Abstract
Mechanisms of cellular and nuclear mechanosensation are unclear, partially because of a lack of methods that can reveal dynamic processes. Here, we present a new concept for a low-cost, three-dimensionally printed device that enables high-magnification imaging of cells during stretch. We observed that nuclei of mouse embryonic skin fibroblasts underwent rapid (within minutes) and divergent responses, characterized by nuclear area expansion during 5% strain but nuclear area shrinkage during 20% strain. Only responses to low strain were dependent on calcium signaling, whereas actin inhibition abrogated all nuclear responses and increased nuclear strain transfer and DNA damage. Imaging of actin dynamics during stretch revealed similar divergent trends, with F-actin shifting away from (5% strain) or toward (20% strain) the nuclear periphery. Our findings emphasize the importance of simultaneous stimulation and data acquisition to capture mechanosensitive responses and suggest that mechanical confinement of nuclei through actin may be a protective mechanism during high mechanical stretch or loading.
Significance
Cells can sense and respond to mechanical cues in their environment. These responses can be rapid, on the timescale of seconds to minutes, and new methods are required for their acquisition and study. We introduce a, to our knowledge, new concept for a three-dimensionally printed cell-stretch device that allows for simultaneous high-resolution imaging while also being low cost and easy to assemble to enable broad applicability. Using this device, we further demonstrated the importance of simultaneous stimulation and data acquisition to elicit mechanosensitive cell behavior as we observed rapid changes in nuclear size and reorganization of actin filaments around the nuclear border in skin cells. Overall, our results suggest that the rapid reorganization of actin during high loads might protect the genome from strain-induced damage.
Introduction
Mechanical cues from the environment are known to have a profound impact on cell fate (1) and cell behavior (2,3), a phenomenon referred to as mechanosensation. Changes in mechanical properties of the extracellular environment due to trauma (4,5), chronic conditions (6, 7, 8), or genetic predispositions (9,10) lead to cellular degeneration and result in a range of pathologies (11). Understanding the mechanisms involved in mechanosensation would help to work toward mitigating these conditions and might allow researchers to direct cell differentiation to generate artificial tissues for drug testing (12) or organ repair (13).
The nucleus is thought to be an essential mechanosensitive organelle because it is tightly connected to all parts of the cytoskeleton through linker of nucleo- and cytoskeleton (LINC) complexes (14,15). Cyclic stretch has been shown to change nuclear morphology (16, 17, 18), induce chromatin condensation (17, 18, 19), increase mechanical resistance of nuclei (16,18,20), and alter gene expression (21,22). Disruption of LINC complexes has shown to inhibit stretch-induced changes in chromatin remodeling (23, 24, 25) and gene expression (21,22), suggesting that strain transfer from the cytoskeleton plays an important role for nuclear mechanosensation. The actin skeleton, in particular, has been shown to be crucial for nuclear responses to dynamic mechanical stimulation (18,21,23,26). Despite these findings, the underlying mechanisms of cellular and nuclear mechanosensation pathways are mostly unclear, partially because of a lack of accessible methods to image cell behavior under mechanical stimulation in real time.
Nuclear responses to mechanical stimulation have been shown to occur within seconds (17,21), which highlights the importance of simultaneous stimulation and data acquisition to understand these processes. However, commercially available cell-stretch devices (e.g., Flexcell) do not allow for the use of high-magnification objectives necessary to elucidate single cell behavior (27). Custom-built devices designed by research groups either lack high-resolution live imaging capability (28,29) or use expensive components such as precise linear actuators (30, 31, 32) or optical traps (33). In addition, designs are often complicated and require special expertise, which overall makes them difficult to replicate for widespread use.
Here, we propose the design of a low-cost TENSCell (tension in cells) device that uses an electromagnetic piston to apply equiaxial stretch to a thin silicone membrane suspended over a water immersion objective (Fig. 1 a; Fig. S1). We used electromagnetic force to drive the membrane deformation because it can be precisely controlled without requiring special components, in our case only needing an electromagnetic coil made in-house and a permanent rare earth magnet attached to the piston. Most device parts were three-dimensionally printed, making the system easy to replicate at a low cost. Only one part, a tapered circular ring that interfaces with the silicone membrane, was machined because of low-friction material requirements. The remaining parts (rare earth magnet, linear rails with carriages, and electronics for control) were generally inexpensive and readily available (Table S1). Using the TENSCell device, we investigated the dynamic response of nuclei from mouse embryonic skin fibroblasts when exposed to cyclic stretch. Our experiments verified that nuclei respond rapidly (within minutes) to mechanical stimulation. To our surprise, we observed opposing nuclear responses during low (5%) or high (20%) strain regiments, as we observed nuclear area shrinkage and chromatin condensation in response to high strain but nuclear area expansion and chromatin decompaction in response to low strain. Our results further suggest that this dichotomous behavior might be mediated through different pathways because only responses to low cyclic strain were dependent on calcium signaling, whereas the actin skeleton was necessary for responses to both low and high strains. Further investigation of actin dynamics during cyclic stretch revealed a similar divergent behavior, with actin filaments shifting toward the nuclear periphery during high loads and toward the cell border during low loads. Actin depolymerization leads to an increase in nuclear strain transfer and DNA damage, overall suggesting that F-actin-dependent nuclear shrinkage might serve as a protective mechanism during high strain exposure.
Figure 1.
TENSCell device allows for precise and repeatable membrane stretch via electromagnetic force. (a) A cross-sectional illustration of the stretch device and its control circuit is shown. A suspended piston containing a permanent magnet moves vertically through an electromagnetic coil. Downward motion of the piston stretches a silicon membrane over a deformation ring, which holds the stretched membrane at a constant distance over a microscope objective. See Fig. S1b for real images of the device. To control the electromagnet, an H-bridge is used to modulate intensity and direction of a constant current (6 A) from a DC power source through low voltage signals using an Arduino microprocessor. On the Arduino, a power-wave-modulation pin (∼, 0–5 V) is used to control the intensity, and a digital pin (D, 0 or 1) is used to control the direction of the current. A USB interface enables control of the Arduino inputs via MATLAB. (b) A distance measurement laser was used to investigate piston movement, and thereby membrane indentation, in response to electromagnetic force as represented by Arduino input voltages. Electromagnetic force could be used for the precise membrane stretch. (c) A sinusoidal function was programmed in MATLAB to generate Arduino inputs from +0.8 to −0.8 V at a frequency of 1 Hz, and piston indentation was recorded over five cycles. Electromagnetic force could be used for precise and repeatable membrane indentation. To see this figure in color, go online.
Methods
TENSCell device fabrication
Our custom-built device was designed to acquire high-magnification images of cells during the application of equiaxial strain while also avoiding the use of expensive materials (Table S1) or complex designs. Two types of three-dimensional (3D) printers were used to fabricate a majority of the components: Objet30 (Computer Aided Technology, Buffalo Grove, IL), using the material VeroClear (OBJ-04055; Computer Aided Technology), and the uPrint SE Plus (311-20200; Computer Aided Technology), using ABS+. Designs for the device components were created as CAD files (Fig. S1 a) using SolidWorks (v. 2018; Dassault Systèmes SolidWorks). All parts were printed at the Integrated Teaching and Learning Laboratory at the University of Colorado Boulder.
The main body consisted of four parts: a deformation ring holder, an electromagnet case, a slider tube, and a piston (Fig. 1 a; Fig. S1 b), which were printed using the uPrint SE Plus. The deformation ring holder was designed to fit into the circular notch of the manual stage of a Nikon Eclipse Ti microscope (Nikon, Tokyo, Japan) by interlocking with two metal wings that otherwise hold the aluminum sample tray and contained an adaptor that encased the microscope objective and held the deformation ring. Within the encasement, the objective had a moving range of ∼8 mm in each direction. The deformation ring was machined from Delrin Acetal Resin (8572K27; McMaster-Carr, Elmhurst, IL) to provide a friction-reduced interface with the silicone membrane. The slider tube fit tightly into the electromagnetic coil and contained three slider rails (NS-01-27; Igus, Cologne, Germany) for friction-reduced vertical movement of the piston, which in turn contained three matching slider carriages (NW-02-27; Igus). A rare earth magnet (R3525; SuperMagnetMan, Birmingham, AL) was attached to the top of the piston to transmit force from the electromagnet below.
The stretch chamber consisted of three parts: a main chamber, a membrane ring, and holding clips (Fig. S1 c), which were printed using the Objet30. The stretch chamber was assembled by placing a 60 × 60 mm silicon elastomer membrane (gloss 0.005; Specialty Manufacturing, St. Paul, MN) straight onto the elevated inner edge of the main chamber, spanning and fixing the membrane with the membrane ring. The membrane ring was secured laterally with three holding clips. To culture cells, a compliant silicon containment ring was fused to the silicon membrane before assembly. Containment rings (dout = 16 mm, din = 11 mm, h = 5 mm, Ain = 100 mm2) were made from polydimethylsiloxane (Sylgard184; Dow Corning, Midland, MI) using a 1:40 mixing ratio to produce soft rings with low mechanical resistance. Circular plastic molds were coated with 3,3,3-trifluoropropyl-trichlorosilane (452807; Sigma-Aldrich, St. Louis, MO) for 1 h under vacuum, after which polydimethylsiloxane was poured into molds and cured over night at 80°C. The contact areas between the silicone membranes and the silicone containment rings were ozone activated for 60 s via corona arc discharge (BD-20; Electro-Technic Products, Chicago, IL), after which rings were pressed onto the membranes, weighted down with a 100 g weight to maintain close contact, and incubated again overnight at 80°C to facilitate bonding. Bonded membranes were sterilized with 70% ethanol and dried, and stretch chambers were assembled.
TENSCell device control
To operate the stretch device, a simple control circuit was designed in which an Arduino microcontroller (DEV-13975; SparkFun Electronics, Niwot, CO) modulated the magnitude and direction of a constant 6 A current from a DC power source (9129B; BK Precision, Yorba Linda, CA) to the coil via an H-bridge (RB-Cyt-132; RobotShop, Mirabel, Quebec, Canada). Two signals from the Arduino to the H-bridge controlled the current flow: A power-wave-modulation pin sending low voltage from 0 to 5 V controlled the current intensity (Fig. S2 a), and a digital pin (either 0 or 1) controlled the direction of the current to allow lifting of the piston in the relaxed state or attracting the piston downwards to intendent the engaged membrane. Arduino inputs were controlled via MATLAB (v. 2018b; The MathWorks, Natick, MA) via a USB interface and the Arduino Support from MATLAB package, and a custom-written code was used to operate the device.
TENSCell device calibration
To measure piston movement and associated membrane indentation in response to electromagnetic fields, a laser distance sensor (LJ-G5001P; Keyence, Osaka, Japan) was pointed vertically at the top of the piston, and changes in vertical movement were recorded via the Keyence LJ-Navigator software (v. 1.7.0.0; Keyence). Particle tracking (34) was used to determine the amount of strain applied to the membrane in response to electromagnetic fields. For strain measurements, containment wells were coated with 2 μm blue fluorescent beads (F8824; Life Technologies, Carlsbad, CA), and images were recorded before and after membrane indentation on an inverted epifluorescence microscope (Ti-Eclipse; Nikon) with a 60× water immersion objective (0.26 μm/pixel) and an EMCCD camera (iXonEM+; Andor Technology, South Windsor, CT). Bead displacements were determined via the particle image velocimetry plugin on ImageJ (v. 1.50e; National Institutes of Health (NIH), Bethesda, MD), and hydrostatic strain was calculated from bead displacements (24) using a custom-written MATLAB code (v. 2018b; The MathWorks). To determine the offset, the Arduino input voltage that kept the piston suspended over the deformation ring against its own weight, the piston was placed in a position in which the membrane would not touch the deformation ring and membrane strains were determined during stepwise reduction of the magnetic field. The offset Arduino input voltage was determined as the input before distinct changes in membrane strain could be observed (Fig. S2 b). Electromagnetic fields produced by the coil (Fig. S6) were modeled using COMSOL (v. 5.2.0.166).
Mouse embryonic skin fibroblast isolation, culture, and pharmacological treatments
Transgenic mice harboring a fluorescence tag at the H2b histone—B6.Cg-Tg (HIST1H2BB/EGFP) 1 Pa/J, Stock No: 006069—were obtained from Jackson Laboratory (Bar Harbor, ME). All animal procedures were performed following Institutional Animal Care & Use Committee approval. Skin from embryonic mice was harvested 18.5 days postconception. Skin was minced, washed with Hank's Balanced Salt Solution (HBSS), and incubated in 35 mm dishes in shallow medium (∼0.5 mL) to avoid floating of the tissue for 4 days, during which fibroblasts extruded from the tissue. After 4 days, remaining tissue was removed (picked out with a pipette), and extruded cells in the dish were detached using TrypLE (Gibco, Gaithersburg, MD) and expanded in culture for another 2 days before being seeded into stretch chambers for experiments. The inner well of assembled stretch chambers was ozone activated for 30 s via corona arc discharge (BD-20; Electro-Technic Products) and coated with 50 μg/mL bovine plasma fibronectin (F1141; Sigma-Aldrich) in a total volume of 250 μL overnight at 37°C. Mouse embryonic skin fibroblasts (MSFs) were seeded into stretch chambers at a density of 80,000 cells/cm2 1 day before experiments. MSFs were extruded and cultured in DMEM (ATCC, Manassas, VA) containing 10% fetal bovine serum (Gibco), 1% penicillin-streptomycin (Gibco), and 25 mM HEPES (Gibco) at 37°C and 5% CO2. To inhibit calcium signaling, MSFs were incubated with 50 μM BAPTA (A4926; Sigma-Aldrich) or 10 μM KN-62 (I2142; Sigma-Aldrich) 1 h before cyclic strain experiments. To disrupt actin polymerization, MSFs were incubated with 2 μM cytochalasin D (C8273; Sigma-Aldrich) 30 min before experiments. Vehicle controls were incubated with 0.001% DMSO (276855; Sigma-Aldrich) 1 h before experiments. To test the use of chromatin dyes, cells were stained with Hoechst (NucBlue Live ReadyProbes; Life Technologies) 30 min before experiments. Stretch experiments were performed at 25°C (room temperature) under humidified conditions.
Live imaging and analysis of nuclear behavior during stretch routines
The stretch device was mounted on an inverted epi-fluorescence microscope (Ti-Eclipse; Nikon) with a 60× water immersion objective (0.26 μm/pixel) and an EMCCD camera (iXonEM+; Andor Technology). The surrounding of the microscope was humidified to prevent the water on the objective from evaporating during the 1 h experiment. Oil immersion is also possible, but water immersion provided better image quality with the silicone membranes used.
Using MATLAB, a sinusoidal control signal (1 Hz) was sent to the Arduino controller, resulting in a cyclic stretch routine of the membrane (Fig. 1 c), with peak strains set to 5% (−0.1 V), 10% (−0.9 V), and 20% (−2.25 V) as determined by the calibration curve. Cells were cyclically stretched for 30 min, followed by 30 min of rest, during which 2 μm z-stacks (0.5 μm steps) of nuclei were acquired. During the rest period, cells experienced no stretch but a constant magnetic field using the offset Arduino input voltage (0.8 V) that keeps the piston suspended at 0% stretch. For acquisition, the cyclic stretch routine was paused for 1 min to allow for a steady imaging plane and fine readjustments in z-position due to water evaporation. Unstretched control cells (0%) were seeded into stretch chambers but kept stationary on the microscope without any magnetic field applied. Unstretched magnetic control cells (MAG) were placed stationary inside the coil without the piston, and a magnetic field corresponding to a 20% sinusoidal stretch routine was applied.
A custom MATLAB code was used to track nuclear outlines via H2b-eGFP and to generate H2b intensity histograms during an image series, from which changes in nuclear area, H2b skewness, and H2b kurtosis were calculated.
To analyze the amount of strain transferred from the membrane to the nucleus after cyclic stretch routines, image stacks of nuclei were acquired under relaxed or stretched conditions, and bulk nuclear strain was calculated from the change in nuclear area using the same MATLAB script as above. Note that nuclear strain transfer could not be accurately analyzed before stretch routines because of the fast response in area decline. MATLAB code is available from the corresponding author upon request.
Perinuclear F-actin and γH2a.x staining and analysis
MSFs were fixed in 4% ice-cold paraformaldehyde (PFA) for 10 min, permeabilized with 1% Triton-X100 in phosphate-buffered saline for 15, min and blocked with 10% NGS, 1% bovine serum albumin in 0.1% PBT (0.1% Tween-20 in phosphate-buffered saline) for 60 min. Primary incubation of Phospho-Histone H2A.X Ser139 (9718S; Cell Signaling Technology, Danvers, MA) was performed at 4°C overnight in 0.1% PBT containing 1% bovine serum albumin at 1:400. Secondary incubation of goat anti-rabbit IgG-AF546 (Life Technologies) was performed in primary incubation buffer for 45 min at 22°C at a dilution of 1:500. F-actin was counterstained with phalloidin-CF405 (Biotium, Fremont, CA) for 30 min at a dilution of 1:30. Staining was performed in containment wells. After staining, membranes were cut out of the containment well using a circular punch and mounted cell-side up onto #1.0 glass slides with ProLong Diamond Antifade Mountant (Life Technologies). Image stacks (5 μm, 1 μm step) of multiple nuclei in a 318 × 318 μm area were acquired on a Nikon A1R confocal microscope using a 40× oil immersion objective (0.31 μm/pixel). A custom MATLAB code was used to track nuclear outlines via H2b-eGFP and to quantify perinuclear F-actin intensities and γH2a.x foci in projected image stacks. Only the projected F-actin within the nuclear outline (apical perinuclear F-actin) was counted in this analysis. To account for variations in staining and imaging (the same imaging settings were used), fluorescence channels were histogram normalized. Perinuclear F-actin intensities were calculated as the sum of normalized phalloidin intensities within a nuclear border. DNA damage foci were determined by detecting 2D peaks in the normalized γH2a.x channel using the MATLAB script FastPeakFind (v. 1.7) previously programmed by Adi Natan. MATLAB code is available from the corresponding author upon request.
LifeAct transfection and analysis of actin dynamics
MSFs were transfected with mRuby-Lifeact-7 using Lipofectamine 3000 (Life Technologies) 18 h after seeding into stretch chambers; mRuby-Lifeact-7 was a gift from Michael Davidson (#54560; Addgene, Cambridge, MA). 1 day after transfection, 2 μm image stacks (0.5 μm steps) of actin (Lifeact) and nuclei (H2b) were acquired during 30 min of cyclic stretch, followed by 30 min of rest (no stretch, static magnetic field using the offset Arduino input +0.8 V). Profile lines were generated with the Plot Profile function in ImageJ (v. 1.50e; NIH) using an eight pixel (= 2.1 μm) thick line. Binned profile analysis was performed using a custom MATLAB code that tracked cell and nuclear outlines during an image series using the Lifeact or H2b fluorescence channel, respectively. Image stacks were rotated to align actin filaments in the horizontal direction, and projections of 8-pixel-thick vertical profile lines that crossed through the nuclear center were extracted for further analysis. During each time step, profile line positions were fixed with respect to the nuclear center. Using the position of the cell and nuclear boundary, profile lines were binned with five bins representing Lifeact intensities from the nuclear center to the inner nuclear border and another five bins representing intensities from the nuclear periphery to the cell border. Corresponding bins from each half of the cell were averaged. MATLAB code is available from the corresponding author upon request.
Statistical analysis
One-way ANOVA with Tukey’s honestly significant difference post hoc test or two-tailed t-test analysis was performed to evaluate statistical significance using JMP Pro12 software (SAS Institute, Cary, NC). Displayed error (SD, standard deviation; SEM, standard error of the mean), number of individual data points (n), and number of independent experiments (exp., if different from n), as well as significances and statistical tests that were used, are indicated in the figure captions.
Results
Cell-stretch device: concept and calibration
To record the dynamic responses of single cells at high magnification during mechanical stimulation, we built a system in which a thin (∼127 μm) silicone membrane is stretched over a microscope objective using an electromagnetic piston (Fig. 1 a; Fig. S1 a). A static deformation ring around the objective allowed for a stable imaging plane in the x, y, and z directions. The silicone membrane was assembled into a stretch chamber (Fig. S1 b) and fused to a compliant silicone ring to form a containment well, e.g., to culture cells. The stretch chamber, in turn, could be engaged into a piston that could move freely up and down in an electromagnetic coil via sliders. The piston contained a rare earth magnet at the top to transfer force from the electromagnet below. When current was applied to the coil, the resulting electromagnetic field lifted the piston up or pushed it down depending on the orientation of the magnetic field. The electromagnetic field, in turn, was controlled through an Arduino microprocessor that modulated intensity and the direction of current from a constant DC power source (Fig. S2 a). A laser displacement sensor was used to show that the electromagnetic field could be used to precisely (Fig. 1 b) and repeatedly (Fig. 1 c) control the position of the piston and, therefore, the indentation of the membrane.
To calibrate the TENSCell device, membranes were coated with fluorescent beads. During membrane stretch, bead displacements were recorded on a microscope to calculate the resulting strain using traction force microscopy (Fig. 2 a; (34)). Because the piston has a weight that would stretch the membrane in the absence of a magnetic field, the Arduino input voltage that produced an electromagnetic field to keep the piston suspended without membrane indentation (offset) was determined to be +0.8 V (Fig. S2 b). For calibration, we recorded bead displacements in response to different Arduino inputs from +0.8 to −3.25 V. The resulting current versus strain calibration curve fit best to a third-order polynomial function because of a mild inflection around 0 V; however, a second-order polynomial function fit almost equally well and was chosen for simplicity (Fig. 2 b). Based on the SD observed, the minimum strain that could be applied reliably is ∼0.5%. The maximum strain that can be applied is dependent on the device properties, more specifically strength of the magnetic field and indentation depth of the deformation ring holder. The maximum strain of the device used in this study is around 29%. Plotting of membrane indentation versus strain from associated Arduino inputs showed a linear relationship, as would be expected from a linear elastic material such as silicone (Fig. 2 c). Analysis of repeated membrane stretch from +0.8 to −0.9 V, corresponding to 10% strain, showed reliable strain application within 0.5% with no indication of declining or increasing trends within 11 repeats (Fig. 2 d). The material properties of silicone elastomers have been well tested, and previous studies have shown that they can be reliably loaded for 102–103 cycles (35). Next, we performed live-cell imaging of cells during cyclic stretch routines to validate the utility of our device.
Figure 2.
TENSCell device calibration and performance using particle tracking. Membrane containment wells were coated with 2-μm-sized fluorescent beads, and membrane strains at different Arduino input voltages were determined from bead displacements. The start point of the calibration was the offset Arduino input voltage (+0.8 V; see Fig. S2b) at which the membrane rests on the deformation ring without stretch. (a) An example of recorded bead displacements and the resulting strain map for an Arduino input of −0.9 V are shown. (b) Calibration curve as determined through bead displacements is shown. The acquired data fit a second-order polynomial function; SD; n = 3. (c) Plotting calculated membrane strains over measured membrane indentations from Fig. 1b showed a linear relationship. (d) Measurements of consecutive membrane indentation for an Arduino input of −0.9 V showed repeatable application of strain within 0.5%. To see this figure in color, go online.
Nuclei of MSFs show dichotomous responses to low and high strain levels
To test the device, we investigated the dynamic behavior of cell nuclei from MSFs from H2b-eGFP harboring mice in response to equiaxial stretch. MSFs were seeded onto fibronectin-coated stretch chambers and stimulated with a sinusoidal stretch routine (1 Hz) for 30 min with peak strains of 5, 10, or 20%. The stretch routine was followed by 30 min of no stimulation (rest) to determine reversibility of any observed responses. In addition to unstretched controls (0%), cells were also subjected to a magnetic field that corresponded to a 20% stretch routine (MAG) under static conditions (no stretch) to assess effects of the magnetic field alone. Image stacks of nuclei were acquired via H2b-eGFP (Fig. 3 a; Videos S1, S2, S3, and S4). Unstretched control cells (0%) showed a continuous but slight decline in nuclear area during the 1 h experiment (Fig. 3 b). This was likely a result of cell toxicity because of light radiation during imaging and performing experiments at room temperature (Methods). In contrast, the areas of nuclei decreased rapidly in response to high strains of 20%, continued decreasing during the 30 min of stretch, and increased again during the 30 min rest period. Rapid here is defined as observing an immediate and significant change based on the time resolution (approximately minutes) of our experiment. Nuclei subjected to 10% cyclic strain also showed a decrease in area, but to a lesser extent and more delayed compared with the 20% strain routine. Surprisingly, at 5% strain, nuclear areas increased during stretch and stayed elevated during rest.
Figure 3.
MSF nuclei show opposing changes in nuclear area and chromatin condensation during low-strain and high-strain cyclic stretch. MSFs were exposed to 30 min of sinusoidal stretch with peak strains of 0, 5, 10, or 20%, followed by 30 min of no stimulation (rest), during which image stacks of nuclei were recorded. Control cells were exposed to the magnetic field alone without stretch (MAG). (a) Images of nuclei recorded via H2b-eGFP are given; scale bars, 5 μm. (b) Relative changes in nuclear area (relative to 0 min) during stretch routines are shown. Nuclear areas decreased in response to high strains (10, 20%), but increased for low strain (5%), whereas exposure to the magnetic field alone (MAG) showed no difference compared to unstretched cells (0%). (c) Difference in H2b histogram kurtosis and skewness (compared with 0 min) during stretch routines is shown. Kurtosis and skewness increased under high-strain routines (10, 20%), whereas they decreased for low strain (5%), indicating elevated or subsided chromatin condensation, respectively; SEM; n > 24 from four exp.; ANOVA: #p < 0.01 for 20% vs. all, $p < 0.01 for 10% vs. all, †p < 0.01 or ‡p < 0.05 for 5% vs. 10%, &p < 0.01 or %p < 0.05 for 20% vs. MAG, 0 and 5%, +p < 0.05 for 5% vs. MAG, ×p < 0.05 for 5% vs. MAG and 0%. To see this figure in color, go online.
We further analyzed changes in chromatin organization by measuring the skewness and kurtosis of chromatin intensity histograms, with a shift toward higher intensities (positive skewness) and narrowing of the histogram peak (positive kurtosis) indicating elevated chromatin compaction (36). Similar to nuclear area, MSF nuclei subjected to 20% cyclic strain showed a rapid (<2 min) increase in both skewness and kurtosis during the stretch interval, followed by a mild decline during the rest period. Nuclei subjected to 5% cyclic strain showed a decrease in skewness and kurtosis during the stretch and rest period (Fig. 3 c). Changes in nuclear area, as well as H2b histogram skewness and kurtosis of MSFs subjected to the magnetic field alone (MAG), closely matched that of unstretched control cells (0%) in absence of a magnetic field, suggesting that the magnetic field had no influence on the observed cell behavior.
To test whether chromatin dyes are suitable to investigate mechanosensitive behavior of nuclei, MSFs were subjected to a 20% strain routine after staining with Hoechst (Fig. S3). Stained nuclei showed a similar dynamic of area decline during stretch, but no recovery during the rest period, compared to unstained nuclei. Additionally, measures of chromatin condensation were reduced during stretch, overall suggesting that chromatin dyes do not alter general nuclear sensitivity to stretch but interfere with chromatin reorganization. Together, these results further confirmed that nuclei can respond rapidly (<2 min) to mechanical stimulation, which emphasizes the need for live imaging capabilities to capture these effects. Moreover, we were surprised to find that nuclei of MSFs showed divergent responses to different levels of strain, as nuclear areas were increased and chromatin compaction decreased for low strains and vice versa for high strains.
Nuclear responses of MSFs to low, but not high, cyclic strain are sensitive to calcium, whereas actin is essential for both
We observed a dichotomous change in nuclear area and chromatin condensation of MSF nuclei in response to high and low cyclic strain. Next, we aimed to investigate whether these responses were mediated through different signaling pathways. Stretch-induced chromatin condensation has been shown to be dependent on calcium signaling (17). Treatment of MSFs with BAPTA (BP) to sequester extracellular calcium or KN-62 (KN) to inhibit intracellular calmodulin signaling abrogated the increase in nuclear area and decrease in chromatin condensation observed for nontreated (NT) and vehicle (VH) control cells during 5% cyclic strain routines (Fig. 4). Conversely, calcium inhibition only minorly effected the decrease in nuclear area and increase in chromatin condensation during 20% cyclic strain routines. Interestingly, both BP and KN treatments interfered with the slight decrease in nuclear area and increase in chromatin condensation observed during static magnetic-field-only routines (MAG).
Figure 4.
The actin skeleton, but not calcium signaling, is required for nuclear responses to high-strain cyclic stretch. MSFs were treated with BAPTA (BP), KN-62 (KN), or cyto D (CD) before being exposed to 30 min of sinusoidal stretch with peak strains of 5, 20, or 0% under the influence of the magnetic field alone (MAG), during which image stacks of nuclei were recorded. (a) Images of nuclei recorded via H2b-eGFP before (0 min) or after (30 min) cyclic stretch routines are given; scale bars, 5 μm. (b) CD treatment inhibited changes in nuclear area and chromatin condensation in response to 5 and 20% cyclic stretch compared to NT or VH control cells. BP or KN treatment abrogated the increase in nuclear area and decrease in chromatin condensation after 5% cyclic stretch but had no effect after 20% cyclic stretch; NT cell data same as Fig. 3 for MAG, 5 and 20%, respectively; SEM; n ≥ 15 from three exp.; ANOVA: ∗p < 0.05, ∗∗p < 0.01. To see this figure in color, go online.
Further, the actin skeleton has been shown to be crucial for mechanosensitive signaling and is thought to be an important structure for forwarding mechanical cues to the nucleus (18,21, 22, 23,26). Treatment of MSFs with the actin depolymerization drug cytochalasin D (cyto D, CD) also abrogated the increase in nuclear area in response to 5% cyclic strain and distinctly increased chromatin condensation (Fig. 4). Interestingly, cyto D treatment inhibited nuclear shrinkage and chromatin condensation during 20% strain routines. In contrast to calcium inhibition, cyto D treatment showed no effect on MSF nuclei during static magnetic-field-only routines. Together, this data suggested that calcium signaling plays a role during low-strain, but not high-strain, stimulation, whereas an intact actin skeleton was crucial for nuclear responses to any magnitude of stretch.
Actin depolymerization increases DNA damage after low and high cyclic strain
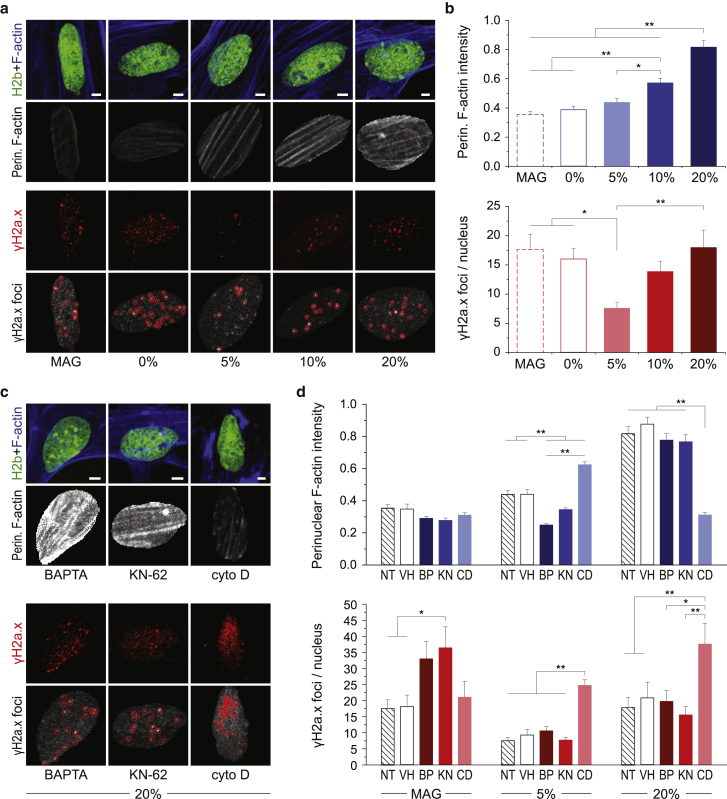
The calcium-independent shrinkage of nuclear areas suggested that there is a different mechanism for cell behavior under high strain compared with low strain. Cyclic stretch has been shown to cause DNA damage (37,38). To test whether actin-mediated nuclear shrinkage under high loads might be a protective mechanism to prevent DNA damage, we stained MSFs for serine-139 phosphorylated H2a.x (γH2a.x) to indicate DNA double-strand breaks (39) and filamentous actin (F-actin) after 30 min of cyclic stretch. F-actin intensities above the nucleus (perinuclear F-actin) increased with strain magnitudes, being only slightly higher after 5% and twice as high after 20% cyclic strain compared to unstretched (0%) and magnetic-field-only control cells (MAG, Fig. 5, a and b). The number of γH2a.x foci per nucleus also increased with increasing levels of strain. Interestingly, DNA damage was as high under static conditions (0%, MAG) as after 20% cyclic stretch.
Figure 5.
Perinuclear F-actin increases with strain magnitude, and actin depolymerization leads to increased DNA damage after low- and high-strain cyclic stretch. MSFs were exposed to 30 min sinusoidal stretch routines with peak strains of 0, 5, or 20%, or under the influence of the magnetic field alone (MAG), after which cells were stained for γH2a.x, as an indicator of DNA double-strand breaks, and F-actin. (a) Stained images of nuclei after stretch routines are given. A custom MATLAB code was used to analyze perinuclear F-actin intensities, using H2b-eGFP as a mask, and to identify γH2a.x foci, as indicated by red circles. (b) Perinuclear F-actin intensities and number of γH2a.x foci increased with strain magnitude; however, the highest levels of DNA damage were observed for static control cells. (c and d) MSFs were treated with BP, KN, or CD before stretch routines. Inhibition of calcium signaling via BP and KN treatment abrogated increases in perinuclear F-actin intensities in response to 5%, but not 20%, cyclic stretch. Actin depolymerization altered F-actin intensities and showed increased number of foci after both 5 and 20% cyclic stretch, whereas DNA damage was only increased for static magnetic-field-only control cells after inhibition of calcium signaling; see Fig. S4a for MAG and 5% images; SEM; n ≥ 150 from three exp.; ANOVA: ∗p < 0.05, ∗∗p < 0.01; all scale bars, 5 μm. To see this figure in color, go online.
Similar to nuclear responses, inhibition of calcium signaling via BP or KN abrogated the increase in perinuclear F-actin intensities after 5% cyclic stretch, but not after 20% cyclic stretch, compared to NT or VH control cells (Fig. 5, c and d; Fig. S4 a). Inhibition of calcium signaling increased DNA damage in static magnetic-field-only controls but showed no difference for cells after 5 or 20% cyclic stretch. In contrast, actin depolymerization via cyto D treatment distinctly increased the number of γH2a.x foci per nucleus after 5 and 20% stretch routines but had no effect on cells exposed to the magnetic field alone. Cyto D treatment also showed no effect on perinuclear F-actin intensities after magnetic-field-only routines. The increase in F-actin intensities after 20% cyclic stretch was abrogated in cyto-D-treated MSFs, as would be expected after actin depolymerization. However, F-actin intensities were increased after 5% cyclic stretch in cyto-D-treated MSFs. Judging from the stained images, this was due to an accumulation of small actin filaments at the nuclear periphery (Fig. S4 a). Further, imaging of nuclei in relaxed or stretched conditions after 30 min of 20% cyclic strain showed that strains transferred to nuclei were higher in cyto-D-treated cells compared to NT cells (Fig. S4 b). Overall, these data showed that perinuclear F-actin increased with increasing levels of strain and actin depolymerization resulted in elevated occurrences of double-strand breaks during high and low cyclic stretch, suggesting that the actin skeleton might play a protective role for the nucleus during high loads.
Live imaging of actin dynamics revealed opposing patterns of reorganization during low and high cyclic strain
Inhibition of actin polymerization abrogated nuclear responses to high- and low-strain routines and increased DNA damage with increasing levels of strain. To further investigate the role of the actin skeleton during stretch-induced changes in cell behavior, we transfected MSFs with a fluorescent F-actin probe (mRuby-Lifeact-7) and acquired image stacks during 30 min of 5 or 20% cyclic stretch followed by 30 min of rest (Video S5). Analysis of 2-μm-thick profile line projections along the minor axis (perpendicular to F-actin) of two example cells showed that Lifeact intensities shifted toward the cell border after 30 min of 5% stretch, whereas they shifted toward the nucleus after 20% (Fig. 6, a and b). To verify these findings in different cells, profile line projections were grouped into bins along their relative distance to the nuclear center, with bins 1–5 representing Lifeact intensities from the nuclear center to the nuclear periphery and bins 6–10 representing intensities from the cytoplasmic site of the nuclear border to the cells border. After 30 min of cyclic stretch, Lifeact intensities were elevated above the nuclear interior (bins 1–4) in cells exposed to both low (5%) and high (20%) levels of strain (Fig. 6 c). However, Lifeact intensities were decreased at the nuclear border (bin 6) and increased toward the cell border (bin 10) in cells after 5% of cyclic stretch, whereas intensities were increased at the nuclear border and decreased at the cell border after 20% of cyclic stretch.
Figure 6.
Lifeact imaging reveals opposing changes of actin reorganization at the cell and nuclear border during low-strain and high-strain cyclic stretch. MSFs were transfected with mRuby-Lifeact-7. The day after, cells were exposed to 30 min of 5 or 20% sinusoidal stretch, followed by 30 min of no stimulation (rest), during which image stacks were recorded. (a) Images of actin (Lifeact) or nuclei (H2b) recorded during the stretch routine are given. White dotted lines represent the center location of profile lines in (b); scale bars, 10 μm. (b) Projections of actin (Lifeact) and nuclear (H2b) profile lines, as indicated in (a), of two example cells before (0 min) or after (30 min) exposure to either 5 or 20% cyclic stretch are given. (c) Changes in Lifeact intensities after 30 min of stretch were binned into relative locations to compare changes in different cells: Bins 1–5 represent intensities from the nuclear center to the inner nuclear border and 6–10 from the nuclear periphery to the cell border. Lifeact intensities shifted from the cell border to the nuclear periphery after 20% cyclic stretch, whereas this trend was inversed after 5% cyclic stretch; SEM; n = 6; t-test (vs. 0 min): ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (d) Changes in Lifeact intensities at the nuclear periphery (bin 6) and cell border (bin 10) over time. Actin reorganization shows dynamics similar to that of nuclear responses; SEM; n = 6; t-test (vs. 0 min): ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. To see this figure in color, go online.
Analysis of Lifeact intensities over time showed a steady decrease or incline of intensities at the nuclear border during the 30 min of stretch in response to low- or high-strain routines, respectively (Fig. 6 c). Intensities stayed declined or elevated during rest at the nuclear border. At the cell border, Lifeact intensities declined rapidly in response to 20% strain, continued decreasing during the stretch period, and raised again during the 30 min of rest, albeit staying lower compared to 0 min. This change in Lifeact intensity was noticeably similar to the dynamics of nuclear area shrinkage after high-strain cyclic stretch, suggesting that F-actin rearrangement might mediate the nuclear response through physical interaction. During 5% cyclic stretch, Lifeact intensities slowly increased at the cell border and declined to reduced levels of intensity, compared with 0 min, during rest. In addition, we observed acute nuclear collapse and actin filament disruption in some cells that were exposed to 20% cyclic stretch (Fig. S5). These observations were more pronounced after the rest period, indicating that the cell died through either necrosis or apoptosis. This supported the notion that elevated strains are a challenge for cell survival. Taken together, we observed that F-actin also showed opposing patterns of reorganization for different levels of strains, shifting away from the nucleus during low-strain and toward the nucleus during high-strain routines. Furthermore, the dynamics of F-actin rearrangements matched that of nuclear responses. Further considering the results from the DNA damage analysis, this suggested that F-actin might mediate the nuclear shrinkage during high loads through physical confinement to provide protection against stretch-induced DNA damage.
Discussion
In this study, we present a, to our knowledge, new TENSCell device concept to image cells at high magnification during mechanical stimulation. The device was constructed using mainly three-dimensionally printed parts and operated through electromagnetic force. This combination made the device easy to replicate through the means of 3D printing while also allowing for the precise control of strains. Another benefit of the design is that a simple control circuit is sufficient to control the flow of current to the electromagnetic coil, which further adds to the ease of use. 3D printing also enables the adjustment of the design to fit different microscope stages or to change device specifications. For example, the current maximum strain the device can achieve (∼29%) is constrained by the depth of the deformation ring holder and strength of the magnetic field. These specifications can easily be changed to fit the user’s needs. Furthermore, at the current configuration we applied equiaxial strain by using a round deformation ring. The geometry of the deformation ring can also be changed to achieve other modes of strain application, e.g., uniaxial.
To test this device, we investigated the response of MSF nuclei to cyclic stretch. Unexpectedly, we observed opposing responses to sinusoidal strain routines with low or high amplitude, as nuclear areas increased and chromatin decompacted for low strains and areas decreased and chromatin condensed for high strains. Previous studies have shown that cyclic uniaxial stretch (3–15%) induces chromatin condensation and nuclear elongation in the direction of stretch in mesenchymal stem cells (17,18). In another study, cyclic force application using a magnetic needle resulted in chromatin decondensation in HeLa cells (23). All groups reported changes in chromatin compaction to be very rapid (immediate within the time resolution of their method) upon stimulation, similar to our findings here. However, to our knowledge, no study has reported dichotomous mechanosensitive behavior of nuclei. Pioneering studies in mechanosensation have shown that mesenchymal stem cells differentiate in accordance with the stiffness of their environment (1) (becoming osteogenic on stiff, myogenic on medium, and neurogenic on soft substrates), which indicated that cells differentiate between intensities of mechanical cues. Still, the underlying mechanosensitive mechanisms, and whether differences in strain magnitudes are processed through either one pathway in a dose-response manner or are the result of different pathways interacting, is not clear. Here, we showed that nuclear responses to low-strain cyclic stretch were dependent on calcium signaling, whereas high-strain responses were mostly not. These results suggest that different mechanisms and pathways could be involved in sensing strains of different magnitudes. One could postulate, for example, that stretch-induced calcium channels are sensitive to small loads but cannot distinguish higher loads because their activation threshold might be saturated quickly. In this scenario, the cytoskeleton might be better suited to sense higher loads. More research would be needed to validate our results and to identify the different mechanisms that are sensitive to different amplitudes of strain.
We also compared the use of the chromatin dye Hoechst with the endogenous H2b-eGFP tag present in the mice that were used as a source for skin fibroblasts. Intercalating chromatin dyes are known to cause DNA damage, inhibited proliferation, and long-term cell toxicity (40,41). However, chromatin dyes are still frequently used for convenience, and information about the use of chromatin dyes for investigating changes in chromatin organization, even on short timescales (<1 h), is limited. In this study, we observed that Hoechst-stained nuclei responded to cyclic stretch (20%) with a reduction in nuclear area similar to unstained nuclei but showed no relaxation during the subsequent resting period. We also observed reduced chromatin compaction in Hoechst-stained nuclei in response to stretch. Because chromatin compaction was measured by histogram kurtosis and skewness, this result could also reflect a difference in intensity distribution between labeling chromatin or H2b histones. It should be noted that, despite different attempts (36,42), the quantification of chromatin compaction from fluorescence markers is not well established yet. Even though more thorough investigation is needed, our results suggest that the use of intercalating chromatin dyes is not suitable to investigate changes in chromatin organization, even on short timescales, during which cytotoxicity and effects on proliferation might be negligible.
Based on our observation of divergent nuclear behavior, it could be hypothesized that different magnitudes of strain represent different modes of operation that are associated with specific challenges for the cell. More specifically, low-strain cyclic stretch might mimic baseline physical activity and drive regular cell activity or maintenance. In turn, high loads might be associated with extreme activity or trauma and trigger a protective mechanism instead. Increased mechanical stress has been shown to cause DNA damage (37,38) and trigger apoptosis (19,38). The observed rapid nuclear contraction during high-strain loads might therefore be a mechanism to protect from strain-induced DNA damage. In this study, we also observed an increase of DNA double-strand breaks with increased levels of strain. Interestingly, the number of double-strand break foci was as high in unstretched control cells as after high levels (20%) of cyclic stretch, suggesting that skin fibroblasts cells perform better under dynamic compared to static conditions. Such findings were not reported by most studies that investigated stretch-induced DNA damage. However, these studies only investigated high loads (15–30%) in aberrant cell culture models (37), which might be less sensitive than primary cells, or focused on oxidative DNA damage in particular (38). In agreement with our results, one study reported that ultraviolet-radiation-induced DNA damage (also measure by γH2a.x) was reduced in NIH-3T3 fibroblasts when subjected to 10% cyclic uniaxial stretch (43). It is likely that skin fibroblasts and other types of cells have an optimal performance under low mechanical stimulation because that reflects the conditions they evolved in inside motile organisms. This might have grave implications considering that cells are usually cultured on rigid plastic or glass. Hence, more work needs to be done to validate and understand this phenomenon. Future work is also needed to address the influence of strain modes in addition to strain magnitude because studies have shown that strain anisotropy can influence cell differentiation, proliferation, apoptosis, and cell polarization, as well as extracellular matrix organization (44,45).
We further observed that the actin skeleton was essential for any nuclear response to stretch. Other studies have reported that stretch-induced changes in chromatin organization (18,23,26) or gene expression (21,22) were abrogated after disruption of the actin skeleton. Here, we observed that perinuclear F-actin increased with strain magnitudes and would therefore not explain the dichotomous nuclear behavior observed for different strains. However, closer investigation of dynamic F-actin reorganization in live cells showed that F-actin shifted away from the nuclear periphery to the cell border during low-strain cyclic stretch and vice versa during high-strain cyclic stretch. Changes in actin reorganization were also observed immediately after cyclic loading (<2 min), particularly during high-strain routines, and their dynamics resembled that of nuclear responses. This suggested an interplay between nucleus and cytoskeleton. Other studies in embryonic fibroblasts also provided strong evidence that the perinuclear actin cap (perinuclear actin above the nucleus) can regulate nuclear morphology in response to mechanical deformation (46,47). These studies also suggest that the actin cap acts to protect the nuclear integrity during deformations. Here, we observed that the occurrence of double-strand breaks was increased during stretch after actin skeleton disruption. Further supporting this hypothesis, we observed that strain transferred from the silicone membrane to the nucleus was increased after actin depolymerizing via cyto D after 30 min of cyclic stretch. Nuclear strain transfer before cyclic strain application could not be accurately assessed because of the rapid decline in nuclear area of cells held in a stretched position (see Methods for details). It has also been shown that depolymerization of actin can inhibit DNA damage repair (48). Therefore, the question whether actin reorganization toward the nuclear periphery reduces nuclear strain transfer remains unanswered. Furthermore, it would be interesting to investigate the role of the LINC complex for the interplay between cytoskeleton and nucleus because LINC complex disruption has been shown to inhibit nuclear responses to mechanical cues (20,22,25) and interfere with cytoskeleton dynamics (25,49).
A disadvantage of the TENSCell device is the potential influence of the magnetic fields used to operate the device on cell behavior. The rare earth magnet was positioned away from the containment well at the opposite side of the piston and had a magnetic field strength of 7 mT at the location of the well (>5 cm from the pole). The distance of the magnet from the well changed only slightly (<1 cm) during stretch and can be considered static (±0.1 mT). In contrast, the electromagnetic coil produces an oscillating field during cyclic stretch with a maximum field strength of ∼25.4 mT at the level of the membrane (Fig. S6) for 20% strain routines (1.0 mT for 5% and −9.3 mT at offset). Studies showed that long-term exposure (1–5 days) to static 6 mT magnetic fields can have a significant but moderate effect on cell survival and cell morphology for some of the cell lines investigated (50,51). Reviews on the effects of static magnetic fields concluded that effects on cell survival and proliferation were absent or minor regardless of the field strength used (52,53). However, it should be noted that static magnetic fields can increase the effect of apoptosis-inducing drugs (50, 51, 52, 53) that have been related to field-induced changes in calcium uptake (54,55). This could also pose a possible explanation for the increase in DNA damage in BP- or KN-treated cells observed in this study. Investigation of the effect of oscillating electromagnetic fields (1–20 mT) showed moderate effects on cancer proliferation over the duration of 5 or 7 days (56,57). These studies used 50 Hz oscillations, which are considered low frequency but are distinctly higher than the 1 Hz used in this study. Research in chick embryos showed that oscillating fields influenced development only upwards of 16 Hz independent of the field strength used (58). Overall, studies on static and oscillatory magnetic fields observed only minor changes in cell behavior after long time (days) exposure. In this study, we found no difference in nuclear responses between unstretched control cells exposed to magnetic field routines or cells in the absence of a magnetic field during the 1 h experimental routine. Special caution should be given for the use of pharmacological agents in the presence of a magnetic field, especially when they negatively affect cell viability.
Author Contributions
Conceptualization, B.S. and C.P.N.; methodology, B.S., A.K.S., I.N., K.C., and C.P.N.; software, B.S.; formal analysis, B.S. and S.E.S.; investigation, B.S., A.K.S., and I.N.; writing—original draft, B.S.; writing—review and editing, all authors; funding acquisition, C.P.N.; resources, C.P.N.; supervision, C.P.N.
Acknowledgments
We thank the Integrated Teaching and Learning Laboratory at the University of Colorado Boulder for help and advice with 3D printing.
This work was supported in part by NIH grants R01 AR063712 and R21 AR066230 and National Science Foundation grant CMMI CAREER 1349735. Patent pending, provisional Patent Application No. 62/876,535.
Editor: Cynthia Reinhart-King.
Footnotes
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2020.03.035.
Supporting Material
References
- 1.Engler A.J., Sen S., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Engler A.J., Carag-Krieger C., Discher D.E. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasqualini F.S., Agarwal A., Parker K.K. Traction force microscopy of engineered cardiac tissues. PLoS One. 2018;13:e0194706. doi: 10.1371/journal.pone.0194706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemphill M.A., Dauth S., Parker K.K. Traumatic brain injury and the neuronal microenvironment: a potential role for neuropathological mechanotransduction. Neuron. 2015;85:1177–1192. doi: 10.1016/j.neuron.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 5.Del Real A., Pérez-Campo F.M., Riancho J.A. Differential analysis of genome-wide methylation and gene expression in mesenchymal stem cells of patients with fractures and osteoarthritis. Epigenetics. 2017;12:113–122. doi: 10.1080/15592294.2016.1271854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thienpont B., Aronsen J.M., Roderick H.L. The H3K9 dimethyltransferases EHMT1/2 protect against pathological cardiac hypertrophy. J. Clin. Invest. 2017;127:335–348. doi: 10.1172/JCI88353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q.J., Chen H.Z., Liu Z.P. The histone trimethyllysine demethylase JMJD2A promotes cardiac hypertrophy in response to hypertrophic stimuli in mice. J. Clin. Invest. 2011;121:2447–2456. doi: 10.1172/JCI46277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter D.R., Beaupré G.S., Schurman D.J. The mechanobiology of articular cartilage development and degeneration. Clin. Orthop. Relat. Res. 2004;427(Suppl):S69–S77. doi: 10.1097/01.blo.0000144970.05107.7e. [DOI] [PubMed] [Google Scholar]
- 9.Jaalouk D.E., Lammerding J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manilal S., Nguyen T.M., Morris G.E. The Emery-Dreifuss muscular dystrophy protein, emerin, is a nuclear membrane protein. Hum. Mol. Genet. 1996;5:801–808. doi: 10.1093/hmg/5.6.801. [DOI] [PubMed] [Google Scholar]
- 11.Lampi M.C., Reinhart-King C.A. Targeting extracellular matrix stiffness to attenuate disease: from molecular mechanisms to clinical trials. Sci. Transl. Med. 2018;10:eaao0475. doi: 10.1126/scitranslmed.aao0475. [DOI] [PubMed] [Google Scholar]
- 12.Huh D., Torisawa Y.S., Ingber D.E. Microengineered physiological biomimicry: organs-on-chips. Lab Chip. 2012;12:2156–2164. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- 13.Rosellini E., Zhang Y.S., Cascone M.G. Protein/polysaccharide-based scaffolds mimicking native extracellular matrix for cardiac tissue engineering applications. J. Biomed. Mater. Res. A. 2018;106:769–781. doi: 10.1002/jbm.a.36272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crisp M., Liu Q., Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aureille J., Belaadi N., Guilluy C. Mechanotransduction via the nuclear envelope: a distant reflection of the cell surface. Curr. Opin. Cell Biol. 2017;44:59–67. doi: 10.1016/j.ceb.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Lee H., Adams W.J., Parker K.K. Cytoskeletal prestress regulates nuclear shape and stiffness in cardiac myocytes. Exp. Biol. Med. (Maywood) 2015;240:1543–1554. doi: 10.1177/1535370215583799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heo S.J., Thorpe S.D., Mauck R.L. Biophysical regulation of chromatin architecture instills a mechanical memory in mesenchymal stem cells. Sci. Rep. 2015;5:16895. doi: 10.1038/srep16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heo S.J., Driscoll T.P., Mauck R.L. Differentiation alters stem cell nuclear architecture, mechanics, and mechano-sensitivity. eLife. 2016;5:e18207. doi: 10.7554/eLife.18207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J., Liu J., Li Y. Caspase-3-mediated cyclic stretch-induced myoblast apoptosis via a Fas/FasL-independent signaling pathway during myogenesis. J. Cell. Biochem. 2009;107:834–844. doi: 10.1002/jcb.22182. [DOI] [PubMed] [Google Scholar]
- 20.Guilluy C., Osborne L.D., Burridge K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 2014;16:376–381. doi: 10.1038/ncb2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tajik A., Zhang Y., Wang N. Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater. 2016;15:1287–1296. doi: 10.1038/nmat4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzer G., Thompson W.R., Rubin J. Cell mechanosensitivity to extremely low-magnitude signals is enabled by a LINCed nucleus. Stem Cells. 2015;33:2063–2076. doi: 10.1002/stem.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyer K.V., Pulford S., Shivashankar G.V. Mechanical activation of cells induces chromatin remodeling preceding MKL nuclear transport. Biophys. J. 2012;103:1416–1428. doi: 10.1016/j.bpj.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh S., Seelbinder B., Neu C.P. Deformation microscopy for dynamic intracellular and intranuclear mapping of mechanics with high spatiotemporal resolution. Cell Rep. 2019;27:1607–1620.e4. doi: 10.1016/j.celrep.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seelbinder B., Ghosh S., Neu C.P. Intra-nuclear tensile strain mediates reorganization of epigenetically marked chromatin during cardiac development and disease. bioRxiv. 2019 doi: 10.1101/455600. [DOI] [Google Scholar]
- 26.Le H.Q., Ghatak S., Wickström S.A. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 2016;18:864–875. doi: 10.1038/ncb3387. [DOI] [PubMed] [Google Scholar]
- 27.Bartalena G., Grieder R., Snedeker J.G. A novel method for assessing adherent single-cell stiffness in tension: design and testing of a substrate-based live cell functional imaging device. Biomed. Microdevices. 2011;13:291–301. doi: 10.1007/s10544-010-9493-3. [DOI] [PubMed] [Google Scholar]
- 28.Harshad K., Jun M., Nguyen N.T. An electromagnetic cell-stretching device for mechanotransduction studies of olfactory ensheathing cells. Biomed. Microdevices. 2016;18:45. doi: 10.1007/s10544-016-0071-1. [DOI] [PubMed] [Google Scholar]
- 29.Toume S., Gefen A., Weihs D. Printable low-cost, sustained and dynamic cell stretching apparatus. J. Biomech. 2016;49:1336–1339. doi: 10.1016/j.jbiomech.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Deguchi S., Kudo S., Sato M. Piezoelectric actuator-based cell microstretch device with real-time imaging capability. AIP Adv. 2015;5:067110. [Google Scholar]
- 31.Huang L., Mathieu P.S., Helmke B.P. A stretching device for high-resolution live-cell imaging. Ann. Biomed. Eng. 2010;38:1728–1740. doi: 10.1007/s10439-010-9968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao Y., Tan X., Liu A.P. Uniaxial cell stretching device for live-cell imaging of mechanosensitive cellular functions. Rev. Sci. Instrum. 2013;84:114304. doi: 10.1063/1.4832977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalut K.J., Höpfler M., Guck J. Chromatin decondensation and nuclear softening accompany Nanog downregulation in embryonic stem cells. Biophys. J. 2012;103:2060–2070. doi: 10.1016/j.bpj.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martiel J.L., Leal A., Théry M. Measurement of cell traction forces with ImageJ. Methods Cell Biol. 2015;125:269–287. doi: 10.1016/bs.mcb.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Neu C.P., Hull M.L., Walton J.H. Error optimization of a three-dimensional magnetic resonance imaging tagging-based cartilage deformation technique. Magn. Reson. Med. 2005;54:1290–1294. doi: 10.1002/mrm.20669. [DOI] [PubMed] [Google Scholar]
- 36.Herbomel G., Grichine A., Usson Y. Wavelet transform analysis of chromatin texture changes during heat shock. J. Microsc. 2016;262:295–305. doi: 10.1111/jmi.12363. [DOI] [PubMed] [Google Scholar]
- 37.Upadhyay D., Correa-Meyer E., Kamp D.W. FGF-10 prevents mechanical stretch-induced alveolar epithelial cell DNA damage via MAPK activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284:L350–L359. doi: 10.1152/ajplung.00161.2002. [DOI] [PubMed] [Google Scholar]
- 38.Mayr M., Hu Y., Xu Q. Mechanical stress-induced DNA damage and rac-p38MAPK signal pathways mediate p53-dependent apoptosis in vascular smooth muscle cells. FASEB J. 2002;16:1423–1425. doi: 10.1096/fj.02-0042fje. [DOI] [PubMed] [Google Scholar]
- 39.Rogakou E.P., Pilch D.R., Bonner W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 40.Durand R.E., Olive P.L. Cytotoxicity, mutagenicity and DNA damage by Hoechst 33342. J. Histochem. Cytochem. 1982;30:111–116. doi: 10.1177/30.2.7061816. [DOI] [PubMed] [Google Scholar]
- 41.Erba E., Ubezio P., D’Incalci M. DNA damage, cytotoxic effect and cell-cycle perturbation of Hoechst 33342 on L1210 cells in vitro. Cytometry. 1988;9:1–6. doi: 10.1002/cyto.990090102. [DOI] [PubMed] [Google Scholar]
- 42.Mascetti G., Carrara S., Vergani L. Relationship between chromatin compactness and dye uptake for in situ chromatin stained with DAPI. Cytometry. 2001;44:113–119. doi: 10.1002/1097-0320(20010601)44:2<113::aid-cyto1089>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 43.Nagayama K., Fukuei T. Cyclic stretch-induced mechanical stress to the cell nucleus inhibits ultraviolet radiation-induced DNA damage. Biomech. Model. Mechanobiol. 2020;19:493–504. doi: 10.1007/s10237-019-01224-3. Published online September 10, 2019. [DOI] [PubMed] [Google Scholar]
- 44.Gould R.A., Chin K., Butcher J.T. Cyclic strain anisotropy regulates valvular interstitial cell phenotype and tissue remodeling in three-dimensional culture. Acta Biomater. 2012;8:1710–1719. doi: 10.1016/j.actbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park J.S., Chu J.S., Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol. Bioeng. 2004;88:359–368. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 46.Khatau S.B., Hale C.M., Wirtz D. A perinuclear actin cap regulates nuclear shape. Proc. Natl. Acad. Sci. USA. 2009;106:19017–19022. doi: 10.1073/pnas.0908686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J.K., Louhghalam A., Kim D.H. Nuclear lamin A/C harnesses the perinuclear apical actin cables to protect nuclear morphology. Nat. Commun. 2017;8:2123. doi: 10.1038/s41467-017-02217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfitzer L., Moser C., Zahler S. Targeting actin inhibits repair of doxorubicin-induced DNA damage: a novel therapeutic approach for combination therapy. Cell Death Dis. 2019;10:302. doi: 10.1038/s41419-019-1546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Auld A.L., Folker E.S. Nucleus-dependent sarcomere assembly is mediated by the LINC complex. Mol. Biol. Cell. 2016;27:2351–2359. doi: 10.1091/mbc.E16-01-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tenuzzo B., Chionna A., Dini L. Biological effects of 6 mT static magnetic fields: a comparative study in different cell types. Bioelectromagnetics. 2006;27:560–577. doi: 10.1002/bem.20252. [DOI] [PubMed] [Google Scholar]
- 51.Chionna A., Dwikat M., Dini L. Cell shape and plasma membrane alterations after static magnetic fields exposure. Eur. J. Histochem. 2003;47:299–308. [PubMed] [Google Scholar]
- 52.Miyakoshi J. Effects of static magnetic fields at the cellular level. Prog. Biophys. Mol. Biol. 2005;87:213–223. doi: 10.1016/j.pbiomolbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Ghodbane S., Lahbib A., Abdelmelek H. Bioeffects of static magnetic fields: oxidative stress, genotoxic effects, and cancer studies. BioMed Res. Int. 2013;2013:602987. doi: 10.1155/2013/602987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fanelli C., Coppola S., Ghibelli L. Magnetic fields increase cell survival by inhibiting apoptosis via modulation of Ca2+ influx. FASEB J. 1999;13:95–102. doi: 10.1096/fasebj.13.1.95. [DOI] [PubMed] [Google Scholar]
- 55.Panagopoulos D.J., Karabarbounis A., Margaritis L.H. Mechanism for action of electromagnetic fields on cells. Biochem. Biophys. Res. Commun. 2002;298:95–102. doi: 10.1016/s0006-291x(02)02393-8. [DOI] [PubMed] [Google Scholar]
- 56.Huang L., Dong L., Xiao D. Effects of sinusoidal magnetic field observed on cell proliferation, ion concentration, and osmolarity in two human cancer cell lines. Electromagn. Biol. Med. 2006;25:113–126. doi: 10.1080/15368370600719067. [DOI] [PubMed] [Google Scholar]
- 57.Pirozzoli M.C., Marino C., Negroni A. Effects of 50 Hz electromagnetic field exposure on apoptosis and differentiation in a neuroblastoma cell line. Bioelectromagnetics. 2003;24:510–516. doi: 10.1002/bem.10130. [DOI] [PubMed] [Google Scholar]
- 58.Juutilainen J., Saali K. Development of chick embryos in 1 Hz to 100 kHz magnetic fields. Radiat. Environ. Biophys. 1986;25:135–140. doi: 10.1007/BF01211737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.