Abstract
Dimethylhydrazine (DMH) is a potent colonic and hepatic carcinogen that is metabolized into oxyradicals causing liver injury and DNA mutations. Matricaria chamomilla is a well-documented medicinal herb that possesses anti-inflammatory, antioxidant and antitumor activities and is commonly used to treat diverse ailments. The present study aimed to reveal the hepatoprotective effects of Matricaria chamomilla aqueous extract during an intermediate stage of colorectal cancer (CRC) in mice. Male Balb/c mice were divided into six groups: group A served as control, group B received chamomile extract (150 mg/Kg b.w.) orally for 12 weeks, and groups C-F received weekly intraperitoneal injections of DMH (20 mg/Kg b.w.) once a week for 12 weeks. In addition to DMH, groups D and F received chamomile during the initiation and post-initiation stages, respectively. Blood and liver samples were collected for biochemical and molecular analyses. The results showed that DMH induced hepatic injury in mice as shown by significant increase in serum aspartate aminotransferase and alanine aminotransferase. The changes in biochemical parameters were accompanied by activation of the Wnt signaling pathway leading to increased hepatocytes proliferation as well as inflammation evidenced by high levels of pro-inflammatory enzymes cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS). The results also showed potential hepatoprotective effects of chamomile extract against DMH-induced liver injury, proliferation and inflammation. Chamomile restored the biochemical and molecular parameters and this improvement was more pronounced in mice pretreated with the extract. In conclusion, chamomile extract may exert its hepatoprotective activities against DMH probably due to the antioxidant, antiproliferative and anti-inflammatory properties of its flavonoids.
Keywords: Dimethylhydrazine, Chamomile, Liver injury, Wnt signaling, COX-2, iNOS, Biochemistry, Biological sciences, Cancer research, Hepatobiliary system, Molecular biology, Oncology, Pharmaceutical science, Microbiology, Pharmaceutical chemistry, Toxicology
Dimethylhydrazine; Chamomile; liver injury; Wnt signaling; COX-2; iNOS; Biochemistry; Biological Sciences; Cancer Research; Hepatobiliary System; Molecular Biology; Oncology; Pharmaceutical Science; Microbiology; Pharmaceutical Chemistry; Toxicology
1. Introduction
Colorectal cancer (CRC) is the third prevalently diagnosed cancer worldwide and has become a major cause of cancer-related mortality [1]. CRC develops from multistep processes that establish accumulating pre-neoplastic lesions in mucosal cells leading to cancer [2]. Due to the observed geographic differences in CRC rates, several epidemiological studies have suggested that diet strongly influences the occurrence of this disease [3]. For instance, Western diets that are rich in fat and red meat constitute major risk factors for CRC development; however, fruit, vegetables, and dietary fibers are commonly associated with a reduced risk of CRC [3,4].
1,2-Dimethylhydrazine (DMH) is a toxic environmental pollutant [5] that has been detected in tobacco [6], some mushrooms and food items [7,8] as well. DMH has been well-documented as a potential carcinogen with selective toxicity for colon and rectum in animal models [9]. Also, it is a powerful hepatocarcinogen that induces oxidative stress, hepatotoxicity, and hepatocellular carcinoma upon its metabolism in the liver [10]. In addition, its metabolites, methyldiazonium ion and a reactive carbonium ion, methylate guanines in DNA forming O6-methyl-deoxyguanosine and N7-methyl-deoxyguanosine, thus inducing genetic mutations in diverse genes such as the adenomatous polyposis coli gene (Apc) and β-catenin gene (Ctnnb1) [11]. These genes are key players of the Wnt pathway, one of the most important and conserved signaling pathways involved in colon and liver cancers [12]. Several studies showed that DMH metabolites cause missense or point mutations in Apc gene and point mutations in the Ctnnb1 gene [13, 14, 15]. In addition, DMH causes the accumulation of pro-inflammatory enzymes such as cyclooxygenase 2 (COX-2) [16] and inducible nitric oxide synthase (iNOS) [17] that play pivotal roles in inflammation and tumor growth in humans and experimental models.
Since conventional and synthetic drugs used in the treatment of diseases, including cancer, have a vast array of unfavorable side effects, there is an increasing worldwide interest in the use of traditional medicinal herbs to treat various diseases [18]. The therapeutic uses of medicinal herbs have many advantages including their safety and easy availability besides being economical and effective [19]. Chamomile, scientifically named by Linnaeus Matricaria chamomilla L., is one of the most commonly used medicinal herbs whose extracts and standardized tea are usually prepared from the dried flowers [20]. Chamomile is a member of the daisy family (Asteraceae) that has been traditionally used in treating wounds, eczema, ulcers, gout, skin irritations, burns, neuralgia, rheumatic pain, hemorrhoids, diaper rash, chicken pox, ear and eye infections, and respiratory disorders [21,22]. In addition, chamomile has been used as a digestive relaxant treating various gastrointestinal disturbances including indigestion, flatulence, diarrhea, motion sickness, anorexia, nausea, and vomiting [23, 24, 25].
Chamomile contains different bioactive constituents such as the blue oil (0.24%–1.9%) containing terpenoids, α-bisabolol and chamazulene, farnesene, spiro-ether quiterpene lactones, hydroxycoumarins, glycosides, flavanoids (apigenin, luteolin, patuletin, and quercetin), coumarins (herniarin and umbelliferone), and terpenoids [26]. Chamomile is widely considered as a sleep-inducer and a mild tranquillizer [27]. Furthermore, some studies suggest that chamomile extracts possess hypoglycemic [28], hepatoprotective [29], antioxidant [30], and antitumor effects against skin, prostate, breast, ovarian, and colorectal cancer [31,32].
Recently, a study by El Joumaa et al. [33] revealed a chemoprotective role of aqueous chamomile extract against the DMH-induced model of CRC. In their study, the chemopreventive and antitumor effects of chamomile were mediated via downregulating the Wnt signaling pathway and mitigating inflammation in the colons of DMH-injected mice. In addition, since chemical carcinogens including DMH require metabolic activation in the liver in order to exert their mutagenic and carcinogenic effects [9], we hypothesized that chamomile extract might exert hepatoprotective effects against DMH-induced carcinogenesis. In this context, the present study was designed to provide a better understanding of the potential action of chamomile extract against DMH-induced hepatocarcinogenicity in mice.
2. Materials and methods
2.1. Chemicals
1,2-Dimethylhydrazine dihydrochloride was obtained from ACROS Organics™ (part of Thermo Fisher Scientific, NJ, USA). Phenylmethanesulfonylchloride (PMSF) was purchased from Roche Diagnostics (Risch-Rotkreuz, Switzerland). All primers were purchased from BIO-RAD® (CA, USA) except GAPDH primers which were synthesized by TIB Molbiol (Berlin, Germany). All other chemicals and reagents used were of high commercial and analytical grades.
2.2. Chamomile extract
Air-dried chamomile flowers of Syrian origin were purchased from a local market in Saida city, Lebanon. The taxonomic identification of this herb was performed by Dr. Salwa Mahmoud Abdul Rahman, Department of Biological Science, Faculty of Science at Beirut Arab University. Chamomile's flowers (2.5 g) were soaked in 100 mL of boiled distilled water (100 °C) and steeped at room temperature for 30 min with occasional stirring. The mixture was then filtered, aliquoted and stored at -20 °C to be used.
2.3. Extraction, UPLC and LC-TSQ-Endura-MS/MS analysis of polyphenols and flavonoids
The aqueous extract was filtered with 0.25 μm Millipore SPE cartridges and diluted 1:10 with LCMS grade water. The resultant crude solution was injected into a UPLC-PDA (Thermo Scientific, MA, USA) using a C18-Hypersil Gold reverse phase column to acquire a fingerprint 3D chromatogram. Gradient elution was performed with 0.1% formic acid in water/acetonitrile at a constant flow rate of 0.285 mL/min and an injection volume of 10 μL. Separation was carried out in 30 min.
A list of 50 common polyphenols and flavonoids (Table 1) was formulated based on a literature review on the constituents of chamomile and culinary herbs [34]. The 50 compounds were then analyzed via direct injection into a UPLC-TSQ-Endura triple Quadruple mass spectrometer (Thermo Scientific, MA, USA) equipped with an ESI source operating in both positive and negative ion mode. In positive ionization mode, the mobile phase used was 10% methanol:water in formic acid at a flow rate of 250 μL/min while in negative ionization mode the same mobile phase was used but without formic acid. The detection and qualitative analysis was carried out based on MRM transitions reported by Vallverdú-Queralt et al. [34] and by PubChem Mass Spectral Data (National Center for Biotechnology information, URL: https://www.ncbi.nlm.nih.gov/pccompound).
Table 1.
List of polyphenols and flavonoids screened for via LC-MS/MS.
| Compound | |
|---|---|
| 1 | alpha-Bisabolol |
| 2 | Chamazulene |
| 3 | Methyl angelate |
| 4 | Angelic acid |
| 5 | Isobutyl angelate |
| 6 | Farnesene |
| 7 | alpha-Pinene |
| 8 | Nobilin |
| 9 | 3-Epinobilin |
| 10 | Bisabolol oxide A |
| 11 | Bisabolol oxide B |
| 12 | Azulene |
| 13 | 4-Hydroxycoumarine |
| 14 | 6-Hydroxycoumarine |
| 15 | 7-Hydroxycoumarine |
| 16 | Luteolin |
| 17 | Patuletin |
| 18 | Herniarin |
| 19 | Apigenine-7-O-glucoside |
| 20 | Apigenin-8-C-glucoside |
| 21 | alpha-Bisabolol acetate |
| 22 | Gallic acid |
| 23 | Vanillic acid-O-hexoside |
| 24 | Syringic acid |
| 25 | Caffeicacid-O-hexoside1 |
| 26 | Neochlorogenic acid |
| 27 | Protocatechuic acid |
| 28 | Caffeicacid-O-hexoside-2 |
| 29 | Homovanillicacid-O-hexoside-1 |
| 30 | Caffeicacid-O-hexoside-3 |
| 31 | p-Hydroxybenzoic acid |
| 32 | Chlorogenic acid |
| 33 | Coumaricacid-O-hexoside-1 |
| 34 | m-Hydroxybenzoic acid |
| 35 | Cryptochlorogenic acid |
| 36 | Homovanillic acid |
| 37 | Caffeic acid |
| 38 | 4-O-p-Coumaroylqunic acid |
| 39 | Coumaric acid-O-hexoside |
| 40 | Vanillic acid |
| 41 | p-Coumaric acid |
| 42 | Ferulic acid |
| 43 | Rutin |
| 44 | Kaempferol-3-O-rutinoside |
| 45 | Kaempferol-3-O-glucoside |
| 46 | Populnetin |
| 47 | Quercetin |
| 48 | Naringenin |
| 49 | Apigenin |
| 50 | Hesperetin |
2.4. Animal model
Healthy 6-week-old male albino Balb/c mice were obtained from Beirut Arab University's animal facility. They were housed under standard laboratory conditions of light (12-hour light/dark cycle), temperature (22 ± 2 °C), and humidity with ad libitum access to standard mouse diet and tap water. Mice were left to acclimate with these conditions for one week before beginning the experiments. Experimental procedures were carried according to the approved guidelines of the Institutional Review Board (IRB) at Beirut Arab University code number 2018A-0033-S-M-0245.
2.5. Experimental design
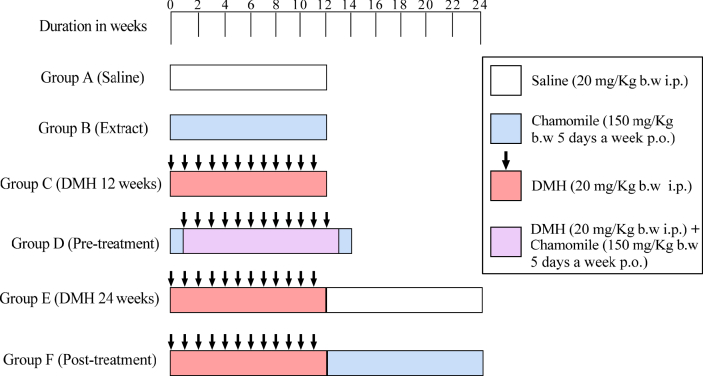
Animals were randomly divided into six experimental groups of 6 mice each. The experimental protocol is shown in Table 2 and schematically represented in Figure 1.
Table 2.
Experimental protocol.
| Group A: Saline | Mice were intraperitoneally (i.p.) injected with saline once per week over a period of 12 weeks. Saline is the vehicle used to dissolve DMH. |
| Group B: Extract | Mice received M. chamomilla aqueous extract only at 150 mg/Kg/ b.w. by gavage (P.O.) 5 days/week for 12 weeks. |
| Group C: DMH 12 weeks | Mice received DMH dissolved in saline (20 mg/Kg b.w., i.p.) once per week over 12 weeks to induce colorectal cancer (CRC). |
| Group D: Pre-treatment | Mice were pre-treated with M. chamomilla extract (150 mg/Kg/ b.w.) starting 1 week before DMH injections and continued till 1 week after the final DMH exposure. |
| Group E: DMH 24 weeks | Mice received DMH for 12 weeks as in group C and left without any treatment for an additional 12 weeks. |
| Group F: Post-treatment | Mice received M. chamomilla extract (150 mg/Kg/ b.w.) starting 1 week after the twelfth DMH injection and continued over additional 12 weeks. |
Figure 1.
Schematic representation of treatment schedule.
The concentration used to induce CRC was based on Gurley, Moser & Kemp [35]. The selected dose of chamomile extract was chosen on the basis of previously published reports and with reference to the average human daily intake [33, 36, 37, 38].
At the end of the treatment, mice were fasted overnight, then sacrificed and their livers were excised for further histological, biochemical and molecular analyses.
2.6. Tissue homogenization and protein quantification
Liver tissues were homogenized in phosphate buffered saline (PBS, pH 7.4) supplemented with 1 mM of the protease inhibitor PMSF at a ratio of 1 g per 5 mL of buffer. The homogenate was then centrifuged for 15 min at 15,000 rpm at 4 °C, and the supernatant was collected and stored at – 80 °C for later use.
Proteins concentration in all tissue homogenates were quantified according to the method of Lowry et al. using BSA (Bovine serum albumin) as a standard [39]. Each sample was run in triplicates. Absorbance of the developed color was assessed spectrophotometrically at 650 nm. Protein concentrations were deduced from the BSA standard curve.
2.7. Enzyme assays for alanine amino transferase (ALT) and aspartate amino transferase (AST)
ALT and AST activities in all tissue homogenates were measured using the GPT (ALT) colorimetric assay kit (cat# BEIS45-E, Spin-React®, Spain) and GOT (AST) assay colorimetric kit (cat# MDBEIS46-P, Spin-React®, Spain), respectively and as recommended by the manufacturer.
2.8. Quantification of Wnt signaling gene expression by RT-PCR
2.8.1. RNA extraction and quantification
Total RNA was extracted from liver homogenates using the RNeasy Plus Mini Kit (catalog # 732-6820, QIAGEN®) according to manufacturer recommendations. In brief, 200 μL of liver tissue homogenate were lyzed in 400 μL of denaturing guanidine-thiocyanate–containing RLT buffer, which inactivates RNases and ensures intact RNA isolation. The lysate was then passed through a gDNA eliminator spin column to eliminate genomic DNA. Ethanol was also added to the lysate in order to create conditions that promote selective binding of RNA to the RNeasy column. The sample was then applied to the RNeasy spin column, where total RNA binds to the membrane and contaminants are efficiently washed away. High-quality RNA was finally eluted in 80 μL RNase-free water.
To check for the integrity of the eluted RNA, samples were electrophoretically separated on 1% agarose and visualized by UV illumination using ethidium bromide staining. RNAs appeared as two sharp bands corresponding to the 28S rRNA and 18 S rRNA. RNA was quantified through its absorbance which was measured at 260 nm. Its purity was assessed from the 260/280 absorbance ratio.
2.8.2. Reverse transcription
RNA was transcribed using the QuantiTect® Reverse Transcription Kit (catalog # 205311, QIAGEN®) according to manufacturer recommendations. In brief, 1.0 μg of RNA samples were incubated in 3 μL gDNA Wipeout Buffer and 9 μL of RNase free water at 42 °C for 2 min to effectively remove contaminating genomic DNA in a total volume of 14 μL. After genomic DNA elimination, RNA samples were reverse transcribed using Quantiscript Reverse Transcriptase (1.5 μL), Quantiscript RT Buffer (6 μL), and RT Primer Mix (1.5 μL) in a final volume of 20 μL. The reaction took place at 42 °C for 15 min and the enzyme was then inactivated at 95 °C for 3 min. Finally, the cDNA obtained were stored at –80 °C for later use.
2.8.3. RT-PCR
The expression of Wnt signaling genes were quantified by RT-PCR using QuantiFast® SYBR® Green PCR Kit (catalog # 204045, QIAGEN®). The amplification reaction was carried out at final volume of 10 μL containing 5 μL of 2x QuantiFast SYBR Green PCR Master Mix, 1 μL (1 μM) of each primer (forward and reverse), 2 μL of cDNA and 1 μL of RNase-free water. Cycling was performed as follows. First, a denaturation step at 95 ° C for 5 min, followed by 45 cycles of denaturation at 95 ° C for 10 s and annealing/extension at 60 ° C for 30s.
Forward (F) and reverse (R) sequences are shown in Table 3 along with expected product size to be amplified (bp).
Table 3.
Sequences of forward and reverse primers used to amplify the selected genes.
| Gene | Primer Sequence | Product size (bp) |
|---|---|---|
| GAPDH |
F: 5′-TGGTGCTCAGTGTAGCCCAG-3′ R: 5′-GGACCTGACCTGCCGTCTAG-3′ |
111 |
| Wnt5a |
F: 5′-CTGGCAGGACTTTCTCAAGG-3′ R: 5′-CTCTAGCGTCCACGAACTCC-3′ |
395 |
| GSK3β |
F: 5′-TCCATTCCTTTGGAATCTGC-3′ R: 5′-CAATTCAGCCAACACACAGC-3 |
236 |
| APC |
F: 5′-TGGAAGTGTGAAAGCATTGATGGAATGTGC-3′ R: 5′-CCACATGCATTACTGACTATTGTCAAG-3′ |
348 |
| β-Catenin |
F: 5′-GCTGACCTGATGGAGTTGGA-3′ R: 5′-GCTACTTGCTCTTGCGTGAA-3′ |
227 |
| Lef1 |
F: 5′-TGAGTGCACGCTAAAGGAGA-3′ R: 5′-ATAATTGTCTCGCGCTGACC-3′ |
160 |
| Tcf4 |
F: 5′-CAAAGAAAGTCCGAAAAGTTCCT-3′ R: 5′-GGCGAGTCCCTGTTGTAGTC-3′ |
88 |
| C-Myc |
F: 5′-TAGTGCTGCATGAGGAGACA-3′ R: 5′-GGTTTGCCTCTTCTCCACAG-3′ |
104 |
| Cyclin D1 |
F: 5′-GGCACCTGGATTGTTCTGTT-3′ R: 5′-CAGCTTGCTAGGGAACTTGG-3′ |
232 |
F: Forward set; R: Reverse set; bp = base pair.
Gene expression was measured by comparative threshold cycle (Ct) method using glyceraldehyde-3 phosphate dehydrogenase (GAPDH) as a reference gene. For each gene, the mean Ct (mCt) values were determined. ΔCt value was determined as the difference between the Ct of gene of interest and the Ct of GAPDH gene. The relative quantity of gene of interest expression compared to GAPDH gene was calculated applying the gene dosage ratio formula (GDR = 2−ΔΔCt) where:
| ΔΔCt = (mCt gene of interest − mCt GAPDH) control sample − (mCt gene of interest − mCt GAPDH) test sample. |
2.9. Quantification of pro-inflammatory enzymes
The level of COX-2 was measured using SimpleStep ELISA® kit (abcam®, MA, USA) according to manufacturer recommendations. The activity of iNOS was measured using Nitric Oxide Synthase Assay Kit (Abnova, CA, USA) according to manufacturer recommendations.
2.10. Statistical analysis
All statistical analyses were performed using Microsoft Excel, and they are shown as mean with standard deviations. Statistical significance was assessed using One-way ANOVA test followed by Tukey test. Graphs were drawn using GraphPad prism software and statistical significance was reported with a p-value < 0.05 considered as significant. Results with ∗∗∗∗ indicate the significance at P < 0.0001, ∗∗∗ at P < 0.001, ∗∗ at P < 0.01, and ∗ at P < 0.05.
3. Results
3.1. Profile of the aqueous chamomile extract
Out of 50 polyphenols and flavonoids screened for, 28 polyphenols were detected in the aqueous extract of the chamomile via direct injection into the MS, whereby detection was confirmed through a signal intensity in excess of e1 (Table 4). Among the detected compounds, the highest signal intensity was in the order of e4 and corresponds to herniarin, chlorogenic acid and ferulic acid. Signals in the order of e3 were observed for alpha-bisabolol, chamazulene, bisabolol oxide B, apigenin-8-C-glucoside, protocatechuic acid, p-hydroxybenzoic acid, homovanillic acid, caffeic acid, vanillic acid, p-coumaric acid, kaempferol-3-O-glucoside, and naringenin.
Table 4.
List of polyphenols and flavonoids present in the chamomile aqueous extract as detected via LC-MS/MS. (CE stands for collision energy, Pos ESI stands for Positive Electrospray Ionization, Neg ESI stands for Negative Electrospray Ionization, and m/z represents mass divided by charge number).
| # | Compound | Ionisation | Exact Mass | m/z | Ions | CE |
|---|---|---|---|---|---|---|
| 1 | alpha-Bisabolol | pos ESI | 222.198 | 223.206 | 205.19, 69.07 | 20 |
| 2 | Chamazulene | pos ESI | 184.125 | 185 | 169 | 5 |
| 3 | alpha-Pinene | pos ESI | 136.125 | 137.13 | 121.1, 105.07 | 40 |
| 4 | 3-Epinobilin | pos ESI | 346.178 | 347.1858 | 247.13, 83.04 | 20 |
| 5 | Bisabolol oxide B | pos ESI | 238.193 | 239.2 | 221.19, 81.07 | 20 |
| 6 | 4-Hydroxycoumarine | pos ESI | 162.032 | 163.0395 | 51.0235, 121.0290, 163.0395 | 40 |
| 7 | 7-Hydroxycoumarine | pos ESI | 162.032 | 163.0395 | 119.0497, 145.0290 | 40 |
| 8 | Luteolin | pos ESI | 286.048 | 287.0556 | 153.0188, 109.0290, 213.0552, 269.0450 | 40 |
| 9 | Herniarin | pos ESI | 176.047 | 177.0552 | 77.0391, 121.0290, 103.0548, 133.0653, 147.0446 | 40 |
| 10 | Apigenin-8C-glucoside | pos ESI | 432.106 | 433.1129 | 415.1, 397.1, 367.1 (10 EV) | 5 |
| 11 | Gallic acid | pos ESI | 170.022 | 171.0293 | 153.0188, 125.0239 | 20 |
| 12 | Syringic acid | pos ESI | 198.053 | 199.0606 | 181.0501 | 20 |
| 13 | Protocatechuicacid | pos ESI | 154.027 | 155.0344 | 109.0290, 137.0239 | 20 |
| 14 | p-Hydroxybenzoicacid | pos ESI | 138.032 | 139.0395 | 121.0290, 95.0503 | 20 |
| 15 | Chlorogenicacid | pos ESI | 354.095 | 355.1029 | 163.0395, 337.0923, 193. 0712, 175.0606 | 20 |
| 16 | m-Hydroxybenzoicacid | pos ESI | 138.032 | 139.0395 | 93.034 | 20 |
| 17 | Homovanillicacid | pos ESI | 182.058 | 183.0657 | 137.0603, 165.0552 | 20 |
| 18 | Caffeicacid | pos ESI | 180.042 | 181.0438 | 135.0446, 163.0395 | 20 |
| 19 | Vanillicacid | pos ESI | 168.042 | 169.0501 | 151.0395, 123.0446 | 20 |
| 20 | p-Coumaricacid | pos ESI | 164.047 | 165.0552 | 91.0544, 147.0446, 119.0497 (30 EV) | 20 |
| 21 | Ferulicacid | pos ESI | 194.058 | 195.0657 | 177.0552, 149.0603 | 20 |
| 22 | Kaempferol-3-O-glucoside | neg ESI | 448.101 | 447.0934 | 284.0237, 255.0294, 227.0341 | 40 |
| 23 | Rosmarinicacid | neg ESI | 360.085 | 359.0772 | 161.0240, 359.0767, 197.0454, 135.0709 | 40 |
| 24 | Populnetin | neg ESI | 286.048 | 285.0415 | 164.9985, 255.0296, 227.0357, 117.0346 | 20 |
| 25 | Quercetin | neg ESI | 302.043 | 301.0373 | 151.0013, 178.9964, 271.0250 | 20 |
| 26 | Naringenin | neg ESI | 272.068 | 271.0606 | 135.0082, 119.0497, 151.0031, 93.0340, 109.0290, 83.0133 | 40 |
| 27 | Apigenin | neg ESI | 270.053 | 269.052 | 117.038, 151.0080 | 30 |
| 28 | Hesperetin | neg ESI | 302.079 | 301.0722 | 136.0169, 151.0042, 164.0118, 285.0403 | 40 |
3.2. Liver-specific injury enzymes
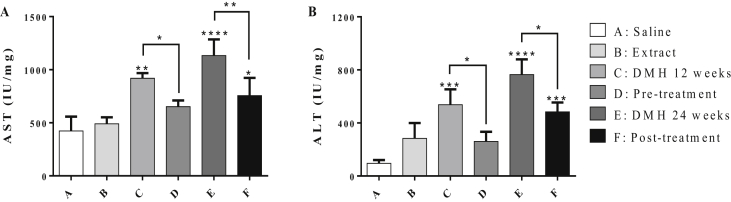
Figure 2 shows the activities of AST and ALT in serum for all groups. DMH induced a significant increase in AST and ALT levels (groups C and E) compared to control group (Group A). Pre-treatment of DMH-injected mice with chamomile (Group D) significantly minimized the liver damage. Significant reduction in the levels of AST and ALT (~30% and 52% respectively, P < 0.05) was obtained as compared to Group C. Likewise, chamomile post-treatment (Group F) significantly reduced the levels of AST and ALT by ~33% (P < 0.01) and 37% (P < 0.05), respectively, compared to the untreated mice in Group E.
Figure 2.
Effect of chamomile on the levels of AST (A) and ALT (B) in liver tissues of treated mice. Data represented are the mean of three determinations ±SD. (∗), (∗∗), (∗∗∗), and (∗∗∗∗) correspond to P < 0.05, 0.01, 0.001, and 0.0001 respectively.
3.3. Gene expression levels of Wnt pathway regulators
3.3.1. Oncogenes: Wnt5a and β-catenin
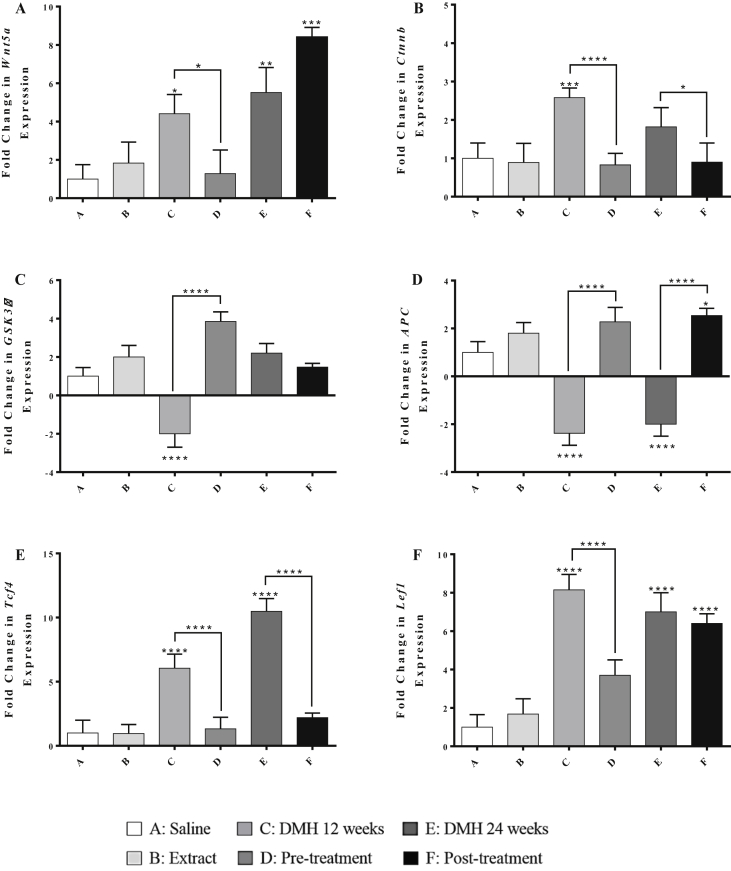
As shown in Figure 3, Panels A and B, DMH administration for 12 weeks (Group C) induced significant upregulations in the expression of Wnt5a by 3.4 folds (P < 0.05) and β-catenin by 1.58 folds (P < 0.001) compared to the control group. Similarly, DMH-treated groups for 24 weeks (Group E) showed a significant upregulation in Wnt5a gene by 4.5 folds (P < 0.01) compared to the control group. However, chamomile pre-treatment of DMH-injected mice (Group D) significantly downregulated the expression level of Wnt5a (3.2-fold decrease, P < 0.05) and β-catenin (1.9-fold decrease, P < 0.0001) genes, compared to those receiving the carcinogen only (Group C). Chamomile post-treatment of DMH-injected mice (Group F) significantly downregulated the expression level of β-catenin (0.9-fold decrease, P < 0.0001) compared to Group E.
Figure 3.
Effect of chamomile on the expression levels of Wnt5a (A), β-catenin (B), GSK3β (C), APC (D), Tcf4 (E), and Lef1 (F) genes in liver tissues of treated mice. Expression levels of treated and control groups were normalized to their respective GAPDH. Fold expression was determined relative to the control. All bars represent mean of three determinations ±SD. (∗), (∗∗), (∗∗∗), and (∗∗∗∗) on bars and on lines drawn upwards, that represent inter-categorical statistical significance, correspond to P < 0.05, <0.01, <0.001, and <0.0001 respectively.
3.3.2. Tumor suppressor genes: GSK3β and APC
In Panels C and D of Figure 3, DMH administration for 12 weeks (Group C) showed decreased expression levels of GSK3β and APC genes by 3 and 3.38 folds (P < 0.0001) respectively, compared to the normal control (Group A). However, pre-treated mice with chamomile extract (Group D) significantly increased the expression level of GSK3β and APC gene by 5.85 and 4.66 folds (P < 0.0001) respectively, compared to Group C. Chamomile, as a post-treatment (Group F), exerted a significant upregulation in the expression levels of APC gene by 4.54 folds in the liver (P < 0.0001) compared to group E.
3.3.3. Transcription factors: Tcf4 and Lef1
DMH administration for 12 weeks (Group C) and 24 weeks (Group E) induced significant upregulation in the expression of Tcf4 gene by 5 folds and 9.4 folds (P < 0.0001) respectively, compared to the control group as illustrated in Panels E and F of Figure 3. These alterations were modulated by chamomile pre- and post-treatments that induced significant downregulations in Tcf4 level by 4.7 folds and 7.3 folds (P < 0.0001) respectively.
As for the Lef1 gene expression, DMH administration for 12 weeks upregulated the level of Lef1 by 2 folds, and this effect was significantly reversed by chamomile pre-treatment which downregulated the expression level of this gene by 2.4 folds (P < 0.0001).
3.4. Gene expression levels of cell cycle regulators
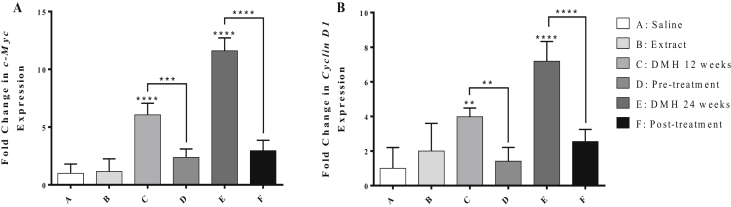
As shown in Figure 4, DMH-treatment for 12 and 24 weeks significantly upregulated the expression level of c-Myc gene by 5 and 10.6 folds (P < 0.0001) respectively, as well as the levels of Cyclin D1 gene by 2.9 (P < 0.01) and 6.2 folds (P < 0.0001) respectively, compared to the control. However, pre-treatment with chamomile extract caused a significant downregulation in the expression level of c-Myc and Cyclin D1 by 3.7 folds (P < 0.001) and 2.5 folds (P < 0.01) respectively, compared to group D. Similarly, chamomile as a post-treatment caused significant downregulation in the expression level of c-Myc gene by 8 folds and Cyclin D1 gene by 4.6 folds (P < 0.0001) respectively compared to group E.
Figure 4.
Effect of chamomile on the expression levels of c-Myc (A) and Cyclin D1 (B) genes in liver tissues of treated mice. Expression levels of treated and control groups were normalized to their respective GAPDH. Fold expression was determined relative to the control. All bars represent mean of three determinations ±SD. (∗), (∗∗), (∗∗∗), and (∗∗∗∗) on bars and on lines drawn upwards, that represent inter-categorical statistical significance, correspond to P < 0.05, <0.01, <0.001, and <0.0001 respectively.
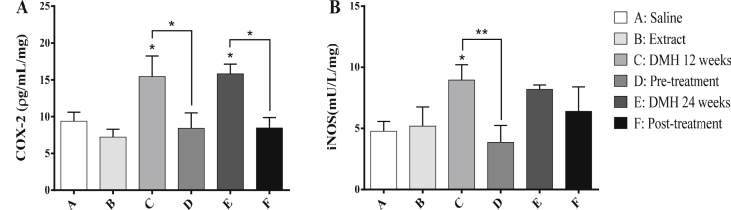
3.5. COX-2 level and iNOS activity
DMH induced a significant elevation in COX-2 level by 40% and 40.7% (P < 0.05) after 12 and 24 weeks respectively, compared to the control group as shown in Figure 5. Chamomile pre and post-treatments (Groups D and F) caused significant decrease in COX-2 level by 45.5% and 46.5% (P < 0.05) respectively, compared to groups C and E. As for iNOS, chamomile pre-treatment of DMH-injected mice (Group D) led to a significant decrease in its activity by 56.7% (P < 0.05) compared to group C.
Figure 5.
Effect of chamomile on the level of COX-2 (A) and activity of iNOS (B) in liver tissues of treated mice. Data are represented as mean ± SD. (∗), (∗∗), (∗∗∗), and (∗∗∗∗) correspond to P < 0.05, 0.01, 0.001, and 0.0001 respectively.
4. Discussion
CRC occurs as a result of many multistep processes that lead to the accumulation of mutations in tumor-suppressor genes and oncogenes [40]. Recently, the role of oxy-radicals during different stages of carcinogenesis has been reported whereby ROS play crucial roles in the metabolic activation of carcinogens. DMH acts as a potent necrogenic hepatic carcinogen that mainly alkylates the hepatocellular DNA leading to hepatocarcinoma [41]. In the liver, DMH is metabolized into the active electrophile, the carbonium ion that elicits DNA mutations and oxidative stress [11]. Thus, this carcinogen has been commonly used as a model to evaluate the effect of therapeutic agents on hepatic molecular and enzymatic alterations that occur during different stages of carcinogenesis [42,43]. Since the conventional treatment methods of CRC share common serious side effects, there is an enduring popularity of herbal medicine mainly due to the ability of herbs to function slowly, effectively and with minimal toxic side effects. Therefore, in this work we aimed to assess the hepatoprotective effects of M. chamomilla extract during an intermediate DMH-induced mouse model of CRC, taking into consideration the activation of Wnt signaling pathway and inflammation.
At the biochemical level, DMH-induced hepatotoxicity was evidenced by significant elevations in the levels of serum ALT and AST that are attributed to liver injury. The increments of serum ALT and AST activities are usually due to their leakage from liver cytosol into blood in cases of necrosis or membrane damage, making these enzyme potent markers of hepatic damage [44]. The present results demonstrated that chamomile extract significantly minimized liver injury via decreasing the levels of AST and ALT. Moreover, our results are in agreement with a study by Mannaa et al. [45] that reported the hepatoprotective effects of chamomile flowers extract against azathioprine-induced liver injury through modulating the levels of ALT and AST enzymes. Similarly, AST and ALT levels were reduced after Matricaria chamomilla treatment in streptozotocin-induced diabetic rats [46]. Likewise, our results are consistent with previous studies where chamomile tea modulated the activity of hepatic cytochrome P450 [47], exerted hepatoprotective activity against paracetamol-induced liver damage in albino rats [29], and reduced hepatic damage and oxidative stress by positively modifying several enzyme systems including AST and ALT in carbon tetrachloride treated rats [48].
At the molecular level, since DMH metabolites were shown to alkylate DNA causing mutations in Apc and β-catenin gene which are key players of the Wnt pathway [11, 12, 13, 14, 15], targeting this signaling pathway reveals insights into novel chemopreventive strategies. In our study, we investigated the altered Wnt signaling through assessing the gene expressions of: Wnt5a, APC, GSK3β, β-catenin, Tcf4 and Lef1, as well as c-Myc and Cyclin D1. Our results showed that this pathway was activated in the liver tissues of DMH mice. Interestingly, pre-treatment with chamomile extract significantly modulated the alterations in this pathway, was more effective than post-treatment, and led to a reduction in hepatic cell proliferation.
It is quite clear from the obtained findings that a significant hepatic damage has been elicited, in the form of elevated AST and ALT levels and activated Wnt signaling pathway, after DMH treatment. While gene changes reflect long term damage attributed to DNA alkylating ability of DMH metabolites [11], chemical changes reflect the continuous hepatic damage caused by DMH metabolites as well as from ROS such as H2O2 released from colonic tumors [49]. A study by Zatrowski and Nathan suggested that tumor cells can further produce substantial amount of H2O2 into the circulation which can then reach the liver for detoxification [50]. Therefore, the liver damage induced by DMH in the present study, especially in post-initiation groups E & F, could be attributed to the excessive generation of H2O2 – by the DMH-induced colon tumors – that has been transferred to the liver for detoxification. Moreover, Burton et al. showed that cancer cells tend to protect themselves and grow by releasing products of lipid peroxidation which also explains the continuous liver damage for weeks after DMH exposure [51].
The putative hepatoprotective and anti-proliferative activities of chamomile extract might be explained at least in part by the well-documented activities of its bioactive compounds. Phenolic compounds, such as flavonoids, tannins, coumarins, lignans, and quinones, are secondary compounds known for their hepatoprotective, antitumor and anti-inflammatory activities [52]. In the present study, the phytochemical screening revealed the presence of flavonoids: quercetin, apigenin, luteolin, rosmarinic acid, caffeic acid, and gallic acid in the aqueous extract of M. chamomilla. The hepatoprotective and anti-proliferative effects of these flavonoids have been extensively studied in the literature. For example, apigenin has been shown to possess cancer-preventive and anti-cancer activities against different types of cancers via inhibiting the Wnt/β-catenin signaling [53,54]. In addition, a recent study by Chiang et al. showed that apigenin exerts anti-hepatoma activities via an apoptotic mechanism that is mediated through the p53-dependent pathway and the induction of p21 expression leading to cell cycle arrest in G2/M phase [55]. Luteolin, another potent flavonoid found in our extract, has been studied for its ability to exert an anticancer activity against HepG2 cells by inducing apoptosis, causing G1 cell cycle arrest, and regulating the expression levels of p21, Bax and caspase-3 [56]. In CRC, it exerts its antitumor effects via inhibiting DMH-induced cell proliferation that involves the Wnt/β-catenin pathway [57]. Also, luteolin was shown to exert potent curative ability through decreasing the activity of different liver enzymes including AST and ALT against hepatocellular carcinoma in rats [58], and against acetaminophen-induced liver injury in mice [59]. Other phytochemicals such as quercetin, rosmarinic acid, caffeic acid, and gallic acid have been well-studied in the literature for their hepatoprotective effects against chemical-induced hepatotoxicity in rodents [60, 61, 62, 63].
Moreover, cancers involve serious complications of inflammation, where the enzymes COX-2 and iNOS provide a link between inflammation and carcinogenesis [64]. The Wnt/β-catenin pathway is known to up-regulate the expression of COX-2 [65]. Additionally, iNOS can stimulate and enhance COX-2 activity through a transcriptional pathway mediated by Wnt/β-catenin [66]. Therefore, based on our signaling results, we went further to investigate the effect of chamomile on COX-2 and iNOS. Our data showed that the pre-treatment of DMH mice with chamomile extract resulted in significant downregulation of COX-2 levels and iNOS activities compared to mice receiving DMH alone. Likewise, our results demonstrate that chamomile acts as COX-2 and iNOS inhibitor, and this is consistent with previous studies that showed that chamomile extract acts as COX-2 inhibitor during gastric damage [67] and iNOS inhibitor in RAW 264.7 macrophages [68]. Moreover, studies by Pandurangan et al. showed that luteolin – a major flavonoid found in chamomile – decreased the expressions of iNOS and COX-2 in AOM-induced CRC in mice [69] and induced growth arrest in colon cancer cells via the Wnt/β-catenin/GSK-3β signaling [70].
More importantly, our findings are in concomitance with a study by El Joumaa et al. [33] who showed that chamomile extract suppressed CRC incidence and progression in DMH-induced mouse model of colorectal carcinogenesis. In that model, chamomile extract inhibited tumor incidence and multiplication, downregulated the Wnt signaling pathway, and mitigated inflammation by modulating the levels of the pro-inflammatory enzymes COX-2 and iNOS in the colonic tissues of DMH-treated mice. Our results extended that work, corroborated the protective effect of chamomile, and provided better understanding of its activities in the liver, whereby it exerted protective effects against DMH-induced damage and carcinogenicity in the liver and subsequently in the colon as proven earlier.
In comparison with other herbs and dietary agents, similar effects were observed in a study by Devasena et al. [49] where a curcumin analog ameliorated the DMH-induced hepatic oxidative stress during colon carcinogenesis. Other studies by Sengottuvelan et al. [71] and Jrah-Harzallah et al. [72] showed the modulatory influence of dietary resveratrol and thymoquinone, respectively, during different phases of DMH-induced hepatic injury and oxidative stress during CRC. Moreover, our results are consistent with a study by Sharma and Sharma which indicated a chemoprotective role of Triphala against DMH-induced carcinogenic damage to mouse livers [73].
In conclusion, M. chamomilla extract is a natural dietary agent with profound biological and pharmacological properties that ameliorate the hepatic damage induced by the carcinogen DMH. Further investigations are needed to provide assessments of oxidative stress and DNA damage caused by DMH. Also, our data open up future work for additivity and/or synergism of chamomile extract with Wnt/COX-2/iNOS inhibitors for the development of more potent therapies with minimal side effect, and identification of the active molecules responsible for antiproliferative and hepatoprotective activities.
Declarations
Author contribution statement
J. Borjac: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
S. Shebbo and R. Kawash: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
M. El Joumaa: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Mármol I., Sánchez-de-Diego C., Pradilla Dieste A., Cerrada E., Rodriguez Yoldi M.J. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017;18(1):197. doi: 10.3390/ijms18010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon K. Colorectal cancer development and advances in screening. Clin. Interv. Aging. 2016;11:967–976. doi: 10.2147/CIA.S109285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marley A.R., Nan H. Epidemiology of colorectal cancer. Int. J. Mol. Epidemiol. Genet. 2016;7(3):105–114. [PMC free article] [PubMed] [Google Scholar]
- 4.Song M., Garrett W.S., Chan A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244–1260. doi: 10.1053/j.gastro.2014.12.035. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamiza O.O., Rehman M.U., Tahir M., Khan R., Khan A.Q., Lateef A., Ali F., Sultana S. Amelioration of 1, 2 Dimethylhydrazine (DMH) induced colon oxidative stress, inflammation and tumor promotion response by tannic acid in Wistar rats. Asian Pac. J. Cancer Prev. 2012;13(9):4393–4402. doi: 10.7314/apjcp.2012.13.9.4393. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y.Y. Chemical studies on tobacco smoke. Quantitative analysis of hydrazine in tobacco and cigarette smoke. Anal. Chem. 1974;46:885–889. doi: 10.1021/ac60343a046. [DOI] [PubMed] [Google Scholar]
- 7.Kostela J.G., Lawrence B.H. Hydrocarbon constituents from white strains of the mushroom Agaricus bisporus. J. Agric. Food Chem. 1981;20(1):185–186. [Google Scholar]
- 8.Wilbert S., Steinbrecher K., Gunderson E. Prevalence of hydrazine derivatives in food and food products. J. Agric. Food Chem. 1990;52:214. [Google Scholar]
- 9.Rosenberg D.W., Giardina C., Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30(2):183–196. doi: 10.1093/carcin/bgn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clair W.H., Billings P.C., Carew J.A., Keller-McGandy C., Newberne P., Kennedy A.R. Suppression of dimethylhydrazine-induced carcinogenesis in mice by dietary addition of the Bowman-Birk protease inhibitor. Cancer Res. 1990;50(3):580–586. [PubMed] [Google Scholar]
- 11.Newell L.E., Heddle J.A. The potent colon carcinogen, 1, 2-dimethylhydrazine induces mutations primarily in the colon. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2004;564(1):1–7. doi: 10.1016/j.mrgentox.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Wang J., Sinha T., Wynshaw-Boris A. Wnt signaling in mammalian development: lessons from mouse genetics. Cold Spring Harb. Perspect. Biol. 2012;4(5):a007963. doi: 10.1101/cshperspect.a007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum C.A., Tanaka T., Zhong X., Li Q., Dashwood W.M., Pereira C., Xu M., Dashwood R.H. Mutational analysis of Ctnnb1 and Apc in tumors from rats given 1, 2-dimethylhydrazine or 2-amino-3-methylimidazo [4, 5-f] quinoline: mutational ‘hotspots’ and the relative expression of β-catenin and c-jun. Mol. Carcinog. 2003;36(4):195–203. doi: 10.1002/mc.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi M., Mutoh M., Kawamori T., Sugimura T., Wakabayashi K. Altered expression of β-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 2000;21(7):1319–1327. [PubMed] [Google Scholar]
- 15.Femia A.P., Luceri C., Toti S., Giannini A., Dolara P., Caderni G. Gene expression profile and genomic alterations in colonic tumours induced by 1, 2-dimethylhydrazine (DMH) in rats. BMC Cancer. 2010;10(1):194. doi: 10.1186/1471-2407-10-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong M., Guda K., Nambiar P.R., Rezaie A., Belinsky G.S., Lambeau G., Giardina C., Rosenberg D.W. Inverse association between phospholipase A 2 and COX-2 expression during mouse colon tumorigenesis. Carcinogenesis. 2003;24(2):307–315. doi: 10.1093/carcin/24.2.307. [DOI] [PubMed] [Google Scholar]
- 17.Sengupta A., Ghosh S., Bhattacharjee S. Dietary cardamom inhibits the formation of azoxymethane-induced aberrant crypt foci in mice and reduces COX-2 and iNOS expression in the colon. Asian Pac. J. Cancer Prev. 2005;6(2):118–122. [PubMed] [Google Scholar]
- 18.Wang C.Z., Calway T., Yuan C.S. Herbal medicines as adjuvants for cancer therapeutics. Am. J. Chin. Med. 2012;40(4):657–669. doi: 10.1142/S0192415X12500498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin T.F., Min Wang Y.Q., Lin Y.M., Wu D. Research progress on chemopreventive effects of phytochemicals on colorectal cancer and their mechanisms. World J. Gastroenterol. 2016;22(31):7058. doi: 10.3748/wjg.v22.i31.7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh O., Khanam Z., Misra N., Srivastava M.K. Chamomile (Matricaria chamomilla L.): an overview. Pharmacogn. Rev. 2011;5(9):82. doi: 10.4103/0973-7847.79103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava J.K., Shankar E., Gupta S. Chamomile: a herbal medicine of the past with a bright future. Mol. Med. Rep. 2010;3(6):895–901. doi: 10.3892/mmr.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKay D.L., Blumberg J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytother Res.: Int. J. Dev. Pharm. Toxicol. Eval. Nat. Prod. Derivat. 2006;20(7):519–530. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- 23.Tubaro A., Zilli C., Redaelli C., Della Loggia R. Evaluation of antiinflammatory activity of a chamomile extract after topical application. Planta Med. 1984;50(4) 359-359. [PubMed] [Google Scholar]
- 24.Gardiner P. Complementary, holistic, and integrative medicine: chamomile. Pediatr. Rev. 2007;28(4):e16. doi: 10.1542/pir.28-4-e16. [DOI] [PubMed] [Google Scholar]
- 25.Sharafzadeh S., Alizadeh O. German and Roman chamomile. J. Appl. Pharmaceut. Sci. 2011;1(10):1–5. [Google Scholar]
- 26.Nováková L., Vildová A., Mateus J.P., Gonçalves T., Solich P. Development and application of UHPLC–MS/MS method for the determination of phenolic compounds in Chamomile flowers and Chamomile tea extracts. Talanta. 2010;82(4):1271–1280. doi: 10.1016/j.talanta.2010.06.057. [DOI] [PubMed] [Google Scholar]
- 27.Shinomiya K., Inoue T., Utsu Y., Tokunaga S., Masuoka T., Ohmori A., Kamei C. Hypnotic activities of chamomile and passiflora extracts in sleep-disturbed rats. Biol. Pharm. Bull. 2005;28(5):808–810. doi: 10.1248/bpb.28.808. [DOI] [PubMed] [Google Scholar]
- 28.Kato A., Minoshima Y., Yamamoto J., Adachi I., Watson A.A., Nash R.J. Protective effects of dietary chamomile tea on diabetic complications. J. Agric. Food Chem. 2008;56(17):8206–8211. doi: 10.1021/jf8014365. [DOI] [PubMed] [Google Scholar]
- 29.Gupta A.K., Misra N. Hepatoprotective activity of aqueous ethanolic extract of Chamomile capitula in paracetamol intoxicated albino rats. Am. J. Pharmacol. Toxicol. 2006;1(1):17–20. [Google Scholar]
- 30.Cvetanović A., Švarc-Gajić J., Mašković P., Savić S., Nikolić L. Antioxidant and biological activity of chamomile extracts obtained by different techniques: perspective of using superheated water for isolation of biologically active compounds. Ind. Crop. Prod. 2015;65:582–591. [Google Scholar]
- 31.Srivastava J.K., Gupta S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J. Agric. Food Chem. 2007;55(23):9470–9478. doi: 10.1021/jf071953k. [DOI] [PubMed] [Google Scholar]
- 32.Al-Dabbagh B., Elhaty I.A., Elhaw M., Murali C., Al Mansoori A., Awad B., Amin A. Antioxidant and anticancer activities of chamomile (Matricaria recutita L.) BMC Res. Notes. 2019;12(1):3. doi: 10.1186/s13104-018-3960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Joumaa M.M., Taleb R.I., Rizk S., Borjac J. Protective effect of Matricaria chamomilla extract against 1,2-dimethylhydrazine-induced colorectal cancer in mice. J. Compl. Integr. Med. 2020:20190143. doi: 10.1515/jcim-2019-0143. (published online ahead of print) [DOI] [PubMed] [Google Scholar]
- 34.Vallverdu-Queralt A., Regueiro J., Martinez-Huelamo M., Rinaldi Alvarenga J.F., Leal L.N., Lamuela-Raventos R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014;154:299–307. doi: 10.1016/j.foodchem.2013.12.106. [DOI] [PubMed] [Google Scholar]
- 35.Gurley K.E., Moser R.D., Kemp C.J. Induction of colon cancer in mice with 1, 2-dimethylhydrazine. Cold Spring Harb. Protoc. 2015 Sep 1;2015(9) doi: 10.1101/pdb.prot077453. pdb-rot077453. [DOI] [PubMed] [Google Scholar]
- 36.Weidner C., Wowro S.J., Rousseau M., Freiwald A., Kodelja V., Abdel-Aziz H., Kelber O., Sauer S. Antidiabetic effects of chamomile flowers extract in obese mice through transcriptional stimulation of nutrient sensors of the peroxisome proliferator-activated receptor (PPAR) family. PLoS One. 2013;8(11):e80335. doi: 10.1371/journal.pone.0080335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehmood M.H., Munir S., Khalid U.A., Asrar M., Gilani A.H. Antidiarrhoeal, antisecretory and antispasmodic activities of Matricaria chamomilla are mediated predominantly through K+-channels activation. BMC Compl. Alternative Med. 2015;15(1):75. doi: 10.1186/s12906-015-0595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prasanna R., Ashraf E.A., Essam M.A. Chamomile and oregano extracts synergistically exhibit antihyperglycemic, antihyperlipidemic, and renal protective effects in alloxan-induced diabetic rats. Can. J. Physiol. Pharmacol. 2017;95:84–92. doi: 10.1139/cjpp-2016-0189. [DOI] [PubMed] [Google Scholar]
- 39.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 40.Armaghany T., Wilson J.D., Chu Q., Mills G. Genetic alterations in colorectal cancer. Gastrointest. Cancer Res. 2012;5(1):19. [PMC free article] [PubMed] [Google Scholar]
- 41.Swenberg J.A., Cooper H.K., Bücheler J., Kleihues P. 1, 2-Dimethylhydrazine-induced methylation of DNA bases in various rat organs and the effect of pretreatment with disulfiram. Cancer Res. 1979;39(2 Part 1):465–467. [PubMed] [Google Scholar]
- 42.Rajeshkumar N.V., Kuttan R. Modulation of carcinogenic response and antioxidant enzymes of rats administered with 1, 2-dimethylhydrazine by Picroliv. Cancer Lett. 2003;191(2):137–143. doi: 10.1016/s0304-3835(02)00203-3. [DOI] [PubMed] [Google Scholar]
- 43.Vinothkumar R., Kumar R.V., Karthikkumar V., Viswanathan P., Kabalimoorthy J., Nalini N. Oral supplementation with troxerutin (trihydroxyethylrutin), modulates lipid peroxidation and antioxidant status in 1, 2-dimethylhydrazine-induced rat colon carcinogenesis. Environ. Toxicol. Pharmacol. 2014;37(1):174–184. doi: 10.1016/j.etap.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 44.McGill M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016;15:817. doi: 10.17179/excli2016-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mannaa F.A., Ibrahim N.A., Ibrahim S.S., Abdel-Wahhab K.G., Hassan N.S., Mohammed S.G. Preventive role of chamomile flowers and fennel seeds extracts against liver injury and oxidative stress induced by an immunosuppressant drug in rats. Hepatoma Res. 2015;1:125–135. [Google Scholar]
- 46.Najla O.A., Olfat A.K., Kholoud S.R., Enas N.D., Hanan S.A. Hypoglycemic and biochemical effects of Matricaria chamomilla leave extract in streptozotocin-induced diabetic rats. J. Health Sci. 2012;2(5):43–48. [Google Scholar]
- 47.Maliakal P.P., Wanwimolruk S. Effect of herbal teas on hepatic drug metabolizing enzymes in rats. J. Pharm. Pharmacol. 2001;53(10):1323–1329. doi: 10.1211/0022357011777819. [DOI] [PubMed] [Google Scholar]
- 48.Aksoy L., Sözbilir N.B. Effects of Matricaria chamomilla L. on lipid peroxidation, antioxidant enzyme systems, and key liver enzymes in CCl4-treated rats. Toxicol. Environ. Chem. 2012;94(9):1780–1788. [Google Scholar]
- 49.Devasena T., Rajasekaran K.N., Menon V.P. Bis-1, 7-(2-hydroxyphenyl)-hepta-1, 6-diene-3, 5-dione (a curcumin analog) ameliorates DMH-induced hepatic oxidative stress during colon carcinogenesis. Pharmacol. Res. 2002;46(1):39–45. doi: 10.1016/s1043-6618(02)00043-9. [DOI] [PubMed] [Google Scholar]
- 50.Zatmoski T.P., Nathan C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 51.Burton G.M., Cheesman K.N., Ingold K.V., Slater T.E. Lipid antioxidant and products of lipid peroxidation as potential tumor protective agents. Biochem. Soc. Trans. 1983;11:261–262. doi: 10.1042/bst0110261. [DOI] [PubMed] [Google Scholar]
- 52.Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines (Basel) 2018;5(3):93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin C.M., Chen H.H., Lin C.A., Wu H.C., Sheu J.J., Chen H.J. Apigenin-induced lysosomal degradation of β-catenin in Wnt/β-catenin signaling. Sci. Rep. 2017;7(1):372. doi: 10.1038/s41598-017-00409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu M., Wang S., Song Y.U., Yao J., Huang K., Zhu X. Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β-catenin signaling pathway. Oncol. Lett. 2016;11(5):3075–3080. doi: 10.3892/ol.2016.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiang L.C., Ng L.T., Lin I.C., Kuo P.L., Lin C.C. Anti-proliferative effect of apigenin and its apoptotic induction in human Hep G2 cells. Cancer Lett. 2006;237(2):207–214. doi: 10.1016/j.canlet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Imran M., Rauf A., Abu-Izneid T., Nadeem M., Shariati M.A., Khan I.A., Imran A., Orhan I.E., Rizwan M., Atif M., Gondal T.A. Luteolin, a flavonoid, as an anticancer agent: a review. Biomed. Pharmacother. 2019;112:108612. doi: 10.1016/j.biopha.2019.108612. [DOI] [PubMed] [Google Scholar]
- 57.Ashokkumar P., Sudhandiran G. Luteolin inhibits cell proliferation during Azoxymethane-induced experimental colon carcinogenesis via Wnt/β-catenin pathway. Invest. N. Drugs. 2011;29(2):273–284. doi: 10.1007/s10637-009-9359-9. [DOI] [PubMed] [Google Scholar]
- 58.Balamurugan K., Karthikeyan J. Evaluation of luteolin in the prevention of N-nitrosodiethylamine-induced hepatocellular carcinoma using animal model system. Indian J. Clin. Biochem. 2012;27(2):157–163. doi: 10.1007/s12291-011-0166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tai M., Zhang J., Song S., Miao R., Liu S., Pang Q., Wu Q., Liu C. Protective effects of luteolin against acetaminophen-induced acute liver failure in mouse. Int. Immunopharm. 2015;27(1):164–170. doi: 10.1016/j.intimp.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Chen X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn. Mag. 2010;6(22):135–141. doi: 10.4103/0973-1296.62900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elufioye T.O., Habtemariam S. Hepatoprotective effects of rosmarinic acid: insight into its mechanisms of action. Biomed. Pharmacother. 2019;112:108600. doi: 10.1016/j.biopha.2019.108600. [DOI] [PubMed] [Google Scholar]
- 62.Janbaz K.H., Saeed S.A., Gilani A.H. Studies on the protective effects of caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomedicine. 2004;11(5):424–430. doi: 10.1016/j.phymed.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Rasool M.K., Sabina E.P., Ramya S.R., Preety P., Patel S., Mandal N., Mishra P.P., Samuel J. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J. Pharm. Pharmacol. 2010;62(5):638–643. doi: 10.1211/jpp.62.05.0012. [DOI] [PubMed] [Google Scholar]
- 64.DiDonato J.A., Mercurio F., Karin M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 2012;246(1):379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 65.Nuñez F., Bravo S., Cruzat F., Montecino M., De Ferrari G.V. Wnt/β-catenin signaling enhances cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer cells. PloS One. 2011;6(4) doi: 10.1371/journal.pone.0018562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y., Borchert G.L., Phang J.M. Polyoma enhancer activator 3, an ets transcription factor, mediates the induction of cyclooxygenase-2 by nitric oxide in colorectal cancer cells. J. Biol. Chem. 2004;279:18694–18700. doi: 10.1074/jbc.M308136200. [DOI] [PubMed] [Google Scholar]
- 67.Ortiz M.I., Fernandez-Martinez E., Soria-Jasso L.E. Isolation, identification and molecular docking as cyclooxygenase (COX) inhibitors of the main constituents of Matricaria chamomilla L. extract and its synergistic interaction with diclofenac on nociception and gastric damage in rats. Biomed. Pharmacother. 2016;78:248–256. doi: 10.1016/j.biopha.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 68.Bhaskaran N., Shukla S., Srivastava J.K., Gupta S. Chamomile: an anti-inflammatory agent inhibits inducible nitric oxide synthase expression by blocking RelA/p65 activity. Int. J. Mol. Med. 2010;26:935–940. doi: 10.3892/ijmm_00000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pandurangan A.K., Kumar S.A., Dharmalingam P., Ganapasam S. Luteolin, a bioflavonoid inhibits azoxymethane-induced colon carcinogenesis: involvement of iNOS and COX-2. Pharmacogn. Mag. 2014;10(Suppl 2):S306–S310. doi: 10.4103/0973-1296.133285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pandurangan A.K., Dharmalingam P., Sadagopan S.K., Ramar M., Munusamy A. Luteolin induces growth arrest in colon cancer cells through involvement of Wnt/β-catenin/GSK-3β signaling. J. Environ. Pathol. Toxicol. Oncol. 2013;32(2) doi: 10.1615/jenvironpatholtoxicoloncol.2013007522. [DOI] [PubMed] [Google Scholar]
- 71.Sengottuvelan M., Senthilkumar R., Nalini N. Modulatory influence of dietary resveratrol during different phases of 1, 2-dimethylhydrazine induced mucosal lipid-peroxidation, antioxidant status and aberrant crypt foci development in rat colon carcinogenesis. Biochim. Biophys. Acta. 2006;1760(8):1175–1183. doi: 10.1016/j.bbagen.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 72.Jrah-Harzallah H., Ben-Hadj-Khalifa S., Almawi W.Y., Maaloul A., Houas Z., Mahjoub T. Effect of thymoquinone on 1, 2-dimethyl-hydrazine-induced oxidative stress during initiation and promotion of colon carcinogenesis. Eur. J. Cancer. 2013;49(5):1127–1135. doi: 10.1016/j.ejca.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 73.Sharma A., Sharma K.K. Chemoprotective role of triphala against 1,2-dimethylhydrazine dihydrochloride induced carcinogenic damage to mouse liver. Indian J. Clin. Biochem. 2011;26(3):290–295. doi: 10.1007/s12291-011-0138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]