identified by the updated search ‐ Choudhary 2010.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion with the third review author. We created a study flow diagram to map out the number of records identified, included and excluded.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted data using the agreed form. We resolved discrepancies through discussion or, when required, we consulted the third review author. We entered data into Review Manager software (RevMan 2012) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact the authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by involving the third author.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the methods as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study all the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We also provided information on whether the intended blinding was effective. Where blinding was not possible, we assessed whether the lack of blinding was likely to have introduced bias. We assessed blinding separately for different outcomes or classes of outcomes. We assessed the methods as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how the possibility of selective outcome reporting bias was examined by us and what we found.
We assessed methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at a high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned to use the mean difference for outcomes measured in the same way between trials and the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Only randomised clinical trials were included in this review. We did not include cluster‐randomised trials or cross‐over trials.
Dealing with missing data
For included studies, levels of attrition were noted. The impact of including studies with high levels of missing data in the overall assessment of treatment effect was explored by using sensitivity analysis. For all outcomes analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau, I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (< 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Where we suspected reporting bias (see ’Selective reporting bias’ above), we attempted to contact study authors to ask them to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, the impact of including such studies in the overall assessment of results was explored by a sensitivity analysis.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses.
Standard peripartum surveillance.
Limited peripartum surveillance.
All the outcomes were used in the subgroup analysis.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2012). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
For significant heterogeneity within subgroups, we used a random‐effects model and explored the heterogeneity by planned sensitivity analysis excluding trials with greater risk of bias, as well as a post hoc sensitivity analysis based on sample size. In view of considerable heterogeneity and differences in adverse events rates between the two subgroups, overall meta‐analyses were not reported for outcomes with significant heterogeneity.
Sensitivity analysis
We conducted a sensitivity analysis by excluding trials with greater risk of bias and examining the effect on the outcomes. A post‐hoc sensitivity analysis was also performed to assess the effect of trial size on heterogeneity
Results
Description of studies
Of 30 studies identified, 14 studies with data on 4435 women met the predefined criteria for inclusion for this update ‐ seeCharacteristics of included studies.
The rate of saline infusion varied between studies. Sadovsky 1989 infused 600 mL in one hour, then 180 mL per hour continuously. Adam 1989 used a single infusion of 1000 mL. Wenstrom 1989 infused 1000 mL over 20 to 40 minutes, repeated six‐hourly. Macri 1992 infused 500 mL initially, then 250 to 500 mL as required to maintain a four‐quadrant amniotic fluid index above 10 cm. Ilagan 1992 infused 500 mL saline. Cialone 1994 infused 600 mL over one hour followed by 150 mL per hour. Eriksen 1994 infused 800 mL over one hour, then 180 mL per hour. Spong 1994 infused 600 mL as a bolus, followed by 3 mL per minute. Moodley 1998 used amnioinfusion with normal saline 10 to 15 mL per minute by gravity via a central venous manometer set and a size eight nasogastric infant feeding tube (1 litre over four hours). Hofmeyr 1998a infused saline 800 mL at 15 mL per minute, them 3 mL per minute maintenance infusion. Mahomed 1998 infused saline 500 mL over 30 minutes then 500 mL at 30 drops per minute. Puertas 2001 used saline via an infusion pump at 600 mL per hour for one hour, then 180 mL per hour until full cervical dilation, or basal uterine pressure increase to 20 mmHg. Rathore 2002 used saline via an FG 8 nasogastric tube, 500 mL over 30 minutes, then 3 mL per minute, to a maximum dose of 1000 mL. Sood 2004 infused saline through a plastic suction catheter (50 cases) or an amnioinfusion catheter (46 cases) until the returning fluid was clear. In most women 1000 mL was infused over 30 to 45 minutes. Fraser 2005 used 800 mL saline over 40 minutes, then at 3 mL per minute to a maximum of 1500 mL. Choudhary 2010 passed transcervical nasogastric tubes cephalad to the baby’s head with infusion of 500 cc’s of normal saline over a period of 30 minutes, followed by 2 mL/min under gravity till time of delivery.
Results of the search
Thirty studies were identified after the search. Of the 30 studies, 14 studies with data on 4435 women met the predefined criteria for inclusion.
Included studies
Fourteen studies were included (Choudhary 2010; Cialone 1994; Eriksen 1994; Fraser 2005; Hofmeyr 1998a; Macri 1992; Mahomed 1998; Moodley 1998; Puertas 2001; Rathore 2002; Sadovsky 1989; Sood 2004; Spong 1994;Wenstrom 1989).
Excluded studies
Sixteen studies were excluded (Adam 1989; Ashfaq 2004; Das 2007; Edwards 1999; Engel 2008; Gonzalez 1998; Gonzalez 2002; Gupta 2011; Ilagan 1992; Khosla 1997; Kirubamani 2000; Lembet 1999; Lo 1993; Mukhopadhyay 2006; Nageotte 1991; Regi 2009).
Risk of bias in included studies
SeeCharacteristics of included studies, particularly the 'Methods' and 'Notes' sections and Figure 1 and Figure 2 for summaries of 'Risk of bias' assessments.
1.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Choudhary 2010, Moodley 1998, Puertas 2001 and Sadovsky 1989 randomly assigned women to two groups according to a list of randomly generated numbers in a sealed envelope assigned to each woman. However, they did not specify how participants were 'randomly' assigned to these groups (e.g. computer‐generated, shuffling envelopes). Cialone 1994 and Spong 1994 used computer‐generated random numbers methods for sequence generation, but they did not describe methods of allocation concealment. Eriksen 1994, Hofmeyr 1998a, Macri 1992, Mahomed 1998, Rathore 2002 and Wenstrom 1989 used computer randomisation and prevented selection bias due to allocation concealment by using sealed envelopes. Fraser 2005 used a centralised computer randomisation service, while Sood 2004 assigned women to each group by a coin toss.
Blinding
Blinding of the intervention was not possible, though some of the outcome measures could be assessed in a blinded fashion. In none of the studies were obstetricians blinded to the treatment, and only Choudhary 2010; Cialone 1994; Fraser 2005; Hofmeyr 1998a; Macri 1992; Mahomed 1998; Puertas 2001; Sadovsky 1989 and Wenstrom 1989 specified that the neonatologists evaluating the babies were blind to the assigned group of each baby.
Incomplete outcome data
Comparability of the groups was compromised by the following exclusions after randomisation: Wenstrom 1989 excluded 5/41 (12%) of women allocated to receive amnioinfusion but who did not (four gave birth spontaneously after 30, 45, 60 and 180 minutes, and one required emergency caesarean section for fetal distress ‐ this information has been included in the analysis for this review in respect of delivery outcomes); Cialone 1994 excluded 7/54 (13%) of the study group who had diabetes (three) or requested withdrawal (four) and 1/59 (1.7%) allocated to the control group who requested withdrawal; Eriksen 1994 excluded 9/139 (6%) women whose records were unavailable or incomplete, and 6/71 (8%) randomised to the amnioinfusion group who delivered before they could receive amnioinfusion. In the study by Hofmeyr et al (Hofmeyr 1998a), one woman in the control group received amnioinfusion (analysis was by intention‐to‐treat), and data were missing on about 7% of outcome measures. In Mahomed 1998, four amnioinfusion allocations were missed and could not be traced. Sood 2004 excluded 3/99 women from the amnioinfusion group in whom amnioinfusion was abandoned because of vaginal bleeding. Fetal heart rate abnormalities before enrolment were present in 48/96 women in the amnioinfusion versus 60/100 in the control group. The perinatal mortality data are difficult to interpret because the reported rate (2.8%) in the amnioinfusion group does not reflect a whole number of the group of 96. In Fraser 2005, 1.2% of women were excluded from the analysis because of loss to follow‐up (amnioinfusion 7/995; control 12/1003), because they did not meet inclusion criteria (breech presentation or multiple pregnancy: two in each group).
Selective reporting
All expected outcomes were reported in one study Choudhary 2010 and a published protocol was available for another study (Fraser 2005). All predefined outcomes were reported (Fraser 2005).
Other potential sources of bias
Spong 1994 do not account for a small discrepancy in group size (43 versus 50) despite using computer‐generated allocation to create equal sized groups. The Cialone 1994 report does not account for a discrepancy in birth weights between the groups. There are thus several methodological shortcomings in all the studies except Fraser 2005.A potential source of bias in this review was the degree of heterogeneity in the individual trials.
Effects of interventions
Settings with standard peripartum surveillance
Primary outcomes
There was no significant reduction in meconium aspiration syndrome (11 studies, 3374 women; average risk ratio (RR) 0.52, 95% confidence interval (CI) 0.26 to 1.06; Heterogeneity: Tau² = 0.56; Chi² = 20.08, df = 9 (P = 0.02); I² = 55%), Analysis 1.1, nor in perinatal deaths (seven studies, 2762 women; RR 1.00, 95% CI 0.29 to 3.45), Analysis 1.15, nor the combined outcome perinatal death or severe morbidity (one study, 1975 women; RR 1.13, 95% CI 0.88 to 1.47), Analysis 1.2. Analgesia use recorded in one trial and first stage of labour duration in two, were not significantly different between groups. The incidence of puerperal infection was not statistically different between the groups in these studies.
1.1. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 1 Meconium aspiration syndrome.
1.15. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 15 Perinatal death.
1.2. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 2 Perinatal death or serious morbidity (post hoc).
Secondary outcomes
There was considerable heterogeneity for several outcomes, in which case a random‐effects model has been used. In two small studies (138 women) with relevant data, heavy meconium staining was virtually eliminated (RR 0.03, 95% CI 0.01 to 0.15), Analysis 1.4. There was a reduction in caesarean sections for fetal distress (eight studies, 2765 women; average RR 0.40, 95% CI 0.19 to 0.86; Heterogeneity: Tau² = 0.70; Chi² = 24.49, df = 7 (P = 0.0009); I² = 71%), Analysis 1.6, and a trend overall (11 studies, 3380 women; average RR 0.78, 95% CI 0.60 to 1.02; Heterogeneity: Tau² = 0.11; Chi² = 32.97, df = 10 (P = 0.0003); I² = 70%), Analysis 1.7. There was a reduction in variable fetal heart rate decelerations (five studies, 2101 women; average RR 0.67, 95% CI 0.47 to 0.96; Heterogeneity: Tau² = 0.10; Chi² = 20.46, df = 4 (P = 0.0004); I² = 80%), Analysis 1.5. There was no statistically significant reduction in five‐minute Apgar score less than seven (seven studies, 2868 women; RR 0.80, 95% CI 0.52 to 1.22), Analysis 1.10. Cord arterial pH less than 7.20 was reduced (seven studies, 1788 women; average RR 0.62, 95% CI 0.40 to 0.96; Heterogeneity: Tau² = 0.23; Chi² = 21.95, df = 6 (P = 0.001); I² = 73%), Analysis 1.11. Meconium below the vocal cords diagnosed by laryngoscopy was reduced (10 studies, 3298 women; RR 0.31, 95% CI 0.18 to 0.53; Heterogeneity: Tau² = 0.46; Chi² = 33.13, df = 9 (P = 0.0001); I² = 73%), Analysis 1.12, as was neonatal ventilation or neonatal intensive care unit admission (three studies, 472 women; RR 0.45, 95% CI 0.23 to 0.90), Analysis 1.13.
1.4. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 4 Heavy meconium staining.
1.6. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 6 Caesarean for fetal distress.
1.7. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 7 Caesarean overall.
1.5. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 5 Variable decelerations.
1.10. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 10 5‐minute Apgar < 7.
1.11. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 11 Umbilical artery pH < 7.20.
1.12. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 12 Meconium below vocal cords.
1.13. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 13 Neonatal ventilation/neonatal intensive care unit admission.
Because in the outcomes with significant heterogeneity, the magnitude of effects tended to be greater in the smaller studies, a post‐hoc sensitivity analysis was performed to assess the effect of trial size on heterogeneity. Successive elimination of trials with fewer than 100, 200 and 300 participants did not eliminate the heterogeneity for the outcomes variable decelerations; caesarean section (for fetal distress and overall); umbilical artery pH less than 7.2; and meconium below vocal cords. For the primary outcome, meconium aspiration syndrome, exclusion of trials with fewer than 200 participants reduced the heterogeneity (I² = 0%: three trials, 2506 women; RR 1.22, 95% CI 0.82 to 1.84 fixed‐effect model). Planned sensitivity analysis excluding trials with greater risk of bias (all trials except Fraser 2005) rendered results showing no benefits from amnioinfusion for any of the outcomes measured. As this trial included considerably more women than the other trials combined, the interpretation of results below has taken account of the negative results of this trial.
Studies with limited peripartum surveillance
Three studies were included (Choudhary 2010; Mahomed 1998; Rathore 2002).
Primary outcomes
In the amnioinfusion group there was a reduction in meconium aspiration syndrome (three studies, 1144 women; average RR 0.17, 95% CI 0.05 to 0.52; Heterogeneity: Tau² = 0.39; Chi² = 2.94, df = 2 (P = 0.23); I² = 32%), Analysis 1.1, and perinatal mortality (three studies, 1151 women; RR 0.24, 95% CI 0.11 to 0.53), Analysis 1.15.
Secondary outcomes
In the amnioinfusion group there was a reduction in caesarean section for fetal distress (three studies, 1147 women; RR 0.38, 95% CI 0.27 to 0.54), Analysis 1.6, and overall (three studies, 1137 women; average RR 0.59, 95% CI 0.41 to 0.84; Heterogeneity: Tau² = 0.06; Chi² = 4.94, df = 2 (P = 0.08); I² = 60%), Analysis 1.7; one minute Apgar scores (two studies, 492 women; RR 0.32, 95% CI 0.19 to 0.54), Analysis 1.9; five‐minute Apgar score less than seven (three studies, 1152 women; RR 0.28, 95% CI 0.14 to 0.53), Analysis 1.10; neonatal ventilation or neonatal intensive care unit admission (two studies, 853 women; RR 0.52, 95% 0.37 to 0.73), Analysis 1.13; and no measurable effect on instrumental delivery or puerperal pyrexia. In one of the studies (Rathore 2002) meconium below the vocal cords was reduced (200 women; RR 0.42, 95% CI 0.21 to 0.83), Analysis 1.12; and in the other (Mahomed 1998) neonatal encephalopathy was reduced (649 women; RR 0.07, 95% CI 0.01 to 0.56), Analysis 1.14. In another study (Choudhary 2010) respiratory distress was reduced.
1.9. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 9 1‐minute Apgar < 7.
1.14. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 14 Neonatal encephalopathy.
Discussion
Summary of main results
Fouteen studies of variable quality (4435 women) are included. Subgroup analysis was performed for studies from settings with limited facilities to monitor the baby's condition during labour and intervene effectively, and settings with standard peripartum surveillance.
Settings with standard peripartum surveillance: there was considerable heterogeneity for several outcomes. There was no significant reduction in the primary outcomes meconium aspiration syndrome, perinatal death or severe morbidity, and maternal death or severe morbidity. There was a reduction in caesarean sections (CSs) for fetal distress but not overall. Meconium below the vocal cords diagnosed by laryngoscopy was reduced, as was neonatal ventilation or neonatal intensive care unit admission, but there was no significant reduction in perinatal deaths or other morbidity. Planned sensitivity analysis excluding trials with greater risk of bias resulted in an absence of benefits for any of the outcomes studied.
Several of the smaller studies reviewed were compromised by exclusion of significant numbers of women from the analyses. In addition, outcomes such as the decision to perform caesarean section might have been influenced by the fact that caregivers were not blind to the group allocation, and may have been more motivated to avoid caesarean section in those women subjected to amnioinfusion. The reported reduction in caesarean sections for fetal distress must be interpreted in the light of large variations among institutions with respect to thresholds for intervention for 'fetal distress'. The rates of caesarean section in the control groups ranged from under 10% to 47.5%. Overall, caesarean sections were not significantly reduced.
As thick meconium staining of the amniotic fluid is usually associated with oligohydramnios, beneficial perinatal outcomes seen in some smaller studies may have been related to correction of oligohydramnios rather than dilution of amniotic fluid. In Spong 1994, the results were generally less favourable than in the other smaller studies. The use of amnioinfusion in 8/50 of the control group who developed fetal heart rate decelerations may have obscured beneficial effects of amnioinfusion related to correction of oligohydramnios. This is in keeping with the finding that prophylactic amnioinfusion for oligohydramnios does not have demonstrable benefits over amnioinfusion used only when fetal heart rate decelerations occur (Novikova 2012).
Settings with limited peripartum surveillance: three studies were included. In the amnioinfusion group there was a reduction in CS for fetal distress and overall; meconium aspiration syndrome (three studies, 1141 women; average RR 0.17, 95% CI 0.05 to 0.52); perinatal mortality (three studies, 1151 women; RR 0.24, 95% CI 0.11 to 0.53) and neonatal ventilation or neonatal intensive care unit admission. In one of the studies, meconium below the vocal cords was reduced and, in the other, neonatal encephalopathy was reduced.
Perinatal outcomes in these under‐resourced settings were worse than in the settings with standard peripartum surveillance (perinatal deaths 22/1000 versus 3.6/1000; meconium aspiration syndrome 6.2% versus 2,2%). Under these conditions, with limited facilities to diagnose fetal distress during labour and intervene effectively, amnioinfusion appears to significantly improve perinatal outcome. It is not possible to be sure whether this improvement is due to dilution of amniotic fluid, or correction of oligohydramnios. Interpretation of the data must take into account methodological shortcomings noted above.
Overall completeness and applicability of evidence
This systematic review is relevant to current day practice of obstetrics and gynaecology, showing the impact of amnioinfusion on meconium aspiration syndrome.
Risks
The trials reviewed are too small to address the possibility of rare but serious maternal side‐effects of amnioinfusion. Several case reports have been published of cardiac failure or amniotic fluid embolism following amnioinfusion, though a causal relationship has not been established. The benefits shown in the trials reviewed need to be weighed against the theoretical small risk of serious maternal complications (seeDibble 1992; Dragich 1991; Hofmeyr 1996; Maher 1994; Wegnelius 1996; Wenstrom 1994).
HIV
The diagnosis of meconium‐stained liquor may be limited by a policy of maintaining intact membranes during labour in areas with a high prevalence of HIV infection. Once meconium‐stained liquor is diagnosed, whether amnioinfusion would increase the risk of vertical HIV transmission to the fetus because of placement of the intrauterine catheter, or reduce the risk by irrigation of the genital tract and dilution of maternal fluids, is not known.
A simplified method of amnioinfusion was shown to be feasible in an under‐resourced labour ward environment.
Quality of the evidence
The quality of evidence reflects the extent to which the confidence in an estimate of effect is adequate to support recommendations (Guyatt 2008). For systematic reviews, the quality of evidence reflects the extent to which the confidence of an estimate of effect is correct (Guyatt 2008). The GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach for assessing quality of evidence in systematic reviews involves making separate ratings for quality of evidence outcomes, and identifies five factors that can lower the quality of the evidence (Guyatt 2008). Considering these five factors in this systematic review; first, the majority of the trials had limitations with allocation concealment and blinding and in accounting for losses to follow‐up (low quality); second, the estimates of treatment effect (heterogeneity) and outcomes across trials were not too different (moderate quality). This suggests similarities in the underlying treatment effects across trials (moderate quality). Third, all trials were randomised trials, comparing amnioinfusion to no intervention (high quality). Fourth, majority of the trials recruited relatively few patients and never reported confidence intervals in their analysis (low quality); and finally, there were no instances of publication bias noted in this systematic review. Therefore, overall, the quality of evidence of this systematic review using the GRADE approach, is of low quality.
Potential biases in the review process
There are some potential biases in the review process. Several of the randomised clinical trials that were included did not blind the investigators, especially obstetricians. This is also another potential for bias in this review. Again, while assessing trials for inclusion, we might have omitted some trials that used amnioinfusion for the reduction of meconium aspiration syndrome, as authors may not have included any of the search terms in their title or abstract. Also, the majority of the trials in this review have methodological limitations capable of increasing the risk of bias, hence compromising the strength of evidence.
Agreements and disagreements with other studies or reviews
Evidence concerning the efficacy of amnioinfusion for reducing the impact of meconium aspiration syndrome is in tandem with the findings from another systematic review (Xu 2007) that was published in the British Journal of Obstetrics and Gynaecology in 2007. In another multicentre, randomised controlled trial with 1998 pregnant women comparing amnioinfusion with standard care, there were no significant differences in composite primary outcome, perinatal death, caesarean delivery, meconium aspiration syndrome (Fraser 2005). The American College of Obstetricians and Gynecologists (ACOG) Committee Opinion 346 concluded that amnioinfusion does not prevent meconium aspiration syndrome (ACOG 2006).
Authors' conclusions
Implications for practice.
Units with standard peripartum surveillance
The evidence reviewed does not support the use of amnioinfusion for meconium‐stained amniotic fluid (as opposed to oligohydramnios‐related fetal heart rate decelerations) in clinical practice, given that it is an invasive procedure and has not been shown to have clear benefits in terms of substantive clinical outcomes, and no benefits were found following sensitivity analysis excluding trials with greater risk of bias.
Units with limited peripartum surveillance
The three studies reviewed showed significant improvements in perinatal outcome with a simplified technique of amnioinfusion. The use of amnioinfusion should be considered for women with meconium‐stained liquor in units with limited facilities for peripartum surveillance and high rates of meconium aspiration syndrome. The reduction in the diagnosis of meconium aspiration syndrome after amnioinfusion in these studies may possibly be due to a reduction in fetal distress related to oligohydramnios (see Cochrane review 'Amnioinfusion for potential or suspected umbilical cord compression in labour' (Hofmeyr 2012)).
Implications for research.
Units with standard peripartum surveillance
Future research should be directed towards the use of amnioinfusion for the correction of oligohydramnios rather than meconium‐stained amniotic fluid.
Units with limited peripartum surveillance
Further research is needed to confirm the results of the three included studies, to determine whether the benefits of amnioinfusion for meconium‐stained liquor may be limited to women with oligohydramnios, to determine the effect of amnioinfusion in women infected with HIV, and to investigate the possible risks of amnioinfusion to the mother.
What's new
| Date | Event | Description |
|---|---|---|
| 1 December 2013 | New citation required but conclusions have not changed | Conclusions not changed. |
| 1 December 2013 | New search has been performed | Search updated. One new trial included (Choudhary 2010) and two new trials excluded (Gupta 2011; Regi 2009). In addition, two studies previously in Studies awaiting classification (Engel 2008; Mukhopadhyay 2006) have now been excluded. Methods updated. |
History
Protocol first published: Issue 2, 1995 Review first published: Issue 2, 1995
| Date | Event | Description |
|---|---|---|
| 2 July 2010 | Amended | Contact details edited. |
| 25 May 2009 | New search has been performed | Search updated. Four new trials included (Fraser 2005; Puertas 2001; Rathore 2002; Sood 2004); two new trials added to Studies awaiting classification (Engel 2008a; Mukhopadhyay 2006a); two previously included trials have now been excluded (Adam 1989; Ilagan 1992) and the previously excluded Alvarez 1999 is now included as another report of Puertas 2001 (confirmed by correspondence with Dr Puertas). Four new trials have been excluded (Ashfaq 2004; Das 2007; Gonzalez 2002; Khosla 1997). Adam 1989, previously included, is now excluded. We have added two additional outcomes post hoc and updated Background references. |
| 14 March 2009 | New citation required and conclusions have changed | Amnioinfusion is not beneficial for babies releasing medium to heavy meconium during labour, except in settings with limited facilities to monitor the baby's condition during labour. A new author helped to update this review. |
| 7 November 2008 | Amended | Review withdrawn from publication. |
| 31 October 2008 | Amended | Converted to new review format. |
| 5 October 2001 | New search has been performed | Five studies have been assessed of which two have been included (Alvarez 1999; Moodley 1998) in this version of the review. Inclusion of these studies has not changed the substantive findings of the review. |
Notes
None.
Acknowledgements
Thanks to Kate Barton for help with translation of Alvarez 1999 and Agnieszka Kimball for help with translation of Engel 2008.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Amnioinfusion for meconium‐stained liquor in labour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Meconium aspiration syndrome | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Standard peripartum surveillance | 11 | 3374 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.26, 1.06] |
| 1.2 Limited peripartum surveillance | 3 | 1144 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.05, 0.52] |
| 2 Perinatal death or serious morbidity (post hoc) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Standard peripartum surveillance | 1 | 1975 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.88, 1.47] |
| 2.2 Limited peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal death or serious morbidity (post hoc) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Standard peripartum surveillance | 1 | 1975 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.49, 2.04] |
| 3.2 Limited peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Heavy meconium staining | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Standard peripartum surveillance | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.01, 0.15] |
| 4.2 Limited peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Variable decelerations | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Standard peripartum surveillance | 5 | 2101 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.47, 0.96] |
| 5.2 Limited peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Caesarean for fetal distress | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Standard peripartum surveillance | 8 | 2765 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.19, 0.86] |
| 6.2 Limited peripartum surveillance | 3 | 1147 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.27, 0.54] |
| 7 Caesarean overall | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Standard peripartum surveillance | 11 | 3380 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.60, 1.02] |
| 7.2 Limited peripartum surveillance | 3 | 1137 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.41, 0.84] |
| 8 1‐minute Apgar < 4 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Standard peripartum surveillance | 2 | 1969 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.67, 1.45] |
| 8.2 Limited peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 1‐minute Apgar < 7 | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Standard peripartum surveillance | 5 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.41, 1.60] |
| 9.2 Limited peripartum surveillance | 2 | 492 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.19, 0.54] |
| 10 5‐minute Apgar < 7 | 10 | 4020 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.39, 0.79] |
| 10.1 Standard peripartum surveillance | 7 | 2868 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.22] |
| 10.2 Limited peripartum surveillance | 3 | 1152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.14, 0.53] |
| 11 Umbilical artery pH < 7.20 | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Standard peripartum surveillance | 7 | 1788 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.40, 0.96] |
| 11.2 Limited peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Meconium below vocal cords | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Standard peripartum surveillance | 10 | 3298 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.18, 0.53] |
| 12.2 Limited peripartum surveillance | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.21, 0.83] |
| 13 Neonatal ventilation/neonatal intensive care unit admission | 5 | 1325 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.38, 0.68] |
| 13.1 Standard peripartum surveillance | 3 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.23, 0.90] |
| 13.2 Limited peripartum surveillance | 2 | 853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.37, 0.73] |
| 14 Neonatal encephalopathy | 2 | 709 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.02, 0.49] |
| 14.1 Standard peripartum surveillance | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.00] |
| 14.2 Limited peripartum surveillance | 1 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.56] |
| 15 Perinatal death | 10 | 3913 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.18, 0.66] |
| 15.1 Standard peripartum surveillance | 7 | 2762 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.29, 3.45] |
| 15.2 Limited peripartum surveillance | 3 | 1151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.11, 0.53] |
| 16 Puerperal pyrexia | 8 | 3859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.65, 1.34] |
| 16.1 Standard peripartum surveillance | 5 | 2711 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.74, 1.78] |
| 16.2 Limited peripartum surveillance | 3 | 1148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.14] |
| 17 Puerperal endometritis | 5 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.60] |
| 17.1 Standard peripartum surveillance | 5 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.60] |
| 17.2 Limited peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Oxytocin augmentation | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Standard peripartum surveillance | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.67, 5.12] |
| 18.2 Limited peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Narcotic analgesic | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Standard peripartum surveillance | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.66, 1.72] |
| 19.2 Limited peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Epidural analgesia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 Standard peripartum surveillance | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.90, 1.99] |
| 20.2 Limited peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 First stage labour (minutes) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1 Standard peripartum surveillance | 2 | 229 | Mean Difference (IV, Fixed, 95% CI) | 32.46 [‐68.78, 133.70] |
| 21.2 Limited peripartum surveillance | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Instrumental vaginal delivery for fetal distress | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 Standard peripartum surveillance | 3 | 2286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.76, 1.55] |
| 22.2 Limited peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Instrumental vaginal delivery | 9 | 2059 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.50, 0.91] |
| 23.1 Standard peripartum surveillance | 6 | 914 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.51, 1.04] |
| 23.2 Limited peripartum surveillance | 3 | 1145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.33, 1.01] |
| 24 Second stage labour (minutes) | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 Standard peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Limited peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Respiratory Distress (post hoc) | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.04, 0.32] |
| 25.1 Standard peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Limited peripartum surveillance | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.04, 0.32] |
| 26 Mean maternal hospital stay (post hoc) | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.17, 1.91] |
| 26.1 Standard peripartum surveillance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Limited peripartum surveillance | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.17, 1.91] |
1.3. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 3 Maternal death or serious morbidity (post hoc).
1.8. Analysis.
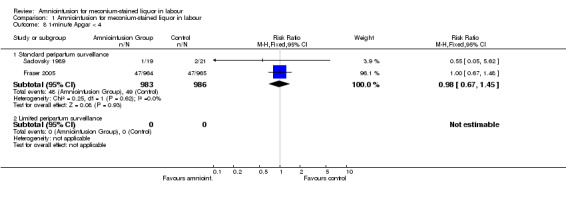
Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 8 1‐minute Apgar < 4.
1.16. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 16 Puerperal pyrexia.
1.17. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 17 Puerperal endometritis.
1.18. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 18 Oxytocin augmentation.
1.19. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 19 Narcotic analgesic.
1.20. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 20 Epidural analgesia.
1.21. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 21 First stage labour (minutes).
1.22. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 22 Instrumental vaginal delivery for fetal distress.
1.23. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 23 Instrumental vaginal delivery.
1.25. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 25 Respiratory Distress (post hoc).
1.26. Analysis.

Comparison 1 Amnioinfusion for meconium‐stained liquor in labour, Outcome 26 Mean maternal hospital stay (post hoc).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Choudhary 2010.
| Methods | Randomly generated number using sealed envelopes. | |
| Participants | Inclusion criteria: term pregnancy (> 37 weeks); singleton pregnancy; cephalic presentation; moderate or thick meconium; adequate pelvis. Exclusion criteria: cord prolapse; persistent fetal bradycardia; presence of chorioamnionitis; antepartum haemorrhage; fetal malpresentation; fetal congenital anomaly; polyhydramnios; maternal cardiac or pulmonary disease; multiple gestations. |
|
| Interventions | Amnioinfusion – Transcervical nasogastric tube was passed cephalad to the baby’s head with infusion of 500 cc’s of normal saline over a period of 30 minutes, followed by 2 mL/min under gravity until time of delivery (n = 146) compared with standard labour management without amnioinfusion (n = 146). All newborns were managed by standard protocol of immediate oropharyngeal suctioning post delivery; tracheal suctioning if not breathing vigorously. | |
| Outcomes | Outcomes: Incidence of caesarean section; maternal hospital stay; maternal febrile morbidity (temperature of > 38°C, 24 hours after delivery); low Apgar score (at 1 and 5 minutes); MAS; perinatal morbidity. | |
| Notes | Trial was carried out in a single teaching hospital in Northern India. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors did not describe how the random sequences were generated (e.g. computer‐generated, shuffling envelopes). |
| Allocation concealment (selection bias) | Low risk | Allocation by sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to outcome assessor (paediatrician). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described by the authors |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Not described by the authors |
Cialone 1994.
| Methods | Computerised randomisation. | |
| Participants | Inclusion criteria: labouring term and post‐term women; uncomplicated antepartum course; singleton vertex presenting fetus; gestation > 36 weeks; moderate to thick meconium assessed clinically. Exclusion criteria: any obstetric risk factor other than meconium. | |
| Interventions | Amnioinfusion of room temperature normal saline 600 mL over 1 hour followed by 150 mL per hour (n = 54), compared with control group (n = 59). Pad weight measured hourly. If vaginal effluent < 100 mL per hour, ultrasound examination performed to exclude overdistension of the uterus. | |
| Outcomes | 'Meconiumcrit', delivery mode, duration of first and second stage, oxytocin, analgesia, 1‐minute Apgar < 7, nuchal cord, maternal infection, meconium below cords, MAS, neonatal ICU admission. | |
| Notes | Discrepancy in birthweights not accounted for. Neonatologists but not obstetricians blinded to group allocation. FHR tracings analysed in a blind fashion. 7 withdrawals from the study group because of diabetes (3) and request (4). 1 withdrawal from the control group on request. Meconium below vocal cords in 33/58 controls according to table vs 34/58 according to text, whereas in previous report of same trial (Cialone 1993), reported in 36/58 controls. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. The method of allocation concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to outcome assessor (neonatologists). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described by the authors |
| Selective reporting (reporting bias) | Unclear risk | Not described by the authors |
| Other bias | Unclear risk | Not described by the authors |
Eriksen 1994.
| Methods | Computer randomisation using sealed packets. | |
| Participants | Inclusion criteria: > 36 weeks' gestation; active labour; thick meconium fluid. Exclusion criteria: multiple gestation; malpresentation; fetal distress on admission; cervical dilation =/> 7 cm; intra‐amniotic infection. | |
| Interventions | Amnioinfusion with 800 mL normal saline at room temperature over 1 hour followed by 180 mL per hour (n = 71), compared with control group (n = 24). | |
| Outcomes | Labour characteristics, fetal distress, operative delivery for fetal distress, meconium below the cords, MAS, fetal acid‐base status, infectious morbidity. | |
| Notes | Of 139 women who consented to the study, 9 were excluded because of incomplete records and 6 because they were randomised to amnioinfusion but delivered before amnioinfusion could be administered. The latter exclusions could have biased results against the study group by excluding those with more rapid labours. There were also somewhat more primiparous women in the study group (35/65 vs 27/59). This could possibly have been due to exclusion of more multips who had rapid labours. The n value for umbilical artery pH is given as the whole group, yet in the text at least 1 infant with MAS had no cord blood result. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described by the authors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described by the authors |
| Selective reporting (reporting bias) | Unclear risk | Not described by the authors |
| Other bias | Unclear risk | Not described by the authors |
Fraser 2005.
| Methods | 1998 women allocation by single, centralised computer‐randomisation service, stratified by site and presence or absence of 3 or more variable FHR decelerations in 30 minutes prior to randomisation. | |
| Participants | Women in labour with: thick meconium‐stained amniotic fluid; single fetus; cephalic presentation; gestational age 36 weeks or more; ruptured membranes; cervical dilation 2‐7 cm; no indication for urgent delivery. Exclusion criteria: suspected major fetal anomaly; chorioamnionitis; placenta praevia; vaginal bleeding; seropositivity for HIV or hepatitis B or C; active genital herpes; polyhydramnios; previous uterine incision other than low transverse; inability to comprehend the consent form. | |
| Interventions | Amnioinfusion: a sterile catheter was introduced transcervically, and 800 mL saline infused over 40 minutes, followed by 2 mL per minute to a maximum of 1500 mL. The infusion was discontinued if the baseline uterine pressure increased by 15 mmHg, the uterus failed to relax between contractions as assessed by palpation, or ultrasound examination revealed polyhydramnios. Control group: no amnioinfusion. Both groups had continuous electronic FHR monitoring and suctioning of the baby's nasopharynx and oropharynx during and after delivery. | |
| Outcomes | Primary outcome: perinatal death and/or moderate or severe MAS, determined by 3 neonatologists blinded to the group allocation. Secondary outcomes included perinatal death or serious morbidity and maternal death or serious morbidity. | |
| Notes | 56 centres in 13 countries, April 1999 to August 2003. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated using a centralised computer‐randomisation service. |
| Allocation concealment (selection bias) | Low risk | Centralised computer randomisation service. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 3 neonatologists were blinded to outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequately described |
| Selective reporting (reporting bias) | Low risk | Protocol published. All predefined outcomes reported. |
| Other bias | Low risk | Adequately described |
Hofmeyr 1998a.
| Methods | Allocation by opaque sealed envelopes in computer‐generated random sequence. Blinding not possible. | |
| Participants | Inclusion criteria: women in labour; moderate or heavy meconium staining of the liquor; gestation 37 weeks or more; singleton cephalic presentation. | |
| Interventions | Amnioinfusion via an Intran or Nelaton intrauterine catheter: 800 mL normal saline at 15 mL per minute, then maintenance of 3 mL per minute (n = 176), compared with no amnioinfusion (n = 176). Electronic FHR monitoring in most cases. 1 woman in the control group received amnioinfusion. Analysis was by intention‐to‐treat. | |
| Outcomes | Primary: caesarean section, MAS diagnosed clinically by paediatrician blind to group allocation, perinatal mortality. Secondary outcomes: assisted delivery; 5‐minute Apgar score < 7; cord pH < 7.2; meconium below cords; X‐ray diagnosis of MAS (amnioinfusion 2/163 vs control 3/161); neonatal ICU admission; neonatal ventilation (0/164 vs 2/163); postpartum temperature =/> 38 degrees centigrade. | |
| Notes | 4 academic hospitals in South Africa. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation by opaque sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not done. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described by the authors |
| Selective reporting (reporting bias) | Unclear risk | Not described by the authors |
| Other bias | Unclear risk | Not described by the authors |
Macri 1992.
| Methods | Sealed envelopes randomised by computer. | |
| Participants | Inclusion criteria: gestation =/> 37 weeks; thick meconium; 4‐quadrant amniotic fluid index < 5 cm; normal FHR pattern; vertex presentation; estimated fetal weight =/> 2500 g; cervical dilation =/< 5 cm; ruptured membranes. Exclusion criteria: vaginal bleeding, chorioamnionitis, fetal anomalies, uterine anomalies, contraindication to labour. | |
| Interventions | Amnioinfusion with 500 mL warmed saline over 20‐30 minutes followed by 250‐500 mL as required to maintain a 4‐quadrant amniotic fluid index above 10 cm (n = 85), compared with control group (n = 85). | |
| Outcomes | Fetal distress, mode of delivery, Apgar scores, umbilical artery pH, meconium in oropharynx, meconium below cords, MAS, chorioamnionitis. | |
| Notes | Neonatologists but not obstetricians stated to have been blinded to group allocation. The women in this report are also included in the report of Schrimmer 1991 on amnioinfusion for oligohydramnios (Paul RH, personal communication) (see review 'Amnioinfusion for potential or suspected umbilical cord compression in labour' (Hofmeyr 2012)). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Allocation by opaque sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described by the authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequately described |
| Selective reporting (reporting bias) | Low risk | Adequately described |
| Other bias | Unclear risk | Not described by the authors |
Mahomed 1998.
| Methods | Allocation by opaque sealed envelopes in computer‐generated random sequence. | |
| Participants | Inclusion criteria: moderate or heavy meconium‐ stained amniotic fluid; singleton cephalic presentation; in labour; gestation 37 weeks or more. Exclusion criteria: indication for immediate delivery; chorioamnionitis; vaginal bleeding; fetal anomaly; maternal cardiac or pulmonary disease. | |
| Interventions | Transcervical amnioinfusion using size 8 nasogastric tube. Normal saline 500 mL infused over 30 minutes, then 500 mL at 2 mL per minute (n = 325). Control group received no amnioinfusion (n = 336). Allocation not blinded. Level of intrapartum surveillance limited by number of midwives in a busy labour ward. FHR auscultated every 30 minutes using Pinard stethoscope or hand‐held doptone FHR detector. Suctioning of the airways at delivery by attending midwives. | |
| Outcomes | Primary outcomes: caesarean section; MAS diagnosed by a paediatrician blind to the group allocation; perinatal mortality. Secondary outcomes: FHR abnormality (amnioinfusion 30/320 vs control 34/334); contractions =/> 40 seconds at 1 hour (206/323 vs 211/324); caesarean section for fetal distress; assisted delivery; 1‐minute Apgar score < 4 ( 8/324 vs 18/336); 5‐minute Apgar score < 7; X‐ray diagnosis of MAS (2/319 vs 9/330); neonatal ICU admission; neonatal ventilation (10/320 vs 34/332); pneumothorax (0/320 vs 3/329); hypoxic ischaemic encephalopathy; > 4 days in neonatal ICU (5/320 vs 10/329); intrapartum and postpartum pyrexia. | |
| Notes | 4 amnioinfusion allocations early in the study unaccounted for. No electronic FHR monitoring. Neonatologist not usually present at the time of delivery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation by opaque sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not done. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described by the authors |
| Selective reporting (reporting bias) | Unclear risk | Not described by the authors |
| Other bias | Unclear risk | Not described by the authors |
Moodley 1998.
| Methods | Randomisation using sealed envelopes. | |
| Participants | Inclusion criteria: singleton, term, cephalic pregnancies in active labour; meconium‐stained amniotic fluid grade 1‐3; normal cardiotocograph. Exclusion criteria: medical or surgical conditions; chorioamnionitis; previous caesarean section. | |
| Interventions | Amnioinfusion with normal saline 10‐15 mL per minute by gravity via a central venous manometer set and a size 8 nasogastric infant feeding tube (1 litre over 4 hours); versus standard care; continuous FHR monitoring. | |
| Outcomes | Mean umbilical artery pH; mean Apgar scores; caesarean section; instrumental delivery; hypoxic ischaemic encephalopathy. | |
| Notes | Durban, South Africa, January to April 1993. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how the randomisation was carried out. |
| Allocation concealment (selection bias) | Low risk | Allocation using sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not done. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described by the authors |
| Selective reporting (reporting bias) | Unclear risk | Not described by the authors |
| Other bias | Unclear risk | Not described by the authors |
Puertas 2001.
| Methods | Randomised sequence in sealed envelopes. | |
| Participants | 206 pregnant women admitted for labour and delivery with moderately or thickly stained amniotic fluid; term pregnancy; no uterine scarring; spontaneous labour or induction of labour for meconium staining of the amniotic fluid; single fetus; vertex presentation; no FHR changes suggesting fetal distress; no vaginal bleeding; and no indication of vertically transmitted infectious disease. | |
| Interventions | Amnioinfusion with 0.9% saline via an infusion pump at 600 mL per hour for 1 hour, then 180 mL per hour until full cervical dilation, or basal uterine pressure increase to 20 mmHg; compared with no amnioinfusion. Continuous FHR monitoring and intrauterine pressure monitoring | |
| Outcomes | Amniotic fluid index change after 1 hour; umbilical cord acid base status; meconium below cords > trace by paediatricians blind to the group allocation; umbilical cord complication; mean umbilical artery pH. | |
| Notes | Granada, Southern Spain. Subdivided into thick (meconium concentration > 15%) and moderate meconium staining. Amnioinfusion stopped in 17 women for uterine hypertonia. Confirmed by correspondence with first author that the data from Alvarez 1999 are included in the final Puertas 2001 report. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly generated numbers in sealed envelopes.' However, method of randomisation not described (e.g. computer‐generated, shuffling envelopes). |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to paediatricians. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described by the authors |
| Selective reporting (reporting bias) | Unclear risk | Not described by the authors |
| Other bias | Unclear risk | Not described by the authors |
Rathore 2002.
| Methods | Computer‐generated random allocation by sequentially numbered sealed opaque envelopes. | |
| Participants | Women in labour; 37 or more weeks' gestation; singleton cephalic presentation; moderate or thick meconium‐stained fluid, or meconium crit > 10%. exclusions criteria: chorioamnionitis; indication for immediate delivery; fetal congenital anomaly, antepartum haemorrhage; polyhydramnios; and maternal cardiac or respiratory disease. | |
| Interventions | Saline amnioinfusion via FG 8 nasogastric tube, 500 mL over 30 minutes, then 3 mL per minute. Maximum dose 1 L. Control group received no amnioinfusion or uterine catheter. No cardiotocography was available. | |
| Outcomes | Caesarean section; meconium at vocal cords; Apgar scores < 7; neonatal ICU admission; respiratory distress, MAS; maternal pyrexia. | |
| Notes | Tertiary level teaching hospital. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Adequate. Sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described by the authors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described by the authors |
| Selective reporting (reporting bias) | Unclear risk | Not described by the authors |
| Other bias | Unclear risk | Not described by the authors |
Sadovsky 1989.
| Methods | 'Random allocation' with sealed envelopes. | |
| Participants | Inclusion criteria: singleton; more than a trace of meconium‐stained liquor; vertex presentation; > 34 weeks; anticipate delivery > 1 hour. Exclusion criteria: malformations; chorioamnionitis; malpresentation; polyhydramnios; cord prolapse; urgent delivery needed; maternal cardiac disease. | |
| Interventions | Amnioinfusion with saline 600 mL over 1 hour then 180 mL per hour (n = 19), compared with control group (n = 21). | |
| Outcomes | Thick meconium on spectrophotometry, labour duration, labour augmentation, analgesia, mode of delivery, umbilical artery pH, meconium below cords, positive pressure ventilation. | |
| Notes | Neither obstetricians nor neonatologists stated to have been blinded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly generated numbers in sealed envelopes.' However, method of randomisation not described (e.g. computer‐generated, shuffling envelopes). |
| Allocation concealment (selection bias) | Low risk | Allocation adequate with sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not done. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described by the authors |
| Selective reporting (reporting bias) | Unclear risk | Not described by the authors |
| Other bias | Unclear risk | Not described by the authors |
Sood 2004.
| Methods | 199 women allocated by flip of a coin. | |
| Participants | Women in labour with thick staining of the amniotic fluid. singleton, vertex presentation; adequate pelvis, cervical dilation > 5 cm, gestational age > 37 weeks. | |
| Interventions | Amnioinfusion via a plastic suction catheter or amnioinfusion catheter with normal saline till the returning fluid was clear. Usually 1000 mL was infused over 30 to 45 minutes. | |
| Outcomes | Persistent FHR decelerations; caesarean section; meconium below neonate's vocal cords; MAS diagnosed by X‐ray, perinatal mortality, hospital stay; uterine atony (6/96 versus 1/100). | |
| Notes | FHR abnormalities before enrolment were present in 48/96 women in the amnioinfusion versus 60/100 in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Flip of a coin. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not feasible. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described by the authors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described by the authors |
| Selective reporting (reporting bias) | Unclear risk | Not described by the authors |
| Other bias | Unclear risk | Not described by the authors |
Spong 1994.
| Methods | 'Randomised' by computer‐generated random number sequence. | |
| Participants | Inclusion criteria: singleton; vertex presentation; 37 or more weeks; moderate to heavy meconium; no variable FHR decelerations. Exclusion criteria: prenatally diagnosed fetal malformations; maternal temperature > 100.4 degrees Fahrenheit; evidence of fetal distress. | |
| Interventions | Amnioinfusion with 600 mL saline bolus followed by 3 mL per minute (n = 43), compared with standard care which included similar amnioinfusion in 8/50 for variable decelerations (n = 50). | |
| Outcomes | Meconium below cords, MAS, Apgar scores, cord pH, neonatal complications, route of delivery, maternal infection. | |
| Notes | Discrepancy in group numbers (43 vs 50), despite use of computer‐generated random number sequence to create equal number groups, not accounted for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised' by computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described by the authors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described by the authors |
| Selective reporting (reporting bias) | Unclear risk | Not described by the authors |
| Other bias | Unclear risk | Not described by the authors |
Wenstrom 1989.
| Methods | Sealed envelopes randomised by computer. | |
| Participants | Inclusion criteria: thick meconium‐stained amniotic fluid. Exclusion criteria: fetal distress, maternal pyrexia. | |
| Interventions | Amnioinfusion of 1000 mL over 20‐40 minutes, repeated 6‐hourly (n = 41), compared with control group (n = 44). | |
| Outcomes | Mode of delivery, Apgar scores, cord arterial pH, meconium below cords, meconium aspiration syndrome, postpartum endometritis. | |
| Notes | 5 study women who did not receive amnioinfusion excluded, of which 4 delivered spontaneously after 30, 45, 60 and 180 minutes and 1 required emergency caesarean section for fetal distress. These exclusions have been included in this review with respect to delivery outcomes. Obstetricians not blinded, but neonatologists blinded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Allocation using sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described by the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neonatologists blinded to outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described by the authors |
| Selective reporting (reporting bias) | Unclear risk | Not described by the authors |
| Other bias | Unclear risk | Not described by the authors |
FHR: fetal heart rate ICU: intensive care unit MAS: meconium aspiration syndrome vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adam 1989 | Excluded because inadequate information to confirm acceptable allocation concealment. |
| Ashfaq 2004 | Excluded because the report does not indicated that allocation was by randomisation. 400 patients were 'equally divided in study and control groups'. Amnioinfusion was associated with very large reductions in caesarean section, low Apgar scores, meconium aspiration syndrome and perinatal death (12/200 vs 36/200). |
| Das 2007 | Comparative study in a low‐resource setting, not randomised. |
| Edwards 1999 | Excluded because comparison was between amnioinfusion with and without antibiotics. |
| Engel 2008 | Excluded because not a randomised trial. Amnioinfusion had no statistically significant effects on the measures outcomes. |
| Gonzalez 1998 | Excluded because no clinically meaningful data. 43 women undergoing amnioinfusion for meconium‐stained liquor were randomly allocated to receive normal saline or lactate Ringer's solution. There were no significant differences in the umbilical artery electrolytes or pH. |
| Gonzalez 2002 | Excluded because no clinically meaningful data. 40 women undergoing amnioinfusion for meconium‐stained liquor were randomly allocated to receive amnioinfusion with normal saline or lactate Ringer's solution. There were no significant differences in the umbilical artery electrolytes or pH, nor were they different from non‐randomised normal controls. |
| Gupta 2011 | Excluded because only abstract available. |
| Ilagan 1992 | Excluded because inadequate information to confirm acceptable allocation concealment. |
| Khosla 1997 | Not a randomised trial. The study group was 25 consecutive patients who gave consent for amnioinfusion and controls were 25 similar patients of the alternative unit where amnioinfusion was not being carried out. |
| Kirubamani 2000 | Excluded because only abstract available, with no figures; discrepancy in group numbers (30 vs 20) not accounted for. |
| Lembet 1999 | Excluded because no data given. Women with thick meconium during labour randomised to transabdominal amnioinfusion (19) vs standard care (20). |
| Lo 1993 | Excluded because allocation was not at random. Of 112 women with moderate or thick meconium‐stained liquor, 63 chose to undergo amnioinfusion. In the amnioinfusion group, there were fewer operative deliveries for fetal distress but not overall, and neonatal outcomes were improved. |
| Mukhopadhyay 2006 | Excluded because allocation was by alternation, not randomised. This study in a low‐resource setting found that with amnioinfusion the rate of caesarean section was one‐half and of meconium below the cords was one‐fifth of that in the control group. |
| Nageotte 1991 | Nageotte 1991 studied 86 women with oligohydramnios and/or thick meconium‐stained liquor. As the reported results relate primarily to problems associated with oligohydramnios, these are considered in the review 'Amnioinfusion for potential or suspected umbilical cord compression in labour' (Hofmeyr 2012). The authors state that there was no significant difference in meconium below the cords, but no figures are given. |
| Regi 2009 | The primary indication for amnioinfusion was fetal heart rate decelerations, not meconium‐stained liquor. See review: "Amnioinfusion for potential or suspected umbilical cord compression in labour". |
vs: versus
Differences between protocol and review
We have added four additional outcomes (perinatal death or severe morbidity; maternal death or severe morbidity; respiratory distress; length of maternal hospital stay) and one sensitivity analysis post hoc and updated Background references.
Contributions of authors
GJ Hofmeyr prepared the original protocol and review. H Xu updated the review following the release of the results of the Fraser 2005 trial, and AC Eke updated the review following the release of the results of the Choudhary 2010 trial. All three review authors checked the data entry and contributed to the writing of the review.
Sources of support
Internal sources
University of the Witwatersrand, South Africa.
External sources
South African Medical Research Council, South Africa.
Declarations of interest
G J Hofmeyr is an author of studies considered for inclusion in the review and has not participated in decisions regarding these studies.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Choudhary 2010 {published data only}
- Choudhary D, Bano I, Ali SM. Does amnioinfusion reduce caesarean section rate in meconium‐stained amniotic fluid. Archives of Gynecology and Obstetrics 2010;282(1):17‐22. [DOI] [PubMed] [Google Scholar]
Cialone 1994 {published data only}
- Cialone PR, Abramowicz JS, Ryan RM, Sinkin RA, Sherer DM. Markedly significant decrease in neonatal morbidity associated with amnioinfusion for labor complicated by particulate meconium. American Journal of Obstetrics and Gynecology 1993;168:319. [DOI] [PubMed] [Google Scholar]
- Cialone PR, Sherer DM, Ryan RM, Sinkin RA, Abramowicz JS. Amnioinfusion during labor complicated by particulate meconium‐stained amniotic fluid decreases neonatal morbidity. American Journal of Obstetrics and Gynecology 1994;170:842‐9. [DOI] [PubMed] [Google Scholar]
Eriksen 1994 {published data only}
- Eriksen N, Hostetter M, Parisi V. Prophylactic amnioinfusion in pregnancies complicated by thick meconium. American Journal of Obstetrics and Gynecology 1994;170:344. [DOI] [PubMed] [Google Scholar]
- Eriksen NL, Hostetter M, Parisi VM. Prophylactic amnioinfusion in pregnancies complicated by thick meconium. American Journal of Obstetrics and Gynecology 1994;171:1026‐30. [DOI] [PubMed] [Google Scholar]
Fraser 2005 {published data only}
- Fraser W, Hofmeyr J, Lede R, Faron G, Alexander S, Goffinet F, et al. An international randomised controlled trial of amnioinfusion for thickly meconium stained amniotic fluid. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S3. [Google Scholar]
- Fraser WD, Hofmeyr J, Lede R, Faron G, Alexander S, Goffinet F, et al. Amnioinfusion for the prevention of the meconium aspiration syndrome. New England Journal of Medicine 2005;353(9):909‐17. [DOI] [PubMed] [Google Scholar]
Hofmeyr 1998a {published data only}
- Gulmezoglu AM, Nikodem V, Hofmeyr GJ. Amniotic fluid index changes after amnioinfusion. Proceedings of the 14th Conference on Priorities in Perinatal Care in South Africa; 1995 March 7‐10; South Africa. 1995:179‐81.
- Hofmeyr GJ, Gulmezoglu AM, Buchmann E, Howarth GR, Shaw A, Nikodem VC, et al. The Collaborative Randomised Amnioinfusion for Meconium Project (CRAMP): 1. South Africa. British Journal of Obstetrics and Gynaecology 1998;105:304‐8. [DOI] [PubMed] [Google Scholar]
- Hofmeyr GJ, Gulmezoglu AM, Nikodem VC. Amnioinfusion for meconium‐staining of the amniotic fluid: A randomized trial. 27th British Congress of Obstetrics And Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:64.
Macri 1992 {published data only}
- Macri CJ, Schrimmer DB, Leung A, Greenspoon JS, Paul RH. Amnioinfusion improves outcome in labor complicated by meconium and oligohydramnios. American Journal of Obstetrics and Gynecology 1991;164:252. [DOI] [PubMed] [Google Scholar]
- Macri CJ, Schrimmer DB, Leung A, Greenspoon JS, Paul RH. Prophylactic amnioinfusion improves outcome of pregnancy complicated by thick meconium and oligohydramnios. American Journal of Obstetrics and Gynecology 1992;67:117‐21. [DOI] [PubMed] [Google Scholar]
Mahomed 1998 {published data only}
- Mahomed K, Mulambo T, Woelk G, Hofmeyr GJ, Gulmezoglu AM. The Collaborative Randomised Amnioinfusion for Meconium Project (CRAMP): 2. Zimbabwe. British Journal of Obstetrics and Gynaecology 1998;105:309‐13. [DOI] [PubMed] [Google Scholar]
Moodley 1998 {published data only}
- Moodley J, Matchaba P, Payne AJ. Intrapartum amnioinfusion for meconium‐stained liquor in developing countries. Tropical Doctor 1998;28:31‐4. [DOI] [PubMed] [Google Scholar]
Puertas 2001 {published data only}
- Alvarez M, Puertas A, Suarez AM, Herruzo A, Miranda JA. Transcervical amnioinfusion in deliveries with meconium‐stained amniotic fluid [Amnioinfusion transcervical en partos con liquido amniotico tenido de meconio]. Progresos de Obstetricia y Ginecologia 1999;42:365‐72. [Google Scholar]
- Puertas A, Carrillo MP, Alvarez M, Canizares JM, Mino M, Malde J. Meconium concentration and amniotic fluid index influence the outcome of amnioinfusion. Minerva Ginecologica 2001;53:321‐30. [PubMed] [Google Scholar]
- Puertas A, Carrillo MP, Molto L, Alvarez M, Sedeno S, Miranda JA. Meconium‐stained amniotic fluid in labor: a randomized trial of prophylactic amnioinfusion. European Journal of Obstetrics & Gynecology and Reproductive Biology 2001;99:33‐7. [DOI] [PubMed] [Google Scholar]
Rathore 2002 {published data only}
- Rathore AM, Singh R, Ramji S, Tripathi R. Randomised trial of amnioinfusion during labour with meconium stained amniotic fluid. BJOG: an international journal of obstetrics and gynaecology 2002;109:17‐20. [DOI] [PubMed] [Google Scholar]
Sadovsky 1989 {published data only}
- Sadovsky Y, Amon E, Bade ME, Petrie RH. Prophylactic amnioinfusion during labor complicated by meconium: a preliminary report. American Journal of Obstetrics and Gynecology 1989;61:613‐7. [DOI] [PubMed] [Google Scholar]
- Sadovsky Y, Amon E, Bade ME, Petrie RH. Prophylactic amnioinfusion during labor complicated by meconium: a preliminary report. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 Feb 1‐4; New Orleans, Louisiana, USA. 1999:24. [DOI] [PubMed]
Sood 2004 {published data only}
- Sood M, Charulata, Dimple D, Aggarwal N, Faridi MM. Amnioinfusion in thick meconium. Indian Journal of Pediatrics 2004;71(8):677‐81. [DOI] [PubMed] [Google Scholar]
Spong 1994 {published data only}
- Spong CY, Ogundipe OA, Ross MG. Prophylactic amnioinfusion for meconium stained amniotic fluid. American Journal of Obstetrics and Gynecology 1994;170:285. [DOI] [PubMed] [Google Scholar]
- Spong CY, Ogunipe OA, Ross MG. Prophylactic amnioinfusion for meconium‐stained amniotic fluid. American Journal of Obstetrics and Gynecology 1994;171:931‐5. [DOI] [PubMed] [Google Scholar]
Wenstrom 1989 {published data only}
- Wenstrom KD, Parsons MT. The prevention of meconium aspiration in labor using amnioinfusion. Obstetrics and Gynecology 1989;73:647‐51. [PubMed] [Google Scholar]
References to studies excluded from this review
Adam 1989 {published data only}
- Adam K, Cano L, Moise KJ. The effect of intrapartum amnioinfusion on the outcome of the fetus with heavy meconium stained amniotic fluid. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 Feb 1‐4; New Orleans, Louisiana, USA. 1989:438.
Ashfaq 2004 {published data only}
- Ashfaq F, Shah AA. Effect of amnioinfusion for meconium stained amniotic fluid on perinatal outcome. Journal of the Pakistan Medical Association 2004;54(6):322‐5. [PubMed] [Google Scholar]
Das 2007 {published data only}
- Das AK, Jana N, Dasgupta S, Samanta B. Intrapartum transcervical amnioinfusion for meconium‐stained amniotic fluid. International Journal of Gynecology & Obstetrics 2007;93:182‐6. [DOI] [PubMed] [Google Scholar]
Edwards 1999 {published data only}
- Edwards RK, Duff P. Prophylactic cephazolin in amnioinfusions administered for meconium‐stained amniotic fluid. Infectious Diseases in Obstetrics and Gynecology 1999;7:153‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engel 2008 {published data only}
- Engel K, Samborska M, Bilar M, Sipak‐Szmigiel O, Ronin‐Walknowska E. Intrapartum amnioinfusion in patients with meconium‐stained amniotic fluid [Amnioinfuzja srodporodowa u pacjentek z obecnoscia smolki w plynie owodniowym]. Ginekologia Polska 2008;79(9):621‐4. [PubMed] [Google Scholar]
Gonzalez 1998 {published data only}
- Gonzalez JL, Martin D, Gardner MO, Mooney S, Curet LB. The effects of the amnioinfused solution on the neonatal electrolytes and acid base. American Journal of Obstetrics and Gynecology 1998;178(1):S43. [Google Scholar]
Gonzalez 2002 {published data only}
- Gonzalez JL, Mooney S, Gardner MO, Martin D, Curet LB. The effects of amnioinfused solutions for meconium‐stained amniotic fluid on neonatal plasma electrolyte concentrations and ph. Journal of Perinatology 2002;22(4):279‐81. [DOI] [PubMed] [Google Scholar]
Gupta 2011 {published data only}
- Gupta K, Das B. Role of intrapartum amnioinfusion in meconium stained amniotic fluid. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:246.
Ilagan 1992 {published data only}
- Ilagan NB, Kazzi GM, Shankaran S, Liang KC, Womack SJ, Bronsteen RA, et al. Transcervical amnioinfusion for the prevention of neonatal meconium aspiration. Pediatric Research 1992;31(4):205A. [Google Scholar]
Khosla 1997 {published data only}
- Khosla AH, Sangwan K, Ahuja SD. Prophylactic amnioinfusion during labor complicated by meconium. Australian and New Zealand Journal of Obstetrics and Gynaecology 1997;37:294‐6. [DOI] [PubMed] [Google Scholar]
Kirubamani 2000 {published data only}
- Kirubamani HN. A fetal therapy in labour with meconium stained liquor. XVI FIGO World Congress of Obstetrics & Gynecology (Book 2); 2000 Sept 3‐8; Washington DC, USA. 2000:125.
Lembet 1999 {published data only}
- Lembet A, Zorlu CG, Yalcin HR, Seckin B, Batioglu S, Gokmen O. Prophylactic transabdominal amnioinfusion during labor with thick meconium: does it work?. European Journal of Obstetrics & Gynecology and Reproductive Biology 1999;86:S80. [Google Scholar]
Lo 1993 {published data only}
- Lo KWK, Rogers M. A controlled trial of amnioinfusion: the prevention of meconium aspiration in labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1993;33:52‐4. [DOI] [PubMed] [Google Scholar]
Mukhopadhyay 2006 {published data only}
- Mukhopadhyay P, Naskar T, Dalui R, Hazra S, Guin K, Bhattacharya D. Role of intrapartum amnioinfusion in meconium stained amniotic fluid. Journal of Obstetrics and Gynecology of India 2006;56(3):230‐2. [Google Scholar]
Nageotte 1991 {published data only}
- Nageotte MP, Bertucci L, Towers CV, Lagrew DL, Modanlou H. Prophylactic amnioinfusion in pregnancies complicated by oligohydramnios: a prospective study. Obstetrics & Gynecology 1991;77:677‐80. [PubMed] [Google Scholar]
Regi 2009 {published data only}
- Regi A, Alexander N, Jose R, Lionel J, Varghese L, Peedicayil A. Amnioinfusion for relief of recurrent severe and moderate variable decelerations in labor. Journal of Reproductive Medicine 2009;54(5):295‐302. [PubMed] [Google Scholar]
Additional references
Abdel‐Aleem 2005
- Abdel‐Aleem H, Amin AF, Shokry M, Radwan RA. Therapeutic amnioinfusion for intrapartum fetal distress using a pediatric feeding tube. International Journal of Gynecology & Obstetrics 2005;90(2):94‐8. [DOI] [PubMed] [Google Scholar]
ACOG 2006
- ACOG Committee Obstetric Practice. ACOG Committee Opinion Number 346, October 2006: amnioinfusion does not prevent meconium aspiration syndrome. Obstetrics and Gynecology 2006;108(4):1053. [DOI] [PubMed] [Google Scholar]
Alvarez 1999
- Alvarez M, Puertas A, Suarez AM, Herruzo A, Miranda JA. Transcervical amnioinfusion in deliveries with meconium‐stained amniotic fluid [Amnioinfusion transcervical en partos con liquido amniotico tenido de meconio]. Progresos de Obstetricia y Ginecologia 1999;42:365‐72. [Google Scholar]
Benifla 1995
- Benifla JL, Goffinet F, Bascou V, Darai E, Proust A, Madelenat P. Transabdominal amnio‐infusion facilitates external version maneouver after initial failure. Six successful attempts. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1995;24:319‐22. [PubMed] [Google Scholar]
Cialone 1993
- Cialone PR, Abramowicz JS, Ryan RM, Sinkin RA, Sherer DM. Markedly significant decrease in neonatal morbidity associated with amnioinfusion for labor complicated by particulate meconium. American Journal of Obstetrics and Gynecology 1993;168:319. [DOI] [PubMed] [Google Scholar]
Davis 1985
- Davis RO, Phillips JB, Harris BA, Huddleston JF. Fatal meconium aspiration syndrome occurring despite airway management considered appropriate. American Journal of Obstetrics and Gynecology 1985;151:731‐6. [DOI] [PubMed] [Google Scholar]
Dibble 1992
- Dibble LA, Elliot JP. Possible amniotic fluid embolism associated with amnioinfusion. Journal of Maternal‐Fetal Medicine 1992;1:263‐6. [Google Scholar]
Dorairajan 2005
- Dorairajan G, Soundararaghavan S. Maternal death after intrapartum saline amnioinfusion‐‐report of two cases. BJOG: an international journal of obstetrics and gynaecology 2005;11:1331‐3. [DOI] [PubMed] [Google Scholar]
Dragich 1991
- Dragich DA, Ross AF, Chestnut DH, Wenstrom KD. Respiratory failure associated with amnioinfusion during labor. Anesthesia & Analgesia 1991;72:549‐51. [DOI] [PubMed] [Google Scholar]
Gelfand 2004
- Gelfand SL, Fanaroff JM, Walsh MC. Controversies in the treatment of meconium aspiration syndrome. Clinics in Perinatology 2004;31:445‐52. [DOI] [PubMed] [Google Scholar]
Ghidini 2001
- Ghidini A, Spong CY. Severe meconium aspiration syndrome is not caused by aspiration of meconium. American Journal of Obstetrics and Gynecology 2001;185:931‐8. [DOI] [PubMed] [Google Scholar]
Gramellini 2003
- Gramellini D, Fieni S, Kaihura C, Piantelli G, Verrotti C. Antepartum amnioinfusion: a review. Journal of Maternal‐Fetal & Neonatal Medicine 2003;14:291‐6. [DOI] [PubMed] [Google Scholar]
Gramellini 2003b
- Gramellini D, Fieni S, Kaihura C, Faiola S, Vadora E. Transabdominal antepartum amnioinfusion. International Journal of Gynecology & Obstetrics 2003;83:171‐8. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hicks 2005
- Hicks P. Systematic review of the risk of uterine rupture with the use of amnioinfusion after previous cesarean delivery. Southern Medical Journal 2005;98:458‐61. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2003
- Hofmeyr GJ, Gülmezoglu AM. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD000941] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2009
- Hofmeyr GJ. Routine ultrasound examination in early pregnancy: is it worthwhile in low‐income countries? . Ultrasound in Obstetrics and Gynecology 2009;34(4):367‐70. [DOI] [PubMed] [Google Scholar]
Hofmeyr 2012
- Hofmeyr GJ, Lawrie. Amnioinfusion for potential or suspected umbilical cord compression in labour. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD000013.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lameier 1993
- Lameier LN, Katz VL. Amnioinfusion: a review. Obstetrical & Gynecological Survey 1993;48:829‐37. [DOI] [PubMed] [Google Scholar]
Maher 1994
- Maher JE, Wenstrom KD, Hauth JC, Meis BJ. Amniotic fluid embolism after saline amnioinfusion: two cases and review of the literature. Obstetrics & Gynecology 1994;83:851‐4. [PubMed] [Google Scholar]
Mahomed 1994
- Mahomed K, Nyoni R, Masona D. Meconium‐staining of the liquor in a low‐risk population. Paediatric and Perinatal Epidemiology 1994;8:292‐300. [DOI] [PubMed] [Google Scholar]
Mitri 1987
- Mitri F, Hofmeyr GJ, Gelderen CJ. Meconium during labour: self‐medication and other associations. South African Medical Journal 1987;71:431‐3. [PubMed] [Google Scholar]
Miyazaki 1983
- Miyazaki FS, Taylor NA. Saline amnioinfusion for relief of prolonged variable decelerations. American Journal of Obstetrics and Gynecology 1983;146:670‐8. [DOI] [PubMed] [Google Scholar]
Nathan 1994
- Nathan L, Levino KJ, Carmody III TJ, Kelly MA, Sherman ML. Meconium: A 1990's perspective on an old obstetric hazard. Obstetrics & Gynecology 1994;83:329‐32. [PubMed] [Google Scholar]
Novikova 2012
- Novikova N, Hofmeyr GJ, Essilfie‐Appiah G. Prophylactic versus therapeutic amnioinfusion for oligohydramnios in labour. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD000176.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pierce 2000
- Pierce J, Gaudier FL, Sanchez‐Ramos L. Intrapartum amnioinfusion for meconium‐stained fluid: meta‐analysis of prospective clinical trials. Obstetrics & Gynecology 2000;95:1051‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schrimmer 1991
- Schrimmer DB, Macri CJ, Paul RH. Prophylactic amnioinfusion as a treatment for oligohydramnios in laboring patients: a prospective, randomized trial. American Journal of Obstetrics and Gynecology 1991;165:972‐5. [DOI] [PubMed] [Google Scholar]
Velaphi 2006
- Velaphi S, Vidyasagar D. Intrapartum and postdelivery management of infants born to mothers with meconium‐stained amniotic fluid: evidence‐based recommendations. Clinical Perinatology 2006;33:29‐42. [DOI] [PubMed] [Google Scholar]
Wax 2004
- Wax JR, Flaherty N, Pinette MG, Blackstone J, Cartin A. Small‐volume amnioinfusion: a potential stimulus of intrapartum fetal heart rate accelerations. American Journal of Obstetrics and Gynecology 2004;190:380‐2. [DOI] [PubMed] [Google Scholar]
Wegnelius 1996
- Wegnelius G, Bergstrom M, Ahlbom L, Thomassen P. A case of life‐threatening pulmonary edema associated with amnioinfusion during labor. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;65(2):237‐9. [DOI] [PubMed] [Google Scholar]
Weismiller 1998
- Weismiller DG. Transcervical amnioinfusion. American Family Physician 1998;57:504‐10. [PubMed] [Google Scholar]
Wenstrom 1994
- Wenstrom KD, Andrews WW, Maher JE. Prevalence, protocols and complications associated with amnioinfusion. American Journal of Obstetrics and Gynecology 1994;170:341. [DOI] [PubMed] [Google Scholar]
Woods 1994
- Woods JR, Glantz JC. Significance of amniotic fluid meconium. In: Creasy RK, Resnick R editor(s). Maternal Fetal Medicine: Principles and Practice. 3rd Edition. Philadelphia: WB Saunders, 1994:413‐22. [Google Scholar]
Xu 2007
- Xu H, Hofmeyr GJ, Roy C, Fraser W. Intrapartum amnioinfusion for meconium‐stained amniotic fluid:a systematic review of randomised controlled trials. BJOG: an international journal of obstetrics and gynaecology 2007;353:383‐90. [DOI] [PubMed] [Google Scholar]
Ziadeh 2000
- Ziadeh SM, Sunna E. Obstetric and perinatal outcomes of pregnancies with term labour and meconium‐stained amniotic fluid. Archives of Gynecology and Obstetrics 2000;264:84‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hofmeyr 1995
- Hofmeyr GJ. Amnioinfusion for meconium‐stained liquor in labour. [ revised 07 October 1993]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Hofmeyr 1996
- Hofmeyr GJ, Gulmezoglu AM, Nikodem VC, Jager M. Amnioinfusion. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;64:159‐65. [DOI] [PubMed] [Google Scholar]
Hofmeyr 2000
- Hofmeyr GJ. Amnioinfusion for meconium‐stained liquor. Current Opinion in Obstetrics and Gynecology 2000;12(2):129‐32. [DOI] [PubMed] [Google Scholar]
Hofmeyr 2002
- Hofmeyr GJ. Amnioinfusion for meconium‐stained liquor in labour. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD000014] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2010
- Hofmeyr GJ, Xu H. Amnioinfusion for meconium‐stained liquor in labour. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD000014.pub3] [DOI] [PubMed] [Google Scholar]


