Abstract
The passage of time dictates the pace at which humans and other organisms age but falls short of providing a complete portrait of how environmental, lifestyle and underlying biological processes contribute to senescence. Two fundamental features of the human experience that change dramatically across the lifespan include social interactions and, for many, patterns of alcohol consumption. Rodent models show great utility for understanding complex interactions among aging, social behavior and alcohol use and abuse, yet little is known about the neural changes in late aging that contribute to the natural decline in social behavior. Here, we posit that aging-related neuroinflammation contributes to the insipid loss of social motivation across the lifespan, an effect that is exacerbated by patterns of repeated alcohol consumption observed in many individuals. We provide a comprehensive review of (i) neural substrates crucial for the expression of social behavior under non-pathological conditions; (ii) unique developmental/lifespan vulnerabilities that may contribute to the divergent effects of low- and high-dose alcohol exposure; and (iii) aging-associated changes in neuroinflammation that may sit at the intersection between social processes and alcohol exposure. In doing so, we provide an overview of correspondence between lifespan/developmental periods between common rodent models and humans, give careful consideration to model systems used to aptly probe social behavior, identify points of coherence between human and animal models, and point toward a multitude of unresolved issues that should be addressed in future studies. Together, the combination of low-dose and high-dose alcohol effects serve to disrupt the normal development and maintenance of social relationships, which are critical for both healthy aging and quality of life across the lifespan. Thus, a more complete understanding of neural systems – including neuroinflammatory processes – which contribute to alcohol-induced changes in social behavior will provide novel opportunities and targets for promoting healthy aging.
Keywords: Aging, development, alcohol, ethanol, neuroinflammation, neuroimmune, social behavior, cytokines, neural circuits, human, rat, mouse
1. Introduction
A fundamental component of the biology of numerous species is an interaction among conspecifics. More broadly, social behaviors can be defined as any modality of communication and/or interaction between two conspecifics of a given species. In humans, social interactions include the detection and processing of social stimuli, bond/relationships formation, and social learning (Cacioppo, Cacioppo, Dulawa, & Palmer, 2014; Dunbar, 2009). Social behaviors are regulated by the social neural networks that process the emotional significance of social stimuli and their rewarding values. Changes in the state of an organism (e.g., altered motivation and/or anxiety) can have substantial influences on social behavior. As individuals age, there is a shift in the nature of social interactions, with reduced social behavior evident in human and animal models. In addition, the use of drugs, such as alcohol, can alter the nature of social interactions. Evidence from human and animal research indicates that both aging and alcohol alter the neuroinflammatory milieu, providing a common mechanism for the role of these factors in social behavior.
Social interactions play a pivotal role in everyday life, significantly influencing physical and mental health outcomes. Importantly, the nature, vigor and outcomes of social behavior and their neural regulation dramatically change over the lifespan. For instance, interactions with peers become particularly important during adolescence, with these interactions having a greater impact on decision-making and behavior of adolescents than adults (Gardner & Steinberg, 2005). Adolescents spend more time interacting with peers than children and adults, with these interactions providing significant positive experiences (Oberle, Guhn, Gadermann, Thomson, & Schonert-Reichl, 2018). During adulthood, a strong social network can produce health benefits evidenced by increased longevity (Tucker, Schwartz, Clark, & Friedman, 1999), enhanced resilience in the face of stress and trauma (Charuvastra & Cloitre, 2008), and faster recovery from different health challenges (Karelina & DeVries, 2011; Seeman, 2000). Social support is consistently identified as a protective factor against stress and trauma (Bonanno, 2004; Charuvastra & Cloitre, 2008; Masten & Obradović, 2006; Southwick, Bonanno, Masten, Panter-Brick, & Yehuda, 2014), emerging as a key component of resilience across the lifespan.
While positive social interactions have a protective value for health throughout the entire lifespan (Colonnello, Petrocchi, Farinelli, & Ottaviani, 2017), exposure to negative social interactions and a lack of social contact may have an adverse effect on mental health (Donovan et al., 2016; Scott, Smith, & Ellis, 2010; Takizawa, Maughan, & Arseneault, 2014). Negative social experiences of being excluded and physically or verbally abused by adolescent peers are associated with social dysfunction (Hawker & Boulton, 2000), while perceived social isolation and social stress are strong predictors of morbidity and mortality in older adults (Bisschop et al., 2003; House, Landis, & Umberson, 1988; Seeman, 2000; Vaillant & Mukamal, 2001). Even in rodent models, prolonged social isolation has been shown to increase propensity to consume alcohol, indicative of the maladaptive effects of social isolation (Karkhanis, Rose, Weiner, & Jones, 2016; McCool & Chappell, 2009).
The social brain is highly vulnerable to adverse life events, with social deficits sometimes representing the first signs of a number of psychiatric disorders (Porcelli et al., 2018). As individuals mature, there are significant changes in the patterns of social behavior, even in those without any signs of psychiatric disorders. In fact, aging is characterized by a narrowing of social circles, preference for interacting with highly familiar individuals, and a decrease in the tendency to establish new social relationships (Amore et al., 2012; Carstensen, 1992; Lang, 2001). In turn, this narrowing of social circles can negatively impact overall well-being as aging-associated debilitation and death of social partners compromises the depth and quality of social engagement (Caruso, Lio, Cavallone, & Franceschi, 2004; Lang, 2001). It is possible that the narrowing of social circles in aged individuals is a product of reduced motivation and increased anxiety related to initiation and maintenance of new social relationships. Understanding the neural mechanisms that contribute to these changes in social behavior may lead to interventions designed to enhance social interactions in aged individuals and in turn increase overall health and well-being. An important, and often overlooked, factor in motivation to engage in, and anxiety related to, social interaction is alcohol. Alcohol is a commonly used drug and potently alters social behavior across the lifespan. Recent human and animal research clearly demonstrates the importance of social factors in alcohol and drug use (Heilig, Epstein, Nader, & Shaham, 2016; Pelloux, Giorla, Montanari, & Baunez, 2019), with social interactions, on the other hand, being sensitive to alcohol (Varlinskaya & Spear, 2015).
Investigation of neural mechanisms involved in social alterations associated either with aging or alcohol use disorders requires experimental models that are less restricted by ethical concerns and allow strict control over several different variables, including exact age, prior social history, alcohol exposure level, etc. Rodent models have proven extremely useful in assessing social behavior alterations associated with aging and alcohol exposure across the lifespan. When addressing the issue of social alterations across the lifespan, it is helpful to define specific ontogenetic periods in humans and animal models (see Introduction to this volume by Deak et al.), while recognizing that the borders/transitions between developmental periods are not bright-line distinctions and may show marked individual variability. Nevertheless, in rodents, a conservative 2 weeks from postnatal days (P) 28 to P42 rats (Spear, 2000) may be roughly analogous to the 10–18 year, early-mid adolescent period in humans, with the ages from P43–P55 or P65 approximating the 18–25 year old period of late adolescence/emerging adulthood in humans (Vetter-O’Hagen & Spear, 2012). It should be noted that, like humans, female rodents typically experience puberty prior to males (i.e., pubertal onset in females is typically ~P35-P37, whereas puberty begins ~P41-P43 in males). Adulthood typically begins in the mid-20’s in humans and after P70 in rats. Humans 65 years of age and older are considered aged adults, which corresponds to a rat age of about 18+ months. Whereas the average lifespan in the U.S.A. is trending toward 80 years (aggregated across sex), most laboratory rodents display typical lifespans of about 2 years, though substantial sex, species and strain-differences are evident (Nadon, 2006; Turturro et al., 1999). Furthermore, when comparisons are made across species, it becomes apparent that humans (relative to many other species, including rodents) spend a considerably greater proportion of their lifespan in early development (see Introduction to this volume by Deak et al.).
Social influences on the use of alcohol and other drugs have been well documented in humans and in animals (Heilig et al., 2016; Pelloux et al., 2019). On the other hand, social interactions are sensitive to alcohol (Varlinskaya & Spear, 2015). For instance, social exclusion in human adolescents is linked to alcohol use (Meisel, Colder, Bowker, & Hussong, 2018), and prolonged social isolation of laboratory rodents during adolescence promotes excessive alcohol consumption (Lesscher et al., 2015) and vulnerability to the development of substance use disorders (Butler et al., 2016). It is still not clear, however, whether social impairments in individuals with alcohol use disorders reflect pre-existing deficits in social functioning or whether social dysfunction is a result of the neurotoxic effects of alcohol. Probably both factors are at play. Indeed, there is some evidence linking high levels of adolescent alcohol use to deficits in social functioning, suggesting that socially enhancing and/or anxiolytic effects of alcohol may play a substantial role in heavy drinking during adolescence (Lewis & O’Neill, 2000). Similar to adolescents, older adults, while experiencing age-associated declines in social interactions, often consume large amounts of alcohol within social occasions for social reward, and drinking-related problems are more likely to occur in these aging individuals (Sacco et al., 2015). It is likely that socially facilitating properties of alcohol play a substantial role in alcohol use and abuse not only during adolescence but also in late adulthood.
Across all ages, the appearance of sickness- or illness-like symptoms due to injury or infection have long been recognized as powerful modulators of social processes. Indeed, reduced social interaction is considered to be a hallmark feature of acute illness, often termed “sickness behaviors” (Dantzer & Kelley, 2007). Sickness-related suppression of social behavior is common to most social species and likely evolved as an evolutionary mechanism to control the spread of infectious disease and promote recovery from infection and/or injury. As such, tell-tale signs of sickness such das overt lethargy, piloerection, fever and shivering responses serve as warning signs to conspecifics to curtail social interaction with the affected individual. In nocturnal animals who tend to be more reliant on olfactory cues, sick individuals emit distinct odor signatures that convey honest information indicative of health status. Importantly, these sickness-related odor cues are independent of other known odor-driven signals that guide behavior of conspecifics such as stress- or reproduction-related signals (Arakawa, Cruz, & Deak, 2011). Thus, acute illness generally—and inflammatory processes specifically – exert a powerful influence over the expression of social behavior.
It should be noted, however, that inflammatory signaling pathways are not just important during acute infection and injury, but also appear to play an important role in many other aspects of physiological function. For example, cytokines, a large class of immune-signaling molecules, fluctuate across the diurnal cycle in the CNS and peripheral organs (Fonken et al., 2015; Fonken, Weil, & Nelson, 2013) and are critical to the initiation of sleep (Krueger, 2008). In addition, cytokines are induced during feeding and likely contribute to satiety processes through modulation of hypothalamic neuropeptides that regulate appetite (Guijarro, Laviano, & Meguid, 2006), especially under conditions in which the consumed food is particularly novel (Hansen, Kapás, Fang, & Krueger, 1998; Hansen, Taishi, Chen, & Krueger, 1998). Stress challenges induce cytokine expression and likely contribute to post-stress recuperative processes (Deak, Kudinova, Lovelock, Gibb, & Hennessy, 2017). Owing to the wide range of physiological roles of cytokines, we have recently hypothesized that inflammatory processes likely govern the expression of social behavior in healthy, typically functioning individuals, rather than just during pathological states of illness or injury (Hennessy, Deak, & Schiml, 2014). Thus, a greater appreciation for the role of inflammation in regulating social behavior has begun to emerge.
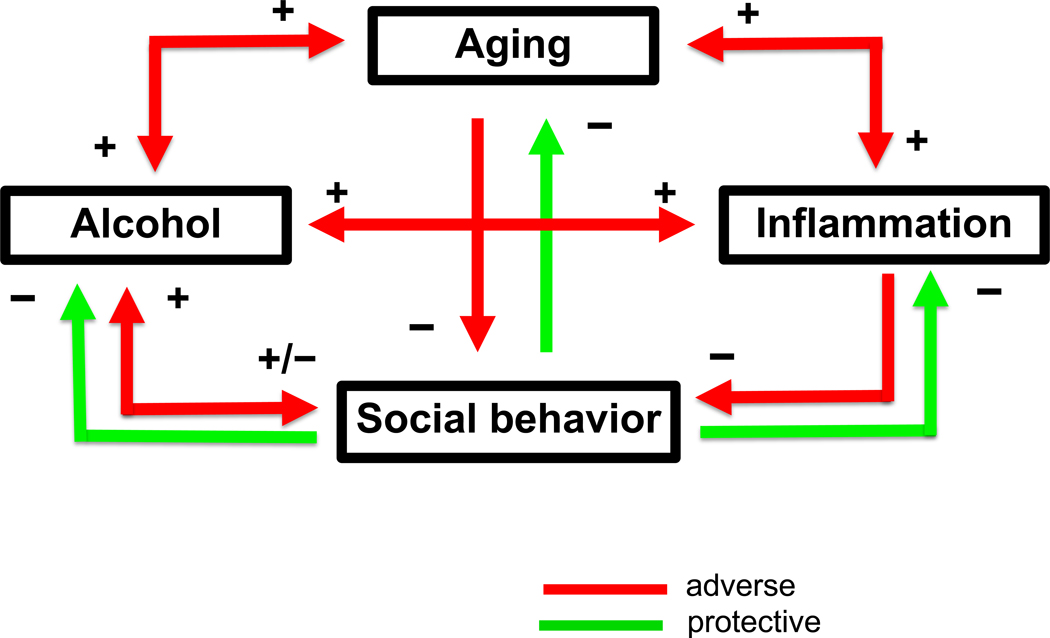
The over-arching goal of this review is to establish the complex, yet critical interactions between sociality, aging and alcohol use and abuse. We posit an instrumental role for neuroinflammation as a key process/mechanism contributing to the adverse health outcomes of disrupted social processes and excessive alcohol consumption as key threats to healthy aging (represented schematically in Figure 2). Social deficits associated with aging and alcohol pose a significant risk to overall health and well-being, therefore making it critical to identify the neural mechanisms contributing to these deficits across the lifespan. Both aging and alcohol abuse are capable of causing immune dysregulation and increased neuroinflammatory signaling (Boule & Kovacs, 2017; Erickson, Grantham, Warden, & Harris, 2019; Norden & Godbout, 2013; Patterson, 2015), however, the role of neuroimmune alterations in social deficits associated with aging and alcohol abuse is still not clear. Animal studies, however, provide extremely valuable information regarding neural circuits and multiple neuromodulatory systems involved in regulation of social behavior, and will be key to unravelling the link between social behavior and immune signaling (Eisenberger, Moieni, Inagaki, Muscatell, & Irwin, 2017). To accomplish this, we review research that is focused on (i) the neural circuits that underlie social behavior and social cognition in humans, (ii) the neural substrates that control the expression of social behavior in animal models across the lifespan, (iii) the relationship between alcohol and social behavior in humans and animal models, and (iv) the possible role of the neuroimmune signaling in social alterations associated with aging and alcohol use.
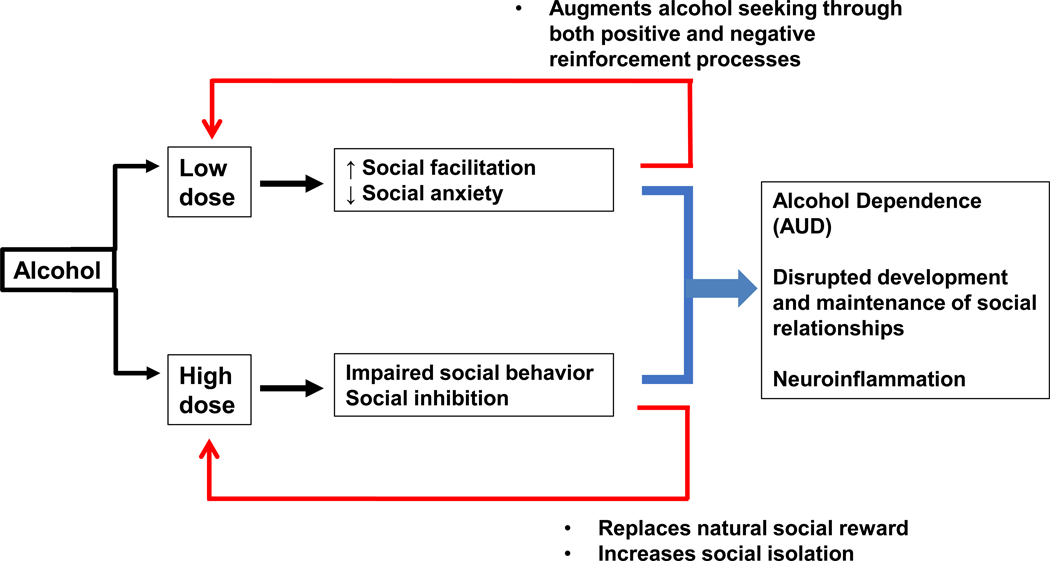
Figure 2: Positive and negative reinforcement processes contributing to pathophysiological relationship between alcohol and social behavior.
Although there is substantial debate regarding the influence of alcohol on aging-related processes, the relative dose, frequency and intervals between alcohol consumption are known to contribute to changes in social behavior in both humans and animal models. In general, low doses of alcohol tend to facilitate social interactions, an effect that likely involves both positive hedonic properties of the drug as well as a reduction in social anxiety in anxiety-prone individuals. In contrast, higher doses of alcohol surpass a threshold for social facilitation and lead to impairments in social behavior and/or social withdrawal, which can in turn lead to social isolation. Together, the combination of low-dose and high-dose alcohol effects conspire to disrupt the normal development and maintenance of social relationships, which are critical for both healthy aging and quality of life across the lifespan. Thus, a more complete understanding of neural systems – including neuroinflammatory processes—which contribute to alcohol-induced changes in social behavior, will provide opportunities and targets for healthy aging.
2.0. Regulation of social behavior
2.1. Human research
2.1.1. Social Brain
Social cognition is a broad term used to describe the complex processes involved in social behavior. Social behavior requires the dynamic integration of perceptual, emotional, and cognitive stimuli into a representation of self and of how others think, feel, and will behave under certain circumstances. Social information processing recruits many cognitive domains, including memory, emotion regulation, perception, and motivation, to name a few. Functional imaging studies have highlighted several proposed networks that are recruited during social tasks, namely the social perception, mentalizing, empathy, and mirror/simulation networks (Kennedy & Adolphs, 2012; Stanley & Adolphs, 2013). The medial prefrontal cortex (mPFC) is clearly involved in social cognition, but it can be divided into the dorsomedial prefrontal cortex (dmPFC), anteromedial prefrontal cortex (amPFC) and ventromedial prefrontal cortex (vmPFC), each of which may serve slightly different roles in social information processing. For example, the dmPFC is particularly important for mentalizing, defined as inferring the intentions and/or thoughts of others. On the other hand, the vmPFC is critical for assigning value to stimuli, particularly those stimuli with emotional components (Lieberman, Straccia, Meyer, Du, & Tan, 2019). There appears to be some overlap in the brain regions involved in social cognition and the default mode network, in particular dorsomedial prefrontal cortex and medial parietal cortex (Schilbach et al., 2012). The amygdala (AMG) is a highly heterogenous structure that is a critical component of several distributed brain networks. As such, it is implicated in multiple processes associated with social cognition, including integrating perceptual information about emotion and social stimuli and projecting to brainstem structures involved in behavioral responses (Kennedy & Adolphs, 2012).
2.1.2. Adolescent-typical neural alterations
Adolescence is a transitional period that involves changes in the hormonal milieu, altered emotionality, increased risk-taking, increased social interaction with peers, and development of executive function, to name a few. These changes are thought to be mediated by development of neural circuitry, particularly in the prefrontal cortex (PFC) and limbic structures, that occur during this time. Human and animal studies have shown that the brain continues to develop through adolescence, with the brains of adolescents demonstrating similar ontogenetic changes across mammalian species (Spear, 2013). A number of recent reviews have focused on specific aspects of maturational changes that occur in the brain during adolescence (Blakemore, 2012; Silveri, 2012; Zimmermann, Richardson, & Baker, 2019). In humans, functional MRI studies have demonstrated differences between adolescents and adults in patterns of activity within certain regions of the social brain, including the AMG, mPFC and anterior cingulate cortex (ACC) (Burnett, Sebastian, Cohen, & Blakemore, 2011). One of the most notable neural alterations of adolescence that may contribute to age-related differences in social interaction is delayed maturation of the PFC (Caballero, Granberg, & Tseng, 2016), which plays an important role in inhibitory control and contrasts with the earlier maturation of the limbic regions such as the nucleus accumbens (NAC) and AMG, which are critical for processing of emotional and rewarding stimuli (Casey & Jones, 2010). The developmental delay in the maturation of PFC regions relative to earlier maturing limbic regions plays a substantial role in adolescent-typical behavior alterations, including adolescent-typical increase in social interaction (Casey, Jones, & Somerville, 2011; Ernst & Fudge, 2009; Steinberg, 2008). Specific cellular and synaptic changes that occur in the PFC during adolescence are reviewed in Caballero et al. (2016).
2.1.3. Alterations associated with aging
Changes in brain structure and function occur naturally across the lifespan, even in individuals without significant neurodegeneration (Lockhart & DeCarli, 2014). While a complete discussion of these changes is beyond the scope of this review, brain regions involved in social information processing are indeed affected by senescence. Many of the brain regions that are recruited during social cognition undergo volumetric changes during senescence. Older age is associated with reduced volume of the lateral PFC, vmPFC, HPC, and PFC white matter (Raz et al., 2005). In addition, the volumetric changes may be moderated by levels of social support. Specifically, increased social support was associated with decreased AMG volume and increased right mPFC thickness, even after correcting for age (Sherman, Cheng, Fingerman, & Schnyer, 2015). Increased right vmPFC thickness and left AMG volume was also associated with better memory for social information in older adults (Cassidy & Gutchess, 2012). Functional imaging during socially relevant tasks provides another important piece of information. There is mixed evidence as to whether performance on social tasks declines with age, with some studies indicating impaired mentalizing performance associated with reduced activation of dmPFC (Moran, Jolly, & Mitchell, 2012) and some studies showing similar mentalizing and self-referencing performance coupled with similar levels of activation of dmPFC, vmPFC, and temporoparietal junction (TPJ) in younger and older adults (Cassidy, Shih, & Gutchess, 2012). Follow-up studies indicated that the emotional valence of social stimuli was important in determining activation of vmPFC and dmPFC, such that activation was increased for negative impressions in younger adults and for positive impressions in older adults (Cassidy, Leshikar, Shih, Aizenman, & Gutchess, 2013).
Altogether, it seems clear that when compared to declines in cognitive performance commonly observed in aging, sociocognitive processing remains relatively spared. This suggests that the decline in social behavior observed in aged individuals may result from altered motivation to engage in social interaction, rather than decrements in cognitive processing related to social stimuli. However, functional imaging studies do not rule out age-related changes in microstructure within these regions or age-related changes in neurochemical process associated with social behavior. To begin to address this question, we must turn to animal models. Animal models have confirmed the role of these regions in social information processing, in addition to providing evidence of the role of certain neurotransmitter and neuropeptide systems in the expression of social reward and social behavior.
2.2. Regulation of social behavior: animal models
2.2.1. Social behavior across the lifespan
There are challenges associated with assessing the underlying neurobiology of social interaction in humans, such as lack of experimental control. This is a place where animal models, rodents in particular, are extremely valuable, owing to a rich history of analyzing the neurobiology of social behaviors. For example, seminal work by Pellis & Pellis (Pellis, Field, Smith, & Pellis, 1997; Pellis & Pellis, 1998), Panksepp (Siviy & Panksepp, 2011), and Vanderschuren (Vanderschuren, Niesink, & Van Ree, 1997) described patterns of juvenile social play behavior and examined the neurobiological underpinnings of social play. On the other hand, monogamous prairie voles (Microtus ochrogaster) have been the focus of research on the neurobiology of adult pair bonds ( Lim and Young, 2006; Ross and Young, 2009; Young et al., 2011). While a complete discussion of these models is beyond the scope of this review, a brief overview provides background for discussing alterations in social behavior associated with either aging or alcohol exposure.
Rodents are naturally very social mammals, although the targets and frequencies of social behavior vary drastically across the lifespan. Early on, social behavior is limited to interactions with the mother as well as with littermates. As rats enter the second and third week of life, there is an increase in social behavior directed towards same-aged conspecifics that typically takes the form of play behavior among peers. In rats, peer-directed social play begins around P 18, peaks around P30–35, and declines as they transition into young adulthood (~P70). Frequency of social play is greater in males than females (Pellis et al., 1997), though there are some strain differences. Social play behavior is manifested through chasing, pouncing, pinning, boxing, and wrestling (Vanderschuren et al., 1997). Social play is essential for normal development of social skills and learning species-typical “rules of social engagement”, since deprivation from peers during this period of heightened play leads to abnormal expression of social, sexual, and aggressive behavior in adulthood (Vanderschuren et al., 1997). Indeed, it is noteworthy that repeated, intermittent assessments of play behavior between two male conspecifics often leads to the establishment of one dominant and one submissive conspecific within the dyad, with these dominance relationships persisting with future social interactions. While often considered maladaptive, assumption of a submissive role in social situations indicates effective social learning and can have protective benefits to the submissive individual. Social interaction itself has a rewarding value: conditioned place preference (CPP) testing indicates that both adolescents and adults prefer to spend time in a compartment that was previously associated with a social partner, although this effect is stronger in adolescents than adults (Douglas, Varlinskaya, & Spear, 2004). Social interactions decline with aging. This has been demonstrated in several strains of rats, including Fischer 344 (Perkins, Vore, Lovelock, Varlinskaya, & Deak, 2018; Perkins et al., 2016), Wistar (Andersen, Zimmer, & Sams-Dodd, 1999; Hunt, Van Nieuwenhuijzen, Chan-Ling, & McGregor, 2011; Markel, Felszeghy, Luiten, & Nyakas, 1995), Sprague-Dawley (Mencio-Wszalek, Ramirez, & Dluzen, 1992; Salchner, Lubec, & Singewald, 2004) and Fischer 344/Brown Norway rats (Shoji & Mizoguchi, 2011). For example, aged males (14–30 months old) display reductions in play behavior (Soffié & Bronchart, 1988), decreased social investigation (Andersen et al., 1999; Markel et al., 1995; Perkins et al., 2016; Salchner et al., 2004; Shoji & Mizoguchi, 2010), and reduced social contact (Hunt et al., 2011; Perkins et al., 2016). It is perhaps important to note that males and females display substantially different break points in late-life reductions in social behavior, with females sustaining higher levels of social behavior much later in life than males (Perkins et al., 2016).
2.2.2. Neural circuitry of social behavior
There are a number of comprehensive reviews on the neurobiological substrates of social play behavior (Auger and Olesen, 2009; Pellis and Pellis, 1998; Pellis et al., 1997; Siviy and Panksepp, 2011; Vanderschuren et al., 1997). Social interaction, including social play, requires the integration of somatosensory cues with complex motor patterns, in addition to involving motivational components. As such, the neural circuits involved in social interaction are complex. Using c-Fos as a reporter of neuronal activity (Herdegen & Leah, 1998), it has been shown that male adolescent rats exhibit increased activation of the dorsal striatum (Gordon, Kollack-Walker, Akil, & Panksepp, 2002; van Kerkhof et al., 2014), ventral striatum (Gordon et al., 2002; van Kerkhof et al., 2014), mPFC (van Kerkhof et al., 2014), bed nucleus of the stria terminalis (BNST) (van Kerkhof et al., 2014), and dorsal raphe (van Kerkhof et al., 2014) following social play, relative to a control group placed into a test chamber alone. A similar study assessed c-Fos expression after testing alone or with an age- and sex-matched partner in male adolescent (P28) rats and found that testing in a social context was associated with increased c-Fos expression in central amygdala (CeA), basolateral amygdala (BLA), BNST, lateral hypothalamus (LH), and lateral septum (LS) (Varlinskaya, Vogt, & Spear, 2013).
Few studies have assessed c-Fos expression following social interaction in adult or aged rats, presumably due to the lower incidence of social interaction at these ages, and the greater cost of conducting studies in older animals. Varlinskaya et al. (2013) found that adolescent and adult rats differed in c-Fos expression following testing alone or with a social partner. Specifically, while many brain regions (described above) were responsive to social testing in adolescents, no such differences were observed in adults. In fact, in adults, there were several brain regions in which c-Fos expression was higher in adults tested alone, such as the ACC, NAC, and locus coeruleus (LC). Another study using c-Fos found that many brain regions were activated by social interaction in adult rats, such as prelimbic cortex (PrL), infralimbic cortex (IL), LS, NAC, paraventricular nucleus of the hypothalamus (PVN), LH, CEA, medial amygdala (MEA), BLA, all regions of the HPC, and several midbrain nuclei (Salchner et al., 2004). Social interaction resulted in c-Fos induction in PrL, MEA, BNST, and CA3, relative to adult context-exposed F344 control rats (Perkins et al., 2017). Furthermore, adult females, who were more socially active, had increased c-Fos induction in the ventrolateral and medial division of the BNST and in CA3, relative to their adult male counterparts (Perkins et al., 2017). Using another immediate early gene, zif268, it was found that adult male Sprague-Dawley rats exhibited higher levels of social interaction, relative to ovariectomized female rats, an effect that was accompanied by increased zif268 mRNA expression in PrL, IL, and striatum. Interestingly, downregulation of zif268 in the PrL of male rats abolished the sex differences observed in social interaction behavior (Stack et al., 2010). Following an interaction with an age-matched conspecific, c-Fos expression in aged male rats was attenuated in the parvocellular region of the PVN, MEA, BLA, and regions of the periaqueductal gray relative to adult male rats tested under the same conditions (Salchner et al., 2004). It could be argued that the brains of aged rats may simply be less activated, but no age differences in c-Fos expression were observed in control rats not exposed to behavioral testing (Boguszewski & Zagrodzka, 2005; Salchner et al., 2004).
Knockout and inactivation studies have provided insight in to the functional role of these brain structures in social play. For example, the mPFC is critical to social play, since neonatal lesions suppressed pinning in juvenile and adult male rats (Schneider & Koch, 2005) and inactivation of the PrL, IL, or medial/ventral oPFC with GABA agonists, such as muscimol or baclofen, suppressed social play in juvenile male rats (Van Kerkhof, Damsteegt, Trezza, Voorn, & Vanderschuren, 2013). The NAC core may serve to inhibit social play, since inactivation of this region increased the duration of social play. This effect that was mediated by GABA, since AMPA/kainite antagonists administered into the NAC had no effect on social play (Van Kerkhof et al., 2013). Using optogenetics, it has been shown that activation of fibers from BLA—ventral HPC (Felix-Ortiz & Tye, 2014) and BLA—mPFC (Felix-Ortiz, Burgos-Robles, Bhagat, Leppla, & Tye, 2016) suppressed social behavior in a resident-intruder paradigm whereas inactivation of the same pathways reduced social behavior.
In summary, social interaction seems to involve a distributed network of brain regions in adolescent and adult rats, in particular prefrontal cortex (PrL and IL), forebrain nuclei (NAC, BNST, LS), and the limbic system (HPC, amygdala: MEA, BLA). Importantly, many of these brain regions have been implicated in social cognition in humans, suggesting a highly conserved network of brain areas that contribute to social behavior. There is some evidence of impaired activation of these brain regions in aged rats, although more work needs to be done to assess whether and how these circuits involved in social interaction are affected by aging.
2.3. Neurochemistry of social behavior
Social behavior is a dynamic and complex process. In rodents, it involves conspecific recognition via olfactory cues, social memory, and motivation. There has been much research in elucidating the roles of various neurotransmitter and neuropeptide systems in these aspects of social behavior. For example, the neuropeptides oxytocin (OT) and vasopressin (AVP) are critically involved in social recognition (Choleris, Clipperton-Allen, Phan, & Kavaliers, 2009; Gabor, Phan, Clipperton-Allen, Kavaliers, & Choleris, 2012; Young, 2002). On the other hand, dopamine (DA), endogenous opioids, and endogenous cannabinoids are involved in social reward and motivation (Trezza, Baarendse, & Vanderschuren, 2010; Trezza & Vanderschuren, 2008b). A brief overview of the role of these systems in social behavior will be provided, and where possible, age-related changes in these systems is discussed.
OT and AVP are often referred to as “social peptides” due to their known role in many aspects of social behavior. There are several comprehensive reviews on OT and AVP (Bredewold & Veenema, 2018; Carter, 2007; Johnson & Young, 2017). OT and AVP are highly conserved peptides produced by magnocellular neurons in the hypothalamus. These neurons project not only to the pituitary gland where they facilitate the release of AVP and OT into general circulation, but also to several forebrain regions. AVP neurons can also be found in the MEA and BNST (Albers, 2015). OT and AVP are stored in large dense core vesicles located throughout the neuron, where they are released as a result of rising intracellular calcium (Johnson & Young, 2017). As such, OT and AVP may be released not only from axon terminals, but also from soma and dendrites, allowing these neuropeptides to have local as well as distal actions (Johnson & Young, 2017). Receptors for OT and AVP are found throughout the brain in areas critically involved in social behavior, including sensory regions, affective and motivational circuits, and regions involved in memory (Johnson & Young, 2017). There are age and sex differences in the levels of these neuropeptides and their receptors (Bredewold & Veenema, 2018), which is not surprising given the sex differences observed in several aspects of social behavior. For example, in the BNST, adult males have more AVP+ cells than adult females; a similar sex difference is evident in the MEA (DiBenedictis, Nussbaum, Cheung, & Veenema, 2017). The number of AVP+ cells was also higher in adults, relative to juveniles (DiBenedictis et al., 2017). OT fiber density does not differ as a function of age or sex (DiBenedictis et al., 2017), but adult males have greater OTR binding in the LS, medial preoptic area (MPOA), MeA, CA1, NAC, and BNST relative to adult females (Dumais, Bredewold, Mayer, & Veenema, 2013). While the OT and AVP systems have been well characterized in the juvenile and adult rat, less is known about these systems in the aged rat. Elevated OT secretion has been observed in aged male Brown-Norway rats (Goudsmit, Fliers, & Swaab, 1988). Plasma concentrations of OT (Keck et al., 2000) and AVP (Keck et al., 2000; Terwel, Markerink, & Jolles, 1992) are elevated in 20–28-month-old male rats, although a more recent study reported decreased plasma OT in 22-month-old male mice (Elabd et al., 2014). Within the brain, an increase in the size of OT neurons in the hypothalamus was observed in aged male Wistar rats (Bazhanova, Grinevich, Danilova, & Chernigovskaya, 1998).
There is mixed evidence in humans about whether aging is associated with alterations in AVP or OT. Post-mortem analysis of human hypothalamus revealed that aging was associated with decreased staining intensity of AVP+ and OT+ cells in the PVN (Calzà, Pozza, Coraddu, Farci, & Giardino, 1997), but an increase in the size of AVP+ neurons in the PVN was observed in women (Ishunina & Swaab, 1999). In addition, there are few studies assessing age differences in OT or AVP in rodent models, none of which have examined these neuropeptides in the context of social interaction. For example, a study by Keck et al. (2000) assessed AVP and OT release using in vivo microdialysis in response to an acute stressor and found that aged rats had elevated release of AVP in PVN, but blunted AVP release in SON; OT release was blunted in PVN (Keck et al., 2000). AVP concentrations in the AMG were decreased in 10-, 20-, and 28-month old, compared to 3-month-old male Wistar rats (Terwel et al., 1992). To our knowledge, no study has looked at regional expression of OT receptors (OTR) or AVP receptors (V1aR) in the aged brain, nor has functional release been assessed in target regions of OT and AVP neurons, such as BNST or MEA.
Manipulation of OT and AVP can influence many aspects of social behavior, including social preference (Lukas et al., 2011), social investigation (Dumais, Alonso, Bredewold, & Veenema, 2016), and social recognition (Choleris et al., 2009; Veenema, Bredewold, & de Vries, 2012). Central OTR antagonist administration attenuated social preference, but direct administration of OTR antagonist into CEA or MEA had no effect on social preference in adult males (Lukas et al., 2011). OTR antagonist delivered into the CEA decreased investigation of a novel juvenile conspecific in the home cage in males, but not females (Dumais, Alonso, Bredewold, et al., 2016). No effects of OTR antagonist were observed on social investigation when administered into the MEA (Dumais, Alonso, Bredewold, et al., 2016) or posterior BNST (Dumais, Alonso, Immormino, Bredewold, & Veenema, 2016). In sum, OT seems to be prosocial, although the specific effects depend on sex and the brain region into which OT (or its antagonists) are administered. The ability to determine if a conspecific has been previously encountered or not is termed social recognition. Peripheral (Hicks et al., 2015) or intracerebroventricular (ICV) AVP (Le Moal, Dantzer, Michaud, & Koob, 1987) enhance social recognition memory. This effect of AVP on social recognition depends, in part, upon activation of V1aR receptors in LS (Veenema et al., 2012). There is also a role for OT in social recognition memory, as OT knockout mice (OTKO) display impaired social recognition memory (Choleris et al., 2003; Ferguson, Aldag, Insel, & Young, 2001), and this effect is reversed by OT administered into the MEA before the original encounter, indicating that OT is important for the acquisition of memory for conspecifics (Ferguson et al., 2001). Social recognition memory is blocked by ICV administration of OT antagonists (Samuelsen & Meredith, 2011) and is increased by OT delivered to the olfactory bulb (Dluzen, Muraoka, Engelmann, & Landgraf, 1998; Dluzen, Muraoka, & Landgraf, 1998), MPOA (Piotr Popik & van Ree, 1991), septum (Popik, Vetulani, & van Ree, 1992), or posterior BNST (Dumais, Alonso, Immormino, et al., 2016) of male rats. OT delivered to the posterior BNST did not enhance social recognition memory in female rats (Dumais, Alonso, Immormino, et al., 2016), although OTR antagonist into posterior BNST impaired social recognition memory in both males and females (Dumais, Alonso, Immormino, et al., 2016). Aged male rodents do display deficits in social recognition memory (Guan & Dluzen, 1994; Prediger, Batista, & Takahashi, 2005; Prediger, De-Mello, & Takahashi, 2006), which could be mediated by changes in olfaction, although olfactory sensitivity does not seem to be impaired in aged rats (Kraemer & Apfelbach, 2004; Mencio-Wszalek et al., 1992). An alternative explanation could be that aging involves changes in behavioral flexibility or motivation that impair social recognition memory. Aged male rats exhibited reduced anogenital sniffing of a receptive female without any changes in general locomotion or ability to locate buried food, suggesting impaired detection of specific olfactory cues or impaired sociosexual motivation (Mencio-Wszalek et al., 1992). Whether age-related changes in social recognition memory are mediated by alterations in OT or AVP remain to be determined.
Social behavior, and social play in particular, is reinforcing, as indicated by studies using conditioned place preference and operant conditioning paradigms (Trezza et al., 2010; Trezza, Campolongo, & Vanderschuren, 2011). The neurotransmitter DA and the endogenous opioids and cannabinoids all play an important role in social reward. For the most part, these systems have been studied in the context of social reward, so it is unclear to what extent they are involved in social behavior in adulthood or aging. Aging does appear to involve changes in social motivation, so it is plausible that aging-related changes in DA, opioids, and/or cannabinoids may influence social behavior.
Endogenous opioids are a large family of peptides, such as enkephalins, dynorphins, and β-endorphin, that act on endogenous μ, δ, and κ opioid receptors (Kieffer, 1995). Opioid receptors can be found peripherally, with the primary function of mediating nociception, and centrally, where they also mediate nociception in addition to a variety of other functions (Kieffer, 1995). For example, central opioid signaling has been implicated in regulating the hedonic value of stimuli, such that they play a major role in the neuroscience of addiction (Koob, 2015; Koob & Le Moal, 1997). Opioid receptors are richly expressed in the cortex, limbic system, and brain stem, with some overlap in the specific subtype of receptor expressed within these brain regions, but also considerable separation (Cross, Hille, & Slater, 1987; Le Merrer, Becker, Befort, & Kieffer, 2009). For example, within the AMG, the μ opioid receptor is most prominent, whereas in the dorsal and ventral striatum, κ receptors are more numerous. Further, the olfactory system contains a high concentration of δ receptors (Le Merrer et al., 2009). Interestingly, opioid receptors can be found in many of the brain structures that mediate social behavior, including the mPFC, BNST, AMG, and ventral striatum (Le Merrer et al., 2009). Further, motivated- and goal-directed behavior is facilitated by the action of endogenous opioids, not only in the context of drug-seeking (Laurent, Morse, & Balleine, 2015), but also in social behavior (Trezza et al., 2010). Specifically, peripheral administration of fentanyl, a μ-opioid receptor agonist, facilitated play whereas administration of a μ-opioid receptor antagonist or κ-opioid receptor agonist suppressed play (Vanderschuren, Niesink, Spruijt, & Van Ree, 1995). Trezza et al. (2011) demonstrated that morphine, an opioid receptor agonist, facilitates play when administered directly into the NAC. This effect was recapitulated by DAMGO (selective μ-opioid receptor agonist) and β-endorphin (endogenous ligand for μ-opioid receptors) and blocked by a selective μ-opioid receptor antagonist (CTAP). Further, intra-NAC CTAP prevented social conditioned place preference (Trezza, Damsteegt, Achterberg, & Vanderschuren, 2011). In addition, a brief social play experience in juvenile rats led to displacement of 3H-diprenorphine, a μ-opioid receptor agonist, suggesting the release of endogenous opioids during social play behavior (Panksepp & Bishop, 1981; Vanderschuren, Niesink, et al., 1995; Vanderschuren, Stein, Wiegant, & Van Ree, 1995). Although it is clear that social reward is mediated, in part, by opioid signaling, less is known about whether this process is altered in aging.
Aging is associated with alterations in endogenous opioid signaling. There is a reduction in opiate receptor binding in the whole brain (Piva, Maggi, Limonta, Dondi, & Martini, 1987), hypothalamus (Piva et al., 1987) frontal poles (Hess, Joseph, & Roth, 1981), and HPC (Hess et al., 1981; Nagahara, Gill, Nicolle, & Gallagher, 1996) of aged rats. In a PET study in aging humans, μ-opioid receptor binding decreased with age in the AMG of women, with no decrease apparent in males and an increase in μ-opioid receptor binding with age in the PFC of men and women (Zubieta, Dannals, & Frost, 1999). Dynorphin, an endogenous opioid peptide that binds to κ-opioid receptors, is elevated in the brain of aged male rats (Kotz, Weldon, Billington, & Levine, 2004) and humans with neurodegenerative diseases (Yakovleva et al., 2007). Prodynorphin, a precursor for dynorphin, is elevated in the HPC, decreased in the AMG, and not different in the cortex or PVN of aged male rats, relative to adult male rats (Kotz et al., 2004). Aged ovariectomized females have decreased dynorphin and increased leu-enkephalin in the HPC (Williams et al., 2011), suggesting that within the aged hippocampus, endogenous opioids may be sexually dimorphic. Age and sex-related changes in dynorphin may also be region-specific, although a thorough characterization of opioid peptides or their receptors has not been conducted in aged females. Age-related increases in dynorphin may be involved in the decline in social behavior during aging, since studies in adolescent rats have demonstrated that administration of a κ-opioid receptor agonist suppresses play (Vanderschuren, Niesink, et al., 1995). Whether or not there are sex differences in opioid signaling as a function of age remain to be seen, as is the role of endogenous opioids in age-related changes in social behavior.
There has been a recent surge in the understanding of the role of endogenous cannabinoids in many aspects of behavior, including social behavior (Achterberg, van Kerkhof, Damsteegt, Trezza, & Vanderschuren, 2015; Manduca et al., 2015; Trezza et al., 2010, 2012). There are two endocannabinoid receptors: CB1 and CB2, primarily located in the central and peripheral nervous systems, respectively (for review, see Pacher et al., 2006; Piomelli, 2003). CB1 receptors are widely expressed and can be found on neurons and microglia in the cortex, HPC, and striatum. CB2 receptors, although few in number in the CNS, are expressed on microglia and neurons, particularly under conditions of increased inflammation (Bonnet & Marchalant, 2015; Di Marzo, Stella, & Zimmer, 2015). These receptors are bound by endogenous cannabinoids, such as anandamide and 2-arachidonoylglycerol (2-AG). In a series of experiments by Trezza & Vanderschuren (Manduca et al., 2015; Trezza et al., 2012; Trezza & Vanderschuren, 2008a, 2008b, 2009), a clear role for endocannabinoids in social play was established. WIN55,212–2, a direct CB1 receptor agonist, attenuated pinning and pouncing in juvenile rats, whereas URB597, a drug that inhibits the enzymatic degradation of endogenous anandamide, increased these behaviors. Importantly, the effects of these drugs were mediated by CB1 and opioid receptors, as they were blocked by the CB1 antagonist SR141716A and naloxone, an opioid receptor antagonist (Trezza & Vanderschuren, 2008a). Similarly, drugs that selectively inhibit anandamide transporters can increase social play, an effect that was attenuated by CB1, opioid, DA antagonists (Trezza & Vanderschuren, 2009). Further, JZL195, a drug that increases anandamide and 2-AG signaling by preventing their hydrolysis, enhanced social behavior in both adolescent and adult rats. However, there was some evidence that this may be through modulation of overall emotionality, as JZL195 also increased anxiety-like behavior in the elevated plus maze at both ages (Manduca et al., 2015). Social play behavior increased anandamide levels within the NAC and AMG, but not the HPC or PFC and this was accompanied by an increase in CB1 receptor phosphorylation in the AMG, but not NAC. Infusion of URB597 (indirect CB1 receptor agonist) directly into the BLA increased social play behavior, an effect that was blocked by the CB1 antagonist SR141716A (Trezza et al., 2012). A recent report demonstrated that stimulation of OT neurons in the PVN increased levels of anandamide in the NAC and that this was involved in social conditioned place preference (Wei et al., 2015). Overexpression of the CB1 receptor specifically within the mPFC decreased social interaction and reduced cognitive flexibility in adult rats (Klugmann, Goepfrich, Friemel, & Schneider, 2011), indicating that cannabinoid influences on social behavior are not exclusive to adolescence.
The endocannabinoid system undergoes several changes during aging in humans and in animal models. Decreased CB1 receptor mRNA expression has been demonstrated in the HPC, BLA, and hypothalamus of aged (> 24 months old) male Wistar rats (Berrendero et al., 1998). Furthermore, levels of 2-AG but not anandamide were reduced in the HPC of aged male mice (Piyanova et al., 2015). The decrease in endocannabinoid signaling has been implicated in cognitive decline, as aged mice that lack CB1 receptors (Cnr1−/−) exhibit marked deficits in water maze acquisition and reversal learning (Albayram et al., 2011) and reward-related learning in the radial arm maze (Albayram, Bilkei-Gorzo, & Zimmer, 2012). One role of cannabinoid signaling is anti-inflammatory: aged Cnr1−/− mice have increased GFAP and Iba1 expression in CA1, markers of astrocytes and microglia, respectively. IL-6, a pro-inflammatory cytokine, was also increased in the HPC of aged Cnr1−/− mice (Albayram et al., 2011). Endocannabinoids, particularly within the AMG, can regulate social behavior. Specifically, indirect CB1 receptor agonists can increase social play in juveniles (Trezza et al., 2012). Altered endocannabinoid signaling in the aged brain may lead to suppression of social behavior in aged animals, and this may occur via increased brain inflammation.
As discussed above, social play is highly rewarding, particularly for adolescents (Trezza et al., 2010). The mesolimbic DA system, comprised of DA neurons in the ventral tegmental area (VTA) that project to the ventral striatum, or NAC, plays a major role in reward processing and reward-seeking behavior, both in the context of drug-seeking (Berridge, Robinson, & Aldridge, 2009; Everitt et al., 2008; Koob, 2015) as well as social reward (Trezza et al., 2010) and affiliative behavior (Carter & Keverne, 2002; Young et al., 2011; Young, 2002). Amphetamine, a DA agonist, suppresses social play, an effect that is mediated by norepinephrine (NE), since amphetamine-induced suppression of social behavior was reversed by RX821002 (α2 receptor antagonist) but not α-flupenthixol (DA receptor antagonist) (Achterberg et al., 2014). Methylphenidate, another DA agonist, suppressed pinning and pouncing, when administered directly into the IL, ACC, BLA, or habenula, an effect that was not driven by changes in locomotor activity. Moreover, methylphenidate did not influence social play when administered directly into the PrL, vmPFC, or NAC shell. Administration of atomoxetine, a NE reuptake inhibitor, recapitulated these effects, suppressing social behavior (Achterberg et al., 2015), suggesting a primary role for NE in the suppression of social play by DA agonists. There is also some evidence that DA influences on social play are sexually dimorphic since social play increases Fos+ DA neurons in the VTA of juvenile females, but not juvenile males suggesting that, at least in females, social play elicits a robust increase in DA tone (Northcutt & Nguyen, 2014). It is clear that DA and NE are involved in the regulation of social play in juveniles, but to what extent DA and NE are involved in social interaction in adult or aged animals is unknown.
DA concentration and synthesis are altered in senescence, although most studies have focused on the substantia nigra pars compacta (Bardou et al., 2014) since this is the locus of DA depletion in Parkinson’s disease. However, DA dysfunction occurs within social behavior circuits as well, including a reduction in DA concentration in the striatum, AMG, and brainstem (Míguez, Aldegunde, Paz-Valiñas, Recio, & Sánchez-Barceló, 1999), a reduction in DA turnover/biosynthesis in the PFC, NAC, AMG, midbrain, HPC, and raphe nuclei (Miura, Qiao, & Ohta, 2002), and impaired DA release (Friedemann & Gerhardt, 1992; Hebert & Gerhardt, 1998). NE concentrations are decreased in the VTA (Hebert & Gerhardt, 1998) and brainstem (Míguez et al., 1999) of 24-month-old rats. These data suggest that reduced social interaction in aged rodents could be due to a reduction in catecholaminergic tone, which in turn suppresses social motivation.
In summary, while much is known about the neural substrates of social play, the mechanisms that mediate social behavior in adult and aged animals have been less investigated. Social play is highly rewarding, and as such, is driven largely by the brain regions, neurotransmitters, and neuropeptides that regulate reward-seeking and motivated behavior. Studies in adult rodents have been less consistent in demonstrating that social interaction itself is rewarding (Douglas et al., 2004). As such, the mechanisms that drive social behavior in adult and aged animals are not likely to be purely reward-related. Social motivation is likely driven by the endogenous opioid and cannabinoid systems, in addition to catecholamine neurotransmitters. On the other hand, social recognition, which is readily displayed in adult animals, and is impaired in aged animals, is mediated largely by the neuropeptides OT and AVP. It remains to be seen whether these neuropeptide systems are altered in aged animals. Future research should assess whether social motivation is decreased in aged animals, and whether this may be driven by alterations in opioid or cannabinoid signaling, in addition to examining age-related alterations in OT and AVP.
3.0. Social behavior and alcohol
3.1. Alcohol use and sensitivity across lifespan
Alcohol remains the most used and abused psychoactive substance worldwide (World Health Organization, 2018). According to the 2017 National Survey on Drug Use and Health (Center for Behavioral Health Statistics and Quality, 2018), in the United States, 86.3% of people over the age of 18 reported drinking alcohol at some point in their lifetime, 70.1% reported drinking in the past year, and 55.9% reported drinking in the past month. The highest rates of alcohol use were reported by 25-year-olds (90.8%, 83.4%, and 67.5% for lifetime, past year, and past month, respectively). Although these rates gradually decline with age, 56.5 % of older adults (≥65 years of age) reported drinking in the past year, and 44.2% reported drinking in the past month. Furthermore, there is some rise in alcohol use among aged and elderly individuals, perhaps in part due to a rapidly aging demographic and greater longevity in industrialized nations (Center for Behavioral Health Statistics and Quality, 2018). Substantial evidence suggests that alcohol use among adults 65+ years of age has been underidentified for decades (Arndt, Clayton, & Schultz, 2011; Johnson, 2000; Kuerbis, Sacco, & Moore, 2014), with alcohol remaining the most commonly used substance among elderly individuals (Arndt et al., 2011; Moore et al., 2009).
Alcohol use typically begins during early adolescence (Faden, 2006; Masten, Faden, Zucker, & Spear, 2009), and according to the World Health Organization (WHO), approximately 155 million 15–19-year-old adolescents worldwide are current drinkers. Results of school surveys demonstrate that in many countries alcohol use begins before the age of 15, and prevalence of alcohol use among 15-year-olds is in the range of 50–70%, with remarkably small differences between boys and girls (World Health Organization, 2018). According to the Monitoring the Future survey, annual prevalence rates of alcohol use in the United States were 23.5%, 43% and 58.5% for 8th, 10th, and 12th graders, respectively (Johnston et al., 2019). Early initiation of alcohol use during adolescence is a risk factor for later alcohol abuse and dependence (Kuntsche, Rossow, Engels, & Kuntsche, 2016). For instance, adolescents who begin drinking at 14 years of age or earlier are 4 times more likely to become alcohol-dependent relative to those who started drinking at 20 years of age or later (Dawson, Goldstein, Patricia Chou, June Ruan, & Grant, 2008; Ehlers, Slutske, Gilder, Lau, & Wilhelmsen, 2006). Recent findings also suggest that fast escalation from the first drink to first intoxication is another risk factor for the emergence of heavy drinking among adolescents (Kuntsche et al., 2016; Morean, Corbin, & Fromme, 2012; Morean et al., 2014; Morean, L’Insalata, Butler, McKee, & Krishnan-Sarin, 2018).
Binge drinking, defined by the National Institute of Alcohol Abuse and Alcoholism as 5 or more drinks in males and 4 or more drinks in females within a 2-hour period that result in blood alcohol levels of ≥80 mg/dL, is the most common pattern of excessive alcohol use in the United States (Patrick, Evans-Polce, & Terry-McElrath, 2019). Although this guideline has been in place for decades and constitutes the legal limit for driving under the influence of alcohol, it is noteworthy that significant impairment occurs at blood alcohol levels considerably lower than 80 mg/dl, and several states have recently passed—or are actively considering—legislation that would reduce this legal limit to 50 mg/dl. In 2017, 26.4% of people aged 18 and over reported that they engaged in binge drinking in the past month (Center for Behavioral Health Statistics and Quality, 2018). Although binge drinking is most common among younger adults aged 18–34 years (Kanny, Naimi, Liu, Lu, & Brewer, 2018), rates of binge drinking are extremely high among older adolescents as well. Approximately 24% of underage (19–20 years) college students reported consumption of 5 or more drinks per occasion (i.e., binge levels of drinking) within the past 2 weeks (Patrick & Terry-McElrath, 2017).
Furthermore, some adolescents and adults drink at levels that are 2 or even 3 times higher than the current binge threshold. For instance, 10% of high school seniors and 19- and 20-year-olds reported consuming 10 or more drinks in a row at least once in the previous 2 weeks, and an additional 4–5% reported consuming 15 or more drinks in a row (Patrick et al., 2013; Patrick & Terry-McElrath, 2017). Those rates are even higher among college students: 12.4% and 5.1% of 19- and 20-year-old college students consumed ≥10 and ≥15 drinks in the previous two weeks, respectively, compared with 9% and 3.5% of 19- to 20-year-olds not attending college (Patrick & Terry-McElrath, 2017). This high-intensity binge drinking peaks around the age of 25 (Terry-McElrath & Patrick, 2016). High-intensity binge patterns of alcohol consumption are thought to be particularly harmful to the developing adolescent brain (Bava & Tapert, 2010; Olsson et al., 2016; Silveri, 2012; Spear, 2018). The early initiation and relatively high use levels of alcohol by adolescents could potentially disrupt maturational changes occurring in the brain at this time in regions critical for cognitive control (B. J. Casey, Getz, & Galvan, 2008), motivational responding, and the processing of rewarding, social, and emotional stimuli (Blakemore, 2012; Mills, Lalonde, Clasen, Giedd, & Blakemore, 2014). Indeed, early onset of drinking has been found to be predictive of alcohol-related problems later in life (Addolorato et al., 2018; Petit, Kornreich, Verbanck, Cimochowska, & Campanella, 2013), with those engaging in even episodic heavy drinking at an early age being more likely to develop alcohol use (Bonomo, 2005; Bonomo, Bowes, Coffey, Carlin, & Patton, 2004; Hingson, Heeren, & Winter, 2006) and affective disorders (Fidalgo, Da Silveira, & Da Silveira, 2008; Lopez, Turner, & Saavedra, 2005; Schmidt, Buckner, & Keough, 2007). These findings clearly demonstrate that early adolescents are extremely vulnerable to the adverse consequences of alcohol. Negative effects of adolescent drinking on brain and behavior are reviewed in several recent publications (Silveri, Dager, Cohen-Gilbert, & Sneider, 2016; Spear, 2018; Squeglia, Jacobus, & Tapert, 2014).
The elderly is another age group that is extremely vulnerable to alcohol. Physiological changes associated with aging (Oslin, 2000), chronic diseases (Moore et al., 2006), and medication use (Moore, Whiteman, & Ward, 2007) make adults ≥65 years of age extremely sensitive to alcohol. Therefore, this age group is at a higher risk of adverse consequences associated with alcohol use relative to younger adults (Moos, Brennan, Schutte, & Moos, 2005). Given enhanced sensitivity of older adults to alcohol effects and outcomes, including functional impairments (Moore, Endo, & Carter, 2003) and increased mortality risk (Holahan, Schutte, Brennan, Holahan, & Moos, 2014; Moore et al., 2006), the National Institute on Alcohol Abuse and Alcoholism (NIAAA) lowered recommended drinking thresholds for adults aged 65 and older (NIAAA, 2016). According to this new guideline, adults over the age of 65 who are healthy and do not take medications should not have more than 3 drinks on a given day and 7 drinks in a week. However, epidemiological studies of alcohol use by older adults from the 2017 National Survey on Drug Use and Health (Center for Behavioral Health Statistics and Quality, 2018) estimated the prevalence of past-year alcohol use to be 56.5% for adults ≥65 years of age. According to this survey, 11% of older adults reported binge drinking, whereas 2.8% reported heavy drinking (i.e., binge drinking on 5 or more days) during the last 30 days. Surprisingly, the frequency of binge drinking was found to be highest among binge drinkers ≥65 years of age, with an average of 5.5 episodes a month compared to all other age groups (Han, Moore, Sherman, Keyes, & Palamar, 2017). It should be noted, however, that studies examining binge drinking and prevalence of alcohol use disorders (AUDs) in individuals above 65 years of age are likely to be biased by subject attrition, as many individuals with more severe AUD may have already died due to alcohol-related accidents, premature organ failure, or other causes due to life-long alcohol use. These and other selective pressures may therefore render the category of “late aging” individuals as a more homogeneous group than younger comparator groups such as adolescents and young adults.
Adolescent drinkers consume almost 2 times more alcohol per occasion than younger adults. For instance, underage drinkers ages 12 to 20 typically consume 4 to 5 drinks per drinking episode, which is nearly double the average of the 2 to 3 drinks usually consumed by adults over the age of 25 (see Chung et al., 2018 for references). This relatively high level of alcohol consumption per drinking episode demonstrated by underage drinkers suggests that adolescents are less sensitive to acute intoxicating effects of ethanol than their more mature counterparts. Our knowledge about adolescent-typical sensitivity to acute alcohol is based almost entirely on animal research due to restrictions on administering alcohol to underage drinkers. The only study that examined the acute effects of alcohol on boys, ages 8 to 15 years (Behar et al., 1983), reported no behavioral signs of intoxication after a dose of alcohol that produced pronounced intoxication in adults.
Animal studies have demonstrated attenuated sensitivity of adolescent rodents to acute intoxicating effects of alcohol (see Spear, 2014 for references and review). For instance, adolescent rats are less sensitive than adults to ethanol-induced sedation (Little, Kuhn, Wilson, & Swartzwelder, 1996; Silveri & Spear, 1998), motor impairment (Ramirez & Spear, 2010; White et al., 2002), and aversion (Anderson, Varlinskaya, & Spear, 2010; Schramm-Sapyta et al., 2010; Vetter-O’Hagen, Varlinskaya, & Spear, 2009). These relative insensitivities of adolescents to intoxicating effects of ethanol cannot be attributable solely to age-related differences in ethanol pharmacokinetics (Spear, 2007), although adolescents demonstrate higher rates of ethanol metabolism (Brasser & Spear, 2002) and elimination (Doremus, Brunell, Varlinskaya, & Spear, 2003; Varlinskaya & Spear, 2004; Walker & Ehlers, 2009). For instance, adolescent rats regain their righting response about twice as rapidly as adults following administration of a sedative dose of ethanol, but, most importantly regaining of the righting reflex occurs at significantly higher levels of ethanol in adolescent than in adult brains (Silveri & Spear, 1998). This finding clearly demonstrates that adolescents are less sensitive to ethanol-induced sedation than their adult counterparts.
Human research suggests that sensitivity to the adverse consequences of alcohol may increase ontogenetically, as adults aged 65 and over are more susceptible to health-related negative effects of alcohol than younger adults. Consequences of acute alcohol intoxication are accentuated in the elderly. Alterations in alcohol absorption, hepatic alcohol metabolism, excretion of alcohol, and susceptibility to alcohol toxicity play a substantial role in age-related enhanced vulnerability to alcohol effects (Seitz & Stickel, 2007). A number of age-related physiological changes contribute to enhanced sensitivity to alcohol in aging populations. These changes include increase in liver size, as well as reductions in numbers of hepatocytes (Sawabe et al., 2006) and hepatic blood flow (Meier & Seitz, 2008). Metabolism of ethanol decreases with age due to age-related decreases in activity of alcohol and acetaldehyde dehydrogenases and cytochrome P-4502E1 (Meier & Seitz, 2008). These aging-related alterations result in higher blood alcohol levels achieved by the elderly relative to younger people after consuming an equal amount of alcohol. The lower volume of body water in older individuals gives less volume for distribution of alcohol, also contributing to higher blood alcohol levels at comparable quantities of alcohol consumed (Cederbaum, 2012). Age-related changes in body composition are important contributors to enhanced vulnerability to alcohol in the elderly. Lean body mass and total volume of distribution of alcohol is negatively correlated with age (Vestal et al., 1977). Therefore, higher peak BACs evident in older individuals relative to their younger counterparts after intake of the same alcohol amount could be related to the smaller volume of distribution and the decreased body mass in the elderly. Aging also affects the ability of the brain and body to adapt to the presence of alcohol (i.e., tolerance). Through a decreased ability to develop tolerance, elderly subjects persist in exhibiting certain effects of alcohol (e.g., motor incoordination, alcohol-induced sedation) at lower doses than younger individuals who demonstrate increases in tolerance with increased consumption (Kalant, 1998). Furthermore, the elderly represents a group with a higher prevalence of neurological disorders, which increases their sensitivity to the effects of ethanol on the central nervous system (Menninger, 2002). The use of medications, as well as high rates of diseases or health conditions in which concurrent alcohol use is not recommended, exacerbate the potential risks of alcohol use in older people. In the US, up to 78% of older adult drinkers use medications that interact with alcohol (Breslow, Dong, & White, 2015).
Enhanced sensitivity to acute ethanol challenge is also evident in aged laboratory rodents after high doses of ethanol (Novier, Diaz-Granados, & Matthews, 2015). For instance, aged males are more sensitive to motor-impairing, sedative, and socially suppressing effects of ethanol relative to their less mature counterparts (Novier et al., 2013; Ornelas, Novier, Skike, Diaz-Granados, & Matthews, 2015; Perkins, Vore et al., 2018). In aged male rats, acute ethanol produced substantial cognitive deficits, evidenced by impaired performance on the water maze (Novier et al., 2013). Enhanced sensitivity to acute ethanol challenge in aging animals could be associated with age differences in ethanol pharmacokinetics and/or absorption and distribution. Indeed, some studies demonstrated slower clearance of high doses of ethanol in aged rats (Ornelas et al., 2015; Perkins, Vore, et al., 2018), whereas others have shown no age differences in BECs following acute administration of lower doses of ethanol, indicating that age differences in ethanol pharmacokinetics are dose-dependent (Matthews & Mittleman, 2017; Novier, Ornelas, Diaz-Granados, & Matthews, 2016; Perkins, Vore, et al., 2018). Age-related reduction in activity of the hepatic enzyme cytochrome P450 (CYP) 2E1, a key factor in ethanol metabolism, may also contribute to elevated BECs evident after acute challenge with high doses of ethanol (Wauthier, Verbeeck, & Calderon, 2004).
3.2. Drinking Motives and Expectancies
Drinking motives are defined as the reasons why people drink, with the assumption that drinking will result in a desired outcome (Cooper, 1994). This concept is important for understanding excessive alcohol use in adolescents and adults. Four drinking motives are generally recognized (Cooper, Kuntsche, Levitt, Barber, & Wolf, 2015; Maclean & Lecci, 2000), with individuals, especially during adolescence, drinking (1) to alleviate problems and worries (coping motives), (2) to have fun and get drunk (enhancement motives), (3) to have a good time at social gatherings (social motives), or (4) to not feel left out (conformity motives). Individuals are drinking either to achieve positive reinforcement (social and enhancement motives) or to experience negative reinforcing effects of alcohol (conformity and coping motives). Drinking motives are either internal (coping, enhancement) or external (social, conformity). The concept of “drinking motives” has been shown to be particularly helpful in explaining why people engage in alcohol use. Drinking motives predict not only the pattern of alcohol use but also the adverse consequences (Cooper et al., 2015).
In adolescents and young adults, frequent drinking has been associated with positive reinforcement (social and enhancement motives), heavy drinking or high-intensity binge drinking with internal motives (enhancement and coping), and alcohol-related consequences with negative reinforcement (coping and conformity motives). Heavy episodic drinking and alcohol consumption for fun and excitement characterize this age group (Gmel, Gaume, Faouzi, Kulling, & Daeppen, 2008; Kuntsche, Wiers, Janssen, & Gmel, 2010). Furthermore, social and enhancement motives amplify each other in early adulthood and predict increases in risky drinking (Labhart, Kuntsche, Wicki, & Gmel, 2017).
In older adults aged ≥65 years, enhancement and social motives are strongly related to frequency of alcohol use (Sacco et al., 2015), in a manner similar to findings in younger adults (Crutzen, Kuntsche, & Schelleman-Offermans, 2013). However, only social and coping motives predict drinking quantity in older adults, suggesting that enhancement motivation is not indicative of heavy drinking later in life. Furthermore, social motives are much stronger predictors of binge drinking and problematic drinking relative to enhancement motives in this age group. If participants consumed alcohol predominantly in social contexts or for social reward it may have led to drinking larger amounts within these social occasions and consequently drinking-related problems were more likely to occur (Sacco et al., 2015). Taken together, the human data demonstrate that social motives for drinking are endorsed by all age groups, with these motives contributing to heavy and problematic drinking.
Alcohol expectancies play a substantial role in alcohol use and abuse as well. Indeed, expectancy for social facilitation from drinking is an important predictor of heavy drinking in adolescence, with young individuals believing that alcohol will make them more confident and relaxed in a social setting (Mackintosh, Earleywine, & Dunn, 2006), and positive expectancies for social facilitation result in heavier drinking among college students (Labrie, Lamb, & Pedersen, 2008). Similar to adolescents, aged individuals believe that alcohol will enhance their social engagement (reviewed in Kelly et al., 2018). Therefore, drinking in adolescents and older adults is strongly linked to social engagement, with socially facilitating consequences of drinking making alcohol more appealing to these 2 age groups (Comeau, Stewart, & Loba, 2001; Ham & Hope, 2003; Kelly et al., 2018; Kuntsche, Knibbe, Gmel, & Engels, 2006; Tomlinson & Brown, 2012).
3.3. Social behavior and ethanol effects: animal studies
Ethanol-associated social facilitation is not restricted to humans, but is also evident in adolescent (Trezza, Baarendse, & Vanderschuren, 2009, 2014; Varlinskaya & Spear, 2002, 2006, 2015; Varlinskaya, Truxell, & Spear, 2015; Willey, Varlinskaya, & Spear, 2009) and aged rats (Perkins, Vore, et al., 2018). Adolescent rats assessed under familiar, low anxiety-provoking circumstances in a social interaction test show this social facilitation (indexed via increases in social behavior) following acute exposure to relatively low doses (0.5–0.75 g/kg) of ethanol administered intraperitoneally (i.p.), an ethanol-induced facilitation of social behavior that is predominantly characterized by an increase in an adolescent-typical behavior of play fighting and is not normally seen in adults (Varlinskaya & Spear, 2015). The doses producing social facilitation in adolescent rats result in BECs from approximately 40 to 80 mg/dl, which is within the moderate to binge consumption range in humans (Eckardt et al., 1998; Spaak et al., 2008). Under normal circumstances, ethanol-induced social facilitation is not evident in older adolescents and adults (Varlinskaya & Spear, 2002, 2006). However, ethanol-induced social facilitation is seen in aged rats, with this facilitating effect of ethanol being sex-dependent: aging females, but not their male counterparts, exhibited social facilitation (Perkins, Vore, et al., 2018).
μ-opioid receptor antagonists can attenuate adolescent-typical social facilitation evident following ethanol administration (Varlinskaya & Spear, 2009), suggesting that this social facilitation is mediated, at least in part, through ethanol-induced release of endogenous ligands for μ opioid receptors or an ethanol-associated enhancement of sensitivity of these receptors to their endogenous ligands. The CB1 cannabinoid receptor antagonist SR141716A and the dopamine receptor antagonist α-flupenthixol can also attenuate socially facilitating effects of ethanol in early adolescent rats, suggesting that ethanol may enhance endocannabinoid activity in brain areas associated with positive reinforcement, which, in turn, may lead to activation of dopaminergic neurotransmission (Trezza et al., 2009). Neural mechanisms involved in ethanol-induced social facilitation evident in aging females still remain to be investigated.
Ethanol-induced social facilitation is seen following administration of low ethanol doses, whereas higher doses of ethanol elicit social inhibition (Morales, Varlinskaya, & Spear, 2014; Varlinskaya & Spear, 2002, 2006; Varlinskaya, Spear, & Spear, 2001). Sensitivity to the socially inhibiting effects of ethanol increases across ontogeny, with aging animals being more sensitive to these effects of ethanol than adults (Perkins, Vore, et al., 2018), and adults being more responsive than adolescents to ethanol-induced social inhibition ( Varlinskaya & Spear, 2002; Varlinskaya, Truxell, & Spear, 2013). Although no sex differences in sensitivity to the socially inhibiting effects of ethanol were evident in adults, aging males were more sensitive to these effects of ethanol than their female counterparts. The observed sex differences in sensitivity to the socially suppressing effects of ethanol in aging animals are likely not a result of altered ethanol pharmacokinetics, since aging males and females showed similar BECs following administration of 0.75 g/kg ethanol (Perkins, Vore, et al., 2018).
Social inhibition can be observed following a high-dose ethanol challenge (4 g/kg given intraperitoneally) when animals were tested at certain time intervals after clearing ethanol from their systems. When tested 3 hours post clearance, adults showed more pronounced social inhibition than their adolescent counterparts. In males, the suppression of social interactions was less apparent 7.5 to 9 hours after clearance than at the earlier post-clearance interval, with no social inhibition evident in females at this later post-clearance interval (Varlinskaya & Spear, 2004). In contrast to adults, adolescent animals pre-exposed to ethanol demonstrated increased levels of play fighting when tested at 8 to 9 hours after clearance, a phenomenon that was not evident at the early post-clearance test interval or in adults at either test interval. Thus, in contrast to what was observed in adults, adolescent males and females became more socially responsive during the hangover phase (Varlinskaya & Spear, 2004). To our knowledge, this is the first time that such a dramatic age-related difference has been reported in the delayed effects of acute ethanol on social behavior.
Sensitivity to the social consequences of ethanol can be modified by prior stress in older adolescents and adults. Specifically, exposure to repeated restraint (5 days, 90 min/day) during late adolescence and adulthood attenuated sensitivity to the social inhibition seen at higher ethanol doses relative to their non-stressed age mates and reinstated a pattern of responsiveness to the social consequences of ethanol characteristic of younger animals. These stressed animals demonstrated an attenuated sensitivity to the social inhibition seen at higher ethanol doses relative to their non-stressed counterparts and ethanol-induced social facilitation not evident in non- stressed age-matched controls (Varlinskaya, Doremus-Fitzwater, & Spear, 2010; Varlinskaya, Truxell, et al., 2013). Whether prior history of stress will alter sensitivity to the social effects of ethanol in aging rats still remains to be investigated.
Repeated exposure to ethanol can modify social behavior as well as ethanol sensitivity, with the observed alterations differing as a function of sex, age of exposure, and exposure-testing time interval. For instance, significant decreases in social preference assessed in a modified social interaction test (Varlinskaya, Spear, & Spear, 1999) were seen in adolescent, but not adult, males and females following ethanol exposure to a dose of 1 g/kg ethanol given intraperitoneally for 7 consecutive days, when these animals were tested 48 hours after the last ethanol dose (Varlinskaya & Spear, 2007). In contrast, both adolescent and adult males showed social deficits 24 hours after cessation of exposure to 4 g/kg ethanol given intraperitoneally every other day for a total of 5 exposures (Broadwater, Varlinskaya, & Spear, 2011).
A different study assessed long-lasting consequences of repeated ethanol exposure in males and females during 2 different periods of adolescence: early-mid adolescence and late adolescence. Social testing of these animals occurred 25 days after repeated exposure to ethanol (3.5 g/kg intragastrically every other day for a total of 11 exposures). Early-mid adolescent intermittent exposure (early AIE) resulted in significant decreases in social investigation and social preference evident in adult males, but not their female counterparts (Varlinskaya, Kim, & Spear, 2017; Varlinskaya, Truxell, & Spear, 2014), whereas late adolescent intermittent exposure (late AIE) had no effects on social behavior (Varlinskaya et al., 2014). These social deficits evident in adult males, but not females following early AIE are related, at least in part, to ethanol-induced alterations in the balance between the brain OT and AVP systems. Intermittent exposure to ethanol during early adolescence decreased OT, but increased AVP V1b receptor neuronal surface expression in the hypothalamus of adult males, but not of females, whereas the selective OT receptor agonist WAY-267464 and the selective AVP V1b receptor antagonist SSR-149415 reversed social deficits associated with early AIE (Dannenhoffer, Kim, Saalfield, Werner, & Spear, 2018). Social deficits were ameliorated either by the selective OT agonist or the V1b receptor antagonist, suggesting that the shift in the balance between the OT and AVP systems, rather than alterations within both systems per se, plays a major role in social deficits induced by intermittent ethanol exposure during early adolescence. This balance can be restored either by activation of the OT system or by suppression of the AVP system.
Furthermore, ethanol-induced facilitation of social investigation and play fighting, reminiscent of that normally seen during adolescence, was evident in adult males after early AIE, whereas control males showed an age-typical inhibition of social behavior. Late AIE made males insensitive to the socially suppressing effects of acute ethanol challenge, suggesting the development of chronic tolerance in these animals. In contrast, females showed little evidence for alterations in sensitivity to acute ethanol challenge following either early or late AIE (Varlinskaya et al., 2014). These results demonstrate a particular vulnerability of young adolescent males to long-lasting detrimental effects of repeated ethanol on social interaction. Retention of adolescent-typical sensitivity to the socially facilitating effects of ethanol could potentially make ethanol especially appealing to these males, therefore promoting relatively high levels of ethanol intake later in life. Further research of long-lasting effects of repeated ethanol on social behavior and sensitivity to social effects of ethanol in aging males and females is needed, given that this age group is more sensitive to the adverse consequences of alcohol than their younger counterparts.
In summary, positive (socially facilitating) and negative (socially anxiolytic) reinforcing properties of low doses of alcohol contribute to the attractiveness of alcohol in age groups that are more sensitive to its detrimental consequences, with alcohol exposure having adverse effects on social behavior (see Figure 3). To date, a substantial volume of experimental research has focused on the acute effects of ethanol on social behavior across lifespan, although much of the work has focused on adolescents and adults. Clearly, more work needs to be done to assess the effects of acute ethanol on social behavior in aging rats. Long-lasting social consequences of chronic ethanol exposure have been poorly investigated, especially in adult and aging animals. It seems clear, however, that high doses of alcohol commensurate with repeated, binge-like alcohol consumption and often characteristic of individuals with AUD disrupts the development and maintenance of positive social interactions, further erodes the social network, and may make individuals more vulnerable to a late-aging associated collapse in their social network. It remains to be assessed whether chronic ethanol exposure of adult and aging animals can alter social behavior and/or make ethanol more attractive for these age groups by enhancing sensitivity to socially facilitating ethanol effects. Further research should assess possible roles of opioid and cannabinoid brain systems in enhanced sensitivity to socially facilitating effects of ethanol evident in adult animals following adolescent ethanol exposure.
Figure 3. Labeling of microglia (iba1) reveals a distributed network of ramified microglia in the normal brain.
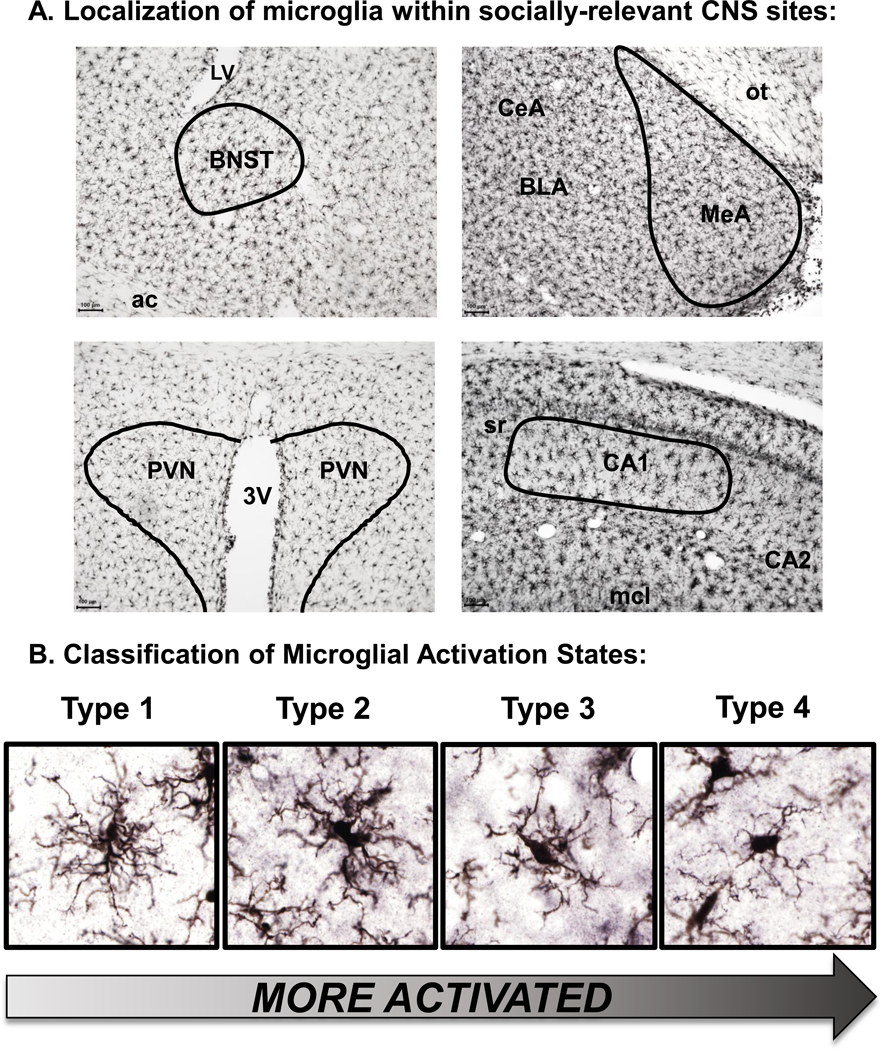
In addition to induced expression of inflammation-related genes and secretory release of inflammatory signaling molecules, microglial morphology has been used as a classical index of neuroinflammation. Panel A illustrates the distributed, grid-like distribution of iba1-labeled cells within the parenchyma of the CNS using DAB staining. Each microglia cell maintains a well-established territory of surveillance evidenced by the length of its processes. Note the substantially greater microglial density observed in the Medial Amygdala (MeA) relative to other structures shown (see Perkins et al., 2018 for more detail). Upon activation, cytoskeletal changes lead to a retraction of thin, elongated processes, a swelling of the cell body, and the appearance of an ameboid state as shown in Panel B. Historically, most studies have manually classified microglia into categories based on their morphological appearance. Special thanks to Paige Marsland for image collection and processing shown in Figure 4.
4.0. Social behavior and neuroinflammation
4.1. Immune challenge: Age differences
The first evidence of immune modulation of social behavior came from studies that reported alterations in social interaction associated with “sickness behavior” (reviewed in Dantzer and Kelley, 2007). “Sickness behavior” is the term used to describe behaviors that emerge as part of the acute phase response - the initial response of the immune system to various potentially infectious agents. Although early notions of sickness suggested that it reflected a state of true debilitation due to pathogenic infection, recent decades have recognized the adaptive value of sickness behaviors in promoting recovery from infection and limiting the spread of infectious disease (Hart, 1988). Consistent with this, a multitude of redundant mechanisms have evolved to sequester infection, with the immediate early aspects of the inflammatory response, often termed the acute phase response, including systemic release of pro-inflammatory factors, such as cytokines [e.g. Interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNFα)]. Release of these factors induces “sickness behavior”, comprised of lethargy, hypoactivity, piloerection, reduced food and water intake (see Dantzer and Kelley, 2007 for review).
Experimental evidence points to a substantial role of centrally acting cytokines in “sickness behavior” (Mccusker & Kelley, 2013), yet the mechanisms of cytokine-induced sickness behavior are somewhat complicated by the immunological protection of the CNS afforded by the Blood-Brain Barrier (BBB). That is, most cytokines are large, lipophobic proteins that do not readily cross the BBB in appreciable quantities, except in areas of the CNS where the BBB is weak or absent (Banks & Erickson, 2010). Instead, circulating cytokines signal through binding their cognate receptors in Gut- and Nerve-Associated Lymphoid Tissue (GALT and NALT, respectively), where immune signals are rapidly converted into afferent neural responses that orchestrate centrally-mediated sickness behaviors. Multiple peripheral nerves are now established as critical immune-to-brain communication pathways, including the vagus, hypoglossal and glossopharyngeal (Goehler et al., 2000; Watkins, Maier, & Goehler, 1995). Notably, however, activation of these neural fibers often elicits increased de novo synthesis of cytokines, prostaglandins, and histamine on the brain side of the BBB, which serve as local effectors to modulate neural circuits involved in coordination of behavioral output (Saper, Romanovsky, & Scammell, 2012). In contrast, descending (efferent) neural fibers from the vagus nerve tend to suppress peripheral inflammation, as evidenced by studies of vagal nerve stimulation (Olofsson, Rosas-Ballina, Levine, & Tracey, 2012). Finally, peripheral immune signals can signal across the cerebral vasculature by binding to receptors on endothelial cells, which can then propagate those immune signals through interactions with pericytes or perivascular microglia, which reside immediately adjacent to the vascular walls (Licinio & Wong, 1997; Schiltz & Sawchenko, 2003; Serrats et al., 2010). Thus, a variety of mechanisms exist for peripheral immune signals to communicate with the CNS, yet it remains a common misperception that circulating cytokines can directly interact with cognate receptors within the parenchyma of the CNS.
Most important in the present context, “sickness behavior” is characterized by reduced social interaction and diminished responsiveness to social stimuli (Hennessy et al., 2014). A commonly used index of “sickness behavior” in laboratory rodents is a reduction of time spent investigating an unfamiliar conspecific (e.g., Arakawa et al., 2009a), with social behavior typically assessed against a novel juvenile conspecific, sometimes in a separate testing room (Fishkin & Winslow, 1997), but most often in the home cage (Abraham & Johnson, 2009; Bluthé, Dantzer, & Kelley, 1992; Godbout et al., 2005; Henry, Huang, Wynne, & Godbout, 2009; Huang, Henry, Dantzer, Johnson, & Godbout, 2008). An immune challenge with the bacterial endotoxin lipopolysaccharide (LPS) – a common method of inducing a predictable course of inflammation in the laboratory - resulted in decreased social exploration and investigation in mice (Abraham & Johnson, 2009; Corona et al., 2010; Fishkin & Winslow, 1997; Godbout et al., 2005; Henry et al., 2009; Wynne, Henry, Huang, Cleland, & Godbout, 2010) and rats (Arakawa, Blandino, & Deak, 2009; Bluthé et al., 1992). In addition, LPS-induced suppression of social behavior in adult laboratory rodents can be blocked with peripheral (Bluthé et al., 1992), and central (Konsman et al., 2008, but also see Abraham and Johnson, 2009) administration of the selective IL-1 receptor antagonist. Suppression of social behavior by LPS can also be attenuated with peripheral administration of cyclooxygenase (COX) inhibitors (Fishkin & Winslow, 1997), corticosteroids (Fishkin & Winslow, 1997), or minocycline, a microglia inhibitor (Henry et al., 2009). Furthermore, central administration of IL-1β induces behavioral changes that are reminiscent of sickness, including decreases in social behavior (Bluthé et al., 1994; Bluthé, Dantzer, & Kelley, 1997). Pre-treatment with the selective IL-1 receptor antagonist administered centrally blocked the effects of systemically administered IL-1β on social behavior, suggesting that the effects of peripheral IL-1β are centrally mediated (Bluthé et al., 1997). Konsman et al. (2008) assessed the role of endogenous activity at brain IL-1 receptors in social alterations induced by LPS in adult male rats. The selective IL-1 receptor antagonist infused into the lateral ventricles 4 hours after LPS injection not only attenuated the reduction in social behavior, a major sign of behavioral depression during sickness, but also blunted c-Fos expression in the amygdala and bed nucleus of the stria terminalis. Furthermore, LPS administration produces neuronal activation in a number of brain regions associated with social behavior, including the extended amygdala, hippocampus and hypothalamus (Frenois et al., 2007). Taken together, these findings suggest that neuroimmune signaling is involved in social alterations associated with an acute immune challenge.
Interestingly, sickness-related changes in social behavior may also involve neuropeptides such as AVP that are known to be instrumental to social behavior regulation. For instance, LPS administration increases c-Fos expression within both OT and AVP-expressing neurons in CNS structures known to be involved in social behavior regulation (Matsunaga et al., 2000). Fever is associated with increased activity of BNST neurons (Mathieson, Federico, Veale, & Pittman, 1989) and electrical stimulation of the BNST reduced fever (Naylor, Pittman, & Veale, 1988). This is consistent with pharmacological studies showing that ICV administration of AVP itself reduced, whereas AVP antagonists enhanced, the expression of sickness behaviors evoked by IL-1 administration (Dantzer, Bluthe, & Kelley, 1991). Furthermore, OT and AVP are involved in both recognition and avoidance of sick conspecifics (Kavaliers & Choleris, 2011). It is perhaps notable that most of these studies have focused largely on male rodents, and significant sex differences in the role of OT and AVP may be evident. Nevertheless, there is clear evidence for neural substrates of social behavior regulation to interact with sickness-related signals.
Although administration of LPS in adult animals produces transient sickness behaviors, with full resolution evident in 6–18 hours depending on the dose (Galic, Riazi, & Pittman, 2012), early life inflammation associated with LPS has long-lasting social consequences. For instance, an acute administration of LPS on P 14 decreased adolescent social behavior in male and female rats at P 40 (Doenni et al., 2016). In contrast, adolescent animals seem to be less affected by this immune challenge relative to their more mature counterparts (e.g., Doremus-Fitzwater et al., 2015; Girard-Joyal et al., 2015), although these age differences in sensitivity are sex-dependent. For instance, exposure to LPS induced a greater and more prolonged sickness response in adult male mice relative to their adolescent counterparts as well as adult females, whereas adolescent female mice displayed greater sickness symptoms than their male counterparts (Sharma et al., 2018). Furthermore, age-related differences in males differed as a function of post-administration time interval: at 2 hours post administration, IL- 1β, TNFα, and IL-6 mRNA expression was higher in adolescents relative to adults, while at 8 hours following LPS challenge IL-1β and IL-6 mRNA expression was more pronounced in adults that in adolescents (Sharma et al., 2018).
Several studies have examined “sickness behavior” produced by endotoxin in aged animals and found that these animals exhibit marked reductions in social behavior following peripheral LPS administration. It took significantly longer for aged animals to recover from an acute inflammatory challenge, with these aged subjects demonstrating social behavior deficits up to 24 hours following LPS administration, a time at which social behavior has begun to return to normal in adult rodents (Abraham & Johnson, 2009; Godbout et al., 2005; Henry et al., 2009; Huang et al., 2008; Wynne et al., 2010). Abraham and Johnson (2009) demonstrated that 24 hours following peripheral LPS administration, adult animals exhibited a 40% reduction in social behavior, whereas aged animals exhibited an 80% reduction in social behavior. In adults, IL-1ra (ICV.) administration did not change social behavior, whereas in aged animals, IL-1ra returned social behavior to levels observed in adults at this time point (Abraham & Johnson, 2009). Increased inflammation is commonly observed in aged animals (Barrientos, Kitt, Watkins, & Maier, 2015; Corona, Fenn, & Godbout, 2012; Jurgens & Johnson, 2012; Norden & Godbout, 2013; Van Overwalle, Baetens, Mariën, & Vandekerckhove, 2014; Wong, 2013), which likely plays a major role in driving the prolonged expression of sickness behavior in late aging.
4.2. Neuroinflammation: aging-associated alterations
Normal, or non-pathological, aging is accompanied by alterations in the immune system in humans (Franceschi & Campisi, 2014; Ostan et al., 2008) as well as animal models (Barrientos et al., 2015; Norden & Godbout, 2013). While a complete discussion of the immune system in aging is beyond the scope of this review, we will highlight a few key findings that are relevant for the expression of social behavior. In addition, we refer the reader to a number of excellent reviews on the topic of inflammation and aging, or “inflammaging” (Barrientos et al., 2015; Eggen, Raj, Hanisch, & Boddeke, 2013; Jurgens & Johnson, 2012; Norden & Godbout, 2013).
The mechanisms that underlie aging-associated neuroinflammation and its role in social decline are not well understood. One possibility is that aging is associated with increased number or reactivity of microglia, resulting in prolonged or excessive release of pro-inflammatory cytokines which serve to suppress social behavior. Microglia are often referred to as resident immune cells of the central nervous system (CNS) distributed throughout all brain regions (Kettenmann, Hanisch, Noda, & Verkhratsky, 2011) with the highest concentrations of these cells evident in substantia nigra, basal ganglia, and hippocampus (Lawson, Perry, Dri, & Gordon, 1990). Microglia play an important role during development as well as in maintaining normal brain function, with microglial cells being involved in a number of pathological brain states and diseases, including neurodegenerative disorders, traumatic brain injuries, psychiatric diseases, as well as drug use and abuse (for reviews see Lacagnina et al., 2017; Wolf et al., 2017). During brain development, microglia are involved in programmed cell death mediated by TNF-α in addition to synaptic pruning (Bilimoria & Stevens, 2015; Filiano, Gadani, & Kipnis, 2015). In adulthood, microglial cells are involved in both synaptic remodeling and neuroprotection across the lifespan (Chen & Trapp, 2015), suggesting an alternative role for microglia in neuroplasticity. Thus, microglia are widely distributed throughout the CNS and their role(s) within the CNS change across the lifespan.
Under normal circumstances, microglia are constantly screening the brain microenvironment (Nimmerjahn, Kirchhoff, & Helmchen, 2005), detecting and responding to pathogenic incursions, cellular damage, excessive cellular activation and other changes that disrupt cellular or brain-wide homeostasis (Li & Barres, 2018). Figure 4 illustrates the distribution of microglia across several key brain areas that are crucial for the expression of social behavior. Upon the detection of pathogens, such as exogenous pathogen-associated molecular patterns (PAMPs) and endogenous danger-associated molecular patterns (DAMPs) by toll-like receptors (TLRs) located on the cell surface (Akira, Uematsu, & Takeuchi, 2006; Jurgens & Johnson, 2012), microglia respond by releasing pro-inflammatory cytokines, such as IL-1β, IFN-γ, TNF-α, and IL-6 (Akira et al., 2006; Vezzani & Viviani, 2015), anti-inflammatory cytokines, such as IL-10 (Heyen, Ye, Finck, & Johnson, 2000), and chemokines (Le Thuc, Blondeau, Nahon, & Rovere, 2015). In addition to detection of pathogens, microglia also respond to inhibitory signals secreted by neighboring neurons, such as fractalkine (CX3CL1) and CD200, both of which serve to maintain microglia in a quiescent state and tonically suppress microglial activation (Cardona et al., 2006). As a result, stimuli and conditions under which microglia become activated are typically indicated by dynamic changes in cell surface receptors such as fractalkine and CD200 receptors, which tend to decrease. Cognate receptors for DAMPs such as TLR4 and CD14, on the other hand, tend to increase in response to microglial activation, and facilitate the subsequent recognition, invagination, digestion and antigen presentation of the invading pathogens. It should be noted, however, that relatively few bacteria and other pathogens are capable of crossing the BBB in the young, intact individual, so microglial recognition of pathogens in the CNS of healthy individuals is exceedingly rare. However, the BBB is highly dynamic, with permeability of substances/pathogens into the CNS varying substantially as a result of peripheral infection (Varatharaj & Galea, 2017), and repeated exposure to stress challenges (Menard et al., 2017). Importantly, microglia are not just responsive to pathogens and DAMPs, but also express receptors for a number of neurotransmitter and neuropeptide signaling systems contributing substantially to the regulation of social behavior, including the DA, NE, endocannabinoid, and opioid brain systems (Kettenmann et al., 2011).
Figure 4. Isolation of individual microglia allows for 3D-reconstruction, surface smoothing, and biometric quantification of activation states.
Analytical tools for isolation of individual microglia within a visual frame have improved dramatically in recent years. Panel A illustrates how a standard fluorescence image of iba1 labelled cells can be image-enhanced to isolate processes associated with individual cells in confocal images. In addition to producing beautiful images, the surface smoothing functions render images with 3-dimensional depth by interpolating gaps in fluorescence and creating a visual representation of cellular membranes, allowing for volumetric analyses of cells to be calculated. In contrast, use of filament reconstruction tools allow for more detailed analysis of processes, including number of branch points, process length, and other biometric properties of microglia that report unbiased, quantitative measures of microglial activation states. Panel B illustrates how the surface-smoothing tool can be utilized for more effective categorization of microglia into Type 1–4 based upon gross cell morphology. These same cells are then displayed in Panel C using 3D-filament reconstruction. In addition to providing color-coding for the z-axis for ease of visualization, this mode reveals some thinner processes that extend beyond the interpolation algorithms of the surface-smoothing function, as well as processes that would be visually obscured by projecting behind the cell of interest. Special thanks to Dr. Cara M. Hueston for collection of confocal images, as well as Dr. Mario Oyola and Paige Marsland for image processing shown in Figure 4.
In the aging brain, microglia become more responsive to pathogens, with this process referred to as microglia priming (Norden & Godbout, 2013). Microglia priming results in an enhanced and prolonged response to an immune challenge (Perry & Holmes, 2014), including increased release of the proinflammatory cytokines IL-1β, TNF-α, and IL-6 (Barrientos, Frank, Watkins, & Maier, 2012; Frank, Barrientos, Hein, et al., 2010; Frank, Barrientos, Watkins, & Maier, 2010; Norden & Godbout, 2013; Richwine et al., 2008). Enhanced proinflammatory activity in the aged brain is not suppressed by the anti-inflammatory cytokines IL-10, TGF-β, or IL-4, suggesting that in the aged brain, microglia priming is insensitive to anti-inflammatory regulation (Norden & Godbout, 2013; Norden, Trojanowski, Walker, & Godbout, 2016). Microglia isolated from the aged brain have enhanced expression of the microglia markers MHCII (Griffin et al., 2006; Henry et al., 2009), CD86 (Griffin et al., 2006), CD68 (Wong et al., 2005), CD11b (Hart, Wyttenbach, Hugh Perry, & Teeling, 2012; Stichel & Luebbert, 2007), and cytokines such as IL-1β (Griffin et al., 2006; Sierra, Gottfried-Blackmore, McEwen, & Bulloch, 2007; Stichel & Luebbert, 2007), and IL-6 (Sierra et al., 2007). These data indicate a shift toward a pro-inflammatory profile in the aged brain. Aging is also accompanied by a significant decline in the expression of fractalkine (CX3CL1), a negative regulator of microglia activation (Fenn et al., 2013). Altered patterns of social behavior evident in CX3CR1 knockout mice provide experimental evidence for a functional role of microglia in social behavior regulation (Zhan et al., 2014). Thus, the reduction of anti-inflammatory cytokines such as IL-10 and IL-4 (Norden & Godbout, 2013; Norden et al., 2016) and reduced expression of fractalkine (CX3CL1), a molecule critical in the shut-off of microglia activation (Fenn et al., 2013), in the aged brain seem to suggest that enhanced inflammation in late aging reflects deficits in shutoff of the neuroimmune response, perhaps even more so than an enhanced pro-inflammatory response.
Although the expression of inflammatory-related cytokines, chemokines and prostaglandins are often used as indices of microglial activation, morphological features of microglia such as the overall shape and complexity of processes vis a vis the cell body are used as a lagging indicator of neuroimmune activation (see Figure 4). That is, in response to acute challenge, microglia first respond with increased/altered expression of inflammation-related genes encoding critical inter-cellular mediators, while at the same time initiating more protracted cytoskeletal changes that lead to the retraction of processes and the transition of the cell body toward an ameboid state. The transition from a ramified (resting) to ameboid (activated) state often emerges a day or more after the initial inflammatory event and can persist for days, weeks or months depending on the nature of the insult. Such morphological changes are typically visualized with antibodies directed against ionized calcium binding adaptor molecule 1 (iba1), a cellular marker that is stably expressed and remarkably unique to monocyte-derived cells, including microglia. A common misnomer in the field, however, is that iba1 expression is increased in response to inflammation, which is not entirely accurate. Actual gene expression for iba1 on a per-cell basis is only modestly influenced by inflammation. What is apparent, however, is that the number of microglia in a given site (i.e., microglial density) increases due to increased influx of iba1-expressing microglia into the site. In addition, the retraction of processes leads to a greater concentration of iba1 protein within cell bodies of activated microglia, thereby increasing the density of iba1 labeling, and as a result, more cells achieve threshold for counting by image analysis programs and/or stereological assessments. Thus, use of cellular labeling approaches to infer microglial activation state is somewhat limited by (i) the delayed nature of morphological changes relative to stimulus onset; (ii) the inability to discriminate between functional M1 (the classically activated, pro-inflammatory phenotype) and M2 (activated, but anti-inflammatory) phenotypes; (iii) the often subjective or biased categorization of microglia based on visual classification; and sole reliance on in vitro or ex vivo techniques (microglial isolation from tissue; tissue slice cultures, etc.) in which the procedures themselves produce substantial changes in microglial activation. With these limitations in mind, assessment of microglial morphology is indeed highly effective at reporting state of inflammation in cases where the evocative circumstances are distributed over the course of days, weeks or months, or when a temporally-discrete stimulus for evoking a neuroimmune response is not apparent (e.g., accumulative effects of aging). Moreover, advances in imaging approaches now provide the ability to perform biometric analyses with greater efficiency and reproducibility, thereby minimizing any remaining sources of inadvertent bias (see Figure 4). That is, using confocal images, a variety of software packages are now available to digitally render, isolate and categorize cells based on morphological features such as number of branch points, process length, volume of cellular “territory”, and other biometric properties. Such approaches show great promise for discovering more subtle differences in cellular morphology that may be particularly advantageous for the study of aging-related changes in neuroinflammation.
To this end, our recent study (Perkins, Piazza, & Deak, 2018) was designed to investigate whether late aging-associated reductions in social behavior was associated with increased number and/or altered activational states of resident microglia within the social behavior neural network (Freeman & Young, 2016; Johnson & Young, 2017; Johnson, Walum, Xiao, Riefkohl, & Young, 2017). In the MEA, aged rats of both sexes had more microglia than their young adult counterparts, whereas within the BNST, similar age differences were evident only in females. When morphological features of microglia were assessed, soma size was increased in the BNST and MEA of aged rats (Perkins, Piazza, et al., 2018), indicative of greater microglial activation among aged rats in sites directly responsible for social behavior regulation. However, it is noteworthy that very few microglia were observed in unchallenged, aged rats that resembled the fully ameboid (type 4) phenotype. Nevertheless, to the extent that microglia morphology is predictive of reactivity to different challenges and subsequent cytokine release, these data suggest that the expression of social behavior in late aging may be adversely influenced by heightened neuroinflammation. It remains to be investigated whether late aging is associated with altered cytokine expression and/or release within brain regions known to be involved in the regulation of social behavior, specifically the PVN, BNST, and MEA.
A new generation of studies has sought to move beyond the simple categorization of microglia based on simple morphology or expression of a few isolated neuroimmune genes. The intent of these studies is to identify a more specific pattern of gene expression profiles that might portray the state of microglia across developmental periods and a range of specific insults. To do this, an increasing number of studies have utilized RNASeq and other gene expression profiling procedures in single-cell preparations. The initial findings have been quite intriguing. For instance, Hammond et al. (2019) performed RNASeq on greater than 76,000 individual microglial cells across early development and late aging, including comparisons with microglia obtained from the injured mouse brain. Expression profiling yielded at least 9 distinct phenotypes of microglia, suggesting that current notions of phenotypic assessments rooted in ex vivo cultures of microglia or classification of cellular morphology alone might underestimate the diversity of microglia present in the aging brain. These studies with emerging technologies will undoubtedly pave the way for greater advances in our understanding of the relationship between neuroinflammation, microglia and social behavior across the lifespan.
Of course, microglia are not the only cells in the CNS that are capable of expressing neuroinflammatory markers, and cooperative interactions among cells must be considered. For instance, microglia are typically considered the “first responders” in the injured CNS, provoking a rapid and forward-propagating neuroimmune response. A now classic study by Raghavendra et al (2003) demonstrated that microglial inhibitors blocked the expression of inflammation and subsequent hyperalgesia when administered prior to injury, but not 24 hr later. Their findings strongly supported the conclusion that microglia produced a rapid, early phase of inflammation-induced hyperalgesia, and that these early immune signals led to activation of astrocytes. By a day or so later, however, microglia-derived cytokines were no longer necessary for the observed hyperalgesia, and the behavioral effects were sustained by astrocytes for a more protracted period of time. In this way, early inflammatory signals appear to get “handed off” from microglia and maintained by astrocytes, underscoring the importance of considering the complex cellular milieu of the CNS. Consistent with this, Bellaver et al. (2017) recently showed that astrocytes recapitulate many of the inflammatory-related changes associated with the aging CNS. Thus, future studies examining the interplay between neuroinflammation and social behavior in late aging will be necessary to examine a broader repertoire of cells that might contribute.
4.3. Neuroinflammation and alcohol
The neuroimmune system is an important target of alcohol, and neuroinflammation plays a substantial role in neural alterations associated with alcohol use and abuse (Bachtell, Jones, Heinzerling, Beardsley, & Comer, 2017; Crews, Lawrimore, Walter, & Coleman, 2017; Erickson et al., 2019; Mayfield, Ferguson, & Harris, 2013). In general, exposure to chronic ethanol is associated with glial activation and increased expression of cytokines and chemokines, including IL-1β, TNF-α, IL-6, CCL2, MIP-1a/ CCL3, and MIP-1b/CCL4 in the brain (Alfonso-Loeches, Pascual-Lucas, Blanco, Sanchez-Vera, & Guerri, 2010; He & Crews, 2008; Lippai et al., 2013; Pascual, Baliño, Alfonso-Loeches, Aragón, & Guerri, 2011), with blockade of IL-1 receptor attenuating ethanol-induced neuroinflammation (Lippai et al., 2013). With the exception of one study (Richey, Doremus-Fitzwater, Buck, & Deak, 2012), the role of neuroimmune signaling in social alterations associated with acute and repeated ethanol has not been investigated. Therefore, in the present review, our main focus is on ethanol-associated neuroimmune changes that may play some role in ethanol effects on social interaction as well as age-related differences in ethanol sensitivity. It seems likely that the neuroimmune system may contribute to withdrawal-related social deficits that emerge within 24–48 hours after an acute high dose ethanol challenge (Richey et al., 2012; Varlinskaya & Spear, 2004) as well as social alterations evident in adult males following adolescent intermittent ethanol exposure (Varlinskaya et al., 2014).
There is some experimental evidence suggesting a potential role for cytokine signaling in sedative effects of ethanol (Blednov, Benavidez, Black, Mayfield, & Harris, 2015; Wu et al., 2011). Therefore, an adolescent-typical decrease in sensitivity to ethanol-induced sedation (Little et al., 1996; Silveri & Spear, 1998) may be related, at least in part, to age differences in the central cytokine response following an acute ethanol challenge. Indeed, some, although not drastic differences between adolescents and adults emerged after acute ethanol challenge. Acute ethanol intoxication (3 hours post-administration) results in elevations of IL-6 and IκBα and suppression of IL-1β and TNFα gene expression in the hippocampus, amygdala and BNST (Doremus-Fitzwater et al., 2014, 2015; Gano, Doremus-Fitzwater, & Deak, 2016). When adolescent (P 31–33) and adult (P 69–71) male Sprague Dawley rats were acutely challenged intraperitoneally with the same ethanol dose, IL-6 and IκBα expression was significantly increased in both ages in the PVN and AMG, with adults exhibiting greater increases in IκBα than adolescents. Intragastric administration of ethanol also increased IL-6 and IκBα expression the PVN, AMG, and HPC, with HPC IL-6 elevated even more so in adults compared to adolescents (Doremus-Fitzwater et al., 2015). Given a potential role of the neuroimmune signaling in ethanol-induced sedation (Blednov et al., 2015; Wu et al., 2011), the observed age-related differences in IκBα and IL-6 may contribute to decreased sensitivity of adolescent rats to ethanol-induced sedation. It is unlikely, however, that increases in IL-6 expression are associated with ethanol-induced sedation, since infusion of IL-6 (25, 50, 100, or 200 ng) into the third ventricle of adult Sprague Dawley males had no effect on any parameters of loss of the righting reflex (Barney, Vore, Gano, Mondello, & Deak, 2019). Nevertheless, it is possible that IL-6 is involved in ethanol-induced sedation affecting brain that were not reached by the infusions, and future studies should test for site-specific effects of IL-6 in adolescents and adults.
In contrast to acute ethanol intoxication-associated increases in IL-6 and IκBα and suppression of IL-1β and TNFα gene expression (Doremus-Fitzwater et al., 2014, 2015; Gano et al., 2016), withdrawal from acute ethanol results in substantial increases in IL-1β and TNFα in the brain of adult male Sprague Dawley rats (Doremus-Fitzwater et al., 2014). In male C57BL/6J mice, effects of acute ethanol challenge (6 g/kg ethanol, i.g.) on microglia and cytokines are also post-injection time interval-dependent, with different patterns evident during intoxication and withdrawal (Walter & Crews, 2017). Ethanol challenge produced biphasic effects on microglial markers, Iba1 and CD68, in the brain, with initial decreases in mRNA levels evident during intoxication and subsequent increases during withdrawal, suggesting microglial activation during acute withdrawal. Activation of microglia resulted in increased brain expression of a number of cytokines (TNFα, Ccl2, IL-1ra, IL-4) at 18 hours post ethanol, with marked changes in mRNA levels accompanied by increased brain protein levels at this time point (Walter & Crews, 2017). One of our earlier studies was designed to directly assess whether social alterations associated with acute ethanol withdrawal can be reversed by blockade of IL-1 receptors. Central administration of the IL-1 receptor antagonist (10, or 100 μg i.c.v.) given 30 minutes prior to behavioral to behavioral testing was ineffective in reversing withdrawal-related social alterations evident in adult male Sprague Dawley rats tested 18 hours following i.p. administration of 4.0 g/kg ethanol (Richey et al., 2012). These results, however, would not entirely preclude the involvement of cytokine signaling in the expression of social alterations associated with ethanol withdrawal. For instance, multiple pro-inflammatory cytokines may in fact be involved in social alterations associated with ethanol withdrawal. If this were case, then IL-10 as a general anti-inflammatory compound that reduces the expression of multiple pro-inflammatory cytokines including IL-1, IL-6, and TNFα (Dantzer, 2004), may be more effective in reducing social alteration associated with ethanol withdrawal. Indeed, withdrawal-related social alterations were reversed by rosiglitazone, an anti-diabetic drug that been shown to have anti-inflammatory properties by reducing NFκB binding and TNFα concentrations (Mohanty et al., 2004). Therefore, further studies should focus on the role of brain cytokines in social alterations evident during ethanol withdrawal.
Neuroimmune signaling may play a substantial role in social alterations associated with chronic ethanol exposure, given that animal studies have shown increases in mRNA levels of pro-inflammatory cytokines and chemokines in brain regions implicated in modulation of reward, affect, and social interaction following repeated ethanol. For instance, exposure of adult male and female Wistar rats to ethanol vapor for 7 days (14 hours on, 10 hours off) have enhanced TNFα expression in the BLA, NAC, and VTA regardless of sex, while IL-6 expression, in contrast to acute ethanol, was reduced in the mPFC of males but not their female counterparts (Baxter-Potter et al., 2017). Following chronic intermittent ethanol exposure that lasted 6 weeks (14 h on: 10 h off), increases in TNFα expression in the NAC as well as increases in IL-6 expression in the vmPFC and NAC were evident in males and females, with this chronic intermittent exposure also increasing CCL2 expression in the BLA and VTA in males but not females (Baxter-Potter et al., 2017). Furthermore, in rats, intermittent ethanol exposure during adolescence (P30-P44, 3 g/kg, i.p., 2 days on/off) has been shown to increase gene expression of TNFα and IL-1β in the PFC, whereas similar ethanol exposure during adulthood did not produce significant changes in these cytokines (Pascual, Pla, Miñarro, & Guerri, 2014).
Adolescent alcohol exposure disrupts excitatory/inhibitory balance in the brain, and pro-inflammatory immune signaling may play a role in these alterations. For instance, TNFα modulates expression of AMPA and NMDA receptors, decreases neuronal GABAA receptor expression (Olmos & Llado, 2014), inhibits astrocytic glutamate uptake (Tilleux & Hermans, 2007), and increases astrocytic glutamate release (Habbas et al., 2015). IL-6 suppresses inhibitory transmission by reducing GABAA and glycine receptor function (Kawasaki, Zhang, Cheng, & Ji, 2008). Therefore, neuroinflammation associated with adolescent alcohol exposure may contribute to the emergence of social alterations through different mechanisms, including disruptions of excitatory/inhibitory balance in brain regions associated with regulation of social behavior.
Surprisingly few studies have specifically tested whether the aged brain is differentially sensitive to ethanol-induced neuroimmune changes compared to young adults. A recent study from our group examined ethanol-induced expression of neuroimmune genes 3 hr after a high dose of ethanol in F344 rats aged 3, 9, 12, and 18 months (Gano, Doremus-fitzwater, & Deak, 2017). As expected, ethanol led to a robust increase in IL-6 and IκBα in young- and middle-aged rats. Although IL-6 levels in particular were similar across all ethanol-challenged groups, a profound aging-related increase in several neuroimmune markers (IL-6, CD14, etc.) among vehicle-injected controls made the aged rats seemingly unresponsive to neuroimmune effects of ethanol challenge, despite the fact that they were behaviorally more sensitive to ethanol-induced loss of righting reflex. Beyond these acute effects, recent studies indicate that life-long alcohol consumption in rodent models led to surprisingly few changes in neuroinflammatory markers in gross dissections of the brain, except in individuals that were co-treated with pyrithiamine to accelerate thiamine depletion, a model of Wernickes-Korsakoff (WKS) syndrome (Toledo Nunes, Vedder, Deak, & Savage, 2019). In these groups, enhanced expression of multiple neuroimmune genes occurred shortly after the onset of seizure activity, the magnitude of which is predictive of behavioral deficits in animal models of WKS (Savage, Hall, & Resende, 2012). These studies provide initial information regarding neuroimmune responses in the aged brain, yet many unresolved issues remain. Future studies will be necessary to better understand (i) dose-dependent effects of ethanol in late aging; (ii) the influence of voluntary ethanol consumption (rather than forced administration) on neuroimmune function in late aging; and (iii) to establish a causal role for neuroimmune signaling factors in ethanol-dependent changes in social behavior.
5.0. Summary and future directions
The over-arching goal of this review article was to bring together several features of late aging that significantly influence quality of life. Late aging is known to be associated with a decline in overall social motivation in humans, an effect that ultimately coalesces into reduced quality of life and a collapse of their supportive social network. Consider, for instance, the observation that stroke outcomes were substantially improved in mice given regular social interaction, an effect that was apparently mediated through the social neuropeptide OT (Karelina et al., 2011). Indeed, other studies have demonstrated a potent, tempering effect of OT on inflammatory processes (Yuan et al., 2016). Although great progress has been made in the delineation of neural, neurohormonal and neuropeptide regulation of social processes in younger animals, remarkably little is known about the mechanisms contributing to the decline in social behavior in late aging. We believe that a deeper pursuit and elaboration of aging-related changes in brain function, specifically beginning with established mechanisms of social behavior regulation, will ultimately reveal critical insights into behavioral and/or pharmacological therapies that might be used to improve quality of life among elderly populations. Because aging is associated with heightened inflammation in brain and elsewhere throughout the body, and inflammation is a major driver of social processes, neuroinflammation may be a likely culprit in the demise of social behavior in late aging. At the same time, it is clear that other, less healthy adaptations in aging humans such as alcohol consumption are tending to increase among the elderly. As the social network collapses and negative affect escalates as a result, the likelihood of greater alcohol consumption in late aging may continue to rise, further threatening social interaction and compromising overall health. Here again, inflammation is well positioned as a broad mechanism to contribute to the unique sensitivities of aged individuals to alcohol. At present, there is not a definitive set of studies that can tie inflammatory mechanisms, evoked by ethanol or other life circumstances, to the decline in social functioning and overall health during senescence. Our hope is that bringing these issues to light may stimulate further studies that will provide an evidence-based, brain-informed approach toward defining promising avenues to improve quality of life in late aging.
Figure 1. Reciprocal interactions between aging, neuroinflammation, alcohol and social behavior.
Aging is characterized by increased neuroinflammation and enhanced sensitivity to adverse effects of alcohol. In turn, alcohol use and inflammation are capable of exacerbating signs of aging. Alcohol abuse is associated with increased neuroinflammatory signaling. Declines in social interactions are associated with aging, alcohol abuse, and inflammation, whereas positive social experiences can diminish alcohol use, reduce cognitive declines associated with aging, and decrease signs of inflammation.
Acknowledgements:
Supported by NIH grant numbers P50AA017823 and R01AG043467 to T.D. and the Center for Development and Behavioral Neuroscience at Binghamton University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies. The authors have no conflicts of interest to declare.
References
- Abraham J, & Johnson RW (2009). Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain, Behavior, and Immunity, 23(3), 396–401. 10.1016/j.bbi.2008.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achterberg EJM, Trezza V, Siviy SM, Schrama L, Schoffelmeer AN, & Vanderschuren LJMJ (2014). Amphetamine and cocaine suppress social play behavior in rats through distinct mechanisms. Psychopharmacology, 231(8), 1503–1515. 10.1016/j.surg.2006.10.010.Use [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achterberg EJM, van Kerkhof LWM, Damsteegt R, Trezza V, & Vanderschuren LJMJ (2015). Methylphenidate and Atomoxetine Inhibit Social Play Behavior through Prefrontal and Subcortical Limbic Mechanisms in Rats. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 35(1), 161–169. 10.1523/JNEUROSCI.2945-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addolorato G, Vassallo GA, Antonelli G, Antonelli M, Tarli C, Mirijello A, … Sestito L (2018). Binge Drinking among adolescents is related to the development of Alcohol Use Disorders: results from a Cross-Sectional Study. Scientific Reports, 8(1), 1–9. 10.1038/s41598-018-29311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, & Takeuchi O (2006). Pathogen Recognition and Innate Immunity. Cell, 124(4), 783–801. 10.1016/j.cell.2006.02.015 [DOI] [PubMed] [Google Scholar]
- Albayram O, Alferink J, Pitsch J, Piyanova A, Neitzert K, Poppensieker K, … Bilkei-Gorzo A (2011). Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proceedings of the National Academy of Sciences of the United States of America, 108(27), 11256–11261. 10.1073/pnas.1016442108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albayram O, Bilkei-Gorzo A, & Zimmer A (2012). Loss of CB1 receptors leads to differential age-related changes in reward-driven learning and memory. Frontiers in Aging Neuroscience, 4(DEC), 1–8. 10.3389/fnagi.2012.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE (2015). Species, sex, and individual differences in the vasotocin/vasopressin system: Relationship to neurochemical signaling in the social behavior neural network. Frontiers in Neuroendocrinology, 49–71. 10.1016/j.surg.2006.10.010.Use [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, & Guerri C (2010). Pivotal Role of TLR4 Receptors in Alcohol-Induced Neuroinflammation and Brain Damage. Journal of Neuroscience, 30(24), 8285–8295. 10.1523/JNEUROSCI.0976-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amore M, Innamorati M, Costi S, Sher L, Girardi P, & Pompili M (2012). Partial androgen deficiency, depression, and testosterone supplementation in aging men. International Journal of Endocrinology, 2012, 1–17. 10.1155/2012/280724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen MB, Zimmer J, & Sams-Dodd F (1999). Specific behavioral effects related to age and cerebral ischemia in rats. Pharmacol Biochem Behav, 62(4), 673–682. https://doi.org/S0091-3057(98)00204-4 [pii] [DOI] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, & Spear LP (2010). Ethanol-Induced Conditioned Taste Aversion in Male Sprague-Dawley Rats : Impact of Age and Stress, 34(12), 2106–2115. 10.1111/j.1530-0277.2010.01307.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, & Deak T (2009). Acute illness induces the release of aversive odor cues from adult, but not prepubertal, male rats and suppresses social investigation by conspecifics. Behavioral Neuroscience, 123(5), 964–978. 10.1037/a0017114 [DOI] [PubMed] [Google Scholar]
- Arakawa H, Blandino P, & Deak T (2009). Central infusion of interleukin-1 receptor antagonist blocks the reduction in social behavior produced by prior stressor exposure. Physiology & Behavior, 98(1–2), 139–146. 10.1016/j.physbeh.2009.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Cruz S, & Deak T (2011). From models to mechanisms: Odorant communication as a key determinant of social behavior in rodents during illness-associated states. Neuroscience and Biobehavioral Reviews, 35(9), 1916–1928. 10.1016/j.neubiorev.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Arndt S, Clayton R, & Schultz SK (2011). Trends in Substance Abuse Treatment 1998 – 2008 : Increasing Older Adult First-Time Admissions for Illicit Drugs. American Journal of Geriatric Psychiatry, 19, 704–711. 10.1097/JGP.0b013e31820d942b [DOI] [PubMed] [Google Scholar]
- Auger AP, & Olesen KM (2009). Brain sex differences and the organization of juvenile social play behavior. Journal of Neuroendocrinology, 21(6), 519–525. 10.1016/j.micinf.2011.07.011.Innate [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Jones JD, Heinzerling KG, Beardsley PM, & Comer SD (2017). Glial and neuroinflammatory targets for treating substance use disorders. Drug and Alcohol Dependence, 180(March), 156–170. 10.1016/j.drugalcdep.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, & Erickson MA (2010). The blood-brain barrier and immune function and dysfunction. Neurobiology of Disease, 37(1), 26–32. 10.1016/j.nbd.2009.07.031 [DOI] [PubMed] [Google Scholar]
- Bardou I, Kaercher RM, Brothers HM, Hopp SC, Royer S, & Wenk GL (2014). Age and duration of inflammatory environment differentially affect the neuroimmune response and catecholaminergic neurons in the midbrain and brainstem. Neurobiology of Aging, 35(5), 1065–1073. 10.1016/j.neurobiolaging.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barney TM, Vore AS, Gano A, Mondello JE, & Deak T (2019). The influence of central interleukin-6 on behavioral changes associated with acute alcohol intoxication in adult male rats. Alcohol, 79, 37–45. 10.1016/j.alcohol.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR, & Maier SF (2012). Aging-related changes in neuroimmune-endocrine function: Implications for hippocampal-dependent cognition. Hormones and Behavior, 62(3), 219–227. 10.1016/j.yhbeh.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Kitt MM, Watkins LR, & Maier SF (2015). Neuroinflammation in the normal aging hippocampus. Neuroscience, 309(March), 84–99. 10.1016/j.neuroscience.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, & Tapert SF (2010). Adolescent Brain Development and the Risk for Alcohol and Other Drug Problems. Neuropsychological Reviews, 20, 398–413. 10.1007/s11065-010-9146-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Potter LN, Henricks AM, Berger AL, Bieniasz KV, Lugo JM, & McLaughlin RJ (2017). Alcohol vapor exposure differentially impacts mesocorticolimbic cytokine expression in a sex-, region-, and duration-specific manner. Neuroscience, 346, 238–246. 10.1016/j.neuroscience.2017.01.015 [DOI] [PubMed] [Google Scholar]
- Bazhanova E, Grinevich V, Danilova O, & Chernigovskaya E (1998). Age-related changes in oxytocinergic neurosecretory cells in the accessory magnocellular neuroendocrine nuclei of the hypothalamus in rats. Neuroscience and Behavioral Physiology, 28(4), 44–46. [DOI] [PubMed] [Google Scholar]
- Behar D, Berg CJ, Rapoport JL, Nelson W, Linnoila M, Cohen M, … Marshall T (1983). Behavioral and Physiological Effects of Ethanol in High- Risk and Control Children : A Pilot Study. Alcoholism: Clinical and Experimental Research, 7(4), 404–410. [DOI] [PubMed] [Google Scholar]
- Bellaver B, Souza DG, Souza DO, & Quincozes-Santos A (2017). Hippocampal Astrocyte Cultures from Adult and Aged Rats Reproduce Changes in Glial Functionality Observed in the Aging Brain. Molecular Neurobiology, 54(4), 2969–2985. 10.1007/s12035-016-9880-8 [DOI] [PubMed] [Google Scholar]
- Berrendero F, Romero J, Garcia-Gil L, Suarez I, De la Cruz P, Ramos JA, & Fernandez-Ruiz JJ (1998). Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochimica et Biophysica Acta, 1407(3), 205–214. 10.1016/S0925-4439(98)00042-8 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, & Aldridge JW (2009). Dissecting components of reward: “liking”, “wanting”, and learning. Current Opinion in Pharmacology, 9(1), 65–73. 10.1016/j.coph.2008.12.014.Dissecting [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilimoria PM, & Stevens B (2015). Microglia function during brain development: New insights from animal models. Brain Research, 1617, 7–17. 10.1016/j.brainres.2014.11.032 [DOI] [PubMed] [Google Scholar]
- Bisschop MI, Kriegsman DMW, van Tilburg TG, Penninx BWJH, van Eijk JTM, & Deeg DJH (2003). The influence of differing social ties on decline in physical functioning among older people with and without chronic diseases: the Longitudinal Aging Study Amsterdam. Aging Clinical and Experimental Research, 15(2), 164–173. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12889849 [DOI] [PubMed] [Google Scholar]
- Blakemore SJ (2012). Development of the social brain in adolescence. JR Soc Med, 105(3), 111–116. 10.1017/CBO9781139565790.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Mayfield J, & Harris RA (2015). Role of interleukin-1 receptor signaling in the behavioral effects of ethanol and benzodiazepines. Neuropharmacology, 95, 309–320. 10.1016/j.neuropharm.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthé RM, Pawlowski M, Suarez S, Parnet P, Pittman Q, Kelley KW, & Dantzer R (1994). Synergy between tumor necrosis factor α and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology, 19(2), 197–207. 10.1016/0306-4530(94)90009-4 [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Dantzer R, & Kelley KW (1992). Effects of interleukin-1 receptor antagonist on the behavioral-effects of lipopolysaccharide in rat. Brain Research, 573(2), 318–320. [DOI] [PubMed] [Google Scholar]
- Bluthé Rose Marie, Dantzer R, & Kelley KW (1997). Central mediation of the effects of interleukin-1 on social exploration and body weight in mice. Psychoneuroendocrinology, 22(1), 1–11. 10.1016/S0306-4530(96)00042-X [DOI] [PubMed] [Google Scholar]
- Boguszewski P, & Zagrodzka J (2005). Expression of c-Fos in response to stressogenic stimuli in the amygdala of old vs. young rats - A preliminary study. Acta Neurobiologiae Experimentalis, 65(2), 191–194. [DOI] [PubMed] [Google Scholar]
- Bonanno GA (2004). Loss, Trauma, and Human Resilience: Have We Underestimated the Human Capacity to Thrive after Extremely Aversive Events? American Psychologist, 59(1), 20–28. 10.1037/0003-066X.59.1.20 [DOI] [PubMed] [Google Scholar]
- Bonnet AE, & Marchalant Y (2015). Potential Therapeutical Contributions of the Endocannabinoid System towards Aging and Alzheimer’s Disease. Aging and Disease, 6(5), 400–405. 10.14336/AD.2015.0617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomo Y (2005). Early onset of drinking increases alcohol use in adulthood: Commentary. Evidence-Based Mental Health, 8(4), 98 10.1136/ebmh.8.4.98 [DOI] [PubMed] [Google Scholar]
- Bonomo YA, Bowes G, Coffey C, Carlin JB, & Patton GC (2004). Teenage drinking and the onset of alcohol dependence: A cohort study over seven years. Addiction, 99(12), 1520–1528. 10.1111/j.1360-0443.2004.00846.x [DOI] [PubMed] [Google Scholar]
- Boule LA, & Kovacs EJ (2017). Alcohol, aging, and innate immunity. Journal of Leukocyte Biology, 102, 41–55. 10.1189/jlb.4RU1016-450R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasser SM, & Spear NE (2002). Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behavioral Neuroscience, 116(2), 305–320. 10.1037/0735-7044.116.2.305 [DOI] [PubMed] [Google Scholar]
- Bredewold R, & Veenema AH (2018). Sex differences in the regulation of social and anxiety-related behaviors: insights from vasopressin and oxytocin brain systems. Current Opinion in Neurobiology, 49, 132–140. 10.1016/j.conb.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow RA, Dong C, & White A (2015). Prevalence of Alcohol-Interactive Prescription Medication Use Among Current Drinkers: United States, 1999 to 2010. Alcoholism: Clinical and Experimental Research, 39(2), 371–379. 10.1111/acer.12633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, & Spear LP (2011). Chronic intermittent ethanol exposure in early adolescent and adult male rats: Effects on tolerance, social behavior, and ethanol intake. Alcoholism: Clinical and Experimental Research, 35(8), 1392–1403. 10.1111/j.1530-0277.2011.01474.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Cohen K, & Blakemore S (2011). The social brain in adolescence : Evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience & Biobehavioral Reviews, 35, 1654–1664. 10.1016/j.neubiorev.2010.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Karkhanis AN, Jones SR, Weiner JL, Stress EL, & Salem W (2016). Adolescent Social Isolation as a Model of Heightened Vulnerability to Comorbid Alcoholism and Anxiety Disorders. Alcoholism: Clinical and Experimental Research, 40(6), 1202–1214. 10.1111/acer.13075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Granberg R, & Tseng KY (2016). Mechanisms contributing to prefrontal cortex maturation during adolescence. Neuroscience and Biobehavioral Reviews, 70, 4–12. 10.1016/j.neubiorev.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, Dulawa S, & Palmer AA (2014). Social neuroscience and its potential contribution to psychiatry. World Psychiatry, 13, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzà L, Pozza M, Coraddu F, Farci G, & Giardino L (1997). Hormonal influences on brain ageing quality: Focus on corticotropin releasing hormone-, vasopressin- and oxytocin-immunoreactive neurones in the human brain. Journal of Neural Transmission, 104(10), 1095–1100. 10.1007/BF01273321 [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, … Ransohoff RM (2006). Control of microglial neurotoxicity by the fractalkine receptor. Nature Neuroscience, 9(7), 917–924. 10.1038/nn1715 [DOI] [PubMed] [Google Scholar]
- Carstensen L (1992). Motivation for social contact across the lifespan: a theory of socioemotional selectivity. Nebraska Symposium on Motivation, 40, 209–254. [PubMed] [Google Scholar]
- Carter CS (2007). Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behavioural Brain Research, 176(1), 170–186. 10.1016/j.bbr.2006.08.025 [DOI] [PubMed] [Google Scholar]
- Carter CS, & Keverne EB (2002). The neurobiology of social affiliation and pair bonding. Hormones, Brain and Behavior, 1, 299–337. [Google Scholar]
- Caruso C, Lio D, Cavallone L, & Franceschi C (2004). Aging, Longevity, Inflammation, and Cancer. Annals of the New York Academy of Sciences, 1028(1), 1–13. 10.1196/annals.1322.001 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, & Galvan A (2008). The adolescent brain. Developmental Review, 28(1), 62–77. 10.1016/j.dr.2007.08.003.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, & Jones R (2010). Neurobiology of the Adolescent Brain and Behavior : Implications for Substance Use Disorders. J Am Acad Child Adolesc Psychiatry, 49(12), 1189–1201. 10.1016/j.jaac.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Jones R, & Somerville L (2011). Braking and accelerating of the adolescent brain. J Res Adolesc, 21(1), 21–33. 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy BS, & Gutchess AH (2012). Structural Variation within the Amygdala and Ventromedial Prefrontal Cortex Predicts Memory for Impressions in Older Adults. Frontiers in Psychology, 3(AUG), 319 10.3389/fpsyg.2012.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy BS, Leshikar ED, Shih JY, Aizenman A, & Gutchess AH (2013). Valence-based age differences in medial prefrontal activity during impression formation. Social Neuroscience, 8(5), 462–473. 10.1080/17470919.2013.832373.Valence-Based [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy BS, Shih JY, & Gutchess AH (2012). Age-related changes to the neural correlates of social evaluation. Social Neuroscience, 7(6), 552–264. 10.1016/j.micinf.2011.07.011.Innate [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum A (2012). Alcohol metabolism. Clinics in Liver Disease, 16(4), 667–685. 10.1016/j.cld.2012.08.002.ALCOHOL [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality, S. A. and M. H. S. A. (2018). Results from the 2017 National Survey on Drug Use and Health: Detailed tables. Retrieved from http://www.samhsa.gov/
- Charuvastra A, & Cloitre M (2008). Social Bonds and Posttraumatic Stress Disorder. Annual Review of Psychology, 59(1), 301–328. 10.1146/annurev.psych.58.110405.085650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, & Trapp BD (2015). Microglia and neuroprotection. Journal of Neurochemistry, 136, 10–17. 10.1111/jnc.13062 [DOI] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, & Kavaliers M (2009). Neuroendocrinology of social information processing in rats and mice. Frontiers in Neuroendocrinology, 30(4), 442–459. 10.1016/j.yfrne.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson J-A, Korach KS, Muglia LJ, Pfaff DW, & Ogawa S (2003). An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proceedings of the National Academy of Sciences of the United States of America, 100(10), 6192–6197. 10.1073/pnas.0631699100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Creswell KG, Bachrach R, Clark DB, & Martin CS (2018). Adolescent Binge Drinking. Alcohol Research, 39(1), 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnello V, Petrocchi N, Farinelli M, & Ottaviani C (2017). Positive Social Interactions in a Lifespan Perspective with a Focus on Opioidergic and Oxytocinergic Systems: Implications for Neuroprotection. Current Neuropharmacology, 15, 543–561. 10.2174/1570159X14666160816120209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau N, Stewart S, & Loba P (2001). The relations of trait anxiety, anxiety sensitivity, and sensation seeking to adolescents’ motivations for alcohol, cigarette, and marijuana use. Addictive Behaviors, 26(6), 803–825. Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed5&NEWS=N&AN=2001423256 [DOI] [PubMed] [Google Scholar]
- Cooper ML (1994). Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychological Assessment, 6(2), 117–128. 10.1037/1040-3590.6.2.117 [DOI] [Google Scholar]
- Cooper ML, Kuntsche E, Levitt A, Barber LL, & Wolf S (2015). Motivational models of substance use: A review of theory and research on motives for using alcohol, marijuana, and tobacco In Oxford Handbook of Substance Use and Substance Use Disorders (pp. 375–421). Oxford, UK: Oxford University Press; 10.1093/oxfordhb/9780199381678.013.017 [DOI] [Google Scholar]
- Corona AW, Fenn AM, & Godbout JP (2012). Cognitive and behavioral consequences of impaired immunoregulation in aging. Journal of Neuroimmune Pharmacology, 7(1), 7–23. 10.1007/s11481-011-9313-4 [DOI] [PubMed] [Google Scholar]
- Corona AW, Huang Y, O’Connor JC, Dantzer R, Kelley KW, Popovich PG, & Godbout JP (2010). Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. Journal of Neuroinflammation, 7(1), 93 10.1186/1742-2094-7-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, & Coleman LG (2017). The role of neuroimmune signaling in alcoholism. Neuropharmacology, 122, 56–73. 10.1016/j.neuropharm.2017.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AJ, Hille C, & Slater P (1987). Subtraction autoradiography of opiate receptor subtypes in human brain. Brain Research, 418(2), 343–348. 10.1016/0006-8993(87)90101-6 [DOI] [PubMed] [Google Scholar]
- Crutzen R, Kuntsche E, & Schelleman-Offermans K (2013). Drinking motives and drinking behavior over time: A full cross-lagged panel study among adults. Psychology of Addictive Behaviors, 27(1), 197–201. 10.1037/a0029824 [DOI] [PubMed] [Google Scholar]
- Dannenhoffer CA, Kim EU, Saalfield J, Werner DF, & Spear LP (2018). Oxytocin and vasopressin modulation of social anxiety following adolescent intermittent ethanol exposure. Psychopharmacology, 235, 3065–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R (2004). Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. European Journal of Pharmacology, 500(1–3), 399–411. 10.1016/j.ejphar.2004.07.040 [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, & Kelley KW (1991). Androgen-dependent vasopressinergic neurotransmission attenuates interleukin-1-induced sickness behavior. Brain Research, 557(1–2), 115–120. 10.1016/0006-8993(91)90123-D [DOI] [PubMed] [Google Scholar]
- Dantzer R, & Kelley KW (2007). Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior, and Immunity, 21(2), 153–160. 10.1016/j.surg.2006.10.010.Use [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Patricia Chou S, June Ruan W, & Grant BF (2008). Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcoholism: Clinical and Experimental Research, 32(12), 2149–2160. 10.1111/j.1530-0277.2008.00806.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T, Kudinova A, Lovelock DF, Gibb BE, & Hennessy MB (2017). A multispecies approach for understanding neuroimmune mechanisms of stress. Dialogues in Clinical Neuroscience, 19, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Stella N, & Zimmer A (2015). Endocannabinoid signalling and the deteriorating brain. Nature Reviews Neuroscience, 16(1), 30–42. 10.1530/ERC-14-0411.Persistent [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBenedictis BT, Nussbaum ER, Cheung HK, & Veenema AH (2017). Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. Journal of Comparative Neurology, 525(11), 2549–2570. 10.1002/cne.24216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Engelmann M, & Landgraf R (1998). The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides, 19(6), 999–1005. 10.1016/S0196-9781(98)00047-3 [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, & Landgraf R (1998). Olfactory bulb norepinephrine depletion abolishes vasopressin and oxytocin preservation of social recognition responses in rats. Neuroscience Letters, 254(3), 161–164. 10.1016/S0304-3940(98)00691-0 [DOI] [PubMed] [Google Scholar]
- Doenni VM, Gray JM, Song CM, Patel S, Hill MN, & Pittman QJ (2016). Deficient adolescent social behavior following early-life inflammation is ameliorated by augmentation of anandamide signaling. Brain, Behavior, and Immunity, 58, 237–247. 10.1016/j.bbi.2016.07.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan NJ, Okereke OI, Vannini P, Amariglio RE, Rentz DM, Marshall GA, … Sperling RA (2016). Association of Higher Cortical Amyloid Burden With Loneliness in Cognitively Normal Older Adults. JAMA Psychiatry, 73(12), 1230–1237. 10.1001/jamapsychiatry.2016.2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Buck HM, Bordner K, Richey L, Jones ME, & Deak T (2014). Intoxication- and Withdrawal-Dependent Expression of Central and Peripheral Cytokines Following Initial Ethanol Exposure. Alcoholism: Clinical and Experimental Research, 38(8), 2186–2198. 10.1111/acer.12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Gano A, Paniccia JE, & Deak T (2015). Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiology and Behavior, 148, 131–144. 10.1016/j.physbeh.2015.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, & Spear LP (2003). Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats, 75, 411–418. 10.1016/S0091-3057(03)00134-5 [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, & Spear LP (2004). Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Developmental Psychobiology, 45(3), 153–162. 10.1002/dev.20025 [DOI] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Bredewold R, & Veenema AH (2016). Role of the oxytocin system in amygdala subregions in the regulation of social interest in male and female rats. Neuroscience, 330, 138–149. 10.1016/j.neuroscience.2016.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Immormino MA, Bredewold R, & Veenema AH (2016). Involvement of the oxytocin system in the bed nucleus of the stria terminalis in the sex-specific regulation of social recognition. Psychoneuroendocrinology, 64, 79–88. 10.1016/j.psyneuen.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, & Veenema AH (2013). Sex differences in oxytocin receptor binding in forebrain regions: Correlations with social interest in brain region- and sex- specific ways. Hormones and Behavior, 64(4), 693–701. 10.1016/j.yhbeh.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Dunbar RIM (2009). The social brain hypothesis and its implications for social evolution. Annals of Human Biology, 36(5), 562–573. 10.1080/03014460902960289 [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, … Aflairs S (1998). Effects of Moderate Alcohol Consumption on the Central Nervous System. Alcoholism: Clinical and Experimental Research, 22(5), 998–1040. [DOI] [PubMed] [Google Scholar]
- Eggen BJL, Raj D, Hanisch UK, & Boddeke HWGM (2013). Microglial phenotype and adaptation. Journal of Neuroimmune Pharmacology, 8(4), 807–823. 10.1007/s11481-013-9490-4 [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, & Wilhelmsen KC (2006). Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcoholism: Clinical and Experimental Research, 30(11), 1856–1865. 10.1111/j.1530-0277.2006.00222.x [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Moieni M, Inagaki TK, Muscatell KA, & Irwin MR (2017). In Sickness and in Health : The Co-Regulation of Inflammation and Social Behavior. Neuropsychopharmacology, 42, 242–253. 10.1038/npp.2016.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, … Conboy IM (2014). Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nature Communications, 5(1762), 1–11. 10.1038/ncomms5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson EK, Grantham EK, Warden AS, & Harris RA (2019). Neuroimmune signaling in alcohol use disorder. Pharmacology Biochemistry and Behavior, 177(December 2018), 34–60. 10.1016/j.pbb.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, & Fudge JL (2009). A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews, 33(3), 367–382. 10.1016/j.neubiorev.2008.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, & Robbins TW (2008). Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1507), 3125–3135. 10.1098/rstb.2008.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden VB (2006). Trends in initiation of alcohol use in the United States 1975 to 2003. Alcoholism: Clinical and Experimental Research, 30(6), 1011–1022. 10.1111/j.1530-0277.2006.00115.x [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, & Tye KM (2016). Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience, 321, 197–209. 10.1016/j.neuroscience.2015.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, & Tye KM (2014). Amygdala Inputs to the Ventral Hippocampus Bidirectionally Modulate Social Behavior. Journal of Neuroscience, 34(2), 586–595. 10.1523/JNEUROSCI.4257-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Smith KM, Lovett-Racke AE, Guerau-de-Arellano M, Whitacre CC, & Godbout JP (2013). Increased micro-RNA 29b in the aged brain correlates with the reduction of insulin-like growth factor-1 and fractalkine ligand. Neurobiology of Aging, 34(12), 2748–2758. 10.1016/j.neurobiolaging.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, & Young LJ (2001). Oxytocin in the medial amygdala is essential for social recognition in the mouse. The Journal of Neuroscience, 21(20), 8278–8285. https://doi.org/21/20/8278 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidalgo TM, Da Silveira ED, & Da Silveira DX (2008). Psychiatric comorbidity related to alcohol use among adolescents. American Journal of Drug and Alcohol Abuse, 34(1), 83–89. 10.1080/00952990701764664 [DOI] [PubMed] [Google Scholar]
- Filiano AJ, Gadani SP, & Kipnis J (2015). Interactions of innate and adaptive immunity in brain development and function. Brain Research, 1617, 18–27. 10.1016/j.brainres.2014.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishkin RJ, & Winslow JT (1997). Endotoxin-induced reduction of social investigation by mice: Interaction with amphetamine and anti-inflammatory drugs. Psychopharmacology, 132(4), 335–341. 10.1007/s002130050353 [DOI] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, & Maier SF (2015). Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain, Behavior, and Immunity, 45, 171–179. 10.1016/j.bbi.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Weil ZM, & Nelson RJ (2013). Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain, Behavior, and Immunity, 34, 159–163. 10.1016/j.bbi.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Franceschi C, & Campisi J (2014). Chronic inflammation (Inflammaging) and its potential contribution to age-associated diseases. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 69, S4–S9. 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, & Maier SF (2010). IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain, Behavior, and Immunity, 24(2), 254–262. 10.1016/j.bbi.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, & Maier SF (2010). Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. Journal of Neuroimmunology, 226(1–2), 181–184. 10.1016/j.jneuroim.2010.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, & Young LJ (2016). Comparative Perspectives on Oxytocin and Vasopressin Receptor Research in Rodents and Primates: Translational Implications. Journal of Neuroendocrinology, 28(4). 10.1111/jne.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, … Castanon N (2007). Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology, 32(5), 516–531. 10.1016/j.psyneuen.2007.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedemann M, & Gerhardt GA (1992). Regional effects of aging on dopaminergic function in the Fischer-344 rat. Neurobiology of Aging, 13, 325–332. [DOI] [PubMed] [Google Scholar]
- Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, & Choleris E (2012). Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behavioral Neuroscience, 126(1), 97–109. 10.1037/a0026464 [DOI] [PubMed] [Google Scholar]
- Galic MA, Riazi K, & Pittman QJ (2012). Cytokines and brain excitability. Frontiers in Neuroendocrinology, 33(1), 116–125. 10.1016/j.yfrne.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano A, Doremus-fitzwater TL, & Deak T (2017). A cross-sectional comparison of ethanol-related cytokine expression in the hippocampus of young and aged Fischer 344 rats. Neurobiology of Aging, 54, 40–53. 10.1016/j.neurobiolaging.2017.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano A, Doremus-Fitzwater TL, & Deak T (2016). Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain Research, 1646, 62–72. 10.1016/j.brainres.2016.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, & Steinberg L (2005). Peer Influence on Risk Taking, Risk Preference, and Risky Decision Making in Adolescence and Adulthood : An Experimental Study. Developmental Psychology, 41(4), 625–635. 10.1037/0012-1649.41.4.625 [DOI] [PubMed] [Google Scholar]
- Girard-Joyal O, Faragher A, Bradley K, Kane L, Hrycyk L, & Ismail N (2015). Age and sex differences in c-Fos expression and serum corticosterone concentration following LPS treatment. Neuroscience, 305, 293–301. 10.1016/j.neuroscience.2015.06.035 [DOI] [PubMed] [Google Scholar]
- Gmel G, Gaume J, Faouzi M, Kulling JP, & Daeppen JB (2008). Who drinks most of the total alcohol in young men - Risky single occasion drinking as normative behaviour. Alcohol and Alcoholism, 43(6), 692–697. 10.1093/alcalc/agn070 [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine a F., Berg BM, Kelley KW, & Johnson RW (2005). Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. The FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 19(10), 1329–1331. 10.1096/fj.05-3776fje [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Hansen MK, Anderson K, Maier SF, & Watkins LR (2000). Vagal immune-to-brain communication: A visceral chemosensory pathway. Autonomic Neuroscience: Basic and Clinical, 85(1–3), 49–59. 10.1016/S1566-0702(00)00219-8 [DOI] [PubMed] [Google Scholar]
- Gordon NS, Kollack-Walker S, Akil H, & Panksepp J (2002). Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Research Bulletin, 57(5), 651–659. 10.1016/S0361-9230(01)00762-6 [DOI] [PubMed] [Google Scholar]
- Goudsmit E, Fliers E, & Swaab DF (1988). Vasopressin and oxytocin excretion in the Brown-Norway rat in relation to aging, water metabolism and testosterone. Mechanisms of Ageing and Development, 44, 241–252. [DOI] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, & Lynch MA (2006). The age-related attenuation in long-term potentiation is associated with microglial activation. Journal of Neurochemistry, 99(4), 1263–1272. 10.1111/j.1471-4159.2006.04165.x [DOI] [PubMed] [Google Scholar]
- Guan X, & Dluzen DEDE (1994). Age related changes of social memory/recognition in male fischer 344 rats. Behavioural Brain Research, 61, 87–90. [DOI] [PubMed] [Google Scholar]
- Guijarro A, Laviano A, & Meguid MM (2006). Hypothalamic integration of immune function and metabolism. Progress in Brain Research, 153, 367–405. 10.1016/S0079-6123(06)53022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbas S, Santello M, Pryce CR, Volterra A, Habbas S, Santello M, … Liaudet N (2015). Neuroinflammatory TNF a Impairs Memory via Article Neuroinflammatory TNF a Impairs Memory via Astrocyte Signaling. Cell, 163, 1730–1741. 10.1016/j.cell.2015.11.023 [DOI] [PubMed] [Google Scholar]
- Ham LS, & Hope DA (2003). College students and problematic drinking : A review of the literature. Clinical Psychology Review, 23, 719–759. 10.1016/S0272-7358(03)00071-0 [DOI] [PubMed] [Google Scholar]
- Hammond TR, Dufort C, Dissing-olesen L, Piao X, Mccarroll SA, Hammond TR, … Wysoker A (2019). Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes Resource Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-. Immunity, 50(1), 253–271. 10.1016/j.immuni.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BH, Moore AA, Sherman S, Keyes KM, & Palamar JJ (2017). Demographic trends of binge alcohol use and alcohol use disorders among older adults in the United States, 2005–2014. Drug and Alcohol Dependence, 170, 198–207. 10.1016/j.drugalcdep.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Kapás L, Fang J, & Krueger JM (1998). Cafeteria diet-induced sleep is blocked by subdiaphragmatic vagotomy in rats. The American Journal of Physiology, 274, R168–74. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9458914 [DOI] [PubMed] [Google Scholar]
- Hansen M, Taishi P, Chen Z, & Krueger JM (1998). Cafeteria feeding induces interleukin-1β mRNA expression in rat liver and brain. American Journal of Physiology, 274(6), R1734–R1739. 10.1152/ajpregu.1998.274.6.r1734 [DOI] [PubMed] [Google Scholar]
- Hart AD, Wyttenbach A, Hugh Perry V, & Teeling JL (2012). Age related changes in microglial phenotype vary between CNS regions: Grey versus white matter differences. Brain, Behavior, and Immunity, 26(5), 754–765. 10.1016/j.bbi.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL (1988). Biological basis of the behavior of sick animals. Neuroscience & Biobehavioral Reviews, 12, 123–137. [DOI] [PubMed] [Google Scholar]
- Hawker DSJ, & Boulton MJ (2000). Twenty Years ‘ Research on Peer Victimization and Psychosocial Maladjustment : A Meta-analytic Review of Cross-sectional Studies. J Child Psychol Psychiat, 41(4), 441–455. [PubMed] [Google Scholar]
- He J, & Crews FT (2008). Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental Neurology, 210(2), 349–358. 10.1016/j.expneurol.2007.11.017.Increased [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MA, & Gerhardt GA (1998). Normal and drug-induced locomotor behavior in aging: Comparison to evoked DA release and tissue content in Fischer 344 rats. Brain Research, 797(1), 42–54. 10.1016/S0006-8993(98)00370-9 [DOI] [PubMed] [Google Scholar]
- Heilig M, Epstein DH, Nader MA, & Shaham Y (2016). Time to connect: bringing social context into addiction neuroscience. Nature Reviews Neuroscience, 17(9), 592–599. 10.1038/nrn.2016.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, & Schiml PA (2014). Sociality and sickness: Have cytokines evolved to serve social functions beyond times of pathogen exposure? Brain, Behavior, and Immunity, 37, 15–20. 10.1016/j.bbi.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, & Godbout JP (2009). Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain, Behavior, and Immunity, 23(3), 309–317. 10.1016/j.bbi.2008.09.002 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdegen T, & Leah JD (1998). Inducible and constitutive transcription factors in the mammalian nervous system: Control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Research Reviews, 28(3), 370–490. 10.1016/S0165-0173(98)00018-6 [DOI] [PubMed] [Google Scholar]
- Hess GD, Joseph JA, & Roth GS (1981). Effect of age on sensitivity to pain and brain opiate receptors. Neurobiology of Aging, 2(1), 49–55. 10.1016/0197-4580(81)90059-2 [DOI] [PubMed] [Google Scholar]
- Heyen JRR, Ye S Finck B. N. ming, & Johnson RW (2000). Interleukin (IL)-10 inhibits IL-6 production in microglia by preventing activation of NF-kB. Molecular Brain Research, 77(1), 138–147. 10.1016/S0169-328X(00)00042-5 [DOI] [PubMed] [Google Scholar]
- Hicks C, Ramos L, Reekie T. a, Narlawar R, Kassiou M, & McGregor IS (2015). WAY 267,464, a non-peptide oxytocin receptor agonist, impairs social recognition memory in rats through a vasopressin 1A receptor antagonist action. Psychopharmacology, 2659–2667. 10.1007/s00213-015-3902-5 [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, & Winter MR (2006). Age at Drinking Onset and Alcohol Dependence. Archives of Pediatrics & Adolescent Medicine, 160(7), 739 10.1001/archpedi.160.7.739 [DOI] [PubMed] [Google Scholar]
- Holahan CJ, Schutte KK, Brennan PL, Holahan CK, & Moos RH (2014). Episodic Heavy Drinking and 20-Year Total Mortality Among Late-Life Moderate Drinkers. Alcoholism: Clinical and Experimental Research, 38(5), 1432–1438. 10.1111/acer.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, & Umberson D (1988). Social relationships and health. Science (New York, N.Y.), 241(4865), 540–545. 10.1126/science.3399889 [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, & Godbout JP (2008). Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiology of Aging, 29(11), 1744–1753. https://doi.org/S0197-4580(07)00176-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GE, Van Nieuwenhuijzen PS, Chan-Ling T, & McGregor IS (2011). “When an old rat smells a cat”: A decline in defense-related, but not accessory olfactory, Fos expression in aged rats. Neurobiology of Aging, 32(4), 737–749. 10.1016/j.neurobiolaging.2009.03.014 [DOI] [PubMed] [Google Scholar]
- Ishunina TA, & Swaab DF (1999). Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus: size changes in relation to age and sex. J Clin Endocrinol Metab, 84(12), 4637–4644. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10599731 [DOI] [PubMed] [Google Scholar]
- Johnson I (2000). ALCOHOL PROBLEMS IN OLD AGE : A REVIEW OF RECENT EPIDEMIOLOGICAL RESEARCH. International Journal of Geriatric Psychiatry, 15, 575–581. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, & Young LJ (2017). Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neuroscience and Biobehavioral Reviews, 76, 87–98. 10.1016/j.neubiorev.2017.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson Z, Walum H, Xiao Y, Riefkohl PC, & Young LJ (2017). Oxytocin receptors modulate a social salience neural network in male prairie voles. Hormones and Behavior, 87, 16–24. 10.1016/j.yhbeh.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L, Miech R, O’Malley P, Bachman J, Schulenberg J, & Patrick M (2019). Monitoring the Future national survey results on drug use, 1975–2017: Overview, key findings on adolescent drug use. Ann Arbor. [Google Scholar]
- Jurgens HA, & Johnson RW (2012). Dysregulated neuronal–microglial cross-talk during aging, stress and inflammation. Experimental Neurology, 233(1), 40–48. 10.1016/j.expneurol.2010.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant H (1998). Research on Tolerance: What Can We Learn From History? Alcoholism: Clinical and Experimental Research, 22(1), 67–76. 10.1111/j.1530-0277.1998.tb03618.x [DOI] [PubMed] [Google Scholar]
- Kanny D, Naimi TS, Liu Y, Lu H, & Brewer RD (2018). Annual Total Binge Drinks Consumed by U.S. adults, 2015. American Journal of Preventive Medicine, 54(4), 486–496. 10.1016/j.amepre.2017.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K, & DeVries A (2011). Modeling social influences on human health. Psychosomatic Medicine, 73(1), 67–74. 10.1097/PSY.0b013e3182002116.Modeling [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K, Stuller KA, Jarrett B, Zhang N, Wells J, Norman GJ, & DeVries AC (2011). Oxytocin Mediates Social Neuroprotection After Cerebral Ischemia. Stroke, 42(12), 3606–3611. 10.1161/STROKEAHA.111.628008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Weiner JL, & Jones SR (2016). Early-life social isolation stress increases kappa opioid receptor responsiveness and downregulates the dopamine system. Neuropsychopharmacology, 41(9), 2263–2274. 10.1038/npp.2016.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, & Choleris E (2011). Sociality, pathogen avoidance, and the neuropeptides oxytocin and arginine vasopressin. Psychological Science, 22(11), 1367–1374. 10.1177/0956797611420576 [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng J, & Ji R (2008). Cytokine Mechanisms of Central Sensitization : Distinct and Overlapping Role of Interleukin-1b, Interleukin-6, and Tumor Necrosis Factor alpha in Regulating Synaptic and Neuronal Activity in the Superficial Spinal Cord. Journal of Neuroscience, 28(20), 5189–5194. 10.1523/JNEUROSCI.3338-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ME, Hatzinger M, Wotjak CT, Landgraf R, Holsboer F, & Neumann ID (2000). Ageing alters intrahypothalamic release patterns of vasopressin and oxytocin in rats. European Journal of Neuroscience, 12(4), 1487–1494. 10.1046/j.1460-9568.2000.00030.x [DOI] [PubMed] [Google Scholar]
- Kelly S, Olanrewaju O, Cowan A, Brayne C, & Lafortune L (2018). Alcohol and older people : A systematic review of barriers, facilitators and context of drinking in older people and implications for intervention design. PLoS ONE, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, & Adolphs R (2012). The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences, 16(11), 559–572. 10.1016/j.tics.2012.09.006.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch U-K, Noda M, & Verkhratsky A (2011). Physiology of microglia. Physiological Reviews, 91(2), 461–553. 10.1152/physrev.00011.2010 [DOI] [PubMed] [Google Scholar]
- Kieffer BL (1995). Recent advances in molecular recognition and signal transduction of active peptides: receptors for opioid peptides. TL - 15. Cellular and Molecular Neurobiology, 15 VN-r(6), 615–635. 10.1007/BF02071128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M, Goepfrich A, Friemel CM, & Schneider M (2011). AAV-Mediated Overexpression of the CB1 Receptor in the mPFC of Adult Rats Alters Cognitive Flexibility, Social Behavior, and Emotional Reactivity. Frontiers in Behavioral Neuroscience, 5(July), 1–10. 10.3389/fnbeh.2011.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Veeneman J, Combe C, Poole S, Luheshi GN, Dantzer R, & Se V (2008). Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. European Journal of Neuroscience, 28, 2499–2510. 10.1111/j.1460-9568.2008.06549.x [DOI] [PubMed] [Google Scholar]
- Koob GF (2015). The dark side of emotion: The addiction perspective. European Journal of Pharmacology, 753, 73–87. 10.1016/j.ejphar.2014.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (1997). Drug abuse: hedonic homeostatic dysregulation. Science, 278(5335), 52–58. 10.1126/science.278.5335.52 [DOI] [PubMed] [Google Scholar]
- Kotz CM, Weldon D, Billington CJ, & Levine AS (2004). Age-related changes in brain proDynorphin gene expression in the rat. Neurobiology of Aging, 25(10), 1343–1347. 10.1016/j.neurobiolaging.2004.02.025 [DOI] [PubMed] [Google Scholar]
- Kraemer S, & Apfelbach R (2004). Olfactory sensitivity, learning and cognition in young adult and aged male Wistar rats. Physiology & Behavior, 81(3), 435–442. 10.1016/j.physbeh.2004.01.012 [DOI] [PubMed] [Google Scholar]
- Krueger JM (2008). The role of cytokines in sleep regulation. Curr Pharm Des, 14(32), 3408–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerbis A, Sacco P, & Moore AA (2014). Substance Abuse Among Older Adults. Clin Geriatr Med, 30, 629–654. 10.1016/j.cger.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, & Engels R (2006). Who drinks and why? A review of socio-demographic, personality, and contextual issues behind the drinking motives in young people. Addictive Behaviors, 31(10), 1844–1857. 10.1016/j.addbeh.2005.12.028 [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Rossow I, Engels R, & Kuntsche S (2016). Is ‘ age at first drink ‘ a useful concept in alcohol research and prevention? We doubt that. Addiction, 111, 957–965. 10.1111/add.12980 [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Wiers RW, Janssen T, & Gmel G (2010). Same Wording, Distinct Concepts ? Testing Differences Between Expectancies and Motives in a Mediation Model of Alcohol Outcomes. Experimental and Clinical Psychopharmacology, 18(5), 436–444. 10.1037/a0019724 [DOI] [PubMed] [Google Scholar]
- Labhart F, Kuntsche E, Wicki M, & Gmel G (2017). Reciprocal Influences of Drinking Motives on Alcohol Use and Related Consequences: A Full Cross-Lagged Panel Study Among Young Adult Men. Behavioral Medicine, 43(4), 277–284. [DOI] [PubMed] [Google Scholar]
- Labrie J, Lamb T, & Pedersen E (2008). Changes in drinking patterns across the transition to college among first-year college males. J Child Adolesc Subst Abuse, 18(1), 1–15. 10.1080/15470650802526500.Changes [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina MJ, Rivera PD, & Bilbo SD (2017). Glial and Neuroimmune Mechanisms as Critical Modulators of Drug Use and Abuse. Neuropsychopharmacology, 42(1), 156–177. 10.1038/npp.2016.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang FR (2001). Regulation of social relationships in later adulthood. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 56(6), P321–P326. 10.1093/geronb/56.6.P321 [DOI] [PubMed] [Google Scholar]
- Laurent V, Morse AK, & Balleine BW (2015). The role of opioid processes in reward and decision-making. British Journal of Pharmacology, 172, 449–459. 10.1111/bph.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, & Gordon S (1990). Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience, 39(1), 151–170. 10.1016/0306-4522(90)90229-W [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, & Kieffer BL (2009). Reward Processing by the Opioid System in the Brain. Physiological Reviews, 89(4), 1379–1412. 10.1152/physrev.00005.2009.Reward [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moal M, Dantzer R, Michaud B, & Koob G (1987). Centrally injected arginine vasopressin (AVP) facilitates social memory in rats. Neuroscience Letters, 77, 353–359. [DOI] [PubMed] [Google Scholar]
- Le Thuc O, Blondeau N, Nahon JL, & Rovere C (2015). The complex contribution of chemokines to neuroinflammation: Switching from beneficial to detrimental effects. Annals of the New York Academy of Sciences, 1351(1), 127–140. 10.1111/nyas.12855 [DOI] [PubMed] [Google Scholar]
- Lesscher HMB, Spoelder M, Rotte MD, Janssen MJ, Hesseling P, Lozeman-van JG, … Vanderschuren LJMJ (2015). Early social isolation augments alcohol consumption in rats. Behavioural Pharmacology, 26, 673–680. 10.1097/FBP.0000000000000165 [DOI] [PubMed] [Google Scholar]
- Lewis BA, & O’Neill HK (2000). ALCOHOL EXPECTANCIES AND SOCIAL DEFICITS RELATING TO PROBLEM DRINKING AMONG COLLEGE STUDENTS. Addictive Behaviors, 25(2), 295–299. [DOI] [PubMed] [Google Scholar]
- Li Q, & Barres BA (2018). Microglia and macrophages in brain homeostasis and disease. Nature Reviews Immunology, 18, 225–242. 10.1038/nri.2017.125 [DOI] [PubMed] [Google Scholar]
- Licinio J, & Wong ML (1997). Pathways and mechanisms for cytokine signaling of the central nervous system. Journal of Clinical Investigation, 100(12), 2941–2947. 10.1172/JCI119846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Straccia MA, Meyer ML, Du M, & Tan KM (2019). Social, self, (situational), and affective processes in medial prefrontal cortex (MPFC): Causal, multivariate, and reverse inference evidence. Neuroscience and Biobehavioral Reviews, 99(November 2018), 311–328. 10.1016/j.neubiorev.2018.12.021 [DOI] [PubMed] [Google Scholar]
- Lim MM, & Young LJ (2006). Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and Behavior, 50(4), 506–517. 10.1016/j.yhbeh.2006.06.028 [DOI] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-jones EA, & Szabo G (2013). Alcohol-induced IL-1b in the brain is mediated by NLRP3 / ASC inflammasome activation that amplifies neuroinflammation. Journal of Leukocyte Biology, 94, 171–182. 10.1189/jlb.1212659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little P, Kuhn C, Wilson WA, & Swartzwelder HS (1996). Differential Effects of Ethanol in Adolescent and Adult Rats. Alcoholism: Clinical and Experimental Research, 20(8), 1346–1351. [DOI] [PubMed] [Google Scholar]
- Lockhart SN, & DeCarli C (2014). Structural Imaging Measures of Brain Aging. Neuropsychology Review, 24(3), 271–289. 10.1007/s11065-014-9268-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez B, Turner RJ, & Saavedra LM (2005). Anxiety and risk for substance dependence among late adolescents/young adults. Anxiety Disorders, 19, 275–294. 10.1016/j.janxdis.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, & Neumann ID (2011). The Neuropeptide Oxytocin Facilitates Pro-Social Behavior and Prevents Social Avoidance in Rats and Mice. Neuropsychopharmacology, 36(11), 2159–2168. 10.1038/npp.2011.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh M, Earleywine M, & Dunn ME (2006). Alcohol expectancies for social facilitation : A short form with decreased bias. Addictive Behaviors, 31, 1536–1546. 10.1016/j.addbeh.2005.11.009 [DOI] [PubMed] [Google Scholar]
- Maclean MG, & Lecci L (2000). A Comparison of Models of Drinking Motives in a University Sample. Psychology of Addictive Behaviors, 14(1), 83–87. 10.1037//0893-164X.14.1.83 [DOI] [PubMed] [Google Scholar]
- Manduca A, Morena M, Campolongo P, Servadio M, Palmery M, Trabace L, … Trezza V (2015). Distinct roles of the endocannabinoids anandamide and 2-arachidonoylglycerol in social behavior and emotionality at different developmental ages in rats. European Neuropsychopharmacology, 25(8), 1362–1374. 10.1016/j.euroneuro.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Markel E, Felszeghy K, Luiten PGM, & Nyakas C (1995). Beneficial effect of chronic nimodipine treatment on behavioral dysfunctions of aged rats exposed to perinatal ethanol treatment. Archives of Gerontology and Geriatrics, 21, 75–88. [DOI] [PubMed] [Google Scholar]
- Masten AS, Faden VB, Zucker RA, & Spear LP (2009). A developmental perspective on underage alcohol use. Alcohol Research & Health, 32(1), 3–16. [PMC free article] [PubMed] [Google Scholar]
- Masten AS, & Obradović J (2006). Competence and resilience in development. Annals of the New York Academy of Sciences, 1094, 13–27. 10.1196/annals.1376.003 [DOI] [PubMed] [Google Scholar]
- Mathieson WB, Federico P, Veale WL, & Pittman QJ (1989). Single-unit activity in the bed nucleus of the stria terminalis during fever. Brain Research, 486(1), 49–55. 10.1016/0006-8993(89)91276-6 [DOI] [PubMed] [Google Scholar]
- Matsunaga W, Miyata S, Takamata A, Bun H, Nakashima T, & Kiyohara T (2000). LPS-induced Fos expression in oxytocin and vasopressin neurons of the rat hypothalamus. Brain Research, 858(1), 9–18. 10.1016/S0006-8993(99)02418-X [DOI] [PubMed] [Google Scholar]
- Matthews DB, & Mittleman G (2017). Age-dependent effects of chronic intermittent ethanol treatment: Gross motor behavior and body weight in aged, adult and adolescent rats. Neuroscience Letters, 657, 146–150. 10.1016/j.neulet.2017.08.012 [DOI] [PubMed] [Google Scholar]
- Mayfield J, Ferguson L, & Harris RA (2013). Neuroimmune signaling : a key component of alcohol abuse. Current Opinion in Neurobiology, 23, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, & Chappell AM (2009). Early social isolation in male long-evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcoholism: Clinical and Experimental Research, 33(2), 273–282. 10.1111/j.1530-0277.2008.00830.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccusker RH, & Kelley KW (2013). Immune – neural connections : how the immune system ʼ s response to infectious agents influences behavior, 84–98. 10.1242/jeb.073411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P, & Seitz HK (2008). Age, alcohol metabolism and liver disease. Curr Opin Clin Nutr Metab Care, 11(1), 21–26. 10.1097/MCO.0b013e3282f30564\r00075197-200801000-00005 [pii] [DOI] [PubMed] [Google Scholar]
- Meisel SN, Colder CR, Bowker JC, & Hussong AM (2018). A Longitudinal Examination of Mediational Pathways Linking Chronic Victimization and Exclusion to Adolescent Alcohol Use. Developmental Psychology, 54(9), 1795–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, … Russo SJ (2017). Social stress induces neurovascular pathology promoting depression. Nature Neuroscience, 20(12), 1752–1760. 10.1038/s41593-017-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencio-Wszalek T, Ramirez VD, & Dluzen DE (1992). Age-dependent changes in olfactory-mediated behavioral investigations in the male rat. Behavioral and Neural Biology, 57, 205–212. [DOI] [PubMed] [Google Scholar]
- Míguez JM, Aldegunde M, Paz-Valiñas L, Recio J, & Sánchez-Barceló E (1999). Selective changes in the contents of noradrenaline, dopamine and serotonin in rat brain areas during aging. Journal of Neural Transmission, 106(11–12), 1089–1098. 10.1007/s007020050225 [DOI] [PubMed] [Google Scholar]
- Mills KL, Lalonde F, Clasen LS, Giedd JN, & Blakemore SJ (2014). Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience, 9(1), 123–131. 10.1093/scan/nss113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura H, Qiao H, & Ohta T (2002). Influence of aging and social isolation on changes in brain monoamine turnover and biosynthesis of rats elicited by novelty stress. Synapse, 46(2), 116–124. 10.1002/syn.10133 [DOI] [PubMed] [Google Scholar]
- Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, … Dandona P (2004). Evidence for a potent antiinflammatory effect of rosiglitazone. Journal of Clinical Endocrinology and Metabolism, 89(6), 2728–2835. 10.1210/jc.2003-032103 [DOI] [PubMed] [Google Scholar]
- Moore AA, Endo JO, & Carter MK (2003). Is there a relationship between excessive drinking and functional impairment in older persons? Journal of the American Geriatrics Society, 51(1), 44–49. 10.1034/j.1601-5215.2002.51008.x [DOI] [PubMed] [Google Scholar]
- Moore AA, Giuli L, Gould R, Hu P, Zhou K, Reuben D, … Karlamangla A (2006). Alcohol use, comorbidity, and mortality. Journal of the American Geriatrics Society, 54(5), 757–762. 10.1111/j.1532-5415.2006.00728.x [DOI] [PubMed] [Google Scholar]
- Moore AA, Karno MP, Grella CE, Lin JC, Warda U, Liao DH, & Hu P (2009). Alcohol, tobacco, and nonmedical drug use in older U.S. adults: Data from the 2001/02 national epidemiologic survey of alcohol and related conditions. Journal of the American Geriatrics Society, 57(12), 2275–2281. 10.1111/j.1532-5415.2009.02554.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AA, Whiteman EJ, & Ward KT (2007). Risks of combined alcohol/medication use in older adults. American Journal Geriatric Pharmacotherapy, 5(1), 64–74. 10.1016/j.amjopharm.2007.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Brennan PL, Schutte KK, & Moos BS (2005). Older adults’ health and changes in late-life drinking patterns. Aging and Mental Health, 9(1), 49–59. 10.1080/13607860412331323818 [DOI] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, & Spear LP (2014). Pre-pubertal gonadectomy and the social consequences of acute ethanol in adolescent male and female rats. Hormones and Behavior, 66(2), 209–219. 10.1016/j.yhbeh.2014.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Jolly E, & Mitchell JP (2012). Social-Cognitive Deficits in Normal Aging. Journal of Neuroscience, 32(16), 5553–5561. 10.1523/JNEUROSCI.5511-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, & Fromme K (2012). Age of First Use and Delay to First Intoxication in Relation to Trajectories of Heavy Drinking and Alcohol-Related Problems During Emerging Adulthood. Alcoholism: Clinical and Experimental Research, 36(11), 1991–1999. 10.1111/j.1530-0277.2012.01812.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Kong G, Camenga DR, Cavallo DA, Connell C, & Krishnan-Sarin S (2014). First drink to first drunk: Age of onset and delay to intoxication are associated with adolescent alcohol use and binge drinking. Alcoholism: Clinical and Experimental Research, 38(10), 2615–2621. 10.1111/acer.12526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, L’Insalata A, Butler ER, McKee A, & Krishnan-Sarin S (2018). Age at drinking onset, age at first intoxication, and delay to first intoxication: Assessing the concurrent validity of measures of drinking initiation with alcohol use and related problems. Addictive Behaviors, 79(September 2017), 195–200. 10.1016/j.addbeh.2017.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadon NL (2006). Exploiting the rodent model for studies on the pharmacology of lifespan extension. Aging Cell, 5(1), 9–15. 10.1111/j.1474-9726.2006.00185.x [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Gill TM, Nicolle M, & Gallagher M (1996). Alterations in opiate receptor binding in the hippocampus of aged Long-Evans rats. Brain Research, 707(1), 22–30. 10.1016/0006-8993(95)01211-7 [DOI] [PubMed] [Google Scholar]
- Naylor AM, Pittman QJ, & Veale WL (1988). Stimulation of vasopressin release in the ventral septum of the rat brain suppresses prostaglandin E1 fever. The Journal of Physiology, 399(1), 177–189. 10.1113/jphysiol.1988.sp017074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, & Helmchen F (2005). Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science, 308(May), 1314–1318. 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- Norden DM, & Godbout JP (2013). Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathology and Applied Neurobiology, 39(1), 19–34. 10.1111/j.1365-2990.2012.01306.x.Microglia [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Trojanowski PJ, Walker FR, & Godbout JP (2016). Insensitivity of Astrocytes to Interleukin-10 Signaling following Peripheral Immune Challenge Results in Prolonged Microglial Activation in the Aged Brain. Neurobiology of Aging, 44, 22–41. 10.1016/j.neurobiolaging.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt KV, & Nguyen JMK (2014). Female juvenile play elicits Fos expression in dopaminergic neurons of the VTA. Behavioral Neuroscience, 128(2), 178–186. 10.1037/a0035964 [DOI] [PubMed] [Google Scholar]
- Novier A, Diaz-Granados JL, & Matthews DB (2015). Alcohol use across the lifespan: An analysis of adolescent and aged rodents and humans. Pharmacology Biochemistry and Behavior, 133, 65–82. 10.1016/j.pbb.2015.03.015 [DOI] [PubMed] [Google Scholar]
- Novier A, Ornelas LC, Diaz-Granados JL, & Matthews DB (2016). Differences in Behavioral Responding in Adult and Aged Rats Following Chronic Ethanol Exposure. Alcoholism: Clinical and Experimental Research, 40(7), 1462–1472. 10.1111/acer.13098 [DOI] [PubMed] [Google Scholar]
- Novier A, Skike CE Van, Diaz-Granados JL, Mittleman G, Matthews DB, Van Skike CE, … Matthews DB (2013). Acute alcohol produces ataxia and cognitive impairments in aged animals: A comparison between young adult and aged rats. Alcoholism: Clinical and Experimental Research, 37(8), 1317–1324. 10.1111/acer.12110 [DOI] [PubMed] [Google Scholar]
- Oberle E, Guhn M, Gadermann AM, Thomson K, & Schonert-Reichl KA (2018). Positive mental health and supportive school environments: A population-level longitudinal study of dispositional optimism and school relationships in early adolescence. Social Science and Medicine, 214(July), 154–161. 10.1016/j.socscimed.2018.06.041 [DOI] [PubMed] [Google Scholar]
- Olmos G, & Llado J (2014). Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators of Inflammation, 2014, 861231 10.1155/2014/861231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson PS, Rosas-Ballina M, Levine YA, & Tracey KJ (2012). Rethinking inflammation: Neural circuits in the regulation of immunity. Immunological Reviews, 248(1), 188–204. 10.1111/j.1600-065X.2012.01138.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson CA, Romaniuk H, Salinger J, Staiger PK, Bonomo Y, Hulbert C, & Patton GC (2016). Drinking patterns of adolescents who develop alcohol use disorders: Results from the Victorian Adolescent Health Cohort Study. BMJ Open, 6(2), 1–10. 10.1136/bmjopen-2015-010455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelas LC, Novier A, Skike CE Van, Diaz-Granados JL, & Matthews DB (2015). The effects of acute alcohol on motor impairments in adolescent , adult , and aged rats. Alcohol, 49(2), 121–126. 10.1016/j.alcohol.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Oslin DW (2000). Alcohol use in late life: Disability and comorbidity. Journal of Geriatric Psychiatry and Neurology, 13(3), 134–140. 10.1177/089198870001300307 [DOI] [PubMed] [Google Scholar]
- Ostan R, Bucci L, Capri M, Salvioli S, Scurti M, Pini E, … Franceschi C (2008). Immunosenescence and immunogenetics of human longevity. NeuroImmunoModulation, 15(4–6), 224–240. 10.1159/000156466 [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, & Kunos G (2006). The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacological Reviews, 58(3), 389–462. 10.1124/pr.58.3.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, & Bishop P (1981). An autoradiographic map of (3H) diprenorphine binding in rat brain: Effects of social interaction. Brain Research Bulletin, 7(4), 405–410. 10.1016/0361-9230(81)90038-1 [DOI] [PubMed] [Google Scholar]
- Pascual M, Baliño P, Alfonso-Loeches S, Aragón CMG, & Guerri C (2011). Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain, Behavior, and Immunity, 25(SUPPL. 1), 80–91. 10.1016/j.bbi.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Pascual M, Pla A, Miñarro J, & Guerri C (2014). Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: A review with reference to human adolescent drinking. Alcohol and Alcoholism, 49(2), 187–192. 10.1093/alcalc/agt164 [DOI] [PubMed] [Google Scholar]
- Patrick ME, Evans-Polce R, & Terry-McElrath YM (2019). Faster escalation from first drink to first intoxication as a risk factor for binge and high-intensity drinking among adolescents. Addictive Behaviors, 92(September 2018), 199–202. 10.1016/j.addbeh.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE, Martz ME, Maggs JL, O’Malley PM, & Johnston LD (2013). Extreme binge drinking among 12th-grade students in the United States: Prevalence and predictors. JAMA Pediatrics, 167(11), 1019–1025. 10.1001/jamapediatrics.2013.2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, & Terry-McElrath YM (2017). High-intensity drinking by underage young adults in the United States. Addiction, 112(1), 82–93. 10.1111/add.13556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL (2015). Immune dysregulation and cognitive vulnerability in the aging brain: Interactions of microglia, IL-1β, BDNF and synaptic plasticity. Neuropharmacology, 96, 11–18. 10.1016/j.neuropharm.2014.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis SM, Field EF, Smith LK, & Pellis VC (1997). Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neuroscience and Biobehavioral Reviews, 21(1), 105–120. 10.1016/0149-7634(95)00060-7 [DOI] [PubMed] [Google Scholar]
- Pellis SM, & Pellis VC (1998). Play fighting of rats in comparative perspective: A schema for neurobehavioral analyses. Neuroscience and Biobehavioral Reviews, 23(1), 87–101. 10.1016/S0149-7634(97)00071-7 [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Giorla E, Montanari C, & Baunez C (2019). Social modulation of drug use and drug addiction. Neuropharmacology, (November 2018), 0–1. 10.1016/j.neuropharm.2019.02.027 [DOI] [PubMed] [Google Scholar]
- Perkins AE, Doremus-Fitzwater TL, Spencer RL, Varlinskaya EI, Conti MM, Bishop C, & Deak T (2016). A working model for the assessment of disruptions in social behavior among aged rats: The role of sex differences, social recognition, and sensorimotor processes. Experimental Gerontology, 76, 46–57. 10.1016/j.exger.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins AE, Piazza M, & Deak T (2018). Stereological Analysis of Microglia in Aged Male and Female Fischer 344 Rats in Socially Relevant Brain Regions. Neuroscience, 377, 40–52. 10.1016/j.neuroscience.2018.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins AE, Vore AS, Lovelock D, Varlinskaya E, & Deak T (2018). Late aging alters behavioral sensitivity to ethanol in a sex-specific manner in Fischer 344 rats. Pharmacology Biochemistry and Behavior, 175(June), 1–9. 10.1016/j.pbb.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins AE, Woodruff ER, Chun LE, Spencer RL, Varlinskaya EI, & Deak T (2017). Analysis of c-Fos induction in response to social interaction in male and female Fisher 344 rats. Brain Research, 1672, 113–121. 10.1016/j.brainres.2017.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, & Holmes C (2014). Microglial priming in neurodegenerative disease. Nature Reviews Neurology, 10(4), 217–224. 10.1038/nrneurol.2014.38 [DOI] [PubMed] [Google Scholar]
- Petit G, Kornreich C, Verbanck P, Cimochowska A, & Campanella S (2013). Why is adolescence a key period of alcohol initiation and who is prone to develop long-term problem use?: A review of current available data. Socioaffective Neuroscience & Psychology, 3(1), 21890 10.3402/snp.v3i0.21890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D (2003). The molecular logic of endocannabinoid signalling. Nature Reviews. Neuroscience, 4(11), 873–884. 10.1038/nrn1247 [DOI] [PubMed] [Google Scholar]
- Piva F, Maggi R, Limonta P, Dondi D, & Martini L (1987). Decrease of mu opioid receptors in the brain and in the hypothalamus of the aged male rat. Life Sciences, 40, 391–398. [DOI] [PubMed] [Google Scholar]
- Piyanova A, Lomazzo E, Bindila L, Lerner R, Albayram O, Ruhl T, … Bilkei-Gorzo A (2015). Age-related changes in the endocannabinoid system in the mouse hippocampus. Mechanisms of Ageing and Development, 150, 55–64. 10.1016/j.mad.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Popik P, Vetulani J, & van Ree JM (1992). Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology, 106(1), 71–74. 10.1007/BF02253591 [DOI] [PubMed] [Google Scholar]
- Popik Piotr, & van Ree JM (1991). Oxytocin but not vasopressin facilities social recognition following injection into the medial preoptic area of the rat brain. European Neuropsychopharmacology, 1(4), 555–560. 10.1016/0924-977X(91)90010-R [DOI] [PubMed] [Google Scholar]
- Porcelli S, Van Der Wee N, van der Werff S, Aghajani M, Glennon JC, van Heukelum S, … Serretti A (2018). Social brain, social dysfunction and social withdrawal. Neuroscience and Biobehavioral Reviews, (September). 10.1016/j.neubiorev.2018.09.012 [DOI] [PubMed] [Google Scholar]
- Prediger R, Batista L, & Takahashi R (2005). Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiology of Aging, 26(6), 957–964. 10.1016/j.neurobiolaging.2004.08.012 [DOI] [PubMed] [Google Scholar]
- Prediger R, De-Mello N, & Takahashi RN (2006). Pilocarpine improves olfactory discrimination and social recognition memory deficits in 24 month-old rats. European Journal of Pharmacology, 531, 176–182. 10.1016/j.ejphar.2005.12.032 [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, & Deleo JA (2003). Inhibition of Microglial Activation Attenuates the Development but Not Existing Hypersensitivity in a Rat Model of Neuropathy. Journal of Pharmacology and Experimental Therapeutics, 306(2), 624–630. 10.1124/jpet.103.052407 [DOI] [PubMed] [Google Scholar]
- Ramirez RL, & Spear LP (2010). Ontogeny of ethanol-induced motor impairment following acute ethanol: Assessment via the negative geotaxis reflex in adolescent and adult rats. Pharmacology Biochemistry and Behavior, 95(2), 242–248. 10.1016/j.pbb.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, … Acker JD (2005). Regional Brain Changes in Aging Healthy Adults: General Trends, Individual Differences and Modifiers. Cerebral Cortex, 15(11), 1676–1689. 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Richey L, Doremus-Fitzwater TL, Buck HM, & Deak T (2012). Acute illness-induced behavioral alterations are similar to those observed during withdrawal from acute alcohol exposure. Pharmacology Biochemistry and Behavior, 103(2), 284–294. 10.1016/j.pbb.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richwine AF, Parkin AO, Buchanan JB, Chen J, Markham J. a., Juraska JM, & Johnson RW (2008). Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology, 33(10), 1369–1377. 10.1016/j.psyneuen.2008.08.003 [DOI] [PubMed] [Google Scholar]
- Ross HE, & Young LJ (2009). Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in Neuroendocrinology, 30(4), 534–547. 10.1016/j.yfrne.2009.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco P, Burruss K, Smith C, Kuerbis A, Harrington D, Moore AA, & Resnick B (2015). Drinking behavior among older adults at a continuing care retirement community: Affective and motivational influences. Aging and Mental Health, 19(3), 279–289. 10.1080/13607863.2014.933307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salchner P, Lubec G, & Singewald N (2004). Decreased social interaction in aged rats may not reflect changes in anxiety-related behaviour. Behavioural Brain Research, 151(1–2), 1–8. 10.1016/j.bbr.2003.07.002 [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, & Meredith M (2011). Oxytocin antagonist disrupts male mouse medial amygdala response to chemical-communication signals. Neuroscience, 180, 96–104. 10.1016/j.neuroscience.2011.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Romanovsky AA, & Scammell TE (2012). Neural circuitry engaged by prostaglandins during the sickness syndrome. Nature Neuroscience, 15(8), 1088–1095. 10.1038/nn.3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LM, Hall JM, & Resende LS (2012). Translational rodent models of Korsakoff syndrome reveal the critical neuroanatomical substrates of memory dysfunction and recovery. Neuropsychology Review, 22(2), 195–209. 10.1007/s11065-012-9194-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawabe M, Saito M, Naka M, Kasahara I, Saito Y, Arai T, … Shirasawa T (2006). Standard organ weights among elderly Japanese who died in hospital, including 50 centenarians. Pathology International, 56(6), 315–323. 10.1111/j.1440-1827.2006.01966.x [DOI] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, & Eickhoff SB (2012). Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PloS One, 7(2), e30920. 10.1371/journal.pone.0030920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz JC, & Sawchenko PE (2003). Signaling the brain in systemic inflammation: The role of perivascular cells. Frontiers in Bioscience, 8, 1321–1329. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Buckner JD, & Keough ME (2007). Anxiety sensitivitiy as a prospective predictor of alcohol use disorders. Behavior Modification, 31(2), 202–219. 10.1177/0145445506297019 [DOI] [PubMed] [Google Scholar]
- Schneider M, & Koch M (2005). Deficient social and play behavior in juvenile and adult rats after neonatal cortical lesion: effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacology, 30(5), 944–957. 10.1038/sj.npp.1300634 [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, … Kuhn CM (2010). Aversive effects of ethanol in adolescent versus adult rats: Potential causes and implication for future drinking. Alcoholism: Clinical and Experimental Research, 34(12), 2061–2069. 10.1111/j.1530-0277.2010.01302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, Smith DR, & Ellis PM (2010). Prospectively Ascertained Child Maltreatment and Its Association With DSM-IV Mental Disorders in Young Adults. Archives of General Psychiatry, 67(7), 712 Retrieved from https://www.lib.uwo.ca/cgi-bin/ezpauthn.cgi?url=http://search.proquest.com/docview/603591461?accountid=15115%5Cnhttp://vr2pk9sx9w.search.serialssolutions.com/?ctx_ver=Z39.88-2004&ctx_enc=info:ofi/enc:UTF-8&rfr_id=info:sid/ProQ%3Anursing&rft_val_fmt=info:o [DOI] [PubMed] [Google Scholar]
- Seeman TE (2000). Health Promoting Effects of Friends and Family on Health Outcomes in Older Adults. American Journal of Health Promotion, 14, 362–371. [DOI] [PubMed] [Google Scholar]
- Seitz HK, & Stickel F (2007). Alcoholic Liver Disease in the Elderly. Clinics in Geriatric Medicine, 23(4), 905–921. 10.1016/j.cger.2007.06.010 [DOI] [PubMed] [Google Scholar]
- Serrats J, Schiltz JC, García-Bueno B, van Rooijen N, Reyes TM, & Sawchenko PE (2010). Dual Roles for Perivascular Macrophages in Immune-to-Brain Signaling. Neuron, 65(1), 94–106. 10.1016/j.neuron.2009.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Rooke J, Kolmogorova D, Melanson B, Mallet JF, Matar C, … Ismail N (2018). Sex differences in the peripheral and central immune responses following lipopolysaccharide treatment in pubertal and adult CD-1 mice. International Journal of Developmental Neuroscience, 71(August), 94–104. 10.1016/j.ijdevneu.2018.07.012 [DOI] [PubMed] [Google Scholar]
- Sherman SM, Cheng Y-P, Fingerman KL, & Schnyer DM (2015). Social Support, Stress, and the Aging Brain. Social Cognitive and Affective Neuroscience, (August). 10.1093/scan/nsv071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji H, & Mizoguchi K (2010). Acute and repeated stress differentially regulates behavioral, endocrine, neural parameters relevant to emotional and stress response in young and aged rats. Behavioural Brain Research, 211(2), 169–177. 10.1016/j.bbr.2010.03.025 [DOI] [PubMed] [Google Scholar]
- Shoji H, & Mizoguchi K (2011). Aging-related changes in the effects of social isolation on social behavior in rats. Physiology & Behavior, 102(1), 58–62. 10.1016/j.physbeh.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, & Bulloch K (2007). Microglia derived from aging mice exhibit and altered inflammatory profiled. Glia, 55, 412–424. 10.1002/glia [DOI] [PubMed] [Google Scholar]
- Silveri MM (2012). Adolescent brain development and underage drinking in the United States: Identifying risks of alcohol use in college populations. Harv Rev Psychiatry, 20(4), 189–200. 10.3109/10673229.2012.714642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Dager AD, Cohen-Gilbert JE, & Sneider JT (2016). Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neuroscience and Biobehavioral Reviews, 70, 244–259. 10.1016/j.neubiorev.2016.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, & Spear LP (1998). Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcoholism: Clinical and Experimental Research, 22(3), 670–676. 10.1111/j.1530-0277.1998.tb04310.x [DOI] [PubMed] [Google Scholar]
- Siviy SM, & Panksepp J (2011). In search of the neurobiological substrates for social playfulness in mammalian brains. Neuroscience & Biobehavioral Reviews, 35(9), 1821–1830. 10.1016/j.neubiorev.2011.03.006 [DOI] [PubMed] [Google Scholar]
- Soffié M, & Bronchart M (1988). Age-related scopolamine effects on social and individual behaviour in rats. Psychopharmacology, 95(3), 344–350. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3137620 [DOI] [PubMed] [Google Scholar]
- Southwick SM, Bonanno GA, Masten AS, Panter-Brick C, & Yehuda R (2014). Resilience definitions, theory, and challenges: interdisciplinary perspectives. European Journal of Psychotraumatology, 5, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaak J, Merlocco AC, Soleas GJ, Tomlinson G, Morris BL, Picton P, … Floras JS (2008). Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. American Journal of Physiology-Heart and Circulatory Physiology, 294(2), H605–H612. 10.1152/ajpheart.01162.2007 [DOI] [PubMed] [Google Scholar]
- Spear Linda P. (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews, 24(4), 417–463. 10.1016/S0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Spear Linda P. (2018). Effects of adolescent alcohol consumption on the brain and behaviour. Nature Reviews Neuroscience, 19(4), 197–214. 10.1038/nrn.2018.10 [DOI] [PubMed] [Google Scholar]
- Spear Linda Patia. (2007). Assessment of adolescent neurotoxicity: Rationale and methodological considerations. Neurotoxicology and Teratology, 29(1), 1–9. 10.1016/j.ntt.2006.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear Linda Patia. (2013). Adolescent neurodevelopment. Journal of Adolescent Health, 52(2 SUPPL.2), S7–S13. 10.1016/j.jadohealth.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear Linda Patia. (2014). Adolescents and alcohol: Acute sensitivities, enhanced intake, and later consequences. Neurotoxicology and Teratology, 41, 51–59. 10.1016/j.ntt.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, & Tapert SF (2014). The effect of alcohol use on human adolescent brain structures and systems. Handbook of Clinical Neurology (1st ed., Vol. 125). b 10.1016/B978-0-444-62619-6.00028-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, & Kabbaj M (2010). Sex Differences in Social Interaction in Rats: Role of the Immediate-Early Gene zif268. Neuropsychopharmacology, 35(2), 570–580. 10.1038/npp.2009.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley DA, & Adolphs R (2013). Toward a Neural Basis for Social Behavior. Neuron, 80(3), 816–826. 10.1016/j.neuron.2013.10.038.Toward [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28(1), 78–106. 10.1016/j.dr.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stichel CC, & Luebbert H (2007). Inflammatory processes in the aging mouse brain: Participation of dendritic cells and T-cells. Neurobiology of Aging, 28(10), 1507–1521. 10.1016/j.neurobiolaging.2006.07.022 [DOI] [PubMed] [Google Scholar]
- Takizawa R, Maughan B, & Arseneault L (2014). Adult health outcomes of childhood bullying victimization: Evidence from a five-decade longitudinal British birth cohort. American Journal of Psychiatry, 171(7), 777–784. 10.1176/appi.ajp.2014.13101401 [DOI] [PubMed] [Google Scholar]
- Terry-McElrath YM, & Patrick ME (2016). Intoxication and binge and high-intensity drinking among US young adults in their mid-twenties. Substance Abuse, 37(4), 597–605. 10.1080/08897077.2016.1178681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwel D, Markerink M, & Jolles J (1992). Age-related changes in concentrations of vasopressin in the central nervous system and plasma of the male Wistar rat. Mechanisms of Ageing and Development, 65, 127–136. [DOI] [PubMed] [Google Scholar]
- Tilleux S, & Hermans E (2007). Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. Journal of Neuroscience Research, 85, 2059–2070. 10.1002/jnr [DOI] [PubMed] [Google Scholar]
- Toledo Nunes P, Vedder LC, Deak T, & Savage LM (2019). A Pivotal Role for Thiamine Deficiency in the Expression of Neuroinflammation Markers in Models of Alcohol-Related Brain Damage. Alcoholism: Clinical and Experimental Research, 43(3), 425–438. 10.1111/acer.13946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson KL, & Brown SA (2012). Self-medication or social learning? A comparison of models to predict early adolescent drinking. Addictive Behaviors, 37(2), 179–186. 10.1016/j.addbeh.2011.09.016 [DOI] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, & Vanderschuren LJMJ (2009). Prosocial Effects of Nicotine and Ethanol in Adolescent Rats Through Partially Dissociable Neurobehavioral Mechanisms. Neuropsychopharmacology, 34(12), 2560–2573. 10.1038/npp.2009.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, & Vanderschuren LJMJ (2010). The pleasures of play: pharmacological insights into social reward mechanisms. Trends in Pharmacological Sciences, 31(10), 463–469. 10.1016/j.tips.2010.06.008.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, & Vanderschuren LJMJ (2014). On the interaction between drugs of abuse and adolescent social behavior. Psychopharmacology, 231(8), 1715–1729. 10.1007/s00213-014-3471-z [DOI] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, & Vanderschuren LJMJ (2011). Evaluating the rewarding nature of social interactions in laboratory animals. Developmental Cognitive Neuroscience, 1(4), 444–458. 10.1016/j.dcn.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJM, & Vanderschuren LJMJ (2011). Nucleus Accumbens mu-Opioid Receptors Mediate Social Reward. Journal of Neuroscience, 31(17), 6362–6370. 10.1523/JNEUROSCI.5492-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Manduca A, Petrosino S, Van Kerkhof L, Pasterkamp RJ, … Vanderschuren LJMJ (2012). Endocannabinoids in amygdala and nucleus accumbens mediate social play reward in adolescent rats. Journal of Neuroscience, 32(43), 14899–14908. 10.1523/JNEUROSCI.0114-12.2012.Endocannabinoids [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, & Vanderschuren LJMJ (2008a). Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology, 197(2), 217–227. 10.1007/s00213-007-1025-3 [DOI] [PubMed] [Google Scholar]
- Trezza V, & Vanderschuren LJMJ (2008b). Cannabinoid and opioid modulation of social play behavior in adolescent rats: Differential behavioral mechanisms. European Neuropsychopharmacology, 18(7), 519–530. 10.1016/j.euroneuro.2008.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, & Vanderschuren LJMJ (2009). Divergent effects of anandamide transporter inhibitors with different target selectivity on social play behavior in adolescent rats. The Journal of Pharmacology and Experimental Therapeutics, 328(1), 343–350. 10.1124/jpet.108.141069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JS, Schwartz JE, Clark KM, & Friedman HS (1999). Age-related changes in the associations of social network ties with mortality risk. Psychology & Aging, 14(4), 564–571. Retrieved from http://queens.ezp1.qub.ac.uk/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med4&AN=10632145 [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, & Hart RW (1999). Growth Curves and Survival Characteristics of the Animals Used in the Biomarkers ofAging Program. Journal of Gerontology: Biological Sciences, 54A(11), B492–501. [DOI] [PubMed] [Google Scholar]
- Vaillant G, & Mukamal K (2001). Successful Aging. American Journal of Psychiatry, 158, 839–847. Retrieved from http://ci.nii.ac.jp/naid/40004418345/en/ [DOI] [PubMed] [Google Scholar]
- Van Kerkhof L, Damsteegt R, Trezza V, Voorn P, & Vanderschuren LJ (2013). Social Play Behavior in Adolescent Rats is Mediated by Functional Activity in Medial Prefrontal Cortex and Striatum. Neuropsychopharmacology, 38(10), 1899–1909. 10.1038/npp.2013.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof L, Trezza V, Mulder T, Gao P, Voorn P, & Vanderschuren LJMJMJ (2014). Cellular activation in limbic brain systems during social play behaviour in rats. Brain Structure and Function, 219(4), 1181–1211. 10.1111/ejn.12353.Functional [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K, Mariën P, & Vandekerckhove M (2014). Social cognition and the cerebellum: A meta-analysis of over 350 fMRI studies. NeuroImage, 86, 554–572. 10.1016/j.neuroimage.2013.09.033 [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Spruijt BM, & Van Ree JM (1995). Mu- and kappa-opioid receptor-mediated opioid effects on social play in juvenile rats. European Journal of Pharmacology, 276(3), 257–266. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7601211 [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, & Van Ree JM (1997). The neurobiology of social play behavior in rats. Neuroscience and Biobehavioral Reviews, 21(3), 309–326. 10.1016/S0149-7634(96)00020-6 [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Stein E. a, Wiegant VM, & Van Ree JM (1995). Social play alters regional brain opioid receptor binding in juvenile rats. Brain Research, 680(1–2), 148–156. 10.1016/0006-8993(95)00256-P [DOI] [PubMed] [Google Scholar]
- Varatharaj A, & Galea I (2017). The blood-brain barrier in systemic inflammation. Brain, Behavior, and Immunity, 60, 1–12. 10.1016/j.bbi.2016.03.010 [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Doremus-Fitzwater TL, & Spear LP (2010). Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacology Biochemistry and Behavior, 96(2), 228–235. 10.1016/j.pbb.2010.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Kim EU, & Spear LP (2017). Chronic intermittent ethanol exposure during adolescence: Effects on stress-induced social alterations and social drinking in adulthood. Brain Research, 1654, 145–156. 10.1016/j.brainres.2016.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2002). Acute Effects of Ethanol on Social Behavior of Adolescent and Adult Rats : Role of Familiarity of the Test Situation. Alcoholism: Clinical and Experimental Research, 26(10), 1502–1511. 10.1097/01.ALC.0000034033.95701.E3 [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2006). Differences in the social consequences of ethanol emerge during the course of adolescence in rats: Social facilitation, social inhibition, and anxiolysis. Developmental Psychobiology, 48(2), 146–161. 10.1002/dev.20124 [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2007). Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicology and Teratology, 29, 23–30. 10.1016/j.ntt.2006.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2009). Ethanol-induced social facilitation in adolescent rats: Role of endogenous activity at mu opioid receptors. Alcoholism: Clinical and Experimental Research, 33(6), 991–1000. 10.1111/j.1530-0277.2009.00920.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2015). Social consequences of ethanol: Impact of age, stress, and prior history of ethanol exposure. Physiology and Behavior, 148, 145–150. 10.1016/j.physbeh.2014.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, & Spear NE (2001). Acute Effects of Ethanol on Behavior of Adolescent Rats : Role of Social Context. Alcoholism: Clinical and Experimental Research, 25(3), 377–385. [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell EM, & Spear LP (2013). Repeated restraint stress alters sensitivity to the social consequences of ethanol differentially in early and late adolescent rats. Pharmacology Biochemistry and Behavior, 113, 38–45. 10.1016/j.pbb.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell EM, & Spear LP (2015). Sex differences in sensitivity to the social consequences of acute ethanol and social drinking during adolescence. Behavioural Brain Research, 282, 6–13. 10.1016/j.bbr.2014.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell E, & Spear LP (2014). Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol, 45(5), 433–444. 10.1016/j.pestbp.2011.02.012.Investigations [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Vogt B. a., & Spear LP (2013). Social context induces two unique patterns of c-Fos expression in adolescent and adult rats. Developmental Psychobiology, 55(7), 684–697. 10.1002/dev.21064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya E, Spear L, & Spear N (1999). Social behavior and social motivation in adolescent rats: role of housing conditions and partner’s activity. Physiology & Behavior Behav, 67(4), 475–482. 10.1016/S0031-9384(98)00285-6 [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2004). Acute Ethanol Withdrawal ( Hangover ) and Social Behavior in Adolescent and Adult Male and Female Sprague-Dawley Rats. Alcoholism: Clinical and Experimental Research, 28(1), 40–50. 10.1097/01.ALC.0000108655.51087.DF [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, & de Vries GJ (2012). Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Hormones and Behavior, 61(1), 50–56. 10.1016/j.immuni.2010.12.017.Two-stage [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal RE, McGuire EA, Tobin JT, Andres R, Norris AH, & Mezey E (1977). Aging and ethanol metabolism. Clinical Pharmacology and Therapeutics, 21(3), 343–354. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen Courtney, Varlinskaya E, & Spear L (2009). Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol and Alcoholism, 44(6), 547–554. 10.1093/alcalc/agp048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, & Spear LP (2012). Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Developmental Psychobiology, 54(5), 523–535. 10.1002/dev.20610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, & Viviani B (2015). Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology, 96(Pt A), 70–82. 10.1016/j.neuropharm.2014.10.027 [DOI] [PubMed] [Google Scholar]
- Walker BM, & Ehlers CL (2009). Age-related differences in the blood alcohol levels of Wistar rats. Pharmacology Biochemistry and Behavior, 91(4), 560–565. 10.1016/j.pbb.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ, & Crews FT (2017). Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. Journal of Neuroinflammation, 14(1), 1–19. 10.1186/s12974-017-0856-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, & Goehler LE (1995). Cytokine-to-brain communication: A review & analysis of alternative mechanisms. Life Sciences, 57(11), 1011–1026. 10.1016/0024-3205(95)02047-M [DOI] [PubMed] [Google Scholar]
- Wauthier V, Verbeeck RK, & Calderon PB (2004). Age-related changes in the protein and mRNA levels of CYP2E1 and CYP3A isoforms as well as in their hepatic activities in Wistar rats. What role for oxidative stress? Archives of Toxicology, 78(3), 131–138. 10.1007/s00204-003-0526-z [DOI] [PubMed] [Google Scholar]
- Wei D, Lee D, Cox CD, Karsten CA, Peñagarikano O, Geschwind DH, … Piomelli D (2015). Endocannabinoid signaling mediates oxytocin-driven social reward. Proceedings of the National Academy of Sciences, 112(45), 14084–14089. 10.1073/pnas.1509795112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae J, Ahmad S, Wilson W, Best P, & Swartzwelder HS (2002). Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacology, Biochemistry, and Behavior, 73, 673–677. [DOI] [PubMed] [Google Scholar]
- Willey AR, Varlinskaya EI, & Spear LP (2009). Social interactions and 50 kHz ultrasonic vocalizations in adolescent and adult rats. Behavioural Brain Research, 202(1), 122–129. 10.1016/j.bbr.2009.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Mitterling KL, Thompson LI, Torres-Reveron A, Waters EM, McEwen BS, … Milner TA (2011). Age- and hormone-regulation of opioid peptides and synaptic proteins in the rat dorsal hippocampal formation. Brain Research, 1379, 71–85. 10.1523/JNEUROSCI.3593-07.2007.Omega-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Boddeke HWGM, & Kettenmann H (2017). Microglia in Physiology and Disease. Annual Review of Physiology, 79(1), 619–643. 10.1146/annurev-physiol-022516-034406 [DOI] [PubMed] [Google Scholar]
- Wong AM, Patel NV, Patel NK, Wei M, Morgan TE, De Beer MC, … Finch CE (2005). Macrosialin increases during normal brain aging are attenuated by caloric restriction. Neuroscience Letters, 390(2), 76–80. 10.1016/j.neulet.2005.07.058 [DOI] [PubMed] [Google Scholar]
- Wong WT (2013). Microglial aging in the healthy CNS: phenotypes, drivers, and rejuvenation. Frontiers in Cellular Neuroscience, 7(March), 22 10.3389/fncel.2013.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2018). Global status report on alcohol and health. Global status report on alcohol. https://doi.org//entity/substance_abuse/publications/global_alcohol_report/en/index.html [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Robertson SA, Coller JK, … Hutchinson MR (2011). Attenuation of microglial and IL-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain, Behavior, and Immunity, 25(SUPPL. 1), S155–S164. 10.1016/j.bbi.2011.01.012 [DOI] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, & Godbout JP (2010). Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behavior and Immunity, 24(7), 1190–1201. 10.1016/j.surg.2006.10.010.Use [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovleva T, Marinova Z, Kuzmin A, Seidah NG, Haroutunian V, Terenius L, & Bakalkin G (2007). Dysregulation of dynorphins in Alzheimer disease. Neurobiology of Aging, 28(11), 1700–1708. 10.1016/j.neurobiolaging.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Young K, Gobrogge K, Liu Y, & Wang Z (2011). The neurobiology of pair bonding: Insights from a socially monogamous rodent. Frontiers in Neuroendocrinology, 32(1), 53–69. 10.1016/j.yfrne.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ (2002). The neurobiology of social recognition, approach, and avoidance. Biological Psychiatry, 51(1), 18–26. 10.1016/S0006-3223(01)01268-9 [DOI] [PubMed] [Google Scholar]
- Yuan L, Liu S, Bai X, Gao Y, Liu G, Wang X, … Wang Z (2016). Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. Journal of Neuroinflammation, 13(1), 77 10.1186/s12974-016-0541-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, … Gross CT (2014). Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nature Neuroscience, 17(3), 400–406. 10.1038/nn.3641 [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Richardson R, & Baker K (2019). Maturational Changes in Prefrontal and Amygdala Circuits in Adolescence: Implications for Understanding Fear Inhibition during a Vulnerable Period of Development. Brain Sciences, 9(3), 65 10.3390/brainsci9030065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Dannals RF, & Frost JJ (1999). Gender and age influences on human brain mu-opioid receptor binding measured by PET. American Journal of Psychiatry, 156(6), 842–848. 10.1176/ajp.156.6.842 [DOI] [PubMed] [Google Scholar]