Abstract
Background
Primary insulin hypersecretion predicts type 2 diabetes (T2DM) independent of insulin resistance. Enhanced β-cell glucose responsivity contributes to insulin hypersecretion. African Americans (AAs) are at a higher risk for T2DM than non-Hispanic Whites (NHWs). Whether AAs manifest primary insulin hypersecretion is an important topic that has not been examined systematically.
Objective
To examine if nondiabetic AA adults have a higher β-cell glucose responsivity compared with NHWs.
Methods
Healthy nondiabetic AA (n = 18) and NHW (n=18) subjects were prospectively recruited. Indices of β-cell function, acute C-peptide secretion (X0); basal (Φ B), first-phase (Φ 1), second-phase (Φ 2), and total β-cell responsivity to glucose (Φ TOT), were derived from modeling of insulin, C-peptide, and glucose concentrations during an intravenous glucose tolerance test. Insulin sensitivity was assessed by the hyperinsulinemic–euglycemic glucose clamp technique.
Results
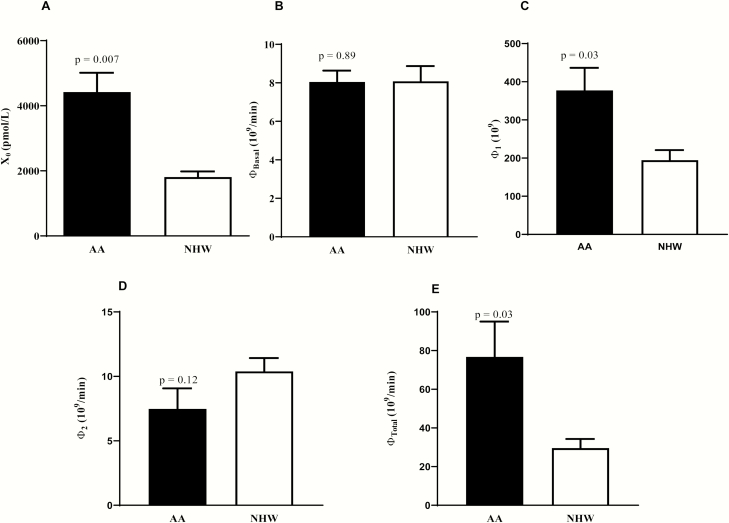
Glucose disposal rate (GDR) during clamp was similar in AAs and NHWs (GDR: [AA] 12.6 ± 3.2 vs [NHW] 12.6 ± 4.2 mg/kg fat free mass +17.7/min, P = .49). Basal insulin secretion rates were similar between the groups. AA had significantly higher X0 (4423 ± 593 vs 1807 ± 176 pmol/L, P = .007), Φ 1 [377.5 ± 59.0 vs 194.5 ± 26.6 (109) P = 0.03], and Φ TOT [76.7 ± 18.3 vs 29.6 ± 4.7 (109/min), P = 0.03], with no significant ethnic differences in Φ B and Φ 2.
Conclusions
Independent of insulin sensitivity, AAs showed significantly higher first-phase and total β-cell responsivity than NHWs. We propose that this difference reflects increased β-cell responsivity specifically to first-phase readily releasable insulin secretion. Future studies are warranted to identify mechanisms leading to primary β-cell hypersensitivity in AAs.
Keywords: African Americans, insulin sensitivity, β-cell function
African Americans (AA) have a disproportionately higher risk for the development of type 2 diabetes mellitus (T2DM) than non-Hispanic Whites (NHW) (1-3). Multiple factors including diet, socioeconomic status, body fat distribution, insulin resistance, and β-cell dysfunction have been proposed to explain the higher risk of T2DM in this population (4-8). Large epidemiological studies in the 1990s reported lower insulin sensitivity in AAs (5, 6), a finding confirmed in subsequent studies (7). Hyperinsulinemia during fasting and following a glucose load has been frequently observed in AAs (7, 9-11). Consistent with the conventionally accepted notion, lower insulin sensitivity has been suggested to be the proximal driver of compensatory increases in β-cell function and hyperinsulinemia in AAs (12-14). However, others have proposed that “primary insulin hypersecretion” may play a role in the pathogenesis of T2DM (15-17). The role of primary insulin hypersecretion in mediating increased T2DM risk in certain ethnic groups is unknown.
Primary hypersecretion is the hypersecretion of insulin that is not a compensatory response to insulin resistance, wherein both insulin resistance and insulin secretion are directly and independently measured (17). Whether AAs manifest primary insulin hypersecretion is an important topic that has not been systematically examined. In a study of obese and nondiabetic AA and white adolescents, insulin sensitivity and β-cell function were directly measured by hyperinsulinemic–euglycemic and hyperglycemic clamp techniques, respectively (18). When compared with white adolescents with similar insulin sensitivity, AA had higher first-phase insulin concentrations, suggesting an accentuated β-cell function (18). However, circulating insulin concentration is also modulated by hepatic extraction of insulin and thus does not directly assess insulin secretion or β-cell glucose sensitivity. Consequently, in this study, we sought to examine if nondiabetic AA adults have a higher β-cell glucose hyperreactivity following an intravenous glucose load when compared with NHW with similar insulin sensitivity measured using hyperinsulinemic euglycemic clamps.
Subjects and Methods
Study design and study subjects
The study protocol was approved by the Institutional Review Board of the National Institute of Diabetes, Digestive and Kidney Diseases and was conducted at the Clinical Research Center, National Institutes of Health in Bethesda, Maryland. Written informed consent was obtained from all subjects. Thirty-six healthy volunteers were prospectively recruited as part of the ongoing Obesity Phenotyping Study (ClinicalTrials.gov identifier NCT00428987). A prospective cross-sectional study design was used to compare AA (n = 18) and NHW (n = 18) adults. Insulin sensitivity was evaluated by the hyperinsulinemic–euglycemic glucose clamp and β-cell function by a frequently sampled intravenous glucose tolerance test (FSIVGTT). Participants over 18 years old with body mass index (BMI) >18.5 kg/m2 and stable weight over the last 3 months were included in the study. They were not on any medications affecting glucose metabolism, insulin sensitivity, or body weight. The exclusion criteria included diagnosis of diabetes mellitus, pregnancy, liver disease, renal insufficiency, and polycystic ovarian syndrome. Participants were admitted for a 3-night visit to the National Institutes of Health Metabolic Research Unit.
Study procedures
Assessment of body composition
Body weight was measured using a digital balance (Scale-Tronix 5702; Scale-Tronix, Carol Stream, IL). Body composition was measured by dual-energy X-ray absorptiometry with a Lunar iDXA scanner (GE Healthcare, Madison WI).
Hyperinsulinemic euglycemic glucose clamp
Insulin sensitivity was evaluated by the glucose clamp technique as previously described (19). Blood glucose concentrations were measured at the bedside every 5 to 10 minutes using a glucose analyzer (YSI 2700 Select; YSI, Yellow Springs, OH). Insulin (regular Humulin; Eli Lilly, Indianapolis, IN) was infused intravenously at 120 mU/m2/min for 3 hours using a calibrated syringe pump (model A-99; Razel Industries, Stamford, CT). During insulin infusion, blood glucose concentrations were maintained at approximately 100 mg/dL by altering the intravenous infusion rate of 20% dextrose. The rate of glucose disposal (M), a measure of insulin sensitivity, was defined as the average of the glucose infusion rate during the steady state (GDR, mg/min) corrected for estimated metabolic body size (fat-free mass + 17.7 kg). The steady-state period of the clamp was defined as a ≥25-minute period, 90 minutes after the initiation of the clamp where the coefficient of variation for plasma glucose and glucose infusion rate was <5%.
Measurement of endogenous glucose production and hepatic insulin resistance
In the healthy fasting state, plasma insulin suppresses hepatic endogenous glucose production (HGP). Hepatic insulin resistance is characterized by attenuated suppression of HGP by insulin. Accordingly, the product of basal HGP and plasma insulin concentration is a surrogate measure of hepatic insulin resistance (20). Lower insulin clearance leads to relative hyperinsulinemia in AAs. Therefore, peripheral insulin levels may not accurately reflect portal insulin concentrations (11, 21). C-peptide is released in equimolar concentrations with insulin and not extracted by the liver. Consequently, peripheral C-peptide levels more accurately reflect portal insulin. Hepatic-insulin resistance index (HIRIBasal) was calculated as product of HGP and fasting C-peptide concentration. Following an overnight fast, stable isotope tracer was used to measure glucose turnover by the tracer dilution method (n = 24). At 05.00 hours, a primed, continuous infusion of [6,6-2H2] glucose (priming dose, 28 µmol/kg; infusion rate, 0.4 µmol/kg/min for 180 minutes) was used to measure basal endogenous glucose production (Cambridge Isotope Laboratories). Euglycemic glucose clamps were then performed, but HGP was not assessed during the steady state of the clamp. Due to interruption in pharmacy compounding services and access to tracers, we could conduct tracer studies in only 24 individuals (n = 12 in each group).
Measurement of β-cell function during a frequently sampled intravenous glucose tolerance test
After a 12-hour overnight fast, an intravenous bolus of glucose (20% dextrose, 0.3 g/kg) was administered. At 20 minutes, subjects were administered an intravenous injection of insulin (0.03 units/kg) over 5 minutes. Blood samples were collected to measure plasma glucose, insulin, and C-peptide at the following time points relative to glucose administration: –10, –1, 0, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 16, 20, 22, 23, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100, 120, 150, and 180 minutes. Acute intravenous injection of glucose stimulates the release of insulin from the rapidly releasable pool and the area under the curve of plasma concentrations of insulin during the first 10 minutes is often termed the acute insulin response (AIRg). Insulin secretory rates were derived by mathematical modeling of C-peptide and glucose concentrations (22, 23). β-Cell parameters derived from the modeling include basal (Φ B), first-phase (Φ 1), second-phase (Φ 2), and total (Φ TOT) sensitivity. In addition, the amount of C-peptide released immediately following glucose administration, X0, was calculated as described (22, 23). Φ 1 is a measure of the capacity of β-cells to respond to the rate of increase in glucose concentration following an intravenous glucose load and Φ 2 reflects the ability of β-cells to deliver new pools of insulin granules to the plasma membrane in response to increases in glucose concentration (22, 23). The parameters and equations underlying the model have been published previously (22, 23). The model is described briefly:
where CP1(t) and CP2(t) are the C-peptide concentrations above basal in the accessible and peripheral compartments, respectively. k01, k12, and k21 are C-peptide kinetic parameters, X is the amount of C-peptide, X(t) is proportional to the secretion rate (SR) normalized to the C-peptide volume of distribution, m is the secretion rate constant, and CPb is the basal C-peptide concentration.
X0 is representative of first-phase secretion and the second phase secretion is denoted by the provision of C-peptide, Y. Y is influenced by the provision rate constant, α, second-phase sensitivity to glucose, β, and glucose (G) concentration above threshold value (h), [G – h].
The secretory rate is:
First-phase , where ΔG is the maximal increase in G; second-phase ; basal(Φ B) = SRb/Gb; and total (Φ TOT) =Ф 2+[Ф 1*ΔG/.
Statistical analyses
Variables with normal distributions are expressed as mean ± standard error of the mean (SEM). Variables with a nonnormal distribution are expressed as median (interquartile range). Comparisons between groups were assessed by independent unpaired t-test or the Wilcoxon–Mann–Whitney test. P < .05 was considered statistically significant. Data were analyzed with JMP version 13.0 (SAS Institute, Cary, NC) and GraphPad Prism 7 (GraphPad Software Inc, La Jolla, CA).
Results
Subject characteristics and metabolic profiles
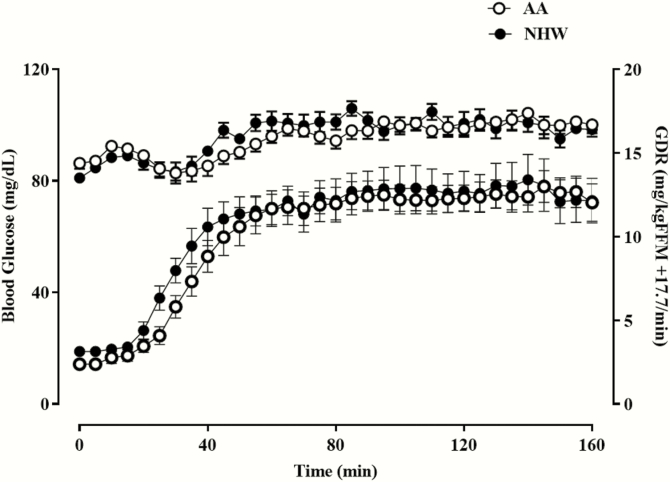
To investigate the ethnic differences in β-cell sensitivity, 18 AA and 18 NHW subjects were compared. The groups were similarly healthy, with no significant differences in percent body fat, total body fat, fat-free mass, visceral fat mass, fasting plasma glucose (FPG) and insulin concentrations, or lipid profile (Table 1). AAs had a significantly greater hemoglobin A1C (HbA1C) (P = .001). Based on clinical history, FPG, and HbA1C, none of our study participants were diabetic. Only 4 AA and 2 NHW subjects had impaired fasting glucose. QUICKI, a surrogate measure of insulin sensitivity based on fasting glucose and insulin concentrations, was not significantly different between the groups (Table 1). Direct measurement of insulin sensitivity by the glucose clamp was similar between the groups (GDR: 12.6 ± 3.2 vs 12.6 ± 4.2 mg/kg fat free mass [FFM] + 17.7/min, P = .49) (Table 1 and Fig. 1). Similarly, fasting endogenous glucose production and the hepatic insulin resistance index (HIRIBasal) did not differ between the groups (Table1). FPG was not significantly related to GDR (AA: r = –0.24, P = .33; NHW: r = –0.30, P = .22). There was no difference in this relationship between the ethnic groups.
Table 1.
Clinical and metabolic characteristics in African American (AA) and non-Hispanic White (NHW) subjects.
| NHW (n = 18) | AA (n = 18) | P value | |
|---|---|---|---|
| Age (years) | 39.5 ± 10.9 | 37.9 ± 12.4 | .65 |
| Sex (female, n) | 9 | 6 | .49 |
| Body mass index (kg/m2) | 28.7 ± 6.9 | 27.8 ± 6.8 | .66 |
| Total body fat (%) | 32.8 ± 13.4 | 27.3 ± 11.2 | .22 |
| Total body fat (kg) | 29.4 ± 17.4 | 24.8 ± 14.3 | .53 |
| Fat-free mass (kg) | 55.0 ± 95.2 | 62.9 ± 12.2 | .06 |
| Visceral adipose tissue (g) | 979 ± 800 | 963 ± 724 | .96 |
| Fasting plasma glucose (mg/dL) | 90 ± 8 | 92 ± 10 | .12 |
| Fasting plasma insulin (mU/L) | 8.5 ± 5.6 | 7.8 ± 5.6 | .09 |
| Hemoglobin A1C (%) | 5.3 ± 0.4 | 5.8 ± 0.3 | .001 |
| Total cholesterol (mg/dL) | 173 ± 37 | 177 ± 29 | .38 |
| LDL cholesterol (mg/dL) | 90 ± 39 | 100 ± 22 | .09 |
| HDL cholesterol (mg/dL) | 64 ± 21 | 61 ± 10 | .98 |
| Triglycerides (mg/dL) | 97 ± 43 | 79 ± 36 | .20 |
| Free fatty acid (mEq/L) | 0.44 ± 0.18 | 0.33 ± 0.15 | .07 |
| Glucose disposal rate (M) (mg/kgFFM +17.7/min) | 12.6 ± 4.2 | 12.6 ± 3.2 | .49 |
| QUICKI | 0.36 ± 0.03 | 0.35 ± 0.04 | .81 |
| Basal HGP (mg/kg/min) | 2.54 ± 0.31 | 2.49 ± 0.34 | .64 |
| Hepatic insulin resistance index (mg/kg/min.ng/mL) | 6.40 ± 3.88 | 7.24 ± 3.33 | .38 |
Data are presented as arithmetic mean ± SD; P values indicate significance for comparisons between ethnic groups.
Abbreviations: n, no. of subjects. QUICKI, quantitative insulin sensitivity check index; HGP, hepatic glucose production.
Figure 1.
Hyperinsulinemic euglycemic glucose clamp studies in AA and NHW subjects. Data shown are the mean ± SEM for blood glucose concentration and glucose infusion rate plotted as a function of time.
Measures of β-cell sensitivity in AA and NHW
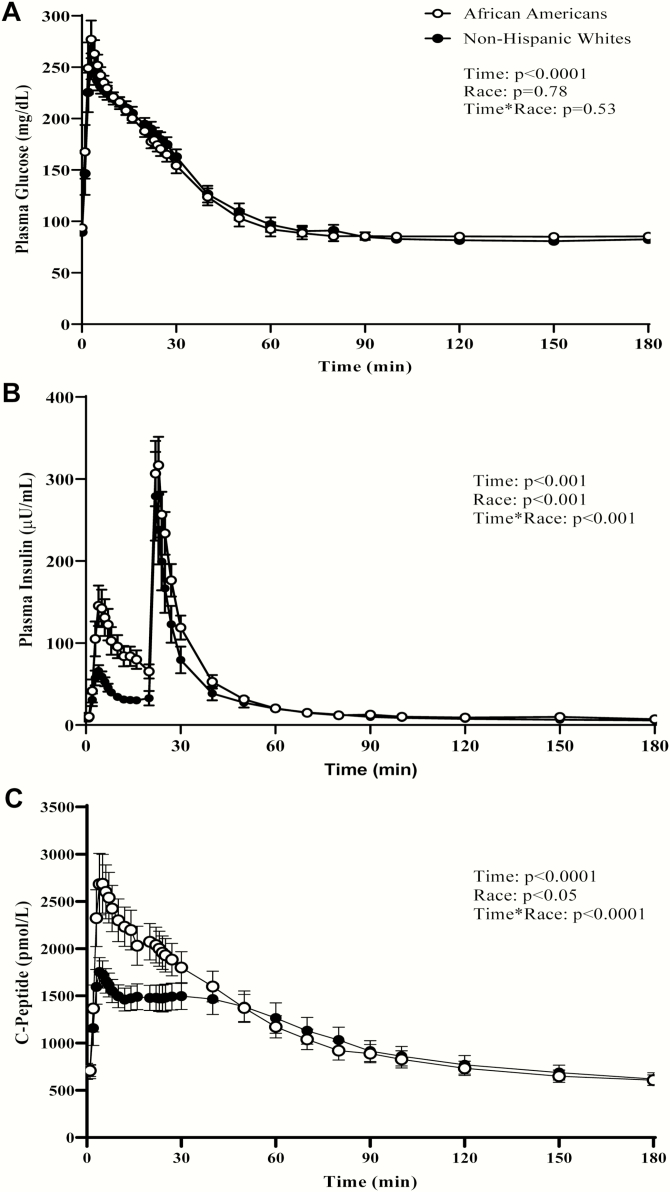
Time courses of plasma glucose, insulin, and C-peptide concentrations during an IVGTT in AA and NHW are shown in Fig. 2. Plasma glucose levels following intravenous glucose administration were not different between the ethnic groups over time (time*race interaction, P = .53) (Fig. 2A). AIRg was higher in AA (mean ± SEM, 910.3 ± 157.2 vs 340.3 ± 41.7 µU/mL/min, P = .01). Early insulin secretion (<30 minutes) was significantly higher in AA than in NHW (race effect, P < .001 and time*race interaction, P < .0001) (Fig. 2B). Baseline plasma C-peptide levels did not differ between the groups (722.6 ± 64.5 vs 687.7 ± 73.4 pmol/L, P = .72) (Fig. 2C). C-peptide AUC0-30min during the first 30 minutes was higher in AAs (62.4 ± 5.7 vs 43.9 ± 3.9 nmol, P < .001), but C-peptide AUC0-180min was not significantly different between the groups during the entire course of the test (P = .70). X0 which represents acute C-peptide release following glucose administration was significantly higher in AAs (Fig. 3A). Mean basal insulin secretion rate, SRb, was similar between the ethnic groups (41.3 ± 3.7 vs 40.5 ± 4.3 pmol/min/L, P = .70). Likewise, Φ B, basal β-cell glucose sensitivity was not significantly different between groups (Fig. 3B) (Table 2). AAs displayed a significantly higher Φ 1 than NHWs (377.5 ± 59.0 vs 194.5 ± 26.6 [109], P = .03) (Fig. 3C) (Table 2). Φ 2 was comparable between the groups (7.5 ± 1.6 vs 10.4 ± 1.0 [109/min], P = .12) (Fig. 3D) (Table 2). Overall β-cell glucose sensitivity, Φ Total, was significantly higher in AAs (76.7 ± 18.3 vs 29.6 ± 4.7 [109/min], P = .03) (Fig. 3E and Table 2). Post hoc sex difference analyses were conducted between and within the 2 ethnic groups to examine differences in indices of insulin sensitivity and β-cell function (Table 2). AA women had a significantly higher GDR, X0, and Φ 1 than AA men. In contrast, NHW men tended to have higher Φ 1 and a higher Φ Total than NHW women. AA women had higher X0, Φ 1, and Φ Total than NHW women, while there were no significant ethnic differences in these parameters between men (Table 2). Furthermore, when the groups were stratified by BMI categories (lean, overweight, or obese), there was no interaction between race and BMI category on any of the parameters considered, suggesting that BMI had no significant modulating effect on the observed ethnic differences (data not shown).
Figure 2.
Plasma levels of glucose (A), insulin (B), and C-peptide (C), in adult African Americans (AA) and non-Hispanic Whites (NHW) during an insulin-modified intravenous glucose test (IM-FSIVGT). Data shown are mean ± SEM. P values for the effects of racial group, time, and the race*time interaction were obtained with repeated-measures analysis of variance with post hoc Bonferroni’s test.
Figure 3.
Model estimates of β-cell function during an insulin-modified intravenous glucose test. X0 (A), Φ Basal (B), Φ 1 (C), Φ 2 (D), and Φ Total (E) in adult African Americans (AA) and non-Hispanic Whites (NHW). Data shown are mean ± SEM. Comparisons between groups were assessed by independent unpaired t-test or The Wilcoxon-Mann-Whitney test.
Table 2.
Measures of insulin sensitivity and β-cell function in African American (AA) and non-Hispanic White (NHW) subjects.
| NHW (n=18) (F/M, 6/12) | AA (n=18) (F/M, 9/9) | P value | |
|---|---|---|---|
| Glucose disposal rate (M) (mg/kgFFM +17.7/min) | 12.6 ± 4.2 | 12.6 ± 3.2 | .49 |
| Females (F) | 12.4 ± 4.9 | 15.1 ± 1.8 | .08 |
| Males (M) | 12.8 ± 3.7 | 11.3 ± 3.1 | .55 |
| P value | .45 | .02 | |
| Hepatic insulin resistance index (mg/kg/min x ng/mL) | 6.40 ± 3.88 | 7.24 ± 3.33 | .38 |
| Females | 6.89 ± 3.83 | 5.79 ± 1.01 | 1.00 |
| Males | 6.09 ± 4.14 | 8.27 ± 4.08 | .27 |
| P value | .71 | .32 | |
| Acute insulin response (µU/mL/min) | 340 ± 177 | 910 ± 667 | .03 |
| Females | 358 ± 186 | 1095 ± 286 | .002 |
| Males | 323 ± 177 | 818 ± 788 | .37 |
| P value | .54 | .22 | |
| X0 (nmol/min/L) | 1.81 ± 0.75 | 4.42 ± 2.44 | .007 |
| Females | 1.62 ± 0.81 | 6.39 ± 1.91 | .002 |
| Males | 1.99 ± 0.67 | 3.35 ± 2.03 | .19 |
| P value | .25 | .02 | |
| Φ Basal (109/min) | 8.08 ± 3.34 | 8.05 ± 2.49 | .89 |
| Females | 9.00 ± 3.89 | 8.39 ± 2.83 | .77 |
| Males | 7.16 ± 2.59 | 7.87 ± 2.42 | .50 |
| P value | .33 | 1.00 | |
| Φ 1 (109) | 194 ± 112 | 377 ± 250 | .03 |
| Females | 150 ± 65 | 629 ± 178 | .002 |
| Males | 238 ± 135 | 251 ± 174 | 1.00 |
| P value | .09 | .003 | |
| Φ 2 (109/min) | 10.39 ± 4.37 | 7.48 ± 6.73 | .12 |
| Females | 11.67 ± 4.87 | 6.98 ± 9.72 | .17 |
| Males | 9.11 ± 3.64 | 7.73 ± 5.19 | .64 |
| P value | .33 | .39 | |
| Φ Total (109/min) | 29.6 ± 19.8 | 76.7 ± 75.3 | .03 |
| Females | 20.4 ± 8.63 | 106.7 ± 93.1 | .002 |
| Males | 38.8 ± 23.9 | 60.4 ± 62.5 | .88 |
| P value | .03 | .13 |
Data are presented as arithmetic mean ± SD; n, no. of subjects. P values indicate significance for comparisons between ethnic groups or between sexes.
Relationships between adiposity and measures of β-cell sensitivity
Φ B was positively related to percent body fat (NHW: r = 0.65, P < .05; AA: r = 0.62, P < .05) and visceral fat mass (NHW: r = 0.70, P < .05; AA: r = 0.56, P < .05). In NHWs, but not AAs, Φ 1, Φ 2, and Φ Total were significantly (all P < .05) correlated with percent body fat (r = –0.50, r = 0.54, and r = –0.57, respectively). There were no significant relationships between Φ 1, Φ 2, and Φ Total and visceral fat mass in either group. Ethnic differences in Φ 1 and Φ Total remained significantly different even after adjusting for percent body fat. There was no significant relationship between AIR and GDR in either ethnic group (AA: r = 0.33, P = 0.16; NHW: r = 0.03, P = .88).
Discussion
In the present study, we demonstrate that AA adults have a higher β-cell glucose hyperreactivity than similarly insulin-sensitive NHWs. These conclusions are based on results from a cohort of AA and NHW in whom insulin sensitivity and insulin secretion were separately measured. We demonstrated early hyperinsulinemia (higher AIRg and X0), greater total β-cell glucose responsivity, and higher Φ 1, a measure of early insulin secretion that is proportional to the rate of increase of glucose in AA. These results suggest that AAs have primary β-cell glucose hypersensitivity, specifically to first-phase insulin secretion in response to a glucose load.
Few studies have examined ethnic differences in β-cell insulin secretion using C-peptide measurements during an FSIVGTT (10, 24). β-Cell glucose responsivity was examined in normoglycemic AA and European American (EA) girls (7-12 years) and women (18-70 years) following an IVGTT (24). AA women had lower SI and higher X0 and Φ 1 than EA women. In this study, higher X0 and β-cell responsivity may thus be a compensatory response to lower insulin sensitivity in AAs. In addition, differences in age, body composition, and hormonal status among prepubertal girls, premenopausal, and postmenopausal women could also have impacted measures of β-cell function. Furthermore, measures of β-cell function and insulin sensitivity were derived from FSIVGTT and not independently assessed in this study (24). In contrast, insulin sensitivity (by hyperinsulinemic–euglycemic clamp) and β-cell function (by hyperglycemic clamp) were directly and independently measured in obese and nondiabetic AA and white adolescents (18). Insulin-mediated glucose disposal (M) was similar in AA and white adolescents, but first-phase insulin concentrations were higher in AAs during the hyperglycemic clamp (by ~63%). Based on these findings, the authors suggested that β-cell function was “upregulated” in AA adolescents. Stefan et al. measured AIR following an intravenous glucose load and insulin sensitivity by the hyperinsulinemic–euglycemic clamp technique in age, sex, and body fat content-matched AA and Caucasians (25). Insulin sensitivity was similar between the groups, but AIR was significantly higher in AA leading the authors to suggest that AA manifest “exaggerated” insulin secretion. Lower hepatic clearance of insulin typically seen in AA (10, 11) may have partly contributed to the higher AIR observed in these studies (18, 25). Since C-peptide levels were not measured in these studies, insulin secretion and β-cell glucose responsivity were not assessed (18, 25). Nevertheless, these studies suggest that first-phase insulin release was upregulated in AA adolescents and adults.
The hyperinsulinemic–euglycemic clamp technique is the gold standard technique for assessing insulin sensitivity in humans. In clamps performed at an insulin dose of 120 mU/m2, the cutoff for defining insulin resistance is an M value of 5.6 mg/kg FFM + 17.7/min (26). Mean glucose disposal rate in our cohort was 12.6 ± 3.7 mg/kg FFM + 17.7/min with no significant difference between the groups (Table 1 and Fig. 1). The sensitivity of insulin-induced HGP suppression is higher than that of peripheral glucose disposal by insulin. Thus, there may be incomplete suppression of HGP at lower clamp insulin infusion rates or in insulin-resistant populations such as AA (27). Studies have previously reported that AAs have fasting and postprandial hyperinsulinemia and are less insulin sensitive (5, 6). The 120 mU/m2/min dose of insulin used for this study was chosen to completely suppress endogenous HGP and to avoid missing potential differences in peripheral insulin sensitivities among subjects.
Hepatic insulin resistance induces β-cell hyperplasia and compensatory insulin hypersecretion (28, 29). Hepatic insulin resistance indices are calculated as the product of HGP and the corresponding plasma insulin concentration either at baseline HIRIBasal (basal HGP × fasting plasma insulin) or during the steady state of the glucose clamp (HIRIClamp) (20, 27). Previously we have shown that HIRIBasal is highly correlated with HIRIClamp (n = 653, r = 0.69, P < .001) (30). Consequently, this index was adequate to measure hepatic insulin resistance in our study. Hepatic resistance index was also similar between the ethnic groups in our cohort (Table 1). Both AA and NHW participants in our cohort were highly insulin sensitive and ideal to study ethnic differences in β-cell function. In the setting of similar peripheral and hepatic insulin sensitivity, we observed a higher AIR, X0, Φ 1, and Φ Total in AA. These findings suggest that upregulation of β-cell function observed in AAs is independent of insulin sensitivity.
Dynamic responsivity indices measure the capacity of β-cells to respond to the rate of increase of glucose concentration following an intravenous (Φ 1) or oral glucose load (Φ D) (22). Static responsivity indices reflect the ability of β-cell to deliver new pool of insulin granules in response to continued increases in glucose concentration (Φ 2 and Φ S) (22). Few studies in women have examined differences in β-cell glucose responsivity in AA and NHW people (24, 31). In healthy, normoglycemic AA adult females, dynamic responsivity indices (Φ 1 and Φ D), but not static responsivity, were higher when compared with their white counterparts (24, 31). Our findings are consistent with these studies, albeit AA subjects in the aforementioned studies were less insulin sensitive than white people. Glucose-induced first-phase insulin secretion is instantaneous and is the result of exocytosis of insulin from primed vesicles docked at the plasma membrane termed the readily releasable pool (RRP). Following intravenous glucose challenge, change in the amount of insulin in RRP (IRRP) is proportional to the product of kRRP (a first-order rate constant) and IRRP (–kRRP × IRRP). First-phase insulin secretion is dependent on the size of RRP (IRRP-basal) at baseline glucose concentration. Xie et al. used a pharmacokinetic (PK/PD) model to examine ethnic differences in glucose-induced insulin response in AA (n = 15) and Caucasian children and adolescents (n = 18) (32). They found that IRRP-basal and the product, kRRP × IRRP was higher by ~63% in AA than in Caucasians. This suggests that even at a younger age, the amount of RRP is higher in AA children than in Caucasians. In contrast to these indices derived from insulin concentrations (eg, AIR) that are confounded by hepatic insulin extraction, Φ 1 is exclusively a measure of β-cell function. The higher Φ 1 suggests that the magnitude of RRP is greater in AA and is independent of adiposity and insulin sensitivity. These findings collectively point to an increased β-cell hypersensitivity to glucose in AA.
In our study, it appears that women, but not men, contribute to the observed higher β-cell insulin secretion and sensitivity in AAs. Not surprisingly, there are no prior studies examining the effects of sex on racial differences in β-cell function. However, as observed in our study, African-Caribbean females had a higher AIRg than African-Caribbean males and white women (8). Our study was not designed to test sex differences in β-cell function. Considering the small sample size of women (n = 6-9) our study lacks the statistical power to test interaction by sex. Post hoc sex difference analyses should be interpreted with caution and our finding should be confirmed by larger studies with an a priori intent aimed at elucidating the effects of sex on β-cell function in this population (33).
The cellular mechanisms and factors that underlie heightened β-cell sensitivity in AA are unknown and not the focus of this study. Amino acids (34), free fatty acids (35), incretins (36), neural tone (37), and expression of cellular soluble N-ethylmaleimide sensitive factor attachment protein receptors (SNARE) (38, 39) have all known to modulate insulin secretion. AIRg is negatively correlated with FPG (40). In our study, there were no ethnic differences in FPG. However, HbA1C was higher in AAs, consistent with other studies in nondiabetic individuals suggesting glycemia-independent ethnic effect on HbA1C (41). Nevertheless, β-cell responsivity measures were still significantly higher in AA after adjusting for HbA1C. Ethnicity is known to modulate the association of adiposity and β-cell function (8, 24). Φ 1, Φ 2, and Φ Total were correlated with percent body fat in NHW but not AA. However, in our study, even after adjusting for percent body fat, AA had a higher Φ 1 and Φ Total than NHW.
Primary insulin hypersecretion has been proposed to precede the development of insulin resistance and subsequently increase the risk for T2DM (15-17, 42-45). Likewise, β-cell “exhaustion” due to increased insulin secretion has also been posited to play an independent role in the pathogenesis of glucose intolerance (46, 47). Insulin sensitivity (on the x-axis) and β-cell function (y-axis) are inversely related and described by a hyperbolic curve. In an normal glucose tolerant individual, decreases in insulin sensitivity is associated with a compensatory increase in β-cell function to achieve “normal” glucose homeostasis. This moving up the curve is referred to as “canalization” and an optimum phenotype would be a stabilization point in the zone of maximum robustness where changes in insulin sensitivity are easily offset by concurrent changes in β-cell function. Indeed, the stabilization points of Europeans and AAs are different, with the former in the most robust optimum zone and the latter occupying an “unstable” extreme point (7, 48, 49). Thus, AAs are more prone to “falling” off this curve if unable to maintain the high degree of first-phase insulin secretion in the face of small changes in insulin sensitivity. Consistent with this hypothesis, insulin secretion following oral glucose load was lower in black Africans than in Europeans with early T2DM (50). However, more research is needed to determine whether therapeutic agents that are known to improve β-cell function such as GLP-1 agonists and thiazolidinediones may be appropriate in early T2DM in AAs (51).
This study has strengths and few limitations. An important strength is that the study explicitly examines ethnic differences in β-cell sensitivity in similarly insulin-sensitive nondiabetic adults assessed independently by glucose clamp technique. One of the limitations is that we did not examine if β-cell function in AAs is also exaggerated following an oral glucose load that is more physiological and includes the incretin effect. Specifically, an oral glucose tolerance test would have been ideal. Although all our subjects were nondiabetic, glucose tolerance status following an oral glucose tolerance test was not assessed directly. Also, ethnicity for both cohorts was self-reported. Lastly, the observational nature and relatively small sample size in each group are additional limitations.
To conclude, independent of insulin sensitivity, AA people showed significantly higher first-phase and total β-cell responsivity than NHW people. We propose that this difference describes variability in the quantity or response intensity of readily releasable insulin following a glucose load. This perspective of β-cell dynamics in AAs and NHWs offers insight into the pathophysiologic mechanisms differentially driving T2DM risk. Future studies are warranted to identify mechanisms leading to primary β-cell hypersensitivity in AAs.
Acknowledgments
Financial Support: Funding is provided by the Intramural Research Program at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland.
Clinical Trial Information: ClinicalTrials.gov Identifier: NCT00428987 (January 30, 2007).
Glossary
Abbreviations
- Φ1
first-phase β-cell responsivity to glucose
- Φ2
second-phase β-cell responsivity to glucose
- ΦB
basal β-cell responsivity to glucose
- ΦTOT
total β-cell responsivity to glucose
- AA
African American
- AIRg
acute insulin response to glucose; FFM, fat free mass
- FPG
fasting plasma glucose
- GDR
glucose disposal rate
- HbA1C
hemoglobin A1C
- HGP
hepatic endogenous glucose production
- HIRIBasal
hepatic-insulin resistance index
- IVGTT
intravenous glucose tolerance test
- NHW
non-Hispanic White
- RRP
readily releasable pool
- T2DM
type 2 diabetes mellitus
- X0
acute C-peptide secretion
Additional Information
Disclosure Summary: The authors have nothing to disclose
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Bancks MP, Kershaw K, Carson AP, Gordon-Larsen P, Schreiner PJ, Carnethon MR. Association of modifiable risk factors in young adulthood with racial disparity in incident type 2 diabetes during middle adulthood. JAMA. 2017;318(24):2457-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283(17):2253-2259. [DOI] [PubMed] [Google Scholar]
- 3. Resnick HE, Valsania P, Halter JB, Lin X. Differential effects of BMI on diabetes risk among black and white Americans. Diabetes Care. 1998;21(11):1828-1835. [DOI] [PubMed] [Google Scholar]
- 4. Piccolo RS, Subramanian SV, Pearce N, Florez JC, McKinlay JB. Relative contributions of socioeconomic, local environmental, psychosocial, lifestyle/behavioral, biophysiological, and ancestral factors to racial/ethnic disparities in type 2 diabetes. Diabetes Care. 2016;39(7):1208-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haffner SM, D’Agostino R, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45(6):742-748. [DOI] [PubMed] [Google Scholar]
- 6. Haffner SM, Howard G, Mayer E, et al. Insulin sensitivity and acute insulin response in African-Americans, non-Hispanic whites, and Hispanics with NIDDM: the Insulin Resistance Atherosclerosis Study. Diabetes. 1997;46(1):63-69. [DOI] [PubMed] [Google Scholar]
- 7. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goff LM, Griffin BA, Lovegrove JA, et al. ; RISCK Study Group Ethnic differences in beta-cell function, dietary intake and expression of the metabolic syndrome among UK adults of South Asian, black African-Caribbean and white-European origin at high risk of metabolic syndrome. Diab Vasc Dis Res. 2013;10(4):315-323. [DOI] [PubMed] [Google Scholar]
- 9. Osei K, Cottrell DA, Harris B. Differences in basal and poststimulation glucose homeostasis in nondiabetic first degree relatives of black and white patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 1992;75(1):82-86. [DOI] [PubMed] [Google Scholar]
- 10. Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med. 1994;11(8):755-762. [DOI] [PubMed] [Google Scholar]
- 11. Piccinini F, Polidori DC, Gower BA, Bergman RN. Hepatic but not extrahepatic insulin clearance is lower in African American than in European American women. Diabetes. 2017;66(10):2564-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42(11):1663-1672. [DOI] [PubMed] [Google Scholar]
- 13. Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J Clin Invest. 1997;100(5):1166-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(Suppl 2):S262-S268. [DOI] [PubMed] [Google Scholar]
- 16. Thomas DD, Corkey BE, Istfan NW, Apovian CM. Hyperinsulinemia: an early indicator of metabolic dysfunction. J Endocr Soc. 2019;3(9):1727-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trico D, Natali A, Arslanian S, Mari A, Ferrannini E. Identification, pathophysiology, and clinical implications of primary insulin hypersecretion in nondiabetic adults and adolescents. JCI Insight. 2018;3(24):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hannon TS, Bacha F, Lin Y, Arslanian SA. Hyperinsulinemia in African-American adolescents compared with their American white peers despite similar insulin sensitivity: a reflection of upregulated beta-cell function? Diabetes Care. 2008;31(7):1445-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muniyappa R, Karne RJ, Hall G, et al. Oral glucosamine for 6 weeks at standard doses does not cause or worsen insulin resistance or endothelial dysfunction in lean or obese subjects. Diabetes. 2006;55(11):3142-3150. [DOI] [PubMed] [Google Scholar]
- 20. Choukem SP, Gautier JF. How to measure hepatic insulin resistance? Diabetes Metab. 2008;34(6 Pt 2):664-673. [DOI] [PubMed] [Google Scholar]
- 21. Osei K, Schuster DP, Owusu SK, Amoah AG. Race and ethnicity determine serum insulin and C-peptide concentrations and hepatic insulin extraction and insulin clearance: comparative studies of three populations of West African ancestry and white Americans. Metabolism. 1997;46(1):53-58. [DOI] [PubMed] [Google Scholar]
- 22. Cobelli C, Toffolo GM, Dalla Man C, et al. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007;293(1):E1-E15. [DOI] [PubMed] [Google Scholar]
- 23. Toffolo G, Cefalu WT, Cobelli C. Beta-cell function during insulin-modified intravenous glucose tolerance test successfully assessed by the C-peptide minimal model. Metabolism. 1999;48(9):1162-1166. [DOI] [PubMed] [Google Scholar]
- 24. Chandler-Laney PC, Phadke RP, Granger WM, et al. Adiposity and β-cell function: relationships differ with ethnicity and age. Obesity 2010;18(11):2086-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stefan N, Stumvoll M, Weyer C, Bogardus C, Tataranni PA, Pratley RE. Exaggerated insulin secretion in Pima Indians and African-Americans but higher insulin resistance in Pima Indians compared to African-Americans and Caucasians. Diabet Med. 2004;21(10):1090-1095. [DOI] [PubMed] [Google Scholar]
- 26. Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 2012;35(7):1605-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15-E26. [DOI] [PubMed] [Google Scholar]
- 28. Escribano O, Guillén C, Nevado C, Gómez-Hernández A, Kahn CR, Benito M. Beta-cell hyperplasia induced by hepatic insulin resistance: role of a liver-pancreas endocrine axis through insulin receptor A isoform. Diabetes. 2009;58(4):820-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Faerch K, Brøns C, Alibegovic AC, Vaag A. The disposition index: adjustment for peripheral vs. hepatic insulin sensitivity? J Physiol. 2010;588(Pt 5):759-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muniyappa R, Tella SH, Sortur S, et al. Predictive accuracy of surrogate indices for hepatic and skeletal muscle insulin sensitivity. J Endocr Soc. 2019;3(1):108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chung ST, Galvan-De La Cruz M, Aldana PC, et al. Postprandial insulin response and clearance among black and white women: the federal women’s study. J Clin Endocrinol Metab. 2019;104(1):181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xie L, Hoffman RP, Veng-Pedersen P. Population analysis of ethnicity and first-phase insulin release. Diabetes Res Clin Pract. 2010;89(3):243-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rich-Edwards JW, Kaiser UB, Chen GL, Manson JE, Goldstein JM. Sex and gender differences research design for basic, clinical, and population studies: Essentials for Investigators. Endocr Rev. 2018;39(4):424-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Floyd JC Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45(9):1487-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crespin SR, Greenough WB 3rd, Steinberg D. Stimulation of insulin secretion by long-chain free fatty acids. A direct pancreatic effect. J Clin Invest. 1973;52(8):1979-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aulinger BA, Vahl TP, Wilson-Pérez HE, Prigeon RL, D’Alessio DA. β-cell sensitivity to GLP-1 in healthy humans is variable and proportional to insulin sensitivity. J Clin Endocrinol Metab. 2015;100(6):2489-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahrén B. Autonomic regulation of islet hormone secretion–implications for health and disease. Diabetologia. 2000;43(4):393-410. [DOI] [PubMed] [Google Scholar]
- 38. Thurmond DC, Gaisano HY. Recent insights into beta-cell exocytosis in Type 2 diabetes. J Mol Biol. 2020;432(5):1310-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oh E, Stull ND, Mirmira RG, Thurmond DC. Syntaxin 4 up-regulation increases efficiency of insulin release in pancreatic islets from humans with and without type 2 diabetes mellitus. J Clin Endocrinol Metab. 2014;99(5):E866-E870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brunzell JD, Robertson RP, Lerner RL, et al. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab. 1976;42(2):222-229. [DOI] [PubMed] [Google Scholar]
- 41. Cavagnolli G, Pimentel AL, Freitas PA, Gross JL, Camargo JL. Effect of ethnicity on HbA1c levels in individuals without diabetes: Systematic review and meta-analysis. Plos One. 2017;12(2):e0171315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carnethon MR, Palaniappan LP, Burchfiel CM, Brancati FL, Fortmann SP. Serum insulin, obesity, and the incidence of type 2 diabetes in black and white adults: the atherosclerosis risk in communities study: 1987-1998. Diabetes Care. 2002;25(8):1358-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Corkey BE. Diabetes: have we got it all wrong? Insulin hypersecretion and food additives: cause of obesity and diabetes? Diabetes Care. 2012;35(12):2432-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dankner R, Chetrit A, Shanik MH, Raz I, Roth J. Basal-state hyperinsulinemia in healthy normoglycemic adults is predictive of type 2 diabetes over a 24-year follow-up: a preliminary report. Diabetes Care. 2009;32(8):1464-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes. 2000;49(12):2094-2101. [DOI] [PubMed] [Google Scholar]
- 46. Hudish LI, Reusch JE, Sussel L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest. 2019;129(10):4001-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brown RJ, Rother KI. Effects of beta-cell rest on beta-cell function: a review of clinical and preclinical data. Pediatr Diabetes. 2008;9(3 Pt 2):14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gibson G. Decanalization and the origin of complex disease. Nat Rev Genet. 2009;10(2):134-140. [DOI] [PubMed] [Google Scholar]
- 49. Diamond J. The double puzzle of diabetes. Nature. 2003;423(6940):599-602. [DOI] [PubMed] [Google Scholar]
- 50. Mohandas C, Bonadonna R, Shojee-Moradie F, et al. Ethnic differences in insulin secretory function between black African and white European men with early type 2 diabetes. Diabetes Obes Metab. 2018;20(7):1678-1687. [DOI] [PubMed] [Google Scholar]
- 51. Consortium R. Lack of durable improvements in beta-cell function following withdrawal of pharmacological interventions in adults with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care. 2019;42(9):1742-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]