Abstract
Aging is a degenerative, biological, time-dependent, universally conserved process thus designed as one of the highest known risk factors for morbidity and mortality. Every individual has its own aging mechanisms as both environmental conditions (75%) and genetics (25%) account for aging. Several theories have been proposed until now but not even a single theory solves this mystery. There are still some queries un-answered to the scientific community regarding mechanisms behind aging. However, oxidative stress theory (OST) is considered one of the famous theories that sees mitochondria as one of the leading organelles which largely contribute to the aging process. Many reactive oxygen species (ROS) are produced endogenously and exogenously that are associated with aging. But the mitochondrial ROS contribute largely to the aging process as mitochondrial dysfunction due to oxidative stress is considered one of the contributors toward aging. Although ROS is known to damage cell machinery, new evidence suggests their role in signal transduction to regulate biological and physiological processes. Moreover, besides mitochondria, other important cell organelles such as peroxisome and endoplasmic reticulum also produce ROS that contribute to aging. However, nature has provided humans with free radical scavengers called antioxidants that protect from harmful effects of ROS. Future predictions regarding aging, biochemical mechanisms involved, biomarkers internal and external factors can be easily done with machine learning algorithms and other computational models. This review explains important aspects of aging, the contribution of ROS producing organelles in aging, importance of antioxidants fighting against ROS, different computational models developed to understand the complexities of the aging.
Keywords: Biological sciences, Bioinformatics, Biochemistry, Pharmaceutical science, Reactive oxygen species, Antioxidants, Mitochondria, Computer models
Biological sciences; Bioinformatics; Biochemistry; Pharmaceutical science; Reactive oxygen species; Antioxidants; Mitochondria; Computer models.
1. Introduction
Aging is a biological, degenerative process. It progresses slowly and is much more complicated to be measured quantitatively. Aging results in functional decline of organisms such as physiological functions with time and hence chances of death and disease rate are increased (Lee and Wei, 2012; Labat-Robert and Robert, 2015; Kauppila et al., 2017).
The increased rate of life expectancy is a consequence of the availability of treatments and better life quality conditions (Suzman et al., 2015). Numerous chronic and non-communicable diseases are responsible for disability and death worldwide (Szentesi et al., 2019).
The average life span for a healthy person is 80 years and aging leads to mortality and pathophysiological conditions (Theurey and Pizzo, 2018). Muhammad ibn Yusuf al-Harawi in 1532 conducted the first documented study on aging in his book “Ainul Hayat”. Nearly 5 centuries have passed, but the cause and mechanism behind aging are still nebulous (Peng et al., 2014). The challenging reality of this century is global aging. According to one study, about 576 million people having 65 years of age and above were present in 2010 (Chaves et al., 2017). By the year 2050, this number will become 1.5 billion (Lara, 2018). Throughout mankind history, aging that is a time-dependent functional decline has provoked excited imagination and curiosity. Hence, to date, the most unanswered scientific question is the biological basis of aging (López-Otín et al., 2013; Jang et al., 2018).
Even though among all organisms, aging is relatively a universally conserved process, but the basic molecular mechanisms remain widely ambiguous (Cui et al., 2012). The activity theory that is an influential early theory reported the definition of successful aging as the maintenance of attitudes and activities of younger and middle age as long as possible (Nyberg and Pudas, 2019). Another study defines "successful aging" as key ideas including longevity, satisfaction, mastery and growth, less disability, independence and energetic life style (Martin et al., 2014).
Process of getting old is quasi-programmed, a continued program that is never turned off. A quasi-program can be defined as a developmental program that continues purposelessly and after completion that would never switch off. And this is what the evolutionary theory predicts. Damage does not cause aging, in fact, aging causes damage, hence the process of aging is not driven by damage (Blagosklonny, 2008). Human body functions are deteriorated by aging, causing diseases and progressive decline to resist stress (Davalli et al., 2016).
According to Biogerontologists, both genetics and environmental conditions account for aging, that can be estimated by the fact that due to genetics 25% individuals get older while 75% individuals are accounted by environmental conditions, that include behavioral patterns (Fernández-Ballesteros et al., 2013). Although some of the mechanisms and aspects of aging involve gene-related processes, however aging is not linked with genes (Labat-Robert and Robert, 2015). Thus, aging is a lengthy procedure attributed to personal and behavioral events and socio-environmental conditions, other than genes. Two factors determine the individual's capacity for aging well-healthy and active: behavioral selections and decisions taken (Fernández-Ballesteros et al., 2013; Labat-Robert and Robert, 2015).
2. Aging theories
Indeed, above 300 theories including many mechanistic and evolutionary theories have been proposed by the scientific community to explain why and how living organisms age and the driving force behind aging, but not even a single theory has been proved to be universally applicative (Pomatto and Davies, 2018). For instance, according to the "somatic mutation" theory, somatic mutation and increased DNA damage largely account for aging, while "telomere loss" theory suggests that with age cellular division capacity associated with progressive telomeres shortening in somatic tissues is decreased. However, the "altered proteins and waste accumulation" theory postulates about association of certain factors with some age-linked ailments, like protein turnover being indispensable to conserve cellular function and accumulation of altered proteins and damaged proteins over time (Theurey and Pizzo, 2018).
Hence, it is considered from all aging theories, from programmed cell death to 'wear-and-tear', that ROS (reactive oxygen species) or free radicals account for age development. The message for research of aging would read and summarized precisely and shrewdly as: It's the free radicals” (Blagosklonny, 2008).
Free radical theory of aging is extremely common concept based on work by Gerschman et al. (1954). They observed that oxygen toxicity is caused by free oxygen radicals. It is assumed that "aging caused by free radicals that produce harmful side attacks on connective tissues and cell components". However, free radical's production normally occurs during cellular metabolism. Therefore, the idea sparked from Harman's theory that cellular damage could be mitigated by the complete removal of so-called harmful molecules, as a consequence, slowing the overall process of aging (Pomatto and Davies, 2018). Unfortunately, skin is the only organ in human body where this theory seems true. The comparison among different organs regarding ROS load indicates that skin contains a higher ROS load as compared to other organs, also in many cases, a clear correlation can be found between a pro-aging effect and the origin of ROS from internal and external insults. The fact that distinguished this organ is that like intrinsic aging, extrinsic aging is also, important (Rinnerthaler et al., 2015). Environmental influences such as ultraviolet (UV) radiation exposure that contributes up to 80% to skin aging defines extrinsic type whereas the corporal changes and genetic factors elaborate the intrinsic aging that happens during normal aging process (Farage et al., 2008; Ferri et al., 2019).
According report stated that free radical theory is not applicable and hence this theory was dismissed in 2014. The argument that support this statement is the participation of ROS to the aging process occurs by the assist of cell's metabolic organization, individual's genotype and protective systems. Hence, as by-products, ROS cause cellular damage (Carocho et al., 2019). So, a new theory “a free radical theory of frailty” was defined that damage due to oxidation associates with both sequential aging and frailty. Frailty is a geriatric perception in which there is the appearance of certain factors such as lack of feeling wellbeing, lesser speed of walking, unplanned loss in body weight, comparatively less grip power, and problems in standing in an older person (Viña, 2019).
Another study indicated that the free radical theory of aging must be modified with the argument that, though oxidants have adverse effects, but they are also involved in signaling pathways and have a role in cellular senescence. Hence, according to this "Organisms age as accumulated ROS dependent damage over time in cells” to “Organisms age as accumulated damage and senescent characteristics, that are oxidants’-dependent, over time in cells” (Clement and Luo, 2019).
Recently, a new concept termed "hormesis," has emerged. According to this concept, the cell response can be improved by low doses of a stressor for an extra damaging state. This may enhance cellular fitness and lifespan. Due to activation homeostatic responses, decreased ROS levels may be advantageous, but damage or aging may occur due to its disproportional augmentation (Barbosa et al., 2018).
2.1. Oxidative stress
Oxidative damage means the accumulation of free radicals due to free radical's over-production that cannot be processed gradually or because of less availability of antioxidants. It leads to a wide range of random and indiscriminate biomolecular damage (Simioni et al., 2018). Term “oxidative stress” was first used in the 1970s & 1980s, for various deleterious processes. However, it was later defined as antioxidants and oxidants imbalance in favor of the oxidants, which potentially leads to deterioration as shown in Figure 1 (Weidinger and Kozlov, 2015). Oxidative stress occurs when the antioxidant buffering capacity is less than the production of pro-oxidant compounds such as ROS (Czerska et al., 2015; Pisoschi and Pop, 2015).
Figure 1.
Free radicals and antioxidants imbalance causes oxidative stress and results in aging, while several endogenous and exogenous ROS sources contribute to aging (Weidinger and Kozlov, 2015).
The overabundance of ROS may initially enhance synthesis of concomitant cytokine and promote inflammation, that can additionally accelerate the ROS formation (Van De Lagemaat et al., 2019).
Oxidative stress takes part in the development of aging and many degenerative and chronic disorders including autoimmune disorders, inflammation, cancer, arthritis, neurodegenerative and cardiovascular diseases. Oxidative stress could actively provoke many abnormalities so it leads to initiate many ailments (Chandrasekaran et al., 2017; Simioni et al., 2018).
Various pathophysiological events could occur due to disruption in bio-signaling because of oxidative stress and subsequent alterations at various levels of life, particularly in old age (Szentesi et al., 2019). The modulation of ROS location, inactivation and production occur continuously in both pathological and physiological conditions, if a fine balance is maintained between oxidant-antioxidant mechanisms (Davalli et al., 2016).
2.1.1. Production of ROS
During the cellular redox process, byproducts are generally produced such as RONS (reactive nitrogen species) as well as ROS (Pham-Huy et al., 2008). These products define the radical and non-radical active compounds of nitrogen and oxygen (Powers et al., 2011).
The exogenous ROS and RONS sources that metabolized as free radicals include water and air pollution, drugs, alcohol, tobacco, heavy or transition metals, radiation and cooking products. The endogenous RONS sources are lipoxygenase, myeloperoxidase (MPO), angiotensin II and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. The major sources of ROS within cell are enzymes (Liguori et al., 2018).
There are multiple enzymes and multiple compartments within the cell where ROS are generated. This includes compartments such as cytoplasm, oxidase and cyclooxygenases, NADPH oxidase at plasma membrane, mitochondria during oxidative phosphorylation and lipid oxidation within peroxisomes. Though the overall oxidative burden comes from these sources in aging, maximum ROS production occurs during oxidative phosphorylation (Dai et al., 2012; Flores-López., 2019).
Apart from these sources, other major sources of endogenous oxidants include cytochrome p450, nitric oxide synthase, monoamine oxidase, many oxidoreductases including xanthine oxidase, mitochondrial respiratory chain (RC) and enzymes responsible for infection and inflammatory responses to stimulate xenobiotic such as NADPH oxidases (Haeri and Knox, 2012). The observations show that oxidants production from these sources has ability to rise with age and varies with pathophysiological conditions (Zhang et al., 2015a, Zhang et al., 2015b). However, endogenous oxidants produce during the lifetime, a continuous process, so contact with internal oxidants sources is much more extensive (Simioni et al., 2018).
Compounds (radicals, non-radicals) with oxygen are potent and able to initiate some kind of detrimental reaction, indicated as ROS. These include hydrogen peroxide (H2O2), superoxide anion (O⁻), hypochlorous acid (HOCl), peroxyl radical (HO2), alkoxyl radical (RO·), hydroperoxyl, singlet oxygen (O2) and hydroxyl radical (HO·) (Lambert and Brand, 2009; Cui et al., 2012). ROS include oxygen derivative and are oxidizing agents either radical or non-radical and/or that are converted into radicals easily (Genova and Lenaz, 2015).
The free radicals are produced when cells need to generate energy by using oxygen, so the ATP production by mitochondria results in free radicals production (Pham-Huy et al., 2008). They are also produced during different aerobic processes such as during intensive physical activity, cellular respiration and microbial infection exposure that involves phagocyte activation (Pisoschi and Pop, 2015). Free radicals were identified in biological systems in the 1950th, after their existence, it was supposed immediately that they are involved in aging and other diverse pathological processes (Lushchak, 2015).
When certain molecules interact with oxygen, free radicals with one or more unpaired electrons in their outermost shell are produced. These active free radicals behave as oxidants or reductants as they are produced by losing or accepting a single electron in cells (Liguori et al., 2018).
Free radicals may have zero, negative or positive charge. The specie that has two unpaired electrons is a radical such as diatomic oxygen O2. While both the electrons have a similar spin quantum number but the location of each electron is in the different π∗ anti-bonding orbital. Thus their low reactivity with non-radical molecules is attributed to this parallel spin. Though, O2 can be converted in to much more reactive singlet oxygen O2, by providing an energy input that can invert spin of one of the unpaired electrons. Hence both the electrons may either form a pair in the similar π∗ orbital or may remain in two different orbitals. The electron addition to an oxygen one at a time can overcome the spin restriction. During normal aerobic metabolism, non-radicals are produced in the body such as H2O2, they are also synthesized in the body during stress conditions (Genova and Lenaz, 2015).
Different types of ROS have distinct properties. Highly reactive oxygen species (hiROS) are harmful for biomolecules. Less reactive oxygen species (loROS) take part in cellular signaling include superoxide and especially hydrogen peroxide (Scialo et al., 2013). The family of highly reactive molecules is called ROS physioma, these are free oxygen radicals (hydroxyl radical; HO·, superoxide anion; O⁻₂) and non-radical oxygen derivatives (hydrogen peroxide; H2O2). Other ROS formed from superoxide radicals such as hydroxyl radicals, hydrogen peroxides which interconvert with reactive nitrogen species (RNS), have similar effects as that of ROS (Davalli et al., 2016).
Various cellular activities are accomplished by ROS such as cell signaling transduction, gene transcription, immune response, cell death, cell survival, differentiation, and inflammation. An equilibrium between oxidation species and antioxidants (AOs) is significant for biological role, growth, adaptation and regulation ((Lushchak, 2015; Weidinger and Kozlov, 2015; Liu et al., 2018; Flores-López et al., 2019).
2.2. Mitochondria and aging
Human mitochondrial DNA (mtDNA) consists of 16, 659 base pairs. There are many mtDNA copies in the mitochondria of mammalian cells. There are 13 proteins, tRNA and rRNA coded by human mtDNA and they are indispensable for the structural and functional preservation of mitochondria (Dröse and Brandt, 2012). Numerous studies proposed that ATP production can be influenced by mutations in mtDNA. Several ailments such as early aging process and neurodegeneration are linked with disturbances in mtDNA integrity (Kawamura et al., 2018). Different key metabolic pathways take place in mitochondria including fatty acid β-oxidation, one-carbon cycle and tri-carboxylic acid (TCA) cycle (Mailloux, 2015; Zhang et al., 2018). It is responsible for the production of cell's 90% energy (Marchi et al., 2012). Disturbances in mitochondrial function is assumed as a symbol of aging (Bolduc et al., 2019).
The mitochondrial ROS production and damage to DNA occurs with the passage of time and eventually results in inability of cell to identify the key role of mitochondria. This fact led to formulate “mitochondrial free radical theory of aging” (MFRTA), though it is quite controversial (Son and Lee, 2019). It was modified, expanded and challenged by many researchers but two fundamental statements were never changed. Firstly, oxidatively damaged macromolecules accumulation with aging due to antioxidant/oxidant imbalance, Secondly, degenerative aging phenotype due to accumulated oxidative damage. First point is well accepted that an imbalance of oxidant/antioxidant in the elderly results in the accumulation of damaged molecules due to oxidative damage while some studies challenged the second point (Zhang et al., 2015a, Zhang et al., 2015b). Currently, MFRTA is well-known theory (Lara, 2018), now termed Oxidative Stress Theory (OST).
Several studies proposed alterations in mtDNA and mitochondria, decreased oxidative phosphorylation, increased structural disintegration, elevated ROS synthesis and hazardous effects on fats, proteins and nucleic acids during aging. Estimated mutation rate of mtDNA is 10 times more than DNA present in nucleus with lesser ability of repairing, lack of histones, such aspects influence aging and cancer (Kammeyer and Luiten, 2015; Haas, 2019).
As the above discussion demonstrates that the main site of ROS synthesis is mitochondria in mammals, thus the mtDNA are more prone to harm because of their presence adjacent to site of ROS production. Consistently, it has been indicated by many studies that mtDNA contains a elevated concentrations of 8-hydroxy-2′-deoxyguanosine 8-oxo-dG as compared to nuclear DNA, 8-oxo-dG is byproduct of oxidation (Cui et al., 2012).
Decline in mitochondrial activity is linked with onset of many diseases (Sun et al., 2016). However, general concept is that the accumulation of random molecular damage due to ROS largely contributes to aging (Blagosklonny, 2008). During aging, damaged mitochondria increase in number and these synthesize greater amount of ROS and less ATP (Stefanatos and Sanz, 2018). The adverse effects due to damaged DNA occurs in cells by transcription, blocking replication, chromosome rearrangements, generation of breaks in one or both strands of DNA (Fakouri et al., 2019).
However, these phenomenon of somatic mtDNA mutations and DNA strand breaks may be induced by progressive oxidation reactions with increase in age. Impairment of RC complexes occurs by the accumulated mtDNA alterations, results in a vicious cycle with the more mitochondrial DNA mutations and elevated ROS synthesis (Bonomini et al., 2015). Thus, current consensus indicates that damaging mitochondria, oxidative stress and ultimately an energetic crisis by ROS accelerates aging and triggers neurodegenerative diseases (Stefanatos and Sanz, 2018). mtDNA damage is associated with aging, but it is unclear whether mitochondrial dysfunction or mtDNA damage is directly involved in vascular aging (Foote et al., 2018).
2.3. Mitochondrial ROS
ROS synthesis in RC was first reported in 1966, which came from the pioneering work of Chance and colleagues (Chance et al., 1979).
Major sources of ROS production are first and third complex of RC, where free radicals are generated when oxygen or other electron acceptors react directly with electrons derived from ubiquinone and NADH. Thus the normal side products of the respiration process are ROS that react with protein, fats and nucleic acid, hence damage these biomolecules (Marchi et al., 2012).
The significant production of ROS occurs by isolated mitochondria via two modes of operation, chiefly from (i) first complex when coenzyme Q is reduced and has large proton motive force (Fp) due to zero production of ATP from mitochondria; (ii) when mitochondrial matrix contains a higher reduced to oxidized ratio of NAD. The amount of O2 •− produced is less when ATP production by mitochondria occurs actively, and as a consequence have a lower NADH/NAD+ and Fp ratio (Murphy, 2008).
In isolated mitochondria, ROS can be produced in up to eleven distinct sites. However, it has been shown that in vivo ROS production takes place only at three sites that are more relevant: respiratory complex CIII (aka Ubiquinol: cytochrome c reductase, complex II (CII aka succinate dehydrogenase) and complex I (CI aka NADH: ubiquinone oxidoreductase) (Stefanatos and Sanz, 2018). Recently it has been investigated that appreciable O2•– formation occurs at complex II (Robb et al., 2013). It is now known that from at least 11 different sites, mammalian mitochondria can produce hydrogen peroxide and/or superoxide. Each site has its own distinct properties and is associated with RC and substrate catabolism (Brand, 2016).
An electrochemical gradient is generated when electrons are sequentially transported from CI or CII to CIII and complex IV. Ultimately transport of protons into inter-membrane part from matrix occurs. During this transmission, hydrogen peroxide (pair-electron transfer) or superoxide (single-electron transfer) are produced by the direct transfer of electrons to oxygen, principally at complex I, II and III, having acceptor as ubiquinone (Eleutherio et al., 2018).
Surprisingly, production of ROS by RC complex IV (cytochrome c oxidase) does not occur, though it reduces oxygen to water, demonstrating that in RC, production of ROS and leakage of electron can be prevented. Production of ROS by CI and CIII are important in regulating various pathological and physiological processes that range from cellular differentiation to damage during reperfusion/ischemia (Reczek and Chandel, 2015; Scialò et al., 2017), while the development of certain types of cancer is mediated by the production of ROS from CII. ROS produced by CIII are divided among matrix and inter-membrane part. ROS formed by CI are send to matrix. This demonstrates that both complexes follow diverse distribution systems (Stefanatos and Sanz, 2018).
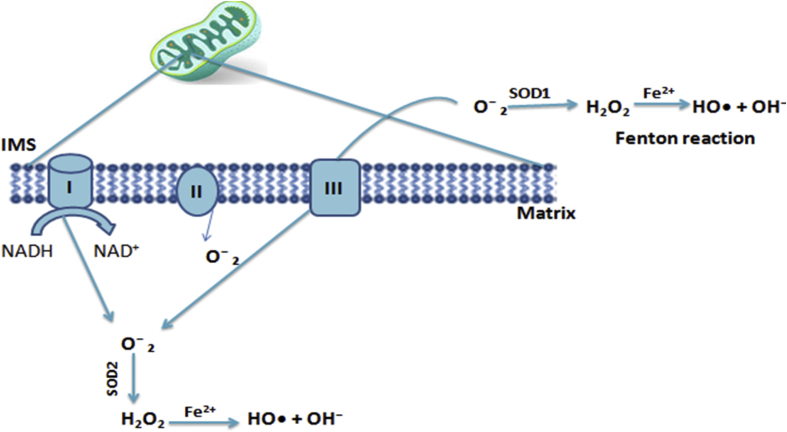
During electrons transport from NADH to CoQ in CI, ROS can be created at both sites, site IQ (CoQ binding site) and site IF (FMN site) in the matrix. ROS production in CII occurs at the IIF site, which is linked with succinate dehydrogenase. Only small amounts of ROS are produced by CIII which are negligible compared to ROS production of CI. ROS produced by CIII are transferred to matrix and into intermembrane space (IMS). The ROS released into the IMS undergo a reaction catalyzed by superoxide dismutase (SOD) i.e., SOD2 in matrix and SOD1 in IMS). There it is converted into H2O2 which has important role in pathophysiological pathways. Superoxide is short-lived, presumably acts where it is produced, and poor membrane-permeability compared to H2O2 that is more stable, uncharged and permeable through aquaporin channels hence more versatile signaling molecule. However, similar to superoxide species, H2O2 produces significant oxidative damage to biomolecules in the presence of free Fe2+. Formation of extremely active OH-radicals occurs when this cation is present as shown in Figure 2 (Wang et al., 2018; Zsurka et al., 2018; Zhao et al., 2019).
Figure 2.
Generation of ROS in the electron transport chain through complex I, II, III and the formation of OH-radicals in the presence of free Fe2+cation by Fenton reaction.
There are at least nine mitochondrial sites where the generation of superoxide anion takes place, a progenitor ROS (Andreyev et al., 2005). Furthermore, superoxide is also produced by a family of membrane-bound enzymes called NADPH oxidases, that by coupling NADPH-derived electrons to oxygen, catalyze controlled O−2 production (Labunskyy and Gladyshev, 2013).
The significance of appreciating the topology of ROS production sites and finding the difference between different ROS especially hydrogen peroxide and superoxide is evident: oxidation-reduction bio-signaling generated by superoxide by site IQ in matrix may vary from such bio-signaling send by superoxide into cytosol by complex IIIQo, additionally, redox signaling by hydrogen peroxide will be different from both these, formed on any side of the inner mitochondrial, indirectly or directly (Brand, 2016).
The partial reduction of approximately 1–5% of the total oxygen that is consumed by mitochondria results in ROS production that is the formation of superoxide anion radical (O−2). One study revealed that 1%–3% of oxygen is converted into free radicals during normal physiologic conditions when consumed by the body, but free radicals production increases promptly beyond metabolically required amounts when oxidative stress occurs (Brawek et al., 2010; Buehler, 2012).
Mitochondrial membrane potential (ΔΨm), activity RC complexes and ROS production rate are strongly related. Hence, if respiration is inhibited and increased ROS production results by the mitochondrial membrane potential dissipation then a reduced rate of free radical generation occurs if uncoupling stimulates drop in ΔΨm. Despite the fact that major ROS generation takes place in RC under the resting situation, generation of (O−2) could also occur by various complexes and matrix proteins such as enzymes (α-ketoglutarate dehydrogenase, pyruvate dehydrogenase, aconitase) Krebs cycle (Angelova and Abramov, 2018).
Researches demonstrated that during the aging, RC function is compromised which might be due to a high level of accumulated ROS from altered metabolic process and weakened antioxidant defense. However, the induction of mitohormesis to prevent age-related diseases at young age and manipulation of RC activity to prolong lifespan in late-life through pharmaceutics will be a promising avenue to pursue (Bouska et al., 2019).
One study indicated that degenerative cellular features of Friedreich's ataxia (FRDA) may appear due to mitochondrial ROS production which is enhanced by the frataxin deficiency in mitochondria. The study suggested that complex IV defect is produced by defective heme synthesis and defective expression of heme and cytochrome c in Complex III and IV results in increased ROS in FRDA cells. Thus, in FRDA, heme defect causes increased mitochondrial ROS and limited cytochrome c and oxidase action, all due to defects in Fe–S centers (ISC). This leads to shortage of heme and ultimately more ROS production, so it is not surprising that defects in mitochondrial pathway for heme happens with increasing age (Napoli et al., 2006).
Recent data indicate that in deteriorating diseases related with aging, the activation of mitochondrial permeability transition pore (mPTP) (an inner membrane protein complex that can form voltage-gated nonselective channel) is triggered by ROS and when mitochondria are overloaded with calcium. The full opening of the mPTP initiates not only further release and production of ROS that may not only harm DNA, phospholipids and proteins but also cause release of matrix DNA to intermembrane space where it is hydrolyzed. This causes depletion of cellular DNA that contributes in accelerating the aging process. It is assumed that aging increases mPTP opening and vice versa. ROS also triggers the activation of mPTP. This can further aggravate clinical features of FRDA as mitochondria itself get damage during oxidative reactions because of activated mPTP. The majority of the ROS and 'mitochondrial dysfunction' effects on lifespan and aging are mediated through the mPTP activation (Rottenberg and Hoek, 2017; Panel et al., 2018).
2.3.1. ROS-induced health benefits
Homeostasis of metabolism relies on the functionality of mitochondria. Mitochondria not only convert the chemical energy of reduced organic compounds into energy currency of the cell; the ATP but also produce numerous biosynthetic intermediates and ROS. ROS damage lipids, proteins, and DNA. However, it is suggested ROS also play a role in signal transduction to regulate biological and physiological processes (Cheng and Ristow, 2013). Oxidative stress leads to pathophysiology and aging. To minimize these deleterious effects, antioxidants are prescribed. However, it is clinically proven that this may not be as efficient as expected. Such supplementations failed to improve health. It is suggested that some molecular aspects of cellular respiration such as ROS are not harmful. ROS exerts positive effects on health during stressful situations, although its mediation and regulation mechanisms are not known (Ristow, 2014).
By limiting glucose and calorie intake, cells provoke mitochondrial metabolism that leads to longevity. Contrary to the findings depicted in Harman's theory of aging, these effects may be attributed to the elevated production of ROS that induces resistance against stress. Actively respiring mitochondria produce ROS that promotes long life. ROS act as biosignaling molecules that initiate internal defense processes that lead to enhanced stress resistance. This type of adaptive reaction is known as named mitochondrial hormesis (mitohormesis). This phenomenon offers a mutual mechanism denominator for bioactive roles of exercise, restricted calorie, and glucose intake (Ristow and Schmeisser, 2011; Ristow and Zarse, 2010).
Survival in saturated oxygen atmosphere demands efficacious physiological approaches to identify and eliminate metabolic by-products; the oxidants. Oxidative tensions are linked with ageing and longevity (Finkel and Holbrook, 2000). Though the behavior of oxidants is haphazard and damaging, their generation is securely controlled in several cellular events. These oxidants are effector molecules for myriad pathways (Finkel, 2003). Aging and related diseases might have common underlying physiological mechanisms as with an increase in age susceptibility to develop numerous diseases also rises. ROS generated during oxidative phosphorylation may affect the rate of aging and exposure to disease (Finkel, 2005).
Under mild stress conditions, mitochondria stimulate signaling pathways in cytosol that affect gene expression, making the cell less vulnerable to expected perturbations. Such a response known as mitohormesis has been extensively studied. Comprehensive knowledge of mitohormesis will highlight the unanimous theory of aging (Yun and Finkel, 2014).
It has been observed by Ristow and Schmeisser (2014) that superoxide, hydrogen peroxide, and other ROS may act as signals to prevent or delay numerous diseases and thus increase longevity. Low concentrations of ROS ameliorate physiological defense systems by initiating adaptable strategies. ROS mediated signaling actions include downstream of insulin/IGF-1 receptor, AMP-dependent kinase (AMPK), target-of-rapamycin (TOR), and sirtuins to end regulation of proteostasis, unfolded protein response (UPR), stem cell maintenance and stress resistance.
Living organisms can produce energy, transform it, and consume organic compounds for growth and reproduction. During bioenergetic, oxidative phosphorylation in eukaryotic cells produce ATP and ROS. Although ROS is known to damage cell machinery, new evidence suggests their role in biosignaling (Shadel and Horvath, 2015). Lu and Finkel (2008) reviewed different studies where entrance into cellular senescence had been assessed when ROS concentrations were moderated by growth in elevated or reduced oxygen level environment or when the antioxidant status was disturbed. It was also observed that senescence caused by oncogene expression is partially facilitated by ROS levels. However, it is not known whether ROS acts randomly or specifically and which specific chemical specie is the actual triggering molecule.
Multiple downstream signaling actions require ROS. When ligand-triggered ROS generation was characterized, it revealed valuable insight into oxidases composition and GTPases-dependent regulation (Finkel, 2001).
Similarly, Holmström and Finkel (2014) stated that redox-dependent bio-signaling encompasses oxidation reduction reactions at cysteine amino acid mostly. For this purpose, the generation of oxidants is ensured due to their role in self-renewal of stem cells, tumorigenesis, immune response, and aging. Some features of the aging process such as senescence, inflammation, and the loss of stem cell activity are provoked by mitochondria. Signaling pathways regulate mitophagy and longevity (Sun et al., 2016).
ROS regulates signaling within the cell by covalent alterations of cysteine residues of specific proteins. Such oxidative modifications can amend enzyme activity. Response to growth factor stimulus and generation of inflammatory responses are subjected to redox regulation. Impaired ROS signaling can lead to numerous diseases (Finkel, 1998, 2011).
Limited data exist regarding redox reactions regulations in non-phagocytic cells, particularly the origin of oxidant generation, and their targets within the cell. Active alterations in the redox environment act as determining factors in cell choices for growth, programmed cell death and (Finkel, 1999).
According to West et al. (2011a), innate immune systems are supported by mitochondria. In mouse macrophage, bactericidal activity is mediated by mitochondrial ROS (mROS). However, the mechanism that links inborn immune signals to mROS generation is still not clear. TLR1, TLR2, and TLR4 are Toll-like receptors that are used to involve mitochondria for ROS synthesis. The biochemical mechanism of elevated ROS synthesis involves TLR signaling adaptor, TRAF6 (tumour necrosis factor receptor-associated factor 6), evolutionarily conserved signaling protein Toll pathways (ECSIT). Additionally, mitochondria regulate antiviral signaling (West et al., 2011b).
It is stated by Schroeder et al. (2013) that extension in life span emerges from adaptive signaling mechanisms influenced by slightly higher ROS levels. Stress-induced signals exist between mitochondria and nucleus and these are involved in the regulation of aging through epigenetic silencing of gene expression, thereby initiate longevity effect.
Further studies are warranted to determine the nature of reactive chemical species that are involved in signal transduction, ligand stimulation, and protein targeting. The mechanisms by which these oxidants transduce signals should be explored to enhance our understanding about different pathophysiological states (Finkel, 2000, 2012).
2.3.2. Mitochondrial homeostasis and mitophagy
Recognition of gene variants responsible for longevity elaborated aging biology. Research in the field of aging has social, therapeutic, and marketable values (Campisi et al., 2019). Numerous biochemical phenomena such as bioenergetics, genomic stability, mitochondrial homeostasis, cellular adjustments under stress, and cell existence have involvement of NAD+, an essential metabolic molecule. Several enzymes involved in brain activity depend upon NAD+. NAD+ and other associated metabolites play a crucial role in neuron's adaptive strategies against stress inducers and corrective counteractions in progressive brain deteriorating conditions (Lautrup et al., 2019). NAD+ depletion is a vital characteristic of aging and it can lead to many chronic diseases. Reduction in NAD+ concentration is observed not only in neurodegenerative diseases but also in aging. Physiological and therapeutic interferences that boost NAD+ levels may delay aging and associated diseases (Fang et al., 2017).
NM signaling (signaling from the nucleus to mitochondria) regulates mitochondrial function and ageing. Nuclear DNA damage is the starter of this signaling, and this DNA accumulates with age. NM signaling system has sirtuins proteins, that ensure DNA and mitochondrial stability (Fang et al., 2016). Nicotinamide adenine dinucleotide (NAD+)-dependent deacylases, sirtuins (SIRT1-7) have extraordinary capabilities to avert diseases and ageing effects. Animal models had better organ physiology, physical strength, disease defiance, and long life when treated to express extra sirtuins (SIRT1 or SIRT6), or given STACS (sirtuin-activators) like resveratrol and SRT2104 or with NAD+ precursors. It was concluded that STAC can be used to treat inflammatory and metabolic diseases (Bonkowski and Sinclair, 2016).
2.4. Other organelles and aging
Many organelles are involved in generation of significant amounts of ROS. Cellular ROS concentrations depend upon mechanisms of ROS generation and this would ultimately result in initiating redox strain and signaling. Most important organelle is peroxisome that is involved in detoxifying and generating hydrogen peroxide. Accordingly, within peroxisomes loss of redox homeostasis correlates with the induction of cellular senescence and increased hydrogen peroxide levels. Lysosomes contribute to the turnover of damaged organelles and proteins (Stefanatos and Sanz, 2018).
The word “peroxisome” was originally derived for any cell organelle which possesses at-least one H2O2-detoxifying enzymes either catalase or H2O2-producing oxidase (Titorenko and Terlecky, 2011). Peroxisomes play a primary role in lipid metabolism. It is suggested that peroxisomes may perform a physiological function in aging. Moreover, it has been demonstrated that mitochondrial redox balance can be influenced by changes in the peroxisomal ROS metabolism (Fransen et al., 2012). Consequently, accelerated aging and stress may be due to loss functional ability of both organelles. Through specific protein complexes, peroxisomes can physically connect with mitochondria. Hence, the functional and structural association of organelles are vital and active sign to regulate aerobic metabolism, but real chemistry will be exposed in upcoming days (Pascual-Ahuir et al., 2017). However, very limited knowledge exist regarding the involvement of peroxisomal ROS (H2O2, NO) in bio-signaling that has fundamental involvement in the pathogenesis of aging and carcinogenesis (Antonenkov et al., 2010).
Peroxisomes by keeping many scavenging enzymes catalase and peroxiredoxins as well as ROS-producing oxidases, not only regulate the intra peroxisomal ROS homeostasis but can also help in the regulation of extra peroxisomal ROS levels within the whole cell (Titorenko and Terlecky, 2011).
For metabolically active tissues such as liver, peroxisomes are not only the sources of ROS production and oxygen utilization (up to 35%). Peroxisomes, the highly oxidative organelles possess multiple antioxidant systems, which are vital for its ROS homeostasis (Pascual-Ahuir et al., 2017). Endoplasmic reticulum (ER) oxidative folding machinery generates H2O2 during protein folding and incorporates ER oxidation-reduction, ER forms contact sites with mitochondria that are known as mitochondria-associated membranes (MAMs) (Rieusset, 2018; Siegenthaler and Sevier, 2019).
It is experimentally estimated that during oxidative protein folding in ER, about 25% of ROS are produced by disulfide bonds. Hence, more ROS are generated from proteins having more disulfide bonds in comparison with the proteins having fewer such bonds (Zeeshan et al., 2016). Two ER enzymes ER oxidoreductin 1 (ERO1) and protein disulfide isomerase (PDI) are considered to be an electron transport chain that is similar to mitochondrial RC because in a nascent secretory protein during formation of a disulfide bond, electrons are lost and are transferred to these enzymes. Hence, results in the formation of hydrogen peroxide as electrons are eventually transmitted from ERO1 to molecular oxygen (Siegenthaler and Sevier, 2019).
ER also contains different systems to restrict the accumulation of ROS, likewise mitochondria. For example, ER contains peroxiredoxins that play important roles in redox signaling such as thiol oxidizers and both hydrogen peroxide sensors (Kritsiligkou et al., 2018). Both glutathione peroxidase and peroxiredoxins, ER-localized enzymes, scavenge hydrogen peroxide to water by donating electrons from PDI to hydrogen peroxide (Siegenthaler and Sevier, 2019).
2.5. Antioxidants
ROS have a role in carrying out the vital physiological processes. Although elevated ROS levels lead to stress and pathology, reduced ROS levels are associated with the normal physiological conditions (Banerjee et al., 2017; Bodega et al., 2019).
Presence of free radicals near tissues and cells induces a series of reactions and activates certain multiple internal defense cellular processes to eliminate ROS (Mirończuk-Chodakowska et al., 2018). The cells and the organism have a specific anti-oxidative protection system to defend themselves (Höhn et al., 2017).
The oxidation of substrate is prevented by antioxidants that are present in smaller amounts in body and hence have a principal role in the defense mechanism (Suleman et al., 2019). Antioxidants quench or inhibit the reactions caused by free radicals and ultimately prevent or delay cellular damage. The existence of the anti-oxidant defense mechanism is universal, although there are variations in antioxidant defenses between different species (Nimse and Pal, 2015).
In humans, three lines of defense have been evolved by nature. They function through different mechanisms as; Inhibiting, Catching and Restoring. The first line of defense that scavenges ROS/RNS involves enzymatic and non-enzymatic antioxidants. They can delay or prevent initiation of different oxidative stresses directly and these include glutathione (GSH), uric acid, vitamin E and vitamin C. The non-enzymatic substances in blood plasma such as transferrin, ferritin, ceruloplasmin and albumin belong to preventive antioxidants and they inhibit new reactive species formation by binding transition metal ions (e.g. copper and iron). Another defense line consists of non-enzymatic antioxidants that serve as an intermediate defense to inactivate oxidants and radicals. The third and last line of defense involves repairing and damage-removing, causing regeneration of damaged bio-molecules from oxidative injury (Lei et al., 2015; Mirończuk-Chodakowska et al., 2018; Neha et al., 2019).
Anti-oxidants terminate oxidative chain reactions by binding with free radicals and donate their electrons to them, hence making free radicals unavailable for further attack. After donating their electrons, the free radical state is attained by antioxidants. Hence they are not harmful because they have the ability to accommodate the change in electrons, without themselves being reactive (Ahmad, 2018).
2.5.1. Endogenous antioxidants
Antioxidant can be divided into enzymatic and nonenzymatic ROS scavengers on the bases of their biological function. The main enzymatic antioxidants are catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase, glutathione reductase, glutamyl Transpeptidase (GT). Non-enzymatic antioxidants are dietary compounds including minerals (selenium, zinc) vitamins (C, E) and also uric acid, ubiquinol, tocopherol, retinol and glutathione (Momtaz and Abdollahi, 2012). Both non-enzymatic and enzymatic antioxidants function in different cellular compartments and against different oxidative species, hence they are complementary to each other. Additionally, they perform their functions with exogenous antioxidant systems in a synergistic way (Petruk et al., 2018). Many fresh and raw foods contain minerals or essential vitamins. Collectively, these nature-derived molecules are known as "phytonutrients" (Bjørklund and Chirumbolo, 2017).
Researches have shown that nuclear factor (erythroid-derived 2)-like 2 (NRF2), fight contrary to stress by transcriptionally up-regulating the genes. NRF2 loss causes unlimited oxidative stress and drive the aging phenotype. Most endogenous antioxidants are expressed only when NRF2 is activated (Aguilar et al., 2016; Schmidlin et al., 2019).
Melatonin together with its metabolites has long been recognized in protecting the cells exposed to toxins or in alleviating the burden of oxidative stress in aging cells. One study reported that melatonin is"always present at the right place within cell" to fight against stress. Recently, it has been demonstrated that high concentration of melatonin are available when abundant free radicals are present (Reiter et al., 2018).
2.5.2. Exogenous anti-oxidants
Human body lacks non-enzymatic anti-oxidants and hardly produces them, hence there are exogenous antioxidants. There are three categories of exogenous non-enzymatic antioxidants: i) mineral elements such as selenium ii) nutritional antioxidants like carotenoids, vitamin E and vitamin C and iii) natural antioxidants: obtained from natural resources commonly known as phytochemicals/phytonutrients (Santos et al., 2018). All the antioxidants are known as Total Antioxidant Capacity (TAC) that reflects a general status of antioxidants (Momtaz and Abdollahi, 2012). Nutritional antioxidants are primarily free radical scavengers but they function in different compartments with different mechanisms. They act as free radical scavengers by 1) decreasing the production of ROS, 2) repairing the oxidized membranes, 3) neutralizing the free radicals, 4) through lipid metabolism in which cholesteryl esters and short-chain free fatty acids neutralize ROS (Simioni et al., 2018).
Though genetic aspects have a great impact on longevity, the environmental factors are also important, however, among them, the diet has the most influence on longevity (Aversa et al., 2016). A study was conducted in healthy subjects aged 65–94 to evaluate the antioxidant status which showed the higher concentration of antioxidant in healthy centenarians, indicating the great importance of diet rich in antioxidants and ultimately healthy centenarians have a lower degree of oxidative stress as compared to aged subjects. After eradication of statistically confounding factors, their vocabulary and memory are tested which showed circulating levels of beta-carotene and vitamin C, both these antioxidants appeared as predictors of their best efficiency (Mecocci et al., 2018).
Furthermore, the importance of diet rich in antioxidants can be determined by the conditions in which endogenous antioxidant systems are believed to be inadequately efficient. This includes conditions such as chronic oxidative stress (Vaiserman et al., 2016) and aging, when performance of endogenous antioxidant system in cell decreased. Hence, centenarians are more affected by oxidative stress (Petruk et al., 2018). Consequently, the combined effects of antioxidant-deficiency and malnutrition make individuals more susceptible to oxidative stress (Liu et al., 2018).
According to Harman's theory (Harman, 2006), exogenous antioxidant sources are very important to hamper the effects of oxidative stress. This includes beta-carotene, coenzyme Q10, vitamins E and C, lipoic acid, glutathione, coenzyme Q10, phytoestrogens, polyphenols, selenium, zinc and manganese. Endogenous antioxidant depletion can be halted by antioxidant supplementation, ultimately mitigating the associated oxidative damage (Liu et al., 2018).
Fruits, vegetables, beverages, tea, coffee, spices, nuts, and cereal products are major sources of plant-derived antioxidants. These are primarily phenolic compounds, vitamins, and flavonoids (Zujko et al., 2015) and alleviate cardiovascular disease, diabetes mellitus and numerous other diseases (Mirończuk-Chodakowska et al., 2018; Khanthapok and Sukrong, 2019; Suleman et al., 2019).
A significant role is played by nutraceuticals and functional foods having antioxidants in delaying the process of aging. A research performed on different aging models has shown the prolonged lifespan by some antioxidants and dietary calorie restriction (Peng et al., 2014). Coumarins and compounds having OH groups are more active for antioxidant activity (Neha et al., 2019). Antioxidants have a heterogeneous group of molecules. The chemical properties and physical location (emulsion interfaces, proximity to membrane phospholipids or in the aqueous phase) determine the anti-oxidative effectiveness of these compounds (Oroian and Escriche, 2015).
Antioxidant supplementation catalyzes the degradation of organic peroxides and hydrogen peroxide, superoxide and detoxifies oxy radicals and finally their conversion into the water, oxygen, and alcohol (Santos et al., 2018). Many antioxidants such as superoxide dismutases, peroxiredoxins, catalases, and reduced glutathione are made by humans themselves. However, in recent years the discovery of per-oxiredoxins is one of the biggest advances. Peroxiredoxins have many fascinating properties, most importantly their ability to act as scavengers of H2O2 in vivo (Halliwell, 2012).
Another study reported the production of different specific mitochondria-targeted antioxidants (such as anti-oxidants conjugated with mitophenyltertbutyline and triphenylphosphonium cation e.g., mitovitamin E, mitophenyltertbutyline and mitoquinone). However, they are under experimental testing and development, and still, their role in inhibiting vascular inflammation and mitigating the effects of oxidative stress in aging has not been evaluated (Ungvari et al., 2010).
Another observation has shown the role of p53 in enhancing the antioxidant gene expression, which accounts for the ability of p53's in regulating the oxidative stress in mice and cells (Vigneron and Vousden, 2010). Different studies have indicated that dried fruits such as dates and peaches contain a higher concentration of corresponding antioxidant activities and bioactive compounds as compared to their respective fresh counterparts. The reason why dried fruits are rich sources of antioxidant polyphenols is that after the process of dehydration antioxidants become concentrated (Chang et al., 2016).
Recently, researchers focused more on replacing synthetic antioxidants with efficacious natural antioxidants (Li et al., 2013). Presently, polysaccharides have been extensively utilized and are obtained from species of Hericium, Lentinula, Ganoderma, Tremella, Pachyme (Zhang et al., 2017). Another study reported the effectiveness of taxanthin (3,3 P-dihydroxyl-4,4P-dioxo-L-carotene) as an anti-oxidant agent. Taxanthin a common lipophilic pigment present in birds, fish and algae and it is more effective than other antioxidant agents e.g., vitamin E and carotenoid (Peng et al., 2014).
Plant-based phytomelatonin (PM) has antioxidant capacities and available as supplement (Ferri et al., 2019). Phytochemical antioxidants have capability to fight against aging and the disorders associated with it. For example, coffee reduced both cognitive and motor deficits in aged rats because it contains high levels of antioxidant phytochemicals. However, antioxidant phytochemicals have various mechanisms through which they show anti-aging activities (Zhang et al., 2015a, Zhang et al., 2015b).
According to the criteria of evidence-based medicine, long-term clinical trials always demonstrated the ineffectiveness of antioxidants, only with the exception of a few studies. Currently, there is little or no impact of antioxidant potions or pills on aging or human health, because from billions of years antioxidant safety is an evolved system that resists being easily tampered with (Schmidt et al., 2015; Gutteridge and Halliwell, 2018).
Although, the effects of endogenous antioxidants (glutathione, catalase, superoxide dismutase) in protecting cells from aging by scavenging free radicals is known but the role of exogenous antioxidant supplements against aging requires more investigations. Several studies have shown that the use of dietary antioxidant supplements cannot increase life span in mammals (Wichansawakun and Buttar, 2019).
Another observation demonstrated that, though a healthy diet is recommended in preventing effects of oxidative stress and is considered indispensable but it remains poorly understood that what's the molecular mechanisms through which nutrients are capable of exerting antioxidant effects (Monti et al., 2019). One study reported that both catalase and synthetic SOD increase life span of cells significantly (Abbas et al., 2017).
Based upon review of literature depicted in this article, antioxidants can be classified as given in Table 1.
Table 1.
Types of antioxidants.
| Endogenous Antioxidants | Exogenous Antioxidants |
|---|---|
|
|
3. Computational models
Almost all species are affected by aging. A variety of controlled machine learning algorithms are used to understand the complexities of the aging. A review of literature by data mining and machine learning has led to following inferences: 1) DNA repair has link to aging, 2) certain proteins have role in aging, 3) life span is associated with programmed cell death, ions transport, membrane receptors, 4) many biomarkers of aging exist. Machine learning algorithms have certain limitations too. Only one study verified computational machine learning data inferences with wet-lab trials. Additional research of validation of results is required in future (Fabris et al., 2017).
Classic theory of aging suggested that detrimental DNA mutations are not eliminated by natural selection in older age. Another theory proposed that genetic basis of aging is adapted to optimize evolutionary passage of selection. By computer modeling (Markov et al., 2018), it was proposed that intrinsic decline of some physiological processes enhances efficacy of natural choices for other processes too. However, they could not elaborate the mechanisms that warrant the spread and sustenance of age-related genes.
ROS and numerous inflammatory processes stimulate neurodegeneration. Advanced computational biology techniques such as CADD (computer aided design) has major contribution in development, characterization and testing of drugs against neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease. CADD was used in a study of secondary plant metabolites in molecular docking. Ligand-based-virtual screening (Random Forest) and structure-based- virtual screening (docking of 469 alkaloids data set from family Apocynaceae was taken from data bank) were done to identify potential structures that could inhibit acetylcholinesterase activity in human (Scotti and Scotti, 2015).
In a study (Putin et al., 2016), a modular ensemble of 21 deep neural networks (DNNs) of variable complexity, assembly and machine learning (ML) technique support vector machines (SVM) were used to assess factors affecting human aging process. The model ensemble found that albumin protein, blood glucose levels, enzyme alkaline phosphatase, urea level red blood cells are significant biomarkers for aging process as shown in Figure 3. Such computer stimulatory approaches may enable incorporation of complex data with multiple computational models to identify risk factors for aging.
Figure 3.
Standardized data sets of blood biochemistry for laboratory analysis, diverse deep neural network with different characteristics pooled collectively in ElasticNet model (Putin et al., 2016).
MicroRNAs (miRNAs) control genetic manifestations through communications with targeted points within mRNAs. These increase mRNA breakdown or block translation. Different miRNAs in skeletal muscle play role in muscle regeneration or degeneration during aging. Role of specific miRNAs in age-related muscle deterioration cannot be defined in the presence of complex underlying mechanisms. Proctor and Goljanek-Whysall (2017) developed a model to understand key regulatory role of miRNA in muscle development, differentiation and wasting. Early shift to differentiation is controlled by miR-1, miR-181 ensures continued shifting. miR-378 is responsible to preserve differentiation, while miR-143 obstructs myogenesis.
Memory is affected badly by aging. Current theories presented for poor memory and aging can be explained by computerized simulation models too. Hoareau et al. (2016) used time-based resource sharing (TBRS) and Serial Order in a Box-Complex Span (SOB-CS) to assess memory functions. Both computational models opposed in defining reasons for memory loss as whether it was time dependent decay or technical interferences in simulation modelling for young adult sample. When again tested for multifaceted span data from young and older adults, both models were incapable to properly interpret older adult records.
4. Conclusion
We all are aware of the fact that a high mortality and morbidity rate are attributed to aging. Aging is a global issue as the number of centenarians increasing worldwide. We cannot deny the reality of being aged but we can convert this time-dependent and natural process of aging, into a healthy aging process by taking certain preventive measures, because the number of healthy centenarians is not very high. As environmental parameters also contribute largely to aging so we should pay attention to regulating the ROS producing environmental factors. Similarly, our diet should be rich in antioxidants such as fresh fruits and vegetables that may help in preserving the equilibrium between oxidants and antioxidants and ultimately healthy aging process. A lot of work is needed for the production of effective exogenous antioxidants from natural sources, though they cannot stop it but may lessen the process of aging hence making it less bad or a healthy one. Future predictions regarding aging, biochemical mechanisms involved, biomarkers internal and external factors can be easily done with machine learning algorithms and other computational models.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abbas G., Salman A., Rahman S.U., Ateeq M.K., Usman M., Sajid S.…Younas T. Aging mechanisms: linking oxidative stress, obesity and inflammation. Matrix Sci. Med. 2017;1(1):30–33. [Google Scholar]
- Aguilar T.A.F., Navarro B.C.H., Pérez J.A.M. 2016. Endogenous Antioxidants: a Review of Their Role in Oxidative Stress A Master Regulator of Oxidative Stress-The Transcription Factor Nrf2: Intech Open. [Google Scholar]
- Ahmad R. Free Radicals, Antioxidants and Diseases, 1. 2018. Introductory chapter: basics of free radicals and antioxidants. [Google Scholar]
- Andreyev A.Y., Kushnareva Y.E., Starkov A. Mitochondrial metabolism of reactive oxygen species. Biochem (Mosc) 2005;70(2):200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- Angelova P.R., Abramov A.Y. Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 2018;592(5):692–702. doi: 10.1002/1873-3468.12964. [DOI] [PubMed] [Google Scholar]
- Antonenkov V.D., Grunau S., Ohlmeier S., Hiltunen J.K. Peroxisomes are oxidative organelles. Antioxidants Redox Signal. 2010;13(4):525–537. doi: 10.1089/ars.2009.2996. [DOI] [PubMed] [Google Scholar]
- Aversa R., Petrescu R.V., Apicella A., Petrescu F.I. One can slow down the aging through antioxidants. Am. J. Eng. Appl. Sci. 2016;9(4) [Google Scholar]
- Banerjee J., Khanna S., Bhattacharya A. MicroRNA regulation of oxidative stress. Oxid. Med. Cell Longev. 2017 doi: 10.1155/2017/2872156. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M.C., Grosso R.A., Fader C.M. Hallmarks of aging: an autophagic perspective. Front. Endocrinol. 2018;9 doi: 10.3389/fendo.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørklund G., Chirumbolo S. Role of oxidative stress and antioxidants in daily nutrition and human health. Natr. J. 2017;33:311–321. doi: 10.1016/j.nut.2016.07.018. [DOI] [PubMed] [Google Scholar]
- Blagosklonny M.V. Aging: ros or tor. Cell Cycle. 2008;7(21):3344–3354. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- Bodega G., Alique M., Puebla L., Carracedo J., Ramírez R. Microvesicles: ROS scavengers and ROS producers. J. Extracell. Vesicles. 2019;8(1):1626654. doi: 10.1080/20013078.2019.1626654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc J.A., Collins J.A., Loeser R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019;132:73–82. doi: 10.1016/j.freeradbiomed.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomini F., Rodella L.F., Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6(2):109. doi: 10.14336/AD.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski M.S., Sinclair D.A. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016;17(11):679-690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouska M., Huang K., Kang P., Bai H. Organelle aging: lessons from model organisms. J. Genet. Genomics. 2019 doi: 10.1016/j.jgg.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Brawek B., Löffler M., Wagner K., Huppertz H.-J., Wendling A.-S., Weyerbrock A.…Feuerstein T.J. Reactive oxygen species (ROS) in the human neocortex: role of aging and cognition. Brain Res. Bull. 2010;81(4-5):484–490. doi: 10.1016/j.brainresbull.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Buehler B.A. The free radical theory of aging and antioxidant supplements: a systematic review. Evid. Based Compl. Alter. 2012;17(3):218–220. [Google Scholar]
- Campisi J., Kapahi P., Lithgow G.J., Melov S., Newman J.C., Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571(7764):183-192. doi: 10.1038/s41586-019-1365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carocho M., Ferreira I.C.F.R., Morales P., Soković M. Oxid Med Cell Longev; 2019. Antioxidants and Prooxidants: Effects on Health and Aging 2018; p. 7971613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran A., Idelchik M.d.P.S., Melendez J.A. Redox control of senescence and age-related disease. Redox Biol. 2017;11:91–102. doi: 10.1016/j.redox.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chang S.K., Alasalvar C., Shahidi F. Review of dried fruits: phytochemicals, antioxidant efficacies, and health benefits. J. Funct. Foods. 2016;21:113–132. [Google Scholar]
- Chaves A.C.S., Fraga V.G., Guimarães H.C., Teixeira A.L., Barbosa M.T., Carvalho M.D.G., Mota A.P.L., Silva I.D.F.O., Caramelli P., Gomes K.B., Alpoim P.N. Estrogen receptor-alpha gene XbaI A> G polymorphism influences short-term cognitive decline in healthy oldest-old individuals. Arquivos de neuro-psiquiatria. 2017;75(3):172–175. doi: 10.1590/0004-282X20170018. [DOI] [PubMed] [Google Scholar]
- Cheng Z., Ristow M. Mitochondria and metabolic homeostasis. Antioxidants Redox Signal. 2013;19(3):240–242. doi: 10.1089/ars.2013.5255. [DOI] [PubMed] [Google Scholar]
- Clement M.V., Luo L. Organismal aging and oxidants beyond macromolecules damage. Proteomics. 2019 doi: 10.1002/pmic.201800400. [DOI] [PubMed] [Google Scholar]
- Cui H., Kong Y., Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012 doi: 10.1155/2012/646354. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerska M., Mikolajewska K., Zielinski M., Gromadzinska J., Wasowicz W. Today’s oxidative stress markers. Med. Pr. 2015;66(3):393–405. doi: 10.13075/mp.5893.00137. [DOI] [PubMed] [Google Scholar]
- Dai D.-F., Rabinovitch P.S., Ungvari Z. Mitochondria and cardiovascular aging. Circ. Res. 2012;110(8):1109–1124. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalli P., Mitic T., Caporali A., Lauriola A., D’Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell Longev. 2016 doi: 10.1155/2016/3565127. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröse S., Brandt U. Springer; 2012. Molecular Mechanisms of Superoxide Production by the Mitochondrial Respiratory Chain Mitochondrial Oxidative Phosphorylation; pp. 145–169. [DOI] [PubMed] [Google Scholar]
- Eleutherio E., de Araujo Brasil A., França M.B., de Almeida D.S.G., Rona G.B., Magalhães R.S.S. Oxidative stress and aging: learning from yeast lessons. Fungal Biol. 2018;122(6):514–525. doi: 10.1016/j.funbio.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Fabris F., Magalhães J.P., Freitas A.A. A review of supervised machine learning applied to ageing research. Biogerontology. 2017;18(2):171–188. doi: 10.1007/s10522-017-9683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakouri N.B., Hansen T.L., Desler C., Anugula S., Rasmussen L.J. From powerhouse to perpetrator—mitochondria in health and disease. Biology. 2019;8(2):35. doi: 10.3390/biology8020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang E.F., Scheibye-Knudsen M., Chua K., Mattson M., Croteau D. Nuclear DNA damage signaling to mitochondria in aging. Nat. Mol. Cell Biol. 2016;17:308–321. doi: 10.1038/nrm.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang E.F., Lautrup S., Hou Y., Demarest T.G., Croteau D.L., Mattson M.P., Bohr V.A. NAD+ in aging: molecular mechanisms and translational implications. Trends Mol. Med. 2017;23(10):899–916. doi: 10.1016/j.molmed.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage M., Miller K., Elsner P., Maibach H. Intrinsic and extrinsic factors in skin ageing: a review. Int. J. Cosmet. Sci. 2008;30(2):87–95. doi: 10.1111/j.1468-2494.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Ballesteros R., Robine J.M., Walker A., Kalache A. Active aging: a global goal. Curr. Gerontol. Geriatr. Res. 2013 doi: 10.1155/2013/298012. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri F., Olivieri F., Cannataro R., Caroleo M.C., Cione E. Phytomelatonin regulates keratinocytes homeostasis counteracting aging process. Cosmetics. 2019;6(2):27. [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species in non-phagocytic cells. J. Leukoc. Biol. 1999;65(3):337–340. doi: 10.1002/jlb.65.3.337. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxygen radicals and signaling. Curr. Opin. Cell Biol. 1998;10(2):248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- Finkel T. Redox-dependent signal transduction. FEBS Lett. 2000;476(1-2):52–54. doi: 10.1016/s0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
- Finkel T. Reactive oxygen species and signal transduction. IUBMB Life. 2001;52(1-2):3–6. doi: 10.1080/15216540252774694. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003;15(2):247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Finkel T. Radical medicine: treating ageing to cure disease. Nat. Rev. Mol. Cell Biol. 2005;6(12):971–976. doi: 10.1038/nrm1763. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194(1):7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by mitochondrial oxidants. J. Biol. Chem. 2012;287(7):4434–4440. doi: 10.1074/jbc.R111.271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Flores-López L.Z., Espinoza-Gómez H., Somanathan R. Silver nanoparticles: electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019;39(1):16–26. doi: 10.1002/jat.3654. [DOI] [PubMed] [Google Scholar]
- Foote K., Reinhold J., Yu E.P., Figg N.L., Finigan A., Murphy M.P., Bennett M.R. Restoring mitochondrial DNA copy number preserves mitochondrial function and delays vascular aging in mice. Aging Cell. 2018;17(4) doi: 10.1111/acel.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen M., Nordgren M., Wang B., Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim. Biophys. Acta. 2012;1822(9):1363–1373. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Genova M.L., Lenaz G. The interplay between respiratory supercomplexes and ROS in aging. Antioxidants Redox Signal. 2015;23(3):208–238. doi: 10.1089/ars.2014.6214. [DOI] [PubMed] [Google Scholar]
- Gerschman R., Gilbert D.L., Nye S.W., Dwyer P., Fenn W.O. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- Gutteridge J.M., Halliwell B. Mini-Review: oxidative stress, redox stress or redox success? Biochem. Biophys. Res. Commun. 2018;502(2):183–186. doi: 10.1016/j.bbrc.2018.05.045. [DOI] [PubMed] [Google Scholar]
- Haas R.H. Multidisciplinary Digital Publishing Institute; 2019. Mitochondrial Dysfunction in Aging and Diseases of Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeri M., Knox B.E. Endoplasmic reticulum stress and unfolded protein response pathways: potential for treating age-related retinal degeneration. J. Ophthalmic Vis. Res. 2012;7(1):45. [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr. Rev. 2012;70(5):257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- Harman D. Alzheimer's disease pathogenesis: role of aging. Ann. N. Y. Acad. Sci. 2006;1067:454-460. doi: 10.1196/annals.1354.065. [DOI] [PubMed] [Google Scholar]
- Hoareau V., Lemaire B., Portrat S., Plancher G. Reconciling two computational models of working memory in aging. Top. Cogn. Sci. 2016;8(1):264–278. doi: 10.1111/tops.12184. [DOI] [PubMed] [Google Scholar]
- Höhn A., Weber D., Jung T., Ott C., Hugo M., Kochlik B.…Castro J.P. Happily (n) ever after: aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017;11:482–501. doi: 10.1016/j.redox.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmström K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15(6):411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- Jang J.Y., Blum A., Liu J., Finkel T. The role of mitochondria in aging. J. Clin. Invest. 2018;128(9) doi: 10.1172/JCI120842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammeyer A., Luiten R. Oxidation events and skin aging. Ageing Res. Rev. 2015;21:16–29. doi: 10.1016/j.arr.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Kauppila T.E., Kauppila J.H., Larsson N.-G. Mammalian mitochondria and aging: an update. Cell Metabol. 2017;25(1):57–71. doi: 10.1016/j.cmet.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Kawamura K., Qi F., Kobayashi J. Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production. J. Radiat. Res. 2018;59(suppl_2):ii91–ii97. doi: 10.1093/jrr/rrx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanthapok P., Sukrong S. Anti-aging and health benefits from Thai food: protective effects of bioactive compounds on the free radical theory of aging. J. Food Health Bioenviron. Sci. 2019;12(1):88–117. [Google Scholar]
- Kritsiligkou P., Rand J.D., Weids A.J., Wang X., Kershaw C.J., Grant C.M. Endoplasmic reticulum (ER) stress–induced reactive oxygen species (ROS) are detrimental for the fitness of a thioredoxin reductase mutant. J. Biol. Chem. 2018;293(31):11984–11995. doi: 10.1074/jbc.RA118.001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labat-Robert J., Robert L. Longevity and aging. Mechanisms and perspectives. Pathol. Biol. 2015;63(6):272–276. doi: 10.1016/j.patbio.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Labunskyy V.M., Gladyshev V.N. Role of reactive oxygen species-mediated signaling in aging. Antioxidants Redox Signal. 2013;19(12):1362–1372. doi: 10.1089/ars.2012.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert A.J., Brand M.D. Springer; 2009. Reactive Oxygen Species Production by Mitochondria Mitochondrial DNA; pp. 165–181. [DOI] [PubMed] [Google Scholar]
- Lara R. Oxidative Stress: In vitro comparative evaluation of the resveratrol modulator capacity in neuro 2-A lines and human leukocyte cells. Curr. Trend. Metabol. 2018;2018:1–8. [Google Scholar]
- Lautrup S., Sinclair D.A., Mattson M.P., Fang E.F. NAD+ in brain aging and neurodegenerative disorders. Cell Metabol. 2019;30(4):630-655. doi: 10.1016/j.cmet.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-C., Wei Y.-H. Springer; 2012. Mitochondria and Aging Advances in Mitochondrial Medicine; pp. 311–327. [DOI] [PubMed] [Google Scholar]
- Lei X.G., Zhu J.-H., Cheng W.-H., Bao Y., Ho Y.-S., Reddi A.R.…Arnér E.S. Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiol. Rev. 2015;96(1):307–364. doi: 10.1152/physrev.00010.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Liu S., Xing R., Li K., Li R., Qin Y.…Li P. Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydr. Polym. 2013;92(2):1991–1996. doi: 10.1016/j.carbpol.2012.11.088. [DOI] [PubMed] [Google Scholar]
- Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D.…Bonaduce D. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Ren Z., Zhang J., Chuang C.-C., Kandaswamy E., Zhou T., Zuo L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak V. Free radicals, reactive oxygen species, oxidative stresses and their classifications. Ukrainian Biochem. J. 2015;87(6):11–18. doi: 10.15407/ubj87.06.011. [DOI] [PubMed] [Google Scholar]
- Lu T., Finkel T. Free radicals and senescence. Exp. Cell Res. 2008;314(9):1918–1922. doi: 10.1016/j.yexcr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailloux R.J. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015;4:381–398. doi: 10.1016/j.redox.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S., Giorgi C., Suski J.M., Agnoletto C., Bononi A., Bonora M.…Poletti F. Mitochondria-ros crosstalk in the control of cell death and aging. J. Signal Transduct. 2012 doi: 10.1155/2012/329635. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov A.V., Barg M.A., Yakovleva E.Y. Can aging develop as an adaptation to optimize natural selection? (Application of computer modeling for searching conditions when the "Fable of Hares" can explain the evolution of aging) Biochemistry (Mosc.) 2018;83(12):1504–1516. doi: 10.1134/S0006297918120088. [DOI] [PubMed] [Google Scholar]
- Martin P., Kelly N., Kahana B., Kahana E., Willcox B.J., Willcox D.C., Poon L.W. Defining successful aging: a tangible or elusive concept? J. Gerontol. 2014;55(1):14–25. doi: 10.1093/geront/gnu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P., Boccardi V., Cecchetti R., Bastiani P., Scamosci M., Ruggiero C., Baroni M. A long journey into aging, brain aging, and Alzheimer’s disease following the oxidative stress tracks. J. Alzheimers Dis. 2018;62(3):1319–1335. doi: 10.3233/JAD-170732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirończuk-Chodakowska I., Witkowska A.M., Zujko M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. POLAND. 2018;63(1):68–78. doi: 10.1016/j.advms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Momtaz S., Abdollahi M. A comprehensive review of biochemical and molecular evidences from animal and human studies on the role of oxidative stress in aging: an epiphenomenon or the cause. Asian J. Anim. Vet. Adv. 2012 [Google Scholar]
- Monti D.M., Rigano M.M., Monti S.M., Peixoto H.S. Role of antioxidants in the protection from aging-related diseases. Oxid. Med. Cell Longev. 2019 doi: 10.1155/2019/7450693. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2008;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli E., Taroni F., Cortopassi G.A. Frataxin, iron–sulfur clusters, heme, ROS, and aging. Antioxidants Redox Signal. 2006;8(3-4):506–516. doi: 10.1089/ars.2006.8.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neha K., Haider M.R., Pathak A., Yar M.S. Medicinal prospects of antioxidants: a review. Eur. J. Med. Chem. 2019 doi: 10.1016/j.ejmech.2019.06.010. [DOI] [PubMed] [Google Scholar]
- Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5(35):27986–28006. [Google Scholar]
- Nyberg L., Pudas S. Successful memory aging. Annu. Rev. Psychol. 2019;70:219–243. doi: 10.1146/annurev-psych-010418-103052. [DOI] [PubMed] [Google Scholar]
- Oroian M., Escriche I. Antioxidants: characterization, natural sources, extraction and analysis. Food Res. Int. 2015;74:10–36. doi: 10.1016/j.foodres.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Panel M., Ghaleh B., Morin D. Mitochondria and aging: a role for the mitochondrial transition pore? Aging Cell. 2018;17(4) doi: 10.1111/acel.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Ahuir A., Manzanares-Estreder S., Proft M. Pro-and antioxidant functions of the peroxisome-mitochondria connection and its impact on aging and disease. Oxid. Med. Cell Longev. 2017 doi: 10.1155/2017/9860841. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C., Wang X., Chen J., Jiao R., Wang L., Li Y.M.…Ma K.Y. Biology of ageing and role of dietary antioxidants. BioMed Res. Int. 2014 doi: 10.1155/2014/831841. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruk G., Del Giudice R., Rigano M.M., Monti D.M. Antioxidants from plants protect against skin photoaging. Oxid. Med. Cell Longev. 2018 doi: 10.1155/2018/1454936. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008;4(2):89. [PMC free article] [PubMed] [Google Scholar]
- Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- Pomatto L.C., Davies K.J. Adaptive homeostasis and the free radical theory of ageing. Free Radic. Biol. Med. 2018;124:420–430. doi: 10.1016/j.freeradbiomed.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers S.K., Ji L.L., Kavazis A.N., Jackson M.J. Reactive oxygen species: impact on skeletal muscle. Comp. Physiol. 2011;1(2):941–969. doi: 10.1002/cphy.c100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor C.J., Goljanek-Whysall K. Using computer simulation models to investigate the most promising microRNAs to improve muscle regeneration during ageing. Sci. Rep. 2017;7(1):12314. doi: 10.1038/s41598-017-12538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putin E., Mamoshina P., Aliper A., Korzinkin M., Moskalev A., Kolosov A., Ostrovskiy A., Cantor C., Vijg J., Zhavoronkov A. Deep biomarkers of human aging: application of deep neural networks to biomarker development. Aging (Albany NY) 2016;8(5):1021. doi: 10.18632/aging.100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek C.R., Chandel N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter R.J., Tan D.X., Rosales-Corral S., Galano A., Zhou X.J., Xu B. Mitochondria: central organelles for melatonin′ s antioxidant and anti-aging actions. Molecules. 2018;23(2):509. doi: 10.3390/molecules23020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieusset J. The role of endoplasmic reticulum-mitochondria contact sites in the control of glucose homeostasis: an update. Cell Death Dis. 2018;9(3):1–12. doi: 10.1038/s41419-018-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnerthaler M., Bischof J., Streubel M.K., Trost A., Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5(2):545–589. doi: 10.3390/biom5020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M., Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Exp. Gerontol. 2010;45(6):410-418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Ristow M., Schmeisser S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011;51(2):327-336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat. Med. 2014;20(7):709–711. doi: 10.1038/nm.3624. [DOI] [PubMed] [Google Scholar]
- Ristow M., Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS) Dose Res. 2014;12(2):288–341. doi: 10.2203/dose-response.13-035.Ristow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb E.L., Christoff C.A., Maddalena L.A., Stuart J.A. Mitochondrial reactive oxygen species (ROS) in animal cells: relevance to aging and normal physiology. Can. J. Zool. 2013;92(7):603–613. [Google Scholar]
- Rottenberg H., Hoek J.B. The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell. 2017;16(5):943–955. doi: 10.1111/acel.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A.L., Sinha S., Lindner A.B. The good, the bad, and the ugly of ROS: new insights on aging and aging-related diseases from eukaryotic and prokaryotic model organisms. Oxid. Med. Cell Longev. 2018 doi: 10.1155/2018/1941285. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlin C.J., Dodson M.B., Madhavan L., Zhang D.D. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 2019;134:702–707. doi: 10.1016/j.freeradbiomed.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H.H., Stocker R., Vollbracht C., Paulsen G., Riley D., Daiber A., Cuadrado A. Antioxidants in translational medicine. Antioxidants Redox Signal. 2015;23(14):1130–1143. doi: 10.1089/ars.2015.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialò F., Fernández-Ayala D.J., Sanz A. Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front. Physiol. 2017;8:428. doi: 10.3389/fphys.2017.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialo F., Mallikarjun V., Stefanatos R., Sanz A. Regulation of lifespan by the mitochondrial electron transport chain: reactive oxygen species-dependent and reactive oxygen species-independent mechanisms. Antioxidants Redox Signal. 2013;19(16):1953–1969. doi: 10.1089/ars.2012.4900. [DOI] [PubMed] [Google Scholar]
- Scotti L., Scotti M.T. Computer aided drug design studies in the discovery of secondary metabolites targeted against age-related neurodegenerative diseases. Curr. Top. Med. Chem. 2015;15(21):2239–2252. doi: 10.2174/1568026615666150610143510. [DOI] [PubMed] [Google Scholar]
- Schroeder E.A., Raimundo N., Shadel G.S. Epigenetic silencing mediates mitochondria stress-induced longevity. Cell Metabol. 2013;17(6):954–964. doi: 10.1016/j.cmet.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel G.S., Horvath T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163(3):560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler K.D., Sevier C.S. Working Together: redox signaling between the endoplasmic reticulum and mitochondria. Chem. Res. Toxicol. 2019;32(3):342–344. doi: 10.1021/acs.chemrestox.8b00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simioni C., Zauli G., Martelli A.M., Vitale M., Sacchetti G., Gonelli A., Neri L.M. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018;9(24):17181. doi: 10.18632/oncotarget.24729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J.M., Lee C. Mitochondria: multifaceted regulators of aging. BMB Rep. 2019;52(1):13. doi: 10.5483/BMBRep.2019.52.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanatos R., Sanz A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018;592(5):743–758. doi: 10.1002/1873-3468.12902. [DOI] [PubMed] [Google Scholar]
- Suleman M., Khan A., Baqi A., Kakar M.S., Ayub M. 2. Antioxidants, its role in preventing free radicals and infectious diseases in human body. Pure Appl. Biol. 2019;8(1):380–388. [Google Scholar]
- Sun N., Youle R.J., Finkel T. The mitochondrial basis of aging. Mol. Cell. 2016;61(5):654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzman R., Beard J.R., Boerma T., Chatterji S. Health in an ageing world—what do we know? Lancet. 2015;385(9967):484–486. doi: 10.1016/S0140-6736(14)61597-X. [DOI] [PubMed] [Google Scholar]
- Szentesi P., Csernoch L., Dux L., Keller-Pintér A. Changes in redox signaling in the skeletal muscle with aging. Oxid. Med. Cell Longev. 2019 doi: 10.1155/2019/4617801. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurey P., Pizzo P. The aging mitochondria. Genes. 2018;9(1):22. doi: 10.3390/genes9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titorenko V.I., Terlecky S.R. Peroxisome metabolism and cellular aging. Traffic. 2011;12(3):252–259. doi: 10.1111/j.1600-0854.2010.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z., Sonntag W.E., Csiszar A. Mitochondria and aging in the vascular system. J. Mol. Med. 2010;88(10):1021–1027. doi: 10.1007/s00109-010-0667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiserman A.M., Lushchak V., Koliada A.K. Anti-aging pharmacology: promises and pitfalls. Ageing Res. Rev. 2016;31:9–35. doi: 10.1016/j.arr.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Van De Lagemaat E.E., De Groot L.C., van den Heuvel E.G. Vitamin B12 in relation to oxidative stress: a systematic review. Nutrients. 2019;11(2):482. doi: 10.3390/nu11020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron A., Vousden K.H. p53, ROS and senescence in the control of aging. Aging (Albany NY) 2010;2(8):471. doi: 10.18632/aging.100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viña J. The free radical theory of frailty: mechanisms and opportunities for interventions to promote successful aging. Free Radic. Biol. Med. 2019;134:690–694. doi: 10.1016/j.freeradbiomed.2019.01.045. [DOI] [PubMed] [Google Scholar]
- Wang Y., Branicky R., Noë A., Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018;217(6):1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger A., Kozlov A.V. Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules. 2015;5(2):472–484. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A.P., Brodsky I.E., Rahner C., Woo D.K., Erdjument-Bromage H., Tempst P., Walsh M.C., Choi Y., Shadel G.S., Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472(7344):476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A.P., Shadel G.S., Ghosh S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011;11(6):389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichansawakun S., Buttar H.S. Elsevier; 2019. Antioxidant Diets and Functional Foods Promote Healthy Aging and Longevity through Diverse Mechanisms of Action the Role of Functional Food Security in Global Health; pp. 541–563. [Google Scholar]
- Yun J., Finkel T. Mitohormesis. Cell Metab. 2014;19(5):757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeshan H.M.A., Lee G.H., Kim H.-R., Chae H.-J. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 2016;17(3):327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Gao Z., Hu C., Zhang J., Sun X., Rong C., Jia L. Antioxidant, antibacterial and anti-aging activities of intracellular zinc polysaccharides from Grifola frondosa SH-05. Int. J. Biol. Macromol. 2017;95:778–787. doi: 10.1016/j.ijbiomac.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Zhang H., Davies K.J., Forman H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Menzies K.J., Auwerx J. The role of mitochondria in stem cell fate and aging. Development. 2018;145(8):dev143420. doi: 10.1242/dev.143420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-J., Gan R.-Y., Li S., Zhou Y., Li A.-N., Xu D.-P., Li H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20(12):21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R.Z., Jiang S., Zhang L., Yu Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling. J. Mol. Med. 2019;44(1):3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsurka G., Peeva V., Kotlyar A., Kunz W.S. Is there still any role for oxidative stress in mitochondrial DNA-dependent aging? Genes. 2018;9(4):175. doi: 10.3390/genes9040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zujko M.E., Witkowska A.M., Waśkiewicz A., Mirończuk-Chodakowska I. Dietary antioxidant and flavonoid intakes are reduced in the elderly. Oxid. Med. Cell Longev. 2015 doi: 10.1155/2015/843173. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]