Abstract
Background:
Aedes aegypti and Ae. albopictus are mosquito vectors of more than 22 arboviruses that infect humans.
Objectives:
Our objective was to develop regional ecological niche models for Ae. aegypti and Ae. albopictus in the conterminous United States and Canada with current observed and simulated climate and land-use data using boosted regression trees (BRTs).
Methods:
We used BRTs to assess climatic suitability for Ae. albopictus and Ae. aegypti mosquitoes in Canada and the United States under current and future projected climates.
Results:
Models for both species were mostly influenced by minimum daily temperature and demonstrated high accuracy for predicting their geographic ranges under the current climate. The northward range expansion of suitable niches for both species was projected under future climate models. Much of the United States and parts of southern Canada are projected to be suitable for both species by 2100, with Ae. albopictus projected to expand its range north earlier this century and further north than Ae. aegypti.
Discussion:
Our projections suggest that the suitable ecological niche for Aedes will expand with climate change in Canada and the United States, thus increasing the risk of Aedes-transmitted arboviruses. Increased surveillance for these vectors and the pathogens they carry would be prudent. https://doi.org/10.1289/EHP5899
Introduction
Mosquito-borne diseases (MBDs) account for approximately 350 million cases of human illness each year (WHO et al. 2017). Approximately 5% of infectious diseases are attributed to diseases transmitted by two mosquito species: Aedes aegypti and Ae. albopictus. These species are vectors for more than 22 arboviruses of global public health importance, including dengue, chikungunya, Zika, Japanese encephalitis, Rift Valley fever, yellow fever, and West Nile viruses (Medlock et al. 2015; Schaffner et al. 2013). With climate change, rising temperature and changes in precipitation patterns (Blunden and Arndt 2019; IPCC 2018) are expected to permit changes to the geographic range of these species, including poleward range expansion in North America (Bonizzoni et al. 2013). Concurrently, it is clear that North American travelers to tropical and subtropical regions can acquire arbovirus infections, with a proportion viremic when they return home and acting as a source of infection for Aedes mosquitoes that may be present (Drebot et al. 2015; Khan et al. 2014; Ogden et al. 2017; Petersen et al. 2016). As a consequence, autochthonous transmission of MBDs previously considered endemic to tropical and subtropical regions may become more frequent in current temperate regions (Ng et al. 2017; Ogden 2017).
During recent years, there have been multiple reports of autochthonous transmission of MBDs leading to localized epidemics of Zika virus, chikungunya virus, and dengue virus infections in humans in the southern continental United States due to the establishment of local Aedes mosquito populations and range expansion (Hahn et al. 2016; Kendrick et al. 2014; Likos et al. 2016; Ramos et al. 2008). Canada’s mosquito surveillance programs are primarily targeted toward mosquitoes carrying endemic pathogens of public health concerns such as West Nile virus and eastern equine encephalitis. However, these surveillance programs are capable of detecting mosquito species exotic to Canada (i.e., Aedes spp.), which results in targeted active surveillance in specific regions. During 2016–2017, Ae. aegypti was found in low abundance during the summer months in southwestern Ontario in Canada; the most northern known occurrence in continental North America in recent years (WECHU 2017). This observation triggered additional active surveillance for Aedes mosquitoes in this region. However, mosquito trapping from 2018 did not identify Ae. aegypti in that region (WECHU 2017). Since its introduction into continental North America in 1985 (Sprenger and Wuithiranyagool 1986), Ae. albopictus is now frequently reported from the U.S. southern to upper Midwestern states, some northeastern states, and southern regions of the northwestern states of the United States and along the Pacific coast (Hahn et al. 2016, 2017; Kraemer et al. 2015a), increasingly pushing through their hypothesized northern boundaries (Nawrocki and Hawley 1987). In Canada, Ae. albopictus was consistently reported in southwestern Ontario from 2016 to 2018 (Nelder and Russell 2019; Awuor et al. 2019, WECHU 2018). Ae. albopictus is currently considered to be locally established in this region (M. Nelder and C. Russell, personal communication).
Changes in temperature are expected to be a major driver of changes in geographic ranges because temperature affects the fundamental biological processes of the mosquitoes, including survival and interstadial development rates (and thus life span) and reproduction rates, which determine where and when populations can persist in particular locations (Couret et al. 2014; Dell et al. 2011; Mordecai et al. 2017). The rate of average global temperature change is now increasing more rapidly (IPCC 2018), and since the year 2000 an accelerated warming has been observed globally (Blunden and Arndt 2019). Canada has warmed twice as fast as the rest of the world, and the Canadian North has warmed three times as fast, over the last seven decades (i.e., 1948–2017) (Zhang et al. 2019).
In recent years, several global and regional models have been developed to describe the current ecological niche and possible geographic distribution of Ae. aegypti and Ae. albopictus mosquitoes (Campbell et al. 2015; Ding et al. 2018; Johnson et al. 2017; Kraemer et al. 2015a; Nawrocki and Hawley 1987). The approaches used ranges from defining thermal limits (Nawrocki and Hawley 1987) and temperature suitability indices (Brady et al. 2014) of Ae. aegypti and Ae. albopictus distributions to statistical and machine learning approaches in order to develop global (Campbell et al. 2015; Ding et al. 2018; Kraemer et al. 2015a) and regional (Johnson et al. 2017) ecological niche models of these species. These are useful guides; however, in many instances, a wide range of time periods (e.g., 1960s to 2016) were considered for Aedes mosquitoes occurrence data to be incorporated to the models (Ding et al. 2018; Johnson et al. 2017; Kraemer et al. 2015a). To our knowledge, model-based assessments of future distributions of these Aedes spp. mosquitoes have only been attempted at the global scale (Kraemer et al. 2019; Ryan et al. 2019), at a regional scale for Ae. albopictus up to 2070 (Ogden et al. 2014), and at a local scale in the northeastern United States (Rochlin et al. 2013). In almost all cases, the recent shifts in the climate that could influence the recent changes in the distribution of mosquitoes and regional climatic variability were not addressed. Our objective was to develop regional ecological niche models for Ae. aegypti and Ae. albopictus in the conterminous United States and Canada with current observed and simulated climate and land-use data using boosted regression trees (BRTs). We then used output from an ensemble simulation of regional climate models (RCMs) to project possible changes to the geographic range of Ae. aegypti and Ae. albopictus, and to the human population at risk of Aedes-borne infections, in Canada and the United States from 2011 to 2100.
Methods
Ecological Niche Modeling Approach
To model the ecological niche of the Aedes mosquitoes, we utilized the BRT model, which is a powerful tool for modeling complex nonlinear dependencies, identifying interactions between predictors, and avoiding over-fitting (Elith et al. 2008). We employed the following steps of data manipulation and analyses to predict the ecological niche suitability: a) We compiled a list of the two Aedes species occurrence data from multiple databases from Canada and the United States from 2001 to 2016. b) We compiled climatic and urban land cover data from 2001 to 2016. c) We developed an ecological niche model using BRTs for the current time period (2001–2016) to obtain climatic and urban land cover predictors of the occurrence of the Aedes species to identify the geographic limits of their ecological niches under the current climate. d) We used projected climatic data under moderate (RCP4.5) and high (RCP8.5) emission scenarios from four RCM simulations to project ecological niches for the Aedes species from 2011 to 2100. Finally, e) we developed ensemble ecological niche models from the individual RCM under future climatic conditions.
Aedes Mosquito Vector Occurrence Data
We reviewed the existing Ae. aegypti and Ae. albopictus occurrence databases in Canada and the United States between 2001 and 2016 and identified multiple sources that were credible and comprehensive. These included the data presented in reports by Kraemer et al. (2015b) and Hahn et al. (2016, 2017) in addition to data from the Centers for Disease Control and Prevention ArboNet surveillance system database (https://wwwn.cdc.gov/arbonet/). Data were also obtained from records held in the following institutions: Mevlabs, Inc., U.S. Army Public Health Command Region–North, and Walter Reed Biosystematics Unit (WRAIR, Division of Entomology) accessed through the VectorMap data portal (http://vectormap.si.edu/dataportal.htm) and also from Windsor-Essex County Health unit vector surveillance (WECHU 2017, 2018). We merged the Aedes mosquito occurrence records from multiple sources after de-duplicating records, cross-checking, and georeferencing in ArcGIS™ (version 10.4.1, (Esri®) (see Excel Tables 1.1 and 1.2). To reduce redundancy of the number of reports across the databases, we included only a single reported geographical information system coordinate of mosquito occurrence in a location per year.
Current Climatic and Urban Land Cover Data
We selected the climatic and urban land cover information in the model based on their known influence on the survival, life span, and reproductive rates of Aedes species mosquitoes. The temperature and vegetation index data was acquired from the Moderate Resolution Imaging Spectroradiometer (MODIS) platform, which captures high-resolution land surface temperature data on a daily basis; average precipitation data from the Modern-Era Retrospective analysis for Research and Applications, version 2 (MERRA-2) (Gelaro et al. 2017), which benefits from the integration of recent forecast model updates; and urban land cover data from the Global Rural-Urban Mapping project (GRUMP), version 1.01, which utilized observations of lights at night to assess urbanicity (see Table S1). Average, minimum, and maximum temperature, including the temperature in the coldest month (January), are known to influence survival and reproduction in both species (Brady et al. 2014; Mordecai et al. 2017). An average daily temperature threshold at 10°C has been linked to breeding and survival in Ae. albopictus (Delatte et al. 2009; Kobayashi et al. 2002), whereas an average daily temperature threshold at 20°C has been linked to larval-to-adult survival in Ae. aegypti under experimental conditions (Rueda et al. 1990). Precipitation is a likely possible determinant of the presence of suitable larval habitats for both species, as this may increase water in containers that hold rainwater, which is an breeding habitat for both species (Morrison et al. 2004). Vegetation in combination with precipitation can serve as a proxy for the availability of breeding habitat (Estallo et al. 2008) and for the survival of adult Aedes mosquitoes (Messina et al. 2016; Sota and Mogi 1992). Urbanization, as captured by urban land cover, acts as a proxy for the density of humans, who are one of the primary hosts for Ae. aegypti (Bargielowski et al. 2013). In contrast, Ae. albopictus has a broader host range and is also commonly found in rural and peri-urban areas (Ponlawat and Harrington 2005). Nevertheless, both mosquito vectors have been shown to lay eggs in artificial breeding grounds in urban areas (e.g., containers with stagnant water) (Li et al. 2014).
Projected Climate and Urbanization Land Cover Data
To project the future ecological niche of the Aedes mosquitoes, we included mean, minimum, and maximum daily temperature; mean daily temperature in the coldest month of the year (January); number of days ; number of days ; and average total monthly precipitation from the data simulated by different RCMs under the two Representative Concentration Pathways (RCPs); (van Vuuren et al. 2011) and using model-derived urban land cover expansion data (Angel et al. 2011). The temperature and precipitation data were extracted from four RCMs with simulations using a 0.44° grid mesh (around a grid resolution): CanRCM4-CanESM2, CRCM5-CanESM2, CRCM5-MPI-ESM-LR, and HIRHAM5-EC-EARTH under moderate (RCP4.5) and high (RCP8.5) RCPs from series of simulated data sets of the North America Coordinated Regional climate Downscaling Experiment (CORDEX) project (Mearns et al. 2017) (see Table S2). These models project climate from 2006 to 2100. For each RCM, data from the 2006–2016 period were used to develop baseline BRT models, and which is hereafter referred to as the 2010 time point. Decision trees from the RCM-specific baseline BRT models were then used to predict Aedes species ecological niches for 30-y time (climatological) periods from 2011 to 2100. There were three time points used to illustrate projected changes: a) 2020, as a climatology computed between 2011 and 2040; b) 2050, as a climatology computed between 2041 and 2070; and c) 2080, as a climatology computed between 2071 and 2100.
We calculated estimated urban land cover expansion from 2000 to 2050 using data presented by Angel et al. (2011). We fitted a linear regression over the North American urban land cover data from 2000 to 2050 due to the linear rate of the predicted urban land cover expansion and extrapolated the rate of urban land cover expansion until 2100. We applied this rate of urban expansion to the currently available global urban regional land cover data set from GRUMPv1 (CIESIN et al. 2017) and developed 30-y average urban land cover data for Canada and the United States from 2011 to 2100.
Boosted Regression Trees
We performed an ensemble BRT modeling procedure similar to that reported by Bhatt et al. (2013) and Gilbert et al. (2014) in order to understand the climatic and urban land-use factors influencing the ecological niche of Ae. aegypti and Ae. albopictus mosquitoes and to project their distribution. This modeling approach is particularly useful in assessing complex nonlinear dependencies, identifying interactions between predictors, and avoiding over-fitting. We developed a bootstrapping algorithm that involved the following series of steps:
We created pseudo controls to match Aedes mosquitoes’ occurrence by randomly generating one pseudo control for each occurrence based on the second-order spatial variation of the known distribution of Aedes mosquitoes (Berman and Diggle 1989).
We developed a master data set by extracting the predictor values intersecting the mosquitoes’ presence and pseudo absence occurrence locations at a resolution in ArcGIS™ (version 10.4.1; (Esri®).
We randomly sampled 80% of the data points from the master data set as a training data set for model building with a 10-fold cross validation and utilized the remaining data points for independent evaluation of the model (evaluation data set).
We developed a BRT model using the training data set by using a stepwise procedure to jointly optimize the number of trees in a model, rate of learning, and the tree complexity. We also included a bag function of 0.5 to facilitate stochasticity in the models (Elith et al. 2008). At this stage, we also performed model simplification to identify the minimum set of predictors required for model building (Elith et al. 2008). Through this process, the vegetation index predictor from both models and the urban land cover predictor from the Ae. aegypti model were excluded as least contributing factors.
We validated the model performance using the evaluation data set to assess the area under curve (AUC).
Finally, we generated mosquito distribution maps by repeating Steps c to e over 120 iterations to generate niche distribution of Aedes mosquito vectors and partial dependency plots with means and 95% confidence intervals (CIs) of the relative influence (Friedman 2001) of the most influential predictors in the model.
Once the probability of an ecological niche was defined, we utilized true skill statistics () (Allouche et al. 2006) to identify a threshold cutoff value for the probability distribution to categorically define the presence or absence of ecological niche models. A probability value greater than or equal to the threshold cutoff defined the presence of suitable ecological niche in a location. When both species had a suitable ecological niche on a geographical location, a niche overlap was considered. These steps were utilized for BRT models both for the current and projected climatic scenarios, except the projected climatic model went through a single iteration in the BRT modeling Step f. Additional descriptions on BRT model fitting, simplification, and R codes are in the Supplemental Material in “Section S9.”
The observed and projected climatic data were derived differently; one captured the observed climatic conditions and the latter was derived from RCM-coupled global climate model (CGCM) driven simulation models. This led us to develop a separate base model using simulated climatic conditions and urban land cover data for the current period and under future climatic conditions. We also considered the fact that the simulated climate data for both RCPs (4.5 and 8.5) close to the current timeline were similar because the model inputs (e.g., greenhouse gas concentration) were similar for this short time window close to the current period (van Vuuren et al. 2011). Therefore, we utilized climatic predictors from the four RCMs (RCP4.5 only) to develop BRT models for the time period 2006–2016 (base model), which was the only available projected data close to the observed climate data (2001–2016) used for BRT models to describe current ecological niche. Decision trees from the respective RCMs were used to simulate ecological niches for the two Aedes mosquitoes for the three climatological time windows (2020, 2050, and 2080). For each time period, we used the RCM-specific baseline BRT model’s AUC in the receiver operating characteristics (ROC) score (Breiner et al. 2015) to estimate a weighted ensemble model. The final estimate maps were generated at a resolution of using R (version 3.5.2; R Development Core Team).
Population Living within the Predicted Aedes Niche
We utilized the projected global population grids from 2011 to 2100 (Jones and O’Neill 2016) to estimate the changes in the proportion of population in Canada and the United States living within the geographical regions’ suitable niche for Aedes mosquitoes. We took a conservative approach and utilized the projected population estimated through moderate Shared Socioeconomic Pathways (SSP2) scenarios, which account for demographic factors, urbanization, education, and other factors such as socioeconomic scenarios (Jones and O’Neill 2016). We calculated the proportion of the population living within the predicted Aedes niche corresponding to the time periods and RCPs. Finally, we performed a robust locally weighted nonparametric regression (Cleveland 1981) to estimate the changes in the proportion of the total population living within the projected ecological niche of Aedes mosquitoes.
Results
Aedes Ecological Niches for the Current Time Period (2001–2016)
We identified 341 unique occurrence data points for Ae. aegypti and 2,954 for Ae. albopictus from five databases. Both species were predominantly found in southern and southeastern regions of the United States. Although Ae. aegypti was sparsely distributed, the Ae. albopictus distribution was heavily concentrated within a region extending from the Central states to the East Coast (see Figure S1). One occurrence for Ae. aegypti and one for Ae. albopictus were in Canada (both in Windsor, ON, Canada) (see Figure S1 and Excel Tables S1 and S2 for a list of the Aedes species identified by occurrence year, state/province, and country).
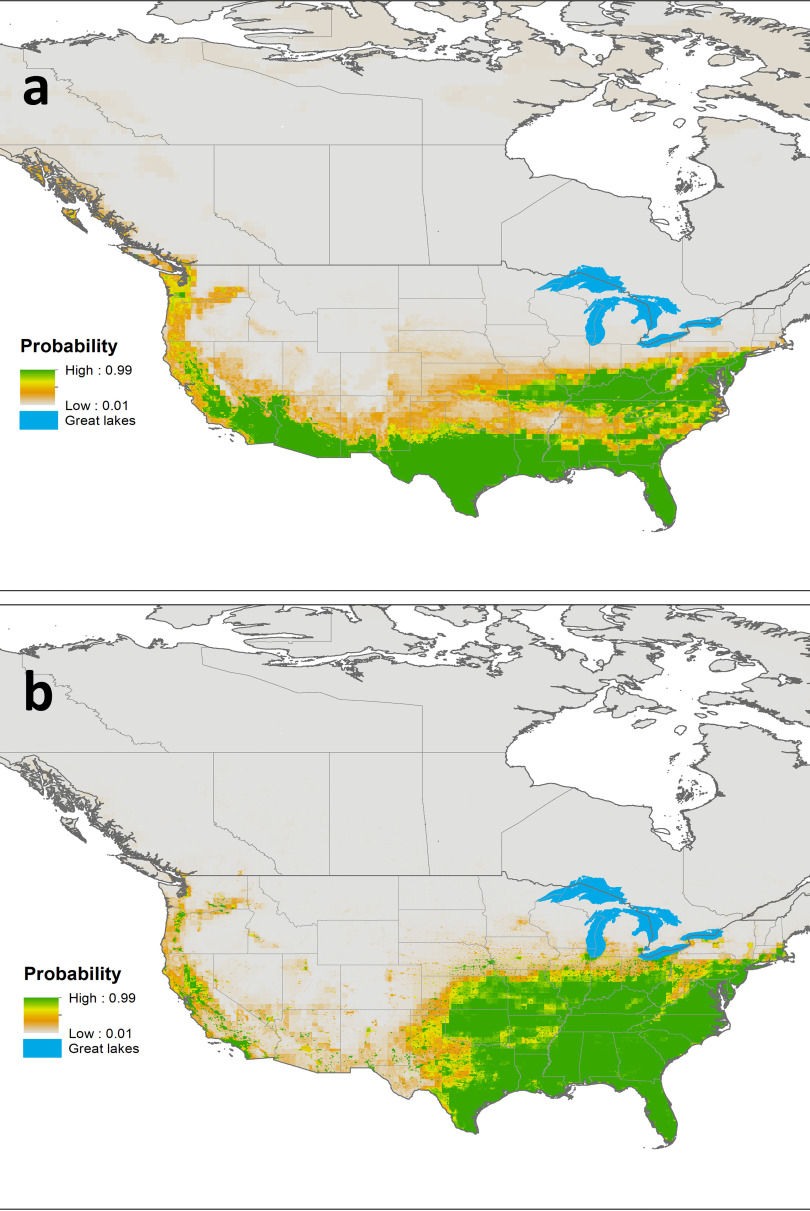
Based on BRTs, the probability of an ecological niche for Ae. aegypti at baseline (2001–2016) was highest in states in the southern and southeastern United States, with a northern boundary from southern New York to Kansas (Figure 1A). In addition, there was a relatively low probability of an ecological niche for Ae. aegypti along the West Coast of Canada and the United States (Figure 1A). The key predictors influencing the niche distribution of Ae. aegypti were average annual minimum daily temperature [relative contribution [ (95% CI: 48.2%, 50.1%)], annual maximum daily temperature [ (95% CI: 12.4%, 13.8%)], and mean daily temperature in January [ (95% CI: 9.7%, 10.2%)]; in combination, these predictors contributed to more than 70% of the regression tree decisions (Tables 1 and 2; see also Figure S2). All three predictors were positively associated with suitability for Ae. aegypti.
Figure 1.
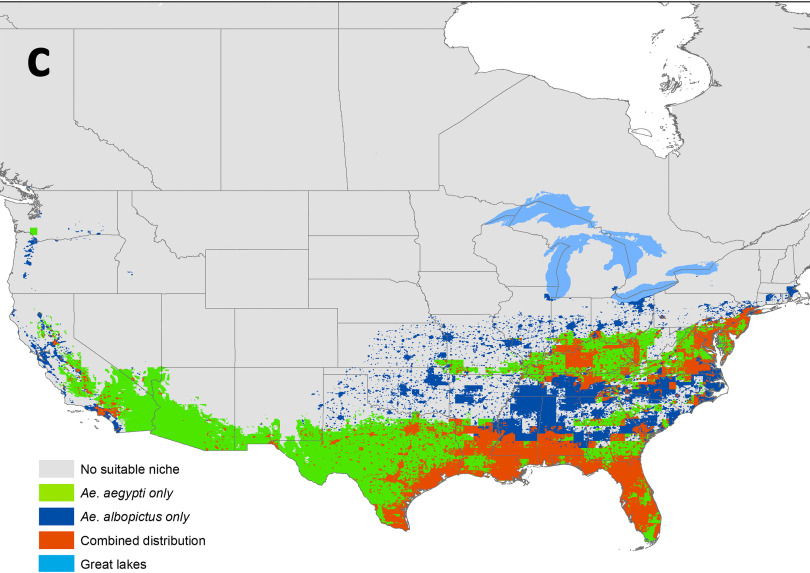
Predicted ecological niche (probability from 0 to 1) for (A) Aedes aegypti and (B) Ae. albopictus mosquitoes, and (C) areas predicted to be an ecological niche for Aedes aegypti [True Skill Statistics (TSS): ], Ae. albopictus (TSS: ), in the continental United States and Canada under current climatic conditions (2001–2016). When both species had a suitable ecological niche in a geographical location, a niche overlap was considered.
Table 1.
Relative contribution (%) of the ecological factors contributing toward predicting the distribution of Aedes aegypti and Ae. albopictus mosquito vectors in Canada and the United States for the time period 2001–2016.
| Climatic and land-use data | Ae. aegypti [mean (95% CI)] | Ae. albopictus [mean (95% CI)] |
|---|---|---|
| Mean minimum daily temperature | 49.2 (48.2, 50.1) | 46.7 (45.8, 47.5) |
| Mean maximum daily temperature | 13.1 (12.4, 13.8) | — |
| Number of days | 3.7 (3.6, 3.8) | 19.6 (18.7, 20.3) |
| Mean daily temperature in January | 10.0 (9.7, 10.2) | 3.6 (3.6, 3.7) |
| Number of days | 9.6 (9.4, 10.3) | 7.9 (7.6, 8.1) |
| Mean total monthly precipitation | 6.0 (5.8, 6.2) | 8.7 (8.6, 8.8) |
| Mean daily temperature | 8.1 (8.0, 8.4) | 5.4 (5.4, 5.5) |
| Urban land cover | — | 8.1 (8.1, 8.2) |
Note: The relative mean contribution and 95% confidence intervals (95% CI) for the covariates in the Aedes spp. models are presented in the respective columns. The covariates that contributed to the model were considered most influential. —, indicates covariate dropout during model simplification process.
Table 2.
Relative contribution (%) of the ecological factors contributing toward predicting the distribution of Aedes aegypti and Ae. albopictus mosquito vectors in Canada and the United States for the time period 2006–2016, using projected climatic data from four regional climatic data models (RCP4.5) and a single boosted regression trees model run.
| Species | Covariates | Regional climatic models | |||
|---|---|---|---|---|---|
| CanRCM4-CanESM2 [RC (%)]a |
CRCM5-CanESM2 [RC (%)]a |
CRCM5-MPI-ESM-LR [RC (%)]a |
HIRHAM5-EC-EARTH [RC (%)]a |
||
| Aedes aegypti | Mean temperature | 8.21 | 51.92 | 20.13 | 30.56 |
| Mean minimum temperature | 3.49 | 2.77 | 8.61 | 36.84 | |
| Mean maximum temperature | 6.41 | 17.16 | 15.45 | 4.48 | |
| Mean January temperature | 43.48 | 11.90 | 13.03 | 0.00 | |
| Number of days | 24.10 | 9.01 | 19.46 | 17.36 | |
| Number of days | 2.56 | 1.63 | 13.69 | 2.11 | |
| Mean precipitation | 4.45 | 1.58 | 3.75 | 3.60 | |
| Urban land cover | 7.34 | 4.03 | 5.87 | 5.04 | |
| Aedes albopictus | Mean temperature | 5.88 | 3.92 | 16.33 | 9.83 |
| Mean minimum temperature | 38.54 | 41.57 | 40.02 | 11.04 | |
| Mean maximum temperature | — | — | — | — | |
| Mean January temperature | 3.41 | 4.14 | 5.33 | 0.00 | |
| Number of days | 34.00 | 33.73 | 19.23 | 52.03 | |
| Number of days | 4.18 | 3.55 | 5.38 | 4.77 | |
| Mean precipitation | 7.47 | 9.11 | 8.67 | 14.36 | |
| Urban land cover | 6.52 | 3.99 | 5.05 | 7.97 | |
Note: The covariates that contributed to the model were considered most influential. —, indicates covariate dropout during model simplification process; RC, relative contribution.
The RC values are rounded to two decimal digits.
The niche for Ae. albopictus extended from southeastern regions of the United States to the south and southwestern borders of Ontario, Canada, and from the East Coast to the Central United States, and sporadically along the West Coast of both Canada and the United States (Figure 1). Primary factors associated with suitability for Ae. albopictus were annual minimum daily temperature [ (95% CI: 45.8%, 47.5%)] and annual average number of days [ (95% CI: 18.7%, 20.3%)]. In addition, the total mean monthly precipitation [ (95% CI: 8.6%, 8.8%)] and urban land cover [ (95% CI: 8.1%, 8.2%)]; all these factors collectively contributed to 83% of the regression tree decisions and were positively associated with suitability for Ae. albopictus (Table 1; see also Figure S3). BRT models validation statistics with external testing data over 120 iterations demonstrated good model fit for both species: Ae. aegypti [ (95% CI: 0.96, 0.97)] and Ae. albopictus [ (95% CI: 0.95, 0.95)].
Figure 1A,B shows a probability distribution between of 0 to 1 for the ecological niche for the two species, and Figure 1C utilized True Skill Statistics to identify a threshold cutoff (see Table S3) for the probability values derived in the models presented in Figure 1A,B to explore ecological niche suitability with a presence–absence indicator and to identify the regions where there could be a niche overlap for both species. The niche overlaps for the mosquitoes were primarily in the southcentral to southeastern states of the United States and sporadically in the southern regions of the Illinois and New York state (Figure 1C). A sparsely distributed niche overlap was also predicted in the southern West Coast (Figure 1C).
Aedes Ecological Niches for the Projected Time Period (2011–2100)
The weighted ensemble models generated from four simulations of four different RCM-CGCM combinations—one RCM used two different boundary conditions from two CGCMs and two other RCMs with each used one CGCM as boundary conditions—had high AUC scores (0.96–0.97 for Ae. albopitus, 0.93–0.98 for Ae. aegypti) and consistency among models (see Table S4). For the simulated climate, the Ae. aegypti models were mostly influenced by mean and maximum daily temperature, temperatures in the coldest month of the year (January), number of days and , minimum daily temperature, and urban land cover. For Ae. albopictus, the models were primarily influenced by minimum daily temperature, number of days , daily mean temperature, total monthly precipitation, and urban land cover (Table 2; see also Figure S4). The ecological niche models using the observed and simulated climatic data for the current time periods (2001–2016 vs. 2006–2016) demonstrated similar distributions for both Aedes species (Figures 1 and 2–5, 2010 panels).
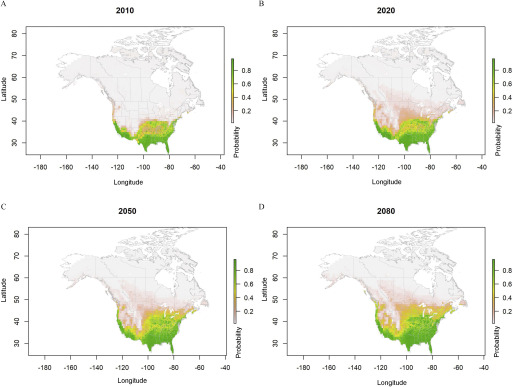
Figure 2.
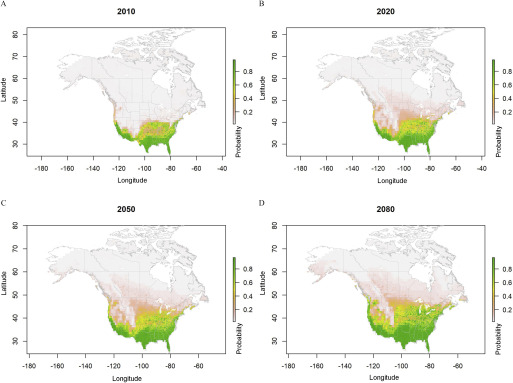
Predicted probabilities for Aedes aegypti ecological niche areas based on ensemble model simulations using four regional climate model data sets (CanRCM4-CanESM2, CRCM5-CanESM2, CRCM5-MPI-ESM-LR, and HIRHAM5-EC-EARTH), under representative concentration pathway (RCP) 4.5 from the year 2006 to 2100. Estimated probabilities shown for (A) 2010, (B) 2020, (C) 2050, and (D) 2080 are climatological conditions averaged over the 2006–2016, 2011–2040, 2041–2070, and 2071–2100 periods, respectively.
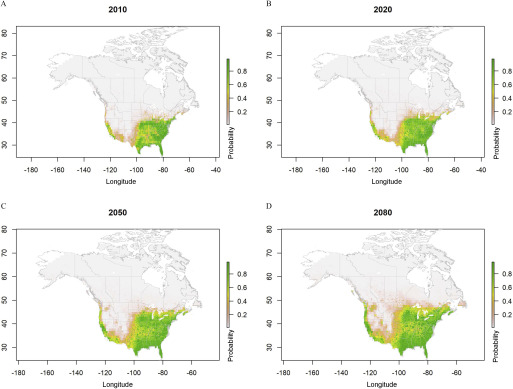
Figure 5.
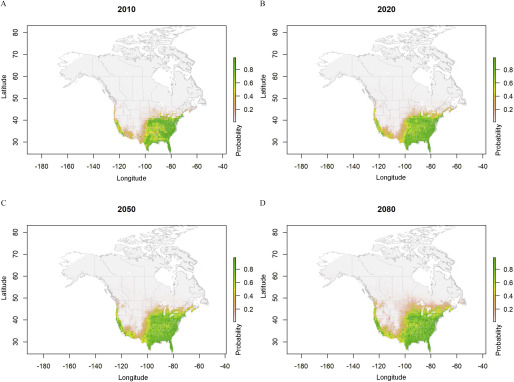
Predicted probabilities for Aedes albopictus ecological niche areas based on ensemble model simulations using four regional climate models data sets (CanRCM4-CanESM2, CRCM5-CanESM2, CRCM5-MPI-ESM-LR, and HIRHAM5-EC-EARTH) under representative concentration pathway (RCP) 8.5, from the year 2006 to 2100. Estimated probabilities shown for (A) 2010, (B) 2020, (C) 2050, and (D) 2080 are climatological conditions averaged over the 2006–2016, 2011–2040, 2041–2070, and 2071–2100 periods, respectively.
Under moderate and high radiative forcing associated with low and high greenhouse gas emissions (respectively, RCP4.5 and RCP8.5), a gradual expansion of suitable niche for both mosquito species in the northern and northeastern parts of the United States reaching the southern provinces of eastern Canada was projected (Figures 2–6). The simulated distribution of the ecological niche of Ae. aegypti under the RCP4.5 scenario expanded to all the coastal regions of the United States bordering Canada and from the southern regions of New York along the East Coast to the Central states by 2100 (Figures 2 and 6). In Canada, the niche expansion was mostly in the province of Ontario, reaching southern Québec by the end of the 21st century and into some locations in British Columbia. Under the high (RCP8.5) radiative forcing scenario for Ae. aegypti, a rapid expansion was predicted during 2020–2050 in the Central states of the United States and to the mid- to northwestern states, shifting from sparse to patchy distribution by the 2080s (Figures 3 and 6).
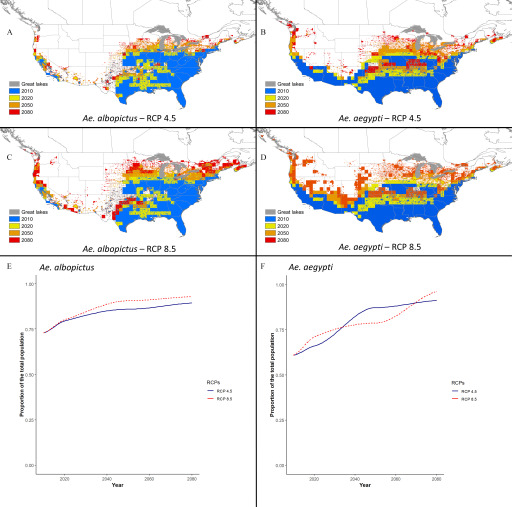
Figure 6.
An overview of the ecological niche expansion of Aedes albopictus and Ae. aegypti mosquitoes from 2006–2100 under moderate (RCP4.5) and high (RCP8.5) representative concentration pathways (RCP) scenarios and the proportion of humans living within the projected ecological niche. (A–D) The ecological niche expansion from 2010 to 2080, with Ae. albopictus, RCP4.5 and RCP8.5, (A and C, respectively) and Ae. aegypti, RCP4.5 and RCP8.5, (B and D, respectively). True Skill Statistics (TSS: for Ae. aegypti, and TSS: for Ae. albopictus) were calculated to determine the cutoffs for the presence of an ecological niche. The 2010 predicted ecological niche was placed as the bottom layer and subsequent additional expansions for the years 2020 to 2080 were stacked on top, the 2080 being the topmost layer. (E) and (F) proportion of total projected human population in the continental United States and Canada living within the projected ecological niche of Aedes mosquitoes [Ae. albopictus (E), Ae. aegypti (F)] from 2010–2100 under the moderate and high RCPs.
Figure 3.
Predicted probabilities for Aedes aegypti ecological niche areas based on ensemble model simulations using four regional climate model data sets (CanRCM4-CanESM2, CRCM5-CanESM2, CRCM5-MPI-ESM-LR, and HIRHAM5-EC-EARTH), under representative concentration pathway (RCP) 8.5, from the year 2006 to 2100. Estimated probabilities shown for (A) 2010, (B) 2020, (C) 2050, and (D) 2080 are climatological conditions averaged over the 2006–2016, 2011–2040, 2041–2070, and 2071–2100 periods, respectively.
The projected distribution of the ecological niche of Ae. albopictus under the RCP4.5 scenario expanded from the current distribution to cover most of the Central to southern regions of the northwestern United States and southern Ontario and Québec in Canada by 2100 (Figures 4 and 6). Under the RCP8.5 scenario and compared with the RCP4.5 scenario, the simulated niche expanded into southern Ontario and Québec by 2080 and was greater in the U.S. Midwest and in southern regions of British Columbia on the Pacific coast (Figures 5 and 6).
Figure 4.
Predicted probabilities for Aedes albopictus ecological niche areas based on ensemble model simulations using four regional climate models data sets (CanRCM4-CanESM2, CRCM5-CanESM2, CRCM5-MPI-ESM-LR, and HIRHAM5-EC-EARTH) under representative concentration pathway (RCP) 4.5, from the year 2006 to 2100. Estimated probabilities shown for (A) 2010, (B) 2020, (C) 2050, and (D) 2080 are climatological conditions averaged over the 2006–2016, 2011–2040, 2041–2070, and 2071–2100 periods, respectively.
Under the RCP4.5 scenario, the percentage of humans living within the projected Ae. aegypti niche in the continental United States and Canada was projected to increase from 66% () in 2020 to 91% () by 2100 (Figure 6F). For Ae. albopictus, the percentage is projected to increase from 79% () in 2020 to 89% () in 2100 (Figure 6E). Compared with the RCP4.5 scenario, the projected populations living within the ecological niche of Aedes mosquitoes were 3% (Ae. albopictus) to 5% (Ae. aegypti) higher under the RCP8.5 scenario (Figure 6E,F).
Discussion
We developed regional ecological niche models for Ae. aegypti and Ae. albopictus mosquitoes in Canada and the United States for up to the year 2100 using current observed and simulated climate from RCMs and urban land cover information. For both species, the predicted ecological niche distribution under the current observed climate during 2001–2016 was primarily influenced by temperature and comprised the south and southeastern regions of the United States. A more northern limit for Ae. albopictus was predicted (Figure 1), as would be expected for this more cold-tolerant species (Nawrocki and Hawley 1987). The predicted ecological niches for Ae. aegypti and Ae. albopictus species based on BRTs models for 2006–2016 demonstrated high accuracy (i.e., AUC values ) and the projected distributions were consistent with previous models (Ding et al. 2018; Johnson et al. 2017; Kraemer et al. 2015a). Although the model using current climatic data was a testing ground for predictor selection and comparing mosquito distribution projections with previously developed simulations, it also enabled us to determine a suitable model structure for the simulated climate data. The utilization of RCMs rather than CGCM simulations allowed us to better explore how regional climatic variations (Di Luca et al. 2013) could influence the two Aedes mosquitoes’ ecological niches under future climatic conditions, rather than using coarse-scale CGCM outputs. Under the projected climates, the degree and direction of projected ecological niche range expansion varied between the species and the RCP scenarios. There was a general trend for northward and northwestern expansion of ecological niches for both species, reaching southcentral regions of Ontario and the southern border of Québec in Canada by 2100. By 2100, a large proportion of the human population in the continental United States and Canada is predicted to be living in the regions where these mosquitoes were projected to have a suitable ecological niche.
The current and simulated ecological niche for Ae. aegypti mosquitoes was mostly influenced by mean, minimum, and maximum daily temperatures and mean temperature in January, with higher temperatures being associated with suitability. Although optimal temperature range plays a substantial role in egg-to-adult survival, permitting local persistence of mosquito populations (Couret et al. 2014; Dell et al. 2011; Mordecai et al. 2017), low winter temperatures prevent Ae. aegypti surviving over winter and will make the difference between the possible occurrence of self-sustaining local populations and transient populations that require reintroduction during summer each year. The phenomenon of likely transient Ae. aegypti populations has been seen in Washington, DC; Maryland; Virginia; and Windsor, Ontario (Eisen and Moore 2013; Lima et al. 2016; WECHU 2017), where the ecological suitability is predicted to be suboptimal for this mosquito, so sporadic observations of the mosquito in these locations may be more consistent with reintroductions in the warmer months. One of the key reasons for this consideration was Ae. aegypti’s poor thermal tolerance: None of the life stages of this species are known to survive below the freezing temperature, even for an overnight duration (Davis 1932). Our findings also demonstrate that once the average minimum daily temperature increased above a threshold of 0°C, the chances of Ae. aegypti’s suitable habitat increased considerably (see Figure S2). This transient population phenomenon has multiple implications: First, with increasing temperatures associated with climate change, the transient Aedes population sites likely mark the regions where mosquito range expansion will occur within near future, and second, surveillance of these areas would be important for monitoring of sporadic autochthonously acquired human cases of diseases transmitted by these mosquito vectors.
Our findings on the current range of the ecological niche of Ae. albopictus was consistent with previous findings on their thermal tolerances. For example, our findings suggest (approximately) to be the lowest thermal limit for Ae. albopictus overwintering (see Figure S3), which has been hypothesized to define the northern limit for this species in North America (Mitchell 1995). Our findings also suggest that additional factors, such as the average number of days , precipitation, and urban land cover are required to ensure egg-to-adult survival, host availability, and ongoing reproduction (Kraemer et al. 2015a; Mordecai et al. 2017). A combined effect of a simulated warming climate on these factors led to projected expansion of Ae. albopictus.
The predicted ecological suitability for the current time period using simulated climate data for the two Aedes species (Figures 2–5, 2010 panels) are consistent with previous studies (Ding et al. 2018; Johnson et al. 2017; Kraemer et al. 2015a; Ogden et al. 2014) and model projections using observed climatic data (Figure 1). However, our projections under climate change scenarios differ from previously published projections with regard to the extent of geographic expansion and the factors that had the greatest influence on predicted expansion (Kraemer et al. 2019; Ryan et al. 2019). The potential differences are primarily due to our use of finer-resolution RCM-based climatic projections using a resolution (Šeparović et al. 2013; Whan and Zwiers 2016), in contrast with previous estimates based on CGCM. For example, the temperature variability due to the effects of topography and land/sea contrasts is not resolved at the scale of a CGCM (Lucas-Picher et al. 2017) using usually a horizontal resolution of around 2° to 3° (1° longitude is approximately ) (Laprise 2008). One key consideration for the CGCM is that climate demonstrates high spatial autocorrelation, resulting in relatively similar characteristics over space. However, when homogeneous surface conditions are encountered, this often leads to an erroneous estimation of the local and regional climatic variations (Šeparović et al. 2013), especially when topographic effects or heterogeneous surface conditions are important forcing factors [see the effects for the precipitation regime over North America in the paper by Diaconescu et al. (2016)]. In comparison with CGCMs, a dynamically downscaled RCM provides a better estimation of the local or regional climate partly because of the higher resolution (Šeparović et al. 2013). Sub-grid–scale features and limitations in the CGCMs could lead to a distorted regional estimation of the predicted ecological conditions of a species and could overinflate the importance of certain climatic parameter contributions in models.
Our study is not without limitations. We utilized four RCM-CGCM simulations and created an ensemble model to minimize variability within individual RCM-CGCM simulations. However, more RCMs could be used for a complete ensemble product given that a matrix of 10–12 models are available from the North American CORDEX archive (Mearns et al. 2017). In addition, higher-resolution grids at 0.22° and 0.1° are available compared with our 0.44° grid. This higher resolution and a more complete matrix of simulations would further capture variability among models and boundary conditions (i.e., CGCMs) to address more precisely a larger range of uncertainties in climate projections and to explore potential robustness or refinement from higher spatial scale information. However, our approach to calculate an ensemble niche model using weighted AUCs of the RCM-specific BRT submodels contribute to addressing uncertainties associated with the individual model outcomes.
Because Ae. aegypti and Ae. albopictus mosquitoes pose a growing public health concern for temperate regions such as the northern United States and southern Canada, models such as those presented here enable us to assess their probable geographical range expansion over time and to identify factors that may contribute to their expansion and the population at risk. Although a high proportion of the continental population are projected to be at risk in the future, socioeconomic conditions in North America and public health infrastructure could limit Aedes spp. mosquito vector outbreaks and impact. On the other hand, the near absence of locally transmitted exotic vector-borne diseases (such as Aedes-borne arbovirus infections) currently in the continental United States and Canada could result in a lack of awareness among the health practitioners and may result in delayed diagnosis, treatment, and outbreak detection (Ng et al. 2019). Studies to assess the impact of non-climatic factors (e.g., socioeconomic conditions, inadequate housing, population-level risk-behaviors such as the degree of outdoor activities) that could be used as a proxy to assess mosquito exposure could help us further quantify the at-risk populations and their potential to predict these mosquito’s ecological distribution.
The presented models have the potential to target vector surveillance in locations of particular interest such as a) locations identified as suitable at present (particularly those at the limits of the predicted mosquito ranges) but where no mosquitoes are currently known to occur, b) locations projected to become suitable in the coming decades, and c) areas where human population densities are high and/or considered particularly vulnerable. Overall, these findings contribute to the development of a rigorous scientific framework to assess the changes in the climatic suitability of mosquito vectors over time and their risk of expansion and subsequent spread that is critical for long-term public health planning in Canada and the United States.
Supplementary Material
Acknowledgments
S.U.K., N.H.O., A.A.F., A.L.G., and V.N. conceptualized the research. S.U.K., N.H.O., A.A.F., P.H.G., G.U.D., A.L.G., and V.N. contributed toward designing the experiment. P.H.G. and G.U.D. pre-organized the projected climatic data sets. S.U.K. analyzed the data, drafted the manuscript and N.H.O., A.A.F., P.H.G., G.U.D., A.L.G., and V.N. reviewed, edited the draft manuscript and consented for final submission.
We thank the Arboviral Disease Branch, Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention for sharing Aedes mosquito occurrence data from the ArboNET database. We acknowledge the World Climate Research Programme’s Working Group on Regional Climate, and the Working Group on Coupled Modelling, former coordinating body of Coordinated Regional climate Downscaling Experiment (CORDEX) and responsible panel for Coupled Model Intercomparison Project Phase 5 (CMIP5 data). We also thank the climate modeling groups for producing and making available their climate model output. We also acknowledge the U.S. Department of Defense Environmental Security Technology Certification Program (ESTCP) for its support of the NA-CORDEX data archive.
Financial support of this work was provided through the Canadian Institutes of Health Research’s (CIHR) Health System Impact Fellowship, with co-funding from the CIHR Institute of Health Services and Policy Research (CIHR-IHSPR), Infection and Immunity (CIHR-III), and the Public Health Agency of Canada.
References
- Allouche O, Tsoar A, Kadmon R. 2006. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43(6):1223–1232, 10.1111/j.1365-2664.2006.01214.x. [DOI] [Google Scholar]
- Angel S, Parent J, Civco DL, Blei A, Potere D. 2011. The dimensions of global urban expansion: estimates and projections for all countries, 2000–2050. Prog Plann 75(2):53–107, 10.1016/j.progress.2011.04.001. [DOI] [Google Scholar]
- Awuor L, Meldrum R, Liberda EN. 2019. Prospects of leveraging an existing mosquito-borne disease surveillance system to monitor other emerging mosquito-borne diseases: a systematic review of West Nile Virus surveillance in Canada (2000–2016). Environ Health Rev 62(3):82–91, 10.5864/d2019-020. [DOI] [Google Scholar]
- Bargielowski IE, Lounibos LP, Carrasquilla MC. 2013. Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proc Natl Acad Sci USA 110(8):2888–2892, PMID: 23359710, 10.1073/pnas.1219599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M, Diggle P. 1989. Estimating weighted integrals of the second‐order intensity of a spatial point process. J R Stat Series B Stat Methodol 51(1):81–92, 10.1111/j.2517-6161.1989.tb01750.x. [DOI] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. 2013. The global distribution and burden of dengue. Nature 496(7446):504–507, PMID: 23563266, 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunden J, Arndt DS. 2019. State of the climate in 2018. Bull Am Meteorol Soc 100(9):Si–S306, 10.1175/2019BAMSStateoftheClimate.1. [DOI] [Google Scholar]
- Bonizzoni M, Gasperi G, Chen X, James AA. 2013. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol 29(9):460–468, PMID: 23916878, 10.1016/j.pt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady OJ, Golding N, Pigott DM, Kraemer MUG, Messina JP, Reiner RC Jr, et al. 2014. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit Vectors 7:338, PMID: 25052008, 10.1186/1756-3305-7-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiner FT, Guisan A, Bergamini A, Nobis MP. 2015. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol Evol 6(10):1210–1218, 10.1111/2041-210X.12403. [DOI] [Google Scholar]
- Campbell LP, Luther C, Moo-Llanes D, Ramsey JM, Danis-Lozano R, Peterson AT. 2015. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos Trans R Soc Lond B Biol Sci 370(1665):20140135, PMID: 25688023, 10.1098/rstb.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIESIN (Center for International Earth Science Information Network), Columbia University, CIDR (CUNY Institute for Demographic Research), IFPRI (International Food Policy Research Institute), World Bank, CIAT (Centro Internacional de Agricultura Tropical). 2017. Global Rural-Urban Mapping Project, version 1 (GRUMPv1): urban extent polygons, revision 01. Palisades, NY: NASA Socioeconomic Data and Applications Center; 10.7927/h4z31wkf [accessed 27 March 2017]. [DOI] [Google Scholar]
- Cleveland WS. 1981. LOWESS: a program for smoothing scatterplots by robust locally weighted regression [TEKST]. Am Stat 35:54, 10.2307/2683591. [DOI] [Google Scholar]
- Couret J, Dotson E, Benedict MQ. 2014. Temperature, larval diet, and density effects on development rate and survival of Aedes aegypti (diptera: Culicidae). PLoS One 9(2):e87468, PMID: 24498328, 10.1371/journal.pone.0087468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NC. 1932. The effects of heat and of cold upon Aëdes (stegomyia) aegypti. Am J Hyg 16:171–191. [Google Scholar]
- Delatte H, Gimonneau G, Triboire A, Fontenille D. 2009. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J Med Entomol 46(1):33–41, PMID: 19198515, 10.1603/033.046.0105. [DOI] [PubMed] [Google Scholar]
- Dell AI, Pawar S, Savage VM. 2011. Systematic variation in the temperature dependence of physiological and ecological traits. Proc Natl Acad Sci USA 108(26):10591–10596, PMID: 21606358, 10.1073/pnas.1015178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Luca A, de Elía R, Laprise R. 2013. Potential for small scale added value of RCM’s downscaled climate change signal. Clim Dyn 40(3–4):601–618, 10.1007/s00382-012-1415-z. [DOI] [Google Scholar]
- Diaconescu EP, Gachon P, Laprise R, Scinocca JF. 2016. Evaluation of precipitation indices over North America from various configurations of regional climate models. Atmosphere Ocean 54(4):418–439, 10.1080/07055900.2016.1185005. [DOI] [Google Scholar]
- Ding F, Fu J, Jiang D, Hao M, Lin G. 2018. Mapping the spatial distribution of Aedes aegypti and Aedes albopictus. Acta Trop 178:155–162, PMID: 29191515, 10.1016/j.actatropica.2017.11.020. [DOI] [PubMed] [Google Scholar]
- Drebot MA, Holloway K, Zheng H, Ogden NH. 2015. Travel-related chikungunya cases in Canada, 2014. Can Commun Dis Rep 41(1):2–5, PMID: 29769913, 10.14745/ccdr.v41i01a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, Moore CG. 2013. Aedes (stegomyia) aegypti in the continental United States: a vector at the cool margin of its geographic range. J Med Entomol 50(3):467–478, PMID: 23802440, 10.1603/me12245. [DOI] [PubMed] [Google Scholar]
- Elith J, Leathwick JR, Hastie T. 2008. A working guide to boosted regression trees. J Anim Ecol 77(4):802–813, PMID: 18397250, 10.1111/j.1365-2656.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- Estallo EL, Lamfri MA, Scavuzzo CM, Almeida FF, Introini MV, Zaidenberg M, et al. 2008. Models for predicting Aedes aegypti larval indices based on satellite images and climatic variables. J Am Mosq Control Assoc 24(3):368–376, PMID: 18939688, 10.2987/5705.1. [DOI] [PubMed] [Google Scholar]
- Friedman JH. 2001. Greedy function approximation: a gradient boosting machine. Ann Stat 29(5):1189–1232, 10.1214/aos/1013203451. [DOI] [Google Scholar]
- Gelaro R, McCarty W, Suárez MJ, Todling R, Molod A, Takacs L, et al. 2017. The Modern-era retrospective analysis for research and applications, version 2 (MERRA-2). J Clim 30(13):5419–5454, PMID: 32020988, 10.1175/JCLI-D-16-0758.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Golding N, Zhou H, Wint GR, Robinson TP, Tatem AJ, et al. 2014. Predicting the risk of avian influenza A H7N9 infection in live-poultry markets across Asia. Nat Commun 5:4116, PMID: 24937647, 10.1038/ncomms5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Eisen RJ, Eisen L, Boegler KA, Moore CG, McAllister J, et al. 2016. Reported distribution of Aedes (stegomyia) aegypti and Aedes (stegomyia) albopictus in the United States, 1995–2016 (diptera: Culicidae). J Med Entomol 53(5):1169–1175, PMID: 27282817, 10.1093/jme/tjw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Eisen L, McAllister J, Savage HM, Mutebi JP, Eisen RJ. 2017. Updated reported distribution of Aedes (stegomyia) aegypti and Aedes (stegomyia) albopictus (diptera: Culicidae) in the United States, 1995–2016. J Med Entomol 54(5):1420–1424, PMID: 28874014, 10.1093/jme/tjx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change). 2018. Summary for policymakers. In: Global Warming of 1.5°C. An IPCC Special Report on the Impacts of Global Warming of 1.5°C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. Masson-Delmotte V, Zhai P, Pörtner HO, Roberts D, Skea J, Shukla PR, et al., eds. Geneva, Switzerland: World Meteorological Organization; https://www.ipcc.ch/sr15/chapter/spm/ [accessed 5 May 2020]. [Google Scholar]
- Johnson TL, Haque U, Monaghan AJ, Eisen L, Hahn MB, Hayden MH, et al. 2017. Modeling the environmental suitability for Aedes (stegomyia) aegypti and Aedes (stegomyia) albopictus (diptera: Culicidae) in the contiguous United States. J Med Entomol 54(6):1605–1614, PMID: 29029153, 10.1093/jme/tjx163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, O’Neill BC. 2016. Spatially explicit global population scenarios consistent with the Shared Socioeconomic Pathways. Environ Res Lett 11(8):084003, 10.1088/1748-9326/11/8/084003. [DOI] [Google Scholar]
- Kendrick K, Stanek D, Blackmore C, Centers for Disease Control and Prevention. 2014. Notes from the field: transmission of chikungunya virus in the continental United States—Florida, 2014. MMWR Morb Mortal Wkly Rep 63(48):1137, PMID: 25474035. [PMC free article] [PubMed] [Google Scholar]
- Khan K, Bogoch I, Brownstein JS, Miniota J, Nicolucci A, Hu W, et al. 2014. Assessing the origin of and potential for international spread of chikungunya virus from the Caribbean. PLoS Curr 6: PMID: 24944846, 10.1371/currents.outbreaks.2134a0a7bf37fd8d388181539fea2da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Nihei N, Kurihara T. 2002. Analysis of northern distribution of Aedes albopictus (diptera: Culicidae) in Japan by geographical information system. J Med Entomol 39(1):4–11, PMID: 11931270, 10.1603/0022-2585-39.1.4. [DOI] [PubMed] [Google Scholar]
- Kraemer MUG, Reiner RC Jr, Brady OJ, Messina JP, Gilbert M, Pigott DM, et al. 2019. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol 4(5):854–863, PMID: 30833735, 10.1038/s41564-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer MUG, Sinka ME, Duda KA, Mylne AQN, Shearer FM, Barker CM, et al. 2015a. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 4:e08347, PMID: 26126267, 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer MUG, Sinka ME, Duda KA, Mylne A, Shearer FM, Brady OJ, et al. 2015b. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci Data 2:150035, PMID: 26175912, 10.1038/sdata.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise R. 2008. Regional climate modelling. J Comput Phys 227(7):3641–3666, 10.1016/j.jcp.2006.10.024. [DOI] [Google Scholar]
- Li Y, Kamara F, Zhou G, Puthiyakunnon S, Li C, Liu Y, et al. 2014. Urbanization increases aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl Trop Dis 8(11):e3301, PMID: 25393814, 10.1371/journal.pntd.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likos A, Griffin I, Bingham AM, Stanek D, Fischer M, White S, et al. 2016. Local mosquito-borne transmission of Zika virus—Miami-Dade and Broward counties, Florida, June–August 2016. MMWR Morb Mortal Wkly Rep 65(38): 1032–1038, PMID: 27684886, 10.15585/mmwr.mm6538e1. [DOI] [PubMed] [Google Scholar]
- Lima A, Lovin DD, Hickner PV, Severson DW. 2016. Evidence for an overwintering population of Aedes aegypti in Capitol Hill neighborhood, Washington, DC. Am J Trop Med Hyg 94(1):231–235, PMID: 26526922, 10.4269/ajtmh.15-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Picher P, Laprise R, Winger K. 2017. Evidence of added value in North American regional climate model hindcast simulations using ever-increasing horizontal resolutions. Clim Dyn 48(7–8):2611–2633, 10.1007/s00382-016-3227-z. [DOI] [Google Scholar]
- Mearns L, McGinnis S, Korytina D, Arritt R, Biner S, Bukovsky M, et al. 2017. The NA-CORDEX dataset, version 1.0. Boulder, CO:NCAR Climate Data Gateway. 10.5065/d6sj1jch [accessed 5 May 2020]. [DOI]
- Medlock JM, Hansford KM, Versteirt V, Cull B, Kampen H, Fontenille D, et al. 2015. An entomological review of invasive mosquitoes in Europe. Bull Entomol Res 105(6):637–663, PMID: 25804287, 10.1017/S0007485315000103. [DOI] [PubMed] [Google Scholar]
- Messina JP, Kraemer MUG, Brady OJ, Pigott DM, Shearer FM, Weiss DJ, et al. 2016. Mapping global environmental suitability for Zika virus. elife 5:e15272, PMID: 27090089, 10.7554/eLife.15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CJ. 1995. Geographic spread of aedes albopictus and potential for involvement in arbovirus cycles in the Mediterranean basin. J Vector Ecol 20:44–58. [Google Scholar]
- Mordecai EA, Cohen JM, Evans MV, Gudapati P, Johnson LR, Lippi CA, et al. 2017. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis 11(4):e0005568, PMID: 28448507, 10.1371/journal.pntd.0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AC, Gray K, Getis A, Astete H, Sihuincha M, Focks D, et al. 2004. Temporal and geographic patterns of Aedes aegypti (diptera: Culicidae) production in Iquitos, Peru. J Med Entomol 41(6):1123–1142, PMID: 15605653, 10.1603/0022-2585-41.6.1123. [DOI] [PubMed] [Google Scholar]
- Nawrocki SJ, Hawley WA. 1987. Estimation of the northern limits of distribution of Aedes albopictus in North America. J Am Mosq Control Assoc 3(2):314–317, PMID: 3504917. [PubMed] [Google Scholar]
- Nelder M, Russell P. 2019. Vector-Borne Diseases, 2018 Summary Report. Public Health Ontario https://www.publichealthontario.ca/-/media/documents/V/2019/vector-borne-diseases-2018.pdf?la=en [accessed 15 December 2019].
- Ng V, Fazil A, Gachon P, Deuymes G, Radojević M, Mascarenhas M, et al. 2017. Assessment of the probability of autochthonous transmission of chikungunya virus in Canada under recent and projected climate change. Environ Health Perspect 125(6):067001, PMID: 28731409, 10.1289/EHP669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng V, Rees EE, Lindsay LR, Drebot MA, Brownstone T, Sadeghieh T, et al. 2019. Could exotic mosquito-borne diseases emerge in Canada with climate change. Can Commun Dis Rep 45(4):98–107, PMID: 31285699, 10.14745/ccdr.v45i04a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH. 2017. Climate change and vector-borne diseases of public health significance. FEMS Microbiol Lett 364(19):fnx186, PMID: 28957457, 10.1093/femsle/fnx186. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Fazil A, Safronetz D, Drebot MA, Wallace J, Rees EE, et al. 2017. Risk of travel-related cases of Zika virus infection is predicted by transmission intensity in outbreak-affected countries. Parasit Vectors 10(1):41, PMID: 28122631, 10.1186/s13071-017-1977-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH, Radojević M, Caminade C, Gachon P. 2014. Recent and projected future climatic suitability of North America for the Asian tiger mosquito Aedes albopictus. Parasit Vectors 7:532, PMID: 25441177, 10.1186/s13071-014-0532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen EE, Staples JE, Meaney-Delman D, Fischer M, Ellington SR, Callaghan WM, et al. 2016. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep 65(2):30–33, PMID: 26796813, 10.15585/mmwr.mm6502e1. [DOI] [PubMed] [Google Scholar]
- Ponlawat A, Harrington LC. 2005. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol 42(5):844–849, PMID: 16363170, 10.1093/jmedent/42.5.844. [DOI] [PubMed] [Google Scholar]
- Ramos MM, Mohammed H, Zielinski-Gutierrez E, Hayden MH, Lopez JLR, Fournier M, et al. 2008. Epidemic dengue and dengue hemorrhagic fever at the Texas–Mexico border: results of a household-based seroepidemiologic survey, December 2005. Am J Trop Med Hyg 78(3):364–369, PMID: 18337327, 10.4269/ajtmh.2008.78.364. [DOI] [PubMed] [Google Scholar]
- Rochlin I, Ninivaggi DV, Hutchinson ML, Farajollahi A. 2013. Climate change and range expansion of the Asian tiger mosquito (Aedes albopictus) in northeastern USA: implications for public health practitioners. PLoS One 8(4):e60874, PMID: 23565282, 10.1371/journal.pone.0060874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda LM, Patel KJ, Axtell RC, Stinner RE. 1990. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (diptera: Culicidae). J Med Entomol 27(5):892–898, PMID: 2231624, 10.1093/jmedent/27.5.892. [DOI] [PubMed] [Google Scholar]
- Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. 2019. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis 13(3):e0007213, PMID: 30921321, 10.1371/journal.pntd.0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner F, Medlock JM, Van Bortel W. 2013. Public health significance of invasive mosquitoes in Europe. Clin Microbiol Infect 19(8):685–692, PMID: 23574618, 10.1111/1469-0691.12189. [DOI] [PubMed] [Google Scholar]
- Šeparović L, Alexandru A, Laprise R, Martynov A, Sushama L, Winger K, et al. 2013. Present climate and climate change over North America as simulated by the fifth-generation Canadian regional climate model. Clim Dyn 41(11–12):3167–3201, 10.1007/s00382-013-1737-5. [DOI] [Google Scholar]
- Sota T, Mogi M. 1992. Interspecific variation in desiccation survival time of Aedes (stegomyia) mosquito eggs is correlated with habitat and egg size. Oecologia 90(3):353–358, PMID: 28313521, 10.1007/BF00317691. [DOI] [PubMed] [Google Scholar]
- Sprenger D, Wuithiranyagool T. 1986. The discovery and distribution of Aedes albopictus in Harris County, Texas. J Am Mosq Control Assoc 2(2):217–219, PMID: 3507493. [PubMed] [Google Scholar]
- van Vuuren DP, Edmonds J, Kainuma M, Riahi K, Thomson A, Hibbard K, et al. 2011. The representative concentration pathways: an overview. Clim Change 109(1–2):5, 10.1007/s10584-011-0148-z. [DOI] [Google Scholar]
- WECHU (Windsor-Essex County Health Unit). 2017. Aedes aegypti mosquito. 2017 enhanced mosquito surveillance. https://www.Wechu.Org/z-health-topics/aedes-aegypti-mosquito [accessed 14 August 2018].
- WECHU. 2018. 2018 adult mosquito surveillance—Ae. albopictus mosquitoes identified. https://www.Wechu.Org/z-health-topics/aedes-albopictus-mosquito [accessed 14 August 2018].
- Whan K, Zwiers F. 2016. Evaluation of extreme rainfall and temperature over North America in CanRCM4 and CRCM5. Clim Dyn 46(11–12):3821–3843, 10.1007/s00382-015-2807-7. [DOI] [Google Scholar]
- WHO (World Health Organization), United Nations Children’s Fund, United Nations Development Programme, World Bank, WHO Special Programme for Research and Training in Tropical Diseases. 2017. Global vector control response 2017–2030. License CC by-NC-SA 3.0 IGO. Geneva, Switzerland: WHO; https://apps.who.int/iris/handle/10665/259205 [accessed 5 May 2020]. [Google Scholar]
- Zhang X, Flato G, Kirchmeier-Young M, Vincent L, Wan H, Wang X, et al. 2019. Changes in temperature and precipitation across Canada. In: Canada’s Changing Climate Report. Bush E, Lemmen DS, eds. Ottawa, ON, Canada: Government of Canada, 112–193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.