Abstract
Cardiovascular disease (CVD) is an increasingly important cause of morbidity and mortality among people living with HIV (PLWH) now that HIV is a manageable chronic disease. Identification and treatment of comorbid medical conditions for PLWH, including CVD and its risk factors, typically lack a critical component of care: integrated care for histories of trauma. Experiences of trauma are associated with increased HIV infection, CVD risk, inconsistent treatment adherence, and poor CVD outcomes. To address this deficit among those at greatest risk and disproportionately affected by HIV and trauma–i.e., Black and Latinx individuals–a novel culturally-congruent, evidence-informed care model, “Healing our Hearts, Minds and Bodies” (HHMB), has been designed to address patients' trauma histories and barriers to care, and to prepare patients to engage in CVD risk reduction. Further, in recognition of the need to ensure that PLWH receive guideline-concordant cardiovascular care, implementation strategies have been identified that prepare providers and clinics to address CVD risk among their Black and Latinx PLWH. The focus of this paper is to describe the hybrid Type 2 effectiveness/implementation study design, the goal of which is to increase both patient and organizational readiness to address trauma and CVD risk among 260 Black and Latinx PLWH recruited from two HIV service organizations in Southern California. This study is expected to produce important information regarding the value of the HHMB intervention and implementation processes and strategies designed for use in implementing HHMB and other evidence-informed programs in diverse, resource-constrained treatment settings, including those that serve patients living in deep poverty. Clinical trials registry: NCT04025463.
Keywords: Cardiovascular disease, HIV, Black and Latinx, Trauma, Effectiveness, Implementation
Cardiovascular disease (CVD) is a serious concern among people living with HIV (PLWH). Antiretroviral therapy (ART) use has increased the survival of PLWH, but CVD mortality is rising in the face of lower overall mortality.1 After adjustment for traditional CVD risk factors, PLWH have almost 50% higher risk for myocardial infarction (MI) than the general population.2,3 The mechanisms underlying the association between HIV infection, ART, and CVD risk are not fully understood, yet factors that may contribute to this heightened risk include: HIV infection is associated with dyslipidemia and inflammation; ART agents increase the risk of diabetes and dyslipidemia; and the HIV infected population is living longer, resulting in higher rates of traditional CVD risk factors (e.g., hypertension, diabetes, hyperlipidemia).4 Among age-matched African American and Latinx patients, including those with or without HIV, traditional CVD risk factors are more common or more poorly controlled than among white patients.5 Additionally, PLWH have high rates of traditional behavioral and social risk factors (e.g., smoking, sedentary lifestyle, low income), and high rates of nontraditional risk factors (e.g., Hepatitis C infection, substance use).6
A history of trauma may adversely affect healthcare utilization and adherence among PLWH as histories of trauma and adversity are associated with decreased healthcare utilization, inconsistent treatment adherence, and engagement in HIV-risk behaviors.7,8 Although PLWH have access to HIV medical care, trauma increases their risk for treatment dropout and non-adherence.9 It is estimated that 50–80% of PLWH with histories of trauma do not fully comply with ART protocols.10 Although the reasons for non-adherence vary, trauma is thought to negatively impact provider-patient relationships. For example, PLWH with severe trauma histories tend to experience medical care as both intrusive and re-traumatizing.9 A lack of supportive clinic environments can increase exposure to trauma-related triggers.11,12 Most PLWH do not disclose past sexual trauma to HIV providers, despite extensive evidence that psychological and behavioral correlates of these experiences compromise HIV and other medical care engagement.13 Additional barriers to effective prevention and treatment efforts include distrust of medical providers and institutions–particularly among African American PLWH–coupled with disparities in quality of care.14 Conversely, trust in one's medical care providers is associated with increased clinic visits, treatment adherence, and greater health and mental health, regardless of ethnicity, suggesting the importance of addressing these issues in HIV treatment paradigms.14
Post-traumatic stress disorder (PTSD) is associated with adverse CVD outcomes, including risk for pulmonary disease and coronary artery disease.15,16 Women develop PTSD two to three times more often than men after experiencing trauma, yet most research on the association between trauma, PTSD, and CVD has been conducted with men.17 Among women, both childhood and adult abuse are associated with increased risk for diabetes, CVD, and chronic pain.18 Gender differences have also been found between low childhood socio-economic status, family instability, and poor health in adulthood. For example, compared with men, the risk of MI or other CVD is more strongly associated with adverse childhood conditions for women.19,20 Both trauma and PTSD have been associated with physiological processes that may affect CVD, including dysregulation in the autonomic nervous system and the hypothalamic-pituitary adrenal axis, resulting in elevations in blood pressure, heart rate, dysregulation of cortisol, increased secretion of catecholamines, and inflammation.21-23 These relationships may be greater for African American and impoverished individuals, particularly women, who experience socioeconomic and racial/ethnic disparities in CVD risk.24 Research is needed to examine the cumulative effects of trauma on CVD, as repeated exposures may increase risk for heart attack and other CVD, even in individuals who display few or no symptoms of PTSD.20,24
Health systems that coordinate and integrate care across HIV and chronic conditions such as CVD for men and women may provide the infrastructure needed to address the complex interplay with trauma symptomatology (e.g., PTSD and depression). A novel, culturally-congruent, evidence-informed care model called, “Healing our Hearts, Minds and Bodies” (HHMB) has been designed to address patients' trauma histories and barriers to care, and to prepare patients to engage in CVD risk reduction. Recognizing the need to ensure that PLWH receive CVD guideline concordant care, implementation strategies have also been developed to prepare providers and clinics to address CVD risk among their HIV-positive patients. The goal of this study is to increase both patient and organizational readiness to address trauma and CVD risk among PLWH. The HHMB study addresses the need for greater patient, provider, and health system focus on CVD risk in PLWH by incorporating both implementation and effectiveness aims using hybrid type 2 effectiveness/implementation study design,25 which have a “dual focus on effectiveness and implementation outcomes; these designs allow for the simultaneous testing or piloting of implementation strategies during what is otherwise an effectiveness trial.”26 The specific aims are as follows:
To assess and enhance organizational readiness for addressing trauma and CVD risk among Black and Latinx PLWH. Specifically, a phased approach will drive the use of implementation strategies designed to educate, monitor, and support providers and staff in adhering to CVD care guidelines;
To use mixed methods to: (a) Evaluate the use and effectiveness of implementation strategies over time, and (b) Identify barriers and facilitators to organizational adoption of guidelines, provider adherence to guidelines, feasibility, and sustainability; and
To evaluate the effect of HHMB on cognitive-behavioral, emotional, and physical outcomes among 260 PLWH, specifically: patient activation, engagement in care, knowledge of CVD risk, adherence to clinicians' recommendations, cardiovascular health, mental health symptoms, and satisfaction with care.
Methods/design
Overview of study design
Drawing on a multi-framework approach articulated by Damschroder and colleagues,27 an adapted version of the Replicating Effective Programs (REP) framework28 will guide the use of implementation strategies and the tailoring of the HHMB intervention within the two participating implementation settings. The Consolidated Framework for Implementation Research (CFIR)29 will be used to guide the evaluation analyses.
The organizational-level sample for this study is the collaborative network of the two clinics, both of which serve large numbers of adult African American or Black (hereafter referred to as Black) and Hispanic, Latino, or Latinx (hereafter referred to as Latinx) men and women ages 18 to 75 with a dianosis of HIV or AIDS. It is expected that 21 staff members across the two agencies will complete the organizational survey and qualitative interviews, although all agency staff will be recruited to participate. The community organization partners are similar to those that would be likely to use HHMB and the organizational implementation strategies when the study is completed. Past collaborations with clinic staff and the directors enhance the potential adoption and sustainability of the HHMB program.
Participants for this study are PLWH at one of two participating agencies who are already in care. We will use a self-controlled case series method design (i.e., patients as their own controls), wherein “only individuals who have experienced an event are included and all time invariant confounding is eliminated.”30 The outcomes for the 260 participants will be measured before and after the intervention program and the difference in outcomes will be evaluated and analyzed. This design is efficient and appropriate particularly when it is difficult to identify an appropriate comparison group. We found in a previous hybrid type 2 implementation/effectiveness study that randomization (to a waitlist control) was not feasible given the hard-to-reach population and the population's vulnerability and need for care immediately upon expressing willingness to participate.31 This design also permits the focus on providing new services rather than recruiting new patients to the clinics for HIV treatment. The latter new population would have to establish trust before assessing their readiness to be involved in their own health care and HIV treatment. As noted above, lack of trust is a factor in non-adherence to medication and to the recommendations of health providers; this study permits the enhancement of trust rather than its initial establishment.
The patient-level sample consists of 260 Black (African American and African) and Latinx men and women recruited from the clinics where they have received care on at least one occasion for testing or treatment. The power analysis is based on a sample size of 260. In Brown et al.'s study, the total Life's Simple Seven (LS7; see below) standard deviation (SD) was 3.1 for Black participants and 3.5 for Hispanic participants.32 Brewer et al.'s study33 found that the mean LS7 scores accounting for all LS7 components (range 0 to 14) were 8.8 (SD 2.5) and 8.2 (SD 2.1) at baseline and 3-month post-intervention, respectively. Using a type I error of 0.05, type II error of 0.2 (or equivalent to power of 80%), and a paired t-test (from baseline to 3 months), a sample size of 260 and total LS7 SD at 3.5 level will enable us to detect a mean of paired difference as small as 0.6 for the primary intervention outcome of total LS7.
Participants are recruited through advertisements at the clinics and staff awareness. Participant eligibility criteria include: 1) 18 and 75 years of age; 2) HIV-positive; 3) expecting to remain in the Los Angeles area for at least five months; 4) agreeing to medical record review for prior CVD-related test results (e.g., high cholesterol, weight gain, a history of diabetes or high blood pressure); and 5) being willing to participate in English or Spanish. To be eligible, clients must also have >0 (i.e., at least one of the six items is endorsed) on the UCLA Life Adversities Screener (LADS), a validated brief assessment of trauma and adversity history that has demonstrated a strong relationship to PTSD symptoms, depression, and anxiety (Liu et al., 201 5).34 Clients must also identify at least one CVD risk factor on the CVD Screener. This screener was derived from the AHA LS7 Screener35 and the Cardiovascular Patient Worksheet, a one-page self-report form (developed for a Veterans Health Administration-based CVD risk reduction implementation study36) that asks clients about CVD-related illnesses, pregnancyrelated CVD complications, and family history, as well as smoking and exercise behaviors. Individuals who participate receive a $25 incentive for each data collection episode (pre-, post-, 3-month follow up) as well as $20 for each HHMB intervention session attended.
Participant exclusion criteria include: 1) known psychiatric, physical or neurological impairment that would limit their effective participation; 2) recent history of a severe illness, sexual or physical abuse that might require sudden medical, psychological and/or legal intervention; 3) unwilling or unable to give consent to participate in the study through completion. Referrals are provided as necessary.
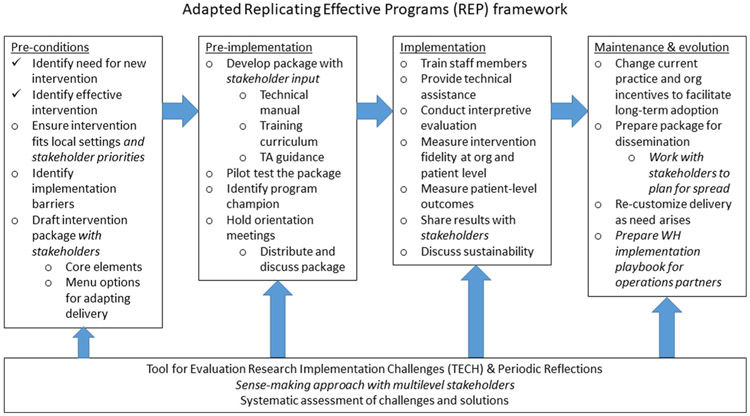
Study activities and procedures are described below according to the phased approach conceptualized in an adapted version of the REP framework (Fig 1). All participants will provide informed consent. The protocol is approved by the University of California Los Angeles Institutional Review Board.
Fig. 1.
Adapted Replicating Effective Programs (REP) framework (adapted from Kilbourne et al.28 and Hamilton et al.36) enhanced with the Tool for Evaluating Research Implementation Challenges64 and Periodic Reflections65
Phase 1: pre-conditions
Three main implementation strategies occur in this phase: (1) draft intervention package, in collaboration with our agency partners and consultant (CG); (2) ensure intervention fits local settings and stakeholder priorities; and (3) identify implementation barriers (baseline evaluation) via qualitative interviews with staff37 and a brief survey assessing staff experience of workload (Maslach Burnout Inventory38), climate (Implementation Climate Scale39), leadership (Implementation Leadership Scale40), and citizenship (Implementation Citizenship Behavior Scale41). Survey and interview findings will be used to characterize the participating organizations at baseline, i.e., before introducing the intervention. This data will allow us to compare and contrast implementation processes over time, and to reflect on implementation outcomes using this “diagnostic”42 contextual data.
Phase 2: pre-implementation
Four implementation strategies are used in this phase: (1) develop intervention package training materials; (2) identify program champions; (3) hold orientation meetings; and (4) pilot test the intervention. After receiving training, facilitators at each intervention site pilot the intervention with the first HHMB group of ~8 participants. Facilitators are rated for their fidelity to the curriculum and given feedback and debriefed after the first HHMB group at each organization. Clients are briefly interviewed regarding comfort and satisfaction with the intervention.
Phase 3: implementation
This phase involves: (1) technical assistance and any additional training; (2) delivery of the HHMB intervention with a target of at least 12 cycles of the five-week intervention over a 26-month implementation phase; (3) client-level data collection (pre-, post-, 3-month follow-up, plus medical record review at one-year post); (4) evaluation; and (5) stakeholder engagement, including discussing plans for maintenance and evolution of the HHMB intervention package.
The Healing our Hearts, Minds and Bodies (HHMB) intervention. HHMB is a psychoeducational, trauma-focused intervention derived from three evidence-informed programs: Healing Our Women (HOW), Emotional Emancipation Circle (EE Circle), and Diabetes Prevention Program (DPP). HOW is an evidence-based intervention originally designed for HIV-positive women targeting sexual risk behavior, medication adherence, and psychological distress.43 Components of HOW were adapted to the study population and components of the EE Circle Model44 were added. EE Circles address the historical and contemporary traumas of enslavement, oppression, racism, and discrimination. These two models combined serve as the primary foundation for addressing the traumatic experiences that the Black and Latinx study population is likely to have experienced in their personal and collective racial group histories.
The final component of the HHMB intervention draws upon relevant concepts in the evidence-based Diabetes Prevention Program.45 In the Action Plan for Health component of the intervention, participants incorporate CVD risk reduction using a CVD scorecard based on the American Heart Association LS7/My Healthy Heart program.46 The scorecard identifies seven biologic and behavioral risk factors for CVD and whether the participant is at goal, in the caution range, or not at goal for each risk factor. Participants choose one risk factor that is out of range to work on (in collaboration with the class facilitator). The classes provide information on all seven CVD risk factors, which are either self-reported (healthy diet, physical activity, smoking) or measured as part of the study (blood pressure control, healthy weight, cholesterol and blood sugar control). Two risk factors (i.e., cholesterol and blood sugar control) are also obtained from the patient's electronic health record available at both agencies. Two composite scores are also constructed for each participant: a 5-item score without blood sugar or lipid levels and a 7-item score consisting of all the items. The 5-item score is measured immediately after the end of the intervention (approximately six weeks after the baseline measurements), as changes in LDL cholesterol and A1c would not be expected in that time period.
HHMB targets cognitive, affective, and behavioral dimensions that ultimately affect the targeted psychological and biological outcomes. For example, within the cognitive dimension, the intervention targets the following skills/practices: the role and disclosure of trauma; identifying triggers to poor health habits and decisions; counter-narratives, schemas, and cognitive self-monitoring. Through expressive writing,47 guided discussion, and group experiential activities, participants explore the role that trauma plays in their psychological and physical functioning, learn to identify triggers from previous trauma that precipitate poor coping and health practices, and develop counter-narratives and new schemas for more effective coping and management of trauma to ameliorate the effects of racism and racial stress on health and wellness. Within the affective domain, the following skills/practices are targeted: relaxation techniques, mindfulness, and self-monitoring of mood states. Participants learn and practice relaxation techniques and a mindfulness approach of attention to the present moment, as well as how to monitor one's own mood states. These practices have demonstrated positive effects on anxiety reduction. Within the cognitive domain, using the EE Circles, participants are guided through a process of critical consciousness about cultural trauma and its effects related to a dramatic loss of identity and meaning and racial group cohesion.48
Although compensation for participation may limit the generalizability of the results, experience indicates that incentives enhance attendance and active participation, which are essential for treatment effectiveness. Therefore, participants are given compensation for each data collection episode and intervention session attended as well as for transportation costs and cellular phone air time minutes to facilitate regular communication. Usual care, services, and referrals are available to all clients before, during, and after the intervention.
The self-report assessment protocol developed for previous studies and pre-programmed using audio computer-assisted self-interviewing (ACASI)49 takes about 90 min to complete. ACASI provides both audio and video presentation of the questions and response options on a laptop computer. ACASI has been shown to significantly decrease social desirability bias. If a participant becomes distressed during assessment, the session is stopped, brief support provided, and the participant is referred for mental health services.
We will also conduct clinical data collection during the intake session to complete the LS7 score (see below). The HHMB consent form requests permission to review clinical data from the electronic health record. The data requested include CVD risk factor processes and intermediate outcomes, including body mass index, blood pressure, hemoglobin A1c (A1c), and lipid profiles.
Baseline and outcome measures
Psychological health.
Depression symptoms are assessed with the 21-item Beck Depression Inventory Il (BDI-11), a self-report questionnaire designed to assess symptoms of Major Depressive Disorder.50 Post traumatic stress symptoms are assessed with the 17-item Post-traumatic Diagnostic Scale (PDS) that yields a reliable sum score.51 General anxiety is assessed with the Patient Health Questionnaire-15 (PHQ-15),52 based on the diagnostic criteria for anxiety. Emotional regulation is assessed with the Short Form of the Difficulties in Emotional Regulation Scale (DERS).53 This 18-item short version is highly correlated with the original 36-item form and demonstrates good validity and reliability. Sleep habits are measured with items from the Pittsburgh Sleep Quality Index (PSQI).54
The main effectiveness outcome is the LS7 score. The LS7 is a set of national goals developed by the American Heart Association to define, monitor, and enhance cardiovascular health through the primary prevention of heart disease and stroke,55 and to track health disparities.32 The LS7 score summarizes control of three health factors (blood pressure, serum lipids, and blood glucose levels) and four health behaviors (physical activity, diet, maintaining a healthy weight, and not smoking). Lower scores are associated with higher all-cause and CVD-related mortality56 and with a higher incidence of CVD,57 stroke,58 heart failure,59 cognitive impairment,60 depressive symptoms,61 and endstage renal disease.62 Diet, physical activity, and smoking are obtained through the survey and blood pressure is measured by the study team at baseline (pre-), approximately one week after completion of the classes (post-), and at three months. A1c and lipids are measured with blood spots at baseline and three months. The A1c is measured with the DCA Vantage® Analyzer (Siemens); non-fasting cholesterol levels (total, LDL, and HDL) and triglycerides are measured using a CardioChek PA Analyzer (CardioChek).
Table 1 details definitions of poor, intermediate, and ideal levels for each LS7 component.55,63 To score each LS7 component, we assign 2 points to the ideal category, 1 point for intermediate values, and zero points to the poor category. Total LS7 score is calculated for each participant by summing the scores for all seven components, with a resulting range from 0 to 14.
Table 1.
Life's simple seven cardiovascular health metrics and definitions.
| Health metric | Poor (scored = 0) | Intermediate (scored = 1) | Ideal (scored = 2) |
|---|---|---|---|
| Blood pressure | SBP ≥ 140 or DBP ≥ 90 mmHg | SBP 120–139 and DBP 80–89 mmHg or treated to ideal goal | SBP < 120 and DBP < 80 mmHg and not on blood pressure medications |
| Total cholesterol | ≥240 mg/dL | 200–239 mg/dL, or treated to ideal goal | <200 mg/dL, and not on lipid lowering medications |
| A1c | ≥5.7% | 5.0–5.6 | <4.9 |
| Body mass index | ≥30 kg/m2 | 25–29.9 kg/m2 | <25 kg/m2 |
| Physical activitya | None | Under 30 min moderate exercise 3 days per week | ≥ 30 min moderate exercise at least 3 days per week |
| Fruit and vegetable servings per day | 0 | 1–4 | ≥5 |
| Smoking | Current | Former, stopped ≤12 months ago | Never smoked or quit >12 months ago |
Abbreviations: SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure.
Healthy diet score is calculated using four components of the Health Eating Index (fruits, vegetables, grains and sodium), a diet quality scale that assesses conformance to federal dietary guidance.
Evaluation.
Several types of assessments are used to track implementation processes, specifically with regard to fidelity, dose, and intensity of intervention. Throughout the intervention, various factors are monitored such as session attendance, session completion, and participant satisfaction with sessions. Facilitators complete short surveys noting adherence to the curriculum, level of participation, specific obstacles that may have arisen, and what components appear to be most/least appropriate for the session's participants. Facilitators also complete overall ratings of each participant's engagement, competency, and knowledge. To assess fidelity, sessions are digitally recorded. A random sample of all session recordings will be reviewed and scored for fidelity to core elements, with a criterion of 80% or more of the total elements considered acceptable.
To assess the impact of implementation challenges, we use the Tool for Evaluating Research Implementation Challenges (TECH),64 which is recommended under three pre-conditions: 1) when “implementation adaptations are expected due to the emergent nature of complex research settings;” 2) the research environment needs to encourage “spontaneous emergent solutions” and creativity; and 3) all research team members must be empowered to participate. The study settings and approach meet these pre-conditions. TECH, which has been used successfully in community studies, is codified into a series of interactive steps: identifying challenges (e.g., through observing day-to-day dynamics, listening to complaints, asking questions, etc.), interpreting the challenges in weekly meetings, generating and testing solution strategies, and, if necessary, addressing regulatory issues. Solution strategies are developed through open dialogue among the team members as well as others who might have perspectives on potential solutions. The TECH is complemented with the periodic reflections method designed to capture implementation progress and document use of implementation strategies (see Fig. 1).65 Approximately 13 months into implementation, key stakeholders are asked to participate in midimplementation qualitative interviews to evaluate the value of the implementation strategies, barriers and facilitators to implementation, and recommendations for the remainder of the implementation phase.
Phase 4: Maintenance and Evolution
Phase 4 involves: (1) evaluating current practices and organizational incentives to facilitate long-term adoption; (2) preparing the intervention package for dissemination; based on feedback from clients and key stakeholders, the team will make adjustments to the intervention package and encourage sites to continue using HHMB, as well as the techniques used to evaluate guideline-concordant care; (3) recustomizing delivery as need arises; depending on implementation and effectiveness outcomes, the organizations may elect to recustomize the intervention and/or the organizational-level implementation strategies; and (4) preparing implementation tip sheets in collaboration with agency partners; depending on implementation and effectiveness outcomes, these sheets will be designed to scale-up and spread the approach to other organizations serving Black and Latinx PLWH.
Data analysis and statistical modeling plan
Qualitative data document how the participating organizations are supported in implementing and sustaining the intervention. The focus is on describing the process of assisting the organizations with implementation and maintenance. This description and summary is generated from interviews and notes from project calls, along with other qualitative data gathered throughout the study.
To evaluate the use and effectiveness of implementation strategies over time, and identify barriers and facilitators to organizational adoption of guidelines, provider adherence to guidelines, feasibility, and sustainability, the Consolidated Framework for Implementation Research (CFIR)29 is used as a guide. CFIR provides an analytic rubric for examining intervention and provider characteristics, as well as inner and outer settings. Data regarding acceptability, barriers, and facilitators are derived from interviews and implementation-focused evaluation measures. Descriptive statistics are used to analyze the survey data given the small sample size and the modest, practical goal of characterizing the agencies in preparation for implementation. In addition, facilitator and participant satisfaction ratings are evaluated to characterize acceptability of the intervention. These ratings are compared both within and across cohorts and organizations.
Potential determinants of adoption and fidelity include but are not limited to training factors, competency at delivering the intervention, types of facilitators, participant gender and racial/ethnic diversity in a given cohort, retention, and satisfaction. These factors are examined when characterizing fidelity across agencies. In addition, based on interviews with facilitators and clients, other determinants may emerge during implementation. There are several possible determinants of maintenance. It is hypothesized that maintenance will be achieved in agencies that have their own trained staff facilitators who deliver the intervention with fidelity and who had positive experiences with the intervention during the implementation phase.
Qualitative data analysis
All interviews are digitally recorded and transcribed by a professional transcription company. Analysis will be conducted using ATLAS. ti, a software package that allows for fluid “interaction” of data types and sources. Using constant comparison analytic methods,66 a preliminary codebook will be developed both deductively using CFIR and inductively from a sub-sample of interviews within and across organizations at baseline. Qualitative findings at baseline are augmented by preliminary analyses of staff-level data from the survey described above, and a baseline profile developed for each agency. These profiles are reviewed by the Implementation Team, and used to tailor implementation strategies at each site (e.g., where there is a high level of burnout, additional attention will be paid to supporting staff and managing workload throughout implementation).
This approach of using baseline data as diagnostic and informative for tailored implementation has been employed in a prior implementation study.67 The codebook will be elaborated upon and adjusted as each round of interviews is reviewed. Interviews will be compared within each organization, across organizations, across different types of respondents, and over time. Additional sources of qualitative data (i.e., meeting minutes, periodic reflections, archival information) will also be included in the data set. The data will be analyzed specifically for barriers to and facilitators of implementation, including but not limited to the ways in which the project's implementation strategies affect adoption, satisfaction, and maintenance. In addition to identifying themes and patterns qualitatively, statistical associations will be examined between important process and outcome variables such as satisfaction with the intervention, fidelity, and retention, and improvement in clinical and behavioral outcomes. Agency profiles will be revisited and further developed at the end of the active implementation phase, and again after implementation, thereby creating a story of implementation at each organization.
Quantitative data analysis
To evaluate the clinical outcomes and the effects of the intervention, systematic data analysis and statistical modeling will be conducted: (1) For univariate analysis, the marginal distribution of each measure obtained will be calculated at each time point and across time. For categorical variables (e.g., gender, and race/ethnicity), the frequency distribution and modes will be calculated. For continuous measures (e.g., blood sugar levels/A1c), the central tendency (mean and median) will be calculated, variation (e.g., standard deviation, kurtosis), percentiles and range. Statistical tests (e.g., Shapiro-Wilk test) will be used to assess normality of the outcomes. The degree and patterns of missingness will be determined, and we will perform imputation (e.g., multiple imputation), if needed. (2) Bivariate analyses of each outcome measure and independent variables will be carried out. Pearson and Spearman rank correlation will be used to correlate predictors with outcome measures. t-tests, analysis of variance (ANOVA) or marginal regressions will be used for continuous measures. The change (delta) of outcome measures between time-points will be calculated, and distributions of the deltas will be carefully examined. The bivariate analyses between the deltas and covariates will be carried out. Marginal repeated measures models will be carried out between each independent measure and outcomes over time. (3) Multivariate repeated measures modeling68,69: To evaluate the relationships between outcome measures and independent variables over time, generalized linear mixed effect models (GLMEM) will be fitted, controlling for age and others factors. Graphical analyses (e.g., parallel plots) will be used to assist in examining the relationship between predictors and outcome measures over time. Potential non-linear relationships between the outcome measures and the continuous predictors will be explored through generalized additive mixed models.
Discussion
This study incorporates several innovative features. First, it shifts current clinical paradigms by focusing on the nexus of HIV, CVD risk, and individual and cultural trauma histories among Black and Latinx individuals in the US, who are disproportionately affected by each of these factors. The use of trauma history screening using an innovative composite trauma exposure risk index captures trauma experiences not typically asked about in healthcare settings. This project is the first to investigate how brief trauma interventions can prepare PLWH to address concurrent health conditions such as CVD risk, particularly within community-based healthcare contexts that are also addressing these health conditions. Importantly, integrated HIV and chronic disease services show promise for better outcomes among PLWH who have co-morbid conditions,70 but to date, there is a dearth of U.S. literature on how to integrate services, how to support guideline-concordant services for co-morbid conditions, and how to ensure that patients are ready to access those services and engage in appropriate care. This study provides an opportunity to shape and facilitate two complementary pathways to improved CVD care: the patient pathway and the organizational pathway. With blended, REP-guided implementation strategies71 supporting both pathways, it is expected that CVD outcomes will be improved and CVD-related services will be strengthened and maintained.
Second, to address multiple co-morbidities, implementation science literature stresses the importance of multifaceted, modular, flexible interventions72-74 rather than interventions focused on singular conditions and/or interventions that pose fidelity challenges in usual care settings. The proposed intervention, Healing our Hearts, Minds and Bodies (HHMB), represents an improvement over existing interventions in that it: addresses multiple, interrelated conditions; draws on a combination of evidence-based, evidence-informed, and culturally congruent approaches to individual and cultural trauma, HIV, and CVD risk43; reduces barriers to accessing CVD care for a diverse cohort of patients with complex needs75,76; is specifically intended for populations most impacted by HIV; is group-based, brief, and tailorable to local context; and can be facilitated by a trained staff member with minimal prior experience of delivering mental health/CVD interventions. As such, it falls within the concept of a “disruptive innovation,”77 with its emphasis on simplicity, accessibility, and potential scalability, should favorable effectiveness and implementation outcomes be achieved.
Third, extensive implementation research across varied settings and with diverse populations78-80 has been used to propose innovative methods to comprehensively capture the complexity of implementation, adaptations to the intervention, and real-time use of implementation strategies that are tailored to local contexts. A persistent challenge in implementation research has been to capture the dynamic nature of change over the course of implementation.81 Our methods for capturing this complexity offer a more ethnographically oriented approach.65
This hybrid implementation/effectiveness study has limitations, including 1) potential for being underpowered to detect significant effects on any one of the LS7 factors, since participants will be working on different factors; 2) lack of generalizability to other PLWH patient populations with histories of trauma; and 3) small number of implementation sites, possibly limiting applicability of implementation strategy implications.
A recent systematic review of programs that integrated CVD, hypertension, and diabetes with HIV services found that these programs showed promise in coordinating care for the rising number of patients living with HIV and CVD or its risk factors, often leveraging existing HIV and AIDS services.70 However, few of these programs were based in the United States, and there was limited evidence of their long-term impact. Health systems, such as our participating clinics, that work to coordinate and integrate care across HIV and chronic conditions such as CVD may provide the infrastructure needed to address the complex interplay of these conditions. Their ability to do so may be strengthened by our intervention's explicit recognition of and care for individual and cultural histories of trauma. Should successful outcomes in this hybrid effectiveness-implementation study be achieved, the intervention and accompanying implementation strategies will be refined to support reach to other vulnerable populations (e.g., Black and Latinx PLWH who are not currently in care, other populations disproportionately affected by HIV) and to more organizations that serve these populations.
Acknowledgements
Funding provided by National Heart, Lung, and Blood Institute (U01 HL142109; PIs: Wyatt, Hamilton, Brown). We would like to thank the participating agencies for their support.
List of Abbreviations
- ACASI
audio computer-assisted self-interviewing
- AHA
American Heart Association
- ANOVA
analysis of variance
- ART
antiretroviral therapy
- BDI
Beck Depression Inventory
- CFIR
Consolidated Framework for Implementation Research
- CVD
cardiovascular disease
- DPP
Diabetes Prevention Program
- DERS
Difficulties in Emotional Regulation Scale
- EE Circle
Emotional Emancipation Circle
- GLMEM
generalized linear mixed effect models
- HDL
high-density lipoprotein
- HHMB
healing our hearts, minds, and bodies
- HIV
human immunodeficiency virus
- HOW
Healing Our Women
- LADS
Life Adversities Screener
- LDL
low-density lipoprotein
- LS7
Life's Simple Seven
- MI
myocardial infarction
- PDS
Post-traumatic Diagnostic Scale
- PHQ
Patient Health Questionnaire
- PLWH
people living with HIV
- PSQI
Pittsburgh Sleep Quality Index
- PTSD
post-traumatic stress disorder
- REP
Replicating Effective Programs
- SD
standard deviation
- TECH
Tool for Evaluating Research Implementation Challenges
Footnotes
Statement of conflict of interest
None of the authors has any conflicts of interests with regard to this publication.
References
- 1.Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol 2016;117(2): 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acutemyocardial infarction. JAMA Intern Med 2013;173(8):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce D, Ani C, Espinosa-Silva Y, et al. Comparison of in-hospital mortality from acute myocardial infarction in HIV sero-positive versus sero-negative individuals. Am J Cardiol 2012;110(8):1078–1084. [DOI] [PubMed] [Google Scholar]
- 4.Boccara F, Lang S, Meuleman C, et al. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol 2013;61(5):511–523. [DOI] [PubMed] [Google Scholar]
- 5.Bell CN, Thorpe RJ Jr, Bowie JV, LaVeist TA. Race disparities in cardiovascular disease risk factors within socioeconomic status strata. Ann Epidemiol 2018;28(3):147–152. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan RC, Kingsley LA, Sharrett AR, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis 2007;45(8):1074–1081. [DOI] [PubMed] [Google Scholar]
- 7.Aaron E, Criniti S, Bonacquisti A, Geller PA. Providing sensitive care for adult HIV-infected women with a history of childhood sexual abuse. J Assoc Nurses AIDS Care 2013;24(4):355–367. [DOI] [PubMed] [Google Scholar]
- 8.Meade CS, Hansen NB, Kochman A, Sikkema KJ. Utilization of medical treatments and adherence to antiretroviral therapy among HIV-positive adults with histories of childhood sexual abuse. AIDS Patient Care STDS 2009;23(4):259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leserman J Role of depression, stress, and trauma in HIV disease progression. Psychosom Med 2008;70(5):539–545. [DOI] [PubMed] [Google Scholar]
- 10.Johnson MO, Catz SL, Remien RH, et al. Theory-guided, empirically supported avenues for intervention on HIV medication nonadherence: findings from the healthy living project. AIDS Patient Care STDS 2003;17(12):645–656. [DOI] [PubMed] [Google Scholar]
- 11.Machtinger EL, Wilson TC, Haberer JE, Weiss DS. Psychological trauma and PTSD in HIV-positive women: a meta-analysis. AIDS Behav 2012;16(8):2091–2100. [DOI] [PubMed] [Google Scholar]
- 12.LeGrand S, Reif S, Sullivan K, Murray K, Barlow ML, Whetten K. A review of recent literature on trauma among individuals living with HIV. Curr HIV/AIDS Rep 2015;12 (4):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watt MH, Dennis AC, Choi KW, et al. Impact of sexual trauma on HIV care engagement: perspectives of female patients with trauma histories in Cape Town, South Africa. AIDS Behav 2016;21(11):3209–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whetten K, Reif S, Whetten R, Murphy-McMillan LK. Trauma, mental health, distrust, and stigma among HIV-positive persons: implications for effective care. Psychosom Med 2008;70(5):531–538. [DOI] [PubMed] [Google Scholar]
- 15.Dong M, Giles WH, Felitti VJ, et al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation 2004;110(13):1761–1766. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer C, Barnow S, Volzke H, John U, Freyberger HJ, Grabe HJ. Trauma, posttraumatic stress disorder, and physical illness: findings from the general population. Psychosom Med 2009;71(9):1012–1017. [DOI] [PubMed] [Google Scholar]
- 17.Olff M, Langeland W, Draijer N, Gersons BP. Gender differences in posttraumatic stress disorder. Psychol Bull 2007;133(2):183–204. [DOI] [PubMed] [Google Scholar]
- 18.Lutwak N, Dill C. Military sexual trauma increases risk of post-traumatic stress disorder and depression thereby amplifying the possibility of suicidal ideation and cardiovascular disease. Mil Med 2013;178(4):359–361. [DOI] [PubMed] [Google Scholar]
- 19.Hamil-Luker J, O’Rand AM. Gender differences in the link between childhood socioeconomic conditions and heart attack risk in adulthood. Demography 2007;44(1): 137–158. [DOI] [PubMed] [Google Scholar]
- 20.O'Rand AM, Hamil-Luker J. Processes of cumulative adversity: childhood disadvantage and increased risk of heart attack across the life course. J Gerontol B Psychol Sci Soc Sci. 2005;60 Spec No 2:117–124. [DOI] [PubMed] [Google Scholar]
- 21.Sumner JA, Kubzansky LD, Elkind MS, et al. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation 2015;132(4):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaccarino V, Bremner JD. Traumatic stress is heartbreaking. Biol Psychiatry 2013;74 (11):790–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health 2005;26:469–500. [DOI] [PubMed] [Google Scholar]
- 24.Lewis TT. Trauma and PTSD: emerging risk factors for cardiovascular disease in women? Circulation 2015;132:227–229. [DOI] [PubMed] [Google Scholar]
- 25.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50(3):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landes SJ, McBain SA, Curran GM. An introduction to effectiveness-implementation hybrid designs. Psychiatry Res 2019;280:112513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damschroder LJ, Reardon CM, AuYoung M, et al. Implementation findings from a hybrid III implementation-effectiveness trial of the Diabetes Prevention Program (DPP) in the Veterans Health Administration (VHA). Implement Sci 2017;12(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilbourne AM, Neumann MS, Pincus HA, Bauer MS, Stall R. Implementing evidence-based interventions in health care: application of the replicating effective programs framework. Implement Sci 2007;2(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ 2016;354:i4515. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton AB, Mittman BS, Campbell D, et al. Understanding the impact of external context on community-based implementation of an evidence-based HIV risk reduction intervention. BMC Health Serv Res 2018;18(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown AF, Liang L-J, Vassar SD, et al. Trends in racial/ethnic and nativity disparities in cardiovascular health among adults without prevalent cardiovascular disease in the United States, 1988 to 2014. Ann Intern Med 2018;168(8):541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brewer LC, Balls-Berry JE, Dean P, Lackore K, Jenkins S, Hayes SN. Fostering African-American improvement in total health (FAITH!): an application of the American Heart Association’s life’s simple 7TM among Midwestern African-Americans. J Racial Ethn Health Disparities 2017;4(2):269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, Prause N, Wyatt GE, et al. Development of a composite trauma exposure risk index. Psychol Assess 2015;27(3):965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Association AH. Life's Simple 7, https://www.heart.org/en/professional/workplace-health/lifes-simple-7 2020. Accessed February 10, 2020. [Google Scholar]
- 36.Hamilton AB, Farmer MM, Moin T, et al. Enhancing mental and physical health of women through engagement and retention (EMPOWER): a protocol for a program ofresearch. Implement Sci 2017;12(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton AB, Finley EP. Qualitative methods in implementation research: an introduction. Psychiatry Res 2019;280:112516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maslach C, Jackson SE, Leiter MP, Schaufeli WB, Schwab RL. Maslach burnout inventory. ,Vol 21 Palo Alto, CA: Consulting psychologists press; 1986. [Google Scholar]
- 39.Ehrhart MG, Aarons GA, Farahnak LR. Assessing the organizational context for EBP implementation: the development and validity testing of the Implementation Climate Scale (ICS). Implement Sci 2014;9(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aarons GA, Ehrhart MG, Farahnak LR. The implementation leadership scale (ILS): development of a brief measure of unit level implementation leadership. Implement Sci 2014;9(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehrhart MG, Aarons GA, Farahnak LR. Going above and beyond for implementation: the development and validity testing of the Implementation Citizenship Behavior Scale (ICBS). Implement Sci 2015;10(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stetler CB, Legro MW, Wallace CM, et al. The role of formative evaluation in implementation research and the QUERI experience. J Gen Intern Med 2006;21(2):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyatt GE, Hamilton AB, Myers HF, et al. Violence prevention among HIV-positive women with histories of violence: healing women in their communities. Womens Health Issues 2011;21(6):S255–S260. [DOI] [PubMed] [Google Scholar]
- 44.Grills CN, Aird EG, Rowe D. Breathe, baby, breathe: clearing the way for the emotional emancipation of black people. Cult Stud <->Crit Methodol 2016;16(3):333–343. [Google Scholar]
- 45.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;2002(346): 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fretts AM, Howard BV, McKnight B, et al. Life’s simple 7 and incidence of diabetes among American Indians: the Strong Heart Family Study. Diabetes Care 2014;37 (8):2240–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pennebaker JW. Putting stress into words: health, linguistic, and therapeutic implications. Behav Res Ther 1993;31(6):539–548. [DOI] [PubMed] [Google Scholar]
- 48.Eyerman R Cultural Trauma: Slavery and the Formation of African American Identity. Cambridge University Press; 2001. [Google Scholar]
- 49.Metzger DS, Koblin B, Turner C, et al. Randomized controlled trial of audio computer-assisted self-interviewing: utility and acceptability in longitudinal studies. Am J Epidemiol 2000;152(2):99–106. [DOI] [PubMed] [Google Scholar]
- 50.Beck AT, Steer RA, Brown GK. Beck Depression Inventory-ii (bdi-ii). San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 51.Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: the Posttraumatic Diagnostic Scale. Psychol Assess 1997;9(4):445. [Google Scholar]
- 52.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64(2):258–266. [DOI] [PubMed] [Google Scholar]
- 53.Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopathol Behav Assess 2004; 26(1):41–54. [Google Scholar]
- 54.Smyth C The Pittsburgh sleep quality index (PSQI). J Gerontol Nurs 1999;25(12):10. [DOI] [PubMed] [Google Scholar]
- 55.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- 56.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation 2012;125(8):987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Folsom AR, Yatsuya H, Nettleton JA, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011;57(16):1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong C, Rundek T, Wright CB, et al. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks and Hispanics: the northern Manhattan study. Circulation 2012;125(24):2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Folsom AR, Shah AM, Lutsey PL, et al. American Heart Association’s Life’s Simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med 2015;128(9):970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thacker EL, Gillett SR, Wadley VG, et al. The American Heart Association Life9s simple 7 and incident cognitive impairment: the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. J Am Heart Assoc 2014;3(3), e000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kronish IM, Carson AP, Davidson KW, Muntner P, Safford MM. Depressive symptoms and cardiovascular health by the American Heart Association’s definition in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. PLoS One 2012;7(12), e52771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muntner P, Judd SE, Gao L, et al. Cardiovascular risk factors in CKD associate with both ESRD and mortality. J Am Soc Nephrol 2013;24(7):1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med 2014;160(8): 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simpson KM, Porter K, McConnell ES, et al. Tool for evaluating research implementation challenges: a sense-making protocol for addressing implementation challenges in complex research settings. Implement Sci 2013;8(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Finley EP, Huynh AK, Farmer MM, et al. Periodic reflections: a method of guided discussions for documenting implementation phenomena. BMC Med Res Methodol 2018;18(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corbin J, Strauss A. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. London: Sage; 1990. [Google Scholar]
- 67.Hamilton AB, Cohen AN, Young AS. Organizational readiness in specialty mental health care. J Gen Intern Med 2010;25(1):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCullagh P, Nelder JA. Generalized Linear Models. 2nd ed. New York: Chapman & Hall; 1989. [Google Scholar]
- 69.McCulloch CE, Searle SR. Generalized, Linear, and Mixed Models. New York: Wiley; 2001. [Google Scholar]
- 70.Haldane V, Legido-Quigley H, Chuah FLH, et al. Integrating cardiovascular diseases, hypertension, and diabetes with HIV services: a systematic review. AIDS Care 2017: 1–13. [DOI] [PubMed] [Google Scholar]
- 71.Cabassa LJ, Gomes AP, Lewis-Fernández R. What would it take? Stakeholders’ views and preferences for implementing a health care manager program in community mental health clinics under health care reform. Med Care Res Rev 2015;72(1):71–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garland AF, Bickman L, Chorpita BF. Change what? Identifying quality improvement targets by investigating usual mental health care. Adm Policy Ment Health Ment Health Serv Res 2010;37(1–2):15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weisz JR, Chorpita BF, Palinkas LA, et al. Testing standard and modular designs for psychotherapy treating depression, anxiety, and conduct problems in youth: a randomized effectiveness trial. Arch Gen Psychiatry 2012;69(3):274–282. [DOI] [PubMed] [Google Scholar]
- 74.Chorpita BF, Weisz JR, Daleiden EL, et al. Long-term outcomes for the child STEPs randomized effectiveness trial: a comparison of modular and standard treatment designs with usual care. J Consult Clin Psychol 2013;81(6):999. [DOI] [PubMed] [Google Scholar]
- 75.Yancy CW. Cardiovascular disease outcomes: priorities today, priorities tomorrow for research and community health. Ethn Dis 2012;22(3 (Suppl 1)). (1-7-S1-12). [PubMed] [Google Scholar]
- 76.Rodriguez CJ, Allison M, Daviglus ML, et al. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States. Circulation 2014;130(7):593–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rotheram-Borus MJ, Swendeman D, Chorpita BF. Disruptive innovations for designing and diffusing evidence-based interventions. Am Psychol 2012;67(6):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamilton AB, Mittman BS, Williams JK, et al. Community-based implementation and effectiveness in a randomized trial of a risk reduction intervention for HIVserodiscordant couples: study protocol. Implement Sci 2014;9:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamilton AB, Brunner J, Cain C, et al. Engaging multilevel stakeholders in an implementation trial of evidence-based quality improvement in VA women’s health primary care. Transl Behav Med 2017;7(3):478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamilton AB, Cohen AN, Glover DL, et al. Implementation of evidence-based employment services in specialty mental health. Health Serv Res 2013;48(6 Pt 2):2224–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci 2013;8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]