Abstract
Background
HPV testing is replacing cytology for cervical cancer screening because of greater sensitivity and superior reassurance following negative tests for the dozen HPV genotypes that cause cervical cancer. Management of women testing positive is unresolved. The need for identification of individual HPV genotypes for clinical use is debated. Also, it is unclear how long to observe persistent infections when precancer is not initially found.
Methods
In the longitudinal NCI-Kaiser Permanente Northern California Persistence and Progression (PaP) Study, we observed the clinical outcomes (clearance, progression to CIN3+, or persistence without progression) of 11,573 HPV-positive women aged 30–65 yielding 14,158 type-specific infections.
Findings
Risks of CIN3+ progression differed substantially by type, with HPV16 conveying uniquely elevated risk (26% of infections with seven-year CIN3+ risk of 22%). The other carcinogenic HPV types fell into 3 distinct seven-year CIN3+ risk groups: HPV18, 45 (13% of infections, risks >5%, with known elevated cancer risk); HPV31, 33, 35, 52, 58 (39%, risks >5%); and HPV39, 51, 56, 59, 68 (23%, risks <5%). In the absence of progression, HPV clearance rates were similar by type, with 80% of infections no longer detected within three years; persistence to seven years without progression was uncommon. The predictive value of abnormal cytology was most evident for prevalent CIN3+, but less evident in follow-up. A woman's age did not modify risk; rather it was the duration of persistence that was important.
Interpretation
HPV type and persistence are the major predictors of progression to CIN3+; at a minimum, distinguishing HPV16 is clinically important. Dividing the other HPV types into three risk-groups is worth considering.
Keywords: HPV genotype; HPV outcome, Clearance; Progression; Persistence
Abbreviations: AGC, Atypical glandular cells; AIS, Adenocarcinoma in-situ; ASC-H+, Atypical squamous cells - cannot exclude HSIL; ASC-US, Atypical squamous cells of undetermined significance; BD, Becton Dickinson; CIN, Cervical intraepithelial neoplasia; HC2, Hybrid Capture 2; HPV, human papillomavirus; KPNC, Kaiser Permanente Northern California; LSIL, Low-grade squamous intraepithelial lesion; NCI, National Cancer Institute; NILM, Negative for intraepithelial lesion or malignancy; PaP, Persistence and Progression; PCR, Polymerase chain reaction; STM, Specimen transport medium
Research in Context.
Evidence before this study
HPV genotypes range substantially in risk of progression to cervical precancer/cancer (defined here as CIN3+), but the clinical value of typing is debated. Within one to two years of exposure, in immunocompetent populations, most cervical HPV infections found on screening are cleared. It is uncertain how long to follow HPV infections without treatment, if a precancer needing treatment is not initially diagnosed.
Added value of this study
This study demonstrated that the initially rapid, then slowing, rates of clearance of most of the individual carcinogenic HPV types are clinically indistinguishable. HPV types varied substantially in cumulative risk and annual rate of progression to precancer. Partial HPV typing is an important part of accurate risk estimation and optimal clinical management: (1) HPV16 is uniquely carcinogenic and should be individually distinguished; (2) HPV18 and HPV45 are higher risk, and especially risky for adenocarcinomas and squamous cancer; (3) HPV31, HPV33, HPV35, HPV52, and HPV58 (HPV16-related types) pose substantially higher risk than (4) HPV39, HPV51, HPV56, HPV59, and HPV68 that pose very little risk if precancer is not immediately found.
Implications of all the available evidence
In a cervical cancer screening program based on HPV testing starting at age 30, the most important predictors of risk of precancer at baseline testing are HPV status (positive versus negative), HPV type group, prevalent versus incident detection of HPV (duration of infection), and high-grade cytology. Persistence of the highest risk HPV types past about two to three years following baseline screening is highly associated with precancer.
Alt-text: Unlabelled box
1. Introduction
Cervical cancer screening is designed to detect treatable cancer precursors (“precancers”, approximated most closely histopathologically as CIN3 and AIS), to prevent cancer mortality and morbidity. Screening strategies are shifting from cytology to carcinogenic HPV testing, due to the superior sensitivity of HPV testing for detection of precancer, providing greater reassurance against cancer when testing is negative. Although HPV testing is increasingly used, important aspects of its use are still unresolved, including optimal management of positive results.
The management of positive results is important because HPV test positivity is generally high, even when testing starts at age 25 or 30 past the pronounced peak of HPV acquisition following initiation of sexual intercourse [1]. HPV infections have two possible initial competing outcomes: clearance or “progression” (development of precancer); infections that neither clear nor progress are said to persist. Most infections are typically benign, and they clear among immunocompetent women, such that invasive diagnostic procedures or universal destructive treatment is not warranted [2]. Waiting for clearance, with repeat testing, would clarify risk of any HPV infection, but is generally not favored. Secondary “triage” testing of HPV-positive women at the time of initial detection is typically recommended.
At present, cytology is frequently used for triage of HPV-positive women, with non-normal results leading to immediate colposcopy. Selective genotyping for the highest risk HPV types is sometimes recommended [3,4]; in which case, detecting HPV16 or HPV18 (the latter of which is sometimes combined with the related virus HPV45) [5] also leads to colposcopy.
While it is known that persistent infections are substantially more likely to yield precancer [6], [7], [8], [9], [10], there is no consensus on how long to follow persistent infections of different types before referral for colposcopically directed biopsy or even treatment when no precancer is found at initial colposcopy.
The large National Cancer Institute/Kaiser Permanente Northern California Persistence and Progression (PaP) study was initiated in 2006 to help inform the natural history of individual HPV types, including the rarer types. In order to guide the use of typing as a triage following primary HPV testing, the study aimed to identify clinically meaningful, type-specific patterns in clearance, progression to precancer, or persistence. Here, we report the main results of the PaP study, and the implications for the greater use of HPV typing in clinical management.
2. Methods
2.1. Study design and population
This is a longitudinal analysis using data from HPV typing of residual specimens that were archived to permit a historical cohort study, following routine HPV testing conducted at Kaiser Permanente Northern California (KPNC). At KPNC, women were tested by Hybrid Capture 2 (HC2) for HPV (as a pool without genotyping) to triage the equivocal cytologic result of atypical squamous cells of undetermined significance (ASC-US) (since 2001) and as a co-test with cytology in women ages 30 and older (since 2003) [11,12]. The HPV Persistence and Progression (PaP) study was created by banking residual discarded cervical specimens collected into specimen transport medium (STM; Qiagen) from women tested by HC2. Opt-out consents were mailed to women; 8% opted out of specimen storage and research testing. Survey sampling techniques were used to account for the differential selection of CIN2+ in the PaP cohort [13]. Specimens were selected from CIN2+ cases (sampling fraction 0.81, weight 1.22) and controls (sampling fraction 0.33, weight 3.07) for HPV genotyping.
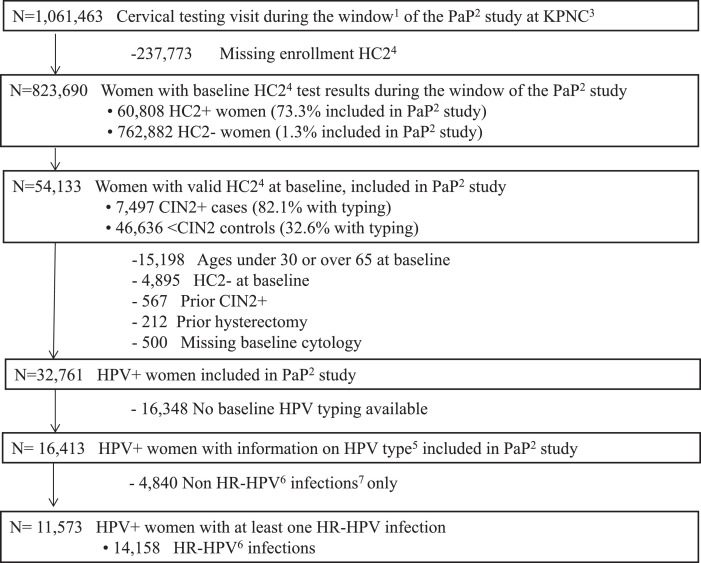
The PaP cohort included specimens from 54,133 women aged 25–65 who were tested by HC2 during the study period (Fig. 1). Women with known history of CIN2+ or hysterectomy prior to baseline were excluded, as were women under 30 or over 65 years old at baseline.
Fig. 1.
Study population in KPNC's Persistence and Progression (PaP) study.
1Cervical testing visits obtained between January 1, 2007- January 31, 2011 2 PaP: Persistence and Progression study 3 KPNC: Kaiser Permanente North California 4 HC2: Hybrid Capture 2 5 HPV typing was done with Linear Array (6910), cobas (6281), MY09/11 (3422), and Onclarity (9953) 6. HR: high risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) 7. Non-oncogenic HPV infections exclude HPV66 8. This first collection was defined as “baseline”. Specimens collected after baseline were referred to as “follow-up”. Only samples with HC2+ results at baseline were used, with no additional follow-up specimens or correction for potential infections missed by HC2.
We then restricted the analytic sample for type-specific analyses to all 14,158 infections genotyped from among the sample of positive HC2 test results at baseline [14]. HPV typing was performed at Roche Molecular Systems (Pleasanton CA) by cobas and Linear Array or at BD Diagnostics (Sparks MD) by Onclarity [14,15]. A smaller group of samples was HPV-typed using either Linear Array or an MY09-MY11 PCR-based method at academic laboratories.
2.2. Variables
A subset of HPV-positive specimens (36%) at baseline had more than one HPV type, complicating interpretation. For example, a woman infected with three types at baseline could clear one type, acquire another, while a third could lead to progression. We chose to deal with misclassification of causal exposure by restricting the main analysis to women with a single type-specific infection at baseline and no indication of incident infection by any other type during follow-up. Supplementary analyses considered possible impact on outcomes due to multiple infections.
The natural history of type-specific infections was analyzed based on positivity of any one (or more) of the assays used, because all of the major HPV DNA tests had roughly equivalent sensitivity for the types they distinguished [16]. The main analyses reported individual estimates for each of the 13 carcinogenic HPV types. Supplementary analyses also addressed pooled results available in current commercial HPV tests used in clinical settings, which involve some grouping of typing data. For Onclarity, the data are grouped as HPV16, HPV33/58, HPV18, HPV31, HPV52, HPV45, HPV35/39/68, HPV51, and HPV56/59/66. For cobas, “HR12” refers to the 14 types in the cobas assay minus HPV16 and HPV18. Of note, HPV66 is included as a common mistake in several HPV assays; it is only extremely rarely carcinogenic though it causes some lesions that look like precancer [17]. We did not consider HPV66 individually in the final analysis, after confirming its low risk of progression (data not shown) and that invasive cervical cancer is extremely rare [18,19].
Mutually exclusive outcomes for this analysis, starting from baseline HPV detection, were: (1) progression of HPV infection to development of precancer, (2) clearance of HPV infection, and (3) persistence of HPV infection (last follow-up time without clearance or progression). Clearance, progression, and persistence are key terms in our discussion but their definition for this analysis is not meant to represent the actual biology of HPV infections. Progression was defined as a histopathologic diagnosis of CIN3+ (CIN3/AIS/cancer) at baseline or any time during follow-up, with CIN2 leading to censoring at diagnosis as “persistent”. Supplementary analyses considered CIN2 as an alternative definition of progression, although CIN2 was de-emphasized because it is a less reliable histopathologic definition of precancer. Clearance was defined as type-specific HPV positivity followed by one or more HPV negative result from either type-specific or group channeled HPV (including HC2 negativity) [20], [21], [22], [23]. Persistence was assigned when neither progression nor clearance were identified by the end of follow-up of that woman.
Possible effect modifiers of risk of progression included observed length of infection, a woman's age, and concurrent cytology results. All analyses started with baseline type-specific HPV infections. We divided infections into two groups based on the immediate previous HC2 results prior to the baseline PaP visit. Infections with known immediately prior negative HC2 (median interval = 1063 days) were labeled "incident". Infections with unknown prior HC2 status or immediately prior positive HC2 results were possibly already persistent and labeled "prevalent”. Age was categorized in three age groups corresponding roughly to pre-, peri‑, and post-menopausal cervical status changes (30–44, 45–54, 55–65). Cytology was reported at the initial visit, and grouped as: NILM, ASC-US/LSIL, or ASC—H, AGC, and HSIL+ (the last three together referred to as high-grade cytologic result).
2.3. Statistical analysis
First, marginal risk models focused on type-specific time to progression for prevalent and incident infections with single and multiple HPV types. This analysis described the cumulative risk of progression by HPV type over seven years; we observed more years of follow-up at the extreme but truncated the analysis to favor stability of estimates with large numbers of women observed. Supplementary marginal analyses stratified risk of progression by cytology, age, and prevalent/incident infection.
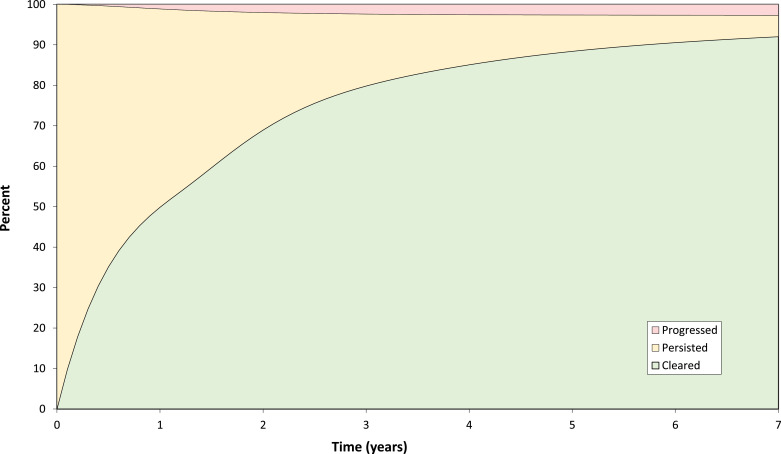
As a complementary analytic approach, competing risk analyses were performed that aimed to guide clinical surveillance. For infections without precancer at baseline, competing risk models estimated competing prospective outcomes (progression, clearance, or persistence) of HPV infections at each visit over seven years of follow-up for which we had sufficiently large numbers to perform a stable analysis. This analysis was designed to mimic the clinical experience of following-up an infection that had not (yet) caused precancer, waiting for resolution of the infection or progression to precancer. In competing risk analyses, the estimates for progression, clearance, and persistence add up to 100% at each time point. Three cumulative percentage curves of the HPV-infection transitions were created using type-specific estimates (Fig. 2). In these analyses, a negative result was labeled as clearance even in the rare event that the type re-appeared and led to CIN3+. Supplementary analyses reclassifying clearance, specifically restricting the outcome to infections without subsequent CIN3+ (data not shown) did not alter our conclusions.
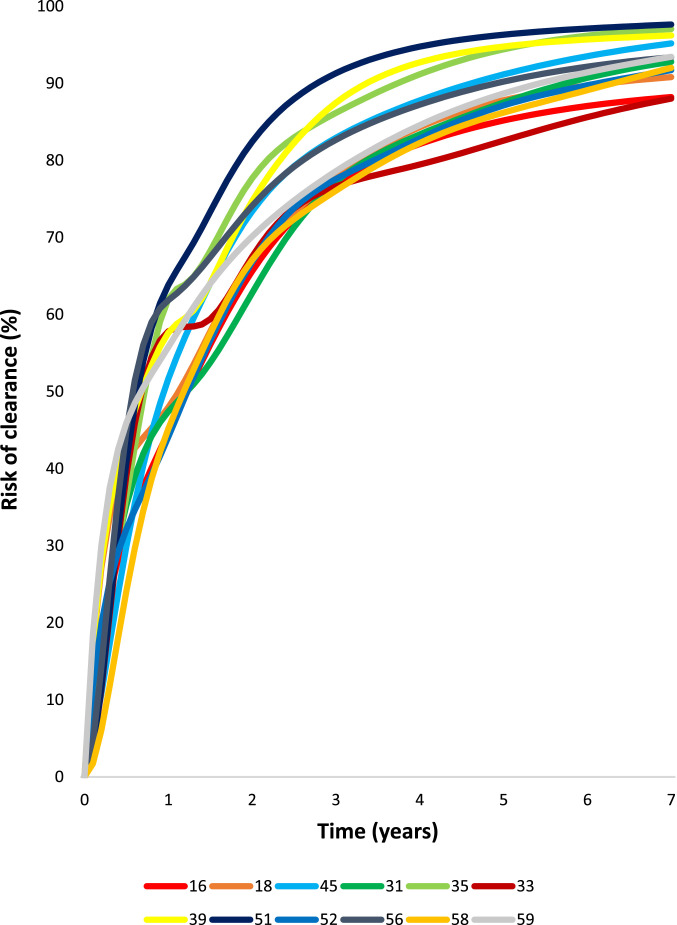
Fig. 2.
Competing cumulative risk of clearance, progression (to CIN3+), and persistence of type-specific [1] HPV infections over 7 years of follow-up.
1Competing cumulative incidences for progression and clearance were calculated as the weighted average of the estimated cumulative incidence rates for each type-specific HPV infection, where weights were based on the subgroup sample size.
Sample-weighted logistic-Cox models (combining logistic regression models with odds ratios for CIN3+ present at baseline and Cox models with hazard ratios for CIN3+ occurring after baseline) were used to estimate the cumulative risk of progression while accounting for differential sampling fractions for CIN2+ [13,24,25]. Methods used in estimation accounted for verification bias, given that colposcopic referral in KPNC depended on cytology and repeat HPV positivity. Time to CIN3+ progression occurred between the last screening visit that ruled out CIN3+ (via colposcopy or via negative HPV with NILM/ASC-US) and the first detected CIN3+ measurement (“interval-censoring”). Similarly, exact time of clearance was not observed and we used the time interval between the last HPV positive measurement and the first HPV negative measurement for each type-specific HPV infection. To estimate the cumulative incidence for progression/clearance over time, we employed a maximum likelihood estimation approach for interval-censored events [8]. Women who completed the study without progressing to precancer, clearing their infection, or loss to follow-up were censored as of their last available positive test, admittedly underestimating true persistence time.
3. Results
3.1. Marginal cumulative risk analysis
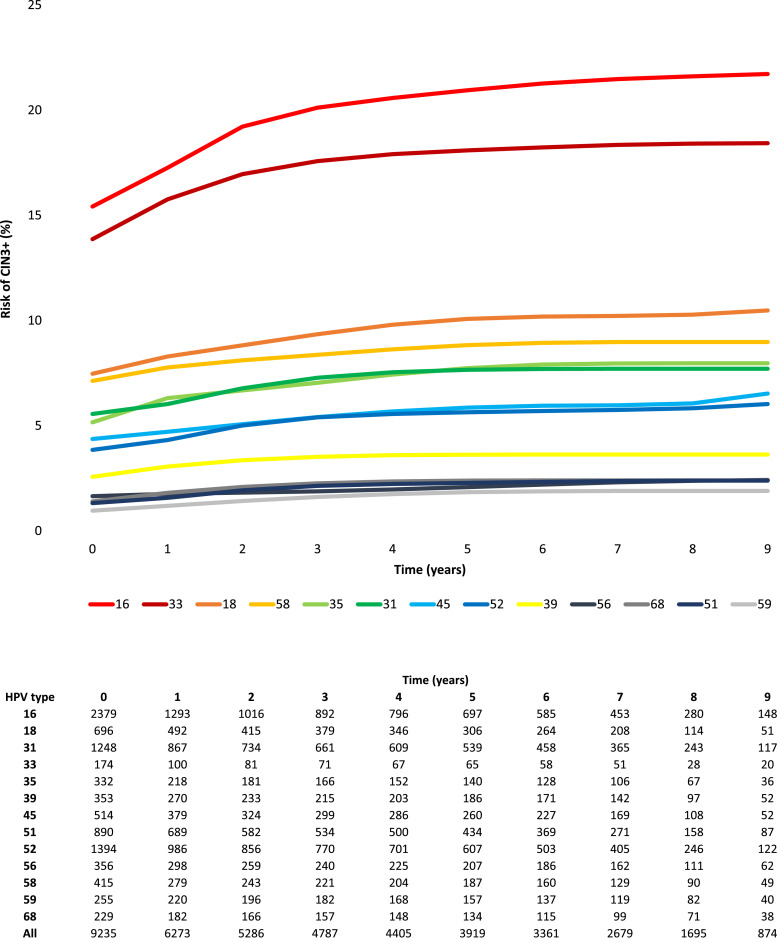
Cumulative risk of progression to CIN3+ varied, as expected, for different HPV types (Fig. 3). The majority (61.2%) of cases were diagnosed at the initial HPV-positive visit (62.8% of the CIN3+ among prevalent infections and 51.5% among incident infections), with a minority of CIN3+ cases identified during follow-up (Table 1). HPV16 had the highest risk of progression and, unlike most other types, it was linked to continually increased cumulative risk during follow-up, reaching a risk of 21.5% by year seven in women with a baseline prevalent infection. HPV33 also showed high cumulative risk, with 18.4% progression by year seven, but it was much less common than HPV16 (1.9% vs. 25.8% of infections) (Supplementary Table 1). All other HPV types had much lower cumulative risks than HPV16 or HPV33, with seven-year risks of about 10% or lower. The non- 16 or 18 HPV types divided naturally in two distinct risk subgroups. HPV types 33, 58, 31, 35, 45, 52 had risks above 5% by the end of follow-up. HPV types 39, 51, 56, 59, and 68 had seven-year risks of CIN3+ under 5%.
Fig. 3.
a. Marginal type-specific cumulative risk of progression to CIN3+ of single HPV infections over 9 years of follow-up. Fig. 3b. Single type-specific HPV infections at risk of progression to CIN3+ over 9 years of follow-up.
Table 1.
Marginal type-specific risk of progression to CIN3+ of incident and prevalent single-type HPV infections, over 7 years of follow-up.
| Length of infection | HPV type1 | Single-type HPV infections2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | CIN3+ cases3 |
Cumulative risk of CIN3+ (%) |
|||||||
| Total | Detected at baseline | Yr 0 | Yr 1 | Yr 3 | Yr 5 | Yr 7 | |||
| Prevalent | 16 | 1907 | 664 | 430 | 18.2 | 20.4 | 23.3 | 24.1 | 24.7 |
| 33 | 130 | 42 | 28 | 16.1 | 18.7 | 20.0 | 20.5 | 20.9 | |
| 18 | 520 | 102 | 60 | 8.1 | 9.0 | 10.2 | 10.9 | 11.0 | |
| 58 | 324 | 60 | 46 | 8.4 | 9.1 | 10.0 | 10.6 | 10.8 | |
| 31 | 949 | 149 | 89 | 6.7 | 7.4 | 8.9 | 9.3 | 9.4 | |
| 35 | 261 | 39 | 19 | 5.5 | 7.0 | 7.9 | 8.8 | 9.1 | |
| 52 | 1073 | 130 | 73 | 4.6 | 5.2 | 6.5 | 6.7 | 6.9 | |
| 45 | 371 | 50 | 35 | 5.3 | 5.7 | 6.3 | 6.7 | 6.8 | |
| 39 | 268 | 20 | 13 | 2.7 | 3.2 | 3.8 | 3.9 | 3.9 | |
| 56 | 253 | 14 | 11 | 2.2 | 2.2 | 2.4 | 2.6 | 2.7 | |
| 51 | 603 | 30 | 15 | 1.6 | 1.9 | 2.5 | 2.6 | 2.6 | |
| 68 | 174 | 7 | 3 | 0.8 | 1.3 | 1.9 | 2.1 | 2.1 | |
| 59 | 189 | 8 | 4 | 1.3 | 1.6 | 1.9 | 2.0 | 2.0 | |
| Prevalent Total | 7022 | 1315 | 826 (62.8%) | ||||||
| Incident | 16 | 472 | 87 | 42 | 6.2 | 7.8 | 9.6 | 10.8 | 10.9 |
| 33 | 44 | 8 | 5 | 7.6 | 9.2 | 11.0 | 11.1 | 11.1 | |
| 18 | 176 | 27 | 16 | 5.6 | 6.2 | 7.1 | 7.8 | 7.9 | |
| 58 | 91 | 6 | 6 | 3.3 | 3.3 | 3.3 | 3.3 | 3.3 | |
| 31 | 299 | 21 | 10 | 2.4 | 2.6 | 3.0 | 3.4 | 3.5 | |
| 35 | 71 | 5 | 4 | 3.8 | 3.8 | 3.8 | 3.8 | 3.8 | |
| 52 | 321 | 15 | 8 | 1.6 | 1.7 | 2.2 | 2.4 | 2.4 | |
| 45 | 143 | 11 | 5 | 2.2 | 2.4 | 3.1 | 3.8 | 4.0 | |
| 39 | 85 | 5 | 3 | 2.1 | 2.5 | 2.8 | 2.8 | 2.8 | |
| 56 | 103 | 3 | 0 | 0.0 | 0.7 | 0.9 | 0.9 | 1.2 | |
| 51 | 287 | 10 | 3 | 0.7 | 0.9 | 1.4 | 1.6 | 1.7 | |
| 68 | 55 | 4 | 3 | 3.4 | 3.4 | 3.4 | 3.4 | 3.4 | |
| 59 | 66 | 2 | 0 | 0.0 | 0.1 | 0.8 | 1.6 | 1.8 | |
| Incident Total | 2213 | 204 | 105 (51.5%) | ||||||
| All infections | Total | 9235 | 1519 | 931 (61.2%) | |||||
Ordered based on descending risk of CIN3+ for prevalent (most common) single type infections.
Risk estimates restricted to single-type HPV infections.
Average time to end of follow-up was 4.1 years for prevalent infections and 4.9 years for incident ones.
Prevalent infections had higher risk of progression and clearer risk stratification by HPV type (Table 1) than incident infections. Type-specific cumulative risk of CIN3+ curves for single infections (Supplementary figure 1A and 1B) showed the most clearly distinguishable type-specific risk estimates, while curves for multiple infections (Supplementary figure 1C and 1D) seemingly averaged rather than summed risk of CIN3+ from different co-infecting HPV types, resulting in a different and less informative type-specific hierarchy.
High-grade cytologic results were highly predictive of prevalent CIN3+ at baseline visits and during follow-up after colposcopy showing <CIN2. In contrast, among women in follow-up following colposcopy showing <CIN2, low-grade cytologic results were less predictive of CIN3+ than knowledge of type-specific persistence (Supplementary Table 2). Once HPV type and length of infection were considered, stratification by age did not show consistent patterns (Supplementary Table 3).
Competing risk analysis
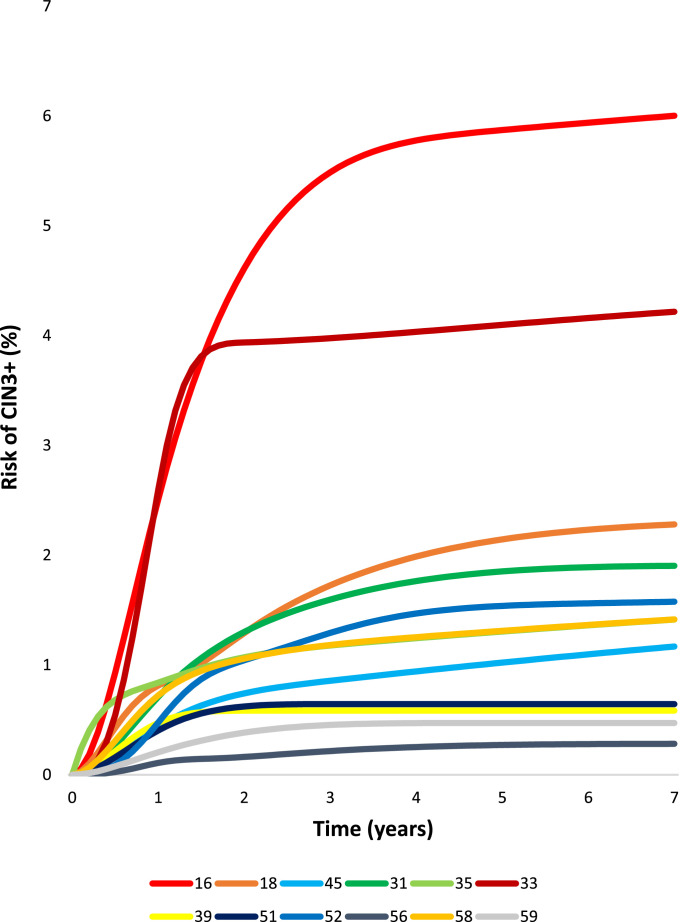
Infections without prevalent CIN2+ could persist, progress, or clear (Fig. 2) during yearly follow-up. By seven years of follow-up, viewed as competing risks (the manner in which clinicians observe infections as they follow individual women), 92.0% of infections cleared and 2.7% progressed. Persistence was uncommon (5.3%). For HPV 16, there was a marked shift towards progression. Although risk estimates for progression were low using the competing risk approach, the type patterns were the same as the marginal analysis presented first (Fig. 4). HPV16 and HPV33 had the highest rates of progression. HPV18 and HPV45 had lower risk but also continued to yield new cases of CIN3+ throughout follow-up. Risks for HPV types 31, 33, 35, 52, 58 continued to increase slightly during follow-up past year three, but HPV types 39, 51, 56, 59, and 68, which together comprised approximately 30% of the infections, were linked to virtually no progression past the initial three years. Although HPV types 16, 33, 18, and 45 continued to yield new cases of CIN3+ over the seven years of follow-up, the annual rates of progression decreased dramatically over the first three years for all HPV types (Supplementary Table 4). By year five, the yearly rates of progression were under 0.1% for most HPV types (except HPV 18) and remained low in the following years.
Fig. 4.
Type-specific competing cumulative incidence rates of progression to CIN3+, for single type-specific HPV infections that did not clear, over 7 years of follow-up, among women without CIN3+ detected at baseline.
In the competing risks approach, the first negative result was counted as clearance. The great majority of HPV infections (79.3%) detected at screening visits cleared within three years (Fig. 5). The different HPV types followed similar patterns and time of clearance, such that the curves and yearly clearance rates were qualitatively the same. (Supplementary Table 4).
Fig. 5.
Type-specific competing cumulative incidence rates of clearance of single type-specific HPV infections that did not progress, over 7 years of follow-up, among women without CIN3+ detected at baseline.
4. Discussion
Our results consolidate previous, less comprehensive analyses, and form the basis for use of HPV typing in cervical cancer screening within immunocompetent populations. The analyses confirm that risk of progression differs substantially by HPV type and can be meaningfully categorized into four groups as: (1) HPV16; (2) HPV18 and HPV45; (3) HPV31, HPV33, HPV52, HPV58, and HPV35 (especially for women of African descent) [26]; and (4) HPV39, HPV51, HPV56, HPV59, HPV68. HPV18 and, to a lesser extent, HPV45 deserve additional consideration because they have a relatively higher risk of cancer, including especially adenocarcinoma, that cannot be seen in intermediate length studies of precancer. We conclude that HPV typing, at the minimum yielding information for HPV16 (probably HPV18, and possibly HPV45), is a useful and worthy aspect of a state-of-the-art HPV test used for primary cervical cancer screening and management.
The time to clearance was similar across types other than HPV16 and HPV33, which tended to clear less often than other HPV types at any given time mainly because of substantially greater competing risk of progression to precancer. A persistent infection that neither cleared nor progressed was ultimately uncommon as recently reported by Dillner [27]. The fate of most HPV infections found at screening was evident within three years, when the great majority of infections had cleared [8]. The majority of cases of CIN3+ were diagnosed at the first HPV screening visit, and progression beyond three years following baseline screening was rare.
Typing based on HPV molecular assays permitted us to observe accurately that over approximately seven years of follow-up, >90% of HPV infections clear, ~3% progress, and ~5% persist. This is contrary to older publications reporting that, if a CIN1 lesion (a poorly reproducible sign of HPV infection) was observed, roughly one-third would progress, one third would persist, and one third would clear [10,22,23]. That older view, and the concept of CIN1–2–3 in general, is made obsolete by improved understanding of HPV natural history and cervical carcinogenesis [28].
Our choice of CIN3/AIS as the proxy for “precancer” was pragmatic, given known likelihood to progress. Restricting to CIN3/AIS undercounts typical clinical endpoints given that those found at CIN2 would have been censored before being counted. However, including CIN2 as a precancerous outcome leads to distorted, exaggerated estimates of the importance of several types at lower risk of cancer. Sensitivity analyses using CIN2 as an alternative case definition showed major differences in HPV type hierarchy, upgrading HPV35 and substantially downgrading HPV16, HPV18, and HPV45 (Supplementary Table 1B). Our data are consistent with previous literature, confirming that it is an inferior surrogate endpoint for cervical cancer risk [36].
We recognize as a limitation that even many cases of CIN3/AIS would not progress to cancer if left untreated (based on relative numbers of precancer to cancer diagnosed in cross-sectional studies). Therefore, true cancer risk posed by various HPV types can be misspecified even when estimated by the prospective risk of CIN3/AIS. For example, our analyses found higher cumulative risk of progression among HPV33 than for HPV18, and for HPV types 31, 35, 52, or 58 than for HPV45, while we know from worldwide case series of >40,000 invasive cancer cases that HPV18 and HPV45 account for more cancers than any of the other types except for HPV16 [8,29]. Both viral methylation and integration into the host genome are particularly common features of HPV18 and HPV45 cervical carcinogenesis, suggesting that there are differences in the evolutionary clades α7 (HPV18 related) and α9 (HPV16 related) types [15,30,31]. Even seven years of follow-up do not permit observation of the cancer risk, which was only barely visible in our data and is clear only in the longest cohorts spanning ten or more years [11,32,33].
A major goal of the analysis was to determine how long to follow repeated HPV testing waiting for infections to clear, if CIN2+ is not initially found. Our main methodological limitation was that we could not estimate absolute time to clearance because of left censoring (unknown history prior to baseline) and because we did not have sufficiently dense timing of visits and were likely to miss the true time of transition between the last positive and first negative results. Therefore, we roughly measured time to clearance using the maximum likelihood approach for interval censored events. Using competing risk models to replicate the clinician's experience, we could conclude with confidence that the patterns of clearance of different HPV genotypes (rapid clearance that gradually slows, as shown in Fig. 5) are virtually indistinguishable and do not merit clinical distinction. We also observed that the inflection point is two to three years of type-specific persistence following first detection in a screening setting (where true time of acquisition is “left censored” and unknown).
The data showed plainly that incidently detected HPV infections pose much lower risk than prevalent infections that might be already persistent (i.e., those that are found prevalently in women 30 and above on first use of HPV screening without knowledge of past history). Thus, HPV risk group and prevalent-incident status combined, if available, permit excellent risk prediction. Of note, our data illustrate patterns among immunocompetent women and are not meant as descriptive curves for all regions in the world.
High-grade cytologic abnormalities correlated highly with prevalent CIN3+. In contrast, subtle distinctions of concurrent cytology (negative, equivocal, low-grade abnormalities) were much less important modulators of risk of CIN3+ than HPV type and length of infection. In screening programs new to HPV testing, cytology can be useful to manage HPV-positive women because of the higher prevalence of pre-existing high-grade lesions. As screening with HPV becomes more established, genotype and the duration of infection, rather than cytologic result (or other factors like a woman's age) could more accurately estimate risk. This finding presages a fundamental shift in the underpinnings of cervical cancer screening, from a morphologic result (cytologic appearance) to a virologic one. The great majority of women being followed for abnormal cervical cancer screening results, either pre- or post-colposcopic examination, have HPV infections or the cytologic and histologic correlates of infection, without evidence of precancer. Our data support that, after excluding prevalent precancer, molecular virologic measurements (HPV presence/absence, HPV type group, HPV prevalence/incidence) are more useful than familiar but subjective clinical tests (minor cytologic distinctions, colposcopic impression, minor histologic diagnoses) for estimating risk of progression to CIN3+.
The role of distinguishing the individual HPV types as part of cervical cancer screening is unresolved and debated internationally. The pioneering Dutch screening program does not use HPV typing at all [34]. The British have recently published data from the HPV Pilot Steering Group showing no additional benefit of typing [35] in a program with good compliance with early recall, while the ARTISTIC trial supports partial typing for primary HPV testing and triage [3,4].
The Australian and US guidelines currently include typing for the two most carcinogenic types, HPV16 and HPV18, as part of determining risk of precancer/cancer. Our data, generalizable to the US context in which loss-to-follow-up is a perennial problem, support consideration of HPV type and duration in the evaluation and follow-up of cervical cancer screening abnormalities. Specifically, it is worth considering extended genotyping (into four risk groups) on the basis of distinct natural histories and risk profiles. However, the eventual decision of how much type distinction is useful should consider cost-effectiveness and be decided by guidelines committees.
Contributions
Study conceptualization and supervision was carried out by M.S., N.W., W.K.K., P.E.C. Sample collection, genotyping, and/or clinical characterization was performed by T.R.B., J.C.G., W.K.K., N.E.P., M-H.M, F.C., R.D.B, T.L., P.E.C., N.W., M.S. Statistical analyses were performed by M.D., N.H., L.C.C., X.C., B.B. Statistical, epidemiologic, and/or clinical expertise was provided by O.C-P., A.H., N.G.C., R.P., X.H., C.D., J.C., J.C.G. The manuscript was drafted and reviewed by all co-authors.
Declaration of competing interest
The following disclosure statements were reported: Dr. Demarco, Dr. Gage, Dr. Schiffman, and Dr. Wentzensen report that the NCI has received masked HPV and cytology test results at no cost from Roche Molecular Systems, BD Diagnostics, and Qiagen for independent evaluations of these technologies. Dr. Raine-Bennett reports other contracts from National Cancer Institute, during the conduct of the study. Dr. Campos reports other salary support from National Cancer Institute, during the conduct of the study, and personal fees from Basic Health International, outside the submitted work. Dr. Coutlee reports grants from Réseau FRSQ SIDA-MI, during the conduct of the study. Dr. Burk reports grants from NIH, during the conduct of the study. Dr. Castle reports discounted or free HPV tests and assays for research from Roche, Becton Dickinson, Cepheid, and Arbor Vita Corporation. Dr. Hyun, Dr. Carter-Pokras, Dr. Cheung, Dr. Chen, Dr. Hammer, Dr. Kinney, Dr. Befano, Dr. Perkins, Dr. He, Dr. Dallal, Dr. Chen, Dr. Poitras, Dr. Lorey, and Dr. Mayrand have nothing to disclose.
Acknowledgments
Acknowledgements
The field effort was a collaboration of the NCI and KPNC and was supported in part by the intramural program of the NCI. The NCI has received HPV and cytology test results at no cost from Roche Molecular Systems and BD Diagnostics for independent evaluations of these technologies.
Funding
The study was funded by the NCI Intramural Research Program, the National Cancer Institute (CA78527), and was made possible by the routine clinical care provided at KPNC. The analysis tool for competing risks was validated by a simulation study, partially supported by Institutional Research Grant IRG #16-183-31 from the American Cancer Society and the MCW Cancer Center. A small portion of the testing costs were funded by the Einstein Cancer Research Centre (P30CA013330) (RDB), Canadian Institutes for Health Research (The Réseau FRQS SIDA-MI); most HPV typing was performed at no cost to the investigators by Roche or BD. The academic investigators directed all aspects of the analysis and interpretation of data without commercial involvement.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100293.
Appendix. Supplementary materials
References
- 1.Schiffman M., Wentzensen N. A suggested approach to simplify and improve cervical screening in the United States. J Low Genit Tract Dis. 2016;20(1):1–7. doi: 10.1097/LGT.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch F.X., Broker T.R., Forman D. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(Suppl 7):H1–31. doi: 10.1016/j.vaccine.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilham C., Sargent A., Kitchener H.C., Peto J. HPV testing compared with routine cytology in cervical screening: long-term follow-up of artistic rct. 2019; 23: 28. [DOI] [PMC free article] [PubMed]
- 4.Gilham C., Sargent A., Peto J. Triaging women with human papillomavirus infection and normal cytology or low-grade dyskaryosis: evidence from 10-year follow up of the artistic trial cohort. BJOG: Int J Obstetr Gynaecol. 2020;127(1):58–68. doi: 10.1111/1471-0528.15957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huh W.K., Ault K.A., Chelmow D. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330–337. doi: 10.1097/AOG.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 6.Castle P.E., Schiffman M., Herrero R. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191(11):1808–1816. [Google Scholar]
- 7.Katki H.A., Cheung L.C., Fetterman B., Castle P.E., Sundaram R. A joint model of persistent human papillomavirus infection and cervical cancer risk: implications for cervical cancer screening. J R Stat Soc Ser A Stat Soc. 2015;178(4):903–923. doi: 10.1111/rssa.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plummer M., Schiffman M., Castle P.E., Maucort-Boulch D., Wheeler C.M., Group A. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195(11):1582–1589. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez A.C., Schiffman M., Herrero R. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100(7):513–517. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffman M., Wheeler C.M., Castle P.E. Atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesion triage study G. human papillomavirus dna remains detectable longer than related cervical cytologic abnormalities. J Infect Dis. 2002;186(8):1169–1172. doi: 10.1086/343816. [DOI] [PubMed] [Google Scholar]
- 11.Schiffman M., Glass A.G., Wentzensen N. A long-term prospective study of type-specific human papillomavirus infection and risk of cervical neoplasia among 20,000 women in the Portland Kaiser cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1398–1409. doi: 10.1158/1055-9965.EPI-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castle P.E., Shaber R., LaMere B.J. Human papillomavirus (HPV) genotypes in women with cervical precancer and cancer at kaiser Permanente northern California. Cancer Epidemiol Biomarkers Prev. 2011;20(5):946–953. doi: 10.1158/1055-9965.EPI-10-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyun N., Cheung L.C., Pan Q., Schiffman M., Katki H.A. Flexible risk prediction models for left or interval-censored data from electronic health records. Ann Appl Stat. 2017;11(2):1063–1084. doi: 10.1214/17-AOAS1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffman M., Hyun N., Raine-Bennett T.R. A cohort study of cervical screening using partial hpv typing and cytology triage. Int J Cancer J Int Cancer. 2016;139(11):2606–2615. doi: 10.1002/ijc.30375. [DOI] [PubMed] [Google Scholar]
- 15.Gage J.C., Schiffman M., Solomon D. Risk of precancer determined by hpv genotype combinations in women with minor cytologic abnormalities. Cancer Epidemiol Biomark Prev. 2013;22(6):1095–1101. doi: 10.1158/1055-9965.EPI-12-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demarco M., Carter-Pokras O., Hyun N. Validation of a human papillomavirus (HPV) DNA cervical screening test that provides expanded hpv typing. J Clin Microbiol. 2018;56(5) doi: 10.1128/JCM.01910-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiffman M., Doorbar J., Wentzensen N. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016;2:16086. doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- 18.IARC. Human papillomaviruses . Distributed for the International Agency for Research on Cancer by the Secretariat of the World Health Organization; 2007. IARC monogr eval carcinog risks HUM: IARC; pp. 1–636. [Google Scholar]
- 19.Schiffman M., Clifford G., Buonaguro F.M. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect. Agents Cancer. 2009;4:8-. doi: 10.1186/1750-9378-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miranda P.M., Silva N.N., Pitol B.C. Persistence or clearance of human papillomavirus infections in women in Ouro Preto, Brazil. Biomed Res Int. 2013;2013 doi: 10.1155/2013/578276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson H., Abrahamowicz M., Tellier P.P. Modifiable risk factors associated with clearance of type-specific cervical human papillomavirus infections in a cohort of university students. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1149–1156. doi: 10.1158/1055-9965.EPI-04-0230. [DOI] [PubMed] [Google Scholar]
- 22.Bulkmans N.W., Berkhof J., Bulk S. High-risk hpv type-specific clearance rates in cervical screening. Br J Cancer. 2007;96(9):1419–1424. doi: 10.1038/sj.bjc.6603653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molano M., Van den Brule A., Plummer M. Determinants of clearance of human papillomavirus infections in colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003;158(5):486–494. doi: 10.1093/aje/kwg171. [DOI] [PubMed] [Google Scholar]
- 24.Cheung L.C., Pan Q., Hyun N. Mixture models for undiagnosed prevalent disease and interval-censored incident disease: applications to a cohort assembled from electronic health records. Stat Med. 2017;36:3583–3595. doi: 10.1002/sim.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landy R., Cheung L.C., Schiffman M. Challenges in risk estimation using routinely collected clinical data: the example of estimating cervical cancer risks from electronic health-records. Prev Med. 2018;111:429–435. doi: 10.1016/j.ypmed.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinheiro M., Gage J., Clifford G. Association of HPV35 with cervical carcinogenesis among women of african ancestry: evidence for co-evolution and implications for control. Int J Cancer. 2020 doi: 10.1002/ijc.33033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dillner J., Rebolj M., Birembaut P. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richart .RM. Proceedings: an assessment of the biology of cervical intraepithelial neoplasia. Proc Natl Cancer Conf. 1972;7:219–222. [PubMed] [Google Scholar]
- 29.(IARC) IAfRoC . 2016. Globocan 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012.http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx [Accessed 01 January 2019] [Google Scholar]
- 30.Wentzensen N., Sun C., Ghosh A. Methylation of HPV18, HPV31, and HPV45 genomes and cervical intraepithelial neoplasia grade 3. J Natl Cancer Inst. 2012;104(22):1738–1749. doi: 10.1093/jnci/djs425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke M.A., Gradissimo A., Schiffman M. Human papillomavirus dna methylation as a biomarker for cervical precancer: consistency across 12 genotypes and potential impact on management of HPV-Positive women. Clin Cancer Res. 2018;24(9):2194–2202. doi: 10.1158/1078-0432.CCR-17-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kjaer S.K., van den Brule A.J., Paull G. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ. 2002;325(7364):572. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H.C., Schiffman M., Lin C.Y. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst. 2011;103(18):1387–1396. doi: 10.1093/jnci/djr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maver P.J., Poljak M. Primary HPV-based cervical cancer screening in Europe: implementation status, challenges, and future plans. Clin Microbiol Infect. 2019 doi: 10.1016/j.cmi.2019.09.006. S1198-743X(19)30491-4. [DOI] [PubMed] [Google Scholar]
- 35.Rebolj M., Brentnall A.R., Mathews C. 16/18 genotyping in triage of persistent human papillomavirus infections with negative cytology in the English cervical screening pilot. Br. J. Cancer. 2019;121(6):455–463. doi: 10.1038/s41416-019-0547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wheeler CM, Hunt WC, Cuzick J. The influence of type-specific human papillomavirus infections on the detection of cervical precancer and cancer: A population-based study of opportunistic cervical screening in the United States. Int J Cancer. 2014;135(3):624–634. doi: 10.1002/ijc.28605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.