Abstract
Background:
Propolis as a natural product has shown beneficial effects on human health. This study was aimed to investigate the chemical compositions and biological activity of three different extracts of propolis from two distinct geographic areas in Iran.
Methods:
The chemical composition of Iranian propolis extracts that were collected in the Spring of 2016 from two provinces in northern Iran: Ardabil and Polur in Mazandaran Province were measured through gas chromatography-mass spectrometry (GC-MS) methods. In addition, antimicrobial activity and cytotoxicity effect on HN5 and LNCaP cell lines were evaluated. The data were analyzed using one-way ANOVA and p<0.05 was considered as significant.
Results:
The GC-MS analysis identified the presence of compounds that belonged to the different groups such as aromatics acids and their related esters, flavonoid and flavonoid derivatives and terpenes. Flavanone was the most dominant compound of flavonoids. The maximum growth inhibition was observed against S. aureus of ethanolic extract of propolis (p<0.05). Moreover, cytotoxicity showed that ethanolic and dichloromethane extracts had more inhibitory effects on cell lines than the water extract.
Conclusion:
The results determined that extracts had the highest percentage of flavonoids. Therefore, it is expected that the synergistic effect of the main components of propolis is related to the increase of biological activity of propolis.
Key Words: Cytotoxicity, Flavonoids, Gas chromatography-mass spectrometry, Propolis
Propolis is a wax-like substance produced by honey bees. It is soft and can be found in colors ranging from yellowish-green to dark brown (1-3). Propolis acts as a disinfectant for bees and an effective agent for preventing the incidence and outbreak of diseases in the beehive (4, 5). Propolis is generally composed of about 50% resins, 30% beeswax, 10% essential fatty acids, 5% pollen, and 5% other organic compounds including vitamins, and minerals. The amount and type of constituting compounds depends on the location and time of collection (1, 6). Different types of resins such as poplars, conifers, birch, pine, alder, willow, and palm were identified in propolis. The best-known variety of propolis is poplar propolis. This propolis is mainly composed of flavonoids, phenolic acids, and their esters, which distinguish it from other propolis. The main compounds associated with the biological activity of propolis include polyphenols, aromatic acids, and diterpenic acids (7-9). The solvents commonly used in propolis extraction are water, methanol, ethanol, chloroform, dichloromethane, ether, and acetone (1, 10). Ethanol is the most used solvent to obtain low-wax propolis extracts that are rich in active biological compounds (11).
With the advancements in separation and purification techniques, other compounds in propolis, including flavonoids, terpenes, phenols and esters, sugars, hydrocarbons, and mineral elements have been identified (10). Flavonoids exhibit a wide range of biological properties and depending on their chemical composition can be classified into flavones, flavonols, flavanones, chalcones, dihydrochalcones, isoflavones, isodihydroflons, flavanes, isoflavanes, and neoflavonoids (12,7). Propolis is known to have positive effects on human health and has been used in traditional medicine since ancient times (13). Studies carried out on propolis extracts from different parts of the worlds have demonstrated the antimicrobial, anti-inflammatory, cytotoxic, and antiparasitic properties and immunomodulatory and anti-leishmanicidal effects (14, 15). Given the potential applications of propolis, this study used the GC/MS analysis method to investigate the chemical composition of ethanol, dichloromethane, and water extracts of propolis of northern Iran and compared the effect of different solvents on biological activity of the extract.
Methods
Propolis samples: This study was approved by the Research Ethics Committee of Babol University of Medical Sciences (MUBABOL.REC.1394020). Samples of crude propolis produced by Apis mellifera bees were collected in the Spring of 2016 from Sabalan Mountains in Ardabil Province (Ardabil city) and Alborz Mountains in Mazandaran Province (Polur). Ethanolic extract of Iranian propolis (EEIP), dichloromethane extract of Iranian propolis (DEIP) and water extract of Iranian propolis (WEIP) were prepared according to the 2018 study by Afrouzan et al. (16).
GC/MS analysis: The EEIP, DEIP and WEIP of propolis samples were analyzed using gas chromatography–mass spectrometry (GC–MS) equipment (7890B-5977B MSD, Agilent). The experimental conditions for the DB-5 MS capillary column were as follows: length=30 m; ID=0.25 mm; film thickness = 0.25 μm; and the carrier gas was helium at a flow rate of 1 ml/min. A sample of 1 μl was injected with an auto sampler in a split ratio of 10:1. The injector temperature was set at 250 °C and the oven temperature was programmed from 50 °C (storage time of 1 min) and increased at a rate of 8°C/min to 120 °C (storage time of 1 min), and then increased at a rate of 6 °C/min to 250°C, finally to 250°C at 15 min. The solvent delay was 0 to 3 min, and the total GC–MS running time was 47 min. Using the National Institute of Standards and Technology (NIST 11 Variant) database, the mass spectrum was used to identify the name, molecular weight, and structure of the components of propolis samples.
Antibacterial activity: Gram-positive strain includes staphylococcus aureus (ATCC 25923), and gram-negative strains include Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 15442), which were provided in lyophilized form by Iranian Research Organization for Science and Technology (IROST). Lyophilized culture of strains was transferred from the stock culture to Brain Heart Infusion (BHI) and Muller Hinton agar (MHA) (Merck, Darmstadt, Germany) and incubated at 37 °C for 24 h. Suspensions of bacteria was prepared according to the turbidity of 0.5 McFarland turbidity (17). The broth micro-dilution method was used to determine minimum inhibitory concentration (MIC) of extracts according to the Clinical and Laboratory Standards Institute guidelines (18).
Cell cultures and cytotoxicity analysis: The cytotoxic effect of extracts was tested on cancer cell lines including the human prostate cancer cell line (LNCaP) and the head and neck carcinoma cell line (HN5). LNCaP and HN5 cell line was obtained from the National Cell Bank of Iran, Pasteur Institute (Tehran, Iran) and was cultured in RPMI 1640 containing L-glutamin and supplemented with 10% fetal calf serum (FBS), and 1% Penstrep (penicillin G 100 IU/ml, streptomycin 100 μg/ml). The cell lines were grown as monolayers in 25 cm2 cell culture flasks at 37°C in a 5% CO2 humidified atmosphere. Cells were treated with 0 up to 500 μg/ml of EEIP, DEIP and WEIP. Each concentration was tested in triplicates along with the control group (without treatment). The cytotoxic effect was measured using the MTT assay after 48 h incubation (12). The obtained OD from the control group was considered as 100% viability.
Statistical analysis: Data were analyzed using GraphPad Prism v 6.07 (GraphPad Software Inc., La Jolla, CA, USA). Results were expressed as the mean± SD. Comparisons between groups was performed via one-way ANOVA. P<0.05 was considered statistically significant.
Results
In particular, EEIP extract of Ardabil showed the compounds of different aromatic acids and their related esters such as benzeneethanol, 3-hydroxy-4-methoxycinnamic acid, 5-phenylthiazolidine and flavonoid (galangin flavanone) and derivatives (pinostrobin chalcone, techtochrysin). Also, it indicated the terpene derivatives (dihydro-. alpha. -terpineol). EEIP extract of Polur had different aromatic acid and corresponding esters such as benzeneethanol, dihydrobenzofuran, 4-hydroxycinnamic acid, cinnamic acid, 4-hydroxy-3-methoxy-, 2,5 dimethoxyterephthalic acid, 3- hydroxy-4-methoxycinnamic acid, methyl 3-(4'-hydroxyphenyl)prop-2-enoate, 1,4- Dihydrophenanthrene, diethylmethylbenzyloxysilane, benzeneacetic acid, methyl ester, as well as flavonoid and flavonoid derivatives (chrysin, pinostrobin chalcone, 5,7 -dihydroxy -dihydroflavone, tectochrysin). The combination of terpenes was not found (table 1).
Table 1.
Compounds identified the extracts of Iranian propolis using GC/MS analysis
| a R.T. min | Compounds |
Composition (%)
* EP EA DP DA WP WA |
|||||
|---|---|---|---|---|---|---|---|
| 5.642 | Dimethyl sulfone | - | - | - | - | - | 5.882 |
| 9.144 | Benzeneethanol | 3.312 | 3.004 | - | - | - | - |
| 11.177 | Dihydrobenzofuran | 1.662 | - | - | 8.45 | 3.411 | |
| 13.327 | 2-Methoxy-4-vinylphenol | - | - | - | - | 1.774 | - |
| 16.057 | 2,2-Diethynylbut-2-ene-1,4-diol | 15.663 | - | - | - | 85.552 | 82.894 |
| 20.313 | -4-Methyl-2-(1-ethylethenyl)-1-cyclopentene-1-carboxaldehyde | - | 1.379 | - | - | - | - |
| 20.365 | 3-Ethyl-8-methyl-2-oxatetracyclo[4.4.0.0(1,4).0(6,8)]decane | - | 1.370 | - | - | - | - |
| 23.260 | Dihydro-.alpha.-terpineol | - | 3.273 | - | - | - | - |
| 23.276 | Rosifoliol | 1.549 | - | - | - | - | - |
| 26.035 | (-)-Elema-1,3,11(13)-trien-12-al | - | 2.104 | - | - | - | - |
| 26.053 | 1,3,6-Octatriene, 3,7-dimethyl-, (E)- | 1.061 | - | - | - | - | - |
| 27.065 | 3,4-Octadiene, 7-methyl- | - | 3.805 | - | - | - | - |
| 27.667 | 9-Dodecenol | - | - | - | 0.765 | - | - |
| 27.672 | Heptylacetylene | - | - | 2.085 | - | - | - |
| 27.746 | 9-Octadecenoic acid (Z)-, methyl ester | - | 4.805 | - | - | - | - |
| 28.742 | Trifluoroacetic acid, n-heptadecyl ester | - | 3.780 | - | - | - | - |
| 28.753 | 4-Hydroxycinnamic acid | 2.549 | 1.395 | - | - | - | - |
| 29.448 | Cinnamic acid, 4-hydroxy-3-methoxy- | 1.003 | - | - | - | 1.233 | 4.264 |
| 29.961 | 2,5 Dimethoxyterephthalic acid | 1.023 | - | - | - | - | - |
| 29.962 | Benzaldehyde, 4,6-dimethoxy-2,3-dimethyl- | - | - | - | 0.809 | - | - |
| 30.219 | 3- Hydroxy-4-methoxycinnamic acid | 0.931 | 4.925 | - | 0.954 | - | - |
| 30.592 | Methyl 3-(4'-hydroxyphenyl)prop-2-enoate | 0.834 | - | - | 0.609 | - | - |
| 31.733 | Aniline, 2,4,6-trimethyl-3-nitro- | - | - | - | 12.975 | - | - |
| 31.785 | 1,4- Dihydrophenanthrene | 7.883 | - | - | - | ||
| 32.172 | Pinostrobin chalcone | 12.820 | 15.051 | 19.710 | 8.595 | - | - |
| 32.253 | 3-Methoxy-4,5-methylenedioxybenzaldehyde | - | - | - | 1.586 | - | - |
| 32.276 | Diethylmethylbenzyloxysilane | 0.877 | - | - | - | - | - |
| 32.408 | Caffeic acid | - | - | - | 5.619 | - | - |
| 33.148 | p-Pentyloxynitrobenzene | - | - | 1.352 | - | - | - |
| 33.426 | 5,7- -dihydroxy -dihydroflavone | 16.855 | - | - | - | - | - |
| 33.359 | Galangin flavanone | - | 36.672 | 57.069 | 36.548 | - | - |
| 34.201 | 1,2-Benzenedicarboxylic acid | - | - | - | - | 4.243 | 3.55 |
| 34.771 | Hydrocinnamic acid | - | - | 1.423 | 2.489 | - | - |
| 34.784 | Methyl phenylacetate | - | - | 1.094 | - | - | - |
| 34.817 | Benzeneacetic acid, methyl ester | 2.899 | - | - | - | - | - |
| 35.059 | 10-hydroxybenzo[j]fluoranthene | - | - | 16.458 | - | - | - |
| 35.145 | Tectochrysin | 13.571 | 14.792 | - | 9.662 | - | - |
| 36.692 | Naringenin | - | - | - | 9.892 | - | - |
| 36.726 | Chrysin | 13.547 | - | - | 9.493 | - | - |
| 39.940 | 5-phenylthiazolidine | - | 3.534 | - | - | - | - |
aRT: Retention time (minutes). * EP: ethanolic extract of polur, EA: ethanolic extract of Ardabil, DP: dichloromethane extract of polur, DA: dichloromethane extract of Ardabil, WP: water extract of polur, WA: water extract of Ardabil
Regarding the DEIP extract of Ardabil propolis, the highest quantity compounds were flavonoids (pinostrobin chalcone, galangin flavanone, naringenin, tectochrysin, and chrysin). Other compounds were aromatic acids and its derivatives (benzaldehyde, 4,6-dimethoxy-2,3-dimethyl-, 3-hydroxy-4-methoxycinnamic acid, aniline, 2,4,6-trimethyl-3-nitro-, 3-methoxy-4,5-methylenedioxybenzaldehyde, caffeic acid and hydrocinnamic acid). Concerning Polur propolis, two types of flavonoids (galangin flavanone and pinostrobin chalcone) had the highest amounts, and the other identified compounds were aromatic acids (p-pentyloxynitrobenzene, hydrocinnamic acid, 10-hydroxybenzo[j]fluoranthene (table 1). In WEIP extract of Ardabil propolis, 2-ethenyl-1,3-benzenediol of aromatic acid had the highest quantity. In case of polur propolis, 85.552% of total was 2,2-diethynylbut-2-ene-1,4-diol. Flavonoids were not found in WEIP extract (table 1). According to these results, the all extracts were composed of aromatic acids and its related esters, flavonoid and flavonoid derivatives. EEIP of Ardabil propolis had little amounts of aromatic acids (11.463% of total) and more than flavonoid and flavonoid derivatives (66.515% of total) compared with Polur propolis 24.908% and 56.793% respectively. Tectochrysin and pinostrobin chalcone were common flavonoids identified in both extracts. In the DEIP extracts, Ardabil propolis had the highest content of flavonoids (76.779% of total). Also, galangin flavanone was the highest quantity compounds in samples. However, ethanolic extract was more efficient solvent for the isolation of phenolic compounds compared to other solvents. In MIC assay, DEIP and EEIP extract of Ardabil propolis at lower concentrations were able to inhibit S. aureus and E. coli compared with Polur propolis. The all extracts (up to a concentration of 1000 µg/ml) were not able to inhibit the growth of P.aeruginosa (table 2). Our data showed that EEIP, DEIP and WEIP were able to induce cytotoxicity in a dose-dependent manner. Significant cytotoxic effects of propolis on HN5 and LNCaP cell lines are shown in figures.1 and 2.
Table 2.
The minimum inhibitory concentration (MIC) values of extracts against strains (values in µg/ml)
|
The Minimum Inhibitory Concentration (MIC) µg/ml
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Samples |
S. aureus
(ATCC 25923)
|
E. coli (
ATCC 25922
)
|
P.aeruginosa
(ATCC 15442)
|
|||||||
| EEIP | DEIP | WEIP | EEIP | DEIP | WEIP | EEIP | DEIP | WEIP | ||
| Ardabil | 250 a | 250 a | 1000 b | 500 | 500 | - | - | - | - | |
| Polur | 250a | 500 b | 1000 b | 1000a | 500b | - | - | - | - | |
| Gentamicin | 250 | 250 | 250 | 500 | 250 | 500 | 1000 | 500 | 1000 | |
| Chloramphenicol | 1 | 4 | 8 | 4 | 8 | 8 | 64 | 64 | 128 | |
Values are mean of three different tests. Means followed by the same letters are not significantly different in each row for each isolates (p<0.05), - >1000 µg/ml.
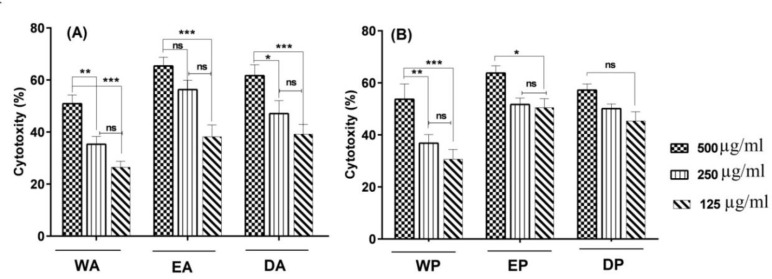
Figure 1.
The cytotoxity of HN5 cell line was measured via MTT assays. The cells were treated with various concentrations for 48 h. The results are presented as a percentage of the control group. The data shown are the mean± SD of three determinations. * (p <0.05), * * (p <0.01), *** (p <0.001) and ns; non-significant
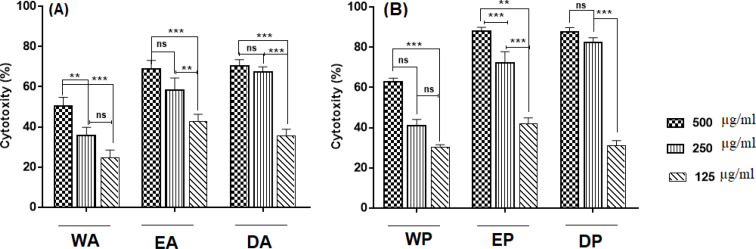
Figure 2.
The cytotoxity of LNCaP cells was measured via MTT assays. The cells were treated with various concentrations for 48 h. The results are presented as a percentage of the control group. The data shown are the mean± SD of three determinations. * * (p <0.01), *** (p <0.001) and ns; non-significant
In the presence of 500 µg/ml of EEIP propolis revealed the highest cytotoxicity on HN5 cell line, the percentage of cell viability decreased to 34.6% for EEIP of Ardabil (EA) and 36.2% for EEIP of Polur (EP) compared to control group (without treatment). Also, 500 µg/ml of EP revealed the highest cytotoxicity on LNCaP cells (87.8 %). A significant difference was found between EA and EP in concentrations of 500 µg/ml and 125 µg/ml (P<0.001) and between DA and DP in concentrations of 500 µg/ml and 250 µg/ml (P<0.01).
Discussion
Considering the use of propolis in the industry, such as food, cosmetics and pharmaceutical industries, the analysis of physicochemical composition of propolis has been considered to determine the quality of this material. The chemical compositions of EEIP, DEIP and WEIP from two Iranian propolis samples were analyzed by GC-MS and showed the presence of compounds that belonged to different groups such as aromatic acids and their related esters, flavonoid and flavonoid derivatives and terpenes.
The results showed that extraction solvent could play an important role in the isolation of bioactive compounds. In this concern, individual compounds were identified in ethanoic extract of Iranian propolis was more than the other solvents. However, both ethanolic and dichloromethane solvents showed a high percentage of flavonoids. Afrouzan et al. analyzed the chemical compositions from four Iranian propolis samples (Morad Beyg, Taleghan, Kalaleh and Chenaran) using GC-MS methods and indicated that the total amount of flavonoids in dichloromethane extracts of propolis was higher than ethanolic extracts (16). Alizadeh et al. investigated the chemical components of Iranian propolis from two origins of Hamadan and Taleghan and showed different amount of aromatic acids )2270 µg/ml and 489 µg/ml respectively) while the amount of phenolic compounds in the two areas was almost the same (1238 µg/ml and 1568 µg/ml, respectively). This difference between levels of aromatic compounds probably related to their geographical origins. Caffeic acid isoprenyl esther (isomer 2) and pinobanksin were the highest quantity compounds found in Hamadan and Taleghan propolis (32.52% and 16.52%, respectively) (19). The chemical components of ethanolic and water extracts of Iranian propolis from Kordkoy indicated that extracts have a high percentage of flavonoids, though the amount of flavonoids in ethanolic extract (15.88%) was more than water extracts (14.87%) (20).
These studies indicated that Iranian propolis is rich in flavonoids and phenolic compounds. These diversity of flavonoid compounds showed the typical pattern of “poplar” propolis (21, 22). Analysis of Ardabil and polur propolis by HPLC in the previous study showed the bioactive compounds included caffeic acid, quercetin, chrysin, galangin, and pinobanksin (12). Therefore, the results of this studies indicated flavonoid compounds commonly present in the poplar type of China, Serbia, Italy, Slovenia and Germany propolis (23-27).
In the current study, the antibacterial activity of EEIP of Ardabil and Polur propolis against S. aureus was higher than Chenaran, MoradBeyg, Kalaleh and Alborz proplis, that previously reported for Iranian propolis, while the growth inhibition zone against E. coli was less. Also, DEIP extracts indicated the growth inhibition zone less than this study against S. aureus and E. coli (16). Also in the previous study, EEIP of Ardabil inhibited the growth of oral streptococci with MIC values ranging from 3.12 to 100μg/ml and indicated good anti-biofilm activities against streptococcus mutans (28). The analysis of the effect of propolis on several bacterial species revealed that propolis is more active against gram-positive bacteria than gram-negative bacteria. It inhibited bacterial motility and enzymatic activity, which can lead to bacteriostatic activity. Also, at high concentrations, exhibit bactericidal capability against various bacterial species (29, 30).
The results of the present study are consistent with the findings reported for the ethanol extract of propolis from Bulgaria, Greece, Turkey, Australian and Algeria, as samples showed a good antibacterial effect against S. aureus, but had a poor effect on gram-negative bacteria (31- 34).
The studies indicate that flavonoids and derivatives can protect cells against cancer (35, 36). However, mechanisms of their protective effect on cells is unclear so far (37). Also, the inhibitory effect of propolis on the growth of cancer cell lines may be related to the production of reactive oxygen species (ROS) resulting to activation of apoptotic caspases in cancer cells (29).
Accordingly, in previous studies we evaluated intracellular ROS induction mechanism involved in the anticancer effects of ethanolic extract of Iranian propolis against MCF-7 and RAW 264.7 cell lines using a flow cytometry method. In both studies, the levels of ROS increased significantly in cell lines in a dose-dependent manner compared with the control group (12, 38). Although in another study, we showed that Iranian propolis could inhibit the growth of KB and A431 cancer cells in a dose-dependent manner, but they had no effect on fibroblast cells compared to the control (28). In line with the results of other studies, cytotoxic activity of different extracts of propolis confirm our findings (39-46).
Taken together, the chemical compositions of EEIP, DEIP and WEIP from two Iranian propolis samples showed the presence of compounds belonging to different groups such as aromatic acids, flavonoids and terpenes. EEIP of Ardabil sample had the highest percentage of flavonoids. Accordingly, it is expected that a high percentage of flavonoids in these extracts cause more biological activity against S. aureus and HN5 and LNCaP cell lines. In conclusion, we suggest that the synergistic effect of the main components of propolis is related to the increase of biological activity of propolis such as antimicrobial and cytotoxity. Further research is necessary to clarify the affecting mechanisms of the beneficial properties of propolis.
Acknowledgments
The authors specially thank Mr. Payam Morakabati for his help for cell culture.
Funding:
This study was financially supported by Babol University of Medical Sciences, Grant NO 9441716.
Conflict of Interest:
There is no conflict of interest.
References
- 1.Wagh VD. Propolis: a wonder bees product and its pharmacological potentials. Adv Pharmacol Sci. 2013;2013:308249. doi: 10.1155/2013/308249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kupchan SM. Recent advances in the chemistry of tumor inhibitors of plant origin. Trans N Y Acad Sci. 1970;32:85–106. doi: 10.1111/j.2164-0947.1970.tb02046.x. [DOI] [PubMed] [Google Scholar]
- 3.Zaccaria V, Curti V, Di Lorenzo A, et al. Effect of green and brown propolis extracts on the expression levels of micrornas, mrnas and proteins, related to oxidative stress and inflammation. Nutrients. 2017;9:1090. doi: 10.3390/nu9101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinotti S, Ranzato E. Propolis: a new frontier for wound healing? Burns Trauma. 2015;3 doi: 10.1186/s41038-015-0010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simone-Finstrom M, Borba RS, Wilson M, Spivak M. Propolis counteracts some threats to honey bee health. Insects. 2017;8:E46. doi: 10.3390/insects8020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pietta PG, Gardana C, Pietta AM. Analytical methods for quality control of propolis. Fitoterapia. 2002;73:S7–20. doi: 10.1016/s0367-326x(02)00186-7. [DOI] [PubMed] [Google Scholar]
- 7.Huang S, Zhang CP, Wang K, Li GQ, Hu FL. Recent advances in the chemical composition of propolis. Molecules. 2014;19:19610–32. doi: 10.3390/molecules191219610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosalec I, Bakmaz M, Pepeljnjak S, Vladimir-Knezevic S. Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharm . 2004;54:65–72. [PubMed] [Google Scholar]
- 9.Bankova VS, Castro SLd, Marcucci MC. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31:3–15. [Google Scholar]
- 10.Ahangari Z, Naseri M, Vatandoost F. Propolis: Chemical Composition and Its Applications in Endodontics. Iran Endod J. 2018;13:285–92. doi: 10.22037/iej.v13i3.20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubiliene L, Laugaliene V, Pavilonis A, et al. Alternative preparation of propolis extracts: comparison of their composition and biological activities. BMC Complement Altern Med. 2015;15:156. doi: 10.1186/s12906-015-0677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asgharpour F, Moghadamnia AA, Kazemi S, et al. Chemical Composition analysis and in vitro investigation of cytotoxic and antioxidative activities of iranian propolis against breast cancer cell line, MCF-7. Chem Select. 2018;3:10857–63. [Google Scholar]
- 13.Pasupuleti VR, Sammugam L, Ramesh N, Gan SH. Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. Oxid Med Cell Longev. 2017;2017:1259510. doi: 10.1155/2017/1259510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sena-Lopes Â, Bezerra FSB, das Neves RN, et al. Chemical composition, immunostimulatory, cytotoxic and antiparasitic activities of the essential oil from Brazilian red propolis. PLoS One. 2018;13:e0191797. doi: 10.1371/journal.pone.0191797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011;133:253–60. doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Afrouzan H, Tahghighi A, Zakeri S, Es-haghi A. Chemical composition and antimicrobial activities of Iranian propolis. Iran Biomed J. 2018;22:50–65. doi: 10.22034/ibj.22.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 18.CLSI. Performance standards for antimicrobial susceptibility testing. 27th ed. CLSI Supplement M100S. Clinical and Laboratory Standards Institute, Wayne, IN. 2017. Available at: https://clsi.org/media/1469/m100s27_sample.pdf.
- 19.Alizadeh AM, Afrouzan H, Dinparast-Djadid N, Sawaya AC, Azizian S, Hemmati HR, et al. Chemoprotection of MNNG-initiated gastric cancer in rats using Iranian propolis. Arch Iran Med. 2015;18:18–23. [PubMed] [Google Scholar]
- 20.Payandan E, Sayyed-alangi SZ, Shamloofar M, Koohsari H. study of chemical composition and efficacy of different extracts of iranian propolis on the microbiological and sensory parameters of minced cyprinus carpio meat at 4°c storage. J Aquatic Food Prod Technol. 2017;26:593–603. [Google Scholar]
- 21.Bankova V. Recent trends and important developments in propolis research. Evid Based Complement Alternat Med. 2005;2:29–32. doi: 10.1093/ecam/neh059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ristivojevic P, Trifkovic J, Andric F, Milojkovic-Opsenica D. Poplar-type propolis: chemical composition, botanical origin and biological activity. Nat Prod Commun. 2015;10:1869–76. [PubMed] [Google Scholar]
- 23.Pellati F, Orlandini G, Pinetti D, Benvenuti S. HPLC-DAD and HPLC-ESI-MS/MS methods for metabolite profiling of propolis extracts. J Pharm Biomed Anal. 2011;55:934–48. doi: 10.1016/j.jpba.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Shi H, Yang H, Zhang X, Yu LL. Identification and quantification of phytochemical composition and anti-inflammatory and radical scavenging properties of methanolic extracts of Chinese propolis. J Agric Food Chem. 2012;60:12403–10. doi: 10.1021/jf3042775. [DOI] [PubMed] [Google Scholar]
- 25.Ristivojevic P, Trifkovic J, Gasic U, et al. Ultrahigh-performance liquid chromatography and mass spectrometry (UHPLC-LTQ/Orbitrap/MS/MS) study of phenolic profile of Serbian poplar type propolis. Phytochemical analysis: PCA. 2015;26:127–36. doi: 10.1002/pca.2544. [DOI] [PubMed] [Google Scholar]
- 26.Mavri A, Abramovic H, Polak T, et al. Chemical properties and antioxidant and antimicrobial activities of Slovenian propolis. Chem Biodivers. 2012;9:1545–58. doi: 10.1002/cbdv.201100337. [DOI] [PubMed] [Google Scholar]
- 27.Chernetsova ES, Bromirski M, Scheibner O, Morlock GE. DART-Orbitrap MS: a novel mass spectrometric approach for the identification of phenolic compounds in propolis. Anal Bioanal Chem. 2012;403:2859–67. doi: 10.1007/s00216-012-5800-6. [DOI] [PubMed] [Google Scholar]
- 28.Asgharpour F, Moghadamnia AA, Zabihi E, et al. Iranian propolis efficiently inhibits growth of oral streptococci and cancer cell lines. BMC Complement Altern Med. 2019;19:266. doi: 10.1186/s12906-019-2677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva-Carvalho R, Baltazar F, Almeida-Aguiar C. Propolis: a complex natural product with a plethora of biological activities that can be explored for drug development. Evid Based Complement Alternat Med. 2015;2015:206439. doi: 10.1155/2015/206439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirzoeva OK, Grishanin RN, Calder PC. Antimicrobial action of propolis and some of its components: the effects on growth, membrane potential and motility of bacteria. Microbiol Res. 1997;152:239–46. doi: 10.1016/S0944-5013(97)80034-1. [DOI] [PubMed] [Google Scholar]
- 31.Seidel V, Peyfoon E, Watson DG, Fearnley J. Comparative study of the antibacterial activity of propolis from different geographical and climatic zones. Phytotherapy Res. 2008;22:1256–63. doi: 10.1002/ptr.2480. [DOI] [PubMed] [Google Scholar]
- 32.Dias LG, Pereira AP, Estevinho LM. Comparative study of different Portuguese samples of propolis: Pollinic, sensorial, physicochemical, microbiological characterization and antibacterial activity. Food Chem Toxicol. 2012;50:4246–53. doi: 10.1016/j.fct.2012.08.056. [DOI] [PubMed] [Google Scholar]
- 33.Velikova M, Bankova V, Sorkun K, Houcine S, Tsvetkova I, Kujumgiev A. Propolis from the Mediterranean region: chemical composition and antimicrobial activity. Z Naturforsch C J Biosci. 2000;55:790–3. doi: 10.1515/znc-2000-9-1019. [DOI] [PubMed] [Google Scholar]
- 34.Massaro CF, Katouli M, Grkovic T, et al. Anti-staphylococcal activity of C-methyl flavanones from propolis of Australian stingless bees (Tetragonula carbonaria) and fruit resins of Corymbia torelliana (Myrtaceae) Fitoterapia. 2014;95:247–57. doi: 10.1016/j.fitote.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15:561–71. doi: 10.1002/ptr.1029. [DOI] [PubMed] [Google Scholar]
- 36.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47–e. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hehlgans S, Lange I, Eke I, Kammerer B, Cordes N. Human head and neck squamous cell carcinoma cell lines are differentially radiosensitised by the honeybee product Propolis. Int J Radiat Biol. 2011;87:243–53. doi: 10.3109/09553002.2010.533248. [DOI] [PubMed] [Google Scholar]
- 38.Asgharpour F, Moghadamnia AA, Motallebnejad M, Nouri HR. Propolis attenuates lipopolysaccharide-induced inflammatory responses through intracellular ROS and NO levels along with downregulation of IL-1β and IL-6 expressions in murine RAW 2647 macrophages. J Food Biochem. 2019;43:e12926. doi: 10.1111/jfbc.12926. [DOI] [PubMed] [Google Scholar]
- 39.Devequi-Nunes D, Machado BAS, Barreto GA, et al. Chemical characterization and biological activity of six different extracts of propolis through conventional methods and supercritical extraction. PLoS One. 2018;13:e0207676. doi: 10.1371/journal.pone.0207676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reis JHO, Barreto GA, Cerqueira JC, et al. Evaluation of the antioxidant profile and cytotoxic activity of red propolis extracts from different regions of northeastern Brazil obtained by conventional and ultrasound-assisted extraction. PLoS One. 2019;14:e0219063. doi: 10.1371/journal.pone.0219063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Utispan K, Chitkul B, Koontongkaew S. Cytotoxic activity of propolis extracts from the stingless bee trigona sirindhornae against primary and metastatic head and neck cancer cell lines. Asian Pac J Cancer Prev. 2017;18:1051–5. doi: 10.22034/APJCP.2017.18.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barlak Y, Deger O, Colak M, et al. Effect of Turkish propolis extracts on proteome of prostate cancer cell line. Proteome Sci. 2011;9:74. doi: 10.1186/1477-5956-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popolo A, Piccinelli LA, Morello S, et al. Antiproliferative activity of brown Cuban propolis extract on human breast cancer cells. Nat Prod Commun. 2009;4:1711–6. [PubMed] [Google Scholar]
- 44.Pratsinis H, Kletsas D, Melliou E, Chinou I. Antiproliferative activity of Greek propolis. J Med Food. 2010;13:286–90. doi: 10.1089/jmf.2009.0071. [DOI] [PubMed] [Google Scholar]
- 45.Eom HS, Lee EJ, Yoon BS, Yoo BS. Propolis inhibits the proliferation of human leukaemia HL-60 cells by inducing apoptosis through the mitochondrial pathway. Nat Prod Res. 2010;24:375–86. doi: 10.1080/14786410903370908. [DOI] [PubMed] [Google Scholar]
- 46.Salehi M, Motallebnejad M, Moghadamnia AA, et al. An intervention airing the effect of iranian propolis on epithelial dysplasia of the tongue: a preliminary study. J Clin Diagn Res. 2017;11:ZC67–ZC70. doi: 10.7860/JCDR/2017/24887.10249. [DOI] [PMC free article] [PubMed] [Google Scholar]