Summary
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes COVID-19 and is spread person-to-person through close contact. We aimed to investigate the effects of physical distance, face masks, and eye protection on virus transmission in health-care and non-health-care (eg, community) settings.
Methods
We did a systematic review and meta-analysis to investigate the optimum distance for avoiding person-to-person virus transmission and to assess the use of face masks and eye protection to prevent transmission of viruses. We obtained data for SARS-CoV-2 and the betacoronaviruses that cause severe acute respiratory syndrome, and Middle East respiratory syndrome from 21 standard WHO-specific and COVID-19-specific sources. We searched these data sources from database inception to May 3, 2020, with no restriction by language, for comparative studies and for contextual factors of acceptability, feasibility, resource use, and equity. We screened records, extracted data, and assessed risk of bias in duplicate. We did frequentist and Bayesian meta-analyses and random-effects meta-regressions. We rated the certainty of evidence according to Cochrane methods and the GRADE approach. This study is registered with PROSPERO, CRD42020177047.
Findings
Our search identified 172 observational studies across 16 countries and six continents, with no randomised controlled trials and 44 relevant comparative studies in health-care and non-health-care settings (n=25 697 patients). Transmission of viruses was lower with physical distancing of 1 m or more, compared with a distance of less than 1 m (n=10 736, pooled adjusted odds ratio [aOR] 0·18, 95% CI 0·09 to 0·38; risk difference [RD] −10·2%, 95% CI −11·5 to −7·5; moderate certainty); protection was increased as distance was lengthened (change in relative risk [RR] 2·02 per m; pinteraction=0·041; moderate certainty). Face mask use could result in a large reduction in risk of infection (n=2647; aOR 0·15, 95% CI 0·07 to 0·34, RD −14·3%, −15·9 to −10·7; low certainty), with stronger associations with N95 or similar respirators compared with disposable surgical masks or similar (eg, reusable 12–16-layer cotton masks; pinteraction=0·090; posterior probability >95%, low certainty). Eye protection also was associated with less infection (n=3713; aOR 0·22, 95% CI 0·12 to 0·39, RD −10·6%, 95% CI −12·5 to −7·7; low certainty). Unadjusted studies and subgroup and sensitivity analyses showed similar findings.
Interpretation
The findings of this systematic review and meta-analysis support physical distancing of 1 m or more and provide quantitative estimates for models and contact tracing to inform policy. Optimum use of face masks, respirators, and eye protection in public and health-care settings should be informed by these findings and contextual factors. Robust randomised trials are needed to better inform the evidence for these interventions, but this systematic appraisal of currently best available evidence might inform interim guidance.
Funding
World Health Organization.
Introduction
As of May 28, 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 5·85 million individuals worldwide and caused more than 359 000 deaths.1 Emergency lockdowns have been initiated in countries across the globe, and the effect on health, wellbeing, business, and other aspects of daily life are felt throughout societies and by individuals. With no effective pharmacological interventions or vaccine available in the imminent future, reducing the rate of infection (ie, flattening the curve) is a priority, and prevention of infection is the best approach to achieve this aim.
SARS-CoV-2 spreads person-to-person through close contact and causes COVID-19. It has not been solved if SARS-CoV-2 might spread through aerosols from respiratory droplets; so far, air sampling has found virus RNA in some studies2, 3, 4 but not in others.5, 6, 7, 8 However, finding RNA virus is not necessarily indicative of replication-competent and infection-competent (viable) virus that could be transmissible. The distance from a patient that the virus is infective, and the optimum person-to-person physical distance, is uncertain. For the currently foreseeable future (ie, until a safe and effective vaccine or treatment becomes available), COVID-19 prevention will continue to rely on non-pharmaceutical interventions, including pandemic mitigation in community settings.9 Thus, quantitative assessment of physical distancing is relevant to inform safe interaction and care of patients with SARS-CoV-2 in both health-care and non-health-care settings. The definition of close contact or potentially exposed helps to risk stratify, contact trace, and develop guidance documents, but these definitions differ around the globe.
Research in context.
Evidence before this study
We searched 21 databases and resources from inception to May 3, 2020, with no restriction by language, for studies of any design evaluating physical distancing, face masks, and eye protection to prevent transmission of the viruses that cause COVID-19 and related diseases (eg, severe acute respiratory syndrome [SARS] and Middle East respiratory syndrome [MERS]) between infected individuals and people close to them (eg, household members, caregivers, and health-care workers). Previous related meta-analyses have focused on randomised trials and reported imprecise data for common respiratory viruses such as seasonal influenza, rather than the pandemic and epidemic betacoronaviruses causative of COVID-19 (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]), SARS (SARS-CoV), or MERS (MERS-CoV). Other meta-analyses have focused on interventions in the health-care setting and have not included non-health-care (eg, community) settings. Our search did not retrieve any systematic review of information on physical distancing, face masks, or eye protection to prevent transmission of SARS-CoV-2, SARS-CoV, and MERS-CoV.
Added value of this study
We did a systematic review of 172 observational studies in health-care and non-health-care settings across 16 countries and six continents; 44 comparative studies were included in a meta-analysis, including 25 697 patients with COVID-19, SARS, or MERS. Our findings are, to the best of our knowledge, the first to rapidly synthesise all direct information on COVID-19 and, therefore, provide the best available evidence to inform optimum use of three common and simple interventions to help reduce the rate of infection and inform non-pharmaceutical interventions, including pandemic mitigation in non-health-care settings. Physical distancing of 1 m or more was associated with a much lower risk of infection, as was use of face masks (including N95 respirators or similar and surgical or similar masks [eg, 12–16-layer cotton or gauze masks]) and eye protection (eg, goggles or face shields). Added benefits are likely with even larger physical distances (eg, 2 m or more based on modelling) and might be present with N95 or similar respirators versus medical masks or similar. Across 24 studies in health-care and non-health-care settings of contextual factors to consider when formulating recommendations, most stakeholders found these personal protection strategies acceptable, feasible, and reassuring but noted harms and contextual challenges, including frequent discomfort and facial skin breakdown, high resource use linked with the potential to decrease equity, increased difficulty communicating clearly, and perceived reduced empathy of care providers by those they were caring for.
Implications of all the available evidence
In view of inconsistent guidelines by various organisations based on limited information, our findings provide some clarification and have implications for multiple stakeholders. The risk for infection is highly dependent on distance to the individual infected and the type of face mask and eye protection worn. From a policy and public health perspective, current policies of at least 1 m physical distancing seem to be strongly associated with a large protective effect, and distances of 2 m could be more effective. These data could also facilitate harmonisation of the definition of exposed (eg, within 2 m), which has implications for contact tracing. The quantitative estimates provided here should inform disease-modelling studies, which are important for planning pandemic response efforts. Policy makers around the world should strive to promptly and adequately address equity implications for groups with currently limited access to face masks and eye protection. For health-care workers and administrators, our findings suggest that N95 respirators might be more strongly associated with protection from viral transmission than surgical masks. Both N95 and surgical masks have a stronger association with protection compared with single-layer masks. Eye protection might also add substantial protection. For the general public, evidence shows that physical distancing of more than 1 m is highly effective and that face masks are associated with protection, even in non-health-care settings, with either disposable surgical masks or reusable 12–16-layer cotton ones, although much of this evidence was on mask use within households and among contacts of cases. Eye protection is typically underconsidered and can be effective in community settings. However, no intervention, even when properly used, was associated with complete protection from infection. Other basic measures (eg, hand hygiene) are still needed in addition to physical distancing and use of face masks and eye protection.
To contain widespread infection and to reduce morbidity and mortality among health-care workers and others in contact with potentially infected people, jurisdictions have issued conflicting advice about physical or social distancing. Use of face masks with or without eye protection to achieve additional protection is debated in the mainstream media and by public health authorities, in particular the use of face masks for the general population;10 moreover, optimum use of face masks in health-care settings, which have been used for decades for infection prevention, is facing challenges amid personal protective equipment (PPE) shortages.11
Any recommendations about social or physical distancing, and the use of face masks, should be based on the best available evidence. Evidence has been reviewed for other respiratory viral infections, mainly seasonal influenza,12, 13 but no comprehensive review is available of information on SARS-CoV-2 or related betacoronaviruses that have caused epidemics, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS). We, therefore, systematically reviewed the effect of physical distance, face masks, and eye protection on transmission of SARS-CoV-2, SARS-CoV, and MERS-CoV.
Methods
Search strategy and selection criteria
To inform WHO guidance documents, on March 25, 2020, we did a rapid systematic review.14 We created a large international collaborative and we used Cochrane methods15 and the GRADE approach.16 We prospectively submitted the systematic review protocol for registration on PROSPERO (CRD42020177047; appendix pp 23–29). We have followed PRISMA17 and MOOSE18 reporting guidelines (appendix pp 30–33).
From database inception to May 3, 2020, we searched for studies of any design and in any setting that included patients with WHO-defined confirmed or probable COVID-19, SARS, or MERS, and people in close contact with them, comparing distances between people and COVID-19 infected patients of 1 m or larger with smaller distances, with or without a face mask on the patient, or with or without a face mask, eye protection, or both on the exposed individual. The aim of our systematic review was for quantitative assessment to ascertain the physical distance associated with reduced risk of acquiring infection when caring for an individual infected with SARS-CoV-2, SARS-CoV, or MERS-CoV. Our definition of face masks included surgical masks and N95 respirators, among others; eye protection included visors, faceshields, and goggles, among others.
We searched (up to March 26, 2020) MEDLINE (using the Ovid platform), PubMed, Embase, CINAHL (using the Ovid platform), the Cochrane Library, COVID-19 Open Research Dataset Challenge, COVID-19 Research Database (WHO), Epistemonikos (for relevant systematic reviews addressing MERS and SARS, and its COVID-19 Living Overview of the Evidence platform), EPPI Centre living systematic map of the evidence, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, relevant documents on the websites of governmental and other relevant organisations, reference lists of included papers, and relevant systematic reviews.19, 20 We handsearched (up to May 3, 2020) preprint servers (bioRxiv, medRxiv, and Social Science Research Network First Look) and coronavirus resource centres of The Lancet, JAMA, and N Engl J Med (appendix pp 3–5). We did not limit our search by language. We initially could not obtain three full texts for evaluation, but we obtained them through interlibrary loan or contacting a study author. We did not restrict our search to any quantitative cutoff for distance.
Data collection
We screened titles and abstracts, reviewed full texts, extracted data, and assessed risk of bias by two authors and independently, using standardised prepiloted forms (Covidence; Veritas Health Innovation, Melbourne, VIC, Australia), and we cross-checked screening results using artificial intelligence (Evidence Prime, Hamilton, ON, Canada). We resolved disagreements by consensus. We extracted data for study identifier, study design, setting, population characteristics, intervention and comparator characteristics, quantitative outcomes, source of funding and reported conflicts of interests, ethics approval, study limitations, and other important comments.
Outcomes
Outcomes of interest were risk of transmission (ie, WHO-defined confirmed or probable COVID-19, SARS, or MERS) to people in health-care or non-health-care settings by those infected; hospitalisation; intensive care unit admission; death; time to recovery; adverse effects of interventions; and contextual factors such as acceptability, feasibility, effect on equity, and resource considerations related to the interventions of interest. However, data were only available to analyse intervention effects for transmission and contextual factors. Consistent with WHO, studies generally defined confirmed cases with laboratory confirmation (with or without symptoms) and probable cases with clinical evidence of the respective infection (ie, suspected to be infected) but for whom confirmatory testing either had not yet been done for any reason or was inconclusive.
Data analysis
Our search did not identify any randomised trials of COVID-19, SARS, or MERS. We did a meta-analysis of associations by pooling risk ratios (RRs) or adjusted odds ratios (aORs) depending on availability of these data from observational studies, using DerSimonian and Laird random-effects models. We adjusted for variables including age, sex, and severity of source case; these variables were not the same across studies. Because between-study heterogeneity can be misleadingly large when quantified by I2 during meta-analysis of observational studies,21, 22 we used GRADE guidance to assess between-study heterogeneity.21 Throughout, we present RRs as unadjusted estimates and aORs as adjusted estimates.
We used the Newcastle-Ottawa scale to rate risk of bias for comparative non-randomised studies corresponding to every study's design (cohort or case-control).23, 24 We planned to use the Cochrane Risk of Bias tool 2.0 for randomised trials,25 but our search did not identify any eligible randomised trials. We synthesised data in both narrative and tabular formats. We graded the certainty of evidence using the GRADE approach. We used the GRADEpro app to rate evidence and present it in GRADE evidence profiles and summary of findings tables26, 27 using standardised terms.28, 29
We analysed data for subgroup effects by virus type, intervention (different distances or face mask types), and setting (health care vs non-health care). Among the studies assessing physical distancing measures to prevent viral transmission, the intervention varied (eg, direct physical contact [0 m], 1 m, or 2 m). We, therefore, analysed the effect of distance on the size of the associations by random-effects univariate meta-regressions, using restricted maximum likelihood, and we present mean effects and 95% CIs. We calculated tests for interaction using a minimum of 10 000 Monte Carlo random permutations to avoid spurious findings.30 We formally assessed the credibility of potential effect-modifiers using GRADE guidance.21 We did two sensitivity analyses to test the robustness of our findings. First, we used Bayesian meta-analyses to reinterpret the included studies considering priors derived from the effect point estimate and variance from a meta-analysis of ten randomised trials evaluating face mask use versus no face mask use to prevent influenza-like illness in health-care workers.31 Second, we used Bayesian meta-analyses to reinterpret the efficacy of N95 respirators versus medical masks on preventing influenza-like illness after seasonal viral (mostly influenza) infection.13 For these sensitivity analyses, we used hybrid Metropolis-Hastings and Gibbs sampling, a 10 000 sample burn-in, 40 000 Markov chain Monte Carlo samples, and we tested non-informative and sceptical priors (eg, four time variance)32, 33 to inform mean estimates of effect, 95% credibility intervals (CrIs), and posterior distributions. We used non-informative hyperpriors to estimate statistical heterogeneity. Model convergence was confirmed in all cases with good mixing in visual inspection of trace plots, autocorrelation plots, histograms, and kernel density estimates in all scenarios. Parameters were blocked, leading to acceptance of approximately 50% and efficiency greater than 1% in all cases (typically about 40%). We did analyses using Stata version 14.3.
Role of the funding source
The funder contributed to defining the scope of the review but otherwise had no role in study design and data collection. Data were interpreted and the report drafted and submitted without funder input, but according to contractual agreement, the funder provided review at the time of final publication. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
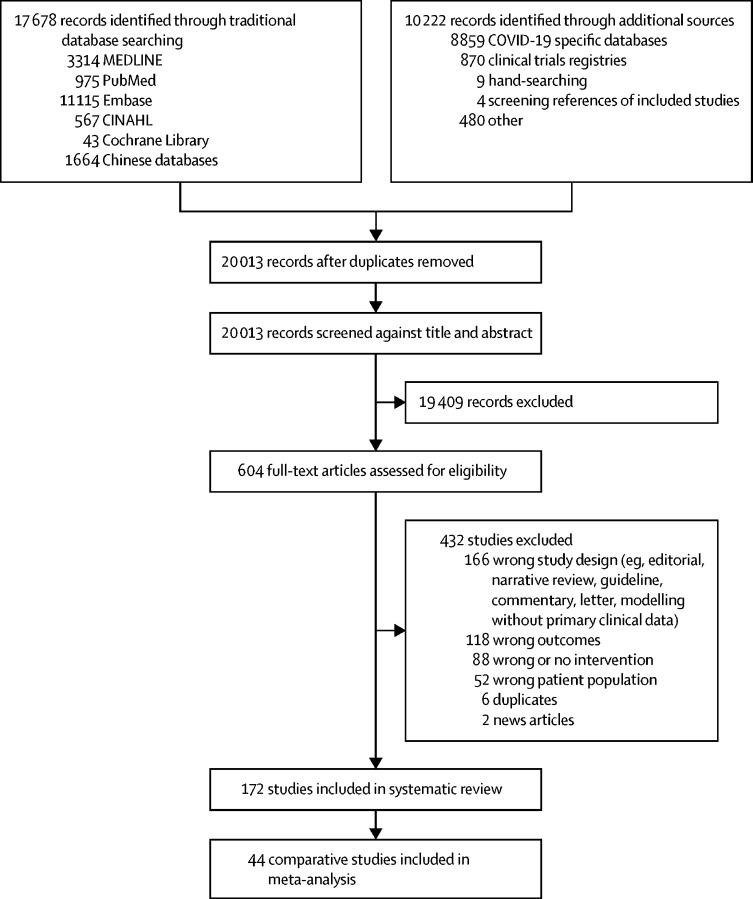
We identified 172 studies for our systematic review from 16 countries across six continents (figure 1; appendix pp 6–14, 41–47). Studies were all observational in nature; no randomised trials were identified of any interventions that directly addressed the included study populations. Of the 172 studies, 66 focused on how far a virus can travel by comparing the association of different distances on virus transmission to people (appendix pp 42–44). Of these 66 studies, five were mechanistic, assessing viral RNA, virions, or both cultured from the environment of an infected patient (appendix p 45).
Figure 1.
Study selection
44 studies were comparative34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77 and fulfilled criteria for our meta-analysis (n=25 697; figure 1; table 1). We used these studies rather than case series and qualitative studies (appendix pp 41–47) to inform estimates of effect. 30 studies34, 37, 41, 42, 43, 44, 45, 47, 48, 49, 50, 51, 53, 54, 55, 56, 58, 59, 60, 61, 64, 65, 66, 67, 68, 69, 70, 72, 74, 75 focused on the association between use of various types of face masks and respirators by health-care workers, patients, or both with virus transmission. 13 studies34, 37, 38, 39, 47, 49, 51, 54, 58, 60, 61, 65, 75 addressed the association of eye protection with virus transmission.
Table 1.
Characteristics of included comparative studies
| n | Country | Setting | Disease caused by virus | Case definition (WHO) | Adjusted estimates | Risk of bias* | |
|---|---|---|---|---|---|---|---|
| Alraddadi et al (2016)34 | 283 | Saudi Arabia | Health care | MERS | Confirmed | Yes | ******** |
| Arwady et al (2016)35 | 79 | Saudi Arabia | Non-health care (household and family contacts) | MERS | Confirmed | No | ****** |
| Bai et al (2020)36 | 118 | China | Health care | COVID-19 | Confirmed | No | ***** |
| Burke et al (2020)37 | 338 | USA | Health care and non-health care (including household and community) | COVID-19 | Confirmed | No | **** |
| Caputo et al (2006)38 | 33 | Canada | Health care | SARS | Confirmed | No | ***** |
| Chen et al (2009)39 | 758 | China | Health care | SARS | Confirmed | Yes | ******* |
| Cheng et al (2020)40 | 226 | China | Non-health care (household and family contacts) | COVID-19 | Confirmed | No | ****** |
| Ha et al (2004)42 | 117 | Vietnam | Health care | SARS | Confirmed | No | ** |
| Hall et al (2014)43 | 48 | Saudi Arabia | Health care | MERS | Confirmed | No | *** |
| Heinzerling et al (2020)44 | 37 | USA | Health care | COVID-19 | Confirmed | No | **** |
| Ho et al (2004)45 | 372 | Taiwan | Health care | SARS | Confirmed | No | ******** |
| Ki et al (2019)47 | 446 | South Korea | Health care | MERS | Confirmed | No | ****** |
| Kim et al (2016)48 | 9 | South Korea | Health care | MERS | Confirmed | No | ***** |
| Kim et al (2016)49 | 1169 | South Korea | Health care | MERS | Confirmed | No | ****** |
| Lau et al (2004)50 | 2270 | China | Non-health care (households) | SARS | Probable | Yes | ****** |
| Liu et al (2009)51 | 477 | China | Health care | SARS | Confirmed | Yes | ***** |
| Liu et al (2020)52 | 20 | China | Non-health care (close contacts) | COVID-19 | Confirmed | No | ******* |
| Loeb et al (2004)53 | 43 | Canada | Health care | SARS | Confirmed | No | ** |
| Ma et al (2004)54 | 426 | China | Health care | SARS | Confirmed | Yes | ********* |
| Nishiura et al (2005)55 | 115 | Vietnam | Health care | SARS | Confirmed | Yes | ******** |
| Nishiyama et al (2008)56 | 146 | Vietnam | Health care | SARS | Confirmed | Yes | ****** |
| Olsen et al (2003)57 | 304 | China | Non-health care (airplane) | SARS | Confirmed | No | ****** |
| Park et al (2004)58 | 110 | USA | Health care | SARS | Confirmed | No | ********** |
| Park et al (2016)59 | 80 | South Korea | Health care | MERS | Confirmed and probable | No | *** |
| Peck et al (2004)60 | 26 | USA | Health care | SARS | Confirmed | No | ********* |
| Pei et al (2006)61 | 443 | China | Health care | SARS | Confirmed | No | ******** |
| Rea et al (2007)62 | 8662 | Canada | Non-health care (community contacts) | SARS | Probable | No | **** |
| Reuss et al (2014)63 | 81 | Germany | Health care | MERS | Confirmed | No | ***** |
| Reynolds et al (2006)64 | 153 | Vietnam | Health care | SARS | Confirmed | No | *** |
| Ryu et al (2019)65 | 34 | South Korea | Health care | MERS | Confirmed | No | ****** |
| Scales et al (2003)66 | 69 | Canada | Health care | SARS | Probable | No | ** |
| Seto et al (2003)67 | 254 | China | Health care | SARS | Confirmed | Yes | ******** |
| Teleman et al (2004)68 | 86 | Singapore | Health care | SARS | Confirmed | Yes | ******** |
| Tuan et al (2007)69 | 212 | Vietnam | Non-health care (household and community contacts) | SARS | Confirmed | Yes | ****** |
| Van Kerkhove et al (2019)46 | 828 | Saudi Arabia | Non-health care (dormitory) | MERS | Confirmed | Yes | ******** |
| Wang et al (2020)41 | 493 | China | Health care | COVID-19 | Confirmed | Yes | **** |
| Wang et al (2020)70 | 5442 | China | Health care | COVID-19 | Confirmed | No | ***** |
| Wiboonchutikul et al (2016)71 | 38 | Thailand | Health care | MERS | Confirmed | No | ***** |
| Wilder-Smith et al (2005)72 | 80 | Singapore | Health care | SARS | Confirmed | No | ******** |
| Wong et al (2004)73 | 66 | China | Health care | SARS | Confirmed | No | ***** |
| Wu et al (2004)74 | 375 | China | Non-health care (community) | SARS | Confirmed | Yes | ******** |
| Yin et al (2004)75 | 257 | China | Health care | SARS | Confirmed | Yes | ****** |
| Yu et al (2005)76 | 74 | China | Health care | SARS | Confirmed | No | ******* |
| Yu et al (2007)77 | 124 wards | China | Health care | SARS | Confirmed | Yes | ******* |
Across studies, mean age was 30–60 years. SARS=severe acute respiratory syndrome. MERS=Middle East respiratory syndrome.
The Newcastle-Ottawa Scale was used for the risk of bias assessment, with more stars equalling lower risk.
Some direct evidence was available for COVID-19 (64 studies, of which seven were comparative in design),36, 37, 40, 41, 44, 52, 70 but most studies reported on SARS (n=55) or MERS (n=25; appendix pp 6–12). Of the 44 comparative studies, 40 included WHO-defined confirmed cases, one included both confirmed and probable cases, and the remaining three studies included probable cases. There was no effect-modification by case-definition (distance pinteraction=0·41; mask pinteraction=0·46; all cases for eye protection were confirmed). Most studies reported on bundled interventions, including different components of PPE and distancing, which was usually addressed by statistical adjustment. The included studies all occurred during recurrent or novel outbreak settings of COVID-19, SARS, or MERS.
Risk of bias was generally low-to-moderate after considering the observational designs (table 1), but both within studies and across studies the overall findings were similar between adjusted and unadjusted estimates. We did not detect strong evidence of publication bias in the body of evidence for any intervention (appendix pp 15–18). As we did not use case series data to inform estimates of effect of each intervention, we did not systematically rate risk of bias of these data. Therefore, we report further only those studies with comparative data.
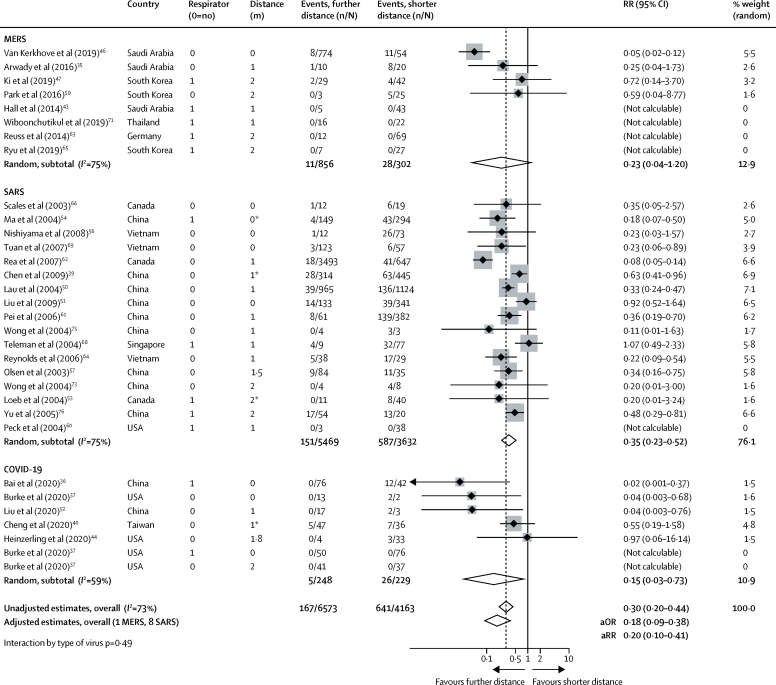
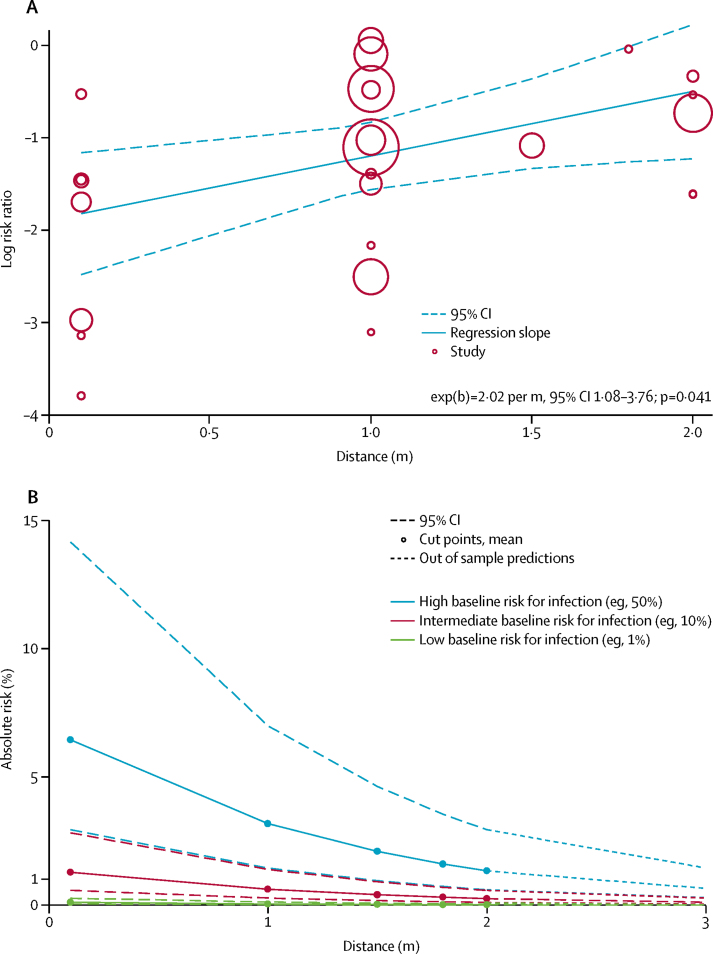
Across 29 unadjusted and nine adjusted studies,35, 36, 37, 39, 40, 43, 44, 46, 47, 50, 51, 52, 53, 54, 56, 57, 59, 60, 61, 62, 63, 64, 65, 66, 68, 69, 71, 73, 76 a strong association was found of proximity of the exposed individual with the risk of infection (unadjusted n=10 736, RR 0·30, 95% CI 0·20 to 0·44; adjusted n=7782, aOR 0·18, 95% CI 0·09 to 0·38; absolute risk [AR] 12·8% with shorter distance vs 2·6% with further distance, risk difference [RD] −10·2%, 95% CI −11·5 to −7·5; moderate certainty; figure 2; table 2; appendix p 16). Although there were six studies on COVID-19, the association was seen irrespective of causative virus (pinteraction=0·49), health-care setting versus non-health-care setting (pinteraction=0·14), and by type of face mask (pinteraction=0·95; appendix pp 17, 19). However, different studies used different distances for the intervention. By meta-regression, the strength of association was larger with increasing distance (2·02 change in RR per m, 95% CI 1·08 to 3·76; pinteraction=0·041; moderate credibility subgroup effect; figure 3A; table 2). AR values with increasing distance given different degrees of baseline risk are shown in figure 3B, with potential values at 3 m also shown.
Figure 2.
Forest plot showing the association of COVID-19, SARS, or MERS exposure proximity with infection
SARS=severe acute respiratory syndrome. MERS=Middle East respiratory syndrome. RR=relative risk. aOR=adjusted odds ratio. aRR=adjusted relative risk. *Estimated values; sensitivity analyses excluding these values did not meaningfully alter findings.
Table 2.
GRADE summary of findings
| Studies and participants | Relative effect (95% CI) |
Anticipated absolute effect (95% CI), eg, chance of viral infection or transmission |
Difference (95% CI) | Certainty* | What happens (standardised GRADE terminology)29 | ||
|---|---|---|---|---|---|---|---|
| Comparison group | Intervention group | ||||||
| Physical distance ≥1 m vs <1 m | Nine adjusted studies (n=7782); 29 unadjusted studies (n=10 736) | aOR 0·18 (0·09 to 0·38); unadjusted RR 0·30 (95% CI 0·20 to 0·44) | Shorter distance, 12·8% | Further distance, 2·6% (1·3 to 5·3) | −10·2% (−11·5 to −7·5) | Moderate† | A physical distance of more than 1 m probably results in a large reduction in virus infection; for every 1 m further away in distancing, the relative effect might increase 2·02 times |
| Face mask vs no face mask | Ten adjusted studies (n=2647); 29 unadjusted studies (n=10 170) | aOR 0·15 (0·07 to 0·34); unadjusted RR 0·34 (95% CI 0·26 to 0·45) | No face mask, 17·4% | Face mask, 3·1% (1·5 to 6·7) | −14·3% (−15·9 to −10·7) | Low‡ | Medical or surgical face masks might result in a large reduction in virus infection; N95 respirators might be associated with a larger reduction in risk compared with surgical or similar masks§ |
| Eye protection (faceshield, goggles) vs no eye protection | 13 unadjusted studies (n=3713) | Unadjusted RR 0·34 (0·22 to 0·52)¶ | No eye protection, 16·0% | Eye protection, 5·5% (3·6 to 8·5) | −10·6% (−12·5 to −7·7) | Low‖ | Eye protection might result in a large reduction in virus infection |
Table based on GRADE approach.26, 27, 28, 29 Population comprised people possibly exposed to individuals infected with SARS-CoV-2, SARS-CoV, or MERS-CoV. Setting was any health-care or non-health-care setting. Outcomes were infection (laboratory-confirmed or probable) and contextual factors. Risk (95% CI) in intervention group is based on assumed risk in comparison group and relative effect (95% CI) of the intervention. All studies were non-randomised and evaluated using the Newcastle-Ottawa Scale; some studies had a higher risk of bias than did others but no important difference was noted in sensitivity analyses excluding studies at higher risk of bias; we did not further rate down for risk of bias. Although there was a high I2 value (which can be exaggerated in non-randomised studies)21 and no overlapping CIs, point estimates generally exceeded the thresholds for large effects and we did not rate down for inconsistency. We did not rate down for indirectness for the association between distance and infection because SARS-CoV-2, SARS-CoV, and MERS-CoV all belong to the same family and have each caused epidemics with sufficient similarity; there was also no convincing statistical evidence of effect-modification across viruses; some studies also used bundled interventions but the studies include only those that provide adjusted estimates. aOR=adjusted odds ratio. RR=relative risk. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. SARS-CoV=severe acute respiratory syndrome coronavirus. MERS-CoV=Middle East respiratory syndrome coronavirus.
GRADE category of evidence; high certainty (we are very confident that the true effect lies close to that of the estimate of the effect); moderate certainty (we are moderately confident in the effect estimate; the true effect is probably close to the estimate, but it is possibly substantially different); low certainty (our confidence in the effect estimate is limited; the true effect could be substantially different from the estimate of the effect); very low certainty (we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect).
The effect is very large considering the thresholds set by GRADE, particularly at plausible levels of baseline risk, which also mitigated concerns about risk of bias; data also suggest a dose–response gradient, with associations increasing from smaller distances to 2 m and beyond, by meta-regression; we did not rate up for this domain alone but it further supports the decision to rate up in combination with the large effects.
The effect was very large, and the certainty of evidence could be rated up, but we made a conservative decision not to because of some inconsistency and risk of bias; hence, although the effect is qualitatively highly certain, the precise quantitative effect is low certainty.
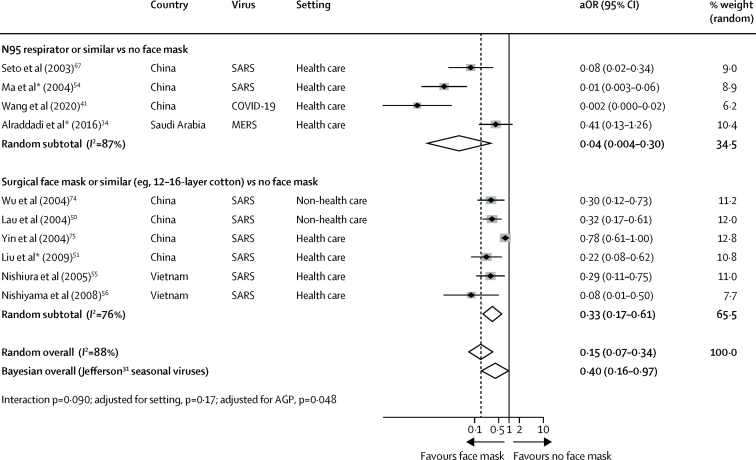
In a subgroup analysis comparing N95 respirators with surgical or similar masks (eg, 12–16-layer cotton), the association was more pronounced in the N95 group (aOR 0·04, 95% CI 0·004–0·30) compared with other masks (0·33, 0·17–0·61; pinteraction=0·090); there was also support for effect-modification by formal analysis of subgroup credibility.
Two studies54, 75 provided adjusted estimates with n=295 in the eye protection group and n=406 in the group not wearing eye protection; results were similar to the unadjusted estimate (aOR 0·22, 95% CI 0·12–0·39).
The effect is large considering the thresholds set by GRADE assuming that ORs translate into similar magnitudes of RR estimates; this mitigates concerns about risk of bias, but we conservatively decided not to rate up for large or very large effects.
Figure 3.
Change in relative risk with increasing distance and absolute risk with increasing distance
Meta-regression of change in relative risk with increasing distance from an infected individual (A). Absolute risk of transmission from an individual infected with SARS-CoV-2, SARS-CoV, or MERS-CoV with varying baseline risk and increasing distance (B). SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. SARS-CoV=severe acute respiratory syndrome coronavirus. MERS-CoV=Middle East respiratory syndrome coronavirus.
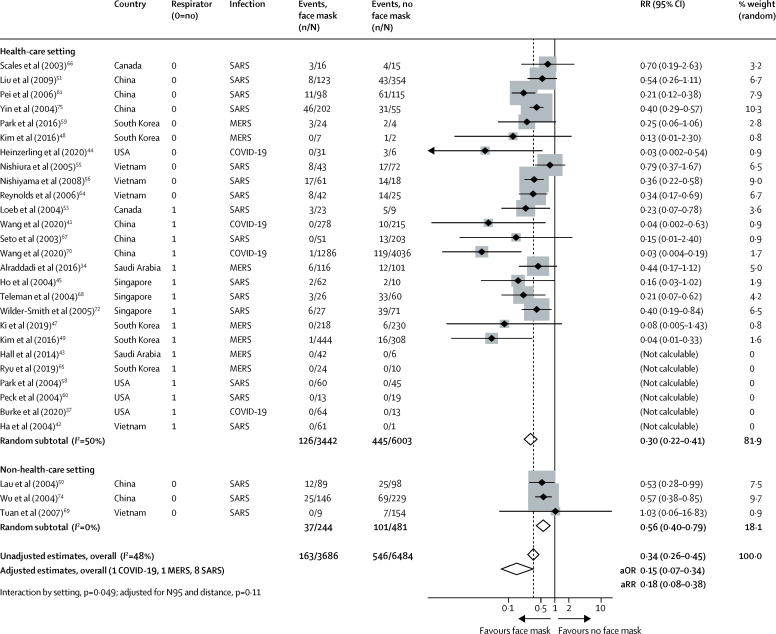
Across 29 unadjusted studies and ten adjusted studies,34, 37, 41, 42, 43, 44, 45, 47, 48, 49, 50, 51, 53, 54, 55, 56, 58, 59, 60, 61, 64, 65, 66, 67, 68, 69, 70, 72, 74, 75 the use of both N95 or similar respirators or face masks (eg, disposable surgical masks or similar reusable 12–16-layer cotton masks) by those exposed to infected individuals was associated with a large reduction in risk of infection (unadjusted n=10 170, RR 0·34, 95% CI 0·26 to 0·45; adjusted studies n=2647, aOR 0·15, 95% CI 0·07 to 0·34; AR 3·1% with face mask vs 17·4% with no face mask, RD −14·3%, 95% CI −15·9 to −10·7; low certainty; figure 4; table 2; appendix pp 16, 18) with stronger associations in health-care settings (RR 0·30, 95% CI 0·22 to 0·41) compared with non-health-care settings (RR 0·56, 95% CI 0·40 to 0·79; pinteraction=0·049; low-to-moderate credibility for subgroup effect; figure 4; appendix p 19). When differential N95 or similar respirator use, which was more frequent in health-care settings than in non-health-care settings, was adjusted for the possibility that face masks were less effective in non-health-care settings, the subgroup effect was slightly less credible (pinteraction=0·11, adjusted for differential respirator use; figure 4). Indeed, the association with protection from infection was more pronounced with N95 or similar respirators (aOR 0·04, 95% CI 0·004 to 0·30) compared with other masks (aOR 0·33, 95% CI 0·17 to 0·61; pinteraction=0·090; moderate credibility subgroup effect; figure 5). The interaction was also seen when additionally adjusting for three studies that clearly reported aerosol-generating procedures (pinteraction=0·048; figure 5). Supportive evidence for this interaction was also seen in within-study comparisons (eg, N95 had a stronger protective association compared with surgical masks or 12–16-layer cotton masks); both N95 and surgical masks also had a stronger association with protection versus single-layer masks.38, 39, 51, 53, 54, 61, 66, 67, 75
Figure 4.
Forest plot showing unadjusted estimates for the association of face mask use with viral infection causing COVID-19, SARS, or MERS
SARS=severe acute respiratory syndrome. MERS=Middle East respiratory syndrome. RR=relative risk. aOR=adjusted odds ratio. aRR=adjusted relative risk.
Figure 5.
Forest plot showing adjusted estimates for the association of face mask use with viral infection causing COVID-19, SARS, or MERS
SARS=severe acute respiratory syndrome. MERS=Middle East respiratory syndrome. RR=relative risk. aOR=adjusted odds ratio. AGP=aerosol-generating procedures. *Studies clearly reporting AGP.
We did a sensitivity analysis to test the robustness of our findings and to integrate all available information on face mask treatment effects for protection from COVID-19. We reconsidered our findings using random-effects Bayesian meta-analysis. Although non-informative priors showed similar results to frequentist approaches (aOR 0·16, 95% CrI 0·04–0·40), even using informative priors from the most recent meta-analysis on the effectiveness of masks versus no masks to prevent influenza-like illness (RR 0·93, 95% CI 0·83–1·05)31 yielded a significant association with protection from COVID-19 (aOR 0·40, 95% CrI 0·16–0·97; posterior probability for RR <1, 98%). Minimally informing (25% influence with or without four-fold smaller mean effect size) the most recent and rigorous meta-analysis of the effectiveness of N95 respirators versus medical masks in randomised trials (OR 0·76, 95% CI 0·54–1·06)13 with the effect-modification seen in this meta-analysis on COVID-19 (ratio of aORs 0·14, 95% CI 0·02–1·05) continued to support a stronger association of protection from COVID-19, SARS, or MERS with N95 or similar respirators versus other face masks (posterior probability for RR <1, 100% and 95%, respectively).
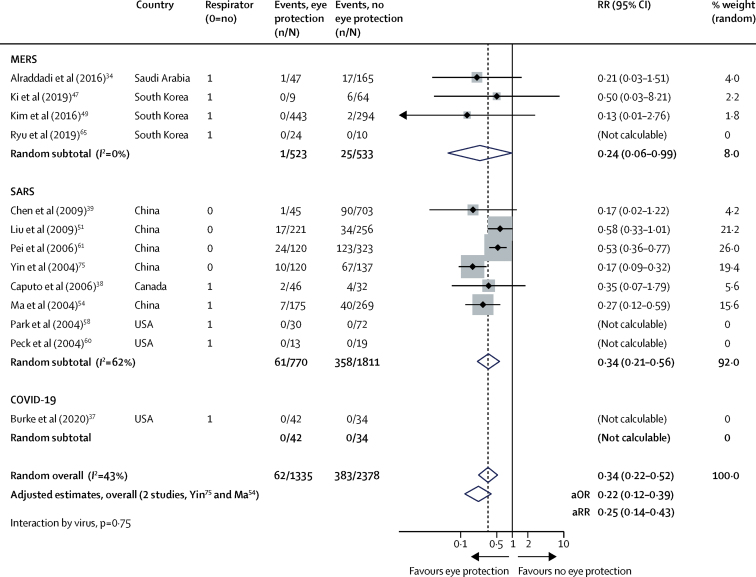
In 13 unadjusted studies and two adjusted studies,34, 37, 38, 39, 47, 49, 51, 54, 58, 60, 61, 65, 75 eye protection was associated with lower risk of infection (unadjusted n=3713, RR 0·34, 95% CI 0·22 to 0·52; AR 5·5% with eye protection vs 16·0% with no eye protection, RD −10·6%, 95% CI −12·5 to −7·7; adjusted n=701, aOR 0·22, 95% CI 0·12 to 0·39; low certainty; figure 6; table 2; appendix pp 16–17).
Figure 6.
Forest plot showing the association of eye protection with risk of COVID-19, SARS, or MERS transmission
Forest plot shows unadjusted estimates. SARS=severe acute respiratory syndrome. MERS=Middle East respiratory syndrome. RR=relative risk. aOR=adjusted odds ratio. aRR=adjusted relative risk.
Across 24 studies in health-care and non-health-care settings during the current pandemic of COVID-19, previous epidemics of SARS and MERS, or in general use, looking at contextual factors to consider in recommendations, most stakeholders found physical distancing and use of face masks and eye protection acceptable, feasible, and reassuring (appendix pp 20–22). However, challenges included frequent discomfort, high resource use linked with potentially decreased equity, less clear communication, and perceived reduced empathy of care providers by those they were caring for.
Discussion
The findings of this systematic review of 172 studies (44 comparative studies; n=25 697 patients) on COVID-19, SARS, and MERS provide the best available evidence that current policies of at least 1 m physical distancing are associated with a large reduction in infection, and distances of 2 m might be more effective. These data also suggest that wearing face masks protects people (both health-care workers and the general public) against infection by these coronaviruses, and that eye protection could confer additional benefit. However, none of these interventions afforded complete protection from infection, and their optimum role might need risk assessment and several contextual considerations. No randomised trials were identified for these interventions in COVID-19, SARS, or MERS.
Previous reviews are limited in that they either have not provided any evidence from COVID-19 or did not use direct evidence from other related emerging epidemic betacoronaviruses (eg, SARS and MERS) to inform the effects of interventions to curtail the current COVID-19 pandemic.13, 19, 31, 78 Previous data from randomised trials are mainly for common respiratory viruses such as seasonal influenza, with a systematic review concluding low certainty of evidence for extrapolating these findings to COVID-19.13 Further, previous syntheses of available randomised controlled trials have not accounted for cluster effects in analyses, leading to substantial imprecision in treatment effect estimates. In between-study and within-study comparisons, we noted a larger effect of N95 or similar respirators compared with other masks. This finding is inconsistent with conclusions of a review of four randomised trials,13 in which low certainty of evidence for no larger effect was suggested. However, in that review, the CIs were wide so a meaningful protective effect could not be excluded. We harmonised these findings with Bayesian approaches, using indirect data from randomised trials to inform posterior estimates. Despite this step, our findings continued to support the ideas not only that masks in general are associated with a large reduction in risk of infection from SARS-CoV-2, SARS-CoV, and MERS-CoV but also that N95 or similar respirators might be associated with a larger degree of protection from viral infection than disposable medical masks or reusable multilayer (12–16-layer) cotton masks. Nevertheless, in view of the limitations of these data, we did not rate the certainty of effect as high.21 Our findings accord with those of a cluster randomised trial showing a potential benefit of continuous N95 respirator use over medical masks against seasonal viral infections.79 Further high-quality research, including randomised trials of the optimum physical distance and the effectiveness of different types of masks in the general population and for health-care workers' protection, is urgently needed. Two trials are registered to better inform the optimum use of face masks for COVID-19 (NCT04296643 [n=576] and NCT04337541 [n=6000]). Until such data are available, our findings represent the current best estimates to inform face mask use to reduce infection from COVID-19. We recognise that there are strong, perhaps opposing, sentiments about policy making during outbreaks. In one viewpoint, the 2007 SARS Commission report stated:
“...recognize, as an aspect of health worker safety, the precautionary principle that reasonable action to reduce risk, such as the use of a fitted N95 respirator, need not await scientific certainty”.80
“...if we do not learn from SARS and we do not make the government fix the problems that remain, we will pay a terrible price in the next pandemic”.81
A counter viewpoint is that the scientific uncertainty and contextual considerations require a more nuanced approach. Although challenging, policy makers must carefully consider these two viewpoints along with our findings.
We found evidence of moderate certainty that current policies of at least 1 m physical distancing are probably associated with a large reduction in infection, and that distances of 2 m might be more effective, as implemented in some countries. We also provide estimates for 3 m. The main benefit of physical distancing measures is to prevent onward transmission and, thereby, reduce the adverse outcomes of SARS-CoV-2 infection. Hence, the results of our current review support the implementation of a policy of physical distancing of at least 1 m and, if feasible, 2 m or more. Our findings also provide robust estimates to inform models and contact tracing used to plan and strategise for pandemic response efforts at multiple levels.
The use of face masks was protective for both health-care workers and people in the community exposed to infection, with both the frequentist and Bayesian analyses lending support to face mask use irrespective of setting. Our unadjusted analyses might, at first impression, suggest use of face masks in the community setting to be less effective than in the health-care setting, but after accounting for differential N95 respirator use between health-care and non-health-care settings, we did not detect any striking differences in effectiveness of face mask use between settings. The credibility of effect-modification across settings was, therefore, low. Wearing face masks was also acceptable and feasible. Policy makers at all levels should, therefore, strive to address equity implications for groups with currently limited access to face masks and eye protection. One concern is that face mask use en masse could divert supplies from people at highest risk for infection.10 Health-care workers are increasingly being asked to ration and reuse PPE,82, 83 leading to calls for government-directed repurposing of manufacturing capacity to overcome mask shortages84 and finding solutions for mask use by the general public.84 In this respect, some of the masks studied in our review were reusable 12–16-layer cotton or gauze masks.51, 54, 61, 75 At the moment, although there is consensus that SARS-CoV-2 mainly spreads through large droplets and contact, debate continues about the role of aerosol,2, 3, 4, 5, 6, 7, 8, 85, 86 but our meta-analysis provides evidence (albeit of low certainty) that respirators might have a stronger protective effect than surgical masks. Biological plausibility would be supported by data for aerosolised SARS-CoV-25, 6, 7, 8 and preclinical data showing seasonal coronavirus RNA detection in fine aerosols during tidal breathing,87 albeit, RNA detection does not necessarily imply replication and infection-competent virus. Nevertheless, our findings suggest it plausible that even in the absence of aerosolisation, respirators might be simply more effective than masks at preventing infection. At present, there is no data to support viable virus in the air outside of aerosol generating procedures from available hospital studies. Other factors such as super-spreading events, the subtype of health-care setting (eg, emergency room, intensive care unit, medical wards, dialysis centre), if aerosolising procedures are done, and environmental factors such as ventilation, might all affect the degree of protection afforded by personal protection strategies, but we did not identify robust data to inform these aspects.
Strengths of our review include adherence to full systematic review methods, which included artificial intelligence-supported dual screening of titles and abstracts, full-text evaluation, assessment of risk of bias, and no limitation by language. We included patients infected with SARS-CoV-2, SARS-CoV, or MERS-CoV and searched relevant data up to May 3, 2020. We followed the GRADE approach16 to rate the certainty of evidence. Finally, we identified and appraise a large body of published work from China, from which much evidence emerged before the pandemic spread to other global regions.
The primary limitation of our study is that all studies were non-randomised, not always fully adjusted, and might suffer from recall and measurement bias (eg, direct contact in some studies might not be measuring near distance). However, unadjusted, adjusted, frequentist, and Bayesian meta-analyses all supported the main findings, and large or very large effects were recorded. Nevertheless, we are cautious not to be overly certain in the precise quantitative estimates of effects, although the qualitative effect and direction is probably of high certainty. Many studies did not provide information on precise distances, and direct contact was equated to 0 m distance; none of the eligible studies quantitatively evaluated whether distances of more than 2 m were more effective, although our meta-regression provides potential predictions for estimates of risk. Few studies assessed the effect of interventions in non-health-care settings, and they primarily evaluated mask use in households or contacts of cases, although beneficial associations were seen across settings. Furthermore, most evidence was from studies that reported on SARS and MERS (n=6674 patients with COVID-19, of 25 697 total), but data from these previous epidemics provide the most direct information for COVID-19 currently. We did not specifically assess the effect of duration of exposure on risk for transmission, although whether or not this variable was judged a risk factor considerably varied across studies, from any duration to a minimum of 1 h. Because of inconsistent reporting, information is limited about whether aerosol-generating procedures were in place in studies using respirators, and whether masks worn by infected patients might alter the effectiveness of each intervention, although the stronger association with N95 or similar respirators over other masks persisted when adjusting for studies reporting aerosol-generating medical procedures. These factors might account for some of the residual statistical heterogeneity seen for some outcomes, albeit I2 is commonly inflated in meta-analyses of observational data,21, 22 and nevertheless the effects seen were large and probably clinically important in all adjusted studies.
Our comprehensive systematic review provides the best available information on three simple and common interventions to combat the immediate threat of COVID-19, while new evidence on pharmacological treatments, vaccines, and other personal protective strategies is being generated. Physical distancing of at least 1 m is strongly associated with protection, but distances of up to 2 m might be more effective. Although direct evidence is limited, the optimum use of face masks, in particular N95 or similar respirators in health-care settings and 12–16-layer cotton or surgical masks in the community, could depend on contextual factors; action is needed at all levels to address the paucity of better evidence. Eye protection might provide additional benefits. Globally collaborative and well conducted studies, including randomised trials, of different personal protective strategies are needed regardless of the challenges, but this systematic appraisal of currently best available evidence could be considered to inform interim guidance.
Acknowledgments
Acknowledgments
This systematic review was commissioned and in part paid for by WHO. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of WHO. We thank Susan L Norris, April Baller, and Benedetta Allegranzi (WHO) for input in the protocol or the final article; Xuan Yu (Evidence Based Medicine Center of Lanzhou University, China), Eliza Poon, and Yuqing (Madison) Zhang for assistance with Chinese literature support; Neera Bhatnagar and Aida Farha (information specialists) for peer-reviewing the search strategy; Artur Nowak (Evidence Prime, Hamilton, ON, Canada) for help with searching and screening using artificial intelligence; and Christine Keng for additional support. DKC is a CAAIF-CSACI-AllerGen Emerging Clinician-Scientist Research Fellow, supported by the Canadian Allergy, Asthma and Immunology Foundation (CAAIF), the Canadian Society of Allergy and Clinical Immunology (CSACI), and AllerGen NCE (the Allergy, Genes and Environment Network).
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
DKC, EAA, SD, KS, SY, and HJS designed the study. SY, SD, KS, and HJS coordinated the study. SY and LH designed and ran the literature search. All authors acquired data, screened records, extracted data, and assessed risk of bias. DKC did statistical analyses. DKC and HJS wrote the report. All authors provided critical conceptual input, analysed and interpreted data, and critically revised the report.
COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors
Argentina—German Hospital of Buenos Aires (Ariel Izcovich); Canada—Cochrane Consumer Executive (Maureen Smith); McMaster University (Mark Loeb, Anisa Hajizadeh, Carlos A Cuello-Garcia, Gian Paolo Morgano, Leila Harrison, Tejan Baldeh, Karla Solo, Tamara Lotfi, Antonio Bognanni, Rosa Stalteri, Thomas Piggott, Yuan Zhang, Stephanie Duda, Derek K Chu, Holger J Schünemann); Southlake Regional Health Centre (Jeffrey Chan); University of British Columbia (David James Harris); Chile—Pontificia Universidad Católica de Chile (Ignacio Neumann); China—Beijing University of Chinese Medicine, Dongzhimen Hospital (Guang Chen); Guangzhou University of Chinese Medicine, The Fourth Clinical Medical College (Chen Chen); China Academy of Chinese Medical Sciences (Hong Zhao); Germany—Finn Schünemann; Italy—Azienda USL–IRCCS di Reggio Emilia (Paolo Giorgi Rossi); Universita Vita-Salute San Raffaele, Milan, Italy (Giovanna Elsa Ute Muti Schünemann); Lebanon—American University of Beirut (Layal Hneiny, Amena El-Harakeh, Fatimah Chamseddine, Joanne Khabsa, Nesrine Rizk, Rayane El-Khoury, Zahra Saad, Sally Yaacoub, Elie A Akl); Rafik Hariri University Hospital (Pierre AbiHanna); Poland—Evidence Prime, Krakow (Anna Bak, Ewa Borowiack); UK—The London School of Hygiene & Tropical Medicine (Marge Reinap); University of Hull (Assem Khamis).
Declaration of interests
ML is an investigator of an ongoing clinical trial on medical masks versus N95 respirators for COVID-19 (NCT04296643). All other authors declare no competing interests.
Contributor Information
Holger J Schünemann, Email: schuneh@mcmaster.ca.
COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors:
Derek K Chu, Elie A Akl, Amena El-harakeh, Antonio Bognanni, Tamara Lotfi, Mark Loeb, Anisa Hajizadeh, Anna Bak, Ariel Izcovich, Carlos A Cuello-Garcia, Chen Chen, David J Harris, Ewa Borowiack, Fatimah Chamseddine, Finn Schünemann, Gian Paolo Morgano, Giovanna E U Muti Schünemann, Guang Chen, Hong Zhao, Ignacio Neumann, Jeffrey Chan, Joanne Khabsa, Layal Hneiny, Leila Harrison, Maureen Smith, Nesrine Rizk, Paolo Giorgi Rossi, Pierre AbiHanna, Rayane El-khoury, Rosa Stalteri, Tejan Baldeh, Thomas Piggott, Yuan Zhang, Zahra Saad, Assem Khamis, Marge Reinap, Stephanie Duda, Karla Solo, Sally Yaacoub, and Holger J Schünemann
Supplementary Material
References
- 1.Worldometer COVID-19 coronavirus pandemic. 2020. https://www.worldometers.info/coronavirus/
- 2.Guo ZD, Wang ZY, Zhang SF. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020 doi: 10.3201/eid2607.200885. published online April 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chia PY, Coleman KK, Tan YK. Detection of air and surface contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in hospital rooms of infected patients. medRxiv. 2020 doi: 10.1101/2020.03.29.20046557. published online April 9. (preprint). [DOI] [Google Scholar]
- 4.Santarpia JL, Rivera DN, Herrera V. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. medRxiv. 2020 doi: 10.1101/2020.03.23.20039446. published online March 26. (preprint). [DOI] [Google Scholar]
- 5.Cheng V, Wong S-C, Chen J. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol. 2020;41:493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong SCY, Kwong RT-S, Wu TC. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. 2020;105:119–127. doi: 10.1016/j.jhin.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faridi S, Niazi S, Sadeghi K. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ong SWX, Tan YK, Chia PY. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qualls N, Levitt A, Kanade N. Community mitigation guidelines to prevent pandemic influenza: United States, 2017. MMWR Recomm Rep. 2017;66:1–34. doi: 10.15585/mmwr.rr6601a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng S, Shen C, Xia N, Song W, Fan M, Cowling BJ. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020;8:434–436. doi: 10.1016/S2213-2600(20)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacIntyre R, Chughtai A, Tham CD, Seale H. COVID-19: should cloth masks be used by healthcare workers as a last resort? April 9, 2020. https://blogs.bmj.com/bmj/2020/04/09/covid-19-should-cloth-masks-be-used-by-healthcare-workers-as-a-last-resort/
- 12.Loeb M, Dafoe N, Mahony J. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 13.Bartoszko JJ, Farooqi MAM, Alhazzani W, Loeb M. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses. 2020 doi: 10.1111/irv.12745. published online April 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schünemann HJ, Moja L. Reviews: rapid! Rapid! Rapid! . . . and systematic. Syst Rev. 2015;4:4. doi: 10.1186/2046-4053-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochrane Training Cochrane handbook for systematic reviews of interventions, version 6. 2019. https://training.cochrane.org/handbook/current
- 16.Guyatt GH, Oxman AD, Vist GE. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19.Jefferson T, Del Mar CB, Dooley L. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;7 doi: 10.1002/14651858.CD006207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Offeddu V, Yung CF, Low MSF, Tam CC. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:1934–1942. doi: 10.1093/cid/cix681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Kunz R. GRADE guidelines, 7: rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Iorio A, Spencer FA, Falavigna M. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 23.Moskalewicz A, Oremus M. No clear choice between Newcastle-Ottawa Scale and Appraisal Tool for Cross-Sectional Studies to assess methodological quality in cross-sectional studies of health-related quality of life and breast cancer. J Clin Epidemiol. 2020;120:94–103. doi: 10.1016/j.jclinepi.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Wells GA, Shea B, O'Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2019. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 25.Sterne JAC, Savović J, Page MJ. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt G, Oxman AD, Akl EA. GRADE guidelines, 1: introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt GH, Thorlund K, Oxman AD. GRADE guidelines, 13: preparing summary of findings tables and evidence profiles—continuous outcomes. J Clin Epidemiol. 2013;66:173–183. doi: 10.1016/j.jclinepi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Santesso N, Carrasco-Labra A, Langendam M. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. J Clin Epidemiol. 2016;74:28–39. doi: 10.1016/j.jclinepi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Santesso N, Glenton C, Dahm P. GRADE guidelines, 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–135. doi: 10.1016/j.jclinepi.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 31.Jefferson T, Jones M, Al Ansari LA. Physical interventions to interrupt or reduce the spread of respiratory viruses, part 1: face masks, eye protection and person distancing—systematic review and meta-analysis. medRxiv. 2020 doi: 10.1101/2020.03.30.20047217. published online April 7. (preprint). [DOI] [Google Scholar]
- 32.Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001;10:277–303. doi: 10.1177/096228020101000404. [DOI] [PubMed] [Google Scholar]
- 33.Goligher EC, Tomlinson G, Hajage D. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA. 2018;320:2251–2259. doi: 10.1001/jama.2018.14276. [DOI] [PubMed] [Google Scholar]
- 34.Alraddadi BM, Al-Salmi HS, Jacobs-Slifka K. Risk factors for Middle East respiratory syndrome coronavirus infection among healthcare personnel. Emerg Infect Dis. 2016;22:1915–1920. doi: 10.3201/eid2211.160920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arwady MA, Alraddadi B, Basler C. Middle East respiratory syndrome coronavirus transmission in extended family, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:1395–1402. doi: 10.3201/eid2208.152015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai Y, Wang X, Huang Q. SARS-CoV-2 infection in health care workers: a retrospective analysis and a model study. medRxiv. 2020 doi: 10.1101/2020.03.29.20047159. published online April 1. (preprint). [DOI] [Google Scholar]
- 37.Burke RM, Balter S, Barnes E. Enhanced contact investigations for nine early travel-related cases of SARS-CoV-2 in the United States. medRxiv. 2020 doi: 10.1101/2020.04.27.20081901. published online May 3. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caputo KM, Byrick R, Chapman MG, Orser BJ, Orser BA. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anaesth. 2006;53:122–129. doi: 10.1007/BF03021815. [DOI] [PubMed] [Google Scholar]
- 39.Chen WQ, Ling WH, Lu CY. Which preventive measures might protect health care workers from SARS? BMC Public Health. 2009;9:81. doi: 10.1186/1471-2458-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng H-Y, Jian S-W, Liu D-P, Ng T-C, Huang W-T, Lin H-H. High transmissibility of COVID-19 near symptom onset. medRxiv. 2020 doi: 10.1101/2020.03.18.20034561. published online March 19. (preprint). [DOI] [Google Scholar]
- 41.Wang X, Pan Z, Cheng Z. Association between 2019-nCoV transmission and N95 respirator use. J Hosp Infect. 2020;105:104–105. doi: 10.1016/j.jhin.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ha LD, Bloom SA, Hien NQ. Lack of SARS transmission among public hospital workers, Vietnam. Emerg Infect Dis. 2004;10:265–268. doi: 10.3201/eid1002.030707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall AJ, Tokars JI, Badreddine SA. Health care worker contact with MERS patient, Saudi Arabia. Emerg Infect Dis. 2014;20:2148–2151. doi: 10.3201/eid2012.141211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heinzerling A, Stuckey MJ, Scheuer T. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient: Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:472–476. doi: 10.15585/mmwr.mm6915e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho KY, Singh KS, Habib AG. Mild illness associated with severe acute respiratory syndrome coronavirus infection: lessons from a prospective seroepidemiologic study of health-care workers in a teaching hospital in Singapore. J Infect Dis. 2004;189:642–647. doi: 10.1086/381558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Kerkhove MD, Alaswad S, Assiri A. Transmissibility of MERS-CoV infection in closed setting, Riyadh, Saudi Arabia, 2015. Emerg Infect Dis J. 2019;25:1802–1809. doi: 10.3201/eid2510.190130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ki HK, Han SK, Son JS, Park SO. Risk of transmission via medical employees and importance of routine infection-prevention policy in a nosocomial outbreak of Middle East respiratory syndrome (MERS): a descriptive analysis from a tertiary care hospital in South Korea. BMC Pulm Med. 2019;19:190. doi: 10.1186/s12890-019-0940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim T, Jung J, Kim SM. Transmission among healthcare worker contacts with a Middle East respiratory syndrome patient in a single Korean centre. Clin Microbiol Infect. 2016;22:e11–e13. doi: 10.1016/j.cmi.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim CJ, Choi WS, Jung Y. Surveillance of the Middle East respiratory syndrome (MERS) coronavirus (CoV) infection in healthcare workers after contact with confirmed MERS patients: incidence and risk factors of MERS-CoV seropositivity. Clin Microbiol Infect. 2016;22:880–886. doi: 10.1016/j.cmi.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lau JTF, Lau M, Kim JH, Tsui HY, Tsang T, Wong TW. Probable secondary infections in households of SARS patients in Hong Kong. Emerg Infect Dis. 2004;10:235–243. doi: 10.3201/eid1002.030626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu W, Tang F, Fang LQ. Risk factors for SARS infection among hospital healthcare workers in Beijing: a case control study. Trop Med Int Health. 2009;14(suppl 1):52–59. [Google Scholar]
- 52.Liu ZQ, Ye Y, Zhang H, Guohong X, Yang J, Wang JL. Analysis of the spatio-temporal characteristics and transmission path of COVID-19 cluster cases in Zhuhai. Trop Geogr. 2020 doi: 10.13284/j.cnki.rddl.003228. published online March 12. [DOI] [Google Scholar]
- 53.Loeb M, McGeer A, Henry B. SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10:251–255. doi: 10.3201/eid1002.030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma HJ, Wang HW, Fang LQ. A case-control study on the risk factors of severe acute respiratory syndromes among health care workers. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:741–744. (in Chinese). [PubMed] [Google Scholar]
- 55.Nishiura H, Kuratsuji T, Quy T. Rapid awareness and transmission of severe acute respiratory syndrome in Hanoi French Hospital, Vietnam. Am J Trop Med Hyg. 2005;73:17–25. [PubMed] [Google Scholar]
- 56.Nishiyama A, Wakasugi N, Kirikae T. Risk factors for SARS infection within hospitals in Hanoi, Vietnam. Jpn J Infect Dis. 2008;61:388–390. [PubMed] [Google Scholar]
- 57.Olsen SJ, Chang HL, Cheung TY. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349:2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- 58.Park BJ, Peck AJ, Kuehnert MJ. Lack of SARS transmission among healthcare workers, United States. Emerg Infect Dis. 2004;10:244–248. doi: 10.3201/eid1002.030793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park JY, Kim BJ, Chung KH, Hwang YI. Factors associated with transmission of Middle East respiratory syndrome among Korean healthcare workers: infection control via extended healthcare contact management in a secondary outbreak hospital. Respirology. 2016;21(suppl 3):89. (abstr APSR6-0642). [Google Scholar]
- 60.Peck AJ, Newbern EC, Feikin DR. Lack of SARS transmission and U.S. SARS case-patient. Emerg Infect Dis. 2004;10:217–224. doi: 10.3201/eid1002.030746. [DOI] [PubMed] [Google Scholar]
- 61.Pei LY, Gao ZC, Yang Z. Investigation of the influencing factors on severe acute respiratory syndrome among health care workers. Beijing Da Xue Xue Bao Yi Xue Ban. 2006;38:271–275. [PubMed] [Google Scholar]
- 62.Rea E, Laflèche J, Stalker S. Duration and distance of exposure are important predictors of transmission among community contacts of Ontario SARS cases. Epidemiol Infect. 2007;135:914–921. doi: 10.1017/S0950268806007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reuss A, Litterst A, Drosten C. Contact investigation for imported case of Middle East respiratory syndrome, Germany. Emerg Infect Dis. 2014;20:620–625. doi: 10.3201/eid2004.131375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reynolds MG, Anh BH, Thu VH. Factors associated with nosocomial SARS-CoV transmission among healthcare workers in Hanoi, Vietnam, 2003. BMC Public Health. 2006;6:207. doi: 10.1186/1471-2458-6-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryu B, Cho SI, Oh MD. Seroprevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) in public health workers responding to a MERS outbreak in Seoul, Republic of Korea, in 2015. Western Pac Surveill Response J. 2019;10:46–48. doi: 10.5365/wpsar.2018.9.3.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scales DC, Green K, Chan AK. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerg Infect Dis. 2003;9:1205–1210. doi: 10.3201/eid0910.030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seto WH, Tsang D, Yung RWH. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1519–1520. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teleman MD, Boudville IC, Heng BH, Zhu D, Leo YS. Factors associated with transmission of severe acute respiratory syndrome among health-care workers in Singapore. Epidemiol Infect. 2004;132:797–803. doi: 10.1017/s0950268804002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tuan PA, Horby P, Dinh PN. SARS transmission in Vietnam outside of the health-care setting. Epidemiol Infect. 2007;135:392–401. doi: 10.1017/S0950268806006996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Q, Huang X, Bai Y. Epidemiological characteristics of COVID-19 in medical staff members of neurosurgery departments in Hubei province: a multicentre descriptive study. medRxiv. 2020 doi: 10.1101/2020.04.20.20064899. published online April 24. (preprint). [DOI] [Google Scholar]
- 71.Wiboonchutikul S, Manosuthi W, Likanonsakul S. Lack of transmission among healthcare workers in contact with a case of Middle East respiratory syndrome coronavirus infection in Thailand. Antimicrob Resist Infect Control. 2016;5:21. doi: 10.1186/s13756-016-0120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilder-Smith A, Teleman MD, Heng BH, Earnest A, Ling AE, Leo YS. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg Infect Dis. 2005;11:1142–1145. doi: 10.3201/eid1107.041165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong TW, Lee CK, Tam W. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis. 2004;10:269–276. doi: 10.3201/eid1002.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu J, Xu F, Zhou W. Risk factors for SARS among persons without known contact with SARS patients, Beijing, China. Emerg Infect Dis. 2004;10:210–216. doi: 10.3201/eid1002.030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yin WW, Gao LD, Lin WS. Effectiveness of personal protective measures in prevention of nosocomial transmission of severe acute respiratory syndrome. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:18–22. [PubMed] [Google Scholar]
- 76.Yu ITS, Wong TW, Chiu YL, Lee N, Li Y. Temporal-spatial analysis of severe acute respiratory syndrome among hospital inpatients. Clin Infect Dis. 2005;40:1237–1243. doi: 10.1086/428735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu IT, Xie ZH, Tsoi KK. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin Infect Dis. 2007;44:1017–1025. doi: 10.1086/512819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verbeek JH, Rajamaki B, Ijaz S. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2019;7 doi: 10.1002/14651858.CD011621.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MacIntyre CR, Wang Q, Seale H. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am J Respir Crit Care Med. 2013;187:960–966. doi: 10.1164/rccm.201207-1164OC. [DOI] [PubMed] [Google Scholar]
- 80.Campbell A. Chapter eight: it's not about the mask: SARS Commission final report, volume 3. December, 2006. http://www.archives.gov.on.ca/en/e_records/sars/report/v3-pdf/Vol3Chp8.pdf
- 81.Webster P. Ontario issues final SARS Commission report. Lancet. 2007;369:264. doi: 10.1016/S0140-6736(07)60130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rimmer A. COVID-19: experts question guidance to reuse PPE. BMJ. 2020;369 doi: 10.1136/bmj.m1577. [DOI] [PubMed] [Google Scholar]
- 83.Mackenzie D. Reuse of N95 masks. Engineering. 2020 doi: 10.1016/j.eng.2020.04.003. published online April 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Greenhalgh T, Schmid MB, Czypionka T, Bassler D, Gruer L. Face masks for the public during the covid-19 crisis. BMJ. 2020;369 doi: 10.1136/bmj.m1435. [DOI] [PubMed] [Google Scholar]
- 85.Bahl P, Doolan C, de Silva C, Chughtai AA, Bourouiba L, MacIntyre CR. Airborne or droplet precautions for health workers treating coronavirus disease 2019? J Infect Dis. 2020 doi: 10.1093/infdis/jiaa189. published online April 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schünemann HJ, Khabsa J, Solo K. Ventilation techniques and risk for transmission of coronavirus disease, including COVID-19: a living systematic review of multiple streams of evidence. Ann Intern Med. 2020 doi: 10.7326/M20-2306. published online May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leung NHL, Chu DKW, Shiu EYC. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.