Abstract
The prolonged lockdown of health facilities providing non‐urgent gamete cryopreservation—as currently recommended by many reproductive medicine entities and regulatory authorities due to the SARS‐CoV‐2 pandemic will be detrimental for subgroups of male infertility patients. We believe the existing recommendations should be promptly modified and propose that the same permissive approach for sperm banking granted for men with cancer is expanded to other groups of vulnerable patients. These groups include infertility patients (eg, azoospermic and cryptozoospermic) undergoing medical or surgical treatment to improve sperm quantity and quality, as well as males of reproductive age affected by inflammatory and systemic auto‐immune diseases who are about to start treatment with gonadotoxic drugs or who are under remission. In both scenarios, the “fertility window” may be transitory; postponing diagnostic semen analysis and sperm banking in these men could compromise the prospects of biological parenthood. Moreover, we provide recommendations on how to continue the provision of andrological services in a considered manner and a safe environment. Our opinion is timely and relevant given the fact that fertility services are currently rated as of low priority in most countries.
Keywords: azoospermia, male infertility, opinion, SARS‐CoV‐2, semen analysis, sperm banking, systemic auto‐immune diseases
1. INTRODUCTION
Severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) is a novel coronavirus and causative agent of COVID‐19, a disease with potentially dangerous implications for human health. The remarkable increase in the number of infections by SARS‐CoV‐2 worldwide raised the prospect of massive hospitalizations that few healthcare systems would be able to deal with. On this basis, governments across the globe have announced the most far‐reaching restrictions on personal freedom in modern history. The urgent need to avoid a collapse in the healthcare system has been the justification for the implemented measures, and reproductive medicine societies, as well as regulatory authorities, decisively followed by issuing guidance based on expert best judgment. The key recommendations for practitioners include suspension of initiation of new fertility treatment and non‐urgent gamete cryopreservation, as well as suspension of elective surgery and non‐urgent diagnostic procedures. 1 , 2 Sperm banking has been rated as of low priority, indicating that clinical harm is very unlikely if postponed for six months. 3 Exceptions are oncological patients who require urgent fertility preservation.
Taking the above mentioned into account, we would like to raise a viewpoint hardly voiced so far. Our concerns are that, first of all, a prolonged lockdown of andrological services will be detrimental to subgroups of male infertility patients. Secondly, the andrological community is uneasy about how to provide optimal care to our patients without compromising safety. We, therefore, propose remedies to mitigate the consequences of a prolonged cessation of andrological services. The aim is to help authorities and healthcare providers identify which patients might be prioritized for the continuation of andrological services in a safe environment.
2. THE PANDEMIC FACTS
At the time of writing (April 21), the global deaths caused by SARS‐CoV‐2 represent approximately one percent of total deaths expected to occur worldwide over the first three months of the current year, with a wide variation in the reported death rates per country (www.worldometers.info/coronavirus). In total, more than 2.5 million infections by SARS‐CoV‐2 have been reported, 95% of which have been defined as mild. Among the severe or critical cases, the overwhelming majority affects people aged 50 and above. By contrast, the reported death rate among individuals of reproductive age remains low, ranging from 0.2% in China to 0.8% in the United States, with an estimated 1.5:1 male to female ratio, mainly affecting those individuals with pre‐existing conditions, including cardiovascular disease, diabetes, chronic respiratory disease, hypertension, obesity, and cancer. 4
3. THE IMPACT OF SARS‐COV‐2 FOR MALES IN NEED OF SPERM BANKING
While it is prudent to advocate temporary social distancing and closure of non‐emergency health services, we do not know how long this pandemic will last. Estimates ranging from 3 to 12 months have been projected, depending on how effective governments implement quarantine measures and how long it takes to achieve herd immunity. Thus, we would like to consider what a prolonged lockdown of clinics providing andrological services might mean for infertility patients. This consideration will focus primarily on priority recommendations for sperm banking and diagnostic semen analysis for patients seeking fertility rather than donors.
The “time” variable is crucial in specific subgroups of infertile males. Besides reproductive‐age oncological patients, loss of time is particularly consequential among patients under medical treatment aimed at improving sperm quantity or quality and in those with inflammatory or auto‐immune diseases who will either start treatment—with potentially gonadotoxic drugs—or are under the “remission window” of such treatment, as explained in more detail below. In both scenarios, the “fertility window” may be transitory and, therefore, the implications of postponing diagnostic semen analysis and sperm banking in these men could permanently compromise the prospects of biological parenthood. Hence, the provision of andrological services cannot be considered a low priority. Our opinion is particularly important given the fact that healthcare providers are reluctant to recommend assisted conception in most cases—using either fresh or frozen‐thawed spermatozoa—as pregnancy might act as a comorbidity in women affected by SARS‐CoV‐2. 5 , 6
3.1. Cancer patients
Up to 30% of male cancer survivors lose their fertility potential after anti‐cancer therapy. 7 Chemotherapy, radiotherapy, and radical surgical procedures might irreversibly impair spermatogenesis and/or ejaculation. Cancer itself can also affect fertility directly (eg, testicular cancer and Hodgkin's lymphoma). 8 Currently, the only reliable method of fertility preservation in reproductive‐aged men with cancer is sperm banking. 9 Sperm banking must be ideally completed before the start of gonadotoxic therapy. Specimens are usually collected by masturbation and ejaculated spermatozoa are cryopreserved using slow or rapid freezing protocols. Before cryopreservation, the specimen undergoes semen analysis, which is used to both assess the baseline sperm variables (eg, count, motility, and morphology) and to plan. After thawing, it is inevitable that sperm parameters are overall reduced, and such specimens have to be used with intrauterine insemination (IUI) or assisted reproductive technology (ART) to allow these patients to have biological children. 10 The costs associated with sperm banking are relatively low and most cancer patients who banked sperm were found to be pleased by having taken that decision. 11
3.2. Azoospermic/cryptozoospermic males
The most vulnerable male infertility patient during the SARS‐CoV‐2 pandemic is probably the non‐obstructive azoospermic (NOA) or cryptozoospermic patient being medically treated to restore or improve spermatogenesis. An example is the patient with hypogonadotropic hypogonadism (HH), in whom azoospermia results from the lack of adequate testicular stimulation by pituitary gonadotropins. 12 In pre‐pubertal and post‐pubertal HH males, gonadotropin treatment increases testicular size, promotes virilization, and restores spermatogenesis (to varying degrees) in up to 90% of patients, with reported pregnancy rates—either by intercourse or with the aid of IUI or ART—of up to 65%. 12 , 13 , 14 However, the treatment duration is long—typically six months or longer—and expensive as well. Moreover, the follow‐up during treatment requires monitoring serum levels of pituitary and sexual hormones, as well as semen parameters. Sperm banking should be considered in men with HH who respond to therapy, that is, have viable spermatozoa in the ejaculate, in particular, when the continuation of gonadotropin therapy during the SARS‐CoV‐2 pandemic is neither possible (eg, due to economic or logistic reasons), nor desired. In patients who have not responded yet and have economic constraints to continue therapy, the medication dose and regimen could be adjusted (eg, decrease hCG dose and suspend FSH injections) to keep intratesticular as well as serum testosterone levels within lower normal limits.
Another example refers to males with NOA due to spermatogenic failure, including those with rare numbers of spermatozoa occasionally found in the ejaculate (cryptozoospermia), accounting for 60% of the azoospermia cases. 15 Although the condition is untreatable, medical therapy has been explored as a way to optimize or induce spermatogenesis and, thus, increase the likelihood of having spermatozoa retrieved surgically or ejaculated. A few cohort studies have shown that spermatozoa can be occasionally found in the ejaculate after the use of medication for boosting intratesticular testosterone production, like hCG injections—alone or combined with FSH injections—, and estrogen receptor modulators, such as tamoxifen. 16 , 17 , 18 , 19 Similar to HH patients, the continuation of gonadotropin therapy is not always possible nor desired in men with NOA due to spermatogenic failure who require sperm utilization or banking. Moreover, immediate ART might not be an option in some countries with strict lockdown measures during the current SARS‐CoV‐2 pandemic. Thus, sperm banking is urged in NOA patients who respond to medical therapy and present sperm in the ejaculate as a way to preserve fertility and allow future ART. Naturally, semen analyses are required to monitor treatment results and identify who is eligible for sperm banking. Patients achieving cryptozoospermia or severe oligozoospermia after treatment may have a short window for sperm cryopreservation as their semen quality could deteriorate. 20 If the opportunity of sperm banking is lost, surgical sperm retrieval will be required, which could inflict both clinical and financial burdens on patients. Nevertheless, sperm retrieval and cryopreservation of testicular spermatozoa should be considered in specific situations of persistent azoospermia when a narrow window of opportunity exists. Sperm retrieval can be performed on an outpatient basis under local/intravenous anesthesia; the procedure is associated with minimal postoperative complications. 21
Along the same lines, varicocelectomy has been used as an attempt to improve spermatogenesis in NOA men with a coexistent varicocele. Spermatogonia type B, pachytene spermatocytes, and early spermatids are vulnerable to heat stress associated with varicocele. 22 In a systematic review comprising 468 NOA patients with varicocele, 44% of the treated patients had viable ejaculated sperm postoperatively, suitable for ICSI or cryopreservation. 23 These patients should also be monitored with semen analyses, and sperm cryopreservation recommended for those with ejaculate spermatozoa due to the risk of relapse to azoospermia. 24
Lastly, loss of fertility due to a late obstruction has been reported in up to 12% and 50% of men with obstructive azoospermia subjected to vasovasostomy and vasoepididymostomy, respectively. 25 , 26 This situation can also occur after the transrectal resection of the ejaculatory ducts. In both scenarios, semen analysis is used to monitor the patency status postoperatively, and sperm banking should be offered to those patients who experience a continuous decrease in sperm count/quality during the follow‐up as a way to avoid future sperm retrievals. 26 , 27
3.3. Infertile men of advanced paternal age
Infertile men of advanced paternal age (eg, >50 years) have occasionally used sperm banking for planning of medically assisted reproduction. 28 , 29 Given that advanced age is a risk factor for SARS‐CoV‐2 complications, and severe SARS‐CoV‐2 illness might be treated with non‐specific anti‐viral drugs with possible gonadotoxic effects, 30 sperm banking could be offered to those patients who are concerned about acquiring the infection.
3.4. Inflammatory and systemic auto‐immune diseases
At present, the prevailing consensus is to allow gamete cryopreservation to continue for oncological patients. However, males at reproductive age affected by non‐oncological conditions (ie, inflammatory bowel diseases and autoimmune disorders) may also need immediate sperm banking. 31 Gonadotoxic drugs (eg, cyclophosphamide, methotrexate, mycophenolate mofetil, and mTOR inhibitors) are commonly used to control the inflammatory process in such patients.
Inflammatory bowel disease (eg, Crohn's disease and ulcerative colitis) mainly affects young adults, and drugs used for treatment (eg, sulfasalazine, azathioprine, and methotrexate) appear to harm sperm quality. 32 The sulfapyridine metabolite of sulfasalazine impairs semen parameters and increases the production of reactive oxygen species. 32 , 33 Moreover, pregnancy‐related complications and the risk of congenital abnormalities might increase when the father had used azathioprine before conception. 34 , 35 , 36 Also, methotrexate (MTX) is an immunosuppressive agent used to treat inflammatory and auto‐immune diseases with known teratogenic effects. Besides, the antifolate mechanism of MTX decreases DNA synthesis and inhibits cellular proliferation, possibly resulting in oligozoospermia. 37
Likewise, young men may be affected by systemic autoimmune diseases (SADs) (eg, systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, ankylosing spondylitis, dermatomyositis, Behçet disease, psoriasis, among others). 38 In these patients, the chronic inflammation could adversely affect the hypothalamic‐pituitary‐testicular axis and the testicles directly, causing impairment of sperm quality and quantity. However, gonadal dysfunction is primarily related to the effects of immunosuppressive therapy (eg, alkylating agents, methotrexate, and mycophenolate mofetil). 39
Among patients with inflammatory bowel disease or SAD considering fertility preservation, sperm banking is usually conditioned to a temporary discontinuation of therapy for at least 3‐4 months. 35 Several patients might have been planning for this “fertility window” for an extended time, which unfortunately occurred during the SARS‐CoV‐2 pandemic. Sperm banking is, therefore, an option for these patients who are concerned about establishing a pregnancy during the SARS‐CoV‐2 pandemic, in particular, those with semen abnormalities who are candidates for ART. On this basis, we would argue that the same permissive approach that has been granted for men with cancer to enable gamete preservation should be extended to male patients with inflammatory and autoimmune diseases.
4. WHAT ARE THE POSSIBLE REMEDIES?
We need to consider the health and psychological consequences of not offering the above patients andrological services. The lockdown of andrological services may have a devastating psychological impact on men undergoing fertility‐related treatment. Like women, men undergoing fertility treatment may also experience anxiety and stress. 40 , 41 This psychological distress can aggravate the feeling of fear and uncertainty imposed by the SARS‐CoV‐2 pandemic, 42 which might have negative consequences for the reproductive outcome.
The damage to the affected patients is difficult to measure, and it will take months, perhaps years before we can assess the broader implications of the current restrictive measures for patients as well as healthcare providers. While we believe that the various lockdowns will slow the spread of SARS‐CoV‐2, a strict lockdown is unlikely to last too long due to its practicality and pitfalls on other aspects of society, mainly economical. Thus, a certain level of risk of infections by SARS‐CoV‐2 is expected because there will be new cases when measures are relaxed, and no vaccine is likely to be available soon. Therefore, not only urgent short‐term responses, but also long‐term measures are essential.
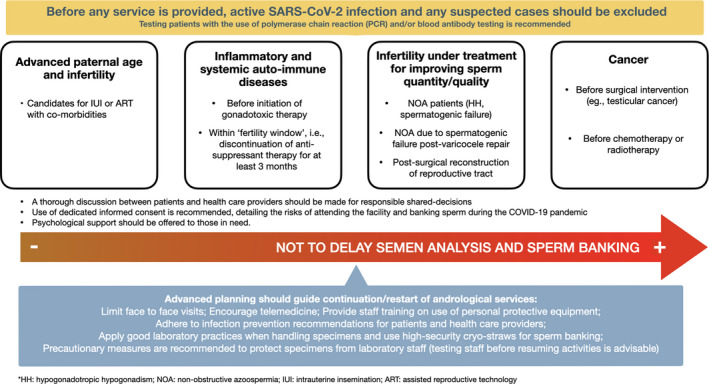
Hence, in this time of uncertainty, denying andrological services from those who need it most might be even worse than the risks of providing them. We, therefore, propose some remedies that we believe might offer fertility providers and patients alike greater autonomy, and that could be used to alleviate the adverse impact of the coronavirus pandemic in the months to come (Figure 1).
Before any service is provided, active SARS‐CoV‐2 infections and suspected cases should be excluded. Testing patients with the use of polymerase chain reaction (PCR) and/or blood antibody testing is recommended before starting sperm banking. Ideally, only samples from patients with negative results or who have acquired herd immunity should be cryopreserved.
-
Andrological services (eg, diagnostic semen analysis and sperm cryopreservation) should not only be available for oncological patients, but also for the group of patients listed below.
Patients with severe male factor infertility under medical or surgical treatment aiming at improving sperm quantity or quality (eg, patients with NOA or cryptozoospermia/severe oligozoospermia, including post‐varicocele repair, and those with evidence of loss of patency after successful surgical reconstruction of the reproductive tract).
Men at reproductive age affected by inflammatory diseases or SADs, that is, before initiation of gonadotoxic therapy or if under the “fertility window” achieved after temporary (at least three months) discontinuation of therapy.
Infertile men older than 50 years, in particular those with comorbidities who are candidates for IUI or ART and are concerned about the risk of acquiring SARS‐CoV‐2 and the possibility of anti‐viral therapy causing gonadotoxic effects.
Surgical sperm retrieval and cryopreservation of testicular spermatozoa should be considered in specific situations (eg., persistent azoospermia) among men with NOA undergoing medical therapy to improve spermatogenesis. In this setting, procedures should be performed, if possible, on an outpatient basis under local anesthesia. The use of electrocautery should be avoided as the surgical smoke might carry the virus if a patient is infected but asymptomatic. Only essential staff should stay in the operating theater, and personal protection measures should be strictly followed as determined by the local healthcare authorities. In closed‐controlled air systems, the airflow might produce an increase in the viral spread from potential asymptomatic patients. Thus, special attention should be given to air quality control, including the use of air filtration systems, particularly in surgical and laboratory areas. 43
Encourage telemedicine and phone counseling for providing instructions about testing and sperm banking.
Adherence to infection prevention recommendations is of utmost importance for patients and health practitioners alike. This advice includes the use of appropriate personal protective equipment (PPE) by healthcare staff, adherence to social distancing measures for healthcare staff and patients, and space out appointments so that no patients are waiting together in the clinic's waiting area. We stress the importance of training staff (receptionists, nurses, technicians, doctors) on PPE needs and usage (please see https://www.cdc.gov/coronavirus/2019‐ncov/hcp/clinic‐preparedness.html ).
Good laboratory practices should be strictly applied when handling sperm/seminal fluid in the andrology laboratory. 44 This advice includes (a) use of class II safety cabinets, 45 which gives protection to the specimen handled as well as the operator performing the work, (b) use of high‐security straws for sperm cryopreservation, as routinely used in most sperm banks, and (c) additional measures to protect the specimens from laboratory staff (eg, use of googles, N95 mask, gown/coverall, and gloves)—who might be asymptomatic for SARS‐CoV‐2.
Technicians/biologists should, ideally, be tested by PCR and/or blood antibody testing before resuming activities, and only staff with negative results or who have acquired herd immunity should perform laboratory duties. If the staff that manipulated specimens get infected, an aliquot of cryopreserved semen samples should be tested (eg, by PCR) because semen samples, cryopreservation media, straws, and pipette tips could have been contaminated by asymptomatic PCR‐positive biologists/technicians.
A thorough discussion between patients and healthcare providers should be made for responsible shared decisions. This advice includes the development and use of a dedicated informed consent, detailing the risks of attending the facility and banking of spermatozoa during the SARS‐CoV‐2 pandemic. Furthermore, psychological support and financial aid might be offered to those in need. The latter might be particularly relevant to patients under economic pressure due to the pandemic who need to afford the costs of semen analysis and sperm banking.
Advanced planning should guide the continuation of andrological services. Working groups and quality managers should determine which patients to prioritize and how working lists should be filled, including staff scheduling.
Figure 1.
SARS‐CoV‐2 pandemic and provision of andrological services: proposal for individualized management
5. PRACTICAL CONSIDERATIONS
During the coming weeks, we should continue to look critically and objectively at the SARS‐CoV‐2 evidence. Although our recommendations are unlikely to create any further burden to the already overwhelmed medical infrastructure, we acknowledge that patients might be reluctant to use andrological services on the basis of fear of being infected or economic reasons. We also realize that much is unknown about SARS‐CoV‐2 and its implication on male reproductive health. The existing data indicate that a subject can be infectious 3‐5 days before the onset of actual symptoms of the viral infection, and the risk of such cases spreading the infection has not been rigorously researched. 46 While testing patients and staff with the use of PCR and/or antibody kits is recommended, the majority of clinics lack prompt access to these tests. Moreover, some countries face a short supply of test kits, which have been made available for symptomatic patients and frontline health providers only. Besides, the accuracy of these tests has been questioned, with some reports suggesting that many of the SARS‐CoV‐2 kits in the market have a false‐negative rate of 30%‐40%. 47 Thus, it remains to be determined how clinics can screen patients and healthcare providers optimally. Likewise, it remains to be decided who—patients or clinics—will assimilate the costs related to testing, PPE, and reduced patient volume due to extra measures instituted to avoid infections. Along these lines, clinics and hospitals providing andrological services have to determine ways of protecting themselves from potential liability issues. Although the overall mortality rate among men at reproductive age remains low, it should be considered that contamination of patients and staff could occur with SARS‐CoV‐2 in the context of asymptomatic shedding. For this reason, it seems sound to advise postponing medical therapy in azoospermic men who had planned to initiate it and who have no pressing concerns (eg, no maternal factors such as advanced maternal age) until it is deemed safe to obtain regular semen analyses, hormone profiles, and banking of spermatozoa. The same reasoning applies to semen analysis and sperm banking in men under therapy who opt to continue on medication till the pandemic ends.
At present, limited data exist about potential routes of SARS‐CoV‐2 infection in respiratory, cardiovascular, digestive, urinary, and reproductive systems. In this regard, data of virus load in semen or testicular biopsies of SARS‐CoV‐2 infected patients is minimal. 48 , 49 , 50 Nevertheless, angiotensin‐converting enzyme 2 (ACE2) receptors, used by the virus to enter host cells, exist in spermatogonia, Sertoli cells, and Leydig cells. 51 , 52 Also, previous reports suggested that other coronaviruses, like the SARS coronavirus, could cause orchitis. 53
As for pregnancy with the use of banked or fresh ejaculate spermatozoa during the SARS‐CoV‐2 pandemic, it has been suggested that pregnant women might be at a higher risk of developing complications, including miscarriage, preeclampsia, and preterm birth. 5 , 6 However, the evidence is still limited, and we, therefore, abstain from making recommendations about the use of fresh or banked spermatozoa for assisted conception during the pandemic until more data are available. Naturally, the use of spermatozoa for assisted conception—either fresh or frozen‐thawed—would not be recommended in most cases if it is confirmed that pregnancy acts as an important comorbidity factor. Notwithstanding these observations, it should be acknowledged that serology testing, once properly validated and widely available, will be helpful to identify immune patients that could be allowed for treatment. 54 These patients have little risk of either pregnancy complications or propagating the disease when attending fertility clinics. Nevertheless, the provision of andrological services should only be undertaken if the medical infrastructure can support them. We reiterate the above recommendations that care should only be restarted if social distancing can be maintained, areas regularly disinfected, and screening for signs and symptoms of the infection undertaken before allowing patients into the facility in accordance with guidance issued by health regulatory authorities.
6. CONCLUSIONS
We propose remedies to mitigate the consequences of a prolonged cessation of andrological services due to the SARS‐CoV‐2 pandemic to vulnerable subgroups of male infertility patients. In a moment when the reorganization of healthcare services is focused on supporting SARS‐CoV‐2 patients who might need critical care, limiting burdens for national health systems could still represent a relevant issue. We advocate that correct identification of the more “time‐sensitive” cases is crucial for regulating the continuation of andrological services, including diagnostic semen analysis and sperm banking. Moreover, we provide recommendations on how to most optimally provide care to our patients—without compromising safety—once andrological services are resumed. The aim is to help authorities and healthcare providers identify which patients might be prioritized during the SARS‐CoV‐2 pandemic for the continuation of andrological services in a safe environment.
CONFLICT OF INTEREST
SCE and CA declare the receipt of unrestricted research grants and lecture fees from Merck outside the submitted work. SEML is an employee of Examenlab Ltd., a university spin‐out company with a commercial interest in sperm DNA damage. FL, NG, JA, AZ, GMC, JK‐B, LB, AH, C‐LC, PV, JH, EA, MC, FCB, RCF, RS, RL, AMM, SKJ, SP, RR, PH, JLY, and AA declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
SCE contributed to the conception, designed the manuscript, and wrote the first draft. JA, NG, FB, GP, AM, C‐LC, PV, JH, MC, JY, and AA wrote sections of the manuscript. All authors contributed to manuscript revision and critical analysis, read and approved the submitted version. The corresponding author takes the final responsibility for the decision to submit the manuscript for publication.
ACKNOWLEDGMENTS
None.
Esteves SC, Lombardo F, Garrido N, et al. SARS‐CoV‐2 pandemic and repercussions for male infertility patients: A proposal for the individualized provision of andrological services. Andrology.2021;9:10–18. 10.1111/andr.12809
REFERENCES
- 1. American Society for Reproductive Medicine (ASRM) . American Society for Reproductive Medicine Patient Management And Clinical Recommendations during The Coronavirus (Covid‐19) Pandemic. 2020. https://www.asrm.org/news‐and‐publications/covid‐19/statements/patient‐management‐and‐clinical‐recommendations‐during‐the‐coronavirus‐covid‐19‐pandemic/ Accessed April 14, 2020.
- 2. European Society for Human Reproduction and Embryology (ESHRE) . Coronavirus Covid‐19: ESHRE statement on pregnancy and conception. 2020.
- 3. European Association of Urology (EAU) COVID‐19 Recommendations . EAU Guidelines Office Rapid Reaction Group: An organization‐wide collaborative effort to adapt the EAU guidelines recommendations to the COVID era. https://uroweb.org/wp‐content/uploads/Combined‐non‐oncology‐COVID‐recommendations.pdf Accessed April 18, 2020. [DOI] [PMC free article] [PubMed]
- 4. COVID‐10 Coronavirus Pandemic . https://www.worldometers.info/coronavirus/ Accessed April 16, 2020.
- 5. Yu N, Li W, Kang Q,, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID‐19 in Wuhan, China: a retrospective, single‐centre, descriptive study. Lancet Infect Dis. 2020;20(5):559‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson MD, Cooper AR, Jungheim ES, Lanzendorf SE, Odem RR, Ratts VS. Sperm banking for fertility preservation: a 20‐year experience. Eur J Obstet Gynecol Reprod Biol. 2013;170:177‐182. [DOI] [PubMed] [Google Scholar]
- 8. Xu R, Centola GM, Tanrikut C. Genitourinary cancer patients have worse baseline semen parameters than healthy sperm bankers. Andrology. 2019;7:449‐453. [DOI] [PubMed] [Google Scholar]
- 9. Agarwal A, Ong C, Durairajanayagam D. Contemporary and future insights into fertility preservation in male cancer patients. Transl Androl Urol. 2014;3:27‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freour T, Mirallie S, Jean M, Barriere P. Sperm banking and assisted reproductive outcome in men with cancer: a 10 years' experience. Int J Clin Oncol. 2012;17:598‐603. [DOI] [PubMed] [Google Scholar]
- 11. Williams DH. Sperm banking and the cancer patient. Ther Adv Urol. 2010;2:19‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fraietta R, Zylberstejn DS, Esteves SC. Hypogonadotropic hypogonadism revisited. Clinics (Sao Paulo). 2013;68(Suppl 1):81‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang L, Zhang SX, Dong Q, Xiong ZB, Li X. Application of hormonal treatment in hypogonadotropic hypogonadism: more than ten years experience. Int Urol Nephrol. 2012;44:393‐399. [DOI] [PubMed] [Google Scholar]
- 14. Behre HM. Clinical Use of FSH in Male Infertility. Front Endocrinol (Lausanne). 2019;10:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esteves SC. Clinical management of infertile men with nonobstructive azoospermia. Asian J Androl. 2015;17:459‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moein MR, Tabibnejad N, Ghasemzadeh J. Beneficial effect of tamoxifen on sperm recovery in infertile men with nonobstructive azoospermia. Andrologia. 2012;44(Suppl 1):194‐198. [DOI] [PubMed] [Google Scholar]
- 17. Shinjo E, Shiraishi K, Matsuyama H. The effect of human chorionic gonadotropin‐based hormonal therapy on intratesticular testosterone levels and spermatogonial DNA synthesis in men with non‐obstructive azoospermia. Andrology. 2013;1:929‐935. [DOI] [PubMed] [Google Scholar]
- 18. Hussein A, Ozgok Y, Ross L, Rao P, Niederberger C. Optimization of spermatogenesis‐regulating hormones in patients with non‐obstructive azoospermia and its impact on sperm retrieval: a multicentre study. BJU Int. 2013;111(3b):E110‐E114. [DOI] [PubMed] [Google Scholar]
- 19. Laursen RJ, Elbaek HO, Povlsen BB, et al. Hormonal stimulation of spermatogenesis: a new way to treat the infertile male with non‐obstructive azoospermia? Int Urol Nephrol. 2019;51:453‐456. [DOI] [PubMed] [Google Scholar]
- 20. Koscinski I, Wittemer C, Lefebvre‐Khalil V, Marcelli F, Defossez A, Rigot JM. Optimal management of extreme oligozoospermia by an appropriate cryopreservation programme. Hum Reprod. 2007;22:2679‐2684. [DOI] [PubMed] [Google Scholar]
- 21. Miyaoka R, Orosz JE, Achermann AP, Esteves SC. Methods of surgical sperm extraction and implications for assisted reproductive technology success. Panminerva Med. 2019;61:164‐177. [DOI] [PubMed] [Google Scholar]
- 22. Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele‐associated male infertility: part 1. Nat Rev Urol. 2012;9:678‐690. [DOI] [PubMed] [Google Scholar]
- 23. Esteves SC, Miyaoka R, Roque M, Agarwal A. Outcome of varicocele repair in men with nonobstructive azoospermia: systematic review and meta‐analysis. Asian J Androl. 2016;18:246‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pasqualotto FF, Sobreiro BP, Hallak J, Pasqualotto EB, Lucon AM. Induction of spermatogenesis in azoospermic men after varicocelectomy repair: an update. Fertil Steril. 2006;85:635‐639. [DOI] [PubMed] [Google Scholar]
- 25. Carbone DJ Jr, Shah A, Thomas AJ Jr, Agarwal A. Partial obstruction, not antisperm antibodies, causing infertility after vasovasostomy. J Urol. 1998;159:827‐830. [PubMed] [Google Scholar]
- 26. Farber NJ, Flannigan R, Li P, Li PS, Goldstein M. The kinetics of sperm return and late failure following vasovasostomy or vasoepididymostomy: a systematic review. J Urol. 2019;201:241‐250. [DOI] [PubMed] [Google Scholar]
- 27. Esteves SC, Miyaoka R, Agarwal A. Surgical treatment of male infertility in the era of intracytoplasmic sperm injection ‐ new insights. Clinics (Sao Paulo). 2011;66:1463‐1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jennings MO, Owen RC, Keefe D, Kim ED. Management and counseling of the male with advanced paternal age. Fertil Steril. 2017;107:324‐328. [DOI] [PubMed] [Google Scholar]
- 29. Bertoncelli Tanaka M, Agarwal A, Esteves SC. Paternal age and assisted reproductive technology: problem solver or trouble maker? Panminerva Med. 2019;61:138‐151. [DOI] [PubMed] [Google Scholar]
- 30. Drobnis EZ, Nangia AK. Antivirals and male reproduction. Adv Exp Med Biol. 2017;1034:163‐178. [DOI] [PubMed] [Google Scholar]
- 31. Choux C, Cavalieri M, Barberet J, et al. Immunosuppressive therapy and fertility preservation: Indications and methods. Rev Med Interne. 2018;39:557‐565. [DOI] [PubMed] [Google Scholar]
- 32. Shin T, Kobori Y, Suzuki K, et al. Inflammatory bowel disease in subfertile men and the effect of mesalazine on fertility. Syst Biol Reprod Med. 2014;60:373‐376. [DOI] [PubMed] [Google Scholar]
- 33. Alonso V, Linares V, Bellés M, et al. Sulfasalazine induced oxidative stress: a possible mechanism of male infertility. Reprod Toxicol. 2009;27:35‐40. [DOI] [PubMed] [Google Scholar]
- 34. Rajapakse RO, Korelitz BI, Zlatanic J, Baiocco PJ, Gleim GW. Outcome of pregnancies when fathers are treated with 6‐mercaptopurine for inflammatory bowel disease. Am J Gastroenterol. 2000;95:684‐688. [DOI] [PubMed] [Google Scholar]
- 35. Palomba S, Sereni G, Falbo A, et al. Inflammatory bowel diseases and human reproduction: a comprehensive evidence‐based review. World J Gastroenterol. 2014;20:7123‐7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nørgård B, Pedersen L, Jacobsen J, Rasmussen SN, Sørensen HT. The risk of congenital abnormalities in children fathered by men treated with azathioprine or mercaptopurine before conception. Aliment Pharmacol Ther. 2004;19:679‐685. [DOI] [PubMed] [Google Scholar]
- 37. Sands K, Jansen R, Zaslau S, Greenwald D. Review article: the safety of therapeutic drugs in male inflammatory bowel disease patients wishing to conceive. Aliment Pharmacol Ther. 2015;41(9):821‐834. [DOI] [PubMed] [Google Scholar]
- 38. Brubaker WD, Li S, Baker LC, Eisenberg ML. Increased risk of autoimmune disorders in infertile men: analysis of US claims data. Andrology. 2018;6:94‐98. [DOI] [PubMed] [Google Scholar]
- 39. Tiseo BC, Cocuzza M, Bonfa E, Srougi M, Silva CA. Male fertility potential alteration in rheumatic diseases: a systematic review. Int Braz J Urol. 2016;42:11‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peterson BD, Newton CR, Feingold T. Anxiety and sexual stress in men and women undergoing infertility treatment. Fertil Steril. 2007;88:911‐914. [DOI] [PubMed] [Google Scholar]
- 41. Boivin J, Schmidt L. Infertility‐related stress in men and women predicts treatment outcome 1 year later. Fertil Steril. 2005;83:1745‐1752. [DOI] [PubMed] [Google Scholar]
- 42. Wang C, Pan R, Wan X, et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID‐19) epidemic among the general population in China. Int J Environ Res Public Health. 2020;17(5):1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Esteves SC, Bento FC. Implementation of air quality control in reproductive laboratories in full compliance with the Brazilian Cells and Germinative Tissue Directive. Reprod Biomed Online. 2013;26:9‐21. [DOI] [PubMed] [Google Scholar]
- 44. Esteves SC, Agarwal A. Explaining how reproductive laboratories work. In: Bento FC, Esteves SC, Agarwal A, eds. Quality Management in ART Clinics: a Practical Guide, 1st edn. New York: Springer; 2013:79‐127. [Google Scholar]
- 45. Ramstorp M. What is a clean room? In: Esteves SC, Varghese A, Worrilow KC eds. Clean Room Technology in ART Clinics: A Practical Guide, 1st edn. Boca Raton: CRC Press; 2017:3‐18. [Google Scholar]
- 46. Yuen KS, Ye ZW, Fung SY, Chan CP, Jin DY. SARS‐CoV‐2 and COVID‐19: The most important research questions. Cell Biosci. 2020;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. West CP, Montori VM, Sampathkumar P. COVID‐19 testing: the threat of false‐negative results. Clin Proc. 2020. Ahead of print April 11, pii: S0025‐6196(20)30365‐7. 10.1016/j.mayocp.2020.04.004 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Song C, Wang Y, Li W, et al. Detection of 2019 novel coronavirus in semen and testicular biopsy specimen of COVID‐19 patients. https://www.medrxiv.org/content/10.1101/2020.03.31.20042333v2.article‐info Accessed April 18, 2020.
- 49. Pan F, Xiao X, Guo J, et al. No evidence of SARS‐CoV‐2 in semen of males recovering from COVID‐1. Fertil Steril. Ahead of print April 17, 10.1016/j.fertnstert.2020.04.024. [DOI] [Google Scholar]
- 50. Paoli D, Pallotti F, Colangelo S, et al. Study of SARS‐CoV‐2 in semen and urine samples of a volunteer with positive naso‐pharyngeal swab. J Endocrinol Invest. 2020. 10.1007/s40618-020-01261-1 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pan PP, Zhan QT, Le F, Zheng YM, Jin F. Angiotensin‐converting enzymes play a dominant role in fertility. Int J Mol Sci. 2013;14:21071‐21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Z, Xu X. scRNA‐seq profiling of human testes reveals the presence of the ACE2 receptor, A target for SARS‐CoV‐2 infection in spermatogonia, leydig and sertoli cells. Cells. 2020;9(4):920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu J, Qi L, Chi X, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006;74:410‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Petherick A. Developing antibody tests for SARS‐CoV‐2. Lancet. 2020;395:1101‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]


