Abstract
Background
Conflicting results on associations between dietary quality and bone have been noted across populations, and this has been understudied in Puerto Ricans, a population at higher risk of osteoporosis than previously appreciated.
Objective
To compare cross-sectional associations between 3 dietary quality indices [Dietary Approaches to Stop Hypertension (DASH), Alternative Health Eating Index (AHEI-2010), and Mediterranean Diet Score (MeDS)] with bone outcomes.
Method
Participants (n = 865–896) from the Boston Puerto Rican Osteoporosis Study (BPROS) with complete bone and dietary data were included. Indices were calculated from validated food frequency data. Bone mineral density (BMD) was measured using DXA. Associations between dietary indices (z-scores) and their individual components with BMD and osteoporosis were tested with ANCOVA and logistic regression, respectively, at the lumbar spine and femoral neck, stratified by male, premenopausal women, and postmenopausal women.
Results
Participants were 59.9 y ± 7.6 y and mostly female (71%). Among postmenopausal women not taking estrogen, DASH (score: 11–38) was associated with higher trochanter (0.026 ± 0.006 g/cm2, P <0.001), femoral neck (0.022 ± 0.006 g/cm2, P <0.001), total hip (0.029 ± 0.006 g/cm2, P <0.001), and lumbar spine BMD (0.025 ± 0.007 g/cm2, P = 0.001). AHEI (score: 25–86) was also associated with spine and all hip sites (P <0.02), whereas MeDS (0–9) was associated only with total hip (P = 0.01) and trochanter BMD (P = 0.007) in postmenopausal women. All indices were associated with a lower likelihood of osteoporosis (OR from 0.54 to 0.75). None of the results were significant for men or premenopausal women.
Conclusions
Although all appeared protective, DASH was more positively associated with BMD than AHEI or MeDS in postmenopausal women not taking estrogen. Methodological differences across scores suggest that a bone-specific index that builds on existing indices and that can be used to address dietary differences across cultural and ethnic minority populations should be considered.
Keywords: osteoporosis, dietary quality, Hispanic/Latino, nutrition, bone
Introduction
Osteoporosis is an emerging public health problem among Puerto Rican adults living on the US mainland. Recent evidence suggests a similar and/or higher prevalence of osteoporosis in this population, compared with non-Hispanic white adults (1). The age-adjusted prevalence of osteoporosis was 8.6% for Puerto Rican men compared with 2.3% for non-Hispanic white men, and 10.7% for Puerto Rican women compared with 10.1% for non-Hispanic white women based on data from the Boston Puerto Rican Osteoporosis Study (BPROS) and NHANES 2005–2010 (1). Osteoporosis increases the risk of fracture and subsequent morbidity, mortality, and reduced quality of life (2); thus, it is imperative to identify factors associated with the prevention of osteoporosis to enhance public health messaging to reduce the prevalence and burden of this disease. Diet is a known major modifiable risk factor for bone health (3, 4) and may, in part, explain disparities in bone outcomes across populations. Few studies have investigated the dietary risk factors for osteoporosis among Puerto Rican adults living on the US mainland (5, 6).
The extant literature on nutrition and osteoporosis suggests that overall dietary quality is critical for bone health (7–11). Dietary indices have been developed to characterize overall dietary quality based on existing nutrition knowledge and/or dietary recommendations for disease prevention (12). The Mediterranean Diet Score (MeDS) and Alternative Health Eating Index (AHEI) are widely used indices, as higher adherence to these dietary patterns has been associated with reduced chronic disease risk and mortality (13–18). Several population-based studies have examined relations between various dietary quality indices, including the MeDS (19–26) and AHEI (22, 26–28) and bone health, with mixed results. Some reported protective findings with higher adherence to dietary indices (19, 20, 22, 26, 27), 1 found a negative association (21), whereas others showed no association with bone mineral density (BMD) or fracture (23–25, 28). Most studies on dietary indices and bone health have been conducted in non-Hispanic populations (19, 20, 22, 23, 25–27, 29–31) and few have focused on Puerto Ricans (5). A limited number of studies have examined Dietary Approaches to Stop Hypertension (DASH) in relation to bone, despite the unique inclusion of foods that are beneficial for bone health. It is currently unclear which dietary pattern would be most beneficial for bone in this population.
The objective of this study was to compare the relation between 3 dietary quality indices (DASH, AHEI-2010, and MeDS) with BMD at the hip and spine, and the prevalence of osteoporosis at the lumbar spine (L2–L4) and/or the femoral neck among Puerto Rican adults aged 47–77 y. To our knowledge, this is the first study to compare widely used dietary quality indices and bone in this population. Understanding how dietary quality relates to bone health is a key strategy for prevention, as osteoporosis is a silent chronic health condition until fracture occurs.
Methods
Study population
BPROS is an ancillary study to the Boston Puerto Rican Health Study (BPRHS), a longitudinal investigation of genetic, sociological, health, and environmental factors associated with health disparities experienced by Puerto Rican adults living in the Greater Boston area (32). Participants in the BPRHS completed a baseline interview (n = 1499) and a 2-y follow-up (n = 1267) interview (Figure 1). Participants were eligible to participate if they self-identified as Puerto Rican, were between the ages of 45–75 y, and lived within the Greater Boston area. Individuals who planned to move from the area within 2 y, had a Mini-Mental State Examination Score ≤10, or were unable to answer questions due to a serious health condition were excluded (32). After the 2-y interview, participants were invited and, if interested, were reconsented to participate in the BPROS (n = 973). A total of 298 BPRHS participants did not participate in the BPROS for the following reasons: 205 declined, 13 moved from the area, 47 had difficulty scheduling the interview, 11 were lost to follow-up, 2 did not participate for health reasons, and 20 had died since their 2-y interview. Four participants did not complete the 2-y interview but were consented for the BPROS. Those who declined to participate in the BPROS were older (60.9 y compared with 58.7 y, P <0.001) and were more likely to have type 2 diabetes (47.8% compared with 40.4%, P = 0.03) compared with those who participated.
FIGURE 1.
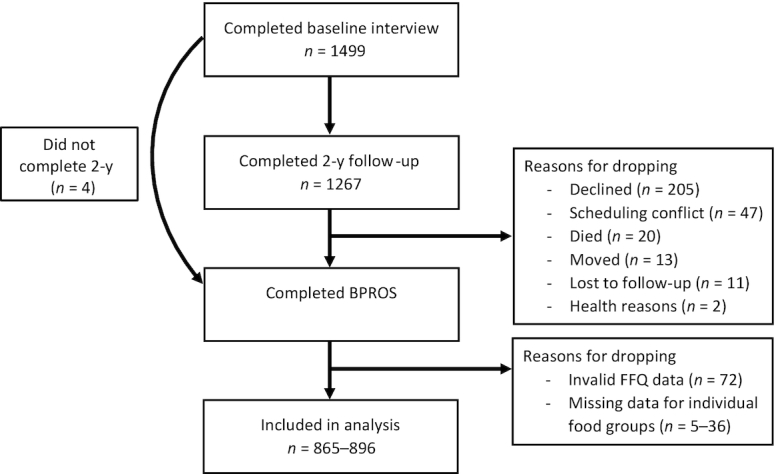
Flow chart of participation from the BPRHS and BPROS. BPROS, Boston Puerto Rican Osteoporosis Study; BPRHS, Boston Puerto Rican Health Study.
Participants enrolled in the BPROS visited the Metabolic Research Unit at the Jean Mayer USDA Human Nutrition Research Center on Aging (HNRCA) at Tufts University to complete an interview, BMD measures, and a blood draw. A trained bilingual phlebotomist collected a fasting blood sample in the participants’ home on the morning after the interview, or as soon as possible thereafter, for biochemical analysis. All participants provided written informed consent. The Institutional Review Boards at Tufts Medical Center, Tufts University, Northeastern University, and the University of Massachusetts Lowell approved this study.
Dietary assessment and dietary pattern methodology
Usual dietary intake over the past year was assessed at baseline, using a semi-quantitative FFQ adapted and validated for use in this population (33). Food intakes, in grams, were collapsed into food groups and mixed dishes were disaggregated and assigned to the appropriate food groups. Average daily nutrient intakes were estimated using the Nutrition Data System for Research software version 2007 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN, USA). Participants with energy intakes <600 kcal or >4800 kcal/d or with >10 questions blank in the FFQ were excluded (n = 72).
Three dietary quality indices were used in this analysis and defined as follows (Table 1):
TABLE 1.
Scoring methodology for the DASH, AHEI-2010, and MeDS dietary indices
| DASH | MeDS | AHEI-2010 | ||||||
|---|---|---|---|---|---|---|---|---|
| Min score | Max score | Median cut-off | Min score | Max score | ||||
| Q1 | Q5 | Men | Women | Mean intake | ||||
| Vegetables, serv/d | 0.64 | 4.25 | Vegetables, serv/d | 1.56 | 1.47 | Vegetables, serv/d | 0 | ≥5 |
| Fruit, serv/d | 0.22 | 2.96 | Fruit, serv/d | 1.07 | 1.07 | Fruit, serv/d | 0 | ≥4 |
| Whole grain, serv/d | 0.08 | 2.43 | Whole grain, serv/d | 0.64 | 0.66 | Whole grain, g/d | ||
| Men | 0 | 75 | ||||||
| Women | 0 | 90 | ||||||
| Low-fat dairy, serv/d | 0.09 | 2.27 | Dairy, serv/d | 1.76 | 1.56 | |||
| Nuts, serv/d | 0.1 | 1.37 | Nuts and legumes, serv/d | 0.77 | 0.65 | Nuts and legumes, serv/d | 0 | ≥1 |
| SS beverages, serv/d | 0.01 | 1.61 | Fish, serv/d | 0.95 | 0.81 | SS beverages, serv/d (including fruit juice) | ≥1 | 0 |
| Red/processed meat, serv/d | 0.17 | 1.97 | Meat, serv/d | 4.70 | 4.01 | Red/processed meat, serv/d | ≥1.5 | 0 |
| Sodium, mg/d | 2172 | 8107 | MUFA:SFA ratio | 1.16 | 1.18 | Sodium, mg/d | Highest decile | Lowest decile |
| Alcohol, drinks/d | ≤ 2 | ≤ 1 | Alcohol, drinks/d | |||||
| Men | ≥3.5 | 0.5–2.0 | ||||||
| Women | ≥2.5 | 0.5–1.5 | ||||||
| PUFA, % energy | ≤2 | ≥10 | ||||||
| EPA + DHA, mg/dL | 0 | 250 | ||||||
| Trans fat, % energy | ≥4 | ≤0.05 | ||||||
AHEI-2010, Alternative Health Eating Index-2010; DASH, Dietary Approaches to Stop Hypertension; MeDS, Mediterranean Diet Score; SS, sugar-sweetened beverages.
DASH was defined following methods by Fung et al. (34). Participants received 1 point for those in the lowest quintile and 5 points for those in the highest quintile of intakes of fruit, vegetables, nuts and legumes, low-fat dairy products, and whole grains, according to quintile rankings. Those with the lowest quintile of intake of sodium, sugar-sweetened beverages, and red and processed meats received a score of 5 and those in the highest quintile received a score of 1. Scores were totaled with a possible score range from 8 (lowest adherence) to 40 (greatest adherence).
AHEI-2010 was defined from 11 food groups or nutrients, with higher intakes of 6 components [vegetables, fruit, whole grains, nuts and legumes, long-chain (n–3) fat (EPA + DHA), and PUFAs], moderate intake of 1 component (alcohol), and lower intakes of 4 components (sugar-sweetened beverages, red and processed meat, trans fat, and sodium) and has been shown to be protective of chronic health conditions (13). Participants received a score between 0 (minimal adherence) and 10 points (maximal adherence) for each component, based on dietary intakes. A sum of all components was calculated and ranged from 0 (poorest dietary quality) to 110 (highest dietary quality), with a higher score representing better adherence.
MeDS was defined following methods by Trichopoulou et al. (35). The score was modified to include whole grains instead of total grains, due to the high intake of refined grains in this population, as described by Mattei et al. (36). The MeDS included 9 food group and nutrient components that were scored using the sex-specific population median adjusted for total energy calculated using the residual method. These included vegetables, fruit, whole grains, nuts and legumes, meat, fish, dairy, MUFA:SFA ratio, and alcoholic beverages. Participants consuming below the median for healthful components received a score of 0 and a score of 1 for above the sex-specific median. For unfavorable components, a score of 0 was assigned for those consuming above the median and a score of 1 for those consuming below the sex-specific median intake. A total score was calculated and ranged from 0 (poorest adherence) to 9 (highest adherence).
Bone outcome measures
Primary outcome variables included BMD at the hip and lumbar spine and osteoporosis at the femoral neck and/or lumbar spine. Hip and lumbar spine BMD (g/cm2) was measured using DXA (GE-Lunar Model Prodigy scanner; General Electric) at the Bone Metabolism Laboratory at the HNRCA at Tufts University. All measurements were obtained following standard protocols; the right hip was scanned unless a previous hip fracture or joint replacement in the right hip was self-reported by the participant. The root mean square precision was 1.31% for BMD measures of the femoral neck and 1.04% for the lumbar spine, as reported elsewhere (37). An external standard (aluminum spine phantom: Lunar Radiation Corp) was scanned weekly to assess stability of the DXA measures. After reviewing all scans with T-scores >4.0, 25 participants’ lumbar spine (L2–L4) measures and 7 participants’ femoral neck measures were excluded, as they were determined to be inaccurate by the study endocrinologist (BD-H).
Osteoporosis was defined as T-score ≤ −2.5 (2.5 SD or more below peak bone mass) and low bone mass as T-score between −1.0 and −2.5 (between 1.0 and 2.5 SD below peak bone mass) at the femoral neck or lumbar spine, as defined by criteria from the WHO (38). Lumbar spine T-scores were estimated using a reference group of 30-y-old non-Hispanic white females from the DXA manufacturer's database (39) and femoral neck T-scores from a reference group of 20–29-y-old non-Hispanic white females from NHANES III (40).
Covariate assessment
Sociodemographic factors including sex and educational attainment were assessed at baseline, through questionnaire. Age was assessed at the BPROS visit. Anthropometric measures, including weight and height, were obtained at the BPROS interview, in duplicate, and an average of the 2 measures was used. BMI was calculated as weight (kg) divided by height (m2). Health behaviors, including smoking and alcohol consumption, were ascertained and categorized as never, past, or current, as assessed at 2-y follow-up. Participants were asked to self-report the use of medication to treat osteoporosis (e.g., Calcitonin, Fosamax, and Didronal) during the BPROS interview. For women only, the use of estrogen preparations either orally or by patch (not including vaginal cream), was obtained and included Premarin, Prempro, Premphase, Estratab, Menest, Estrace, Ogen (Ortho-est), Estraderm (Vivelle), Evista, and/or other medication. Data on menopause (yes or no) and estrogen preparations (yes or no) were used to classify women as estrogenic (premenopausal or currently taking exogenous estrogen) or nonestrogenic (postmenopausal and not taking exogenous estrogen). Serum 25(OH) vitamin D concentration (ng/mL) was measured from fasting blood samples collected during the BPROS interview, and by extraction with radioimmunoassay (RIA) with a 25I RIA Packard Q7 COBRA II Gamma Counter (catalog no. 68100E; DiaSorin, Inc.). The intra-assay and interassay CVs were 10.8% and 9.4%, respectively.
Statistical analysis
All analyses were conducted using SAS software (version 9.4, SAS Institute). All variables and distributions were examined for normality. Sample size varied across dietary patterns, due to missing data for individual food groups (n = 865–896). Means (SEs) and frequencies of sociodemographic factors and health behaviors were calculated by quintile of the DASH, AHEI, and MeDS, using chi-square and ANOVA, for men, premenopausal women (including postmenopausal estrogen users), and postmenopausal women (excluding estrogen users). Dietary quality indices were standardized by converting each score to z-scores. Multivariable linear regression models were used to examine associations between each index and BMD at hip and spine sites in men, and pre- and postmenopausal women. Multivariable logistic regression was used to model relations between dietary quality indices and osteoporosis (yes/no) at the femoral neck and lumbar spine in men and postmenopausal women only, as the number of premenopausal women with osteoporosis was too low (n = 3). Multivariable logistic regression models were also used to investigate associations between individual components of each index and the odds of osteoporosis for men and postmenopausal women. An unadjusted model was initially assessed. Model 1 was adjusted for age. Model 2 was adjusted for model 1 plus BMI and height. Model 3 was adjusted for model 2 plus education, smoking status, alcohol use, season of BMD measurement, osteoporosis medication use, calcium intake, and serum vitamin D. DASH and AHEI models were also adjusted for total energy intake (MeDS is based on population medians adjusted for total energy) (36) and alcohol consumption only for DASH, as alcohol is included as a component of the AHEI and MeDS scores.
Results
Characteristics between the highest compared with lowest quintile of each dietary index were examined for men, and premenopausal (including postmenopausal estrogen users) and postmenopausal women (excluding estrogen users). Results were similar for each dietary index, and therefore, data only for the DASH index are presented in Table 2. The prevalence of osteoporosis was 8.8% for Puerto Rican men and 11.2% for Puerto Rican women. Compared with the lowest quintile, men in the highest quintile of the DASH, MeDS, and AHEI (56.7 ± 1.2 y compared with 62.1 ± 1.0 y; 55.4 ± 1.3 y compared with 60.8 ± 0.97 y; 57.8 ± 0.97 y compared with 62.4 ± 1.2 y) and postmenopausal women in the highest quintile of the DASH index (59.5 ± 0.8 y compared with 63.6 ± 0.6 y) were older. A greater percentage of postmenopausal women in the highest quintile of the MeDS had less than an 8th grade education than those in the lowest quintile (57% compared with 38.7%). Premenopausal women in the highest quintile of the DASH index were less likely to be currently smoking, and men and pre- and postmenopausal women in the highest quintile had a higher intake of dietary calcium compared with those in the lowest quintile (P <0.05 for all, Table 2). More men and pre- and postmenopausal women in the highest compared with lowest quintile of the MeDS and AHEI were less likely to be heavy alcohol consumers (P <0.01 for all). Premenopausal women in the highest compared with lowest quintile of the AHEI had a higher intake of dietary calcium (1287 ± 94 mg/d compared with 846 ± 92 mg/d). Postmenopausal women in the highest quintile of the DASH and AHEI had a higher BMD of the trochanter (0.814 ± 0.011 g/cm2 compared with 0.752 ± 0.015 g/cm2; 0.807 ± 0.012 g/cm2 compared with 0.749 ± 0.014 g/cm2, P <0.05) and total hip (1.01 ± 0.012 g/cm2 compared with 0.951 ± 0.017 g/cm2; 1.01 ± 0.014 g/cm2 compared with 0.940 ± 0.016 g/cm2, P <0.05), and a lower prevalence of osteoporosis (5.3% compared with 17.6%; 4.6% compared with 19.3%, P <0.05, respectively) compared with the lowest quintile. There were no differences in postmenopausal status, use of estrogen or osteoporosis medication, total energy intake, or serum 25(OH) vitamin D concentration for the highest compared with lowest quintile of the DASH, AHEI, and MeDS.
TABLE 2.
Sociodemographic and health factors by highest (Q5) and lowest quintile (Q1) of the DASH for men, and premenopausal and postmenopausal women
| DASH | ||||||
|---|---|---|---|---|---|---|
| Men (n = 244) | Postmenopausal women1 (n = 507) | Premenopausal women2 (n = 139) | ||||
| Q1 (11–19) | Q5 (28–36) | Q1 (12–19) | Q5 (28–38) | Q1 (12–19) | Q5 (28–37) | |
| Age, y | 56.7 ± 1.2 | 62.1 ± 1.0** | 59.5 ± 0.8 | 63.6 ± 0.6*** | 52.0 ± 0.7 | 54.4 ± 0.8 |
| <8th grade educational level, % | 57.5 | 62.1 | 44.6 | 50.4 | 70.6 | 77.4 |
| Current smoker, % | 40.0 | 19.4 | 24.3 | 11.5 | 41.2 | 6.5** |
| Alcohol consumption, % | ||||||
| None within past year | 50.0 | 43.9 | 59.5 | 58.8 | 47.1 | 41.9 |
| Moderate | 42.5 | 45.5 | 35.1 | 40.5 | 44.1 | 51.6 |
| Heavy | 7.5 | 10.6 | 5.4 | 1.0 | 8.8 | 6.5 |
| BMI, kg/m2 | 29.3 ± 0.9 | 31.3 ± 0.7 | 32.9 ± 0.8 | 32.7 ± 0.6 | 30.8 ± 1.1 | 34.6 ± 1.2 |
| Height, cm | 166 ± 1.1 | 167 ± 0.8 | 155 ± 0.7 | 154 ± 0.5 | 156 ± 1.0 | 157 ± 1.2 |
| Postmenopausal, % | — | — | 100 | 100 | 5.9 | 12.9 |
| Use of estrogen medication, % | — | — | 0 | 0 | 5.9 | 16.1 |
| Use of medication to treat osteoporosis, % | 2.5 | 1.5 | 12.2 | 9.2 | 0 | 0 |
| Total energy, kcal | 2230 ± 131 | 2359 ± 101 | 2184 ± 102 | 1874 ± 76 | 2404 ± 135 | 1891 ± 141 |
| Dietary calcium, mg/d | 775 ± 86 | 1198 ± 66** | 948 ± 70 | 1215 ± 52* | 825 ± 81 | 1179 ± 85* |
| Serum 25-OH vitamin D, ng/mL | 18.3 ± 1.1 | 18.6 ± 0.86 | 19.6 ± 0.90 | 20.6 ± 0.68 | 17.6 ± 1.2 | 21.9 ± 1.2 |
| Bone mineral density, g/cm2 | ||||||
| Trochanter | 0.874 ± 0.024 | 0.907 ± 0.018 | 0.752 ± 0.015 | 0.814 ± 0.011** | 0.849 ± 0.020 | 0.853 ± 0.020 |
| Femoral neck | 0.963 ± 0.024 | 1.00 ± 0.018 | 0.868 ± 0.015 | 0.911 ± 0.011 | 0.983 ± 0.021 | 0.999 ± 0.022 |
| Total hip | 1.05 ± 0.026 | 1.09 ± 0.020 | 0.951 ± 0.017 | 1.01 ± 0.012* | 1.07 ± 0.022 | 1.07 ± 0.022 |
| Lumbar spine | 1.19 ± 0.030 | 1.23 ± 0.023 | 1.09 ± 0.019 | 1.14 ± 0.015 | 1.25 ± 0.028 | 1.23 ± 0.029 |
| Osteoporosis; femoral neck or lumbar spine, % | 12.5 | 5.9 | 17.6 | 5.3* | 5.9 | 0 |
Postmenopausal women excluding estrogen users.
Premenopausal women, including postmenopausal estrogen users.
ANOVA for continuous and chi-square for categorical variables were used. Means ± SE and frequencies are presented. * P value of <0.05, ** P <0.01, and ***P <0.001 between the lowest and highest quintile of each pattern.
DASH, Dietary Approaches to Stop Hypertension.
Higher adherence to DASH was significantly associated with a higher BMD at the trochanter (β: 0.026 ± 0.006, P <0.001), femoral neck (β: 0.022 ± 0.006, P <0.001), total hip (β: 0.029 ± 0.006, P <0.001), and lumbar spine (β: 0.025 ± 0.007, P = 0.001) for postmenopausal women, after adjusting for sociodemographic, anthropometric, and lifestyle factors, as well as the use of osteoporosis medication and season of BMD measurement (Table 3). Higher adherence to MeDS was significantly associated with a higher BMD at the trochanter (β: 0.015 ± 0.005, P = 0.007) and total hip (0.015 ± 0.006, P = 0.01, Table 4) among postmenopausal women only. Higher adherence to the AHEI was associated with a higher BMD at the trochanter (β: 0.018 ± 0.006, P = 0.001), femoral neck (β: 0.015 ± 0.006, P = 0.01), total hip (0.021 ± 0.006, P = 0.001), and lumbar spine (β: 0.017 ± 0.008, P = 0.02, Table 5) among postmenopausal women only.
TABLE 3.
Cross-sectional associations of DASH index z-scores and bone mineral density (g/cm2) for men, and premenopausal and postmenopausal women
| DASH | ||||||
|---|---|---|---|---|---|---|
| Men (n = 244) | Premenopausal women1 (n = 139) | Postmenopausal women2 (n = 507) | ||||
| β ± SE | P | β ± SE | P | β ± SE | P | |
| Trochanter | ||||||
| Unadjusted | 0.005 ± 0.009 | 0.57 | 0.0007 ± 0.009 | 0.94 | 0.021 ± 0.006 | <0.001 |
| Model 1 | 0.003 ± 0.010 | 0.72 | 0.004 ± 0.010 | 0.65 | 0.027 ± 0.006 | <0.001 |
| Model 2 | −0.004 ± 0.009 | 0.70 | −0.003 ± 0.002 | 0.79 | 0.025 ± 0.005 | <0.001 |
| Model 3 | −0.004 ± 0.010 | 0.65 | −0.005 ± 0.010 | 0.69 | 0.026 ± 0.006 | <0.001 |
| Femoral neck | ||||||
| Unadjusted | 0.004 ± 0.010 | 0.68 | 0.005 ± 0.010 | 0.62 | 0.015 ± 0.006 | 0.01 |
| Model 1 | 0.007 ± 0.010 | 0.50 | 0.010 ± 0.010 | 0.31 | 0.022 ± 0.006 | <0.001 |
| Model 2 | 0.001 ± 0.009 | 0.90 | 0.005 ± 0.010 | 0.63 | 0.021 ± 0.005 | <0.001 |
| Model 3 | 0.006 ± 0.010 | 0.59 | 0.009 ± 0.012 | 0.46 | 0.022 ± 0.006 | <0.001 |
| Total hip | ||||||
| Unadjusted | 0.007 ± 0.010 | 0.51 | 0.002 ± 0.011 | 0.83 | 0.022 ± 0.007 | <0.001 |
| Model 1 | 0.007 ± 0.011 | 0.52 | 0.008 ± 0.011 | 0.49 | 0.030 ± 0.006 | <0.001 |
| Model 2 | 0.001 ± 0.010 | 0.90 | −0.002 ± 0.01 | 0.83 | 0.029 ± 0.006 | <0.001 |
| Model 3 | −0.001 ± 0.010 | 0.89 | 0.001 ± 0.013 | 0.92 | 0.029 ± 0.006 | <0.001 |
| Lumbar spine | ||||||
| Unadjusted | 0.016 ± 0.012 | 0.17 | −0.011 ± 0.013 | 0.41 | 0.018 ± 0.007 | 0.02 |
| Model 1 | 0.010 ± 0.012 | 0.41 | −0.004 ± 0.013 | 0.76 | 0.024 ± 0.007 | 0.001 |
| Model 2 | 0.003 ± 0.012 | 0.78 | −0.010 ± 0.013 | 0.46 | 0.022 ± 0.007 | 0.002 |
| Model 3 | 0.003 ± 0.013 | 0.80 | 0.007 ± 0.016 | 0.67 | 0.025 ± 0.007 | 0.001 |
Premenopausal women, including postmenopausal estrogen users.
Postmenopausal women, excluding estrogen users.
Multivariable linear regression models were used. Unadjusted model includes the dietary quality index only. Model 1 was adjusted for age and sex. Model 2 was adjusted for model 1 plus BMI and height. Model 3 was adjusted for model 2 plus smoking status, season of bone mineral density measurement (fall, winter, spring, summer), osteoporosis medication use (yes/no), and calcium intake (mg/d), total energy intake, alcohol consumption, serum vitamin D status (mg/dL).
DASH, Dietary Approaches to Stop Hypertension.
TABLE 4.
Cross-sectional associations of MeDS z-scores and bone mineral density (g/cm2) for men, and premenopausal and postmenopausal women
| MeDS (n = 865) | ||||||
|---|---|---|---|---|---|---|
| Men (n = 244) | Premenopausal women1 (n = 139) | Postmenopausal women2(n = 507) | ||||
| β ± SE | P | β ± SE | P | β ± SE | P | |
| Trochanter | ||||||
| Unadjusted | 0.010 ± 0.009 | 0.27 | −0.005 ± 0.011 | 0.60 | 0.014 ± 0.006 | 0.02 |
| Model 1 | 0.009 ± 0.009 | 0.32 | −0.004 ± 0.010 | 0.67 | 0.014 ± 0.006 | 0.014 |
| Model 2 | 0.005 ± 0.009 | 0.56 | −0.002 ± 0.009 | 0.83 | 0.015 ± 0.005 | 0.004 |
| Model 3 | 0.005 ± 0.009 | 0.58 | −0.005 ± 0.010 | 0.54 | 0.015 ± 0.005 | 0.007 |
| Femoral neck | ||||||
| Unadjusted | 0.010 ± 0.009 | 0.31 | −0.006 ± 0.010 | 0.53 | 0.010 ± 0.006 | 0.10 |
| Model 1 | 0.011 ± 0.010 | 0.24 | −0.005 ± 0.010 | 0.61 | 0.011 ± 0.006 | 0.07 |
| Model 2 | 0.007 ± 0.009 | 0.42 | −0.004 ± 0.010 | 0.68 | 0.010 ± 0.005 | 0.08 |
| Model 3 | 0.007 ± 0.009 | 0.46 | −0.007 ± 0.010 | 0.47 | 0.010 ± 0.005 | 0.07 |
| Total hip | ||||||
| Unadjusted | 0.012 ± 0.010 | 0.23 | −0.004 ± 0.011 | 0.70 | 0.014 ± 0.007 | 0.044 |
| Model 1 | 0.012 ± 0.010 | 0.23 | −0.003 ± 0.011 | 0.80 | 0.014 ± 0.007 | 0.030 |
| Model 2 | 0.007 ± 0.010 | 0.45 | −0.0003 ± 0.01 | 0.97 | 0.016 ± 0.006 | 0.009 |
| Model 3 | 0.007 ± 0.010 | 0.47 | −0.003 ± 0.011 | 0.76 | 0.015 ± 0.006 | 0.01 |
| Lumbar spine | ||||||
| Unadjusted | 0.015 ± 0.012 | 0.21 | −0.006 ± 0.014 | 0.67 | 0.009 ± 0.008 | 0.23 |
| Model 1 | 0.011 ± 0.012 | 0.38 | −0.005 ± 0.013 | 0.74 | 0.009 ± 0.008 | 0.20 |
| Model 2 | 0.006 ± 0.012 | 0.61 | −0.006 ± 0.013 | 0.64 | 0.009 ± 0.007 | 0.19 |
| Model 3 | 0.001 ± 0.012 | 0.90 | −0.008 ± 0.014 | 0.57 | 0.008 ± 0.007 | 0.28 |
Premenopausal women including postmenopausal estrogen users.
Postmenopausal women, excluding estrogen users.
Multivariable linear regression models were used. Unadjusted model includes the dietary quality index only. Model 1 was adjusted for age and sex. Model 2 was adjusted for model 1 plus BMI and height. Model 3 was adjusted for model 2 plus smoking status, season of bone mineral density measurement (fall, winter, spring, summer), osteoporosis medication use (yes/no), and calcium intake (mg/d), serum vitamin D status (mg/dL).
MeDS, Mediterranean Diet Score.
TABLE 5.
Cross-sectional associations of AHEI z-scores and bone mineral density (g/cm2) for men, and premenopausal and postmenopausal women
| AHEI (n = 865) | ||||||
|---|---|---|---|---|---|---|
| Men (n = 237) | Premenopausal women1 (n = 133) | Postmenopausal women2 (n = 489) | ||||
| β ± SE | P | β ± SE | P | β ± SE | P | |
| Trochanter | ||||||
| Unadjusted | 0.009 ± 0.010 | 0.35 | −0.006 ± 0.010 | 0.51 | 0.018 ± 0.006 | 0.003 |
| Model 1 | 0.008 ± 0.010 | 0.42 | −0.004 ± 0.010 | 0.71 | 0.020 ± 0.006 | <0.001 |
| Model 2 | 0.002 ± 0.009 | 0.83 | −0.006 ± 0.009 | 0.51 | 0.021 ± 0.005 | <0.001 |
| Model 3 | 0.004 ± 0.010 | 0.67 | −0.007 ± 0.012 | 0.56 | 0.018 ± 0.006 | 0.001 |
| Femoral neck | ||||||
| Unadjusted | 0.005 ± 0.010 | 0.61 | −0.004 ± 0.010 | 0.66 | 0.013 ± 0.006 | 0.03 |
| Model 1 | 0.008 ± 0.010 | 0.44 | −0.0004 ± 0.010 | 0.97 | 0.015 ± 0.006 | 0.007 |
| Model 2 | 0.005 ± 0.010 | 0.62 | −0.004 ± 0.010 | 0.66 | 0.016 ± 0.005 | 0.004 |
| Model 3 | 0.007 ± 0.010 | 0.51 | −0.002 ± 0.013 | 0.86 | 0.015 ± 0.006 | 0.01 |
| Total hip | ||||||
| Unadjusted | 0.011 ± 0.010 | 0.32 | −0.006 ± 0.011 | 0.59 | 0.020 ± 0.007 | 0.004 |
| Model 1 | 0.011 ± 0.011 | 0.30 | −0.001 ± 0.011 | 0.90 | 0.022 ± 0.007 | <0.001 |
| Model 2 | 0.005 ± 0.010 | 0.86 | −0.006 ± 0.011 | 0.59 | 0.024 ± 0.006 | 0.001 |
| Model 3 | 0.007 ± 0.011 | 0.54 | 0.0003 ± 0.014 | 0.98 | 0.021 ± 0.006 | 0.001 |
| Lumbar spine | ||||||
| Unadjusted | 0.011 ± 0.013 | 0.39 | −0.016 ± 0.013 | 0.24 | 0.015 ± 0.008 | 0.05 |
| Model 1 | 0.005 ± 0.013 | 0.67 | −0.011 ± 0.014 | 0.41 | 0.017 ± 0.007 | 0.02 |
| Model 2 | 0.004 ± 0.013 | 0.77 | −0.014 ± 0.014 | 0.29 | 0.017 ± 0.008 | 0.03 |
| Model 3 | −0.007 ± 0.014 | 0.62 | 0.0008 ± 0.018 | 0.96 | 0.017 ± 0.008 | 0.02 |
Premenopausal women, including postmenopausal estrogen users.
Postmenopausal women, excluding estrogen users.
Multivariable logistic regression models were used. Unadjusted model includes the dietary quality index only. Model 1 was adjusted for age and sex. Model 2 was adjusted for model 1 plus BMI and height. Model 3 was adjusted for model 2 plus smoking status, season of bone mineral density measurement (fall, winter, spring, summer), osteoporosis medication use (yes/no), and calcium intake (mg/d), total energy intake, serum vitamin D status (mg/dL).
AHEI, Alternative Health Eating Index-2010.
DASH and AHEI were associated with a lower likelihood of osteoporosis for postmenopausal women only, after adjusting for all covariates. MeDS approached significance after adjusting for all covariates (Table 6). Estrogenic women were not included in the analysis, due to the limited number with osteoporosis (n = 3). Among postmenopausal women, higher adherence to DASH was associated with 46% lower odds of osteoporosis at the lumbar spine and/or the femoral neck (OR: 0.54, 95% CI: 0.39, 0.75); higher adherence to MeDS and AHEI were also associated with a lower likelihood of osteoporosis (OR: 0.75, 95% CI: 0.56, 1.01 and OR: 0.64, 95% CI: 0.46, 0.88, respectively).
TABLE 6.
ORs and 95% CIs for the associations of DASH z-scores and osteoporosis at the lumbar spine and/or femoral neck for men and postmenopausal women1
| DASH | MeDS | AHEI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 244) | Postmenopausal women (n = 507) | Men (n = 244) | Postmenopausal women (n = 507) | Men (n = 237) | Postmenopausal women (n = 489) | |||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Osteoporosis | ||||||||||||
| Unadjusted | 0.82 | 0.52, 1.28 | 0.66 | 0.50, 0.87 | 0.88 | 0.56, 1.37 | 0.77 | 0.58, 1.01 | 0.72 | 0.44, 1.17 | 0.68 | 0.51, 0.90 |
| Model 1 | 0.84 | 0.53, 1.33 | 0.58 | 0.44, 0.78 | 0.90 | 0.58, 1.40 | 0.76 | 0.57, 1.00 | 0.74 | 0.45, 1.21 | 0.65 | 0.49, 0.87 |
| Model 2 | 0.82 | 0.50, 1.35 | 0.60 | 0.45, 0.81 | 0.93 | 0.58, 1.50 | 0.75 | 0.56, 0.99 | 0.72 | 0.42, 1.25 | 0.65 | 0.48, 0.87 |
| Model 3 | 0.71 | 0.40, 1.29 | 0.54 | 0.39, 0.75 | 0.85 | 0.51, 1.42 | 0.75 | 0.56, 1.01 | 0.75 | 0.41, 1.36 | 0.64 | 0.46, 0.88 |
Postmenopausal women excluding estrogen users.
Multivariable logistic regression models were used. Unadjusted model includes the dietary quality index only. Model 1 was adjusted for age. Model 2 was adjusted for model 1 plus BMI and height. Model 3 was adjusted for model 2 plus smoking status, season of bone mineral density measurement (fall, winter, spring, summer), osteoporosis medication use (yes/no), and calcium intake (mg/d), serum vitamin D status (mg/dL).
AHEI, Alternative Health Eating Index-2010; DASH, Dietary Approaches to Stop Hypertension; MeDS, Mediterranean Diet Score.
For DASH, MeDS, and AHEI, the whole grains (servings/d) component was associated with a lower likelihood of osteoporosis in postmenopausal women (OR: 0.66, 95% CI: 0.52, 0.83; OR: 0.36, 95% CI: 0.19, 0.67; and OR: 0.77, 95% CI: 0.62, 0.96, Table 7). For DASH only, higher low-fat dairy and lower red and processed meat and sodium were also associated with a lower likelihood of osteoporosis in postmenopausal women. For AHEI, higher vegetable score and a lower percentage of energy from trans fat were associated with a lower likelihood of osteoporosis in postmenopausal women. No other statistically significant associations were observed between diet score components and prevalence of osteoporosis.
TABLE 7.
ORs and 95% CIs for the associations of individual components and osteoporosis at the lumbar spine or the femoral neck sites for each dietary index1,2,3
| DASH | MeDS | AHEI | ||||||
|---|---|---|---|---|---|---|---|---|
| Men | Postmenopausal women | Men | Postmenopausal women | Men | Postmenopausal women | |||
| Component | OR (95%CI) | OR (95% CI) | Component | OR (95% CI) | OR (95% CI) | Component | OR (95% CI) | OR (95% CI) |
| Vegetables, servings/d | 0.80 (0.51, 1.2) | 0.82 (0.65, 1.03) | Vegetables, servings/d | 0.57 (0.18, 1.8) | 0.98 (0.55, 1.7) | Vegetables, servings/d | 0.86 (0.63, 1.2) | 0.86 (0.75, 1.0) |
| Fruit, servings/d | 0.81 (0.54, 1.2) | 0.91 (0.73, 1.1) | Fruit, servings/d | 0.64 (0.21, 1.9) | 0.91 (0.51, 1.6) | Fruit, servings/d | 0.94 (0.72, 1.2) | 0.89 (0.76, 1.03) |
| Whole grains, servings/d | 0.97 (0.65, 1.4) | 0.66 (0.52, 0.83) | Whole grains, g/d | 0.73 (0.25, 2.1) | 0.36 (0.19, 0.67) | Whole grains, g/d | 0.98 (0.65, 1.5) | 0.77 (0.62, 0.96) |
| Low-fat dairy, servings/d | 0.79 (0.52, 1.2) | 0.77 (0.61, 0.98) | Dairy, servings/d | 0.62 (0.20, 2.0) | 1.3 (0.68, 2.4) | |||
| Nuts, servings/d | 1.0 (0.67, 1.6) | 1.0 (0.79, 1.2) | Nuts and legumes, servings/d | 0.99 (0.34, 2.9) | 0.73 (0.41, 1.3) | Nuts and legumes, servings/d | 0.96 (0.78, 1.2) | 0.98 (0.88, 1.1) |
| Sugar-sweetened beverages, servings/d | 0.93 (0.62, 1.4) | 0.87 (0.70, 1.1) | Fish, servings/d | 3.1 (0.92, 10.2) | 0.83 (0.51, 1.3) | Sugar-sweetened beverages, servings/d | 0.93 (0.79, 1.1) | 0.98 (0.89, 1.1) |
| Red and processed meat, servings/d | 1.0 (0.51, 2.0) | 0.69 (0.48, 0.97) | Meat, servings/d | 2.0 (0.62, 6.5) | 0.29 (0.44, 1.4) | Red and processed meat, servings/d | 0.82 (0.65, 1.04) | 0.92 (0.81, 1.04) |
| Sodium, mg/d | 1.0 (0.51, 2.0) | 0.69 (0.48, 0.97) | MUFA:SFA ratio | 0.50 (0.16, 1.6) | 1.2 (0.68, 2.2) | Sodium, mg/d | 1.1 (0.79, 1.6) | 0.81 (0.65, 1.02) |
| Alcohol, drinks/d | 0.81 (0.23, 2.6) | 0.79 (0.42, 1.5) | Alcohol, drinks/d | 0.97 (0.82, 1.2) | 0.94 (0.84, 1.1) | |||
| PUFA, % of energy Long-chain n–3 fats | 1.2 (0.98, 1.5) | 0.91 (0.81, 1.02) | ||||||
| Trans fat, % energy | 0.73 (0.47, 1.1) | 0.74 (0.55, 1.0) | ||||||
*Denotes significant 95% CI.
Multivariable logistic regressions were used. Models were adjusted for age, education, BMI, height, smoking status, season of bone mineral density measurement (fall, winter, spring, summer), osteoporosis medication use (yes/no), and calcium intake (mg/d), serum vitamin D status (mg/dL). DASH and AHEI were also adjusted for total energy intake. DASH was also adjusted for alcohol consumption.
Postmenopausal women excluding estrogen users.
AHEI, Alternative Health Eating Index-2010; DASH, Dietary Approaches to Stop Hypertension; MeDS, Mediterranean Diet Score.
Discussion
In this population of older Puerto Rican adults, DASH and AHEI-2010 indices were associated with a lower likelihood of osteoporosis among postmenopausal women (not taking estrogen), whereas the MeDS approached significance. No associations were seen among men, and there were too few estrogenic women to evaluate. Higher DASH adherence was associated with a higher BMD at all hip sites and the lumbar spine, and was more strongly associated with a lower likelihood of osteoporosis than the AHEI-2010 or MeDS. Our findings suggest that a DASH diet may be particularly important in maintaining bone health for postmenopausal women not taking estrogen. Postmenopausal women have lower circulating estrogen, which leads to bone deterioration and progressive bone loss, placing them at increased risk of osteoporosis (41). Thus, it is possible that a healthier dietary pattern, such as DASH, may mitigate bone loss in the environment of lower estrogen among women. In comparison, men and premenopausal women do not appear to experience bone loss at the same accelerated rate and may be less likely to respond to other external stimuli, such as diet. The interaction by estrogen status is corroborated by work from the Aberdeen Prospective Osteoporosis Screening Study, where individual nutrients were associated with bone among peri- and postmenopausal women, but not premenopausal women (42).
The DASH, AHEI-2010, and MeDS indices have many similarities in that they emphasize vegetables, fruit, whole grains, nuts, and legumes (13, 34, 35), which are known to be important for bone health (9, 43). The DASH diet also promotes low-fat dairy as beneficial (34) and the DASH and AHEI-2010 include limiting sodium (13, 35). These 2 dietary components may contribute to the DASH being more strongly, positively associated with bone, as dairy is an important component of a dietary quality index for bone, particularly for those with adequate vitamin D status (6). Sodium may also be key, as higher intakes increase urinary calcium excretion, which can lead to poor bone outcomes (44). Several individual low-fat dairy components of the DASH diet were inversely associated with osteoporosis among postmenopausal women in this study. Lower sodium intake represented by the DASH sodium component appeared protective, as did lower red and processed meat consumption. A recent review reported that negative associations between meat consumption and bone health may be due to the fact that it is often consumed as part of a Western dietary pattern (45). A red meat protein pattern was shown to be related to the lowest BMD among adults from the Framingham Osteoporosis Study, compared with other animal sources of protein (46). The implementation of a DASH dietary pattern has been shown to reduce blood pressure and reduce the risk of type 2 diabetes, cancer, and cardiovascular disease (47). This reduction of chronic disease may indirectly benefit bone health. Although few studies have examined DASH directly in relation to bone, those that have suggest beneficial effects. Two dietary interventions with a DASH diet showed improvements in serum bone turnover markers (48) and reduced serum calcitrol (49), and 1 longitudinal cohort study showed an inverse association, although not significant, of hip fracture with a higher DASH diet score (22).
Several studies of dietary quality indices and bone outcomes have focused on the MeDS (19–21, 23), although other dietary quality indices have also been examined (22, 24–26, 28). Overall, findings from studies on dietary quality indices and bone measures (BMD, fracture, bone turnover markers) have been inconsistent, with some showing favorable associations (19, 20, 22, 26, 27, 29, 31), others reporting null associations (23, 24, 28, 30), and 1 study reporting a negative relation (21). Conflicting findings across studies may be due to differences in methodology in creating the dietary indices, such as decisions on which foods and beverages to include in food groupings, and criteria used to assess how closely an individual adheres to a specific dietary pattern. Some dietary quality indices, such as MeDS and DASH, are based on population-based cut-offs (sex-specific median intake adjusted for total energy and quintiles) whereas others (e.g., AHEI-2010) are based on existing nutrition knowledge and dietary recommendations. The different methodologies used to define the cut-offs may explain differences in point estimates of the individual components (e.g., whole grain and vegetables) with bone outcomes. For example, the whole grain component of DASH and MeDS, but not for AHEI, was associated with a lower likelihood of osteoporosis in our study. The DASH and MeDS cut-offs are based on the population distribution of intakes, whereas the AHEI is based on an a priori cut-off. Further, some dietary quality indices use dichotomous cut-offs (e.g., MeDS), compared with normative or ordinal cut-offs (AHEI, DASH) for each of the individual components, which impacts the range of scoring. Therefore, the degree to which an individual is classified as below or above the recommended amount for each component of the dietary index may not accurately represent differences in dietary quality.
Due to inconsistent results across populations, few studies have compared commonly recommended indices with BMD and fracture outcomes in adolescents/young adults (24, 25) and older adults (22, 26). In 90,014 postmenopausal women from the Women's Health Initiative cohort study, greater adherence to a Mediterranean diet was associated with a lower risk of hip fracture, and there was a nonsignificant inverse association between higher DASH and AHEI-2010 scores and hip fracture (22). In a case-control study, 4 dietary quality indices [AHEI, HEI-2005, Diet Quality Index-International, and alternate Mediterranean Diet Score (aMed)] were inversely associated with hip fracture in 1452 older Chinese adults; however, the authors note that the aMed was preferable, based on the ability to calculate the score and its interpretability (26). No significant results were observed for studies comparing dietary indices among young adults (24, 25). Many dietary quality indices are designed to assess adherence to dietary recommendations for preventing chronic conditions for the general US population, and do not account for the unique traditional patterns of ethnic and minority populations. This is also true of dietary quality indices that are developed for specific regions, such as the Mediterranean diet, as these scores may not allow for the inclusion of traditional or culturally specific foods (50).
Our analysis demonstrates that, within a US mainland Puerto Rican population using a culturally tailored FFQ to estimate dietary intake, DASH was the strongest predictor of BMD, relative to MeDS or AHEI. This is notable, because the DASH dietary pattern emphasizes nutrients such as potassium and fiber and limits sodium intake (34). Potassium and magnesium in fruit and vegetables may promote lower acid load and positive calcium balance, favoring bone formation over resorption (9, 43). Greater fruit and vegetable variety has been related to lower inflammation, another risk factor for osteoporosis (51), in this population (52).
The strengths of this study include a large cohort of Puerto Rican adults living on the US mainland, a population at high risk of osteoporosis, with comprehensive assessment of sociodemographic, lifestyle, and health factors related to bone. Dietary data were collected using an FFQ that was validated for use in this population. This study is limited by its cross-sectional nature and is subject to possible reverse causation. Further, results were stratified by sex and estrogen status; however, power to detect associations among men and premenopausal estrogenic women was limited, due to the small sample size. Thus, data should be interpreted cautiously, given the unbalanced sample size between men, and estrogenic and nonestrogenic women. Future studies are needed to investigate relations between dietary quality and bone loss in a larger cohort with greater numbers of men, premenopausal women, and postmenopausal women taking estrogen.
In conclusion, several dietary quality indices were associated with bone outcomes in a population of older Puerto Rican adults, with DASH more strongly associated than MeDS or AHEI-2010, particularly in postmenopausal women. This contributes to a growing body of literature on dietary quality and bone health, but also highlights limitations of dietary indices for ethnic and minority groups. Given the inconsistent findings among dietary quality indices and bone in the extant literature, there is a need for the development of a bone index that can be used to identify individuals at risk and provide recommendations for dietary improvement across cultural and ethnic minority groups. Further research is needed to examine the impact of these indices on changes in bone measures.
Acknowledgments
The authors’ responsibilities were as follows—SEN and KLT: designed the research; SEN, KMM, JM, JLG, BD-H, and SB: conducted the research; SEN and JLG: performed the statistical analysis; SEN: wrote the manuscript; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
This research was funded by the NIH (P01 AG023394, P50 HL105185, R01 AG027087). SEN is supported by K01 AR067894.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the NIH.
Data described in the manuscript, code book, and analytic code will be made available upon request pending approval of a manuscript proposal found at https://www.uml.edu/Research/UML-CPH/Research/bprhs/.
Abbreviations used: AHEI-2010, Alternative Healthy Eating Index-2010; BMD, bone mineral density; BPROS, Boston Puerto Rican Osteoporosis Study; BPRHS, Boston Puerto Rican Health Study; DASH, Dietary Approaches to Stop Hypertension; HNRCA, Human Nutrition Research Center on Aging; MeDS, Mediterranean Diet Score; RIA, radioimmunoassay.
References
- 1. Noel SE, Mangano KM, Griffith JL, Wright NC, Dawson-Hughes B, Tucker KL. Prevalence of osteoporosis and low bone mass among Puerto Rican older adults. J Bone Miner Res. 2018;33(3):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kanis JA, McCloskey E, Johansson H, Oden A, Leslie WD. FRAX((R)) with and without bone mineral density. Calcif Tissue Int. 2012;90(1):1–13. [DOI] [PubMed] [Google Scholar]
- 3. Ahmadieh H, Arabi A. Vitamins and bone health: beyond calcium and vitamin D. Nutr Rev. 2011;69(10):584–98. [DOI] [PubMed] [Google Scholar]
- 4. New SA. Exercise, bone and nutrition. Proc Nutr Soc. 2001;60(2):265–74. [DOI] [PubMed] [Google Scholar]
- 5. Bhupathiraju SN, Lichtenstein AH, Dawson-Hughes B, Hannan MT, Tucker KL. Adherence to the 2006 American Heart Association Diet and lifestyle recommendations for cardiovascular disease risk reduction is associated with bone health in older Puerto Ricans. Am J Clin Nutr. 2013;98(5):1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mangano KM, Noel SE, Sahni S, Tucker KL. Higher dairy intakes are associated with higher bone mineral density among adults with sufficient vitamin D status: results from the Boston Puerto Rican Osteoporosis Study. J Nutr. 2019;149(1):139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heaney RP. Dairy and bone health. J Am Coll Nutr. 2009;28(Suppl 1):82S–90S. [DOI] [PubMed] [Google Scholar]
- 8. Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int. 2012;23(1):1–16. [DOI] [PubMed] [Google Scholar]
- 9. New SA. Intake of fruit and vegetables: implications for bone health. Proc Nutr Soc. 2003;62(4):889–99. [DOI] [PubMed] [Google Scholar]
- 10. Tucker KL. Osteoporosis prevention and nutrition. Curr Osteoporos Rep. 2009;7(4):111–17. [DOI] [PubMed] [Google Scholar]
- 11. Tucker KL, Morita K, Qiao N, Hannan MT, Cupples LA, Kiel DP. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: the Framingham Osteoporosis Study. Am J Clin Nutr. 2006;84(4):936–42. [DOI] [PubMed] [Google Scholar]
- 12. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 13. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Onvani S, Haghighatdoost F, Surkan PJ, Larijani B, Azadbakht L. Adherence to the Healthy Eating Index and Alternative Healthy Eating Index dietary patterns and mortality from all causes, cardiovascular disease and cancer: a meta-analysis of observational studies. J Hum Nutr Diet. 2017;30(2):216–26. [DOI] [PubMed] [Google Scholar]
- 15. George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF, Tinker LF, Vitolins M, Neuhouser ML. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women's Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol. 2014;180(6):616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr. 2014;144(6):881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dinu M, Pagliai G, Casini A, Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr. 2018;72(1):30–43. [DOI] [PubMed] [Google Scholar]
- 18. Soltani S, Jayedi A, Shab-Bidar S, Becerra-Tomas N, Salas-Salvado J. Adherence to the Mediterranean diet in relation to all-cause mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Adv Nutr. 2019;; 10(6):1029–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benetou V, Orfanos P, Pettersson-Kymmer U, Bergström U, Svensson O, Johansson I, Berrino F, Tumino R, Borch KB, Lund E et al.. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24(5):1587–98. [DOI] [PubMed] [Google Scholar]
- 20. Chen GD, Dong XW, Zhu YY, Tian HY, He J, Chen YM. Adherence to the Mediterranean diet is associated with a higher BMD in middle-aged and elderly Chinese. Sci Rep. 2016;6:25662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feart C, Lorrain S, Ginder Coupez V, Samieri C, Letenneur L, Paineau D, Barberger-Gateau P. Adherence to a Mediterranean diet and risk of fractures in French older persons. Osteoporos Int. 2013;24(12):3031–41. [DOI] [PubMed] [Google Scholar]
- 22. Haring B, Crandall CJ, Wu C, LeBlanc ES, Shikany JM, Carbone L, Orchard T, Thomas F, Wactawaski-Wende J, Li W et al.. Dietary patterns and fractures in postmenopausal women: results from the Women's Health Initiative. JAMA Intern Med. 2016;176(5):645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kontogianni MD, Melistas L, Yannakoulia M, Malagaris I, Panagiotakos DB, Yiannakouris N. Association between dietary patterns and indices of bone mass in a sample of Mediterranean women. Nutrition. 2009;25(2):165–71. [DOI] [PubMed] [Google Scholar]
- 24. Monjardino T, Lucas R, Ramos E, Barros H. Associations between a priori-defined dietary patterns and longitudinal changes in bone mineral density in adolescents. Public Health Nutr. 2014;17(1):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whittle CR, Woodside JV, Cardwell CR, McCourt HJ, Young IS, Murray LJ, Boreham CA, Gallagher AM, Neville CE, McKinley MC. Dietary patterns and bone mineral status in young adults: the Northern Ireland Young Hearts Project. Br J Nutr. 2012;108(8):1494–504. [DOI] [PubMed] [Google Scholar]
- 26. Zeng FF, Xue WQ, Cao WT, Wu BH, Xie HL, Fan F, Zhu HL, Chen YM. Diet-quality scores and risk of hip fractures in elderly urban Chinese in Guangdong, China: a case-control study. Osteoporos Int. 2014;25(8):2131–41. [DOI] [PubMed] [Google Scholar]
- 27. Dai Z, Butler LM, van Dam RM, Ang LW, Yuan JM, Koh WP. Adherence to a vegetable-fruit-soy dietary pattern or the Alternative Healthy Eating Index is associated with lower hip fracture risk among Singapore Chinese. J Nutr. 2014;144(4):511–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zagarins SE, Ronnenberg AG, Gehlbach SH, Lin R, Bertone-Johnson ER. Are existing measures of overall diet quality associated with peak bone mass in young premenopausal women?. J Hum Nutr Diet. 2012;25(2):172–9. [DOI] [PubMed] [Google Scholar]
- 29. de Jonge EA, Kiefte-de Jong JC, de Groot LC, Voortman T, Schoufour JD, Zillikens MC, Hofman A, Uitterlinden AG, Franco OH, Rivadeneira F. Development of a food group-based diet score and its association with bone mineral density in the elderly: The Rotterdam Study. Nutrients. 2015;7(8):6974–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamidi M, Tarasuk V, Corey P, Cheung AM. Association between the Healthy Eating Index and bone turnover markers in US postmenopausal women aged ≥ 45 y. Am J Clin Nutr. 2011;94(1):199–208. [DOI] [PubMed] [Google Scholar]
- 31. Shivappa N, Hebert JR, Karamati M, Shariati-Bafghi SE, Rashidkhani B. Increased inflammatory potential of diet is associated with bone mineral density among postmenopausal women in Iran. Eur J Nutr. 2016;55(2):561–8. [DOI] [PubMed] [Google Scholar]
- 32. Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148(5):507–18. [DOI] [PubMed] [Google Scholar]
- 34. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–20. [DOI] [PubMed] [Google Scholar]
- 35. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–608. [DOI] [PubMed] [Google Scholar]
- 36. Mattei J, Sotos-Prieto M, Bigornia SJ, Noel SE, Tucker KL. The Mediterranean diet score is more strongly associated with favorable cardiometabolic risk factors over 2 years than other diet quality indexes in Puerto Rican adults. J Nutr. 2017;147(4):661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White J, Harris SS, Dallal GE, Dawson-Hughes B. Precision of single vs bilateral hip bone mineral density scans. J Clin Densitom. 2003;6(2):159–62. [DOI] [PubMed] [Google Scholar]
- 38. Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–75. [DOI] [PubMed] [Google Scholar]
- 39. Kelly T. Bone mineral density reference databases for American men and women. J Bone Miner Res. 1990;5(Suppl 1):s249. [Google Scholar]
- 40. Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–89. [DOI] [PubMed] [Google Scholar]
- 41. Brown JP, Albert C, Nassar BA, Adachi JD, Cole D, Davison KS, Dooley KC, Don-Wauchope A, Douville P, Hanley DA et al.. Bone turnover markers in the management of postmenopausal osteoporosis. Clin Biochem. 2009;42(10–11):929–42. [DOI] [PubMed] [Google Scholar]
- 42. Macdonald HM, New SA, Golden MH, Campbell MK, Reid DM. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr. 2004;79(1):155–65. [DOI] [PubMed] [Google Scholar]
- 43. Brandao-Burch A, Utting JC, Orriss IR, Arnett TR. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif Tissue Int. 2005;77(3):167–74. [DOI] [PubMed] [Google Scholar]
- 44. Teucher B, Dainty JR, Spinks CA, Majsak-Newman G, Berry DJ, Hoogewerff JA, Foxall RJ, Jakobsen J, Cashman KD, Flynn A et al.. Sodium and bone health: impact of moderately high and low salt intakes on calcium metabolism in postmenopausal women. J Bone Miner Res. 2008;23(9):1477–85. [DOI] [PubMed] [Google Scholar]
- 45. Perna S, Avanzato I, Nichetti M, D'Antona G, Negro M, Rondanelli M. Association between dietary patterns of meat and fish consumption with bone mineral density or fracture risk: a systematic literature. Nutrients. 2017;9(9): pii:E1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mangano KM, Sahni S, Kiel DP, Tucker KL, Dufour AB, Hannan MT. Bone mineral density and protein-derived food clusters from The Framingham Offspring Study. J Acad Nutr Diet. 2015;115(10):1605–13. e1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2015;115(5):780–800. e785. [DOI] [PubMed] [Google Scholar]
- 48. Lin PH, Ginty F, Appel LJ, Aickin M, Bohannon A, Garnero P, Barclay D, Svetkey LP. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. 2003;133(10):3130–6. [DOI] [PubMed] [Google Scholar]
- 49. Hassoon A, Michos ED, Miller ER, Crisp Z, Appel LJ. Effects of different dietary interventions on calcitriol, parathyroid hormone, calcium, and phosphorus: results from the DASH trial. Nutrients. 2018;10(3): pii:E367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sotos-Prieto M, Mattei J. Mediterranean diet and cardiometabolic diseases in racial/ethnic minority populations in the United States. Nutrients. 2018;10(3): pii:E352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immun Ageing. 2005;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bhupathiraju SN, Tucker KL. Greater variety in fruit and vegetable intake is associated with lower inflammation in Puerto Rican adults. Am J Clin Nutr. 2011;93(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


